
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 04 October 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.930266
This article is part of the Research Topic Sex and Gender Effects on Power, Status, Dominance, and Leadership – An Interdisciplinary Look at Human and Other Mammalian Societies View all 22 articles
Females dominate a subset of the males in a minority of mammalian species despite male-biased sexual dimorphism. How this may arise is suggested by a computational model, DomWorld. The model represents male-biased sexual dimorphism through the males’ greater initial dominance and higher intensity of aggression, meaning that fights initiated by males have a greater impact than those by females. The model shows that female dominance over males increases with a greater proportion of males in the group. This happens because when males are involved in a larger fraction of fights this results in greater hierarchical differentiation (i.e., steepness). This causes rank overlap between the sexes (i.e., partial female dominance). We test the validity of these processes in vervet monkeys (Cercopithecus pygerythrus), a primate species with partial female dominance. We confirm that the proportion of males in the group is significantly positively correlated with the degree of dominance by females over males and with the steepness of the hierarchy among males exclusively, but not with the steepness of the hierarchy among all adults of the group. The steepness in male hierarchies correlated positively with female dominance over males in these groups. We show that steeper hierarchies among vervet males resulted from male-to-male fights being a larger proportion of the fights among all adults of the group. We conclude that the higher frequency of male intrasexual aggression favors female dominance in vervet monkeys. We also show that females received coalitionary support when they were in conflict with a male, mainly from other females, and that this favors female dominance in this species, but this does not explain why partial female dominance increased with the proportion of males in the group. We advocate further investigation of the influence of male intrasexual aggression on the degree of female dominance over males in other species with partial female dominance.
Females are seldom dominant over males in competitive interactions in mammals (Holekamp and Engh, 2009). This is usually explained by males being larger than females and better armed (e.g., longer canines; Clutton-Brock, 2016), the prior attribute hypothesis (Chase et al., 2002). Indeed, in species in which females show complete dominance over males (e.g., spotted hyena, Crocutta crocutta: Tilson and Hamilton, 1984; several lemur species: Kappeler, 1993) sexual dimorphism is biased toward females (spotted hyena: Swanson et al., 2013), or non-significant (lemurs: Kappeler, 1990; rock hyrax, Procavia capensis: Koren et al., 2006). However, there are cases in which females—despite being smaller than males—show partial dominance over males (e.g., bonobos, Pan paniscus: Paoli et al., 2006; vervet monkeys, Chlorocebus pygerythrus: Struhskarer, 1967; Smuts, 1987; Hemelrijk et al., 2008; Young et al., 2017; capuchin monkeys, Sapajus spp.: Izar et al., 2021). The existence of ‘partial female dominance’ (Smuts, 1987) has been explained through social dynamics, such as coalitions of females against males (Smuts, 1987; White and Wood, 2007; Tokuyama and Furuichi, 2016), a reduction of aggression by males to females in exchange for sexual access named the docile male hypothesis (Surbeck and Hohmann, 2013), and frequent victimization of males by other males, so that they become low in rank via the self-reinforcing effects of winning and losing fights (the winner-loser effect), where a high intensity of aggression and a high proportion of males in the group lead to a larger degree of female dominance (Hemelrijk et al., 2008). The winner-loser effect implies that individuals are more likely to win a fight after winning a previous one, and vice versa for losing (Chase, 1982).
Female dominance over a subset of males was shown to emerge through the self-reinforcing effects of winning and losing fights in a computational model of dominance interactions in groups, based on self-organization, DomWorld (Hemelrijk, 1999). In the model, individuals start the simulation with an internal dominance value that determines their probability to win fights. Here, individuals with higher dominance values are more likely to win fights against individuals with lower dominance values. The winner-loser effect is reflected by the change in the dominance value of each individual after a fight, with the winner increasing its dominance value and the loser decreasing it. To reflect the sexual dimorphism of primates with males having a larger body size and better armament than females, males start in the simulation with a higher dominance value than females and their aggression is more intense. The initial dominance values of all individuals of the same sex are equal. The intensity of aggression influences the magnitude of the change of the dominance value of the two combatants after a fight. Fights started by a male result in greater change of dominance of both opponents than those started by a female, reflecting the higher intensity of aggression by males than females. We include more information about the equations underlying dominance interactions in DomWorld in the Supplementary material. In the model, partial female dominance over males develops despite females starting the simulation with lower dominance than males. It develops specifically when males were more intense in their aggression than females (the same pattern was not found for lower intensity of aggression by males), and more strongly the greater the proportion of males in the group (Hemelrijk et al., 2008). This happens because during the simulation, the dominance hierarchy of both sexes becomes steeper, i.e., the internal dominance values of each sex become more differentiated, when proportionally more males are present in the group, due to the higher intensity of aggression by males than females (Hemelrijk et al., 2008). Thus, in groups with a higher proportion of males, some males will drop down the hierarchy below some females because of the increased proportion of defeats from other males they have experienced, and some females will end up dominating some males without necessarily having ever fought against them (Hemelrijk et al., 2003). The winner-loser effect, on which the computational model is based, has been found across taxa (e.g., Hsu et al., 2009; Hirschenhauser et al., 2013; Kar et al., 2016), such as primates (Barchas and Menzosa, 1984; Eaton, 1984; Franz et al., 2015) including humans (Page and Coates, 2017). The self-reinforcing effect of winning or losing a single fight lasts up to 2 months in some species (Lan and Hsu, 2011).
So far, empirical support for the positive relationship between the degree of female dominance over males and the proportion of males in the group and has been found in (1) macaques; in a few groups of rhesus macaques, Macaca mulatta, and between several species of despotic macaques, Macaca spp. (Hemelrijk et al., 2008), (2) multiple groups of wild vervet monkeys (Hemelrijk et al., 2020), and (3) multiple groups of wild capuchin monkeys (Izar et al., 2021). Although these positive relationships support the predictions of the computational model DomWorld, we still need to investigate the dynamics proposed by the model to explain how this relationship arises in real life.
In line with the computational model DomWorld, we test whether (a) males are more intense in their aggression than females (necessary condition), (b) a larger proportion of males in the group leads to more fights in which males are involved (first prediction), (c) this leads to steeper hierarchies among all adults (second prediction), and (d) steeper hierarchies result in a larger proportion of males being subordinate to one or more females of high rank (i.e., partial female dominance, third prediction). We refer to this as the DomWorld Hypothesis.
Alternatively, a larger proportion of males in the group may result in stronger competition for mates among males due to the relatively lower availability of females. Therefore, males may become more intense in their aggression toward other males. Increased intra-sexual competition among males may result in a steeper hierarchy among males, but the steepness of the hierarchy among females would not be particularly influenced by the increased male competition and thus the hierarchy among all adults would be less affected. A steeper hierarchy among males would imply that males are sinking in the hierarchy below an increasing proportion of females, increasing the degree of partial female dominance over males in a similar way as proposed by the computational model, DomWorld. We refer to these processes as the Male Competition Hypothesis.
We investigate these hypotheses in wild vervet monkeys in an enlarged dataset (with two more years of behavioral observations) compared to the one used by Hemelrijk et al. (2020) where they showed that the degree of female dominance increased with the proportion of males in the group. Our dataset consists of 22 group-year data points (from four groups of vervet monkeys). Vervet monkeys are an ideal study species because they meet the requirements underlying the DomWorld hypothesis (Hemelrijk et al., 2020; Izar et al., 2021) by showing partial female dominance over males (Hemelrijk et al., 2020), male-biased sexual dimorphism (Turner et al., 2018), intense aggression (Cheney and Seyfarth, 1990), and a large range of sex ratios, due to frequent male dispersal between groups and years (Cheney and Seyfarth, 1990).
Furthermore, we explore the role of coalitions between females against males in causing the partial female dominance in wild vervet monkeys. They are thought to maintain partial dominance of females over males in bonobos, regardless of the species’ male-biased sexual dimorphism (Parish, 1996; Tokuyama and Furuichi, 2016) and help females win fights against larger males in several other species with male-biased sexual dimorphism, including vervet monkeys (reviewed in Smuts and Smuts, 1993). In vervet monkeys, Hemelrijk et al. (2020) showed that the proportion of intersexual fights in which a female received support from another female increased with the proportion of males in the group. This was considered to be a side effect of the higher rank of females in the group (thus lowering their risk when attacking) and was not considered as a potential reason why females are high ranking (Hemelrijk et al., 2020). Here, however, we argue that once females occupy higher positions in the hierarchy, female coalitions against males may further increase partial female dominance over males in those groups. Thus, a stronger tendency for females to form coalitions with other females against males in groups with proportionally more males may help explain why female dominance is higher in these groups. We refer to this hypothesis as the Female Coalition Hypothesis.
In vervet monkeys, the sexes are dimorphic with males on average 1.4 times the weight of females (Turner et al., 2018), more muscular, and with canines on average 1.3 times the length of those of females (Bolter and Zihlman, 2003). Males disperse from the natal group when they reach sexual maturity (around 4 to 5 years-old), and subsequently disperse multiple times in their lifespan (Cheney and Seyfarth, 1990). In the new group they fight to establish their place in the dominance hierarchy (Cheney and Seyfarth, 1990). Females are philopatric and inherit their rank based on the rank of their mother (i.e., matrilineal society, Cheney and Seyfarth, 1990).
Data were collected at the Inkawu Vervet Project (IVP) in the Mawana Game Reserve, South Africa, on four neighboring groups of wild vervet monkeys, named Ankhase, Baie Dankie, Kubu, and Noha. There is heterogeneity of vegetation both within the home range of each single group and between different groups, from areas of dense vegetation to areas with more sparse vegetation dominated by Acacia species. Data of social interactions were collected by ad libitum sampling (Altmann, 1974) from January 2011 to December 2019, after monkeys were habituated to human presence in ten meters vicinity in 2010. Researchers at the IVP are trained in collecting behavioral data and are required to pass a test for identifying all the monkeys they are collecting data on, as well as to perform periodical inter-observer tests ensuring that data collection is standardized among all researchers. Males were considered adult after emigrating from their natal group for the first time, and females after giving birth to their first offspring.
In our analyses we focused on adults. For being included in the analysis, individuals of both sexes had to be present in the group as adults for at least 6 months per year. For the DomWorld Hypothesis and the Male Competition Hypothesis, the analyses included only dyadic interactions, excluding interactions with support from a third party. For the Coalitionary Support Hypothesis, we also included agonistic interactions between adults receiving support from a juvenile or an adult. Regardless of the duration and complexity of the interaction, and regardless of eventual support from third parties, the individual that showed as its last behavior an act that was clearly aggressive (“stare,” “attack,” “grab,” “displacement,” “bite,” “hit,” “chase,” “aggressive call,” “steal food,” and “hand on head”) was noted as the winner and the individual showing a clearly submissive behavior as the loser of the interaction (“avoid,” “jump,” “crawl,” “leave,” “retreat,” “flee,” and “scream”) (Hemelrijk et al., 2020). In the following cases, the interactions were not used because they were not unequivocally defined. (1) An individual spontaneously showed submission to another or there was no reaction from the victim to an (attempted) aggression. (2) One or both individuals displayed a last behavior that was not clearly submissive or aggressive (e.g., “undetermined vocalization”). (3) The last behavior of both individuals was similar—both were aggressive or submissive. Note that supporters involved in triadic interactions to help one of the two opponents were not counted as winning or losing interactions.
Following these criteria, we analyzed 4578 dyadic interactions from 123 distinct individuals—69 females and 54 males—from four groups, over a period of up to 9 years, with a total of 22 group—year points (Table 1). The average number of adults per group was 14.7 (standard deviation = 6.8), number of adults per group ranged from six to 32 individuals, and group size varied between years (Table 1). Females were usually the most abundant sex, with an average proportion of males in the group of 0.31 (standard deviation = 0.12); the group with the smallest proportion of males was Noha in 2011, with one male and nine females (0.10), while the one with the largest proportion was Baie Dankie in 2014, with eight males and seven females (0.53).
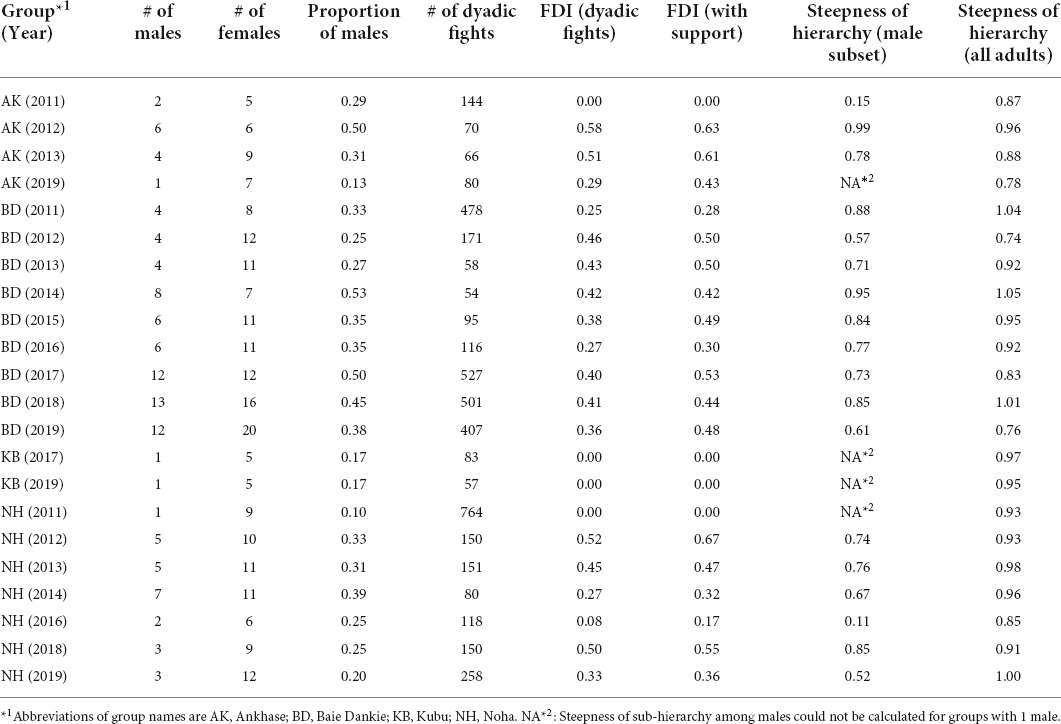
Table 1. Information on groups per year (in parentheses) regarding the number (#) of adults of each sex, the proportion of males, the number of dyadic fights, the degree of female dominance (i.e., Female Dominance Index) from dyadic interactions and when interactions with support were added to the calculation of the dominance hierarchy, and the steepness of the hierarchy of all adults and of males only.
For the intensity of aggression, we classified interactions with physical contact (hit, bite, chase, grab, steal food, or hand on head) as severe and interactions without (stare, displace, and aggressive call) as mild. For each aggressive interaction, we considered only the initiator to be responsible for the intensity of the aggression. If an individual reacted with a severe aggressive behavior after receiving severe aggression, its behavior was not counted as being severe aggression, since it was responding to severe aggression received from another individual instead of causing it. Also, if an individual escalated a mild interaction with a severe behavior, it was counted as showing severe aggression even though it did not initiate the interaction.
We measured the intensity of aggression in two ways. (1) The average proportion of severe fights of the total number of fights per individual, and (2) the average absolute number of severe fights per individual. We used both metrics to compare the intensity of aggression of males to females in each group-year point.
We investigated the intrasexual competition among males by two metrics. The first concerned intensity of competition, namely the proportion of intrasexual severe fights by males of all severe aggressive acts by males against adults. This metric quantified which part of their intense aggression males directed to other males, thus controlling for any differences in the frequency of aggression per year related to socioecological factors. The second metric concerned the relative frequency of competition among males from that among all adults (ignoring its intensity), namely the proportion of male-to-male fights of all group fights among adults. This metric quantified how often male-to-male aggression occurred in a group during each year, controlling for different baseline frequencies of aggression among adults that may differ among groups and years due to socioecological variables.
To determine the dominance hierarchy among adults, we organized competitive interactions in matrices with the winners in the rows and losers in columns. Interaction matrices were calculated per group and per year, excluding group-year points with less than 50 dyadic competitive interactions, as done by Hemelrijk et al. (2020). We calculated the rank of each individual by the average dominance index, ADI (Hemelrijk et al., 2005). It is the average proportion of winning by an individual from all its group members excluding those with whom it did not interact competitively.
We quantified the degree of female dominance in each group-year point by the Female Dominance Index (Hemelrijk et al., 2008). Here, we make use of both intrasexual and intersexual aggressive interactions. The Female Dominance Index represents the proportion of males that each female is dominant over, averaged over all females in the group; this value ranges from 0 (complete male dominance; i.e., zero female dominance over males) to 1 (complete female dominance; i.e., all females are dominant over all males). If a female and a male are equally dominant, the male is counted as being co-dominant in the calculation of the Female Dominance Index by contributing half a unit (0.5).
We calculated the steepness of the hierarchy as the slope of the linear regression between the ordinal rank of the individuals and the dominance index of each individual (de Vries et al., 2006). Because unknown relationships were present in the interaction matrices of our dataset and the steepness measure based on the normalized David’s score is strongly biased by this (Klass and Cords, 2011), we based the steepness measure on the normalized average dominance index instead, as it was less affected by unknown relationships (Saccà et al., 2022). We measured the hierarchical steepness, and investigated the effect for both all adults of the group and for exclusively the adult males among all adults of the group (i.e., the male sub-hierarchy). In the male sub-hierarchy, each male kept his dominance index (thus his relative rank) as calculated for the hierarchy of all adults. We used these values of the dominance indices instead of the dominance indices derived from competitive interactions among males only, in order to account for the influence via the winner-loser effect that all fights (intersexual fights, as well as fights among males and among females) have on males’ cardinal and ordinal ranks. Thus, we test the hierarchical differentiation (i.e., steepness) among males including the dynamics of interaction among all adults. It should be noted that in multiple species (among which vervet monkeys) the rank order of same sex individuals was highly correlated between dominance hierarchies based on interactions among the same sex only and on among both sexes (Kappeler et al., 2022). When only a single male was present in a group, it was impossible to calculate the steepness of the hierarchy among males and therefore, this group-year point was excluded from the analyses (so, four group-year points were excluded).
We analyzed the relation between the degree of female dominance over males and the proportion of males in our enlarged dataset, in which we added 2 years of observation for the four groups in the Mawana reserve to the data used by Hemelrijk et al. (2020). We did this because this relation is essential to our analyses, and although the relation was shown by Hemelrijk et al. (2020) to be positive and significant it needed confirmation with our updated and enlarged dataset. We used a Generalized Linear Mixed Model (GLMM from now on) assuming a beta-binomial distribution of the response variable, the Female Dominance Index, similarly to Hemelrijk et al. (2020), with the proportion of males in the group as the explanatory variable and with the group of each group-year point as the random part of our model. We tried to fit a GLMM adding also the effect of the years to the random part of the model, but we could not because this led to problems in model convergence.
We investigated whether the steepness of the hierarchy, either among all adults or the subset of males only from the hierarchy among all adults (the male sub-hierarchy), can explain the aforementioned relationship as it was proposed by the DomWorld Hypothesis or by the Male Competition Hypothesis. We tested our hypotheses by analyzing the relationships between three variables: the proportion of males in the group, the hierarchical steepness (of either all adults or the subset of males), and the degree of female dominance over males (Female Dominance Index).
To investigate the DomWorld Hypothesis, we investigated the relation between the hierarchical steepness among all adults and the proportion of males in the group. In the DomWorld Hypothesis, this relation is expected to be positive and significant. We tested this in a Linear Mixed Model (LMM from now on), with the hierarchical steepness as the response variable and the group and year of each group-year point as random effects. We assumed a Gaussian distribution for the hierarchical steepness because the steepness of the group can reach values over one, which is a characteristic of the steepness measure when it is calculated for interaction matrices in which not all relationships are known (Saccà et al., 2022). Next, we tested whether the Female Dominance Index was related to the steepness of hierarchy among adults. We expect this relationships to be positive and significant, if the hypothesis is correct. Here, we used a GLMM assuming a beta-binomial distribution of the response variable, the Female Dominance Index, with the steepness of hierarchy among adults as the explanatory variable and the group of each group-year point as the random part. We could not include the year as a random effect because of problems in model convergence.
We investigated whether males and females differ in the intensity of aggression, since the DomWorld Hypothesis assumes that males are more intense in their aggression than females. For this we compared two metrics of intensity of aggression: the average proportion of severe fights of the total number of fights per individual and the average number of severely aggressive interactions per individual. We compared these two measures between the sexes for each group-year point using a non-parametric test, namely the Wilcoxon signed-rank test, because the distribution of the differences between the values of males and those of females for both metrics of intensity of aggression was not normal (based on the Shapiro-Wilk test for normality).
When examining the relation between the proportion of fights with males (of all fights among adults) with the proportion of males in the group, we used a GLMM assuming a beta-binomial distribution of the response variable, the proportion of fights with males (of fights among all adults), and the year and group of each group-year point as the random part. We expect that the proportion of fights with males increases with the proportion of males. When testing whether the proportion of male fights is related with the steepness of the group hierarchy, we used a LMM with the steepness of the hierarchy among adults as the response variable, and the random effects for the years and groups of each group-year point, and according to the DomWorld Hypothesis we expect the steepness to increase with the proportion of males.
In the Male Competition Hypothesis, we tested the relation between the steepness of the male sub-hierarchy, namely the hierarchy among males (when males were interacting with all adult group members) with the proportion of males in the group. According to the Male Competition Hypothesis, we expect the steepness of males to increase with the proportion of males in the group. Here, we used a LMM, with the steepness of the sub-hierarchy among males in the group as our response variable, and with the random part of the model composed of the effect of group and year for each group-year point. We assumed a Gaussian distribution because the steepness could theoretically reach values higher than one. Next, we tested the relation between the Female Dominance Index and the steepness of hierarchy in the subset of males using a GLMM assuming a beta-binomial distribution of the response variable, the Female Dominance Index. In line with the Male Competition Hypothesis, we expect female dominance over males to increase with increased steepness of the male sub-hierarchy.
We tested whether greater steepness of the hierarchy among males with a larger proportion of males may be due to an increase of intrasexual competition among males via more intense or frequent aggression among males. Thus we investigated the relation between the intensity and the frequency of male intrasexual aggression with the proportion of males in the group by using two GLMMs for two metrics of male intrasexual competition (intensity and frequency). We also investigated with two GLMMs the relation between the steepness of male sub-hierarchy and either intensity or frequency of male intrasexual competition.
In one GLMM we assumed a beta-binomial distribution for the proportion of male-to-male severe fights of all male severe fights (indicating the intensity of male intrasexual competition) and the proportion of males in the group was the explanatory variable, with the random part of the model formed by the year of each group-year point. We tried to fit a model with also the random effect of group, but we found that our fitted model showed significant quantile deviations in the plot of the residuals versus predicted values (DHARMa package for R: Hartig, 2022). In the other GLMM we assumed a Gaussian distribution for the hierarchical steepness among the subset of males separately (the male sub-hierarchy), with the proportion of male-to-male severe fights of all severe fights by males as the explanatory variable and the group and year of each group-year point as random factors.
Similarly, in another GLMM we assumed a beta-binomial distribution for the proportion of male-to-male fights of all fights among adults (i.e., representing male intrasexual competition), and using the proportion of males in the group as the explanatory variable, and the effects of group and year as the random part of the model. In the other LMM we assume a Gaussian distribution for the hierarchical steepness of the subset of males, and the proportion of male-to-male fights of all fights among adults was the explanatory variable, with the random part of the models being the effects of group and year for each group-year point.
When analyzing the Male Competition Hypothesis, we used group-year points with at least two males.
To test the Female Coalition Hypothesis, we investigated whether the Female Dominance Index calculated for the dataset including support in fights differed from the one without support in fights. We calculated the difference between the Female Dominance Index per group-year point when coalitionary support is included minus when it is excluded. A positive value means that partial female dominance increased in the group-year point when including support versus when not. We investigated whether the average difference differed from zero with a LMM with the difference in FDI values between group-years with and without coalitionary support as the response variable, no explanatory variable and the random effects of groups and years for each group-year point. The value and significance of the intercept of this model represent the difference between groups in female dominance over males when support is added. We did so to include the random effects of group and year for each group-year point in the analyses. We also investigated whether coalitionary support caused a larger degree of female dominance with an increasing proportion of males in the group than without coalitionary support. In our LMM, we assumed a Gaussian distribution for the difference in the values of the FDI between group-year points with and without coalitionary support as the response variable, and the proportion of males in the group as the explanatory variable and the random effects of group and year.
All analyses were conducted in R, version 4.2.0 (R Core Team, 2022). To fit all our Generalized Linear Mixed Model and Linear Mixed Models we used the package glmmTMB (Brooks et al., 2017). For model diagnostics, in our Supplementary material, we show QQ-plots and plots of fitted residuals versus predicted residuals with relevant statistical analyses, obtained from the package DHARMa (Hartig, 2022). For model performance statistics, we calculate pseudo R2 values using the package MuMIn (Bartoń, 2022) and we perform likelihood ratio tests (LRT) comparing our full models to the same models without the explanatory variable, to test whether the change in likelihood was significant. For the LRT as well as for the Wilcoxon signed-rank tests and the Shapiro-Wilk tests we used the base package of R. Figures were made using the package ggplot2 (Wickham, 2016).
The degree of female dominance over males, the Female Dominance Index, increased significantly with the proportion of males in the group (GLMM, 22 group-year points, β = 2.41, SE = 1.20, P = 0.044, Figure 1A) in line with earlier results from Hemelrijk et al. (2020) on a smaller dataset. To detect the processes underlying this relationship we study three hypotheses, the DomWorld Hypothesis, the Male Competition Hypothesis, and the Female Coalition Hypothesis.
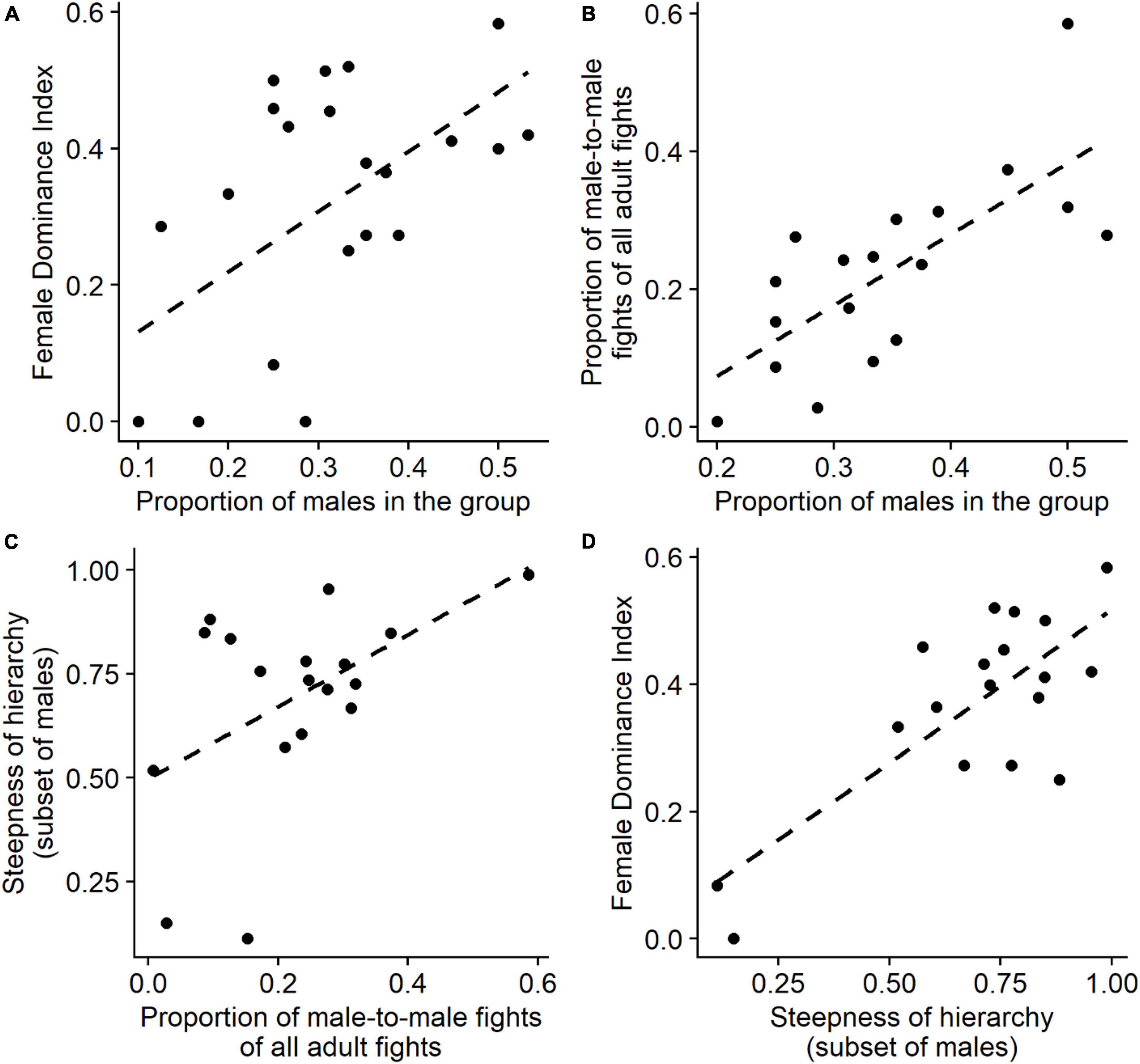
Figure 1. Summary of relevant relations for the Male Competition Hypothesis: (A) proportion of males in the group versus degree of female dominance (i.e., female dominance index, FDI) (22 group-year points). (B) The proportion of male-to-male fights of all adult fights (relative frequency of male intrasexual competition) versus the proportion of males in the group. (C) The steepness of hierarchy among the subset of adult males versus the proportion of male-to-male fights of all adult fights (i.e., relative frequency of male intrasexual competition). (D) The degree of female dominance (i.e., FDI) versus the steepness of hierarchy among the subset of adult males. Plots (B–D) concern only group-year points with at least two males (18 group-year points). Trend lines (dotted lines) are calculated for (ordinary least squares) linear regressions, using the function “geom_smooth” of the R package “ggplot2” (Wickham, 2016). For more accurate statistical analyses, see text.
The steepness of the hierarchy among all adults did not increase significantly with the proportion of males (LMM, 22 group-year points, Slope = 0.17, SE = 0.15, P = 0.255) nor with the Female Dominance Index (GLMM, 22 group-year points, β = −0.19, SE = 1.19, P = 0.871). Therefore, the steepness of the hierarchy among all adults did not explain the larger degree of female dominance in the group-years when the proportion of males in the group increased.
Although the proportion of fights with males of all fights among adults increased significantly when the proportion of males increased (GLMM, 22 group-year points, β = 4.40, SE = 1.01, P < 0.001), the increased proportion of fights among males was not related to the hierarchical steepness among adults (LMM, 22 group-year points, slope = 0.05, SE = 0.11, P = 0.649). This led to the question of whether the assumption was met that males were more intense in their aggression than females.
Although there was a trend that the average proportion of severe fights of all fights by males was higher than that by females, it was not significant (Wilcoxon signed-rank: 22 group-year points, V = 184, P = 0.063, Supplementary Figure 1, median: 9.0% for males versus 7.4% for females). Neither did males and females differ significantly in their average number of severe interactions per individual (Wilcoxon signed-rank test: 22 group year points, V = 131, P = 0.602, Supplementary Figure 1, median: 2.2 for males versus 1.8 for females).
The Female Dominance Index increased significantly with the steepness of the sub-hierarchy among males (GLMM, 18 group-year points with at least two males, β = 1.69, SE = 0.59, P = 0.004, Figure 1D) and the steepness of male sub-hierarchy increased significantly with the proportion of males in the group (LMM, 18 group-years with at least two males, slope = 1.36, SE = 0.49, P = 0.005). Therefore, the degree of female dominance may increase with the proportion of males in the group via the increased steepness of the male sub-hierarchy.
Both intensity and frequency of intrasexual aggression among males may lead to the increase in steepness of hierarchy in the subset of males. Concerning the intensity of male intrasexual aggression, males did not direct a significantly larger proportion of their intensely aggressive actions to other males when the proportion of males in the group increased (GLMM, 18 group-year points with at least two males, β = 3.54, SE = 2.03, P = 0.081), and the proportion of male-to-male severely aggressive interactions was not significantly related to the steepness of male hierarchy (LMM, 18 group-year points with at least two males, slope = 0.38, SE = 0.20, P = 0.061).
Concerning the relative frequency of male intrasexual aggression, a larger proportion of fights among adults were between males when the proportion of males in the group increased (GLMM, 18 group-year points with at least two males, β = 6.82, SE = 1.44, P < 0.001, Figure 1B), and a larger proportion of male-to-male fights was positively related to hierarchical steepness when studying the subset of males among the adults (LMM, 18 group-year points with at least two males, β = 0.67, SE = 0.35, P = 0.014, Figure 1C).
Considering fights between a male and a female in which one or more external individuals intervened (there were 265 intersexual fights with support), support was overwhelmingly given to females (239 times, 90% of cases). In the intersexual fights in which the female received support, this was provided mostly by a single adult female (111 times out of 239, 44% of cases), and less often by a single adult male (79 times, 33% of cases).
The degree of female dominance over males, the Female Dominance Index (FDI), increased significantly when including support in fights versus when not and considering the random effects of group and year (LMM, 22 group-year points, intercept = 0.056, SE = 0.010, P < 0.001, Figure 2). Results did not change when calculating the average difference in FDI irrespectively of random effects, likely because they had very little effect. Namely, on average per group-year point FDI increase by 0.056 (SE = 0.010), with a minimum of 0 and a maximum of 0.15. In all group-year points, female dominance was equal or higher when including fights with support. However, the difference between female dominance values when including fights with support or not was not significantly greater the higher the proportion of males in the group (LMM, 22 group-year points, slope = −0.06, SE = 0.09, P = 0.515).
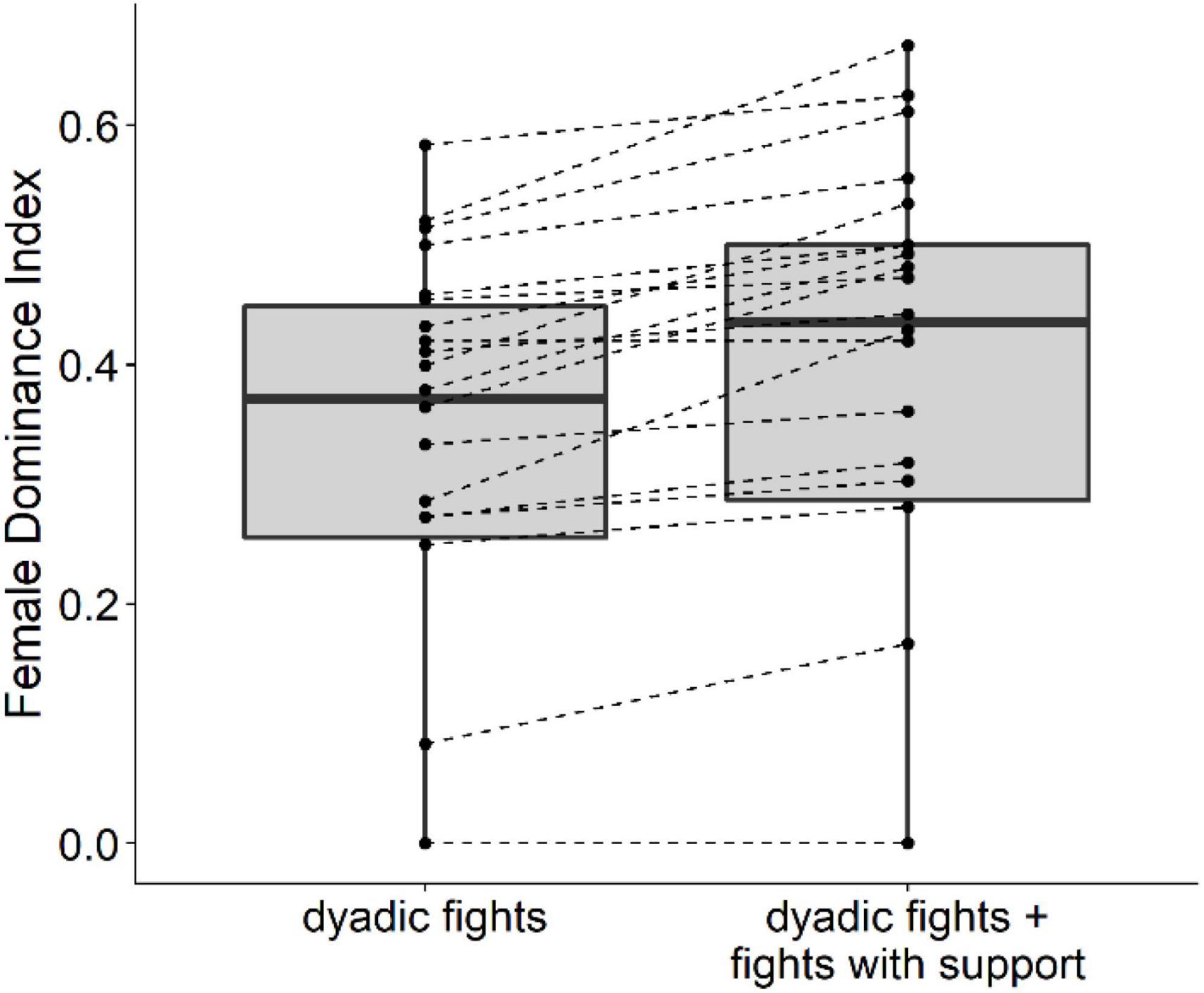
Figure 2. The Female Dominance index of the dominance hierarchy calculated from only dyadic fights (left) versus the hierarchy with the inclusion of fights with support (right). Note that support was seldom received from more than one individual (23 cases from 239 cases; from two or more females in 5 cases, from two or more males in 8 cases, and from two or more individuals of both sexes in 6 cases, in 2 cases, support was received from a coalition of a female and unidentified juveniles, and in 2 cases from a coalition of a male and unidentified juveniles). In 26 cases in which a female received support in an intersexual fight the sex of the supporter could not be identified.
We confirm that the degree of female dominance over males increases with the proportion of males in four groups of wild vervet monkeys, in line with earlier findings by Hemelrijk et al. (2020). We found neither evidence that the steepness of the hierarchy among adults increased with the proportion of males in the group, nor that the degree of female dominance over males increased with the steepness of the hierarchy among adults, despite being suggested by DomWorld (DomWorld Hypothesis, see Figure 3). A requirement for the DomWorld hypothesis is that males are more intense in their aggression than females. However, we did not find evidence for this in vervet monkeys when we categorize severe aggression as fights with physical contact. Thus, this requirement of the computational model may be missing, which may explain that the predictions of the DomWorld Hypothesis were not met. However, it should be noted that male vervets are larger and better armed than females, thus making their fights with physical contact more menacing by default. Consequently, even without physical contact their attacks are probably perceived as more severe than those by females due to the threat of escalating the conflict.
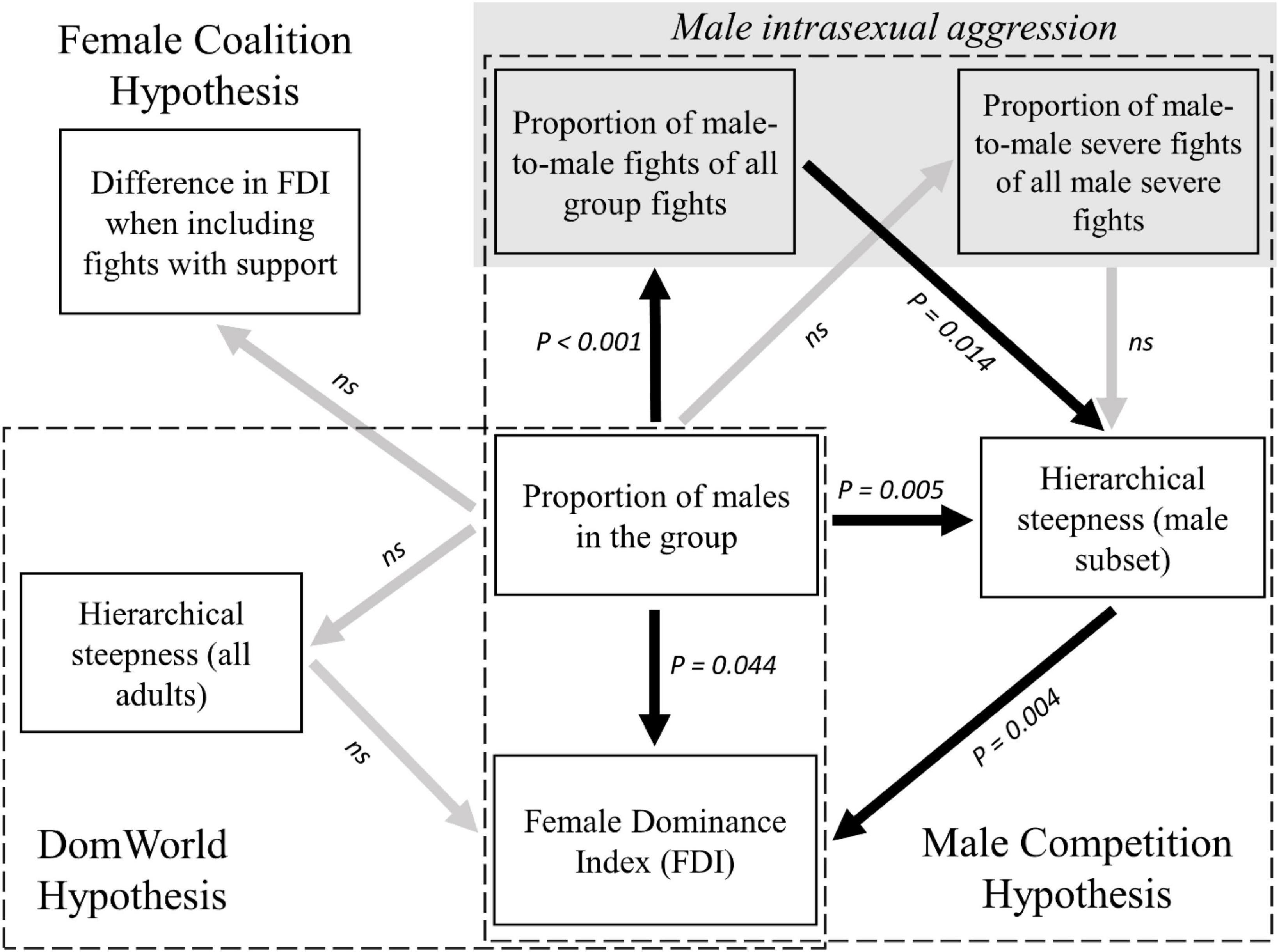
Figure 3. Summary of correlations related to the degree of female dominance (i.e., Female Dominance Index, FDI) in vervet monkeys. Correlations are represented as arrows pointing from the response variable to the explanatory variable. The statistical significance of each correlation is noted next to the arrow (with not significant values noted as ns). Significant correlations are noted as black arrows, while non-significant correlations as gray arrows. All significant correlations are positive.
Instead, the steepness of the hierarchy of the subset of males increased significantly with the proportion of males in the group and it was positively related to the degree of female dominance over males. This is in line with the Male Competition Hypothesis and may explain the higher degree of female dominance in group-year points with proportionally more males as being due to more males dropping down the hierarchy below a larger proportion of females. The steeper male hierarchy within the hierarchy of all adults, when the proportion of males in the group increased, may be due to an increase in frequency of male intrasexual conflicts, and was not related to an increase in the intensity of male intrasexual fights (Male Competition Hypothesis, see Figure 3).
The Male Competition Hypothesis resembles the DomWorld Hypothesis, because the self-organization processes underlying both hypotheses are similar. In the DomWorld Hypothesis males sink down the hierarchy below more females because the intensity of fights initiated by males is higher than initiated by females, and a higher proportion of males in the group results in more fights initiated by males and thus a steeper hierarchy for the whole group. In vervet monkeys, males probably sink down the hierarchy below more females because males fight more frequently among themselves the higher the proportion of males in the group, and thus the male hierarchy becomes steeper. In the model, a higher number of male-male fights when the group comprises more males would be expected from a simple self-organization process where more males being present in the group results in more male-male encounters by chance and thus, potentially, more fights among males. The same self-organization process could be expected in groups of wild primates. In vervet monkeys, the same increase in male-male fights with the proportion of males in the group may also reflect mating competition, as the presence of more males limits the access to females. The self-organization process and low availability of females can favor competition among males in a synergistic way. The self-organization processes underlying the Male Competition Hypothesis and the DomWorld Hypothesis differ in the absence of the requirement that males are more intense than females in their aggression in the Male Competition Hypothesis. Thus, the Male Competition Hypothesis can be relevant even in species in which sexual dimorphism is absent (e.g., lemurs: Kappeler, 1990) or in favor of females (e.g., spotted hyena: Swanson et al., 2013), where we do not expect males to be more intense in their aggression than females.
The similarities between the DomWorld Hypothesis and the Male Competition Hypothesis can be illustrated by the work by Izar et al. (2021). The study shows that in three study species of capuchin monkeys (Sapajus libidinosus, S. nigritus, and S. xanthosternos) not only did the Female Dominance Index increase with the proportion of males in the group, but so did the proportion of male-male aggression of total male aggression (Izar et al., 2021). However, Izar and colleagues did not distinguish between the effect that male fights have on the hierarchy of all adults (our DomWorld Hypothesis) and on the adult male sub-hierarchy (our Male Competition Hypothesis). If males are more intense in their aggression than females, an increase in male-male aggression (and thus in the steepness of male sub-hierarchies, the Male Competition Hypothesis) may still happen in combination with a larger proportion of male fights of all group fights (and thus in steeper hierarchies for the whole group, the DomWorld Hypothesis). Consequently, these two hypotheses are not mutually exclusive, and whether they happen jointly or independently probably depends on the characteristics of the species, and warrants further investigation.
Although coalitionary support by females to other females in fights between the sexes enhanced partial female dominance in vervet monkeys (Figure 2 and Table 1), female dominance did not increase more when females received more support when the proportion of males in the group was larger (Female Coalition Hypothesis, see Figure 3). Therefore, coalitions by females did not cause the increase in female dominance with the increased proportion of males in the group (Female Coalition Hypothesis). These coalitions may instead be a by-product of the already higher status that females enjoy in groups with a larger proportion of males, as proposed by Hemelrijk et al. (2020). Males provided support to females in one third of intersexual conflicts in which a female received support from a third party. Females may recruit males with incentives to helpful males and disincentives to un-cooperative ones, as they do in intergroup fights (Arseneau-Robar et al., 2016b; or males may help females in exchange for increased mating success, as also observed in the context of intergroup encounters (Arseneau et al., 2015; Arseneau-Robar et al., 2016a).
In conclusion, our study of vervet monkeys partially supports the self-organization processes from DomWorld, as we give evidence that male intrasexual competition increases with the proportion of males in the group and favors partial female dominance in a way that could be explained by self-organization processes also present in the computational model. The increase in male-male competition when the proportion of males in the group increases could be due to self-organization processes, to male competition due to lower female availability, or a combination of self-organization and male competition, and may be a widespread phenomenon in group-living animals. Future studies should try to disentangle the effect that the self-organization process and mating competition have on the increase in male intrasexual competition, with its interaction with hierarchical steepness and the degree of female dominance over males. This could be done by testing the role of sex ratio alongside other causes of mating competition such as number of fertile females and mating seasons, in relation to competition among males, hierarchical steepness and partial female dominance over males.
The data and R script to replicate results of this manuscript are available at https://doi.org/10.34894/IMWDGX.
This animal study was reviewed and approved by Ezemvelo KZN Wildlife, South Africa.
CH and TS contributed to conception and design of the study. TS wrote the first draft of the manuscript and performed statistical analyses. EW was responsible for the acquisition of data. GG supervised statistical analyses. All authors contributed to interpretation of data, manuscript revision, and read and approved the submitted version.
This project was funded by the Swiss National Science Foundation (31003A_159587 and PP00P3_170624) along with the Branco Weiss Fellowship–Society in Science (granted to EW).
We thank the van der Walt family for permission to conduct the study on their land and the whole IVP team for their help and support in the field. We are grateful to the editor and reviewers for their constructive comments.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.930266/full#supplementary-material
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour 49, 227–267. doi: 10.1163/156853974X00534
Arseneau, T. J. M., Taucher, A. L., van Schaik, C. P., and Willems, E. P. (2015). Male monkeys fight in between-group conflicts as protective parents and reluctant recruits. Anim. Behav. 110, 39–50. doi: 10.1016/j.anbehav.2015.09.006
Arseneau-Robar, T. J. M., Taucher, A. L., Müller, E., Van Schaik, C., Bshary, R., and Willems, E. P. (2016b). Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc. R. Soc. B Biol. Sci. 283:20161817. doi: 10.1098/rspb.2016.1817
Arseneau-Robar, T. J. M., Müller, E., Taucher, A. L., Van Schaik, C. P., and Willems, E. P. (2016a). Male food defence as a by-product of intersexual cooperation in a non-human primate. Sci. Rep. 6:35800. doi: 10.1038/srep35800
Barchas, P. R., and Menzosa, S. D. (1984). “Emergent hierarchical relationships in rhesus macaques: An application of Chase’s model,” in Social hierarchies: Essays towards a sociophysiological perspective, ed. P. R. Barchas (Westport: Greenwood Press), 81–95.
Bartoń, K. (2022). MuMIn: Multi-model inference. R package version 1.46.0. Available online at: https://cran.r-project.org/package=MuMIn (accessed September 1, 2022).
Bolter, D. R., and Zihlman, A. L. (2003). Morphometric analysis of growth and development in wild-collected vervet monkeys (Cercopithecus aethiops), with implications for growth patterns in Old World monkeys, apes and humans. J. Zool. 260, 99–110. doi: 10.1017/S0952836903003522
Brooks, M. E., Kristense, K., van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB Balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/RJ-2017-066
Chase, I. D. (1982). Behavioral sequences during dominance hierarchy formation in chickens. Science 216, 439–440. doi: 10.1126/science.216.4544.439
Chase, I. D., Tovey, C., Spangler-Martin, D., and Manfredonia, M. (2002). Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl. Acad. Sci. U.S.A. 99, 5744–5749. doi: 10.1073/pnas.082104199
Cheney, D. L., and Seyfarth, R. M. (1990). How monkeys see the world: Inside the mind of another species. Chicago, IL: University of Chicago Press. doi: 10.7208/chicago/9780226218526.001.0001
de Vries, H., Stevens, J. M. G., and Vervaecke, H. (2006). Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 71, 585–592. doi: 10.1016/j.anbehav.2005.05.015
Eaton, G. G. (1984). Aggression in adult male primates: A comparison of confined Japanese macaques and free-ranging olive baboons. Int. J. Primatol. 5, 145–160. doi: 10.1007/BF02735738
Franz, M., McLean, E., Tung, J., Altmann, J., and Alberts, S. C. (2015). Self-organizing dominance hierarchies in a wild primate population. Proc. R. Soc. B Biol. Sci. 282:20151512. doi: 10.1098/rspb.2015.1512
Hartig, F. (2022). DHARMa: Residual diagnostics for hierarchical (Multi-Level/Mixed) regression models. R package version 0.4.5. Available online at: http://florianhartig.github.io/DHARMa/ (accessed September 1, 2022).
Hemelrijk, C. K. (1999). An individual-orientated model of the emergence of despotic and egalitarian societies. Proc. R. Soc. B Biol. Sci. 266, 361–369. doi: 10.1098/rspb.1999.0646
Hemelrijk, C. K., Wantia, J., and Dätwyler, M. (2003). Female co-dominance in a virtual world: Ecological, cognitive, social and sexual causes. Behaviour 140, 1247–1273. doi: 10.1163/156853903771980585
Hemelrijk, C. K., Wantia, J., and Gygax, L. (2005). The construction of dominance order: Comparing performance of five methods using an individual-based model. Behaviour 142, 1043–1064. doi: 10.1163/156853905774405290
Hemelrijk, C. K., Wantia, J., and Isler, K. (2008). Female dominance over males in primates: Self-organisation and sexual dimorphism. PLoS One 3:e2678. doi: 10.1371/journal.pone.0002678
Hemelrijk, C. K., Wubs, M., Gort, G., Botting, J., and van de Waal, E. (2020). Dynamics of intersexual dominance and adult sex-ratio in wild vervet monkeys. Front. Psychol. 11:839. doi: 10.3389/fpsyg.2020.00839
Hirschenhauser, K., Gahr, M., and Goymann, W. (2013). Winning and losing in public: Audiences direct future success in Japanese quail. Horm. Behav. 63, 625–633. doi: 10.1016/j.yhbeh.2013.02.010
Holekamp, K. E., and Engh, A. L. (2009). “Reproductive skew in female-dominated mammalian societies,” in Reproductive skew in vertebrates: Proximate and ultimate causes, eds R. Hager and C. Jones (Cambridge: Cambridge University Press), 53–83. doi: 10.1017/CBO9780511641954.005
Hsu, Y., Lee, I. H., and Lu, C. K. (2009). Prior contest information: Mechanisms underlying winner and loser effects. Behav. Ecol. Sociobiol. 63, 1247–1257. doi: 10.1007/s00265-009-0791-9
Izar, P., Fernández-Bolaños, M., Seex, L., Gort, G., Suscke, P., Tokuda, M., et al. (2021). Female emancipation in a male dominant, sexually dimorphic primate under natural conditions. PLoS One 16:e0249039. doi: 10.1371/journal.pone.0249039
Kappeler, P. M. (1990). Female dominance in lemur catta: More than just female feeding priority? Folia Primatol. 55, 92–95. doi: 10.1159/000156504
Kappeler, P. M. (1993). “Female dominance in primates and other mammals,” in Perspectives in ethology, eds P. Bateson, P. Klopfer, and N. Thompson (New York, NY: Plenum Press), 143–158.
Kappeler, P. M., Huchard, E., Baniel, A., Canteloup, C., Charpentier, M. J. E., Cheng, L., et al. (2022). Sex and dominance: How to assess and interpret intersexual dominance relationships in mammalian societies. Front. Ecol. Evol. 10:918773. doi: 10.3389/fevo.2022.918773
Kar, F., Whiting, M. J., and Noble, D. W. A. (2016). Influence of prior contest experience and level of escalation on contest outcome. Behav. Ecol. Sociobiol. 70, 1679–1687. doi: 10.1007/s00265-016-2173-4
Klass, K., and Cords, M. (2011). Effect of unknown relationships on linearity, steepness and rank ordering of dominance hierarchies: Simulation studies based on data from wild monkeys. Behav. Process. 88, 168–176. doi: 10.1016/j.beproc.2011.09.003
Koren, L., Mokady, O., and Geffen, E. (2006). Elevated testosterone levels and social ranks in female rock hyrax. Horm. Behav. 49, 470–477. doi: 10.1016/j.yhbeh.2005.10.004
Lan, Y. T., and Hsu, Y. (2011). Prior contest experience exerts a long-term influence on subsequent winner and loser effects. Front. Zool. 8:28. doi: 10.1186/1742-9994-8-28
Page, L., and Coates, J. (2017). Winner and loser effects in human competitions. Evidence from equally matched tennis players. Evol. Hum. Behav. 38, 530–535. doi: 10.1016/j.evolhumbehav.2017.02.003
Paoli, T., Palagi, E., and Tarli, S. (2006). Reevaluation of dominance hierarchy in bonobos. Am. J. Phys. Anthropol. 130, 116–122. doi: 10.1002/ajpa.20345
Parish, A. (1996). Female relationships in bonobos (Pan paniscus): Evidence for bonding, cooperation, and female dominance in a male-philopatric species. Hum. Nat. 7, 61–96. doi: 10.1007/BF02733490
R Core Team (2022). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Saccà,, T., Gort, G., van de Waal, E., and Hemelrijk, C. K. (2022). Reducing the bias due to unknown relationships in measuring the steepness of a dominance hierarchy. Anim. Behav.
Smuts, B. B. (1987). “Gender, aggression and influence,” in Primate societies, ed. B. Smuts (Chicago, IL: University of Chicago Press), 400–412.
Smuts, B. B., and Smuts, R. W. (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: Evidence and theoretical implications. Adv. Study Behav. 22, 1–63. doi: 10.1016/S0065-3454(08)60404-0
Struhskarer, T. T. (1967). Social structure among vervet monkeys (Cercopithecus aethiops). Behaviour 29, 83–121. doi: 10.1163/156853967X00073
Surbeck, M., and Hohmann, G. (2013). Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767–1780. doi: 10.1007/s00265-013-1584-8
Swanson, E. M., McElhinny, T. L., Dworkin, I., Weldele, M. L., Glickman, S. E., and Holekamp, K. E. (2013). Ontogeny of sexual size dimorphism in the spotted hyena (Crocuta crocuta). J. Mammal. 94, 1298–1310. doi: 10.1644/12-MAMM-A-277.1
Tilson, R. L., and Hamilton, W. J. (1984). Social dominance and feeding patterns of spotted hyaenas. Anim. Behav. 32, 715–724. doi: 10.1016/S0003-3472(84)80147-5
Tokuyama, N., and Furuichi, T. (2016). Do friends help each other? Patterns of female coalition formation in wild bonobos at Wamba. Anim. Behav. 119, 27–35. doi: 10.1016/j.anbehav.2016.06.021
Turner, T. R., Schmitt, C. A., Cramer, J. D., Lorenz, J., Grobler, J. P., Jolly, C. J., et al. (2018). Morphological variation in the genus Chlorocebus: Ecogeographic and anthropogenically mediated variation in body mass, postcranial morphology, and growth. Am. J. Phys. Anthropol. 166, 682–707. doi: 10.1002/ajpa.23459
White, F. J., and Wood, K. D. (2007). Female feeding priority in bonobos, Pan paniscus, and the question of female dominance. Am. J. Primatol. 69, 837–850. doi: 10.1002/ajp.20387
Wickham, H. (2016). Ggplot2: Elegant Graphics for Data Analysis. Available online at: https://ggplot2.tidyverse.org (accessed September 1, 2022).
Keywords: female dominance over males, male intrasexual aggression, sexual competition, female coalitions, sex ratio
Citation: Saccà T, Gort G, van de Waal E and Hemelrijk CK (2022) Male intrasexual aggression and partial dominance of females over males in vervet monkeys. Front. Ecol. Evol. 10:930266. doi: 10.3389/fevo.2022.930266
Received: 27 April 2022; Accepted: 14 September 2022;
Published: 04 October 2022.
Edited by:
Hope Klug, University of Tennessee at Chattanooga, United StatesReviewed by:
Jeremy Morris, Wofford College, United StatesCopyright © 2022 Saccà, Gort, van de Waal and Hemelrijk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tommaso Saccà, dC5zYWNjYUBydWcubmw=
†These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.