- 1State Key Laboratory of Continental Dynamics, Shaanxi Key Laboratory of Early Life and Environments and Department of Geology, Northwest University, Xi’an, China
- 2Department of Zoology, University of Cambridge, Cambridge, United Kingdom
†Isoxys is a worldwide distributed bivalved arthropod known almost exclusively from Cambrian Burgess Shale-type Lagerstätten. Outline analyses using 34 specimens of the iconic large bivalved arthropod †Isoxys minor from the Cambrian Stage 3 (~518 Ma) Qingjiang biota and the Cambrian Stage 4 Guanshan biota, interpret that they are the same species and there is a very slight difference in the shape of the outlines of the carapaces between the two biotas. This suggests that environment might be driving intraspecific variation. Quantitative analysis of shape changes during growth using 51 specimens of †I. minor from the Qingjiang biota, reveals that its valves gradually elongate and the ratio of cardinal spines and spherical eyes relative to the valve length significantly decreases during postembryonic development. †I. minor has proportionally large cardinal spines and eyes in the earliest stages, and this allometric growth is beneficial for self-protection and foraging, which may have improved the survival rate of individuals with these characters. In addition, two of the specimens document the evidence of brood care in †I. minor, and the egg cluster occupies almost the entire dorsal region under the carapace. Compared to other early Paleozoic egg-carrying arthropods, †I. minor broods have the highest number (~300 per clutch) of small (Ø, ~0.5 mm) eggs. Since the ovigerous individuals are almost half the size of the adults, †I. minor may have possessed reproductive ability during the early life stage. The results indicate that spines played an antipredatory role for †I. minor, and that it followed an r-strategy of reproducing with many individuals at an early stage. †I. minor also represents the earliest diverging arthropod from which brood care has been documented.
1. Introduction
†Isoxys arthropod is a widely distributed taxon of the Cambrian marine ecosystem, with a large bivalved carapace bearing two prominent cardinal spines, which is occurred in Cambrian Series 2 and 3 and has a widespread paleogeographical distribution (Williams et al., 1996; Vannier and Chen, 2000; Stein et al., 2010; Fu et al., 2011; Legg and Vannier, 2013; Liu et al., 2018). Hitherto, up to 22 species of †Isoxys have been recorded from Australia (Glaessner, 1979; García-Bellido et al., 2009a), North America (Simonetta and Delle Cave, 1975; Butterfield and Nicholas, 1994; Williams et al., 1996; Briggs et al., 2008; García-Bellido et al., 2009b), North Greenland (Stein et al., 2010; Nielsen et al., 2017), France (Vannier et al., 2005), Spain (Richter and Richter, 1927; Wang, 2014), Russia (Ivantsov, 1990, 2005), and China (Hou, 1987; Shu et al., 1995; Luo et al., 1999, 2006, 2008; Zhao et al., 2005, 2011; Wang et al., 2010; Fu et al., 2011, 2014; Wen et al., 2015; Liu et al., 2018; Du et al., 2020). It is widely distributed in the continental shelf, as well as in deep-water slope areas, recovered from deposits ranging between 30° N and 30° S paleolatitude (Vannier and Chen, 2000; Vannier et al., 2009; Huang and Wang, 2014; Liu et al., 2018).
Hundreds of †Isoxys minor specimens have been reported in the Guanshan biota making it the dominant †Isoxys species in this biota (Luo et al., 2008; Wang et al., 2012; Huang and Wang, 2014). This species was initially recognized as †I. auritus (Luo et al., 1999), but due to its carapace morphology being quite different from that of †I. auritus, in particular the morphology of the cardinal spines, size of carapaces and surface ornamentation, Luo et al. in 2006 considered it as an unidentified species (Luo et al., 2006). †Isoxys minor was established by Luo and Hu in 2008 (Luo et al., 2008) and subsequently revised by various authors (Wang et al., 2012; Hu et al., 2013; Huang and Wang, 2014; Wang, 2014). Although a high number of specimens have been obtained from the Guanshan biota, only a few have revealed the soft anatomy (Hu et al., 2013; Huang and Wang, 2014).
In this study, carapace outlines of †I. minor specimens from the Qingjiang and Guanshan biotas are compared. The quantitative analysis of new specimens of †I. minor is recovered from the Qingjiang biota (Fu et al., 2019). Meanwhile, the ontogeny of this species is described in detail. In addition, exceptionally preserved specimens of †I. minor which carried an extremely large number of small eggs, are also described for the first time. The growth strategy and brooding strategy of †I. minor may serve as a key factor in driving this taxon as the most abundant †Isoxys species in both Qingjiang and Guanshan paleoecosystems. Given the phylogenetic position of †Isoxys – as an early diverging deuteropod (e.g., Fu et al., 2022) – †I. minor may represent the earliest diverging deuteropod to utilize an egg-carrying brooding strategy.
2. Materials and methods
2.1. Materials
A total of 51 specimens of †I. minor from the Qingjiang biota were analyzed in this study. Among which, 20 specimens had the trunk appendages preserved, and 13 had eyes preserved. These materials were collected from the middle member of the Shuijingtuo formation (Cambrian Series 2, Stage 3) at the Jinyangkou (prefix JY) section, Changyang County, Hubei Province, South China (Fu et al., 2019; Ma et al., 2021; Li et al., 2022). These soft-bodied fossils are preserved as dark organic carbon on fresh grey shale (Fu et al., 2019). All the studied specimens are deposited in the Shaanxi Key Laboratory of Early Life and Environments (LELE) and Department of Geology, Northwest University (NWU), Xi’an, China.
2.2. Fossil preparation and imaging
All specimens here were gathered from the laminated calcareous claystones (Fu et al., 2019). Some were further prepared with fine needles at high magnification using a Nikon SMZ 100 stereomicroscope. The fossils were photographed with a Canon EOS 5D Mark II digital camera under an incandescent lamp, controlled for remote shooting with EOS Utility 3.2. The line drawings were prepared with the Corel Draw X9 software (Bouton, 2008). All images were processed in Adobe Photoshop CC to make minor adjustments to contrast, exposure, colour balance and sharpness (Press, 2010).
2.3. Terminology
The morphological terms used for the description of †Isoxys minor are in general accordance with those used by previous authors to describe the morphology of Isoxys (Williams et al., 1996; Vannier and Chen, 2000; García-Bellido et al., 2009a; Fu et al., 2011; Aria and Caron, 2015).
2.4. Comparison of carapace outlines from the Guanshan and Qingjiang biotas
A total of 34 intact, laterally compressed specimens without deformation were selected for outline analyses. We focused on 25 specimens from the Qingjiang biota and nine previously figured specimens from the Guanshan biota (Wang et al., 2012; Huang and Wang, 2014) in this analysis (Figure 1A; Supplementary Table S1). All selected specimens were imaged in lateral view with the anterior oriented to the left; those oriented to the right were mirrored. Photographs of specimens were converted to black silhouettes on a white background using Inkscape, and saved as.jpg files (Supplementary Figure S1). These were imported into the R environment using the import_jpg function (Momocs package; Bonhomme et al., 2014; R Core Team, 2021) and converted to outlines (Supplementary Data 1). Outlines were scaled, centered, and resampled at 64-point resolution, and subjected to elliptical Fourier analysis (efourier function, Momocs package), retaining harmonics that achieve 99.9% of the total harmonic power. Eliptical Fourier analysis results were then visualized using a principal components analysis. A hierarchical clustering analysis (CLUST function, Momocs package) was then used to group carapaces by shape similarity.

Figure 1. Analysis of †Isoxys minor carapace shape and comparison between the Guanshan and Qingjiang biotas. (A) Outlines of all specimens from the Qingjiang and Guanshan biotas analyzed in this study. Specimen number at centre of carapace, with outline color indicating biota. (B) Principal component analysis (PCA) visualizing PC1 and PC2 of results of elliptical Fourier analysis on †I. minor carapace outline shapes. (C) Hierarchical clustering results on elliptical Fourier analysis of †Isoxys minor carapace shapes. (D) Principal component analysis (PCA) visualizing PC1 and PC3 of results of elliptical Fourier analysis on †I. minor carapace outline shapes. Bars in bottom right of panels (B,D), indicate contribution to overall variation of PCs, with plotted PCs in dark grey.
2.5. Quantification and statistical analysis
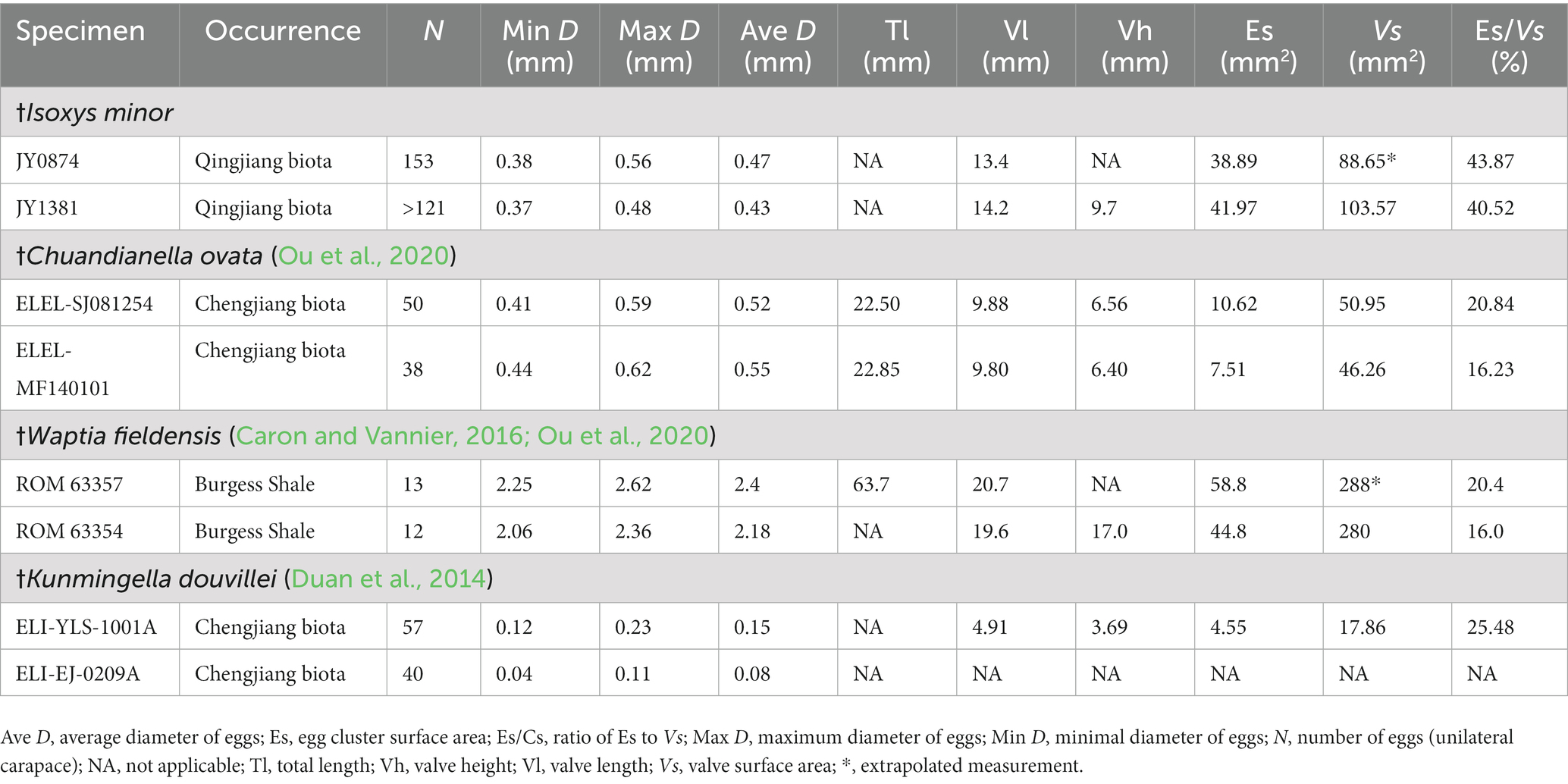
Size measurements were taken from the photographs using the software ImageJ 1.8.0 (Burger and Burge, 2006). Six parameters were documented, including the total length of the carapace, the length of the valve and cardinal spines, the height of the valve, and the diameter of the eyes (Supplementary Table S2). The valve length was measured with exclusion of’ the two cardinal spines. Regression analysis of the length (independent variable) and the height (dependent variable) of the valve was performed in Microsoft Excel (Figure 2D; Supplementary Table S3). The violin plot (i.e., box plots made of Kernel density distributions) of the carapace and cardinal spines was performed with Power BI (Figure 2E).
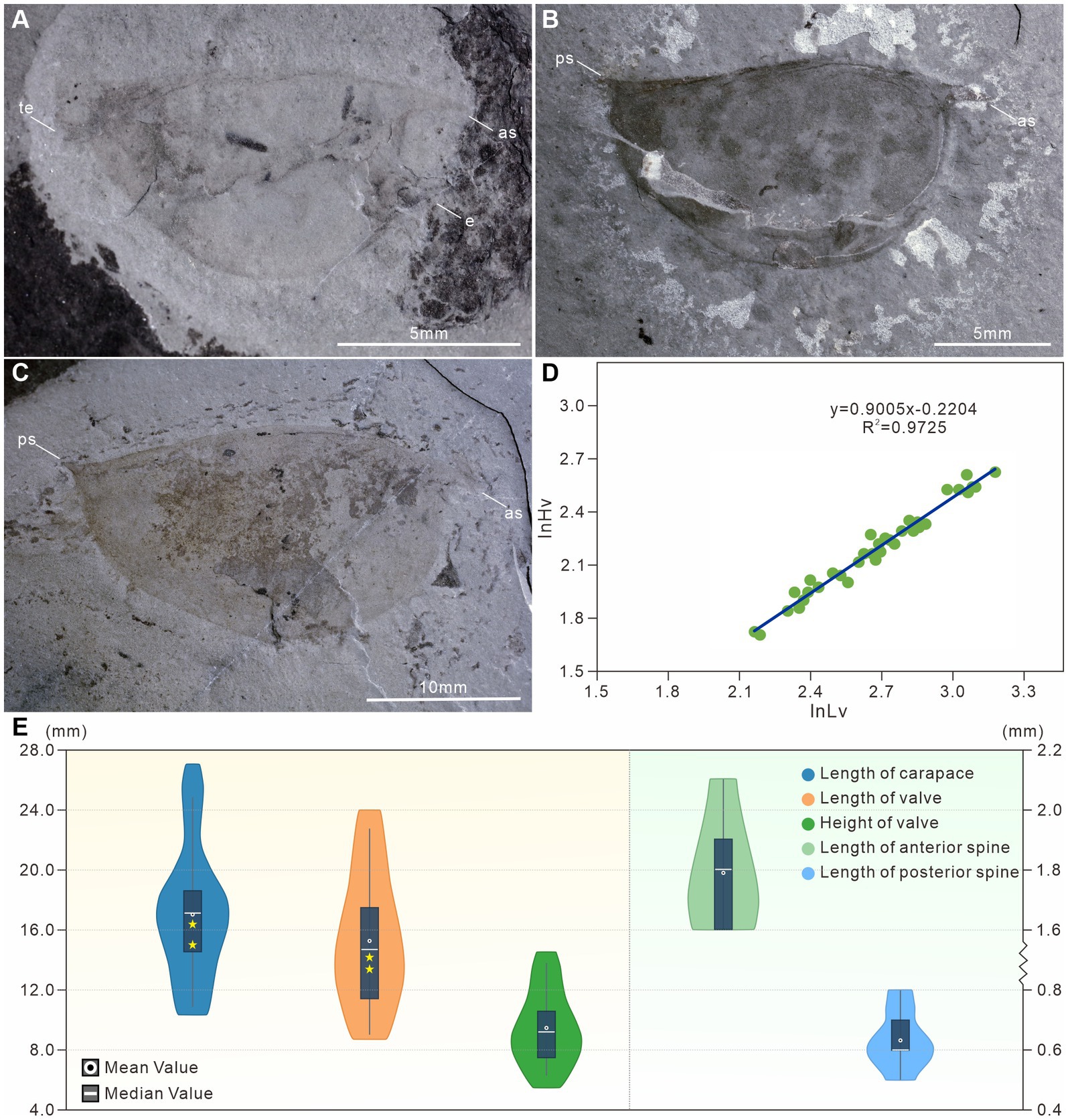
Figure 2. Statistical analysis of †Isoxys minor from the Qingjiang biota. (A) The smallest specimen with complete carapace, indistinct eyes and telson, JY1287. (B) The medium specimen with complete carapace, JY1123. (C) The largest specimen with complete carapace, JY1005. (D) Ontogenetic relationship between the length (Lv) and height (Hv) of †I. minor valve, showing the slow increase of the height with respect to the length. Regression analysis of Lv and Hv: lnLv = −0.2204 + 0.9005lnHv (N = 36, R2 = 0.9725). N, number of specimens; R2, the determination coefficient. (E) The violin plot of the valve and cardinal spines of †I. minor. as, anterior spine; e, eye; ps, posterior spine; te, telson.
3. Results
3.1. Outline analysis and hierarchical clustering
Nineteen harmonics were retained for the elliptical Fourier analysis. Principal components 1, 2, and 3 retained just under 90% of the total variation (60.1, 19.2, and 9.5% respectively). Carapaces have greater height relative to length and more robust spines with increasing values of Principal component 1 (PC1), and the anterior spine changes orientation from pointing dorsally to ventrally from negative values to positive values across this axis (Figure 1B). Spine length increases with PC2 (Figure 1B), and PC3 describes finer changes in the carapace outline (Figure 1D). Guanshan and Qingjiang specimens are separated in the PCA space, due to non-overlapping PC1 values. Guanshan carapaces have more negative PC1 values than those from Qingjiang. Guanshan and Qingjiang carapaces overlap across PC2 and PC3. The cluster analysis did not separate all Guanshan and Qingjiang specimens, instead a subset of Guanshan specimens cluster within the Qingjiang specimens (Figure 1C).
3.2. Ontogeny of †Isoxys minor
51 specimens from the Qingjiang biota were quantified and statistically analyzed to unravel the morphological changes in carapaces and soft bodies during ontogeny.
3.2.1. Carapace
The carapace length of specimens in the study ranges from 10.4 mm (JY1287) to 27.1 mm (JY1005), and the valve length ranges from 8.7 mm to 24 mm (Figures 2A,C,E). Dorsal margin clearly curved, ventral outline semicircular, the maximum height being in the mid-length of carapace. The valve L:H ratio is an average of 1.5 to 1.7 (Figures 2A–C). Regression analysis of the length of the valve against the height of the valve was performed using the 36 complete laterally compressed specimens. We analyzed the ontogenetic relationship of †I. minor by the allometric equation Y = aXb (a, the slope of the line; b, the allometric coefficient), and linearized the equation to lnY = lna + blnX. The result shows the valve of †Isoxys grew allometrically (Lv–Hv: ln Lv = −0.2204 + 0.9005 ln Hv; b < 1, N = 36, R2 = 0.9725; N, number of specimens; R2, the determination coefficient), gradually elongating during postembryonic development (Figure 2D).
The anterior spine is long and slightly curved, with lengths ranging from 1.6 mm to 2.1 mm (Figures 2C,E, 3). The ratio of anterior spine to valve length in the smallest individuals of †I. minor is 18.4%. This decreases significantly during ontogeny to a minimum ratio of 7.8% (Figures 2A,C, 3). The posterior spine is short and upturned, ranging from 0.5 mm to 0.8 mm in length (Figures 2A–C,E, 3). The ratio of posterior spine to valve length in the smallest individuals of †I. minor is 5.6%. This decreases significantly during ontogeny to a minimum ratio of 2.6% (Figures 2A–C,E, 3). Thus growth of cardinal spines was also allometric, with those of small individuals nearly the same length as those of the large individuals.
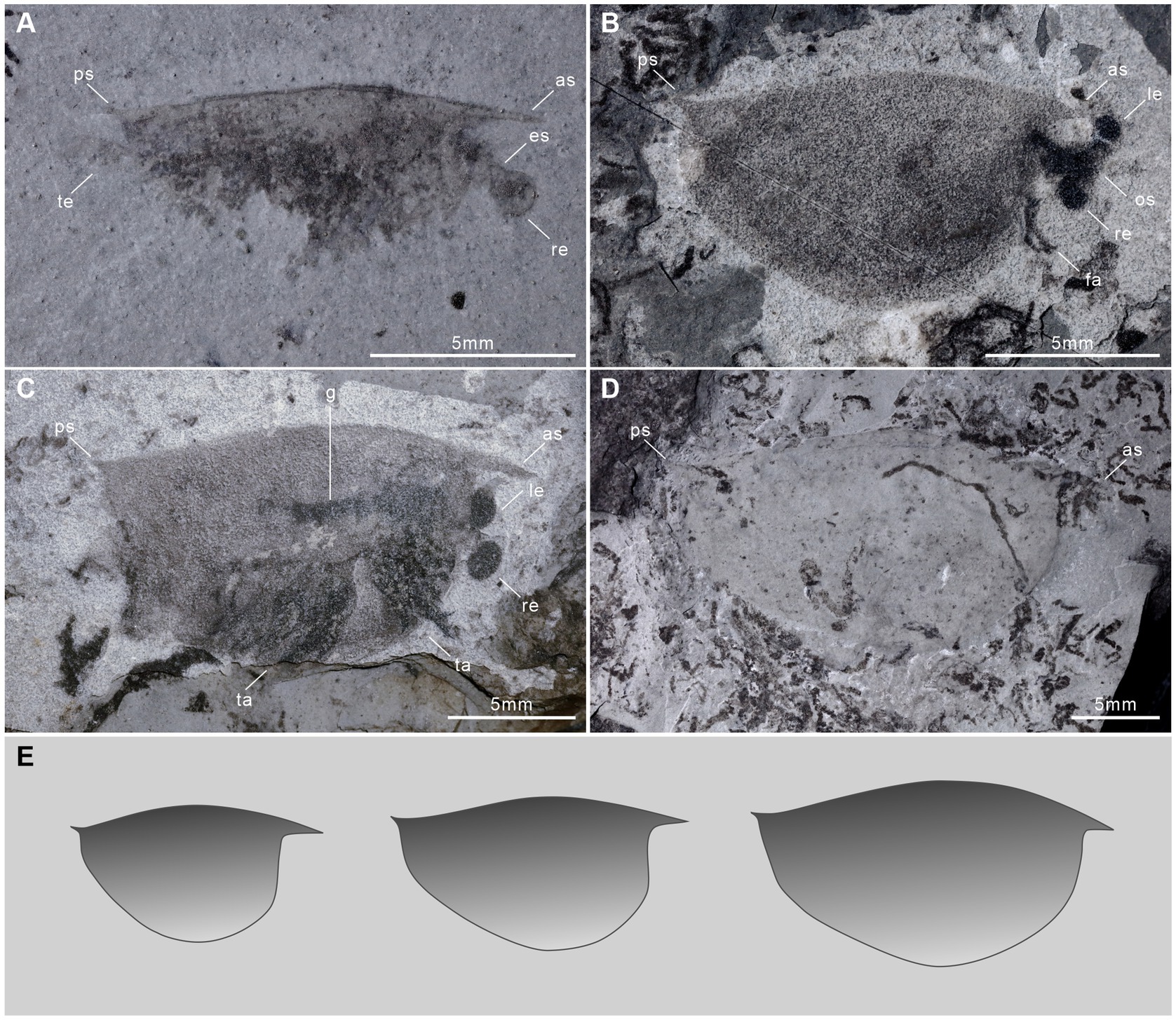
Figure 3. †Isoxys minor specimens of varying sizes from the Qingjiang biota. (A) The small specimen with complete cardinal spines and indistinct telson, showing eye stalk and eye sphere, JY1401. (B) The small specimen with complete carapace, a pair of frontal appendages, paired compound eyes and the ocular segment, JY1681A. (C) The medium specimen with distinct paired eyes, trunk appendages, and gut, JY1290A. (D) The large specimen with complete carapace, JY1491. es, eye stalk; fa, frontal appendages; g, gut; le, left eye; os, ocular segment; re, right eye; ta, trunk appendages. (E) The outline of the carapace of a small, medium and large †I. minor changes through ontogeny.
3.2.2. Eyes
A pair of large spheroidal eyes projects anteroventrally (Figures 3A–C). The spherical eye is connected to the head with a stalk, whose length is equal to, and its width is proximately half of the diameter of the eye (Figures 3A,B). Eye size remained almost constant during ontogeny (Figures 3A–C), and thus was largest in relation to the body size for the smallest individuals of the species (diameter ca. 16.1% of valve height and 11.1% of the body length) (Figures 2A, 3A).
3.3. Egg clusters in †Isoxys minor
Clusters of ovoid objects are present in two of the 51 specimens. In both specimens, these objects occur in the anterior and middle of the body beneath the bivalved carapace (Figures 4A,D,E). Their consistent location between the anterodorsal part of the body and the inner wall of the carapace, as well as the similar size of each ovoid object, all suggest that these clusters are eggs. The eggs are particularly prominent in JY0874 (Figures 4A–C). Clusters seem to consist of a single layer of eggs with no or limited overlap among eggs. Eggs of JY1381 are poorly preserved with more than 121 eggs in the cluster, while those of JY0874 are preserved more clearly, with 153 eggs in the cluster (Table 1). Extrapolation from previous reports on the egg-carrying behavior of Cambrian bivalved arthropods, considering the number of eggs beneath left and right valves to be approximately equal, the total number of †I. minor eggs may reach 306 per female (Caron and Vannier, 2016; Ou et al., 2020). Clusters of eggs occupy about 43.87 and 40.52% of the lateral surface area of the valves of each specimen (Figures 4A,B,E). Egg diameter does not vary much within individual clusters, nor between the two specimens, with means of 0.41 mm and 0.47 mm, representing a ratio of ca. 2.9% (JY1381) and 3.5% (JY0874) of the valve length (Table 1). The valve length of egg-bearing females is 13.4 mm (JY0874) and 14.2 mm (JY1381). The egg-carrying individuals are small compared to other †I. minor from the Qingjiang biota – the carapace length is about half that of the largest specimen of this species (Figure 2E).
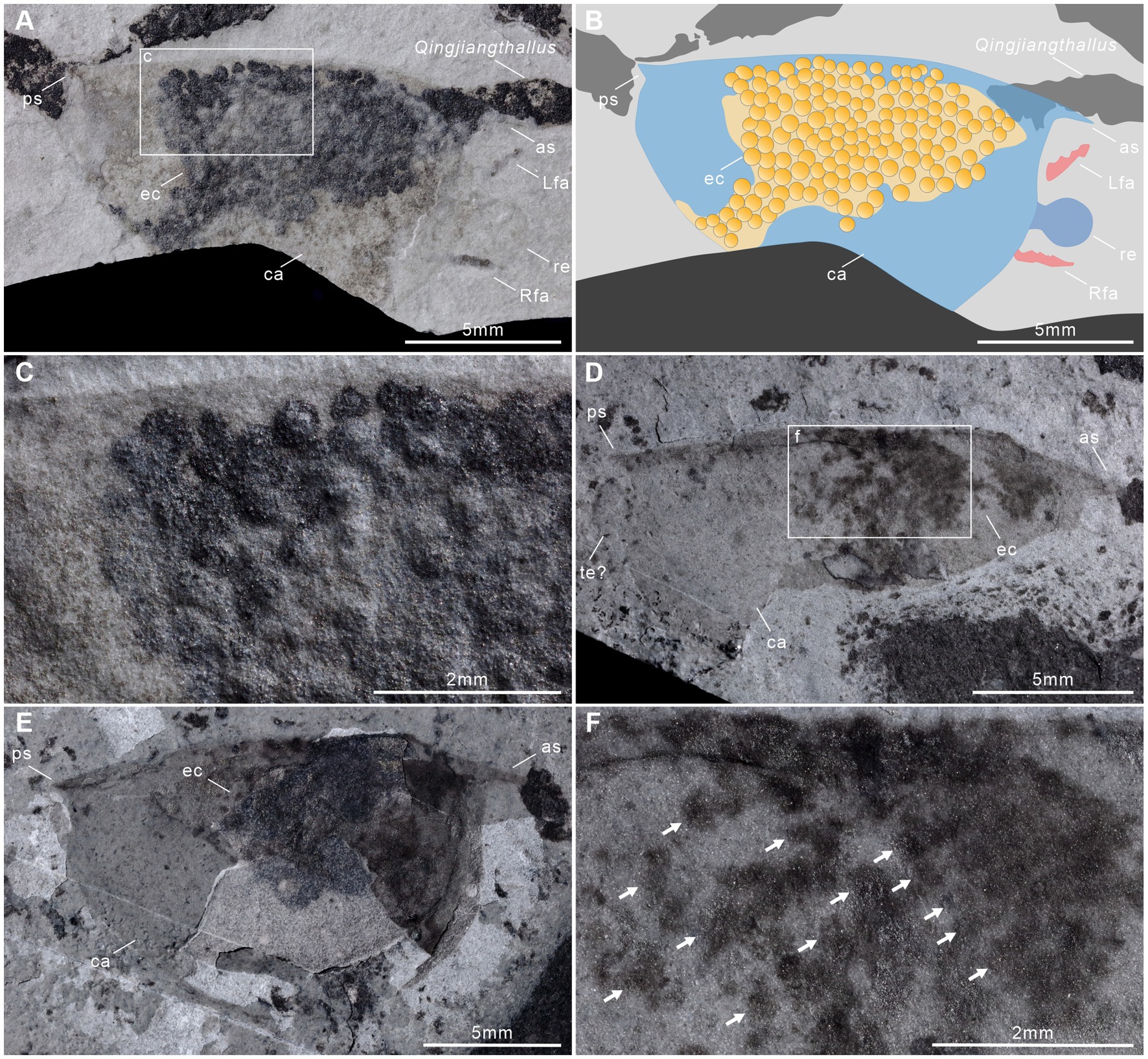
Figure 4. Egg clusters in †Isoxys minor from the Qingjiang biota. (A) Laterally compressed specimen (JY0874) with eggs, surrounded by the red alga (Qingjiangthallus). (B) Interpretative drawing of (A). (C) Detail of egg cluster in (A). (D) The incomplete specimen (JY1381B) with poorly-preserved eggs. (E) The counterpart of (D) with complete carapace, JY1381A. (F) Detail of egg cluster in (D). ca, carapace; ec, egg cluster; Lfa, left frontal appendage; Rfa, right frontal appendage.
4. Discussion
4.1. Intraspecific variation in †Isoxys minor
The carapaces of specimens from the Qingjiang and the Guanshan biotas have many same morphological characteristics: (i) valves nearly semicircular (slightly pentagonal), dorsal margin curved, (ii) anterior spines long and strong, posterior spines short and slightly upturned, and (iii) carapace surface smooth (Wang et al., 2012). The outline analysis demonstrates that the specimens from the Guanshan biota are typically slenderer and display a different orientation of the anterior spine than those from the Qingjiang biota (Figures 1B,D). These differences are very slight, and, in fact, our hierarchical clustering analysis (Figure 1C) did not support them being two different species. Such differences are, therefore, considered as intraspecific variation. Other morphological differences visualized in the PCA space, such as the relative length of spines to body length, are shown to change during ontogeny (section below).
The Wulongqing Formation that hosts the Guanshan biota likely represents a shallower setting than most other Cambrian Konservat-Lagerstätten (Hu et al., 2013; Chen et al., 2019, 2020; Ding et al., 2020; Zhao et al., 2020). By contrast, the part of the Shuijingtuo Formation that hosts the Qingjiang biota represents a distal shelf setting, further from the coast and deeper than most other Cambrian Konservat-Lagerstätten (Fu et al., 2019). These two environments may have placed distinct selection pressures on †I. minor, both, environmentally, and ecologically, that could possibly have influenced these slight morphological differences. The energy of the environment would be expected to be higher in shallower waters (Jiao et al., 2021), thus the observed differences in carapace morphology perhaps may reflect slightly improved streamlining required in higher energy environments and/or to escape from predators, while the upturned and longer anterior spines may have also enhanced antipredation or defense. In addition, the changes of carapaces of †I. minor may also facilitate its extensive vertical migration in the water column and improve swimming efficiency, such as the external structure of pelagic halocypridid ostracods plays a role in retarding sinking in the water column (Vannier and Chen, 2000; Perrier et al., 2007).
4.2. Allometric growth of †Isoxys minor
Among Cambrian bivalved arthropods, allometric growth is relatively common, for instance the carapace development of †Isoxys communis (García-Bellido et al., 2009a) and Tuzoia bispinosa (Wen et al., 2015), the ontogeny and trunk segmentation of †Isoxys auritus (Fu et al., 2014), and the development of the posterior tagma of †Chuandianella ovata (Liu et al., 2022). By contrast, isometric growth is rare in Cambrian bivalved arthropod postembryonic development, having been only reported in the carapace of †Branchiocaris? Yunnanensis (Wu et al., 2016) and †C. ovata (Liu et al., 2021).
In †Isoxys minor, allometry is evident in the changing shape of the valve outline, and the relative sizes of eyes and spines to valve length. Both of these latter two features reach the size of those of large individuals early on in ontogeny (Figures 3A–C). †Isoxys is a visual predator whose eyes are very effective in order to recognize small prey and provide sufficient depth perception (Vannier et al., 2009; Schoenemann and Clarkson, 2011). Thus, the allometry of eyes of †I. minor may indicate that it acquired good vision early on to facilitate predation by small individuals. Generally, the cardinal spines of †Isoxys are thought to play an indirect role in locomotion and be helpful in defending against predators (Williams et al., 1996; Vannier and Chen, 2000; Luo et al., 2006; Liu et al., 2018; Pates et al., 2021). For †Isoxys minor, the allometric growth indicates that the spines more likely played a defensive rather than hydrodynamic role. The relatively larger size of the spines in smaller individuals indicates a defensive or deterrent role, which became reduced during ontogeny. This is comparable to the spiny carapaces of some extant malacostracan larvae (e.g., Vannier and Chen, 2000, Figure 11), which deter predation by fish but do not play a clear hydryodynamic role (Morgan, 1989; Vannier and Chen, 2000; García-Guerrero et al., 2006). By contrast, hydrodynamic streamlining would become more important at larger sizes, at higher Reynolds numbers. In †Isoxys minor, spines are shorter relative to body size in larger individuals, indicating that the changing morphology with growth in this species does not lead to improved hydrodynamic performance.
4.3. Brood care of †Isoxys minor
Since male egg-carrying is generally a rare phenomenon in extant arthropods (Trumbo, 2012; Caron and Vannier, 2016), it is most likely that the egg-carrying specimens represent mature females. The two ovigerous individuals from the Qingjiang biota are smaller and the carapace length is about half that of the largest specimen of this species (Figure 2E). This suggests that †I. minor may have possessed reproductive ability during comparatively early stages of development, and kept growing after becoming sexually mature. Detection of potential sexual dimorphism is precluded by the small sample size.
Egg clusters of †I. minor represent another record of brood care using carapaces in early arthropods. The dark stain around the egg clusters might be the remnants of mucus or other adhesive substances extruded from the eggs. There is no evidence of egg stalks or ovigerous setae in †I. minor, but the compact arrangement of eggs indicates that they may be attached to the inner wall of the carapace, similar to what has been observed in waptiids and extant branchiopod crustaceans (e.g., Limnadia texana) (Caron and Vannier, 2016; Ou et al., 2020). This location might have provided the eggs with optimal protection against physical damage, predators, and might have formed a suitable microenvironment to limited parasite or fungal infestation (Caron and Vannier, 2016). Movement of the trunk appendages might also have generated water currents between the body and the carapace to ensure ventilation over egg clusters (Ou et al., 2020). A coeval bivalved arthropod †Kunmingella douvillei carried eggs using three pairs of posterior appendages, although the type of its brood care was different from †I. minor, the carapaces also provided protection (Duan et al., 2014). Therefore, the bivalved carapace is pivotal to egg protection and incubation.
4.4. Reproductive trade-off in Cambrian arthropods
Trade-offs play a crucial role in the reproduction of organisms, which enhance their adaptations to the environment to obtain the maximum available resources by choosing between the offspring number and energy allocated to individual offspring (e.g., nutrition, parental care, etc.). Different organisms differ not only in exoskeleton and soft anatomy but also in their egg production. By comparing the brood care in Cambrian arthropods, we explored the evidence of trade-offs in reproduction of different taxa.
Egg clutches of †I. minor are attached between the inner surface of the carapace and the body, like †Waptia fieldensis and †C. ovata (Caron and Vannier, 2016; Ou et al., 2020; Liu, 2022). By contrast, the eggs of †K. douvillei are carried by the endopods and gathered ventrally (Duan et al., 2014). In spite of the similar valve length and egg size of ovigerous specimens between †I. minor and †C. ovata, †I. minor (≤306 per clutch; Ø, ~0.45 mm) has approximately three times as many eggs as †C. ovata (≤100 per clutch; Ø, ~0.5 mm) (Table 1). In addition, the brooded egg mass occupies a larger proportion (up to 40%) of †I. minor valve surface (Es/Vs), and a similar and small proportion (ca. 20–25%) of the valve surface of other Cambrian arthropods, which might correspond to their maximum brooding capacity. †W. fieldensis has a small clutch size of up to 26 eggs with a large mean diameter (Ø, ~2.0 mm) in well-preserved specimens, which is obviously different from the other three species (Figure 5; Table 1).
Our findings identify the distinct brooding strategy of the Cambrian arthropods: a mature female of †I. minor can brood an extremely high number of small eggs, those of †C. ovata and †K. douvillei brooded a relatively large number of small eggs, whereas those of †W. fieldensis carried a low number of large eggs (Figure 5). The largest number of small eggs might have allowed †I. minor to maximize the offspring number while investing minimum resources in each one. By comparison, the large and yolky eggs in †W. fieldensis might supply offspring with greater nutrient during embryonic development in response to adverse environmental factors (Fox and Czesak, 2000). Hence, †W. fieldensis greatly reduces the egg number, while increasing the brood care and the probability for individual offspring to survive to adulthood. Cambrian arthropods reproduced with trade-offs between the quantity and quality of their offspring: †W. fieldensis chooses to invest more resources for offspring; †I. minor, †C. ovata, and †K. douvillei choose to increase the offspring number to improve individual survival, and in particular †I. minor is an extreme case of this strategy in Cambrian arthropods where egg clutches have been observed.
4.5. Survival strategy of †Isoxys minor and evolutionary implications of brood care
A dominant species is one that has high abundance compared to other species in the same community, having proportionate effects on environmental conditions, community diversity, and ecosystem function (Avolio et al., 2019). †I. minor is the dominant species of †Isoxys in both Qingjiang and Guanshan biotas. At present, a total of 85 †Isoxys specimens have been identified from the Qingjiang biota, among which 51 are recognized as †I. minor (ca. 60% of †Isoxys). According to previous reports, there are more than 300 †I. minor specimens from the Guanshan biota (up to 96.7% of †Isoxys), while no more than 10 specimens of other species (Huang and Wang, 2014; Wang, 2014). Based on the study of †I. minor from the Qingjiang biota, we assumed that it may have become the dominant species in the two biotas by improving its interspecific competitiveness through allometric growth and r-strategy of reproducing: (i) cardinal spines and eyes have reached the size of those of large individuals in the early stage, which is beneficial for self-protection and foraging, thus increasing small individual survival, (ii) it may have possessed reproductive ability during the early stage of development, allowing it to move quickly into breeding, and (iii) it improves the survival rate of offspring by maximizing egg production. To sum up, these three aspects may have contributed to the dominance of †I. minor in the interspecific competition, and thus it gradually became the dominant species in the two biotas.
The position of †Isoxys as an early diverging deuteropod (e.g., Fu et al., 2022) also makes this taxon the earliest diverging arthropod from which brood care has been described. Brood care has appeared very early in arthropod evolution well before the first mandibulates (e.g., Waptia, Chuandianella) appeared. At the same time, primitive arthropods seem to favor r-strategy of reproducing, and with the evolution of arthropods, parents gradually reduce the number of offspring and invest more in quality. Thus, this life strategy appears to have had a deep root in the phylum, and evolved multiple times within the group.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DF conceived the project. JM interpreted the fossil material and wrote the draft manuscript. SP was involved in analyzing the fossil morphology and discussing the results. YW, WL, CL, YHW, and MZ were involved in discussing the method and the results. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by funds from the Natural Science Foundation of China (41930319, 41890844, and 42242201), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000), and 111 Project (D17013), Natural Science Basic Research Plan of Shaanxi Province (2022JC-DW5-01). SP was supported by a Herchel Smith Postdoctoral Fellowship (University of Cambridge).
Acknowledgments
The authors are grateful to Jean Vannier, Yu Liu, and Brigitte Schoenemann for constructive comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1174564/full#supplementary-material
References
Aria, C., and Caron, J. B. (2015). Cephalic and limb anatomy of a new Isoxyid from the burgess shale and the role of “stem bivalved arthropods” in the disparity of the frontalmost appendage. PLoS One 10:e0124979. doi: 10.1371/journal.pone.0124979
Avolio, M. L., Forrestel, E. J., Chang, C. C., La Pierre, K. J., Burghardt, K. T., and Smith, M. D. (2019). Demystifying dominant species. New Phytol. 223, 1106–1126. doi: 10.1111/nph.15789
Bonhomme, V., Picq, S., Gaucherel, C., and Claude, J. (2014). Momocs: outline analysis using R. J. Stat. Softw. 56, 1–24. doi: 10.18637/jss.v056.i13
Briggs, D. E. G., Lieberman, B. S., Hendricks, J. R., Halgedahl, S. L., and Jarrard, R. D. (2008). Middle Cambrian arthropods from Utah. J. Paleontol. 82, 238–254. doi: 10.1666/06-086.1
Butterfield, N. J., and Nicholas, C. J. (1994). Exceptionally preserved fossils from the lower-middle Cambrian mount cap formation, Northwest Territories, Canada. Palaeontology Newsletter 24:16.
Caron, J. B., and Vannier, J. (2016). Waptia and the diversification of brood care in early arthropods. Curr. Biol. 26, 69–74. doi: 10.1016/j.cub.2015.11.006
Chen, F., Brock, G. A., Zhang, Z., Laing, B., Ren, X., and Zhang, Z. (2020). Brachiopod-dominated communities and depositional environment of the Guanshan Konservat-Lagerstätte, eastern Yunnan, China. J. Geol. Soc. 178, 1–19. doi: 10.1144/jgs2020-043
Chen, F., Zhang, Z., Betts, M. J., Zhang, Z., and Liu, F. (2019). First report on Guanshan biota (Cambrian stage 4) at the stratotype area of Wulongqing formation in Malong County, eastern Yunnan, China. Geosci. Front. 10, 1459–1476. doi: 10.1016/j.gsf.2018.09.010
Ding, Y., Liu, J., and Chen, F. (2020). Ichnology, palaeoenvironment, and ecosystem dynamics of the early Cambrian (stage 4, series 2) Guanshan biota, South China. Geol. J. 55, 77–94. doi: 10.1002/gj.3360
Du, K., Ortega-Hernández, J., Yang, J., Yang, X., Guo, Q., Li, W., et al. (2020). A new early Cambrian Konservat-Lagerstätte expands the occurrence of burgess shale-type deposits on the Yangtze platform. Earth Sci. Rev. 211:103409. doi: 10.1016/j.earscirev.2020.103409
Duan, Y., Han, J., Fu, D., Zhang, X., Yang, X., Komiya, T., et al. (2014). Reproductive strategy of the bradoriid arthropod Kunmingella douvillei from the lower Cambrian Chengjiang Lagerstätte, South China. Gondwana Res. 25, 983–990. doi: 10.1016/j.gr.2013.03.011
Fox, C. W., and Czesak, M. E. (2000). Evolutionary ecology of progeny size in arthropods. Annu. Rev. Entomol. 45, 341–369. doi: 10.1146/annurev.ento.45.1.341
Fu, D., Legg, D. A., Daley, A. C., Budd, G. E., Wu, Y., and Zhang, X. (2022). The evolution of biramous appendages revealed by a carapace-bearing Cambrian arthropod. Phil. Trans. R. Soc. B 377:20210034. doi: 10.1098/rstb.2021.00342022
Fu, D., Tong, G., Dai, T., Liu, W., Yang, Y., Zhang, Y., et al. (2019). The Qingjiang biota—a burgess shale-type fossil Lagerstätte from the early Cambrian of South China. Science 363, 1338–1342. doi: 10.1126/science.aau8800
Fu, D., Zhang, X., Budd, G. E., Liu, W., and Pan, X. (2014). Ontogeny and dimorphism of Isoxys auritus (Arthropoda) from the early Cambrian Chengjiang biota, South China. Gondwana Res. 25, 975–982. doi: 10.1016/j.gr.2013.06.007
Fu, D., Zhang, X., and Shu, D. (2011). Soft anatomy of the early Cambrian arthropod Isoxys curvirostratus from the Chengjiang biota of South China with a discussion on the origination of great appendages. Acta Palaeontol. Pol. 56, 843–852. doi: 10.4202/app.2010.0090
García-Bellido, D. C., Paterson, J. R., Edgecombe, G. D., Jago, J. B., Gehling, J. G., and Lee, M. S. Y. (2009a). The bivalved arthropods Isoxys and Tuzoia with soft-part preservation from the lower Cambrian Emu Bay shale Lagerstätte (Kangaroo Island, Australia). Palaeontology 52, 1221–1241. doi: 10.1111/j.1475-4983.2009.00914.x
García-Bellido, D. C., Vannier, J., and Collins, D. (2009b). Soft-part preservation in two species of the arthropod Isoxys from the middle Cambrian burgess shale of British Columbia, Canada. Acta Palaeontol. Pol. 54, 699–712. doi: 10.4202/app.2009.0024
García-Guerrero, M. U., Rodríguez, A., and Hendrickx, M. E. (2006). Larval development of the eastern Pacific anomuran crab Porcellana cancrisocialis (Crustacea: Decapoda: Anomura: Porcellanidae) described from laboratory reared material. J. Mar. Biol. Assoc. UK 86, 1123–1132. doi: 10.1017/S002531540601410X
Glaessner, M. F. (1979). Lower Cambrian Crustacea and annelid worms from Kangaroo Island, South Australia. Alcheringa 3, 21–31. doi: 10.1080/03115517908565437
Hou, X. (1987). Early Cambrian large bivalved Arthropoda from Chengjiang, Eastern Yunnan. Acta Palaeontol. Sinica 26, 286–298. [in Chinese with English abstract]
Hu, S., Zhu, M., Luo, H., Steiner, M., Zhao, F., Li, G., et al. (2013). The Guanshan biota. Yunnan Science and Technology Press, Yunnan.
Huang, D., and Wang, Y. (2014). The soft anatomy of Isoxys minor from the Guanshan fauna, lower Cambrian of Southwest China. Palaeoworld 23, 225–228. doi: 10.1016/j.palwor.2014.10.006
Ivantsov, A. I. (1990). Pervye hakhodki fillokarid v nizhnem kembrii Iakutii. Palaeontologičeskij Žurnal 34, 130–132. [in Russian]
Ivantsov, A. I. (2005). “Tardipolypodians, 112–113” in Unique Sinsk localities of early Cambrian organisms (Siberian platform). ed. A. G. Ponomarenko (Moscow: Nauka) [in Russian]
Jiao, D., Pates, S., Lerosey-Aubril, R., Ortega-Hernández, J., Yang, J., Lan, T., et al. (2021). The endemic radiodionts of the Cambrian stage 4 Guanshan biota of South China. Acta Palaeontol. Pol. 66, 255–274. doi: 10.4202/app.00870.2020
Legg, D. A., and Vannier, J. (2013). The affinities of the cosmopolitan arthropod Isoxys and its implications for the origin of arthropods. Lethaia 46, 540–550. doi: 10.1111/let.12032
Li, R., Cui, L., Fu, D., and Zhang, X. (2022). A new red alga preserved with possible reproductive bodies from the 518-million-year-old Qingjiang biota. J. Syst. Evol. 1–11. doi: 10.1111/jse.12942
Liu, C. (2022). Cambrian arthropod Chuandianella ovata: morphology, taxonomy, ontogeny, ecology, mineralization and phylogeny. PhD thesis, Northwest University. [in Chinese with English abstract]
Liu, C., Fu, D., and Zhang, X. (2021). Phosphatic carapace of the waptiid arthropod Chuandianella ovata and biomineralization of ecdysozoans. Palaeontology 64, 755–763. doi: 10.1111/pala.12570
Liu, C., Fu, D., and Zhang, X. (2022). Developmental dynamics is revealed in the early Cambrian arthropod Chuandianella ovata. iScience 25:103591. doi: 10.1016/j.isci.2021.103591
Liu, S., Peng, J., Wen, R., and Liang, B. (2018). New data for Isoxys of the Balang Fauna (Cambrian stage 4), South China. Bull. Geosci. 93, 147–162. doi: 10.3140/bull.geosci.1673
Luo, H., Fu, X., Hu, S., Li, Y., Chen, L., You, T., et al. (2006). New bivalved arthropods from the early Cambrian Guanshan fauna in the Kunming and Wuding area. Acta Palaeontol. Sin. 45, 460–472. [in Chinese with English summary]
Luo, H., Li, Y., Hu, S., Fu, X., Hou, S., Liu, X., et al. (2008). Early Cambrian Malong Fauna and Guanshan Fauna from Eastern Yunnan, China. Yunnan: Yunnan Science and Technology Press.
Luo, H., Hu, S., Chen, L., Zhang, S., and Tao, Y. (1999). Early Cambrian Chengjiang Fauna from Kunming region. 129 pp. Yunnan Science of Technology Press, Kunming. [in Chinese with English summary]
Ma, J., Lin, W., Liu, C., Sun, A., Wu, Y., Wu, Y., et al. (2021). A new bivalved arthropod from the Cambrian (stage 3) Qingjiang biota expands the palaeogeographical distribution and increases the diversity of Tuzoiidae. J. Geol. Soc. 179:jgs2020-229. doi: 10.1144/jgs2020-229
Morgan, S. G. (1989). Adaptive significance of Spination in estuarine crab Zoeae. Ecology 70, 464–482. doi: 10.2307/1937551
Nielsen, M. L., Rasmussen, J. A., and Harper, D. A. T. (2017). Sexual dimorphism within the stem-group arthropod Isoxys volucris from the Sirius Passet Lagerstätte, North Greenland. Bull. Geol. Soc. Den. 65, 47–58. doi: 10.37570/bgsd-2017-65-04
Ou, Q., Vannier, J., Yang, X., Chen, A., Mai, H., Shu, D., et al. (2020). Evolutionary trade-off in reproduction of Cambrian arthropods. Sci. Adv. 6, eaaz3376–eaaz3405. doi: 10.1126/sciadv.aaz3376
Pates, S., Daley, A. C., Legg, D. A., and Rahman, I. A. (2021). Vertically migrating Isoxys and the early Cambrian biological pump. Proc. R. Soc. B 288:20210464. doi: 10.1098/rspb.2021.0464
Perrier, V., Vannier, J., and Siveter, D. J. (2007). The Silurian pelagic myodocope ostracod Richteria migrans. Earth Environ. Sci. Trans. R. Soc. Edinb. 98, 151–163. doi: 10.1017/S1755691007006147
Press, A. (2010). Adobe Photoshop CS5- classroom in a book. J. Vis. Commun. Med. 34, 125–152. doi: 10.3109/17453054.2011.604842
R Core Team. (2021). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/
Richter, R., and Richter, E. (1927). Eine Cruseacee (Isoxys carbonelii n. sp.) in den Archaeocyathus-Bildungen der Sierra Morena und ihre stratigraphische Beurteilung. Senckenbergiana 9, 188–195.
Schoenemann, B., and Clarkson, E. N. (2011). Eyes and vision in the Chengjiang arthropod Isoxys indicating adaptation to habitat. Lethaia 44, 223–230. doi: 10.1111/j.1502-3931.2010.00239.x
Shu, D., Zhang, X., and Geyer, G. (1995). Anatomy and systematic affinities of the lower Cambrian bivalved arthropod Isoxys auritus. Alcheringa 19, 333–342. doi: 10.1080/03115519508619512
Simonetta, A. M., and Delle Cave, L. D. (1975). The Cambrian non-trilobite arthropods from the burgess shale of British Columbia. A study of their comparative morphology, taxonomy and evolutionary significance. Palaeontogr. Ital. 69, 1–37.
Stein, M., Peel, J. S., Siveter, D. J., and Williams, M. (2010). Isoxys (Arthropoda) with preserved soft anatomy from the Sirius Passet Lagerstätte, lower Cambrian of North Greenland. Lethaia 43, 258–265. doi: 10.1111/j.1502-3931.2009.00189.x
Trumbo, S. T. (2012). “Patterns of parental care in invertebrates” in The evolution of parental care. eds. N. J. Royle, P. T. Smiseth, and M. Kölliker. First ed (Oxford University Press, New York), 81–100.
Vannier, J., and Chen, J. (2000). The early Cambrian colonization of pelagic niches exemplified by Isoxys (Arthropoda). Lethaia 33, 295–311. doi: 10.1080/002411600750053862
Vannier, J., García-Bellido, D. C., Hu, S., and Chen, A. (2009). Arthropod visual predators in the early pelagic ecosystem: evidence from the burgess shale and Chengjiang biotas. Proc. R. Soc. Lond. B Biol. Sci. 276, 2567–2574. doi: 10.1098/rspb.2009.0361
Vannier, J., Williams, M., Alvaro, J. J., Vizcaïno, D., Monceret, S., and Monceret, E. (2005). New early Cambrian bivalved arthropods from southern France. Geol. Mag. 142, 751–763. doi: 10.1017/S0016756805001093
Wang, Y. (2014). The diversity and systematics of Isoxys and Tuzoia from the Cambrian of China. PhD thesis, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Science. [in Chinese with English abstract].
Wang, Y., Huang, D., and Lieberman, B. S. (2010). New Isoxys (Arthropoda) from the Cambrian, Mantou Fromation, Shandong Province. Acta Palaeontol. Sinica 49, 398–406. [in Chinese with English abstract]
Wang, Y., Huang, D., Liu, Q., and Hu, S. (2012). Isoxys from the Cambrian Guanshan Fauna, Yunnan Province. Earth Sci. J China University Geosci. 37, 156–164. doi: 10.3799/dpkx.2012.So.016
Wen, R., Zhao, Y., and Peng, J. (2015). Morphology and ontogeny of Tuzoia bispinosa from the Kaili biota (Cambrian stage 5) of eastern Guizhou, China. Palaeoworld 24, 61–70. doi: 10.1016/j.palwor.2014.12.005
Williams, M., Siveter, D. J., and Peel, J. S. (1996). Isoxys (Arthropoda) from the early Cambrian Sirius Passet Lagerstätte, North Greenland. J. Paleontol. 70, 947–954. doi: 10.1017/S0022336000038646
Wu, Y., Fu, D., Zhang, X., Daley, A. C., and Shu, D. (2016). Dimorphism of Bivalved arthropod Branchiocaris? Yunnanensis from the early Cambrian Chengjiang biota, South China. Acta Geol. Sinica 90, 818–826. doi: 10.1111/1755-6724.12725
Zhao, J., Li, Y., Selden, P. A., and Cong, P. (2020). New occurrence of the Guanshan Lagerstätte (Cambrian series 2, stage 4) in the Kunming area, Yunnan, Southwest China, with records of new taxa. Alcheringa An Australasian J. Palaeontol. 44, 343–355. doi: 10.1080/03115518.2020.1781257
Zhao, Y., Zhu, M., Babcock, L. E., and Peng, J. (2011). The Kaili biota-marine organisms from 508 million years ago. 251 Guizhou Science and Technology Press, Guiyang.
Keywords: Cambrian, Qingjiang biota, Isoxys, allometry, brood care
Citation: Ma J, Pates S, Wu Y, Lin W, Liu C, Wu Y, Zhang M and Fu D (2023) Ontogeny and brooding strategy of the early Cambrian arthropod Isoxys minor from the Qingjiang biota. Front. Ecol. Evol. 11:1174564. doi: 10.3389/fevo.2023.1174564
Edited by:
Allison Daley, Universitéde Lausanne, SwitzerlandReviewed by:
Jean Vannier, Université Claude Bernard Lyon 1, FranceYu Liu, Yunnan University, China
Brigitte Schoenemann, University of Cologne, Germany
Copyright © 2023 Ma, Pates, Wu, Lin, Liu, Wu, Zhang and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongjing Fu, ZGpmdUBud3UuZWR1LmNu
 Jiaxin Ma1
Jiaxin Ma1 Stephen Pates
Stephen Pates Dongjing Fu
Dongjing Fu