
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 29 June 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.940606
This article is part of the Research TopicEffects of Non-Random Sources of Alteration on Biodiversity and Ecosystem FunctioningView all 19 articles
 Peng Gu1
Peng Gu1 Zhaochang Zhang1
Zhaochang Zhang1 Jing Liu2
Jing Liu2 Tao Wang3
Tao Wang3 Yunxing Xiao2
Yunxing Xiao2 YangJinzhi Yu2
YangJinzhi Yu2 Hengfeng Miao1
Hengfeng Miao1 Yumiao Zhang2
Yumiao Zhang2 Fei Liao2
Fei Liao2 Kunlun Yang1*
Kunlun Yang1* Qi Li2*
Qi Li2*At present, there is little research on the impact of small hydropower stations on aquatic biodiversity. In order to investigate whether the existence of small hydropower stations has a significant impact on the aquatic biodiversity of their watersheds, we conducted a systematic study on the abundance of plankton, benthic animal, fish and microorganism in the watersheds of 15 small hydropower stations in Qionglai City. The results showed that 59 species of phytoplankton from 3 divisions, 16 species of zooplankton from 4 categories, 25 species of benthic animal from 3 phyla and 30 species of fish were found in the study basin. The analysis of the physical and chemical indicators of water bodies and the distribution characteristics of aquatic organisms found that the operation of small diversion-type power stations in Qionglai City changed part of the aquatic habitat in the basin, with a greater impact on the activities of large aquatic animals (fish) and a smaller impact on plankton and microorganism, and the intensity of the impact was shown as fish > benthic animal > plankton > microorganism. The small hydropower stations in this study have an impact but not significant on the aquatic biodiversity in the Baimo and Wenjing River in the Qionglai City, and this study provides a data reference for the comprehensive assessment of the environmental impact of small hydropower stations.
Since 1950s, as an important clean and renewable energy, hydropower has played an important role in electricity supply, poverty reduction, rural economy and social development (Li et al., 2022), which is an indispensable guarantee for achieving the goals of “carbon peaking” and “carbon neutrality”. At the same time, small hydropower station projects are widely used in rural areas and remote mountainous areas because of their small investment, low risk, stable benefits and low operating costs (Fu et al., 2008). By 2021, more than 47,000 small hydropower stations have been built in China, with an annual power generation of 250 billion kilowatt-hours, equivalent to saving more than 70 million tons of standard coal and reducing carbon dioxide emissions by 180 million tons per year (Peng et al., 2022). It has also been pointed out that the construction of small hydropower stations has negative ecological impacts, such as affecting fish migration and reproduction (Cao, 2019); limiting the spread of animals and plants (Rivinoja et al., 2010); altering the hydraulic flow characteristics of the river (Guo et al., 2015); and changing the structure and genetic diversity of biological communities (Sundermann et al., 2011; Liu, 2020; Costea et al., 2021).
The impact of small hydropower stations on the aquatic environment can be monitored by aquatic biodiversity. As one of the main indicators of water environment assessment, aquatic biodiversity plays an important role in ecological restoration and health assessment of river basins (Sun et al., 2013; Gao et al., 2015), while the health of aquatic biota can directly or indirectly reflect the health of the water environment. Phytoplankton, zooplankton and large aquatic organisms are widely used in water environment monitoring and assessment. For example, Wei et al. (2012) analyzed the water quality of the Erhai Sea by investigating the species, number and distribution of phytoplankton; Zhao et al. (2008) evaluated and analyzed the status of the water ecological environment in the Huaihe River basin by investigating the composition and abundance of phytoplankton, zooplankton and benthic animal. Fish cover all trophic levels of consumer ecology and provide a powerful tool for assessing aquatic environments (Lin et al., 2021); plankton are an important link in the material and energy transfer of aquatic ecosystems, with phytoplankton playing a vital role in the food chain as the first trophic level (Zhang et al., 2010); zooplankton participate in the material cycle of aquatic ecosystems by feeding on phytoplankton, bacteria and other organisms (Bi et al., 2001; Li et al., 2004); benthic organisms are an essential part of the aquatic food web and provide the bait base for most fish in rivers, and are closely related to the ecological taxa and zonal composition of riverine fish (Šarauskienė et al., 2021); in addition, the species and abundance of microbial communities can reflect changes in aquatic ecosystems. Overall, the aquatic organisms mentioned above can indicate the health of the aquatic environment at a certain level (Xu, 1996; Araújo et al., 2000; Jakovčev-Todorović et al., 2005; Salmaso et al., 2006; Li, 2022).
At present, the assessment of aquatic ecological environment based on aquatic biodiversity is mainly focused on large and medium-sized reservoirs and hydropower stations (Otahelová and Valachovič, 2003; Bredenhand and Samways, 2009; Andrianova, 2020; Chen et al., 2020), and fewer studies have been reported on the disturbance of aquatic biodiversity by small hydropower stations. Therefore, it is meaningful to explore the impact of small hydropower stations on the characteristics, diversity and functional groups of aquatic communities to improve the ecological impact assessment index system of small hydropower stations. This study investigated the impacts of the construction of 15 small hydropower stations in Qionglai on the diversity of aquatic organisms (fish, plankton, benthic animal and microoganism). With a view to provide basic data and theoretical basis for the development of small hydropower stations and ecological environmental protection of river basins.
Qionglai City is located in Sichuan Province of western China, at the upper reaches of the Yangtze River, and has been “the first state in southern Sichuan” since ancient times. There are numerous small hydropower stations in the Baimo and Wenjing River basins in Qionglai.
Since the sampling conditions were available during the low-water seasons, in December 2021, we collected samples from a total of 16 points at 15 small hydropower stations and the confluence of watersheds in the Baimo and Wenjing River basins in Qionglai City. The river is divided into four watersheds (Q1-Q4) according to its connectivity and location (Table 1). Among them, S0 is the confluence of rivers, and the remaining 15 sampling points are all diversion-type power stations. The geographical distribution of sampling points is shown in Figure 1, S1-S4 belong to basin Q1, S5 belongs to basin Q2, S6-S9 belong to basin Q3 and S10-S15 belong to basin Q4.
The collection of fish resources included field sampling and visiting surveys, on-site identification of fish specimens as far as possible, determination of biological basic data, looking through common fish atlases to determine fish species, and taking scales and other materials for age identification. For the identification of fish, reference the Chinese Fish Atlas (Li, 2015) to determine the species of fish.
1 liter of water sample was collected with glass water sampler into polyethylene bottles, stored in 1.5% Lugol’s solution and brought back to the experiment for analysis. Firstly, it needed to settle for 48 h, and then using a siphon device, the water sample was concentrated to 30 mL, 0.1 mL of the shaken concentrated water sample was taken on a plankton counting plate, and the morphological analysis of the algal species was observed by microscopic counting method under a light microscope. For the identification of algae, reference the The system, taxonomy and ecology of Chinese freshwater algae (Hu and Wei, 2006).
Fifty liters of water sample were collected at the same place, and the mixed water samples were filtered through a No. 25 plankton net (pore size of 64 μm), and finally the concentrated solution was transferred to a polyethylene bottle, and the samples were stored in 5% formaldehyde solution, protected from light and low temperature. The shaken concentrated water samples were taken 0.1 mL on a plankton counting plate, covered with a coverslip, and the samples were identified and counted for species under a light microscope. Referring to the Atlas of Freshwater Microorganisms (Zhou, 2005) for classification and identification of species.
Benthic animals were collected by a mud collector for 3-5 times, with the sampling area of 0.09 m2. And the samples in the mud collector were passed through a 40 mesh sieve, washed and picked out as suspected benthic animals, and loaded into specimen bottles containing 30% alcohol. The samples were brought back to the laboratory and the benthic animals were then picked out on dissecting trays and stored in 75% alcohol at 4°C. The alcohol was replaced once a week pending identification. For quantitative benthic analysis, sample species identification and enumeration were carried out under a light microscope, mainly with reference to the Atlas of Chinese Animals (Tong, 1982), and identified to species.
The collected microbial water samples were filtered with a water body extractor (0.45 μm filter membrane) for microbial enrichment, and if the samples are turbid, centrifugation is used for enrichment. The enriched microbial water samples were sent to Shanghai Personalbio Technology Co., Ltd., for testing, and the microbial community-related data were obtained by high-throughput sequencing (HTS) and polymerase chain reaction (PCR) amplification of 16sRNA gene-specific fragments.
Physical and chemical properties of water quality and spatial distribution of phytoplankton density and biomass use Personalbio for analysis. Distribution of each sampling point at different division levels uses Origin 2022 for analysis.
Water samples from S0, which is at the confluence of Baimo and Wenjing River, and 3 representative power stations (S4, S9, and S15) are selected to measure physical and chemical indexes, and the comparison maps of pH, water temperature (WT), conductivity (Cond), dissolved oxygen (DO) and transparency of each sampling site are drawn (Figure 2). As can be seen from Figure 2, the pH of water in the study basin varies between 8.5 and 8.8, belonging to weakly alkaline water quality; WT varies between 11.6°C and 14.3°C; Cond varies from 30.3 ms/m to 43.0 ms/m; DO varies between 12.99 ppm and 13.5 ppm; transparency varies between 0.4 m and 1.3 m. There are no significant differences in pH, WT, and DO among the sampling sites.
The results of the fish survey are detailed in Supplementary Table 1. There are 30 species of fish in total. Among them, 6 species are obtained by on-site sampling, namely Misgurnus anguillicaudatus, Monopterus albus, Pelteobagrus fulvidraco, Cyprinus carpio, Pseudorasbora parva and Carassius auratus. A total of 10 species are found after checking the data, mainly including Rhodeus sinensis, Rhodeus ocellatus, Abbottina rivularis and Hemibarbus maculatus, etc. Through the interview survey, it is found that there are 14 kinds of fish, including Silurus asotus, Hypophthalmichthys molitrix, Silurus meridionalis and Aristichthys nobilis, etc.
A total of 6 species of fish are collected on site, among which Misgurnus anguillicaudatus, Cyprinus carpio and Carassius auratus are highly resistant to pollution and are medium pollution zone indicator fish, indicating that there may be a certain degree of pollution in the watershed. Zhu et al. (2021) collected 71 species of fish in the nearshore Jingjiang section of the Yangtze River near the mouth; Wang et al. (2020) collected 64 species of fish in the Xinzhou waters of Anqing of the Yangtze River; Zheng et al. (2019) collected 56 species of fish in the Wuhan section of the Yangtze River, the fish resources in the survey basin are relatively small compared to the aquatic resources in the upper reaches of the Yangtze River, it is possible that the construction of small hydropower stations has led to changes in river volume and flow rate, which has affected the survival and reproduction of fish (Sun and Liu, 2020), reducing the space in the water column and affecting the species composition of fish (Benejam et al., 2016). The construction of small hydropower stations has a greater impact on fish diversity than other aquatic organisms.
The results of the phytoplankton survey in the study basin are shown in Table 2. The phytoplankton shows Q3 > Q4 > Q1 > Q2 in terms of species abundance and diversity. The proportion of phytoplankton in the three divisions of Bacillariophyta, Cyanophyta and Chlorophyta in the four watersheds is shown in Figure 3. The Bacillariophyta is mainly found in the basin Q1 and Q4, especially in the 4 sampling sites S1, S2, S10, and S11. And the Cyanobacteria and Chlorophyta are mainly distributed in the Q3 basin.
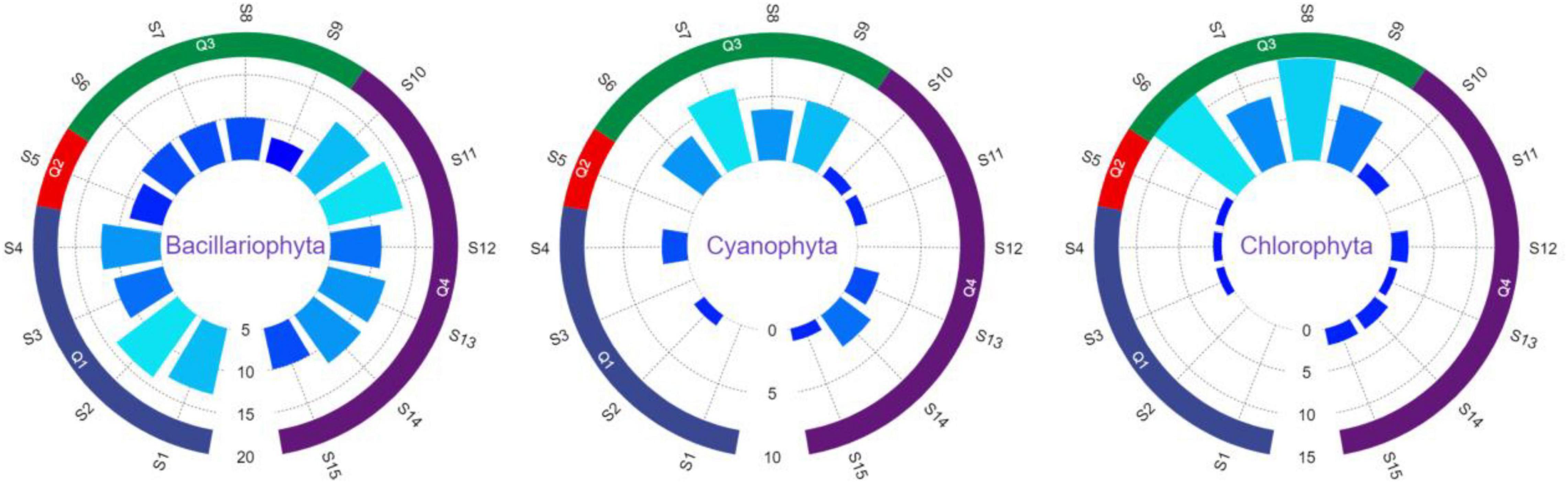
Figure 3. The proportion of phytoplankton in the divisions of Bacillariophyta, Cyanophyta, and Chlorophyta in the four watersheds.
A total of 26 species of common phytoplankton are found in Q1, among which the Bacillariophyta is the most abundant with 22 species, mainly including Diploneis elliptica and Navicula sp., etc., followed by Chlorophyta, Cyanobacteria with 2 species each, namely, Scenedesmus bijugatus, Ulothrix sp. and Pseudoanabaena sp., Aphanocapsa sp. A total of 10 species of common phytoplankton are found in the basin Q2, among which Bacillariophyta are the most abundant with 9 species, mainly including Cymbella affinis and C. cymbiformis, etc., followed by Chlorophyta with only 1 species of C. fracta. A total of 32 species of common phytoplankton are found in Q3, among which 14 species of Chlorophyta, mainly including Scenedesmus quadricauda, etc., followed by the Bacillariophyta with 11 species, mainly including Fragilaria capucina, etc., there are 7 species of Cyanobacteria, mainly including Phormidium faveolarum, etc. A total of 28 species of common phytoplankton are found in Q4, among which Bacillariophyta is the most abundant with 22 species, mainly including Diploneis elliptica and Navicula sp., etc., followed by 3 species each of Cyanobacteria and Chlorophyta. In general, The largest relative abundance is in the Bacillariophyta, with 55.9%, followed by the Chlorophyta, with 28.8%, and the Cyanophyta, with 15.3% (Supplementary Table 2).
The distribution of phytoplankton population density and biomass at each sampling point is shown in Figure 4. Quantitative analysis statistics of phytoplankton from 15 sampling sites shows that the density of phytoplankton ranges from 8.0 pcs/L to 7869.1 pcs/L, with an average of 2967.1 pcs/L. The highest sampling point is S6 with 7869.1 pcs/L, the second highest is S9 with 7396.8 pcs/L and the lowest is S5 with 8 pcs/L. The biomass of phytoplankton ranges from 0.0071 mg/L to 0.9908 mg/L, with an average of 0.3468 mg/L. The highest sampling point is S6 with 0.9908 mg/L, the second highest is S8 with 0.9227 mg/L, and the lowest is S5 with 0.0071 mg/L. The difference between sampling zones is significant (p < 0.05). Phytoplankton density in Q1 ranges from 11 pcs/L to 15 pcs/L, with an average of 12.8 pcs/L and biomass ranges from 0.0194 mg/L to 0.0234 mg/L, with an average of 0.0215 mg/L; phytoplankton density and biomass of Q2 are 8 pcs/L and 0.0071 mg/L, respectively; phytoplankton density in Q3 ranges from 7228.5 pcs/L to 7869.1 pcs/L, with an average of 7450.8 pcs/L and biomass ranges from 0.1759 mg/L to 0.9908 mg/L, with an average of 0.6838 mg/L; phytoplankton density in Q4 ranges from 2004.3 pcs/L to 2979.9 pcs/L, with an average of 2445.5 pcs/L and biomass ranges from 0.1759 mg/L to 0.2796 mg/L, with an average of 0.2507 mg/L.
A total of 59 species of 3 phytoplankton divisions are identified in the study basin. Tan et al. identified 95 phytoplankton species from 6 phyla in five sections from the upper reaches of the Yangtze River to Jiangjin (Tan et al., 2017); Min et al. found 79 phytoplankton species from 6 phyla in the Yangtze River source area (Min et al., 2020); Cui et al. recorded 175 phytoplankton species from 8 phyla in the main stream and its tributaries in the Yangtze River source area (Cui et al., 2020). In comparison, fewer phytoplankton species were found in this survey. The phytoplankton in the Q1, Q2, and Q4 basins are dominated by Bacillariophyta, occupying 84.6, 90.0, and 78.6%, respectively, consistent with the theory that Bacillariophyta dominate the phytoplankton of many lowland rivers (Devercelli and O’Farrell, 2013). The Bacillariophyta has the largest biomass in winter (56.1%), followed by the Chlorophyta (29.8%), which is similar to the results of Tian et al. (2017) and Wu et al. (2014). Generally speaking, diatoms is the dominant species in clean water, green algae is the representative of medium-sized eutrophic water, and blue algae is the dominant species in eutrophic water (Zhen and Zhang, 2017). Green algae and blue algae accounted for 15.4% in Q1, 10.0% in Q2, 65.6% in Q3 and 21.4% in Q4 basin, which indicated that there is some pollution in this area.
Through identification, a total of 16 species of zooplankton in 4 categories are detected. And they are Protozoa, Cladocera, Copepoda and Rotifera, respectively. The largest relative abundance is Protozoa (43.8%), followed by Rotifera (31.3%), Copepoda (18.8%), and Cladocera (6.3%). The distribution of zooplankton species in the study basin in each phylum is shown in Figure 5. A total of 7 common zooplankton species in 4 categories are detected in Q1. The Protozoa includes Arcella discoides and Epistylis sp.; the Rotifera includes Lepadella patella and Keratella cochlearis; the Copepoda includes Cyclopoid larva and Nauplius; the Cladocera includes Moina sp. 6 common zooplankton species in 4 categories are detected in Q2. The Protozoa includes Areclla vulgaris, Centropyxis aerophile and Paramecium caudatum; the Rotifera includes Keratella cochlearis, Notholca squamula and Brachionus calyciflorus. 4 common zooplankton species in 2 categories are detected in Q3, among which the Protozoa is the dominant phylum with 3 species, including Difflugia sp., Centropyxis aculeata and Arcella discoides; 1 species of Copepoda is Nitocra sp. 8 common zooplankton species in 4 categories are detected in Q4, among which Rotifera is the dominant phylum with 3 species, including Lepadella patella, Filinia longiseta and Keratella cochlearis; the Protozoa includes Arcella discoides and Epistylis sp.; the Copepoda includes Cyclopoid larva and Nauplius; the Cladocera includes Moina sp. (Supplementary Table 3).
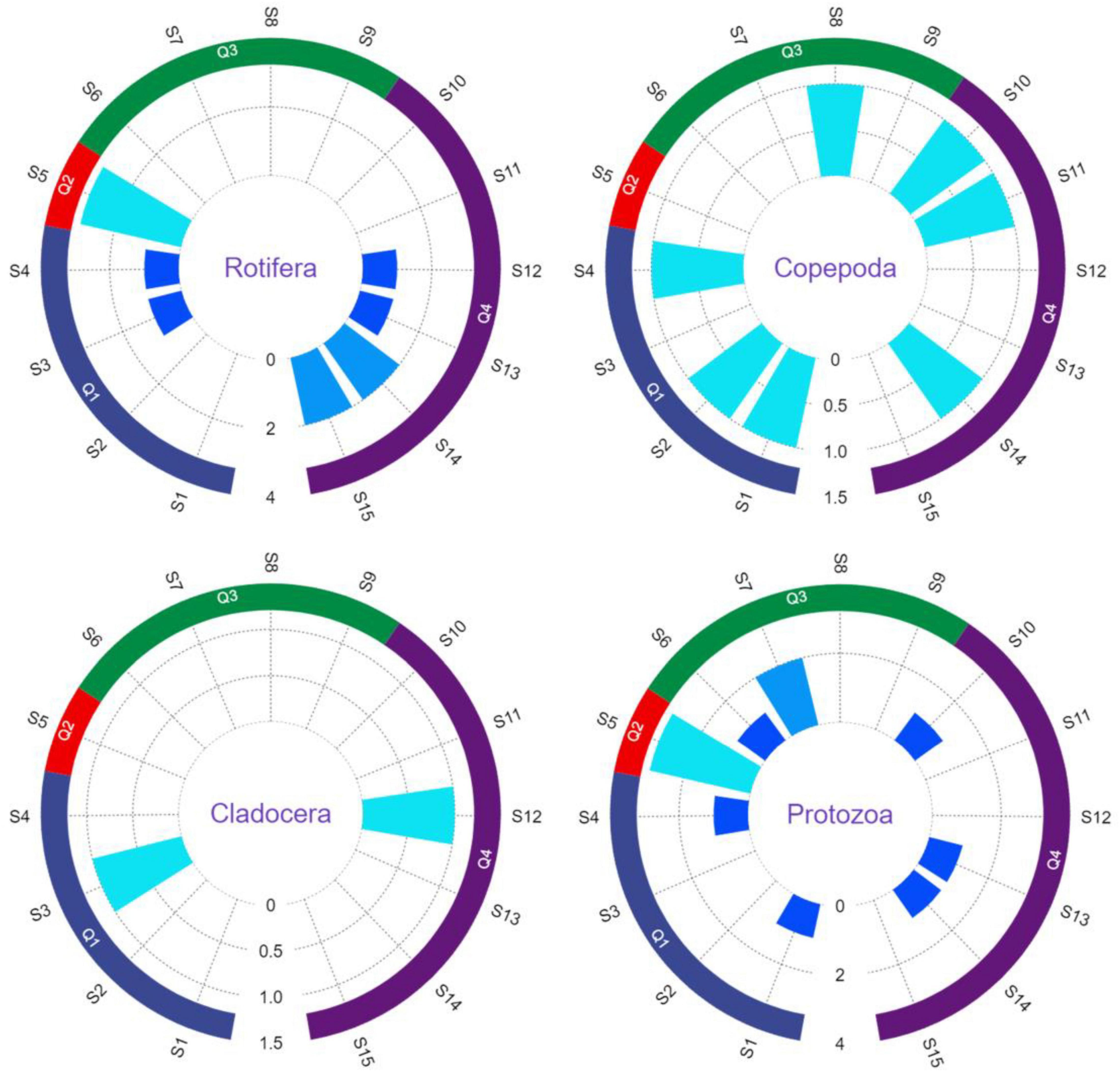
Figure 5. The proportion of zooplankton in the phyla of Rotifera, Copepoda, Cladocera, and Protozoa in the four watersheds.
The results show that Protozoa and Rotifera dominate the study basin, with Arcella discoides and Keratella cochlearis being the most widely distributed. Q3 has the lowest number of zooplankton species (4 species) and Q4 has the highest number of zooplankton species (8 species). Among other Yangtze River basins, Sun et al. (2021) collected 74 species of zooplankton in the middle reaches of the Yangtze River; Dai et al. collected 74 species of zooplankton in 4 categories in the Yangtze River Dolphin Protection Zone in Zhenjiang, Jiangsu (Dai et al., 2011), Dai et al. (2019) collected 46 species of zooplankton in 4 categories in the Xinzhou waters of Anqing, Yangtze River, in contrast, the zooplankton in the surveyed watersheds are less diverse and less abundant than those in the other basins of the Yangtze River, and species diversity is low, probably because the water temperature in the Qionglai area is low (11.2°C-14.3°C), which is not conducive to the growth and reproduction of zooplankton (Sun et al., 2021), while the steeper slope and faster current in the Qionglai area make it difficult for phytoplankton to survive, and the number of zooplankton feeding on phytoplankton is thus reduced (Liu et al., 2017).
The species distribution of each phylum of benthic animals is shown in Figure 6. Among them, Arthropoda is widely distributed in all watersheds, with the most in watershed Q3, while only 1 species of Annelida is detected in Q3, and 1 species of Mollusca. 10 species of 2 common phyla are detected in the Q1 watershed, among which the Arthropoda is the dominant phylum with 9 species, mainly including the Baetis sp. and Tipula sp., etc.; followed by the Mollusca, with 1 species, which is Limnoperna fortunei. Q2 watershed detected common benthic animals 1 phylum 4 species, for the Arthropoda, including stonefly, Rhyacophila sp., etc. Q3 watershed detected common benthic animals 2 phylum 16 species, among which the Arthropoda is the dominant phylum with 15 species, including Ephemera sp. and Baetis sp., etc., followed by the Annelida with 1 species, which is Limnodrilus hoffmeisteri. 9 species of 1 common phylum were detected in the Q4 watershed, the Arthropoda, including Corixidae sp. and Baetis sp., etc. (Supplementary Table 4).
The survey results show that the benthic animals are mainly composed of Arthropoda, with the most widespread distribution of Baetis sp., Tipula sp., Polypedilum sp., orthocladius sp., and Corixidae sp. Studies have shown that Ephemeroptera are sensitive groups, mostly distributed in clean water, while Chironomidae and Limnodrilus are pollution-resistant groups, generally distributed in polluted areas (Wu et al., 2011). These three kinds of benthic animals are found in Qionglai watershed, of which Ephemeroptera account for 10.0% and Chironomidae account for 40.0% in Q1; Ephemeroptera account for 50.0% in Q2; Ephemeroptera account for 18.8%, Chironomidae and Limnodrilus account for 31.3% in Q3; Ephemeroptera account for 11.1% and Chironomidae accounted for 55.6% in Q4, therefore, there is a certain degree of pollution in the basin under investigation.
A total of 23 species from three phyla of benthic animals are identified in the study basin. Zhang et al. (2022a) collected 38 species of macrobenthic animals from 3 phyla in the Qiaobian River, a first-class tributary of the Yichang section of the Yangtze River; Zhang et al. (2022b) collected 31 species of benthic animals from 3 phyla in the Huangbai River basin, a first-class tributary on the left bank of the middle reaches of the Yangtze River; Li et al. (2020) collected 28 species of benthic animals from 3 phyla in the Qiaobian River, a tributary of the Yangtze River (Li et al., 2020). In contrast, the species abundance of benthic animals in the surveyed watershed is low and the overall resources are poor. It may be due to the construction of small hydropower stations that the water pollution is aggravated, the water depth and water flow velocity are changed (Premstaller et al., 2017), at the same times, the responses of different benthic animals to environmental changes are different (Ren et al., 2015), so the biomass and abundance of macrobenthos are decreased.
The samples are clustered by euclidean distance of species composition data for UPGMA (default clustering algorithm), and the top 20 species are selected to draw species composition heat maps at the phylum level (Figure 7A) and genus level (Figure 7B). At the phylum level, Planctomycetes, WPS-2 and Armatimonadetes are highly expressed in S0; Dependentiae and Elusimicrobia are highly expressed in S4; Proteobacteria and Bacteroidetes are highly expressed in S9. Verrucomicrobia, Margulisbacteria, Chlamydiae, Nitrospirae, Chloroflexi and Hydrogenedentes are highly expressed in S15. At the genus level, Polynucleobacter, TRA3-20, Candidatus_Planktophila and Candidatus_Methylopumilus are highly expressed in S0; Limnohabitans is highly expressed in S4; Nevskia, NS11-12_marine_group, Sediminibacterium, NS9_marine_group, Alkanibacter, Panacagrimonas and Sphingorhabdus are highly expressed in S9; Tychonema_CCAP_1459-11B, Rhodoferax and Pedobacter are highly expressed in S15. Microbial diversity and species abundance are high at all sampling sites, and the construction of small hydropower stations had a low impact on microorganisms due to their small size and wide distribution, and because they are less affected by environmental factors than benthic invertebrates (Vilmi et al., 2020).
Alpha diversity analysis of microorganisms in water samples from 4 sampling points at S0 which at the confluence of Wenjing and Baimo River and three representative power stations (S4, S9, and S15) are performed, and the results are shown in Figure 8. From the Chao1 index, the number of species is high at all sampling sites, of which S9 has the highest number of sampling sites. The Shannon index, Simpson index and Observed species index show that the species richness and diversity of each sampling site are high, and the species are evenly distributed, among which the species richness of S15 is higher than the other 3 sampling sites.
Beta diversity analysis shows (Figure 9) that S4 is more similar to the top 10 species in terms of the abundance to S0. The relative abundance of hgcl_clade (27.30%) and Sporichthyaceae (8.17%) are higher in S0; the relative abundance of hgcl_clade (21.55%) and Chloroplast (20.70%) are higher in S4; the relative abundance of NS11.12_marine_group (32.70%) is the highest in S9; the relative abundance of Chloroplast (18.53%) and hgcl_clade (6.59%) are higher in S15.
This study of the impact of small hydropower stations on aquatic organisms in the Qionglai watershed, which is located in the upper reaches of the Yangtze River, shows that the biodiversity of the Baimo and Wenjing River in Qionglai is at a low level, and the results of the fish diversity survey shows relatively few fish resources and poor species diversity, with only 6 species of fish found in the field sampling, mainly including Cyprinidae; 23 species of benthic animals are detected, and Arthropoda (91.3%) is dominant; the number of zooplankton species is less and the diversity is low, and only 16 species are detected in the study watershed; the biomass of Bacillariophyta accounts for the largest proportion of phytoplankton (56.1%), followed by Chlorophyta (29.8%) and Cyanobacteria (4%); both the diversity and species abundance of microorganisms are high, and the impact of small hydropower stations is the lowest. In summary, small hydropower stations have an impact on the biodiversity of the study watershed, showing an order of fish > benthic > plankton > microorganism.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
PG, TW, and YX contributed to the conception and design of the study. YY and YZ organized the database. FL performed the statistical analysis. HM and JL wrote the first draft of the manuscript. ZZ, KY, and QL wrote sections of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
This study was supported by the Chengdu University of Technology Research Startup Fund (10912-KYQD2021-01944), Jiangsu Natural Science Foundation (BK20211035) and Open Fund of State Key Laboratory of Geohazard Prevention and Geoenviroment Protection (SKLGP2022K023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.940606/full#supplementary-material
Andrianova, A. V. (2020). Assessment of the ecological state of the boguchany reservoir by zoobenthos organisms. Earth Environ. Sci. 548:072054. doi: 10.1088/1755-1315/548/7/072054
Araújo, F. G., Williams, W. P., and Bailey, R. G. (2000). Fish assemblages as indicators of water quality in the middle thames estuary, England (1980–1989). Estuar. Coasts 23, 305–317. doi: 10.2307/1353323
Benejam, L., Saura-Mas, S., Bardina, M., Solà, C., Munné, A., and García-Berthou, E. (2016). Ecological impacts of small hydropower plants on headwater stream fish: from individual to community effects. Ecol. Freshw. Fish. 25, 295–306. doi: 10.1111/eff.12210
Bi, H. S., Sun, S., Gao, S. W., Zhang, G. T., and Zhang, F. (2001). The characteristics of zooplankton community in the Bohai Sea III. The distribution of abundance and seasonal dynamics of major taxa except copepod. Acta Ecol. Sinica. 21, 513–521. doi: 10.3321/j.issn:1000-0933.2001.04.001
Bredenhand, E., and Samways, M. J. (2009). Impact of a dam on benthic macroinvertebrates in a small river in a biodiversity hotspot: cape floristic region, south africa. J. Insect. Conserv. 13, 297–307. doi: 10.1007/s10841-008-9173-2
Cao, W. X. (2019). Water Ecological Restoration in the Cascade Development of Hydropower in the Upper Reaches of the Yangtze River. Technol. Econ. Changjiang. 2019, 5–10. doi: 10.19679/j.cnki.cjjsjj.2019.0201
Chen, X. J., He, D., Zhou, L. F., Cao, Y. F., and Li, Z. J. (2020). Influence of hydropower stations on the water microbiota in the downstream of Jinsha River. China. PeerJ. 8:e9500. doi: 10.7717/peerj.9500
Costea, G., Pusch, M. T., Bănăduc, D., Cosmoiu, D., and Curtean-Bănăduc, A. (2021). A review of hydropower plants in Romania: distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sustain. Energy Rev. 145:111003. doi: 10.1016/j.rser.2021.111003
Cui, Y. X., Zhang, J., Chai, Y. B., Yang, C. L., Lu, D., Jia, J., et al. (2020). Research on Phytoplankton Community in the Source Region of the Yangtze River. Environ. Sci. Technol. 43, 20–25. doi: 10.19672/j.cnki.1003-6504.2020.01.004
Dai, L. L., Gong, Y. C., Feng, W. S., and Yu, Y. H. (2011). Community structure of zooplankton and its relationship to survivability of yangtze dolphin in zhenjiang yangtze dolphin nature reserve, jiangsu province. J. Hydroecol. 32, 30–36. doi: 10.15928/j.1674-3075.2011.05.001
Dai, P., Wang, Y. P., Kuang, Z., Lin, D. Q., Yang, Y. P., Liu, S. L., et al. (2019). Community structure of zooplankton and its relation with environmental factors in Xinzhou water. Anqing section of Yangtze River. J. Anhui. Agr. Univ. 46, 623–631. doi: 10.13610/j.cnki.1672-352x.20191013.017
Devercelli, M., and O’Farrell, I. (2013). Factors affecting the structure and maintenance of phytoplankton functional groups in a nutrient rich lowland river. Limnologica 43, 67–78. doi: 10.1016/j.limno.2012.05.001
Fu, X. C., Tang, T., Jiang, W. X., Li, F. Q., Wu, N. C., Zhou, S. C., et al. (2008). Impacts of small hydropower plants on macroinvertebrate communities. Acta Ecol. Sinica. 28, 45–52. doi: 10.3321/j.issn:1000-0933.2008.01.005
Gao, Y., Pan, J. Z., Li, Y., He, S. W., and Wu, X. D. (2015). Spatio-temporal distribution of phytoplankton and environmental factors in the north part of Lake Gehu(Jiangsu) after muti-treatment. J. Lake. Sciences. 27, 649–656. doi: 10.18307/2015.0413
Guo, W. J., Zhao, W. H., and Wang, Z. H. (2015). Effects of Cascade Diversion-type Hydropower Stations on Benthic Macroinvertebrate Communities. J. Yangtze. River. Sci. Res. Insti. 32, 87–93. doi: 10.3969/j.issn.1001-5485.2015.06.016
Hu, H. J., and Wei, Y. X. (2006). The System, Taxonomy and Ecology of Chinese Freshwater Algae. China Science Publishing & Media Ltd.
Jakovčev-Todorović, D., Paunović, M. M., Stojanović, B., Simić, V. M., Dikanović, V., and Veljković, A. (2005). Observation of the quality of Danube water in the Belgrade region based on benthic animals during periods of high and low water conditions in 2002. Arch. Biol. Sci. 57, 237–242. doi: 10.2298/ABS0503237J
Li, J. J., Li, W. M., Su, Y. F., Sun, X. Y., and Hu, W. (2020). Community structure of macroinvertebrates and water quality bioassessment in Qiaobian River, a tributary of Yangtze River. Acta Sci. Circumst. 40, 3020–3027. doi: 10.13671/j.hjkxxb.2020.0120
Li, K. Z., Yin, J. Q., and Huang, L. M. (2004). Dynamic Variations of Community Structre and Quantity of Zooplankton in Zhujiang River Estuary. J. Trop. Oceanogr. 24, 60–68. doi: 10.3969/j.issn.1009-5470.2005.05.007
Li, M., Gong, Q. C., and Pan, J. H. (2022). Evaluation and Strategic Transformation of China’s Small Hydropower under the Goal of Carbon Neutrality. J. Beijing. Univ. Techno. 22, 86–104. doi: 10.12120/bjutskxb202202086
Li, W. K. (2022). The response of bacterioplankton community to water nutritional condition in Poyang Lake. Nancang. Univ. 2022:3. doi: 10.27232/d.cnki.gnchu.2021.002965
Lin, P. C., Gao, X., Liu, F., Li, M. Z., and Liu, H. Z. (2021). Ecological Assessment of the Yangtze River Environment Based on Fish Species Richness. Acta Hydrobiol. Sinica 45, 1385–1389. doi: 10.7541/2021.2021.0269
Liu, M. D., Li, P. F., Zeng, Z. G., Huang, C., and Liu, S. P. (2017). The characteristic of phytoplankton community structure in Anqing section of the yangtze river. Freshw. Fish. 47, 29–36. doi: 10.13721/j.cnki.dsyy.2017.04.005
Liu, R. X. (2020). Study on the assessment index system and its application of Small Hydropower’s eco-environmental impact. Zhejiang. Univ. 2021:12. doi: 10.27461/d.cnki.gzjdx.2020.001231
Min, M., Chai, Y. B., Chen, X. F., Xi, W. B., and Lu, D. (2020). Relation Between Phytoplankton Community Characteristics and Water Environmental Factors in the Source Region of Yangtze River. J. Yangtze. River. Sci. Res. Inst. 37, 28–33. doi: 10.11988/ckyyb.20191167
Otahelová, H., and Valachovič, M. (2003). Distribution of macrophytes in different water-bodies (habitats) influenced by the Gabčíkovo hydropower station (Slovakia) – present status. Large Rivers 14, 97–115. doi: 10.1127/lr/14/2003/97
Peng, W. Q., Han, Z., Wang, S. Y., Liu, C., and Dong, F. (2022). Discussion on some problems of small scaled hydropower rivers ecological recovery. China. W. Resour. 7, 40–44.
Premstaller, G., Cavedon, V., Pisaturo, G. R., Schweizer, S., Adami, V., and Righetti, M. (2017). Hydropeaking mitigation project on a multi-purpose hydro-scheme on Valsura River in South Tyrol/Italy. Sci. Total. Environ. 574, 642–653. doi: 10.1016/j.scitotenv.2016.09.088
Ren, H. Q., Yuan, X. Z., Liu, H., Zhang, Y. W., and Zhou, S. B. (2015). The effects of environment factors on community structure of benthic invertebrate in rivers. Acta Ecol. Sinica. 35:9. doi: 10.5846/stxb201306241759
Rivinoja, P., Calles, O., Karlsson, S., and Lundstrm, S. (2010). Effects of small scale hydropower on aquatic fauna. Report 4. Uppsala: Swedish University of Agricultural Sciences
Salmaso, N., Morabito, G., Buzzi, F., Garibaldi, L., Simona, M., and Mosello, R. (2006). Phytoplankton as an indicator of the water quality of the deep lakes south of the alps. Hydrobiologia 563, 167–187. doi: 10.1007/s10750-005-0003-1
Šarauskienė, D., Adžgauskas, G., Kriaučiūnienė, J., and Jakimavičius, D. (2021). Analysis of Hydrologic Regime Changes Caused by Small Hydropower Plants in Lowland Rivers. Water 13:1961. doi: 10.3390/w13141961
Sun, D. D., and Liu, Y. (2020). Study on the Post Evalution of the Impact of Hydropower Project on Aquatic Ecology. Sichuan. Water. Power. 39, 120–123.
Sun, X. M., Liu, S. P., Chen, D. Q., Wei, J. M., Yu, Q., et al. (2021). Plankton community structure and its relationship with environmental factors in the middle reaches of the Yangtze River. Freshw. Fish. 51, 3–12. doi: 10.13721/j.cnki.dsyy.2021.03.001
Sun, Z. Q., Shi, X. L., Xu, L. L., Meng, X. W., and Liu, G. J. (2013). The Protozoan Community Structure and its Response to the Change of Water Quality in a Typical Wetland Landscape in Summer. Acta Hydrobiol. Sinica. 37, 290–299. doi: 10.7541/2013.17
Sundermann, A., Antons, C., Cron, N., Lorenz, A. W., Hering, D., and Haase, P. (2011). Hydromorphological restoration of running waters: effects on benthic invertebrate assemblages. Freshw. Boil. 56, 1689–1702. doi: 10.1111/j.1365-2427.2011.02599.x
Tan, Q., Ma, Q. Q., Li, B. B., Lv, H. J., Fu, M., and Yao, W. Z. (2017). Ecological health assessment of the upper reaches of the Yangtze River, based on biotic integrity index of phytoplankton. Freshw. Fish. 47, 97–104.
Tian, W., Zhang, H. Y., Wang, Z. Y., Zhang, J., Miao, S. M., and Zhao, L. (2017). Phytoplankton diversity effects on community biomass and temporal stability in lake nansihu. China. Environ. Sci. 37, 319–327. doi: 10.3969/j.issn.1000-6923.2017.01.039
Vilmi, A., Zhao, W., Picazo, F., Li, M., Heino, J., Soininen, J., et al. (2020). Ecological processes underlying the community assembly of aquatic microscopic and macroscopic organisms under contrasting climates in the Tibetan Plateau biodiversity hotspot. Sci. Total. Environ. 702:134974. doi: 10.1016/j.scitotenv.2019.134974
Wang, Y. P., Kuang, Z., Lin, D. Q., Li, P. J., Yang, Y. P., and Liu, K. (2020). Community structure and species diversity of fish around the Xinzhou shoal in the Anqing section of the Yangtze River. China. Acta Ecologica. Sin. 40, 2417–2426. doi: 10.5846/stxb201901160130
Wei, Z. H., Zhang, L. X., Yang, S. K., Lv, X. J., Zhu, J., and Dou, J. S. (2012). Community structure and seasonal succession of phytoplankton in Erhai lake. J. Hydroecol. 33, 21–25. doi: 10.15928/j.1674-3075.2012.04.002
Wu, X. W., Liang, Y. A., Li, Y. M., Xue, F., Luo, R., Yu, Z. Y., et al. (2014). Survey and evaluation of the hydrobiology in the dam area of xuanlai hydropower station on nanpeng river. J. Yunnan. Agr. Univ. 29, 806–814. doi: 10.3969/j.issn.1004-390X(n).2014.06.004
Wu, Z. S., Cai, Y. J., Chen, Y. W., Shao, X. Y., and Gao, J. F. (2011). Assemblage structure investigation of macrozoobenthos and water quality bioassessment of the main river systems in Taihu Basin. J. Lake. Sci. 23, 686–694. doi: 10.18307/2011.0504
Xu, M. Q. (1996). Evaluation of Self-purification Efficiency of Fuhe Stream-Baiyangdian Lake through Zooplankton. Acta Hydrobiol. Sin. 1996, 212–220. doi: 10.1007/BF02951625
Zhang, K., Li, W. M., Chen, S. S., Xiong, W. W., Zhang, X. T., and Liu, Z. J. (2022b). River Health Assessment Based on Macroinvertebrates in Huangbai River, a Tributary of Yangtze River, China. Resour. Environ. Yangtze.Basin. 126:107686
Zhang, M., Yu, Y., Qian, S. Q., Li, D. M., and Kong, F. X. (2010). Phytoplankton community structure and biodiversity in summer Yunnan-Guizhou Plateau lakes. J. Lake. Sci. 22, 829–836. doi: 10.18307/2010.0604
Zhang, X. T., Li, W. M., Zhang, K., Xiong, W. W., Chen, S. S., and Li, Z. J. (2022a). Spatiotemporal distribution of macroinvertebrate functional feeding groups in Qiaobian River, a tributary of Yangtze River in Yichang. Acta Ecol. Sinica. 42, 2559–2570. doi: 10.5846/stxb202107061806
Zhao, C. S., Xia, J., Wang, G. S., Yan, W. J., Liu, M., Zhang, H. T., et al. (2008). Evaluation and analysis on aquatic ecology and environmental quality of Huai River basin. Chin. J. Environ. Eng. 2, 1698–1704. doi: 10.1007/s11356-022-20252-8
Zhen, M. Y., and Zhang, J. F. (2017). Composition and Characteristics of Phytoplankton in Typical Small and Medium Size Rivers Hydropower Station in Guangdong Province. Guangdong. Water. Resour. Hydropower. 8, 30–35.
Zheng, Y. T., Han, P., Ni, J. R., and Xiong, M. H. (2019). Studies on Structure of Fish Community and Species Diversity in Wuhan Section of the Yangtze River. J. Basic. Sci. Eng. 27, 24–35. doi: 10.16058/j.issn.1005-0930.2019.01.003
Keywords: small hydropower station, fish, plankton, benthic animal, microorganism
Citation: Gu P, Zhang Z, Liu J, Wang T, Xiao Y, Yu Y, Miao H, Zhang Y, Liao F, Yang K and Li Q (2022) Effects of Small Hydropower Stations Along Rivers on the Distribution of Aquatic Biodiversity. Front. Ecol. Evol. 10:940606. doi: 10.3389/fevo.2022.940606
Received: 10 May 2022; Accepted: 10 June 2022;
Published: 29 June 2022.
Edited by:
Chao Wang, Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, ChinaCopyright © 2022 Gu, Zhang, Liu, Wang, Xiao, Yu, Miao, Zhang, Liao, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kunlun Yang, eWFuZ2t1bmx1bkBqaWFuZ25hbi5lZHUuY24=; Qi Li, bGlxaTIxQGNkdXQuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.