
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 22 June 2022
Sec. Paleontology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.911512
This article is part of the Research TopicA Fossil View of Insect Evolution: Integrating Paleontological Evidence to Explore the Origins of Insect BiodiversityView all 10 articles
Ptiliidae is a group of distinctly miniaturized staphylinoid beetles with a scarce fossil record. Here, we report a new ptiliid genus and species, Crenossidium slipinskii Li, Newton and Cai gen. et sp. nov., from mid-Cretaceous amber from northern Myanmar. Crenossidium can be attributed to the subfamily Nossidiinae based on the hind wing morphology, which has also been confirmed through phylogenetic analyses. Crenossidium differs from other extant nossidiine genera in the combination of the wide apical maxillary palpomeres, posteriorly widest pronotal disk, (almost) contiguous procoxae, fewer setae along wing margin, and multidentate pygidium.
urn:lsid:zoobank.org:pub:8038D763-6856-4AC5-972C-E20D636137EE.
The family Ptiliidae is a cosmopolitan beetle group in Staphylinoidea. The number of described ptiliid species increased fast in the past several years (e.g., 77 new species of Cissidium Motschulsky in a single article: Darby, 2020). Currently, there are 1,015 valid species known (Newton, 2019, updated by A.F. Newton through March 2022). Ptiliidae is well-known for their minute body size. The smallest member of this family, which is also the smallest beetle and the smallest free-living insect, Scydosella musawasensis Hall, has an adult body length of 325 μm (Polilov, 2015). Even the largest members do not exceed 3 mm in length (Polilov, 2016). The adults of Ptiliidae are distinctively characterized by their feather-like hind wings (Polilov et al., 2019a; Petrov et al., 2020), which are effectively adapted for flight under miniaturization (Farisenkov et al., 2022).
The sister relationship between Ptiliidae and Hydraenidae has long been recognized, supported by multiple lines of morphological and molecular evidence (e.g., Beutel and Leschen, 2005; Lawrence et al., 2011; Mckenna et al., 2015). Their relationship with other staphylinoids has also become rather certain based on several recent phylogenomic studies (Zhang et al., 2018; McKenna et al., 2019; Cai et al., 2022). However, the internal relationships within Ptiliidae are much less clear. The subfamily Nossidiinae, now considered as the deepest branch in Ptiliidae, was formally established only in 2019, based initially on larval characters (Sörensson and Delgado, 2019) and quickly confirmed by adult characters plus molecular data (Polilov et al., 2019b). In the most recent version of the Handbook of Zoology (Hall, 2016), three subfamilies were recognized (Acrotrichinae, Cephaloplectinae, and Ptiliinae). However, based on new phylogenetic analyses, Polilov et al. (2019b) placed all the groups except Nossidiinae (originally in Ptiliinae) in the subfamily Ptiliinae. Seven tribes were recognized within Ptiliinae by Polilov et al. (2019b), although Ptiliini and Discheramocephalini were still likely to be paraphyletic, and the inclusion of Ptenidiini in Ptiliinae was not fully confirmed (Polilov et al., 2019b: Figure 8).
The fossil record of Ptiliidae is very scarce. A few members have been reported from various Cenozoic deposits, although all of them were either placed in extant genera or not determined to the generic level (e.g., Polilov and Perkovsky, 2004; Shockley and Greenwalt, 2013). The only described Mesozoic fossil (Kekveus jason Yamamoto et al.) comes from the mid-Cretaceous Burmese amber (Yamamoto et al., 2018). Kirejtshuk and Azar (2013) additionally mentioned three undescribed specimens from the Early Cretaceous Lebanese amber. In this study, we describe the second member of Ptiliidae from Burmese amber, which also represents the first fossil of the subfamily Nossidiinae.
The Burmese amber specimen studied herein (Figures 1–4) originated from amber mines near Noije Bum (26°20' N, 96°36' E), Hukawng Valley, Kachin State, northern Myanmar. Zircon U-Pb dating has suggested an age of ~99 Ma for the amber-producing strata (Shi et al., 2012), although Mao et al. (2018) suggested that it might be slightly younger than the actual age. Based on the biota of Burmese amber, the Burma Terrane, where the amber mines are located, has been suggested to have a Gondwanan origin (Poinar, 2019). During the mid-Cretaceous, the Burma Terrane was probably an isolated island at near-equatorial latitude based on paleomagnetic evidence (Westerweel et al., 2019).

Figure 1. Crenossidium slipinskii Li, Newton and Cai gen. et sp. nov., holotype, NIGP180053, under incident or transmitted light. (A) Habitus, dorsal view. (B) Habitus, ventral view. (C) Left hind wing, dorsal view. (D) Right hind wing, dorsal view. Scale bars: 200 μm.
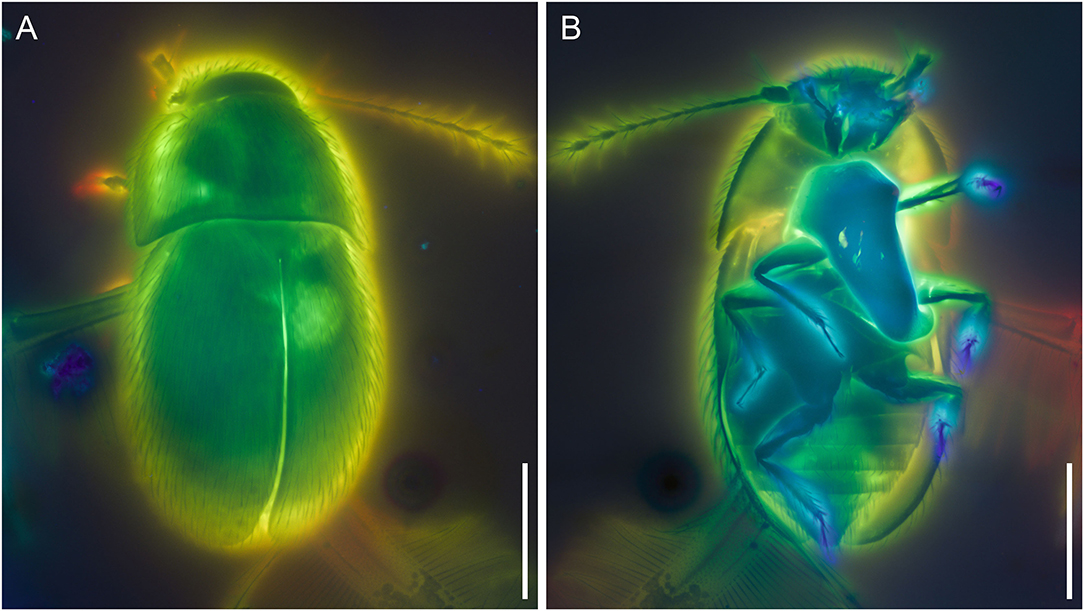
Figure 2. Crenossidium slipinskii Li, Newton and Cai gen. et sp. nov., holotype, NIGP180053, under confocal microscopy, with depth color coding. (A) Dorsal view. (B) Ventral view. Scale bars: 200 μm.
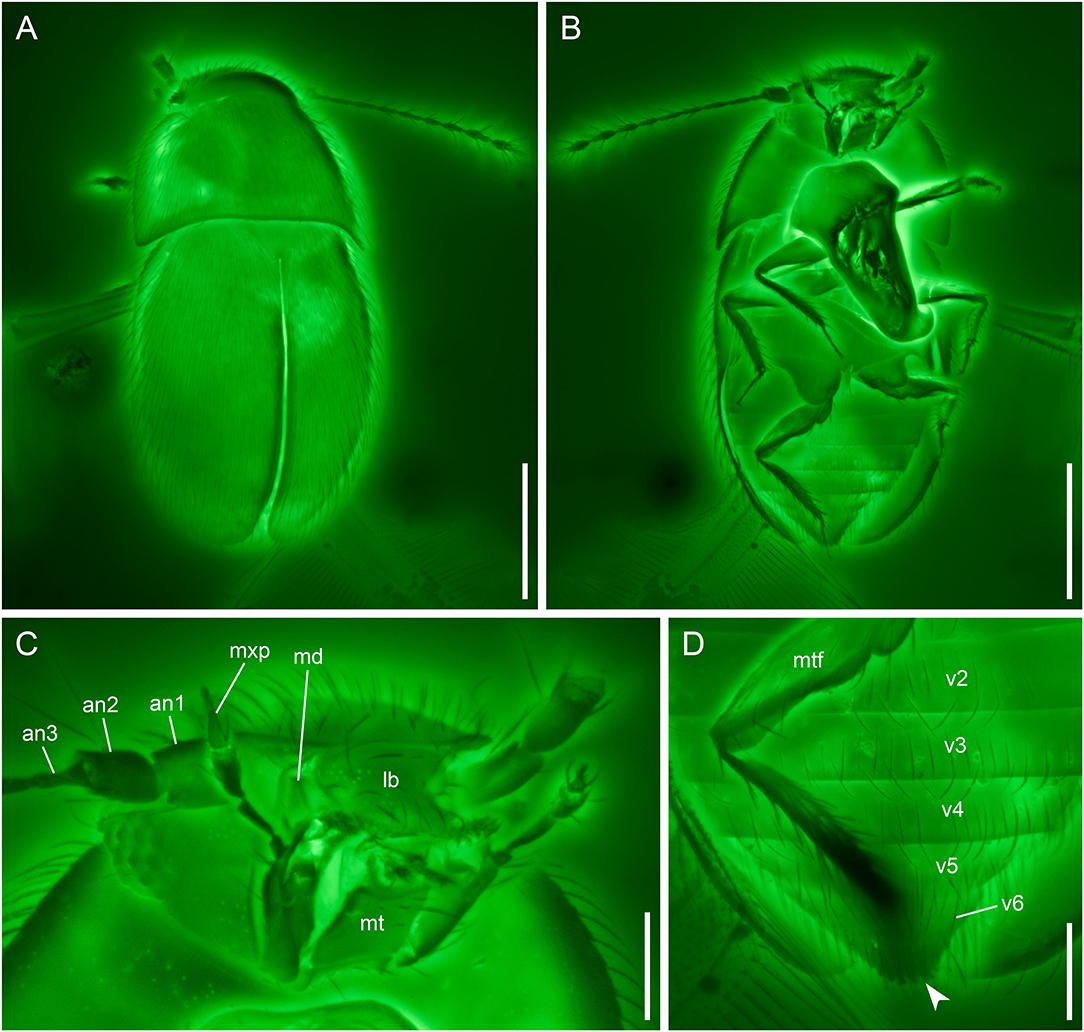
Figure 3. Crenossidium slipinskii Li, Newton and Cai gen. et sp. nov., holotype, NIGP180053, under confocal microscopy. (A) Habitus, dorsal view. (B) Habitus, ventral view. (C) Mouthparts, ventral view. (D) Abdomen, ventral view, showing the multidentate pygidium (arrowhead). an1–3, antennomeres 1–3; lb, labrum; md, mandible; mt, mentum; mtf, metafemur; mxp, maxillary palp; v1–6, ventrites 1–6. Scale bars: 200 μm in (A,B), 50 μm in (C,D).

Figure 4. X-ray microtomographic reconstruction of Crenossidium slipinskii Li, Newton and Cai gen. et sp. nov., holotype, NIGP180053, with hind wings removed in (A–D). (A) Dorsal view. (B) Ventral view. (C) Lateral view. (D) Posterodorsal view. (E) Anterodorsal view. Scale bar: 200 μm.
The specimen is deposited in the Nanjing Institute of Geology and Paleontology (NIGP), Chinese Academy of Sciences, Nanjing, China. The amber piece was trimmed with a small table saw, ground with emery papers of different grit sizes, and finally polished with polishing powder.
Brightfield images were taken with a Zeiss Discovery V20 stereomicroscope. Confocal images were obtained with a Zeiss LSM710 confocal laser scanning microscope, using the 488 nm Argon laser excitation line (Fu et al., 2021). Brightfield images were stacked with Helicon Focus 7.0.2 and Adobe Photoshop CC. Confocal images were stacked with color-coding for depth in ZEN 3.4 (Blue Edition), or semi-manually stacked in Helicon Focus 7.0.2 and Adobe Photoshop CC. Microtomographic data were obtained with a Zeiss Xradia 520 Versa 3D X-ray microscope at the micro-CT laboratory of NIGP and analyzed in VGStudio MAX 3.0. Scanning parameters were as follows: isotropic voxel size, 1.7807 μm; power, 3 W; acceleration voltage, 40 kV; exposure time, 6 s; projections, 3001. Images were further processed in Adobe Photoshop CC to adjust brightness and contrast.
To evaluate the systematic placement of the new species, constrained morphological phylogenetic analyses were performed under Bayesian inference and weighted parsimony. The data matrix (Supplementary Data 1, 2) was mainly derived from a previously published dataset (Polilov et al., 2019b). The vein CuA (character 54) was originally coded as absent for Nossidium Erichson, Motschulskium Matthews and Sindosium Johnson by Polilov et al. (2019b), but according to Polilov et al. (2019a), the posterior vein in the petiole of these species was interpreted as CuA. Thus, the coding of this character was amended accordingly in our analyses. The Bayesian molecular tree (their Figures 8 and S9) by Polilov et al. (2019b) was used as the constraining backbone tree. For taxa with both morphological and molecular data, their interrelationships were fixed according to the molecular tree. The fossil and other extant taxa without molecular data were allowed to move freely across the reference tree (e.g., Fikáček et al., 2020).
The Bayesian analysis was performed using MrBayes 3.2.6 (Ronquist et al., 2012). Two Markov chain Monte Carlo (MCMC) analyses were run simultaneously, each with one cold chain and three heated chains. Trees were sampled every 1,000 generations. Analyses were stopped when the average standard deviation (SD) of split frequencies remained below 0.01. The first 25% of sampled trees were discarded as burn-in, and the remains were used to build a majority-rule consensus tree.
The parsimony analysis was performed under implied weights with R 4.1.0 (R Core Team, 2021) and the R package TreeSearch 1.0.1 (Smith, 2021). Parsimony analyses achieve the highest accuracy under a moderate weighting scheme (i.e., when concavity constants, K, are between 5 and 20) (Goloboff et al., 2018; Smith, 2019). Therefore, the concavity constant was set to 12 here, as suggested by Goloboff et al. (2018).
The trees were drawn with the online tool iTOL 6.5.2 (Letunic and Bork, 2021) and graphically edited with Adobe Illustrator CC 2017.
Order Coleoptera Linnaeus, 1758
Suborder Polyphaga Emery, 1886
Superfamily Staphylinoidea Latreille, 1802
Family Ptiliidae Erichson, 1845
Subfamily Nossidiinae Sörensson and Delgado, 2019
Crenossidium slipinskii sp. nov.
The generic name is a combination of “Cretaceous” and “Nossidium”, the type genus of Nossidiinae. The name is neuter in gender.
Hind wings with comparatively wide wing blade; petiole with two veins; wing margin with fewer than 130 setae; anterior edge of petiole almost without setae; posterior edge of petiole with setae (Figures 1C,D). Maxillary palpomere 4 more than half as wide at base as palpomere 3 (Figure 3C). Pronotum widest basally (Figure 4A). Prosternal process narrow; procoxae (almost) touching (Figure 4B). Pygidium apically multidentate (Figure 3D).
Holotype, NIGP180053 (Figures 1–4), mid-Cretaceous (upper Albian to lower Cenomanian; Shi et al., 2012; Mao et al., 2018) from amber mine near Noije Bum Village, Tanai Township, Myitkyina District, Kachin State, Myanmar.
The species is named after Dr. Stanisław Adam Ślipiński, an internationally recognized coleopterist.
As for the genus.
Body small, about 0.69 mm long and 0.35 mm wide.
Head with vertex somewhat declined. Compound eyes well-developed. Antennal grooves absent. Antennae 11-segmented, generally filiform; antennomeres 1–2 moderately enlarged; antennomeres 3–8 slender and elongate, with similar width; antennomeres 9–11 forming a loose club; antennomere 9 about 1.5 × as wide as 8; antennomeres 10–11 about twice as wide as 8. Mandibles distinctly retracted. Maxillary palps 4-segmented; palpomere 3 thicker than other segments, oval; palpomere 4 aciculate, more than half as wide at base as palpomere 3. Mentum rectangular, with subparallel sides.
Pronotal disk widest basally, gradually narrowed anteriorly; lateral edges convex, weakly serrate; posterior angles moderately produced posteriorly; surface smooth, without clear elevations or impressions. Prosternal process probably narrow. Procoxae touching apically.
Scutellar shield triangular. Elytra complete, apically rounded; lateral edges weakly serrate in anterior half; elytral epipleura present only in anterior half. Mesoventrite with a longitudinal keel medially. Metaventrite posteriorly with a bifurcate process between metacoxae. Metacoxae almost contiguous; plates developed proximally.
Legs short and slender. Femora not strongly flattened. Tibiae with relatively dense setae. Tarsi of typical ptiliid type, cylindrical, apparently 2-segmented. Pretarsal claws simple, equal in size.
Hind wings of typical nossidiine type (see Polilov et al., 2019a); wing blade comparatively wide; petiole with two veins (ScP+RA and CuA); wing margin with about 126 setae; anterior edge of petiole with a single seta; posterior edge of petiole with setae.
Abdomen with six ventrites (presumably sternites III–VIII). Hind margin of pygidium (presumably tergite X) with several clearly marked small teeth.
Crenossidium gen. nov. can be placed in Ptiliidae based on the following characters: antennomere 3 inserted in the apex of antennomere 2 and distinctly narrower than the latter (Figure 3C; Yavorskaya et al., 2017: Figure 4), antennal club 3-segmented, apical maxillary palpomere aciculate (Figure 3C; Yavorskaya et al., 2017: Figure 2C), and hind wings feather-like (wing blade narrower than the length of setae).
Based on the analysis of Polilov et al. (2019b), Ptiliidae can be divided into two subfamilies, Nossidiinae and Ptiliinae. Nossidiinae contains only four extant genera (Sörensson and Delgado, 2019), and is believed to have retained many “primitive” characters. The hind wing morphology of Crenossidium corresponds well with Nossidiinae. They share the presence of two veins in the petiole, edges of petiole with setae, and a comparatively wide wing blade (Figures 1C,D; Polilov et al., 2019a: Figures 1A,B, 2B) (petiole with a single vein, edges of petiole without setae, and wing blade narrower than half length of setae in Ptiliinae). The nossidiine affinity of Crenossidium is also confirmed through formal phylogenetic analyses (Figure 5, Supplementary Figure 1).
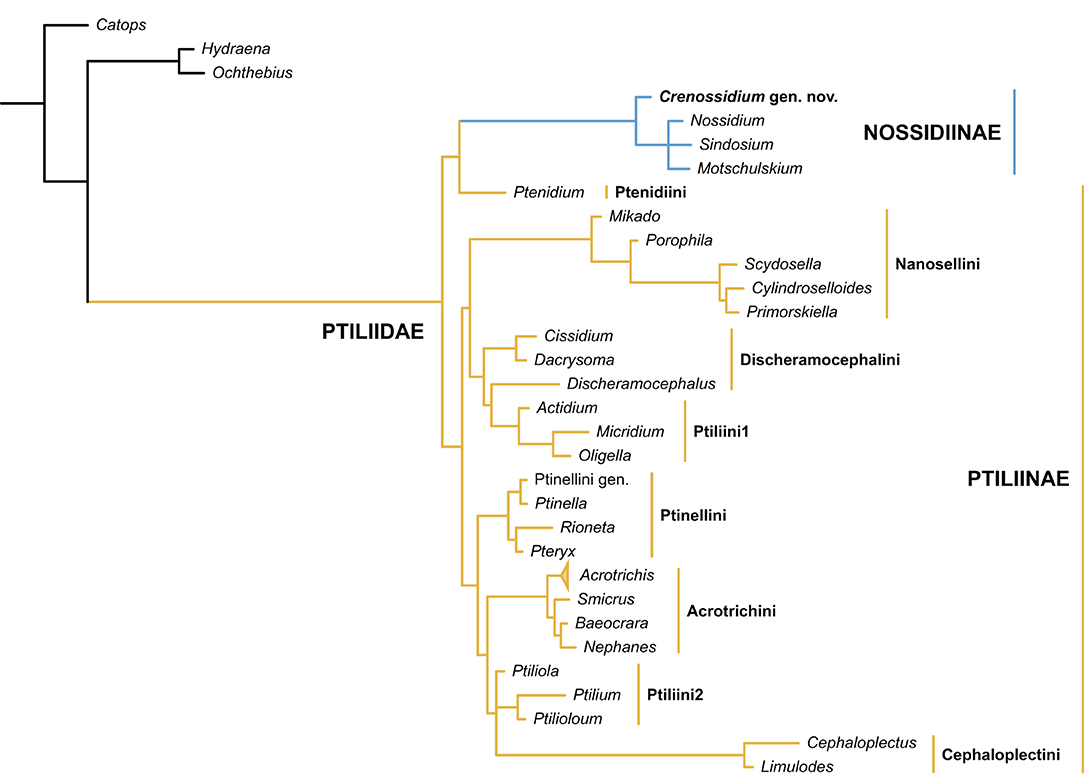
Figure 5. Suggested placement of Crenossidium slipinskii Li, Newton and Cai gen. et sp. nov. within Ptiliidae. Tree resulting from the Bayesian analysis constrained by a molecular backbone tree.
The accurate position of Crenossidium within Nossidiinae is not yet resolved, although it might be sister to all the remaining genera sampled, as suggested by the Bayesian analysis (Figure 5). There are fewer than 130 setae along the hind wing margin in Crenossidium. By contrast, according to Polilov et al. (2019b), the three extant nossidiine terminals sampled in the analysis have more than 200 setae along the wing margin (although in some species the setae may be somewhat fewer than 200; e.g., Darby, 2016: Figure 4E; Maté Nankervis, 2020: Figure 2F). The anterior margin of the petiole is free of setae in Crenossidium and Sindosium (actually, with a single seta), while setae are present on this portion in Nossidium and Motschulskium (Polilov et al., 2019a). Crenossidium shares a multidentate pygidium with Nossidium (Figure 3D; Darby, 2015: Figure 11), whereas the pygidium is bidentate in Motschulskium (Hall, 2000: Figure 53.17) and toothless in Sindosium and Bicavella Johnson (Johnson, 2007). Crenossidium differs from Motschulskium additionally in pronotum widest basally (widest in the middle region in Motschulskium; Polilov et al., 2019b: Figure S1F). However, Nossidium has a moderately wide prosternal process, which clearly separates the procoxae (Polilov et al., 2019b: Figure S1D; though there might be some interspecific variation, A.F. Newton, personal observation), whereas the prosternal process is much narrower with procoxae (almost) touching in Crenossidium (Figure 4B), as well as in the other three extant genera (Johnson, 2007; Polilov et al., 2019b). Crenossidium is distinctive in Ptiliidae by its relatively wide apical maxillary palpomere, which is almost as wide at base as the penultimate one. In most of the modern nossidiines we examined, the palpomere 4 is at most one-third of the maximum width of palpomere 3, and to our knowledge, no modern member in the whole family has maxillary palpomere 4 distinctly wider than the half width of palpomere 3.
The divergence between Ptiliidae and Hydraenidae has been dated to the Early Jurassic, approximately 178–198 Ma (Cai et al., 2022). Therefore, it is reasonable to expect that Ptiliidae might have already been highly diversified at least in the late Mesozoic. However, the Mesozoic fossil record of Ptiliidae is extremely scarce. Only one species has been described from Burmese amber before the current study (Yamamoto et al., 2018), with the addition of a few mentioned presences from Lebanese and Burmese ambers (Rasnitsyn and Ross, 2000; Grimaldi et al., 2002; Kirejtshuk and Azar, 2013; though the unproportionally high specimen number of Ptiliidae mentioned by Grimaldi et al., 2002 seems to be dubious). Indeed, miniaturized groups are often insufficiently documented in the fossil record (e.g., Cai et al., 2019). The minute size probably impeded their preservation as adpression fossils. Even if they have been successfully preserved as adpressions, most fossil collectors would not be able to notice them. Thus, it further emphasizes the importance of amber fossils in alleviating the bias against miniaturized taxa.
The original contributions presented in the study are included in the article/Supplementary Material. The original micro-CT and confocal data are available in Zenodo repository at https://doi.org/10.5281/zenodo.6527975. Further inquiries can be directed to the corresponding author/s.
C-YC and Y-DL conceived the study. C-YC and D-YH acquired and processed the specimen. Y-DL acquired and processed the photomicrographs, performed the analyses, and drafted the manuscript, to which AFN and C-YC contributed. All authors commented on the manuscript and gave final approval for publication.
Financial support was provided by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000), the National Natural Science Foundation of China (42072022 and 41688103), and the Second Tibetan Plateau Scientific Expedition and Research project (2019QZKK0706).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Su-Ping Wu for technical help in micro-CT reconstruction, and Rong Huang and Yan Fang for technical help with confocal imaging.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.911512/full#supplementary-material
Beutel, R. G., and Leschen, R. A. B. (2005). Phylogenetic analysis of Staphyliniformia (Coleoptera) based on characters of larvae and adults. Syst. Entomol. 30, 510–548. doi: 10.1111/j.1365-3113.2005.00293.x
Cai, C., Lawrence, J. F., Yamamoto, S., Leschen, R. A. B., Newton, A. F., Ślipiński, A., et al. (2019). Basal polyphagan beetles in mid-Cretaceous amber from Myanmar: biogeographic implications and long-term morphological stasis. Proc. R. Soc. B. 286, 20182175. doi: 10.1098/rspb.2018.2175
Cai, C., Tihelka, E., Giacomelli, M., Lawrence, J. F., Ślipiński, A., Kundrata, R., et al. (2022). Integrated phylogenomics and fossil data illuminate the evolution of beetles. R. Soc. Open Sci. 9, 211771. doi: 10.1098/rsos.211771
Darby, M. (2015). Nossidium katyae, a new species of Bolivian Ptiliidae (Coleoptera), was described and figured. Zootaxa. 4048, 436–440. doi: 10.11646/zootaxa.4048.3.8
Darby, M. (2016). New species and records of Costa Rican featherwing beetles (Coleoptera: Ptiliidae). Zootaxa. 4184, 41–51. doi: 10.11646/zootaxa.4184.1.2
Darby, M. (2020). A revision of Cissidium Motschulsky (Coleoptera: Ptiliidae) with seventy seven new species. Eur. J. Taxon. 622, 1–188. doi: 10.5852/ejt.2020.622
Farisenkov, S. E., Kolomenskiy, D., Petrov, P. N., Engels, T., Lapina, N. A., Lehmann, F. O., et al. (2022). Novel flight style and light wings boost flight performance of tiny beetles. Nature. 602, 96–100. doi: 10.1038/s41586-021-04303-7
Fikáček, M., Beutel, R. G., Cai, C., Lawrence, J. F., Newton, A. F., Solodovnikov, A., et al. (2020). Reliable placement of beetle fossils via phylogenetic analyses–Triassic Leehermania as a case study (Staphylinidae or Myxophaga?). Syst. Entomol. 45, 175–187. doi: 10.1111/syen.12386
Fu, Y.-Z., Li, Y.-D., Su, Y.-T., Cai, C.-Y., and Huang, D.-Y. (2021). Application of confocal laser scanning microscopy to the study of amber bioinclusions. Palaeoentomology. 4, 266–278. doi: 10.11646/palaeoentomology.4.3.14
Goloboff, P. A., Torres, A., and Arias, J. S. (2018). Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics. 34, 407–437. doi: 10.1111/cla.12205
Grimaldi, D. A., Engel, M. S., and Nascimbene, P. C. (2002). Fossiliferous Cretaceous amber from Myanmar (Burma): its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit. 3361, 1–71.
Hall, W. E. (2000). “Ptiliidae Erichson, 1845,” in American Beetles. Vol. 1. Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia, eds R. H. Arnett and M. C. Thomas (Boca Raton: CRC Press), 233–246.
Hall, W. E. (2016). “Ptiliidae Erichson, 1845,” in Handbook of Zoology, Arthropoda: Insecta, Coleoptera, beetles, Vol. 1: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim). 2nd Edition, eds R. G. Beutel and R. A. B. Leschen (Berlin: Walter de Gruyter), 345–356.
Johnson, C. (2007). Studies on Ptiliidae (Col.) from the Solomon Islands, 1. Entomol. Mon. Mag. 143, 213–227.
Kirejtshuk, A. G., and Azar, D. (2013). Current knowledge of Coleoptera (Insecta) from the Lower Cretaceous Lebanese amber and taxonomical notes for some Mesozoic groups. Terr. Arthropod Rev. 6, 103–134. doi: 10.1163/18749836-06021061
Lawrence, J. F., Ślipiński, A., Seago, A. E., Thayer, M. K., Newton, A. F., and Marvaldi, A. E. (2011). Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 61, 1–217. doi: 10.3161/000345411X576725
Letunic, I., and Bork, P. (2021). Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296. doi: 10.1093/nar/gkab301
Mao, Y., Liang, K., Su, Y., Li, J., Rao, X., Zhang, H., et al. (2018). Various amberground marine animals on Burmese amber with discussions on its age. Palaeoentomology. 1, 91–103. doi: 10.11646/palaeoentomology.1.1.11
Maté Nankervis, J. F. (2020). Description of two new species of Sindosium Johnson, 2007 from Australia (Coleoptera, Ptiliidae). Zootaxa. 4895, 528–540. doi: 10.11646/zootaxa.4895.4.4
Mckenna, D. D., Farrell, B. D., Caterino, M. S., Farnum, C. W., Hawks, D. C., Maddison, D. R., et al. (2015). Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Syst. Entomol. 40, 35–60. doi: 10.1111/syen.12093
McKenna, D. D., Shin, S., Ahrens, D., Balke, M., Beza-Beza, C., Clarke, D. J., et al. (2019). The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. USA. 116, 24729–24737. doi: 10.1073/pnas.1909655116
Newton, A. F. (2019). “StaphBase: Staphyliniformia world catalog database (version Nov 2018),” in Catalogue of Life Checklist (Nov 2018), eds O. Bánki, Y. Roskov et al. Available online at: https://www.catalogueoflife.org/.
Petrov, P. N., Farisenkov, S. E., and Polilov, A. A. (2020). Miniaturization re-establishes symmetry in the wing folding patterns of featherwing beetles. Sci. Rep. 10, 16458. doi: 10.1038/s41598-020-73481-7
Poinar, G. (2019). Burmese amber: Evidence of Gondwanan origin and Cretaceous dispersion. Hist. Biol. 31, 1304–1309. doi: 10.1080/08912963.2018.1446531
Polilov, A. A. (2015). How small is the smallest? New record and remeasuring of Scydosella musawasensis Hall, 1999 (Coleoptera, Ptiliidae), the smallest known free-living insect. ZooKeys. 526, 61–64. doi: 10.3897/zookeys.526.6531
Polilov, A. A. (2016). “Structure of the principal groups of microinsects. III. Featherwing Beetles (Coleoptera: Ptiliidae),” in At the Size Limit-Effects of Miniaturization in Insects (Cham: Springer), 77–133. doi: 10.1007/978-3-319-39499-2_5
Polilov, A. A., and Perkovsky, E. E. (2004). New species of Late Eocene feather-winged beetles (Coleoptera, Ptiliidae) from the Rovno and Baltic amber. Paleontol. J. 38, 664–668.
Polilov, A. A., Reshetnikova, N. I., Petrov, P. N., and Farisenkov, S. E. (2019a). Wing morphology in featherwing beetles (Coleoptera: Ptiliidae): features associated with miniaturization and functional scaling analysis. Arthropod Struct. Dev. 48, 56–70. doi: 10.1016/j.asd.2019.01.003
Polilov, A. A., Ribera, I., Yavorskaya, M. I., Cardoso, A., Grebennikov, V. V., and Beutel, R. G. (2019b). The phylogeny of Ptiliidae (Coleoptera: Staphylinoidea)—the smallest beetles and their evolutionary transformations. Arthropod Syst. Phylogeny. 77, 433–455.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org
Rasnitsyn, A. P., and Ross, A. J. (2000). A preliminary list of arthropod families present in the Burmese amber collection at The Natural History Museum, London. Bull. Nat. Hist. Mus. Lond. (Geol.). 56, 21–24.
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shi, G., Grimaldi, D. A., Harlow, G. E., Wang, J., Wang, J., Yang, M., et al. (2012). Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 37, 155–163. doi: 10.1016/j.cretres.2012.03.014
Shockley, F. W., and Greenwalt, D. (2013). Ptenidium kishenehnicum (Coleoptera: Ptiliidae), a new fossil described from the Kishenehn oil shales, with a checklist of previously known fossil ptiliids. Proc. Entomol. Soc. Wash. 115, 173–181. doi: 10.4289/0013-8797.115.2.173
Smith, M. R. (2019). Bayesian and parsimony approaches reconstruct informative trees from simulated morphological datasets. Biol. Lett. 15, 20180632. doi: 10.1098/rsbl.2018.0632
Smith, M. R. (2021). TreeSearch: morphological phylogenetic analysis in R. bioRxiv. doi: 10.1101/2021.11.08.467735
Sörensson, M., and Delgado, J. A. (2019). Unveiling the smallest–systematics, classification and a new subfamily of featherwing beetles based on larval morphology (Coleoptera: Ptiliidae). Invertebr. Syst. 33, 757–806.
Westerweel, J., Roperch, P., Licht, A., Dupont-Nivet, G., Win, Z., Poblete, F., et al. (2019). Burma Terrane part of the Trans-Tethyan arc during collision with India according to palaeomagnetic data. Nat. Geosci. 12, 863–868. doi: 10.1038/s41561-019-0443-2
Yamamoto, S., Grebennikov, V. V., and Takahashi, Y. (2018). Kekveus jason gen. et sp. nov. from Cretaceous Burmese amber, the first extinct genus and the oldest named featherwing beetle (Coleoptera: Ptiliidae: Discheramocephalini). Cretac. Res. 90, 412–418. doi: 10.1016/j.cretres.2018.05.016
Yavorskaya, M., Beutel, R. G., and Polilov, A. (2017). Head morphology of the smallest beetles (Coleoptera: Ptiliidae) and the evolution of sporophagy within Staphyliniformia. Arthropod Syst. Phylogeny. 75, 417–434.
Keywords: Ptiliidae, fossil, Burmese amber, Mesozoic, phylogeny
Citation: Li Y-D, Newton AF, Huang D-Y and Cai C-Y (2022) The First Fossil of Nossidiinae From Mid-Cretaceous Amber of Northern Myanmar (Coleoptera: Ptiliidae). Front. Ecol. Evol. 10:911512. doi: 10.3389/fevo.2022.911512
Received: 02 April 2022; Accepted: 09 May 2022;
Published: 22 June 2022.
Edited by:
Li Tian, China University of Geosciences Wuhan, ChinaReviewed by:
Alexey Polilov, Lomonosov Moscow State University, RussiaCopyright © 2022 Li, Newton, Huang and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen-Yang Cai, Y3ljYWlAbmlncGFzLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.