
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 26 October 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1151913
This article is part of the Research Topic Origin, Conservation, and Restoration of the Threatened European Grassland Ecosystem in the Anthropocene View all 16 articles
The development of urban areas now requires the integration of biodiversity issues, and this leads to better consideration of their seminatural habitats. Among these habitats, urban grasslands subjected to mowing management practices are commonly promoted over lawns to enhance biodiversity in cities. Despite their ecological value, relatively little attention has been paid to the effects of urban grassland management regimes or the landscape contexts of these habitats in terms of biodiversity. This study aims to investigate the effects of mowing practices and the landscape context of urban grasslands on species diversity and composition and the ecological strategies of plant communities. In this study, 66 sites (mown grasslands) were selected in the Angers and Rennes conurbations of western France according to their management practices (regarding mowing) and landscape gradient (more or less urbanized). The results show that mowing practices and landscape composition did not affect the richness or diversity of plant species but significantly influenced the composition of communities. Partitioning analysis showed that landscape composition explained twice as much of the variance in plant species composition as mowing practices did. Landscape composition favors plant species according to their strategies, preferential habitats, and life spans. Furthermore, diversification of management practices limits the establishment of nonnative species and induces a wider range of functional strategies, as late mowing favors competitors and disfavors stress-tolerant species. Nevertheless, management practices need to be put into perspective in the context of urban grasslands. Thus, this research brings new perspectives to recommendations for the management of urban green spaces.
Among seminatural habitats in Europe, grasslands present important challenges for biodiversity conservation (Lindborg et al., 2008; Napoleone et al., 2021), especially due to their high species richness (Habel et al., 2013). In contrast to agricultural grasslands, urban grasslands remain understudied (Cochard et al., 2019) in spite of their contribution to urban biodiversity (McKinney, 2002; Fischer et al., 2013; Vega and Küffer, 2021; Mugnai et al., 2023). Urban grasslands also provide valuable ecosystem services such as improvement of human well-being (Wu, 2014), climate regulation, or recreational use (Zhao et al., 2020). The practical application of these services takes the form of nature-based solutions, such as the implantation of grasslands to reduce urban heat islands (Zhao et al., 2020). Urban grasslands are associated with different uses because their establishment results from various urban planning choices (e.g., parks, residential areas, land reserves, derelict areas) or constraints (e.g., flood-prone areas, roadsides) (Klaus, 2013). Urban grasslands can be defined as seminatural herbaceous green spaces located in moderately to strongly urbanized areas and managed by city stakeholders. Unlike other herbaceous green spaces in cities, such as lawns, urban grasslands are characterized by relatively low-intensity management practices that aim to limit the establishment of bushes and woody plants (Politi Bertoncini et al., 2012), maintain open herbaceous habitats for biodiversity, and promote seminatural aesthetic elements in cities. The most common type of urban grasslands are urban meadows, managed by mowing (Humbert et al., 2012; Klaus, 2013) and neither fertilized nor sprayed with pesticides. Because of the absence of productive goals, urban grasslands tend to be mowed late in the season, but management choices by stakeholders regarding the timing and frequency of mowing may vary widely, depending on their objectives.
Recent changes in urban management practices, especially the generalization of lower-intensity management, have been developed ahead of scientific knowledge and bring new questions about the effects of low-intensity management of urban herbaceous spaces on plant biodiversity (Aronson et al., 2017). Regarding mowing, beneficial effects can be expected from moderate disturbance frequency (e.g., a maximum of two cuts per year), as this is known to maintain relatively high plant species richness (Parr and Way, 1988; Bakker et al., 2002). However, most studies still compare the biodiversity of contrasting types of urban green spaces rather than focusing on urban grasslands per se (Sehrt et al., 2020). In other types of herbaceous habitats, the effect of mowing frequency has mainly been studied by comparing lawns, which do not harbor much biodiversity due to their intensive management (Klaus, 2013), to urban grasslands (Norton et al., 2019; Sehrt et al., 2020). Urban grasslands with reduced mowing frequency were found to harbor higher plant species richness and the occurrence of typical meadow species when compared with intensively managed lawns (Sehrt et al., 2020). To our knowledge, the impact of mowing timing on urban grasslands also remains underinvestigated. In agricultural grasslands, late mowing is known to allow plant species to produce more seeds (Smith et al., 2002; Leng et al., 2011; Chaudron et al., 2016) and to complete their life cycle (Jantunen et al., 2007), which could favor competitive strategies (Johansen et al., 2019). However, existing literature has found contradictory effects of late mowing on biodiversity. It can affect species richness either negatively if delayed from spring to fall or positively if delayed from spring to summer (Grime, 2001; Humbert et al., 2012; Chaudron et al., 2016). There is thus a gap in the body of knowledge regarding the effects of the diverse mowing regimes applied by city stakeholders, in terms of both frequency and timing, on the diversity of plant communities in urban grasslands.
Beyond the effects of local management practices, the flora of urban grasslands is also likely to be influenced by their landscape context. Urbanization (defined as the process of growth and densification of urban areas) is now considered one of the major threats to biodiversity (Foley et al., 2005; Grimm et al., 2008). Urbanization induces several mechanisms, including habitat destruction, that cause the fragmentation and spatial isolation of remnant habitat patches (Williams et al., 2009; Aronson et al., 2017). Such changes have been found to decrease the frequency and abundance of native plant species (Hooftman et al., 2021) in favor of exotic species (Thompson and Jones, 1999; Smart et al., 2005; Hahs et al., 2009). For urban grasslands, although the degree of spatial isolation of grasslands did not appear to induce changes in the composition of plant communities (Cochard et al., 2017), the high occurrence of buildings and other impervious surfaces in the urban matrix was found to increase the risk of extinction for native species (Williams et al., 2006). High degrees of urbanization in the landscape context of urban grasslands are likely to affect their flora through the deleterious impacts of pollution (chemical and light) and human preferences (e.g., the introduction of exotic species) (McDonnell and Pickett, 1990; Williams et al., 2009). This leads to the selection of perennial plant species and species adapted to human disturbances (Hill et al., 2002; Lososová et al., 2006; Godefroid and Koedam, 2007). Moreover, studies have shown that plants in urban contexts tend to be more tolerant of, or favored by, high soil fertility and alkalinity (Knapp et al., 2009; Williams et al., 2015; Tautenhahn et al., 2008; Hill et al., 2002; Pyšek, 1995; Roy et al., 1999). Thus, identification of the drivers of plant species diversity in urban grasslands requires not only consideration of the local management regimes applied by stakeholders but also of the degree of urbanization in the landscape context of grasslands.
Therefore, the goal of the present paper is to investigate the effects of management regimes (mowing) and landscape composition along a rural-urban gradient on plant communities in urban grasslands. More specifically, our objectives are (1) to investigate the effects of management regimes (mowing date and frequency), the urban matrix composition in urban grasslands’ landscape context, and the relative influence of these two drivers on plant species composition and diversity in urban grasslands, and (2) to examine the effects of management regimes and landscape context characteristics on plant species richness and plant strategies.
Based on the literature, it is expected that urban grassland plant communities may vary in richness, diversity, and composition according to both management practices and landscape composition. However, management practices are expected to be more likely to affect communities by favoring species according to their ecological strategies, while landscape composition should favor species according to their life span and their preferential habitat. Furthermore, we expect to find few nonnative species (Albrecht and Haider, 2013; Cochard et al., 2019).
The study was carried out in two urban areas of western France: Angers (47°28′N, 0°33′W) which covers 667 km² and contains 302,000 inhabitants, and Rennes (48°06′N, 1°40′W), which covers 705 km² and contains 460,000 inhabitants (INSEE 2022 https://www.insee.fr) in the “Zone Atelier Armorique”. Both urban areas belong to the Armorican massif and are characterized by a temperate oceanic climate (average annual rainfall in Rennes: 677 mm, Angers: 668 mm; average annual temperature in Rennes: 11.7°C, Angers: 11.9°C). In the two cities, the bedrock is acidic (mainly schist and altered granite). The two cities consist of a high-built town center surrounded by low-built suburban areas, in which seminatural areas (woodlands, rivers and lakes, grasslands, and green spaces) are present. The cities are embedded within a landscape of hedgerow networks connecting mosaics of annual crops, grasslands, woodlands, and hedges.
Urban grasslands were selected in the two urban areas with the aim of sampling grasslands distributed along a landscape gradient more or less urbanized and characterized by contrasting management regimes with regard to mowing practices. The procedure used to select the sampled grasslands is described in the following sections.
Land-cover maps of the two conurbations were produced using QGIS software (different versions from 2020 to 2022, http://qgis.org), using French public spatial databases and satellite or aerial images to obtain similar information for both cities. First, we collected vector data of buildings, roads, forests, woods, hedgerows, and water land cover from the high-resolution BD TOPO 3-0® database (IGN, 2018) and vector data for agricultural land uses (crops, temporary and permanent grasslands) from the Graphical Parcel Register database (RPG, 2018; www.data.gouv.fr). To classify the unassigned surfaces, we calculated the Normalized Difference Vegetation Index (NDVI) from SPOT 6-7 satellite images and infrared aerial images: higher values were considered to indicate herbaceous surfaces and lower values to indicate bare soils or mineral surfaces.
These different maps were merged to produce the final continuous land-cover maps of the two conurbations. The proportion of buildings and road surfaces is a common proxy for urbanization (Hill et al., 2002; Géron et. al., 2021). Thus, all buildings, road surfaces, and other nonvegetated areas were grouped and considered “impervious surfaces”. The map of impervious surfaces was then rasterized at 5 m × 5 m resolution. Moving window analysis was then used to characterize pixels according to the proportion of impervious surfaces in their landscape context (across a 500-m buffer area) in the two urban areas using CHLOE software (Boussard and Baudry, 2017). This led to the production of a final map (at 5 m × 5 m resolution) where the value of each pixel corresponded to the proportion of impervious surfaces in its surroundings. This map was used to characterize the landscape gradients in the two conurbations (Figure 1).
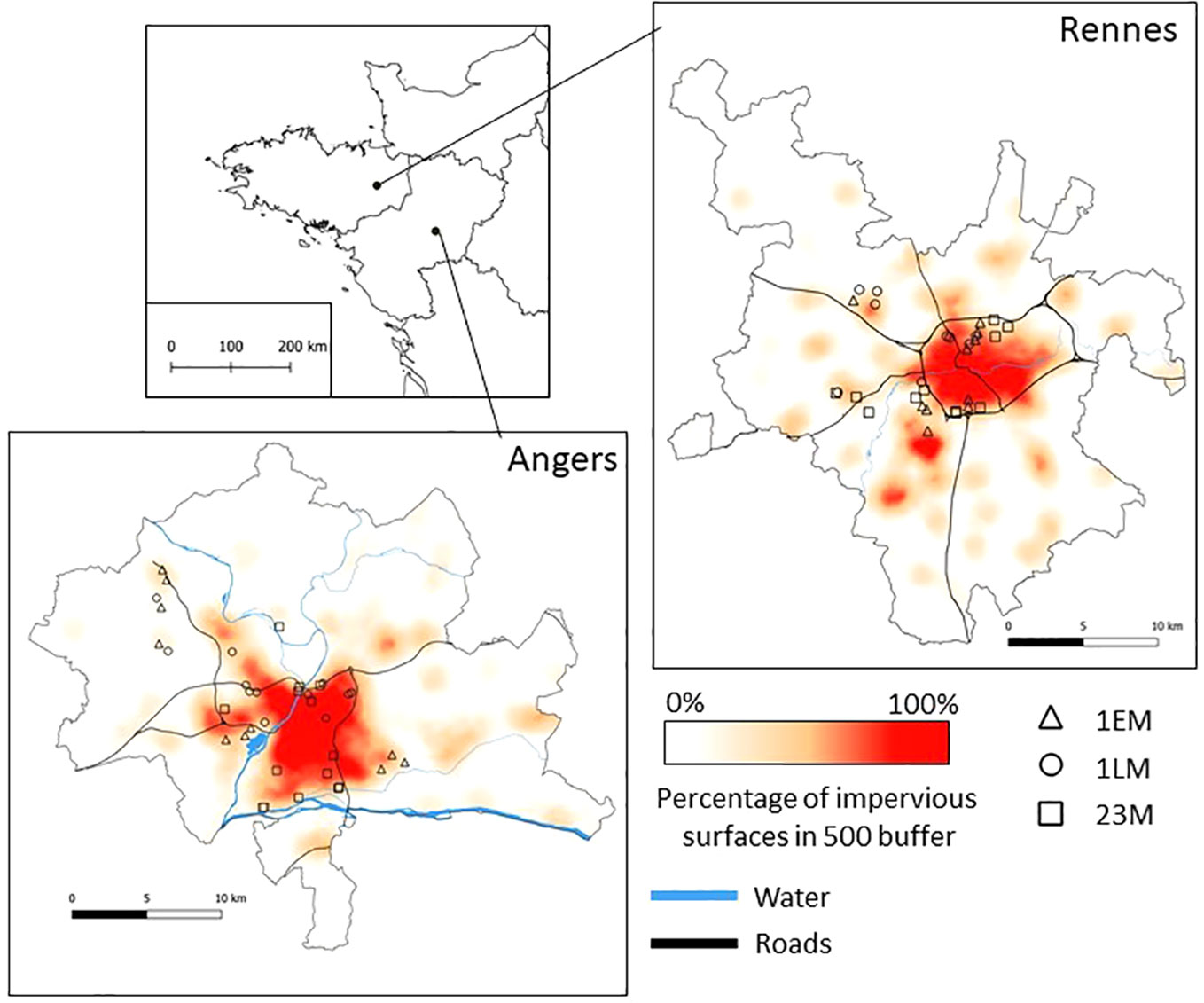
Figure 1 Geographical location of the two study areas and the studied grasslands with indications of management practices (mown two or three times a year (23M), mown early once a year (1EM), and mown late once a year (1LM)). Proportion of impervious surfaces was calculated in a 500-m radius around 5*5 m grids.
We also calculated the proportions of three other land-cover classes, i.e., crops, woody, and herbaceous surfaces, to describe the two urban areas. To characterize artificial habitats other than impervious surfaces, annual crops and temporary agricultural grasslands were grouped into “crops”. Permanent grasslands and other nonagricultural surfaces (i.e., wastelands, herbaceous green spaces, and roadsides) were grouped as “herbaceous surfaces” (Cochard et al., 2019; Pithon et al., 2021). Forests, woods, and hedgerows were grouped as “woody surfaces”.
Interviews with city green space managers of the two urban areas were performed in order to identify the management practices they use for mowed urban grasslands (grazed grasslands were excluded) and to identify potential sites for sampling. Three management practices that are commonly applied on urban grasslands were retained in this study: one late mowing a year (1LM) performed after July to achieve biodiversity goals, one early mowing a year (1EM) between May and July, and mowing twice or three times a year (23M) with the first mowing between May and July. Subsequently, 149 potential sites were identified where one of these management practices was applied.
By crossing data on grassland management regimes and the map of landscape gradient in the two conurbations, 66 mowed grasslands (Figure 2) managed by cities were selected: 36 in the Angers area and 30 in the Rennes area. Selected grasslands were distributed along a gradient of impervious surfaces ranging from 2% to 83% at 500 m. In total, 24 of the selected grasslands were under a 1EM management regime, and 21 grasslands were under 1LM or 23M management regimes. Only grasslands larger than 0.5 ha with fixed management for at least 5 years were selected to avoid the effects of recent perturbations. We also excluded wet habitats to restrict the study to grasslands under mesophilic conditions.
In order to ensure independence between management practices and landscape context characteristics during site selection, a Tukey’s test was performed to test for differences in the proportions of impervious, woody, herbaceous, and cropped surfaces according to mowing management regimes. No significant differences were found (Figure 3).
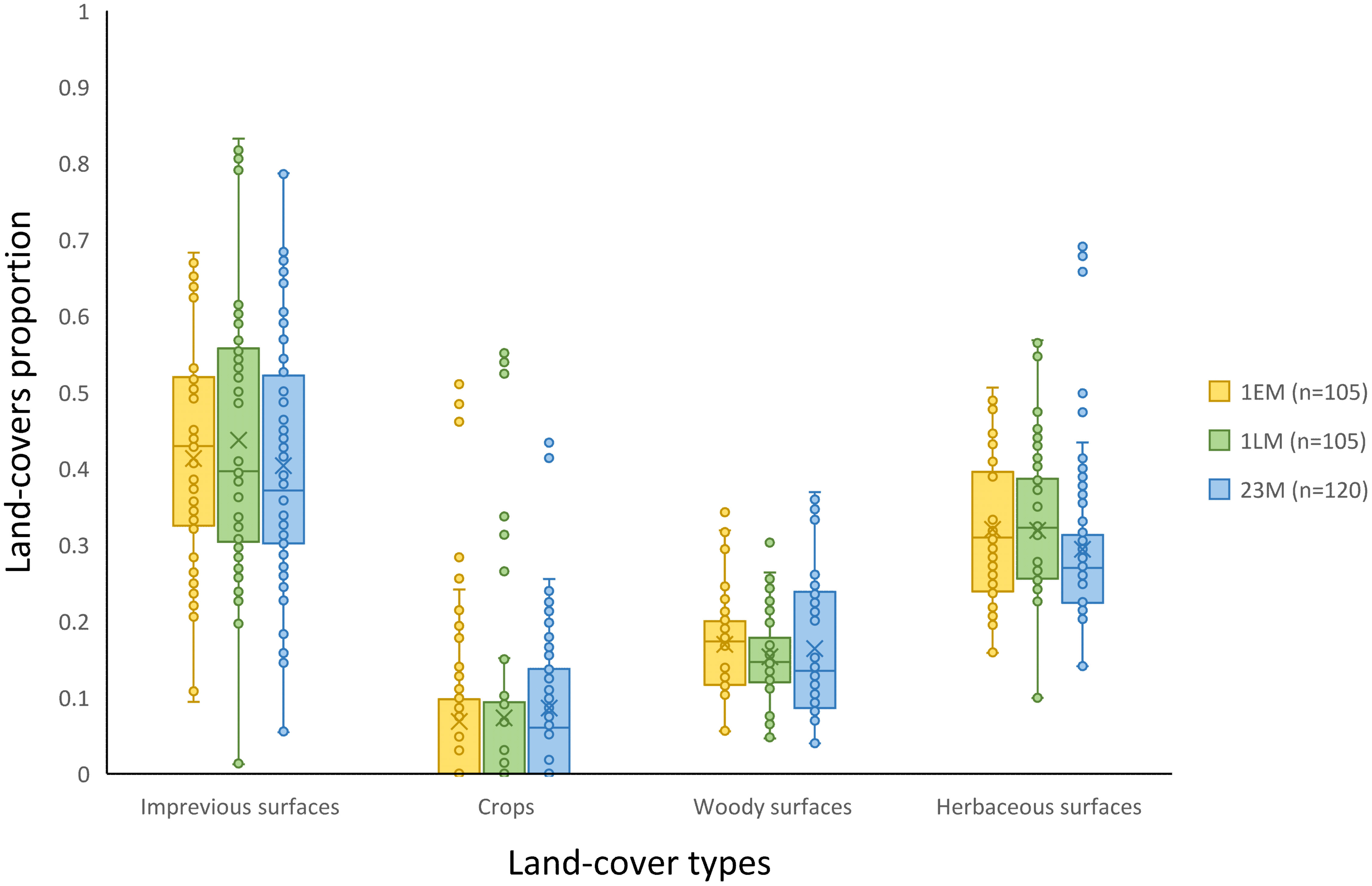
Figure 3 Comparison of four land-cover proportions (impervious surfaces, crops, woody surfaces, and herbaceous surfaces) within a 500-m radius of grasslands according to their management practices (mown two or three times a year (23M), mown early once a year (1EM), and mown late once a year (1LM)). Tukey’s test did not show any significant differences in the proportion of each land-cover type between management practices (p-value > 0.05).
In each selected grassland, five plots (330 plots in total) of 8 m² (2 m by 4 m) were defined with at least 5 m between each plot and from the grassland edges. In each plot, all plant species were recorded using the nomenclature defined by Tison and de Foucault (2014). An adjusted Braun-Blanquet scale method (Braun-Blanquet, 1932; Van der Maarel, 2005) was used to estimate species abundance in each plot. The fieldwork was performed in May 2021 in Angers and May 2022 in Rennes, before the first mowing of the grasslands.
Beyond the effects of mowing practices and landscape context on species richness and diversity, studying functional composition helps to facilitate an understanding of how plant communities respond to these variables. Thus, we collected information on plant traits and adaptive strategies. First, we collected competitor/stress-tolerator/ruderal (CSR) strategies for each species according to the database provided by Grime et al. (1988). We transformed CSR strategies into three variables that corresponded to the coordinates of each plant species in Grime’s triangle: competitors (C), stress tolerators (S), and ruderals (R). A habitat preference, adapted from Julve’s (2015) categories, was assigned to each species. Species characterizing meadows and grasslands (including swards or lawns) were considered grassland species, and those related to wastelands and crops were considered anthropogenic habitat species (Klotz et al., 2002). Finally, for each species, Ellenberg scores for reaction, nitrogen, and light were extracted from Julve’s (2015) database.
Using land-cover maps of impervious surfaces and other land covers, the landscape context of each plot was described across a circular buffer with a 500-m radius according to the proportions of impervious surfaces (mean = 41%, SD = 17%, range from 2% to 83%), woody surfaces (mean = 16%, SD = 8%, range from 4% to 37%), crops (mean = 8%, SD = 11%, range from 0% to 55%), and herbaceous surfaces (mean = 31%, SD = 10%, range from 10% to 69%).
In addition, these four landscape metrics were calculated across circular buffers with radii of 250 m and 1,000 m. However, landscape metrics were highly correlated among the three radius sizes (Spearman correlation rs > 0.7; Supplementary Figure S1), suggesting high redundancy between the three spatial scales. For this reason, only the scale of 500 m was used in analyses, as it was found to optimally reflect the responses of plant communities to landscape structure (Jackson and Fahrig, 2012; Jackson and Fahrig, 2015).
Canonical correspondence analysis (CCA) was performed on plant assemblages of the sampled plots to scale to test the effects of management type (one late mowing, one early mowing, and two to three mowings) and the four calculated landscape characteristics (proportions of impervious, cropped, woody, and herbaceous surfaces across a 500-m buffer area). This multivariate method is appropriate for measuring variation in plant species assemblages according to their environment (Ter Braak, 1986). To limit the strong effect of rare species, only species detected in more than 5% of plots were retained in the analyses. First, a separate CAA was undertaken to test each management and landscape variable individually. Correlations between significant variables (p-value < 0.05) were checked using the Pearson method to avoid multicollinearity. No variables were highly correlated (r < 0.7). Subsequently, variance partitioning was performed using partial CCA in order to evaluate the relative contribution of mowing practices and landscape metrics. The forward selection procedure was used to select significant mowing and landscape variables (p-value < 0.05) (Lepš and Šmilauer, 2003). To avoid site effects, sites were computed as block factors (a block of five replicates per grassland). All variables were significant in the global CCA. All CCAs were performed in CANOCO 5 (Ter Braak and Smilauer, 2012).
Generalized linear mixed models (GLMM) were used to test the effect of management type (one late mowing 1LM; one early mowing 1EM; two or three mowing 23M) and the same four landscape variables (proportions of land cover types) as in the CCA on plant species diversity (species richness and Shannon diversity index) as well as plant functional traits and strategies (CSR mean score, mean trophic level and species richness for habitat preferences and life span). The CSR score and Ellenberg index were weighed by abundance. The GLMMs were run using the lmer function of the lme4 package (Bates et al., 2015). Simple effects were tested in all models at the plot scale. Sites and urban areas were used as random effects in the models. Landscape metrics were standardized (mean = 0, SD = 1) in order to compare the effect strength (or relative importance) of the coefficients across variables. All independent variables respected the Gaussian distribution except for the proportion of crops that were log-transformed. For each plant variable, an averaged model was produced using the MuMin package (Barton, 2009); this included all models with delta AICc values lower than 15. We checked for multicollinearity in the models using the variance inflation factor (VIF) and the performance package (version 0.10.0, Lüdecke et al., 2021). The proportion of wood was excluded in the models, as this variable led to a high VIF (> 5). Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality.
A total of 153 species were observed in the 330 plots. The most common species were Holcus lanatus L. (85%), Dactylis glomerata L. (67%), Arrhenatherum elatius L. (65%), and Rumex acetosa L. (60%). Only three exotic species were found: Oenothera sp. (1%), Briza maxima L. (0.6%), and Hordeum vulgare L. (0.3%). The mean species richness found in 8 m² plots was 15.4 (SD = 4.13, max = 34, min = 6). The cumulative mean species richness in each grassland plot was 29 (SD = 7.05, max = 52, min = 11). The most abundant species were Holcus lanatus L. (frequency of 51% with a plot abundance higher than 25%), Arrhenatherum elatius L. (36%), Anthoxantum odoratum L. (18%), and Dactylis glomerata L. (18%).
The arrangement of the 59 most frequent species along the two first axes of the CCA is shown in Figure 4. The forward selection procedure included the four land-cover proportions in the landscape group of the variance, partitioning CCA and mowing practices into a second group. The first two axes explained 39.5% of the relationships between plant species assemblages and environmental variables. All selected environmental variables explained 19.2% of the total variation. The first axis opposed plots surrounded by a high proportion of impervious surfaces within 500 m to plots mowed early and surrounded by high proportions of woods and herbaceous surfaces within 500 m. Plots surrounded by a high proportion of impervious surfaces were characterized by several grasses (i.e., Avena barbata Pott., Trisetum flavescens L., and Vulpia bromoides L.) and typical grassland dicotyledons such as Vicia sativa L. and Myosotis discolor Pers. Daucus carota L., while plots surrounded by a high proportion of woods and herbaceous surfaces were characterized by a wood species, Stellaria graminea L., and many frequently occurring grasses (Holcus lanatus L., Anthoxanthum odoratum L., Poa pratensis L. and Bromus hordeaceus L.).

Figure 4 Biplot of canonical correspondence analysis representing environmental variables selected by the forward selection procedure and plot position (left) and a scatterplot of the 20 most influential species (right). For full names, see Supplementary Table S1. Arrows represent continuous landscape variables (“Proportion of Imper” for impervious surfaces, “Crops” for crop surfaces, “Wood” for woody surfaces, and “Herb” for herbaceous surfaces), and the red triangle represents mown variables (mown two or three times a year (23M), mown early once a year (1EM), and mown late once a year (1LM)) resulting from the forward selection procedure as shown. The color of the plots corresponds to mowing practices (23M plots in dark blue, 1EM plots in green, and 1LM plots in light blue).
The second axis was related to management practices and opposed plots under late mowing regimes to plots that were mowed two or three times a year. It also distinguished agricultural landscape contexts from landscapes with high proportions of herbaceous and woody surfaces, independently of the proportion of impervious surfaces (Figure 4). No grasses were associated with late mowing, but some species occurring in wastelands were found (Cirsium arvense (L.) Scop., Rumex crispus L.). Grasslands mowed only once early in the year were associated with the typical grassland species Poaceae (Holcus lanatus L., Anthoxanthum odoratum L., Poa pratensis L., and Bromus hordeaceus L.), while grasslands mowed two or three times a year were associated with lawn species (Hypochaeris radicata L. and Plantago lanceolata L.).
Variance partitioning showed that landscape context explained 13.2% (p-value < 0.01) of the total variance, while management practices explained 5.7% (p-value < 0.01) of the total variance (Figure 5). This represents, respectively, 68.6% and 29.7% of the explained variance. The interaction between landscape and management variables explained less than 1% of the total variance.
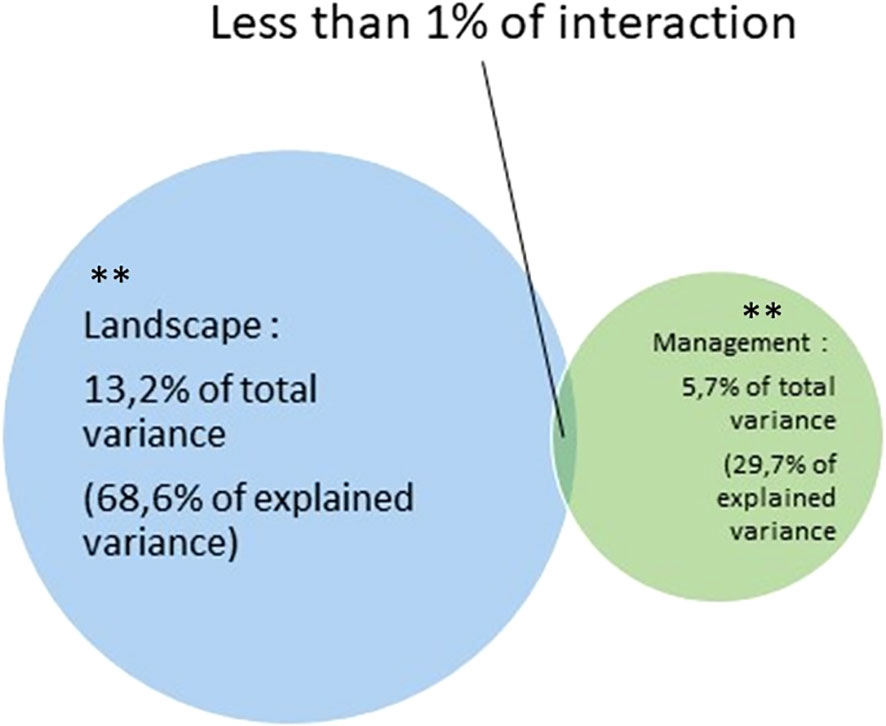
Figure 5 Venn diagram representation of CCA variance partitioning due to land-cover proportion and mowing practices. Both landscape and management variance explanations were significant (**: p-value < 0.01).
GLMM did not show any significant effect of management practices or landscape composition on species richness, Shannon index, Ellenberg index, or life form species richness (Supplementary Table S2).
The GLMM performed for plant strategies showed a significant effect of management type on plant CSR scores (Table 1). Scores for competitors in late-mown grasslands (1LM) were 8% higher than in early-mown grasslands (1EM) and 11% higher than in grasslands that were mowed two or three times a year (23M) (Figure 6). Late-mown grasslands also contained fewer stress-tolerant species than early-mown grasslands and grasslands which were mowed two or three times a year (Figure 6). No significant difference was found between early mown grasslands and grasslands mown twice or three times a year for both stress-tolerant and competitor plant species (Table 1). Management type did not have significant effects on ruderal score, the species richness of grassland and anthropogenic habitats, or the species richness of annual species.
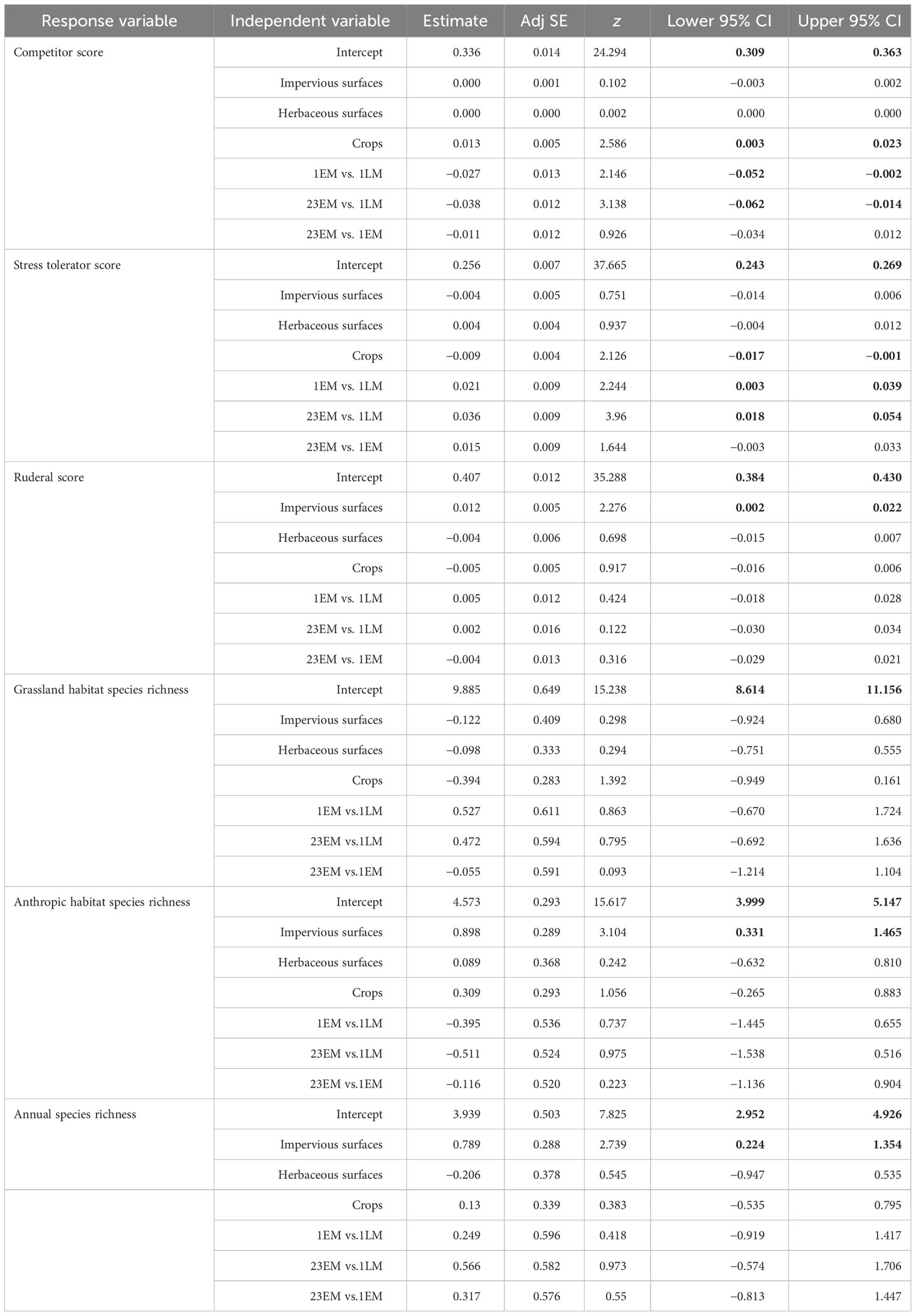
Table 1 Final mean models for the effect of mown practices and land-cover proportion in a 500-m radius on CSR strategy score, optimal habitat, and annual species richness. Significant results, i.e. estimates whose 95% confidence interval do not include zero, are in bold.
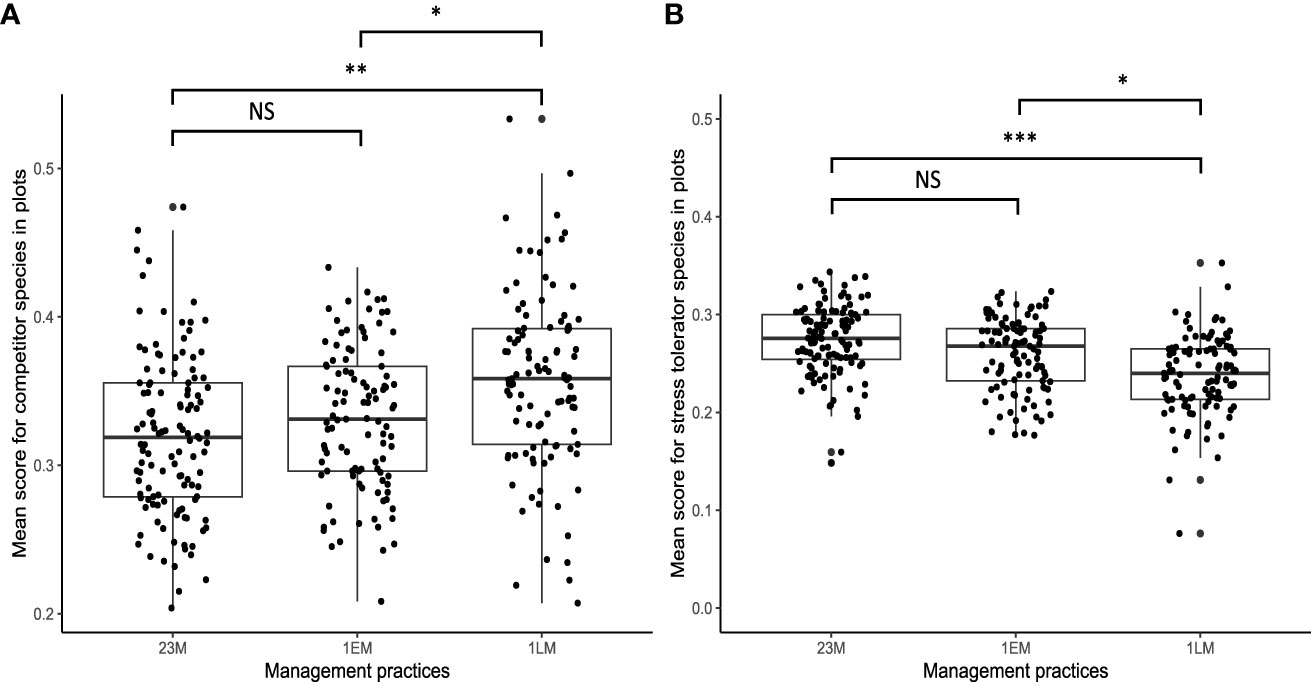
Figure 6 Graphical representation of the significant effect of mowing practices (mown two or three times a year (23M), mown early once a year (1EM), and mown late once a year (1LM)) on competitor score (A) and stress tolerator score (B) (n = 330) according to mean model results (*p < 0.05; **p < 0.01; ***p < 0.001).
GLMM also showed a significant response of plant CSR scores to two landscape variables (Table 1). A higher proportion of impervious surfaces within a 500-m radius resulted in more ruderal plant species (Figure 7), while the proportion of crops within 500 m had a positive effect on competitor scores in plant communities and a negative effect on stress-tolerant species. The proportion of impervious surfaces also had a significant and positive effect on the species richness of annual species and of species associated with anthropogenic habitats (Table 1; Figure 8).
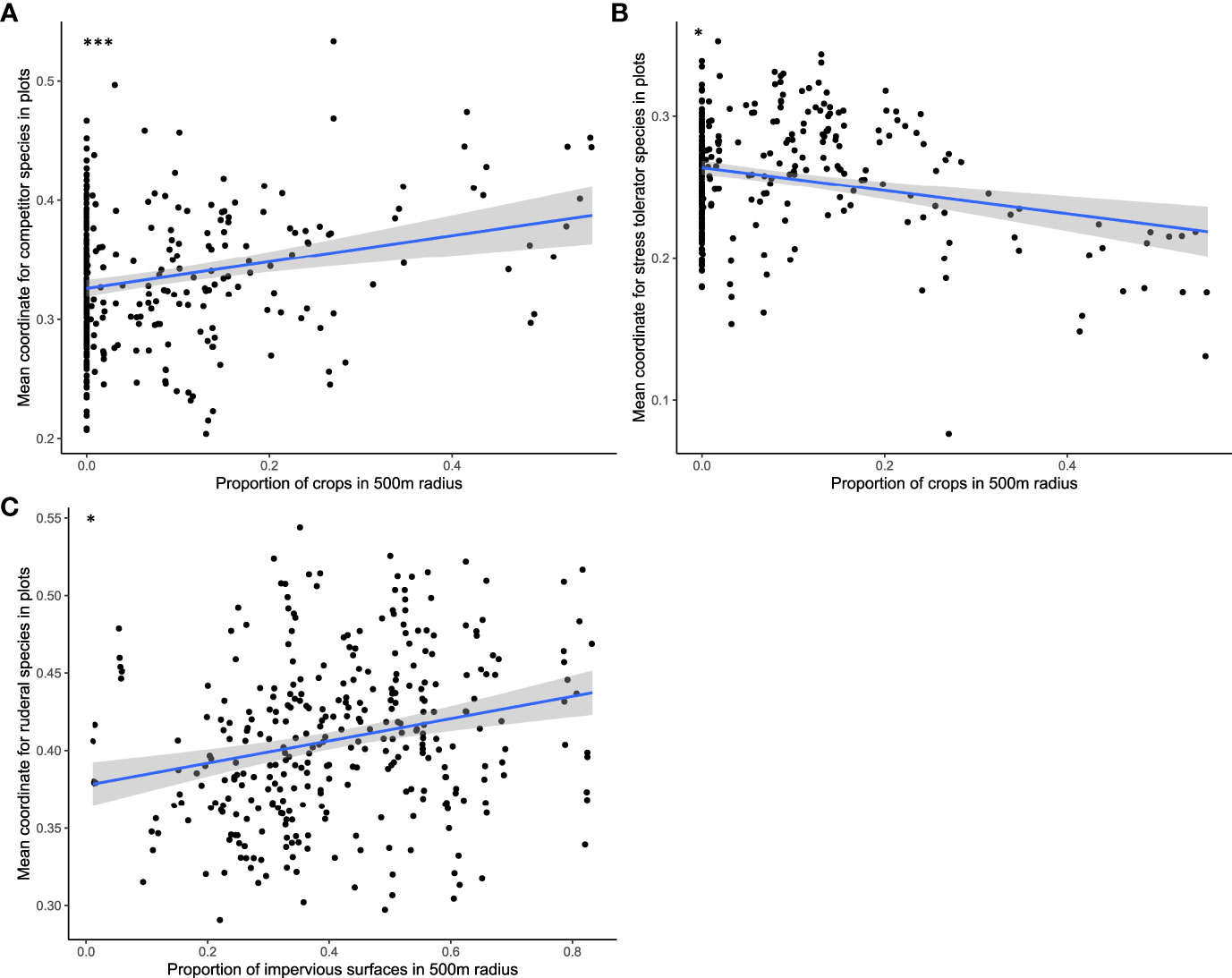
Figure 7 Graphical representation of significant effects of crop proportion on competitor score (A) and stress tolerator score (B) and of impervious surface proportion on ruderal score (C) according to mean model results (*p < 0.05; **p < 0.01; ***p < 0.001).
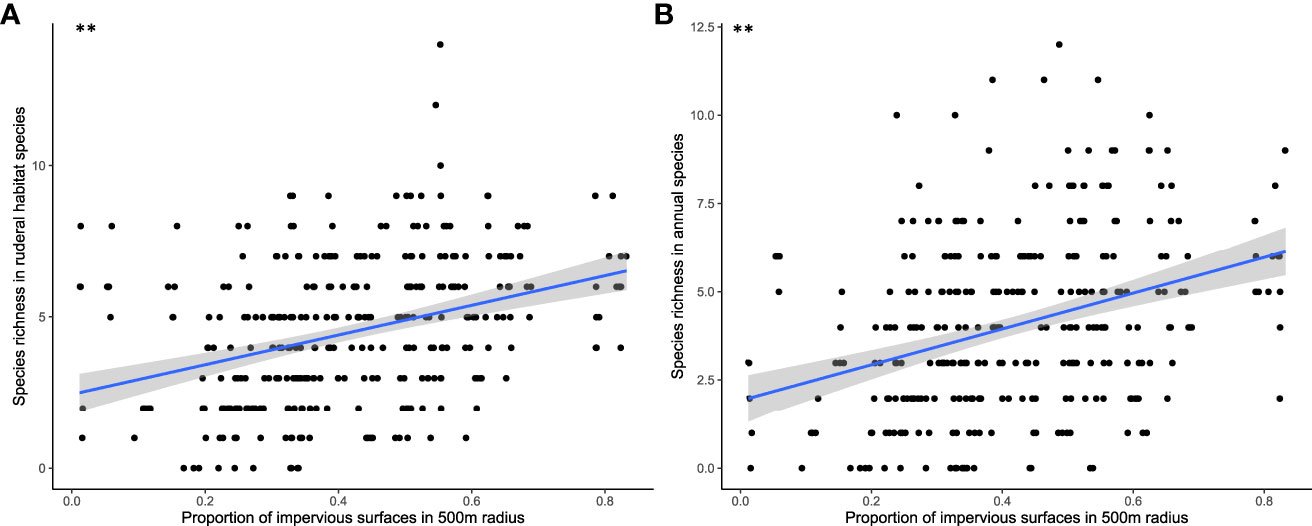
Figure 8 Graphical representation of impervious surface proportion on species richness of anthropogenic habitat plants (A) and annuals (B) according to mean model results (*p < 0.05; **p < 0.01; ***p < 0.001).
The total species richness (153 species) found in the 66 urban grasslands appeared to be relatively important in the studied region, especially in comparison to herbaceous green spaces with higher management intensity (i.e., mowed up to 20 times a year) in the same urban area (Chollet et al., 2018). Compared with other urban habitats, this species richness represents three times the species richness found in Paris lawns (Bertoncini et al., 2012) and is similar to species richness in the woodlands of Angers and Rennes (Vallet et al., 2008). This highlights the ecological importance of ordinary grassland habitats in urbanized areas, which previous studies have also found (Muratet et al., 2007; Threlfall et al., 2016). Furthermore, only three exotic species were observed in the present study. This result contradicts other studies that have found high occurrences of these species in urban habitats (Pyšek, 1998; Kowarik, 2008). Unlike unmanaged habitats such as wasteland, which harbors many nonnative species (Albrecht and Haider, 2013), grassland under low-intensity management practices such as mowing could maintain relatively competitive flora that may prevent the installation of nonnative species. Our study confirms the value of urban meadows for the conservation of native species in cities (English et al., 2022).
No effect of mowing practices or landscape composition was found on plant species richness and diversity in urban grasslands. We expected that lower management frequency (i.e., one cut a year compared to two or three cuts a year), as well as late mowing, would lead to higher species richness and diversity, as other studies have highlighted in the context of agricultural grasslands (Smith et al., 2000; Gaujour et al., 2012). However, our focus on a narrow range of mowing intensities (i.e., one early cut, one late cut, or two to three cuts a year) may explain the lack of response of plant richness and diversity to grassland management. In contrast, previous studies showing the effects of mowing regimes on plant species richness have compared more contrasting intensities of management practice in urban herbaceous spaces from lawns to grasslands (Socher et al., 2012; Rudolph et al., 2017; Norton et al., 2019; Sehrt et al., 2020). Another hypothesis could be that the effects of management practices on species richness found in the literature might be related to the presence of high proportions of nonnative plants. Indeed, such species have been found by other studies to represent up to 20% of plant species richness in unmanaged habitats (Muratet et al., 2007). In the present study, the lack of species gain related to the installation of nonnative species might then explain the absence of an effect of mowing regimes on species richness and diversity.
Moreover, the lack of a significant effect of landscape composition on plant richness or diversity may be explained by the length of the landscape gradient considered in the present study. Indeed, the studied urban grasslands were distributed along a gradient ranging from moderately urbanized (2%) to highly urbanized landscapes (83%), because these extensive habitats are less frequent in rural landscapes (Fischer et al., 2013; Horák et al., 2022). Although many studies have found significant increases in plant richness in highly to moderately urbanized landscapes (McKinney, 2008), the impact of urbanization on species richness is nevertheless likely to strongly vary according to the geographical location of cities, historical and economic factors, and the spatial scale considered (McKinney, 2008). In the specific context of the present study, the landscape gradient considered may be too narrow to detect any variability in plant richness or diversity. In contrast to species richness and diversity, we found that the species composition of plant communities in urban grasslands was significantly influenced by local mowing practices and landscape composition. Analysis of plant species assemblages showed that intensively managed grasslands (i.e., mowed two to three times a year), as opposed to grasslands mowed once late in the year, were especially characterized by plant species that are typical of lawns. The strength of the influence of mowing practices on floristic composition found in the present study is comparable with that found in another study carried out in an agricultural context (Barbaro et al., 2004). This highlights the potential for the implementation of late mowing practices to add value by enhancing the composition of plant communities in urban grasslands. However, the difference in species composition between extensively and frequently mown grasslands was not associated with an increase in species richness or diversity. This suggests that there is no optimum in our range of management practices and that, on the contrary, the diversification of urban grassland management practices can lead to a wider range of floristic composition.
Plant species assemblages in urban grasslands were, more importantly, impacted by the composition of the surrounding landscapes. Grasslands with a high proportion of herbaceous and woody habitats in their surroundings were characterized by contrasting species assemblages in comparison with grasslands embedded in landscapes with many impervious surfaces. Along an urban-rural gradient, Cochard et al. (2019) have also observed an effect of landscape composition on the plant species assemblages of extensively managed road verges, and many grassland species seemed to be negatively affected by the increasing proportion of built-up areas in the landscape. In rural contexts, many studies have found an effect of landscape patterns on plant diversity and composition (Schmucki et al., 2012; Olsen et al., 2018; Kimberley et al., 2021). In Schmucki et al. (2012), the presence of seminatural elements in the surroundings seemed to affect the composition of plant communities and increase local plant species richness (Schmucki et al., 2012), thus contrasting with agricultural landscape contexts (Loos et al., 2021).
In urban contexts, plant communities are the result of different specific filters applied to the global species pool (Williams et al., 2009; Aronson et al., 2016), on which factors acting at both landscape and local scales induce selective pressures (Williams et al., 2015). In this study, we found an influence of both landscape composition and local management practices on species assemblages, with a greater influence of variations in landscape composition than of local mowing practices. The differences in plant community composition observed between urban and rural grasslands can be linked to the increased effect of environmental change in the city center compared to suburban areas (i.e., water stress, pollution, and the urban heat island effect) (Williams et al., 2009). It could also be explained by the effects of landscape configuration that were not specifically considered in the present study. Habitat fragmentation (e.g., escribed via the mean proximity size index) and landscape complexity (e.g., edge density and largest patch index) can strongly affect plant composition in urban contexts (Peng et al., 2019). Habitat fragmentation was found to increase the biotic homogenization of plant communities (Zeeman et al., 2017), while landscape complexity has been shown to be positively related to plant species diversity (Peng et al., 2019). Although we did not specifically address the potential effects of landscape configuration, it has been shown in landscape ecology that landscape composition and configuration are highly correlated (Fahrig, 2003; Fahrig et al., 2011). In an urban context, higher levels of urbanization can also be associated with higher habitat fragmentation and landscape complexity (Yeh and Huang, 2009; Buyantuyev and Wu, 2010; Peng et al., 2019). Thus, the changes in plant communities found along our landscape gradients may be partially explained by variations in landscape configuration underlying landscape composition. However, distinguishing the respective effects of these two landscape components is difficult (Fahrig, 2003; Fahrig et al., 2011). Other landscape factors can act as filters for plant communities. Habitat transformation and fragmentation might also filter species, especially in relation to the dispersal strategies that determine their capacity to reach a suitable environment. Although such processes remain unclear (Williams et al., 2015), previous studies have suggested that wind-dispersed species might be more likely to disappear in urban environments (Williams et al., 2005; Sodhi et al., 2008; Knapp et al., 2010), and isolated urban grasslands may limit seed arrival (Fischer et al., 2013). Thus, further study of seed rain composition in urban contexts is needed to better understand how the landscape changes induced by urbanization drive the selection of species plant strategies in urban habitats.
Although mowing practices and landscape composition did not influence plant species richness in our sample, they had a significant effect on species composition by favoring certain plant strategies. In agreement with our hypothesis, an effect of mowing practices on plant CSR strategy was found. Indeed, there were more competitive species (such as Dactylis glomerata L. and Arrhenatherum elatius L.) in late-mown than in early-mown grasslands, however frequently they were mowed (1EM or 23M). One cut before flowering could prevent plant reproduction (Gaujour et al., 2012), and a cut before seed exports could select a competitive strategy (Johansen et al., 2019). In addition, vegetation observed under late mowing conditions was denser and could prevent the establishment of new plants (Smith and Haukos, 2002), thus favoring the development of competitor species. Stress-tolerant species (such as Lotus corniculatus L.) were less observed in late-mown grasslands compared to early-mown grasslands (1EM and 23M). This result indicates that early management of grasslands may cause more disturbance (Kahmen et al., 2002).
Beyond the effects of local management practices on plant strategies, our results show that urban grasslands harbored less stress-tolerant and more competitive plant species when surrounded by high crop coverage in their landscape context, which contradicts the results of a previous study showing enhanced competitor strategy in more urbanized contexts (Chocholoušková and Pyšek, 2003). However, considering the complexity of urban systems and the various methods used to assess CSR strategies, the response of CSR plant traits to urbanization remained unclear (Williams et al., 2015). Actually, both types of plant strategy (i.e., stress tolerators and competitors) might reflect not only plant responses to landscape composition but also the degree of grassland disturbance (Herben et al., 2018). Indeed, a high score for ruderal species was observed in grasslands in more urbanized landscapes, as shown in Knapp et al. (2009). Since this strategy is associated with disturbed habitats, urban grasslands seem to undergo more disturbance in highly urbanized landscape contexts. In addition, the richness of annual species and of species associated with anthropogenic habitats was also higher in grasslands in more urban contexts, confirming the hypothesis that grasslands located in the most urbanized landscapes tend to be more disturbed.
Our study illustrates the importance of considering factors that act at both local and landscape scales when identifying the drivers of plant communities in urban grasslands. This study also provides evidence that assessing effects in terms of species richness is not sufficient and that community composition must also be considered. Accordingly, although mowing practices and landscape composition did not influence plant species richness or diversity, we found that they were both important drivers of the composition of plant species assemblages. Nevertheless, they affected plant communities in different ways and to different extents. Landscape composition had the strongest influence; this was mostly characterized by effects on CSR strategies, preferential habitats, and the life span of plant species. This highlights the important role of landscape composition in filtering plant species communities in urban grasslands in comparison with local drivers. Our study therefore provides evidence that favors rethinking urban grassland planning not only by changing local management practices but also by promoting the maintenance of seminatural green spaces in the landscape context. Our findings also support changes in management practices for herbaceous vegetation in cities, favoring extensive management practices such as late mowing. Such practices, which are becoming increasingly common in cities (Watson et al., 2020; Marshall et al., 2023), should be promoted. More generally, the importance of urban grasslands in contributing to biodiversity conservation and ecosystem services has been neglected (Klaus, 2013). Given the plant diversity observed in urban grasslands, their restoration or establishment should be promoted as a nature-based solution to provide high-quality habitat for animal species. This is particularly important for pollinating arthropods such as Lepidoptera and Hymenoptera, which are also highly affected by urban environments (Kurylo et al., 2020; Horák et al., 2022). In this context, this study contributes to addressing conservation challenges in the urban context by highlighting the importance of not separating grassland management from urban planning considerations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CG, AB, SA, VB, and HD conceived and designed the study. CG gathered and analyzed the data with the help of SA, AB, VB, and HD. CG, AB, SA, VB, and HD wrote the manuscript. All authors contributed to the article and approved the submitted version.
This study was financed by “l’Institut Agro Rennes Angers” and the “Conseil Régional des Pays de la Loire”.
We thank M. Durieu, P. Collignon, and F. Glodt for their help in collecting field data and T. Rodier for spatial data analysis. We also thank “Angers Loire Métropole”, “Rennes Métropole”, “Zone Atelier Armorique”, and all stakeholders involved for their help in sampling the sites.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1151913/full#supplementary-material
Albrecht H., Haider S. (2013). Species diversity and life history traits in calcareous grasslands vary along an urbanization gradient. Biodivers. Conserv. 22, 2243–2267. doi: 10.1007/s10531-013-0437-0
Aronson M. F. J., Lepczyk C. A., Evans K. L., Goddard M. A., Lerman S. B., MacIvor J. S., et al. (2017). Biodiversity in the city: key challenges for urban green space management. Front. Ecol. Environ. 15, 189–196. doi: 10.1002/fee.1480
Aronson M. F. J., Nilon C. H., Lepczyk C. A., Parker T. S., Warren P. S., Cilliers S. S., et al. (2016). Hierarchical filters determine community assembly of urban species pools. Ecology 97, 2952–2963. doi: 10.1002/ecy.1535
Bakker J. P., Elzinga J. A., de Vries Y. (2002). Effects of long-term cutting in a grassland system: perspectives for restoration of plant communities on nutrient-poor soils. Appl. Veg. Sci. 5, 107–120. doi: 10.1111/j.1654-109X.2002.tb00540.x
Barbaro L., Dutoit T., Anthelme F., Corcket E. (2004). Respective influence of habitat conditions and management regimes on prealpine calcareous grasslands. J. Environ. Manage. 72, 261–275. doi: 10.1016/j.jenvman.2004.05.006
Barton (2009) MuMIn : multi-model inference. Available at: http://r-forge.r-project.org/projects/mumin/https://cir.nii.ac.jp/crid/1572824499154168192 (Accessed November 25, 2022).
Bates D., Mächler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Soft. 67, 1–48. doi: 10.18637/jss.v067.i01
Bertoncini A., Machon N., Pavoine S., Muratet A. (2012). Local gardening practices shape urban lawn floristic communities. Landsc. Urban Plan. 105, 53–61. doi: 10.1016/j.landurbplan.2011.11.017
Boussard H., Baudry J. (2017). Chloe4.0: A software for landscape pattern analysis. https://bagap.rennes.hub.inrae.fr/productions/logiciels.
Braun-Blanquet J. (1932). Plant sociology: the study of plant communities. 1st ed (New York and London: McGraw-Hill).
Buyantuyev A., Wu J. (2010). Urban heat islands and landscape heterogeneity: linking spatiotemporal variations in surface temperatures to land-cover and socioeconomic patterns. Landscape Ecol. 25, 17–33. doi: 10.1007/s10980-009-9402-4
Chaudron C., Chauvel B., Isselin-Nondedeu F. (2016). Effects of late mowing on plant species richness and seed rain in road verges and adjacent arable fields. Agric. Ecosyst. Environ. 232, 218–226. doi: 10.1016/j.agee.2016.03.047
Chocholoušková Z., Pyšek P. (2003). Changes in composition and structure of urban flora over 120 years: a case study of the city of Plzeň. Flora – Morphol. Distribution Func. Ecol. Plants 198, 366–376. doi: 10.1078/0367-2530-00109
Chollet S., Brabant C., Tessier S., Jung V. (2018). From urban lawns to urban meadows: Reduction of mowing frequency increases plant taxonomic, functional and phylogenetic diversity. Landsc. Urban Plan. 180, 121–124. doi: 10.1016/j.landurbplan.2018.08.009
Cochard A., Pithon J., Braud F., Beaujouan V., Bulot A., Daniel H. (2019). Intraspecific trait variation in grassland plant communities along urban-rural gradients. Urban Ecosyst. 22, 583–591. doi: 10.1007/s11252-019-0827-5
Cochard A., Pithon J., Jagaille M., Beaujouan V., Pain G., Daniel H. (2017). Grassland plant species occurring in extensively managed road verges are filtered by urban environments. Plant Ecol. Divers. 10, 217–229. doi: 10.1080/17550874.2017.1350764
English J., Barry K. E., Wood E. M., Wright A. J. (2022). The effect of urban environments on the diversity of plants in unmanaged grasslands in Los Angeles, United States. Front. Ecol. Evol. 10. doi: 10.3389/fevo.2022.921472
Fahrig L. (2003). Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. System. 34, 487–515. doi: 10.1111/j.1461-0248.2010.01559.x
Fahrig L., Baudry J., Brotons L., Burel F. G., Crist T. O., Fuller R. J., et al. (2011). Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 14, 101–112. doi: 10.1111/j.1461-0248.2010.01559.x
Fischer L., Lippe M., Kowarik I. (2013). Urban land use types contribute to grassland conservation: The example of Berlin. Urban For. Urban Green. 12, 263–272. doi: 10.1016/j.ufug.2013.03.009
Foley J. A., DeFries R., Asner G. P., Barford C., Bonan G., Carpenter S. R., et al. (2005). Global consequences of land use. Science 309, 570–574. doi: 10.1126/science.1111772
Gaujour E., Amiaud B., Mignolet C., Plantureux S. (2012). Factors and processes affecting plant biodiversity in permanent grasslands. A review. Agron. Sustain. Dev. 32, 133–160. doi: 10.1007/s13593-011-0015-3
Géron C., Lembrechts J. J., Borgelt J., Lenoir J., Hamdi R., Mahy G., et al. (2021). Urban alien plants in temperate oceanic regions of Europe originate from warmer native ranges. Biol. Invasions 23, 1765–1779. doi: 10.1007/s10530-021-02469-9
Godefroid S., Koedam N. (2007). Urban plant species patterns are highly driven by density and function of built-up areas. Landsc. Ecol. 22, 1227–1239. doi: 10.1007/s10980-007-9102-x
Grime J. P. (2001) Plant strategies, vegetation processes, and ecosystem properties. Available at: https://www.cabdirect.org/cabdirect/abstract/20013069580 (Accessed May 17, 2021).
Grime J. P., Hodgson J. G., Hunt R. (1988). Comparative Plant Ecology. (Dordrecht: Springer). doi: 10.1007/978-94-017-1094-7
Grimm N. B., Faeth S. H., Golubiewski N. E., Redman C. L., Wu J., Bai X., et al. (2008). Global change and the ecology of cities. Science 319, 756–760. doi: 10.1126/science.1150195
Habel J. C., Dengler J., Janišová M., Török P., Wellstein C., Wiezik M. (2013). European grassland ecosystems: threatened hotspots of biodiversity. Biodivers. Conserv. 22, 2131–2138. doi: 10.1007/s10531-013-0537-x
Hahs A. K., McDonnell M. J., McCarthy M. A., Vesk P. A., Corlett R. T., Norton B. A., et al. (2009). A global synthesis of plant extinction rates in urban areas. Ecol. Lett. 12, 1165–1173. doi: 10.1111/j.1461-0248.2009.01372.x
Herben T., Klimešová J., Chytrý M. (2018). Philip Grime’s fourth corner: are there plant species adapted to high disturbance and low productivity? Oikos 127, 1125–1131. doi: 10.1111/oik.05090
Hill M. O., Roy D. B., Thompson K. (2002). Hemeroby, urbanity and ruderality: bioindicators of disturbance and human impact. J. Appl. Ecol. 39, 708–720. doi: 10.1046/j.1365-2664.2002.00746.x
Hooftman D., Kimberley A., Cousins S. A. O., Escribano-Avila G., Honnay O., Krickl P., et al. (2021). Dispersal limitation, eutrophication and propagule pressure constrain the conservation value of Grassland Green Infrastructure. Biol. Conserv. 258, 109152. doi: 10.1016/j.biocon.2021.109152
Horák J., Šafářová L., Trombik J., Menéndez R. (2022). Patterns and determinants of plant, butterfly and beetle diversity reveal optimal city grassland management and green urban planning. Urban For. Urban Green. 73, 127609. doi: 10.1016/j.ufug.2022.127609
Humbert J.-Y., Pellet J., Buri P., Arlettaz R. (2012). Does delaying the first mowing date benefit biodiversity in meadowland? Environ. Evid. 1, 9. doi: 10.1186/2047-2382-1-9
Jackson H. B., Fahrig L. (2012). What size is a biologically relevant landscape? Landsc. Ecol. 27, 929–941. doi: 10.1007/s10980-012-9757-9
Jackson H. B., Fahrig L. (2015). Are ecologists conducting research at the optimal scale? Glob. Ecol. Biogeogr. 24, 52–63. doi: 10.1111/geb.12233
Jantunen J., Saarinen K., Valtonen A., Saarnio S. (2007). Flowering and seed production success along roads with different mowing regimes. Appl. Veg. Sci. 10, 285–292. doi: 10.1111/j.1654-109X.2007.tb00528.x
Johansen L. Q., Westin A., Wehn S., Iuga A., Ivascu C. M., Kallioniemi E., et al. (2019). Traditional semi-natural grassland management with heterogeneous mowing times enhances flower resources for pollinators in agricultural landscapes. Glob. Ecol. Conserv. 18, e00619. doi: 10.1016/j.gecco.2019.e00619
Julve (2015) Baseflor. Index botanique, écologique et chorologique de la flore de France, version 2015. Available at: http://perso.wanadoo.fr/philippe.julve/catminat.htm.
Kahmen S., Poschlod P., Schreiber K.-F. (2002). Conservation management of calcareous grasslands. Changes in plant species composition and response of functional traits during 25 years. Bio. Conserv. 104, 319–328. doi: 10.1016/S0006-3207(01)00197-5
Kimberley A., Hooftman D., Bullock J. M., Honnay O., Krickl P., Lindgren J., et al. (2021). Functional rather than structural connectivity explains grassland plant diversity patterns following landscape scale habitat loss. Landsc. Ecol. 36, 265–280. doi: 10.1007/s10980-020-01138-x
Klaus V. H. (2013). Urban grassland restoration: A neglected opportunity for biodiversity conservation. Restor. Ecol. 21, 665–669. doi: 10.1111/rec.12051
Klotz S., Kühn I., Durka W., für Naturschutz D. B., Briemle G. (2002). BIOLFLOR - eine Datenbank mit biologisch-ökologischen Merkmalen zur Flora von Deutschland . Münster: BfN-Schriftenvertrieb im Landwirtschaftsverl.
Knapp S., Kühn I., Bakker J. P., Kleyer M., Klotz S., Ozinga W. A., et al. (2009). How species traits and affinity to urban land use control large-scale species frequency. Divers. Distrib. 15, 533–546. doi: 10.1111/j.1472-4642.2009.00561.x
Knapp S., Kühn I., Stolle J., Klotz S. (2010). Changes in the functional composition of a Central European urban flora over three centuries. Perspect. Plant Ecol. Evol. Syst. 12, 235–244. doi: 10.1016/j.ppees.2009.11.001
Kowarik I. (2008). ““On the role of alien species in urban flora and vegetation,”,” in Urban Ecology: An International Perspective on the Interaction Between Humans and Nature. Eds. Marzluff J. M., Shulenberger E., Endlicher W., Alberti M., Bradley G., Ryan C., et al (Boston, MA: Springer US), 321–338. doi: 10.1007/978-0-387-73412-5_20
Kurylo J. S., Threlfall C. G., Parris K. M., Ossola A., Williams N. S. G., Evans K. L. (2020). Butterfly richness and abundance along a gradient of imperviousness and the importance of matrix quality. Ecol. Appl. 30, e02144. doi: 10.1002/eap.2144
Leng X., Musters C. J. M., de Snoo G. R. (2011). Effects of mowing date on the opportunities of seed dispersal of ditch bank plant species under different management regimes. J. Nat. Conserv. 19, 166–174. doi: 10.1016/j.jnc.2010.11.003
Lepš J., Šmilauer P. (2003). Multivariate Analysis of Ecological Data using CANOCO. (Cambridge: Cambridge University Press). doi: 10.1017/CBO9780511615146
Lindborg R., Bengtsson J., Berg Å., Cousins S. A. O., Eriksson O., Gustafsson T., et al. (2008). A landscape perspective on conservation of semi-natural grasslands. Agric. Ecosyst. Env. 125, 213–222. doi: 10.1016/j.agee.2008.01.006
Loos J., Krauss J., Lyons A., Föst S., Ohlendorf C., Racky S., et al. (2021). Local and landscape responses of biodiversity in calcareous grasslands. Biodivers. Conserv. 30, 2415–2432. doi: 10.1007/s10531-021-02201-y
Lososová Z., Chytrý M., Kühn I., Hájek O., Horáková V., Pyšek P., et al. (2006). Patterns of plant traits in annual vegetation of man-made habitats in central Europe. Perspect. Plant Ecol. Evol. Syst. 8, 69–81. doi: 10.1016/j.ppees.2006.07.001
Lüdecke D., Ben-Shachar M. S., Patil I., Waggoner P., Makowski D. (2021). performance: an R package for assessment, comparison and testing of statistical models. J. Open Source Software 6, 3139. doi: 10.21105/joss.03139
Marshall C. A. M., Wilkinson M. T., Hadfield P. M., Rogers S. M., Shanklin J. D., Eversham B. C., et al. (2023). Urban wildflower meadow planting for biodiversity, climate and society : An evaluation at King’s College, Cambridge. Ecol. Solut. Evid. 4, e12243. doi: 10.1002/2688-8319.12243
McDonnell M. J., Pickett S. T. A. (1990). Ecosystem structure and function along urban-rural gradients: an unexploited opportunity for ecology. Ecology 71, 1232–1237. doi: 10.2307/1938259
McKinney M. L. (2002). Urbanization, Biodiversity, and Conservation: The impacts of urbanization on native species are poorly studied, but educating a highly urbanized human population about these impacts can greatly improve species conservation in all ecosystems. BioScience 52, 883–890. doi: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2
McKinney M. L. (2008). Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 11, 161–176. doi: 10.1007/s11252-007-0045-4
Mugnai M., Ferretti G., Gesuelli E., Nuti L., Di Natale S., Corti E., et al. (2023). Site dependence of local variations in taxonomic and functional diversity of plant communities in semi-natural dry grasslands. Plant Ecol. 224, 95–111. doi: 10.1007/s11258-022-01282-1
Muratet A., Machon N., Jiguet F., Moret J., Porcher E. (2007). The role of urban structures in the distribution of wasteland flora in the greater Paris area, France. Ecosystem 10, 661–671. doi: 10.1007/s10021-007-9047-6
Napoleone F., Giarrizzo E., Burrascano S. (2021). Habitat conservation state and plant diversity respond to different drivers in semi-natural grasslands. J. Veg. Sci. 32, e13055. doi: 10.1111/jvs.13055
Norton B., Bending G., Clark R., Corstanje R., Dunnett N., Evans K., et al. (2019). Urban meadows as an alternative to short mown grassland: effects of composition and height on biodiversity. Ecol. Appl. 29, 1095–1115. doi: 10.1002/eap.1946
Olsen S. L., Evju M., Endrestøl A. (2018). Fragmentation in calcareous grasslands: species specialization matters. Biodivers. Conserv. 27, 2329–2361. doi: 10.1007/s10531-018-1540-z
Parr T. W., Way J. M. (1988). Management of roadside vegetation: the long-term effects of cutting. J. Appl. Ecol. 25, 1073–1087. doi: 10.2307/2403767
Peng Y., Mi K., Wang H., Liu Z., Lin Y., Sang W., et al. (2019). Most suitable landscape patterns to preserve indigenous plant diversity affected by increasing urbanization: A case study of Shunyi District of Beijing, China. Urban Forest. Urban Greening 38, 33–41. doi: 10.1016/j.ufug.2018.11.004
Pithon J. A., Duflot R., Beaujouan V., Jagaille M., Pain G., Daniel H. (2021). Grasslands provide diverse opportunities for bird species along an urban-rural gradient. Urban Ecosyst. 24, 1281–1294. doi: 10.1007/s11252-021-01114-6
Pyšek P. (1995). “Approaches to studying spontaneous settlement flora and vegetation in central Europe: A review”, in Urban Ecology As the Basis of Urban Planning. Ed. Sukopp H., Numata M., Huber A. (Amsterdam: SPB Academic Publishing), 23–29.
Pyšek P. (1998). Alien and native species in Central European urban floras: a quantitative comparison. J. Biogeogr. 25, 155–163. doi: 10.1046/j.1365-2699.1998.251177.x
Roy D. B., Hill M. O., Rothery P. (1999). Effects of urban land cover on the local species pool in Britain. Ecography 22, 507–517. doi: 10.1111/j.1600-0587.1999.tb01279.x
Rudolph M., Velbert F., Schwenzfeier S., Kleinebecker T., Klaus V. H. (2017). Patterns and potentials of plant species richness in high- and low-maintenance urban grasslands. Appl. Veg. Sci. 20, 18–27. doi: 10.1111/avsc.12267
Schmucki R., Reimark J., Lindborg R., Cousins S. A. O. (2012). Landscape context and management regime structure plant diversity in grassland communities. J. Ecol. 100, 1164–1173. doi: 10.1111/j.1365-2745.2012.01988.x
Sehrt M., Bossdorf O., Freitag M., Bucharova A. (2020). Less is more! Rapid increase in plant species richness after reduced mowing in urban grasslands. Basic Appl. Ecol. 42, 47–53. doi: 10.1016/j.baae.2019.10.008
Smart S. M., Bunce R. G. H., Marrs R., LeDuc M., Firbank L. G., Maskell L. C., et al. (2005). Large-scale changes in the abundance of common higher plant species across Britain between 1978, 1990 and 1998 as a consequence of human activity: Tests of hypothesised changes in trait representation. Bio. Conserv. 124, 355–371. doi: 10.1016/j.biocon.2004.12.013
Smith L. M., Haukos D. A. (2002). Floral diversity in relation to playa wetland area and watershed disturbance. Conserv. Bio. 16, 964–974. doi: 10.1046/j.1523-1739.2002.00561.x
Smith R. S., Shiel R. S., Millward D., Corkhill P. (2000). The interactive effects of management on the productivity and plant community structure of an upland meadow: an 8-year field trial. J. Appl. Ecol. 37, 1029–1043. doi: 10.1046/j.1365-2664.2000.00566.x
Smith R. S., Shiel R. S., Millward D., Corkhill P., Sanderson R. A. (2002). Soil seed banks and the effects of meadow management on vegetation change in a 10-year meadow field trial. J. Appl. Ecol. 39, 279–293. doi: 10.1046/j.1365-2664.2002.00715.x
Socher S. A., Prati D., Boch S., Müller J., Klaus V. H., Hölzel N., et al. (2012). Direct and productivity-mediated indirect effects of fertilization, mowing and grazing on grassland species richness. J. Ecol. 100, 1391–1399. doi: 10.1111/j.1365-2745.2012.02020.x
Sodhi N. S., Koh L. P., Peh K. S.-H., Tan H. T. W., Chazdon R. L., Corlett R. T., et al. (2008). Correlates of extinction proneness in tropical angiosperms. Divers. Distrib. 14, 1–10. doi: 10.1111/j.1472-4642.2007.00398.x
Tautenhahn S., Heilmeier H., Götzenberger L., Klotz S., Wirth C., Kühn I. (2008). On the biogeography of seed mass in Germany – distribution patterns and environmental correlates. Ecography 31, 457–468. doi: 10.1111/j.0906-7590.2008.05439.x
Ter Braak C. J. F. (1986). Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 67, 1167–1179. doi: 10.2307/1938672
Ter Braak C. J. F., Smilauer P. (2012). CANOCO reference manual and user’s guide : Software for ordination (version 5.0). Ithaca USA: Microcomputer Power.
Thompson K., Jones A. (1999). Human population density and prediction of local plant extinction in Britain. Conserv. Biol. 13, 185–189. doi: 10.1046/j.1523-1739.1999.97353.x
Threlfall C. G., Ossola A., Hahs A. K., Williams N. S. G., Wilson L., Livesley S. J. (2016). Variation in vegetation structure and composition across urban green space types. Front. Ecol. Evol. 4. doi: 10.3389/fevo.2016.00066
Vallet J., Daniel H., Beaujouan V., Rozé F. (2008). Plant species response to urbanization: comparison of isolated woodland patches in two cities of North-Western France. Landscape Ecol. 23, 1205–1217. doi: 10.1007/s10980-008-9293-9
Van der Maarel E. (2005). “Vegetation ecology - an overview,”. In Vegetation Ecologie, ed. van de Maarel E. (Oxford: Blackwell Publishing), 1–51.
Vega K. A., Küffer C. (2021). Promoting wildflower biodiversity in dense and green cities: The important role of small vegetation patches. Urban For. Urban Green. 62, 127165. doi: 10.1016/j.ufug.2021.127165
Watson C. J., Carignan-Guillemette L., Turcotte C., Maire V., Proulx R. (2020). Ecological and economic benefits of low-intensity urban lawn management. J. Appl. Ecol. 57, 436–446. doi: 10.1111/1365-2664.13542
Williams N. S. G., Hahs A. K., Vesk P. A. (2015). Urbanisation, plant traits and the composition of urban floras. Perspect. Plant Ecol. Evol. Syst. 17, 78–86. doi: 10.1016/j.ppees.2014.10.002
Williams N. S. G., Morgan J. W., McCarthy M. A., McDonnell M. J. (2006). Local extinction of grassland plants: the landscape matrix is more important than patch attributes. Ecology 87, 3000–3006. doi: 10.1890/0012-9658(2006)87[3000:LEOGPT]2.0.CO;2
Williams N. S. G., Morgan J. W., Mcdonnell M. J., Mccarthy M. A. (2005). Plant traits and local extinctions in natural grasslands along an urban-rural gradient: Plant extinctions along an urban-rural gradient. J. Ecol. 93, 1203–1213. doi: 10.1111/j.1365-2745.2005.01039.x
Williams N. S. G., Schwartz M. W., Vesk P. A., McCarthy M. A., Hahs A. K., Clemants S. E., et al. (2009). A conceptual framework for predicting the effects of urban environments on floras. J. Ecol. 97, 4–9. doi: 10.1111/j.1365-2745.2008.01460.x
Wu J. (2014). Urban ecology and sustainability: The state-of-the-science and future directions. Landscape Urban Plann. 125, 209–221. doi: 10.1016/j.landurbplan.2014.01.018
Yeh C.-T., Huang S.-L. (2009). Investigating spatiotemporal patterns of landscape diversity in response to urbanization. Landscape Urban Plann. 93, 151–162. doi: 10.1016/j.landurbplan.2009.07.002
Zeeman B. J., McDonnell M. J., Kendal D., Morgan J. W. (2017). Biotic homogenization in an increasingly urbanized temperate grassland ecosystem. J. Veg. Sci. 28, 550–561. doi: 10.1111/jvs.12507
Keywords: herbaceous green spaces, low intensity mowing, urbanization, plant traits, biodiversity conservation, meadows
Citation: Gros C, Bulot A, Aviron S, Beaujouan V and Daniel H (2023) Both management practices and landscape influence plant communities in urban grasslands. Front. Ecol. Evol. 11:1151913. doi: 10.3389/fevo.2023.1151913
Received: 26 January 2023; Accepted: 04 October 2023;
Published: 26 October 2023.
Edited by:
Thierry Dutoit, UMR7263 Institut Méditerranéen de Biodiversité et D’écologie marine et continentale (IMBE), FranceReviewed by:
Hong Hanh Nguyen, Senckenberg Research Institute and Natural History Museum Frankfurt, GermanyCopyright © 2023 Gros, Bulot, Aviron, Beaujouan and Daniel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clément Gros, Y2xlbWVudC5ncm9zQGluc3RpdHV0LWFncm8uZnI=; Hervé Daniel, aGVydmUuZGFuaWVsQGluc3RpdHV0LWFncm8uZnI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.