
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Ecol. Evol. , 10 August 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1187711
 John Heydinger1,2*
John Heydinger1,2*Community-based conservation of the desert-adapted lions takes place within the semi-arid and arid environments of northwest Namibia. This area is primarily designated as communal conservancies, a form of community-based natural resource management (CBNRM). The article describes the activities of the Lion Rangers program, a CBNRM organization, emphasizing how the use of remote sensing techniques, including the Spatial Analysis and Report Tools (SMART) software and mobile-app, GPS/satellite collars, and trail cameras, contribute to lion monitoring and limiting human–lion conflict (HLC). Remote sensing data are being integrated with historical and sociological research, with applicable lessons for lion conservation and conservation of other problem-causing species.
Community-based natural resource management (CBNRM) of the desert-adapted lions (Panthera leo) presents an array of challenges to local communities. Within the semi-arid and arid environments of the Kunene Region (northwest Namibia), lions were historically not monitored on a wide scale. In 2017, Namibia’s Ministry of Environment, Forestry and Tourism released the Human Lion Conflict Management Plan for North West Namibia (NW Lion Plan) (GRN, 2017), which prioritizes limiting human–lion conflict (HLC) to support the survival of the northwest lion population. The NW Lion Plan defines HLC as “any event in which lions harm or destroy human life or their domestic livestock, or in which wild lions are injured, captured or destroyed as a result of a perceived threat to humans or their property” (GRN, 2017: 5). Among the recommendations adopted from this plan was the formation of the Lion Rangers program1, a community-based organization whereby locals take responsibility for monitoring lions and managing, mitigating, and preventing HLC whenever possible.
This Perspective focuses on the Lion Rangers program, emphasizing certain approaches to limiting HLC in northwest Namibia and the application of remote sensing technologies for lion monitoring and conservation in this largely unfenced, rugged landscape. Based upon the work of the Lion Rangers and ongoing research, HLC is revealed to be chronic, yet situational and locally specific (Heydinger, 2020a; Heydinger, 2021). Integrating remote sensing technologies into locally-centered HLC interventions is part of adaptive management to lessen HLC where eradication is not possible. Different remote sensing techniques are reviewed. I close with a brief discussion of the lessons learned, including the importance of historical research as part of forging locally-appropriate and durable community-centered lion conservation, both for northwest Namibia and elsewhere.
Desert-adapted lions occupy an area of approximately 40,000 km2. This includes 11 communal area conservancies (Anabeb, Doro!Nawas, Ehi-rovipuka, ǂKhoadi-ǁHoas, Omatendeka, Orupupa, Puros, Sesfontein, Sorris, Torra and Tsiseb) as well as the Hobatere, Etendeka, and Palmwag tourism concessions, and part of the Skeleton Coast National Park (Stander, 2007; Figure 1). This area consists of varied landscapes including the northern Namib Desert, rugged mountains, and gravel plains bisected by east-to-west ephemeral riverbeds. Northwest Namibia’s basaltic soil is shallow, rocky, and relatively unproductive (Mendelsohn et al., 2002). Rainfall is generally low (50–250 mm per year) and erratic, increasing from west to east. During the wet season (January–May) rains fall in brief, localized downpours. Prey species, including gemsbok (Oryx gazella), mountain zebra (Equus zebra), giraffe (Giraffa camelopardalis), springbok (Antidorcas marsupialis) and kudu (Tragelaphus strepsiceros) maintain seasonal movements driven by patchy rainfall and subsequent available grasses and browse. Other iconic desert-adapted species include black rhinoceros (Diceros bicornis) and elephant (Loxodonta africana). During the dry season (June–December) prey often congregate in riverbeds. Due to an intensive government borehole-drilling program during the 1970s, much of the region is considered grazing-, not water-limited (Bollig, 2020). From 2000 to 2010, the region experienced a relatively wet period, resulting in wildlife and livestock increases. From 2011 to 2017, extensive drought caused the decline of indicator prey species by as much as 60% and livestock by as much as 67% (Heydinger et al., 2019). Since that time, a modest increase in rainfall appears to be leading to recovering wildlife numbers (NACSO, 2022).
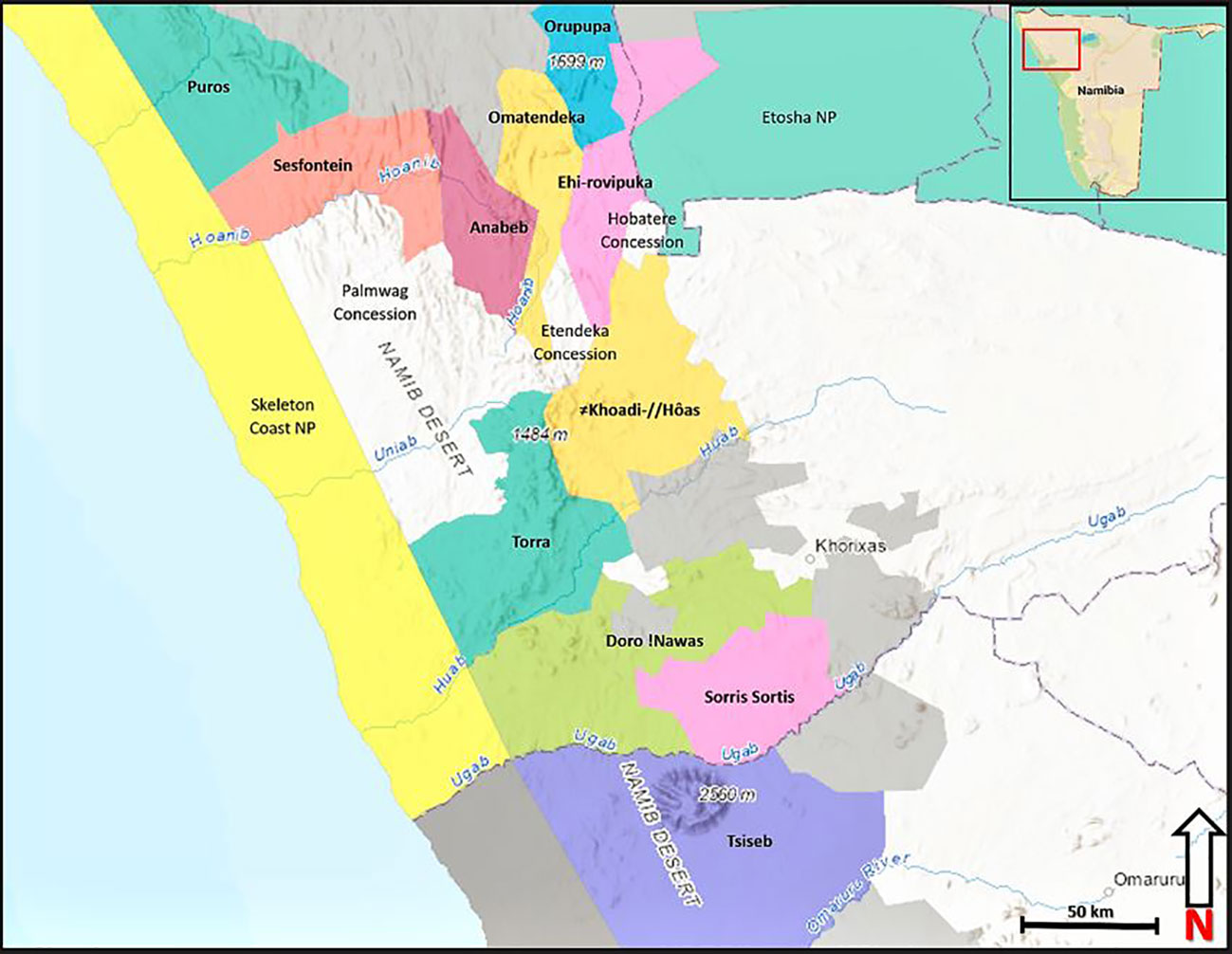
Figure 1 Core lion-range conservancies and neighboring government lands within the Kunene Region, northwest Namibia. Colors of each conservancy and relevant government lands are illustrative.
Core lion-range conservancies are home to approximately 19,300 rural residents, primarily Otjiherero and Damara-speaking peoples. Most are small-scale pastoralists for whom drought and predation represent significant threats to livelihoods. Due to limited economic alternatives, livestock farming will remain the main source of income with the region for the foreseeable future (Atlas of Namibia Team, 2022). Lions alone account for approximately 20% of livestock losses (Heydinger et al., 2019). Household incomes are generally low and insecure. Forty percent of Kunene Region residents earn ≤ US$1/day, while twenty-three percent earn ≤ US$0.73/day (NNPC, 2012). Livelihoods have been further hampered by a downturn in tourism receipts stemming from the COVID-19 pandemic (Lendelvo et al., 2020). Additionally, Kunene has Namibia’s highest drop-out rate: only fifty-five percent of residents complete primary school by age seventeen (UNICEF, 2013).
The desert-adapted lions are iconic, demonstrating unique grouping patterns (Stander, 2018) and movements (GRN, 2017) within northwest Namibia’s semi-arid and arid environments. This relatively small, though stable, population – estimated between 56–60 individuals during a recently-completed northwest lion population survey (Heydinger and Muzuma, in press) – has experienced dramatic fluctuations during the past 30-plus years. From a low of perhaps 20 individuals in the late 1990s, to an estimated high of 180 in 2015 (GRN, 2017), population numbers appear to track with changing rainfall patterns and subsequent prey numbers, as well as resulting from the concerted efforts of communal conservancies and their residents.
The dramatic recovery of lions in northwest Namibia from 1997 to 2015 coincided with the growth of Namibia’s communal conservancy system. A form of CBNRM, communal conservancies are self-identified communities, where locals are granted conditional ownership of wildlife and stand to receive economic benefits via consumptive and non-consumptive use (Jones, 2001). As part of a counter-hegemonic conservation movement that gained momentum in the late 1980s to early 1990s, communal conservancies aim to overcome some of the social, political, and economic injustices stemming from wildlife conservation-oriented interventions during Africa’s colonial era (Owen-Smith, 2010), including the forced removal of people from protected areas (Dowie, 2009). By design conservancies incorporate mixed land-use, including livestock husbandry and wildlife conservation. There are currently 86 communal conservancies in Namibia. The country’s conservancy system is considered among the most highly-regarded examples of successful CBNRM (Dressler et al., 2010).
The challenge facing CBNRM of lions in northwest Namibia is one of costs versus benefits, revealing a certain paradox for conservancies: lions impose costs on many livestock owners without providing the benefits to match. Under Namibia’s Nature Conservation Amendment Act (No. 5/1996), communal area residents maintain limited rights to “huntable game” species via their conservancy. However, lions are designated as a protected species (Nature Conservation Act No. 4/1975), and thus not subject to standard hunting regulations. Heydinger et al. (in press) examined how conservancies constrain residents’ ability to manage and benefit from lions, revealing that many residents question the justification for living alongside lions. HLC, which overwhelmingly occurs at night and outside of livestock enclosures while livestock are grazing away from homesteads, imposes significant costs upon livestock-owning farmers in conservancies. Surveys of communal farmers estimated that livestock losses from HLC have imposed an average cost of US$ 2,985 per household in recent years, while losses from all forms of human–carnivore conflict stand at US$ 10,151 over the same period (average estimated value of a cow: US$ 468; sheep: US$ 86; goat: US$ 109; donkey: US$ 70) (Heydinger et al., 2019). Rated among Namibia’s poorest regions, extant economic and social challenges facing residents are exacerbated when lions destroy livestock, endangering households’ abilities to meet day-to-day needs such as school and clinic fees. Surveyed communal farmers overwhelmingly (84%) feel they do not benefit from living alongside lions. Yet, a majority (75%) state their desire for lions to persist within their conservancy, primarily so future generations may see wild lions (Heydinger et al., 2019). Furthermore, the Namibian government has consistently averred its commitment to maintaining a free-ranging lion population in the area (GRN, 2017), thus rendering HLC somewhat inevitable.
The tenuous relationship between communal farmers and lions, and between lions and the aims of conservancies, has engendered high levels of retaliatory and preventive killing of lions. Since 2000, retaliatory killings have accounted for 89% of recorded lion (non-cub) mortalities (GRN, 2017) – with more than 130 lions killed during this period (Stander, 2010; GRN, 2017). Such mortalities, biased towards male lions, may also be skewing the age structure of males towards sub-adults: nearly half of males (n = 10/21) are non-dispersed subadults (Stander, 2010; Heydinger and Muzuma, in press). High numbers of HLC may be driven by a lack of available prey species (Tavolaro et al., 2022), whose numbers declined by as much as 60% from 2010 to 2019 due primarily to the effects of drought (Heydinger et al., 2019; NACSO, 2020).
Emphasizing the importance of CBNRM as a mechanism for limiting persistent HLC and fostering durable lion conservation, the NW Lion Plan established the need to activate and capacitate the Lion Rangers. The goal of the program and associated research is to support a sustainable lion population in light of HLC. Objectives to reach this goal include limiting HLC to support livelihoods and promote the continued existence of the local lion population. Selected by conservancy management, employed by their conservancies, and capacitated by the Lion Rangers program, Lion Rangers are tasked with monitoring lion movements, providing timely information to farmers and conservancy personnel regarding lion presence, behavior, and ecology, while supporting local livelihoods by limiting HLC. Among other objectives of the NW Lion Plan, the Lion Rangers have been tasked with developing a standardized monitoring system, establishing best practices for HLC mitigation, and creating new mechanisms for reducing HLC on communal lands. Based upon social surveys (Heydinger et al., 2019) and extensive on-the-ground experience, it is believed these combined initiatives will increase local tolerance for living alongside lions, provided the financial costs of living with lions can be reduced (Heydinger et al., 2019; Heydinger et al., in press). Within the eleven core lion-range conservancies are 49 Lion Rangers. Field deployment, as well as monitoring, data collection, and conflict intervention approaches were initially based upon ecological research from Desert Lion Conservation2, while also adapting lessons from the successful Lion Guardians3 program in East Africa (Dolrenry et al., 2016), as well as the anti-poaching work of the northwest Namibia’s Save the Rhino Trust4 (Muntifering et al., 2015). Since the program’s activation in 2018, field tactics, HLC interventions, and research methods have been adapted to the unique challenge of monitoring and conserving the desert-adapted lions. Among these are the massive ranges covered by individual desert-adapted lions – the largest ever recorded (Stander, 2018) – as well as the difficulties of moving across the rugged landscape, lack of fencing, and expectations that humans, livestock, and lions will live alongside one another (Heydinger, 2020b; NACSO, 2020).
To monitor lions and minimize HLC, the Lion Rangers and program researchers draw upon three remote sensing methods. They include use of the mobile app-based Spatial Monitoring and Reporting Tool (SMART)5, fitting a high proportion of lions with GPS/satellite collars with Early-Warning capabilities, and deployment of camera-trap arrays to better understand lion presence and other large carnivore presence within core habitat patches.
Collecting relevant, timely information concerning lion movements and HLC presents a considerable challenge in northwest Namibia. Distances are vast and many areas are difficult to access. Figure 2 shows the recent expansion of lion-range in the region. Lion Rangers perform foot- and vehicle-based patrols across communal conservancies composing the core range of the desert-adapted lions. Patrol observations are recorded on the SMART Mobile app.
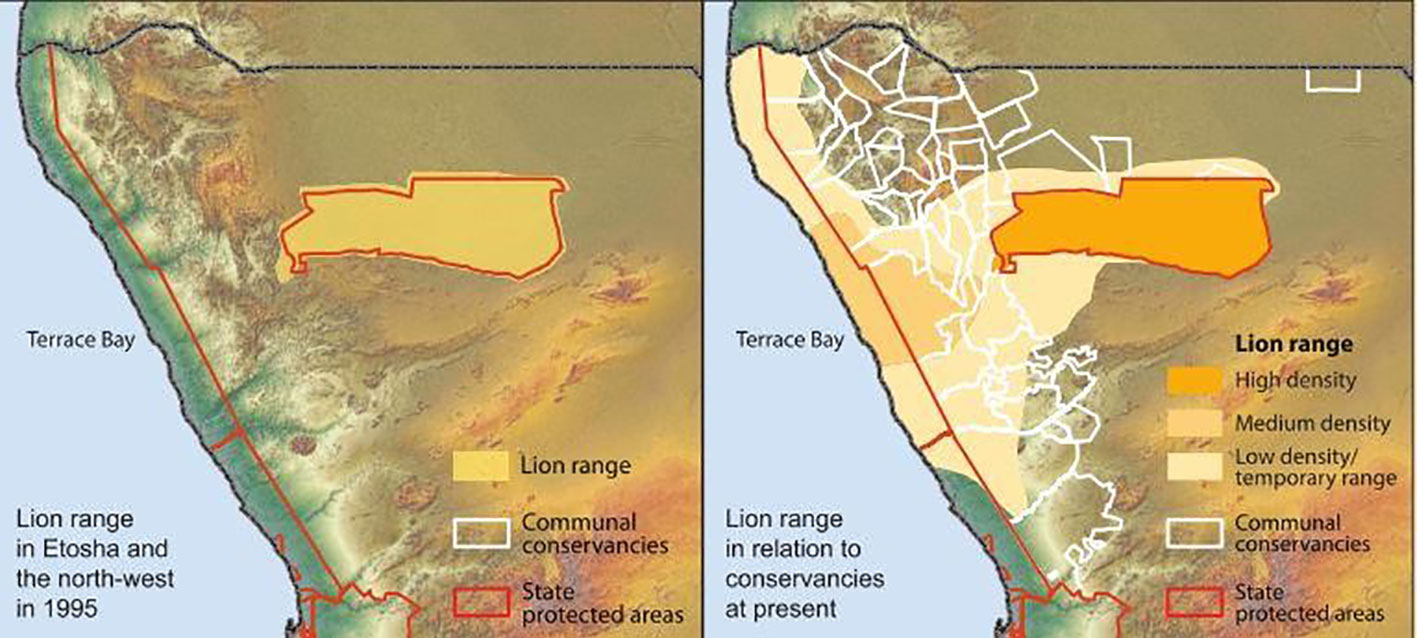
Figure 2 Lion range expansion in northwest Namibia, 1995–2015. Reprinted with permission from NACSO (2016: 40).
The SMART platform consists of a set of software and analysis tools designed to help collect, store, analyze and visualize a wide range of incoming environmental data. SMART enables rapid collection and transfer of relevant environmental data, primarily focused on lion and livestock movements, HLC, and assessing ranger performance on an ongoing basis. These data are used by program leadership as part of an adaptive management approach to ranger deployment and lion monitoring. In brief, Lion Rangers collect standardized data on the SMART Mobile app while performing regular and as-needed patrols. Data are exported via SMART Connect – either through the local cellular network or via WIFI to a centralized server. The Lion Ranger Program Administrator compiles and analyzes these data, creating monthly reports which are fed back to the Rangers, conservancy management, and government. Figure 3 visualizes the SMART workflow, from ranger patrol and data collection to the use of these data as part of adaptive management.
Beginning in April 2022, Lion Rangers began recording all patrols using SMART. By the end of 2022, a total of 5,928 separate patrols (3,550 foot; 2,378 vehicle) were completed, representing nearly 34,000 km walked and nearly 26,000 hours spent in the field monitoring lion movements. Data captured include patrol effort, lion and other carnivore sightings, prey sightings, information on livestock encountered, human–wildlife conflict incidents, animal carcasses, tracks, dung/scats, human settlements, human signs including illegal activities, the presence of fenced livestock enclosures (kraals), water bodies, and community meetings. SMART is also being used to assess Ranger performance, supporting important incentives. In 2022, the program celebrated the achievements of the top-three performing Lion Rangers with awards and prizes recognizing their demonstrated commitment to lion conservation. In just six months (May–October 2022) Uaroua Kaidue, Esau Matundu, and Uezekandavi Nguezeeta recorded 271 foot patrols, totaling more than 2,700 km. As an innovative part of community-based lion conservation, SMART is providing quantifiable evidence of the too-often-overlooked efforts of the people living alongside and conserving lions.
SMART data provide a picture of HLC. Since the deployment of SMART (May 2020), Lion Rangers have responded to 84 human-wildlife conflict incidents, including 44 HLC incidents, at which 293 livestock were killed. This represents a recent decline in HLC incidents. Since the inception of the Lion Rangers program in 2018, HLC incidents have decreased by more than 33% annually (2016: n = 126; 2019: n = 102; 2021: n = 82; 2022: n = 47) (Lion Rangers, unpublished data). Given increased Lion Ranger deployment during this period, these data represent both important reductions in conflict and improved reporting. Since the activation of the Lion Rangers program, illegal lion killing has been eliminated in Anabeb, Doro!Nawas, Puros, Orupupa, Sorris Sorris, and Tsiseb, with only two killings in Omatendeka, and a single killing in both Sesfontein, and Torra. Where illegal killings have occurred, these are leading to increased law enforcement. Since 2021, the Lion Rangers have assisted with six arrests and one conviction following lion killing incidents.
Finally, SMART played a critical role in the recently-completed Northwest Lion Population Survey. From 6 November 2022 to 6 January 2023, the Lion Rangers partnered with Namibia’s Ministry of Environment, Forestry and Tourism, as well as staff from the Namibian Lion Trust6, to perform the first-ever scientific count of lions inhabiting northwest Namibia. Based on SMART records, the Lion Rangers covered approximately 43,530 km, including 6,399 km on foot and 37,231 km by vehicle over a 54-day period. Logging 331 total SMART patrols, the Lion Rangers recorded 80 individual lion sightings, as well as landscape-wide abundance data on ten prey species, six additional large carnivores, plus elephant and black rhino. Using SMART in conjunction with related geospatial software, data from the survey are being analyzed and visualized by the Lion Rangers research team and will be made publicly-available in late-2023 (Heydinger and Muzuma, in press).
Very-high Frequency (VHF), and later GPS/satellite collaring of elusive, wide-ranging wild animals has been an important part of population biology since the 1960s (Benson, 2010). Lions have been collared across sub-Saharan Africa since that time, primarily for research purposes (Schaller, 1972; Somerville, 2019). By the 1990s lion collaring in Namibia’s Etosha National Park was common place. Beginning in 1997, Namibian Philip “Flip” Stander began collaring lions inhabiting communal lands in northwest Namibia (Stander, 2018). To date more than 100 lions have been fitted with VHF and GPS/satellite collars in the region.
Increasing availability of GPS/satellite collars at relatively affordable prices, makes these remote sensing tools an important part of lion monitoring. As of April 2023, there are 45 active GPS/satellite collars fitted to desert-adapted lions. Based upon the recently completed Northwest Lion Population Survey, this represents approximately 65% of adult (non-cub) lions, which, as a percentage of the total population, makes this the most comprehensively collared free-ranging lion population in Africa. Lions prioritized for collaring include those occupying home ranges adjacent to livestock grazing areas and other lions considered to be at high risk for HLC, such as dispersing subadult males, and females with increasing hunting responsibilities to feed cubs. Most individuals fall within one of these categories.
Lion collars in northwest Namibia are programmed to provide location fixes every four hours during the day, and every two hours at night – increasing to every hour when lions penetrate designated geofence boundaries. Location fixes are relayed via the iridium satellite network to a secure online interface, visible to area researchers and key government officials. Geofence polygons have been manually created with input from researchers, regional government staff, and the Lion Rangers, emphasizing areas where lions are likely to encounter humans and livestock. These boundaries are easily re-drawn to reflect changing livestock movements, as lion home ranges change, or when rainfall alters the geography of available grazing. Geofences have primarily been created to improve human and livestock safety. When a lion collar crosses a geofence boundary, automated SMS notifications are sent to area Lion Rangers and designated livestock owners. These alerts contain the lion’s unique identification number as well as its distance and direction from the nearest farming area. Since the inception of the geofence alert system in 2020, more than 6,800 automated SMS notifications have been sent, across every core lion-range conservancy. Anecdotal evidence from the Lion Rangers and other community members indicates these alerts are often forwarded to other residents, providing many people with information regarding lion presence near livestock grazing areas. As in other, similar alert programs (Weise et al., 2019), exact GPS locations of lions are not provided.
Geofence alerts are only part of a broader Early-Warning System, created to keep Lion Rangers, researchers, community members, and key government personnel informed about lion movements. Five Lion Ranger Rapid Response Teams are responsible for responding to potential or actual conflict when lions within geofence boundaries acutely threaten livestock. Each Rapid Response Team has full-time use of a 4×4 vehicle, enabling them to move across the landscape at speed. Each vehicle is outfitted with a mobile “rover” unit. These rovers are linked to the iridium satellite network, enabling Rapid Response Teams to receive geofence alerts and query individual lion collar locations, even in areas without cellular coverage. Rover units can also communicate with each other as well as preselected cell phone numbers, enabling timely communication between Lion Rangers and other program personnel.
Finally, collar locations are automatically communicated to Early-Warning Towers, which have been deployed to key HLC hotspot farms (Figure 4). Standing 4–5 meters tall, these towers are constantly scanning for radio-frequency identification (RFID) tags affixed to lion collars. When the tags are detected the Early-Warning Towers alert farmers, via bright lights and sirens mounted on the tower, that collared lions are nearby. This enables farmers to make informed decisions about lion presence, including whether to move or kraal their livestock. Since the towers work round-the-clock, they inform farmers when lions are approaching during the night, serving as a back-up when geofence alerts and Rapid Response are insufficient. Currently there 14 Early-Warning Towers across the landscape. To date no lion has penetrated a livestock kraal where Early-Warning Towers are operational. As funding allows, chain-link and aluminum “predator-proof” kraals are also erected at HLC hotspots. To date 97 predator-proof kraals have been built, with only one incident of lions getting into such kraals.
Recently completed surveys among 339 livestock owners reveal favorable impressions of the Early-Warning System, as well as of the Lion Rangers and Rapid Response Teams. However, more work is needed to inform community members about this system. 48% (n = 157) of respondents have a positive overall attitude towards the Early-Warning System, though 41% (n = 133) stated they did not know about it. Similarly, 48% (n = 162) have a positive attitude towards the Lion Rangers, though 47% (n = 158) were either neutral or did not know about them. Rapid Response Teams were viewed positively by 39% (n = 131) of respondents, while 51% (n = 170) stated they were either neutral or unsure (IRDNC, 2022).
The Early-Warning System, from the creation of the online server and geofence boundaries, to the rover units and Early-Warning Towers, has been designed and the components manufactured by the Namibian-based firm Wide Horizons Aerial Technologies, who began partnering with Desert Lion Conservation in 2014.
Motion-activated cameras taking high-quality pictures (camera traps) augment the ability of researchers to identify unknown lions, while also providing Lion Rangers with greater information about the interactions between lions and other large carnivores. Since they were first used to estimate tiger populations (Karanth and Nichols, 1998), camera traps have been increasingly recognized as an effective tool for monitoring large carnivores and estimating their abundance (Balme et al., 2009; Williams et al., 2021). Since May 2021, the Lion Rangers research team has deployed camera trap arrays surrounding key waterpoints and along lion movement corridors in the Anabeb, Omatendeka, and Ehi-rovipuka conservancies, and within the Hobatere, Etendeka, and Palmwag tourism concessions. Each of the target areas were identified as those frequently containing numerous lions, based upon Lion Rangers’ expertise. Cameras are generally mounted on trees or rocky outcrops, between 80–200 cm high, 2–3 meters from game trails or other paths such as 4×4 tracks, with camera lenses positioned to record images at approximately the flank height of an adult lion (70–90 cm). To maximize detection probability (Hofmeester et al., 2021), a “camera-blitz” approach was used, whereby all 80-plus available cameras are deployed together along movement corridors and near waterholes. Specific camera placement is decided upon by researchers and the Lion Rangers, reading visible tracks of lions, other large carnivores, and prey, prioritizing photographic captures of lions. Each photograph is categorized according to date, time, and location.
Since their initial deployment, cameras have been active for 11,419 camera days (number of cameras × number of days deployed), recording 43,737 images of lions, large carnivores, and target prey species. This includes 591 lion images. Adjusted for camera effort, these data provide important insight into relative landscape use by lions. Across four camera trap arrays, lions were present in between 0.002% and 0.09% of all camera trap photos. Analysis of the ecological and management implications of different relative lion presence is ongoing.
Scrutiny of camera trap images enables program researchers to identify individual lions. Given the low overall population and extremely low density (0.11 lions/100km2) (Heydinger and Muzuma, in press), time and location of each photographic capture, along with demographic markers such as sex and age, as well as diagnostic markings such as ear tears, scars, and whether the lion is collared, combine to enable our research team to differentiate among individuals with a high degree of confidence. This both provides greater information to researchers and the Lion Rangers in terms of lion movements, as well as being useful for identifying individuals to be targeted for collaring.
While cameras were deployed to maximize lion photographic captures, these data are also providing the first intensive visual record of leopard (Panthera pardus) (n = 41 images), spotted hyena (Crocuta crocuta) (n = 200), brown hyena (Hyaena brunnea) (n = 209), and cheetah (Acinonyx jubatus) (n = 16) in northwest Namibia. This has implications for refining Lion Rangers operations and management of area carnivores. For example, unexpectedly high number of brown hyena photos in mountainous areas have necessitated refining Lion Rangers field methods, including paying greater attention to differences in spotted hyena and brown hyena tracks, to gauge more accurately the presence and abundance of each. Camera data are also contributing to a renewed interest by government in implementing a northwest hyena population survey. Forthcoming publications are examining the likelihood of photographic capture of lions and other large carnivores given different site covariates, as well as carnivore–prey spatial overlap. Because hyena, cheetah, and leopard all are responsible for greater numbers of human–carnivore conflict than lions are, though they are not killed in retaliation as often (Heydinger et al., 2019), monitoring the movements of these species is also an important part of limiting the economic effects of human–carnivore conflict.
Each remote sensing data stream forms part of the information needed for the Lion Rangers to mitigate, manage, and prevent HLC. Emphasizing local involvement via the use of CBNRM, is central to minimizing the costs of living alongside lions while increasing locals’ role in their conservation. SMART patrol data provide timely information and landscape-wide data on the movements of lions, other large carnivores, and prey species, as well as the deployment of the Lion Rangers themselves and changing locations of livestock herds, human infrastructure, and conflict incidents. Over time we anticipate these data will become a useful longitudinal picture of environmental and human covariates. GPS/satellite collars are providing an increasingly comprehensive picture of lion movements. This allows researchers and the Lion Rangers to increasingly “know” the region’s lions; resulting in improved monitoring and even anticipating certain lion movements. Paired with the multi-level Early-Warning System and work of the Lion Rangers and Rapid Response Teams, collar information is helping limit HLC, thereby decreasing the financial costs of living with lions. Camera trap data augments these remote sensing approaches, serving as the backbone of further lion and other large carnivore research, while also helping monitor wildlife movements in key areas.
Crucially, remote sensing data are interpreted, and subsequent interventions are designed, considering historical and social research information, which informs the goals of desert-adapted lion conservation. Foremost among these is a commitment to CBNRM-based approaches. To meet the goals of CBNRM remote sensing data must be collected and disseminated in a socially-inclusive manner. SMART empowers the Lion Rangers as the frontline monitors of wildlife and related resources. Lion Rangers decide what environmental variables are worth recording, and are part of an iterative process to refine the mobile app, making it more user-friendly and representative of their daily activities. Though data analysis occurs using technical computer programs, monthly reports are provided to each conservancy. This maintains an open line of communication between researchers, technical staff, the Lion Rangers, and conservancy management.
Through the Early-Warning System, lion movement data are directly communicated to the Lion Rangers and other conservancy personnel, who are empowered to make intervention and management decisions. This is important for community-based management and is fostering accountability. Researchers are accountable for collaring conflict-causing lions as well as maintaining the Early-Warning System. Lion Rangers and other conservancy personnel are accountable for disseminating relevant information and responding to actual and potential HLC. Camera data augments these other data streams, providing spatially-explicit information on numerous species, while also suggesting new research topics for minimizing human–carnivore conflict.
Remote sensing techniques are also supporting local livelihoods. In addition to limiting livestock losses, the Lion Ranger program provides employment, linking the presence of lions to income. By providing training in topics such as wildlife tracking, first aid, drug and alcohol awareness, and law-enforcement techniques, the program improves the skills and increases the social stature of the Lion Rangers. Data from SMART, collars, and trail cameras are also contributing to a Wildlife Credits project (Wildlife Credits, 2023), whereby conservancies receive monetary compensation for lion presence (Heydinger et al., 2022). These funds offset the financial costs of living with lions, while encouraging conservation performance by forging positive economic linkages between lions and communal area residents.
In northwest Namibia, as it is in other places, HLC is simultaneously timeless, as well as situational and locally specific (Heydinger, 2020a; Heydinger, 2021). Where humans, livestock, and lions all inhabit unfenced communal lands, HLC will not be eradicated (Heydinger, 2020b). A livestock-based economy, coupled with interest among government and conservationists for desert-adapted lions to persist on communal lands, ensures its perpetuation. Rather, interventions must address environmental and social factors.
In vast arid rangelands, it is infeasible for under-resourced farmers to always monitor livestock. Simultaneously, desert-adapted lions are highly-mobile, maintaining massive home-ranges and irregular grouping patterns punctuated by periods where prides split into smaller groups, often for weeks or months (GRN, 2017; Stander, 2018). Heydinger et al. (in press) have examined some of the unique aspects of desert-adapted lion ecology and how these affect HLC. During times of strain, such as the depths of the dry season, when prey are scarce, or are scattered following patchy rainfall events, lions struggle to meet energetic requirements. When this happens, they often move far-afield, encountering livestock, who are also forced to trek extensively for browse and grazing.
A common refrain among frustrated conservationists includes some version of “why don’t farmers kraal their livestock every night?” In unfenced areas of low productivity, this is not always possible. One Sesfontein pastoralist responds succinctly: when asked why her livestock often remain outside the kraal at night, she responded that stock tramping back and forth over long distances for grazing, and subject to overcrowding inside the kraal, will lose 25 to 30 percent of their body condition. By comparison, if they are allowed to sleep in the field, perhaps they will be killed by carnivores (see Heydinger, 2021). Such an experience matches the challenges facing arid pastoralists elsewhere (van Sittert, 1998) and appears to broadly reflect many farmers’ attitudes. Furthermore, many livestock owners do not have the resources to erect and maintain reliably secure enclosures.
Difficulties kraaling livestock and inability of communal farmers to proactively respond to lion presence are tied to historical and contemporary socio-economic inequalities. Lack of financial resources, stemming from legacies of White supremacy still experienced by many residents, and unequal access to firearms, ammunition, and industrial poisons exacerbate the challenges of HLC faced by many residents (see: Heydinger, 2020a; Heydinger, 2021). Such challenges are not new: many contemporary complaints resemble historical ones: HLC persists in northwest Namibia because it is among the few places where lions, livestock, and people are expected to share unfenced lands (Heydinger, 2021; Heydinger et al., in press). Largely due to colonial and apartheid-era policies, Namibia’s extant lion range largely overlaps lands historically set aside for Black inhabitants, while commercial, overwhelmingly White-owned, farmlands are free of lions and most large carnivores (NCE, 2022).
The emphasis on using socially-inclusive approaches and incentives to limiting human–wildlife conflict and conserving large and dangerous mammals has proven to be scalable and transferable to other settings (e.g. Mishra et al., 2003; Muntifering et al., 2015; Dolrenry et al., 2016). Though our interventions are tailored to lions in northwest Namibia, remote sensing methods can help limit conflict with other carnivores and human–wildlife conflict elsewhere. Forthcoming research will examine the presence and livelihood effects of spotted and brown hyena in the region. Accurate and timely monitoring of wildlife movements can provide affected communities with the tools they need to mitigate, manage, and hopefully prevent human–wildlife conflict. Applications and interventions should be tailored not only to ecological, but to social circumstances, including political and economic considerations. This can help redress historical legacies of land and resource dispossession tied to wildlife conservation (Brockington, 2002; Dowie, 2009; Owen-Smith, 2010). Where effective, such technologically-sophisticated, yet socially-inclusive, approaches may support durable community-based conservation.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
The animal study was reviewed and approved by Namibia Council of Research, Science and Technology. Written informed consent was obtained from the individual(s) for the publication of any identifiable images or data included in this article.
The author confirms being the sole contributor of this work and has approved it for publication.
Thanks to Namibia’s Ministry of Environment, Forestry and Tourism (MEFT) and Namibia Council of Research Science and Technology for overseeing this work. Thanks to Mathilde Brassine for reviewing a draft and to the Reviewers and Editor. Thanks to the Community Conservation Fund of Namibia, Conservation Travel Foundation, Green Climate Fund of Namibia, KfW German Development Bank, International Union for the Conservation of Nature, Lion Recovery Fund, Oliver Adolph and Family, Natural Selection, Ultimate Safaris, University of Minnesota Lion Center, and WWF-Namibia for supporting the Lion Rangers work and research. Thanks to MEFT-Directorate of Scientific Services and Game Capture for assisting with lion collaring. Thanks to all partnering conservancies.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Atlas of Namibia Team (2022). Atlas of Namibia: its land, water and life (Windhoek: Namibia Nature Foundation).
Balme G. A., Hunter L. T. B., Slotow R. (2009). Evaluating methods for counting cryptic carnivores. J. Wildl. Manage. 73, 433–441. doi: 10.2193/2007-368
Benson E. (2010). Wired Wilderness: Technologies of Tracking and the Making of Modern Wildlife (Baltimore: Johns Hopkins University Press).
Bollig M. (2020). Shaping the African Savannah: From Capitalist Frontier to Arid Eden in Namibia (Cambridge: Cambridge University Press).
Brockington D. (2002). Fortress Conservation: The Preservation of Mkomazi Game Reserve, Tanzania (Oxford: Oxford University Press).
Dolrenry S., Hazzah L. N., Frank L. G. (2016). Conservation and monitoring of a persecuted African lion population by Maasai warriors. Conserv. Biol. 30, 467–475. doi: 10.1111/cobi.12703
Dowie M. (2009). Conservation Refugees: The Hundred-Year Conflict between Global Conservation and Native Peoples (Cambridge: MIT Press).
Dressler W., Busher B., Schoon M., Brockington D., Haynes T., Kull C. A., et al. (2010). From hope to crisis and back again? A critical history of the global CBNRM narrative. Environ. Conserv. 37, 5–15. doi: 10.1017/S0376892910000044
GRN. (2017). Human-Lion Conflict Management Plan for North West Namibia (Namibian Ministry of Environment, Forestry and Tourism, Windhoek, Namibia).
Heydinger J. (2020a). “Vermin”: predator eradication as an expression of white supremacy in colonial Namibia 1921–1952. J. South. Afr. Stud. 46, 91–108. doi: 10.1080/03057070.2020.1708011
Heydinger J. (2020b). Humans, Livestock, and Lions in Northwest Namibia. [PhD thesis] (Macquarie University/University of Minnesota).
Heydinger J. (2021). Human-lion conflict and the reproduction of white supremacy in Northwest Namibia. Afr. Stud. Rev. 64, 909–937. doi: 10.1017/asr.2021.72
Heydinger J., Diggle R., Stuart-Hill G., Dierkes K., Packer C. (2022). Differentiated payments for ecosystem services based on estimated prey consumption by lions within communal conservancies in Northwest Namibia. Ecosyst. Serv. 53, 101403. doi: 10.1016/j.ecoser.2021.101403
Heydinger J., Muzuma U. Report on the Population Survey of the Free-ranging Lions of Northwest Namibia, with Results and Recommendations (Windhoek, Namibia: Namibia Ministry of Envionrment, Forestry and Tourism). doi: 10.1016/j.biocon.2019.06.003
Heydinger J., Muzuma U., Tsaneb J. “CBNRM and the desert-adapted lion: centering local perspectives to limit human-lion conflict,” in Conservation in East and Southern Africa: People, Policy, Practice. Eds. Anderson D., Bollig M. (Cambridge University Press). Available at: http://lionrangers.org/wp-content/uploads/2022/09/HeydingerMuzumaTsaneb_CBNRMandDesertAdaptedLions.pdf.
Heydinger J., Packer C., Tsaneb J. (2019). Desert-adapted lions on communal land: Surveying the costs incurred by, and perspectives of, communal-area livestock owners in Northwest Namibia. Biol. Conserv. 236, 496–504.
Hofmeester T. R., Thorsen N. H., Cromsigt J. P. G. M., Kindberg J., Andren H., Linnell J. D. C., et al. (2021). Effects of camera-trap placement and number on detection of members of a mammalian assemblage. Ecosphere 12, e03662. doi: 10.1002/ecs2.3662
IRDNC. (2022). Human-wildlife conflict mitigation survey (Integrated Rural Development and Nature Conservation, Windhoek, Namibia).
Jones B. T. B. (2001). “African wildlife & Livelihoods: the promise and performance of community conservation,” in The Evolution of a Community-based Approach to Wildlife Management at Kunene, Namibia. Eds. Hulme D., Murphree M. W. (Oxford: James Currey), 160–176.
Karanth K. U., Nichols J. D. (1998). Estimation of tiger densities in India using photographic captures and recaptures. Ecology 79, 2852–2862. doi: 10.1890/0012-9658(1998)079[2852:EOTDII]2.0.CO;2
Lendelvo S., Mechtilde P., Sullivan S. (2020). A perfect storm? COVID-19 and community-based conservation in Namibia. Namibian J. Environ. 4, 1–15. http://hdl.handle.net/11070/3027.
Mendelsohn J., Jarvis A., Roberts C., Robertson T. (2002). Atlas of Namibia: a portrait of the land and its people (Cape Town: David Philip).
Mishra C., Allen P., McCarthy T., Madusudan M. D., Bayarjargal A., Prins H. (2003). The role of incentive programs in conserving the snow leopard. Cons. Biol. 17, 1512–1520. doi: 10.1111/j.1523-1739.2003.00092.x
Muntifering J. R., Linklater W. L., Clark S. G., Khob S., Kasaona J. K., Uiseb K., et al. (2015). Harnessing values to save the rhinoceros: insights from Namibia. Oryx, 1–8. doi: 10.1017/S0030605315000769
NACSO. (2016). The state of community conservation in Namibia: a review of communal conservancies, community forests and other CBNRM initiatives; annual report 2016. Namibian Association of CBNRM Support Organizations, MET/NACSO, Windhoek, Namibia.
NACSO. (2022). Game counts in North-West Namibia. Namibian Association of CBNRM Support Organizations. Available at: https://www.nacso.org.na/sites/default/files/North%20West%20Game%20Count-Regional%202022%20final.pdf (Accessed 7 February 2023).
NACSO. (2020). The state of community conservation in Namibia (Annual Report 2018). Namibian Association of CBNRM Support Organizations. MET/NACSO, Windhoek, Namibia.
NCE. (2022). Carnivore Status and Red List of the Terrestrial Carnivores of Namibia (Namibia Chamber of Environment, Large Carnivore Management Association of Namibia, Ministry of Environment, Forestry and Tourism, Windhoek, Namibia).
Owen-Smith G. (2010). An Arid Eden: A Personal Account of Conservation in the Kaokoveld (Johannesburg: Jonathan Ball).
Schaller G. B. (1972). The Serengeti Lion: a study in predator-prey relations (Chicago: University of Chicago Press).
Somerville K. (2019). Humans and Lions: Conflict, Conservation, and Coexistence (London: Routledge).
Stander P. E. (2007). Behaviour-ecology and Conservation of desert-adapted Lions; 2007 Progress Report of the Kunene Lion Project, Namibia (Windhoek). https://www.the-eis.com/viewfile.php?pth=data/literature/Behaviour_ecologyandConservationofdesert_adaptedLions.pdf
Stander P. E. (2010). The impact of male-biased mortality on the population structure of the desert-adapted lions of Namibia (Namibia: Desert Lion Conservation Report. Windhoek).
Tavolaro F. M., Woodgate Z., Brown C., Redpath S. M., O’Riain M. J. (2022). Multispecies study of patterns and drivers of wildlife impacts on human livelihoods in communal conservancies. Conserv. Sci. Pract., 4 e12773. doi: 10.1111/csp2.12773
UNICEF (2013). Regional education Analysis for Namibia. UNICEF, Windhoek, Namibia. https://www.unicef.org/namibia/MoE-UNICEF_2013_Regional_education_analysis_Namibia_combined1.pdf.
van Sittert L. (1998). Keeping the Enemy at Bay”: the extermination of wild carnivora in the Cape Colony 1889-1910. Environ. Hist. 3, 333–356.
Weise F. J., Hauptmeier H., Stratford K. J., Hayward M. W., Aal K., Heuer M., et al. (2019). Lions at the gates: trans-disciplinary design of an early warning system to improve human-lion coexistence. Front. Ecol. Evol. 6, 1–19. doi: 10.3389/fevo.2018.00242
Wildlife Credits (2023). Available at: https://wildlifecredits.com/ (Accessed 7 February).
Keywords: remote sensing, CBNRM, lions, Panthera leo, Kunene, desert-adapted lions
Citation: Heydinger J (2023) Community conservation and remote sensing of the desert-adapted lions in northwest Namibia. Front. Ecol. Evol. 11:1187711. doi: 10.3389/fevo.2023.1187711
Received: 16 March 2023; Accepted: 12 July 2023;
Published: 10 August 2023.
Edited by:
Robert A. Montgomery, University of Oxford, United KingdomReviewed by:
Lovemore Sibanda, Cheetah Conservation Project Zimbabwe, ZimbabweCopyright © 2023 Heydinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Heydinger, aGV5ZGluZ2VyakBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.