
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 08 August 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.955663
Functional trait-based plant ecology is often used to study plant survival strategies and growth processes. In this work, the variation regularity of functional traits and their correlations were studied in Abies georgei var. smithii seedlings of different seedling ages found along the altitude gradient (3,800–4,400 m) in Sejila Mountain, Southeast Tibet. The following functional traits of seedlings in five age classes were determined: above-ground functional traits∼leaf thickness (T), leaf area (LA), specific leaf area (SLA), and leaf dry matter content (LDMC); below-ground functional traits∼specific stem length (SSL), specific root length (SRL), specific root surface area (SRA), root tissue density (RTD), and root dry matter content (RDMC). Results showed that (1) except for LDMC, most of the functional traits of the seedlings at different altitudes showed a regular change trend over time. The changes in traits caused by seedling age had significant effects on other traits (p < 0.05). Altitude only had significant effects on T, LA, SLA, SRA, RTD, and RDMC (p < 0.05). (2) The correlation between the above- and below-ground traits was more significant in 5-6-year-old seedlings than in other age classes (p < 0.05). Principal component analysis (PCA) results showed that LA and SLA were the dominant traits of fir seedlings in five age categories Pearson correlation analysis indicated a correlation between RTD and above-ground traits, thus validating the correlation between the above- and below-ground traits of seedlings of Abies georgei var. smithii of different ages. (3) Available potassium, total potassium, and total organic carbon (TOC) had the greatest influence on the traits of 5-6-year-old seedlings. This study revealed that the functional traits of Abies georgei var. smithii seedlings at different altitudesdynamically change with seedling age. The findings help in understanding the growth strategies of seedlings during early development. Future research on the combination of soil factors and seedling traits will provide a theoretical basis for artificial cultivation and protection of native vegetation.
Seedlings are at the most vulnerable growth stage in the plant life cycle and are highly susceptible to environmental stress, such as drought, light and nutrient deficiency (Valladares et al., 2000; Chaves et al., 2003; Leck et al., 2008; Onoda et al., 2008; Poorter et al., 2009). Mortality at the seedling stage is often due to abiotic and biotic factors, such as topographic factors, physical and chemical properties of the soil (Ding et al., 2011; Grossnickle, 2012; Laughlin et al., 2015; Coelho et al., 2018). Functional traits are powerful indicators of the establishment and survival of ecological strategies for plant selection (Wright et al., 2004; Freschet et al., 2010; De la Riva et al., 2016a). Trait-based approaches have been used to elucidate seedling colonization and survival patterns (Larson et al., 2014; Zirbel and Brudvig, 2020). The relative importance of abiotic and biotic factors changes with the plant age, thus leading to modifications in plant growth strategies (Henn and Damschen, 2021). Therefore, some researchers considered the effect of seedling age on the changes in plant survival strategies and studied the alterations in traits with seedling age (Niinemets, 2004, 2005; Boege and Marquis, 2005; Moriuchi and Winn, 2005; Poorter et al., 2012). However, trait-based ecological studies mainly focus on mature plant traits (Pérez-Harguindeguy et al., 2013) and less on seedlings. As a particularly vulnerable life stage of plants, the successful colonization of seedlings is critical for the establishment of future forests (Johnson et al., 2011). Ontogeny reflects an important driver of changes in above- and below-ground traits (Mason et al., 2013; Mitchell and Bakker, 2014; Freschet et al., 2021), which are indicators of ecological strategies for plant selection (Wright et al., 2004; Freschet et al., 2010; De la Riva et al., 2016a). Therefore, understanding the variation of seedling traits with age may be helpful to further understand the ecological strategies of seedling colonization.
Trait-based ecological research has two main purposes: (1) one is to balance plant traits for resource acquisition and improved self-viability (Wright et al., 2004; Chave et al., 2009; Weemstra et al., 2016). Traits from different tissues should be coordinated within species (Reich, 2014; Diaz et al., 2016). Many researchers supported the trait coordination hypothesis. For example, a spectrum of plant economics that combines leaf, stem, and root traits was proposed to explain ecological strategies for plant resource acquisition (Freschet et al., 2010; Pérez-Ramos et al., 2012; Reich, 2014; De la Riva et al., 2016b). Studies found that the correlations between traits at the seedling stage may differ from those at the mature stage (Mason et al., 2013; Laughlin et al., 2017; Harrison and LaForgia, 2019). However, previous reports focused only on the traits at the mature stage. Accurately assessing the traits, growth, and development of seedlings appears impossible (Henn and Damschen, 2021). Although many researchers studied the relationship between above- and below-ground traits (Ryser, 1996; Wahl and Ryser, 2000; Craine et al., 2001; Tjoelker et al., 2005), only a few considered seedling age in their analysis. Determining the relationship between ontogeny and functional traits is fundamental to understanding plant ecological strategies (Grime et al., 1997; Garnier et al., 2016). Therefore, understanding how the correlations among traits change with age is crucial in studying the ecological strategies of seedlings. (2) Another purpose of trait-based ecological research is to correlate the performance of plant functional traits in the environment (Violle et al., 2007). Environmental factors act as a filter that determines which individuals with a certain trait will survive in a special habitat (Keddy, 1992; Bu et al., 2019). Soil factors can directly affect plant growth (Baker et al., 2009) and cause plants to develop specific functional traits while growing in a particular site (Gourlet-Fleury et al., 2011). For example, plants in poor soils have low specific leaf area (SLA) and high leaf dry matter content (LDMC) (Ordonez et al., 2009; Jager et al., 2015). On the contrary, plants in fertile soils have high SLA and specific root length (SRL) and other traits associated with low tissue density (Poorter et al., 2008; Martinez-Vilalta et al., 2010; Wright et al., 2010; Mommer et al., 2011; Hajek et al., 2013; Weemstra et al., 2016). Furthermore, shifts in plant life strategies are important in predicting their response to environmental changes. Plants may exhibit different survival strategies at different stages due to specific environmental factors (Yang and Rudolf, 2010). The seedling stage is the most sensitive period for plants to respond to the environment, and the growth at this stage reflects the seedling’s viability and adaptability to the environment (Gill and Jackson, 2000). Understanding how seedling traits respond to local environmental factors can help improve breed plant species.
In the gradient study of plant adaptation to environmental characteristics, altitude gradient gradually replaces latitude gradient as a model template (Rahbek, 2005), and altitude and altitude-related environmental changes (climate, soil factors, etc.) can induce adaptive traits in plants, thereby directly or indirectly controlling local ecosystem processes (Violle et al., 2007; Paillex et al., 2013). Previous studies have shown that the growth of species at high altitudes is limited by climatic severity and resource availability (Lomolino, 2001). Among them, soil nutrients, as an important resource for plant growth and development, vary significantly at different altitudes (Wilcox and Nichols, 2008). Therefore, altitude gradients are a suitable natural platform when exploring the effects of environmental factors on plant traits (Dunne et al., 2004; Malhi et al., 2010).
Abies georgei var. smithii is the dominant population of the natural dark coniferous forest in Sejila Mountains in Southeastern Tibet and typically distributed at an elevation of 3,600-4,390 m. It is a dominant species of alpine timberline with strong tolerance to cold and barren soil, and plays an important role in maintaining biodiversity and sustaining forestry ecosystems in the Plateau (Guo and Zhang, 2015). However, global climate change causes the deterioration of the ecological environment of forest land, limits natural regeneration, shrinks the natural distribution area of fir forests, and decreases the population of this species (Liang et al., 2010; Wang et al., 2014). Hence, artificial cultivation is an inevitable trend in the future. Trait-based ecological approaches are applied to understand the survival mechanisms of successfully established seedlings and may improve our ability to grow a species ex situ. Therefore, this study aimed to: (1) analyze the changing trends of the traits of seedlings of A. georgei var. smithii in five age classes at different altitudes; (2) find evidence for the trade-off between the above- and below-ground functional traits of seedlings adapting to high-altitude habitats; and (3) determine the effect of key soil factors on the growth of A. georgei var. smithii seedlings and link these factors to seedling traits.
The study site is located in Sejila Mountain (93° 12’95°–35’E, 29° 10’–30° 15’ N) in Nyingchi City, Tibet Autonomous Region, China (Figure 1). Sejila Mountain is close to the branch of the Yarlung Zangbo River (Niyang River Basin), has an altitude of 2,100–5,300 m, and is a part of the Nyenqing Tanggula Mountains (Zhou et al., 2015). This region is characterized by typical warm temperate and temperate mountain climates with distinct dry and wet seasons. The annual temperature ranges from –13.98°C to 9.23°C, and the annual average temperature is –0.73°C (Wang et al., 2019). Rainfall mainly occurs from June to September, and precipitation does not exceed 1,000 mm and accounts for 80% of the total annual precipitation received by the area. The frost less period is as long as 6 months, the total sunshine duration is as long as 1,151 h, and the humidity is between 60 and 80% (Duan et al., 2020). The study area and location of seedling sampling sites are shown in Figure 1.
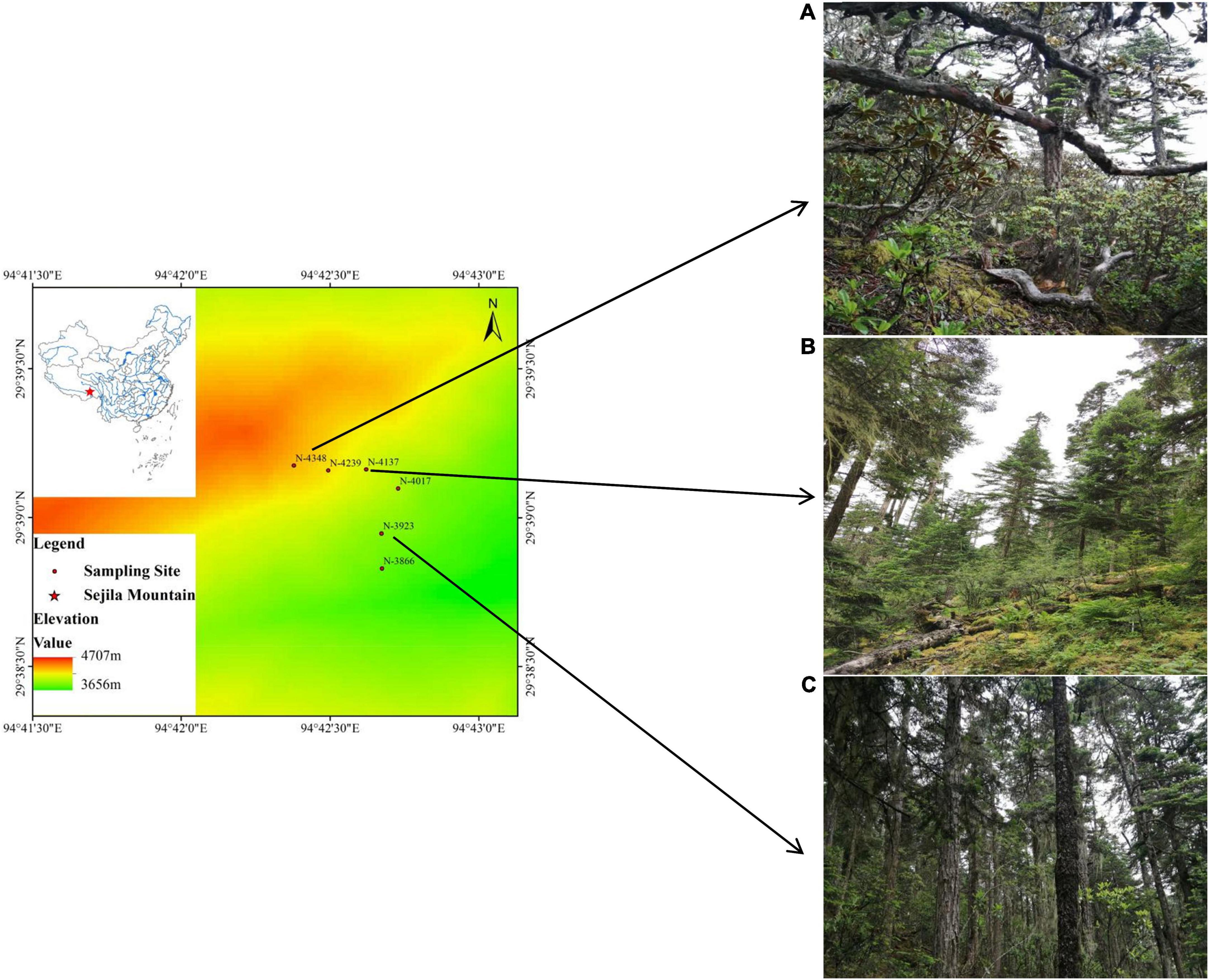
Figure 1. Location of seedling sampling sites. Three photos of sampling plots in (A) 4,348 m, (B) 4,137 m, and (C) 3,923 m.
A transect with a width of 100 m was created in the continuous gradient range from 3,800 to 4,400 m above sea level, and six 50 m × 50 m plots were set at intervals of 100 m along the transect. At each 50 m × 50 m plots, four 5 m × 5 m shrub survey quadrats and four 1 m × 1 m herb survey quadrats were set up. The basic information of each plot (location, elevation, dominant species) were recorded, and the species diversity index was showed in Supplementary Table 1.
Seedlings were collected using the full excavation method (Williams et al., 2019) and divided into five age classes: 1–2 years old, 3–4 years old, 5–6 years old, 7–8 years old, and 9–10 years old. Seedling age was determined by branch color, flower body, and bud scale markers (Duarte et al., 1999; Deng et al., 2018). Five seedlings of each age class were collected from each plot, and the distance between seedlings of the same age must be at least 3 m. The whole seedling of the A. georgei var. smithii was dug out and then marked. For the digging of old seedlings, tools such as scrapers and brushes were used to gently pluck the roots along the lateral root extension until the root ends were exposed. This method can avoid measurement errors caused by other plant root disturbance and seedling root damage. The seedlings were placed in moist airtight plastic bags and stored in cool boxes to keep the leaves saturated with water until processing. The samples were processed quickly (usually within 4 h) in the laboratory at the research site. The basic characteristics of seedlings collected from different altitudes are shown in Supplementary Table 2.
Three relatively young, healthy, fully expanded leaves were selected from each age group of seedlings (Cornelissen et al., 2003; Shen et al., 2019). Immediately after the leaf surface was cleaned, the leaves were weighed to determine their saturated fresh mass. The leaves were then scanned with a scanner (EPSON V370, China), and the leaf area (LA, cm2) was determined using ImageJ software (version 1.43u, United States). Leaf thickness (T, cm) was measured once on each side of the main vein at the widest part of each leaf while avoiding large secondary veins using a micrometer (Shen et al., 2019). All the leaves were baked in an oven at 60°C to constant weight (48 h) and then weighed to determine their dry mass. The following formulas were used to determine leaf functional traits: SLA = leaf area/leaf dry mass (cm2.g–1), LDMC = leaf dry mass/leaf fresh mass (g.g–1) (Shen et al., 2014). Specific stem length (SSL, cm. g–1) was calculated as the ratio of stem length (from stem base to stem tip) to stem dry weight (excluding leaves and shoots). These leaf and diameter traits are referred to as “above-ground traits.”
In brief, 1–3 fine roots (non-woody fine roots, diameter < 2 mm) with intact terminal branches were collected (Kubisch et al., 2015; Liese et al., 2017), washed carefully, spread out in a purified water bath, and scanned (EPSON STD4800, United States) to obtain the sample images. Root average diameter (DIAM, mm), total root length, surface area, and volume were measured (Kubisch et al., 2015; Erktan et al., 2016; Liese et al., 2017; Weemstra et al., 2017). After scanning, the excess water on the roots was blotted with filter paper, and the fresh mass of the roots was measured with a balance. The roots were then placed in an oven at 70°C for at least 72 h until constant weight to determine their dry mass. The following formulas were used to determine root functional traits: RTD = root dry mass/root volume (g.cm–3), SRL = root length/root dry mass (cm.g–1) (Kong et al., 2014), SRA = root surface area/root dry mass (cm2.g–1) (Hajek et al., 2013), RDMC = root dry mass/root fresh mass (g.g–1) (Garbowski et al., 2021). These root traits are called “below-ground traits.” All abbreviations of functional traits used in this study are described in Table 1.
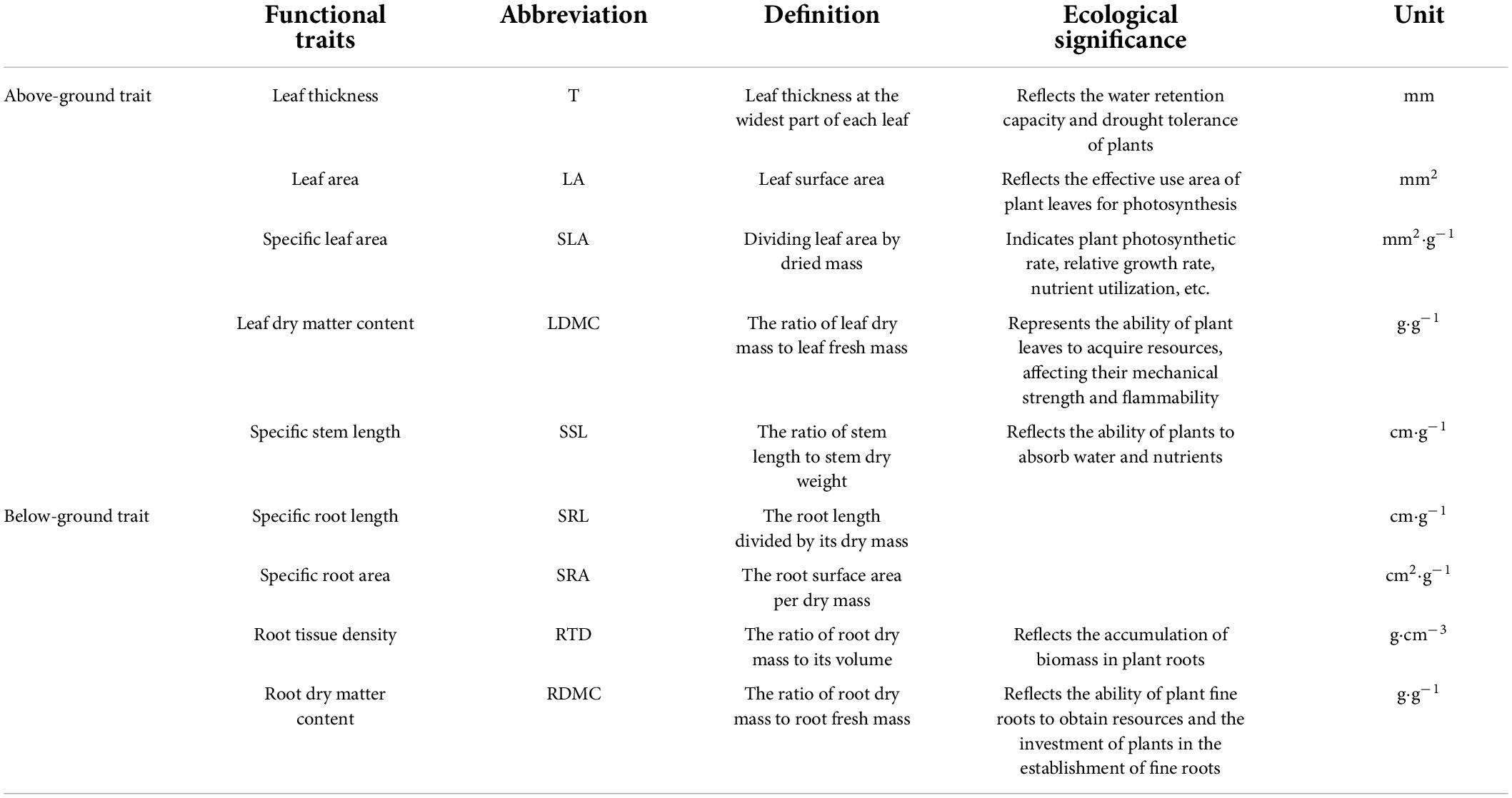
Table 1. The definition, abbreviation, and unit for 9 functional traits of Abies georgei var. smithii seedlings.
The soil near the roots of the seedlings was also collected, and three samples at 100–300 g each were collected from each plot. The soil samples were placed in plastic valve bags and sent to the laboratory for processing. Their physical and chemical properties were determined after air drying. After stones and visible plant roots were removed, the soil samples were passed through a 0.25 mm screen. Soil water content (SWC) was measured by the drying method (Chang et al., 2012). Total organic carbon (TOC) was determined by the dry combustion method at 500°C (Storer, 1984). Total nitrogen (TN) and total phosphorus (TP) were determined by Kjeldahl and NaOH alkali fusion and molybdenum–antimony anti-colorimetric methods (Sparks et al., 1996), respectively. Total potassium (TK) and available potassium (AK) were determined by NaOH melt-flame photometry and 1 mol/L ammonium acetate extraction–flame photometry (Gammon, 1951), respectively. Available phosphorus (AP) was determined via an offline extraction column (Jakmunee and Junsomboon, 2009). Nitrate nitrogen (NO3–-N,Ni-N) was determined by the phenol disulfonic acid colorimetry method (Haby, 1989). Ammonium nitrogen (NH4+-N, Am-N) was extracted with 1.2 mol/L KCl via the indophenol blue colorimetric method (Dorich and Nelson, 1983). Particulate organic carbon (POC) was assayed using the method of Garten et al. (1999). Easily oxidized organic carbon (EOC) was assessed using the determination method of Chen et al. (2017). Dissolved organic carbon (DOC) was determined using the method of Fang et al. (2014). The physical and chemical properties of soils at different altitudes are shown in Supplementary Table 3.
All variables were first assessed for normality. A standardized major axis regression method (SMA) was used to calculate the regression slope and determination coefficient (R2) of the regression mode in Soft Standardized Major Axis Tests and Routines (SMATR Version 2.0) to test the relationships between seedling age and functional traits at different elevations (statistical routines described by Warton et al., 2006). Two-way ANOVA was used to analyze the effects of altitude, age classes, and their interaction on the traits of fir seedlings. Pearson correlation analysis was performed using Bonferroni correction to explore the relationship between above- and below-ground traits. Principal component analysis (PCA) was performed using nine functional traits to obtain a comprehensive view of the functional traits of seedlings of A. georgei var. smithii in different age categories and determine their functional strategies. Significant differences between means were determined by one-way ANOVA with Tukey’s test. All statistical values were considered significant at p < 0.05. Statistical analyses were conducted using Excel 2013 and SPSS 26.0. (IBM, United States). All charts depicting variations in parameters were generated using Origin 2021 (OriginLab, Northampton, MA, United States).
The relationship between seedling traits and soil properties was investigated by redundancy analysis (RDA)-constrained ranking of experimental data using Canoco 5.0 (Microcomputer Power, Ithaca, NY, United States). RDA analysis has two matrices: species data, which include nine functional traits, and environmental data, which consist of 12 soil properties. All the data of different dimensions were normalized before classification. In the ordination plot, the length of the vector (indicated by the red arrows for each environmental variable) represents the magnitude of the environmental factors relative to the explanatory trait (blue arrows). The angle between the two arrows indicates the relationship between soil properties and seedling traits. An angle between 0° and 90° indicates a positive correlation between these two variables, a 90° angle indicates no significant correlation, and an angle between 90° and 180° represents a negative correlation.
The functional traits of all A. georgei var. smithii seedlings at different altitude gradients varied with their age classes (Figure 2 and Supplementary Tables 3, 4). For the above-ground functional traits, the SLA and SSL of fir seedlings decreased gradually with the increase in age classes (Figures 2D,E). Meanwhile, T, LA, and LDMC showed the opposite trend (Figures 2A-C). For below-ground functional traits, SRL, SRA, and RDMC all decreased gradually (Figures 2F,G,I), and RTD increased gradually with the increase in age classes (Figure 2H). In addition, SSL (R2 = 0.66 ± 0.084), SRL (R2 = 0.614 ± 0.136), and RTD (R2 = 0.673 ± 0.208) had a good fit with seedling age, and T (R2 = 0.181 ± 0.163), LDMC (R2 = 0.15 ± 0.062), and RDMC (R2 = 0.207 ± 0.217) had a low degree of fit with seedling age (Supplementary Table 5). LDMC had the lowest fitting degree with forest age among the nine functional traits, indicating that it was highly affected by seedling age; this finding was verified by two-way ANOVA (Table 2). Age classes had significant effects on the remaining eight seedling traits (p < 0.05), but altitude only had significant effects on T, LA, SLA, SRA, RTD, and RDMC (p < 0.05). The interaction of T, LA, SLA, SSL, SRA, and RTD showed an extremely significant effect (p < 0.001).
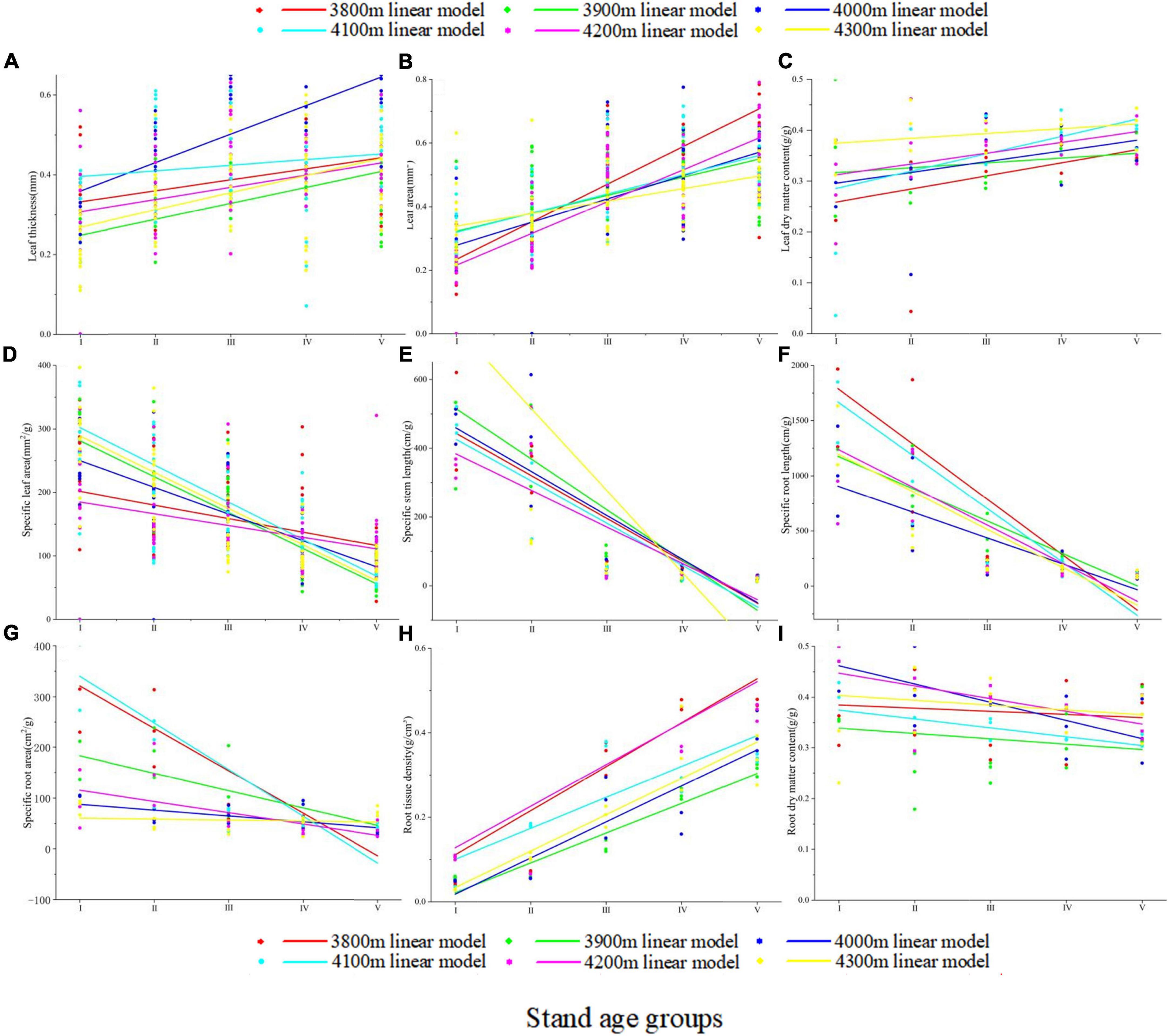
Figure 2. Variation trends of 9 functional traits over time. Lines represent linear models of seedling traits at each elevation, with colors corresponding to different elevations. (A) Leaf thickness (T); (B) leaf area (LA); (C) leaf dry matter content (LDMC); (D) specific leaf area (SLA); (E) specific stem length (SSL); (F) specific root length (SRL); (G) specific root area (SRA); (H) root tissue density (RTD); (I) root dry matter content (RDMC).
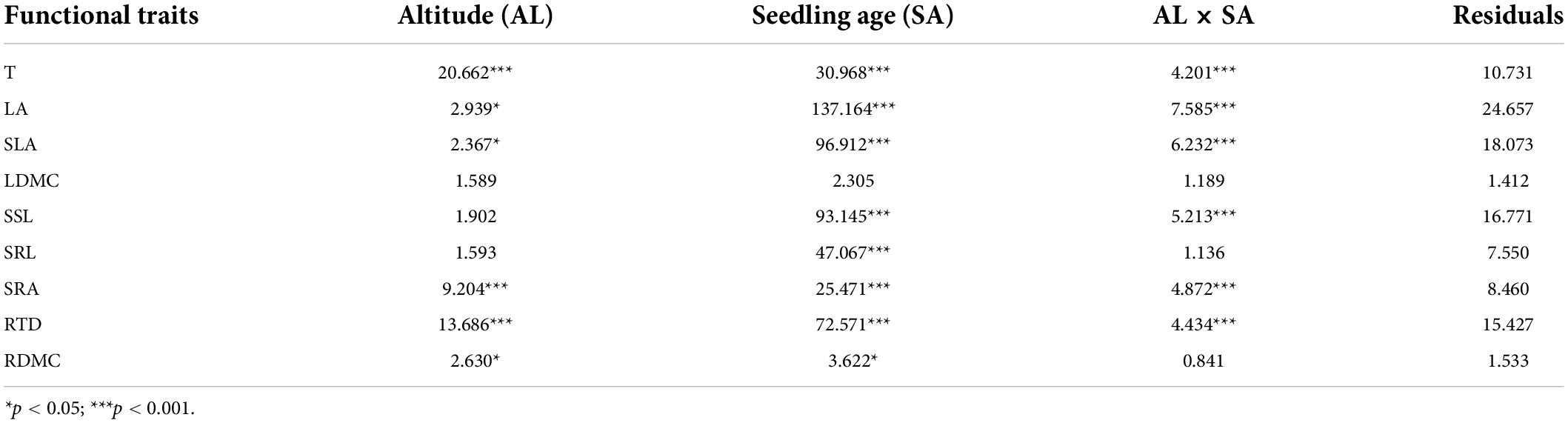
Table 2. Effects of altitude, seedling age and the interaction between altitude and seedling age on the functional traits of Abies georgei var. smithii seedlings.
Although the load of some functional traits along the principal component axis varied at different time points, qualitative PCA interpretations suggested that similar trade-offs existed between traits across the ontogeny stages. To discuss the PCA results, we chose to keep the first two axes because they explained > 50% of the total variance value of the trait across all time points. We discussed traits with eigenvalues > | 0.7| on the PC axis. As shown in the PCA ordination axis (Figure 3 and Supplementary Table 6), PC 1 explained 36.2, 31.9, 52.8, 35.8, and 36.3% of the data variance in the five age classes of seedling development. The traits that highly contributed to PC 1 were mainly LA and SLA. Meanwhile, PC 2 explained 24.5, 25.6, 13.2, 20.5, and 20.1% of the data variation of seedling development in the five age classes. SRA highly contributed to PC 2 in the first two seedling stages, and T highly contributed to PC 2 in the last three stages.
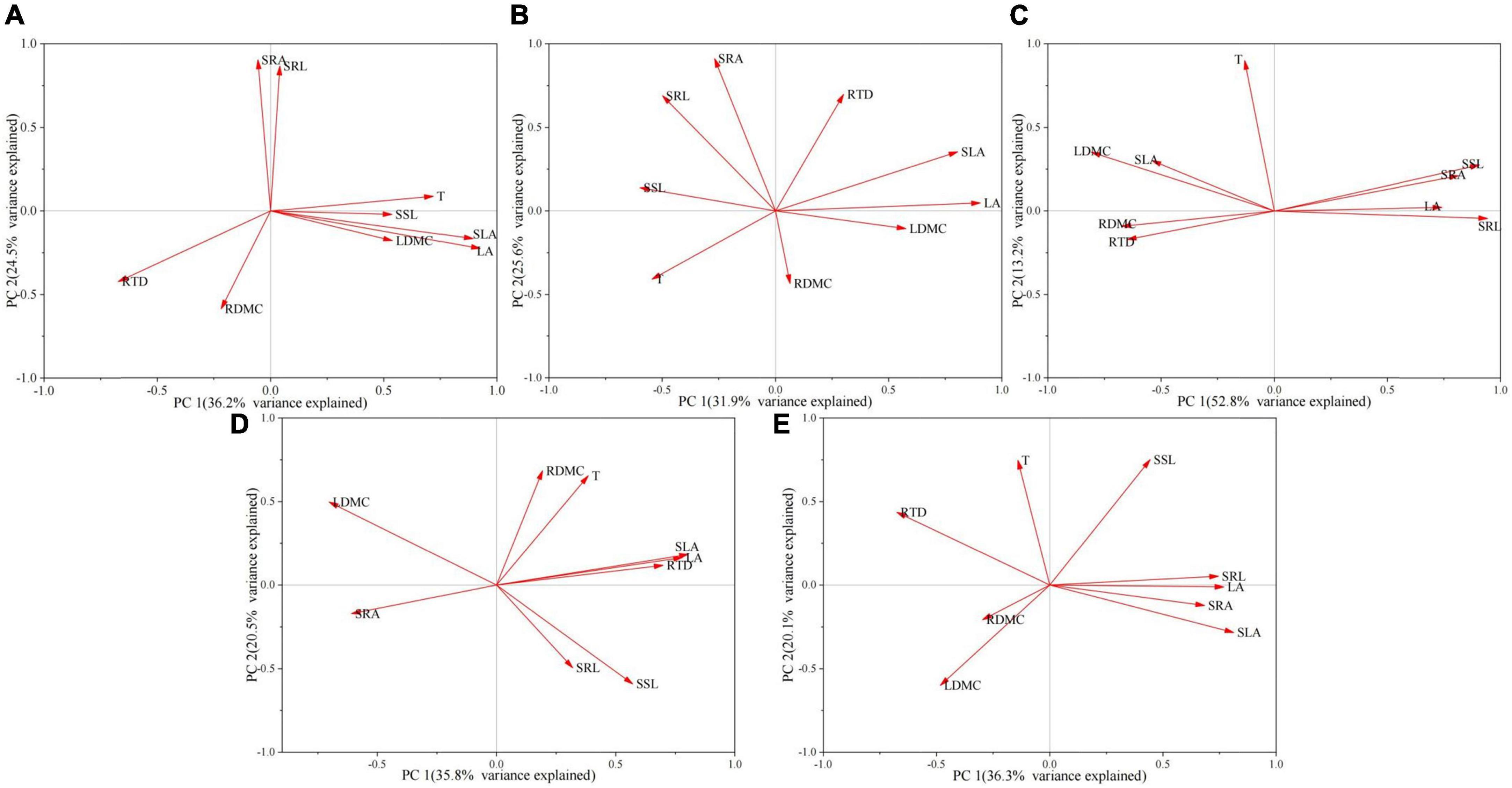
Figure 3. Principal component analysis of functional traits of Abies georgei var. smithii seedlings in different age classes. (A) 1–2 years old, (B) 3–4 years old, (C) 5–6 years old, (D) 7–8 years old, (E) 9–10 years old.
Some above-ground traits were correlated with below-ground traits at various ontogeny stages (Figure 4 and Supplementary Table 7). The correlation between the above- and below-ground traits of 1-4-year-old seedlings was low, and only the RTD of the 1-2-year-old seedlings was significantly negative correlated with LA (r = –0.551, p < 0.05), SLA (r = –0.471, p < 0.05), SSL (r = –0.477, p < 0.05). However, a turning point occurred at the third stage of seedling development (5–6 years old), and the correlation between above- and below-ground traits became highly significant. Except for T, the above-ground traits and below-ground traits showed different degrees of correlation. LA and SSL were significantly positively correlated with SRL (r = 0.693, p < 0.05; r = 0.835, p < 0.05) and SRA (r = 0.649, p < 0.05; r = 0.782, p < 0.05). RTD was negatively correlated with LA (r = –0.478, p < 0.01) but significantly negatively correlated with SSL (r = –0.669, p < 0.05). LDMC was significantly negatively correlated with SRL (r = –0.743, p < 0.05) and SRA (r = –0.514, p < 0.05) but significantly positively correlated with RDMC (r = 0.606, p < 0.05). The correlation between the above- and below-ground traits in the 7-10-year-old seedlings was reduced. In particular, only SLA (7-8 years old) was significantly positively correlated with SRL (r = 0.471, p < 0.05) and SRA (r = 0.584, p < 0.05), RTD (7-8 years old) was significantly negatively correlated with LA (r = –0.512, p < 0.05) and SLA (r = –0.570, p < 0.05). The RTD of 9-10-year-old seedlings was significantly positively correlated with LA (r = 0.532, p < 0.05) and SLA (r = 0.536, p < 0.05). In conclusion, except for the 3-4-year-old seedlings (Figure 4 and Supplementary Table 7), strong correlations between RTD and above-ground functional traits (LA and SLA) were found in the remaining seedlings. These correlations may be an ecological strategy for the trade-off between above- and below-ground traits adopted by A. georgei var. smithii seedlings to adapt to their high-altitude habitats.
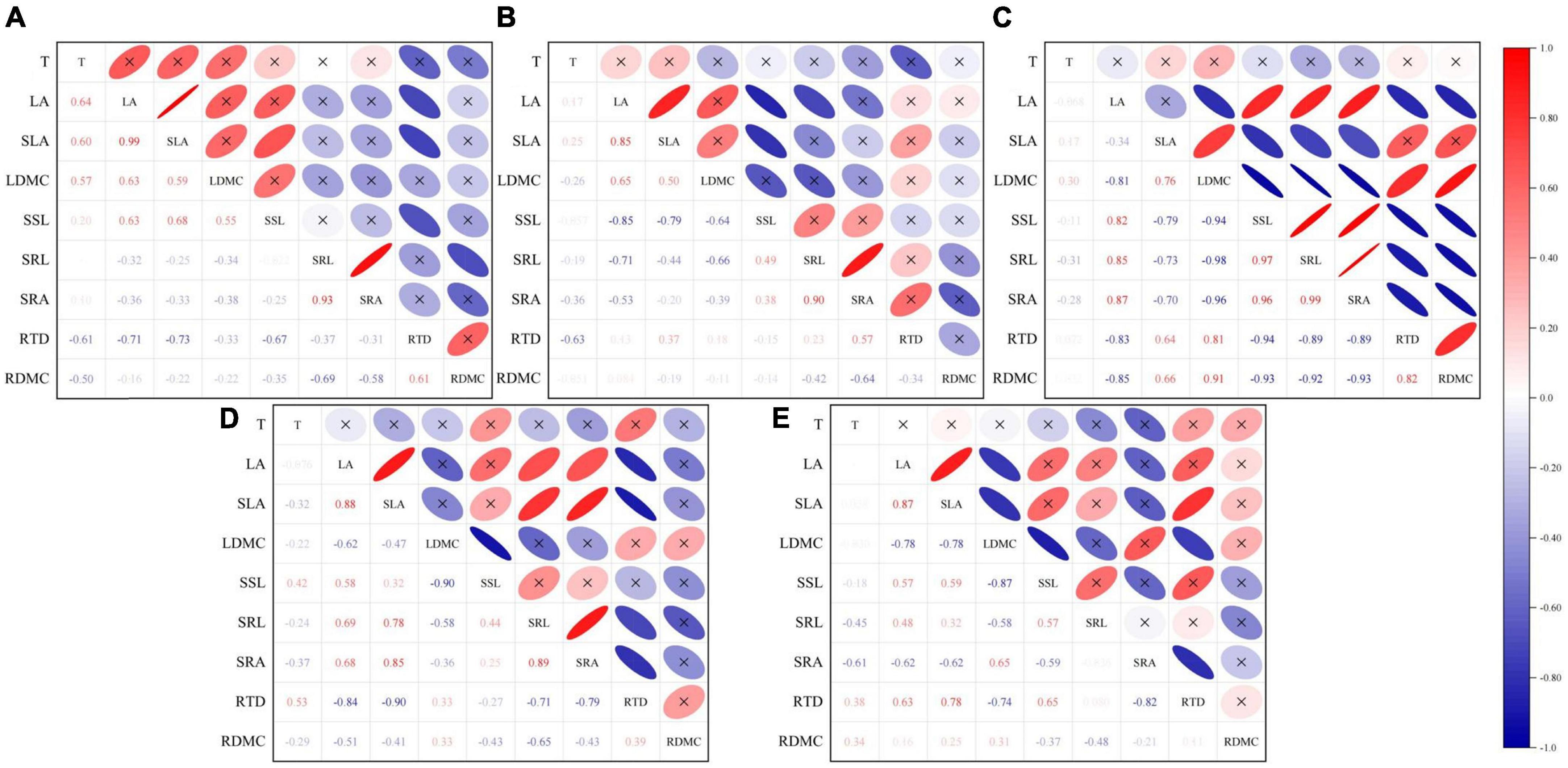
Figure 4. Correlation analysis between functional traits of Abies georgei var. smithii seedlings in different age classes (Pearson). (A) 1–2 years old, (B) 3–4 years old, (C) 5–6 years old, (D) 7–8 years old, (E) 9–10 years old. Red represents positive correlation and blue represents negative correlation. The darker the color, the higher the correlation.
The most significant correlations between above-ground and below-ground traits were found in the 5-6-year-old seedlings. To identify the main environmental factors affecting the functional traits of 5-6-year-old seedlings, we used RDA to explore the response laws of these functional traits to different soil factors. Prior to RDA, we performed detrended correspondence analysis on the seedling trait data. The results showed that the four ranking axes of the seedling trait data were all less than 3.0; therefore, the RDA method could be applied. The cumulative contribution rate of soil factors to the first two axes of seedling functional traits was 84.82%. Therefore, the first two ranking axes can be used for RDA to reflect the correlation between soil factors and seedling traits (Figure 5). In general, the traits of 5- 6-year-old seedlings were affected by soil factors at different degrees (Figure 5 and Table 3). The soil factors that highly contributed to seedling traits were AK (24.9%), TK (15.4%), and TOC (13.5%); among which, AK had a significant effect on seedling traits (p < 0.05). According to the angle of the vectors in Figure 5, LDMC, RDMC, and RTD were positively correlated with AK, TOC, and TK. Meanwhile, SSL, SRL, and SRA were negatively correlated with AK, TOC, and TK. SLA and LA were positively correlated with TOC, AK, TOC, and TK. T was positively correlated with TOC and AK but negatively correlated with TK.
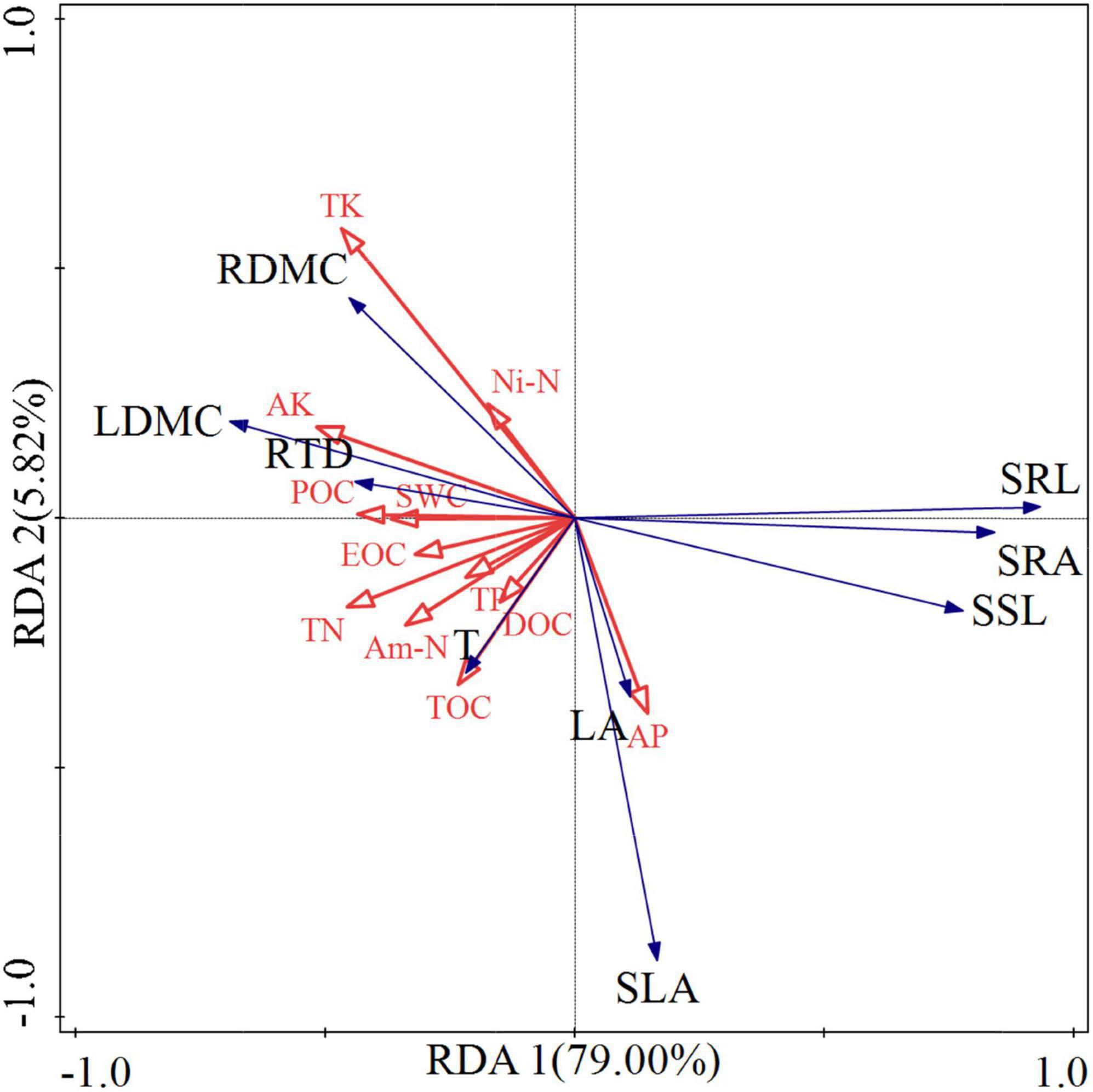
Figure 5. Redundancy analysis of the association of soil factors with 9 functional traits. These two axes illustrate the two principal components of the multiple linear regression residuals from x to y. Red arrows indicate environmental factors and blue arrows indicate functional traits. The intersection angle between the vector lines represents the correlation between the corresponding variables, where an acute angle indicates a positive correlation, an obtuse angle indicates a negative correlation, and a right angle indicates no correlation. SWC, Soil water content; TOC, Total organic carbon; TN, Total nitrogen; TP, Total phosphorus; TK, Total potassium; AK, available potassium; AP, Available phosphorus; Ni-N, NO3––N, Nitrate nitrogen; Am-N, NH4+–N, Ammonium nitrogen; POC, Particulate organic carbon; EOC, Easily oxidized organic carbon; DOC, Dissolved organic carbon.
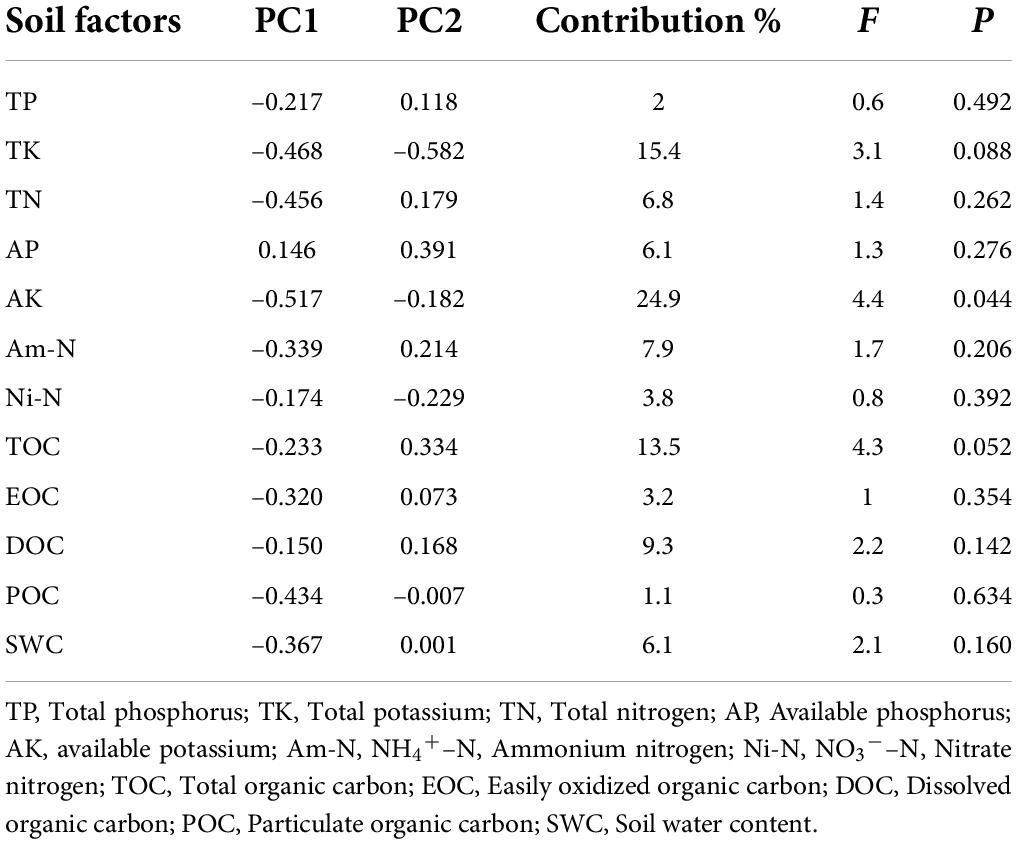
Table 3. The vector value, contribution rate, F value and P-value of soil factors on the RDA ranking axis.
The functional traits of A. georgei var. smithii seedlings varied over time in their own way but in the same direction at different elevations. We divided all functional traits into above- and below-ground traits to further explore their correlations.
Among the above-ground traits, SLA and SSL had the same change trend and gradually decreased with the increase of seedling age (Figures 2D,E). This finding may be related to the growth strategy of plants of choosing to increase their stem and leaf input. Old plants need to increasingly invest in their stem to allow their leaves to get sunlight (Poorter et al., 2012). Similarly, the gradual decrease in SLA with the increasing age classes was due to the increased input for leaves and was also consistent with the gradual increase in LDMC with the increasing age classes (Figure 2C). The decrease in SLA and increase in LDMC with increasing of seedling age is a shift in leaf strategy in old plants (Wright et al., 2004; Reich, 2014). Plants are inclined to increase inputs to structural defense sites as their age increasing (Barton and Koricheva, 2010), and this change is highly pronounced in seedlings to achieve an increased viability of resource acquisition (Dayrell et al., 2018). As above-ground traits of seedlings, T and LA may not be considered in some studies. We found that T and LA gradually increased with the seedling age. Large T and LA indicate a high photosynthesis rate (Juárez-López et al., 2008). This finding showed that the photosynthesis of A. georgei var. smithii seedlings increases with age class, which is considered as their way of adjusting their growth strategies.
Compared with the above-ground traits, the below-ground traits of seedlings are more critical because they are the basis for seedling colonization and resource acquisition (Padilla and Pugnaire, 2007; Harrison and LaForgia, 2019). We observed that only the RTD increased gradually with the increasing of seedling age (Figure 2H). RTD is associated with plant selection for growth strategies that conserve resource utilization (Pérez-Ramos et al., 2012; Kramer-Walter et al., 2016; Roumet et al., 2016) and with tolerance to low resource conditions (Kramer-Walter et al., 2016; Bristiel et al., 2019). A. georgei var. smithii seedlings choose the growth strategy of conservative resource utilization so that they can survive in the low-resource environment of high cold and high altitude. For the gradually decrease in the SRL, SRA, and RDMC of A. georgei var. smithii seedlings with the increasing of seedling age (Figures 2F,G,I), we speculated that this phenomenon was related to the root biomass. SRL and SRA reflect the absorptive capacity of roots under unit biomass input (Eissenstat et al., 2000; Alvarez-Uria and Körner, 2011). Seedlings increase the input to the root system in the early stage and improved the ability of the root system to absorb water and nutrients to obtain the resources needed for growth and development (Krasowski and Caputa, 2005). However, this ability may gradually decrease with the increase in age class mainly because the resource input of seedlings is inclined to the shoot (Mensah et al., 2016). As an intrinsic manifestation of biomass accumulation, RDMC is associated with the arid growth environment of plants (Zwicke et al., 2015; De la Riva et al., 2017). In a water-free environment, RDMC gradually decreases with the increasing plant age mainly because plants chose a growth strategy of conservative resource utilization (Garbowski et al., 2021). In the present study, the area where A. georgei var. smithii seedlings grow has the characteristics of mountain warm temperate and temperate climates and an annual precipitation exceeding 1,000 mm (Zhou et al., 2015); hence, moisture is not the main factor affecting the growth of A. georgei var. smithii seedlings. The reason why RDMC gradually decreased with the increase in age class may be consistent with the reason that RTD gradually increased with the increasing of seedling age, that is, A. georgei var. smithii seedlings choose the growth strategy of conservative resource utilization.
We found that seedling age had significant effects on most functional traits (Table 2), indicating that ontogeny affects seedling functional traits. According to studies evaluating the effect of ontogeny on seedling traits, most traits change in species-specific manner over time, and age affects intraspecific variation in seedling traits (Garbowski et al., 2021; Henn and Damschen, 2021) which were consistent with our findings in Tibet. In the present study, altitude had an extremely significant effect on T, SRA, and RTD (Table 2). Altitude variation has a gradient effect on multiple environmental factors (temperature and water), and this effect has practical significance for understanding the response of plants to climate change at the seedling stage (Hu et al., 2016). Rather than a direct phenological response to some environmental variables, the remarkably effect of altitude on leaf thickness may be the result of natural selection (Hovenden and Schoor, 2003). Altitude had a significant effect on root surface area because plants improve their resource utilization to assess and adapt to severe environmental changes caused by altitude changes (Xiang et al., 2021). In addition, the change in temperature caused by the altitude prompts the plant to change its input strategy to the root system, and the root biomass change affects the root tissue density; these phenomena may explain why altitude has an extremely significant effect on root tissue density (Eissenstat et al., 2000; Craine et al., 2001).
Above-ground traits are good predictors of below-ground traits, and these two types of traits are highly correlated with each other (Shen et al., 2019). According to PCA and Pearson correlation analysis (Figures 3, 4), no strong dynamic trade-off occurred between above- and below-ground traits in seedlings of all five age classes, and the relationship between above- and below-ground traits was highly pronounced in 5-6-year-old seedlings (Figure 4 and Supplementary Table 7). Ontogeny leads to changes in functional traits (Quero et al., 2008; Spasojevic et al., 2014; Lasky et al., 2015), we speculated that the functional traits of A. georgei var. smithii seedlings change with the increasing of seedling age. When the seedling age reaches a certain critical point, the frequency of significant correlation between above- and below-ground traits reaches the peak value. Furthermore, plant growth strategies are fairly consistent throughout early development (Henn and Damschen, 2021). Similar PCA results were observed at all five age groups: the traits affecting the PC 1 axis were mainly LA and SLA, and the traits affecting the PC 2 axis were mainly SRA and T (Supplementary Table 3). We also observed some pairwise inter-trait relationships at each age class. Strong correlations were observed between RTD and multiple above-ground traits, which may be valid evidence for the correlations between above- and below-ground traits in A. georgei var. smithii seedlings. We found that RTD was significantly associated with SLA across multiple age groups (Supplementary Table 5). According to a previous study, the traits of different plant organs (roots, diameters, and leaves) should be coordinated (Reich, 2014). RTD and SLA have a certain degree of coordination, thus reflecting the plant economics spectrum related to different organs and ontogeny (Reich, 2014; Kramer-Walter et al., 2016).
Environmental factors are the main drivers of plant functional traits (Loreau and Naeem, 2001; Kichenin et al., 2013; Hu et al., 2014), and some key environmental variables can be used to explain plant trait changes at local scales (Grime, 2006). In the genealogy theory of plant economics, Reich predicts that the traits of different plant organs should be consistent with resource gradients (Reich, 2014), that is, when environmental factors change, the plant traits change accordingly. Therefore, we speculated that when key environmental factors vary, the traits of different organs are also altered. On the basis of the results of PCA and Pearson correlation analysis (Supplementary Tables 6, 7), the soil conditions of the habitat of 5–6-year-old seedlings should be paid more attention to protect seedling renewal and colonization. Therefore, we propose a third objective to identify key soil factors affecting the growth of 5–6-year-old A. georgei var. smithii seedlings.
Crucial soil nutrients (N, P, and K) are major ecological factors driving plant functional trait responses (Lu et al., 2010; Pérez-Ramos et al., 2012; Santiago et al., 2012; Record et al., 2016). We found that K and TOC were the main limiting soil factors for the growth of 5–6-year-old A. georgei var. smithii seedlings (Table 3). Most of the traits in the present study were related to biomass. K increases the photosynthetic rate of seedlings (Pasquini and Santiago, 2012); when the photosynthetic rate of a plant is enhanced, its biomass increases rapidly (Perrin and Mitchell, 2013). Therefore, we can infer that the values of the traits positively correlated with biomass increase with K and vice versa. AK and TK were positively correlated with LDMC, RDMC, and RTD but negatively correlated with SSL, SRL, SRA, SLA, and LA. The reason may be that potassium increases the photosynthetic rate of seedlings and affects the biomass of different organs of seedlings. A significant relationship has been observed between plant biomass and soil carbon content (Smith et al., 2014; Purcell, 2016; Bu et al., 2019). Different from K, TOC was positively correlated with SLA and LA (Figure 5). Soil organic carbon content affects leaf traits mainly because the latter determine the energy and nutrient sources of soil biota (Wardle et al., 2004); this relationship explains why TOC is positively correlated with SLA and LA.
Although some traits of A. georgei var. smithii seedlings did not change significantly among elevations, most of them showed a regular trend with the increasing of seedling age. The significant correlation between RTD and above-ground traits (SLA and LA) is evidence for the trade-off between the above- and below-ground functional traits of A. georgei var. smithii seedlings to adapt to high-altitude habitats. Measuring adult plant traits is suitable for comparing mature species; however, these same traits are not necessarily representative of seedling traits (Henn and Damschen, 2021). Therefore, our study on A. georgei var. smithii seedlings complements the previous works on A. georgei var. smithii in the seedling stage. This field of research is important for understanding its complete survival strategy. In addition, soil factors accounted for 84.82% of the variation in the functional traits of 5–6-year-old seedlings. This finding indicated that soil properties are important factors affecting the growth of 5–6-year-old A. georgei var. smithii seedlings. Therefore, the role of soil factors should be carefully considered when taking artificial cultivation and actual protection measures.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XZ: software, methodology, and writing – original draft. NZ: writing – original draft. CZ: methodology and conceptualization. JL and XW: conceptualization. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (31960256) and the central government guided local projects of China (XZ202101YD0016C), and the Independent Research Project of Science and Technology Innovation Base in Tibet Autonomous Region and the Study on Ecological Characteristics of Seedlings and Rhizosphere Soil of Abies georgei var. smithii Forest on the Elevation Gradient in Sejila Mountain.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.955663/full#supplementary-material
Alvarez-Uria, P., and Körner, C. (2011). Fine root traits in adult trees of evergreen and deciduous taxa from low and high elevation in the alps. Alpine Bot. 121, 107–112. doi: 10.1007/s00035-011-0092-6
Baker, T. R., Phillips, O. L., Laurance, W. F., Pitman, N., Almeida, S., Arroyo, L., et al. (2009). Do species traits determine patterns of wood production in Amazonian forests? Biogeosciences 6, 297–307. doi: 10.5194/bg-6-297-2009
Barton, K. E., and Koricheva, J. (2010). The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am. Nat. 175, 481–493. doi: 10.1086/650722
Boege, K., and Marquis, R. J. (2005). Facing herbivory as you grow up:the ontogeny of resistance in plants. Trends Ecol. Evol. 20, 441–448. doi: 10.1016/j.tree.2005.05.001
Bristiel, P., Roumet, C., Violle, C., and Volaire, F. (2019). Coping with drought: root trait variability within the perennial grass Dactylis glomerata captures a trade-off between dehydration avoidance and dehydration tolerance. Plant Soil 434, 327–342. doi: 10.1007/s11104-018-3854-8
Bu, W., Zhang, C., Huang, J., Zang, R., and Wang, J. (2019). The influences of disturbance histories and soil properties on aboveground biomass through plant functional traits in a tropical rainforest. Forests 10:774. doi: 10.3390/f10090774
Chang, R., Fu, B., Liu, G., Yao, X., and Shuai, W. (2012). Effects of soil physicochemical properties and stand age on fine root biomass and vertical distribution of plantation forests in the loess plateau of china. Ecol. Res. 27, 827–836. doi: 10.1007/s11284-012-0958-0
Chave, J., Coomes, D., Jansen, S., Lewis, S. L., Swenson, N. G., and Zanne, A. E. (2009). Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366. doi: 10.1111/j.1461-0248.2009.01285.x
Chaves, M. M., Maroco, J. P., and Pereira, J. S. (2003). Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 30, 239–264.
Chen, X., Yang, Q., Chen, Z., Xue, Y. U., Yang, Q., and Lei, J. (2017). Distribution of soil easily oxidized organic carbon and its response to soil factors in the tropical coastal forest of hainan island, china. J. Central South Univ. For. Technol. 37, 140–145.
Coelho, M. S., Carlos, P. P., Pinto, V. D., Meireles, A., Negreiros, D., Morellato, L. P. C., et al. (2018). Connection between tree functional traits and environmental parameters in an archipelago of montane forests surrounded by rupestrian grasslands. Flora 238, 51–59.
Cornelissen, J. H. C., Lavorel, S., Garnier, E., Diaz, S., Buchmann, N., Gurvich, D. E., et al. (2003). A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380. doi: 10.1071/bt02124
Craine, J. M., Froehle, J., Tilman, D. G., Wedin, D. A., and Iii, F. (2001). The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93, 274–285. doi: 10.2307/3547305
Dayrell, R. L. C., Arruda, A. J., Pierce, S., Negreiros, D., Meyer, P. B., Lambers, H., et al. (2018). Ontogenetic shifts in plant ecological strategies. Funct. Ecol. 32, 2730–2741. doi: 10.1111/1365-2435.13221
De la Riva, E. G., Francisco, L., Pérez-Ramos Ignacio, M., Maraón, T., Sandra, S. M., and Ricardo, D. D. (2017). The importance of functional diversity in the stability of Mediterranean shrubland communities after the impact of extreme climatic events. J. Plant Ecol. 10, 281–293. doi: 10.1093/jpe/rtw027
De la Riva, E. G., Pérez-Ramos Ignacio, M., Tosto, A., Navarro-Fernandez, C. M., Olmo, M., Maranon, T., et al. (2016a). Disentangling the relative importance of species occurrence, abundance and intraspecific variability in community assembly: a trait-based approach at the whole-plant level in Mediterranean forests. Oikos 125, 354–574. doi: 10.1111/oik.01875
De la Riva, E. G., Tosto, A., Pérez-Ramos Ignacio, M., Navarro-Fernandez, C. M., Olmo, M., Anten, N. P. R., et al. (2016b). A plant economics spectrum in mediterranean forests along environmental gradients: is there coordination among leaf, stem and root traits? J. Veg. Sci. 27, 187–199. doi: 10.1111/jvs.12341
Deng, L., Guan, J., and Zhang, W. (2018). Response of root morphological characteristics of quercus liaotungensis seedlings to environmental gradients. Acta Ecol. Sin. 38, 5739–5749.
Diaz, S., Kattge, J., Cornelissen, J. H. C., Wright, I. J., Lavorel, S., Dray, S., et al. (2016). The global spectrum of plant form and function. Nature 529, 167–173. doi: 10.1038/nature16489
Ding, J., Wu, Q., Yan, H., and Zhang, S. R. (2011). Effects of topographic variations and soil characteristics on plant functional traits in a subtropical evergreen broad-leaved forest. Biodivers. Sci. 19, 158–167.
Dorich, R. A., and Nelson, D. W. (1983). Direct colorimetric measurement of ammonium in potassium chloride extracts of soils. Soil Sci. Soc. Am. J. 47, 833–836. doi: 10.2136/sssaj1983.03615995004700040042x
Duan, F., Fang, J. P., and Zhou, C. N. (2020). Study on the relationship between the release characteristics of organic carbon from litters and soil organic carbon pool in tibetan primitive dark coniferous fores. J. Soil Water Conserv. 34, 349–355.
Duarte, C. M., Thampanya, U., Terrados, J., Geertz-Hansen, O., and Fortes, M. D. (1999). The determination of the age and growth of se asian mangrove seedlings from internodal counts. Mangr. Salt Marsh. 3, 251–257. doi: 10.1023/A:1009967401337
Dunne, J. A., Saleska, S. R., Fischer, M. L., and Harte, J. (2004). Integrating experimental and gradient methods in ecological climate change research. Ecology 85, 904–916. doi: 10.1890/03-8003
Eissenstat, D. M., Wells, C. E., Yanai, R. D., and Whitbeck, J. L. (2000). Building roots in a changing environment: implications for root longevity. New Phytol. 147, 33–42. doi: 10.1046/j.1469-8137.2000.00686.x
Erktan, A., Cecillon, L., Graf, F., Roumet, C., Legout, C., and Rey, F. (2016). Increase in soil aggregate stability along a Mediterranean successional gradient in severely eroded gully bed ecosystems: combined effects of soil, root traits and plant community characteristics. Plant Soil 398, 121–137. doi: 10.1007/s11104-015-2647-6
Fang, H. J., Cheng, S. L., Yu, G. R., Xu, M. J., and Wang, Y. S. (2014). Experimental nitrogen deposition alters the quantity and quality of soil dissolved organic carbon in an alpine meadow on the qinghai-tibetan plateau. Appl. Soil Ecol. 81, 1–11. doi: 10.1016/j.apsoil.2014.04.007
Freschet, G. T., Cornelissen, J. H. C., Van Logtestijn, R. S. P., and Aerts, R. (2010). Evidence of the ‘plant economics spectrum’ in a subarctic flora. J. Ecol. 98, 362–373. doi: 10.1111/j.1365-2745.2009.01615.x
Freschet, G. T., Pagès, L., Iversen, C., Comas, L., and Mccormack, M. L. (2021). A starting guide to root ecology: strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements. New Phytol. 232, 973–1122. doi: 10.1111/nph.17572
Gammon, N. (1951). Determination of total potassium and sodium in sandy soils by flame photometer. Soil Sci. 71, 211–214. doi: 10.1097/00010694-195103000-00009
Garbowski, M., Johnston, D. B., and Brown, C. S. (2021). Leaf and root traits, but not relationships among traits, vary with ontogeny in seedlings. Plant Soil 460, 247–261. doi: 10.1007/s11104-020-04790-z
Garnier, E., Navas, M. L., and Grigulis, K. (2016). Plant functional diversity: organism traits, community structure, and ecosystem properties. Oxford: Oxford Scholarship Online.
Garten, C. T., Post, W. M., Hanson, P. J., and Cooper, L. W. (1999). Forest soil carbon inventories and dynamics along an elevation gradient in the southern appalachian mountains. Biogeochemistry 45, 115–145. doi: 10.1023/A:1006121511680
Gill, R. A., and Jackson, R. B. (2000). Global patterns of root turnover for terrestrial ecosystems. New Phytol. 147, 13–31. doi: 10.1046/J.1469-8137.2000.00681.X
Gourlet-Fleury, S., Rossi, V., Rejou-Mechain, M., Freycon, V., Fayolle, A., Saint-André, L., et al. (2011). Environmental filtering of dense-wooded species controls above-ground biomass stored in African moist forests. J. Ecol. 99, 981–990. doi: 10.1111/j.1365-2745.2011.01829.x
Grime, J. P. (2006). Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. J. Veg. Sci. 17, 255–260. 44.x doi: 10.1111/j.1654-1103.2006.tb024
Grime, J. P., Thompson, K., Hunt, R., Hodgson, J. G., Cornelissen, J. H. C., Rorison, I. H., et al. (1997). Integrated screening validates pri-mary axes of specialisation in plants. Oikos 79, 259–281. doi: 10.2307/3546011
Grossnickle, S. C. (2012). Why seedlings survive: influence of plant attributes. New For. 43, 711–738. doi: 10.1007/s11056-012-9336-6
Guo, Q. Q., and Zhang, W. H. (2015). Sap flow of Abies georgei var. smithii and its relationship with the environment factors in the Tibetan subalpine region, China. J. Mountain Sci. 12, 1372–1382. doi: 10.1007/s11629-015-3618-3
Haby, V. A. (1989). Soil no3-n analysis in ca(oh)2 extracts by the chromotropic acid method. Soil Sci. Soc. Am. J. 53, 308–310. doi: 10.2136/sssaj1989.03615995005300010059x
Hajek, P., Hertel, D., and Leuschner, C. (2013). lntraspecific variation in root and leaf traits and leaf-root trait linkages in eight aspen demes (Populus tremula and P. tremuloides). Front. Plant Sci. 4:415. doi: 10.3389/Fpls.2013.00415
Harrison, S., and LaForgia, M. (2019). Seedling traits predict drought- induced mortality linked to diversity loss. Proc. Natl. Acad. Sci. U.S.A. 116, 5576–5581. doi: 10.1073/pnas.1818543116
Henn, J. J., and Damschen, E. I. (2021). Plant age affects intraspecific variation in functional traits. Plant Ecol. 222, 669–680. doi: 10.1007/s11258-021-01136-2
Hovenden, M. J., and Schoor, J. K. V. (2003). Nature vs nurture in the leaf morphology of southern beech, Nothofagus cunninghamii (nothofagaceae). New Phytol. 161, 585–594. doi: 10.1046/j.1469-8137.2003.00931.x
Hu, R. Z., Du, Z. Q., Liu, S., and Shi, J. W. (2016). Fine root morphology characteristics of Larix principis-rupprechtii along an elevation gradient. Chin. J. Ecol. 35, 1248–1253.
Hu, Y. S., Yao, X. Y., and Liu, Y. H. (2014). The functional traits of forests at different succession stages and their relationship to terrain factors in Changbai Mountains. Acta Ecol. Sin. 34, 5915–5924.
Jager, M. M., Richardson, S. J., Bellingham, P. J., Clearwater, M. J., and Laughlin, D. C. (2015). Soil fertility induces coordinated responses of multiple independent functional traits. J. Ecol. 103, 374–385. doi: 10.1111/1365-2745.12366
Jakmunee, J., and Junsomboon, J. (2009). Determination of available phosphorus in soils by using a new extraction procedure and a flow injection amperometric system. Talanta 79, 1076–1080. doi: 10.1016/j.talanta.2009.01.028
Johnson, D. M., Mcculloh, K. A., and Reinhardt, K. (2011). “The earliest stages of tree growth: development, physiology and impacts of microclimate,’ in Size and age-related changes in tree structure and function, eds F. C. Meinzer, B. Lachenbruch, and T. E. Dawson (Berlin: Springer).
Juárez-López, F. J., Alfonso, E., and Sonia, M. (2008). Ontogenetic changes in stomatal and biochemical limitations to photosynthesis of two co-occurring mediterranean oaks differing in leaf life span. Tree Physiol. 3, 367–374. doi: 10.1093/treephys/28.3.367
Keddy, P. A. (1992). Assembly and response rules: Two goals for predictive community ecology. J. Veg. Sci. 3, 157–164. doi: 10.2307/3235676
Kichenin, E., Wardle, D. A., Peltzer, D. A., Morse, C. W., and Freschet, G. T. (2013). Contrasting effects of plant inter and intraspecific variation on community-level trait measures along an environmental gradient. Funct. Ecol. 27, 1254–1261. doi: 10.1111/1365-2435.12116
Kong, D. L., Ma, C. G., Zhang, Q., Li, L., Chen, X. Y., Zeng, H., et al. (2014). Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 203, 863–872. doi: 10.1111/nph.12842
Kramer-Walter, K. R., Bellingham, P. J., Millar, T. R., Rob, D. Smissen, Sarah, J. R., et al. (2016). Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 104, 1299–1310. doi: 10.1111/1365-2745.12562
Krasowski, M. J., and Caputa, A. (2005). Relationships between the root system size and its hydraulic properties in white spruce seedlings. New For. 30, 127–146. doi: 10.1007/s11056-005-7482-9
Kubisch, P., Hertel, D., and Leuschner, C. (2015). Do ectomycorrhizal and Arbuscular mycor-rhizal temperate tree species systematically differ in root order-related fine root morphology and biomass? Front. Plant Sci. 6:64. doi: 10.3389/fpls.2015.00064
Larson, J. E., Sheley, R. L., Hardegree, S. P., Doescher, P. S., and James, J. J. (2014). Seed and seedling traits affecting critical life stage transitions and recruitment outcomes in dryland grasses. J. Appl. Ecol. 52, 199–209. doi: 10.1111/1365-2664.12350
Lasky, J. R., Bachelot, B., Muscarella, R., Schwartz, N., Forero, M. J., Nytch, C. J., et al. (2015). Onto-genetic shifts in trait-mediated mechanisms of plant community assembly. Ecology 96, 2157–2169. doi: 10.1890/14-1809.1
Laughlin, D. C., Lusk, C. H., Bellingham, P. J., Burslem, D. F. R. P., Simpson, A. H., and Kramer-Walter, K. R. (2017). Intraspecific trait variation can weaken interspecific trait correlations when assessing the whole- plant economic spectrum. Ecol. Evol. 7, 8936–8949. doi: 10.1002/ece3.3447
Laughlin, D. C., Richardson, S. J., Wright, E. F., and Bellingham, P. J. (2015). Environmental filtering and positive plant litter feedback simultaneously explain correlations between leaf traits and soil fertility. Ecosystems 18, 1269–1280.
Leck, M. A., Parker, V. T., Simpson, R. L., and Simpson, R. S. (2008). Seedling ecology and evolution. Cambridge: Cambridge University Press.
Liang, E. Y., Wang, Y. F., Xu, Y., Liu, B. M., and Shao, X. (2010). Growth variation in Abies georgei var. smithii along altitudinal gradients in the sygera mountains, southeastern tibetan plateau. Trees 24, 363–373. doi: 10.1007/s00468-009-0406-0
Liese, R., Alings, K., and Meier, I. C. (2017). Root branching is a leading root trait of the plant economics spectrum in temperate trees. Front. Plant Sci. 8:315. doi: 10.3389/fpls.2017.00315
Lomolino, M. V. (2001). Elevation gradients of species-density: historical and prospective views. Glob Ecol Biogeogr. 10, 3–13. doi: 10.1046/j.1466-822x.2001.00229.x
Loreau, M., and Naeem, P. (2001). Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. doi: 10.1126/science.1064088
Lu, X. K., Mo, J. M., Gilliam, F. S., Zhou, G. Y., and Fang, Y. T. (2010). Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Glob. Change Biol. 16, 2688–2700. doi: 10.1111/j.1365-2486.2010.02174.x
Malhi, Y., Silman, M., Salinas, N., Bush, M., Meir, P., and Saatchi, S. (2010). Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Global Change Biol. 16, 3171–3175. doi: 10.1111/j.1365-2486.2010.02323.x
Martinez-Vilalta, J., Mencuccini, M., Vayreda, J., and Retana, J. (2010). Interspecific variation in functional traits, not climatic differences among species ranges, determines demographic rates across 44 temperate and Mediterranean tree species. J. Ecol. 98, 1462–1475. doi: 10.1111/j.1365-2745.2010.01718.x
Mason, C. M., McGaughey, S. E., and Donovan, L. A. (2013). Ontogeny strongly and differentially alters leaf economic and other key traits in three diverse Helianthus species. J. Exp. Bot. 64, 4089–4099. doi: 10.1093/jxb/ert249
Mensah, S., Kakaï, R. G., and Seifert, T. (2016). Patterns of biomass allocation between foliage and woody structure: the effects of tree size and specific functional traits. Ann. For. Res. 59, 49–60. doi: 10.15287/afr.2016.458
Mitchell, R. M., and Bakker, J. D. (2014). Intraspecific trait variation driven by plasticity and ontogeny in Hypochaeris radicata. PLoS One 9:e109870. doi: 10.1371/journal.pone.0109870
Mommer, L., Visser, E., Ruijven, J. V., Caluwe, H. D., Pierik, R., and Kroon, H. D. (2011). Contrasting root behaviour in two grass species: a test of functionality in dynamic heterogeneous conditions. Plant Soil 344, 347–360. doi: 10.1007/s11104-011-0752-8
Moriuchi, K. S., and Winn, A. A. (2005). Relationships among growth, development and plastic response to environment qualityin a perennial plant. New Phytol. 166, 149–158. doi: 10.1111/j.1469-8137.2005.01346.x
Niinemets, U. (2004). Adaptive adjustments to light in foliage and whole-plant characteristics depend on relative age in the perennial herb Leontodon hispidus. New Phytol. 162, 683–696. doi: 10.1111/j.1469-8137.2004.01071.x
Niinemets, U. (2005). Key plant structural and allocation traits depend on relative age in the perennial herb Pimpinella saxifraga. Ann. Bot. 96, 323–330. doi: 10.1093/aob/mci180
Onoda, Y., Schieving, F., and Anten, N. P. (2008). Effects of light and nutrient availability on leaf mechanical properties of Plantago major: a conceptual approach. Ann. Bot. 101, 727–736. doi: 10.1093/aob/mcn013
Ordonez, J. C., Van Bodegom, P. M., Witte, J. P. M., Wright, I. J., Reich, P. B., and Aerts, R. (2009). A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Glob. Ecol. Biogeogr. 18, 137–149. doi: 10.1111/j.1466-8238.2008.00441.x
Padilla, F. M., and Pugnaire, F. I. (2007). Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 21, 489–495. doi: 10.1111/j.1365-2435.2007.01267.x
Paillex, A., Dolédec, S., and Castella, E. (2013). Functional diversity in a large river floodplain: anticipating the response of native and alien mac-roinvertebrates to the restoration of hydrological connectivity. J. Appl. Ecol. 50, 97–106. doi: 10.1111/1365-2664.12018
Pasquini, S. C., and Santiago, L. S. (2012). Nutrients limit photosynthesis in seedlings of a lowland tropical forest tree species. Oecologia 168, 311–319. doi: 10.1007/s00442-011-2099-5
Pérez-Harguindeguy, N., Diaz, S., Garnier, E., Lavorel, S., Poorter, H., Jaureguiberry, P., et al. (2013). New handbook for standardised measurement of plant functional traits worldwide. Austral. J. Bot. 64:715. doi: 10.1071/BT12225
Pérez-Ramos, I. M., Roumet, C., Cruz, P., Blanchard, A., Autran, P., and Garnier, E. (2012). Evidence for a “plant community econom-ics spectrum” driven by nutrient and water limitations in a Mediterranean rangeland of southern France. J. Ecol. 100, 1315–1327. doi: 10.1111/1365-2745.12000
Perrin, P. M., and Mitchell, F. (2013). Effects of shade on growth, biomass allocation and leaf morphology in european yew (taxus baccata l.). Eur. J. For. Res. 132, 211–218. doi: 10.1007/s10342-012-0668-8
Poorter, H., Niinemets, Ü, Poorter, L., Wright, I. J., and Villar, R. (2009). Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 182, 565–588. doi: 10.1111/j.1469-8137.2009.02830.x
Poorter, H., Niklas, K. J., Reich, P. B., Oleksyn, J., Poot, P., and Mommer, L. (2012). Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental con-trol. New Phytol. 193, 30–50. doi: 10.1111/j.1469-8137.2011.03952.x
Poorter, L., Wright, S. J., Paz, H., Ackerly, D. D., Condit, R., Ibarra-Manriques, G., et al. (2008). Are functional traits good predictors of demographic rates? Evidence from five Neotropical forests. Ecology 89, 1908–1920. doi: 10.1890/07-0207.1
Purcell, A. (2016). Functional trait variation along a hydrological gradient and trait-based predictions of the composition of a wetland plant community. Hamilton: University of Waikato.
Quero, J. L., Go’mez-Aparicio, L., Zamora, R., and Maestre, F. T. (2008). Shifts in the regeneration niche of an endangered tree (Acer opalus ssp. granatense) during ontogeny: using an ecological concept for application. Basic Appl. Ecol. 9, 635–644. doi: 10.1016/j.baae.2007.06.012
Rahbek, C. (2005). The role of spatial scale and the perception of large-scale species-richness patterns. Ecol. Lett. 8, 224–239. doi: 10.1111/j.1461-0248.2004.00701.x
Record, S., Kobe, R. K., Vriesendorp, C. F., and Finley, A. O. (2016). Seedling survival responses to conspecific density, soil nutrients, and irradiance vary with age in a tropical forest. Ecology 97, 2406–2415. doi: 10.1002/ecy.1458
Reich, P. B. (2014). The world-wide ‘fast-slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301. doi: 10.1111/1365-2745.12211
Roumet, C., Birouste, M., Picon-Cochard, C., Ghestem, M., Osman, N., Vrignon-Brenas, S., et al. (2016). Root structure function relationships in 74 species: evidence of a root economics spectrum related to carbon economy. New Phytol. 210, 815–826. doi: 10.1111/nph.13828
Ryser, P. (1996). The importance of tissue density for growth and life span of leaves and roots: a comparison of five ecologically contrasting grasses. Funct. Ecol. 10, 717–723. doi: 10.2307/2390506
Santiago, L. S., Wright, S. J., Harms, K. E., Yavitt, J. B., Korine, C., Garcia, M. N., et al. (2012). Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J. Ecol. 100, 309–316. doi: 10.1111/j.1365-2745.2011.01904.x
Shen, Y., Gilbert, G. S., Li, W., Fang, M., and Yu, S. (2019). Linking aboveground traits to root traits and local environment: implications of the plant economics spectrum. Front. Plant Sci. 10:1412. doi: 10.3389/fpls.2019.01412
Shen, Y., Santiago, L. S., Shen, H., Ma, L., Lian, J. Y., Cao, H. L., et al. (2014). Determinants of change in subtropical tree diameter growth with ontogenetic stage. Oecologia 175, 1315–1324. doi: 10.1007/s00442-014-2981-z
Smith, S. W., Woodin, S. J., Pakeman, R. J., Johnson, D., and Van, D. W. R. (2014). Root traits predict decomposition across a landscape-scale grazing experiment. New Phytol. 203, 851–862. doi: 10.1111/nph.12845
Sparks, D. L., Page, A., Helmke, P., Loeppert, R., Soltanpour, P., Tabatabai, M., et al. (1996). Methods of Soil Analysis. Part 3-Chemical Methods. Washington, DC: Soil Science Society of America Inc.
Spasojevic, M. J., Yablon, E. A., Oberle, B., and Myers, J. A. (2014). Onto-genetic trait variation influences tree community assembly across environmental gradients. Ecosphere 5, 1–20. doi: 10.1890/ES14-000159.1
Storer, D. A. (1984). A simple high sample volume ashing procedure for determination of soil organic matter. Commun. Soil Sci. Plant Anal. 15, 759–772. doi: 10.1080/00103628409367515
Tjoelker, M. G., Craine, J. M., Wedin, D., Reich, P. B., and Tilman, D. (2005). Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 167, 493–508. doi: 10.1111/j.1469-8137.2005.01428.x
Valladares, F., Wright, S. J., Lasso, E., Kitajima, K., and Pearcy, R. W. (2000). Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 81, 1925–1936.
Violle, C., Navas, M. L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., et al. (2007). Let the concept of trait be functional! Oikos 116, 882–892. doi: 10.1111/j.2007.0030-1299.15559.x
Wahl, S., and Ryser, P. (2000). Root tissue structure is linked to ecological strategies of grasses. New Phytol. 148, 459–471. doi: 10.1046/j.1469-8137.2000.00775.x
Wang, G. X., Ran, F., Chang, R. Y., Yang, Y., Luo, J., and Fan, J. R. (2014). Variations in the live biomass and carbon pools of abies georgei along an elevation gradient on the tibetan plateau, china. For. Ecol. Manage. 329, 255–263. doi: 10.1016/j.foreco.2014.06.023
Wang, W., Xu, W., Collett, J. L., Liu, D., and Liu, X. (2019). Chemical compositions of fog and precipitation at sejila mountain in the southeast tibetan plateau, china. Environ. Pollut. 253, 560–568. doi: 10.1016/j.envpol.2019.07.055
Wardle, D. A., Bardgett, R. D., Klironomos, J. N., Setälä, H., Van Der Putten, W. H., and Wall, D. H. (2004). Ecological linkages between aboveground and belowground biota. Science 304, 1629–1633. doi: 10.1126/science.1094875
Warton, D. I., Wright, I. J., Falster, D. S., and Westoby, M. (2006). Bivariate line-fitting methods for allometry. Biol. Rev. Camb. Philos. Soc. 81, 259–291. doi: 10.1017/S1464793106007007
Weemstra, M., Mommer, L., Visser, E. J. W., van Ruijven, J., Kuyper, T. W., Mohren, G. M. J., et al. (2016). Towards a multidimensional root trait framework: a tree root review. New Phytol. 211, 1159–1169. doi: 10.1111/nph.14003
Weemstra, M., Sterck, F. J., Visser, E. J. W., Kuyper, T. W., Goudzwaard, L., and Mommer, L. (2017). Fine-root trait plasticity of beech (Fagus sylvatica) and spruce (Picea abies) forests on two contrasting soils. Plant Soil 415, 175–188. doi: 10.1007/s11104-016-3148-y
Wilcox, D. A., and Nichols, S. J. (2008). The effects of water-level fluctuations on vegetation in a lake Huron wetland. Wetlands 28, 487–501. doi: 10.1672/07-129.1
Williams, A., George, S., Birt, H., Daws, M. I., and Tibbett, M. (2019). Sensitivity of seedling growth to phosphorus supply in six tree species of the australian great western woodlands. Austral. J. Bot. 67, 1–30. doi: 10.1071/BT18247
Wright, I. J., Reich, P. B., Westoby, M., Ackerly, D. D., Baruch, Z., Bongers, F., et al. (2004). The worldwide leaf economics spectrum. Nature 428, 821–827. doi: 10.1038/Nature02403
Wright, S. J., Kitajima, K., Kraft, N. J. B., Reich, P. B., Wright, I. J., Bunker, D. E., et al. (2010). Functional traits and the growth-mortality trade-off in tropical trees. Ecology 91, 3664–3674. doi: 10.1890/09-2335.1
Xiang, X., Huang, Y. M., Yang, C. Y., Li, Z. Q., Chen, H. Y., Pan, Y. P., et al. (2021). Effect of altitude on community-level plant functional traits in the Qinghai Lake Basin, China. Chin. J. Plant Ecol. 45, 456–466.
Yang, L. H., and Rudolf, V. H. W. (2010). Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett. 13, 1–10. doi: 10.1111/j.1461-0248.2009.01402.x
Zhou, C. N., Ren, Y. H., Ma, H. P., and Guo, Q. Q. (2015). Research on soil organic carbon pool of typical natural dark coniferous forests in sygara mountains in tibet. Environ. Sci. Technol. 38, 1–7.
Zirbel, C. R., and Brudvig, L. A. (2020). Trait-environment interactions affect plant establishment success during restoration. Ecology 101:e02971. doi: 10.1002/ecy.2971
Keywords: seedlings, functional traits, altitude, Abies georgei var. smithii, seedling age
Citation: Zhang X, Zhao N, Zhou C, Lu J and Wang X (2022) Seedling age of Abies georgei var. smithii reveals functional trait coordination in high-altitude habitats in southeast tibet. Front. Ecol. Evol. 10:955663. doi: 10.3389/fevo.2022.955663
Received: 29 May 2022; Accepted: 11 July 2022;
Published: 08 August 2022.
Edited by:
Roger Guevara, Instituto de Ecología (INECOL), MexicoReviewed by:
Diego F. Angulo, Scientific Research Center of Yucatán (CICY), MexicoCopyright © 2022 Zhang, Zhao, Zhou, Lu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenni Zhou, Y2hlbm5pMjAxOEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.