
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 25 August 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1062764
This article is part of the Research TopicConservation in Contested LandscapesView all 4 articles
 Ariful Khan1
Ariful Khan1 Md Rezaul Karim2
Md Rezaul Karim2 Mohammed1
Mohammed1 Mohammad Golam Kibria1,3
Mohammad Golam Kibria1,3 Karishma Sinha4
Karishma Sinha4 Fahmida Sultana1
Fahmida Sultana1 Sharif A. Mukul5,6,7*
Sharif A. Mukul5,6,7* Mohammed A. S. Arfin-Khan1,8
Mohammed A. S. Arfin-Khan1,8The relationship between ecosystem functions and plant functional traits has been well documented and is considered to be one of the most vital topics in ecology. However, the correlation between anthropogenic disturbance and tree functional trait diversity remains largely unclear. The present study investigates the role of anthropogenic disturbance on selected tree functional traits in Ratargul Swamp Forest (RSF) – the only remnant tropical freshwater swamp forest ecosystem in Bangladesh. We established 50 plots and collected six plant traits, i.e., tree height, specific leaf area (SLA), wood density (WD), leaf dry matter content (LDMC), seed mass, and bark thickness. A two-way analysis of variance (ANOVA) was carried out to test the interaction between plant functional traits and anthropogenic disturbance in RSF. Our study found that anthropogenic disturbance has a significant (p< 0.05) influence on deciduous swamp forest tree species’ functional traits but none on evergreen tree species’ functional traits. For deciduous trees, disturbance has a significant positive effect on CWM.SLA (p< 0.05) and CWM.Height (p< 0.05) and a negative impact on CWM.Bark-thickness and CWM.Seed-mass (p< 0.05). There were no significant effects of disturbance on CWM.WD and CWM.LDMC. We believe the present study will contribute toward improving our understanding of the effect of disturbances on tree functional trait diversity in tropical human-dominated landscapes where anthropogenic pressure is increasing at an unprecedented rate. It will also function as an essential conservation for related decision-making of Ratargul Swamp Forest in northeastern Bangladesh and elsewhere in the tropics with a similar context.
Human-induced disturbance is considered to be the primary cause of the degradation of forest habitats and associated biodiversity loss around the world (Fischer and Lindenmayer, 2007; Kowarik and Säumel, 2011; Moreno-Mateos et al., 2017; Pyles et al., 2018). Anthropogenic disturbance above a certain level (i.e., agricultural activities, urbanization, deforestation, etc.) has always resulted in the disruption of the natural ecological functioning of a healthy ecosystem (Uddin et al., 2013; Scanes, 2018). Before leading up to the eventual dismantling of biodiversity, some of the impacts faced due to anthropogenic disturbance by a functioning ecosystem include biotic homogenization and negative impact on floral and faunal functional diversity (Bruelheide et al., 2018). Abiotic conditions also change, including hydrological regimes, soil properties, and temperature regimes, all of which play a vital role in the distribution of species which ultimately reduces ecological services provided by an ecosystem (McKinney, 2006; Alamgir et al., 2015; Blouin et al., 2019; Cao and Natuhara, 2020; Yinan et al., 2021).
Swamp forests are forested wetlands that have been subjected to continued disturbance around the world (Cole et al., 2015), hampering their ability to provide key ecosystem services and functions (Roy et al., 2019; Hanisch et al., 2020). Plant functional traits, on the other hand, are traits that represent morphological, physiological, or phenological attributes of plants, indirectly influencing the fitness of an individual plant in an ecosystem through administering effects on growth, reproduction, and survival (Violle et al., 2007). Environmental conditions influence plant functional distribution in an ecosystem (Sutton-Grier et al., 2011; Fyllas et al., 2020); this is a critical field of study as it provides information on different strategies of resource use and acquisition by plants based on environmental factors (Scheres and van der Putten, 2017). Evaluating the effects of disturbance on plant traits also provide a representation of the overall status of a forest’s health (Bernard-Verdier et al., 2012; Sultana et al., 2023). Clear knowledge of how organisms interact with their environment, with each other, and how traits vary in an environment depending on ecological gradients are also essential to determine particular traits (Naeem and Wright, 2003; Bruelheide et al., 2018). According to Gross et al. (2007), hard and soft traits are the two plant trait groups that can yield information on local persistence, community structure, and response to environmental changes (Cornelissen et al., 2003; Lavorel and Garnier, 2002; Gillison et al., 2013; Wullschleger et al., 2014; Goodness et al., 2016). A study on resource-acquisitive and conservative traits by Poorter et al. (2015) and King et al. (2006) suggests that acquisitive traits such as height and SLA are less resistant to disturbances, while resource-conservative traits are more resistant to disturbance, resulting in a wider distribution of conservative traits.
Plant functional traits vary across different functional types, such as evergreen, deciduous, and light-demanding shade-tolerant species, studies of which are essential to evaluate the dynamic distribution of vegetation in an ecosystem (Fyllas et al., 2012; Atkin et al., 2015). Evergreen species compared to deciduous species are able to withstand anthropogenic disturbance of the ecosystem due to some superior plant traits, but they are also able to tolerate low disturbance regimes (Greenwood et al., 2017; Karim et al., 2023). A study on cavity resistance and nutrient-use efficiency of evergreen species suggests that this species is resistant to disturbed habitats (Brodribb et al., 2012) although recent studies show that cavity resistance and nutrient-use efficiency do not represent the whole hydraulic strategy of a plant (Anderegg, 2015; Gleason et al., 2016). On the other hand, deciduous species perform better than evergreen species under ideal resource conditions (Berendse and Scheffer, 2009). Recent studies investigated the variation among functional traits in different functional ecosystems (Sharmin et al., 2016; Fyllas et al., 2020) as functional traits mediate the performance of the ecosystem (Diaz et al., 2004). (Kattge et al., 2011) Most of these studies, however, were conducted in relatively less complex grassland ecosystems (Roscher et al., 2012; Zuo et al., 2012) with only a few conducted in forests or wetlands (Roy et al., 2019).
In Bangladesh, the impact of anthropogenic disturbance on plant functional traits is yet to be investigated in forested ecosystems, particularly in a wetland like Ratargul Swamp Forest (RSF), which is a special type of ecosystem in the country with great conservation value (Mukul et al., 2018). In the present study, we investigated differential trait responses of deciduous and evergreen species in RSF with regard to anthropogenic disturbances. By anthropogenic disturbances, here, we refer to illegal tree felling/removal and subsequent canopy openings, decreases in plant population and densities, disrupted regeneration of species, the decrease in tree species richness and tree abundance, alterations in forest structure, and finally, the decrease in tree functional diversity (Sultana et al., 2023). Our objectives were to: (1) construct a functional trait distribution map of RSF and (2) determine how anthropogenic disturbances influence plant functional trait conditions in RSF. Our study will complement existing research gaps in Bangladesh and will improve our understanding of the role of anthropogenic disturbances on functional trait diversity in Ratargul Swamp Forest, the only remaining freshwater swamp forest ecosystem in Bangladesh.
This study was conducted in Ratargul Swamp Forest (RSF), which was declared to be a Reserved Forest under the Assam Forest Act in 1932 with an area of 118.55 ha (Choudhury et al., 2004). Geographically, located between 25°00.025′×0384;N latitude and 91°58.180′×0384; E longitude, RSF is the only freshwater swamp forest in the Sylhet region of northeast Bangladesh (Figure 1).
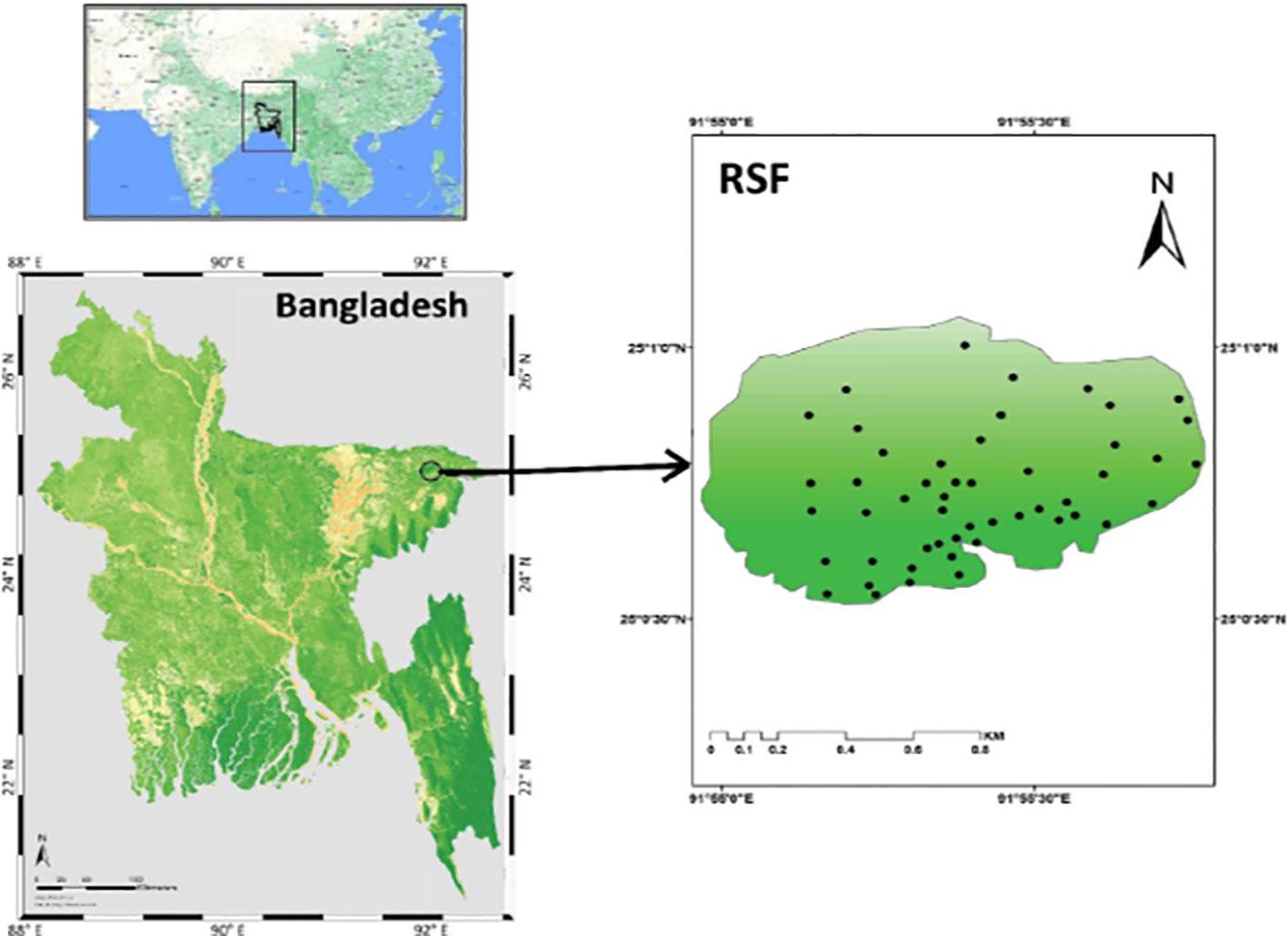
Figure 1 The location map of Ratargul swamp forest (RSF) in the Sylhet region of northeast Bangladesh (left) with sampling plots (right).
As it is a swamp forest, the terrain of RSF is not uniform. Most of the land is submerged underwater throughout the year, while the remaining land is plain or low land (Choudhury et al., 2004). The area is situated 35 meters above sea level (asl). The soil is a gray, solid, silty clay loam with a clayey texture (Choudhury et al., 2004). Agricultural fields surrounding the RSF are loamy in general. At the end of the rainy season and during the dry season, soils in the forest area easily turn dry and hard. The soil moisture varies from 18.7 to 37.1% (Sharmin et al., 2016). The mean maximum temperature is approximately 32°C. On average, RSF receives 1250 mm of rain per annum, and relative humidity is approximately 74% during December, while in July–August, it is over 90% (Choudhury et al., 2004). The forest has a canopy coverage of 80% with approximately 15 m canopy height. RSF has two major strata, where the upper story is covered by Koroch (Pongamia pinnata) and the understory is covered by Murta or patipata (Schumannianthus dichotomus) along with other endemic species (Choudhury et al., 2004).
A total of 50 20 m×20 m square plots were laid in the RSF in December 2019. Species, traits, and environment data for this study were collected through intensive field surveys where the locations of the plots were selected randomly with the help of ArcGIS 10.5 software to avoid any sampling bias (Figure 1). The geographic coordinates of each plot were recorded at the center of each plot using a hand-held Garmin GPS. All the trees with a diameter at breast height (dbh) greater than and/or equal to 10 cm were identified with their appropriate taxonomic identity and counted for each plot. The main trunk of trees that possessed more than a single stem was measured at the narrowest point below the branches. High-buttressed trees were measured at approximately 30 cm above the convergence of the protrusions of the buttresses on the bowl. We recorded the number of cut stems and tree canopy openings in a plot as an anthropogenic disturbance factor although we did not assess the timing and rate of the disruption due to unreliable data. A densitometer was taken in the center of the plot to measure the tree canopy coverage. The canopy coverage of the shrub and herb species was, however, measured via visual estimation (i.e., ocular estimate). For the ocular estimate, the average observation of two people was considered (Karim et al., 2020).
We recorded information on six functional traits, i.e., plant height, specific leaf area (SLA), leaf dry matter content (LMDC), wood density (WD), seed mass, and bark thickness, from each of our study plots. Altogether, 511 individual trees were samples from all of our plots following the protocols described in Pérez-Harguindeguy et al. (2013). Tree height was measured with Suunto PM-5 clinometers with the shortest distance between the upper pick point of the tree and ground level, which is measured by a meter (Garnier et al., 2013). The height of each tree standing at dbh ≥ 10 cm was measured from each sample plot. For SLA measurement, fresh leaves were collected from a separate plot. Relatively young but fully expanded leaves presenting to the sun and leaves in shade were gathered from each plot. More than one leaf was collected from each tree. Wood samples were collected by an increment borer. To calculate leaf area, a scanner and ImageJ software were used (Glozer, 2008). SLA was measured as the leaf area of a fresh leaf divided by oven-dry mass (m2/kg). After collecting green leaves, fresh weight was immediately measured. The leaves were then oven-dried for 72 hours at 65–70°C temperature in the laboratory. Oven-dry weight was measured by a digital weighing scale. LDMC was calculated by dividing the oven-dry weight (mg) of a leaf by its fresh weight (g), expressed in mg/g.
Wood density was measured from the dry weight of wood divided by its fresh volume, and samples were oven-dried for 72 hours at 105°C temperature. Afterward, dry weight was measured in milligrams (mg) with a digital weighing scale. The following formula was used to measure the volume of the wood sample: V = πr2h, where V = volume, r = Radius of the fresh wood sample, and h = length of the fresh wood sample. Similar individuals, i.e., fully grown healthy adult trees that had their foliage exposed to full sunlight were sampled for leaf traits and plant height. For the bark thickness of trees, five random measurements of individual trees are made with a thickness gauge. At least 10 mature and live seeds were collected from individuals of a given species (Pérez-Harguindeguy et al., 2013).
The disturbance index (DI) was calculated according to Saimun et al. (2021):
Where DI is the disturbance index, CS is the percentage of cut stems calculated as the number of cut stems in a plot divided by the total stem of that plot, COT is the canopy openness of the tree, and COSH is the canopy openness of shrub and herb species of the plot in percentage.
This DI measures large-scale biomass that integrates the effects of all types of disturbance through recent history, like fuelwood collection, illicit felling, wind throw, logging, or fire. This is important because disturbance is expected to affect forest plots at relatively longer time scales.
To characterize trait dissemination in a community, both the mean and variance of trait values weighted by species abundances are examined. The community-weighted mean (CWM) for each trait t was calculated as the mean of species trait values, ti, of the S species in each plot, with each species, i, weighted by its relative abundance, pi (Violle et al., 2007):
Community-weighted mean values were computed in R-3.5.1 software by using the FD package.
Community-weighted mean trait usually describes the overall functional trait value of the total community by adjusting individual species trait values with the abundance of respective species (Diaz et al., 2007). In the present study, to measure community-weighted trait value, we calculated SLA, LDMC, plant height, WD, seed mass, and bark thickness for all individuals within the sample plots using the “FD” package (Ziout et al., 2017). The empirical Bayesian Kriging method was used to make spatial predictions about the community-weighted mean trait distribution in Ratargul Swamp Forest. To perform kriging spatial analysis, we used ArcGIS software (version 10.4.1).
All statistical analysis was performed in the R-3.6.1 environment (R Core Team, 2019; Pinheiro et al., 2020). Analysis of variance (ANOVA) was used in combination with linear models to test the effects of anthropogenic disturbance on the functional trait diversity under packages “nlme”. The results of the comparison were presented by AB trend line using the “ggplot2” package. Principal component analysis was done by package “factoextra” in R. Pearson correlation was measured in the “data analysis” plugin in Microsoft Excel (version 365).
We found a total of 511 individual trees belonging to five species under five genera and five families in our study plots in RSF. In RSF, Koroch (Pongamia pinnata) was the dominant tree species with 302 individuals. Among other tree species, 140 was Hijal (Barringtonia acutangular), 36 was Puti jaam (Syzygium formosum), 11 was Barun (Crateva magna), and 3 was Jarul (Lagerstroemia speciosa).
The distribution, intensity of disturbance, and plant functional traits in RSF are shown in Figure 2. The intensity of disturbance was higher in the northern and southern sides compared to the eastern and western parts of RSF (Figure 2A). Human disturbance is lower in the northern and southern parts of RSF. Among the three resource-acquisitive traits, the intensity of CWM-height and CWM-SLA showed similar patterns in the eastern and western parts of the study area, with the highest values. The northern and southern areas had the lowermost values (Figures 2B, C). The eastern region followed by the extreme western area represents comparatively vigorous species present in RSF. Moreover, our study found that Koroch and Hijal trees required less time to regrow due to greater abundance in the area. The intensity of CWM-SeedMass, another acquisitive trait, showed higher values in the northern and southern areas compared to the eastern and western parts of the area (Figure 2D). The corner of northern and southern areas represents comparatively higher values of CWM-SeedMass because of the high presence of Barun, Puti jam, and the negligible presence of Koroch. These species are highly stress tolerant and can survive under a high-disturbance regime.
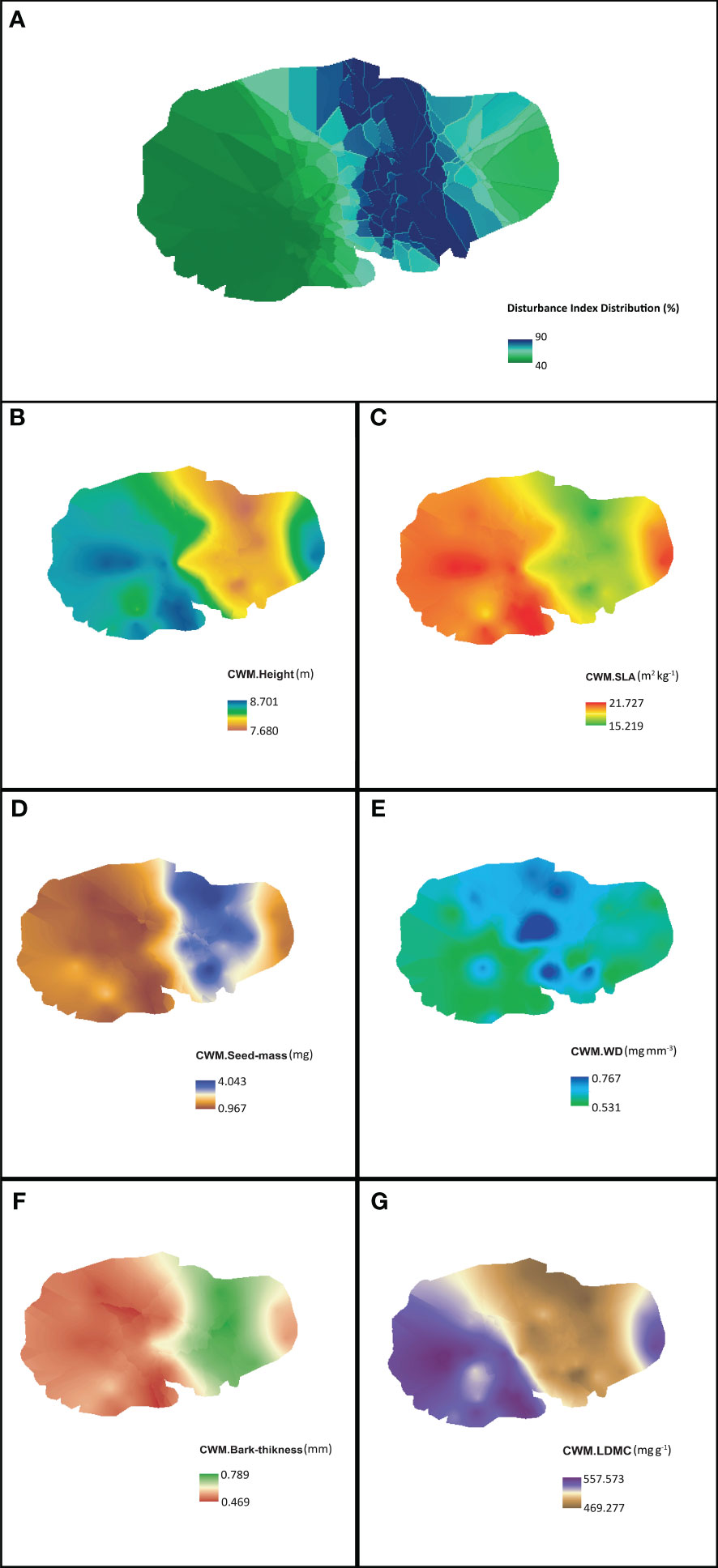
Figure 2 Spatial allocation of (A) disturbance index (%) and functional trait diversity in the study sites of north-east Bangladesh. Resource-acquisitive traits are (B–D), and resource-conservative traits are (E–G).
In terms of the resource-conservative traits, CWM-bark-thickness and CWM-WD indicated higher values toward the north–southern part and lower values toward the eastern and western areas of RSF (Figures 2E, F), while CWM-LDMC showed the opposite pattern with the eastern and western parts having the lowest values and north–southern part having the highest values (Figure 2G). The corner of the north–southern part represents comparatively higher values of CWM-bark-thickness and CWM-WD due to a higher density of Barun and Puti jaam. Human disturbance is also higher there than in the eastern and western parts. Fishing, the movement of tourists, and the presence of a tourist watch tower is the main reason for the disturbance. Again, higher wood density and bark thickness values contribute to enhanced plant survival. CWM-LDMC was comparatively higher in the eastern and western parts compared to the north-southern areas mainly due to a greater presence of Koroch and Hijol trees.
Our results indicated that anthropogenic disturbance is associated with functional traits at the plot level (Figures 3A, B). Evergreen species traits, PCA 1 (Dim1) and PCA 2 (Dim2), demonstrated 63.9% and 17.9% total variation of the study findings (Figure 3A). This indicated that disturbance accounted for 81.8% variation in functional traits within the studied plots. On the other hand, PCA 1 (Dim1) and PCA 2 (Dim2) demonstrated 67.2% and 17.1% of total variation in functional traits (Figure 3B). This indicated that together, disturbance accounted for 84.3% variation in functional traits in the studied plots. In evergreen species (Figure 3A), CWM of bark-thickness showed the weakest association with disturbance whereas CWM of wood density showed the closest association with disturbance; similar results were also found in the case of deciduous species (Figure 3).
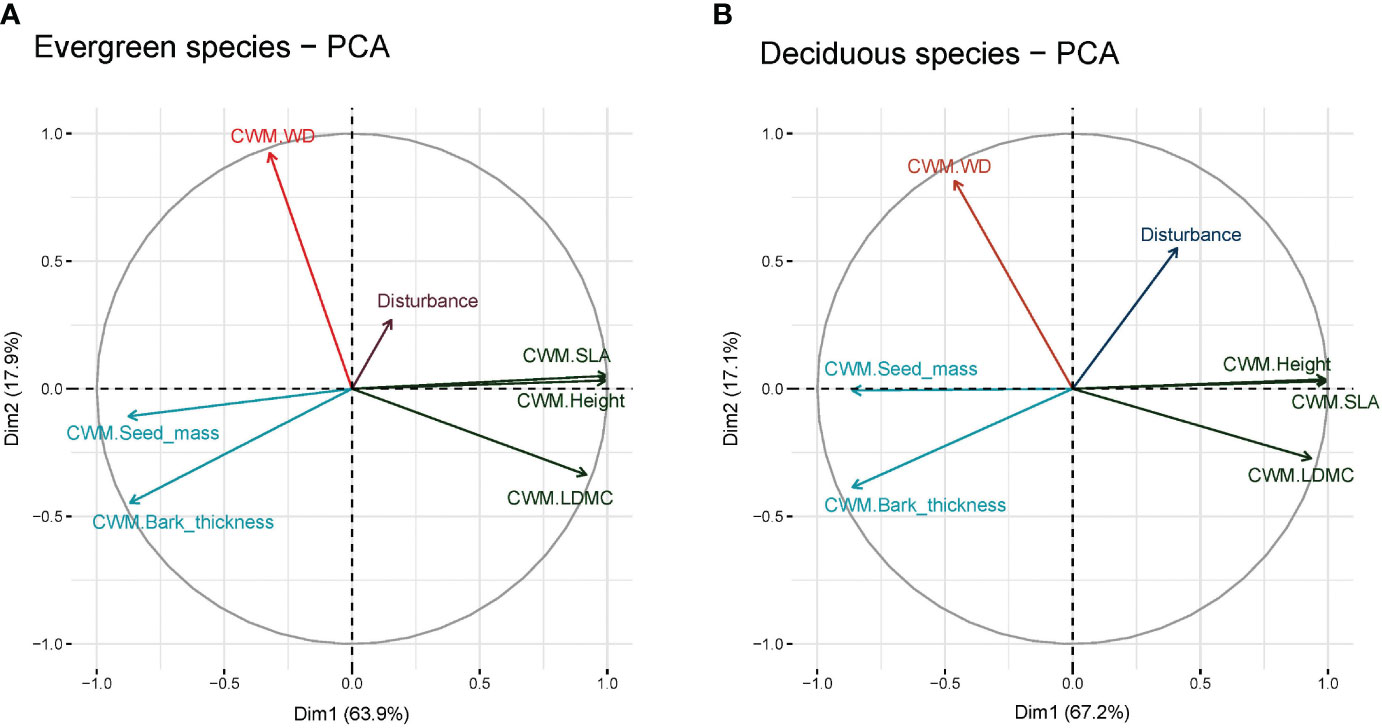
Figure 3 Principal components analysis (PCA) of (A) evergreen species and (B) deciduous species. Here, CWM, Community-weighted mean; SLA, Specific leaf area; WD, Wood density; and LDMC, Leaf dry matter content.
The anthropogenic disturbance had a significant relationship with deciduous species whereas it showed no significant relationship with evergreen species (Figure 4; Table 1). In terms of deciduous species, disturbance had significant positive effects (p<0.05) on CWM-Height (m) and CWM-SLA (kg m−2) (Figures 4A, B). It was also positively correlated with disturbance and CWM-height (m) and disturbance and CWM-SLA (kg m−2) with a correlation coefficient of r = 0.34 and r = 0.35, respectively (Table 1). Disturbance had significant negative effects (p< 0.05) on CWM-bark thickness (mm) and CWM-seed mass (mg) (Figures 4E, F). It was negatively correlated with CWM-bark thickness (mm) and slightly correlated with CWM-seed mass (mg) with correlation coefficients of r = −0.36 and r = −0.33, respectively (Table 1). On the other hand, disturbance had no significant effects on CWM-LDMC (mg g−1) and CWM-WD (mg mm−3) (Figures 4C, D). Disturbance showed no correlation with CWM-LDMC (mg g−1) (r = 0.23, p = 0.15) and CWM-WD (mg mm−3) (r = 0.0064, p = 0.96) (Table 1).
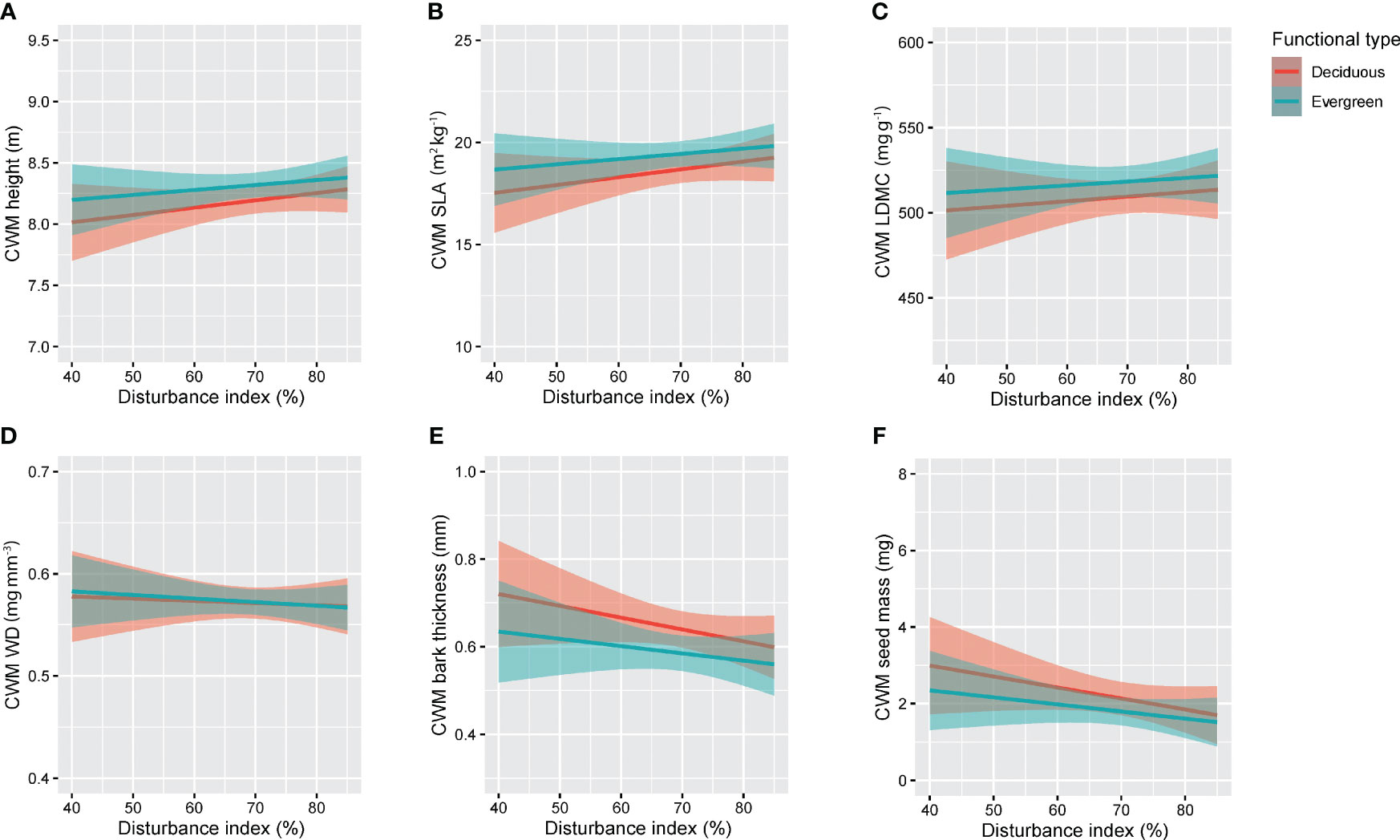
Figure 4 The relationship between anthropogenic disturbance and functional type of plant species in our study in RSF in northeast Bangladesh: (A) CWM height and Disturbance index, (B) CWM SLA and Disturbance index, (C) CWM LDMC and Disturbance index, (D) CWM WD and Disturbance index, (E) CWM bark thickness and Disturbance index, and (F) CWM seed mass and Disturbance index.
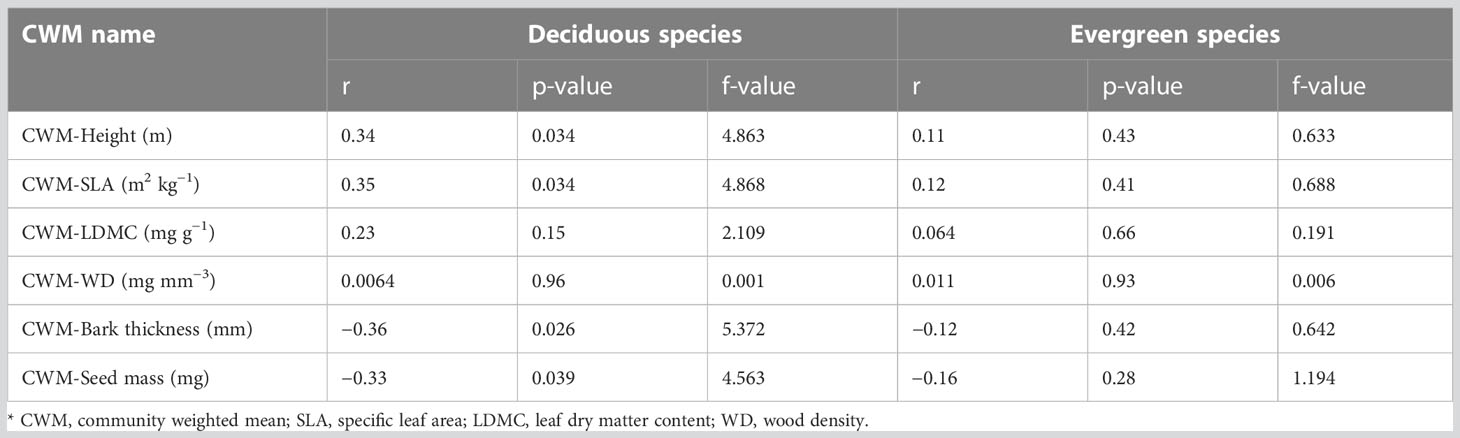
Table 1 ANOVA table of disturbance associated with functional traits under two functional types in RSF in northeast Bangladesh.
Surprisingly, in our study, disturbance showed no significant effect on evergreen tree species: CWM-Height (m) (r = 0.11, p = 0.43), CWM-SLA (kg m−2) (r = 0.12, p = 0.41), CWM-LDMC (mg g−1) (r = 0.064, p = 0.66), CWM-WD (mg mm−3) (r = 0.011, p = 0.93), CWM-bark thickness (mm) (r = −0.12, p = 0.47), and CWM-seed mass (mg) (r = −0.16, p = 0.28) (Figure 4; Table 1).
The present study showed that the community-weighted mean of SLA and tree height had higher trait values in the eastern and western areas compared to the northern and southern parts of RSF. The eastern and western parts showed more resilience (and better ecosystem health) and required less time to grow during various disturbance events sometimes also leading to increased nutrient availability. Cornelissen et al. (2003) also reported similar results wherein areas under less disturbance contributed more nutrients and were comparatively more robust, whereas Moles et al. (2014) reported a minimum community-weighted mean of SLA and height of trees that survived under extreme disturbance events. The community-weighted mean of LDMC and WD was also higher in the southern and northern parts compared to the eastern and western areas. High values of the community-weighted mean of LDC and WD were directly related to poor habitat constitution and highly disturbed areas, respectively. Cruz et al. (2010) reported similar results where a highly disturbed area contributed to poor nutrients and was comparatively less healthy. Poorter et al. (2008) also reported that higher values of wood density and leaf dry matter content contributed to an enhanced plant survival rate. The community-weighted mean of bark thickness and seed mass was found to be higher in the northern and southern parts compared to the eastern and western areas of RSF. Bark thickness which is directly related to the fire regime, fungal attack, and seed size (mass) is an important indicator of plant fitness (Pausas et al., 2014). However, the IAWA Journal (2007) reported that the dead outer bark protects the tree from fires, pathogens, and herbivores; reduces water loss; and provides structural support. Seemi and Shaukat (2010) showed that seed mass is directly related to plant fitness. Eastern and western parts of RSF were more susceptible to fire breakage and fungal and pathogen attacks (Wright et al., 2004).
In our study, specific leaf area and tree height showed a significantly positive correlation with anthropogenic disturbance which is in accordance with the study by Herben et al. (2018) and Ribeiro et al. (2019). Anthropogenic disturbance affecting the nutrient concentration, photosynthesis, and concentration rates of a plant community explains this relationship pattern between disturbance, tree height, and leaf area (Finegan et al., 2015). On the other hand, we found that bark thickness is negatively correlated with anthropogenic disturbance, which is similar to the findings of Graves et al. (2014). We also found seed mass negatively correlated with disturbance, which is similar to the findings of Castro et al. (2008). Grubb et al. (2013), on the other hand, found a positive relationship between plant functional traits and disturbance. Seed mass takes some time to adapt to anthropogenic disturbances in a forest area. This may explain the negative relationship of seed mass with disturbance in our study which may turn positive in due time or with tree maturity.
Resource-conservative traits such as leaf dry matter content and wood density conserve resources to survive in a stressed environment (King et al., 2006). Contrary to Ribeiro et al. (2019), leaf dry matter content and wood density were not affected by disturbance in the present study.
Our study found that evergreen species were not significantly affected by anthropogenic disturbance, whereas deciduous species were significantly affected. Similarly, Bonfil et al. (2004) found that evergreen species were more resistant to disturbance compared to deciduous species. With the increase in disturbance intensity, the CWM changed from conservative traits to more acquisitive traits (Carreño-Rocabado et al., 2012). The present study also found that disturbance had a significant positive effect on deciduous species’ acquisitive traits (SLA and tree height and a negative significant effect on acquisitive seed mass). We also found that disturbance had a negative significant effect on the conservative trait of bark thickness. Sfair et al. (2018) and Graves et al. (2014) reported similar results, where disturbance had a significant effect on acquisitive traits versus conservative traits. It was also expected that a higher disturbance would lead to a decrease in the CWM of other acquisitive traits but instead, there was a significant increase. Conservative traits survived under stressful conditions compared to acquisitive traits (King et al., 2006). The community-weighted mean of SLA and height increased with disturbance intensity, but bark thickness and seed mass dropped (Table 1; Figure 4), implying an increase in primary productivity along with carbon and other nutrient cycling in the short term. Kaul et al. (2010) found that in the early stages of forest regeneration, an increase in fast-growing species was accompanied by an increase in primary productivity. Species with low community-weighted mean of SLA and high WD, on the other hand, were projected to influence primary productivity over longer periods because they increased their abundance at the expense of fast-growing species.
Functional trait-based approaches are imperative for improving our understanding of plant functions to manage forest ecosystems efficiently. Study on plant functional trait variability which changes across functional types within plant communities is also crucial to comprehend ecosystem processes such as nutrient cycling and plant species’ ability to adapt to the impacts of human-induced climate change. The present study addresses the anthropogenic disturbance effects on functional traits. The findings of our study will improve our understanding of ecosystem functioning and aid in the implementation of site-specific conservation strategies in Ratargul Swamp Forest and elsewhere. Furthermore, our study illustrated the importance of plant functional traits and alterations in functional traits that might be responsible for changes in the ecosystem process within Ratargul Swamp Forest. Future studies should consider other environmental parameters, such as soil nutrient availability, soil pH, water availability, and light intensity which may provide a more comprehensive and meaningful understanding of ecosystem processes in the area with broader implications for other tropical regions with similar socio-ecological context and ecosystems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AK, MGK, SM, and MA-K: conceptualization, experimental design, and methodology. AK, MRK, and M: data collection. AK, MRK, MGK, KS, FS, SM, and MA-K: data processing and writing. MA-K and MGK: funding acquisition. All authors contributed to the article and approved the submitted version.
Field data collection and laboratory analysis of this project were funded by Oxfam (UK) in Bangladesh.
We thank the Department of Forestry and Environmental Science, Shahjalal University of Science and Technology, Bangladesh, for providing us with the laboratory space and other facilities for trait sample processing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alamgir M., Mukul S. A., Turton S. M. (2015). Modelling spatial distribution of critically endangered Asian elephant and Hoolock gibbon in Bangladesh forest ecosystems under a changing climate. Appl Geogr. 60, 10–19. doi: 10.1016/j.apgeog.2015.03.001
Anderegg W. R. L. (2015). Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 205 (3), 1008–1014. doi: 10.1111/nph.12907
Atkin O. K., Bloomfield K. J., Reich P. B., Tjoelker M. G., Asner G. P., Bonal D., et al. (2015). Global variability in leaf respiration in relation to climate, plant functional types and leaf traits. New Phytol. 206, 614–636. doi: 10.1111/nph.13253
Berendse F., Scheffer M. (2009). The angiosperm radiation revisited, an ecological explanation for Darwin’s “abominable mystery”. Ecol. Lett. 12 (9), 865–872. doi: 10.1111/j.1461-0248.2009.01342.x
Bernard-Verdier M., Navas M. L., Vellend M., Violle C., Fayolle A., Garnier E. (2012). Community assembly along a soil depth gradient: Contrasting patterns of plant trait convergence and divergence in a Mediterranean rangeland. J. Ecol. 100 (6), 1422–1433. doi: 10.1111/1365-2745.12003
Blouin D., Pellerin S., Poulin M. (2019). An increase in non-native species richness leads to biotic homogenization in vacant lots of a highly urbanized landscape. Urban Ecosyst. 22 (5), 879–892. doi: 10.1007/s11252-019-00863-9
Bonfil C., Cortés P., Espelta J. M., Retana J. (2004). The role of disturbance in the co-existence of the evergreen Quercus ilex and the deciduous Quercus cirripedes. J. Veg. Sci. 15 (3), 423–430. doi: 10.1111/j.1654-1103.2004.tb02280.x
Brodribb T. J., Pittermann J., Coomes D. A. (2012). Elegance versus Speed: Examining the Competition between Conifer and Angiosperm Trees. Int. J. Plant Sci. 173 (6), 673–694. doi: 10.1086/666005
Bruelheide H., Dengler J., Purschke O., Lenoir J., Jiménez-Alfaro B., Hennekens S. M., et al. (2018). Global trait–environment relationships of plant communities. Nat. Ecol. Evol. 2, 1906–1917. doi: 10.1038/s41559-018-0699-8
Cao Y., Natuhara Y. (2020). Effect of anthropogenic disturbance on floristic homogenization in the floodplain landscape: insights from the taxonomic and functional perspectives. Forests 11 (10), 1036. doi: 10.3390/f11101036
Carreño-Rocabado G., Peña-Claros M., Bongers F., Alarcón A., Licona J.-C., Poorter L. (2012). Effects of disturbance intensity on species and functional diversity in a tropical forest. J. Ecol. 100 (6), 1453–1463.
Castro J., Reich P. B., Sanchez-MIranda A., Guerrero J. D. (2008). Evidence that the negative relationship between seed mass and relative growth rate is not physiological but linked to species identity: a within-family analysis of Scots pine. Tree Physiol. 28 (7), 1077–1082. doi: 10.1093/treephys/28.7.1077
Choudhury J. K., Biswas S. R., Islam S. M., Rahman O., Uddin S. N. (2004). Biodiversity of Ratargul Swamp Forest, Sylhet (Dhaka, Bangladesh: IUCN- The World Conservation Union).
Cole L. E. S., Bhagwat S. A., Willis K. J. (2015). Long-term disturbance dynamics and resilience of tropical peat swamp forests. J. Ecol. 103 (1), 16–30. doi: 10.1111/1365-2745.12329
Cornelissen J., Lavorel S., Garnier E. B., Diaz S., Buchmann N., Gurvich D., et al. (2003). Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380. doi: 10.1071/BT02124
Cruz P., Quadros F. L. F., De, Theau J. P., Frizzo A., Jouany C., Duru M., et al. (2010). Leaf traits as functional descriptors of the intensity of continuous grazing in native grasslands in the south of Brazil. Rangeland Ecol. Manage. 63 (3), 350–358. doi: 10.2111/08-016.1
Diaz S., Hodgson J., Thompson K., Cabido M., Cornelissen J., Jalili A., et al. (2004). The plant traits that drive ecosystems: Evidence from three continents. J. Veg. Sci. 15, 295–304. doi: 10.1111/j.1654-1103.2004.tb02266
Diaz S., Lavorel S., de Bello F., Quétier F., Grigulis K., Robson T. M. (2007). Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. U States America. doi: 10.1073/pnas.0704716104
Finegan B., Peña-Claros M., de Oliveira A., Ascarrunz N., Bret-Harte M. S., Carreño-Rocabado G., et al. (2015). Does functional trait diversity predict above-ground biomass and productivity of tropical forests. Testing three alternative hypotheses J. Ecol. 103 (1), 191–201.
Fischer J., Lindenmayer D. B. (2007). Landscape modification and habitat fragmentation: a synthesis. Global Ecol. Biogeogr. 16 (3), 265–280. doi: 10.1111/j.1466-8238.2007.00287.x
Fyllas N. M., Michelaki C., Galanidis A., Evangelou E., Zaragoza-Castells J., Dimitrakopoulos P. G., et al. (2020). Functional trait variation among and within species and plant functional types in mountainous mediterranean forests. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00212
Fyllas N. M., Quesada C. A., Lloyd J. (2012). Deriving Plant Functional Types for Amazonian forests for use in vegetation dynamics models. Perspect. Plant Ecol. Evol. Syst. 14 (2), 97–110. doi: 10.1016/j.ppees.2011.11.001
Garnier E., Lavorel S., Poorter H., Jaureguiberry P., Cornwell W. K., Craine J. M., et al (2013). New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234 doi: 10.1071/BT12225_CO
Gillison A. N., Bignell D. E., Brewer K. R. W., Fernandes E. C. M., Jones D. T., Sheil D., et al. (2013). Plant functional types and traits as biodiversity indicators for tropical forests: two biogeographically separated case studies including birds, mammals, and termites. Biodivers. Conserv. 22 (9), 1909–1930. doi: 10.1007/s10531-013-0517-1
Gleason S. M., Westoby M., Jansen S., Choat B., Hacke U. G., Pratt R. B., et al. (2016). Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world's woody plant species. New Phytol. 209, 123–136. doi: 10.1111/nph.13646
Glozer K. (2008) Protocol for Leaf Image Analysis – Surface Area. Available at: https://ucanr.edu/sites/fruittree/files/49325.pdf (Accessed November 14, 2022).
Goodness J., Andersson E., Anderson P. M. L., Elmqvist T. (2016). Exploring the links between functional traits and cultural ecosystem services to enhance urban ecosystem management. Ecol. Indic. 70, 597–605. doi: 10.1016/j.ecolind.2016.02.031
Graves S. J., Rifai S. W., Putz F. E. (2014). Outer bark thickness decreases more with height on stems of fire-resistant than fire-sensitive Floridian oaks (Quercus spp.; Fagaceae). Am. J. Bot. 101 (12), 2183–2188. doi: 10.3732/ajb.1400412
Greenwood S., Ruiz-Benito P., Martínez-Vilalta J., Lloret F., Kitzberger T., Allen C. D., et al. (2017). Tree mortality across biomes is promoted by drought intensity, lower wood density, and higher specific leaf area. Ecol. Lett. 20 (4), 539–553. doi: 10.1111/ele.12748
Gross N., Suding K., Lavorel S. (2007). Leaf dry matter content and lateral spread predict response to land use change for six subalpine grassland species. J. Veg. Sci. 18, 289-300. doi: 10.1111/j.1654-1103.2007.tb02540.x
Grubb P. J., Bellingham P. J., Kohyama T. S., Piper F. I., Valido A. (2013). Disturbance regimes, gap-demanding trees, and seed mass related to tree height in warm temperate rain forests worldwide. Biol. Rev. 88 (3), 701–744. doi: 10.1111/brv.12029
Hanisch M., Schweiger O., Cord A. F., Volk M., Knapp S. (2020). Plant functional traits shape multiple ecosystem services, their trade-offs, and synergies in grasslands. J. Appl. Ecol. 57 (8), 1535–1550. doi: 10.1111/1365-2664.13644
Herben T., Klimešová J., Chytrý M. (2018). Effects of disturbance frequency and severity on plant traits: An assessment across a temperate flora. Funct. Ecol. 32 (3), 799–808. doi: 10.1111/1365-2435.13011
IAWA Journal. (2007). Esau's Plant Anatomy – Meristems, Cells, and Tissues of the Plant Body – Their Structure, Function, and Development. Third Edition Vol. 13. Ed. Evert R. (Hoboken, New Jersey, USA: Wiley Interscience, John Wiley & Sons), 601 pp.
Karim M. R., Mukul S. A., Zahira R. B., Saimun M. S. R., Arfin-Khan M. A. S. (2023). The role of protected areas co-management in enhancing resistance and resilience of deciduous forest ecosystem to extreme climatic events in Bangladesh. J. Environ. Manage. 326, 116800. doi: 10.1016/j.jenvman.2022.116800
Karim M. R., Sultana F., Saimun M. S. R., Mukul S. A., Arfin-Khan M. A. S. (2020). Plant diversity and local rainfall regime mediate soil ecosystem functions in tropical forests of north-east Bangladesh. Environ. Adv. 2, 100022. doi: 10.1016/j.envadv.2020.100022
Kattge, DÍAz S., Lavorel S., Prentice I. C., Leadley P., BÖNisch G., et al. (2011). TRY – a global database of plant traits. Global Change Biol. 17, 2905–2935. doi: 10.1111/j.1365-2486.2011.02451.x
Kaul M., Mohren G. M. J., Dadhwal V. K. (2010). Carbon storage and sequestration potential of selected tree species in India. Mitig. Adapt. Strateg. Global Change 15 (5), 489–510. doi: 10.1007/s11027-010-9230-5
King D. A., Davies S. J., Tan S., Noor N. S. M. (2006). The role of wood density and stem support costs in the growth and mortality of tropical trees. J. Ecol. 94 (3), 670–680. doi: 10.1111/j.1365-2745.2006.01112.x
Kowarik I., Säumel I. (2007). Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspect. Plant Ecol. Evol. Syst. 8 (4), 207–237. doi: 10.1016/j.ppees.2007.03.002
Lavorel S., Garnier E. (2002). Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol.. 16 (5), 545–556. doi: 10.1046/j.1365-2435.2002.00664.x
McKinney M. L. (2006). Urbanization is a major cause of biotic homogenization. Biol. Conserv. 127 (3), 247–260. doi: 10.1016/j.biocon.2005.09.005
Moles A. T., Perkins S. E., Laffan S. W., Flores-Moreno H., Awasthy M., Tindall M. L., et al. (2014). Which is a better predictor of plant traits: temperature or precipitation? J. Veg. Sci. 25, 1167–1180. doi: 10.1111/jvs.12190
Moreno-Mateos D., Barbier E. B., Jones P. C., Jones H. P., Aronson J., López-López J. A., et al. (2017). Anthropogenic ecosystem disturbance and the recovery debt. Nat. Commun. 8 (1), 14163. doi: 10.1038/ncomms14163
Mukul S. A., Biswas S. R., Rashid A. Z. M. M. (2018). “Biodiversity in Bangladesh,” in Global Biodiversity (Vol. 1: Selected Countries in Asia). Ed. Pullaiah T. (Canada: Apple Academic Press/CRC Press), 93–107.
Naeem S., Wright J. P. (2003). Disentangling biodiversity effects on ecosystem functioning: Deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 6 (6), 567–579. doi: 10.1046/j.1461-0248.2003.00471.x
Pinheiro J., Bates D., Roy S. D., Sarkar D. (2020). nlme: linear and nonlinear mixed effects models. R package version 3. 1–149. Available at: https://cran.r-project.org/web/packages/nlme/index.html.
Pausas J. G., Bradstock R., Keith D., Keeley J. E., Gcte T., Ecology S., et al. (2014). Plant Functional Traits about Fire in Crown-Fire Ecosystems. Ecology 85 (4), 1085–1100. doi: 10.1890/02-4094
Pérez-Harguindeguy N., Díaz S., Garnier E., Lavorel S., Poorter H., Jaureguiberry P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234. doi: 10.1071/BT12225
Poorter L., van der Sande M., Thompson J., Arets E., Alarcón A., Álvarez-Sánchez F., et al. (2015). Diversity enhances carbon storage in tropical forests. Global Ecol. Biogeogr. 24, 1314-1328. doi: 10.1111/geb.12364
Poorter L., Wright S. J., Paz H., Ackerly D. D., Condit R., Ibarra-Manríquez G., et al. (2008). Are functional traits good predictors of demographic rates? Evidence from five neotropical forests Ecology 89 (7), 1908–1920. doi: 10.1890/07-0207.1
Pyles M. V., Prado-Junior J. A., Magnago L. F. S., de Paula A., Meira-Neto J. A. A. (2018). Loss of biodiversity and shifts in aboveground biomass drivers in tropical rainforests with different disturbance histories. Biodivers. Conserv. 27 (12), 3215–3231. doi: 10.1007/s10531-018-1598-7
R Core Team (2019). R Foundation for Statistical Computing (Vienna: R: A language and environment for statistical computing).
Ribeiro E. M. S., Lohbeck M., Santos B. A., Arroyo-Rodríguez V., Tabarelli M., Leal I. R. (2019). Functional diversity and composition of Caatinga woody flora are negatively impacted by chronic anthropogenic disturbance. J. Ecol. 107 (5), 2291–2302. doi: 10.1111/1365-2745.13177
Roscher C., Schumacher J., Gubsch M., Lipowsky A., Weigelt A., Buchmann N., et al. (2012). Using plant functional traits to explain diversity–productivity relationships. PloS One 7 (5), e36760. doi: 10.1371/journal.pone.0036760
Roy M.-C., Azeria E. T., Locky D., Gibson J. J. (2019). Plant functional traits as indicators of the ecological condition of wetlands in the Grassland and Parkland of Alberta, Canada. Ecol. Indic. 98, 483–491. doi: 10.1016/j.ecolind.2018.11.021
Saimun M. S. R., Karim M. R., Sultana F., Arfin-Khan M. A. S. (2021). Multiple drivers of the tree and soil carbon stock in the tropical forest ecosystems of Bangladesh. Trees Forests People, 5, 100108. doi: 10.1016/j.tfp.2021.100108
Scanes C. G. (2018). “Human activity and habitat loss: destruction, fragmentation, and degradation,” in Animals and Human Society. Eds. Scanes C. G., Toukhsati S. R. (Cambridge, USA:Academic Press), 451–482. doi: 10.1038/nature22010
Scheres B., van der Putten W. H. (2017). The plant perceptron connects environment to development. Nature 543 (7645), 337–345.
Seemi A., Shaukat S. S. (2010). Effect of seed mass variations on the germination and survival of three desert annuals. Pakistan J. Bot. 42 (4), 2813–2825.
Sfair J. C., De Bello F., De Frana T. Q., Baldauf C., Tabarelli M. (2018). Chronic human disturbance affects plant trait distribution in a seasonally dry tropical forest. Environ. Res. Lett. 13 (2), 025005. doi: 10.1088/1748-9326/aa9f5e
Sharmin M., Dey S., Chowdhury S. (2016). Relationship between Diversity and Productivity at Ratargul Fresh Water Swamp Forest in Bangladesh. J. For. Environ. Sci. 32 (3), 291–301. doi: 10.7747/JFES.2016.32.3.291
Sultana F., Karim M. R., Arfin-Khan M. A. S., Mukul S. A. (2023). Rainfall modifies the disturbance effects on regulating ecosystem services in tropical forests of Bangladesh. Forests 14:272. doi: 10.3390/f14020272
Sutton-Grier A. E., Wright J. P., McGill B. M., Richardson C. (2011). Environmental conditions influence the plant functional diversity effect on potential denitrification. PloS One 6 (2), e16584. doi: 10.1371/journal.pone.0016584
Uddin M. B., Steinbauer M. J., Jentsch A., Mukul S. A., Beierkuhnlein C. (2013). Do environmental attributes, disturbances and protection regimes determine the distribution of exotic plant species in Bangladesh forest ecosystems? Forest Ecology and Management 303, 72–80. doi: 10.1016/j.foreco.2013.03.052
Violle C., Navas M. L., Vile D., Kazakou E., Fortunel C., Hummel I., et al. (2007). “Let the concept of trait be functional!,” Oikos. 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Wright I. J., Reich P., Westoby M., Ackerly D. D., Baruch Z., Bongers F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/nature02403
Wullschleger S. D., Epstein H. E., Box E. O., Euskirchen E. S., Goswami S., Iversen C. M., et al. (2014). Plant functional types in Earth system models: past experiences and future directions for application of dynamic vegetation models in high-latitude ecosystems. Ann. Bot. 114 (1), 1–16. doi: 10.1093/aob/mcu077
Yinan H., Chen G., Cobb R. C., Zhao K., Meentemeyer R. K. (2021). Forest landscape patterns shaped by interactions between wildfire and sudden oak death disease. For. Ecol. Manage. 486, 118987. doi: 10.1016/j.foreco.2021.118987
Ziout A., Salah B., Azab A., Alkahtani M., Biswas N. (2017). Environmental performance of air conditioner remanufacturing. Paper presented at 2nd International Conference on Energy and Indoor Environment for Hot Climates – ASHRAE held in Doha, Qatar, 85–91.
Keywords: tree functional trait, wood density, specific leaf area, disturbance, community weighted mean
Citation: Khan A, Karim MR, Mohammed, Kibria MG, Sinha K, Sultana F, Mukul SA and Arfin-Khan MAS (2023) Anthropogenic disturbance modifies tree functional traits in the only remnant swamp forest of Bangladesh. Front. Ecol. Evol. 11:1062764. doi: 10.3389/fevo.2023.1062764
Received: 06 October 2022; Accepted: 24 July 2023;
Published: 25 August 2023.
Edited by:
Lourdes Morillas, University of Lisbon, PortugalReviewed by:
Tanmoy Dey, Bangladesh Forest Research Institute, BangladeshCopyright © 2023 Khan, Karim, Mohammed, Kibria, Sinha, Sultana, Mukul and Arfin-Khan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sharif A. Mukul, bXVrdWxAZWRzLnVpdS5hYy5iZA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.