
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 27 April 2023
Sec. Behavioral and Evolutionary Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1034941
Human-altered landscapes may act as an environmental filter benefiting species or individuals with specific sets of capacities or behaviors. Yet the effects of human activity on culturally transmitted traits in animals are still poorly understood. Combining song recordings and simulated territory intrusions, we investigated whether songs (a cultural trait) and aggressiveness (a personality trait) in small ground finches (Geospiza fuliginosa) differed along a gradient of human activity levels (high-low-high) spanning two habitats with contrasting levels of rainfall (arid lowlands, humid highlands). We found that more common syllable types were more prevalent in arid lowland sites and at sites with high human activity. The number of syllables per song, song duration, song tempo and song rhythmicity did not differ across habitats or levels of human activity. During simulated territorial intrusions, small ground finches living in areas with higher levels of human activity and in the arid lowlands (regardless of human activity) showed the strongest aggressive response compared to those living in areas with lower levels of human activity or in the humid highlands. Thus, prevalence of aggression and syllable commonness correlated with each other across sites. Our results support the idea that resource distribution and human-impacted environments may select jointly for specific behavioral phenotypes such as aggression as well as common cultural traits.
Environments are changing at unprecedented speed due to human activities, which imposes challenges for many species. Animal behavioral responses to such changes may influence evolutionary processes by determining which individuals survive and reproduce under altered conditions (Sih et al., 2011; Chapple et al., 2012), which in turn can have long-term consequences for biodiversity. Such responses may be driven by pressures within the environments or selection for individuals with traits that make them better suited to colonize changing environments (Marzluff and Neatherlin, 2006; Carrete and Tella, 2010; Cote et al., 2010; Hu and Cardoso, 2010; Holtmann et al., 2017). However, the ultimate and proximate mechanisms behind such responses, as well as the role of individual differences in behavior associated with population persistence in rapidly changing environments, remain poorly understood (Maspons et al., 2019).
Human activities and human-altered landscapes are now recognized as ecological filters benefiting certain species or individuals with specific sets of characteristics, capacities, or behaviors (e.g., Silva et al., 2016; Callaghan et al., 2019; Patankar et al., 2021). Compared with their more rural counterparts, individuals living near humans are generally more aggressive toward conspecifics (Coss et al., 2002; Scales et al., 2011; Baxter-Gilbert and Whiting, 2019), more prone to take risks when facing predators (Møller, 2010; Scales et al., 2011; Tryjanowski et al., 2016; Ducatez et al., 2017; Holtmann et al., 2017; Gotanda, 2020), more exploratory (Charmantier et al., 2017), and differ in their signaling (Hu and Cardoso, 2010; Luther and Derryberry, 2012; Kunc and Schmidt, 2021) and dispersal (Partecke and Gwinner, 2007) behaviors. Such behavioral differences can also correlate with greater innovativeness and cognitive performance that may lead to adaptive responses to challenging environments (see Sol et al., 2005; Lowry et al., 2013; Lee and Thornton, 2021).
Risk-taking behaviors such as territorial aggression are predicted to be particularly important to determine the survival or success of an individual when faced with environmental changes, such as human-altered landscapes (see Lapiedra et al., 2017). The higher levels of territorial aggression in urban habitats seen for instance in several songbird species (e.g., Evans et al., 2010; Scales et al., 2011; Foltz et al., 2015; Myers and Hyman, 2016; Hardman and Dalesman, 2018) is likely due to a combination of factors. In particular, anthropogenic noise (e.g., Phillips and Derryberry, 2018; Akçay et al., 2020), higher conspecific density (e.g., Fokidis et al., 2011) or variation in the availability of resources, such as food or nesting spaces (e.g., Fox et al., 1981; Scales et al., 2013) may lead to higher levels of boldness and aggressiveness in human-altered landscapes, which in turn may elevate the level of competition for territories.
Bird song is a culturally learned trait, which has a very important function in territory establishment and maintenance (Searcy and Beecher, 2009; Logue, 2021). Indeed, there is evidence from several species of songbirds that singing locally common song types (i.e., conformity in song types) correlates positively with components of individual fitness such as territory tenure and mating success (Payne, 1982; O'Loghlen and Rothstein, 1995; Beecher et al., 2000; Demko et al., 2016). Such correlations may come about because common songs (i) may better transmit in the local habitat than uncommon songs (Morton, 1975), (ii) be indicators of genotypes better suited to the local habitat and thus be preferred by mates (Baker, 1975; Stewart and MacDougall-Shackleton, 2008), (iii) serve as honest signals of song learning ability (Rothstein and Fleischer, 1987) or (iv) facilitate cooperative behaviors between territory owners and serve as signals of alliances toward third parties (Beecher et al., 2020). Therefore, individuals singing common song types may be favored during territory establishment and maintenance, particularly when competition for territories is higher, as expected in human-altered landscapes. For instance, in a study by Laiolo and Tella (2005), song sharing between neighboring male Dupont’s larks (Chersophilus duponti) increased with anthropogenic habitat fragmentation, suggesting that heightened competition for the limited suitable territories may select for high cultural conformity in song. This study remains one of only a handful of studies investigating differences in discrete cultural elements in human-altered landscapes (but see Deoniziak and Osiejuk, 2019; Brewer and Fudickar, 2022).
The islands of the Galapagos archipelago represent an excellent opportunity to investigate the effects of human-induced environmental changes on cultural and behavioral traits. Human settlement in the Galapagos islands is recent, having occurred over the past 200 years and especially since the 1950s (see Watson et al., 2010), and most islands have small and localized urban centers. The endemic Darwin’s finches are one of the best examples of adaptive radiation, where a single species evolved into 17 known species that exploit different ecological niches (Grant and Grant, 2002, 2008, 2014a; Kleindorfer et al., 2022). Darwin’s finches are opportunistic breeders that strongly depend on rainfall for breeding (Grant and Boag, 1980; Gibbs and Grant, 1987; Hau et al., 2004; Kleindorfer, 2007a; Camacho et al., 2019), and studies have shown differences in diet (De León et al., 2019), microbiota (Knutie et al., 2019), morphology (Hendry et al., 2006; De Leon et al., 2011), breeding success (Harvey et al., 2021) and tolerance toward humans (Gotanda, 2020) between Darwin’s finches living near or away from humans. Specifically, a previous study showed that overall, small ground finches (Geospiza fuliginosa) living near humans were more tolerant toward humans (i.e., took longer to initiate flight in response to a human approaching) than finches in areas less exposed to humans. Interestingly, the study also found that among the populations studied, the finches in the town of Puerto Velasco Ibarra on Floreana Island (one of the sites in the present study) were the least tolerant toward humans (Gotanda, 2020). The author suggested that Puerto Velasco Ibarra (a town of about ~140 permanent residents) may simply be too small to be considered an “urban” site to investigate the impacts of urbanization on the finches’ behavioral adaptations (Gotanda, 2020).
In this study, we investigated whether song syllable type (a culturally learned trait) and aggressiveness (territorial response to simulated conspecific intruders) in small ground finches differed along a gradient of human activity levels (high-low-high) spanning 20 km and two habitats with contrasting levels of rainfall (and thus resources; arid lowlands and humid highlands) on Floreana Island. The only road on the island passes from the lowlands to the highlands, and the areas differ in resource availability (food and availability of nest sites). The majority of lowland human activity occurs in the town, Puerto Velasco Ibarra, where 90% of the houses are found, and near the highland farms, where 90% of the food-growing agricultural land is managed, which is adjacent to Asilo de la Paz, the main tourist site on the island for day-trip visitors. The two main areas rich in food for birds are the town in the lowlands and the agricultural zone (near Asilo de la Paz) in the highlands. The agricultural zone has the most fruit-bearing trees, but some can also be found in town near individual family homes. In addition, inhabitants in town often dry their crops collected from the agricultural zone in open areas, and captive tortoises near Asilo de la Paz produce dung that attracts many insects. Human impacts in the town and Asilo de la Paz, however, differ in scope as the habitat structure of the town is highly modified (with different types of trees, more open spaces, fewer shrubs, additional noise from human activities, traffic, etc.), while the Asilo de la Paz tourist site (where many finches co-occur near the tortoise holding pens) is exposed to humans walking along trails and noise from the adjacent agricultural lands.
We predicted that small ground finches living in areas with higher levels of human activity would have the strongest territorial response if human environments or human-provided food select for more aggressive behavioral phenotypes (in line with Evans et al., 2010; Scales et al., 2011; Foltz et al., 2015; Myers and Hyman, 2016; Hardman and Dalesman, 2018). Additionally, we predicted that birds living in the lowlands, with lower rainfall and more patchy natural resources (food and availability of nest sites), would have the strongest territorial response if natural resource limitation selects for more aggressive behavioral phenotypes (see Foltz et al., 2015). In a previous study, we found that singing common song syllable types was associated with aggressiveness in three species of Darwin’s finches including small ground finches (Colombelli-Négrel et al., unpublished data). We therefore also predicted a greater prevalence of song with the common syllable type in areas with higher levels of human activity and in the lowlands, and song with rare or uncommon syllable types in areas with lower levels of human activity and in the highlands.
We conducted all recordings and simulated territorial intrusions at the start of the breeding season (January–February) in 2020 and 2022 on Floreana Island (Galapagos archipelago). Floreana island has the longest record of human settlement in the Galapagos archipelago and currently has ~140 permanent inhabitants (Lack, 1947a; Sulloway, 1982; Steadman, 1986). The highland is characterized by agricultural areas with Cedrela trees and a humid Scalesia forest (rich in nesting resources as many finches prefer to nest in Scalesia trees) located at the base of the Cerro Pajas volcano (01°17′S, 90°27′W) ~ 300–400 m above sea level and adjacent to the agricultural zone and the main tourist area, Asilo de la Paz (01°18′S, 90°27′W). Rainfall in the highland varies between 600 and 2,300 mm per year (Ben-Yosef et al., 2017; Conservancy, 2022; see also Common et al., 2022). The lowlands are adjacent to the town of Puerto Velasco Ibarra (01°16′S, 90°29′W) ~0–150 m above sea level and the vegetation is dominated by Palo Santo (Bursera graveolens) and Acacia (Parkinsonia aculeata and Scutia spicata; Dvorak et al., 2017). Rainfall in the lowlands varies between 100 and 700 mm per year (Common et al., 2022; Conservancy, 2022). Vegetation surveys across eight 100 m-transects in February 2020 in the highlands and lowlands showed that the two habitats differed in tree density and hence in the number of available nest sites: we found 12 trees in the lowlands (none of them Scalesia) during our transects vs. 240 trees (including 167 Scalesia) in the highlands.
To assess differences in levels of human activity across our study sites, we counted the number of vehicles per hour at each site in February 2022 between 6 am and 10 am on two different days. We counted on average 12.5 vehicles per hour in Puerto Velasco Ibarra (hereafter referred to Lowlands 1), 0.12 vehicles per hour in the arid zone outside the town (hereafter referred to Lowlands 2), 0 vehicles per hour in Cerro Pajas Volcano (hereafter referred to Highlands 1), and 7 vehicles per hour in Asilo de la Paz (hereafter referred to Highlands 2). Each vehicle had 4 to 10 passengers. On the same days, we also counted the number of tourists and locals seen during the day. We counted 60 tourists/locals per day and 95 tourists/locals at night in Lowlands 1, 0 local/tourist per day in Lowlands 2, 0 local/tourist per day in Highlands 1, and 58 tourists/locals per day and 30 locals per night in Highlands 2 (Table 1).
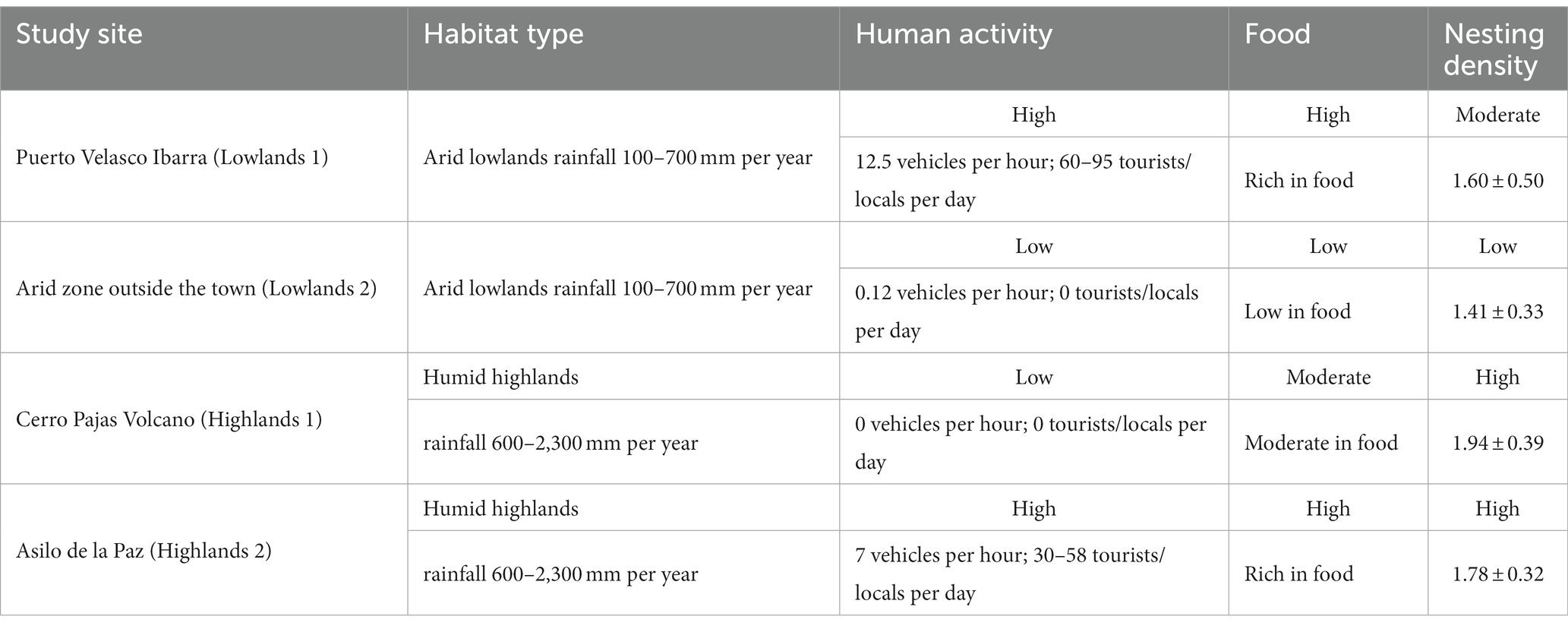
Table 1. Summary of the differences in habitats, levels of human activity, food, and small ground finch nesting densities (average number of active nests within 20 m) across our four study sites.
We conducted all our field work along a gradient of human activity levels (high-low-high) spanning two rainfall-influenced habitats (arid lowlands and humid highlands). Our gradient started in the lowland town of Puerto Velasco Ibarra (lower rainfall, higher level of human activity, rich in food; Lowlands 1), extended from the town edge through the lowland arid zone (lower rainfall, lower level of human activity, low in food and nesting sites; Lowlands 2), along the road to the highland forest trail to Cerro Pajas Volcano (higher rainfall, lower level of human activity, rich in nesting sites; Highlands 1) and finished at the highland tourist destination of Asilo de la Paz (higher rainfall, higher level of human activity, rich in food and nesting sites; Highlands 2; Figure 1; Table 1).
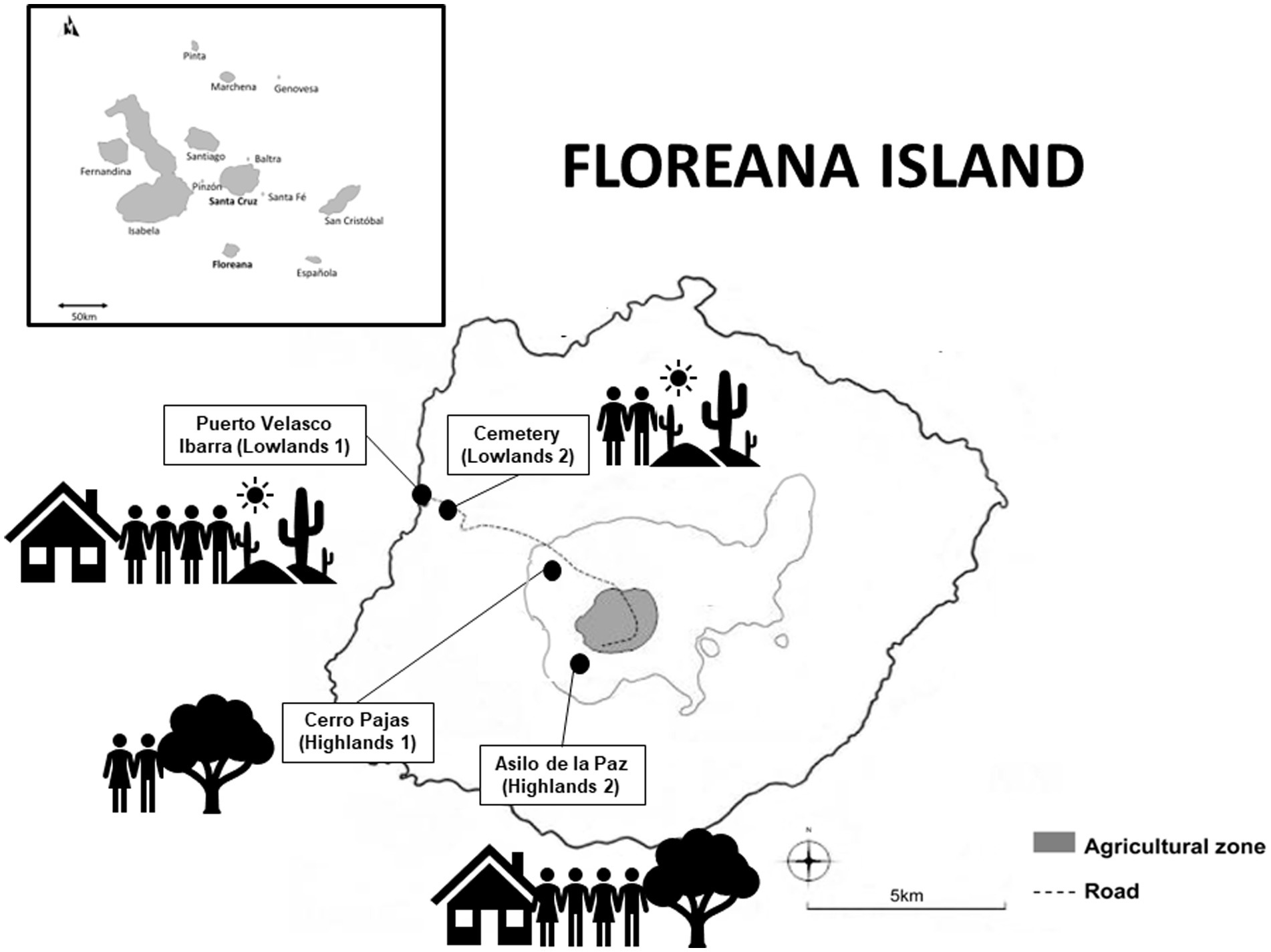
Figure 1. Map illustrating the urban gradient used in this study. The gradient started in the lowland town of Puerto Velasco Ibarra (lower rainfall, higher level of human activity; referred to as Lowlands 1), extended from the town edge through the lowland arid zone (lower rainfall, lower level of human activity; referred to as Lowlands 2), along the road to the highland forest trail to Cerro Pajas Volcano (higher rainfall, lower level of human activity; referred to as Highlands 1), and finished at the highland tourist destination of Asilo de la Paz (higher rainfall, higher level of human activity; referred to as Highlands 2).
We recorded and tested territorial small ground finch males occurring in both the lowlands and highlands (Petren et al., 2005; Grant and Grant, 2008; Grant and Grant, 2014b; Lamichhaney et al., 2015; Kleindorfer et al., 2019). Ground finches typically feed on seeds but are often described as “imperfect generalists” because they also feed on a wide variety of foods, such as invertebrates and plant material (Tebbich et al., 2004; De León et al., 2014; Loo et al., 2019; Kleindorfer et al., 2022). Male age in Darwin’s finches can be estimated by the proportion of black body plumage, which increases with each annual molt: yearling males have no black (black 0) while fully black males (black 5) are at least 5 years old (Grant, 1990; Kleindorfer, 2007b; Langton and Kleindorfer, 2019). At the start of the breeding season, which occurs after high rainfall (Kleindorfer and Dudaniec, 2020), males build a display nest and sing to attract prospective mates (Lack, 1947b; Christensen et al., 2006; Kleindorfer, 2007b). We located all nests by systematic searches in the study area—using cues such as singing males, nest building behavior or pair activity—and recorded the location of each nest using a Garmin GPS 64 (Garmin Ltd., Australia).
In 2020, we recorded 70 singing males (14 males at Lowlands 1, 22 males at Lowlands 2, 20 males at Highlands 1, and 14 males at Highlands 2) at their nest between 0600 and 1,100 (peak of song activity) as wave files using either (1) a Zoom H6 recorder (Zoom North America, United States) and a Sennheiser ME67 directional microphone (Sennheiser electronic GmbH & Co., United States) or (2) a Marantz PMD 661 Solid State recorder (Sound United, LLC, United States) and a Sennheiser ME66/K6 directional microphone (24 kHz sampling rate, 16-bit depth). We visualized all recordings with Raven Pro 1.6 Sound Analysis Software (Cornell Lab of Ornithology Bioacoustics Research Program, Ithaca, New York) using the Hann algorithm (DFT = 512 samples; frequency resolution = 124 Hz; time resolution = 11.6 ms; frame overlap = 50%). We categorized syllable types as previously described in Darwin’s finches (Bowman, 1983; Ratcliffe and Grant, 1985; Gibbs, 1990; Colombelli-Négrel and Kleindorfer, 2021). Each individual small ground finch sings a trilled song that consists of several repetitions of a single syllable type (Bowman, 1979, 1983; Grant, 1984; Millington and Price, 1985; Grant and Grant, 1996; Figure 2). A male only sings one syllable type throughout its life. This syllable is learned early in life and does not change subsequently (Grant and Grant, 1996). We recorded nine different syllable types in our population of small ground finches (Colombelli-Négrel et al., unpublished data using 578 songs from 70 males; Figure 2). Previous analyses showed that the complete repertoire of syllable types was recorded for the species within our sample size (Colombelli-Négrel et al., unpublished data). Syllable types recorded from the individuals in the present study were defined as being “common” (types IV, V, and VI) or “uncommon” (types I, II, III, VII, VIII, IX) in the population based on a previous study in which uncommon syllable types were sung by less than 20% of the recorded males (Colombelli-Négrel et al., unpublished data). From the recordings, we also noted for each male: (1) the average number of syllables per song, (2) the average song duration (s), (3) the average song tempo (sometimes referred to as the “pace” of the song or trill rate in trilled songs; the average number of syllables per second), and (4) the average song rhythmicity, calculated as the average duration of silence between two syllables divided by the average syllable duration (Sasahara et al., 2015).
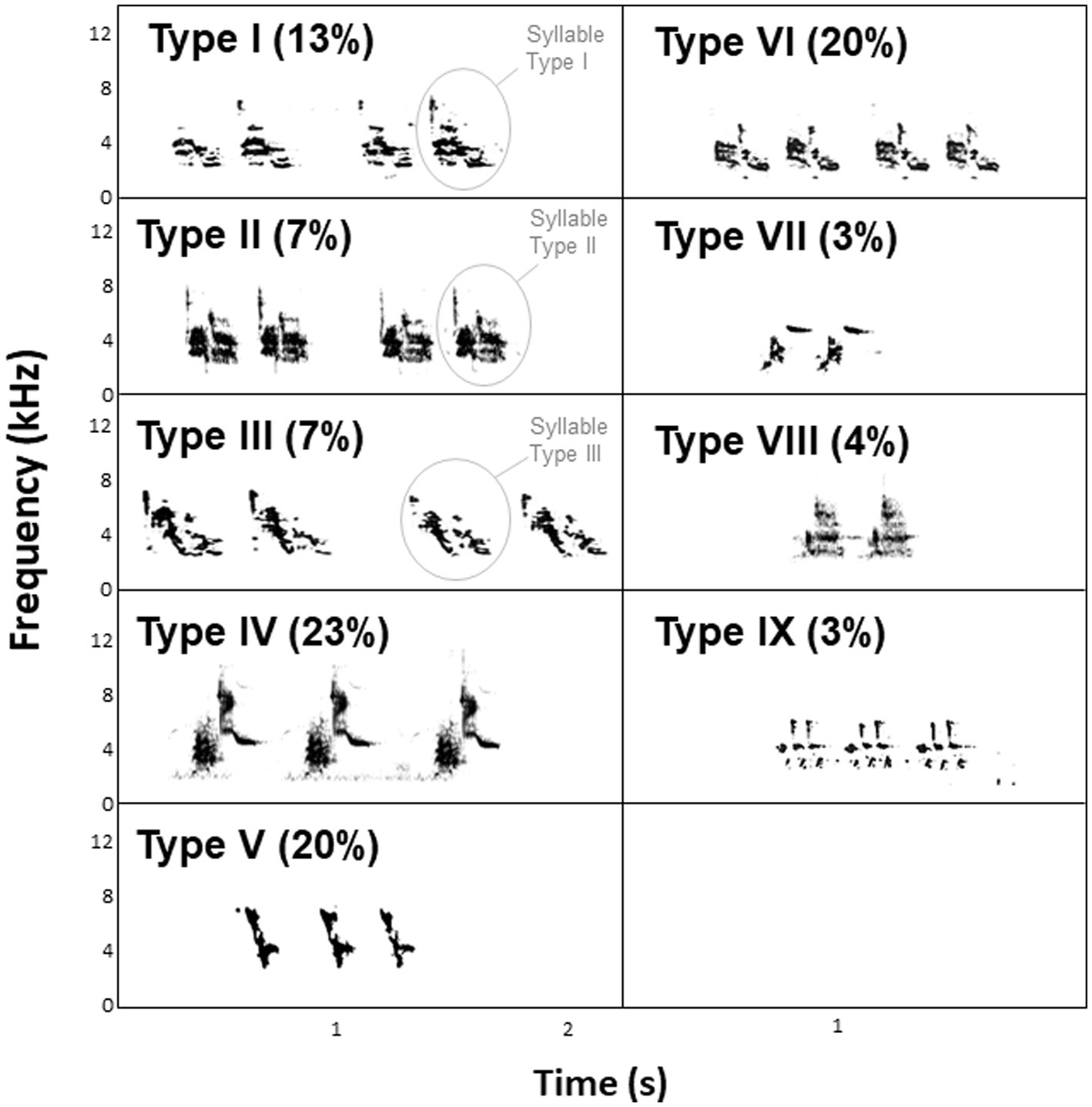
Figure 2. Spectrograms and percentages of the different syllable types recorded in small ground finches on Floreana Island in 2020.
We prepared all song playback tracks for the simulated intrusions using previously recorded conspecific songs with a good signal-to-noise ratio. All songs used for the playback were recorded on Floreana Island in 2020 as described above; all individuals used for the intrusions differed from those used in the song analyses. We used a high pass filter to remove sounds < 1.5 kHz and saved the tracks as uncompressed 16-bit broadcast wave files (.wav). We prepared a total of 18 different playback tracks using songs from 18 different birds. Each playback track was played on average 1.8 ± 0.4 times; range 1–8 times. Each track consisted of 3 min: 1 min of playback (six evenly spaced songs from the same individual) followed by 1 min of silence, and then a repeat of 1 min of songs. We then transferred these playback tracks onto an Apple iPod (Apple Inc., United States). We did not use a control (heterospecific) treatment in this study as previous experiments using Darwin’s finches on Floreana showed very little response to our control treatment (yellow warbler, Setophaga petechia aureola, songs; Colombelli-Négrel and Kleindorfer, 2021), with birds rarely responding within the 3 min of playback (average latency 146 s out of 180 s) and remaining on average 9.32 m from the speaker.
In 2020 and 2022, we tested the response of 82 territorial males (47 in 2020 and 35 in 2022: 14 at Lowlands 1, 16 at Lowlands 2, 22 at Highlands 1, and 30 at Highlands 2) to the simulated intruder (via song playback) of the same species and the same location (i.e., we played Lowlands 1 songs to males tested at Lowlands 1, Lowland 2 songs to males from Lowland 2 and so on) but located at least three territories away. Out of the 82 males, we tested 30 males with an uncommon syllable type (a syllable defined as uncommon in the population as described above) and 52 males with a common syllable type (a syllable defined as common in the population as described above). We did all simulated intrusions via playback of songs between 0600 and 1,100, which corresponds to the peak of song activity. We never started playback until the male was observed within a 20 m radius of its nest prior to the experiment. All birds were tested once. Once we located a singing male and its nest, we placed the playback speaker (Sony XB12 Extra Bass Portable Bluetooth Speaker, Sony Australia Limited or Soundcore Icon Mini Bluetooth Speaker, Anker Technology Ltd., United Kingdom; frequency response 20 Hz–20 kHz) and iPod on the ground 5 m from the nest and started a randomly chosen playback track of a conspecific song (played at ~80 dB at 1 m, measured with a VLIKE VL6708 sound-level meter). One observer posted within the focal male’s territory (~10 m from the speaker) narrated the trial using a Marantz PMD 661 Solid State recorder (Sound United, LLC, United States) and a Sennheiser ME66/K6 directional microphone (Sennheiser electronic GmbH & Co., United States) or a Zoom H6 recorder (Zoom North America, United States) and a Sennheiser ME67 directional microphone. During the 3 min of playback, we recorded: (1) the latency (s; latency of first approach or song within 20 m); (2) the minimum distance (m; minimum distance between the focal bird and the speaker); (3) the time at 1 m (total time (s) spent within 1 m of the speaker during the 3 min of playback); (4) the number of flights over the speaker (crosses); as well as (5) the total number of songs produced by the male during the 3 min of playback.
We used SPSS version 25.0 for Windows (SPSS Inc., Chicago, IL, United States) for all statistical analyses. Data are shown as means ± S.E.
We analyzed difference in the prevalence of syllable types (common, uncommon) across the four study sites using a Chi-squared test. We quantified differences in song characteristics (number of syllables per song, song duration, song tempo and song rhythmicity) in relation to “habitat” (lowlands, highlands), “human activity” (low, high) and the interaction “habitat × human activity” using MANOVAs and Bonferroni post hoc pairwise comparisons.
We used principal component analysis (PCA, correlation matrix) with Varimax rotation to reduce playback responses (minimum distance, latency, time within 1 m, number of flights over the speaker, and number of songs produced during the playback period), which produced two principal components with eigenvalues > 1 (Kaiser-Meyer-Olkin = 0.73; Bartlett’s test: χ2(10) = 91.96, p < 0.0001): PC1 (which we label PCA Physical Behaviors) with an eigenvalue of 2.40 and accounting for 48% of variance and PC2 (which we label PCA Vocal Behaviors, as it primarily depends on number of songs) with an eigenvalue of 1.12 and accounting for 22% of variance (Table 2). High PCA scores indicated a strong response to playback (a shorter latency to respond, a shorter minimum distance, more time spent close the speaker, many flights over the speaker, many songs produced). We analyzed playback response with a generalized linear mixed model (GLMM; PCA Physical Behaviors) and a general linear model (GLM; PCA Vocal Behaviors). Both models had normal distributions (identity link) and included “year” (2020, 2022), “habitat” (lowlands, highlands), “human activity” (low, high), the interaction effect “habitat × human activity,” playback syllable (common vs. uncommon), and “male black” (as an indication of male age; categorized between 0 and 5 as mentioned above) as fixed factors. We included “stimulus ID” as a random effect when analyzing PCA Physical Behaviors (GLMM) but not PCA Vocal Behaviors (GLM) due to the lack of a positive definite covariance matrix of the random effect when “stimulus ID” was added to the model.
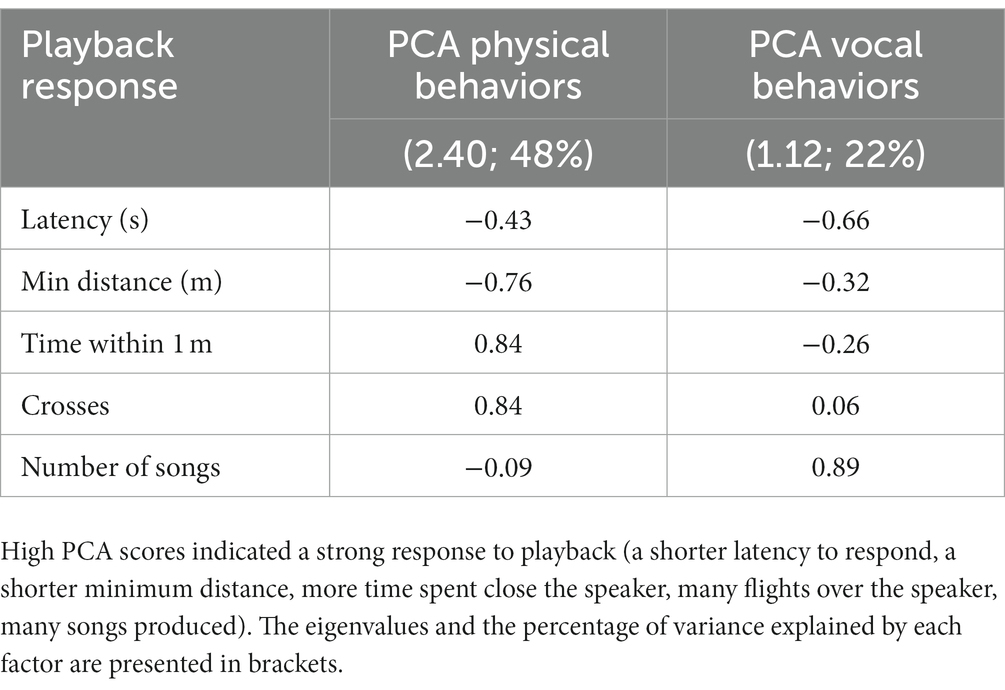
Table 2. Factor loadings from principal component analysis (PCA) of the small ground finches’ responses to playback (n = 82 individuals).
The prevalence of common syllable types differed across study sites [χ2(3) = 7.90, p = 0.048; Figure 3], with common syllable types being more prevalent at Lowlands 1, Lowlands 2, and Highlands 2 compared to Highlands 1 site. The number of syllables per song, song duration, song tempo and song rhythmicity did not differ across habitats or levels of human activity (all p > 0.06).
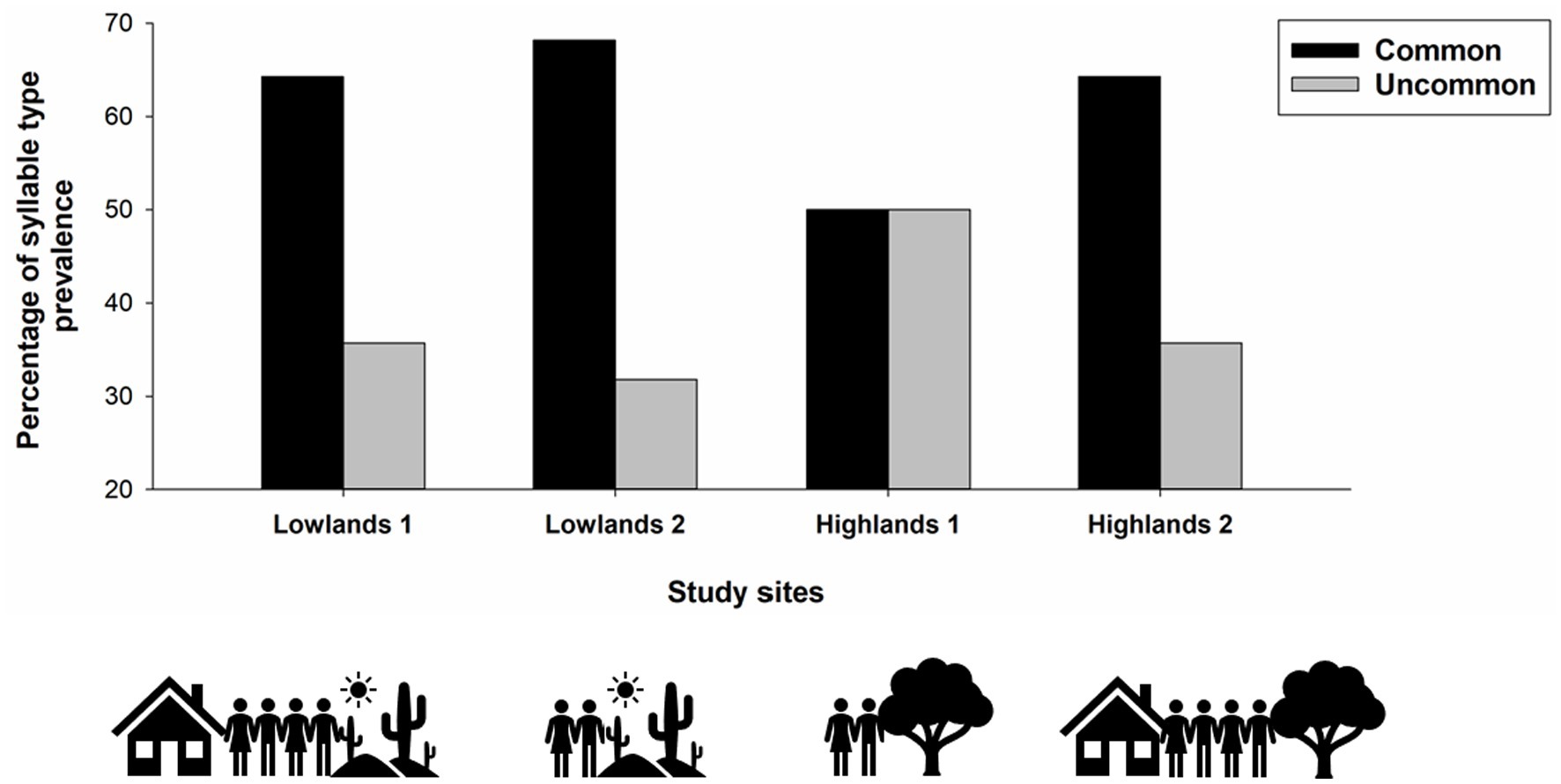
Figure 3. Prevalence of syllable types (common, uncommon) across study sites (Lowlands1, Lowlands 2, Highlands 1, Highlands 2). Small ground finches in our study sang IX different syllable types and syllable type was defined as “common” (types IV, V, and VI) or “uncommon” (types I, II, III, VII, IIX, IX) based on a previous study in which uncommon syllable types were sung by fewer than 20% of the recorded males (Colombelli-Négrel et al., unpublished data).
During our simulated territorial intrusions, 75 males (91%) approached the speaker within less than 10 m. We found significant main effects of habitat, year, and levels of human activity but no interaction effect of habitat × human activity on behavioral response to playback (PCA Physical Behaviors; GLMM: Figure 4; Table 3). Specifically, finches living in the lowlands were more aggressive than finches living in the highlands, irrespective of human activity (Figure 4; Table 3). Within each habitat (lowland and highland), birds were more aggressive in areas with more human activity (Lowlands 1 and Highlands 2) compared to areas with less human activity (Lowlands 2 and Highlands 1; Figure 4; Table 3). Behavioral responses were stronger in 2020 compared to 2022 (Table 3). Behavioral responses did not differ in relation to playback syllable or male black (Table 3). The random effect stimulus ID did not significantly account for any variance within PCA Physical Behaviors (Wald Z = 0.81; p = 0.42).
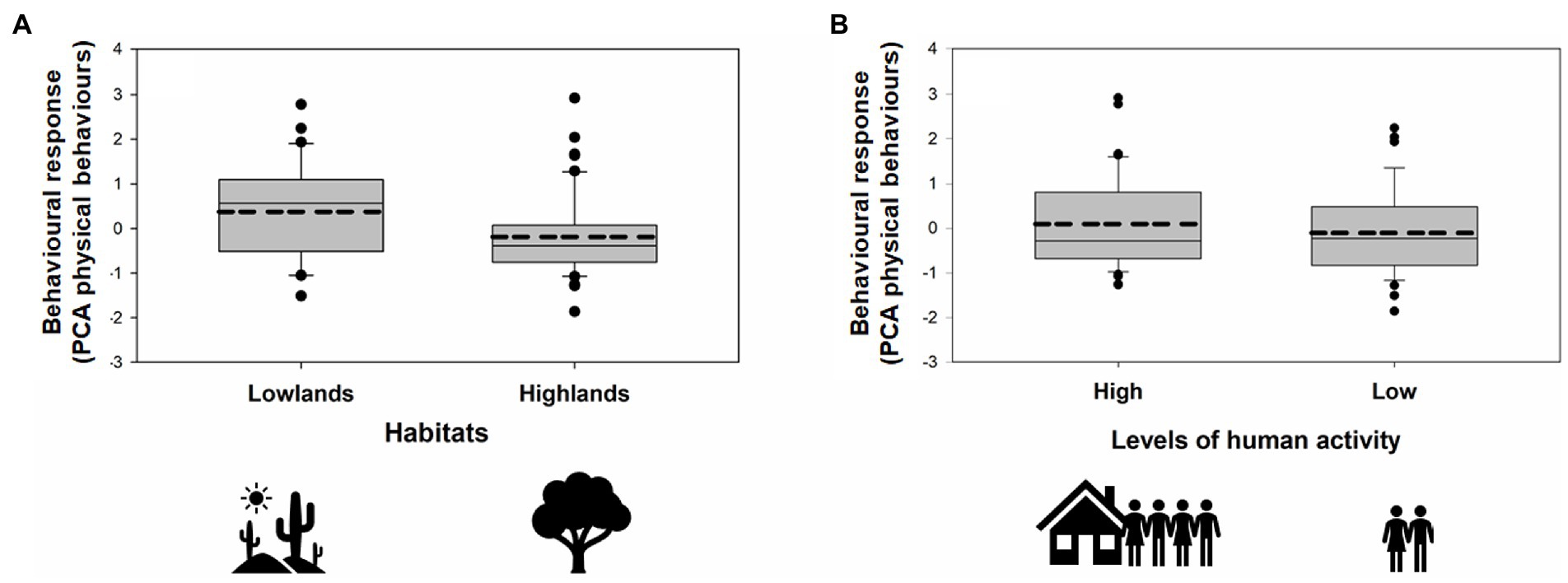
Figure 4. Behavioral playback responses of 82 resident males to the playback of conspecific intruder songs in relation to (A) habitat (lowlands, highlands) and (B) levels of human activity (high, low). The responses are shown as PCA scores (PCA Physical Behaviors) whereby higher scores indicate a stronger response to playback (a shorter latency to respond, a shorter minimum distance, more time spent close the speaker, many flights over the speaker). Horizontal lines within the boxes represent the means (hashed lines) and medians (straight lines). The upper and lower limits of the boxes show the 75th and 25th percentiles, respectively. Black circles indicate outliers.
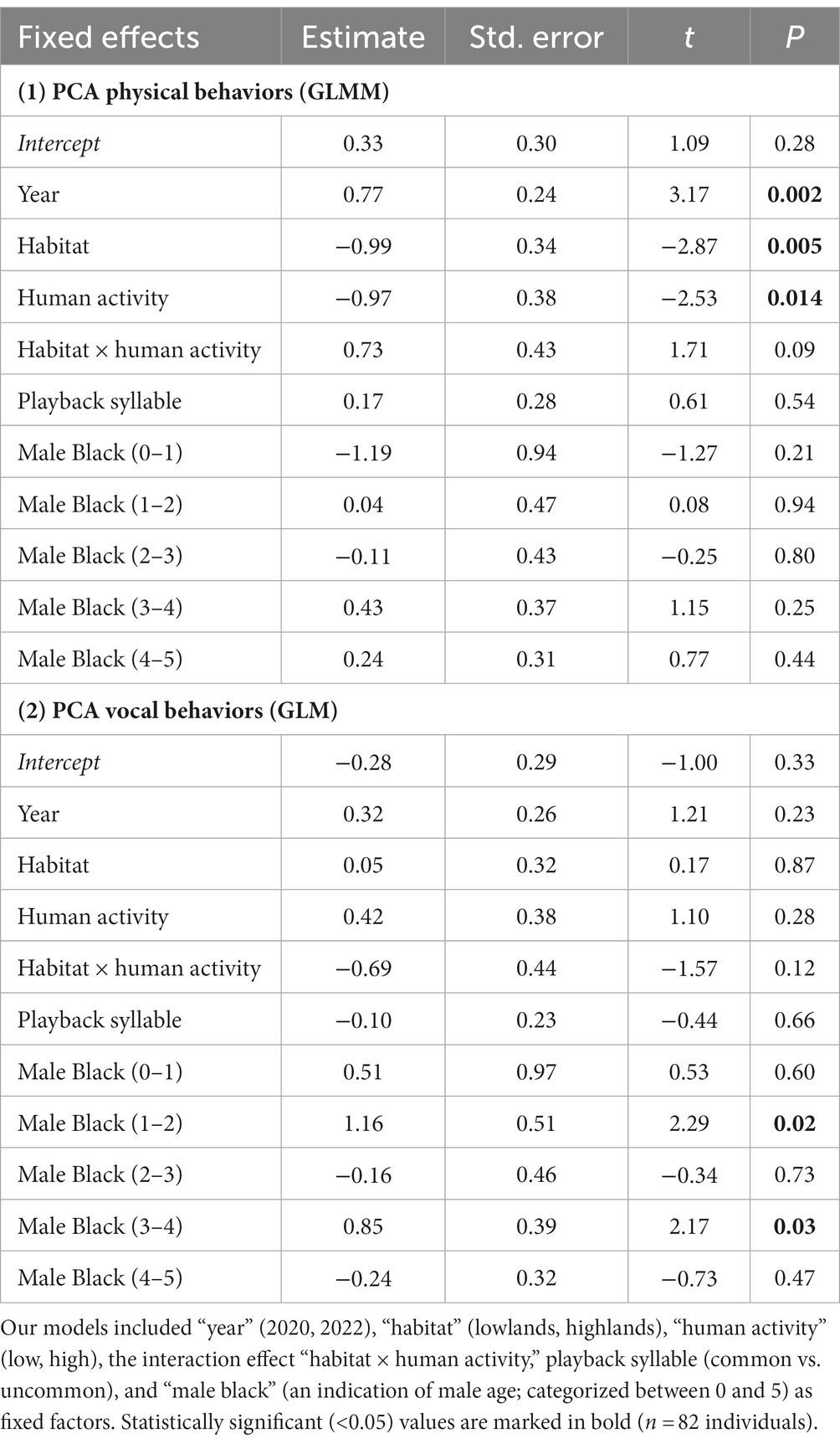
Table 3. Output from the GLMM and GLM testing whether year, habitat, human activity, habitat × human activity correlated with playback response in small ground finches.
During simulated territorial intrusions, 61 males (74%) sang at least one song. Vocal response to playback (PCA Vocal Behaviors) did not differ across years, levels of human activity, habitat or in relation to playback syllable (GLM: Table 3). Males categorized as 1 year old and 3 year old responded with significantly more vocalizations than older males (Table 3).
While human activities can have negative consequences for biodiversity (Shochat et al., 2010), some species can thrive in human-altered ecosystems. In this study, we provide the first evidence that a culturally learned signal (syllable type) that correlates with a behavioral trait (aggressiveness; Colombelli-Négrel et al., unpublished data) may be favored in human-affected environments. Specifically, we found that small ground finches living in human impacted areas and in the arid lowlands had the strongest behavioral response to stimulated territory intrusions. We also found that at sites with a higher percentage of males singing one of the three common syllables, males also had the strongest aggressiveness responses during playback. Our results add to the growing number of studies showing the importance of resource distribution and human-altered environments as potential selective forces in the evolution of differences between birds living near or away from humans (e.g., Evans et al., 2010; Scales et al., 2013; Foltz et al., 2015; Hardman and Dalesman, 2018; Gotanda, 2020).
Similar to previous studies showing that birds living near humans were more aggressive than those living away from humans (e.g., Evans et al., 2010; Scales et al., 2011; Foltz et al., 2015; Myers and Hyman, 2016; Hardman and Dalesman, 2018), we found that small ground finches living in areas with more human activity (Lowlands 1, Highlands 2) showed stronger behavioral response to playback compared to those living in areas with less human activity (Lowlands 2, Highlands 1). This may be due to the fact that human impacted areas have more resources like food and water (e.g., Fox et al., 1981; Scales et al., 2013), which is the case on Floreana island (Lowlands 1) where the lowland town has water and many human food sources (inhabitants often dry their crops collected from the agricultural zone in the open) while the highland agricultural areas and the tortoise breeding center near Asilo de la Paz (Highlands 2) have large reservoirs of water and many tortoises that produce dung and attract insects. In addition, the town and agricultural areas are more likely to be impacted by noise from vehicles, or even generators, than the more rural areas (e.g., Phillips and Derryberry, 2018; Akçay et al., 2020). While both ideas remain to be tested to explain the observed differences in aggressiveness between our populations, our results support the idea that even small urban centers or the mere occurrence of human activity can influence birds and their behavior (see also Gutzwiller and Anderson, 1999; Fernández-Juricic, 2002).
Regardless of human activity, we found that ground finches living in the arid lowlands had the strongest behavioral response to a simulated intruder, suggesting that natural resource distribution or human-provided food may also select for more aggressive behavioral phenotypes (see Foltz et al., 2015). Non-random distribution of resources across the landscape can result in heterogeneity in territory or individual quality, which in turn may correlate with aggressive response in territory owners (Scales et al., 2013; Foltz et al., 2015). Higher quality territories may be subject to higher levels of intrusions by conspecifics (Piper et al., 2000; Ferrer et al., 2015), thereby resulting in higher levels of aggressive defense (Piper et al., 2022). High aggressiveness may also be driven by the supplementation of more human-related food, which could increase body condition and thus aggressiveness (see Foltz et al., 2015). In support of this idea, color banded lowland birds nesting outside of the town center in the arid Lowlands 2 site were often seen foraging in town near human dwellings.
Differences in song frequencies (kHz) in relation to human activities have been more commonly investigated than differences in cultural distribution of signals, such as learned syllable types (e.g., Seger-Fullam et al., 2011; Slabbekoorn, 2013; Moiron et al., 2015). Still, one study reports that birds living near humans were more complex singers (i.e., had larger syllable repertoires; Deoniziak and Osiejuk, 2019), while another study reports that they sang songs with fewer elements than forest birds (Nemeth and Brumm, 2009). Considering the importance of song and element or syllable type for territory defense and mate attraction (Catchpole and Slater, 2008), understanding how cultural aspects of bird songs may vary with the level of human activity could help predict which individuals may survive in human-altered environments.
There are several potential explanations for why the cultural distribution of signals may be different in human-impacted habitats. First, habitat fragmentation resulting from human activities may restrict connectivity between populations, and thus cultural transmission (Laiolo and Tella, 2005; Laiolo and Tella, 2007). Second, individuals may be surrounded by different conspecific densities (Fokidis et al., 2011), which would influence whom they learn their cultural traits from Grant and Grant (1997). Finally, differences in cultural traits may simply be a side effect of selection for other behavioral traits, such as higher aggressiveness (Coss et al., 2002; Scales et al., 2011; Baxter-Gilbert and Whiting, 2019), which may be a favorable trait in modified environments. For instance, social enforcement of conformity may be stronger in populations with higher aggressiveness levels (Lachlan et al., 2004). In this study, we found that more common syllable types were produced in areas where birds were also most aggressive, which aligns with previous research showing that males singing common syllable types had the strongest aggressive response to simulated territory intrusions (Colombelli-Négrel et al., unpublished data). Syllable types in Darwin’s finches do not seem to influence breeding success, but could have important fitness consequences for individuals if, for example, common syllable types elicit stronger aggressive responses from conspecifics than uncommon syllable types (Colombelli-Négrel et al., unpublished data). Despite the fact that our current study did not use the same birds for our song and playback analyses, it suggests that variation in song syllable types may be the result of selection for particular behavioral phenotypes, an idea that warrants further investigation.
While we found differences in the prevalence of common syllable types across our study sites, we found no differences in the number of syllables per song, song duration, song tempo or song rhythmicity between habitats or levels of human activity. One study suggested that acoustic signals may better transmit in human-altered (i.e., noisy) environments if produced at a faster rate (Redondo et al., 2013). Yet studies investigating temporal variation in relation to levels of human activities have found mixed results. For example, Potvin et al. (2011) and Hill et al. (2018) found that birds living near humans sang songs with slower syllable rate or longer inter-syllable rate compared to rural birds, while Nemeth and Brumm (2009) found that birds living near humans sang shorter songs than forest birds, and Brewer and Fudickar (2022) found no temporal differences. Further investigation into how human-altered environments may affect temporal aspects of bird songs is needed.
In conclusion, this study provides the first evidence that human-altered environments and resource distribution may select for behavioral phenotypes that carry a cultural tag. Interestingly, it should be noted that the small ground finches are expanding into highlands previously occupied by sharp-beaked ground finch (Geospiza difficilis; see Galligan and Kleindorfer, 2010), a species now extinct on Floreana island (Grant et al., 2005). The distribution of the new small ground finch populations on Floreana island—with persistence of (source) lowland small ground finch populations having different traits compared with (purported colonist) highland populations—suggests selective factors shaping behaviors and culture.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by Flinders University Animal Welfare Committee (E480-19).
DC-N, ÇA, and SK conceived the idea, designed the study, and collected the data. DC-N analyzed the data and wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Australian Research Council (DP190102894) and the University of Vienna.
We thank the Galapagos National Park for permission to conduct research (permit no. PC-02–20) and the Charles Darwin Foundation for logistical support. We also thank the community of Floreana Island for their invaluable support. Special thanks to Lauren Common, Jody O’Connor, David Arango Roldán, Jefferson García Loor, Mario Gallego-Abenza, Alena Hohl, Leon Hohl, Andrew Katsis, Petra Pesak, and Verena Puehringer-Sturmayr for their help with data collection. Many thanks to Lauren Common for the illustrations. This publication is contribution number 2467 of the Charles Darwin Foundation of the Galapagos Islands.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akçay, Ç., Porsuk, Y. K., Avşar, A., Çabuk, D., and Bilgin, C. C. (2020). Song overlapping, noise, and territorial aggression in great tits. Behav. Ecol. 31, 807–814. doi: 10.1093/beheco/araa030
Baker, M. C. (1975). Song dialects and genetic differences in white-crowned sparrows (Zonotrichia leucophrys). Evolution 29, 226–241. doi: 10.1111/j.1558-5646.1975.tb00203.x
Baxter-Gilbert, J. H., and Whiting, M. J. (2019). Street fighters: bite force, injury rates, and density of urban Australian water dragons (Intellagama lesueurii). Austral Ecol. 44, 255–264. doi: 10.1111/aec.12670
Beecher, M. D., Akçay, Ç., and Campbell, S. E. (2020). Birdsong learning is mutually beneficial for tutee and tutor in song sparrows. Anim. Behav. 166, 281–288. doi: 10.1016/j.anbehav.2020.05.015
Beecher, M. D., Campbell, S. E., and Nordby, J. C. (2000). Territory tenure in song sparrows is related to song sharing with neighbours, but not to repertoire size. Anim. Behav. 59, 29–37. doi: 10.1006/anbe.1999.1304
Ben-Yosef, M., Zaada, D. S., Dudaniec, R. Y., Pasternak, Z., Jurkevitch, E., Smith, R. J., et al. (2017). Host-specific associations affect the microbiome of Philornis downsi, an introduced parasite to the Galápagos Islands. Mol. Ecol. 26, 4644–4656. doi: 10.1111/mec.14219
Bowman, R. I. (1979). Adaptive morphology of song dialects in Darwin's finches. J. Ornithol. 120, 353–389. doi: 10.1007/BF01642911
Bowman, R. I. (1983). “The evolution of song in Darwin’s finches” in Patterns of evolution in Galapagos organisms. eds. R. I. Bowman, M. Berson, and A. E. Leviton (San Francisco, CA: American Association for the Advancement of Science. Pacific Division), 237–537.
Brewer, D. E., and Fudickar, A. M. (2022). A preliminary comparison of a songbird’s song repertoire size and other song measures between an urban and a rural site. Ecol. Evol. 12:e8602. doi: 10.1002/ece3.8602
Callaghan, C. T., Major, R. E., Wilshire, J. H., Martin, J. M., Kingsford, R. T., and Cornwell, W. K. (2019). Generalists are the most urban-tolerant of birds: a phylogenetically controlled analysis of ecological and life history traits using a novel continuous measure of bird responses to urbanization. Oikos 128, 845–858. doi: 10.1111/oik.06158
Camacho, C., Beausoleil, M.-O., Rabadán-González, J., and Richard, R. (2019). Nest building by Darwin’s finches as an overlooked seed dispersal mechanism. J. Trop. Ecol. 35, 43–45. doi: 10.1017/S0266467418000378
Carrete, M., and Tella, J. L. (2010). Individual consistency in flight initiation distances in burrowing owls: a new hypothesis on disturbance-induced habitat selection. Biol. Lett. 6, 167–170. doi: 10.1098/rsbl.2009.0739
Catchpole, C. K., and Slater, P. J. B. (2008). Bird song: Biological themes and variations. Cambridge: Second Edition. Cambridge University Press.
Chapple, D. G., Simmonds, S. M., and Wong, B. B. (2012). Can behavioral and personality traits influence the success of unintentional species introductions? Trends Ecol. Evol. 27, 57–64. doi: 10.1016/j.tree.2011.09.010
Charmantier, A., Demeyrier, V., Lambrechts, M., Perret, S., and Grégoire, A. (2017). Urbanization is associated with divergence in pace-of-life in great tits. Front. Ecol. Evol. 5:53. doi: 10.3389/fevo.2017.00053
Christensen, R., Kleindorfer, S., and Robertson, J. (2006). Song is a reliable signal of bill morphology in Darwin's small tree finch Camarhynchus parvulus, and vocal performance predicts male pairing success. J. Avian Biol. 37, 617–624. doi: 10.1111/j.0908-8857.2006.03684.x
Colombelli-Négrel, D., and Kleindorfer, S. (2021). Behavioural response to songs between genetically diverged allopatric populations of Darwin’s small tree finch in the Galápagos. J. Evol. Biol. 34, 816–829. doi: 10.1111/jeb.13783
Common, L. K., Sumasgutner, P., Sumasgutner, S. C., Colombelli-Négrel, D., Dudaniec, R. Y., and Kleindorfer, S. (2022). Temporal and spatial variation in sex-specific abundance of the avian vampire fly (Philornis downsi). Parasitol. Res. 121, 63–74. doi: 10.1007/s00436-021-07350-1
Conservancy, G. (2022). Galapagos vital signs: a satellite-based environmental monitoring system for the Galapagos archipelago. Available at: https://www.galapagosvitalsigns.org/ (Accessed 06 May 2022).
Coss, R. G., Marks, S., and Ramakrishnan, U. (2002). Early environment shapes the development of gaze aversion by wild bonnet macaques (Macaca radiata). Primates 43, 217–222. doi: 10.1007/BF02629649
Cote, J., Clobert, J., Brodin, T., Fogarty, S., and Sih, A. (2010). Personality-dependent dispersal: characterization, ontogeny and consequences for spatially structured populations. Philos. Trans. R. Soc. B 365, 4065–4076. doi: 10.1098/rstb.2010.0176
De León, L., Podos, J., Gardezi, T., Herrel, A., and Hendry, A. (2014). Darwin's finches and their diet niches: the sympatric coexistence of imperfect generalists. J. Evol. Biol. 27, 1093–1104. doi: 10.1111/jeb.12383
De Leon, L. F., Raeymaekers, J. A. M., Bermingham, E., Podos, J., Herrel, A., and And Hendry, A. P. (2011). Exploring possible human influences on the evolution of Darwin's finches. Evolution 65, 2258–2272. doi: 10.1111/j.1558-5646.2011.01297.x
De León, L. F., Sharpe, D. M., Gotanda, K. M., Raeymaekers, J. A., Chaves, J. A., Hendry, A. P., et al. (2019). Urbanization erodes niche segregation in Darwin's finches. Evol. Appl. 12, 1329–1343. doi: 10.1111/eva.12721
Demko, A. D., Reitsma, L. R., and Staicer, C. A. (2016). Repertoire structure, song sharing, reproductive success, and territory tenure in a population of Canada warblers (Cardellina canadensis) in Central New Hampshire. Can. J. Zool. 94, 283–290. doi: 10.1139/cjz-2015-0213
Deoniziak, K., and Osiejuk, T. S. (2019). Habitat-related differences in song structure and complexity in a songbird with a large repertoire. BMC Ecol. 19, 1–11. doi: 10.1186/s12898-019-0255-7
Ducatez, S., Audet, J.-N., Rodriguez, J. R., Kayello, L., and Lefebvre, L. (2017). Innovativeness and the effects of urbanization on risk-taking behaviors in wild Barbados birds. Anim. Cogn. 20, 33–42. doi: 10.1007/s10071-016-1007-0
Dvorak, M., Nemeth, E., Wendelin, B., Herrera, P., Mosquera, D., Anchundia, D., et al. (2017). Conservation status of landbirds on Floreana: the smallest inhabited Galápagos Island. J. Field Ornithol. 88, 132–145. doi: 10.1111/jofo.12197
Evans, J., Boudreau, K., and Hyman, J. (2010). Behavioural syndromes in urban and rural populations of song sparrows. Ethology 116, 588–595. doi: 10.1111/j.1439-0310.2010.01771.x
Fernández-Juricic, E. (2002). Can human disturbance promote nestedness? A case study with breeding birds in urban habitat fragments. Oecologia 131, 269–278. doi: 10.1007/s00442-002-0883-y
Ferrer, M., Morandini, V., and Newton, I. (2015). Floater interference reflects territory quality in the Spanish Imperial eagle Aquila adalberti: a test of a density-dependent mechanism. Ibis 157, 849–859. doi: 10.1111/ibi.12289
Fokidis, H. B., Orchinik, M., and Deviche, P. (2011). Context-specific territorial behavior in urban birds: no evidence for involvement of testosterone or corticosterone. Horm. Behav. 59, 133–143. doi: 10.1016/j.yhbeh.2010.11.002
Foltz, S. L., Ross, A. E., Laing, B. T., Rock, R. P., Battle, K. E., and Moore, I. T. (2015). Get off my lawn: increased aggression in urban song sparrows is related to resource availability. Behav. Ecol. 26, 1548–1557. doi: 10.1093/beheco/arv111
Fox, S. F., Rose, E., and Myers, R. (1981). Dominance and the acquisition of superior home ranges in the lizard Uta stansburiana. Ecology 62, 888–893. doi: 10.2307/1936985
Galligan, T. H., and Kleindorfer, S. (2010). Loss of assortative pairing following colonization of a new environment by Darwin’s small ground finch (Geospiza fuliginosa). Evol. Ecol. Res. 12, 751–760.
Gibbs, H. L. (1990). Cultural evolution of male song types in Darwin's medium ground finches, Geospiza fortis. Anim. Behav. 39, 253–263. doi: 10.1016/S0003-3472(05)80869-3
Gibbs, H. L., and Grant, P. R. (1987). Ecological consequences of an exceptionally strong El Niño event on Darwin's finches. Ecology 68, 1735–1746. doi: 10.2307/1939865
Gotanda, K. M. (2020). Human influences on antipredator behaviour in Darwin’s finches. J. Anim. Ecol. 89, 614–622. doi: 10.1111/1365-2656.13127
Grant, B. (1984). The significance of song variation in a population of Darwin's finches. Behaviour 89, 90–116. doi: 10.1163/156853984X00056
Grant, B. R. (1990). The significance of subadult plumage in Darwin's finches, Geospiza fortis. Behav. Ecol. 1, 161–170. doi: 10.1093/beheco/1.2.161
Grant, P. R., and Boag, P. T. (1980). Rainfall on the Galapagos and the demography of Darwin’s finches. Auk 97, 227–244. doi: 10.1093/auk/97.2.227
Grant, B. R., and Grant, P. R. (1996). Cultural inheritance of song and its role in the evolution of Darwin's finches. Evolution 50, 2471–2487. doi: 10.2307/2410714
Grant, P. R., and Grant, B. R. (1997). Hybridization, sexual imprinting, and mate choice. Am. Nat. 149, 1–28. doi: 10.1086/285976
Grant, P. R., and Grant, B. R. (2002). Adaptive radiation of Darwin's finches. Am. Sci. 90, 130–139. doi: 10.1511/2002.10.130
Grant, P. R., and Grant, B. R. (2008). How and why species multiply: The radiation of Darwin's finches. Princeton: Princeton University Press.
Grant, P. R., and Grant, B. R. (2014a). 40 years of evolution: Darwin's finches on Daphne Major Island. San Francisco, CA: Princeton University Press.
Grant, P. R., and Grant, B. R. (2014b). Synergism of natural selection and introgression in the origin of a new species. Am. Nat. 183, 671–681. doi: 10.1086/675496
Grant, P. R., Grant, B. R., Petren, K., and Keller, L. F. (2005). Extinction behind our backs: the possible fate of one of the Darwin's finch species on Isla Floreana, Galapagos. Biol. Conserv. 122, 499–503. doi: 10.1016/j.biocon.2004.09.001
Gutzwiller, K. J., and Anderson, S. H. (1999). Spatial extent of human-intrusion effects on subalpine bird distributions. Condor 101, 378–389. doi: 10.2307/1370001
Hardman, S. I., and Dalesman, S. (2018). Repeatability and degree of territorial aggression differs among urban and rural great tits (Parus major). Sci. Rep. 8, 1–12. doi: 10.1038/s41598-018-23463-7
Harvey, J. A., Chernicky, K., Simons, S. R., Verrett, T. B., Chaves, J. A., and Knutie, S. A. (2021). Urban living influences the nesting success of Darwin’s finches in the Galápagos Islands. Ecol. Evol. 11, 5038–5048. doi: 10.1002/ece3.7360
Hau, M., Wikelski, M., Gwinner, H., and Gwinner, E. (2004). Timing of reproduction in a Darwin's finch: temporal opportunism under spatial constraints. Oikos 106, 489–500. doi: 10.1111/j.0030-1299.2004.13206.x
Hendry, A. P., Grant, P. R., Grant, B. R., Ford, H. A., Brewer, M. J., and Podos, J. (2006). Possible human impacts on adaptive radiation: beak size bimodality in Darwin's finches. Proc. R. Soc. Lond. B 273, 1887–1894. doi: 10.1098/rspb.2006.3534
Hill, S. D., Aryal, A., Pawley, M. D., and Ji, W. (2018). So much for the city: urban–rural song variation in a widespread Asiatic songbird. Integr Zool 13, 194–205. doi: 10.1111/1749-4877.12284
Holtmann, B., Santos, E. S., Lara, C. E., and Nakagawa, S. (2017). Personality-matching habitat choice, rather than behavioural plasticity, is a likely driver of a phenotype–environment covariance. Proc. R. Soc. B 284:20170943. doi: 10.1098/rspb.2017.0943
Hu, Y., and Cardoso, G. C. (2010). Which birds adjust the frequency of vocalizations in urban noise? Anim. Behav. 79, 863–867. doi: 10.1016/j.anbehav.2009.12.036
Kleindorfer, S. (2007a). The ecology of clutch size variation in Darwin’s small ground finch Geospiza fuliginosa: comparison between lowland and highland habitats. Ibis 149, 730–741. doi: 10.1111/j.1474-919X.2007.00694.x
Kleindorfer, S. (2007b). Nesting success in Darwin's small tree finch, Camarhynchus parvulus: evidence of female preference for older males and more concealed nests. Anim. Behav. 74, 795–804. doi: 10.1016/j.anbehav.2007.01.020
Kleindorfer, S., Colombelli-Négrel, D., Common, L. K., O’connor, J. A., Peters, K. J., Katsis, A. C., et al. (2022). Functional traits and foraging behaviour: Avian vampire fly larvae change the beak and fitness of their Darwin’s finch hosts. Funct. Ecol 36, 1806–1817. doi: 10.1111/1365-2435.14061
Kleindorfer, S., and Dudaniec, R. Y. (2020). Hybridization fluctuates with rainfall in Darwin’s tree finches. Biol. J. Linn. Soc. 130, 79–88. doi: 10.1093/biolinnean/blaa029
Kleindorfer, S., Fessl, B., Peters, K. J., and Anchundia, D. (2019). Field guide. Resident land birds of Galapagos. Santa Cruz, Ecuador: Charles Darwin Foundation.
Knutie, S. A., Chaves, J. A., and Gotanda, K. M. (2019). Human activity can influence the gut microbiota of Darwin's finches in the Galapagos Islands. Mol. Ecol. 28, 2441–2450. doi: 10.1111/mec.15088
Kunc, H. P., and Schmidt, R. (2021). Species sensitivities to a global pollutant: a meta-analysis on acoustic signals in response to anthropogenic noise. Glob. Chang. Biol. 27, 675–688. doi: 10.1111/gcb.15428
Lachlan, R. F., Janik, V. M., and Slater, P. J. (2004). The evolution of conformity-enforcing behaviour in cultural communication systems. Anim. Behav. 68, 561–570. doi: 10.1016/j.anbehav.2003.11.015
Laiolo, P., and Tella, J. L. (2005). Habitat fragmentation affects culture transmission: patterns of song matching in Dupont's lark. J. Appl. Ecol. 42, 1183–1193. doi: 10.1111/j.1365-2664.2005.01093.x
Laiolo, P., and Tella, J. L. (2007). Erosion of animal cultures in fragmented landscapes. Front. Ecol. Environ. 5, 68–72. doi: 10.1890/1540-9295(2007)5[68:EOACIF]2.0.CO;2
Lamichhaney, S., Berglund, J., Almén, M. S., Maqbool, K., Grabherr, M., Martinez-Barrio, A., et al. (2015). Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 518, 371–375. doi: 10.1038/nature14181
Langton, A., and Kleindorfer, S. (2019). Minimum longevity and age-related male plumage in Darwin’s finches on Floreana Island. J. Ornithol. 160, 351–361. doi: 10.1007/s10336-019-01626-1
Lapiedra, O., Chejanovski, Z., and Kolbe, J. J. (2017). Urbanization and biological invasion shape animal personalities. Glob. Chang. Biol. 23, 592–603. doi: 10.1111/gcb.13395
Lee, V. E., and Thornton, A. (2021). Animal cognition in an urbanised world. Front. Ecol. Evol. 9:633947. doi: 10.3389/fevo.2021.633947
Logue, D. M. (2021). Countersinging in birds. Adv Study Behav 53, 1–61. doi: 10.1016/bs.asb.2021.03.001
Loo, W. T., García-Loor, J., Dudaniec, R. Y., Kleindorfer, S., and Cavanaugh, C. M. (2019). Host phylogeny, diet, and habitat differentiate the gut microbiomes of Darwin’s finches on Santa Cruz Island. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-54869-6
Lowry, H., Lill, A., and Wong, B. B. (2013). Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537–549. doi: 10.1111/brv.12012
Luther, D. A., and Derryberry, E. P. (2012). Birdsongs keep pace with city life: changes in song over time in an urban songbird affects communication. Anim. Behav. 83, 1059–1066. doi: 10.1016/j.anbehav.2012.01.034
Marzluff, J. M., and Neatherlin, E. (2006). Corvid response to human settlements and campgrounds: causes, consequences, and challenges for conservation. Biol. Conserv. 130, 301–314. doi: 10.1016/j.biocon.2005.12.026
Maspons, J., Molowny-Horas, R., and Sol, D. (2019). Behaviour, life history and persistence in novel environments. Philos. Trans. R. Soc. B 374:20180056. doi: 10.1098/rstb.2018.0056
Millington, S. J., and Price, T. D. (1985). Song inheritance and mating patterns in Darwin's finches. Auk 102, 342–346. doi: 10.2307/4086777
Moiron, M., González-Lagos, C., Slabbekoorn, H., and Sol, D. (2015). Singing in the city: high song frequencies are no guarantee for urban success in birds. Behav. Ecol. 26, 843–850. doi: 10.1093/beheco/arv026
Møller, A. P. (2010). Interspecific variation in fear responses predicts urbanization in birds. Behav. Ecol. 21, 365–371. doi: 10.1093/beheco/arp199
Morton, E. S. (1975). Ecological sources of selection on avian sounds. Am. Nat. 109, 17–34. doi: 10.1086/282971
Myers, R. E., and Hyman, J. (2016). Differences in measures of boldness even when underlying behavioral syndromes are present in two populations of the song sparrow (Melospiza melodia). J. Ethol. 34, 197–206. doi: 10.1007/s10164-016-0465-9
Nemeth, E., and Brumm, H. (2009). Blackbirds sing higher-pitched songs in cities: adaptation to habitat acoustics or side-effect of urbanization? Anim. Behav. 78, 637–641. doi: 10.1016/j.anbehav.2009.06.016
O'Loghlen, A. L., and Rothstein, S. I. (1995). Culturally correct song dialects are correlated with male age and female song preferences in wild populations of brown-headed cowbirds. Behav. Ecol. Sociobiol. 36, 251–259. doi: 10.1007/BF00165834
Partecke, J., and Gwinner, E. (2007). Increased sedentariness in European blackbirds following urbanization: a consequence of local adaptation? Ecology 88, 882–890. doi: 10.1890/06-1105
Patankar, S., Jambhekar, R., Suryawanshi, K. R., and Nagendra, H. (2021). Which traits influence bird survival in the city? A review. Land 10:92. doi: 10.3390/land10020092
Payne, R. B. (1982). Ecological consequences of song matching: breeding success and intraspecific song mimicry in indigo buntings. Ecology 63, 401–411. doi: 10.2307/1938958
Petren, K., Grant, P. R., Grant, B. R., and Keller, L. F. (2005). Comparative landscape genetics and the adaptive radiation of Darwin's finches: the role of peripheral isolation. Mol. Ecol. 14, 2943–2957. doi: 10.1111/j.1365-294X.2005.02632.x
Phillips, J. N., and Derryberry, E. P. (2018). Urban sparrows respond to a sexually selected trait with increased aggression in noise. Sci. Rep. 8, 1–10. doi: 10.1038/s41598-018-25834-6
Piper, W. H., Lee, K. R., and Hoover, B. (2022). Territory holders are more aggressive towards older, more dangerous floaters. Behav. Ecol. Sociobiol. 76, 1–11. doi: 10.1007/s00265-022-03131-7
Piper, W. H., Tischler, K. B., and Klich, M. (2000). Territory acquisition in loons: the importance of take-over. Anim. Behav. 59, 385–394. doi: 10.1006/anbe.1999.1295
Potvin, D. A., Parris, K. M., and Mulder, R. A. (2011). Geographically pervasive effects of urban noise on frequency and syllable rate of songs and calls in silvereyes (Zosterops lateralis). Proc. R. Soc. Lond. B 278, 2464–2469. doi: 10.1098/rspb.2010.2296
Ratcliffe, L. M., and Grant, P. R. (1985). Species recognition in Darwin's finches (Geospiza, Gould). III. Male responses to playback of different song types, dialects and heterospecific songs. Anim. Behav. 33, 290–307. doi: 10.1016/S0003-3472(85)80143-3
Redondo, P., Barrantes, G., and Sandoval, L. (2013). Urban noise influences vocalization structure in the H ouse W ren T roglodytes aedon. Ibis 155, 621–625. doi: 10.1111/ibi.12053
Rothstein, S. I., and Fleischer, R. C. (1987). Vocal dialects and their possible relation to honest status signalling in the brown-headed cowbird. Condor 89, 1–23. doi: 10.2307/1368756
Sasahara, K., Tchernichovski, O., Takahasi, M., Suzuki, K., and Okanoya, K. (2015). A rhythm landscape approach to the developmental dynamics of birdsong. J. R. Soc. Interface 12:20150802. doi: 10.1098/rsif.2015.0802
Scales, J., Hyman, J., and Hughes, M. (2011). Behavioral syndromes break down in urban song sparrow populations. Ethology 117, 887–895. doi: 10.1111/j.1439-0310.2011.01943.x
Scales, J., Hyman, J., and Hughes, M. (2013). Fortune favours the aggressive: territory quality and behavioural syndromes in song sparrows, Melospiza melodia. Anim. Behav. 85, 441–451. doi: 10.1016/j.anbehav.2012.12.004
Searcy, W. A., and Beecher, M. D. (2009). Song as an aggressive signal in songbirds. Anim. Behav. 78, 1281–1292. doi: 10.1016/j.anbehav.2009.08.011
Seger-Fullam, K. D., Rodewald, A. D., and Soha, J. A. (2011). Urban noise predicts song frequency in northern cardinals and American robins. Bioacoustics 20, 267–276. doi: 10.1080/09524622.2011.9753650
Shochat, E., Lerman, S. B., Anderies, J. M., Warren, P. S., Faeth, S. H., and Nilon, C. H. (2010). Invasion, competition, and biodiversity loss in urban ecosystems. Bioscience 60, 199–208. doi: 10.1525/bio.2010.60.3.6
Sih, A., Ferrari, M. C., and Harris, D. J. (2011). Evolution and behavioural responses to human-induced rapid environmental change. Evol. Appl. 4, 367–387. doi: 10.1111/j.1752-4571.2010.00166.x
Silva, C. P., Sepúlveda, R. D., and Barbosa, O. (2016). Nonrandom filtering effect on birds: species and guilds response to urbanization. Ecol. Evol. 6, 3711–3720. doi: 10.1002/ece3.2144
Slabbekoorn, H. (2013). Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim. Behav. 85, 1089–1099. doi: 10.1016/j.anbehav.2013.01.021
Sol, D., Duncan, R. P., Blackburn, T. M., Cassey, P., and Lefebvre, L. (2005). Big brains, enhanced cognition, and response of birds to novel environments. PNAS 102, 5460–5465. doi: 10.1073/pnas.0408145102
Steadman, D. W. (1986). Holocene vertebrate fossils from Isla Floreana, Galápagos, (Washington, DC: Smithsonian Institution Press) 1–103.
Stewart, K. A., and MacDougall-Shackleton, E. A. (2008). Local song elements indicate local genotypes and predict physiological condition in song sparrows Melospiza melodia. Biol. Lett. 4, 240–242. doi: 10.1098/rsbl.2008.0010
Sulloway, F. J. (1982). The beagle collections of Darwins's finches (Geospizinae). Bulletin of the British museum (natural history). Fortschr. Zool. 43, 49–94.
Tebbich, S., Taborsky, M., Fessl, B., Dvorak, M., and Winkler, H. (2004). Feeding behavior of four arboreal Darwin's finches: adaptations to spatial and seasonal variability. Condor 106, 95–105. doi: 10.1093/condor/106.1.95
Tryjanowski, P., Møller, A. P., Morelli, F., Biaduń, W., Brauze, T., Ciach, M., et al. (2016). Urbanization affects neophilia and risk-taking at bird-feeders. Sci. Rep. 6, 1–7. doi: 10.1038/srep28575
Keywords: animal communication, cultural signals, territory defense, Darwin’s finches, urbanization, urban ecology
Citation: Colombelli-Négrel D, Akçay Ç and Kleindorfer S (2023) Darwin’s finches in human-altered environments sing common song types and are more aggressive. Front. Ecol. Evol. 11:1034941. doi: 10.3389/fevo.2023.1034941
Received: 02 September 2022; Accepted: 05 April 2023;
Published: 27 April 2023.
Edited by:
Alejandro Ariel Ríos-Chelén, Autonomous University of Tlaxcala, MexicoReviewed by:
J. Roberto Sosa-Lopez, National Polytechnic Institute (IPN), MexicoCopyright © 2023 Colombelli-Négrel, Akçay and Kleindorfer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sonia Kleindorfer, U29uaWEua2xlaW5kb3JmZXJAdW5pdmllLmFjLmF0
†ORCID: Diane Colombelli-Négrel orcid.org/0000-0002-9572-1120
Çağlar Akçay orcid.org/0000-0002-9572-1120
Sonia Kleindorfer orcid.org/0000-0001-5130-3122
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.