- 1Zhejiang Provincial Key Laboratory of Biometrology and Inspection and Quarantine, College of Life Sciences, China Jiliang University, Hangzhou, China
- 2Sterling International, Inc., Spokane, WA, United States
The tea aphid (Toxoptera aurantii Boyer de Fonscolombe) is an important tea plant pest insect worldwide. The parasitoid wasp, Aphelinus sp., is one of the most important natural enemies of the tea aphid in China. Unfortunately, Aphelinus sp. alone cannot effectively control the outbreaks of the aphid under natural conditions. In this study, 27 volatile compounds from tea aphid-injured tea shoots, tea flowers, aphid sex pheromones, or body rinses were selected and tested in Y-tube olfactometer assays to find potential attractants of the parasitoid wasp, Aphelinus sp. Based on the Y-tube assay results, the following three attractant mixtures were formulated and further tested in the field. Attractant-1 (HIPV-based) included trans-2-hexenal (10−6 g/ml), β-ionone (10−6 g/ml), allyl isothiocyanate (10−4 g/ml), trans-2-pentenal (10−2 g/ml), and jasmone (10−2 g/ml) at equal loading volume of their solutions. Attractant-2 (with aphid sex-pheromone and body rinse compounds) included nepetalactone (10−6 g/ml), 2,5-hexanedione (10−4 g/ml), benzaldehyde (10−2 g/ml), eicosane (10−2 g/ml), and heptadecane (10−2 g/ml) at equal loading volume of their solutions. Attractant-3 (partial combination of Attractant-1 and Attractant-2) included nepetalactone (10−4 g/ml), benzaldehyde (10−2 g/ml), jasmone (10−2 g/ml), trans-2-hexenal (10−6 g/ml), eicosane (10−2 g/ml), and heptadecane (10−2 g/ml) at equal loading volume of their solutions. Field trials showed that Attractant-3 was much more attractive to the parasitic wasps than Attractant-1 and Attractant-2. From late August to late September the controlled release of Attractant-3 effectively attracted Aphelinus sp. to parasitize and colonize the aphid populations in the treated tea plantations, resulting in a progressive decrease of the tea aphid abundances/populations in the fall. The continued enhanced parasitism of overwintered aphids by Aphelinus sp. further reduced this population during the next spring tea harvest season. This approach may present an environmentally sound, non-insecticidal control tactic against tea aphids using synthetic semiochemicals.
Introduction
The tea aphid, Toxoptera aurantii Boyer de Fonscolombe, is an important pest insect widely distributed in tea plantations in China, Japan, India, Sri Lanka, and Kenya (Das and Kakoty, 1992; Sudoi and Rotich, 1997; Shizuoka Plant Epidemic Prevention Association, 2001; Hazarika et al., 2009; Zeng and Wu, 2014; Ghosh et al., 2015). It is also one of the major citrus pests worldwide (Guidolin and Cônsoli, 2018; Wang et al., 2019). In the middle and lower reaches of the Yangtze river, China, the tea plant shoots sprout four times per year (late February to early May; late May to June; July to early August; and September to early November) and are always colonized by the populations of tea aphids. There are 25–35 aphid generations per year with two major population peaks occurring in early May and mid-September, respectively. During the peak periods, large populations of aphid adults, nymphs, and eggs can be found on tender tea shoots each with one bud and five leaves. Both adults and nymphs can suck the sap from the young leaves, buds, and even the tender stems, and excrete large amounts of honeydew onto the lower leaf surface, promoting the prevalence of sooty mold disease and inhibiting photosynthesis. Tea shoots are the raw materials for processing top-grade commercial teas. The injury of tea shoots by aphids would not only distort the appearance of commercial teas but also deteriorate their overall quality. Tea market values in recent years have been reduced by >30% due to the aphid infestation alone according to a survey carried out in Anji County, Zhejiang Province, China.
Normally, insecticides are not recommended for controlling tea aphids to avoid serious residues in the tea shoots/leaves. However, once outbreaks of the aphid occur, spraying pesticides might be the only effective control option available. An entomopathogenic fungus, Verticillum lecanii (Zimm.) could infect the tea aphid and the red wax scale, Ceroplastes rubens (Maskell) in tea plantations (Wang et al., 2008; Wang and Wang, 2011). Two species of Zoophthora spp. also infected the aphid and might occasionally cause small-scale epidemics with a prevalence rate ranging from 1 to 8% (Han, 1995). Timely plucking of the tender tea shoots might also be a measure to control the tea aphids (Zeng and Wu, 2014). Over the past few years, yellow sticky boards alone or baited with chemical attractants have been used to trap the aphids in some tea plantations in China (Han et al., 2012). In practice, many species of aphids can cause serious damage to crops to the point that triggers insecticide sprays (Fakhouri et al., 2021). However, there are many species of natural enemies of tea aphids in the tea plantations including the Asian lady beetle, Harmonia axyridis (Pallas) that consists of various subspecies and varieties: H. axyridis (Pallas) var. novemdecimpunctata Falderman, H. axyridis (Pallas) var. spectabilis Falderman, H. axyridis (Pallas) var. conspicua Falderman, and H. axyridis (Pallas) ab. bimaculata Falderman (Zeng and Wu, 2014). Other major natural enemies include Coccinella septempunctata L., Chrysopa septempunctata Wesmael, C. sinica Tjeder, and the egg parasitoids, Aphidius sp. and Aphelinus sp. Of which, only L. axyridis was approved to be able to control the tea aphid populations to some degree by showing its population fluctuation in accordance with that of aphids in springs and summers of certain years (Zeng and Wu, 2014). Unfortunately, under natural conditions, these natural enemies in tea plantations by themselves cannot effectively suppress tea aphid populations below the economical threshold (Zeng and Wu, 2014).
Tea shoots infested by aphids released volatile compounds as a synomone to attract the natural enemies of the tea aphids. For instance, benzaldehyde, trans-2-hexenal, and indoles elicited strong electrophysiological responses from antennae of Coccinella septempunctata, Chrysopa sinica, and Aphidius sp. (Han and Chen, 2002a). Sex pheromone of the tea aphid was identified as (4aS,7S,7aR)-nepetalactone (I) and (1R,4aS,7S,7aR)-nepetalactol (II) at 4.3–4.9:1 ratio (Han et al., 2014). It was also reported that nepetalactone or nepetalactol significantly attracted Chrysopa septempunctata (Zhang et al., 2006). An attractant blend consisting of nepetalactol, α-farnesene, and benzaldehyde at a 6:2:2 ratio effectively attracted C. septempunctata to prey on tea aphids (Cui et al., 2015).
Wasps that commonly parasitize aphids belong to Aphidiinae and Aphelinidae (Wang Z. Z. et al., 2019). Of which, Aphelinus spp. have been reported to be major parasitoid wasps of many aphid species, including the tea aphids. Thus, Aphelinus wasps might be important and potential biological control agent candidates. In fact, within the tea plantation community, Aphelinus sp. (exact specie status to be determined) is a key egg/nymph parasitoid of tea aphids with a high parasitism rate during late August and September (Zeng and Wu, 2014). Usually, individuals of Aphelinus sp. are highly mobile and easily disperse to other locations, and they could not follow closely and effectively the tea aphid populations in the tea plantations. This raised the question of if one could develop an efficient attractant lure to attract the natural populations of Aphelinus sp. to the tea plantations and retain them for effective control of the tea aphids.
Various research reported successful foraging behaviors of parasitoids, including Aphelinus spp., that were modulated by specific volatile compounds emitted from the host-infested plants, such as the herbivore-induced plant volatiles (HIPVs) as a synomone. Parasitoids are attracted by these volatiles in a quite specific way (Ismail et al., 2021); for instance, both the quality and quantity of HIPVs affected the host selection behaviors of parasitic wasps. In addition, the composition and concentration of such volatiles are partially dependent on the density of the insect prey. Based on field observations, Aphelinus sp. parasitizes the eggs and nymphs of tea aphids, and its parasitism rate has risen with the increase of tea aphid population density to some extent. Gregg et al. proposed that the highly effective attractant components can be derived from one or several host plant species, host plants of different families, or even animals (Gregg et al., 2010). Application of synthetic sex pheromone components [(4aS,7S,7aR)-nepetalactone and (1R,4aS,7S,7aR)-nepetalactol] of aphids in an alfalfa field for three consecutive years yielded a reduction in the population of Acyrthosiphon pisum Harris and an increase in parasitism rates of the aphids by Aphidius ervi Haliday and Praon barbatum Mackauer (Nakashima et al., 2016).
In this study, we undertook screening and assaying using both lab and field research, examining several groups of volatile compounds from different natural sources, including tea shoots infested by aphids, tea flowers, aphid body surface rinses, and the aphid sex pheromone components for finding effective semiochemical-based attractants of the parasitoid wasp, Aphelinus sp., which might have potential as an environmentally sound control measure for combating the economically important tea aphids.
Methods
Behavioral assay on attraction of Aphelinus sp. to volatiles from tea shoot-aphid complexes and tea aphid-injured shoots and odors of tea aphids
Insects
Since the founding of the Experimental Tea Plantation of China Jiliang University 20 years ago, both the tea aphid (T. aurantii) and the parasitoid wasp, Aphelinus sp., commonly existed on the plantation. During the tests, tender tea shoots bearing different stages of tea aphids were cut from tea plants in the plantation and placed in the glass beaker filled with water in the lab. The tea shoots with the aphids parasitized by Aphelinus sp. were also cut and inserted into the glass beakers (with water supply to the cut ends) for keeping the tea shoots alive in the lab. All the tea shoots in the glass beakers were placed in artificial weather boxes, and the developmental progress of live aphids and parasitized aphids were regulated by temperature and humidity control until the aphids and the wasps were ready for testing. Newly emerged virgin wasps were collected and placed in 500 ml clear bottles each sealed with a double layer gauze to prevent escape. A cotton ball dipped with 10% honey solution and hang from gauze supplied nutrition to the wasps, and they were allowed to mate freely. The species status of this Aphelinus wasp is still not yet confirmed. However, up to date only one species of the genus Aphelinus was found in the Chinese tea plantations, thus “Aphelinus sp.” is used to represent the target parasitoid wasp species in the current paper.
Y-tube olfactometer assays
Y-tube olfactometer assays were conducted according to the previous descriptions with some minor modifications (Mu et al., 2012). The base and two arms of the Y-tube olfactometer were all 10 cm long with an angle of 120° between the two arms. The opening of the base was connected to a vacuum pump. Each of the two arms was connected to an odor source bottle or a control (CK) bottle, a humidification bottle, an activated carbon filter, and a flowmeter. Plant materials or semiochemicals in the vial, used as the odor source, were placed in the odor source bottle connected to one arm. The other arm was set as the CK arm connected to the CK bottle. Air was pumped from the base of the Y-tube, and the flow rate in each arm was 100 ml/min. Two minutes of pumping was performed before the test to ensure the Y-tube system was running with stable and consistent airflows from both arms. For each testing run, one 1-day old female adult wasp was introduced into the opening of the Y-tube base using a tube. The parasite wasp was allowed to choose between the odor source arm and the CK arm. The wasp was discarded if there was no response within 5 min. Each wasp was only used once. A total of 20 adult wasps were tested against each odor source (plant materials or semiochemicals) treatment. After every 10 wasps were tested, the Y-tube was cleaned with a cotton ball soaked with ethanol and dried. The odor source arm and the CK arm were then shifted to avoid positional bias. Data were collected on the number of wasps that chose the odor source arm and the CK arm. After one odor source was tested, the Y-tube, odor source bottle, control bottle, and all other glass parts were all cleaned with potassium dichromate solution and dried at 120°C for 2 h. The activated carbon used for filtering the air was re-activated by blowing air at 100°C for 4 h, then was cooled and sealed in a glass bottle at ambient temperature for later use. All Y-tube assays were conducted from 9:30 am to 15:30 pm at 25 ± 3°C, 70 ± 5% RH, and 3,200–3,600 lux.
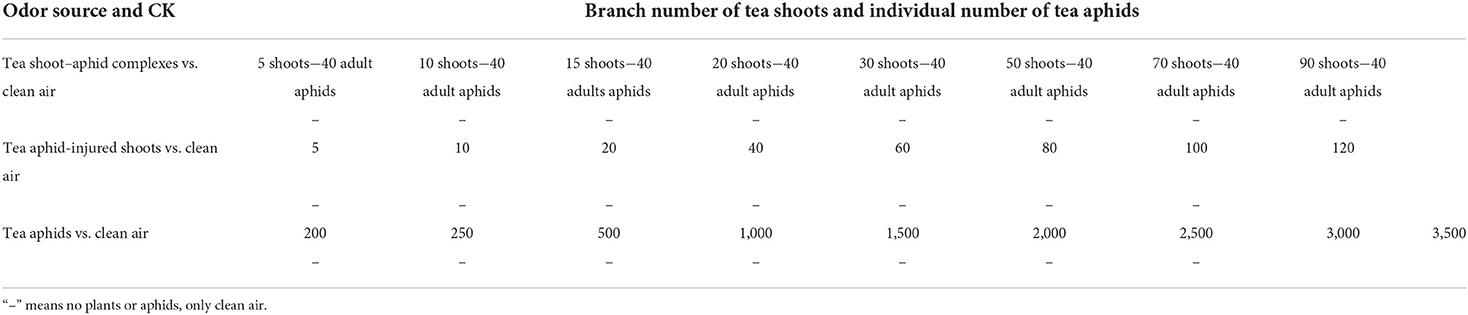
Determining attraction of Aphelinus sp. to different dosages of tea shoot–aphid complexes, tea aphid-injured shoots, and tea aphids
To determine the attraction of Aphelinus sp. to the volatiles from each of three types of candidate odor sources, i.e., tea shoot–aphid complexes, tea aphid-injured shoots, and tea aphids, they were tested in the Y-tube olfactometer against the clean air (as CK). Their tested dosages as well as the pairing details are listed in Table 1. Each tested tea shoot from the tea shoot–aphid complexes or tea aphid-injured shoots was about 7 cm long with one bud and three leaves, weighing appropriately 0.7 g. Tea shoot–aphid complexes and tea aphid-injured shoots were fetched from the experimental tea plantations just before the testing, then the adults, nymphs, and eggs on the latter were lightly cleared away with a small brush. The tested tea aphids, including both live adults and nymphs, were taken from the tea aphid populations reared in the lab artificial weather box. A total of 20 adult wasps were tested against each odor source treatment.
Comparing attraction efficacy of Aphelinus sp. to the three odor sources
To compare the relative attraction of the wasp Aphelinus sp. to three different odor sources, the following pairs were set up and tested in the Y-tube olfactometer:
(1) 50 tea shoot-aphid complexes (i.e., 50 shoots with 40 aphids) vs. 50 fresh-cut tea shoots;
(2) 50 tea aphid-injured shoots vs. 50 fresh-cut tea shoots;
(3) 0 fresh-cut tea shoots vs. clean air.
A total of 20 adult wasps were tested for each odor source pairing treatment.
Lab behavioral assays on the attraction of Aphelinus sp. to four types of synthetic semiochemical odor sources
Semiochemicals derived/identified from odor sources 1 and 2
Odor source 1: Tea shoot–aphid complexes (Han and Chen, 2002b); Odor source 2: tea flowers (Han et al., 2007). Following identified volatiles from odor sources 1 and 2 were chosen for the behavioral assays: 1. linalool; 2. trans-2-hexenal; 3. cis-3-hexen-1-ol; 4. benzaldehyde; 5. methyl salicylate; 6. geraniol; 7. indole; 8. cis-3-hexenyl acetate; 9. methyl jasmonate; 10. trans-2-pentenal; 11. cis-2-pentene-1-ol; 12. allyl isothiocyanate; 13. nerol; 14. ocimene; 15. acetophenone; 16. pentanol; 17. hexanol; 18. 1-pentene-3-ol; 19. jasmone; 20. β-ionone; 21. terpinene; 22. benzyl ethanol; 23. terpineol; 24. cedrol; 25. 3-carene; 26. humulene; 27. benzyl alcohol.
The above-mentioned 27 synthetic compounds were purchased (see Supplementary Table S1, for their sources and purities) and dissolved in n-hexane to make up solutions as test odor sources at dosages of 10−2, 10−4, and 10−6 g/ml, respectively. In addition, two synthetic mixtures, Mixture 1 (a mixture of linalool, trans-2-hexenal, and cis-3-hexen-1-ol at equal loading ratio), and Mixture 2 (a mixture of trans-2-hexenal, cis-3-hexen-1-ol, 2-penten-1-ol, trans-2-pentenal, cis-3-hexenyl acetate, n-pentanol, n-hexanol, and 1-penten-3-ol at equal loading ratio), were also tested. n-Hexane (chromatographical purity) was used as solvent control (CK).
Semiochemicals derived/identified from odor sources 3 and 4
Odor source 3: Tea aphid body surface rinses (Han, 2001) with both n-hexane and ether; Odor source 4: Tea aphid sex pheromone components [(4aS,7S,7aR)-nepetalactone and (1R, 4aS,7S,7aR)-nepetalactol] (Han et al., 2014).
Following nine volatile compounds from odor sources 3 and 4 were selected for the behavioral assays: a. benzaldehyde; b. 2,5-hexanedione; c. linalool; d. E-2-hexenoic acid; e. heptadecane; f. undecane; g. eicosane; h. (4aS,7S,7aR)-nepetalactone; i: (1R, 4aS,7S,7aR)-nepetalactol. These nine compounds were purchased or obtained (see Supplementary Table S1) and dissolved in n-hexane to formulate into solutions as test odor sources for the following assays at dosages of 10−2, 10−4, and 10−6 g/ml, respectively.
Formulations of three attractant mixtures
Based on the above-mentioned behavioral assay results, the following three Aphelinus sp. attractant mixture candidates from different odor sources were formulated:
Attractant-1 is derived from the odor sources 1 and 2, including equal ratio (1:1:1:1:1) of trans-2-hexenal (10−6 g/ml), β-ionone (10−6 g/ml), allyl isothiocyanate (10−4 g/ml), trans-2-pentenal (10−2 g/ml) and jasmone (10−2 g/ml) in hexane; Attractant-2 is derived from odor sources 3 and 4, composed of equal ratio (1:1:1:1:1) of nepetalactone (10−6 g/ml), 2,5-hexanedione (10−4 g/ml), benzaldehyde (10−2 g/ml), eicosane (10−2 g/ml), heptadecane (10−2 g/ml) in hexane; Attractant-3, a partial combination of Attractant-1 and Attractant-2, consisted of equal ratio (1:1:1:1:1:1) of nepetalactone (10−4 g/ml), benzaldehyde (10−2 g/ml), jasmone (10−2 g/ml), trans-2-hexenal (10−6 g/ml), eicosane (10−2 g/ml), and heptadecane (10−2 g/ml) in hexane.
Field bioassays of wasp attractant candidate lures
Preparation of three attractant lures
A 0.75 ml of Attractant-1,−2, or−3 solution was loaded onto a strip of felt (4.5 cm long × 1.5 cm wide × 1.0 cm thick), followed by adding 0.50 ml 2.0 × 10−3 g/ml butylated hydroxytoluene (BHT) as the antioxidant, and then was sealed in a PVC bag (4.6 × 1.6 × 1.1 cm; 100 μm in film thickness). The loaded attractant lures (Attractant-1, Attractant-2, and Attractant-3 dispensers) were stored at −20°C until use. For a Z-jasmone alone lure, 0.75 ml of 10−2 g/ml Z-jasmone (with 0.50 ml 2.0 × 10−3 g/ml BHT added) was loaded onto the same size of felt sealed in the same size PVC bag. For a CK lure, 0.75 ml n-hexane alone (with 0.50 ml 2.0 × 10−3 g /ml BHT added) was used.
Effect of three attractants on the parasitism of tea aphids by Aphelinus sp.
Field trial 1
On August 26, 2019, a large block of an organic tea plantation was selected in Songyang county in Zhejiang province in China, which was further divided into 25 tea plantation plots. Each tea plot was severely infested by the tea aphids with an average of six infested shoots per 1 m tea row, and >20 aphids per infested shoot. Each plot was 25 m long × 25 m wide with >20 m between two neighboring plots. Every five plots were randomly assigned to Attractant-1, Attractant-2, Attractant-3 plots, Z-jasmone plot, and CK lure plot, respectively, resulting in a total of five replicates of each treatment or CK. In each plot, attractant lures or CK dispensers were attached to branches using twist-ties in the upper layer of the tea plant brushes with a distance of 8 m between two neighboring attractant lures or CK dispensers.
All the tea plants in the experimental plots were grown by double row closed planting with row spacing being 1.5 m and plant spacing being 33 cm. The height of tea plants was about 90 cm. Their cultivars were local group cultivars. Tea aphids reached a population peak on September 10, 2019, in these plots. On September 20, five sampling points were selected in each plot based on the five-point sampling method (Tang, 2020), with each point being a 5 m tea plant row. About 100 tea aphids were randomly surveyed at each sampling point, and the numbers of tea aphids parasitized by Aphelinus sp. at each point were recorded.
Field trial 2
On September 1, 2019, a large block of a non-organic tea plantation was chosen in the Meijiawu Village in Hangzhou City in Zhejiang province, China, which was further divided into 20 tea plantation plots. Each plot was also seriously infested by the tea aphids with an average of five infested shoots per 1 m tea row, and > 20 aphids per infested shoot. Each plot was 25 m long × 25 m wide with >20 m between two neighboring plots. Every four plots were randomly assigned to Attractant-1, Attractant-2, Attractant-3 plots, and CK lure plot, respectively, resulting in a total of five replicates of each treatment or CK. In each plot, attractant lures or CK dispensers were attached to branches using twist-ties in the upper layer of the tea plant brushes of every row at a distance of 8 m from each other.
All the tea plants in the experimental plots were grown by double row closed planting with row spacing being 1.5 m and plant spacing being 33 cm. The height of tea plants was around 90 cm. Their cultivars were “Longjin” group cultivars. In these tea plantation plots, the tea aphid population reached its peak on September 15. On September 25, five sampling points were chosen in each plot according to the five-point sampling method (Tang, 2020) with every point being a 5 m tea plant row. About 100 tea aphids were randomly surveyed at each sampling point, and the numbers of tea aphids parasitized by Aphelinus sp. at each sampling point were recorded.
Control efficacy via parasitization of aphids by Aphelinus sp. in the Attractant-3 plots vs. the control efficacy in the insecticide spay plots
On August 25, 2020, a large block of a non-polluted tea plantation was chosen in Songyang county in Zhejiang province of China, which was divided into 16 tea plantation plots. All selected plots were infested by tea aphids with an average of seven infested shoots per 1 m tea row, and > 20 aphids per infested shoot. Each plot was 37 × 37 m with >20 m between two neighboring plots. Every four tea plantation plots were randomly chosen and assigned to the following treatments: attractant A, attractant B, pesticide spray, and untreated control (CK), resulting in a total of four replicates of each attractant treatment or CK.
First, based on the checkerboard sampling method (Tang, 2020), 10 sampling points were selected from each plot, with each point being a 5 m tea row. The number of aphid-injured shoots at each sampling point was counted for each plot. Subsequently, in each plot of attractant A, a total of 20 attractant lures (Attractant-3) were attached by twist-ties to branches in the upper layer of the tea plant brushes at a distance of 8 m between two neighboring lures. Meanwhile, in each plot of attractant B, a total of 10 Attractant-3 lures were also attached by twist-ties to branches in the upper layer of the tea plant brushes at a distance of 12 m from each other. In each pesticide spray plot, 10% wettable imidacloprid powder was sprayed on all tea rows at a dosage of 20 g/plot (Zhengzhou Agro-chemical Limited Company, China). Neither pesticide nor attractant was applied in the four CK plots.
On August 27, 2020, the numbers of aphid-infested shoots were counted by the checkerboard sampling method (Tang, 2020) (10 sampling points in each plot, one point being a 5-m tea row). The same counting effort was repeated on September 10, 2020, and September 25, 2020. After the survey on September 25, 2020, the attractant lures in each attractant plot A and plot B were refreshed, and imidacloprid was re-sprayed in each pesticide plot. The numbers of aphid-infested shoots were counted two more times in the same year on October 25, 2020, and November 25, 2020, and two more times the next year on February 25, 2021, and March 25, 2021. The following formula was used for the calculation of the control efficacy.
Reduction ratio = (infested shoot number before treatment – infested shoot number after treatment)/infested shoot number before treatment × 100%
Control efficacy (adjusted reduction rate) = (reduction rate in treatment plot– reduction rate in untreated CK plot)/(1– reduction rate in untreated CK plot) × 100%.
Statistical analysis
DPS© Data Processing System (Tang, 2020) was used. For the Y-tube bioassays, the χ2 test was performed to determine the significance of the difference between the number of wasps choosing the odor source arm and the number choosing the CK arm. Based on the number of parasitic wasps choosing the odor source arm and the corresponding dosages of plant materials or semiochemicals, correlation equations (formula) with R2 and p-values were established and calculated.
Based on data acquired by the five-point sampling method, the parasitism rate of aphids by the wasp in each of the five sampling points was calculated. Then the average parasitism rates among various attractant treatments and CK (Field trial 1 on September 20, and Field trial 2 on September 25) were analyzed by one-way analysis of variance (ANOVA) (after checking the assumption of homogeneity of variance), followed by Duncan's multiple range test at α = 0.05.
Field data on infested shoot reduction effect among Attractant A plot, Attractant B plot, spraying pesticide plot, and CK plot on the three sampling dates (September 10, September 25, and October 25 in 2020) was also analyzed by one-way ANOVA and Duncan's test.
Results
Volatiles from tea shoot-aphid complexes and tea aphid-injured shoots, and the odor of tea aphids significantly attract Aphelinus sp.
Volatiles from tea shoot–aphid complexes significantly attracted Aphelinus sp. in a dosage-dependent manner, with the 40 adult aphids plus 50 aphid-injured shoots being the most attractive (Table 2). The correlation between the number of Aphelinus sp. responded (Y), the number of tea aphids (X1), and the number of injured shoots (X2) was:
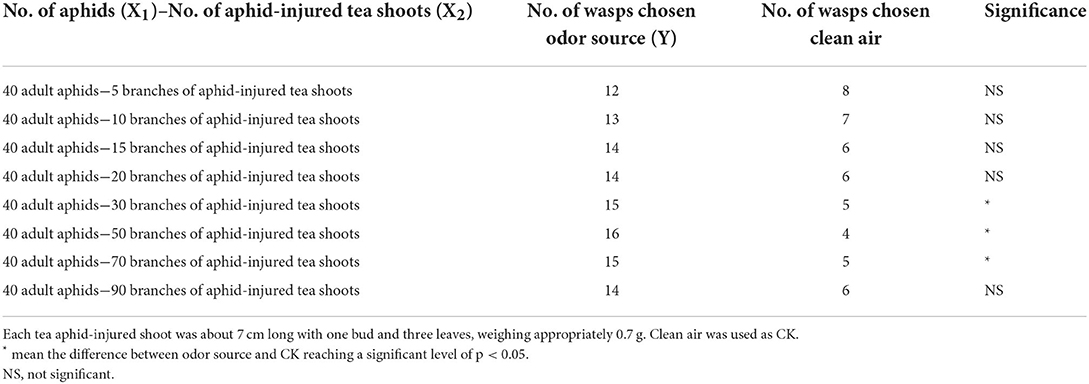
Table 2. Number of Aphelinus sp. attracted to tea shoot–aphid complexes vs. clean air in Y-tube olfactometer assay.
Volatiles from aphid-injured tea shoots also significantly attracted Aphelinus sp. in a dose-dependent manner with the 80 infested shoots being the most attractive. Further increase in the number of infested shoots led to a reduced attraction or no attraction (Table 3). The correlation between the number of Aphelinus sp. responded (Y) and the number of injured shoots (X) was:
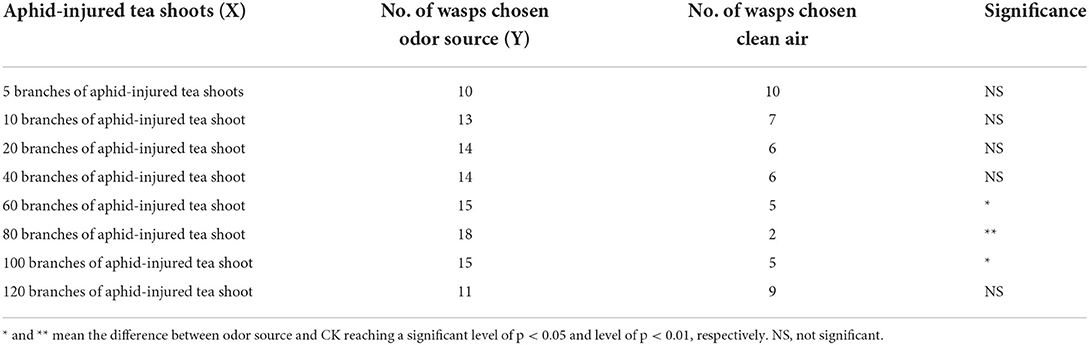
Table 3. Number of Aphelinus sp. attracted to aphid-injured tea shoots vs. clean air in Y-tube olfactometer assay.
The odor of tea aphids significantly attracted Aphelinus sp. in a dose-dependent manner too, with 1,000 tea aphids being the most attractive. Further increase in the number of tea aphids led to a reduced attraction or no attraction (Table 4). The correlation between the number of Aphelinus sp. responded (Y) and the number of tea aphids (X) was:
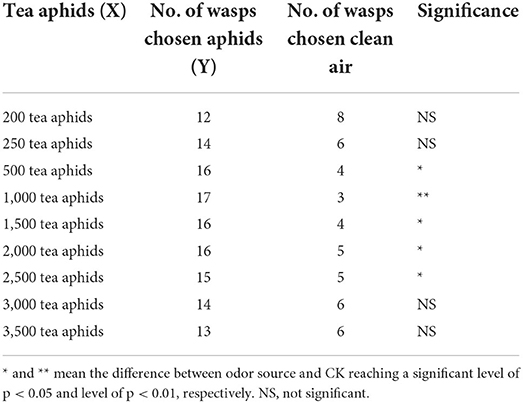
Table 4. Number of Aphelinus sp. attracted to tea aphids vs. clean air in Y-tube olfactometer assay.
Volatiles from tea shoot–aphid complexes or aphid-injured shoots attract Aphelinus sp. significantly more than those from fresh-cut tea shoots
Volatiles of fresh-cut tea shoots appeared to slightly, but not significantly attract Aphelinus sp. when compared to the clean air control. However, both tea shoot–aphid complexes and aphid-injured shoots were significantly more attractive to the testing wasps than were fresh-cut tea shoots (Table 5).
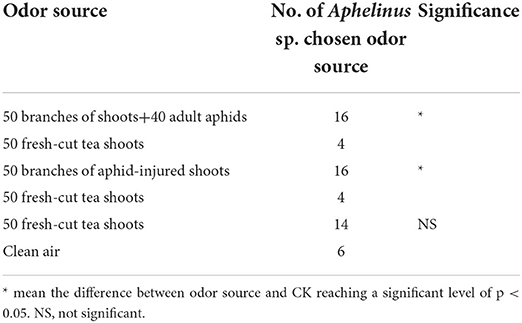
Table 5. Number of Aphelinus sp. attracted to tea shoot–aphid complexes or aphid-injured shoots vs. fresh-cut tea shoots plus fresh-cut tea shoots vs. clean air.
Various volatile components from aphid-injured shoots and tea flowers significantly attract Aphelinus sp. and formulation of Attractant-1
The Y-tube olfactometer assays showed that eight odor sources, i.e., trans-2-hexenal (10−6 g/ml), β-ionone (10−6 g/ml), allyl isothiocyanate (10−4 g/ml), ocimene (10−4 g/ml), terpinene (10−4 g/ml), cis-3-hexen-1-ol (10−2 g/ml), trans-2-pentenal (10−2 g/ml), and jasmone (10−2 g/ml) were significantly more attractive to Aphelinus sp. than was the CK (clean air). A similar attraction was also observed for the odor source 28 (Mixture-1) (Figure 1). Thus, the wasp Attractant-1 was formulated by mixing an equal volume of the following five chemical solutions: trans-2-hexenal (10−6 g/ml), β-ionone (10−6 g/ml), allyl isothiocyanate (10−4 g/ml), trans-2-pentenal (10−2 g/ml), and jasmone (10−2 g/ml).
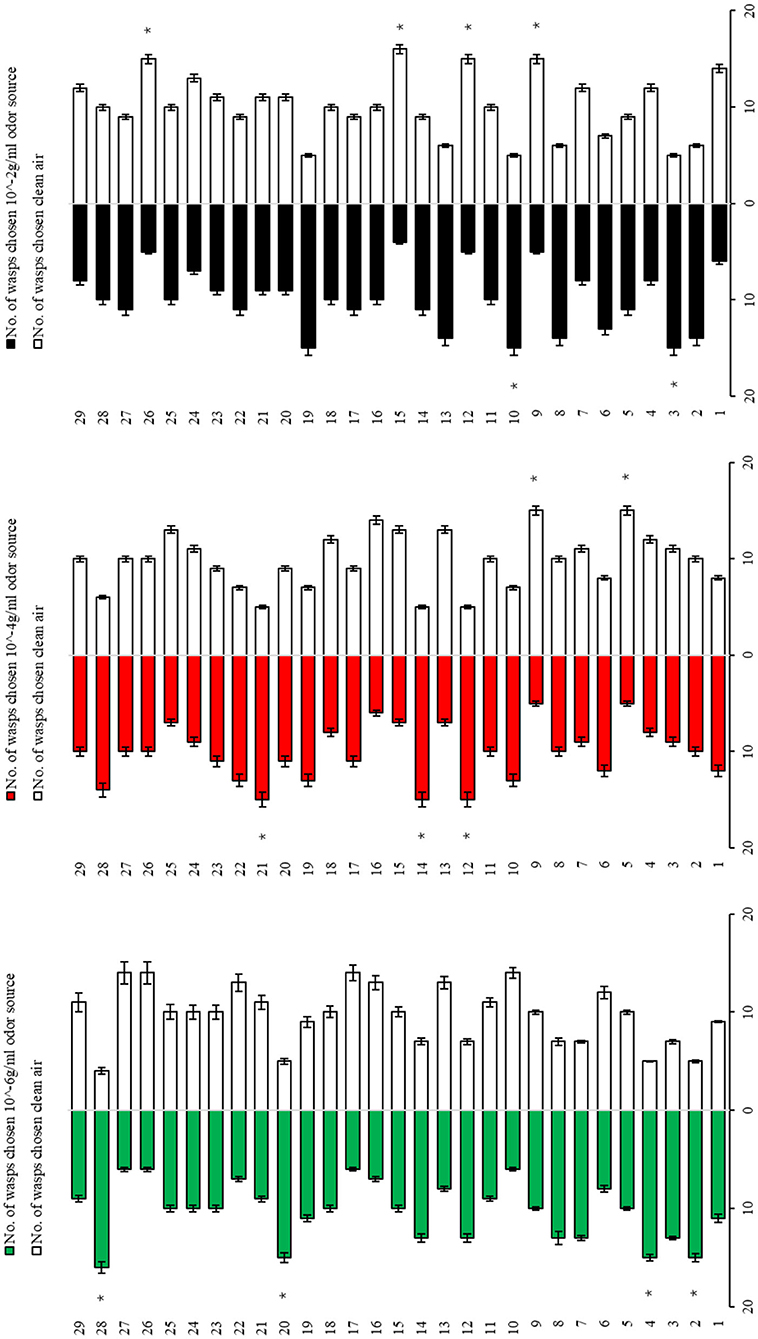
Figure 1. Attraction of Aphelinus sp. to three dosages of 27 synthetic components (plus two mixtures) identified from tea shoot–aphid complexes and/or tea flowers. 1, linalool; 2, trans-2-hexenal; 3, cis-3-hexen-1-ol; 4, benzaldehyde; 5, methyl salicylate; 6, geraniol; 7, indole; 8, cis-3-hexenyl acetate; 9, methyl jasmonate; 10, trans-2-pentenal; 11, cis-2-pentene-1-ol; 12, allyl isothiocyanate; 13, nerol; 14, ocimene; 15, acetophenone; 16, pentanol; 17, hexanol; 18, 1-pentene-3-ol; 19, jasmone; 20, β-ionone; 21, terpinene; 22, benzyl ethanol; 23, terpineol; 24, cedrol; 25, 3-carene; 26, humulene; 27, benzyl alcohol; 28, Mixture 1: a mixture of linalool, trans-2-hexenal, and cis-3-hexen-1-ol; 29, Mixture 2: a mixture of trans-2-hexenal, cis-3-hexen-1-ol, 2-penten-1-ol, trans-2-pentenal, cis-3-hexenyl acetate, n-pentanol, n-hexanol, and 1-penten-3-ol. *mean the difference between the number of wasps to choose odor source and the number of wasps to choose CK (clean air) reaches a significant level of p < 0.05.
Various volatile compounds from solvent rinses of tea aphid body surface and tea aphid sex pheromone significantly attract Aphelinus sp. and formulation of Attractant-2
The Y-tube olfactometer assays showed that 11 odor sources, i.e., nepetalactone (10−6 g/ml), nepetalactol (10−6 g/ml), benzaldehyde (10−4 g/ml), 2,5-hexanedione (10−4 g/ml), nepetalactone (10−4 g/ml), nepetalactol (10−4 g/ ml), benzaldehyde (10−2 g/ml), heptadecane (10−2 g/ml), eicosane (10−2 g/ml), nepetalactone (10−2 g/ml), and nepetalactol (10−2 g/ml) significantly attracted Aphelinus sp. (Figure 2). Thus, Attractant-2 was prepared and formulated by mixing equal volume of the following five chemical solutions: nepetalactone (10−6 g/ml), 2,5-hexanedione (10−4 g/ml), benzaldehyde (10−2 g/ml), eicosane (10−2 g/ml), and heptadecane (10−2 g/ml). In addition, a partial combination of Attractant-1 and Attractant-2, consisted of equal ratio (1:1:1:1:1:1) of nepetalactone (10−4 g/ml), benzaldehyde (10−2 g/ml), jasmone (10−2 g/ml), trans-2-hexenal (10−6 g/ml), eicosane (10−2 g/ml) and heptadecane (10−2 g/ml) in hexane, was formulated as Attractant-3.
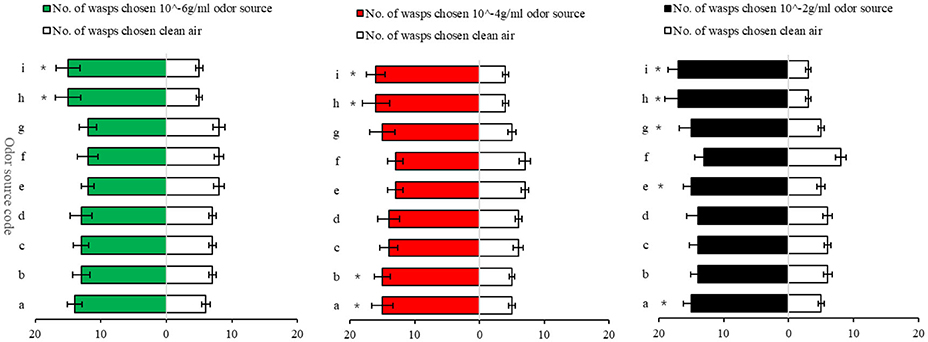
Figure 2. Attraction of Aphelinus sp. to seven synthetic volatile components identified from tea aphid body rinses and 2 synthetic tea aphid sex pheromone components. a, benzaldehyde; b, 2,5-hexanedione; c, linalool; d, E-2-hexenoic acid; e, heptadecane; f, undecane; g, eicosane; h, (4aS,7S,7aR)-nepetalactone; I, (1R, 4aS,7S,7aR)-nepetalactol. *mean the difference between the number of wasps to choose odor source and the number of wasps to choose CK (clean air) reaches a significant level of p < 0.05.
Effect of three attractant mixtures on the parasitism rates of tea aphids by Aphelinus sp.
Field trial 1
The results of field trial 1 (September 20, 2019) showed that average parasitism rates of tea aphids by Aphelinus sp. in Attractant-3 plots, Attractant-1 plots, Attractant-2 plots, Z-jasmone plots, and CK plots were 53.8, 50.2, 49.5, 17.2, and 9.7%, respectively. There was a significant treatment effect (ANOVA; F = 995.5, df = 24, p < 0.05). The effect of Attractant-3 was significantly superior to others, with Z-jasmone being the least effective treatment (Table 6).
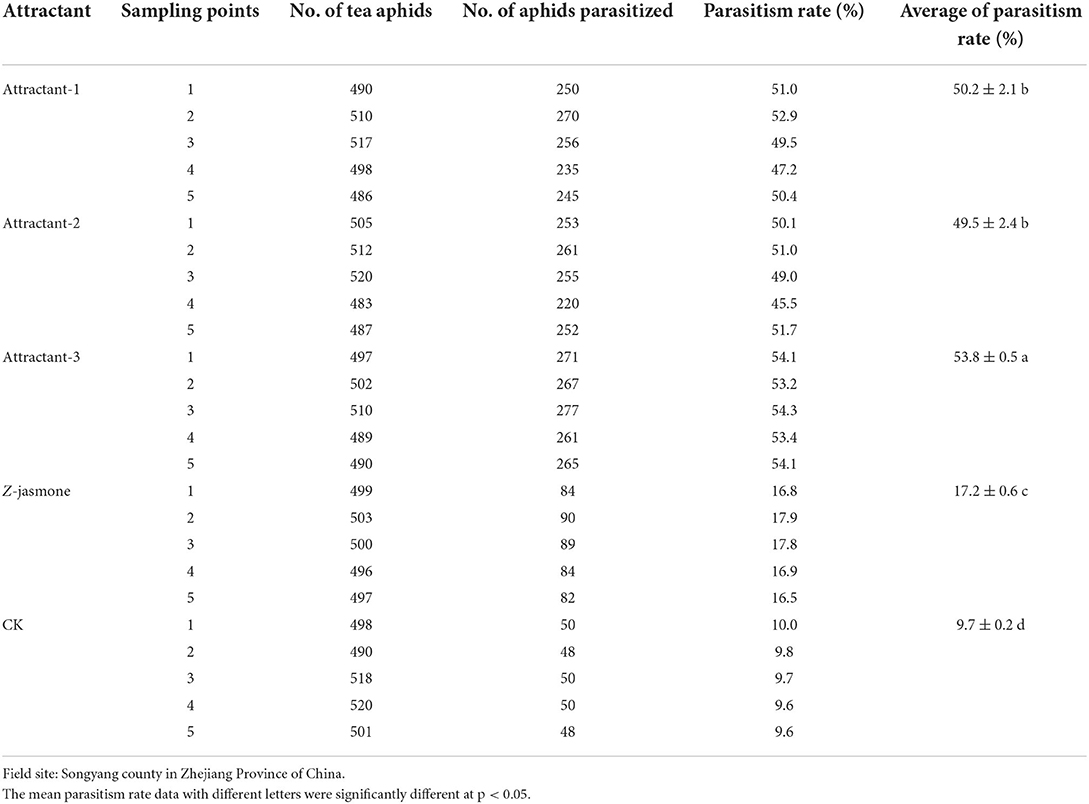
Table 6. Parasitism rates of tea aphids by Aphelinus sp. enticed and regulated by three attractant mixtures and Z-jasmone in comparison with untreated CK (Field trial 1).
Field trial 2
The field survey showed that average parasitism rates of tea aphids by Aphelinus sp. in Attractant-3 plots, Attractant-1 plots, Attractant-2 plots, and CK plots were 32.7, 30.2, 29.3, and 6.1%, respectively, on September 25, 2019. There was a significant treatment effect (ANOVA; F = 421.4, df = 19, p < 0.01). The average parasitism rate in the Attractant-3 plots was the highest, which was significantly higher than that in the CK plots (Table 7).
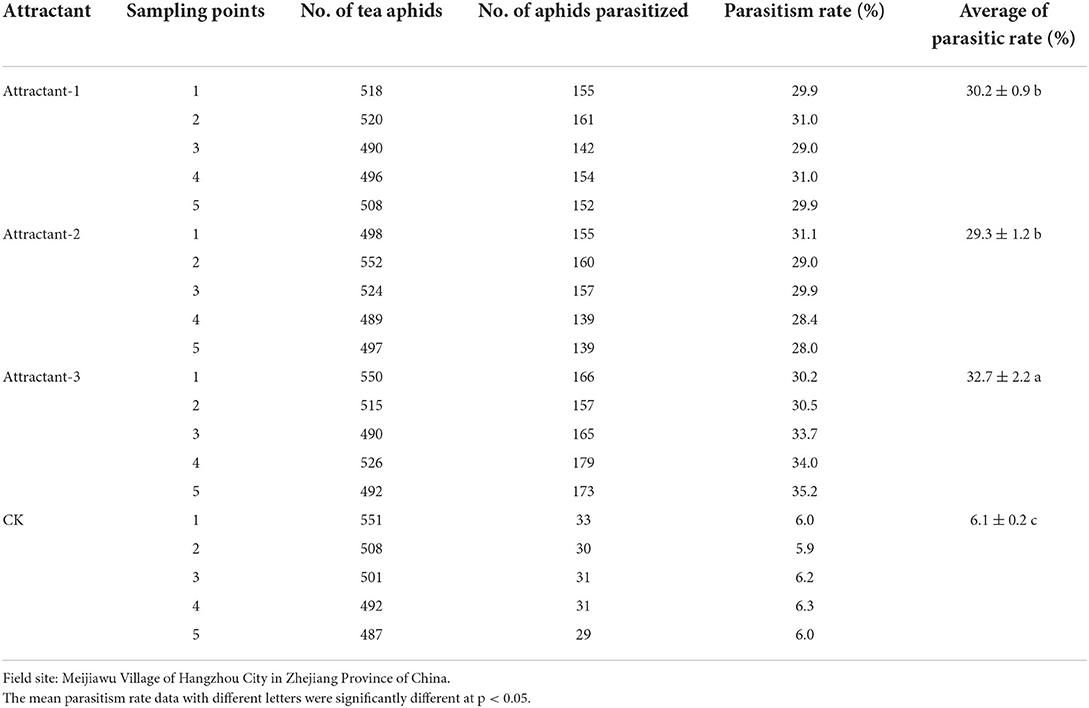
Table 7. Parasitism rates of tea aphids by Aphelinus sp. enticed and regulated by three attractant mixtures in comparison with untreated CK (Field trial 2).
Aphid control efficacy comparison between attractant-3 plots and pesticide spray plots
As shown in Figure 3, the approximate numbers of aphid-infested tea shoots were surveyed in the field trial plots of Attractants A and B, spraying pesticide and CK before the treatments started. After insecticide spray, the number of infested tea shoots sharply declined on August 27, 2020, while those in the plots of Attractants A and B, as well as the plots of CK continued to increase. On September 10, 2020, the number of infested shoots in the plots of Attractants A and B, and the plots of CK reached a peak. Possibly due to the parasitism by Aphelinus sp., the average numbers of aphid-infested shoots in the Attractant A plots decreased by 22.2% on September 10, and by 49.3% on September 25, respectively, compared with those in the CK plots. In the meantime, the average number of aphid-infested shoots gradually rose in pesticide sprayed plots from August 27 to September 25, 2020.
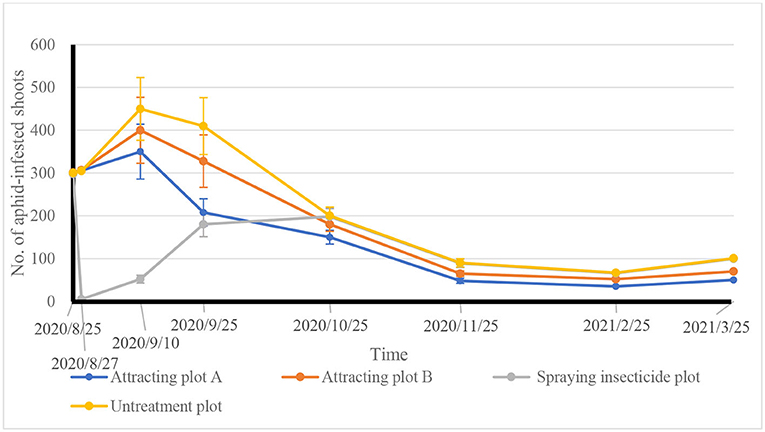
Figure 3. Tea aphid population fluctuations within Attractant plot A and plot B, spraying pesticide plot and CK plot.
On September 25, the lures in the Attractant A and Attractant B plots were all refreshed and insecticide was also re-applied in the pesticide plots, which reduced the number of infested shoots in each treatment plot. After October 25, 2020, a similar trend in the number of infested shoots appeared in pesticide sprayed plots and CK plots (Figure 3).
On October 25, the average numbers of aphid-infested shoots in the Attractant A and Attractant B plots decreased in turn by 25.0 and 10%, respectively, compared with that in the CK plots (or g pesticide sprayed plots). Moreover, the difference in the average numbers of aphid-infested shoots from the Attractant A plots, Attractant B plots, pesticide spray plots, and CK plots reached the level of p < 0.05 for both October 25 and November 25 sampling dates.
The adjusted reduction rate of aphid-infested tea shoots in the Attractant A plots was 47% on November 25, when the tea aphids would be on the point of going to hibernation and overwintering, and their average infested shoot numbers fell approximately 50% compared to that of CK plots (or pesticide spray plots). From February 25, 2021, the spring tea shoots were plucked and processed in Songyang county, and the aphids started to rapidly reproduce. Around March 25, the aphid population entered the 1st peak period of the year, and the adjusted reduction rate of infested tea shoots in Attractant A and Attractant B plots decreased in sequence by 50.7 and 30.9%, respectively, compared to the CK plots (or pesticide sprayed plots) (Figure 3). Aphid control efficacy in Attractant A plots was higher than that in Attractant B plots because the density of Attractant-3 lures in the former was markedly higher than that in the latter.
Discussion
Tea plants are a type of evergreen shrubs that constitute closed and stable communities in which a great number of pests and their natural enemies inhabit. Tea aphids have always been one of the most important pest insects in tea plantations in China, Japan, India, Sri Lanka, and Kenya with frequent outbreaks (Agarwala and Bhattacharya, 1995). Although abundant natural enemies exist in tea plantations, it is really difficult to artificially and effectively utilize them to suppress the tea pests. Aphelinus sp. is a major egg/nymph parasitoid of tea aphids with a relatively high parasitism rate during late August and September (Zeng and Wu, 2014). However, under natural conditions, Aphelinus sp. wasps cannot keep pace with the tea aphid populations, therefore by themselves will not effectively suppress tea aphid populations below the economical threshold. The discovery and development of a powerful wasp attractant might attract and retain a high population of Aphelinus sp. into the tea plantations and will significantly increase the parasitism of tea aphids by the wasps, especially during the fall season. The high parasitism by the wasps in fall would lower overwintering and next spring populations of the tea aphid, significantly reducing damage to the tea shoots and leaves in spring, which is the best harvest season for the most expensive and top-grade of spring teas.
In this study, three synthetic mixture candidates (Attractant-1, Attracatnt-2, and Attractant-3), formulated based on the active compounds identified from aphid-injured tea shoots, tea flowers, aphid sex pheromones, and body rinses showed the significant attraction to Aphelinus sp. in both lab and field bioassays, with Attractant-3 being the most attractive and effective. Employment of Attractant-3 dispensers in two different tea plantations significantly increased the parasitism rates of tea aphids by Aphelinus sp. wasps by 44.1% in the organic tea plantation (with 9.7% in the CK plots of the field trail 1) and 26.6% in the non-organic tea plantation (with 6.1% in the CK plots of the field trail 2), respectively, during the late September 2019. The disparity in the parasitism rates of the same treatment (Attractant-3) between these two tea plantations (field trials 1/2) is mainly due to their management type (organic vs. non-organic) and the difference in the abundances of both tea aphids and Aphelinus sp. wasps.
Employing semiochemicals to attract natural enemies for enhanced biological control against pest insects is a complicated process (Turlings and Erb, 2018; Mair and Ruther, 2019), especially for insects like aphids that reproduce rapidly and are migratory. To effectively suppress tea aphids at their peak density, a sufficient number of Aphelinus sp. are needed. Thus, the continuous release of active wasp attractants at certain high rates might be necessary before the tea aphid population reaches its peak. In this study, fiber-based felt was used as the attractant holding substrate, which could be used to hold a high dose of attractant, and the PVC film was used as a controlled-release membrane for a long-lasting release of the active wasp attractant in tea plantations (Cui et al., 2015).
The active attraction distance of semiochemicals and the movement pattern of Aphelinus sp. can be affected by the plant traits in the field. Thus, these factors should be considered in studying insect feeding behaviors and plant-pest-natural enemy interactions, and application protocols of insect attractant dispensers. Aartsma et al. found that Cotesia parasitic wasp could find plants that release HIPVs 10–20 m away in the Brassica oleracea–Pieris brassicae–Cotesia glomerata interaction system they studied (Aartsma et al., 2019). Plants that release HIPVs at a high release rate can extend this distance to >20 m. In this study, we found that Aphelinus sp. can be attracted within the distance between two neighboring lures/dispensers (8 m or 12 m) within the row, with an 8-m distance being better than the 12-m one.
Aphelinus is a group of important parasitic wasp species in crop fields (Pons et al., 2011; Takada et al., 2011; Elliott et al., 2014; Zeng and Wu, 2014; Lee et al., 2015; Leblanc and Brodeur, 2018; Wang Z. Z. et al., 2019), but only one species (Aphelinus sp.) was reported in tea plantations in China so far. The mass propagation and release of Aphelinus sp. as a biocontrol agent have not been reported yet. In this study, Aphelinus sp. was found to be attracted to the tea plantations baited with synthetic synomone attractants, which enhanced the parasitization of the tea aphids by the parasitic wasps. This approach not only decreased aphid populations in the fall but also suppressed the overwintering and next spring aphid populations; therefore, effectively reducing the aphid damage to the spring teas. To the best of our knowledge, this is the first report on using synomone compounds to modulate Aphelinus sp. behavior for enhanced biological control of tea aphids. It may represent a novel and promising perspective on the prevention or control of tea aphids.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
Conceived of the experiments and supervision: BH. Methodology: SH and BH. Data analysis: YW, SH, and MW. Writing—original draft preparation: BH, YW, and SH. Reviewing, revising, and editing: BH and Q-HZ. All authors have read and agreed to publish the manuscript.
Funding
This work was supported by the Zhejiang Provincial Key Research and Development Program of China (2020C02026), the National Natural Science Foundation of China (32072626 and 32001910), the Zhejiang Provincial Fundamental and Public Welfare of China (LGN20C140005), the Fundamental Research Funds for the Provincial Universities of Zhejiang (2021YW41), the Science and Technology Project of State Administration for Market Regulation (S2021MK0009), and the Team Scientific Special Commissioner Project of Zhejiang Provincial Department of Science and Technology (2020.1-2024.12, SY-1).
Conflict of interest
Author Q-HZ was employed by Sterling International, Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.958871/full#supplementary-material
References
Aartsma, Y., Leroy, B., Werf, W., Dicke, M., Poelman, E., and Bianchi, F. (2019). Intraspecific variation in herbivore-induced plant volatiles influences the spatial range of plant-parasitoid interactions. Oikos 128, 77–86. doi: 10.1111/oik.05151
Agarwala, B. K., and Bhattacharya, S. (1995). Seasonal abundance of black citrus aphid Toxoptera aurantii in North-East India: role of temperature. Proc. Indian Natl. Sci. Acad. 5, 377–382.
Cui, L., Zhang, X. T., Zhou, N. N., Ye, H. X., Yu, J. Z., Zhu, Y., et al. (2015). Behavioral responses of Chrysopa septempunctata to synomones of tea plants and sex pheromones of aphids: effectiveness on tea aphid control. Acta Entomol. Sin. 35, 1537–1546. doi: 10.5846/stxb201402050214
Das, S. C., and Kakoty, N. N. (1992). Biological studies on tea aphids, Toxoptera aurantii Boyer, and its natural enemy complex. Two Bud 39, 29–33.
Elliott, N. C., Backoulou, G. F., Giles, K. L., and Royer, T. A. (2014). Aphids and parasitoids in wheat and nearby canola fields in central Oklahoma. Southwest. Entomol. 39, 23–28. doi: 10.3958/059.039.0103
Fakhouri, K. E., Sabraoui, A., Kehel, Z., and Bouhssini, M. E. (2021). Population dynamics and yield loss assessment for pea aphid, Acyrthosiphon pisum (Harris) (Homoptera: Aphididae), on lentil in Morocco. Insects 12, 1080. doi: 10.3390/insects12121080
Ghosh, A., Das, A., Lepcha, R., Majumdar, K., and Baranwal, V. K. (2015). Identification and distribution of aphid vectors spreading Citrus tristeza virus in Darjeeling hills and Dooars of India. J. Asia Pac. Entomol. 18, 601–605. doi: 10.1016/j.aspen.2015.07.001
Gregg, P. C., Socorro, A. P. D., and Henderson, G. S. (2010). Development of a synthetic plant volatile-based attracticide for female noctuid moths. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 49, 21–30. doi: 10.1111/j.1440-6055.2009.00734.x
Guidolin, A. S., and Cônsoli, F. L. (2018). Diversity of the most commonly reported facultative symbionts in two closely-related aphids with different host ranges. Neotrop Entomol. 47, 440–446. doi: 10.1007/s13744-017-0532-0
Han, B. Y. (1995). On the pathogenic characteristics of insect pathogens and their application in tea pest control. J. Fujian Tea 4, 32–34.
Han, B. Y. (2001). Attractive activity to natural enemies and component analysis of the rinses from tea aphid body surface. Acta Entomol. Sin. 44, 541–547. doi: 10.16380/j.kcxb.2001.04.023
Han, B. Y., and Chen, Z. M. (2002a). Behavioral and electrophysiological responses of natural enemies to synomones from tea shoots and kairomones from tea aphids, Toxoptera aurantii. J. Chem. Ecol. 28, 2203–2219. doi: 10.1023/a:1021045231501
Han, B. Y., and Chen, Z. M. (2002b). Composition of the volatiles from intact and mechanically pierced and tea aphid-tea shoot complexes and their attraction to natural enemies of the tea aphid. J. Agric. Food Chem. 50, 2571–2575. doi: 10.1021/jf010681x
Han, B. Y., Wang, M. X., Zheng, Y. C., Niu, Y. Q., Pan, C., Cui, L., et al. (2014). Sex pheromone of the tea aphid, Toxoptera aurantii (Boyer de Fonscolombe) (Hemiptera: Aphididae). Chemoecology 24, 179–187. doi: 10.1007/s00049-014-0161-6
Han, B. Y., Zhang, Q. H., and Byers, J. A. (2012). Attraction of the tea aphid, Toxoptera aurantii, to combinations of volatiles and colors related to tea plants. Entomol. Exp. Appl. 144, 258–269. doi: 10.1111/j.1570-7458.2012.01303.x
Han, B. Y., Zhou, P., Cui, L., and Fu, J. Y. (2007). Characterization of the key aromatic constituents in tea flowers of elite Chinese tea cultivars. Int. J. Tea Sci. 6, 31–36.
Hazarika, L. K., Bhuyan, M., and Hazarika, B. N. (2009). Insect pests of tea and their management. Annu. Rev. Entomol. 54, 267–284. doi: 10.1146/annurev.ento.53.103106.093359
Ismail, M., Zanolli, P., Muratori, F., and Hance, T. (2021). Aphids facing their parasitoids: a first look at how chemical signals may make higher densities of the pea aphid Acyrthosiphon pisum less attractive to the parasitoid Aphidius ervi. Insects 12, 878. doi: 10.3390/insects12100878
Leblanc, A., and Brodeur, J. (2018). Estimating parasitoid impact on aphid populations in the field. Biol. Control 119, 33–42. doi: 10.1016/j.biocontrol.2018.01.002
Lee, S., Kim, I. K., Park, Y. K., Choi, C. W., and Byun, B. K. (2015). Preliminary survey of indigenous parasites associated with Phyllocnistis citrella Stainton (Lepidoptera, Gracillariidae) in Jeju, Korea. J. Asia Pac. Biodivers. 8, 371–374. doi: 10.1016/j.japb.2015.09.002
Mair, M. M., and Ruther, J. (2019). Chemical ecology of the parasitoid wasp genus Nasonia (Hymenoptera, Pteromalidae). Front. Ecol. Evol. 7, 184. doi: 10.3389/fevo.2019.00184
Mu, D., Cui, L., Ge, J., Wang, M. X., Liu, L. F., Yu, X. P., et al. (2012). Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoasca vitis. Insect Sci. 19, 229–238. doi: 10.1111/j.1744-7917.2011.01476.x
Nakashima, Y., Ida, T. Y., Powell, W., Pickett, J. A., Birkett, M. A., Taki, H., et al. (2016). Field evaluation of synthetic aphid sex pheromone in enhancing suppression of aphid abundance by their natural enemies. BioControl 61, 485–496. doi: 10.1007/s10526-016-9734-3
Pons, X., Lumbierres, B., and Antoni, R. (2011). Parasitoid complex of alfalfa aphids in an IPM intensive crop system in northern Catalonia. J. Pest Sci. 84, 437–445. doi: 10.1007/s10340-011-0383-0
Shizuoka Plant Epidemic Prevention Association (2001). Diagnostic Chart of Crop Pests and Diseases (Enlarged Edition). Shizuoka: Starlight Printing Co. Ltd. 227–228.
Sudoi, V., and Rotich, F. (1997). The rearing of hoverfly Xanthogramma aegyptium (Diptera: Syrphidae) for use as a biocontrol agent in controlling citrus aphids Toxoptera aurantii (Homoptera: Aphidae) in tea. Tea 18, 42–44.
Takada, H., Nakamura, C., and Miyazaki, M. (2011). Parasitoid spectrum (Hymenoptera: Braconidae; Aphelinidae) of the soybean aphid Aphis glycines (Homoptera: Aphididae) in Japan and Indonesia (Java and Bali). Entomol. Sci. 14, 216–219. doi: 10.1111/j.1479-8298.2010.00422.x
Tang, Q. (2020). DPS© Data Processing System Volume III Specialized Statistics and Miscellanea (Fifth Edition). Beijing: Science Press.
Turlings, T. C. J., and Erb, M. (2018). Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 63, 433–452. doi: 10.1146/annurev-ento-020117-043507
Wang, L. D., and Wang, X. Q. (2011). Epizooticological modeling for four strains of Lecanicillium (Verticillium) lecanii against tea aphid Toxoptera aurantia. Wuyi Sci. J. 27, 69–73. doi: 10.15914/j.cnki.wykx.2011.00.005
Wang, X. Q., Jiang, R. L., Lin, D. L., and Wang, L. D. (2008). Epizooticology for four strains of Lecanicillium (Verticillium) lecanii against tea aphid Toxoptera aurantia. Chin. J. Biol. Control 24, 32–35. doi: 10.16409/j.cnki.2095-039x.2008.s1.006
Wang, Y., Ding, M., Du, Y. M., and Huang, A. J. (2019). Phylogenetic relationship and characterization of the complete mitochondrial genome of the black citrus aphid, Aphis aurantii (Hemiptera: Aphididae). Mitochondrial DNA Part B 4, 3567–3568. doi: 10.1080/23802359.2019.1674208
Wang, Z. Z., Liu, Y. Q., Shi, M., Huang, J. H., and Chen, X. X. (2019). Parasitoid wasps as effective biological control agents. J. Integr. Agric. 18, 705–715. doi: 10.1016/S2095-3119(18)62078-7
Zeng, M. S., and Wu, G. Y. (2014). Images of Tea Plant Diseases and Pests and Their Natural Enemies in Fujiang Province of China. Beijing: China Agricultural Science and Technology Press.
Keywords: tea aphid, Aphelinus sp., attractant, enhanced parasitism, synthetic semiochemicals
Citation: Wu Y, Han S, Wang M, Zhang Q-H and Han B (2022) Control of tea aphids via attracting the parasitic wasp, Aphelinus sp. with synthetic semiochemicals. Front. Ecol. Evol. 10:958871. doi: 10.3389/fevo.2022.958871
Received: 01 June 2022; Accepted: 27 July 2022;
Published: 23 August 2022.
Edited by:
Li Chen, Hebei University, ChinaReviewed by:
Yong Zhang, Institute of Plant Protection (CAAS), ChinaJohannes Stökl, University of Bayreuth, Germany
Copyright © 2022 Wu, Han, Wang, Zhang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoyu Han, aGFuYmFveXVAY2psdS5lZHUuY24=
 Yiqi Wu1
Yiqi Wu1 Qing-He Zhang
Qing-He Zhang Baoyu Han
Baoyu Han