
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 20 April 2023
Sec. Population, Community, and Ecosystem Dynamics
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1081385
 Melina Panta1
Melina Panta1 Bijaya Dhami1
Bijaya Dhami1 Bikram Shrestha2,3*
Bikram Shrestha2,3* Nishan Kc1
Nishan Kc1 Nirjala Raut1
Nirjala Raut1 Yajna Prasad Timilsina1
Yajna Prasad Timilsina1 Bir Bahadur Khanal Chhetri1
Bir Bahadur Khanal Chhetri1 Sujan Khanal1
Sujan Khanal1 Hari Adhikari4
Hari Adhikari4 Sona Varachova5
Sona Varachova5 Pavel Kindlmann2,5
Pavel Kindlmann2,5The Chinese pangolin (Manis pentadactyla) has experienced a rapid population decline throughout its distribution. In Nepal, it mostly occurs outside protected areas; therefore, habitat degradation due to anthropogenic activities is one of the major threats to its survival. However, the scarcity of information on the ecology and distribution of pangolins impedes evidence-based conservation of this species in Nepal. Its habitat preferences and distribution and the factors influencing people’s attitude to its conservation were studied in Gorkha District in central Nepal. Thirteen transects, each 0.5 km in length, were used for recording burrows indicating the presence of pangolin. In total, 124 burrows were recorded, of which 38 were new and 86 were old, which indicated a clumped distribution. Based on the highest percentage frequency of occurrence, most burrows occurred between 650 and 800 m a.s.l., in areas with a south-facing aspect, with moderate canopy cover, in forest, red soil and gentle terrain. The logistic regression model revealed that habitat type, soil type, crown cover, terrain, and distance to water were the most important factors affecting pangolin presence. In total, 87 households and 9 key informants were interviewed using questionnaires to determine the people’s knowledge of pangolins and attitude to their conservation. More than 50% of the respondents had seen pangolin in the areas studied and had a general knowledge of their habitat and benefits. However, most of them were unaware that it was illegal to hunt pangolins and were involved in opportunistic hunting for meat consumption. Pangolins were mostly recorded in forest at altitudes 650–800 m a.s.l., with moderate canopy cover, red soil, and close to a source of water; habitat, soil, canopy cover, terrain, and distance to water were statistically significantly associated with the presence of pangolin burrows. This study revealed that an increase in public awareness (mainly through education) would help to increase the likelihood of pangolin survival. These results can also serve as guidelines for protecting pangolin habitats for use by local authorities.
Recent increases in anthropogenic activities, such as hunting and trade (Duffy et al., 2016; Esmail et al., 2020), deforestation and forest fires (Fearnside, 2005), habitat fragmentation (Primack, 2014), infrastructure intervention (Dirzo and Raven, 2003), and agricultural expansion (Laurance et al., 2014) are becoming a key threat to biodiversity conservation. Species highly vulnerable to extinction are more prone to these threats (Pereira et al., 2004), in particular small mammals with a highly specialized ecological niche and less ecological flexibility (Büchi and Vuilleumier, 2014; de Mattos et al., 2021). Hence, both the management and conservation of threatened wildlife necessarily require information on ecological drivers that influence their habitat preferences and distributions (Balakrishnan and Easa, 1986; Aarts et al., 2008; Bajaj and Amali, 2019).
The important ecological factors include altitude, slope, aspect, leaf litter, disturbances (e.g., proximity to settlements and frequency of fire) and vegetation or food resources (e.g., trees and grass). These factors may affect long-term forest composition, structure, or function (Franklin et al., 2002; Palik et al., 2002; Hessburg et al., 2016) and compromise resilience to subsequent disturbance (Radeloff et al., 2000; Hessburg et al., 2019; Leclerc et al., 2021).
The presence and absence of species outside protected areas are directly or indirectly related to anthropogenic activities (Nash et al., 2016; Hairong et al., 2022). The action plans of policymakers and conservationists are based on scientific findings. However, the conservation of endangered species and their habitats is only possible when local people are aware of the ecological importance of these species and/or the wildlife conservation rules and regulations are strictly implemented by governmental organizations (Dirzo and Raven, 2003; Duffy et al., 2016; Archer et al., 2020).
Understanding the perceptions and attitudes of local people toward threatened wildlife is an essential element of any conservation plan (Gillingham and Lee, 1999; Ngonidzashe Mutanga et al., 2015; Nash et al., 2016; Vannelli et al., 2019; Sharma et al., 2020a), as species conservation is always initiated at the local level (Kumssa and Bekele, 2014; Epanda et al., 2019). In addition, knowledge and awareness of wildlife can enhance people’s view of conservation (Schlegel and Rupf, 2010; Sharma et al., 2019). Because of wildlife’s direct and indirect benefits for humans, such as ecosystem services and income generation through tourism, people may desire to protect wildlife even if it can potentially harm them (Schlegel and Rupf, 2010; Sharma et al., 2019). Therefore, it is crucial to understand people’s perception of conservation success (Newmark et al., 1993; Katrina, 2000; Ebua et al., 2011).
The Chinese pangolin (Manis pentadactyla) occurs in several Asian countries: Nepal, Bangladesh, Bhutan, China, Hong Kong, India, Lao PDR, Myanmar, Taiwan, Thailand, and Vietnam (Challender et al., 2019). It occurs below 2,000 m a.s.l. in Nepal (Baral and Shah, 2008; Jnawali et al., 2011) and in primary and secondary tropical and subtropical rainforests, bamboo, limestone, mixed coniferous, and broadleaf forests, and agricultural land and nearby human-dominated areas (Gurung, 1996; Wu et al., 2003; Katuwal et al., 2017). It is nocturnal, elusive, non-aggressive, solitary, insectivorous (primarily feeding on ants and termites), and digs burrows in search of prey and for shelter and protection from predators (Wu et al., 2004; Gaubert, 2011; Bao et al., 2013). It provides important ecosystem services by controlling pests and improving soil structure and composition (Laundré and Reynolds, 1993; Swart et al., 1999).
The Chinese pangolin population has declined significantly throughout its distribution during recent decades due to hunting and poaching for its meat and scales, primarily targeted for international trade and driven by demand from China and Vietnam (Newton et al., 2008; Heinrich et al., 2017; Zhang et al., 2017; Challender et al., 2019). Therefore, this species is listed as “Critically Endangered” in the International Union for Conservation of Nature (IUCN) Red List of Threatened Species (Challender et al., 2019) and in Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES, 2020).
In Nepal, this species is listed as endangered in the National Red List of Mammals (Jnawali et al., 2011) and strictly protected under the National Parks and Wildlife Conservation (NPWC) Act 1973 (Government of Nepal, 1973). Despite its protection status, illegal trade in pangolins is reported to have increased in Nepal because of the growing demand for body parts in international markets (Department of National Parks and Wildlife Conservation, 2018; Ghimire et al., 2020; Bashyal et al., 2021). It is found in 25 districts of Nepal, the majority of which are outside protected areas, including Gorkha District in central Nepal (Department of National Parks and Wildlife Conservation, 2018; Sharma et al., 2020b). The extent of the area of pangolin habitat outside protected areas has raised concerns about anthropogenic threats to this species survival (Khatiwada et al., 2020). The scarcity of information on the ecology and distribution of the Chinese pangolin impedes evidence-based conservation of this species in Nepal (Department of National Parks and Wildlife Conservation, 2018; Khatiwada et al., 2020). There have been many attempts in a range of countries to quantify the illegal hunting and trade in the Chinese pangolin (Katuwal et al., 2015; Nijman et al., 2016; Zhang et al., 2017; Ullmann et al., 2019; Sharma et al., 2020a; Bashyal et al., 2021). However, there are only a few robust studies that focus on its habitat preferences and distribution at national and regional levels (Wu et al., 2003; Bhandari and Chalise, 2014; Dorji et al., 2020; Acharya et al., 2021; Shrestha et al., 2021).
Community interviews is the most cost-effective way of obtaining an insight into the people’s level of knowledge of pangolins, their interactions with humans, site-specific threats, and attitude to conservation (Turvey et al., 2014; Nash et al., 2016; Willcox et al., 2019; Archer et al., 2020; Sharma et al., 2020a). Demographic and socio-economic conditions may influence people’s perception of conservation (Duncker and Gonçalves, 2017). In addition, their perception varies with age, gender and education (Romanach et al., 2007; Tomićević et al., 2010; Mutanga et al., 2017) and is also affected by culture, tradition and knowledge of the legal provisions for species conservation (Sharma et al., 2019).
Therefore, the aim of this study is to determine the habitat preference and distribution of Chinese pangolins and assess the level of the local people’s knowledge and perception of pangolin conservation in Gorkha District, Nepal.
Figure 1 shows a conceptual framework of the study. In the field research, thirteen transects were established, each 0.5 km long, which were searched for indirect signs (active/old burrows) and any other evidence of pangolin presence. We have distinguished active (new) burrows (freshly dug soil, footprints and pangolin feces nearby), and old burrows (spider webs and dead tree leaves inside). When a burrow was found, a circle of 5 m radius with its center at the point where the animal or sign of its presence was located (“presence circle”) was established, an additional similar circle was set up at least 100 m from the former circle in a randomly chosen direction (“comparative circle”) and seven important ecological parameters were measured in all these circles. Based on this data, the distribution of pangolin in the area, the ecological parameters most frequently associated with the presence of pangolins, and the significance of each of the ecological parameters measured for the selection of individual categories of this ecological parameter by pangolin were determined.
Using community interviews, we have determined the people’s knowledge and attitudes to pangolin. The respondents were asked about their understanding of pangolin’s legal protection status, their interest in pangolin conservation and the principal threats to its survival.
The findings of this study will be useful for the wildlife and forestry departments of Nepal, as well as other relevant authorities, for developing action plans and management strategies for the long-term conservation of this species.
The Gorkha District is well known as a source of pangolins (Department of National Parks and Wildlife Conservation, 2018). Several individuals have been rescued by the district forest office in this area, which includes community forests, but there is little information on the ecology of pangolins living there. Thus, this study was carried out in Shahid Lakhan Rural Municipality of Gorkha District (27.927032° N, 84.658404° E) in the central region of Nepal (Figure 2). Bhimsen Rural Municipality surrounds this municipality to the north, Gandaki Rural Municipality to the east and Gorkha Municipality to the west. Within Shahid Lakhan Rural Municipality, two areas (Bunkot and Namjung) were selected (Figure 2), covering an area of 44.60 km2. The sites studied included community forests, private forests and farmland. The main tree species include Shorea robusta, Castanopsis indica, Schima wallichii, and Acacia catechu. Herbaceous vegetation includes Imperata cylindrica, Hyparrhenia hirta, and Nephrolepis cordifolia. The main castes in the district are Brahmin, Chhetri, Dalit, Gurung, Magar, Newar, Damai, Kami, and Sarki.
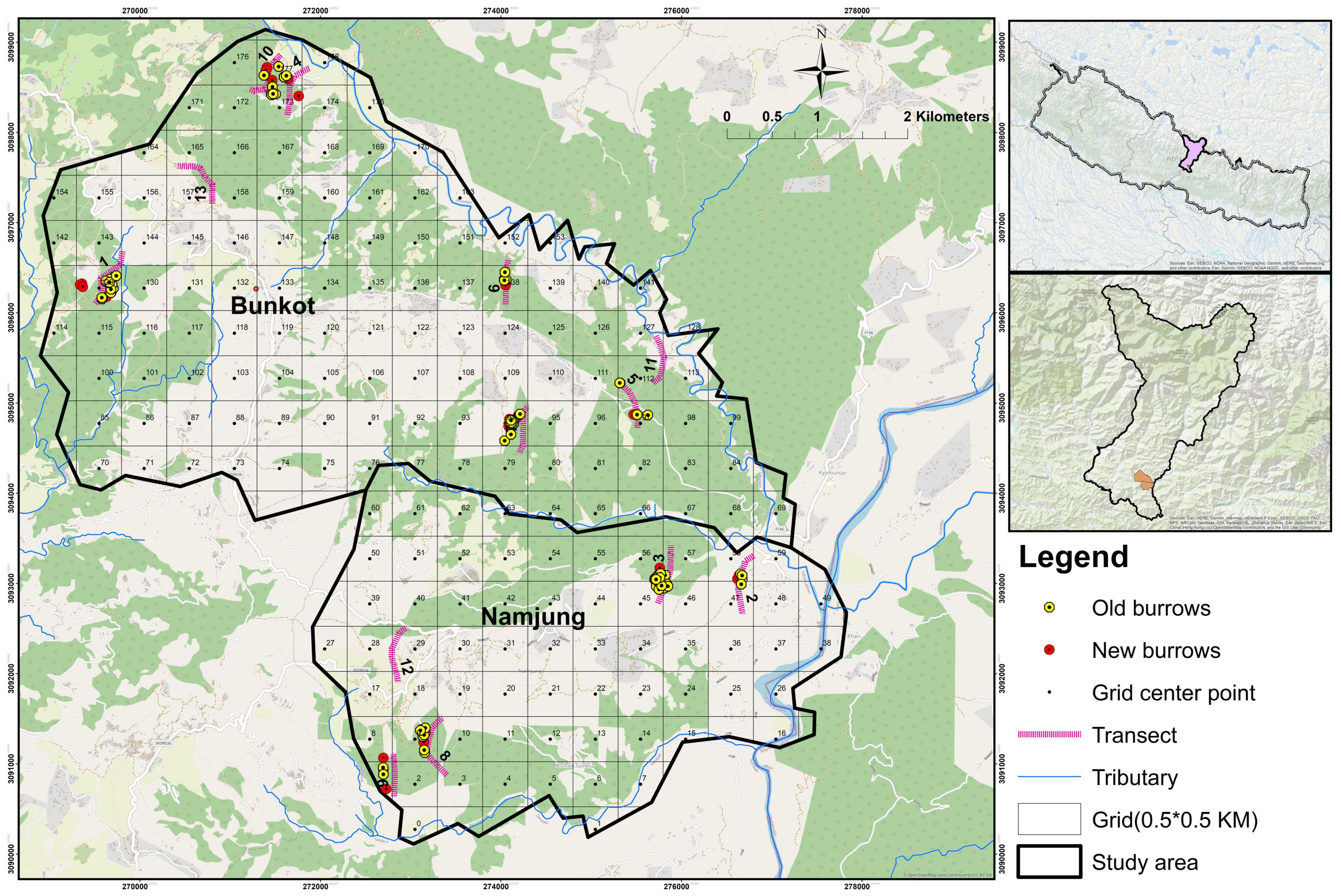
Figure 2. Map of Gorkha District, showing the distributions of old and new burrows, and locations of the transects in the areas studied.
Based on a preliminary survey and discussions with local people, areas for the extensive survey were identified. This study was carried out in February 2019. Thirteen transects were established, each 0.5 km long, which were searched for indirect signs (active/old burrows) and any other evidence of pangolin presence (Figure 2). There was a minimum distance of 100 m between adjacent transects in order to avoid overlapping the sites sampled. The transects were carefully chosen to include all habitats based on their relative size. The pangolin burrows were identified based on the characteristics described by Suwal (2011), Department of National Parks and Wildlife Conservation (2018), and Suwal et al. (2020). If a burrow had freshly dug soil, footprints and pangolin feces nearby, it was categorized as an active (new) burrow, whereas burrows with spider webs and dead tree leaves inside were categorized as old burrows (Suwal, 2011). Burrow locations were determined using a handheld Garmin eTrex 30 GPS.
When a (fresh or old) pangolin burrow was found, a circle of 5 m radius with its center at the point where the animal or sign of its presence was located (“presence circle”) was established, following the method of Yahnke (2006) and Bernard et al. (2014), which is the most appropriate in this situation. For each of these circles, an additional similar circle was set up at least 100 m from the former circle in a randomly chosen direction (“comparative circle”), according to Neupane et al. (2022). These plots were samples of average habitat, independent of the presence/absence of the pangolin. Many of the “comparative circles,” therefore, also contained a burrow.
All the “presence circles” and “comparative circles,” which contained burrows indicating pangolin presence, were categorized as “used plots”; those which did not were categorized as “habitat availability plots.” Thus, in total, there were 124 “used plots” and 36 “habitat availability plots.” In all of the “used plots” and “habitat availability plots” seven important ecological parameters were measured (Table 1). These ecological parameters were selected on the basis of previous studies (Wu et al., 2003; Bhandari and Chalise, 2014; Dorji et al., 2020; Acharya et al., 2021; Shrestha et al., 2021). We did not distinguish between old and active burrows in the analyses, as both indicated a recent presence of pangolin.
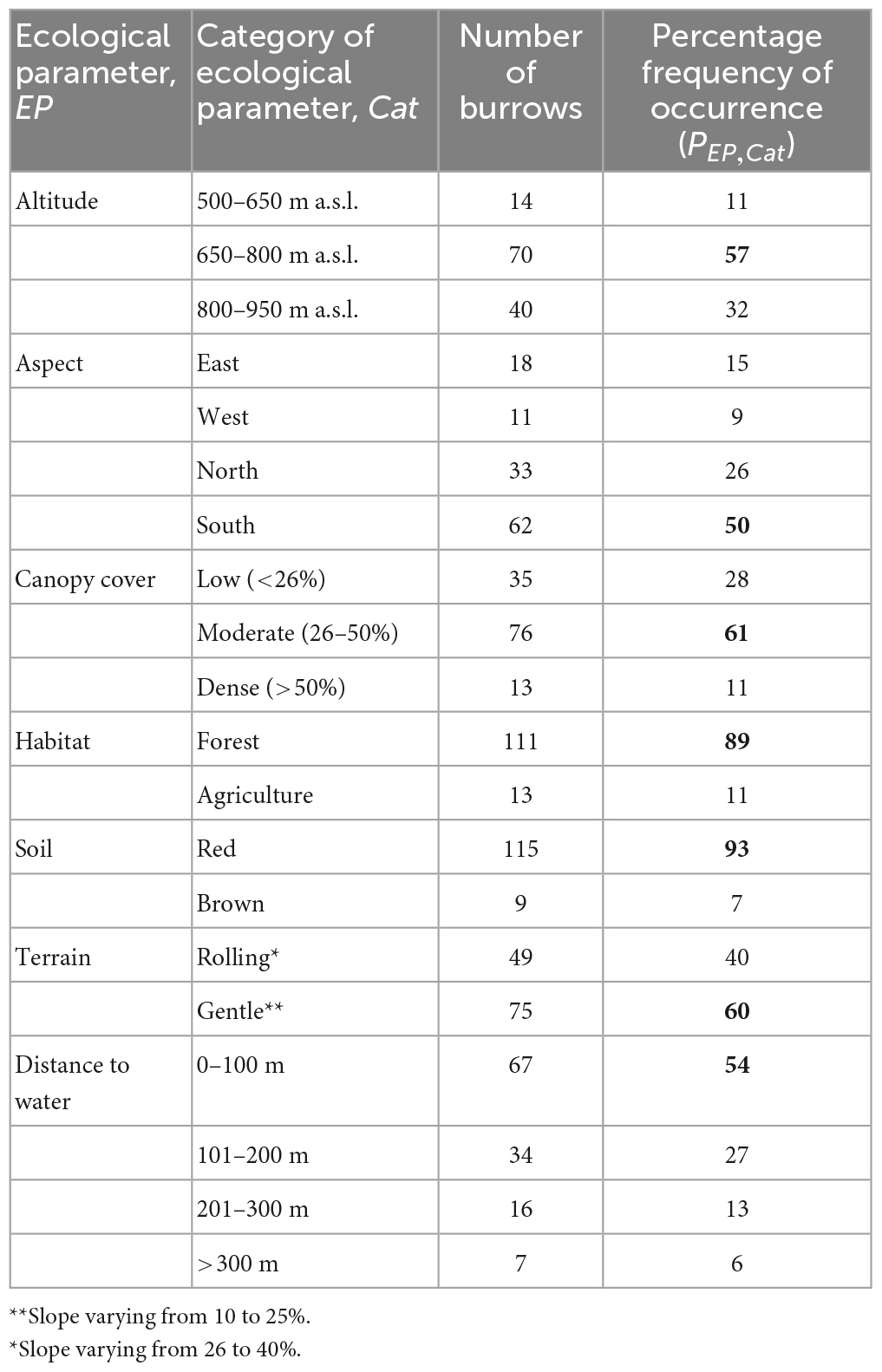
Table 1. Percentage frequency of pangolin burrows recorded for the seven ecological parameters measured in the area studied; for each category, the number of burrows and the percentage of the total number of burrows recorded in the area studied, the most often recorded are in bold.
The households and names of the family heads of all 145 households living within the 3 km buffer zone surrounding the forests in the areas studied were obtained from the village development committee. It was not possible to interview all households because the areas were remote and settlements scattered; therefore, only 87 (60%) of these households were randomly selected for interview. None of them refused to participate in this research.
This survey aimed to determine the people’s knowledge and attitudes to pangolin. It consisted of semi-structured questionnaire (Newton et al., 2008), which was pretested on 5% of the households. The completed questionnaires (see Supplementary material) were followed by face-to-face interviews with the respondents. A random sampling method was used to select potential respondents (Koirala et al., 2012).
Particular households were located with the help of a local field assistant. For the survey, the head of the family was interviewed, and in case of his being unavailable (which does happen and must not exclude the family) the person holding this position in his absence was interviewed. All the respondents completed a questionnaire, which records the demographic (age and gender) and socio-economic status (occupation, education, and ethnicity) of each respondent, which were assigned to different categories, as shown in Table 3.
During the survey, respondents were asked whether they could identify a pangolin based on color photographs, had seen one or any evidence of its presence in the last 2 years, or had knowledge of it and its ecological role. They were also asked about their understanding of pangolin’s legal protection status, their interest in pangolin conservation and the principal threats to its survival. In addition, a survey (n = 9) of the views of members of local government bodies, staff of the divisional forest office, ethnic communities, concerned authorities, police, and traditional healers was completed. During this survey, information on the pangolin’s abundance and major threats to its survival were documented. The questionnaires were recorded in Nepalese or the local language and then translated into English.
In the first analysis, the distribution of pangolin in the area studied was determined by calculating the variance to mean ratio (S2/a), where x is the number of burrows (signs) per transect, a the mean of the x values, and S2 = the variance, (Odum, 1971) as follows:
To determine the ecological parameters most frequently associated with the presence of pangolins, a second analysis for each ecological parameter, EP, was done: the percentage frequency of occurrence (PEP, Cat) for each category, Cat, of ecological parameter, EP, was carried out following Pokharel (1996) and Wu et al. (2003):
It must be noted, however, that because the spatial extent of individual categories of the ecological parameters is unknown and it is not possible to measure it and the value of PEP, Cat reflects the spatial extent of each category for each ecological parameter.
In the third analysis, logistic regression was used to estimate the significance of each of the ecological parameters for the selection of individual categories of this ecological parameter by pangolin using all the plots (both “use plots” and “habitat availability plots”) and all data from all the areas studied (Agresti, 2007) in SPSS (IMB Corp, 2015). The dependent variable was the presence/absence of pangolin burrows in plots (set equal to 1 for “use plots” and 0 for “habitat availability plots”), and the independent variables were the values of the seven ecological parameters measured (Manly et al., 2002).
Multicollinearity problems were initially checked for using the variance inflation factor (VIF), which indicated no problems (VIF not >10) (Bowerman and O’Connell, 1990). The P-value of the beta coefficient in the likelihood ratio testing the effect of the independent variable (ecological parameter) at a 5% significance level then revealed whether the corresponding independent variable (ecological parameter) significantly affected pangolin presence or not.
To summarize: the first analysis determined the spatial aggregation of burrows with no consideration of the ecological parameters, the second analysis revealed the relative number of burrows in each category of each environmental parameter but nothing about the preference of pangolin for individual categories and the third analysis, whether there were significant differences in the numbers of burrows recorded for the categories of each environmental parameter.
In the case of social data, respondent variables were noted and used for the analysis of people’s attitude to pangolin conservation. These variables were age (18–35, 36–55, and ≥56 years), gender (male and female) and education (illiterate and literate). The Chi-square test was used to determine whether there are significant differences in the attitudes of the respondents associated with the above variables at the 5% significance level (∝= 0.05).
In total, 124 burrows were recorded: 38 were new and 86 old. The distribution of burrows at different altitudes was clumped, which is indicated by the variance to mean ratio: S2/a = 2.29 > 1. This is also depicted in Figure 2. The highest numbers of burrows were recorded in Rana Khola, Danda Gaun Community Forest, Jugepani Pakha, and Dhurseni and the lowest numbers in Bhalayo Bas Community Forest, Atmare Bhanjyang, Jhagare, and Shikhar Fedi. No burrows were recorded in Magar and Gurung.
Table 1 shows the percentage frequency, PEP, Cat, of pangolin burrows for each of the seven ecological parameters considered. Most of the burrows were recorded at altitudes of between 650 and 800 m a.s.l., in areas with a south-facing aspect, with moderate canopy cover, red soil, gentle terrain, and distance to water <100 m.
The logistic regression model indicates that type of habitat, soil, crown cover, terrain, and distance to water are significantly associated with the distribution of pangolin (Table 2). The highly positive values of the unstandardized beta coefficient, B, in the logistic regression (Table 3) indicate (judging from B in Table 2 and the numbers of burrows in Table 1) that pangolin are strongly associated with: (i) forest and agricultural land; (ii) red and brown soil; (iii) moderate crown cover (26–50%); and (iv) gentle and rolling terrain.
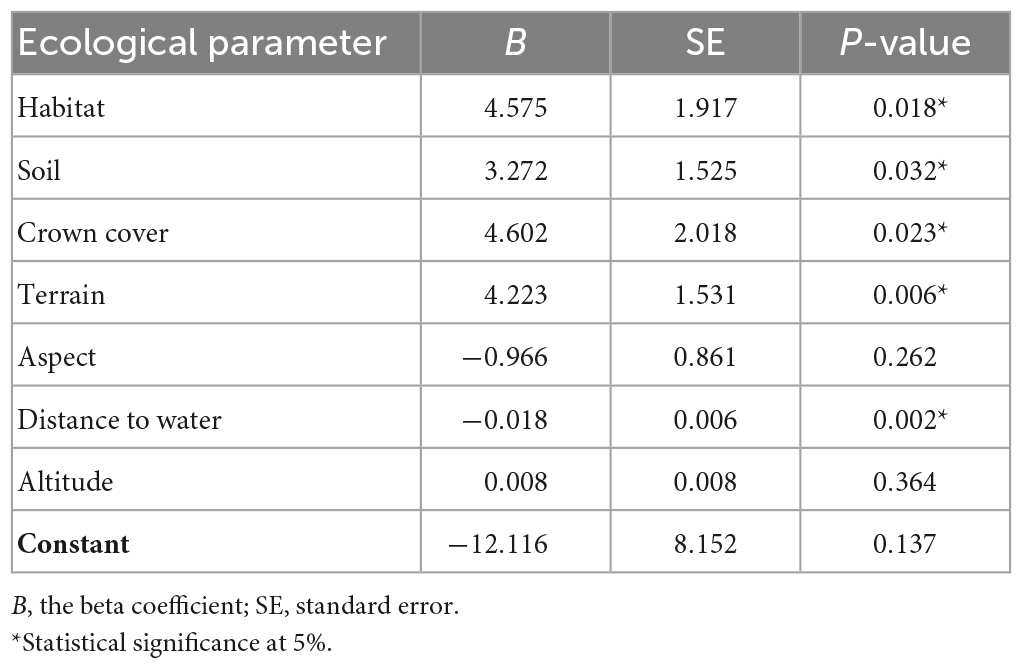
Table 2. Estimates and statistics for the model predicting the relationship between habitat variables and the presence of burrows, according to the likelihood ratio testing of the parameters of the logistic regression model.
Table 3 lists the sociodemographic characteristics of 87 respondents, in terms of gender, age, occupation, education, and ethnicity.
The questionnaire revealed that of the respondents:
• 16% had seen pangolin many times, 36% only once, 33% never, and 15% had only seen and eaten pangolin meat.
• 53% knew about the habitat and benefits of pangolin, 17% knew something and 30% knew nothing.
• 59% did not know that pangolin is a protected species in Nepal and that it is illegal to hunt them, and that there were laws governing the conservation of pangolin.
• 36% had used pangolin products, like rings, necklaces and bracelets.
• 34% knew that the abundance of pangolin was decreasing, 19% that its population was stable and the rest nothing about the population trend in the area studied.
• 29% agreed that hunting was a serious threat to pangolins, 51% disagreed and thought that developmental works, such as extensions to roads and buildings, were the major threats, and the remaining respondents were neutral.
Table 4 lists the responses to the question regarding whether public awareness helps to conserve the habitat of pangolin. There are statistically significant differences in responses based on gender and education. Most males agreed with the statement, whereas female respondents were nearly all neutral. Most literate respondents agreed with the statement, whereas fewer illiterate respondents supported the statement. Responses based on the habitat age were not statistically significant.
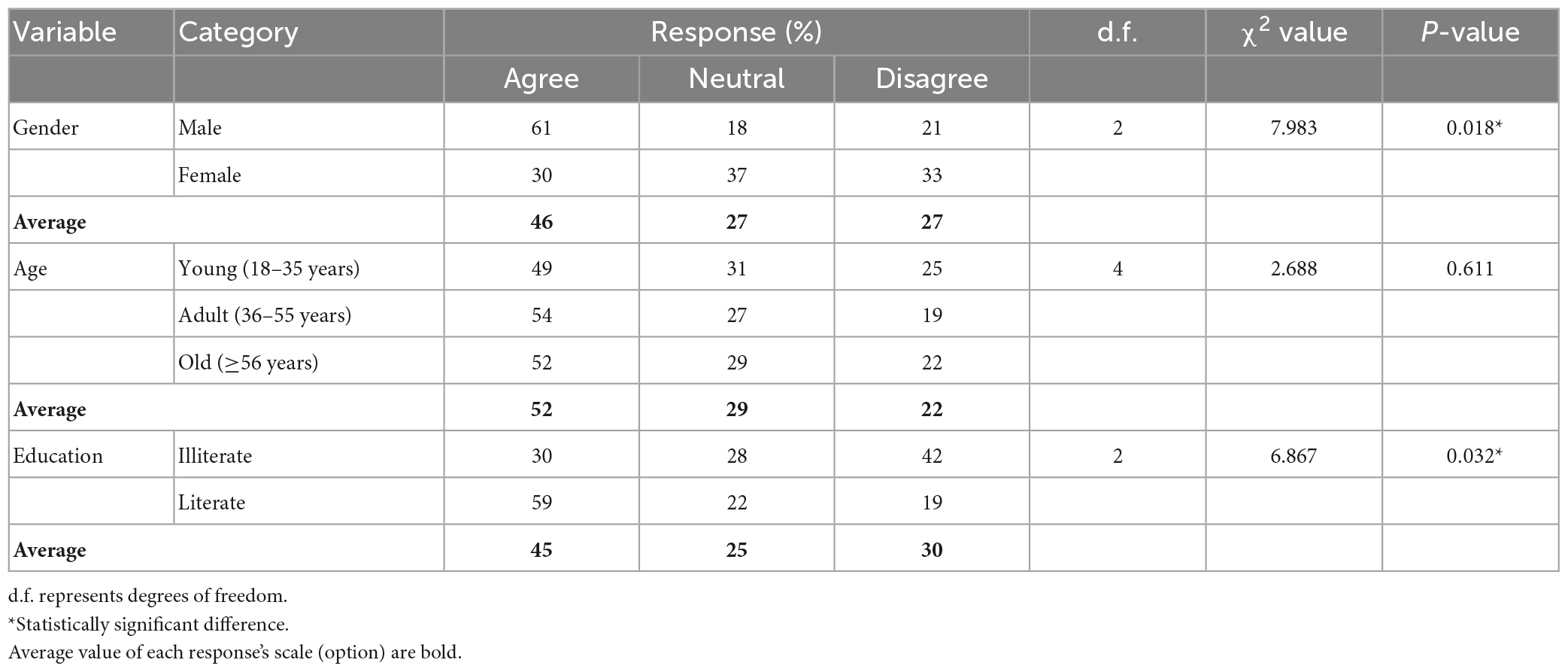
Table 4. Respondents’ responses to the statement “Public awareness helps to conserve the habitat of pangolin”.
Table 5 lists the responses of respondents to the question: do you have an interest in pangolin conservation. There are statistically significant differences based on age and gender. Surprisingly, the numbers of responses of literate and illiterate groups are not statistically significantly different.
Based on the distribution of burrows pangolin distribution is not uniform. Similarly, Suwal (2011), Bhandari and Chalise (2014), and Dhami et al. (2023) report non-uniform distribution of burrows on slopes of all aspects. So, all of the data support the idea that the distribution of pangolin burrows, in the absence of a consideration of the ecological parameters associated with their presence, is clumped.
This study revealed that forest is the preferred habitat of pangolin, with burrows mostly recorded in S. robusta followed by S. wallichii forests. This is in accord with other studies, which report burrows mostly in forested areas (Gurung, 1996; Acharya, 2001; Bhandari and Chalise, 2014; Katuwal et al., 2017: Suwal et al., 2020; Dhami et al., 2023). In forests, termites are very abundant (Ackerman et al., 2009), which could account for the high pangolin occurrence (Swart et al., 1999). In addition, Acharya (2001) report pangolin burrows mostly in S. wallichii and pine forests. So, all of this data support the idea that pangolin prefer forest habitats.
This study revealed that pangolin prefers red soil, which accords with the results of Dhami et al. (2023) reported for Gorkha and Shrestha et al. (2021) for Chitwan, Nepal. In contrast, various studies indicate that pangolin prefers brown soil (Suwal, 2011; Bhandari and Chalise, 2014; Suwal et al., 2020). Heath (1992) for Fujian and Jiangxi provinces in China reports a preference for acidic or yellowish-red soil, which supports the findings of the current study. Thus, it is likely that the “preferred” may be dependent on availability.
This study revealed that pangolins are strongly associated with moderate canopy cover, which is in accordance with the results of Bhandari and Chalise (2014), Dorji et al. (2020), and Dhami et al. (2023). A possible reason for this might be that soils below moderate canopy cover are dry and consist of relatively undecomposed leaf litter and a greater amount of dead twigs and branches (Bhandari and Chalise, 2014), whereas the soil where the crown cover is above 60% is moist with a thick layer of decomposing leaf litter, which is negatively associated with the availability of ants and termites. However, this contradicts Suwal et al. (2020) and Shrestha et al. (2021), who report that pangolins prefer dense crown cover. Therefore, this needs further research.
Wu et al. (2003) report a low number of burrows on slopes greater than 30%, which supports the results presented here of most burrows recorded on moderate slopes. A similar observation is also reported by Bhandari and Chalise (2014) in the Nagarjun Forest in the Shivapuri Nagarjun National Park in Nepal; Sharma et al. (2020c) in Gaurishankar Conservation Area and Ramechhap District in Nepal; and Acharya et al. (2021) in Kavrepalanchok District in Nepal. Pangolin is mostly associated with gentle slopes of between 10° and 30° on which they can easily hunt and feed on ants and termites (Acharya et al., 2021), as well as gentle slopes with an abundance logs that are rich in their preferred prey (Sharma et al., 2020c).
A preference for a certain aspect in different areas might be influenced by climatic conditions, availability of food and degree of human interference (Suwal, 2011). In this study, a high frequency of burrows was recorded on south-facing slopes, which is also reported by Gurung (1996) and Acharya (2001). In contrast, Suwal (2011) reports more burrows on an east-facing slope. A plausible reason for the differing distribution of pangolin burrows on slopes of different aspects in different studies might be that pangolins prefer exposure to direct sunshine, independent of aspect.
Pangolin occurrence is negatively associated with distance to water within a habitat (Katuwal et al., 2017). Thus, similar to the results of the studies by Shrestha et al. (2021) and Dhami et al. (2023), pangolins prefer to live near to a source of water. The reason could be that they need to drink water frequently (Suwal, 2011) in order to regulate their body temperature.
In the current study, burrows were not recorded above 950 m a.s.l., because of the human settlements there and the absence of forest vegetation. A similar observation is also reported by Dhami et al. (2023) for the Gorkha District in Nepal, where the majority of burrows were recorded at altitudes between 450 and 750 m. Bhandari and Chalise (2014) report that pangolins prefer low altitudes, but live mostly at mid-altitudes during winter and Gathorne-Hardy et al. (2001) and Hemachandra et al. (2014) report that termite abundance decreases with increase in altitude, which could be why the number of burrows decreases with increase in altitude. Thus, all the above data supports the idea that pangolin mainly occurs at mid-altitudes.
Very few people knew that pangolins could be a source of income or aware of laws regarding its conservation although it is a protected species in Nepal. According to the fifth amendment of the NPWC Act, there is a provisional fine of NPR 100,000 to NPR 500,000 or jail sentence of 1–10 years or both for the illegal killing, buying and selling and transportation of body parts of protected species (GoN/DNPWC, 2017). In the areas studied, some people used it for meat and medicinal purposes as its meat is considered to be a delicacy and its scales to have curative properties if kept in a cattle shed (Mohapatra et al., 2015). Most of the rural roads that are being constructed are in forests and according to informants they have seen five pangolins in excavator buckets. Thapa et al. (2014) also report that the increase in development works may directly threat the habitat of pangolin. A similar result is that of Katuwal et al. (2017), who report that the construction of footpaths for daily agriculture activities in pangolin habitat directly exposes them to humans resulting in an increase in hunting and poaching.
According to Nash et al. (2016), only 2% of the respondents in Hainan, China, were aware of the medicinal uses of pangolin. However, in Sindhupalchowk District in Nepal, 22% knew that the purpose of the illegal trade in pangolins was their medicinal use (Sharma et al., 2020b). Katuwal et al. (2015) report that 16% of respondents in eastern Nepal believe that pangolin meat can cure gastrointestinal disorders, heart problems, backache, and pain during pregnancy. In addition to its medicinal value, superstitious beliefs are also an important factor in most regions of Nepal because encountering pangolins is believed to bring bad luck (Thapa et al., 2014; Katuwal et al., 2015; Ghimire et al., 2020; Khatiwada et al., 2020).
Anthropogenic activity is the primary threat to pangolin conservation; this finding is similar to that of Baral and Dahal (2022). However, if habitat loss and fragmentation is not greatly reduced or stopped the abundance of pangolins will continue to decrease (Zhang et al., 2022).
Although the majority of respondents in Nepal’s central and eastern regions were aware that pangolin hunting and poaching are illegal (Katuwal et al., 2015; Sharma et al., 2020b), 30.2% had consumed pangolin meat in eastern Nepal (Katuwal et al., 2015). It is mostly unemployed youths aged 16–35 that are involved in the illegal hunting and poaching of pangolins (Katuwal et al., 2015; Ghimire et al., 2020), as it is an easy means of obtaining money (Paudel et al., 2020). Most farmers are unaware of the ecological services provided by pangolin in Nepal (Sharma et al., 2020b). Pangolins are regarded as natural pest control agents, as it is estimated that an adult pangolin consumes more than 70 million insects (mainly ants and termites) annually (Shi and Wang, 1985).
Many local people, on the other hand, claim that burrows reduce crop yield and degrade the aesthetic value of the farmland and report that clusters of burrows can cause landslides during the rainy season (Khatiwada et al., 2020). In Nepal, land conversion for agriculture is expanding (Burton et al., 1989; Paudel et al., 2013), which is accompanied by an increase in the use of insecticides and pesticides (Diwakar et al., 2008; Sharma et al., 2012), potentially reducing the availability of prey for pangolin. Generally there is little or no wildlife conservation in unprotected compared to protected areas (Rodrigues et al., 2004). In the context of Nepal, most pangolin habitat is not in protected areas (Department of National Parks and Wildlife Conservation, 2018), which increases the risk of interaction of this species with local communities.
This study revealed that most respondents did not know anything about pangolins. Suwal et al. (2022) similarly report that the majority surveyed had no knowledge of the ecological, reproductive and behavioral characteristics of this species. Despite the increase in pangolin-related publications in recent years, substantial gaps in knowledge persist in the global literature on various aspects of this species (Heighton and Gaubert, 2021). This includes a dearth of research on pangolin ecology in Nepal, as highlighted by Dhami et al. (2023).
Based on this study, there were significant differences in the extent to which respondents were interested in pangolin conservation depending on their gender and age. Male respondents were more likely to be interested in pangolin conservation, which can be attributed to the fact that males have greater exposure to diverse activities due to better access to education and job opportunities, whereas females are typically confined to household chores and have limited access to such opportunities (Yamamoto et al., 2019). Similarly, old respondents were more interested in pangolin conservation than the young and young adults. Yang et al. (2015) hypothesize that this phenomenon may be attributed to the greater frequency of forest habitat and wildlife encounters in the daily routines of older than younger generations.
This study revealed significant difference across the gender and age groups regarding the question whether public awareness helps to conserve pangolin habitat. Men and educated people may have attended many conservation events hosted by various government and non-governmental organizations, depending on their jobs and obligations. This may help to explain why the male and literate group of participants responded favorably to the aforementioned statement as compared to the female and illiterate group. Thus, pangolin conservation programs should be launched in all the pangolin hotspots, like participatory campaigns in the local community, schools, and radio programs (Goldstein, 2003; Hong et al., 2017) specially designed for females and illiterate people.
Protecting the habitat of pangolins outside protected areas requires people to have a positive perception of endangered animals (Archer et al., 2020). Moreover, conserving these areas as either a national park, community forest or special management unit would safeguard the existence of pangolin in Nepal. Some ecotourism activities can be developed in the area studied, which would help local people economically and improve their attitude towards pangolins (Ngonidzashe Mutanga et al., 2015; Epanda et al., 2019).
The habitat preferences of pangolin, people’s knowledge of this species and attitude to pangolin conservation were recorded in Gorkha District, Nepal.
Pangolins were mostly recorded in forest at altitudes between 650 and 800 m a.s.l., with moderate canopy cover, red soil, and close to a source of water. As this species is also found in agricultural fields, local farmers should be encouraged to reduce the threat to pangolin by adopting a pangolin-friendly management of their fields. Habitat, soil, canopy cover, terrain, and distance to water are statistically significantly associated with the presence of pangolin burrows. Thus, conservation should concentrate on maintaining and increasing the size of these areas. There could also be other factors that affect pangolin presence, which were not considered in this study, such as ecological interactions between pangolins, disturbance, predators, and other bioclimatic factors.
More than half of the respondents were aware of the benefits associated with pangolin, but most did not know that it is a protected species in Nepal. The respondents were positive and supportive of the presence and survival of this endangered species in their area, but despite this some of the people were indirectly involved in opportunistic hunting, especially for acquiring meat for food (and thus getting an additional income). Apart from this, the construction of roads in forests was the main threat to pangolin. People’s attitudes to pangolin conservation varied significantly depending on gender, age and education, because respondents were not satisfied with the conservation initiatives being practiced and wanted a specific program related to the conservation of pangolin. This indicates there is support for initiation of specific educational programs that bring the attention of local people to the issues of poaching and forest fires, and increase their understanding of the conservation value of this mammal. Community groups should be formed to help protect pangolins from emerging threats and help with their recovery in the wild. The importance of pangolin research and conservation following from this research is nicely illustrated in Choo et al. (2022).
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the animal study because research was conducted using transects survey through indirect signs. It was neither physically captured or handled the animals nor any invasive procedures applied. Ethical approval was not necessary for this study on human participants because interviewee names and their socioeconomic information was kept confidential. The participants provided their written informed consent to participate in this study. All the protocols followed and methods applied in the research were within the standard framework of Institute of Forestry, Tribhuvan University, Nepal.
MP and BD collected and analyzed the data. MP, BD, NK, and SK wrote the first draft of the manuscript. BD and SK prepared the Figure 2 using GIS. NR, YT, and BK supervised the research and wrote the first version of the manuscript, which was reviewed by BS, HA, SV, and PK. All authors contributed to the article and approved the version submitted.
This study was funded by the Division of Forest Office, Gorkha (grant no. 15/12/2018). BS and PK were supported by the CzechGlobe institutional grant.
We are thankful to the Division of Forest Office, Gorkha, for providing financial support, research permission, and excellent working conditions. Similarly, we appreciate the help of Ms. Deepika Adhikari, Mr. Prabin Shrestha, and local people for their contribution and generous support during the fieldwork. We thank Prof. Tony Dixon for editing the English of this manuscript. We also thank reviewers for providing constructive comments and suggestions to improve this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1081385/full#supplementary-material
Aarts, G., MacKenzie, M., McConnell, B., Fedak, M., and Matthiopoulos, J. (2008). Estimating space-use and habitat preference from wildlife telemetry data. Ecography 31, 140–160. doi: 10.1111/j.2007.0906-7590.05236.x
Acharya, P. M. (2001). “Status and distribution of pangolin in the Nagarjun forest in Central Nepal,” in Workshop on Asian Pangolin, Taipei, 17–18.
Acharya, S., Sharma, H. P., Bhattarai, R., Poudyal, B., Sharma, S., and Upadhaya, S. (2021). Distribution and habitat preferences of the Chinese Pangolin Manis pentadactyla (Mammalia: Manidae) in the mid-hills of Nepal. J. Threat. Taxa 13, 18959–18966. doi: 10.11609/jott.3952.13.8.18959-18966
Ackerman, I. L., Constantino, R., Gauch, H. G., Lehmann, J., Riha, S. J., and Fernandes, E. C. M. (2009). Termite (Insecta: Isoptera) species composition in a primary rain forest and agroforests in Central Amazonia. Biotropica 41, 226–233. doi: 10.1111/j.1744-7429.2008.00479.x
Agresti, A. (2007). An introduction to categorical data analysis, 2nd Edn. New York, NY: John Wiley & Sons. doi: 10.1002/0470114754
Archer, L. J., Papworth, S. K., Apale, C. M., Corona, D. B., Gacilos, J. T., Amada, R. L., et al. (2020). Scaling up local ecological knowledge to prioritise areas for protection: Determining Philippine pangolin distribution, status and threats. Glob. Ecol. Conserv. 24:e01395. doi: 10.1016/j.gecco.2020.e01395
Bajaj, S., and Amali, D. G. B. (2019). “Species Environmental Niche Distribution Modeling for Panthera Tigris Tigris ‘Royal Bengal Tiger’Using Machine Learning,” in Emerging research in computing, information, communication and applications, eds N. Shetty, L. Patnaik, H. Nagaraj, P. Hamsavath, and N. Nalini (Singapore: Springer), 251–263. doi: 10.1007/978-981-13-5953-8_22
Balakrishnan, M., and Easa, P. S. (1986). Habitat preferences of the larger mammals in the Parambikulam Wildlife Sanctuary, Kerala, India. Biol. Conserv. 37, 191–200. doi: 10.1016/0006-3207(86)90081-9
Bao, F., Wu, S., Su, C., Yang, L., Zhang, F., and Ma, G. (2013). Air temperature changes in a burrow of Chinese pangolin, Manis pentadactyla, in winter. J. Vertebr. Biol. 62, 42–47. doi: 10.25225/fozo.v62.i1.a6.2013
Baral, B., and Dahal, S. (2022). Assessment of people’s perception towards Chinese pangolin Manis pentadactyla conservation in Mandandeupur of Kavrepalanchowk, Central Nepal. J. Bombay Nat. Hist. Soc. 119.
Bashyal, A., Shrestha, N., Dhakal, A., Khanal, S. N., and Shrestha, S. (2021). Illegal trade in pangolins in Nepal: Extent and network. Glob. Ecol. Conserv. 32:e01940. doi: 10.1016/j.gecco.2021.e01940
Bernard, H., Baking, E. L., Giordano, A. J., Wearn, O. R., and Ahmad, A. H. (2014). Terrestrial mammal species richness and composition in three small forest patches within an oil palm landscape in Sabah, Malaysian Borneo. Mamm. Stud. 39, 141–154. doi: 10.3106/041.039.0303
Bhandari, N., and Chalise, M. K. (2014). Habitat and distribution of Chinese pangolin (Manis pentadactyla Linnaeus, 1758) in Nagarjun Forest of Shivapuri Nagarjun National Park, Nepal. Nepal. J. Zool. 2, 18–25.
Bowerman, B. L., and O’Connell, R. T. (1990). Linear statistical models: An applied approach. Pacific Grove, CA: Brooks/Cole.
Büchi, L., and Vuilleumier, S. (2014). Coexistence of specialist and generalist species is shaped by dispersal and environmental factors. Am. Nat. 183, 612–624. doi: 10.1086/675756
Burton, S., Shah, P. B., and Schreier, H. (1989). Soil degradation from converting forest land into agriculture in the Chitawan district of Nepal. Mt. Res. Dev. 9, 393–404. doi: 10.2307/3673587
Challender, D., Wu, S., Kaspal, P., Khatiwada, A., Ghose, A., Ching-Min Sun, N., et al. (2019). Manis pentadactyla (errata version published in 2020). The IUCN Red List of Threatened Species 2019: E.T12764A168392151, 8235.
Choo, S. W., Chong, J. L., Gaubert, P., Hughes, A. C., O’Brien, S., Chaber, A. L., et al. (2022). A collective statement in support of saving pangolins. Sci. Tot. Environ. 824:153666. doi: 10.1016/j.scitotenv.2022.153666
CITES (2020). CITES Appendices I, II, and III 28 August 2020. Available online at: https://cites.org/sites/default/files/eng/app/2020/E-Appendices-2020-08-28.pdf (accessed August 10, 2022).
de Mattos, I., Zimbres, B., and Marinho-Filho, J. (2021). Habitat specificity modulates the response of small mammals to habitat fragmentation, loss, and quality in a neotropical Savanna. Front. Ecol. Evol. 9:751315. doi: 10.3389/fevo.2021.751315
Department of National Parks and Wildlife Conservation (2018). Pangolin Conservation Action Plan for Nepal (2018-2022). Kathmandu: Department of National Parks and Wildlife Conservation and Department of Forests.
Dhami, B., Neupane, B., Devkota, B. P., Maraseni, T., Sadadev, B. M., Bista, S., et al. (2023). Factors affecting the occupancy of Chinese pangolins (Manis pentadactyla) suggest a highly specialized ecological niche. Ecosphere 14:e4356. doi: 10.1002/ecs2.4356
Dirzo, R., and Raven, P. H. (2003). Global state of biodiversity and loss. Annu. Rev. Environ. Res. 28, 137–167. doi: 10.1146/annurev.energy.28.050302.105532
Diwakar, J., Prasai, T., Pant, S. R., and Jayana, B. L. (2008). Study on major pesticides and fertilizers used in Nepal. Sci. World 6, 76–80. doi: 10.3126/sw.v6i6.2638
Dorji, D., Chong, J. L., and Dorji, T. (2020). Habitat preference and current distribution of Chinese Pangolin (Manis pentadactyla L. 1758) in Dorokha Dungkhag, Samtse, southern Bhutan. J. Threat. Taxa 12, 16424–16433. doi: 10.11609/jott.5839.12.11.16424-16433
Duffy, R., St John, F. A. V., Büscher, B., and Brockington, D. (2016). Toward a new understanding of the links between poverty and illegal wildlife hunting. Conserv. Biol. 30, 14–22. doi: 10.1111/cobi.12622
Duncker, L. C., and Gonçalves, D. (2017). Community perceptions and attitudes regarding wildlife crime in South Africa. Int. J. Environ. Chem. Ecol. Geol. Geophys. Eng. 11, 144–150.
Ebua, V. B., Agwafo, T. E., and Fonkwo, S. N. (2011). Attitudes and perceptions as threats to wildlife conservation in the Bakossi area, South West Cameroon. Int. J. Biodivers. Conserv. 3, 631–636.
Epanda, M. A., Fotsing, A. J. M., Bacha, T., Frynta, D., Lens, L., Tchouamo, I. R., et al. (2019). Linking local people’s perception of wildlife and conservation to livelihood and poaching alleviation: A case study of the Dja biosphere reserve. Cameroon. Acta Oecol. 97, 42–48. doi: 10.1016/j.actao.2019.04.006
Esmail, N., Wintle, B. C., t Sas-Rolfes, M., Athanas, A., Beale, C. M., Bending, Z., et al. (2020). Emerging illegal wildlife trade issues: A global horizon scan. Conserv. Lett. 13:e12715. doi: 10.1111/conl.12715
Fearnside, P. M. (2005). Deforestation in Brazilian Amazonia: History, rates, and consequences. Conserv. Biol. 19, 680–688. doi: 10.1111/j.1523-1739.2005.00697.x
Franklin, J. F., Spies, T. A., Van Pelt, R., Carey, A. B., Thornburgh, D. A., Berg, D. R., et al. (2002). Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manage. 155, 399–423. doi: 10.1016/S0378-1127(01)00575-8
Gathorne-Hardy, F., and Syaukani Eggleton, P. (2001). The effects of altitude and rainfall on the composition of the termites (Isoptera) of the Leuser Ecosystem (Sumatra, Indonesia). J. Trop. Ecol. 17, 379–393. doi: 10.1017/S0266467401001262
Ghimire, P., Raut, N., Khanal, P., Acharya, S., and Upadhaya, S. (2020). Species in peril: Assessing the status of the trade in pangolins in Nepal. J. Threat. Taxa 12, 15776–15783. doi: 10.11609/jott.5698.12.8.15776-15783
Gillingham, S., and Lee, P. C. (1999). The impact of wildlife-related benefits on the conservation attitudes of local people around the Selous Game Reserve, Tanzania. Environ. Conserv. 26, 218–228. doi: 10.1017/S0376892999000302
Goldstein, W. (2003). Communication, education and public awareness for protected areas west Asia and northern Africa. Workshop report September 2003. Gland: IUCN.
GoN/DNPWC (2017). 5th amendment of national park and wildlife conservation Act 1973. Available online at: https://dnpwc.gov.np/media/rules/सरकषत_कषतरक_ऐन_नयम_सगल-२०७४.pdf (accessed June 23, 2022).
Government of Nepal (1973). National parks and wildlife conservation act. Nepal: The Nepal Law Commission, Government of Nepal.
Gurung, J. B. (1996). A pangolin survey in Royal Nagarjung Forest in Kathmandu. Nepal. Tigerpap. 23, 29–32.
Hairong, D., Xiaoliang, Z., Minghai, Z., Xiangdong, R., and Lee, T. M. (2022). Spatial distribution and conservation strategies of large carnivores in human-dominated landscape: A case study of Asiatic Black Bear in Jilin, China. Front. Ecol. Evol. 10:882282. doi: 10.3389/fevo.2022.882282
Heighton, S. P., and Gaubert, P. (2021). A timely systematic review on pangolin research, commercialization, and popularization to identify knowledge gaps and produce conservation guidelines. Biol. Conserv. 256:109042. doi: 10.1016/j.biocon.2021.109042
Heinrich, S., Wittman, T. A., Ross, J. V., Shepherd, C. R., Challender, D. W. S., and Cassey, P. (2017). The Global Trafficking of Pangolin: A comprehensive summary of Seizures and trafficking routes from 2010-2015. Available online at: https://www.traffic.org/site/assets/files/1606/global-pangolin-assessment.pdf
Hemachandra, I. I., Edirisinghe, J. P., Karunaratne, W. A. I. P., Gunatilleke, C. V. S., and Fernando, R. H. S. S. (2014). Diversity and distribution of termite assemblages in montane forests in the Knuckles Region, Sri Lanka. Int. J. Trop. Insect Sci. 34, 41–52. doi: 10.1017/S174275841300043X
Hessburg, P. F., Miller, C. L., Parks, S. A., Povak, N. A., Taylor, A. H., Higuera, P. E., et al. (2019). Climate, environment, and disturbance history govern resilience of western North American forests. Front. Ecol. Evol. 7:239. doi: 10.3389/fevo.2019.00239
Hessburg, P. F., Spies, T. A., Perry, D. A., Skinner, C. N., Taylor, A. H., Brown, P. M., et al. (2016). Tamm review: Management of mixed-severity fire regime forests in Oregon, Washington, and Northern California. For. Ecol. Manage. 366, 221–250. doi: 10.1016/j.foreco.2016.01.034
Hong, S., Do, Y., Kim, J. Y., Cowan, P., and Joo, G. J. (2017). Conservation activities for the Eurasian otter (Lutra lutra) in South Korea traced from newspapers during 1962-2010. Biol. Conserv. 210, 157–162. doi: 10.1016/j.biocon.2017.03.010
Jnawali, S. R., Baral, H. S., Lee, S., Acharya, K. P., Upadhyay, G. P., Pandey, M., et al. (2011). The Status of Nepal’s Mammals: The National Red List Series-IUCN. Bangkok: Department of National Parks and Wildlife.
Katrina, B. (2000). People, parks, forests or fields: A realistic view of tropical forest conservation. Land Use Policy 12, 137–144. doi: 10.1016/0264-8377(94)00013-P
Katuwal, H. B., Neupane, K. R., Adhikari, D., Sharma, M., and Thapa, S. (2015). Pangolins in eastern Nepal: Trade and ethno-medicinal importance. J. Threat. Taxa 7, 7563–7567. doi: 10.11609/JoTT.o4202.7563-7
Katuwal, H. B., Sharma, H. P., and Parajuli, K. (2017). Anthropogenic impacts on the occurrence of the critically endangered Chinese pangolin (Manis pentadactyla) in Nepal. J. Mammal. 98, 1667–1673. doi: 10.1093/jmammal/gyx114
Khatiwada, A. P., Suwal, T. L., Wright, W., Roe, D., Kaspal, P., Thapa, S., et al. (2020). Community conservation in Nepal–opportunities and challenges for pangolin conservation. Pangolins 2020, 395–409. doi: 10.1016/B978-0-12-815507-3.00025-3
Koirala, R. K., Aryal, A., Parajuli, A., and David, A. (2012). Human-common leopard (Panthera pardus) conflict in lower belt of Annapurna Conservation Area, Nepal. J. Res. Conserv. Biol. 1, 5–12.
Kumssa, T., and Bekele, A. (2014). Attitude and perceptions of local residents toward the protected area of Abijata-Shalla Lakes National Park (ASLNP), Ethiopia. J. Ecosyst. Ecogr. 4:138. doi: 10.1155/2014/295362
Laundré, J. W., and Reynolds, T. D. (1993). Effects of soil structure on burrow characteristics of five small mammal species. Gt. Basin Nat. 53, 358–366.
Laurance, W. F., Sayer, J., and Cassman, K. G. (2014). Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 29, 107–116. doi: 10.1016/j.tree.2013.12.001
Leclerc, J.-C., Brante, A., and Viard, F. (2021). Rapid recovery of native habitat-builders following physical disturbance on pier pilings offsets colonization of cryptogenic and non-indigenous species in a Chilean port. Mar. Environ. Res. 163:105231. doi: 10.1016/j.marenvres.2020.105231
Manly, B. F. J., McDonald, L. L., Thomas, D. L., MacDonald, T. L., and Erickson, W. P. (2002). Resource selection by animals. statistical design and analysis for field studies, 2nd Edn. London: Kluwer Academic Publisher.
Mohapatra, R. K., Panda, S., Acharjyo, L. N., Nair, M. V., and Challender, D. W. (2015). A note on the illegal trade and use of pangolin body parts in India. Traff. Bull. 27, 33–40. doi: 10.1007/s12639-015-0653-5
Mutanga, C. N., Muboko, N., and Gandiwa, E. (2017). Protected area staff and local community viewpoints: A qualitative assessment of conservation relationships in Zimbabwe. PLoS One 12:e0177153. doi: 10.1371/journal.pone.0177153
Nash, H. C., Wong, M. H. G., and Turvey, S. T. (2016). Using local ecological knowledge to determine status and threats of the Critically Endangered Chinese pangolin (Manis pentadactyla) in Hainan, China. Biol. Conserv. 196, 189–195. doi: 10.1016/j.biocon.2016.02.025
Neupane, B., Dhami, B., Bista, S., Sadadev, B. M., Regmi, S., Shrestha, S., et al. (2022). Ecological factors determining barking deer distribution and habitat use in the mid-hills of Nepal. Front. Ecol. Evol. 10:894369. doi: 10.3389/fevo.2022.894369
Newmark, W. D., Leonard, N. L., Sariko, H. I., and Gamassa, D.-G. M. (1993). Conservation attitudes of local people living adjacent to five protected areas in Tanzania. Biol. Conserv. 63, 177–183. doi: 10.1016/0006-3207(93)90507-W
Newton, P., Van Thai, N., Roberton, S., and Bell, D. (2008). Pangolins in peril: Using local hunters’ knowledge to conserve elusive species in Vietnam. Endanger. Spec. Res. 6, 41–53. doi: 10.3354/esr00127
Ngonidzashe Mutanga, C., Vengesayi, S., Gandiwa, E., and Muboko, N. (2015). Community perceptions of wildlife conservation and tourism: A case study of communities adjacent to four protected areas in Zimbabwe. Trop. Conserv. Sci. 8, 564–582. doi: 10.1177/194008291500800218
Nijman, V., Zhang, M. X., and Shepherd, C. R. (2016). Pangolin trade in the mong la wildlife market and the role of myanmar in the smuggling of pangolins into China. Glob. Ecol. Conserv. 5, 118–126. doi: 10.1016/j.gecco.2015.12.003
Palik, B. J., Mitchell, R. J., and Hiers, J. K. (2002). Modeling silviculture after natural disturbance to sustain biodiversity in the longleaf pine (Pinus palustris) ecosystem: Balancing complexity and implementation. For. Ecol. Manage. 155, 347–356. doi: 10.1016/S0378-1127(01)00571-0
Paudel, B., Pandit, J., and Reed, B. (2013). Fragmentation and conversion of agriculture land in Nepal and Land Use Policy 2012. Germany: University Library of Munich.
Paudel, K., Potter, G. R., and Phelps, J. (2020). Conservation enforcement: Insights from people incarcerated for wildlife crimes in Nepal. Conserv. Sci. Pract. 2:e137. doi: 10.1111/csp2.137
Pereira, H. M., Daily, G. C., and Roughgarden, J. (2004). A framework for assessing the relative vulnerability of species to land-use change. Ecol. Appl. 14, 730–742. doi: 10.1890/02-5405
Pokharel, C. P. (1996). Food habit and habitat utilization of swamp deer (Cervus duvauceli duvauceli) in Royal Bardia National Park, Nepal. Ph.D. thesis. Kirtipur: Tribhuvan University.
Primack, R. B. (2014). Essentials of conservation biology, 6th Edn. Sunderland, MA: Sinauer. Associates.
Radeloff, V. C., Mladenoff, D. J., and Boyce, M. S. (2000). Effects of interacting disturbances on landscape patterns: Budworm defoliation and salvage logging. Ecol. Appl. 10, 233–247. doi: 10.1890/1051-0761(2000)010[0233:EOIDOL]2.0.CO;2
Rodrigues, A. S. L., Akcakaya, H. R., Andelman, S. J., Bakarr, M. I., Boitani, L., Brooks, T. M., et al. (2004). Global gap analysis: Priority regions for expanding the global protected-area network. Bioscience 54, 1092–1100. doi: 10.1641/0006-3568(2004)054[1092:GGAPRF]2.0.CO;2
Romanach, S. S., Lindsey, P. A., and Woodroffe, R. (2007). Determinants of attitudes towards predators in central Kenya and suggestions for increasing tolerance in livestock dominated landscapes. Oryx 41, 185–195. doi: 10.1017/S0030605307001779
Schlegel, J., and Rupf, R. (2010). Attitudes towards potential animal flagship species in nature conservation: A survey among students of different educational institutions. J. Nat. Conserv. 18, 278–290. doi: 10.1016/j.jnc.2009.12.002
Sharma, D. R., Thapa, R. B., Manandhar, H. K., Shrestha, S. M., and Pradhan, S. B. (2012). Use of pesticides in Nepal and impacts on human health and environment. J. Agric. Environ. 13, 67–74. doi: 10.3126/aej.v13i0.7590
Sharma, H. P., Belant, J. L., and Shaner, P.-J. L. (2019). Attitudes towards conservation of the Endangered red panda Ailurus fulgens in Nepal: A case study in protected and non-protected areas. Oryx 53, 542–547. doi: 10.1017/S0030605317000990
Sharma, H. P., Rimal, B., Zhang, M., Sharma, S., Poudyal, L. P., Maharjan, S., et al. (2020a). Potential distribution of the critically endangered Chinese pangolin (Manis pentadactyla) in different land covers of Nepal: Implications for conservation. Sustainability 12:1282. doi: 10.3390/su12031282
Sharma, S., Sharma, H. P., Katuwal, H. B., and Belant, J. L. (2020b). Knowledge of the Critically Endangered Chinese pangolin (Manis pentadactyla) by local people in Sindhupalchok, Nepal. Glob. Ecol. Conserv. 23:e01052. doi: 10.1016/j.gecco.2020.e01052
Sharma, S., Sharma, H. P., Chaulagain, C., Katuwal, H. B., and Belant, J. L. (2020c). Estimating occupancy of Chinese Pangolin (Manis pentadactyla) in a protected and non-protected area of Nepal. Ecol. Evol. 10, 4303–4313. doi: 10.1002/ece3.6198
Shrestha, A., Bhattarai, S., Shrestha, B., and Koju, N. P. (2021). Factors influencing the habitat choice of pangolins (Manis spp.) in low land of Nepal. Ecol. Evol. 11, 14689–14696. doi: 10.1002/ece3.8156
Suwal, T. L. (2011). Status, distribution, behaviour and conservation of pangolins in private and community forest of Balthali in Kavre, Nepal. Ph.D. thesis. Kirtipur: Tribhuwan University.
Suwal, T. L., Gurung, S., Shrestha, M. B., Ingram, D. J., and Pei, K. J. (2022). Human dimensions of pangolin conservation: Indigenous and local knowledge, ethnozoological uses, and willingness of rural communities to enhance pangolin conservation in Nepal. J. Ethnobiol. 42. doi: 10.2993/0278-0771-42.3.7
Suwal, T. L., Thapa, A., Gurung, S., Aryal, P. C., Basnet, H., Basnet, K., et al. (2020). Predicting the potential distribution and habitat variables associated with pangolins in Nepal. Glob. Ecol. Conserv. 23:e01049. doi: 10.1016/j.gecco.2020.e01049
Swart, J. M., Richardson, P. R. K., and Ferguson, J. W. H. (1999). Ecological factors affecting the feeding behaviour of pangolins (Manis temminckii). J. Zool. 247, 281–292. doi: 10.1111/j.1469-7998.1999.tb00992.x
Thapa, P., Khatiwada, A. P., Nepali, S. C., and Paudel, S. (2014). Distribution and conservation status of Chinese pangolin (Manis pentadactyla) in Nangkholyang VDC, Taplejung, Eastern Nepal. Am. J. Zool. Res. 2, 16–21.
Tomićević, J., Shannon, M. A., and Milovanović, M. (2010). Socio-economic impacts on the attitudes towards conservation of natural resources: Case study from Serbia. For. Policy Econ. 12, 157–162. doi: 10.1016/j.forpol.2009.09.006
Turvey, S. T., Fernández-Secades, C., Nuñez-Miño, J. M., Hart, T., Martinez, P., Brocca, J. L., et al. (2014). Is local ecological knowledge a useful conservation tool for small mammals in a Caribbean multicultural landscape? Biol. Conserv. 169, 189–197. doi: 10.1016/j.biocon.2013.11.018
Ullmann, T., Veríssimo, D., and Challender, D. W. S. (2019). Evaluating the application of scale frequency to estimate the size of pangolin scale seizures. Glob. Ecol. Conserv. 20:e00776. doi: 10.1016/j.gecco.2019.e00776
Vannelli, K., Hampton, M. P., Namgail, T., and Black, S. A. (2019). Community participation in ecotourism and its effect on local perceptions of snow leopard (Panthera uncia) conservation. Hum. Dimens. Wildl. 24, 180–193. doi: 10.1080/10871209.2019.1563929
Willcox, D., Nash, H. C., Trageser, S., Kim, H. J., Hywood, L., Connelly, E., et al. (2019). Evaluating methods for detecting and monitoring pangolin (Pholidata: Manidae) populations. Glob. Ecol. Conserv. 17:e00539. doi: 10.1016/j.gecco.2019.e00539
Wu, S. B., Liu, N. F., Ma, G. Z., Xu, Z. R., and Chen, H. (2003). Habitat selection by Chinese pangolin (Manis pentadactyla) in winter in Dawuling Natural Reserve. Mammalia 67, 493–502. doi: 10.1515/mamm-2003-0403
Wu, S., Liu, N., Zhang, Y., and Ma, G. Z. (2004). Assessment of threatened status of Chinese Pangolin (Manis pentadactyla). Chin. J. Appl. Environ. Biol. 10, 456–461.
Yahnke, C. J. (2006). Habitat use and natural history of small mammals in the central Paraguayan Chaco. Mastozool. Neotrop. 13, 103–116.
Yamamoto, Y., Matsumoto, K. I., Kawata, K., and Kaneko, S. (2019). Gender-based differences in employment opportunities and wage distribution in Nepal. J. Asian Econ. 64:101131. doi: 10.1016/j.asieco.2019.07.004
Yang, H., Harrison, R., Yi, Z. F., Goodale, E., Zhao, M. X., and Xu, J. C. (2015). Changing perceptions of forest value and attitudes toward management of a recently established nature reserve: A case study in southwest China. Forests 6, 3136–3164. doi: 10.3390/f6093136
Zhang, F., Wu, S., and Cen, P. (2022). The past, present and future of the pangolin in Mainland China. Glob. Ecol. Conserv. 33:e01995. doi: 10.1016/j.gecco.2021.e01995
Keywords: Chinese pangolin, Manis pentadactyla, habitat preference, critically endangered mammal, conservation effort
Citation: Panta M, Dhami B, Shrestha B, Kc N, Raut N, Timilsina YP, Khanal Chhetri BB, Khanal S, Adhikari H, Varachova S and Kindlmann P (2023) Habitat preference and distribution of Chinese pangolin and people’s attitude to its conservation in Gorkha District, Nepal. Front. Ecol. Evol. 11:1081385. doi: 10.3389/fevo.2023.1081385
Received: 27 October 2022; Accepted: 28 March 2023;
Published: 20 April 2023.
Edited by:
Yixin Zhang, Soochow University, ChinaReviewed by:
Mukesh Thakur, Zoological Survey of India, IndiaCopyright © 2023 Panta, Dhami, Shrestha, Kc, Raut, Timilsina, Khanal Chhetri, Khanal, Adhikari, Varachova and Kindlmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bikram Shrestha, YmlrcmFtb25lQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.