
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 07 February 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1082661
This article is part of the Research Topic Theories, Methods, and Practices of Wetland Degradation and Restoration View all 17 articles
 Haipeng Dong1
Haipeng Dong1 Lihong Xie1†
Lihong Xie1† Hongjie Cao1
Hongjie Cao1 Yu Zhang1†
Yu Zhang1† Yingnan Liu1†
Yingnan Liu1† Junhui Xing1†
Junhui Xing1† Xiaoling Fu1†
Xiaoling Fu1† Jianbo Wang1†
Jianbo Wang1† Dayong Han2‡
Dayong Han2‡ Haixiu Zhong1
Haixiu Zhong1 Chunyu Luo1
Chunyu Luo1 Yi Qu1
Yi Qu1 Hongwei Ni3‡
Hongwei Ni3‡ Jifeng Wang1*‡
Jifeng Wang1*‡Plants utilize different strategies in different environments to maximize population expansion. Understanding plant reproductive strategies in heterogeneous habitats is therefore important for explaining plant ecological adaptability, and for effectively managing and conserving ecosystems. We wanted to explore the reproductive strategy transformation of D. angustifolia in heterogeneous habitats, as well as the environmental factors driving and affecting its reproductive characteristics. To do this we measured the reproductive characteristics of D. angustifolia, as well as the soil physical and chemical properties of these heterogeneous habitats. The density, biomass per unit area, and proportion of aboveground biomass in swampy meadows were significantly higher compared to other habitats. The proportion of rhizome node buds gradually increased from swampy to typical to miscellaneous grass meadows, while the proportion of tillering node buds decreased. The allocation of sexual reproduction within D. angustifolia populations was significantly and positively correlated with plant rhizome biomass and negatively correlated with the number of tillering node buds. The propagation strategies of D. angustifolia in heterogeneous habitats were consistent with CSR theory (Competitor, Stress-tolerator, and Ruderal). The proportions of inflorescence (2.07 ± 0.52%; 1.01 ± 0.15%) and root (23.8 ± 1.5%; 19.6 ± 1.4%) biomass in miscellaneous and typical meadows were high, which tended toward the “Ruderal” adaptation strategy. In swampy meadow, D. angustifolia invested mostly in vegetative growth to produce tiller node buds (14426.67 buds/m2; 46%) and ramets (1327.11 ± 102.10 plants/m2), which is characteristic of the “Competitor” strategy. Swamp D. angustifolia resisted flooding by maintaining a resource balance in its body, and was therefore biased toward the “Stress-tolerator” strategy. Environmental factors accounted for 74.63% of reproductive characteristic variation, in which the interpretative proportions of soil water content, dissolved organic carbon, ammonia nitrogen, and nitrate nitrogen were significant (p < 0.01). When soil water content, dissolved organic carbon, and nitrate nitrogen increased, D. angustifolia tended toward the C strategy; in contrast, when soil water content decreased, amine nitrogen and available phosphorus increased, and D. angustifolia tended toward the R strategy. In a stressful environment, the escape mechanism constitutes an increased rhizome and sexual reproduction investment. In contrast, for suitable habitats, tillering node buds increased in order to expand the population via new plant production, which was the propagation strategy of D. angustifolia in heterogeneous habitats.
Wetlands are important ecosystems formed by an interaction between water and land, and play a vitally important role in regulating the water cycle, reducing greenhouse gas emissions, breaking down pollution, and protecting biodiversity (Chen et al., 2018; Zhang et al., 2019). The Sanjiang Plain wetland is the largest freshwater wetland distribution area in northeastern China, and is essential for protecting biodiversity and maintaining an ecological balance (Zhao, 1999; Dong et al., 2022). In recent years, the scale of irrigation has expanded due to the need for agricultural development, and groundwater levels have subsequently experienced an annual decrease (Chen et al., 2018; Qu et al., 2022). Wetland water levels have been lowering continuously, and the wetlands themselves have in turn changed from swamps to swampy-, typical-, or miscellaneous grass meadows (Dong et al., 2022; Luo et al., 2022). As a result, these wetland ecosystems have become spatially heterogeneous. In ecology, spatial heterogeneity is characterized by ecosystem patchiness and changes in environmental gradients (Dong, 2011). Ecosystem patchiness shows that resource levels within patches are relatively consistent; however, large differences exist between patches (Wijesinghe and Handel, 1994). The widespread existence of environmental heterogeneity increases the difficulty in which plants obtain and utilize essential resources (Benton et al., 2003; Chu et al., 2020; Luo et al., 2022). The uptake and utilization of necessary resources for plant growth are linked to environmental suitability, which thus enables plants to develop adaptive strategies for efficiently accessing the necessary resources (Benton et al., 2003; Dong, 2011).
Plants have developed adaptive strategies toward their environments to cope with inter-and intraspecific competition during all their life stages (Sugiyama and Bazzaz, 1998; Pierce et al., 2013, 2017). The study of reproduction has been at the heart of evolutionary ecology (Hodgson et al., 1999; Rüger et al., 2018). The CSR theory, proposed by Grime (1974), greatly promoted the development of a new stage of life-history strategy research, namely from qualitative description to quantitative analysis. A plant life history strategy is the most suitable trade-off strategy between the environment and plant characteristics that gradually formed over long-term evolution (Grime, 2001; Caccianiga et al., 2006). Both environmental and genetic traits influence plant life-history strategies. According to plant productivity levels and the stress intensity produced by the environment, CSR theory classifies plant ecological strategies into three types: C-competitors, S-tolerators, and R-ruderals. C-competitors generally have good competitiveness and plasticity, and their energy utilization is mainly directed toward monopolizing resources quickly in fertile habitats to obtain the highest population competitiveness (Grime, 1974; Dayrell et al., 2018). S-tolerators, which have low population growth rates, short statures, and infrequent flowering events, invest energy into ecological adaptation behaviors, such as stress resistance (Reich, 2014; Dayrell et al., 2018). R-ruderals have a good colonization capacity, and are characterized by rapid germination and flowering rates, which allow them to quickly invest resources and energy into reproduction (Pierce et al., 2017; Dayrell et al., 2018). After years of development and validation, CSR strategies have been heavily supplemented. Hodgson et al. (1999) provide a method for calibrating the classification of CSR strategies: (1) the degree of species dominance in productive and undisturbed habitats provides a C-selection index, (2) variability in leaf traits known to reflect conservative lifestyles provides an S-selection index, and (3) species frequency in potentially productive but disturbed habitats provides an R-selection index (Hodgson et al., 1999). Pierce et al. (2013) used specific quantitative traits for analysis, and applied the method to general vascular plants, while simultaneously adapting it to the comparison of species within a colony and individuals within a population. Based on functional ecology theory, principal component analysis (PCA) is used to transform functional trait variation into a triangular order corresponding to CSR theory, which has a theoretical and practical application value (Pierce et al., 2013).
Reproduction is an important part of a plant’s life history. Not only is it the basis of population formation, development, and evolution, but also of the condition of ecosystem evolution (Chen and Wang, 2014; Rüger et al., 2018; Chen et al., 2019). Different plants use different reproduction methods to maintain and expand their populations (Jackson et al., 1985). Resource allocation theory holds that the biomass fixed by photosynthesis is quantitative, and that plants cannot invest an equal amount of biomass into each trait (Cornwell et al., 2014). Thus, trade-offs must be made between traits for plants to adapt to the environment (Jackson et al., 1985). Increased resource allocation to one trait may therefore decrease resource allocation to another trait (Hodgson et al., 1999). For example, a trade-off exists between clonal growth and sexual reproduction such that an increased investment in sexual reproduction will reduce the investment in clonal reproduction (Levins, 1968). This trade-off may be determined by environmental conditions, plant size, population density, and heredity (Abrahamson, 1975; Reekie, 1991). Díaz posited that the trade-off among functional traits determines plant ecological countermeasures (Díaz et al., 2016), and that ecological countermeasures are the basic conditions required to adapt to habitat diversity. The study of plant ecological responses has long been a vital element in plant ecology (Pierce et al., 2012). Only through the rational coordination of resource allocation among traits can plants achieve optimal allocation in complex ecosystems, which provides a competitive advantage for survival and reproduction (Pierce et al., 2017).
Deyeuxia angustifolia is a typical perennial, rhizomatous Poaceae species, and is widely distributed in northeastern China, the Russian Far East, Mongolia, Korea, and Japan. In China, D. angustifolia is the most concentrated in the Sanjiang Plain (Ma, 1995; An et al., 2018; Ni et al., 2022). Under suitable environmental conditions, D. angustifolia mainly relies on vegetative reproduction. This produces a large area consisting of a single, dominant community, and sexual reproduction only occurs to produce offspring. In the past, D. angustifolia research mainly focused on physiological and ecological characteristics, grassland productivity, and the effects of nitrogen deposition (An et al., 2018; Song et al., 2019; Wang et al., 2019). However, a few studies focused on D. angustifolia population reproduction in heterogeneous habitats, and more specifically on plant sexual-and asexual reproduction characteristics, as well as reproduction strategies. Four wetland types were studied: swamp, swampy meadow, typical meadow, and miscellaneous grass meadow. These were selected to analyze the reproductive characteristics and trade-offs of D. angustifolia, and to explore the following: (1) whether D. angustifolia reproductive characteristics significantly change in heterogeneous habitats; (2) whether D. angustifolia ecological strategies change in heterogeneous habitats; and (3) which environmental factors dominate the changes in the reproductive characteristics of D. angustifolia. This study reveals D. angustifolia ecological strategy transformations in heterogeneous habitats, and provides references and a scientific basis for wetland restoration.
The Honghe National Nature Reserve, of Heilongjiang Province (47°42′18″ – 47°52′07″ N, 133°34′38″ – 133°46′29″ E), is located in the northeastern part of the Sanjiang Plain, China (Dong et al., 2022; Luo et al., 2022). The study area has a temperate monsoon climate with an average annual temperature of 2.5°C and an annual average precipitation of 558 mm, which occurs mainly from July to September (Yang et al., 2012; Shi et al., 2015). Furthermore, the elevation is 50–60 m above sea level, with a slope of only 1/1000–1/300. Because of the low and relatively flat terrain, river curvature is large and water flow is unsmooth (Qu et al., 2018). The water level, soil, and nutrients of the wetlands in the Honghe Reserve are very heterogenous (Yang et al., 2012; Qu et al., 2022). A large area of natural wetland, such as typical meadow, swampy meadow, or proper swamp, occurs within the reserve, as well as an island forest which is distributed in the higher lying areas (Dong et al., 2022; Qu et al., 2022).
The sample plots were located in four different habitats, namely: perennial swamp (SW), seasonal swampy meadow (SM), typical meadow (TM) with occasional stagnant water, and miscellaneous grass meadow (MG) on the forest edge. The dominant species in SW is Carex pseudocuraica, while the subdominant species is D. angustifolia, and the soil is a typical swamp soil (Shi et al., 2015; Dong et al., 2022). In SM and TM, D. angustifolia is the dominant species and has an aggregated distribution, while the soil is a typical meadow soil. MG is located on the edge of a Populus davidiana forest, and D. angustifolia is the dominant species, accompanied by Spiraea salicifolia, Sanguisorba tenuifolia, and Anemone dichotoma. Finally, MG soil is a typical white serous soil (Qu et al., 2018; Dong et al., 2022; Luo et al., 2022; Qu et al., 2022).
In the Sanjiang Plain, rapid D. angustifolia growth starts in May, while seeds mature in mid-to-late July; above-ground biomass reaches its maximum at the end of July (Song et al., 2019; Dong et al., 2022). In contrast, rhizomes continue to grow and produce offspring through rhizome buds. At the end of September, underground biomass reaches its maximum. Hereafter, overwintering buds are produced and the number of underground buds reaches its maximum. In mid-July 2020, when above-ground D. angustifolia biomass peaked, nine 1 m x 1 m quadrats were randomly placed within each habitat. In each quadrat, five sexual and five asexual D. angustifolia plants, each exhibiting healthy growth, were selected. The above-ground parts for each plant were studied by specifically measuring their heights, whereafter the plants were divided into inflorescences, stems, and leaves.
At the beginning of October 2020, nine 1 m x 1 m quadrats were randomly placed within each habitat. In each quadrat, a 0.25 m x 0.25 m sub-quadrat was further placed in the center. The above-and underground D. angustifolia parts were excavated together. First, we counted the number of aboveground plants. Underground rhizomes were then separated from excavated plants, their fibrous roots removed, and the number of tiller node buds and rhizome buds of all plants recorded. Lastly, plant parts were dried at 60°C, until a constant weight was obtained, and then weighed. Aboveground biomass was calculated as the sum of inflorescence, stem, and leaf biomass, while total biomass was calculated as the sum of above and belowground biomass. Furthermore, rhizome biomass was calculated as the biomass of the underground parts without fibrous roots, while rhizome bud biomass was calculated as the sum of rhizome internode buds and rhizome apical buds (Figure 1 a). Tiller node buds were considered as all buds on the nodes between roots and aboveground parts (Figure 1 b). Rhizome length was calculated as the total length of all rhizomes connected end-to-end. The total number of buds was calculated as the sum of all rhizome and tiller node buds. The proportion of rhizome biomass was calculated as rhizome biomass divided by total biomass, while the proportion of aboveground biomass was calculated as the aboveground biomass divided by total biomass. Finally, the proportion of inflorescence biomass/reproductive allocation was calculated as inflorescence biomass divided by total biomass.
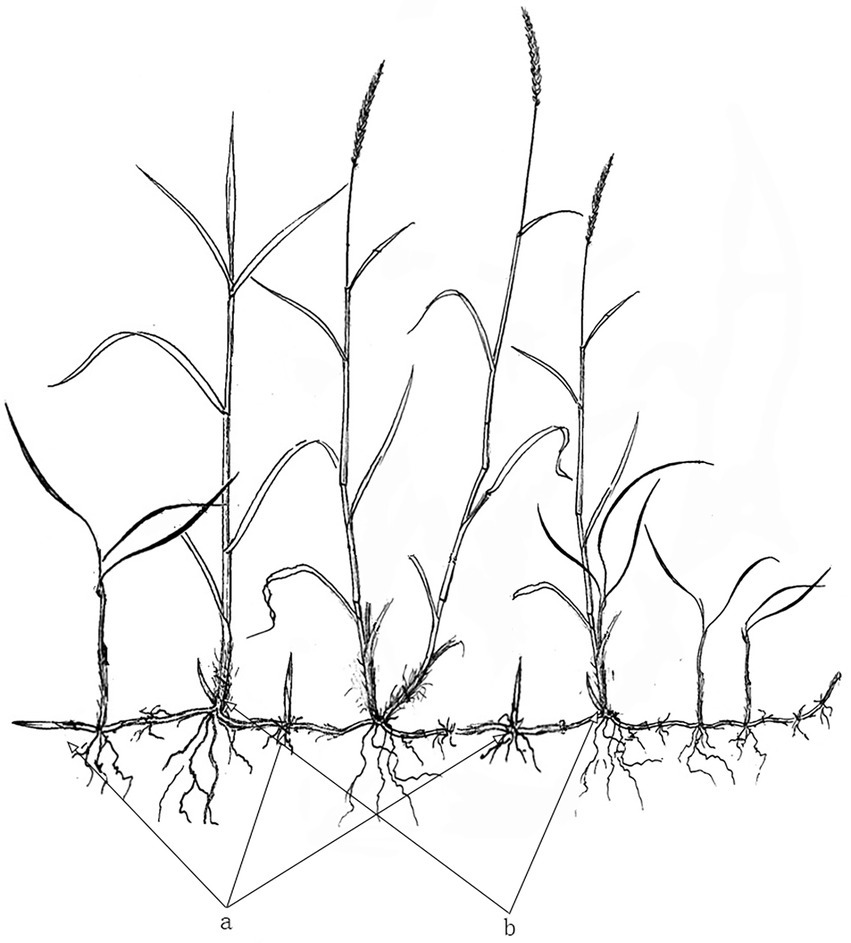
Figure 1. General morphology of various buds of Deyeuxia angustifolia. a, rhizome bud; b, tiller node bud.
Soil factor determination was performed according to the Soil Agrochemical Analysis Method (Lu, 2010) and Soil Agrochemical Analysis (Bao, 2000). In each quadrat, we first removed 5 cm of ground litter, then collected soil at a depth of 5–25 cm and mixed it into a soil sample, removed all plant roots inside the soil sample, placed the sample in a plastic bag, and then removed a part of the soil for water content determination. The other part of the soil was air-dried naturally, whereafter the soil index was determined after sieving.
Soil water content (WC) was calculated using the drying method. Fresh soil samples were weighed in aluminum boxes and oven-dried at 105°C to a constant weight.
Soil pH (pH) was measured in a soil slurry at a soil-to-water ratio of 1:2.5 by using a calibrated pH meter with a combination electrode.
For available phosphorus (AP), 5 g of soil sample was added to 25 ml of 0.5 mol·L−1 NaHCO3 and vortexed for 30 min. The filtrate was then used to determine the concentration of available phosphorus by colorimetry (Ade et al., 2018).
For total phosphorus (TP), 0.25 g of soil sample was added to 2 g NaOH, and after melting and washing 5 ml molybdenum-antimony anti-color agent was added to determine phosphorus content by a colorimetric method.
Available potassium (AK) was determined using 5 g air-dried soil by flame emission technology after vortex and filtration with 1 mol·L−1 NH4OAc.
Total potassium (TK) was determined after 0.25 g of dried soil was melted by NaOH and diluted with HCl and H2SO4, whereafter total potassium concentration was measured by flame emission technology.
Dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) were determined by extraction with 0.5% mol·L−1 CaCl2 using a TOC/TN analyzer (Shang et al., 2016).
For ammonia nitrogen (AN) and nitrate nitrogen (NN), soil NH4+-N and NO3−-N were extracted using a 2 mol·L−1 KCl solution with a 1 h vortex period. After extraction, NH4+-N and NO3−-N were determined using an AA3 continuous flow chemical analyzer (Table 1).
All statistical analyses were performed using Excel 2019 and SPSS 25.0. The one-way ANOVA and Least Significant Difference (LSD) methods were used to analyze differences in soil physicochemical properties, plant height, density, biomass, and reproductive characteristics of the different habitats. A correlation analysis was performed to analyze rhizome characteristics, and plots were created with Origin 2018. The R software, with the ‘corrplot’ package, was used to draw the correlation diagram of reproductive characteristics, and for analyzing the correlation between sexual and asexual reproduction characteristics. The trend correspondence analysis (DCA) was performed with the R software vegan package. The first axis value was less than 3.0, and a redundancy analysis (RDA) was therefore used to analyze the relationship between reproductive characteristics and soil factors. The results were plotted with the ggplot package.
Total biomass was greatly affected by environmental changes. Biomass per unit area and plant density were the highest (1246.1 ± 88.6 g, 1327.1 ± 102.1 plants/m2) in SM, followed by TM (927.7 ± 60.9 g, 870.2 ± 68.5 plants /m2), with the lowest being SW (256.6 ± 17.4 g, 166.22 ± 12.5 plants/m2) (Table 2). There were no significant differences in biomass and density between MG (327.1 ± 18.9 g, 168.9 ± 10.4 plants/m2) and SW. According to the biomass allocation analysis, the resources invested in the aboveground parts were the highest in SM (88.2 ± 0.9%), while the highest underground investment occurred in MG (23.8 ± 1.5%). There was no significant difference between SW and TM. The proportion of sexual reproduction differed between habitats. The absolute proportion of sexual reproduction was the highest in MG (2.07 ± 0.52%) and the lowest in SW (0.33 ± 0.04%). There was no significant difference in the proportion of absolute investment in sexual reproduction between TM and SM (p > 0.05).
The width of the columns and the numbers at the top denote the bud density of Deyeuxia angustifolia, and different colors indicate the proportion of different types of buds.
Deyeuxia angustifolia showed a trade-off between biomass allocation and reproductive allocation in heterogeneous habitats, and the proportion of asexual reproductive buds differed between these habitats. The proportion of tiller node buds was the highest in SM (46.3%) and the lowest in MG (11.4%) (Figure 2). The total number of buds, as well as the number of all bud kinds, differed between heterogeneous habitats. The total number of buds, as well as rhizome and tiller node bud densities, were the highest in SM; specifically, rhizome and tiller node bud densities were 12186.11 ± 573.70 buds/m2 and 10355.56 ± 840.18 buds/m2, respectively. Rhizome and tiller node bud densities were lowest in SW and MG, with 1633.33 ± 136.93 buds/m2 and 558.33 ± 30.46 buds/m2, respectively. Differences also existed in individual biomass and number of buds per plant between heterogeneous habitats, and individual biomass specifically differed significantly between habitats (p < 0.05; Table 3). Individual biomass was the highest in MG (1.942 ± 0.037 g) and the lowest in SW (0.851 ± 0.017). The number of rhizome buds, rhizome length, and rhizome biomass were all lowest in SM (6.18 ± 0.58, 17.68 ± 1.6, 0.11 ± 0.01), while those in MG were the highest (17.45 ± 2.22, 52.8 ± 4.03, 0.47 ± 0.04). By increasing the investment in rhizomes, D. angustifolia tends toward a “guerrilla-type” clonal configuration for population expansion, which manifests mainly in an increased number of rhizome buds, as well as increased rhizome biomass. Therefore, the number of rhizome buds, and rhizome biomass, can reflect changing trends in clonal configuration during habitat change. The number of rhizome buds, as well as rhizome biomass, in each habitat was significantly and positively correlated with rhizome length (Figure 3), where regression slope represents the rate at which the number of rhizome buds and rhizome biomass increased with increasing rhizome length. The regression slopes for the four habitats were MG – 0.508, 0.0098, TM – 0.273, 0.0078, SM – 0.257, 0.0044, and SW – 0.255, 0.0072, respectively. The slope of a single regression line cannot fully explain the rhizome strength for all four habitats; that is, the ability of the rhizome to expand outward through clonal reproduction. Thus, we defined rhizome strength as the product of the slopes of the two regression lines. The rhizome strengths of the four habitats were: MG – 0.0050, TM – 0.0020, SM – 0.0011, SW – 0.0018.
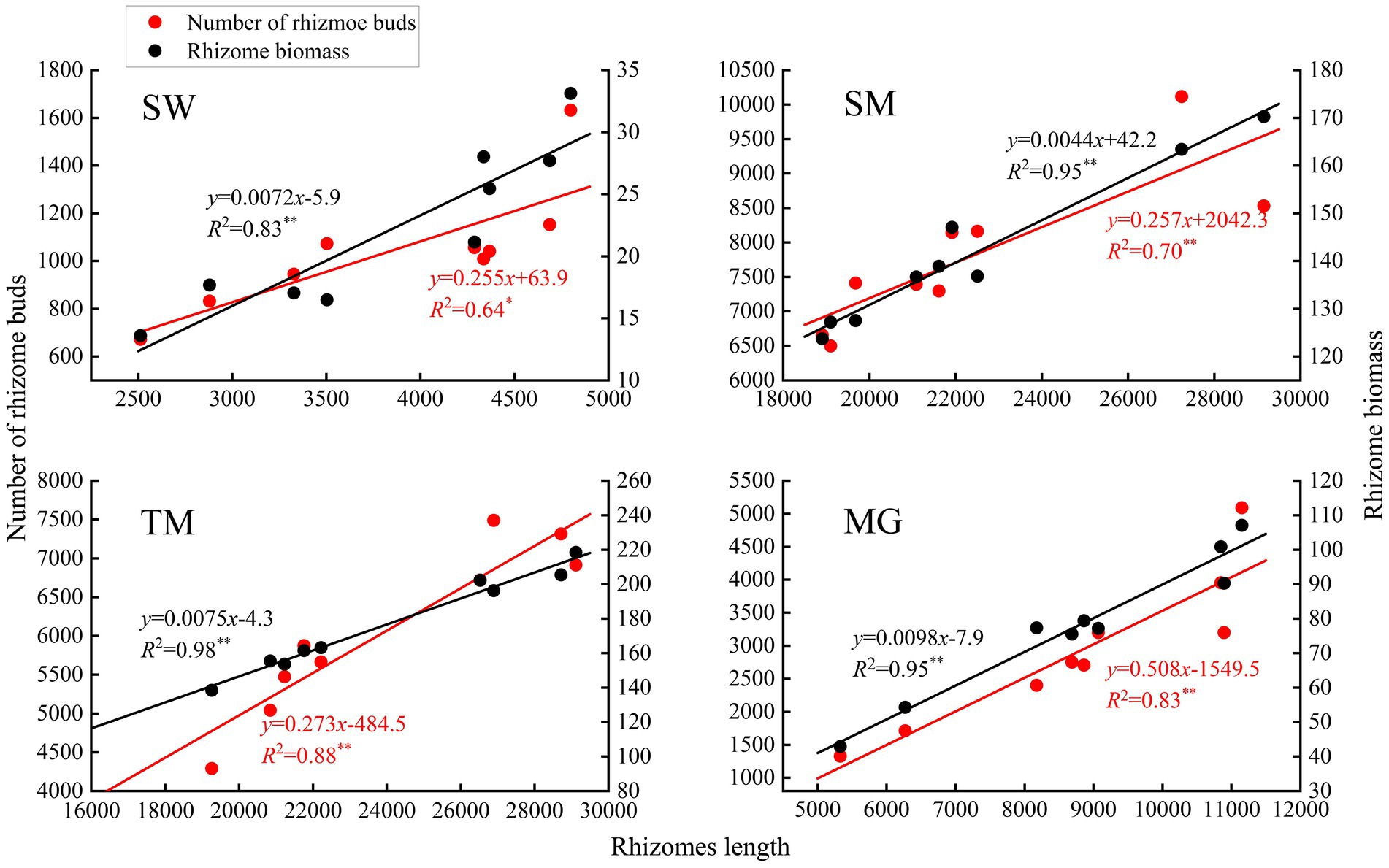
Figure 3. Regression of rhizome bud number, rhizome biomass and rhizomes length of D. angustifolia in four habitats.
The correlation analysis between sexual and asexual reproductive characteristics of D. angustifolia showed a significant positive correlation among the proportion of rhizome buds, spacer length, number of rhizome buds per plant, rhizome biomass per plant, and rhizome length per plant (p < 0.001; Figure 4). The number of rhizome buds per plant, rhizome biomass per plant, and rhizome length per plant were significantly and negatively correlated with the number of tiller node buds per plant, as well as tiller node bud and density (p < 0.01). There was a significant positive correlation between sexual reproductive allocation and inflorescence biomass, but not sexual plant biomass. Sexual reproductive allocation was positively correlated with rhizome length per plant, rhizome biomass per plant, proportion of rhizome buds, number of rhizome buds per plant, and spacer length (p < 0.01). Sexual reproductive allocation was significantly and negatively correlated with the number of tiller node buds per plant (p < 0.05). Inflorescence biomass and sexual plant biomass were positively correlated with population rhizome biomass, but had no correlation with the number of rhizome buds per plant or rhizome biomass per plant.
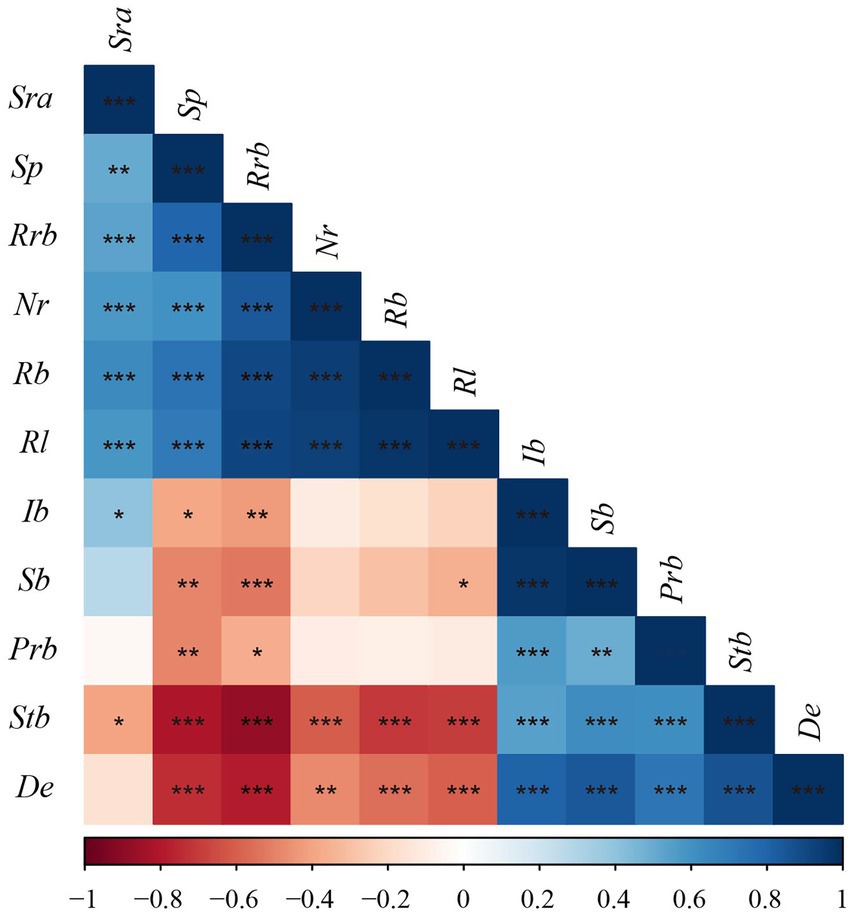
Figure 4. Pearson correlation among reproductive characteristics of D. angustifolia. Rl, rhizome length per plant; Nr, number of rhizome buds per plant; Sp, spacer; Rrb, proportion of rhizome bud; Rb, rhizome biomass per plant; Stb, tiller node bud per plant; Sra, sexual reproduction allocation; De, density; Prb, population rhizome biomass; Ib, inflorescence biomass; Sb, sexual plant biomass. * indicate the level of correlation, *p < 0.05; **p < 0.01; ***p < 0.001.
A redundancy analysis of D. angustifolia reproductive characteristics and soil factors (Figure 5) showed that the first two axes explained, by 74.63%, the influence of soil factors on the reproductive characteristics of D. angustifolia in which the number of tiller nodes per plant was mainly positively affected by WC and AK. It was negatively correlated with NN, DON, pH, AN, and AP (Table 4). Rhizome length, number of rhizome buds per plant, spacer length, and proportion of rhizome buds were all positively affected by AP and negatively affected by NN. Sexual plant biomass, rhizome biomass, and inflorescence biomass were positively correlated with TK, AP, AN, DON, and pH, but negatively correlated with WC (p < 0.001), while AP was weakly correlated with plant reproductive characteristics (p < 0.05). No correlation was found between sexual reproductive allocation and other environmental factors (p > 0.05).
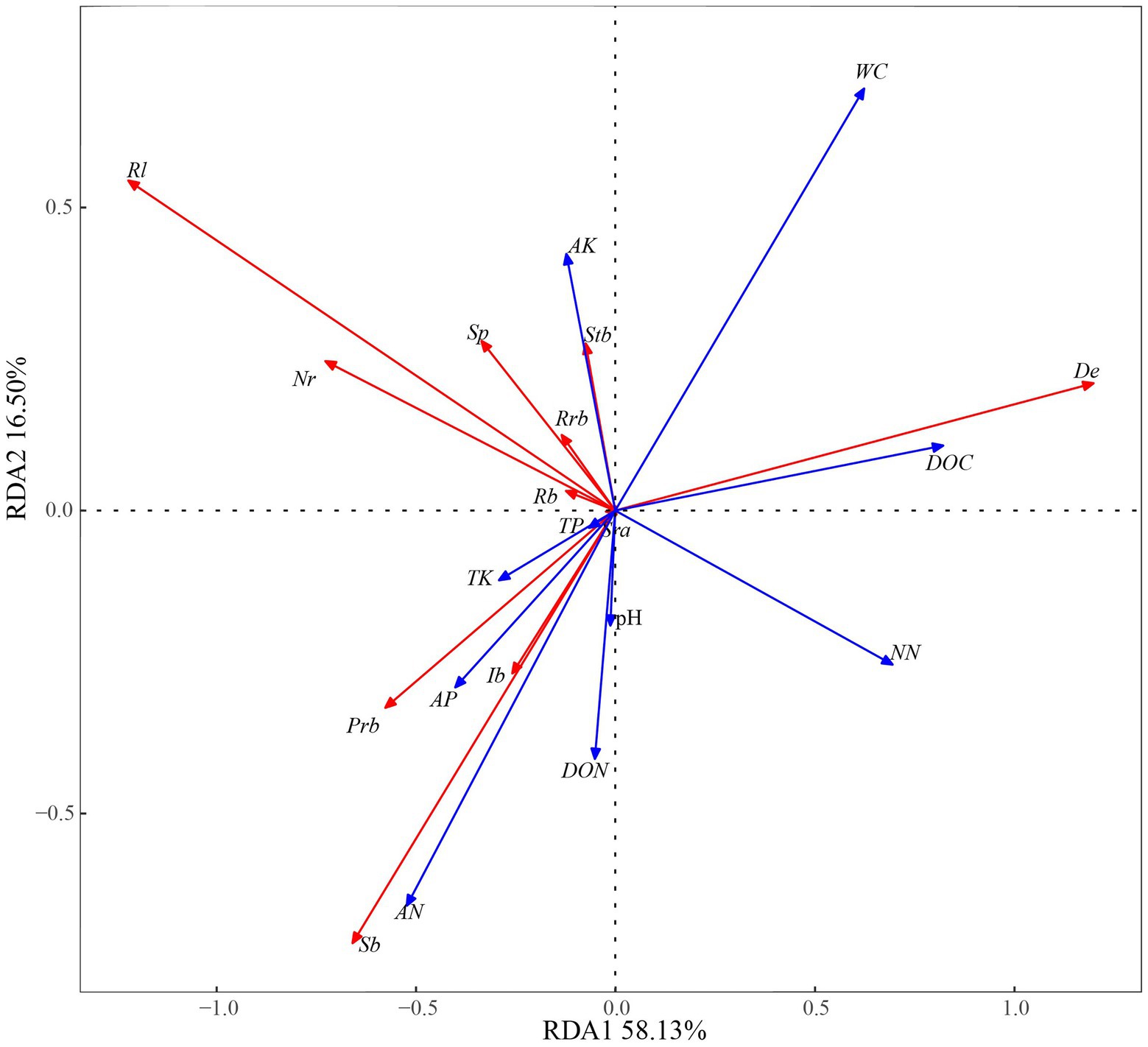
Figure 5. Relationship between reproductive characters and soil factors in redundancy analyses. Rl, rhizome length per plant; Nr, number of rhizome buds per plant; Sp, spacer; Rrb, proportion of rhizome bud; Rb, rhizome biomass per plant; Stb, tiller node bud per plant; Sra, sexual reproduction allocation; De, density; Prb, population rhizome biomass; Ib, inflorescence biomass; Sb, sexual plant biomass. pH, Soil pH; AP, Available phosphorus; TP, Total phosphorus; TK, Total kalium; AK, Available kalium; DOC, Dissolved organic carbon; DON, Dissolved organic nitrogen; NN, Nitrate nitrogen; AN, Ammonia nitrogen; WC, Soil water content.
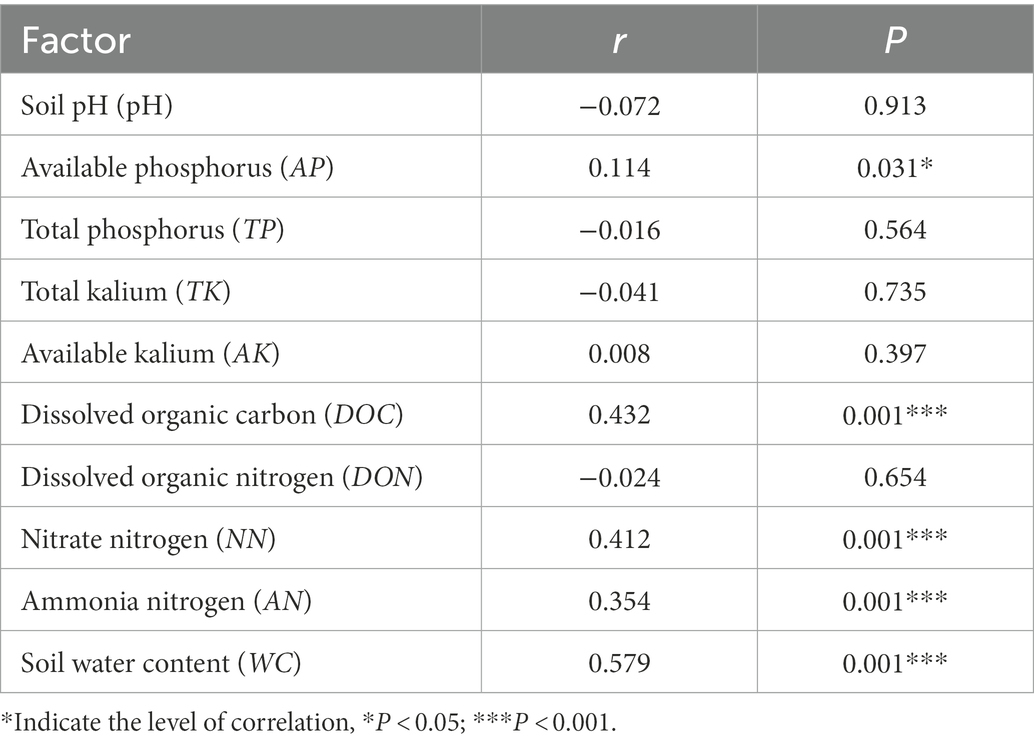
Table 4. Mantel test for the correlation between reproductive characteristics and environmental factors of D. angustifolia.
Mantel test results for each environmental factor showed that the effects of DOC, NN, AN, and WC on D. angustifolia reproductive characteristics were highly significant (p < 0.001). AP also had a significant influence on the reproductive characteristics of D. angustifolia (p < 0.001; Table 4). Finally, DON, AK, TK, TP, and pH did not significantly affect the reproductive characteristics of D. angustifolia.
Deyeuxia angustifolia is a clonal, rhizomatous plant type with well-developed underground rhizomes (Shi et al., 2015; Dong et al., 2022). At the end of each growing season, energy is directed toward rhizome growth, and rhizomes produce terminal-and node buds which develop into new plants (Hutchings and Wijesinghe, 1997; Dong et al., 2022). Rhizomes occupy a specific niche space by elongating away from the mother plant (Dong, 2011). In addition, tiller node buds can also develop into new plants. Therefore, rhizome bud growth is closely related to outward population expansion, while tiller node buds are related to the population’s ability to colonize new areas (Chu et al., 2020). Plants develop a variety of life history patterns during environmental adaptation. Specifically, out of all four habitats, the water level of SW was too high, which seriously restricted D. angustifolia growth, consequently leading to aboveground biomass and plant density being the lowest (Tables 2, 3). The water level of SM was slightly lower, and flooding stress therefore reduced. In this case, biomass per unit area, population density, and all the various bud densities were maximized. Rhizome strength was the highest in MG and the lowest in SM (Figure 3). Wetland clone plant rhizomes are mainly used for population expansion. Thus, stronger rhizomes give D. angustifolia an enhanced ability to expand its population (Dong, 2011; Shi et al., 2015; Chu et al., 2020). From SM to TM to MG, D. angustifolia density and biomass gradually decreased. When D. angustifolia occurred in TM and MG under drought stress, or in SW under flooding stress, the density of all bud types decreased, while the proportion of rhizome node buds, the number of rhizome buds, rhizome length, and rhizome biomass per plant increased. Whether at population or individual level, D. angustifolia showed a guerrilla-type clonal configuration in adverse environments. On the one hand, an increased roots biomass increased access to resources and therefore enhanced population competitiveness (Hutchings and Wijesinghe, 1997). On the other hand, rhizomes enabled an escaped from unfavorable habitats (Wijesinghe and Handel, 1994). This result is consistent with Chen et al. (2014) for growth and reproductive responses of two water-holding plants to different water level gradients. Therefore, in a restricted environment, D. angustifolia will increase investment in growth and clonal reproduction, thereby reducing the number of individuals per unit area to adapt to environmental stress (Mclntyre et al., 2009; Dong et al., 2022). This enables it to adapt to a wide range of water gradients.
Life history theory predicts a trade-off between resource allocation among different functional traits (Pierce et al., 2012, 2013; Rüger et al., 2018). This means that, when the total amount of resources is certain, increasing resource allocation to one trait will inevitably reduce resource allocation to other traits (Zhang, 2004; Mclntyre et al., 2009; Dong et al., 2022). Due to competition for nutrient elements, photosynthetic nutrients, and other resources between clonal growth and sexual reproduction, an increased investment in sexual reproduction may weaken the investment in clonal reproduction, and vice versa (Dong, 2011; Shi et al., 2015; Lu et al., 2022). In this study, a significant positive correlation existed between sexual reproductive allocation and rhizome biomass per plant, as well as a significant negative correlation between sexual reproductive allocation and tiller node bud number (Figure 4). In terms of reproductive strategy, an investment in sexual reproduction increases with increased environmental stress, while the investment in asexual reproduction (rhizomes) also increased. In contrast, the investment toward producing tillers and sprouts to form new plants reduced. In a more suitable habitat, D. angustifolia increased tiller node output to reduce sexual reproduction input, increased the tillers formed by tiller node bud germination, reduced the production of rhizomes and rhizome node buds, and reduced the investment in sexual reproduction (Figure 2). We can thus classify D. angustifolia functional traits into two categories: 1) outgoing rhizomes, rhizome buds, and sexual reproductive characteristics, and 2) in-situ growing tillering node buds and population density (Chu et al., 2020). D. angustifolia tended toward an “outgoing guerrilla” clonal configuration in stressed environments at both population and individual levels, and thereby produced an escape mechanism. In suitable habitats, D. angustifolia tended toward an in-situ growing “phalanx” clonal configuration (Jung et al., 2010; Zhang et al., 2022). This is different from Li et al. (2018) on the decrease in sexual reproduction and increase in asexual reproduction of Vallisneria spinulosa in stressful environments. The main reason is that different plants have different adaptation strategies to environmental changes.
Plant functional traits result from adaptation to different environments in the long-term evolutionary process, and can objectively express plant adaptability toward external environments (Mclntyre et al., 2009; Jung et al., 2010). In this study, the ecological strategies of D. angustifolia and its changing functional characteristic in the four habitats correspond to the CSR strategy proposed by British ecologist Grime (1974). D. angustifolia growing in MG and TM corresponded to R-strategies. It allocated resources to reproduction and population expansion mainly by devoting more resources to sexual reproduction and prolonging rhizomes to produce nodal buds (Munoz et al., 2016). It was therefore inclined toward the “Ruderal” adaptation strategy, characterized by a good expanding ability and large investment in reproduction (Grime, 1974). This is similar to Cerabolini et al. in potentially fertile, but disturbed, habitats, where many plants tended toward the R-strategy (Cerabolini et al., 2010). In MG and TM, the investment in sexual reproduction increased (2.07 ± 0.52%; 1.01 ± 0.15%), and rhizomes from asexual reproduction tended to guerrilla clonal growth (23.8 ± 1.5%; 19.6 ± 1.4%) so that plants could quickly expand from stressed sites to more suitable habitats (Pierce et al., 2013). D. angustifolia growing in SM corresponded to the C-strategy, in which it invested more resources into vegetative growth; that is, by increasing tiller nodes buds and ramets to occupy most of the available resources so that resource utilization could be optimized. The C-strategy dominated in favorable habitats and enabled the maintenance of a relatively high rate of resource acquisition in crowded, fertile, and undisturbed habitats (Grime, 2001; Cerabolini et al., 2010). The progeny formed by increasing tiller node bud germination, produced by clonal reproduction, increased resource utilization efficiency. The clonal configuration tended to the “Competitor” type. D. angustifolia therefore corresponded to the S-strategy in SW habitats. Due to the serious stress caused by excessive flooding, most of its resources were spent on maintaining survival, which was mainly reflected in short statured plants with few branches, a low flowering and seed setting rate, and other adaptive characteristics. Most of the previous studies analyzed the functional traits of multiple species and classified plants into various CSR strategies (Caccianiga et al., 2006; Pierce et al., 2013). In this study, we analyzed the functional traits of D. angustifolia under fertile, disturbed, and stressed habitats, and found that the adaptation strategies of D. angustifolia populations in these different habitats were consistent with the three CSR strategy types.
In heterogeneous habitats, plants can maximize access to resources by adjusting their resource allocation patterns, thus enabling them to have a wider ecological range and higher tolerance (Wijesinghe and Handel, 1994). Plant organs (roots, stems, leaves, flowers, and fruits) are formed by meristem differentiation (Cornwell et al., 2014; Johnson et al., 2016), which exhibits a high level of plasticity. Organ composition changes dramatically when affected by the external environment. Plant resource allocation varies with the environment, and functional traits are prioritized differently to maximize adaptability to different environments (Johnson et al., 2016). The main problem affecting performance during wetland degradation is the lack of adequate soil water, which affects soil nutrient conditions (Dong et al., 2022). By applying the Mantel test to reproductive characteristics and soil environmental factors, it was found that soil DON, DOC, AN, NN, WC, and AP were important factors driving the differences in reproductive characteristics between heterogeneous habitats in the Sanjiang Plain. In this study, WC was the most important limiting factor, and WC was negatively correlated with rhizome biomass, sexual plant biomass, and inflorescence biomass. This indicated that, when WC decreases, D. angustifolia increases its investment in rhizome and sexual reproduction. On the one hand, water absorption can be enhanced by increasing the number of rhizome fibrous roots, and on the other hand, the investment in rhizomes and seeds can be increased to avoid environmental stress. When WC decreased, and AN and AP increased, D. angustifolia tended toward the R-strategy. Some studies have shown that increased nitrogen levels promote plant sexual reproduction (Hu et al., 2012; Chen et al., 2019). In this study, AN and DON promoted the biomass of sexual plants, while increased WC reduced this effect (Li et al., 2018; Dong et al., 2022). NN and DOC were significantly and positively correlated with plant density, and when WC, DON, and NN increased simultaneously, D. angustifolia tended toward the C-strategy. This result differed from Tomlinson and O'connor (2004). This may be because, in an environment with sufficient soil nutrients, the reproduction strategy of D. angustifolia tended to increase the number of plants per unit area through the germination of tiller node buds instead of escaping by increasing rhizome investment (Wang et al., 2019; Dong et al., 2022). DOC can be quickly transformed into other components in the soil to provide nutrition for plants. In the habitats with a relatively high soil WC, such as SM, this can promote litter decomposition and increase the content of soluble organic carbon in the soil, which in turn promotes D. angustifolia growth (Chen et al., 2019).
This study focused on the process of habitat change along the SM-TM-MG gradient of heterogeneous habitats. Specifically, D. angustifolia produced an escape mechanism from unfavorable environments by increasing individual plant biomass, sexual reproductive investment, and rhizome input, and reducing biomass per unit area, tiller node buds, and plant density. The quantitative variation in reproductive characters, and the trade-off relationship between them, reflected the adaptation strategy of D. angustifolia to changes in environmental factors in heterogeneous habitats. In MG and TM, D. angustifolia invested more in sexual reproduction and rhizome extension, and tended toward the R-adaptation strategy. Clonal configuration tended to be guerrilla clonal configuration. In SM, D. angustifolia invested more in vegetative growth, and tended to the C-adaptation strategy and intensive clonal configuration. D. angustifolia resisted the flooded environment by maintaining a balance of resources, and tended toward the S-adaptation strategy. The reproductive strategy of D. angustifolia in heterogeneous habitats conforms to CSR theory. Among all the environmental factors, WC had the most significant effect on the reproductive characteristics of D. angustifolia. In the arid MG environment, it maintained a high uptake of AN and DON, and increased plant biomass to maintain its adaptation to environmental drought stress. In SM, which had enough water, it increased the number of individuals per unit area to maximize the use of DOC and other nutrients, and to maintain maximum productivity. This study provides a scientific explanation for understanding the ecological adaptation strategies of wetland plants in heterogeneous habitats.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
HD, HC, JW, YL and HN designed the study. HD, DH, QY and HZ collected the data. HD, LX, YZ and XF analyzed the data. HD, CL, JW and JX lead the writing with all co-authors. All authors gave final approval for publication.
This research was supported by the Youth Innovation Foundation of Heilongjiang Academy of Science (No. CXJQ2022ZR01), the Central Government Guides Local Science and Technology Development Special Projects (No. ZY20B15), the Heilongjiang Academy of Sciences Double-mention Wild Goose Array Special Program Project-Leading Talents (STYZ2023ZR01), and the Applied Technology Research and Development Program of Heilongjiang Province (GA19C006-5).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrahamson, W. G. (1975). Reproductive strategies in dewberries. Ecology 56, 721–726. doi: 10.2307/1935508
Ade, L. J., Hu, L., Zi, H. B., Wang, C. T., Lerdau, M., and Dong, S. K. (2018). Effect of snowpack on the soil bacteria of alpine meadows in the Qinghai-Tibetan Plateau of China. Catena 164, 13–22. doi: 10.1016/j.catena.2018.01.004
An, Y., Gao, Y., Tong, S. Z., Lu, X. G., Wang, X. H., Wang, G. D., et al. (2018). Variations in vegetative characteristics of Deyeuxia angustifolia wetlands following natural restoration in the Sanjiang plain, China. Ecol. Eng. 112, 34–40. doi: 10.1016/j.ecoleng.2017.12.022
Benton, T. G., Vickery, J. A., and Wilson, J. D. (2003). Farmland biodiversity: is habitat heterogeneity the key? Trends Ecol. Evolut. 18, 182–188. doi: 10.1016/S0169-5347(03)00011-9
Caccianiga, M., Luzzaro, A., Pierce, S., Ceriani, R. M., and Cerabolini, B. (2006). The functional basis of a primary succession resolved by CSR classification. Oikos 112, 10–20. doi: 10.1111/j.0030-1299.2006.14107.x
Cerabolini, B. E. L., Brusa, G., Ceriani, R. M., Andreis, R. D., Luzzaro, A., and Pierce, S. (2010). Can CSR classification be generally applied outside Britain? Plant Ecol. 210, 253–261. doi: 10.1007/s11258-010-9753-6
Chen, X. S., Deng, Z. M., Xie, Y. H., Li, F., and Li, X. (2014). Differential growth and vegetative reproduction by two co-occurring emergent macrophytes along a water Table gradient. Pak. J. Bot. 46, 881–886.
Chen, T., and Wang, Y. F. (2014). Response of reproductive characteristics of Saussurea macrota Franch. To elevation at eastern Qinhai-Tibetan plateau. Chin. J. Ecol. 33, 3216–3221. doi: 10.13292/j.1000-4890.2014.0282
Chen, L., Wang, L., Yang, X. G., Song, N. P., Li, Y. F., Su, Y., et al. (2019). Reproductive characteristics of Artemisia scoparia and the analysis of the underlying soil drivers in a desert steppe of China. Chin. J. Plant Ecol. 43, 65–76. doi: 10.17521/cjpe.2018.0211
Chen, H., Zhang, W. C., Gao, H. R., and Nie, N. (2018). Climate change and anthropogenic impacts on wetland and agriculture in the Songnen and Sanjiang plain, Northeast China. Remote Sens. 10:356. doi: 10.3390/rs10030356
Chu, L. S., Li, H. Y., and Yang, Y. F. (2020). Vegetative reproduction characteristics of Leymus chinensis in heterogeneous habitats in songnen plain, China. Chin. J. Appl. Ecol. 31, 83–88. doi: 10.13287/j.1001-9332.202001.001
Cornwell, W. K., Westoby, M., Falster, D. S., Fitzjohn, R. G., O’Meara, B. C., Pennell, M. W., et al. (2014). Functional distinctiveness of major plant lineages. J. Ecol. 102, 345–356. doi: 10.1111/1365-2745.12208
Dayrell, R. L. C., Arruda, A. J., Pierce, S., Negreiros, D., Meyer, P. B., Lambers, H., et al. (2018). Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 32, 2730–2741. doi: 10.1111/1365-2435.13221
Díaz, S., Kattge, J., Cornelissen, J. H. C., Wright, L. J., Lavorel, S., Dray, S., et al. (2016). The global spectrum of plant form and function. Nature 529, 167–171. doi: 10.1038/nature16489
Dong, H. P., Cao, H. J., Xie, L. H., Huang, Q. Y., Yang, L. B., Ni, H. W., et al. (2022). Effects of water levels in heterogeneous habitats on sexual reproductive allocation of Deyeuxia angustifolia. Chin. J. Appl. Ecol. 33, 378–384. doi: 10.13287/j.1001-9332.0202.004
Grime, J. P. (1974). Vegetation classification by reference to strategies. Nature 250, 26–31. doi: 10.1038/250026a0
Grime, J. P. (2001). Plant Strategies, Vegetation Processes and Ecosystem Properties, 2nd. Chichester: Wiley.
Hodgson, J. G., Wilson, P. J., Hunt, R., Grime, J. P., and Thompson, K. (1999). Allocating CSR Plant Functional Types: A Soft Approach to a Hard Problem. Oikos 85, 282–294. doi: 10.2307/3546494
Hu, Z. X., Mulholland, M. R., Duan, S. S., and Xu, N. (2012). Effects of nitrogen supply and its composition on the growth of Prorocentrum donghaiense. Harmful Algae 13, 72–82. doi: 10.1016/j.hal.2011.10.004
Hutchings, M. J., and Wijesinghe, D. K. (1997). Patchy habitats, division of labour and growth dividends in clonal plants. Trends Ecol. Evol. 12, 390–394. doi: 10.1016/S0169-5347(97)87382-X
Jackson, J. B. C., Buss, L. W., Cook, R. E., and Ashmun, J. W. (1985). Population Biology and Evolution of Clonal Organisms. New Haven: Yale University Press: 259–296.
Johnson, B. G., Verburg, P. S., and Arnone, J. A. (2016). Plant species effects on soil nutrients and chemistry in arid ecological zones. Oecologia 182, 299–317. doi: 10.1007/s00442-016-3655-9
Jung, V., Violle, C., Mondy, C., Hoffmann, L., and Muller, S. (2010). Intraspecific variability and trait-based community assembly. J. Ecol. 98, 1134–1140. doi: 10.1111/j.1365-2745.2010.01687.x
Levins, R. (1968). Evolution in Changing Environments. Princeton: Princeton University Press: 27–85.
Li, L., Lan, Z. H., Chen, J. K., and Song, Z. P. (2018). Allocation to clonal and sexual reproduction and its plasticity in Vallisneria spinulosa along a water-depth gradient. Ecosphere 9:e02070. doi: 10.1002/ecs2.2070
Lu, Y. T., Liu, H. L., Chen, Y. F., Zhang, L., Kudusi, K., and Song, J. H. (2022). Effects of drought and salt stress on seed germination of ephemeral plants in desert of Northwest China. Front. Ecol. Evol. 10, 01–16. doi: 10.3389/fevo.2022.1026095
Luo, C. Y., Fu, X. L., Zeng, X. Y., Cao, H. J., Wang, J. F., Ni, H. W., et al. (2022). Responses of remnant wetlands in the Sanjiang Plain to farming-landscape patterns. Ecol. Indic. 135:108542. doi: 10.1016/j.ecolind.2022.108542
Ma, K. P. (1995). Studies on the structure and function of Calamagrostis angustifolia grassland ecosystem I. the basic characteristics of plant community and environment. Chin. Bull. Bot. 12, 1–8.
Mclntyre, S., Lavorel, S., Landsberg, J., and Forbes, T. D. A. (2009). Disturbance response in vegetation–towards a global perspective on functional traits. J. Veg. Sci. 10, 621–630. doi: 10.2307/3237077
Munoz, F., Violle, C., and Cheptou, P. O. (2016). CSR ecological strategies and plant mating systems: outcrossing increases with competitiveness but stress-tolerance is related to mixed mating. Oikos 125, 1296–1303. doi: 10.1111/oik.02328
Ni, B., Zhao, W., Zuo, X. H., You, J., Li, Y. L., Li, J. N., et al. (2022). Deyeuxia angustifolia Kom. Encroachment changes soil physicochemical properties and microbial Community in the alpine tundra under climate change. Sci. Total Environ. 813:152615. doi: 10.1016/j.scitotenv.2021.152615
Pierce, S., Brusa, G., Sartori, M., and Cerabolini, B. E. L. (2012). Combined use of leaf size and economics traits allows direct comparison of hydrophyte and terrestrial herbaceous adaptive strategies. Ann. Bot. 109, 1047–1053. doi: 10.1093/aob/mcs021
Pierce, S., Brusa, G., Vagge, I., and Cerabolini, B. E. L. (2013). Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 27, 1002–1010. doi: 10.1111/1365-2435.12095
Pierce, S., Negreiros, D., Cerabolini, B. E. L., Kattge, J., Díaz, S., Kleyer, M., et al. (2017). A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 31, 444–457. doi: 10.1111/1365-2435.12722
Qu, Y., Luo, C. Y., Zhang, H. Q., Ni, H. W., and Xu, N. (2018). Modeling the wetland restorability based on natural and anthropogenic impacts in Sanjiang Plain, China. Ecol. Indic. 91, 429–438. doi: 10.1016/j.ecolind.2018.04.008
Qu, Y., Zheng, Y. M., Gong, P., Shi, J. L., Li, L. P., Wang, S. D., et al. (2022). Estimation of wetland biodiversity based on the hydrological patterns and connectivity and its potential application in change detection and monitoring: a case study of the Sanjiang Plain, China. Sci. Total. Environ. 805:150291. doi: 10.1016/j.scitotenv.2021.150291
Reekie, E. (1991). Cost of seed versus rhizome production in Agropyron repens. Can. J. Bot. 69, 2678–2683. doi: 10.1139/b91-336
Reich, P. B. (2014). The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301. doi: 10.1111/1365-2745.12211
Rüger, N., Comita, L. S., Condit, R., Purves, D., Rosenbaum, B., Visser, M. D., et al. (2018). Beyond the fast–slow continuum: demographic dimensions structuring a tropical tree community. Ecol. Lett. 21, 1075–1084. doi: 10.1111/ele.12974
Shang, W., Wu, X. D., Zhao, L., Yue, G. Y., Zhao, Y. H., Qiao, Y. P., et al. (2016). Seasonal variations in labile soil organic matter fractions in permafrost soils with different vegetation types in the Central Qinghai-Tibet Plateau. Catena 137, 670–678. doi: 10.1016/j.catena.2015.07.012
Shi, F. X., Song, C. C., Zhang, X. H., Mao, R., Guo, Y. D., and Gao, F. Y. (2015). Plant zonation patterns reflected by the differences in plant growth, biomass partitioning and root traits along a water level gradient among four common vascular plants in freshwater marshes of the Sanjiang Plain, Northeast China. Ecol. Eng. 81, 158–164. doi: 10.1016/j.ecoleng.2015.04.054
Song, Y. Y., Song, C. C., Ren, J. S., Zhang, X. H., and Jiang, L. (2019). Nitrogen input increases Deyeuxia angustifolia litter decomposition and enzyme activities in a marshland ecosystem in Sanjiang Plain, Northeast China. Wetlands 39, 549–557. doi: 10.1007/s13157-018-1102-x
Sugiyama, S., and Bazzaz, F. A. (1998). Size dependence of reproductive allocation: the influence of resource availability, competition and genetic identity. Funct. Ecol. 12, 280–288. doi: 10.1046/j.1365-2435.1998.00187.x
Tomlinson, K. W., and O'connor, T. G. (2004). Control of tiller recruitment in bunchgrasses: uniting physiology and ecology. Funct. Ecol. 18, 489–496. doi: 10.1111/j.0269-8463.2004.00873.x
Wang, X., Tong, S. Z., Li, Y. Z., Qi, Q., Zhang, D. J., Lyu, X. G., et al. (2019). Plant diversity performance after natural restoration in reclaimed Deyeuxia angustifolia wetland. Chin. Geogr. Sci. 29, 437–445. doi: 10.1007/s11769-019-1043-1
Wijesinghe, D. K., and Handel, S. N. (1994). Advantages of clonal growth in heterogeneous habitats: an experiment with Potentilla simplex. J. Ecol. 82, 495–502. doi: 10.2307/2261258
Yang, W. J., Cheng, H. G., Hao, F. H., Ouyang, W., Liu, S. Q., and Lin, C. Y. (2012). The influence of land-use change on the forms of phosphorus in soil profiles from the Sanjiang Plain of China. Geoderma 189–190, 207–214. doi: 10.1016/j.geoderma.2012.06.025
Zhang, D. Y. (2004). Plant Life-History Evolution and Reproductive Ecology Edited. Beijing: Science Press: 67–98.
Zhang, D. J., Qi, Q., Wang, X. H., Tong, S. Z., Lv, X. G., An, Y., et al. (2019). Physiological responses of Carex schmidtii Meinsh to alternating flooding-drought conditions in the Momoge wetland, Northeast China. Aquat. Bot. 153, 33–39. doi: 10.1016/j.aquabot.2018.11.010
Zhang, D. J., Xia, J. B., Sun, J. K., Dong, K. K., Shao, P. S., Wang, X. H., et al. (2022). Effect of wetland restoration and degradation on nutrient trade-off of Carex schmidtii. Front. Ecol. Evol. 9, 1–14. doi: 10.3389/fevo.2021.801608
Keywords: Sanjiang Plain wetland, heterogeneous habitats, propagation strategies, tradeoff, Deyeuxia angustifolia
Citation: Dong H, Xie L, Cao H, Zhang Y, Liu Y, Xing J, Fu X, Wang J, Han D, Zhong H, Luo C, Qu Y, Ni H and Wang J (2023) Propagation strategies of Deyeuxia angustifolia in heterogeneous habitats. Front. Ecol. Evol. 11:1082661. doi: 10.3389/fevo.2023.1082661
Received: 28 October 2022; Accepted: 16 January 2023;
Published: 07 February 2023.
Edited by:
Haitao Wu, Northeast Institute of Geography and Agroecology (CAS), ChinaReviewed by:
Pengshuai Shao, Binzhou University, ChinaCopyright © 2023 Dong, Xie, Cao, Zhang, Liu, Xing, Fu, Wang, Han, Zhong, Luo, Qu, Ni and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jifeng Wang, ✉ d2pmZW5nODhAMTYzLmNvbQ==
†These authors have contributed equally to this work and share last authorship
‡These authors have contributed equally to this work and share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.