- 1Department of Theoretical and Applied Sciences, Università degli Studi dell'Insubria, Varese, Italy
- 2Royal (Dick) School of Veterinary Studies, University of Edinburgh, Scotland, United Kingdom
- 3Istituto Zooprofilattico Sperimentale della Lombardia e dell'Emilia Romagna, Brescia, Italy
- 4Department of Veterinary Medicine and Animal Sciences, Università degli Studi di Milano, Lodi, Italy
- 5School of Biological and Behavioural Sciences, Queen Mary University of London, London, United Kingdom
The eastern gray squirrel (Sciurus carolinensis) has been labeled as one of the 100 worst invasive alien species by the IUCN. In Europe, the species has been introduced to Britain, Ireland and Italy, and its subsequent spread has resulted in wide-scale extinction of native Eurasian red squirrels (Sciurus vulgaris) from the areas colonized by the gray squirrel. This replacement of a native by an alien competitor is one of the best documented cases of the devastating effects of biological invasions on native fauna. To understand how this replacement occurs, we present a systematic review of the literature on competition and interactions between red and gray squirrels. We describe the patterns of red and gray squirrel distribution in those parts of Europe where gray squirrels occur and summarize the evidence on the different processes and mechanisms determining the outcome of competition between the native and alien species including the influence of predators and pathogens. Some of the drivers behind the demise of the red squirrel have been intensively studied and documented in the past 30 years, but recent field studies and mathematical models revealed that the mechanisms underlying the red-gray paradigm are more complex than previously thought and affected by landscape-level processes. Therefore, we consider habitat type and multi-species interactions, including host-parasite and predator-prey relationships, to determine the outcome of the interaction between the two species and to better address gray squirrel control efforts.
1. Introduction
Species introduced into new environments may become invasive and impact native species through competitive exclusion, niche displacement, predation, parasitism, disease, hybridization, introgression or combinations of these processes (Jeschke and Strayer, 2005; Mazzamuto et al., 2021). In some cases, the native species may become extinct, and it is recognized that invasive species pose a serious threat to biodiversity across the world (e.g., Pyšek et al., 2020; Tedeschi et al., 2022). Here we consider the introduction of the North American gray squirrel (Sciurus carolinensis) into Europe and its replacement of the native Eurasian red squirrel (S. vulgaris). It will become evident from the studies reviewed in this paper that ideas, theories, and research methods for studying competition between red and gray squirrels, the red squirrel-gray squirrel paradigm, emerged in the 1990s and have been considerably added to over the last 20–30 years.
1.1. The introduction of gray squirrels into Europe
In 1876, four gray squirrels from North America were introduced to Henbury Park, Cheshire, England (Middleton, 1931; Shorten, 1954). This was the beginning of a series of introductions of gray squirrels to Britain, Ireland and Italy over the ensuing 150 years. The gray squirrel is native to the eastern and midwestern United States of America and to some southerly provinces in Canada. It is a resilient and adaptable species that has not only been introduced to Britain, Ireland and Italy, but also to other places around the world, such as Melbourne, Ballarat and Adelaide in Australia (now extinct, Peacock, 2009), and South Western Cape, South Africa (Gurnell, 1987), as well as western Canada (Larsen, 2016) and several places in the western United States (Tran et al., 2022). After the first introduction to Britain, there were at least 8 further introductions form North America and approximately 19 secondary introductions or translocations to different places within Britain (including several translocations to Scotland) and Ireland until 1929 (Middleton, 1931; Shorten, 1954). Many of the first recorded introductions to Britain, Ireland and later to Italy were made by landowners to embellish their large country estates. DNA profiling approaches in Britain, Ireland and Italy indicate that human help has been a critical factor in the successful spread of this species (Signorile et al., 2014b, 2016).
Often the numbers of gray squirrels introduced to a location were low, in single figures. Shorten (1954) noted that many small introductions throughout the country may have gone unrecorded and hypothesized that the escape of gray squirrels kept as pets might have played a relevant role in the spread of the species. As well as the wider countryside, gray squirrels readily moved into towns and cities and were frequently recorded, often in high numbers, in parks and common areas (Shorten, 1954; Merrick et al., 2016; Lawton et al., 2020). Despite an increasing number of attempts to control gray squirrels (Gurnell and Pepper, 2016), by the mid-1980s, gray squirrels were to be found throughout mainland central and southern England and Wales, and in the Central Lowlands in Scotland (Lloyd, 1983; Gurnell, 1987). Thereafter, their range continued to expand into northern England, southern and central Scotland and up the east coast of Scotland to Aberdeen (with a separate introduction to the city). This range expansion was not straightforward because of concerted efforts by various organizations and volunteers, especially in the north of England and Scotland, to control gray squirrels for red squirrel conservation (Seward and O'Hare, 2015; Shuttleworth et al., 2020). Thus, especially at the local scale, gaps occur within their range, sometimes temporary, as gray squirrels are removed (Gurnell et al., 2014).
Gray squirrels were first introduced to Ireland when six pairs were translocated from the Woburn Estate in England to Castle Forbes in County Longford in 1911 (Lawton et al., 2015). Subsequently there have been 19 distribution surveys of gray (and red) squirrels in Ireland between 1932 and 2019 (O'Teangana et al., 2000a; for full list see Lawton et al., 2020). By 2007 gray squirrels had colonized the eastern half of the island, although absences in previously occupied areas in the midlands were noted, and this has been attributed to the re-appearance of the pine marten, Martes martes (Sheehy and Lawton, 2014; Lawton et al., 2015). Twining et al. (2022a) analysis of survey data indicates that the range of the gray squirrel decreased by 41% between 2007 and 2019 with a commensurate increase of 51% in the range of the red squirrel and a 205% increase in range of the pine marten. Further, emphasizing the impact of pine martens on gray squirrels, gray squirrels had disappeared from 85% of the 100 km2 grid squares occupied by both species in 2007 during the following 12 years (Twining et al., 2022a).
Gray squirrels were introduced from North America into Italy from 1948 onwards and the first introduction (1948) was of four individuals from Washington D.C. to Candiolo, close to the Stupinigi forests just south of Turin (Currado et al., 1987; Bertolino and Genovesi, 2003; Bertolino, 2008). A second introduction was to Genoa Nervi in 1966 and thereafter many illegal releases and translocations, some recorded and many not, occurred through to 2010 (Bertolino, 2008; Martinoli et al., 2010; Signorile et al., 2014a). In particular, the many populations registered in Lombardy by 2010 (see Table 1 and Figure 1 in Martinoli et al., 2010), all of unknown origin, have subsequently increased their abundance and distribution over the past 12 years, causing a strong reduction and/or extinction of local red squirrel populations, despite (limited) control attempts in some areas during the LIFE EC-SQUARE LIFE09/NAT/IT/000095 “rossoscoiattolo” project. In fact, gray squirrel control and/or red squirrel conservation measures in Italy have been sporadic and without any continuous effort, with additional pressure for “no action” by animal welfare groups exacerbating the situation (Lioy et al., 2019). Necessary, but by itself insufficient, legal restrictions, implemented first at national and subsequently at the EU-level (Bertolino et al., 2013), included a trade-ban and the prohibition to keeping gray squirrels in captivity. These measures were an important step to prevent further illegal releases but were unsuccessful in stopping illegal translocations (authors unpubl. data). Despite these serious issues, some local eradication actions of the invasive alien species (IAS) have succeeded (in Perugia, La Morgia et al., 2017 and in Genoa Nervi, Scapin et al., 2019). Further information on introductions to Piedmont, Lombardy, Umbria and Veneto are provided by Bertolino et al. (2015), but detailed records of introductions are often lacking or fragmentary.
Although little is known about the fate of many introductions, there is evidence that many were successful (Shorten, 1954; Signorile et al., 2016). The general pattern appears to be that, after a period of consolidation from a small founding population, gray squirrels start to spread from their places of introduction. A typical scenario can be seen from the first introduction of gray squirrels to the Stupinigi forests (Piedmont) in Italy in 1948. Gray squirrels only started to expand their range in the late 1970s, when animals dispersed mainly along river corridors and started to colonize the highly fragmented surrounding landscape (Wauters et al., 1997; Bertolino et al., 2014). The Piedmont metapopulation's expansion was monitored until 2015 (Bertolino et al., 2014): in this period gray squirrels increased their range from 900 km2 in 1990 to more than 2000 km2 in 2012.
Many introductions and translocations of gray squirrels over wide geographic areas in Britain, Ireland and Italy over many years have made it difficult to contain the spread of the invading species. There have been some hard-won successes, such as the removal of gray squirrels from the island of Anglesey (Wales), or ongoing control of gray squirrels in parts of the north of England, Scotland, Ireland and Italy (Gurnell et al., 2014; Seward and O'Hare, 2015; Bertolino et al., 2016). Landscape features may slow down spread, such as movement to the west of the River Shannon in Ireland (Lawton et al., 2020), although as discussed above, this may at least in part be predator related. At the same time, tree and river corridors have facilitated dispersal through farmland, moorland or fragmented habitats (Bertolino et al., 2014; Shuttleworth et al., 2016).
1.2. Effects of gray squirrel introduction on native red squirrels
The invading gray squirrels are in competition with native red squirrels. As gray squirrels have spread, they have replaced red squirrels in most habitats in a process that appears inevitable without some form of human intervention, and with the possible exception of the return of the pine marten in Ireland and more recently parts of Scotland (see Section 5.4). In simple terms, red squirrels eventually disappear as the gray squirrels expand their range. The replacement may however not be immediate, and some habitats, such as commercial Sitka spruce (Picea sitchensis) dominated forests in Britain (also see Halliwell et al., 2016), may provide refuges for red squirrels, assuming proper management of tree species diversity and forest age structure to ensure a dependable food supply. In Italy, after the first introduction in Stupinigi (Piedmont), between 1970 and 2010 local red squirrel populations disappeared and went extinct in 62% of tetrads (ca. 1,689 km2) in the 2016 km2 area colonized by gray squirrels (Bertolino et al., 2014).
This red squirrel-gray squirrel paradigm has received a considerable amount of attention in the last 50 years, and, based on a systematic review of the literature, we ask the question: “Is the ecological replacement of red by gray squirrels really inevitable?” We particularly look at the mechanisms of competition between the two species and to what extent they are mediated by pathogens as well as predators. In fact, we consider the evidence for interference, exploitation, and apparent competition. We also consider whether human intervention can reverse the outcome of competition.
2. Methods
2.1. Literature search
A systematic literature search was undertaken in April 2022 following the PRISMA protocols (Page et al., 2021). We used Web of Science, Scopus and Google Scholar to conduct a literature search using the following terms: (“red squirrel*” OR “Sciurus vulgaris”) AND (“gray squirrel*” OR “gray squirrel*” OR “Sciurus carolinensis”) AND (“interaction*” OR “competition”). All results listed on Web of Science and Scopus were collected, while only the first 200 articles of Google Scholar were included, following the threshold of relevance used in other reviews (Lison et al., 2020; Fingland et al., 2022).
2.2. Inclusion criteria
We only included articles written in English and published in peer-reviewed journals; we did not collect theses, book chapters, reports and conference proceedings. There were no restrictions regarding the year of publication. The screening was conducted using the following criteria:
(1) the title or abstract specified “(Eurasian) red squirrel” or “Sciurus vulgaris” and “(Eastern) or (North American) grey or gray squirrel” or “Sciurus carolinensis” as being the focal species, and that the study investigated a form of competition and/or interaction between them (e.g., disease-mediated competition, food/space competition, predator effects);
(2) the title or abstract characterized studies as being important to understand red-gray squirrel relationships (e.g., landscape or species management and control, changing distribution patterns). Hence, single species studies not revealing patterns or information relevant for competition and/or interaction were excluded. Only the key findings from the final set of selected papers were collated and reported.
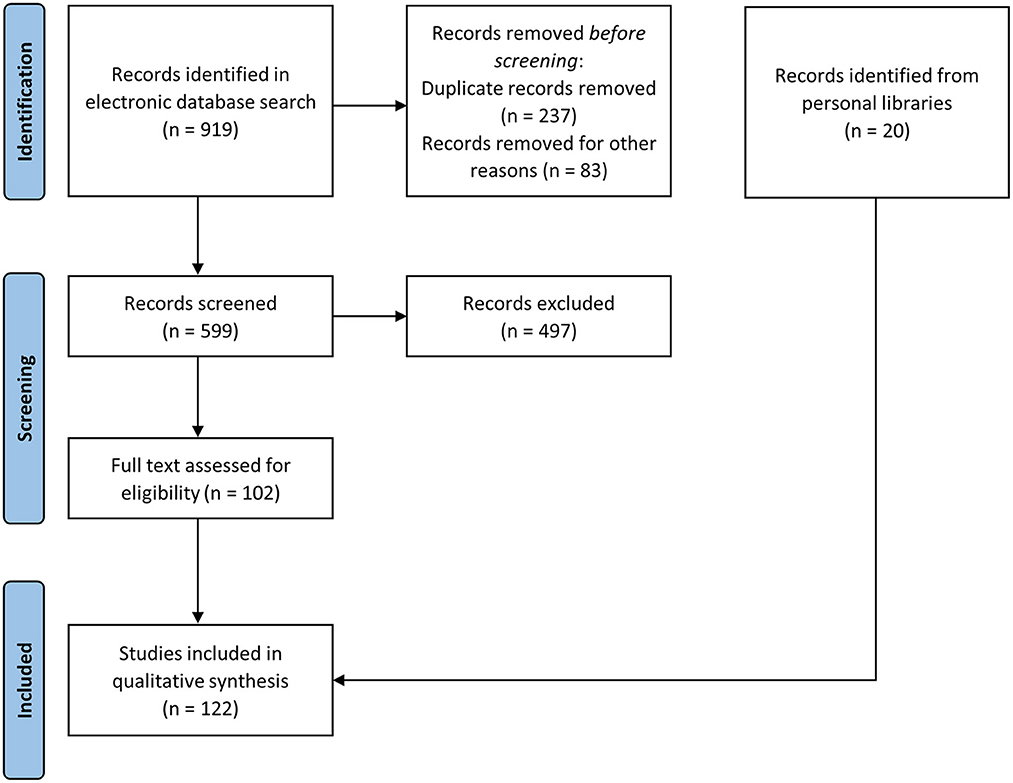
We collected a total of 599 articles, of which 102 were retained after screening. We added 6 articles and 14 publications that did not appear in our search but which were present in our personal libraries and relevant for the objectives of the present systematic review. The PRISMA flowchart is provided as Figure 1, and the list of the publications included in the qualitative synthesis is presented in Supplementary material, with the extra 20 publications highlighted in bold.
3. The study of interspecific competition
In relation to the mechanisms of interference and exploitation competition in the replacement of red by gray squirrels in Europe, little has been added since the review by Gurnell and colleagues published in 2015 (Gurnell et al., 2015a). In fact, the 13 publications on this topic selected for this paper are dated between 1986 and 2004, with the exception of one on gray squirrel dynamics from 2015 (Goldstein et al., 2015).
To date, two main types of field study design have been used to explore the interspecific competition between the two species: (1) Comparative studies investigating the same phenotypic, spatial or demographic parameters in multiple single-species (only red squirrel and only gray squirrel) study areas (populations), taking into account broad habitat types (deciduous woods, pine-dominated conifer forests, spruce-dominated conifer plantations); (2) Studies using a pseudo-experimental replicated design of red-only (control) sites and red-gray (experimental) sites, comparing behavior, foraging and feeding, space use, activity patterns and demographic processes of red squirrels between control and experimental situation and between red and gray squirrels in the experimental sites. Although both designs produce mainly correlative relationships, the second approach, comparing data between red-only and red-gray sites, of similar habitat-type, is more likely to reveal cause-effect patterns. Earlier studies on single-species situations of both squirrel species in deciduous and coniferous (pine/spruce) habitats show the importance of habitat type for species-specific demographic processes and overall densities (Kenward and Hodder, 1998). Several authors suggest that food competition alone could be sufficient to explain the replacement, at least in oak-dominated forests, where acorns crops provide gray squirrels with a food refuge, that is avoided, or marginally exploited, by red squirrels (e.g., Kenward and Holm, 1993; Kenward et al., 1998).
4. Context and differences between the two species
4.1. The role of body size
Among competing mammal species, being larger and heavier has physiological benefits related to thermoregulation and body fat reserves that might affect the outcome of competition. Morphologically, gray squirrels are larger and heavier than red squirrels. Comparing the relationship between shin length (average 70.5 mm in red and 78.0 mm in gray squirrels) and body mass (average over different seasons: 277–303 g in red and 542–659 g in gray squirrels), gray squirrels are proportionally heavier for their body size than red squirrels (79–138% greater weight against the 35% difference expected; Kenward and Tonkin, 1986). In deciduous and mixed forests with a high availability of large tree seeds (acorns, beechnuts, chestnuts, and hazelnuts) this is associated with a greater capacity to accumulate subcutaneous body fat in autumn when high-energy food resources are abundant. In fact, where autumn weight increase in red squirrels is about 10%, gray squirrels can increase their body mass by 17–23% from the seasonal low in summer to peak mass in late autumn in deciduous habitats (Kenward and Tonkin, 1986; Wauters and Dhondt, 1989; Koprowski, 1994; Lurz et al., 2005). In contrast, in large man-made spruce-dominated conifer plantations in northern England, neither red nor gray squirrels increased their body mass in the autumn-winter period (Lurz and Lloyd, 2000). In coniferous forests fat accumulation is likely to be less pronounced than in mixed deciduous woods because autumn and winter food supplies are more predictable, and maneuverability to feed on cones in the canopy is important (Lurz and Lloyd, 2000; Wauters et al., 2007; Tranquillo et al., 2022). The different body shapes and interspecific differences in fat accumulation capacity are most likely genetically determined, since studies on size and weight variation in red squirrels over a variety of habitat types suggest local selection for smaller or larger size, based on quality and quantity of primary food resources (tree seeds) (Wauters et al., 2007; Cox et al., 2020; Tranquillo et al., 2022).
Gray squirrels have higher Daily Energy Expenditure (DEE) than red squirrels. However, the difference between the two species was not separable from the predicted effect of body mass on DEE (Bryce et al., 2001), and their DEEs were in accordance with allometric predictions based on body mass and ambient temperature. DEEs for gray squirrels varied with body mass, fluctuating between a low of 300 kJ day−1 to a maximum of 1,150 kJ day−1 with most values between 500 and 700 kJ day−1. The autumn mean DEE for gray squirrels was about 200 kJ day−1 higher than the winter mean (Bryce et al., 2001). Red squirrels showed less seasonal difference, with the highest DEE in spring and the lowest in autumn (spring 389.8 ± 122 kJ day−1; autumn 314 ± 281 kJ Day−1; Bryce et al., 2001). These authors argued that the higher energy requirements of gray squirrels, linked to higher consumption of limited high-quality food resources, could exert greater interspecific competition than the intraspecific competition red squirrels undergo in the natural situation (areas without grays), driving populations of the native species where they co-occur with the IAS to such low numbers that they are no longer viable. Hence, gray squirrels have a morphological advantage to cope with fluctuating deciduous seed-crops; this, together with their physiological (higher capacity to digest tannins; Kenward and Holm, 1993) and behavioral adaptations (female kin-groups, intensive caching, better spatial memory and problem-solving ability, Gurnell et al., 2001; Chow et al., 2018), contributed to the observed faster replacement of red by gray squirrels in broadleaf habitats (compared to conifer) in Northern Italy and the British Isles. Replacement of red by gray squirrels is therefore complex, highly context dependent and loss rates are influenced by habitat type (and thus also food availability), morphological as well as behavioral adaptations and disease (see also disease-mediated competition in Section 5.3 and community level impacts in Section 8).
4.2. Environmental factors and niche overlap
The role of habitat type as environmental driver of the replacement process and the speed of replacement has long been recognized (e.g., Gurnell and Pepper, 1993; O'Teangana et al., 2000b; Di Febbraro et al., 2013). Red squirrels thrive in Scots pine dominated woodlands and in large, conifer forests with a high proportion of Norway spruce (Kenward et al., 1998; Wauters et al., 2004, 2008; Lurz et al., 2005; Santicchia et al., 2018); gray squirrels reach high densities in oak dominated woodlands and in suburban or even urban areas (Kenward and Holm, 1993; Gurnell, 1996; Kenward et al., 1998; Santicchia et al., 2018, 2019). It should be noted, that red squirrel populations also do well in broadleaf habitats in the absence of the invader (e.g., North Italy, Wauters et al., 2001b; Belgium, Wauters et al., 2004; Isle of Wight, Gurnell et al., 2015b) although they live at a similar range of densities as in conifer woodlands and at lower densities than gray squirrels in similar habitats (Kenward and Holm, 1993; Gurnell, 1996; Kenward et al., 1998). Similarly, large-scale surveys of presence/absence of the two species in the landscape tend to find associations of red squirrels with larger and conifer dominated forests and of gray squirrels with deciduous woods, parklands, and gardens (e.g., O'Teangana et al., 2000b in Northern Ireland). For red squirrel persistence, the degree of habitat fragmentation and the distance to the nearest gray squirrel population can be important factors (O'Teangana et al., 2000b).
These habitat effects raise the question to what extent there is niche partitioning in space- and habitat use. In an upland mixed-species conifer plantation in N. England, the habitat niche (tree species used) overlap between red and gray squirrels was high (75%). Red squirrels selected pure Sitka spruce stands and used mixed Sitka spruce-Scots pine; but they avoided the pure Scots pine stand, heavily used by gray squirrels, and potentially the “best” habitat for reds. Patterns of both intra-specific and inter-specific core-area overlap further confirmed that intraspecific (and intrasexual) competition among adult red squirrels was mainly determining red squirrel spacing behavior at this red-gray site, and that interspecific competition with the IAS had only a limited impact (Wauters et al., 2000). Hence, there was only limited space- or habitat-niche partitioning between the two species, suggesting a poor capacity for red squirrels to avoid interspecific resource competition at least at spatial and habitat levels in both conifer-dominated and broadleaf-dominated woodlands and forests (Wauters et al., 2000, 2002a).
A different example of at least partial niche partitioning was observed in Craigvinean Forest in Scotland where the two species co-existed for up to 30 years (Bryce et al., 2002), again suggesting the importance of landscape and habitat composition in determining the rate of replacement. Although the home ranges of red and gray squirrels overlapped, gray squirrels selected riparian corridors of mixed woodland with deciduous trees, while native reds favored foraging in Norway spruce trees within their home range (Bryce et al., 2002). Overall, niche-overlap was considerable (77%) and the authors did not find evidence for fine-scale (temporal) avoidance between the two species (Bryce et al., 2002). Their work suggests that determining the spatial scale at which some form of co-existence occurs can be informative.
To what extent did this high niche-overlap (ca. 75%) and lack of spatial segregation between red and gray squirrels in conifer plantations affect squirrel demography? Although the red-gray site had, overall, a higher tree-seed production, red squirrel densities (Minimum Number Alive) did not differ between red-only (0.17 to 0.30/ha) and red-gray sites (0.26 to 0.33/ha). But, even in these upland conifer forests dominated by Sitka spruce, gray squirrel density was about 4 times higher (0.92 to 1.1/ha) and they tended to have higher breeding rates than the native congener (44% of red squirrel females breeding in both spring and summer, against, respectively, 70 and 54% of gray squirrel females; Wauters et al., 2000; Gurnell et al., 2004). There were no differences in red squirrel adult survival or female breeding rate between the two populations, but juvenile recruitment in the red-gray site (13%) was much lower than in the red-only site (50%). Hence, in conifer plantations, adult red squirrels seemed to be little affected by the high number of grays residing in the study area, and the key demographic result of interspecific competition for food and space was the reduced juvenile and subadult recruitment (Wauters et al., 2000; Gurnell et al., 2004).
4.3. Foraging and seed-caching behavior
Comparing tree-species selection by the two species in mixed deciduous woods in N. Italy, the relative, seasonal use of tree species differed between red and gray squirrels. Gray squirrels positively selected and used oaks more frequently than native red squirrels (ca. 41% by reds, 63% by grays), while red squirrels foraged often in walnut trees (Juglans regia) in autumn. Because of greater specialization in feeding on oak buds, flowers, insects (spring-summer), and acorns (autumn) by the alien species, it had a narrower niche width than the native one: overall niche overlap between the two species was 0.73 in spring-summer and 0.69 in autumn-winter (Wauters et al., 2002a). Diets of both red and gray squirrels were highly variable, but whereas acorns are high-energy staple food for grays, red squirrels eat only one to a few acorns in a day and acorns are never preferred over other hard mast (Wauters et al., 2001a); this seems related to their low ability to tolerate and neutralize acorn polyphenols (high tannin contents in acorns, Kenward and Holm, 1993). In captive-held gray and red squirrels kept on an acorn diet, the former thrived while the latter had a comparative digestive efficiency of only 59% and several animals died during the experiment (Kenward and Holm, 1993).
Gray squirrels spend more time on the ground than reds, and, having evolved in North American deciduous woods with large-seeded trees (e.g., oaks, hickory, and black walnut), cache (and retrieve) larger amounts of acorns and other nuts (Kenward and Tonkin, 1986; Wauters et al., 2002a,b). However, in mixed broadleaf woodlands in their European range, each year in autumn, both species scatterhoard numerous hazelnuts (Corylus avellana), chestnuts (Castanea spp.), beechnuts (Fagus sylvatica), and acorns (Quercus spp.) in small caches in the ground, retrieving these high-energy food items between late autumn and the following spring (Koprowski, 1994; Wauters and Casale, 1996; Wauters et al., 2001a, 2002b). The recovery and consumption of these cached seeds enhances summer reproduction of female red squirrels (Wauters et al., 1995). Foraging more on the ground, gray squirrels could potentially pilfer and partially deplete seeds cached by red squirrels, since naïve individuals are known to find and retrieve seeds cached by other squirrels (in most cases of the same species), by visual observation or by olfactory cues (Leaver et al., 2007; Robin and Jacobs, 2022).
Red squirrels show marked seasonal variation in both the proportion of ground foraging (more frequent in autumn-winter and in spring) and in tree-species niche width: the latter is smallest in spring when in mixed deciduous woods they spend a lot of time in oaks feeding on flowers and caterpillars (Wauters et al., 2001a, 2002a). Both red and gray squirrels were frequently observed moving and feeding on the ground (reds 27 and 37% of observations in spring-summer and autumn-winter respectively; grays 29 and 43% respectively; Wauters et al., 2002a). Consequently niche-overlap for ground vs. tree use was extremely high (0.98 in spring-summer; 0.94 in autumn-winter), and the proportion of ground foraging by red squirrels varied greatly between habitats, in relationship to the availability of cacheable autumn tree-seeds (hazelnuts, chestnuts, beechnuts, and walnuts) (Wauters and Casale, 1996; Wauters et al., 2002a,b).
4.4. Personality and problem solving
The relative occurrence of different personality traits in red squirrels, measured through arena tests by recording the behavior of the animals placed in a standardized box-shaped arena with a controlled environment (see Mazzamuto et al., 2019), was studied in sites with only red squirrels and sites with gray squirrels also present (Wauters et al., 2019). It was found that the presence of gray squirrels did not influence the proportion of time spent in behaviors related to the personality traits activity or shyness. However, sociability behaviors were expressed more in sites with the presence of gray squirrels (Wauters et al., 2019). These results could indicate a fitness advantage for more sociable red squirrels in the red-gray sites. However, none of the personality traits were correlated with the probability to survive to the next year, or with the probability for females to produce a litter (Wauters et al., 2019). That personality-fitness relationships were not affected by the presence or absence of gray squirrels suggests that the higher expression of sociability by red squirrels in the red-gray sites was not due to natural selection, but probably the result of context-related benefits when co-existing with gray squirrels. In general, more studies are needed to understand whether interspecific competition influences the selection of personality phenotypes which would allow native species to cope more efficiently with invasive alien species.
Few studies have looked at interspecific differences in memory or problem-solving abilities. In a preliminary study on few individuals, Macdonald (1997) showed that gray squirrels' spatial memory was accurate, allowing them to precisely relocate cache sites compared to less long-lasting memory of red squirrels. In a more recent study, Chow et al. (2018), compared the level of behavioral flexibility among invasive gray and native red squirrels using two food extraction tasks, an easy and a difficult one. Both species showed flexibility and the solution time for solving the easy task was comparable between the native and alien species (Chow et al., 2018). But a higher proportion of gray (93%) than red squirrels (50%) solved the problem at the first attempt. Likewise, more gray than red squirrels solved the more difficult task but, for the individuals that did solve it, efficiency was comparable between the two species. Overall, the authors suggested that the on average more successful problem-solving ability exhibited by gray squirrels could have facilitated invasion success and become a competitive advantage in replacing native red squirrels.
5. Mechanisms and outcomes of interspecific competition
5.1. Interference competition
The Interference Competition Hypothesis (ICH) was long retained a likely mechanism behind the replacement of native red by alien gray squirrels. The ICH suggests three ways in which gray squirrels could directly interact with native red squirrels: direct aggressive interactions; interrupting red squirrel mating chases; or forcing native squirrels to actively avoid areas of the woodland intensively used by the invasive congener (Wauters and Gurnell, 1999). These authors explicitly tested six predictions of this hypothesis, using data on activity patterns, behavior and reproductive performance of red squirrels, and comparing them between a red-only and a red-gray site in Italy (Wauters and Gurnell, 1999).
Red squirrels only slightly increased time spent interacting with other squirrels (both species in the Experimental site) and interactions with gray squirrels were rarely observed. Moreover, where, in both sites, most within-sex (males with males or females with females) interactions between two red squirrels were aggressive, this was not the case for encounters between a red and a gray squirrel of the same sex. Hence, the prediction that gray squirrels replaced reds by direct aggressive interactions was rejected (Wauters and Gurnell, 1999). The same study also showed that gray squirrels did not interfere with red squirrel mating chases, or affected mating success.
Activity patterns (time spent active/hour) of both species were similar in all seasons (in the red-gray site) and red squirrels' activity rhythm did not differ between the two study sites. In the red-gray site in N. Italy, correlations of the proportion of time spent active in each 1-h period between red and gray squirrels were high in all seasons (all r > 0.73; see Figure 1 in Wauters et al., 2002a). Hence, red squirrels did not avoid the invasive congener by shifting their activity to times of day when gray squirrels were less active (Wauters and Gurnell, 1999; Wauters et al., 2002a). Finally, detailed analyses of red squirrel home-range and core-area size, intra- and interspecific core-area overlap and between-year shifts in core-areas of red squirrels in relation with the amount of overlap with gray squirrels, indicated that any observed shifts of red squirrel core-areas were not caused by a local increase in gray squirrel density. Furthermore, sex-specific core-area overlap among red squirrels did not differ from the same sex-specific combinations between red and gray squirrels (details in Table 5 in Wauters and Gurnell, 1999; see also Wauters et al., 2002a). In other words, red squirrels did not avoid gray squirrels more strongly than they avoided other red squirrels. The conclusion to these studies was that there was no home-range niche partitioning between the two squirrel species and no evidence to support the ICH. Thus, interference competition can be dismissed as a mechanism behind the replacement process.
5.2. Exploitation competition
Exploitation competition is a form of direct competition between species for (limited) resources, such as food or nest sites. Red and gray squirrels eat similar foods, but do gray squirrels exploit certain shared foods more efficiently, reducing their availability to red squirrels? Red squirrels spent more time foraging in red-only than in red-gray sites in N. Italy in spring and in winter, but the absolute differences were small (less than 20 min day−1; Wauters et al., 2001a). In both sites, native squirrels selected smaller, early maturing seeds in late spring and summer, and larger later maturing nuts in autumn. Overall, there was no clear evidence that interspecific competition for important food resources in the presence of gray squirrels caused large changes in red squirrel food choice. However, estimating average daily rates of energy intake (DEI) by red squirrels in three periods (autumn, early winter, and late winter), DEI was higher in the red-only than in the red-gray site in early winter (December), but not in the other seasons (Wauters et al., 2001a). Hence, food competition with gray squirrels seemed to have a limited impact on the energy-intake of adult red squirrels.
Regardless of this, observations on caching and retrieval behavior of radio-tagged squirrels of both species revealed some interesting findings. For example, gray squirrels mainly cached acorns, walnuts and black walnuts, but they also retrieved hazelnuts and walnuts that were cached by red squirrels (Wauters et al., 2002b). Also, and although the total seed-energy cached by red squirrels did not differ between the red-only and red-gray sites the average ratio of seed-energy recovered to seed-energy cached by red squirrels was estimated at 99.8% at the red-only site, but only 66% at the red-gray site (Table 2 in Wauters et al., 2002b). At the individual level, daily energy-intake from consumption of cached seeds was not related to interspecific core-area overlap in winter; however, in the following spring, when caches were gradually depleted, red squirrels with high interspecific core-area overlap had lower daily energy-intake than low-overlap squirrels (Figure 1b in Wauters et al., 2002b). In addition, spring body mass of red squirrels was negatively correlated with % core-area overlap with the congener. In summary, the pilfering of red squirrels' food caches by the IAS seems to result in a reduced energy-intake and loss of body mass, mainly in spring, of those animals that were strongly overlapped by gray squirrels (Wauters et al., 2002b). Whether the opposite also occurs (red squirrels pilfering gray squirrels' seed caches) still needs to be investigated.
How do these differences in food choice and energy-intake affect phenotype? Comparing right hind foot length (index of body size) and body mass of red squirrels between the red-only and the red-gray sites, both in coniferous and deciduous habitats, individuals of the native species were larger and weighed more in the absence than in the presence of the IAS (Wauters et al., 2000, 2001a). However, differences in body mass between the sites were mainly explained by the variation in foot length; hence adult red squirrels weighed less when co-occurring with gray squirrels because they were smaller than in the control areas without the alien congener (Wauters et al., 2000, 2001a).
5.3. Disease-mediated apparent competition
Parasite-mediated (or disease-mediated) competition is an asymmetric form of apparent (indirect) competition (Holt and Bonsall, 2017) that may arise between two host species when they share a common parasite (sensu Anderson and May, 1979) that has a differential impact on them (Hudson and Greenman, 1998). Biological invasions may represent an optimal scenario for such parasite-mediated interactions to occur, as alien and native species will have likely co-evolved with different enemies and will thus have a different susceptibility and tolerance to shared pathogens, either native or introduced (Dunn et al., 2012; Lymbery et al., 2014). It is likely that an alien parasite introduced by the invading host will be more virulent to naïve native species that lack any previous exposure to it. In this case, if spillover occurs, the parasite will facilitate the invader, leading to a so-called disease-mediated invasion (Strauss et al., 2012).
5.3.1. Squirrel poxvirus
One of the most prominent and well-studied cases of disease-mediated invasions involves the SQPV, a viral infection mediating the competition between red and gray squirrels in Britain and Ireland. Although outbreaks of an epidemic skin disease causing high morbidity and mortality in red squirrels had been reported in England since the 1930s (Middleton, 1930), it was only in the early 2000s that a set of epidemiological (Sainsbury et al., 2000), clinical (Tompkins et al., 2002) and modeling studies (Rushton et al., 2000; Tompkins et al., 2003) confirmed that SQPV dynamics played a role in the replacement of red squirrels by gray squirrels in Britain and Ireland.
SQPV was first isolated in the 1980s and mistakenly identified as a parapoxvirus (Scott et al., 1981), but based on genetic analyses has later been classified as a new genus within the Poxviridae family (McInnes et al., 2006; Obon et al., 2011; Darby et al., 2014). The available evidence points toward a North American origin of the virus (McInnes et al., 2015). The disease has been reported in Britain and Ireland only after gray squirrels had been introduced and it has never been recorded in continental Europe (McInnes et al., 2006). As mentioned above, the virus initially emerged and spread increasingly faster through England and Wales (Shorten, 1954), reaching southern Scotland in 2005 together with gray squirrels expanding from the south (McInnes et al., 2009). In Ireland, the first seropositive gray squirrels were reported in the 1990s, but it was only a decade later that diseased red squirrels were detected (McInnes et al., 2013; Stritch et al., 2015). Conversely, SQPV does not appear to be present in Italian gray squirrel populations and no diseased red squirrels have ever been reported in the country either (Romeo et al., 2019).
In red squirrels, infection by SQPV induces severe skin lesions located mostly on the face and limbs and characterized by exudative and ulcerative dermatitis which progresses to haemorragic scabbing (Tompkins et al., 2002; Fiegna et al., 2016). Both experimental infections (Tompkins et al., 2002; Fiegna et al., 2016) and observational field studies (Carroll et al., 2009) showed that SQPV is highly virulent to red squirrels, with a short incubation period (<15 days) and a rapid disease progression (10–15 days) that usually results in death. Although red squirrels can occasionally recover from the infection (Tompkins et al., 2002; Sainsbury et al., 2008; Shuttleworth et al., 2014), retrospective serology indicates that only about 8% of exposed red squirrels will survive an epidemic (Chantrey et al., 2014). Ballingall et al. (2016) observed a strongly reduced diversity in the major histocompatibility complex (MHC, a cluster of genes involved in antigen recognition and essential for the vertebrate acquired immune response) of UK red squirrel populations compared to continental ones and hypothesized this may be among the causes for such a high susceptibility to SQPV.
On the contrary, except for sporadic cases, gray squirrels normally show no clinical signs of the disease (Tompkins et al., 2002; Atkin et al., 2010) and seroprevalence in infected populations often exceeds 50% (Sainsbury et al., 2000; Bruemmer et al., 2010; Chantrey et al., 2019). Moreover, Chantrey et al. (2019) showed that SQPV infection is chronic in most gray squirrels (>80%) and viral loads may persist over time, implying that they may remain infectious for months. Skin lesions of infected red squirrels typically contain a large number of viral particles (Atkin et al., 2010; Fiegna et al., 2016) and the virus is shed also in the feces and urine (Collins et al., 2014). Shedding of SQPV by asymptomatic gray squirrels is lower and appears to occur mostly at the forearm, where vibrissae and sebaceous glands used for scent-marking are located (Atkin et al., 2010; Dale et al., 2016; Chantrey et al., 2019). Transmission can occur therefore through direct contact between individuals (e.g., during fights, mating, or grooming) and by environmental contamination of scent-marking posts, feeding sites, and dreys (Atkin et al., 2010; Bruemmer et al., 2010; Fiegna et al., 2016). Ectoparasitic arthropods can act as mechanical vectors (Collins et al., 2014), but the contribution of this route of transmission to disease circulation is thought to be negligible (Sainsbury et al., 2008; Carroll et al., 2009; Atkin et al., 2010; Chantrey et al., 2019).
At the population level, the vastly different impact of SQPV on the two species normally results in gray squirrel populations acting as reservoirs for the virus, with outbreaks in red squirrel populations occurring through spillover of the virus from infected gray squirrels moving into their area (McInnes et al., 2013; Chantrey et al., 2014; Shuttleworth et al., 2022). Although SQPV is not necessary for the ecological replacement of the native species by the invasive congener, modeling studies have shown that the in the presence of the disease the process can be up to 25 times more rapid (Rushton et al., 2006; see Section 6). In essence, SQPV facilitates the spatial exclusion of their native competitor representing a prominent example of disease-mediated invasions (Strauss et al., 2012).
5.3.2. The parasite Strongyloides robustus
While SQPV plays no part in the replacement of red squirrels by gray squirrels in Italy, there is emerging evidence that the nematode Strongyloides robustus does. The natural range of S. robustus is the whole North American region, where this gastro-intestinal parasite is commonly reported in several squirrel species (e.g., Davidson, 1976; O'Brien et al., 2022). The presence of this nematode was first reported in Europe in culled gray squirrels from Italy in 2014 (Romeo et al., 2014b). To the best of our knowledge, S. robustus has never been detected in other parts of the gray squirrels' European introduction range (Oldham, 1961; Scantlebury et al., 2010; McGowan et al., 2014).
Adult S. robustus inhabit the upper intestinal tract of squirrels and shed their eggs into the environment with the host feces. Once the eggs hatch, infective larvae actively seek a host and infect it by skin penetration. Inter- and intraspecific transmission is thus likely to occur on the ground, in particular at seed-caching sites, but also in dreys (Bartlett, 1995). After the first report of S. robustus in gray squirrels in Italy, an observational study comparing the macroparasite community of red squirrels in Italian red-only and red-gray sites showed that the native rodent harbored S. robustus as well, but only in the presence of the invader, strongly suggesting that the parasite is acquired by red squirrels through spillover from gray squirrels (Romeo et al., 2015). Prior to this, previous surveys on red squirrels carried out in Europe had never reported the alien parasite, describing instead a species poor endoparasite community largely dominated by a single, lowly-pathogenic nematode species, the oxyurid Trypanoxyuris sciuri (Hugot, 1984; Shimalov and Shimalov, 2002; Romeo et al., 2013). Prevalence of infection of S. robustus in gray and red squirrels was found to be similar (56.6 and 61.2%, respectively; Romeo et al., 2014b, 2015), indicating that the parasite was well-adapted to the new host. Additionally, red squirrels shed a much higher number of S. robustus eggs with feces than gray squirrels (Romeo et al., 2014a, 2020, 2021; Santicchia et al., 2020). This suggested that, in contrast to gray squirrels, they might not be able to control the infection since the immune response against Strongyloides spp. is known to suppress helminth fecundity (Wilkes et al., 2004; Viney et al., 2006).
A first piece of evidence demonstrating a detrimental effect of S. robustus on the native species came when Santicchia et al. (2020) showed that infection by the alien nematode altered behavior in red squirrels. More specifically, they revealed that infection by S. robustus correlated with a reduced expression of costly personality traits, namely the amount of activity displayed in the arena test. A more recent study (Romeo et al., 2021) demonstrated that spillover of S. robustus negatively affects red squirrels' individual fitness. From a long-term study over 9 years, the authors showed that, although the alien nematode does not have a detectable impact on red squirrels' weight or reproductive success, it ultimately reduces their survival. Future modeling studies could disclose the extent to which S. robustus spillover adds to the detrimental effect of resource competition and its impact at population level.
Red squirrels act as competent hosts for S. robustus, shedding even more eggs than gray squirrels (Romeo et al., 2021). Consequently, once the parasite is introduced in a population, it will circulate among red squirrels even after sympatric gray squirrels are removed. However, S. robustus eggs are not particularly resistant to low temperatures and drought (Viney and Lok, 2007), and in areas where red squirrels had gone locally extinct, the parasite is likely to disappear from the environment after its primary host has been removed. Therefore, the risks of infecting red squirrels should they return will be low.
5.3.3. Other pathogens
During the last decades, other pathogens have been identified in the two squirrel species in Europe, including the presence of leprosy in red squirrels in Britain and Ireland (e.g., Greenwood and Sanchez, 2002; LaRose et al., 2010; Prediger et al., 2017; Wibbelt et al., 2017; Schilling et al., 2019; Schulze et al., 2020). However, there is currently no evidence of other infections playing a role in the interaction between the two squirrel species. For instance, outbreaks of gastro-intestinal disease caused by an adenovirus have caused high mortality in red squirrel populations in Britain and Ireland (Martínez-Jiménez et al., 2011), but although gray squirrels may carry the virus, so far there is no evidence that they play a pivotal role in its circulation (Everest et al., 2012, 2014). Both red and gray squirrels are also commonly infected by coccidian protozoa (>90% prevalence), however studies in England (Ball et al., 2014) and Italy (Hofmannova et al., 2016) have shown that the two squirrels harbor different coccidia species and no interspecific transmission appears to be occurring. Also, gray and red squirrels share ectoparasites: the North American flea Orchopeas howardii is carried along by the invader in both Britain and Ireland (Scantlebury et al., 2010; McGowan et al., 2014), and the Eurasian flea Cerathophyllus sciurorum in Italy (Romeo et al., 2013, 2014a). However, whether they mediate the interaction between the two species is still unknown. Finally, it is worth mentioning that the presence of gray squirrels was found to increase chronic stress in individuals of the native species (Santicchia et al., 2020; discussed in Section 5.5). It is thus possible that gray squirrels indirectly increase susceptibility to disease of red squirrels through stress-mediated effects on immunity (Martin, 2009). Although there are no studies investigating this potential mechanism in squirrels yet, this represents an intriguing possibility to be addressed in future research.
5.4. Predator-mediated apparent competition
Predator-mediated apparent competition occurs when a shared predator influences the outcome of competition between two prey species.
In 2014, Sheehy and Lawton (2014) investigated the regional reduction in gray squirrel presence in the Irish midlands, where the distribution of the alien species had crashed over an estimated area of 9,000 km2 and red squirrels were returning after an absence of 30 years. At the same time, pine marten recolonized much of the area and there was a positive correlation between pine marten and red squirrel occurrence, whereas the correlation between pine martens and gray squirrels was negative. This dramatic impact of a native predator on red-gray squirrel interactions was also confirmed in a follow-on study in Scotland where Sheehy et al. (2018) similarly demonstrated a highly asymmetrical impact of recovering pine marten populations on red and gray squirrels with gray squirrel distribution being strongly negatively affected. A synthesis of available data by Twining et al. (2022a) argues that: “evolutionary naivety of invasive species to native predators and lack of local refuges results in higher predation of naive compared to coevolved prey”. The authors also suggest that a depauperate vertebrate predator population has increased ecosystem vulnerability to non-native species invasion. They raise concern about gray squirrels persisting in urban and suburban areas, where pine marten cannot establish, that may act as source populations for continuous immigration into more natural habitats (Twining et al., 2021).
In short, studies over the past decade on the role of pine marten in the outcome of interspecific and disease mediated competition between red and gray squirrels indicate that the recovery of pine marten populations in Britain and Ireland can hinder replacement of red by gray squirrels or locally even reverse the process through the reduction or exclusion of the invasive species (Table 1). This will depend, however, on different ecological variables such as forest and woodland suitability for pine marten establishment and for supporting red squirrel populations, landscape structure enhancing pine marten dispersal and colonization of new areas, and the degree of urbanization of a region (Slade et al., 2022a,b; Twining et al., 2022a). So far, urbanized areas are not colonized by pine marten, while gray squirrels thrive in urban and suburban woodlands, parks and gardens. Therefore, these areas are likely to act as sources in a potential source-sink dynamics from where gray squirrels will continuously disperse toward rural habitats (Table 1). Multi-species population dynamics modeling (Slade et al., 2022b) suggests that fluctuations in the abundance of other prey species (in particular voles) can create predator-prey dynamics that are temporarily more favorable for gray squirrels (switch to abundant high density vole prey with concomitant low marten pressure on gray squirrels and thus increased competition with red squirrels and higher disease transmission, Table 1). Whether such temporal changes in abundance of alternative prey can influence the red-gray squirrel system needs further empirical evidence.
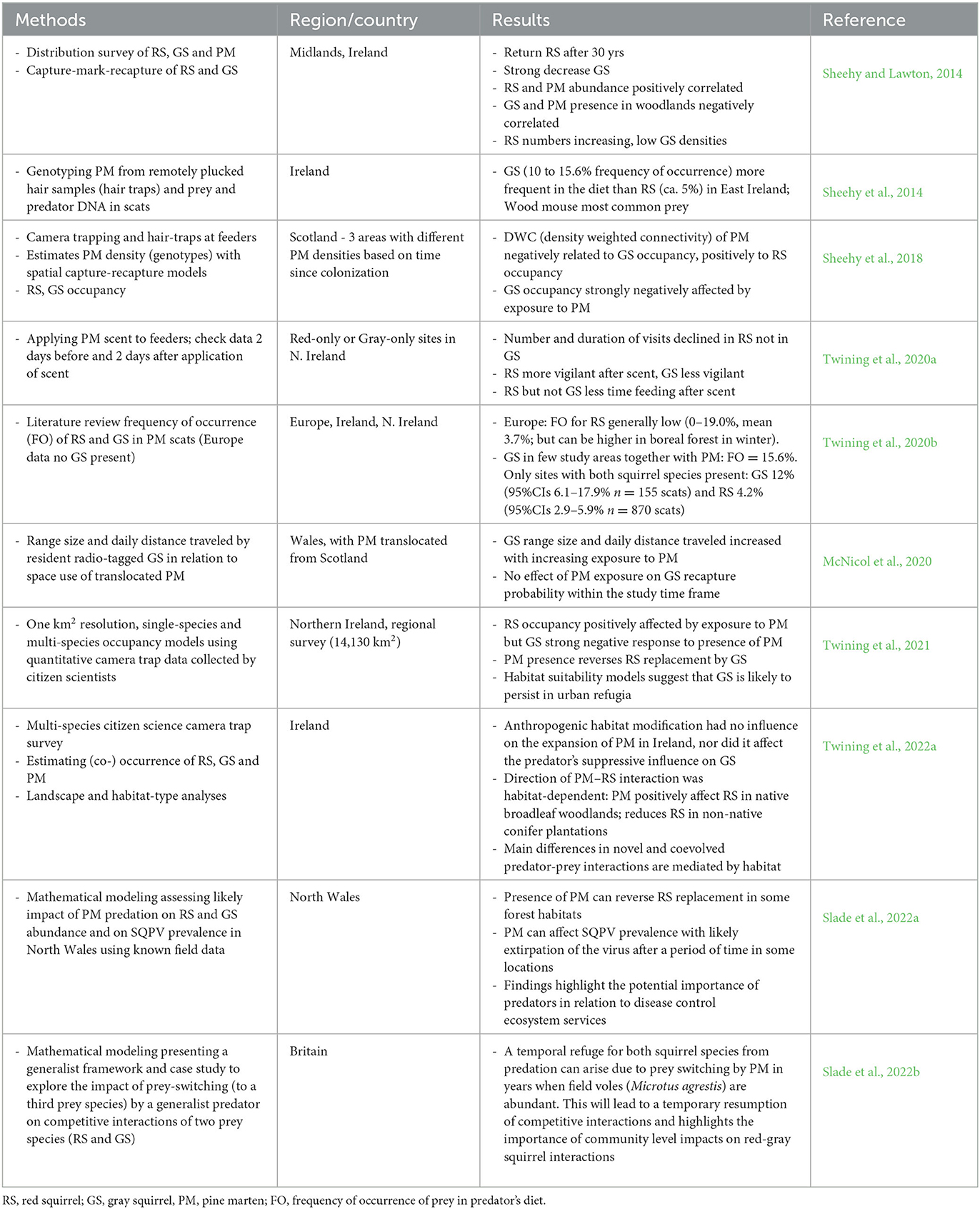
Table 1. Summary of studies in Britain and Ireland that support the role of pine marten in the outcome of predator-mediated competition between red and gray squirrels.
5.5. Interspecific competition and physiological stress
Gray squirrels can be considered as an environmental stressor when they invade red squirrel areas. Boonstra (2013) defined an environmental stressor as an unpredictable and/or noxious environmental stimulus that threatens (or is perceived to threaten) survival and/or homeostasis. Vertebrates cope with those stimuli using a suite of behavioral and physiological responses that allow them to maintain or return to homeostasis and reduce fitness loss. Short-term elevation (acute stress) of glucocorticoids (GCs), namely, cortisol and cortisone via activation of the hypothalamic–pituitary–adrenal (HPA) axis, help individuals to cope with life-threatening events (Romero, 2004; Breuner et al., 2013), while a long-term production of GCs (chronic stress), due to a prolonged or repeated exposure to stressor, might result in negative effects on survival and reproduction (Romero, 2004; Bonier et al., 2009; Cyr and Romero, 2009). In free-living animals, fecal glucocorticoid metabolites concentrations (FGMs) have been used as an estimate of long-term physiological stress (Boonstra, 2013; Palme, 2019). This measure has been validated for the Eurasian red squirrel (Dantzer et al., 2016).
By comparing FGMs between red squirrels from red-only (control) sites and animals from red-gray sites (red squirrel sites colonized by gray squirrels), Santicchia et al. (2018) found that FGMs (260 samples from 166 individuals) were on average three times higher in the latter (red-gray n = 135, mean ± SD = 78,133 ± 61,074 ng g−1 dry feces; red-only n = 125, 24,890 ± 20,566 ng g−1 dry feces), and this increase in FGMs was similar for both sexes. Moreover, comparing FGMs in animals from the same populations before and after stressor initiation, in other words, before and after the first gray squirrels colonized these sites, FGMs in the native red squirrels increased with respect to pre-colonization concentrations (see Figure 2 in Santicchia et al., 2018). This difference over time was conversely not found in control areas where no colonization by the alien species occurred (Figure 2 in Santicchia et al., 2018). These findings suggested that the presence of gray squirrels contributed to the increase in FGMs in the red squirrels, thus acting as an environmental stressor. These results were further supported by the removal of gray squirrels which resulted in a significant decrease in FGMs in co-occurring red squirrels over a 2-months interval (for details see Table 1 in Santicchia et al., 2018).
Habituation in the red squirrel stress response to the presence of gray squirrels has been observed over a longer time period (Santicchia et al., 2022a). FGMs of red squirrels increased following the early stages of invasion by gray squirrels, but then gradually decreased over time in a curvilinear way. FGMs in individual red squirrels decreased more markedly as they experienced a prolonged contact with invasive gray squirrels, whereas for those with little experience with the invasive species there was no or very little reduction in FGMs. On average, it took red squirrels about a year to habituate physiologically to the presence of gray squirrels (Santicchia et al., 2022a). These studies highlighted that the increase in physiological stress in red squirrels, caused by the presence of the invasive gray squirrel, should be considered as one of the factors that can affect the outcome of interspecific competition between these species, worsening the impact of exploitation competition on body mass/growth and possibly making red squirrels more susceptible to infections.
The physiological responses discussed above may covary with behavioral responses and this association is called a coping-style (Koolhaas et al., 1999). Individuals can be considered proactive with low GCs and a bold, more active-explorative, more aggressive personality; or reactive with high GCs and a more shy, less explorative and less aggressive personality. In theory, when a population of a native species comes in contact with an invasive species the associations between physiological and behavioral responses (i.e., coping-style) as well as among personality traits (i.e., behavioral syndrome, sensu Sih et al., 2004), might be disrupted in response to the pressure imposed by the presence of the competing invasive species (Sih et al., 2012). A recent study on red squirrels found that variation in FGMs was not correlated with any of the personality traits considered (activity, exploration, and a measure of sociability derived from arena tests); this was similar in study sites with only red squirrels and in sites where also gray squirrel occurred (see Figure 2 in Santicchia et al., 2022b). Conversely, they found a strong correlation among the personality traits in the study sites with only red squirrels, but no such correlation was found where red squirrels had to compete with the invasive congener (see Figure 2 in Santicchia et al., 2022b), suggesting a disruption of the behavioral syndrome caused by, or as a consequence of, the presence of the invasive competitor.
5.6. Interspecific competition and the ultimate effect on red squirrel population dynamics
Population dynamics of red squirrels in natural situations (no IAS present) are mainly determined by the abundance of tree seed-crops in different types of forest habitat (Kenward and Holm, 1993; Kenward et al., 1998; Wauters et al., 2004, 2008). In these studies, carried out in areas without pine martens, most population processes are bottom-up, with important seed-crops (hazelnuts in British oak-hazel woods; chestnuts or beechnuts; Scots and/or Corsican pine cone -crops; Norway spruce; and Arolla pine seed-crops) significantly affecting reproductive success, recruitment rates, and in some populations adult survival rates and/or immigration rates. However, as squirrel abundance increases, density-dependent dispersal and/or recruitment tend to reduce population growth rate (with negative per-capita growth rate at high density; Wauters et al., 2004), reducing density fluctuations and regulating the abundance around an equilibrium density for a particular habitat. The role predation might play in single species (red or gray squirrels) or mixed species dynamics, and how it interacts with bottom-up processes, requires further study. There is evidence from human-modified landscapes in Northern Ireland that habitat may influence the interactions between pine martens and red squirrels in the absence of gray squirrels (Twining et al., 2022b). Red squirrels were negatively affected by the presence of pine martens in large, structurally simple conifer plantations but not so in broadleaf woodlands. In contrast, pine martens suppressed gray squirrels regardless of habitat (see Section 5.4). Evidence for the influence of predators on red and/or gray squirrels in Italy is lacking, and the studies on exploitation or parasite-mediated competition were carried out in areas without pine martens.
Comparisons of red squirrel population processes (reproductive rate, recruitment, and adult survival) between red-only and red-gray study sites in both deciduous (in north Italy) and coniferous habitats (in north England), showed that adult survival in the native species was not reduced when gray squirrels were present (Gurnell et al., 2004). Breeding rate (% females producing a litter) of female red squirrels in spring was also similar between both sites, but there was much lower summer breeding in the red-gray (ca. 20%) than in the red-only sites (ca 50%). This resulted in no, or very few, females raising two litters/year when gray squirrels were present. Reduced summer breeding could be related to the lower body mass of females, particularly in late spring-early summer when they need to enter oestrus, possibly caused by gray squirrels pilfering food caches of sympatric red squirrels (Wauters et al., 2001a, 2002b; Gurnell et al., 2004). Moreover, recruitment of red squirrels (all age-classes but mainly juveniles) was strongly reduced in the presence of gray squirrels compared to red-only sites (Italy: 12% against 26%; England: 13% against 50%), and recruitment decreased with increasing gray squirrel density. This is probably the most important demographic process behind the replacement of red by gray squirrels, with recruitment no longer compensating for emigration and mortality. Further studies are needed on why juvenile red squirrels do not settle near their birthplace when gray squirrels are present, and whether it is related to competition for space and higher mortality, lower foraging efficiency, slower growth rates and/or increases in stress in young red squirrels.
Within this basal scenario, the other described mechanisms may or may not operate to accelerate (disease-mediated competition) or hinder/reverse (predator-mediated competition) the replacement of the native species by the invader (Figure 2).
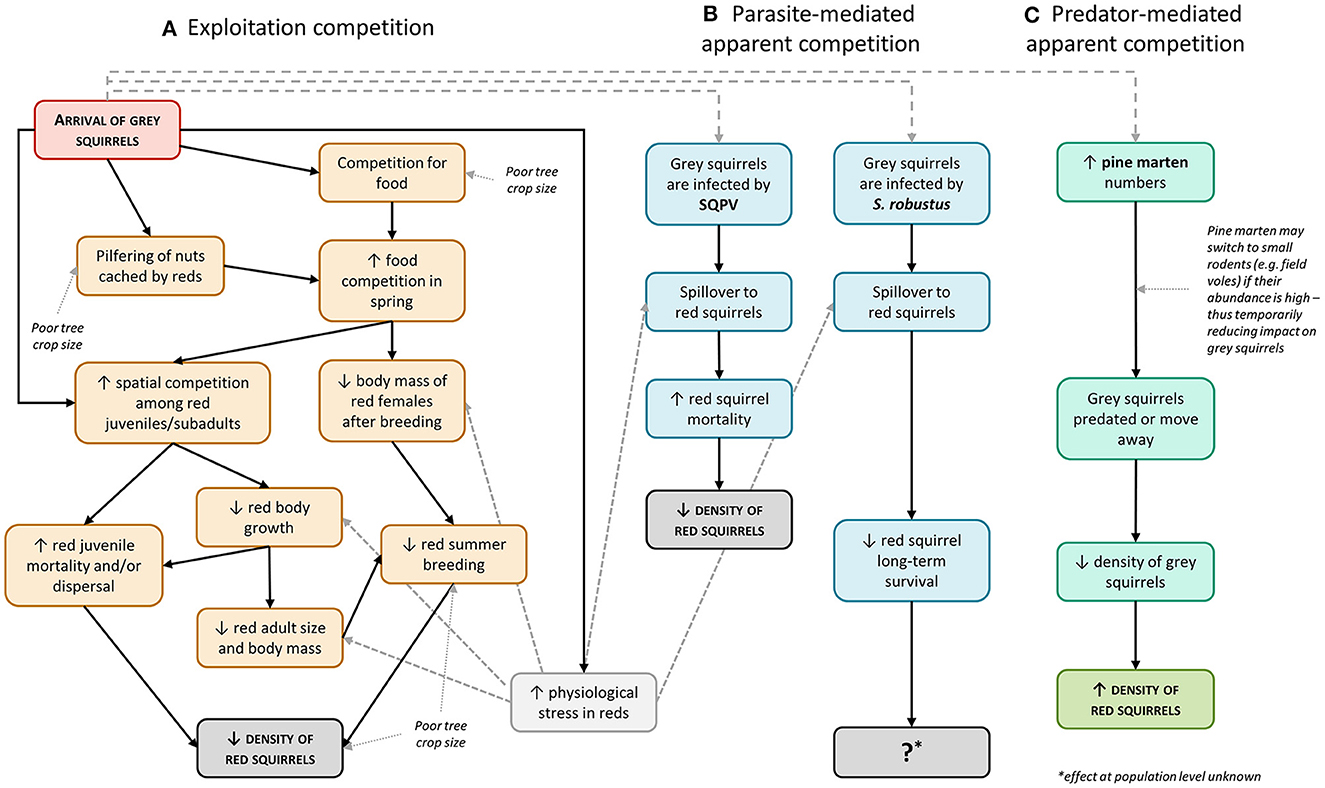
Figure 2. Principal potential pathways of the mechanisms and the factors influencing the interspecific competition between red and gray squirrels (modified and expanded from Gurnell et al., 2015a). Solid lines indicate cause-effect relationships; dotted lines indicate external factors that may influence such relationships. Big-dashed lines indicate that the arrival of gray squirrels can potentially induce parasite- or predator-mediated apparent competition. Gray squirrels may not be infected with either SQPV or S. robustus. The effects of predator-mediated competition do not apply to urban areas and an abundance of small rodents such as voles and mice may provide a temporal refuge for both squirrel species as pine martens switch to feeding on these species (Slade et al., 2022b). Finally, small-dashed lines indicate where physiological stress may influence juvenile body growth and/or body condition of adults and contribute to increase the effects of parasites on red squirrel hosts.
6. Distribution data, mathematical models, and the red-gray squirrel paradigm
Surveys to determine the distribution of gray and red squirrels go back to Middleton (1930) with his interest in the status of the introduced gray squirrel in Britain. Since then, surveys in Britain and Ireland and more recently Italy have been carried out at intervals to understand the changing distributions of the two species (Shorten, 1946; Lloyd, 1983; O'Teangana et al., 2000b; Martinoli et al., 2010). Both professionals and volunteers have been involved in surveys using a variety of methods including sightings, feeding signs, drey counts, hair-tubes, camera traps, and of late the use of phone apps for recording observations (Gurnell et al., 2014; Flaherty and Lawton, 2019). Gurnell et al.'s (2014) analysis of 20 years (1991–2010) of presence-absence records of both species collected by volunteers in northern England and Scotland at a 4 km2 scale revealed a complex pattern with a general trend in the expansion of gray squirrels and contraction of red squirrels but with local reversals and the slowing of red squirrel decline over time. This probably reflected the increasing efforts to control gray squirrels in the North of England and Scotland. The study highlighted the shortfalls of volunteer data in terms of different sampling designs, varying volunteer effort and gaps in geographic coverage pointing to the need for reliable, systematic information on changes in distribution and trends (Gurnell et al., 2014).
While repeated surveys allow the glimpse of a possible future based on continuing trends, the mechanisms underlying the changes in the observed population dynamics may not be uncovered. Although simplifications of reality, mathematical models offer a method to understand and reveal the key processes in real systems. Moreover, small effects at the individual level such as low pathogenic parasitism can reveal unexpected effects at population level. Red and gray squirrel model systems have been used to analyze changes in distribution and abundance, the effects of competition, the impact of management, and the respective roles of habitat, disease or predators on their relative abundance in space and time (Rushton et al., 2002; Bell et al., 2009; Gosso et al., 2012; Di Febbraro et al., 2013; White et al., 2014, 2016; Jones et al., 2017; Travaglia et al., 2020). Non-spatial models have been used to assess the key competitive and disease interactions between species and with habitat and predators (Tompkins et al., 2003; Bell et al., 2009; Roberts and Heesterbeek, 2021), but do not consider heterogeneity in terms of habitat quality and linkage as in real landscapes. In addition, deterministic models such as the Lotka-Volterra type competition-diffusion model used by Okubo et al. (1989) do not factor in stochasticity and variability typical of real-life systems and can be unrealistic (e.g., Barbara et al., 2018).
Subsequently the spatially explicit population dynamics model developed by Rushton et al. (1997) attempted to simulate the observed historical distribution changes in red and gray squirrels with existing woodland distribution, in this case using East Anglia in England as an example. However, their findings suggested that factors other than competition were contributing to red squirrel decline. Thereafter, Rushton et al. (2000), Tompkins et al. (2003), and Bell et al. (2009) expanded the competition model to include the effects of SQPV. Their individual findings highlighted the critical role of disease in the decline of red squirrels in Britain and Ireland (Tompkins et al., 2003; Rushton et al., 2006).
Attempts to simulate red-gray squirrel population dynamics (with and without disease) also benefited from the fact that squirrel habitat (trees and shrubs) can be mapped (including remote sensing methods) and that squirrel ecology such as mortality or fecundity in relation to different tree seed crops is relatively well understood and researched (Bertolino et al., 2008). Similarly, control effort is generally recorded and disease in terms of the presence of SQPV can be inferred. A more difficult aspect is the determination of realistic values for competitive interactions, which has been estimated from known changes in red and gray squirrel distribution (e.g., Jones et al., 2017). This led to the further development and application of spatially explicit, individual-based, population dynamics models, linked to “real-landscapes”, used to explore the risk of SQPV spread along a network of woodlands and dispersal corridors into Kielder Forest, Northumberland (the first English Squirrel reserve; Gurnell et al., 2006). Similar models have also been used to simulate the likely gray squirrel expansion in Italy (Lurz et al., 2001; Tattoni et al., 2005, 2006; Bertolino et al., 2008), as well as Ireland, and to inform disease, habitat and gray squirrel population management (White et al., 2014; Goldstein et al., 2016; Jones et al., 2017; Slade et al., 2020, 2021). Current frameworks are starting to address multi-species and ecosystem impacts such as the presence of a predator and/or the availability of different prey species on red-gray squirrel disease-mediated competition and population dynamics (Roberts and Heesterbeek, 2021; Slade et al., 2022a,b). The interesting outcome of these analyses shows that the persistence and effect of SQPV is strongly dependent on different interactions acting on diverse trophic levels and highlight the need to investigate this system using a multidimensional approach.
7. Can management and control reverse the situation?
Throughout Britain, Ireland and Italy, red squirrels persist in their natural habitat if gray squirrels are absent. A good example of this is the Isle of Wight, a 384 km2 red squirrel stronghold off the southern coast of Britain, where gray squirrels abound on the nearby mainland (Gurnell et al., 2015b). Thus, if gray squirrels can be prevented from entering an area or can be removed from an area, red squirrels will remain or return if there are habitat connections to red squirrel source areas or red squirrels are reintroduced (e.g., Lawton et al., 2015). Red squirrels may also persist in an area in the presence of gray squirrels if numbers of the latter are kept low using appropriate gray squirrel culling methods (Schuchert et al., 2014; Santicchia et al., 2018). Gray squirrel removal can also help minimize the spread of SQPV to red squirrel populations (Schuchert et al., 2014; Bertolino et al., 2016). Culling methods involve shooting, kill trapping or, the most popular method, live trapping followed by euthanasia (see Bertolino et al., 2016; Gurnell and Pepper, 2016). It is noteworthy that programmes of gray squirrel removal for red squirrel conservation must be year-round, otherwise gray squirrels can return in as little as 3 months (Lawton and Rochford, 2007). This strategy is not the same as that used for tree damage prevention whereby the aim is to minimize numbers in and around damage vulnerable woodland during the main damage season, April to July (Gurnell and Pepper, 2016). Damage to trees by bark removal is particularly serious and costly, affecting timber quality and forest regeneration, as well as the aesthetic appearance of individual trees (Derbridge et al., 2016; Nichols and Gill, 2016).
There are societal sensitivities and public opposition to culling gray squirrels in some areas (e.g., Bertolino et al., 2016, 2021; Dunn et al., 2021). As a consequence, Scapin et al. (2019) report on a gray squirrel surgical sterilization procedure adopted in Italy that will enable captured animals to be returned to the wild rather than killed, and in Britain, research on gray squirrel fertility control is ongoing as a form of non-lethal management to control gray squirrel populations (e.g., Croft et al., 2021). In this respect, the adoption of contraceptive agents (Yoder et al., 2011) and gene-drive technology as humane, efficient, and cost-effective methods of controlling gray squirrels has also been suggested (Faber et al., 2021), although a discussion of inherent risks with this approach is overdue. Other research is being carried out on a vaccine for SQPV to reduce the effects of the virus on the replacement of red by gray squirrels in Britain and Ireland (McInnes et al., 2015).
A mix of habitats, some more favorable to red squirrels, may further enable the native species to persist for a longer time (several decades, Bryce et al., 2002). Managing habitats and landscapes to this end is a medium to longer term strategy that can be adopted, for example in red squirrel strongholds in Britain and Ireland (Lurz et al., 2003; Gurnell et al., 2015b). We should also add, as mentioned elsewhere, that in parts of Ireland and Scotland the recovery of the pine marten appears to have led to a decline in the gray squirrels with a concomitant return of the red squirrel, providing a natural, biological control of the invasive species in non-urban habitats (Sheehy et al., 2014, 2018; Lawton et al., 2020; Twining et al., 2021, 2022a,b). This has encouraged the reintroduction of pine martens in Britain to places such as Wales and the Forest of Dean, in the west of England (McNicol et al., 2020; MacPherson and Wright, 2021). There is currently no information that pine or beech martens (Martes foina) are influencing red squirrel-gray squirrel competition in Italy (e.g., Twining et al., 2022a).
Although it sounds simple enough to remove gray squirrels, it is a complex, resource and human-effort intensive exercise, often involving large numbers of dedicated volunteers and requiring political engagement and the support of the local people (Bertolino et al., 2016; Robinson and Shuttleworth, 2019). Unless gray squirrels are eradicated, the control effort needs to be maintained because gray squirrels can recolonise a cleared area within a few months. Where gray squirrels have infiltrated an area or region three key red squirrel conservation strategies have been proposed (Table 2; see also Gurnell and Pepper, 1993). These approaches are not independent, and their application will depend on local and regional circumstances. One problem that has been encountered is restricted access to private properties where, for one reason or another, landowners oppose gray squirrel control actions (Bertolino et al., 2021). Gray squirrels in large urban areas may also be a problem. Cities may act as refugia and be a source for colonizing areas cleared of gray squirrels outside the urban area or, for example, where pine martens are making a comeback in parts of the Republic of Ireland, Northern Ireland or Scotland (Twining et al., 2020a, 2021). Culling gray squirrels in large urban areas will be complex and require close collaboration between local government, control agencies and local people; eradication may be particularly difficult to achieve in these situations. In fact, there are few examples of successful gray squirrel eradication from defined areas, although gray squirrel control measures in Wales, northern England, Scotland and Italy involving a mix of the above strategies are helping the red squirrel to persist or even return in areas where they would otherwise disappear in the wake of the advance of gray squirrels (e.g., Gurnell et al., 2014). As mentioned previously, documented examples of gray squirrel eradication include the Isle of Anglesey, a 714 km2 island off the north-west coast of Wales, and the urban area of Genoa Nervi in Liguria, Italy (Bertolino et al., 2016; Robertson et al., 2016).
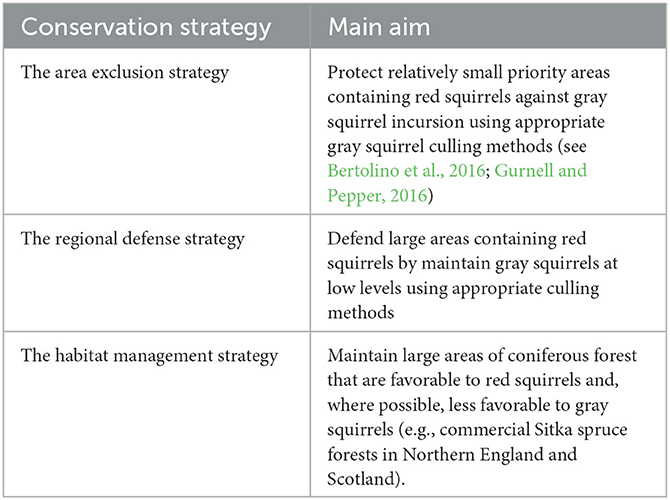
Table 2. The three key conservation strategies proposed in the United Kingdom (from Gurnell and Pepper, 1993).
8. Synthesis
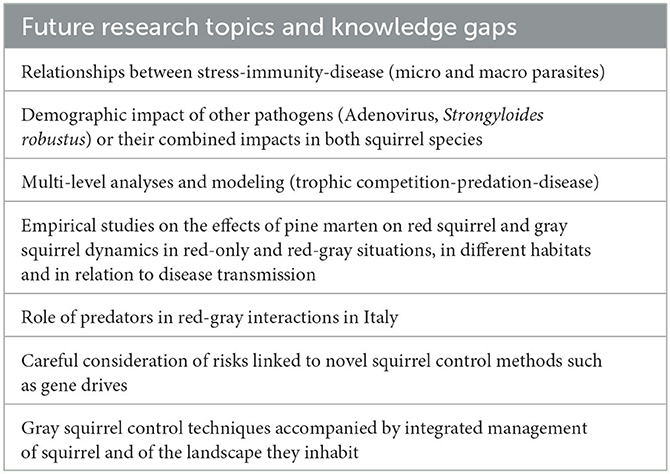
North American gray squirrels were introduced, deliberately or inadvertently, many times to several locations in Britain and Ireland from 1876, and to Italy from 1948. As they spread from their places of introduction, they invariably caused the decline or extinction of native red squirrels, providing a classic example of interspecific competition. In this systematic review we have considered the factors that influence the replacement process such as body size, behavior and physiology, population size, habitat and landscape features, and the mechanisms involved. We showed that interference competition is of little importance and that the outcome of the interaction between the two species is mainly determined by exploitation competition and apparent competition, the latter revealing often complex host-parasite and/or predator-prey interactions that can further depend on alternative prey species, landscape composition and ecosystem integrity. There are some differences between the replacement process in Britain, Ireland and Italy. SQPV virus has been a major influence in the speed of replacement in Britain and Ireland but is not present in Italy. Recent work in Italy has pointed to the likely importance of other pathogens and physiological stress, which require further study in Britain and Ireland (Table 3). Outside of urban areas, the recovery of pine martens in Ireland and Scotland appears to lead to a reduction of gray squirrels with a return of red squirrels. At present, there is no such evidence of such a predator effect occurring in Italy. These recent community-level studies, including the role of predators, highlight that progress toward solutions to problems arising from non-native species invasions require multidisciplinary approaches (Table 3). In the case of red and gray squirrels, this has involved zoologists, ecologists, foresters, veterinarians, microbiologists, geneticists, and mathematicians. Importantly, our review also illustrates that even in this well-studied model system, there are still knowledge gaps, and despite decades of research we do not have a complete picture of all mechanisms involved (Table 3). Linked to this, and no doubt typical for other species-systems as well, there has frequently been a lack of funding for replicate studies making meta-analyses approaches difficult. On a practical note, various types of management interventions, some well-established, others being designed, aim to reduce or remove gray squirrels in order to conserve red squirrels or to decrease gray squirrel damage to trees. Such interventions are complex and resource intensive,
with variable application and limited success across the European range of the invasive species. Last but not least, gray squirrel management and efforts for red squirrel conservation should attain wide public support, indicating the importance of sound and scientifically based communication to policy makers, stakeholders and the public as a whole. Only by considering all these aspects can we hope to save the native red squirrel from wide-scale extinction.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LW, FS, PL, CR, and JG developed the systematic review structure. FS did the identification of the records. FS, LW, JG, PL, and CR did the screening and the assessment of the studies. CR, PL, JG, FS, and LW wrote the first draft of the manuscript. NF and AM commented and edited drafts. All authors contributed and agreed on the final version.
Acknowledgments
We thank the three reviewers and the handling editor for their constructive comments which helped us to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor MM declared a past co-authorship with the authors LW, FS, AM, and CR.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1083008/full#supplementary-material
References
Anderson, R. M., and May, R. M. (1979). Population biology of infectious diseases: Part I. Nature 280, 361–367. doi: 10.1038/280361a0
Atkin, J. W., Radford, A. D., Coyne, K. P., Stavisky, J., and Chantrey, J. (2010). Detection of squirrel poxvirus by nested and real-time PCR from red (Sciurus vulgaris) and grey (Sciurus carolinensis) squirrels. BMC Vet. Res. 6, 33. doi: 10.1186/1746-6148-6-33
Ball, S. J., Daszak, P., Sainsbury, A. W., and Snow, K. R. (2014). Coccidian parasites of red squirrels (Sciurus vulgaris) and grey squirrels (Sciurus carolinensis) in England. J. Nat. Hist. 48, 1225–1230. doi: 10.1080/00222933.2013.867376
Ballingall, K. T., McIntyre, A., Lin, Z. Z., Timmerman, N., Matthysen, E., Lurz, P. W., et al. (2016). Limited diversity associated with duplicated class II MHC-DRB genes in the red squirrel population in the United Kingdom compared with continental Europe. Conserv. Genet. 17, 1171–1182. doi: 10.1007/s10592-016-0852-3
Barbara, F., La Morgia, V., Parodi, V., Toscano, G., and Venturino, E. (2018). Analysis of the incidence of poxvirus on the dynamics between red and grey squirrel. Mathematics. 6, 113. doi: 10.3390/math6070113
Bartlett, C. (1995). Morphology, homogonic development, and lack of a free-living generation in Strongyloides robustus (Nematoda, Rhabditoidea), a parasite of North American sciurids. Folia Parasitol. 42, 102–114.
Bell, S. S., White, A., Sherratt, J. A., and Boots, M. (2009). Invading with biological weapons: the role of shared disease in ecological invasion. Theor. Ecol. 2, 53–66. doi: 10.1007/s12080-008-0029-x
Bertolino, S. (2008). Introduction of the American grey squirrel (Sciurus carolinensis) in Europe: a case study in biological invasion. Curr. Sci. 95, 903–906.
Bertolino, S., Balduzzi, A., Martinoli, A., Carlini, E., Carnevale, P., Picco, L., et al. (2013). “Banning squirrels from the pet trade in Italy. Aliens the invasive species bulletin,” in Newsletter of the IUCN/SSC Invasive Species Specialist Group 33, 44–46.
Bertolino, S., Cordero di Montezemolo, N., Preatoni, D., Wauters, L. A., and Martinoli, A. (2014). A grey future for Europe: Sciurus carolinensis is replacing native red squirrels in Italy. Biol. Inv. 16, 53–62. doi: 10.1007/s10530-013-0502-3
Bertolino, S., and Genovesi, P. (2003). Spread and attempted eradication of the grey squirrel (Sciurus carolinensis) in Italy, and consequences for the red squirrel (Sciurus vulgaris) in Eurasia. Biol. Conserv. 109, 351–358. doi: 10.1016/S0006-3207(02)00161-1
Bertolino, S., Lurz, P., Shuttleworth, C., Martinoli, A., and Wauters, L. A. (2016). “The management of grey squirrel populations in Europe: evolving best practice,” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C. Shuttleworth, P. Lurz, and J. Gurnell (Suffolk, England: European Squirrel Initiative) 495–514.
Bertolino, S., Lurz, P. W. W., Sanderson, R., and Rushton, S. R. (2008). Predicting the spread of the American grey squirrel (Sciurus carolinensis) in Europe: A call for a co-ordinated European approach. Biol. Conserv. 141, 2564–2575. doi: 10.1016/j.biocon.2008.07.017
Bertolino, S., Martinoli, A., Paoloni, D., Marsan, A., and Wauters, L. A. (2015). The grey squirrel in Italy: impacts and management, in Red Squirrels: Ecology, Conservation and Management in Europe, eds. C. Shuttleworth, P. Lurz, and M. Hayward (Suffolk, England: European Squirrel Initiative) 163–173.
Bertolino, S., Vimercati, G., Paoloni, D., Martinoli, A., Wauters, L., Genovesi, P., et al. (2021). Restricted access to private properties limits management of invasive alien species: A literature review and case studies. J. Environ. Manage. 297, 113318. doi: 10.1016/j.jenvman.2021.113318
Bonier, F., Martin, P. R., Moore, I. T., and Wingfield, J. C. (2009). Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642. doi: 10.1016/j.tree.2009.04.013
Boonstra, R. (2013). Reality as the leading cause of stress: Rethinking the impact of chronic stress in nature. Funct. Ecol. 27, 11–23. doi: 10.1111/1365-2435.12008
Breuner, C. W., Delehanty, B., and Boonstra, R. (2013). Evaluating stress in natural populations of vertebrates: total CORT is not good enough. Funct. Ecol. 27, 24–36. doi: 10.1111/1365-2435.12016
Bruemmer, C. M., Rushton, S. P., Gurnell, J., Lurz, P. W. W., Nettleton, P., Sainsbury, A. W., et al. (2010). Epidemiology of squirrelpox virus in grey squirrels in the UK. Epidemiol. Infect. 138, 941–950. doi: 10.1017/S0950268810000816
Bryce, J., Johnson, P. J., and Macdonald, D. W. (2002). Can niche use in red and grey squirrels offer clues for their apparent coexistence? J. Appl. Ecol. 39, 875–887. doi: 10.1046/j.1365-2664.2002.00765.x
Bryce, J. M., Speakman, J. R., Johnson, P. J., and MacDonald, D. W. (2001). Competition between Eurasian red and introduced Eastern grey squirrels: the energetic significance of body-mass differences. Proc. R. Soc. B 268, 1731–1736. doi: 10.1098/rspb.2001.1700
Carroll, B., Russell, P., Gurnell, J., Nettleton, P., and Sainsbury, A. W. (2009). Epidemics of squirrelpox virus disease in red squirrels (Sciurus vulgaris): Temporal and serological findings. Epidemiol. Infect. 137, 257–265. doi: 10.1017/S0950268808000836
Chantrey, J., Dale, T., Jones, D., Begon, M., and Fenton, A. (2019). The drivers of squirrelpox virus dynamics in its grey squirrel reservoir host. Epidemics 28, 100352. doi: 10.1016/j.epidem.2019.100352
Chantrey, J., Dale, T. D., Read, J. M., White, S., Whitfield, F., Jones, D., et al. (2014). European red squirrel population dynamics driven by squirrelpox at a gray squirrel invasion interface. Ecol. Evol. 4, 3788–3799. doi: 10.1002/ece3.1216
Chow, P. K. Y., Lurz, P. W. W., and Lea, S. E. G. (2018). A battle of wits? Problem-solving abilities in invasive eastern grey squirrels and native Eurasian red squirrels. Anim. Behav. 137, 11–20. doi: 10.1016/j.anbehav.2017.12.022
Collins, L. M., Warnock, N. D., Tosh, D. G., McInnes, C., Everest, D., Montgomery, W. I., et al. (2014). Squirrelpox Virus: Assessing Prevalence, Transmission and Environmental Degradation. PLoS ONE 9, e89521. doi: 10.1371/journal.pone.0089521
Cox, P. G., Morris, P. J. R., Hennekam, J. J., and Kitchener, A. C. (2020). Morphological and functional variation between isolated populations of British red squirrels (Sciurus vulgaris). J. Zool. 312, 271–283. doi: 10.1111/jzo.12829
Croft, S., Aegerter, J. N., Beatham, S., Coats, J., and Massei, G. (2021). A spatially explicit population model to compare management using culling and fertility control to reduce numbers of grey squirrels. Ecol. Modell. 440, 109386. doi: 10.1016/j.ecolmodel.2020.109386
Currado, I., Scaramozzino, P., and Brussino, G. (1987). Note sulla presenza dello scoiattolo grigio (Sciurus carolinensis gmelin, 1788) in piemonte (Rodentia: Sciuridae). Ann. Fac. Sci. Agr. Univ. Torino. 19, 307–331.
Cyr, N. E., and Romero, L. M. (2009). Identifying hormonal habituation in field studies of stress. Gen. Comp. Endocrinol. 161, 295–303. doi: 10.1016/j.ygcen.2009.02.001
Dale, T. D., Watts, P. C., Jones, D., Pounder, K., Everest, D. J., Begon, M. E., et al. (2016). Enhancement of wildlife disease surveillance using multiplex quantitative PCR: development of qPCR assays for major pathogens in UK squirrel populations. Eur. J. Wildl. Res. 62, 589–599. doi: 10.1007/s10344-016-1031-z
Dantzer, B., Santicchia, F., van Kesteren, F., Palme, R., Martinoli, A., and Wauters, L. A. (2016). Measurement of fecal glucocorticoid metabolite levels in Eurasian red squirrels (Sciurus vulgaris): effects of captivity, sex, reproductive condition, and season. J. Mammal. 97, 1385–1398. doi: 10.1093/jmammal/gyw095
Darby, A. C., McInnes, C. J., Kjær, K. H., Wood, A. R., Hughes, M., Martensen, P. M., et al. (2014). Novel host-related virulence factors are encoded by squirrelpox virus, the main causative agent of epidemic disease in red squirrels in the UK. PLoS ONE 9, e96439. doi: 10.1371/journal.pone.0096439
Davidson, W. R. (1976). Endoparasites of selected populations of gray squirrels (Sciurus carolinensis) in the southeastern United States. Proc. Helminthol. Soc. Wash. 43, 211–217.
Derbridge, J., Pepper, H., and Koprowski, J. (2016). Economic damage by invasive grey squirrels in Europe, in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P., Lurz, and J., Gurnell (Woodbridge, Suffolk, UK: European Squirrel Initiative) 393–405.
Di Febbraro, M., Lurz, P. W. W., Genovesi, P., Maiorano, L., Girardello, M., and Bertolino, S. (2013). The use of climatic niches in screening procedures for introduced species to evaluate risk of spread: A case with the American Eastern Grey Squirrel. PLoS ONE 8, e66559. doi: 10.1371/journal.pone.0066559
Dunn, A. M., Torchin, M. E., Hatcher, M. J., Kotanen, P. M., Blumenthal, D. M., Byers, J. E., et al. (2012). Indirect effects of parasites in invasions. Funct. Ecol. 26, 1262–1274. doi: 10.1111/j.1365-2435.2012.02041.x
Dunn, M., Marzano, M., and Forster, J. (2021). The red zone: Attitudes towards squirrels and their management where it matters most. Biol. Cons. 253, e108869. doi: 10.1016/j.biocon.2020.108869
Everest, D. J., Shuttleworth, C. M., Grierson, S. S., Duff, J. P., Jackson, N., Litherland, P., et al. (2012). Systematic assessment of the impact of adenovirus infection on a captive reintroduction project for red squirrels (Sciurus vulgaris). Vet. Rec. 171, 617. doi: 10.1136/vr.100617
Everest, D. J., Shuttleworth, C. M., Stidworthy, M. F., Grierson, S. S., Duff, J. P., and Kenward, R. E. (2014). Adenovirus: an emerging factor in red squirrel Sciurus vulgaris conservation. Mammal Rev. 44, 225–233. doi: 10.1111/mam.12025
Faber, N. R., McFarlane, G. R., Gaynor, R. C., Pocrnic, I., Whitelaw, C. B. A., and Gorjanc, G. (2021). Novel combination of CRISPR-based gene drives eliminates resistance and localises spread. Scient. Rep., 11, 3719. doi: 10.1038/s41598-021-83239-4
Fiegna, C., Dagleish, M. P., Coulter, L., Milne, E., Meredith, A., Finlayson, J., et al. (2016). Host-pathogen dynamics of squirrelpox virus infection in red squirrels (Sciurus vulgaris). Vet. Microbiol. 182, 18–27. doi: 10.1016/j.vetmic.2015.10.012
Fingland, K., Ward, S. J., Bates, A. J., and Bremner-Harrison, S. (2022). A systematic review into the suitability of urban refugia for the Eurasian red squirrel Sciurus vulgaris. Mammal Rev. 52, 26–38. doi: 10.1111/mam.12264
Flaherty, M., and Lawton, C. (2019). The regional demise of a non-native invasive species: the decline of grey squirrels in Ireland. Biol. Inv. 21, 2401–2416. doi: 10.1007/s10530-019-01987-x
Goldstein, E. A., Butler, F., and Lawton, C. (2015). Frontier population dynamics of an invasive squirrel species: Do introduced populations function differently than those in the native range? Biol. Inv. 17, 1181–1197. doi: 10.1007/s10530-014-0787-x
Goldstein, E. A., Butler, F., and Lawton, C. (2016). Modeling future range expansion and management strategies for an invasive squirrel species. Biol. Inv. 18, 1431–1450. doi: 10.1007/s10530-016-1092-7
Gosso, A., La Morgia, V., Marchisio, P., Telve, O., and Venturino, E. (2012). Does a larger carrying capacity for an exotic species allow environment invasion? - some considerations on the competition of red and grey squirrels. J. Biol. Syst. 20, 221–234. doi: 10.1142/S0218339012500131
Greenwood, A. G., and Sanchez, S. (2002). Serological evidence of murine pathogens in wild grey squirrels (Sciurus carolinensis) in North Wales. Vet. Rec. 150, 543–546. doi: 10.1136/vr.150.17.543
Gurnell, J. (1996). The effects of food availability and winter weather on the dynamics of a grey squirrel population in Southern England. J. Appl. Ecol. 33, 325–338. doi: 10.2307/2404754
Gurnell, J., Blackett, T., Butler, H., Lurz, P., Magris, L., and Shuttleworth, C. (2015b). “British red squirrel strongholds: challenges for conservation,” in Red Squirrels: Ecology, Conservation and Management in Europe, eds. C.M., Shuttleworth, P.W.W., Lurz, and M.W., Hayward (Suffolk, England: European Squirrel Initiative) 211–231.
Gurnell, J., Lurz, P. W. W., and Bertoldi, W. (2014). The changing patterns in the distribution of red and grey squirrels in the North of England and Scotland between 1991 and 2010 based on volunteer surveys. Hystrix 25, 83–89. doi: 10.4404/hystrix-25.2-9988
Gurnell, J., Lurz, P. W. W., and Wauters, L. A. (2015a). “Years of interactions and conflict in Europe: competition between Eurasian red squirrels and North American grey squirrels,” in Red Squirrels: Ecology, Conservation and Management in Europe, eds. C.M., Shuttleworth, P.W.W., Lurz, and M.W., Hayward (Suffolk, England: European Squirrel Initiative) 19–37.
Gurnell, J., and Pepper, H. (1993). A critical look at conserving the British red squirrel Sciurus vulgaris. Mammal Rev. 25, 125–136. doi: 10.1111/j.1365-2907.1993.tb00424.x
Gurnell, J., and Pepper, H. (2016). “The control and management of grey squirrel populations in Britain,” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P. Lurz, and J., Gurnell (Suffolk, England: European Squirrel Initiative) 407–436.
Gurnell, J., Rushton, S. P., Lurz, P. W. W., Sainsbury, A. W., Nettleton, P., Shirley, M. D. F., et al. (2006). Squirrel poxvirus: Landscape scale strategies for managing disease threat. Biol. Conserv. 131, 287–295. doi: 10.1016/j.biocon.2006.04.009
Gurnell, J., Wauters, L. A., Lurz, P. W. W., and Tosi, G. (2004). Alien species and interspecific competition: effects of introduced eastern grey squirrels on red squirrel population dynamics. J. Anim. Ecol. 73, 26–35. doi: 10.1111/j.1365-2656.2004.00791.x
Gurnell, J., Wauters, L. A., Preatoni, D., and Tosi, G. (2001). Spacing behaviour, kinship, and population dynamics of grey squirrels in a newly colonized broadleaf woodland in Italy. Can. J. Zool. Lond. 79, 1533–1543. doi: 10.1139/z01-109
Halliwell, E., Shuttleworth, C., Cartmel, S., Lloyd, I., and Jenkins, R. (2016). “Experiences of grey squirrel management in an upland conifer forest,” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P., Lurz, and J., Gurnell (Suffolk, England: European Squirrel Initiative) 453–472.
Hofmannova, L., Romeo, C., Stohanzlova, L., Jirsova, D., Mazzamuto, M. V., Wauters, L. A., et al. (2016). Diversity and host specificity of coccidia (Apicomplexa: Eimeriidae) in native and introduced squirrel species. Eur. J. Protistology 56, 1–14. doi: 10.1016/j.ejop.2016.04.008
Holt, R. D., and Bonsall, M. B. (2017). Apparent Competition. Ann. Rev. Ecol. Evol. Syst. 48, 447–471. doi: 10.1146/annurev-ecolsys-110316-022628
Hudson, P., and Greenman, J. (1998). Competition mediated by parasites: biological and theoretical progress. Trends Ecol. Evol. 13, 387–390. doi: 10.1016/S0169-5347(98)01475-X
Hugot, J.-P. (1984). Sur le genre Trypanoxyuris (Oxyuridae, Nematoda). I: Parasites de Sciuridés: sous-genre Rodentoxyuris. Bull. Muséum Natl. Hist. Nat. Sect. Zool. Biol. Écologie Anim. 6, 711–720. doi: 10.5962/p.285910
Jeschke, J. M., and Strayer, D. L. (2005). Invasion success of vertebrates in Europe and North America. Proc. Natl. Acad. Sci. USA 102, 7198–7202. doi: 10.1073/pnas.0501271102
Jones, H., White, A., Lurz, P., and Shuttleworth, C. (2017). Mathematical models for invasive species management: Grey squirrel control on Anglesey. Ecol. Model. 359, 276–284. doi: 10.1016/j.ecolmodel.2017.05.020
Kenward, R. E., and Hodder, K. H. (1998). Red squirrels (Sciurus vulgaris) released in conifer woodland: the effects of source habitat, predation and interactions with grey squirrels (Sciurus carolinensis). J. Zool. Lond. 244, 23–32. doi: 10.1111/j.1469-7998.1998.tb00003.x
Kenward, R. E., Hodder, K. H., Rose, R. J., Walls, C. A., Parish, T., Holm, J. L., et al. (1998). Comparative demography of red squirrels (Sciurus vulgaris) and grey squirrels (Sciurus carolinensis) in deciduous and conifer woodland. J. Zool. Lond. 244, 7–21. doi: 10.1111/j.1469-7998.1998.tb00002.x
Kenward, R. E., and Holm, J. L. (1993). On the replacement of the red squirrel in britain - a phytotoxic explanation. Proc. R. Soc. B 251, 187–194. doi: 10.1098/rspb.1993.0028
Kenward, R. E., and Tonkin, J. M. (1986). Red and Grey squirrels: some behavioural and biometric differences. J. Zool. Lond. 209, 279–281. doi: 10.1111/j.1469-7998.1986.tb03583.x
Koolhaas, J. M., Korte, S. M., De Boer, S. F., Van Der Vegt, B. J., Van Reenen, C. G., Hopster, H., et al. (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 23, 925–935. doi: 10.1016/S0149-7634(99)00026-3
La Morgia, V., Paoloni, D., and Genovesi, P. (2017). Eradicating the grey squirrel Sciurus carolinensis from urban areas: an innovative decision-making approach based on lessons learnt in Italy. Pest Manage. Sci. 73, 354–363. doi: 10.1002/ps.4352
LaRose, J. P., Meredith, A. L., Everest, D. J., Fiegna, C., McInnes, C. J., Shaw, D. J., et al. (2010). Epidemiological and postmortem findings in 262 red squirrels (Sciurus vulgaris) in Scotland, 2005 to 2009. Vet. Rec. 167, 297–302. doi: 10.1136/vr.c4196
Larsen, K. (2016). “Grey squirrels in Western Canada: history repeats itself (again, and again),” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P., Lurz, and J., Gurnell (Suffolk, England: European Squirrel Initiative) 19–34.
Lawton, C., Flaherty, M., Goldstein, E. A., Sheehy, E., and Carey, M. (2015). Irish Squirrel Survey 2012 Irish Wildlife Manuals, No. 89. National Parks and Wildlife Service, Department of Arts, Heritage and the Gaeltacht, Ireland.
Lawton, C., Hanniffy, R., Molloy, V., Guilfoyle, C., Stinson, M., and Reilly, E. (2020). All-Ireland squirrel and pine marten survey 2019. Irish Wildlife Manuals, No. 121. National Parks and Wildlife Service, Department of Culture, Heritage and the Gaeltacht, Ireland.
Lawton, C., and Rochford, J. (2007). The recovery of grey squirrel (Sciurus carolinensis) populations after intensive control programmes. Biol. Environm.: Proc. R. Irish Acad. 107B, 19–29. doi: 10.1353/bae.2007.0014
Leaver, L., Hopewell, L., Caldwell, C., and Mallarky, L. (2007). Audience effects on food caching in grey squirrels (Sciurus carolinensis): evidence for pilferage avoidance strategies. Anim. Cognition. 10, 23–27. doi: 10.1007/s10071-006-0026-7
Lioy, S., Marsan, A., Balduzzi, A., Wauters, L., Martinoli, A., and Bertolino, S. (2019). The management of the introduced grey squirrel seen through the eyes of the media. Biol. Inv. 21, 3723–3733. doi: 10.1007/s10530-019-02084-9
Lison, F., Jimenez-Franco, M. V., Altamirano, A., Haz, A., Calvo, J. F., and Jones, G. (2020). Bat ecology and conservation in semi-arid and arid landscapes: a global systematic review. Mammal Rev. 50, 52–67. doi: 10.1111/mam.12175
Lloyd, H. (1983). Past and present distributions of red and grey squirrels. Mammal Rev. 13, 69–80. doi: 10.1111/j.1365-2907.1983.tb00269.x
Lurz, P. W. W., Geddes, N., Lloyd, A. J., Shirley, M. D. F., Rushton, S. P., and Burlton, B. (2003). Planning a red squirrel conservation area: Using a spatially explicit population dynamics model to predict the impact of felling and forest design plans. Forestry 76, 95–108. doi: 10.1093/forestry/76.1.95
Lurz, P. W. W., Gurnell, J., and Magris, L. (2005). Sciurus vulgaris. Mammalian Species 769, 1–10. doi: 10.1644/1545-1410(2005)7690001:SV2.0.CO;2
Lurz, P. W. W., and Lloyd, A. J. (2000). Body weights in grey and red squirrels: do seasonal weight increases occur in conifer woodland? J. Zool. 252, 539–543. doi: 10.1111/j.1469-7998.2000.tb01237.x
Lurz, P. W. W., Rushton, S. P., Wauters, L. A., Bertolino, S., Currado, I., Mazzoglio, P., et al. (2001). Predicting grey squirrel expansion in North Italy: a spatially explicit modelling approach. Landsc. Ecol. 16, 407–420. doi: 10.1023/A:1017508711713
Lymbery, A. J., Morine, M., Kanani, H. G., Beatty, S. J., and Morgan, D. L. (2014). Co-invaders: The effects of alien parasites on native hosts. Int. J. Parasitol. Parasites Wildl. 3, 171–177. doi: 10.1016/j.ijppaw.2014.04.002
Macdonald, I. M. V. (1997). Field experiments on duration and precision of grey and red squirrel spatial memory. Anim. Behav. 54, 879–891. doi: 10.1006/anbe.1996.0528
MacPherson, J., and Wright, P. (2021). Long-term strategic recovery plan for pine martens in Britain. Hertfordshire, England: Vincent Weir Trust.
Martin, L. B. (2009). Stress and immunity in wild vertebrates: timing is everything. Gen. Comp. Endocrinol. 163, 70–76. doi: 10.1016/j.ygcen.2009.03.008
Martínez-Jiménez, D., Graham, D., Couper, D., Benk,ö, M., Schöniger, S., Gurnell, J., et al. (2011). Epizootiology and pathologic findings associated with a newly described adenovirus in the red squirrel, Sciurus Vulgaris. J. Wildl. Dis. 47, 442–454. doi: 10.7589/0090-3558-47.2.442
Martinoli, A., Bertolino, S., Preatoni, D., Balduzzi, A., Marsan, A., Genovesi, P., et al. (2010). Headcount 2010: The multiplication of the grey squirrel introduced in Italy. Hystrix 21, 127–136. doi: 10.4404/hystrix-21.2-4463
Mazzamuto, M. V., Cremonesi, G., Santicchia, F., Preatoni, D., Martinoli, A., and Wauters, L. A. (2019). Rodents in the arena: a critical evaluation of methods measuring personality traits. Ethol. Ecol. Evol. 31, 38–58. doi: 10.1080/03949370.2018.1488768
Mazzamuto, M. V., Wauters, L. A., and Koprowski, J. L. (2021). Exotic pet trade as a cause of biological invasions: the case of tree squirrels of the genus callosciurus. Biology 10, 1046. doi: 10.3390/biology10101046
McGowan, N. E., Marks, N. J., McInnes, C. J., Deane, D., Maule, A. G., and Scantlebury, M. (2014). Effects of Parasitism and Morphology on Squirrelpox Virus Seroprevalence in Grey Squirrels (Sciurus carolinensis). PLoS ONE 9, e83106. doi: 10.1371/journal.pone.0083106
McInnes, C., Deane, D., and Fiegna, C. (2015). Squirrelpox virus: origins and the potential for its control, in Red Squirrels: Ecology, Conservation and Management in Europe, Eds. C., Shuttleworth, P., Lurz, and M. Hayward (Woodbridge, Suffolk, UK: European Squirrel Initiative) 251–264.
McInnes, C. J., Coulter, L., Dagleish, M. P., Deane, D., Gilray, J., Percival, A., et al. (2013). The emergence of squirrelpox in Ireland. Anim. Conserv. 16, 51–59. doi: 10.1111/j.1469-1795.2012.00570.x
McInnes, C. J., Coulter, L., Dagleish, M. P., Fiegna, C., Gilray, J., Willoughby, K., et al. (2009). First cases of squirrelpox in red squirrels (Sciurus vulgaris) in Scotland. Vet. Rec. 164, 528–531. doi: 10.1136/vr.164.17.528
McInnes, C. J., Wood, A. R., Thomas, K., Sainsbury, A. W., Gurnell, J., Dein, F. J., et al. (2006). Genomic characterization of a novel poxvirus contributing to the decline of the red squirrel (Sciurus vulgaris) in the UK. J. Gen. Virol. 87, 2115–2125. doi: 10.1099/vir.0.81966-0
McNicol, C. M., Bavin, D., Bearhop, S., Ferryman, M., Gill, R., Goodwin, C. E. D., et al. (2020). Translocated native pine martens Martes martes alter short-term space use by invasive non-native grey squirrels Sciurus carolinensis. J. Appl. Ecol. 57, 903–913. doi: 10.1111/1365-2664.13598
Merrick, M., Evans, K., and Bertolino, S. (2016). “Urban grey squirrel ecology, associated impacts, and management challenges,” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P., Lurz, and J., Gurnell (Suffolk, England: European Squirrel Initiative) 57–77.
Middleton, A. D. (1930). The Ecology of the American Grey Squirrel (Sciurus carolinensis Gmelin) in the British Isles. Proc. Zool. Soc. Lond. 100, 809–843. doi: 10.1111/j.1096-3642.1930.tb01000.x
Middleton, A. D. (1931). The Grey Squirrel: The Introduction and Spread of the American Grey Squirrel in the British Isles, its Habits, Food, and Relations with the Native Fauna and Country. London: Sidgwick and Jackson.
Nichols, C., and Gill, R. (2016). “Bark stripping behaviour by the grey squirrel, Sciurus carolinensis,” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P., Lurz, and J. Gurnell (Woodbridge, Suffolk, UK: European Squirrel Initiative) 369–390.
Obon, E., Juan-Sallés, C., McInnes, C. J., and Everest, D. J. (2011). Poxvirus identified in a red squirrel (Sciurus vulgaris) from Spain. Vet. Rec. 168, 86–86. doi: 10.1136/vr.d204
O'Brien, P. P., Bowman, J., Newar, S. L., and Garroway, C. J. (2022). Testing the parasite-mediated competition hypothesis between sympatric northern and southern flying squirrels. Int. J. Parasitol. Parasites Wildl. 17, 83–90. doi: 10.1016/j.ijppaw.2021.11.001
Okubo, A., Maini, P. K., Williamson, M. H., and Murray, J. D. (1989). On the spatial spread of the grey squirrel in Britain. Proc. R. Soc., Lond B 238, 113–125. doi: 10.1098/rspb.1989.0070
Oldham, J. N. (1961). Studies on parasites of the grey squirrel (Sciurus carolinensis Gmelin) from south eastern England. I. Helminth parasites. J. Helminthol. 127–130. doi: 10.1017/S0022149X0001765X
O'Teangana, D., Reilly, S., Montgomery, W. I., and Rochford, J. (2000a). Distribution and status of the Red Squirrel (Sciurus vulgaris) and Grey Squirrel (Sciurus carolinensis) in Ireland. Mammal Rev. 30, 45–56. doi: 10.1046/j.1365-2907.2000.00054.x
O'Teangana, D., Russ, J. M., Mathers, R. G., and Montgomery, W. I. (2000b). Habitat associations of the red squirrel Sciurus vulgaris and grey squirrel S. carolinensis in Northern Ireland. Biol. Environm. 100, 27–33.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10, 89. doi: 10.1186/s13643-021-01626-4
Palme, R. (2019). Non-invasive measurement of glucocorticoids: advances and problems. Physiol. Behav. 199, 229–243. doi: 10.1016/j.physbeh.2018.11.021
Peacock, D. (2009). The Grey Squirrel Sciurus carolinensis in Adelaide, South Australia: Its introduction and eradication. Victor. Natur. 126, 150–155.
Prediger, J., Horčičková, M., Hofmannová, L., Sak, B., Ferrari, N., Mazzamuto, M. V., et al. (2017). Native and introduced squirrels in Italy host different Cryptosporidium spp. Eur. J. Protistol. 61, 64–75. doi: 10.1016/j.ejop.2017.09.007
Pyšek, P., Hulme, P. E., Simberloff, D., Bacher, S., Blackburn, T. M., Carlton, J. T., et al. (2020). Scientists' warning on invasive alien species. Biol. Rev. 95, 1511–1534. doi: 10.1111/brv.12627
Roberts, M. G., and Heesterbeek, J. A. P. (2021). Infection dynamics in ecosystems: on the interaction between red and grey squirrels, pox virus, pine martens and trees. J. Royal Soc. Interface 18, 551. doi: 10.1098/rsif.2021.0551
Robertson, P. A., Adriaens, T., Lambin, X., Mill, A., Roy, S., Shuttleworth, C. M., et al. (2016). The large-scale removal of mammalian invasive alien species in Northern Europe. Pest Manag. Sci. 73, 273–279. doi: 10.1002/ps.4224
Robin, A. N., and Jacobs, L. F. (2022). The socioeconomics of food hoarding in wild squirrels. Curr. Opin. Behav. Sci. 45, 101139. doi: 10.1016/j.cobeha.2022.101139
Robinson, N., and Shuttleworth, C. (2019). Invasive Alien Species Colonisation Prevention: Your Guide to Early Detection and Rapid Response. Newark, UK: The Royal Society of Wildlife Trusts.
Romeo, C., Ferrari, N., Lanfranchi, P., Saino, N., Santicchia, F., Martinoli, A., et al. (2015). Biodiversity threats from outside to inside: effects of alien grey squirrel (Sciurus carolinensis) on helminth community of native red squirrel (Sciurus vulgaris). Parasitol. Res. 114, 2621–2628. doi: 10.1007/s00436-015-4466-3
Romeo, C., McInnes, C. J., Dale, T. D., Shuttleworth, C., Bertolino, S., Wauters, L. A., et al. (2019). Disease, invasions and conservation: no evidence of squirrelpox virus in grey squirrels introduced to Italy. Anim. Conserv. 22, 14–23. doi: 10.1111/acv.12433
Romeo, C., Pisanu, B., Ferrari, N., Basset, F., Tillon, L., Wauters, L. A., et al. (2013). Macroparasite community of the Eurasian red squirrel (Sciurus vulgaris): poor species richness and diversity. Parasitol. Res. 112, 3527–3536. doi: 10.1007/s00436-013-3535-8
Romeo, C., Piscitelli, A. P., Santicchia, F., Martinoli, A., Ferrari, N., and Wauters, L. A. (2021). Invading parasites: spillover of an alien nematode reduces survival in a native species. Biol. Inv. 23, 3847–3857. doi: 10.1007/s10530-021-02611-7
Romeo, C., Wauters, L. A., Cauchie, S., Martinoli, A., Matthysen, E., Saino, N., et al. (2014a). Faecal egg counts from field experiment reveal density dependence in helminth fecundity: Strongyloides robustus infecting grey squirrels (Sciurus carolinensis). Parasitol. Res. 113, 3403–3408. doi: 10.1007/s00436-014-4005-7
Romeo, C., Wauters, L. A., Ferrari, N., Lanfranchi, P., Martinoli, A., Pisanu, B., et al. (2014b). Macroparasite fauna of alien grey squirrels (Sciurus carolinensis): Composition, variability and implications for native species. PLoS ONE 9, e88002. doi: 10.1371/journal.pone.0088002
Romeo, C., Wauters, L. A., Santicchia, F., Dantzer, B., Palme, R., Martinoli, A., et al. (2020). Complex relationships between physiological stress and endoparasite infections in natural populations. Curr. Zool. 66, 449–457. doi: 10.1093/cz/zoaa029
Romero, L. M. (2004). Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255. doi: 10.1016/j.tree.2004.03.008
Rushton, S. P., Gurnell, J., Lurz, P. W. W., and Fuller, R. M. (2002). Modeling impacts and costs of gray squirrel control regimes on the viability of red squirrel populations. J. Wildl. Mngmt. 66, 683–697. doi: 10.2307/3803135
Rushton, S. P., Lurz, P. W. W., Fuller, R., and Garson, P. J. (1997). Modelling the distribution of the red and grey squirrel at the landscape scale: a combined GIS and population dynamics approach. J. Appl. Ecol. 34, 1137–1154. doi: 10.2307/2405227
Rushton, S. P., Lurz, P. W. W., Gurnell, J., and Fuller, R. (2000). Modelling the spatial dynamics of parapoxvirus disease in red and grey squirrels: a possible cause of the decline in the red squirrel in the UK? J. Appl. Ecol. 37, 997–1012. doi: 10.1046/j.1365-2664.2000.00553.x
Rushton, S. P., Lurz, P. W. W., Gurnell, J., Nettleton, P., Bruemmer, C., Shirley, M. D. F., et al. (2006). Disease threats posed by alien species: the role of a poxvirus in the decline of the native red squirrel in Britain. Epidemiol. Infect. 134, 521–533. doi: 10.1017/S0950268805005303
Sainsbury, A. W., Deaville, R., Lawson, B., Cooley, W. A., Farelly, S. S. J., Stack, M. J., et al. (2008). Poxviral disease in red squirrels sciurus vulgaris in the UK: Spatial and temporal trends of an emerging threat. EcoHealth 5, 305–316. doi: 10.1007/s10393-008-0191-z
Sainsbury, A. W., Nettleton, P., Gilray, J., and Gurnell, J. (2000). Grey squirrels have high seroprevalence to a parapoxvirus associated with deaths in red squirrels. Anim. Conserv. 3, 229–233. doi: 10.1111/j.1469-1795.2000.tb00107.x
Santicchia, F., Dantzer, B., van Kesteren, F., Palme, R., Martinoli, A., Ferrari, N., et al. (2018). Stress in biological invasions: Introduced invasive grey squirrels increase physiological stress in native Eurasian red squirrels. J. Anim. Ecol. 87, 1342–1352. doi: 10.1111/1365-2656.12853
Santicchia, F., Romeo, C., Ferrari, N., Matthysen, E., Vanlauwe, L., Wauters, L. A., et al. (2019). The price of being bold? Relationship between personality and endoparasitic infection in a tree squirrel. Mamm. Biol. 97, 1–8. doi: 10.1016/j.mambio.2019.04.007
Santicchia, F., Wauters, L. A., Dantzer, B., Palme, R., Tranquillo, C., Preatoni, D., et al. (2022b). Native species exhibit physiological habituation to invaders: a reason for hope. Proc. R. Soc. B 289, 20221022. doi: 10.1098/rspb.2022.1022
Santicchia, F., Wauters, L. A., Piscitelli, A. P., Dongen, S. V., Martinoli, A., Preatoni, D., et al. (2020). Spillover of an alien parasite reduces expression of costly behaviour in native host species. J. Anim. Ecol. 89, 1559–1569. doi: 10.1111/1365-2656.13219
Santicchia, F., Wauters, L. A., Tranquillo, C., Villa, F., Dantzer, B., Palme, R., et al. (2022a). Invasive alien species as an environmental stressor and its effects on coping style in a native competitor, the Eurasian red squirrel. Horm. Behav. 140, 105127. doi: 10.1016/j.yhbeh.2022.105127
Scantlebury, M., Maher McWilliams, M., Marks, N. J., Dick, J. T. A., Edgar, H., and Lutermann, H. (2010). Effects of life-history traits on parasite load in grey squirrels. J. Zool., Lond. 282, 246–255. doi: 10.1111/j.1469-7998.2010.00734.x
Scapin, P., Ulbano, M., Ruggiero, C., Balduzzi, A., Marsan, A., Ferrari, N., et al. (2019). Surgical sterilization of male and female grey squirrels (Sciurus carolinensis) of an urban population introduced in Italy. J. Vet. Med. Sci. 81, 641–645. doi: 10.1292/jvms.18-0319
Schilling, A.-K., Avanzi, C., Ulrich, R. G., Busso, P., Pisanu, B., Ferrari, N., et al. (2019). British red squirrels remain the only known wild rodent host for leprosy bacilli. Front. Vet. Sci. 6. doi: 10.3389/fvets.2019.00008
Schuchert, P., Shuttleworth, C. M., McInnes, C. J., Everest, D. J., and Rushton, S. P. (2014). Landscape scale impacts of culling upon a European grey squirrel population: can trapping reduce population size and decrease the threat of squirrelpox virus infection for the native red squirrel? Biol. Inv. 16, 2381–2391. doi: 10.1007/s10530-014-0671-8
Schulze, V., Lurz, P. W. W., Ferrari, N., Romeo, C., Steele, M. A., Marino, S., et al. (2020). Search for polyoma-, herpes-, and bornaviruses in squirrels of the family Sciuridae. Virol. J. 17, 42. doi: 10.1186/s12985-020-01310-4
Scott, A. C., Keymer, I. F., and Labram, J. (1981). Parapoxvirus infection of the red squirrel (Sciurus vulgaris). Vet. Rec. 109, 202. doi: 10.1136/vr.109.10.202
Seward, A., and O'Hare, S. (2015). “The importance of community support and participation for regional red squirrel conservation: a case study from northern England,” in Red Squirrels: Ecology, Conservation and Management in Europe, eds. C., Shuttleworth, P. Lurz, and M. Hayward (Suffolk, England: European Squirrel Initiative) 299–316.
Sheehy, E., and Lawton, C. (2014). Population crash in an invasive species following the recovery of a native predator: the case of the American grey squirrel and the European pine marten in Ireland. Biodiv. Conserv. 23, 753–774. doi: 10.1007/s10531-014-0632-7
Sheehy, E., O'Meara, D. B., O'Reilly, C., Smart, A., and Lawton, C. (2014). A non-invasive approach to determining pine marten abundance and predation. Eur. J. Wildl. Res. 60, 223–236. doi: 10.1007/s10344-013-0771-2
Sheehy, E., Sutherland, C., O'Reilly, C., and Lambin, X. (2018). The enemy of my enemy is my friend: native pine marten recovery reverses the decline of the red squirrel by suppressing grey squirrel populations. Proc. R. Soc. B 285, doi: 10.1098/rspb.2017.2603
Shimalov, V.V., and Shimalov, V.T. (2002). Helminth fauna of the red squirrel (Sciurus vulgaris Linnaeus, 1758) in Belorussian Polesie. Parasitol. Res. 88, 1008–1008. doi: 10.1007/s004360100437
Shorten, M. (1946). A Survey of the Distribution of the American Grey Squirrel (Sciurus carolinensis) and the British Red Squirrel (S. vulgaris leucourus) in England and Wales in 1944-5. J. Anim. Ecol. 15, 82–92. doi: 10.2307/1628
Shuttleworth, C., Halliwell, E., and Robertson, P. (2016). “Identifying incursion pathways, early detection responses and management actions to prevent grey squirrel range expansion: an island case study in Wales,” in The Grey Squirrel: Ecology and Management of an Invasive Species in Europe, eds. C., Shuttleworth, P., Lurz, and J., Gurnell (Suffolk, England: European Squirrel Initiative) 475–492.
Shuttleworth, C. M., Everest, D., Holmes, P., Bell, S., and Cripps, R. (2022). An opportunistic assessment of the impact of squirrelpox disease outbreaks upon a red squirrel population sympatric with grey squirrels in Wales. Animals 12, 99. doi: 10.3390/ani12010099
Shuttleworth, C. M., Everest, D. J., McInnes, C. J., Greenwood, A., Jackson, N. L., Rushton, S., et al. (2014). Inter-specific viral infections: Can the management of captive red squirrel collections help inform scientific research? Hystrix Ital. J. Mammal. 25, 18–24.
Shuttleworth, C. M., Robinson, N., Halliwell, E. C., Clews-Roberts, R., Peek, H., Podgornik, G., et al. (2020). Evolving grey squirrel management techniques in Europe. Manage. Biol. Inv. 11, 747–761. doi: 10.3391/mbi.2020.11.4.09
Signorile, A., Paoloni, D., and Reuman, D. (2014a). Grey squirrels in central Italy: a new threat for endemic red squirrel subspecies. Biol. Inv. 16, 2339–2350. doi: 10.1007/s10530-014-0668-3
Signorile, A. L., Reuman, D. C., Lurz, P. W. W., Bertolino, S., Carbone, C., and Wang, J. (2016). Using DNA profiling to investigate human-mediated translocations of an invasive species. Biol. Cons. 195, 97–105. doi: 10.1016/j.biocon.2015.12.026
Signorile, A. L., Wang, J., Lurz, P. W. W., Bertolino, S., Carbone, C., and Reuman, D. C. (2014b). Do founder size, genetic diversity and structure influence rates of expansion of North American grey squirrels in Europe? Divers. Distrib. 20, 918–930. doi: 10.1111/ddi.12222
Sih, A., Bell, A., and Johnson, J. C. (2004). Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. doi: 10.1016/j.tree.2004.04.009
Sih, A., Cote, J., Evans, M., Fogarty, S., and Pruitt, J. (2012). Ecological implications of behavioural syndromes: ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. doi: 10.1111/j.1461-0248.2011.01731.x
Slade, A., White, A., Kortland, K., and Lurz, P. W. W. (2020). An assessment of long-term forest management policy options for red squirrel conservation in Scotland. Hystrix 31, 137–147. doi: 10.4404/hystrix-00351-2020
Slade, A., White, A., Kortland, K., and Lurz, P. W. W. (2021). Natural strongholds for red squirrel conservation in Scotland. Nat. Cons. 43, 93–108. doi: 10.3897/natureconservation.43.62864
Slade, A., White, A., Lurz, P. W. W., Shuttleworth, C., and Lambin, X. (2022b). A temporal refuge from predation can change the outcome of prey species competition. Oikos 2022, e08565, doi: 10.1111/oik.08565
Slade, A., White, A., Lurz, P. W. W., Shuttleworth, S., Tosh, D. G., and Twining, J. P. (2022a). Indirect effects of pine marten recovery result in benefits to native prey through suppression of an invasive species and a shared pathogen. Ecol. Modell. 476, 110216. doi: 10.1016/j.ecolmodel.2022.110216
Strauss, A., White, A., and Boots, M. (2012). Invading with biological weapons: the importance of disease-mediated invasions. Funct. Ecol. 26, 1249–1261. doi: 10.1111/1365-2435.12011
Stritch, C., Naulty, F., Zintl, A., Callanan, J. J., McCullough, M., Deane, D., et al. (2015). Squirrelpox virus reservoir expansion on the east coast of Ireland. Eur. J. Wildl. Res. 61, 483–486. doi: 10.1007/s10344-015-0909-5
Tattoni, C., Preatoni, D. G., Lurz, P. W. W., Rushton, S. P., Tosdi, G., Wauters, L. A., et al. (2005). Using GRASS and Spatial Explicit Population dynamics Modelling as a conservation tool to manage grey squirrel (Sciurus carolinensis) in northern Italy. Int. J. Geoinform. 1, 71–77.
Tattoni, C., Preatoni, D. G., Lurz, P. W. W., Rushton, S. P., Tosi, G., Bertolino, S., et al. (2006). Modelling the expansion of a grey squirrel population: implications for squirrel control. Biol. Inv. 8, 1605–1619. doi: 10.1007/s10530-005-3503-z
Tedeschi, L., Biancolini, D., Capinha, C., Rondinini, C., and Essl, F. (2022). Introduction, spread, and impacts of invasive alien mammal species in Europe. Mammal Rev. 52, 252–266. doi: 10.1111/mam.12277
Tompkins, D. M., Sainsbury, A. W., Nettleton, P., Buxton, D., and Gurnell, J. (2002). Parapoxvirus causes a deleterious disease in red squirrels associated with UK population declines. Proc. R. Soc. Lond. B 269, 529–533. doi: 10.1098/rspb.2001.1897
Tompkins, D. M., White, A. R., and Boots, M. (2003). Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 6, 189–196. doi: 10.1046/j.1461-0248.2003.00417.x
Tran, T. T., Carter, B. E., and Castillo Vardaro, J. A. (2022). Predicted threats to a native squirrel from two invading species based on citizen science data. Biol. Inv. 24, 3539–3553. doi: 10.1007/s10530-022-02859-7
Tranquillo, C., Wauters, L. A., Santicchia, F., Preatoni, D., and Martinoli., A. (2022). Living on the edge: morphological and behavioral adaptations to a marginal high-elevation habitat in an arboreal mammal. Integr. Zool. 2022, 12678. doi: 10.1111/1749-4877.12679
Travaglia, E., La Morgia, V., and Venturino, E. (2020). Poxvirus, red and grey squirrel dynamics: is the recovery of a common predator affecting system equilibria? Insights from a predator-prey ecoepidemic model. Discr. Contin. Dynam. Syst. B 25, 2023–2040. doi: 10.3934/dcdsb.2019200
Twining, J., Lawton, C., White, A., Sheehy, E., Hobson, K., Montgomery, W., et al. (2022b). Restoring vertebrate predator populations can provide landscape-scale biological control of established invasive vertebrates: Insights from pine marten recovery in Europe. Global Change Biol. 28, 5368–5384. doi: 10.1111/gcb.16236
Twining, J. P., Montgomery, W. I., Price, L., Kunc, H. P., and Tosh, D. G. (2020b). Native and invasive squirrels show different behavioural responses to scent of a shared native predator. Royal Soc. Open Sci. 7, 191841. doi: 10.1098/rsos.191841
Twining, J. P., Montgomery, W. I., and Tosh, D. G. (2020a). The dynamics of pine marten predation on red and grey squirrels. Mamm. Bio. 100, 285–293. doi: 10.1007/s42991-020-00031-z
Twining, J. P., Montgomery, W. I., and Tosh, D. G. (2021). Declining invasive grey squirrel populations may persist in refugia as native predator recovery reverses squirrel species replacement. J. Appl. Ecol. 58, 248–260. doi: 10.1111/1365-2664.13660
Twining, J. P., Sutherland, C., Reid, N., and Tosh, D. G. (2022a). Habitat mediates coevolved but not novel species interactions. Proc. R. Soc., Lond. B 289. doi: 10.1098/rspb.2021.2338
Viney, M. E., and Lok, J. B. (2007). “Strongyloides spp.,” in WormBook Online Review C Elegans Biology 1–15. doi: 10.1895/wormbook.1.141.1
Viney, M. E., Steer, M. D., and Wilkes, C. P. (2006). The reversibility of constraints on size and fecundity in the parasitic nematode Strongyloides ratti. Parasitology 133, 477–483. doi: 10.1017/S003118200600062X
Wauters, L. A., and Casale, P. (1996). Long-term scatterhoarding in the red squirrel (Sciurus vulgaris). J. Zool. Lond 238, 195–207. doi: 10.1111/j.1469-7998.1996.tb05389.x
Wauters, L. A., and Dhondt, A. A. (1989). Variation in length and body weight of the red squirrel in two different habitats. J. Zool. Lond. 217, 93-106. doi: 10.1111/j.1469-7998.1989.tb02477.x
Wauters, L. A., Githiru, M., Bertolino, S., Molinari, A., Tosi, G., and Lens, L. (2008). Demography of alpine red squirrel populations in relation to fluctuations in seed crop size. Ecography 31, 104–114. doi: 10.1111/j.2007.0906-7590.05251.x
Wauters, L. A., and Gurnell, J. (1999). The mechanism of replacement of red by grey squirrels: a test of the interference competition hypothesis. Ethology 105, 1053–1071. doi: 10.1046/j.1439-0310.1999.10512488.x
Wauters, L. A., Gurnell, J., Currado, I., and Mazzoglio, P. (1997). Grey squirrel Sciurus carolinensis management in Italy - squirrel distribution in a highly fragmented landscape. Wildl. Bio. 3, 117–124. doi: 10.2981/wlb.1997.014
Wauters, L. A., Gurnell, J., Martinoli, A., and Tosi, G. (2001a). Does interspecific competition with introduced grey squirrels affect foraging and food choice of Eurasian red squirrels? Anim. Behav. 61, 1079–1091. doi: 10.1006/anbe.2001.1703
Wauters, L. A., Gurnell, J., Martinoli, A., and Tosi, G. (2002a). Interspecific competition between native Eurasian red squirrels and alien grey squirrels: does resource competition occur? Behav. Ecol. SocioBiol. 52, 332–341. doi: 10.1007/s00265-002-0516-9
Wauters, L. A., Gurnell, J., Preatoni, D., and Tosi, G. (2001b). Effects of spatial variation in food availability on spacing behaviour and demography of Eurasian red squirrels. Ecography 24, 525–538. doi: 10.1034/j.1600-0587.2001.d01-208.x
Wauters, L. A., Lurz, P. W. W., and Gurnell, J. (2000). The effects of interspecific competition by grey squirrels (Sciurus carolinensis) on the space use and population dynamics of red squirrels (S. vulgaris) in conifer plantations. Ecol. Res. 15, 271–284. doi: 10.1046/j.1440-1703.2000.00354.x
Wauters, L. A., Matthysen, E., Adriaensen, F., and Tosi, G. (2004). Within-sex density dependence and population dynamics of red squirrels Sciurus vulgaris. J. Anim. Ecol. 73, 11–25. doi: 10.1111/j.1365-2656.2004.00792.x
Wauters, L. A., Mazzamuto, M. V., Santicchia, F., Van Dongen, S., Preatoni, D. G., and Martinoli, A. (2019). Interspecific competition affects the expression of personality-traits in natural populations. Sci. Rep. 9, 11189. doi: 10.1038/s41598-019-47694-4
Wauters, L. A., Suhonen, J., and Dhondt, A. A. (1995). Fitness consequences of hoarding behaviour in the Eurasian red squirrel. Proc. R. Soc. Lond B. 262, 277–281. doi: 10.1098/rspb.1995.0206
Wauters, L. A., Tosi, G., and Gurnell, J. (2002b). Interspecific competition in tree squirrels: do introduced grey squirrels (Sciurus carolinensis) deplete tree seeds hoarded by red squirrels (S. vulgaris)? Behav. Ecol. SocioBiol. 51, 360–367. doi: 10.1007/s00265-001-0446-y
Wauters, L. A., Vermeulen, M., Van Dongen, S., Bertolino, S., Molinari, A., Tosi, G., et al. (2007). Effects of spatio-temporal variation in food supply on red squirrel Sciurus vulgaris body size and body mass and its consequences for some fitness components. Ecography 30, 51–65. doi: 10.1111/j.0906-7590.2007.04646.x
White, A., Bell, S. S., Lurz, P. W. W., and Boots, M. (2014). Conservation management within strongholds in the face of disease-mediated invasions: red and grey squirrels as a case study. J. Appl. Ecol. 51, 1631–1642. doi: 10.1111/1365-2664.12274
White, A., Lurz, P. W. W., Bryce, J., Tonkin, M., Ramoo, K., Bamforth, L., et al. (2016). Modelling disease spread in real landscapes: Squirrelpox spread in Southern Scotland as a case study. Hystrix 27, 75–82.
Wibbelt, G., Tausch, S. H., Dabrowski, P. W., Kershaw, O., Nitsche, A., and Schrick, L. (2017). Berlin squirrelpox virus, a new poxvirus in red squirrels, Berlin, Germany. Emerg. Infect. Dis. 23, 1726–1729. doi: 10.3201/eid2310.171008
Wilkes, C. P., Thompson, F. J., Gardner, M. P., Paterson, S., and Viney, M. E. (2004). The effect of the host immune response on the parasitic nematode Strongyloides ratti. Parasitology 128, 661–669. doi: 10.1017/S0031182004005062
Yoder, C. A., Mayle, B. A., Furcolow, C. A., Cowan, D. P., and Fagerstone, K. A. (2011). Feeding of grey squirrels (Sciurus carolinensis) with the contraceptive agent DiazaCon (TM): effect on cholesterol, hematology, and blood chemistry. Integr. Zool. 6, 409–419. doi: 10.1111/j.1749-4877.2011.00247.x
Keywords: exploitation competition, apparent competition, interspecific competition, Sciurus vulgaris, Sciurus carolinensis, mathematical models, biological invasions
Citation: Wauters LA, Lurz PWW, Santicchia F, Romeo C, Ferrari N, Martinoli A and Gurnell J (2023) Interactions between native and invasive species: A systematic review of the red squirrel-gray squirrel paradigm. Front. Ecol. Evol. 11:1083008. doi: 10.3389/fevo.2023.1083008
Received: 28 October 2022; Accepted: 15 March 2023;
Published: 31 March 2023.
Edited by:
Maria Vittoria Mazzamuto, University of Wyoming, United StatesReviewed by:
Colin Lawton, University of Galway, IrelandTim Adriaens, Research Institute for Nature and Forest (INBO), Belgium
Copyright © 2023 Wauters, Lurz, Santicchia, Romeo, Ferrari, Martinoli and Gurnell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Santicchia, Zi5zYW50aWNjaGlhQHVuaW5zdWJyaWEuaXQ=
 Lucas A. Wauters
Lucas A. Wauters Peter W. W. Lurz2
Peter W. W. Lurz2 Francesca Santicchia
Francesca Santicchia Claudia Romeo
Claudia Romeo Nicola Ferrari
Nicola Ferrari Adriano Martinoli
Adriano Martinoli John Gurnell
John Gurnell