
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 27 April 2023
Sec. Behavioral and Evolutionary Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fevo.2023.1000531
This article is part of the Research Topic The Importance of Olfaction in Intra- and Interspecific Communication, Volume II View all 7 articles
Avian courtship behaviour is essential to attract potential mating partners. Courtship behaviours can involve displays of different sensory modes. Sex discrimination is a crucial step and in many bird species, sexes differ in acoustic and visual traits, allowing sex discrimination. It has been shown only recently that in some species of Estrildid Finches, chemical cues are involved in social communication. Here, we investigated whether olfaction also plays a role in sex discrimination in Estrildid Finches. Investigating olfactory sex preferences as an indicator behaviour in six different Estrildid Finch species, we aimed to understand whether sex- and/or species-specific differences in olfactory preferences exists and whether olfactory sex preferences correspond to species-specific differences in sex-specific acoustic and visual displays, e.g., singing, plumage dimorphisms and courtship dance. Olfactory sex preferences were tested in a Y-Maze test. We found differences in scent preferences among the different species of Estrildid Finches. We discussed the behavioural pattern with respect to other species-specific traits. And their potential implications in a broader mate choice context.
Avian courtship behaviour is involved in attracting potential mating partners, and is thus ultimately involved in increasing the chance to reproduce and to transfer genes to the next generation (Andersson, 2019). Courtship displays may allow individuals to identify and choose between potential mating partners, and in some cases to maintain a pair bond. Avian courtship behaviours can involve displays of different sensory modes; from visual signals, such as plumage pattern or colouration (e.g., Goodwin, 1982; Naguib and Nemitz, 2007; Soma and Garamszegi, 2015), acoustic signals, such as bird song (e.g., Catchpole and Slater, 2003; Riebel, 2009), to olfactory signals (e.g., Caro and Balthazart, 2010; Whittaker et al., 2013; Caspers et al., 2015a; Krause et al., 2018; Grieves et al., 2022), and specific movements, such as courtship dances (e.g., Goodwin, 1982; Zann, 1996; Soma and Garamszegi, 2015).
These different modalities of partner attraction can be displayed simultaneously or sequentially by either one or both sexes. Whereas the use of visual and acoustic signals as courtship displays has been rather intensively studied in birds (Catchpole and Slater, 2003; Andersson, 2019), the use and potential role of olfactory cues during intersexual communication has only recently gained attention (Caro et al., 2015; Grieves et al., 2022). It is known that the olfactory phenotype is sex specific in a large proportion of investigated species (reviewed in Grieves et al., 2022), for example in mallards (Jacob et al., 1979), blue tits (Caspers et al., 2022), dark eyed juncos (Whittaker et al., 2010), and song sparrows (Grieves et al., 2019) and that in many cases these differences are particularly present during the breeding season (Grieves et al., 2022). Whether individuals make use of olfactory cues for sex recognition and/or whether species-specific differences exist are, however, less well understood. Evidence for olfactory sex discrimination has been described in a few species, such as in dark-eyed juncos (Whittaker et al., 2011) and spotless starlings (Amo et al., 2012), in which individuals, irrespective of their own sex, spent more time with the males’ odour. In other species, such as black-capped chickadees, Carolina chickadees and song sparrows, both sexes spent more time with the odour of the opposite sex and female budgerigars, as well as female crimson rosellas, have been found to show a preference for the male odour (Zhang et al., 2010; Mihailova et al., 2014; Grieves et al., 2019, 2022). Thus, odours are used for sex discrimination in some bird species. Whether general patterns have evolved, however, is unknown yet.
By presenting male and female odours simultaneously, we investigated sex preferences in six different Estrildid Finch species: Zebra Finches, Bengalese Finches, Diamond Firetails, Red-browed Finch, Red Avadavats, and Magpie Manakins, all relatively broad distributed among the taxon of the Estrildid Finches (Arnaiz-Villena et al., 2009). Sex preferences based on olfactory cues have, to our knowledge, not been addressed in this taxon, but Estrildid Finches are quite promising study systems in this context, as some members of this taxon are well known to use olfactory cues (e.g., Zebra Finches and Bengalese Finches) in intraspecific communication (Krause et al., 2012, 2014; Caspers et al., 2015a,2017; Golüke et al., 2019, 2021). Furthermore, in Estrildid Finches, species show a certain variability in courtship displays, i.e., in plumage colouration, song characteristics and courtship dance and courtship displays usually include multimodal signalling (Goodwin, 1982; Soma and Garamszegi, 2015). Estrildid Finch species are distributed among three continents: Asia, Africa, and Australia (Arnaiz-Villena et al., 2009). Interestingly, males of all Estrildid Finches perform a courtship song as well as a courtship dance (Goodwin, 1982; Soma and Garamszegi, 2015). However, females’ courtship performance is highly variable within the taxon. In some species, females also perform courtship songs and/or courtship dances. Female Red Avadavats (Amandava amandava), for example, perform courtship songs and courtship dance (Harrison et al., 1962), while in female Bengalese Finches (Lonchura striata) both are absent (Soma and Garamszegi, 2015). With respect to visual signals, again a dimorphism in plumage colouration appears only in some Estrildid Finch species. For example, in the Zebra Finch (Taeniopygia guttata), males have colourful plumage ornamentation (Zann, 1996; Naguib and Nemitz, 2007), while Diamond Firetails (Stagonopleura guttata) have no clear plumage dimorphism (Goodwin, 1982; Soma and Garamszegi, 2015).
Although some Estrildid Finches have been shown to use olfactory cues in a variety of contexts (e.g., Krause et al., 2018; Golüke et al., 2019, 2021), little is known about the use of olfactory cues during courtship or mate attraction. To close this knowledge gap, we tested sex preferences by presenting olfactory cues in six Estrildid Finch species (Figure 1). We tested individuals in a Y-maze for their preference of the odour of a same-sex conspecific or an opposite-sex conspecific. We further expect olfactory sex preferences of the Estrildid finch species to be linked to other characteristic of these species such as e.g., courtship behaviour or plumage dimorphisms. With this, we aimed to gather a broader understanding of olfactory sex preferences in Estrildid Finches.
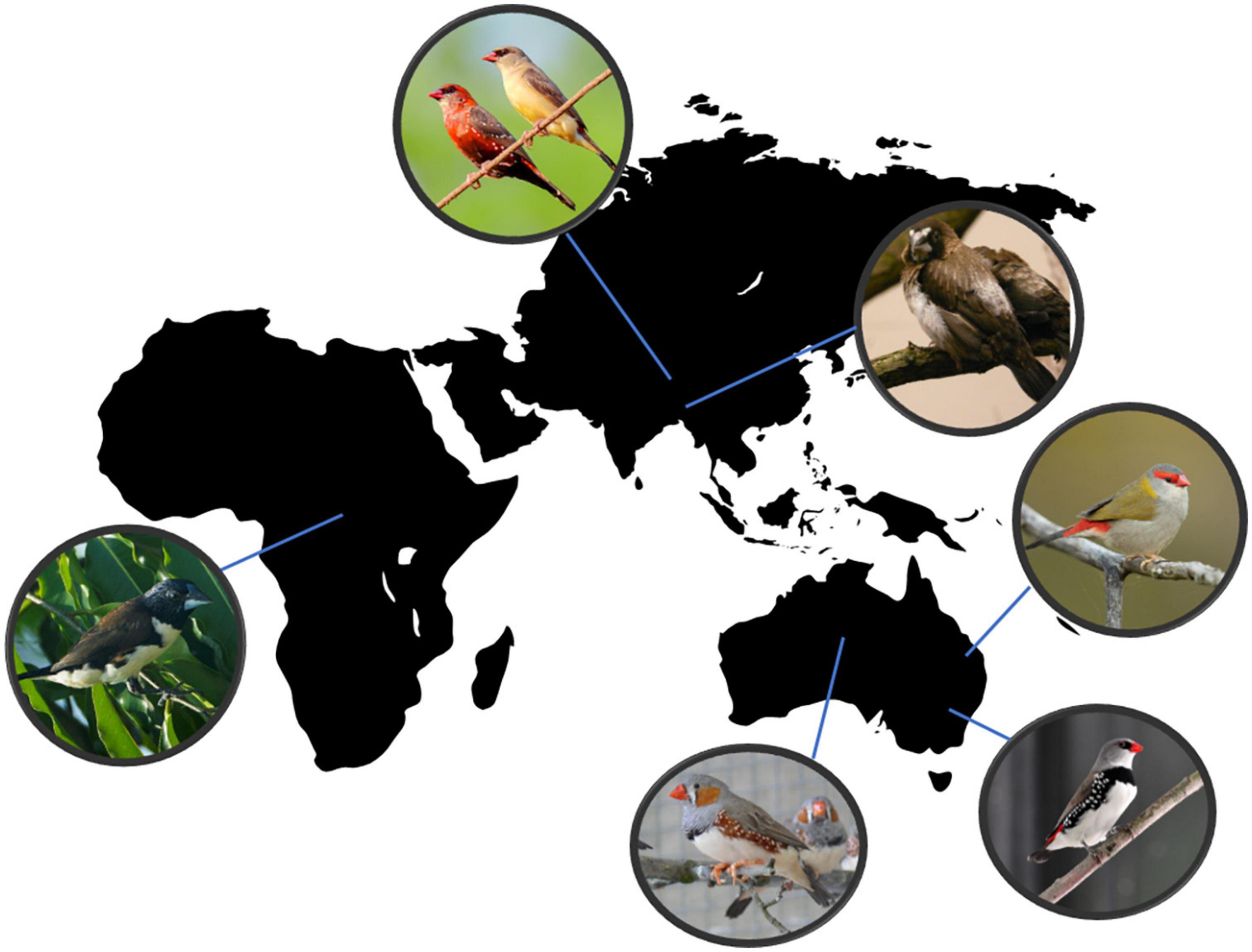
Figure 1. The three continents, Africa, Asia, and Australia, from where the six examined Estrildid Finch species originated. From left in clockwise direction these species are the Magpie Manikin (Lonchura fringilloides; photo modified after Francesco Veronesi from Italy, CC BY-SA 2.0, via Wikimedia Commons); the Red Avadavat (Amandava amandava; photo modified after Shantanu Kuveskar - Own work, CC BY 4.0, https://commons.wikimedia.org/w/index.php?curid=56884220); the Bengalese Finch (Lonchura striata var domestica; photo by ETK); the Red-browed Finch (Neochmia temporalis; photo modified after JJ Harrison (https://www.jjharrison.com.au/) - Own work, CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=86319921); the Diamond Firetail (Stagonopleura guttata; photo by Ulrich Pörschmann), and the Zebra Finch (Taeniopygia guttata; photo by EK).
We tested a total of 181 birds of six different Estrildid Finch species (Figure 1), Zebra Finch [Taeniopygia guttata; 100 birds: 50 males (m) and 50 females (f)], Diamond Firetail (Stagonopleura guttata; 30 birds: 15 m, 15 f), Bengalese Finch (Lonchura striata var domestica; 20 birds: 10 m, 10 f), Red-browed Finch (Neochmia temporalis; 10 birds: 4 m, 6 f), Red Avadavat (Amandava amandava; 12 birds: 6 m, 6 f) and Magpie Manakin (Lonchura fringilloides; 9 birds: 4 m, 5 f), in an olfactory Y-maze test. We presented each bird with the scent of two conspecifics, (i) of the own sex and (ii) of the opposite sex.
All birds were housed for several weeks in single-sex groups (2–4 birds per cage, cage size 83 cm × 30 cm × 40 cm) and were fed with ad libitum standard seed mix, and two times a week germinated standard seeds. The adult Zebra Finches were taken from the domesticated lab stock (Forstmeier et al., 2007; Hoffman et al., 2014) of Bielefeld University, Bielefeld. Zebra Finches are an Australian Estrildid Finch species (Goodwin, 1982) distributed nearly over the entire continent (Immelmann, 1965; Goodwin, 1982; Zann, 1996). They form monogamous pairs and breed in large colonies (Zann, 1996). A plumage dimorphism between males and females is apparent (Zann, 1996), males show several colorful secondary sexual ornaments, which represent condition-dependent sexually selected ornaments (Naguib and Nemitz, 2007). Zebra Finches are well known for their use of olfactory cues in intraspecific communication, such as the recognition of conspecifics (Krause et al., 2014), nest recognition (Caspers and Krause, 2011; Caspers et al., 2013, 2015a), kin recognition (Krause et al., 2012), or in parent-offspring recognition (Caspers et al., 2017; Golüke et al., 2019, 2021). The adult Diamond Firetails were from the Bielefeld lab stock (Krause et al., 2014; Schmelz et al., 2015; Krause and Caspers, 2018). Diamond Firetails are also Australian Estrildid Finches (Goodwin, 1982), with no sexual plumage dimorphism. They are far less social in breeding and non-breeding contexts than Zebra Finches (Birke, 1974; Robiller, 1979; Zann, 1996; Restall, 1997; Nicolai, 2001). Diamond Firetails differ in their olfactory phenotype from Zebra Finches (Krause et al., 2014), but do not show any sign of conspecific recognition, when given the odour of a conspecific or a heterospecific (Krause et al., 2014). Bengalese Finches originated from the Bielefeld lab stock (Krause and Caspers, 2012; Schmelz et al., 2015). Bengalese Finches are a gregarious Estrildid species originating from Asia (Goodwin, 1982), which do not have a sexual plumage dimorphism. As in Zebra Finches, female Bengalese Finches show a preference for their nest odour during the nestling phase of their chicks (Krause and Caspers, 2012). For Red-browed Finches, Red Avadavats, and Magpie Manakins, we had less than 20 individuals per species for testing. These three Estrildid Finch species originated from all three continents where Estrildid Finches are distributed (Africa, Asia, and Australia) (Goodwin, 1982; Arnaiz-Villena et al., 2009). We tested ten Red-browed Finches, which are Australian Estrildid Finches (Goodwin, 1982) with no sexual plumage dimorphism (Goodwin, 1982). Furthermore, we tested twelve Red Avadavats, which represent an Asian Estrildid Finch species with a sexual dimorphism during reproduction only (Goodwin, 1982). Last we tested nine individuals of the Magpie Manikins, a monomorphic African Estrildid Finch species (Goodwin, 1982).
We used an olfactory Y-maze two-choice apparatus (Krause et al., 2014). We conducted the experiments with the olfactory Y-maze choice tests in a separate room to ensure that the birds were not distracted from visual, acoustic and other than the experimental olfactory cues from conspecifics. The Y-maze set up and main experimental protocol are described in Krause et al. (2014). Briefly, the Y-maze consists of three arms in the shape of a capital Y, one which contains the start box and two arms, in which the odour stimuli are presented. A fan, placed at the end of each odour stimuli arm, created an airflow transporting the odour through the arm towards the start arm. Before the start of each experiment, the focal bird, i.e., either male or female, was allowed to habituate in the start-box of the Y-maze for 5 min. Thereafter, the start-box was opened and the birds had up to 10 min time to make a choice in the Y-maze, i.e., entering one of the two arms. Air-flows coming out of each arm contained different odour stimuli of (a) a conspecific male and (b) a conspecific female odour. Sides where odours were presented were alternated across experiments. Odour stimuli were changed between tested animals. Odour samples were collected directly prior to testing by placing the donor birds (conspecifc male/female) each in a clean 100% cotton bag (10 cm × 14 cm; L-Shop-Tea, Dortmund, Germany, #XT007) to impregnate the cotton bag with the individual scent profiles (Krause et al., 2014; Caspers et al., 2015b,2017). At a specific day, each bird was either only donor or focal bird. To rule out the possibility that a bird’s preference was influenced by familiarity, we used birds as odour donors which were not housed in the same cage as the focal bird. Each bird used in the study was used as a focal and as a donor animal in a randomised order. The focal bird’s choice was determined by its’ first choice, i.e., which arm of the Y-maze it entered first (Bonadonna and Sanz-Aguilar, 2012; Krause et al., 2014). If a bird did not enter one of the arms within the 10 minutes, we repeated the experiment once again the next day (Krause et al., 2014). In case the bird still did not make a choice, the decision was recorded as “no choice.” After each experiment, the Y-maze was cleaned with 70%-ethanol. We handled all birds with fresh nitrile gloves.
We measured for each bird the first choice for one of the two stimulus scents, i.e., conspecifics’ male and conspecifics’ female odour. We investigated potential species-specific sex preferences patterns for the three species with sample sizes larger than N ≥ 20 (Table 1). To analyse potential differences with respect to the sex of the choosing birds, we used a Woolf’s test for stratified data using a 2 × 2 × 3 table (2 = sex, 2 = (choice for female/for male), 3 = species). Species comparison was calculated with a Cochran-Mantel-Haenszel test. The tests were calculated using the package “vcd” (Meyer et al., 2006) in R 4.0.3 (R Core Team, 2020). For the species with N < 20 only descriptive data is reported.
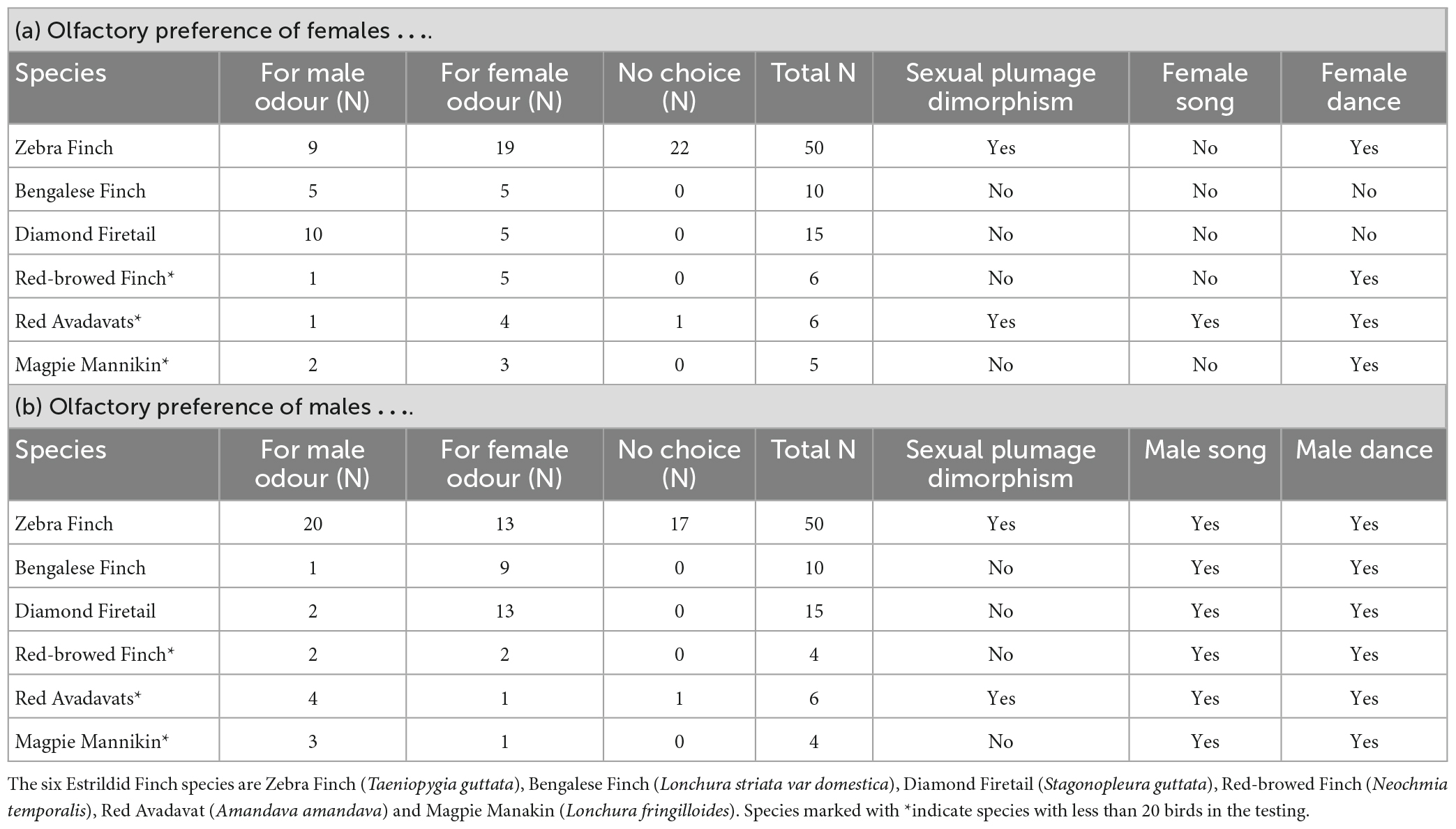
Table 1. Results from the olfactory Y-maze test and selected species-specific characteristics from the six Estrildid Finch species used in the study as extracted from the literature (see text for references) for (a) females and (b) males.
After the experiments, all birds remained in our lab stocks or were returned to the stocks from a local breeder. The experiments were performed in 2014 at Bielefeld University in accordance with the current laws of the country at that time. Housing of birds was conducted with the permission of the Gesundheits-, Veterinär- und Lebensmittelüberwachungsamt, Stadt Bielefeld, Germany (no. 530.421630-1, 18/04/2002 and no. 530.4, 27.07.2014). All birds were monitored daily.
Female Zebra Finches have chosen most often the female odour, while male Zebra Finches have chosen more often the male odour in the Y-Maze. Bengalese Finch females have chosen the male and the female odour similar often, and male Bengalese Finches have chosen most often the female odour. Both sexes of Diamond Firetails have chosen most often the odour of the opposite sex. The comparison between sexes of choosing birds showed that the patterns differ with respect to sex (Woolf-test on Homogeneity of Odds Ratios, χ2 = 4.25, df = 1, p = 0.039; Figures 2A, B). The descriptive choice preference pattern of Zebra Finches, Bengalese Finches and Diamond Firetails in the olfactory Y-maze are shown in Table 1.
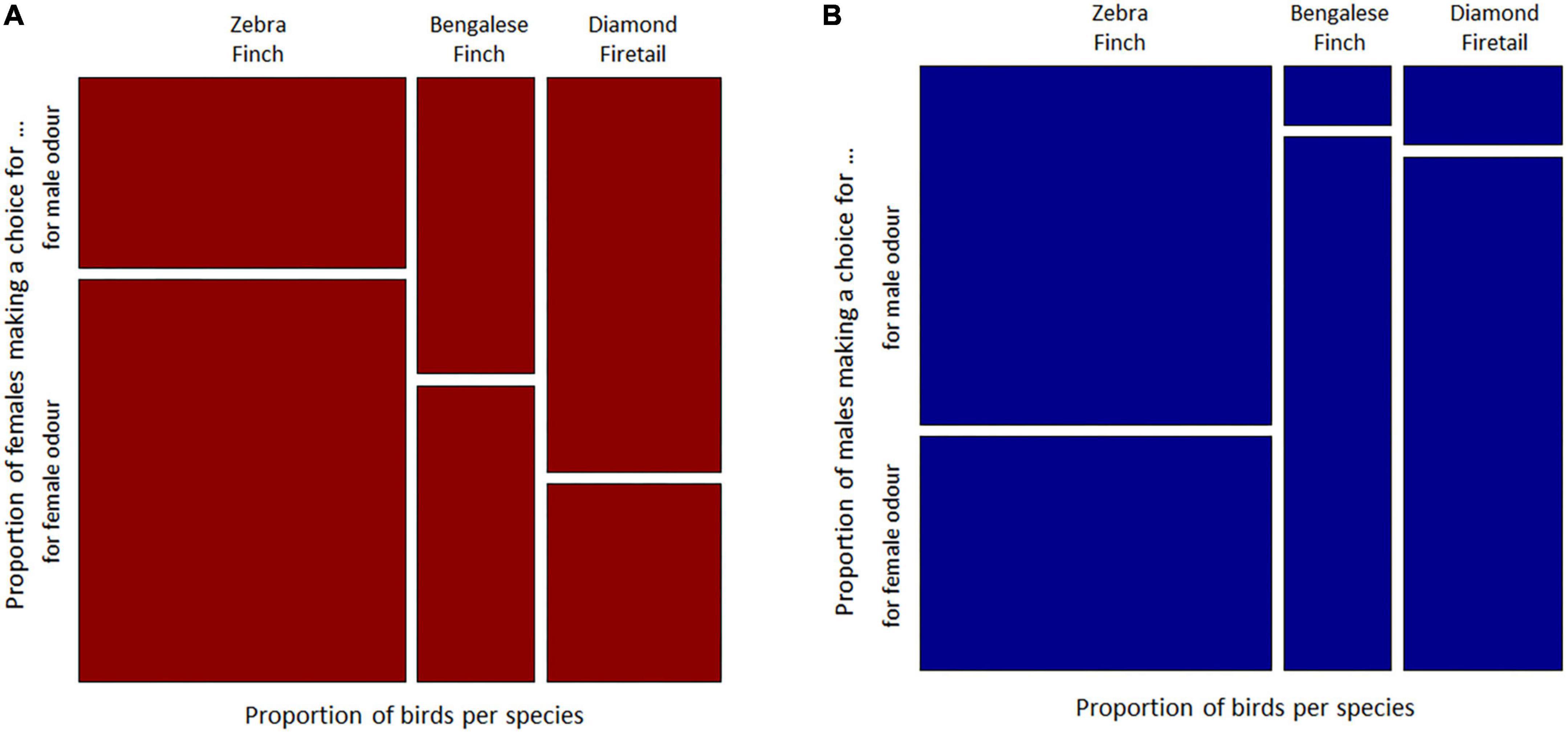
Figure 2. Visualisation of the olfactory preference behaviour of three species of Estrildid Finches Zebra Finches, Bengalese Finches and Diamond Firetail, scale to proportions of choices made for conspecifics male or conspecifics female odour in the Y-maze respectively for (A) focal female and (B) focal males of each respective species.
However, between species no significant difference was found in the choice preference patterns (Cochran-Mantel-Haenszel test, M2 = 2.02, df = 2, p = 0.36: Figures 2A, B).
Red-browed Finch females preferred mainly the female odours, while males preferred both sexes similarly often. In Red Avadavats and Magpie Mannikins, males and females chose in the majority of cases the odour of the same sex. However, from all three species the number of tested birds is rather low and therefore these findings should only be considered carefully (Table 1).
There is no hint that any of the two male courtship traits, i.e., male song and male dance correlates with the overall Y-maze choice preference (Figure 3). While the same is true for female song, we observe that the absence of female dance correlates with a preference for the opposite sex odour (Figure 3).
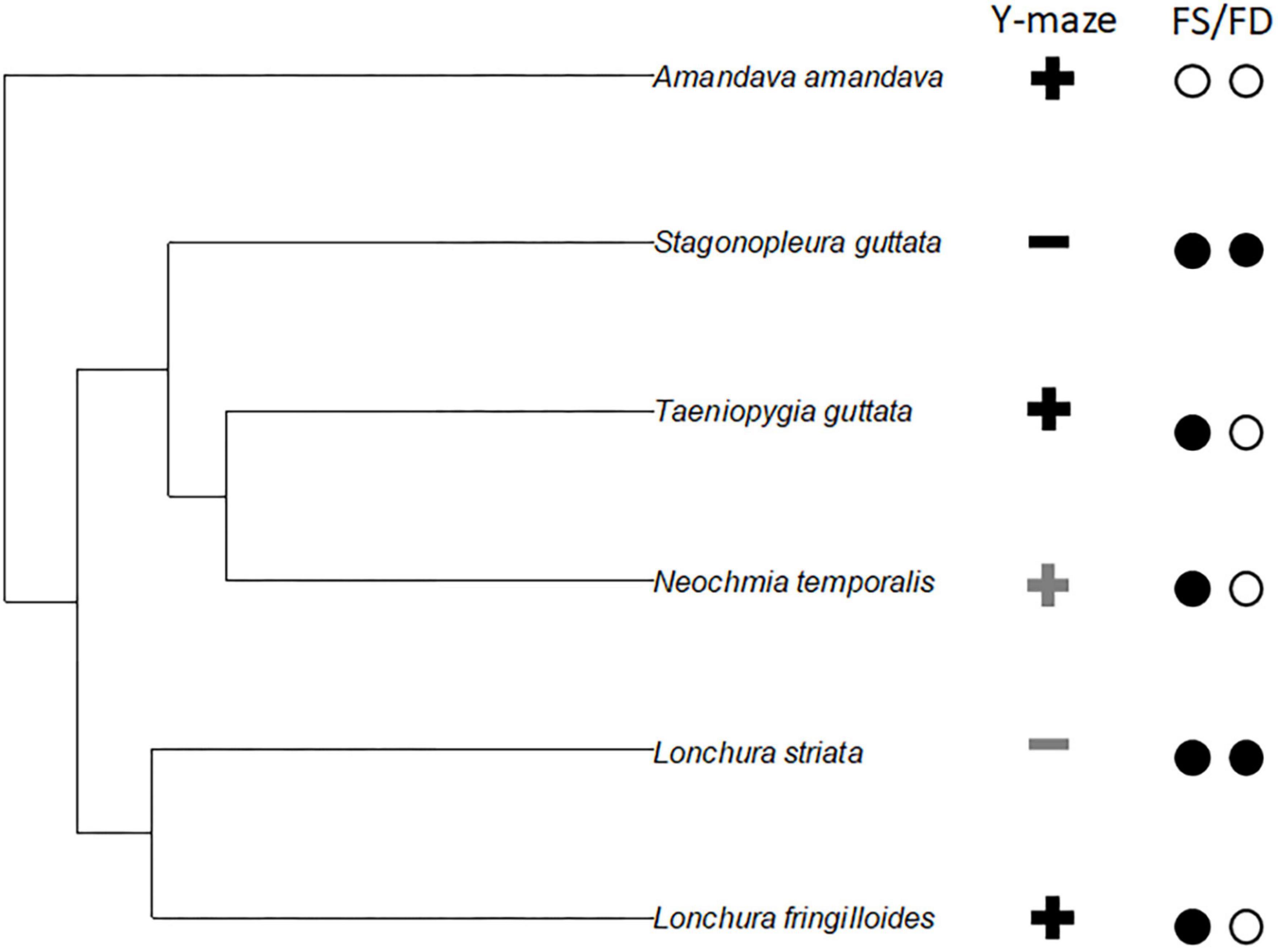
Figure 3. An exemplary phylogenetic tree for the six used Estrildid Finch species, Zebra Finch (Taeniopygia guttata), Diamond Firetail (Stagonopleura guttata), Bengalese Finch (Lonchura Striata var domestica), Red-browed Finch (Neochmia temporalis), Red Avadavat (Amandava amandava), and Magpie Manakin (Lonchura fringilloides). For the exemplary phylogenetic tree, a random Hackett tree (Jetz et al., 2012) was plotted using the R-package “ape” (Paradis et al., 2004). The symbols in the columns on the right indicate for the respective species the following. Y-maze: “+” indicates that birds from that species chose more likely same sexed birds, if coloured in grey this is only true for one of the two sexes and the other sex was not different. “−” indicates that birds from that species chose more likely opposite sexed birds, if coloured in grey this is only true for one of the two sexes and the other sex was not different. The open circles indicate the presence of a female song/female dance (FS/FD in this species, while the black circles indicate its absence.
Comparative studies of olfactory sex preferences will help us to understand general patterns in courtship behaviours. Therefore, we tested whether males and females of six species of Estrildid Finches show preferences for the odour of same sex or opposite sex conspecifics. The six species of Estrildid Finches differed in several characteristics with respect to their courtship related traits, as plumage dimorphism and the presence/absence of courtship dance.
We found sex-specific differences in the preference towards a same-sex conspecific or an opposite-sex conspecific odour among the six Estrildid Finch species and interesting different patterns among species. Zebra Finches of both sexes showed a stronger preference for the scent of same-sex conspecifics; while Diamond Firetails of both sexes preferred the odour of opposite-sex conspecifics. A similar pattern was found in male Bengalese Finches. Thus, sex of the focal individual significantly affects odour preferences and this was slightly different between different species.
Furthermore, we found that the choice behaviour corresponded to the absence of female dance. We observed that birds from species that possess no female courtship dance, i.e., Diamond Firetails and Bengalese Finches, showed more likely a preference for the odour of the opposite-sex conspecific, whereas individuals from species with female courtship dance preferred more likely the odour of same-sex conspecifics. There was no hint that any of the other courtship traits considered here, such as the presence of female song, or visual plumage dimorphism correspond with the olfactory preferences found in our tests. Although this is only a first hint, it might be a starting point for follow up studies.
Female dance is suggested to play a considerable role in communication within a pair as well as in communication to conspecifics outside the pair bond, i.e., to signal pair-bonding to other conspecifics (Soma and Garamszegi, 2015). Particularly, it has been suggested that dancing and dance complexity during courtship resulted from selection pressures for behavioural coordination between the sexes (Soma and Garamszegi, 2015). In the presence of female dance, one might speculate that body odours are not used for mate attraction and mate recognition as the visual presentation and movements are more important, whereas in species without female dance, it can be speculated that body odours are alternatively used for mate attraction. As we do not have any knowledge about the motivation of the birds it might also be possible that individuals use olfactory cues to avoid individuals of a certain sex. Independent of the motivation of the tested individuals, our results indicate that Estridild Finches, at least Zebra Finches, Benaglese Finches, Diamond Firetails, and presumuably Red Avadavats show sex specific differences in body odour preferences. Body odours might be used during the complex multimodal signalling in mate choice in Estridild Finches (Soma and Garamszegi, 2015). In line with earlier argumentation, the use of body odours evolved probably independently from male courtship patterns (Soma and Garamszegi, 2015).
Interestingly, the two species that lack female dance and show olfactory preference for the opposite sex, i.e., Bengalese Finches and Diamond Firetails are substantially different in their social life style. Bengalese Finches, and respectively their wild ancestors, are gregarious birds that live outside the breeding season in flocks with up to 100 birds (Robiller, 1979; Restall, 1997; Schmelz et al., 2015). While Diamond Firetails have much smaller flock sizes of only 20–30 birds (Robiller, 1979; Restall, 1997; Schmelz et al., 2015). Thus, sociality cannot explain the observed olfactory preference for opposite sex conspecifics.
Other explanations for the observed olfactory preferences can of course not excluded, as there might be many other underlying confounding or explanatory factors, e.g., random differences in domestication of the species, about which is for most of the species relatively less known. Also, general differences in e.g., fear or exploration behaviour might influence the results, although we tried our best with the experimental design to minimise such effects. Nevertheless, it is interesting that only in Zebra Finches a considerable large amount of birds made no choice. Although our measure, the first choice in a Y-maze/T-maze is an established method in olfaction research (Bonadonna et al., 2003a,b; Bonadonna and Nevitt, 2004; Whittaker et al., 2011; Bonadonna and Sanz-Aguilar, 2012; Zhang et al., 2013; Krause et al., 2014), in future studies, it would be worth taking more parameters from the birds behaviour into account, as for example, are vocalisation or courtship displays conducted in the maze.
Taken together, it is important to note that our study can only be a first, hopefully, inspiring first step to investigate among species differences in olfactory choice preferences within the family of Estrildid Finches. We could only test six out of more than 130 species (Goodwin, 1982). Ideally, more species will be added in future studies to get a comprehensive understanding of the potential link between sex-specific body odours and other courtship displays. Nevertheless, our results show sex- as well as potentially species-specific differences in olfactory preferences of same or opposite sex conspecifics. Sex specific differences in olfactory cues have been found in a variety of bird species, including blue tits (Caspers et al., 2022), song sparrows (Grieves et al., 2019), mallards (Jacob et al., 1979), or dark eyed juncos (Whittaker et al., 2010). Sex specific differences are particularly prominent during the breeding season (Grieves et al., 2022) and found to be mainly driven by females (Whittaker and Hagelin, 2021). All birds used in our study were not breeding, however, we do not have any knowledge about the hormonal state of these birds, i.e., cannot support, nor rule out, whether the birds were in reproductive mode. In addition, we have not done any chemical analyses and thus are not able to determine chemically, whether sex-specific differences in the olfactory phenotype occur and whether potential differences are driven by females, i.e., through a higher diversity of substances, or a higher concentration of substances (Whittaker and Hagelin, 2021).
Differences in olfactory sex preferences, as found in our study, are also known from mammals (e.g., Eisenberg and Kleiman, 1972; Jänig et al., 2022) and other vertebrates (e.g., Shohet and Watt, 2004; Galeotti et al., 2007; Caspers and Steinfartz, 2011). Furthermore, multimodal signal integration can also be found in other vertebrate taxa (e.g., Voigt et al., 2008; Starnberger et al., 2014; Ronald et al., 2020; Carreira Bruinjé et al., 2022).
Our results suggest (i) that sex- as well as potentially species-specific differences occur in the olfactory preferences in Estrildid Finches, as tested for Zebra Finches, Diamond Firetails and Bengalese Finches, and it should be addressed in the future if and how other Estrildid Finch species would refine or complete this pattern. Furthermore, our results show that (ii) sex specific body odours occur also in Estridild Finches, as it has been shown for many other bird species (Grieves et al., 2022). Thus, in future research on mate choice in Estrildid Finches it is worth taking the importance of olfaction into account.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethical review and approval was not required for the animal study because after the experiments, all birds remained in our lab stocks or were returned to the stocks from a local breeder. The experiments were performed in 2014 at Bielefeld University in accordance with the current laws of the country at that time. Housing of birds was conducted with the permission of the Gesundheits-, Veterinär- und Lebensmittelüberwachungsamt, Stadt Bielefeld, Germany (no. 530.421630-1, 18/04/2002 and no. 530.4, 27.07.2014). All birds were monitored daily.
EK, OK, and BC conceived and designed the study and wrote the manuscript. EK, MP, and BC conducted the experiments. EK and BC conducted the analysis. All authors contributed to the article and approved the submitted version.
EK was funded by a fellowship of the Volkswagen Foundation under its Evolutionary Biology Initiative (85994). BC was funded by a Freigeist Fellow of the Volkswagen Foundation.
We thank the two reviewers for extremely helpful comments to an earlier version of the manuscript. We also thank the animal caretakers at Bielefeld University for the bird management and U. Baßfeld for providing some birds.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amo, L., Avilés, J. M., Parejo, D., Peña, A., Rodríguez, J., and Tomás, G. (2012). Sex recognition by odour and variation in the uropygial gland secretion in starlings. J. Anim. Ecol. 81, 605–613. doi: 10.1111/j.1365-2656.2011.01940.x
Andersson, M. (2019). Sexual selection. Princeton NJ: Princeton University Press. doi: 10.2307/j.ctvs32s1x
Arnaiz-Villena, A., Ruiz-del-Valle, V., Gomez-Prieto, P., Reguera, R., Parga-Lozano, C., and Serrano-Vela, I. (2009). Estrildinae finches (Aves, Passeriformes) from Africa, South Asia and Australia: A molecular phylogeographic study. Open Ornith. J. 2, 29–36. doi: 10.2174/1874453200902010029
Birke, L. I. (1974). Social facilitation in the Bengalese finch. Behaviour 48, 111–122. doi: 10.1163/156853974X00282
Bonadonna, F., and Nevitt, G. A. (2004). Partner-specific odor recognition in an Antarctic seabird. Science 306, 835–835. doi: 10.1126/science.1103001
Bonadonna, F., and Sanz-Aguilar, A. (2012). Kin recognition and inbreeding avoidance in wild birds: The first evidence for individual kin-related odour recognition. Anim. Behav. 84, 509–513. doi: 10.1016/j.anbehav.2012.06.014
Bonadonna, F., Cunningham, G. B., Jouventin, P., Hesters, F., and Nevitt, G. A. (2003a). Evidence for nest-odour recognition in two species of diving petrel. J. Exp. Biol. 206, 3719–3722. doi: 10.1242/jeb.00610
Bonadonna, F., Hesters, F., and Jouventin, P. (2003b). Scent of a nest: Discrimination of own-nest odours in Antarctic prions, Pachyptila desolata. Behav. Ecol. Sociobiol. 54, 174–178. doi: 10.1007/s00265-003-0610-7
Caro, S. P., and Balthazart, J. (2010). Pheromones in birds: Myth or reality? J. Comp. Physiol. A 196, 751–766. doi: 10.1007/s00359-010-0534-4
Caro, S. P., Balthazart, J., and Bonadonna, F. (2015). The perfume of reproduction in birds: Chemosignaling in avian social life. Horm. Behav. 68, 25–42. doi: 10.1016/j.yhbeh.2014.06.001
Carreira Bruinjé, A., de Alencar Paiva, T. M., and Costa, G. C. (2022). Multimodal female mate choice in a polymorphic flat rock lizard. Behav. Ecol. Sociobiol. 76:77. doi: 10.1007/s00265-022-03181-x
Caspers, B. A., and Krause, E. T. (2011). Odour-based natal nest recognition in the zebra finch (Taeniopygia guttata), a colony-breeding songbird. Biol. Lett. 7, 184–186. doi: 10.1098/rsbl.2010.0775
Caspers, B. A., and Steinfartz, S. (2011). Preference for the other sex: Olfactory sex recognition in terrestrial fire salamanders (Salamandra salamandra). Amphib. Reptil. 32, 503–508. doi: 10.1163/156853811X603265
Caspers, B. A., Gagliardo, A., and Krause, E. T. (2015a). Impact of kin odour on reproduction in zebra finches. Behav. Ecol. Sociobiol. 69, 1827–1833. doi: 10.1007/s00265-015-1995-9
Caspers, B. A., Hagelin, J., Bock, S., and Krause, E. T. (2015b). An easy method to test odour recognition in songbird hatchlings. Ethology 121, 882–887. doi: 10.1111/eth.12400
Caspers, B. A., Hagelin, J. C., Paul, M., Bock, S., Willeke, S., and Krause, E. T. (2017). Zebra Finch chicks recognise parental scent, and retain chemosensory knowledge of their genetic mother, even after egg cross-fostering. Sci. Rep. 7:12859. doi: 10.1038/s41598-017-13110-y
Caspers, B. A., Hoffman, J. I., Kohlmeier, P., Krüger, O., and Krause, E. T. (2013). Olfactory imprinting as a mechanism for nest odour recognition in zebra finches. Anim. Behav. 86, 85–90. doi: 10.1016/j.anbehav.2013.04.015
Caspers, B. A., Marfull, R., Dannenhaus, T., Komdeur, J., and Korsten, P. (2022). Chemical analysis reveals sex differences in the preen gland secretion of breeding Blue Tits. J. Ornithol. 163, 191–198. doi: 10.1007/s10336-021-01921-w
Catchpole, C. K., and Slater, P. J. (2003). Bird song: Biological themes and variations. Cambridge: Cambridge University Press.
Eisenberg, J. F., and Kleiman, D. G. (1972). Olfactory communication in mammals. Annu. Rev. Ecol. Syst. 3, 1–32. doi: 10.1146/annurev.es.03.110172.000245
Forstmeier, W., Segelbacher, G., Mueller, J., and Kempenaers, B. (2007). Genetic variation and differentiation in captive and wild zebra finches (Taeniopygia guttata). Mol. Ecol. 16, 4039–4050. doi: 10.1111/j.1365-294X.2007.03444.x
Galeotti, P., Sacchi, R., Rosa, D. P., and Fasola, M. (2007). Olfactory discrimination of species, sex, and sexual maturity by the Hermann’s tortoise Testudo hermanni. Copeia 2007, 980–985. doi: 10.1643/0045-8511(2007)7[980:ODOSSA]2.0.CO;2
Golüke, S., Bischof, H. J., and Caspers, B. A. (2021). Nestling odour modulates behavioural response in male, but not in female zebra finches. Sci. Rep. 11:712. doi: 10.1038/s41598-020-80466-z
Golüke, S., Bischof, H. J., Engelmann, J., Caspers, B. A., and Mayer, U. (2019). Social odour activates the hippocampal formation in zebra finches (Taeniopygia guttata). Behav. Brain Res. 364, 41–49. doi: 10.1016/j.bbr.2019.02.013
Grieves, L. A., Bernards, M. A., and MacDougall-Shackleton, E. A. (2019). Wax ester composition of songbird preen oil varies seasonally and differs between sexes, ages, and populations. J. Chem. Ecol. 45, 37–45. doi: 10.1007/s10886-018-1033-2
Grieves, L. A., Gilles, M., Cuthill, I. C., Székely, T., MacDougall-Shackleton, E. A., and Caspers, B. A. (2022). Olfactory camouflage and communication in birds. Biol. Rev. 97, 1193–1209. doi: 10.1111/brv.12837
Harrison, C. J. O., Nicolai, J., Immelmann, K., and Wolters, H. E. (1962). Solitary song and its inhibition in some Estrildidae. J. Ornithol. 103, 369–379. doi: 10.1007/BF01676599
Hoffman, J. I., Krause, E. T., Lehmann, K., and Krüger, O. (2014). MC1R genotype and plumage colouration in the zebra finch: Population structure generates artefactual associations. PLoS One 9:e86519. doi: 10.1371/journal.pone.0086519
Jacob, J., Balthazart, J., and Schoffeniels, E. (1979). Sex differences in the chemical composition of uropygial gland waxes in domestic ducks. Biochem. Syst. Ecol. 7, 149–153. doi: 10.1016/0305-1978(79)90024-3
Jänig, S., Kücklich, M., Kulik, L., Zetzsche, M., Weiß, B. M., and Widdig, A. (2022). Olfactory inspection of female reproductive states in chimpanzees. Front. Ecol. Evol. 10:884661. doi: 10.3389/fevo.2022.884661
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K., and Mooers, A. O. (2012). The global diversity of birds in space and time. Nature 491, 444–448. doi: 10.1038/nature11631
Krause, E. T., and Caspers, B. A. (2012). Are olfactory cues involved in nest recognition in two social species of Estrildid finches? PLoS One 7:e36615. doi: 10.1371/journal.pone.0036615
Krause, E. T., and Caspers, B. A. (2018). Do diamond Firetails (Stagonopleura guttata) recognise the scent of their nest as other Estrildid finches do? Emu Aust. Ornithol. 118, 375–380. doi: 10.1080/01584197.2018.1459727
Krause, E. T., Bischof, H. J., Engel, K., Golüke, S., Maraci, Ö, Mayer, U., et al. (2018). Olfaction in the zebra finch (Taeniopygia guttata): What is known and further perspectives. Adv. Study Behav. 50, 37–85. doi: 10.1016/bs.asb.2017.11.001
Krause, E. T., Brummel, C., Kohlwey, S., Baier, M. C., Müller, C., Bonadonna, F., et al. (2014). Differences in olfactory species recognition in the females of two Australian songbird species. Behav. Ecol. Sociobiol. 68, 1819–1827. doi: 10.1007/s00265-014-1791-y
Krause, E. T., Krüger, O., Kohlmeier, P., and Caspers, B. A. (2012). Olfactory kin recognition in a songbird. Biol. Lett. 8, 327–329. doi: 10.1098/rsbl.2011.1093
Meyer, D., Zeileis, A., and Hornik, K. (2006). The Strucplot framework: Visualizing multi-way contingency tables with vcd. J. Stat. Softw. 17, 1–48.
Mihailova, M., Berg, M. L., Buchanan, K. L., and Bennett, A. T. (2014). Odour-based discrimination of subspecies, species and sexes in an avian species complex, the crimson rosella. Anim. Behav. 95, 155–164. doi: 10.1016/j.anbehav.2014.07.012
Naguib, M., and Nemitz, A. (2007). Living with the past: Nutritional stress in juvenile males has immediate effects on their plumage ornaments and on adult attractiveness in zebra finches. PLoS One 2:e901. doi: 10.1371/journal.pone.0000901
Nicolai, J. (2001). Prachtfinken: Australien, Ozeanien, Südostasien, 3rd Edn. Stuttgart: Verlag Eugen Ulmer.
Paradis, E., Claude, J., and Strimmer, K. (2004). APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. doi: 10.1093/bioinformatics/btg412
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Riebel, K. (2009). Song and female mate choice in zebra finches: A review. Adv. Study Behav. 40, 197–238. doi: 10.1016/S0065-3454(09)40006-8
Robiller, F. (1979). Prachtfinken: Vögel von drei kontinenten. Berlin: VEB Deutscher Landwirtschaftsverlag.
Ronald, K. L., Zhang, X., Morrison, M. V., Miller, R., and Hurley, L. M. (2020). Male mice adjust courtship behavior in response to female multimodal signals. PLoS One 15:e0229302. doi: 10.1371/journal.pone.0229302
Schmelz, M., Krüger, O., Call, J., and Krause, E. T. (2015). A Comparison of spontaneous problem solving abilities in three estrildid finch (Taeniopygia guttata, Lonchura striata var. domestica, Stagonopleura guttata) species. J. Comp. Psychol. 129, 356–365. doi: 10.1037/a0039646
Shohet, A. J., and Watt, P. J. (2004). Female association preferences based on olfactory cues in the guppy, Poecilia reticulata. Behav. Ecol. Sociobiol. 55, 363–369. doi: 10.1007/s00265-003-0722-0
Soma, M., and Garamszegi, L. Z. (2015). Evolution of courtship display in Estrildid finches: Dance in relation to female song and plumage ornamentation. Front. Ecol. Evol. 3:4. doi: 10.3389/fevo.2015.00004
Starnberger, I., Preininger, D., and Hödl, W. (2014). The anuran vocal sac: A tool for multimodal signalling. Anim. Behav. 97, 281–288. doi: 10.1016/j.anbehav.2014.07.027
Voigt, C. C., Behr, O., Caspers, B., von Helversen, O., Knörnschild, M., Mayer, F., et al. (2008). Songs, scents, and senses: Sexual selection in the greater sac-winged bat, Saccopteryx bilineata. J. Mammal. 89, 1401–1410. doi: 10.1644/08-MAMM-S-060.1
Whittaker, D. J., and Hagelin, J. C. (2021). Female-based patterns and social function in avian chemical communication. J. Chem. Ecol. 47, 43–62. doi: 10.1007/s10886-020-01230-1
Whittaker, D. J., Gerlach, N. M., Soini, H. A., Novotny, M. V., and Ketterson, E. D. (2013). Bird odour predicts reproductive success. Anim. Behav. 86, 697–703. doi: 10.1016/j.anbehav.2013.07.025
Whittaker, D. J., Richmond, K. M., Miller, A. K., Kiley, R., Bergeon Burns, C., Atwell, J. W., et al. (2011). Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav. Ecol. 22, 1256–1263. doi: 10.1093/beheco/arr122
Whittaker, D. J., Soini, H. A., Atwell, J. W., Hollars, C., Novotny, M. V., and Ketterson, E. D. (2010). Songbird chemosignals: Volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav. Ecol. 21, 608–614. doi: 10.1093/beheco/arq033
Zann, R. A. (1996). The Zebra Finch–A synthesis of field and laboratory studies. Oxford: Oxford University Press.
Zhang, J. X., Wei, W., Zhang, J. H., and Yang, W. H. (2010). Uropygial gland-secreted alkanols contribute to olfactory sex signals in budgerigars. Chem. Senses 35, 375–382. doi: 10.1093/chemse/bjq025
Keywords: Estridild Finches, avian olfaction, olfactory communication, sex recognition, mate choice, Zebra Finch, Bengalese Finch
Citation: Krause ET, Paul M, Krüger O and Caspers BA (2023) Olfactory sex preferences in six Estrildid Finch species. Front. Ecol. Evol. 11:1000531. doi: 10.3389/fevo.2023.1000531
Received: 22 July 2022; Accepted: 11 April 2023;
Published: 27 April 2023.
Edited by:
Magdalena Ruiz Rodriguez, University of Granada, SpainReviewed by:
Elia Gatto, University of Ferrara, ItalyCopyright © 2023 Krause, Paul, Krüger and Caspers. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. Tobias Krause, dG9iaWFzLmtyYXVzZUBmbGkuZGU=; Barbara A. Caspers, YmFyYmFyYS5jYXNwZXJzQHVuaS1iaWVsZWZlbGQuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.