- Hainan Academy of Forestry (Hainan Academy of Mangrove), Haikou, China
Pest herbivory regulation is one of the key functions provided by diverse ecosystems, especially when compared to species depauperate agro-ecosystems and in the context of increased pest outbreaks due to global change. The dilution effect of host diversity on insect herbivory suggests that mixed plantations are feasible for regulating pest herbivory in agroecosystems. Dilution effect of increased plant diversity on insect herbivory has been widely observed, yet little is known about how it may change with plant phylogenetic relatedness and herbivore specialization, especially at the community level. Here, we compared herbivore richness, abundance, and degree of herbivory (i.e., the ratio of trees damaged by pest and total trees in a given area) among the two monocultures and the four mixed plantations in Wenchang city, Hainan Province, China. We also respectively assessed the effects of phylogenetically close or distant species on generalist and specialist herbivores in monocultures and mixture. We found that increasing the number of phylogenetically closely-related tree species could dilute generalist herbivore richness, abundance, and degree of herbivory but amplify specialist herbivore richness, abundance, and degree of herbivory. In contrast, increasing the number of phylogenetically distant tree species increased generalist herbivore richness, abundance, and degree of herbivory, while reducing specialist herbivore richness, abundance, and degree of herbivory. Our results suggest that plant phylogenetic relatedness and herbivore specialization can indeed interact to influence pest control efficiency when using mixed plantations to manage pest herbivory in agroecosystems. Thus, both herbivore specialization and plant phylogenetic relatedness should be taken into account in the management of agro-ecosystems and biodiversity conservation with respect to herbivory.
1. Introduction
The anthropogenic acceleration of biodiversity loss (Pimm et al., 2014) and its detrimental effects on ecosystem functions remain one of the major concerns in ecology (Tilman et al., 2014). Pest herbivory regulation is one of the key functions provided by diverse ecosystems, which is especially important for agro-ecosystems in the context of increased pest outbreaks due to global change (Jactel et al., 2012; Klapwijk et al., 2012; Castagneyrol et al., 2014; Andrew and Hill, 2017). Given the widely acknowledged phenomenon that increasing plant diversity can have a dilution effect (i.e., increasing plant diversity results in decreased pest herbivory) on insect herbivory, mixed plantations can potentially provide a key ecosystem service for the regulation of pest herbivory in mono-plantation in agroecosystems (Castagneyrol et al., 2014). However, the magnitude of the dilution effect of plant diversity on pest herbivory is known to vary according to the composition of plant and herbivore communities (Jactel and Brockerhoff, 2007; Vehvilainen et al., 2007; Barbosa et al., 2009). Moreover, evidence refuting this effect (i.e., that increasing plant diversity leads to increased pest herbivory) has also been found in other studies (Schuldt et al., 2015; Zhang et al., 2017; Grossman et al., 2018). As a result, it remains unclear whether increasing plant diversity through mixed plantations can be used to help manage pest herbivory in agroecosystems.
Recent synthesis studies highlight two primary factors that may be responsible for the lack of consensus regarding plant diversity-herbivory relationships (Jactel and Brockerhoff, 2007; Castagneyrol et al., 2014). First, host specificity of herbivores, particularly herbivory by specialist species (feeding on a specific plant genus), is usually reduced in diverse communities compared to monocultures (but see Plath et al., 2012). In contrast, the response of generalist species (feeding on different plant genus) is asynchronous in terms of its direction and magnitude (Schuldt et al., 2015). The second possibility regards the plant diversity being examined. Previous studies have shown that plant functional and phylogenetic diversity of the host community, and the relative abundance of the focal plant species, is more important than plant species richness in regulating the magnitude of herbivory (Mouquet et al., 2012). Moreover, herbivore specificity and plant phylogenetic relatedness interact to regulate the intensity of herbivory (Castagneyrol et al., 2014).
Till now, the associational resistance hypothesis has been recognized as the primary mechanism driving the dilution effect of increased plant diversity on specialist herbivory (Root, 1973; Agrawal et al., 2006; Lewinsohn and Roslin, 2008). This hypothesis claims that specialist herbivores prefer homogeneous neighbors to the same host plants for increase their herbivore loads (Lewinsohn and Roslin, 2008). As such, mixing closely related plant species with a mono-plantation may amplify herbivory caused by specialist herbivores. In contrast, mixing phylogenetically distant plant species with mono-plantations may dilute the effects from specialist herbivores (Figure 1). Likewise, the associational susceptibility hypothesis has been considered key to explaining the dilution effect of increased plant diversity on generalist herbivory (Unsicker et al., 2008; Plath et al., 2012). The hypothesis assumes that generalist herbivores require heterospecific neighbors to a focal plant to increase their herbivore load (Plath et al., 2012). As a result, mixing phylogenetically distant plant species with mono-plantation consumed by generalist herbivores may amplify herbivory due to generalist herbivores. In contrast, mixing closely related plant species with mono-plantations can dilute generalist herbivory (Figure 1). To the best of our knowledge, however, little is known about whether and how herbivore specificity and plant phylogenetic relatedness interact to alter the efficiency of mixed plantation on controlling herbivore in monoculture agroecosystem.
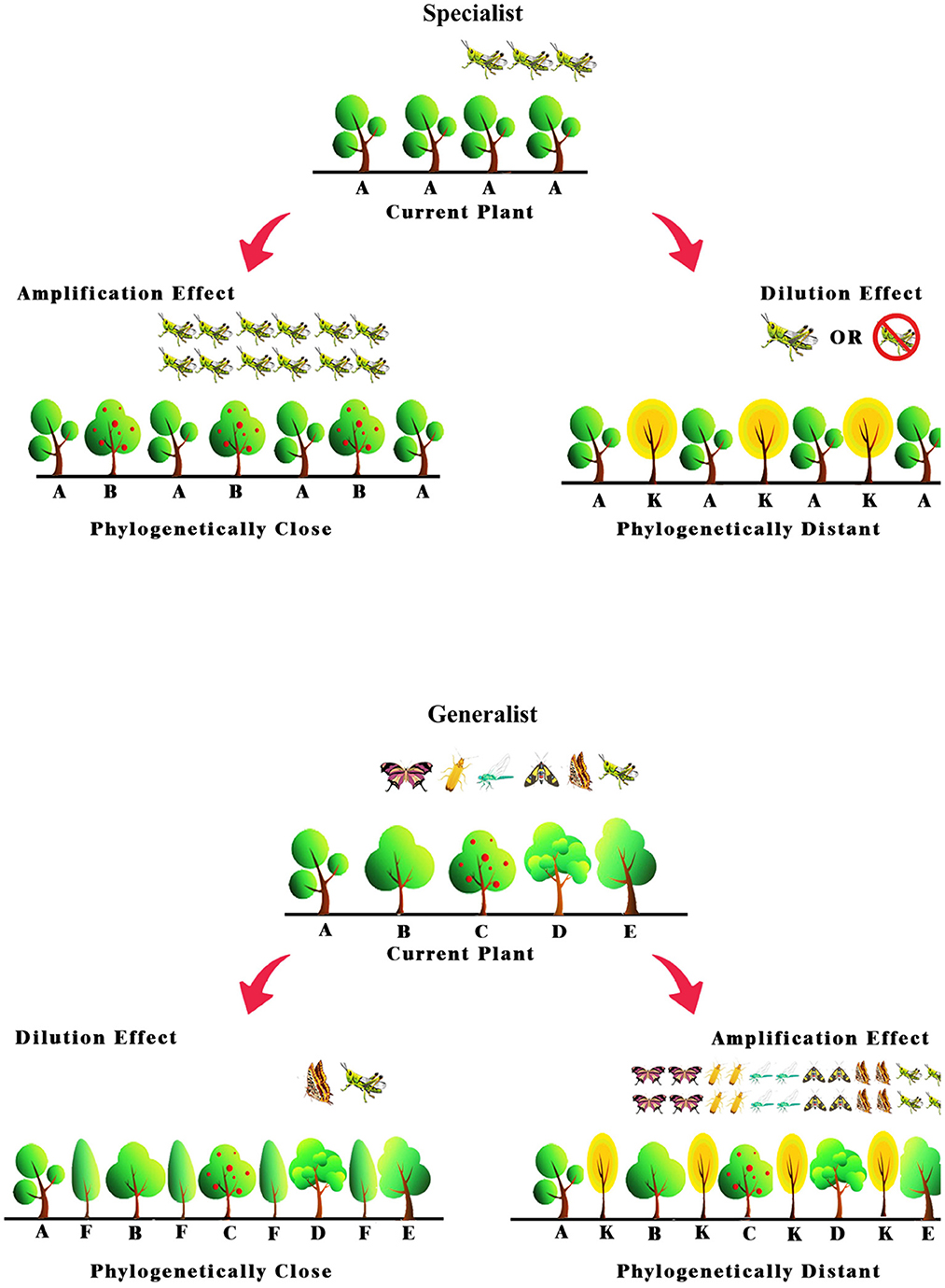
Figure 1. Hypothesized dilute and amplified effects of mixing closely and distantly related tree species on specialist and generalist pest herbivory respectively.
Current knowledge regarding the effect of plant phylogenetic diversity on generalist vs. specialist herbivores is largely based on comparisons of generalist and specialist herbivore intensity between monoculture and mixed plantations (Castagneyrol et al., 2014). By doing so, however, herbivore-herbivore interactions are largely overlooked. In fact, more complexity and even inverse patterns may emerge when multiple hosts and herbivores are taken into account as in the realty. For instance, phylogenetic relatedness of a host community may have different effect on the mean intensity of herbivory for multiple generalist herbivores and a specific herbivore respectively. An assemblage of phylogenetically distant plants may harbor more generalist herbivore species, and may also suffer from a higher level of herbivory through the sampling effect (i.e., higher possibility of attracting herbivores with high herbivory) (Schuldt et al., 2014). Similarly, phylogenetically similar species can also trigger many new specialist species, which may also lead to higher specialist herbivory via the sampling effect. As a result, pest species diversity (species richness and abundance) and the herbivore intensity by the targeted generalist or specialist herbivores should be compared simultaneously among monocultures and mixed plantations. However, experimental studies addressing the specificity of multiple herbivores and their community composition at the community level remain scarce.
Here, we sampled two monocultures in Wenchang City, Hainan Province, China. One was one subspecies of Cocos nucifera (we called “green Cocos nucifera”) stand and the other is a pure stand of Pinus elliottii. Based on 20 years' pest herbivore survey in this area, “green Cocos nucifera” is attacked by generalist herbivores (e.g., Brontispa longissima, Rhynchophorus ferrugineus, Oryctes rhinoceros, Diocalandra frument, Aspidiotus destucto, and Aleurocanthus spiniferus) (Lin et al., 2010). In contrast, Pinus elliottii is attacked merely by a specialist herbivore (Dendrolimus puntatus) (Xu et al., 2014). We mixed ten tree species which are phylogenetically distant with the two monocultures (Supplementary Figure S1) to simulate the influence of mixture of phylogenetically distant species on generalist and specialist pest herbivory respectively. These ten tree species are Litsea glutinosa, Calophyllum inophyllum, Casuarina equisetifolia, Rapsnea linearis, Rhodomyrtus tomentosa, Psychotria rubra, Clerodendrum cyrtophyllum, Zanthoxylum piperitum, Wikstroemia indica, and Atalantia buxifoli (Supplementary Figure S1). We also mixed another subspecies of Cocos nucifera (we called “red Cocos nucifera”) with the pure “green Cocos nucifera” stand “red Cocos nucifera” is phylogenetically close to “green Cocos nucifera” stand (Supplementary Figure S1), so we use this design to assess the effect of mixing phylogenetically close species on generalist pest herbivory. Similarly, since Pinus caribaea is phylogenetically close to Pinus elliottii (Supplementary Figure S1), we mixed Pinus caribaea with the pure Pinus elliottii stand to assess the impacts of mixing phylogenetically similar species on specialist pest herbivory too. Specifically, we compared herbivore richness, abundance, and degree of herbivory (i.e., the ratio of trees damaged by pest and total trees in a given area) among the two monocultures and the four mixed plantations. This experimental design aims to test: (1) if the addition of distantly related plant species can amplify herbivory by generalist herbivores, but dilute herbivory caused by specialist herbivores in a diverse host community; and (2) if adding closely related species to a host community can dilute herbivory caused by generalist herbivores, but amplify herbivory caused by specialist herbivores.
2. Materials and methods
2.1. Study sites
Our study site included four mixed stands (mixed stand 1, 110°57′34″E, 19°43′58″N; mixed stand 2, 110°56′39″E, 19°44′4″N; mixed stand 3, 110°58′34″E, 19°44′23″N and mixed stand 4: 110°49′24″E, 19°32′29″N), one monoculture pure “green Cocos nucifera” stand (110°51′15″E, 19°34′58″N), and one monoculture pure Pinus elliottii stand (110°43′19″E, 19°34′01″N) in Wenchang City, Hainan Province, China (Supplementary Figure S2). These sites have a mean annual temperature (1978–2010) of 23.9 °C, with a low monthly mean temperature of 16.2°C in January and high of 33°C in August. Average annual precipitation is 1,729 mm, most of which occurs from April to October.
One hundred stems from each of ten species (i.e., Litsea glutinosa, Calophyllum inophyllum, Casuarina equisetifolia, Rapsnea linearis, Rhodomyrtus tomentosa, Psychotria rubra, Clerodendrum cyrtophyllum, Zanthoxylum piperitum, Wikstroemia indica, and Atalantia buxifoli) were randomly mixed with 1,000 individuals of “green Cocos nucifera” in mixed stand 1 and 1,000 individuals of Pinus elliottii in mixed stand 2. Thus, this planting was used to test the influence of mixing phylogenetically distant plant species on generalist and specialist pest herbivory individually. In mixed stand 3, 1,000 individuals of “green Cocos nucifera” and 1,000 individuals of “red Cocos nucifera” were planted to determine the influence of mixing phylogenetically similar plant species on generalist pest herbivory. In mixed stand 4, 1,000 individuals of Pinus elliottii and 1000 individuals of Pinus caribaea were therefore randomly mixed to assess the impact of mixing phylogenetically similar plant species on specialist pest herbivory. Pure “green Cocos nucifera” and Pinus elliottii stands of at least 1000 ha were established in Wenchang for nearly 30 years. Pure “green Cocos nucifera” and Pinus elliottii stands, mixed stand 1, 2, 3, and 4 have been planted for at least 20 years, and their planting densities were all kept at 500 stems per hectare. Since all six stands were very near the ocean, the local government forbids any human management (e.g., pesticide spraying and fertilization), thereby to avoid any potential ocean pollution.
2.1.1. Measurements of degree of herbivory for “green Cocos nucifera” and Pinus elliottii
We randomly selected three 20 × 20 m2 subplots from each of the six stands described above. The subplots within each stand were at least 500 m apart from one another and from the edges of the stands. We then checked all “green Cocos nucifera” and Pinus elliottii trees in the three 20 × 20 m2 subplots from each of the six stands to see how many “green Cocos nucifera” and Pinus elliottii trees were damaged by pests. Examples of different types of pest herbivory in “green Cocos nucifera” and Pinus elliottii stands are shown in Supplementary Figure S3. Then we calculated the degree of herbivory as the ratio of trees damaged by a pest by the total number of trees found in each of the three 20 × 20 m2 subplots within each of the six plantation stands.
2.1.2. Insect species richness and abundance trapping
Following Xue et al. (2021), we used 18 insect nets trap that were 50 cm in diameter and 160 cm in length to collect all flying insects during the day from July 26–28, 2019 in the three 20 × 20 m2 subplots from each of the six stands. We also used 18 mercury lamps (450W) and 18 white cloths (2 × 3 m) (Supplementary Figure S4) to trap nocturnal insects from 8:00 pm to 10:00 pm during the same period. All non-larval herbivore insects were sorted and identified to species, and the abundance of each species was also recorded within each subplot.
2.1.3. Constructing phylogenetic tree of the 12 tree species and two subspecies of Cocos nucifera
The chloroplast genome of the 12 tree species and two subspecies of Cocos nucifera (“green Cocos nucifera” and “red Cocos nucifera”) were measured to quantify the phylogenetic relationships of the 12 tree species and two subspecies of Cocos nucifera. The chloroplast genome for Pinus elliottii, Casuarina equisetifolia, Clerodendrum cyrtophyllum, Litsea glutinosa, Rhodomyrtus tomentosa, Zanthoxylum piperitum, and Wikstroemia indica can be directly found in NCBI. Thus, we only sequenced chloroplast genome for “green Cocos nucifera” and “red Cocos nucifera”, Pinus caribaea, Calophyllum inophyllum, Rapsnea linearis, Psychotria rubra, and Atalantia buxifolia. Detail descriptions of the chloroplast genome for these seven tree species or subspecies were described in detail in Supplementary Table S1. A maximum likelihood tree (Supplementary Figure S1) was then constructed using RAxML (Stamatakis, 2014).
2.2. Statistical methods
Since species richness and abundance are discrete random variables (count data here), we used the generalized linear mixed effect model (GLMM) with Poisson error to test whether phylogenetic relatedness, herbivore specialization and their interactive effects can significantly influence degree of herbivory, total insect species richness and abundance, and total herbivore insect species richness and abundance. Specifically, we used a GLMM model [Degree of herbivory or Richness or Abundance ~ herbivore specialization × phylogenetic relatedness + (1|plantation type)]. Here richness and abundance indicate total insect or herbivore species richness and insect or herbivore insect abundance respectively. Herbivore specialization was either generalist or specialist. Phylogenetic relatedness coded phylogenetically close and distant individually. Plantation type indicated the six stands (green Cocos nucifera stand, mixed stand 1, mixed stand 3, Pinus elliottii stand, mixed stand 2, and mixed stand 4).
3. Results
The GLMM model results demonstrated that herbivore specialization, phylogenetic relatedness and their interactive effects all significantly influenced degree of herbivory, total insect species richness and abundance and total herbivore insect species richness and abundance (p of GLMM < 0.05, Supplementary Table S2). Our GLMM model results showed that degree of herbivory of “green Cocos nucifera” in mixed stand 1 was 1.63 times higher than that in the pure “green Cocos nucifera” stand (Figure 2A). The degree of herbivory of Pinus elliottii decreased from 43% in the pure Pinus elliottii stand to 0% in mixed stand 2 (Figure 2C). Degree of herbivory of “green Cocos nucifera” in mixed stand 3 was 1.82 times lower than that in the pure “green Cocos nucifera” stand, while the degree of herbivory of Pinus elliottii in mixed stand 4 was 1.44 times higher than that in the pure Pinus elliottii stand (Figures 2B, D).
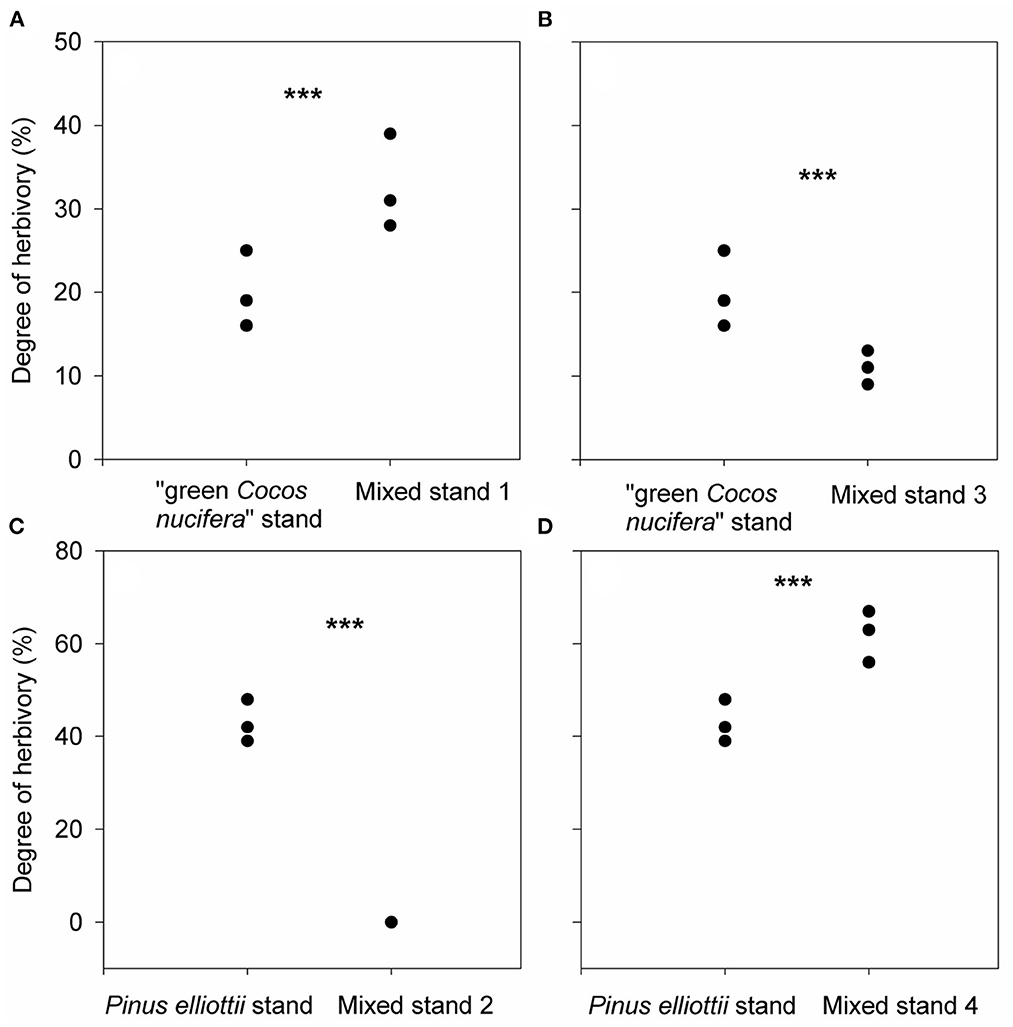
Figure 2. Differences in degree of herbivory [the percentage of trees damaged by a pest (%)] of “green Cocos nucifera” and Pinus elliottii between mixed stand 1 and the pure “green Cocos nucifera” stand (A), mixed stand 2 and the pure Pinus elliottii stand (C), mixed stand 3 and the pure “green Cocos nucifera” stand (B), and mixed stand 4 and the pure Pinus elliottii stand (D), respectively. ***indicates p < 0.05, results from our generalized linear mixed effect model with Poisson error.
We found 25 herbivore species in the pure “green Cocos nucifera” stand, all of which were generalist feeders (Figure 3A, Supplementary Figure S2, Supplementary Table S3). They also emerged in mixed stand 1 (Figure 3C, Supplementary Table S3), but their total abundance in mixed stand 1 increased to 1.4 times higher than that in the pure “green Cocos nucifera” stand (Figures 3B, D). Moreover, 10 generalist herbivore species that were absent from the pure Cocos nucifera stand appeared in mixed stand 1 (Figure 3A, Supplementary Table S3). In contrast, although one new generalist species emerged (Figure 4A, Supplementary Table S3), only 14 out of the 25 generalist herbivore species observed in the pure “green Cocos nucifera” stand were found in mixed stand 3 (Figure 4C, Supplementary Table S3). Moreover, herbivore abundances in mixed stand 3 decreased 2-fold compared with the pure “green Cocos nucifera” stand (Figures 4B, D, Supplementary Table S3).
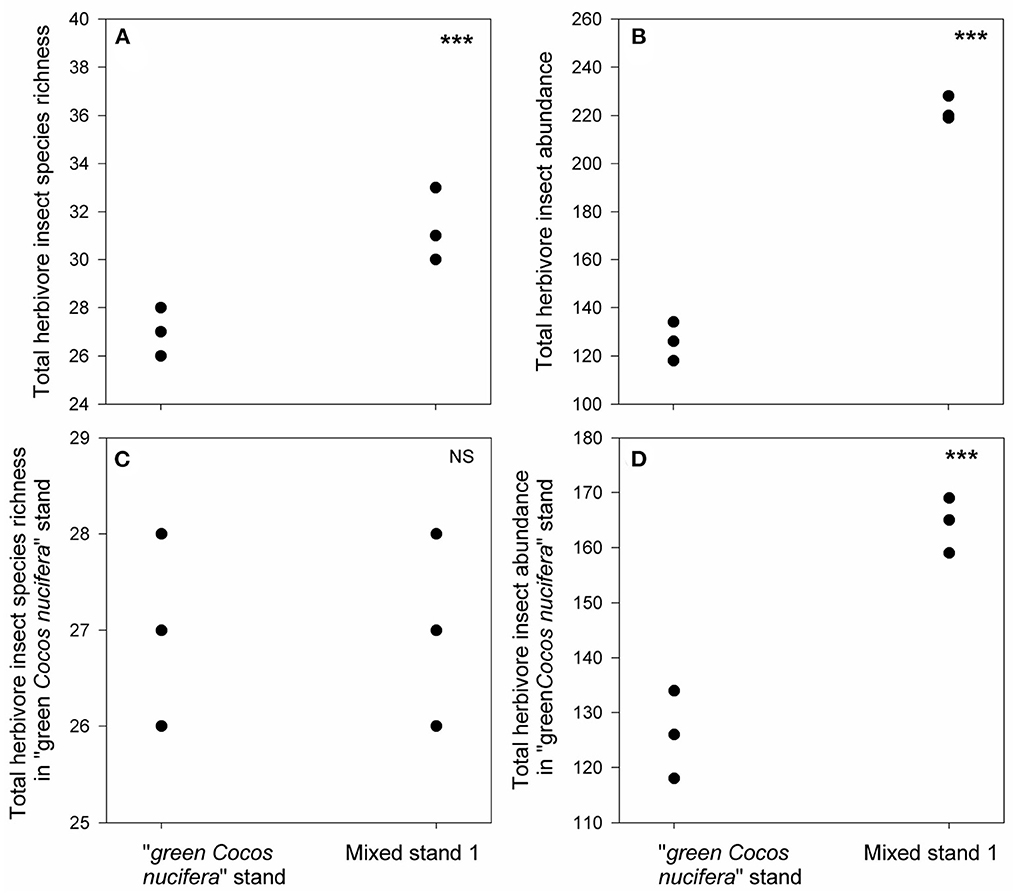
Figure 3. Differences in total herbivore insect species richness (A), total herbivore insect abundance (B), total herbivore insect species richness in “green Cocos nucifera” stand (C) and their abundance (D) between mixed stand 1 and “green Cocos nucifera” stand. *** indicates p < 0.05, results from our generalized linear mixed effect model with Poisson error.
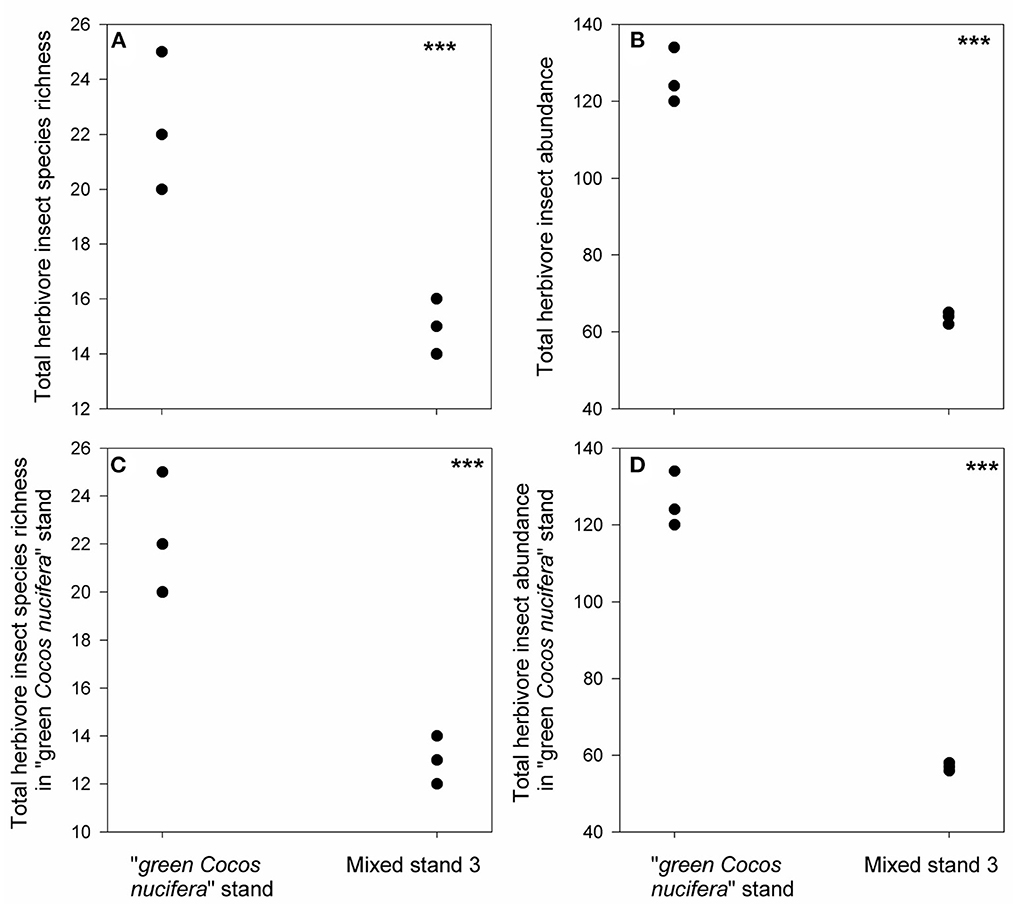
Figure 4. Differences in total herbivore insect species richness (A), total herbivore insect abundance (B), total herbivore insect species richness in “green Cocos nucifera” stand (C) and their abundance (D) between mixed stand 3 and “green Cocos nucifera” stand. ***indicates p < 0.05, results from our generalized linear mixed effect model with Poisson error.
In the Pinus elliottii stand we only found one specialist herbivore species (Dendrolimus puntatus), which was absent from mixed stand 2 (Figure 5C, Supplementary Figure S2, Supplementary Table S3). However, another 24 generalist herbivore species were found in mixed stand 2, leading to both higher total herbivore species richness and total abundance than those found in the Pinus elliottii stand (Figures 5A, B, D, Supplementary Table S3). In contrast, in mixed stand 4, one more new specialist herbivore (Dioryctria splendidella) emerged, except for Dendrolimus puntatus (Figures 6A, C, Supplementary Table S3). Moreover, the abundance of Dendrolimus puntatus increased 2-fold compared to that in the mono-Pinus elliottii stand (Figures 6B, D, Supplementary Table S3).
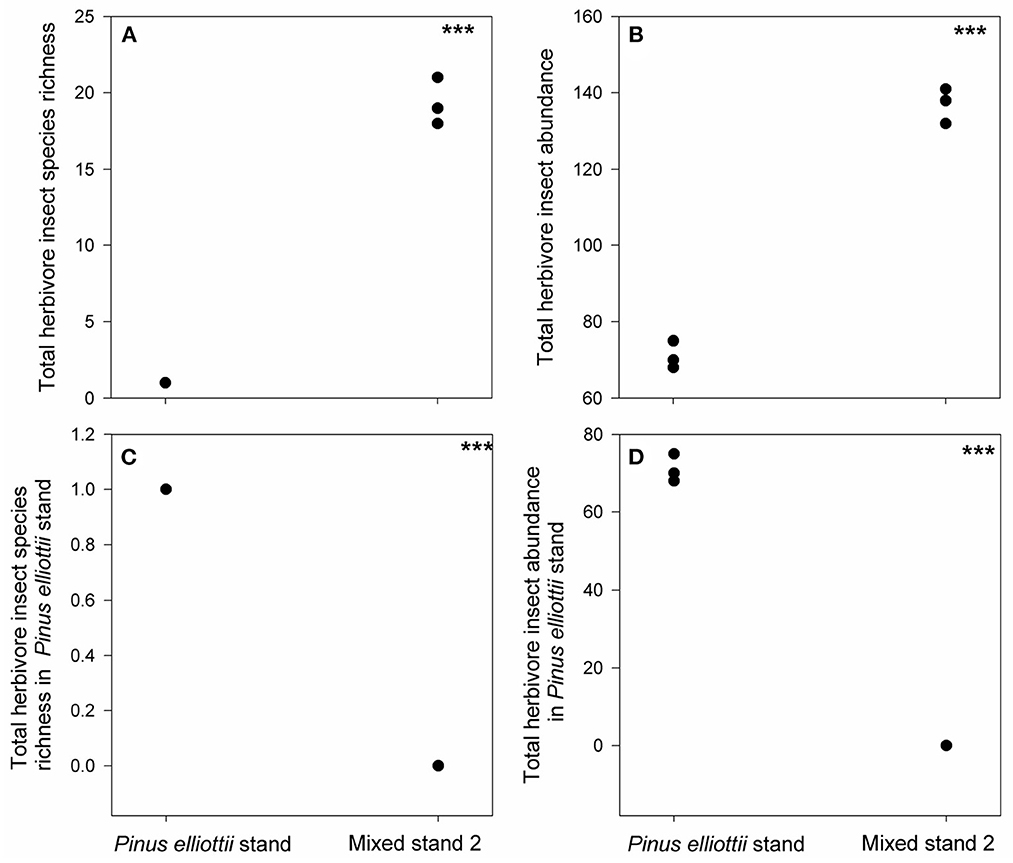
Figure 5. Differences in total herbivore insect species richness (A), total herbivore insect abundance (B), total species richness of herbivore insects found in Pinus elliottii stand (C) and their abundance (D) between mixed stand 2 and Pinus elliottii stand. ***indicates p < 0.05, results from our generalized linear mixed effect model with Poisson error.
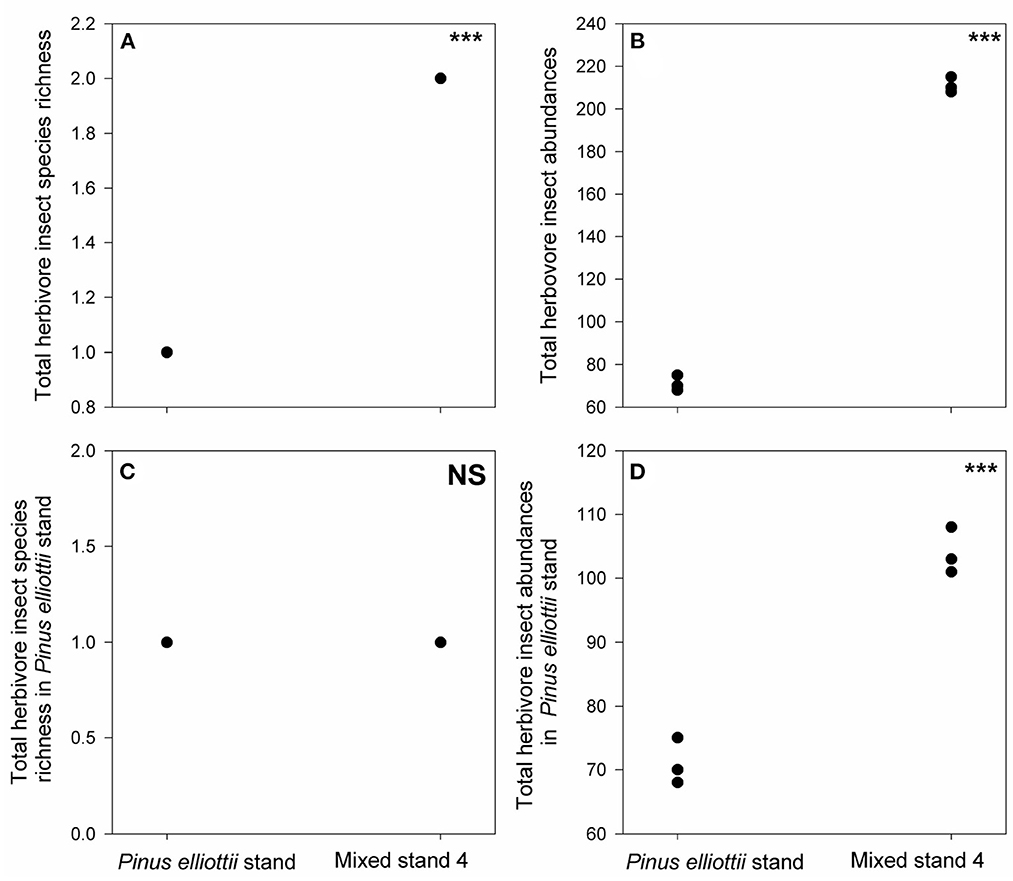
Figure 6. Differences in total herbivore insect species richness (A), total herbivore insect abundance (B), total species richness of herbivore insects in Pinus elliottii stand (C) and their abundance (D) between mixed stand 4 and Pinus elliottii stand. *** indicates p < 0.05 and NS (non-significant) indicates p > 0.05, results from our generalized linear mixed effect model with Poisson error.
4. Discussion
Herbivores play key functions in ecosystems across the world, and multiple herbivore species can interact to regulate ecosystem processes and functions (Schemske et al., 2009). Hence, studies focusing on herbivory of a single focal plant species and by a single herbivore species may provide misleading information regarding biodiversity conservation and agroecosystem management. By investigating herbivory from multiple herbivores and across six different plant communities, we found that the addition of phylogenetically distant plants and the mixture of phylogenetically close plants can have opposite effects on herbivory. Specifically, mixing closely related tree species diluted herbivory of generalist pest herbivores, but amplified the impact from specialist pest. In contrast, mixing phylogenetically distant tree species enhanced the herbivory of generalist pest while diluting the herbivory effects from specialist pest. As mixed plantations are widely acknowledged as a useful management strategy, our results highlight the need to carefully consider the combined effects of multiple herbivore types on overall herbivore load and damage. Our results also emphasized the importance of which plants being added into monoculture forests.
By comparing mixed stands 1 and 3 with the pure “green Cocos nucifera” stand, we found that increasing the number of distantly related tree species can increase herbivory from generalist pest (i.e., increasing “green Cocos nucifera” generalist pest degree of herbivory). This is consistent with some previous studies (Schuldt et al., 2010, 2012, 2014) which find that increasing plant diversity can dilute pest herbivore. This finding can be explained, in part, by the widely acknowledged “associational susceptibility hypothesis,” which claims that generalist herbivores need many distantly related plants to increase their herbivore load, thereby increasing herbivore intensity (i.e., increased damage percentage) (Brown and Ewel, 1987; Jactel and Brockerhoff, 2007; Unsicker et al., 2008; Plath et al., 2012). For instance, more distantly related plants in a community may benefit the growth of generalist herbivores via dietary mixing (Bernays et al., 1994). Likewise, high diversity stands may increase damage from non-preferred host species due to spillover effects from preferred hosts (Power and Mitchell, 2004). However, we propose an alternative explanation, which is that phylogenetically distant plant species may support many new generalist herbivore species and increase total abundance of generalists found in monocultures, which may in turn lead to a higher possibility of obtaining high generalist herbivory intensity (i.e., sampling effect). Indeed, in mixed stand 1, the number of generalist herbivores was 1.4 times greater than the pure “green Cocos nucifera” stand. Moreover, 10 new generalist herbivore species were found in mixed stand 1.
We found that increasing the number of closely related tree species can reduce the generalist degree of herbivory in stand of pure “green Cocos nucifera”. This can also be explained by the associational susceptibility hypothesis. Nevertheless, another potential reason is that phylogenetically similar plant species may result in new generalist species for “green Cocos nucifera”, which may in turn compete with or directly prey upon the generalist pest of “green Cocos nucifera”. Indeed, although one new generalist species emerged, 11 generalist pest herbivore species in the pure “green Cocos nucifera” stand disappeared in mixed stand 3. Moreover, herbivore abundance in mixed stand 3 decreased 2-folds compared with the pure “green Cocos nucifera” stand.
By comparing mixed stands 2 and 4 with the pure Pinus elliottii stand, we found the effects of phylogenetic relatedness on herbivory differed for specialist pest herbivores compared with generalist pest herbivores. For Pinus elliottii, the degree of herbivory of the specialist pest, Dendrolimus puntatus, was reduced to zero when it was mixed with ten phylogenetically distant tree species (i.e., mixed stand 2). This result suggests that mixed plantation of phylogenetically distant plant species can have a clear dilution effect on herbivory intensity from specialist pest. This pattern can be explained by the associational resistance hypothesis, which claims that specialist herbivores prefer more homogeneous neighbors to their preferred host plants to increase their loads (Root, 1973; Agrawal et al., 2006; Lewinsohn and Roslin, 2008). Thus, increasing the numbers of phylogenetically distant tree species will dilute specialist herbivores, thereby decreasing the abundance of specialist herbivores (Tahvanainen and Root, 1972; Barbosa et al., 2009). However, another possibility is that phylogenetically distant tree species are favored by generalist herbivores that may compete with or directly prey upon the specialist herbivores (Brown and Ewel, 1987). Indeed, we observed 24 generalist herbivore species in mixed stand 2, but we cannot observe original specialist pest (Dendrolimus puntatus) anymore.
The degree of herbivory of Pinus elliottii by the specialist pest herbivore was 1.44 times greater when it was mixed with phylogenetically close tree species (mixed stand 4), thus providing support for the associational resistance hypothesis. However, it is also highly possible that phylogenetically close tree species (Pinus caribaea) can trigger new specialists, which in turn results in higher specialist degree of herbivory via sampling effects. Indeed, we ended up sampling a new specialist (Dioryctria splendidella) in mixed stand 4.
5. Conclusions
Our study provides clear evidence that herbivore specialization and plant phylogenetic relatedness interact to influence the efficiency of mixed plantations on the regulation of pest herbivory in agroecosystems. Mixing phylogenetically distant tree species with monocultures enhanced herbivory by generalist herbivores, while simultaneously diluting herbivory by specialist herbivores. In contrast, mixing phylogenetically close tree species with monocultures can give rise to totally opposite influences on generalist and specialist herbivory. Our results may differ with those observed on a single focal plant species attacked by a single herbivore species. Although investigations in other agroecosystems are needed to test the generality of the conclusion, our results suggest that both herbivore specialization and plant phylogenetic relatedness should be taken into account when using mixed plantations to manage pest herbivory in agroecosystems.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
QY and XC: conceptualization, methodology, writing—original draft preparation, and writing—reviewing and editing. Both authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Special Technical Innovation Project of Provincial Scientific Research Institutes (jscx202023).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1187996/full#supplementary-material
References
Agrawal, A. A., Lau, J. A., and Hambäck, P. A. (2006). Community heterogeneity and the evolution of interactions between plants and insect herbivores. Quart. Rev. Biol. 81, 349–376. doi: 10.1086/511529
Andrew, N. R., and Hill, S. J. (2017). Effect of climate change on insect pest management. Environ. Pest Manage. 2017, 195–223. doi: 10.1002/9781119255574.ch9
Barbosa, P., Hines, J., Kaplan, I., Martinson, H., Szczepaniec, A., Szendrei, Z., et al. (2009). Associational resistance and associational susceptibility: having right or wrong neighbors. Ann. Rev. Ecol. Evolut. System. 40, 1–20. doi: 10.1146/annurev.ecolsys.110308.120242
Bernays, E. A., Bright, K. L., and Gonzalez, N. (1994). Dietary mixing in a generalist herbivore: tests of two hypotheses. Ecology 75, 1997–2006. doi: 10.2307/1941604
Brown, B. J., and Ewel, J. J. (1987). Herbivory in complex and simple tropical successional ecosystems. Ecology 68, 108–116. doi: 10.2307/1938810
Castagneyrol, B., Jactel, H., Vacher, C., Brockerhoff, E. G., and Koricheva, J. (2014). Effects of plant phylogenetic diversity on herbivory depend on herbivore specialization. J. Appl. Ecol. 51, 134–141. doi: 10.1111/1365-2664.12175
Grossman, J. J., Cavender-Bares, J., Reich, P. B., Montgomery, R. A., and Hobbie, S. E. (2018). Neighborhood diversity simultaneously increased and decreased susceptibility to contrasting herbivores in an early stage forest diversity experiment. J. Ecol. 107, 1492–1505. doi: 10.1111/1365-2745.13097
Jactel, H., and Brockerhoff, E. G. (2007). Tree diversity reduces herbivory by forest insects. Ecol. Lett. 10, 835–848. doi: 10.1111/j.1461-0248.2007.01073.x
Jactel, H., Petit, J., Desprez-Loustau, M. L., Delzon, S., Piou, D., Battisti, A., et al. (2012). Drought effects on damage by forest insects and pathogens: a meta-analysis. Global Change Biol. 18, 267–276. doi: 10.1111/j.1365-2486.2011.02512.x
Klapwijk, M. J., Myres, A. M. P., Battisti, A., and Larsson, S. (2012). “Assessing the impact of climate change on outbreak potential,” in Insect Outbreaks Revisited, eds P. Barbosa, D. K. Letourneau and A. A. Agrawal 429–450. doi: 10.1002/9781118295205.ch20
Lewinsohn, T. M., and Roslin, T. (2008). Four ways towards tropical herbivore megadiversity. Ecol. Lett. 11, 398–416. doi: 10.1111/j.1461-0248.2008.01155.x
Lin, M., Han, Y., Li, W., Liu, F., Xu, W., Ao, S., et al. (2010). Monitoring and survey on pest insects and diseases of coconut trees in Hainan. Plant Quarant. 24, 21–24.
Mouquet, N., Devictor, V., Meynard, C. N., Munoz, F., Bersier, L. F., Chave, J., et al. (2012). Ecophylogenetics: advances and perspectives. Biol. Rev. 87, 769–785. doi: 10.1111/j.1469-185X.2012.00224.x
Pimm, S. L., Jenkins, C. N., Abell, R., Brooks, T. M., Gittleman, J. L., Joppa, L. N., et al. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752–1246752. doi: 10.1126/science.1246752
Plath, M., Dorn, S., Riedel, J., Barrios, H., and Mody, K. (2012). Associational resistance and associational susceptibility: specialist herbivores show contrasting responses to tree stand diversification. Oecologia 169, 477–487. doi: 10.1007/s00442-011-2215-6
Power, A. G., and Mitchell, C. E. (2004). Pathogen spillover in disease epidemics. Am. Natural. 164, S79–S89. doi: 10.1086/424610
Root, R. B. (1973). Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecol. Monog. 43, 95–124. doi: 10.2307/1942161
Schemske, D. W., Mittelbach, G. G., Cornell, H. V., Sobel, J. M., and Roy, K. (2009). Is there a latitudinal gradient in the importance of biotic interactions? Ann. Rev. Ecol. Evol. System. 40, 245–269. doi: 10.1146/annurev.ecolsys.39.110707.173430
Schuldt, A., Assmann, T., Bruelheide, H., Durka, W., Eichenberg, D., Härdtle, W., et al. (2014). Functional and phylogenetic diversity of woody plants drive herbivory in a highly diverse forest. New Phytol. 202, 864–873. doi: 10.1111/nph.12695
Schuldt, A., Baruffol, M., Böhnke, M., Bruelheide, H., Härdtle, W., Lang, A. C., et al. (2010). Tree diversity promotes insect herbivory in subtropical forests of south-east China. J. Ecol. 98, 917–926. doi: 10.1111/j.1365-2745.2010.01659.x
Schuldt, A., Bruelheide, H., Durka, W., Eichenberg, D., Fischer, M., Kröber, W., et al. (2012). Plant traits affecting herbivory on tree recruits in highly diverse subtropical forests. Ecol. Lett. 15, 732–739. doi: 10.1111/j.1461-0248.2012.01792.x
Schuldt, A., Bruelheide, H., Härdtle, W., Assmann, T., Li, Y., Ma, K., et al. (2015). Early positive effects of tree species richness on herbivory in a large-scale forest biodiversity experiment influence tree growth. J. Ecol. 103, 563–571. doi: 10.1111/1365-2745.12396
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatic 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Tahvanainen, J. O., and Root, R. B. (1972). The influence of vegetational diversity on the population ecology of a specialized herbivore, Phyllotreta cruciferae (Coleoptera: Chrysomelidae). Oecologia 10, 321–346. doi: 10.1007/BF00345736
Tilman, D., Isbell, F., and Cowles, J. M. (2014). Biodiversity and ecosystem functioning. Ann. Rev. Ecol. Evol. System. 45, 471–490. doi: 10.1146/annurev-ecolsys-120213-091917
Unsicker, S. B., Oswald, A., Kohler, G., and Weisser, W. W. (2008). Complementarity effects through dietary mixing enhance the performance of a generalist insect herbivore. Oecologia 156, 313–324. doi: 10.1007/s00442-008-0973-6
Vehvilainen, H., Koricheva, J., and Ruohomaki, K. (2007). Tree species diversity influences herbivore abundance and damage: meta-analysis of long-term forest experiments. Oecologia 152, 287–298. doi: 10.1007/s00442-007-0673-7
Xu, L. N., Xue, Y., Wang, X. Y., Su, X. F., and Lin, Z. P. (2014). The survey and control strategy of Pinus elliottii × Pinus oaribaea. Trop. Forestry 2, 21–23. doi: 10.3969/j.issn.1672-0938.2014.02.006
Xue, Y., Robert, J., Liu, X., Wang, X. Y., Su, S. F., Tan, Z. Y., et al. (2021). Rocket launching activities are associated with reduced insect species richness and abundance in two types of tropical plantations around the Wenchang Satellite Launch Center, southern China. Ecol. Indic. 127, 17751. doi: 10.1016/j.ecolind.2021.107751
Keywords: phylogenetic relatedness, herbivore specialization, plant-insect herbivore interaction amplification effect, dilution effect, generalist, herbivory, specialist
Citation: Yang Q and Chen X (2023) Plant phylogenetic relatedness and herbivore specialization interact to determine pest biocontrol efficiency in mixed plantations. Front. Ecol. Evol. 11:1187996. doi: 10.3389/fevo.2023.1187996
Received: 16 March 2023; Accepted: 11 April 2023;
Published: 28 April 2023.
Edited by:
Xiang Liu, Lanzhou University, ChinaCopyright © 2023 Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohua Chen, OTY1ODE5ODMzQHFxLmNvbQ==
 Qingqing Yang
Qingqing Yang Xiaohua Chen*
Xiaohua Chen*