- 1Lushan Botanical Garden, Jiangxi Province and Chinese Academy of Sciences, Jiujiang, Jiangxi, China
- 2Terrestrial Ecology Unit, Department of Biology, Ghent University, Ghent, Belgium
- 3Department of Biology, Norwegian University of Science and Technology, Trondheim, Norway
Introduction: Plant chemical defenses can influence the distribution, community composition, and abundance of soil biota. Urbanization plays a key role in shaping soil biotic communities either directly through changes in soil properties or indirectly via changes in plant characteristics such as defense traits. The effects of urbanization and plant defenses on the abundance and structure of aboveground plant-associated communities have been studied, yet their effects on belowground root-associated communities are poorly understood.
Methods: Here we sampled white clover (Trifolium repens L.) leaves and roots along urban–rural gradients in the cities of Antwerp and Ghent, Belgium. We measured production of hydrogen cyanide (HCN) in leaves, a known defense trait against herbivores, and abundances of different feeding guilds of nematodes associated with the roots.
Results: We found that HCN production decreased with increasing levels of urbanization in both cities. Urbanization was significantly correlated with shifts in root-associated nematode community structure in Antwerp but not in Ghent. Responses of nematode feeding guilds and trophic groups to urbanization were highly dependent on the clovers’ HCN production, especially in Ghent. Changes in nematode channel ratio in Antwerp indicated that urban root-associated nematode communities of white clover were more strongly dominated by fungivorous nematodes.
Discussion: Our results demonstrate that urbanization is driving changes in a plant phenotypic trait and in the community structure of root-associated nematodes, as well as that both changes interact. Plant defense mechanisms could thus help elucidate the effects of urbanization on root-associated biota communities. As strong differences existed between the two studied cities, the particular properties of cities should be taken into account to better understand the direction and strength of phenotypic trait changes driven by urbanization.
1. Introduction
Urbanization has become a global driver of both environmental and biodiversity change due to the human population boom and city expansion worldwide (United Nations, 2019). Urbanization results in the replacement of natural habitats by impervious surface (McDonnell et al., 2009). Urban soils are thus dramatically altered, physically and chemically (Lehmann and Stahr, 2007). The composition and structure of the soil food web commonly aggregated in the rhizosphere are often directly affected by human-induced changes in soil properties (Geisen et al., 2019), such as agricultural intensification and urbanization (Pothula et al., 2019), and indirectly through changes in plant traits, including plant defenses (Hawlena and Zaguri, 2016). Both urbanization and plant defenses can influence aboveground communities (Miles et al., 2019; Qu et al., 2022), yet much less is known about their effects on soil biota.
Nematodes dominate the soil food web and function as useful indicators of soil condition regarding environmental disturbance (Yeates, 2003; Ferris, 2010). Specifically, nematodes occupy various trophic positions and have diverse feeding habits. They include bacterivores, fungivores, herbivores, omnivores and predators (Yeates et al., 1993). Several studies have demonstrated that nematodes are affected by urbanization, but not all functional groups are affected in the same way (Pothula et al., 2019; Li et al., 2022). Generally, urbanization decreases the overall abundance of nematodes. Abundances of omnivorous and predatory nematodes at higher trophic levels decrease in urbanized sites, while nematodes at lower trophic levels (i.e., herbivores, bacterivores, fungivores) often display opposite trends (Pavao-Zuckerman and Coleman, 2007). This may be attributed to differences in the sensitivity to environmental perturbances, with higher sensitivity of nematodes at higher trophic levels (Bongers and Ferris, 1999). In addition, nematode indices based on the presence and abundance of taxa and trophic groups are useful to evaluate the structure and function of the soil food web (Ferris et al., 2001). For example, the Nematode Channel Ratio (NCR) indicates the predominant decomposition pathway in the soil. The NCR varies from zero to one, with increasing values indicating a relative shift from bacterial to fungal decomposition (Ferris et al., 2001; Yeates, 2003). NCR values were reported to not change (Li et al., 2011) or decrease with increased urbanization levels (Pouyat et al., 1994; Pavao-Zuckerman and Coleman, 2007). However, the underlying mechanisms in relation to specific urban drivers behind these patterns remain unclear.
There are, so far, few studies linking patterns in soil communities to drivers associated with urbanization. Urban land use has profound effects on soil physiochemical properties. Herbivorous nematodes can benefit from urban soils that are sandy with lower clay content but also from rural soils with higher content of organic materials (Li et al., 2011). Besides, urban soils experienced highest heavy metal pollution compared to rural and suburban soils due to industrial, construction and agroforestry development in the city (Pouyat et al., 1994; Li et al., 2011; Ugarte and Taylor, 2020). Metal pollution immediately reduced total nematode abundance and the abundance of omnivores and carnivores but increased the NCR (Li et al., 2006; Pen-Mouratov et al., 2010; Šalamún et al., 2012). Moreover, soil management practices such as fertilization and tillage are key drivers of soil nematode community composition (Biswal, 2022; Li et al., 2022). Furthermore, the movement of nematodes strongly depends on soil water content (Freckman et al., 1987). Additionally, any management related to soil moisture can have important effects on nematode communities. Importantly, the key drivers of nematode abundance differ among trophic groups, with bacterivores and fungivores sensitive to soil characteristics, while plant feeders are strongly affected by vegetation traits (van den Hoogen et al., 2019). However, except for Francini et al. (2018), who documented effects of plant identity on abundance and composition of soil nematode communities within an urban context, few studies have investigated whether the effect of urbanization on soil nematode communities is related to changes in plant traits. Here, we studied the role of plant traits, more specifically secondary metabolites, in structuring root-associated nematode assemblages.
Plant secondary metabolites are recognized as a major defense mechanism against herbivores and therefore play a key role in shaping the structure of biotic communities (Bezemer and van Dam, 2005; van Dam and Heil, 2011). The rhizosphere community can be influenced by secondary metabolites released from root exudates and from leaf litter returned to the soil (Chomel et al., 2016; Tsunoda and van Dam, 2017; Zhang et al., 2019). These secondary metabolites can act as attractants, repellents or inhibitors for herbivorous nematodes (Abgrall et al., 2018; Sikder and Vestergård, 2020), and further influence the overall nematode community through trophic cascades. Plant metabolites also have strong influences on soil microorganisms and therefore on microbes and microbivorous nematodes and, consequently, indirectly on the omnivorous and predatory nematodes that feed on them (Tsunoda and van Dam, 2017). Generally, responses of nematode abundance and community structure to leaf litter highly depends on plant species identity and associated differences in litter quality (Cesarz et al., 2013). However, secondary metabolites in leaves showed variation among populations along urbanization gradients (Thompson et al., 2016; Moreira et al., 2018; Santangelo et al., 2020). Such variation can have an impact on belowground multi-trophic interactions (van Geem et al., 2016). We therefore expected that urbanization can indirectly influence nematode communities through its effects on plant traits.
White clover (Trifolium repens L., Fabaceae) is a cosmopolitan herb and can be found in nearly all temperate cities. It is capable of cyanogenesis, that is the ability to release hydrogen cyanide (HCN) upon tissue damage (Hughes, 1991). HCN not only defends against small generalist herbivores aboveground, including snails, slugs and insects (Burgess and Ennos, 1987; Thompson and Johnson, 2016) but also suppresses nematodes in the soil (Dutta et al., 2019). For example, incorporating white clover leaf material into soil caused a 45% reduction in severity of root galling by Meloidogyne hapla (Widmer and Abawi, 2002). Furthermore, there was a strong correlation between the concentration of HCN in sudangrass leaves incorporated into soil and the reduction of M. hapla root-galling severity (Widmer and Abawi, 2002). Recent work reported that urbanization has driven parallel clines of HCN frequency in white clover populations in 47% of cities across 160 cities globally, with 39% of cities showing a declined frequency of HCN in urban populations (Santangelo et al., 2022). Such urban–rural clines were unrelated to leaf herbivory at least in some cities (Thompson et al., 2016), but whether they were related to root-associated nematode communities is unknown.
In this study, we explored the relationships between root-associated nematode communities and plant secondary metabolites along urban–rural gradients in the cities of Antwerp and Ghent, Belgium. We determined whether the abundance and community composition of these nematodes changed with HCN frequency in white clover leaves and urbanization levels to address four hypotheses. As urbanization drives homogenization of the environment among distinct cities (Groffman et al., 2014) and the intensity of urbanization is positively related to city size (Bettencourt and West, 2010), and given the prevailing trend of declined HCN frequencies in urban areas worldwide (Santangelo et al., 2022), we hypothesize that (1) the HCN frequencies would decline with urbanization in the two cities, and that the strength of the decline would be stronger in the larger city (Antwerp). Considering that HCN possibly reduces litter quality with potential cascading effects across trophic groups, (2) HCN frequency would reduce the abundance of nematodes for all feeding types and trophic groups. Because of less-variable environmental conditions including impervious surface among urban populations within cities compared to rural populations (Santangelo et al., 2022), (3) we hypothesize a stronger negative effect of HCN in rural areas on root-associated nematode communities. Furthermore, (4) we expect the more disturbance-sensitive predatory and omnivorous nematodes to respond more strongly to urbanization.
2. Materials and methods
2.1. Study system
White clover (Trifolium repens) is a perennial herbaceous legume that is native to Eurasia and has been introduced to temperate regions globally as a nitrogen-fixing forage crop and soil stabilizer (Burdon, 1983; Kjærgaard, 2003). White clover is typically found in grazed or mowed pastures, lawns, meadows and roadsides where it can maintain large and dense populations (Burdon, 1983). In addition to sexual reproduction, T. repens reproduces clonally, with horizontal stems (i.e., stolons) that spread along the ground. A single clone can be up to 0.5 m in diameter (Burdon, 1983).
Cyanogenesis occurs as a discrete Mendelian polymorphism in white clover, with cyanogenic (HCN present) and acyanogenic (HCN absent) plants co-occurring in many natural populations (Daday, 1958). The molecular genetics of the cyanogenesis, controlled by two individual Mendelian genes, has recently been documented in detail (Olsen et al., 2007, 2013; Olsen and Small, 2018). Ac/ac determines the synthesis of cyanogenic glucosides (linamarin and lotaustralin) in the cell vacuole; Li/li determines the synthesis of hydrolysing enzyme (linamarase/β-glucosidase) in the cell wall. For both Ac/ac and Li/li, the dominant allele expresses the cyanogenic precursor. Therefore, plants with at least one functional allele at each locus are cyanogenic and produce HCN upon tissue damage, for example due to herbivore attacks. Both cyanogenic glucosides and linamarase are synthesized during shoot growth and stored in the mature leaves (Collinge and Hughes, 1982). Hence, the roots and seedlings before shoot emergence are not cyanogenic.
2.2. Study sites and sampling design
The study was conducted in two cities (Antwerp and Ghent) in Belgium in north-west Europe (Supplementary Figure S1). Belgium is a highly urbanized country (average human population density: 380.8 inhabitants/km2; Eurostat, 2021), and the landscape of both sites is composed of highly urbanized, agricultural, and seminatural areas.
From September to November 2018, we selected 40 white clover populations that were spaced at least 200 meters apart from each other along an urban–rural gradient within each city. For each population, we recorded the coordinates at the center using a hand-held GPS device (± 5 m accuracy) and collected 10 to 20 stolons that had three to four intact leaves. Stolons had a minimum distance of 1.5 m between each other to minimize the probability of collecting the same clonal genotype. Afterwards, roots were excavated at several locations and pooled to obtain a total fresh weight of up to 6 g. In total, we sampled stolons from 697 and 664 plants and roots from 24 and 27 populations in Antwerp and Ghent, respectively. Samples of stolons and roots (per population) were separately placed in plastic bags and preserved in a cooler with ice bags until being transported to a fridge at 4°C in the lab. Stolons were individually transferred to 2 ml microcentrifuge tubes and preserved at−80°C until HCN assay.
As urbanization involves many factors that characterize different physical environments, we used the proportion of built-up cover (%BUC) as a proxy for urbanization and BUC only included all buildings but not roads, parking infrastructure and pavements. This proxy was determined from digital maps in a GIS software for a nested series of spatial scales (50, 100, 200, 400, 800, 1,600, and 3,200 m radii, Supplementary Figure S1; Merckx et al., 2018) surrounding the center of each population and expressed as a continuous variable. Meanwhile, populations with BUC higher than 15% within 3,200 m surrounding each population were assigned to “urban,” otherwise to “rural.”
2.3. Cyanogenesis analysis
To assess the frequency of cyanogenesis in natural T. repens populations, we detected the absence/presence of HCN in each plant/stolon using Feigl-Anger assays based on a color change reaction (Feigl and Anger, 1996; Gleadow et al., 2011). We took samples out of the freezer, let them thaw, and put a single intact leaf (three 1-cm-wide leaflets) of each stolon in wells of 96-well plates. Subsequently, we added 80 μl of distilled water to each well and macerated tissue samples using pipette tips. The plates were covered with Feigl-Anger test paper and put in an incubator at 37°C for 3 h. Cyanogenic plants were indicated by a blue color.
2.4. Nematode analysis
Roots were separated from bulk soil and rinsed with tap water. If fresh roots were more than 3 g, they were divided into two portions and separately weighed to the nearest 0.001 g. One portion was used to extract nematodes, and another one to determine the relationship between dry and fresh root weights. Otherwise, all roots were used for nematode extraction. Nine out of 24 populations in Antwerp, and 20 out of 27 populations in Ghent, had extra roots for determining the dry weight. These roots were oven-dried at 50°C for 72 h and weighed to the nearest 0.001 g. For Antwerp, this relationship was fitted well by a linear regression (dry root weight = 0.018 + 0.1547* fresh root weight, R2 = 0.86, n = 9). For Ghent, among urban populations where estimates were needed, the best fitting relationship was dry root weight = 0.1743*1.3896fresh root weight (R2 = 0.62, n = 7). According to this relationship, we could predict dry weight of roots used for nematode extraction.
Nematodes were extracted using the Baermann funnel technique (Whitehead and Hemming, 1965). Extractions were collected three times with approximately 15 ml every day in three consecutive days and kept in a fridge at 4°C for 3 days until identification. Nematodes were counted and identified to each of five feeding guilds based on mouthpart, cuticle, and pharynx morphology (Yeates et al., 1993): bacterial-feeder (BF), fungal-feeder (FF), plant-feeder (PF), predator (PR) and omnivore (OM). A small proportion (less than 2% across the two cities) of nematodes was difficult to identify and categorized as unknown. Total abundance of nematodes in each population was the sum of the five feeding guilds and the unknown group. Nematode Channel Ratio is estimated by the ratio of bacterial-feeders to bacterial-feeders plus fungal-feeders, NCR = BF/(BF + FF), and has values between 0 (totally fungal-dominated) and 1 (totally bacterial-dominated) (Yeates, 2003). To depict trophic levels of the root-associated food web based on its nematode community, we defined PF as the first trophic-level (TL1), BF and FF as the second trophic-level (TL2), and PR and OM as the third trophic-level (TL3) (Peralta et al., 2020).
2.5. Statistics
We performed all statistical analyzes (Supplementary Figure S2) in R 3.6.3 (R Development Core Team, 2018).
2.5.1. Spatial scale of urbanization and general approach
We tested for each response variable at which spatial scale of urbanization the corresponding model explained most variation. We plotted R2 values from linear models (LMs) or PERMANOVA, and Nagelkerke’s pseudo-R2 from generalized linear models (GLMs) for each response variable (see below) against the seven scales (50–3,200 m radii) at which urbanization was evaluated (Supplementary Figures S3–S5). For each response variable analyzed both across and within the cities of Antwerp and Ghent, we decided the best scale with the highest R2 values for at least in two of these three analyzes. Afterwards, p values from either type-III Chi2 Wald tests or F tests for effects of independent variables were estimated by the function ‘Anova’ (‘car’ package) for GLMs and LMs, respectively.
2.5.2. Patterns of frequency of HCN
The frequency of HCN was calculated as the proportion of all tested plants that was cyanogenic within each population. To examine whether the proportion of HCN-producing plants varied with urbanization levels and across cities, we fitted a series of GLMs at each of the seven spatial scales, with city, urbanization level, and their interaction as predictors. A ‘quasibinomial’ distribution was used to account for overdispersion of binomial data.
2.5.3. Nematode abundance and nematode channel ratio
We first tested whether the influence of urbanization and HCN frequency on nematode abundance differed between the cities of Antwerp and Ghent, with an individual GLM for each of the seven scales. Nematodes were tested in terms of feeding type, trophic level, and total abundance. Predictors included city, urbanization level, HCN frequency, and all possible interactions. Dry weight of roots used for nematode extraction was included in the models as a covariate. A negative binomial distribution was used to account for overdispersion of the count data. Similarly, the influence of urbanization level, HCN frequency and their interaction on NCR was tested by LMs. Afterwards, to better illustrate the interactions among the city effect and those of other predictors, we examined how urbanization, HCN frequency and their interaction affected nematode abundance and NCR within each city, performing a similar series of GLMs and LMs, respectively, but leaving city out as a predictor. To meet the assumptions of normality of the model residuals, NCR was square transformed while analyzed across cities and within the city of Ghent.
2.5.4. Nematode community composition
To detect differences in feeding-guild composition of root-associated nematode community (expressed as abundance per gram of dry root weight) between the cities of Antwerp and Ghent, we conducted a permutational multivariate analysis of variance (PERMANOVA, 9,999 permutations) with city as only predictor, based on Bray–Curtis dissimilarity distances using the function ‘adonis2’ from the package ‘vegan’. We tested for multivariate homogeneity of dispersions via the function ‘betadisper’ (‘vegan’ package). Afterwards, to gain insights into the effects of urbanization and HCN frequency on nematode community composition within each city, a set of type II PERMANOVAs was conducted for each of the seven spatial scales. As the interactive effect between urbanization and HCN frequency was not significant for each city, only their main effects were retained in the final models. Finally, the patterns of the nematode community composition across cities and within each city were visualized using separate non-metric multi-dimensional scaling (NMDS) ordination, and urbanization and HCN frequency were overlaid on NMDS plots within each city via the function ‘envfit’ (‘vegan’ package).
3. Results
3.1. Variation in HCN frequencies along the urbanization gradients
The frequency of HCN in the two cities consistently and significantly decreased with increasing urbanization levels measured within a radius of 800 m (χ2 = 17.9, p < 0.001), but their slopes were not significantly different (χ2 = 1.78, p = 0.182; Figure 1; Supplementary Table S1). On average 24% of Antwerp clover plants versus 18% of plants in Ghent expressed HCN. The difference between intercepts of both cities was statistically significant (χ2 = 4.52, p = 0.034; Figure 1; Supplementary Table S1).
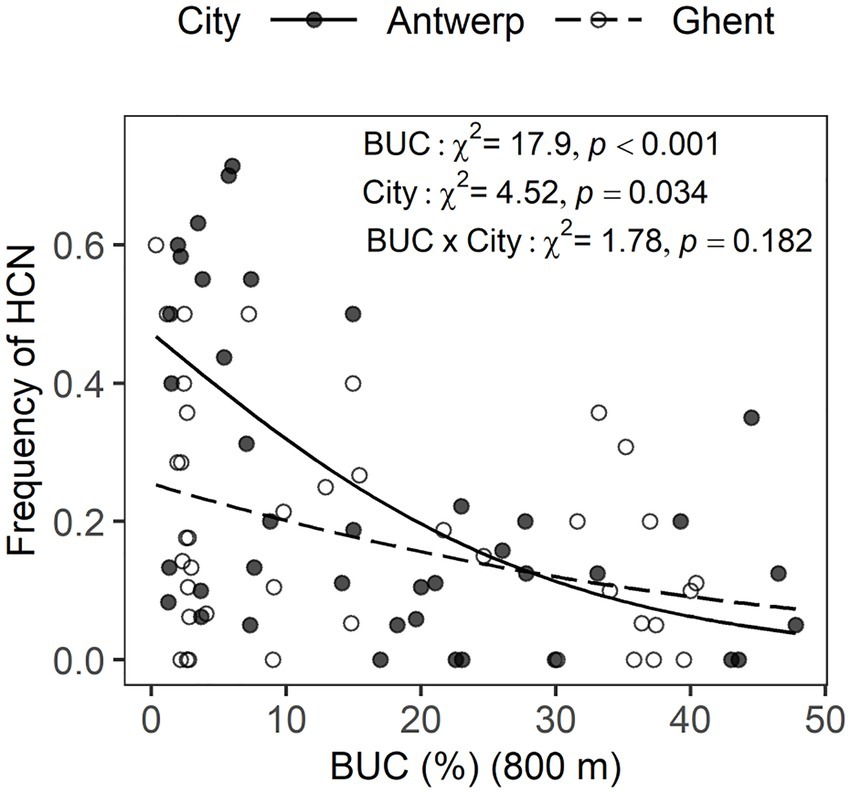
Figure 1. Frequency of HCN within populations of Trifolium repens along urbanization gradients (BUC) within a 800-meter radius in the cities (City) of Antwerp and Ghent. A quasibinomial generalized linear model was fitted and model R2 is 0.63. Number of sampled populations in each city is 40. For each predictor, χ2 and p value from type-III Chi2 Wald tests are shown on the plot, while estimates and standard errors are shown in Supplementary Table S1.
3.2. Characteristics of the root-associated nematode community
The main effect of city as well as its two-way and three-way interactions with urbanization and HCN frequency were significant for most variables, especially for root-feeding and total nematode abundance (Table 1), indicating that their patterns were specific to each city. In Antwerp, abundances of plant-feeding nematodes and total nematodes decreased with an increasing HCN frequency, and this decrease for plant feeders was only significant in locations at low urbanization levels (Figures 2A,B; Table 1).
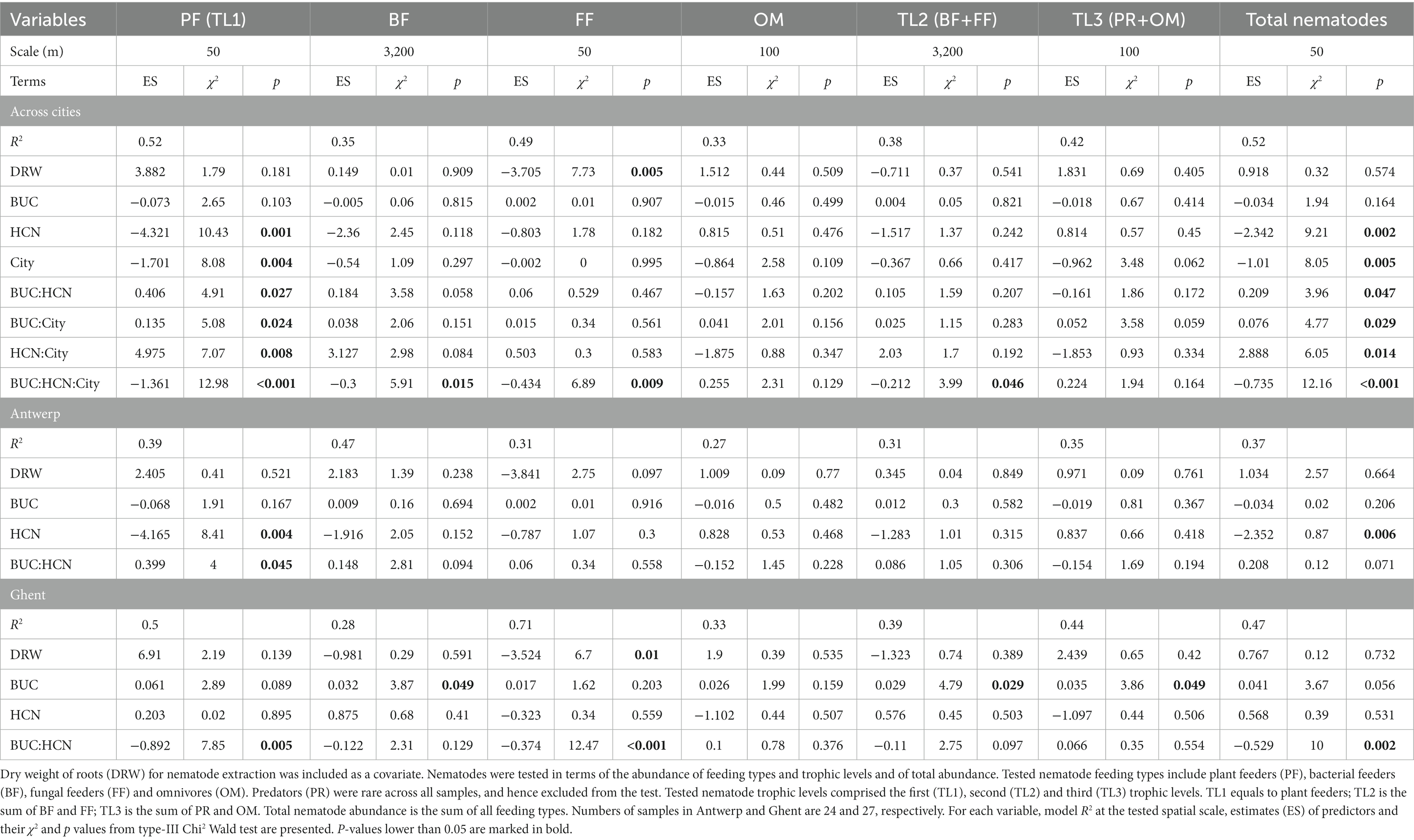
Table 1. Outputs of negative-binomial generalized linear models that test the effects of HCN frequency (HCN) and urbanization level (BUC) and their interaction on root-associated nematode abundance across and within the cities (City) of Antwerp and Ghent.
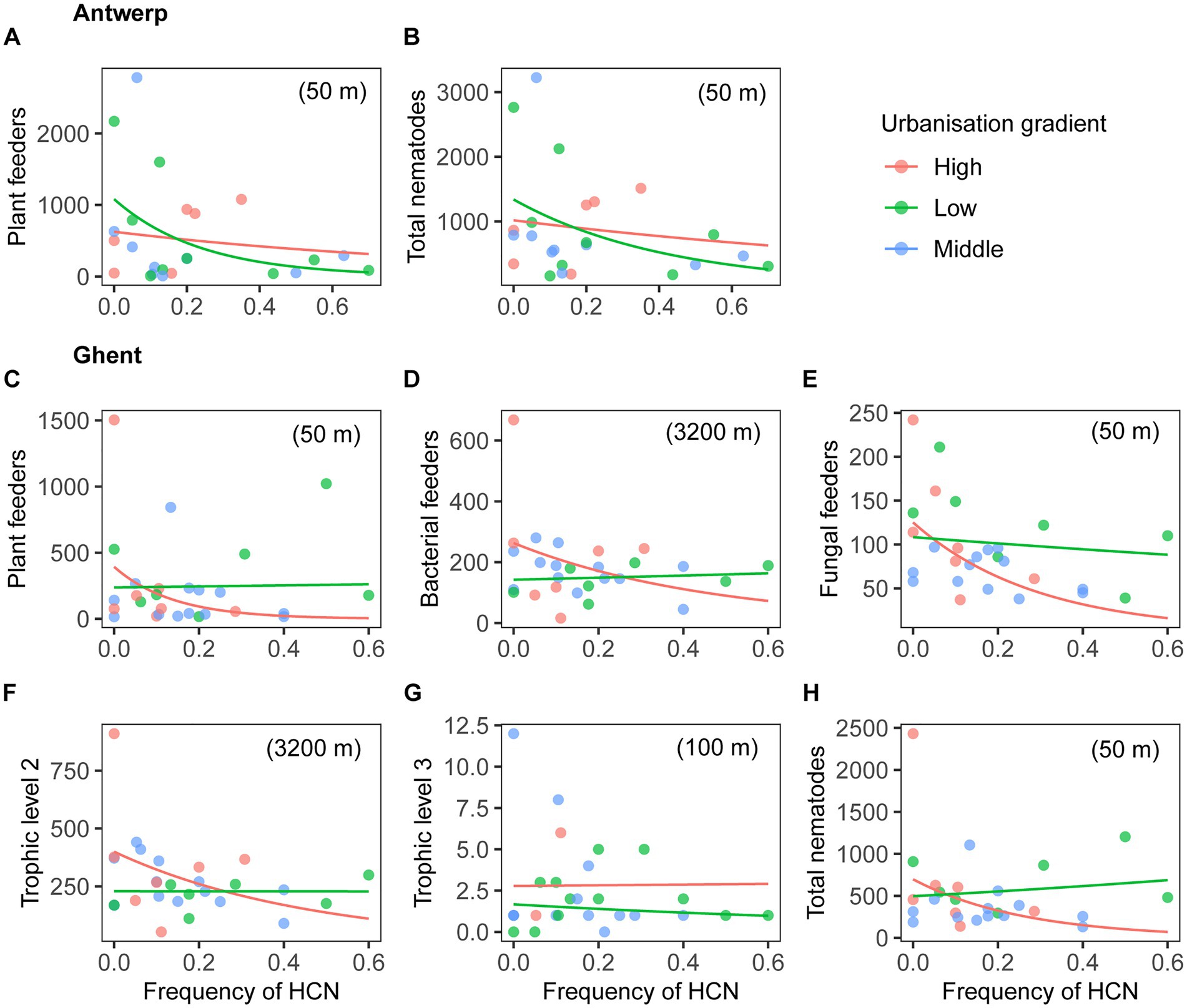
Figure 2. Root-associated nematode abundance as a function of urbanization gradient and frequency of HCN in the city of Antwerp (A,B) and Ghent (C–H). Negative binomial generalized linear models were fitted, with dry weight of roots for nematode extraction as a covariate. Nematode abundances were tested regarding feeding types, trophic levels and total nematodes. Trophic level 2 is the sum of bacterial and fungal feeders; Trophic level 3 is the sum of predators and omnivores. Nematode abundance of tested groups whose models showed significant effects of at least one factor at p < 0.05 (see the detailed results, Table 1) is plotted against frequency of HCN, for a mean value of root dry biomass. To illustrate the interaction effect of urbanization and frequency of HCN, nematode abundance at low (the first quartile value, green lines) and high (the third quartile value, red lines) levels of urbanization is predicted. Similarly, data points with urbanization values lower than the first quartile, between the first and third quartile, and higher than the third quartile are indicated in green, blue, and red, respectively. The scale of urbanization at which nematode abundance was best explained by urbanization and frequency of HCN is shown in between parentheses.
In Ghent, the abundance of PF (Figure 2C), FF (Figure 2E) and total nematodes (Figure 2H) showed similar responses to urbanization gradients and HCN frequencies (Table 1). Their abundance significantly decreased with increasing frequency of HCN in more urbanized locations, whereas it changed very little in less urbanized areas. However, abundance of BF (Figure 2D), TL2 (Figure 2F), and TL3 (Figure 2G) in general, did not vary significantly with HCN frequency but was greater at higher levels of urbanization (Table 1). Note that the patterns of BF and TL2, too, represented a decrease in abundance with increasing HCN frequency only in more urban sites, even though the interaction between the effects of urbanization and HCN frequency was not significant here. Except for FF across cities and within Ghent, dry weight of roots for nematode extraction did not affect nematode abundance (Table 1).
The trend of NCR along the urban–rural gradient depended on the city (Supplementary Table S2). In Antwerp, NCR significantly decreased with increased urbanization levels and was independent of HCN frequency (Figure 3A; Supplementary Table S2). In Ghent, neither urbanization level nor HCN frequency affected NCR among clover populations (Figure 3B; Supplementary Table S2).
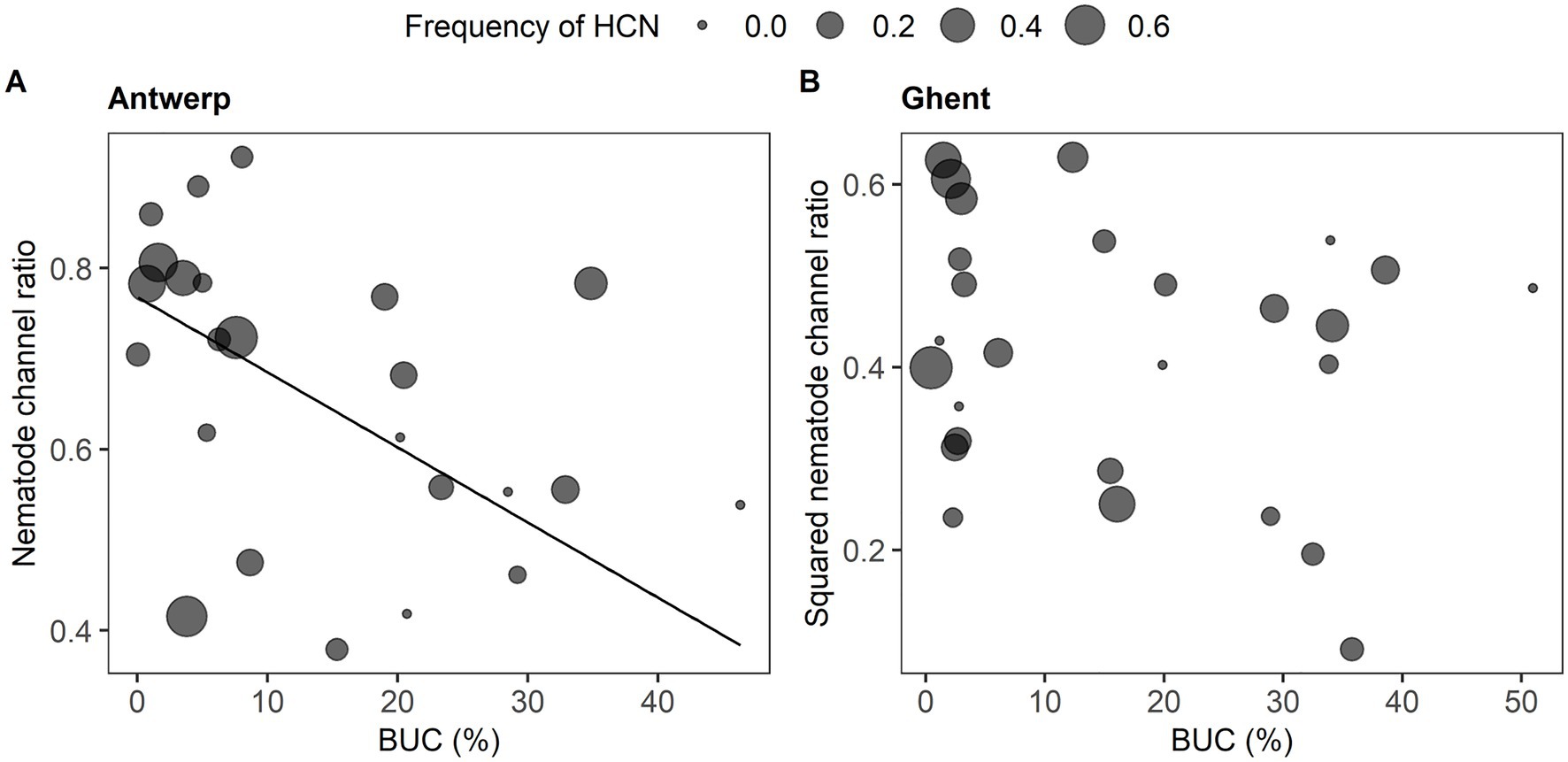
Figure 3. Linear relationship of nematode channel ratio with urbanization (BUC) within a 200-meter radius in the cities of Antwerp (A) and Ghent (B). The effects of predictors are presented in Supplementary Table S3. Nematode channel ratio is plotted against BUC values and the size of dots corresponds to the proportion of HCN-producing plants. The fitted line for the significant predictor of BUC at p < 0.05 is based on its modeled intercept and slope.
Nematode feeding guild composition in T. repens roots was significantly different between Antwerp and Ghent (PERMANOVA: pseudo-F1,49 = 2.49, p = 0.02, R2 = 0.05), with a marginally larger dispersion in the city of Antwerp (Betadisper: F1,49 = 3.06, p = 0.09; Figure 4A). Urbanization level (PERMANOVA: pseudo-F1,21 = 3.6, p = 0.03, R2 = 0.13) but not HCN frequency showed a significant effect on the feeding guild structure of the nematode community in Antwerp (Figure 4B), while neither of them had a significant effect on that in Ghent (Figure 4C; Supplementary Table S3).
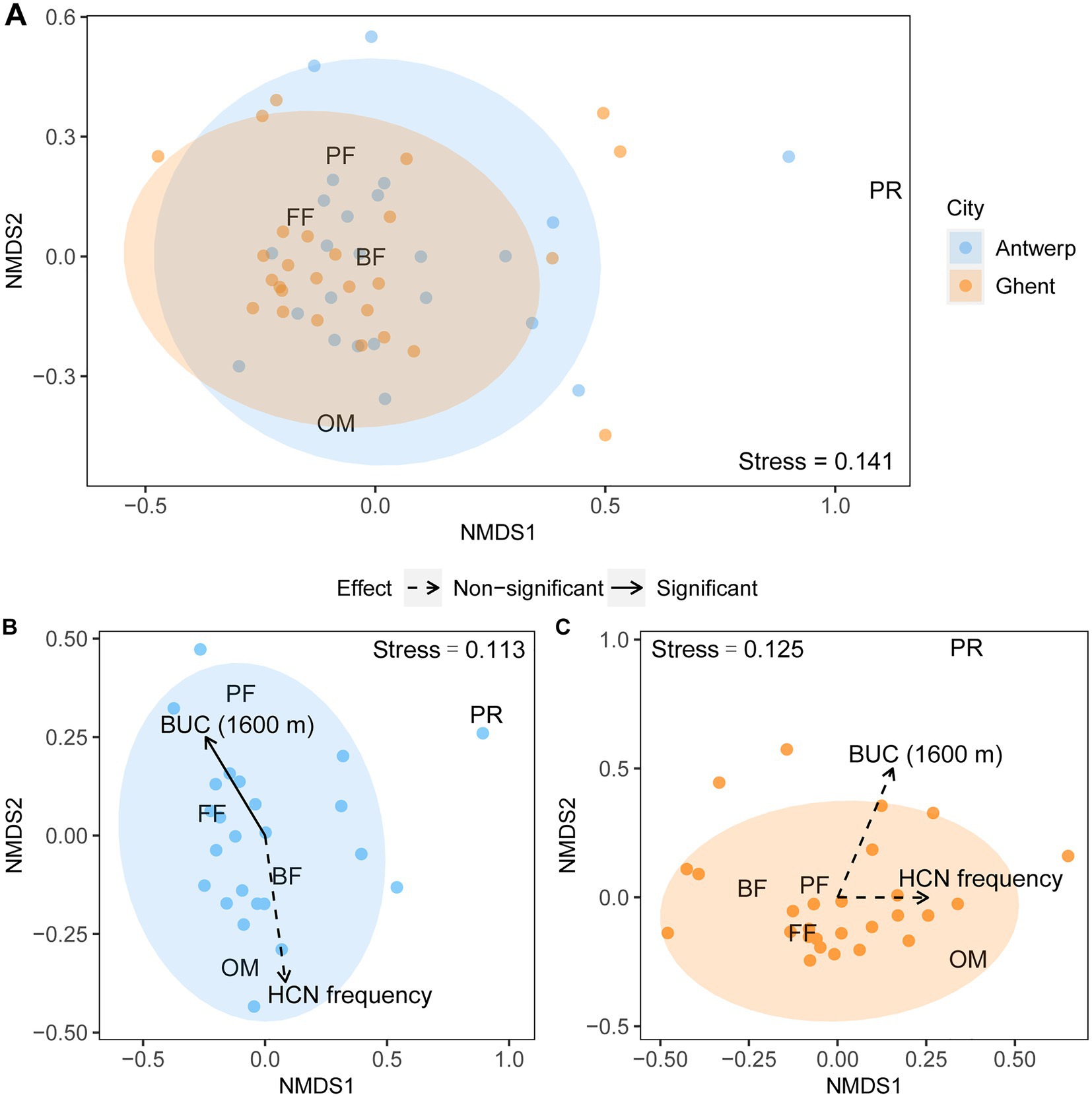
Figure 4. Nematode feeding-guild composition in the roots of Trifolium repens across the cities of Antwerp and Ghent and environmental factors for each of them. (A) Non-metric multidimensional scaling (NMDS) plot showing compositional difference of nematode feeding guilds between Antwerp and Ghent. Explanatory variables of urbanization gradient (BUC) within the radius of 1,600 meter and frequency of HCN production (HCN frequency) are superimposed for Antwerp (B) and Ghent (C). Sample size for Antwerp and Ghent is 24 and 27, respectively. Nematode feeding guilds are bacterial feeders (BF), fungal feeders (FF), plant feeders (PF), predators (PR) and omnivores (OM). Effects of both environmental variables on the nematode community composition were evaluated by PERMANOVA (Supplementary Table S3), and significant and non-significant effects are indicated in solid and dashed lines, respectively. The stress levels of NMDS 2D plots are shown.
4. Discussion
We assessed clines of HCN frequency in T. repens along urban-rural gradients in the cities of Antwerp and Ghent and compared the relationships among urbanization, HCN frequency, and the functional structure of nematode assemblages associated with the clover roots. As expected, HCN frequency decreased along a gradient of increasing urbanization in both cities. However, the decline in Antwerp, which is the more urbanized and larger, was not significantly stronger than that in Ghent. We mainly observed negative relationships between HCN frequency and nematodes from the most urbanized clover populations in Ghent yet from the less urbanized populations in Antwerp. Conversely, predators and omnivores together were more abundant in urbanized sites in Ghent. Urban soils in Antwerp were less bacterially dominated as indicated by a lower nematode channel ratio.
4.1. Patterns of clines in cyanogenesis
The consistent decrease in cyanogenesis in white clover populations along gradients from rural to urban environments in Antwerp and Ghent was in line with the trend in 47% of cities throughout the world (Santangelo et al., 2022), confirming that urbanization is driving the evolution of clover populations. Besides, Santangelo et al. (2022) concluded that drought stress and vegetation cover were the major environmental factors driving the strength of clines. Due to the urban-heat-island effect, urban areas at such temperate latitudes are often warmer and drier than rural sites throughout the year (Merckx et al., 2018). Considering that cyanogenesis functions as an antiherbivore defense, an alternative explanation is that herbivory may play a role in generating urban–rural clines in HCN. As a low frequency of HCN production was found in urban clover populations, these clover populations may either experience less herbivory, if herbivores control HCN production, or more herbivory, if herbivores select undefended plants. Although Thompson et al. (2016) did not establish a causal link between leaf herbivory and HCN along an urbanization gradient in the city of Toronto, they highlighted that the role of herbivory in driving HCN clines should be further investigated.
In contrast with our hypothesis, the parallel declines along rural–urban gradients in both cities were statistically equally steep. Environmental changes such as impervious surface along rural–urban gradients adopted by this study were more parallel (Santangelo et al., 2022). Given the proximity of Antwerp and Ghent, other local environments rather than impervious surface itself are more likely to regulate the strength of the observed HCN clines. Even though the extent of urbanization in Antwerp is larger than in Ghent, this difference was apparently not strong enough to cause a significant difference in micro-environments and hence in HCN clines.
4.2. Root-associated nematode community composition
We frequently observed interactions between urbanization and HCN frequency in their relationship with the abundance of different feeding groups of root-associated nematodes. This highlights the important roles of both plant defense and urbanization in the trophic structure of these nematode communities.
4.2.1. Urbanization effect
The effect of urbanization on the functional composition of root-associated nematode communities was highly city-specific in our study. City size determines most properties of the city, such as the extent of urbanization and the characteristics of local environments (Bettencourt et al., 2007; Uchida et al., 2021). The larger the city, the stronger the intensity of urbanization and the more complex the environmental heterogeneity. The feeding-guild composition of root-associated nematode communities was influenced by urbanization only in Antwerp. We speculate that this could be due to pollutants (Li et al., 2011), which are more common in relatively larger cities (Pavao-Zuckerman and Coleman, 2007). The shift in root-associated nematode community structure in Antwerp was possibly caused by the slightly larger dispersion of different feeding guilds, rather than the responses of individual feeding guilds to urbanization. Related environmental factors such as soil water content plays a key role in nematode assemblages (Amossé et al., 2016) but its role is highly guild-specific (Vandegehuchte et al., 2015). Besides, our findings of increased overall abundance of predators and omnivores (TL3) with increasing urbanization levels in Ghent indicate that urbanization can modify the functional composition of the root-associated food web through changes in abundances of trophic groups. This is contrary to our hypothesis (4) based on the general rule that nematodes at higher trophic levels are more sensitive to urban soils (Georgieva et al., 2002; Pavao-Zuckerman and Coleman, 2007; Li et al., 2011). A plausible explanation could be that these nematodes track the increased abundance of microbivorous nematodes (TL2) with urbanization, which are a main part of their diet. This would suggest that bottom-up forces outweigh any top-down disturbance effects on these higher tropic levels.
Urbanization had mixed effects on the nematode channel ratio. In Antwerp, root-associated food webs of white clover switched to a fungal-dominated decomposition system in more urbanized areas, which contrasts with studies reporting a less fungal dominance in urban soil food webs (Pouyat et al., 1994; Pavao-Zuckerman and Coleman, 2007). However, the nematode channel ratio did not vary across the urban–rural gradients in Ghent, which was in line with the work conducted by Li et al. (2011). Soils exposed to heavy industrial pollution have been shown to be dominated by bacterial-based decomposition processes (Pen-Mouratov et al., 2010), which suggests that urban soils in Antwerp did not experience heavy pollution, probably because industries were mainly close to ports of rural sites. In addition, frequent fertilization in agriculture and grassland ecosystems in rural areas can stimulate the growth of bacteria (Bittman et al., 2005) and, hence increase the number of bacterivorous nematodes (Hu and Qi, 2010; Pan et al., 2010), which could explain the higher NCR observed in less urbanized locations in Antwerp. Moreover, frequent and intensive mowing in more urbanized sites increases leaf litter in the soil and the resulted higher amount of decaying residues could support a higher fungal biomass and fungivorous nematodes (Nakamoto and Tsukamoto, 2006; Deguchi et al., 2007). Finally, unmeasured variables could have covaried differently with the urbanization gradients in Ghent and Antwerp, and resulted in the different responses of NCR to urbanization in both cities.
4.2.2. Plant defense
In line with our hypothesis (2), HCN frequency reduced the abundance of most of functional and trophic groups of nematodes in rural areas of Antwerp and in urban areas of Ghent, which suggests that the feeding-guild patterns of root-associated nematode communities could be partially attributed to plant chemical defense. HCN can have negative impacts on root feeders (Dutta et al., 2019). The components of cyanogenesis in white clover are also synthesized in shoots, but roots are not cyanogenic (Collinge and Hughes, 1982). As there is no evidence that HCN in shoots of white clover can be directly transported to roots, the possible effects of HCN on soil biota may occur when the defensive chemicals in the leaves of white clover could be transported into soil through degradation of litter. In this case, the decreased abundance of herbivores observed in both cities may have been the indirect consequence of such litter effects. We further speculate that in Ghent, the HCN-containing leaf litter may have decreased the abundance of bacteria and fungi in the rhizosphere, causing a decline in the abundance of bacterial and fungal feeders, or have directly affected these two nematode feeding types via fumigation with HCN (Dutta et al., 2019). Lower fungal and bacterial feeder abundances may in turn have caused predators and omnivores to increasingly feed on plant feeders. This hypothesis would need further experimental validation, and does not address the question why nematodes in Ghent seem to react most strongly to HCN in urban sites where the proportion of HCN-producing plants is lowest, in contrast with our hypothesis (3). Perhaps nematode communities become less sensitive to HCN through adaptation if it is returned to soil in larger amounts. Nevertheless, some evidence suggest that the quality of plant litter can drive belowground communities. For example, stem litter of the invasive Spartina alterniflora stimulated the growth of nematodes, particularly bacterivores, due to its higher quality than that of the native Phragmites australis (Chen et al., 2007). Besides, S. alterniflora that decayed faster had lower values of structure index and maturity index than P. australis, which means nematode communities around the former plants were dominated by colonizers with shorter longevity, smaller size and more sensitive to disturbance. This is in accordance with the observation that labile litter induced a more bacterially dominated energy channel than recalcitrant litter (Vauramo and Setälä, 2010). Therefore, the litter input with high quality may cause bacterial-based and active belowground biota communities. However, in Antwerp urban white clover with low HCN frequency and correspondingly high-quality litter harbored fungal-dominated communities. This is not in line with the idea that HCN is more antibacterial than antifungal, because the activity of the cyanide-inducible enzyme cyanide hydratase, which can detoxify HCN, appears to only occur in fungi not in bacteria (Fry and Evans, 1977; Wang et al., 1992). It thus seems that HCN frequency and NCR in Antwerp were driven by one or more unmeasured factors associated with urbanization.
5. Conclusion
This study showed that the relationship between urbanization and root-associated nematode feeding-guild composition was modified by the presence of a plant defense molecule, most likely via changes in the quality and palatability of leaf litter entering the soil system. Therefore, these results confirm that nematodes are good bioindicator of environmental changes. Although it is challenging to identify the specific urbanization drivers shifting nematode communities, we suggest that future studies incorporating plant traits and relevant environmental variables will contribute to interpreting the consequences of urbanization for soil communities and the ecosystem functions and services they provide.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JQ, DB, and MLV conceived the study and designed methodology. JQ collected and analyzed the data and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the China Scholarship Council (CSC, Grant no. 201606910051).
Acknowledgments
We are grateful to Pieter Vantieghem and Viki Vandomme for help with field sampling. We thank Femke Batsleer for urbanization quantification and creating the study map. We also thank Marc T. J. Johnson from University of Toronto Mississauga and the Global Urban Evolution project for sharing protocol for the determination of white clover (Trifolium repens) HCN cyanotype using Feigl-Anger Assay and for providing Feigl-Anger test papers.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1113671/full#supplementary-material
References
Abgrall, C., Forey, E., Mignot, L., and Chauvat, M. (2018). Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 127, 100–109. doi: 10.1016/j.soilbio.2018.09.016
Amossé, J., Dózsa-Farkas, K., Boros, G., Rochat, G., Sandoz, G., Fournier, B., et al. (2016). Patterns of earthworm, enchytraeid and nematode diversity and community structure in urban soils of different ages. Eur. J. Soil Biol. 73, 46–58. doi: 10.1016/j.ejsobi.2016.01.004
Bettencourt, L. M. A., Lobo, J., Helbing, D., Kühnert, C., and West, G. B. (2007). Growth, innovation, scaling, and the pace of life in cities. Proc. Natl. Acad. Sci. U. S. A. 104, 7301–7306. doi: 10.1073/pnas.0610172104
Bettencourt, L., and West, G. (2010). A unified theory of urban living. Nature 467, 912–913. doi: 10.1038/467912a
Bezemer, T. M., and van Dam, N. M. (2005). Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol. Evol. 20, 617–624. doi: 10.1016/j.tree.2005.08.006
Biswal, D. (2022). Nematodes as ghosts of land use past: elucidating the roles of soil nematode community studies as indicators of soil health and land management practices. Appl. Biochem. Biotechnol. 194, 2357–2417. doi: 10.1007/s12010-022-03808-9
Bittman, S., Forge, T. A., and Kowalenko, C. G. (2005). Responses of the bacterial and fungal biomass in a grassland soil to multi-year applications of dairy manure slurry and fertilizer. Soil Biol. Biochem. 37, 613–623. doi: 10.1016/j.soilbio.2004.07.038
Bongers, T., and Ferris, H. (1999). Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 14, 224–228. doi: 10.1016/S0169-5347(98)01583-3
Burgess, R. S. L., and Ennos, R. A. (1987). Selective grazing of acyanogenic white clover: variation in behaviour among populations of the slug Deroceras reticulatum. Oecologia 73, 432–435. doi: 10.1007/BF00385261
Cesarz, S., Ruess, L., Jacob, M., Jacob, A., Schaefer, M., and Scheu, S. (2013). Tree species diversity versus tree species identity: driving forces in structuring forest food webs as indicated by soil nematodes. Soil Biol. Biochem. 62, 36–45. doi: 10.1016/j.soilbio.2013.02.020
Chen, H. L., Li, B., Fang, C. M., Chen, J. K., and Wu, J. H. (2007). Exotic plant influences soil nematode communities through litter input. Soil Biol. Biochem. 39, 1782–1793. doi: 10.1016/j.soilbio.2007.02.011
Chomel, M., Guittonny-Larchevêque, M., Fernandez, C., Gallet, C., DesRochers, A., Paré, D., et al. (2016). Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J. Ecol. 104, 1527–1541. doi: 10.1111/1365-2745.12644
Collinge, D. B., and Hughes, M. A. (1982). Developmental and physiological studies on the cyanogenic glucosides of white clover, Trifolium repens L. J. Exp. Bot. 33, 154–161. doi: 10.1093/jxb/33.1.154
Daday, H. (1958). Gene frequencies in wild populations of Trifolium repens L. III. World distribution. Heredity 12, 169–184. doi: 10.1038/hdy.1958.22
Deguchi, S., Shimazaki, Y., Uozumi, S., Tawaraya, K., Kawamoto, H., and Tanaka, O. (2007). White clover living mulch increases the yield of silage corn via arbuscular mycorrhizal fungus colonization. Plant Soil 291, 291–299. doi: 10.1007/s11104-007-9194-8
Dutta, T. K., Khan, M. R., and Phani, V. (2019). Plant-parasitic nematode management via biofumigation using brassica and non-brassica plants: current status and future prospects. Curr. Plant Biol. 17, 17–32. doi: 10.1016/j.cpb.2019.02.001
Eurostat. (2021). Population Density by NUTS 2 Region. Available at: https://ec.europa.eu/eurostat/databrowser/view/tgs00024/default/table?lang=en (Accessed August 21, 2021).
Feigl, F., and Anger, V. (1996). Replacement of benzidine by copper ethylacetoacetate and tetra base as spot-test reagent for hydrogen cyanide and cyanogen. Analyst 91, 282–284. doi: 10.1039/AN9669100282
Ferris, H. (2010). Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 42, 63–67.
Ferris, H., Bongers, T., and de Goede, R. G. M. (2001). A framework for soil food web diagnostics: extension of the nematode faunal analysis concept. Appl. Soil Ecol. 18, 13–29. doi: 10.1016/S0929-1393(01)00152-4
Francini, G., Hui, N., Jumpponen, A., Kotze, D. J., Romantschuk, M., Allen, J. A., et al. (2018). Soil biota in boreal urban greenspace: responses to plant type and age. Soil Biol. Biochem. 118, 145–155. doi: 10.1016/j.soilbio.2017.11.019
Freckman, D. W., Whitford, W. G., and Steinberger, Y. (1987). Effect of irrigation on nematode population dynamics and activity in desert soils. Biol. Fertil. Soils 3-3, 3–10. doi: 10.1007/BF00260571
Fry, W. E., and Evans, P. H. (1977). Association of formamide hydro-lyase with fungal pathogenicity to cyanogenic plants. Phytopathology 77, 1001–1006. doi: 10.1094/phyto-67-1001
Geisen, S., Wall, D. H., and van der Putten, W. H. (2019). Challenges and opportunities for soil biodiversity in the anthropocene. Curr. Biol. 29, R1036–R1044. doi: 10.1016/J.CUB.2019.08.007
Georgieva, S. S., McGrath, S. P., Hooper, D. J., and Chambers, B. S. (2002). Nematode communities under stress: the long-term effects of heavy metals in soil treated with sewage sludge. Appl. Soil Ecol. 20, 27–42. doi: 10.1016/S0929-1393(02)00005-7
Gleadow, R. M., Bjarnholt, N., Jørgensen, K., Fox, J., and Miller, R. (2011). “Cyanogenic glycosides” in Research Methods in Plant Sciences Volume 1: Soil Allelochemicals. eds. S. S. Narwal, L. Szajdak, and D. A. Sampietro (Venice, CA: Stadium Press LLC), 283–310.
Groffman, P. M., Cavender-Bares, J., Bettez, N. D., Grove, J. M., Hall, S. J., Heffernan, J. B., et al. (2014). Ecological homogenization of urban USA. Front. Ecol. Environ. 12, 74–81. doi: 10.1890/120374
Hawlena, D., and Zaguri, M. (2016). Fear and below-ground food-webs. Soil Biol. Biochem. 102, 26–28. doi: 10.1016/j.soilbio.2016.06.019
Hu, C., and Qi, Y. C. (2010). Effect of compost and chemical fertilizer on soil nematode community in a Chinese maize field. Eur. J. Soil Biol. 46, 230–236. doi: 10.1016/j.ejsobi.2010.04.002
Hughes, M. A. (1991). The cyanogenic polymorphism in Trifolium repens L. (white clover). Heredity 66, 105–115. doi: 10.1038/hdy.1991.13
Kjærgaard, T. (2003). A plant that changed the world: the rise and fall of clover 1000-2000. Landsc. Res. 28, 41–49. doi: 10.1080/01426390306531
Lehmann, A., and Stahr, K. (2007). Nature and significance of anthropogenic urban soils. J. Soils Sediments 7, 247–260. doi: 10.1065/jss2007.06.235
Li, Q., Jiang, Y., and Liang, W. J. (2006). Effects of heavy metals on soil nematode communities in the vicinity of metallurgical factory. J. Entomol. Sci. 18, 323–328.
Li, X. P., Liu, T., Li, H. X., Geisen, S., Hu, F., and Liu, M. Q. (2022). Management effects on soil nematode abundance differ among functional groups and land-use types at a global scale. J. Anim. Ecol. 91, 1770–1780. doi: 10.1111/1365-2656.13744
Li, Q., Zhong, S., Li, F. P., Lou, Y. L., and Liang, W. J. (2011). Nematode community structure as bioindicator of soil heavy metal pollution along an urban-rural gradient in southern Shenyang, China. Int. J. Environ. Pollut. 45, 297–309. doi: 10.1504/IJEP.2011.040276
McDonnell, M. J., Hahs, A. K., and Breuste, J. H. (2009). Ecology of Cities and Towns: A Comparative Approach. Cambridge: Cambridge University Press.
Merckx, T., Souffreau, C., Kaiser, A., Baardsen, L. F., Backeljau, T., Bonte, D., et al. (2018). Body-size shifts in aquatic and terrestrial urban communities. Nature 558, 113–116. doi: 10.1038/s41586-018-0140-0
Miles, L. S., Breitbart, S. T., Wagner, H. H., and Johnson, M. T. J. (2019). Urbanization shapes the ecology and evolution of plant-arthropod herbivore interactions. Front. Ecol. Evol. 7:310. doi: 10.3389/fevo.2019.00310
Moreira, X., Abdala-Roberts, L., Berny Mier y Teran, J. C., Covelo, F., de la Mata, R., Francisco, M., et al. (2018). Impacts of urbanization on insect herbivory and plant defences in oak trees. Oikos 128, 113–123. doi: 10.1111/oik.05497
Nakamoto, T., and Tsukamoto, M. (2006). Abundance and activity of soil organisms in fields of maize grown with a white clover living mulch. Agric. Ecosyst. Environ. 115, 34–42. doi: 10.1016/j.agee.2005.12.006
Olsen, K. M., Kooyers, N. J., and Small, L. L. (2013). Recurrent gene deletions and the evolution of adaptive cyanogenesis polymorphisms in white clover (Trifolium repens L.). Mol. Ecol. 22, 724–738. doi: 10.1111/j.1365-294X.2012.05667.x
Olsen, K. M., and Small, L. L. (2018). Micro- and macroevolutionary adaptation through repeated loss of a complete metabolic pathway. New Phytol. 219, 757–766. doi: 10.1111/nph.15184
Olsen, K. M., Sutherland, B. L., and Small, L. L. (2007). Molecular evolution of the Li/li chemical defence polymorphism in white clover (Trifolium repens L.). Mol. Ecol. 16, 4180–4193. doi: 10.1111/j.1365-294X.2007.03506.x
Pan, F. J., McLaughlin, N. B., Yu, Q., Xue, A. G., Xu, Y. L., Han, X. Z., et al. (2010). Responses of soil nematode community structure to different long-term fertilizer strategies in the soybean phase of a soybean-wheat-corn rotation. Eur. J. Soil Biol. 46, 105–111. doi: 10.1016/j.ejsobi.2010.01.004
Pavao-Zuckerman, M. A., and Coleman, D. C. (2007). Urbanization alters the functional composition, but not taxonomic diversity, of the soil nematode community. Appl. Soil Ecol. 35, 329–339. doi: 10.1016/j.apsoil.2006.07.008
Pen-Mouratov, S., Shukurov, N., and Steinberger, Y. (2010). Soil free-living nematodes as indicators of both industrial pollution and livestock activity in Central Asia. Ecol. Indic. 10, 955–967. doi: 10.1016/j.ecolind.2010.02.005
Peralta, G., Dickie, I. A., Yeates, G. W., and Peltzer, D. A. (2020). Community- and trophic-level responses of soil nematodes to removal of a non-native tree at different stages of invasion. PLoS One 15:e0227130. doi: 10.1371/journal.pone.0227130
Pothula, S. K., Grewal, P. S., Auge, R. M., Saxton, A. M., and Bernard, E. C. (2019). Agricultural intensification and urbanization negatively impact soil nematode richness and abundance: a meta-analysis. J. Nematol. 51, 1–17. doi: 10.21307/jofnem-2019-011
Pouyat, R. V., Parmelee, R. W., and Carreiro, M. M. (1994). Environmental effects of forest soil-invertebrate and fungal densities in oak stands along an urban-rural land use gradient. Pedobiologia 38, 385–399.
Qu, J., Bonte, D., and Vandegehuchte, M. L. (2022). Phenotypic and genotypic divergence of plant–herbivore interactions along an urbanization gradient. Evol. Appl. 15, 865–877. doi: 10.1111/eva.13376
R Development Core Team. (2018). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: R Development Core Team. https://www.r-project.org/. (Accessed April, 2018).
Šalamún, P., Renčo, M., Kucanová, E., Brázová, T., Papajová, I., Miklisová, D., et al. (2012). Nematodes as bioindicators of soil degradation due to heavy metals. Ecotoxicology 21, 2319–2330. doi: 10.1007/s10646-012-0988-y
Santangelo, J. S., Ness, R. W., Cohan, B., Fitzpatrick, C. R., Innes, S. G., Koch, S., et al. (2022). Global urban environmental change drives adaptation in white clover. Science 375, 1275–1281. doi: 10.1126/science.abk0989
Santangelo, J. S., Thompson, K. A., Cohan, B., Syed, J., Ness, R. W., and Johnson, M. T. J. (2020). Predicting the strength of urban-rural clines in a Mendelian polymorphism along a latitudinal gradient. Evol. Lett. 4, 212–225. doi: 10.1002/evl3.163
Sikder, M. M., and Vestergård, M. (2020). Impacts of root metabolites on soil nematodes. Front. Plant Sci. 10:1792. doi: 10.3389/fpls.2019.01792
Thompson, K. A., and Johnson, M. T. J. (2016). Antiherbivore defenses alter natural selection on plant reproductive traits. Evolution 70, 796–810. doi: 10.1111/evo.12900
Thompson, K. A., Renaudin, M., and Johnson, M. T. J. (2016). Urbanization drives the evolution of parallel clines in plant populations. Proc. Biol. Sci. 283:20162180. doi: 10.1098/rspb.2016.2180
Tsunoda, T., and van Dam, N. M. (2017). Root chemical traits and their roles in belowground biotic interactions. Pedobiologia 65, 58–67. doi: 10.1016/j.pedobi.2017.05.007
Uchida, K., Blakey, R. V., Burger, J. R., Cooper, D. S., Niesner, C. A., and Blumstein, D. T. (2021). Urban biodiversity and the importance of scale. Trends Ecol. Evol. 36, 123–131. doi: 10.1016/j.tree.2020.10.011
Ugarte, C. M., and Taylor, J. R. (2020). Chemical and biological indicators of soil health in Chicago urban gardens and farms. Urban Agric. Reg. Food Syst. 5:e20004. doi: 10.1002/uar2.20004
United Nations. (2019). Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420). New York: United Nations. Press.
van Dam, N. M., and Heil, M. (2011). Multitrophic interactions below and above ground: en route to the next level. J. Ecol. 99, 77–88. doi: 10.1111/j.1365-2745.2010.01761.x
van den Hoogen, J., Geisen, S., Routh, D., Ferris, H., Traunspurger, W., Wardle, D. A., et al. (2019). Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198. doi: 10.1038/s41586-019-1418-6
van Geem, M., Gols, R., Raaijmakers, C. E., and Harvey, J. A. (2016). Effects of population-related variation in plant primary and secondary metabolites on aboveground and belowground multitrophic interactions. Chemoecology 26, 219–233. doi: 10.1007/s00049-016-0222-0
Vandegehuchte, M. L., Sylvain, Z. A., Reichmann, L. G., de Tomasel, C. M., Nielsen, U. N., Wall, D. H., et al. (2015). Responses of a desert nematode community to changes in water availability. Ecosphere 6, 1–15. doi: 10.1890/ES14-00319.1
Vauramo, S., and Setälä, H. (2010). Urban belowground food-web responses to plant community manipulation – impacts on nutrient dynamics. Landsc. Urban Plan. 97, 1–10. doi: 10.1016/j.landurbplan.2010.04.004
Wang, P., Matthews, D. E., and VanEtten, H. D. (1992). Purification and characterization of cyanide hydratase from the phytopathogenic fungus Gloeocercospora sorghi. Arch. Biochem. Biophys. 298, 569–575. doi: 10.1016/0003-9861(92)90451-2
Whitehead, A. G., and Hemming, J. R. (1965). A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann. Appl. Biol. 55, 25–38. doi: 10.1111/j.1744-7348.1965.tb07864.x
Widmer, T. L., and Abawi, G. S. (2002). Relationship between levels of cyanide in sudangrass hybrids incorporated into soil and suppression of Meloidogyne hapla. J. Nematol. 34, 16–22.
Yeates, G. W. (2003). Nematodes as soil indicators: functional and biodiversity aspects. Biol. Fertil. Soils 37, 199–210. doi: 10.1007/s00374-003-0586-5
Yeates, G. W., Bongers, T., de Goede, R. G. M., Freckman, D. W., and Georgieva, S. S. (1993). Feeding habits in soil nematode families and genera - an outline for soil ecologists. J. Nematol. 25, 315–331.
Keywords: cyanogenesis, nematodes, plant secondary metabolites, urbanization, white clover
Citation: Qu J, Bonte D and Vandegehuchte ML (2023) Hydrogen cyanide, a key plant defense, as a potential driver of root-associated nematode communities along urbanization gradients. Front. Ecol. Evol. 11:1113671. doi: 10.3389/fevo.2023.1113671
Edited by:
Jana Růžičková, ELKH-ELTE-MTM Integrative Ecology Research Group, HungaryReviewed by:
Winfried Voigt, Friedrich Schiller University Jena, GermanyEric Yee, Johns Hopkins University, United States
Copyright © 2023 Qu, Bonte and Vandegehuchte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiao Qu, cXVqaWFvQGxzYmcuY24=
†ORCID: Jiao Qu https://orcid.org/0000-0002-3199-0372
Dries Bonte https://orcid.org/0000-0002-3320-7505
Martijn L. Vandegehuchte https://orcid.org/0000-0003-1283-4654
 Jiao Qu
Jiao Qu Dries Bonte
Dries Bonte Martijn L. Vandegehuchte
Martijn L. Vandegehuchte