
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 31 August 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1225466
This article is part of the Research Topic Biodiversity Conservation and Sustainable Development of Protected Areas View all 12 articles
 Yaoyao Zhang1
Yaoyao Zhang1 Xuechun Zhao1,2*
Xuechun Zhao1,2* Lingjun Wang3
Lingjun Wang3 Zhaoyi Wang1
Zhaoyi Wang1 Honggang Shuai1
Honggang Shuai1 Yuefeng Wang1
Yuefeng Wang1 Baocheng Jin1
Baocheng Jin1 Chao Chen1*
Chao Chen1*Tree base sprouting is the main reproduction and expansion mode of Rhododendron plants. By leveraging the plot survey method, the species composition, community renewal, and species diversity in three Rhododendron shrub communities in control, and before and after clearcutting (CK, D3yr, and D6yr, respectively) were studied. Moreover, the dissimilarity of Rhododendron communities in CK, D3yr and D6yr were analyzed. The results showed that there were 26 plant species belonging to 14 families and 22 genera, in 3 communities in total, with 19 species of shrub plants and 7 species of herbaceous plants. The number of species increased from 13 in CK to 23 in D3yr and then decreased to 20 in D6yr. The height and coverage of D3yr and D6yr reached 39.3% and 58.9% of that of CK, respectively. The relative height of CK, D3yr, and D6yr was 43.79%, 65.4%, and 58.54%, respectively. The coverage of D3yr and D6yr reached 60.8% and 114.70% of that of CK, respectively. The relative coverage of CK, D3yr, and D6yr was 19.05%, 83.24%, and 77.32%, respectively. The important value of Rhododendron plants in the communities increased from 0.42 in CK to 0.74 in D3yr, and then decreased to 0.67 in D6yr. The α diversity in the shrub layer of D3yr and D6yr were generally lower than those of CK except Pielou evenness index. The β diversity indicates that the similarity between CK and D3yr was lower, that between CK and D6yr was moderate, and that between D3yr and D6yr was higher. The sprouting height and coverage of Rhododendron plants was significantly correlated with age and sprouting time. The sprouting ability of Rhododendron plants increased first and then decreased with age, while the sprouting ability of Rhododendron plants with age of 10–12 years was the strongest. Clearcutting measures can improve the dominance of Rhododendron plants in the communities, promote the sprouting and renewal of Rhododendron population, and accelerate the succession rate of communities.
Rhododendron is one of the largest genera of angiosperms with more than 1200 species worldwide, which have important ecological and socio-economic applications of ornamental, cultural, scientific, economic, and medicinal value (MacKay and Gardiner, 2017; Li et al., 2018; Ahmad et al., 2021). The genus is mostly distributed in subtropical evergreen broad-leaved mountain forests, mixed coniferous broad-leaved forests, coniferous forests, and dark coniferous forests at altitudes varying from 1000 to 3800 m (Fang and Min, 1995). It only forms a single species of Rhododendron shrub or Rhododendron dwarf forest in certain high mountains above the tree line (Sun, 2002). Guizhou Province is located at the edge of the distribution center of modern Rhododendron (Hengduan Mountain region in China) and its transition zone along the eastern distribution range (Dai et al., 2020). More than 110 species of Rhododendron plants are distributed naturally in Guizhou Province (Dai et al., 2020). Northwest Guizhou Province is a vital area for the distribution of Rhododendron, with six subgenera and more than fifty species accounting for approximately 50% of the total Rhododendron population in Guizhou. The Baili Rhododendron National Nature Reserve at the junction of Qianxi and Dafang counties is the most representative, with 43 species of Rhododendron plants (Wang et al., 2010; Chen et al., 2013). It is a typical representative of the largest and contiguously distributed natural wild Rhododendron communities in the world (Rong et al., 2009) which may play an important role in understanding Rhododendron shrub ecosystem processes, succession, and shrub management. However, both the quantity of seedlings and the renewal rate of Rhododendron plants are low (Yang et al., 2020a). Meanwhile, it is easy for Rhododendron plants to be replaced by highly competitive trees and other shrubs because of succession (Bian et al., 2006). Coupled with global changes in recent years, Rhododendron plants, particularly some narrowly distributed Rhododendron species, have begun to decline or even die (Ma et al., 2014; Liu et al., 2019; Yu et al., 2019; Bitayan et al., 2021).
Sprouting renewal primarily refers to dry base sprouting and underground stem sprouting (Lu et al., 2021). New plants are formed via dormant or adventitious buds from underground stems and stubble of trees to achieve forest renewal (Vesk and Westoby, 2004). Sprouting plants can use nutrients in the soil more effectively through their strong root system, which usually grows faster than seedlings and has stronger adaptability (Kauffman, 1991; Chen et al., 2019). Owing to the weak competitiveness and high canopy density of Rhododendron shrub communities in northwest Guizhou, seed renewal of the Rhododendron population is difficult, and tree-based sprouting renewal is the main mode of reproduction and expansion (Kong et al., 2019). At present, the primary measures to promote tree-based sprouting renewal are controlled forest fires, clearcutting and other natural disasters such as hurricane, mudslides (Li, 1992; Luoga et al., 2004; Subedi et al., 2019). However, forest fires are harmful as these reduce the sprouting ability of shrub communities (Tang et al., 2001). In contrast, planned clearcutting promotes the growth of shrub tillering branches, regenerates and rejuvenates plant clusters, and increases the canopy width and biomass (Shang et al., 2020). Following clearcutting, sprouting plants usually grow faster than seedlings (Vesk and Westoby, 2004).
Research on sprouting renewal of Rhododendron plants in global started late at present; further, research on sprouting and stress tolerance of wild Rhododendron plants is limited, decreasing the promotion and utilization of wild Rhododendron plants resources (MacKay and Gardiner, 2016). Although several studies have reported renewal of Rhododendron shrubs by disturbance, altitude, and climate changes (Singh et al., 2019; Choudhary et al., 2021; Jia et al., 2021), there is limited knowledge regarding the sprouting renewal of Rhododendron plants by clearcutting. In this study, the effects of rational clearcutting on the sprouting renewal of Rhododendron communities were studied to determine the effect of clearcutting–sprouting on the role of Rhododendron plants in Rhododendron shrub communities. The objectives were to (1) Can clearcutting promote the community renewal of Rhododendron shrubs? (2) Does age affect the response of Rhododendron shrubs to clearcutting? (3) Can the sprouting and regeneration of Rhododendron be predicted after clearcutting?
The study area is in the Tiaohuapo Scenic Spot in the Baili Rhododendron National Nature Reserve, Guizhou Province, China (27°23′22′′N and 105°51′52′′E), near the Jiaozi Mountain, at an altitude of 1700–1900 m. The climate of the region can be characterized as subtropical humid monsoon climate, with an annual average temperature and precipitation of 11.8°C, and 1150.4 mm, respectively, a frost-free period of 257 days, and an annual sunshine duration of 1335.5 h. Zonal vegetation is dominated by the evergreen broad-leaved mountain forest. The existing vegetation is primarily Rhododendron shrub species exhibiting succession and transitional characteristics (Li and Chen, 2005; Jiang et al., 2015). Among Rhododendron shrubs, R. delavayi, R. annae, and Lyonia ovalifolia are the main dominant species. The soil type is primarily yellow soil with an acidic pH (4.61–5.32).
In August 2015, three 20 m × 20 m fixed quadrats were set in the study area to represent the control community (CK), and three 5 m × 5 m survey quadrats were set diagonally in each quadrat. The species, plant number, branch number, height, and canopy width of the shrubs as well as the species and number of the herbaceous plants in the quadrats were recorded. Each Rhododendron shrub in the fixed quadrat was labelled.
In conjunction with the guidelines of Management Office of the Baili Rhododendron National Nature Reserve, all plants in the fixed quadrat were deforested and recovered. The shrubs in the fixed quadrat were deforested in December 2015 and the stubble height was recorded as approximately 15 cm. The number of stubbles was also recorded. The age of shrubs was determined based on the rings of the main pile of shrubs. All other plants were deforested and eliminated.
The same method was used to survey and record the sprouting number, height, and canopy width of Rhododendron shrubs in August 2018 and August 2021. Moreover, the species number, height, and canopy width of other shrubs, as well as the species and number of herbaceous plants in the quadrats, were also surveyed. The communities surveyed in 2018 and 2021 were denoted as D3yr (Community 3 years after clearcutting) and D6yr (Community 6 years after clearcutting), respectively.
IV of the shrub layer for plant species in the three annual communities (CK, D3yr, and D6yr) was calculated using the equation (Wang et al., 2023)
where RH denotes the relative height, RC denotes the relative coverage, and RD denotes the relative density.
The Margalef richness index (R), Simpson diversity index (D), Shannon–Wiener diversity index (H), and Pielou evenness index (J) were determined to measure the α-diversity of the three communities using the equations (Zhang et al., 2017)
where Pi denotes the relative IV of the ith species, S denotes the number of species, and N denotes the number of individual plants in the quadrat.
The Cody index (βc), Whittaker index (βws), Jaccard index (Cj), and Sorenson index (Cs) were determined to measure the β-diversity of the three communities before and after clearcutting using the equations (Baselga, 2010)
where βc and βws denote the dissimilarity measures, Cj and Cs denote the similarity measures, g(H) denotes the number of species increased along the time gradient H, L(H) denotes the number of plant species existing in the previous community but lost in the next community, ms denotes the total number of species recorded in the three communities, ma denotes the average number of species in each community, a denotes the number of species in community A, b denotes the number of species in community B, and j denotes the number of species shared by communities A and B.
The ratio between the number of Rhododendron sprouts and stumps after clearcutting was determined to measure the ability of Rhododendron plant to rejuvenate and renew using the equation
where RS denotes sprouting ability, ES denotes the number of sprouts, and NS denotes the number of stubbles.
SPSS 26.0 software was used to measure the correlation (Pearson correlation) and linear regression analysis between the height and coverage as well as clearcutting time and age of Rhododendron. Analysis of variance was performed (p < 0.05 indicates significant differences) to determine the difference in IV of community species and biodiversity indices between the different stages of Rhododendron shrubs. All plots were prepared using SigmaPlot 14.0.
The three communities comprised 26 species, including nineteen species of shrubs and seven species of herbaceous plants, belonging to 14 families and 22 genera (Tables 1, 2). Ericaceae, Rosaceae, and Theaceae accounted for the highest number of species, specifically 19.23%, 15.38%, and 11.54% of the total, respectively. Three species of Rhododendron plants accounted for 11.54% of the total species.
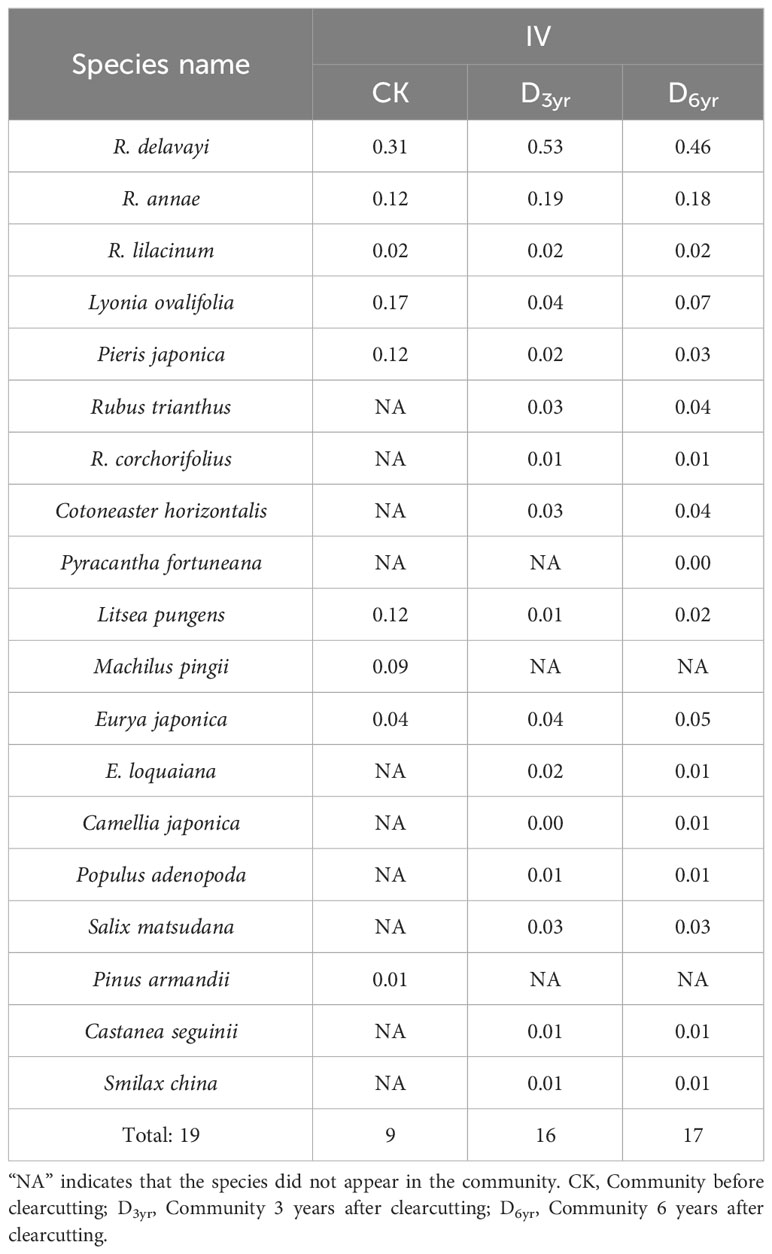
Table 2 Effects of clearcutting on importance values (IVs) of species in the shrub layer of Rhododendron shrub communities.
CK comprised 13 species belonging to seven families (including Ericaceae, Lauraceae, Theaceae, and Poaceae) and 11 genera. Four species were identified in the herbaceous layer and nine in the shrub layer of which five viz., R. delavayi, L. ovalifolia, R. annae, and P. japonica of Ericaceae and L. pungens of Lauraceae exhibited IVs > 0.1 (0.31, 0.17, 0.12, 0.12, and 0.12, respectively). R. delavayi was the dominant species in the shrub layer of CK, and L. ovalifolia was the subdominant species.
D3yr comprised the highest number of species, with 23 species from 12 families (including Ericaceae, Rosaceae, Theaceae, and Poaceae) and 19 genera. There were seven species in the herbaceous layer and 16 in the shrub layer of which two, R. delavayi and R. annae of Ericaceae, exhibited IVs of > 0.1 (0.53 and 0.19, respectively). R. delavayi was the dominant species in the shrub layer of D3yr, and R. annae was the subdominant species.
D6yr comprised 20 species belonging to nine families (including Ericaceae, Rosaceae, Theaceae, and Poaceae) and 16 genera. There were three species in the herbaceous layer and seventeen in the shrub layer. Consistent with D3yr, two species in the shrub layer, R. delavayi and R. annae, exhibited IVs > 0.1 (0.46 and 0.18, respectively). R. delavayi was the dominant species in the shrub layer of D6yr, and R. annae was the subdominant species.
In the CK→D3yr→D6yr community sequence, the height and coverage of Rhododendron shrubs first decreased and then increased (Figure 1). The height decreased significantly from 191.87 cm in CK to 75.61 cm in D3yr and then recovered to 116.71 cm in D6yr. The coverage decreased significantly from 68.46% in CK to 37.89% in D3yr and then increased significantly to 73.28% in D6yr. The relative height increased from 43.79% in CK to 65.40% in D3yr and then decreased to 58.54% in D6yr. The relative coverage increased from 49.05% in CK to 83.24% in D3yr and then decreased to 77.32% in D6yr. The height of Rhododendron species in the three communities were significantly different (p < 0.05). The coverage in D3yr was significantly different from that in CK and D6yr (p< 0.05). The relative height and coverage in CK were both significantly different from those in D3yr and D6yr (p < 0.05).
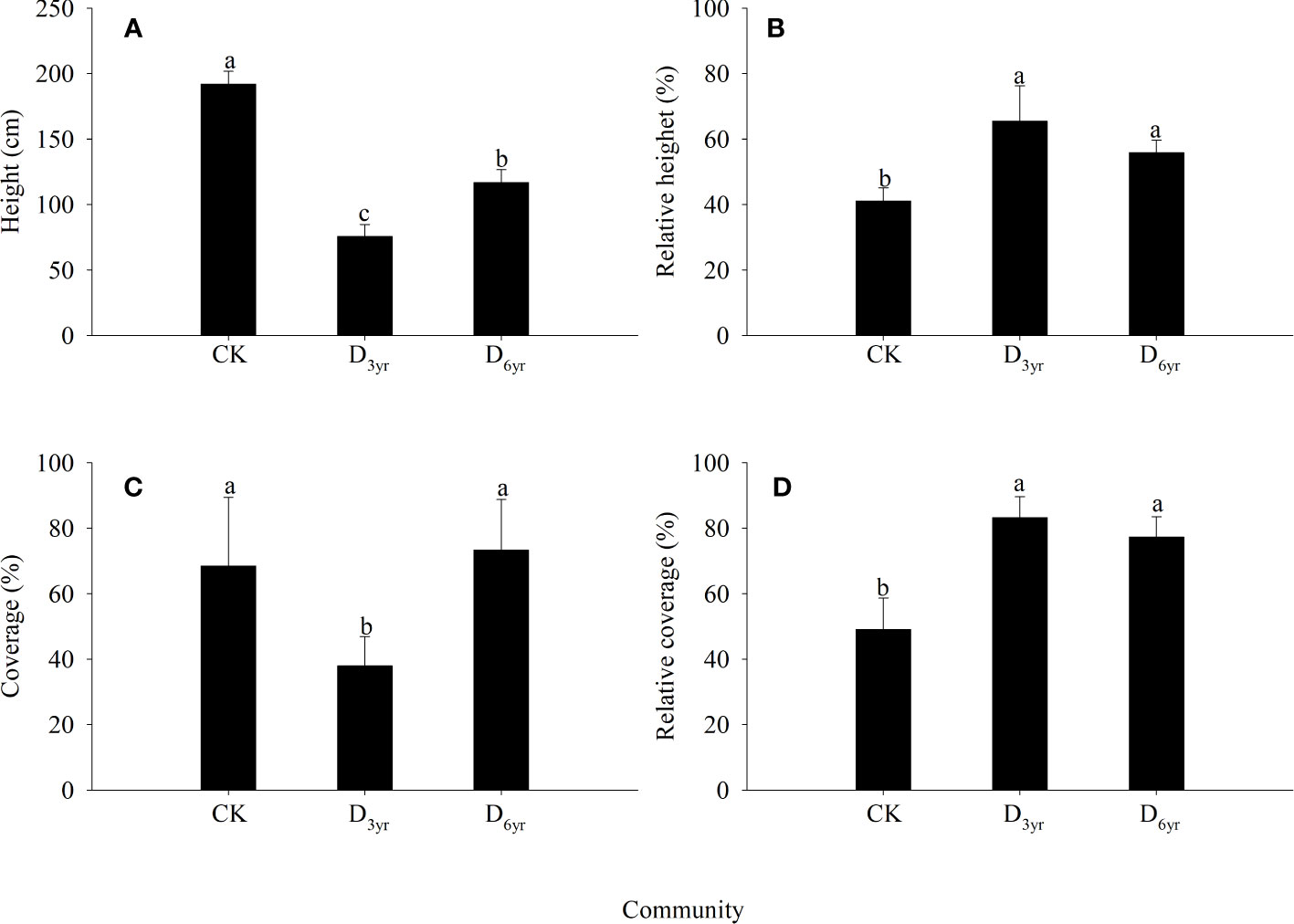
Figure 1 Effects of clearcutting on the height and coverage of Rhododendron shrubs. Height (A); Relative height (B); Coverage (C); Relative coverage (D). (A–C) indicate significant difference (p< 0.05). CK, control; D3yr, community in 2018; D6yr, community in 2021.
The Margalef richness index values of species in the shrub layers of the three communities were significantly different (p < 0.05) (Figure 2). However, the differences in the Simpson diversity, Shannon–Wiener diversity, and Pielou evenness indices were not significant (p > 0.05). The values of all four indices were the highest in CK and lowest in D3yr. The values of the Margalef richness, Simpson diversity, Shannon–Wiener diversity, and Pielou evenness indices of species in D3yr were 61.15%, 80.30%, 84.79%, and 96.61% of that of species in CK, respectively.
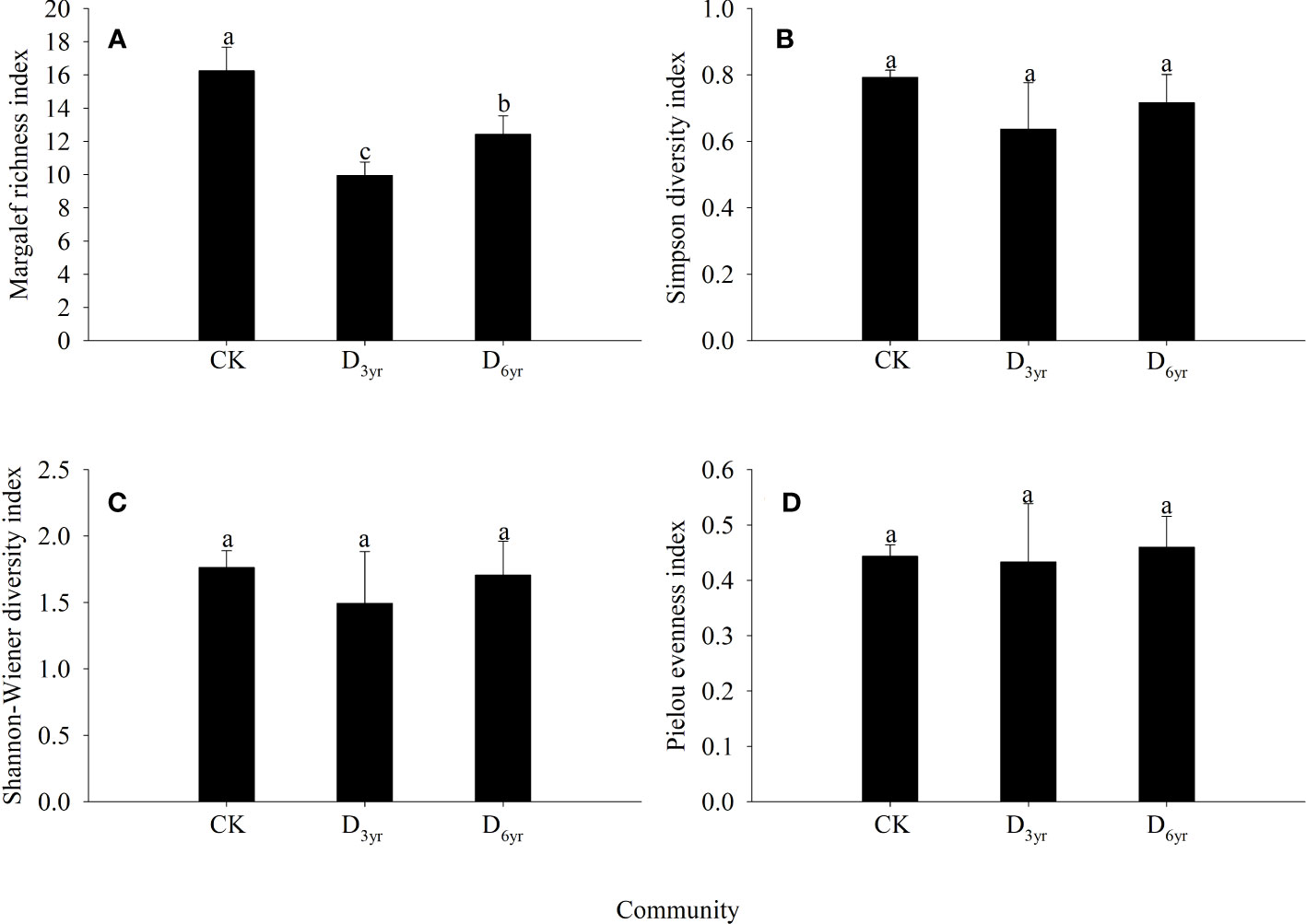
Figure 2 Effects of clearcutting on α-diversity of Rhododendron shrub communities. Margalef richness index (A); Simpson diversity index (B); Shannon–Wiener diversity index (C); Pielou evenness index (D). (A–C) significant difference (p< 0.05). CK, control; D3yr, community in 2018; D6yr, community in 2021.
As CK→D3yr→D6yr progressed, the dissimilarity coefficients of the three communities showed a decreasing trend (Table 3). Both Cody and Whittaker indices were the highest in CK and D3yr (3.83 and 1.65, respectively) and the lowest in D3yr and D6yr (0.83 and 1.20, respectively). The similarity indices, the Jaccard and Sorenson indices, were the highest in D3yr and D6yr (0.87 and 0.93, respectively) and the lowest in CK and D3yr (0.38 and 0.55, respectively). The similarity between CK and D3yr was low, that between CK and D6yr was moderate, and that between D3yr and D6yr was high.

Table 3 Effects of clearcutting on the β-diversity index of species in Rhododendron shrub communities.
The height and coverage as well as the sprouting time and age of Rhododendron shrubs were significantly and positively correlated (p < 0.01). The correlation degree of sprouting time was higher than that of the age of Rhododendron shrubs (Table 4).
The sprouting time (t) and age (a) are the two main factors that affect clearcutting sprouting of Rhododendron shrubs. The linear relationships between sprouting height (h), sprouting coverage (c), sprouting time (t), and age (a) of Rhododendron shrubs after clearcutting were well fitted (R² = 0.65, p < 0.01 and R² = 0.50, p < 0.01, respectively) (Table 5).

Table 5 Regression model of sprouting height, sprouting coverage, and sprouting time of Rhododendron shrubs.
The height of Rhododendron shrubs in CK, D3yr, and D6yr showed a gradual increasing trend with age (Figure 3). Compared with CK, the height of Rhododendron shrubs in D3yr and D6yr was lesser and increased slowly. The coverage rates of Rhododendron shrubs in the three communities also increased gradually with increasing age. The coverage rates of Rhododendron shrubs in D3yr and D6yr at the early stage were higher than those in CK.
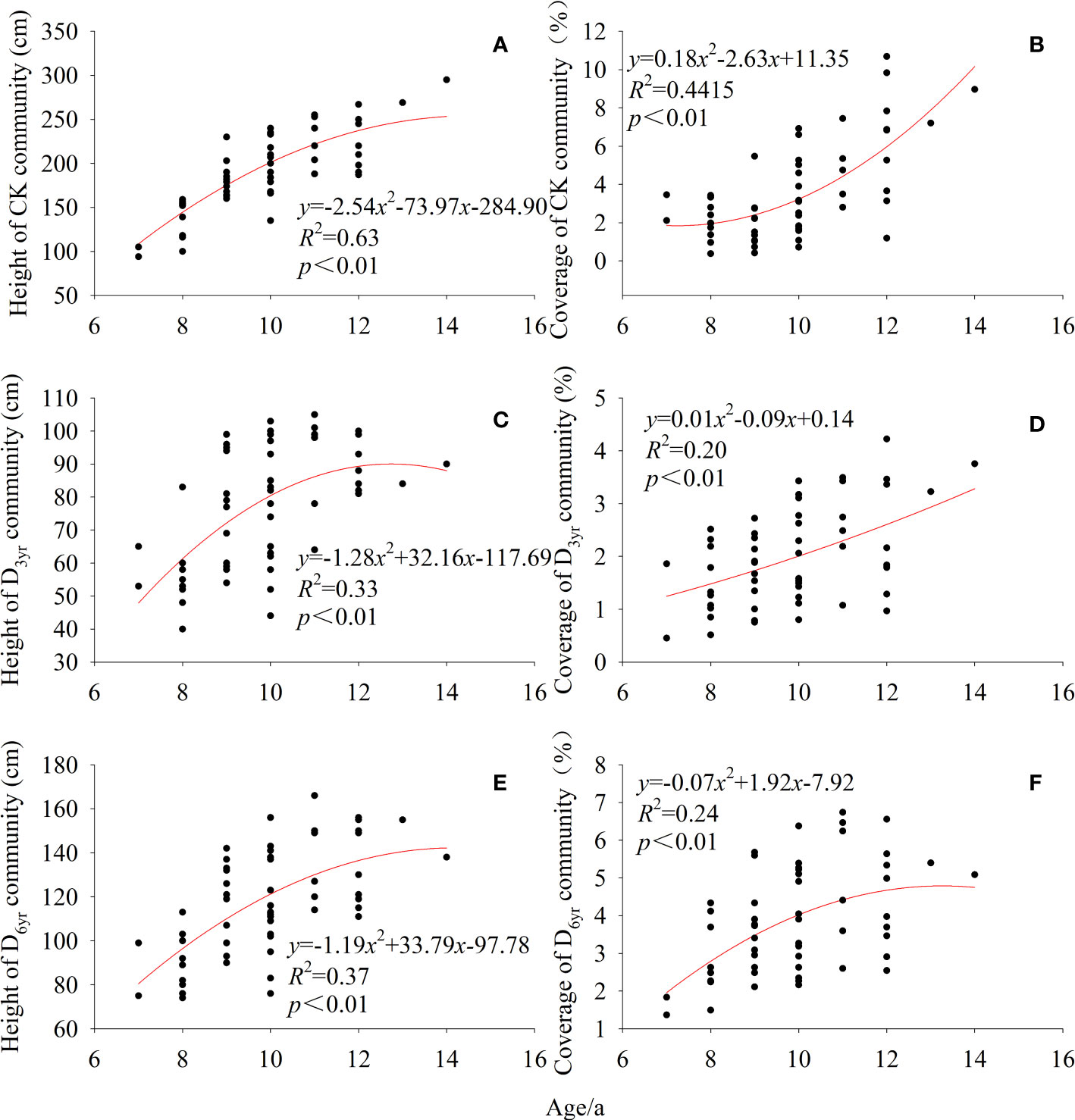
Figure 3 Variation in height and coverage of Rhododendron shrubs with age. Height of CK community (A); Coverage of CK community (B); Height of D3yr community (C); Coverage of D3yr community (D); Height of D6yr community (E); Coverage of D6yr community (F). CK, control; D3yr, community in 2018; D6yr, community in 2021.
To determine the sprouting ability at different ages, the age of Rhododendron shrubs was divided into three classes (I, II, and III). The sprouting ability of Rhododendron shrubs first increased and then decreased with age (Figure 4). Class II Rhododendron shrubs had the strongest sprouting ability. Moreover, the sprouting ability of class III Rhododendron shrubs was significantly different from that of classes I and II shrubs (p < 0.05).
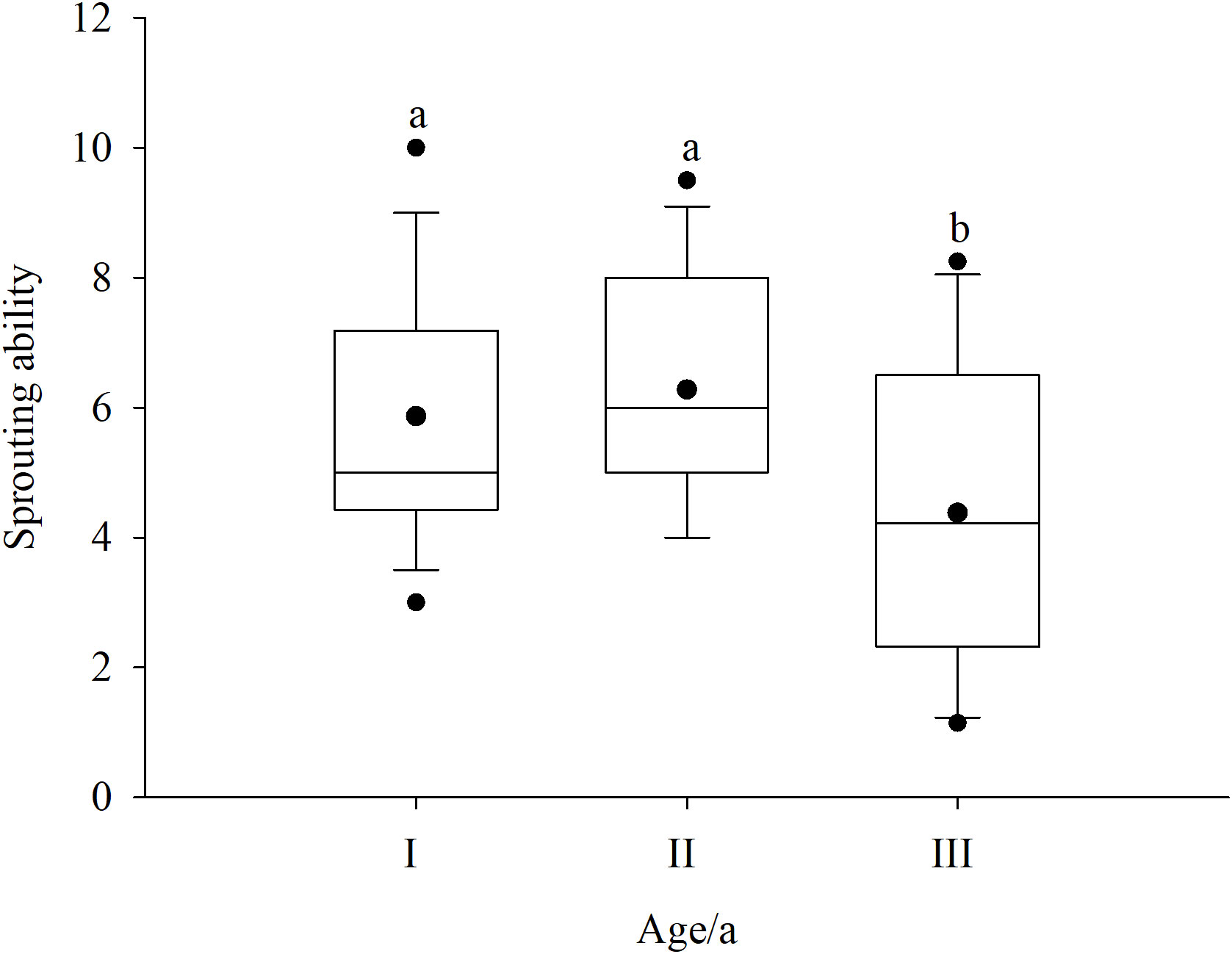
Figure 4 Effect of age of Rhododendron shrubs on sprouting ability. I, 7–9 years; II, 10–12 years; and III, > 12 years. a and b denote significant difference (p < 0.05).
Species composition reflects the structure, dynamic changes, and succession characteristics of a community and plays a decisive role in community diversity (Gilliam, 2007; Liu et al., 2020). In this study, 26 species belonging to 14 families and 22 genera were identified in the three communities studied (Table 1). The number of species in CK was the lowest probably because the canopy density of Rhododendron shrubs was high and the litter layer was thick, which reduced the migration and colonisation of Rubus trianthus, Cotoneaster horizontalis, Eurya loquaiana, Populus adenopoda, belonging to the Rosaceae, Theaceae, Salicaceae, as well as herbaceous plants. However, clearcutting weakened interspecies competition by reducing canopy density (Jones et al., 2019). Consequently, a large number of herbaceous plants, and species belonging to Rosaceae, Theaceae, and Salicaceae migrated; thus, D3yr had the highest number of species. Subsequently, Rhododendron shrubs gradually occupied the upper space, the canopy density increased, and herbaceous plants gradually disappeared. Therefore, the number of species in D6yr was lower compared with that in D3yr.
The height and coverage of plants in communities are important indicators for evaluating the plant growth status (Chen et al., 2014). In this study, the height and coverage of plants in communities after clearcutting were significantly higher than those of plants in the seed renewal communities (Figure 1), primarily because the large number of sprouting branches produced by Rhododendron shrubs could occupy more space by utilizing the resources, hence promoting rapid formation of community hierarchy. Post clearcutting, the sprouting coverage of Rhododendron shrubs in D6yr was higher than that of shrubs in CK, primarily because clearcutting promoted shrub tillering and expanded individual canopy width. After clearcutting, the relative height of Rhododendron shrubs in D3yr and D6yr also increased significantly, probably because Rhododendron shrubs occupied the upper space of the whole shrub community after clearcutting, playing a dominant role in light absorption and utilization. The absence of other tall trees and shrub vegetation enabled Rhododendron plants roots to utilize soil nutrients more effectively. Therefore, clearcutting resulted in the domination of Rhododendron plants in the community, with vigorous population renewal and a stronger self-sustaining ability of the population.
As a basic feature of a community, biodiversity is a key factor driving the dynamics and processes of an ecosystem and can indicate the habitat status, composition structure, and distribution pattern of a community (Liu and Bra, 1998; Tilman et al., 2012). In this study, the evenness index of the communities changed after clearcutting (Figure 2). The index value was relatively large, indicating that the heterogeneity of the study area was poor. This was also determined by the characteristics of the sprouting branches of Rhododendron shrubs. Although clearcutting increased the number of species, the α-diversity was still lower than that in CK before clearcutting. This may be because Rhododendron plants has a strong sprouting ability. After clearcutting, Rhododendron plants is likely to occupy space in the form of sprouting branches to inhibit the migration and colonisation of foreign species (Grant and Loneragan, 1999; Kruger and Midgley, 2001). Therefore, Rhododendron plants with its strong sprouting abilities can reduce species diversity and species turnover in the community to a certain extent.
β-Diversity is usually expressed as the turnover rate of biological species in different habitats. A high β-diversity index indicates a low level of species similarity between different habitats or ecosystems (Yang et al., 2020b). The results showed that the similarity between CK and D3yr was low, that between CK and D6yr was moderate, and that between D3yr and D6yr was high (Table 3). The Cody and Whittaker indices were higher before and after clearcutting, whereas the Jaccard and Sorenson indices were lower, indicating that clearcutting changed the species composition and community structure of Rhododendron. The similarity between CK and D3yr was low but that between CK and D6yr was moderate, indicating that with the progression of CK→D3yr→D6yr, the number of common plant species first decreased and then increased. Subsequently, the similarity among species composition also first decreased and then increased, indicating that Rhododendron plants could recover to the pre-clearcutting community level in a short time through clearcutting–sprouting renewal.
In Rhododendron shrub communities, both seedlings and sprouting seedlings can undergo population renewal. Nevertheless, sprouting seedlings are an important source of population dynamics (Vesk and Westoby, 2004). Due to the strong sprouting ability of Rhododendron plants, deforested communities rapidly self-substitute, which not only affects the community structure but also has an important impact on population dynamics. Clearcutting–sprouting renewal avoids the problems of very low sprouting and survival rates as well as weak seedling competitiveness. The results of this study similarly showed that the dominance of the Rhododendron population in D3yr and D6yr after sprouting was greater than that in CK before clearcutting. The growth recovery of Rhododendron plants after clearcutting is completely dependent on the amount of stubble, root tiller, and rhizome, whereas the number of sprouts is a common index to characterize the strength of sprouting (Chi et al., 2019). Although the sprouting ability of Rhododendron plants was strong in this study, the recovery ability first increased and then decreased with the age of the Rhododendron shrubs. The sprouting ability of Rhododendron shrubs of age 10–12 years was the strongest (Figure 4). This may be associated with the aging of plants, an excessively large root system, and a lack of rhizome growth, which led to partial necrosis of the underground root system.
The community succession of Rhododendron plants does not deviate far from the original direction because of its strong sprouting ability and the effect of the ecological niche after clearcutting (Ahmad et al., 2021). However, the communities in this study had a high number and proportion of Rhododendron shrubs initially, which were in situ substituted with a similarly high number, accelerating the recovery to the original structure and succession rate of the community. Therefore, the shrub layer of Rhododendron communities after clearcutting was primarily composed of sprouting branches of Rhododendron, and the final direction of succession was subtropical evergreen broad-leaved forest. Compared with seed renewal and succession, the clearcutting–sprouting succession of Rhododendron plants takes a shorter time to achieve maturity (Loucks, 1970; Chen et al., 2019).
In total, 26 species from 22 genera and 14 families were found in the three communities. Clearcutting promoted the migration and colonisation of the families Rosaceae, Theaceae, and Salicaceae and other herbaceous plants, whereas the relative height and coverage of shrubs in the communities increased significantly. After clearcutting, there was a slight change in community evenness and a decrease in the α-diversity. The β-diversity showed that clearcutting improved the dominance of Rhododendron plants in the community and promoted sprouting renewal of Rhododendron populations. Moreover, the sprouting ability of Rhododendron shrubs of age 10–12 years was the strongest. Clearcutting did not affect the direction of community succession, but could accelerate the succession rate. Owing to the short observation period of clearcutting and sprouting of Rhododendron communities in this study, it was not possible to accurately predict the rate of population renewal and community succession of Rhododendron shrubs. In subsequent studies, multiple clearcutting modes and Rhododendron population succession stages will be established in combination with the competitive ability of the Rhododendron population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YZ, XZ, and LW contributed to the conception and design of the study. HS confirmed and guided the study. ZW, BJ, and HS carried out the experiments. YW performed the statistical analysis and wrote the first draft of the manuscript. YZ, XZ, and CC guided writing sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was funded by the Science and Technology Department of Guizhou Province (Qian Ke He Zhicheng [2020]1Y076 and [2021]Yiban503, QKHPTRC-CXTD[2022]011).
We acknowledge the Baili Rhododendron National Nature Reserve and laboratory of the Department of Grassland Science, Guizhou University. We thank Xin Liu, Guiying Liu, Yini Wang, and Xiaolong Tian for their valuable suggestions and help with the laboratory analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad P. I., Puni L., Pandey R., Pala N. A., Rather M. M., Rashid M., et al. (2021). Influence of different treatments and techniques on rooting behaviour of Rhododendron arboreum Sm. In Indian Himalayas. Acta Ecol. Sin. 41 (4), 332–335. doi: 10.1016/j.chnaes.2021.01.001
Baselga A. (2010). Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 19 (1), 134–143. doi: 10.1111/j.1466-8238.2009.00490.x
Bian C. M., Jin Z. X., Zhang J. H., Jin J. D. (2006). Response of seed germination of Rhododendron fortunei to drought stress. Bull. Bot. Res. 26 (6), 718–721. doi: 10.7525/j.issn.1673-5102.2006.06.016
Bitayan M., Cervantes S. S., Napaldet J. T. (2021). Morpho-anatomical characterization of Rhododendron subsessile Rendle, an endangered species of the Cordillera Central Range, Philippines. J. For. Res. 32, 241–247. doi: 10.1007/s11676-019-01087-5
Chen Z. G., Batu N. C., Xu Z. Y., Hu Y. F. (2014). Measuring grassland vegetation cover using digital camera images. Acta Pratac. Sin. 23 (6), 20–27. doi: 10.11686/cyxb20140603
Chen X., Huang J. Y., Huang C. L., Chen X. (2013). Study on the macromorphological characters of Rhododendron (Ericaceae) in Northwestern Guizhou. Seed. 32 (8), 51–55. doi: 10.16590/j.cnki.1001-4705.2013.08.077
Chen S. Q., Wei X. L., An M. T., Kong D. M. (2019). Species diversity of plant communities at different succession stages in Baili Rhododendron Nature Reserve of Guizhou. Acta Bot. Boreali-Occident. Sin. 39 (7), 1298–1306. doi: 10.7606/j.issn.1000-4025.2019.07.1298
Chi X. L., Wang Q. G., Guo Q., Yang X., Tang Z. Y. (2019). Sprouting characteristics of communities during succession in an ever-green broad-leaved forest on Gutian Mountain, East China. Biodivers. Sci. 27 (1), 24–32. doi: 10.17520/biods.2018222
Choudhary S., Thakur S., Majeed A., Bhardwaj P. (2021). Adaptability of Rhododendrons in high altitude habitats. J. For. Res. 32 (2), 449–460. doi: 10.1007/s11676-019-01081-x
Dai X. Y., Yang C. H., Yang B., Chen P., Ma Y. P. (2020). A new species of Rhododendron (Ericaceae) from Guizhou, China. Phytokeys. 146 (10), 53–59. doi: 10.3897/phytokeys.146.51342
Fang R. Z., Min T. L. (1995). The floristic study on the genus Rhododendron. Plant Divers. 17 (4), 359–379. doi: ir.kib.ac.cn:8080/handle/151853/12313
Gilliam F. S. (2007). The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience. 57 (10), 845–858. doi: 10.1641/B571007
Grant C. D., Loneragan W. A. (1999). The effects of burning on the understorey composition of 11–13-year-old rehabilitated bauxite mines in Western Australia–Vegetation characteristics. Plant Ecol. 145, 291–305. doi: 10.1023/A:1009821128075
Jia Z. Z., Li W. J., Tian A., Wang J. G. (2021). Ecological influence of tourism disturbance on the characteristics of different communities in Baili Rhododendron Forest. Acta Ecol. Sin. 41 (11), 4641–4649. doi: 10.5846/stxb201907021387
Jiang H., He B., Luo Q. (2015). A study of the relationship between the distribution of bryophytes and the environmental factors in Baili Azalea National Forest Park. Ecol. Sci. 34 (4), 31–36. doi: 10.14108/j.cnki.1008-8873.2015.04.006
Jones G. L., Tomlinson M., Owen R., Scullion J., Winters A., Jenkins T., et al. (2019). Shrub establishment favoured and grass dominance reduced in acid heath grassland systems cleared of invasive Rhododendron pontic. Sci. Rep. 9, 2239. doi: 10.1038/s41598-019-38573-z
Kauffman J. B. (1991). Survival by sprouting following fire in tropical forests of the eastern amazon. Biotropica 23 (3), 219–224. doi: 10.2307/2388198
Kong D. M., Ran J. C., An M. T., Cui X. Y., Chen S. Q. (2019). Analysis on structure characteristics and regeneration trend of typical Rhododendron communities at Baili Rhododendron Nature Reserve. Non-wood For. Res. 37 (4), 112–119. doi: 10.14067/j.cnki.1003-8981.2019.04.015
Kruger L. M., Midgley J. J. (2001). The influence of resprouting forest canopy species on richness in Southern Cape forests, South Africa. Glob. Ecol. Biogeogr. 10, 567–572. doi: 10.1046/j.1466-822x.2001.00264.x
Li W. J., Chen X. A. (2005). Preliminary study on structure and regeneration of Rhododendron delavayi population in Baili Azalea Forest Park. Guizhou Sci. 23 (3), 46–49. doi: 10.3969/j.issn.1003-6563.2005.03.011
Li T. Q., Liu X. F., Li Z. H., Ma H., Wan Y. M., Liu X. X., et al. (2018). Study on reproductive biology of Rhododendron longipedicellatum: a newly discovered and special threatened plant surviving in limestone habitat in southeast Yunnan China. Front. Plant Sci. 9 (1). doi: 10.3389/fpls.2018.00033
Liu Q., Bra S. (1998). Long-term effects of clear-felling on vegetation dynamics and species diversity in a boreal pine forest. Biodivers. Conserv. 7 (2), 207–220. doi: 10.1023/A:1008836502640
Liu D., Guo Z. L., Cui X. Y., Fan C. N. (2020). Comparison of five associations of Taxus cuspidata and their species diversity. Biodivers. Sci. 28 (3), 340–349. doi: 10.17520/biods.2019112
Liu D. T., Sun W. B., Ma Y. P., Fang Z. D. (2019). Rediscovery and conservation of the Critically Endangered Rhododendron griersonianum in Yunnan, China. Oryx 53 (1), 14. doi: 10.1017/S0030605318001278
Loucks O. L. (1970). Evolution of diversity, efficiency, and community stability. Am. Zool. 10 (1), 17–25. doi: 10.1093/icb/10.1.17
Lu M. Z., Du H., Song T. Q., Peng W. X., Liu K. P., Su L., et al. (2021). Characteristics of sprouting in woody plants in evergreen-deciduous broadleaf karst forest in Mulun National Nature Reserve. Acta Ecol. Sin. 41 (15), 6182–6190. doi: 10.5846/stxb202004291045
Luoga E. J., Witkowski E., Balkwill K. (2004). Regeneration by coppicing (resprouting) of miombo (African savanna) trees in relation to land use. For. Ecol. Manage. 189 (1-3), 23–35. doi: 10.1016/j.foreco.2003.02.001
Ma Y. P., Nielsen J., Chamberlain D. F., Li X. Y. (2014). The conservation of Rhododendrons is of greater urgency than has been previously acknowledged in China. Biodivers. Conserv. 23 (12), 3149–3154. doi: 10.1007/s10531-014-0764-9
MacKay M., Gardiner S. E. (2016). A model for determining ex situ conservation priorities in big genera is provided by analysis of the subgenera of Rhododendron (Ericaceae). Biodivers. Conserv. 26 (1), 189–208. doi: 10.1007/s10531-016-1237-0
MacKay M., Gardiner S. E. (2017). Geographic analysis of Red List Rhododendron (Ericaceae) taxa by country of origin identifies priorities for ex situ conservation. Blumea 62 (2), 103–120. doi: 10.3767/blumea.2017.62.02.05
Rong L., Chen X., Wang X. C. (2009). Leaf anatomical characters and its ecological adaptation of 13 species of Rhododendron in Baili Azalea Area. J. Anhui Agric. Sci. 37 (3), 1084–1088. doi: 10.13989/j.cnki.0517-6611.2009.03.113
Shang K. K., Zhang G. W., Song N. N. (2020). Effects of different cutting intensities on phytoremediation of heavy metal for flowering shrubs. J. Northeast For. Univ. 48 (5), 50–54+61. doi: 10.13759/j.cnki.dlxb.2020.05.010
Singh N., Ram J., Tewari A., Yadav R. (2019). Phenological events along the elevation gradient and effect of climate change on Rhododendron Arboreum Sm. in Kumaun Himalaya. BioScience 37 (4), 112–119. doi: 10.18520/CS/V108/I1/106-110
Subedi S. C., Ross M. S., Sah J. P., Redwine J., Baraloto C. (2019). Trait-based community assembly pattern along a forest succession gradient in a seasonally dry tropical forest. Ecosphere 10 (4), e02719. doi: 10.1002/ecs2.2719
Sun H. (2002). Tethys retreat and Himalayas-Hengduanshan Mountains uplift and their significance on the origin and development of the Sino-Himalayan elements and Alpine flora. Acta Bot. Yunnanica 24 (6), 671–688. doi: 10.3969/j.issn.2095-0845.2002.06.001
Tang Y., Fong Z. L., Cao M. (2001). Forest regeneration by sprouting in slash and burn field, Xishuangbanna. J. Northeast For. Univ. 2001 (4), 64–66. doi: 10.3969/j.issn.1000-5382.2001.04.019
Tilman D., Reich P. B., Isbell F. (2012). Biodiversity impacts ecosystem productivity as much as resources, disturbance, or herbivory. Proc. Natl. Acad. Sci. U. S. A. 109 (26), 10394–10397. doi: 10.1073/pnas.1208240109
Vesk P. A., Westoby M. (2004). Sprouting ability across diverse disturbances and vegetation types worldwide. J. Ecol. 92 (2), 310–320. doi: 10.1111/j.0022-0477.2004.00871.x
Wang Z. C., Chen X., Ren Z. M., Zhou J. W., Chen X., An M., et al. (2010). Analysis on phylogenesis of Rhododendron L. plant located in hundred Rhododendron protection zone of Guizhou and flowering characteristics in molecular level. Seed 29 (12), 72–76. doi: 10.16590/j.cnki.1001-4705.2010.12.076
Wang Z. Y., Wang L. J., Tian X. L., Zhang Q., Wang J. G., Shuai H. G., et al. (2023). Characteristics and species diversity of different Rhododendron shrub communities in northwest Guizhou. Acta Ecol. Sin. 43 (2), 693–701. doi: 10.5846/stxb202101310336
Yang J. W., Liu X. Q., Peng X. H., Wang M., Zhen L. Y., Liu S., et al. (2020b). Plant diversity and soil physical properties in different degree of rocky desertification in northern Hubei Province. Res. Soil Water Conserv. 27 (6), 100–106+115. doi: 10.13869/j.cnki.rswc.2020.06.015
Yang B., Yuan C. J., Dai X. Y., Yang C. H., Chen Z. P., Guo Y., et al. (2020a). Characteristics of wild populations and their communities of endemic plant Rhododendron bailiense in Guizhou Province. J. Plant Resour. Environ. 29 (4), 61–68. doi: 10.3969/j.issn.1674-7895.2020.04.08
Yu F. Y., Wang T. J., Groen T. A., Skidmore A. K., Yang X. F., Ma K. P., et al. (2019). Climate and land use changes will degrade the distribution of Rhododendrons in China. Sci. Total Environ. 659, 515–528. doi: 10.1016/j.scitotenv.2018.12.223
Keywords: Rhododendron shrub, clearcutting, species composition, species diversity, community renewal
Citation: Zhang Y, Zhao X, Wang L, Wang Z, Shuai H, Wang Y, Jin B and Chen C (2023) Effects of clearcutting on species composition and community renewal of Rhododendron shrubs in northwest Guizhou Province, China. Front. Ecol. Evol. 11:1225466. doi: 10.3389/fevo.2023.1225466
Received: 19 May 2023; Accepted: 17 August 2023;
Published: 31 August 2023.
Edited by:
Weiguo Sang, Minzu University of China, ChinaReviewed by:
Suresh Chandra Subedi, Arkansas Tech University, United StatesCopyright © 2023 Zhang, Zhao, Wang, Wang, Shuai, Wang, Jin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuechun Zhao, eGN6aGFvQGd6dS5lZHUuY24=; Chao Chen, Y2hlbmNAZ3p1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.