- 1College of Life Sciences and Academy for Multidisciplinary Studies, Capital Normal University, Beijing, China
- 2Beijing No. 50 Middle School, Beijing, China
- 3Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC, United States
- 4Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China
The extant kempynines, a strict “southern group,” are confined to South America and Australia, while their most fossil relatives are abundantly recorded in the Northern Hemisphere. This pattern of the biogeographic distribution implies the complicated evolutionary scenario of Kempyninae. Herein, a new northern species Arbusella platyptera Ma et Wang, sp. nov. is described from the Jiulongshan Formation in Daohugou, Inner Mongolia, China. Additionally, a key to the extinct species and extant genera of Kempyninae is provided. Integrating all extant and most fossil genera of Kempyninae, we conducted phylogenetic analyses to explore the inner relationships of Kempyninae for the first time. The results corroborate the monophyly of Kempyninae and retrieve three clades within the subfamily, namely, two northern fossil genera (†Arbusellla + †Jurakempynus), constituting the basalmost clade and three other northern fossil genera (†Sauktangida + †Mirokempynus + †Ponomarenkius), forming a monophylic clade, which is sister to the third clade that includes all extant southern genera and the southern fossil genus of †Euporismites. Also, the extant kempynines were hypothesized to evolve independently from their northern Mesozoic relatives. The Dispersal-vicariance (DIVA) analysis revealed a northern and prepangean origin of Kempyninae, and the northern ancestral kempynines first colonized the Southern Hemisphere before the split of Pangea. Our results expose a more complicated evolutionary scenario of the insects with a long evolutionary history and provide new insights into the formation of distribution patterns in current relictual insects.
Introduction
Kempyninae, a subfamily of Osmylidae, currently comprises 11 genera, including three extant genera, namely, Kempynus Navás, 1912; Australysmus Kimmins, 1940; and Euosmylus Krüger, 1913, and eight fossil genera, namely, †Euporismites Tillyard, 1916; †Cretosmylus Makarkin, 1990; †Jurakempynus Wang et al., 2011b; †Kempynosmylus Makarkin, 2014; †Sauktangida Khramov, 2014a; †Arbusella Khramov, 2014b; †Ponomarenkius Khramov et al., 2017; and †Mirokempynus Ma et al., 2020a (Winterton et al., 2019; Ma et al., 2020a). The extant Kempyninae, as an absolute “southern group,” is restricted to Australia, New Zealand, Argentina, and Chile in South America (Kimmins, 1940; New, 1983; Martins et al., 2016), of which most genera are confined to a single area except for the genus Kempynus, occurring in all areas. Contradictory to the extant lineages, the fossil kempynines were almost entirely documented from the Northern Hemisphere in the Mesozoic, with the exception of one Cenozoic fossil species Euporismites balli from Queensland, which appears to be more closely related to the extant species in distribution (Lambkin, 1987; Makarkin, 1990, 2014; Wang et al., 2011a; Khramov, 2014a,b; Khramov et al., 2017; Ma et al., 2020a). The distribution pattern of fossil and extant lineages indicates the evolutionary process, and the historical biogeographic dynamics of Kempyninae may be more complicated (Wang et al., 2011b). Furthermore, the recent phylogenetic study of Osmylidae proposed the northern subfamily Osmylinae be sister to the other four southern subfamilies, i.e., Kempyninae, Eidoporisminae, Porisminae, and Stenosmylinae, instead of the current northern distributed groups, i.e., Protosmylinae and Spilosmylinae (Winterton et al., 2017). These results implied an earlier pre-Pangaea origin of Osmylidae and introduced a more complicated evolutionary scenario, and as a result, the current distributions of extant Kempyninae should not be simply attributed to the Gondwanan biogeographic events. To address the issues regarding the historical vicariance biogeographic distributions of Kempyninae, it is essential to study the phylogeny of Kempyninae by combining all fossil and extant lineages.
Herein, we describe a new species, Arbusella platyptera Ma et Wang, sp. nov., from the Middle Jurassic Jiulongshan Formation in Daohugou, Inner Mongolia, China. This new species can be assigned to Kempyninae based on the following apomorphic characters: The M space in the hind wing is extremely broad and sinuate cross-veins are arranged in the intramedial area to produce two rows of irregular cells, which are regarded as an apomorphy of Kempyninae (Winterton et al., 2019; Ma et al., 2020a). To investigate the inner relationships of Kempyninae, we conducted a phylogenetic analysis including all extant and most fossil genera. The phylogenetic results corroborated the monophyly of the subfamily, and the historical biogeography of Kempyninae was outlined by using the DIVA analysis. This study first investigated the historical biogeography of Kempyninae under the phylogenetic framework, and then promoted our understanding of this particular insect group.
Materials and Methods
Material
The type specimen was collected from the Jiulongshan Formation of the Middle Jurassic at Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China, and deposited in the Key Laboratory of Insect Evolution and Environmental Changes, College of Life Sciences and Academy for Multidisciplinary Studies, Capital Normal University, Beijing (CNUB; Dong Ren, Curator). The specimen was observed and photographed by using a stereoscopic microscope, Nikon SMZ25 with an attached Nikon DS-Ri2 digital camera system. The line drawing was produced using the Adobe Photoshop CC and Adobe Illustrator CC software. The terminology for wing venation and genitalia follows Breitkreuz et al. (2017) and Winterton et al. (2019).
Wing vein abbreviations are as follows: C, costa; Sc, subcosta; RA, anterior branch of radius; RP, posterior branch of radius; MA, anterior branch of media; MP, posterior branch of media; CuA, anterior cubitus; CuP, posterior cubitus; A, anal veins. Other abbreviations involved are as follows: J1, Early Jurassic; J2, Middle Jurassic; J3, Late Jurassic; O1, late Paleocene-early Eocene; AUS, Australia; CHN, China; KG, Kyrgyzstan; KZ, Kazakhstan; MGL, Mongolia; NZL, New Zealand; SA, South America.
Phylogenetic Analyses
Taxa Sampling
Nine genera of Kempyninae were sampled in the phylogenetic analyses, including three extant genera, namely, Kempynus, Euosmylus, and Australysmus, and six extinct genera, namely, †Arbusella, †Euporismites, †Jurakempynus, †Mirokempynus, †Ponomarenkius, and †Sauktangida (refer to Table 1 for more details). Three genera are selected as outgroups, including †Vetosmylus (a fossil genus of Osmylinae), Osmylus (the type genus of Osmylinae), and Heterosmylus (an extant genus of Protosmylinae). Two nominal kempynine genera, Cretosmylus Makarkin, 1990, and Kempynosmylus Makarkin, 2014, from the Baissa of Lower Cretaceous were not included in the analysis due to the absence of the key features (Makarkin, 1990, 2014), and their kempynine affinity requires further investigation.
Phylogenetic Analyses
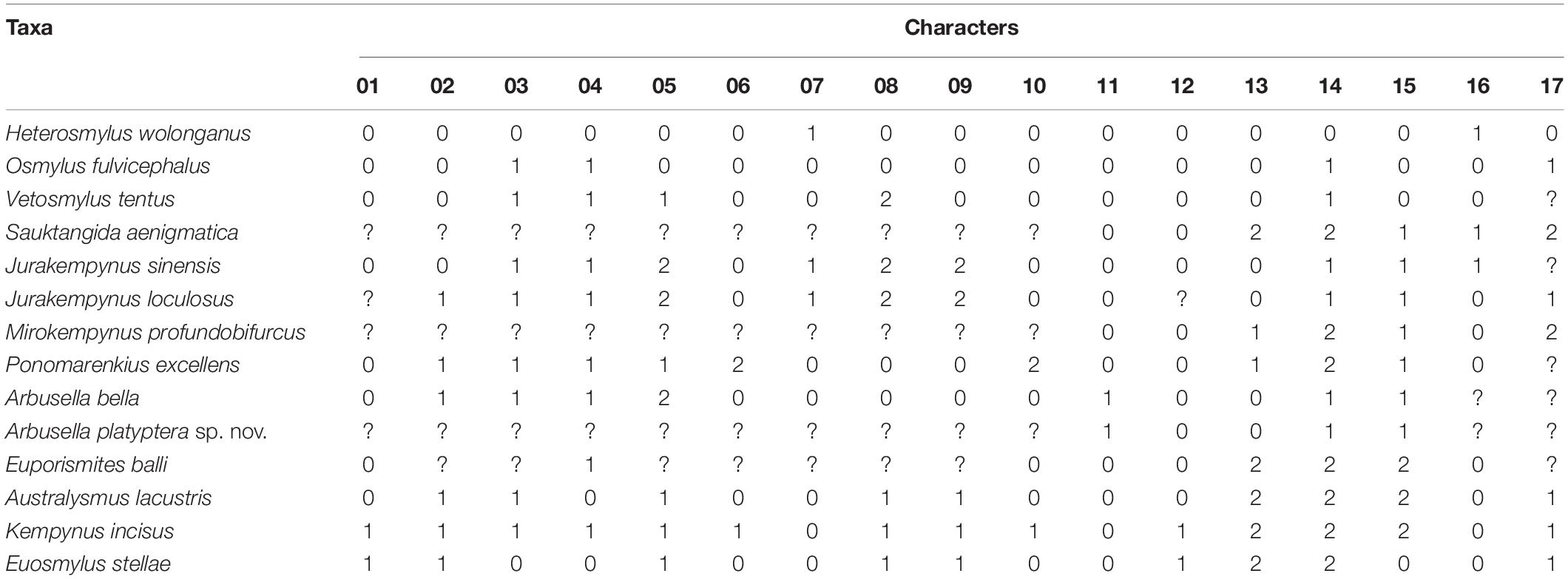
As most fossil taxa of Kempyninae were established based on the characters of wings, we primarily adopted the wing characters in the phylogenetic analysis, including ten characters for the forewing and seven characters for the hind wing. The list of characters and character states for the phylogenetic analyses of Kempyninae are outlined as follows. The data matrix of characters used in the phylogenetic analyses is shown in Table 2. The missing data were coded as “?” The character matrix was edited using NDE 0.5.0 (Page, 2001). The parsimony analyses were performed with Nona/WinClada using a heuristic search that employed a TBR + TBR search strategy, holding 10,000 trees and 1,000 replicates of random additions (Nixon, 2002).
Biogeographic Analysis
To trace the historical biogeographic distributions, the ancestral distribution reconstruction of Kempyninae was conducted with RASP 4.0 using the dispersal-vicariance optimization model, which is generally used in the analyses of ancestral reconstruction (Yu et al., 2010, 2015; Liu et al., 2012). Based on the current phylogenetic analysis and the previous study (Winterton et al., 2017), the sampled tree was compiled to declare a fully resolved topology of kempynine genera. The DIVA analysis was performed with default settings except for the maximum number of areas in ancestral ranges being constrained to three, considering the widest kempynine genus Kempynus distributed in three areas, i.e., South America, Australia, and New Zealand.
List of Characters and Character States for the Phylogenetic Analyses
Morphological character states are scored 0–2 and ? (0 = plesiomorphic state; 1–2 = apomorphic state; ? = state unknown). As the phylogenetic analysis was conducted for fossil and extant kempynine genera, the sampled characters were primarily selected from the wings that were most shared by fossil and extant taxa.
Characters of Forewing
1. An outline of the apical margin. 0, normal or slightly convex; 1, distinctly concave. The normal outer margin occurs in the most sampled genera. State 1 exists as an apomorphy in two extant genera: Kempynus and Euosmylus, whose shape of forewings is evidently modified into the falcate shape.
2. Position of M forking. 0, proximally branched, before (or corresponding to) the RP1 point from RP; 1, distinctly beyond the RP1 point from RP, and often present in-between RP1 and RP2 from RP. The position of M fork stably occurs before RP1 among Protosmylinae, Spilosmylinae, and most fossil kempynines. Although the sampled Osmylinae outgroups Osmylus fulvicephalus and Vetosmylus tentus have the proximally branched M, state 1 is also common in some extant species, implying the distinct variation in the position of the M fork within the subfamily. Among Kemypinae, M fork commonly located at the in-between of RP1 and RP2 from RP.
3. Subcostal veinlets. 0, simple, no distal forks; 1, many distal forks present. Simple subcostal veinlets are present in six subfamilies of Osmylidae. State 1 occurs in some genera of the subfamilies Kempyninae and Osmylinae.
4. The number of RP branches. 0, lesser, not more than 10; 1, numerous, more than 10. Number of RP branches is variable in Osmylidae, but state 0 is common in most families of Neuroptera. State 1 occurs in Kempyninae (except Euosmylus and Australysmus) and Osmylinae.
5. Arrangement of RP cross-veins. 0, two or more gradate series are present; 1, one gradate series is present; 2, irregular, gradate series is absent. State 0 occurs in the outgroups, Heterosmylus and Osmylus. In Vetosmylus and most extant kempynines, RP cross-veins generally form a row of gradate series. The irregularly arranged cross-veins in the radial sector are common in the fossil kempynine species.
6. Forewing MP. 0, branched pectinately distally; 1, branched dichotomously near the middle of the wing; 2, branched proximally (near the wing base). State 0 is common in most species of Osmylidae. State 1 occurs in a few extant species of Kempynus. State 2 only occurs in Ponomarenkius.
7. Length of CuP. 0, more than three-quarters the length of CuA, approximating equal length with CuA; 1, about half the length of CuA, and less than three-quarters the length of CuA. State 0 commonly occurs in Kempyninae and Osmylinae. State 1 occurs in Jurakempynus and Heterosmylus.
8. Forewing CuA. 0, branched pectinately distally; 1, branched dichotomously distally; 2, branched complicatedly near the middle of the wing. The pectinately branched CuA is common in Osmylidae and is considered as a synapomorphy of the family (Winterton et al., 2019; Ma et al., 2020a). State 1 only occurs in extant species of Kempyninae. State 2 only occurs in Jurakempynus.
9. Length of A1.0, approximately half the length of CuP, and less than three quarters; 1, less than or approximately half the length of CuP; 2, more than three-quarters the length of CuP. The moderate length of A1 (approximately half the length of CuP) occurs in most extant Osmylidae. In the Kempyninae, Al is distinctly beyond half the length of CuP, of which two states are detected, i.e., approximately three-quarters the length of CuP and approximately equal length of CuP. State 1 only occurs in extant species of Kempyninae. State 2 commonly occurs in most fossil kempynines.
10. Space between M branches. 0, normal or slightly dilated, with a single row of cells; 1, obviously broadened, with a single row of cells; 2, strongly broadened, with multiple rows of cells. State 0 occurs commonly in all subfamilies except Kempyninae. State 1 is distinct in some species of Kempynus that are apomorphies of the genus. State 2 only occurs in Ponomarenkius.
Characters of Hind Wing
11. The interlinked cross-veins between costal veinlets. 0, absent; 1, present. State 0 is common in Osmylidae, and state 2 only occurs in Arbusella within Kempyninae.
12. RP branches. 0, branched regularly, straight or slightly bent near wing margin; 1, strongly curved in distal half of RP, and slightly sinuous in distal part. State 0 occurs commonly in five subfamilies: Osmylinae, Protosmylinae, Gumillinae Porisminae and Spilosmylinae. State 1 is distinct in Kempynus and Euosmylus.
13. Pattern of MP branches. 0, branched pectinately in distal; 1, branched proximally near the wing base; 2, branched dichotomously in distal. State 0 is common in outgroups and most species of Osmylidae. State 1 only occurs in two fossil genera Mirokempynus and Ponomarenkius. State 2 generally presents in other kempynine genera.
14. Space between M branches. 0, both branches parallel, obviously un-broadened; 1, distinctly broadened from the middle; 2, gradually widened from the base to the distal. State 0 occurs in most subfamilies of Osmylidae except Kempyninae and Osmyninae. State 1 occurs in Osmylinae and some fossil genera of Kempyninae. State 2 is common in Kempyninae, which is regarded as an apomorphy of the subfamily.
15. Arrangement of cells between M branches. 0, a single row of cells present; 1, multiple rows of cells occupying the entire area; 2, multiple rows of cells present in distal half of MP. Multiple rows of cells only occur in Kempyninae, which is an apomorphy of this subfamily. State 1 occurs in almost all fossil species of Kempyninae. State 2 only occurs to Kempynus, Euosmylus and Euporismites.
16. Length of A1.0, about equal to or less than half the length of CuP; 1, more than half the length of CuP. State 0 occurs in most species of Osmylidae. State 1 only occurs to Sauktangidas, Jurakempynus and Heterosmylus.
17. Pattern of A3.0, A3 simple, directly reaching the margin; 1, A3 fused with the first branch of A2 and forming a small loop; 2, A3 well-development, with some pectinated branches. State 0 occurs in the outgroup of Heterosmylus and also occurs in a few genera of Gumilinae. State 1 is present in Osmylinae and Kempyninae (e.g., Euosmylus). State 2 is common within the fossil osmylids.
Results and Discussion
Systematic Paleontology
Order NEUROPTERA Linnaeus, 1758
Family OSMYLIDAE Leach, 1815
Subfamily KEMPYNINAE Carpenter, 1943
Genus ARBUSELLA Khramov, 2014b
Type species. Arbusella bella Khramov, 2014b
Included species. Arbusella bella Khramov, 2014b; Arbusella magna Khramov et al., 2017; Arbusella platyptera Ma et Wang, sp. nov.
Keys to the Extinct Species and Extant Genera of Kempyninae
1. HW: MP proximally branched and forming deep forking…………2
- HW: MP forked at the middle or in distal half of wing……………3
2. HW: MA and MP forming the similar deep branches, three rows of regular cells present in medial region…………. †Ponomarenkius Khramov et al., 2017 (CHN, J2)
- HW: The branches of MA and MP are different: MA branched distally, and MP proximally forked………………… †Mirokempynus Ma et al., 2020a (CHN, J2)
3. HW: cross-veins between MA and MP sinuous, and forming multiple rows of cells before branching of MP………………………4
- HW: cross-veins between MA and MP relatively straight, only forming a single row of cells before branching of MP…………………13
4. HW: MP forked close to the middle, each branch of MP with pectinated or dichotomous branches………………….†Sauktangida Khramov, 2014a (KG, J1)
- HW: MP only forming the distal branches…………………….5
5. HW: the presence of an additional row of gradate series between the subcostal veinlets ……………… 6 (†Arbusella Khramov, 2014b)
- HW: the absence of the gradate series between the subcostal veinlets………………………………… 8 (†Jurakempynus Wang et al., 2011b)
6. Presence of large eye spots in hind wing……………… 7
- Absence of large eye spots in the hind wing; the presence of three tint longitudinal stripes in the forewing……. †Arbusella magna Khramov et al., 2017 (CHN, J2)
7. HW: Presence of two large eye spots, CuA forming more pectinate branches (ca. 12)…………. †Arbusella bella Khramov, 2014b (KZ, J3)
- HW: Presence of three large eye spots, CuA with less pectinate branches (ca. 6)……. Arbusella platyptera Ma et Wang, sp. nov. (CHN, J2)
8. HW: M region with two relatively regular rows of quadrangular cells; CuP with deeply dichotomic branches……………. †Jurakempynus bellatulus Wang et al., 2011b (CHN, J2)
- HW: M region with 2–3 irregular rows of cells; CuP with many pectinated branches……. 9
9. HW: CuP with relatively few branches, only forming 4–5 branches…………… †Jurakempynus sinensis Wang et al., 2011b (CHN, J2)
- HW: CuP with numerous branches, more than 9 branches…………. 10
10. HW: M region relatively narrow with an additional short and narrow row of cells…………. †Jurakempynus sublimis Khramov, 2014b (MGL, J3)
- HW: M region without an additional short and narrow row of cells…………. 11
11. HW multi-row cells appeared only in distal half of the M widened region…………. †Jurakempynus epunctatus Wang et al., 2011b (CHN, J2)
- HW multi-row cells occupied almost all the M widened regions before MP distal fork……. 12
12. FW: A2 with relatively few and short branches; A2 shorter than half of A1……………………. †Jurakempynus arcanus Khramov, 2014b (KZ, J3)
- FW: A2 with relatively numerous and long branches; A2 longer than half of A1…………… †Jurakempynus loculosus Ma et al., 2020a (CHN, J2)
13. HW: MP deeply forked at the mid-length of wing; gradate series absent from both wings………………. †Euporismites Tillyard, 1916 (AUS, P1)
- HW: MP with dichotomous branched beyond the mid-length of wing; outer gradate series distinct on both wings……………… 14
14. HW: M branches widely forked, cross-veins between them somewhat sinuous……………………. Kempynus Navás, 1912 (AUS, NZL, and SA)
- HW: M branches normally forked, cells between them relatively quadrate……………….15
15. Forewings distinctly falcate, membrane strongly patterned; relative small insect (wingspan ca. 20–30 mm)…………Euosmylus Krüger, 1913 (NZL)
- Forewings not or slightly falcate, membrane-less patterned and scattered with sporadic spots; relatively large insect (wingspan > 40 mm)………………. Australysmus Kimmins, 1940 (AUS)
Arbusella platyptera Ma and Wang, sp. nov. (Figure 1).
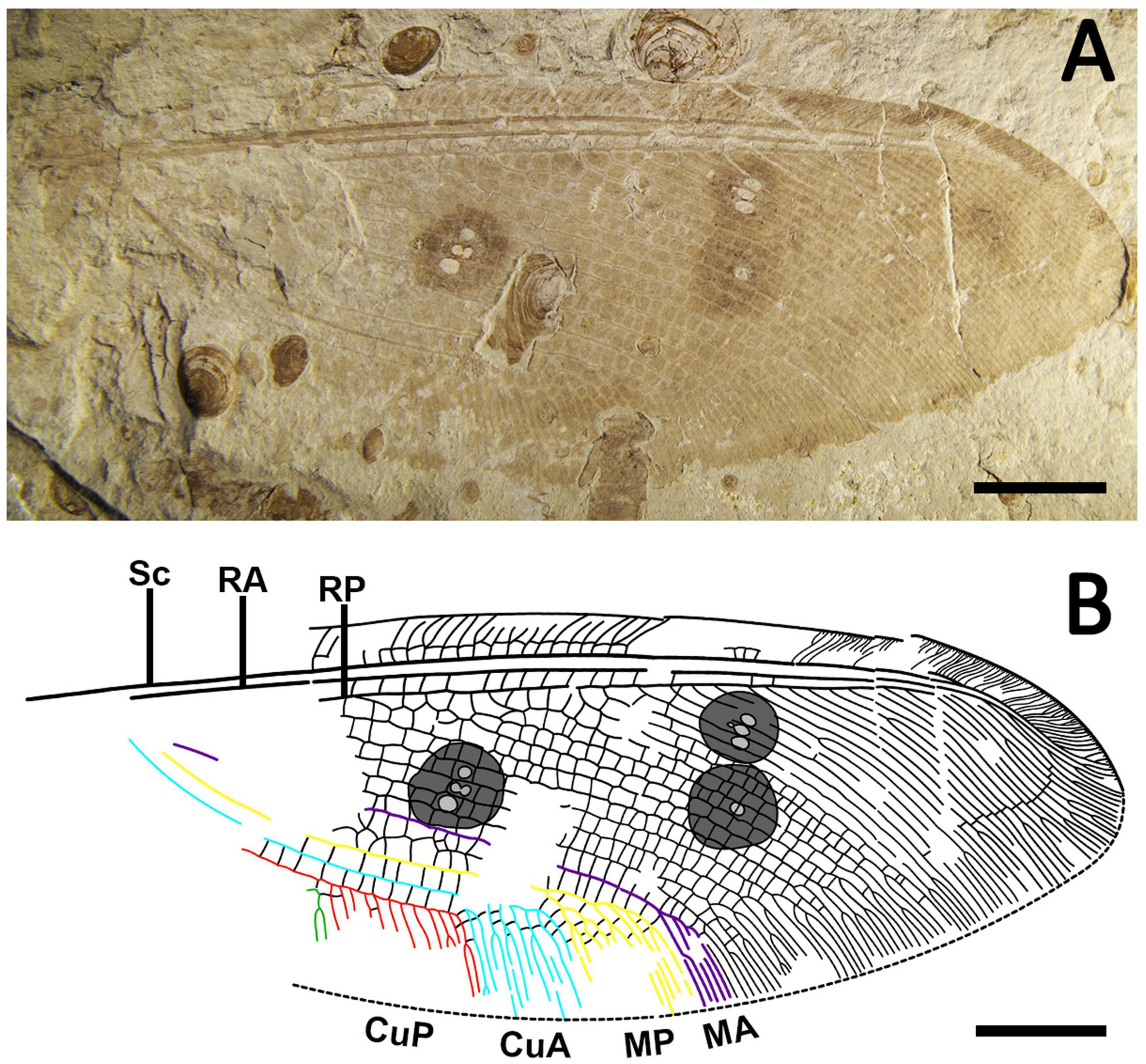
Figure 1. Lance lacewing Arbusella platyptera Ma and Wang, sp. nov. (holotype, CNU-NEU-NN2020005) from the Middle Jurassic of Jiulongshan Formation in Daohugou, China. (A) Photograph of the hind wing. (B) Line drawing of the hind wing. Scale bars are 10 mm.
Diagnosis. Large hind wing with length exceeding 40 mm and width exceeding 15 mm; membrane with three large eye spots, one located at proximal one-third of wing, and the other two closely spaced and located at distal one-third of wing; numerous and sinuate cross-veins arranged in the two-third of radial region forming densely distributed cells with irregular sizes and shapes, and the outer well-defined gradate series; MP with irregularly arranged pectinated branches in the distal, and the first branch forming dichotomous branches; CuA with 6 pectinated branches.
Etymology. From the Greek combination of platy- and -ptera, referring to the broad wing.
Type material. Holotype CNU-NEU-NN2020005. Only the hind wing is preserved.
Type locality and age. Daohugou Village, Shantou Township, Ningcheng County, Inner Mongolia, China; Jiulongshan Formation, Aalenian/Bajocian boundary, Middle Jurassic.
Description. Hind wing ca. 43.8 mm in length, 16.6 mm in width. The proximal part of the hind wing is incompletely preserved.
Hind wing (Figure 1): membrane nearly transparent except for three distinct eye spots; trichosors well-developed along the margin; subcostal veinlets dichotomously forked from the pterostigma region to the apex; subcostal cross-veins regularly arranged and forming a row of gradate series in-between subcostal veinlets; RP with more than 25 branches, and each RP branch with irregular distal bifurcations; the region between MA and MP dilated visibly in the middle, with two rows of irregular cells in-between; MA only forming three simple primary branches in distal; CuP with 11 pectinated branches; A1 relatively straight and long, about one-half of CuP.
Remarks. The hind wing is significant to the generic taxonomy of Kempyninae, and many fossil genera were established based on the characters of the hind wing (Winterton et al., 2019). The genus Arbusella was first erected by Khramov based on a species from the Late Jurassic of Kazakhstan, which was most characterized by the presence of a row of gradate series in-between subcostal veinlets (Khramov, 2014a). In spite of the absence of forewing, the new specimen can be clearly assigned to the genus of Arbusella for sharing this character. It is noted that the hind wing length of the new specimen exceeds 40 mm long as preserved, which is the hitherto known largest fossil kempynine. Although the hind wings of two other Arbusella species are preserved partially, it can still be concluded that the two species are distinctly smaller than the new specimen according to the lengths of their forewings, i.e., 22.5 mm long in A. bella and ca. 37 mm long in A. magna. Comparing two Arbusella species, the new specimen can be easily distinguished from A. bella by three eye spots in the hind wing and CuA with 6–7 branches (cf. two large eye spots and 12 branches in A. bella). As only the partial hind wing of A. magna was preserved, the new specimen was just separated from the latter species by one apomorphic character, i.e., a large eye spot present in the middle of the hind wing vs. distinctly absent in A. magna (Figure 1, Khramov, 2017: figure 4B). Considering the significant differences between the new specimen and the known Arbusella species, we established a new species for it, and it is hoped that more evidence could be found to further corroborate the taxonomic status of the new species.
Phylogenetic Results
The phylogenetic analyses resulted in one most parsimonious tree (tree length = 35; consistency index = 0.74; retention index = 0.76), which is shown in Figure 2. Based on the phylogenetic results, all fossil and extant kempynine genera were well grouped as a monophyly, which was supported by two synapomorphic characters: the distal branched M in the forewing (Cha. 2: 1) and multiple rows of cells between M branches in the hind wing (Cha. 15: 1). The latter character has been considered as a diagnostic feature of Kempyninae (Winterton et al., 2019; Ma et al., 2020b). The Kempyninae was divided into two primary clades, namely, clade A, consisting of two fossil genera Arbusella + Jurakempynus, and clade B + C, consisting of the other genera (Figure 2). The grouping of Arbusella and Jurakempynus was anticipated for their homologous venation, which was supported by one synapomorphy: the absence of gradate series in the forewing (Cha. 5: 2). The other kempynine genera were well grouped, which was supported by two apomorphic characters of the hind wing: distal branched MP (Cha. 13: 2) and the gradually divergent M branches (Cha. 14: 2). Two clades were identified in this grouping, namely, clade B, comprising three other Mesozoic fossil genera, Sauktangida, Mirokempynus, and Ponomarenkius, and clade C, comprising the only Cenozoic genus of Eupoismites and all extant genera (Figure 2). In clade B, the genus Sauktangida from the Early Jurassic is sister to Mirokempynus and Ponomarenkius, which is supported by one synapomorphy: well-developed A2 with several pectinated branches (Cha. 17:2). The grouping of two Middle Jurassic genera, Mirokempynus and Ponomarenkius was also anticipated for sharing the distinct pattern of MP branches in the hind wing (Cha. 13: 1). The four southern genera were well grouped (clade C), which was supported by three apomorphic characters: distally dichotomously branched CuA (Cha. 8: 1), A1 approximately the half the length of CuP (Cha. 9: 1); the multiple rows of cells present in the distal half of MP (Cha. 15: 2). However, the inner relationships of these southern genera were not well resolved except for the grouping of Kempynus and Euosmylus that were supported by two apomorphic characters: the modified outer margin of the forewing (Cha. 1: 1) and distinctly curved RP branches (Cha. 12: 1) (Figure 2).
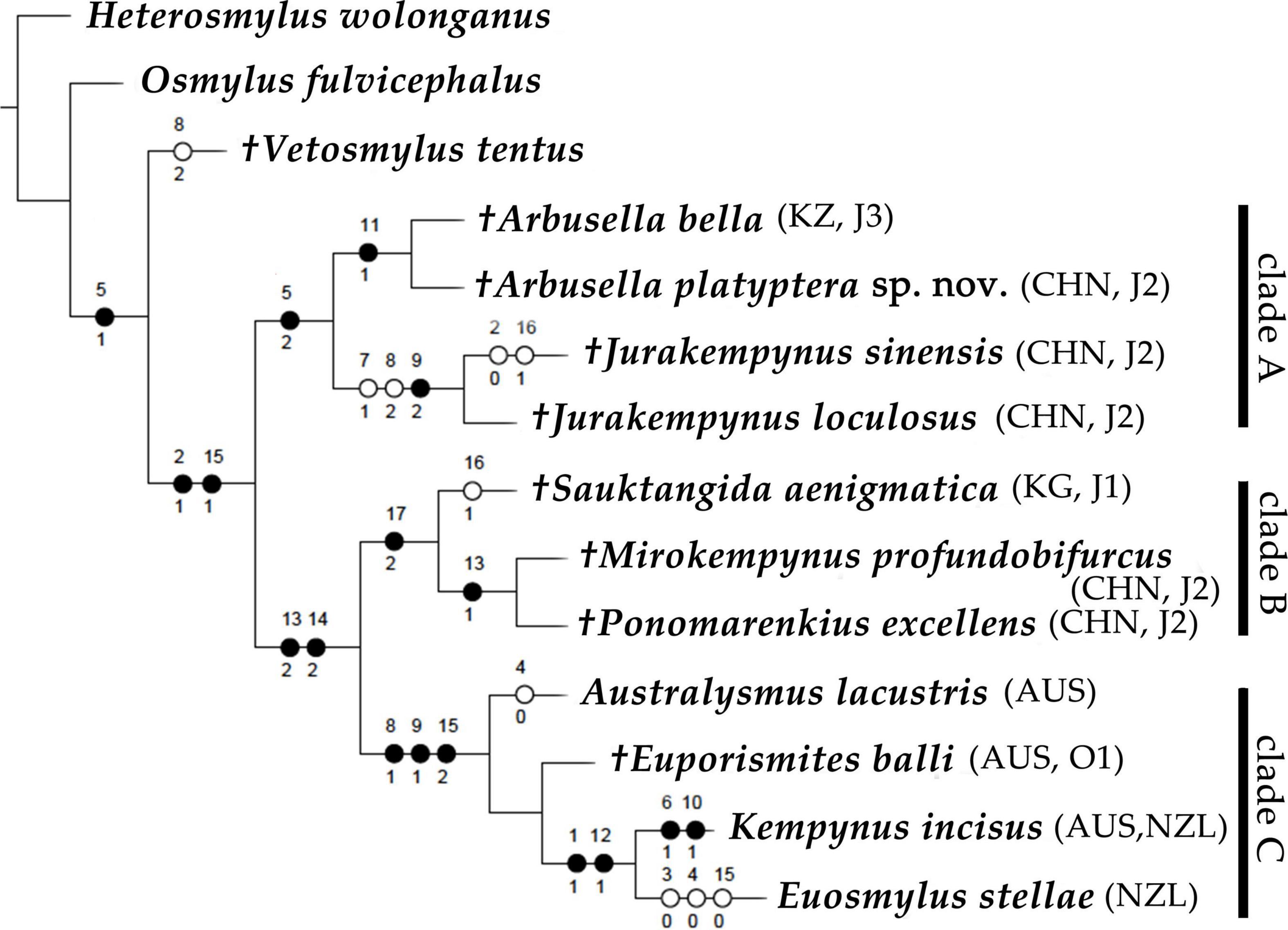
Figure 2. Most parsimonious tree of Kempyninae from phylogenetic analyses (tree length = 35 steps; consistency index, CI = 0.74; retention index, RI = 0.76); ●, apomorphic characters; ○, parallelisms and reversals.
Discussion
Considering the distribution pattern of extant lineages, Kempyninae unequivocally belongs to a typical Gondwanan group, especially for the occurrence of the Cenozoic genus Euporismites, also from Australia. However, the occurrences of diverse fossil kempynines from the Northern Hemisphere imply that the evolution of this group should have a more complicated scenario. Based on the results of phylogenetic analyses (Figure 2), the northern Mesozoic kempynines occupied the earliest divergence of the group (Figure 2: Clades A, B), which strongly implies an earlier and northern origin of this subfamily. Two questions have arisen: how and when the northern ancestors migrated to the austral areas, and how the extant kempynines shaped the current distribution pattern.
It is noted that the ancestral distribution reconstruction from DIVA analysis acquired multiple ancestral distributions, in which Australia was consistently aligned with the Northern distributions (Figure 3A: node 17). The genus Sauktangida, as the earliest fossil record of Kempyninae, in the phylogenetic tree (Figure 2), is not the most basal clade of Kempyninae, and instead, along with two Middle Jurassic genera, constitutes a derived clade that is sister to the southern lineages. The results strongly implied an earlier and prepangean origin of Kempyninae, and the ancestors first migrated to the future Gondwana before the breakup of Pangea [no later than 174 Mya (Dietz and Holden, 1970; Cox, 2000)]. This point partly agrees with the results of molecular dating that concluded the divergence time of major clades of Osmylidae was before the breakup of Pangaea (Early to Mid-Triassic, 247 Mya) (Winterton et al., 2017). In this hypothesis, the northern and southern kempynines are considered to evolve independently after the separation of Laurasia and Gondwana. As Laurasia was an intact landmass (Dietz and Holden, 1970; Cox, 2000), the northern kempynine ancestors were able to achieve a widespread distribution by an early dispersal over land (Figure 3A: nodes 10, 12), resulting in the remarkable diversity of Kempyninae in North Hemisphere. Although we could not determine the origin place of Kempyninae, it is evident that the early colonization had been proceeding, considering the widespread distribution of Jurassic kempynines in the Northern Hemisphere. It is noteworthy that no convincing Cretaceous kempynines were reported except for several equivocal records (Makarkin, 1990, 2014). Also, it is still an enigma for the ultimate disappearance of these kempynine antiques in the Northern Hemisphere. Considering the stable Laurasia landmass, the extinction of kempynines in the Northern Hemisphere was possibly not related to the plate drift but more possibly to the subsequent climate changes.
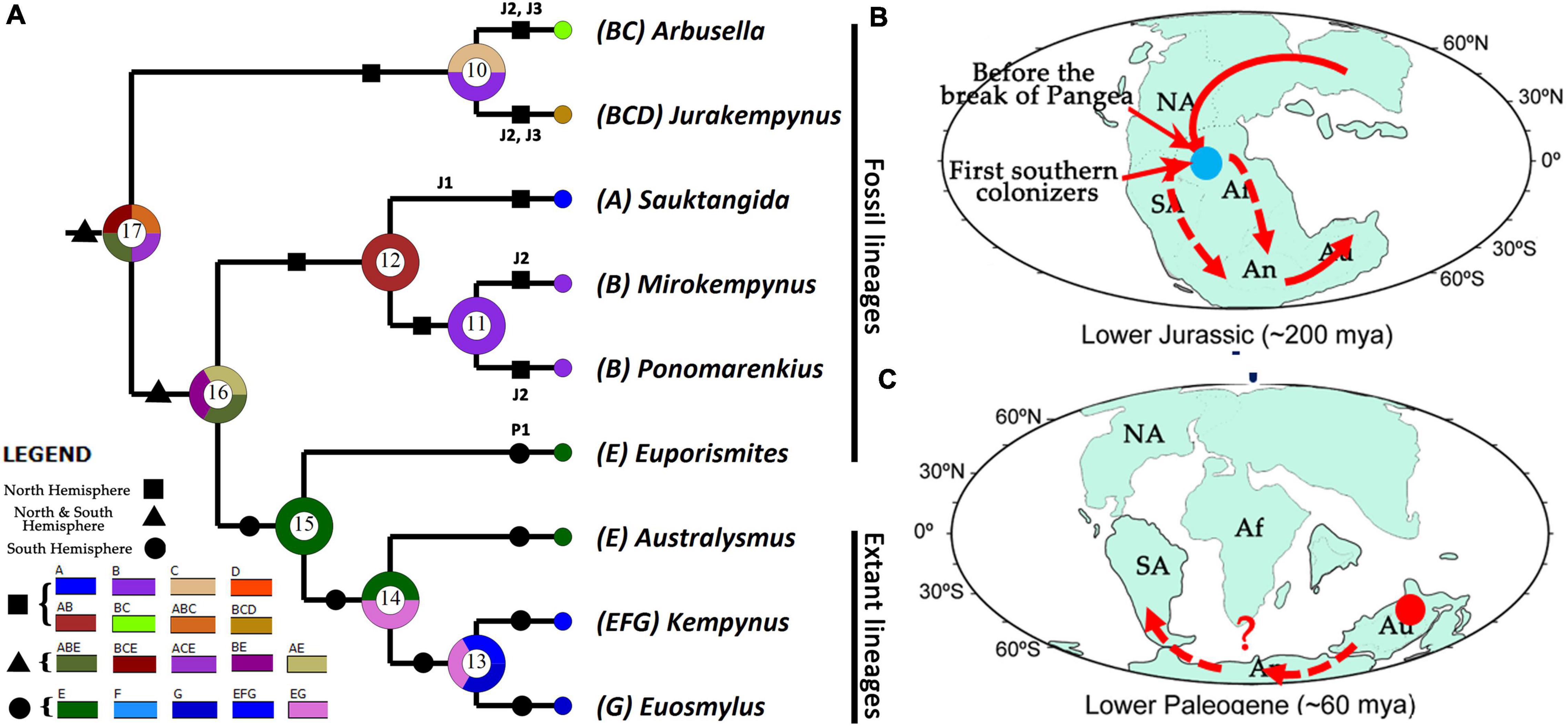
Figure 3. Ancestral distribution reconstruction and putative dispersal routes of Kempyninae. (A) Ancestral distribution reconstruction of kempynine genera from DIVA analysis (A, Sauk-Tanga of early Jurassic, Kyrgyzstan; B, Daohugou of middle Jurassic, China; C, Karatau of later Jurassic, Kazakhstan; D, Shar-Teg of late Jurassic, Mongolia; E, Australia; F, South America; G, New Zealand). (B,C) Hypothesized historical dispersal routes of kempynine ancestors (NA, North America; SA, South America; Af, Africa; An, Antarctica; Au, Australia).
It is bewildering that the absence of the Mesozoic fossil kempynines in the Southern Hemisphere (Khramov, 2017), which hindered our understanding to further trace the history of Kempyninae. It is noted that the youngest known kempynine fossil is from the Redbank Plains Formation of the late Paleocene-early Eocene in Australia [ca. 55 Mya (Tillyard, 1916; Rozefelds et al., 2016)], and it means that the ancestors of Kempyninae should have reached the southern destination no later than this time. Based on the current analyses, it is noted that all southern kempynines are grouped into a single clade (Figure 2), which shared a common ancestral distribution in DIVA analysis, i.e., Australia (Figure 3A: node 15). Considering the prepangean origin of Kempyninae, it is easy to conclude that the first southern colonizers could disperse southerly through South America or Africa to the southernmost destination (Figure 3B). It means that the extant kempynines should have no direct ancestor-descendent relationships with their northern Mesozoic relatives, but instead descended from the “first southern ancestors.” It should be mentioned that the most widespread genus Kempynus [occurs in Australia, New Zealand, and South America (Argentina and Chile)] and a New Zelanian endemic genus Euosmylus were placed in the most derived clade (Figure 2). If considering the northern origin of Kempyninae, it is easy to deduce that the northerly American lineages should represent the earlier divergence, and the Australian lineages should be a more derived clade. If so, the Kempynus should be the basalmost lineage of Kempyninae. Herein, an alternative hypothesis is proposed to explain this contradiction: the extant kempynines of Southern America should derive from Australia, which means that the extant kempynines possibly re-dispersed to South America following their Mesozoic ancestors’ route, representing a recent Paleocene-Eocene dispersal event (Figure 3C). Nevertheless, this hypothesis is far from being supported just based on the current evidence. It is hoped that a future phylogeny of extant kempynines, especially for the inclusion of all Kempynus species, could provide a deep insight into this question.
As the relic of ancient lineages, it is vital to trace the history by combining both fossil and extant groups. Although it is far from eventually revealing the evolutionary history of Kempyninae due to the currently limited evidence, the results still exposed a more complicated scenario of Kempyninae, which could direct the future research to the insects.
Conclusion
In this study, we described a new species Arbusella platyptera Ma et Wang, sp. nov. from the Jiulongshan Formation in Daohugou, Inner Mongolia, China. A key to the extinct species and extant genera of Kempyninae is provided. Combining all extant and most fossil genera, we performed phylogenetic and dispersal-vicariance analyses to trace the history of Kempyninae for the first time. The results corroborated the monophyly of Kempyninae and revealed a northern and prepangean origin for the subfamily. Our results also indicated that the Mesozoic kempynine and the extant lineages had no direct ancestor-descendent relationships. The results expose a more complicated evolutionary scenario of the relic insects and provide new insight into understand the historical evolution of insects that have a long history.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
YM contributed to conceptualization, formal analysis, and writing—original draft. CS contributed to writing—original draft. DR contributed to funding acquisition, investigation, resources, supervision, and writing—original draft. YW contributed to conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, and writing—original draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China, grant numbers: 31970383 and 31730087; the Beijing Natural Science Foundation, grant number: 5192002; the Academy for Multidisciplinary Studies of Capital Normal University; the Capacity Building for Sci-Tech Innovation–Fundamental Scientific Research Funds, grant number: 19530050144; the Program For Changjiang Scholars and Innovative Research Team in University, grant number: Irt-17r75, and the Support Project of High Level Teachers in Beijing Municipal Universities, grant number: IDHT20180518.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank the two reviewers’ critical comments to improve this study.
References
Breitkreuz, L. C. V., Winterton, S. L., and Engel, M. S. (2017). Wing tracheation in Chrysopidae and other Neuroptera (Insecta): a resolution of the confusion about vein fusion. Am. Mus. Novit. 3890, 1–44. doi: 10.1206/3890.1
Cox, C. B. (2000). Plate tectonics, seaways and climate in the historical biogeography of mammals. Mem. Inst. Oswaldo Cruz 95, 509–516. doi: 10.1590/S0074-02762000000400012
Dietz, R. S., and Holden, J. C. (1970). Reconstruction of Pangaea: breakup and dispersion of continents, Permian to present. J. Geophys. Res. 75, 4939–4956. doi: 10.1029/JB075i026p04939
Khramov, A. V. (2014a). Early osmylids (Neuroptera: Osmylidae) from the lower–middle Jurassic of Kyrgyzstan. Russ. Entomol. J. 23, 53–60. doi: 10.15298/rusentj.23.1.07
Khramov, A. V. (2014b). Lacewings of the family Osmylidae (Insecta: Neuroptera) from the Upper Jurassic of Asia. Paleontol. J. 48, 300–309. doi: 10.1134/S0031030114030095
Khramov, A. V., Liu, Q., and Zhang, H. (2017). Mesozoic diversity of relict subfamily Kempyninae (Neuroptera: Osmylidae). Hist. Biol. 31, 938–946. doi: 10.1080/08912963.2017.1411351
Kimmins, D. E. (1940). A revision of the osmylid subfamilies Stenosmylinae and Kalosmylinae (Neuroptera). Novit. Zool. 42, 165–201.
Lambkin, K. J. (1987). A re-examination of Euporismites balli Tillyard from the Palaeocene of Queensland (Neuroptera: Osmylidae: Kempyninae). Neuroptera Int. 4, 295–300.
Liu, X., Wang, Y., Shih, C., Ren, D., and Yang, D. (2012). Early evolution and historical biogeography of fishflies (Megaloptera: Chauliodinae): implications from a phylogeny combining fossil and extant taxa. PLoS One 7:e40345. doi: 10.1371/journal.pone.0040345
Ma, Y. M., Shih, C. K., Ren, D., and And Wang, Y. J. (2020a). New lance lacewings (Osmylidae: Kempyninae) from the Middle Jurassic of Inner Mongolia, China. Zootaxa 4822, 94–100. doi: 10.11646/zootaxa.4822.1.4
Ma, Y. M., Shih, C. K., Ren, D., and Wang, Y. J. (2020b). A new genus of lance lacewings from the Middle Jurassic of Inner Mongolia, China. Acta Palaeontol. Pol. 65, 363–369. doi: 10.4202/app.00691.2019
Makarkin, V. N. (1990). A new fossil genus and species of Osmylidae from the Lower Cretaceous of East Siberia (Neuroptera). Dtsch. Entomol. Z. 37, 101–103. doi: 10.1002/mmnd.19900370121
Makarkin, V. N. (2014). A new fossil genus of Osmylidae (Neuroptera) from the Early Cretaceous of Baissa. Transbaikalia 278, 8–12.
Martins, C. C., Ardila-Camacho, A., and Aspöck, U. (2016). Neotropical osmylids (Neuroptera, Osmylidae): three new species of Isostenosmylus Krüger, 1913, new distributional records, redescriptions, checklist and key for the Neotropical species. Zootaxa 4149, 1–66. doi: 10.11646/zootaxa.4149.1.1
New, T. R. (1983). A revision of the Australian Osmylidae: Kempyninae (Insecta: Neuroptera). Aust. J. Zool. 31, 393–420. doi: 10.1071/ZO9830393
Nixon, K. C. (2002). WinClada ver. 1.00.08. Ithaca, NY: KC Nixon. doi: 10.1016/B978-0-08-050625-8.50005-5
Page, R. (2001). NDE (NEXUS Data Editor for Windows): Version 0.5.0. Glasgow: University of Glasgow.
Rozefelds, A. C., Dettmann, M. E., Clifford, H. T., and Lewis, D. (2016). Macrofossil evidence of early sporophyte stages of a new genus of water fern Tecaropteris (Ceratopteridoideae: Pteridaceae) from the Paleogene Redbank Plains Formation, southeast Queensland, Australia. Alcheringa 40, 1–11. doi: 10.1080/03115518.2015.1069460
Tillyard, R. J. (1916). Studies in Australian Neuroptera. No. II. Descriptions of new genera and species of the families Osmylidae, Myrmeleontidae, and Ascalaphidae. Proc. Linn. Soc. N. S. W. 41, 41–70. doi: 10.5962/bhl.part.15306
Wang, Y. J., Liu, Z. Q., Ren, D., and Shih, C. K. (2011b). New Middle Jurassic kempynin osmylid lacewings from China. Acta Palaeontol. Pol. 56, 865–869. doi: 10.4202/app.2010.0050
Wang, Y. J., Winterton, S. L., and Liu, Z. Q. (2011a). Phylogeny and biogeography of Thyridosmylus (Neuroptera: Osmylidae). Syst. Entomol. 36, 330–339. doi: 10.1111/j.1365-3113.2010.00565.x
Winterton, S. L., Martins, C. C., Makarkin, V., Ardila-Camacho, A., and And Wang, Y. J. (2019). Lance lacewings of the world (Neuroptera: Archeosmylidae, Osmylidae & Saucrosmylidae): a review of living and fossil genera. Zootaxa 4581, 1–99. doi: 10.11646/zootaxa.4581.1.1
Winterton, S. L., Zhao, J., Garzón-Orduña, I., Wang, Y. J., and Liu, Z. Q. (2017). Phylogeny of lance lacewings (Neuroptera: Osmylidae). Syst. Entomol. 42, 555–574. doi: 10.1111/syen.12231
Yu, Y., Harris, A. J., Blair, C., and He, X. J. (2015). RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Mol. Phylogenet. Evol. 87, 46–49. doi: 10.1016/j.ympev.2015.03.008
Keywords: new species, phylogeny, evolution, fossil, biogeography, DIVA
Citation: Ma Y, Shih C, Ren D and Wang Y (2022) A New Jurassic Kempynine Species With Notes on Historical Distributions of Kempyninae Integrated Both Fossil and Extant Taxa (Neuroptera: Osmylidae). Front. Ecol. Evol. 10:920255. doi: 10.3389/fevo.2022.920255
Received: 14 April 2022; Accepted: 09 May 2022;
Published: 03 June 2022.
Edited by:
Phil Barden, New Jersey Institute of Technology, United StatesReviewed by:
Alexander Khramov, Russian Academy of Sciences (RAS), RussiaYuyu Wang, Agricultural University of Hebei, China
Copyright © 2022 Ma, Shih, Ren and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjie Wang, d2FuZ3lqb3NteUBmb3htYWlsLmNvbQ==
 Yiming Ma
Yiming Ma Chungkun Shih
Chungkun Shih Dong Ren
Dong Ren Yongjie Wang
Yongjie Wang