
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
METHODS article
Front. Ecol. Evol., 06 April 2023
Sec. Chemical Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1128514
This article is part of the Research TopicMicrobial Volatiles and CommunicationView all 6 articles
Microbes communicate with each other using a wide array of chemical compounds, including volatile organic compounds (VOCs). Usually, such volatile-mediated interactions are studied by growing two different microbes in a shared, confined environment and by subsequently collecting and analyzing the emitted VOCs by gas chromatography. This procedure has several drawbacks, including artificial volatile overaccumulation and potential oxygen limitation, as well as the impossibility to assign a producer to the compounds newly emitted during the interaction. To address these challenges, we have developed a novel system specifically designed to analyze volatile-mediated interactions allowing for sequential unidirectional exposure of a “receiver” microorganism to the VOCs of an “emitter” microorganism. Using hermetically sealed systems connected to an air compressor, a constant unidirectional airflow could be generated, driving emitted volatiles to be absorbed by a collection charcoal filter. Thus, our developed system avoids artificial overaccumulation of volatile compounds and lack of oxygen in the headspace and enables the univocal assignment of VOCs to their producers. As a proof of concept, we used this newly developed experimental setup to characterize the reaction of plant growth-promoting and biocontrol fungus (Trichoderma simmonsii) to the perception of VOCs emitted by two plant pathogens, namely Botrytis cinerea and Fusarium oxysporum. Our results show that the perception of each pathogen's volatilome triggered a specific response, resulting in significant changes in the VOCs emitted by Trichoderma. Trichoderma's volatilome modulation was overall stronger when exposed to the VOCs from Fusarium than to the VOCs from Botrytis, which correlated with increased siderophore production when co-incubated with this fungus. Our newly developed method will not only help to better understand volatile-mediated interactions in microbes but also to identify new molecules of interest that are induced by VOC exposure, as well as the putative-inducing signals themselves.
Volatile-mediated interactions are involved in many processes for microbe–microbe communication. Volatiles emitted by microbes can affect receiving organisms in different ways. They can affect their growth and development, their motility, they can attract or repel, as well as inhibit or promote the growth of individuals from the same species or other kingdoms including animals and plants (Schmidt et al., 2016; Sharifi and Ryu, 2018; Bruisson et al., 2020; Farh and Jeon, 2020). In the past few years, evidence showed that once a microorganism is affected by external volatiles, the composition of its own volatilome (i.e., the sum of all the volatiles it emits) may also change; some compounds will therefore be detected in higher vs. lower abundances, while others will only be detected or in contrast will no longer be detected as a result of the interaction (Rybakova et al., 2017, 2022). Until now, such interactions have only been studied in confined environments with the partners sharing the same headspace, allowing the reciprocal influence of the two partners. This method despite its effectiveness has several limitations. The culture of microbes in a closed and often very small environment may lead to oxygen deprivation and overaccumulation of several volatiles that would not occur in natural conditions, with unknown consequences on the behavior of the emitting microbes. In addition, these systems usually rely on solid-phase microextraction (SPME) fibers, which as a passive trapping technique does not allow the reliable acquisition of quantitative data (Xu and Ouyang, 2019). Finally, when a new compound is detected upon volatile-mediated interaction between both partners, it frequently is not possible to identify the emitter since both organisms are grown in the same headspace, with the exception of species-specific compounds whose origin can be traced back because of their specificity (Weisskopf et al., 2021). To overcome these limitations, we describe in this study a new system to study volatile-mediated communication, which allows the unidirectional exposure of one microbe to the volatilome of another by growing each of these organisms in small Teflon chambers called “microcosms” connected in a series by a unidirectional airflow generated by a compressor, which solves the problem of oxygen deprivation. The microbe located downstream (“receiver”) is then unilaterally exposed to the VOCs emitted by the microbe located upstream (“emitter”). The volatiles emitted are then carried by the constant airflow which prevents their overaccumulation and driven into an active charcoal filter which will trap all the volatiles. This method, thus, makes it possible to study unidirectional volatile-mediated interactions between two partners, characterizing first the effect of one on the other, and then the reciprocal effect after switching the order of the two connected microcosms. Since this system requires both organisms to grow in similar conditions (e.g., temperature), we also tested an alternative setup where VOCs are first collected in a single microcosm setup and then supplied to the exposed partner (in dissolved form) in another microcosm that can be incubated in different conditions.
In the present study, we used this new microcosm setup to study the volatile-mediated interaction between the plant beneficial fungus T. simmonsii and two phytopathogenic fungi, B. cinerea and F. oxysporum. Earlier studies have demonstrated the potential of several Trichoderma species as biocontrol agents and plant growth promoters (Alfiky and Weisskopf, 2021; Joo and Hussein, 2022), and these effects on plants and their pathogens are also partly due to the emission of active volatiles affecting neighboring organisms (Lee et al., 2016). In particular, Trichoderma species can produce antifungal molecules that inhibit plant pathogens including Botrytis and Fusarium (Amin et al., 2010; Kottb et al., 2015; Joo and Hussein, 2022) and produce some of these molecules in reaction to exposure to pathogens' volatiles (Zhang et al., 2014; Li et al., 2018). We, therefore, wondered whether Trichoderma would be able to perceive the volatiles of either phytopathogenic fungus or it would react in a similar or specific manner to the different blends emitted. In addition to the emission of volatile, another important factor for rhizosphere colonization and plant protection against pathogenic fungi is the ability to acquire sparingly soluble iron via the secretion of high-affinity iron chelators called siderophores (Li et al., 2015; Saha et al., 2016). We, therefore, also assessed whether Trichoderma would react to the volatiles emitted by either phytopathogenic fungus by increasing its siderophore release. This study shows that our newly developed directional volatile exposure setup is suitable to identify changes in the volatilome composition after exposure to external volatiles, and Trichoderma reacted differentially to the volatiles of Fusarium and Botrytis both in terms of the volatilome composition and siderophore production.
Trichoderma simmonsii TAA11 and Botrytis cinerea strain BMM provided by Brigitte Mauch-Mani (University of Neuchatel, Switzerland) were grown in potato dextrose agar (PDA) (Roth), a rich medium favorable for volatile production in fungi and were incubated at 18°C with a 12 h light/12 h dark photoperiod. Fusarium oxysporum f. sp. conglutinans strain 699 (ATCC 58110) was grown on PDA in the dark at 28°C. For volatile collection, all fungi were grown from one 5 mm mycelium plug in 5 cm glass Petri dishes filled with 20 ml of PDA. F. oxysporum and B. cinerea were incubated 4 days prior to the experiments, while T. simmonsii was incubated 1 day prior to the experiments due to its faster growth rate, for the mycelium of each organism to completely cover the culture medium and starts its maturation at the beginning of the experiment. The same medium was used at each step for all organisms in order to avoid nutrient differences affecting their behavior since volatile production is particularly sensitive to culture media.
The volatile collection was performed using a modified closed-loop stripping apparatus (CLSA) (Schulz et al., 2004; Groenhagen et al., 2013; Hunziker et al., 2015; Bruisson et al., 2019). Glass Petri dishes containing the emitter strain and the receiver strain were placed in two hermetically sealed home-built PTFE microcosms connected to each other by PTFE tubing (de Vrieze et al., 2015). The box containing the receiver strain was also connected to a collection filter containing activated charcoal to trap volatiles, while the box containing the emitter strain was connected to an ACO-388D electromagnetic air compressor (Hailea®, China) generating a constant airflow of 0.5 ml/min reaching the emitter strain first, then the receiver strain, and finally the collection filter. To prevent contaminations from volatiles present in the environment, an air-purifying filter was placed between the air compressor and the first microcosm (Figure 1). In this system, the strain downstream was exposed to the volatiles emitted by the strain upstream, but the volatiles emitted by the strain downstream could not affect the strain upstream. After 2 days of incubation under these conditions, the collection filter was removed, and the volatiles trapped were recovered using three consecutive washes of 25 μl of dichloromethane (DCM) for volatile desorption, prior to the quadrupole time-of-flight mass spectrometry (GC/QTOF-MS) analysis. Volatiles collected from the same setup but using only one box containing one of the organisms were used as the control for the comparison of the volatilome compositions. For each modality, two independent experiments were performed for a total of n = 7 replicates in the end.
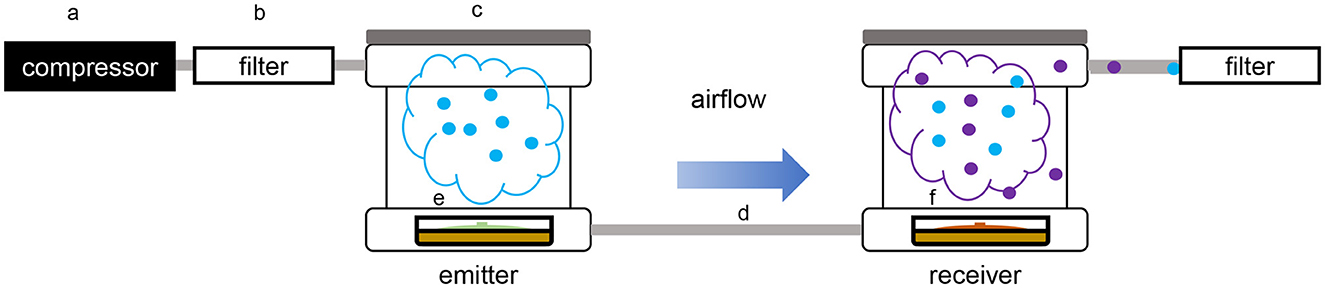
Figure 1. Schematic overview of the dual microcosm apparatus used for volatile exposition and collection. (a) Air compressor; (b) active charcoal filter; (c) PTFE box (microcosm); (d) PTFE pipe; (e) glass Petri dish containing the emitter organism; (f) glass Petri dish containing the receiver organism.
While using the dual microcosm setup has many advantages, since it is the better way to simulate a natural exposure, this setup may not always be a viable solution to study such interactions. In some cases, each partner needs to be incubated in different conditions to reach an optimal growth rate or volatile production, or in order to produce some targeted volatiles. To answer this hindrance, an alternative setup was tested, which consisted of exposing the receiver strain to a solution containing the total volatilome of the emitter strain previously collected and dissolved in methanol. In this single microcosm setup, the same procedure as described earlier was followed, but the strains were incubated with three PTFE septa on each of which 50 μl of the emitter strain's volatilome dissolved in methanol were spotted (Figure 2). The volatilome profiles were then compared to those obtained from plates of T. simmonsii incubated with drops of pure methanol which served as unexposed control samples, as well as to the profiles recollected from a drop containing only the emitter's volatiles (without the receiving organism plate).
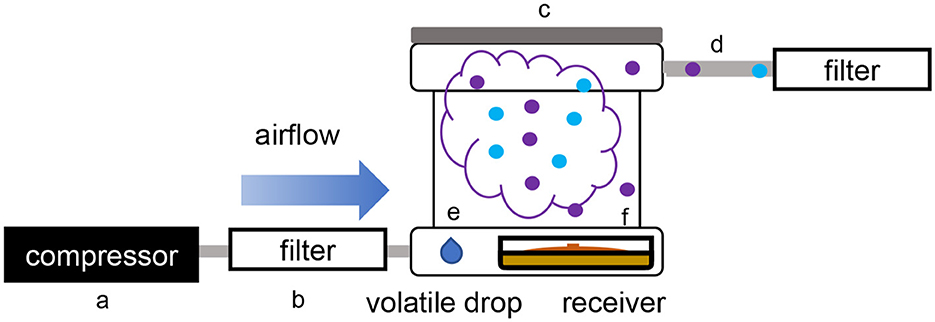
Figure 2. Schematic overview of the single microcosm apparatus used for volatile exposure and collection. (a) Air compressor; (b) active charcoal filter; (c) PTFE box (microcosm); (d) PTFE pipe; (e) three drops of methanol containing the dissolved volatilome from the emitter strain; (f) glass Petri dishes containing the receiver organism.
The total volatiles were analyzed by gas chromatography with quadrupole time-of-flight mass spectrometry (GC/QTOF-MS), using a 7890B GC system connected to a 7250 GC/Q-TOF (Agilent) allowing a detection limit down to the femtogram. Samples were injected in an HP-5 ms column (30 m; 0.25 mm inside diameter; 0.25 μm film; Agilent) using the following parameters: He flow, 4 ml/min; injection volume, 2 μl splitless; transfer line, 300°C; injector, 250°C; electron energy, 70 eV and the following program: 5 min at 50°C, then increase of 5°C/min to 320°C, and hold for 1 min. The mass spectra were acquired in the centroid mode (m/z: 20–400, 3 scans/s). The retention time index (RI) was calculated using an alkane retention standard solution injected using the same parameters.
All GC/QTOF data were analyzed using MzMine 2.53 after converting the raw data files from the “.D” format to the “.mzdata” format. Mass detection was performed prior to chromatogram building and deconvolution using the Automated Data Analysis Pipeline (ADAP) algorithm, and spectral deconvolution was then performed with the Multivariate Curve Resolution method (Du et al., 2020). All features were aligned using the RANdom Sample Consensus method (RANSAC) prior to statistical analysis using the MetaboAnalyst platform (Supplementary Table S1). Volatile identification was performed using NIST MS software.
For the volatile exposure using dissolved volatiles, the absence of the effect of pure methanol on fungal growth was assessed with dual assays using two-compartment Petri dishes. One compartment was filled with PDA, and the other compartment was filled with water agar. A total of three 50 μl drops of pure methanol were deposited on the water agar side, 1 day after placing a plug of T. simmonsii mycelium and 4 days after placing a plug of B. cinerea or F. oxysporum mycelium in the compartment containing the PDA (the time for T. simmonsii was shorter due to its faster growth). In the control plates, the fungi were exposed to sterile water. In total, 4 days after the solvent deposition, pictures were taken, and the growth area was measured using ImageJ software. The effect on growth was assessed by comparing the average growth area of the control and test plates.
The absence of effect of pure methanol on volatile production was assessed by comparing the volatilome composition of T. simmonsii, B. cinerea, and F. oxysporum exposed to three drops of 50 μl of methanol with their volatilome composition after exposure to sterile water in the single microcosm setup (Figure 2).
The chrome azurol S (CAS) agar medium for siderophore production visualization was prepared according to Neilands (1987) and was used for a “sandwich plate” assay (Li et al., 2018). In this assay, PDA plates were used to incubate one plug of B. cinerea or F. oxysporum 4 days prior to the experiment, and CAS agar plates were used to incubate a plug of T. simmonsii 1 day prior to the experiment. The CAS agar plates were then placed on top of the plates containing either B. cinerea or F. oxysporum and sealed together with surgical tape, so the pathogens and T. simmonsii could only influence each other through their emitted volatiles. Four of each combination were carried out (n = 4), and the experiment was performed two times. Pictures were taken 2 weeks after incubation at 18°C with a 12 h light/12 h dark photoperiod, for final visualization. The control consisted of CAS agar plates with a plug of T. simmonsii exposed to non-inoculated PDA plates.
To explore the responses of B. cinerea and F. oxysporum to volatiles emitted by T. simmonsii, we analyzed the volatilome composition of the plant-beneficial fungus T. simmonsii and the two phytopathogenic fungi, B. cinerea and F. oxysporum, when grown alone or when exposed to volatiles emitted by other fungi.
We used a newly developed dual microcosm system allowing for continuous exposure of one interacting organism to the volatiles of another organism (see material and methods and Figure 1) to mimic conditions that the organisms would encounter in their natural environment, where volatiles are released gradually and in increasing concentrations as the emitting organism grows or comes closer.
This setup was used to assess whether the volatilome of Trichoderma would change upon exposure to volatiles emitted by phytopathogenic fungi. We compared the volatiles emitted by Trichoderma when grown alone or when exposed to external volatiles. The volatiles collected after exposing Trichoderma to the pathogens' volatiles were significantly different from the sum of volatilomes emitted by each organism incubated alone (Figures 3A, Bi, ii). Overall, significant modifications occurred in the Trichoderma volatilome composition after exposure to any of the two pathogens. These differences are highlighted in the principal component analysis, where clear separations between exposed and non-exposed Trichoderma could be seen with both Fusarium and Botrytis (Figure 3A). In all cases, the three groups (volatilomes from pathogens, non-exposed Trichoderma, and exposed Trichoderma) clustered in three distinct groups while also displaying some overlap indicating similarities. These observations were confirmed in the clustering analysis and the heatmaps. The heatmaps focus only on the significantly different features between groups, which yielded three coherent clusters for the three different groups and highlighted features that were either upregulated or downregulated in exposed vs. unexposed Trichoderma (Figure 3B).
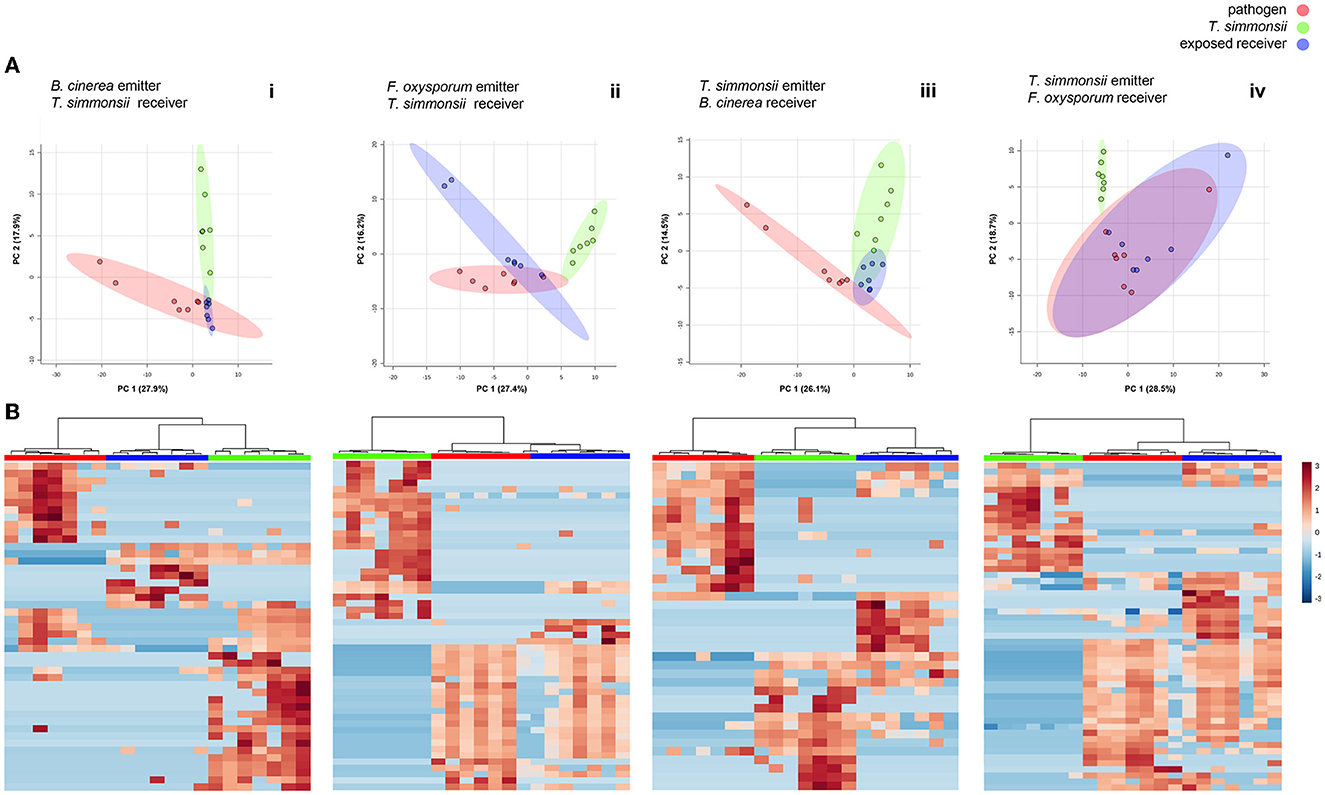
Figure 3. Differences in volatilome profiles between volatilomes of fungi incubated alone vs. exposed to their interactor's volatilome in a dual microcosm setup (n = 7). (A) Principal Component Analysis (PCA) plots of volatilome profiles; each dot represents one volatilome sample and each circle represents the 95% confidence interval. (B) Heatmap representing the hierarchical clustering of volatiles with significantly modified abundance between the different groups. Each line represents a mass feature (volatile compound) and each column represents a sample. The color indicates the normalized intensity and the dendrograms display the similarity based on the Pearson algorithm.
This first comparative analysis showed that the exposure of Trichoderma to volatiles from the two pathogens led to two different types of effects on its volatilome as follows: (i) some metabolites were less abundant after exposure, some of which were even below the detection threshold, (ii) others were more abundant after exposure, with some metabolites even only detected after exposure. These two contrasting effects could be observed in Trichoderma volatilomes after exposure regardless of the pathogen used as the emitter, but the types of compounds affected as well as their numbers were different depending on the emitting fungus (Table 1). Overall, Botrytis volatiles triggered a slightly higher number of modifications in Trichoderma volatilome than Fusarium volatiles (16 vs. 13). Exposure to both phytopathogenic fungi led to decreased abundance of a large set of compounds (14 for Botrytis vs. 10 for Fusarium) but in an emitter-specific manner, with only three compounds commonly downregulated upon exposure to both fungi: furfural (CAS number: 98-01-1), propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester (25265-77-4), and (-)-aristolene (6831-16-9). In contrast, only few volatiles were increased in their emission after exposure to Botrytis (2) or Fusarium (3), with no overlap between them (Table 1).
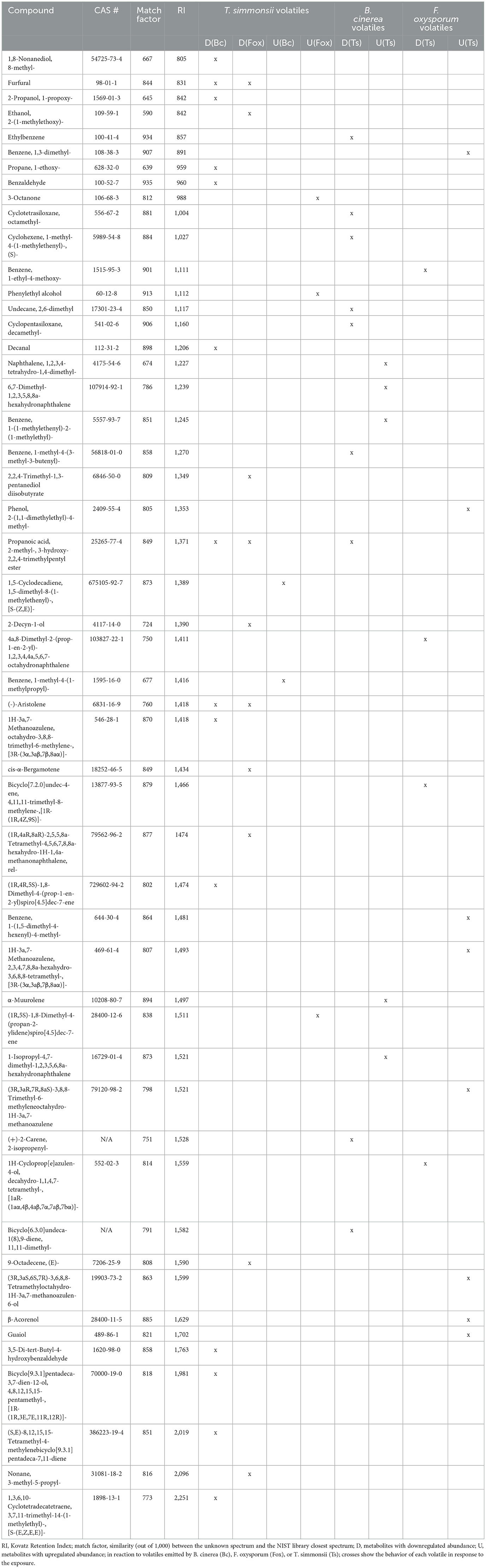
Table 1. List of volatiles emitted by Trichoderma, Botrytis, and Fusarium with significantly modified abundance after exposure to fungal volatiles.
To compare the influence of each partner's volatiles on the volatilome composition of the other partner, we simply switched the order in which the microcosms were interconnected (see Figure 1) and monitored the volatilome changes observed in either Fusarium or Botrytis when exposed to Trichoderma volatiles, to compare them with the previously acquired data on Trichoderma exposed to either interacting fungus. In these conditions, the volatilome of all fungi was modified upon exposure to each other's volatiles with similar reactions as those described previously, decreased vs. increased abundance of specific compounds (Figures 3A, Biii, iv; Table 1). In this configuration, PCA plots show that Botrytis reacted more strongly to the exposure to Trichoderma volatiles than Fusarium, whose volatilomes exhibited an important overlap in the absence or presence of Trichoderma volatiles. However, a clear separation of these two groups was achieved if PC3 (not shown but responsible for 9.9% of the variance) was taken into consideration. As for the previous results, all samples from the same treatments clustered together in the heatmaps when only significant modifications were considered. Even though Trichoderma volatiles triggered a comparable number of modifications in Botrytis and Fusarium (14 vs. 12), the reactions were quite different between the two organisms: Botrytis reacted by decreasing the abundance of nine compounds (against four for Fusarium) and by increasing the abundance of five compounds (against eight for Fusarium). These results highlight once again that exposure to external volatiles leads to specific reactions for each organism. Interestingly, one volatile compound previously shown to be commonly downregulated by Trichoderma after exposure to both Botrytis and Fusarium (Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester, CAS 25265-77-4) and was also downregulated by Botrytis upon exposure to Trichoderma.
Overall, most of the volatiles from the emitter fungi were found in the two groups with a similar abundance, which is expected as the volatiles emitted by the organism incubated in the first microcosm should in principle also be pumped into the collection filter (Figure 1). However, for all partner pairs analyzed, several compounds produced by the emitter were no longer detectable in the exposed sample (Table 2). The list of these elusive compounds shows that from the complex blend emitted by Trichoderma, only two volatiles disappeared in the interaction with Botrytis, while this happened to seven compounds in the interaction with Fusarium. Notably, furfural disappeared in both cases. When Trichoderma was exposed to volatiles from Botrytis and Fusarium, this led to the disappearance of eight vs. five volatiles, respectively, for each phytopathogen.
To determine whether the microcosm system was suitable, to study reactions in organisms exposed to synthetic volatiles dissolved in chemical solvents, we repeated our investigation using a single microcosm setup in search of similar effects. This variation allowed the exposure of Trichoderma with drops of methanol containing dissolved volatiles previously collected from either phytopathogenic fungus (see material and methods and Figure 2). We first verified that methanol itself had neither any adverse effect on the growth of Trichoderma (Supplementary Figure S1) nor on fungal volatile emission (Supplementary Figure S2). Results showed that the profiles previously observed in the dual microcosm setup were also present in these conditions. For both pairs of fungi, all volatilomes clustered in three different groups while also displaying some overlap indicating similarities in both the PCA and the heatmaps (Figure 4).
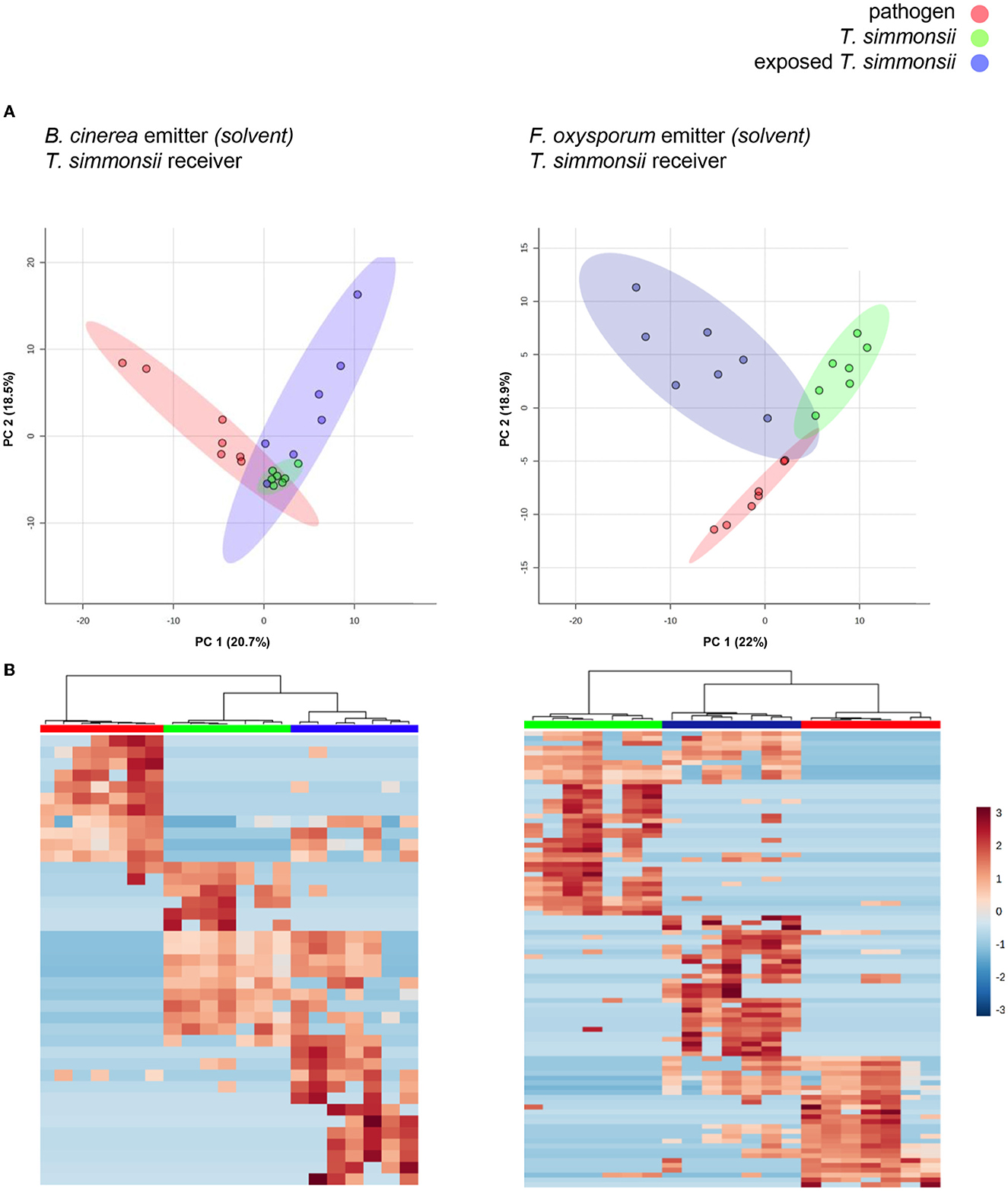
Figure 4. Differences in volatilome profiles between Trichoderma grown alone or exposed to the volatilomes of either Botrytis or Fusarium dissolved in methanol (single microcosm setup). (A) Principal Component Analysis (PCA) plots of volatilome profiles; each dot represents one volatilome sample and each circle represents the 95% confidence interval. (B) Heatmap representing the hierarchical clustering of volatiles with significantly different abundances between the different groups. Each line represents a mass feature (volatile compound) and each column represents a sample. The color indicates the normalized intensity of the respective volatiles and the dendrograms display the similarity based on the Pearson algorithm. Volatilomes from Trichoderma originate from mycelial cultures grown either with or without exposure to the volatiles from Botrytis or Fusarium, while the volatilomes from both phytopathogens originate from microcosms containing previously collected volatiles resuspended in solvent (see material and methods for more details).
Overall, a stronger reaction of Trichoderma was observed when exposed to Fusarium volatiles (19 significant changes) than when exposed to Botrytis volatiles (11 significant changes) (Table 3). Both phytopathogens reduced the abundance of a similar number of compounds including the same three common volatiles (CAS numbers 98-01-1, 25265-77-4, and 6831-16-9) identified previously in the dual microcosm setup. The exposure to Fusarium triggered an increased abundance of more compounds than the exposure to Botrytis (11 against 4, respectively). Acetophenone, which had not been detected in the dual microcosm setup, was the only compound that was commonly upregulated by Trichoderma in response to the volatiles from both phytopathogenic fungi (Table 3).
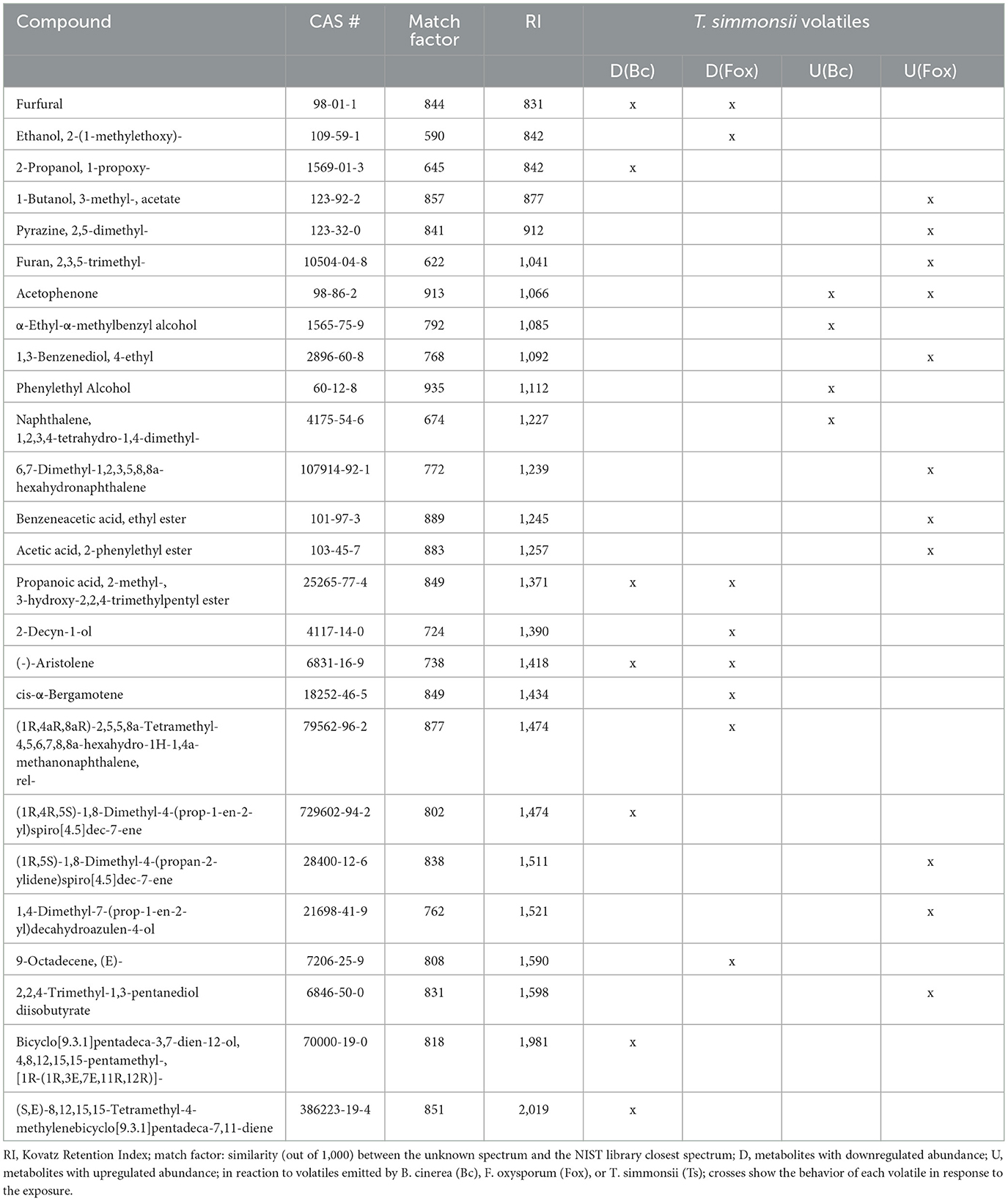
Table 3. List of volatiles emitted by Trichoderma with significantly modified abundance after exposure to Botrytis and Fusarium volatiles.
Despite their similarities, the results obtained with the two different setups show several discrepancies when compared systematically (Table 4). Interestingly, most of the volatiles showing lesser emission by Trichoderma upon exposure to either phytopathogenic fungus were detected in both setups. Thus, seven of the compounds inhibited by Botrytis out of 14 differentially abundant volatiles, and eight of the compounds inhibited by Fusarium out of 10 were identified in both conditions (Figure 5). In contrast, compounds whose abundance was increased by the exposure were mostly different in both setups. Only one of these compounds, promoted by Fusarium {(1R,5S)-1,8-Dimethyl-4-(propan-2-ylidene)spiro[4.5]dec-7-ene, CAS number: 28400-12-6} was identified in both setups. It is also striking that much more volatiles emitted by Trichoderma increased when this fungus was exposed to Fusarium volatiles in the single microcosm setup compared with the dual microcosm setup (11 against 3, see also Figure 5). The same trend with a smaller difference was also observed in Botrytis (four against two, Figure 5). This higher emission of volatiles upon sensing of Fusarium and Botrytis volatiles dissolved in methanol contrasted with Trichoderma's reaction to gradual exposure to volatiles directly emitted by the fungi in the dual microcosm setup, where only few volatiles displayed higher emission.
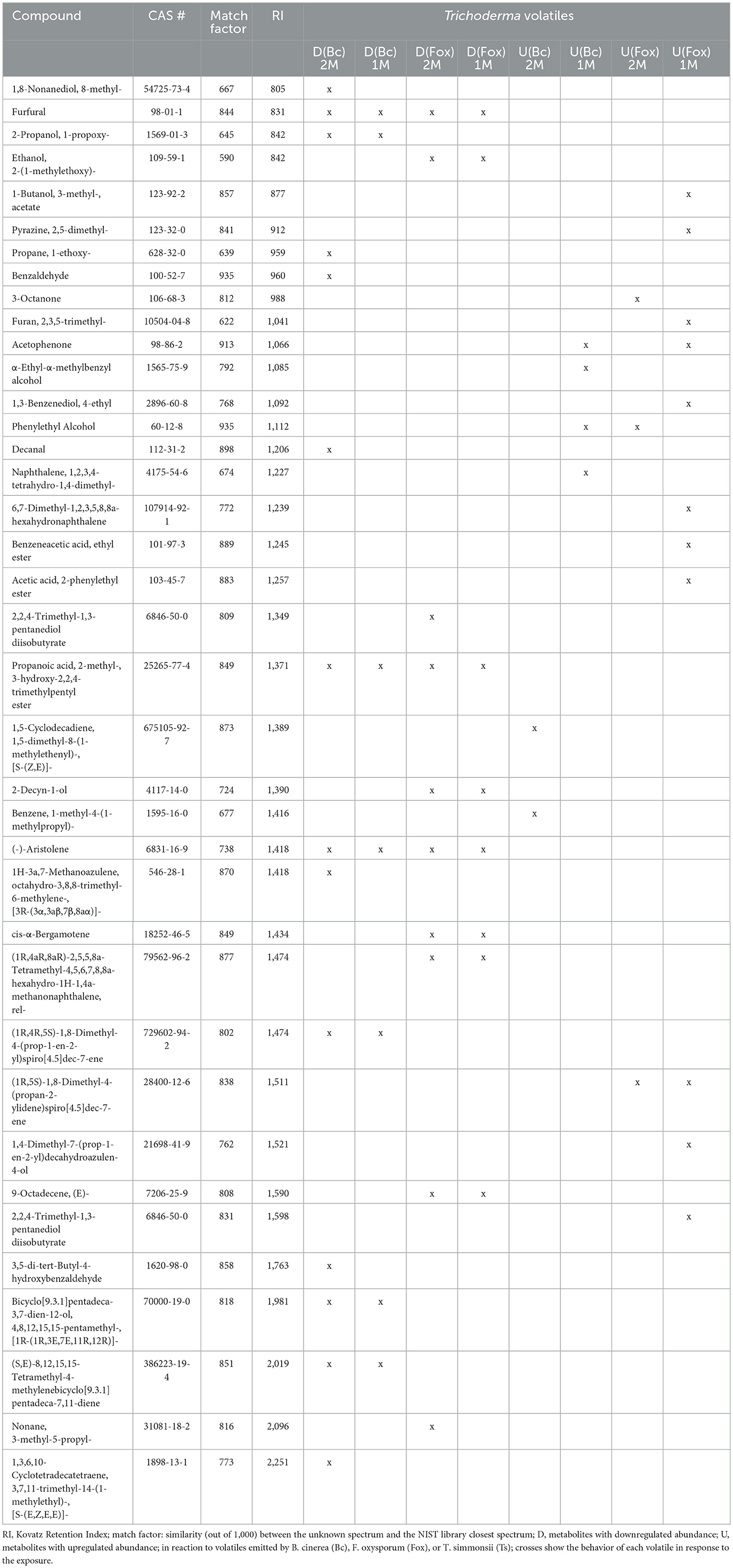
Table 4. Comparative list of compounds with significantly modified abundance after exposure to phytopathogenic fungi using the dual microcosm setup (2M) or the single microcosm setup (1M).
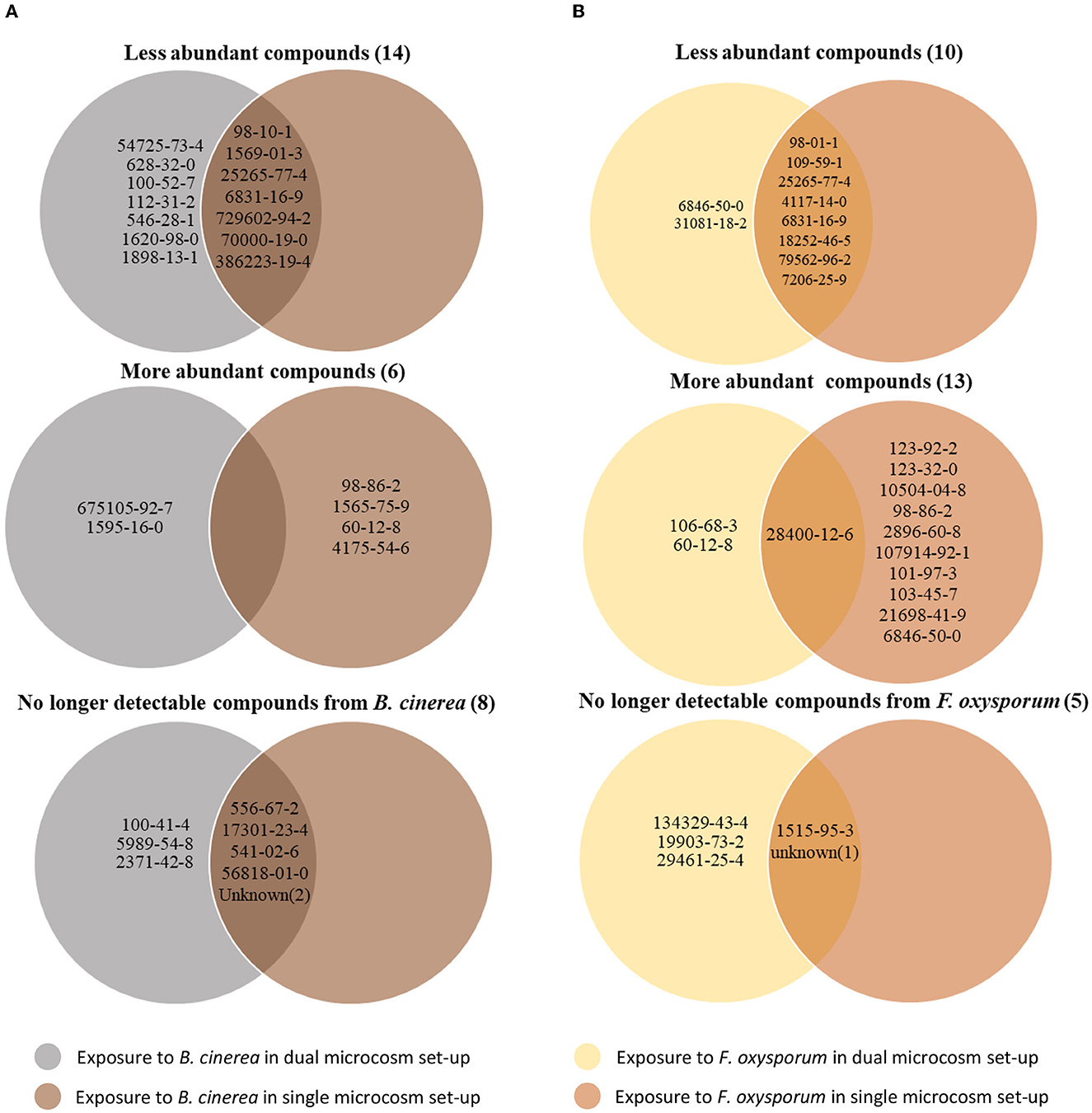
Figure 5. Trichoderma volatile modifications after exposure to Botrytis or Fusarium volatilomes using the two different microcosm systems. For each interaction pattern, the Venn diagram displays the CAS number of compounds with significantly modified abundance after exposure of Trichoderma to Botrytis (A) or Fusarium (B) volatiles diluted in methanol or directly emitted from another microcosm.
Regarding the compounds produced by the emitting fungus which were no longer detectable after exposure, most of them were identified in both setups when Botrytis was the emitter (five out of eight). When Fusarium was used as the emitter strain, two out of five compounds were no longer detectable in both setups (Figure 5; Supplementary Table S2). Both methods of exposure (gradual, live exposure vs. instant exposure with solvent-dissolved volatiles), therefore, generally led to similar changes and results, especially in terms of downregulation of volatile emission or in absorption (disappearance) of emitting organisms' volatiles, while compounds upregulated upon the perception of the emitters' volatiles greatly differed between both methods.
In both methods of exposure, we observed a stronger reaction of Trichoderma to the volatiles emitted by Fusarium than to the volatiles emitted by Botrytis (see e.g., PCAs in Figures 3–5). We wondered whether beyond the volatilome changes, Trichoderma would also show other reactions upon detection of Fusarium volatiles. To investigate this question, we compared the capacity of volatiles from both phytopathogenic fungi to trigger siderophore production in Trichoderma using a sandwich plate assay, which allowed both interacting fungi to interact with each other only through their volatiles. After 2 weeks of incubation, Trichoderma reacted to the perception of Fusarium by increasing its production of siderophores, while this increase was not observed when co-incubated with Botrytis or with empty PDA medium, which confirmed the stronger reaction of Trichoderma to the volatiles emitted by Fusarium compared with those emitted by Botrytis. This halo attesting to the presence of siderophore suggests that the volatiles emitted by Fusarium can influence the behavior of Trichoderma causing it to secrete more siderophores diffusing into a larger area (Figure 6). When using dissolved volatiles from F. oxysporum instead of the actively growing culture, a similar increase in siderophore production was observed (Supplementary Figure S3).
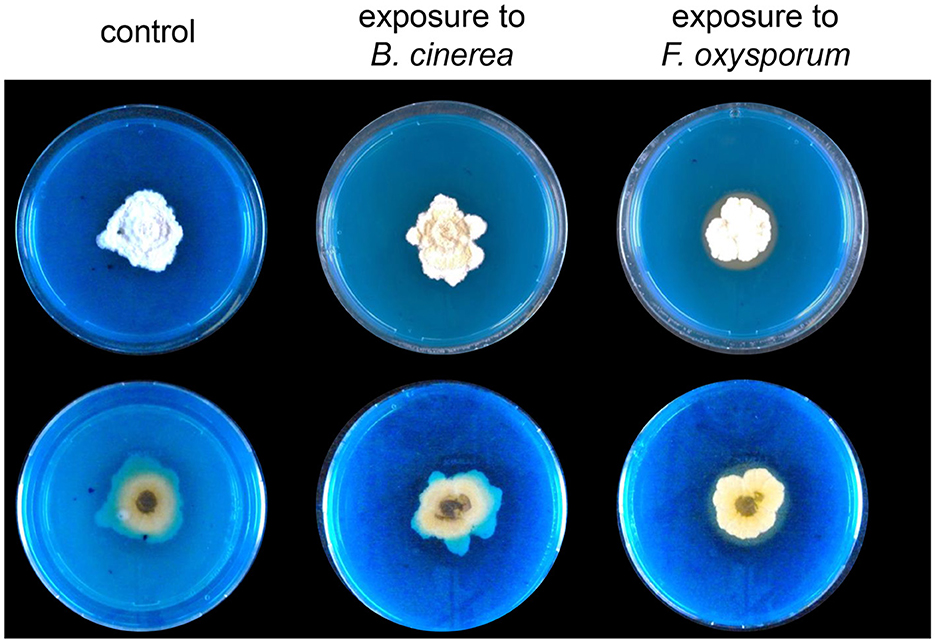
Figure 6. Representative pictures of (top) and (bottom) views of Trichoderma grown on CAS medium and exposed to Botrytis or Fusarium volatiles for 2 weeks in a sandwich plate assay (n = 4). The orange color shows the presence of siderophores secreted by Trichoderma. Control plates were exposed to PDA.
The method developed in this study shows that unilateral exposure is a powerful tool to study volatile-mediated interactions. The setup reduces the complexity of reciprocal interactions between two organisms by sequentially exposing one organism to the volatiles emitted by the other. It also allows more accurate reproduction of the environmental conditions in which these interactions take place, where emitted volatiles can easily spread and where a constant supply of oxygen is provided by the atmosphere, unlike the standard hermetic systems commonly used to study such interactions. Since it is admitted that low volatile concentrations are sufficient for a receiver strain to detect the presence of an interacting organism and trigger significant reactions (Schulz-Bohm et al., 2017; Sharifi et al., 2018; Sá et al., 2022), it is highly probable that the accumulation of artificially high concentrations of compounds that occurs in hermetically closed systems triggers reactions that are not representative of those taking place in nature. This problem of volatile overaccumulation is solved here by the application of constant airflow, and despite the constant “washing” of volatiles it causes, our results show that strong reactions of the receiving organisms can nonetheless be observed, e.g., with substantial changes in the volatilomes of the receiving strains as previously reported with closed systems (Barbieri et al., 2005; Rybakova et al., 2017; Tyc et al., 2017; Sharifi et al., 2022). When used in its single microcosm form, this setup allows the collection of the whole volatilome of an emitting organism grown in specific conditions, which can be used for a receiving organism grown in different conditions while still offering the benefits described earlier.
Our results demonstrate that all three interacting fungi, whether they are phytopathogenic or beneficial, can be affected by the presence of foreign volatiles which results in a change in the composition of their own volatilome, but that each organism displays a specific response to the same stimulus. The three fungi used in this study are Ascomycetes and share a large number of common compounds in their volatilomes when grown without an external volatile exposure. However, they all showed a unique and specific pattern in response to an external volatile exposure. For all three fungi, the main reaction to exposure was a decreased emission of many volatiles, an observation that was consistent in the two systems we tested (dual microcosm setup vs. single microcosm setup using the emitter's volatiles in dissolved form). Despite the overall strong specificity of the reaction, few compounds displayed similar modification patterns in the different interacting fungal pairs. One of these was the aldehyde furfural (98-01-1), which was produced by all three tested fungi and which showed decreased emission by Trichoderma in response to the volatiles from both pathogens, and decreased emission by each of the pathogens in response to Trichoderma volatiles. This suggests that this widespread volatile might play an important role in interspecific fungal communication. Furfural can be produced by plant-associated microbes (Jeleń and Wasowicz, 1998) and is known to be a potent fungicide of interest for crop protection in view of its lack of toxicity for human and environmental health (Zeitsch, 2000). This compound is known to inhibit the growth of Trichoderma species, F. oxysporum, and B. cinerea (Jung et al., 2007; El-Mougy et al., 2008; Sharip et al., 2016). The reduction in furfural abundance could then be a strategy to avoid overaccumulation of this toxic compound that could otherwise reach a threshold of toxicity for these organisms. Beyond furfural, two other compounds emitted by Trichoderma, (-)-aristolene (6831-16-9) and propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester (5265-77-4), responded in the same way to the exposure to the volatiles of both pathogens. (-)-Aristolene (CAS number: 6831-16-9) is a sesquiterpenoid that is found in plant essential oils with demonstrated antifungal activity (Juárez et al., 2016; Souza et al., 2016), while, to our knowledge, no information on the biological activity of propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester (CAS number: 25265-77-4) is available. Since these three compounds showed reduced abundance upon exposure and in view of the antifungal activity of two of them, we can speculate that the emitter's volatiles might have specifically triggered a response leading to reduced emission of compounds that would be harmful to their own development. However, since many more compounds beyond the three mentioned here were downregulated in the receiving organisms upon volatile perception, such decreased emission could also represent a hiding strategy from the receiver, or even an energy-saving strategy to reallocate resources on more essential activities upon sensing a prospective competitor. Consistently with this latter hypothesis, Trichoderma produced more siderophores and secreted them into a larger area around its colony after exposure to Fusarium volatiles, most likely to secure the scarce iron resources and gain a significant advantage for medium colonization over its competitor. Both Fusarium and Trichoderma are soil inhabitants, and it is, therefore, possible that they have co-evolved as natural competitors for iron. This would explain the presence of siderophore response to Fusarium volatiles and the absence of siderophore response to volatiles emitted from a pathogen of the aerial parts such as Botrytis.
In contrast to the abundant compounds showing decreased emission after volatile exposure, few compounds showed an increased emission in the dual microcosm setup, among which several are potential or known antimicrobial or antifungal compounds, such as 3-octanone, phenylethyl alcohol, and α-muurolene (CAS numbers: 106-68-3, 60-12-8, and 10208-80-7) (Zhu et al., 2011; Lin et al., 2012; Liu et al., 2014; Naik, 2018). We observed a higher number of compounds with increased emission in the single microcosm setup in which Trichoderma was exposed to total volatilomes dissolved in a solvent drop. This increase in the number of more abundant compounds could be the result of the instant release of highly concentrated volatiles instead of the continuous exposure to lower and changing concentrations we generated in the dual microcosm setup. This single microcosm setup would then trigger stronger stress and therefore a stronger reaction, in accordance with our hypothesis that overaccumulation of compound leads to different reactions, which are likely less representative than those observed in more natural conditions. Nevertheless, similar to the dual microcosm setup, several of the compounds that showed increased emission were antimicrobial or antifungal, such as 1-butanol-3-methyl-acetate, 2,5-dimethyl-pyrazine, acetophenone, or phenylethyl alcohol (CAS numbers 123-92-2, 123-32-0, 98-86-2, and 60-12-8) (Mo and Sung, 2007; Zhu et al., 2011; Ma et al., 2013; Ando et al., 2015; Janssens et al., 2019). This suggests that this setup could be used to detect and identify new molecules of interest or new applications for known molecules that are usually missed in drug-discovery projects on pure cultures since they need a triggering signal to be induced. In terms of putative signaling compounds, one category of volatiles detected in our new directional experimental setup is of particular interest, those which, although produced by the emitter strain located upstream, were no longer detected after the exposure. Several hypotheses can explain this absence, e.g., (i) they were absorbed by the culture media of the receiver strain, (ii) they were transformed into other compounds after a spontaneous (chemical) reaction with volatiles emitted by the receiver strain, as reported in a previous study (Kai et al., 2018), or (iii) they were absorbed by the receiver strain. In the latter case, these volatiles could have been metabolized and acted as a trigger causing the reactions described earlier. Once again, these “disappearing” emitter volatiles were specific according to the emitter strain but also to the receiver strain, which likely rules out a mere absorption into the medium of the receiver strain. There was a strong overlap in terms of these putative signaling volatiles in both microcosm setups we compared, which also favors a scenario where the receiver strains would absorb (and potentially metabolize) them rather than a scenario where they would interact with the receiver volatiles to form new compounds, especially since very few new compounds were observed and only one of them was commonly detected in both setups. To our knowledge, no information regarding the ability of these compounds to act as signal molecules is available in the literature, but future studies, using these identified putative signals as pure compounds and testing whether they induced phenotypic changes (such as the volatilome modulation or siderophore production), will bring a conclusive answer to this question. The siderophore experiment shows that the volatiles collected from our setup can induce the same reaction as the one observed when Trichoderma is incubated with Fusarium. It should then be possible to easily identify the compound(s) involved in the reaction since they most likely belong to the compounds absorbed by the Trichoderma upon exposure.
Our setup still has some limitations, the main one being due to its strict unilateral exposure, which prevents the reciprocal influence of both partners. Indeed, the compounds whose production has been increased in response to volatile exposure in the receiver strain are likely to also trigger an effect on the emitter strain. This latter reaction (resulting in an emitter's modified volatilome) could then lead to another reaction of the receiver, itself leading to further changes in a “chain reaction.” To address this issue, our setup could be used to expose a receiver fungus with the volatiles emitted by another fungus previously exposed to its volatiles, in search of differences compared with the exposure to volatiles emitted by a non-exposed fungus.
Beyond the fungus–fungus interaction example selected as proof of concept in this study, this new experimental setup could not only be used to gain insight into the interactions occurring between other microbes such as bacteria but also into interkingdom communication, such as volatile-mediated interactions between plants and microbes (Farré-Armengol et al., 2016; Bouwmeester et al., 2019). With minor adaptations, it would be, for example, possible to study the effects of pathogens' volatiles on small plants inoculated or not with beneficial microbes. This could help to reach a better understanding of microbe-mediated plant defense mechanisms and may lead to the identification of new compounds triggering effective plant protection against diseases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
SB, AA, and LW designed the research. SB and AA performed the experiments with help from FL'H. SB analyzed the data and wrote the manuscript with help from LW, AA, and FL'H. All authors contributed to the article and approved the submitted version.
Financial support from the Swiss National Science Foundation (grants 179310 and 207917 to LW) is gratefully acknowledged.
The authors are grateful to Dr. Pierre-Marie Allard and Emmanuel Defossez from the metabolomic platform of the University of Fribourg for their technical help with data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1128514/full#supplementary-material
Supplementary Table S1. List of compounds detected in gas chromatography for each organism (exposed or unexposed) used in this study with the dual microcosm setup. row m/z: largest mass-to-charge ratio of the feature; RI: Kovatz Retention Index; mean area: average of peak area of each sample (n = 7); compound: name of the compound (for compounds whose abundance has been significantly changed in this study).
Supplementary Table S2. Comparative list of compounds with significant modified abundance after exposure to phytopathogenic fungi using dual microcosm set-up (2M) or single microcosm set-up (1M). RI: Kovatz Retention Index; match factor: similarity (out of 1000) between the unknown spectrum and the NIST library closest spectrum; D: metabolites with down-regulated abundance; U: metabolites with up-regulated abundance; in reaction to volatiles emitted to B. cinerea (Bc), F. oxysporum (Fox) or T. simmonsii (Ts); crosses show the behavior of each volatile in response to the exposure.
Supplementary Figure S1. Trichoderma, Botrytis and Fusarium growth was evaluated 4 days after exposure to 3 drops of 50 μL methanol and compared to their control exposed to 3 drops of 50 μL sterile water (n = 3). (A) Representative pictures of each fungus exposed to water or methanol; (B) average of each surface covered by the mycelium of each fungus after 4 days of incubation. Student's t-test showed no significant difference between methanol and water exposure for each fungus.
Supplementary Figure S2. Effect of 3 drops of 50 μL of pure methanol on Trichoderma, Botrytis and Fusarium placed in a single microcosm set-up. Graphs show the Univariate Analysis Result for each metabolite between samples exposed to water and samples exposed to methanol (n = 4). Grey dots: metabolites showing no significant differences were observed.
Supplementary Figure S3. Representative pictures of top and bottom view of Trichoderma grown on CAS medium and exposed to recovered Fusarium volatiles solubilized in methanol for two weeks in a sandwich plate assay. The orange color shows the presence of siderophores secreted by Trichoderma n = 4. Control plates were exposed to PDA.
Alfiky, A., and Weisskopf, L. (2021). Deciphering Trichoderma–plant–pathogen interactions for better development of biocontrol applications. J. Fungi 7, 1–18. doi: 10.3390/jof7010061
Amin, F., Razdan, V. K., Mohiddin, F. A., Bhat, K. A., and Sheikh, P. A. (2010). Effect of volatile metabolites of Trichoderma species against seven fungal plant pathogens in-vitro. J. Phytol. 2, 34–37.
Ando, H., Kurata, A., and Kishimoto, N. (2015). Antimicrobial properties and mechanism of volatile isoamyl acetate, a main flavour component of Japanese sake (Ginjo-shu). J. Appl. Microbiol. 118, 873–880. doi: 10.1111/jam.12764
Barbieri, E., Gioacchini, A. M., Zambonelli, A., Bertini, L., and Stocchi, V. (2005). Determination of microbial volatile organic compounds from Staphylococcus pasteuri against Tuber borchii using solid-phase microextraction and gas chromatography/ion trap mass spectrometry. Rapid Commun. Mass Spectrometry 19, 3411–3415. doi: 10.1002/rcm.2209
Bouwmeester, H., Schuurink, R. C., Bleeker, P. M., and Schiestl, F. (2019). The role of volatiles in plant communication. Plant J. 100, 892–907. doi: 10.1111/tpj.14496
Bruisson, S., Berg, G., Garbeva, P., and Weisskopf, L. (2020). “Volatile Interplay Between Microbes: Friends and Foes,” in Bacterial Volatile Compounds as Mediators of Airborne Interactions (Singapore: Springer Singapore), 215–235. doi: 10.1007/978-981-15-7293-7_9
Bruisson, S., Zufferey, M., L'Haridon, F., Trutmann, E., Anand, A., Dutartre, A., et al. (2019). Endophytes and epiphytes from the grapevine leaf microbiome as potential biocontrol agents against phytopathogens. Front. Microbiol. 10, 2726. doi: 10.3389/fmicb.2019.02726
de Vrieze, M., Pandey, P., Bucheli, T. D., Varadarajan, A. R., Ahrens, C. H., Weisskopf, L., et al. (2015). Volatile organic compounds from native potato-associated Pseudomonas as potential anti-oomycete agents. Front. Microbiol. 6, e1295. doi: 10.3389/fmicb.2015.01295
Du, X., Smirnov, A., Pluskal, T., Jia, W., and Sumner, S. (2020). “Metabolomics data preprocessing using ADAP and MZmine 2,” in Methods in Molecular Biology, Vol. 2104 (Humana Press Inc), 25–48. doi: 10.1007/978-1-0716-0239-3_3
El-Mougy, N., El-Gamal, N., Mohamed, M., and Abdel-Kader, M. (2008). Furfural approaches as control measures against root rot and root-knot incidence of tomato under greenhouse and field conditions. J. Plant Protect. Res. 48, 93–105. doi: 10.2478/v10045-008-0010-0
Farh, M. E. A., and Jeon, J. (2020). Roles of fungal volatiles from perspective of distinct lifestyles in filamentous fungi. Plant Pathol J. 36, 193–203. doi: 10.5423/PPJ.RW.02.2020.0025
Farré-Armengol, G., Filella, I., Llusia, J., and Peñuelas, J. (2016). Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 21, 854–860. doi: 10.1016/j.tplants.2016.06.005
Groenhagen, U., Baumgartner, R., Bailly, A., Gardiner, A., Eberl, L., Schulz, S., et al. (2013). Production of bioactive volatiles by different burkholderia ambifaria strains. J. Chem. Ecol. 39, 892–906. doi: 10.1007/s10886-013-0315-y
Hunziker, L., Bönisch, D., Groenhagen, U., Bailly, A., Schulz, S., and Weisskopf, L. (2015). Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 81, 821–830. doi: 10.1128/AEM.02999-14
Janssens, T. K. S., Tyc, O., Besselink, H., de Boer, W., and Garbeva, P. (2019). Biological activities associated with the volatile compound 2,5-bis(1-methylethyl)-pyrazine. FEMS Microbiol. Lett. 366, 1–10. doi: 10.1093/femsle/fnz023
Jeleń, H., and Wasowicz, E. (1998). Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev. Int. 14, 391–426. doi: 10.1080/87559129809541170
Joo, J. H., and Hussein, K. A. (2022). Biological control and plant growth promotion properties of volatile organic compound-producing antagonistic Trichoderma spp. Front. Plant Sci. 13, 897668. doi: 10.3389/fpls.2022.897668
Juárez, Z. N., Bach, H., Sánchez-Arreola, E., Bach, H., and Hernández, L. R. (2016). Protective antifungal activity of essential oils extracted from Buddleja perfoliata and Pelargonium graveolens against fungi isolated from stored grains. J. Appl. Microbiol. 120, 1264–1270. doi: 10.1111/jam.13092
Jung, K.-H., Yoo, S. K., Moon, S.-K., and Lee, U.-S. (2007). Furfural from pine needle extract inhibits the growth of a plant pathogenic fungus, Alternaria mali. Mycobiology 35, 39. doi: 10.4489/MYCO.2007.35.1.039
Kai, M., Effmert, U., Lemfack, M. C., and Piechulla, B. (2018). Interspecific formation of the antimicrobial volatile schleiferon. Sci. Rep. 8, 1–6. doi: 10.1038/s41598-018-35341-3
Kottb, M., Gigolashvili, T., Großkinsky, D. K., and Piechulla, B. (2015). Trichoderma volatiles effecting Arabidopsis: from inhibition to protection against phytopathogenic fungi. Front. Microbiol. 6, 995. doi: 10.3389/fmicb.2015.00995
Lee, S., Yap, M., Behringer, G., Hung, R., and Bennett, J. W. (2016). Volatile organic compounds emitted by Trichoderma species mediate plant growth. Fungal Biol. Biotechnol 3, 1–14. doi: 10.1186/s40694-016-0025-7
Li, N., Alfiky, A., Wang, W., Islam, M., Nourollahi, K., Liu, X., et al. (2018). Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Front. Microbiol. 9, 1–15. doi: 10.3389/fmicb.2018.02614
Li, R.-X., Cai, F., Pang, G., Shen, Q.-R., Li, R., and Chen, W. (2015). Solubilisation of phosphate and micronutrients by Trichoderma harzianum and its relationship with the promotion of tomato plant growth. PLoS ONE 10, e0130081. doi: 10.1371/journal.pone.0130081
Lin, J., Dou, J., Xu, J., and Aisa, H. A. (2012). Chemical composition, antimicrobial and antitumor activities of the essential oils and crude extracts of Euphorbia macrorrhiza. Molecules 17, 5030–5039. doi: 10.3390/molecules17055030
Liu, P., Cheng, Y., Yang, M., Liu, Y., Chen, K., Long, C. A., et al. (2014). Mechanisms of action for 2-phenylethanol isolated from Kloeckera apiculata in control of Penicillium molds of citrus fruits. BMC Microbiol. 14, 242. doi: 10.1186/s12866-014-0242-2
Ma, Y. T., Fan, H. F., Gao, Y. Q., Li, H., Zhang, A. L., and Gao, J. M. (2013). Natural products as sources of new fungicides (I): synthesis and antifungal activity of acetophenone derivatives against phytopathogenic fungi. Chem. Biol. Drug Des. 81, 545–552. doi: 10.1111/cbdd.12064
Mo, E. K., and Sung, C. K. (2007). Phenylethyl alcohol (PEA) application slows fungal growth and maintains aroma in strawberry. Postharvest Biol. Technol. 45, 234–239. doi: 10.1016/j.postharvbio.2007.02.005
Naik, B. S. (2018). Volatile hydrocarbons from endophytic fungi and their efficacy in fuel production and disease control. Egypt J Biol Pest Control 28, 1–9. doi: 10.1186/s41938-018-0072-x
Rybakova, D., Müller, H., Olimi, E., Schaefer, A., Cernava, T., and Berg, G. (2022). To defend or to attack? Antagonistic interactions between Serratia plymuthica and fungal plant pathogens, a species-specific volatile dialogue. Front. Sustain. Food Syst. 6, 1020634. doi: 10.3389/fsufs.2022.1020634
Rybakova, D., Rack-Wetzlinger, U., Cernava, T., Schaefer, A., Schmuck, M., and Berg, G. (2017). Aerial warfare: a volatile dialogue between the plant pathogen Verticillium longisporum and its antagonist Paenibacillus polymyxa. Front. Plant Sci. 8, 1–10. doi: 10.3389/fpls.2017.01294
Sá, C., Matos, D., Cardoso, P., and Figueira, E. (2022). Do volatiles affect bacteria and plants in the same way? growth and biochemical response of non-stressed and cd-stressed Arabidopsis thaliana and Rhizobium E20-8. Antioxidants 11, 2303. doi: 10.3390/antiox11112303
Saha, M., Sarkar, S., Sarkar, B., Sharma, B. K., Bhattacharjee, S., and Tribedi, P. (2016). Microbial siderophores and their potential applications: a review. Environ. Sci. Pollut. Res. 23, 3984–3999. doi: 10.1007/s11356-015-4294-0
Schmidt, R., Etalo, D. W., Jager, V., de, Gerards, S., Zweers, H., Boer, W., et al. (2016). Microbial small talk: volatiles in fungal-bacterial interactions. Front. Microbiol. 6, 1–12. doi: 10.3389/fmicb.2015.01495
Schulz, S., Fuhlendorff, J., and Reichenbach, H. (2004). Identification and synthesis of volatiles released by the myxobacterium Chondromyces crocatus. Tetrahedron. 60, 3863–3872. doi: 10.1016/j.tet.2004.03.005
Schulz-Bohm, K., Martín-Sánchez, L., and Garbeva, P. (2017). Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 8, 1–10. doi: 10.3389/fmicb.2017.02484
Schwyn, B., and Neilands, J. B. (1987). Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160, 47–56. doi: 10.1016/0003-2697(87)90612-9
Sharifi, R., Jeon, J. S., and Ryu, C. M. (2022). Belowground plant-microbe communications via volatile compounds. J. Exp. Bot. 73, 463–486. doi: 10.1093/jxb/erab465
Sharifi, R., Lee, S. M., and Ryu, C. M. (2018). Microbe-induced plant volatiles. New Phytol. 220, 684–691. doi: 10.1111/nph.14955
Sharifi, R., and Ryu, C. M. (2018). Revisiting bacterial volatile-mediated plant growth promotion: Lessons from the past and objectives for the future. Ann. Bot. 122, 349–358. doi: 10.1093/aob/mcy108
Sharip, N. S., Ariffin, H., Hassan, M. A., Nishida, H., and Shirai, Y. (2016). Characterization and application of bioactive compounds in oil palm mesocarp fiber superheated steam condensate as an antifungal agent. RSC Adv. 6, 84672–84683. doi: 10.1039/C6RA13292H
Souza, C. M. C., Junior, S. A. P., Moraes, T. D. S., Damasceno, J. L., Mendes, S. A., Dias, H. J., et al. (2016). Antifungal activity of plant-derived essential oils on Candida tropicalis planktonic and biofilms cells. Med. Mycol. 54, 515–523. doi: 10.1093/mmy/myw003
Tyc, O., de Jager, V. C. L., van den Berg, M., Gerards, S., Janssens, T. K. S., Zaagman, N., et al. (2017). Exploring bacterial interspecific interactions for discovery of novel antimicrobial compounds. Microb. Biotechnol. 10, 910–925. doi: 10.1111/1751-7915.12735
Weisskopf, L., Schulz, S., and Garbeva, P. (2021). Microbial volatile organic compounds in intra-kingdom and inter-kingdom interactions. Nat. Rev. Microbiol. 19, 391–404. doi: 10.1038/s41579-020-00508-1
Xu, J., and Ouyang, G. (2019). “Extraction | Solid-phase microextraction,” in Encyclopedia of Analytical Science (Amsterdam: Elsevier), 100–108. doi: 10.1016/B978-0-12-409547-2.14552-4
Zeitsch, K. J. (ed). (2000). “14. Applications of furfural,” in The Chemistry and Technology of Furfural and Its Many by-Products Sugar Series. (Elsevier), 98–103. doi: 10.1016/S0167-7675(00)80014-7
Zhang, F., Yang, X., Ran, W., and Shen, Q. (2014). Fusarium oxysporum induces the production of proteins and volatile organic compounds by Trichoderma harzianum T-E5. FEMS Microbiol. Lett. 359, 116–123. doi: 10.1111/1574-6968.12582
Keywords: volatile-mediated interaction, Trichoderma, Fusarium, Botrytis, microcosm, unilateral volatile exposure, GC-MS
Citation: Bruisson S, Alfiky A, L'Haridon F and Weisskopf L (2023) A new system to study directional volatile-mediated interactions reveals the ability of fungi to specifically react to other fungal volatiles. Front. Ecol. Evol. 11:1128514. doi: 10.3389/fevo.2023.1128514
Received: 20 December 2022; Accepted: 07 March 2023;
Published: 06 April 2023.
Edited by:
Dani Lucas-Barbosa, Research Institute of Organic Agriculture (FiBL), SwitzerlandReviewed by:
Chien-Jui Huang, National Chiayi University, TaiwanCopyright © 2023 Bruisson, Alfiky, L'Haridon and Weisskopf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sébastien Bruisson, c2ViYXN0aWVuLmJydWlzc29uQHVuaWZyLmNo; Laure Weisskopf, bGF1cmUud2Vpc3Nrb3BmQHVuaWZyLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.