- 1Programa de Pós-Graduação em Ciências Ambientais, Universidade Federal de Alfenas, Alfenas, Brazil
- 2Departamento de Silvicultura, Universidad de Concepción, Concepción, Chile
- 3Independent Researcher, São Paulo, Brazil
- 4Centro de Ciências Naturais e Humanas, Universidade Federal do ABC (UFABC), Santo André, Brazil
Termites are one of the most relevant groups for recycling nutrients and keeping the flow of energy in ecosystems. Although their role as lignocellulose decomposers is the focus of studies, they also act as dung recyclers, but their importance in this process is poorly understood. Here we performed manipulation experiments to determine dung removal by termites in forest remnants and cattle pastures in a fragmented Atlantic Forest landscape. We used wire bags of different mesh sizes placed along transects in three forest fragments and pastures for 10 days to compare the contribution of termites and other coprophagous macrodetritivores to dung removal. Our results indicated that termites removed more dung in pastures than in the forest fragments. In addition, dung beetle exclusion significantly reduced the percentage of dung removal within forest fragments, but not on pastures, indicating termites are important dung recyclers in pastures.
Introduction
Most natural environments have been currently modified by human population growth causing different degrees of habitat loss (Fahrig, 2019). This loss in turn impacts biodiversity and causes the disruption of several ecological processes (Veldkamp et al., 2020). Due to a growing human population, cattle production has increased significantly over the years, which contributed about 12–18% of total greenhouse gas emissions, mainly through gut fermentation and excreta. In addition, livestock dung is also involved in eutrophication and acidification of natural ecosystems (Cai et al., 2021).
Termites (Blattodea) are ecosystem engineers, modulating the balance of carbon between the soil and the atmosphere through lignocellulose decomposition mediated by symbiotic microorganisms (Brune, 2014). The construction of tunnel networks and nests by these insects alters the physical and chemical properties of the soil through bioturbation and deposition of organic material, promoting growth and modifying the community structure of plants in the surrounding soils (Jouquet et al., 2011; Ashton et al., 2019; Griffiths et al., 2021; Myer et al., 2021). Basal groups of termites (called lower termites) are primarily xylophagous and rely primarily on flagellate protozoans for lignocellulose digestion (Brune, 2014). However, the evolutionary success of the Termitidae (the most diverse and numerous family of termites, called higher termites) is attributed to the loss of protozoans and the acquisition of specialized lignocellulolytic bacterial lineages that allowed diet diversification, including wood, grass, soil, litter, lichen, and fungi (Bourguignon et al., 2011; Brune and Dietrich, 2015). In tropical environments, termites are the main macrodetritivores, decomposing half of deadwood in rainforests and more than 30% of the litter in savannas (Veldhuis et al., 2017; Griffiths et al., 2019; Sundsdal et al., 2020).
Dung removal is an important ecological function provided by some macrodetritivores that benefits to the environment through the mobilization of carbon and nitrogen into the soil, reduction of greenhouse gasses and ammonia (Cai et al., 2021), and fly pest suppression (Nichols et al., 2008; Sitters et al., 2014). Dung removal have been usually attributed to dung beetles (Scarabeinae) (Nichols et al., 2008); however, several termite species are also known to feed on a wide range of herbivore mammal dung; the majority of these termites belong to the grass and litter feeding guilds (Freymann et al., 2008) and are likely attracted to the fibrous plant material, nitrogen, and water content of feces (Anderson and Coe, 1974; Freymann et al., 2008).
Brazil is one of the largest producers of livestock in the world (OECD and FAO, 2021). Expanding cattle production requires additional land and water, in addition to increasing excrement output. The destruction of forests through the expansion of grazing land for livestock is one of the main drivers of decreased biodiversity (Sano et al., 2008; Freitas et al., 2010). The loss of coprophagous organisms, particularly termites and dung beetles (Braga et al., 2012; Cancello et al., 2014), may bring further issues to the environment due to their key role in removing dung and the other indirect ecosystem services they exert that were listed above (Nichols et al., 2008; Cai et al., 2021). However, there is no information on how habitat degradation simultaneously affects these organisms and their ecological role in dung removal. In this study, we established a manipulative experiment to quantify and compare the relative contribution of dung removal by termites and other coprophagous macrodetritivores to pastures and forest fragments in an Atlantic Forest landscape.
Materials and methods
Study site
We conducted this study in the rural areas of Alfenas (21°25′45″ S; 45°56′50″ W), a municipality in the southern part of the Brazilian state of Minas Gerais (Supplementary Table 1). This region was originally covered by Atlantic Forest but agricultural development resulted in a largely fragmented landscape (Figure 1). The Atlantic Forest is considered a biodiversity hotspot due to its high diversity and endemism (Myers et al., 2000). However, it is a domain severely fragmented and threatened with less than 28% of the original areas remaining (Rezende et al., 2018). Experiments were conducted during January–March 2014 in three forest fragments and three deforested areas converted in cattle pastures. These sites were selected using digital image processing of the satellite Sino-Brazilian CBERS-2B with a resolution of 20 m following two criteria: (i) Similarity of spectral attributes such as color and texture and (ii) the presence of natural cover forest in a circular buffer of 1 km radius from the center of selected sites. Forest fragments contains semi decidual vegetation (Carneiro et al., 2014) while the pastures, dominated by Brachiaria grasses were not exposed to cattle grazing to avoid interference with dung removal experiments. Sampling did not involve any endangered species and the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA), a Brazilian Ministry of the Environment’s enforcement agency, provided authorization for termite sampling (SISBIO n° 33269). All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
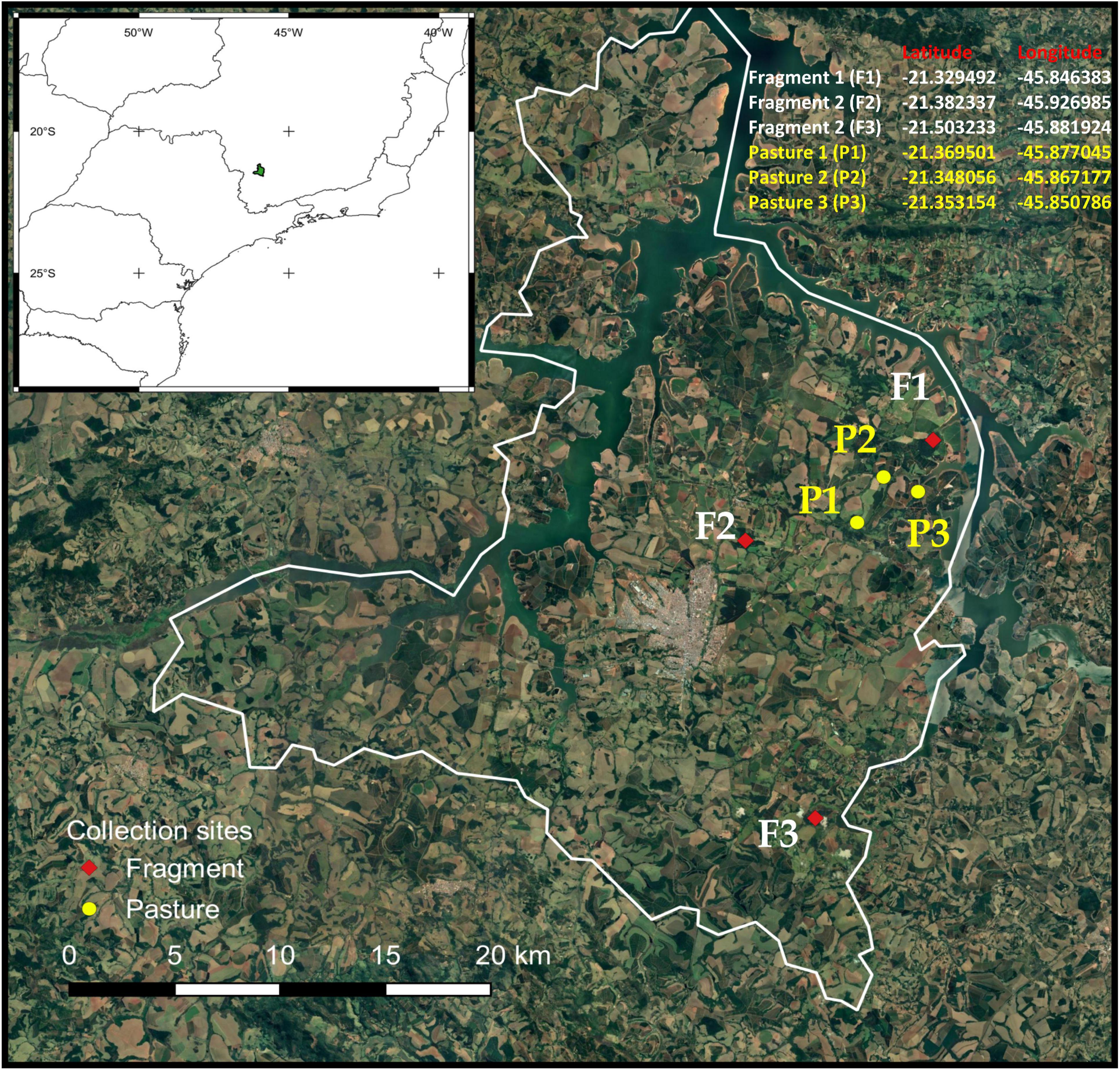
Figure 1. Map of study site and experimental design. Dung removal was evaluated in the Alfenas region of the southeastern Brazil, in the state of Minas Gerais.
Termites associated with cattle dung
Fresh dairy (115 samples) and dry-cow dung pats (183 samples) were collected at cattle grazing pastures 1 km apart from the experimental areas. Termites were carefully removed using forceps and brought to the lab where they were identified to the lowest possible taxon.
Dung removal
We set up this experiment using circular arenas of 60 cm in diameter limited by a fence of nylon with 20 cm in height to avoid dung transportation outside the arenas. In the study site, we found that, apart from termites, the most abundant coprophagous macrodetritivores were dung beetles (Alves, 2015). To assess dung removal by termites, we used 3 mm wire mesh to produce “only termite accessible dung bags” because termite species found in cattle dung have a body width of 1.52 ± 0.39 mm (mean ± SD) (Araujo, 1970; Coles De Negret and Redford, 1982; Redford, 1984; Krishna et al., 2013). Because the smallest dung beetle species found in this study have body width of 3.47 ± 0.34 mm (N = 24) (Supplementary Table 2), 3 mm dung bags allow termites to feed on the dung, effectively excluding dung beetles (Howison et al., 2016). Each bag was filled with 200 g of a mixture of herbivore (cow) and omnivore (pig) dung, obtained from local farmers and placed at the center of the arenas. Both cow and pig dung are effective attractants to a wide range of coprophagous organisms dependent on the dung of different mammal feeding guilds (Nichols et al., 2008). Two additional experiments with “dung bags” of 1 mm (macrodetritivores inaccessible) and 20 mm (macrodetritivores accessible) wire mesh were used in combination with “only termite accessible dung bags” to evaluate the contribution of all coprophagous macrodetritivores to dung removal (Table 1).
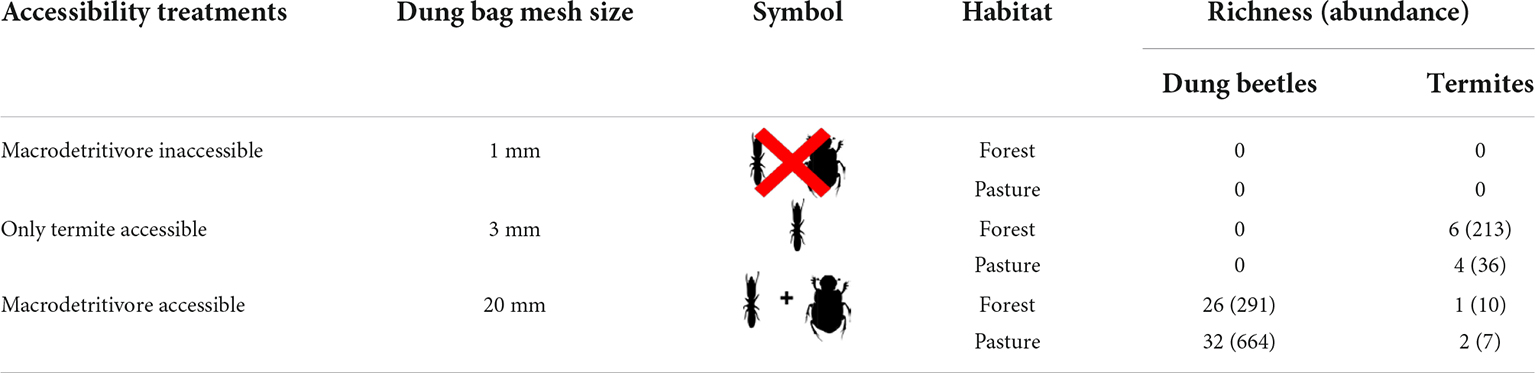
Table 1. Richness and overall abundance of dung beetles and termites for the different treatments and habitat types.
Each sample was protected from rain by covering it with a 30 cm diameter plastic plate placed 15 cm above it, as a roof. The arenas were placed along three 120 m transects at each habitat type. Transects were placed at the center of the site and perpendicular to one of the borders. In each transect, we randomly deployed 12 arenas (four for each treatment) separated by 10 m. We had a total of 216 arenas (forest n = 108, pastures n = 108) in 18 transects across two habitat types in six sites (Figure 1). Dung removal was measured after 10 days. Only samples with termite or dung beetle activity (presence of these insects, termite galleries, dung beetle tunnels) were considered in the analyses. At the start of the experiment, we estimated the moisture content of twenty dung subsamples by determining their fresh and dry weight and used this value to calculate the initial dry biomass and the relative weight loss of each sample, following to Sitters et al. (2014). Dry weights were obtained oven drying fresh dung samples at 60°C for 24 h. Dung removal was calculated as a percentage of dry weight loss after 10 days for each experimental treatment. Termites and dung beetles were carefully removed from dung samples in the bags and identified to the lowest possible taxon using taxonomic keys (Vaz-De-Mello et al., 2011; Rocha et al., 2017) and comparison with a reference collection from the Museu de Zoologia da Universidade de São Paulo (MZUSP) and the Invertebrate Ecology and Conservation Laboratory, at the Universidade Federal de Lavras (UFLA), Brazil, respectively.
Statistical analyses
All the analysis and graphics were generated using R version 3.4.4 (R Core Team, 2019). We examined differences in richness between pastures and forest fragments through individual-based accumulation curves (Chao et al., 2014) using de iNEXT package (Hsieh et al., 2016). A permutational multivariate analysis of variance (PERMANOVA) was used to evaluate differences of termites and dung beetle community composition between habitats (Anderson, 2001). Singletons and doubletons were excluded from the analyses. We fitted beta regressions models with a logit link function using the betareg function (Cribari-Neto and Zeileis, 2010) to test for differences of the percentage of dung removal between habitat types and the experimental treatments. Explanatory variables to affect the outcome were the type of habitat and macrodetritivore accessibility. We carried out least square mean analyses for multiple comparisons to evaluated the effect of model predictors using the emmeans function of the lsmeans package (Lenth, 2016). The significant threshold was P < 0.05.
Results
Dung samples in the 3 mm mesh bags were colonized by termites and micro arthropods (mites and springtails), while samples in the 20 mm bags also attracted dung beetles. Dung samples in the 1 mm bags were colonized only by micro-arthropods. Simultaneous colonization by termites and dung beetles was observed in only 12 bags. We collected eight species of termites from the dung bags. Ruptitermes reconditus (Apicotermitinae) was found in both pastures and forest fragments; however, it was the most abundant species in forest fragments). Three species were found exclusively in forest fragments and one in the pastures. In the areas with cattle activity, we found seven species of termites in 36.6% of the dry dung pats, with a higher incidence of R. reconditus and Procornitermes araujoi (Syntermitinae) (Supplementary Table 3). Fresh wet dung pats were not colonized by termites. On the other hand, 40 dung beetle species were collected. Uroxys sp. (Ateuchini) and Dichotomius bos (Coprini) were the most abundant species in forest fragments and pastures, respectively (Supplementary Table 2).
Our results indicated that richness (Figures 2A,B) and species composition of termite and dung beetles were not affected by habitat loss (Termites: F = 0.43; P = 0.50; Dung-beetles: F = 4.63; P = 0.10; PERMANOVA), but the abundance of these insects on pastures decreased (Figures 2C–F). Dung removal has also been impacted by reduction of forest cover and macrodetritivore suppression. For instance, exclusion of dung beetles significantly reduced the percentage of dung removal within forest fragments (Z = –17.78; P < 0.001) but not on pastures (Z = –0.89; P = 0.375). On the other hand, termites removed more dung from pastures than from forest fragments (Z = 6.33; P < 0.001). Finally, dung samples in the macrodetritivore-inaccessible bags showed a non-significant weight reduction (Z = 1.29; P = 0.198) (Supplementary Tables 4, 5 and Figure 3).
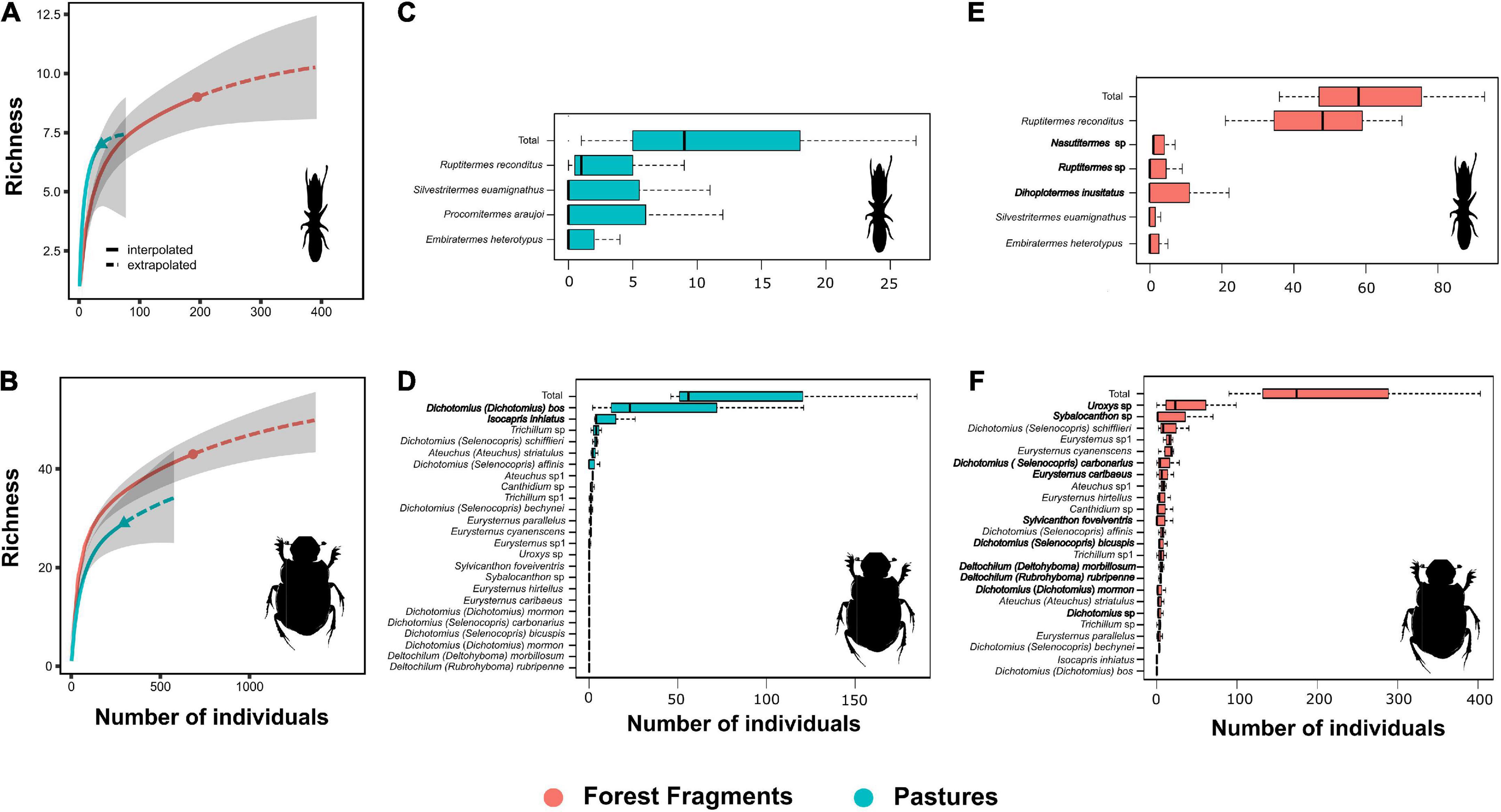
Figure 2. (A,B) Individual-based species accumulation curves across forest fragments and pastures. Curves were plotted based on data grouped across all sites. The solid line shows predictions based on interpolation and the dashed part shows predictions based on extrapolation. Ninety-five percent confidence intervals are shown as shaded areas. (C–F) Species abundance for pastures (blue boxes) and forest fragments (red boxes). Boxplots report median, upper and lower quartiles, and maximum and minimum values. Habitat specialists are marked in bold.
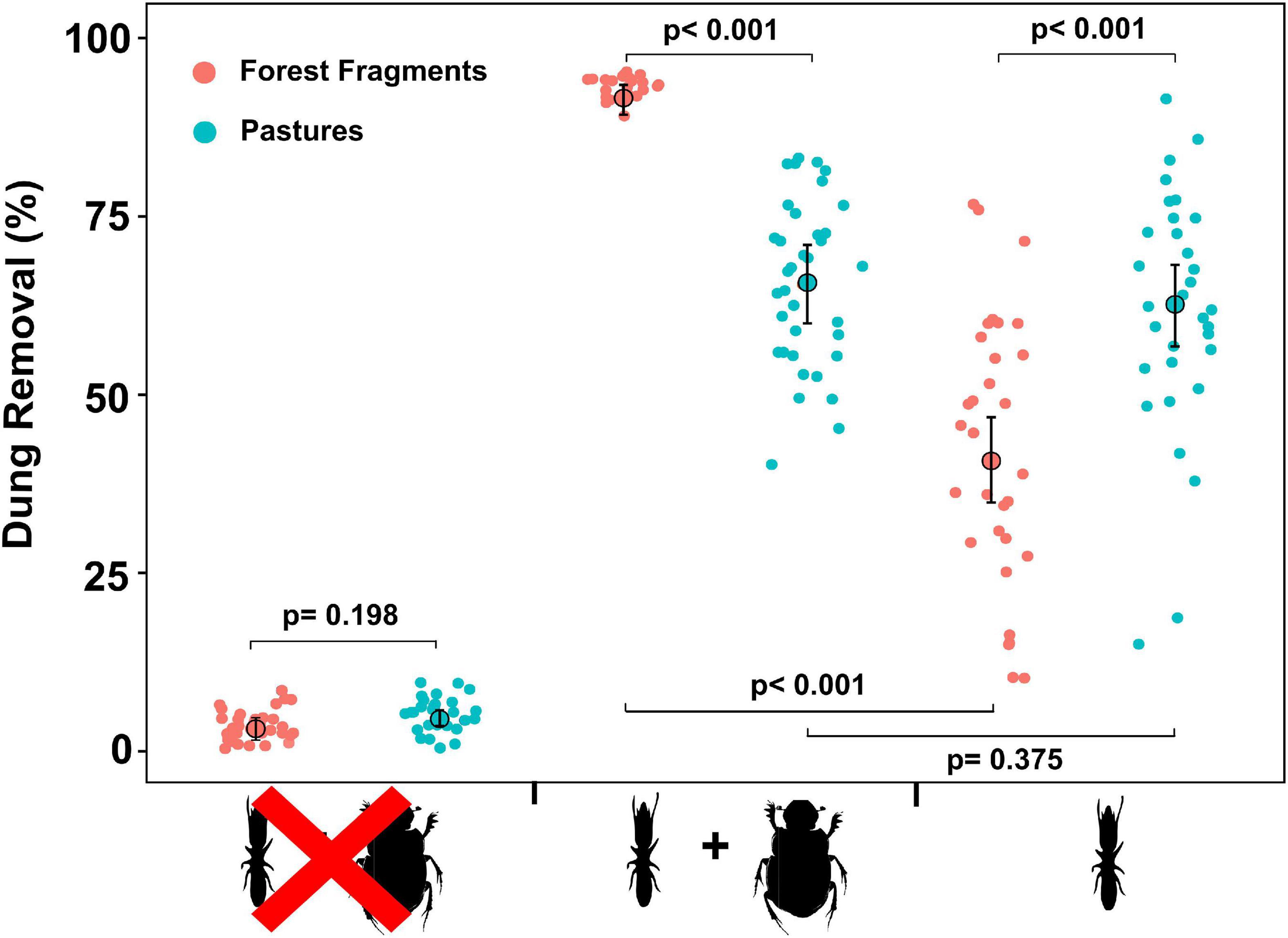
Figure 3. Percentage of dung removal among different experimental treatments (see Table 1 for description) in the two habitat types. The plot shows de modeled mean (GLMM) represented by large circles, the modeled 95% confidence intervals of each mean (error bars), and the model-adjusted individual response values, represented by the small dots and the P-values of the least square mean analyses for pairwise comparisons.
Discussion
In this study, we demonstrate that termites play an important role in dung removal, particularly in pasture areas, where these insects were the main dung removals. Savannah termites are more tolerant to habitat perturbation due to their cryptic lifestyle, their thermoregulated nests or by their habit of foraging at night when temperature and humidity are suitable for these insects (Coles De Negret and Redford, 1982; Korb, 2003). In this study, the absence of tree cover excluded forest-restricted dung beetle species as found in other ecosystems (Halffter and Arellano, 2002). Although the experimental setup did not allow us to quantify the dung removal by dung beetles alone, the exclusion of these insects significantly reduced dung removal within the forest fragments, but had no effect on pastures. It is possible that as dung beetle abundance decreases, termites assume a similar role in the ecosystem, thereby mitigating the effects of habitat loss on dung beetle abundance. These results support that the functional role of termites in removal of mammalian dung is widely underestimated (Freymann et al., 2008), especially in disturbed habitats.
Savannas and pastures in Brazil are populated by a wide variety of termite feeding guilds (Carrijo et al., 2009) and our results showed that termite dung removal was higher on the pastures than in forest fragments, despite the decreasing abundance of these insects in the dung bags in this habitat in comparison with forest fragments. Due to the high temperatures and low humidity, termites from savannas and pastures forage mainly at night to avoid dehydration. Dung bags were collected during daylight hours, which may explain the lower abundance of termite foragers observed in the pastures. Termites within forest fragments, on the other hand, are naturally protected against desiccation. Finally, in the absence of dung beetles and termites, physical weathering, the activity of micro arthropods and microbes account for only 5% of dung removal.
In the pastures with cattle activity, termites were only found in dry dung pats. This can be explained by the following factors. First, most adult dung beetles feed on fresh dung (Holter and Scholtz, 2007) and termite behavior may change in response to competition from dung beetles. The second possible explanation is the tolerance of termites to climatic conditions in the pastures, which could also allow these insects to use dry dung pats for a longer period. In addition, dung beetles, due to their narrow thermal tolerances, cannot cope with the drastic temperature and humidity changes characteristic of deforested habitats (Barragán et al., 2014; Gómez-Cifuentes et al., 2017); a third possible explanation is fiber, the main component of dry cattle dung (Holter, 2016) that could act as an extra source of grass and litter and thus attract termites; and finally, dung may provide termites with nitrogen to supplement their low-nitrogen diet (Higashi et al., 1992). Although there is no information on the interaction between dung beetle and termites, the small number of samples colonized by both insects suggests that they may be mutually exclusive (Gould et al., 2001).
Although this work is constrained by a relatively short duration and low number of sites, this is the first study to show the relative contribution of termites to dung removal under the influence of habitat degradation. A better understanding of termites’ role in dung removal could be achieved by collecting samples at night when termites forage, and more species and individuals could be registered. To further disentangle climatic effects of season, additional experiments of dung removal with a longer duration could be established. Our results support the utility of termites as focal taxa in cattle pastures. The expansion of livestock in Brazil is expected to continue growing over the next decade (OECD and FAO, 2021) leading to increased deforestation and cattle-dung accumulation. Although termites from pastures in Brazil are often considered pests and their nests are removed by local farmers, dung removal by termites in livestock areas could reduce harmful emissions of ammonia and greenhouse gasses. Termite benefits are also linked to nutrient cycling and pedoturbation that help preserve soils (Herrick and Lal, 1996) and conservation of these insects should be a priority.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AA designed the study. AA and FA performed the experiments. AA, FA, and DA-O analyzed the data. AA, MR, and DA-O wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the São Paulo Research Foundation (FAPESP), (grant nos. #2015/21497-6 and 2018/22839-6). FA was a grant fellow of the Coordination for the Improvement of Higher Education Personnel (CAPES). DA-O was grateful for the FONDECYT postdoctoral project (grant no. 3190381).
Acknowledgments
We are thankful to the Laboratório de Ecologia e Conservação de Invertebrados of the Universidade Federal de Lavras for supporting dung beetle identification. We are thankful to Johana Rincones for comments on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.982602/full#supplementary-material
References
Alves, F. R. (2015). Interações e funções ecológicas desempenhadas por Scarabaeinae (Coleoptera: Scarabaeidae) e térmitas (Isoptera) em áreas de pastagem e fragmentos de Mata Atlântica no sul de Minas Gerais, Brasil. Available online at: https://bdtd.unifal-mg.edu.br:8443/handle/tede/875 (accessed on Jul 23, 2022).
Anderson, J. M., and Coe, M. J. (1974). Decomposition of elephant dung in an arid, tropical environment. Oecologia 141, 111–125. doi: 10.1007/BF00344902
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 26, 32–46. doi: 10.1111/j.1442-9993.2001.01070.pp.x
Araujo, R. L. (1970). “Termites of the Neotropical Region,” in Biology of Termites, eds K. Krishna and F. M. Weesner (London: Academic Press), 527–576.
Ashton, L. A., Griffiths, H. M., Parr, C. L., Evans, T. A., Didham, R. K., Hasan, F., et al. (2019). Termites mitigate the effects of drought in tropical rainforest. Science 363, 174–177. doi: 10.1126/science.aau9565
Barragán, F., Moreno, C. E., Escobar, F., Bueno-Villegas, J., and Halffter, G. (2014). The impact of grazing on dung beetle diversity depends on both biogeographical and ecological context. J. Biogeogr. 41, 1991–2002. doi: 10.1111/JBI.12351
Bourguignon, T., Šobotník, J., Lepoint, G., Martin, J. M., Hardy, O. J., Dejean, A., et al. (2011). Feeding ecology and phylogenetic structure of a complex neotropical termite assemblage, revealed by nitrogen stable isotope ratios. Ecol. Entomol. 36, 261–269. doi: 10.1111/j.1365-2311.2011.01265.x
Braga, R. F., Korasaki, V., Audino, L. D., and Louzada, J. (2012). Are Dung Beetles Driving Dung-Fly Abundance in Traditional Agricultural Areas in the Amazon? Ecosystem 157, 1173–1181. doi: 10.1007/S10021-012-9576-5
Brune, A. (2014). Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180. doi: 10.1038/nrmicro3182
Brune, A., and Dietrich, C. (2015). The Gut Microbiota of Termites: Digesting the Diversity in the Light of Ecology and Evolution. Annu. Rev. Microbiol. 69, 145–166. doi: 10.1146/annurev-micro-092412-155715
Cai, Y., Tang, R., Tian, L., and Chang, S. X. (2021). Environmental impacts of livestock excreta under increasing livestock production and management considerations: Implications for developing countries. Curr. Opin. Environ. Sci. Heal. 24:100300. doi: 10.1016/j.coesh.2021.100300
Cancello, E. M., Silva, R. R., Vasconcellos, A., Reis, Y. T., and Oliveira, L. M. (2014). Latitudinal Variation in Termite Species Richness and Abundance along the Brazilian Atlantic Forest Hotspot. Biotropica 46, 441–450. doi: 10.1111/BTP.12120
Carneiro, E., Mielke, O. H. H., Casagrande, M. M., and Fiedler, K. (2014). Community structure of skipper butterflies (Lepidoptera, Hesperiidae) along elevational gradients in brazilian atlantic forest reflects vegetation type rather than altitude. PLoS One 9:e108207. doi: 10.1371/journal.pone.0108207
Carrijo, T. F., Brandão, D., de Oliveira, D. E., Costa, D. A., and Santos, T. (2009). Effects of pasture implantation on the termite (Isoptera) fauna in the Central Brazilian Savanna (Cerrado). J. Insect. Conserv. 13, 575–581. doi: 10.1007/s10841-008-9205-y
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K., et al. (2014). Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Coles De Negret, H. R., and Redford, K. H. (1982). The biology of nine termite species (Isoptera: Termitidae) from the Cerrado of Central Brazil. Psyche 89, 81–106.
Cribari-Neto, F., and Zeileis, A. (2010). Beta regression in R. J. Stat. Softw. 34, 1–24. doi: 10.18637/jss.v034.i02
Fahrig, L. (2019). Habitat fragmentation: A long and tangled tale. Glob. Ecol. Biogeogr. 28, 33–41. doi: 10.1111/geb.12839
Freitas, S. R., Hawbaker, T. J., and Metzger, J. P. (2010). Effects of roads, topography, and land use on forest cover dynamics in the Brazilian Atlantic Forest. For. Ecol. Manag. 259, 410–417. doi: 10.1016/j.foreco.2009.10.036
Freymann, B. P., Buitenwerf, R., DeSouza, O., and Olff, H. (2008). The importance of termites (Isoptera) for the recycling of herbivore dung in tropical ecosystems: A review. Eur. J. Entomol. 105, 165–173. doi: 10.14411/eje.2008.025
Gómez-Cifuentes, A., Munevar, A., Gimenez, V. C., Gatti, M. G., and Zurita, G. A. (2017). Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina. J. Insect Conserv. 21, 147–156. doi: 10.1007/s10841-017-9964-4
Gould, K. A., Herrick, J. E., and Lezama, H. (2001). Refuse to Refuge: Dry Season Use and Modification of Cattle Dung by Subterranean Termites in Guanacaste. Costa Rica. Biotropica 33, 121–130. doi: 10.1111/J.1744-7429.2001.TB00162.X
Griffiths, H. M., Ashton, L. A., Evans, T. A., Parr, C. L., and Eggleton, P. (2019). Termites can decompose more than half of deadwood in tropical rainforest. Curr. Biol. 29, R118–R119. doi: 10.1016/j.cub.2019.01.012
Griffiths, H. M., Eggleton, P., Hemming-Schroeder, N., Swinfield, T., Woon, J. S., Allison, S. D., et al. (2021). Carbon flux and forest dynamics: Increased deadwood decomposition in tropical rainforest tree-fall canopy gaps. Glob. Change Biol. 27, 1601–1613. doi: 10.1111/gcb.15488
Halffter, G., and Arellano, L. (2002). Response of Dung Beetle Diversity to Human–induced Changes in a Tropical Landscape. Biotropica 34, 144–154. doi: 10.1111/J.1744-7429.2002.TB00250.X
Herrick, J. E., and Lal, R. (1996). Dung decomposition and pedoturbation in a seasonally dry tropical pasture. Biol. Fertil. Soils 23, 177–181.
Higashi, M., Abe, T., and Burns, T. P. (1992). Carbon—nitrogen balance and termite ecology. Proc. R. Soc. London. Ser. B Biol. Sci. 249, 303–308. doi: 10.1098/rspb.1992.0119
Holter, P. (2016). Herbivore dung as food for dung beetles: Elementary coprology for entomologists. Ecol. Entomol. 41, 367–377. doi: 10.1111/een.12316
Holter, P., and Scholtz, C. H. (2007). What do dung beetles eat? Ecol. Entomol. 32, 690–697. doi: 10.1111/J.1365-2311.2007.00915.X
Howison, R. A., Berg, M. P., Smit, C., van Dijk, K., and Olff, H. (2016). The Importance of Coprophagous Macrodetritivores for the Maintenance of Vegetation Heterogeneity in an African Savannah. Ecosystems 19, 674–684. doi: 10.1007/s10021-016-9960-7
Hsieh, T. C., Ma, K. H., and Chao, A. (2016). iNEXT: Interpolation and extrapolation for species diversity. R package version 2.0.8. R-project.
Jouquet, P., Traoré, S., Choosai, C., Hartmann, C., and Bignell, D. (2011). Influence of termites on ecosystem functioning. Ecosystem services provided by termites. Eur. J. Soil Biol. 47, 215–222. doi: 10.1016/j.ejsobi.2011.05.005
Korb, J. (2003). Thermoregulation and ventilation of termite mounds. Naturwissenschaften 90, 212–219. doi: 10.1007/s00114-002-0401-4
Krishna, K., Grimaldi, D. A., Krishna, V., and Engel, M. S. (2013). Treatise on the Isoptera of the world. Bull. Am. Museum Nat. Hist. 377, 1–200.
Lenth, R. V. (2016). Least-Squares Means: The R Package lsmeans. J. Stat. Softw. 69, 1–33. doi: 10.18637/jss.v069.i01
Myer, A., Myer, M. H., Trettin, C. C., and Forschler, B. T. (2021). The fate of carbon utilized by the subterranean termite Reticulitermes flavipes. Ecosphere 12:e03872. doi: 10.1002/ecs2.3872
Myers, N., Mittermeler, R. A., Mittermeler, C. G., Da Fonseca, G. A. B., and Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. doi: 10.1038/35002501
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., and Favila, M. E. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 141, 1461–1474. doi: 10.1016/j.biocon.2008.04.011
R Core Team (2019). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Redford, K. H. (1984). The Termitaria of Cornitermes cumulans (Isoptera, Termitidae) and Their Role in Determining a Potential Keystone Species. Biotropica 16, 112–119. doi: 10.2307/2387842
Rezende, C. L., Scarano, F. R., Assad, E. D., Joly, C. A., Metzger, J. P., Strassburg, B. B. N., et al. (2018). From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 16, 208–214. doi: 10.1016/j.pecon.2018.10.002
Rocha, M. M., Morales-Corrêa e Castro, A. C., Cuezzo, C., and Cancello, E. M. (2017). Phylogenetic reconstruction of Syntermitinae (Isoptera, Termitidae) based on morphological and molecular data. PLoS One 12:e0174366. doi: 10.1371/journal.pone.0174366
Sano, E. E., Rosa, R., Brito, J. L. S., and Ferreira, L. G. (2008). Mapeamento semidetalhado do uso da terra do Bioma Cerrado. Pesqui. Agropecuária Bras. 43, 153–156. doi: 10.1590/S0100-204X2008000100020
Sitters, J., Maechler, M. J., Edwards, P. J., Suter, W., and Olde Venterink, H. (2014). Interactions between C: N: P stoichiometry and soil macrofauna control dung decomposition of savanna herbivores. Funct. Ecol. 28, 776–786. doi: 10.1111/1365-2435.12213
Sundsdal, A., Graae, B. J., Speed, J. D. M., Bukombe, J., Mtweve, P. J., Arneberg, M. K., et al. (2020). Teatime in the Serengeti: Macrodetritivores sustain recalcitrant plant litter decomposition across human-modified tropical savannahs. Plant Soil 456, 241–258. doi: 10.1007/s11104-020-04704-z
Vaz-De-Mello, F. Z., Edmonds, W. D., Ocampo, F. C., and Schoolmeesters, P. (2011). A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2854, 1–73. doi: 10.11646/zootaxa.2854.1.1
Veldhuis, M. P., Laso, F. J., Olff, H., and Berg, M. P. (2017). Termites promote resistance of decomposition to spatiotemporal variability in rainfall. Ecology 98, 467–477. doi: 10.1002/ECY.1658
Keywords: Isoptera, forest fragmentation, cattle dung, Atlantic forest, beetles, ecological roles
Citation: Alves FR, Aguilera-Olivares D, Rocha MM and Arab A (2022) Termites are the main dung removals in a degraded landscape in Brazil. Front. Ecol. Evol. 10:982602. doi: 10.3389/fevo.2022.982602
Received: 30 June 2022; Accepted: 23 August 2022;
Published: 15 September 2022.
Edited by:
José F. Fontanari, University of São Paulo, BrazilReviewed by:
Ervin Humprey Duran-Bautista, University of the Amazon, ColombiaCaroline Franco, University of Oxford, United Kingdom
Copyright © 2022 Alves, Aguilera-Olivares, Rocha and Arab. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Arab, YWxiZXJ0b2FyYWJAZ21haWwuY29t
†ORCID: Daniel Aguilera-Olivares, orcid.org/0000-0003-0776-0275; Alberto Arab, orcid.org/0000-0003-0009-6658
 Frederico R. Alves1
Frederico R. Alves1 Daniel Aguilera-Olivares
Daniel Aguilera-Olivares Mauricio Martins Rocha
Mauricio Martins Rocha Alberto Arab
Alberto Arab