
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 04 August 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.972942
This article is part of the Research Topic Adaptive Evolution of Organs Size in Cold-Blooded Animals View all 6 articles
Phenotypic variation of morphological and physiological traits is assumed to be generated from spatial heterogeneity in environments, and it has been regarded as an important concern domain in evolutionary biology. Organs display markedly size variation among populations along environmental gradients and this variation is associated with changes in oxygen supply and energy demands. Here, we investigated geographical variation in the relative size of organs (i.e., brain, heart, lung, gallbladder, livers, spleen, kidneys, and digestive tract) among 14 populations of Andrew’s toad (Bufo andrewsi) transcending an elevational range from 864 to 2,367 m, and spanning 8° latitude. We found that although the relative sizes of the eight specific organs varied significantly among populations, none organ size was affected by altitude and latitude. However, based on the combined the new data and published data we found a negative relationship between the relative size of the heart and latitude, contrasting to the Hesse’s rule. We also found that the relative size of livers was positively linked to latitude, suggesting that more energy demands and intakes due to slower metabolism in high latitude shaped the evolution of larger livers.
The theory of phenotypic plasticity states that organisms change their morphology and physiological function as an adaptive response to environmental conditions (Stearns, 1989; Clifton et al., 2020; Peng et al., 2022). Phenotypic plasticity in morphological and physiological traits is a common phenomenon across animal taxa (Tucker and Horvath, 1973; Hammond et al., 1999; Piersma et al., 1999; Naya et al., 2009; Zhong et al., 2017; Balciauskas et al., 2020; Donihue et al., 2021; Huang et al., 2021; Liang et al., 2021; Zamora-camacho, 2021; Zedda et al., 2021; Giacomini et al., 2022; Hinds et al., 2022). Organ’s size can reflect the traits of structures with certain morphology and independent physiological functions within an organism, and it’ variation also undergo certain adaptations to respond to environmental changes (Hammond et al., 2001; Chen et al., 2021; Jiang et al., 2022a). Adaptive strategies of organs responding to the change in temperature and rainfall across geographical gradients were related to food ingestion and the distribution of nutrition digested from food and oxygen consumption in metabolism (Hansson, 1971; Bonnet et al., 1998; Piersma et al., 1999; Dudczak et al., 2022). Indeed, there is evidence that environmental changes affect the development of organ sizes in vertebrates (Piersma et al., 1999; Liu et al., 2011, 2014; Lou et al., 2013; Jin et al., 2016; Ma et al., 2016; Yang et al., 2017; Zhong et al., 2017; Tang et al., 2018; Bláha et al., 2021).
Variation of organ sizes are positively associated with the energetic storage because organisms need energy to reproduce and survive over periods when the available food is limited (Jonsson et al., 1997). Many studies have shown that organ sizes are correlated with the changes in energetic status based on the short-term and long-term sustainable metabolic rates (Chappell et al., 1988; Daan et al., 1990; Hammond and Wunder, 1991; Loeb et al., 1991). Several hypotheses have suggested to explain the relationship between energetic status and organ size. For instance, the brain was regarded as an important costly organ, and two main hypotheses have been proposed to explain the relationship between brain size variation and environmental selection pressures (Striedter, 2005; Liu et al., 2022). The cognitive buffer hypothesis (CBH) predicts a major cognitive advantage of a relatively large brain for foraging and avoiding predators in changing environments (Lefebvre et al., 1997). By contrast, the expensive brain framework (EBF) states that relative brain size is reduced in fluctuating environments when the developmental costs are over the cognitive benefits of large brains (van Woerden et al., 2010; Luo et al., 2017). Moreover, the Hesse’s rule also explains variation in heart size among populations where individuals living in colder and hypoxic conditions have larger hearts than individuals inhabiting warmer and hyperoxic conditions (Hesse et al., 1937). Indeed, following the prediction of the Hesse’s rule, the size of the heart displays pronounced size variation in species along altitudinal gradients (Hock, 1964; Hammond et al., 1999, 2001; Naya et al., 2009; Müller et al., 2014).
Previous studies have shown that environmental factors such as temperature and rainfall can promote variations in organ sizes among populations in anurans (Jönsson et al., 2009; Jiang et al., 2015; Zhao et al., 2019). For example, supporting the prediction of the EBF, there is a positive correlation between brain size and activity season length among Bufo andrewsi populations (Jiang et al., 2015). The common frogs (Rana temporaria) from the north have larger livers than southern conspecifics due to more requirement of energy (Jönsson et al., 2009). The digestive tract length in Andean toads (Bufo spinulosus) declines with increasing altitude (Naya et al., 2009) whereas the Yunnan frog (Pelophylax pleuraden) exactly shows the opposite direction (Lou et al., 2013). Besides, the heart and lung mass increase with altitude in the spot-legged treefrog (Polypedates megacephalus) among populations, supporting the prediction of the Hesse’s rule (Zhong et al., 2017). Hence, the environmental pressures leading to differences in requirement of energy were regarded as the key powers in shaping variations in organ sizes across populations in species of frogs.
The Andrew’s toad (B. andrewsi) is a medium-sized anuran species that lives in the subtropical forests of the Hengduan Mountains, China, at elevations ranging from 750 to 3,500 m (Fei and Ye, 2001). Toward high altitudes, this species attains a larger size at metamorphism, longer longevity, and larger body size at slower growth rates (Liao and Lu, 2012; Liao et al., 2015, 2016). Although ecological factors have been suggested to shape variations in brain size and limb muscles among populations along an altitudinal gradient (Jiang et al., 2015; Yang et al., 2017; Zhao et al., 2019), the variation in organ size (e.g., brain, heart, lung, gallbladder, livers, spleen, kidneys, digestive tract) in this species across total geographical ranges at the intraspecific level is as yet unexplored. Here, we investigated patterns and possible causes of geographical variation in organ size among B. andrewsi populations. We first examined the predictions of the CBH and EBF by investigating the relationship between brain size and altitude and/or latitude. We further tested whether the heart size variation followed the Hesse’s rule. Finally, we tested geographical variations in other organ size (e.g., lung, gallbladder, livers, spleen, kidneys, digestive tract) among populations.
A total of 355 male toads were collected from 14 populations in southwestern China between 2017 and 2019 (Figure 1 and Supplementary Table 1). All the toads were sampled each year between the end of March and the beginning of April during the breeding season. We captured all individuals within each sampling site at night using a 12-V flashlight, and confirmed their sexes through their secondary sexual traits (e.g., nuptial pads in males and eggs in females). After being kept at room temperature for 12–24 h in the rectangular tank (1 × 0.5 × 0.8 m, L × W × H) filled with fresh water (2 cm deep) in the laboratory, all individuals were sacrificed by single-pithing (Yu et al., 2018; Zhao et al., 2019). The body size (snout–vent length, SVL) of each individual was measured to the nearest 0.1 mm using a vernier caliper. Subsequently, all specimens were fixed in 4% phosphate-buffered formalin (Zeng et al., 2016). All measurements were taken blindly by identifying specimens by ID number without knowledge of the species’ identity. After a maximum of 2 months of preservation, the brain, heart, lungs, gallbladder, liver, spleen, kidneys, digestive tract were dissected. All organs except the digestive tract and brain were placed in a thermostat drier (60°C) for 48 h and measured their dry mass to the nearest 0.1 mg with an electronic balance. The digestive tract length was measured to the nearest 0.1 mm using a vernier caliper. There is evidence that the length of preservation time has a non-significant effect on organ size in a frog of species (Zhong et al., 2017). The Animal Ethics Committee at China West Normal University (AECCWNU) approved the sacrifice of animals for the reported experiments.
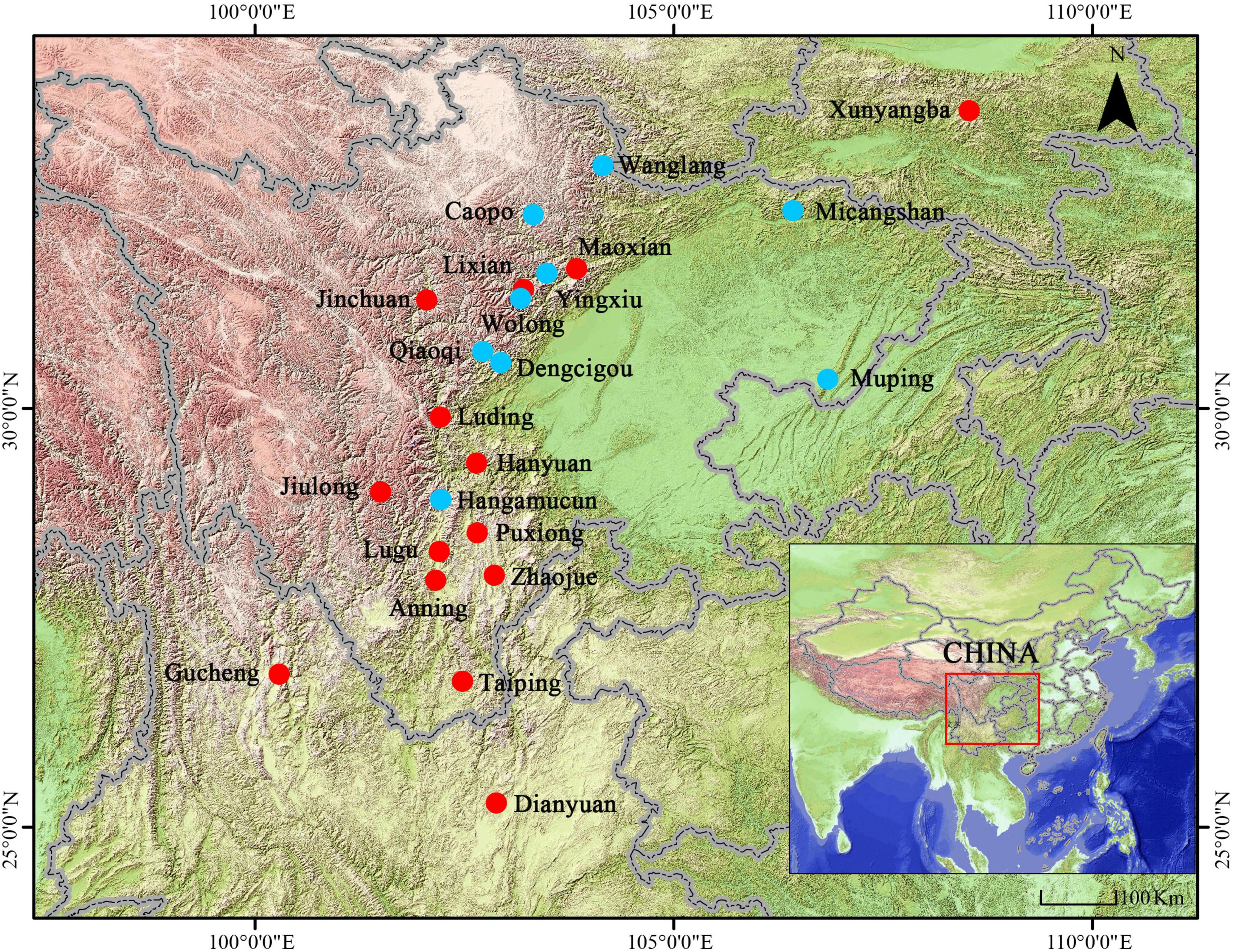
Figure 1. Map depicting the study sites for 24 Bufo andrewsi populations. Red circles indicate sampling sites in this time and blue circles indicate sampling sites in published papers.
We took digital images of the dorsal, ventral, left and right sides of the brain with a Motic Images 3.1 digital camera mounted on a Moticam 2006 light microscope at a 400 × magnification (Liao et al., 2022). For dorsal and ventral views, we ensured that the view of the brain being photographed was horizontal and that the brain was symmetrically positioned such that one hemisphere did not appear larger than the other. For paired regions, we measured only the width of the right hemisphere and the volume estimate was doubled. The length (L), width (W), and height (H) of the brain were measured from the digital photographs using a tpsDig2 Software. The definition of brain was given as the greatest distance and exhibited the used landmarks. Volumetric estimates of brains were then obtained through an ellipsoid model: volume = (L × W × H) π/(6 × 1.43); (see details in Jiang et al., 2015). The sizes of brains were measured three times and very high intra-measurer repeatability was found for anurans of species.
All analyses were performed using R software 4.2.0 (R Core Team, 2022; also see Jiang et al., 2022b). Prior to the analyses, all continuous variables were scaled by 1,000 and log10-transformed to meet the assumption of normality and enhance the homogeneity of variances. To analyze the differences in organ size among populations, we used one-way analyses of variance (ANOVA) for each organ separately, controlling for body size as a covariate. A previous study has demonstrated that latitude and/or altitude affect the differences in organ size in this species (Zhao et al., 2017). We thus used linear mixed models (LMMs) to assess the effect of geographical gradients (e.g., latitude and altitude) on organ size, with the population as a random effect, and body size as a covariate. The mean values for different organ sizes were also calculated for each population. Because we gain insight into variations in organ sizes across total distributed region of the toad, we analyzed the relationship between the average size of organs and geographical gradients (altitude and latitude) at the population level using a multiple regression analysis in combination with data extracted from previously published literatures (Supplementary Table 2; Jiang et al., 2015; Ma et al., 2016; Zhao et al., 2019). Finally, we analyzed the relationship between the relative size of all organs and both temperature and rainfall of each site among all populations.
The size of all organs with mean ± SE for all populations was shown in Supplementary Table 2. One-way ANOVA revealed that in analyses of covariance controlling for body size (all P < 0.001), the size of the eight specific organs differed significantly among populations (Table 1). The LMMs revealed that variation in the relative size of all organs was independent on altitude and/or latitude at the level of individuals (all P > 0.05; Table 2).
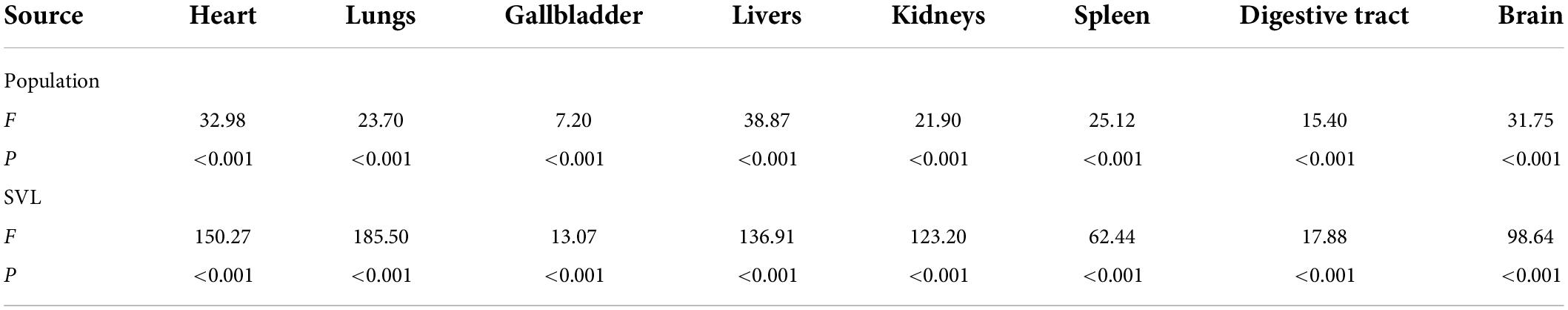
Table 1. The variations of organs size in Bufo andrewsi among 14 populations when correcting for body size using one-way analyses of variance.
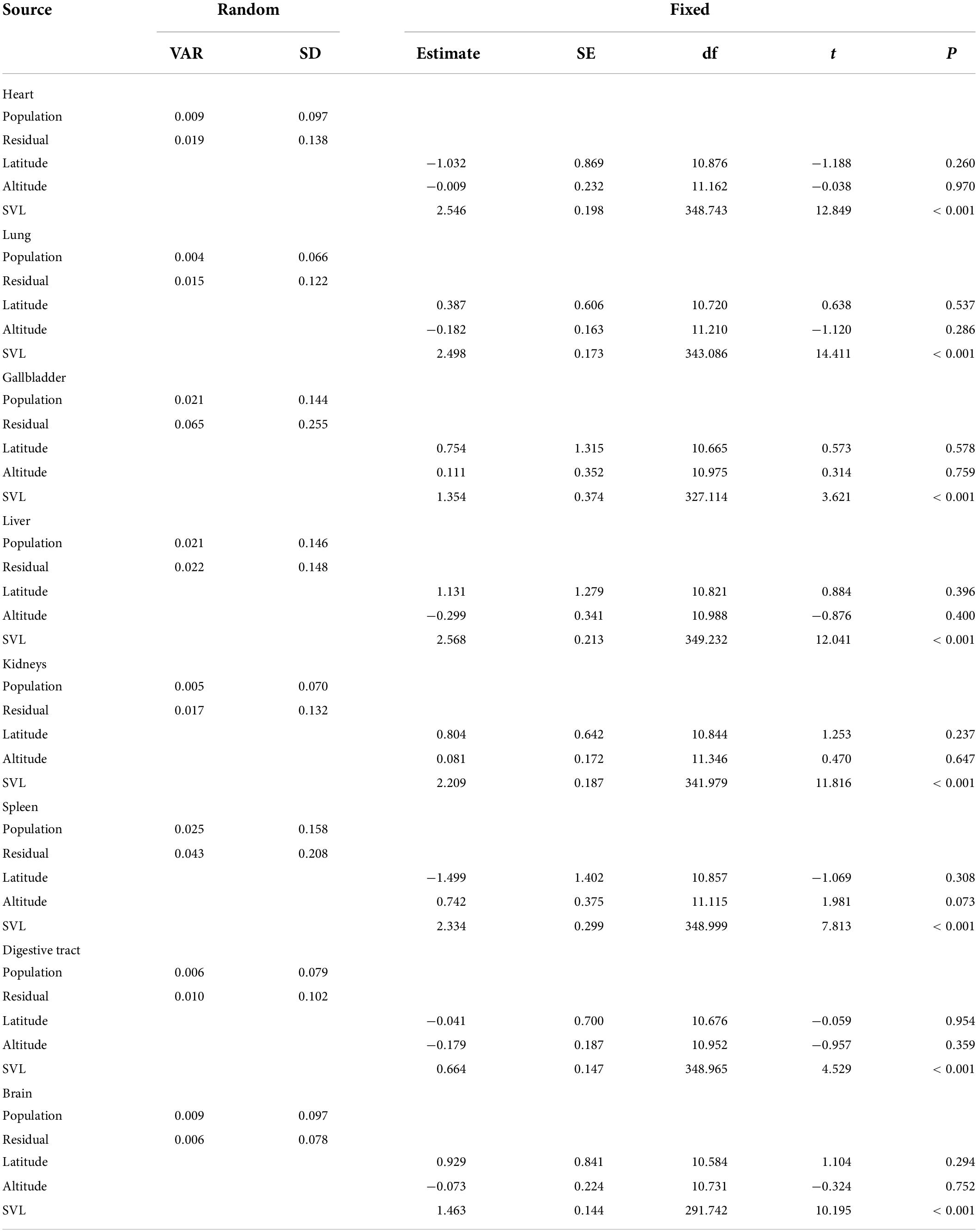
Table 2. The effects of altitude, latitude, and population on variation in organ size across 14 populations of the Andrew’s toads when correcting for snout–vent length (SVL) using linear mixed models (LMMs).
We further examined altitudinal and/or latitudinal variation in organ size at the population level based on the published data. After controlling for body size, the relative size of heart was negatively correlated with latitude (Figure 2A; t = −2.459, n = 24, P = 0.023), but not vary significantly with altitude (t = 0.264, n = 24, P = 0.794). Inconsistent with the relative size of heart, we found a significant effect of latitude on the variation of the size of livers (Figure 2B; t = 2.284, n = 24, P = 0.034). We did not find convincing evidence for altitudinal and/or latitudinal variation in the other organ size (Table 3). Moreover, none organ size showed a correlation with both temperature and rainfall within each site among all populations (Supplementary Table 3).
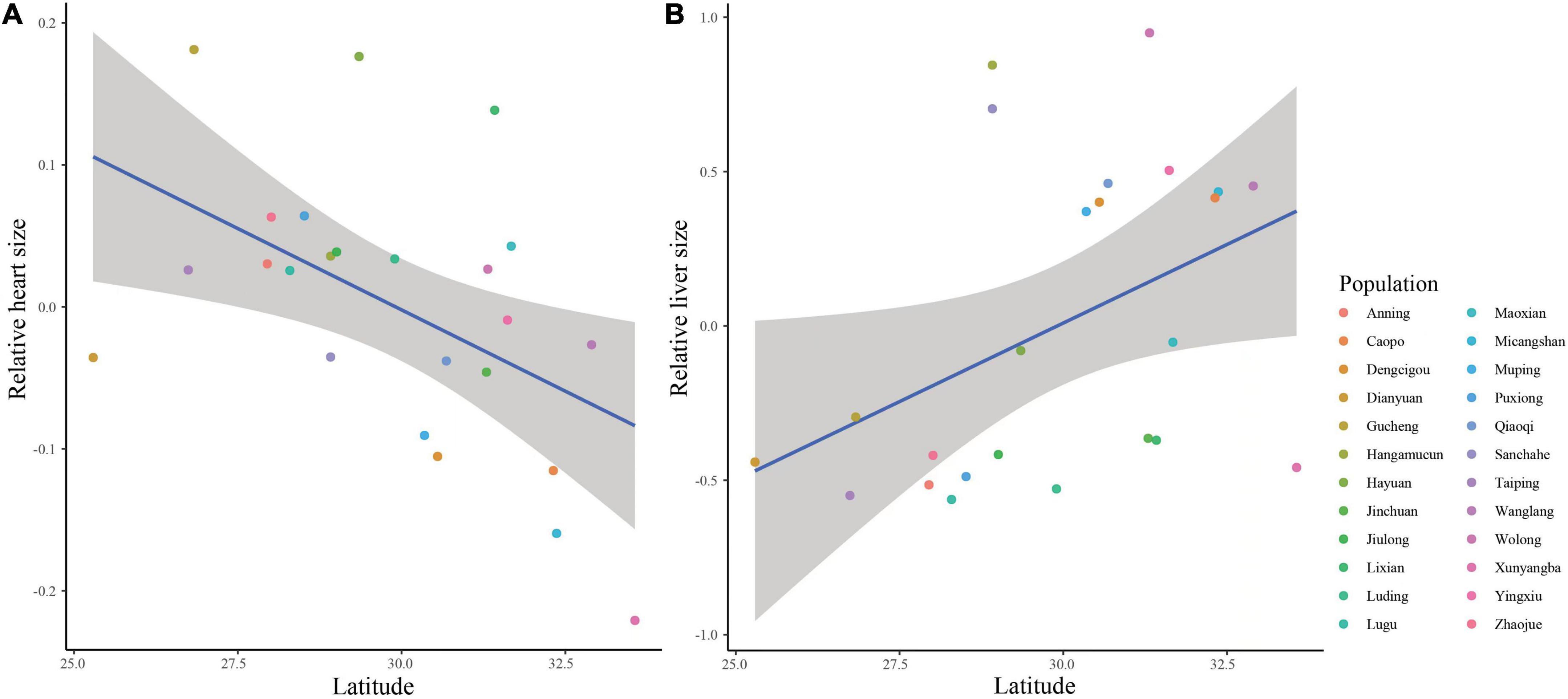
Figure 2. The relationships between latitude and relative size of heart (A) and livers (B) in Bufo andrewsi among 24 populations.
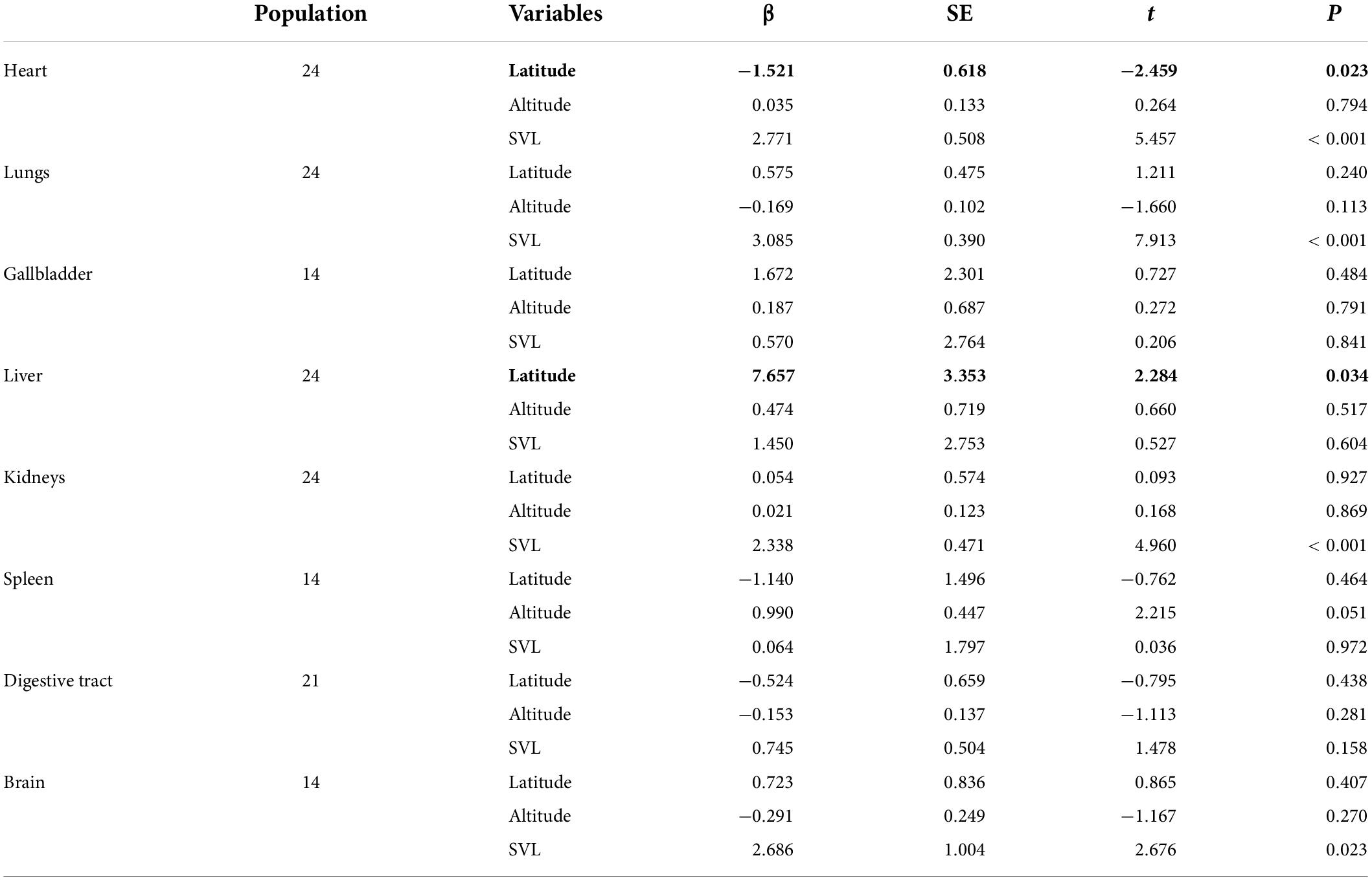
Table 3. The relationship between altitude and latitude and variation in mean organ size across all populations of the Andrew’s toads when correcting for snout–vent length (SVL).
We demonstrate the significant differences in relative size of organs (brain, heart, lungs, gallbladder, livers, kidney, spleen, and digestive tract) among populations. However, we find that altitude and/or latitude are not correlated with the relative size of all organs across populations. Based on published data on the average size of organs, we find that the relative size of the heart is negatively correlated with latitude whereas the relative size of the livers increases with increasing latitude. Our findings suggest that the heart size variation of the toad contrasts with the prediction of Hesse’s rule. We find that none organ size is linked to temperature and rainfall across populations. In what follows, we discuss our findings associated with what has previously been known from intraspecific studies the variation in size of organs in anurans.
The relatively larger brains with higher cognitive abilities can deal with novel environmental challenges in animals (Sol and Lefebvre, 2000; Sol et al., 2008). However, the brain is a high metabolic organ, and its energetic costs need to be overcome to maintain a larger brain (van Woerden et al., 2011; Jiang et al., 2021). A previous study has indicated that there is significant seasonality-dependent population variation in absolute and relative brain size in B. andrewsi (Jiang et al., 2015). In this study, we found that relative brain size differed significantly among populations. Amphibians living at lower altitudes and/or latitudes have more access to food than amphibians living at higher altitudes and/or latitudes, which can be explained by the CBH (Luo et al., 2017). As a result, individuals with lower altitudes and/or latitudes for foraging, growth, and reproduction by decreasing hibernation were expected to have relatively larger brains. Indeed, Jiang et al. (2015) found that higher altitudes and/or latitudes lead to smaller brain sizes in B. andrewsi due to the short active season. However, the observed variation in relative brain size was not correlated with altitude and latitude among B. andrewsi populations, which do not support both the CBH and EBF.
Animals may experience limitations to aerobic activities (i.e., exercise and heat production) to adapt to the less oxygen supply at higher altitudes (Chappell et al., 1988). There are evidences for the Hesse’s rule that hearts are larger at high altitudes than at low altitudes (Hock, 1964; Hammond et al., 1999; Zhong et al., 2017). However, the relative size of heart does not increase with increasing altitudes or latitudes among ten B. andrewsi populations (Zhao et al., 2019). Here we found that relative size of heart decreased with increasing latitude among 24 populations, which contrasted the Hesse’s rule.
In anurans, the mass and glycogen contents of the livers display a positive correlation with altitudes and/or latitudes because more energy stores are related to more uncertain environments (McNamara and Houston, 1990; Jönsson et al., 2009; Zhong et al., 2017). Consequently, the larger livers at higher altitudes and/or latitudes are expected to result from the more energy storage demands during hibernation and at post-hibernation emergence. Although individuals from higher altitudes and/or latitudes do not display relatively larger livers among 10 populations (Zhao et al., 2019), we found that individuals from higher latitudes had relatively larger livers in B. andrewsi based on the combined the new data and published data across all populations. This pattern suggested that individuals living at higher latitudes generally had more energy demands and intakes. Moreover, the lower temperatures and oxygen supply at higher altitudes can explain the smaller kidneys because of the overall lower metabolism (Tucker and Horvath, 1973). Consistent with the previous findings (Zhao et al., 2019), we did not find larger kidneys at higher altitudes and/or latitudes in this toad, which suggested that variations in kidney size cannot be explained by the oxygen supply.
Animals can adjust digestive tract morphology to adapt to food habits and quality under changing environments (Crump and Franklin, 2005; Naya and Bozinovic, 2006; Naya et al., 2009; Lou et al., 2013; Ma et al., 2016). Indeed, individuals at higher-altitude populations consuming greater plant materials exhibit longer gut than individuals at lower-altitude populations mainly predating on seeds in rats (Hansson, 1985; Hammond et al., 1999). For B. spinulosus, higher temperatures result in decreasing the animal-based food availability and increasing the plant-based foods (Naya et al., 2009). Meanwhile, there is a positive correlation between the length of the digestive tract and temperature among populations in B. andrewsi (Zhao et al., 2019). However, the digestive tract length was not correlated with altitude and/or latitude among 21 populations, suggesting that food habits with animal-based food availability in the toads for all populations did not affect variation in digestive tract length. We also did not find the relative size of lungs, gallbladder, and spleen increasing with altitude and/or latitude across populations.
In sum, our findings indicate that altitude and/or latitude do not affect the relative size of organs except for the heart and livers. Brain size variation cannot be explained by both the CBH and EBF. The increased latitude results in relatively smaller heart, which contrasts the prediction of the Hesse’s rule. The larger livers in higher-latitude individuals suggest more energy demands and intakes due to slower metabolism. Temperature and rainfall within each site do not affect the relative size of heart and livers, suggesting that the other environmental factors such as oxygen supply is likely to shape variation in heart and livers.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was reviewed and approved by the animal study was reviewed and approved by China West Normal University. All experiments were performed by the relevant guidelines and regulations.
XZ, CC, and YJ participated in laboratory work, data analysis, and manuscript drafting. CC, LZ, and LJ conducted data analysis and visual representation of the data. All authors contributed to the article and approved the submitted version.
Financial support was provided by the National Natural Science Foundation of China (31970393) and the Key Project of Natural Sciences Foundation of Sichuan Province (22NSFSC0011).
We would like to thank CL Mai, JP Yu, and ZP Mi for their assistance in data collection during the experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.972942/full#supplementary-material
Balciauskas, L., Amshokova, A., Balciauskiene, L., Benedek, A. M., Cichocki, J., Csanády, A., et al. (2020). Geographical clines in the size of the herb field mouse (Apodemus uralensis). Integr. Zool. 15, 55–68. doi: 10.1111/1749-4877.12407
Bláha, M., Patoka, J., Japoshvili, B., Let, M., Buøiè, M., Kouba, A., et al. (2021). Genetic diversity, phylogenetic position and morphometric analysis of Astacus colchicus (Decapoda, Astacidae): A new insight into Eastern European crayfish fauna. Integr. Zool. 16, 368–378. doi: 10.1111/1749-4877.12493
Bonnet, X., Shine, R., Naulleau, G., and Vacher–Vallas, M. (1998). Sexual dimorphism in snakes: Different reproductive roles favour different body plans. Proc. R. Soc. B 265, 179–183. doi: 10.1098/rspb.1998.0280
Chappell, M. A., Hayes, J. P., and Snyder, L. R. G. (1988). Hemoglobin polymorphisms in deer mice (Peromyscus maniculatus): Physiology of beta–globin variants and alpha–globin recombinants. Evolution 42, 681–688. doi: 10.1111/j.1558-5646.1988.tb02486.x
Chen, C., Jiang, Y., Jin, L., and Liao, W. B. (2021). No evidence for effects of ecological and behavioral factors on eye size evolution in anurans. Front. Ecol. Evol. 9:755818. doi: 10.3389/fevo.2021.755818
Clifton, I. T., Chamberlain, J. D., and Gifford, M. E. (2020). Role of phenotypic plasticity in morphological differentiation between water snake populations. Integr. Zool. 15, 329–337. doi: 10.1111/1749-4877.12431
Crump, R. L., and Franklin, C. E. (2005). Arousal and re–feeding rapidly restores digestive tract morphology following aestivation in green–striped burrowing frogs. Comp. Biochem. Phys. A 142, 451–460. doi: 10.1016/j.cbpa.2005.09.013
Daan, S., Masman, D., and Groenewold, A. (1990). Avian basal metabolic rates: Their association with body composition and energy expenditure in nature. Am. J. Physiol. 259:R333–40. doi: 10.1152/ajpregu.1990.259.2.R333
Donihue, C. M., Daltry, J. C., Challenger, S., and Herrel, A. (2021). Population increase and changes in behavior and morphology in the Critically Endangered Redonda ground lizard (Pholidoscelis atratus) following the successful removal of alien rats and goats. Integr. Zool. 16, 379–389. doi: 10.1111/1749-4877.12500
Dudczak, A. C., De La Torre, G. M., Euclydes, L., and Campião, K. M. (2022). The roles of anurans in antagonistic networks are explained by life-habit and body-size. Integr. Zool. 17, 530–542. doi: 10.1111/1749-4877.12586
Fei, L., and Ye, C. Y. (2001). The Colour Handbook Of Amphibians Of Sichuan. Beijing: China Forestry Publishing House.
Giacomini, G., Herrel, A., Chaverri, G., Brown, R. P., Russo, D., Scaravelli, D., et al. (2022). Functional correlates of skull shape in Chiroptera: Feeding andecholocation adaptations. Integr. Zool. 17, 430–442. doi: 10.1111/1749-4877.12564
Hammond, K. A., Roth, J., Janes, D. N., and Dohm, M. R. (1999). Morphological and physiological responses to altitude in deer mouse (Peromyscus maniculatus). Physiol. Biochem. Zool. 75, 613–622. doi: 10.1086/316697
Hammond, K. A., Szewczak, J., and Król, E. (2001). Effects of altitude and temperature on organ phenotypic plasticity along an altitudinal gradient. J. Exp. Biol. 204, 1991–2000. doi: 10.1242/jeb.204.11.1991
Hammond, K. A., and Wunder, B. A. (1991). The role of diet quality and energy need in the nutritional ecology of a small herbivore, Microtus ochrogaster. Physiol. Zool. 64, 541–567. doi: 10.1086/physzool.64.2.30158190
Hansson, L. (1971). Estimates of the productivity of small mammals in a South Swedish spruce plantation. Ann. Zool. Fenn. 8, 118–126.
Hansson, L. (1985). Geographic differences in bank voles, Clethrionomys glareolus, in relation to ecogeographical rules and possible demographic and nutritive strategies. Ann. Zool. Fenn. 22, 319–328.
Hesse, R., Allee, W. C., and Schmidt, K. P. (1937). Ecological Animal Geography. New York, NY: John Wiley and SonsInc. doi: 10.2307/1436962
Hinds, L. A., Henry, S., Van de Weyer, N., Robinson, F., Ruscoe, W. A., and Brown, P. R. (2022). Acute oral toxicity of zincphosphide: An assessment for wild house mice (Mus musculus). Integr. Zool. [Epub ahead of print]. doi: 10.1111/1749-4877.12666
Hock, R. J. (1964). “Physiological responses of deer mice to various native altitudes,” in The Physiological Effects of High Altitude, ed. W. H. Weihe (New York, NY: Macmillan), 59–72. doi: 10.1016/B978-1-4831-6699-5.50013-9
Huang, S., Li, G., Pan, Y., Song, M., Zhao, J., Wan, X., et al. (2021). Density–induced social stress alters oxytocin and vasopressin activities in the brain of a small rodent species. Integr. Zool. 16, 149–159. doi: 10.1111/1749-4877.12467
Jiang, A., Zhong, M. J., Xie, M., Lou, S. L., Jin, L., Robert, J., et al. (2015). Seasonality and age is positively related to brain size in Andrew’s toad (Bufo andrewsi). Evol. Biol. 42, 339–348. doi: 10.1007/s11692-015-9329-4
Jiang, Y., Chen, C., Mi, Z. P., and Liao, W. B. (2022a). Testing the role of environmental harshness and sexual selection in limb muscle mass in anurans. Front. Ecol. Evol. 10:879885. doi: 10.3389/fevo.2022.879885
Jiang, Y., Chen, C., and Liao, W. B. (2022b). Anuran interorbital distance variation: The role of ecological and behavioral factors. Integr. Zool. [Epub ahead of print]. doi: 10.1111/1749-4877.12653
Jiang, Y., Wang, J. Y., Huang, X. F., Mai, C. L., and Liao, W. B. (2021). Brain size evolution in small mammals: Test of the expensive tissue hypothesis. Mammalia 85, 455–461. doi: 10.1515/mammalia-2019-0134
Jin, L., Yang, S. N., Liao, W. B., and Lüpold, S. (2016). Altitude underlies variation in the mating system, somatic condition, and investment in reproductive traits in male Asian grass frogs (Fejervaryalimnocharis). Behav. Ecol. Sociobiol. 70, 1197–1208. doi: 10.1007/s00265-016-2128-9
Jönsson, K. I., Herczeg, G., O’Hara, R. B., Söderman, F., ter Schure, A. F. H., Larsson, P., et al. (2009). Sexual patterns of prebreeding energy reserves in the common frog Rana temporaria along a latitudinal gradient. Ecography 32, 831–839. doi: 10.1111/j.1600-0587.2009.05352.x
Jonsson, N., Jonsson, B., and Hansen, L. P. (1997). Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon (Salmo salar). J. Anim. Ecol. 66, 425–436. doi: 10.2307/5987
Lefebvre, L., Whittle, P., Lascaris, E., and Finkelstein, A. (1997). Feeding innovations and forebrain size in birds. Anim. Behav. 53, 549–560. doi: 10.1006/anbe.1996.0330
Liang, T., Meiri, S., and Shi, L. (2021). Sexual size dimorphism in lizards: Rensch’s rule, reproductive mode, clutch size, and line fitting method effects. Integr. Zool. 10.1111/1749-4877.12569 [Epub ahead of print]. doi: 10.1111/1749-4877.12569
Liao, W. B., Jiang, Y., Li, D. Y., Jin, L., Zhong, M. J., Qi, Y., et al. (2022). Cognition contra camouflage: How the brain mediates predator-driven crypsis evolution. Sci. Adv. 8:eabq1878.
Liao, W. B., Liu, W. C., and Merilä, J. (2015). Andrew meets Rensch: Sexual size dimorphism and the inverse of Rensch’s rule in Andrew’s toad (Bufo andrewsi). Oecologia 177, 389–399. doi: 10.1007/s00442-014-3147-8
Liao, W. B., and Lu, X. (2012). Adult body size = f (initial size +/ growth rate ×/ age): Explaining the proximate cause of Bergman’s cline in a toad along altitudinal gradients. Evol. Ecol. 26, 579–590. doi: 10.1007/s10682-011-9501-y
Liao, W. B., Luo, Y., Lou, S. L., Lu, D., and Jehle, R. (2016). Geographic variation in life–history traits: Growth season affects age structure, egg size and clutch size in Andrew’s toad (Bufo andrewsi). Front. Zool. 13:6. doi: 10.1186/s12983-016-0138-0
Liu, J., Zhou, C. Q., and Liao, W. B. (2014). Evidence for neither the compensation hypothesis nor the expensive–tissue hypothesis in Carassius auratus. Anim. Biol. 64, 177–187. doi: 10.1163/15707563-00002437
Liu, Y. H., Liao, W. B., Zhou, C. Q., Mi, Z. P., and Mao, M. (2011). Asymmetry of testes in Guenther’s frog, Hylarana guentheri (Anuar: Ranidae). Asian Herpetol. Res. 2, 234–239. doi: 10.3724/SP.J.1245.2011.00234
Liu, Y. T., Wu, Z. J., and Liao, W. B. (2022). Large-brained birds display lower extra-pair paternity. Integr. Zool. [Epub ahead of print]. doi: 10.1111/1749-4877.12636
Loeb, S. C., Schwab, R. G., and Demment, M. W. (1991). Responses of pocket gophers (Thomomys bottae) to changes in diet quality. Oecologia 86, 542–551. doi: 10.1007/BF00318321
Lou, S. L., Li, Y. H., Jin, L., Mi, Z., Liu, W., Liao, W., et al. (2013). Altitudinal variation in digestive tract length in Yunnan pond frog (Pelophylax pleuraden). Asian Herpetol. Res. 4, 263–267. doi: 10.3724/SP.J.1245.2013.00263
Luo, Y., Zhong, M. J., Huang, Y., Li, F., Liao, W. B., Kotrschal, A., et al. (2017). Seasonality and brain size are negatively associated in frogs: Evidence for the expensive brain framework. Sci. Rep. 7:16629. doi: 10.1038/s41598-017-16921-1
Ma, X. H., Zhong, M. J., Jin, L., Mi, Z. P., and Liao, W. B. (2016). Digestive tract adaptation associated with temperature and precipitation in male Bufo andrewsi. Anim. Biol. 66, 279–288. doi: 10.1163/15707563-00002504
McNamara, J. M., and Houston, A. I. (1990). The value of fat reserves and the tradeoff between starvation and predation. Acta. Biotheor. 38, 37–61. doi: 10.1007/BF00047272
Müller, J., Bässler, C., Essbauer, S., Schex, S., Müller, D. W. H., Opgenoorth, L., et al. (2014). Relative heart size in two rodent species increases with elevation: Reviving Hesse’s rule. J. Biogeogr. 41, 2211–2220. doi: 10.1111/jbi.12365
Naya, D. E., and Bozinovic, F. (2006). The role of ecological interactions on the physiological flexibility of lizards. Funct. Ecol. 20, 601–608. doi: 10.1111/j.1365-2435.2006.01137.x
Naya, D. E., Claudio, V., and Francisco, B. (2009). Gut size variation among Bufo spinulosus populations along an altitudinal (and dietary) gradient. Ann. Zool. Fenn. 46, 16–20. doi: 10.5735/086.046.0102
Peng, Z. W., Zhang, L. X., and Lu, X. (2022). Global gaps in age data based on skeletochronology for amphibians. Integr. Zool. 1–12. doi: 10.1111/1749-4877.12584
Piersma, T., Gudmundsson, G. A., and Lilliendahl, K. (1999). Rapid changes in the size of different functional organ and muscle groups during refueling in a long–distance migrating shorebird. Physiol. Biochem. Zool. 72, 405–415. doi: 10.1086/316680
R Core Team (2022). R: A Language And Environment For Statistical Computing. Vienna: R Foundation for Statistical Computing.
Sol, D., Bacher, S., Reader, S. M., and Lefebvre, L. (2008). Brain size predicts the success of mammal species introduced into novel environments. Am. Nat. 172:S63–S71. doi: 10.1086/588304
Sol, D., and Lefebvre, L. (2000). Behavioral flexibility predicts invasion success in birds introduced to New Zealand. Oikos 90, 599–605. doi: 10.1034/j.1600-0706.2000.900317.x
Stearns, S. C. (1989). The evolutionary significance of phenotypic plasticity. Bioscience 39, 436–445. doi: 10.2307/1311135
Tang, T., Luo, Y., Huang, C. H., Liao, W. B., and Huang, W. C. (2018). Variation in somatic condition and testis mass in Feirana quadranus along an altitudinal gradient. Anim. Biol. 68, 277–288. doi: 10.1163/15707563-17000142
Tucker, A., and Horvath, S. M. (1973). Relationship between organ weight and blood flow in rats adapted to simulated high altitude. Aerosp. Med. 44, 1036–1039.
van Woerden, J. T., van Schaik, C. P., and Isler, K. (2010). Effects of seasonality on brain size evolution: Evidence from strepsirrhine primates. Am. Nat. 176, 758–767. doi: 10.1086/657045
van Woerden, J. T., Willems, E. P., van Schaik, C. P., and Isler, K. (2011). Large brains buffer energetic effects of seasonal habitats in catarrhine primates. Evolution 66, 191–199. doi: 10.1111/j.1558-5646.2011.01434.x
Yang, S. N., Huang, X. F., Zhong, M. J., and Liao, W. B. (2017). Geographical variation in limb muscle mass of the Andrew’s toad (Bufo andrewsi). Anim. Biol. 67, 17–28. doi: 10.1163/15707563-00002518
Yu, X., Zhong, M. J., Li, D. Y., Jin, L., Liao, W. B., Kotrschal, A., et al. (2018). Large–brained frogs mature later and live longer. Evolution 72, 1174–1183. doi: 10.1111/evo.13478
Zamora-camacho, F. J. (2021). Sex and habitat differences in size and coloration of an amphibian’s poison glands match differential predator pressures. Integr. Zool. [Epub ahead of print]. doi: 10.1111/1749-4877.12597
Zedda, M., Sathe, V., Chakraborty, P., Palombo, M.R., and Farina, V. (2021). A first comparison of bone histomorphometry in extant domestic horses (Equus caballus) and a Pleistocene Indian wild horse (Equus namadicus). Integr. Zool. 15, 448–460. doi: 10.1111/1749-4877.12444
Zeng, Y., Lou, S. L., Liao, W. B., Jehle, R., and Kotrschal, A. (2016). Sexual selection impacts brain anatomy in frogs and toads. Ecol. Evol. 6, 7070–7079. doi: 10.1002/ece3.2459
Zhao, L., Chen, C., and Liao, W. B. (2017). No evidence for trade–off between clutch size and egg size in the spot–legged treefrog (Polypedates megacephalus). North West. J. Zool. 13, 58–62.
Zhao, L., Mai, C. L., Liu, G. H., and Liao, W. B. (2019). Altitudinal implications in organ size in the Andrew’s toad (Bufo andrewsi). Anim. Biol. 69, 365–376. doi: 10.1163/15707563-00001068
Keywords: phenotypic plasticity, Hesse’s rule, organ size, Bufo andrewsi, oxygen supply
Citation: Zhu X, Chen C, Jiang Y, Zhao L and Jin L (2022) Geographical variation of organ size in Andrew’s toad (Bufo andrewsi). Front. Ecol. Evol. 10:972942. doi: 10.3389/fevo.2022.972942
Received: 19 June 2022; Accepted: 18 July 2022;
Published: 04 August 2022.
Edited by:
Cui Wang, University of Helsinki, FinlandReviewed by:
Bao-jun Sun, Institute of Zoology (CAS), ChinaCopyright © 2022 Zhu, Chen, Jiang, Zhao and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhao, bGl6aGFvXzY4OEAxNjMuY29t; Long Jin, bG9uZ2ppbjA3QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.