
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol., 03 February 2023
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1017361
This article is part of the Research TopicDrivers of Small-Mammal Community Structure in Tropical SavannasView all 14 articles
Small-mammal faunas of tropical savannas consist of endemic assemblages of murid rodents, small marsupials, and insectivores on four continents. Small mammals in tropical savannas are understudied compared to other tropical habitats and other taxonomic groups (e.g., Afrotropical megafauna or Neotropical rainforest mammals). Their importance as prey, ecosystem engineers, disease reservoirs, and declining members of endemic biodiversity in tropical savannas compels us to understand the factors that regulate their abundance and diversity. We reviewed field studies published in the last 35 years that examined, mostly experimentally, the effects of varying three primary endogenous disturbances in tropical savanna ecosystems—fire, large mammalian herbivory (LMH), and drought—on abundance and diversity of non-volant small mammals. These disturbances are most likely to affect habitat structure (cover or concealment), food availability, or both, for ground-dwelling small mammalian herbivores, omnivores, and insectivores. Of 63 studies (included in 55 published papers) meeting these criteria from the Afrotropics, Neotropics, and northern Australia (none was found from southern Asia), 29 studies concluded that small mammals responded (mostly negatively) to a loss of cover (mostly from LMH and fire); four found evidence of increased predation on small mammals in lower-cover treatments (e.g., grazed or burned). Eighteen studies concluded a combination of food- and cover-limitation explained small-mammal responses to endogenous disturbances. Only two studies concluded small-mammal declines in response to habitat-altering disturbance were caused by food limitation and not related to cover reduction. Evidence to date indicates that abundance and richness of small savanna mammals, in general (with important exceptions), is enhanced by vegetative cover (especially tall grass, but sometimes shrub cover) as refugia for these prey species amid a “landscape of fear,” particularly for diurnal, non-cursorial, and non-fossorial species. These species have been called “decreasers” in response to cover reduction, whereas a minority of small-mammal species have been shown to be “increasers” or disturbance-tolerant. Complex relationships between endogenous disturbances and small-mammal food resources are important secondary factors, but only six studies manipulated or measured food resources simultaneous to habitat manipulations. While more such studies are needed, designing effective ones for cryptic consumer communities of omnivorous dietary opportunists is a significant challenge.
Tropical and subtropical savanna ecosystems (TSE) cover 20% of the land area of the Neotropics, sub-Saharan Africa, Southern Asia, and Northern Australia (Bond, 2016) and contribute around 30% of terrestrial primary productivity, globally (Grace et al., 2006). The pre-anthropocene structure of tropical savannas was dependent on the effects of endogenous disturbances, primarily fire and large-mammalian herbivory, which maintained the grassy understory and high native biodiversity (Buisson et al., 2019; Andersen, 2021). In general, fire is more frequent in TSE than in any other biome type (He et al., 2019). The native biodiversity of each region and type of TSE includes an assemblage of small mammals (< 1 kg), including rodents, small marsupials, and members of several insectivorous eutherian orders that primarily live on and just under the ground surface. The one small-mammal taxon in common to TSE on all four continents are native species and genera of the most diverse of mammalian families, Muridae (Mammal Diversity Database, 2022). Small mammals are important and potentially abundant components of tropical savannas and play ecologically important roles including ecosystem engineers (e.g., by digging burrows and tunnel systems that are used by many commensals and help to aerate savanna soils), insect and plant regulators, food sources for mammalian, reptilian, and avian predators, and reservoirs for realized and potential zoonotic diseases (e.g., Wurm, 1998; Hagenah and Bennett, 2013; Byrom et al., 2014; Limongi et al., 2016; Lamberto and Leiner, 2019; Teman et al., 2021). TSE small rodents are important reservoirs for diseases that are and may be zoonotic, including the bacterial diseases bubonic plague and Bartonellosis, hantaviruses (which cause various hemorrhagic fevers), and adenoviruses (one of which causes Lassa fever; Lecompte et al., 2006; Luis et al., 2013; Young et al., 2014).
Small mammals are highly influenced by habitat changes driven by common endogenous disturbances of TSE (mostly fire, grazing, and drought) and their effects on both vegetative cover and food availability (Seymour and Joseph, 2019). These factors may affect small-mammal species and communities differentially, however, in the distinct types of TSE around the world. Of the three major endogenous disturbances under which TSE evolved (Buisson et al., 2019), megafaunal extinctions culminating ca. 11,000 years ago have greatly reduced the native large-mammal herbivory disturbance component from savannas in Australia (although recent predator control has allowed kangaroos to increase to the point of overgrazing in temperate grasslands; Mills et al., 2020) and the Neotropics. In contrast, substantial populations of native large mammalian herbivores (LMH) remain in portions of the Afrotropics and Southern Asia. Domestic livestock on open range have arguably partially replaced the role of native LMH in many tropical savannas (but their interactive effects are complex; Riginos et al., 2012; Archibald and Hempson, 2016). Insect herbivory may have expanded particularly in Neotropical savannas after Pleistocene megafaunal extinctions (Costa et al., 2008), thereby limiting the extent of competitive release (i.e., primary production available, instead, to small-mammal herbivores). Exclusion of aboriginal fire regimes, in addition to LMH extirpation (as well as misguided afforestation efforts; Kumar et al., 2020), have allowed woody encroachment, non-native plant invasions, loss of biodiversity, and ironically, in some cases, greatly increased extent and intensity of wildfire.
The goals of restoring native biodiversity and stability or resilience to a tropical savanna require a determination of the deeper history of natural disturbance regimes in each of the TSE worldwide (Buisson et al., 2019), which may be difficult to determine in those areas depleted of native megafauna and in all areas because of the direct and indirect effects of global climate change and atmospheric CO2 increases (Bond, 2016). The tremendous primary productivity of tropical C4 grasses (Buisson et al., 2019) is largely what supports the massive native LMH community of savanna protected areas in East Africa, for example (as much as 75 kg/ha; Augustine, 2010), and this herbivore community both responds strongly to fire (Kimuyu et al., 2017) and can affect fire behavior in complex ways (Young et al., 2022). For millennia, mobile pastoralists have inserted domestic cattle into this fire-herbivory interaction and have enriched and diversified African savannas in unique ways (Charles et al., 2016; Martin et al., 2018). Where humans have reduced native LMH, productivity of otherwise intact savanna may be available for consumption by domestic LMH and/or by small consumers such as insects and small mammals—both of which can undergo irruptions—or it may be lost by catastrophic wildfires.
In the present review, we survey field studies from the past 35 years of how endogenous disturbances have affected small-mammal community structure (abundance, diversity, and species composition) via changes to their habitats in TSE globally, including Southern Asian, Afrotropical, Australian tropical, and Neotropical savannas. Here, we examine the results and conclusions of published field studies of ground-level habitat variation, mostly experimental, to determine whether the cover (=microhabitat selection) hypothesis or the food-competition (=resource availability) hypothesis is better supported when the major endogenous disturbances of tropical savannas (mostly grazing and fire, but also including shrub encroachment, soil enrichment, and drought) alter the ground-level environments of the native small mammals. This dichotomy largely aligns with a comparison of the strength of bottom-up vs. top-down limitation of small mammals, because loss of cover has often been linked to loss of concealment and consequent increased mortality from predation (Stobo-Wilson et al., 2020).
We expected fire to be a focus of such studies in all regions, because all tropical savannas are fire-prone and fire-adapted (Andersen et al., 2003; Figure 1). We expected herbivory by domestic LMH also to be a common factor, but we expected studies of native LMH to be based mostly in Africa. For example, it is known that when native and domestic LMH are experimentally excluded from savanna plots in East Africa, small-mammal abundance can increase as much as 20-fold for some species, and species richness can roughly double (Bergstrom et al., 2018). Control plots from such experiments have comparatively little above-ground biomass, resembling in that regard recently burned areas compared to unburned controls. These recently burned plots are often avoided by many (but not all) small mammals, just as heavily grazed plots are (Yarnell et al., 2007). It is possible that greater use by small mammals of savanna patches with greater above-ground biomass, particularly of grass, could reflect either increased forage availability or increased cover and concealment from predators. It follows that declines in small-mammal abundance or biomass in response to plant-biomass reduction by LMH could be explained by either the forage hypothesis, predicting a direct exploitative competitive effect; or the cover hypothesis, predicting an indirect interaction, in which LMH grazing and fire (the latter, in the short term) would both have similar indirect effects on small-mammal abundance or occupancy (Kutt and Woinarski, 2007; Hagenah et al., 2009). These effects would be mediated by the predation-risk perception behavior of the small mammals. Alternatively, fire can restore a flush of nutrients to the new grass growth and actually increase foraging by small folivorous mammals. We expect this positive impact of fire on small mammals to manifest in the medium or long term, even if not in the short term.
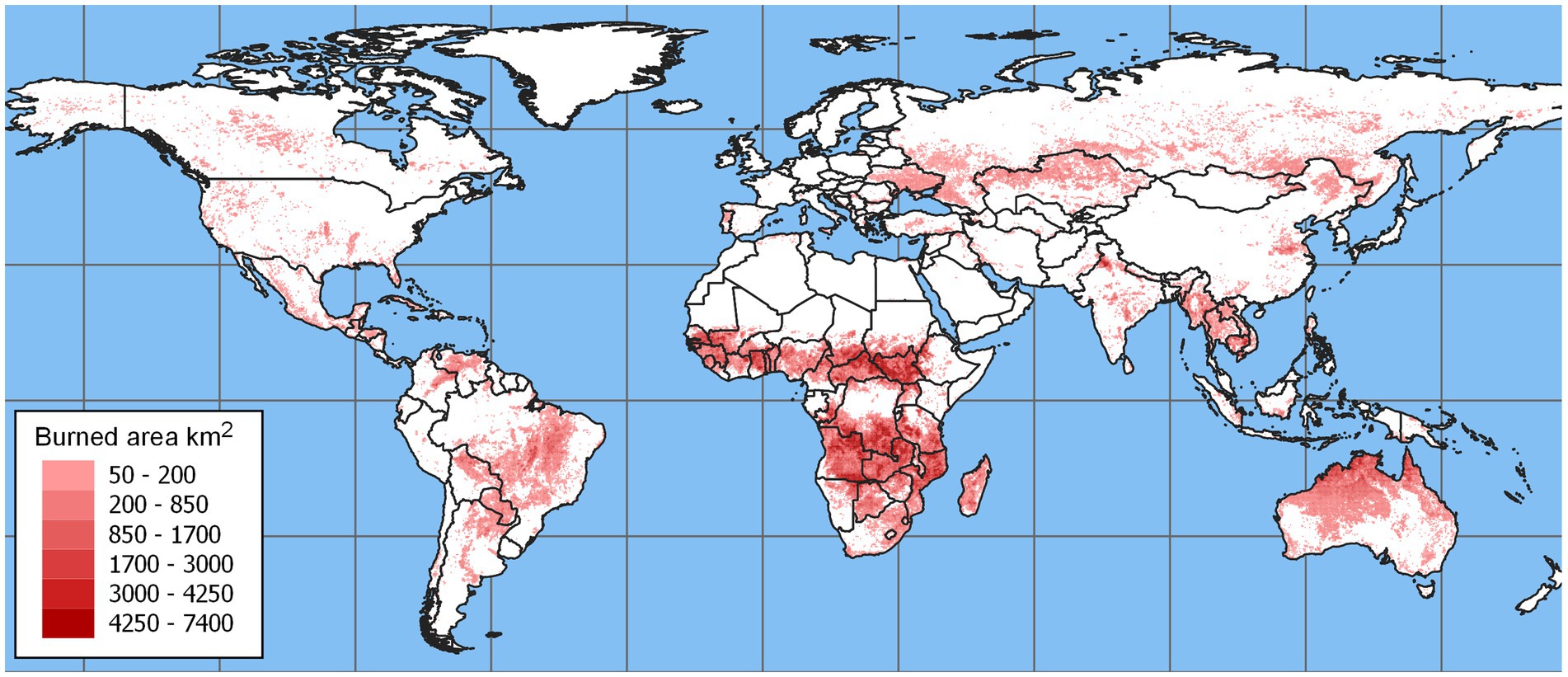
Figure 1. Satellite-derived data showing the annual total area burned in the world based on a time-series of rasters from 2008 to 2021. These data indicate that large fires are conspicuously concentrated in regions where tropical savannas occur, including Central Africa, Northern Australia, and the Cerrado of Brazil. The burned area is for all fire types and represents the total area (hectares) in each 0.25 degree x 0.25 degree grid cell. This figure was generated with MODIS/MCD64A1 data (Giglio et al. 2021).
Our goal was a thorough if not exhaustive classical literature review, which had not previously been undertaken on this topic. We began by assembling published papers on field studies in TSE of potential habitat disturbance effects on non-volant small-mammal abundance, occupancy, vital rates, foraging behavior (excluding dietary shifts but including, e.g., time spent in disturbed vs. control habitat patch), and species diversity in tropical savannas already known to us. We added any additional relevant titles cited by these papers. We then conducted keyword searches on Web of Science, SCOPUS, Academic Search Complete (EbscoHost), JSTOR, Google Scholar, and Google to find additional peer-reviewed published field studies. We found titles from this set on Google Scholar and examined “cited by” papers, adding new titles that were relevant. After reading abstracts, we eliminated reviews, studies of primarily arboreal mammals or volant mammals or mammals >1 kg, or studies conducted primarily in forest or dense woodland (e.g., gallery forest). We also did not include studies that were primarily food supplementation experiments (rather than habitat manipulation experiments). We eliminated otherwise relevant studies (of small, non-volant, and terrestrial mammals in tropical savannas) where no habitat component was either explicitly varied or where no habitat variation could be inferred (as it might be, for example, in recently burned experimental plots vs. unburned controls in the same habitat). We also eliminated studies (mostly in the Neotropics) that examined differences in small-mammal community structure among different macrohabitats (e.g., grassland vs. thorn-scrub vs. woodland, which occur on sites with different edaphic factors and are not seral stages of each other), even when habitat components (e.g., grass, shrub, and tree cover) were quantified and random, replicated sampling was done. Finally, we excluded the few studies where the response variables were community nestedness or modularity, as these were not directly comparable to those studies included in the set.
The final set of potentially pertinent papers totaled 137: 67 African, 41 Australian, 28 Neotropical, zero Asian, and one multi-region (these are included in Supplementary Table S2 along with other studies providing background on the TSEs in which the studies were conducted). Upon more careful reading of each study’s methods, we reduced the set of papers scorable by our criteria (examination of effects of ground-level habitat changes resulting from endogenous disturbances on abundance, occupancy, vital rates, activity, and/or richness of non-volant small mammals in TSE) to 55: 21 African, 19 Australian, and 15 Neotropical. A few of these 55 papers reported results for two or three of our target explanatory variables, so we summarized each such case as a substudy (hereafter “study”), and this brought our total to 63 studies (Supplementary Table S1). We included studies that presented original results on individual species as well as assemblages, and in the latter, response variables related to community structure (species composition, relative abundance, and diversity) were often included. In our analysis, we did not include reviews (or any data presented therein), republication of previously published results, or studies that were merely descriptive.
We tracked whether experiments were manipulative (ME; experimenter creates the variation in a replicated design with controls) or comparative mensurative (CM; nature creates the variation, and the experimenter imposes a replicated, randomized sampling design with a priori knowledge of that variation; see Hurlbert, 1984; McGarigal and Cushman, 2002). Regardless, we deemed both ME and CM studies experimental. If the range of variation in one or more explanatory variables (e.g., occurrence of fire and presence of LMH) was not known a priori but discovered only after data were collected, then such a study was deemed observational (Obs), or non-experimental. We noted where studies were otherwise of an experimental nature (i.e., an explanatory variable was manipulated) but did not meet, or barely met, the expected minimum of two replicates (randomized sampling units) per treatment and control, because such studies are not experiments (Krebs, 2014).
For each relevant study, we recorded whether an explanatory variable related to one or more of the following habitat treatments was manipulated or studied: fire, LMH removal, drought or precipitation, shrub removal or encroachment, soil enrichment, and soil type. Where applicable, we recorded effect sizes and significance levels of treatments on response variables including both per-species and overall small-mammal abundance, occupancy, residency time [e.g., at feeding trays for optimal giving-up density (GUD; sensu Brown, 1988) studies], and in a few cases population vital rates. Also where applicable, we recorded the same results for community indices of species diversity of small mammals. We used these summaries of results, usually in concurrence with the authors’ conclusions as stated in their Discussion sections (except where we found errors in reporting or interpretation by authors of their own results), to score whether the authors concluded that the primary limiting factor of small mammals was herbaceous or woody cover (the microhabitat selection hypothesis, in which the small mammal seeks cover for concealment from predators and/or favorable microclimatic conditions) or food (the resource availability hypothesis), or some combination of the two (identified as “both,” cf. Figure 2). The “both” categorization included studies whose authors concluded that both cover- and food-related limiting factors probably operated in conjunction as well as those studies in which one or more species were found to be food-limited and one or more other species were found to be cover-limited. In other cases, results either did not find significant changes in small-mammal metrics (see above) related to habitat treatments or did not control for confounding variables (e.g., by adequate replication); these studies were scored as no-effect or inconclusive. To avoid undue complexity, where habitat treatments showed significant effects on small mammals overall, and/or one small-mammal species but not others in the same study, we did not subdivide the study further and report separate results (e.g., Figure 2); rather, we here report the results and likely proximate cause(s) for the species found to respond significantly and include expanded, detailed conclusions regarding other species in Supplementary Table S1.
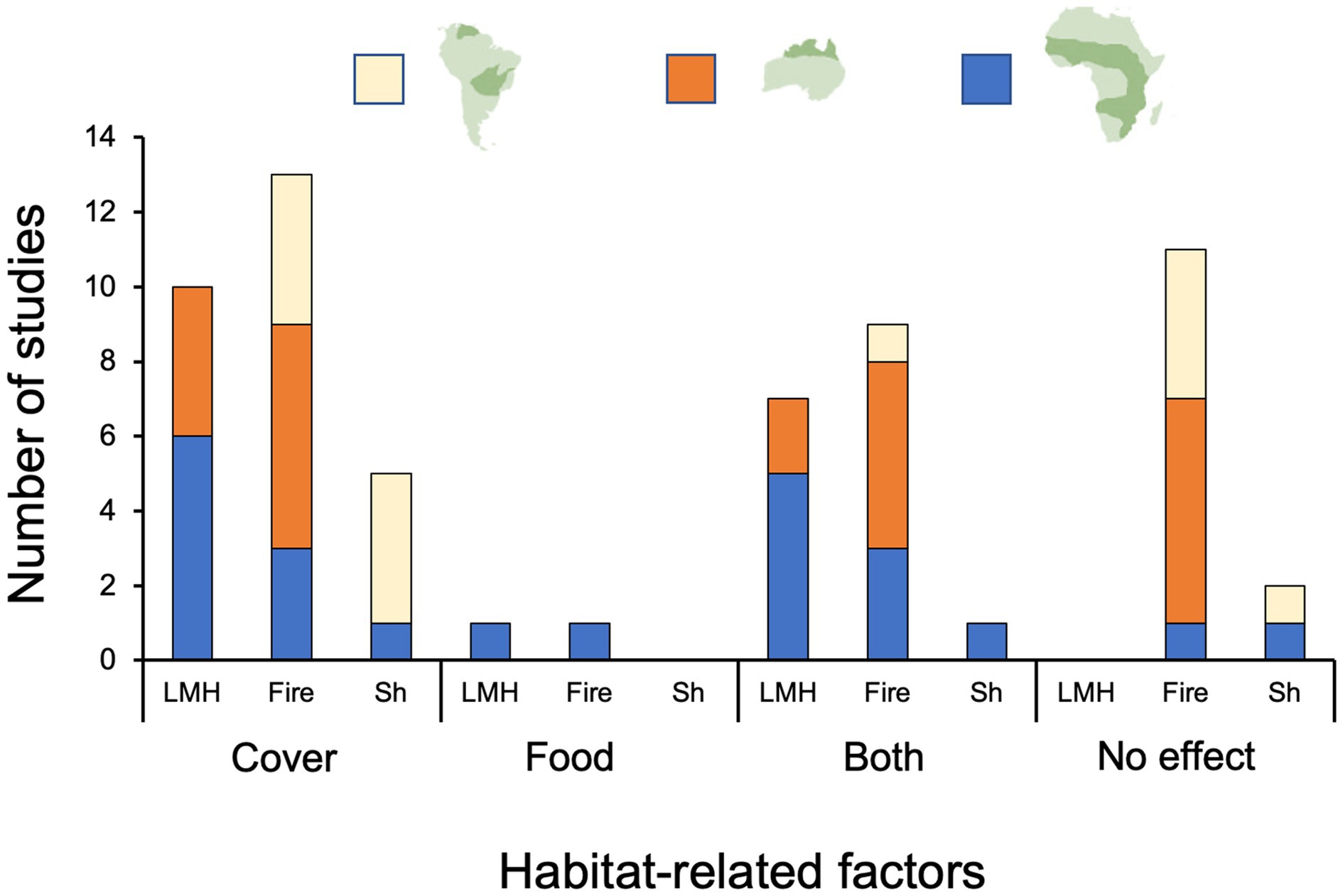
Figure 2. Number of published field studies from each region of tropical and subtropical savannas (beige: Neotropical; orange: northern Australian; blue: Afrotropical) in which either a conclusion was reached about which habitat-related factor—cover, food, or both—was the primary limiting factor for species or communities of non-volant small mammals or in which either no treatment effect was found or results were inconclusive (“No effect”). Studies scored “Both” included those in which some species were primarily limited by cover and others by food, as well as studies where there was evidence of both factors limiting small mammals (see Supplementary Table S1 for details). Manipulated or naturally varying factors affecting ground-level habitats and resources for small mammals were: (1) large mammalian herbivore reduction and/or removal (LMH; n = 18), (2) fire (Fi; n = 33), (3) shrub encroachment or reduction/removal (Sh; n = 8). This compilation includes substudies within individual papers for a total sample of 63. Additionally, two studies in Africa and one in South America assessed drought or rainfall seasonality effects (not included in the figure). The African studies indicated the role of “Cover” and “Both” while the South American study was inconclusive. Soil type (ST) and soil enrichment (SE)—which both concluded enhanced cover increased overall small-mammal abundance—were not included in the graph either, as only one study examined each of these treatment types (see “Summaries of Findings and Research Gaps by Region” and Supplementary Table S1).
Only six of the 63 studies (9.5%) of the effects of habitat manipulations on small mammals also simultaneously manipulated or otherwise tested changes in food quantity or quality or tested for dietary shifts related to the habitat variation (Supplementary Table S1). Of these six, three were inconclusive in that either the results were not significant or no direct linkage between habitat change and food availability or diet was established. The remaining three studies concluded that both cover (concealment from predators) and food resources were limiting factors of one or more small-mammal species that showed significant differences in abundance between/among habitat treatments (Supplementary Table S1). However, none of these studies of mostly omnivorous small mammals manipulated or measured changes in more than one food type, i.e., plant forage and insects, leaving alternative explanations for abundance changes a possibility in every case. Consequently, for all conclusive studies, we considered carefully the authors’ logical arguments and citations of other studies within their systems and involving the same small-mammal species to characterize what was most likely the primary limiting factor or factors.
We found no relevant field studies from savannas of southern Asia. For Afrotropical, Australian tropical, and Neotropical savannas, we found 21, 19, and 15 peer-reviewed papers, respectively that met our criteria. Some papers in each of these three regions tested multiple explanatory variables, yielding 63 studies overall: 24 for the Afrotropics, 24 for tropical Australia, and 15 for the Neotropics (Supplementary Table S1). Of these, 14 studies were inconclusive, and 49 studies reached definitive conclusions as to which factor(s) primarily limited either small mammals as a group or at least one species, singly; overall, 29 of these (58%) concluded that factor was cover, two (4%) that it was food resources, and 18 (37%) that it was a combination of food or cover (i.e., either some species were food-limited and others cover-limited, or both limiting factors played a partial role in limiting one or more species; Figure 2; Supplementary Table S1 reports categorized results of each study). Conclusions of studies from the Afrotropics aligned with this percentage allocation of limiting factors fairly closely and had a more diverse representation of explanatory variables than studies from the other two regions. Studies from Australian tropical savannas were nearly evenly divided between cover and “both” as limiting factors and, with one exception, explored only fire and LMH as factors impacting small-mammal habitat. Factors evaluated in the Neotropical savanna studies included fire, shrub encroachment, prey availability, and drought and concluded overwhelmingly (eight of 10 conclusive studies) that small mammals were limited by cover (Figure 2).
Effect sizes (in terms of treatment means) were not reported in many of the studies we reviewed (including 22 studies of fire and six studies of LMH). We did not glean effect sizes from regression analyses, which pertains to most of the conclusive Neotropical studies; but seven of nine of those studying fire reported significant R2. There were samples of >10 each of studies examining fire and LMH as explanatory variables that reported treatment effect sizes on small-mammal abundance (Supplementary Table S1). Significant effects of these two treatments on abundance ranged from 1.4 to 16 for individual studies, with the following mean effects ± S.E. from studies of fire (3.7 ± 0.6, n = 11) and LMH (5.0 ± 1.3, n = 12; multiple effect sizes from single studies were reported as a single arithmetic mean per study).
Studies from Afrotropical savannas were unique among the three regions in that diverse and abundant assemblages of native wild LMH were on the landscape in most cases, and their effects on terrestrial small-mammal habitat could potentially be distinguished from that of livestock. Ten experimental studies examined LMH-mediated habitat effects on small mammals and one directly manipulated grass height, as wild or domestic LMH would do. Of those 11 experimental studies, five concluded that small mammals responded positively to LMH removal primarily because of increased cover (or, in the one case, directly to experimentally increased cover as manifested by grass height; Bowland and Perrin, 1989; Saetnan and Skarpe, 2006; Young et al., 2015; Banasiak and Shrader, 2016; Bergstrom et al., 2018; Figure 3A; Supplementary Table S1). Of the four of these studies that experimentally reduced or removed LMH, three showed positive responses of small mammals to increased grass height and coverage, whereas one study failed to measure grass height but showed positive response to increased grass cover. Five of the 11 studies (scored “both”; Figure 2; Supplementary Table S1) either concluded that both cover and food were likely to have played a role in the habitat-mediated limitation of small mammals or could not rule out either the food-competition or cover (concealment) mechanism as a factor (Yarnell et al., 2007; Hagenah et al., 2009; Kuiper and Parker, 2013; Okullo et al., 2013; Long et al., 2017). Hagenah et al. (2009) was the only one of the published studies in our set from Africa reporting on small-mammal abundance or occupancy as a response variable that explicitly tested for quantity and quality of food resources simultaneous to their habitat manipulations (Supplementary Table S1), yet even this study did not directly examine insect abundance, which could have been affected by LMH treatment and could in turn have affected omnivorous small mammals.
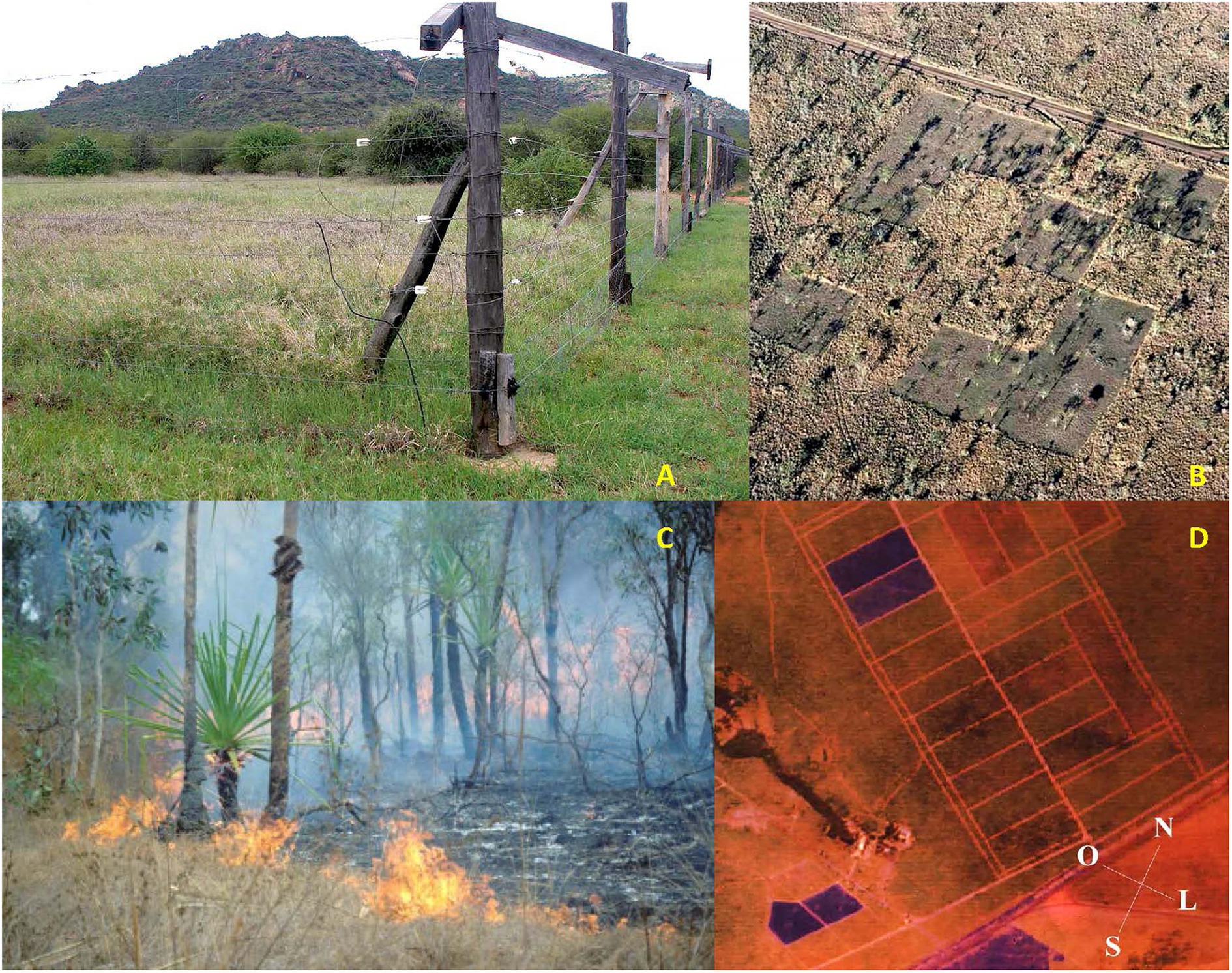
Figure 3. (A) Experimental LMH-exclusion plot on a glade (enriched soils of an abandoned cattle boma) on red sandy loam site at Mpala Research Center on the Laikipia Plateau of central Kenya. Electric fencing keeps LMH out but allows free passage of small rodents, shrews, and elephant shrews. Area outside the fence is an adjacent control plot. Overall abundance and diversity of small mammals was significantly greater inside exclosure than in the greener but shorter grazing lawn of the adjacent control (Bergstrom et al., 2018). Photo by BB. (B) Aerial photo of experimental “patchy” prescribed burn plot on whistling-thorn Acacia (Acacia drepanolobium) black cotton vertisol savanna habitat atop the escarpment at Mpala Research Center on the Laikipia Plateau of central Kenya. The 16-ha square area is divided into 1-ha pixels, nine of which have been burned, and seven unburned, and is surrounded by unburned matrix habitat. Small-mammal abundance was greater in unburned pixels, even 1–4 years after this photo was taken (Bergstrom et al., 2018). Photo courtesy of R. L. Sensenig. (C) Early-dry season wildfire on tropical savanna at Kapalga Research Station, Kakadu National Park, northern Australia (image taken by B. McKaige), which tends to be less severe and have less deleterious effects on small mammals than late-dry-season wildfire, from Griffiths and Brook (2015). (D) Aerial view of experimental plots of the “Fire Project” (Dias and Miranda, 2010), conducted between 1991 and 2011, in the Brazilian savanna (Cerrado). Rectangular areas in the image were 10-ha experimental plots covering typical Cerrado vegetation, burned with different fire frequencies (including every 2 years, every 4 years, and unburned control plots). Dark rectangles had just been burned when the image was taken (<1 month after burning). Two studies evaluated in the present review were conducted as part of the “Fire Project” (Vieira, 1999; Vieira and Briani, 2013).
Additionally, Yarnell et al. (2007) showed that small-mammal abundance was positively related to grass coverage and grass height, which both fire and grazing reduce in the short term. Moreover, Long et al. (2017) found that small mammals responded negatively to increased bare-soil coverage and decreased tree cover, although they failed to measure grass height. In the only one of these 11 experimental LMH-removal studies that concluded food availability (i.e., resource competition with LMH) was the primary limiting factor (Keesing, 1998), again, the study failed to measure grass height and found no differences in vegetative cover variables among the LMH treatment plots (which later surveys of the same plots did find; Young et al., 2015). Although an observational study, Muck and Zeller (2006) offered evidence that small-mammal abundance was positively correlated with grass height and coverage and that grazing by cattle leaves a basal layer of vegetation through which small mammals can tunnel, whereas grazing by wild ungulates and domestic sheep removes that layer.
Overall small-mammal responses to manipulated LMH, fire, and other habitat perturbations were not necessarily uniform across species in multi-species studies in which species were analyzed separately. Some African savanna small mammals were shown to prefer open or bare-soil habitats and had different responses to vegetative variables in some LMH experiments, as compared to other species and to overall small-mammal abundance or occupancy (which generally favored increased cover). This includes gerbils of certain species (Blaum et al., 2006), as well as elephant shrews and the murid mouse Steatomys pratensis (Saetnan and Skarpe, 2006). Some common small mammals, including spiny mice (Acomys spp.), did not respond significantly to LMH removal and its attendant increase in grass height and vegetative cover, probably because these small murid rodents preferentially occurred in or near rock outcrops and relied on rock crevices for cover (Bergstrom et al., 2018).
Of six experimental studies on small mammal responses to fire in African savannas, one was inconclusive (MacFayden et al., 2012), three concluded both cover and food were, or could be, limiting, and two concluded primarily cover was limiting (Supplementary Table S1; Figure 2). These disparate conclusions probably were influenced by the fact that species-specific life histories, in part, determined responses to fire, making some small mammals fire-positive and others fire-negative (Plavsic, 2014). Moreover, amount of time since the fire affected mammals’ responses (Yarnell et al., 2007), as did complex interactions with precipitation (Plavsic, 2014) and grazing (Yarnell et al., 2007; Bergstrom et al., 2018). The widespread, disturbance-tolerant, opportunistic feeding and often human-commensal multimammate mouse (Mastomys natalensis; see Avenant, 2000) showed no response to fire in a study where the cover-sensitive, diurnal grass rat (Arvicanthis niloticus; see Bergstrom et al., 2018) preferred unburned plots (Manyonyi et al., 2020; Supplementary Table S1 critiques authors’ erroneous conclusion that M. natalensis showed a significant response). The aforementioned inconclusive study (MacFayden et al., 2012) discussed the complex and unpredictable relationship of M. natalensis to fire. In addition to the experimental studies, suggestive non-experimental evidence that increased nutritional value of burned savanna becomes an overriding advantage for cover-sensitive small mammals several months after a burn was provided for one southern African savanna mouse (Steatomys pratensis; Monadjem, 1999). Heavy grazing by abundant native LMH was found to delay positive post-fire responses by small mammals for years (Bergstrom et al., 2018; Figure 3B), whereas high rainfall was found to speed up that outcome (Yarnell et al., 2007). An observational study using radiotelemetry found that small elephant shrews increased their use of thickets after surrounding grass cover was removed by fire (Yarnell et al., 2008).
Three experimental studies varied shrub cover in southern African savannas and recorded effects on native murid rodents: Blaum et al. (2006) found that three open-country gerbil species (murid rodents of Tribe Gerbillurini) avoided shrub cover because of increased food availability in open microhabitats, whereas another gerbil and a striped mouse (Rhabdomys pumilio) preferred intermediate shrub cover, where grass height was also greatest; and Loggins et al. (2019) found that most rodents had greater foraging activity in proximity to shrub cover where, again, grass height was tallest, but that Mastomys natalensis was cover-neutral or even cover-averse. Lloyd and Vetter (2019) found that shrub encroachment caused a shift in food habits in the generalist rodent Rhabdomys pumilio, but the study was otherwise inconclusive regarding the response variables for which we screened (see the section Methods).
Of five experimental studies examining livestock grazing effects on small mammals, three showed species-specific responses to increased herbaceous vegetative cover following destocking. In one, a smaller mouse (Pseudomys delicatulus) was grazing tolerant, whereas small-mammal abundance, generally, and abundance of two larger Pseudomys spp., P. desertor and P. gracilicaudatus, responded positively to increased cover (Kutt and Gordon, 2012). Two LMH studies were two-factor experiments including fire. In one, the endangered Pseudomys desertor declined due to cover loss, whether by grazing or fire. In contrast, P. delicatulus, an open-ground species, found enhanced forage after fire but responded negatively to grazing, and fire and grazing combined were synergistically deleterious (Kutt and Woinarski, 2007). Four smaller rodents and dasyurid marsupials showed stronger positive responses to destocking than larger species (one increasing about 10-fold 3 years after destocking). These positive responses could be explained by either increased cover mediated by feral cat predation or increased food resources, as allowed by the investigators (Legge et al., 2011). A large-sample observational study (n = 94) found that total small-mammal abundance and diversity increased, and mortality due to feral cats decreased, as three types of cover—rock, shrub, and perennial grass—increased, and as livestock grazing was reduced (Radford et al., 2021). A 13-year natural experiment with varying levels of livestock grazing and fire extent and intensity concluded that fire and domestic LMH each suppressed small mammals (murids and small dasyurid marsupials) and had a negative synergistic—but not additive—effect on overall small-mammal abundance and richness (but opposite effects on P. delicatulus compared to the four other commonly caught species; Legge et al., 2019). An observational study found that brush-tailed rabbit-rat occupancy was negatively associated with increased cattle stocking and concomitant decreased shrub cover and increased feral cat activity (Davies et al., 2017).
Of 17 studies of fire effects conducted in Northern Australia, 12 of which were experimental, six concluded that loss of cover explained significant small-mammal responses (Kutt and Woinarski, 2007; Legge et al., 2008; Kutt and Gordon, 2012; Leahy et al., 2015; Davies et al., 2017; Radford et al., 2021), five concluded that both cover and food resources were partly important as limiting factors (Lawes et al., 2015; McDonald et al., 2016; Ondei et al., 2020; Radford et al., 2020; Penton et al., 2021), and six studies were inconclusive (Pardon et al., 2003; Woinarski et al., 2004; Griffiths and Brook, 2014; Abom et al., 2016; Davies et al., 2018; Legge et al., 2019; Supplementary Table S1, Figure 2). One study found 90% direct mortality of small mammals from intense fires, with the individuals surviving being in unburned refuges (Legge et al., 2008). Two experimental studies were done at the Kapalga experimental site, where both fire frequency and fire intensity were manipulated, although none of them reported habitat (vegetation) variables; these collectively showed that severe declines in survival and recruitment of northern brown bandicoot (Isoodon macrourus) were exacerbated by more frequent fires and more intense fires (which occur later in the dry season; Pardon et al., 2003; Griffiths and Brook, 2015; Figure 3C). Varying fire intensity had no effect on grassland melomys (Melomys burtoni). Experimental studies on tropical savannas on Melville Island showed overall abundance of seven taxa of small mammals was greater with less frequent fire and a landscape consisting of mostly long-unburned patches (Davies et al., 2018); northern brown bandicoot and pale field mouse (Rattus tunneyi) had significantly greatest abundance in triennially burned patches (compared to annually burned and long-unburned), whereas grassland melomys had significantly greatest abundance in long-unburned patches, and other species’ abundances did not vary among fire frequencies.
Most of the Australian fire studies found indirect mortality after fires (e.g., due to loss of cover and increased predation; Leahy et al., 2015). The rock rat, Zyzomus pedunculatus, preferred burned areas, because it sought cover in rock crevices but foraged on early-successional herbaceous plants; whereas Pseudomys desertor preferred unburned grass hummocks both for cover and for forage (McDonald et al., 2016; which was the only Australian study to simultaneously measure food resources). A unique study using radio-tracking confirmed: (1) increased mortality by predation in burned areas, and (2) that mammals were not food-limited in burned areas because they did not lose body mass over time (Leahy et al., 2015). Two studies concluded that fire, especially of large extent and high intensity, altered vegetative structure, and simplified habitats, which has led to widespread endangerment of tropical savanna small mammals and reduced community diversity; this was probably due both to loss of cover and concomitant increased predation and to loss of food resources such as fruits and seeds (Lawes et al., 2015; Ondei et al., 2020). In an aforementioned study, which varied both LMH and fire, richness of small-mammal species was most affected by large-scale (100 km2) fire events and abundance by meso-scale fire (1 km2); and, once again, P. delicatulus had opposite (i.e., positive) responses to fire compared to the other commonly caught small mammals (Legge et al., 2019).
Two non-climate-change related anthropogenic impacts have made fires more catastrophic and thus more deleterious to native Australian small mammals: (1) decline of patchy, aboriginal fires, and (2) culling of water buffalo (Bubalus bubalis, which kept grasses clipped) in wet savannas (Ondei et al., 2020). Although focusing on a different set of mammals than most studies we reviewed, Penton et al. (2021) found that two larger, semi-arboreal rodents (brush-tailed rabbit-rat, Conilurus penicillatus, and black-footed tree-rat, Mesembriomys gouldii), which den in tree cavities (borne by larger, older trees, which are negatively affected by frequent, intense fires and other disturbances), were not limited by abundance of those trees or their cavities but rather by shrub cover (which is reduced by fire and livestock grazing) as protection against predators while they forage and move on the ground.
The 15 Neotropical studies meeting our criteria included only one manipulative experiment (Durigan et al., 2020; which had the bare minimum of two replicates), but 11 others were CM and three were Obs. Most of these studies investigated the effects of plant cover (mainly altered by fire events) on rodent populations. Two studies evaluated fire’s effects on food availability and thereby on rodent populations, but without measuring vegetative variables (Layme et al., 2004; Vieira and Briani, 2013). Using regression designs to investigate the same population of the murid Necromys lasiurus in an Amazonian savanna, two studies concluded that densities and population growth of this omnivorous rodent were strongly correlated with availability of invertebrate prey (Layme et al., 2004; which, however, was not related to vegetation structure in a study that examined both food and cover variables; Ghizoni et al., 2005).
Neotropical vegetative cover studies evaluated small-mammal responses at distinct habitat scales. At microhabitat scale (capture stations or sampling points spread over trapping areas ranging from 1.8 to 7.7 ha), there were mixed responses from murid rodents. Necromys lasiurus and Cerradomys scotti from open areas (shrubby grasslands and savannas with 10–60% of tree cover) responded positively to grass height, and Oxymcyterus roberti responded positively to forb ground cover, tree cover, and shrub cover, whereas Calomys tener responded negatively to grass height (Henriques and Alho, 1991; Vieira et al., 2005; Rocha et al., 2011). At a larger scale (40 plots spread over 200 km2), N. lasiurus densities and population growth rates in Amazonian savannas were significantly associated with principal component analysis (PCA) axes representing increasing tree, shrub, and small-grass cover (PC1) and increasing tall-grass cover (PC2) (Ghizoni et al., 2005). For community responses, the effects of habitat structure variables on community structure were evaluated in seven sites of Cerrado rocky fields by Santos and Henriques (2010), who reported a significant relationship between plant complexity (a PCA axis representing a gradient with an increase in plant height and in number of plants) and community composition (first Multidimensional Scaling—MDS axis) but no clear relation between habitat structure and small mammal richness.
Nine of our 15 Neotropical studies evaluated effects of fire on small savanna mammals. These studies varied in terms of temporal and spatial scale analyzed and in relation to the analytical approach used, which precluded unambiguous summaries of fire effects on small mammals. Some common patterns, however, were highlighted. Direct mortality caused by fire was not commonly found, probably because small mammals found refuge in burrows (mainly made by armadillos; Vieira and Marinho-Filho, 1998; Vieira, 1999). Short-term responses to fire were reported for some species, including the murid rodents Oxymycterus roberti and Necromys lasiurus in grasslands, which showed abrupt reductions in abundance after fire (fire caused a drastic reduction of grass cover). Abundance of both murid species increased as grass recovered after fire (Vieira and Marinho-Filho, 1998). In typical Neotropical savanna habitats, at local scales (burned areas of 10 ha), population size of N. lasiurus reduced drastically up to 4–6 months after fire, generally recovering to pre-fire numbers after this period (Vieira, 1999; Owen, 2013; Vieira and Briani, 2013; Figure 3D). This post-fire reduction in abundance was not caused solely by food limitation, as invertebrates were still available in burned areas (Vieira and Briani, 2013). Necromys lasiurus, at much larger spatial scales (distinct 4-ha plots covering about 100 km2), did not show significant changes in population densities related to fire-induced abrupt reduction in plant cover (Layme et al., 2004).
Responses of small-mammal communities to fire changed depending on the time elapsed since fire when they were evaluated. Briani et al. (2004) evaluated six sites with distinct fire histories and reported a strong negative correlation (r = −0.81) between community abundance and time since the last fire (1–26 years) in typical Neotropical savanna (i.e., cerrado sensu stricto). Studies conducted at small temporal scales (up to 1 year after fire), however, failed to show reduction in overall abundance or richness of small mammals (Vieira, 1999; Durigan et al., 2020). On the whole, fire in the Cerrado affected small-mammal communities mainly by temporary changes in dominance patterns within communities occurring in habitat types with sparse to moderate tree cover (Vieira, 1999). In areas of Cerrado dry woodlands, however, fire tended to increase species homogenization. The occurrence of fire in Cerrado dry woodlands (locally known as “cerradão”) reduced tree cover and increased patchiness of these formations, allowing open-area terrestrial rodents to invade such habitats (Camargo et al., 2018).
While not directly examining fire, Furtado et al. (2021) found that shrub encroachment resulting from years of fire suppression in the southeastern Cerrado is changing the small-mammal community composition from open-country or grassland specialists (e.g., Necromys lasiurus and Cryptonanus spp.) to closed-canopy forest specialists (e.g., Didelphis albiventris and Oligoryzomys nigripes).
Unlike the Afrotropics, Neotropical and Australian tropical savannas have not had a significant native LMH presence for roughly the past 10,000 and 45,000 years, respectively (Malhi et al., 2016). The role of extinct megaherbivores may have been replaced, at least partially, by domestic herbivores, and although this issue has been investigated somewhat in Australian tropical savanna small-mammal studies, it has not been adequately investigated with respect to small-mammal habitat in Neotropical savannas. Another future research need for Neotropical savanna small-mammal habitat responses is manipulative field experiments on the effects of endogenous disturbances on small-mammal community structure, of which we found only one (and that with only two replicates; Supplementary Table S1). In African savannas, there is some evidence of additive negative effects of cattle and native LMH on vegetative cover and small-mammal abundance (Bergstrom et al., 2018), and cattle grazing in the absence of native LMH can be more conducive to cover-sensitive small mammals than native LMH grazing in the absence of cattle (Muck and Zeller, 2006). We recognize, however, that stocking rates and grazing strategies can vary widely between regions and cultures and interact differently with different habitats, vegetation, climates, and native fauna. Very few studies exist that examine effects of differential stocking rates on any native fauna, much less on small mammals (Wells et al., 2021). A recent global review found that domestic LMH grazing suppresses a wide variety of native bird and mammal populations but found very few studies focused on small mammals (Schieltz and Rubenstein, 2016).
In different ways, LMH grazing and browsing, fire, and drought can all be considered disturbances that reduce above-ground plant biomass and alter structure, nutrition, and species composition of tropical savanna plant communities, and this in turn affects the small-mammal community. Partial to complete shrub removal (which can mirror effects of fire or heavy browsing in Africa) was found indirectly to cause grass-cover reduction, which caused most small rodents to decrease their foraging time (Loggins et al., 2019). Although some species of small mammals respond positively to these disturbances [e.g., some gerbils and the generally disturbance-tolerant Mastomys spp. in Africa, Calomys expulsus (= callosus) in the Cerrado, Pseudomys delicatulis in Australian tropical savannas, and other open-ground inhabitants, which, importantly, are nocturnal and have cursorial and/or burrowing adaptations that aid their escape from predators], our review found that most species, and small-mammal abundance, overall, responded negatively, at least in the short term, to these disturbances (see also Andersen et al., 2005; Griffiths and Brook, 2015; Stobo-Wilson et al., 2020; Andersen, 2021). Thus, a “landscape of fear” (Laundré et al., 2010) on heavily grazed and recently burned areas may cause many less-cursorial small mammals to avoid them or reduce their time spent there due to a perception of increased predation risk, and from some evidence an actual increase in predation risk (Leahy et al., 2015). Supporting this conclusion is that, across three tropical continents, the commonality of negative response by most non-volant savanna small mammals to these disparate types of disturbances was most often concluded to be primarily or at least partially related to loss of cover. The best direct evidence of increased predation (especially by feral cats) being the mechanism by which reduced cover from fire and/or LMH increases mortality rates and extirpations of small mammals comes from Australian TSE studies (Frank et al., 2014; McGregor et al., 2014, 2015; Hohnen et al., 2016; Tuft et al., 2021). But in the other two TSE regions, this same conclusion can be inferred from two frequent observations: (1) most small mammals avoid recently burned areas and heavily grazed areas even though forage palatability and nutrient content is usually higher in these areas (Monadjem, 1999; Yarnell et al., 2007), and (2) in small murid rodents, the affinity to high-cover sites is particularly strong among the few diurnal species (e.g., Arvicanthis niloticus; Bergstrom et al., 2018; Manyonyi et al., 2020 and Necromys lasiurus; Ghizoni et al., 2005; Vieira et al., 2005). These diurnal rodents are most susceptible to predation by visual hunters such as birds of prey, and their fine-scale foraging and home ranges are often limited precisely by the boundaries of these “overgrown” refuges (Vieira et al., 2005; Whittington-Jones et al., 2008; Bergstrom et al., 2018).
Most studies concluding that loss of cover was the predominant reason for small-mammal declines due to LMH grazing or recent fire (as did the majority of those we reviewed) did not rule out shortage of food resources as a contributing or simultaneous factor, especially for omnivorous small mammals for which fruit, seeds, and insects are important in the diet (see Bergstrom, 2013; Vieira and Briani, 2013). Taller grass protected from grazing in Kenyan (Pringle et al., 2007) and South African (Jonsson et al., 2010), savannas has been found to have greater arthropod diversity and abundance. Somewhat contrastingly, in Neotropical savannas, the temporary post-fire replacement of the diurnal N. lasiurus by nocturnal rodents of the genus Calomys as dominant species was related to both a reduction in plant cover and a relatively higher availability of invertebrates (compared with plant resources) in just-burned areas (Vieira, 1999; Owen, 2013; Vieira and Briani, 2013). The diurnal Necromys lasiurus is probably more affected by the fire-induced reduction in plant cover and consequent increase in risk of predation by visually oriented raptors. In contrast, the nocturnal Calomys tener and especially C. expulsus are probably less affected by the reduction in plant cover and able to take advantage of the invertebrate increase, consuming more invertebrates and becoming more abundant after burning than before burning (Vieira and Briani, 2013).
Reduction of conifer seeds as a food source in burned Australian savannas was mentioned as a possible explanation for reduced small-mammal abundance in addition to loss of cover (Lawes et al., 2015). The grass hummock specialist in Australian tropical savannas, Pseudomys desertor, was found to prefer unburned grass hummocks within a fire matrix for both food and protection from feral cat predation (McDonald et al., 2016), the latter because it was thought cats would have reduced hunting efficacy in this habitat structure.
Very few studies in our sample concluded that food reduction, alone, and not cover loss, or a combination of the two, was the primary limiting factor for small mammals when habitats underwent fire, drought, or heavy grazing (recall that only six studies measured or manipulated food resources simultaneous to habitat manipulations or measurements). Only one African study that experimentally reduced LMH grazing concluded, inferentially, that food availability was the primary limiting factor for small mammals when LMH were present (Keesing, 1998). In part, this inference was based on the lack of difference in percent vegetative cover between recently established experimental and control plots, but the study did not measure a variable that many others have found critical—grass height. Consider that a close-cropped lawn may have 100% coverage of herbaceous vegetation but 0% visual cover to reduce perception of (or, indeed, actual) vulnerability to predation (see Figure 3A). In fact, grazing lawns are a feature of African savannas with a history of nomadic pastoralism (Veldhuis et al., 2014) and are also a feature of recently burned Australian tropical savannas where livestock are grazed (Bond and Keeley, 2005). In the only African experimental LMH study that explicitly tested both food quantity and quality and vegetative cover and structure, Hagenah et al. (2009) showed how meso-herbivores remove the higher food-value lawn grasses, whereas larger LMH reduce the height of taller, less nutritious grasses that are more useful for visual cover; therefore, a full complement of native African LMH can reduce both food and cover resources for small mammals.
Nine separate studies from African savannas, some of them experimentally manipulating multiple habitat-altering factors, concluded that those manipulations (grazing, fire, and drought) did significantly reduce grass (or vegetation) height, and that grass-height differences between treatments did significantly affect small-mammal species and/or communities (Blaum et al., 2006; Muck and Zeller, 2006; Saetnan and Skarpe, 2006; Plavsic, 2014; Yarnell et al., 2007; Kuiper and Parker, 2013; Banasiak and Shrader, 2016; Bergstrom et al., 2018; Loggins et al., 2019). All of these studies concluded that cover was either the primary, or an important contributing factor explaining the response of the small-mammal assemblage and of the individual species, but especially of those species whose responses to grass height were positive (and thus were grazing-negative and short-term fire-negative). It is important to note that some studies in our review (e.g., Durigan et al., 2020; Supplementary Table S1) found no effect of fire on small-mammal species richness or abundance.
In studies reviewed herein, as a rule, endogenous disturbances in TSEs—especially fire, LMH, and drought—reduced small-mammal abundance or occupancy at least in the short term, due to loss of cover, which led either to loss of concealment from predators and/or unfavorable microclimates. Increased predation in burned and heavily grazed areas in Australian tropical savannas has been established by several studies detailed in this review, including direct confirmation of that predation by radiotelemetry. Under anthropogenically exacerbated disturbance regimes that Australia is currently experiencing, with attendant loss of vegetative cover, feral cat populations are increasing in its tropical savannas (Davies et al., 2020), and increased predation on declining small mammals has been linked to intensively burned areas (Leahy et al., 2015). To a much greater degree (as currently known) than in African or Neotropical TSE, endemic TSE small mammals in Australia are declining, and many of them are endangered (Woinarski et al., 2011). So it is particularly important to understand what landscape factors combine to offer them the best chance of recovery. One prescription is to preserve unburned and ungrazed refuges of vegetative cover for at least 4 years (Radford et al., 2015, 2021). This will be critical as long as climate change-driven drought and wildfire, and uncontrolled feral cat predation, continue unabated (Woolley et al., 2019; Hale et al., 2021).
Twenty of the studies we reviewed reported effect sizes, overall, on small mammals averaging 4–5-fold in response to LMH and fire in tropical savannas. Small mammals’ generally negative responses (as noted previously, certain species are “increasers” in response to disturbance) to both disturbances point to both positive and negative consequences: positive in the prevention of irruptions of some small-mammal species that are important reservoirs of zoonotic disease; and negative in that some once-common species of TSE small mammals are now threatened by loss of understory habitat (cover); that community diversity is being suppressed; and that threats to already rare, cryptic, or understudied small mammal species will emerge. Native small mammals have coevolved with large mammalian herbivory and natural (often aboriginal) fire in all geographic regions of TSE, but increased intensity and frequency of fire under current and forecast conditions of anthropogenic climate change—in the face of negative synergistic effects of overgrazing by domestic LMH, and of drought—pose a challenge for us to monitor, much less predict how small mammals will fare under this new reality.
In all regions, managers should increase use of prescribed fire to forestall catastrophic wildfires, and researchers should establish refuges against domestic LMH (and more experimental exclosures against native LMH) to begin to understand the potential diversity of small mammals that exists in TSE. For example, more than 60 new species of rodents were described from the Afro-Malagasy region between 1989 and 2018 (Taylor et al., 2019). The most speciose genus of mammal is the shrew genus Crocidura (Family Soricidae, Order Eulipotyphla), of which an unknown number (though more than 100) of species occur in Africa, many of which are not identifiable morphologically and are little known ecologically, because they are difficult even to census. But they are important secondary consumers and prey species of TSE; some are also known reservoirs for hantavirus and other zoonotic disease, and one is critically endangered (Igbokwe et al., 2019). As much as additional study of the effects of endogenous disturbances on murid rodents of TSE is needed, the need for similar studies of these cryptic shrews—of which almost no ecological studies exist—is even greater. Similarly, 118 species of small mammals occur across the Cerrado savanna region of Brazil, with local richness as high as 26, yet N. lasiurus represented >20% of individuals captured in the aggregate of 96 field studies (Mendonça et al., 2018); this, and only a handful of other small-mammal species are the focus of habitat-related studies we found for the current review, meaning that responses to habitat perturbations by most native Cerrado small-mammal species are unknown. Importantly, Furtado et al. (2021) fills some of this knowledge gap for seven species of small mammals of the Cerrado. Finally, as we mentioned earlier, we found no studies of habitat effects on small mammals from South Asian TSE at all.
We recognize the limitations of this narrative, first-ever global review of field studies on this topic. Only 14 of the 63 studies we reviewed were manipulative experiments. Experimental designs, spatial and temporal scales of the study, field methodologies, analytical techniques, and degree of replication varied widely among those experiments. For all studies, whether experimental or not, the nature of herbivory varies by species of large mammal, and the behavior of fire varies across vegetative biomes and with temporally varying environmental conditions in any given biome. The above disparities make direct comparisons among studies challenging. Finally, there is every possibility that one or more forms of publication bias (Lortie et al., 2007) constrains the sample of field datasets that is published and, if published, is discoverable via our search methods. This may mean that studies that found no treatment effect are especially underrepresented. It is our hope that this initial review will inspire further studies, especially experimental ones that fill the abovementioned gaps and that, in future, a more systematic review and meta-analysis of experimental findings may be possible, enabling clearer conclusions of how endogenous disturbances affect small-mammal community structure in all TSE regions.
BB conceived the review and wrote much of the first draft, with the contribution of EV. BB and EV conferred on and contributed to several revised drafts. BB, EV, and SS compiled potential studies, and BB and EV decided on which to include and proofed the manuscript. SS compiled the initial spreadsheet and bibliography. All authors contributed to the article and approved the submitted version.
The Valdosta State University Foundation financed publication costs of this article. Rossano M. Ramos generated the map for Figure 1.
Two reviewers made helpful suggestions on a previous version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2023.1017361/full#supplementary-material
SUPPLEMENTARY TABLE S1 | Spreadsheet summarizing methods and findings of 63 published field studies of effects of endogenous disturbances on small-mammal communities in tropical savanna ecosystems. Details of explanatory and response variables, study type, disturbance type, effects and effect sizes, and authors’ conclusions about mechanism(s) for significant responses.
SUPPLEMENTARY TABLE S2 | Bibliography on habitat responses of tropical savanna small-mammal communities, and related studies and sources, including other reviews.
LMH, Large mammalian herbivores; TSE, Tropical savanna ecosystems.
Abom, R., Parsons, S. A., and Schwarzkopf, L. (2016). Complex mammal species responses to fire in a native tropical savannah invaded by non-native grader grass (Themeda quadrivalvis). Biol. Invasions 18, 3319–3332. doi: 10.1007/s10530-016-1224-0
Andersen, A. N. (2021). Faunal responses to fire in Australian tropical savannas: insights from field experiments and their lessons for conservation management. Divers. Distrib. 27, 828–843. doi: 10.1111/ddi.13198
Andersen, A. N., Cook, G. D., Corbett, K. K., Douglas, M. M., Eager, R. W., Russell-Smith, J., et al. (2005). Fire frequency and biodiversity conservation in Australian tropical savannas: implications from the Kapalga fire experiment. Austral. Ecol. 30, 155–167. doi: 10.1111/j.1442-9993.2005.01441.x
Andersen, A., Cook, G. D., and Williams, R. J. (eds.) (2003). Fire in Tropical Savannas: The Kapalga Experiment. Ecological Studies. New York: Springer-Verlag
Archibald, S., and Hempson, G. P. (2016). Competing consumers: contrasting the patterns and impacts of fire and mammalian herbivory in Africa. Philos. Trans. Roy. Soc. B. Biol. Sci. 371:20150309. doi: 10.1098/rstb.2015.0309
Augustine, D. J. (2010). Response of native ungulates to drought in semi-arid Kenyan rangeland. Afr. J. Ecol. 48, 1009–1020. doi: 10.1111/j.1365-2028.2010.01207.x
Avenant, N. L. (2000). Small mammal community characteristics as indicators of ecological disturbance in the Willem Pretorius nature reserve, Free State, South Africa. S. Afr. J. Wildl. Res. 30, 26–33. doi: 10.10520/EJC117089
Banasiak, N., and Shrader, A. M. (2016). Similarities in perceived predation risk prevent temporal partitioning of food by rodents in an African grassland. J. Mammal. 97, 483–489. doi: 10.1093/jmammal/gyv192
Bergstrom, B. J. (2013). Would east African savanna rodents inhibit woody encroachment? J. Mammal. 94, 436–447. doi: 10.1644/12-MAMM-A-146.1
Bergstrom, B. J., Sensenig, R. L., Augustine, D. J., and Young, T. P. (2018). Searching for cover: soil enrichment and herbivore exclusion, not fire, enhance African savanna small-mammal abundance. Ecosphere 9:e02519. doi: 10.1002/ecs2.2519
Blaum, N., Rossmanith, E., and Jeltsch, F. (2006). Land use affects rodent communities in Kalahari savannah rangelands. Afr. J. Ecol. 45, 189–195. doi: 10.1111/j.1365-2028.2006.00696.x
Bond, W. J., and Keeley, J. E. (2005). Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends Ecol. Evol. 20, 387–394. doi: 10.1016/j.tree.2005.04.025
Bowland, A. E., and Perrin, M. R. (1989). The effect of overgrazing on the small mammals in Umfolozi game reserve. Zeitschrift für Säugetierkunde 54, 251–260.
Briani, D. C., Palma, A. R. T., Vieira, E. M., and Henriques, R. P. B. (2004). Post-fire succession of small mammals in the Cerrado of Central Brazil. Biodivers. Conserv. 13, 1023–1037. doi: 10.1023/B:BIOC.0000014467.27138.0b
Brown, J. S. (1988). Patch use as an indicator of habitat preference, predation risk, and competition. Behav. Ecol. Sociobiol. 22, 37–47. doi: 10.1007/BF00395696
Buisson, E., Stradic, S. L., Silveira, F. A. O., Durigan, G., Overbeck, G. E., Fidelis, A., et al. (2019). Resilience and restoration of tropical and subtropical grasslands, savannas, and grassy woodlands. Biol. Rev. 94, 590–609. doi: 10.1111/brv.12470
Byrom, A. E., Craft, M. E., Durant, S. M., Nkwabi, A. J. K., Metzger, K., Hampson, K., et al. (2014). Episodic outbreaks of small mammals influence predator community dynamics in an east African savanna ecosystem. Oikos 123, 1014–1024. doi: 10.1111/oik.00962
Camargo, A. C. L., Barrio, R. O. L., Camargo, N. F., Mendonça, A. F., Ribeiro, J. F., Rodrigues, C. M. F., et al. (2018). Fire affects the occurrence of small mammals at distinct spatial scales in a neotropical savanna. Eur. J. Wildl. Res. 64, 1–11. doi: 10.1007/s10344-018-1224-8
Charles, G., Porensky, L. M., Riginos, C., Veblen, K. E., and Young, T. P. (2016). Herbivore effects on productivity vary by guild: cattle increase mean productivity while wildlife reduce variability. Ecol. Appl. 27, 143–155. doi: 10.1002/eap.1422
Costa, A. N., Vasconcelos, H. L., Vieira-Neto, E. H. M., and Bruna, E. M. (2008). Do herbivores exert top-down effects in Neotropical savannas? Estimates of biomass consumption by leaf-cutter ants. J. Veg. Sci. 19, 849–854. doi: 10.3170/2008-8-18461
Davies, H. F., Maier, S. W., and Murphy, B. P. (2020). Feral cats are more abundant under severe disturbance regimes in an Australian tropical savanna. Wildl. Res. 47:624. doi: 10.1071/WR19198
Davies, H. F., McCarthy, M. A., Firth, R. S. C., Woinarski, J. C. Z., Gillespie, G. R., Andersen, A. N., et al. (2017). Top-down control of species distributions: feral cats driving the regional extinction of a threatened rodent in northern Australia. Divers. Distrib. 23, 272–283. doi: 10.1111/ddi.12522
Davies, H. F., McCarthy, M. A., Rioli, W., Puruntatameri, J., Roberts, W., Kerinaiua, C., et al. (2018). An experimental test of whether pyrodiversity promotes mammal diversity in a northern Australian savanna. J. Appl. Ecol. 55, 2124–2134. doi: 10.1111/1365-2664.13170
Dias, B. F. S., and Miranda, H. S. (2010). “O Projeto Fogo (the long-term Cerrado fire experiment in Brasilia)” in Efeitos do Regime de Fogo Sobre a Estrutura de Comunidades de Cerrado: Projeto Fogo. ed. H. S. Miranda (Brasília, Brazil: IBAMA), 15–22.
Durigan, G., Pilon, N. A. L., Abreu, R. C. R., Hoffmann, W. A., Martins, M., Fiorillo, B. F., et al. (2020). No net loss of species diversity after prescribed fires in the Brazilian savanna. Front. For. Glob. Change 3:13. doi: 10.3389/ffgc.2020.00013
Frank, A. S. K., Johnson, C. N., Potts, J. M., Fisher, A., Lawes, M. J., Woinarski, J. C. Z., et al. (2014). Experimental evidence that feral cats cause local extirpation of small mammals in Australia's tropical savannas. J. Appl. Ecol. 51, 1486–1493. doi: 10.1111/1365-2664.12323
Furtado, L. O., Felicio, G. R., Lemos, P. R., Christianini, A. V., Martins, M., and Carmignotto, A. P. (2021). Winners and losers: how woody encroachment is changing the small mammal community structure in a neotropical savanna. Front. Ecol. Evol. 9:774744. doi: 10.3389/fevo.2021.774744
Ghizoni, I. R. Jr., Layme, V. M. G., Lima, A. P., and Magnusson, W. E. (2005). Spatially explicit population dynamics in a declining population of the tropical rodent Bolomys lasiurus. J. Mammal. 86, 677–682. doi: 10.1644/1545-1542(2005)086[0677:SEPDIA]2.0.CO;2
Giglio, L., Justice, C., Boschetti, L., and Roy, D. (2021). MODIS/Terra+Aqua Burned Area Monthly L3 Global 500m SIN Grid V061, distributed by NASA EOSDIS Land Processes DAAC. Available at: https://doi.org/10.5067/MODIS/MCD64A1.061 (Accessed January 21, 2023).
Grace, J., José, J. S., Meir, P., Miranda, H. S., and Montes, R. A. (2006). Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 33, 387–400. doi: 10.1111/j.1365-2699.2005.01448.x
Griffiths, A. D., and Brook, B. W. (2014). Effect of fire on small mammals: A systematic review. Int J Wildland Fire 23, 1034–1043. doi: 10.1071/WF14026
Griffiths, A. D., and Brook, B. W. (2015). Fire impacts recruitment more than survival of small mammals in a tropical savanna. Ecosphere 6, art99–art22. doi: 10.1890/ES14-00519.1
Hagenah, N., and Bennett, N. C. (2013). Mole rats act as ecosystem engineers within a biodiversity hotspot, the cape fynbos. J. Zool. 289, 19–26. doi: 10.1111/j.1469-7998.2012.00958.x
Hagenah, N., Prins, H. H. T., and Olff, H. (2009). Effects of large herbivores on murid rodents in a south African savanna. J. Trop. Ecol. 25, 483–492. doi: 10.1017/S0266467409990046
Hale, S., Mendoza, L., Yeatman, T., Cooke, R., Doherty, T., Nimmo, D., et al. (2021). Evidence that post-fire recovery of small mammals occurs primarily via in situ survival. Divers. Distrib. 28, 404–416. doi: 10.1111/ddi.13283
He, T., Lamont, B. B., and Pausas, J. G. (2019). Fire as a key driver of Earth's biodiversity. Biol. Rev. 94, 1983–2010. doi: 10.1111/brv.12544
Henriques, R., and Alho, C. J. R. (1991). Microhabitat selection by two rodent species in the Cerrado Central Brazil. Mammalia 55, 49–56. doi: 10.1515/mamm.1991.55.1.49
Hohnen, R., Tuft, K., Mcgregor, H. W., Legge, S., Radford, I. J., and Johnson, C. N. (2016). Occupancy of the invasive feral cat varies with habitat complexity. PLoS One 11:e0152520. doi: 10.1371/journal.pone.0152520
Hurlbert, S. H. (1984). Pseudoreplication and the design of ecological field experiments. Ecol. Monogr. 54, 187–211. doi: 10.2307/1942661
Igbokwe, J., Nicolas, V., Oyeyiola, A., Obadare, A., Adesina, A. S., Awodiran, M. O., et al. (2019). Molecular taxonomy of Crocidura species (Eulipotyphla: Soricidae) in a key biogeographical region for African shrews, Nigeria. C. R. Biol. 342, 108–117. doi: 10.1016/j.crvi.2019.03.004
Jonsson, M., Bell, D., Hjältén, J., Rooke, T., and Scogings, P. F. (2010). Do mammalian herbivores influence invertebrate communities via changes in the vegetation? Results from a preliminary survey in Kruger National Park, South Africa. Afric. J. Range Forage Sci. 27, 39–44. doi: 10.2989/10220111003703468
Keesing, F. (1998). Impacts of ungulates on the demography and diversity of small mammals in Central Kenya. Oecologia 116, 381–389. doi: 10.1007/s004420050601
Kimuyu, D. M., Sensenig, R. L., Chira, R. M., Githaiga, J. M., and Young, T. P. (2017). Spatial scales influence long-term response of herbivores to prescribed burning in a savanna ecosystem. Int. J. Wildland Fire 26, 287–295. doi: 10.1071/WF16152
Kuiper, T. R., and Parker, D. M. (2013). Grass height is the determinant of sheep grazing. effects on small mammals in a savanna ecosystem. Rangeland J. 35, 403–408. doi: 10.1071/RJ13063
Kumar, D., Pfeiffer, M., Gaillard, C., Langan, L., Martensa, C., and Scheitera, S. (2020). Misinterpretation of Asian savannas as degraded forest can mislead management and conservation policy under climate change. Biol. Conserv. 241:108293. doi: 10.1016/j.biocon.2019.108293
Kutt, A. S., and Gordon, C. E. (2012). Variation in terrestrial mammal abundance on pastoral and conservation land tenures in north-eastern Australian tropical savannas. Anim. Conserv. 15, 416–425. doi: 10.1111/j.1469-1795.2012.00530.x
Kutt, A. S., and Woinarski, J. C. Z. (2007). The effects of grazing and fire on vegetation and the vertebrate assemblage in a tropical savanna woodland in north-eastern Australia. J. Trop. Ecol. 23, 95–106. doi: 10.1017/S0266467406003579
Lamberto, J., and Leiner, N. O. (2019). Broad-headed spiny rats (Clyomys laticeps) as ecosystem engineers in the Brazilian savannah. J. Zool. 309, 60–68. doi: 10.1111/jzo.12684
Laundré, J. W., Hernández, L., and Ripple, W. J. (2010). The landscape of fear: ecological implications of being afraid. Open Ecol. J. 3, 1–7. doi: 10.2174/1874213001003030001
Lawes, M. J., Murphy, B. P., Fisher, A., Woinarski, J. C. Z., Edwards, A. C., and Russel-Smith, J. (2015). Small mammals decline with increasing fire extent in northern Australia: evidence from long-term monitoring in Kakadu National Park. Int. J. Wildland Fire 24:712. doi: 10.1071/WF14163
Layme, V. M. G., Lima, A. P., and Magnusson, W. E. (2004). Effects of fire, food availability and vegetation on the distribution of the rodent Bolomys lasiurus in an Amazonian savanna. J. Trop. Ecol. 20, 183–187. doi: 10.1017/S0266467403001263
Leahy, L., Legge, S. M., Tuft, K., McGregor, H. W., Barmuta, L. A., Jones, M. E., et al. (2015). Amplified predation after fire suppresses rodent populations in Australia’s tropical savannas. Wildl. Res. 42, 705–771. doi: 10.1071/WR15011
Lecompte, E., Fichet-Calvet, E., Daffis, S., Koulemou, K., Sylla, O., Kourouma, F., et al. (2006). Mastomys natalensis and Lassa fever, West Africa. Emerg. Infect. Dis. 12, 1971–1974. doi: 10.3201/eid1212.060812
Legge, S., Kennedy, M. S., Lloyd, R., Murphy, S. A., and Fisher, A. (2011). Rapid recovery of mammal fauna in the Central Kimberley, northern Australia, following the removal of introduced herbivores. Anim. Ecol. 36, 791–799. doi: 10.1111/j.1442-9993.2010.02218.x
Legge, S., Murphy, S., Heathcote, J., Flaxman, E., Augusteyn, J., and Crossman, M. (2008). The short-term effects of an extensive and high intensity fire on vertebrates in the tropical savannas of the Central Kimberley, northern Australia. Wildl. Res. 35, 33–43. doi: 10.1071/WR07016
Legge, S., Smith, J. G., James, A., Tuft, K. D., Webb, T., and Woinarski, J. C. Z. (2019). Interactions among threats affect conservation management outcomes: livestock grazing removes the benefits of fire management for small mammals in Australian tropical savannas. Conserv. Sci. Pract. 1:e52. doi: 10.1111/csp2.52
Limongi, J. E., Oliveira, R. C., Guterres, A., Costa Neto, S. F., Fernandes, J., Vicente, L. H., et al. (2016). Hantavirus pulmonary syndrome and rodent reservoirs in the savanna-like biome of Brazil's southeastern region. Epidemiol. Infect. 144, 1107–1116. doi: 10.1017/s095026881500237x
Lloyd, K. J., and Vetter, S. (2019). Generalist trophic ecology in a changing habitat: The case of the four-striped mouse in a woody-encroached savannah. African Journal of Ecology 57, 371–381. doi: 10.1111/aje.12613
Loggins, A. A., Shrader, A. M., Monadjem, A., and McCleery, R. A. (2019). Shrub cover homogenizes small mammals’ activity and perceived predation risk. Sci. Rep. 9:16857. doi: 10.1038/s41598-019-53071-y
Long, R. A., Wambua, A., Goheen, J. R., Palmer, T. M., and Pringle, R. M. (2017). Climatic variation modulates the indirect effects of large herbivores on small-mammal habitat use. J. Anim. Ecol. 86, 739–748. doi: 10.1111/1365-2656.12669
Lortie, C. J., Aarssen, L. W., Budden, A. E., Koricheva, J. K., Leimu, R., and Tregenza, T. (2007). Publication bias and merit in ecology. Oikos 116, 1247–1253. doi: 10.1111/j.0030-1299.2007.15686.x
Luis, A. D., Hayman, D. T. S., O’Shea, T. J., Cryan, P. M., Gilbert, A. T., Pulliam, J. R. C., et al. (2013). A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. R. Soc. B 280:20122753. doi: 10.1098/rspb.2012.2753
Macfayden, S., Gibson, R. H., Symondson, W. O. C., and Memmott, J. (2012). Landscape structure influences modularity patterns in farm food webs: consequences for pest control. Ecol. Appl. 21, 516–524. doi: 10.1890/09-2111.1
Malhi, Y., Doughty, C. E., Galetti, M., Smith, F. A., Svenning, J., and Terborgh, J. W. (2016). Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. 113, 838–846. doi: 10.1073/pnas.1502540113
Mammal Diversity Database (2022). Mammal diversity database (version 1.5) [Data set]. Zenodo. Available at: https://zenodo.org/record/5945626#.Yfr3HvhOlaQ
Manyonyi, A. M., Mariki, S. B., Mnyone, L. L., Belman, S. R., and Mulungu, L. S. (2020). Effects of prescribed burning on rodent community ecology in Serengeti National Park. J. Verteb. Biol. 69:20001.1-13. doi: 10.25225/jvb.20001
Martin, F., Reid, R. B., Goldstein, S., Storozum, M., Wreschnig, A., Hu, L., et al. (2018). Ancient herders enriched and restructured African grasslands. Nature 561, 387–390. doi: 10.1038/s41586-018-0456-9
McDonald, P. J., Stewart, A., Schubert, A. T., Nano, C. E. M., Dickman, C. R., and Luck, G. W. (2016). Fire and grass cover influence occupancy patterns of rare rodents and feral cats in a mountain refuge: implications for management. Wildl. Res. 43, 121–129. doi: 10.1071/WR15220
McGarigal, K., and Cushman, S. A. (2002). Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecol. Appl. 12, 335–345. doi: 10.1890/1051-0761(2002)012[0335:CEOEAT]2.0.CO;2
McGregor, H. W., Legge, S., Jones, M. E., and Johnson, C. N. (2014). Landscape management of fire and grazing regimes alters the fine-scale habitat utilisation by feral cats. PLoS One 9:e109097. doi: 10.1371/journal.pone.0109097
McGregor, H., Legge, S., Jones, M. E., and Johnson, C. N. (2015). Feral cats are better killers in open habitats, revealed by animal-borne video. PLoS One 10:e0133915. doi: 10.1371/journal.pone.0133915
Mendonça, A., Percequillo, A. R., Camargo, N. F., Ribeiro, J. F., Palma, A. R. T., Oliveira, L. C., et al. (2018). Cerrado small mammals: abundance and distribution of marsupials, lagomorphs, and rodents in a Neotropical savanna. Ecology 99:1900. doi: 10.1002/ecy.2367
Mills, C. H., Waudby, H., Finlayson, G., Parker, D., Cameron, M., and Letnic, M. (2020). Grazing by over-abundant native herbivores jeopardizes conservation goals in semi-arid reserves. Glob. Ecol. Conserv. 24:e01384. doi: 10.1016/j.gecco.2020.e01384
Monadjem, A. (1999). Population dynamics of Mus minutoides and Steatomys pratensis (Muridae: Rodentia) in a subtropical grassland in Swaziland. Afr. J. Ecol. 37, 202–210. doi: 10.1046/j.1365-2028.1999.00169.x
Muck, C., and Zeller, U. (2006). Small mammal communities on cattle and game grazing areas in Namibia. Afr. Zool. 41, 215–223. doi: 10.1080/15627020.2006.11407357
Okullo, P., Greve, P. M. K., and Moe, S. R. (2013). Termites, large herbivores, and herbaceous plant dominance structure small mammal communities in savannahs. Ecosystems 16, 1002–1012. doi: 10.1007/s10021-013-9663-2
Ondei, S., Prior, L. D., McGregor, H. W., Reid, A. M., Johnson, C. N., Vigilante, T., et al. (2020). Small mammal diversity is higher in infrequently compared with frequently burnt rainforest–savanna mosaics in the North Kimberley, Australia. Wildl. Res. 48, 218–229. doi: 10.1071/WR20010
Owen, R. D. (2013). Ecology of small terrestrial mammals in an isolated Cerrado patch, eastern Paraguay: communities, species, and effects of ENSO, precipitation, and fire. Mastozool. Neotrop. 20, 97–112.
Pardon, L. G., Brook, B. W., Griffiths, A. D., and Braithwaite, R. W. (2003). Determinants of survival for the northern brown bandicoot under a landscape-scale fire experiment. J. Anim. Ecol. 72, 106–115. doi: 10.1046/j.1365-2656.2003.00686.x
Penton, C., Davies, H. F., Radford, I., Woolley, L., Murphy, B. P., and Rangers, T. (2021). A hollow argument: understory vegetation and disturbance determine abundance of hollow-dependent mammals in an Australian tropical savanna. Front. Ecol. Evol. 9:739550. doi: 10.3389/fevo.2021.739550
Plavsic, M. J. (2014). Proximate and ultimate drivers of small-mammal recolonization after fire: microhabitat conditions, rainfall, and species traits. Anim. Conserv. 17, 573–582. doi: 10.1111/acv.12124
Pringle, R. M., Young, T. P., Rubenstein, D. I., and McCauley, D. J. (2007). Herbivore-initiated interaction cascades and their modulation by productivity in an African savanna. Proc. Natl. Acad. Sci. 104, 193–197. doi: 10.1073/pnas.0609840104
Radford, I. J., Corey, B., Carnes, K., Shedley, E., Mccaw, L., and Woolley, L.-A. (2021). Landscape-scale effects of fire, cats, and feral livestock on threatened savanna mammals: unburnt habitat matters more than pyrodiversity. Front. Ecol. Evol. 9:739817. doi: 10.3389/fevo.2021.739817
Radford, I. J., Gibson, L. A., Corey, B., Carnes, K., and Fairman, R. (2015). Influence of fire mosaics, habitat characteristics and cattle disturbance on mammals in fire prone savanna landscapes of the northern Kimberley. PLoS One 10:e0130721. doi: 10.1371/journal.pone.0130721
Radford, I. J., Woolley, L. A., Corey, B., Vigilante, T., Wunambal Gaambera Aboriginal Corporation, Hatherley, E., et al. (2020). Prescribed burning benefits threatened mammals in northern Australia. Biodivers. Conserv. 29, 2985–3007. doi: 10.1007/s10531-020-02010-9
Riginos, C., Porensky, L. M., Veblen, K. E., Odadi, W. O., Sensenig, R. L., Kimuyu, D., et al. (2012). Lessons on the relationship between livestock husbandry and biodiversity from the Kenya long-term exclosure experiment (KLEE). Pastoral. Res. Pol. Pract. 2:10. doi: 10.1186/2041-7136-2-10
Rocha, C. R., Ribeiroa, R., Takahashi, F. S. C., and Marinho-Filhoa, J. (2011). Microhabitat use by rodent species in a central Brazilian cerrado. Mamm. Biol. 76, 651–653. doi: 10.1016/j.mambio.2011.06.006
Saetnan, E. R., and Skarpe, C. (2006). The effect of ungulate grazing on a small mammal community in southeastern Botswana. Afr. Zool. 41, 9–16. doi: 10.1080/15627020.2006.11407331
Santos, R. A. L., and Henriques, R. P. B. (2010). Spatial variation and the habitat influence in the structure of communities of small mammals in areas of rocky fields in the Federal District. Biota Neotropica 10, 31–38. doi: 10.1590/S1676-06032010000100002
Schieltz, J. M., and Rubenstein, D. I. (2016). Evidence based review: positive versus negative effects of livestock grazing on wildlife. What do we really know? Environ. Res. Lett. 11:113003. doi: 10.1088/1748-9326/11/11/113003
Seymour, C., and Joseph, G. (2019). “Ecology of smaller animals associated with savanna woody plants” in Savanna Woody Plants and Large Herbivores. eds. P. F. Scogings and M. Sankaran (Hoboken, NJ: John Wiley and Sons, Inc).
Stobo-Wilson, A. M., Stokelda, D., Einodera, L. D., Davies, H., Fisher, F., Hilla, A., et al. (2020). Bottom-up and top-down processes influence contemporary patterns of mammal species richness in Australia's monsoonal tropics. Biol. Conserv. 247:108638. doi: 10.1016/j.biocon.2020.108638
Taylor, P. J., Denys, C., and Cotterill, F. P. D. (2019). Taxonomic anarchy or an inconvenient truth for conservation? Accelerated species discovery reveals evolutionary patterns and heightened extinction threat in afro-Malagasy small mammals. Mammalia 83, 313–329. doi: 10.1515/mammalia-2018-0031
Teman, S. J., Stevens, N., Monadjem, A., Fletcher, R. J., Austin, J. D., and McCleery, R. (2021). Savanna rodents’ selective removal of an encroaching plant’s seeds increased with grass biomass. Front. Ecol. Evol. 9:676572. doi: 10.3389/fevo.2021.676572
Tuft, K., Legge, S., Frank, A. S. K., James, A. I., May, T., Page, E., et al. (2021). Cats are a key threatening factor to the survival of local populations of native small mammals in Australia’s tropical savannas: evidence from translocation trials with Rattus tunneyi. Wildl. Res. 48, 654–662. doi: 10.1071/WR20193
Veldhuis, M. P., Howison, R. A., Fokkema, R. W., Tielens, E., and Olff, H. (2014). A novel mechanism for grazing lawn formation: large herbivore-induced modification of the plant–soil water balance. J. Ecol. 102, 1506–1517. doi: 10.1111/1365-2745.12322
Vieira, E. M. (1999). Small mammal communities and fire in the Brazilian Cerrado. J. Zool. 249, 75–81. doi: 10.1111/J.1469-7998.1999.TB01061.X
Vieira, E. M., and Briani, D. C. (2013). Short-term effects of fire on small rodents in the Brazilian Cerrado and their relation with feeding habits. Int. J. Wildland Fire 22, 1063–1071. doi: 10.1071/WF12153
Vieira, E. M., Iob, G., Briani, D. C., and Palma, A. R. T. (2005). Microhabitat selection and daily movements of two rodents (Necromys lasiurus and Oryzomys scotti) in Brazilian Cerrado, as revealed by a spool-and-line device. Mamm. Biol. 70, 359–365. doi: 10.1016/j.mambio.2005.08.002
Vieira, E. M., and Marinho-Filho, J. (1998). Pre- and post-fire habitat utilization by rodents of Cerrado from Central Brazil. Biotropica 30, 491–496. doi: 10.1111/j.1744-7429.1998.tb00086.x
Wells, H. B. M., Kimuyu, D. M., Odadi, W. O., Dougill, A. J., Stringer, L. C., and Young, T. P. (2021). Wild and domestic savanna herbivores increase smaller vertebrate diversity, but less than additively. J. Appl. Ecol. 58, 953–963. doi: 10.1111/1365-2664.13843
Whittington-Jones, G. M., Bernard, R. T. F., and Parker, D. M. (2008). Bushclumps as refugia for small mammals in two eastern cape conservation areas. Afr. Zool. 43, 273–276. doi: 10.1080/15627020.2008.11657244
Woinarski, J. C. Z., Armstrong, M., Price, O., McCartney, J., Griffiths, A. D., and Fisher, A. (2004). The terrestrial vertebrate fauna of Litchfield National Park, Northern Territory: monitoring over a 6-year period and response to fire history. Wildl. Res. 31, 587–510. doi: 10.1071/WR03077
Woinarski, J. C. Z., Legge, S., Fitzsimons, J. A., Traill, B. J., Burbidge, A. A., Fisher, A., et al. (2011). The disappearing mammal fauna of northern Australia: context, cause, and response. Conserv. Lett. 4, 192–201. doi: 10.1111/j.1755-263X.2011.00164.x
Woolley, L., Geyle, H. M., Murphy, B. P., Legge, S., Palmer, R., Dickman, C. R., et al. (2019). Introduced cats Felis catus eating a continental fauna: inventory and traits of Australian mammal species killed. Mammal Rev. 49, 354–368. doi: 10.1111/mam.12167
Wurm, P. A. S. (1998). A surplus of seeds: high rates of post-dispersal seed predation in a flooded grassland in monsoonal Australia. Aust. J. Ecol. 23, 385–392. doi: 10.1111/j.1442-9993.1998.tb00743.x
Yarnell, R. W., Metcalfe, D. J., Dunstone, N., Burnside, N., and Scott, D. M. (2008). The impact of fire on habitat use by the short-snouted elephant shrew Elephantulus brachyrhynchus in north west province. Afric. Zool. 43, 45–52. doi: 10.1080/15627020.2008.11407405
Yarnell, R. W., Scott, D. M., Chimimba, C. T., and Metacalfe, D. J. (2007). Untangling the roles of fire, grazing, and rainfall on small mammal communities in grassland ecosystems. Oecologia 154, 387–402. doi: 10.1007/s00442-007-0841-9
Young, H. S., Dirzo, R., Helgen, K. M., McCauley, D. J., Billeter, S. A., Kosoy, M. Y., et al. (2014). Declines in large wildlife increase landscape-level prevalence of rodent-borne disease in Africa. Proc. Natl. Acad. Sci. 111, 7036–7041. doi: 10.1073/pnas.1404958111
Young, T. P., Kimuyu, D. N., LaMalfa, E. M., Werner, C. M., Jones, C., Masudi, P., et al. (2022). Effects of large mammalian herbivory, previous fire, and year of burn on fire behavior in an African savanna. Ecosphere 13:e3980. doi: 10.1002/ecs2.3980
Keywords: climate change, endogenous disturbance, fire, grazing, landscape of fear, murid rodents, shrub encroachment, zoonotic reservoirs
Citation: Bergstrom BJ, Scruggs SB and Vieira EM (2023) Tropical savanna small mammals respond to loss of cover following disturbance: A global review of field studies. Front. Ecol. Evol. 11:1017361. doi: 10.3389/fevo.2023.1017361
Received: 11 August 2022; Accepted: 10 January 2023;
Published: 03 February 2023.
Edited by:
Mariana M. Vale, Federal University of Rio de Janeiro, BrazilReviewed by:
Marcelo Weber, Federal University of Santa Maria, BrazilCopyright © 2023 Bergstrom, Scruggs and Vieira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bradley J. Bergstrom, ✉ YmVyZ3N0cm1AdmFsZG9zdGEuZWR1; Emerson M. Vieira, ✉ ZW12aWVpcmFAdW5iLmJy
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.