
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 20 October 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.945886
This article is part of the Research Topic Forest Adaptation to Extreme Environments and Climate Changes View all 5 articles
Acute and extreme weather events can cause considerable damage to the tissues of trees, including stem death and branch or leaf distortion, which may limit their survival and reproduction. In January 2016, a rare cold spell impacted the coastal forests of subtropical China. Using post-hoc assessments, we evaluated the morphological and physiological response of Chinese tallow (Triadica sebifera L.) to the extreme cold spell in two distinct ecoregions, one (Xiangshan, China) representing the cold spell impacted zone and the other (Taizhou, China) representing the non-affected zone. To determine if the extreme cold events impacted the vigor of Chinese tallow, we assessed differences in growth rate, leaf characteristics, and leaf gas exchange. As age may affect tree morphological and physiological response to stress, we grouped subject trees into three distinct cohorts, namely, seedlings (1–2 years old), young-aged (5–6 years old), and middle-aged (10–12 years old). Our results suggest that although tree height and diameter did not differ, leaf area expansion and leaf mass were reduced in the impacted zone. In seedling and young-aged trees, the cold spell significantly reduced leaf net photosynthetic (An), transpiration rates (Tr), stomatal conductance (Gs) and water use efficiency (WUE) while leaf intercellular CO2 concentration (Ci), vapor pressure deficit (Vpd), and intercellular CO2 pressure (Ci-Pa) increased. In contrast, the middle-aged group was less responsive to the cold spell. Across all cohorts, the event did not affect leaf temperature (Tleaf), but the activity of superoxide dismutase (SOD) and peroxidase (POD) decreased. We also detected increases of leaf malondialdehyde (MDA) and free proline (Pro) contents in young-aged and middle-aged groups. Hence, the extreme cold spell caused remarkable negative effects on the morphological and physiological traits of Chinese tallow. Redundancy analysis revealed that the cold spell also impacted the subsequent recovery process of damaged Chinese tallow by reducing the ability of leaf to utilize microenvironmental resources (radiation, air humidity, and CO2) for gas exchange. Results from this study are important to strengthen our understanding of Chinese tallow responding to extreme cold stress within its native range, also be helpful to predict the distributions of Chinese tallow in its invasive range where it has devastating impacts to coastal ecosystems in the southeast US.
The frequency and duration of extreme climatic events (severe storms, droughts, floods, and ice storms etc.) are predicted to increase due to the accelerated climate change, causing serious damages to ecological communities (Boucek et al., 2016; Konisky et al., 2016; Liu et al., 2017). Severe winter weather events, including frigid temperatures and ice storms, are among the most injurious natural disturbances to forest ecosystems (Zhou et al., 2011). Many biological processes require certain temperatures for optimal functioning and temperatures below these thresholds would significantly impact plant performance (Larcher, 1981). Discrete weather occurrences that exceed the acclimation capacity of a species could result in large-scale mortality events (Gutschick and BassiriRad, 2003; Smith, 2011). On a larger scale, the lack of tolerance to low temperatures often drive the abundance and distribution of tree species. For example, low temperatures limit the elevational and latitudinal distribution of hardwood forests in the eastern US (Irland, 2000; Kollas et al., 2014). Most studies to date on the effects of freezing weather events on forests have focused on timber loss for commercially important trees, while species-specific response and its underlying mechanisms are seldom revealed, even though the functional traits of individual trees may play a fundamental role in stand-level recovery of forests (Lafon, 2006; Bragg, 2016; Priebe et al., 2018).
Trees have developed an array of mechanisms that enable them to recover and grow following the negative consequences of cold stress. This includes genetic, morphological, and physiological adaptations to cold (Basuchaudhuri, 2014). For genotypes to be cold-adapted, they must experience cold temperatures periodically and over relatively long time (Larcher, 2005). While morphological and physiological cold-adaptive changes are more important to reduce damage-risk in short period, for instance, smaller stature and higher concentrations of protective chemical compounds would help trees to strengthen cold resistance and avoid more severe damage (Körner, 2016). Among them, leaf characteristics, gas exchange, and biochemical oxidative stress responses are considered the most important factors for determining recovery rates of trees to extreme cold (dos Santos et al., 2006). Leaves are the most sensitive organ to respond to temperature variations, therefore, leaf morphological and physiological traits are closely related to cold stress acclimation and subsequently, affect the survival and growth of trees (López de Heredia et al., 2009). Tree age may also influence damage susceptibility and recovery rates from severe winter weather. During ice storms, younger or smaller trees are prone to bending, while older and larger individuals are more likely to have canopy loss or snapped boles (Lafon, 2006; Lu et al., 2020). Reports of ice damage on trees have focused on forests in Europe and North America. While limited information exists, on the morphological and physiological response of subtropical China tree species to severe cold, an area where extreme winter weather events are historically infrequent (Seischab et al., 1993; Pisaric et al., 2008).
Coastal forests in China are primarily plantations that distributed along the shoreline. These coastal plantations provide important ecosystem services such as windbreaks, soil conservation, and regional climate regulation (Xie et al., 2018). But they are facing frequent extreme climatic events due to their sea-land transitional zonation (Obura, 2006; Mattsson et al., 2009; Fernandes et al., 2018). In this region, strong winds, severe storms, and floods are ranked as the primary sources of natural disturbance. The significance of these natural disturbance events have attracted much attention over the last several decades, but few studies have reported the impact of severe winter weather (Strimbeck et al., 2015; Xie et al., 2018). In January 2016, a rare, extreme cold spell with freezing precipitation in the form of heavy snow and ice, affected subtropical China, with Zhejiang province being one of the most affected areas. The lowest temperature reached −20.4°C in some mountainous areas, greatly exceeding the average minimum temperature of winters of last decade (−2.6°C), also created a new record of the lowest temperature (the former record was −17.4°C in January 1976, from Meteorological Bureau of Zhejiang Province) (Chen et al., 2017; Supplementary Figure 1). The cold spell caused observable injury and death to aboveground vegetation, especially to some trees, in which heavy snow bent branches or branches were withered, and substantial numbers of leaves were fallen or distorted by cold stress.
Chinese tallow [Triadica sebifera (L.) Small] is native to southeastern China, where it is prized and utilized as an ornamental species, or used for pharmaceutical goods and forest products. Chinese tallow is also important to subtropical coastal forests due to its strong adaptability to saline-alkali stress and salt-spray (Conner, 1994; Yang et al., 2012; Chen et al., 2013; Paudel and Battaglia, 2015). However, in the southeastern US, Chinese tallow is considered one of the most aggressive invasive tree species in coastal forests and grasslands (Pile et al., 2017). Chinese tallow is also considered cold-sensitive, which may limit its expansion in its native and invasive ranges (Jubinsky and Anderson, 1996; Bruce et al., 1997; Gan et al., 2009). However, only limited studies have evaluated the effects of freezing temperatures on the growth and physiological responses of Chinese tallow and more information is needed to test the presumed “temperature-limited” range expansion assumption (Pattison and Mack, 2008; Gan et al., 2009; Pile et al., 2017). Considering the similarity in latitude and climate between southeastern China and the southeastern US, the potential of Chinese tallow to sustain or resume growth following severe winter weather in the Zhejiang province of China could help predictive estimates of its potential invasive distribution in the US and inform adaptive climate modeling for China’s coastal plantation forests. Following the severe cold spell in subtropical China in 2016, significant damages to Chinese tallow were observed thus, providing a great opportunity to study the resistance and recovery of Chinese tallow to severe cold and winter precipitation under natural conditions.
In this study, we focus on the morphological and physiological response of three cohort ages of Chinese tallow to the severe cold spell. We assessed changes in growth rate, leaf traits, leaf gas exchange, and alterations to the antioxidant enzyme system. We compared the responses of Chinese tallow impacted by the cold spell (Xiangshan) to Chinese tallow not affected (Taizhou), in Zhejiang Province, China. The objectives of this study were to (1) evaluate the growth (stem height and diameter) and morphological (leaf area and mass) response of Chinese tallow following damage from the cold spell by cohort age; (2) determine differences in leaf gas exchange and biochemical (superoxide dismutase, peroxidase, malondialdehyde, and free proline) responses of Chinese tallow by age cohort; (3) explore the relationships between microenvironmental environment (light availability, humidity, and CO2 concentrations) and leaf gas exchange in cold spell impacted and non-affected zones; and (4) assess the degree of recovery in damaged Chinese tallow 1 year following the cold spell. Since Chinese tallow has limited native and invasive distributions in coastal subtropical regions of China and the US due to its perceived cold temperature limitations, we hypothesize: (1) the acute severe cold spell will impact the morphological and physiological recovery of Chinese tallow through decreasing gas exchange rates and disrupting the internal biochemical self-regulating system; and (2) damage sustained from a single extreme cold spell will have long-term impacts on the recovery of Chinese tallow.
The experiment was conducted in Xiangshan county and Taizhou city of Zhejiang Province, China. Both areas represent the typical north subtropical marine monsoon climate. Xiangshan has a mean annual air temperature of 16.5°C and a mean annual precipitation of 1,415 mm, while Taizhou’s mean annual air temperature and precipitation are 16.9°C and 1,632 mm, respectively. Soils at both sites are Ultisols. Both sites have an afforestation history of Chinese tallow, Xiangshan is north of the historic native range of Chinese tallow with plantations beginning in the 1990s, while more southerly Taizhou is within its native range. In Xiangshan, Chinese tallow is mainly distributed along roadsides and farmlands, with some residents planting them for their economic value, resulting in its relatively dispersed distribution in the landscape. In contrast, Chinese tallow in Taizhou is primarily distributed in coastal forests where they are used as the main tree species for shelterbelts (Supplementary Figure 2).
In January 2016, the temperature began to drop dramatically from 20th parallel as a strong cold air front moved south from the north. Accompanying the cold front was moderate to heavy snow in Zhejiang province, with the maximum snow depth exceeding 20 cm. The lowest air temperature in Xiangshan was recorded on 25 January (−7.8°C) and ranged from −4.6 to −7.8°C between 20–28 January, 2016 (Figure 1). The severe winter weather event resulted in massive observable mortality and degeneration of coastal forests in Xiangshan. Many sub-tropical trees are semi-deciduous or tardily deciduous, losing foliage for short periods before regrowth occurs or retaining leaves into the winter, greatly increasing their vulnerability to severe cold or ice glaze. In our study region, beech sheoak (Casuarina equisetifolia L.), swamp mahogany (Eucalyptus robusta Smith), and Chinese tallow were among the most impacted tree species. Chinese tallow was observed to have living leaves upon the onset of the cold spell allowing for visual assessments of damage. In coastal South Carolina, USA, leaves of Chinese tallow have been observed to remain on the stem at least until late December with leaf emergence and expansion in early March (Pile Knapp, personal observation).
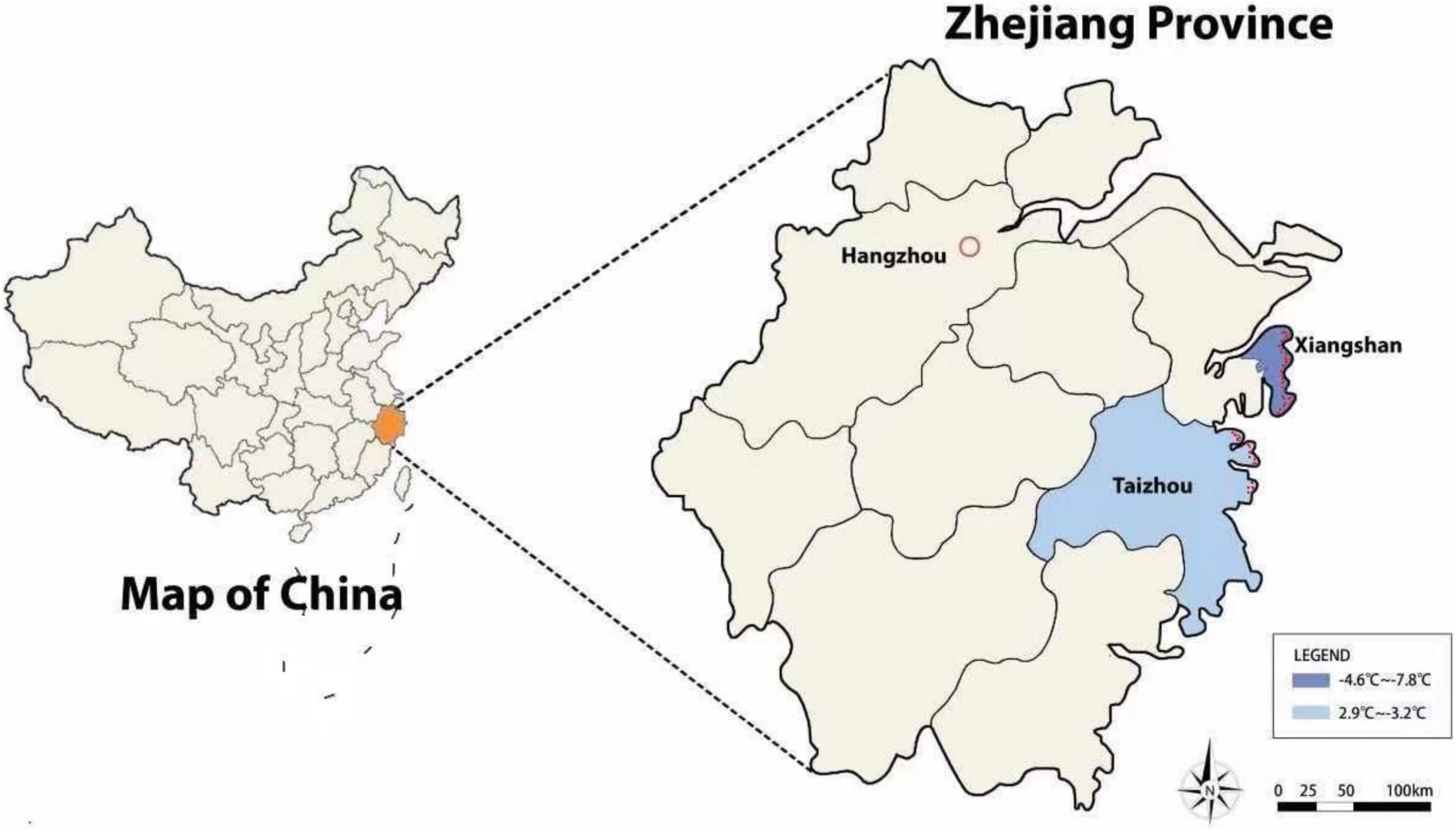
Figure 1. Minimum air temperatures of the experimental zones from 20–28 January of 2016. The red points along the coastline represent specific sampling sites.
In March 2016, we conducted a survey along the coast, starting in northern Xiangshan and ending in southern Taizhou. The purpose of the survey was to determine the extent of Chinese tallow damage caused by the cold spell. Chinese tallow in Xiangshan county was obviously damaged, with observable characteristics like withered and dead branches, browning and distorted leaves. However, in Taizhou, we saw minimal damage to Chinese tallow. Therefore, we established Xiangshan county as the cold spell affected zone and Taizhou city as the non-affected zone. Since we conducted the survey shortly after the event, we focused exclusively on Chinese tallow that were damaged but living to determine growth and physiological response to the cold spell. Subsequently, we did not record the overall mortality resulting from the event, which would have been difficult to discern without pre-storm information on individual trees. To determine the severity of damage to living Chinese tallow in Xiangshan, we categorized damage into four classes:
(1) Crown damage, the crown was removed or partially broken;
(2) Stem damage, the stem was noticeably bent, but the crown was nearly complete;
(3) Branch damage, the crown, and stem were undamaged, but more than 35% of branches were dead or withered;
(4) Leaf damage, the crown, stem, and branches were undamaged, but and the remaining leaves showed noticeable signs of deformation or discoloration.
If a tree suffered multiple types of damages, it was categorized into the most severe class (severity = crown damage > stem damage > branch damage > leaf damage).
As age is a factor in tree growth and development following damage, we delineated three age cohorts according to the afforestation history and the overall age distribution of Chinese tallow in both zones. This resulted in the following cohorts: “seedlings” (1∼2 years old), “young-aged” (5∼6 years old), and “middle-aged” (10∼12 years old). Since the majority Chinese tallow were planted within the last decade, we found fewer older Chinese tallow in the study area. Therefore, an older age cohort (>12 years) was not included but we did observe less damage to older Chinese tallow after the cold spell when compared to the younger cohorts included in our study. Although Chinese tallow is relatively short-lived (<100 years) and reaches reproductive maturity quickly (<3 years) (Scheld et al., 1984; Pile et al., 2017), these subjective classes represent only the general age of Chinese tallow in our study region but do not represent individual life-stage or fecundity.
We selected 42 sites in Xiangshan and 16 sites in Taizhou to determine the morphological and physiological response of Chinese tallow to the severe cold spell (Figure 1). In each site, one or two plot (20 m × 20 m) was established to take samples depending on the number of Chinese tallow in the plot, since the coastal forests were predominantly mixtures, accompanying tree species included Ligustrum compactum, Sapindus mukorossi, Koelreuteria bipinnata, etc. A total of 689 damaged Chinese tallow stems in Xiangshan and 316 healthy stems in Taizhou were located in March 2016, then we assigned the damaged stems into corresponding damage classes as described above. Among them, 541 (78.5%) stems sustained leaf damage, thus we focused mainly on the response of leaves to the cold spell.
In the same sample period, we randomly selected 15 stems from each cohort in both zones (n = 90) and labeled them with numbered tags, and recorded stem height and diameter at breast height (DBH). For seedlings, we measured the diameter 10 cm above the ground (“seedling diameter”). From each stem, we sampled ten leaves from the outer edge of the tree crown and transported them immediately to the lab in a sealed plastic bag to measure leaf area and fresh leaf mass. Leaf area was determined with a leaf area analyzer (MRS-9600TFU2L, Shanghai), and fresh leaf mass was determined with a highly precise electronic scale (ES200, China).
One year later, in May 2017, we remeasured the DBH (seedling diameter), height, leaf area, and fresh leaf mass of each stem using the same methods. To assess recovery rates, we calculated the relative growth rates from 2016 to 2017. To determine the physiological response of Chinese tallow to the cold spell, we measured leaf gas exchange and biochemical constituents.
To determine leaf gas exchange, we selected 3–5 fully expanded leaves from the eastern outer edge of the tree crown at approximately the same height for each tree in a cohort to minimize microclimatic differences among the sampled leaves. Leaf photosynthesis was measured with a LI-COR 6400XT (Li-Cor Inc., Lincoln, NE, USA) using the standard leaf chamber under ambient light conditions. Measurements were conducted during 8:30–11:30 a.m., and care was taken to not damage the leaves. Leaf gas exchange measurements recorded included leaf net photosynthesis rate (An), stomatal conductance (Gs), intercellular CO2 concentration (Ci), transpiration rate (Tr), vapor pressure deficit (Vpd), leaf temperature (Tleaf), the ratio of intercellular CO2 concentration to air CO2 concentration (Ci/Ca), and intercellular CO2 pressure (Ci-Pa). Leaf water use efficiency (WUE) was calculated as An/Tr. We also recorded the microenvironmental conditions with the LI-COR 6400XT, including photosynthetically active radiation (PAR), relative air humidity (RHsfc), and ambient air CO2 concentration (C2sfc).
To determine biochemical compounds and oxidative stress response to the severe cold spell, fresh leaves were collected and pooled from four sides of the 15 individual trees for each cohort, to assay the activity of antioxidant enzymes and contents of osmoregulation substance. An ice bucket was used to store and transport leaf samples to the laboratory within 8 h. Leaves were washed with pure water and excess moisture was removed. Leaf samples were homogenized with ice-cold phosphate buffer (0.1 M, pH 7.5) that contains 0.5 mM EDTA, using prechilled pestle and mortar. The homogenate was then transferred to tubes and centrifuged at 4°C at 15,000 rpm for 15 min. The supernatant was transferred to 30 ml tubes and referred to as enzyme extract (Weisany et al., 2012). The activity of superoxide dismutase (SOD) and peroxidase (POD) were determined with the method of Liu et al. (2014). The content of malondialdehyde (MDA) was determined using the thiobarbituric acid reaction, as described by Weisany et al. (2012). The content of free proline (Pro) was determined, according to Li (2000).
Chi-square tests were used to determine if the overall damage rates caused by the four damage types differed between tree age groups. To determine growth response following the severe cold spell to Chinese tallow by age cohort (Objective 1), we calculated relative growth rates (RGR) of DBH, height, leaf area, and leaf fresh mass as:
Where X was the measured value of DBH, height, leaf area and leaf fresh mass of each subject tree, respectively.
To determine the morphological and physiological response of Chinese tallow by age cohort (Objective 2), we compared RGRs, leaf gas exchange, biochemical compounds, and oxidative stress response between cohorts with one-way ANOVA followed by Tukey’s HSD (Honestly Significant Difference) test. To explore the relationship of the severe cold spell on leaf gas exchange, biochemical compounds, and oxidative stress response of Chinese tallow between experimental zones, an independent-sample t-test was used. The above statistical analyses were conducted using SPSS 20.0 (IBM, Chicago, IL, USA) and Origin 9.1 (OriginLab, New York, NY, USA), with significance levels set at P < 0.05. All data were evaluated for normality and homogeneity (Kolmogorov-Smirnov-Test) prior to performing the statistical analyses. To determine the relationship between microenvironmental conditions and leaf gas exchange of Chinese tallow between the two experimental zones, a redundancy analysis (RDA) was conducted with Canoco 5.0. Two matrices representing the microenvironmental conditions (PAR, RHsfc, C2sfc) and leaf gas exchange (An, Gs, Ci, Tr, Vpd, Tleaf, Ci/Ca, Ci-Pa, WUE) were constructed separately for each experimental zone before conducting the constrained analysis. Changes in total variation that the first two axes explained and the positional relationship between the explanatory variables reveal which and how the microenvironment affected leaf gas exchange of Chinese tallow (Smilauer and Leps, 2014).
Seedlings of Chinese tallow were least impacted by the cold spell, while the young-aged and middle-aged cohorts were more impacted by the extreme cold spell (Table 1). Leaf damage was the principal injury type across all cohorts. Crown damage and branch damage were more common in middle-aged trees than in young-aged trees (Table 1).
One year following the severe winter weather event, the cold spell presented constraining effects on individual growth of Chinese tallow. Although, relative height and DBH growth rates did not differ between those in the impacted zone and non-impacted zone, leaf area and mass significantly differed between the two zones. Specifically, seedling and young-aged cohorts both had decreased leaf mass while the young-aged cohort also exhibited reduced leaf area expansion in the cold spell impacted zone. However, the middle-aged cohort was not affected by the extreme cold spell for these recorded parameters (Table 2).
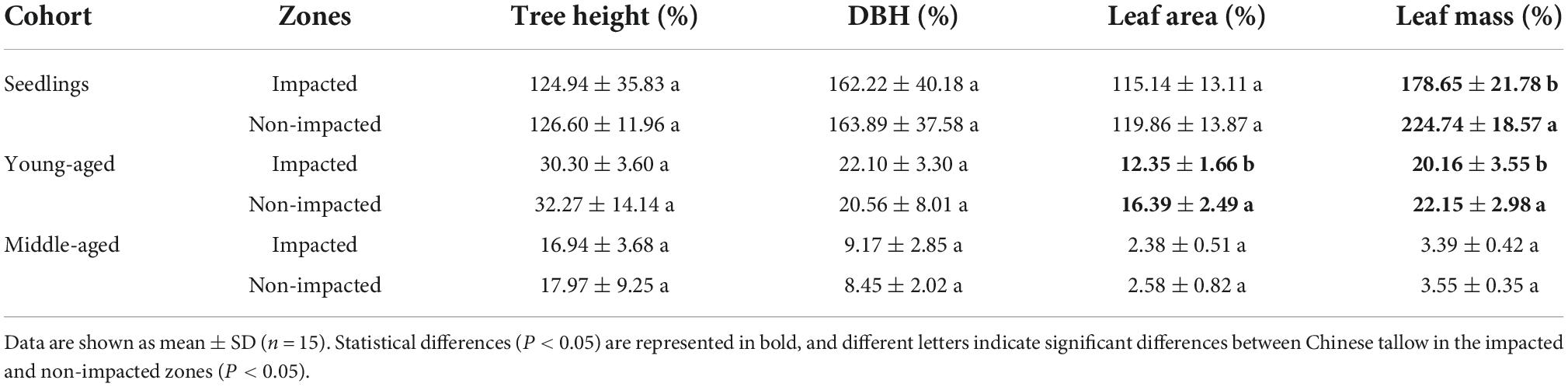
Table 2. Relative tree growth and leaf characteristics changes of Chinese tallow in cold spell impacted (Taizhou city) and non-impacted (Xiangshan county) zones by cohort age.
The extreme cold spell had significant impacts on leaf gas exchange. Among the evaluated variables, leaf An was significantly reduced across all cohorts in the impacted zone while Gs decreased only in young-aged and middle-aged cohorts (P < 0.05, Supplementary Tables 1, 2 and Figures 2A,B). Leaf Ci, Ci-Pa, and Ci/Ca increased in seedlings and young-aged cohorts, while Vpd increased only in the middle-aged cohort within the impacted zone (P < 0.05) (Figures 2C,H,I). Leaf Tr and the WUE of the young-aged cohort were reduced in the impacted zone (P < 0.05) (Figures 2D,E). No effect was detected on Tleaf in any cohort (Figure 2G). Regardless of the cold spell effects, as tree cohort age increased, leaf An, Gs, Tr, and WUE increased and leaf Ci, Ci-Pa, and Ci/Ca decreased (P < 0.05). However, leaf Vpd and Tleaf did not differ by cohort (Figure 2).
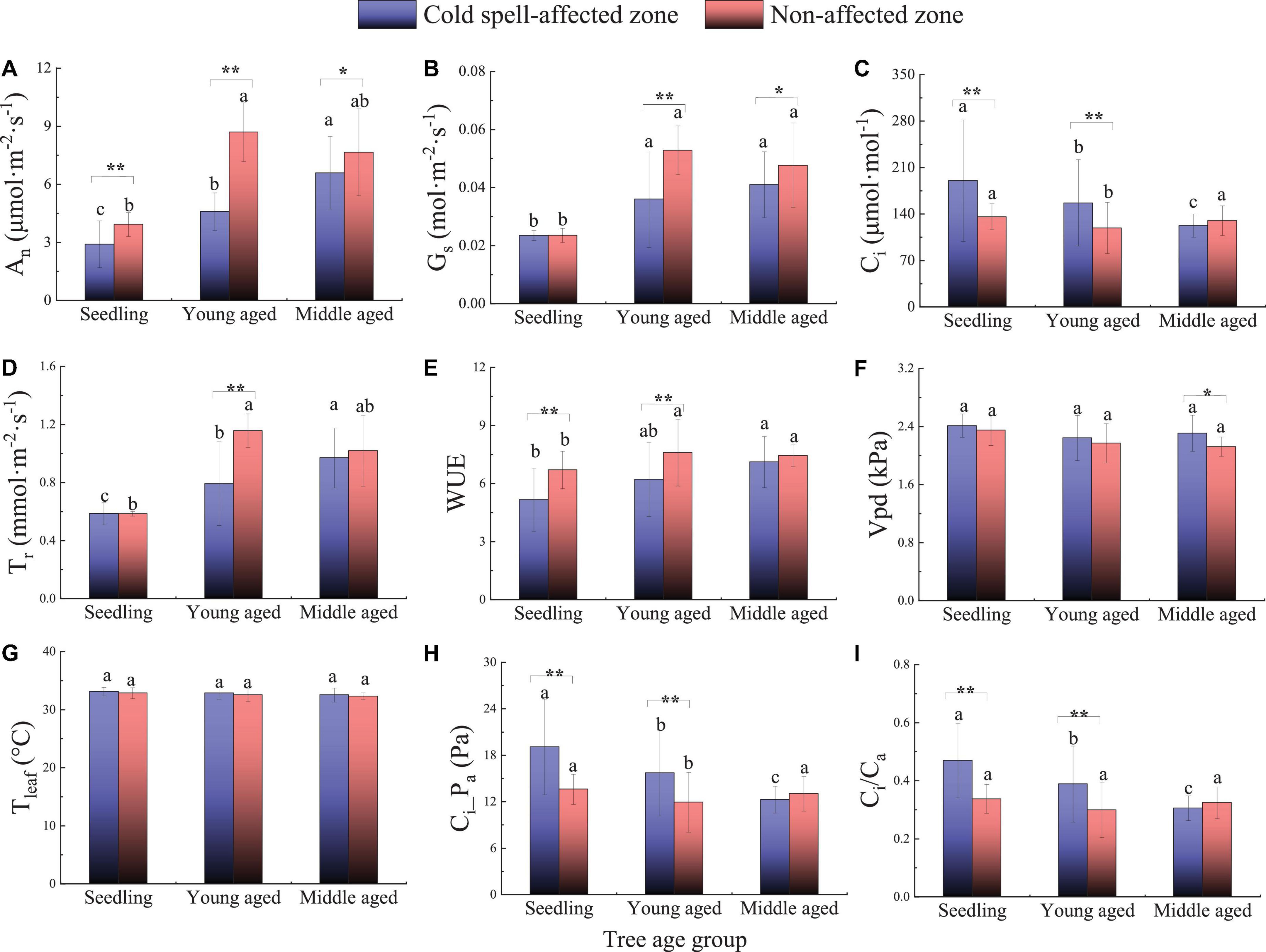
Figure 2. Leaf gas exchange of Chinese tallow in the cold spell impacted and non-impacted zones by cohort. Different lowercase letters indicate significant differences between cohorts (P < 0.05). Asterisks with brackets indicate significant differences between cold spell impact zone and non-impacted zone within the cohort (*P < 0.05; **P < 0.01). (A) leaf net photosynthesis rate (An); (B) stomatal conductance (Gs); (C) intercellular CO2 concentration (Ci); (D) transpiration rate (Tr); (E) water use efficiency (WUE); (F) vapor pressure deficit (Vpd); (G) leaf temperature (Tleaf); (H) the ratio of intercellular CO2 concentration to air CO2 concentration (Ci/Ca); (I) intercellular CO2 pressure (Ci-Pa).
The extreme cold spell had significant effects on leaf biochemical compounds and oxidative stress response of Chinese tallow. Leaf MDA and Pro contents were higher in the young-aged and middle-aged cohorts in the cold spell impacted zone (P < 0.05) (Figures 3A,D). Across all cohorts, the activities of SOD and POD were both reduced in the impacted zone (P < 0.05) (Figures 3B,C). Regardless of the cold spell impacts, as cohort age increased, SOD, POD, and Pro increased while the content of MDA decreased.
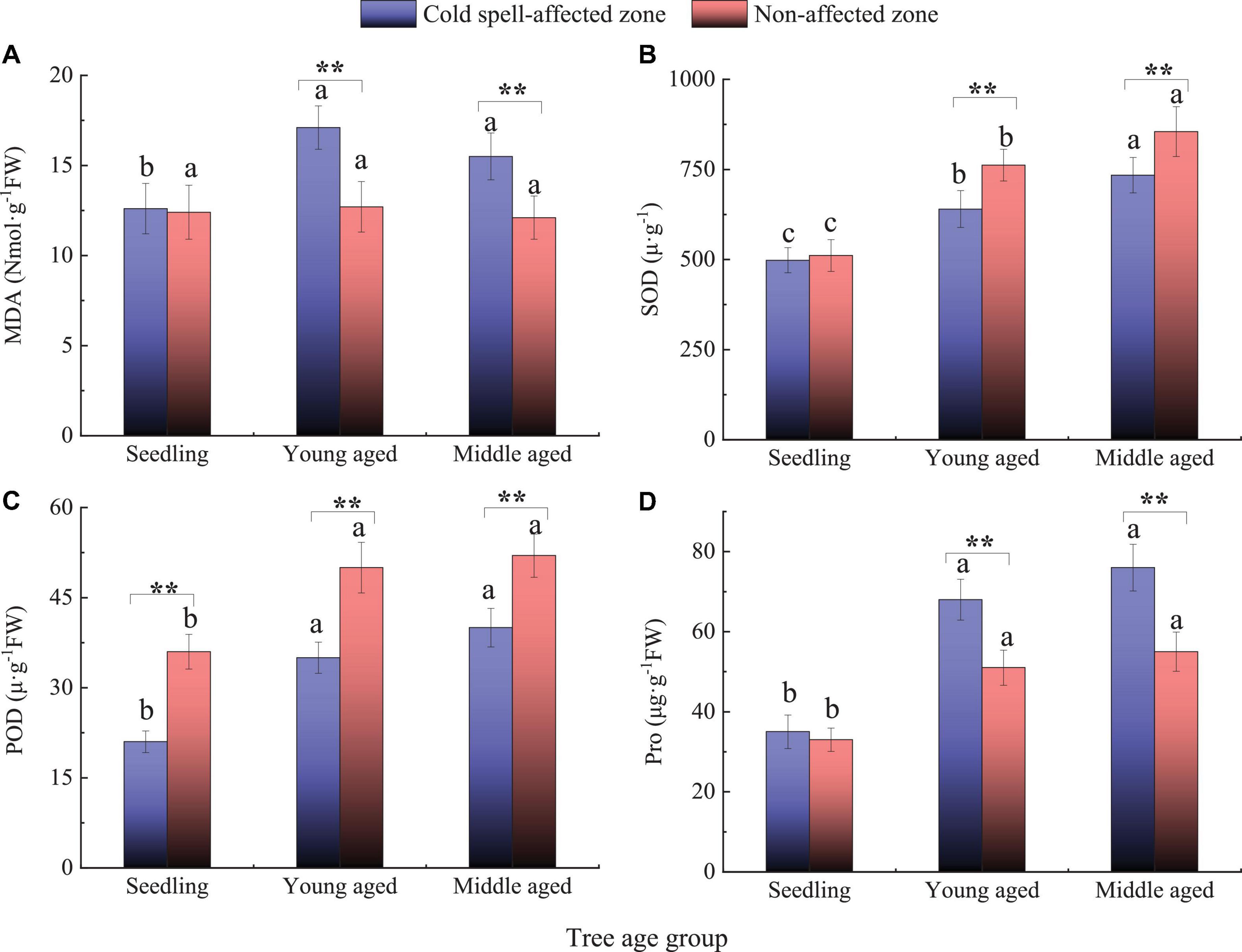
Figure 3. Leaf biochemical compounds and oxidative stress response in the cold spell impacted and non-impacted zones. Different lowercase letters indicated significant differences between cohorts (P < 0.05). Asterisks with brackets indicate significant differences between cold spell impacted zone and non-impacted zone within the cohort (*P < 0.05; **P < 0.01). (A) malondialdehyde (MDA); (B) superoxide dismutase (SOD); (C) peroxidase (POD); (D) free proline (Pro).
Based on the constrained RDA, microenvironmental conditions affected the leaf gas exchange of Chinese tallow following the extreme cold spell. Within the cold spell impacted zone, the total variation was 8.18, and the explanatory variables accounted for 19.2% of the total variance, with the first two axes explaining only 18.6% of the total variance. RHsfc was strongly related to An, Tr, Gs, WUE, Ci, Ci-Pa, and Ci/Ca, whereas PAR and C2sfc showed weak correlations with Vpd and Tleaf (Figure 4A and Supplementary Table 3). While the RDA analysis in the non-impacted zone indicated that the total variation was 2.4 and that the explanatory variables accounted for 75.3% of the total variance. The first two axes explained 73.4% of the total variance. RHsfc and PAR were strongly correlated with An, Tr, Gs, and WUE. C2sfc was associated with Ci, Ci-Pa, and Ci/Ca, but weakly related to Vpd and Tleaf (Figure 4B and Supplementary Table 3). The decreased total variance that the environmental factors explained and the reduced correlation between microenvironmental conditions and leaf gas exchange in the impacted zone indicate that the extreme cold spell resulted in reduced leaf utilization rates of microenvironmental resources for gas exchange, especially using RHsfc and PAR for leaf An and Tr (Figure 4).
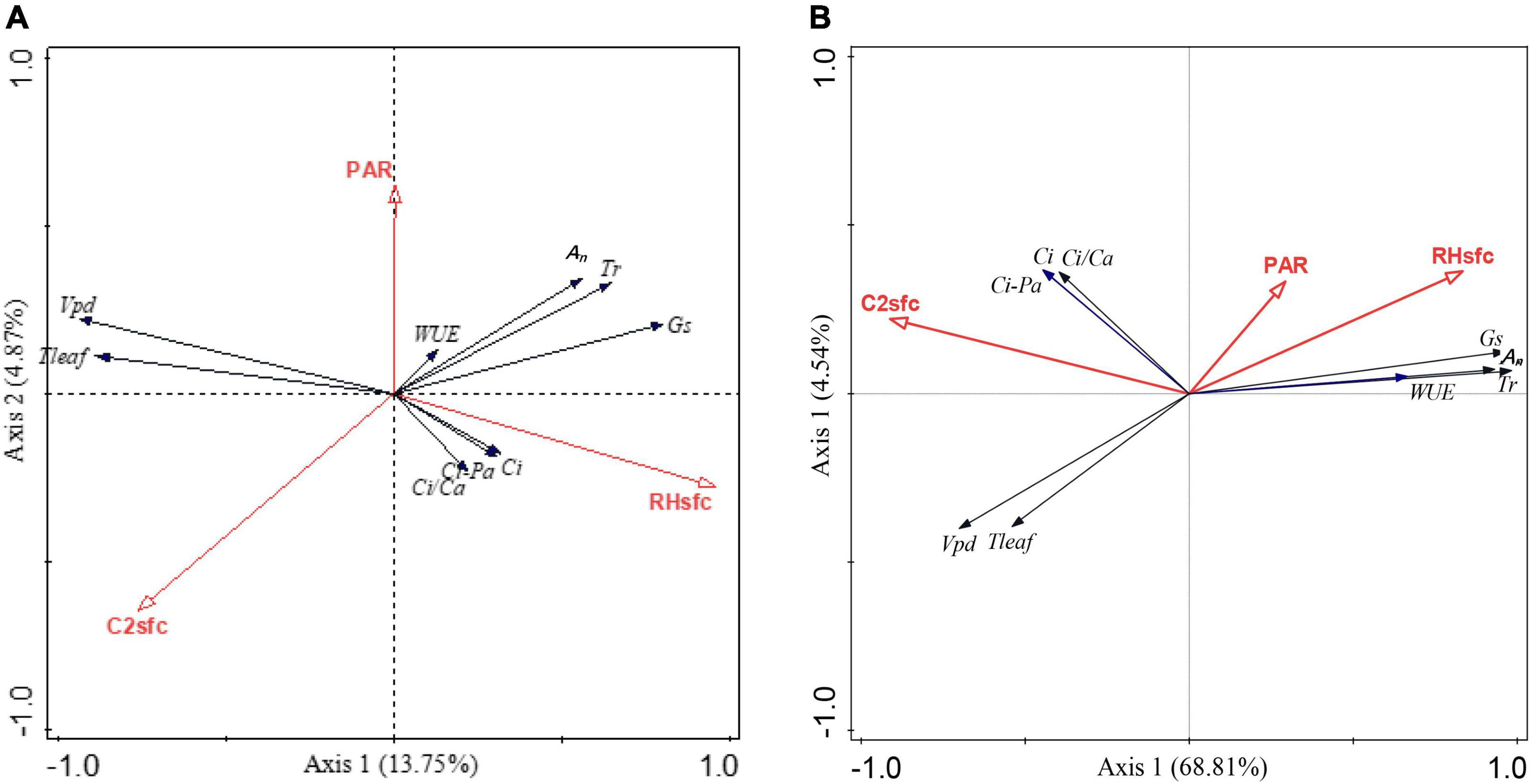
Figure 4. Effects of microenvironment resources on leaf gas exchange based on redundancy analysis (RDA). (A) Cold spell impacted zone; (B) non-impacted zone. An, leaf net photosynthesis rate; Gs, stomatal conductance; Ci, intercellular CO2 concentration; Tr, transpiration rate; Vpd, vapor pressure deficit; Tleaf, leaf temperature; Ci/Ca, the ratio of intercellular CO2 concentration to air CO2 concentration; Ci-Pa, intercellular CO2 pressure; WUE, leaf water use efficiency; PAR, photosynthetically active radiation; RHsfc, relative air humidity; C2sfc, air CO2 concentration.
Extreme cold spells, severe winter storms, and freezing precipitation not only cause immediate injury to trees, but also affect the long-term recovery and regeneration of damaged stems (Aroca et al., 2001). In this study, the severe cold spell brought prevailing damage to Chinese tallow with different aged cohorts in Xiangshan. Although instant tree mortality was rare, storm-related damages were common. We observed reducing trends of height and diameter growth as well as significantly smaller leaf area and leaf mass in cold spell impacted Chinese tallow individuals. We also detected a significant reduction of leaf gas exchange in comparison to the Chinese tallow in the non-impacted zone, such as declines in leaf An, Gs, Tr, and WUE, coupled with increased Ci, Vpd, Ci-Pa, and Ci/Ca. Leaf biochemical compounds and oxidative stress response also reacted to the event, in which leaf MDA and Pro contents increased while the activities of SOD and POD decreased. These findings suggest that the extreme cold spell impacted the growth and recovery of Chinese tallow in multiple aspects. Tree growth and critical metabolic activity were both significantly restrained by the cold event, the recovery rates were slow even after an adaptation period of 1 year. Low air temperatures especially restrict tree species with southerly distributions in the northern hemisphere like Chinese tallow, where the respiration, root growth, and phenophases can be affected by uncommon cold weather. Therefore, greater attention is required to understand winter weather effects on the ecological adaptions of southerly distributed tree species (Campbell et al., 2007; Augspurger, 2013; Zhu et al., 2014), especially when increased climate variability may result in more frequent and injurious winter weather events.
Although height and diameter growth were not significantly reduced from the cold spell, these metrics of tree growth were lower in the cold impacted zone. Leaf area and leaf mass of Chinese tallow were significantly reduced by the cold spell when compared with the non-affected zone. Smaller leaf area and mass could be attributed to the combined effects of reduced photosynthate and stronger defense mechanisms resulting from low-temperature stress. Plants living in cold environments evolve corresponding morphological characteristics to avoid being damaged when they are under cold stress (Bragg and Shelton, 2010). These adaptations include shrinking leaf area to minimize leaf exposure to freezing temperatures and to reduce water loss (Anjum et al., 2011; Zheng et al., 2011). Similar to other studies linking plant defensive reactions to damage or stress, we observed decreased activities of SOD and POD in leaves of cold spell affected Chinese tallow. Decreased SOD and POD activity would reduce functionality of the active oxygen scavenging system and accumulate MDA (Kong et al., 2014). The elevated Pro content observed in our study in cold affected Chinese tallow serves as osmotic regulator to improve cold resistance. This is an important metabolic process to defend against cold stress and to maintain internal physiological stability (Ashraf et al., 2012; Muchate et al., 2016).
Freezing temperatures generally disrupt the functioning of the primary components involved in the photosynthetic process, including thylakoid electron transportation, carbon reduction cycle, and variation in stomatal conductance (Li et al., 2004). Ice formation inside the plant cells can break plant cell membranes and compromise enzyme activity (such as Rubisco), resulting in direct damage to the cells and cause leaf water deficit (Allen and Ort, 2001; Zheng et al., 2011). As a result of the extreme cold spell, these breakages and changes were responsible for the substantial losses in leaf An, Gs, Tr, and WUE. As observed in this study, reduced An can be attributed to stomatal factors like decreased stomatal conductance and transpiration but can also be attributed to non-stomatal factors, including changes in photochemical efficiency, RuBP carboxylase activity, and RuBP regeneration, which will have a more severe impact on leaf photosynthesis (Rouhi et al., 2007; Guo et al., 2009). Additionally, cold spell-exposed Chinese tallow had notably increased leaf Ci, Vpd, Ci-Pa, and Ci/Ca, which may resulted from decreased photosynthetic CO2 consumption and subsequent accumulation of intercellular CO2 within the leaves. As a response to freezing precipitation, leaf Ci can be also regulated by the partial closure of leaf stomates, which also leads to an increased leaf Ci (Pagter et al., 2013; Fonteyne et al., 2016).
We detected a weakened relationship between the microenvironment conditions and leaf gas exchange of Chinese tallow following the extreme cold spell. This demonstrates a reduced capacity of Chinese tallow to fully recover metabolic capacities even after 1 year following the event. For cold spell-impacted Chinese tallow, leaf photosynthesis had already reached the light saturation point, but this point was still below the light intensity in the environment. Thus, resulting in a reduced relationship between PAR, C2sfc, and photosynthetic ability (Wan et al., 2009; Liu et al., 2013). There was a strong relationship between RHsfc and photosynthesis, suggesting that humidity was the key factor affecting the photosynthetic ability of Chinese tallow. Humidity also influences the light reaction of photosynthesis, because water is one of the raw materials for photosynthesis and regulates the opening and closing of leaf stomata and CO2 uptake (Ge et al., 2011; Moshelion et al., 2015).
Based on the results from this study, we can draw a first-step conclusion that the resistance and adaptability of Chinese tallow to freezing temperatures are greater than might have been assumed by its current native and invasive distributions. Even with incredibly low ambient air temperatures of −7.8°C, we did not detect cold-induced mortality to cold spell-impacted Chinese tallow. However, gas exchange, biochemical compounds, and oxidative stress response were still suppressed 1 year after the cold spell, indicating that recovery of the damaged stems was slow and the complete recovery is still on-going. Studies conducted in the southeastern US invasive range suggest that Chinese tallow may be limited by temperatures as low as −12°C (Gan et al., 2009), which also confirmed the underestimated cold tolerance of Chinese tallow (Park et al., 2012). Future projects should monitor the long-term impacts on the recovery of Chinese tallow affected by extreme cold. Understanding the adaptive capacity to climate extremes will be critical for determining its potential distribution in native, expanded, and invasive ranges.
Coastal forest frequently faces threats from both natural and anthropogenic disturbances. Southerly distributed tree species that are not well-adapted to freezing temperatures and precipitation may sustain more severe damage from these acute pulse disturbance events. Therefore, it is critical to understand their cold-resistant capabilities and adaptive mechanisms. Changes in either cold-resistant traits of trees or the severities of climatic disturbances can influence the abundance and distribution of tree species at a regional scale. Besides, the community structure of coastal forests, especially species composition, height and width of shelterbelt, also influences the response of tree species to extreme cold spells. Evergreen broadleaf trees were more susceptible to low temperature, with higher mortality and damage than other species in the forest overstory (Lu et al., 2020). Higher trees might be more easily affected by the ice storm considering the concomitant strong wind in the coastal areas, but wider shelterbelts had advantage over narrow belts to resist the ice storm, since the microhabitat temperature inside the shelterbelts were higher than periphery, resulting an overall lower damage rate of the coastal forest (Wang, 2014). Currently, expanding the native range of Chinese tallow in southeastern China for coastal protection is still warranted, but more studies are needed to understand the cold resistance mechanisms of Chinese tallow under both experimental and field conditions. Long-term monitoring programs should track winter weather-stressed individuals for susceptibility to secondary or compounding tree health concerns, including drought, pests and pathogens.
The 2016 extreme cold spell had significant effects on Chinese tallow in coastal forests of subtropical China. We observed obvious physiological adjustments in cold spell-impacted Chinese tallow, such as reduced leaf area and leaf mass, decreased leaf gas exchange and strengthened biochemical self-regulating and oxidative stress response. These cold-induced adaptations would be helpful to the recovery and resistance of cold stressed Chinese tallow. Across cohorts, the young-aged Chinese tallow were more immediately susceptible to the cold spell. Redundancy analysis showed that the cold spell impacted the ability of leaves to use water and CO2 for photosynthesis, thus slowing the recovery rate of cold spell damaged Chinese tallow even after a year. Therefore, the extreme cold event caused the degeneration of Chinese tallow, although few stems had direct cold-induced mortality, periodic occurrences of severe winter events storms may affect the long-term survival and reproduction of Chinese tallow. When tending damaged Chinese tallow stands in shelterbelt plantations, age-dependent recovery manipulations are highly encouraged, and results that combine research at multiple-scales are highly needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
HX carried out the fieldwork and laboratory analysis, prepared figures, and wrote the manuscript. LP and GW revised the manuscript. MY contributed substantially to the study design and supervised the field and laboratory personnel. All authors contributed to the article and approved the submitted version.
This project was funded by the National Key Research and Development Project (2019YFE0118900), the National Natural Science Foundation of China (32101506), the cooperative program in forest science between Zhejiang Province and the Chinese Academy of Forestry (16204002), and Jiyang College of Zhejiang A&F University under Grant No. RQ1911F09.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.945886/full#supplementary-material
Allen, D. J., and Ort, D. R. (2001). Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 6, 36–42. doi: 10.1016/S1360-1385(00)01808-2
Anjum, S., Xie, X., Wang, L., Saleem, M. F., Man, C., and Lei, W. (2011). Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 6, 2026–2032.
Aroca, R., Irigoyen, J. J., and Sanchez-Diaz, M. (2001). Photosynthetic characteristics and protective mechanisms against oxidativestress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Sci. 161, 719–726. doi: 10.1016/S0168-9452(01)00460-5
Ashraf, M. A., Ashraf, M., and Shahbaz, M. (2012). Growth stage-based modulation in antioxidant defense system and proline accumulation in two hexaploid wheat (Triticum aestivum L.) cultivars differing in salinity tolerance. Flora 207, 388–397. doi: 10.1016/j.flora.2012.03.004
Augspurger, C. K. (2013). Reconstructing patterns of temperature, phenology, and frost damage over 124 years: Spring damage risk is increasing. Ecology 94, 41–50. doi: 10.1890/12-0200.1
Basuchaudhuri, P. (2014). Cold tolerance in rice cultivation. CRC press. New York, NY: United States of America. doi: 10.1201/b16873
Boucek, R. E., Gaiser, E. E., Liu, H., and Rehage, J. S. (2016). A review of subtropical community resistance and resilience to extreme cold spells. Ecosphere 7:e01455. doi: 10.1002/ecs2.1455
Bragg, D. C. (2016). Initial mortality rates and extent of damage to loblolly and longleaf pine plantations affected by an ice storm in South Carolina. For. Sci. 62, 574–585. doi: 10.5849/forsci.15-177
Bragg, D. C., and Shelton, M. G. (2010). Recovery of planted loblolly pine 5 years after severe ice storms in Arkansas. South. J. Appl. For. 34, 13–20. doi: 10.1093/sjaf/34.1.13
Bruce, K. A., Cameron, G. N., Harcombe, P. A., and Jubinsky, G. (1997). Introduction, impact on native habitats, and management of a woody invader, the Chinese tallow tree, Sapium sebiferum (L.) Roxb. Nat. Areas J. 17, 255–260.
Campbell, C., Atkinson, L., Zaragoza-Castells, J., Lundmark, M., Atkin, O., and Hurry, V. (2007). Acclimation of photosynthesis and respiration is asynchronous in response to changes in temperature regardless of plant functional group. N. Phytol. 176, 375–389. doi: 10.1111/j.1469-8137.2007.02183.x
Chen, L., Tiu, C. J., Peng, S., and Siemann, E. (2013). Conspecific plasticity and invasion: Invasive populations of Chinese tallow (Triadica sebifera) have performance advantage over native populations only in low soil salinity. PLoS One 8:e74961. doi: 10.1371/journal.pone.0074961
Chen, Z., Dong, D., and Wan, X. (2017). Analysis of a severe cold wave weather process in Zhejiang province in January 2016. J. Zhejiang Meteorol. 38, 11–14. (in Chinese with English abstract).
Conner, W. H. (1994). The effect of salinity and waterlogging on growth and survival of baldcypress and Chinese tallow seedlings. J. Coast. Res. 10, 1045–1049.
dos Santos, U. M. Jr., de Carvalho, G. J. F., and Feldpausch, T. R. (2006). Growth, leaf nutrient concentration and photosynthetic nutrient use efficiency in tropical tree species planted in degraded areas in central Amazonia. For. Ecol. Manage. 226, 299–309. doi: 10.1016/j.foreco.2006.01.042
Fernandes, A., Rollinson, C. R., Kearney, W. S., Dietze, M. C., and Fagherazzi, S. (2018). Declining radial growth response of coastal forests to hurricanes and nor’easters. J. Geophys. Res. Biogeosci. 123, 832–849. doi: 10.1002/2017JG004125
Fonteyne, S., Lootens, P., Muylle, H., Van den Ende, W., De Swaef, T., Reheul, D., et al. (2016). Chilling tolerance and early vigour-related characteristics evaluated in two Miscanthus genotypes. Photosynthetica 54, 295–306. doi: 10.1007/s11099-016-0193-y
Gan, J., Miller, J. H., Wang, H., and Taylor, J. W. (2009). Invasion of tallow tree into southern US forests: Influencing factors and implications for mitigation. Can. J. For. Res. 39, 1346–1356. doi: 10.1139/X09-058
Ge, Z., Zhou, X., Kellomäki, S., Wang, K., Peltola, H., and Martikainen, P. J. (2011). Responses of leaf photosynthesis, pigments and chlorophyll fluorescence within canopy position in a boreal grass (Phalaris arundinacea L.) to elevated temperature and CO2 under varying water regimes. Photosynthetica 49, 172–184. doi: 10.1007/s11099-011-0029-8
Guo, W., Zheng, J., Zhang, Z., Chen, W., and Guo, Y. (2009). Effects of short-term chilling stress on the photosynthetic physiology of fingered citrons (Citrus medica var. sarcodactylis Swingle). Acta Ecol. Sin. 29, 2286–2293. (in Chinese with English abstract).
Gutschick, V. P., and BassiriRad, H. (2003). Extreme events as shaping physiology, ecology, and evolution of plants: Toward a unified definition and evaluation of their consequences. N. Phytol. 160, 21–42. doi: 10.1046/j.1469-8137.2003.00866.x
Irland, L. C. (2000). Ice storms and forest impacts. Sci. Total Environ. 262, 231–242. doi: 10.1016/S0048-9697(00)00525-8
Jubinsky, G., and Anderson, L. C. (1996). The invasive potential of Chinese tallow-tree (Sapium sebiferum Roxb) in the southeast. Castanea 61, 226–231.
Kollas, C., Körner, C., and Randin, C. F. (2014). Spring frost and growing season length co-control the cold range limits of broad-leaved trees. J. Biogeogr. 41, 773–783. doi: 10.1111/jbi.12238
Kong, J., Dong, Y., Xu, L., Liu, S., and Bai, X. (2014). Role of exogenous nitric oxide in alleviating iron deficiency-induced peanut chlorosis on calcareous soil. J. Plant Interact. 9, 450–459. doi: 10.1080/17429145.2013.853327
Konisky, D. M., Hughes, L., and Kaylor, C. H. (2016). Extreme weather events and climate change concern. Clim. Change 134, 1–15. doi: 10.1007/s10584-015-1555-3
Körner, C. (2016). Plant adaptation to cold climates. F1000Res. 5 (F1000FacultyRev):2769. doi: 10.12688/f1000research.9107.1
Lafon, C. W. (2006). Forest disturbance by ice storms in Quercus forests of the southern Appalachian Mountains. USA. Ecoscience 13, 30–43. doi: 10.2980/1195-6860(2006)13[30:FDBISI]2.0.CO;2
Larcher, W. (1981). “Chapter 14: Physiological processes limiting plant productivity,” in Effects of low temperature stress and frost injury on plant productivity (Oxford: Butterworth-Heinemann), 253–269. doi: 10.1016/B978-0-408-10649-8.50018-6
Larcher, W. (2005). Climatic constraints drive the evolution of low temperature resistance in woody plants. J. Agric. Meteorol. 61, 189–202. doi: 10.2480/agrmet.61.189
Li, H. (2000). Plant Physiology and Biochemistry experimental principles and techniques. Beijing: Higher Education Press, 148–219.
Li, X., Wang, X., Meng, Q., and Zou, Q. (2004). Factors limiting photosynthetic recovery in sweet pepper leaves after short-term chilling stress under low irradiance. Photosynthetica 42, 257–262. doi: 10.1023/B:PHOT.0000040598.48732.af
Liu, D., Estiarte, M., Ogaya, R., Yang, X., and Peñuelas, J. (2017). Shift in community structure in an early-successional Mediterranean shrubland driven by long-term experimental warming and drought and natural extreme droughts. Glob. Change Biol. 23, 4267–4279. doi: 10.1111/gcb.13763
Liu, R., Zhang, D., He, X., Nirasawa, S., Tatsumi, E., and Liu, H. (2014). The relationship between antioxidant enzymes activity and mungbean sprouts growth during the germination of mungbean seeds treated by electrolyzed water. Plant Growth Regul. 74, 83–91. doi: 10.1007/s10725-014-9899-7
Liu, X., Fan, Y., Long, J., Wei, R., Kjelgren, R., Gong, C., et al. (2013). Effects of soil water and nitrogen availability on photosynthesis and water use efficiency of Robinia pseudoacacia seedlings. J. Environ. Sci. 25, 585–595. doi: 10.1016/S1001-0742(12)60081-3
López de Heredia, U., Valbuena-Carabaña, M., Córdoba, M., and Gil, L. (2009). Variation components in leaf morphology of recruits of two hybridising oaks [Q. petraea (Matt.) Liebl. and Q. pyrenaica Willd.] at small spatial scale. Eur. J. For. Res. 128, 543–554. doi: 10.1007/s10342-009-0302-6
Lu, D., Pile, L. S., Yu, D., Zhu, J., Bragg, D. C., and Wang, G. G. (2020). Differential responses of tree species to a severe ice storm and their implications to forest composition in the southeast United States. For. Ecol. Manage. 468:118177. doi: 10.1016/j.foreco.2020.118177
Mattsson, E., Ostwald, M., Nissanka, S. P., Holmer, B., and Palm, M. (2009). Recovery and protection of coastal ecosystems after tsunami event and potential for participatory forestry CDM-Examples from Sri Lanka. Ocean Coast. Manage. 52, 1–9. doi: 10.1016/j.ocecoaman.2008.09.007
Moshelion, M., Halperin, O., Wallach, R., Oren, R. M., and Way, A. (2015). Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 38, 1785–1793. doi: 10.1111/pce.12410
Muchate, N. S., Nikalje, G. C., Rajurkar, N. S., Suprasanna, P., and Nikam, T. D. (2016). Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 82, 371–406. doi: 10.1007/s12229-016-9173-y
Obura, D. (2006). Impacts of the December 26 2004 tsunami in eastern Africa. Ocean Coast. Manage. 49, 873–888. doi: 10.1016/j.ocecoaman.2006.08.004
Pagter, M., Petersen, K. K., and Kjaer, K. H. (2013). Direct and indirect effects of shoot-and/or root-chilling stress on growth, photosynthesis, and osmotic root water uptake in Kalanchoe blossfeldiana poelln. ‘Molly’. J. Hortic. Sci. Biotechnol. 88, 571–579. doi: 10.1080/14620316.2013.11513008
Park, I., DeWalt, S. J., Siemann, E., and Rogers, W. E. (2012). Differences in cold hardiness between introduced populations of an invasive tree. Biol. Invasions 14, 2029–2038. doi: 10.1007/s10530-012-0209-x
Pattison, R. R., and Mack, R. N. (2008). Potential distribution of the invasive tree Triadica sebifera (Euphorbiaceae) in the United States: Evaluating climex predictions with field trials. Glob. Change Biol. 14, 813–826. doi: 10.1111/j.1365-2486.2007.01528.x
Paudel, S., and Battaglia, L. L. (2015). The role of light, soil and human factors on the probability of occurrence of an invasive and three native plant species in coastal transitions of coastal Mississippi, USA. J. Plant Ecol. 8, 491–500. doi: 10.1093/jpe/rtu045
Pile, L. S., Wang, G. G., Stovall, J. P., Siemann, E., Wheeler, G. S., and Gabler, C. A. (2017). Mechanisms of Chinese tallow (Triadica sebifera) invasion and their management implications-A review. For. Ecol. Manage. 404, 1–13. doi: 10.1016/j.foreco.2017.08.023
Pisaric, M. F. J., King, D. J., Macintosh, A. J. M., and Bemrose, R. (2008). Impact of the 1998 ice storm on the health and growth of sugar maple (Acer saccharum Marsh.) dominated forests in Gatineau Park, Quebec. J. Torrey Bot. Soc. 135, 530–539. doi: 10.3159/08-RA-053R.1
Priebe, J. E., Powers, M. D., and Cole, E. C. (2018). Species, tree size, and overstory environment affect likelihood of ice storm damage to understory trees in a mature Douglas-fir forest. For. Ecol. Manage. 409, 777–788. doi: 10.1016/j.foreco.2017.11.041
Rouhi, V., Samson, R., Lemeur, R., and Van Damme, P. (2007). Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environ. Exp. Bot. 59, 117–129. doi: 10.1016/j.envexpbot.2005.10.001
Scheld, H. W., Cowles, J. R., Engler, C. R., and Kleiman, R. E. B. (1984). “Seeds of the Chinese tallow tree as a source of chemicals and fuels,” in Fuels and chemicals from oil seeds: Technology and policy options, eds E. B. Schultz Jr. and R. P. Morgan (Boulder, CO: Westview Press), 81–101. doi: 10.1201/9780429044779-7
Seischab, F. K., Bernard, J. M., and Eberic, M. D. (1993). Glaze storm damage to western New York forest communities. Bull. Torrey Bot. Club 120, 64–72 doi: 10.2307/2996665
Smilauer, P., and Leps, J. (2014). Multivariate analysis of ecological data using CANOCO 5, 2th Edn. Cambridge: Cambridge University Press, 362. doi: 10.1017/CBO9781139627061
Smith, M. D. (2011). An ecological perspective on extreme climatic events: A synthetic definition and framework to guide future research. J. Ecol. 99, 656–663. doi: 10.1111/j.1365-2745.2011.01798.x
Strimbeck, G. R., Schaberg, P. G., Fossdal, C. G., Schröde, W. P., and Kjellsen, T. D. (2015). Extreme low temperature tolerance in woody plants. Front. Plant Sci. 6:00884. doi: 10.3389/fpls.2015.00884
Wan, S., Jia, Z., and Yang, B. (2009). Relationship between diurnal changes of alfalfa net photosynthetic rate and environmental factors. Acta Agrectir Sin. 17, 27–31. (in Chinese with no English abstract).
Wang, W. (2014). Monitoring and evaluation of ecological effects of metasequoia glyptostroboides shelter forest in muddy coastal area. MA thesis. Beijing: Chinese Academy of Forestry. (in Chinese with no English abstract).
Weisany, W., Sohrabi, Y., Heidari, G., Siosemardeh, A., and Ghassemi-Golezani, K. (2012). Changes in antioxidant enzymes activity and plant performance by salinity stress and zinc application in soybean (Glycine max L.). Plant Omics J. 5, 60–67.
Xie, H., Wang, G. G., and Yu, M. (2018). Ecosystem multifunctionality is highly related to the shelterbelt structure and plant species diversity in mixed shelterbelts of eastern China. Glob. Ecol. Conserv. 16:e00470. doi: 10.1016/j.gecco.2018.e00470
Yang, D., Wan, F., Gu, T., and Li, M. (2012). Study on the selection of afforestation species for coastal protective in Shanghai city. J. Nanjing For. Univ. 36, 95–100. (in Chinese with English abstract).
Zheng, G., Tian, B. O., Zhang, F., Tao, F., and Li, W. (2011). Plant adaptation to frequent alterations between high and low temperatures: Remodelling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ. 34, 1431–1442. doi: 10.1111/j.1365-3040.2011.02341.x
Zhou, B., Gu, L., Ding, Y., Shao, L., Wu, Z., Yang, X. et al. (2011) The great 2008 chinese ice storm its socioeconomic-ecological impact and sustainability lessons learned. Bull. Am. Meteorol. Soc. 92, 47–60. doi: 10.1175/2010BAMS2857.1
Keywords: extreme cold spell, leaf characteristics, gas exchange, leaf biochemistry, native range
Citation: Xie H, Pile Knapp LS, Yu M and Wang GG (2022) Morphological and physiological response of Chinese tallow (Triadica sebifera) to an extreme cold spell in subtropical, coastal forests of China. Front. Ecol. Evol. 10:945886. doi: 10.3389/fevo.2022.945886
Received: 17 May 2022; Accepted: 06 October 2022;
Published: 20 October 2022.
Edited by:
Robert Guralnick, University of Florida, United StatesReviewed by:
Shishir Paudel, Phipps Conservatory and Botanical Gardens, United StatesCopyright © 2022 Xie, Pile Knapp, Yu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongtao Xie, eGllaG9uZ3Rhb2RlQDEyNi5jb20=; Mukui Yu, eXVtdWt1aUBjYWYuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.