
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 22 July 2022
Sec. Conservation and Restoration Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.914623
This article is part of the Research TopicBiodiversity Conservation and Ecological Function Restoration in Freshwater EcosystemsView all 16 articles
The social and environmental impacts of large dams are quantifiable and have been well documented, while small dams have often been presumed to be less environmentally damaging than large dams. The purpose of this study was to analyze longitudinal gradients in environmental, hydrodynamic variables and their impact on phytoplankton function, within a cascade of four reservoirs (XuanMiaoGuan, XMG; TianFuMiao, TFM; XiBeiKou, XBK; ShangJiaHe, SJH) and one reservoir bay (Huangbohe Bay, HBH), located from upstream to downstream in the Huangbo River, Hubei Province, China. Our results showed that water temperature, total nitrogen, and soluble silicate increased along the cascade reservoir system, while the concentration of dissolved oxygen and total phosphorus decreased. We identified 16 phytoplankton functional groups, and the predominant groups, including D (Synedra and Stephanodiscus hantzschii), E (Dinobryon divergens), Lo (Dinoflagellate: Peridinium bipes and Peridiniopsis), X2 (Chroomona), and Y (Cryptomonas), changed longitudinally from up to down in the cascade reservoirs. The number of dominant functional groups increased along the longitudinal gradient, indicating that the function of the phytoplankton community was more stable. Functional group D was the dominant phytoplankton functional group among the four reservoirs, and Lo group was dominant except SJH. The phytoplankton functional groups in the HBH have been completely changed due to the backwater jacking of the main stream of the Yangtze River. Euphotic depth, suspended solids, and nutrients were apparently the key factors driving variations in phytoplankton functional groups among the reservoirs. Notably, the patterns we observed were not all consistent with the cascading reservoir continuum concept (CRCC) that typically characterizes large rivers. Thus, our findings contribute to the further theoretical development of the CRCC, which may not apply widely to all cascade systems.
River is the major conjunction of terrestrial and aquatic ecosystems and has important ecological functions such as water supply, flow regulation, and moderation of climate (Costanza et al., 1997; Cai et al., 2003). However, the constructed dam that fragmented the river ecosystem leads to modifications of the river’s original conditions and water dynamics, resulting in changes in abiotic and biotic compartments (Zhao et al., 2017; Liu et al., 2020; Castro et al., 2021). Cascade reservoirs can maximize the utilization of water resources such as water supply, seasonal flood regulation, electricity production, and navigation (Rosenberg et al., 2000; WCD, 2000).
The cascade dams ignore the long-term environmental impacts (McCartney et al., 2000). Dams cause considerable changes in surrounding basins by disrupting the continuous gradient of the rivers (Nogueira et al., 2018; Xiao et al., 2019). Several hypotheses have been proposed to depict and interpret the longitudinal variations in physical, chemical, and biological attributes of river ecosystems. Various studies have shown that the longitudinal distribution of functional feeding groups only partially follows the Reservoir Continuum Concept (RCC) framework (Tomanova et al., 2007; Jiang et al., 2011; Jelil et al., 2021). In contrast, the serial discontinuity concept (SDC) emphasizes that non-free-flowing rivers, such as regulated streams, often have discontinuous changes in riverine geomorphology, as well as biological populations and communities (Ward and Stanford, 1983). The longitudinal connectivity has been severely disrupted by transformation of the rivers into cascade reservoirs (Miranda et al., 2008; Wu et al., 2010). Research of these aquatic systems has emphasized the isolated reservoirs, with no specific study focused on the spatial distribution along a single river basin (Miranda et al., 2008). Nevertheless, studies in such cascades considering step-like continuous systems are rare despite their obvious hydrological and suspected functional interconnectivity downstream. A subsequent addition to lotic theory, the cascading reservoir continuum concept (CRCC) shows connectivity since it deals with data concerning biotic and abiotic among the serial reservoirs (Barbosa et al., 1999; Da Silva et al., 2005). The downstream reservoirs are affected by the features of the upstream reservoirs. CRCC has provided some theoretical postulates about the effects of upstream reservoirs on the downstream ones. However, CRCC was proposed in the study of large rivers, and those concerning small cascade reservoirs are still rare.
Previous studies have considered the impact of cascade reservoirs from the aspects of water quality and biological community changes, and those concerning functional groups were rare (Rodgher et al., 2005; Perbiche-Neves and Nogueira, 2010; Nogueira et al., 2018; Wu et al., 2021). For example, the study on phytoplankton in cascade reservoirs has been carried out in Brazilian Iguacu River and Lancang-Mekong River in China (Li et al., 2013; Nogueira et al., 2018). As important primary producers in ecosystems, phytoplankton is considered to be a natural bioindicator for its complex and rapid responses to fluctuations in environmental conditions, and is well suited to study the impact of the construction of cascade reservoirs (Lewitus, 2002; Darchambeau et al., 2014; Wentzky et al., 2020). The Reynolds functional approach is more effective in evaluating the phytoplankton responses to changes in environmental conditions and has been widely used in the temperate and subtropical areas of the world (Kruk et al., 2002; Reynolds et al., 2002). But this approach in small cascade reservoirs is still not well documented, especially considering that they are not more important due to the social and environmental benefits (Wang et al., 2020; Zaidel et al., 2021). Consequently, the effects of these cascade systems on phytoplankton functional groups still require attention (Moura et al., 2013).
This study of phytoplankton ecology in reservoirs along the Huangbo River Basin (HRB) in Central China has two main objectives. First, a single river system is selected to study the impact of cascade reservoirs on phytoplankton functional groups. Moreover, our results are helpful to clarify the changing trend of phytoplankton functional groups in cascade reservoir system and enrich the CRCC theory.
Huangbo River, with a length of 162 km, is one of the first-grade branches between Three Gorges Reservoir and Gezhou Reservoir at the north shore of the Xiling Gorge of Yangtze River, located in Yichang, Hubei Province, China (30°42′–31°29′ N, 110°08′–111°34′ E; Figure 1). The HR drains a basin of 1931.5 km2 and flows from north to south, entering the Yangtze River 1.5 km upstream of the Gezhou Dam. The region occupied by the HRB has a subtropical monsoon climate with a hot rainy summer. The mean annual temperature is 16.9°C, with the highest mean temperature in July and lowest in January; the annual frost-free period is 223–273 days; mean annual precipitation and runoff are approximately 1,101 mm and 28.4 m3⋅s–1, respectively (Wei et al., 2021).
The HR is a low-lying river that runs from the north to the south. It is canyon-shaped with the highest altitude at 1,962 m, lowest at 61.5 m, and a relative height of 1,895.5 m. A series of reservoirs, such as XuanMiaoGuan (XMG), TianFuMiao (TFM), XiBeiKou (XBK), and ShangJiaHe (SJH), were constructed in the HR to meet the demand for water for agricultural irrigation in eastern Yichang from 1966 to 2005. The total installed capacity of cascade hydropower stations reaches about 6.6 × 104 kW, and the total annual power generation is 2.2 × 108 kWh. After the impoundment of the Gezhouba Reservoir (GZBR) was filled to an altitude of 66 m above sea level in June 2003, the lower 10.4 km stretch of this river became Huangbohe Bay (HBH, a representative bay of GZBR), with a flow velocity of 1.8–3.6 × 10–3 m⋅s–1 (Bao et al., 2021). Basic habitat characteristics of the cascade reservoirs (Bay) of HRB are shown in Table 1.
Samples were collected every 3 months during the dry (October, January) and rainy (April, July) seasons from 2011 to 2012. Collections were taken at 18 sampling stations distributed along different reservoirs of the main mid-lower course of the HRB. The selected stations are shown in Figure 1. Integrated water samples for analyses of water quality and phytoplankton community were collected at 0.5 m below the surface with a 5-L plexiglass water sampler. Excluding SJH in January due to bad weather and harsh road conditions, samples were collected at each site, resulting in a total of 69 samples. Water samples for nutrients were kept cool and shaded in acid-cleaned plastic containers, before being transported to laboratory. An Environmental Monitoring System probe (YSI 6600EDS, United States) was used to measure water temperature (WT) and dissolved oxygen (DO), pH, specific conductance (Cond), and turbidity (Turb) at 0.5 m below the water surface. Water depth was also measured by YSI 6600EDS. Water transparency was measured with Secchi Disk, and suspended solids were concentrated by filtering a known volume of water through a weighed pre-ignited glass fiber filter (Whatman type GF/C). An additional known volume of water was filtered through the GF/C for chlorophyll a (chl. a) determination (Xu et al., 2009). All the filters were immediately placed in a dark cooler and stored at (−20°C) until the laboratory analysis.
In the laboratory, the following variables were measured. Different forms of nitrogen (TN, NO3-N, NH4-N), phosphorus (TP, PO4-P), and silicon (SiO2-Si) were measured using Skalar (San++, The Netherlands; Shen et al., 2014). Total organic carbon (TOC) and dissolved organic carbon (DOC) were measured using Shimadzu (TOC-VCPH, Japan). Suspended solids (TSS) and their two fractions, volatile (VSS) and non-volatile fractions (NVSS), were measured according to a Standard Operating Procedure for Total Suspended Solids Analysis (Xu et al., 2009). The Chl. a concentration was determined using a spectrophotometer (Shimadzu UV-1601, Japan) by measuring the absorbance of the extract at various wavelengths (750, 663, 645, and 630 nm; Cai, 2007).
Samples for phytoplankton analysis were preserved with 5% formalin and neutral Lugol’s solution, then isolated through sedimentation for 48 h and concentrated to a final volume of 30 ml, and at least 100 random transects for over 300 cells of phytoplankton were counted in 0.1 ml under an Olympus CX21 microscope at 400 × magnification (Huang et al., 1999; Cai, 2007). Taxa identification was done according to Hu and Wei (2006) and John et al. (2002). Algal biomass was calculated using the approximation of cell morphology to regular geometric shapes, assuming that the fresh weight unit is expressed in mass, where 1 mm3⋅L–1 = 1 mg⋅L–1 (Huang et al., 1999; Wang et al., 2011). We combined species contributing > 5% to the total biomass into functional groups using the criteria of Padisák et al. (2009) and Shen et al. (2014).
We calculated the euphotic zone depth (Zeu) as 2.7 times the Secchi depth (Cole, 1994). In the absence of stratification for small reservoirs, mixing depth (Zmix) was taken equal to the average depth of the reservoir (Naselli-Flores, 2000). The ratio between the euphotic and mixing depths (Zeu: Zmix) was used as a measure of light availability (Shatwell et al., 2012). Relative water column stability (RWCS) was calculated following Padisák et al. (2003): RWCS = (Db - Ds)/(D4 - D5), where Db is the density of the bottom water, Ds is the water density at surface, a depth of 1 m was artificially considered “surface water.” D4 and D5 are the densities of water at 4 and 5°C, respectively. Phytoplankton data were log(x + 1) transformed to reduce the effects of extreme values, and the relationship between functional groups and environmental factors was analyzed using the CANOCO 4.5 software (Lepš and Šmilauer, 2003; Shen et al., 2014). A forward selection of environmental factors was applied to avoid using collinear environmental factors in the same constrained ordination model. An initial detrended corresponded analysis suggested that redundancy analysis (RDA) should be used because the length of the first axis exceeded 3 SDs. The relationships between functional groups and environmental variables were also assessed by Pearson correlations with IBM SPSS Statistics 20.0 (IBM, Chicago, IL, United States).
Reservoirs varied widely in most of the environmental variables. Significant differences in the nutrient parameters along the cascade reservoirs were observed during the sampling periods (p < 0.01; Table 2). TN (range from 0.94 to 2.71 mg⋅L–1) and SiO2-Si (1.7 to 4.5 mg⋅L–1) showed a progressive increase in the last two reservoirs, especially HBH (Figure 2A), NO3-N (0.79–2.05 mg⋅L–1), TOC (1.18–1.73 mg⋅L–1), and DOC (1.07–1.68 mg⋅L–1; except a little higher values in TFM) showed a slight increase from the upper part to the mid and lower sections of the cascade reservoirs, while TP and PO4-P in the four upper reservoirs showed a slight decrease in this longitudinal pattern (Table 1 and Figure 2A). As for the highest concentrations of nutrients, the mean concentrations of NH4-N and TP were 45 and 123.9 μg⋅L–1, respectively. We found that changes in the water quality of the first three reservoirs are relatively stable, while HBH has decreased, which may be mainly affected by the backwater jacking of the main stream of the Yangtze River.
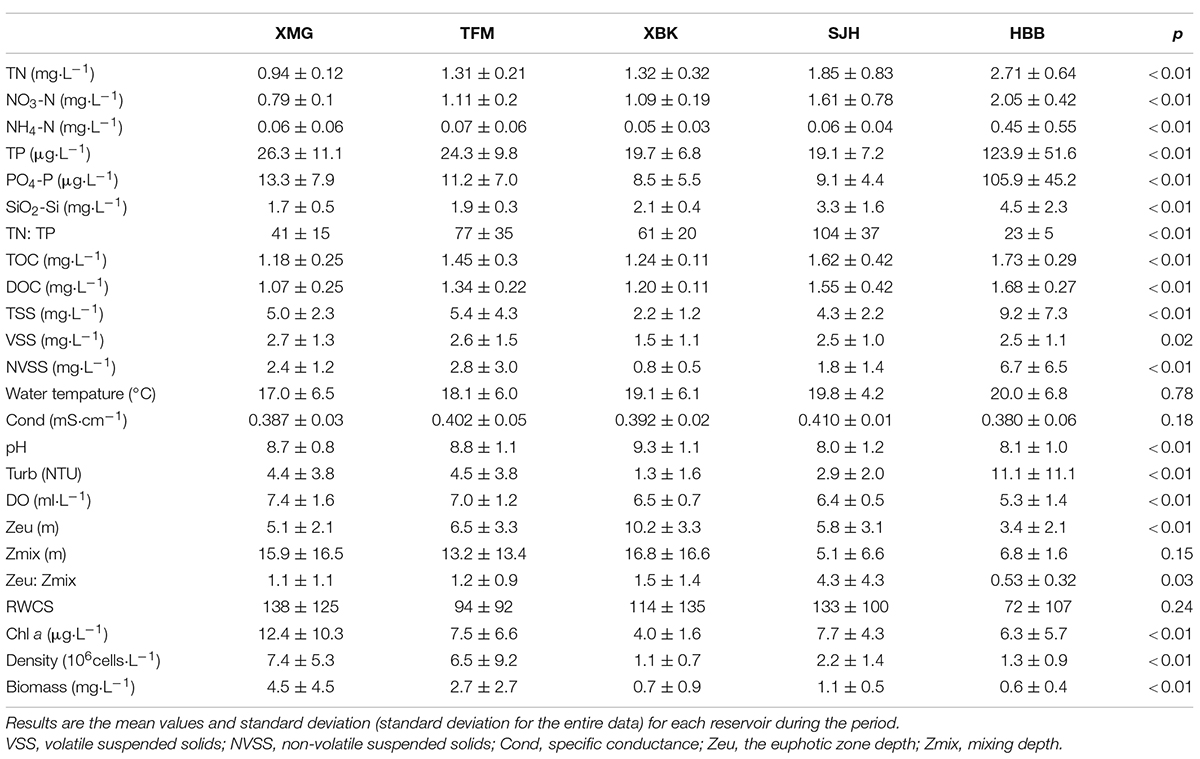
Table 2. The summary statistics of environmental variables and results of non-parametric test between the cascade reservoirs.
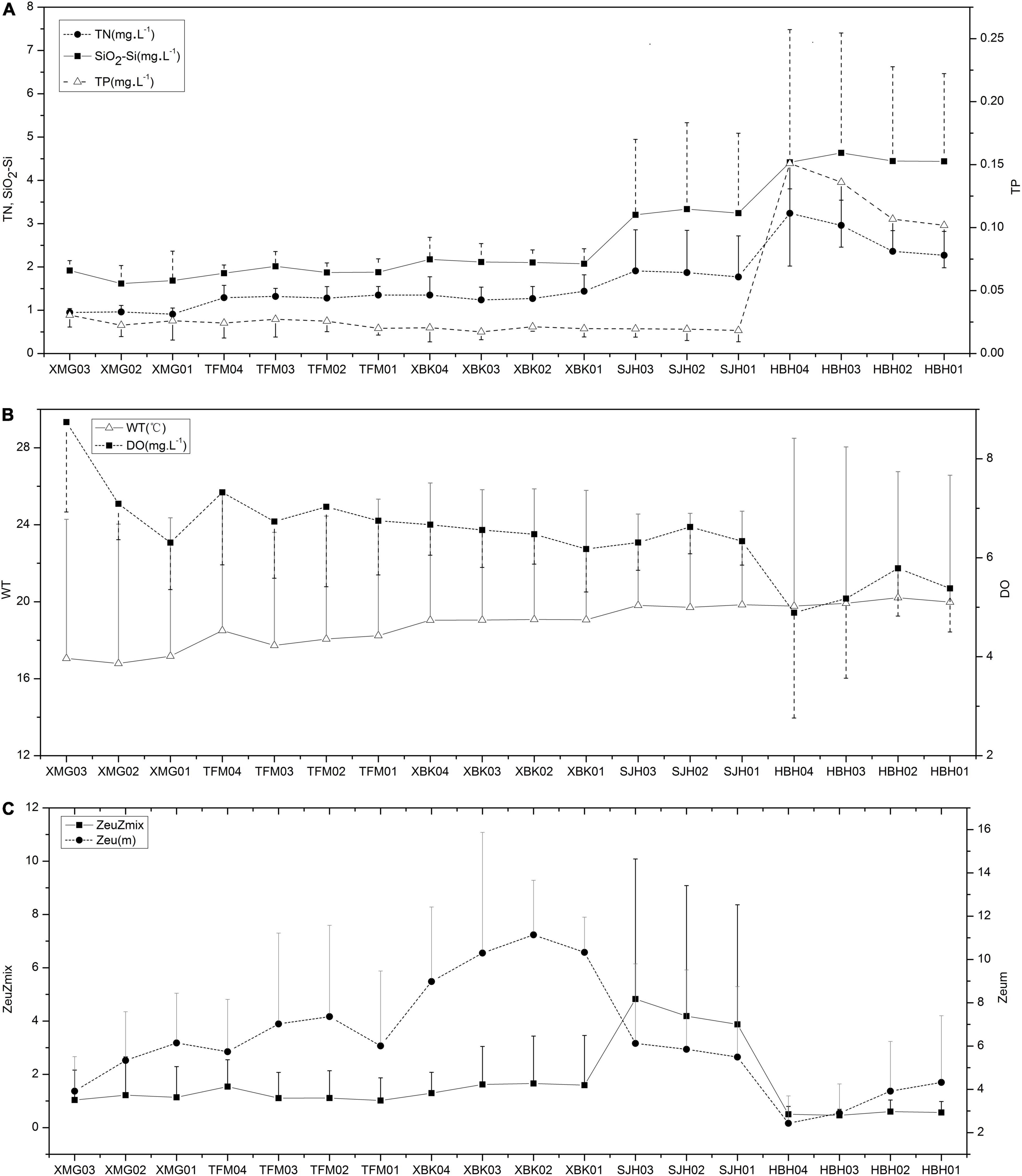
Figure 2. Spatial variation of environmental variables in the cascade reservoirs (A) TN, SiO2-Si and TP; (B) WT and DO; (C) Zeu, Zmix and Zeu: Zmix.
There was no significant variation among the four abiotic variables (WT, Cond, Zmix, and RWCS). Remarkable variations existed between the other physical variables (TSS, VSS, NVSS, pH, Turb, DO, Zeu and Zeu: Zmix) along the cascade system (Table 2). Surface WT increased slightly down the cascade (range from 17 to 20°C). DO decreased slowly from 7.4 to 5.3 ml. L–1 (Figure 2B). The mean specific conductance of the reservoirs was about 0.4 mS⋅cm–1. The highest RWCS was 409 in XBK in July, while the lowest (−2) in TFM in January. The mean suspended solids (TSS, VSS, and NVSS were 2.2, 1.5, and 0.8 mg⋅L–1, respectively) and Turb (1.3 NTU) were lower in the XBK. The highest value of Zeu (10.2 m) was recorded in the XBKR, for the longest retention time (115.5 days) in the cascade reservoirs (Table 1). Higher Zeu were always present in front of the dam in the four reservoirs (Figure 2C). The reservoirs (except HBH) showed stratification in April and July of 2012, with high light availability (Zeu: Zmix > 1), and the lowest value of Zmix was 1 m.
According to the results of principal component analysis (PCA; Figure 3), the first PCA axis explained 53.33% of the variance in the environmental variables, and the second axis explained 24.20% of the variance. The PCA results showed that all samples in each reservoir were separated independently into five groups by the phytoplankton density, DO, RWCS, pH, Zeu, WT, bottom WT, TN, SiO2-Si, and TSS. Furthermore, distinct spatial pattern was detected in the PCA ordination diagram (Figure 3B).
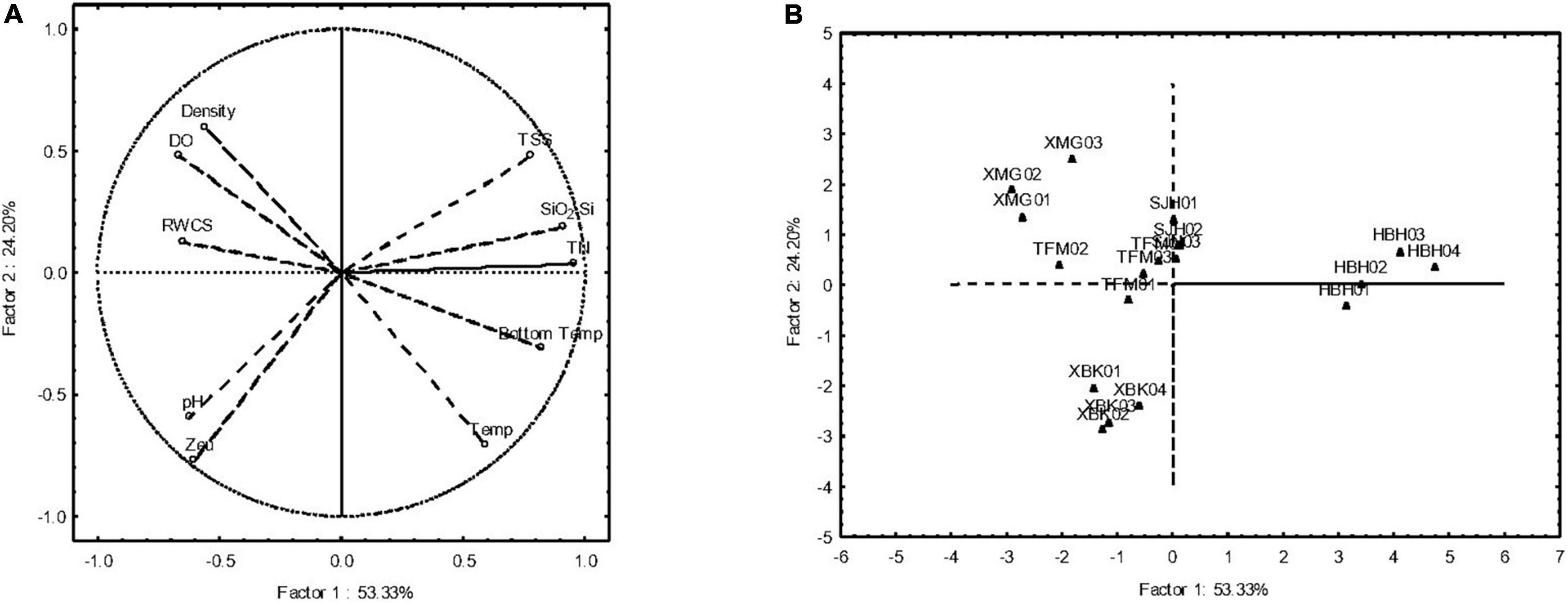
Figure 3. Ordination diagram of PCA for environmental variables and phytoplankton community characteristics (A) and samples (B).
The 124 algal species identified in the cascading reservoirs are members of the following seven major taxonomic categories: Bacillariophyta (55), Chlorophyta (37), Cyanophyta (10 taxa), Cryptophyta (7), Pyrrophyta (10), Chrysophyta (3), and Euglenophyta (2). In the subtropical cascade reservoirs, 80 species were sorted into seven major taxonomic categories and belonged to 16 functional groups using the functional approach (>5% of the total biomass) described by Reynolds (1984) and Reynolds et al. (2002). The 43 descriptor species (>10% of the total biomass) were members of 13 functional groups (Table 3). The typical traits of these functional groups are exhibited in Table 3. Phytoplankton biomass was characteristically low and varied widely among reservoirs (Figure 4). Biomass ranged from 0.05 to 16.2 mg⋅L–1, with higher biomass in the upstream (XMG and TFM) and lower in the downstream (XBK, SJH, and HBH). Phytoplankton functional groups occurred in the cascade reservoirs, with one or more species contributing to their compositions (Figure 4A). Groups D, E, Lo, X2, and Y were classified as dominant phytoplankton functional groups (Figure 4).
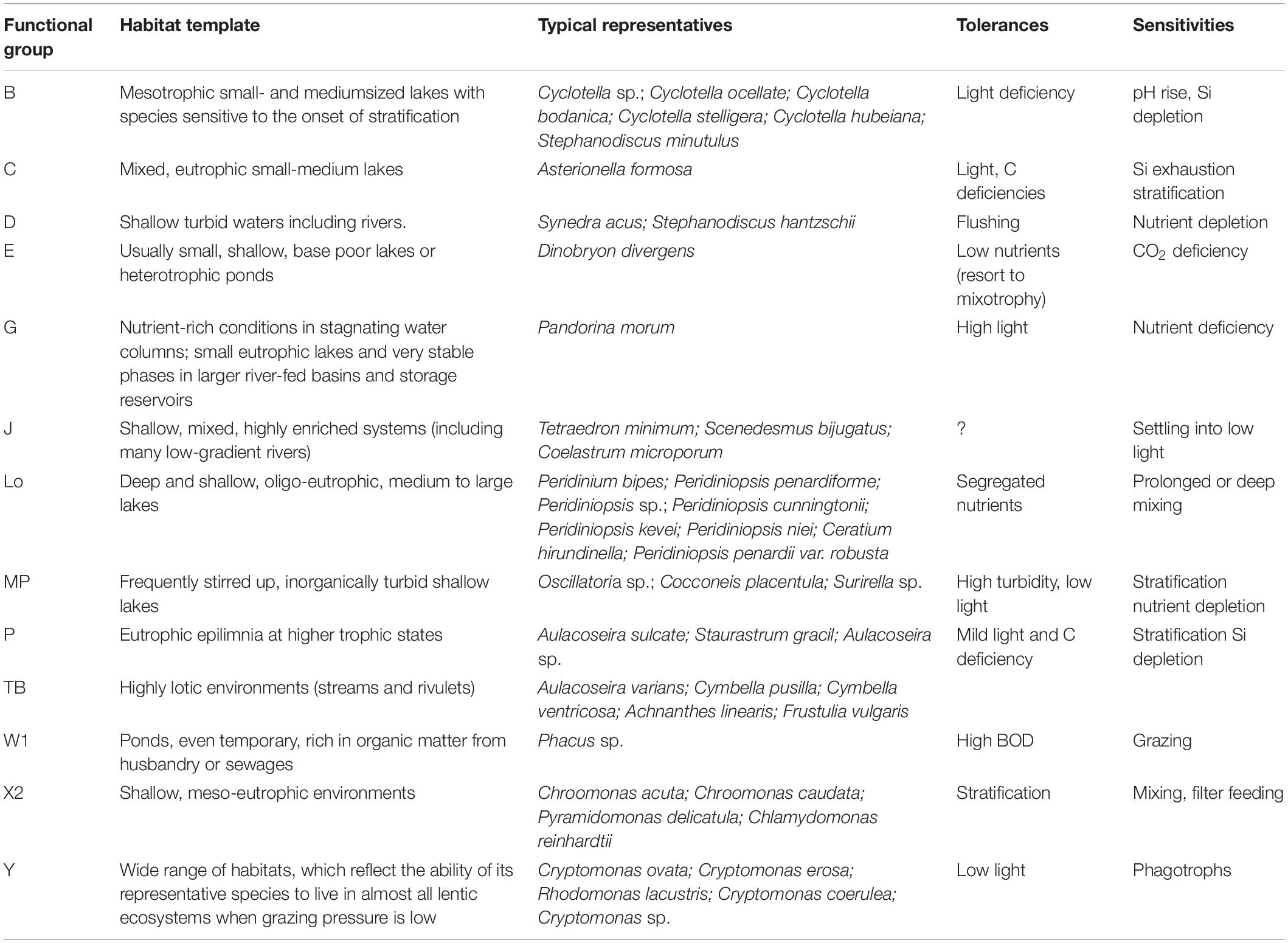
Table 3. Trait of phytoplankton functional groups detected in the cascade reservoirs quoted from Reynolds et al. (2002) and Padisák et al. (2009).
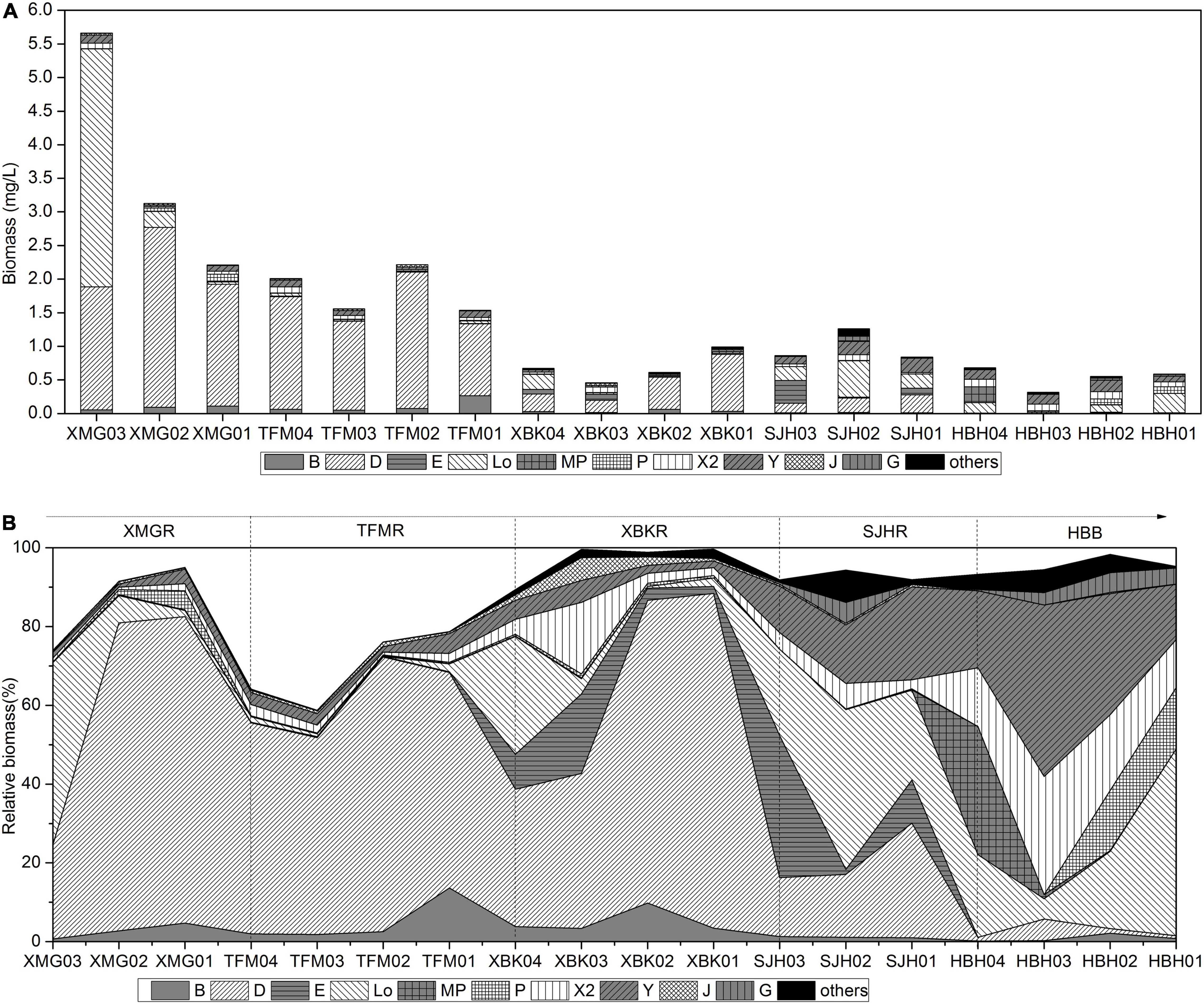
Figure 4. Longitudinal variation of the biomass (A) and relative biomass (%) (B) of the main phytoplankton functional groups in the cascade reservoirs.
The diatom of group D (Synedra acus and S. hantzschii) was the most important in quantity, sharing this importance with the dinoflagellates P. bipes and Peridiniopsis (Peridiniopsis sp., Peridiniopsis kevei, Peridiniopsis penardiforme, Peridiniopsis cunningtonii, Peridiniopsis niei, and Ceratium hirundinella) in group Lo of phytoplankton community in the XMG. Biomass was dominated by groups Lo (46.3%) and D (23.9%) upstream of the reservoir (XMG03). Group D also dominated in the TFM, XBK, and SJH with over 20% of the contribution. The dominant and co-dominant groups in the riverine zone of XBK were groups D (34.1%), Lo (16.9%), and X2 (14.5%), formed by small Chroomonas (Chroomonas acuta and Chroomonas caudate) and Chlorophyta (Pyramidomonas delicatula, Chlamydomonas reinhardtii). The biomass of groups X2 and Lo decreased while D increased downstream of the XBK reservoir; the dominance of X2 and Lo groups was supplanted by the D group. In SJH, groups Lo and D dominated (28.4%, 20% of biomass) though the dominance of group D declined. Group E (D. divergens) and the crytophyceans in group Y (Cryptomonas ovata, Cryptomonas erosa, Cryptomonas sp., Cryptomonas coerulea, and Rhodomonas lacustris) were the high biomass contributors. In HBH, Lo, X2 (C. acuta, C. caudate, P. delicatula, C. reinhardtii) and Y supplanted D group became dominant biomass contributors. Despite low biomass contributions, groups MP (Oscillatoria sp., Cocconeis placentula, and Surirella sp.) and P (Aulacoseira sulcata, Aulacoseira sp., and Staurastrum gracile) were also important as they were present in the upper reach (MP) and the river mouth region (Figure 4A).
Redundancy analysis was the appropriate method for linear ordination (gradient lengths of the first two axes are 2.73 and 2.88, respectively). The results of the RDA ordination for phytoplankton functional groups and environmental variables on axes 1 and 2 are shown in Figure 5A. The Monte Carlo test revealed that the first canonical axis and all canonical axes were significant (F = 52.32, p = 0.002; F = 9.94, p = 0.002; 499 random permutation).
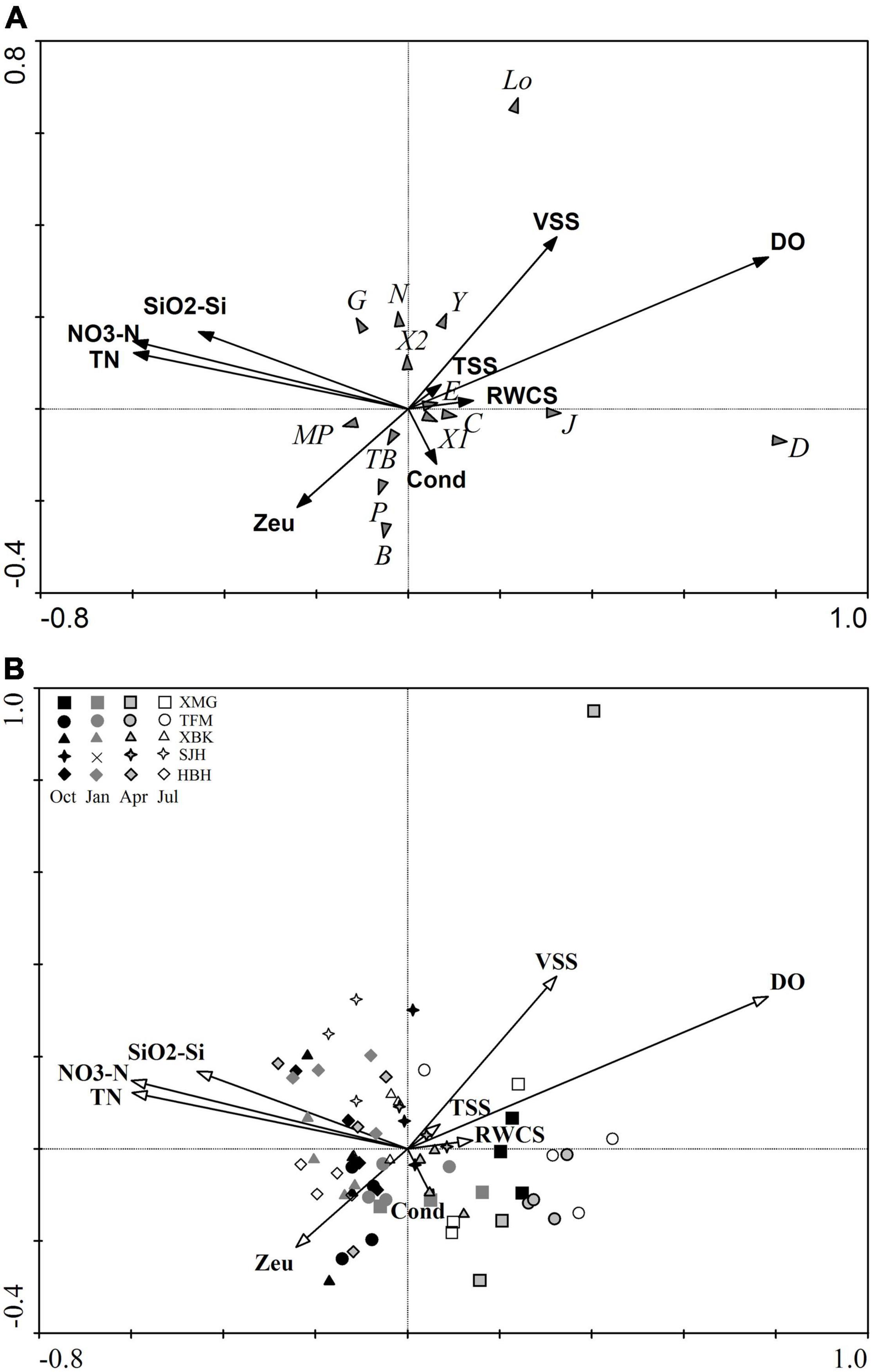
Figure 5. Ordination diagram of RDA for environmental variables and phytoplankton functional groups (A) and samples (B).
The correlations for the first (0.84) and second (0.70) axes were high (Table 4), indicating a strong relationship between phytoplankton and selected environmental factors. All canonical axes cumulatively explained 99.43% of the total variation in RDA, and the first two redundancy axes jointly accounted for 97% of the species–environmental variables relation (axis 1: 78.0%; axis 2: 21.0%; Table 4). Nine significant variables of environmental data were screened by forward selection, DO (F = 28.98, p = 0.001), TN (F = 5.76, p = 0.005), TSS (F = 4.98, p = 0.007), NO3-N (F = 5.44, p = 0.012), VSS (F = 4.82, p = 0.013), Cond (F = 4.48, p = 0.012), Zeu (F = 3.84, p = 0.022), SiO2-Si (F = 3.54, p = 0.033), and RWCS (F = 3.37, p = 0.029). These variables accounted for 58.5% variance of species data for the first two axes (axis 1: 47.0%; axis 2: 11.5%). The first RDA axis was mainly positively correlated to DO (0.66) and negatively to TN (−0.50), NO3-N (−0.50), and SiO2-Si (−0.38). Axis 2 was positively correlated with VSS (0.26; Figure 5A).
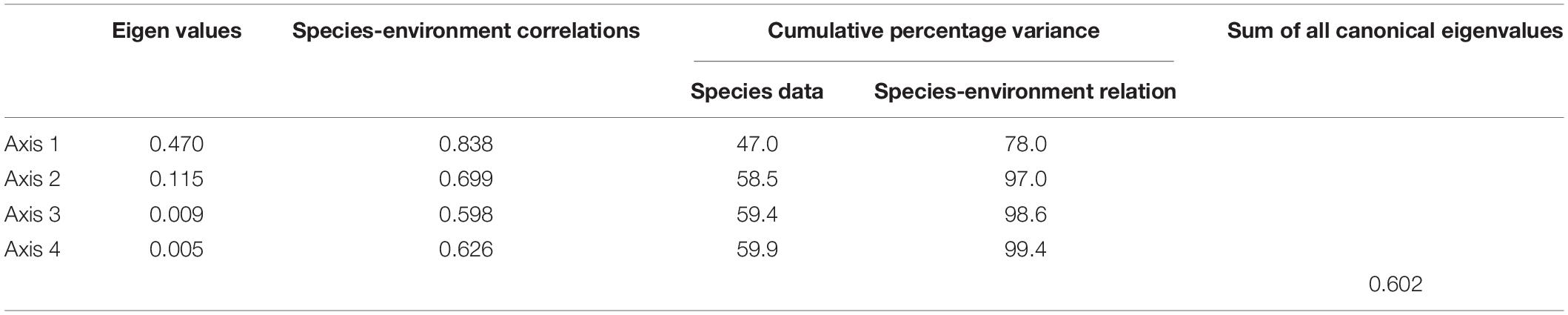
Table 4. Redundancy analysis results for phytoplankton functional groups and environmental variables.
The spatiotemporal dynamic changes of phytoplankton functional groups in cascade reservoirs of the Huangbo River were well presented in the RDA diagram (Figure 5B). The first RDA axis illustrates differences between upstream and downstream regions. Upstream reservoirs showed high concentrations of DO while the downstream regions were characterized by high nitrogen nutrient levels (TN, NO3-N, and SiO2-Si). Pearson correlations found significant correlations (p < 0.05) between the biomass of main functional groups and most of the examined physicochemical factors (WT, DO, Zeu, nutrients, suspended solids, and hydrodynamic conditions; Table 5). The biomass of groups X2 and Y was negatively correlated with Zeu and TN:TP ratio and positively correlated with WT, TOC, DOC, and VSS. Group Y was also positively correlated with TP, PO4-P, and TSS and negatively correlated with pH and Zeu: Zmix. In contrast, the biomass of group D was positively correlated with DO and VSS and negatively correlated with nutrients (N, P, Si, and C). The significant environmental variables for group Lo were DO and VSS; for groups MP and P, significant environmental variables were WT, Turb, SiO2-Si, TSS, and NVSS; for E, significant environmental variables were Zeu, Zeu: Zmix, and TN:TP ratios. Total phytoplankton biomass was significantly related to DO, Zeu, Zmix, TN, NO3-N, SiO2-Si, TSS, and VSS.
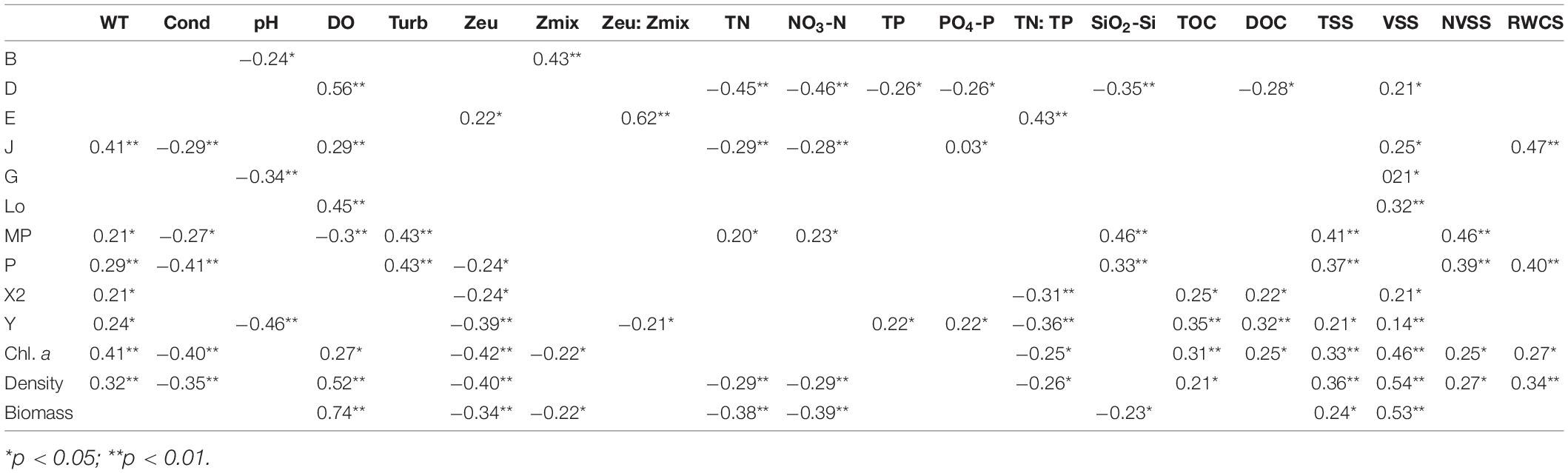
Table 5. Significant Pearson correlations between the biomass of the 10 main phytoplankton functional groups and the biomass of total phytoplankton, and 20 environmental variables (n = 69).
Significant differences in major environmental factors existed between different locations by RDA, which are not displayed in this study. In XMG, pH and DO were the principal environmental factors related to phytoplankton community and accounted for 73.4% variance of species data in the first two axes (F = 12.4, p = 0.002). In TFM, however, the central environmental factors changed into Zeu: Zmix, TN, RWCS, and Turb and explain 86% of the species data (F = 17.3, p = 0.002); SiO2-Si, NO3-N, and Cond replaced the previous factors as the important factors in XBK with a probability are 59% (F = 5.8, p = 0.008). Phytoplankton growth is limited by Cond, pH, and Zeu: Zmix in SJH, where the phytoplankton could also be explained as 64% (F = 4.5, p = 0.002); No remarkable factor was selected in the RDA to explain phytoplankton distribution in HBH (p > 0.05).
It is reported that the capture of nutrients by reservoirs with low retention time along the basin would increase the nutrient level in the downstream of the cascade. In the Tietê River, Brazil, the uppermost reservoir in a series of nine impoundments captured most of the nutrients released from São Paulo, Brazil (Barbosa et al., 1999), and decreased linearly with descent down the cascade. In the Iguaçu River, Brazil, Da Silva et al. (2005) observed that nutrients decreased in a downstream direction while in Tennessee River, United States, nutrients increased (Miranda et al., 2008), but nutrient ratios changed, reflecting nutrient-specific gradients along the cascade reservoirs. The values of TP also showed a decreased gradient, while TN and SiO2-Si increased along the longitudinal cascade reservoir system in the Huangbo River, China. The nutrient levels in the cascade reservoirs may be recorded as a result of specific watershed differences in morphometric features, retention time, hydrodynamic conditions, external loads, and retention by dams (Barbosa et al., 1999; Rodgher et al., 2005). Like nutrients, other water quality characteristics are affected by impoundments and exhibit gradients along cascade reservoirs. The values of DO displayed descending gradients in Tennessee River (Miranda et al., 2008) and Huangbo River; however, surface WT revealed an ascending gradient variation along the longitudinal cascading system in Tietê River (Barbosa et al., 1999), Iguaçu River (Da Silva et al., 2005), and Huangbo River.
The discontinuity also presented in the system, and there was a sharp decline in PO4-P, TOC, DOC, and suspended solids in XBK, with the same trends for chl. a, density, and biomass of phytoplankton. In addition, the light condition and RWCS were also interrupted by dams. In general, the proposed concept of CRCC was partly verified in the reservoirs in Huangbo River unlike the established gradients in Tietê River, Tennessee River, and Iguaçu River (Barbosa et al., 1999; Da Silva et al., 2005; Miranda et al., 2008). In contrast, more attention from limnologists was paid to longitudinal patterns within reservoirs than among reservoirs, leading to informational deficiencies in the literature (Miranda et al., 2008). As a consequence, we could not predict which limnological characteristic parameter will show continuous longitudinal gradients along the cascade reservoirs. Longitudinal gradients may reset and restart multiple times as the river travels through a different basin (Barbosa et al., 1999; Miranda et al., 2008). Thus, the exact patterns in Huangbo River may not apply directly to other cascade systems.
In the cascade reservoirs, physicochemical conditions were successfully described by the predominant coexistent phytoplankton species. We found that groups D, E, Lo, X2, and Y occurred in the cascade reservoir system, however, with relatively different contributions. D group was more important in the former three reservoirs, while E, Lo, and Y groups became more representative in XBK, SJH, and HBH. The relative biomass of D group decreased in SJH and HBH, while the Lo, X2, and Y groups increased along the longitudinal cascade reservoirs from upstream to downstream. In view of the above description, we concluded that the longitudinal distribution characteristics of phytoplankton in the cascade reservoirs of the Huangbo River could be summarized as groups D and Lo in XMG, only D in TFM, and D, Lo, E groups in XBK, while in SJH, the dominant groups were Lo, D, E, and Y, based on biomass changes of functional groups. Due to the influence of jacking from the mainstream of GZB, the phytoplankton in HBH has changed greatly compared with the four reservoirs and Y, Lo, and X2 groups dominant in HBH, which was consistent with the study of Xiangxi bay (Wang et al., 2011).
D group was represented by lanceolate, pinnate diatoms (Synedra), and centric diatom (S. hantzschii), which were also the main contributors of the group on this occasion. D group was more important in the upper three reservoirs and exhibited a higher biomass over the reservoirs, commonly found in shallow, turbid waters, with species sensitive to nutrient consumption (Reynolds et al., 2002; Shen et al., 2014). Although the group typically occurs in spring (Yin et al., 2011), it was observed in the other seasons in our study. In fact, diatoms can become dominant in the flooding and mixed waters because dissolved silicate is replenished from deeper water (Stević et al., 2013; Yi et al., 2020). As a C-strategist, this small-celled and fast-growing species is tolerant of mixing and low light (Reynolds, 2006). In this study, group D was well adapted to high VSS though strongly negatively correlated with nutrients (C, N, P, and Si) and light conditions. As reported in the lower Salado River (Argentina), group D was also directly correlated with Cond in the region (Devercelli and Farrell, 2013).
Group E, with the representative species of D. divergens, can tolerate low nutrients (resort to mixotrophy) but is sensitive to CO2 deficiency (Reynolds et al., 2002). This group is wildly distributed in the northern hemisphere and reported in moderately to very nutrient-rich ponds and lakes (John et al., 2002; Hu and Wei, 2006). However, the correlation analysis showed its significant relation with light availability and TN:TP ratio. Many different species of Dinobryon are distributed widely in different nutritional water, and most of them were recorded once and predominantly occurred in clear-water lakes and ponds with low nutrient contents, low temperature, and high transparency (Feng, 2008).
Groups Lo and Y became more representative in SJH and HBH. The Lo group mainly comprises dinoflagellates species in the reservoirs. The representative species of group Lo were P. niei, P. bipes, C. hirundinella, and Peridiniopsis penardii var. robusta. This group was usually observed in mesotrophic lakes, tolerated segregated nutrients, and sensitive to prolonged or deep mixing as emphasized by Reynolds et al. (2002). According to former research, most of the dinoflagellates were capable of using their slower swimming velocities to perform diel vertical migration where nutrient-depleted conditions occurred in aquatic ecosystems (Xu et al., 2010a; Shen et al., 2014). High temperature resulted in group Lo in a Mediterranean reservoir (Becker et al., 2010) and Lake Sakadaš along the Danube (Stević et al., 2013). In this study, group Lo had no significant correlation to WT and nutrients. Concerning P. niei, high abundances have been found in Danjiangkou Reservoir and Three Gorges Reservoir, leading to bloom in some periods (Xu et al., 2010b; Shen et al., 2014). This species can be a dominant species in winter and spring for a short time, and is widely distributed in waters whose trophic status varies from oligotrophic to eutrophic (Amorim and Moura, 2022).
The Y group was characterized by C. ovata and C. erosa, occurred in the system, and dominated in the downstream throughout the year. These two species have similar ecological requirements, which is well adapted to live in small, low light, and enriched lakes (Reynolds et al., 2002). The relatively high surface to volume ratio of cryptomonads facilitates their rapid absorption of nutrients and fast growth (Bovo-Scomparin and Train, 2008). The group Y was dominated by wide suitable habitat during the mixing period, where there was low grazing pressure and a quiet water body (Becker et al., 2010). In this study, group Y was characterized by low Zeu and TN:TP ratio, high concentration of TP, PO4-P, TOC, and DOC. Thus, in HBH, lowest eutrophic depth and TN:TP ratio and the highest nutrients contribute to the high biomass. The results obtained from RDA analysis also showed a virtual correspondence to Rychtecký and Znachor, 2011 in the Římov Reservoir, Czech Republic, and a young canyon reservoir in Southwest China (Liao et al., 2020).
The habitat template for group X2 is shallow, meso-eutrophic environment with species-tolerant stratification but sensitive to mixing regime and filter feeding (Padisák et al., 2009). The representative species of the group are C. acuta and C. caudata, existing in all waters along the Huangbo River. This group presented a high biomass only in the downstream area, but never became dominant in the cascade reservoirs except in HBH. These small unicellular flagellates are generally considered as “C” strategists with high surface/volume ratio (Bovo-Scomparin and Train, 2008), high intrinsic growth rates, and high metabolic activity and low light requirement in common (Grime, 1977; Reynolds, 1988). However, they differ in sedimentation velocity and silica requirement (Mieleitner and Reichert, 2008). X2 group was positively correlated with VSS, nutrients (TN, NO3-N, and SiO2-Si), and negatively correlated with Zeu and Cond, which was not consistent with observations in the Middle Paraná River (Devercelli, 2006; Devercelli and Farrell, 2013). The present habitat for X2 is well consistent with what RDA indicated and also with shallow eutrophic lakes of Huaihe River, China (Yi et al., 2020).
It is widely recognized that spatial heterogeneity plays a functional role in aquatic ecosystems (Dutilleul and Legendre, 1993; Becker et al., 2010), and phytoplankton communities are regulated mainly by hydrology, WT, the availability of light, nutrients, and mixing conditions (Costa et al., 2009; Rigosi and Rueda, 2012; Shen et al., 2014). In some reservoirs, although both internal and external variables determine the structure of the phytoplankton community, physical variables and hydrodynamics generally predominate (Rangel et al., 2012; Xu et al., 2021). Phytoplankton community structure was driven by light and nutrient, which have illuminated by Reynolds (2006). The results also highlighted the hydrodynamic conditions, light availability, mixing regime and nutrients as the main factors related to the phytoplankton functional groups in the cascade reservoirs. In terms of importance, nutrients seemed to play a more important role in the four reservoirs in this study. The results verified by the correlations between the biomass and environmental factors. The importance of environmental factors showed spatial differences in terms of influence. The major environmental factors shape phytoplankton in different waters for the main functional groups. Heterogeneity in the distribution of phytoplankton was clearly demonstrated as in Mangueira Lake (Crossetti et al., 2013). Reservoirs were traditionally considered as an independent system, separated from the surrounding watersheds and other parts of the river basin (Miranda et al., 2008). Despite the shortcomings of the paradigm, our research was restricted to five reservoirs in phytoplankton assemblages and physicochemical variables, and obvious longitudinal gradients were identified in the cascade reservoir system of the Huangbo River. In this study, the effects of the cascading dams on phytoplankton assemblages were obvious and more complex than those in a single reservoir (Amorim and Moura, 2022). The operations of cascade reservoirs can cause eutrophication of downstream reservoirs, longitudinal connectivity loss in RCC, and cascading and accumulation effects on phytoplankton communities.
Instead of seeing each part of the reservoir as independent, we regarded them as an interdependent system. For the spatial pattern, the biomass of functional groups was interfered by the strong influence of the dams, especially the series of dams in the study region. The number of dominant functional groups increased along the longitudinal gradient, indicating that the function of the phytoplankton community was more stable. Functional group D was the dominant phytoplankton functional group among the four reservoirs, and Lo group was dominant except SJH. Among the main environmental variables, the depth of euphotic layer, nutrients were the limiting factors of algae growth, and the effects of nutrients were apparently more important. Furthermore, the study confirmed that CRCC existed in the cascade reservoirs of Huangbo River, which were not completely consistent with it in large rivers. The phytoplankton functional groups in the HBH have been completely changed due to the backwater jacking of the main stream of the Yangtze River.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
HS and QC conceived the ideas and designed the study. HS and LT sampled the phytoplankton. HS, LY, LT, and QC analyzed the data and led wrote the manuscript. All authors contributed, and approved it for publication.
This study was funded by the National Key R&D Program of China (2018YFD0900806), the Shandong Provincial Natural Science Foundation (ZR2016CL05), the Technology Projects (A) Category, Chinese Academy of Sciences (XDA23040500), and the State Key Laboratory FEBL Research (2019FBZ01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amorim, C. A., and Moura, A. D. N. (2022). Habitat templates of phytoplankton functional groups in tropical reservoirs as a tool to understand environmental changes. Hydrobiologia 849, 1095–1113. doi: 10.1007/s10750-021-04750-3
Bao, Y., Hu, M., Wang, D., Wu, X., Wang, Y., Li, Z., et al. (2021). Distribution and pollution assessment of nutrients and heavy metals in sediments of the cascade reservoirs in Huangbai River. Ecol. Environ. Sci. 30, 1005–1016. doi: 10.16258/j.cnki.1674-5906.2021.05.013
Barbosa, F., Padisák, J., Espíndola, E., Borics, G., and Rocha, O. (1999). The Cascading Reservoir Continuum Concept (CRCC) and its Application to the River Tietê-basin, São Paulo State, Brazil. Leiden: Backhuys Publishers.
Becker, V., Caputo, L., Ordóñez, J., Marcé, R., Armengol, J., Crossetti, L. O., et al. (2010). Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 44, 3345–3354. doi: 10.1016/j.watres.2010.03.018
Bovo-Scomparin, V. M., and Train, S. (2008). Long-term variability of the phytoplankton community in an isolated floodplain lake of the Ivinhema River State Park. Brazil. Hydrobiologia 610, 331–344. doi: 10.1007/s10750-008-9448-3
Cai, Q. (2007). Protocols for Standard Observation and Measurement in Aquatic Ecosystems. Beijing: Chinese Environmental Science Press.
Cai, Q., Tang, T., and Liu, J. (2003). Several research hotspots in river ecology. Chin. J. Appl. Ecol. 14, 1573–1577.
Castro, L. D., Arago, D., Colares, L., Palheta, L., Mayko, D., Fernandes, L. M., et al. (2021). Dam promotes downriver functional homogenization of phytoplankton in a transitional river-reservoir system in amazon. Limnology 22, 245–257. doi: 10.1007/s10201-021-00650-6
Costa, L. S., Huszar, V., and Ovalle, A. R. (2009). Phytoplankton functional groups in a tropical estuary: hydrological control and nutrient limitation. Estuar. Coast. 32, 508–521. doi: 10.1007/s12237-009-9142-3
Costanza, R., D’Arge, R., Groot, R. D., Farber, S., Grasso, M., Hannon, B., et al. (1997). The value of the world’s ecosystem services and natural capital. Nature 387, 253–260. doi: 10.1016/S0921-8009(98)00020-2
Crossetti, L. O., Becker, V., Cardoso, L., Rodrigues, L. R., Costa, L., and Motta-Marques, D. D. (2013). Is phytoplankton functional classification a suitable tool to investigate spatial heterogeneity in a subtropical shallow lake? Limnologica 43, 157–163. doi: 10.1016/j.limno.2012.08.010
Da Silva, C. A., Train, S., and Rodrigues, L. C. (2005). Phytoplankton assemblages in a Brazilian subtropical cascading reservoir system. Hydrobiologia 537, 99–109. doi: 10.1007/s10750-004-2552-0
Darchambeau, F., Sarmento, H., and Descy, J. (2014). Primary production in a tropical large lake: the role of phytoplankton composition. Sci. Total Environ. 473, 178–188. doi: 10.1016/j.scitotenv.2013.12.036
Devercelli, M. (2006). Phytoplankton of the Middle Paraná River during an anomalous hydrological period: a morphological and functional approach. Hydrobiologia 563, 465–478. doi: 10.1007/s10750-006-0036-0
Devercelli, M., and Farrell, O. I (2013). Factors affecting the structure and maintenance of phytoplankton functional groups in a nutrient rich lowland river. Limnologica 43, 67–78. doi: 10.1016/j.limno.2012.05.001
Dutilleul, P., and Legendre, P. (1993). Spatial heterogeneity against heteroscedasticity: an ecological paradigm versus a statistical concept. Oikos 66, 152–171. doi: 10.2307/3545210
Grime, J. P. (1977). Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 111, 1169–1194. doi: 10.2307/2460262
Hu, H., and Wei, Y. (2006). The Freshwater Algae of China: Systematic, Taxonomy and Ecology. Beijing: Science Press.
Huang, X., Chen, W., and Cai, Q. (1999). Survey, Observation and Analysis of Lake Ecology. Standard Methods for Observation and Analysis in Chinese Ecosystem Research Network, Series V. Beijing: Science Press.
Jelil, S. N., Gaykar, A., Girkar, N., Ben, C., and Ramesh, K. (2021). Mammal persistence along riparian forests in western india within a hydropower reservoir 55 years post construction. Front. Ecol. Evol. 9:643285. doi: 10.3389/fevo.2021.643285
Jiang, X., Jing, X., Xie, Z., and Chen, Y. (2011). Longitudinal patterns of macroinvertebrate functional feeding groups in a Chinese river system: a test for river continuum concept (RCC). Quatern. Int. 244, 289–295. doi: 10.1016/j.quaint.2010.08.015
John, D. M., Whitton, B. A., and Brook, A. J. (2002). The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae. Cambridge: Cambridge University Press.
Kruk, C., Mazzeo, N., Lacerot, G., and Reynolds, C. (2002). Classification schemes for phytoplankton: a local validation of a functional approach to the analysis of species temporal replacement. J. Plankton Res. 24, 901–912. doi: 10.1093/plankt/24.9.901
Lepš, J., and Šmilauer, P. (2003). Multivariate Analysis of Ecological Data Using CANOCO. Cambridge: Cambridge University Press.
Lewitus, A. J. (2002). Eutrophication processes in coastal systems: origin and succession of plankton blooms and effects on secondary production in gulf coast estuaries. Copeia 1, 248–249.
Li, J., Dong, S., Liu, S., Yang, Z., Peng, M., and Zhao, C. (2013). Effects of cascading hydropower dams on the composition, biomass and biological integrity of phytoplankton assemblages in the middle Lancang-Mekong River. Ecol. Eng. 60, 316–324. doi: 10.1016/j.ecoleng.2013.07.029
Liao, N., Li, H., You, L., Chen, M., and Zhang, Y. (2020). Succession of phytoplankton functional groups and driving variables in a young canyon reservoir. Int J Environ. Sci. Te. 1, 1–14. doi: 10.1007/s13762-020-02949-w
Liu, L., Yang, Z., Delwiche, K. B., Long, L., and Lorke, A. (2020). Spatial and temporal variability of methane emissions from cascading reservoirs in the upper Mekong river. Water Res. 186:116319. doi: 10.1016/j.watres.2020.116319
McCartney, M. P., Sullivan, C., Acreman, M. C., and McAllister, D. E. (2000). Ecosystem Impacts of Large Dams. Thematic Review II, Prepared for IUCN/UNEP/WCD. Available online at: https://www.researchgate.net/profile/Matthew-Mccartney-3/publication/45165880_Ecosystem_Impacts_of_Large_Dams/links/0deec538c8d836760c000000/Ecosystem-Impacts-of-Large-Dams.pdf
Mieleitner, J., and Reichert, P. (2008). Modelling functional groups of phytoplankton in three lakes of different trophic state. Ecol. Model. 211, 279–291. doi: 10.1016/j.ecolmodel.2007.09.010
Miranda, L. E., Habrat, M. D., and Miyazono, S. (2008). Longitudinal gradients along a reservoir cascade. T. Am. Fish. Soc. 137, 1851–1865. doi: 10.1577/T07-262.1
Moura, A. N., Severiano, J. S., Tavares, N., and Dantas, E. W. (2013). The role of a cascade of reservoirs and seasonal variation in the phytoplankton structure in a tropical river. Brazil J. Biol. 73, 291–298. doi: 10.1590/S1519-69842013000200009
Naselli-Flores, L. (2000). Phytoplankton assemblages in twenty-one sicilian reservoirs: relationships between species composition and environmental factors. Hydrobiologia 424, 1–11. doi: 10.1023/A:1003907124528
Nogueira, M. G., Ferrareze, M., Moreira, M. L., and Gouvêa, R. M. (2018). Phytoplankton assemblages in a reservoir cascade of a large tropical-subtropical river (SE. Brazil). Brazil J. Biol. 70, 781–793. doi: 10.1590/s1519-69842010000400009
Padisák, J., Barbosa, F., Koschel, R., and Krienitz, L. (2003). Deep layer cyanoprokaryota maxima in temperate and tropical lakes. Arch. Hydrobiol. 58, 175–199.
Padisák, J., Crossetti, L. O., and Naselli-Flores, L. (2009). Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621, 1–19. doi: 10.1007/s10750-008-9645-0
Perbiche-Neves, G., and Nogueira, M. G. (2010). Multi-dimensional effects on Cladoceran (Crustacea, Anomopoda) assemblages in two cascade reservoirs in Southeast Brazil. Lakes. Reserv. Res. Manag. 15, 139–152. doi: 10.1111/j.1440-1770.2010.00429.x
Rangel, L. M., Silva, L. H., Rosa, P., Roland, F., and Huszar, V. L. (2012). Phytoplankton biomass is mainly controlled by hydrology and phosphorus concentrations in tropical hydroelectric reservoirs. Hydrobiologia 693, 13–28. doi: 10.1007/s10750-012-1083-3
Reynolds, C. S. (1984). Phytoplankton periodicity: the interactions of form, function and environmental variability. Freshw. Biol. 14, 111–142. doi: 10.1111/j.1365-2427.1984.tb00027.x
Reynolds, C. S. (1988). Functional Morphology and the Adaptive Strategies of Freshwater Phytoplankton. Growth and Reproductive Strategies of Freshwater Phytoplankton. Cambridge: Cambridge University Press.
Reynolds, C. S. (2006). Ecology of Phytoplankton. Cambridge: Cambridge University Press, doi: 10.1017/CBO9780511542145
Reynolds, C. S., Vera, H., Carla, K., Luigi, N. F., and Sergio, M. (2002). Towards a functional classification of the freshwater phytoplankton. J. Plankton. Res. 24, 417–428. doi: 10.1093/plankt/24.5.417
Rigosi, A., and Rueda, F. J. (2012). Hydraulic control of short-term successional changes in the phytoplankton assemblage in stratified reservoirs. Ecol. Eng. 44, 216–226. doi: 10.1016/j.ecoleng.2012.04.012
Rodgher, S., Espíndola, E., Rocha, O., Fracácio, R., Pereira, R., and Rodrigues, M. (2005). Limnological and ecotoxicological studies in the cascade of reservoirs in the Tietê river (São Paulo. Brazil. Brazil. J. Biol. 65, 697–710. doi: 10.1590/S1519-69842005000400017
Rosenberg, D. M., McCully, P., and Pringle, C. M. (2000). Global-scale environmental effects of hydrological alterations: introduction. Bioscience 50, 746–751. doi: 10.1641/0006-3568(2000)050[0746:GSEEOH]2.0.CO;2
Rychtecký, P., and Znachor, P. (2011). Spatial heterogeneity and seasonal succession of phytoplankton along the longitudinal gradient in a eutrophic reservoir. Hydrobiologia 663, 175–186. doi: 10.1007/s10750-010-0571-6
Shatwell, T., Nicklisch, A., and Köhler, J. (2012). Temperature and photoperiod effects on phytoplankton growing under simulated mixed layer light fluctuations. Limnol. Oceanogr. 57, 541–553. doi: 10.4319/lo.2012.57.2.0541
Shen, H., Li, B., Cai, Q., Han, Q., Gu, Y., and Qu, Y. (2014). Phytoplankton functional groups in a high spatial heterogeneity subtropical reservoir in China. J. Great Lakes res. 40, 859–869. doi: 10.1016/j.jglr.2014.09.007
Stević, F., Mihaljević, M., and Špoljarić, D. (2013). Changes of phytoplankton functional groups in a floodplain lake associated with hydrological perturbations. Hydrobiologia 709, 143–158. doi: 10.1007/s10750-013-1444-6
Straškraba, M., and Tundisi, J. G. (1999). Reservoir Water Quality Management. Guidelines of Lake Management, vol. 9. Kusatsu: International Lake Environment Committee.
Tomanova, S., Tedesco, P. A., Campero, M., Van Damme, P. A., Moya, N., and Oberdorff, T. (2007). Longitudinal and altitudinal changes of macroinvertebrate functional feeding groups in neotropical streams: a test of the River Continuum Concept. Fund. Appl. Limnol. 170, 233–241. doi: 10.1006/jmbi.1999.3310
Wang, J., Ding, C., Tao, J., Jiang, X., and He, D. (2020). Damming affects riverine macroinvertebrate metacommunity dynamics: insights from taxonomic and functional beta diversity. Sci. Total Environ. 763:142945. doi: 10.1016/j.scitotenv.2020.142945
Wang, L., Cai, Q., Tan, L., and Kong, L. (2011). Phytoplankton development and ecological status during a cyanobacterial bloom in a tributary bay of the Three Torges Reservoir. China. Sci. Total Environ. 409, 3820–3828. doi: 10.1016/j.scitotenv.2011.06.041
Ward, J. V., and Stanford, J. A. (1983). “The serial discontinuity concept of lotic ecosystems,” in Dynamics of Lotic Ecosystems, eds T. D. Fontaine and S. M. Bartell (Ann Arbor: Ann Arbor Science Publishers). 29–42.
Wei, K., Zeng, X., Wang, C., Peng, Z., Wang, J., Zhou, F., et al. (2021). Phosphate distribution and sources in the waters of huangbai river, China: using oxygen isotope composition of phosphate as a tracer. Environ. Sci. Pollut. Res. 28, 29732–29741. doi: 10.1007/s11356-021-12808-x
Wentzky, V. C., Tittel, J., Jger, C. G., Bruggeman, J., and Rinke, K. (2020). Seasonal succession of functional traits in phytoplankton communities and their interaction with trophic state. J. Ecol. 108, 1649–1663. doi: 10.1111/1365-2745.13395
Wu, D., Zhao, Y., Cheng, L., Zhou, Z., and Yuan, Q. (2021). Activity and structure of methanogenic microbial communities in sediments of cascade hydropower reservoirs, southwest China. Sci. Total Environ. 786:147515. doi: 10.1016/j.scitotenv.2021.147515
Wu, N., Tang, T., Fu, X., Jiang, W., Li, F., Zhou, S., et al. (2010). Impacts of cascade run-of-river dams on benthic diatoms in the Xiangxi river. China Aquat. Sci. 72, 117–125. doi: 10.1007/s00027-009-0121-3
Xiao, X., Chen, X., Zhang, L., Lai, R., and Liu, J. (2019). Impacts of small cascaded hydropower plants on river discharge in a basin in southern china. Hydrol. Proc. 33, 1420–1433. doi: 10.1002/hyp.13410
Xu, H., Yan, M., Long, L., Ma, J., Ji, D., Liu, D., et al. (2021). Modeling the effects of hydrodynamics on thermal stratification and algal blooms in the Xiangxi Bay of three gorges reservoir. Front. Ecol. Evol. 8:610622. doi: 10.3389/fevo.2020.610622
Xu, Y., Cai, Q., Shao, M., Han, X., and Cao, M. (2009). Seasonal dynamics of suspended solids in a giant subtropical reservoir (china) in relation to internal processes and hydrological features. Quatern. Int. 208, 138–144. doi: 10.1016/j.quaint.2008.12.019
Xu, Y., Cai, Q., Wang, L., Kong, L., and Li, D. (2010a). Diel vertical migration of Peridiniopsis niei, a new species of dinoflagellates in an eutrophic bay of Three-Gorge Reservoir. China Aquat. Ecol. 44, 387–395. doi: 10.1007/s10452-009-9298-8
Xu, Y., Wang, L., Cai, Q., and Ye, L. (2010b). Temporal coherence of chlorophyll a during a spring phytoplankton bloom in Xiangxi bay of Three-Gorges Reservoir. China Int. Rev. Hydrobiol. 94, 656–672.
Yi, Q., Wan, K., Pan, Y., Xie, K., Zhang, X., and Wang, Q. (2020). Driving factors of phytoplankton functional groups in the shallow eutrophic lakes of lowland areas of Huaihe River (China). Environ. Sci. Pollut. Res. 27, 13930–13938. doi: 10.1007/s11356-020-07924-z
Yin, D., Zheng, L., and Song, L. (2011). Spatio-temporal distribution of phytoplankton in the Danjiangkou Reservoir, a water source area for the South-to-North Water Diversion Project (Middle Route), China. Chin. J. Oceanol. Limn. 29, 531–540. doi: 10.1007/s00343-011-0120-9
Zaidel, P. A., Roy, A. H., Houle, K. M., Lambert, B., and Smith, C. (2021). Impacts of small dams on stream temperature. Ecol. Indic. 120:106878. doi: 10.1016/j.ecolind.2020.106878
Keywords: longitudinal heterogeneity, continuum, phytoplankton functional group, cascade reservoirs, Huangbo River
Citation: Shen H, Ye L, Cai Q and Tan L (2022) Longitudinal Variations in Physiochemical Conditions and Their Consequent Effect on Phytoplankton Functional Diversity Within a Subtropical System of Cascade Reservoirs. Front. Ecol. Evol. 10:914623. doi: 10.3389/fevo.2022.914623
Received: 07 April 2022; Accepted: 22 June 2022;
Published: 22 July 2022.
Edited by:
Xiaodong Qu, China Institute of Water Resources and Hydropower Research, ChinaReviewed by:
Fen Guo, Guangdong University of Technology, ChinaCopyright © 2022 Shen, Ye, Cai and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghua Cai, cWhjYWlAaWhiLmFjLmNu; Lu Tan, dGFubHVAaWhiLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.