- 1Instituto de Investigaciones en Ecosistemas y Sustentabilidad, Universidad Nacional Autónoma de México, Morelia, Mexico
- 2Instituto de Ecología, Universidad Nacional Autónoma de México, Mexico City, Mexico
Dung beetles are recognized as providers of important ecosystem functions, most of which are derived from the removal of vertebrate dung from the soil surface. These insects occur in nearly all terrestrial biomes but are most diverse in the humid tropics. Several of the ecological functions attributed to dung beetles are related to their direct and indirect interactions with plants. Among these functions, the secondary dispersal of seeds defecated by mammals has received the most attention in tropical forests. Nonetheless, while several aspects of secondary seed dispersal by dung beetles are relatively well understood, others remain understudied or have not been addressed at all. Thus, a broad generalization about the effects of secondary seed dispersal by dung beetles on plant fitness remains somewhat elusive. Furthermore, other effects of dung beetle activity on tropical plants have received very little attention. A few studies have shown that through their behaviors of dung burial and soil-excavation, dung beetles can shape seed bank structure and dynamics. Also, though numerous greenhouse studies and field experiments in agricultural lands and temperate grasslands have shown that dung beetle activity increases plant nutrient uptake and yield, it is uncertain whether such effects are common in tropical forests. Here, we review and synthesize our current knowledge on how dung beetles affect tropical forest plants by dispersing defecated seeds, shaping the structure and dynamics of seed banks, and influencing the performance of understory seedlings. We focus on the Neotropics, where most studies on the effects of dung beetles on tropical forest plants have been carried out, but we also show results from other regions and biomes, to present a more general picture of these beetle-plant interactions. Throughout the review we emphasize aspects that need more research to allow generalizations and point out those questions that remain unanswered. We hope that this review will stimulate more research about the fascinating interactions between dung beetles and plants in tropical ecosystems.
Introduction
Dung beetles are conspicuous insects found in terrestrial ecosystems of all continents, except Antarctica (Hanski and Cambefort, 1991). Adults and larval beetles feed on animal feces, preferring the dung of mammals. Through their feeding and nesting behaviors, most dung beetle species remove feces from the surface and incorporate it into the soil. The ecological consequences of this activity are manyfold, from soil conditioning and increased plant yield, to livestock parasite control and secondary seed dispersal, among others (Nichols et al., 2008; Scholtz et al., 2009). Dung beetle communities reach their highest abundance and diversity in tropical forests and savannas, where the mammal faunas that provide their main food resource also display their highest richness and biomass (Hanski and Cambefort, 1991). Because several of the ecological functions attributed to dung beetles can affect plants, it is believed that these insects can play an important role in structuring tropical plant communities (Andresen and Feer, 2005). However, except for the secondary dispersal of seeds defecated by mammals, most studies quantifying the effects of dung beetle activity on plants have been carried out in productive systems and/or temperate regions and have mostly focused on aspects related to soil conditioning (Nichols et al., 2008; Scholtz et al., 2009). The lack of empirical evidence often leads to the assumption that effects of dung beetles measured in other study systems (e.g., greenhouse experiment on temperate grasses) can be extrapolated to natural conditions in tropical forests. However, such an assumption is not justified because the effects of dung beetle activity on plants are context dependent (Slade et al., 2011; Griffiths et al., 2016; Urrea-Galeano et al., 2021).
Dung beetle functions performed at any given site depend on the composition of the local dung beetle community, which varies strongly among ecosystems, regions, and continents (Hanski and Cambefort, 1991), as well as the type and degree of habitat disturbance (Fuzessy et al., 2021a; López-Bedoya et al., 2022). Most tropical dung beetle species belong to the subfamily Scarabaeinae (often referred to as the ‘true dung beetles’), although other dung-feeding beetle taxa (Aphodiinae, Geotrupidae) are important in other bioregions (Scholtz et al., 2009). Furthermore, dung beetle species vary in their nesting, feeding, and dung-relocating behaviors (Halffter and Edmonds, 1982), which also affects their functional impact in ecosystems. Though definitions can vary according to authors (Tonelli, 2021), three general behavioral groups are distinguished: (i) dwellers (including both non-nesting species as well as endocoprid nesters) use the dung directly at the source; (ii) rollers relocate dung portions by rolling them away from the source and then burying them in underground tunnels and chambers; and (iii) tunnellers or burrowers relocate dung portions underneath or very close to the dung source, also into underground tunnels and chambers. Most tropical dung beetles are rollers and tunnellers.
Through the burial of dung in underground tunnels, dung beetles can have direct or indirect interactions with plants. Direct interactions occur through the fortuitous manipulation of seeds that are imbedded in dung or in the soil. On the other hand, indirect interactions between beetles and plants, are those driven by the changes that beetle activity can cause in the biological, chemical and/or physical properties of the soil. In some rare cases dung beetles can also have effects that are not a consequence of their dung-relocation behavior, including the pollination of plant species with decay-scented flowers (Nichols et al., 2008), and the use of acorns as food/nesting resource (i.e., seed predation or seed dispersal when the embryo is not killed; Pérez-Ramos et al., 2007, 2013). In this review, we focus only on the effects that are derived from the dung-relocation activity of dung beetles (Figure 1; Table 1), because they are the most widespread. First, we review the secondary dispersal of seeds imbedded in dung (Secondary seed dispersal), which is the ecological function of dung beetles that has received the most attention in tropical forests, particularly in the Neotropics. Then, we present the findings of those few studies that have addressed the effects that dung beetles can have on the structure and dynamics of tropical soil seed banks (Seed banks). Finally, we evaluate our knowledge about the indirect effects of dung beetle activity on plant nutrient uptake and performance in tropical forests (Plant performance). The aims of this review are to present a concise synthesis of our understanding about these three interactions between dung beetles and tropical forest plants, to point out research gaps, to discuss some methodological aspects, and to encourage researchers to critically question and assess whether the functions of dung beetles can be extrapolated among different study systems.
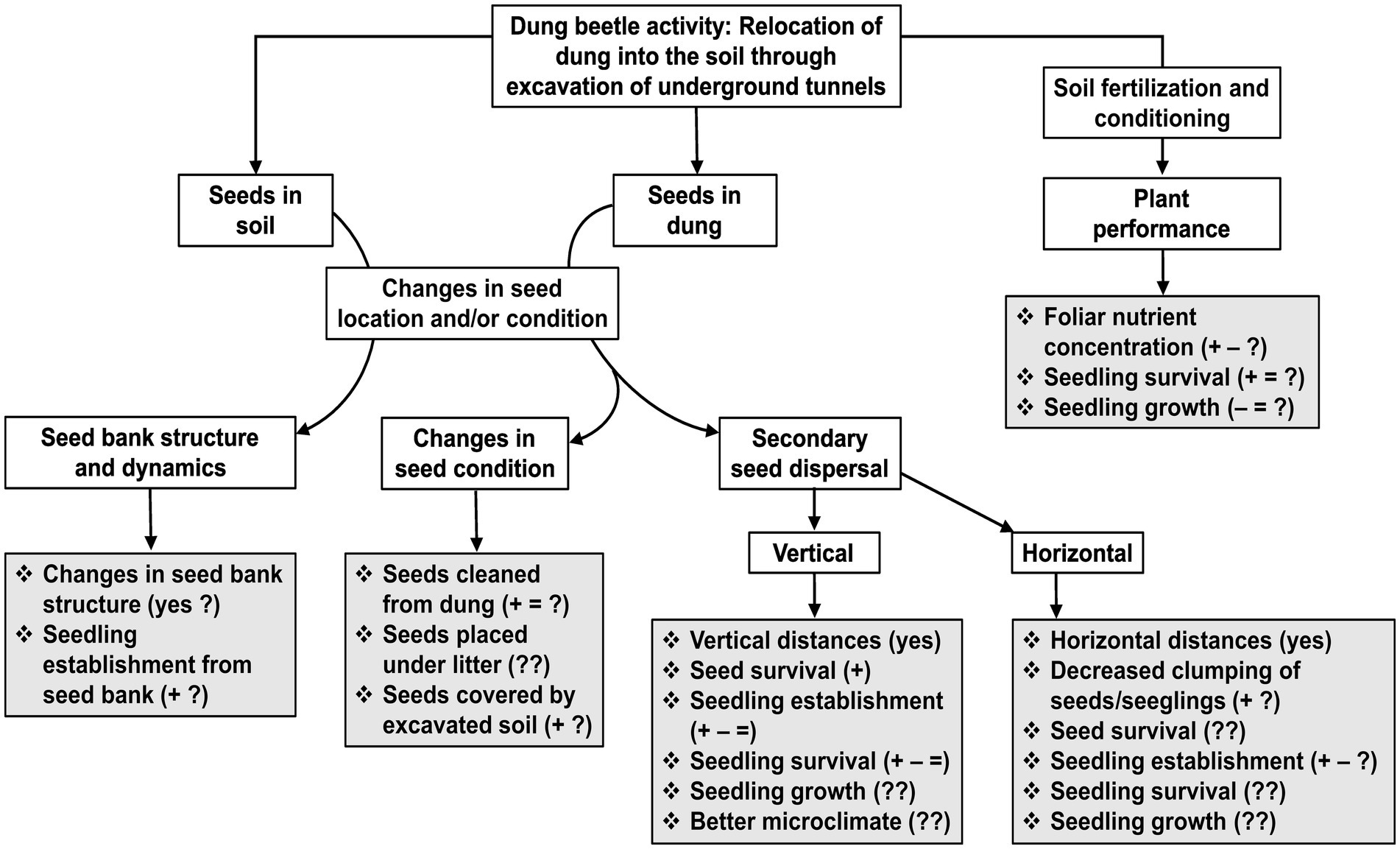
Figure 1. Flowchart showing the consequences that dung beetle activity can have on plants that are reviewed in this article (Secondary seed dispersal; Seed banks; and Plant performance). The activity of dung beetles that we focus on, is the relocation of animal feces (mostly mammal dung) from the soil surface into deeper soil layers, which in turn occurs through the excavation of soil to build underground tunnels and chambers for feeding and nesting. The consequences for plants result from a direct interaction between dung beetles and seeds (seeds in dung and seeds in soil), and from an indirect interaction between beetles and plants, which is mediated through soil fertilization and conditioning. Grey boxes show specific responses that have been measured (those followed by “yes”, “+”, “–“, and/or “=”), or have been suggested to occur. Signs indicate positive (+), negative (−), and no effect (=) of dung beetles reported for each of the responses; question marks indicate that a response has been little studied (?), or not at all (??). Studies that have assessed one or more of these responses are shown in Table 1. For secondary seed dispersal, information for specific plant species can be found in Supplementary Table S1.
Secondary seed dispersal
Secondary seed dispersal by dung beetles occurs when they move seeds that have been defecated by a fruit-eating vertebrate (i.e., the primary disperser). Beetles are attracted to the defecations and when they relocate portions of it, seeds present in the dung are incidentally relocated as well. From the beetles’ perspective, seeds are useless ‘contaminants’; thus, they may exclude seeds prior or during dung-relocation. Seeds dispersed by beetles may be buried by them (vertical dispersal) and may be moved some horizontal distance away from the site of deposition (horizontal dispersal). One or both movements can occur and can have consequences for seed fate. Secondary seed dispersal by dung beetles was initially reported in a greenhouse experiment, in which burial of seed-containing cattle dung by dung beetles promoted seedling establishment of a temperate prairie grass (Wicklow et al., 1984). A few years later, the first field study quantifying some aspects of secondary seed dispersal by dung beetles in a tropical forest was published (Estrada and Coates-Estrada, 1986, 1991), and seven years elapsed before the next study (Shepherd and Chapman, 1998). Since then, the publication stream has been steady, though modest, yielding a total of 71 articles (not including reviews) worldwide between 1984 and June 2022, which assess some aspect of secondary seed dispersal by dung beetles, 83% of them in tropical biomes (Figure 2).
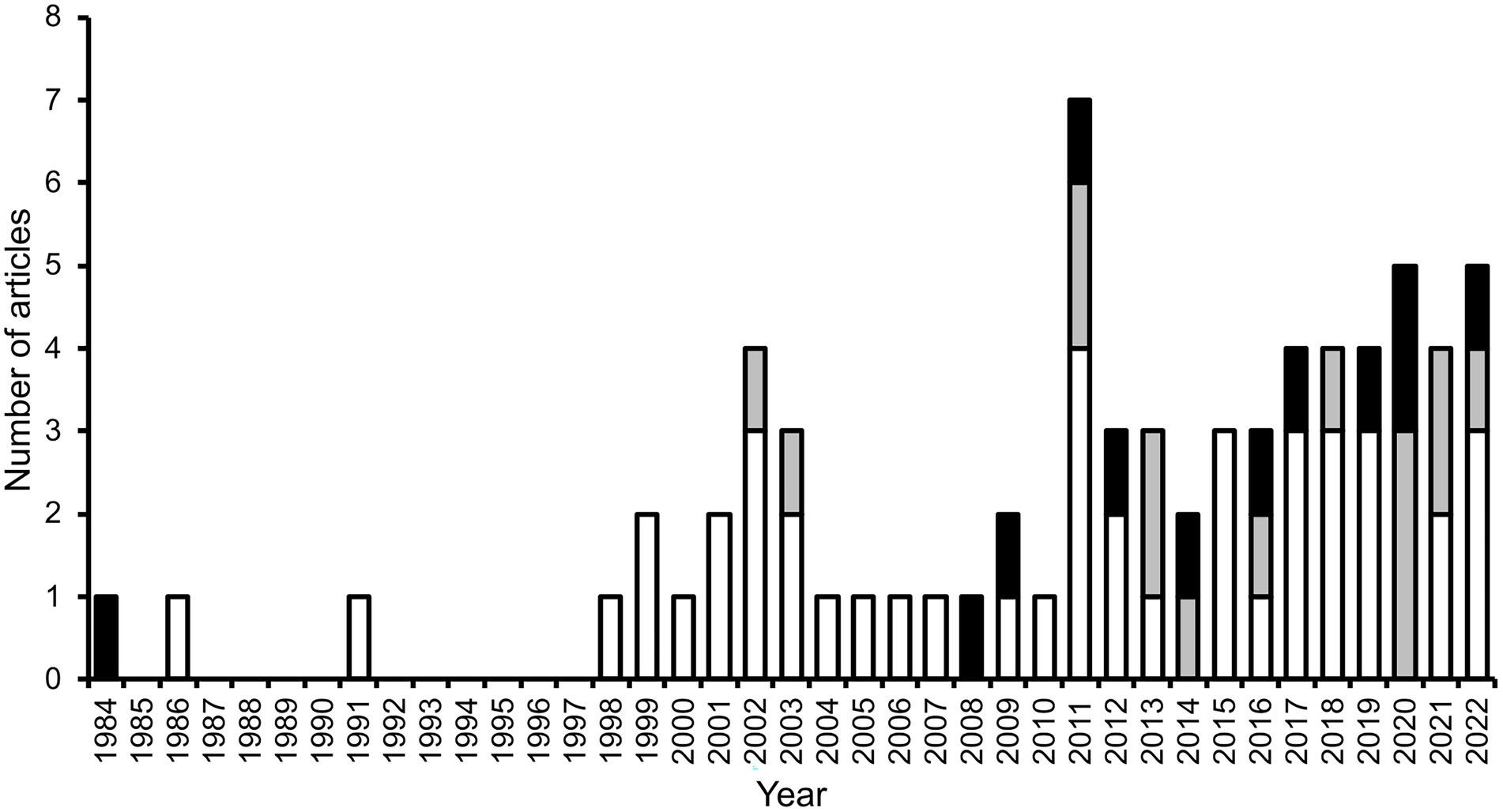
Figure 2. Number of scientific articles (excluding reviews) published per year, which include information on some aspect related to the secondary dispersal by dung beetles of seeds embedded in the feces of vertebrates (mostly mammal dung), in tropical (white and grey bars) and extratropical biomes (black bars). Grey bars represent studies that use dung beetles as a focal taxon in applied biodiversity conservation research (all carried out in the tropics; see Biodiversity studies using dung beetles as a focal taxon).
Why did secondary seed dispersal by dung beetles catch the interest of tropical ecologists in the 90s? The following lines of ecological evidence had to align for this to occur: (i) that the great majority of tropical woody plants depend on frugivorous birds and mammals for primary seed dispersal (Howe and Smallwood, 1982); (ii) that the effectiveness of a primary disperser depends not only on the quantity of seeds dispersed, but also on the quality of dispersal, which is related to how seeds are handled and deposited (Schupp, 1993); (iii) that it is necessary to assess post-dispersal seed fate to determine seed dispersal quality and have a better understanding of the seed dispersal process (Chapman, 1989); and (iv) that the dung surrounding seeds dispersed by mammals can affect post-dispersal seed fate by attracting rodent seed predators (Janzen, 1982) and dung beetles (Estrada and Coates-Estrada, 1986). Thus, it was realized that dung beetles processing the feces of mammalian frugivores were likely to affect the post-dispersal fate of defecated seeds, and therefore seed dispersal effectiveness. Indeed, the mammal-seed-beetle interaction was defined by Vander Wall and Longland (2004) as a ‘diplochory’, i.e., a system in which the primary and secondary dispersal vectors are different (i.e., mammal and dung beetle, respectively), and often confer different advantages to the plant. According to these authors, while the mammal allows the seed to escape an area of low survival probability near the parent plant, the dung beetles move the seeds deterministically to microsites that favor seed fate (i.e., respectively ‘Escape Hypothesis’ and ‘Directed Dispersal Hypothesis’ sensu Howe and Smallwood, 1982).
Almost two decades ago, a first review on secondary seed dispersal by dung beetles synthesized our initial understanding of this interaction and pointed out many research gaps (Andresen and Feer, 2005). A few years later, two publications that reviewed the ecological functions of dung beetles, also included accounts on secondary seed dispersal (Nichols et al., 2008; Scholtz et al., 2009). Since then, many more studies have assessed different aspects of secondary seed dispersal by dung beetles. In the next subsections we try to summarize old and new insights about this plant–animal interaction. First, we review the factors that determine whether and how a seed is secondarily dispersed by dung beetles. Second, we present an overview of the consequences of secondary seed dispersal by beetles for plants and discuss to what extent they can be generalized. Third, we describe how secondary seed dispersal is currently often included in sampling protocols of applied biodiversity conservation research that uses dung beetles as a focal taxon. Throughout the text, we point out how methodological choices may affect the results we obtain when quantifying secondary seed dispersal by dung beetles.
Factors influencing secondary seed dispersal by dung beetles
There is tremendous variation in the patterns of secondary seed dispersal by dung beetles: (i) all or none of the seeds in a defecation may be relocated by dung beetles, with all the possibilities in between (Andresen and Feer, 2005); (ii) most seeds buried by beetles are found at depths ≤ 10 cm, but some may be buried as deeply as 40 cm (Griffiths et al., 2015); (iii) most seeds are moved short horizontal distances (≤ 50 cm), but some can be moved a few meters in tropical forests (e.g., Estrada and Coates-Estrada, 1991) and up to 20 m in African savannas (Kunz and Krell, 2011). Also, while some of the seeds dispersed vertically remain inside the dung portions buried by beetles, others (particularly larger seeds) are excluded from the dung portions at some point during burial (Andresen and Feer, 2005; Stanbrook et al., 2017).
Many factors can influence if and how a defecated seed is secondarily dispersed by beetles. Some of these have been assessed often, while others only a few times (Table 1A). It is important to mention that all studies quantify the vertical dispersal of seeds (i.e., seed burial), but fewer quantify horizontal dispersal. Also, we want to point out that in experiments that only aim to measure the probability and distances of seed movement without determining the subsequent fate of those seeds (i.e., whether they die, germinate, or establish as seedlings), it is very common to use seed mimics (Andresen, 2002a). Seed mimics are usually plastic beads, though other types of mimics can also be used. Dung beetles show the same behavior towards all dung ‘contaminants’, be they real or artificial seeds. Using seed mimics has many methodological advantages for assessing secondary seed dispersal by dung beetles: they are not removed by granivorous animals; their characteristics, such as size and shape, can be controlled; large numbers can be deployed; they can be reused etc. However, since measuring the fate of seeds dispersed by beetles is necessary to determine if secondary seed dispersal has a positive effect on plant fitness or not (see next section), the usefulness of seed mimics is limited.
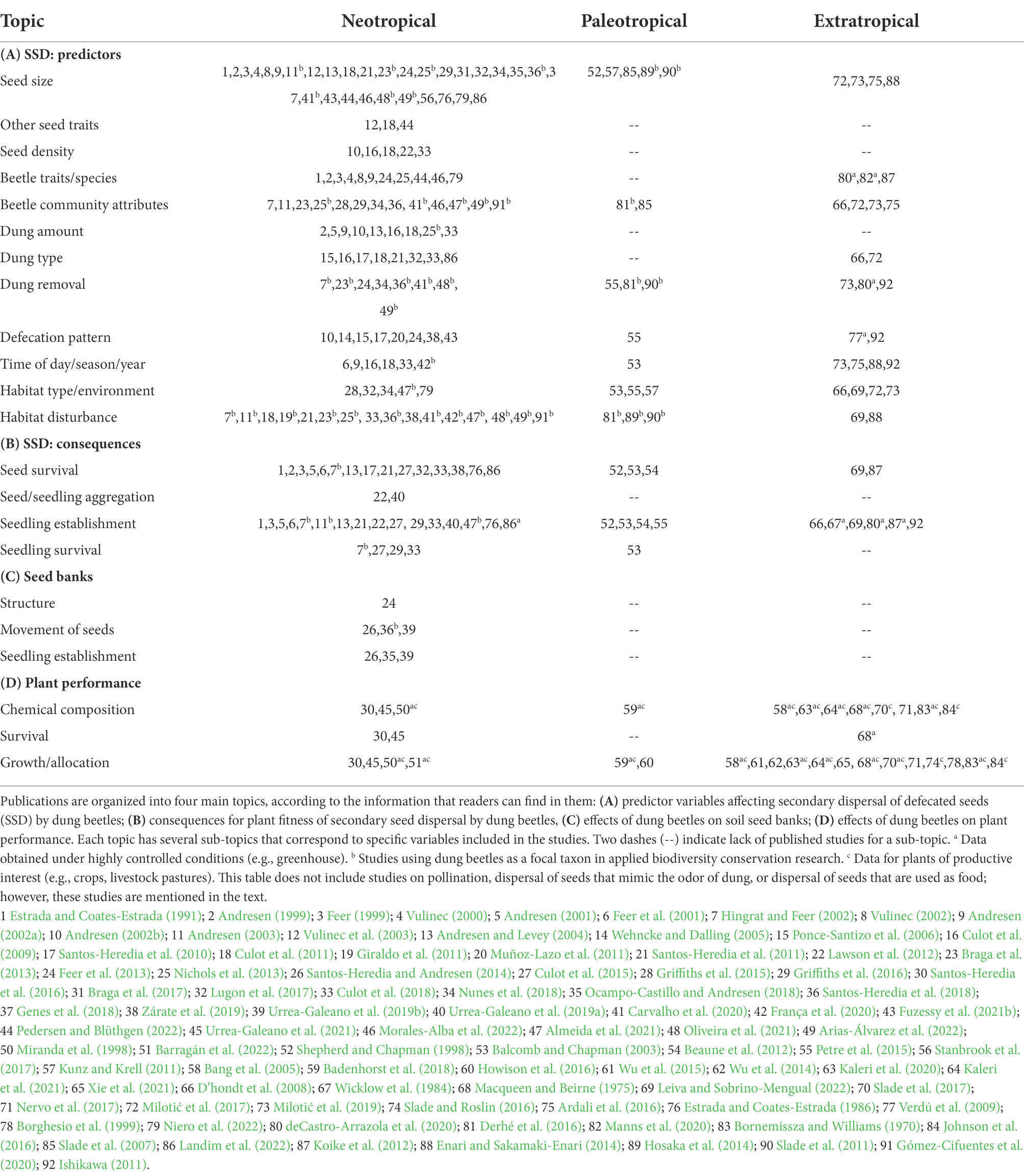
Table 1. List of publications (excluding reviews) that include information on the effects of dung beetle activity on plants in three realms: Neotropical, Paleotropical, and Extratropical.
Two of the factors that most consistently affect secondary seed dispersal by dung beetles are seed size and beetle size. The relationships are driven by the facts that seeds are dung contaminants from the beetle’s perspective and that larger beetles relocate larger portions of dung. Thus, secondary seed dispersal by dung beetles is negatively related to seed size, and positively related to beetle size. In other words: smaller seeds are relocated more often, more deeply, and to larger horizontal distances, than larger seeds, and seeds have a higher chance of being secondarily dispersed when handled by larger beetles. However, some exceptions occur, as not all studies have found an effect of seed size (e.g., no effect of seed size on burial probability: Culot et al., 2011; no effect of seed size on all secondary seed dispersal variables: Hosaka et al., 2014; no effect of seed size on burial depth: Andresen and Levey, 2004). Exceptions are probably due to methodological aspects, such as the range of seed sizes used in studies, relative to the size distribution of the beetles at the study site. One study determined that the maximum seed size that is dispersed by beetles approximates the beetle’s body length (Pedersen and Blüthgen, 2022). So, for example, if in a study all seed sizes used are smaller or larger than the largest beetles, an effect of seed size on secondary seed dispersal might be more difficult to detect or non-existent. What is important, however, is that researchers choose seed sizes that are realistic (e.g., dung beetles are likely to encounter them in vertebrate’s feces in their study site) and that allow them to answer their research questions. Also, one must consider that even for seeds of the same size variability in dispersal probabilities and distances can be very high, and thus large sample sizes are necessary to statistically confirm biological trends.
Other seed characteristics, aside from seed size, may also affect secondary seed dispersal, but evidence is scant. For example, one study found that spherical seeds are more likely to be buried by beetles than elongated ones (Culot et al., 2011), but two other studies found no effect of seed shape (Vulinec et al., 2003; Pedersen and Blüthgen, 2022). Additionally, one study found that large pubescent seeds are more likely to be incorporated into the dung portions relocated by beetles than large seeds that are smooth (Pedersen and Blüthgen, 2022). The latter relationship is explained by the fact that dung is more likely to stay attached on the surface of pubescent seeds. Indeed, the authors found that very large pubescent seeds with a thin layer of feces on their surface are seemingly mistaken by dung beetles to be piles of dung and buried as such. Such ‘secondary seed dispersal by mistake’ had also previously been reported in African savannas (Kunz and Krell, 2011). But the ‘ultimate deception’ occurs in at least two plant species of the South African fynbos, which have dung-smelling seeds that emit volatiles found in herbivore feces (Midgley et al., 2015, 2021). These seeds, without having been defecated by any frugivore, attract dung beetles that roll and bury them, to later abandon them when the deception is discovered. For these seeds, dung beetles are acting as primary dispersers of fallen seeds (sensu Vander Wall et al., 2005), rather than secondary seed dispersers. We do not yet know if secondary seed dispersal by mistake and/or primary seed dispersal through fecal mimicry are common in certain ecosystems and/or plant taxa.
As with seed traits, other dung beetle traits aside from size can also influence secondary seed dispersal. One trait that has received relatively more attention is the nesting and dung-relocation behavior of beetles (see Introduction). In general, it is considered that dwellers do not play a role in secondary seed dispersal of defecated seeds, that tunnellers bury more seeds than rollers, and that rollers are more likely to move seeds to greater horizontal distances than tunnellers (Andresen and Feer, 2005). It is also argued that rollers are more selective than tunnellers of similar size, i.e., they tend to exclude larger seeds from the dung portion they relocate more often than tunnellers (Feer et al., 2013). While these broad patterns are likely accurate, we are probably still missing much detailed knowledge, considering that beetle nesting and dung-relocation behaviors can vary tremendously among species within each of the three general behavioral categories (Halffter and Edmonds, 1982; Hanski and Cambefort, 1991). For example, beetles of the Neotropical genus Eurysternus, which technically belong to the rollers, are sometimes considered functional dwellers (e.g., Feer et al., 2013; Griffiths et al., 2016). The truth is, that beetles in this genus, which can be very abundant, process dung very differently from rollers and dwellers, as they relocate dung balls just underneath the dung source (Halffter and Edmonds, 1982). Thus, these beetles, which have been classified as marginally or not at all involved in secondary seed dispersal (Vulinec, 2000), might actually have a positive effect on seed fate by hiding them from predators at shallow depths that are optimal for germination and seedling establishment (e.g., Griffiths et al., 2016; see next section). Also, many species of tunnellers push fragments of dung, showing a behavior that, in terms of secondary seed dispersal, has some characteristics of tunnellers (they are less selective, excluding fewer seeds) and some of rollers (they move smaller portions of dung but to larger horizontal distances; Culot et al., 2011). Finally, while several studies have assessed the secondary seed dispersal capacities of particular beetle species (Estrada and Coates-Estrada, 1991; Vulinec, 2000; Vulinec, 2002; Andresen, 2002a; Vulinec et al., 2003; Koike et al., 2012; Feer et al., 2013; deCastro-Arrazola et al., 2020; Manns et al., 2020; Niero et al., 2022), the number of species compared is usually low (1–10) and experimental manipulations probably alter beetle behavior (i.e., beetles are placed in relatively narrow cylinders manually filled with soil). Thus, this is an area of many research opportunities.
In addition to beetle and seed traits, dung amount, dung type, seed density, and the spatial defecation pattern can also affect secondary seed dispersal by dung beetles. These are interrelated factors, as they all depend on the characteristic of each species of frugivorous vertebrate (e.g., size, behavior, diet). With the exception of one study, which assessed secondary seed dispersal for seeds defecated by a bird species (guan; Landim et al., 2022), all others have focused on seeds in mammalian dung, particularly that of primates. Consistently, studies comparing different amounts of dung while controlling for the other factors have found that seeds surrounded by more feces have a higher probability of secondary seed dispersal and are often buried more deeply. On the other hand, two studies comparing dung types while controlling for the other factors found differences in secondary seed dispersal (Ponce-Santizo et al., 2006; Santos-Heredia et al., 2011), while one did not (Culot et al., 2009). Yet, another study found an effect of dung type in one experiment, but not in another, which was attributed to seasonal differences in the frugivores’ diets, which in turn affected dung texture (Santos-Heredia et al., 2010). Studies comparing the spatial distribution of dung (clumped vs. scattered), while controlling dung type and amount, have either found no effect on secondary seed dispersal (Andresen, 2002b; Ponce-Santizo et al., 2006) or have found that seeds in clumped defecations are buried more often and more deeply (Santos-Heredia et al., 2010). Some studies have used an integrative approach for comparing the secondary dispersal of seeds defecated by different frugivores, in which all characteristics associated with the defecation pattern of each species are mimicked (Lugon et al., 2017; Landim et al., 2022) or the secondary dispersal is measured in situ where seeds are defecated, with very little manipulation (Culot et al., 2009, 2018). These studies have found differences in secondary seed dispersal among the frugivore species being compared. These differences were strongly driven by the effect of dung amount, but also by dung texture (Lugon et al., 2017), and the number of seeds in a dung pile (Culot et al., 2009, 2018).
The deposition of dung may not only be aggregated in space, but also in time. This occurs when mammals defecate in the same sites (often sites used for resting) recurrently over time, creating a ‘latrine effect’. A latrine effect occurs when, due to the recurrent defecations, certain biotic and/or abiotic characteristics of the habitat (e.g., density of seedlings, soil nutrients) differ between latrines and non-latrine sites (Whitworth et al., 2019). Only two studies have evaluated secondary seed dispersal by dung beetles in latrines, finding that seed burial by beetles was higher in latrines than non-latrines (Fuzessy et al., 2021b) and higher in latrines used more frequently vs. less frequently (Feer et al., 2013). This is an area of interest for future research, given that the habitat heterogeneity generated by the latrine behavior of certain mammals may be accentuated by the differential activity of dung beetles (see also Seed banks).
Other environmental factors that can either influence the composition of the dung beetle assemblage attracted to a seed-containing defecation (e.g., season of the year, time of day, vegetation characteristics) and/or dung beetle behavior (e.g., soil type, soil compaction, soil water content) can also affect secondary seed dispersal but have been explored very little (Table 1A), or not at all. On the other hand, the effects of habitat disturbance and dung beetle community attributes (e.g., species richness) are increasingly being related to secondary seed dispersal due to the tremendous popularity of dung beetles as a focal taxon in biodiversity studies. We will come back to this topic below (see Biodiversity studies using dung beetles as a focal taxon).
The consequences of secondary seed dispersal by dung beetles
The immediate consequences for seeds embedded in the dung that is processed by dung beetles are potential changes in their location and condition. However, what is relevant to know are the long-term consequences of those changes. Unfortunately, we know much less about these long-term consequences than we know about the immediate ones, given that relatively few studies on secondary seed dispersal by dung beetles have followed seed fate until seedling establishment and even fewer assess seedling survival (Table 1B; Supplementary Table S1).
In terms of location, as already explained, dung beetles may bury seeds and/or they may move them horizontally away from the original site of deposition. The best studied consequences of secondary dispersal on seed fate are those related to seed burial (Supplementary Table S1). There is a strong consensus in the literature that seed burial by dung beetles increases seed survival by lowering the probability of seed predation. However, researchers also agree that seeds buried too deeply may suffer negative effects because seedling establishment is hindered. While the first effect (lower seed predation) can be generalized to most plant species, the second cannot, since the range of depths from which a seed can emerge as seedling varies strongly among plant species and biomes (Gallagher, 2014). To some extent, the negative effect of seed burial is likely related to seed size, with larger seeds suffering less from non-emergence of seedlings than smaller seeds, in general. However, even for large tropical seeds, there is high variability regarding this negative effect, but with no clear relationship to seed size (Andresen and Levey, 2004; Culot et al., 2015, 2018). Thus, other seed/seedling functional traits (e.g., type of germination, type of cotyledons) are likely important too in determining whether a seed buried by dung beetles will be able to emerge as seedling, or not (Andresen and Feer, 2005). Additionally, the challenge of following the long-term fate of small seeds (< 3 mm), pointed out long ago (Andresen and Feer, 2005), has not yet been completely solved. Also, we know little about the specific mechanisms that hinder seedling establishment from a seed buried by dung beetles. In some cases, buried seeds seem to germinate well but the elongating seedling is unable to emerge (e.g., Andresen and Levey, 2004), in other cases germination itself may be hindered, or buried seeds may suffer higher rates of mortality due to pathogen attack or other causes (Lugon et al., 2017). Unfortunately, most studies on seed burial by dung beetles are not designed to determine which mechanism is responsible, since germination is quantified indirectly by assessing seedling establishment (Supplementary Table S1). Overall, the species-dependent variation in seedling establishment from seeds buried by dung beetles strongly limits our ability to generalize whether secondary seed dispersal by these insects has mostly a net positive effect on plant fitness, or not.
Seed burial can also affect seed fate through other mechanisms. Since the first studies on secondary seed dispersal by dung beetles, it was argued that seeds buried by dung beetles might encounter microclimatic conditions (e.g., temperature, moisture) that could favor their survival and/or germination (Wicklow et al., 1984; Andresen and Feer, 2005; Nichols et al., 2008). While the effects of the microclimate on the germination of buried seeds has been studied (Gallagher, 2014), they have not been assessed for seeds buried by dung beetles. In tropical forests, encountering better microclimatic conditions when buried could be of particular importance for plant species whose seeds’ viability quickly decreases when they lose moisture. This potential effect of seed burial by dung beetles may also be more relevant in tropical dry forests or secondary forests (e.g., Culot et al., 2018), where conditions on the soil surface can be harsh (high temperature, low moisture). Similarly, in soils that are highly compacted (e.g., grasslands used by large domestic or wild herbivores), seed burial by dung beetles may be crucial for seed germination and seedling establishment. Future studies will need to test whether this often-cited advantage of secondary seed dispersal by dung beetles occurs or not.
Another positive aspect of secondary seed dispersal by dung beetles that was proposed early on, but for which we also lack empirical evidence, is increased plant fitness due to the reduction of density-dependent processes, such as predation and/or competition (Andresen and Feer, 2005; Nichols et al., 2008). Mammal defecations can often contain large numbers of seeds; thus, it has been argued that redistribution of those seeds through dung beetle activity would diminish the degree of clumping and consequently improve seed survival, seedling establishment, and, eventually, seedling performance. While many studies on secondary seed dispersal by dung beetles report horizontal movement of seeds, the effects on seed fate have not been assessed. Two studies have experimentally proven that dung beetle activity indeed decreases the spatial aggregation of seeds deposited in dung and of the seedlings that establish from them (Lawson et al., 2012; Urrea-Galeano et al., 2019a). However, of the four plant species tested (two in each study; Supplementary Table S1), only one showed a higher probability of seedling establishment in plots with dung beetle activity (Lawson et al., 2012), while for the other three species the effect was negative. The challenge in these types of studies is to design experiments that allow us to disentangle the different effects of secondary seed dispersal by dung beetles on seed fate and seedling establishment, which occur simultaneously in the field, and some of which may only be detectable after longer periods of time (Lawson et al., 2012; Urrea-Galeano et al., 2019a). So, for example, in the studies mentioned above, the negative effect of dung beetle activity on seedling establishment might have been caused by seed burial, and not by the horizontal dispersal of the seeds. Alternatively, diminished spatial aggregation due to horizontal dispersal might have positive effects on seedling survival or growth that are only detectable in the long term.
Aside from the vertical and horizontal movement of seeds by dung beetles, other more subtle changes in seed location and/or condition could also affect seed fate (Braga et al., 2017). For example, seeds are often moved by beetles from an exposed location on the soil, to a location under the leaf litter (e.g., Zárate et al., 2019), particularly when moved by rollers. In other cases, although seeds are not buried by beetles, they nonetheless end up covered by the soil that beetles excavate when building underground tunnels and chambers for dung burial (Braga et al., 2017). It is possible that seeds in these conditions might experience the positive effect of reduced seed predation and/or improved microclimate, while avoiding the negative effect of being buried too deeply to establish as seedlings, but we lack the information to confirm this. However, a recent study in a Mediterranean savanna used for cattle grazing, showed that seedling establishment was much higher for acorns falling on cattle dung than for acorns falling on the ground, due to the covering of acorns with soil excavated by dung beetles (Leiva and Sobrino-Mengual, 2022). Though this phenomenon is not secondary seed dispersal sensu stricto, given that the acorns where not defecated by cattle but rather fell from the parental crown on top of a dung pad, the result is the same as described by Braga et al. (2017) for defecated seeds, and may be considered as ‘passive seed burial’ by dung beetles (Leiva and Sobrino-Mengual, 2022).
Another important change in seed condition that is a consequence of dung beetle activity, though not necessarily associated to secondary seed dispersal, is the ‘cleaning’ of seeds. After dung removal by beetles, seeds that are not buried by them remain on the soil surface, often in the same location of original deposition, but without dung. We know very little about the positive and negative effects that the dung surrounding seeds can have on seeds and seedlings (Traveset et al., 2007) and consequently we do not know the effects of seed cleaning. The little information we have for tropical forests, shows that seeds embedded in dung may suffer higher mortality due to seed predators (Janzen, 1982) and seed pathogens (Jones, 1994). Nonetheless, for seeds defecated by certain mammal species, the feces may have a protective effect. This possibly occurs in the case of seeds dispersed by tapirs, as studies have suggested that the dung of this mammal, which disintegrates slowly, may protect seeds against desiccation, vertebrate predation, and invertebrate parasitism (Rios and Pacheco, 2006; Lugon et al., 2017). Even seeds that are buried by dung beetles may or may not be embedded in dung, as beetles re-process the dung portions during burial, often removing seeds, which then remain in the tunnels but not imbedded in dung (Stanbrook et al., 2017). Buried seeds that are embedded in dung have been reported to suffer higher seed predation when compared to buried seeds not surrounded by dung (Andresen, 1999). On the other hand, seeds buried with the dung may encounter a boost of nutrients upon germinating, which might enhance seedling establishment and/or survival (Traveset et al., 2007). To our knowledge, only two studies have assessed the latter effects of secondary seed dispersal by dung beetles, finding a negative effect of the dung on germination (Fuzessy et al., 2021b) and no effect on seedling establishment (Griffiths et al., 2016; Fuzessy et al., 2021b). Again, this is a topic that needs to be investigated further.
Biodiversity studies using dung beetles as a focal taxon
Since dung beetles were proposed as an ideal animal group for analyzing and monitoring biodiversity in modified tropical landscapes 30 years ago (Halffter and Favila, 1993), they have become tremendously popular as a focal taxon in these types of studies (Nichols and Gardner, 2011; Fuzessy et al., 2021a; López-Bedoya et al., 2022). More recently, many of these studies have started quantifying ecological functions of dung beetles, in addition to community attributes, and assessing the relationships between both types of variables. While dung removal is the most frequently measured function (Raine and Slade, 2019), secondary seed dispersal is now often included in sampling protocols too (Figure 2; Table 1). In addition to being used as a focal taxon in applied biodiversity conservation research, beetles are also used as a model taxon in studies that focus on understanding the relationship between biodiversity and ecosystem functioning (i.e., BEF studies). In these two types of biodiversity studies, researchers often use seed mimics instead of real seeds, which allows for a quick and easy quantification of secondary seed dispersal in experimental mesocosms (e.g., Braga et al., 2013). The caveat, however, is that it is taken as a fact that secondary seed dispersal is an ecological function that has positive effects on plant regeneration, which, as we have seen, is not something that we can yet generalize. We are not arguing that biodiversity studies using dung beetles as a focal taxon should stop measuring secondary seed dispersal, we are merely asking researchers to be prudent with their justifications and interpretations. Better still, researchers could use real seeds rather than (or in addition to) seed mimics to assess the true effect of secondary seed dispersal on plant regeneration in their study systems (e.g., Andresen, 2003; Griffiths et al., 2016).
We have learned interesting lessons from biodiversity studies using dung beetles as a focal taxon. First, secondary seed dispersal is often correlated to one or more attributes of the dung beetle community (e.g., species richness, abundance, biomass, functional diversity, community weighted means of functional traits, etc.). However, which community attribute has the strongest relationship with secondary seed dispersal is still a matter of contention, and most likely context-specific (Nichols et al., 2013; Griffiths et al., 2015; Derhé et al., 2016). Second, while secondary seed dispersal is a consequence of dung removal, these two functions are not always positively correlated (e.g., Carvalho et al., 2020). Thus, inferences about secondary seed dispersal should not be reached based on dung removal rates. Third, methodological choices may in part be responsible for discrepancies among studies (Raine et al., 2020). For example, it is common that, in the same study, different amounts or types of dung are used to measure secondary seed dispersal and to sample the dung beetle community, which can create spurious relationships (Nichols et al., 2013). Furthermore, the dung in pitfall traps remains attractive during a long period of time (24 h or more), whereas dung piles in secondary seed dispersal experiments are usually buried within few hours. Consequently, the dung beetle assemblage captured in a pitfall trap, is probably not very representative of the assemblage responsible for processing a dung pile. This problem can be avoided by using modified pitfall traps, in which beetles are allowed to bury the dung inside the trap (Culot et al., 2011), or by using an experimental setup that captures the individual beetles responsible for processing the dung and relocating the seeds (Griffiths et al., 2015, 2016). The latter method is labor-intensive but yields very precise data for relating community metrics and functions.
Finally, we want to draw attention to the way secondary seed dispersal is measured in many biodiversity studies, which does not yield an estimate that is independent of dung removal; this, in our opinion, is inadequate. Studies usually deploy large piles of dung (e.g., 100–200 g) that contain a known number (or weight) of plastic beads used as seed mimics. Generally, not all dung has been removed by beetles by the time secondary seed dispersal is measured (usually after 24–48 h). The remaining dung is then collected, the beads still imbedded in it are counted, and by subtraction, all the beads not found in the dung are considered as having been dispersed by beetles. Sometimes, only beads that are not on the soil surface are considered as dispersed by beetles, but the percentages of secondary seed dispersal are still calculated with respect to the total number of beads originally placed in the dung pile (e.g., Gómez-Cifuentes et al., 2020), thus yielding an estimate that is dependent on dung removal. We argue that to have a measure of secondary seed dispersal that is independent of dung removal, secondary seed dispersal should only be quantified for the portion of dung that was buried by beetles. For example, if a pile of 100 g containing 100 seed mimics was used in an experiment, and after 48 h 70 g of dung remained on the soil surface containing 60 seeds, then secondary seed dispersal should be assessed for the 40 seeds that were in the 30 g of dung that were buried by beetles. Then, for those 40 seeds, one should determine which ones were moved by beetles (horizontally and/or vertically), and only those should constitute the quantity of seeds dispersed.
Seed banks
As seen in the previous section, dung beetles can affect the fate of seeds through secondary seed dispersal and through other less studied mechanisms. For example, as already mentioned, seeds can be cleaned of dung (Braga et al., 2017), covered by excavated soil (i.e., passive burial; Braga et al., 2017; Leiva and Sobrino-Mengual, 2022), and in some cases eaten by dung beetles (Pérez-Ramos et al., 2007, 2013). However, in addition to their effect on the fate of individual seeds, dung beetles can have community-wide effects by shaping seed banks.
Seed banks play important roles in driving plant-community composition and dynamics (Gallagher, 2014). The characteristics of soil seed banks vary tremendously among ecosystems. While persistent soil seed banks are common in temperate biomes due to long dormancies of many seed species, seed banks in tropical forests tend to be transient, as few plant species have prolonged dormancy (Garwood, 1989). Thus, the effects that dung beetles may have on plant communities through their interactions with seed banks will also differ among ecosystems and regions. However, we know next to nothing about how dung beetle activity drives seed bank structure and dynamics, either in tropical forests or in any other biome, although their potential influence had been suggested more than once (D’hondt et al., 2008; Pouvelle et al., 2009; Koike et al., 2012).
Dung beetles could influence seed bank structure and/or dynamics through at least four potential mechanisms: (1) through the burial of seeds (either vertical secondary dispersal or passive burial) they incorporate seeds into the underground layers of the seed bank (Feer et al., 2013); (2) through their soil-excavation behavior, they move seeds that are buried in the soil, both upwards and downwards, which may promote or hinder germination (Urrea-Galeano et al., 2019b); (3) through their activity in the dung-soil interface they create irregularities in the soil surface that may facilitate the incorporation of small seeds into the soil, either through gravity or hygroscopic self-burying mechanisms (e.g., Verdú et al., 2009); and (4) through soil bioturbation and dung burial, they may create conditions that stimulate the germination of buried seeds (Urrea-Galeano et al., 2019b).
The little we know about the effects of dung beetles on soil seed banks comes from four studies (five publications) carried out in the Neotropics (Table 1C). First, a study in French Guiana described seed bank variability in monkey latrines (i.e., sites in the understory where monkeys defecate recurrently) associated to dung beetle activity (Feer et al., 2013). Researchers found that the abundance and species richness of small seeds buried in the soil were higher in latrines vs. non-latrine sites, and higher in latrines used by monkeys more frequently than those used less frequently. While the monkeys were responsible for the higher numbers of seeds reaching the soil surface, the authors argue that it was mostly due to dung beetle activity that those seeds were buried, and thus the structure of the seed bank shaped. Through a field experiment, the authors also found that seed burial activity by beetles was higher in frequently-used latrines than in those used less frequently. Though not focused on seed banks, a study in Brazil and a study in Spain found a similar pattern, with more seeds buried actively by beetles in monkey latrines (Fuzessy et al., 2021b), and more seeds buried passively after dung beetle activity in rabbit latrines (Verdú et al., 2009), than in non-latrine sites. Overall, it seems that dung beetle activity plays an important role in shaping the seed banks in mammal latrines, but more studies are needed.
Second, the other three studies, carried out in different tropical forests in Mexico, have shown that dung beetle activity enhances the establishment of seedlings originating from the natural seed bank (Santos-Heredia and Andresen, 2014; Ocampo-Castillo and Andresen, 2018; Urrea-Galeano et al., 2019b). In these studies, mammal dung was placed on the forest floor inside small circular plots (~ 30 cm diameter) where dung beetles could enter but were forced (by a small perimeter fence) to bury all dung within the plot. Control plots had no dung added to them, and seed rain was excluded from all plots. After several months, the number of seedlings establishing in plots with access to dung beetles was statistically higher than in control plots, in the three studies. While various mechanisms could be responsible for enhancing seedling establishment from the seed bank after dung beetle activity, the spatial re-distribution of buried seeds seems to be one of them, particularly the upward movement of buried seeds to more superficial layers or even to the surface (i.e., seed exhumation; Santos-Heredia and Andresen, 2014; Santos-Heredia et al., 2018). To test this mechanism, in one of these studies, seeds of two plant species were buried at known depths (3–10 cm) in experimental cylinders, a dung pile was placed on the soil surface and beetles were allowed to bury the dung (Urrea-Galeano et al., 2019b). In these cylinders, compared to the two controls (no dung added, and dung added but beetles excluded), seedling establishment was higher. Overall, there seems to be enough evidence to suggest that dung beetle activity affects tropical seed bank dynamics by promoting the germination of buried seeds, but again, more studies are needed to assess the generality of this effect and to determine the specific mechanisms driving it.
Plant performance
When dung beetles bury feces, they fertilize and bioturbate the soil. These actions modify the chemical, biological and physical properties of the soil (e.g., higher availability of nutrients, increased aeration and permeability, enhanced microbial activity, etc.), which in turn may improve plant nutrient uptake and plant productivity (see references in Nichols et al., 2008 and in Scholtz et al., 2009). Evidence for these effects comes from greenhouse studies and controlled field experiments with crops and/or temperate grasslands (Table 1D). In tropical forests, it has generally been assumed that similar positive effects on plants must also occur, but until recently no study had tested this assumption.
In a study in Brazil, researchers found that for one plant species, seedlings established from seeds buried by dung beetles survived better in plots where dung beetles had buried dung, compared to plots where dung was added but beetles were excluded (Griffiths et al., 2016). That same year, a study in Mexico found that seedlings of one plant species established in the forest understory had higher phosphorous concentrations in their leaves in plots where beetles buried small dung piles placed at their base, compared to seedlings in plots with no dung, and in plots with dung added but beetles excluded (Santos-Heredia et al., 2016). These studies gave us the first data suggesting that dung beetle activity might change the soil environment in a way that favors nutrient uptake and survival of seedlings established in the tropical forest understory. To gather more evidence, a third study in a different Mexican rainforest assessed the effects of dung beetle activity for the seedlings of six plant species, measuring foliar nutrients, growth, and survival (Urrea-Galeano et al., 2021). However, contrary to the previous results, no positive effect of dung beetle activity was detected for any of the variables in any of the seedling species. Furthermore, a negative effect of dung beetle activity was found for seedling growth. So, back to ground zero.
Whether dung beetle activity, through soil fertilization and/or bioturbation, has positive effects on the performance of tropical forest plants remains an unanswered question. At this point, to guide future studies, we can only summarize some recommendations that have been previously voiced by us or others (Nichols et al., 2008; Griffiths et al., 2016; Santos-Heredia et al., 2016; Urrea-Galeano et al., 2021): (i) to avoid extrapolating into natural conditions of tropical forests, results obtained in other study systems and regions; (ii) to carry out field experiments in tropical forest that would allow us to distinguish the effects that dung beetle activity has through fertilization vs. bioturbation on plant performance; (iii) to empirically measure the changes that feces burial by beetles causes in the tropical forest soil (i.e., physical, chemical, and biological changes) to better understand the mechanisms driving plant responses; (iv) to measure other responses in plants, such as herbivory and pathogen attack, since these plant antagonists are known to prefer plants with higher nutrient content; (v) to replicate these studies in forests that vary in soil characteristics (e.g., soil fertility, soil compaction, texture), and with plant species that differ in their functional traits (e.g., seed reserves, shade tolerance). There is much work to be done here.
Conclusion
To conclude, we want to emphasize two interrelated take-home messages. First, dung beetles are very abundant in tropical forests, and the large amounts of dung produced by forest mammals are buried by them within hours (Hanski and Cambefort, 1991). Thus, the impact that dung beetles can have on plants, through their direct and indirect interactions with seeds, seedlings, and even mature individuals, is potentially large. It has been suggested that, given the patchy distribution of mammal feces, dung beetles probably contribute to creating spatial heterogeneity in soil conditions and plant regeneration niches, and may even facilitate the co-existence of plant species (Nichols et al., 2008; Griffiths et al., 2016; Urrea-Galeano et al., 2019b). However, we still lack the necessary information that would allow us to estimate the true ecological impact of dung beetle interactions with tropical forest plants. Second, dung beetles have become a tremendously popular focal taxon in biodiversity studies that assess the effects of anthropogenic disturbances in tropical forests. The use of dung beetles is often justified by the ecological functions attributed to them, including their potentially positive effects on plants. These ecological functions can be services of huge economic impact in agricultural systems (e.g., Lopez-Collado et al., 2017), and so, much of what we know about dung beetle functions comes from such systems. However, except for secondary seed dispersal, we have neglected to accurately quantify the ecological consequences that dung beetle activity has in tropical forests. It is important to fill these gaps because we know that dung beetle communities vary tremendously among ecosystems, regions, and continents (Hanski and Cambefort, 1991), and that so do the ecological impacts of their activity (e.g., Milotić et al., 2017, 2019). Thus, as previously stressed, extrapolating results among regions and study systems is problematic and should be avoided (Slade et al., 2011; Koike et al., 2012; Griffiths et al., 2016; Urrea-Galeano et al., 2021). We finish with an invitation for young researchers to tackle the many questions that remain unanswered in the fascinating network of interactions between mammal dung, dung beetles, soil, and plants.
Author contributions
EA wrote the manuscript with feedback from LAUG. LAUG reviewed the literature and produced Figure 2, Table 1, and Supplementary Table S1, with feedback from EA. EA and LAUG produced Figure 1. All authors contributed to the article and approved the submitted version.
Acknowledgments
LAUG thanks the Dirección General de Asuntos del Personal Académico (DGAPA) at the Universidad Nacional Autónoma de México (UNAM) for a postdoctoral fellowship during the time this manuscript was prepared.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.979676/full#supplementary-material
References
Almeida, H. A., Antonini, Y., Tavares Junior, C., Braga, R. F., da Silva, P. G., and Beiroz, W. (2021). Dung beetles can sow: the potential of secondary seed dispersers to assist ecological restoration. Ecol. Entomol. 47, 181–191. doi: 10.1111/een.13100
Andresen, E. (1999). Seed dispersal by monkeys and the fate of dispersed seeds in a Peruvian rain forest. Biotropica 31, 145–158. doi: 10.1111/j.1744-7429.1999.tb00125.x
Andresen, E. (2001). Effects of dung presence, dung amount and secondary dispersal by dung beetles on the fate of Micropholis guyanensis (Sapotaceae) seeds in Central Amazonia. J. Trop. Ecol. 17, 61–78. doi: 10.1017/S0266467401001043
Andresen, E. (2002a). Dung beetles in a central Amazonian rainforest and their ecological role as secondary seed dispersers. Ecol. Entomol. 27, 257–270. doi: 10.1046/j.1365-2311.2002.00408.x
Andresen, E. (2002b). Primary seed dispersal by red howler monkeys and the effect of defecation patterns on the fate of dispersed seeds. Biotropica 34, 261–272. doi: 10.1111/j.1744-7429.2002.tb00537.x
Andresen, E. (2003). Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 26, 87–97. doi: 10.1034/j.1600-0587.2003.03362.x
Andresen, E., and Feer, F. (2005). “The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests,” in Seed Fate: Predation, Dispersal and Seedling Establishment. eds. P.-M. Forget, J. E. Lambert, P. E. Hulme, and S. B. Vander Wall (Wallingford: CABI Publishing).
Andresen, E., and Levey, D. J. (2004). Effects of dung and seed size on secondary dispersal, seed predation, and seedling establishment of rain forest trees. Oecologia 139, 45–54. doi: 10.1007/s00442-003-1480-4
Ardali, E. O., Tahmasebi, P., Bonte, D., Milotić, T., Pordanjani, I. R., and Hoffmann, M. (2016). Ecological sustainability in rangelands: the contribution of dung beetles in secondary seed dispersal (case study: Chaharmahal and Bakhtiari province, Iran). Eur. J. Sustain. Dev. 5:133. doi: 10.14207/ejsd.2016.v5n3p133
Arias-Álvarez, G. A., Vanegas-Alarcón, D. A., García-Hernández, A. L., Santos-Heredia, C., and Andresen, E. (2022). Efecto de la cobertura vegetal en escarabajos coprófagos (Coleoptera: Scarabaeinae) y sus funciones ecológicas en un bosque andino de Colombia. Rev. Biol. Trop. 70, 53–66. doi: 10.15517/rev.biol.trop.v70i1.47849
Badenhorst, J., Dabrowski, J., Scholtz, C. H., and Truter, W. F. (2018). Dung beetle activity improves herbaceous plant growth and soil properties on confinements simulating reclaimed mined land in South Africa. Appl. Soil Ecol. 132, 53–59. doi: 10.1016/j.apsoil.2018.08.011
Balcomb, S. R., and Chapman, C. A. (2003). Bridging the gap: influence of seed deposition on seedling recruitment in a primate–tree interaction. Ecol. Monogr. 73, 625–642. doi: 10.1890/02-4036
Bang, H. S., Lee, J.-H., Kwon, O. S., Na, Y. E., Jang, Y. S., and Kim, W. H. (2005). Effects of paracoprid dung beetles (Coleoptera: Scarabaeidae) on the growth of pasture herbage and on the underlying soil. Appl. Soil Ecol. 29, 165–171. doi: 10.1016/J.APSOIL.2004.11.001
Barragán, F., Douterlungne, D., Ramírez-Hernández, A., Gelviz-Gelvez, S. M., Guzmán Miranda, A. V., and Rodas Ortíz, J. P. (2022). The rolling dung master: an ecosystem engineer beetle mobilizing soil nutrients to enhance plant growth across a grassland management intensity gradient in drylands. J. Arid Environ. 197:104673. doi: 10.1016/j.jaridenv.2021.104673
Beaune, D., Bollache, L., Bretagnolle, F., and Fruth, B. (2012). Dung beetles are critical in preventing post-dispersal seed removal by rodents in Congo rain forest. J. Trop. Ecol. 28, 507–510. doi: 10.1017/S0266467412000466
Borghesio, L., Luzzatto, M., and Palestrini, C. (1999). Interactions between dung, plants and the dung fauna in a heathland in northern Italy. Pedobiologia 43, 97–109.
Bornemissza, G. F., and Williams, C. H. (1970). An effect of dung beetle activity on plant yield. Pedobiologia 10, 1–7.
Braga, R. F., Carvalho, R., Andresen, E., Anjos, D. V., Alves-Silva, E., and Louzada, J. (2017). Quantification of four different post-dispersal seed deposition patterns after dung beetle activity. J. Trop. Ecol. 33, 407–410. doi: 10.1017/S0266467417000335
Braga, R. F., Korasaki, V., Andresen, E., and Louzada, J. (2013). Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. PLoS One 8:e57786. doi: 10.1371/journal.pone.0057786
Carvalho, R. L., Andresen, E., Barônio, G. J., Oliveira, V. H. F., Louzada, J., and Braga, R. F. (2020). Is dung removal a good proxy for other dung beetle functions when monitoring for conservation? A case study from the Brazilian Amazon. Ecol. Indic. 109:105841. doi: 10.1016/j.ecolind.2019.105841
Chapman, C. A. (1989). Spider monkey sleeping sites: use and availability. Am. J. Primatol. 18, 53–60. doi: 10.1002/ajp.1350180106
Culot, L., Huynen, M.-C., Gérard, P., and Heymann, E. W. (2009). Short-term post-dispersal fate of seeds defecated by two small primate species (Saguinus mystax and Saguinus fuscicollis) in the Amazonian forest of Peru. J. Trop. Ecol. 25, 229–238. doi: 10.1017/S0266467409005860
Culot, L., Huynen, M.-C., and Heymann, E. W. (2015). Partitioning the relative contribution of one-phase and two-phase seed dispersal when evaluating seed dispersal effectiveness. Methods Ecol. Evol. 6, 178–186. doi: 10.1111/2041-210X.12317
Culot, L., Huynen, M.-C., and Heymann, E. W. (2018). Primates and dung beetles: two dispersers are better than one in secondary forest. Int. J. Primatol. 39, 397–414. doi: 10.1007/s10764-018-0041-y
Culot, L., Mann, D. J., Muñoz Lazo, F. J. J., Huynen, M.-C., and Heymann, E. W. (2011). Tamarins and dung beetles: an efficient diplochorous dispersal system in the Peruvian Amazonia. Biotropica 43, 84–92. doi: 10.1111/j.1744-7429.2010.00655.x
D’hondt, B., Bossuyt, B., Hoffmann, M., and Bonte, D. (2008). Dung beetles as secondary seed dispersers in a temperate grassland. Basic Appl. Ecol. 9, 542–549. doi: 10.1016/j.baae.2007.11.002
deCastro-Arrazola, I., Hortal, J., Noriega, J. A., and Sánchez-Piñero, F. (2020). Assessing the functional relationship between dung beetle traits and dung removal, burial, and seedling emergence. Ecology 101:e03138. doi: 10.1002/ecy.3138
Derhé, M. A., Murphy, H., Monteith, G., and Menéndez, R. (2016). Measuring the success of reforestation for restoring biodiversity and ecosystem functioning. J. Appl. Ecol. 53, 1714–1724. doi: 10.1111/1365-2664.12728
Enari, H., and Sakamaki-Enari, H. (2014). Synergistic effects of primates and dung beetles on soil seed accumulation in snow regions. Ecol. Res. 29, 653–660. doi: 10.1007/s11284-014-1152-3
Estrada, A., and Coates-Estrada, R. (1986). “Frugivory by howling monkeys (Aluoatta palliata) at Los Tuxtlas, Mexico: dispersal and fate of seeds,” in Frugivores and Seed Dispersal. eds. A. Estrada and T. H. Fleming (Dordrecht: Dr W. Junk Publishers).
Estrada, E., and Coates-Estrada, R. (1991). Howler monkeys (Alouatta palliata), dung beetles (Scarabaeidae) and seed dispersal: ecological interactions in the tropical rain forest of Los Tuxtlas. Mexico. J. Trop. Ecol. 7, 459–474. doi: 10.1017/S026646740000585X
Feer, F. (1999). Effects of dung beetles (Scarabaeidae) on seeds dispersed by howler monkeys (Alouatta seniculus) in the French Guianan rain forest. J. Trop. Ecol. 15, 129–142. doi: 10.1017/S0266467499000711
Feer, F., Julliot, C., Simmen, B., Pierre-Michel, F., Bayart, F., and Chauvet, S. (2001). La régénération, un processus multi-étape au résultat imprévisible: l’exemple d’une Sapotaceae en forêt de Guyane française. Rev Écol 56, 119–145. Available at: https://hal.archives-ouvertes.fr/hal-03530049
Feer, F., Ponge, J.-F., Jouard, S., and Gomez, D. (2013). Monkey and dung beetle activities influence soil seed bank structure. Ecol. Res. 28, 93–102. doi: 10.1007/s11284-012-1006-9
França, F. M., Ferreira, J., Vaz-de-Mello, F. Z., Maia, L. F., Berenguer, E., Ferraz Palmeira, A., et al. (2020). El Niño impacts on human-modified tropical forests: consequences for dung beetle diversity and associated ecological processes. Biotropica 52, 252–262. doi: 10.1111/btp.12756
Fuzessy, L. F., Benítez-López, A., Slade, E. M., Bufalo, F. S., Magro-de-Souza, G. C., Pereira, L. A., et al. (2021a). Identifying the anthropogenic drivers of declines in tropical dung beetle communities and functions. Biol. Conserv. 256:109063. doi: 10.1016/j.biocon.2021.109063
Fuzessy, L. F., Sobral, G., and Culot, L. (2021b). Linking howler monkey ranging and defecation patterns to primary and secondary seed dispersal. Am. J. Primatol. 84:e23354. doi: 10.1002/ajp.23354
Gallagher, R. S. (2014). Seeds: The Ecology of Regeneration in Plant Communities. Wallingford: CABI Publishing.
Garwood, N. C. (1989). “Tropical soil seed bank: a review,” in Ecology of Soil Seed Banks. eds. M. A. Leck, R. L. Simpson, and V. T. Parker (New York: Academic Press).
Genes, L., Fernandez, F. A. S., Vaz-de-Mello, F. Z., da Rosa, P., Fernandez, E., and Pires, A. S. (2018). Effects of howler monkey reintroduction on ecological interactions and processes. Conserv. Biol. 33, 88–98. doi: 10.1111/cobi.13188
Giraldo, C., Escobar, F., Chará, J. D., and Calle, Z. (2011). The adoption of silvopastoral systems promotes the recovery of ecological processes regulated by dung beetles in the Colombian Andes. Insect Conserv. Divers. 4, 115–122. doi: 10.1111/j.1752-4598.2010.00112.x
Gómez-Cifuentes, A., Vespa, N., Semmartín, M., and Zurita, G. (2020). Canopy cover is a key factor to preserve the ecological functions of dung beetles in the southern Atlantic Forest. Appl. Soil Ecol. 154:103652. doi: 10.1016/j.apsoil.2020.103652
Griffiths, H. M., Bardgett, R. D., Louzada, J., and Barlow, J. (2016). The value of trophic interactions for ecosystem function: dung beetle communities influence seed burial and seedling recruitment in tropical forests. Proc. R. Soc. B 283:20161634. doi: 10.1098/rspb.2016.1634
Griffiths, H. M., Louzada, J., Bardgett, R. D., Beiroz, W., França, F., Tregidgo, D., et al. (2015). Biodiversity and environmental context predict dung beetle-mediated seed dispersal in a tropical forest field experiment. Ecology 96, 1607–1619. doi: 10.1890/14-1211.1
Halffter, G., and Edmonds, W. D. (1982). The Nesting Behavior of Dung Beetles (Scarabaeinae): An Ecological and Evolutive Approach. Ciudad de México: Man and the Biosphere Program UNESCO.
Halffter, G., and Favila, M. E. (1993). The Scarabaeinae (Insecta: Coleoptera), an animal group for analysing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biol. Int. 27, 15–21.
Hingrat, Y., and Feer, F. (2002). Effets de la fragmentation forestière sur l’activité des coléoptères coprophages: dispersion secondaire des graines en Guyane française. Rev Écol 57, 165–179. Available at: https://hal.archives-ouvertes.fr/hal-03530078
Hosaka, T., Niino, M., Kon, M., Ochi, T., Yamada, T., Fletcher, C., et al. (2014). Effects of logging road networks on the ecological functions of dung beetles in peninsular Malaysia. For. Ecol. Manag. 326, 18–24. doi: 10.1016/j.foreco.2014.04.004
Howe, H. F., and Smallwood, J. (1982). Ecology of seed dispersal. Annu. Rev. Ecol. Syst. 13, 201–228. doi: 10.1146/annurev.es.13.110182.001221
Howison, R. A., Berg, M. P., Smit, C., van Dijk, K., and Olff, H. (2016). The importance of coprophagous macrodetritivores for the maintenance of vegetation heterogeneity in an African savannah. Ecosystems 19, 674–684. doi: 10.1007/s10021-016-9960-7
Ishikawa, H. (2011). Effects of dung beetles on seedling emergence from herbaceous seeds in the dung of sika deer (Cervus nippon) in a temperate Japanese grassland ecosystem. Ecol. Res. 26, 725–734. doi: 10.1007/s11284-011-0831-6
Janzen, D. H. (1982). Removal of seeds from horse dung by tropical rodents: influence of habitat and amount of dung. Ecology 63, 1887–1900. doi: 10.2307/1940128
Johnson, S. N., Lopaticki, G., Barnett, K., Facey, S. L., Powell, J. R., and Hartley, S. E. (2016). An insect ecosystem engineer alleviates drought stress in plants without increasing plant susceptibility to an above-ground herbivore. Funct. Ecol. 30, 894–902. doi: 10.1111/1365-2435.12582
Jones, M. B. (1994). Secondary seed removal by ants, beetles, and rodents in a neotropical moist forest. M. S. Thesis. Gainesville: University of Florida.
Kaleri, A. R., Ma, J., Abro, S. A., Faqir, Y., Nabi, F., Hakeem, A., et al. (2021). Dung beetle improves soil bacterial diversity and enzyme activity and enhances growth and antioxidant content of chinese cabbage (brassica rapa ssp. pekinensis). J. Soil Sci. Plant Nutr. 21, 3387–3401. doi: 10.1007/s42729-021-00614-w
Kaleri, A. R., Ma, J., Jakhar, A. M., Hakeem, A., Ahmed, A., Napar, W. P. F., et al. (2020). Effects of dung beetle-amended soil on growth, physiology, and metabolite contents of bok choy and improvement in soil conditions. J. Soil Sci. Plant Nutr. 20, 2671–2683. doi: 10.1007/s42729-020-00333-8
Koike, S., Morimoto, H., Kozakai, C., Arimoto, I., Soga, M., Yamazaki, K., et al. (2012). The role of dung beetles as a secondary seed disperser after dispersal by frugivore mammals in a temperate deciduous forest. Acta Oecol. 41, 74–81. doi: 10.1016/j.actao.2012.04.009
Kunz, B. K., and Krell, F.-T. (2011). Habitat differences in dung beetle assemblages in an African savanna–forest ecotone: implications for secondary seed dispersal. Integr. Zool. 6, 81–96. doi: 10.1111/j.1749-4877.2011.00240.x
Landim, A. R., Fernandez, F. A. S., and Pires, A. (2022). Primate reintroduction promotes the recruitment of large-seeded plants via secondary dispersal. Biol. Conserv. 269:109549. doi: 10.1016/j.biocon.2022.109549
Lawson, C. R., Mann, D. J., and Lewis, O. T. (2012). Dung beetles reduce clustering of tropical tree seedlings. Biotropica 44, 271–275. doi: 10.1111/j.1744-7429.2012.00871.x
Leiva, M. J., and Sobrino-Mengual, G. (2022). Cattle dung and bioturbation by dung beetles improve oak seedling establishment in Mediterranean silvopastoral ecosystems. New For. doi: 10.1007/s11056-022-09922-0
López-Bedoya, P. A., Bohada-Murillo, M., Ángel-Vallejo, M. C., Audino, L. D., Davis, A. L. V., Gurr, G., et al. (2022). Primary forest loss and degradation reduces biodiversity and ecosystem functioning: a global meta-analysis using dung beetles as an indicator taxon. J. Appl. Ecol. 59, 1572–1585. doi: 10.1111/1365-2664.14167
Lopez-Collado, J., Cruz-Rosales, M., Vilaboa-Arroniz, J., Martínez-Morales, I., and Gonzalez-Hernandez, H. (2017). Contribution of dung beetles to cattle productivity in the tropics: a stochastic-dynamic modeling approach. Agric. Syst. 155, 78–87. doi: 10.1016/J.AGSY.2017.05.001
Lugon, A. P., Boutefeu, M., Bovy, E., Vaz-de-Mello, F. Z., Huynen, M.-C., Galetti, M., et al. (2017). Persistence of the effect of frugivore identity on post-dispersal seed fate: consequences for the assessment of functional redundancy. Biotropica 49, 293–302. doi: 10.1111/btp.12418
Macqueen, A., and Beirne, B. P. (1975). Effects of cattle dung and dung beetle activity on growth of beardless wheatgrass in British Columbia. Can. J. Plant Sci. 55, 961–967. doi: 10.4141/cjps75-152
Manns, S., Holley, J. M., Hemmings, Z., and Andrew, N. R. (2020). Behavioral ecology and secondary seed dispersal by two roller dung beetles, Sisyphus rubrus (Paschalidis, 1974) and Sisyphus spinipes (Thunberg, 1818) (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 74, 849–859. doi: 10.1649/0010-065X-74.4.849
Midgley, J. J., White, J. D. M., Johnson, S. D., and Bronner, G. N. (2015). Faecal mimicry by seeds ensures dispersal by dung beetles. Nat. Plants 1:15141. doi: 10.1038/nplants.2015.141
Midgley, J. J., White, J. D. M., Scholtz, C. H., and Johnson, S. D. (2021). Seed dispersal by dung beetles in Ceratocaryum pulchrum (Restionaceae): another example of faecal mimicry in plants. South Afr. J. Bot. 137, 365–368. doi: 10.1016/j.sajb.2020.11.004
Milotić, T., Baltzinger, C., Eichberg, C., Eycott, A. E., Heurich, M., Müller, J., et al. (2019). Functionally richer communities improve ecosystem functioning: dung removal and secondary seed dispersal by dung beetles in the Western Palaearctic. J. Biogeogr. 46, 70–82. doi: 10.1111/jbi.13452
Milotić, T., Quidé, S., Van Loo, T., and Hoffmann, M. (2017). Linking functional group richness and ecosystem functions of dung beetles: an experimental quantification. Oecologia 183, 177–190. doi: 10.1007/s00442-016-3756-5
Miranda, C. H. B., Do Santos, J. C. C., and Bianchin, I. (1998). Contribution of Onthophagus gazella to soil fertility improvement by bovine fecal mass incorporation into the soil. 1: greenhouse studies. Rev. Bras. Zootec. 27, 681–685.
Morales-Alba, A., Morales, I., and Alvarado, F. (2022). Bigger and stronger bury deeper: the role of dung beetles as secondary seed dispersers in the northern Colombian Andes. Int. J. Trop. Insect Sci. 42, 2259–2268. doi: 10.1007/s42690-022-00748-z
Muñoz-Lazo, F. J. J., Culot, L., Huynen, M.-C., and Heymann, E. W. (2011). Effect of resting patterns of tamarins (Saguinus fuscicollis and Saguinus mystax) on the spatial distribution of seeds and seedling recruitment. Int. J. Primatol. 32, 223–237. doi: 10.1007/s10764-010-9463-x
Nervo, B., Caprio, E., Celi, L., Lonati, M., Lombardi, G., Falsone, G., et al. (2017). Ecological functions provided by dung beetles are interlinked across space and time: evidence from 15N isotope tracing. Ecology 98, 433–446. doi: 10.1002/ecy.1653
Nichols, E. S., and Gardner, T. A. (2011). “Dung beetles as a candidate study taxon in applied biodiversity conservation research,” in Ecology and Evolution of Dung Beetles. eds. L. W. Simmons and T. J. Ridsdill-Smith (Chichester: John Wiley & Sons).
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M. E., et al. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 141, 1461–1474. doi: 10.1016/j.biocon.2008.04.011
Nichols, E., Uriarte, M., Peres, C. A., Louzada, J., Braga, R. F., Schiffler, G., et al. (2013). Human-induced trophic cascades along the fecal detritus pathway. PLoS One 8:e75819. doi: 10.1371/journal.pone.0075819
Niero, M. M., Batilani-Filho, M., and Medina Hernández, M. I. (2022). Comparative analysis of the ecological functions of dung removal and seed dispersal among two telecoprid and two paracoprid dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Coleopt. Bull. 76, 221–231. doi: 10.1649/0010-065X-76.2.221
Nunes, C. A., Braga, R. F., de Moura Resende, F., de Siqueira Neves, F., Cortes Figueira, J. E., and Fernandes, G. W. (2018). Linking biodiversity, the environment and ecosystem functioning: ecological functions of dung beetles along a tropical elevational gradient. Ecosystems 21, 1244–1254. doi: 10.1007/s10021-017-0216-y
Ocampo-Castillo, J., and Andresen, E. (2018). Interacciones entre semillas y escarabajos del estiércol (Scarabaeinae) en un bosque tropical seco. TIP Rev. Espec. En Cienc. Quím.-Biológicas 21, 24–33. doi: 10.1016/j.recqb.2017.08.003
Oliveira, Y. F., Oliveira, C. M., and Frizzas, M. R. (2021). Changes in land use affect dung beetle communities but do not affect ecosystem services in the Cerrado of Central Brazil. Ecol. Entomol. 46, 973–987. doi: 10.1111/een.13034
Pedersen, K. M., and Blüthgen, N. (2022). Seed size and pubescence facilitate secondary dispersal by dung beetles. Biotropica 54, 215–225. doi: 10.1111/btp.13052
Pérez-Ramos, I. M., Marañón, T., Lobo, J. M., and Verdú, J. R. (2007). Acorn removal and dispersal by the dung beetle Thorectes lusitanicus: ecological implications. Ecol. Entomol. 32, 349–356. doi: 10.1111/j.1365-2311.2007.00874.x
Pérez-Ramos, I. M., Verdú, J. R., Numa, C., Marañón, T., and Lobo, J. M. (2013). The comparative effectiveness of rodents and dung beetles as local seed dispersers in Mediterranean oak forests. PLoS One 8:e77197. doi: 10.1371/journal.pone.0077197
Petre, C.-A., Zinque, M.-H., Tagg, N., Beudels-Jamar, R.-C., Haurez, B., Josso, J.-F., et al. (2015). Differences in dung beetle activity at western gorilla defecation sites in South-East Cameroon: implications for establishment of Uapaca spp. seedlings. J. Trop. Ecol. 31, 165–174. doi: 10.1017/S0266467414000753
Ponce-Santizo, G., Andresen, E., Cano, E., and Cuarón, A. D. (2006). Dispersión primaria de semillas por primates y dispersión secundaria por escarabajos coprófagos en Tikal, Guatemala. Biotropica 38, 390–397. doi: 10.1111/j.1744-7429.2006.00144.x
Pouvelle, S., Jouard, S., Feer, F., Tully, T., and Ponge, J.-F. (2009). The latrine effect: impact of howler monkeys on the distribution of small seeds in a tropical rain-forest soil. J. Trop. Ecol. 25, 239–248. doi: 10.1017/S0266467409005987
Raine, E. H., Mikich, S. B., Lewis, O. T., and Slade, E. M. (2020). Linking dung beetle-mediated functions to interactions in the Atlantic forest: sampling design matters. Biotropica 52, 215–220. doi: 10.1111/btp.12722
Raine, E. H., and Slade, E. M. (2019). Dung beetle–mammal associations: methods, research trends and future directions. Proc. R. Soc. B 286:20182002. doi: 10.1098/rspb.2018.2002
Rios, R. S., and Pacheco, L. F. (2006). The effect of dung and dispersal on postdispersal seed predation of Attalea phalerata (Arecaceae) by bruchid beetles. Biotropica 38, 778–781. doi: 10.1111/j.1744-7429.2006.00209.x
Santos-Heredia, C., and Andresen, E. (2014). Upward movement of buried seeds: another ecological role of dung beetles promoting seedling establishment. J. Trop. Ecol. 30, 409–417. doi: 10.1017/S0266467414000376
Santos-Heredia, C., Andresen, E., Del Val, E., Zárate, D. A., Nava Mendoza, M., and Jaramillo, V. J. (2016). The activity of dung beetles increases foliar nutrient concentration in tropical seedlings. Biotropica 48, 565–567. doi: 10.1111/btp.12364
Santos-Heredia, C., Andresen, E., and Stevenson, P. (2011). Secondary seed dispersal by dung beetles in an Amazonian forest fragment of Colombia: influence of dung type and edge effect. Integr. Zool. 6, 399–408. doi: 10.1111/j.1749-4877.2011.00261.x
Santos-Heredia, C., Andresen, E., and Zárate, D. A. (2010). Secondary seed dispersal by dung beetles in a Colombian rain forest: effects of dung type and defecation pattern on seed fate. J. Trop. Ecol. 26, 355–364. doi: 10.1017/S0266467410000192
Santos-Heredia, C., Andresen, E., Zárate, D. A., and Escobar, F. (2018). Dung beetles and their ecological functions in three agroforestry systems in the Lacandona rainforest of Mexico. Biodivers. Conserv. 27, 2379–2394. doi: 10.1007/s10531-018-1542-x
Scholtz, C. H., Davis, A. L. V., and Kryger, U. (2009). Evolutionary Biology and Conservation of dung Beetles. Bulgaria: Pensoft Publishers.
Schupp, E. W. (1993). Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio 107-108, 15–29. doi: 10.1007/bf00052209
Shepherd, V. E., and Chapman, C. A. (1998). Dung beetles as secondary seed dispersers: impact on seed predation and germination. J. Trop. Ecol. 14, 199–215. doi: 10.1017/S0266467498000169
Slade, E. M., Kirwan, L., Bell, T., Philipson, C. D., Lewis, O. T., and Roslin, T. (2017). The importance of species identity and interactions for multifunctionality depends on how ecosystem functions are valued. Ecology 98, 2626–2639. doi: 10.1002/ecy.1954
Slade, E. M., Mann, D. J., and Lewis, O. T. (2011). Biodiversity and ecosystem function of tropical forest dung beetles under contrasting logging regimes. Biol. Conserv. 144, 166–174. doi: 10.1016/j.biocon.2010.08.011
Slade, E. M., Mann, D. J., Villanueva, J. F., and Lewis, O. T. (2007). Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J. Anim. Ecol. 76, 1094–1104. doi: 10.1111/j.1365-2656.2007.01296.x
Slade, E. M., and Roslin, T. (2016). Dung beetle species interactions and multifunctionality are affected by an experimentally warmed climate. Oikos 125, 1607–1616. doi: 10.1111/oik.03207
Stanbrook, R., Raisin, C., and Vulinec, K. (2017). Observations on the tunneling behavior and seed dispersal efficacy of copris nubilosus Kohlmann, Cano, and Delgado (Coleoptera: Scarabaeinae: Coprini). Coleopt. Bull. 71, 777–780. doi: 10.1649/0010-065X-71.4.777
Tonelli, M. (2021). Some considerations on the terminology applied to dung beetle functional groups. Ecol. Entomol. 46, 772–776. doi: 10.1111/een.13017
Traveset, A., Robertson, A. W., and Rodríguez-Pérez, J. (2007). “A review on the role of endozoochory in seed germination” in Seed Dispersal. Theory and its Application in a Changing World. eds. A. J. Dennis, E. W. Schupp, R. J. Green, and D. A. Westcott (Oxon: CABI Publishing)
Urrea-Galeano, L. A., Andresen, E., Coates, R., Mora Ardila, F., Del Val, E., and Nava Mendoza, M. (2021). Dung beetle activity had no positive effect on nutrient concentration or performance of established rainforest seedlings. Biotropica 53, 808–819. doi: 10.1111/btp.12934
Urrea-Galeano, L. A., Andresen, E., Coates, R., Mora Ardila, F., Díaz Rojas, A., and Ramos-Fernández, G. (2019a). Horizontal seed dispersal by dung beetles reduced seed and seedling clumping, but did not increase short-term seedling establishment. PLoS One 14:e0224366. doi: 10.1371/journal.pone.0224366
Urrea-Galeano, L. A., Andresen, E., Coates, R., Mora Ardila, F., and Ibarra-Manríquez, G. (2019b). Dung beetle activity affects rain forest seed bank dynamics and seedling establishment. Biotropica 51, 186–195. doi: 10.1111/btp.12631
Vander Wall, S. B., Forget, P.-M., Lambert, J., and Hulme, P. (2005). “Seed fate pathways: filling the gap between parent and offspring,” in Seed Fate: Predation, Dispersal and Seedling Establishment. eds. P.-M. Forget, J. E. Lambert, P. E. Hulme, and S. B. Vander Wall (Wallingford: CABI Publishing).
Vander Wall, S. B., and Longland, W. S. (2004). Diplochory: are two seed dispersers better than one? Trends Ecol. Evolution 19, 155–161. doi: 10.1016/j.tree.2003.12.004
Verdú, J. R., Numa, C., Lobo, J. M., Martínez-Azorín, M., and Galante, E. (2009). Interactions between rabbits and dung beetles influence the establishment of Erodium praecox. J. Arid Environ. 73, 713–718. doi: 10.1016/j.jaridenv.2009.02.008
Vulinec, K. (2000). Dung beetles (Coleoptera: Scarabaeidae), monkeys, and conservation in Amazonia. Fla. Entomol. 83, 229–241. doi: 10.2307/3496341
Vulinec, K. (2002). Dung beetle communities and seed dispersal in primary forest and disturbed land in Amazonia. Biotropica 34, 297–309. doi: 10.1111/j.1744-7429.2002.tb00541.x
Vulinec, K., Edmonds, W. D., and Mellow, D. J. (2003). Biological and taxonomic notes on a rare phanaeine dung beetle, phanaeus alvarengai Arnaud (Coleoptera: Scarabaeidae). Coleopt. Bull. 57, 353–357. doi: 10.1649/639
Wehncke, E. V., and Dalling, J. W. (2005). Post-dispersal seed removal and germination selected tree species dispersed by Cebus capucinus on Barro Colorado island, Panama. Biotropica 37, 73–80. doi: 10.1111/j.1744-7429.2005.03037.x
Whitworth, A., Whittaker, L., Huarcaya, R. P., Flatt, E., Lopez Morales, M., Connor, D., et al. (2019). Spider monkeys rule the roost: ateline sleeping sites influence rainforest heterogeneity. Animals 9:1052. doi: 10.3390/ani9121052
Wicklow, D. T., Kumar, R., and Lloyd, J. E. (1984). Germination of blue grama seeds buried by dung beetles (Coleoptera: Scarabaeidae). Environ. Entomol. 13, 878–881. doi: 10.1093/ee/13.3.878
Wu, X., Griffin, J. N., and Sun, S. (2014). Cascading effects of predator–detritivore interactions depend on environmental context in a Tibetan alpine meadow. J. Anim. Ecol. 83, 546–556. doi: 10.1111/1365-2656.12165
Wu, X., Griffin, J. N., Xi, X., and Sun, S. (2015). The sign of cascading predator effects varies with prey traits in a detrital system. J. Anim. Ecol. 84, 1610–1617. doi: 10.1111/1365-2656.12403
Xie, M., Wu, X., and Sun, S. (2021). Interspecific interactions between burrowing dung beetles and earthworms on yak dung removal and herbage growth in an alpine meadow. Soil Ecol. Lett. 3, 94–102. doi: 10.1007/s42832-020-0059-x
Keywords: bioturbation, ecological functions, secondary seed dispersal, seed bank, seedlings, Scarabaeinae, soil conditioning
Citation: Andresen E and Urrea-Galeano LA (2022) Effects of dung beetle activity on tropical forest plants. Front. Ecol. Evol. 10:979676. doi: 10.3389/fevo.2022.979676
Edited by:
Mario Favila, Instituto de Ecología (INECOL), MexicoReviewed by:
César M. A. Correa, Federal University of Mato Grosso do Sul, BrazilAmy Dunham, Rice University, United States
Malva Isabel Medina Hernández, Federal University of Santa Catarina, Brazil
Copyright © 2022 Andresen and Urrea-Galeano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ellen Andresen, YW5kcmVzZW5AY2llY28udW5hbS5teA==
 Ellen Andresen
Ellen Andresen Lina Adonay Urrea-Galeano
Lina Adonay Urrea-Galeano