- 1Division of Science, Yale-NUS College, Singapore, Singapore
- 2Department of Biological Sciences, National University of Singapore, Singapore, Singapore
- 3School of BioSciences, University of Melbourne, Melbourne, VIC, Australia
- 4Animal Behaviour Group, Department of Environment and Genetics, La Trobe University, Melbourne, VIC, Australia
- 5Gansu Key Laboratory of Biomonitoring and Bioremediation for Environmental Pollution, School of Life Sciences, Lanzhou University, Lanzhou, China
Diverse animals including snakes, spiders and phasmids sway in response to abiotic and biotic factors. Recent research on swaying in phasmids suggest they may adopt distinctive swaying to reduce detection from predators. This view was recently challenged, by interpreting swaying behavior as serving a balancing function related to postural sway and not a form of anti-predator behavior. We dispute this interpretation as the reanalysis of data for balance was based on an erroneous perception of the upright posture of the insects, contrary to the initial study and natural history observations. We present observations collected from four species of more than 300 phasmids over a three-day period and show that the insects seldom adopt an upright posture (4% of observations). While we appreciate that attempts to reinterpret data form a central role of the scientific method, we urge caution when inferring biological function without an accurate knowledge of the species’ natural history. Investigations of signals in motion require great care to ensure they are interpreted in a natural environment and context.
Introduction
Animals move for various purposes, such as to forage, find mates, deter rivals and predators and to seek shelter. This movement may draw unwanted attention from natural enemies. It is thus particularly surprising that many species sway, a non-perambulatory movement which involves the lateral rocking of the body, while the legs remain stationary and in contact with the substrate (Bian et al., 2016). This behavior is particularly prevalent in phasmids, but also occurs in diverse taxa, including spiders, mantids and snakes (Fleishman, 1985; Jackson, 1985; Watanabe and Yano, 2009; Tan and Elgar, 2021). Several lines of thought suggest that swaying behavior has a signaling function, providing offensive or defensive mechanisms to improve foraging success or the likelihood of attack from predators. For example, Portia spiders sway their palps, legs and bodies to evade detection when they approach their arachnid prey, as the movements give Portia the appearance unlike that of a spider or an animal that imposes a threat (Jackson, 1985). Vine snakes Oxybelis aeneus oscillate forward and backward, presumably to mimic vegetation movement (Fleishman, 1985). In mantids, swaying can reduce detection by predators, cannibalistic conspecifics and prey (Watanabe and Yano, 2009, 2012, 2013). Several species of phasmids are reported to sway (Rupprecht, 1971; Bian et al., 2016; Pohl et al., 2022), with recent research on phasmids consistent with the view that phasmids adopt swaying to reduce detection (Bian et al., 2016; Pohl et al., 2022). Thus, swaying provides phasmids with a form of concealment between revealing behaviors through motion masquerade, which is the matching of an animal’s motion to environmental motion, such that the animal resembles an inanimate object and prevents detection by an observer (Fleishman, 1985). Through the interpretation of swaying behavior in phasmids, this article emphasizes the importance of inferring biological function with an accurate knowledge of the species’ natural history.
Swaying, hanging and perching
Recently, swaying behavior has been interpreted as serving a balancing function (Kelty-Stephen, 2018; Cuthill et al., 2019), based on reanalysis by Kelty-Stephen (2018) using the data presented in Bian et al. (2016). Bian et al. (2016) examined the movement of the phasmid Extatosoma tiartum in response to wind cues. While the insects would sway in response to wind stimulus, the frequency of swaying declined over time. The number of sways was higher in variable wind conditions compared with constant wind conditions, suggesting that the insects react to environmental cues such as wind stimulus and modify their swaying behavior in response. In the presence of plants in the background, insect swaying was consistent with the movement of the wind-blown plants. Reanalyzing part of the same dataset, Kelty-Stephen (2018) proposed that the swaying behavior allows the insects to achieve stability in response to wind-like stimulation. Kelty-Stephen (2018) further proposes that these data provide evidence for multifractal complexity in postural stabilization under wind-like stimulation and point to similarities between phasmid and human postural sway. Kelty-Stephen (2018) proposed that the reduction in sway exhibited by phasmids can be explained by non-linear interactions across time consistent with tensegrity principles and dismissed Bian et al.’s (2016) suggestion of anti-predator behavior on the part of the insects. Such a balancing function could be more relevant to perching than hanging insects, with the latter relying on gravity to remain stable.
We do not seek to question Kelty-Stephen’s (2018) analysis. However, we must point out that the author has foremost, incorrectly characterized the phasmids described in Bian et al. (2016) to ‘perch upon a branch’ (Kelty-Stephen, 2018, p. 8), when they were, in fact, hanging from a branch (Figures 1A,B). It is unclear why Kelty-Stephen (2018) took this perspective on phasmid swaying behavior and whether this inaccuracy influenced the author’s interpretations. While phasmids can walk when perching, it is uncommon. E. tiaratum (Phasmatidae) typically hangs from perches, rarely spending time ‘upright’. We found a similar pattern for four species of phasmids that we had collected from the field, maintained in the laboratory and observed over a three-day period (Figures 1C–F; Table 1). Hanging from the top or side of the enclosure, host plant or even another phasmid was the overwhelming position observed, while perching was relatively rare (60 out of 1,343 observations, 4%). Swaying behavior was not ubiquitous across these four species: swaying was common in Lonchodes brevipes (Diapheromeridae), occasionally observed in Calvisia flavopennis (Lonchodidae) and Marmessoidea rosea (Lonchodidae), and rarely observed in Haaniella echinata (Heteropterygidae). Indeed, our study (Pohl et al., 2022) indicate that even for the same species (L. brevipes), individuals at different life stages appear to sway to different extents. More nuanced studies in the future are crucial to understand the factors affecting swaying behavior, but the appropriate biological context to consider stabilizing mechanisms in such systems is of a hanging organism.
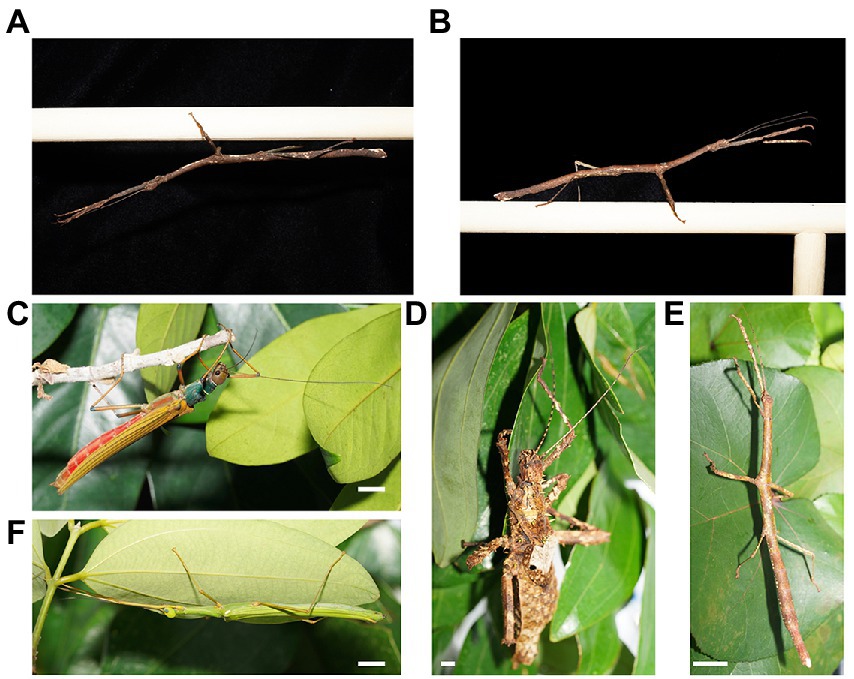
Figure 1. Phasmid Lonchodes brevipes (A) hanging and (B) perched from a dowel. Phasmids at rest: (C) Calvisia flavopennis; (D) Haaniella echinata; (E) Lonchodes brevipes; (F) Marmessoidea rosea. Only female adult insects are represented; white line represents 10 mm in each panel. Images are presented as taken in real life without image rotation. Photos by Eunice J. Tan.
Interpreting behavior
We find it surprising that the author misrepresented the work in this way, particularly as the hanging phasmids considered in Bian et al. (2016) contrasts with upright focal organisms in references on postural sway (e.g., Straube et al., 1987; Clayton et al., 2003; Hutchinson et al., 2007; Munafo et al., 2016; Dewolf et al., 2021). Demonstrating tensegrity principles in a hanging organism seems novel and worthy of further attention; but evaluation of the significance and broader implications of these findings are relevant only when the behavior is placed in the correct context. Kelty-Stephen (2018) was, perhaps, too quick to dismiss the potential for sway to represent an adaptive behavior to avoid predators. We do not claim here that the data provides definitive evidence that it does, nor did Bian et al. (2016). However, we argue that Kelty-Stephen (2018) dismisses the possibility based on a limited consideration of the broader investigation, and we do not wish for this premature and inaccurate characterization to propagate (e.g., Cuthill et al., 2019).
In characterizing Bian et al.’s (2016) study, Kelty-Stephen (2018) correctly describes how phasmids decreased swaying over time and that these data were from trials in which no plants were present. It is from this account alone that Kelty-Stephen (2018) rejects the adaptation explanation, suggesting that decreasing sway could be regarded as “a morbid wish to stand out to predators” (p. 16). This is a flawed proposition because there were no plants present in the trial so continuing to sway would also offer no camouflage benefit. What Kelty-Stephen (2018) did not mention is that the aim of the initial experiment by Bian et al. (2016) was to determine whether wind initiates swaying in hanging insects; which was clearly demonstrated. A second experiment subsequently showed that insects swayed more under variable wind, which is a more natural wind stimulus, compared with constant wind as used in the first experiment. Together, these two experiments by Bian et al. (2016) showed that wind initiates swaying and that insects can control swaying. The presence of both wind and insect control of swaying are necessary for a putative motion camouflage explanation; however, neither were undertaken in the presence of plants and make no attempt to relate swaying behavior with plant movement.
Similarities between phasmid and plant movement was investigated in a third experiment. Here insects and plants were filmed in natural conditions and in many circumstance the movement of both matched in the frequency domain. There were some exceptions that serve to highlight the potential for non-moving objects to standout from moving ones, but also to prompt consideration of the circumstance that do and do not lead to swaying in natural environments. These are outlined in Bian et al. (2016) and highlight the complex factors that contribute to animal behavior, a point which seems to have alluded Kelty-Stephen (2018). Thus, contrary to Kelty-Stephen’s (2018) assertion that Bian et al. (2016) “failed to find evidence that phasmids exploited the wind in the way they predicted” (p. 16), the complete dataset in Bian et al. (2016) showed that there most certainly is the potential for insects to benefit from swaying in wind and enough evidence was provided to take the next step, which is to confirm such behavior confers a survival advantage for the insects. Such investigations necessarily require an understanding of predator motion vision systems and behavior.
Conclusion
The range of taxa from phasmids to spiders and snakes that adopt swaying behavior suggests that this behavior may have an adaptive function. To uncover the adaptive function of swaying, further investigations are necessary to examine the circumstances in which individuals within species do and do not sway. We presented several offensive and defensive functions of swaying, which need not be mutually exclusive (Jackson, 1985; Watanabe and Yano, 2009, 2012, 2013; Bian et al., 2016; Tan and Elgar, 2021). We dispute Kelty-Stephen’s (2018) conclusion following reanalysis of the data presented in Bian et al. (2016) and the following assertions, as the reanalysis was based on flawed assumptions – contrary to both the diagrams presented in Bian et al. (2016), and natural history observations, the species hangs rather than perches on the vegetation. A key challenge for studies of animal behavior is to understand the function of animal behaviors. We urge caution when inferring function without sufficient/accurate natural history knowledge, often best achieved by collaboration. Particularly for the investigation of signals in motion such as swaying, great care must be taken to interpret the signals in the natural environment and context.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ET and ME conceptualized the project. ET collected the data. ET, ME, and RP wrote the draft. All authors contributed to the manuscript revision, read, and approved the final manuscript.
Funding
This work was supported by Ministry of Education, Singapore, and Yale-NUS College Start-up Grant to ET.
Acknowledgments
We thank Jay Wong for helping with the observations on the phasmids.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bian, X., Elgar, M. A., and Peters, R. A. (2016). The swaying behavior of Extatosoma tiaratum: motion camouflage in a stick insect? Behav. Ecol. 27, 83–92. doi: 10.1093/beheco/arv125
Clayton, H. M., Bialski, D. E., Lanovaz, J. L., and Mullineaux, D. R. (2003). Assessment of the reliability of a technique to measure postural sway in horses. Am. J. Vet. Res. 64, 1354–1359. doi: 10.2460/ajvr.2003.64.1354
Cuthill, I. C., Matchette, S. R., and Scott-Samuel, N. E. (2019). Camouflage in a dynamic world. Curr. Opin. Behav. Sci. 30, 109–115. doi: 10.1016/j.cobeha.2019.07.007
Dewolf, A. H., Ivanenko, Y. P., Mesquita, R. M., and Willems, P. A. (2021). Postural control in the elephant. J. Exp. Biol. 224:jeb243648. doi: 10.1242/jeb.243648
Fleishman, L. J. (1985). Cryptic movement in the vine Snake Oxybelis aeneus. Copeia 1985, 242–245. doi: 10.2307/1444822
Hutchinson, D., Ho, V., Dodd, M., Dawson, H. N., Zumwalt, A. C., Schmitt, D., et al. (2007). Quantitative measurement of postural sway in mouse models of human neurodegenerative disease. Neuroscience 148, 825–832. doi: 10.1016/j.neuroscience.2007.07.025
Jackson, R. R. (1985). A web-building jumping spider. Sci. Am. 253, 102–115. doi: 10.1038/scientificamerican0985-102
Kelty-Stephen, D. G. (2018). Multifractal evidence of nonlinear interactions stabilizing posture for phasmids in windy conditions: a reanalysis of insect postural-sway data. PLoS One 13:e0202367. doi: 10.1371/journal.pone.0202367
Munafo, J., Curry, C., Wade, M. G., and Stoffregen, T. A. (2016). The distance of visual targets affects the spatial magnitude and multifractal scaling of standing body sway in younger and older adults. Exp. Brain Res. 234, 2721–2730. doi: 10.1007/s00221-016-4676-7
Pohl, S., Bungum, H. Z., Lee, K., Bin Sani, M., Poh, Y., Wahab, R., et al. (2022). Age and appearance shape behavioural responses of phasmids in a dynamic environment. Front. Ecol. Evol. 9:767940. doi: 10.3389/fevo.2021.767940
Rupprecht, R. (1971). Bewegungsmimikry bei Carausius morosus Br. (Phasmida). Experientia 27, 1437–1438. doi: 10.1007/BF02154275
Straube, A., Brandt, T., and Probst, T. (1987). Importance of the visual cortex for postural stabilization: inferences from pigeon and frog data. Hum. Neurobiol. 6, 39–43.
Tan, E. J., and Elgar, M. A. (2021). Motion: enhancing signals and concealing cues. Biol. Open 10:bio058762. doi: 10.1242/bio.058762
Watanabe, H., and Yano, E. (2009). Behavioral response of Mantid Hierodula patellifera to wind as an Antipredator strategy. Ann. Entomol. Soc. Am. 102, 517–522. doi: 10.1603/008.102.0323
Watanabe, H., and Yano, E. (2012). Behavioral response of male mantid Tenodera aridifolia (Mantodea: Mantidae) to windy conditions as a female approach strategy. Entomol. Sci. 15, 384–391. doi: 10.1111/j.1479-8298.2012.00535.x
Keywords: swaying, phasmids, adaptive function, anti-predator, natural history
Citation: Tan EJ, Elgar MA, Bian X and Peters RA (2023) Interpreting animal behaviors – A cautionary note about swaying in phasmids. Front. Ecol. Evol. 11:1065789. doi: 10.3389/fevo.2023.1065789
Edited by:
Nicola Marples, Trinity College Dublin, IrelandReviewed by:
Rodrigo Willemart, University of São Paulo, BrazilNathalia Ximenes, University of São Paulo, Brazil, in collaboration with reviewer RW
Copyright © 2023 Tan, Elgar, Bian and Peters. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eunice J. Tan, ✉ eW5jdGplQG51cy5lZHUuc2c=
 Eunice J. Tan
Eunice J. Tan Mark A. Elgar
Mark A. Elgar Xue Bian4,5
Xue Bian4,5 Richard A. Peters
Richard A. Peters