
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 08 December 2022
Sec. Population, Community, and Ecosystem Dynamics
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.972176
This article is part of the Research TopicNeotropical Dung Beetle Diversity: Ecological, Historical, and Anthropogenic PerspectivesView all 11 articles
Beetles of the subfamily Scarabaeinae are commonly used as ecological indicators in studies about the effects of environmental changes. We analyzed the influence of the type of habitat, vegetation, mammals (as food resource), and temperature on dung beetle metacommunities in subtropical native forests and Pinus monocultures to evaluate the factors driving these assemblages. In the summer of 2018/2019, we sampled 12 areas in Southern Brazil, six Pinus monocultures and six native forests. We performed a dispersal test, applying a marking-recapture method. Some recaptures occurred in different habitats, showing low dispersal between habitats. We recorded behavioral activities confirming the use of both native forest and Pinus areas. The metrics did not reflect the difference in the environmental quality of the areas regarding species richness and diversity in different habitats. This shows that these metrics are not the best when using dung beetle assemblages as ecological indicators of biodiversity loss resulting from land-use changes, requiring complementing the analysis with composition analysis methods. When we partitioned beta diversity between habitats, we observed a dissimilarity between Pinus monocultures and native forest assemblages due to species substitution, with many species contributing to the dissimilarity between habitats. In our structural equation models, the influence of environmental factors on metacommunities showed no predictor related to dung beetle richness, but several variables influenced their abundance.
Beetles of the subfamily Scarabaeinae, commonly called dung beetles, have been used in several studies as ecological indicators of habitat disturbance since their first proposal by Halffter and Favila 1993 (Davis and Sutton 1998; Davis et al. 2001; Gardner et al. 2008; Audino et al. 2014; Sarmiento-Garcés and Hernández 2021). The advantages of using this taxon are many since they are abundant in a wide range of terrestrial ecosystems (Mcgeoch et al., 2002; Davis et al., 2004; Hernández et al., 2014), are easy to sample, have a quick response to environmental disturbance (Gardner et al., 2008), and, especially, because of their species specificity to different habitats (Hanski and Cambefort, 1991; Scholtz et al., 2009). They can also be used to explore species-functioning relationships (Sarmiento-Garcés and Hernández, 2021), as they provide ecosystem functions by actively participating in nutrient cycling, promoting soil aeration, and removal of decaying organic matter (Halffter and Matthews, 1966; Nichols et al., 2008).
We can interpret habitat fidelity of dung beetles as an evolutionary response to the high interspecific competition for resources, which are often limited and ephemeral (Hanski and Cambefort, 1991). These beetles use dung or other organic debris, such as carcasses and some decaying fruits, as food resources. High competition greatly influences their assemblage structure (Simmons and Ridsdill-Smith, 2011). Furthermore, there is also a correlation between dung beetles’ richness and mammals abundance and richness because of the amount and diversity of available resources (Halffter and Matthews, 1966; Estrada et al., 1999; Davis et al., 2002; Andresen and Laurance, 2007; Nichols et al., 2009; Barlow et al., 2010; Bogoni et al., 2016). Dung beetles have developed foraging techniques by using their olfactory cues to rapidly locate and choose resources, depending on each resource’s type, distance, and nutritional quality (Hanski and Cambefort, 1991). Nevertheless, most species of this group are generalist in feeding strategies (Larsen et al., 2008; Frank et al., 2018; Giménez Gómez et al., 2018).
In local assemblages, the high diversity of dung beetles is related to niche differentiation due to evolutionary pressures of interspecific competition, which led to behavior variation according to resource allocation, time of activity, body size, and other intraspecific differences such as sex and age (Hanski and Cambefort, 1991). The adaptive evolution inside the group allows the occupation and preference of many species for a specific type of habitat, with some exclusive to forest areas and others typical of open areas, like savannas and meadows (Hanski and Cambefort, 1991). These features of habitat partition allow dung beetles to occupy many types of environments, having a high degree of fidelity for a biotope or phytophysiognomy (Klein, 1989; Driscoll and Weir, 2005). Some studies show that some forest specialist species do not leave their habitat even with the supply of food resources in open areas nearby (Klein, 1989; Larsen et al., 2008).
The high competition and ephemeral nature of food resources suggest that dung beetles are probably good dispersers (Roslin and Viljanen, 2011). However, according to some studies, Scarabaeinae species with different sets of ecological traits differ in mean movement rate (Howden and Nealis, 1975; Peck and Forsyth, 1982), with only a few species traveling longer distances in the same habitat (Arellano et al., 2008; Da Silva and Hernández, 2015). This dispersion ability in searching for resources through poorly suited habitats and the tolerance to remain in sub-optimal environments can be an important factor in their species reproduction. Some species from open areas may even have a higher tolerance to microclimatic changes, being able to enter and inhabit degraded forests and vice versa, changing the assemblage composition of these areas. As a result, forested areas adjacent to open habitats can present a high turnover of species composition, where open habitat specialists increase the alpha diversity of these disturbed places (Arellano and Halffter, 2003; Gardner et al., 2008). Although remnants of native forests allow forest-associated dung beetles and other animals to survive in patchy landscapes (Halffter and Arellano, 2002; Arellano and Halffter, 2003; Andresen, 2005; Campos and Hernández, 2015), habitat modification is often related to species loss, especially the ones with larger body size (Gardner et al., 2008; Batilani-Filho and Hernández, 2017; Sarmiento-Garcés and Hernández, 2021).
When using dung beetles as ecological indicators, we hope to obtain reliable measurements and interpretations regarding changes in environmental conditions by their presence and abundances in a particular area (Nichols and Gardner, 2011). For that, richness and diversity indexes are often used as measures to assess assemblage changes, considering different species as equal in their contribution to ecosystem functioning (Barragán et al., 2011). However, when we try to understand how dung beetle assemblages vary between habitats (such as natural and anthropogenic), including species compositon, it is possible to better understand how human actions can transform its dynamics, structure, and behavior.
We also point here to the importance of considering the spatial scale when using dung beetles as ecological indicators since they can disperse between different areas. In this study, we try to look beyond the diversity of each location, seeking a better understanding of the dynamics of these assemblages and the metacommunities formed between them. In metacommunity ecology, the local scale and a combination of local and regional processes matter to understand patterns of species abundance, occurrence, composition, and diversity in different scales of space and time (Chase et al., 2020). The formation of dung beetle metacommunities is strongly marked by the habitat (model known as species sorting). Still, it can also follow the mass effect model, where the rescue of species from competitive exclusion is marked by dispersal of individuals between areas with different environmental qualities (Leibold et al., 2004). In this case, individuals depart from sites considered to be of better quality to areas of worse resource quality, resulting in some environments working as sources and others as sinks. The mass effect can be more significant in species with high dispersal capacity or smaller spatial extensions due to habitat proximity, regardless of environmental quality (Heino et al., 2015).
Many studies indicate the exotic trees from the genus Pinus as invaders and their potential to inhibit the growth of other plant species, negatively affecting local and regional biodiversity (Rejmánek and Richardson, 1996; Brewer, 1998; Ledgard, 2001; Buckley et al., 2005; Richardson, 2006; Essl et al., 2011; Gundale et al., 2014). Studies regarding the composition and dynamics of dung beetle assemblages in Pinus monocultures shows that some species can inhabit mature Pine plantation areas (Peyras et al., 2013; Pryke et al., 2013). Furthermore, when forestry plantations are connected to natural forest, a negative effect may not be registered (López-Bedoya et al., 2021). It is possible that certain aspects of the environmental characteristics of Pinus monocultures, such as temperature and canopy cover, may not show such discrepant microclimatic changes when compared to native forest areas, as occurs in open fields. There are studies that showed high diversity and abundance of dung beetles in land-uses that preserve tree canopy (Bustamante-Sánchez et al., 2004; Braga et al., 2012; Gómez-Cifuentes et al., 2017; Giménez Gómez et al., 2018). Therefore, in cases where habitats modified for forestry use present similar microclimatic conditions to native habitats, it would be important to understand under which conditions dung beetles can be used as ecological indicators.
This paper hypothesizes that the local assemblages of dung beetles in Pinus monocultures and the native forest remnants are connected by dispersion constituting metacommunities. The factors that allow dung beetles to inhabit both Pinus monocultures and native forest areas would be the presence of food resources, as a product of the transit of mammals and attendance of domestic animals, and similar microclimatic conditions suitable for their occurrence. Therefore, we aim to understand if the structuring of local dung beetle assemblages in Pinus monocultures resembles the nearby native forest dung beetle assemblages. We first verified the species’ dispersal and fidelity to the different habitats. Then we looked for the factors that may drive them, relating dung beetle assemblages to factors known to influence their ecology, such as food resources availability, microclimate conditions, and vegetation structure variables (Halffter and Arellano, 2002).
We developed this study in the microregion of Tabuleiro in the State of Santa Catarina, southern Brazil, which includes the counties of Anitápolis, Rancho Queimado, Alfredo Wagner, Águas Mornas, and São Bonifácio (27°54′25.25″ S, 49°10′48.3″ W). This region has rugged topography, with elevation ranging between 440 and 1,000 m a.s.l., and native vegetation mainly composed of a dense ombrophilous forest. The landscape is a heterogeneous mosaic of forest patches that vary in size, density, and connectivity, immersed in a matrix of forestry, pastures, and small crop fields. The climate is Cfa according to the Köppen-Geiger classification, with rainfall well distributed in average annual rainfall of 1,700 mm.
We selected six sample sites that presented two landscape components in this region, Pinus monocultures and a native forest area (Figure 1). Thus, sampling was performed in six areas with Pinus monocultures (P1, P2, P3, P4, P5, P6) paired with six native forest areas (F1, F2, F3, F4, F5, F6), totaling 12 sampling areas. We selected the sites based on their accessibility and degree of isolation of the Pinus monocultures in relation to the forest fragments. Thereby, three of the Pinus monocultures areas (P1, P2, and P3) were connected to the native forest and the other three monocultures (P4, P5, P6) were at least 60 meters away from the native forest areas. There were open fields with small bushes between the three areas apart. All six areas were at least 1 km apart from each other. We conducted the fieldwork during November–December of 2018 and January–February 2019.

Figure 1. General view of the landscape and the six sample sites, each with two areas of different habitat (one of Pinus monocultures and one of native forest), in Santa Catarina, southern Brazil.
We placed 10 attraction traps in each area to test if dung beetles build nests for reproduction in both habitats and the dispersion between different habitats (Pinus monocultures and native forest). Those traps, named “nesting houses,” consist of PVC pipes buried vertically on the ground, with an opening on top for free access of the dung beetles. The traps were filled with soil and dog feces as a food resource. The nesting houses were placed 10 meters from each other at 10 meters from the edge of the habitat (Supplementary Figure S1). We applied the following marking and release protocol after 48 h of exposure: first, we cleaned and identified the collected Scarabaeinae, then marked them with scarification on the pronotum using a dental drill added to a small battery, subsequently releasing them near the trap in the same areas where they were captured. The species were identified by comparison using a reference collection of regional species from the Entomological Collection Mítia Heusi-Silveira of the Universidade Federal de Santa Catarina (Hernández et al., 2019). The scarification marking technique used is considered noninvasive and does not risk being lost by the insect as some paints (Wuerges and Hernández, 2020). The mark had the shape of a line and allowed us to identify the habitat where the beetle was first found, Pinus monocultures on the left side of the pronotum, and native forest on the right. We marked only species with body lengths of 4 mm or higher. We replicated the marking-release protocol once after a month from the first sampling. After 3 weeks from that second sampling, we conducted one last attempt to recapture marked individuals.
With the nesting house data, we first confirmed the use of the different habitats (Pinus monocultures and native forest) by the dung beetle species found there. Afterward, we calculated recapture rates for each species with one or more recaptured individuals. We then analyzed the recaptures that occurred outside the original marking habitats to observe the dispersion of the dung beetles between the different habitats (Pinus monocultures and native forests).
Parallel to the first experiment of the nesting houses, we installed 10 pitfall traps in the same 12 areas to capture dung beetles (Supplementary Figure S1). We placed those pitfall traps 40 meters away from the nesting houses, remaining 50 meters from the edge of sampled areas. The sampling design consisted of 10 pitfall traps distributed in pairs into two parallel transects, one pair trap spaced 100 meters apart from the other to avoid pseudoreplication. Paired traps were spaced 10 m apart and had different types of bait, one with human feces (20 g) and the other with two-day rotten meat (20 g), for the attraction of both coprophagous and necrophagous species, respectively. The pitfall traps consisted of plastic containers (15 cm diameter × 20 cm depth), buried with the top edge leveled to the ground, allowing insects to fall in. All traps contained water (300 ml) and neutral detergent, with a plastic lid supported by wooden sticks placed approximately 10 cm above their opening for rain protection. The lid prevented overflow and supported the bait. We replicated sampling three times, once a month, during 3 months (December 2018 and January–February 2019) on all 12 areas. All traps remained in the field for 48 h, after which we took the collected material to the Laboratório de Ecologia Terrestre Animal (LECOTA/UFSC). There, we mounted dung beetle individuals on entomological pins, dried in an oven (40°C for 48 h), identified, and included them in the Coleção Entomológica Mítia Heusi-Silveira from the Centro de Ciências Biológicas of the Universidade Federal de Santa Catarina. The expert Dr. Fernando Vaz-de-Mello, from the Universidade Federal de Mato Grosso, Brazil, confirmed the identifications.
We classified species into generalists and specialists of the two habitats using a multinomial classification model based on an iterative program (CLAM). The program estimated the species’ relative abundance in the two types of habitats (Pinus monocultures and native forest), allowing a robust statistical classification of habitat specialists and generalists without excluding rare species (Chazdon et al., 2011). We used the R package “vegan” for this analysis (Oksanen et al., 2020).
We used rarefaction and extrapolation curves with the effective number of species to compare and estimate species richness, diversity, and sample sufficiency between different habitats of each site. This method is based on Hill numbers and sets up intervals of confidence around species richness (q = 0), Shannon entropy (q = 1), and Simpson dominance (q = 2; Chao et al., 2014; Hsieh et al., 2016). The baseline sample size was the highest or double of the smallest sample size, the interval of confidence was 95%, and the analysis was performed using the R package “iNEXT.”
To verify differences in composition among assemblages of native forest and Pinus monocultures, we used the Bray-Curtis index of dissimilarity, partitioned into two components: balanced variation in abundance and abundance gradients. The balanced variation in abundance is related to individuals of some species in one site substituted by the same number of individuals of different species in another site. The second component of the partition concerns the loss of individuals from one site to another (Baselga, 2013). We used the R package “betapart” for this analysis. Subsequently, we compared the dissimilarities between the sites with adjacent habitats and the ones with habitats apart using a t-test to see if there were major differences among the areas.
Finally, we partitioned total beta diversity into species contributions to beta diversity (SCBD), which is the degree of variation of individual species across the study area, to test the relative importance of each species affecting beta-diversity patterns per site. The analysis was based on abundance data (Legendre and De Cáceres, 2013). All analyses were performed in the R 3.6.3 program (R Core Team, 2020).
We used camera traps to record mammalian presence inside the areas for dung beetle resource availability. We placed one camera trap in the central point of each area during dung beetle sampling and checked the batteries every 20 days. The cameras were active for a minimum of 30 days and a maximum of 60 days. We only considered the records made during 1 month for all locations. After that period, we identified the mammals from the photographs. The mammalogist Dr. Mauricio E. Graipel from the Universidade Federal de Santa Catarina, Brazil, confirmed the species identification. After the mammals identification, we calculated an approximation on the abundance of mammals as a way of checking the resources available in each area. For that, we considered only records of mammals from the same species that had at least one-hour difference each and the number of individuals of the same species in each record when in groups.
We measured environmental variables on each area related to vegetation using an adapted point-centered quarter method (Cottam and Curtis, 1956). We placed a plastic pipe cross in the center of every two sampling points in each study area, dividing them into four quadrants: northwest, southwest, southeast, and northeast. Then, we measured the distance from the nearest tree, from the nearest shrub, and their height for each quadrant. Additionally, we visually estimated the percentage of vegetation cover and bare ground in 1m2 plots in each quadrant. We considered the shrubs with a minimum height of 1 m and, for trees, the height over 1 m and the diameter at breast height over five centimeters. We measured the circumferences and distances with a tape measure.
Temperatures (in oC) were measured throughout the experiment using an environmental thermometer (datalogger) installed in the central point of each sampling site, buried in the ground. Geographical coordinate data (UTM) of each site and the sampled points were obtained using a manual GPS. We used the Google Earth Path software to measure the altitude and size of the sites and the distance between the isolated sites of Pinus monocultures and native forest.
We used structural equation models (SEM) to evaluate the relationships of environmental factors on dung beetle assemblages (Grace, 2006; Shipley, 2016). Thus, we built a conceptual model employing dung beetle richness and abundance as response variables. For the explanatory variables, we had temperature, vegetation structure (trees for tall vegetation structure, shrubs for middle vegetation structure, and herb cover for the ground level), and the number of times mammals were recorded (as a form to measure the amount of food resources available to dung beetles). This model could determine which explanatory variables would influence other variables, with hierarchical submodels influencing the final result. The regressions between variables were performed using Piecewise SEM in the R 3.6.3 program (R Core Team, 2020). All variables with a probability below 0.05 were included in a structural frame, where we estimated the coefficient for each equation in the model. Then, we highlighted the positive and negative relationships using arrows, with sizes according to the coefficient value of each variable relationship.
We marked and released 883 live individuals belonging to 19 species, all captured in the nesting houses (Supplementary Table S1). We recorded feeding balls from telecoprids and tunnels with paracoprids inside the traps, indicating that dung beetles use both native forests and Pinus monocultures to feed and bury resources for nesting. The species with the highest number of marked and released individuals were Dichotomius sericeus (Harold, 1867)(236), Canthon rutilans cyanescens Harold, 1868 (133), Coprophanaeus saphirinus (Stürm, 1828) (122), and Dichotomius assifer (Eschscholtz, 1822) (102). During 20 to 76 days, we recaptured 18 individuals from three species only: Canthon rutilans cyanescens, Dichotomius sericeus, and Dichotomius assifer, with an overall recapture rate of 3.82 (Table 1).
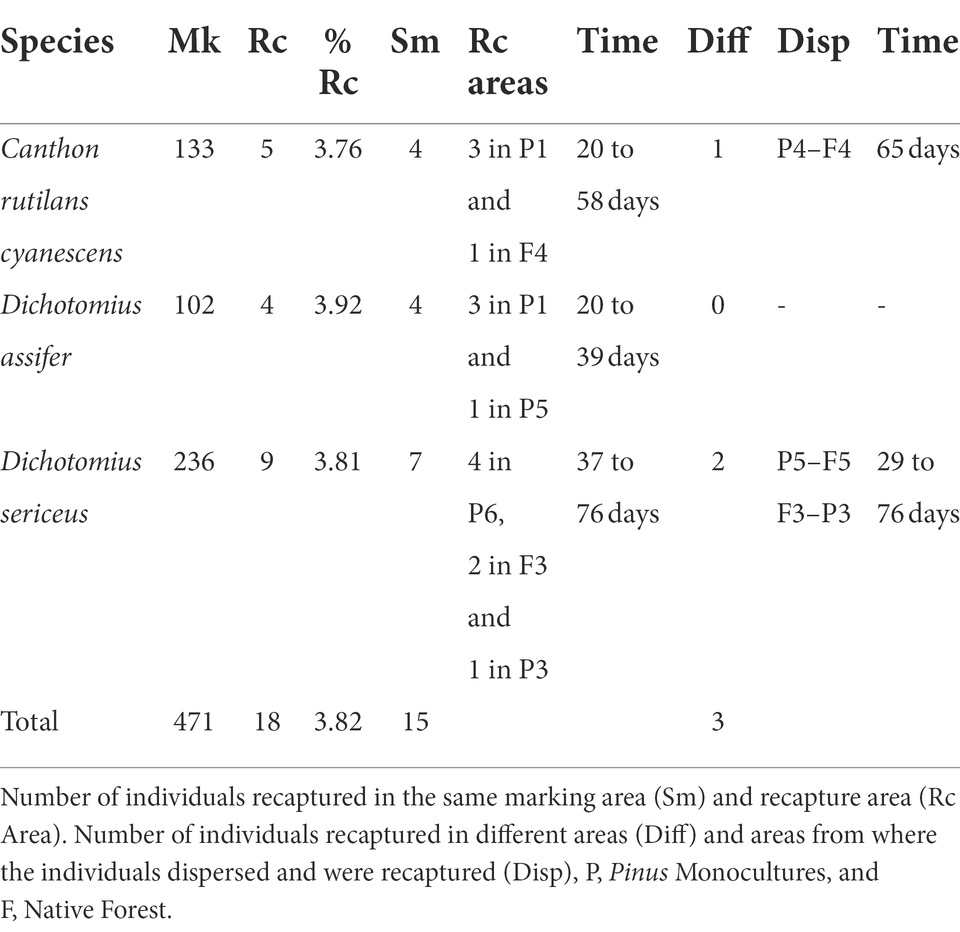
Table 1. Number of marked (Mk) and recaptured (Rc) individuals and total recapture rate (% Rc) per species.
Of those individuals, 15 were recaptured in the same marking areas, 12 in Pinus monocultures and three inside native forests. Three individuals from two species were found in different areas from where they were marked, showing their ability to move between habitats, marked on Pinus monocultures and found in native forests (Table 1). One hundred and thirty-three individuals of the species Canthon rutilans cyanescens were marked five were recaptured. Among these, three were recaptured in the same marking area (Pinus monocultures P1) and one was found in a different habitat, being marked in the Pinus monocultures area and recaptured 2 months later 180 meters into native forest area (from P4 to F4). For D. assifer, 102 individuals were marked and four were recaptured, all in the same marking areas: inside Pinus monocultures (three in P1 and one in P5), of which one was recaptured in 20 days, another in 36, and the last two in 58 days. Dichotomius sericeus presented the highest number of individuals marked (236) and recaptured (9). Seven of these were found in the same marking areas, both in Pinus monocultures and native forests (four in P6, one in P3, and two in F3). The other two individuals were found in areas different from where they were first captured and marked, going from Pinus monocultures to native forest and vice-versa (from F3 to P3 at a distance of 180 meters, and from P5 to F5, at 130 m). One of the recaptures happened in a pitfall trap (from F3 to P3). These results show the species dispersion capacity inside and between both habitats.
We collected 3,222 dung beetles belonging to 41 species (Supplementary Table S2). The three most abundant species were Dichotomius sericeus (19.11%), Eurysternus inflexus (Germar, 1824) (14.83%), and Deltochilum morbillosum Burmeister, 1848 (13.22%), which together represented 47.16% of the total individuals captured. The rare species, with only one individual collected, were Canthidium aff. taurinum (Harold, 1867), Canthidium femoratum Boucomont, 1935 and Canthon oliverioi (Pereira and Martínez, 1956). Scatonomus fasciculatus Erichson, 1835 and Sulcophanaeus radamanthus (Harold, 1875) had two individuals captured each.
Seven of the collected species were found in all sampled areas: Deltochilum morbillosum, Dichotomius assifer, Dichotomius sericeus, Canthidium aff. trinodosum (Boheman, 1858), Coprophanaeus saphirinus, Eurysternus inflexus, and Phanaeus splendidulus (Fabricius, 1781). In contrast, we collected six species in only one of the 12 areas: Dichotomius opalescens (Felsche, 1910) (P4), Canthidium aff. taurinum (F3), Canthidium dispar (Harold, 1867; F2), Canthidium femorale (F2), Canthon oliverioi (F2), and Sulcophanaeus radamanthus (F2).
According to the multinomial classification analyses, only four species were native forest specialists: Canthidium aff. trinodosum, Canthon angularis Harold 1868, Paracanthon aff. rosinae Balthasar, 1942, and Uroxys terminalis Waterhouse, 1891, and seven were Pinus monocultures specialists: Canthidium sp.1, Canthon lividus seminitens Harold 1868, Canthon rutilans cyanescens, Deltochilum multicolor Balthasar, 1939, Deltochilum rubripenne (Gory, 1831), Eurysternus inflexus, and Onthophagus tristis Harold, 1873. This analysis showed 16 habitat generalist species inhabiting both Pinus monocultures and native forest habitats, reaffirming that many species occupy both habitats. Fourteen species were considered too rare to be confidently classified (Figure 2).
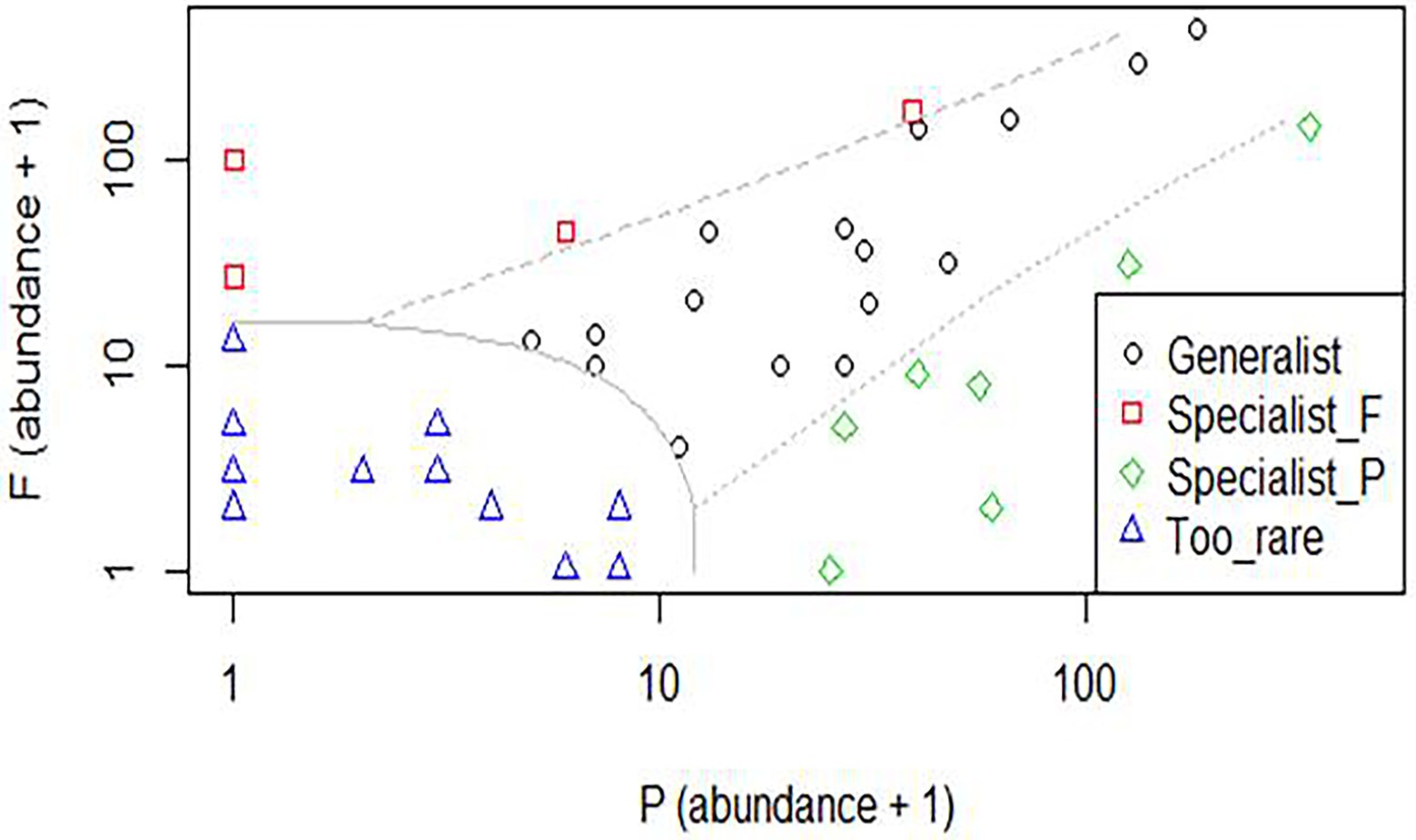
Figure 2. Species classification with data collected in habitats of native forest and Pinus monocultures in the State of Santa Catarina, southern Brazil. Specialist_F, native forest specialist; Specialist_P, Pinus monocultures specialist.
The abundance of dung beetles found per type of habitat was 1,351 individuals from 32 species in the Pinus monocultures and 1,844 dung beetles from 38 species in the native forest areas (Supplementary Figure S1). The extrapolated species accumulation curves for each area showed sampling sufficiency since all curves reached the asymptote (sample coverage over 95% for all the sampling areas). Species richness (q = 0) was similar between Pinus monocultures and native forests in all six sites, with overlapping intervals of confidence (Figure 3).
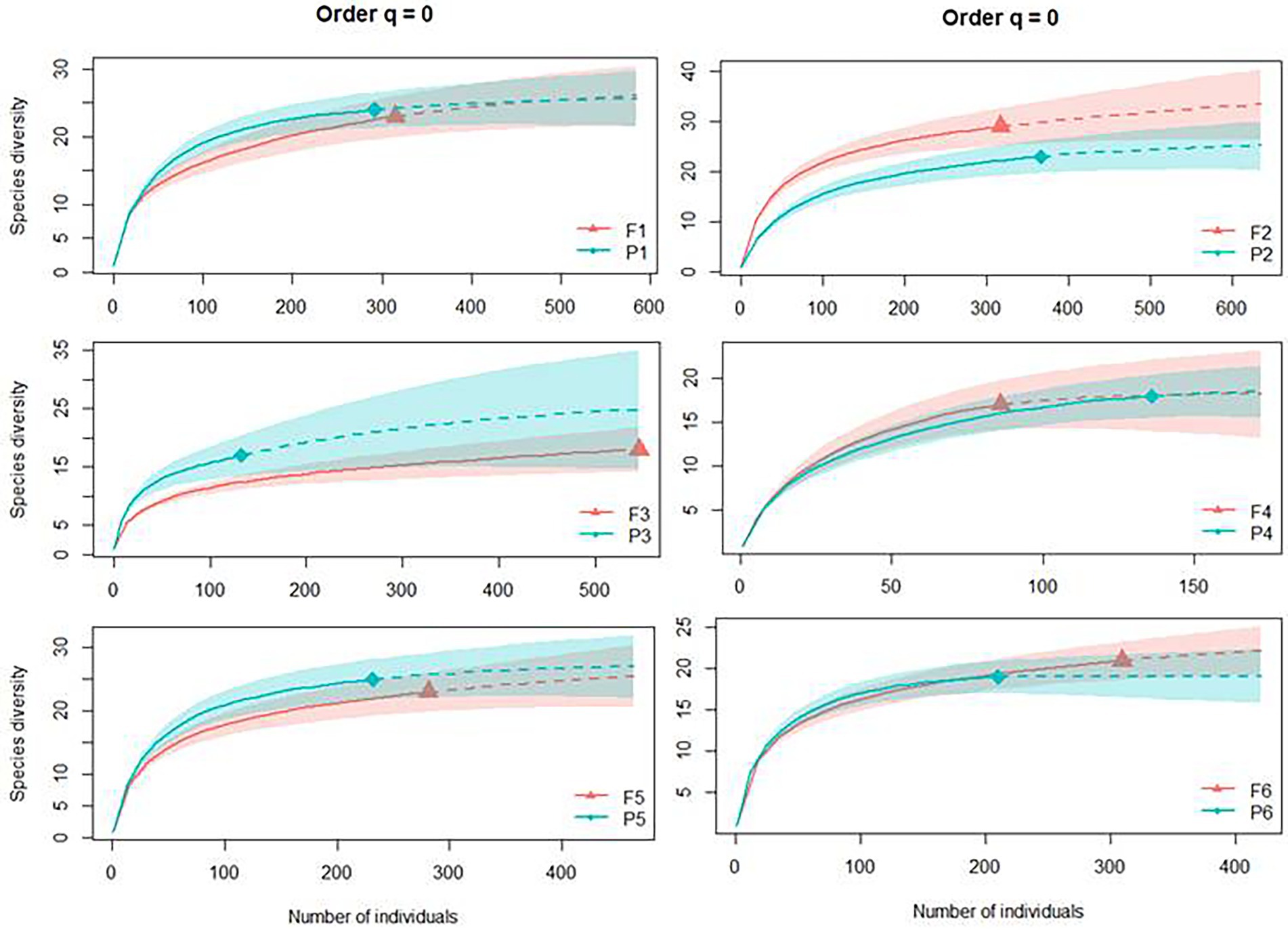
Figure 3. Individual-based rarefaction (solid lines) and extrapolation (dashed lines, up to double the smallest sample size) of dung beetle species richness for Hill number (q = 0) for each site, where F represents native forests and P represents Pinus monocultures.
Including abundance data in the analyses (Shannon entropy exponential, q = 1), we can see that the number of typical species was the same in four of the six sites, with the same diversity measure between Pinus monocultures and native forest. Only Sites 2 and 3 presented opposite patterns, with the first having higher diversity in the forest (F2 with 15.12 typical species and P2 with 6.24) and the second in Pinus monocultures (P3 with 10.45 typical species and F3 with 5.51; Figure 4).
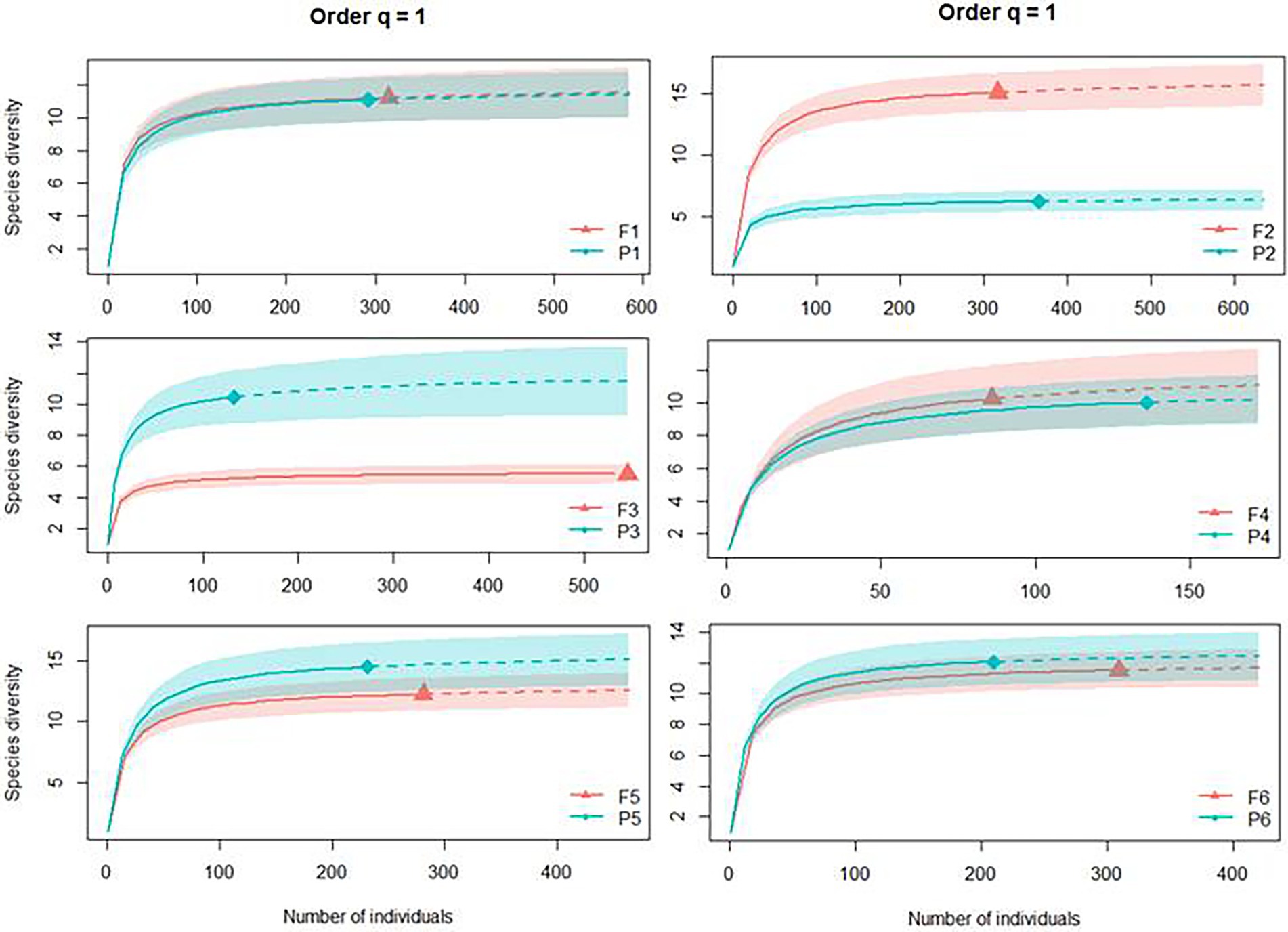
Figure 4. Individual-based rarefaction (solid lines) and extrapolation (dashed lines, up to double the sample size) of Dung beetle diversity for Hill numbers (q = 1) for each site, where F represents native forests and P represents Pinus monocultures.
The areas of Pinus monocultures and native forest statistically presented the same number of dominant species in four of the six sites from this study (Site 1, 4, 5, and 6) based on the Simpson’s dominance analyses (q = 2; abundant species). Site 2 presented more dominant species in the native forest habitat (F2 with 9.82 and P2 with 3.15), and Site 3 had more dominant species in the Pinus monocultures (F3 with 3.28 dominant species and P3 with 7.93; Figure 5).
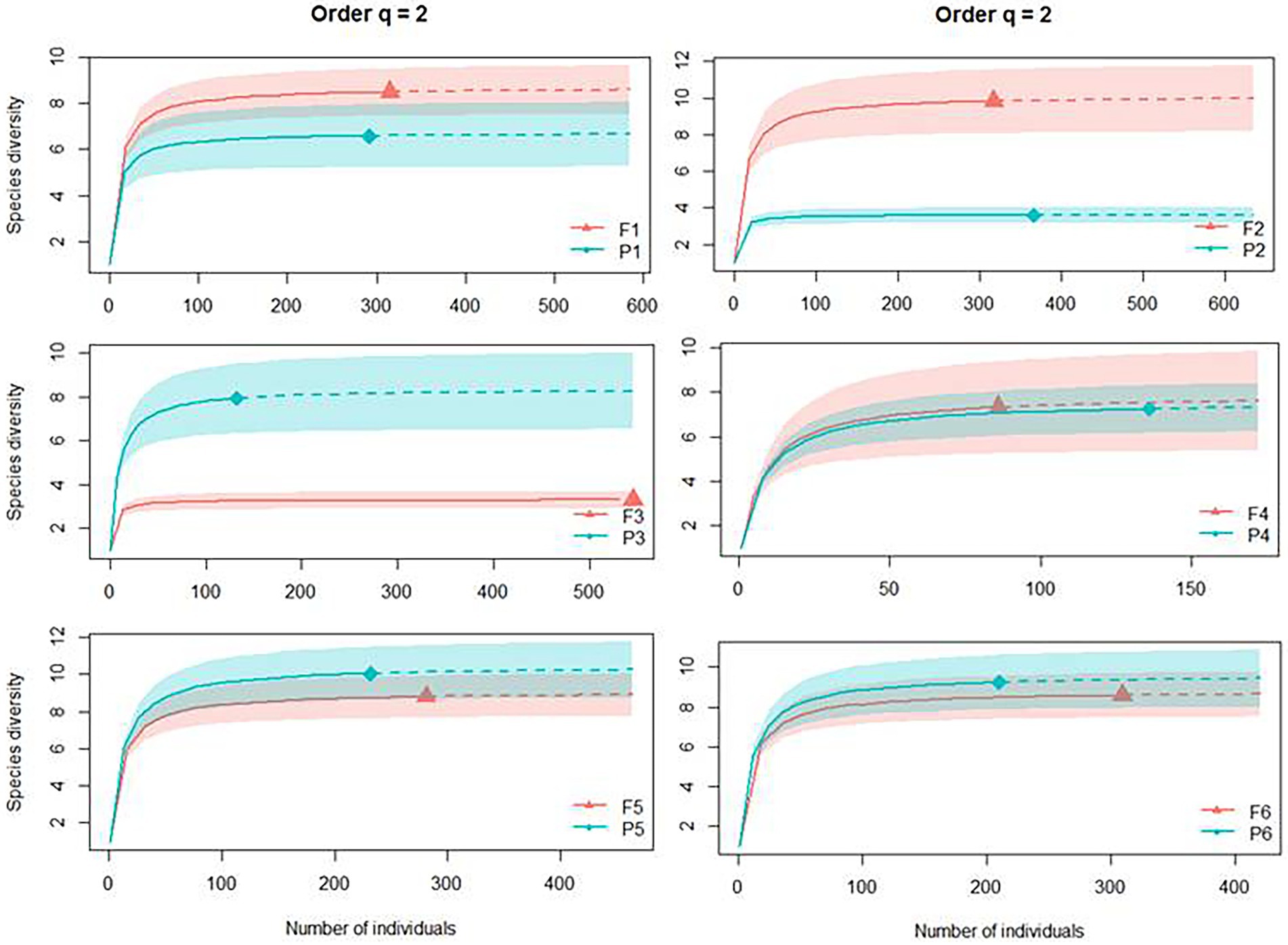
Figure 5. Individual-based rarefaction (solid lines) and extrapolation (dashed lines, up to double the sample size) of Dung beetle diversity for Hill numbers (q = 2) for each site, where F represents native forests and P represents Pinus monocultures.
Unlike the diversity analysis, which shows the two habitats with similar richness, beta diversity was very high between them. The Bray–Curtis dissimilarity index indicated a greater difference between the assemblages that inhabit Pinus monocultures and native forests, varying between 47% and 72% of dissimilarity (Supplementary Table S3). The partitioning of beta diversity showed that the dissimilarity due to balanced variation in abundance is higher in all areas (except in Site 3), showing that species composition and relative abundance of the assemblages in native forest areas differ from Pinus monocultures.
The dissimilarity did not vary according to the proximity of native forest and Pinus monocultures since both adjacent areas (Mean: 0.563, Sites 1, 2, and 3) and areas apart (Mean: 0.574, Sites 4, 5, and 6) had the same dissimilarity values when compared using a t-test (t = 0.104, df = 3.99, value of p = 0.92).
The analysis of species contribution to beta diversity (SCBD) indicated that 24 species are important contributors to beta diversity, whether for just one site or more (Supplementary Table S4). All SCBD values ranged from 0.035 to 0.299, and 14 species contributed to beta diversity above the overall mean (0.102). The species that most contributed to beta diversity were Eurysternus inflexus and Onthophagus tristis, more abundant in Pinus monocultures than in native forests, and Canthidium aff. trinodosum and Paracanthon aff. rosinae, more abundant in the native forest habitat (Supplementary Table S4). All 11 species classified as specialists are present in the SCBD results, reinforcing the multinomial classification analysis previously carried out (Figure 4). However, the SCBD analysis had 13 more species contributing to overall beta diversity, identified as generalists or too rare in the multinomial classification analysis.
As a result of camera trap records, we obtained 92 records of mammals belonging to 13 species (Supplementary Table S5). Nine species were native, with few records, occurring mainly in native forests. Three exotic species showed a large occurrence in Pinus areas, demonstrating the large supply of food resources that these habitats offer to dung beetles. These are: Bos taurus Linnaeus 1758, Equus caballus Linnaeus 1758, and Canis lupus familiaris Linnaeus 1758, the first two considered large.
The result obtained in measuring environmental variables, temperature, altitude, and tree density assessment (calculated as the average distance between trees) were very similar between Pinus monoculture and native forests (Supplementary Table S6). In the shrubs density assessment, we got lower values of distance between shrubs in the native forests when compared to Pinus monocultures, showing greater density of shrubs in those areas (understory). Lastly, the green cover percentage of the soil varied widely between areas, without following any apparent pattern.
The overall SEM model showed that none of the environmental factors significantly affected dung beetle richness, but several influenced dung beetle abundances (Figure 6). The variables temperature, vegetation structure (trees and herbs), and mammal abundance positively influenced the abundance of dung beetles. Temperature, with the most significant relationship with dung beetle abundance. Vegetation structure (shrubs) and mammal richness also influenced dung beetle abundance but in a negative way. Trees, shrubs and mammal richness had positive effect on the amount of resources.
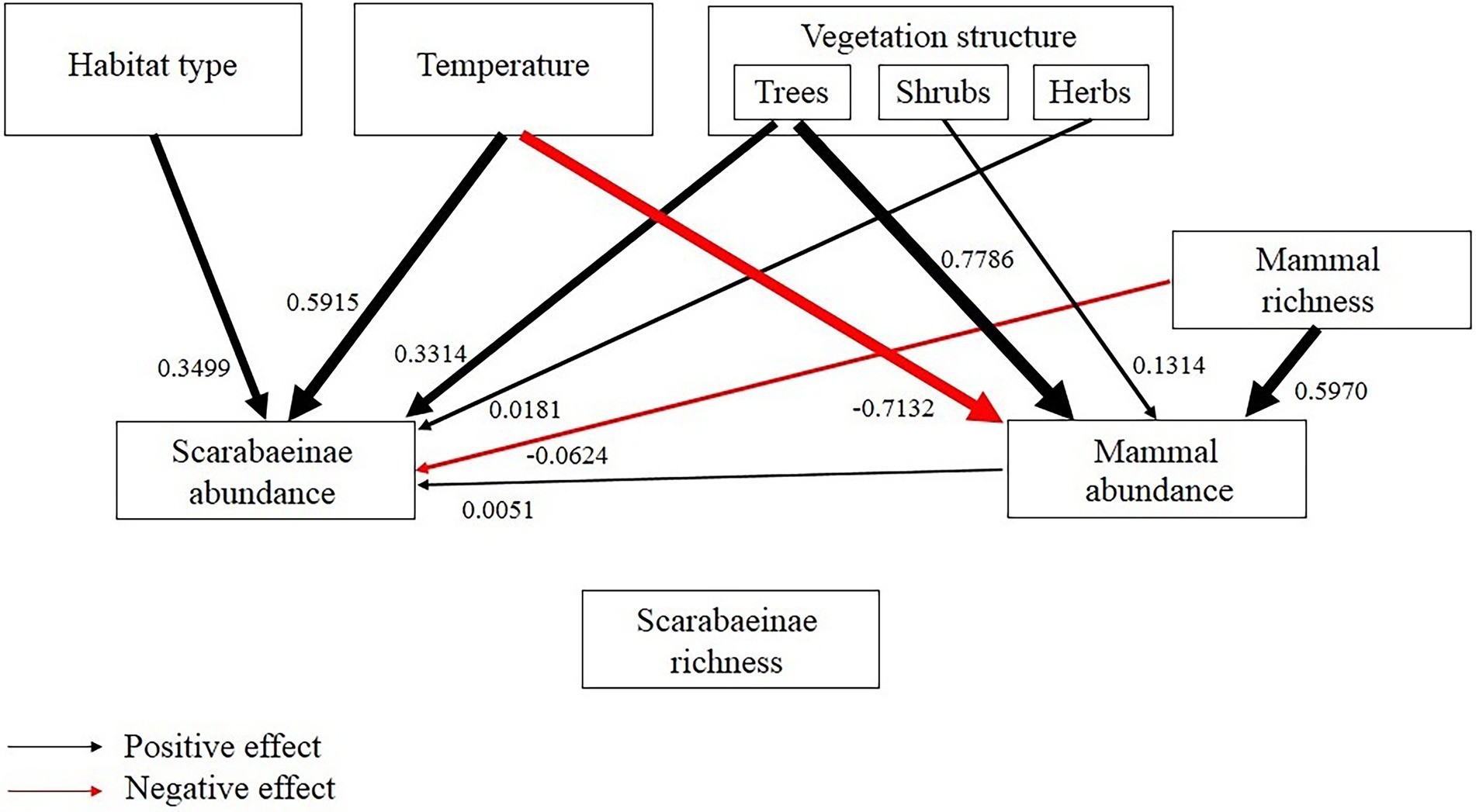
Figure 6. Structural equation model for dung beetle richness and abundance. The arrows indicate the significant influence of environmental factors and resources (with their respective coefficients) on dung beetle abundance. Black arrows denote positive relationships, and red arrows denote negative relationships.
The results from the marking-recapture experiment show a species sorting effect in dung beetle metacommunities, in which niche has more influence than dispersal, with low movement rates. We observed some movement of individuals between the habitats. Still, most dispersions were inside the same habitat, showing that dung beetle dispersal was insufficient to highly expand their distributions but was enough for the individuals to track the alternative resources present in the Pinus monocultures. Although we have a low rate of individuals recaptured, the recapture rate was similar to results presented in other studies (Arellano et al., 2008; Noriega and Acosta, 2011; Da Silva and Hernández, 2015). In addition to the dispersal between areas, the species found inside the nesting houses were effectively using the habitats since we registered the presence of feeding balls and tunnels within those traps in both habitats (Pinus monocultures and native forest).
The transit of dung beetles between one habitat and the other shows that they move through areas of Pinus monocultures and native forests, interacting and connecting with those assemblages. We can state that the coexistence of species within a regional level occurred due to the niche differentiation between them, causing high beta diversity (Leibold et al., 2004), differences that were not pointed out by richness and diversity indices. The species richness and diversity of dung beetles had close values in both Pinus monocultures and native forest habitats. A study in areas close to those of the present work, related to dung beetle taxonomic and functional diversity among native forests and altered subtropical habitats, also showed that forests and Pinus monocultures have similar richness values, different from open habitats (fields), which presented a great decrease of species richness and individual abundance (Sarmiento-Garcés and Hernández, 2021). These results contrast with the severe decline in biodiversity observed in other studied areas that suffered higher alteration levels (Nichols et al., 2007; López-Bedoya et al., 2022).
Then, considering species abundance and richness, as shown in the extrapolation curve analysis, it is impossible to observe significant differences between assemblages of different habitats, not being a reliable and sufficient approach. Moreover, even in cases where these measures serve to assess changing patterns of diversity, they remain limited in describing which species are lost and how this loss can alter ecosystem dynamics (Nichols et al., 2007). That’s because ecological indicators should provide reliable and interpretable information on the ecological consequences of human activities for a measured component of biodiversity (Nichols and Gardner, 2011). In the diversity partitioning analysis, we can see that there are differences in both assemblages, which are mainly due to the species variation in abundance. The Bray-Curtis index analysis shows that part of the dissimilarity between the areas of Pinus monocultures and native forests is due to the abundance of some species that prefer one or another habitat. This occurs because of the balance or shift of species between the areas. The composition of the assemblages is similar to a certain point, where some species become more specific to forest areas and others more linked to Pinus monocultures sites. These species, presented in the multinominal classification analyses as habitat-specific, are few compared to those considered generalists or too rare to be classified.
Furthermore, the structural equation models showed no influence of environmental factors or resource availability on dung beetle richness, with very close averages among habitats. On the other hand, many factors like habitat type, temperature, vegetation structure (trees and herbs), and mammal abundance positively influenced dung beetle abundance, with more emphasis on temperature. Thus, we can see important features for the maintenance of the dung beetles, such as proper temperature and the presence of resources. It is interesting to reflect on the presence of exotic animals, such as cattle, horses, and dogs, which greatly contributed to the occurrence and mammal richness, especially in the Pinus monocultures. These domestic animals can have a major effect on dung beetles assemblages, being an alternative as a potential source of resources, reflecting the mammal abundance in altered habitats. In this way, the dung beetle assemblage in the Pinus monocultures areas is affected by mammal composition, habitat structure, and spatial distance (Barlow et al., 2010; Bogoni et al., 2016).
The presence of exotic domestic mammal species mainly in monoculture areas reaffirms the anthropization of these areas. In addition, among all species recorded, two are large (Bos taurus and Equus caballus), contributing even more to the supply of resources. Many studies have shown that there is a positive relationship between dung beetles and the richness of omnivorous mammals, where the majority of the individuals being attracted by the feces of mammals from this trophic group (Estrada et al., 1993; Filgueiras et al., 2009; Bogoni et al., 2016). We took the opportunity to reflect on the limitations we found when analyzing mammal abundance as the amount of resources available because we know that the size of the identified mammals is not standard, with species of large, medium, and small size. Other points not included are the different trophic groups of mammals present in the areas (herbivores, carnivores, and omnivores) and necrophagous dung beetle preference for arthropod carcasses (Bogoni et al., 2016; Gimenéz Gómez et al., 2021).
Some studies suggest that very few forest dung beetles can extend their activity into strongly altered forests or natural open habitats (Nummelin and Hanski, 1989; Halffter and Arellano, 2002; Gardner et al., 2008; Gries et al., 2011). However, our results suggest that Pinus monocultures provide habitat for forest dung beetles. Canopy openness is an influential variable that structures dung beetle assemblages across all habitats and types of plantation (Hernández and Vaz-De-Mello, 2009; Barlow et al., 2010; Da Silva et al., 2018). Canopy cover can influence both soil humidity and surface temperature, which might affect the survival and reproduction of dung beetles and food availability and attractiveness (Gries et al., 2011). Thus, the conservation of either native or exotic canopy can determinate whether highly diverse dung beetle assemblages and their ecological functions are preserved or not (Giménez Gómez et al., 2018).
We remember the risk that Pinus monocultures pose to biodiversity in Brazil, which has more than 7 million hectares in homogeneous reforestation, with Pinus being one of the most representative in the southern region of the country (Anuário estatístico de base florestal para o estado de Santa Catarina, 2019). In addition to being exotic, the species has a high invasive potential and is well documented worldwide (Richardson, 2006). Additionally, some of its features, such as short juvenile period and numerous small, winged seeds that characterize them as pioneers in their native range, are responsible for their invasiveness (Rejmánek, 1996; Richardson, 2006). This way, Pinus trees can severely impact the local biota and ecosystem processes, such as changes in water and fire regimes (Simberloff et al., 2010).
In conclusion, through this work, we observed the lack of indication of the approach of dung beetle richness and diversity in Pinus monocultures and native forest, and to reflect on the need to complement the method with other composition analysis when using dung beetles as ecological indicators. This is due to the differences in the composition of the assemblages (species that prefer different types of habitats) being demonstrated only when we calculate beta diversity. We must consider this when using dung beetles as ecological indicators since we found similar indices in very different areas in terms of biodiversity. Thus, our results demonstrate that the use of species richness and diversity indices alone may not show real differences between assemblages in areas with distinct habitats, not reflecting the real environmental quality of the sites. Therefore, we must consider the differences in species composition of the assemblages between habitats. In this case, analyses like the ones of dissimilarity, SCBD, and multinomial classification complement each other, contributing to better understanding the dung beetle metacommunities. Still, according to these results, our structural model overall SEM shows that dung beetle richness in both types of habitats is not influenced by environmental factors, although dung beetle abundance is. We also conclude that there was no relationship between the composition and abundance of dung beetle assemblages in monocultures to the distance of the native forest areas in this study. However, the presence of native forests and other habitats very likely provides individuals to the Pinus monocultures. On the other hand, these monocultures have characteristics similar to the native forests, such as temperature, humidity, canopy cover, and alternative food resources, which allow the permanence of the beetles. We also emphasize that even though the Pinus monocultures allow the permanence of dung beetle assemblages with a richness similar to those found in areas of native forests, we should consider the invasive potential of this exotic species and the inhibitory effect on native plants, as well as other possible negative impacts on animal species in the region. Also, the high availability of food resources in Pinus monoculture areas with domestic animals is not an exclusive feature of all monocultures. The monoculture areas in this study belong to small farmers, with some presence of shrubs of other plant species (understory) and are used for purposes other than logging (such as resin extraction and cattle raising). Thus, we can consider that other Pinus monoculture areas, which are larger, more isolated, and aimed only at logging, with high plantation turnover, can provide a much less suitable environment for dung beetles. In this case, the richness and diversity indexes would most likely be more effective.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
We are grateful to the farmers and landowners for allowing us to enter their property for the fieldwork. We thank the members of the Laboratório de Ecologia Terrestre Animal (LECOTA-UFSC) for the help and support, Pedro Giovani da Silva (UFMG) and Eduardo Giehl (UFSC) for their help in the analyses, Fernando Vaz-de-Mello (UFMT) for confirming dung beetle identifications and Maurício Graipel (UFSC) for his help with mammal identifications. We also thank the financial support of the Programa de Pós Graduação em Ecologia (POSECO/UFSC), the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) for the scholarship awarded to TROSC, and the Conselho Nacional de Desenvolvimento Científico e Tecnológico, Ministério da Ciência, Tecnologia e Inovação, Brazil (CNPq) for a Productivity Grant awarded to MIMH (Process: 307437/2017-5).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.972176/full#supplementary-material
Andresen, E. (2005). Effects of season and vegetation type on community organization of dung beetles in a tropical dry forest. Biotropica 37, 291–300. doi: 10.1111/j.1744-7429.2005.00039.x
Andresen, E., and Laurance, S. (2007). Possible indirect effects of mammal hunting on dung beetle assemblages in Panama. Biotropica 39, 141–146. doi: 10.1111/j.1744-7429.2006.00239.x
Anuário estatístico de base florestal para o estado de Santa Catarina (2019). Associação Catarinense de Empresas Florestais. Lages, SC, Brasil.
Arellano, L., and Halffter, G. (2003). Gamma diversity: derived from and a determinant of alpha diversity and beta diversity. An analysis of three tropical landscapes. Acta Zoológica Mexicana 90, 27–76.
Arellano, L., León-Cortés, J. L., and Ovaskainen, O. (2008). Patterns of abundance and movement in relation to landscape structure: a study of a common scarab (Canthon cyanellus cyanellus) in southern Mexico. Landsc. Ecol. 23, 69–78. doi: 10.1007/s10980-007-9165-8
Audino, L. D., Louzada, J., and Comita, L. (2014). Dung beetles as indicators of tropical forest restoration success: is it possible to recover species and functional diversity? Biol. Conserv. 169, 248–257. doi: 10.1016/j.biocon.2013.11.023
Barragán, F., Moreno, C. E., Escobar, F., Halffter, G., and Navarrete, D.. (2011). Negative impacts of human land use on dung beetle functional diversity. PLoS One 6:e17976. doi: 10.1371/journal.pone.0017976
Barlow, J., Louzada, J., Parry, L., Hernández, M. I. M., Hawes, J., Peres, C. A., et al. (2010). Improving the design and management of forest strips in human-dominated tropical landscapes: a field test on Amazonian dung beetles. J. Appl. Ecol. 47, 779–788. doi: 10.1111/j.1365-2664.2010.01825.x
Baselga, A. (2013). Separating the two components of abundance-based dissimilarity: balanced changes in abundance vs. abundance gradients. Methods Ecol. Evol. 4, 552–557. doi: 10.1111/2041-210X.12029
Batilani-Filho, M., and Hernández, M. I. M. (2017). Decline of ecological functions performed by dung beetles in areas of Atlantic Forest and contribution of rollers and tunnellers in organic matter removal. Environ. Entomol. 46, 784–793. doi: 10.1093/ee/nvx091
Bogoni, J. A., Graipel, M. E., De Castilho, P. V., Fantacini, F. M., Kuhnen, V. V., Luiz, M. R., et al. (2016). Contributions of the mammal community, habitat structure, and spatial distance to dung beetle community structure. Biodivers. Conserv. 25, 1661–1675. doi: 10.1007/s10531-016-1147-1
Braga, R. F., Korasaki, V., Audino, L. D., and Louzada, J. (2012). Are dung beetles driving dung-fly abundance in traditional agricultural areas in the Amazon? Ecosystems 15, 1173–1181. doi: 10.1007/s10021-012-9576-5
Brewer, J. (1998). Patterns of plant species richness in a wet slash-pine (Pinus elliottii) savanna. J. Torrey Botan. Soc. 125, 216–224. doi: 10.2307/2997219
Buckley, Y. M., Brockerhoff, E., Langer, L., Ledgard, N., North, H., and Rees, M. (2005). Slowing down a pine invasion despite uncertainty in demography and dispersal. J. Appl. Ecol. 42, 1020–1030. doi: 10.1111/j.1365-2664.2005.01100.x
Bustamante-Sánchez, M. A., Grez, A. A., and Simonetti, J. (2004). Descomposición de heces y sus coleópteros asociados en un bosque templado fragmentado. Rev. Chil. Hist. Nat. 77, 107–120. doi: 10.4067/S0716-078X2004000100009
Campos, R., and Hernández, M. I. M. (2015). The importance of maize management on dung beetle communities in Atlantic Forest fragments. PLoS One 10:e0145000. doi: 10.1371/journal.pone.0145000
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K., et al. (2014). Rarefaction and extrapolation with hill numbers: a framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67. doi: 10.1890/13-0133.1
Chase, J. M., Jeliazkov, A., Ladouceur, E., and Viana, D. S. (2020). Biodiversity conservation through the lens of metacommunity ecology. Ann. N. Y. Acad. Sci. 1469, 86–104. doi: 10.1111/nyas.14378
Chazdon, R. L., Chao, A., Colwell, R. K., Lin, S., Norden, N., Letcher, S. G., et al. (2011). A novel statistical method for classifying habitat generalists and specialists. Ecology 92, 1332–1343. doi: 10.1890/10-1345.1
Cottam, G., and Curtis, J. T. (1956). The use of distance measures in phytosociological sampling. Ecology 37, 451–460.
Da Silva, P. G., and Hernández, M. I. M. (2015). Spatial patterns of movement of dung beetle species in a tropical forest suggest a new trap spacing for dung beetle biodiversity studies. PLoS One 10:e0126112. doi: 10.1371/journal.pone.0126112
Da Silva, P., Lobo, J., Hensen, M., Vaz-De-Mello, F., and Hernández, M. I. M. (2018). Turnover and nestedness in subtropical dung beetle assemblages along an elevational gradient. Divers. Distrib. 24, 1277–1290. doi: 10.1111/ddi.12763
Davis, A., Holloway, J., Huijbregts, H., Krikken, J., Kirk-Spriggs, A., and Sutton, S. (2001). Dung beetles as indicators of change in the forest of northern Borneo. J. Appl. Ecol. 38, 593–616. doi: 10.1046/j.1365-2664.2001.00619.x
Davis, A. L. V., Scholtz, C. H., Dooley, P. W., Bham, N., and Kryger, U. (2004). Scarabaeine dung beetles as indicators of biodiversity, habitat transformation and pest control chemicals in agro-ecosystems. S. Afr. J. Sci. 100, 415–424.
Davis, A. L. V., Scholtz, C. H., and Philips, T. K. (2002). Historical biogeography of Scarabaeinae dung beetle. J. Biogeogr. 29, 1217–1256. doi: 10.1046/j.1365-2699.2002.00776.x
Davis, A. J., and Sutton, S. L. (1998). The effects of rainforest canopy loss on arboreal dung beetles in Borneo: implications for the measurement of biodiversity in derived tropical ecosystems. Divers. Distrib. 4, 167–173. doi: 10.1046/j.1472-4642.1998.00017.x
Driscoll, D. A., and Weir, T. (2005). Beetle response to habitat fragmentation depends on ecological traits, habitat conditions and remnant size. Conserv. Biol. 19, 182–194. doi: 10.1111/j.1523-1739.2005.00586.x
Essl, F., Mang, T., Dullinger, S., Moser, D., and Hulme, P. (2011). Macroecological drivers of alien conifer naturalizations worldwide. Ecography 34, 1076–1084. doi: 10.1111/j.1600-0587.2011.06943.x
Estrada, A., Anzures, A. D., and Coates-Estrada, R. (1999). Tropical rain forest fragmentation, howler monkeys (Alouatta palliata) and dung beetles at los Tuxtlas, Mexico. Am. J. Primatol. 48, 253–262. doi: 10.1002/(SICI)1098-2345(1999)48:4<253::AID-AJP1>3.0.CO;2-D
Estrada, A., Halffter, G., Coates-Estrada, R., and Merrit, D. A.Jr. (1993). Dung beetles attracted to mammalian herbivore (Alouatta palliata) and omnivore (Nasua narica) dung in tropical rain forest of Los Tuxlas, Mexico. J. Trop. Ecol. 9, 45–54.
Filgueiras, B. K. C., Liberal, C. N., Aguiar, C. D. M., Hernández, M. I. M., and Iannuzzi, L. (2009). Attractivity of omnivore, carnivore and herbivore mammalian dung to Scarabaeinae (Coleoptera, Scarabaeidae) in a tropical Atlantic rainforest remnant. Rev. Bras. Entomol. 53, 422–427.
Frank, K., Krell, F.-T., Slade, E. M., Raine, E. H., Chiew, L. Y., Schmitt, T., et al. (2018). Global dung webs: high trophic generalism of dung beetles along the latitudinal diversity gradient. Ecol. Lett. 21, 1229–1236. doi: 10.1111/ele.13095
Gardner, T. A., Hernández, M. I. M., Barlow, J., and Peres, C. A. (2008). Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J. Appl. Ecol. 45, 883–893. doi: 10.1111/j.1365-2664.2008.01454.x
Giménez Gómez, V. C., Verdú, J. R., Vaz-De-Mello, F. Z., and Zurita, G. A. (2018). Influence of land use on the trophic niche overlap of dung beetles in the semideciduous Atlantic forest of Argentina. Insect Conserv. Divers. 11, 554–564. doi: 10.1111/icad.12299
Giménez Gómez, V. C., Verdú, J. R., Velazco, S., and Zurita, G. A. (2021). Dung beetle trophic ecology: are we misunderstanding resources attraction? Ecol. Entomol. 46: 552–561. doi: 10.1111/een.13001
Gómez-Cifuentes, A., Muneva, A., Giménez, V. C., Gatti, M. G., and Zurita, G. A. (2017). Influence of land use on the taxonomic and functional diversity of dung beetles (Coleoptera: Scarabaeinae) in the southern Atlantic forest of Argentina. J. Insect Conserv. 21, 147–156. doi: 10.1007/s10841-017-9964-4
Grace, J. B. (2006). Structural equation modeling and natural systems. England: Cambridge University Press.
Gries, R., Louzada, J., Almeida, S., Macedo, R., and Barlow, J. (2011). Evaluating the impacts and conservation value of exotic and native tree afforestation in Cerrado grasslands using dung beetles. Insect Conserv. Divers. 5, 175–185. doi: 10.1111/j.1752-4598.2011.00145.x
Gundale, M. J., Pauchard, A., Langdon, B., Peltzer, D. A., Maxwell, B. D., and Nuñez, M. A. (2014). Can model species be used to advance the field of invasion ecology? Biol. Invasions 16, 591–607. doi: 10.1007/s10530-013-0610-0
Halffter, G., and Arellano, L. (2002). Response of dung beetle diversity to human-induced changes in a tropical landscape. Biotropica 34, 144–154. doi: 10.1111/j.1744-7429.2002.tb00250.x
Halffter, G., and Favila, M. E. (1993). The Scarabaeinae an animal group for analysing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biol. Int. 27, 15–21.
Halffter, G., and Matthews, E. G. (1966). The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomológica Mexicana 12, 1–312.
Heino, J., Melo, A. S., and Bini, L. M. (2015). Reconceptualising the beta diversity-environmental heterogeneity relationship in running water systems. Freshw. Biol. 60, 223–235. doi: 10.1111/fwb.12502
Hernández, M. I. M., Barreto, P. S. C. S., Costa, V. H., Creão-Duarte, A. J., and Favila, M. E. (2014). Response of a dung beetle assemblage along a reforestation gradient in Restinga forest. J. Insect Conserv. 18, 539–546. doi: 10.1007/s10841-014-9645-5
Hernández, M. I. M., Da Silva, P. G., Niero, M. M., Alves, V. M., Bogoni, J. A., Brandl, A. L., et al. (2019). Ecological characteristics of Atlantic Forest dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae) in the state of Santa Catarina, Southern Brazil. Coleopt. Bull. 73, 693–709. doi: 10.1649/0010-065X-73.3.693
Hernández, M. I. M., and Vaz-De-Mello, F. Z. (2009). Seasonal and spatial species richness variation of dung beetle (Coleoptera, Scarabaeidae s. str.) in the Atlantic Forest of southeastern Brazil. Revista Brasileira de Entomologia 53, 607–613.
Howden, H. F., and Nealis, V. G. (1975). Effects of clearing in a tropical rain forest on the composition of the coprophagous scarab beetle fauna (Coleoptera). Biotropica 7, 77–83.
Hsieh, T. C., Ma, K. H., Chao, A., and Mcinerny, G. (2016). iNEXT: an R package for rarefaction and extrapolation of species diversity (hill numbers). Methods Ecol. Evol. 7, 1451–1456. doi: 10.1111/2041-210X.12613
Klein, B. (1989). Effects of forest fragmentation on dung and carrion beetle communities in Central Amazonia. Ecology 70, 1715–1725. doi: 10.2307/1938106
Larsen, T. H., Lopera, A., and Forsyth, A. (2008). Understanding trait-dependent community disassembly: dung beetles, density functions, and forest fragmentation. Conserv. Biol. 22, 1288–1298. doi: 10.1111/j.1523-1739.2008.00969.x
Ledgard, N. (2001). The spread of lodgepole pine (Pinus contorta Dougl.) in New Zealand. For. Ecol. Manage. 141, 43–57. doi: 10.1016/S0378-1127(00)00488-6
Legendre, P., and De Cáceres, M. (2013). Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecol. Lett. 16, 951–963. doi: 10.1111/ele.12141
Leibold, M. A., Holyoak, M., Mouquet, N., Amarasekare, P., Chase, J. M., Hoopes, M. F., et al. (2004). The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613. doi: 10.1111/j.1461-0248.2004.00608.x
López-Bedoya, P. A., Bohada-Murillo, M., Ángel-Vallejo, M. C., Audino, L. D., Davis, A. L. V., Gurr, G., et al. (2022). Primary forest loss and degradation reduces biodiversity and ecosystem functioning: a global meta-analysis using dung beetles as an indicator taxon. J. Appl. Ecol. 59, 1572–1585. doi: 10.1111/1365-2664.14167
López-Bedoya, P. A., Magura, T., Edwards, F. A., Edwards, D. P., Rey-Benayas, J. M., Lövei, G. L., et al. (2021). What level of native beetle diversity can be supported by forestry plantations? A global synthesis. Insect Conserv. Divers. 14, 736–747. doi: 10.1111/icad.12518
Mcgeoch, M. A., Rensburg, B. J., and Botes, A. (2002). The verification and application of bioindicators: a case study of dung beetles in a savanna ecosystem. J. Appl. Ecol. 39, 661–672. doi: 10.1046/j.1365-2664.2002.00743.x
Nichols, E., and Gardner, T. A. (2011). “Dung beetles as a candidate study taxon in applied biodiversity conservation research,” in Ecology and evolution of dung beetles. eds. L. W. Simmons and T. J. Ridsdill-Smith (Oxford: Wiley-Blackwell), 267–291.
Nichols, E., Gardner, T. A., Peres, C. A., and Spector, S. (2009). Co-declining mammals and dung beetles: an impending ecological cascade. Oikos 118, 481–487. doi: 10.1111/j.1600-0706.2009.17268.x
Nichols, E., Larsen, T., Spector, S., Davis, A. L., Escobar, F., Favila, M., et al. (2007). Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol. Conserv. 137, 1–19. doi: 10.1016/j.biocon.2007.01.023
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., and Favila, M. E. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 141, 1461–1474. doi: 10.1016/j.biocon.2008.04.011
Noriega, J. A., and Acosta, A. (2011). Population size and dispersal of Sulcophanaeus Leander (Coleoptera: Scarabaeidae) on riverine beaches in the Amazonian region. J. Trop. Ecol. 27, 111–114. doi: 10.1017/S0266467410000581
Nummelin, M., and Hanski, I. (1989). Dung beetles of the Kibale Forest, Uganda: comparison between virgin and managed forests. J. Trop. Ecol. 5, 349–352. doi: 10.1017/S0266467400003758
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., et al. (2020). Vegan: community ecology package. R package version 2, 5–7.
Peck, S. B., and Forsyth, A. (1982). Composition, structure, and competitive behaviour in a guild of Ecuadorian rain forest dung beetles (Coleoptera; Scarabaeidae). Can. J. Zool. 60, 1624–1634. doi: 10.1139/z82-213
Peyras, M., Vespa, N. I., Bellocq, M. I., and Zurita, G. A. (2013). Quantifying edge effects: the role of habitat contrast and species specialization. J. Insect Conserv. 17, 807–820. doi: 10.1007/s10841-013-9563-y
Pryke, J. S., Roets, F., and Samways, M. J. (2013). Importance of habitat heterogeneity in remnant patches for conserving dung beetles. Biodivers. Conserv. 22, 2857–2873. doi: 10.1007/s10531-013-0559-4
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rejmánek, M. (1996). A theory of seed plant invasiveness: the first sketch. Biol. Conserv. 78, 171–181. doi: 10.1016/0006-3207(96)00026-2
Rejmánek, M., and Richardson, D. M. (1996). What attributes make some plant species more invasive? Ecology 77, 1655–1661. doi: 10.2307/2265768
Richardson, D. M. (2006). Pinus: a model group for unlocking the secrets of alien plant invasions? Preslia 78, 375–388.
Roslin, T., and Viljanen, H. (2011). “Dung beetle populations: structure and consequences,” in Ecology and evolution of dung beetles. eds. L. W. Simmons and T. J. Ridsdill-Smith (Oxford: Wiley-Blackwell), 221–244.
Sarmiento-Garcés, R., and Hernández, M. I. M. (2021). A decrease in taxonomic and functional diversity of dung beetles impacts the ecosystem function of manure removal in altered subtropical habitats. PLoS One 16:e0244783. doi: 10.1371/journal.pone.0244783
Scholtz, C. H., Davis, A. L. V., and Kryger, U. (2009). Evolutionary biology and conservation of dung beetles. Moscow: Pensoft Publishers.
Shipley, B. (2016). Cause and correlation in biology: A user’s guide to path analysis, structural equations and causal inference with R. 2nd Edn. Vol. 314. England: Oxford University Press.
Simberloff, D., Nuñez, M. A., Ledgard, N. J., Pauchard, A., Richardson, D. M., Sarasola, M., et al. (2010). Spread and impact of introduced conifers in South America: lessons from other southern hemisphere regions. Austral Ecol. 35, 489–504. doi: 10.1111/j.1442-9993.2009.02058.x
Simmons, L. W., and Ridsdill-Smith, T. J. (2011). Ecology and evolution of dung beetles. Oxford: Wiley-Blackwell.
Keywords: alpha and beta diversity, biodiversity conservation, bioindicators, dispersion, ecology
Citation: Simões-Clivatti TRO and Hernández MIM (2022) Ecological indication metrics on dung beetles metacommunities in native forests and Pinus monocultures. Front. Ecol. Evol. 10:972176. doi: 10.3389/fevo.2022.972176
Received: 17 June 2022; Accepted: 10 November 2022;
Published: 08 December 2022.
Edited by:
Mario Favila, Instituto de Ecología (INECOL), MexicoReviewed by:
Eduardo Périco, Universidade do Vale do Taquari - Univates, BrazilCopyright © 2022 Simões-Clivatti and Hernández. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Malva Isabel Medina Hernández, bWFsdmEubWVkaW5hQHVmc2MuYnI=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.