- 1School of Biological Sciences, Louisiana Tech University, Ruston, LA, United States
- 2Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, United States
- 3Department of Biology, Wake Forest University, Winston-Salem, NC, United States
Assessing stress in wild populations is important in many ecological and conservation contexts because the physiological responses of individuals to stressors can be used to identify at-risk populations and the ability to respond appropriately to stressors is related to individual quality and fitness. Yet, one of the great challenges in ecophysiology is linking physiological measures in wild animal populations with changes in individual fitness. Here, we examined two indices of stress, namely, circulating baseline corticosterone concentration ([Cort]) and the heterophil:lymphocyte (H/L) ratio, in a long-lived seabird, the Nazca booby (Sula granti) and their relationship with current individual state and subsequent survival and residual and lifetime reproductive success. [Cort] was related to sex, age, and current reproductive effort in in that males, older birds, and birds currently engaged in a breeding attempt birds had higher [Cort]. [Cort] was negatively associated with survival to the next breeding season. The H/L ratio was not associated with the current state of birds but predicted cohort-specific long-term survival. Lifespan and reproductive performance are correlated in Nazca boobies; therefore, our results suggest that the H/L ratio may be useful as an indicator of overall fitness, while [Cort] can be used to predict current or near-term fitness in this species. We further propose the H/L [or neutrophil/lymphocyte (N/L)] Ratio-Fitness Hypothesis, which posits that this ratio is repeatable within individuals and are negatively associated with fitness. This hypothesis needs to be tested in Nazca boobies and other species, and when supported by empirical evidence, then these ratios could be a powerful monitoring tool for assessing population health or identifying at-risk populations.
1. Introduction
Assessing stress in wild populations is important in many ecological and conservation contexts because the physiological responses of individuals to stressors can be used to identify at-risk populations (Wikelski and Cooke, 2006; Fefferman and Romero, 2013), and the ability to respond appropriately to stressors is related to individual quality and fitness (Romero et al., 2009; Angelier et al., 2010). Environmental and anthropogenic disturbances activate the vertebrate neuroendocrine hypothalamic–pituitary–adrenal (HPA) axis, resulting in the production and release of glucocorticoids (GCs). Elevated GCs act within a relatively short time frame to help vertebrates cope with the immediate stressor by modulating energy allocation, metabolism, behavior, and immune function (Romero, 2004; Martin, 2009). Thus, GCs are a primary mediator of vertebrate allostasis or the ability of individuals to cope with changing environments (McEwen and Wingfield, 2003). Therefore, baseline GCs should be an indicator of how well an individual copes with its environment when individuals are sampled under the same conditions (Bonier et al., 2009a).
Many hypotheses have been proposed to explain the relationship between baseline GCs and fitness (Schoenle et al., 2018); however, three non-mutually exclusive hypotheses have emerged as the broadest (Breuner and Berk, 2019). The “Cort-Fitness Hypothesis” predicts that individuals with elevated baseline GCs have low fitness (integrating survival and reproductive success), because baseline GCs increase with environmental challenges, and fitness decreases with increasing environmental challenges (Bonier et al., 2009a). While this hypothesis is supported by the literature, a major exception often occurs during reproduction, giving rise to the “Cort-Adaptation Hypothesis” (Bonier et al., 2009a). This modified version of the Cort-Fitness Hypothesis predicts that when the environmental challenge is associated with reproduction, elevated baseline GCs correlate with increased reproductive success because high-quality individuals can increase their allostatic load (indicated by baseline GCs) beyond those of low-quality individuals. Finally, the “Cort-Tradeoff Hypothesis” represents the long-standing view that GCs mediate tradeoffs between reproduction and survival; thus, elevated GCs will increase survival but decrease reproductive success (Breuner and Berk, 2019). Originally applied to stress-induced GCs, this hypothesis has since expanded to include baseline GCs (Schoenle et al., 2018).
Life-history strategies appear to be a major determinant of the effects of GCs on fitness, with short-lived species generally providing support for the Cort-Adaptation Hypothesis and long-lived species supporting the Cort-Tradeoff Hypothesis (Bókony et al., 2009; Hau et al., 2010; Breuner and Berk, 2019). However, few studies have tested these hypotheses in long-lived animals, because long-term (i.e., > 10 years) data sets on lifetime reproductive success and survival can be challenging to obtain. Long-lived species, such as seabirds, are largely able to escape adult mortality due to predation. Because of this, they demonstrate a shift toward adult self-maintenance and an extended reproductive lifespan and away from short-term reproductive effort (Apanius et al., 2008). Thus, environmental stressors (e.g., low food availability) typically result in decreased reproductive success, but no change in adult survival until stressors become severe or prolonged (Kitaysky et al., 2007). Previous work in seabirds has revealed that GC secretion is an indicator of nutritional stress during the breeding season, as it increases with challenging environmental conditions and declining food supply (Kitaysky et al., 2010; Satterthwaite et al., 2010; Will et al., 2020; Shimabukuro et al., 2023) and decreases with experimental supplemental feeding (Schultner et al., 2013). Nutritional stress, and thus GC secretion, then negatively predicts reproductive success (Kitaysky et al., 2007) and adult survival when breeding in poor food conditions (Kitaysky et al., 2010; Satterthwaite et al., 2010) in long-lived seabirds, in general supporting the Cort-Fitness Hypothesis.
Although GCs are frequently measured in wild animals, their collection and interpretation pose many challenges. Because GCs increase rapidly in circulation in response to stressors, collection of baseline GCs is extremely time-sensitive (typically requiring collection within 3 min of disturbance for birds) (Romero and Reed, 2005). Moreover, “normal” GC concentrations vary by breeding state, season, age, sex, time of day, and recent social interactions, as well as exposure to stressors (Romero and Wingfield, 2016). Finally, low baseline GC concentrations could indicate a low level of HPA axis activation or suppression of the HPA axis due to acclimation to a stressor, chronic exposure to long-term stressors (Johnstone et al., 2012), or increasing age (Heidinger et al., 2006, 2008).
Given these difficulties in obtaining and interpreting baseline GCs, ecologists increasingly have turned to leucocyte profiles, specifically the heterophil/lymphocyte ratio (H/L) in birds and reptiles, or the neutrophil/lymphocyte (N/L) ratio in fish, amphibians, and mammals, as a measure of stress in wild animals. Leucocytes are redistributed to different areas of the body in response to elevated GCs. Specifically, lymphocytes leave circulation (i.e., lymphopenia), while heterophils or neutrophils enter circulation (i.e., heterophilia or neutrophilia Dhabhar et al., 1996; Davis et al., 2008). This redistribution of leucocytes is slower than changes in circulating GC concentrations, sometimes occurring up to 24h after stressor exposure (Davis and Maney, 2018). Thus, the H/L(N/L) ratio is easier to obtain for wild populations because sampling is far less time-sensitive than for GC concentrations. Although both circulating GCs and H/L(N/L) ratios typically change after exposure to an acute stressor, these indices may not be interchangeable because they are not always correlated at baseline due to the differences in the timing of the onset of these changes (reviewed by Davis and Maney, 2018). This had led some authors to argue that these metrics reflect different types of stressors (Müller et al., 2011) or that leucocyte profiles are better suited for assessing chronic stress (Davis and Maney, 2018) while GCs may be better for assessing current acute stressors (Romero and Wingfield, 2016; Davis and Maney, 2018). In addition, the H/L ratio may be an important evolutionary and life-history trait in birds because a phylogenetic analysis found a negative relationship between the H/L ratio and lifespan at the order and superfamily level (Minias, 2019).
In this study, we examined baseline corticosterone (the primary avian GC) and the H/L ratio in a long-lived seabird, the Nazca booby (Sula granti; Anderson and Apanius, 2003) during the early breeding season. We evaluated the relationships between these indices of stress, current individual state, and subsequent survival and reproductive success to test the Cort-Fitness, Cort-Adaptation, and Cort-Tradeoff hypotheses.
2. Materials and methods
2.1. Field site and sample collection
Adult Nazca boobies were sampled in the breeding colony at Punta Cevallos, Española, and Galápagos Islands, Ecuador from 24 November to 5 December 2009 (see Apanius et al., 2008 for a detailed description of the site). Nazca boobies breed from October to May or June of the following calendar year (Huyvaert and Anderson, 2004); therefore, sampling was performed early in the 2009–2010 breeding season. Birds at Punta Cevallos have been banded and monitored for survival since 1984. Monitoring of the reproductive success of banded individuals began in 1992. This continuing effort has allowed the accumulation of detailed longitudinal data on survival and reproduction in this species (e.g., Tompkins and Anderson, 2019, 2021) because site fidelity is essentially 100% (Huyvaert and Anderson, 2004).
Adult birds were caught and restrained by hand from 0300h to 0520h, which is the period of the highest circadian corticosterone concentration and when birds are least likely to be disturbed by external events (Tarlow et al., 2003a). Blood samples (1–2 ml; ≤ 1% of body weight) were collected by brachial venipuncture, allowed to clot at ambient temperature for 2–4 h in 1.5-ml microfuge tubes, and then centrifuged at 6000 rpm for 5 min. The serum was then transferred to a clean 1.5-ml cryovial and frozen in the field in liquid nitrogen. Samples were transported from the field in liquid nitrogen and then stored at −80°C until laboratory analysis. Sampling time from the moment the bird was captured was recorded. If sampling took longer than 3 min, then any further blood collection was put into a second 1.5-ml microfuge, labeled as “>3 min”, and treated as described previously. At the time of sampling, one drop of fresh blood was used to make blood smears on microscope slides that were air-dried and fixed in methanol in the field (Fudge, 2000). Smears were kept at ambient temperature until laboratory processing.
We sampled birds across all age classes present in the colony, but particularly focused on birds that fledged during the 2002–2003 breeding season, which were 7 years old in 2009–2010. Young Nazca boobies return to the breeding colony between the ages of 2 and 7 years (Maness and Anderson, 2013; Champagnon et al., 2018). Thus, the 2002–2003 cohort was sampled toward the beginning of their reproductive years, with relatively little opportunity for the selective disappearance of low-quality adults to limit their representation in the sample. The adult sex ratio is male-biased in the study colony (Maness et al., 2007) due to the lower survival probability of females during the juvenile period (Maness and Anderson, 2013). Efforts were made to sample equal numbers of males and females.
2.2. Sample processing
Total bound and unbound circulating corticosterone concentration ([Cort]) was measured by quantitative competitive enzyme immunoassay (Enzo Life Sciences/Assay Designs, Cat. No. ADI-901-097), validated for use with Nazca booby serum for accuracy, precision, cross-reactivity, and parallelism in measurements (Grace et al., 2011). Serum was used directly in the enzyme immunoassay as described by Grace and Anderson (2014), and samples were run in duplicate. All corticosterone assays were performed by JKG and samples were part of a larger sampling effort, which were all analyzed simultaneously. For all samples in this larger dataset, the efficiency of immunoassay in measuring known quantities of corticosterone (using the supplied corticosterone standard diluted in stripped chicken serum) averaged 100% (SD = 6.6, N = 23). The immunoassay detection limit was 0.078 nmol/L, and average intra- and inter-assay coefficients of variation were 3.4% (SD = 0.5%, N = 190) and 6.2% (SD = 1.3%, N = 30), respectively. Because the primary antibody in the assay did not cross-react to a significant degree with other circulating steroids, all measures are called “Cort” measurements. Blood smears were stained using a Hemacolor® Staining Kit (EMD Millipore Corp., product code 65044), following manufacturer protocols. Stained slides were viewed at 400X magnification to estimate the total white blood cell count (TWBC; Fudge, 2000). Smears were then viewed at 1000X magnification for differential blood cell counts following Fudge (2000). All blood cell counts were performed by MRH.
2.3. Statistical analyses
[Cort] and H/L measurements were examined for outliers using the Dixon outlier range statistic, which identifies the most extreme value at the upper or lower limit as an outlier if D/R > 0.3, where D = |extreme value – next nearest value| and R is the range of all values (Dixon, 1983). Reference intervals for [Cort] and the H/L ratio in Nazca boobies were calculated with MedCalc Statistical Software (version 20.216, Ostend, Belgium) using non-parametric estimation when the sample size was large and the robust method when the sample size was small (Geffré et al., 2011). Adult Nazca boobies were categorized by their breeding status when sampled as follows: non-breeder (no breeding attempt in 2009–2010), pre-breeder (started a breeding attempt after sampling), incubating an egg, brooding a chick, breeder that failed at the egg stage, and breeder that failed at the chick stage. The effect of age, sex, sampling date, and breeding status on [Cort] or the H/L ratio was determined with general linear models.
Short-term effects of [Cort] on survival probability in the year following sampling and long-term effects of both [Cort] and H/L ratio on average annual survival probability over the next 11 years (2009–2010 to 2021–2022) were evaluated by fitting data from an annual band-resight survey (BRS) to capture–mark–recapture (CMR) models. CMR models estimated apparent annual survival probabilities from individual recapture histories while controlling imperfect detection (Lebreton et al., 1992) and were used to control detection probabilities that were less than one in Nazca boobies (Townsend et al., 2007; Champagnon et al., 2018; and lower in females). Recapture histories covered all breeding seasons from 2009–2010 to 2021–2022 except 2020–2021 (details of the BRS in Huyvaert and Anderson, 2004; Champagnon et al., 2018).
Effects of [Cort] and H/L ratio on survival were modeled separately because of a difference in sample size (N = 565 birds for [Cort], N = 85 birds for H/L ratio). Initially, previous results were used to construct a base model: female Nazca boobies have slightly lower annual survival than males do (Champagnon et al., 2018; Tompkins and Anderson, 2019), and each sex shows actuarial senescence starting in the late teens. Sex differences in the rate and timing of actuarial senescence are slight (Tompkins and Anderson, 2019), and, given our small sample size, we started with a base model predicting survival by sex (a two-level factor) and age (a continuous variable), but not their interaction. Age effects were modeled using a threshold function allowing slope estimates to change (e.g., become steeper) after age 16 (following Tompkins and Anderson, 2019). Detection probabilities were sex-specific. [Cort] or H/L ratio was added as a continuous predictor of annual survival probability to our base model, and their performance was evaluated using AICc-based model comparison (Burnham and Anderson, 2010). For the [Cort] model set, a third candidate model was included, fitting an interaction between [Cort] and a two-level factor differentiating the 2009–2010 to 2010–2011 interval from all other years. This interaction tested the hypothesis that [Cort] predicts survival to the next breeding season but does not affect survival over subsequent intervals.
Initially, analyses were run on data from the 2002–2003 cohort (N = 285 birds with [Cort] measurements, 38 birds with H/L measurements). Analyses were then repeated on data from all cohorts combined (N = 565 birds with [Cort] measurements, 85 birds with H/L measurements).
Models were constructed using a logit link function and fit using the program MARK (White and Burnham, 1999) to generate maximum-likelihood estimates of survival and detection parameters. We ran MARK through the RMark interface (v.3.0.0; Laake, 2013) in R (v.4.2.2; R Core Team, 2022). We evaluated the overall fit of our base model on data from the [Cort] data subsets (all cohorts combined vs. the 2002–2003 cohort only) using the median ĉ method in program MARK; goodness-of-fit testing was performed on the base model, and not on our most complex model, because individual covariates, like [Cort] and H/L ratio, are not allowed using the median c method. Estimated overdispersion parameters (ĉ) were close to 1 for the base models (for [Cort]), suggesting a reasonable fit (ĉ = 1.09 [95% CI: 1.08–1.10] and ĉ = 1.20 [95% CI: 1.05–1.46] for all cohorts vs. the 2002–2003 cohort data subsets). We mean-centered [Cort] before analysis.
Effects of [Cort] and H/L ratio on reproductive performance were evaluated using “lifetime” reproductive success (LRS), calculated as the sum of offspring produced through the 2019–2020 breeding season. Although 50% of the 2002–2003 cohort were still alive in the final year of the study (age 17 in 2019–2020), reproductive senescence begins in the mid-teens (Anderson and Apanius, 2003; Tompkins and Anderson, 2019), and offspring production through age 17 is highly correlated with lifetime reproductive success in older cohorts (males: Pearson's r = 0.96, d.f. = 1,504, P < 0.01; females: Pearson's r = 0.97, d.f. = 1,202, P < 0.01; data from cohorts 1984–1987 and 1992–1995).
Nazca boobies raise at most one offspring per breeding season (Humphries et al., 2006). Reproductive success for each parent, in each breeding season, was assigned based on daily monitoring of banded offspring late in the breeding cycle. Rarely, a nestling/fledgling will die after monitoring ends but before reaching independence and we adjusted reproductive success based on the recovery of offspring bands/carcasses during the following two breeding seasons (following Tompkins and Anderson, 2021). Offspring raised in 2007–2008 were excluded from lifetime reproductive success estimates because reproductive success was not monitored for some study colony subsections. We excluded 17 boobies with [Cort] measurements from analyses (6% of the total) because they occupied a colony subsection that was not monitored for reproductive success in 2017–2018 through 2019–2020 (location information from the annual band resight survey identified these individuals) for a final sample size of 268 birds in the 2002 cohort (38 for the H/L ratio) and 557 birds in all cohorts combined (85 for the H/L ratio).
As was done for survival, the effects of [Cort] or H/L ratio on reproductive performance were assessed separately, first for members of the 2002–2003 cohort and then for the full dataset. Effects of [Cort] and H/L ratio on lifetime reproductive success for the 2002–2003 cohort were evaluated in R using linear models (LMs) with a Gaussian error distribution and an identity link function. Lifetime reproductive success for the 2002–2003 cohort was predicted by sex (a two-level factor) and either the H/L ratio or [Cort]. The magnitude of slope estimates and span of 95% CIs relative to zero were used to evaluate support for [Cort] and the H/L ratio as predictors of lifetime reproductive success. Finally, because lifetime reproductive success is a count variable, we verified that our results were unchanged when fit with a GLM with Poisson errors and a log link function (Zuur et al., 2009). The same approach was used for the data from all cohorts combined, except using linear mixed models (LMMs; using package lme4; Bates et al., 2015) and including cohort as a random intercept in addition to the fixed effects (sex and either [Cort] or the H/L ratio).
The baseline [Cort] and H/L ratio sampled in the 2009–2010 breeding season may not reflect individual state earlier in life, and we repeated our reproductive performance analyses using summed reproductive success from 2009–2010 onward as the response variable (“residual reproductive success”) using GLMs (for the 2002–2003 cohort) and GLMMs (for all cohorts) with Poisson errors and a log link function. Cohort was included as a random intercept, accounting for cohort-level differences in residual reproductive success due to the age when stress indices were measured or to other factors. Finally, we verified that including individuals with incomplete LRS information (still alive at the end of the study) did not affect our results by repeating our evaluation of stress effects on reproductive performance (for LRS and residual reproductive performance) using data restricted to cohorts 1994–1995 and earlier (N = 158 for [Cort], N = 35 for the H/L ratio). A few members (8%) of the 1994–1995 cohort were still alive in 2019–2020 (and even lower percentages for the 1992–1993 and 1993–1994 cohorts), but successfully producing an offspring at age 25 or older is extremely rare (only four cases in our long-term data).
3. Results
The 2009–2010 breeding season was a poor year for reproductive success in the colony at Punta Cevallos. In 27 years of regular nest monitoring by our group, only two other breeding seasons, which were years of strong El Niño Southern Oscillation warm events (Clifford and Anderson, 2001; Champagnon et al., 2018), had lower reproductive success (Figure 1). This indicates that the birds in our colony experienced greater-than-usual environmental stressors associated with reduced food availability and/or prey quality (Champagnon et al., 2018) at the time of sampling.
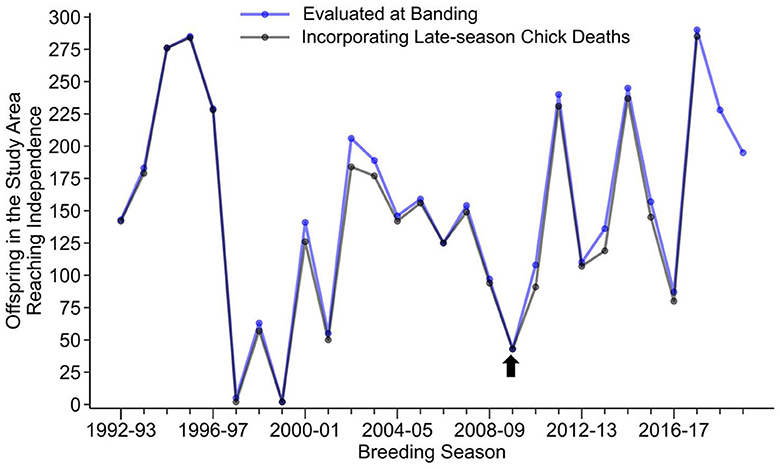
Figure 1. Reproductive success, as indicated by the production of an independent offspring, of Nazca booby breeding pairs located in an area of the colony designated as the “Study-Area”, where all nests are monitored regardless of the banding status of the parents (Huyvaert and Anderson, 2004). Only the 1997–1998 and 1999–2000 breeding seasons had lower reproductive success than the year of sample collection in the current study (2009–2010, indicated by the arrow). Production of independent offspring was assessed at two different time points during the breeding season, at the time the chick was banded (blue line), which was typically done at the 1% down developmental stage (Maness et al., 2011), and at a later time that would include late-season chick deaths (blue line; Tompkins and Anderson, 2021).
One outlier was identified for baseline [Cort] and was subsequently removed for measurement of reference intervals. However, we had no indication that this value was due to sampling or analytical error, so we retained this sample for further analyses. Tests were run with and without this sample to see whether the results changed and, as none did, the results we present included this value. The reference interval (RI) for baseline [Cort] as measured by the non-parametric method was 25.66 (90% CI = 24.87–28.7) – 278.63 (90% CI = 258.12–295.91) nmol/L. In total, 14 birds had elevated [Cort] (i.e., above the upper RI limit) and 14 birds had values below the lower RI limit. Those with high [Cort] were 2.5 times more likely to be in older age classes, and those with low values were 4.7 times more likely to be female and 11.2 times more likely to be in younger age classes (odds ratio 95% CI = 1.0–7.6; 1.8–11.2; and 4.8–26.3, respectively). Comparison of our baseline [Cort] with other published studies of sulids revealed that our values were higher than most, except for oiled and breeding northern gannets (Morus bassanus) (Table 1).
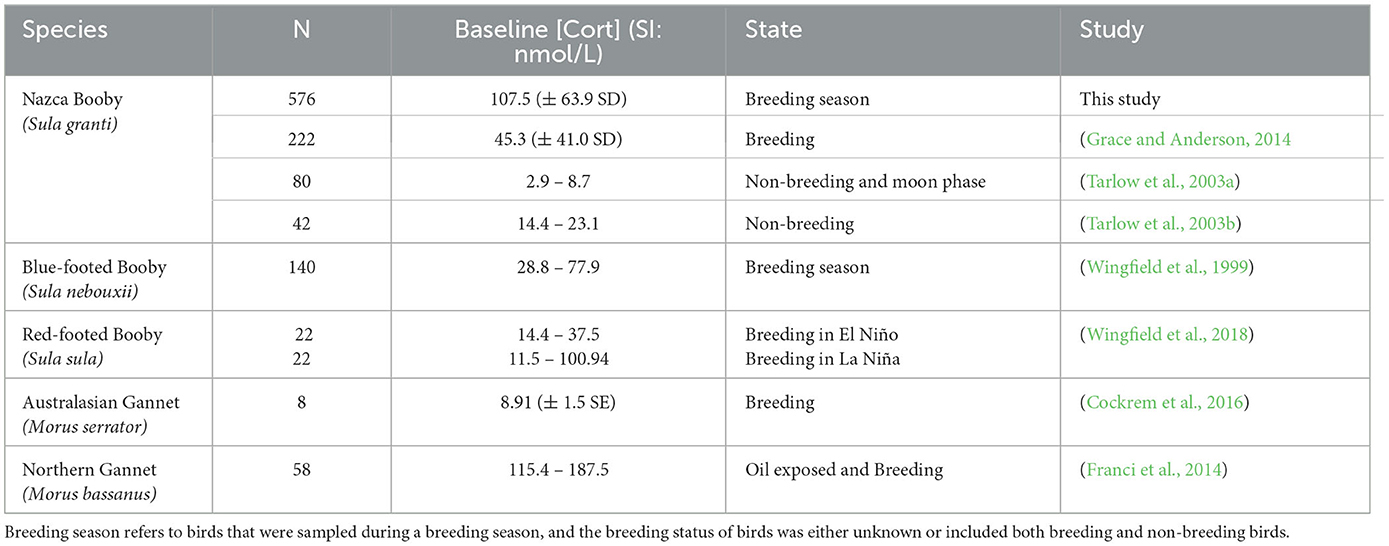
Table 1. Baseline [Cort] values (converted to standard international (SI) units if needed) and sample sizes (N) reported in studies of sulids.
No outliers were identified for the H/L ratio or individual cell count. In total, 11 slides were counted twice to assess the reliability of the white blood cell count. Intraclass correlation coefficients were calculated using two-way random-effects models (ICC; Liljequist et al., 2019) and were TWBC ICC = 0.93 (95% CI = 0.74–0.98), lymphocyte count ICC = 0.93 (95% CI = 0.73–0.98), heterophil count ICC = 0.88 (95% CI = 0.57–0.97), eosinophil count ICC = 0.85 (95% CI = 0.46–0.97), basophil count ICC = 0.85 (95% CI = 0.45–0.96), and monocyte count ICC = 0.78 (95% CI = 0.19–0.94). The RI for the H/L ratio as estimated by the robust method ranged from 0.15 (90% CI = 0.13–0.18) to 1.15 (90% CI = 0.99–1.39). RIs for TWBC and individual cell types are available in Supplementary Table 1. Three birds had low H/L ratios, and two had high ratios. The sample size of birds falling outside the RI limits for the H/L ratio was too small to reliably calculate odds ratios for group membership. Our H/L ratio values were lower than values reported in other studies of sulids (Table 2). However, most studies did not report variability measures in this index or did not calculate the H/L ratio from their differential white blood cell counts (Table 2), which makes comparisons difficult. We also had a substantially larger sample size than the other studies (Table 2).
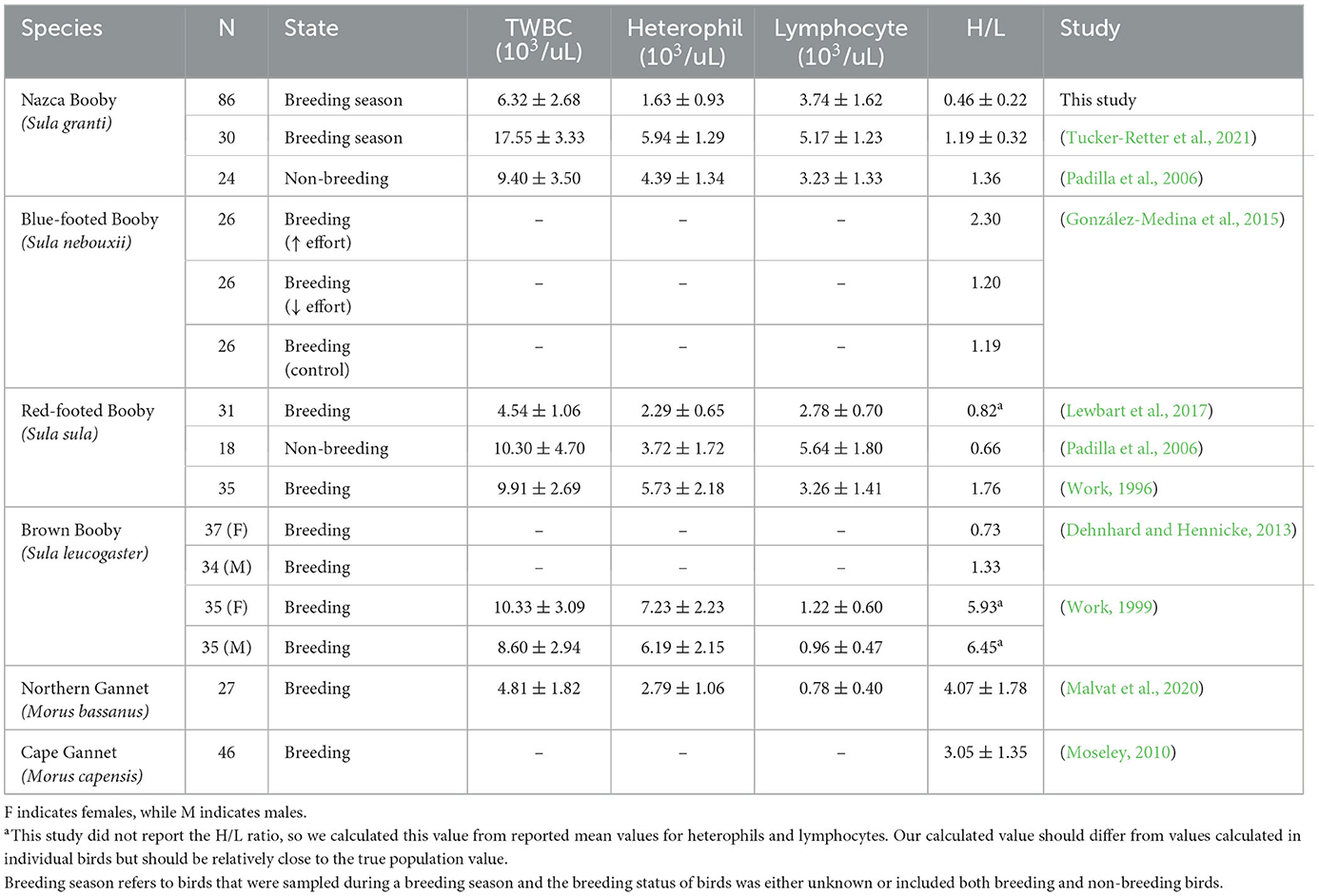
Table 2. The H/L ratio, sample size (N), state at sampling, total white blood cell count (TWBC), heterophil count, and lymphocyte count of other studies in adult sulids.
The baseline [Cort] and H/L ratio were natural log-transformed to meet normality assumptions, with 1 added to the H/L ratio before log transformation to avoid negative values for ratios that were < 1.0. Sampling time was >3 min for 24 birds; however, all samples were collected within 5 min of capture. The mean baseline [Cort] was not different between birds sampled in ≤ 3 min (Ln(mean) = 4.50; 95% CI = 4.45–4.55) and those sampled in 3–5 min (Ln(mean) = 4.63; 95% CI = 4.38–4.87; F1,27 = 0.99, P = 0.32); therefore, all samples were combined for all analyses.
3.1. Effects of the current state on stress indices
Model ranking (Burnham et al., 2011) of baseline [Cort] included two top additive models: sex, age, and reproductive state and reproductive state and age (Table 3). The relative importance of the variables included in the top models was as follows: reproductive state = 0.87, age = 0.87, and sex = 0.52. Males had a higher baseline [Cort] than females; birds that were currently engaged in a breeding attempt had a higher baseline [Cort] than other groups; and age was positively associated with baseline [Cort] (Figures 2A–C). Models including sex, reproductive state, age, and date and additive combinations of these predictors did not explain more variation in the H/L ratio than the null model (Supplementary Table 2). No association between baseline [Cort] and H/L ratio (Pearson's r = −0.08; P = 0.54) or between [Cort] and any white blood cell type count (data not shown; P > 0.45) was found.
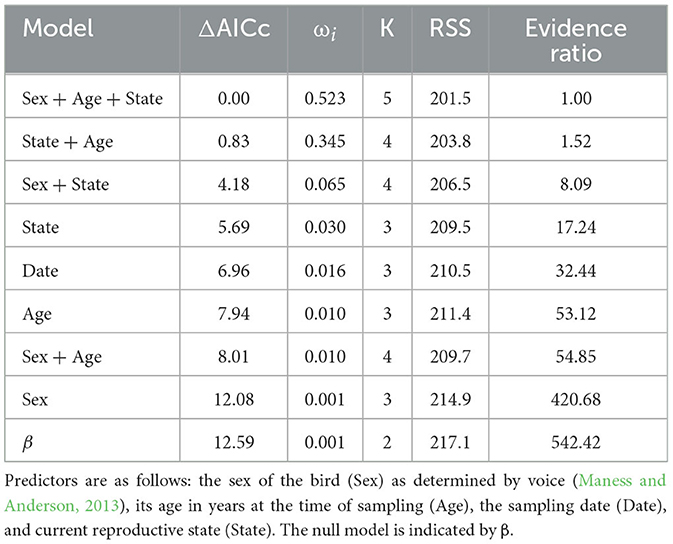
Table 3. Model ranking of potential predictors of baseline [Cort] in adult Nazca boobies showing the model tested, the change in Akaike information criterion corrected for small sample sizes (ΔAICc), the model weight (ωi), number of predictors in the model (K), the residual sums of squares of the model (RSS), and evidence ratio (Burnham et al., 2011).
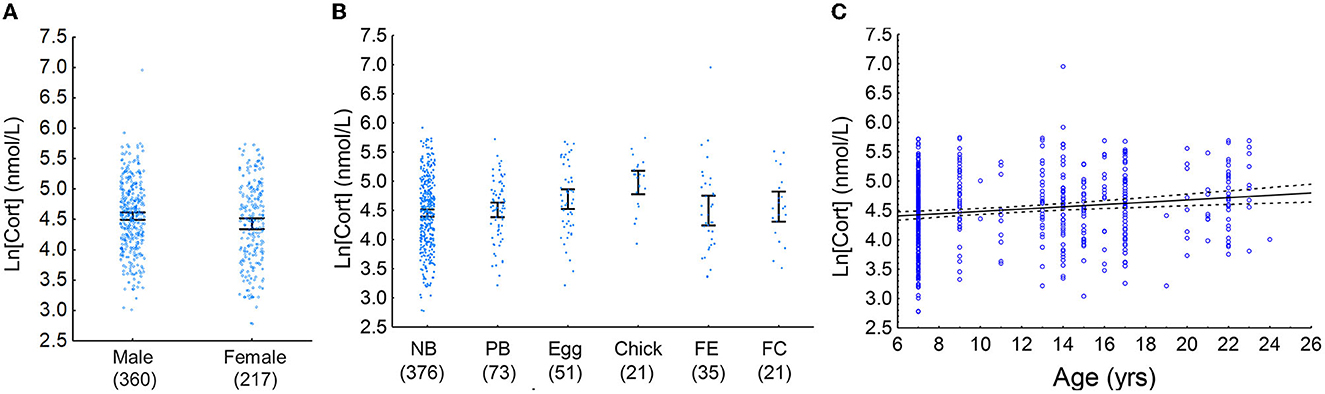
Figure 2. (A) Mean male and female baseline [Cort]. Males had a higher mean [Cort] than females (partial eta2 = 0.011). Samples sizes are in parentheses below the x-axis, blue circles indicate individual data points, and error bars are 95% confidence intervals. (B) Mean baseline [Cort] of adult Nazca boobies in various reproductive states at the time of sampling. NB, non-breeders; PB, pre-breeders; Egg, incubating an egg, Chick, incubating a small chick; FE, nesting attempt failed at the egg stage; FC, nesting attempt failed after eggs had hatched. Sample sizes are in parentheses below the x-axis, blue circles indicate individual data points, and error bars are 95% confidence intervals. Birds that were currently breeding (Egg and Chick) had higher baseline [Cort] than all other groups (partial eta2 = 0.037). (C) Baseline [Cort] increased with increasing age in Nazca boobies (partial eta2 = 0.024). Blue circles are individual data points, the black line is the line of best fit, and the dashed lines are 95% confidence intervals.
3.2. Effects of stress indices on subsequent survival
For the 2002–2003 cohort, baseline [Cort] did not influence survival: the base model outperformed all others (Table 4A) and coefficient estimates describing the effect of [Cort] on annual survival probability immediately after the study (β = −0.77 [95% CI: −2.74–1.21]) and in later years (β = −0.03 [95% CI: −0.34–0.29]) were not distinct from zero. Although a candidate model including baseline [Cort] falls < 2 ΔAICc units from the top model (Table 4), this does not indicate support for the predictor: when a candidate model is identical to the top model except for the addition of one predictor, that model will be < 2 ΔAICc units from the top simply because the penalty for the additional complexity is low (2 AIC units) and not because the additional predictor is supported (Burnham and Anderson, 2010).
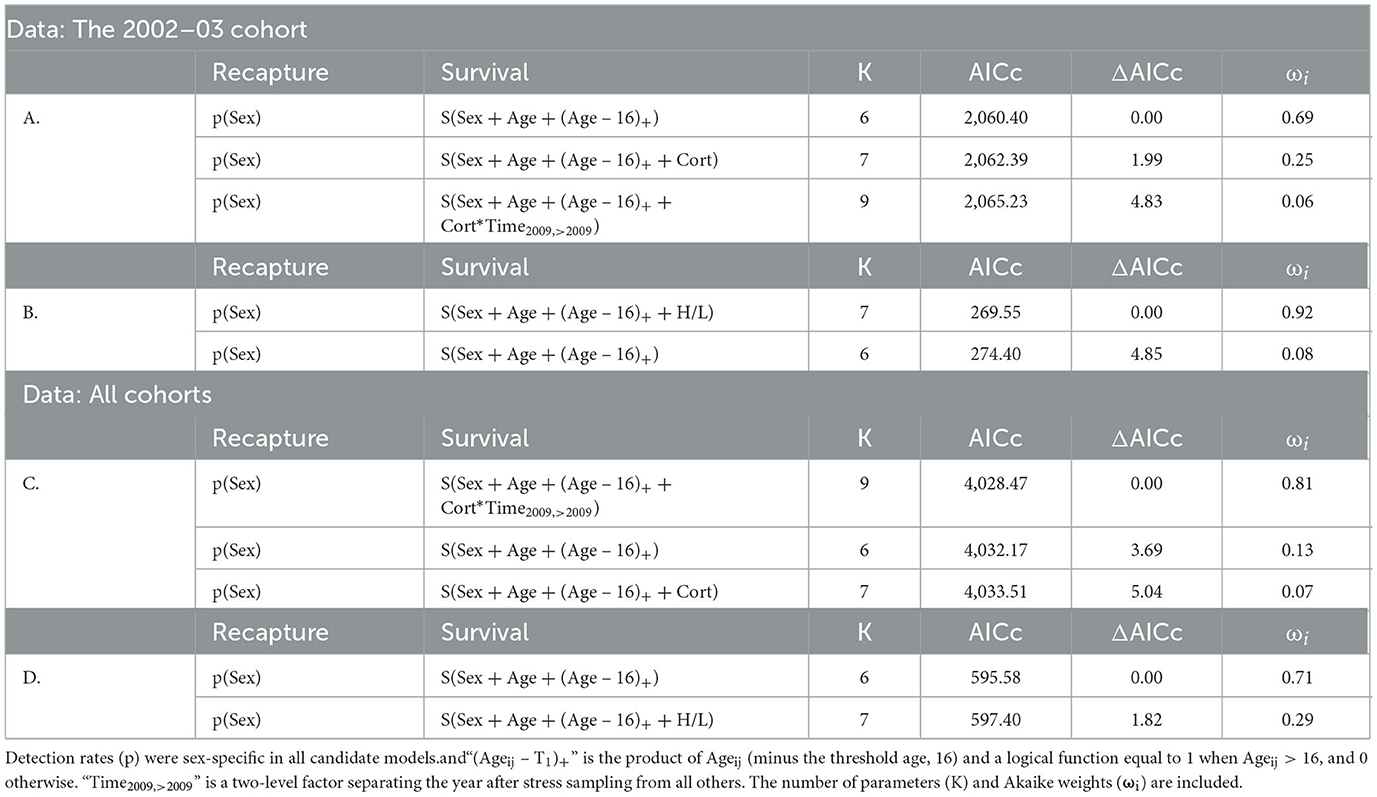
Table 4. Candidate models evaluating the H/L ratio and [Cort] as predictors of Nazca booby survival (S), ranked by AICc.
In contrast, the H/L ratio negatively affected annual survival probabilities (β = −2.80 [95% CI: −4.86 – −0.74], Figure 3, Table 4B). Lower average annual survival probabilities for individuals with high H/L ratio (Figure 3) accumulated across the 11-year period of our study to exert large effects on expected lifespan (Figure 4). We did not attempt to distinguish between immediate and long-term effects of the H/L ratio on annual survival probability because of our small sample size.
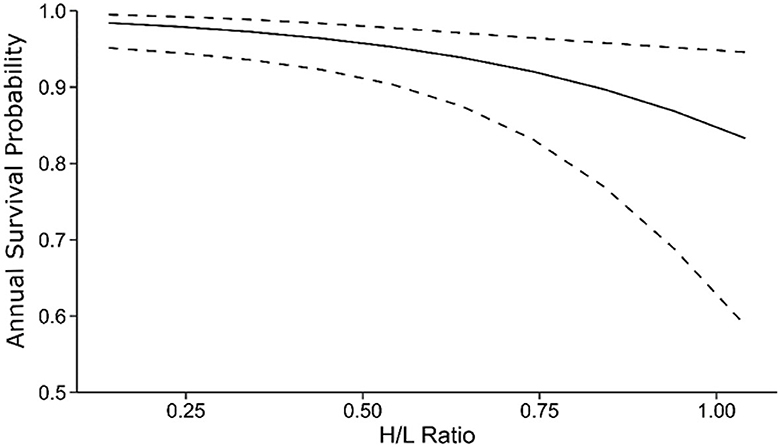
Figure 3. Negative effects of the H/L ratio on annual survival probability of the 2002–2003 (focal) cohort. Dashed lines show the 95% CI around the model-estimated relationship. Predictions are from the best-supported model in Table 1. Age was set to 10, and sex was set to female for plotting (effects of the H/L ratio are not sex-specific). Note that the lower boundary of the y-axis is at 0.5.
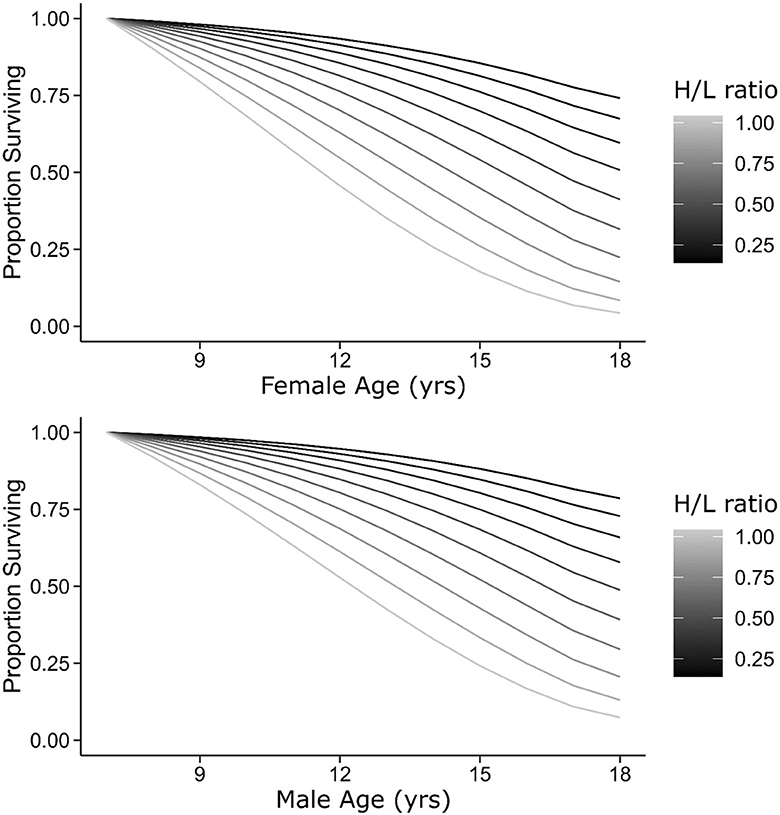
Figure 4. Survivorship curves for females (top) and males (bottom) of the 2002–2003 cohort as a function of the H/L ratio. Curves begin at age 7, the age at which the H/L ratio was sampled. Lines show predicted values from a model including age (as a threshold function), sex, and the H/L ratio as predictors of annual survival probability (see Methods).
When data from all sampled cohorts were included, [Cort] appeared as a predictor within the best-supported model and interacts with a two-level factor distinguishing effects on survival in the year after the study from those affecting all subsequent years (Table 4, Figure 5). [Cort] negatively affected the probability of survival in the year after the study (β = −0.87 [−1.71 – −0.05]) but not beyond (β = −0.04 [95% CI: −0.23–0.15]; Table 4C). In contrast with results from data restricted to the 2002–2003 cohort, the H/L ratio was not an important predictor of survival when data for all sampled cohorts were combined (Table 4D), although a negative slope estimate was maintained (β = −0.30 [95% CI: −1.50–0.91]).
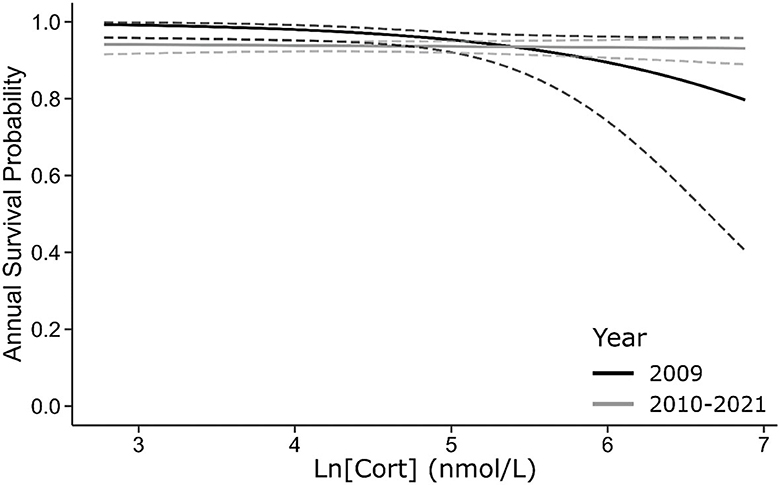
Figure 5. For all sampled cohorts, baseline [Cort] negatively covaries with the probability of surviving from 2009–2010 to the following year (black line) but has no effect on annual survival probability over subsequent intervals (gray line). Dashed lines are 95% CI.
3.3. Effects of stress indices on reproduction
Because 2009–2010 was a poor year for reproductive success in the entire colony (Figure 1), few sampled birds produced a fledgling (54 out of 576 individuals with baseline [Cort] measured, a 9% success rate). Therefore, we could not assess the effect of [Cort] or H/L ratio on the current reproductive effort.
Neither baseline [Cort] nor the H/L ratio predicted lifetime reproductive success for the 2002-2003 cohort (β = −0.01 [95% CI: −0.52–0.49] and β = −1.32 [95% CI: −5.37-2.72], respectively). Visual inspection of residual plots did not indicate a lack of fit, and results were identical using Poisson GLMs. Extending the data to include all sampled cohorts also showed no effect of [Cort] (β = 0.08 [95% CI: −0.26–0.42]) or H/L ratio on lifetime reproductive success (β = −1.24 [95% CI: −3.63–1.24]).
As with lifetime reproductive success, the baseline [Cort] and H/L ratio did not predict residual reproductive success for the 2002–2003 cohort or for all cohorts combined (coefficients reported in Supplementary Table 3). Finally, restricting the data to cohorts with (nearly) complete information on lifetime and residual reproductive success (cohorts 1994–1995 and earlier) did not affect our results: across all versions of our analyses, we document no association between stress indices and reproduction (Supplementary Table 3).
4. Discussion
4.1. Corticosterone
In our study, the baseline [Cort] reflected the current state of sampled birds in that males, older birds, and actively breeding birds had higher [Cort] than females, younger birds, and currently non-breeding birds (Figure 2). Increased baseline GCs during reproductive events are relatively well documented in birds and other vertebrates (reviewed in Bonier et al., 2009a) including Nazca boobies (Grace and Anderson, 2014), and in other long-lived seabirds when food is scarce (Kitaysky et al., 2007, 2010; Shimabukuro et al., 2023). This finding supports one tenet of the Cort-Adaptation Hypothesis that predicts a positive correlation between allostatic load (measured by baseline GCs) and reproductive activities (Bonier et al., 2009a). However, the three predictors associated with baseline [Cort] (sex, age, and current reproductive state) in our study only explained approximately 7% of the variability in [Cort]. Several studies have shown that nutritional stress raises circulating [Cort] in seabirds and is more strongly associated with [Cort] than reproduction alone (Kitaysky et al., 2007, 2010; Schultner et al., 2013; Will et al., 2020; Shimabukuro et al., 2023). Thus, the challenging environmental conditions in the sampling year (Figure 1) may have elevated baseline [Cort] of all birds (Table 1) overwhelming trends between [Cort] and other indicators of allostatic load (Sorenson et al., 2017). Indeed, Nazca boobies at our study site had a higher baseline [Cort] in March during the 2009–2010 breeding season than in March of the 2008–2009 season (see Supplementary Material 1 of Grace and Anderson, 2014), indicating that nutritional stress was probably quite high.
Regarding survival and reproductive success, we found mixed support for the Cort-Fitness hypothesis and no support for the Cort-Adaptation or Cort-Tradeoff hypotheses. The baseline [Cort] negatively predicted the immediate survival of birds in our study, supporting the Cort-Fitness hypothesis (Bonier et al., 2009a) and contradicting expectations of the Cort-Tradeoff hypothesis (Breuner and Berk, 2019). Birds with elevated [Cort] were less likely to survive to the following year when all cohorts were considered, and the effect of age at the time of sampling was controlled (Figure 5), similar to findings in other seabirds (Kitaysky et al., 2007; Schultner et al., 2013). This effect was not seen when considering only the 2002–2003 cohort, although the sign and magnitude of the coefficient estimate are similar in the two datasets (−0.77 vs. −0.87). The larger confidence intervals surrounding the estimate for the 2002–2003 cohort alone probably reflect the extremely low mortality rates for 7-year-old boobies (Anderson and Apanius, 2003; Tompkins and Anderson, 2019).
However, baseline [Cort] was not associated with long-term survival or lifetime reproductive success in our study species, which contradicts the predictions of all three hypotheses. Relationships between baseline [Cort] and reproductive success are well studied in birds but with varying conclusions. Most studies, predominantly in passerine birds, support the Cort-Adaptation hypothesis (e.g., Ouyang et al., 2011; Burtka et al., 2016) or the Cort-Tradeoff Hypothesis (Bókony et al., 2009; Hau et al., 2010), while some of those in seabirds support the Cort-Fitness Hypothesis (e.g., Kitaysky et al., 2007), but others have found no relationship between [Cort] and reproductive success (e.g., Schoenle et al., 2017), like our own study. This may, in part, be due to relationships with reproductive success being specific to the reproductive stage at the time of [Cort] measurement (e.g., incubation vs. chick-rearing; Bonier et al., 2009b; Fischer et al., 2020). Most studies also only examine reproductive success during a single breeding season (e.g., Ouyang et al., 2011; Burtka et al., 2016; Schoenle et al., 2017; Fischer et al., 2020) and not lifetime reproductive success, which may be a more comprehensive indicator of fitness and a stronger test of these hypotheses. Although we were unable to evaluate short-term reproductive success in this study, our finding that [Cort] predicted short-term survival in conjunction with the results of these previous studies suggests that baseline [Cort] reflects current nutritional stress (Kitaysky et al., 2007, 2010; Shimabukuro et al., 2023) and thus correlates with short-term proxies of fitness, but may correlate less well with long-term indicators of fitness. Future studies in other species should investigate relationships between [Cort] and both short-term and lifetime reproductive success to evaluate this hypothesis.
Together, our results suggest that the baseline [Cort] reflects the current, transient physiological state of Nazca boobies (i.e., current reproductive activities and immediate survival) and does not reflect long-term differences in individual quality or fitness. This finding concurs with previous work indicating that GCs reflect current acute stressors such as environmental conditions (Kitaysky et al., 2007, 2010; Satterthwaite et al., 2010; Schultner et al., 2013; Romero and Wingfield, 2016; Davis and Maney, 2018; Shimabukuro et al., 2023). Moreover, the year-to-year repeatability of baseline [Cort] is low in our study species after controlling for other environmental factors (i.e., sex, reproductive status, mass, and year) (Grace and Anderson, 2014), providing no support for an assumption of the Cort-Fitness and Cort-Adaptation hypotheses that baseline [Cort] reflects inherent, repeatable differences in the overall quality of individuals in a population (e.g., Angelier et al., 2010). Instead, Grace and Anderson (2014) found that maximum stress induced [Cort] was highly repeatable in Nazca boobies, thus this metric could be a better indicator of individual quality differences than baseline [Cort] in our study species. In addition, maximum acute stress-induced [Cort] in seabirds may reflect the long-term nutritional state of birds (Kitaysky et al., 2007), reflecting differences in foraging ability or long-term reduction in food quantity or quality (Champagnon et al., 2018).
4.2. H/L ratio
In contrast to [Cort], the H/L ratio was a strong negative predictor of long-term survival for the 2002–2003 cohort but did not reflect the current state in our study, as it had no association with sex, age, or reproductive activities. The effect of the H/L ratio on survival was not apparent when all cohorts were considered. This is perhaps due to the selective disappearance of low-quality phenotypes from older cohorts, which would leave a biased subset of relatively low H/L ratio individuals at the time of sampling (e.g., Nussey et al., 2008; Bouwhuis et al., 2009). Although we found no relationship between the H/L ratio and age in this study (Supplementary Table 2), the power of this analysis was low (β = 0.10) due to the low number of older birds in the dataset.
Longevity has been found to be an important component of lifetime reproductive success in many species (Brown, 1988; Newton, 1989; Oring et al., 1991; Kruuk et al., 1999; Heidinger et al., 2021) including Nazca boobies (Townsend et al., 2007). However, we did not find any relationship between the H/L ratio and reproductive success. This may be because lifetime reproductive success was probably incomplete for our youngest and largest cohort. The number of individuals that fledged from the colony in 2002–2003 indicates that this was a good year for fledging success in the colony (Figure 1). Fledglings produced during this breeding season were heavier, larger, had faster nestling growth rates, and had higher juvenile survival rates than other cohorts (Maness and Anderson, 2013). These favorable rearing conditions likely influence the fitness of this cohort (e.g., Van de Pol et al., 2006) and could influence when these individuals begin to senesce. The 2002–2003 cohort was 17 years old during the last year of the nest monitoring effort (2019–2020). These birds should be entering reproductive senescence (Anderson and Apanius, 2003; Tompkins and Anderson, 2019), but 50% were still alive, so our lifetime reproductive success estimate may be incomplete in this cohort. Further monitoring of the reproductive success of this focal cohort is needed to determine whether the H/L ratio corresponds with complete lifetime reproductive success, as would be expected when the H/L ratio reflects the individual quality. If true, then the H/L ratio would seem to be a good indicator of fitness in Nazca boobies, in a similar manner to the Cort-Fitness hypothesis, at least when it is measured in young individuals. Alternatively, the H/L ratio may indicate differential investment in the innate and acquired arms of the immune system (Martin et al., 2006; Martin, 2009; Brace et al., 2017), creating a tradeoff between survival probabilities and reproductive success similar to that of the Cort-Tradeoff hypothesis. However, this tradeoff is not supported by our data because we found no relationship between the H/L ratio and lifetime or residual reproductive success in the focal cohort or in the entire dataset.
Few studies have attempted to correlate the H/L or N/L ratios with fitness in animal populations. The H/L ratio is associated with aspects of fitness in selectively bred domestic chickens (Gallus gallus), for which low H/L ratios were associated with greater egg production, heavier eggs with higher fertility and hatchability, and heavier chicks with lower mortality rates (Al-Murrani et al., 2006). In wild animals, studies that have attempted to link the H/L or N/L ratio and fitness have been short-term or used proxies for fitness-like body condition (e.g., Xuereb et al., 2012) male ornamentation (e.g., Lebigre et al., 2012) or chick weight (e.g., Parejo and Silva, 2009). If the H/L ratio reflects inherent individual quality, then the H/L ratio should be repeatable from year to year after controlling for confounding environmental variables, similar to the Cort-Fitness Hypothesis. This possibility needs to be assessed in Nazca boobies, but evidence from a truncation selection experiment (using the 99% confidence interval for H/L ratio to select breeders) showed that the H/L ratios are heritable in domestic chickens (Al-Murrani et al., 2006), suggesting individual repeatability in birds. Similarly, the N/L ratio was found to be repeatable in three populations of wild roe deer (Capreolus capreolus) over an 8- or 9-year interval (Carbillet et al., 2019). Further studies are needed to assess both the repeatability of individual H/L and N/L ratios and their correlates with fitness in animal populations.
5. Conclusion
One of the great challenges in ecophysiology is linking physiological measures in wild animal populations with changes in individual fitness. Here, we assessed two indicators of stress in a wild long-lived bird, namely, baseline [Cort] and H/L ratio, to determine whether these metrics corresponded with individual performance. [Cort] corresponded with the current conditions and activities (e.g., breeding) that the animal was engaged in (Figure 2) and predicted near-term survival probability (Figure 5). The H/L ratio predicted long-term survival in our youngest focal cohort (Figures 3, 4). Our results suggest that the H/L ratio may be a good indicator of long-term fitness in Nazca boobies because survival and reproductive performance are known to correlate in this species (Townsend et al., 2007). Our results also support findings from other seabirds (e.g., Kitaysky et al., 2007, 2010) that suggest that baseline [Cort] may be a good indicator of the current state and near-term performance. We propose a corresponding hypothesis to the Cort-Fitness Hypothesis: the H/L(N/L) Ratio-Fitness Hypothesis. This hypothesis predicts that H/L or N/L ratios reflect the underlying quality of an individual, such that lower values predict higher fitness. An assumption of the hypothesis is that these ratios should be repeatable across years. This hypothesis needs to be tested in this and other species and, if supported by empirical evidence, then these ratios could be a powerful monitoring tool for assessing population health or identifying at-risk populations. Continued monitoring of reproductive success in study birds that are still alive and assessment of the repeatability of the H/L ratio is needed to demonstrate the applicability of this hypothesis in our study system.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: ZSR Library, http://hdl.handle.net/10339/102187.
Ethics statement
This animal study was reviewed and approved by Wake Forest University Institutional Animal Care and Use Committee. This study was permitted under Wake Forest University IACUC (protocol A08-029) and Galápagos National Park, and adheres to NIH and the Ornithological Council's standards for animal use in research.
Author contributions
TM: conceptualization, supervision of lab analyses, data analysis, writing, and manuscript preparation. JG: supervision of fieldwork, sample collection, performed baseline [Cort] analysis, writing, and manuscript preparation. MH: performed blood cell counts, data analysis, and manuscript preparation. ET: data analysis, writing, and manuscript preparation. DA: conceptualization, supervision, writing, and manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
We received funding from DA: Division of Environmental Biology, grant/award numbers: DEB 0235818, DEB 0842199, DEB 1354473, DEB 93045679, DEB 9629539, and DEB 98-06606; Wake Forest University; National Geographic Society; and TM: College of Applied and Natural Sciences, Louisiana Tech University.
Acknowledgments
We thank the Galápagos National Park Service for permission to work in the Park; the Charles Darwin Research Station, and TAME Airline, for logistical support; and the National Science Foundation, National Geographic Society, Wake Forest University, and Louisiana Tech University for research funding. We thank the reviewers for insightful comments that improved the manuscript. This publication is contribution number 2522 of the Charles Darwin Foundation for the Galápagos Islands.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1172904/full#supplementary-material
References
Al-Murrani, W. K., Al-Rawi, A. J., Al-Hadithi, M. F., and Al-Tikriti, B. (2006). Association between heterophil/lymphocyte ratio, a marker of “resistance” to stress, and some production and fitness traits in chickens. Br. Poult. Sci. 47, 443–448. doi: 10.1080/00071660600829118
Anderson, D. J., and Apanius, V. (2003). Actuarial and reproductive senescence in a long-lived seabird: preliminary evidence. Exp. Gerontol. 38, 757–760. doi: 10.1016/S0531-5565(03)00104-9
Angelier, F., Wingfield, J. C., Weimerskirch, H., and Chastel, O. (2010). Hormonal correlates of individual quality in a long-lived bird: a test of the “corticosterone-fitness hypothesis”. Biol. Lett. 6, 846–849. doi: 10.1098/rsbl.2010.0376
Apanius, V. A., Westbrock, M. W., and Anderson, D. J. (2008). Reproduction and immune homeostasis in a long-lived seabird, the Nazca booby (Sula granti). Ornithol. Monogr. 65, 1–46. doi: 10.1525/om.2008.65.1.1
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, ei01. doi: 10.18637/jss.v067.i01
Bókony, V., Lendvai, Á. Z., Likér, A., Angelier, F., Wingfield, J. C., Chastel, O., et al. (2009). Stress response and the value of reproduction: are birds prudent parents? Am. Nat. 173, 589–598. doi: 10.1086/597610
Bonier, F., Martin, P. R., Moore, I. T., and Wingfield, J. C. (2009a). Do baseline glucocorticoids predict fitness? Trends Ecol. E24, 634–642. doi: 10.1016/j.tree.2009.04.013
Bonier, F., Moore, I. T., Martin, P. R., and Robertson, R. J. (2009b). The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol. 163, 208–213. doi: 10.1016/j.ygcen.2008.12.013
Bouwhuis, S., Sheldon, B. C., Verhulst, S., and Charmantier, A. (2009). Great tits growing old: Selective disappearance and the partitioning of senescence to stages within the breeding cycle. Proc. R. Soc. B Biol. Sci. 276, 2769–2777. doi: 10.1098/rspb.2009.0457
Brace, A. J., Lajeunesse, M. J., Ardia, D. R., Hawley, D. M., Adelman, J. S., Buchanan, K. L., et al. (2017). Costs of immune responses are related to host body size and lifespan. J. Exp. Zool. Part A Ecol. Integr. Physiol. 327, 254–261. doi: 10.1002/jez.2084
Breuner, C. W., and Berk, S. A. (2019). Using the van noordwijk and de jong resource framework to evaluate glucocorticoid-fitness hypotheses. Integr. Comp. Biol. 59, 243–250. doi: 10.1093/icb/icz088
Brown, D. (1988). Components of Lifetime Reproductive Success. Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. Chicago: Univeristy of Chicago Press, and 439–453.
Burnham, K. P., and Anderson, D. R. (2010). Model Selection and Multimodel Inference. 2nd Edn. New York, NY: Springer-Verlag.
Burnham, K. P., Anderson, D. R., and Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. doi: 10.1007/s00265-010-1029-6
Burtka, J. L., Lovern, M. B., and Grindstaff, J. L. (2016). Baseline hormone levels are linked to reproductive success but not parental care behaviors. Gen. Comp. Endocrinol. 229, 92–99. doi: 10.1016/j.ygcen.2016.03.010
Carbillet, J., Rey, B., Lavabre, T., Chaval, Y., Merlet, J., Débias, F., et al. (2019). The neutrophil to lymphocyte ratio indexes individual variation in the behavioural stress response of wild roe deer across fluctuating environmental conditions. Behav. Ecol. Sociobiol. 73, 1–14. doi: 10.1007/s00265-019-2755-z
Champagnon, J., Lebreton, J. D., Drummond, H., and Anderson, D. J. (2018). Pacific Decadal and El Niño oscillations shape survival of a seabird. Ecology 99, 1063–1072. doi: 10.1002/ecy.2179
Clifford, L., and Anderson, D. (2001). Food limitation explains most clutch size variation in the Nazca booby. J. Anim. Ecol., 539–545. doi: 10.1046/j.1365-2656.2001.00521.x
Cockrem, J. F., Candy, E. J., Potter, M. A., and Machovsky-Capuska, G. E. (2016). Corticosterone responses to capture and restraint in australasian gannets, morus serrator, at cape kidnappers, New Zealand. Emu - Austral Ornithol. 116, 86–90. doi: 10.1071/MU15012
Davis, A. K., and Maney, D. L. (2018). The use of glucocorticoid hormones or leucocyte profiles to measure stress in vertebrates: what's the difference? Methods Ecol. E9, 1556–1568. doi: 10.1111/2041-210X.13020
Davis, A. K., Maney, D. L., and Maerz, J. C. (2008). The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct. Ecol. 22, 760–772. doi: 10.1111/j.1365-2435.2008.01467.x
Dehnhard, N., and Hennicke, J. C. (2013). Leucocyte profiles and body condition in breeding brown boobies and red-tailed tropicbirds: effects of breeding stage and sex. Aust. J. Zool. 61, 178. doi: 10.1071/ZO12123
Dhabhar, F. S., Miller, A. H., McEwen, B. S., and Spencer, R. L. (1996). Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J. Immunol. 157, 1638–1644. doi: 10.4049/jimmunol.157.4.1638
Fefferman, N. H., and Romero, L. M. (2013). Can physiological stress alter population persistence? A model with conservation implications. Conserv. Physiol. 1, 1–13. doi: 10.1093/conphys/cot012
Fischer, D., Marrotte, R. R., Chin, E. H., Coulson, S., and Burness, G. (2020). Maternal glucocorticoid levels during incubation predict breeding success, but not reproductive investment, in a free-ranging bird. Biol. Open 9, 1–10. doi: 10.1242/bio.045898
Franci, C. D., Guillemette, M., Pelletier, É., Chastel, O., Bonnefoi, S., Verreault, J., et al. (2014). Endocrine status of a migratory bird potentially exposed to the Deepwater Horizon oil spill: A case study of northern gannets breeding on Bonaventure Island, Eastern Canada. Sci. Total Environ. 474, 110–116. doi: 10.1016/j.scitotenv.2013.12.006
Geffré, A., Concordet, D., Braun, J. P., and Trumel, C. (2011). Reference value advisor: a new freeware set of macroinstructions to calculate reference intervals with microsoft excel. Vet. Clin. Pathol. 40, 107–112. doi: 10.1111/j.1939-165X.2011.00287.x
González-Medina, E., Castillo-Guerrero, J. A., Santiago-Quesada, F., Villegas, A., Masero, J. A., Sánchez-Guzmán, J. M., et al. (2015). Regulation of breeding expenditure in the blue-footed booby, Sula nebouxii : an experimental approach. Anim. Behav. 108, 9–16. doi: 10.1016/j.anbehav.2015.06.025
Grace, J. K., and Anderson, D. J. (2014). Corticosterone stress response shows long-term repeatability and links to personality in free-living Nazca boobies. Gen. Comp. Endocrinol. 208, 39–48. doi: 10.1016/j.ygcen.2014.08.020
Grace, J. K., Dean, K., Ottinger, M. A., and Anderson, D. J. (2011). Hormonal effects of maltreatment in Nazca booby nestlings: implications for the “cycle of violence”. Horm. Behav. 60, 78–85. doi: 10.1016/j.yhbeh.2011.03.007
Hau, M., Ricklefs, R. E., Wikelski, M., Lee, K., and Brawn, J. D. (2010). Corticosterone, testosterone and life-history strategies of birds. Proc. Biol. Sci. 277, 3203–3212. doi: 10.1098/rspb.2010.0673
Heidinger, B. J., Kucera, A. C., Kittilson, J. D., and Westneat, D. F. (2021). Longer telomeres during early life predict higher lifetime reproductive success in females but not males. Proc. R. Soc. B Biol. Sci. 288, 560. doi: 10.1098/rspb.2021.0560
Heidinger, B. J., Nisbet, I. C. T., and Ketterson, E. D. (2006). Older parents are less responsive to a stressor in a long-lived seabird: a mechanism for increased reproductive performance with age? Proc. Biol. Sci. 273, 2227–2231. doi: 10.1098/rspb.2006.3557
Heidinger, B. J., Nisbet, I. C. T., and Ketterson, E. D. (2008). Changes in adrenal capacity contribute to a decline in the stress response with age in a long-lived seabird. Gen. Comp. Endocrinol. 156, 564–568. doi: 10.1016/j.ygcen.2008.02.014
Humphries, C. A., Arevalo, V. D., Fischer, K. N., and Anderson, D. J. (2006). Contributions of marginal offspring to reproductive success of Nazca booby (Sula granti) parents: Tests of multiple hypotheses. Oecologia 147, 379–390. doi: 10.1007/s00442-005-0264-4
Huyvaert, K., and Anderson, D. (2004). Limited dispersal by Nazca boobies (Sula granti). J. Avian Biol. 1, 46–53. doi: 10.1111/j.0908-8857.2004.03131.x
Johnstone, C. P., Reina, R. D., and Lill, A. (2012). Interpreting indices of physiological stress in free-living vertebrates. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 182, 861–879. doi: 10.1007/s00360-012-0656-9
Kitaysky, A. S., Piatt, J. F., Hatch, S. A., Kitaiskaia, E. V., Benowitz-Fredericks, Z. M., Shultz, M. T., et al. (2010). Food availability and population processes: severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct. Ecol. 24, 625–637. doi: 10.1111/j.1365-2435.2009.01679.x
Kitaysky, A. S., Piatt, J. F., and Wingfield, J. C. (2007). Stress hormones link food availability and population processes in seabirds. Mar. Ecol. Prog. Ser. 352, 245–258. doi: 10.3354/meps07074
Kruuk, L. E. B., Clutton-Brock, T. H., Rose, K. E., and Guinness, F. E. (1999). Early determinants of lifetime reproductive success differ between the sexes in red deer. Proc. R. Soc. London. Ser. B Biol. Sci. 266, 1655–1661. doi: 10.1098/rspb.1999.0828
Laake, J. L. (2013). RMark: An R Interface for analysis of capture-recapture data with MARK. AFSC Processed Report 2013-01. Seattle, WA: National Oceanic and Atmospheric Administration. p. 25.
Lebigre, C., Alatalo, R. V., Kilpimaa, J., Staszewski, V., and Siitari, H. (2012). Leucocyte counts variation and measures of male fitness in the lekking Black Grouse. J. Ornithol. 153, 95–102. doi: 10.1007/s10336-011-0701-6
Lebreton, J. D., Burnham, K. P., Clobert, J., and Anderson, D. R. (1992). Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62, 67–118. doi: 10.2307/2937171
Lewbart, G. A., Ulloa, C., Deresienski, D., Regalado, C., Muñoz-Pérez, J.-P., Garcia, J., et al. (2017). Health status of red-footed boobies (Sula sula) determined by hematology, biochemistry, blood gases, and physical examination. J. Zoo Wildl. Med. 48, 1230–1233. doi: 10.1638/2017-0031.1
Liljequist, D., Elfving, B., and Skavberg Roaldsen, K. (2019). Intraclass correlation – A discussion and demonstration of basic features. PLoS ONE 14, e0219854. doi: 10.1371/journal.pone.0219854
Malvat, Z., Lynch, S. A., Bennison, A., and Jessopp, M. (2020). Evidence of links between haematological condition and foraging behaviour in northern gannets (Morus bassanus). R. Soc. Open Sci. 7, 192164. doi: 10.1098/rsos.192164
Maness, T., and Anderson, D. (2013). Predictors of juvenile survival in birds. Ornithol. Monogr. 78, 1–55. doi: 10.1525/om.2013.78.1.1
Maness, T., Westbrock, M., and Anderson, D. (2007). Ontogenic sex ratio variation in Nazca boobies ends in male-biased adult sex ratio. Waterbirds 30, 10–16. doi: 10.1675/1524-4695(2007)030[0010:OSRVIN]2.0.CO;2
Maness, T., Westbrock, M., Feeley, K., and Anderson, D. (2011). Offspring sex and duration of post-fledging parental care in the sexually size dimorphic Nazca Booby (Sula granti). Neotrop. Ornithol. 22, 347–359.
Martin, L. B. (2009). Stress and immunity in wild vertebrates: Timing is everything. Gen. Comp. Endocrinol. 163, 70–76. doi: 10.1016/j.ygcen.2009.03.008
Martin, L. B., Hasselquist, D., and Wikelski, M. (2006). Investment in immune defense is linked to pace of life in house sparrows. Oecologia 147, 565–575. doi: 10.1007/s00442-005-0314-y
McEwen, B. S., and Wingfield, J. C. (2003). The concept of allostasis in biology and biomedicine. Horm. Behav. 43, 2–15. doi: 10.1016/S0018-506X(02)00024-7
Minias, P. (2019). Evolution of heterophil/lymphocyte ratios in response to ecological and life-history traits: A comparative analysis across the avian tree of life. J. Anim. Ecol. 88, 554–565. doi: 10.1111/1365-2656.12941
Moseley, C. (2010). Comparing body condition and foraging ecology of two populations of Cape Gannets on Bird and Malgas Islands (Master's thesis, University of Cape Town).
Müller, C., Jenni-Eiermann, S., and Jenni, L. (2011). Heterophils/Lymphocytes-ratio and circulating corticosterone do not indicate the same stress imposed on Eurasian kestrel nestlings. Funct. Ecol. 25, 566–576. doi: 10.1111/j.1365-2435.2010.01816.x
Nussey, D. H., Coulson, T., Festa-Bianchet, M., and Gaillard, J. M. (2008). Measuring senescence in wild animal populations: towards a longitudinal approach. Funct. Ecol. 22, 393–406. doi: 10.1111/j.1365-2435.2008.01408.x
Oring, L. W., Colwell, M. A., and Reed, J. M. (1991). Lifetime reproductive success in the spotted sandpiper (Actitis macularia): sex differences and variance components. Behav. Ecol. Sociobiol. 28, 425–432. doi: 10.1007/BF00164124
Ouyang, J. Q., Sharp, P. J., Dawson, A., Quetting, M., and Hau, M. (2011). Hormone levels predict individual differences in reproductive success in a passerine bird. Proc. Biol. Sci. 278, 2537–2545. doi: 10.1098/rspb.2010.2490
Padilla, L., Whiteman, N., and Merkel, J. (2006). Health assessment of seabirds on Isla Genovesa, Galápagos Islands. Ornithol. Monogr. 60, 86–97. doi: 10.1642/0078-6594(2006)60[86:HAOSOI]2.0.CO;2
Parejo, D., and Silva, N. (2009). Immunity and fitness in a wild population of Eurasian kestrels Falco tinnunculus. Naturwissenschaften 96, 1193–1202. doi: 10.1007/s00114-009-0584-z
Romero, L. M. (2004). Physiological stress in ecology: lessons from biomedical research. Trends Ecol. E19, 249–255. doi: 10.1016/j.tree.2004.03.008
Romero, L. M., Dickens, M. J., and Cyr, N. E. (2009). The reactive scope model - A new model integrating homeostasis, allostasis, and stress. Horm. Behav. 55, 375–389. doi: 10.1016/j.yhbeh.2008.12.009
Romero, L. M., and Reed, J. M. (2005). Collecting baseline corticosterone samples in the field: Is under 3 min good enough? Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 140, 73–79. doi: 10.1016/j.cbpb.2004.11.004
Romero, L. M., and Wingfield, J. C. (2016). Tempests, Poxes, Predators, and People: Stress in Wild Animals and How They Cope. New York, NY, USA: Oxford University Press.
Satterthwaite, W. H., Kitaysky, A. S., Hatch, S. A., Piatt, J. F., and Mangel, M. (2010). Unifying quantitative life-history theory and field endocrinology to assess prudent parenthood in a long-lived seabird. Evol. Ecol. Res. 12, 779–792.
Schoenle, L. A., Dudek, A. M., Moore, I. T., and Bonier, F. (2017). Red-winged blackbirds (Agelaius phoeniceus) with higher baseline glucocorticoids also invest less in incubation and clutch mass. Horm. Behav. 90, 1–7. doi: 10.1016/j.yhbeh.2017.02.002
Schoenle, L. A., Zimmer, C., and Vitousek, M. N. (2018). Understanding context dependence in glucocorticoid–fitness relationships: the role of the nature of the challenge, the intensity and frequency of stressors, and life history. Integr. Comp. Biol. 58, 777–789. doi: 10.1093/icb/icy046
Schultner, J., Kitaysky, A. S., Welcker, J., and Hatch, S. (2013). Fat or lean: adjustment of endogenous energy stores to predictable and unpredictable changes in allostatic load. Funct. Ecol. 27, 45–55. doi: 10.1111/j.1365-2435.2012.02058.x
Shimabukuro, U., Takahashi, A., Okado, J., Kokubun, N., Thiebot, J., Will, A., et al. (2023). Across the North Pacific, dietary-induced stress of breeding rhinoceros auklets increases with high summer Pacific Decadal Oscillation index. Mar. Ecol. Prog. Ser. 708, 177–189. doi: 10.3354/meps14276
Sorenson, G. H., Dey, C. J., Madliger, C. L., and Love, O. P. (2017). Effectiveness of baseline corticosterone as a monitoring tool for fitness: a meta-analysis in seabirds. Oecologia 183, 353–365. doi: 10.1007/s00442-016-3774-3
Tarlow, E. M., Hau, M., Anderson, D. J., and Wikelski, M. (2003a). Diel changes in plasma melatonin and corticosterone concentrations in tropical Nazca boobies (Sula granti) in relation to moon phase and age. Gen. Comp. Endocrinol. 133, 297–304. doi: 10.1016/S0016-6480(03)00192-8
Tarlow, E. M., Wikelski, M., and Anderson, D. J. (2003b). Correlation between plasma steroids and chick visits by nonbreeding adult Nazca boobies. Horm. Behav. 43, 402–407. doi: 10.1016/S0018-506X(03)00011-4
Tompkins, E. M., and Anderson, D. J. (2019). Sex-specific patterns of senescence in Nazca boobies linked to mating system. J. Anim. Ecol. 88, 986–1000. doi: 10.1111/1365-2656.12944
Tompkins, E. M., and Anderson, D. J. (2021). Breeding responses to environmental variation are age- and trait-dependent in female Nazca boobies. Ecology 102, 3457. doi: 10.1002/ecy.3457
Townsend, H. M., Maness, T. J., and Anderson, D. J. (2007). Offspring growth and parental care in sexually dimorphic Nazca boobies (Sula granti). Can. J. Zool. 85, 686–694. doi: 10.1139/Z07-047
Tucker-Retter, E. K., Velsey-Gross, Z., Deresienski, D., Ulloa, C., Muñoz-Pérez, J.-P., Skehel, A., et al. (2021). Health status of Nazca boobies (Sula granti) on Daphne Major Island in the Galápagos determined by hematology, biochemistry, and physical examination. J. Zoo Wildl. Med. 52, 136. doi: 10.1638/2020-0136
Van de Pol, M., Bruinzeel, L. W., Heg, D., Van der Jeugd, H. P., and Verhulst, S. (2006). A silver spoon for a golden future: long-term effects of natal origin on fitness prospects of oystercatchers (Haematopus ostralegus). J. Anim. Ecol. 75, 616–626. doi: 10.1111/j.1365-2656.2006.01079.x
White, G. C., and Burnham, K. P. (1999). Program MARK: survival estimation from populations of marked animals. Bird Study 46, S120–S139. doi: 10.1080/00063659909477239
Wikelski, M., and Cooke, S. J. (2006). Conservation physiology. Trends Ecol. E21, 38–46. doi: 10.1016/j.tree.2005.10.018
Will, A., Takahashi, A., Thiebot, J. B., Martinez, A., Kitaiskaia, E., Britt, L., et al. (2020). The breeding seabird community reveals that recent sea ice loss in the Pacific Arctic does not benefit piscivores and is detrimental to planktivores. Deep. Res. Part II Top. Stud. Oceanogr. 181–182 104902. doi: 10.1016/j.dsr2.2020.104902
Wingfield, J. C., Hau, M., Boersma, P. D., Romero, L. M., Hillgarth, N., Ramenofsky, M., et al. (2018). Effects of El Niño and La Niña Southern Oscillation events on the adrenocortical responses to stress in birds of the Galapagos Islands. Gen. Comp. Endocrinol. 259, 20–33. doi: 10.1016/j.ygcen.2017.10.015
Wingfield, J. C., Ramos-Fernandez, G., Nuñez-de la Mora, A., and Drummond, H. (1999). The effects of an “El Niño” southern oscillation event on reproduction in male and female blue-footed boobies, Sula nebouxii. Gen. Comp. Endocrinol. 114, 163–172. doi: 10.1006/gcen.1998.7243
Work, T. M. (1996). Weights, hematology, and serum chemistry of seven species of free-ranging tropical pelagic seabirds. J. Wildl. Dis. 32, 643–657. doi: 10.7589/0090-3558-32.4.643
Work, T. M. (1999). Weights, hematology, and serum chemistry of free-ranging brown boobies (Sula leucogaster) in Johnston Atoll, Central Pacific. J. Zoo Wildl. Med. 30, 81–84.
Xuereb, A., Row, J. R., Brooks, R. J., MacKinnon, C., and Lougheed, S. C. (2012). Relation between parasitism, stress, and fitness correlates of the eastern foxsnake (Pantherophis gloydi) in Ontario. J. Herpetol. 46, 555–561. doi: 10.1670/10-259
Keywords: seabird, Sula granti, stress response, glucocorticoids, allostasis, H/L ratio, survival, lifetime reproductive success
Citation: Maness TJ, Grace JK, Hirchak MR, Tompkins EM and Anderson DJ (2023) Circulating corticosterone predicts near-term, while H/L ratio predicts long-term, survival in a long-lived seabird. Front. Ecol. Evol. 11:1172904. doi: 10.3389/fevo.2023.1172904
Received: 24 February 2023; Accepted: 15 May 2023;
Published: 21 June 2023.
Edited by:
Todd Jason McWhorter, University of Adelaide, AustraliaReviewed by:
Alexander Kitaysky, University of Alaska Fairbanks, United StatesCeliwe Ngcamphalala, University of Cape Town, South Africa
Copyright © 2023 Maness, Grace, Hirchak, Tompkins and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Terri J. Maness, dG1hbmVzc0BsYXRlY2guZWR1; Jacquelyn K. Grace, amFjcXVlbHluLmdyYWNlQGFnLnRhbXUuZWR1
 Terri J. Maness
Terri J. Maness Jacquelyn K. Grace
Jacquelyn K. Grace Michael R. Hirchak
Michael R. Hirchak Emily M. Tompkins
Emily M. Tompkins David J. Anderson3
David J. Anderson3