
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Ecol. Evol., 16 June 2023
Sec. Behavioral and Evolutionary Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1004384
This article is part of the Research TopicDuetting and Turn-Taking Patterns of Singing Mammals: From Genes to Vocal Plasticity, and BeyondView all 14 articles
The evolution of human language is a topic that has received undiminished attention. Numerous hypotheses for the origin of human language have been proposed, including gestural communication found specifically among apes. This study advances the hypothesis that human evolution, including human language development, is three-pronged: prosocial, cognitive, and collaborative. Duetting and turn-taking in primates are used as pivotal examples of how bonding leads to joint action and collaboration. It points out that such vocal behavior itself may be a crucial precursor of language evolution in the sense that it is explicitly focused on a conspecific. Some current hypotheses have acknowledged duetting as an important perceptual and behavioral example of synchronicity. Some forms of synchronized behavior, as found in duetting, synchronized dance, or even shared song, were perhaps crucial evolutionary steps preceding the evolution of human language. Duetting signifies more than that, however, because it is an observable and significant cognitive investment that signals attention toward a partner. This study also advances the hypothesis that affect and cognition would have needed to precede any form of duetting or signs of affiliation such as grooming. Hence, this study, asking what duetting in primates signifies in evolutionary terms, takes a multidisciplinary and multimodal approach to suggest important affective and cognitive steps in the evolution of human language and speech, the chief of which is prosociality. Prosociality, as an attitude and awareness of another, be this as a friend or partner for whom one can do favors or whom one can help, is a model for collaboration and cooperation, and also increased cognition.
Duetting exemplifies a significant step in the evolution of language for several reasons. It is usually a time-sensitive vocal activity performed by a pair of closely connected individuals. It further requires coordination of vocal production and a degree of vocal flexibility. In duetting, listening is a key element in the switch from self- to other-oriented and affiliative behavior that may signal cooperation on a broader scale (i.e., beyond duetting). Such behavior may be termed “prosocial”. Prosociality has often been understood as a main facilitating driver of cooperation (Martin et al., 2021). Coordination in the sense of prosociality, unlike empathy, carries no direct cost to the actor but presupposes a positive attitude toward another and doing things together, even supporting others (Silk, 2007).
Accommodation of the behavior and even needs of others may then develop into a new awareness and affective sensibilities toward others for which a new cognitive framework may be needed. Since duetting is an exemplification of one of the most basic forms of joint vocal action of committed pairs, it will be discussed in light of prosocial tendencies.
The literature seems to agree that during the last 2 million years, hominins had become more and more socially complex animals in comparison to other primates (Dunbar, 2014). According to James Baldwin's insights (called the Baldwin effect) evolution by natural selection occurs in three stages: (1) the appearance of new environmental challenges, (2) the adoption of a new behavior through learning (natural selection favoring cognitive plasticity), and (3) new genetically based predispositions when natural selection favors individuals that exhibit a particular adaptive behavior (Podlipniak, 2017). Certainly, the first stage can be readily reconstructed, i.e., the changing physical environments in which early humans moved (Suzuki, 1970). Africa was drying, vegetation reducing, leaving a band of ill-equipped hominins surviving on the savannah exposed to formidable predators. Confronting such new environmental challenges, as Baldwin argued, would lead to the invention of new behavior and this might have forged how individuals acquired synchronizing behavior and collaboration and probably did so as the best or even only chance of survival (Klein, 1977; Caley et al., 2018).
Baldwin's third stage (new genetically based predispositions when natural selection favors individuals that exhibit a particular adaptive behavior), such as the shift to more prosocial, even verbal communication, may have been a step too far for chimpanzees and even bonobos. Chimpanzees, although many attempts were made, could not be taught, or made to speak (Gardner and Gardner, 1969; Gardner et al., 1989). Hence, acquiring the ability first to be able to articulate sounds in the sequence required physiological and cognitive changes (brain nuclei to process information), memory, and the ability to expand vocal expression. Vocal convergence, in which adjustments to one sound type result in similarities between individuals, occurs in a wider range of mammalian orders including primates, mole-rats, goats, and mice (Janik and Knörnschild, 2021). Duetting is part of these parameter adjustments. Learning for a purpose, most likely for cooperation, might well have been a crucial element that fostered the human species' survival and eventually might also have led to the development of human language.
I argue in this study that new environmental pressures forced the development and expression of innovative and new socio-psychological traits and that prosociality is a key characteristic as a driver for this change. Duetting is used as an example of one possible tipping point toward prosociality and eventually cooperation.
To develop these ideas, the study will first introduce the prosocial hypothesis along with hypotheses on human language evolution and then present duetting as a special case of vocal interactive behavior that leads to cooperation and cognitive expansion, and it finally shows why prosociality has an important place, or might well be the lynchpin, in the evolution of human language.
The evolution of human language has been of undiminished interest and has been pursued by vastly different scholarly disciplines and, sometimes, these disciplines either do not read each other's conclusions and insights or their respective conclusions expose chasms. For instance, biology-based evolutionary theories and linguistic explanations concerning human language evolution have often been at loggerheads with each other (Hockett, 1959; Cadková, 2015). The alleged uniqueness of the human language proposed by 19th-century linguists was irreconcilable with evolutionary theory. Darwin (1859) certainly outraged Oxford University linguist Friedrich Max Müller who proclaimed that “language is the Rubicon which divides man from beast, and no animal will ever cross it” (cited by Fitch, 2013). Müller was not the only critic. Later, researchers adopted an a priori position arguing that primates were incapable of engaging in vocal learning (i.e., did not possess the ability to modify acoustic and syntactic sounds and were unable to imitate sounds and words) and hence primate communication was far inferior to that of humans, and to suggest otherwise was indefensible (Penn and Povinelli, 2007). As recently as 20 years ago, some linguists still expressed the belief that animals only produced sounds whose signal inventories “are limited and not subject to cultural modification” or, more precisely, animals were only able to produce innate sounds (Studdert-Kennedy, 2000). Studdert-Kennedy and Goldstein (2003) further argued that human language is defined by the dissociation of sound and meaning and has no precedence in animal vocalizations. Dissociation is seen as a critical discontinuity that separates human language from other primate systems of vocal communication (Studdert-Kennedy and Goldstein, 2003).
Not surprisingly, despite the controversies about primate linguistic abilities, comparative research into the origins of human language has focussed on the primate line, the closest extant relatives of humans (Fedurek and Slocombe, 2011; Wheeler and Fischer, 2012; Townsend and Manser, 2013; Levinson, 2016; Vonk, 2020). Some primates, particularly apes, actually show a great diversification of communicative acts, from gestures (Liebal and Call, 2012; Hobaiter et al., 2022) to body movements (Gasser and Arbib, 2019), from singular vocal acts to sustained vocal expressions (Liebal and Oña, 2018), and, finally, to joint vocal actions (Sekulic and Chivers, 1986; Baker-Médard et al., 2013) and even “song”; the latter largely limited to gibbons (Geissmann, 2000), Malagasy Indri, Indri indri (Maretti et al., 2010; De Gregorio et al., 2019), titi monkeys, of the following three genera: Cheracebus, Callicebus, and Plecturocebus (Adret et al., 2018; Aldrich et al., 2023), and Sulawesi tarsiers (MacKinnon and MacKinnon, 1980; Clink et al., 2022). These various and diverse examples of communicative behavior in primates have provided a rich canvas as starting points for human language origins, be they initially gestural or vocal (Deacon, 2003).
Indeed, theories of language evolution have proposed a vast range of different possibilities, be this via gestures, music, and rhythm (alternatives to be discussed later) but the puzzle remains how the switch from non-speaking great apes to speaking humans could have occurred. We know now that apes can form concepts and abstract ideas concerning the passage of time (Patterson, 1978). Through American Sign Language, ample evidence has been accumulated that apes use this means of communication to create new meanings, invent new signs, and combine words in ways that create a message (Miles, 1990, 1994). And Koko, a gorilla, showed that he was able to remember past events and plan or imagine the future (Patterson, 1978). Experience, memory, and learning produced new outcomes. Barton (1998) and later Barrett and Henzi (2005) explained that, as primates formed larger and socially more cohesive groups, their perceptual system needed to be enhanced to process details of dynamic social stimuli, such as facial expression, posture, gaze direction, and the like (Barton, 1998).
Significantly, research has shown that proto-language or gestures in great apes are mapped to specific areas of the brain used in human language such as Wernicke's and Broca's areas (Cantalupo and Hopkins, 2001). Interestingly though, the greatest expansion of the primate brain over evolutionary time apparently occurred in the visual cortex (in particular, area V1; Das and Gilbert, 1995), in the parvocellular region, which is associated with the analysis of fine detail and color in diurnal primates (Harting et al., 1973; Smaers and Vanier, 2019). Also largely located in layer V1 of the visual cortex are the recently discovered spindle cells, probably unique to great apes and the human brain (Banovac et al., 2021). There is some evidence that specialized spindle cells project to highly specific motor centers “controlling vocalization, facial expression, or autonomic function” (Nimchinsky et al., 1999). Perceptual abilities and the ability to discriminate vocal and facial expressions are certainly of benefit when subtleties in communication and an understanding of the emotions and intentions of others are increasingly important. Gesturing is a non-linguistic act but, as had been shown time and again, it can convey very specific meaning. Pointing, in particular, has often been identified as a key behavior for understanding the development of language and theory of mind (Camaioni et al., 2004). More of this later. Recent work has also identified a “primate mosaic brain evolution” (De Casien and Higham, 2019). The authors concluded that these were in the area of sensory and cognitive specializations that enabled effective communication even at a non-linguistic level (De Casien and Higham, 2019).
The apes' proven physiological inability to speak required morphological changes. Such changes included the dropping of the larynx before speech could occur and this led to humans' ability to speak (Lieberman, 1985). This theory had lost some traction in favor of suggesting different processes. Nishimura et al. (2022), for instance, have now shown that important physiological changes did occur but in an unexpected direction. The adaptations involved a process of shedding anatomical features of the vocal apparatus via structural simplifications: the laryngeal air sacs of great apes disappeared (Trenbeath, 2021) and as humans evolved, they also lost the standard primate laryngeal feature of thin upward projections of the vocal folds, and they considered these the crucial adaptations for speech (Nishimura et al., 2022).
As recent research has shown, however, some primate and avian vocal abilities are far more complex and varied than once thought (Kaplan, 2014), starting, in primates, with the discovery of referential signals in vervet monkeys, Chlorocebus pygerythrus (Seyfarth et al., 1980; Seyfarth and Cheney, 1986), continuing with the discovery of referential food grunts in chimpanzees, Pan troglodytes (Watson et al., 2015), food calls in common marmosets, Callithrix jacchus (Rogers et al., 2018), and the vocal modifications found in pygmy marmosets, Cebuella pygmaea (Snowdon, 2018). Great apes and even new world monkeys (such as black-fronted titi monkeys, Callicebus nigrifrons: Caesar and Zuberbuehler, 2012; and white-faced capuchin monkey, Cebus imitator: Coss et al., 2019) have been shown to use referential gesturing and vocalizations. Indeed, the detailed linguistically based studies of the 1980s and 1990s confirmed that apes were able to learn American Sign Language (Gardner et al., 1989; Miles, 1994). They understood words, commands, and objects, even showed some sense of grammar (Greenfield and Savage-Rumbaugh, 1990), and were able to count (Boysen and Bernston, 1989). This was confirmed for all four great ape species (bonobos, Pan paniscus, and chimpanzees, P. troglodytes: Savage-Rumbaugh, 1984; gorillas, Gorilla gorilla: Patterson, 1978; orangutans, Pongo abelii: Miles, 1990). While some avian species trump some of the primate skills (from chickens being able to count (Rugani et al., 2011) to the ability to understand speech (Pepperberg, 2007), the point here is that there are many precursors to human language evolution, be this conceptually and semantically, and thus cognitively already present in primates (Lameira, 2017).
By the 1990s, experts in the field spoke openly about the “minds” of great apes, rather than about “cognition” (Russon et al., 1996). In primates, this abstract ability to be able to deal with symbolic representations of language and thus display complex cognitive processes led to a host of detailed investigations both of behavior and of the structure and function of the primate brain (Maestripieri, 1999; Reader and Laland, 2002). Such investigations and comparative studies between primates and humans continue to this day and have clarified differences (Palomero-Gallagher and Zilles, 2019) and similarities (Miller et al., 2021) between the brain of apes and the human brain. The discoveries of mental time travel (conceiving of past, present, and future) conveyed in sign language added depth to the view that apes are cognitively very advanced, can readily cope with abstract concepts, and imbue gestures with meaning (Leavens, 2004; Liebal and Call, 2012; Fröhlich and Hobaiter, 2018; Hobaiter et al., 2022). Cognitive features of primate behavior, such as cooperation, have also been identified as essential qualities for human language evolution (Williams et al., 2022).
Prosocial behavior has long been of central concern and research interest in human psychology, partly because adolescents who show weakly developed prosocial behavior tend to display several behavioral problems (Card et al., 2008; Carson, 2013). The prosocial hypothesis proposed here is that human evolution and human language development depend on a three-pronged model of key pillars: prosocial, cognitive, and collaborative actions. It is not a combination of those three elements but a sequential development, i.e., of prosocial behavior leading to sharing of cognitive insights and eventually collaborative actions. These advances tended to offer or help solve a range of environmental and inter- or intra-group challenges. The argument of the prosocial hypothesis is well in line with other hypotheses of complex, often multilevel social structures (Cronin, 2012; Sewall, 2015; Aureli and Schino, 2019; Kappeler et al., 2019; Morrison et al., 2020), communication, cognition (Sewall, 2015), and, importantly, cooperation (Jaeggi and Gurven, 2013) as drivers of evolution. They also fit well with the human self-domestication hypothesis (Hare et al., 2012). As Hare (2017) states: the human self-domestication hypothesis entails (a) selection for prosocial behavior linked to derived human cooperative-communicative abilities and (b) the domestication syndrome in human morphology, physiology, development, and cognition, as seen in other self-domesticated species (such as dogs).
However, there are some contradictory and unresolved problems between the studies of primatology and anthropology. The occurrence of prosociality in animal studies has spawned two main hypotheses, called the cooperative breeding hypothesis and the self-domesticated hypothesis. According to Amici et al. (2014), the cooperative breeding hypothesis, at least in primates, predicts low levels of prosociality when specific species are non-cooperative breeders, while the self-domestication hypothesis predicts high levels of prosocial behavior because self-domestication presumes high levels of tolerance of each other (Amici et al., 2014). Humans and callitrichid monkeys are the only primate species described as cooperative breeders, so they should show high levels of prosocial behavior and they do (Martin et al., 2021). All great apes should show low levels of prosocial behavior as Amici and colleagues found when they tested chimpanzees, bonobos, gorillas, orangutans, tufted capuchin monkeys (Sapajus apella), and Geoffroyi's spider monkeys (Ateles geoffroyi). Indeed, Amici and colleagues found little to no prosocial behavior in any of the great apes and New World monkeys they studied. This very much runs counter to other research results but, importantly, also to hominin evolution that has argued repeatedly that prosociality, indeed the human ability to support each other, is an essential precondition for the success of humans, perhaps the main reason why this species survived and thrived (Hare, 2017).
The results by Amici et al. (2014) showing little evidence of prosocial behavior especially in the four great ape species may be explicable by different circumstances and housing as well as gender. However, their results have been duplicated. Three years after the publication of their results, Verspeek et al. (2022) conducted experiments with bonobos and equally found no evidence of prosocial behavior, confirming the results and conclusion of Amici et al. (2014).
However, these results run counter to the prediction that self-domesticated primates should show high levels of prosocial behavior. The anthropological literature on human evolution from the Lower Paleolithic (ca 1.5 million to 200,000 years ago) to the Holocene Epoch (11,700 years ago to the present) periods strongly argues that later humans are self-domesticated (one hypothesis of prosociality) and, by the time of the Holocene, show strong prosocial behavior. The human self-domestication hypothesis (HSD) (Hare and Tomasello, 2005; Hare et al., 2012; Hare, 2017) seems very convincing and supports evolutionary trends also in other species, especially dogs (Hare, 2017). The assumption is, of course, that the nearest relatives to early humans, chimpanzees and bonobos, should share the same traits of prosociality and high levels of mutual tolerance or even spontaneous altruism, as has been described in humans, and certainly high levels of tolerance were found in children and chimpanzees (Warneken et al., 2007; Warneken, 2015). Such incompatible results give at least pause for thought.
Equally, evolutionary theories, such as Darwin's and Baldwin's, suggest that environmental pressures led to the invention of a new behavior by means of learning (natural selection favoring cognitive plasticity) and gradually an increase in cognitive abilities in humans. However, more social pressure does not always require more cognitive ability but can lead to more subdivision of tasks and a lowering of individual cognitive ability (Fedorova et al., 2017).
The first imperative would seem to be that individuals had to bond with conspecifics in some social way. The social brain hypothesis Dunbar proposed in the late 1990s (Dunbar, 1998) was at first designed to explain why primates had unusually large brains for body size compared to all other vertebrates: He attributed this to their complex social system but later he extended this hypothesis to human evolution (Dunbar, 2014). The social brain hypothesis that Dunbar developed largely seemed to explain the expansion of cognitive abilities particularly in the primate line and chiefly in chimpanzees. It did not necessarily explain the evolution of complex communication and prosociality in humans until the human self-domestication hypothesis was developed and tested, showing that apart from the physical, physiological, and other changes, self-domestication selected for high prosociality (Cieri et al., 2014; Hare, 2017).
The prosocial hypothesis advanced in this study does not conflict with the social brain hypothesis (Dunbar, 1998) or the hypothesis of a gestural origin of human language (Corballis, 2002, 2010). Both, as well as several others, rightly emphasize the gradual sequencing of psycho-social developments, including nuances of communicative behavior. The term “communication” is chosen deliberately here. In agreement with Fitch (2020), even sophisticated, vocalized (referential signaling), or verbalized (human speech) communication does not address the cognitive richness of concepts that may or may not be expressed in words and may not leave measurable behavioral evidence. Also, the multifarious, at times instantly changeable, and flexible interactions between environment and organism need to remain firmly in view. We know that extant apes are capable of distinguishing gestures, facial expressions, and vocal information and, presumably, so was the hominid and hominin brain.
Even gene expressions can change relatively quickly. Wiles et al. (2005) gave as an example the genetic ability of mammals to synthesize vitamin C in the body. But in primates, by a process called genetic redistribution, this gene expression was eliminated so that, from then on, the only way to acquire vitamin C had to occur exogenously. A second example, a purely morphological change, was provided by Darwin's Galapagos finches. Darwin concluded that consistent environmental differences in different habitats in the Galapagos promoted directional natural selection on resident finches for optimal beak morphology. This process has produced more than a dozen distinct species of finches, all unique to the archipelago, further cementing Darwin's idea of natural selection (Grant, 2017).
Thus, in psycho-cognitive developments, both behavioral synchronicity (the ability to match the behavior of another, be this in movement, sound, and mood) and prosocial inclinations need to precede the development of intentional acts toward conspecifics (see Table 1) and create a niche for enhancing cognitive abilities in what has been termed “emotional intelligence” (Salovey and Mayer, 1990). Communication is a very important part of this but so is finding a reason for extended communication, namely the emergence of “other-directedness”, of the importance of a partner or a group for one's survival.
Table 1 should be read from left to right as a cumulative and dynamic development toward prosocial and affiliative behavior. Note that the Australian shingleback lizard (Tiliqua rugosa) is a monogamous lizard but the pair separates outside the breeding season. Only those species are included here with pairs also staying together outside the breeding season and that particular condition alone limits the number of species included in animal bonds. Table 1 also indicates that the life history data of species, including their reproductive strategies, are important variables.
The point of this study revolves largely around two main social characteristics of any form of cooperation (one is biparental care and the other is prosociality) as two imperative milestones in the evolution of human bonding (Launay et al., 2016) and human language. However, biparental care in humans has a slim evolutionary base. When, for instance, examining reproductive strategies in fish, reptiles, and amphibians (Andrew-DeWoody et al., 2000), or even mammals, the number of species across classes of animals remaining paired for years is very small indeed. While Table 1 has identified species from marine life to a broad range of land animals, pair bonds, let alone monogamous life-long bonds, are overall very rare in any vertebrates, except for birds. In birds, at least 95–97% of more than 10,000 avian species pair bond with a mate and jointly raise their offspring (Cockburn, 2006).
In mammals, according to Clutton-Brock (1991), only about 5%, including some primates such as marmosets (Burkart and van Schaik, 2020; Martin et al., 2021), meerkats, wild dogs, and certain species of mice, form lifelong pair bonds or even short-term pair bonds and practice biparental care. But the 5% of mammals that practice biparental care still tend to live in troops, groups, prides, or packs, in which the breeding pair typically consists of the alpha male and the alpha female. Hence, the social configuration of pair bonding of two humans and the evolution of complex communication systems, including language, in humans, have few direct evolutionary predecessors, and, with some exceptions, the various elements required for creating a prosocial context are often not in the one species together.
Birds and humans thus have in common that they both raise their offspring as pairs (biparental care) or raise offspring cooperatively and even join forces in group defense. Cooperation and bonding in hominin evolution may not be an innovation de novo but evidence of such social relationships and task coordination still offers challenges to our understanding of their developments, be this in humans or birds (Issa et al., 2023).
Great apes generally provide many variations in mating and alliance systems, however, making meaningful comparisons with human society more difficult. Of course, the mating system of a species does not always mirror its social system (Dixson, 2009). By and large, orangutans are solitary (Kaplan and Rogers, 2000), western lowland gorilla (Gorilla gorilla gorilla) groups with several females and offspring are usually ruled by a single silverback (Forcina et al., 2019), and, with some variations, mountain gorillas, Gorilla beringei beringei, although classified as one male group may be up to 40% multimale groups (Robbins, 1999; Morrison et al., 2020). Chimpanzees live in multimale and multifemale social groups and may strongly compete with and aggressively fight other groups, and bonobos have a matriarchal system (Sommer et al., 2011). At some stage, the human social organization may have been the closest to that of gorillas, living with them in forests (White et al., 2009) or having moved to savannahs (the oldest established hypothesis on human bipedalism; Senut et al., 2018) or, as has also been suggested, living largely near water and exploiting its resources (Stewart, 1994; Finlayson, 2014). As Schacht and Kramer (2019) noted recently, consensus on a human-typical mating system remains elusive. “While a simple classification would be useful for cross-species comparisons, monogamous, polyandrous, and polygynous marriage systems exist across contemporary human societies” (Schacht and Kramer, 2019).
In discussing various mating systems, respective benefits for offspring are worth mentioning here. For instance, there is evidence that stable, socially monogamous pairs or stable small family groups in whatever species or class of animal create a safe and largely stress-free emotional and learning environment (Raposa et al., 2016) strengthening survival and long-term health. In many species with these characteristics, there is also an extra social layer—that of socializing juveniles. As I have explored elsewhere (Kaplan, 2020a, 2023), such environments encourage extensive social play behavior that is intimate, communicative, and creative (Bateson and Martin, 2013), and this is usually regarded as beneficial for the individual concerned. Whatever one might call the effects of play: they are now recognized as generating positive emotions (rats: Panksepp, 2005; Pellis and Pellis, 2007; Vanderschuren et al., 2016; ravens: Osvath and Sima, 2014; primates: Loizos, 2017). Positive emotions are themselves reinforcing to seek similar contact in future. Hence, regardless of how social interactions proceeded to evolve into human language—be this via gestures, music (song and dance), drumming, whistling, or extension of referential vocal signals—it required motivation first to even get to a position of seeking expansion of any form of communication.
Second, Table 1 is meant to emphasize the centrality of evolving prosocial behavior in the formation and maintenance of strong affiliative bonds. “Prosociality”, as already mentioned, has been highly topical in the field of psychology for some time (Luengo-Kanacri et al., 2021), especially in human developmental studies (Spataro et al., 2020). The social circumstances for the absence or presence of prosociality (which may be variable and flexible) continue to be explored, especially the consequences when a well-defined profile of prosociality is absent or weakly developed (Donald et al., 2021). However, its role in animal communication and bond strength (a) in flexibly functioning pairs, families, and animal communities and (b) as a trigger for the intentional sharing of goods, such as food (Feistner and McGrew, 1989; Jaeggi and Gurven, 2013; Güroglu et al., 2014), in communication and ultimately in human language evolution is rarely considered. This is surprising, given the question: why are we the only primate that can speak? remains an open question.
I am suggesting a domino effect from joint action to prosocial tendencies, generating more detailed communication, leading in turn to an increase in differentiated acts of communication in both referential signals (vocal and gestural) as well as semantic content. Another point to be made here is that even the most occasional acts of prosocial behavior in great apes mean that prosocial behavior is, and most likely was, an option in primate culture.
Furthermore, prosocial behavior is causally linked to the evolution of human language because language is more than a linguistic manifestation. It is a tool for a continuing motivation to address a conspecific or partner. To achieve and maintain such motivation, both emotional and cognitive complexity needs to have developed and, if already present, increased further. In agreement with the dynamic systems paradigm (see Shanker and King, 2002; King, 2009), converging feelings and intentions among partners may continue to be enhanced in a dynamic of ongoing negotiation at inter- and intra-personal levels, leading perhaps to closer bonds. The latter is a claim of the involvement of emotions, recently discussed by Dukes et al. (2021).
Another point that at times seems to have been lost in debates between selfish and prosocial actions within pairs and groups of primates is to consider evolutionary principles: Whatever format of skills, communication, or affiliations is more sustainable, these traits are more likely to survive via natural selection. In some cases, they may even develop further, be this at the cellular level, in morphology, physiology, or even chemistry. Exhuberant morphological features are generally associated with food acquisition. Well-known examples are the elongated middle finger of one of the Madagascar's nocturnal lemurs, the Aye-Aye, Daubentonia madagascariensis (Sterling and McCreless, 2007), or the exaggerated beak length of the sword-billed hummingbird, Ensifera ensifera (Abrahamczyk et al., 2014), or, as Darwin described, the diversification of beak strength in finches in different environments. While the finch model of natural selection is well-known and can explain so many other variations in biology, it should be applied rigorously to behavior because the same evolutionary principles ought to apply.
Duetting, a vocal manifestation of synchronicity, is one of the most studied vocal behaviors in mammals and birds and occurs in many forms, referred to as antiphonal singing, turn-taking, counter-calling, or counter-singing. Some of these exchanges are expressed between males. Whatever the dyadic composition, most interactions between two members of the same species are between male and female partners.
In the broadest sense, duetting and counter-singing are vocal behaviors that exist in many songbirds, in some primates, but also in Alston's singing mouse, Scotinomys teguina (Neff, 2019), Klipspringer antelopes, Oreotragus oreotragus (Tilson and Norton, 1981), the maned wolf (Ferreira et al., 2022), whales (sperm whale, Physeter macrocephalus: Schulz et al., 2008; long-finned pilot whale, Globicephala melas: Courts et al., 2020; reviewed in Vanderhoff and Bernal Hoverud, 2022), amphibians (chorus frogs such as spring peepers, Pseudacris crucifer; Forester and Harrison, 1987; south African clawed frog, Xenopus laevis: Tobias et al., 1998; Legler's stream frog, Ptychohyla legleri: Etzel et al., 2020), toadfish, Tetraodontidae (Vieira et al., 2021), and even in a range of invertebrates (Bailey, 2003; Henry et al., 2013).
Duetting in the narrowest sense is defined as a temporally coordinated interactive vocalization between two adults, usually of established pair bonds. Such vocal exchanges tend to have specific temporal patterns and may overlap even substantially while, in birds, few or no overlaps occur. Taking turns, as Banerjee and Vallentin (2022) noted, requires a fast sensory perception of the sender's vocal output but also the precise control of the responder's vocal onset. During these interactions, participants simultaneously plan upcoming vocalizations while listening to respond as early as possible without interrupting the initiator of the duet (Levinson and Torreira, 2015; Banerjee and Vallentin, 2022). Many avian duets fit into this characterization. Duets consist of calls or syllables in rapidly produced vocalizations and even these can be void of specific meaning (Arriaga and Jarvis, 2013; Dahlin and Benedict, 2014; Barón Birchenall, 2016). Any of the turn-taking vocalizations can be defined as an orderly exchange of communicative vocal signals that may or may not overlap.
However, among those primates that are mated pairs in stable monogamous bonds and are in stable territories, duetting is a rare social phenomenon and involves clear-cut examples of closely temporally matched vocalizations. We know only of a few diverse primate families—Tarsiidae, Indriidae, Lemuridae, Hylobatidae, Cercopithecidae, and Pitheciidae—to which these conditions apply (e.g., Tarsius spp.; indri Indri indri; Mentawai langur, Presbytis potenziani; and gibbons, Hylobatidae). After studying the duetting and vocal behavior of some of these taxa Haimoff (1986) concluded that the occurrence of duetting in these primate species and the similarities found in the acoustical features of their vocal behavior, represented a case of functional convergence. Such convergence was possibly a result of their evolution of a common social organization or similar ecological niche (Haimoff, 1986). To my knowledge, this conclusion has not been challenged to date.
Duetting can have several functions, some of which might even be present in one single species (Dahlin and Benedict, 2014). These are mate-guarding (Dowling and Webster, 2018; Dolotovskaya et al., 2020), to signify and or strengthen partnerships (Mèndez-Càrdenas and Zimmermann, 2009; Smith et al., 2010; Singletary and Tecot, 2020), and may serve as an indicator of the presence of a well-versed territorial defense team that may send a warning to potential intruders (Adret et al., 2018; Amorim et al., 2022). In sperm whales, Schulz et al. (2008) studied the frequent exchanges of short sequences of clicks, termed codas. They found that the sequencing of exchanges into duet-like chains with overlapping and matching functions reinforced social bonds between whales, which is attributed to the same or very similar function to duetting as in primates or birds.
The act of duetting also seems to have some measurable, “feel-good” consequences for the participating partners, be this in hormonal changes in oxytocin and vasopressin and increased brain-to-brain synchrony in frontal and pre-frontal brain areas (Amodio and Frith, 2006; Reindl et al., 2018), confirmed in bats (Zhang and Yartsev, 2019; Rose et al., 2021), primates (Smith et al., 2010), and human studies (Atzil et al., 2012; Bales et al., 2021).
We thus have some cumulative evidence that duets are largely partner and pair dependent and contribute to the bond, be this in inhibition driven by auditory feedback (Coleman et al., 2021: plain-tailed wrens, Pheugopedius euophrys) or in very precise timing but different frequencies (Hoffmann et al., 2019: white-browed sparrow-weavers, P. mahali). The evidence also suggests that, over time, coordination of duetting improves in timing and auditory adjustments to the partner's specific auditory characteristics of their part of the duet. In my research of duetting in wild free-ranging magpie larks (Grallina cyanoleuca), the duets I recorded of a local pair in coastal New South Wales, Australia (Coordinates 30.5869° S, 153.0001° E), were not just timed precisely but the segments of each partner were near identical (Figure 1). In one of the rare longitudinal studies of duetting, Hall and Magrath (2007) showed that, in magpie larks at least, duets in newly established pairs were not precisely timed and their vocalizations would even overlap. By contrast, in well-established pairs, timing became very precise in all measures. Presumably, a potential territorial invader can audibly ascertain whether a pair is well-established and has perfected the art of territorial defense or the pair was newly formed and relatively inexperienced in which case its territorial claims could be challenged. In this avian species at least, duetting has a dual function as a form of mate-guarding and as a warning for potential intruders that they are dealing with well-experienced pairs (Vanderhoff and Bernal Hoverud, 2022). In most cases, the coordination of a song tends to have a leader and a follower. The partner who maintains the rhythm becomes the leader and the partner who maintains the synchrony of the joint behavior becomes the follower, arguing that maintaining synchrony requires greater adaptation (Hoffmann et al., 2019).
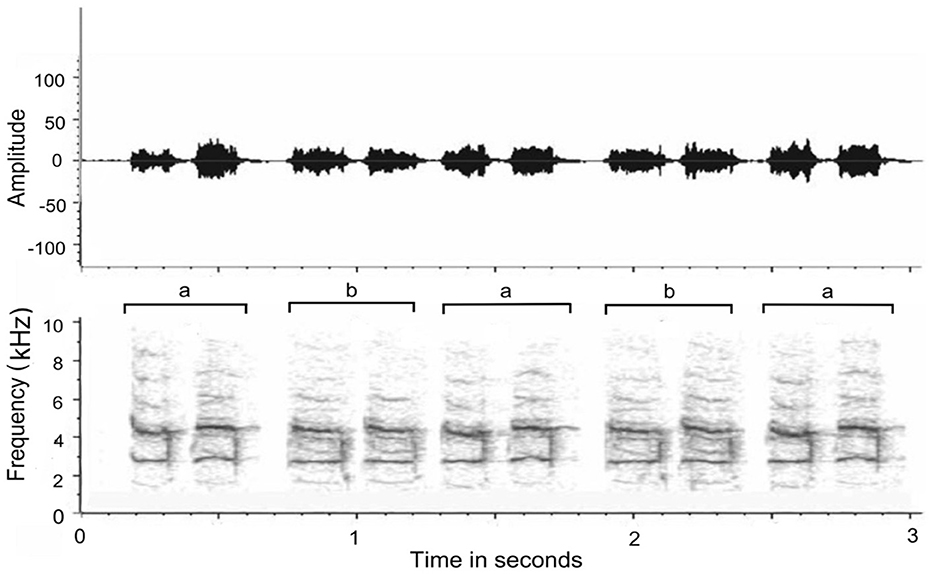
Figure 1. Waveform envelope (Top) and spectrogram (Bottom) of magpie lark duet (a) male; (b) female (author's recording). Note that the morphology of each call is matched almost exactly by the partner and the time intervals between initiated call and reply is reduced to split seconds. The miniscule time delay between a and b was not due to great distance or hesitancy by the replying bird but a function of the position of the remote-control microphone (closer to male) and wind direction (toward female).
Interestingly, in one of the larger nocturnal sportive lemurs (Lepilemur edwardsi) that Smith et al. (2010) studied, pair partners synchronized behavioral activities, especially after duetting. In other words, duetting is not an isolated skill but one that, in mammals and birds at least, is a well-evolved expression of social rules and bonds. The latter may readily lead to ever-increasing invention of sound symbols, i.e., sounds with semantic meaning (Vonk, 2020).
Much has been made of the gestural origin of human language and for good reason. In apes, some 80 gestural referential signals have been identified (Leavens and Hopkins, 1998). As was mentioned before, apes trained in American Sign Language were able to show human researchers that they were capable of thinking of the past and the future (theory of mind), and of being linguistically innovative by making new combinatory words and even sentences (Corballis, 2002, 2010; Hobaiter et al., 2022). These discoveries were significant in showing that concepts and theory of mind existed in apes before the evolution of human language and that these were applied intentionally and directed toward another individual or group.
One might argue (with some justification) that duetting is a very weak link to human language evolution especially when compared to the rich conceptual and symbolic range of ape gestures. With some exceptions (Clarke et al., 2006; Andrieu et al., 2020), duetting tends not to carry complex and personal messages as gestures can. But this is not the point. Lifting out any vocal behavior in extant species is providing a static snapshot of how and how far each species has taken its cognitive and affective abilities.
The question is why an expanded need for more communication arose in the first place, what its motivation was, and in what specific social context vocal communication eventually arose. Equally, the question remains as to why language as speech had to come about at all. A static snapshot may discover the extent of the cognitive achievements of a species, but it needs an evolutionary, dynamic perspective to address the question as to why and how vocal signals developed to the extent to which they did in humans.
It is generally agreed that biological changes can be due to mechanisms such as natural selection, random genetic drift (Santangelo et al., 2018; Miles et al., 2019), sexual selection (Kuijper et al., 2012), and other extraneous events or features (such as climate change: traditional food sources dwindling and changes in environmental topography; Veit, 2021). Such changes are responses and adaptations vital for enhanced chances of survival.
The changes that occurred in the hominin brain are structurally and functionally substantial. After investigating the differences in the brains of chimpanzees and humans, Ardesch et al. (2019) concluded, “…[our] findings suggest an evolutionary shift in the human brain toward investment of neural resources in multimodal connectivity facilitating neural integration, combined with an increase in language-related connectivity supporting functional specialization”. The question is what possible internal or environmental factors could have made this happen? And how could language acquisition be achieved within the organisms' own biology and available social skills and resources?
In this regard, duetting is an important milestone, even if only shared by a few species among primates. This is not related to the less than frequent vocal displays of duets but for another reason: duetting can show the very point when adaptive behavior, that initially might have evolved for ecological reasons, can flip onto a cognitive and affective plane. First, unlike transitory mimicry of movement or sound, courtship dance rituals, or pre-copulatory synchronicity to establish a bond or common interests, this kind of synchrony investing in cooperative behavior means that such bonds have already been established. Such specific ongoing bonding practices may lead to further expressions of cooperative, prosocial, and even empathetic behavior (Hove and Risen, 2009). This is so because the partner has become a “significant other” and is given careful attention.
Clearly, the longitudinal study of magpie lark duetting, cited above (Hall and Magrath, 2007), showed that learning was involved when the duetting signals matched more closely after a year than they did initially. Mastering precision requires close listening to and comprehending the other's rhythm, tempo, frequency, emphases, and even length of the duet. Nuances of duetting can vary substantially in terms of developmental plasticity (Adret, 2022), and calls can be sophisticated and distinct in expression (Clink et al., 2021) or may not seem sophisticated at all but are nevertheless significant as a collective behavior (Logue and Krupp, 2016).
This ability to create precise duetting is well-supported by identified brain mechanisms that allow such processes to occur. For instance, Okobi et al. (2019) pointed out that acoustic communication such as duetting often requires rapid modification of motor output in response to sensory cues. When they examined the vocal exchanges in Alston's singing mouse Scotinomys teguina, they found that males could modify their singing behavior on a sub-second time course that resembled “both traditional sensorimotor tasks and even conversational speech” in humans.
Two summary points about duetting can be made here in relation to the concept of synchronicity. First, duetting is just one manifestation of synchronicity, if a powerful one when the communication is intentional and practiced. Second, duetting is overwhelmingly found in stable and long-term relationships (Dahlin and Benedict, 2014). In pair duetting, “tuning in” to the bonded partner more than suggests that there is some flexibility both to innovate, learn, and adjust and to fit more directly with the vocal expressions of the bonded partner (Haraway and Maples, 1998; Oller and Griebel, 2008, 2021). Given these sustained observations, it becomes more plausible to suggest that some types of vocal behavior can lead to complex sociality and cognition (Roberts and Roberts, 2020).
Synchronizing, as discussed above, denotes the precise timing and coordination of movements to coincide with those of another (Bernieri and Rosenthal, 1991). Coordination is socially not far removed from synchronizing behavior and thus plays a fundamental role in social interaction (Yu and Tomonaga, 2015), and such coordination can be a crucial step toward voluntary, intentional cooperation (Valdesolo et al., 2010; Michael et al., 2020). Unlike courtship dances or pre-copulatory synchronicity to establish a bond or common interests, this kind of synchrony investing in cooperative behavior presumes that such bonds have already been established. Past research has shown that synchronicity can also be tested behaviorally because it is interactional and observable (Hoehl et al., 2020).
Results of many studies confirm that the brain of great apes and hominins, while expanding substantially from earlier primates (Smaers et al., 2017), did not do so uniformly, identifying some high-expanding areas within the forebrain (Sneve et al., 2019). According to Sneve et al. (2019), especially the brain of Homo habilis marked transverse expansion of the cerebrum and the frontal and parieto-occipital parts, and increases in the mass of the frontal and parietal lobes and the two major cerebral areas governing spoken language (Tobias, 1987). One notes also, that while brain mass increased, estimated body mass did not change appreciably (Table 2).
Such an increase in neocortical neurons comes with a high metabolic cost. Sneve et al. (2019) believed that the “capacity of high-expanding cortex to connect flexibly with various specialized brain ‘networks' suggests an involvement in ‘supramodal' cognition”. Whatever is implied in this statement, it is clear that some of these expanding cortical areas are associated with language function in humans. For instance, both in humans and extant great apes, strong asymmetries are present at the population level in the frontal cortex, including a left hemisphere dominant asymmetry of the planum temporale, and in the brain region of Wernicke's area (Figure 2), which supports a critical component of speech production (Gannon et al., 1998; Hopkins et al., 1998; Spocter et al., 2010). Also, the sulci within the inferior frontal cortex, which contains Broca's area, displays left hemisphere dominant asymmetry in both humans and great apes (Sherwood et al., 2008; Hill et al., 2010). Both areas are specific to language and speech. Such patterns of select cortical expansions happened also during human evolution (Hill et al., 2010). In other words, the primate brain was already rather well-equipped to handle cooperation and coordinate activities in ways that required cognitive flexibility.
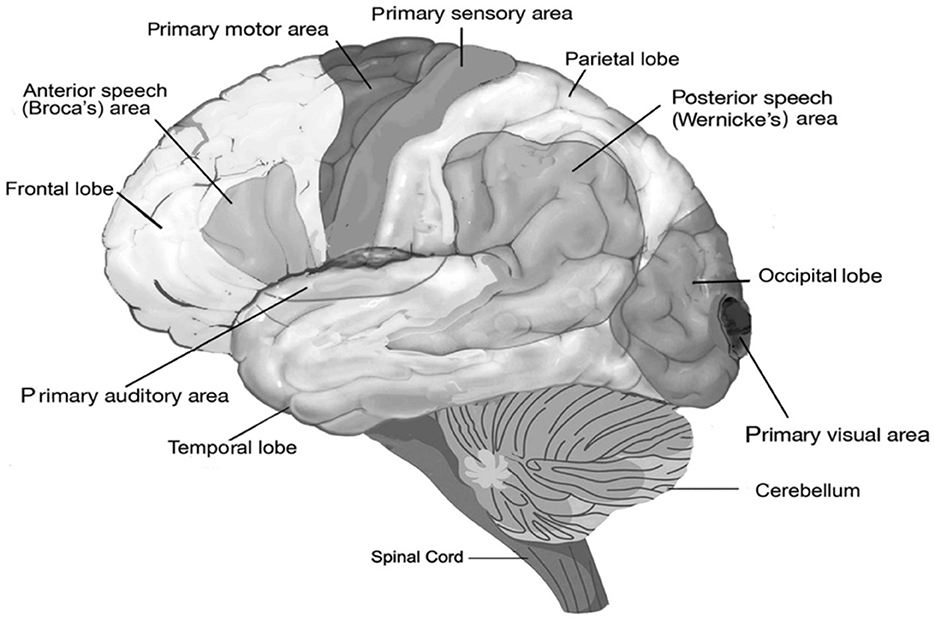
Figure 2. Functional areas of the human brain. The diagram shows the areas for speech and the location of basic perceptual areas (audition, vision, and primary sensory area) and motor area, as well as the anterior Broca's area and posterior Wernicke's areas, both of which are indispensable for speech and for which homologs have been found in non-human primates (Hopkins, 2022).
Those substantial expansions of some brain areas have come at a cost, however. The brain has been described as the most “expensive” part of the body (called “the expensive brain hypothesis”, see Isler and van Schaik, 2009), demanding substantially higher energy input than the rest of the body. The “expense” is one of the nutritional requirement because neurons use up to 10 times more energy than body cells (Yu et al., 2014). We know from humans and mammals that energy consumption in the brain accounts for over 20% of total oxygen metabolism (Watts et al., 2018) and neurons consume 75–80% of energy produced in the brain (Hyder et al., 2013). The expensive brain hypothesis argues that the increased length and difficulties to raise an offspring usually lower the number of offspring that can be raised, which can lead to a creeping extinction, a process whereby replenishment of offspring falls below the death rate.
Such metabolic and cytoarchitectural changes in the brain would likely have occurred only if (a) there were substantial evolutionary pressures for new adaptations, (b) the “cost” could be offset by some external compensatory benefits and action, i.e., co-opt others to help protect and raise offspring, and (c) incurred benefits including higher survival rates of self and offspring. To have some negotiated position with a partner, family, or group to feed and care for an individual for a long period also raises the stakes as to the quality of social bonds and responsiveness to a partner—any close social bond or commitment thus involves the communication of some kind, creating a fertile social framework for the expression of emotions and the expansion of cognitive abilities.
Older theories of animal behavior tended to imply, influenced by the views of the French philosopher René Descartes (1596–1650), that animals were mere automata without minds, morality, language, or general intelligence (Thomas, 2020). In this instinct-dominated model, any behavioral expression by an animal was not based on choice but was elicited by a present stimulus that determined the frequency and form of the response. The behavioral form is the same from episode to episode of its elicitation and across animals of the same kind (Epstein, 1982; Miller, 2013). Against the affect-based theories, Richard Lazarus had argued from the 1960s onwards (Lazarus, 1982) that cognitive processes precede emotional ones, establishing a clear link between cognition and emotions well before neuroscience could confirm the brain processes involved. He argued that cognitive processes generate, influence, and shape the emotional response in every species that react with emotion.
According to the Lazarus doctrine, cognition is not a postscript to emotions, but for any species, no matter how limited its cognitive abilities may be, any event or encounter in the environment undergoes some evaluative process first. This suggested that most organisms, as far as tested, should come with an array of cognitive skills. This has since been confirmed experimentally. For instance, tests of young chicks have shown some abilities to form abstract concepts using geometrical cues (Vallortigara et al., 1990; Tommasi and Vallortigara, 2004). Indeed, young chickens were found to come equipped with a “package” of conceptual skills in geometry, physics, and mathematics (Vallortigara et al., 2010). Among non-vertebrates, similar cognitive skills were identified. For instance, bees can acquire the ability to deal with conceptual relationships such as “above” and “below”, “same,” “different,” “larger than,” and “better than,” among others (Avarguès-Weber et al., 2011; Avarguès-Weber and Giurfa, 2013), and were recently shown to solve numerical cognition tasks (MaBouDi et al., 2021), but they may do so using quite different neural processes than birds or other vertebrates (Kaplan, 2015), and octopuses have multiple cognitive abilities that have now been identified (Amodio, 2019; Mather, 2022).
Hence, following several decades of research, it is now generally agreed that all of these elements described above—a basis in natural physics, mathematics, geometry, and natural psychology (for a review of these four pillars of animal cognition, see Vallortigara et al., 2010) is present in primates, many other mammals, birds, and even some insects so far tested.
The evaluative processes that animals may undertake, as Lazarus (1982) had argued, however, did not imply anything about deliberate reflection, rationality, or awareness but suggested that responses are based on learning and recall of previous and similar situations (accessible memory). Social learning undoubtedly plays a key role, both in an ecological and a psychological sense (Whiten and de Waal, 2018). Part of that learning process is taking note of someone else and, if a partner, that someone else may even be openly acknowledged by signs of affection (preening, for instance), in responding to requests, or in simple forms, by just walking in step, mirror imaging movements.
Both at functional levels and one that involves regulation of emotions in some way, duetting utilizes one single modality (audition), largely because individuals may be visually separated from one another (Smith et al., 2010). Duets may function as ways to reassure the two partners of their current location, be an example of mate-guarding or warn a potential intruder against invasion (Grafe and Bitz, 2004; Marshall-Ball et al., 2006). However, turn-taking in communication can happen in wider contexts and in visual contact with each other. Animals, be they diurnal or nocturnal, operate in a multi-sensory world (Partan and Marler, 1999; Hiramatsu et al., 2009). In addition to auditory information, individuals may simultaneously be exposed to and respond to visual and olfactory cues that may either confirm and strengthen the information received or contradict or annul information received in another modality (there is food but there is also a predator—a negative sensory input). Such stimuli combined may produce very different outcomes in behavior (Zhou et al., 2010). New World monkeys, such as the common marmoset (Callithrix jacchus), have a well-developed olfactory system and display a range of olfaction-based social behavior (Epple, 1993; Lazaro-Perea et al., 1999). As yet, however, there are too few studies that address the effects on the behavior of multi-modal signaling or incidental information on the response choices.
In our laboratory, we tested the idea of whether predator-naive marmosets (Callithrix j. jacchus) would show aversion to and withdrawal from fecal odors of predators and curiosity (approach) to food-based odors and found that marmosets perceive and respond to specific olfactory information and that olfaction may be more important for a broad range of functions not previously considered (Kemp and Kaplan, 2012). Although the importance of olfaction gradually declined in the primate line, it is worth remembering that olfaction has played an important role in perception apart from vision and audition (red-bellied tamarins: Caine and Weldon, 1989; cotton-top tamarins: Buchanan-Smith et al., 1993; wild mousse lemurs: Kappel et al., 2011). In the few research projects in which multimodal perception and responses have been investigated in detail, performance and success (whatever the measure might have been) tend to be enhanced by multimodal signaling. Rek and Magrath (2020), for instance, showed that visual display enhances vocal duet production in Australian magpie-larks, Grallina cyanoleuca.
Facial expressions, as visual stimuli, belong to another form of non-verbal communication that is shared by many primates, all apes, and humans, because we share the same facial musculature with the apes (Burrows, 2008). These expressions have been studied extensively, starting with Richard Andrew's first very detailed account (Andrew, 1963) and followed by an unbroken plethora of research publications until now, be this of great apes, some other primates, or humans (apes: van Lawick-Goodall, 1968; Parr and Waller, 2006; Kret et al., 2020; macaques: Hinde and Rowell, 1962; Partan, 2002; marmosets: Epple, 1967; Stevenson and Poole, 1976). We were interested to see how well marmosets could “read” the facial expressions of their cage mates and devised video footage, played back on large screens behind a food dish, and then tested whether specific facial responses to food and predator-related stimuli might act as social signals to conspecifics (Kemp and Kaplan, 2013). We recorded two contrasting facial expressions (fear and pleasure) as separate sets of video clips and then presented food together with these images of cage mates. Results showed that the expression of a fearful face on the screen significantly reduced time spent near the food bowl compared to the duration of staying near the food bowl when a face showing pleasure was screened.
These multifarious non-verbal forms of communication in addition to gestural signals (Fröhlich and Hobaiter, 2018) remind one that all these aspects of primate and human social life act in unison, in one body and often simultaneously, providing a rich palate of possible emotions, messages, and intentions to be interpreted by the recipient (Kret et al., 2020).
The central cognitive task lies in the ability of the partner, offspring, or wider group to read these signals correctly and in conjunction with one another (Fröhlich and van Schaik, 2018). Waller's objection to viewing these communicative acts together is that they may have different underlying cognitive processes (Waller et al., 2013). Processing simultaneous signals can be far more challenging than one might suspect. The combinatory signals allow for strong messages in the negative and positive sense (Crivelli and Fridlund, 2018) by providing tools for deception (Gyger and Marler, 1988), contradictions, ambiguities, and misunderstandings—a possibility that does not improve with the evolution of speech (Herman et al., 2022). The understanding of non-verbal messages is supported by the brain's mirroring system that is shaped by individual experience. Tight links, therefore, exist between action and perception, both within an individual and between several individuals (Roelfsema et al., 1997; Dinstein et al., 2007; Schippers et al., 2010).
Michael Corballis has been particularly persuasive over the years in his argument that gestural communication was the forerunner of human language evolution (Corballis, 2002, 2010). Many have agreed with him, and they have been supported by further evidence, as already mentioned, showing homologous areas of the human brain for speech production (Broca's area) and for language comprehension (Wernicke's area) are found in great apes and macaque brains (Cantalupo and Hopkins, 2001; Gil-da-Costa et al., 2006). Infants make pointing gestures spontaneously from an early age (Liszkowski et al., 2004), a key to understanding the development of language and theory of mind (Butterworth, 2003; Camaioni et al., 2004). Others have argued that the act of pointing is a complex cultural and cognitive behavior (Kita, 2003).
Undoubtedly, such evidence of referential gesturing adds to the duetting paradigm of coordinated action involving a conspecific. However, it is not enough to explain the substantial expansion of the hominin brain and the actual development of human speech because gesturing itself is already a clear sign of motivation to expand communication. The question is rather, what events, ecological and social circumstances, prompted and motivated the expansion of communication and cooperation in partners and groups.
To be in synchrony with another individual on a specific task may be the beginning of some ongoing collaboration (Duguid and Melis, 2020) and thus create openings for entering into some level of the ongoing bond. When Heyes (2009) summarized her research interest in imitation and mimicry in human development, she might as well have spoken about prosociality in primates, birds, and humans. Heyes said: “Imitation is an important and intriguing neurocognitive process: a process that bridges the gap between one mind and another; that powers cognitive and social development in infancy and childhood; that promotes empathy, cooperation and well-being in our relationships with others” (Heyes, 2009). How these variables might interact is presented in Figure 3, showing that cognition is both shaped by learning or knowledge already gained and memorized and by perception-action systems (Savage et al., 2020). Evolving sociality, synchronicity of movement, body or facial expression, or synchronized vocalizations, such as duetting, increases the chances for further communicative acts, including the development of a gestural repertoire that is shaped and enhanced by cognitive abilities.
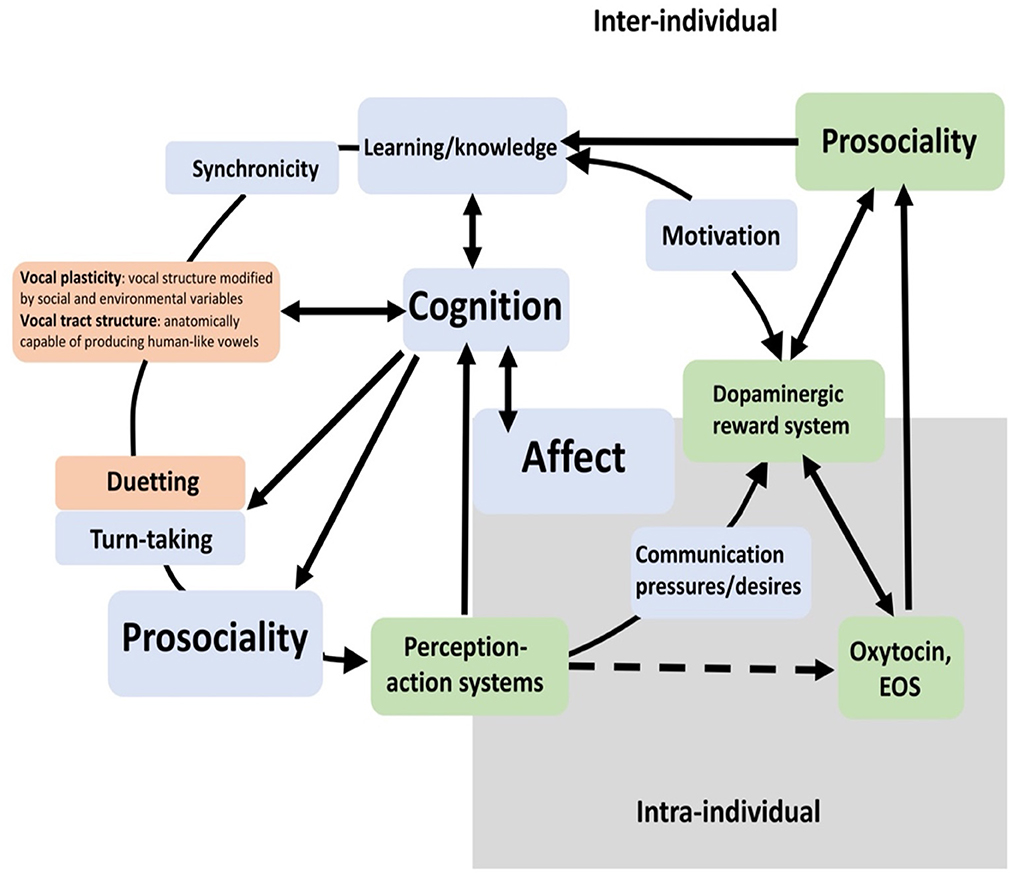
Figure 3. Prosociality in context—Adapted from Savage et al. (2020) (in green) and argument included from Snowdon (2018) (in orange). EOS refers to Endogenous Opioid System; see also the article by Hurlemann and Marsh (2019) which provides an overview of the neurobiology of prosocial behavior and the modulatory role of oxytocin in human prosociality.
Note that, in Figure 3, prosociality is not featured as central to this diagram but it occurs two times: as part of the affective system and as part of the cognitive system. The cognitive system relies on perception and then relays its emotional response via a network of prefrontal nuclei for learning and memory to action a response. Figure 3 also shows that motivation is influenced and reinforced by emotions which in turn are regulated by a set of reward hormones: the dopaminergic reward system, the endogenous opioid system, and oxytocin (Savage et al., 2020). These systems also regulate moods and behavior in humans. However, they can only become active and functional if the individual has developed an ability to identify and respond to social cues, such as gaze and head or body orientation, clearly beneficial for the survival of any social animal, even in fish (Leadner et al., 2021).
Prosocial tendencies represent the next cognitive leap (post simple synchrony) in that a conspecific, or a group of conspecifics, come to constitute valuable “others” and are recognized as having their personalities, needs, moods, and demands. In addition, prosocial tendencies seem to require some basic form of bonding with another individual or a group beyond a mother–infant bond (that, in birds, can be achieved by imprinting, McCabe, 2019). Prosociality is sometimes referred to as “self-other resonance” to emphasize the interactive nature of this trait (Christov-Moore and Iacoboni, 2016) and is as much a social, emotional, and a cognitive process.
In human developmental psychology, prosociality has been a key topic for research into children's and adolescent behavior (Ferraro, 2019), but it is relatively rarely considered in animals. One of the reasons why it is so central in human developmental psychology is defined by the behavioral damage done to adolescent individuals in whom “prosocial” attitudes are missing or are poorly developed (Meehan et al., 2019).
However, de Waal and Suchak (2010) discussed prosociality in non-human primates at some length and emphasized the difference between empathy and prosociality, as is also used in this study. In brief, empathy is the capacity of the observer to feel with and indirectly experience the emotional state or even pain of the observed, while prosocial responses can be entirely unselfconscious, unaware, and consist of spontaneous helpful acts that demand no reciprocity. Prosociality and empathy have in common that either may be readily expressed within the social network of the observer or, in rare cases, may also be extended to strangers (Norscia and Palagi, 2011; Decety et al., 2016).
In 2015, a specialist in evolutionary anthropology wrote an article that argued for human uniqueness on the grounds of three inherently human characteristics: an evolved advanced cognition, hyper-prosociality, and psychology for social learning (Marean, 2015). The narrative about human evolution and the development of human language as a set of linear prehistoric events seems to border on story-telling and certainly suggests an over-simplification, based on fossil finds that are possibly chronologically tens or even hundreds of thousands of years apart (de León et al., 2021). Nevertheless, fossil finds so far indicate that there was a modern human lineage in Africa (Templeton, 2002; Carotenuto et al., 2016; Husson et al., 2022), at least one archaic African lineage (Hammer et al., 2011), and two archaic Eurasian lineages, Neanderthals and Denisovans (Mithen, 2006; Petr et al., 2020; de León et al., 2021). Certainly, the hypotheses of human evolution are getting more complex. The more fossil skulls are found and the more improved techniques of dating them in the 21st century, the less clear are the genetic and anatomical elements, involving consideration of admixtures and radiations which have made some evolutionary trajectories more confusing and unresolved (Lieberman, 2001). It is clear, however, that there was a substantial increase in brain volume from chimps and bonobos to Homo habilis and to Homo erectus, as shown in Table 2.
In between the estimated departure dates of hominins from Africa, there are long periods without any fossil evidence of any kind, in which various human groups would presumably have moved about, probably in small isolated bands. Genetically, socially and cognitively, much could have transpired. All hominin lineages eventually went extinct, leaving one single remaining homo member of the large family tree and perhaps its survival was contingent on precisely the qualities that were described in this study. From very different perspectives, the present paper and Marean's article have arrived at the same conclusion of the centrality of cognition, prosociality, and the ability to work closely together, be these primates or humans.
Assuming the above is correct, the gap in explaining human language evolution is still large and might remain an open question. One can agree with Marean that the surviving branch was an “anomaly” in so far, as it was the only branch surviving despite tough climatic conditions and the species' very poor physical attributes. Humans, compared to other primates, had no fur to protect themselves from insects, from cold or heat, had poor climbing ability, only average speed in running, no claws, and little physical strength against any predators. But they did get one advantage: a large brain equipped for problem-solving and close cooperation, both enough to survive.
Human language is an arbitrary construct, and all bands of humans developed their own. One of the oldest living cultures in the world, Australia's Aboriginal culture, consisted of more than 250 nations and could boast as many languages, most of them bearing no similarity to each other (Blevins, 2001; Dixon and Dixon, 2011), except for the additional many dialects. There is no reason to think that all human communities developed language at the same time or had similar vocabulary sizes or even names for the same concepts or objects (Blevins, 2001; Dixon and Dixon, 2011).
An argument, rarely raised but possibly of substantial importance is to consider life histories in hominin species. Based on available evidence, John L. Locke and Barry Bogin did exactly that: they calculated the mean age of eruption of the first permanent molar and built the length of childhood around such available physical data. According to Locke and Bogin (2006), stages of childhood gradually lengthened from Homo habilis (3.8 years), early Homo erectus (4.5 years), and late Homo erectus (5.0 years) to Homo sapiens (6.2 years). Juvenile and adolescent stages also lengthened from 12 years in Homo habilis to 17 years in Homo sapiens (Locke and Bogin, 2006).
Lengthening childhood and juvenile stages over time suggests an increased biparental or family group commitment to protect and food-support their offspring for an ever-increasing period. We know from primates under group or biparental care, as well as from biparental care in avian species with protracted “childhoods”, that the offspring seem to get three main benefits from this delay in maturation: 1, protection (low-stress levels); 2, long learning time; and 3, more play time with other juveniles fostering prosocial development. These social conditions, as I have shown elsewhere (Kaplan, 2020b), tend to correlate with growing large brains. In chimpanzees, offspring are typically weaned at ~4 years of age, and thereafter the immatures of the western chimpanzees (Pan troglodytes verus), a subspecies of the genus Pan troglodytes, continue to associate with their mothers for up to 10 years beyond weaning (Samuni et al., 2020). From studies of both wild and captive gibbons, it is thought that gibbons reach sexual maturity at about 6–8 years of age, and the siamang (Hylobates syndactylus) at about 8–9 years (Geissmann, 1991). Similarily, in birds, some cockatoos reach sexual maturity when they are 6–8 years of age. To them and other avian species with similar life histories, the benefits tend to be identical to those in long-nurtured primates and hominin societies, such as longevity, cognitive complexity, and strong social bonds (Kaplan, 2019).
Finally, as Arbib (2013) rightly pointed out: “language” is not speech. Arbib (2013) and others before and since have seen song and dance as a bridge between music and language. The latter can exist as speech or in signs and can exploit voice, hands, and face (be this via voice utterances, whistles, drumming, clapping, and gesturing) using hearing and/or vision so that there is always a duality of patterning. To this day, there are sign languages, many whistled languages (Meyer, 2008), and also drum languages (Seifart et al., 2018; Ros, 2021). And there is dance combining rhythm, sound, and even song and movement. Laland et al. (2016) reminded us that dance has representational properties that “rely on the dancers' ability to imitate particular people, animals or events, as well as the audience's ability to recognize these correspondences.” The beginnings of language might well have occurred via imitation and mimicry of animals and were expressed in music and dance. Both are ubiquitous among humans (Lewis, 2009; Knight and Lewis, 2017). Mimicry of sound (entrainment to a musical beat) or of body movement (dance) is suggestive of the capabilities of motor and vocal imitation (Fitch, 2016; Laland et al., 2016; Fink et al., 2021). Mimicry of sounds, songs, and dance may first have evolved from imitated movements (say of animals they have seen and might have hunted) to communicate socially relevant information about them accurately. Indeed, such information could have been conveyed in many ways, be this via gestures, pointing, sound imitation, or even dancing. These articulations may well be processed by a similar neural network as those responsible for vocal learning in songbirds (Schuppe et al., 2022). Darwin thought that different aspects of language were acquired sequentially and possibly over vast stretches of evolutionary time. Vocal actions needed partners, such as in duetting (Clink and Lau, 2020; Clink et al., 2020) or turn-taking (Takahashi et al., 2016), joint-calling as in choruses (Mitani and Gros-Louis, 1998; De Gregorio et al., 2021, 2022), and referential signaling addressed to a conspecific or a family group and groups (Seyfarth et al., 1980; Snowdon, 2020; Vonk, 2020). And in such partnerships in dyadic or group vocalizations and movements, coalitions and partnerships were forged that could solve problems and innovate.
Tobias et al. (2016) argued that communal signaling (which includes duetting and choruses) is perhaps the most complex and least understood form of communication in social animals. They used Bayesian phylogenetic models to test whether acoustic communal signals are explained by a range of life history and environmental variables across 10,328 bird species worldwide and estimated that duets and choruses occur in some 1830 (18%), and in these, evolutionary transitions between communal signaling and solo signaling were “not explained by latitude, migration, climate, or habitat, and only weakly correlated with cooperative breeding. Instead, they are most strongly associated with year-round territoriality, typically in conjunction with stable social bonds” (Logue and Hall, 2014; Tobias et al., 2016).
I suspect that in some cases, if not all, prosociality was a vital step toward communicating with others on a broader basis, be this out of necessity or to share information that was about matters not immediately visible. Beyond the speculative, the neurobiological and anatomical evidence and the behavior of extant vertebrates, especially primates, have provided mounting evidence of the importance of the development of prosociality which makes its centrality in human language evolution very plausible.
In his treatise The Expression of the Emotions in Man and Animals (Darwin, 1872) and in chapters 2 and 3 of The Descent of Man (Darwin, 1871), Darwin talked about attention and imitation and he argued that if an individual can attend to something then it is possible for that individual either to imitate what it has seen or to be taught to do something (Kaplan and Rogers, 2004). Duetting and synchronized movements are both hallmarks of communication and group affiliations known in the primate line and particularly evident in many songbird species.
Furthermore, there is an ancestral social behavior network within the basal forebrain and midbrain that is common to all vertebrates from teleosts to birds and mammals and a mesolimbic reward system that forms a larger social decision-making network (Goodson, 2005; O'Connell and Hofmann, 2011). At the very least, one can say that a path to express and develop the ability for adaptive social behavior toward conspecifics has been in existence in ancient and well-preserved networks of the brain. Many research projects have also shown that interpersonal synchrony increases affiliation and increases cooperative behavior (Hove and Risen, 2009; Reddish et al., 2013). Note, however, that the social and vocal aspects of behavior can be mutually reinforcing. In a study of vocal behavior in bonobos, the researchers concluded that social bonds drive vocal exchanges (Levréro et al., 2019).
To have identified some potential sources for precursors of the evolution of human language should not be seen at the exclusion of many other evolutionary elements that might well have played into such a momentous innovation as speech. One might well speculate that any form of “language” in humans was evolutionarily a late development, suggested by complex activation of brain areas when such communicative acts occur. Kaan and Swaab (2002) found neuroimaging support for arguing that syntactical processing of multimodal information does not just recruit one specific brain area. Instead, a network of areas including Broca's area and anterior, middle, and superior areas of the temporal lobes are involved. Okobi et al. (2019) identified the neural control needed for duetting. Although this applied to Alston's singing mice, the model has been proposed as an emerging vocalization model also for duets in primates (Neff, 2019). Indeed, in primates, duetting happens to be one of the most convincing examples of vocal flexibility. How else would bonded couples achieve their voiced synchrony if it were not for the ability to adjust any specific features in vocal production, be they syntactical, rhythmic, or in frequency.
Anatomically, the road from pre-speech to speech in the hominid line was not blocked by the inability for vocal learning in primates or for lack of ability to form concepts, think of things past, and even plan a future. Primates and specifically great apes and some New World monkeys have shown remarkable cognitive abilities in solving problems and vocal learning.
Whether the gestural thesis of the origin of human language might explain the evolution of speech is not the point of argument here. The language might as well have developed via music and dance as said above. Moreover, “language” did not always result in speech as has also been pointed out above. These evolving systems of complex communication all reflect forms of self-expression as well as stable, communally agreed, unambiguous vocal labels for objects or concepts. While they well describe how rich in communicative abilities they may be, none of them show why any of them would have evolved in the first place.
The argument here has focussed on the possible motivators for the evolutionary precursors of such manifestations of communication.
First, in evolution, change tends to happen when an organism is stressed to fulfill its basic needs and/or when a small change in behavior or physiology gives one species a significant advantage over another. The hominid line had a poor record in meeting the challenges. All hominid ancestors eventually went extinct except Homo sapiens, suggesting that substantial innovations were needed to make this last hominin species viable. Studies on stress responses in modern humans interestingly found that stress triggers social approach behavior, which operates as a potent stress-buffering strategy, thereby providing evidence for the context and triggers in prosocial behavior, also referred to as the tend-and-befriend hypothesis (Von Dawans et al., 2012). How much speech has to do with it is yet another question.
Second, one constraint in the formulations of theories on human language evolution has been the need to remain focussed on one major variable, such as gestural origins, vocal synchronisations, and concept of musicality or dance. Hence, theories have tended to be single-focused on one singular candidate as a precursor of human language evolution. However, focus on any of these visual or vocal social expressions (and their expansions) is a focus on vocal expressions that are all, to varying degrees, outcomes in the communicative refinement of expressions of vocal and movement behavior.
Instead, this paper has posed the question of what impetus could have led to any of these impressive self-expressions and communicative complexities. It has been the contention of this study to ask why such outcomes occurred at all and which evolutionary steps had to precede these developments. As Hoehl et al. (2020) argued: Synchronizing benefits arise from an increased predictability of incoming signals and include many positive outcomes ranging from basic information processing at the individual level to the bonding of dyads and larger groups. Cooperative behavior, starting with specific vocal expressions such as duetting, fostered social cohesion (Launay et al., 2016). To achieve some synchronicity in duetting, as has been shown in many studies mentioned here, requires vocal flexibility. A recent study of lar gibbons, Hylobates lar (Raimondi et al., 2023), revealed not only substantial sophistication in the gibbon's rhythmic vocal expressions but showed that isochrony, at the core of human musicality, is present in lar gibbon duetting. Raimondi et al. (2023) found that gibbons are more isochronous when duetting than singing solo, achieving a higher-than-chance degree of synchrony in their duets because of this ability to rhythmically adjust their part of the duet and coordinate it (Raimondi et al., 2023).
In conclusion, the evolution of prosocial behavior may well be the vital precondition for, and the motivational link to, any expansion of cognition and communication and ultimately causally related to the evolution of human language. Furthermore, evidence that has been provided in the duetting literature of primates and dolphins is the degree of flexibility in vocal exchanges. The remarkable vocal communication among dolphins has no bearing on human language evolution but is a case of convergent evolution. Their social behavior also showed consistency in some other social factors, comparable with primates (King et al., 2022). Indeed, in dolphins and some avian species, the same or very similar basic biological and social factors can be observed: high cognitive ability, strong social bonds, and a high degree of vocal flexibility and individuality as the vocal labeling of dolphins (King et al., 2018). Clink et al. (2022) discovered flexibility in vocal exchanges of Gursky's spectral tarsier, Tarsius spectrum gurskyae. They rightly argued that vocal flexibility (and individuality) is a precursor to human language, and it evolved early in the primate lineage and long before the emergence of modern humans (Clink et al., 2022).
It seems from the physical evidence on record that joint actions led to more cooperation, more communication, further brain growth, better problem-solving, and a more secure place for humans in the natural environment, despite the many physical inadequacies of the modern human species. The motivation to pursue shared goals and indulge in creative models of ever-expanding communication, eventually language, also has to do with the extensive reward system the brain provided. This probably came about because positive rewards accompanied acts and attitudes of prosociality and this, in turn, helped increase affiliative bonds.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The author confirms being the sole contributor of this work and has approved it for publication.
This project was funded by the University of New England, Armidale, NSW, Australia.
I thank Dr. Patrice Adret for excellent editorial support, am very grateful to my university for funding this project, and am thankful for the very helpful comments on this paper by Prof. Lesley Rogers.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abrahamczyk, S., Souto-Vilarós, D., and Renner, S. S. (2014). Escape from extreme specialization: passionflowers, bats and the sword-billed hummingbird. Proc. R. Soc. B: Biol. Sci. 281, 20140888. doi: 10.1098/rspb.2014.0888
Adret, P. (2022). Developmental plasticity in primate coordinated song: parallels and divergences with duetting songbirds. Front. Ecol. Evol. 10, 862196. doi: 10.3389/fevo.2022.862196
Adret, P., Dingess, K. A., Caselli, C. B., Vermeer, J., Martínez, J., and Luna Amancio, J. C. (2018). Duetting patterns of titi monkeys (Primates, Pitheciidae: Callicebinae) and relationships with phylogeny. Animals 8, 178–211. doi: 10.3390/ani8100178
Aldrich, B. C., Feddema, K., Fourage, A., Nekaris, K. A.I., and Shanee, S. (2023). “Primate portrayals: narratives and perceptions of primates in entertainment,” in Primates in Anthropogenic Landscapes,eds T. McKinney, S. Waters, and M. A. Rodrigues (Cham: Springer), 307–326.
Amici, F., Visalberghi, E., and Call, J. (2014). Lack of prosociality in great apes, capuchin monkeys and spider monkeys: convergent evidence from two different food distribution tasks. Proc. R. Soc. B:Biol. Sci. 281, 1699. doi: 10.1098/rspb.2014.1699
Amodio, D. M., and Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277. doi: 10.1038/nrn1884
Amodio, P. (2019). Octopus intelligence: the importance of being agnostic. Anim. Senti. 4, 20. doi: 10.51291/2377-7478.1507
Amorim, P. S., Diniz, P., Rossi, M. F., and Guaraldo, A. C. (2022). Out of sight, out of mind: dear enemy effect in the rufous hornero, Furnarius rufus. Anim. Behav. 187, 167–176. doi: 10.1016/j.anbehav.2022.03.010
Andrew, R. J. (1963). The origin and evolution of the calls and facial expressions of the primates. Behaviour 20, 1–107. doi: 10.1163/156853963X00220
Andrew-DeWoody, J., Fletcher, D. E., David Wilkins, S., Nelson, W. S., and Avise, J. C. (2000). Genetic monogamy and biparental care in an externally fertilizing fish, the large mouth bass (Micropterus salmoides). Proc. R. Soc. Series B Biol. Sci. 267, 2431–2437. doi: 10.1098/rspb.2000.1302
Andrieu, J., Penny, S. G., Bouchet, H., Malaivijitnond, S., Reichard, U. H., et al. (2020). White-handed gibbons discriminate context-specific song compositions. PeerJ 8, e9477. doi: 10.7717/peerj.9477
Arbib, M. A. (2013). Language, Music, and the Brain. A Mysterious Relationship. Cambridge, MA; London: MIT Press.
Ardesch, D. J., Scholtens, L. H., Li, L., Preuss, T. M., Rilling, J. K., and van den Heuvel, M. P. (2019). Evolutionary expansion of connectivity between multimodal association areas in the human brain compared with chimpanzees. PNAS 116, 7101–7106. doi: 10.1073/pnas.1818512116
Arriaga, G., and Jarvis, E. D. (2013). Mouse vocal communication system: are ultrasounds learned or innate? Brain Lang. 124, 96–116. doi: 10.1016/j.bandl.2012.10.002
Atzil, S., Hendler, T., Zagoory-Sharon, O., Winetraub, Y., and Feldman, R. (2012). Synchrony and specificity in the maternal and the paternal brain: relations to oxytocin and vasopressin. J. Am. Acad. Child Adolesc. Psychiatry 51, 798–811. doi: 10.1016/j.jaac.2012.06.008
Aureli, F., and Schino, G. (2019). Social complexity from within: how individuals experience the structure and organization of their groups. Behav. Ecol. Sociobiol. 73, 6. doi: 10.1007/s00265-018-2604-5
Avarguès-Weber, A., Dyer, A. G., and Giurfa, M. (2011). Conceptualization of above and below relationships by an insect. Proc. R. Soc. B 278, 898–905. doi: 10.1098/rspb.2010.1891
Avarguès-Weber, A., and Giurfa, M. (2013). Conceptual learning by miniature brains. Proc. R. Soc. B 280, 1907. doi: 10.1098/rspb.2013.1907
Bailey, W. J. (2003). Insect duets: underlying mechanisms and their evolution. Physiol. Entomol. 28, 157–174. doi: 10.1046/j.1365-3032.2003.00337.x
Baker-Médard, M. S., Baker, M. C., and Logue, D. M. (2013). Chorus song of the Indri (Indri indri: Primates, Lemuridae): group differences and analysis of within-group vocal interactions. e-scholarship Publishing, University of California. Available online at: https://escholarship.org/uc/item/0gg070fd
Bales, K. L., Ardekani, C. S., Baxter, A., Karaskiewicz, C. L., Kuske, J. X., et al. (2021). What is a pair bond? Horm. Behav. 136, 105062. doi: 10.1016/j.yhbeh.2021.105062
Banerjee, A,., and Vallentin, D. (2022). Convergent behavioral strategies and neural computations during vocal turn-taking across diverse species. Curr. Opin. Neurobiol. 73, 102529. doi: 10.1016/j.conb.2022.102529
Banovac, I., Sedmak, D., Judaš, M., and Petanjek, Z. (2021). Von Economo neurons–primate-specific or commonplace in the mammalian brain?. Front. Neur. Circ. 89, 714611. doi: 10.3389/fncir.2021.714611
Barón Birchenall, L. (2016). Animal communication and human language: an overview. Intern. J Comp. Psych. 29, 1–26. doi: 10.46867/ijcp.2016.29.00.07
Barrett, L., and Henzi, P. (2005). The social nature of primate cognition. Proc. R. Soc. B 272, 1865–1875. doi: 10.1098/rspb.2005.3200
Barton, R. A. (1998). Visual specialization and brain evolution. Proc. R. Soc. B 265, 1933–1937. doi: 10.1098/rspb.1998.0523
Bateson, P. P. G., and Martin, P. (2013). Play, Playfulness, Creativity and Innovation. Cambridge: Cambridge University Press.
Bernieri, F., and Rosenthal, R. (1991). “Interpersonal coordination: behavior matching and interactional synchrony,” in Fundamentals of Nonverbal Behavior, eds R. S. Feldman, and B. Rime (Cambridge: Cambridge University Press,) 401–431.
Blevins, J. (2001). Nhanda: An Aboriginal Language of Western Australia. University of Hawai'i Press.
Boysen, S. T., and Bernston, G. G. (1989). Numerical competence in a chimpanzee (Pan troglodytes). J. Comp. Psychol. 10323, 23. doi: 10.1037/0735-7036.103.1.23
Buchanan-Smith, H. M., Anderson, D. A., and Ryan, C. W. (1993). Responses of cotton-top tamarins (Saguinus oedipus) to faecal scents of predators and non-predators. Anim. Welf. 2, 17–32. doi: 10.1017/S0962728600015438
Burkart, J. M., and van Schaik, C. P. (2020). Marmoset prosociality is intentional. Anim.Cogn. 23, 581–594. doi: 10.1007/s10071-020-01363-6
Burrows, A. M. (2008). The facial expression musculature in primates and its evolutionary significance. Bioessays 30, 212–225. doi: 10.1002/bies.20719
Butterworth, G. (2003). “Pointing is the royal road to language for babies,” in Pointing: Where Language, Culture, and Cognition Meet, ed S. Kita (Mahwah, NJ: Erlbaum), 9–33.
Caesar, C., and Zuberbuehler, K. (2012). Referential alarm calling behaviour in New World primates. Curr. Zool. 58, 680–697. doi: 10.1093/czoolo/58.5.680
Caine, N. G., and Weldon, P. J. (1989). Responses by red-bellied tamarins (Saguinus labiatus) to fecal scents of predatory and non-predatory neotropical mammals. Biotropica 21, 186–189. doi: 10.2307/2388709
Caley, T., Extier, T., Collins, J. A., Schefuß, E., Dupont, L., Malaizé, B., et al. (2018). A two-million-year-long hydroclimatic context for hominin evolution in southeastern Africa. Nature 560, 76–79. doi: 10.1038/s41586-018-0309-6
Camaioni, L., Perucchini, P., Bellagamba, F., and Colonnesi, C. (2004). The role of declarative pointing in developing a theory of mind. Infancy 5, 291–308, doi: 10.1207/s15327078in0503_3
Cantalupo, C., and Hopkins, W. D. (2001). Asymmetric Broca's area in great apes. Nature 414, 505. doi: 10.1038/35107134
Card, N. A., Stucky, B. D., Sawalani, G. M., and Little, T. D. (2008). Direct and indirect aggression during childhood and adolescence: a meta-analytic review of gender differences, intercorrelations, and relations to maladjustment. Child Dev. 79, 1185–1229. doi: 10.1111/j.1467-8624.2008.01184.x
Carotenuto, F., Tsikaridze, N., Rook, L., Lordkipanidze, D., Longo, L., Condemi, S., et al. (2016). Venturing out safely: the biogeography of Homo erectus dispersal out of Africa. J. Hum. Evol. 95, 1–12. doi: 10.1016/j.jhevol.2016.02.005
Carson, D. C. (2013). Perceptions of prosocial and delinquent peer behavior and the effect on delinquent attitudes: a longitudinal study. J. Crim. Just. 41, 151–161. doi: 10.1016/j.jcrimjus.2013.01.005
Christov-Moore, L., and Iacoboni, M. (2016). Self-other resonance, its control and prosocial inclinations: brain-behavior relationships. Hum. Brain Mapp. 37, 1544–1558. doi: 10.1002/hbm.23119
Cieri, R. L., Churchill, S. E., Franciscus, R. G., Tan, J., and Hare, B. (2014). Craniofacial feminization, social tolerance, and the origins of behavioral modernity. Curr. Anthropol. 55, 419–443. doi: 10.1086/677209
Clarke, E., Reichard, U. H., and Zuberbühler, K. (2006). The syntax and meaning of wild gibbon songs. PLoS ONE 1, e73. doi: 10.1371/journal.pone.0000073
Clink, D. J., Comella, I. A., Tasirin, J. S., and Klinck, H. (2022). Tarsier islands: exploring patterns of variation in tarsier duets from offshore islands of North Sulawesi. Am. J. Primat. e23410. doi: 10.1002/ajp.23410
Clink, D. J., and Lau, A. R. (2020). Adherence to Menzerath's Law is the exception (not the rule) in three duetting primate species. R. Soc. Open Sci. 7, 201557. doi: 10.1098/rsos.201557
Clink, D. J., Tasirin, J. S., and Klinck, H. (2020). Vocal individuality and rhythm in male and female duet contributions of a nonhuman primate. Curr. Zool. 66, 173–186. doi: 10.1093/cz/zoz035
Clink, D. J., Zafar, M., Ahmad, A. H., and Lau, A. R. (2021). Limited evidence for individual signatures or site-level patterns of variation in male northern gray gibbons (Hylobates funereus) Duet Codas. Intern. J. Primatol. 42, 896–914. doi: 10.1007/s10764-021-00250-2
Clutton-Brock, T. (1991). The Evolution of Parental Care. Princeton, NJ: Princeton University Press.
Cockburn, A. (2006). Prevalence of different modes of parental care in birds. Proc. R. Soc. B 273, 1375–1383. doi: 10.1098/rspb.2005.3458
Coleman, M. J., Day, N. F., Rivera-Parra, P., and Fortune, E. S. (2021). Neurophysiological coordination of duet singing. PNAS 118, e2018188118. doi: 10.1073/pnas.2018188118
Corballis, M. C. (2002). From Hand to Mouth: The Origins of Language. Princeton, NJ: Princeton University Press.
Corballis, M. C. (2010). Language as gesture. Hum. Mov. Sci. 28, 556–565. doi: 10.1016/j.humov.2009.07.003
Coss, R. G., Cavanaugh, C., and Brennan, W. (2019). Development of snake-directed antipredator behavior by wild white-faced capuchin monkeys: III. The signaling properties of alarm-call tonality. Am. J. Primatol. 81, e22950. doi: 10.1002/ajp.22950
Courts, R., Erbe, C., Wellard, R., Boisseau, O., Jenner, K. C., and Jenner, M. N. (2020). Australian long-finned pilot whales (Globicephala melas) emit stereotypical, variable, biphonic, multi-component, and sequenced vocalisations, similar to those recorded in the northern hemisphere. Sci. Rep. 10, 1–14. doi: 10.1038/s41598-020-74111-y
Crivelli, C., and Fridlund, A. J. (2018). Facial displays are tools for social influence. Trends Cogn. Sci. 22, 388–399. doi: 10.1016/j.tics.2018.02.006
Cronin, K. A. (2012). Prosocial behaviour in animals: the influence of social relationships, communication and rewards. Anim. Behav. 84, 1085–1093. doi: 10.1016/j.anbehav.2012.08.009
Dahlin, C. R., and Benedict, L. (2014). Angry birds need not apply: a perspective on the flexible form and multifunctionality of avian vocal duets. Ethology 120, 1–10. doi: 10.1111/eth.12182
Darwin, C. (1859). The Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. London: John Murray.
Das, A., and Gilbert, C. D. (1995). Receptive field expansion in adult visual cortex is linked to dynamic changes in strength of cortical connections. J. Neurophysiol. 74, 779–792. doi: 10.1152/jn.1995.74.2.779
De Casien, A. R., and Higham, J. P. (2019). Primate mosaic brain evolution reflects selection on sensory and cognitive specialization. Nat. Ecol. Evol. 3, 1483–1493. doi: 10.1038/s41559-019-0969-0
De Gregorio, C., Carugati, F., Estienne, V., Valente, D., Raimondi, T., Torti, V., et al. (2021). Born to sing! Song development in a singing primate. Curr. Zool. 67, 585–596. doi: 10.1093/cz/zoab018
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evo. 34, 205–219. doi: 10.1080/03949370.2021.2015451
De Gregorio, C., Zanoli, A., Valente, D., Torti, V., Bonadonna, G., Randrianarison, R. M., et al. (2019). Female indris determine the rhythmic structure of the song and sustain a higher cost when the chorus size increases. Curr. Zool. 65, 89–97. doi: 10.1093/cz/zoy058
de León, M. S. P., Bienvenu, T., Marom, A., Engel, S., Tafforeau, P., Warren, J. L. A., et al. (2021). The primitive brain of early Homo. Science 372, 165–171. doi: 10.1126/science.aaz0032
de Waal, F. B. M., and Suchak, M. (2010). Prosocial primates: Selfish and unselfish motivations. Philos. Trans. R. Soc. B 365, 2711–2722. doi: 10.1098/rstb.2010.0119
Deacon, T. W. (2003). “Multilevel selection in a complex adaptive system: the problem of language origins,” in Evolution and Learning: The Baldwin Effect Reconsidered, eds B. H. Weber, and D. J. Depew (Cambridge, MA: MIT Press).
Decety, J., Bartal, I. B.-A., Uzefovsky, F., and Knafo-Noam, A. (2016). Empathy as a driver of prosocial behaviour: highly conserved neurobehavioural mechanisms across species. Philos. Trans. R. Soc. B 371, 20150077. doi: 10.1098/rstb.2015.0077
Dinstein, I., Hasson, U., Rubin, N., and Heeger, D. J. (2007). Brain areas selective for both observed and executed movements. J. Neurophysiol. 198, 1415–1427. doi: 10.1152/jn.00238.2007
Dixon, R. M., and Dixon, R. M. (2011). The Languages of Australia. Melbourne, VIC: Cambridge University Press.
Dixson, A. F. (2009). Sexual Selection and the Origins of Human Mating Systems. Oxford: Oxford University Press.
Dolotovskaya, S., Walker, S., and Heymann, E. W. (2020). What makes a pair bond in a Neotropical primate: female and male contributions. R. Soc. Open Sci. 7, 191489. doi: 10.1098/rsos.191489
Donald, J. N., Bradshaw, E. L., Conigrave, J. H., Parker, P. D., Byatt, L. L., and Noetel, M. (2021). Paths to the light and dark sides of human nature: a meta-analytic review of the prosocial benefits of autonomy and the antisocial costs of control. Psychol. Bull. 147, 921. doi: 10.1037/bul0000338
Dowling, J., and Webster, M. S. (2018). Acoustic and physical mate guarding have different effects on intruder behaviour in a duetting songbird. Anim. Behav.135, 69–75. doi: 10.1016/j.anbehav.2017.11.011
Duguid, S., and Melis, A. P. (2020). How animals collaborate: underlying proximate mechanisms. Wiley Interdisc. Rev. Cogn. Sci. 11, e1529. doi: 10.1002/wcs.1529
Dukes, D., Abrams, K., Adolphs, R., Ahmed, M. E., Beatty, A., Berridge, K. C., et al. (2021). The rise of affectivism. Nat. Hum. Behav. 5, 816–820. doi: 10.1038/s41562-021-01130-8
Dunbar, R. I. M. (1998). The social brain hypothesis. Evol. Anthrop. 6, 178–190. doi: 10.1002/(SICI)1520-6505(1998)6:5<178::AID-EVAN5>3.0.CO;2-8
Epple, G. (1967). Vergleichende Untersuchungen über Sexual-und Sozialverhalten der Krallenaffen (Hapalidae). Folia Primatol. 7, 37–65. doi: 10.1159/000155095
Epple, G. (1993). “Making sense out of scents: species differences in scent glands, scent-marking behaviour, and scent-marking composition in the Callitrichidae,” in Marmosets and Tamarins: Systematics, Behaviour and Ecology, ed A. B. Rylands (Oxford: Oxford University Press), 123–151.
Epstein, A. N. (1982). “Instinct and motivation as explanations for complex behavior,” in The Physiological Mechanisms of Motivation, ed D. W. Pfaff (New York, NY: Springer), 25–58.
Etzel, R., Cornish, M., Kifer, M. S., Nuñez, L., Valladao, G., and Folt, B. (2020). Subterranean advertisement and duet calling behavior in Ptychohyla legleri (Legler's Stream Frog). Alytes 37, 57–61. doi: 10.6084/m9.figshare.11797191.v1
Fedorova, N., Evans, C. L., and Byrne, R. W. (2017). Living in stable social groups is associated with reduced brain size in woodpeckers (Picidae). Biol. Lett. 13, 20170008. doi: 10.1098/rsbl.2017.0008
Fedurek, P., and Slocombe, K. E. (2011). Primate vocal communication: a useful tool for understanding human speech and language evolution? Human Biol. 83, 153–173. doi: 10.3378/027.083.0202
Feistner, A. T., and McGrew, W. C. (1989). Food-sharing in primates: a critical review. Persp. in Primate Biol. 3, 21–36.
Ferraro, F. R. (2019). Special issue on psychology of prosocial behavior. Curr. Psych. 38, 909. doi: 10.1007/s12144-019-00336-4
Ferreira, L. S., Sábato, V., Pinheiro, T. A., Neto, E., Rocha, L. H., Baumgarten, J., et al. (2022). Long-distance counter calling in maned wolves: friends or foes? Animal 12, 1081. doi: 10.3390/ani12091081
Fink, B., Bläsing, B., Ravignani, A., and Shackelford, T. K. (2021). Evolution and functions of human dance. Evol. Human Behav. 42, 351–360. doi: 10.1016/j.evolhumbehav.2021.01.003
Fitch, W. T. (2013). “Musical protolanguage: Darwin's theory of language evolution revisited,” in Birdsong, Speech, and Language: Exploring the Evolution of Mind and Brain, eds R. C. Berwick and N. Chomsky (Cambridge, MA: MIT Press), 489–504.
Fitch, W. T. (2016). Dance, music, meter and groove: a forgotten partnership. Front. Hum. Neurosci. 10, 64. doi: 10.3389/fnhum.2016.00064
Fitch, W. T. (2020). Animal cognition and the evolution of human language: why we cannot focus solely on communication. Philos. Trans. R. Soc. B 375, 20190046. doi: 10.1098/rstb.2019.0046
Forcina, G., Vallet, D., Le Gouar, P. J., Bernardo-Madrid, R., Illera, G., et al. (2019). From groups to communities in western lowland gorillas. Proc. R. Soc. B 286, 20182019. doi: 10.1098/rspb.2018.2019
Forester, D. C., and Harrison, W. K. (1987). The significance of antiphonal vocalisation by the spring peeper, Pseudacris crucifer (Amphibia, Anura). Behaviour 103, 1–15. doi: 10.1163/156853987X00233
Fröhlich, M., and Hobaiter, C. (2018). The development of gestural communication in great apes. Behav. Ecol. Sociobiol. 72, 1–14. doi: 10.1007/s00265-018-2619-y
Fröhlich, M., and van Schaik, C. P. (2018). The function of primate multimodal communi-cation. Anim. Cogn. 21, 619–629. doi: 10.1007/s10071-018-1197-8
Fuentes, A. (2000). Hylobatid Communities: Changing views on pair bonding and social organization in hominoids. Yrbk Phys. Anthrop. 43, 33–60. doi: 10.1002/1096-8644(2000)43:31+<33::AID-AJPA3>3.0.CO;2-D
Gannon, P. J., Holloway, R. L., Broadfield, D. C., and Braun, A. R. (1998). Asymmetry of chimpanzee planum temporale: humanlike pattern of Wernicke's brain language area homolog. Science 279, 220–222. doi: 10.1126/science.279.5348.220
Gardner, A. R.A., Gardner, B. T., and van Cantfort, T. E. (1989). Teaching Sign Language to Chimpanzees. Albany, NY: State University of New York Press).
Gardner, R. A., and Gardner, B. T. (1969). Teaching sign language to a chimpanzee: a standardized system of gestures provides a means of two-way communication with a chimpanzee. Science 165, 664–672. doi: 10.1126/science.165.3894.664
Gasser, B., and Arbib, M. (2019). A dyadic brain model of ape gestural learning, production and representation. Anim. Cogn. 22, 519–534. doi: 10.1007/s10071-018-1228-5
Geissmann, T. (1991). Reassessment of age of sexual maturity in gibbons (hylobates spp.). Am. J. Primatol. 23, 11–22. doi: 10.1002/ajp.1350230103
Geissmann, T. (2000). “Gibbon songs and human music from an evolutionary perspective” in The Origins of Music, eds N. L. Wallin, B. Merker, and S. Brown (Cambridge, MA: The MIT Press). Ch.7.
Gil-da-Costa, R., Martin, A., Lopes, M. A., Munoz, M, Fritz, J. B., and Braun, A. R. (2006). Species-specific calls activate homologs of Broca's and Wernicke's areas in the macaque. Nat. Neurosci. 9, 1064–1070. doi: 10.1038/nn1741
Goodson, J. L. (2005). The vertebrate social behaviour network: Evolutionary themes and variations. Horm. Behav. 48, 11–22. doi: 10.1016/j.yhbeh.2005.02.003
Grafe, T. U., and Bitz, J. H. (2004). Functions of duetting in the tropical boubou, Laniarius aethiopicus: territorial defence and mutual mate guarding. Anim. Behav. 68, 193–201. doi: 10.1016/j.anbehav.2003.11.003
Grant, P. R. (2017). Ecology and Evolution of Darwin's Finches (Princeton Science Library Edition). Princeton, NJ: Princeton University Press.
Greenfield, P. M., and Savage-Rumbaugh, E. S. (1990). “Grammatical combination in Pan paniscus: processes of learning and invention in the evolution and development of language,” in “Language” and Intelligence in Monkeys and Apes: Comparative Developmental Perspectives, ed S. T.Parker (Cambridge: Cambridge UP), 540–578.
Güroglu, B., van den Bos, W., and Crone, E. A. (2014). Sharing and giving across adolescence: an experimental study examining the development of prosocial behaviour. Front. Psych. 5, 291. doi: 10.3389/fpsyg.2014.00291
Gyger, M., and Marler, P. (1988). Food calling in the domestic fowl, Gallus gallus: the role of external referents and deception. Anim. Behav. 36, 358–365. doi: 10.1016/S0003-3472(88)80006-X
Haimoff, E. H. (1986). Convergence in the duetting of monogamous old world primates. J. Human Evol. 15, 51–59. doi: 10.1016/S0047-2484(86)80065-3
Hall, M. L., and Magrath, R. D. (2007). Temporal coordination signals coalition quality. Curr. Biol. 17, R406–R407. doi: 10.1016/j.cub.2007.04.022
Hammer, M. F., Woerner, AE, Mendez, F. L., Watkins, J. C., and Wall, J. D. (2011). Genetic evidence for archaic admixture in Africa. PNAS 108, 15123–15128 doi: 10.1073/pnas.1109300108
Haraway, M. M., and Maples, E. G. (1998). Flexibility in the species-typical songs of gibbons. Primates 39, 1–12. doi: 10.1007/BF02557739
Hare, B. (2017). Survival of the Friendliest: homo sapiens evolved via selection for prosociality. Annu. Rev. Psych. 68, 155–186. doi: 10.1146/annurev-psych-010416-044201
Hare, B., and Tomasello, M. (2005). Human-like social skills in dogs? Trends Cogn. Sci. 9, 439–444. doi: 10.1016/j.tics.2005.07.003
Hare, B., Wobber, V., and Wrangham, R. (2012). The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Anim. Behav. 83, 573–585. doi: 10.1016/j.anbehav.2011.12.007
Harting, J. K., Glendenning, K. K., Diamond, I. T., and Hall, W. C. (1973). Evolution of the primate visual system: anterograde degeneration studies of the tecto-pulvinar system. Am. J. Phys. Anthrop. 38, 383–392. doi: 10.1002/ajpa.1330380237
Henry, C. S., Brooks, S. J., Duelli, P., Johnson, J. B., Wells, M. M., and Mochizuki, A. (2013). Obligatory duetting behaviour in the Chrysoperla carnea-group of cryptic species (Neuroptera: Chrysopidae): its role in shaping evolutionary history. Biol. Rev. 88, 787–808. doi: 10.1111/brv.12027
Herman, H., Purba, R., Sijabat, P. A., Saputra, N., and Van Thao, N. (2022). Investigating the realization of speech function in a speech through systemic functional linguistics perspective. Script J. Ling. Engl. Teach. 7, 31–41. doi: 10.24903/sj.v7i1.917
Heyes, C. (2009). Evolution, development and intentional control of imitation. Philos. Trans. R. Soc. B 364, 2293–2298. doi: 10.1098/rstb.2009.0049
Hill, J., Inder, T., Neil, J., Dierker, D., Harwell, J., and Van Essen, D. (2010). Similar patterns of cortical expansion during human development and evolution. PNAS 107, 13135–13140. doi: 10.1073/pnas.1001229107
Hinde, R. A., and Rowell, T. E. (1962). Communication by postures and facial expressions in the rhesus monkey (Macaca mulatta). Proc. Zool. Soc. London 138, 1–21. doi: 10.1111/j.1469-7998.1962.tb05684.x
Hiramatsu, C., Melin, A. D., Aureli, F., Schaffner, C. M., Vorobyev, M., and Kawamura, S. (2009). Interplay of olfaction and vision in fruit foraging of spider monkeys. Anim. Behav. 77, 1421–1426. doi: 10.1016/j.anbehav.2009.02.012
Hobaiter, C., Graham, K. E., and Byrne, R. W. (2022). Are ape gestures like words? Outstanding issues in detecting similarities and differences between human language and ape gesture. Philos. Transact. R. Soc. B 377, 20210301. doi: 10.1098/rstb.2021.0301
Hockett, C. F. (1959). “Animal ≪languages≫ and human language,” in The Evolution of Man's Capacity for Culture, ed J. N. Spuhler (Detroit: Wayne State UP), 32–39.
Hoehl, S., Fairhurst, M., and Schirmer, A. (2020). Interactional synchrony: signals, mechanisms, and benefits. Soc.Cogn. Affect. Neurosci. 16, 5–18. doi: 10.1093/scan/nsaa024
Hoffmann, S., Trost, L., Voigt, C., Leitner, S., Lemazina, A., and Sagunsky, H. (2019). Duets recorded in the wild reveal that inter-individually coordinated motor control enables cooperative behavior. Nat. Commun. 10, 2577. doi: 10.1038/s41467-019-10593-3
Hopkins, W. D. (2022). Neuroanatomical asymmetries in nonhuman primates in the homologs to Broca's and Wernicke's areas: a mini-review. Emerg. Top. Life Sci. 6, 271–284. doi: 10.1042/ETLS20210279
Hopkins, W. D., Marino, L., Rilling, J. K., and MacGregor, L. A. (1998). Planum temporale asymmetries in great apes as revealed by magnetic resonance imaging (MRI). NeuroRep 9, 2913–2918. doi: 10.1097/00001756-199808240-00043
Hove, M. J., and Risen, J. L. (2009). It's all in the timing: interpersonal synchrony increases affiliation. Soc. Cogn. 27, 949–960. doi: 10.1521/soco.2009.27.6.949
Hurlemann, R., and Marsh, N. (2019). Unraveling the role of oxytocin in the motivational structure of conflict. Behav. Brain Sci. 42, e126. doi: 10.1017/S0140525X19000785
Husson, L., Salles, T., Lebatard, A. E., Zerathe, S., Braucher, R., Noerwidi, S., et al. (2022). Javanese Homo erectus on the move in SE Asia circa 1.8 Ma. Sci. Rep. 12, 1–12. doi: 10.1038/s41598-022-23206-9
Hyder, F., Rothman, D. L., and Bennett, M. R. (2013). Cortical energy demands of signaling and nonsignaling components in brain are conserved across mammalian species and activity levels. PNAS 110, 3549–3554. doi: 10.1073/pnas.1214912110
Isler, K., and van Schaik, C. P. (2009). The expensive brain: a framework for explaining evolutionary changes in brain size. J Hum. Evol. 57, 392–400. doi: 10.1016/j.jhevol.2009.04.009
Issa, M. M., Sikora, B., Rusiecki, S., and Osiejuk, T. S. (2023). The Yellow-breasted Barbet (Trachyphonus margaritatus) introduces vocal duets and choruses with a specific multimodal signal, during territorial advertisement. J. Ornithol. 164, 183–192. doi: 10.1007/s10336-022-02016-w
Jaeggi, A. V., and Gurven, M. (2013). Natural cooperators: food sharing in humans and other primates. Evol. Anthrop. Issues News Rev. 22, 186–195. doi: 10.1002/evan.21364
Janik, V. M., and Knörnschild, M. (2021). Vocal production learning in mammals revisited. Phil. Transact. Roy. Soc. B. 376, 20200244. doi: 10.1098/rstb.2020.0244
Kaan, E., and Swaab, T. Y. (2002). The brain circuitry of syntactic comprehension. Trends Cogn. Sci. 6, 350–356. doi: 10.1016/S1364-6613(02)01947-2
Kaplan, G. (2015). Bird Minds. Cognition and Behaviour of Australian Native Birds. Melbourne, VIC: CSIRO Publishing.
Kaplan, G. (2019). Bird Bonds. Sex, Mate-choice and Cognition in Australian Native Birds. Sydney, NSW: Pan Macmillan.
Kaplan, G. (2020a). Long-term attachments and complex cognition in birds and humans are linked to pre-reproductive prosociality and cooperation. Constructing a hypothesis. Ann. Cogn. Sci. 4,127–142. doi: 10.36959/447/347
Kaplan, G. (2020b). Play behaviour, not tool using, relates to brain mass in a sample of birds. Sci. Rep. 0, 20437. doi: 10.1038/s41598-020-76572-7
Kaplan, G. (2023). The evolution of social play in songbirds, parrots and cockatoos - emotional or highly complex cognitive behaviour or both? Neurosci. Biobehav. Rev. [In Preparation].
Kaplan, G., and Rogers, L. J. (2000). The Orang-Utans. Their Evolution, Behavior, and Future. Cambridge, M: Perseus Publishing.
Kaplan, G., and Rogers, L. J. (2004). “Charles Darwin and animal behaviour,” in Encyclopaedia of Animal Behaviour, 3 vols), eds M. Bekoff, and J. Goodall (Westport CT: Greenwood Publishing), Introductory essay to vol. 2, 471–479.
Kappel, P., Hohenbrink, S., and Radespiel, U. (2011). Experimental evidence for olfactory predator recognition in wild mouse lemurs. Am. J. Primatol. 73, 928–938. doi: 10.1002/ajp.20963
Kappeler, P. M., Clutton-Brock, T., Shultz, S., and Lukas, D. (2019). Social complexity: patterns, processes, and evolution. Behav. Ecol. Sociobiol. 73, 5. doi: 10.1007/s00265-018-2613-4
Kemp, C., and Kaplan, G. (2012). Olfactory cues modify and enhance responses to visual cues in the common marmoset (Callithrix jacchus). J. Primatol. 1, 102. doi: 10.4172/jpmt.1000102
Kemp, C., and Kaplan, G. (2013). Facial expressions in common marmosets (Callithrix jacchus) and their use by conspecifics. Anim. Cogn. 16, 773–788. doi: 10.1007/s10071-013-0611-5
King, B. J. (2009). The Dynamic Dance: Nonvocal Communication in African Great Apes. Boston, MA: Harvard University Press.
King, S. L., Connor, R. C., and Montgomery, S. H. (2022). Social and vocal complexity in bottlenose dolphins. Trends Neurosci. 45, 881–883. doi: 10.1016/j.tins.2022.09.006
King, S. L., Friedman, W. R., Allen, S. J., Gerber, L., Jensen, F. H., et al. (2018). Bottlenose dolphins retain individual vocal labels in multi-level alliances. Curr. Biol. 28, 1993–1999. doi: 10.1016/j.cub.2018.05.013
Klein, R. G. (1977). The ecology of early man in Southern Africa: the relationship between man and environment is traced through 3 million years of Southern African prehistory. Science 197, 115–126. doi: 10.1126/science.197.4299.115
Knight, C., and Lewis, J. (2017). Wild voices: mimicry, reversal, metaphor, and the emergence of language. Curr. Anthrop. 58, 435–453. doi: 10.1086/692905
Kret, M. E., Prochazkova, E., Sterck, E. H., and Clay, Z. (2020). Emotional expressions in human and non-human great apes. Neurosci. Biobehav. Rev. 115, 378–395. doi: 10.1016/j.neubiorev.2020.01.027
Kuijper, B., Pen, I., and Weissing, F. J. (2012). A guide to sexual selection theory. Ann. Rev. Ecol. Evol. Systemat. 43, 2012. doi: 10.1146/annurev-ecolsys-110411-160245
Laland, K., Wilkins, C., and Clayton, N. (2016). The evolution of dance. Curr. Biol. 26, R5–R9. doi: 10.1016/j.cub.2015.11.031
Lameira, A. R. (2017). Bidding evidence for primate vocal learning and the cultural substrate for speech evolution. Neurosci. Biobehav. Rev. 83, 429–439. doi: 10.1016/j.neubiorev.2017.09.021
Launay, J., Tarr, B., and Dunbar, R. I. M. (2016). Synchrony as an adaptive mechanism for large-scale human social bonding. Ethology. 122, 779–789. doi: 10.1111/eth.12528
Lazaro-Perea, C., Snowdon, C. T., and de Fatima Arruda, M. (1999). Scent-marking behavior in wild groups of common marmosets (Callithrix jacchus). Behav. Ecol. Sociobiol. 46, 313–324. doi: 10.1007/s002650050625
Lazarus, R. (1982). Thoughts on the relations between emotions and cognition. Am. Psych. 37, 1019–1024. doi: 10.1037/0003-066X.37.9.1019
Leadner, K., Sekely, L., Klein, R. M., and Gabay, S. (2021). Evolution of social attentional cues: evidence from the archerfish. Cognition 207, 104511. doi: 10.1016/j.cognition.2020.104511
Leavens, D. A. (2004). Manual deixis in apes and humans. Interact. Stud. 5, 387–408. doi: 10.1075/is.5.3.05lea
Leavens, D. A., and Hopkins, W. D. (1998). Intentional communication by chimpanzees: a cross-sectional study of the use of referential gestures. Dev. Psychol. 34, 813–822. doi: 10.1037/0012-1649.34.5.813
Levinson, S. C. (2016). Turn-taking in human communication–origins and implications for language processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Levinson, S. C., and Torreira, F. (2015). Timing in turn-taking and its implicationsfor processing models of language. Front. Psychol. 6, 731. doi: 10.3389/fpsyg.2015.00731
Levréro, F., Touitou, S., Frédet, J., Nairaud, B., Guéry, J. P., and Lemasson, A. (2019). Social bonding drives vocal exchanges in bonobos. Sci. Rep. 9, 711. doi: 10.1038/s41598-018-36024-9
Lewis, J. (2009). “As well as words: Congo Pygmy hunting, mimicry, and play,” in The Cradle of Language Vol.12: African Perspectives, Series: Studies in the Evolution of Language, eds R. Botha and C. Knight (Oxford: Oxford University Press), 236–256.
Liebal, K., and Call, J. (2012). The origins of non-human primates' manual gestures. Philos. Trans. R. Soc. B Biol. Sci. 367, 118–128. doi: 10.1098/rstb.2011.0044
Liebal, K., and Oña, L. (2018). Different approaches to meaning in primate gestural and vocal communication. Front. Psychol. 9, 478. doi: 10.3389/fpsyg.2018.00478
Lieberman, D. E. (2001). Another face in our family tree. Nature 410, 419–420. doi: 10.1038/35068648
Lieberman, P. (1985). On the evolution of human syntactic ability. Its pre-adaptive Bases—Motor control and speech. J. Hum. Evol. 14, 657–668. doi: 10.1016/S0047-2484(85)80074-9
Liszkowski, U., Carpenter, M., Henning, A., Striano, T., and Tomasello, M. (2004). Twelve-month-olds point to share attention and interest. Dev. Sci. 7, 297–307. doi: 10.1111/j.1467-7687.2004.00349.x
Locke, J. L., and Bogin, B. (2006). Language and life history: a new perspective on the development and evolution of human language. Behav. Brain Sci. 20, 259–325. doi: 10.1017/S0140525X0600906X
Logue, D. M., and Hall, M. L. (2014). Migration and the evolution of duetting in songbirds. Proc. R. Soc. B 281, 20140103. doi: 10.1098/rspb.2014.0103
Logue, D. M., and Krupp, D. B. (2016). Duetting as a collective behavior. Front. Ecol. Evol. 4, 7. doi: 10.3389/fevo.2016.00007
Loizos, K. (2017). A Multiscale Computational Modeling Platform for Design and Analysis of Electrical Neural Stimulation (Doctoral Dissertation). The University of Utah.
Luengo-Kanacri, B. P., Eisenberg, N., Tramontano, C., Zuffiano, A., Caprara, M. G., Regner, E., et al. (2021). Measuring prosocial behaviors: psychometric properties and cross-national validation of the prosociality scale in five countries. Front. Psych. 12, 693174. doi: 10.3389/fpsyg.2021.693174
MaBouDi, H., Barron, A. B., Li, S., Honkanen, M., Loukola, O. J., Peng, F., et al. (2021). Non-numerical strategies used by bees to solve numerical cognition tasks. Proc. R. Soc. B 288, 20202711. doi: 10.1098/rspb.2020.2711
MacKinnon, J., and MacKinnon, K. (1980). The behavior of wild spectral tarsiers. Int. J. Primat. 1, 361–379. doi: 10.1007/BF02692280
Maestripieri, D. (1999). “Primate social organization, gestural repertoire size, and communication dynamics: a comparative study of macaques,” in The Evolution of Language: Assessing the Evidence from Nonhuman Primates, ed B. J. King (Santa Fe, NM: School of American Research).
Marean, C. W. (2015). An evolutionary anthropological perspective on modern human origins. Annu. Rev. Anthrop. 44, 533–556. doi: 10.1146/annurev-anthro-102313-025954
Maretti, G., Sorrentino, V., Finomana, A., Gamba, M., and Giacoma, C. (2010). Not just a pretty song: an overview of the vocal repertoire of Indri indri. J. Anthrop. Sci. 88, 151–165.
Marshall-Ball, L., Mann, N., and Slater, P. J.B. (2006). Multiple functions to duet singing: hidden conflicts and apparent cooperation. Anim. Behav. 71, 823–831. doi: 10.1016/j.anbehav.2005.05.021
Martin, J. S., Koski, S. E., Bugnyar, T., Jaeggi, A. V., and Massen, J. J. (2021). Prosociality, social tolerance and partner choice facilitate mutually beneficial cooperation in common marmosets, Callithrix jacchus. Anim. Behav. 173, 115–136. doi: 10.1016/j.anbehav.2020.12.016
Mather, J. (2022). The case for octopus consciousness: temporality. Neuro Sci. 3, 245–261. doi: 10.3390/neurosci3020018
McCabe, B. J. (2019). Visual imprinting in birds: behavior, models, and neural mechanisms. Front. Physiol. 10, 658. doi: 10.3389/fphys.2019.00658
Meehan, A. J., Maughan, B., and Barker, E. D. (2019). Health and functional outcomes for shared and unique variances of interpersonal callousness and low prosocial behavior. J. Psychopathol. Behav. Assess. 41, 353–365. doi: 10.1007/s10862-019-09756-9
Mèndez-Càrdenas, M. G., and Zimmermann, E. (2009). Duetting—a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol. 139, 523–532. doi: 10.1002/ajpa.21017
Meyer, J. (2008). Typology and acoustic strategies of whistled languages: phonetic comparison and perceptual cues of whistled vowels. J. Int. Phonet. Assoc. 38, 69–94. doi: 10.1017/S0025100308003277
Michael, J., McEllin, L., and Felber, A. (2020). Prosocial effects of coordination–What, how and why? Acta Psychol. 207, 103083. doi: 10.1016/j.actpsy.2020.103083
Miles, H. L. (1990). “The cognitive foundations for reference in a signing orangutan” in “Language” and Intelligence in Monkeys and Apes: Comparative Developmental Perspectives. eds S. T. Parker, and K. R. Gibson (Cambridge: Cambridge University Press), 511–539.
Miles, H. L. (1994). “Chantek: the language ability of an enculturated orangutan (Pongo pygmaeus),” in Proc. Intern. Orang Utan Conf . (Davis, CA: UCLA).
Miles, L. S., Rivkin, L. R., Johnson, M. T., Munshi-South, J,., and Verrelli, B. C. (2019). Gene flow and genetic drift in urban environments. Mol. Ecol. 28, 4138–4151. doi: 10.1111/mec.15221
Miller, E. N., Hof, P. R., Sherwood, C. C., and Hopkins, W. D. (2021). The paracingulate sulcus is a unique feature of the medial frontal cortex shared by great apes and humans. Brain Behav. Evol. 96, 26–36. doi: 10.1159/000517293
Miller, M. R. (2013). Descartes on animals revisited. J. Philos. Res. 38, 89–114. doi: 10.5840/jpr2013386
Mitani, J. C., and Gros-Louis, J. (1998). Chorusing and call convergence in chimpanzees: Tests of three hypotheses. Behaviour. 135, 1041–1064.
Morrison, R. E., Eckardt, W., Stoinski, T. S., and Brent, L. J. N. (2020). Comparing measures of social complexity: larger mountain gorilla groups do not have a greater diversity of relationships. Proc. R. Soc. B 287, 20201026. doi: 10.1098/rspb.2020.1026
Neff, E. P. (2019). Neural control of duets between Alston's singing mice, an emerging vocalization model. Lab. Anim. 48, 137–137. doi: 10.1038/s41684-019-0293-y
Nimchinsky, E. A., Gilissen, E., Allman, J. M., Perl, D. P., Erwin, J. M., and Hof, P. R. (1999). A neuronal morphologic type unique to humans and great apes. PNAS 96, 5268–5273. doi: 10.1073/pnas.96.9.5268
Nishimura, T., Tokuda, I. T., Miyachi, S., Dunn, J. C., Herbst, C. T., and Imai, H. (2022). Evolutionary loss of complexity in human vocal anatomy as an adaptation for speech. Science 377, 760–763. doi: 10.1126/science.abm1574
Norscia, I., and Palagi, E. (2011). Yawn contagion and empathy in Homo sapiens. PLoS ONE 6, e28472. doi: 10.1371/journal.pone.0028472
O'Connell, L. A., and Hofmann, H. A. (2011). The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. doi: 10.1002/cne.22735
Okobi, D. E. Jr., Banerjee, A., Matheson, A. M. M., Phelps, S. M., and Long, M. A. (2019). Motor cortical control of vocal interaction in neotropical singing mice. Science 363, 983–988. doi: 10.1126/science.aau9480
Oller, D. K., and Griebel, U. (2021). Functionally flexible signaling and the origin of language. Front. Psych. 11, 626138. doi: 10.3389/fpsyg.2020.626138
Oller, D. K., and Griebel, U., (eds.). (2008). Evolution of Communicative Flexibility: Complexity, Creativity, and Adaptability. Cambridge, MA: MIT Press.
Osvath, M., and Sima, M. (2014). Sub-adult ravens synchronize their play: a case of emotional contagion. Anim.Behav. Cogn. 1, 197–205. doi: 10.12966/abc.05.09.2014
Palomero-Gallagher, N., and Zilles, K. (2019). Differences in cytoarchitecture of Broca's region between human, ape and macaque brains. Cortex 118, 132–153. doi: 10.1016/j.cortex.2018.09.008
Panksepp, J. (2005). Beyond a joke: from animal laughter to human joy? Science 308, 62–63. doi: 10.1126/science.1112066
Parr, L. A., and Waller, B. M. (2006). Understanding chimpanzee facial expression: insights into the evolution of communication. Soc. Cogn. Affect. Neurosci. 1, 221–228. doi: 10.1093/scan/nsl031
Partan, S. R. (2002). Single and multichannel signal composition: facial expressions and vocalizations of rhesus macaques (Macaca mulatta). Behaviour 139, 993–1027. doi: 10.1163/15685390260337877
Partan, S. R., and Marler, P. (1999). Communication goes multimodal. Science 283, 1272–1273. doi: 10.1126/science.283.5406.1272
Patterson, F. G. (1978). “Linguistic capabilities of a lowland gorilla,” in Sign Language and Language Acquisition in Man and Ape: New Dimensions in Comparative Pedolinguistics, ed F. C. C. Peng (Boulder: Westview Press), 161–201.
Pellis, S. M., and Pellis, V. C. (2007). Rough-and-tumble play and the development of the social brain. Curr. Dir. Psychol. Sci. 16, 95–98. doi: 10.1111/j.1467-8721.2007.00483.x
Penn, D. C., and Povinelli, D. J. (2007). On the lack of evidence that non-human animals possess anything remotely resembling a ‘theory of mind'. Philos. Trans. R. Soc. B 362, 731–744. doi: 10.1098/rstb.2006.2023
Pepperberg, I. M. (2007). Grey parrots do not always ‘parrot': the roles of imitation and phonological awareness in the creation of new labels from existing vocalizations. Lang. Sci. 29, 1–13. doi: 10.1016/j.langsci.2005.12.002
Petr, M., Hajdinjak, M., Fu, Q., Essel, E., Rougier, H., Crevecoeur, I., et al. (2020). The evolutionary history of Neanderthal and Denisovan Y chromosomes. Science 369, 1653–1656. doi: 10.1126/science.abb6460
Podlipniak, P. (2017). The role of the Baldwin effect in the evolution of human musicality. Front. Neurosci. 11, 542. doi: 10.3389/fnins.2017.00542
Raimondi, T., Di Panfilo, G., Pasquali, M., Zarantonello, M., Favaro, L., Ravignani, A., et al. (2023). Isochrony and rhythmic interaction in ape duetting. Proc. R. Soc. B 290, 20222244. doi: 10.1098/rspb.2022.2244
Raposa, E. B., Laws, H. B., and Ansell, E. B. (2016). Prosocial behavior mitigates the negative effects of stress in everyday life. Clin. Psychol. Sci. 4, 691–698. doi: 10.1177/2167702615611073
Reader, S. M., and Laland, K. N. (2002). Social intelligence, innovation, and enhanced brain size in primates. PNAS 99, 4436–4441. doi: 10.1073/pnas.062041299
Reddish, P., Fischer, R., and Bulbulia, J. (2013). Let's dance together: synchrony, shared intentionality and cooperation. PLoS ONE 8, e71182. doi: 10.1371/journal.pone.0071182
Reindl, V., Gerloff, C., Scharke, W., and Konrad, K. (2018). Brain-to-brain synchrony in parent-child dyads and the relationship with emotion regulation revealed by fNIRS-based hyperscanning. NeuroIm 178, 493–502. doi: 10.1016/j.neuroimage.2018.05.060
Rek, P., and Magrath, R. D. (2020). Visual displays enhance vocal duet production and the perception of coordination despite spatial separation of partners. Anim. Behav. 168, 231–241. doi: 10.1016/j.anbehav.2020.08.002
Robbins, M. M. (1999). Male mating patterns in wild multimale mountain gorilla groups. Anim. Behav. 57, 1013–1020. doi: 10.1006/anbe.1998.1063
Roberts, A. I., and Roberts, S. G.B. (2020). Communicative roots of complex sociality and cognition. Biol. Rev. 1, 51–73. doi: 10.1111/brv.12553
Roelfsema, P. R., Engel, A. K., König, P., and Singer, W. (1997). Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nat. Rev. Neurosci. 385, 157–161. doi: 10.1038/385157a0
Rogers, L. J., Stewart, L., and Kaplan, G. (2018). Food calls in common marmosets, Callithrix jacchus, and evidence that one is functionally referential. Animal 8, 99. doi: 10.3390/ani8070099
Ros, S. (2021). Rhythm-speech correlations in a corpus of senegalese drum language. Front. Commun. 6, 643683. doi: 10.3389/fcomm.2021.643683
Rose, M. C., Styr, B., Schmid, T. A., Elie, J. E., and Yartsev, M. M. (2021). Cortical representation of group social communication in bats. Science 374, eaba9584. doi: 10.1126/science.aba9584
Rugani, R., Regolin, L., and Vallortigara, G. (2011). Summation of large numerousness by newborn chicks. Front. Psychol. 2, 179. doi: 10.3389/fpsyg.2011.00179
Russon, A. E., Bard, K. A., and Parker, S. T., (eds.). (1996). Reaching into Thought: The Minds of Great Apes. Cambridge. Cambridge University Press.
Salovey, P., and Mayer, J. D. (1990). Emotional intelligence. Imag. Cogn. Person. 9, 185–211. doi: 10.2190/DUGG-P24E-52WK-6CDG
Samuni, L., Tkaczynski, P., Deschner, T., Löhrrich, T., Wittig, R. M., and Crockford, C. (2020). Maternal effects on offspring growth indicate post-weaning juvenile dependence in chimpanzees (Pan troglodytes verus). Front. Zool. 17, 1–12. doi: 10.1186/s12983-019-0343-8
Santangelo, J. S., Johnson, M. T., and Ness, R. W. (2018). Modern spandrels: the roles of genetic drift, gene flow and natural selection in the evolution of parallel clines. Proc. R. Soc. B Biol. Sci. 285, 20180230. doi: 10.1098/rspb.2018.0230
Savage, P. E., Loui, P., Tarr, B., Schachner, A., Glowacki, L, and Fitch, W. T. (2020). Music as a coevolved system for social bonding. Behav. Brain Sci. 44, e59. doi: 10.31234/osf.io/qp3st
Savage-Rumbaugh, E. S. (1984). “Pan paniscus and Pan troglodytes: contrast in preverbal communicative competence,” in The Pygmy Chimpanzee: Evolutionary Biology and Behavior, ed R. L. Susman (New York, NY: Plenum Press), 395–413.
Schacht, R., and Kramer, K. L. (2019). Are we monogamous? A review of the evolution of pair-bonding in humans and its contemporary variation cross-culturally. Front. Ecol. Evol. 230. doi: 10.3389/fevo.2019.00230
Schippers, M. B., Roebroeck, A., Renken, R., Nanetti, L., and Keysers, C. (2010). Mapping the information flow from one brain to another during gestural communication. PNAS. 107, 9388–9393. doi: 10.1073/pnas.1001791107
Schulz, T. M., Whitehead, H., Gero, S., and Rendell, L. (2008). Overlapping and matching of codas in vocal interactions between sperm whales: insights into communication function. Anim. Behav. 76, 1977–1988. doi: 10.1016/j.anbehav.2008.07.032
Schuppe, E. R., Cantin, L., Chakraborty, M., Biegler, M. T., Jarvis, E. R., Chen, C.-C., et al. (2022). Forebrain nuclei linked to woodpecker territorial drum displays mirror those that enable vocal learning in songbirds. PLoS Biol. 20, e3001751. doi: 10.1371/journal.pbio.3001751
Seifart, F., Meyer, J., Grawunder, S., and Dentel, L. (2018). Reducing language to rhythm: amazonian Bora drummed language exploits speech rhythm for long-distance communication. R. Soc. Open Sci. 5, 170354. doi: 10.1098/rsos.170354
Sekulic, R., and Chivers, D. J. (1986). The significance of call duration in howler monkeys. Int. J. Primat. 7, 183–190. doi: 10.1007/BF02692317
Senut, B., Pickford, M., Gommery, D., and Sègalen, L. (2018). Palaeoenvironments and the origin of hominid bipedalism. Hist. Biol. 30, 284–296. doi: 10.1080/08912963.2017.1286337
Sewall, K. B. (2015). Social complexity as a driver of communication and cognition. Integr. Comp. Biol. 55, 384–395. doi: 10.1093/icb/icv064
Seyfarth, R. M., and Cheney, D. L. (1986). Vocal development in vervet monkeys. Anim. Behav. 34, 1640–1658. doi: 10.1016/S0003-3472(86)80252-4
Seyfarth, R. M., Cheney, D. L., and Marler, P. (1980). Monkey responses to three different alarm calls: classification and semantic communication. Science 210, 801–803. doi: 10.1126/science.7433999
Shanker, S. G., and King, B. J. (2002). The emergence of a new paradigm in ape language research: beyond interactionism. Behav. Brain Sci. 25, 646–651. doi: 10.1017/S0140525X02510116
Sherwood, C. C., Subiaul, F., and Zawidzki, T. W. (2008). A natural history of the human mind: tracing evolutionary changes in brain and cognition. J. Anat. 212, 426–454. doi: 10.1111/j.1469-7580.2008.00868.x
Silk, J. B. (2007). The strategic dynamics of cooperation in primate groups. Adv. Study of Behav. 37, 1–41.
Singletary, B., and Tecot, S. (2020). Multimodal pair-bond maintenance: a review of signalling across modalities in pair-bonded nonhuman primates. Am. J. Primatol. 82, e23105. doi: 10.1002/ajp.23105
Smaers, J. B., Gómez-Robles, A., Parks, A. N., and Sherwood, C. C. (2017). Exceptional evolutionary expansion of prefrontal cortex in great apes and humans. Curr. Biol. 27, 714–720. doi: 10.1016/j.cub.2017.01.020
Smaers, J. B., and Vanier, D. R. (2019). Brain size expansion in primates and humans is explained by a selective modular expansion of the cortico-cerebellar system. Cortex 118, 292–305. doi: 10.1016/j.cortex.2019.04.023
Smith, A. S., Ågmo, A., Birnie, A. K., and French, J. A. (2010). Manipulation of the oxytocin system alters social behavior and attraction in pair-bonding primates, Callithrix penicillata. Horm. Behav. 57, 255–262. doi: 10.1016/j.yhbeh.2009.12.004
Sneve, M. H., Grydeland, H., Rosa, M. G., Paus, T., Chaplin, T., and Fjell, A. M. (2019). High-expanding regions in primate cortical brain evolution support supramodal cognitive flexibility. Cerebr. Cortex 29, 3891–3901. doi: 10.1093/cercor/bhy268
Snowdon, C. T. (2018). Cognitive components of vocal communication: a case study. Animal 8, 126. doi: 10.3390/ani8070126
Snowdon, C. T. (2020). Vervet monkey alarm calls: Setting the historical context. Anim. Behav. Cogn. 7, 87–94. doi: 10.26451/abc.07.02.02.2020
Sommer, V., Bauer, J., Fowler, A., and Ortmann, S. (2011). “Patriarchal chimpanzees, matriarchal bonobos: Potential ecological causes of a Pan dichotomy,” in Primates of Gashaka, eds V. Sommer, and C. Ross (New York, NY: Springer), 469–501.
Spataro, P., Calabrò, M., and Longobardi, E. (2020). Prosocial behaviour mediates the relation between empathy and aggression in primary school children. Eur. J. Dev. Psychol. 17, 727–745. doi: 10.1080/17405629.2020.1731467
Spocter, M. A., Hopkins, W. D., Garrison, A. R., Bauernfeind, A. L., Stimpson, C. D., Hof, P. R., et al. (2010). Wernicke's area homologue in chimpanzees (Pan troglodytes) and its relation to the appearance of modern human language. Proc. R. Soc. B Biol. Sci. 277, 2165–2174. doi: 10.1098/rspb.2010.0011
Sterling, E. J., and McCreless, E. E. (2007). “Adaptations in the aye-aye: a review,” in Lemur: Ecology and Adaptation, eds L. Gould (New York, NY: Sauther Springer Science+Business Media LLC), 159–184.
Stevenson, M. F., and Poole, T. B. (1976). An ethogram of the common marmoset (Calithrix jacchus jacchus): general behavioural repertoire. Anim. Behav. 24, 428–451. doi: 10.1016/S0003-3472(76)80053-X
Stewart, K. M. (1994). Early hominid utilisation of fish resources and implications for seasonality and behaviour. J. Hum. Evol. 27, 229–245. doi: 10.1006/jhev.1994.1044
Studdert-Kennedy, M. (2000). “Evolutionary implications of the particulate principle: imitation and the dissociation of phonetic form from semantic function,” in The Evolutionary Emergence of Language: Social Function and the Origins of Linguistic Form, eds C. Knight, M. Studdert-Kennedy, and J. Hurford (Cambridge: CUP), 161–176. doi: 10.1017/CBO9780511606441.011
Studdert-Kennedy, M., and Goldstein, L. (2003). Launching language: the gestural origin of discrete infinity. Stud. Evol. Lang. 3, 235–254. doi: 10.1093/acprof:oso/9780199244843.003.0013
Suzuki, A. (1970). An ecological study of Chimpanzees in a Savanna woodland. Primates 10, 103–148. doi: 10.1007/BF01730979
Takahashi, D. Y., Fenley, A. R., and Ghazanfar, A. A. (2016). Early development of turn-taking with parents shapes vocal acoustics in infant marmoset monkeys. Philos. Trans. R. Soc. B 371, 20150370. doi: 10.1098/rstb.2015.0370
Thomas, E. (2020). Descartes on the animal within, and the animals without. Can. J. Philos. 50, 999–1014. doi: 10.1017/can.2020.44
Tilson, R. L., and Norton, P. M. (1981). Alarm duetting and pursuit deterrence in an African antelope. Am. Nat. 118, 455–462. doi: 10.1086/283840
Tobias, J. A., Sheard, C., Seddon, N., Meade, A., Cotton, A. J., and Nakagawa, S. (2016). Territoriality, social bonds, and the evolution of communal signalling in birds. Front. Ecol. Evol. 4, 74. doi: 10.3389/fevo.2016.00074
Tobias, M. L., Viswanathan, S. S., and Kelley, D. B. (1998). Rapping, a female receptive call, initiates male–female duets in the South African clawed frog. PNAS 95, 1870–1875. doi: 10.1073/pnas.95.4.1870
Tobias, P. V. (1987). The brain of Homo habilis: a new level of organization in cerebral evolution. J. Hum. Evol. 16, 741–761. doi: 10.1016/0047-2484(87)90022-4
Tommasi, L., and Vallortigara, G. (2004). Hemispheric processing of landmark and geometric information in male and female domestic chicks (Gallus gallus). Behav. Brain Res. 155, 85–96. doi: 10.1016/j.bbr.2004.04.004
Townsend, S. W., and Manser, M. B. (2013). Functionally referential communication in mammals: the past, present and the future. Ethology 119, 1–11. doi: 10.1111/eth.12015
Trenbeath, C. (2021). Yakety Sacs: Laryngeal Air Sac Usage in Great Apes, Master of Science in Integrative Biology. Kennesaw, GA: Kennesaw State University.
Valdesolo, P., Ouyang, J., and De Steno, D. (2010). The rhythm of joint action: synchrony promotes cooperative ability. J. Exp. Soc. Psychol. 46, 693–695. doi: 10.1016/j.jesp.2010.03.004
Vallortigara, G., Chiandetti, C., Rugani, R., Sovrano, V. A., and Regolin, L. (2010). Animal cognition. Wiley Interdisc. Reviews: Cogn. Sci. 1, 882–893. doi: 10.1002/wcs.75
Vallortigara, G., Zanforlin, M., and Pasti, G. (1990). Geometric modules in animal's spatial representation: a test with chicks. J. Comp. Psych. 104, 248–254. doi: 10.1037/0735-7036.104.3.248
van Lawick-Goodall, J. (1968). The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim. Behav. Monogr. 1, 61-IN12. doi: 10.1016/S0066-1856(68)80003-2
Vanderhoff, E. N., and Bernal Hoverud, N. (2022). Perspectives on antiphonal calling, duetting and counter-singing in non-primate mammals: an overview with notes on the coordinated vocalizations of bamboo rats (Dactylomys spp., Rodentia: Echimyidae). Front. Ecol. Evol. 10, 906546. doi: 10.3389/fevo.2022.906546
Vanderschuren, L. J., Achterberg, E. M., and Trezza, V. (2016). The neurobiology of social play and its rewarding value in rats. Neurosci. Biobehav. Rev. 70, 86–105. doi: 10.1016/j.neubiorev.2016.07.025
Veit, W. (2021). Scaffolding natural selection. Biol. Theory 17, 163–180. doi: 10.1007/s13752-021-00387-6
Verspeek, J., van Leeuwen, E. J. C., Laméris, D. W., Staes, N., and Stevens, J. M. G. (2022). Adult bonobos show no prosociality in both prosocial choice task and group service paradigm. PeerJ. 10, e12849. doi: 10.7717/peerj.12849
Vieira, M., Amorim, M. C., and Fonseca, P. J. (2021). Vocal rhythms in nesting Lusitanian toadfish, Halobatrachus didactylus. Ecol. Inform. 63, 101281. doi: 10.1016/j.ecoinf.2021.101281
Von Dawans, B., Fischbacher, U., Kirschbaum, C., Fehr, E., and Heinrichs, M. (2012). The social dimension of stress reactivity: acute stress increases prosocial behavior in humans. Psych. Sci. 23, 651–660. doi: 10.1177/0956797611431576
Vonk, J. (2020). Forty years on from the question of referential signals in nonhuman communication. Anim. Behav. Cogn. 7, 82–86. doi: 10.26451/abc.07.02.01.2020
Waller, B. M., Liebal, K., Burrows, A. M., and Slocombe, K. E. (2013). How can a multimodal approach to primate communication help us understand the evolution of communication? Evol. Psychol. 11, 147470491301100305. doi: 10.1177/147470491301100305
Warneken, F. (2015). Precocious prosociality: Why do young children help? Child Dev. Perspect. 9, 1–6. doi: 10.1111/cdep.12101
Warneken, F., Hare, B., Melis, A. P., Hanus, D., and Tomasello, M. (2007). Spontaneous altruism by chimpanzees and young children. PLoS Biol. 5, e184. doi: 10.1371/journal.pbio.0050184
Watson, S. K., Townsend, S. W., Schel, A. M., Wilke, C., Wallace, E. K., Cheng, L., et al. (2015). Vocal learning in the functionally referential food grunts of chimpanzees. Curr. Biol. 25, 495–499. doi: 10.1016/j.cub.2014.12.032
Watts, M. E., Pocock, R., and Claudianus, C. (2018). Brain energy and oxygen metabolism: emerging role in normal function and disease. Front. Mol. Neurosci. 11, 216. doi: 10.3389/fnmol.2018.00216
Wheeler, B. C., and Fischer, J. (2012). Functionally referential signals: a promising paradigm whose time has passed. Evol. Anthrop. Issues News Rev. 21, 195–205. doi: 10.1002/evan.21319
White, T. D., Asfaw, B., Beyene, Y., Hailie-Selassie, Y., Lovejoy, C. O., Suwa, G., et al. (2009). Ardipithecus ramidus and the paleobiology of early hominids. Science 326, 75–86. doi: 10.1126/science.1175802
Whiten, A., and de Waal, E. (2018). The pervasive role of social learning in primate lifetime development. Behav. Ecol. Sociobiol. 72, 1–16. doi: 10.1007/s00265-018-2489-3
Wiles, J., Watson, J., Tonkes, B., and Deacon, T. W. (2005). Transient phenomena in learning and evolution: genetic assimilation and genetic redistribution. Artif. Life 11, 177–188. doi: 10.1162/1064546053279026
Williams, L., Shultz, S., and Jensen, K. (2022). The primate workplace: cooperative decision-making in human and non-human primates. Front. Ecol. Evol. Sect. Behav. Evo. Ecol. 10, 887187. doi: 10.3389/fevo.2022.887187
Wrangham, R. (1979). On the evolution of ape social systems. Soc. Sci. Info 18, 336–368. doi: 10.1177/053901847901800301
Yu, L., and Tomonaga, M. (2015). Interactional synchrony in chimpanzees: examination through finger tapping experiment. Sci. Rep. 5, 1–9. doi: 10.1038/srep10218
Yu, Y., Karbowski, J., Sachdev, R. N. S., and Feng, J. (2014). Effect of temperature and glia in brain size enlargement and origin of allometric body-brain size scaling in vertebrates. BMC Evo. Biol. 14, 178–192. doi: 10.1186/s12862-014-0178-z
Zhang, W., and Yartsev, M. M. (2019). Correlated neural activity across the brains of socially interacting bats. Cell 178, 413–428. doi: 10.1016/j.cell.2019.05.023
Keywords: prosociality, duetting, cooperation, synchronicity, cognition and emotions in primates, human language evolution, communication (vocal, gestural)
Citation: Kaplan G (2023) Evolution of human language: duetting as part of prosociality and cognition. Front. Ecol. Evol. 11:1004384. doi: 10.3389/fevo.2023.1004384
Received: 27 July 2022; Accepted: 25 April 2023;
Published: 16 June 2023.
Edited by:
Patrice Adret, Universidad Autónoma Gabriel René Moreno, BoliviaReviewed by:
Leonardo Francisco Barón Birchenall, Corporación Universitaria Minuto de Dios, ColombiaCopyright © 2023 Kaplan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gisela Kaplan, Z2thcGxhbkB1bmUuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.