- 1School of Biosciences, The University of Melbourne, Melbourne, VIC, Australia
- 2Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Burwood, VIC, Australia
Darwin argued a role for sexual selection in the evolution of male sensory structures, including insect antennae, the strength of which will depend upon the importance of early arrival at receptive females. There is remarkable variation in the nature and degree of sexual dimorphism in moth antennae, with males of some species having spectacular, feathery antennae. Although it is widely assumed that these elaborate structures provide greater sensitivity to chemical signals (sex pheromones), the factors underlying the interspecific diversity in male antennal structure and size are poorly understood. Because male antennal morphology may be affected by several female life–history traits, including flight ability, we conducted a phylogenetic comparative analysis to test how these traits are linked, using data from 93 species of moths across 11 superfamilies. Our results reveal that elaborate antennae in males have evolved more frequently in species where females are monandrous. Further, female loss of flight ability evolved more frequently in species where males have elaborate antennae. These results suggest that elaborate antennae have evolved in response to more intense male competition, arising from female monandry, and that the evolution of elaborate antennae in males has, in turn, shaped the evolution of female flightlessness.
Introduction
Signalling is a crucial component of reproduction for mobile diecious species, playing a role in both bringing mates together and in facilitating mechanisms of pre–mating sexual selection (e.g., Darwin, 1871; Andersson, 1994; Rosenthal, 2017). The latter is responsible for the evolution of an extraordinary diversity of conspicuous, sexually selected signals, across the range of sensory modalities, and has attracted very extensive research interest. In many moths, females use sex pheromones (olfactory signals) to advertise their location to mate searching males. Although long–distance location–revealing sex pheromones are typically not regarded as being subject to sexual selection [see Johansson and Jones (2007)], they may cause sexual selection to act on male receptor organs (Darwin, 1871; Elgar et al., 2019).
Darwin (1871) suggested that sexual selection will favour improvements in “organs of sense” if that improves the likelihood of male mating success in a competitive environment. Darwin did not explicitly mention antennae as “organs of sense,” because pheromones and the odour receptors located on antennal sensilla (e.g., Hansson, 1995) were not known at that time (Elgar et al., 2019). Nevertheless, his perspective suggests that selection will favour males with antennal features that improve the speed of detection of sex pheromones if that allows males to locate females more quickly than rival males. The taxonomically widespread sexual dimorphism in insect antennal morphology (e.g., Schneider, 1964; Elgar et al., 2018), together with several lines of empirical evidence, are consistent with this perspective (Elgar et al., 2019). Field experiments on a sexually dimorphic moth, the gum-leaf skeletonizer Uraba lugens, demonstrated that males with longer antennae were more likely to detect lower amounts of pheromone (Johnson et al., 2017b), and laboratory experiments with the same species revealed that larvae developing in higher densities (thereby anticipating greater reproductive competition as adults) resulted in males with larger antennae and testes (Johnson et al., 2017a), and in females releasing more attractive sex pheromone (Pham et al., 2020).
Sexual dimorphism in some species of moths is remarkably striking, with the elaborate, feathery, pennate antennae of males contrasting with the simple threadlike or filiform antennae of females (Schneider, 1964; Young, 1997). Phylogenetic comparative analyses indicate that while elaborate antennae are linked with larger body size, suggesting a functional cost to these structures, this pattern is not necessarily consistent with a greater capacity to detect chemical signals, because larger females might be expected to release larger quantities of pheromone (Symonds et al., 2012). On the other hand, in species where males have elaborate antennae, there is a negative correlation between male abundance and male antennal length. This result suggests that lower population densities with concomitantly lower concentrations of pheromone, may select for larger antennae in males, at least in species where males have elaborate antennae (Symonds et al., 2012). Regardless, males of most moth species possess relatively simple filiform antennae, whilst females still emit long–distance sex pheromones. This suggests that the strength of selection on male antennal morphology is linked to other factors that determine the importance of quickly locating a female, and thus rapidly detecting her sex pheromone. A previous comparative analysis of male elaborate antennae in geometrid moths (Javoiš et al., 2019) found that they were more likely to be found in species where females were capital breeders (i.e., that eclose with greater body reserves already available for breeding). Javoiš et al. (2019) suggested that such a strategy may be associated with traits that make them more difficult for males to locate quickly, such as reduced mobility.
There is emerging interest in the effects of movement on the production and detection of signals and cues, although research is largely confined to visual and auditory sensory modalities (Tan and Elgar, 2021). Nevertheless, movement may be consequential for chemical signalling: female moths may adjust the detectability of their sex pheromones by selecting different kinds of locations where they call (emit pheromones). For example, pheromones released in closed habitats may be less easily detected, and females may compensate by moving to a more open location (Murlis et al., 1992). Clearly this option is not available to less mobile, flightless female moths. Females are flightless in roughly 1% of lepidopteran species, although it is taxonomically widespread, occurring in 25 families (Sattler, 1991). Female wing loss in these species varies from a complete loss of wings (aptery) to retaining full sized wings but with a loss of function (Tweedie, 1976; Sattler, 1991). Flightlessness is associated with winter–active adults and spring feeding, as well as high host breadth (Hunter, 1995). In most flightless species, the females are unable to move far and remain on or near their pupation site throughout their typically short adult life (Sattler, 1991). Hackman (1966) noted that these locations may not necessarily be optimal for calling and suggested that this selects for greater sensitivity to the pheromone in males. The converse may also be possible: that loss of flight evolved in part due to the speed with which highly sensitive males can locate females.
Here we use a phylogenetic comparative approach to examine several potential selection pressures favouring male antennal complexity in moths, including flightlessness and other life–history traits. Specifically, we ask whether certain female traits that potentially reduce their reproductive window (i.e., monandry, short lifespan, and the stage of egg development) are associated with the evolution of elaborate male antennae. Our hypothetical framework is that rapid location of a female would be favoured under female monandry, or when females eclose with a full complement of eggs (proovigeny): slower males may arrive after a female has mated and is no longer receptive, or after she has commenced ovipositing, and thus their sperm will fertilise fewer eggs (Jervis et al., 2005; Shuker, 2014). We hypothesise that these female life–history traits would likely increase the level of competition among males and increase the strength of selection favouring elaborate antennae, because such antennae should increase the male’s likelihood of locating the female more quickly.
Subsequently, we explore the links between male antennal morphology, female life-history and the evolution of female flightlessness. We specifically test the hypothesis proposed by Hackman (1966) that female flightlessness will be associated with the need for greater male sensitivity to sex pheromone, and hence with male elaborate antennae.
Materials and methods
Data collection
We collected information on female mating strategy, male antenna type, female flight ability, egg development, oviposition behaviour, lifespan, and fecundity for 93 species of moths from 11 superfamilies. The species were selected based on the availability of these data, which were collated from various sources including published literature, field guides and online lepidopterist resources (see Table 1).
We characterised male antennae as either simple or elaborate: the former is filiform or ciliate, whereas elaborate antennae have one or more side branches (pectinate, bipectinate, quadripectinate). Information on male antennae was obtained from published descriptions or images from various resources (see Table 1 for a full list). Females were designated as capable of flying (macropterous) or flightless: flightless females may still have wings, but do not fly (e.g., Lymantria dispar) or may be brachypterous (small remnant wings) or apterous (wingless).
Mating strategies of females were classified as either monandrous or polyandrous, using information on female remating frequencies (mating frequency of males was not taken into account, as less information is available on this). Monandrous species are those with a remating frequency of 30% or less. This is a conservative value compared with rates previously used to categorise monandry [for example, <50% in Arnqvist et al. (2000), and <40% in Torres–Vila et al. (2002)]. Where detailed data on remating frequency were not available, we followed published qualitative descriptions of species as being either monandrous (including mostly monandrous) or polyandrous.
Females were classified as either synovigenic (continuing to form eggs during their adult life) or proovigenic (eclosing as an adult with a full complement of mature eggs). Species where females eclose with some mature eggs but produce more as an adult were classified as synovigenic. We also distinguished between females that oviposit eggs singly, in multiple small clutches of eggs, or in a single clutch. We then made this classification binary by combining the data for species in the latter two categories who did not lay eggs singly, as this was necessary for statistical analysis (see below). The fecundity and lifespan of the females were obtained from published papers, and we included the midpoint if a range was given. Fecundity was the total reported number of eggs laid over the lifespan. Female lifespan was the average number of days as an adult, as observed in natural field populations where possible. Finally, as body size may affect aspects of mating behaviour (Blanckenhorn, 2000) and male antennal morphology (Symonds et al., 2012), we also obtained measures of the maximum wingspan to use as a proxy for body size (Miller, 1977, 1997). We included male wingspan if the wingspan of both sexes were reported, and the midpoint of the range if a range was reported.
Phylogenetic comparative analysis
We used phylogenetic comparative methods to control for common ancestry (Harvey and Pagel, 1991) in our analysis of evolutionary associations between traits. There is no single phylogeny that incorporates all the species in our sample, and genetic data covering these species is sufficiently poor to make phylogenetic estimation unreliable. Following recommendations by Beaulieu et al. (2012), we compiled a composite phylogeny from the published phylogenies of the families included in our sample. The full tree, along with character mapping for binary traits, is presented in Figure 1.
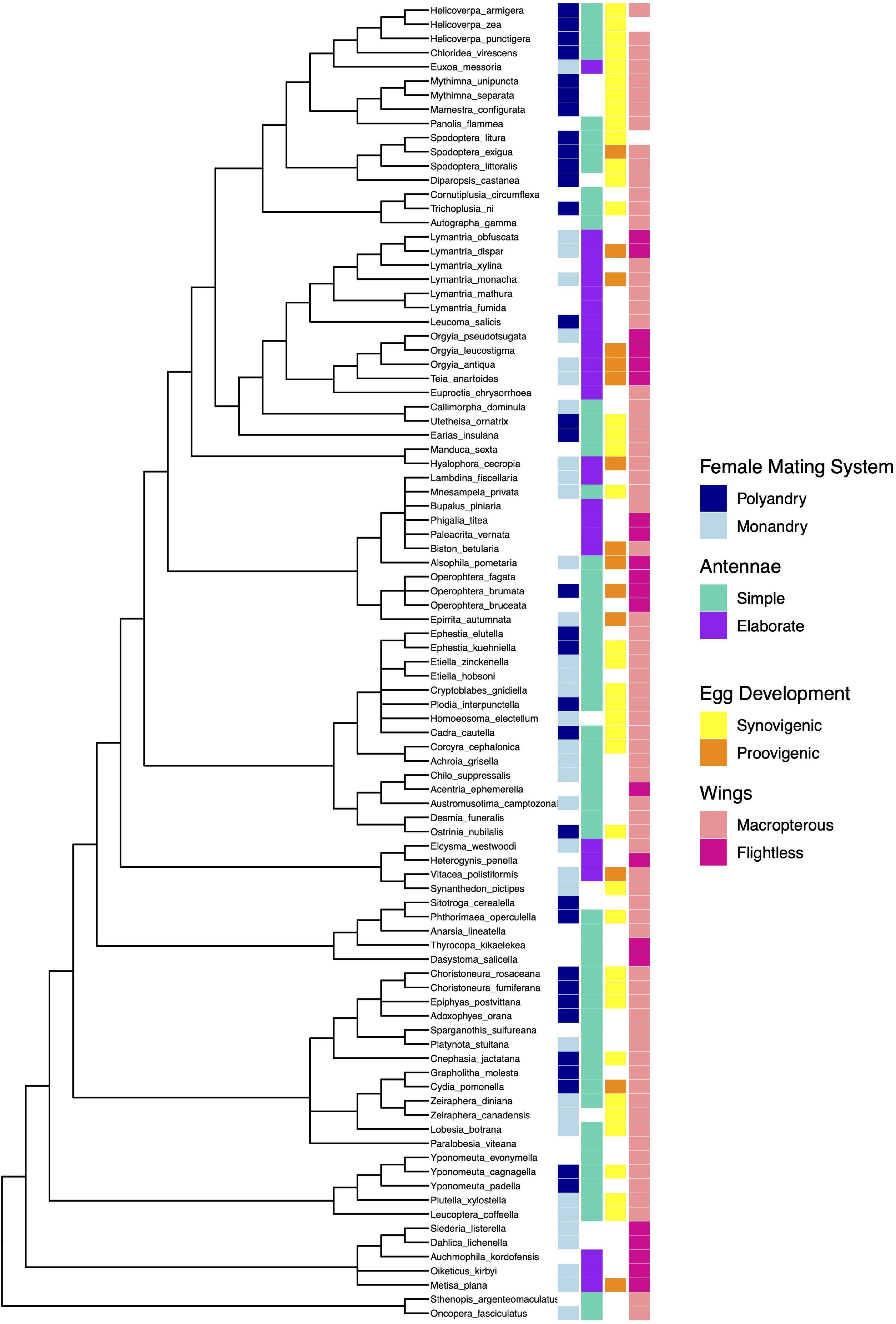
Figure 1. Composite phylogeny of the moth species used in the analysis, with character states of categorical traits indicated (missing blocks indicate the character state was unknown). Branch lengths are for illustrative purposes only, and do not represent the lengths used for analysis.
The main framework for the phylogeny was the superfamilial tree constructed by Heikkilä et al. (2015). The superfamilies Hepialoidea, Sesoidea, and Zygaenoidea each contained two species only, and so further resolution was not necessary. Any species from the same genus were also grouped together. We used the phylogeny from Regier et al. (2012b) to resolve relationships within the Pyraloidea. No further details were available for the Phycitinae family, leaving this group of eight species as a polytomy except for those species from the same genus, which were grouped together. The Tortricoidea were resolved to species level where possible following Regier et al. (2012a). Within the Tortricoidea, Paralobesia viteana was absent from all published phylogenies and therefore its position was left unresolved as a basal polytomy within this clade. The Archipini was also lacking detail on species from our analysis leaving those four species unresolved. Sohn et al. (2013) was used to fully resolve the Yponomeutoidea phylogeny, whereas Löfstedt et al. (1991) was used to resolve the three species in the genus Yponomeuta. The Bombycoidea phylogeny was taken from Mutanen et al. (2010). Mutanen et al. (2010) also provided further resolution to the Psychidae, although two species were missing rendering this group not fully resolved. Yamamoto and Sota (2007) and Sihvonen et al. (2011) were used to resolve the Geometroidea to subfamily, but no further details were available for all species present in our phylogeny leaving the six species of the Ennominae unresolved as well as the three species in the genus Operophtera. The Noctuidae were resolved using Zahiri et al. (2013), and the Lymantria phylogeny was taken from Sutrisno (2014). The superfamily Gracillariidae was fully resolved using the phylogeny from Regier et al. (2013). Relationships within the Gelechiidae were taken from Kaila et al. (2011) and Heikkilä et al. (2014).
In the absence of branch length information, all branches were assigned equal length (=1). The exception to this rule was cases where there remained uncertainty over the branching pattern; any polytomies were arbitrarily resolved but the resolved branches were assigned minimal branch lengths of 0.00001, giving them negligible weight in the analyses. This resolution was necessary because the phylogenetic comparative analysis approach requires fully dichotomously resolved phylogenies.
Following Ives and Garland (2014), we ran multiple tests to improve the strength of our inferences. These tests included generalised linear mixed models with Bayesian estimation (MCMCglmm) (Hadfield and Nakagawa, 2010) and the concentrated changes test (Maddison, 1990). The MCMCglmm models were conducted using the MCMCglmm package in R (Hadfield, 2010; R Core Team, 2014). To determine which traits are linked to our dependent variables when controlling for phylogenetic relatedness, all independent variables of interest were tested using MCMCglmm in separate models where wingspan was included as the covariate to control for body size. Specificially we performed one set of tests with antennal morphology as the dependent variable, coded as simple (1) and elaborate (2) antennae. In these tests the independent variables were female mating system (monandrous/polyandrous), egg development (proovigenic/synovigenic), and lifespan. In the second set of tests the dependent variable was female wing type coded as macropterous (1) or flightless (2). In these tests the independent predictors were female mating system, lifespan, antenna type, egg development, oviposition strategy (single eggs laid/multiple eggs clutches), and fecundity.
These models cannot incorporate missing data; therefore, the data sets were reduced to include only those species where all data were available for the variables being tested in each analysis. These tests require a prior to be set, and when the response variable is categorical in nature the prior for the residual variance should be fixed. For our analyses, we set the residual variance to 1 and used a χ2 distribution with 1 degree of freedom (v = 1, nu = 1000, alpha.mu = 0, alpha.V = 1) for the random effects variance, as suggested by Villemereuil et al. (2013). We used the categorical family response, and used the slice function to improve mixing. To obtain adequate mixing with low autocorrelation, 5 × 107 iterations were used with a 10,000 burnin period, followed by sampling every 2,500 iterations (thinning). The R code used in this procedure is provided in the Supplementary material.
The Concentrate Changes Test (CCT) was used to investigate the co–evolution of two binary discrete characters across a phylogeny, using the computer program MacClade 4 (Maddison and Maddison, 2005). CCTs examine changes from one state to another in a character of interest (the dependent variable) and whether these are more concentrated in lineages that have evolved a particular state of interest in another character (the independent variable) than would be expected by chance (determined by comparison with 1,000 permutations of the dependent variable on the phylogeny). When characters are mapped onto the phylogeny, the most parsimonious reconstruction of the character is applied for all species, including those with missing data, allowing us to leave all 93 species in each analysis. CCTs require a fully resolved phylogeny and we used MacClade to randomly resolve any polytomies 20 times in our composite phylogeny, performing the analysis on each tree individually, thereby yielding a mean p-value ± SEM. For each pair of traits, the tests were run in both directions, with the independent and dependent traits switched, to determine the order of evolution. We also applied the test only to changes where the parsimonious resolution of the evolution of the dependent was unequivocal. These tests lack the statistical power of MCMCglmm, and cannot control for body size, but unlike the MCMCglmm, they do provide information about the direction of evolution (i.e., which correlated trait preceded the evolution of the other), and possible patterns of causality.
Results
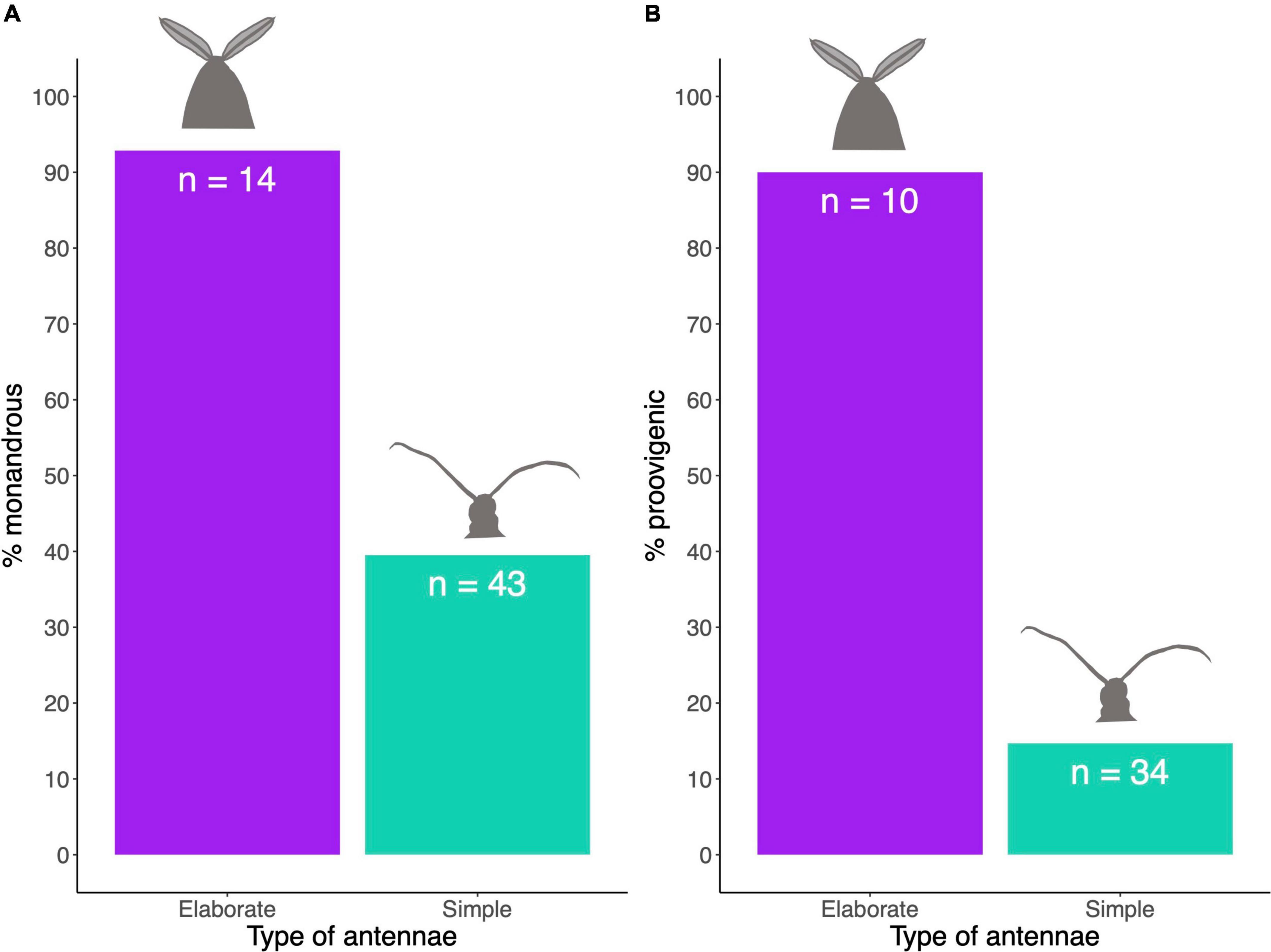
We found a significant association between antennal type and female mating system (MCMCglmm analysis: Table 2), with the concentrated changes test indicating weakly (p = 0.062) that elaborate male antennae evolve more frequently in species where females are monandrous (CCT analysis: Table 3). Indeed, most species in our analysis with elaborate male antennae have monandrous females, whereas most species with simple male antennae have polyandrous females (Figure 2A). Similarly, male antenna type is also associated with egg development pattern, with elaborate male antennae being associated with proovigenic females (Table 2). Indeed, within our sample of species with elaborate male antennae, only one is synovigenic, producing eggs as an adult (Figure 2B). However, the CCT analysis suggests (again weakly: p = 0.081) that proovigeny has evolved more frequently in species where males have elaborate antennae (Table 3). The type of male antennae was not associated with female lifespan (Table 2).
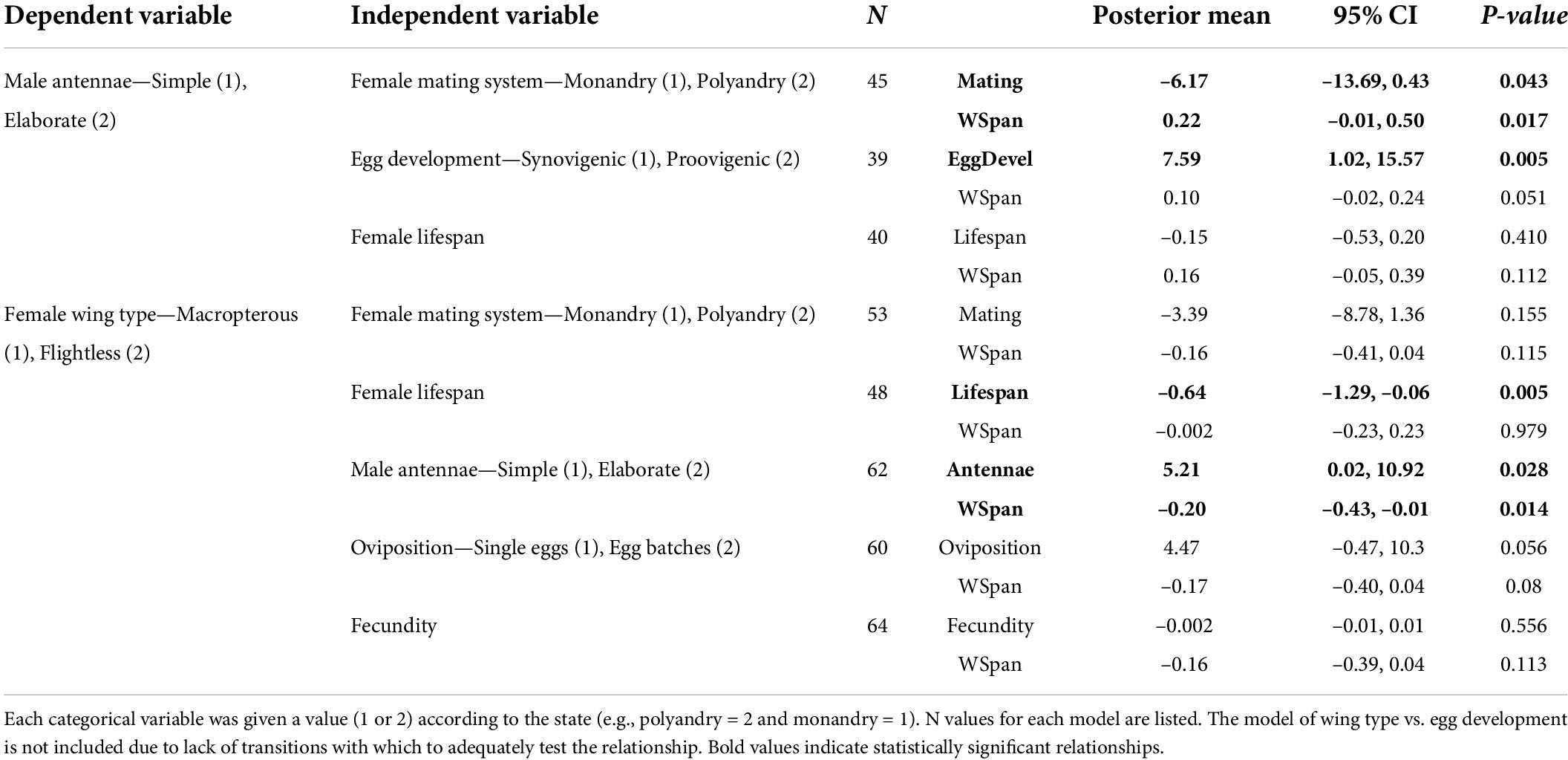
Table 2. Results from the MCMCglmm analysis comparing the dependent variable (male antennal type, female wing type) to the independent variable, controlling for body size (male wingspan).
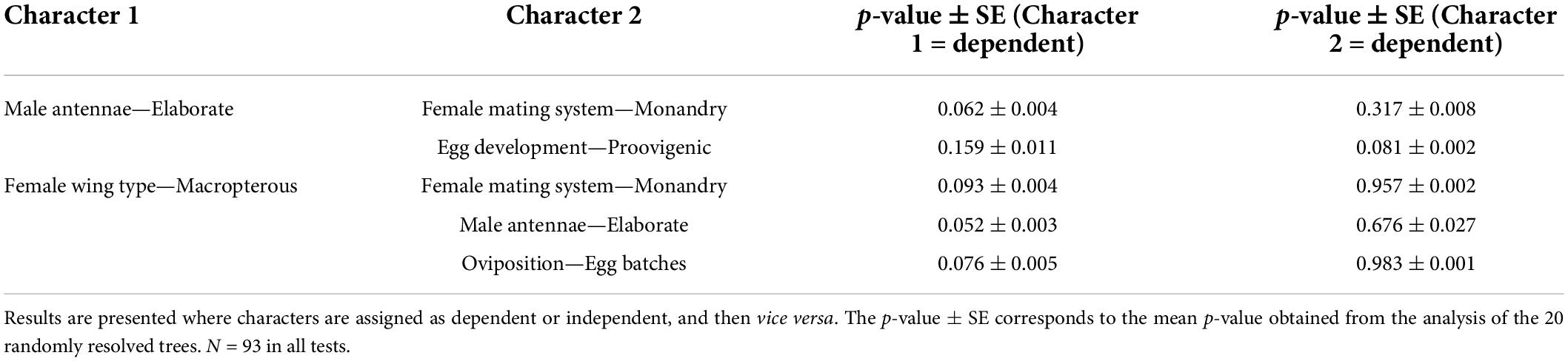
Table 3. Results from the concentrated changes test investigating evolutionary associations between discrete characters with the state of interest listed.
We found a link between female flight ability and male antenna type (Table 2), with the CCT analysis suggesting that female flightlessness is more likely to have evolved in species where males have elaborate antennae, rather than vice versa (Table 3 and Figure 3C). Female flightlessness is also associated with a shorter female lifespan (Table 2), and the association between flight ability and oviposition strategy (single eggs vs. batches) approached significance in the MCMCglmm analysis (p = 0.056, Table 2 and Figure 3D). The CCT results suggest that flightlessness has evolved more often in species where females lay eggs in clutches (Table 3). While all the flightless species in our sample are proovigenic (Figure 3B), the lack of transitions from synovigenic to proovigenic severely limits the power for the MCMCglmm analysis, and these analyses did not converge, so we do not include these tests in our analysis. Although most flightless species have monoandrous females (Figure 3A), the phylogenetic comparative analyses found no significant association between female flight ability and mating system, or between flight ability and fecundity (Tables 2, 3).
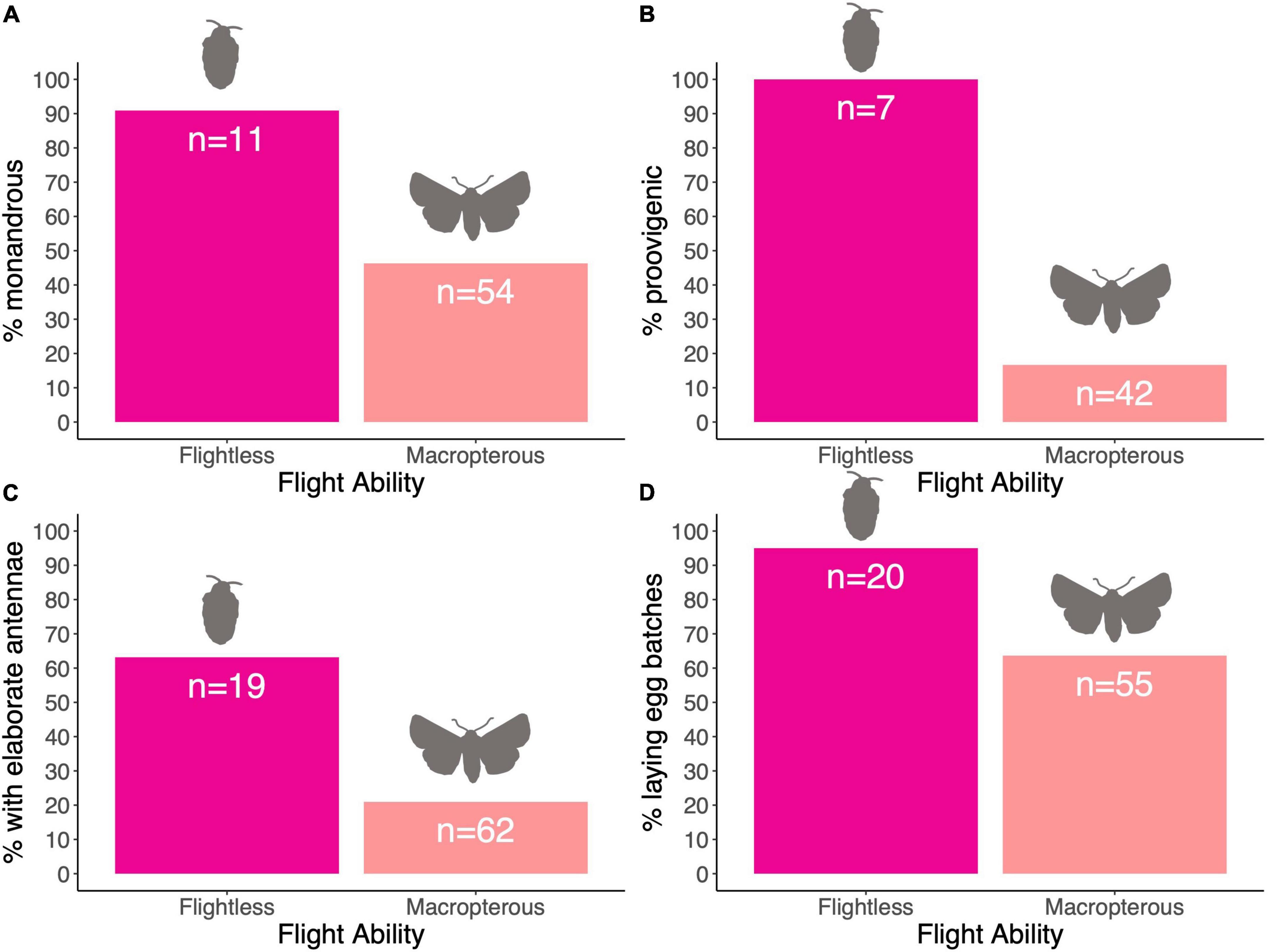
Figure 3. (A–D) Percentage of species with particular traits relative to the flight ability of female.
Discussion
Prevailing wisdom states that elaborate antennae in males evolved to increase their ability to detect odours (specifically the sex pheromone of females). However, this hypothesis is contradicted by the considerable number of moth species where males do not possess elaborate antennae, despite most species utilising long-distance female sex pheromones. Our results suggest a more nuanced version of the hypothesis where ability to detect and locate females quickly is advantageous in some species: elaborate male antennae being more common in species where females are monandrous, proovigenic, or flightless. Concentrated changes tests indicate that it is more likely that the evolution of elaborate male antennae is concentrated lineages where females are monoandrous rather than vice versa (suggesting that the mating system evolved prior to the antennal morphology). Similarly, that the CCTs suggest that female flightlessness is more likely to have evolved in lineages where elaborate male antennae had already evolved rather than the opposite, which argues against the Hackman (1966) hypothesis that female flightlessness selects for males with more sensitive antennae. These trends provide some insights into the conditions leading to the evolution of both male elaborate antennae and female flightlessness, and more generally highlight how olfactory signal perception can be linked with movement.
Darwin’s (1871) conjecture that sexual selection favours male sensory receptor traits that improve the ability to detect and locate females more quickly is supported by the strong association between complex male antennal structure and female monandry. Female monandry places a premium on males being able to rapidly locate virgin females, whose numbers may decline during the male’s adult lifespan, and larger or more elaborate male antennae apparently facilitate this process [see also Johnson et al. (2017b)]. This interpretation implicitly assumes that elaborate antennae bestow greater sensory capabilities, and this has been widely assumed in previous analyses of antennal morphology in moths (Symonds et al., 2012; Javoiš et al., 2019). However, while elaborate antennae might facilitate the trapping of air flow and hence chemical compounds (Loudon and Koehl, 2000), the direct evidence that they have higher sensitivity is surprisingly rare. In this context exceptions to the patterns outlined above can be informative. For example, selection may still favour elaborate male antennae in the polyandrous satin moth Leucoma salicis because females initially oviposit a large clutch of eggs, and subsequently lay smaller clutches (Wagner and Leonard, 1979), which places a premium on males locating virgin females. In this context, it is interesting that there is a relationship between egg development strategy and male antennal morphology, with proovigeny (where females eclose with their full complement of eggs) being associated with elaborate male antennae.
Although the large majority (>90%) of species with elaborate male antennae have monandrous females, males have filiform antennae in 17 of the 30 monandrous species in our sample. In these species, perhaps males with filiform antennae utilise other means of improving sensitivity in detecting female pheromones. For example, detectability may be improved by increasing the number and/or density of sensilla, changing the distribution of the sensilla (Keil, 1989; Lee and Strausfeld, 1990), or adjusting the angle of antennal scales (Wang et al., 2018). Additionally, features of the adult population, such as high population density (Symonds et al., 2012) or male flight speed, may relax selection on rapid detection. Finally, male mating system may also affect the evolution of antennal morphology, although the nature of the relationship between typical male mating frequency and the strength of selection for greater detection capacity seems unlikely to be independent of other factors, including female mating strategy. Consequently it may be that it is overall mating strategy (monogamy vs. polygamy) that is the stronger determinant of selection on male antennal morphology.
Female flightlessness is thought to allow females to invest more resources in egg production, thereby yielding higher fecundity (Tweedie, 1976; Roff, 1990; Sattler, 1991). Our analyses revealed that female flightlessness is associated with a shorter adult lifespan and confining oviposition to a single clutch of eggs, an unsurprising pattern because laying eggs singly or across multiple clutches would require an ability to disperse to different oviposition sites (Sattler, 1991). However, there was no significant correlation between female flightlessness and fecundity after controlling for phylogeny and body size. Hunter (1995) reported a positive correlation between female flightlessness and fecundity, but this pattern was phylogenetically constrained, and the correlation was no longer significant after taking account of phylogeny. Our analysis supports that finding. Nevertheless, we cannot rule out the possibility that females can improve reproductive success by increasing egg size rather than egg number. Indeed, the flight ability of female tussock moth Orgyia thyellina varies seasonally: individuals eclosing in autumn have reduced wings whilst those emerging in summer have fully functional wings (Kimura and Masaki, 1977). The flightless females produce much larger eggs than females that emerge in summer, a strategy that may improve the survival of eggs that diapause over winter. Males of O. thyellina have elaborate, bipectinate antennae (Table 1), and it would be interesting to know if there is a similar seasonal pattern in antennal size.
While our data broadly support the idea that the presence of elaborate antennae in males favoured the evolution of female flightlessness, there are seven species within our data set where females are flightless, and yet males have simple, filiform antennae (Figure 3). Interestingly, females in five of these species are still mobile, either by walking or hopping (Contant, 1988; Sattler, 1991; Medeiros and Gillespie, 2011), and females of the other two species have unusual biological features. Females of Acentria ephemerella are aquatic and the loss of flight is likely to be an adaptation that supports this lifestyle (Miler, 2008), highlighting that the evolution of female flightlessness is not linked exclusively with issues associated with mate search. The second species indicates that the strength of selection through mate search can override the relationship between elaborate antennae and flightlessness: around 80% of Alsophila pometaria females are pseudogamous asexuals (Mitter and Klun, 1987), who produce asexual offspring after mating, resulting in a strongly female–biased population. So, while the loss of flight has likely evolved due to similar pressures affecting other species in our data set, the pseudogamous nature of this species may relax selection favouring elaborate male antennae.
To conclude, our findings suggest that the communication and mating systems of moths are inherently associated, with elaborate male antennae being associated with lineages where females are monandrous, suggesting that necessity to detect females quickly has selected for more sensitive males. In turn male elaborate antennae may have subsequently driven the evolution of female life-history and flightlessness where having sensitive males promoted selection for complete development of eggs at the expense of movement capability. A key aspect of this narrative is that elaborate antennae in males is associated with greater sensitivity, an assumption that still needs to be more thoroughly tested in moths.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
TJ assembled the comparative data set. TJ and MS conducted the comparative statistical analyses. All authors conceived and designed the research, interpreted the data, prepared and edited the manuscript, and approved the submitted version.
Funding
We thank the Australian Research Council (DP0987360) for financial support.
Acknowledgments
We thank Nina Wedell and the late Matthew Gage for their helpful comments on an earlier version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.919093/full#supplementary-material
References
Abdullah, M., Sarnthoy, O., Chaeychomsri, S., and Sarnthoy, O. (2000). Comparative study of artificial diet and soybean leaves on growth, development and fecundity of beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). Nat. Sci. 34, 339–344.
Abernathy, R. L., Teal, P. E. A., and Tumlinson, J. H. (1994). Age and crowding affects the amount of sex pheromone and the oviposition rates of virgin and mated females of Helicoverpa zea (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 87, 350–354. doi: 10.1093/aesa/87.3.350
Akter, T., Jahan, M., and Bhuiyan, M. (2013). Biology of the angoumois grain moth, Sitotroga cerealella (Oliver) on stored rice grain in laboratory condition. J. Asia. Soc. Bangladesh Sci. 39, 61–67. doi: 10.3329/jasbs.v39i1.16034
Ammunét, T., Klemola, N., Heisswolf, A., and Klemola, T. (2009). Larval parasitism of the autumnal moth reduces feeding intensity on the mountain birch. Oecologia 159, 539–547. doi: 10.1007/s00442-008-1240-6
Anderson, P., and Lofqvist, J. (1996). Asymmetric oviposition behaviour and the influence of larval competition in the two pyralid moths Ephestia kuehniella and Plodia interpunctella. Oikos 76, 47–56. doi: 10.2307/3545747
Anderson, P., Hansson, B. S., Nilsson, U., Han, Q., Sjöholm, M., Skals, N., et al. (2007). Increased behavioral and neuronal sensitivity to sex pheromone after brief odor experience in a moth. Chem. Senses 32, 483–491. doi: 10.1093/chemse/bjm017
Anwar, M., Ashraf, M., and Arif, M. D. (1973). Mating, oviposition and gamma sterilization of the spotted bollworm of cotton, Earias insulana. Entomol. Exp. Appl. 16, 478–482. doi: 10.1111/j.1570-7458.1973.tb00299.x
Arnqvist, G., Edvardsson, M., Friberg, U., and Nilsson, T. (2000). Sexual conflict promotes speciation in insects. Proc. Nat. Acad. Sci. U S A. 97, 10460–10464. doi: 10.1073/pnas.97.19.10460
Ascher, K. R. S., Eliyahu, M., Gurevitz, E., and Renneh, S. (1983). Rearing the honeydew moth, Cryptoblabes gnidiella, and the effect of diflubenzuron on its eggs. Phytoparasitica 11, 195–198. doi: 10.1007/BF02980691
Ashworth, J. R. (1993). The biology of Ephestia elutella. J. Stored Prod. Res. 29, 199–205. doi: 10.1016/0022-474X(93)90001-K
Atanassov, A., and Shearer, P. W. (2005). Peach extrafloral nectar impacts life span and reproduction of adult Grapholita molesta (Busck) (Lepidoptera: Tortricidae). J. Agri. Urban Entomol. 22, 41–47.
Australian moths online (2018). CSIRO Australia. Available online at: https://moths.csiro.au (accessed September 2018).
Bakker, A. C., Roessingh, P., and Menken, S. B. J. (2008). Sympatric speciation in Yponomeuta: no evidence for host plant fidelity. Entomol. Exp. Appl. 128, 240–247. doi: 10.1111/j.1570-7458.2008.00684.x
Baltensweiler, W. (1993). A contribution to the explanation of the larch bud moth cycle, the polymorphic fitness hypothesis. Oecologia 93, 251–255. doi: 10.1007/BF00317678
Baltensweiler, W., and Fischlin, A. (1988). “The larch budmoth in the alps,” in Dynamics of Forest Insect Populations, ed. A. Berryman, (Berlin: Springer). doi: 10.1007/978-1-4899-0789-9_17
Barbour, D. A. (1988). The pine looper in britain and Europe,” in Dynamics of Forest Insect Populations, ed. A. Berryman, (Berlin: Springer). doi: 10.1007/978-1-4899-0789-9_15
Barnes, M. J. C. (2002). Moths of the Grenadines. Available online at http://www.mbarnes.force9.co.uk/grenadinesmoths/grenadineshome.htm.
Batra, S. W. T. (1977). Bionomics of the aquatic moth Acentropus niveus, a potential biological control agent for Eurasian water milfoil and Hydrilla. J. N. Y. Entomol. Soc. 85, 143–152.
Beaulieu, J. M., Ree, R. H., Cavender–Bares, J., Weiblen, G. D., and Donoghue, M. J. (2012). Synthesizing phylogenetic knowledge for ecological research. Ecology 93, S4–S13. doi: 10.1890/11-0638.1
Bentley, W. J., and Coviello, R. L. (2012). “Leaf–Eating lepidoptera in north american vineyards,” in Arthropod Management in Vineyards, ed N. J. Bostanian, C. Vincent, and R. Isaacs, (Berlin: Springer). doi: 10.1007/978-94-007-4032-7_13
Benz, G. (1969). Influence of mating, insemination, and other factors on oögenesis and oviposition in the moth Zeiraphera diniana. J. Insect Physiol. 15, 55–71. doi: 10.1016/0022-1910(69)90212-1
Bergh, J. C. (2012). “Grape root borer,” in Arthropod Management in Vineyards, ed N. J. Bostanian, C. Vincent, and R. Isaacs. (Berlin: Springer). doi: 10.1007/978-94-007-4032-7_16
Bezzerides, A. L., Iyengar, V. K., and Eisner, T. (2008). Female promiscuity does not lead to increased fertility of fecundity in an Arctiid moth (Utetheisa ornatrix). J. Insect Behav. 21, 213–221. doi: 10.1007/s10905-008-9121-8
Blanckenhorn, W. U. (2000). The evolution of body size: what keeps organisms small? Q. Rev. Biol. 75, 385–407. doi: 10.1086/393620
Boughton, A. J., Wu, J., and Pemberton, R. W. (2007). Mating biology of Austromusotima camptozonale (Lepidoptera: Crambidae), a potential biological control agent of Old World climbing fern, Lygodium microphyllum (Schizaeaceae). Florida Entomol. 90, 509–517. doi: 10.1653/0015-4040(2007)90[509:MBOACL]2.0.CO;2
Bradley, J. D., Tremewan, W. G., and Smith, A. (1973). British Tortricoid Moths – Cochylidae and Tortricidae: Tortricinae. London: The Ray Society.
Bradley, J. D., Tremewan, W. G., and Smith, A. (1979). British tortricid moths, Tortricidae: Olethreutinae. London: The Ray Society.
Brandt, L. S. E., Ludwar, B. C., and Greenfield, M. D. (2005). Co–occurrence of preference functions and acceptance thresholds in female choice: mate discrimination in the lesser wax moth. Ethology 111, 609–625. doi: 10.1111/j.1439-0310.2005.01085.x
Buckingham, G. R., and Ross, B. M. (1981). Notes on the biology and host specificity of Acentria nivea (= Acentropus niveus). J. Aqua. Plant Manag. 19, 32–36.
Bugguide (2017). Iowa state university. Available online at: https://bugguide.Net (accessed September 2018).
Buse, A., and Good, J. (1996). Synchronization of larval emergence in winter moth (Operophtera brumata L.) and budburst in pedunculate oak (Quercus robur L.) under simulated climate change. Ecol. Entomol. 21, 335–343. doi: 10.1046/j.1365-2311.1996.t01-1-00001.x
Butler, L. (1985). Biology of the half–wing geometer, Phigalia titea Cramer (Geometridae), as a member of a looper complex in West Virginia. J. Lep. Soc. 39, 177–186.
Capinera, J. L. (2008). “Armyworm, Pseudaletia unipuncta (Haworth) (Lepidoptera: Noctuidae),” in Encyclopedia of Entomology, ed. J. P. Capinera, (Berlin: Springer). doi: 10.1007/978-1-4020-6359-6
Cardé, R. T., and Baker, T. C. (1984). “Sexual communication with pheromones,” in Chemical Ecology of Insects, eds W. J. Bell, and R. T. Cardé, (London: Chapman and Hall Ltd), 355–383. doi: 10.1007/978-1-4899-3368-3_13
Cardé, R. T., and Hagaman, T. E. (1983). Influence of ambient and thoracic temperatures upon sexual behaviour of the gypsy moth, Lymantria dispar. Physiol. Entomol. 8, 7–14. doi: 10.1111/j.1365-3032.1983.tb00327.x
Carroll, A. L. (1994). Interactions between body size and mating history influence the reproductive success of males of a tortricid moth, Zeiraphera canadensis. Can. J. Zool. 72, 2124–2132. doi: 10.1139/z94-284
Carroll, A. L., and Quiring, D. T. (1993). Interactions between size and temperature influence fecundity and longevity of a tortricid moth, Zeiraphera canadensis. Oecologia 93, 233–241. doi: 10.1007/BF00317676
Carter, D. J. (1984). Pest Lepidoptera of Europe: with Special Reference to the British Isles. Dordrecht: Dr W. Junk Publishers.
Carter, N. E. (2004). Status of forest Pests in New Brunswick in 2003. Fredericton, NB: New Brunswick Department of Natural Resources.
Cha, D. H., Nojima, S., Hesler, S. P., Zhang, A., Linn, C. E. Jr, Roelofs, W. L., et al. (2008). Identification and field evaluation of grape shoot volatiles attractive to female grape berry moth (Paralobesia viteana). J. Chem.Ecol. 34, 1180–1189. doi: 10.1007/s10886-008-9517-0
Chao, J. T., Lu, S. S., Chen, Y. M., Jaung, L. M., Koh, C. N., and Yeh, W. C. (2001). How fecund is Lymantria xylina Swinhoe (Lepidoptera: Lymantriidae) in Taiwan? Taiwan J. Forest Sci. 16, 259–266.
Charles, J. G., Kean, J. M., and Chhagan, A. (2006). Developmental parameters and voltinism of the painted apple moth, Teia anartoides (Lepidoptera: Lymantriidae) in New Zealand. New Zealand Entomol. 29, 27–36. doi: 10.1080/00779962.2006.9722138
Charlton, R. E., Kanno, H., Collins, R. D., and Cardé, R. T. (1993). Influence of pheromone concentration and ambient temperature on flight of the gypsy moth, Lymantria dispar, in a sustained–flight wind tunnel. Physiol. Entomol. 18, 349–362. doi: 10.1111/j.1365-3032.1993.tb00608.x
Cheng, H. (1972). Oviposition and longevity of the dark–sided cutworm, Euxoa messoria (Lepidoptera: Noctuidae), in the laboratory. Can. Entomol. 104, 919–925. doi: 10.4039/Ent104919-6
Cheraghian, A. (2013). “Pine moth Bupalus piniarius Linnaeus Lepidoptera: geometridae,” in A Guide for Diagnosis Detection Of Quarantine Pests. (Islamic Republic of Iran: Bureau of Plant Pest Surveillance and Pest Risk Analysis).
Chijikwa, M. (2012). “Effects of intercropping systems on incidence and damage to cotton by Diaparopsis castanea Hampson (Lepidoptera: Actiidae),” in Magoye, Mazabuka District of Zambia. M.Sc. thesis, University Of Zambia: Zambia.
Coll, M., Gavish, S., and Dori, I. (2000). Population biology of the potato tuber moth, Phthorimaea operculella (Lepidoptera: Gelechiidae), in two potato cropping systems in Israel. Bull. Entomol. Res. 90, 309–315. doi: 10.1017/S0007485300000432
Contant, H. (1988). Modification of Microclimate by the Blueberry Leaf–tier, Cheimophila salicella (Hbn.) (Lepidoptera: Oecophoridae). M.Sc. thesis, University of British Columbia: Vancouver, BC.
Cook, K. A., and Weinzierl, R. (2004). Corn earworm (Helicoverpa zea). Insect Fact Sheet. Intergrated Pest Management. Champaign, IL: University of Illinois.
Cook, P. A., and Gage, M. J. (1995). Effects of risks of sperm competition on the numbers of eupyrene and apyrene sperm ejaculated by the moth Plodia interpunctella (Lepidoptera: Pyralidae). Behav. Ecol. Sociobiol. 36, 261–268. doi: 10.1007/BF00165835
Cook, P. A., and Wedell, N. (1999). Non–fertile sperm delay female remating. Nature 397, 486–486. doi: 10.1038/17257
Coombs, M., Del Socorro, A., Fitt, G., and Gregg, P. (1993). The reproductive maturity and mating status of Helicoverpa armigera, H. punctigera and Mythimna convecta (Lepidoptera: Noctuidae) collected in tower–mounted light traps in northern New South Wales, Australia. Bull. Entomol. Res. 83, 529–534. doi: 10.1017/S000748530003995X
Covell, C. V. J. (2005). A Field Guide to Moths of Eastern North America. Martinsville, VA: Virginian Museum of Natural History.
Cuong, N., and Cohen, M. (2003). Mating and dispersal behaviour of Scirpophaga incertulas and Chilo suppressalis (Lepidoptera; Pyralidae) in relation to resistance management for rice transformed with Bacillus thuringiensis toxin genes. Int. J. Pest Manag. 49, 275–279. doi: 10.1080/0967087031000101052
Damos, P., and Savopoulou–Soultani, M. (2008). Temperature–dependent bionomics and modeling of Anarsia lineatella (Lepidoptera: Gelechiidae) in the laboratory. J. Econ. Entomol. 101, 1557–1567. doi: 10.1093/jee/101.5.1557
Darwin, C. (1871). The Descent of Man and Selection in Relation to Sex. London: Murray. doi: 10.5962/bhl.title.2092
Delisle, J., Picimbon, J. –F., and Simard, J. (2000). Regulation of pheromone inhibition in mated females of Choristoneura fumiferana and C. rosaceana. J. Insect Physiol. 46, 913–921. doi: 10.1016/S0022-1910(99)00198-5
DePew, L. J. (1988). “Insecticides for control of sunflower moth larvae on sunflower,” in Keeping up with Research 95 (Manhattan, NY: Agricultural Experimental Station, Kansas State University). doi: 10.4148/2378-5977.7304
Deutsch, A. E., Rodriguez–Saona, C. R., Zalapa, J. E., and Steffan, S. A. (2015). Temperature–mediated development thresholds of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberries. Environ. Entomol. 44, 400–405. doi: 10.1093/ee/nvu062
Dharmadhikari, P. R., Ramaseshiah, G., and Achan, P. D. (1985). Survey of Lymantria obfuscata and its natural enemies in India. Entomophaga 30, 399–408. doi: 10.1007/BF02372346
Diamond, S. E., Hawkins, S. D., Nijhout, H. F., and Kingsolver, J. G. (2010). Evolutionary divergence of field and laboratory populations of Manduca sexta in response to host–plant quality. Ecol. Entomol. 35, 166–174. doi: 10.1111/j.1365-2311.2009.01166.x
Dustan, G. (1964). Mating behaviour of the oriental fruit moth, Grapholitha molesta (Busck) (Lepidoptera: Olethreutidae). Can Entomol. 96, 1087–1093. doi: 10.4039/Ent961087-8
Edmonds, R. P., Borden, J. H., Angerilli, N. P. D., and Rauf, A. (2000). A comparison of the developmental and reproductive biology of two soybean pod borers, Etiella spp. in Indonesia. Entomol. Exp. Appl. 97, 137–147. doi: 10.1046/j.1570-7458.2000.00724.x
Elgar, M. A., Johnson, T. L., and Symonds, M. R. E. (2019). Sexual selection and organs of sense: Darwin’s neglected insight. Anim. Biol. 69, 63–82. doi: 10.1163/15707563-00001046
Elgar, M. A., Zhang, D., Wang, Q., Wittwer, B., Pham, H., Johnson, T. L., Freelance, C. B., and Coquilleau, M. (2018). Insect antennal morphology: the evolution of diverse solutions to odorant perception. Yale J. Biol. Med. 91, 457–469.
Elzinga, J. A., Chevasco, V., Grapputo, A., and Mappes, J. (2011). Influence of male mating history on female reproductive success among monandrous Naryciinae (Lepidoptera: Psychidae). Ecol. Entomol. 36, 170–180. doi: 10.1111/j.1365-2311.2010.01258.x
Encyclopedia of Life (2018). Available online at: http://eol.org (accessed September 2018).
Engqvist, L., Cordes, N., Schwenniger, J., Bakhtina, S., and Schmoll, T. (2014). Female remating behavior in a lekking moth. Ethology 120, 662–671. doi: 10.1111/eth.12237
Etman, A., El-Sayed, F., Eesa, N., and Moursy, L. (1988). Laboratory studies on the development, survival, mating behaviour and reproductive capacity of the rice moth, Corcyra cephalonica (Stainton) (Lep., Galleriidae). J. Appl. Entomol. 106, 232–240. doi: 10.1111/j.1439-0418.1988.tb00588.x
European and Mediterranean Plant Protection Organization (2005). Lymantria mathura. EPPO Bull. 35, 464–467. doi: 10.1111/j.1365-2338.2005.00875.x
Evenden, M. L., Delury, L. E., Judd, G. J. R., and Borden, J. H. (2003). Assessing the mating status of male obliquebanded leafrollers Choristoneura rosaceana (Lepidoptera: Tortricidae) by dissection of male and female moths. Ann. Ent. Soc Am. 96, 217–224. doi: 10.1603/0013-8746(2003)096[0217:ATMSOM]2.0.CO;2
Fadamiro, H. Y., and Baker, T. C. (1999). Reproductive performance and longevity of female european corn borer, Ostrinia nubilalis: effects of multiple mating, delay in mating, and adult feeding. J. Insect Physiol. 45, 385–392. doi: 10.1016/S0022-1910(98)00137-1
Fisher, R. A., and Ford, E. B. (1947). The spread of a gene in natural conditions in a colony of the moth Panaxia dominula. Heredity 1, 143–174. doi: 10.1038/hdy.1947.11
Foster, S. P., and Ayers, R. H. (1996). Multiple mating and its effects in the lightbrown apple moth, Epiphyas postvittana (Walker). J. Insect Physiol. 42, 657–667. doi: 10.1016/0022-1910(96)00012-1
Foster, S. P., and Howard, A. J. (1999). The effects of source dosage, flight altitude, wind speed, and ground pattern on the sex pheromone–mediated flight manoeuvres of male lightbrown apple moth, Epiphyas postvittana (Walker). N. Z. J. Zool. 26, 97–104. doi: 10.1080/03014223.1999.9518181
Frago, E., Selfa, J., Pujade–Villar, J., Guara, M., and Bauce, É. (2009). Age and size thresholds for pupation and developmental polymorphism in the browntail moth, Euproctis chrysorrhoea (Lepidoptera: Lymantriidae), under conditions that either emulate diapause or prevent it. J. Insect Physiol. 55, 952–958. doi: 10.1016/j.jinsphys.2009.06.013
Furniss, R. L., and Carolin, V. M. (1977). Western Forest Insects. Washington, D.C.: US Department of Agriculture, Forest Service. doi: 10.5962/bhl.title.131875
Gage, M. J. G. (1995). Continuous variation in reproductive strategy as an adaptive response to population density in the moth Plodia interpunctella. Proc. Roy. Soc. B 261, 25–30. doi: 10.1098/rspb.1995.0112
Gerling, D., and Schwartz, A. (1974). Host selection by Telenomus remus, a parasite of Spodoptera littoralis eggs. Entomol. Exp. Appl. 17, 391–396. doi: 10.1111/j.1570-7458.1974.tb00357.x
Gilligan, T. M., and Epstein, M. E. (2014). Platynota stultana factsheet. Tortricids Agricultural Importance. Available online at https://idtools.org/id/leps/tortai/Platynota_stultana.htm (accessed March 2022).
Grant, G. G., Liu, W., Slessor, K. N., and Abou–Zaid, M. M. (2006). Sustained production of the labile pheromone component, (Z,Z)–6,9–Heneicosadien–11–one, from a stable precursor for monitoring the whitemarked tussock moth. J. Chem. Ecol. 32, 1731–1741. doi: 10.1007/s10886-006-9105-0
Grijpma, P., Belde, J. J. M., and van der Werf, D. C. (1987). Artificial diets and rearing of the nun moth, Lymantria monacha. Entomol. Exp. Appl. 45, 219–225. doi: 10.1111/j.1570-7458.1987.tb01087.x
Groenen, F., and Baixeras, J. (2013). The “Omnivorous Leafroller”, Platynota stultana Walsingham, 1884 (Tortricidae: Sparganothini), a new moth for Europe. Nota Lepidop. 36, 53–55.
Gu, H. N., and Danthanarayana, W. (2000). Variations in life history traits and flight capacity among populations of the light brown apple moth Epiphyas postvittana (Walker) (Lepidoptera : Tortricidae). Austral Ecol. 25, 571–579. doi: 10.1046/j.1442-9993.2000.01056.x
Guerreiro Filho, O. (2006). Coffee leaf miner resistance. Brazilian J. Plant Physiol. 18, 109–117. doi: 10.1590/S1677-04202006000100009
Gupta, R., and Tara, J. S. (2013). Biological studies of I Walker (Lepidoptera: Lymantridae) on apple plantations (Malus domestica Borkh.) in Jammu region of J and K, India. Munis Entomol. Zool. 8, 749–755.
Hadfield, J. D. (2010). MCMC methods for multi–response generalized linear mixed models: the MCMCglmm R package. J. Stat. Soft. 33, 1–22. doi: 10.18637/jss.v033.i02
Hadfield, J. D., and Nakagawa, S. (2010). General quantitative genetic methods for comparative biology: phylogenies, taxonomies and multi–trait models for continuous and categotical characters. J. Evol. Biol. 23, 494–508. doi: 10.1111/j.1420-9101.2009.01915.x
Harakly, F. A. (1975). Biological studies on the loopers Autographa gamma (L.) and Cornutiplusia circumflexa (L.) (Lep., Noctuidae) infesting truck crops in Egypt. J. Appl. Entomol. 78, 285–290. doi: 10.1111/j.1439-0418.1975.tb04182.x
Harris, A. (1988). A first record of the painted apple moth Teia anartoides (Lepidoptera: Lymantriidae) intercepted in New Zealand. N. Z. Entomol. 11, 68–70. doi: 10.1080/00779962.1988.9722540
Hart, M. (2006). The Role of Sonic Signals in the Sexual Communication of Peach Twig Borers, Anarsia lineatella, Zeller (Lepidoptera: Gelechiidae). M.Sc. thesis, Biological Sciences Department. Simon Fraser University: Burnaby, BC.
Harvey, P. H., and Pagel, M. D. (1991). The Comparative Method in Evolutionary Biology. Oxford: Oxford University Press.
Hattori, I., and Sato, A. (1983). Mating and oviposition of the limabean pod borer, Etiella zinckenella (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 18, 511–516. doi: 10.1303/aez.18.511
Haukioja, E., and Neuvonen, S. (1985). The relationship between size and reproductive potential in male and female Epirrita autumnata (Lep., Geometridae). Ecol. Entomol. 10, 267–270. doi: 10.1111/j.1365-2311.1985.tb00723.x
Hausmann, A., and Viidalepp, J. (2012). Larentinae I. Denmark: Apollo Books. doi: 10.1163/9789004260979
Hébert, C., and Brodeur, J. (2013). “Lambdina fiscellaria (Guenee), hemlock looper (Lepidoptera: Geometridae),” in Biological Control Programmes in Canada 2001–2012, eds P. G. Mason and D. R Gillespie (Wallingford: CABI International), 203–207. doi: 10.1079/9781780642574.0203
Hébert, C., Jobin, L., Auger, M., and Dupont, A. (2003). Oviposition traps to survey eggs of Lambdina fiscellaria (Lepidoptera: Geometridae). J. Econ. Entomol. 96, 768–776. doi: 10.1093/jee/96.3.768
Heikkilä, M., Mutanen, M., Kekkonen, M., and Kaila, L. (2014). Morphology reinforces proposed molecular phylogenetic affinities: a revised classification for Gelechioidea (Lepidoptera). Cladistics 30, 563–589. doi: 10.1111/cla.12064
Heikkilä, M., Mutanen, M., Wahlberg, N., Sihvonen, P., and Kaila, L. (2015). Elusive ditrysian phylogeny: an account of combining systematized morphology with molecular data (Lepidoptera). BMC Evol. Biol. 15:260. doi: 10.1186/s12862-015-0520-0
Hendrikse, A. (1986). The courtship behaviour of Yponomeuta padellus. Entomol. Exp. Appl. 42, 45–55. doi: 10.1111/j.1570-7458.1986.tb02186.x
Hirai, K. (1984). Migration of Pseudaletia separata Walker (Lepidoptera: Noctuidae): considerations of factors affecting time of taking–off and flight period. Appl. Entomol. Zool. 19, 422–429. doi: 10.1303/aez.19.422
Hora, K. H., and Roessingh, P. (1999). Oviposition in Yponomeuta cagnagellus: the importance of contact cues for host plant acceptance. Physiol. Entomol. 24, 109–120. doi: 10.1046/j.1365-3032.1999.00120.x
Horak, M., and Komai, F. (2006). Olethreutine Moths of Australia (Lepidoptera: Tortricidae). Melbourne, Vic: CSIRO Publishing. doi: 10.1071/9780643094086
Howlader, M. A., and Gerber, G. H. (1986). Effects of age, egg development, and mating on calling behaviour of the bertha armyworm, Mamestra configurata (Lepidoptera: Noctuidae). Can. Entomol. 118, 1221–1230. doi: 10.4039/Ent1181221-12
Hunter, A. F. (1995). The ecology and evolution of reduced wings in forest macrolepidoptera. Evol. Ecol. 9, 275–287. doi: 10.1007/BF01237773
Identification guide of Japanese Moths (2018). Available online at: http://www.jpmoth.org.
Ioriatti, C., Lucchi, A., and Varela, L. G. (2012). “Grape berry moths in western European vineyards and their recent movement into the New World,” in Arthropod Management in Vineyards. eds N. J. Bostanian, C. Vincent, and R. Isaacs (Berlin: Springer). doi: 10.1007/978-94-007-4032-7_14
Ives, A. R., and Garland, T. Jr. (2014). “Phylogenetic regression for binary dependent variables,” in Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology, ed. L. Z. Garamszegi, (Berlin: Springer). doi: 10.1007/978-3-662-43550-2_9
Iyengar, V. K., and Eisner, T. (1999). Heritability of body mass, a sexually selected trait, in an arctiid moth (Utetheisa ornatrix). Proc. Natl. Acad. Sci. U S A. 96, 9169–9171. doi: 10.1073/pnas.96.16.9169
Iyengar, V. K., and Eisner, T. (2002). Parental body mass as a determinant of egg size and egg output in an arctiid moth (Utetheisa ornatrix). J. Insect Behav. 15, 309–318.
Iyengar, V. K., Reeve, H. K., and Eisner, T. (2002). Paternal inheritance of a female moth’s mating preference. Nature 419, 830–832. doi: 10.1038/nature01027
Jallow, M. F., Zalucki, M. P., and Fitt, G. P. (1999). Role of chemical cues from cotton in mediating host selection and oviposition behaviour in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 38, 359–366. doi: 10.1046/j.1440-6055.1999.00131.x
Javoiš, J., Davis, R. B., and Tammaru, T. (2019). A comparative morphometric study of sensory capacity in geometrid moths. J. Evol. Biol. 32, 380–389. doi: 10.1111/jeb.13422
Javois, J., Tammaru, T., and Käär, M. (2005). Oviposition in an eruptive moth species, Yponomeuta evonymellus, is insensitive to the population density experienced during the larval period. Entomol. Exper. Applic. 115, 379–386. doi: 10.1111/j.1570-7458.2005.00265.x
Jervis, M. A., Boggs, C. L., and Ferns, P. N. (2005). Egg maturation strategy and its associated trade–offs: a synthesis focusing on Lepidoptera. Ecol. Entomol. 30, 359–375. doi: 10.1111/j.0307-6946.2005.00712.x
Jia, F. Y., and Greenfield, M. D. (1997). When are good genes good? variable outcomes of female choice in wax moths. Proc. R. Soc. B 264, 1057–1063. doi: 10.1098/rspb.1997.0146
Jiao, X., Xuan, W., and Sheng, C. (2006). Effects of delayed mating and male mating history on longevity and reproductive performance of the rice stem borer, Chilo suppressalis (Walker)(Lep., Pyralidae). J. Appl Entomol. 130, 108–112. doi: 10.1111/j.1439-0418.2006.01036.x
Jiménez–Pérez, A., and Wang, Q. (2004a). Effect of body weight on reproductive performance in Cnephasia jactatana (Lepidoptera: Tortricidae). J. Insect Behav. 17, 511–522. doi: 10.1023/B:JOIR.0000042538.19559.09
Jiménez–Pérez, A., and Wang, Q. (2004b). Sexual selection in Cnephasia jactatana (Lepidoptera : Tortricidae) in relation to age, virginity, and body size. Ann. Entomol. Soc. 97, 819–824. doi: 10.1603/0013-8746(2004)097[0819:SSICJL]2.0.CO;2
Jiménez–Pérez, A., Wang, Q., and Markwick, N. P. (2002). Adult activity patterns of Cnephasia jactatana (Lepidoptera: Tortricidae). N. Z. Plant Protect. 55, 374–379. doi: 10.30843/nzpp.2002.55.3935
Johansson, B. G., and Jones, T. M. (2007). The role of chemical communication in mate choice. Biol Rev. 82, 265–289. doi: 10.1111/j.1469-185X.2007.00009.x
Johnson, T. L., Symonds, M. R. E., and Elgar, M. A. (2017a). Anticipatory flexibility: larval population density in moths determines male investment in antennae, wings and testes. Proc. R. Soc. B 284:20172087. doi: 10.1098/rspb.2017.2087
Johnson, T. L., Symonds, M. R. E., and Elgar, M. A. (2017b). Sexual selection on receptor organ traits: younger females attract males with longer antennae. Sci. Nat. 104:44. doi: 10.1007/s00114-017-1466-4
Jonko, C. (2004–2022). Lepidoptera mundi. Available online at: https://lepidoptera.eu (accessed March 2022).
Jordan, T. A. (2014). Surveillance of Grape Berry Moth, Paralobesia viteana Clemens (Lepidoptera: Tortricidae), in Virginia vineyards. Ph.D. thesis, VIrginia Polytechnic Institute and State University: Blacksburg, VA.
Kaila, L., Mutanen, M., and Nyman, T. (2011). Phylogeny of the mega–diverse Gelechioidea (Lepidoptera): adaptations and determinants of success. Mol. Phylo. Evol. 61, 801–809. doi: 10.1016/j.ympev.2011.08.016
Karalius, V., and Bûda, V. (1995). Mating delay effect on moths’ reproduction: correlation between reproduction success and calling activity in female Ephestia kuehniella, Cydia pomonella, Yponomeuta cognagellus (Lepidoptera: Pyralidae). Pheromones 5, 169–190.
Kehat, M., and Gordon, D. (1975). Mating, longevity, fertility and fecundity of the cotton leaf–worm, Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae). Phytoparasitica 3, 87–102. doi: 10.1007/BF03158291
Kehat, M., and Gordon, D. (1977). Mating ability, longevity and fecundity of the spiny bollworm, Earias insulana (Lepidoptera: Noctuidae). Entomol. Exp. Appl. 22, 267–273. doi: 10.1111/j.1570-7458.1977.tb02716.x
Keil, T. A. (1989). Fine structure of the pheromone–sensitive sensilla on the antenna of the hawkmoth, Manduca sexta. Tissue Cell 21, 139–151. doi: 10.1016/0040-8166(89)90028-1
Kelly, P. M., Sterling, P. H., Speight, M. R., and Entwistle, P. F. (1988). Preliminary spray trials of a nuclear polyhedrosis virus as a control agent for the brown–tail moth, Euproctis chrysorrhoea (L.) (Lepidoptera: Lymantriidae). Bull. Entomol. Res. 78, 227–234. doi: 10.1017/S0007485300012992
Kimura, T., and Masaki, S. (1977). Brachypterism and seasonal adaptation in Orgyia thyellina (Lepidoptera, Lymantriidae). Kontyû 45, 97–106.
Kingan, T. G., Thomas–Laemont, P. A., and Raina, A. K. (1993). Male accessory gland factors elicit change from ‘virgin’ to ‘mated’ behaviour in the female corn earworm moth Helicoverpa zea. J. Exp. Biol. 183, 61–76. doi: 10.1242/jeb.183.1.61
Kipp, R. M, Larson, J., and Fusaro, A. (2022). Acentria ephemerella Olivier, 1791, U.S. Ann Arbor, MI: Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL, and NOAA Great Lakes Aquatic Nonindigenous Species Information System.
Knight, A. L. (2007). Multiple mating of male and female codling moth (Lepidoptera: Tortricidae) in apple orchards treated with sex pheromone. Environ. Entomol. 36, 157–164. doi: 10.1603/0046-225X(2007)36[157:MMOMAF]2.0.CO;2
Koshio, C. (1996). Reproductive behaviour of the white–tailed zygaenid moth, Elcysma westwoodii (Lepidoptera, Zygaenidae). 2. Female mating strategy. J. Ethol. 14, 21–25. doi: 10.1007/BF02350088
Koshio, C., Muraji, M., Tatsuta, H., and Kudo, S. (2007). Sexual selection in a moth: effect of symmetry on male mating success in the wild. Behav. Ecol. 18, 571–578. doi: 10.1093/beheco/arm017
Kristensen, N. P. (2003). Lepidoptera, Moths and Butterflies Morphology, Physiology, and Development. Berlin: Walter de Gruyter.
Lau, T. F. S., Gross, E. M., and Meyer–Rochow, V. B. (2007). Sexual dimorphism and light/dark adaptation in the compound eyes of male and female Acentria ephemerella (Lepidoptera: Pyraloidea: Crambidae). Eur. J. Entomol. 104, 459–470. doi: 10.14411/eje.2007.066
Lawrence, L. A., and Bartell, R. J. (1972). The effect of age on calling behaviour of virgin females of Epiphyas postvittana (Lepidoptera) and on their pheromone content and ovarian development. Entomol. Exp. Appl. 15, 455–464. doi: 10.1111/j.1570-7458.1972.tb00233.x
Leather, S. R. (1984). The effect of adult feeding on the fecundity, weight loss and survival of the pine beauty moth, Panolis flammea. Oecologia 65, 70–74. doi: 10.1007/BF00384464
Leather, S. R. (1994). The effect of temperature on oviposition, fecundity and egg hatch in the pine beauty moth, Panolis flammea (Lepidoptera: Noctuidae). Bull. Entomol. Res. 84, 515–520. doi: 10.1017/S0007485300032752
Lee, J. –K., and Strausfeld, N. J. (1990). Structure, distribution and number of surface sensilla and their receptor cells on the olfactory appendage of the male moth Manduca sexta. J. Neurocytol. 19, 519–538. doi: 10.1007/BF01257241
Lepiforum e. V. (2018). Bestimmung von Schmetterlingen (Lepidoptera) und ihren Praimaginalstadien. Available online at: https://lepiforum.org (accessed September 2018).
Lim, H., and Greenfield, M. D. (2008). Female arctiid moths, Utetheisa ornatrix, orient towards and join pheromonal choruses. Anim. Behav. 75, 673–680. doi: 10.1016/j.anbehav.2007.07.021
Löfstedt, C., Herrebout, W. M., and Menken, S. B. (1991). Sex pheromones and their potential role in the evolution of reproductive isolation in small ermine moths (Yponomeutidae). Chemoecology 2, 20–28. doi: 10.1007/BF01240662
Loudon, C., and Koehl, M. A. R. (2000). Sniffing by a silkworm moth: wing fanning enhances air penetration through and pheromone interception by antennae. J. Exp. Biol. 203, 2977–2990. doi: 10.1242/jeb.203.19.2977
Maddison, D. R., and Maddison, W. P. (2005). MacClade 4, Analysis of Phylogeny and Character Evolution. Version 4.08a.
Maddison, W. P. (1990). A method for testing the correlated evolution of two binary characters: are gains or losses concentrated on certain branches of a phylogenetic tree? Evolution 44, 539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x
Madge, P. (1954). A field study on the biology of the underground grass caterpillar, Oncopera fasciculata (Walker) (Lepidoptera: Hepialidae), in South Australia. Aust. J. Zool. 2, 193–204. doi: 10.1071/ZO9540193
Magalhaes, S. T. V., Guedes, R. N. C., Demuner, A. J., and Lima, E. R. (2008). Effect of coffee alkaloids and phenolics on egg–laying by the coffee leaf miner Leucoptera coffeella. Bull. Entomol. Res. 98, 483–489. doi: 10.1017/S0007485308005804
Mahmoud, M. E. E. (2015). Host Range, Behaviour, Natural Enemies and Control of the Acacia Bagworm (Auchmophila kordofensis Rebele) in North Kordofan (Sudan). M.Sc. thesis, University of Khartoum: Khartoum.
Makee, H., and Saour, G. (2001). Factors influencing mating success, mating frequency, and fecundity in Phthorimaea operculella (Lepidoptera: Gelechiidae). Environ. Entomol. 30, 31–36. doi: 10.1603/0046-225X-30.1.31
Malinen, P. (2007). Etiella zinckenella. Available online at: http://www.insects.fi (accessed September 2018).
Marcotte, M., Delisle, J., and McNeil, J. N. (2006). Impact of male mating history on the postmating resumption of sexual selection receptivity and lifetime reproductive success in Choristoneura rosaceana females. Physiol. Entomol. 31, 227–233. doi: 10.1111/j.1365-3032.2006.00510.x
Marks, R. (1976). Mating behaviour and fecundity of the red bollworm Diparopsis castanea Hmps. (Lepidoptera Noctuidae). Bull. Entomol. Res. 66, 145–158. doi: 10.1017/S0007485300006568
Mazor, M., and Dunkelblum, E. (2005). Circadian rhythms of sexual behavior and pheromone titers of two closely related moth species Autographa gamma and Cornutiplusia circumflexa. J. Chem. Ecol. 31, 2153–2168. doi: 10.1007/s10886-005-6082-7
Mazzei, P., Morel, D., and Panfili, R. (1999–2022). Moths and Butterflies of Europe and North Africa. Available online at: https://www.leps.it (accessed March 2022).
Mbata, G. N., and Ramaswamy, S. B. (1990). Rhythmicity of sex pheromone content in female Heliothis virescens: impact on mating. Physiol. Entomol. 15, 423–432. doi: 10.1111/j.1365-3032.1990.tb00531.x
McCormack, G. (2007). Cook Island Biodiversity Database. Rarotonga: Cook Island Natural Heritage Trust.
McNamara, K. B., Elgar, M. A., and Jones, T. M. (2009a). Adult responses to larval population size in the Almond moth, Cadra cautella. Ethology 116, 39–46. doi: 10.1111/j.1439-0310.2009.01714.x
McNamara, K. B., Elgar, M. A., and Jones, T. M. (2009b). Large spermatophores reduce female receptivity and increase male paternity success in the almond moth, Cadra cautella. Anim. Behav. 77, 931–936. doi: 10.1016/j.anbehav.2009.01.007
McNeil, J. N., and Delisle, J. (1989). Host plant pollen influences calling behavior and ovarian development of the sunflower moth, Homoeosoma electellum. Oecologia 80, 201–205. doi: 10.1007/BF00380151
Medeiros, M. J., and Gillespie, R. G. (2011). Biogeography and the evolution of flightlessness in a radiation of Hawaiian moths (Xyloryctidae: Thyrocopa). J. Biogeog. 38, 101–111. doi: 10.1111/j.1365-2699.2010.02402.x
Menken, S. B., Herrebout, W., and Wiebes, J. (1992). Small ermine moths (Yponomeuta): their host relations and evolution. Ann. Rev. Entomol. 37, 41–66. doi: 10.1146/annurev.en.37.010192.000353
Michereff, M. F. F., Filho, M. M., and Vilela, E. F. (2007). Mating behavior of the coffee leaf–miner Leucoptera coffeella (Lepidoptera: Lyonetiidae). Neotropical Entomol. 36, 376–382. doi: 10.1590/S1519-566X2007000300005
Michereff, M. F. F., Vilela, E. F., Michereff, M., Nery, D. M. S., and Thiebaut, J. T. (2004). Effects of delayed mating and male mating history on the reproductive potential of Leucoptera coffeella (Lepidoptera : Lyonetiidae). Agric. Forest Entomol. 6, 241–247. doi: 10.1111/j.1461-9555.2004.00227.x
Miler, O. (2008). The Aquatic Moth Acentria ephemerella as a Key Species in Submerged Aquatic Vegetation: Direct and Trait–mediated Interactions with Predators and Food Plants. Ph.D thesis, University of Konstanz: Konstanz.
Millar, J. G., Giblin, M., Barton, D., Reynard, D. A., Neill, G. B., and Underhill, E. W. (1990). Identification and field testing of female–produced sex pheromone components of the spring cankerworm, Paleacrita vernata Peck (Lepidoptera: Geometridae). J. Chem. Ecol. 16, 3393–3409. doi: 10.1007/BF00982106
Miller, W. E. (1977). Wing measure as a size index in Lepidoptera: the family Olethreutidae. Ann. Entomol. Soc. Amer. 70, 253–256. doi: 10.1093/aesa/70.2.253
Miller, W. E. (1997). Body weight as related to wing measure in hawkmoths (Sphingidae). Lepid. Soc. 51, 91–92.
Mitter, C., and Klun, J. (1987). Evidence of pheromonal constancy among sexual and asexual females in a population of fall cankerworm, Alsophila pometaria (Lepidoptera: Geometridae). J. Chem. Ecol. 13, 1823–1831. doi: 10.1007/BF01013231
Molet, T. (2013). CPHST Pest Datasheet for Cryptoblabes gnidiella. Washington, D.C: USDA–APHIS–PPQ–CPHST.
Molinari, F., and Zanrei, O. (2004). Studies on some developmental parametres of Anarsia lineatella Zell. reared on artificial diet. IOBC WPRS Bull. 27, 29–36.
Moth Photographers Group (2020). Available onlne at: http://mothphotographersgroup.msstate.edu (accessed March 2022).
Moths of Canada (2016). Canadian biodiversity information facility. Available online at: https://www.cbif.gc.ca (accessed December 2016).
Murlis, J., Elkinton, J. S., and Carde, R. T. (1992). Odor plumes and how insects use them. Ann. Rev. Entomol. 37, 505–532. doi: 10.1146/annurev.en.37.010192.002445
Mutanen, M., Wahlberg, N., and Kaila, L. (2010). Comprehensive gene and taxon coverage elucidates radiation patterns in moths and butterflies. Proc. R. Soc. Lond. B 277, 2839–2848. doi: 10.1098/rspb.2010.0392
Mutuura, A., and Freeman, T. N. (1966). The North American species of the genus Zeiraphera. J. Res. Lep. 5, 153–176. doi: 10.5962/p.266928
Nabi, M., and Harrison, R. (1983). Activity of sperm and fertility in the potato moth, Phthorimaea operculella. J. Insect Physiol. 29, 431–435. doi: 10.1016/0022-1910(83)90071-9
Nagata, K., Tamaki, Y., Noguchi, H., and Yushima, T. (1972). Changes in sex pheromone activity in adult females of the smaller tea tortrix moth Adoxophyes fasciata. J. Insect Physiol. 18, 339–346. doi: 10.1016/0022-1910(72)90132-1
Naito, A., Iqbal, A., and Hattori, I. (1986). Notes on the morphology and distribution of Etiella hobsoni (Butler), a new soybean podborer in Indonesia, with special reference to comparisons with Etiella zinckenella (Treitschke) (Lepidoptera: Pyralidae). Appl. Entomol. Zool. 21, 81–88. doi: 10.1303/aez.21.81
Naseri, B., Fathipour, Y., Moharramipour, S., and Hosseininaveh, V. (2009). Comparative life history and fecundity of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) on different soybean varieties. Entomol. Sci. 12, 147–154. doi: 10.1111/j.1479-8298.2009.00310.x
Nozato, K. (1982). Effect of group size, and silicate fertilizer application on the population density of Chilo suppressalis Walker (Lepidoptera: Pyralidae). Japanese J. Entomol. Zool. 26, 242–248.
Ochieng–Odero, J. (1992). The effect of three constant temperatures on larval critical weight, latent feeding period, larval maximal weight and fecundity of Cnephasia jactatana (Walker) (Lepidoptera: Tortricidae). J. Insect Physiol. 38, 127–130. doi: 10.1016/0022-1910(92)90041-B
Olien, W. C., Smith, B. J., and Hegwood C. P. Jr (1993). Grape root borer: a review of the life cycle and strategies for integrated control. HortScience 28, 1154–1156. doi: 10.21273/HORTSCI.28.12.1154
Olsen, A. R. (1995). “Moths (Insecta: Lepidoptera),” in Fundamentals of Microanalytical Entomology: A Practical Guide to Detecting and Identifying Filth in Foods, ed A. Olsen (Boca Raton, FL: CRC Press).
Park, Y. I., Ramaswamy, S. B., and Srinivasan, A. (1998). Spermatophore formation and regulation of egg maturation and oviposition in female Heliothis virescens by the male. J. Insect Physiol. 44, 903–908. doi: 10.1016/S0022-1910(98)00065-1
Parker, G. A., Lessells, C. M., and Simmons, L. W. (2013). Sperm competition games: a general model for precopulatory male–male competition. Evolution 67, 95–109. doi: 10.1111/j.1558-5646.2012.01741.x
Pearson, G. A., Dillery, S., and Meyer, J. R. (2004). Modelling intra–sexual competition in a sex pheromone system: how much can female movement affect female mating success? J. Theor. Biol. 231, 549–555. doi: 10.1016/j.jtbi.2004.07.010
Pham HT, McNamara KB, Elgar MA. (2020). Socially cued anticipatory adjustment of female signalling effort in a moth. Biol. Lett. 16:20200614. doi: 10.1098/rsbl.2020.0614
Phillips, J. H. H., and Proctor, J. R. (1969). Studies of fecundity and behaviour of the oriental fruit moth, Grapholitha molesta (Lepidoptera: Tortricidae), on the Niagara peninsula of Ontario. Can. Entomol. 101, 1024–1033. doi: 10.4039/Ent1011024-10
Polavarapu, S., Lonergan, G., Peng, H., and Neilsen, K. (2001). Potential for mating disruption of Sparganothis sulfureana (Lepidoptera: Tortricidae) in cranberries. J. Econ. Entomol. 94, 658–665. doi: 10.1603/0022-0493-94.3.658
Potter, K. A., Davidowitz, G., and Woods, H. A. (2011). Cross–stage consequences of egg temperature in the insect Manduca sexta. Funct. Ecol. 25, 548–556. doi: 10.1111/j.1365-2435.2010.01807.x
Powell, J. A., and Opler, P. A. (2009). Moths of western North America. Berkeley, CA: University of California Press. doi: 10.1525/9780520943773
Quiring, D. T. (1994). Diel activity pattern of a nocturnal moth, Zeiraphera canadensis, in nature. Entomol. Exp. Appl. 73, 111–120. doi: 10.1111/j.1570-7458.1994.tb01845.x
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Raijmann, L. E., and Menken, S. B. J. (2000). Temporal variation in the genetic structure of host-associated populations of the small ermine moth Yponomeuta padellus (Lepidoptera, Yponomeutidae). Biol. J. Linn. Soc. 70, 555–570. doi: 10.1111/j.1095-8312.2000.tb00217.x
Raine, J. (1966). Life history of Dasystoma salicellum Hnn. (Lepidoptera: Oecophoridae) a new pest of blueberries in British Columbia. Can. Entomol. 98, 331–334. doi: 10.4039/Ent98331-3
Ramaswamy, S. B. (1990). Periodicity of oviposition, feeding, and calling by mated female Heliothis virescens in a field cage. J. Insect Behav. 3, 417–427. doi: 10.1007/BF01052118
Reed, D. K., Mikolajczak, K. L., and Krause, C. R. (1988). Ovipositional behavior of lesser peachtree borer in presence of host–plant volatiles. J. Chem. Ecol. 14, 237–252. doi: 10.1007/BF01022544
Regier, J. C., Brown, J. W., Mitter, C., Baixeras, J., Cho, S., Cummings, M. P., et al. (2012a). A molecular phylogeny for the leaf–roller moths (Lepidoptera: Tortricidae) and its implications for classification and life history evolution. PLoS One 7:e35574. doi: 10.1371/journal.pone.0035574
Regier, J. C., Mitter, C., Solis, M., Hayden, J. E., Landry, B., Nuss, M., et al. (2012b). A molecular phylogeny for the pyraloid moths (Lepidoptera: Pyraloidea) and its implications for higher-level classification. Syst. Entomol. 37, 635–656. doi: 10.1111/j.1365-3113.2012.00641.x
Regier, J. C., Mitter, C., Zwick, A., Bazinet, A. L., Cummings, M. P., Kawahara, A. Y., et al. (2013). A large–scale, higher–level, molecular phylogenetic study of the insect order Lepidoptera (moths and butterflies). PLoS One 8:e58568. doi: 10.1371/journal.pone.0058568
Rhainds, M., and Gries, G. (1998). Density and pupation site of apterous female bagworms, Metisa plana (Lepidoptera: Psychidae), influence the distribution of emergant larvae. Can. Entomol. 130, 603–613. doi: 10.4039/Ent130603-5
Rhainds, M., Gries, G., and Castrillo, G. (1995). Pupation site affects the mating success of small but not large female bagworms, Oiketicus kirbyi (Lepidoptera: Psychidae). Oikos 74, 213–217. doi: 10.2307/3545650
Rhainds, M., Gries, G., and Min, M. M. (1999). Size– and density–dependent reproductive success of bagworms, Metisa plana. Entomol. Exp. Appl. 91, 375–383. doi: 10.1046/j.1570-7458.1999.00505.x
Richards, A., Speight, M., and Cory, J. (1999). Characterization of a nucleopolyhedrovirus from the vapourer moth, Orgyia antiqua (Lepidoptera Lymantriidae). J. Invert. Path. 74, 137–142. doi: 10.1006/jipa.1999.4870
Richerson, J. V., Cameron, E. A., and Brown, E. A. (1976). Sexual activity of the gypsy moth. Am. Midl. Nat. 95, 299–312. doi: 10.2307/2424395
Riddiford, L. M., and Ashenhurst, J. B. (1973). The switchover from virgin to mated bahaviour in female cecropia moths: the role of the bursa copulatrix. Biol. Bull. 144, 162–171. doi: 10.2307/1540153
Riemann, J. G. (1986). Reproductive potential and other aspects of the biology of the sunflower moth, Homoeosoma electellum (Hulst) (Lepidoptera, Pyralidae). J. Kansas Entomol. Soc. 59, 32–36.
Robison, D., Abrahamson, L., Raffa, K., and White, E. (1998). Spruce budworm (Lepidoptera: Tortricidae) field fecundity: new insights into its estimation and use. Forest Ecol. Manag. 106, 73–81. doi: 10.1016/S0378-1127(97)00225-9
Roff, D. A. (1990). The evolution of flightlessness in insects. Ecol. Monog. 60, 389–421. doi: 10.2307/1943013
Ronkay, L., and Behounek, G. (2015). Nyungwe lepidoptera diversity project. Available online at: http://driegrain.be (accessed November 2015).
Rosenthal, G. G. (2017). Mate Choice: The Evolution of Sexual Decision Making from Microbes to Humans. Princeton, NJ: Princeton University Press.
Royer, L., and McNeil, J. N. (1993). Male investment in the European corn borer, Ostrinia nubilalis (Lepidoptera: Pyralidae): impact on female longevity and reproductive performance. Funct. Ecol. 7, 209–215. doi: 10.2307/2389889
Sadek, M. M. (2001). Polyandry in field–collected Spodoptera littoralis moths and laboratory assessment of the effects of male mating history. Entomol. Exp. Appl. 98, 165–172. doi: 10.1046/j.1570-7458.2001.00771.x
Schneider, D. (1964). Insect antennae. Ann. Rev. Entomol. 9, 103–122. doi: 10.1146/annurev.en.09.010164.000535
Schneider, J. C. (1980). The role of parthenogenesis and female aptery in microgeographic, ecological adaptation in the fall cankerworm, Alsophila pometaria Harris (Lepidoptera: Geometridae). Ecology 61, 1082–1090. doi: 10.2307/1936827
Sedlacek, J. D., Weston, P. A., and Barney, R. J. (1995). Lepidoptera and Psocoptera. New York, NY: Marcel Dekker, Inc.
Seth, R., and Sharma, V. (2002). Growth, development, reproductive competence and adult behaviour of Spodoptera litura (Lepidoptera: Noctuidae) reared on different diets. Eval. Lepidoptera Population Suppression Radiation Induced Sterility 1283, 15–22.
Shorey, H., Andres, L., and Hale, R. (1962). The biology of Trichoplusia ni (Lepidoptera: Noctuidae). I. life history and behavior. Ann. Entomol. Soc. Am. 55, 591–597. doi: 10.1093/aesa/55.5.591
Shuker, D. M. (2014). “Sexual selection theory,” in The Evolution of Insect Mating Systems, eds D. M. Shuker, and L. W. Simmons, (Oxford: Oxford University Press), 20–25. doi: 10.1093/acprof:oso/9780199678020.003.0002
Sihvonen, P., Mutanen, M., Kaila, L., Brehm, G., Hausmann, A., and Staude, H. S. (2011). Comprehensive molecular sampling yields a robust phylogeny for geometrid moths (Lepidoptera: Geometridae). PLoS One 6:e20356. doi: 10.1371/journal.pone.0020356
Skuhravy, V. (1987). A review of research on the nun moth (Lymantria monacha L.) conducted with pheromone traps in Czechoslovakia, (1973–1984). Anz Schädlingskde Pflanzenschutz Umweltschutz 60, 96–98. doi: 10.1007/BF01906038
Snäll, N., Tammaru, T., Wahlberg, N., Viidalepp, J., Ruohomäki, K., Savontaus, M., et al. (2007). Phylogenetic relationships of the tribe Operophterini (Lepidoptera, Geometridae): a case study of the evolution of female flightlessness. Biol. J. Linn. Soc. 92, 241–252. doi: 10.1111/j.1095-8312.2007.00834.x
Sohn, J. –C., Regier, J. C., Mitter, C., Davis, D., Landry, J. –F., Zwick, A., et al. (2013). A molecular phylogeny for Yponomeutoidea (Insecta, Lepidoptera, Ditrysia) and its implications for classification, biogeography and the evolution of host plant use. PLoS One 8:e55066. doi: 10.1371/journal.pone.0055066
Sorenson, C. J., and Gunnell, F. H. (1955). Biology and control of the peach twig borer (Anarsia lineatella Zeller) in Utah. Bulletin No. 379. UAES Bulletins: China
St. Laurent, R., and McCarthy, R. (2016). Asian Defoliators. National Plant Diagnostic Network. Training and education. Available online at: https://www.firstdetector.org/sites/firstdetector.org/files/pdf/AsianDefoliator_lookalikes_show.pdf (accessed March 2022).
Steinbauer, M. J. (2005). How does host abundance affect oviposition and fecundity of Mnesampela privata? Environ. Entomol. 34, 281–291. doi: 10.1603/0046-225X-34.2.281
Steinbauer, M. J., McQuillan, P. B., and Young, C. J. (2001). Life history and behavioural traits of Mnesampela privata that exacerbate population responses to eucalypt plantations: comparisons with Australian and outbreak species of forest geometrid from the Northern Hemisphere. Austral Ecol. 26, 525–534. doi: 10.1046/j.1442-9993.2001.01130.x
Suckling, D., Barrington, A., Chhagan, A., Stephens, A., Burnip, G., Charles, J., et al. (2007). “Eradication of the Australian painted apple moth Teia anartoides in New Zealand: trapping, inherited sterility, and male competitiveness,” in Area–wide Control of Insect Pests, eds M. J. B. Vreysen, A. S. Robinson, and J. Henrichs. Springer: Berlin.
Sutrisno, H. (2014). Molecular phylogeny of Indonesian Lymantria Tussock moths (Lepidoptera: Erebidae) based on COI gene sequences. J. Species Res. 3, 7–16. doi: 10.12651/JSR.2014.3.1.007
Svard, L., and McNeil, J. N. (1994). Female benefit, male risk – polyandry in the true armyworm Pseudaletia unipuncta. Behav. Ecol. Sociobiol. 35, 319–326. doi: 10.1007/BF00184421
Swaby, J. A., Daterman, G. E., and Sower, L. L. (1987). Mating behavior of douglas–fir tussock moth, Orgyia pseudotsugata (Lepidoptera: Lymantriidae), with special reference to effects of female age. Ann. Entomol. Soc. Am. 80, 47–50. doi: 10.1093/aesa/80.1.47
Symonds, M. R. E., Johnson, T. L., and Elgar, M. A. (2012). Pheromone production, male abundance, body size, and the evolution of elaborate antennae in moths. Ecol. Evol. 2, 227–246. doi: 10.1002/ece3.81
Talekar, N. S., and Shelton, A. M. (1993). Biology, ecology, and management of the diamondback moth. Ann. Rev. Entomol. 38, 275–301. doi: 10.1146/annurev.en.38.010193.001423
Tamhankar, A. J. (1995). Host influence on mating behavior and spermatophore reception correlated with reproductive output and longevity of female Earias insulana (Biosduval) (Lepidoptera: Noctuidae). J. Insect Behav. 8, 499–511. doi: 10.1007/BF01995322
Tammaru, T., Esperk, T., and Castellanos, I. (2002). No evidence for cost of being large in females of Orgyia spp. (Lepidoptera, Lymantriidae): larger is always better. Oecologia 133, 430–438. doi: 10.1007/s00442-002-1057-7
Tammaru, T., Ruohomäki, K., and Saikkonen, K. (1996). Components of male fitness in relation to body size in Epirrita autumnata (Lepidoptera, Geometridae). Ecol. Entomol. 21, 185–192. doi: 10.1111/j.1365-2311.1996.tb01186.x
Tan, E. J., and Elgar, M. A. (2021). Motion: enhancing signals and concealing cues. Biol. Open 10:bio058762, doi: 10.1242/bio.058762
Tang, H., Li, J., Wu, J., Gao, H., and Zhang, S. (2014). Development characteristics and controlling countermeasures of rice stem borer, Chilo suppressalis (walker). Ag. Sci. Technol. 15, 843–845.
Tanhuanpää, M., Ruohomäki, K., and Kaitaniemi, P. (2003). Influence of adult and egg predation on reproductive success of Epirrita autumnata (Lepidoptera: Geometridae). Oikos 102, 263–272. doi: 10.1034/j.1600-0706.2003.12546.x
Tarlack, P., Mehrkhou, F., and Mousavi, M. (2015). Life history and fecundity rate of Ephestia kuehniella (Lepidoptera: Pyralidae) on different wheat flour varieties. Arch. Phytopath. Plant Protect. 48, 95–103. doi: 10.1080/03235408.2014.882135
Thakur, B., Chakrabarti, S., and Mattu, V. K. (2016). Re–description of the adults of indian gypsy moth Lymantria obfuscata Walker (Lepidoptera: Lymantriidae) in Himachal Pradesh, India. Munis Entomol. Zool. 11, 105–113.
Thurston, G. S., and MacGregor, J. D. (2003). Body size–realized fecundity relationship of whitemarked tussock moth. Can. Entomol. 135, 583–586. doi: 10.4039/n02-104
Tingle, F. C., and Mitchell, E. R. (1991). Effect of oviposition deterrents from elderberry on behavioral responses by Heliothis virescens to host–plant volatiles in flight tunnel. J. Chem. Ecol. 17, 1621–1631. doi: 10.1007/BF00984693
Tisdale, R. A., and Sappington, T. W. (2001). Realized and potential fecundity, egg fertility, and longevity of laboratory–reared female beet armyworm (Lepidoptera: Noctuidae) under different adult diet regimes. Ann. Entomol. Soc. Am. 94, 415–419. doi: 10.1603/0013-8746(2001)094[0415:RAPFEF]2.0.CO;2
Torres–Vila, L. M., Gragera, J., Rodríguez–Molina, M. C., and Stockel, J. (2002). Heritable variation for female remating in Lobesia botrana, a usually monandrous moth. Anim. Behav. 64, 899–907. doi: 10.1006/anbe.2003.2000
Torres–Vila, L. M., Rodriguez–Molina, M. C., and Jennions, M. D. (2004). Polyandry and fecundity in the Lepidoptera: can methodological and conceptual approaches bias outcomes? Behav. Ecol. Sociobiol. 55, 315–324. doi: 10.1007/s00265-003-0712-2
Torres–Vila, L. M., Rodríguez–Molina, M. C., Gragera, J., and Bielza–Lino, P. (2001). Polyandry in Lepidoptera: a heritable trait in Spodoptera exigua Hübner. Heredity 86, 177–183. doi: 10.1046/j.1365-2540.2001.00821.x
Tuskes, P. M., Tuttle, J. P., and Collins, M. M. (1996). The Wild Silk Moths of North America: A Natural History of the Saturniidae of the United States and Canada. Ithaca, NY: Cornell University Press,. doi: 10.7591/9781501738005
Van der Pers, J., Cuperus, P., and Den Otter, C. (1980). Distribution of sense organs on male antennae of small ermine moths, Yponomeuta spp.(Lepidoptera: Yponomeutidae). Int. J. Insect Morph. Embryol. 9, 15–23. doi: 10.1016/0020-7322(80)90032-X
Van Dongen, S., Matthysen, E., Sprengers, E., and Dhondt, A. A. (1998). Mate selection by male winter moths Operophtera brumata (Lepidoptera, Geometridae): adaptive male choice or female control? Behaviour 135, 29–42. doi: 10.1163/156853998793066401
Van Dongen, S., Sprengers, E., Lofstedt, C., and Matthysen, E. (1999). Fitness components of male and female winter moths (Operophtera brumata L.) (Lepidoptera, Geometridae) relative to measures of body size and asymmetry. Behav. Ecol. 10, 659–665. doi: 10.1093/beheco/10.6.659
Vegliante, F. (2005). Larval head anatomy of Heterogynis penella (Zygaenoidea, Heterogynidae), and a general discussion of caterpillar head structure (Insecta, Lepidoptera). Acta Zool. 86, 167–194. doi: 10.1111/j.1463-6395.2005.00198.x
Villemereuil, P., Gimenez, O., and Doligez, B. (2013). Comparing parent–offspring regression with frequentist and Bayesian animal models to estimate heritability in wild populations: a simulation study for Gaussian and binary traits. Meth. Ecol. Evol. 4, 260–275. doi: 10.1111/2041-210X.12011
Wagner, T. L., and Leonard, D. E. (1979). Aspects of mating, oviposition, and flight in the satin moth, Leucoma salicis (Lepidoptera, Lymantriidae). Can. Entomol. 111, 833–840. doi: 10.4039/Ent111833-7
Walker, K. (2005). Summer fruit tortrix (Adoxophyes orana). Available online at: https://www.padil.gov.au (accessed March 2022).
Walker, P. W., and Allen, G. R. (2010). Mating frequency and reproductive success in an income breeding moth, Mnesampela privata. Entomol. Exp. Appl. 136, 290–300. doi: 10.1111/j.1570-7458.2010.01028.x
Walker, P. W., and Allen, G. R. (2011). Delayed mating and reproduction in the autumn gum moth Mnesampela privata. Ag. Forest Entomol. 13, 341–347. doi: 10.1111/j.1461-9563.2011.00524.x
Wallace, E., Albert, P., and McNeil, J. (2004). Oviposition behavior of the eastern spruce budworm Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae). J. Insect Behav. 17, 145–154. doi: 10.1023/B:JOIR.0000028565.41613.1a
Wallner, W. E. (1989). “Overview of pest lymantriidae of North America,” in Proceedings: Lymantriidae: a Comparison of Features of New and Old World Tussock Moths, eds W. E. Wallner, and, K. A. McManus (New Haven, CT: USDA Forest Service), 65–79.
Wang, Q., Shang, Y., Hilton, D., Inthavong, K., Zhang, D., and Elgar, M. A. (2018). Antennal scales improve signal detection efficiency for moth communication. Proc. R. Soc. Lond. B 285:20172832. doi: 10.1098/rspb.2017.2832
Wang, X. P., Fang, Y. –L., and Zhang, Z. N. (2005). Effect of male and female multiple mating on the fecundity, fertility, and longevity of diamondback moth, Plutella xylostella. J. Appl. Entomol. 129, 39–42. doi: 10.1111/j.1439-0418.2005.00931.x
Ward, K. E., and Landolt, P. J. (1995). Influence of multiple matings on fecundity and longevity of female cabbage looper moths (Lepidoptera: Noctuidae). Ann. Entomol. Soc. Am. 88, 768–772. doi: 10.1093/aesa/88.6.768
Watson, A., Whalley, P. E. S., and Duckworth, W. D. (1975). The Dictionary of Butterflies and Moths in Color. New York, NY: McGraw–Hill.
Watson, L., and Dallwitz, M. J. (2003–2022). Insects of Britain and Ireland: the genera of Lepidoptera– Noctuidae. Available online at: https://www.delta-intkey.com/britin/noc/intro.htm (accessed March 2022).
Watson, R., and Hill, M. (1985). Pests of maize in New Zealand. maize - management to market. Agronomy Soc. New Zealand Special Publ. 4, 47–58.
WCCP (1995). “Western committee on crop pests,” in Proceedings of the Minutes of the 34th Annual Meeting (Columbia: British Columbia).
Webster, R., and Cardé, R. (1982). Influence of relative humidity on calling behaviour of the female European corn borer moth (Ostrinia nubilalis). Entomol. Exp. Appl. 32, 181–185. doi: 10.1111/j.1570-7458.1982.tb03200.x
Webster, R., and Cardé, R. (1984). The effects of mating, exogenous juvenile hormone and a juvenile hormone analogue on pheromone titre, calling and oviposition in the omnivorous leafroller moth (Platynota stultana). J. Insect Physiol. 30, 113–118. doi: 10.1016/0022-1910(84)90114-8
Weihman, S. (2005). Monitoring and Control Tactics for Grape Root Borer Vitacea polistiformis Harris (Lepidoptera: Sesiidae) in Florida vineyards. M.Sc. thesis, Gainesville, FL: University of Florida.
West, R. J., and Bowers, W. W. (1994). Factors affecting calling behavior by Lambdina fiscellaria fiscellaria (Lepidoptera: Geometridae), under field conditions. Environ. Entomol. 23, 122–129. doi: 10.1093/ee/23.1.122
Wong, T. T. Y., Clevelan, ML., and Davis, D. G. (1969). Sex attraction and mating of lesser peach tree borer moths. J. Econ. Entomol. 62:789. doi: 10.1093/jee/62.4.789
Wysoki, M., Yehuda, S. B., and Rosen, D. (1993). Reproductive behavior of the honeydew moth, Cryptoblabes gnidiella. Invert. Reprod. Develop. 24, 217–223. doi: 10.1080/07924259.1993.9672355
Xu, J., Wang, Q., and He, X. (2008). Emergence and reproductive rhythms of Ephestia kuehniella (Lepidoptera: Pyralidae). N. Z. Plant Protect. 61, 277–282. doi: 10.30843/nzpp.2008.61.6806
Yamamoto, S., and Sota, T. (2007). Phylogeny of the Geometridae and the evolution of winter moths inferred from a simultaneous analysis of mitochondrial and nuclear genes. Mol. Phyl. Evol. 44, 711–723. doi: 10.1016/j.ympev.2006.12.027
Yen, S. –H., Solis, M. A., and Goolsby, J. A. (2004). Austromusotima, a new Musotimine genus (Lepidoptera: Crambidae) feeding on old world climbing fern, Lygodium microphyllum (Schizaeaceae). Ann. Entomol. Soc. Am. 97, 397–410. doi: 10.1603/0013-8746(2004)097[0397:AANMGL]2.0.CO;2
Zacharuk, R. Y. (1985). “Antennae and sensilla,” in Comprehensive Insect Physiology Biochemistry and Pharmacology, eds G. A. Kerkut, and, L. I. Gilbert (London: Pergamon Press).
Zahiri, R., Lafontaine, D., Schmidt, C., Holloway, J. D., Kitching, I. J., Mutanen, M., et al. (2013). Relationships among the basal lineages of Noctuidae (Lepidoptera, Noctuoidea) based on eight gene regions. Zool. Scripta 42, 488–507. doi: 10.1111/zsc.12022
Zalucki, M. P., Daglish, G., Firempong, S., and Twine, P. (1986). The biology and ecology of Heliothis armigera and H. punctigera in Australia: what do we know? Aust. J. Zool. 34, 779–814. doi: 10.1071/ZO9860779
Zhu, J., Chastain, B. B., Spohn, B. G., and Haynes, K. F. (1997). Assortative mating in two pheromone strains of the cabbage looper moth, Trichoplusia ni. J. Insect Behav. 10, 805–817. doi: 10.1023/B:JOIR.0000010414.28494.9a
Keywords: sex pheromone, sexual selection, mating system, antennal morphology, flightless moth, mate location
Citation: Johnson TL, Elgar MA and Symonds MRE (2022) Movement and olfactory signals: Sexually dimorphic antennae and female flightlessness in moths. Front. Ecol. Evol. 10:919093. doi: 10.3389/fevo.2022.919093
Received: 13 April 2022; Accepted: 03 August 2022;
Published: 26 August 2022.
Edited by:
Jennifer M. Gleason, University of Kansas, United StatesReviewed by:
Gerhard Johannes Gries, Simon Fraser University, CanadaAstrid T. Groot, University of Amsterdam, Netherlands
Copyright © 2022 Johnson, Elgar and Symonds. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark A. Elgar, bS5lbGdhckB1bmltZWxiLmVkdS5hdQ==; Matthew R. E. Symonds, bWF0dGhldy5zeW1vbmRzQGRlYWtpbi5lZHUuYXU=
 Tamara L. Johnson1
Tamara L. Johnson1 Mark A. Elgar
Mark A. Elgar Matthew R. E. Symonds
Matthew R. E. Symonds