
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 04 April 2023
Sec. Population, Community, and Ecosystem Dynamics
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1113823
This article is part of the Research TopicThe Effects of Climate Change and Anthropogenic Activities on Patterns, Structures and Functions of Terrestrial EcosystemsView all 18 articles
Introduction: Scutellaria baicalensis is rich in bioactive flavonoid, which are widely used in clinical therapy. Many environmental factors, such as water and temperature, affect gene expression and secondary metabolites accumulation in plants.
Methods: In this study, to explore the effect of drought stress on the accumulation of flavonoids and gene expression in S. baicalensis seedlings, 4-week-old Scutellaria baicalensis seedlings were treated with different concentrations of PEG6000 to simulate drought stress. The contents of four root-specific flavones (baicalein, wogonin, baicalin, and wogonoside) in samples under different treatments were quantitatively analyzed by high performance liquid chromatography (HPLC). The expression levels of flavonoid biosynthesis-related genes (PAL1, PAL2, CHS, and UBGAT) were determined by real-time quantitative PCR (qRT-PCR). Also, a correlation analysis between flavonoid contents and gene expression levels was made.
Results: The HPLC results revealed that 5 and 10% PEG6000 treatments significantly increased the content of four flavonoids, with 5% PEG 6000 treatment being the most beneficial to the flavonoids accumulation. The qRT-PCR results showed that PAL2 and CHS gene expressions differed significantly in different organs, while PAL1 and UBGAT had poor organ-specific. For genes in roots, the expression of PAL1 and UBGAT was the highest in 5% PEG6000 treatment, and PAL2 and CHS were the highest in 10% PEG6000 treatment. Compared with other concentrations of PEG6000, 5 and 10% PEG6000 were more advantageous for gene expression. Collectively, PEG6000 at a low concentration promoted the accumulation of flavonoids and the expression of related genes. Additionally, the correlation results demonstrated that PAL1, PAL2, CHS, and UBGAT genes in roots stimulated the formation and accumulation of the four flavonoids to varying degrees, while the exception of PAL2 gene expression in roots was negatively correlated with wogonin content.
Discussion: This study for the first time investigated the effect of drought stress on the downstream gene UBGAT in S.baicalensis seedlings as well as the correlation between gene expression and flavonoid content in S. baicalensis seedlings under drought stress, providing a new sight for studying the effects of drought stress on flavonoid accumulation and related gene expression in S. baicalensis.
Scutellaria baicalensis (Baikal skullcap), known locally as Huangqin, is a perennial herb native to East Asia (Vrabec et al., 2022). Its dried roots have been an important herb in traditional Chinese medicine for a long time (Wu et al., 2022; Zhou et al., 2022). It has significant therapeutic effects on a wide range of illnesses, including various cancers, inflammation, diabetic nephropathy, oxidative stress (Men et al., 2021; Zhou et al., 2021). Baicalein, wogonin, baicalin, and wogonoside are called as root-specific flavones of S. baicalensis (Zhao et al., 2018). There is a lack of 4′-hydroxy (4′-deoxyflavone) in ring B, which is mainly responsible for the pharmacological activities of S. baicalensis (Zhao et al., 2016). Baicalein has been shown to suppress lipopolysaccharide-induced acute lung injury, ameliorate osteoporosis, and exert an antitumor effect on cervical cancer (Cai et al., 2021; Luo et al., 2021; Jiang et al., 2022). It has been reported that wogonin inhibits cardiac hypertrophy and alleviates kidney tubular epithelial injury (Lei et al., 2021; Shi et al., 2021). Baicalin has been proven to have remarkable effects on various cancer treatments and cardiovascular, hepatic, and renal protection (Singh et al., 2021; Yang et al., 2021; Zhao et al., 2021). Wogonoside can also treat cancer and inflammation (Gu et al., 2021; Huang et al., 2021). Thus, the accumulation of root-specific flavones is an essential aspect of studying S. baicalensis.
Flavonoid synthesis and accumulation depend on the crucial enzymes in their synthesis pathway (Zhao et al., 2018; Wang S. et al., 2019). Studying crucial enzyme genes of the biosynthetic pathway can provide a theoretical basis for the molecular mechanism of active flavonoid accumulation. Flavonoids are usually synthesized through the phenylpropanoid pathway (Ahmed et al., 2021). Their biosynthetic routes are shown in Figure 1. Phenylalanine ammonia-lyase (PAL) catalyzes the first and rate-limiting step of the phenylpropanoid pathway, which provides precursors for a diversity of secondary metabolites, including flavonoids (Liu et al., 2006). It converts primary metabolism into secondary metabolism (Olsen et al., 2008). Chalcone synthase (CHS) is a crucial enzyme that catalyzes the first rate-limiting step of the flavonoid biosynthesis pathway (Wang et al., 2018). UDP-glucuronate (baicalein 7-O-glucuronosyltransferase, UBGAT) is responsible for catalyzing the formation of flavonoid glycosides. Nagashima analyzed the properties of UBGAT purified from cultured cells of S. baicalensis and catalyzed the reaction with various glycosides. The outcomes demonstrated that it only reacted with UDP-glucuronide, baicalein, and wogonin as substrates (Nagashima et al., 2000). Studies show that the flavonoid content of the planted S. baicalensis is lower than that of wild resources (Li et al., 2018; Tian et al., 2018). Therefore, exploring the method to improve the flavonoid content of cultivated medicinal materials is conducive to protecting the wild medicinal resources and preventing the wild resources from being excessively excavated. It has been proven crucial gene expression correlates with flavonoid accumulation (Ma et al., 2014). Accordingly, it is of practical significance for protecting medicinal plant resources and improving the quality of herbs to explore active compound accumulation and crucial enzyme gene expression.
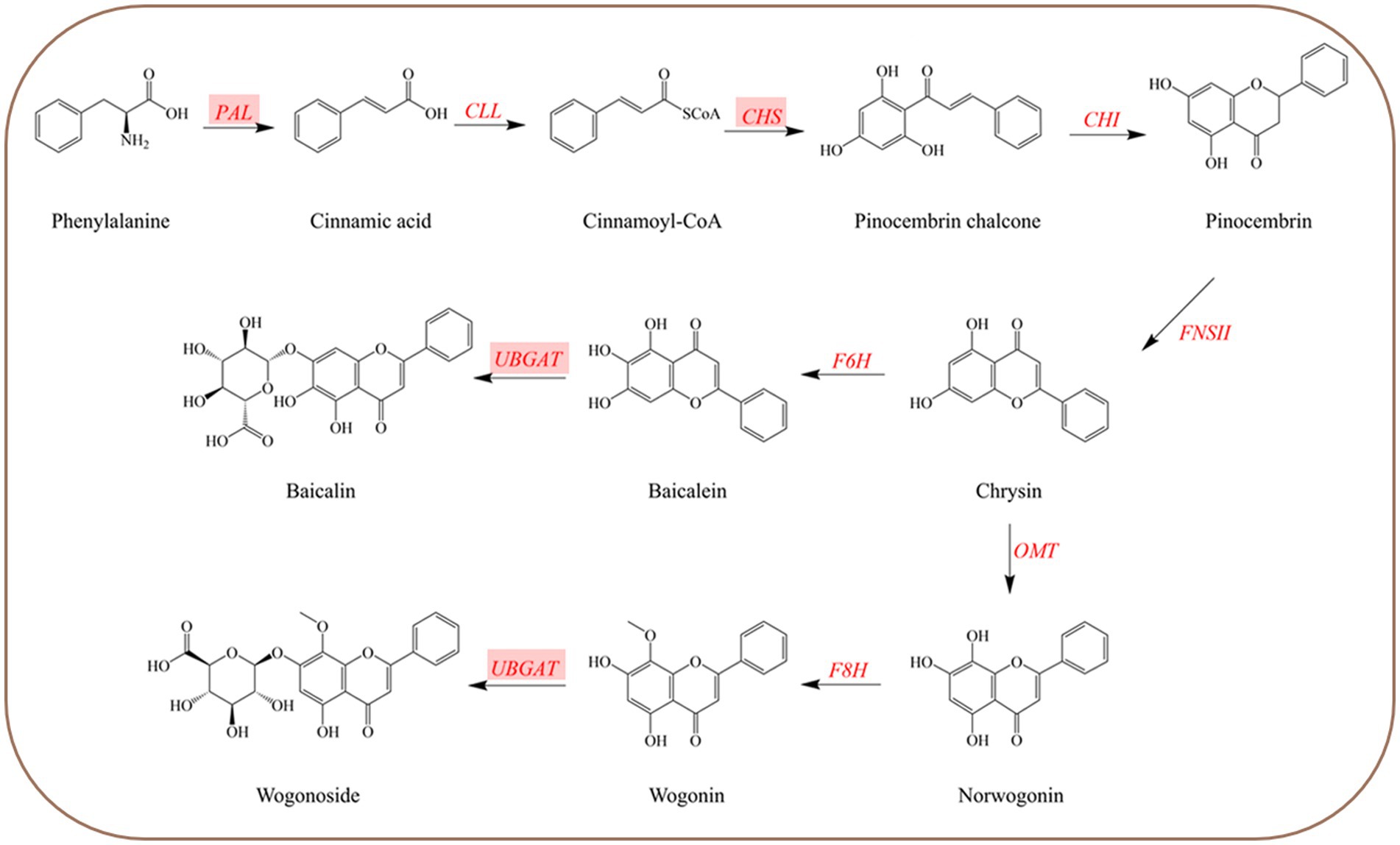
Figure 1. Biosynthetic pathways of four root-specific flavones (baicalin, baicalein, wogonin, and wogonoside) in Scutellaria baicalensis. Highlighted genes are the studied genes in this paper.
Abiotic factors are fundamental components of the environment that determine plant distribution and productivity (Zhang H. et al., 2022). In nature, plants are constantly challenged by adverse abiotic environmental conditions such as drought, heat, and excess salt levels in the soil, which negatively affect crop productivity (Xie et al., 2021; Mahecha et al., 2022; Paparella et al., 2022). The gene expression and bioactive component accumulation are affected by environmental stress (Xu et al., 2020; Jiang et al., 2021; Xu et al., 2021). Accumulating evidence has proven that appropriate drought stress has the potential to promote the production of bioactive metabolites like flavonoids (Jia et al., 2018; Jia and Wang, 2019; Yang et al., 2020; Zhang et al., 2020). Thus, comprehending how plants respond to abiotic stresses and adverse environmental conditions is critical for protecting the ecology and evolution of living systems.
Polyethylene glycol (PEG) is composed of a large molecular size and is nontoxic (Chen et al., 2020). It can reduce the water potential of the surrounding environment of the cells, causing and cause drought (Wang et al., 2020). PEG6000 is often used to simulate drought stress treatments (Jian et al., 2022; Tang et al., 2022). Thus, this study proposed the hypothesis that appropriate drought treatment can promote the accumulation of flavonoids and gene expression in S. baicalensis seedlings. PEG6000 was used to treat seedlings to simulate drought stress. The accumulation of four bioactive root-specific flavones in roots during drought stress was determined by high performance liquid chromatography (HPLC). Simultaneously, the expression of four crucial genes related to flavonoid synthesis in the roots, stems, and leaves of S. baicalensis was determined by real-time quantitative PCR (qRT-PCR). And the effect of drought on the expression of related genes was comprehensively analyzed. This study would provide meaningful information on the accumulation of compounds in S. baicalensis and insights into the study of quality improvement under stressed conditions.
The seeds of S. baicalensis were collected from Inner Mongolia, China. Healthy seeds were selected for the pot experiment. They were sown in a 16.8 cm × 15.5 cm (height × upper diameter) pot and cultured in an artificial illumination incubator (25°C, 16 h of light, and 8 h of dark light).
Compounds wogonoside (CAS: 51059–44-0), baicalin (CAS: 21967–41-9), wogonin (CAS: 632–85-9), and baicalein (CAS: 491–67-8) were procured from Yuanye (Shanghai, China) and had purity >98%. Acetonitrile (CAS: 75–05-8, HPLC) and formic acid (CAS:6449-79-2, LC/MS) were acquired from Fischer Scientific (Hampton, NJ, United States). The remaining reagents were bought locally and were of analytical grade.
Four-week-old healthy seedlings were selected to be subjected to the follow-up treatment. There were six groups in each of the three replicates (three pots per replicate, 30 seedlings per pot). The six experimental groups were treated with 0, 5, 10, 15, 20, and 25% PEG 6000 under drought stress (Meher and Shivakrishna, 2018; Wang Z.Y. et al., 2019; Batool et al., 2022). The drought stress experiment lasted 5 days, and the solution was poured every other day for three times. Immediately after the experiment, the seedlings were frozen in liquid nitrogen and stored at −80°C.
Thirty seedlings of similar growth were selected per replicate for each treatment for content determination. Dried the roots of S. baicalensis at 55–60°C and rushed them (Zhang L.W. et al., 2022). The powder was precisely weighed to approximately 0.1 g and placed in a triangular flask. Then, flavonoids from S. baicalensis were extracted by the extraction method optimized by our research group. 1 mL of 50% ethanol was added. The sample was accurately weighed and extracted with an ultrasonic step for 23 min. The sample was then filtered through a 0.22 μm filter.
The content of flavonoids in S. baicalensis was determined by HPLC established by our research group. The mobile phase was acetonitrile (A) −0.1% formic acid aqueous solution (B). The gradient elution was used under the following conditions: 0.01–4 min, 10–20% A; 4–12 min, 20–22% A; 12–22 min, 22–24% A; 22–49 min, 24–28% A; 49–52 min, 28–35% A; 52–60 min, 35–45% A; 60–64 min, 45% ~ A; 64–70 min, 55–10% A; 70–85 min, 10% A. The volume flow rate was 0.8 mL/min. The detection wavelength was 274 nm. The injection volume was 10 μL. The column temperature was 30°C.
Total RNA was extracted from the roots, stems, and leaves of S. baicalensis according to TRNzol Universal Total RNA Extraction Reagent Operation Guide (Tiangen, Beijing, China). RNase-free water was used to dissolve the purified RNA. Using the extracted RNA as a template, cDNA was obtained by reverse transcription based on the instructions of the Evo M-MLV Mix Kit with gDNA Clean for qPCR kit (AGBio, Hunan, China) and carried out qRT-PCR following the instructions of the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) using an SYBR Green-based PCR assay. The information on primers used in this research is shown in Table 1. The expression levels of PAL1, PAL2, CHS, and UBGAT were determined using CT values and calculated using the 2–△△Ct method (Liu et al., 2019). Each qRT-PCR reaction technique was three replicates. Each experiment has three biological repetitions.
The HPLC results were monitored and analyzed through Lab Solutions 5.92 (Shimadzu Scientific Instruments INC.). GraphPad Prism 8.3.0 (GraphPad Software INC.) and Adobe Illustrator 2020 (Adobe INC.) were applied to mapping analyses. OriginPro 2021 (OriginLab Corporation.) and IBM SPSS® Statistics 25 (International Business Machines Corporation) were used in this research work for data evaluation. All the biological samples were three replicates.
The significance of the Levene variance homogeneity test was greater than the significance level of 0.05 (Table 2), so it can be considered that the variance between sample data is homogeneous. The result indicated that one-way ANOVA could be used. The significance of the F value for all four compounds was 0.000, which was lower than the significance level of 0.05 (Table 2). It demonstrated that drought stress significantly affected the accumulation of four flavonoids.
In the LSD test, the mean difference results of each group showed that different PEG6000 concentrations treatments could lead to significant differences in flavonoid content. There was no significant difference in baicalein between 15 and 25% PEG6000 groups, but there were significant differences among other groups. For baicalin, there was no significant difference between 15, 20, and 25% PEG6000, but there were significant differences between other groups. As to wogonin and wogonoside, different treatments resulted in significant differences among all groups.
The contents of baicalein, wogonin, baicalin, and wogonoside were shown in Figure 2. The concentrations of baicalein under drought stress of 10% PEG6000 and 15% PEG6000 were 8.124 and 7.502 mg/g, respectively, which increased by 20.44 and 11.22% compared with the control group (0% PEG6000). Compared with 0% PEG6000, baicalin in the treated group of 10% PEG6000 and 15% PEG6000 was 68.72 and 35.86% higher. Correspondingly, the content of wogonoside increased by 82.75 and 56.59%, respectively, under the two treatments. Compared with the control group, the 5% PEG6000 group increased the content of wogonin by 45.81%.
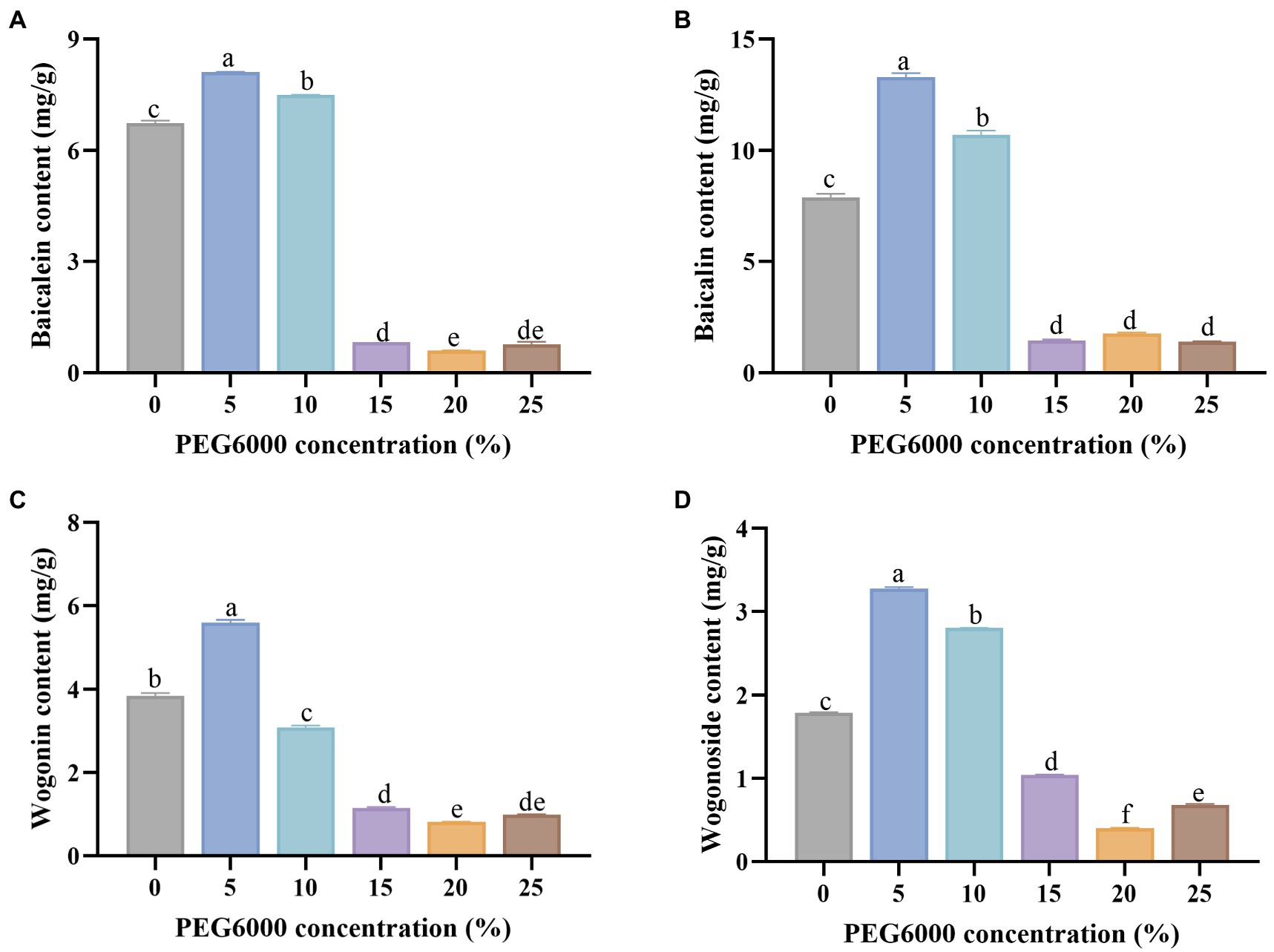
Figure 2. The contents of baicalein (A), baicalin (B), wogonin (C), and wogonoside (D) under different concentrations of PEG6000 solution treatment. Different letters indicate significant differences by t-test between different treatment (p < 0.05).
Under drought stress, the RNA of the samples was extracted and the RNA quality is shown in Supplementary Figure S1 and Supplementary Table S1. Then the expression of four crucial enzyme genes was detected in different organs of S. baicalensis (Figure 3). The effects of different organs of S. baicalensis and the concentration of PEG6000 on gene expression were analyzed by multivariate analysis of variance (Table 3). Results of tests of between-subjects effects revealed that the effects of different concentrations of PEG6000 on CHS, PAL1, PAL2, and UBGAT were significant, and the expression levels of CHS, PAL1, and PAL2 were significantly different in various organs of S. baicalensis.
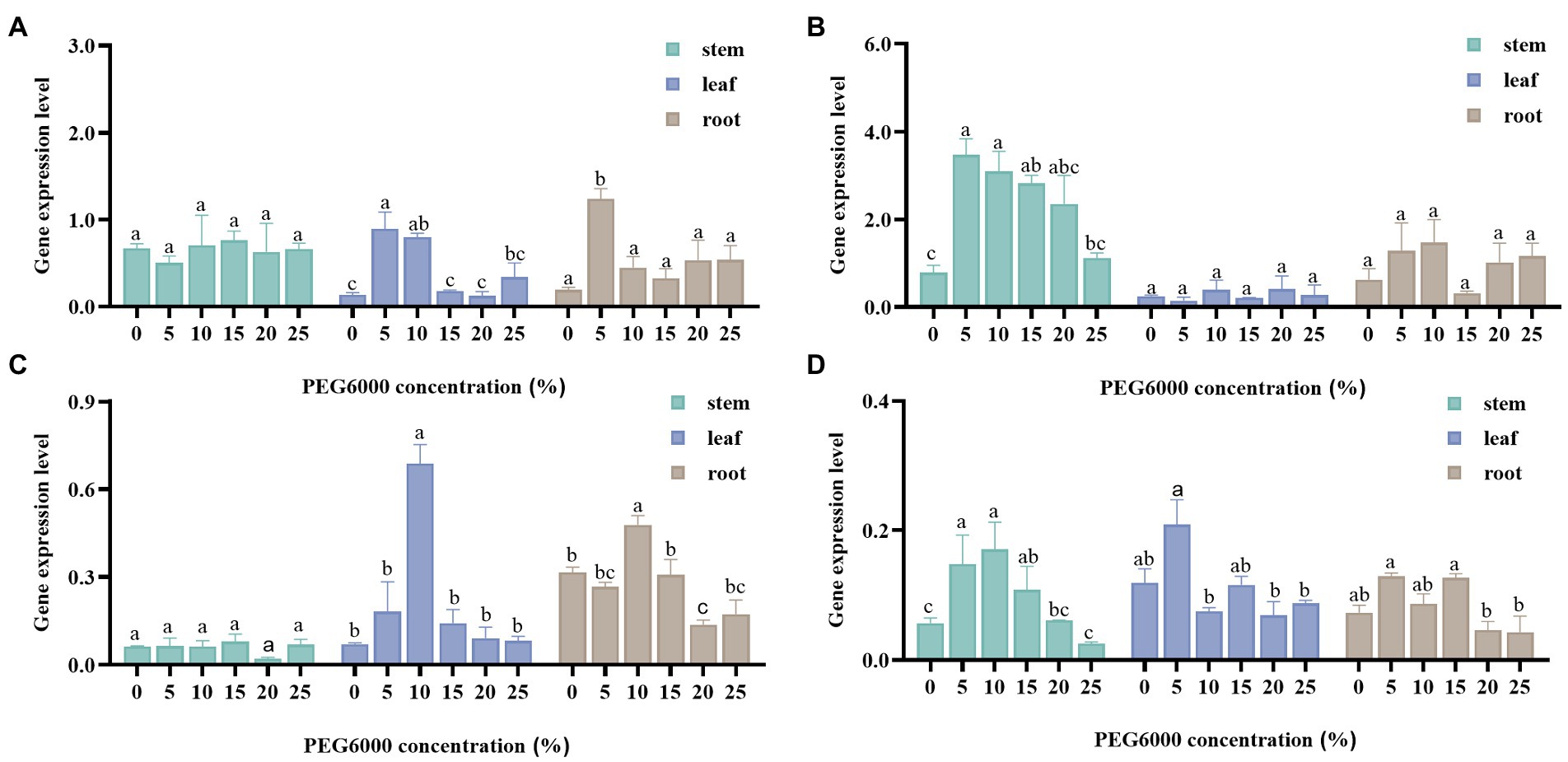
Figure 3. The results of the expression levels of PAL1 (A), PAL2 (B), CHS (C), and UBGAT (D) in stems, leaves, and roots under drought stress. Different letters indicate significant differences by t-test between different treatment (p < 0.05).
The LSD results suggested that the relative expression of CHS was significantly different from other treatments when treated with 10% PEG6000. Compared with other treatment groups (except 10%), the relative expression of PAL1 in the 5% PEG6000 treatment group was significantly different. Between the 5% PEG6000 treatment group and the other treatment concentration groups, the relative expression of UBGAT was considerably different. In the case of PAL2, the 10% PEG6000 treatment group was significantly different from the control group, as were the 15% PEG6000 and 25% PEG6000 treatment groups. In addition, the gene expression levels of genes in roots treated with different concentrations of PEG6000 were compared to explore the best treatment of four genes that affect the production of root-specific flavones. For PAL1 and UBGAT, gene expression was highest when 5% treatment was used.
Meanwhile, the highest expression of PAL1 and UBGAT genes was observed in the 5% treatment, and the highest expression of PAL2 and CHS genes was observed in the 10% treatment, suggesting that low concentration (5 and 10%) of PEG6000 treatment was preferable for the expression of the four crucial enzyme genes. To further explore the gene function, these two concentrations of PEG6000 can be selected to treat samples to increase gene expression.
Pearson correlation method was used to analyze the association between gene expression and flavonoid accumulation by OriginPro 2021 (Figure 4). The findings demonstrated that the amounts of the four flavonoid components were highly positively connected, which indicated that the accumulation patterns of the four flavonoid components were similar under the treatment of PEG6000.
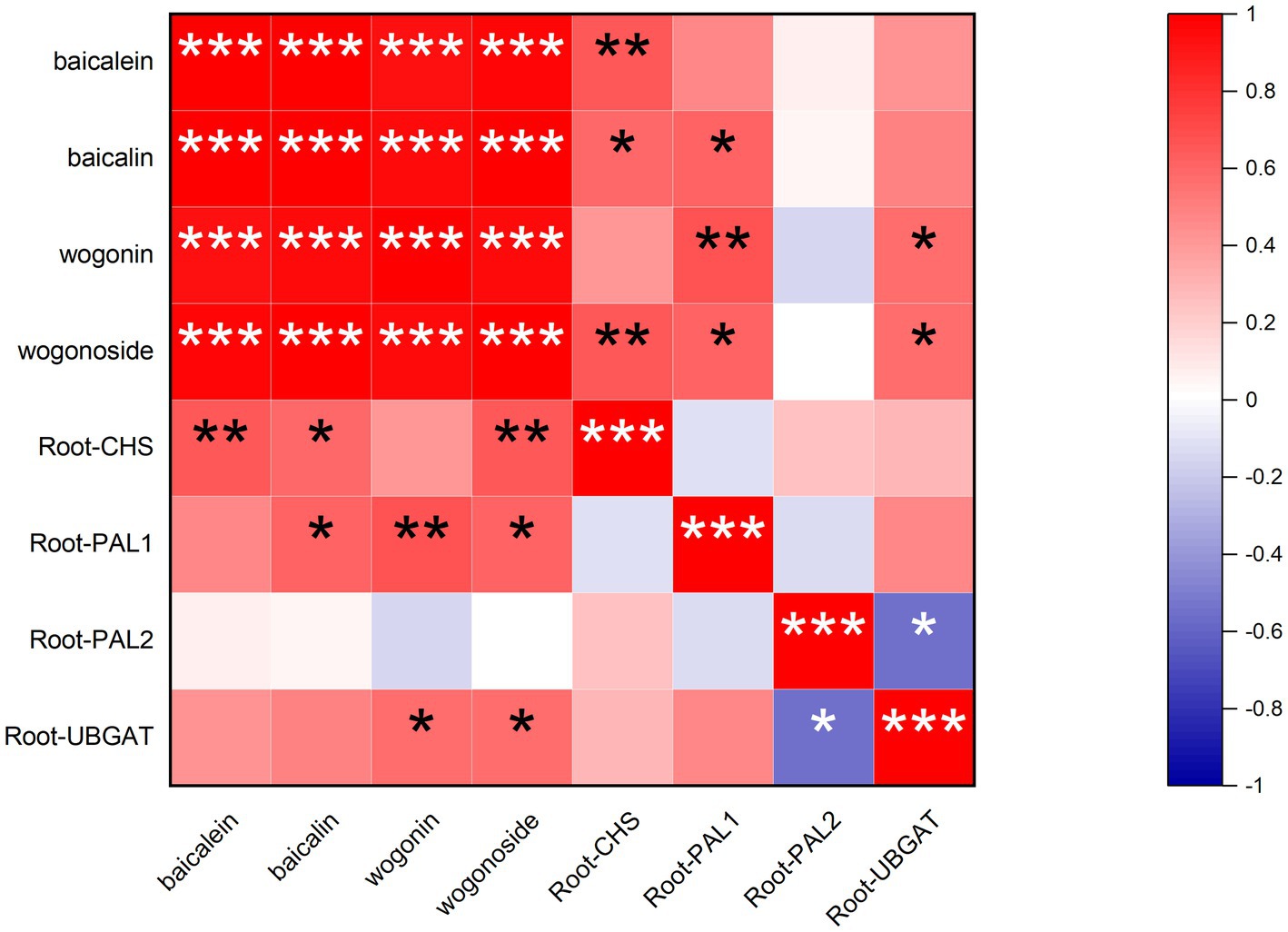
Figure 4. The results of correlation analysis between the indicators in this study. The correlation type is Pearson. *p < 0.05, **p < 0.01, and ***p < 0.001.
The expression of the CHS in roots was positively correlated with the content of baicalein, which indicated that the CHS could promote the accumulation of baicalein. The expression of CHS and PAL1 in roots was positively correlated with baicalin content, which indicated that these genes played a critical role in regulating the accumulation of baicalin content. Correlation analysis showed that PAL1 and UBGAT in roots were also essential regulators of wogonin accumulation. Moreover, PAL1, CHS, and UBGAT in roots had significant effects on the accumulation of wogonoside. The most crucial enzyme gene expression positively impacted the amount of flavonoid. Only PAL2 and wogonin have a negative correlation. It was speculated that PAL2 might inhibit the accumulation of wogonin.
Plants respond physiologically to environmental changes to maintain their individual or reproductive fitness. Flavonoid is a very significant class of secondary metabolites. Their synthesis and accumulation are related to the growing environment of plants, and they are involved in the process of plant resistance (Rodriguez-Calzada et al., 2019). They are essential protective substances for plants against the adverse external environment. Secondary metabolism is crucial for coping with environmental changes and maintaining plant homeostasis. Compared with primary metabolism, it is more susceptible to environmental changes (Yang et al., 2020). Previous studies have confirmed drought stress influenced flavonoid accumulation in S. baicalensis. The effects of PEG6000 stress on the accumulation of baicalin and baicalein in the suspension system of S. baicalensis were studied (Yang et al., 2011). Cheng et al., showed the accumulation of baicalin in the root of S. baicalensis under drought stress through controlled irrigation (Cheng et al., 2018). However, these studies only focused on the content of baicalin and baicalein. Wang et al., studied the PEG effect on the contents of baicalin, wogonoside, baicalein, and wogonin, while the object was suspension cells (Wang et al., 2020). Therefore, the response of four root-specific flavones in seedlings to drought stress was studied in this study. The results showed that proper drought stress could promote the accumulation of flavonoids in S. baicalensis. However, when the concentration of PEG6000 was increased, the content of flavonoids in S. baicalensis decreased significantly (Figure 2). This could be due to extreme drought reducing the plant’s metabolic capacity. The best concentration for accumulating root-specific flavones out of all those tested was 5%.
Many interconnected metabolic branches constitute the biosynthesis pathway of plant flavonoids, which involves multiple enzyme genes whose expression is highly dependent on tissue type and responses to internal or external signals (Sun et al., 2022). The synthesis and accumulation of flavonoids depend on crucial enzymes in its synthesis pathway (Ahmad et al., 2019). These enzymes are usually located at the bifurcation of the metabolic branch in the plant secondary metabolites synthesis pathway and can further determine the metabolic flow direction (Zhao et al., 2018; Wang S. et al., 2019). In this study, PEG6000 was used to treat S. baicalensis seedlings to explore how drought stress affected the related gene expression. The data showed that crucial enzyme genes in the flavonoid biosynthesis pathway responded to abiotic stress differently. The effects of progressive drought stress on the expression patterns of key enzymes upstream of baicalin biosynthesis were investigated during the vegetative period in 2-year-old S. baicalensis. And it was found that appropriate drought treatment resulted in increased gene expression, and over-treatment caused decreased expression (Cheng et al., 2018). This result is similar to our results, but it does not study the downstream key enzyme genes. Yang et al., studied the effects of PEG6000 stress on the expression of PAL, CHS, and UBGAT genes in the S. baicalensis suspension system. It was found that 10% PEG treatment of suspension cells significantly increased the expression of PAL and 20% PEG treatment significantly increased the expression of UBGAT, while there was no significant change in the expression of CHS, which was different from our results (Yang et al., 2011). In this paper, seedlings of S. baicalensis were used for the study. The newly discovered genes PAL1, PAL2, and UBGAT of S. baicalensis were reported. And the effect of drought stress on the downstream gene UBGAT in S.baicalensis seedlings was studied for the first time. The highest expression levels of four genes in roots were found at 5 and 10%, respectively, which indicated that these concentrations were more favorable for gene expression (Figure 3). These findings shed light on variations in the secondary metabolism of medicinal plants.
The biosynthesis of plant secondary metabolites is regulated by the spatial and temporal expression of biosynthesis genes (Lan et al., 2016). LSD results revealed significant differences in the relative expression levels of the PAL2 and CHS genes among organs. Expression results displayed that the expression level of PAL2 showed the highest gene expression level in stems, followed by the root and, finally, the leaf (Figures 3B,C). It may be because PAL is closely related not only to flavonoid synthesis but also to lignin formation. And the lignification of stems and roots was higher than that of leaves (Li et al., 2019). The expression of CHS in roots was the highest (Figure 3C). Generally, flavonoid biosynthesis genes were more strongly expressed in roots with higher flavonoid concentrations. As the medicinal organs, the taproots of Polygonum minus had a higher expression level of CHS (Roslan et al., 2012). The only organs in purple-fleshed sweet potato for anthocyanin biosynthesis are the purple tuberous roots, where CHS expression levels are significantly higher than in any other organ (Mano et al., 2007). However, the relative expression of the UBGAT gene differed significantly only between leaf and root, and the relative expression of the PAL1 gene only differed significantly between stem and leaf (Figures 3A,D). It means that the expression of these two genes was not organ-specific. PAL is the first rate-limiting enzyme in the metabolism of phenylpropanes. CHS is the first rate-limiting enzyme in flavonoid synthesis. Both catalyze the rate-limiting step and affect the speed and direction of the metabolic pathway. And UBGAT is responsible for synthesizing aglycone into glycoside. Therefore, this study selected these four genes as representatives to study the effects of drought stress. Moreover, there are other key enzymes (e.g. CLL, CHI, F8H, OMT, and FNSII) in the flavonoid biosynthesis pathway (Lu et al., 2022). Among them, CHI and OMT have been proven to be able to respond to drought stress in other species (Castellarin et al., 2007; Gharibi et al., 2019). Subsequent experiments may be able to carry out more extensive research on the genes related to flavonoid biosynthesis in S. baicalensis.
There is some previous information on the effect of drought on the flavonoids or genes of S. baicalensis. However, up to now, there is no study on the correlation between the four root-specific flavones and crucial gene expression in S. baicalensis seedlings under drought stress. Therefore, we studied the correlation for the first time and found that there was a positive correlation between gene expression and flavonoid content, except for the negative correlation between PAL2 and wogonin. And the correlation between the expression of CHS, PAL1, and UBGAT and flavonoid content was more significant (Figure 4). Therefore, it is suggested that CHS, PAL1, and UBGAT made a greater contribution to the drought-promoted flavonoid biosynthesis in S. baicalensis seedlings. These results showed that the increase in flavonoid content is related to the expression of the enzymes. Proving the relationship between flavonoids and gene expression may provide a theoretical basis for the gene function research. Given that 5% is the most suitable concentration for flavonoid accumulation and gene expression, subsequent experiments can use this as the optimal concentration to treat samples, and in-depth screen and verify gene function by multiple methods including comparing transcriptome and genetic engineering.
Altogether, this study found that drought stress had a significant effect on the accumulation of four flavonoids as well as the expression of four related genes in the medicinal and edible plant S. baicalensis. These findings deepen the understanding of the drought stress regulating function in S. baicalensis, which regulates the accumulation of flavonoids and gene expression. Overall, this research establishes a theoretical foundation for further exploration into the molecular mechanism of S. baicalensis in response to abiotic stresses, and it provides new insights into the metabolic regulation mechanism of S. baicalensis flavonoids.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/genbank/, HQ847728, AB008748, OP018668, OP018669, and OP018670.
PL contributed to the methodology and writing of the original draft. DJ and CL contributed to the project administration, supervision, and draft review. GR, FW, and JC were responsible for resource collection. All authors contributed to the article and approved the submitted version.
This work was supported by the project National Natural Science Foundation of China (81903740).
This work gratefully acknowledges the financial support provided by the project National Natural Science Foundation of China (81903740).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1113823/full#supplementary-material
Ahmad, N., Liu, J. Y., Tian, X., Noman, M., Jameel, A., Yao, N., et al. (2019). Overexpression of a novel cytochrome P450 promotes flavonoid biosynthesis and osmotic stress tolerance in transgenic Arabidopsis. Gene 10:756. doi: 10.3390/genes10100756
Ahmed, U., Rao, M. J., Qi, C., Xie, Q., Noushahi, H. A., Yaseen, M., et al. (2021). Expression profiling of flavonoid biosynthesis genes and secondary metabolites accumulation in Populus under drought stress. Molecules 26:5546. doi: 10.3390/molecules26185546
Batool, M., El-Badri, A. M., Wang, Z. K., Mohamed, I. A. A., Yang, H. Y., Ai, X. Y., et al. (2022). Rapeseed Morpho-physio-biochemical responses to drought stress induced by PEG-6000. Agronomy 12:579. doi: 10.3390/agronomy12030579
Cai, P., Lu, Y., Yin, Z. F., Wang, X. H., Zhou, X. X., and Li, Z. K. (2021). Baicalein ameliorates osteoporosis via AKT/FOXO1 signaling. Aging 13, 17370–17379. doi: 10.18632/aging.203227
Castellarin, S. D., Pfeiffer, A., Sivilotti, P., Degan, M., Peterlunger, E., and Di Gaspero, G. (2007). Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 30, 1381–1399. doi: 10.1111/j.1365-3040.2007.01716.x
Chen, Z. K., Xu, W. W., Nie, J., Khan, A., Cao, C. G., and Li, P. (2020). Drought stress intensity, duration and its resistance impact on rice (ORYZA sativa l.) seedling. Appl. Ecol. Environ. Res. 18, 469–486. doi: 10.15666/aeer/1801_469486
Cheng, L., Han, M., Yang, L. M., Yang, L., Sun, Z., and Zhang, T. (2018). Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crop. Prod. 122, 473–482. doi: 10.1016/j.indcrop.2018.06.030
Gharibi, S., Tabatabaei, B. E. S., Saeidi, G., Talebi, M., and Matkowski, A. (2019). The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.F. Phytochemistry 162, 90–98. doi: 10.1016/j.phytochem.2019.03.004
Gu, Q., Zhu, C. H., Wu, X., Peng, L. H., Huang, G. Y., and Hu, R. (2021). Wogonoside promotes apoptosis and ER stress in human gastric cancer cells by regulating the IRE1 alpha pathway. Exp. Ther. Med. 21:10. doi: 10.3892/etm.2021.9842
Huang, H., Li, X., Li, Y., Liu, L., Zhu, H. W., Cao, W., et al. (2021). Wogonoside inhibits TNF receptor-associated factor 6 (TRAF6) mediated-tumor microenvironment and prognosis of pancreatic cancer. Ann. Transl. Med. 9:15. doi: 10.21037/atm-21-4164
Jia, X., Sun, C. S., and Li, G. Y. (2018). Effect of drought stress on the growth and physiological characteristics and the accumulation of Astragaloside IV secondary metabolites of Astragalus membranaceus(Fisch.)var.mongholicus(Bge.)Hsiao. Acta Botan. Boreali-Occiden. Sin. 38, 501–509. doi: 10.7606/j.issn.1000-4025.2018.03.0501
Jia, X., and Wang, X. Q. (2019). Effects of salt and drought cross stress on growth and development and accumulation of Total flavonoids in Astragalus membranaceus var.mongholicus seedlings. J. Chin. Med. Mater. 42, 1215–1221. doi: 10.13863/j.issn1001-4454.2019.06.002
Jian, H. J., Sun, H. N., Liu, R. R., Zhang, W. Z., Shang, L. N., Wang, J. C., et al. (2022). Construction of drought stress regulation networks in potato based on SMRT and RNA sequencing data. BMC Plant Biol. 22:17. doi: 10.1186/s12870-022-03758-8
Jiang, D., Li, P., Yin, Y., Ren, G. X., and Liu, C. S. (2021). Molecular cloning and functional characterization of UGTs from Glycyrrhiza uralensis flavonoid pathway. Int. J. Biol. Macromol. 192, 1108–1116. doi: 10.1016/j.ijbiomac.2021.09.136
Jiang, C., Zhang, J. C., Xie, H. W., Guan, H. T., Li, R., Chen, C. X., et al. (2022). Baicalein suppresses lipopolysaccharide-induced acute lung injury by regulating Drp1-dependent mitochondrial fission of macrophages. Biomed. Pharmacother. 145:14. doi: 10.1016/j.biopha.2021.112408
Lan, X., Quan, H., Xia, X., Yin, W., and Zheng, W. (2016). Molecular cloning and transgenic characterization of the genes encoding chalcone synthase and chalcone isomerase from the Tibetan herbal plant Mirabilis himalaica. Biotechnol. Appl. Biochem. 63, 419–426. doi: 10.1002/bab.1376
Lei, L., Zhao, J., Liu, X. Q., Chen, J., Qi, X. M., Xia, L. L., et al. (2021). Wogonin alleviates kidney tubular epithelial injury in diabetic nephropathy by inhibiting PI3K/ Akt/NF-kappa B signaling pathways. Drug Design Dev. Therap. 15, 3131–3150. doi: 10.2147/DDDT.S310882
Li, E., Liu, Y., Liu, Y., Jiang, S., and Wang, Y. (2018). Simultaneous determination of six components in Scutellaria baicalensisGeorgi from different districts by HPLC. Chin. J. Hosp. Pharm. 38, 946–948. doi: 10.13286/j.cnki.chinhosppharmacyj.2018.09.10
Li, G., Wang, H., Cheng, X., Su, X., Zhao, Y., Jiang, T., et al. (2019). Comparative genomic analysis of the PAL genes in five Rosaceae species and functional identification of Chinese white pear. PeerJ 7:e8064. doi: 10.7717/peerj.8064
Liu, F. R., Xie, L. F., Yao, Z. Y., Zhou, Y. L., Zhou, W. F., Wang, J. H., et al. (2019). Caragana korshinskii phenylalanine ammonialyase is up-regulated in the phenylpropanoid biosynthesis pathway in response to drought stress. Biotechnol. Biotechnol. Equip. 33, 842–854. doi: 10.1080/13102818.2019.1623718
Liu, R. R., Xu, S. H., Li, J. L., Hu, Y. L., and Lin, Z. P. (2006). Expression profile of a PAL gene from Astragalus membranaceus var. Mongholicus and its crucial role in flux into flavonoid biosynthesis. Plant Cell Rep. 25, 705–710. doi: 10.1007/s00299-005-0072-7
Lu, Y. M., Cao, B., Su, Y. Y., Yang, J. J., Xue, Y., Zhang, M., et al. (2022). Inter-specific differences of medicinal bioactive products are correlated with differential expressions of key enzyme genes in Scutellaria baicalensis and Scutellaria viscidula. Ind. Crop. Prod. 189:13. doi: 10.1016/j.indcrop.2022.115758
Luo, Y. H., Wang, M. Y., Zhang, L., Jia, W. N., Wengu, E. Z., Xia, J. Y., et al. (2021). Baicalein exerts antitumor effect in cervical cancer. Mater. Express 11, 1347–1353. doi: 10.1166/mex.2021.2042
Ma, D. Y., Sun, D. X., Wang, C. Y., Li, Y. G., and Guo, T. C. (2014). Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 80, 60–66. doi: 10.1016/j.plaphy.2014.03.024
Mahecha, M. D., Bastos, A., Bohn, F. J., Eisenhauer, N., Feilhauer, H., Hartmann, H., et al. (2022). Biodiversity loss and climate extremes — study the feedbacks. Nature 612, 30–32. doi: 10.1038/d41586-022-04152-y
Mano, H., Ogasawara, F., Sato, K., Higo, H., and Minobe, Y. (2007). Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweet potato. Plant Physiol. 143, 1252–1268. doi: 10.1104/pp.106.094425
Meher, P., and Shivakrishna, K. A. Reddy and D. M. Rao (2018). "Effect of PEG-6000 imposed drought stress on RNA content, relative water content (RWC), and chlorophyll content in peanut leaves and roots." Saudi J. Biol. Sci. 25, 285–289. doi: 10.1016/j.sjbs.2017.04.008
Men, L. H., Pi, Z. F., Hu, M. X., Liu, S., Liu, Z. Q., Song, F. R., et al. (2021). Serum metabolomics coupled with network pharmacology strategy to explore therapeutic effects of Scutellaria Baicalensis Georgi on diabetic nephropathy. Chin. J. Anal. Chem. 49:13.
Nagashima, S., Hirotani, M., and Yoshikawa, T. (2000). Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. Cell suspension cultures. Phytochemistry 53, 533–538. doi: 10.1016/S0031-9422(99)00593-2
Olsen, K. M., Lea, U. S., Slimestad, R., Verheul, M., and Lillo, C. (2008). Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J. Plant Physiol. 165, 1491–1499. doi: 10.1016/j.jplph.2007.11.005
Paparella, F., D’Agostino, D., and Burt, J. (2022). Long–term, basin–scale salinity impacts from desalination in the Arabian/Persian gulf. Sci. Rep. 12:20549. doi: 10.1038/s41598-022-25167-5
Rodriguez-Calzada, T., Qian, M. J., Strid, A., Neugart, S., Schreiner, M., Torres-Pacheco, I., et al. (2019). Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (capsicum annuurn L.). Plant Physiol. Biochem. 134, 94–102. doi: 10.1016/j.plaphy.2018.06.025
Roslan, N. D., Yusop, J. M., Baharum, S. N., Othman, R., Mohamed-Hussein, Z.-A., Ismail, I., et al. (2012). Flavonoid biosynthesis genes putatively identified in the aromatic plant Polygonum minus via expressed sequences tag (EST) analysis. Int. J. Mol. Sci. 13, 2692–2706. doi: 10.3390/ijms13032692
Shi, X. W., Zhang, B., Chu, Z. L., Han, B. J., Zhang, X. P., Huang, P., et al. (2021). Wogonin inhibits cardiac hypertrophy by activating Nrf-2-mediated antioxidant responses. Cardiovasc. Ther. 2021:13. doi: 10.1155/2021/9995342
Singh, S., Meena, A., and Luqman, S. (2021). Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 164:21. doi: 10.1016/j.phrs.2020.105387
Sun, G., Liu Jianyu, W., Found, Z. Q., Xintong, M., Xinyue, Z., Yingqi, H., et al. (2022). Correlation analysis between expression of CYP450s gene in Carthamus tinctorius and total flavonoids content in young leaves under stress. Chin. Tradit. Herb. Drug 53, 222–230. doi: 10.7501/j.issn.0253-2670.2022.01.025
Tang, D. F., Quan, C. Q., Lin, Y., Wei, K. H., Qin, S. S., Liang, Y., et al. (2022). Physio-morphological, biochemical and transcriptomic analyses provide insights into drought stress responses in Mesona chinensis Benth. Front. Plant Sci. 13:20. doi: 10.3389/fpls.2022.809723
Tian, T., Han, R., and Liang, Z. (2018). Study on HPLC fingerprint of Scutellaria baicalensis Georgi from different habitats and different years. J. Zhejiang Agri. Sci. 59, 370–376. doi: 10.16178/j.issn.0528-9017.20180304
Vrabec, R., Vokurkova, D., Tumova, L., and Cheel, J. (2022). Ex-vivo immune-stimulating activity of Scutellaria baicalensis and its major flavonoids on human immune cells. Rec. Nat. Products 16, 188–193. doi: 10.25135/rnp.264.21.02.1976
Wang, Z. Y., Pan, X., Meng, Z. J., Gao, Y., Dang, X. H., and Wang, J. (2019). Response of Chamecytisus palmensis to drought stress induced by polyethylene glycol during germination. J. Plant Nutr. 42, 2814–2823. doi: 10.1080/01904167.2019.1659335
Wang, S., Wang, R. S., Liu, T., Lv, C. G., Liang, J. W., Kang, C. Z., et al. (2019). CYP76B74 catalyzes the 3 -hydroxylation of Geranylhydroquinone in Shikonin biosynthesis. Plant Physiol. 179, 402–414. doi: 10.1104/pp.18.01056
Wang, Z. B., Yu, Q. B., Shen, W. X., El Mohtar, C. A., Zhao, X. C., and Gmitter, F. G. (2018). Functional study of CHS gene family members in citrus revealed a novel CHS gene affecting the production of flavonoids. BMC Plant Biol. 18:13. doi: 10.1186/s12870-018-1418-y
Wang, B., Zhang, T. X., Du, H. W., Zhao, Q., and Meng, X. C. (2020). Effect of PEG on secondary metabolites in suspension cells of Scutellaria baicalensis georgi. Acta Med. Mediterr. 36, 2307–2312. doi: 10.19193/0393-6384_2020_4_359
Wu, Q., Zhao, W. L., Chen, G. L., Yao, A. R., Yang, C., Zhao, Y., et al. (2022). Mechanistic study of endothelium independent vasodilation effects of wogonin. Indian J. Exp. Biol. 60, 34–40. doi: 10.56042/ijeb.v60i01.58773
Xie, H. Y., Li, M. R., Chen, Y. J., Zhou, Q. P., Liu, W. H., Liang, G. L., et al. (2021). Important physiological changes due to drought stress on oat. Front. Ecol. Evol. 9:14. doi: 10.3389/fevo.2021.644726
Xu, Z. C., Wang, M., Ren, T. T., Li, K. Y., Li, Y. Q., Marowa, P., et al. (2021). Comparative transcriptome analysis reveals the molecular mechanism of salt tolerance in Apocynum venetum. Plant Physiol. Biochem. 167, 816–830. doi: 10.1016/j.plaphy.2021.08.043
Xu, Z., Zhou, J., Ren, T., Du, H., Liu, H., Li, Y., et al. (2020). Salt stress decreases seedling growth and development but increases quercetin and kaempferol content inApocynum venetum. Plant Biol. 22, 813–821. doi: 10.1111/plb.13128
Yang, J. Y., Li, M., Zhang, C. L., and Liu, D. (2021). Pharmacological properties of baicalin on liver diseases: a narrative review. Pharmacol. Rep. 73, 1230–1239. doi: 10.1007/s43440-021-00227-1
Yang, L. L., Yang, L., Yang, X., Zhang, T., Lan, Y. M., Zhao, Y., et al. (2020). Drought stress induces biosynthesis of flavonoids in leaves and saikosaponins in roots of Bupleurum chinense DC. Phytochemistry 177:112434. doi: 10.1016/j.phytochem.2020.112434
Yang, Z. C., Yuan, Y., Chen, M., Shuai, L. F., Xiao, Q., and Lin, S. F. (2011). Effects of PEG stress on flavonoids accumulation and related gene expression in suspension of Scutellaria baicalensis. Zhongguo Zhong Yao Za Zhi 36, 2157–2161. doi: 10.4268/cjcmm20111601
Zhang, Q. Q., Li, Y. G., Su, Y. L., and Chen, G. L. (2020). Accumulation of flavonoids in different organs of Astragalus membranaceus (Fisch.) Bge.Var.mongholicus (Bge.) Hsiao and Astragalus membranaceus (Fisch.) Bge seedlings under drought stress. Acta Botanica Boreali-Occidentalia Sinica. Acta Botan. Boreali-Occiden. Sin. 40, 1201–1208. doi: 10.7606/j.issn.1000-4025.2020.07.1201
Zhang, L. W., Zhang, X. M., Begum, N., Xia, P. G., Liu, J. L., and Liang, Z. S. (2022). Effects of different processing methods based on different drying conditions on the active ingredients of salvia miltiorrhiza Bunge. Molecules 27:4860. doi: 10.3390/molecules27154860
Zhang, H., Zhu, J., Gong, Z., and Zhu, J.-K. (2022). Abiotic stress responses in plants. Nat. Rev. Genet. 23, 104–119. doi: 10.1038/s41576-021-00413-0
Zhao, Q., Cui, M. Y., Levsh, O., Yang, D. F., Liu, J., Li, J., et al. (2018). Two CYP82D enzymes function as flavone hydroxylases in the biosynthesis of root-specific 4 '-Deoxyflavones in Scutellaria baicalensis. Mol. Plant 11, 135–148. doi: 10.1016/j.molp.2017.08.009
Zhao, Q., Zhang, Y., Wang, G., Hill, L., Weng, J. K., Chen, X. Y., et al. (2016). A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2:e1501780. doi: 10.1126/sciadv.1501780
Zhao, F. C., Zhao, Z. X., Han, Y. R., Li, S. J., Liu, C. L., and Jia, K. (2021). Baicalin suppresses lung cancer growth phenotypes via miR-340-5p/NET1 axis. Bioengineered 12, 1699–1707. doi: 10.1080/21655979.2021.1922052
Zhou, X., Fu, L., Wang, P. L., Yang, L., Zhu, X. S., and Li, C. G. (2021). Drug-herb interactions between Scutellaria baicalensis and pharmaceutical drugs: insights from experimental studies, mechanistic actions to clinical applications. Biomed. Pharmacother. 138:18. doi: 10.1016/j.biopha.2021.111445
Keywords: Scutellaria baicalensis, drought stress, gene expression, qRT-PCR, HPLC, flavonoids
Citation: Li P, Ren G, Wu F, Chen J, Jiang D and Liu C (2023) Root-specific flavones and critical enzyme genes involved in their synthesis changes due to drought stress on Scutellaria baicalensis. Front. Ecol. Evol. 11:1113823. doi: 10.3389/fevo.2023.1113823
Received: 13 January 2023; Accepted: 08 March 2023;
Published: 04 April 2023.
Edited by:
Wei Zhao, Institute of Geographic Sciences and Natural Resources Research (CAS), ChinaReviewed by:
Wei Song, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Li, Ren, Wu, Chen, Jiang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunsheng Liu, bWF4X2xpdWNzQDI2My5uZXQ=; Dan Jiang, amlhbmdkYW4xMDI3QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.