- 1Mara Hyena Project, Nairobi, Kenya
- 2Department of Integrative Biology, Michigan State University, East Lansing, MI, United States
- 3Ecology, Evolution and Behavior Program, Michigan State University, East Lansing, MI, United States
- 4Department of Ecology and Evolutionary Biology (EEB), University of Colorado Boulder, Boulder, CO, United States
- 5Department for the Ecology of Animal Societies, Max Planck Institute for Animal Behavior, Konstanz, Germany
- 6Center for the Advanced Study of Collective Behavior, Universität Konstanz, Konstanz, Germany
Introduction: Dominance relationships in which females dominate males are rare among mammals. Mechanistic hypotheses explaining the occurrence of female dominance suggest that females dominate males because (1) they are intrinsically more aggressive or less submissive than males, and/or (2) they have access to more social support than males.
Methods: Here, we examine the determinants of female dominance across ontogenetic development in spotted hyenas (Crocuta crocuta) using 30 years of detailed behavioral observations from the Mara Hyena Project to evaluate these two hypotheses.
Results: Among adult hyenas, we find that females spontaneously aggress at higher rates than males, whereas males spontaneously submit at higher rates than females. Once an aggressive interaction has been initiated, adult females are more likely than immigrant males to elicit submission from members of the opposite sex, and both adult natal and immigrant males are more likely than adult females to offer submission in response to an aggressive act. We also find that adult male aggressors are more likely to receive social support than are adult female aggressors, and that both adult natal and immigrant males are 2–3 times more likely to receive support when attacking a female than when attacking another male. Across all age classes, females are more likely than males to be targets of aggressive acts that occur with support. Further, receiving social support does slightly help immigrant males elicit submission from adult females compared to immigrant males acting alone, and it also helps females elicit submission from other females. However, adult females can dominate immigrant males with or without support far more often than immigrant males can dominate females, even when the immigrants are supported against females.
Discussion: Overall, we find evidence for both mechanisms hypothesized to mediate female dominance in this species: (1) male and female hyenas clearly differ in their aggressive and submissive tendencies, and (2) realized social support plays an important role in shaping dominance relationships within a clan. Nevertheless, our results suggest that social support alone cannot explain sex-biased dominance in spotted hyenas. Although realized social support can certainly influence fight outcomes among females, adult females can easily dominate immigrant males without any support at all.
Introduction
Dominance hierarchies are common in animal societies and have profound fitness consequences for individuals of many different species (Strauss et al., 2022). Interestingly, in some animals, one sex is typically dominant over the other, prompting questions about the evolutionary and mechanistic origins of this sex bias. Male dominance, where males exert power or influence over females, is very common in mammals and has thus been studied extensively (Carpenter, 1942; Darwin et al., 1981; Drews, 1993). A diverse array of traits facilitates male dominance, including larger body size (Cassini, 2020), superior weaponry (Rico-Guevara and Hurme, 2019), higher androgen concentrations (Nelson, 2005), and more frequent and intense expression of aggressive behavior (Nelson, 2005). These sexually dimorphic, male-biased traits are often correlated (e.g., male aggression levels and circulating testosterone; Muller, 2017), and in most cases, they provide an advantage in both intra- and inter-sexual competition (Nelson, 2005).
Female dominance, where females exert power or influence over males, is uncommon in mammals but occurs in various Malagasy primates (Lewis, 2020), two species of mole rats (Cryptomys hottentotus and Heterocephalus glaber; Holekamp and Engh, 2009), and spotted hyenas (Crocuta crocuta; Kruuk, 1972). Compared to the factors influencing male dominance, those that mediate female dominance in mammalian societies remain poorly understood. Nevertheless, three proximate mechanisms leading to female dominance over males have been proposed: (1) intrinsic attributes or sex-based differences in the ability to use force (e.g., body size, physical strength, aggressiveness; Watts et al., 2009); (2) extrinsic, or derived, attributes, including sex-based differences in social support (e.g., coalition and alliance partners; Vullioud et al., 2019), and (3) leverage or sex-based differences in resources that cannot be taken by force (e.g., fertilizable eggs; Lewis, 2020).
Spotted hyenas offer an ideal system in which to examine the phenomenon of female dominance, as adult female dominance over adult males has been consistently observed in this species. Given that hyenas are a gregarious species, it is also possible to use them to test some of the hypotheses identified above (Kruuk, 1972; East et al., 2003; Holekamp and Strauss, 2020). Spotted hyenas live in mixed-sex matrilineal societies called ‘clans,’ which are characterized by low within-group relatedness, female philopatry, and male dispersal (Smale et al., 1997; Van Horn et al., 2004; Holekamp et al., 2012). Past studies of spotted hyenas have supported both the intrinsic attributes hypothesis (Frank, 1986) and the social support hypothesis (Vullioud et al., 2019).
Although we were unable to assess the leverage hypothesis in this study, we examined both the intrinsic attributes hypothesis and the social support hypothesis. According to our interpretation of the intrinsic attributes hypothesis, behavioral and physiological differences between the sexes contribute to female dominance in hyenas. Under this hypothesis, females have enhanced fighting abilities due to selection favoring females who can obtain priority of access to resources for themselves or their offspring (Watts et al., 2009; Clutton-Brock and Huchard, 2013), and these abilities then support females in achieving intersexual dominance. As adults, female-spotted hyenas aggress at higher rates and intensities than immigrant males when attacking lower-ranking hyenas (McCormick et al., 2021). Additionally, more aggressive behavior is associated with superior reproductive success among females (Watts et al., 2009; Yoshida et al., 2016; McCormick and Holekamp, 2022) but not among male spotted hyenas (East and Hofer, 2001). This suggests that aggressiveness may be selected for in females, but not necessarily in males, and this could give females an edge over males in contests of dominance. In further support of this hypothesis, sex differences in aggressive behavior in spotted hyenas emerge early in life during the neonatal period (Smale et al., 1995; Golla et al., 1999; Wahaj and Holekamp, 2006; Benhaiem et al., 2012).
The social support hypothesis suggests that differential social support allows females to dominate males (Vullioud et al., 2019). Under this hypothesis, female dominance over adult males arises because females have more social support than males, driven by male-biased dispersal that leads adult males to join a new clan where they lack kinship or social ties. Consistent with this hypothesis, Vullioud et al. (2019) found that the outcomes of dyadic interactions between spotted hyenas in Ngorongoro Crater, Tanzania, were better predicted by a proxy for social support than by the sexes of the fight contestants or the differences between them in body size. In this study, social support was approximated by an algorithm that used a combination of kinship, dispersal status, maternal pedigree, and physical location relative to the center of each hyena’s home range to estimate which of the contestants was more likely to receive social support from other hyenas that could potentially arrive during the agonistic encounter. Additional support for this hypothesis comes from work demonstrating that support from social allies during agonistic encounters aids in rank acquisition (Engh et al., 2000) and facilitates rank reversals among adult females (Strauss and Holekamp, 2019).
Here, we interrogated both the intrinsic attributes hypothesis and the social support hypothesis in explaining the tendency for females to dominate males among spotted hyenas. Although these hypotheses are not mutually exclusive, they are often characterized as being in conflict (e.g., Vullioud et al., 2019), despite the aforementioned evidence supporting both hypotheses. Here, we clarified the contributions of these different mechanisms to female-biased dominance using a 30-year dataset on spotted hyenas in Kenya. Regarding the intrinsic attributes hypothesis, we added to the work of McCormick et al. (2021) by investigating unsolicited aggressive and submissive behavior across age and sex classes. Regarding the social support hypothesis, we added to the work of Vullioud et al. (2019) on the potential for receiving social support by investigating realized social support during both successful and unsuccessful spontaneous agonistic behavior.
To test the predictions of both hypothesized mechanisms, we focused initially on agonistic interactions among adult hyenas, where female dominance is most clearly expressed. We examined four aspects of agonistic interactions: (1) the initiation of agonistic interactions by producing spontaneous aggressive or submissive behaviors, (2) the propensity for aggressive acts to be successful, as indicated by eliciting submission from the target animal (e.g., successfully dominating the recipient of the aggression), (3) the likelihood of receiving social support during an aggressive act, and (4) in opposite-sex group-mates, the effect of realized social support on the success of aggression in eliciting a submissive response from the targeted animal. Predictions made by the two hypotheses about these four aspects of agonistic interactions are presented in Table 1. Notably, the two hypotheses make contrasting predictions about the behavior of adult natal males. The intrinsic attributes hypothesis predicts that natal males should behave more similarly to immigrant males than females because of intrinsic sex differences in adult hyenas, whereas the social support hypothesis predicts that natal males should behave more similarly to females than immigrant males because of the greater potential for receiving social support enjoyed by natal individuals (Vullioud et al., 2019; Table 1). Table 1 also presents two additional predictions that follow from the hypothesis that social support is a primary driver of sex differences in dominance in hyenas: (1) natal males and females should receive more social support than immigrant males and (2) receiving social support should help females and natal males successfully elicit submission from immigrant males. After testing these hypotheses in adults, we considered an ontogenetic perspective by examining determinants of female-biased dominance in hyenas that were yet to reach adulthood.
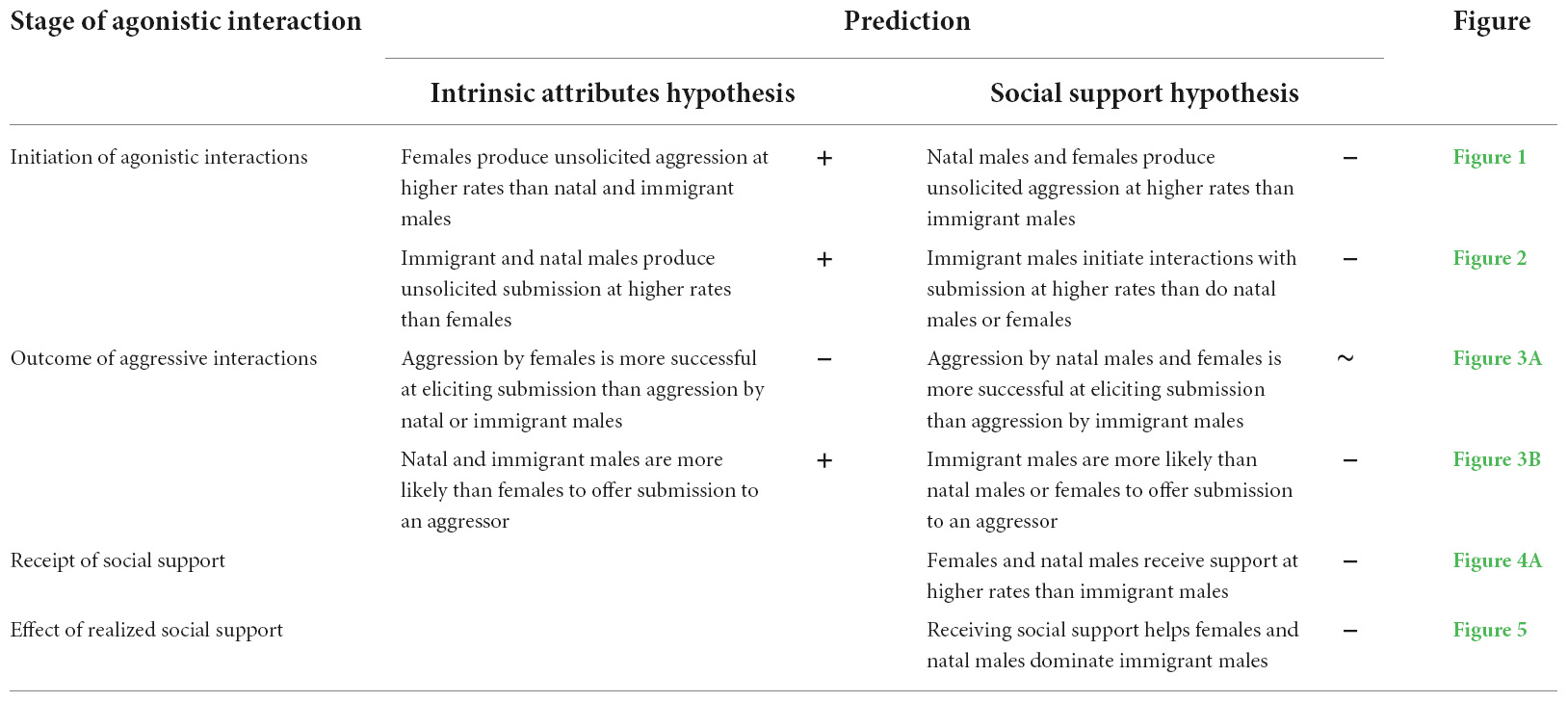
Table 1. Predictions at different stages of agonistic interaction made by the two hypotheses under investigation, with symbols indicating whether results from this study support (+), fail to support (−), or show mixed support (∼) for each prediction.
Materials and methods
Study species
Female spotted hyenas invest heavily in the rearing of offspring (East et al., 2009; Watts et al., 2009; Laubach et al., 2021). They usually bear litters of 1 or 2 cubs; when twin cubs are born, neonatal females dominate males in 67–84% of mixed-sex twin litters (Smale et al., 1995; Golla et al., 1999; Wahaj and Holekamp, 2006; Benhaiem et al., 2012). During the first 2 years of life, juveniles of both sexes assume the social ranks and entire social networks of their mothers (Smale et al., 1993; Holekamp and Smale, 1998; Strauss et al., 2020; Ilany et al., 2021). Young animals of both sexes generally retain their maternal rank as long as they remain in the natal clan, resulting, on average, in parity between the sexes with respect to dominance rank among cubs and subadults. Full-blown female dominance over males emerges after reproductive maturity and male dispersal.
One to 6 years after becoming reproductively mature, most male spotted hyenas disperse to join new social groups (Smale et al., 1997; Höner et al., 2007), a process that induces a suite of physiological, behavioral, and social changes (Holekamp and Sisk, 2003). It also generates two classes of adult males in most hyena clans: immigrant males who have arrived from other clans and adult natal males who have not yet dispersed. In the context of the matrilineal hierarchy, immigrant males are lower ranking than all females and natal males in the group (East and Hofer, 2001); however, immigrant males have been found to sire the vast majority of offspring within our study system (Engh et al., 2002; Van Horn et al., 2004). Immigration into a new clan by a male spotted hyena coincides with an increase in the frequency with which he exhibits extreme submissive behavior (Smale et al., 1997); it also coincides with an elevation in circulating testosterone concentrations and onset of adult testicular function (Holekamp and Sisk, 2003; Curren et al., 2013). Finally, by joining a new group of unfamiliar conspecifics, dispersing males not only experience a drastic decline in their priority of access to food resources (Smale et al., 1997) but they also lose most or all of their established social relationships (Vullioud et al., 2019).
Study population
In this study, we used data collected between 1988 and 2018 from three clans of spotted hyenas inhabiting the Maasai Mara National Reserve, Kenya. Individual hyenas were identified by their unique spots and other marks, such as scars and ear damage. The sex of each individual was determined based on the shape of the glans of its erect phallus (Frank et al., 1990), and ages of natal animals were determined to ± 7 days based on cub appearance when first seen (Holekamp et al., 1996). We classified hyenas in their first year of life as cubs; these individuals are largely dependent on their mothers for food and on communal dens for refugia (Holekamp and Smale, 1998). These communal dens differ from natal dens, where female hyenas give birth and rear offspring for the first 2–5 weeks of life in seclusion (East et al., 1989; Boydston et al., 2006). We classified hyenas in their second year of life as subadults; these individuals are weaned on average at 13 months, and they no longer use communal dens but remain heavily dependent on their mothers for access to food and protection (Watts et al., 2009). We classified hyenas of 2 years and older as adults; hyenas of both sexes are physiologically able to breed at 2 years (Glickman et al., 1992). As a result, there are three categories of resident adult hyenas within each clan: females, natal males that have not yet dispersed, and immigrant males that have successfully left their natal clan to join a new one. Here, a dispersing adult male was considered to have successfully immigrated into a new clan after he was observed in the clan’s territory for at least 6 months and observed interacting with clan residents at least 3 times (Engh et al., 2002). In this population, 59.8% (SD = 15.4%) of adult males are immigrants.
Observations were made daily from vehicles for 3–4 h around dawn and again around dusk. We defined an observation session as observing 1 or more hyenas separated from others by at least 200 m (Holekamp et al., 1997; Yoshida et al., 2016). In each session, we identified all hyenas present, and we used all-occurrence sampling (Altmann, 1974) to record all acts of aggressive and submissive behavior and the responses to these acts. Such acts were considered unsolicited (spontaneous) if they were not immediately preceded by a prior aggressive act. We restricted our analyses to observation sessions in which 2 or more hyenas were present, and we excluded observation sessions that occurred at natal dens, as mothers typically hide natal dens well and minimize interactions with clan mates. We also excluded sessions under 10 min, as these sessions comprised mostly observations of inactive or sleeping hyenas.
Social ranks were assigned yearly based on wins and losses in agonistic encounters between individuals within the study groups. For each year, individual ranks from the prior year were updated based on the outcomes of observed agonistic encounters in that year. Individuals under the age of 13 months at the start of the year were assigned their mother’s rank (Strauss et al., 2020). Individuals first joining the group or first becoming old enough to have ranks calculated were assigned an initial rank based on their arrival and tenure in the clan (for immigrants; East and Hofer, 2001) or their mother’s rank (for natal individuals); the initial rank was then updated based on observed agonistic interactions during that year (Strauss and Holekamp, 2019). To account for the variation in group size, rank was standardized within each year to range from -1 to 1.
Calculating rates of unsolicited aggressive and submissive behaviors
To assess rates of unsolicited aggressive and submissive behavior, we counted the number of aggressive or submissive acts emitted by each individual present in each observation session. These included observation sessions where individuals were typically active within the observation period but did not direct any unsolicited aggressive or submissive acts toward other hyenas present, therefore resulting in a count of zero within the observation session. Aggressive behaviors included intention movements to attack, threats, attack behaviors without bodily contact, and physical contact that might result in injury. Submissive behaviors included appeasement signals such as flattening the ears back against the head or head-bobbing, postural changes such as folding the entire body into a submissive posture with tail down between the legs, and “groveling,” or crawling on one’s belly and carpals (Kruuk, 1972). Descriptions of all agonistic behaviors in our dataset can be found in Supplementary material. Regarding aggressive behavior, we only included acts of spontaneous aggression and did not count acts of aggression that were immediate responses to a prior aggressive act directed at the focal individual, such as counterattacks (n = 861 of 80,597 aggressive acts, or 1.07%). Regarding unsolicited submissive behavior, we only included spontaneous submissive acts that were emitted in the absence of an immediately preceding aggressive act directed at the focal animal.
Calculating dominance
An individual was considered to successfully dominate another individual during an agonistic encounter if the recipient of an aggressive act emitted a submissive response. This resulted in a binary variable, successful vs. unsuccessful aggressive acts, indicating whether or not the recipient hyena emitted a submissive response to a threat or attack. It should be noted that, if a recipient did not respond with submissive behavior, it did not necessarily mean that the aggressor was dominated; instead, it simply meant that the threat was not successful in eliciting a submission from the recipient.
Calculating realized social support
An individual was considered to have been supported during an agonistic interaction if another hyena present during an observation session either acted simultaneously with it to attack the target animal or joined in an ongoing attack. This resulted in a binary factor, supported vs. unsupported aggression, indicating whether or not the aggressor received support from 1 or more clan mates during an agonistic encounter. We used this binary variable of realized social support to assess sex differences in the likelihood of receiving social support during an agonistic encounter and to assess the effect of realized social support on dominance. If both individuals attacked a target animal simultaneously, both were considered supported actors, and each was included as a separate observation in the dataset. Individuals that joined after the initial act of aggression were not included as actors in the dataset, as these joining individuals were not initiators of the conflict.
Modeling rates of aggression and submission
To compare variation in spontaneous aggressive and submissive behavior between sexes and among stages of ontogeny, we built separate mixed models for each age class (cubs, subadults, adults) that included the sex of the acting individual (“actor”) as the independent variable and counts of aggressive and submissive behaviors as the dependent variables. Note that for all adult models we had three categorical variables for actor and recipient sexes: adult female, adult natal male, and adult immigrant male. The number of hyenas present in the observation session was included as a covariate to control for opportunities to interact and known effects of group size on rates of social behavior (McCormick and Holekamp, 2022). The standardized rank of the actor was also included as a covariate to control for known effects of social rank on dominance behavior (McCormick and Holekamp, 2022). The duration of the observation session in minutes was included as a log offset to account for individual variation in the time observed. The observation session ID was included as a random intercept to account for non-independence of measurements within sessions, and actor ID was included as a random intercept to account for non-independence of measurements of individual variation in aggressive behavior.
Models were built using a zero-inflated Poisson approach within the glmmTMB package in R (Brooks et al., 2017), and we reported estimated incidence rate ratios (IRR) in which we set females as the reference group. These IRR values are calculated from exponentiating the model estimates comparing males to the female reference category, such that an IRR of 2 would be interpreted as males exhibiting the modeled behavior 2 times more often than females.
Modeling dominance and realized social support
To ascertain whether successfully dominating another group member was driven by the sex of the actor or the sex of the recipient, we built logistic regression models including actor sex and recipient sex as independent variables and dominance (successful vs. unsuccessful act of aggression) as the binary dependent variable. This allowed for both intra- and inter-sexual comparisons of whether or not an aggressive act elicited a submissive response. We included an interaction term in our models to test whether the effect of actor sex on dominance depends on recipient sex and vice versa. As random intercepts, we included an observation session ID (see above) and a dyad ID of paired actors and recipients, to account for repeated interactions between the same individuals.
To assess whether members of one sex received more social support than members of the other sex, we built logistic regression models that included actor sex and recipient sex as independent variables and realized support (supported vs. unsupported act of aggression) as the binary dependent variable. We again included an interaction between actor sex and recipient sex as a fixed effect, as well as observation session ID and dyad ID as random intercepts.
To determine whether realized social support during an agonistic encounter affected the supported hyena’s ability to successfully dominate a member of the opposite sex, we built logistic regression models that included actor sex, recipient sex, and realized support (supported vs. unsupported act of aggression) as independent variables and dominance (successful vs. unsuccessful act of aggression) as the binary dependent variable. We also included a three-way interaction between actor sex, recipient sex, and support, as well as observation session ID and dyad ID as random intercepts.
To address our research questions, we built a separate mixed model for each actor age class (adults, subadults, and cubs) to compare the effect of sex on dominance and realized social support throughout ontogeny. Aggressive acts were separated by actor age and filtered to require actors to aggress upon individuals of their own or older age classes; for example, the subadult model included subadult actors and both subadult and adult recipients. All models for dominance and realized social support were logistic regression models fit using the glmer function in the lme4 package (Bates et al., 2015). If we found a significant interaction between any explanatory variables at α = 0.05, we further stratified our analyses based on both actor sex and recipient sex to assess their joint effects on, dominance, and realized social support.
All models were built using R software (R Core Team, 2021). All models were tested for violations of dispersion, within-group deviation of uniformity, homogeneity of variance (Levene Test), and influence of outliers using the DHARMa package (Hartig and Lohse, 2022). The inclusion of relevant random intercepts was checked by calculating the intraclass correlation coefficients (ICCs). If a random intercept did not account for a sufficient variation in the model (ICC < 0.1), then it was dropped. Finally, all models were assessed using two-tailed tests with an alpha set at 0.05.
Results
Initiation of agonistic interactions
Spontaneous aggressive behavior
Among adults, comparisons of incidence rate ratios (IRRs) revealed that both adult immigrant males (IRR = 0.551; 95% CI = 0.482, 0.633, p < 0.001; Figure 1), and adult natal males (IRR = 0.644; 95% CI = 0.568, 0.729; p < 0.001; Figure 1) emitted spontaneous aggressive acts at approximately half the rate of adult females. We found no sex difference in aggression rates among either cubs (IRR = 0.890; 95% CI = 0.729, 1.085; p = 0.249; Figure 1) or subadults (IRR = 1.034; 95% CI = 0.891, 1.199; p = 0.482; Figure 1). Here, we used 103,063 observations of 305 females across the three age classes, 370 natal males across the three age classes, and 152 adult immigrant males to compare sex differences in aggressive behavior. A summary of the data for counts of spontaneous aggressive behaviors can be found in Supplementary Table 1.
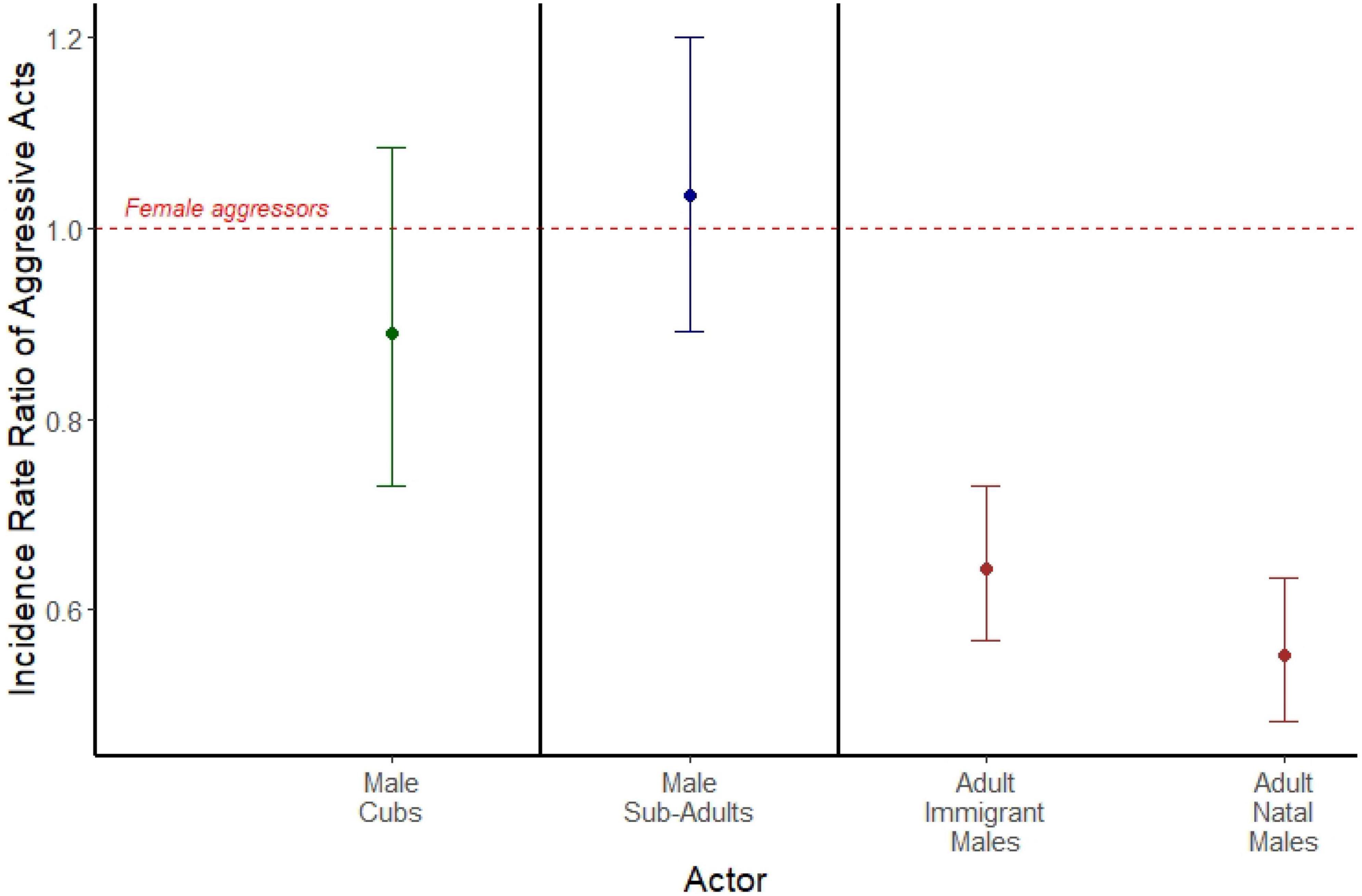
Figure 1. Incidence rate ratios of spontaneous aggressive acts emitted by male cubs (green), male subadults (blue), adult immigrant males (brown), and adult natal males (brown). Each is compared to a female aggressor reference group of the same age class, represented by the red dashed line. Points represent the estimated incidence rate ratios from three separate mixed models separated by bold black lines (actors who are cubs, actors who are subadults, and actors who are adults), and error bars represent 95% confidence intervals around the incidence rate ratio.
Spontaneous submissive behavior
Among adults, adult immigrant males emitted spontaneous acts of submission roughly 60% more often than did adult females (IRR = 1.611; 95% CI = 1.388, 1.872; p < 0.001; Figure 2), and adult natal males roughly 70% more often than adult females (IRR = 1.708; 95% CI = 1.499, 1.946; p < 0.001; Figure 2). We found no sex difference in submission rates among either cubs (IRR = 0.897; 95% CI = 0.740, 1.086; p = 0.265; Figure 2) or subadults (IRR = 0.982; 95% CI = 0.799, 1.208; p = 0.866; Figure 2). Here, we used 81,681 observations of 316 females across the three age classes, 366 natal males across the three age classes, and 156 adult immigrant males. A summary of the data for counts of spontaneous submissive behaviors can be found in Supplementary Table 1.
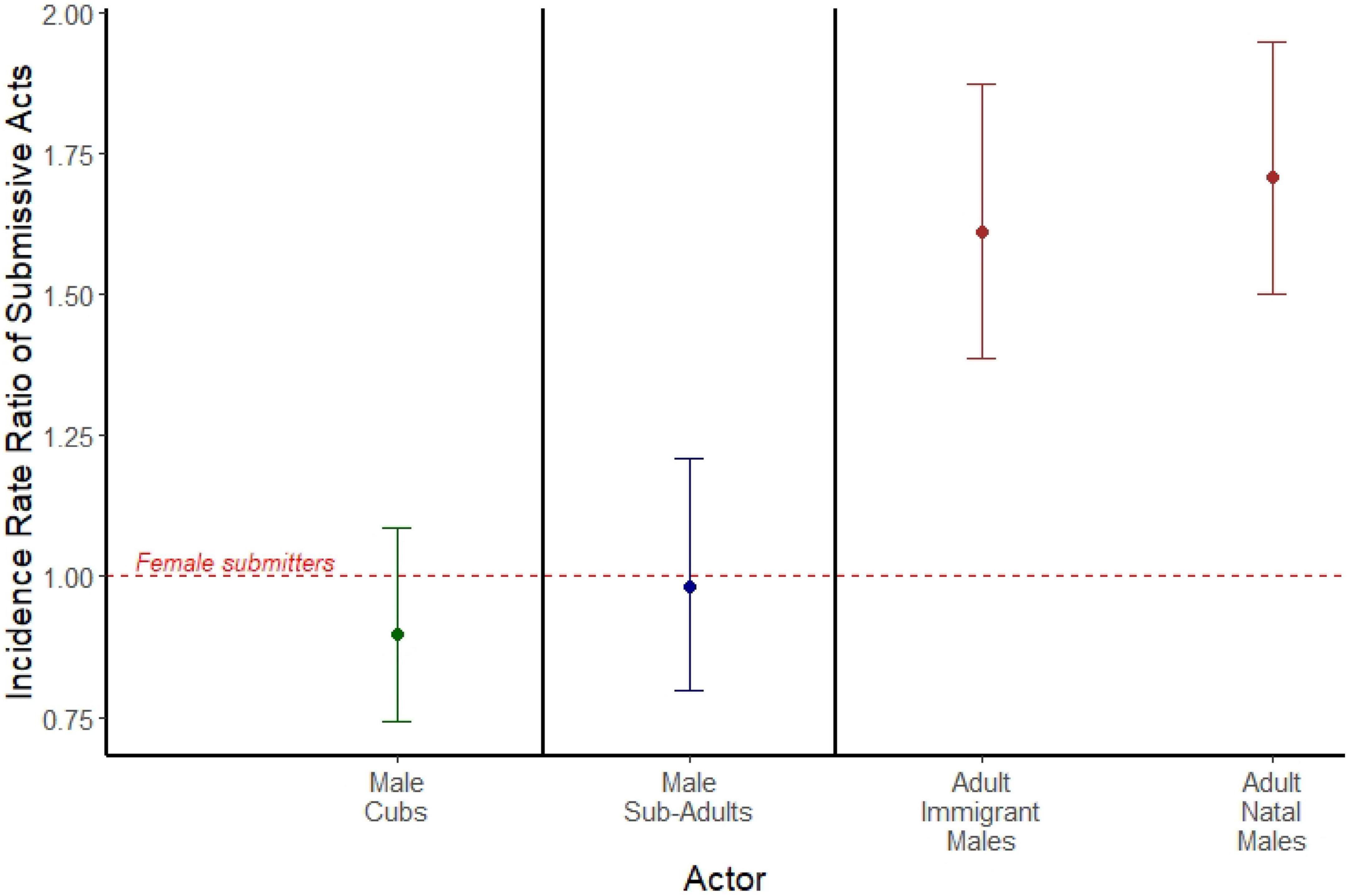
Figure 2. Incidence rate ratios of spontaneous submissive acts emitted by male cubs (green), male subadults (blue), adult immigrant males (brown), and adult natal males (brown). Each is compared to a female actor reference group of the same age class, represented by the red dashed line. Points represent the estimated incidence rate ratios from three separate mixed models separated by bold black lines (actors who are cubs, actors who are subadults, and actors who are adults), and error bars represent 95% confidence intervals around the incidence rate ratio.
Outcome of aggressive interactions
A summary of the number of acting individuals broken down by sex and age class for the following models of aggressive interactions and support can be found in Supplementary Table 2. In our initial model of dominance (successful vs. unsuccessful act of aggression), we found a significant interaction between actor sex and recipient sex in the model for adults (Supplementary Figure 1 and Supplementary Table 3). Given the significant interaction, we assessed the effect of actor sex on dominance while stratifying on recipient sex, and we assessed the effect of recipient sex on dominance while stratifying on actor sex. For consistency, we replicated this stratified model structure for cubs and subadults as well. Here, and in all remaining models, we used 79,736 observations of 366 females across the three age classes, 410 natal males across the three age classes, and 219 adult immigrant males.
Eliciting submission
In our models stratified by recipient sex (Figure 3A and Supplementary Table 4), we investigated the effect of actor sex on the odds of an actor eliciting a submissive response. Among adults, when recipients were females, adult immigrant male actors were considerably less likely than adult female actors to elicit a submissive response (OR = 0.122; 95% CI = 0.083, 0.179; p < 0.001; Figure 3A), but adult natal male actors were just as likely as adult female actors to elicit a submissive response (OR = 0.875; 95% CI = 0.674, 1.136; p = 0.317; Figure 3A). Adult immigrant males, adult natal males, and adult females initiating an aggressive act were equally likely to receive a submissive response from either immigrant or natal male recipients (Figure 3A and Supplementary Table 4). In both cubs and subadults, males and females initiating an aggressive act were equally likely to receive a submissive response from recipients regardless of recipient sex (Figure 3A and Supplementary Table 4).
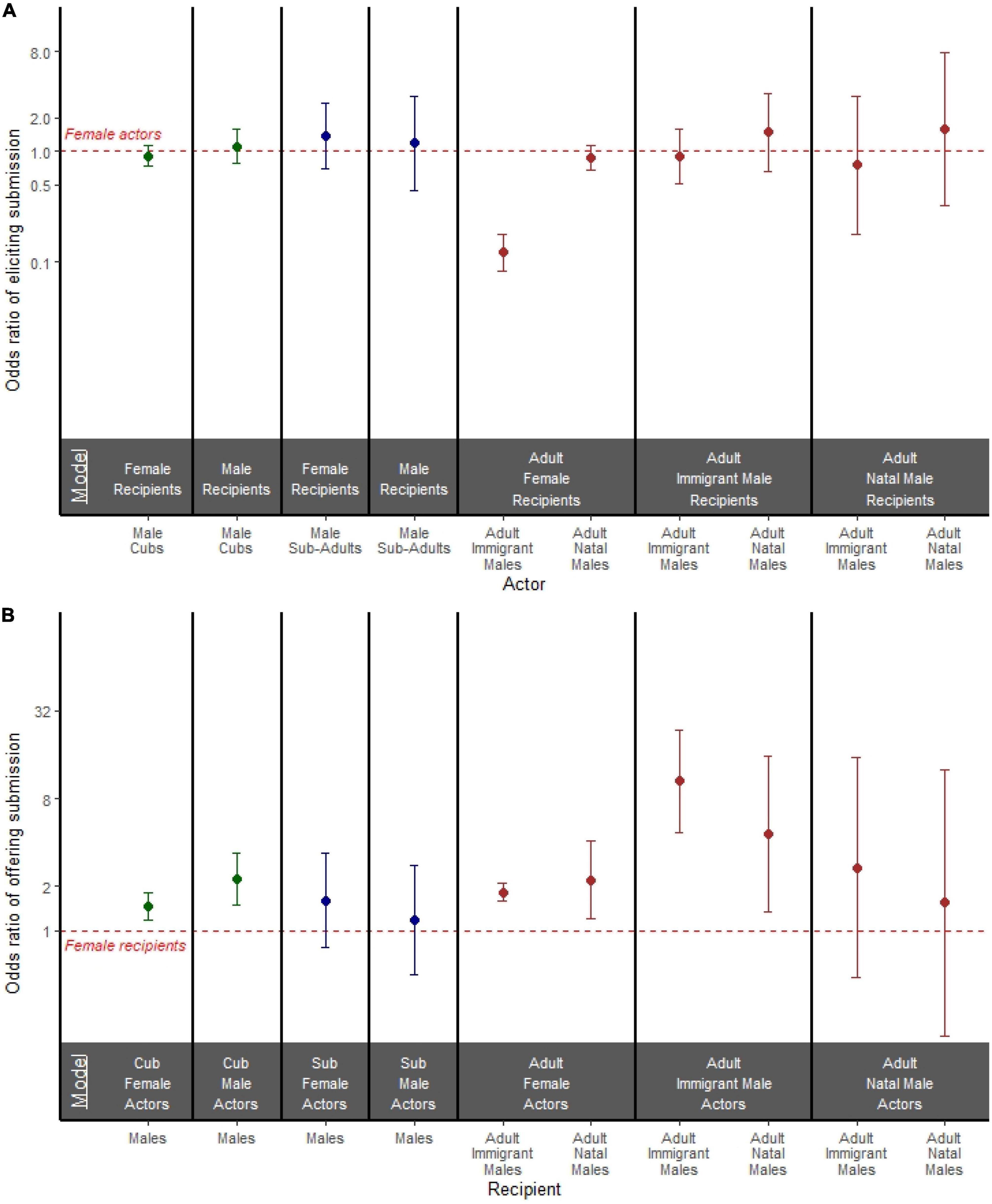
Figure 3. (A) Odds ratio of an actor eliciting a submissive response in models stratified by both actor age and recipient sex. (B) Odds ratio of a recipient offering a submissive response to aggression in models stratified by both actor age and actor sex. (A,B) Models are separated by bold black lines, and age is depicted by color where cubs are green, subadults are blue, and adults are brown. Points represent the odds ratio and error bars represent 95% confidence intervals around the odds ratio. Each point is compared to a female reference group of the same age class represented by the red dashed line.
Offering submission in response to aggressive acts
In our models stratified by actor sex (Figure 3B and Supplementary Table 5), we investigated the effect of recipient sex on the odds of the recipient responding with submissive behavior. Among adults, when the aggressors were females, both adult immigrant male recipients (OR = 1.831; 95% CI = 1.589, 2.108; p < 0.001) and adult natal male recipients (OR = 2.207; 95% CI = 1.197, 4.067; p = 0.011) were more likely to submit than adult female recipients (Figure 3B). When adult immigrant males were the aggressors, both adult immigrant male recipients (OR = 10.536; 95% CI = 4.719, 23.520; p < 0.001) and adult natal male recipients (OR = 4.607; 95% CI = 1.355, 15.664; p = 0.014) were more likely to submit than adult female recipients (Figure 3B). When adult natal males were the aggressors, both sexes were equally likely to offer a submissive response (Figure 3B and Supplementary Table 5).
Among subadults, recipient sex was not associated with a difference in offering submission: male recipients were just as likely as female recipients to offer a submissive response to both female aggressors and male aggressors (Figure 3B and Supplementary Table 5). Among cubs, male recipients were more likely than female recipients to offer a submissive response to both female aggressors (OR = 1.461; 95% CI = 1.190, 1.795; p < 0.001; Figure 3B) and male aggressors (OR = 2.255; 95% CI = 1.504, 3.381; p < 0.001; Figure 3B).
Receipt of social support
Next, we inquired whether there were sex differences in receiving social support or being targeted by coalitionary social support during spontaneous aggressive acts. In our initial models of realized social support (supported vs. unsupported act of aggression), there was no significant interaction between actor sex and recipient sex in any models across the three age classes (cub, subadult, and adult), so the interaction term was not included in the final models and main effects were reported (Supplementary Figure 2 and Supplementary Table 6). A summary of the number of acting individuals broken down by sex, age class, and support can be found in Supplementary Table 2. For consistency with prior results, we stratified our models on actor sex and recipient sex to report and display odds ratios of the effects of actor sex and recipient sex separately.
Receiving social support
Among adult actors, we found that both adult immigrant males (OR = 3.606; 95% CI = 2.990, 4.340; p < 0.001) and adult natal males (OR = 2.565; 95% CI = 2.147, 3.065; p < 0.001) were 2–3 times more likely than adult females to receive social support during aggressive interactions (Figure 4A). Male and female actors were equally likely to receive social support during attacks among both cubs (OR = 1.010; 95% CI = 0.857, 1.190; p = 0.906) and subadults (OR = 1.120; 95% CI = 0.935, 1.330; p = 0.223; Figure 4A).
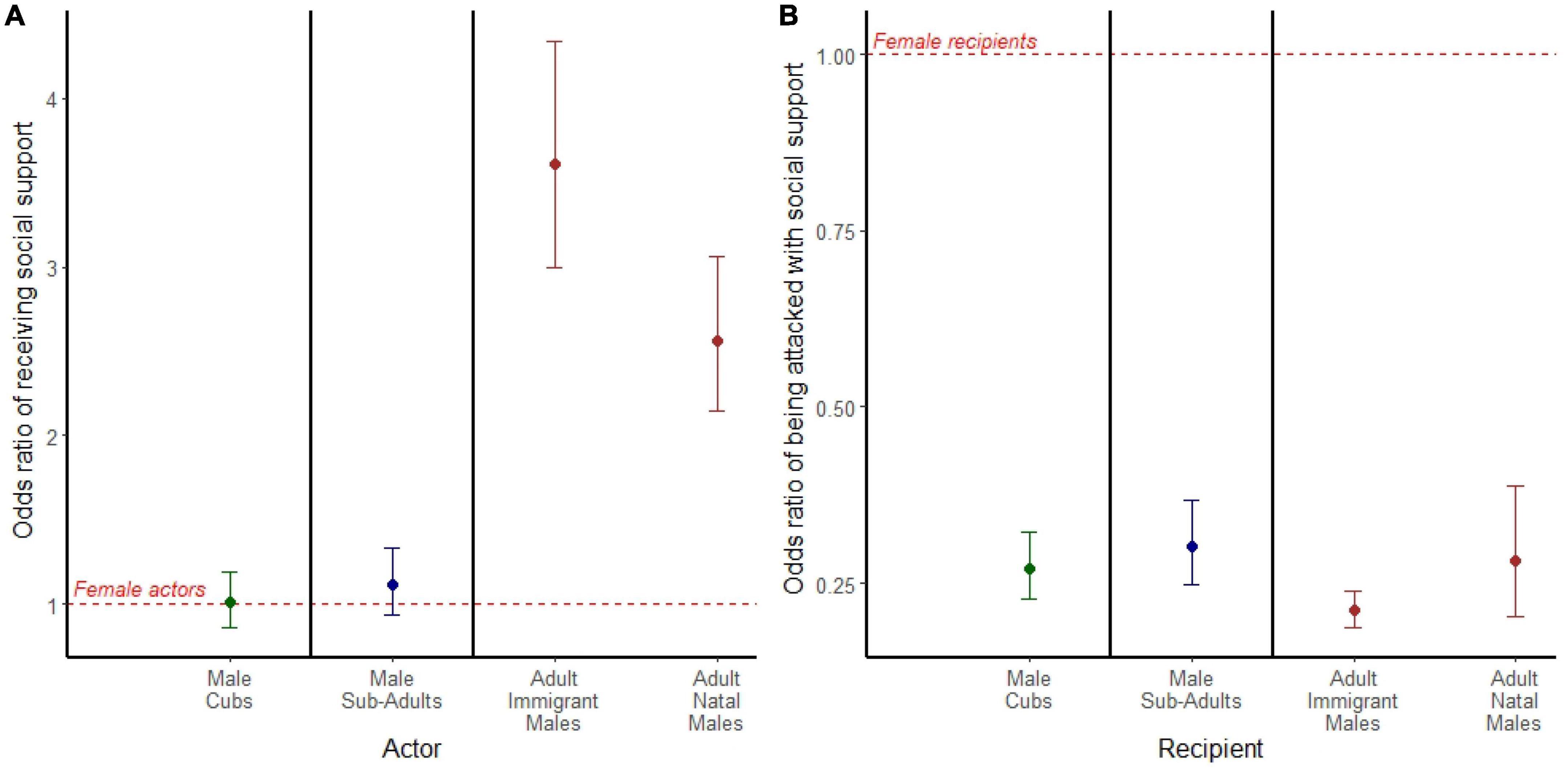
Figure 4. (A) Odds ratio of the acting aggressor receiving social support in aggression. (B) Odds ratio of an individual being targeted by an actor with social support. (A,B) Models are separated by bold black lines, and age is depicted by color where cubs are green, subadults are blue, and adults are brown. Points represent the odds ratio and error bars represent 95% confidence intervals around the odds ratio. Each point is compared to a female reference group of the same age class represented by the red dashed line.
Being targeted by socially supported aggressors
In all age classes, females were more likely than males to be the targets of aggression when actors were supported (Figure 4B). Among adult recipients, both adult immigrant males (OR = 0.211; 95% CI = 0.186, 0.239; p < 0.001) and adult natal males (OR = 0.281; 95% CI = 0.203, 0.389; p < 0.001) were less likely than adult females to be targets of socially supported aggressors (Figure 4B). Male recipients were also less likely than females to be targets of socially supported aggressors among both cubs (OR = 0.271; 95% CI = 0.226, 0.323; p < 0.001; Figure 4B) and subadults (OR = 0.301; 95% CI = 0.247, 0.367; p < 0.001; Figure 4B).
Effect of realized social support
Finally, we inquired whether realized social support during an aggressive encounter was associated with dominance outcomes. In our initial model of dominance (successful vs. unsuccessful act of aggression), we found a significant three-way interaction between actor sex, recipient sex, and realized social support in the model for adults (Supplementary Figure 3 and Supplementary Table 7). Given the significant interaction, we stratified the data by both actor sex and recipient sex to assess the effect of realized social support on whether or not an actor was successful in a dominance interaction with a recipient of the opposite sex. For consistency, we replicated this stratified model structure for our models of cubs and subadults as well. A summary of the number of acting individuals broken down by sex and age class for the following models can be found in Supplementary Table 2. In these results and figures, the reference group is unsupported actors, such that each model compares supported vs. unsupported actors of the same sex and age class. We report inter-sex comparisons of the effect of realized social support on dominance outcomes in Figure 5 and in the text below, and we report all other comparisons in Supplementary Table 8.
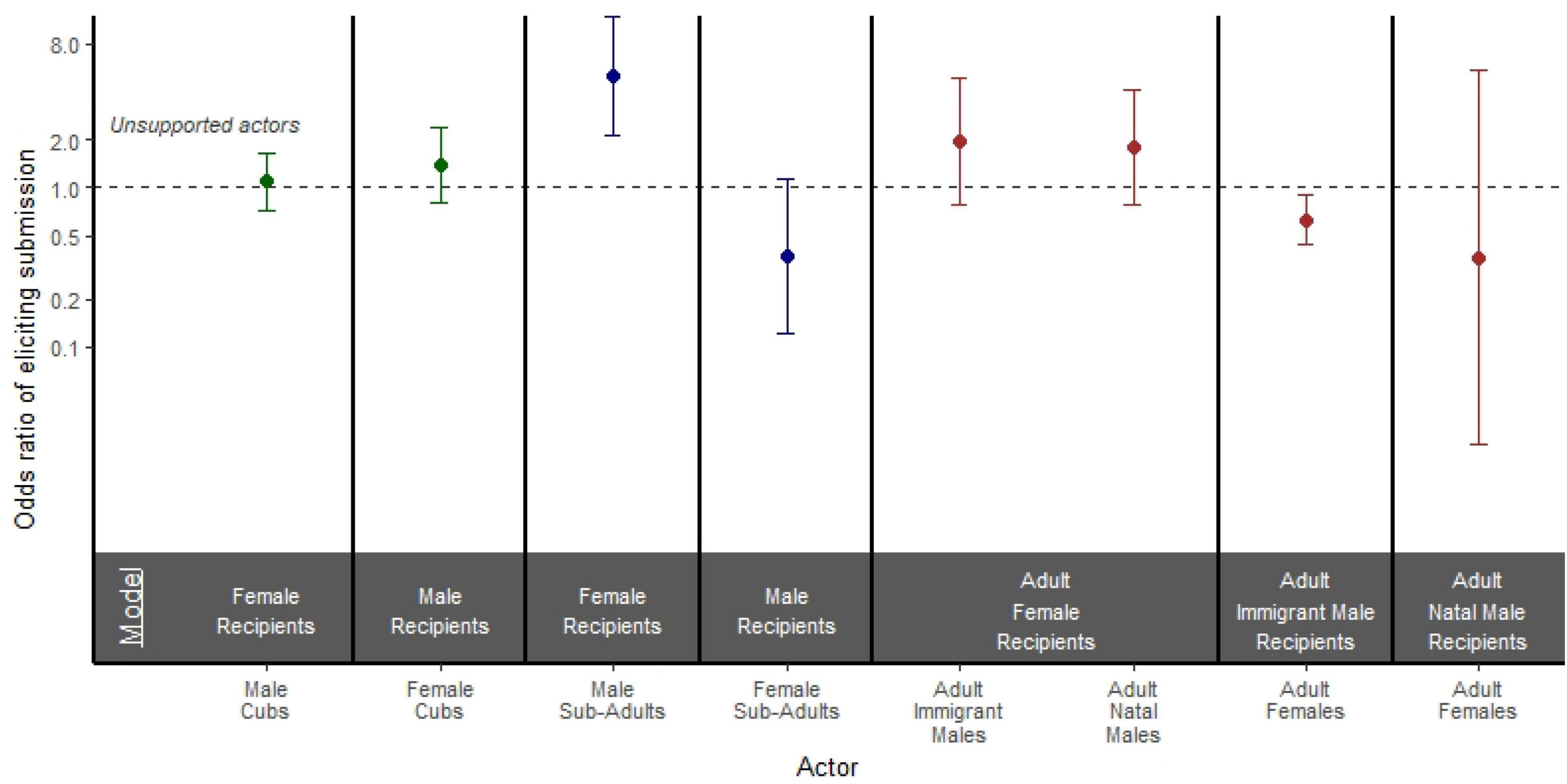
Figure 5. Odds ratio of a supported versus unsupported actor eliciting a submissive response from a recipient of the opposite sex in models stratified by both actor age and actor sex. Models are separated by bold black lines, and age is depicted by color where cubs are green, subadults are blue, and adults are brown. Points represent the odds ratio and error bars represent 95% confidence intervals around the odds ratio. Each point is compared to an unsupported actor reference group of the same age and sex class represented by the black dashed line.
Among adults, we found that support had no effect on how likely adult immigrant males (OR = 1.954; 95% CI = 0.778, 4.906; p = 0.153) or adult natal males (OR = 1.789; 95% CI = 0.776, 4.123; p = 0.171) were to elicit a submissive response from adult females (Figure 5). Interestingly, supported adult females were actually less likely than unsupported adult females to elicit submissive responses from adult immigrant males (OR = 0.629; 95% CI = 0.441, 0.897; p = 0.011; Figure 5). When adult females aggressed on adult natal males, there was no effect of support on the outcome of the aggression (OR = 0.361; 95% CI = 0.023, 5.432; p = 0.461; Figure 5). Among subadult aggressors, supported subadult males were more likely than unsupported subadult males to elicit submissive responses from female recipients (OR = 5.050; 95% CI = 2.145, 11.886; p < 0.001), but support had no effect on the odds of subadult females eliciting a submissive response from males (OR = 0.374; 95% CI = 0.122, 1.147; p = 0.086; Figure 5). Finally, among cubs, there was no effect of support on the odds of male aggressors eliciting a submissive response from females (OR = 1.101; 95% CI = 0.726, 1.670; p = 0.651), or on the odds of female aggressors eliciting a submissive response from males (OR = 1.377; 95% CI = 0.798, 2.373; p = 0.250; Figure 5).
Discussion
Here, we evaluated intrinsic attributes and social support as two non-mutually exclusive hypotheses explaining sex-biased dominance in spotted hyenas. Our results failed to unequivocally support either hypothesis alone, but instead, we found evidence implicating both mechanisms in sex-biased dominance in this species.
Our analysis of unsolicited aggressive and submissive behavior supports the intrinsic attributes hypothesis. We found that, without provocation, adult females were more aggressive than both natal and immigrant adult males (Figure 1), and that both types of adult males were more submissive than adult females (Figure 2). Among cub and subadult hyenas, patterns of intrinsic behavioral differences between the sexes were less clear, suggesting that these strong sex differences in the propensity to emit aggressive and submissive behaviors emerge primarily during adulthood. Our results support the earlier conclusion by Watts et al. (2009) and McCormick et al. (2021) that female spotted hyenas are the more aggressive sex, even after controlling for rank and subgroup size as factors influencing opportunities to act aggressively. Further, our analyses of unsolicited aggressive behavior also support prior work by Yoshida et al. (2016) on a much smaller dataset, where females were observed to be more aggressive toward members of the opposite sex than males, and where females were more aggressive toward males than males were to other males. Our result that adult males were more likely than adult females to submit without any observed provocation suggests another important intrinsic difference between the sexes, one that was also documented earlier by Smale et al. (1993). The fact that adult natal males were more submissive than adult females indicates that this pattern of adult male submissiveness is not driven purely by dispersal-induced changes in social support.
Our analysis of the outcomes of agonistic interactions revealed mixed support for both the social support and the intrinsic attributes hypothesis. Contrary to the predictions of the intrinsic attributes hypothesis, aggressing females were not more likely than aggressing natal males to receive submissive responses from their targets (Figure 3A). Instead, the only difference between the sexes over ontogeny was the reduced ability of adult immigrant males to elicit submission from adult females, which could be due to reduced social support available to immigrants. However, in support of the intrinsic attributes hypothesis, both adult immigrant males and adult natal males were more likely than adult females to submit to aggressors (Figure 3B). If this male-biased pattern of submission was driven exclusively by the actor’s and recipient’s potential for receiving social support, then immigrant males should have differed significantly from natal individuals of both sexes, because only immigrants had changed clans and lost their social support (Vullioud et al., 2019). However, as early as the first year of life, we observed that males were more likely than females to submit to aggressors, regardless of the sex of the aggressor (Figure 3B). These findings, particularly when considered in light of the striking sex differences in dominance within mixed-sex twin litters (Smale et al., 1995; Golla et al., 1999; Wahaj and Holekamp, 2006; Benhaiem et al., 2012), suggest that the behavioral tendencies associated with female dominance start to emerge well before male dispersal.
Our analysis of realized social support and its effect on the outcomes of aggressive interactions suggest that social support alone is insufficient to explain sex differences in dominance in this population of spotted hyenas. If social support was the basis of female dominance over males, we expected to see that females would receive support at higher rates than males. Instead, adult females were considerably more likely than either adult immigrant males or adult natal males to act alone as aggressors (Figure 4A). A prior study found that females engage in coalitionary aggression more frequently than males (Smith et al., 2010), which might reflect that either females are more likely to receive support than males or females are more likely to engage in aggression than males. Our results clearly indicated that this pattern was driven by higher rates of aggression by females but a lower probability of receiving social support per aggressive act. The social support hypothesis also predicted that receiving support aids females in dominating males, but we found that adult females elicited submissive responses from adult immigrant and adult natal males when acting alone just as readily as when acting with support (Figure 5). Despite the lack of evidence for the social support hypothesis as the sole determinant of female dominance within this population of spotted hyenas, our results indicated that social support did shape agonistic interactions in some interesting ways. Realized social support improved the likelihood of adult females successfully dominating other adult females (Supplementary Figure 3A), supporting previous work which revealed that coalitionary aggression is an important mechanism producing rank change among female hyenas (Strauss and Holekamp, 2019). Most strikingly, although not part of our a priori predictions, we found an interesting pattern where hyenas of all age and sex classes were more likely to act alone when aggressing against males than females (Figure 4B). We interpreted this pattern as supporting both of the hypotheses under investigation: social support is most needed when acting against females, who are intrinsically more threatening adversaries.
Interestingly, realized social support slightly increased the likelihood that cubs and subadults would receive a submissive response during an agonistic encounter compared to cubs and subadults acting without support within our full models that included actor sex, recipient sex, and support (Supplementary Figures 3B,C and Supplementary Table 7). Some of this support may be mothers helping cubs win fights as part of the process of rank acquisition in the clan’s dominance hierarchy (Engh et al., 2000; East et al., 2009; Strauss et al., 2020), particularly when subadult males are interacting with females (Supplementary Figure 3C). However, winning fights by female cubs was clearly also affected by the male recipient’s tendency to concede defeat more readily than females when attacked (Supplementary Figures 1A, 3A).
Altogether, our results point to social support and intrinsic sex differences as dual influences on dominance in this species. This contrasts with prior work, which reported that only the social support hypothesis explained sex differences in dominance in spotted hyenas (Vullioud et al., 2019), and multiple factors may explain this discrepancy. First, the two studies differed considerably in study design and methodology. For instance, we examined the behavior of adults, cubs, and subadults separately in this study (finding some interesting variation in dominance behavior across ontogeny), whereas prior work analyzed subadults and adults together. Most notably, Vullioud et al. (2019) included interactions between individuals from different social groups as well as within-group interactions, but we elected to focus only on within-group interactions because the factors influencing agonistic interaction outcomes within and between groups are often different (Majolo et al., 2020, [but see Vullioud et al., 2019]). Second, the two studies differed in the specific predictions tested: here, we tested predictions based on both potential for receiving social support and the realized social support individuals actually experience, whereas Vullioud et al. (2019) focused only on the potential for receiving social support. We made this choice because we felt that measuring realized social support was the most direct way of addressing its effect, but we agree with Vullioud et al. (2019) that the potential for receiving social support can influence interaction outcomes even if that social support is ultimately not delivered. A productive next step would be to investigate the relationship between potential and realized social support. If potential social support has as large an effect on interaction outcomes as realized social support, this may cause realized social support to appear to have a limited effect (e.g., Figure 5).
In addition to differences in study design, differences in the conclusions between the two studies might be due to population-level behavioral differences across the highly variable Serengeti–Mara ecosystem (Ginsberg et al., 1996). A productive avenue for further clarifying the basis of sex-biased dominance in this species would be to directly compare the behavior of natal males in these two populations to understand potential population-level differences. Additionally, given our findings that realized social support does aid adult immigrant males and subadult natal males in eliciting submission from female recipients, a further avenue of research would be to clarify exactly who, based on sex, rank, genetic relationships, and/or social networks, is supporting these males against females in a female-dominated society.
Finally, we conclude that multiple processes are likely to influence dominance in spotted hyenas, as also occurs in many other species (Lewis, 2020; Dehnen et al., 2022). We considered the phenomenon of female dominance in spotted hyenas in light of the general framework suggested by Lewis (2002) for assessing female power in animal societies. Lewis (2002) divides power into two categories, dominance and leverage, depending on the nature of the asymmetry between actor and recipient that generates power; in this framework, ‘dominance’ describes an asymmetry in physical capacities affecting the ability to use force. Female dominance is intrinsic among adult hyenas insofar as females are more aggressive than males (Figure 1), and males are more submissive (Figure 2) and concede defeat much more readily than females (Figure 3B). However, dominance in this species is also based on social support, which helps cubs elicit submission from larger hyenas and helps adult females win fights against other adult females (Engh et al., 2000; Strauss and Holekamp, 2019). Thus, greater aggressiveness may be an intrinsic trait that enhances a female hyena’s likelihood of winning fights with group mates, but the number of kin or other social allies available as potential supporters to a particular aggressor also affect its ability to win fights (Smith et al., 2010; Vullioud et al., 2019). We found that realized social support helped individuals of both sexes across age classes to dominate formidable females; in particular, realized social support helped immigrant males elicit submission from adult females, which they were very unlikely to achieve without support (Supplementary Figure 3). Overall, the results of our study add considerably to prior work demonstrating how support is crucial to rank acquisition (Holekamp and Smale, 1991; Engh et al., 2000; East et al., 2009), how it reinforces the established kin-structured dominance hierarchy among natal individuals (Smith et al., 2010; Holekamp et al., 2012; Vullioud et al., 2019), and how it influences competition among females (Strauss and Holekamp, 2019).
Data availability statement
The datasets generated during and/or analyzed during the course of this study are available in the Dryad repository: https://doi.org/10.5061/dryad.w6m905qsw.
Ethics statement
The animal study was reviewed and approved by Michigan State University Institutional Animal Care and Use Committee (IACUC Protocol 202200047, expiring 2/11/2025).
Author contributions
SM contributed to the conceptualization, methodology, curation of data, coding, analyses, visualization, writing of the manuscript, and supervision. ES, ZL, and TM contributed to the writing of the manuscript, methodology, analysis, and visualization. KH helped to conceptualize the project, contributed to data, funding, and other resources, and helped to draft the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by U.S. National Science Foundation (NSF) Grants OISE1853934 and IOS1755089 to KH. We also received support for this work from NSF Grant OIA 0939454. SM was supported by OISE1853934, a University Distinguished Fellowship from Michigan State University, and a Graduate Research Fellowship from NSF. ES was supported by the Alexander von Humboldt Foundation. ZL was supported by the National Science Foundation DBI 2010607.
Acknowledgments
We thank the Kenyan National Commission for Science, Technology and Innovation, the Narok County Government, the Mara Conservancy, the Kenya Wildlife Service, Naboisho Conservancy, and the Wildlife Research Training Institute for the permission to conduct our long-term study. We thank all those who assisted with data collection in the field and with data entry and manipulation in the lab. We also thank the Michigan State University Integrative Biology Department chair, Tom Getty, and the Michigan State University Ecology, Evolution, and Behavior Program director, Elise Zipkin, for publication fee funding support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.934659/full#supplementary-material
References
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour 49, 227–266. doi: 10.1163/156853974X00534
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67:1. doi: 10.18637/jss.v067.i01
Benhaiem, S., Hofer, H., Kramer-Schadt, S., Brunner, E., and East, M. L. (2012). Sibling rivalry: Training effects, emergence of dominance and incomplete control. Proc. R. Soc. B Biol. Sci. 279, 3727–3735. doi: 10.1098/rspb.2012.0925
Boydston, E. E., Kapheim, K. M., and Holekamp, K. E. (2006). Patterns of den occupation by the spotted Hyaena (Crocuta crocuta). Afr. J. Ecol. 44, 77–86. doi: 10.1111/j.1365-2028.2006.00618.x
Brooks, M. E., Kristensen, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/rj-2017-066
Carpenter, C. R. (1942). Sexual behavior of free ranging rhesus monkeys (Macaca mulatta). I. Specimens, procedures and behavioral characteristics of estrus. J. Comp. Psychol. 33, 113–142. doi: 10.1037/h0058655
Cassini, M. H. (2020). A mixed model of the evolution of polygyny and sexual size dimorphism in mammals. Mammal Rev. 50, 112–120. doi: 10.1111/mam.12171
Clutton-Brock, T., and Huchard, E. (2013). Social competition and its consequences in female mammals. J. Zool. 289, 151–171. doi: 10.1111/jzo.12023
Curren, L. J., Weldele, M. L., and Holekamp, K. E. (2013). Ejaculate quality in spotted hyenas: Intraspecific variation in relation to life-history traits. J. Mammal. 94, 90–99. doi: 10.1644/12-MAMM-A-057.1
Darwin, C., Bonner, J. T., and May, R. M. (1981). The descent of man, and selection in relation to sex (REV-Revised). Princeton, NJ: Princeton University Press.
Dehnen, T., Arbon, J. J., Farine, D. R., and Boogert, N. J. (2022). How feedback and feed-forward mechanisms link determinants of social dominance. Biol. Rev. 97, 1210–1230. doi: 10.1111/brv.12838
Drews, C. (1993). The concept and definition of dominance in animal behaviour. Behaviour 125, 283–313. doi: 10.1163/156853993X00290
East, M. L., and Hofer, H. (2001). Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav. Ecol. 12, 558–568. doi: 10.1093/beheco/12.5.558
East, M. L., Burke, T., Wilhelm, K., Greig, C., and Hofer, H. (2003). Sexual conflicts in spotted hyenas: Male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc. R. Soc. B Biol. Sci. 270, 1247–1254. doi: 10.1098/rspb.2003.2363
East, M. L., Höner, O. P., Wachter, B., Wilhelm, K., Burke, T., and Hofer, H. (2009). Maternal effects on offspring social status in spotted hyenas. Behav. Ecol. 20, 478–483. doi: 10.1093/beheco/arp020
East, M., Hofer, H., and Turk, A. (1989). Functions of birth dens in spotted hyaenas (Crocuta crocuta). J. Zool. 219, 690–697. doi: 10.1111/j.1469-7998.1989.tb02614.x
Engh, A. L., Esch, K., Smale, L., and Holekamp, K. E. (2000). Mechanisms of maternal rank “inheritance” in the spotted Hyaena, Crocuta crocuta. Anim. Behav. 60, 323–332. doi: 10.1006/anbe.2000.1502
Engh, A. L., Funk, S. M., Van Horn, R. C., Scribner, K. T., Bruford, M. W., Libants, S., et al. (2002). Reproductive skew among males in a female-dominated mammalian society. Behav. Ecol. 13, 193–200. doi: 10.1093/beheco/13.2.193
Frank, L. G. (1986). Social organization of the spotted Hyaena Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 34, 1510–1527. doi: 10.1016/S0003-3472(86)80221-4
Frank, L. G., Glickman, S. E., and Powch, I. (1990). Sexual dimorphism in the spotted Hyaena (Crocuta crocuta). J. Zool. 221, 308–313. doi: 10.1111/j.1469-7998.1990.tb04001.x
Ginsberg, J., Sinclair, A. R. E., and Arcese, P. (1996). Serengeti II: Dynamics, management and conservation of an ecosystem. J. Anim. Ecol. 65, 534–537. doi: 10.2307/5794
Glickman, S. E., Frank, L. G., Pavgi, S., and Licht, P. (1992). Hormonal correlates of “masculinization” in female spotted hyaenas (Crocuta crocuta). 1. Infancy to sexual maturity. J. Reprod. Fertil. 95, 451–462. doi: 10.1530/jrf.0.0950451
Golla, W., Hofer, H., and East, M. L. (1999). Within-litter sibling aggression in spotted hyaenas: Effect of maternal nursing, sex and age. Anim. Behav. 58, 715–726. doi: 10.1006/anbe.1999.1189
Hartig, F., and Lohse, L. (2022). Package “DHARMa” − residual diagnostics for hierarchical (multi-level / mixed) regression models. Available online at: https://CRAN.R-project.org/package=DHARMa (accessed September 8, 2022).
Holekamp, K. E., and Engh, A. L. (2009). “Reproductive skew in female-dominated mammalian societies,” in Reproductive skew in vertebrates: Proximate and ultimate causes, eds R. Hager and C. B. Jones (Cambridge: Cambridge University Press), 53–83.
Holekamp, K. E., and Sisk, C. L. (2003). Effects of dispersal status on pituitary and gonadal function in the male spotted hyena. Horm. Behav. 44, 385–394. doi: 10.1016/j.yhbeh.2003.06.003
Holekamp, K. E., and Smale, L. (1991). Dominance acquisition during mammalian social development: The “inheritance” of maternal rank. Integr. Comp. Biol. 31, 306–317. doi: 10.1093/icb/31.2.306
Holekamp, K. E., and Smale, L. (1998). Behavioral development in the spotted hyena. BioScience 48, 997–1005. doi: 10.2307/1313456
Holekamp, K. E., and Strauss, E. D. (2020). Reproduction within a hierarchical society from a female’s perspective. Integr. Comp. Biol. 60, 753–764. doi: 10.1093/icb/icaa068
Holekamp, K. E., Cooper, S. M., Katona, C. I., Berry, N. A., Frank, L. G., and Smale, L. (1997). Patterns of association among female spotted hyenas (Crocuta crocuta). J. Mammal. 78, 55–64. doi: 10.2307/1382638
Holekamp, K. E., Smale, L., and Szykman, M. (1996). Rank and reproduction in the female spotted Hyaena. J. Reprod. Fertil. 108, 229–237. doi: 10.1530/jrf.0.1080229
Holekamp, K. E., Smith, J. E., Strelioff, C. C., Van Horn, R. C., and Watts, H. E. (2012). Society, demography and genetic structure in the spotted hyena. Mol. Ecol. 21, 613–632. doi: 10.1111/j.1365-294X.2011.05240.x
Höner, O. P., Wachter, B., East, M. L., Streich, W. J., Wilhelm, K., Burke, T., et al. (2007). Female mate-choice drives the evolution of male-biased dispersal in a social mammal. Nature 448, 798–801. doi: 10.1038/nature06040
Ilany, A., Holekamp, K. E., and Akçay, E. (2021). Rank-dependent social inheritance determines social network structure in spotted hyenas. Science 373, 348–352. doi: 10.1126/science.abc1966
Kruuk, H. (1972). The spotted hyena: A study of predation and social behavior. Chicago, IL: University of Chicago Press.
Laubach, Z. M., Greenberg, J. R., Turner, J. W., Montgomery, T. M., Pioon, M. O., Sawdy, M. A., et al. (2021). Early-life social experience affects offspring DNA methylation and later life stress phenotype. Nat. Commun. 12, 1–15. doi: 10.1038/s41467-021-24583-x
Lewis, R. J. (2002). Beyond dominance: The importance of leverage. Q. Rev. Biol. 77, 149–164. doi: 10.1086/343899
Lewis, R. J. (2020). Female power: A new framework for understanding “female dominance” in lemurs. Folia Primatol. 91, 48–68. doi: 10.1159/000500443
Majolo, B., deBortoli Vizioli, A., Martínez-Íñigo, L., and Lehmann, J. (2020). Effect of group size and individual characteristics on intergroup encounters in primates. Int. J. Primatol. 41, 325–341. doi: 10.1007/s10764-019-00119-5
McCormick, S. K., and Holekamp, K. E. (2022). Aggressiveness and submissiveness in spotted hyaenas: One trait or two? Anim. Behav. 186, 179–190. doi: 10.1016/j.anbehav.2022.01.012
McCormick, S. K., Holekamp, K. E., Smale, L., Weldele, M. L., Glickman, S. E., and Place, N. J. (2021). Sex Differences in spotted hyenas. Cold Spring Harb. Perspect. Biol. 14:a039180. doi: 10.1101/cshperspect.a039180
Muller, M. N. (2017). Testosterone and reproductive effort in male primates. Horm. Behav. 91, 36–51. doi: 10.1016/j.yhbeh.2016.09.001
Nelson, R. J. (2005). Biology of aggression. Oxford: Oxford University Press. doi: 10.1093/acprof:oso/9780195168761.001.0001
R Core Team (2021). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Rico-Guevara, A., and Hurme, K. J. (2019). Intrasexually selected weapons. Biol. Rev. 94, 60–101. doi: 10.1111/brv.12436
Smale, L., Frank, L. G., and Holekamp, K. E. (1993). Ontogeny of dominance in free-living spotted hyaenas: Juvenile rank relations with adult females and immigrant males. Anim. Behav. 46, 467–477. doi: 10.1006/anbe.1993.1214
Smale, L., Holekamp, K. E., Weldele, M., Frank, L. G., and Glickman, S. E. (1995). Competition and cooperation between litter-mates in the spotted hyaena, Crocuta crocuta. Anim. Behav. 50, 671–682. doi: 10.1016/0003-3472(95)80128-6
Smale, L., Nunes, S., and Holekamp, K. E. (1997). Sexually dimorphic dispersal in mammals: Patterns, causes, and consequences. Adv. Study Behav. 26, 181–250. doi: 10.1016/S0065-3454(08)60380-0
Smith, J. E., Van Horn, R. C., Powning, K. S., Cole, A. R., Graham, K. E., Memenis, S. K., et al. (2010). Evolutionary forces favoring intragroup coalitions among spotted hyenas and other animals. Behav. Ecol. 21, 284–303. doi: 10.1093/beheco/arp181
Strauss, E. D., and Holekamp, K. E. (2019). Social alliances improve rank and fitness in convention-based societies. Proc. Natl. Acad. Sci. U.S.A. 116, 8919–8924. doi: 10.1073/pnas.1810384116
Strauss, E. D., Curley, J. P., Shizuka, D., and Hobson, E. A. (2022). The centennial of the pecking order: Current state and future prospects for the study of dominance hierarchies. Philos. Trans. R. Soc. B Biol. Sci. 377:20200432. doi: 10.1098/rstb.2020.0432
Strauss, E. D., Shizuka, D., and Holekamp, K. E. (2020). Juvenile rank acquisition is associated with fitness independent of adult rank. Proc. R. Soc. B Biol. Sci. 287, 4–6. doi: 10.1098/rspb.2019.2969
Van Horn, R. C., Engh, A. L., Scribner, K. T., Funk, S. M., and Holekamp, K. E. (2004). Behavioural structuring of relatedness in the spotted hyena (Crocuta crocuta) suggests direct fitness benefits of clan-level cooperation. Mol. Ecol. 13, 449–458. doi: 10.1046/j.1365-294X.2003.02071.x
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., and Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nat. Ecol. Evol. 3, 71–76. doi: 10.1038/s41559-018-0718-9
Wahaj, S. A., and Holekamp, K. E. (2006). Functions of sibling aggression in the spotted hyaena, Crocuta crocuta. Anim. Behav. 71, 1401–1409. doi: 10.1016/j.anbehav.2005.11.011
Watts, H. E., Tanner, J. B., Lundrigan, B. L., and Holekamp, K. E. (2009). Post-weaning maternal effects and the evolution of female dominance in the spotted hyena. Proc. R. Soc. B Biol. Sci. 276, 2291–2298. doi: 10.1098/rspb.2009.0268
Keywords: dominance, intrinsic sex differences, social support, aggressive behavior, submissive behavior
Citation: McCormick SK, Laubach ZM, Strauss ED, Montgomery TM and Holekamp KE (2022) Evaluating drivers of female dominance in the spotted hyena. Front. Ecol. Evol. 10:934659. doi: 10.3389/fevo.2022.934659
Received: 02 May 2022; Accepted: 02 November 2022;
Published: 21 December 2022.
Edited by:
Elise Huchard, UMR 5554 Institut des Sciences de l’Evolution de Montpellier (ISEM), FranceReviewed by:
Eve Davidian, Leibniz Institute for Zoo and Wildlife Research, GermanyAlice Baniel, Stony Brook University, United States
Copyright © 2022 McCormick, Laubach, Strauss, Montgomery and Holekamp. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Kevin McCormick, bWNjb3IxODdAbXN1LmVkdQ==
 S. Kevin McCormick
S. Kevin McCormick Zachary M. Laubach
Zachary M. Laubach Eli D. Strauss
Eli D. Strauss Tracy M. Montgomery
Tracy M. Montgomery Kay E. Holekamp
Kay E. Holekamp