- 1School of Archaeology and Museology, Sichuan University, Chengdu, China
- 2Department of Anthropology, Washington University in St. Louis, St. Louis, MO, United States
- 3School of History, Zhengzhou University, Zhengzhou, China
For centuries, hunting and herding of large bovids were important for human survival at high altitudes on the Tibetan Plateau. However, little is known about past human relations with iconic plateau animals, such as takins (Budorcas taxicolor Hodgson, 1850) or yaks (Bos grunniens Linnaeus, 1766). Takins were widely hunted historically for subsistence and social reasons, but an understanding of ancient relationships has been hampered by the difficulty of distinguishing takins from other large wild or domestic bovids, e.g., gaurs (Bos gaurus), yaks, cattle (Bos taurus), water buffalo (Bubalus bubalis). Through the comparative and systematic study of modern specimens, comprising 80 mandibles and 53–78 skeletons curated across five institutions in China and the United States, this research proposes a new set of osteomorphological criteria for differentiating large bovids from the Tibetan Plateau and tests previously published criteria. The results show that takins can be easily differentiated from yaks, cattle, gaurs, and water buffalos using readily identifiable shape differences, non-metric characteristics, and specific landmarks of mandibular teeth and post-cranial elements. Criteria with especially high-reliability scores include mandibular teeth and 14 postcranial elements: scapula, humerus, femur, tibia, fibula, metapodials, lunate, scaphoid, magnum, unciform, and astragalus. Providing a reproducible field method for distinguishing takins from other large bovids in this region, the osteomorphological criteria established in this study will further archaeological investigations of Holocene hunting on the Tibetan Plateau, as well as early usage of domesticated yaks and cattle. These criteria can also be used in conservation to aid field identification of illegally hunted takins.
Introduction
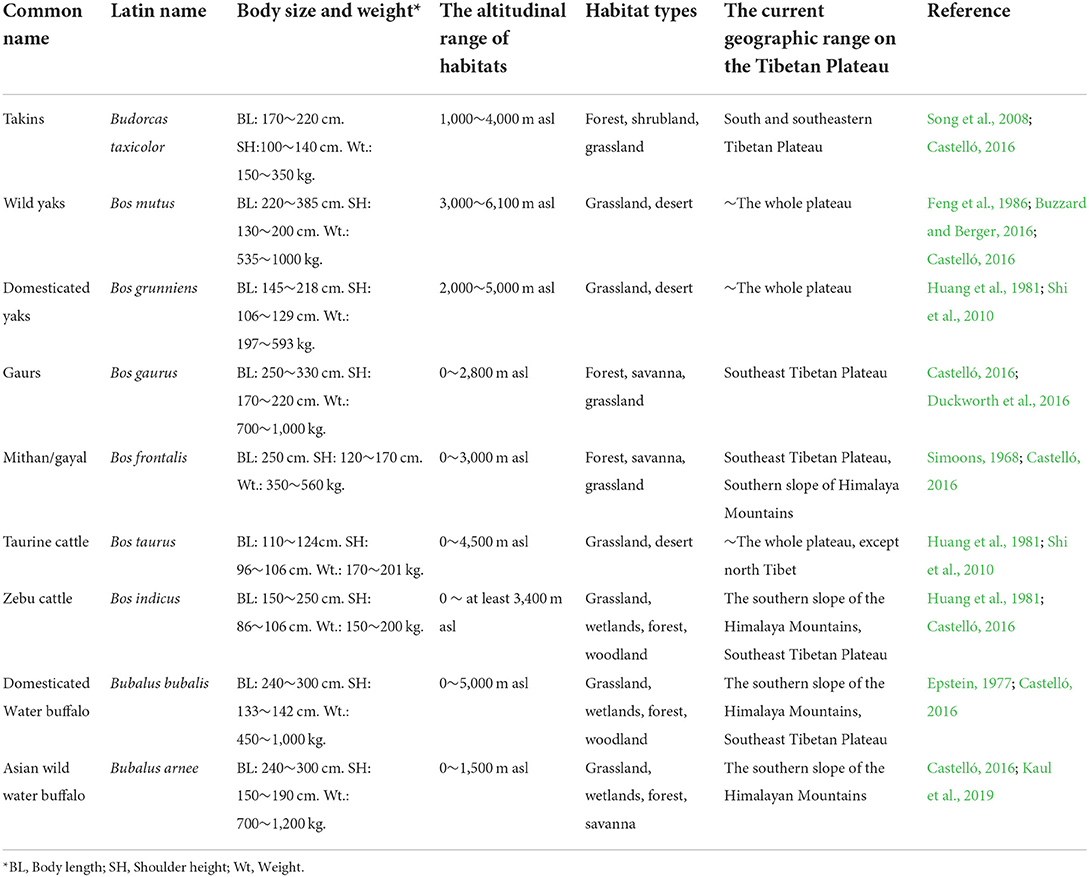
Hunting of wild animals of the Tibetan Plateau (e.g., Tibetan argali [Ovis ammon], blue sheep [Pseudois nayaur], Tibetan antelope [Panthalops hodgsonii], Tibetan gazelle [Procapra picticaudata], takins [Budorcas taxicolor], wild yaks [Bos mutus], and gaurs [Bos gaurus] and herding of domestic sheep [Ovis aries], goats [Capra hircus], taurine cattle [Bos taurus] and yaks [Bos grunniens]) have played a significant role in human survival at high altitudes on the Tibetan Plateau (Cheng et al., 1984; Goldstein and Beall, 1990; Schaller, 2000; Dong et al., 2016; Zhang, 2016; Ren, 2017; Wang, 2017; Zhang et al., 2019; Chen et al., 2020; Wang et al., 2020). Given the iconic nature of plateau animals, such as takins or yaks, however, we know especially little about ancient human relations with large bovids. Takins, also known as “cattle chamois” (Order Artiodactyla, Family Bovidae, Subfamily Caprinae), have been widely hunted in recent centuries for meat and hides, as well as for trade and social and ritual purposes (Feng et al., 1986, p. 234; Guo, 2004; Aiyadurai et al., 2010; Rao et al., 2011; Chen et al., 2020). As browsers, takins have a broad range across the plateau and are adapted to forests, shrublands, and subalpine meadows between 1,000 and 5,000 m a.s.l. (Neas and Hoffmann, 1987; Song et al., 2008; Castelló, 2016) (Table 1). Best documented in the northwest and southeast Tibetan Plateau and Himalayan regions during the historical period, takins migrate seasonally between high (>2,500 m a.s.l.) and low altitude areas for food and to visit salt licks (Wu et al., 1990; Zeng et al., 2008).
Ethnographic records make it clear that people of the southeast Tibetan Plateau and Himalayan regions have had a long history of interactions with wild takins and know their behavior well (Feng et al., 1986, p. 234; Guo, 2004; Aiyadurai et al., 2010; Rao et al., 2011; Chen et al., 2020). Active in herds of 10–50 individuals, takins create obvious paths during regular seasonal migrations, making them more easily tracked and successfully hunted. Several historic studies record mass hunting, with sometimes more than 10 takins trapped at one time (Wu et al., 1990; Guo, 2004). However, the nature of ancient relationships between hunters and the takins is poorly understood. Although large bovids are present at many sites, including Shannashuzhan (>1000 identifiable specimens, Chen et al., 2020), Xiariyamakebu, and others (e.g., Huang and Leng, 1985; Zhou, 1999; He and Chen, 2006; Dong et al., 2016; Zhang et al., 2019), the difficulty of distinguishing among takins and other large wild or domestic bovids has hampered zooarchaeological understanding of this group. Takins are members of the subfamily Caprina but are relatively large, making it possible for fragments of their bones to be confused with those of wild yaks (Bos mutus), domesticated yaks, cattle, gaurs (Bos gaurus), gayal/mithan (domesticated/semi-domesticated gaurs, Bos frontalis), wild water buffalo (Bubalus arnee), and domesticated water buffalo (Bubalus bubalis) (Table 1; Figure 1). Takins were identified at Xiariyamakebu (~3,300 Cal BP), where early sheepherders hunted takins as well as deer (Dong et al., 2016). Takins have not, however, been definitively identified on hunter-gatherer/farmer sites. Analyses that allow more detailed identification of large bovids will allow scholars to determine whether the presence of takins or other large bovids on sites has been masked by identification at broad taxonomic levels (e.g., large bovids).
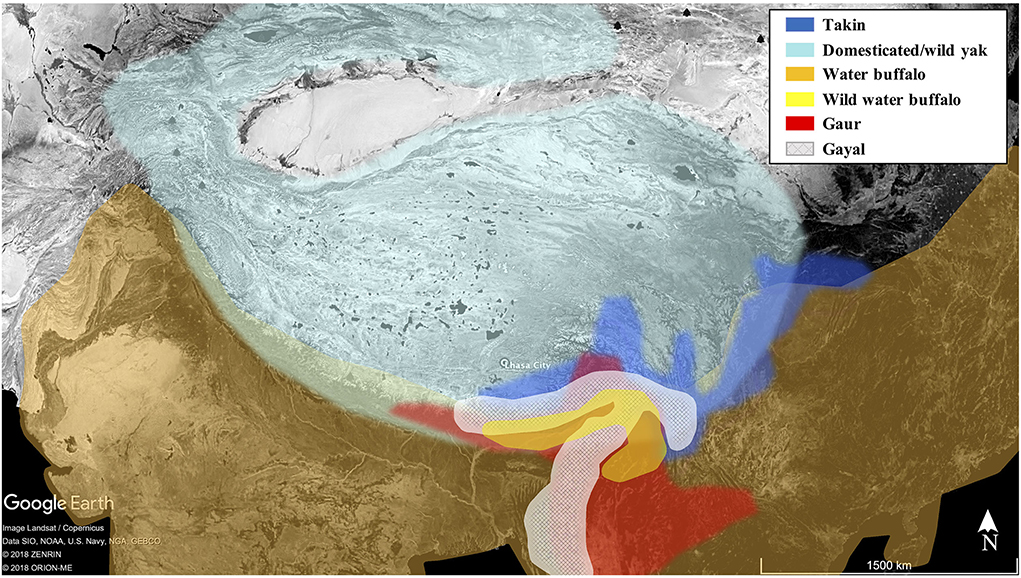
Figure 1. Approximate distributions of extant large bovids of the Tibetan Plateau and vicinity (Data from Simoons, 1968; Zhang, 1989; Song et al., 2008; Buzzard and Berger, 2016; Duckworth et al., 2016; Gilbert et al., 2018; Kaul et al., 2019). Cattle (predominantly taurine) are found throughout the region.
Biomolecular methods (i.e., ZooMS and ancient DNA analyses) have recently contributed to the taxonomic identification of archaeological faunas. They have been instrumental in the analysis of fragmentary or indeterminate specimens. Sampling for these studies is still reliant, however, on baseline osteomorphological taxonomic analyses that characterize variation (e.g., taxonomic, body part, taphonomic) in large zooarchaeological assemblages and is undertaken in laboratories. Osteomorphological analyses are irreplaceable in field zooarchaeological studies, non-destructive, portable, and no/low-cost. However, building research scaffolds for baseline osteomorphological analyses of the Tibetan Plateau wild taxa is challenging. Due to overhunting (food, markets, products), large wild bovids are vulnerable or even threatened with regional extinction (Song et al., 2008; Buzzard and Berger, 2016; Duckworth et al., 2016; Kaul et al., 2019) extinction. As a result, necessary conservation restrictions on collecting skeletal remains make it difficult for regional institutes to build comparative reference collections of wild Tibetan mammals. To lay the foundation for regional zooarchaeological research and to create a baseline for further biomolecular analyses, studies are needed on the comparative osteomorphology of large bovids from the Tibetan Plateau held in global museums and research institute collections.
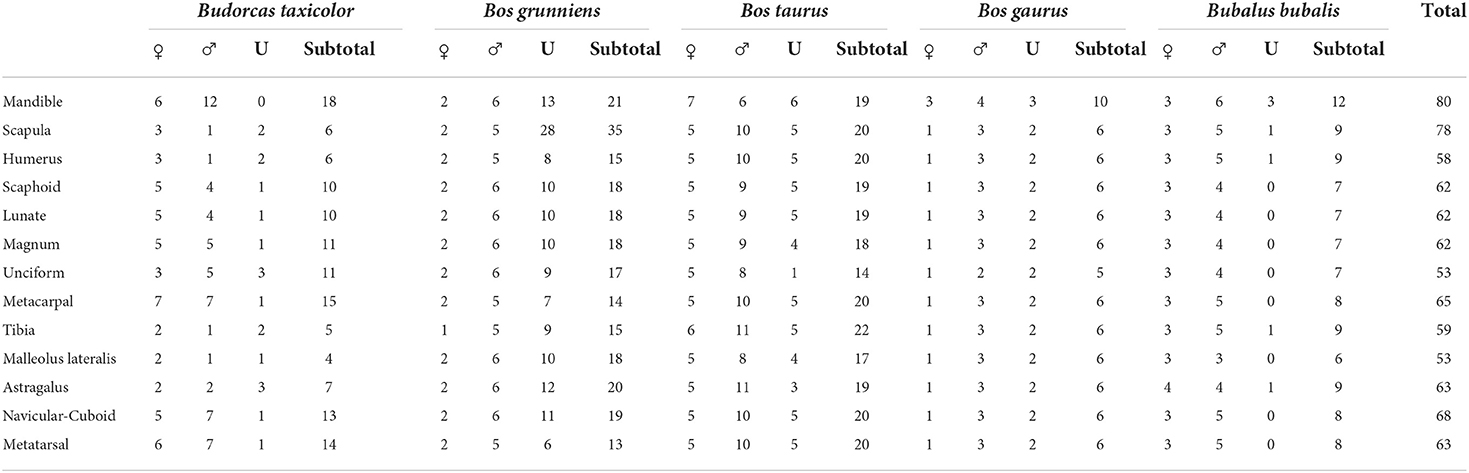
Here, we present comparative osteomorphological criteria for distinguishing takins mandibular teeth and post-cranial skeletons from four regionally common Bovini species: yaks (Bos grunniens), taurine cattle (Bos taurus), gaurs (Bos gaurus), and domesticated water buffalos (Bubalus bubalis), focusing on readily identifiable shape differences (non-metric) that will facilitate field identification. This research builds on the few previous studies that mention osteomorphological characteristics for distinguishing cranial and a few post-cranial skeletal elements of takins from other members of the Bovini (e.g., Lydekker, 1907; Wu et al., 1990; Gentry, 1992; Von Den Driesch, 1995; Vrba and Schaller, 2000). These have shown high potential for differentiating varied large bovids on the Tibetan Plateau, but more comprehensive studies are needed that include takins and other taxa and larger sample sizes (see Lyman, 2019 regarding general sample sizes). To do this, we conducted a systematic study of 80 mandibles and 68 skeletons of modern takins, yaks, gaurs, cattle, and water buffalo held at five institutes in China and the United States. This allowed us to test previously published osteomorphological criteria for differentiating closely related large regional bovids and to propose a set of new criteria for the identification of takins.
Methods and materials
The baseline for this study was provided by the review of previously published osteomorphological criteria for differentiating takins from other large bovids, including domesticated yaks (Vrba and Schaller, 2000), cattle (Wu et al., 1990), and European bison (Bison bonasus) (Gentry, 1992). Criteria from the global osteological literature on differentiation among bovids and the Bovini following paleontological and zooarchaeological perspectives (e.g., Lawrence, 1951; Olsen, 1960; Higham, 1975; Peters, 1986; Balkwill and Cumbaa, 1992; Gee, 1993; Von Den Driesch, 1995; Li, 2019) that might be useful for Tibetan taxa.
We studied mandibular teeth and post-cranial skeletal elements (Table 2) of modern takins (18 mandibles and 4–13 skeletons), domesticated yaks (18 mandibles and 13–35 skeletons), gaurs (15 mandibles and six skeletons), taurine cattle (19 mandibles and 17–22 skeletons), and water buffalo (14 mandibles and 7–9 skeletons) at the American Museum of Natural History (AMNH), the National Museum of Natural History at the Smithsonian Institution (NMNH), the Field Museum (FMNH), the Henan Provincial Institute of Cultural Heritage and Archaeology (Henan), and the Shaanxi Provincial Institute of Archaeology (Shaanxi) (Appendix 1). Specimens were mostly historical and collected during the 19–20th centuries from Asian, American, and European countries (e.g., China, the United States, Russia, India, and Vietnam). Bos indicus was excluded as well-identified specimens were not widely available. Taxonomies are debated for many of the taxa studied, but here we follow zooarchaeological convention (Gentry et al., 2004) for domestic taxa and the IUCN (e.g., Song et al., 2008) and Wilson and Reeder (2005) for wild taxa. This study aimed to identify major shape differences, distinct characters, and landmarks. Measurements are included in Appendix 2. The strength of this sample is that it represents a significant portion of known world holdings for Tibetan taxa. Weaknesses are that some elements samples remain small (e.g., tibia and fibula). In such cases, we only included obvious character differences that scored highly in reliability testing. An issue common to most bovid morphological research is that it focuses on historically collected animals (wild caught or shot). No animals were collected for this study). Without destructive ancient DNA testing (which is currently not possible), undetected hybridization could not be ruled out. Similarly, records did not always exist regarding the populations from which these animals were collected. As a result, the study's goal was to identify clear trends evident in the sample as a whole. Twenty-two elements were studied for each taxon, excluding only the cranium, ribs, vertebra, and phalanges. We included most carpals and tarsals because these small, dense bones are often recovered from sites and have a history of successful discrimination among bovids but are often excluded from identification studies. Anatomical nomenclature used followed Gentry (1992), paleontological convention, and English landmark terms from the Nomenclature (2017). Biometric data was collected on all measurable specimens following Von Den Driesch (1976). Our notation system for pilot criteria was straightforward, designed to recognize clear results rather than degrees of similarity. Z. Zhang carried out all direct museum observations. He started by systematically comparing several specimens from each species to create a set of pilot criteria (two to nine distinguishable characters) that would allow discrimination of each takins element from elements of other taxa, including yaks, cattle, gaurs, and domestic water buffalo. The pilot criteria were examined using all available physical specimens (assigned “1” when a pilot criterion was unambiguous, a “0” when a pilot criterion did not work, or a “0.5” if a character appeared intermediate). Proposed criteria (“1”) were further interrogated through interdependent examination of digital images by FM.
To synthesize and examine the reliability of our observations, we used quantification tools introduced by Hanot and Bochaton (2018). This includes the number of observations (N Obs), which tallies the number of specimens examined in this study, the number of attributions (NA), which tallies the number of specimens assigned “1” and “0” designations, the number of correct attributions (NC), which tallies the number of specimens that were assigned “1,” the percentage of assessment (PA = NA/N Obs*100 reflects the difficulty of defining the certain character clearly and the variability of the criterion), and the correct identification rate (CIR = NC/NA*100, reflects the reliability of the criterion). In this article, we report only criteria with total PA and CI. Both are ≥80% (raw datasheets are included in Appendix 3).
Results
We found reliable criteria (with one to four distinguishable characteristics) for mandibular teeth and fourteen postcranial elements: scapula, humerus, magnum (carpale II + III), unciform (carpi IV), scaphoid (carpi radiale), lunate (carpi intermediate), metacarpal, femur, tibia, fibula (malleolus lateralis), metatarsal, navicular-cuboid (centroquartale), and calcaneus, astragalus. We did not find reliable identification criteria for the ulna, radius, cuneiform (carpi ulnare), and patella (see Appendix 4 for characteristics examined in this study that were found to be unreliable). There were only three atlas and four-axis specimens of takins available in the collections we visited, so these two elements were excluded in this study. Here we describe the most robust criteria.
Mandibular teeth
TE1 (Figure 2). The buccal outlines of lower molars (N Obs of total = 75, PA = 99%, CIR = 100%): In occlusal view, the outlines of the buccal side of takins molars are more pointed (as shown in landmark TE1 in Figure 2) (N Obs = 23, PA = 96%, CIR = 100%) than those of Bovini species, which are more bowed or rounded (N Obs = 52, PA = 100%, CIR = 100%).
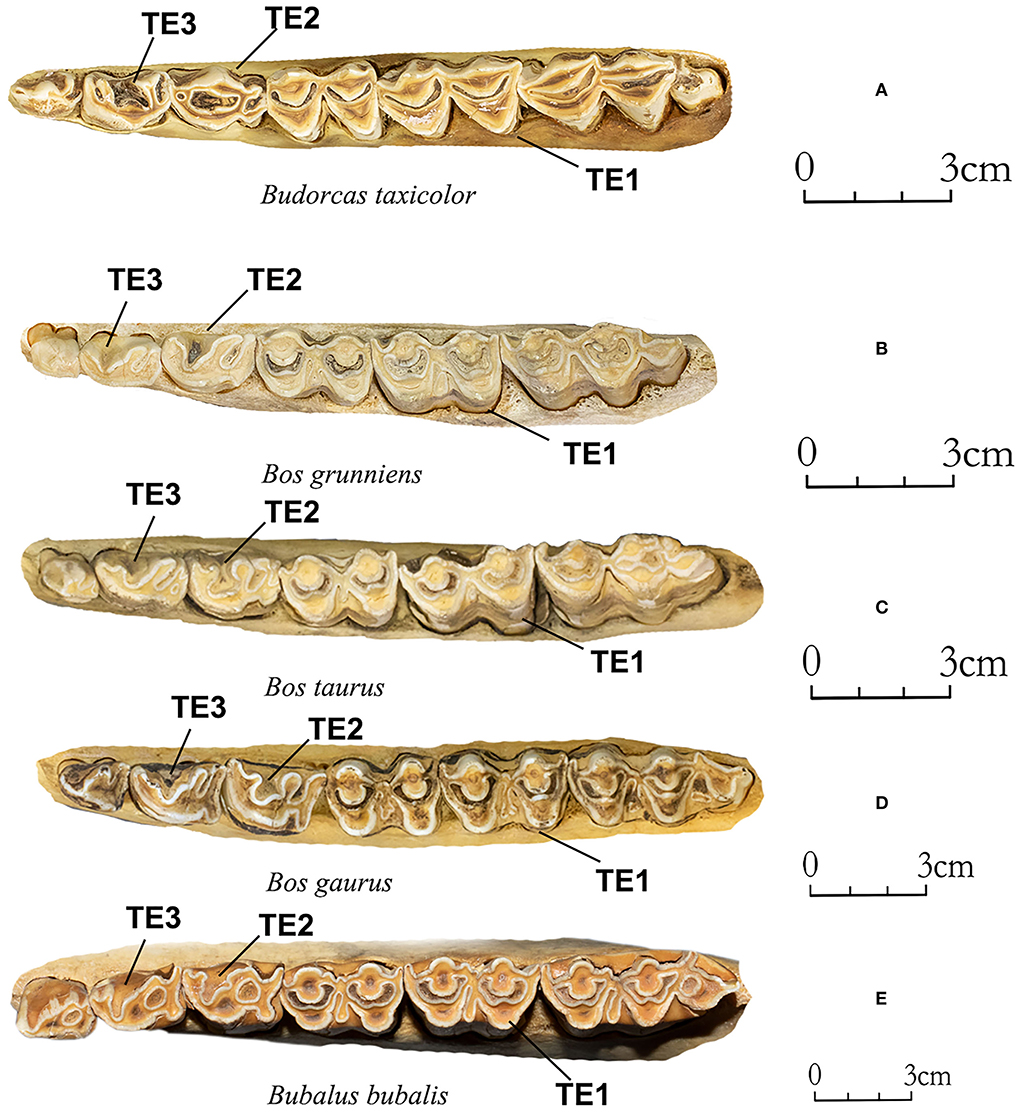
Figure 2. Mandibular teeth of takins and Bovini. (A) Budorcas taxicolor (AMNH–M−57016), (B) Bos grunniens (AMNH–M−22909). (C) Bos taurus (AMNH–M−22734). (D) Bos gaurus (AMNH–M−112979). (E) Bubalus bubalis (AMNH–M−54766). Specimens of Bos gaurus and Bubalus bubalis were from the right side; we filled the photos horizontally to be consistent with the sides of other specimens on this figure. Photograph of each specimen was taken individually using a DSLR (APS–C) camera with a 35 mm lens. Photosgraph were grouped using graphic software.
TE2 (Figure 2). The lower fourth premolar (N Obs of total = 70, PA = 86%, CIR = 100%): The metaconid of takins (N Obs of takins = 23, PA = 96%, CIR = 100%) fuses or almost fuses with the paraconid and forms a closed rectangle but is open on P4 of Bovini species (N Obs of Bovini = 47, PA = 81%, CIR = 100%). This characteristic has been previously described by Gentry (1992) as a characteristic for distinguishing takins and European bison.
TE3 (Figure 2). The lower third premolar (N Obs = 74, PA = 97%, CIR = 99%): Metaconid and paraconid of P3 of takins form a restricted “U” shape (N Obs of takins = 23, PA = 96%, CIR = 95%) but form an open “V” shape on Bovini species (N Obs of Bovini = 51, PA = 98%, CIR = 100%).
Scapula
SC1 (N Obs of total = 79, PA = 85%, CIR = 94%, Figures 3F–J). A medial notch is present in the lateral border of the glenoid cavity in the four Bovini species (N Obs = 72, PA = 83%, CIR = 90%) but absent in takins (N Obs = 7, PA = 100%, CIR = 100%).
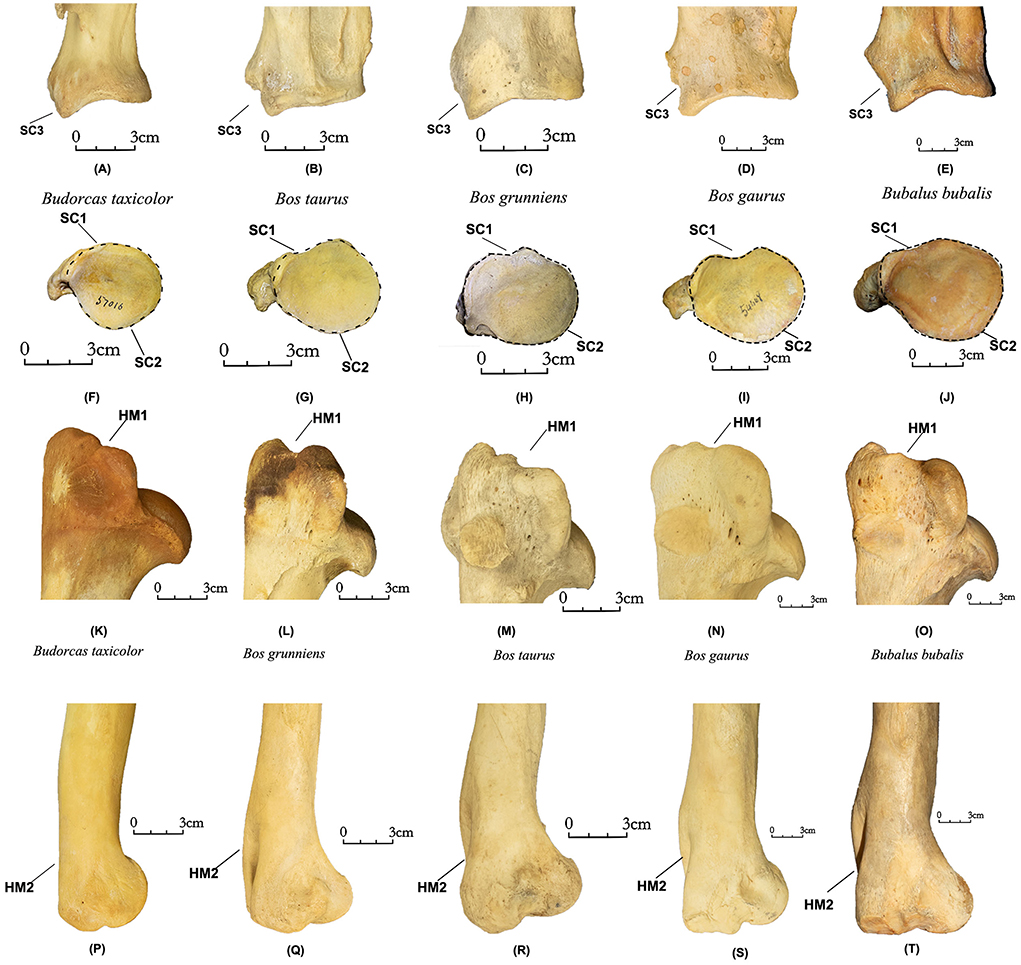
Figure 3. Scapula and humerus of takins and Bovini. Lateral view of the scapula: (A) Budorcas taxicolor (AMNH–M−57016), (B) Bos taurus (AMNH−77932). (C) Bos grunniens (AMNH−10263). (D) Bos gaurus (AMNH−54470). (E) Bubalus bubalis (AMNH–M−54766). Cranioventral view of the scapula: (F) Budorcas taxicolor (AMNH–M−57016), (G) Bos taurus (AMNH−77932). (H) Bos grunniens (AMNH−10263). (I) Bos gaurus (AMNH−54469). (J) Bubalus bubalis (AMNH–M−54766). Lateral view of proximal humerus: (K) Budorcas taxicolor (AMNH–M−57016), (L) Bos grunniens (AMNH–M−22909). (M) Bos taurus (AMNH−10731). (N) Bos gaurus (AMNH−112979). (O) Bubalus bubalis (AMNH–M−54765). Medial view of distal humerus: (P) Budorcas taxicolor (AMNH–M−57016), (Q) Bos grunniens (AMNH–M−22909). (R) Bos taurus (AMNH−10731). (S) Bos gaurus (AMNH−112979). (T) Bubalus bubalis (AMNH–M−54765).
SC2 (N Obs of total = 79, PA = 96%, CIR = 99%, Figures 3A–E). The outline of the glenoid cavity is more rounded in takins (N Obs = 7, PA = 86%, CIR = 100%) but more oval in four Bovini species (N Obs = 72, PA = 97%, CIR = 99%).
SC3 (N Obs of total = 79, PA = 100%, CIR = 100%, Figures 3F–J). In the lateral view, the coracoid process in takins is prominent, and there is no notch between the lateral border of the glenoid cavity and the coracoid process (N Obs = 7, PA = 100%, CIR = 100%). The coracoid process is much reduced in some Bovini (e.g., Bos grunniens), and there is a notch visible between the lateral border of the glenoid cavity and the coracoid process in the other four Bovini species (N Obs = 72, PA = 100%, CIR = 100%).
Humerus
HM1 (N Obs of total = 53, PA = 92%, CIR = 92%, Figures 3K–O). The proximal border of the greater trochanter exhibits a more open single notch in takins (N Obs = 7, PA = 100%, CIR = 100%). This area has a more restricted notch or shows dual notches in the other four Bovini species (N Obs = 46, PA = 91%, CIR = 90%).
HM2 (N Obs of total = 60, PA = 100%, CIR = 100%, Figures 3P–T). The posterior border of the medial condyle is flat in takins (N Obs = 7, PA = 100%, CIR = 100%) but is curved in the other four Bovini species (N Obs = 53, PA = 100%, CIR = 100%).
Scaphoid
SP1 (N Obs of total = 60, PA = 100%, CIR = 98%, Figures 4A–E). In the articular surface of the lunate, distal small articular facets are much reduced in takins (N Obs = 11, PA = 100%, CIR = 91%) and only cover the anterior end. The same articular facets are more prominent and cover both distal parts of the anterior and posterior ends of the scaphoid in the other four Bovini species (N Obs = 49, PA = 100%, CIR = 100%).
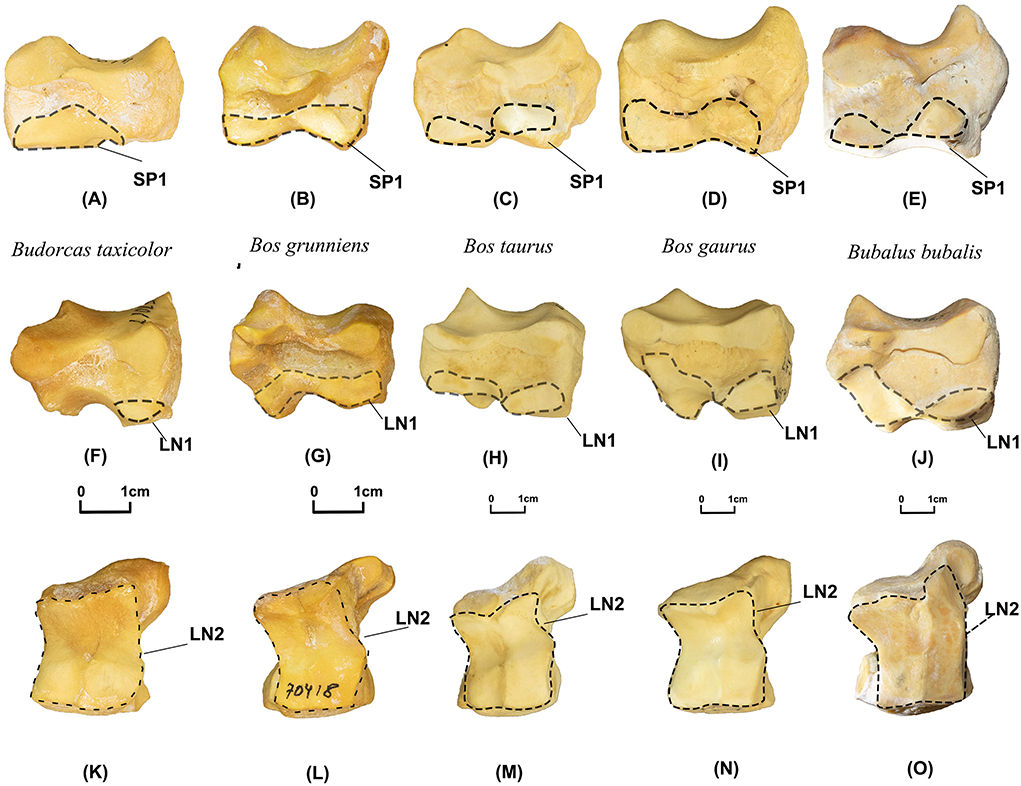
Figure 4. Scaphoid and lunate of takins and Bovini. Scaphoid: (A) Budorcas taxicolor (AMNH–M−57014), (B) Bos grunniens (AMNH–M−22909). (C) Bos taurus (AMNH–M−100031). (D) Bos gaurus (AMNH–M−54470). (E) Bubalus bubalis (AMNH–M−54765). View of the articular surface for scaphoid of lunate: (F) Budorcas taxicolor (AMNH−57017), (G) Bos grunniens (AMNH–M−70418). (H) Bos taurus (AMNH−245658). (I) Bos gaurus (AMNH–M−112979). (J) Bubalus bubalis (AMNH–M−54765). Distal view of lunate: (K) Budorcas taxicolor (AMNH−57017), (L) Bos grunniens (AMNH–M−70418). (M) Bos taurus (AMNH−245658). (N) Bos gaurus (AMNH–M−112979). (O) Bubalus bubalis (AMNH–M−54765).
Lunate
LN1 (N Obs of total = 60, PA = 100%, CIR = 99%, Figures 4F–J). In view of the articular surface for the scaphoid, two small distal articular facets are more reduced in takins (N Obs = 10, PA = 100%, CIR = 90%) than in the other four Bovini species (N Obs = 50, PA = 100%, CIR = 100%).
LN2 (N Obs of total = 61, PA = 100%, CIR = 97%, Figures 4K–O). In the distal view, the outline of the distal articular facet is more rectangular in takins (N Obs = 11, PA = 100%, CIR = 91%) but more irregular in the other four Bovini species (N Obs = 50, PA = 100%, CIR = 98%).
Magnum
MG1 (N Obs of total = 65, PA = 100%, CIR = 98%, Figures 5A–E). In the proximal view, a deep depression is present on the posterior border of the medial articular facet in takins (N Obs = 13, PA = 100%, CIR = 100%) but nearly absent in the other four Bovini species (N Obs = 52, PA = 100%, CIR = 98%).
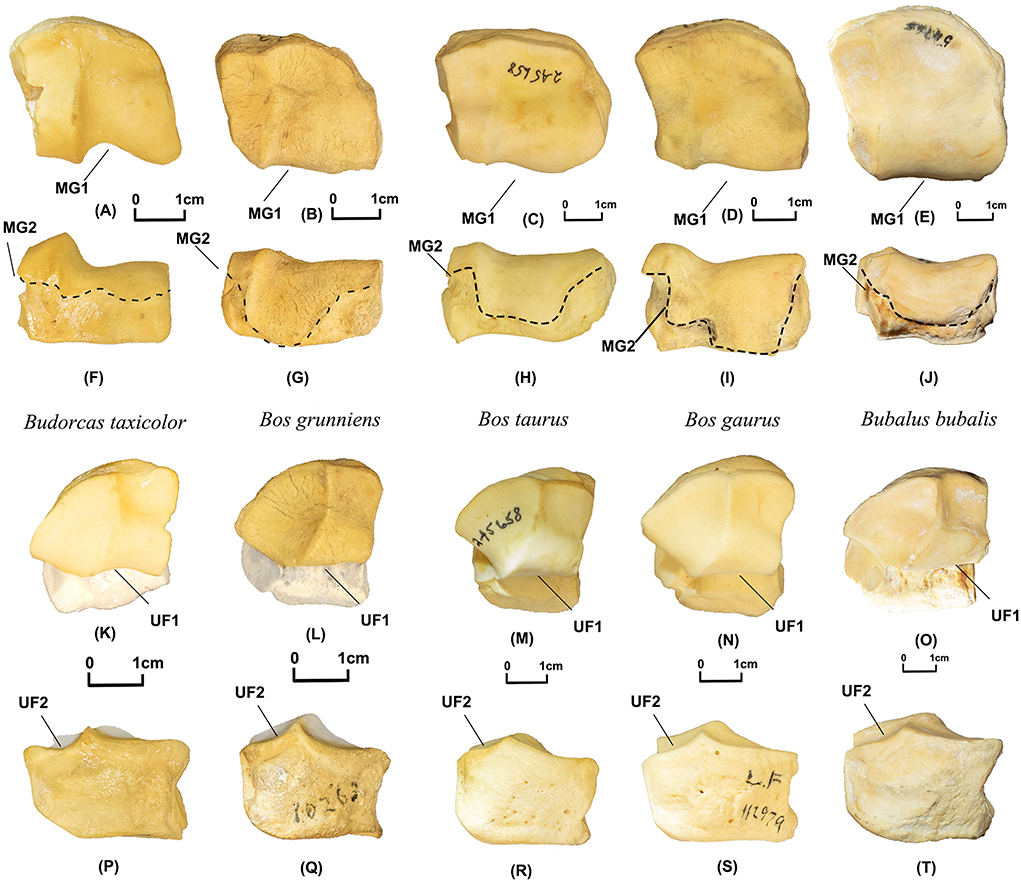
Figure 5. Magnum and unciform of takins and Bovini. Proximal view of magnum: (A) Budorcas taxicolor (AMNH−57017), (B) Bos grunniens (AMNH–MO−10263). (C) Bos taurus (AMNH−245658). (D) Bos gaurus (AMNH–M−54469). (E) Bubalus bubalis (AMNH–M−54765). Posterior view of magnum: (F) Budorcas taxicolor (AMNH−57017), (G) Bos grunniens (AMNH–MO−10263). (H) Bos taurus (AMNH−245658). (I) Bos gaurus (AMNH–M−54469). (J) Bubalus bubalis (AMNH–M−54765). Proximal view of unciform: (K) Budorcas taxicolor (AMNH−57017), (L) Bos grunniens (AMNH–MO−10263). (M) Bos taurus (AMNH−245658). (N) Bos gaurus (AMNH–M−112979). (O) Bubalus bubalis (AMNH–M−54765). Posterior view of unciform: (P) Budorcas taxicolor (AMNH−57017), (Q) Bos grunniens (AMNH–MO−10263). (R) Bos taurus (AMNH−245658). (S) Bos gaurus (AMNH–M−112979). (T) Bubalus bubalis (AMNH–M−54765).
MG2 (N Obs of total = 64, PA = 100%, CIR = 100%, Figures 5F–J). In the posterior view, the proximal articular facet is much reduced in takins (N Obs = 13 PA = 100%, CIR = 100%). The proximal articular facets are farther extended distally in the other four Bovini species (N Obs = 51, PA = 100%, CIR = 100%).
Unciform
UF1 (N Obs of total = 53, PA = 100%, CIR = 96%, Figures 5K–O). In proximal view, the posterior border of the medial articular facet is much more curved in takins (N Obs = 11, PA = 100%, CIR = 100%) but relatively flat in the other Bovini species (N Obs = 42, PA = 100%, CIR = 95%).
UF2 (N Obs of total = 53, PA = 100%, CIR = 100%, Figures 5P–T). In the anterior view, a deep depression is present at the border of the lateral articular facet in takins (N Obs = 11, PA = 100%, CIR = 100%) but absent in the other Bovini species (N Obs = 42, PA = 100%, CIR = 100%).
Metacarpal
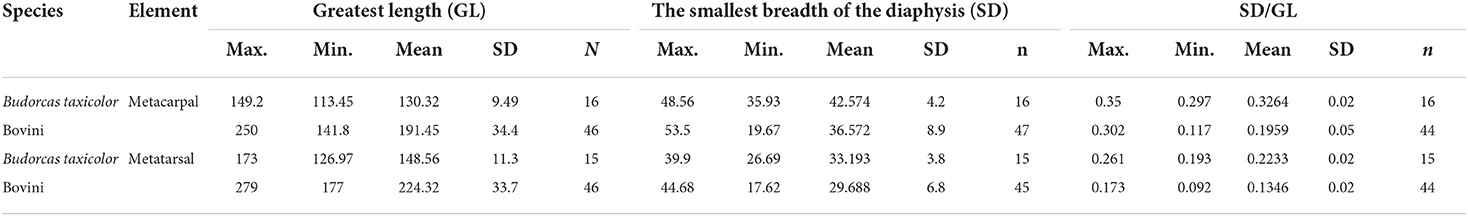
MC1 (N Obs of total = 68, PA = 100%, CIR = 100%). The overall shape of the takins metacarpal is the stoutest in all five species (Table 3). This characteristic has been pointed out by Lydekker (1907) as the most remarkable feature of the takins skel. It has been suggested by Wu et al. (1990), Vrba and Schaller (2000), and Gentry (1992) as a characteristic for distinguishing takins from cattle, yaks, or European bison.
MC2 (N Obs of total = 63, PA = 100%, CIR = 100%, Figures 6A–E). The notch between proximal articular facets is much reduced or nearly absent in takins (N Obs = 16, PA = 100%, CIR = 100%) compared to the other four Bovini species (N Obs = 47, PA = 100%, CIR = 100%).
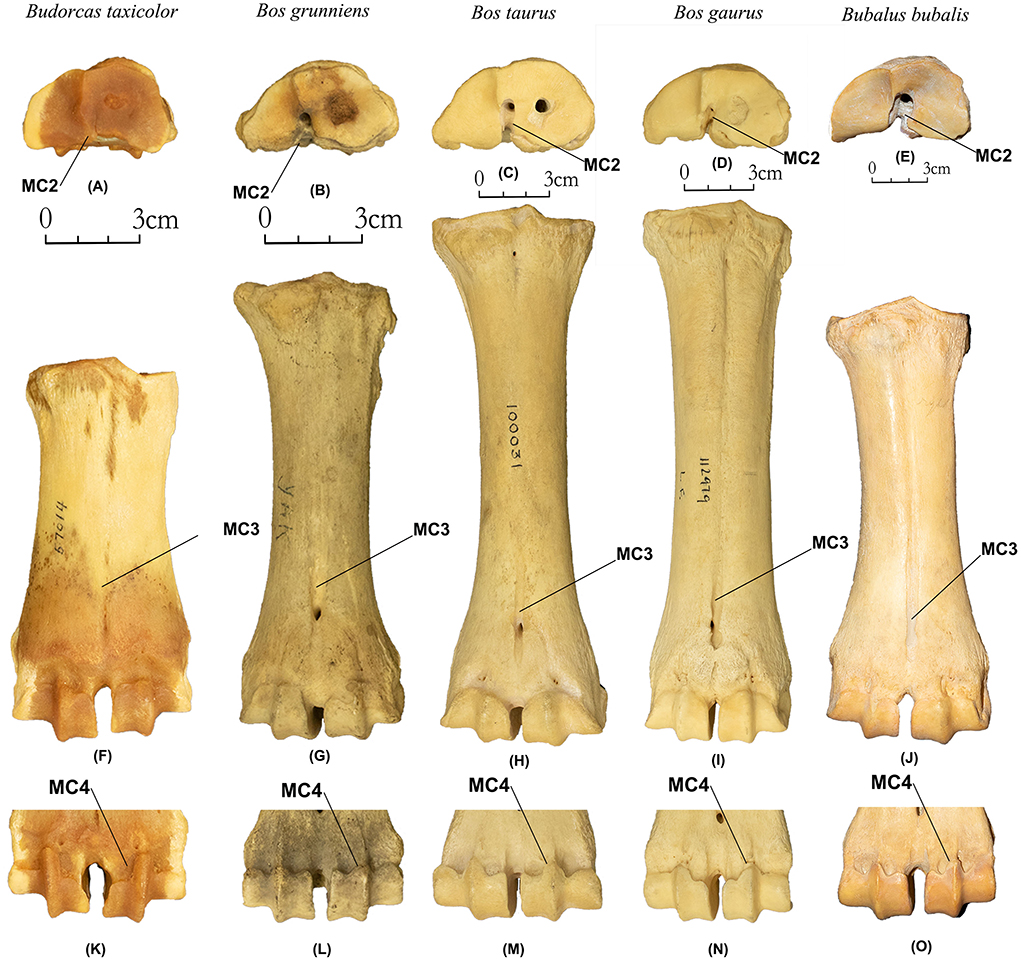
Figure 6. Metacarpal of takins and Bovini. Proximal view: (A) Budorcas taxicolor (AMNH−57014), (B) Bos grunniens (AMNH–M−22909). (C) Bos taurus (AMNH–M−77932). (D) Bos gaurus (AMNH–M−112979). (E) Bubalus bubalis (AMNH–M−54766). Anterior view: (F) Budorcas taxicolor (AMNH–), (G) Bos grunniens (AMNH–M−22909). (H) Bos taurus (AMNH–M−77932). (I) Bos gaurus (AMNH–M−112979). (J) Bubalus bubalis (AMNH–M−54766). Posterior view of distal end: (K) Budorcas taxicolor (AMNH−57014), (L) Bos grunniens (AMNH–M−22909). (M) Bos taurus (AMNH–M−77932). (N) Bos gaurus (AMNH–M−112979). (O) Bubalus bubalis (AMNH–M−54766).
MC3 (N Obs of total = 66, PA = 100%, CIR = 95%, Figures 6F–J). The anterior longitudinal groove is much shallower in takins (N Obs = 16, PA = 100%, CIR = 100%) than in the other four Bovini species (N Obs = 50, PA = 100%, CIR = 94%). This characteristic has been previously described by Gentry (1992) as a characteristic for distinguishing takins and European bison.
MC4 (N Obs of total = 67, PA = 100%, CIR = 97%, Figures 6K–O). In the posterior view, the trochlea projection is much farther extended toward the proximal end in takins (N Obs = 16, PA = 100%, CIR = 100%) than in the other four Bovini species (N Obs = 51, PA = 100%, CIR = 96%).
Tibia
TB1 (N Obs of total = 53, PA = 100%, CIR = 100%, Figures 7A–E). The craniolateral notches in takins (N Obs = 6, PA = 100%, CIR = 100%) are much more restricted than in the other four Bovini species (N Obs = 47, PA = 100%, CIR = 100%).
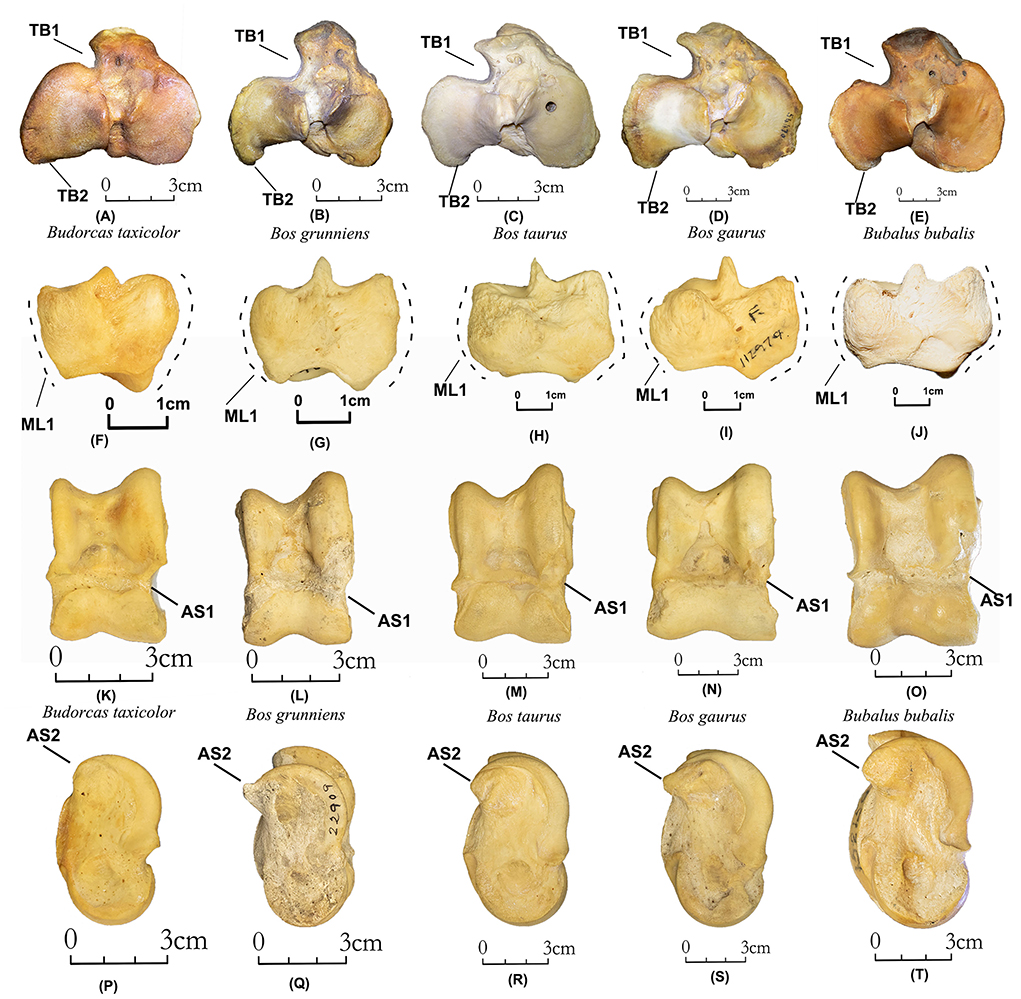
Figure 7. Tibia, malleolus lateralis, and astragalus of takins and Bovini. Proximal view of the tibia: (A) Budorcas taxicolor (AMNH−57016), (B) Bos grunniens (AMNH–M−22909). (C) Bos taurus (AMNH–M−77932). (D) Bos gaurus (AMNH–M−54470). (E) Bubalus bubalis (AMNH–M−54766). Lateral view of malleolus lateralis: (F) Budorcas taxicolor (AMNH−57016), (G) Bos grunniens (AMNH–M−90186). (H) Bos taurus (AMNH−245658). (I) Bos gaurus (AMNH–M−112979). (J) Bubalus bubalis (Henan−77). Dorsal view of astragalus: (K) Budorcas taxicolor (AMNH−57017), (L) Bos grunniens (AMNH–M−22909). (M) Bos taurus (AMNH–M−100031). (N) Bos gaurus (AMNH–M−54470). (O) Bubalus bubalis (AMNH–M−54766). Medial view of astragalus: (P) Budorcas taxicolor (AMNH−57017), (Q) Bos grunniens (AMNH–M−22909). (R) Bos taurus (AMNH–M−100031). (S) Bos gaurus (AMNH–M−54470). (T) Bubalus bubalis (AMNH–M−54766).
TB2 (N Obs of total = 56, PA = 98%, CIR = 98%, Figures 7A–E). The caudolateral border of the lateral condyle in takins (N Obs = 6, PA = 83%, CIR = 100%) is flat in takins but is curved in the other four Bovini species (N Obs = 50, PA = 100%, CIR = 98%).
Malleolus lateralis
ML1 (N Obs of total = 54, PA = 100%, CIR = 96%, Figures 7F–J). In the lateral view, the anterior and posterior borders of the malleolus lateralis of takins (N Obs = 5, PA = 100%, CIR = 100%) form a more flared outline than in the other four Bovini species (N Obs = 49, PA = 100%, CIR = 96%).
Astragalus
AS1 (N Obs of total = 63, PA = 100%, CIR = 98%, Figures 7K–O). In dorsal view, the middle of the lateral border is more medially concave in takins (N Obs = 7, PA = 100%, CIR = 86%) than in other Bovini species (N Obs = 56, PA = 100%, CIR = 100%).
AS2 (N Obs of total = 63, PA = 100%, CIR = 98%, Figures 7P–T). In the medial view, the proximo-planter projection is rounded in takins (N Obs = 7, PA = 100%, CIR = 86%) but more pointed in other Bovini species (N Obs = 56, PA = 100%, CIR = 100%).
Navicular-Cuboid
NC1 (N Obs of total = 71, PA = 100%, CIR = 100%, Figures 8A–E). The length of the articular facet for the calcaneum is much shorter in takins (N Obs = 16, PA = 100%, CIR = 100%) than in the other four Bovini species (N Obs = 55, PA = 100%, CIR = 100%).

Figure 8. Navicular-cuboid and metatarsal of takins and Bovini. Proximal view of navicular-cuboid: (A) Budorcas taxicolor (AMNH–M−57016), (B) Bos grunniens (AMNH–MO−10263). (C) Bos taurus (AMNH–M−100031). (D) Bos gaurus (AMNH–M−112979). (E) Bubalus bubalis (AMNH–M−54766). Anterior view of the metatarsal: (F) Budorcas taxicolor (AMNH–M−57014), (G) Bos taurus (AMNH−245658). (H) Bos grunniens (AMNH–M−22909). (I) Bos gaurus (AMNH–M−54470). (J) Bubalus bubalis (AMNH–M−54765). Posterior view of the metatarsal: (K) Budorcas taxicolor (AMNH–M−57014), (L) Bos taurus (AMNH−245658). (M) Bos grunniens (AMNH–M−22909). (N) Bos gaurus (AMNH–M−54470). (O) Bubalus bubalis (AMNH–M−54765).
Metatarsal
MT1 (N Obs of total = 63, PA = 100%, CIR = 100%). The overall shape of the metatarsal of the takins is the stoutest among all five species studied (Table 3). This characteristic has been pointed out by Lydekker (1907) as the most remarkable feature of the takins skeleton.
MT2 (N Obs of total = 65, PA = 100%, CIR = 100%, Figures 8F–J). The posteromedial portion of the proximal articular surface is much more raised in takins (N Obs = 16, PA = 100%, CIR = 100%) than in four Bovini species (N Obs = 49, PA = 100%, CIR = 100%). This characteristic has been previously described by Gentry (1992) as a characteristic for distinguishing takins and European bison.
MT3 (N Obs of total = 66, PA = 100%, CIR = 95%, Figures 8F–J). The anterior longitudinal groove is much shallower in takins (N Obs = 16, PA = 100%, CIR = 81%) than in the other four Bovini species (N Obs = 50, PA = 100%, CIR = 100%).
MT4 (N Obs of total = 65, PA = 100%, CIR = 100%, Figures 8K–O). In the posterior view, the projection of the trochlea is considerably farther extended toward the proximal end in takins (N Obs = 16, PA = 100%, CIR = 100%) than in the other four Bovini species (N Obs = 49, PA = 100%, CIR = 100%).
Discussion and conclusion
This study provides a reproducible method for distinguishing takin bones and bone fragments from those of other bovines, including domesticated yaks (Bos grunniens), taurine cattle (Bos taurus), gaurs (Bos gaurus), and domesticated water buffalo (Bubalus bubalis) present on the Tibetan Plateau or Himalayan region with 25 new characteristics identified on 13 skeletal elements. Our findings demonstrate that characters of the takins humerus, tibia, lunate, magnum, unciform, metacarpal, cuboid-navicular, and metatarsal are especially reliable, with quantitative assessments of reliability higher than 95% (percentage of assessment and correct identification rates (following Hanot and Bochaton, 2018). This is also true of dental characteristics, including the buccal outlines of lower molars and cusps of the lower third and fourth premolars. We also consider identifications of characters of the scapula, distal humerus, tibia, fibula, and astragalus robust (PA and CIR >95%) despite smaller samples because of the magnitude of differences, e.g., lateral border of the glenoid cavity and the coracoid process are continuous on takins scapula but are discontinuous on those of other taxa (Figure 3).
It is noteworthy that in addition to characters that score above 95%, there are characters with scores higher than 80% that we think are still useful. These include characters TE2 on teeth, scapula (SC1, SC2), humerus (HM1), lunate (LN2,) SP1, metacarpal (MC3), tibia (TB2), astragalus (AS1, AS2), and metatarsal (MT3). We suggest, however, that they should not be relied on alone but rather considered as supporting evidence to complement characters scoring higher than 95% during taxonomic identification. We also found characteristics of the femur and calcaneus that show potential for distinguishing takins from Bovini (Appendix 5) but that need to be tested further due to small sample sizes.
In sum, carpals and tarsals made up a high proportion of the identifiable elements identified. We conducted the first work on the scapula, humerus, lunate, scaphoid, magnum, unciform, tibia, fibula, astragalus, and navicular-cuboid. Since these are dense elements commonly found in archaeological assemblages, these criteria will be useful. We also verified that the mandibular teeth and metapodial characteristics (i.e., TE2, MC1, MC3, MT1, and MT2) suggested by previous scholars (Lydekker, 1907; Wu et al., 1990; Gentry, 1992; Vrba and Schaller, 2000) are reliable for distinguishing takins from other Bovini. Due to limitations in the time that we were allowed to stay at each institute, the variable qualities of comparative specimens, and in some cases, the difficulty of identifying the characteristics mentioned in previous publications (e.g., phylogenic analyses without visual examples), we were not able to make an exhaustive list of reliable characteristics at this stage. There are undoubtedly others that could be added to those listed in this study. Some characteristics worth future attention include some suggested by previous studies that involved takins (Appendix 6) and others that have been suggested as helpful in distinguishing other members of the family Bovidae (e.g., Olsen, 1960; Higham, 1975; Peters, 1986; Balkwill and Cumbaa, 1992; Joglekar et al., 1994; Haraniya et al., 2016; Li, 2019). Future research is also needed on four species of Bovini known in the Tibetan Plateau and Himalayan regions that we could not include due to difficulties obtaining sufficient specimens, namely wild yaks (Bos mutus), gayal/mithan (Bos frontalis), zebu cattle (Bos indicus), and Asian wild water buffalo (Bubalus arnee). Furthermore, although complete teeth and bones of members of the family Cervidae are readily distinguishable from the Bovidae (e.g., Hillson, 2005 for tooth morphology, Lawrence, 1951; Brown and Gustafson, 2000 for post-cranial elements), highly fragmented takin remains might be confused with those of large cervids (e.g., Rusa unicolor, Cervus elaphus, and C. albirostris) in the region. To establish a comprehensive taxonomic identification guide for the zooarchaeology of the Tibetan Plateau and Himalayan regions, more exhaustive examinations of local faunal specimens are needed.
The osteomorphological criteria established here hold great promise for future archaeological investigations of changing hunting patterns through time and across the Tibetan Plateau. Success in distinguishing takins, which were known as a common prey of recent local hunters (e.g., Guo, 2004 and Wu et al., 1990), from other large bovids in this region, will also facilitate understanding of ancient relationships and studies of early usage of domesticated cattle on the Tibetan Plateau and yak domestication. Beyond its application in archaeology, our study will contribute to the conservation of threatened species. The non-destructive method proposed by our study can be readily applied to the field identification of takin bones in remote areas or markets where remains resulting from illegal hunting for bushmeat have been reported in some regions. Over-hunting has been one of the main threats to takins' survival, whose population is decreasing and who are listed as vulnerable by IUCN (Song et al., 2008).
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
ZZ, FM, and FL contributed to conception and design of the study. ZZ collected and analyzed the data and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This study was supported by the National Science Foundation (Grant No. 2017247, co-PIs: Xinyi Liu and ZZ), the National Social Science Fund of China (Grant No. 18CKG013, PI: FL), Zhengzhou University (Grant No. XKZDJC202006, PI: Qingzhu Liu), and the Source of China and Songshan Civilization Academic Research Institute in Zhengzhou (Grant No. Q2017-5, PI: FL).
Acknowledgments
We thank the following people for their help during research visits: Sara Ketelsen, Marisa Surovy, Eleanor Hoeger, and Neil Duncan at the American Museum of Natural History, John Ososky and Darrin Lunde at the National Museum of Natural History at the Smithsonian Institution, Juan Wang (now at the University of Science and Technology of China) and Yanfeng Hou at Henan Provincial Institute of Cultural Heritage and Archaeology, Songmei Hu at Shaanxi Provincial Institute of Archaeology; Adam Ferguson and John Phelps Jr. at the Field Museum. We also thank Xinyi Liu and Qingzhu Liu for financial supports. ZZ thanks Li Chen and Huan Zhang for facilitating Zhang's visit to the American Museum of Natural History. We are grateful to the reviewers for their insightful and constructive comments on this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.956858/full#supplementary-material
References
Aiyadurai, A., Singh, N. J., and Milner-Gulland, E. (2010). Wildlife hunting by indigenous tribes: a case study from Arunachal Pradesh, north-east India. Oryx 44, 564–572. doi: 10.1017/S0030605309990937
Balkwill, D., and Cumbaa, S. L. (1992). Guide to the identification of postcranial bones of Bos taurus and Bison bison. Can. Museum Nat. doi: 10.5962/bhl.title.128454
Brown, C. L., and Gustafson, C. E. (2000). A Key to Postcranial Skeletal Remains of Cattle/Bison, Elk, and Horse. Pullman: Washington State University Laboratory of Anthropology.
Buzzard, P., and Berger, J. (2016). Bos mutus. The IUCN Red List of Threatened Species 2016. e.T2892A101293528. doi: 10.2305/IUCN.UK.2016-2.RLTS.T2892A101293528.en
Castelló, J. R. (2016). Bovids of the World: Antelopes, Gazelles, Cattle, Goats, Sheep, and Relatives, Princeton; Oxford: Princeton University Press. doi: 10.1515/9781400880652
Chen, N., Ren, L., Du, L., Hou, J., Mullin, V. E., Wu, D., et al. (2020). Ancient genomes reveal tropical bovid species in the Tibetan Plateau contributed to the prevalence of hunting game until the late Neolithic. Proc. Natl. Acad. Sci. U. S. A. 117, 202011696. doi: 10.1073/pnas.2011696117
Cheng, H., Ni, Z., Sun, S., Yu, X., and Chen, D. (1984). Agricultural Geography of Xizang (西藏农业地理), Beijing, Science Press.
Dong, G., Ren, L., Jia, X., Liu, X., Dong, S., Li, H., et al. (2016). Chronology and subsistence strategy of Nuomuhong culture in the Tibetan Plateau. Quat. Int. 426, 42–49. doi: 10.1016/j.quaint.2016.02.031
Duckworth, J. W., Sankar, K., Williams, A. C., Samba Kumar, N., and Timmins, R. J. (2016). Bos gaurus. The IUCN Red List of Threatened Species 2016. e.T2891A46363646. doi: 10.2305/IUCN.UK.2016-2.RLTS.T2891A46363646.en
Gee, H. (1993). The distinction between postcranial bones of Bos primigenius Bojanus, 1827 and Bison priscus Bojanus, 1827 from the British Pleistocene and the taxonomic status of Bos and Bison. Jo. Quat. Sci. 8, 79–92. doi: 10.1002/jqs.3390080107
Gentry, A. (1992). The subfamilies and tribes of the family Bovidae. Mammal Rev. 22, 1–32. doi: 10.1111/j.1365-2907.1992.tb00116.x
Gentry, A., Clutton-Brock, J., and Groves, C. P. (2004). The naming of wild animal species and their domestic derivatives. J. Archaeol. Sci. 31, 645–651. doi: 10.1016/j.jas.2003.10.006
Gilbert, M., Nicolas, G., Cinardi, G., Van Boeckel, T. P., Vanwambeke, S. O., Wint, G. R., et al. (2018). Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Scient. Data. 5, 1–11. doi: 10.1038/sdata.2018.227
Goldstein, M. C., and Beall, C. M. (1990). Nomads of Western Tibet: the Survival of a Way of Life. Berkeley; Los Angeles: Univ of California Press.
Guo, G. (2004). Study on the relationships between local people and wildlife in Medog, Tibet, China. Shanghai, China: East China Normal University, 50–51.
Hanot, P., and Bochaton, C. (2018). New osteological criteria for the identification of domestic horses, donkeys and their hybrids in archaeological contexts. J. Archaeol. Sci. 94, 12–20. doi: 10.1016/j.jas.2018.03.012
Haraniya, K., Joglekar, P., and Goyal, P. (2016). Osteological keys for differentiating among hind limb bones of Bos indicus, Bubalus bubalis, and Boselaphus tragocamelus. Heritage 4, 482–502.
He, K., and Chen, J. (2006). Identification report of faunal remains from Haxiu site, Maerkang (马尔康哈休遗址出土动物骨骼鉴定报告). Chengdu Kaogu Faxian (成都考古发现), 13.
Higham, C. (1975). Non Nok Tha: The Faunal Remains From the 1966 and 1968 Excavations at Non Nok Tha, Northeastern Thailand. University of Otago, Department of Anthropology.
Huang, W., and Leng, J. (1985). “Identification of faunal remains from karuo site and research on Tibetan Plateau Climate (卡若遗址兽骨鉴定与高原气候的研究),” in Karuo: A Neolithic Site in Tibet (昌都卡若), eds E. Tong, J. Leng, S. Hou, and Suolangwangdui (eds.) (Beijing: Cultural Relics Publishing House).
Huang, W., Meng, Y., Cai, Q., and Deng, L. (1981). Domestic Animals of Xizang (西藏家畜), Beijing, Science Press.
Joglekar, P., Thomas, P., Matsushima, Y., and Pawankar, S. (1994). Osteological differences between the forelimb bones of Ox (Bos indicus), Buffalo (Bubalus bubalis) and Nilgai (Boselaphus tragocamelus). J. Bombay Vet. Coll. 5, 17–20.
Kaul, R., Williams, A. C., rithe, k., Steinmetz, R., and Mishra, R. (2019). Bubalus arnee. The IUCN Red List of Threatened Species 2019. e.T3129A46364616. doi: 10.2305/IUCN.UK.20191.RLTS.T3129A46364616.en
Lawrence, B. (1951). Part I, Mammals Found at the Awatovi Site: Post-cranial Skeletal Characters of Deer, Pronghorn, and Sheep-goat with Notes on Bos and Bison. Cambridge: Peabody Museum of American Archaeology and Ethnology.
Li, F. (2019). “Differences in osteological morphology between cattle (Bos taurus) and Yak (Bos grunniens) (黄牛与牦牛骨骼形态的对比观察),” in Zooarchaeology vol. 3: Proceedings of Zhengzhou 2016. International Committee Meeting of ICAZ and “Archaeolozoology: Global Developments and Chinese Perspectives” (动物考古第3辑), eds Archaeology HPIOCHA (Beijing: Cultural Relics Press).
Lydekker, R. (1907). The Game Animals of India, Burma, Malaya, and Tibet: Being a New and Revised Edition of'The Great and Small Game of India, Burma, and Tibet,' R. London: Ward, Limited. doi: 10.5962/bhl.title.16137
Lyman, R. L. (2019). Assumptions and protocol of the taxonomic identification of faunal remains in zooarchaeology: a North American perspective. J. Archaeol. Method Theory 26, 1376–1438. doi: 10.1007/s10816-019-09414-0
Neas, J. F., and Hoffmann, R. S. (1987). Budorcas taxicolor. Mamm. Species. 277, 1–7. doi: 10.2307/3503907
Nomenclature, T. I. C. O. V. G. A. (2017). Nomina Anatomica Veterinaria, 6th Edn. Editorial Committee Hannover (Germany), Columbia, MO (USA), Ghent (Belgium), Sapporo (Japan).
Peters, J. (1986). Osteomorphology and Osteometry of the Appendicular Skeleton of African Buffalo, Syncerus Caffer (SPARRMAN, 1779) and Cattle, Bos Primigenius F. Taurus BOJANUS, 1827. Ghent: Laboratory of Palaeontology, State University-Ghent.
Rao, M., Zaw, T., Htun, S., and Myint, T. (2011). Hunting for a living: Wildlife trade, rural livelihoods and declining wildlife in the Hkakaborazi National Park, North Myanmar. Environ. Manag. 48, 158–167. doi: 10.1007/s00267-011-9662-z
Ren, L. (2017). A study on animal exploitation strategies from the late Neolithic to Bronze Age in northeastern Tibetan Plateau and its surrounding areas, China (Ph.D.). Lanzhou: Lanzhou University.
Schaller, G. B. (2000). Wildlife of the Tibetan Steppe. Chicago; London: University of Chicago Press.
Shi, D., Duoji-Ciren, Pubu-Ciren, Jiang, Y., Nima-Qunzong, Bianzhen, et al. (2010). Genetic resources of livestock and poultry breeds in the Tibetan Autonomous Region (西藏禽畜品种遗传资源). Beijing: China Agricultural University Press.
Simoons, F. J. (1968). A Ceremonial Ox of India: The Mithan in Nature, Culture and History. With Notes on the Domestication of Common Cattle, by Frederick J. Simoons, with the Assistance of Elizabeth S. Simoons. Illustrations by Gene M. Christman, Milwaukee. London: University of Wisconsin Press.
Song, Y. -L., Smith, A. T., and MacKinnon, J. (2008). Budorcas taxicolor. The IUCN Red List of Threatened Species 2008. e.T3160A9643719. doi: 10.2305/IUCN.UK.2008.RLTS.T3160A9643719.en
Von Den Driesch, A. (1976). A guide to the measurement of animal bones from archaeological sites: as developed by the Institut für Palaeoanatomie, Domestikationsforschung und Geschichte der Tiermedizin of the University of Munich. Cambridge: Peabody Museum Press.
Von Den Driesch, A. (1995). Wild life in Ancient Khingar, Mustang. Archaeological evidence for locally extinct animal species in the Dzong Khola Valley. Northern Nepal: Archaeofauna.
Vrba, E., and Schaller, G. (2000). Phylogeny of Bovidae based on behavior, glands, skulls, and postcrania. Antelopes Deer Relat. 203, 222.
Wang, J., Xia, H., Yao, J., Shen, X., Cheng, T., Wang, Q., et al. (2020). Subsistence strategies of prehistoric hunter-gatherers on the Tibetan Plateau during the Last Deglaciation. Sci. China Earth Sci. 63, 395–404. doi: 10.1007/s11430-019-9519-8
Wang, Y. (2017). Identifying the Beginnings of Sheep Husbandry in Westen China. Cambridge: University of Cambridge.
Wilson, D. E., and Reeder, D. M. (2005). Mammal Species of the World: A Taxonomic and Geographic Reference. Baltimore: JHU press.
Wu, J., Han, Y., Qu, H., Liu, S., Zhu, X., Jia, J., et al. (1990). The Chinese Takin (中国羚牛). Beijing: China Forestry Publication House.
Zeng, Z.-G., Skidmore, A. K., Song, Y.-L., Wang, T.-J., and Gong, H.-S. (2008). Seasonal altitudinal movements of golden takin in the Qinling Mountains of China. J. Wildlife Manag. 72, 611–617. doi: 10.2193/2007-197
Zhang, Z. (2016). Animal Resources Use in Prehistoric Tibetan Plateau (Master Thesis). Chengdu: Sichuan University.
Zhang, Z., Chen, Z., Marshall, F., Lü, H., Lemoine, X., Wangyal, T., et al. (2019). The importance of localized hunting of diverse animals to early inhabitants of the Eastern Tibetan Plateau at the Neolithic site of Xiaoenda. Quat. Int. 529, 38–46. doi: 10.1016/j.quaint.2019.09.019
Keywords: takin, Bovini, Tibetan Plateau, zooarchaeology, comparative osteomorphology
Citation: Zhang Z, Li F and Marshall F (2022) Comparative osteomorphological criteria for differentiating mandibular teeth and post-cranial skeletons of takins (Budorcas taxicolor) from Bovini species on Tibetan Plateau. Front. Ecol. Evol. 10:956858. doi: 10.3389/fevo.2022.956858
Received: 30 May 2022; Accepted: 29 August 2022;
Published: 10 October 2022.
Edited by:
Shinya Shoda, University of York, United KingdomReviewed by:
Manabu Uetsuki, Teikyo University, JapanAshleigh Haruda, University of Oxford, Oxford, United Kingdom
Copyright © 2022 Zhang, Li and Marshall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Li, bGl2Zmx5QDE2My5jb20=
 Zhengwei Zhang
Zhengwei Zhang Fan Li
Fan Li Fiona Marshall2
Fiona Marshall2