
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 09 December 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.931226
This article is part of the Research Topic Sex and Gender Effects on Power, Status, Dominance, and Leadership – An Interdisciplinary Look at Human and Other Mammalian Societies View all 22 articles
Intersexual dominance, which is measured by the probability that members of one sex elicit submission of members of the other sex during agonistic interactions, is often skewed in favor of males. However, even in sexually dimorphic species, several factors may influence intersexual dominance. Here, we use an 8-year dataset to examine the dynamics of intersexual dominance in wild-living mandrills (Mandrillus sphinx). Mandrills exhibit an extreme male-biased sexual size dimorphism but females show pronounced kin-differentiated social relationships and occasionally form coalitions against males. We established intersexual hierarchies across consecutive 6-month time blocks, representing either mating or birth seasons. Although females appeared to outrank 11% of males, they elicited male submission in only 2% of agonistic interactions against males. This discrepancy is likely due to the temporary residency of most males in the exceptionally large mandrill groups, the sexually coercive male mating strategies and the scarce number of agonistic interactions within most dyads, that may limit hierarchical inferences. In a second step, we found that the intersexual hierarchy mixes the intrasexual ones respecting their respective order. Females outranked mostly young and old males during the mating (vs. birth) season and social integration was positively correlated to dominance status in both sexes. In a third step, we found that females win more conflicts against young or old males which are closer to them in the intersexual hierarchy. These results extend our understanding of female-male dominance relationships by indicating that female mandrills occasionally outrank males who are considerably larger than them, and that a combination of demographic and social factors can influence the intersexual hierarchy.
Intersexual hierarchies reflect sexual asymmetries in the outcome of agonistic interactions, which are often biased toward males. Despite their importance for the social structure, mating strategies and life-history of a species (Parker, 2006), studies of dominance hierarchies have long been restricted to intrasexual contexts (Ellis, 1995; Davidian et al., 2022), sometimes considering by default that all males are dominant over all females (Lewis, 2018). However, recent studies that have quantified intersexual dominance via the construction of intersexual hierarchies draw a more nuanced and dynamic landscape (Lewis, 2020; Davidian et al., 2022; Kappeler et al., 2022), where intersexual dominance varies along a continuum, including more balanced female-male dominance relationships (e.g., Hemelrijk et al., 2020). Except for a handful of well-known species with female-biased dominance [bonobos (Pan paniscus): Parish et al., 2000; most lemurs: Kappeler, 1993; Petty and Drea, 2015; spotted hyenas (Crocuta crocuta): Kruuk, 1972], there is a growing list of species with circumstantial or contextual female dominance over males (primates: Dunham, 2008; Hemelrijk et al., 2008; Ferrari, 2009; Izar et al., 2021; small mammals: Murie and Harris, 1988; Koren et al., 2006; Hewitt et al., 2009; birds: Smith, 1982; Jawor, 2000; see also Hand, 1986).
Our understanding of why intersexual dominance is biased toward females in some species, and toward males in others is still fragmentary and often relies on taxon-specific hypotheses (Kappeler and Fichtel, 2015; Lewis, 2018; Davidian et al., 2022). In addition, there are still several sources of uncertainty concerning the structural properties of intersexual dominance hierarchies. First, it has long been unclear whether intrasexual dominance rank predicts intersexual rank because intrasexual agonistic interactions may target different resources and dominance relationships may be established and maintained through different mechanisms depending on the sex (e.g., inherited vs. fight-based hierarchies; Clutton-Brock and Huchard, 2013b; Tibbetts et al., 2022). A recent analysis including several mammal species indicates, however, that the rank order of same-sex individuals is often conserved in intersexual hierarchies (Kappeler et al., 2022). Second, dominance hierarchies based on matrices constructed with different methods can lead to different results (Lewis, 2002). Agonistic interactions may have different forms and variable outcomes. Although standardized methods to quantify intersexual dominance have been recently proposed (Kappeler et al., 2022), previous studies often mix, for example “undecided” (i.e., not systematically followed by submissive behaviors) and “decided” interactions or even aggressive and submissive behaviors (e.g., Hemelrijk et al., 2020) to construct dominance hierarchies which may affect the comparability of results. Aggression is often expressed under tensed circumstances during social conflicts while submission is regularly, and often spontaneously, expressed in ritualized contexts outside situations of social tension, and may constitute more reliable cues of perceived dominance relationships (Kappeler et al., 2022). Additionally, in species with dominance biased toward males, females may threaten or direct aggression toward males during conflicts or tensed situations when they are aroused, while they may be unable to elicit male submission in routine situations (French et al., 2013). In contrast, in Verreaux sifakas (Propithecus verreauxi) where females are strictly dominant over males, nearly 90% of spontaneous submissions are expressed by males toward females, while females win only about 1/3 of intersexual conflicts (Lewis et al., 2022). Overall, females may appear more or less dominant over males in social hierarchies built using different types of social interactions.
Apart from aggressiveness or physical characteristics (e.g., size or strength), demographic and ecological factors may also influence the dynamics of intersexual dominance hierarchies within groups or populations (Chase et al., 2002; Lewis, 2002; Hewitt et al., 2009; Young et al., 2017; Hemelrijk et al., 2020). First, the group sex-ratio influences the intersexual dominance and females outrank more males when the number of males in the group increases (Hemelrijk et al., 2020; Lewis, 2020; Izar et al., 2021). In primates, female dominance over males may emerge from the so-called “winner and loser effects” where more males in a group fight more, causing more losses and injuries in subordinate males who may eventually submit to females (Hemelrijk et al., 2008; but see also Bonabeau, 1999). Alternatively, this effect may reflect the dynamics of “mating markets” (Noë and Hammerstein, 1994; Gumert, 2007), where fluctuating sex-ratios affect the relative sex-based leverage gained by fertile females, as the relative value of fertilizable eggs increases with their rarity, i.e., when there are less fertile females for a larger number of males. Therefore, females who control the access to their eggs—a valuable resource for males that cannot be taken by force—(Lewis, 2002, 2018, 2020) may have increased intersexual dominance as males might be more cooperative or compliant in order to gain access to fertile females. Accordingly, females may be more or less dominant depending on their reproductive state: for example, female mouse lemurs win more intersexual conflicts during the reproductive season than outside of it (Hohenbrink et al., 2016), and in many monogamous birds, females are more dominant over males when they are sexually receptive (Smith, 1980). Finally, social support and coalitions can also influence the outcome of agonistic interactions in different taxa (Weiß and Kotrschal, 2004; Markham et al., 2015), and may shape emerging hierarchies and reinforce established ones (Bissonnette et al., 2009; Strauss and Holekamp, 2019; Vullioud et al., 2019). In mammals, female philopatry is frequent (Greenwood, 1980), and philopatric females often ground their dominance relationships on social support (Clutton-Brock and Huchard, 2013a), which may further influence the outcome of intersexual interactions, as shown in spotted hyenas (Vullioud et al., 2019). However, few studies have examined the influence of social support on intersexual dominance in species where males are generally dominant over females. Overall, while new evidence indicates that intersexual dominance can be flexible and context-dependent, we know little regarding the extent and determinants of such variation, i.e., the ecology of intersexual dominance.
In this study, we investigate intersexual dominance relationships in mandrills (Mandrillus sphinx). Mandrills are primates of the Cercopithecidae family living in polygynandrous groups including hundreds of individuals (Abernethy et al., 2002). They are seasonal breeders and most males enter the group at the onset of the mating season and leave afterwards, with only a few males remaining in the group during the birth season (Brockmeyer et al., 2015). Male-male competition is severe in this species (Setchell, 2016) and results in high reproductive skew, with 60–70% of reproductions monopolized by the alpha male (Charpentier et al., 2005, 2020). Mandrills exhibit extreme sexual size dimorphism: males are on average 3.4 times heavier than females (Setchell et al., 2001) while they display upper canines almost 5 times longer than females (Leigh et al., 2008). Male mandrills attain adult size and mass and show a major increase in mounts of fully swollen females (likely to be fertile) around the age of 9–10 years (Setchell et al., 2001). Their dominance rank increases with age, peaks from 11 to 16 and falls again after 16 years (Setchell et al., 2006b). Adult males are sexually coercive (Smit et al., 2022) and mate-guard females when they display maximally turgescent sexual swellings around ovulation (Setchell et al., 2005a). Females are philopatric and form differentiated social bonds and linear matrilineal hierarchies, with maternally inherited ranks that are relatively stable across their lives (Setchell et al., 2002). Despite the large physical asymmetries between sexes in mandrills, females can form coalitions against males, sometimes exceptionally violent (Setchell et al., 2006a).
In a first step, we investigate the dynamics of intersexual dominance in mandrills at the “population level,” indexed by the percentage of males outranked by an average female in consecutive 6-month time blocks over an 8-year study period. We build intersexual dominance matrices using only dyadic decided interactions (i.e., when one opponent exhibited submission) and we compute dominance hierarchies based on (i) all submissive behaviors (whether or not they follow aggression) and (ii) ritualized submissive behaviors only (in the absence of aggression), to test if intersexual dominance varies across behavioral contexts. We predict females to outrank less males when the social hierarchy is based on ritualized submissive interactions only, compared to a dataset comprising aggressive interactions occurring during aggressive encounters. In a second step, we investigate factors that could influence the probability that a given female outranks a given male. We expect that (i) intrasexual dominance rank position predicts the position of an individual in the intersexual hierarchy, (ii) females outrank more males during the mating season, when they are sexually receptive and thus have more leverage, and when the group includes more males. We further expect that (iii) more socially integrated individuals are more dominant over the other sex as they have more social support, and that (iv) females have a higher probability to outrank males who are not in their prime (the age range when males are the strongest). In a final step, we investigate factors that may influence the probability of a female to win an intersexual conflict. We predict that (i) the greater the rank difference between the opponents (i.e., the more dominant the male and the more subordinate the female) the lower the probability for the female to win, (ii) sexually receptive females win more conflicts than females in other reproductive states, given that they have more sex-related leverage, and (iii) females have a higher probability to win conflicts against males who are not in their prime.
We studied a natural population of mandrills living in a private park located in Southern Gabon. The population was established in 2002 after the release of 36 captive individuals initially housed in CIRMF (Centre International de Recherches Médicales de Franceville, Gabon). Another 29 individuals were released in 2006 (Peignot et al., 2008; Charpentier et al., 2020). Wild male mandrills were observed to join the group to reproduce, starting in 2003. A field research project (Mandrillus Project) was set-up in early 2012 to monitor the ecology, life-history and behavior of the population. Only 6 adult females out of 230 individuals (from which 79 were adult females) of the group were captive-born in late 2021. All the individuals of the population are individually recognized and daily censused.
We used behavioral, demographic and life-history data collected from April 2013 to September 2021 on 93 adult females (aged 4 years and older) and 35 subadult and adult males (aged 9 years and older). We included subadult males (aged 9–10 years) because males at this stage are fully-grown and have usually entered the male-male competition and started mating with females (Setchell and Dixson, 2002; Setchell et al., 2005b). When the exact birth date was not known or approximated to a few days, we estimated it using body condition and patterns of tooth eruption and wear (Galbany et al., 2014).
We divided the study period in 6-month time blocks roughly equating to the mating (April-September) and birth (October-March) seasons (Dezeure et al., 2022). When an individual turned adult during a season (6-month time block), we included it for the whole season.
We calculated a monthly group sex ratio (SR) as the number of adult females present in the group divided by the number of subadult and adult males that were censused in the group that month for at least a day. We also calculated the mean SR of each 6-month time block. Because the number of males largely varies between the mating vs. the birth season, season (birth vs. mating) and group sex-ratio are correlated (Spearman's rank correlation: rho = 0.59, p = 0.02) and we thus used these two effects in alternative models.
Behavioral observations were made by observers blind to the focus of this study. We considered only dyadic interactions between adult females and adult or subadult males. Ad libitum observations and 5-min focal sampling (Altmann, 1974) were performed daily by recording, inter alia, affiliative and agonistic interactions between group members. In this study, we used four submissive behaviors: (i) “avoidance”: when an individual A walks away from an individual B who is approaching, (ii) “displacement”: when A walks away from B and B takes the place of A, (iii) “escape”: when A flees away from B who expresses aggression and (iv) “submissive vocalization”: when A emits a typical submissive vocalization after B expresses aggression. We calculated intra- and intersexual hierarchies based on two different datasets: a “full dataset” that comprised all submissive interactions (which were preceded by an aggressive event, or not) and a “ritualized dataset” that included only submissive interactions that were not preceded by an aggressive event. These two datasets allowed to test for potential discrepancies in dominance hierarchies due to methodological differences. We, however, did not use “undecided” aggressive interactions, i.e., those that were not followed by a submissive event, and which may not capture intersexual dominance relationships accurately.
We considered a total of 2,768 h (40,678 focal observations) of focal data in addition to ad libitum observations. For the analyses based on the full dataset, we used interactions from both focal and ad libitum observations to increase our sample size. In the ritualized dataset, we used only interactions from focal observations to filter out submissive behavior that followed an aggressive event. In the linear regression models performed below, we used only the full dataset given the similarity of the results based on these two different datasets (see results). For the analysis of intersexual conflicts, we selected from the full dataset all the dyadic interactions between an adult female and an adult male, recorded during focal observations, for which the outcome was unambiguous, i.e., when only one of the two individuals exhibited an aggression (mild threats were not included) followed by a submission from the other individual.
We used the functions DS and ISIranks from the R package EloRating (Neumann et al., 2011) to compute the intersexual hierarchies with both normalized David's score (David, 1987) and I&SI (de Vries, 1998) to evaluate whether these two rank estimates yielded different results suggesting potential methodological biases. David's score is calculated for each individual based on the observed dyadic proportions of wins. Namely, the number of dyadic wins (where the opponent submits to the focal individual) is divided by the sum of dyadic wins and losses (where the focal individual submits to the opponent) over other groupmates (for a formal definition, see David, 1987). As such, the difference among two individuals' scores is more informative than the difference between their ordinal ranks, and reflects the extent of asymmetry in dominance-based power between these individuals. We used normalized David's scores because they correct for the possibility that the observed outcomes occur by chance. Such a possibility is calculated on the basis of a binomial distribution with each animal having an equal chance of winning or losing each agonistic interaction (de Vries et al., 2006). This correction is crucial when the number of interactions greatly differs among dyads, like in our study group. However, David's score may be sensitive to missing data (non-interacting dyads; Neumann et al., 2011). Indeed, in our dataset, almost two out of three (62 ± 15%; ±SD) intersexual dyads never interacted agonistically, on average, while this figure was 32 ± 17 and 71 ± 10%, respectively, in male-male and female-female dyads (Supplementary Table S1). Consequently, intrasexual agonistic interactions may be highly influential in inference of intersexual hierarchies. To evaluate this possibility, we built intersexual hierarchies based on (1) intra- and intersexual agonistic interactions, and (2) intersexual interactions only. Second, we also used I&SI, another dominance index that generates the most parsimonious ordinal rank (the ordinal rank that deviates the least from linearity) based on multiple randomizations (N = 500) that re-order individual ranks from an interaction matrix (de Vries, 1998). Due to this iterative process, the resulting order may include more than one “solution” in the form of several equally likely rank orders. Whenever needed, we averaged the rank of each individual across these equally likely solutions (as per Kappeler et al., 2022). Finally, due to the randomization process, whenever we re-ran the algorithm, the resulting hierarchy could be slightly different (Supplementary Figure S1B). Due to the high level of correlation between David's scores and I&SI within (see results) and across species (Kappeler et al., 2022) and the greater stability of hierarchies based on David's scores (Sánchez-Tójar et al., 2018), we used normalized David's scores (David, 1987) for downstream analyses. In order to examine the robustness of the resulting intersexual hierarchy, we also used two randomization tests which are described in the Supplementary Figure S1. The number of individuals, the interactions among them and the percentage of interacting dyads (over all the possible dyads) are also shown in Supplementary Table S1.
During focal samplings, grooming events and their duration were recorded. For each 6-month time block, we used the total time of grooming recorded among females to create a female-only grooming directed network (function graph_from_data_frame from the package igraph; Csardi and Nepusz, 2006). We calculated the in-degree of each female from the above networks, as a proxy of social integration and support. We used only the number of females grooming (and not groomed by) a female, because we consider these individuals more likely to offer their support during agonistic interactions. Similarly, we calculated the total (in and out) degree of each male in networks including only intersexual grooming interactions (number of females grooming or being groomed by each male). For males, we considered all (given and received) grooming interactions in order to capture better male integration with the females of the group, rather than female support to males.
The reproductive state of each adult female was determined on a near-daily basis based on sexual swelling size (scaled from 0 to 3 by increments of 0.5) and patterns of gestation and lactation. During an estrous cycle of a female mandrill, the perineal swelling inflates for some days and reaches maximal swelling size around ovulation where it remains maximal for a few days before deflating. Each female was classified as: “non-swollen” (i.e., in the non-fertile phase of the cycle that does not fall within the following three categories), “swollen” (i.e., exhibiting an inflating or maximal perineal sexual swelling), “pregnant” (i.e., exhibiting a characteristic pregnancy swelling and/or if the female gave birth less than 163–190 days after a given day; average gestation length: mean ±SD: 175.0 ± 4.7 days; Dezeure et al., 2022) or “lactating” (i.e., nursing a ≤6 month-old infant, without having resumed cycling).
First, at the level of the population, we ran a Spearman's rank correlation test to study whether the intersexual hierarchy differed when using either David's score or I&SI. We then compared the percentage of males dominated by an average female in the two datasets (including all submissive interactions vs. only ritualized submissive interactions) using a Spearman's rank correlation test.
Second, at the dyad level, we ran a generalized linear model (GLMM) with a binomial distribution and a logit function to test whether the probability of a given female to outrank a given male was influenced by the following fixed factors: female and male intrasexual ranks and ages, female's in-degree in the female-only social network and its corresponding quadratic term (suggested following a graphical exploration of the data), and male's total (in and out) degree in the social network including only intersexual grooming interactions and either season or sex ratio. Female, male and dyad identity and the year were fitted as random factors.
Third, at the interaction (conflict) level, we ran a GLMM with a binomial distribution and a logit function to test whether the probability to unambiguously (only one of the two individuals exhibits aggression followed by a submission from the other individual) win an intersexual conflict (1/0; response variable) for a given female was influenced by the following fixed factors: the rank difference between opponents (male and female David's scores in the intersexual hierarchy), the age of the female, the age of the male and the corresponding quadratic term, and female's reproductive state. The female, male and dyad identity and the year were fitted as random factors.
We ran the above tests and models in R version 4.0.3 with the functions cor.test of the package stats and glmmTMB from the package glmmTMB (Magnusson et al., 2017). We used the Anova function of the package car (Fox et al., 2009) to test the significance of all fixed factors and we computed their 95% confidence intervals. We used the performed correlation tests to detect potential multicollinearities and we validated the performed models using the package DHARMa (Hartig and Lohse, 2020).
Female mandrills elicited male submissive behaviors in 2.4 ± 2.1% (±SD) of intersexual agonistic interactions. However, we found that females can outrank males, with a female outranking, on average, 11.3 ± 6.2 % (±SD) of males (results based on the full dataset and David's score or 18.2 ± 8.1% based on I&SI). We found a positive correlation between David's score and I&SI metrics across 6-month time blocks (Spearman's rank correlation, rho = 0.53, p = 0.03; see also Kappeler et al., 2022). When we calculated the hierarchy 500 times using David's score, each time randomly selecting 50% of all agonistic interactions, an average female outranked 12.1 ± 0.8% (mean ± SD) of males (Supplementary Figure S1A). When we calculated the hierarchy 500 times using I&SI, we found that no iteration resulted in an intersexual hierarchy where all females are outranked by all males and an average female outranked 16.1 ± 1.5% (mean ± SD) of males (Supplementary Figure S1B) across all iterations.
When we calculated the intersexual hierarchy using only intersexual agonistic interactions, the percentage of males outranked by an average female was similar to the percentage resulting from the calculation of the hierarchy with both intra- and intersexual agonistic interactions (11.5 ± 6.0 %; results based on David's score). Additionally, we found a strong positive correlation between the average number of males dominated by a female when using all submissive interactions and when using only ritualized submissive interactions (Spearman's rank correlation, rho = 0.72, p = 0.001 when we used David's score). When we used only ritualized submissive interactions to build the intersexual hierarchy, a female appeared to outrank, on average, 9.3 ± 5.1% (±SD; results based on David's score; see previous paragraph for the results based on the full dataset) of males.
Despite these congruent hierarchies resulting from various metrics and datasets, the discrepancy between the percentage of intersexual interactions where a male showed submission toward a female and the percentage of males dominated by an average female appears puzzling. In addition, in 92.7 ± 6.0% of female-dominant intersexual dyads, the two individuals never interacted agonistically, while in male-dominant dyads the corresponding percentage was 60.3 ± 14.4%. From a total of 5,433 intersexual dyads, only 1,844 interacted agonistically at least once, including 1,805 male-dominant dyads and only 39 female-dominant dyads. In these 39 dyads, males showed submission, on average, in 18.8 ± 3.8% of the interactions while in male-dominant dyads, males showed submission in 2.3 ± 12.9% of the interactions.
A given female had a significantly higher probability to outrank a male when she was high-ranking and when he was low-ranking, in comparison to any other combination of rank-sex class (Figure 1A and Table 1). The probability for a female to outrank a male was significantly higher when male degree (number of female grooming partners) was lower (Figure 1B) and female in-degree (number of female partners grooming her) was higher although this relationship was not linear (Figure 1B and Table 1), suggesting that females need at least a certain number of female partners in order to have higher chances to outrank a male. In addition, a female had a higher probability to outrank a male during the mating (than birth) season (Table 1) or when the group sex ratio was male-biased (i.e., when there were more males in relation to females in the group; Chisq = 18.687, p < 0.001). Female age was not significant but a female had a higher probability to outrank younger and older males than males in their prime (Figure 2 and Table 1).
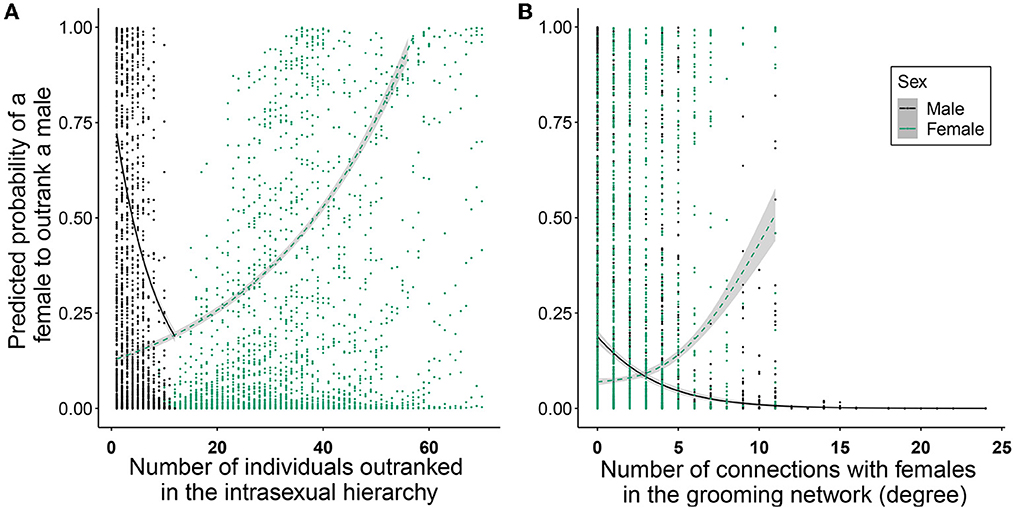
Figure 1. Factors influencing the probability for a female to outrank a male as a function of the (A) female (green-dashed line) and male (black) intrasexual rank (number of individuals outranked) and (B) female (green-dashed line) and male (black) number of connections (degree for males and in-degree for females) in the grooming networks. The fitted values of the GLMM are shown on the y-axis and shaded areas show 95% confidence intervals.
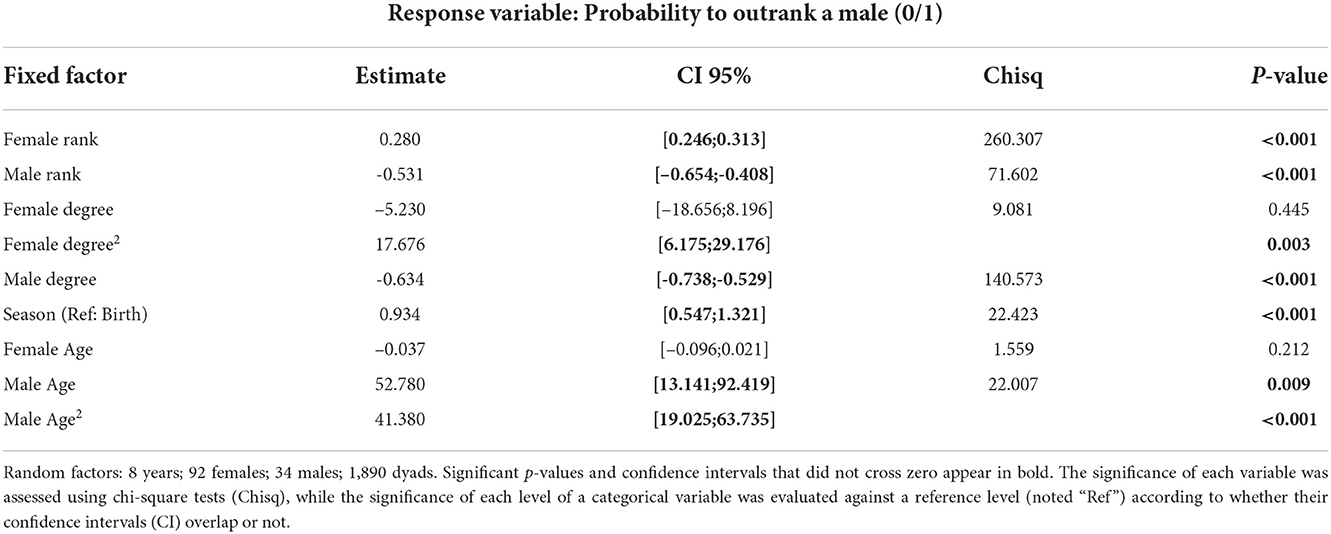
Table 1. Factors affecting the probability for a female to outrank a male (Number of observations: 5,433 dyad.seasons).
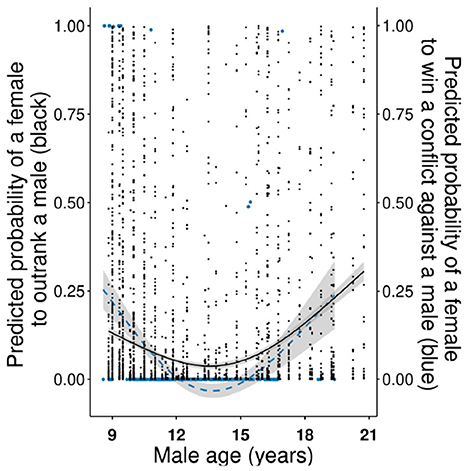
Figure 2. Male age in relation to the probability for a female to outrank a male (black) and win a conflict against a male (blue-dashed line). The fitted values of the GLMMs are shown on the y-axes and shaded areas show 95% confidence intervals. For graphical purposes the quadratic term of male age is shown, although its effect on the probability to win an intersexual conflict was marginally non-significant (p = 0.093).
The probability for a given female to win a conflict (only 11 out of 382 intersexual conflicts were won by females) against a given male was higher for younger males than for males in their prime and tended to increase again when the male was older (marginally non-significant effect of the quadratic term; Figure 2 and Table 2). In addition, a female tended to win more intersexual conflicts when the rank difference of the heterosexual dyad in the intersexual hierarchy was small (marginally non-significant effect; Table 2). Female age and reproductive state did not influence the results.
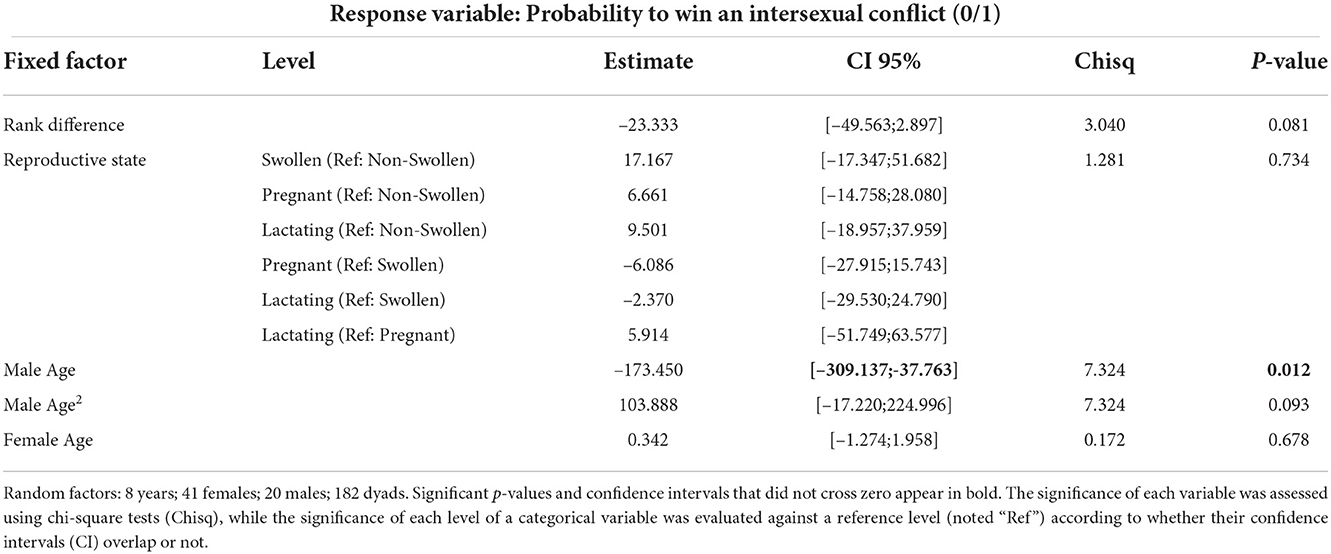
Table 2. Factors affecting the probability for a female to win a conflict against a male (Number of intersexual conflicts: 382).
In this study, we find that adult female mandrills can occasionally elicit male submissive behaviors, and can outrank males, despite being much smaller and traditionally considered strictly dominated by males. We further find that the intersexual hierarchy represents an interdigitation of the female and male intrasexual hierarchies, is not sensitive to the behavioral dataset used for its construction, and fluctuates with sociodemographic variables such as breeding seasonality and group-sex-ratio. Below, we first discuss the biological relevance of our hierarchical inferences, before envisaging potential factors affecting the dynamics of female-male dominance relationships.
We used various methods, including David's score (David, 1987) and I&SI (de Vries, 1998), and different datasets to establish intersexual hierarchies. The average percentage of males outranked by an average female revolved around 9–16% independently of the dataset or method used to infer hierarchies. However, such a result is at odds with the percentage of agonistic interactions where females elicited male submissive behaviors, which is closer to 2% overall. Such discrepancy may reflect methodological problems or biological processes potentially specific to our study system, or a combination both; although similar discrepancies have also been reported in strictly female-dominant species, like Verreaux sifakas where females win the minority of intersexual agonistic interactions suggesting that females can indeed outrank males though they lose most fights against them (Lewis et al., 2022). Regarding methodological problems, mandrills live in exceptionally large groups and in dense forests with low visibility, making it difficult to observe interactions between all group members. Additionally, intersexual aggression is characterized by relatively low severity (Smit et al., 2022) and thus it might be easily overlooked outside focal observations. As a result, a majority (62%) of the intersexual dyads in our study group were never observed interacting agonistically (Supplementary Table S1), and such proportion is highest (92%) in those dyads where the female was found to outrank the male. This may have generated instability in the inferred hierarchies, and questions our finding that females can actually outrank some males. Specifically, establishing the hierarchy between two individuals A and B from a large group relies on direct interactions between A and B, but also on indirect interactions opposing A and B to other groupmates. When there are many missing cells in interaction matrices, indirect interactions may weigh more than direct ones in hierarchical inferences, which may generate a situation where the rank order between two individuals does not reflect the outcome of the few interactions recorded between them.
Some biological aspects of our study system may accentuate these methodological challenges. First, the seasonal changes affecting the demography of the group, with many males entering the group at the onset of the mating season and leaving afterwards (Abernethy et al., 2002; Brockmeyer et al., 2015), necessarily generates high instability in the male as well as the intersexual hierarchy. It is likely that there are frequent rank reversals, at least in the first half of the season, and some immigrants may remain peripheral in the days following their arrival, time to assess the social landscape. They may minimize interactions, during this period, with both male and female groupmates, which may be the typical—but transient—period where they occasionally show submission toward females. While alternative methods, like Elo-rating (Neumann et al., 2011), for calculating dominance hierarchies would, in theory, be better suited to establish hierarchies in such a system, they require highly resolved interaction matrices which are far too challenging to obtain in such large groups. Finally, the frequent use of sexual coercion by high-ranking male mandrills (Smit et al., 2022) may also result in asymmetrical patterns of interactions between male-dominant and female-dominant dyads, and explain why there are lower rates of interactions—and more missing data—in female-dominant than male-dominant dyads. While males may often direct aggression to subordinate females in male-dominant dyads in a context of sexual coercion (Smit et al., 2022), females may not bother about harassing those males they outrank. Indeed, male-dominant dyads interact agonistically over twice as often as female-dominant ones (3.3 ± 4.8 vs. 1.6 ± 1.1 times; ±SD). Altogether, these results highlight the caution needed when interpreting the biological relevance of hierarchies emerging from datasets with high number of non-interacting dyads and in our case, female mandrills may occasionally outrank males but potentially in a lower frequency than our results indicate.
Nevertheless, our results show that females can occasionally elicit male submissive behaviors and suggest that strict male dominance is unlikely in this species. Despite the apparently low predictive power of hierarchical inferences at the dyadic level, the temporal fluctuations of the average percentage of males outranked by females may still reflect genuine changes in the temporal dynamics of intersexual dominance. In line with this, we find that females outrank more males during the mating than during the birth season. Such seasonal changes may be related to variation in individual reproductive states and associated needs (Murie and Harris, 1988; Jawor, 2000), motivation or leverage (Lewis, 2002, 2018; Davidian et al., 2022). In particular, when females have some reproductive control (i.e., control over when and with whom to mate), as in most lemurs (Hohenbrink et al., 2016; Lewis et al., 2022), they typically have more leverage when sexually receptive because males who try to mate with them may avoid to aggress them (Lewis, 2002; Davidian et al., 2022). Yet, additional results show that sexually receptive female mandrills are not more likely to win conflicts against males compared to females in other reproductive states, possibly because they have low reproductive control due to frequent sexual coercion (Smit et al., 2022). Instead, this result may reflect demographic changes due to the influx of male mandrills in the social group at the beginning of that season. An increased number of males may lead to frequent male-male fights, with some males falling below some females at the bottom of the hierarchy, a so-called “winner-loser” effect which is known to affect intersexual dominance in other species (Hemelrijk et al., 2008, 2020).
Alternatively, temporal changes in intersexual dominance may reflect deeper changes in the social dynamics of mandrill groups across seasons. During the mating season, when males are more numerous, they may adopt alternative reproductive tactics. High-ranking resident male mandrills may compete to mate-guard ovulatory females, while low-ranking immigrants may remain transient and peripheral and try to get sneaky matings (similarly to rock hyraxes Bar Ziv et al., 2016) without establishing clear dominance relationships with females. Our result that less socially integrated males are less dominant over females, and that females preferentially outrank young and old males support this interpretation. Overall, those males who take an active part to the social dynamics of the group may simultaneously rise in rank, while females may only outrank those males who may lack the confidence or motivation to confront females or rivals, as may occur in other mammals (Van Schaik and Paul, 1996; Mysterud et al., 2003; Silk et al., 2020). Finally, female mandrills outrank more males when they are more socially integrated in the female social networks, which may reflect males' reluctance to confront well-connected females who may support each other against males (Setchell et al., 2006a). Such coalitions have also been observed in other primates living in polygynandrous groups where females are philopatric (geladas: Dunbar, 1975; Guinea baboons (Papio papio): Goffe et al., 2016) and may often contribute to counter-balance male-biased dominance in species where physical asymmetries between sexes are extensive.
This study contributes to a growing body of evidence that draws a more dynamic landscape of female-male dominance relationships, where intersexual dominance can fluctuate across time and contexts. Our results suggest that females may outrank a small proportion of males in a highly dimorphic nonhuman primate, although this remains to be confirmed due to limits in the resolution of our datasets. We further found that females outrank more males during the mating season and when they are high-ranking and more socially integrated; while they preferentially outrank poorly socially integrated males with low competitive abilities. These results point to the importance of social integration and seasonal breeding, and of associated demographic and motivational shifts in males and females, to explain the dynamics of intersexual dominance, and contribute to a new area aimed at understanding the dynamics of female-male power struggles at an individual scale.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GitLab https://gitlab.com/nksmt/mandrills2.
The animal study was reviewed and approved by CENAREST Institute (permit number, AR0060/18/MESRS/CENAREST/CG/CST/CSAR).
NS, EH, and MC designed the study and contributed to writing the manuscript. NS performed the statistical analyses and wrote the first version of the manuscript. MC, NS, and BN contributed to data collection and database management. All authors contributed to the article and approved the submitted version.
This study was funded by several grants that allowed long-term collection of data: Deutsche Forschungsgemeinschaft (DFG, KA 1082-20-1), SEEG Lékédi (INEE-CNRS) and Agence Nationale de la Recherche (ANR SLEEP 17-CE02-0002 to MC; ANR ERS-17-CE02-0008378 to EH) and State Scholarships Foundation (IKY) scholarship program from the proceeds of the Nic. D. Chrysovergis bequest to NS.
We thank (i) all past and present field assistants who collect daily behavioral and physiological data on the study population of the Mandrillus Project, (ii) Patrícia Izar and two reviewers and (iii) the SODEPAL-COMILOG society for their logistical support. This is a Project Mandrillus publication number 29 and ISEM 2022-301 SUD.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.931226/full#supplementary-material
Abernethy, K. A., White, L. J. T., and Wickings, E. J. (2002). Hordes of mandrills (Mandrillus sphinx): extreme group size and seasonal male presence. J. Zool. 258, 131–137. doi: 10.1017/S0952836902001267
Altmann, J. (1974). Observational study of behavior: sampling methods. Behaviour 49, 227–266. doi: 10.1163/156853974X00534
Bar Ziv, E., Ilany, A., Demartsev, V., Barocas, A., Geffen, E., and Koren, L. (2016). Individual, social, and sexual niche traits affect copulation success in a polygynandrous mating system. Behav. Ecol. Sociobiol. 70, 901–912. doi: 10.1007/s00265-016-2112-4
Bissonnette, A., de Vries, H., and van Schaik, C. P. (2009). Coalitions in male barbary macaques, macaca sylvanus: strength, success and rules of thumb. Anim. Behav. 78, 329–335. doi: 10.1016/j.anbehav.2009.05.010
Bonabeau, E. (1999). Dominance orders in animal societies: the self-organization hypothesis revisited. Bull. Math. Biol. 61, 727–757. doi: 10.1006/bulm.1999.0108
Brockmeyer, T., Kappeler, P. M., Willaume, E., Benoit, L., Mboumba, S., and Charpentier, M. J. (2015). Social organization and space use of a wild mandrill (Mandrillus sphinx) group: mandrill social organization and space use. Am. J. Primatol. 77, 1036–1048. doi: 10.1002/ajp.22439
Charpentier, M., Peignot, P., Hossaert-McKey, M., Gimenez, O., Setchell, J. M., and Wickings, E. J. (2005). Constraints on control: Factors influencing reproductive success in male mandrills (Mandrillus sphinx). Behav. Ecol. 16, 614–623. doi: 10.1093/beheco/ari034
Charpentier, M. J. E., Harté, M., Poirotte, C., de Bellefon, J. M., Laubi, B., Kappeler, P. M., et al. (2020). Same father, same face: deep learning reveals selection for signaling kinship in a wild primate. Sci. Adv. 6, eaba3274. doi: 10.1126/sciadv.aba3274
Chase, I. D., Tovey, C., Spangler-Martin, D., and Manfredonia, M. (2002). Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl. Acad. Sci. U.S.A. 99, 5744–5749. doi: 10.1073/pnas.082104199
Clutton-Brock, T., and Huchard, E. (2013a). Social competition and its consequences in female mammals: female reproductive competition in mammals. J. Zool. 289, 151–171. doi: 10.1111/jzo.12023
Clutton-Brock, T. H., and Huchard, E. (2013b). Social competition and selection in males and females. Philos. Trans. R. Soc. B Biol. Sci. 368, 20130074. doi: 10.1098/rstb.2013.0074
Csardi, G., and Nepusz, T. (2006). The igraph software package for complex network research. Inter. J. Compl. Syst. 1695, 1–9.
David, H. A. (1987). Ranking from unbalanced paired-comparison data. Biometrika 74, 432–436. doi: 10.1093/biomet/74.2.432
Davidian, E., Surbeck, M., Lukas, D., Kappeler, P. M., and Huchard, E. (2022). The eco-evolutionary landscape of power relationships between males and females. Trends Ecol. Evolut. 37, 706–718. doi: 10.1016/j.tree.2022.04.004
de Vries, H. (1998). Finding a dominance order most consistent with a linear hierarchy: a new procedure and review. Anim. Behav. 55, 827–843. doi: 10.1006/anbe.1997.0708
de Vries, H., Stevens, J. M., and Vervaecke, H. (2006). Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592. doi: 10.1016/j.anbehav.2005.05.015
Dezeure, J., Charpentier, M. J., and Huchard, E. (2022). Fitness effects of seasonal birth timing in a long-lived social primate living in the equatorial forest. Anim. Behav. 185, 113–126. doi: 10.1016/j.anbehav.2022.01.002
Dunham, A. E. (2008). Battle of the sexes: cost asymmetry explains female dominance in lemurs. Anim. Behav. 76, 1435–1439. doi: 10.1016/j.anbehav.2008.06.018
Ellis, L. (1995). Dominance and reproductive success among nonhuman animals: a cross-species comparison. Ethol. Sociobiol. 16, 257–333. doi: 10.1016/0162-3095(95)00050-U
Ferrari, S. F. (2009). “Social hierarchy and dispersal in free-ranging buffy-headed marmosets (Callithrix flaviceps),” in The Smallest Anthropoids, eds S. M. Ford, L. M. Porter, and L. C. Davis (Boston, MA: Springer US), 155–165.
Fox, J. (2009). Car: Companion to Applied Regression (R Package Version 1.2-14). Available online at: http://cran.r-project.org/web/packages/car/
French, J. A., Mustoe, A. C., Cavanaugh, J., and Birnie, A. K. (2013). The influence of androgenic steroid hormones on female aggression in ‘atypical' mammals. Philos. Trans. R. Soc. B Biol. Sci. 368, 20130084. doi: 10.1098/rstb.2013.0084
Galbany, J., Romero, A., Mayo-Alesón, M., Itsoma, F., Gamarra, B., Pérez-Pérez, A., et al. (2014). Age-related tooth wear differs between forest and savanna primates. PLoS ONE 9, e94938. doi: 10.1371/journal.pone.0094938
Goffe, A. S., Zinner, D., and Fischer, J. (2016). Sex and friendship in a multilevel society: behavioural patterns and associations between female and male guinea baboons. Behav. Ecol. Sociobiol. 70, 323–336. doi: 10.1007/s00265-015-2050-6
Greenwood, P. J. (1980). Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. doi: 10.1016/S0003-3472(80)80103-5
Gumert, M. D. (2007). Payment for sex in a macaque mating market. Anim. Behav. 74, 1655–1667. doi: 10.1016/j.anbehav.2007.03.009
Hand, J. L. (1986). Resolution of social conflicts: dominance, egalitarianism, spheres of dominance, and game theory. Q. Rev. Biol. 61, 201–220. doi: 10.1086/414899
Hartig, F. (2020). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.3, Vol. 3.
Hemelrijk, C. K., Wantia, J., and Isler, K. (2008). Female dominance over males in primates: self-organisation and sexual dimorphism. PLoS ONE 3, e2678. doi: 10.1371/journal.pone.0002678
Hemelrijk, C. K., Wubs, M., Gort, G., Botting, J., and van de Waal, E. (2020). Dynamics of intersexual dominance and adult sex- ratio in wild vervet monkeys. Front. Psychol. 11, 839. doi: 10.3389/fpsyg.2020.00839
Hewitt, S. E., Macdonald, D. W., and Dugdale, H. L. (2009). Context-dependent linear dominance hierarchies in social groups of European badgers, Meles meles. Anim. Behav. 77, 161–169. doi: 10.1016/j.anbehav.2008.09.022
Hohenbrink, S., Schaarschmidt, F., Bünemann, K., Gerberding, S., Zimmermann, E., and Radespiel, U. (2016). Female dominance in two basal primates, Microcebus murinus and Microcebus lehilahytsara: Variation and determinants. Anim. Behav. 122, 145–156. doi: 10.1016/j.anbehav.2016.10.008
Izar, P., Fernández-Bola nos, M., Seex, L., Gort, G., Suscke, P., Tokuda, M., et al. (2021). Female emancipation in a male dominant, sexually dimorphic primate under natural conditions. PLoS ONE 16, e0249039. doi: 10.1371/journal.pone.0249039
Jawor, J. M. (2000). Female dominance and aggressive behaviors in house sparrow flocks. Auk 117, 4. doi: 10.1093/auk/117.3.799
Kappeler, P. M. (1993). Female dominance in primates and other mammals. Perspect. Ethol. 10, 143–158.
Kappeler, P. M., and Fichtel, C. (2015). Eco-evo-devo of the lemur syndrome: did adaptive behavioral plasticity get canalized in a large primate radiation? Front. Zool. 12(Suppl. 1), S15. doi: 10.1186/1742-9994-12-S1-S15
Kappeler, P. M., Huchard, E., Baniel, A., Canteloup, C., Charpentier, M. J. E., Cheng, L., et al. (2022). Sex and dominance: how to assess and interpret intersexual dominance relationships in mammalian societies. Front. Ecol. Evolut. 10, 918773. doi: 10.3389/fevo.2022.918773
Koren, L., Mokady, O., and Geffen, E. (2006). Elevated testosterone levels and social ranks in female rock hyrax. Horm. Behav. 49, 470–477. doi: 10.1016/j.yhbeh.2005.10.004
Leigh, S. R., Setchell, J. M., Charpentier, M., Knapp, L. A., and Wickings, E. J. (2008). Canine tooth size and fitness in male mandrills (Mandrillus sphinx). J. Hum. Evol. 55, 75–85. doi: 10.1016/j.jhevol.2008.01.001
Lewis, R., Bueno, G. L., and Di Fiore, A. (2022). Variation in female leverage: the influence of kinship and market effects on the extent of female power over males in Verreaux's Sifaka. Front. Ecol. Evolut. 10, 851880. doi: 10.3389/fevo.2022.851880
Lewis, R. J. (2002). Beyond dominance: the importance of leverage. Q. Rev. Biol. 77, 149–164. doi: 10.1086/343899
Lewis, R. J. (2018). Female power in primates and the phenomenon of female dominance. Annu. Rev. Anthropol. 47, 533–551. doi: 10.1146/annurev-anthro-102317-045958
Lewis, R. J. (2020). Female power: a new framework for understanding “female dominance” in lemurs. Folia Primatol. 91, 48–68. doi: 10.1159/000500443
Magnusson, A., Skaug, H., Nielsen, A., Berg, C., Kristensen, K., Maechler, M., et al. (2017). Package ‘glmmtmb'. R Package Version 0.2.0.
Markham, A. C., Lonsdorf, E. V., Pusey, A. E., and Murray, C. M. (2015). Maternal rank influences the outcome of aggressive interactions between immature chimpanzees. Anim. Behav. 100, 192–198. doi: 10.1016/j.anbehav.2014.12.003
Murie, J. O., and Harris, M. A. (1988). Social interactions and dominance relationships between female and male Columbian ground squirrels. Can. J. Zool. 66, 1414–1420. doi: 10.1139/z88-207
Mysterud, A., Holand, Ø., Røed, K. H., Gjøstein, H., Kumpula, J., and Nieminen, M. (2003). Effects of age, density and sex ratio on reproductive effort in male reindeer (Rangifer tarandus). J. Zool. 261, 341–344. doi: 10.1017/S0952836903004114
Neumann, C., Duboscq, J., Dubuc, C., Ginting, A., Irwan, A. M., Agil, M., et al. (2011). Assessing dominance hierarchies: validation and advantages of progressive evaluation with Elo-rating. Anim. Behav. 82, 911–921. doi: 10.1016/j.anbehav.2011.07.016
Noë, R., and Hammerstein, P. (1994). Biological markets: supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behav. Ecol. Sociobiol. 35, 1–11. doi: 10.1007/BF00167053
Parish, A. R., De Waal, F. B. M., and Haig, D. (2000). The other “Closest Living Relative”: how bonobos (Pan paniscus) challenge traditional assumptions about females, dominance, intra- and intersexual interactions, and hominid evolution. Ann. N. Y. Acad. Sci. 907, 97–113. doi: 10.1111/j.1749-6632.2000.tb06618.x
Parker, G. (2006). Sexual conflict over mating and fertilization: an overview. Philos. Trans. R. Soc. B Biol. Sci. 361, 235–259. doi: 10.1098/rstb.2005.1785
Peignot, P., Charpentier, M. J., Bout, N., Bourry, O., Massima, U., Dosimont, O., et al. (2008). Learning from the first release project of captive-bred mandrills Mandrillus sphinx in Gabon. Oryx 42, 136. doi: 10.1017/S0030605308000136
Petty, J. M. A., and Drea, C. M. (2015). Female rule in lemurs is ancestral and hormonally mediated. Sci. Rep. 5, 9631. doi: 10.1038/srep09631
Sánchez-Tójar, A., Schroeder, J., and Farine, D. R. (2018). A practical guide for inferring reliable dominance hierarchies and estimating their uncertainty. J. Anim. Ecol. 87, 594–608. doi: 10.1111/1365-2656.12776
Setchell, J. M. (2016). Sexual Selection and the differences between the sexes in Mandrills (Mandrillus sphinx). Am. J. Phys. Anthropol. 159, 105–129. doi: 10.1002/ajpa.22904
Setchell, J. M., Charpentier, M., and Wickings, E. J. (2005a). Mate guarding and paternity in mandrills: factors influencing alpha male monopoly. Anim. Behav. 70, 1105–1120. doi: 10.1016/j.anbehav.2005.02.021
Setchell, J. M., Charpentier, M., and Wickings, E. J. (2005b). Sexual selection and reproductive careers in mandrills (Mandrillus sphinx). Behav. Ecol. Sociobiol. 58, 474–485. doi: 10.1007/s00265-005-0946-2
Setchell, J. M., and Dixson, A. F. (2002). Developmental variables and dominance rank in adolescent male mandrills (Mandrillus sphinx). Am. J. Primatol. 56, 9–25. doi: 10.1002/ajp.1060
Setchell, J. M., Knapp, L. A., and Wickings, E. J. (2006a). Violent coalitionary attack by female mandrills against an injured alpha male. Am. J. Primatol. 68, 411–418. doi: 10.1002/ajp.20234
Setchell, J. M., Lee, P. C., Wickings, E. J., and Dixson, A. F. (2001). Growth and ontogeny of sexual size dimorphism in the mandrill (Mandrillus sphinx). Am. J. Phys. Anthropol. 115, 349–360. doi: 10.1002/ajpa.1091
Setchell, J. M., Lee, P. C., Wickings, E. J., and Dixson, A. F. (2002). Reproductive parameters and maternal investment in Mandrills (Mandrillus sphinx). Int. J. Primatol. 23, 51–68. doi: 10.1023/A:1013245707228
Setchell, J. M., Wickings, E. J., and Knapp, L. A. (2006b). Life history in male mandrills (Mandrillus sphinx): physical development, dominance rank, and group association. Am. J. Phys. Anthropol. 131, 498–510. doi: 10.1002/ajpa.20478
Silk, J. B., Städele, V., Roberts, E. K., Vigilant, L., and Strum, S. C. (2020). Shifts in male reproductive tactics over the life course in a polygynandrous mammal. Curr. Biol. 30, 1716.e3–1720.e3. doi: 10.1016/j.cub.2020.02.013
Smit, N., Baniel, A., Roura-Torres, B., Amblard-Rambert, P., Charpentier, M. J. E., and Huchard, E. (2022). Sexual coercion in a natural mandrill population. Peer Commun. J. 2:e36. doi: 10.24072/pcjournal.134
Smith, S. M. (1980). Henpecked males: the general pattern in monogamy? J. Field Ornithol. 51, 55–64.
Smith, S. M. (1982). Raptor "Reverse" dimorphism revisited: a new hypothesis. Oikos 39, 118. doi: 10.2307/3544542
Strauss, E. D., and Holekamp, K. E. (2019). Social alliances improve rank and fitness in convention-based societies. Proc. Natl. Acad. Sci. U.S.A. 116, 8919–8924. doi: 10.1073/pnas.1810384116
Tibbetts, E. A., Pardo-Sanchez, J., and Weise, C. (2022). The establishment and maintenance of dominance hierarchies. Philos. Trans. R. Soc. B Biol. Sci. 377, 20200450. doi: 10.1098/rstb.2020.0450
Van Schaik, C., and Paul, A. (1996). Male care in primates: does it ever reflect paternity? Evolut. Anthropol. 5, 152–156. doi: 10.1002/(SICI)1520-6505(1996)5:5<152::AID-EVAN3>3.0.CO;2-H
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., and Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nat. Ecol. Evolut. 3, 71–76. doi: 10.1038/s41559-018-0718-9
Weiß, B. M., and Kotrschal, K. (2004). Effects of passive social support in juvenile greylag Geese (Anser anser): a study from fledging to adulthood. Ethology 110, 429–444. doi: 10.1111/j.1439-0310.2004.00979.x
Keywords: intersexual dominance, hierarchy, agonistic interactions, social bonds, mandrills
Citation: Smit N, Ngoubangoye B, Charpentier MJE and Huchard E (2022) Dynamics of intersexual dominance in a highly dimorphic primate. Front. Ecol. Evol. 10:931226. doi: 10.3389/fevo.2022.931226
Received: 28 April 2022; Accepted: 23 November 2022;
Published: 09 December 2022.
Edited by:
Patricia Izar, University of São Paulo, BrazilReviewed by:
Nicolas Chaline, University of São Paulo, BrazilCopyright © 2022 Smit, Ngoubangoye, Charpentier and Huchard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolaos Smit, c25pa29zQHR1dGFub3RhLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.