
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 10 January 2023
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.908560
This article is part of the Research Topic Social Functions of Bat Vocalizations View all 15 articles
 Lara C. Marggraf1,2*
Lara C. Marggraf1,2* Oliver Lindecke2,3,4
Oliver Lindecke2,3,4 Christian C. Voigt2,3
Christian C. Voigt2,3 Gunārs Pētersons5
Gunārs Pētersons5 Silke L. Voigt-Heucke3,6
Silke L. Voigt-Heucke3,6In late summer, migratory bats of the temperate zone face the challenge of accomplishing two energy-demanding tasks almost at the same time: migration and mating. Both require information and involve search efforts, such as localizing prey or finding potential mates. In non-migrating bat species, playback studies showed that listening to vocalizations of other bats, both con-and heterospecifics, may help a recipient bat to find foraging patches and mating sites. However, we are still unaware of the degree to which migrating bats depend on con-or heterospecific vocalizations for identifying potential feeding or mating opportunities during nightly transit flights. Here, we investigated the vocal responses of Nathusius’ pipistrelle bats, Pipistrellus nathusii, to simulated feeding and courtship aggregations at a coastal migration corridor. We presented migrating bats either feeding buzzes or courtship calls of their own or a heterospecific migratory species, the common noctule, Nyctalus noctula. We expected that during migratory transit flights, simulated feeding opportunities would be particularly attractive to bats, as well as simulated mating opportunities which may indicate suitable roosts for a stopover. However, we found that when compared to the natural silence of both pre-and post-playback phases, bats called indifferently during the playback of conspecific feeding sounds, whereas P. nathusii echolocation call activity increased during simulated feeding of N. noctula. In contrast, the call activity of P. nathusii decreased during the playback of conspecific courtship calls, while no response could be detected when heterospecific call types were broadcasted. Our results suggest that while on migratory transits, P. nathusii circumnavigate conspecific mating aggregations, possibly to save time or to reduce the risks associated with social interactions where aggression due to territoriality might be expected. This avoidance behavior could be a result of optimization strategies by P. nathusii when performing long-distance migratory flights, and it could also explain the lack of a response to simulated conspecific feeding. However, the observed increase of activity in response to simulated feeding of N. noctula, suggests that P. nathusii individuals may be eavesdropping on other aerial hawking insectivorous species during migration, especially if these occupy a slightly different foraging niche.
Animals living in temperate zones are exposed to drastic variations in environmental conditions due to a pronounced climatic seasonality. These fluctuations affect prey abundance and habitat suitability, and as a consequence, many species migrate to more favorable areas (Milner-Gulland et al., 2011). Yet, migration is also an energetically challenging task where easy access to relevant information about profitable resources, e.g., foraging and resting opportunities, may be advantageous or even life-saving (Newton and Brocki, 2008; Goodale et al., 2010). In some animals of the temperate zone, e.g., in many species of bats, the timing of migration may also overlap with mating activities. These species are confronted with both the challenge of finding sufficient food for fueling the energy-demanding migratory journey with the search for a suitable mating partner at the same time. Information from the environment and from conspecifics, or even heterospecifics may be key for the optimal decision-making in such dual challenge situations (Schoener, 1971; Clark and Mangel, 1984; Budaev et al., 2019).
In Europe, Nathusius’ pipistrelle bats (Pipistrellus nathusii) and common noctules (Nyctalus noctula) move within short time from familiar locations of their summer area to areas they may know, poorly know or even do not know (e.g., stopover sites) with temporally and spatially unpredictable availability of food and roosts (Hedenström, 2009). A recent study showed that P. nathusii exhibited high metabolic rates during migratory transit flights, even when flying at an energetically optimal speed (Troxell et al., 2019). To cover the elevated energy demands of transit flights, P. nathusii use a ‘mixed-fuel strategy’ based on oxidizing ingested insect proteins from insects caught en route (“aerial refueling”) and fatty acids from their body reserves (Voigt et al., 2012). Although P. nathusii depend on insects as an oxidative fuel for migration, they rarely engage in foraging while flying in an actual migration corridor (Voigt et al., 2018). Instead, they seem to forage first at nightfall and then launch into the sky to proceed their migration route. However, P. nathusii is well known to also engage in courtship and mating activities at the locations of their daytime stopovers along the migration routes where also temporal harems may be formed (Schmidt, 1994a,b; Furmankiewicz, 2003; Jahelková and Horáček, 2011). It can be assumed that social cues, i.e., male courtship calls motivate susceptible females to break migratory transit flights at night. Thus, both of these energy and time demanding life-history stages, mating and migration, are largely, seasonally overlapping in P. nathusii, and also in some other migratory bat species, such as Soprano pipistrelles (P. pygmaeus), common noctules (N. noctula) and Leisler’s bats (N. leisleri; Dietz et al., 2009).
A solution to the problem of finding profitable foraging sites, suitable mating partners or a roost for resting could either be active communication with other bats via directed social calls (Furmankiewicz et al., 2011), or passive information transfer, i.e., eavesdropping on foraging or courtship behavior of other bats. Indeed, eavesdropping on echolocation calls has been documented for several bat species (e.g., Barclay, 1982; Gillam, 2007; Dechmann et al., 2013; Übernickel et al., 2013; Cvikel et al., 2015; Gager, 2019; Roeleke et al., 2020). At the same time, listening bats which use vocalizations from other bats for additional information acquisition may save energy because echolocation is energetically costly at high intensities (Currie et al., 2020). This is by extending their own range of perception using other bats as a mobile sensory network, i.e., their calls, to detect distant or clumped insect patches, etc., (Fenton, 2003; Jones and Siemers, 2011; Cvikel et al., 2015; Roeleke et al., 2020; Roeleke et al., 2022). This is facilitated by characteristic, stereotypic repetitions of echolocation calls, so-called feeding buzzes emitted by hunting bats (Schnitzler and Kalko, 2001). Eavesdropping on con-and heterospecific echolocation calls, including feeding buzzes, may also help to avoid situations where competition over limited (prey) resources is high (Roeleke et al., 2018). Additionally, flying bats may also locate suitable resting sites by eavesdropping on inadvertent echolocation calls emitted by roosting bats (Ruczyński et al., 2007). Finally, active information transfer with respect to social vocalizations, such as courtship calls or songs, has also been demonstrated for bats. For example, playback experiments showed that bats use social calls to actively coordinate group-foraging (Wilkinson and Boughman, 1998). Further, female bats may use male songs to find potential mates (Knörnschild et al., 2017) and possibly suitable roosts. In summary, both passive and active acoustic information transfers represent a prominent behavior in many bat species. Yet, it is unknown whether either active or passive acoustic information is of relevance in the dual challenge situation of bat migration, when bats might trade potential feeding and social activities with the straight continuation of migratory flights.
The purpose of our study was to determine whether or not either form of acoustic information transfers, active or passive, play a role for migratory P. nathusii during transit flights. We hypothesized that during migration, bats of this species utilize eavesdropping on feeding buzzes to localize promising foraging patches (passive information transfer by another forager) and, secondly, that P. nathusii listen to courtship vocalizations in order to detect suitable mating partners and roosts for stopovers (active information transfer by conspecifics). We therefore predicted P. nathusii to be attracted to feeding buzzes during migratory transit flights and to courtship calls, and thus demonstrate positive phonotaxis accompanied by an increase in bat calls. Further, we assumed that P. nathusii would be more attracted to calls of their own species than to calls of heterospecifics, such as N. noctula. We used feeding calls and courtship calls for this as well. However, based on similar energetic challenges during migratory transit flights, and the fact that both species are insectivorous, we would expect P. nathusii to respond positively, i.e., with increased activity due to N. noctula calls. In contrast to this, we predicted that P. nathusii would not increase activity at the migration corridor when courtship calls of heterospecific N. noctula are played back, i.e., bats would ignore those calls or even show negative phonotaxis through a decrease in activity.
This is the first study to elucidate if and how broadcast acoustic information of bat vocalizations is weighed by actively migrating bats, especially when their need of finding suitable foraging patches and mating partners coincide seasonally and a decision is crucial for both survival (optimal migration) and fitness (optimal mating).
We carried out field work next to ‘Pape Bird Ringing Station’ (56.1667, 21.0059, henceforth abbreviated as PBRS) at the Latvian coastline of the Baltic Sea from 12th of August to 3rd of September 2015. This field site lies within a well-known flight corridor for coastal bat migration used, in particular, by P. nathusii, P. pygmaeus and N. noctula (Pētersons, 2004; Lindecke et al., 2019). To the best of our knowledge, PBRS is solely used as a migration corridor as we are not aware of any mating roosts and courting males in this area. We conducted all experiments on a small clearing at a dune, 100 meter inland off the Baltic Sea shore. Migrating bats traverse it, flying along the shore from North to South (Lindecke et al., 2015, 2019). Because of strongly directional flights, we expected to never encounter an animal twice at the experimental site. In support of this notion, we have never encountered any recaptures of the same banded individual within one season.
In our playback experiment, we used two functional vocalization types and two stimulus species: foraging call sequences with a “feeding buzz” in the end and sequences of courtship calls, both of P. nathusii (focal species) and N. noctula (control; Figure 1).
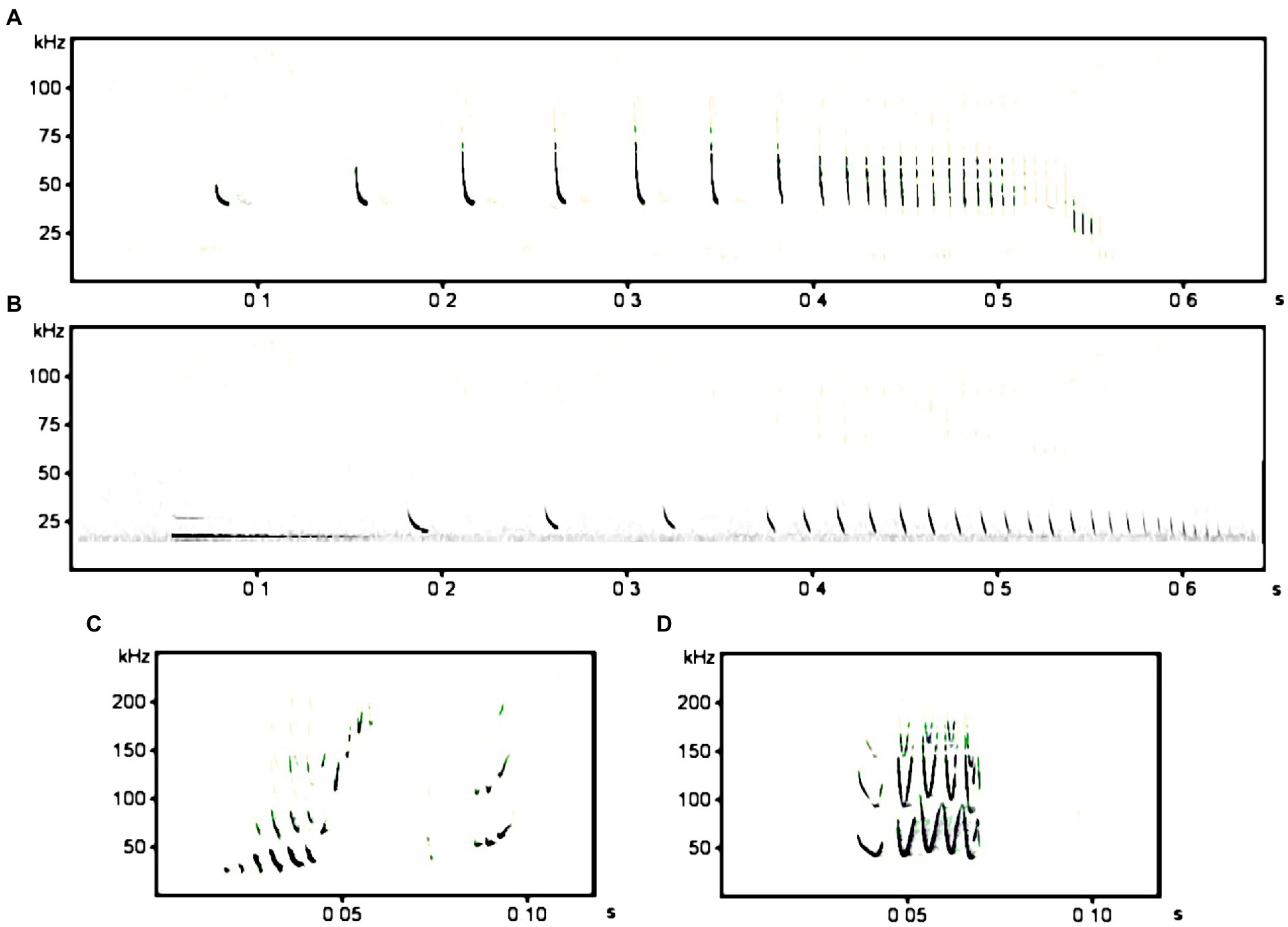
Figure 1. Spectrograms (frequency (kHz) in relation to time (s)) of examples for the stimulus type “feeding buzzes” of Pipistrellus nathusii (A) and Nyctalus noctula (B). The courtship vocalization of a male P. nathusii (C) and the main motif of a male N. noctula courtship song (D).
We only chose recordings with a good signal to noise ratio. To create the playbacks of feeding buzzes, we selected sequences from data recorded in the surroundings of Dedelow, Brandenburg, Germany (53.3631, 13.8085) from May to September 2013 and 2014, i.e., from an area in 575 km airline distance southwesterly to our experimental site at PBRS. This sampling region is well within the European mating area of P. nathusii (Schmidt, 1994a,b) and, in particular, bats passing PBRS may stopover there (ringing data, see, e.g., Pētersons, 2004). We created files of equal length for every single feeding buzz sequence to about 0.6 s by cutting a sequence after the end of the final buzz and from that point backwards until 0.6 s were reached. Every single sequence consisted of a search phase, followed by an approach phase and the final buzz phase (Figures 1A,B). Final playback files with a 1 min duration were created by randomly selecting five 0.6 s sequences which were then replicated in a loop. In total, every 1 min file contained 100 feeding buzzes. This way, we produced 8 individual playback files for each species. For the second vocalization type, the courtship vocalizations, we used files that were also recorded in northeastern Germany during the mating seasons 2010 and 2011 (for detailed information see Voigt-Heucke et al., 2016). For P. nathusii, we used vocalizations produced as part of the advertisement song (Figure 1C) and for N. noctula the most common motif of noctule courtship song (Figure 1D). About 30 individual song motifs per file were randomly pasted together for each species including species-specific characteristics like natural pause lengths between the song motifs. Those sequences were then repeated to obtain a file of 1 min total length. This way, we also obtained 8 individual playback files for each species. Altogether we had 32 different playback files, consisting of 8 files with feeding buzzes and 8 files with courtship calls of P. nathusii and 8 files with feeding buzzes and 8 files with courtship calls of N. noctula. We treated final playback files with a high-pass filter at 10 kHz and a low-pass filter at 125 kHz to eliminate background noise. Additionally, peak amplitudes of playback files were separately normalized to 75%. All playback files were created using Avisoft SAS Lab Pro (Avisoft Bioacoustics; Raimund Specht, Berlin, Germany).
We placed an ultrasound speaker (ScanSpeak, Avisoft Bioacoustics) and an ultrasound microphone (Avisoft condenser ultrasound microphone CM16, Avisoft Bioacoustics) in 3 m distance to each other to broadcast playback sequences and simultaneously monitor vocal responses of passing bats. The speaker was placed about 3.5 m above ground; hanging on a dead branch of a pine tree. The microphone was placed in front of the speaker at a height of 1.5 m and orientated upwards. Playbacks were broadcast with an USG Player 116 (Avisoft Bioacoustics, Berlin, Germany), recorded onto RECORDER USGH (Avisoft Bioacoustics, Berlin, Germany) and an ultrasound speaker (Ultrasonic Dynamic Speaker ScanSpeak, Avisoft Bioacoustics, Berlin, Germany).
Filenames of the broadcast playback files, time and weather conditions were listed simultaneously for differentiation between vocal responses of broadcast playbacks later on in the analysis of the spectrograms. Playback volume was maximized without clipping, resulting in maximum playback amplitudes of 97 ± 2 dB SPL at 0° and 100 cm (mean ± SD). Assuming a bat hearing threshold of 20 dB, low playback frequencies (20 kHz) can be audible over 91 m while higher frequencies (50 kHz) that suffer stronger atmospheric attenuation can reach over 28.5 m at 20°C and 70% relative humidity (calculations based on Urick, 1983). All recordings were conducted using a 16 bit resolution and a 250 kHz sampling rate. We determined the detection range of the microphone for most common echolocation calls (frequency of maximum energy: 37–42 kHz) of P. nathusii was 32 m at 97.2 dB SPL (max. Output of the speaker), 0° and 100 cm distance to the speaker. Louder calls of up to 107 dB would be detectable further away, but are still within the range of our playbacks without considering effects of air temperature, relative humidity, position to the bat in relation to the microphone and intensity adjustments by emitting bats according to targets (R. Specht, Avisoft, pers. comm.; see also Barataud, 2015, pp. 38ff; Adams et al., 2012). However, the relatively high flight speed of P. nathusii in Pape of 6.9 m/s (Troxell et al., 2019) suggests that many bats in the pre-and post-playbackphases passed the location of the speaker, yet never heard any of the playback files; with such speed, bats may have left the audible range (37–42 kHz; 60 m) in approximatly 10 s.
Broadcast playback files consisted of three periods: a 1-min pre-playback period, in which we recorded the baseline for bat activity; a 1-min playback period, in which we presented a playback stimulus and during which we recorded immediate changes to the stimuli; and a 1-min post-playback period, to verify that there was a constant activity of bats passing. We assumed that Nathusius’ pipistrelles migrate at a speed of 6.9 m/s at the experimental site (Troxell et al., 2019) and thus, we ruled out that the same individuals were exposed to all three playback periods. We broadcast two stimulus types: feeding buzzes and courtship vocalizations of two species (resulting in 4 different playback files per playback trial, see Figure 2). In each playback phase, one of the four playback files was broadcast and for each experimental trial, the order of playback stimulus presentation was randomized. In total, one playback trial was 12 min long and started only if we detected calls of P. nathusii with our ultrasonic detector. Each night of the experimental season, we measured wind speed as a proxy for the likelihood of migratory activity approximately 30 min after sunset. Playback trials were run subsequently, if wind force was below 8 m/s as migrating bats usually stop flying at high wind speed (Rydell et al., 2010; Voigt et al., 2018).

Figure 2. Scheme of one experimental trial consisting of two playback stimulus types (feeding buzzes and courtship calls) of the two species (P. nathusii and N. noctula) resulting in four different playback files.
All playback trials were conducted at the same location at PBRS, starting approximately 30 min after sunset. Ideally, playback trials were run throughout the night, except bad weather hindered us from conducting experiments. The likelihood of presenting a playback to the same individual was negligible as bats continuously migrate toward the South (Lindecke et al., 2015; Voigt et al., 2018) and, thus pseudo-replication was avoided. The number of playback trials that we were able to conduct differed between 1 to 15 trials per night with the length of a break ranging between 1 min and 3 h 28 min due to changing weather conditions and general bat activity. We conducted 117 Trials in total. For our subsequent analysis we used 140 playback files.
We intended that the here adopted experimental design would enable direct comparison with previous studies on bats that ran outside of the migration season or with non-migratory species. However, we acknowledge the risk that bats during migration could be less responsive to playbacks if their focus is more toward a quick transit between stopover sites instead of spending time with foraging and/or social interactions.
For further analysis, we only included recordings in which vocal activity of P. nathusii was present in all periods of our experiment in order to control for a constant activity, i.e., constant bat passings during any experiment. The presence of vocal activity meant at least 1 echolocation call of P. nathusii (range: 1–724, median: 69.5). Due to this we post-hoc gathered variable numbers of recordings for each stimulus type. However, this resulted in 72 recordings for playbacks of P. nathusii (32 experimental files for feeding buzzes and 40 files for courtship calls, respectively) and 68 recordings for playbacks of N. noctula (33 experimental files for feeding buzzes, and 35 files for courtship calls). We counted the number of all echolocation calls (EC) in each of the three periods of a playback experiment to quantify the vocal response of P. nathusii to the different stimulus types. For each of these periods, we also counted the number of recorded feeding buzzes (from here on abbreviated with FB) and social calls (from here on abbreviated with SC), however without analyzing them later, because of their low sample size. We use the terms EC and EC activity (or SC) synonymously to the number of EC (SC) recorded. All these synonymous terms therefore represent the same response measure in our playback experiment. All acoustical analyzes of experimental recordings were performed using Avisoft SAS Lab Pro (Avisoft Bioacoustics) spectrograms (Hamming window, 512 FFT length, 50% overlap).
We tested for normal distribution using Shapiro–Wilk-Tests, which revealed a non-normally distributed dataset. To test for differences in vocal responses for each presented stimulus type, we compared the number of EC across time periods (pre-, play-, and post-playback period) using Friedman-Tests. In the presence of significant differences, we used Nemenyis Tests as post-hoc tests. All statistical analyzes were conducted in R (Version 0.98.1103 – © 2009–2014 RStudio, Inc.). The significance level was set to 5%.
We conducted playback experiments on 19 nights over the course of 3 weeks. From 140 experimental playbacks, we collected 39,012 calls, consisting of 39,012 EC (99.9%), including 30 FB (0.08%) and 8 SC (0.02%). The median number of recorded calls per trial was 69.5 EC (range: 1–724), 0 FB (range: 0–5), and 0 SC (range: 0–6).
The vocal response of P. nathusii to the stimulus types quantified as the number of EC differed between the pre-playback period and playback period for two stimulus types of two different species. More precisely, while hearing the playback of conspecific courtship calls, we recorded less EC of P. nathusii compared to the pre-playback period (Friedman-Test; n = 40, χ22 = 5.92, p = 0.05; post-hoc Nemenyis test p = 0.05; Figure 3). The number of EC decreased by 9.06% between pre-playback and playback period. EC activity of migrating P. nathusii did not vary in response to heterospecific courtship calls when compared between the periods prior, during and after the presentation of the playback stimulus (Friedman-Test: n = 34, χ22 = 2.68, p = 0.26; Figure 3).
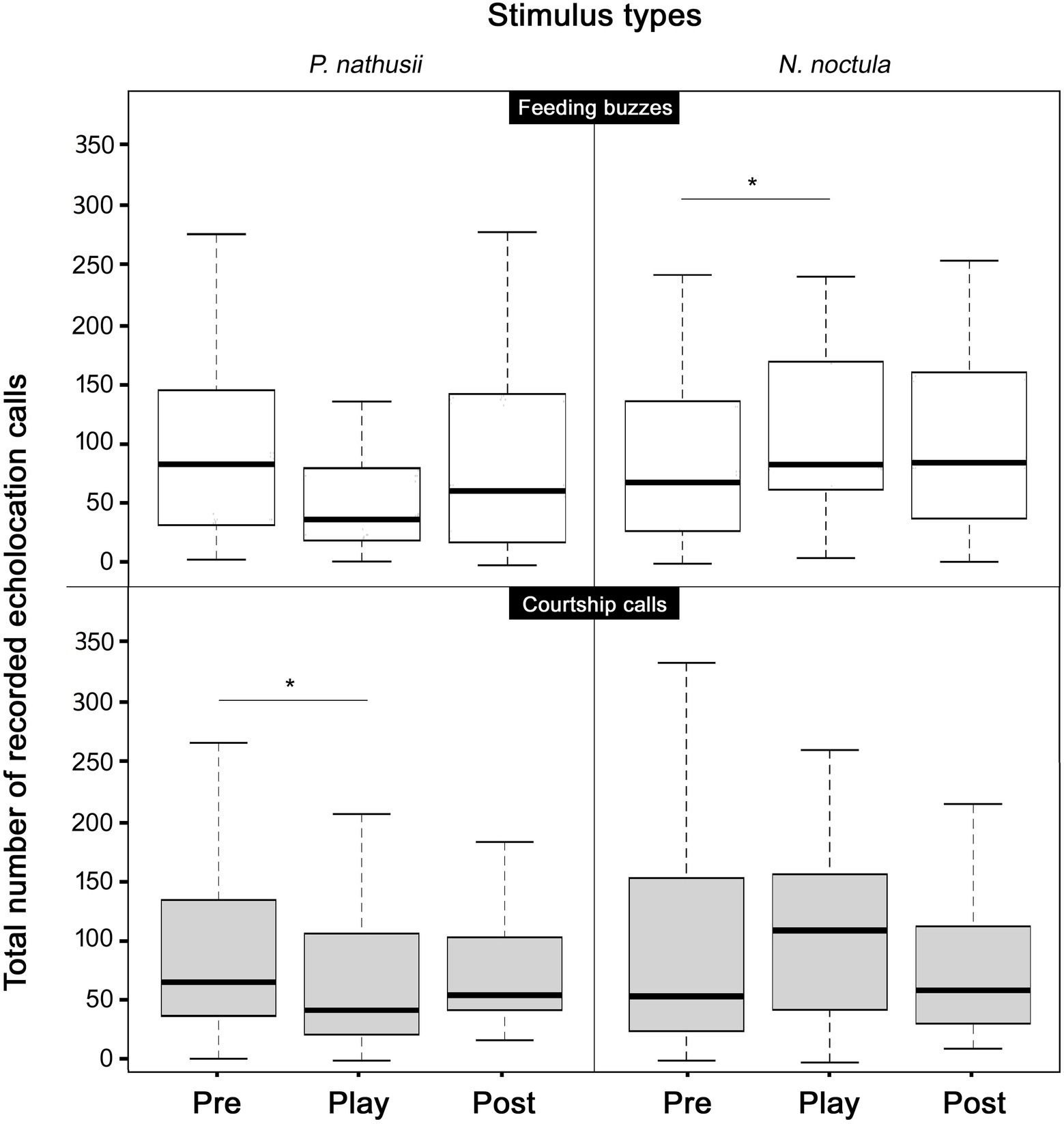
Figure 3. Vocal responses of Nathusius’ bats (P. nathusii) prior, during and after the playback of broadcasted stimulus types, i.e., feeding buzzes and courtship calls of their own (left graphs) and a heterospecific species (N. noctula, right graphs). Solid black lines in the center of boxes represent the median, the borders of boxes are 25 and 75 percentiles; whiskers represent the 5 and 95 percentiles. Note, that no changes of acoustic activity levels, and decreases thereof respectively, suggest that the majority of bats passed the speaker location quickly, i.e., without a reduction of their migration speed. In result, the majority of bats will have experienced only a single playback phase. Significant differences between playback periods are indicated by a line associated with an asterisk (* = p < 0.05).
In contrast to the behavioral response in the presentation of courtship calls, P. nathusii showed more EC activity during the playback of heterospecific feeding buzzes compared to the pre-playback period (Friedman-Test; n = 32, χ22 = 7.56, p < 0.02; post-hoc Nemenyis test p = 0.016; Figure 3). The number of EC increased by 14.94% between pre-playback and playback period. EC activity of migrating P. nathusii did not vary in response to conspecific feeding buzzes when compared between the periods prior, during and after the presentation of the playback stimulus (Friedman-Test; n = 32, χ22 = 4.39, p = 0.11; Figure 3).
In our study, we investigated the acoustic response by means of the acoustic activity of echolocation calls of Nathusius’ bats (P. nathusii) to simulated feeding and courtship activities of con-and heterospecifics during the annual life-history stage of migration at a major migration corridor for bats in Europe, the coast of the Baltic Sea in Latvia. We expected P. nathusii to be attracted to playbacks of FB and to courtship calls of conspecifics during migratory transit flights. We argued that a (simulated) high feeding activity may indicate profitable foraging patches with high insect densities; a valuable resource for migrating bats that encounter high energy demands during migration (Voigt et al., 2016; Costantini et al., 2019; Troxell et al., 2019; Currie et al., 2020). Previous studies demonstrated that some free ranging bat species approach playbacks of conspecific, and even heterospecific FB. For instance, P. nathusii were found to approach loudspeakers that broadcast EC and FB of conspecifics and heterospecifics during late spring and early summer (Dorado-Correa et al., 2013); which is the time when P. nathusii females give birth and wean their young. Approaching behavior was also found for P. nathusii in response to broadcasts of courtship calls in August and September, yet in the non-migratory population of Northern Ireland at the edge of the species distribution range (Russ and Racey, 2007). Our study is therefore the first to look at the response behavior of Nathusius’ bats to conspecific and heterospecific calls during migration.
We found the general EC activity of P. nathusii decreased during the playback of conspecific courtship calls, but not conspecific feeding buzzes. Thus, contrary to our predictions, P. nathusii appeared to ignore acoustically simulated feeding locations and even avoid courtship locations. The observed increase in acoustic activity in response to presented stimuli in earlier studies led to the widely accepted conclusion that bats seem to be generally attracted by FB and SC (Russ and Racey, 2007; Dechmann et al., 2009; Dorado-Correa et al., 2013; for bat species from other geographic and phylogenetic backgrounds see, e.g., Gillam, 2007; Übernickel et al., 2013). However, none of these studies were conducted in a migratory context and thus, previous studies targeted test animals with different motivations compared to our study. Intriguingly, Roeleke et al. (2018) applied our playback files in their study on N. noctula–P. nathusii interactions in Germany. They observed that N. noctula increased local activity in response to playbacks of P. nathusii in early summer when insect densities are high, but reduced their activity in late summer prior to migration onset. Interestingly, in closely related Common pipistrelles, P. pipistrellus, Jonker et al. (2010) also found no attraction to the broadcast of conspecific FB and Voigt-Heucke et al. (2016) obtained the same result to the broadcast of SC in studies conducted between August and September. In contrast to P. nathusii, however, P. pipistrellus moves seasonally over short distances only (~20 km; Hutterer et al., 2005). Yet, unlike in our experiments, these authors observed no decrease in the acoustic activity of Common pipistrelles (aversion) in response to playbacks. Recently and contrary to our study, Reyes and Szewczak (2022) found out that a migratory species from the American continent, Lasiurus cinereus, can be attracted by their own social calls during fall and spring migration as it increases capture success. In our study, we rule out that the aversive behavior of P. nathusii in response to conspecific playbacks of courtship call stimuli might have resulted from an unnatural character of stimuli. Exactly the same stimuli were used in Voigt-Heucke et al. (2016) and similar stimuli were used in the playback of noctule courtship calls. Yet P. nathusii - in the experimental set-up presented here - did not respond with an increase or decrease in vocal activity to this heterospecific stimulus, i.e., bats were neither attracted nor repelled by those playbacks. Like in other playback studies with bats, we remain unaware about the exact number of individuals that we tested in our experiment or the sex and age of the recorded bats. Therefore, we cannot make any inferences about the specific behavior of bat individuals, but rather conjecture about the response behavior of P. nathusii in general. However, in conclusion of our data collection, we realized that the length of playback phases might be reduced in future studies: In the case of migrating bats at PBRS, which may fly 6.9 m/s (Troxell et al., 2019), the number of bats being exposed to silence and a call-playback phase is lower when compared with other playback studies where potentially less transient bats were exposed to two or even three playback phases. A decision between paired and non-paired tests was therefore harder to make, yet an undetermined number of bats will still have experienced the switch from one phase to another. Hence, paired analyzes appear advisable for our data. Irrespective of whether bats are sedentary or migrating, only a second method of observation seems to allow a clear assessment of whether individual bats behave differently after/around playback phase changes, e.g., thermal imaging. This is true for any playback-study and, in sum, the measurement will not always (i.e., for every bat) be based on paired measures, yet it is a possibility that individual bats are recorded in two or even three phases if they remain in the catchment area of the speaker and/or microphone. Keeping these limitations in mind, our study on migrating bats reveals interesting patterns that can be interpreted as aversion to conspecific vocalizations.
Surprisingly, our data revealed an increase in P. nathusii EC activity in response to the broadcast of heterospecific FB during the playback period, but no change in EC activity in response to playbacks of conspecific FB. This finding contradicts our prediction that during migration eavesdropping on foraging conspecifics might be a strategy to save time and energy. In theory, bats should make use of highly profitable foraging patches that we simulated by the playback of FB. Such acoustic cues should increase the likelihood of finding prey when conspecific bats act as an array of sensors (Gillam, 2007; Cvikel et al., 2015; Roeleke et al., 2020). Yet, even though eavesdropping may allow bats to broaden their own range of perception, its use does not necessarily involve advantages only, e.g., bats may need to direct their attention toward conspecifics, and are thus not able to detect prey items at the same time, consequently using more energy for flight maneuvers in order to avoid collision (Amichai et al., 2015). Therefore, we speculate that P. nathusii at our study site were not attracted to FB of their own species, because they were anticipating disadvantages from hunting in proximity of unfamiliar conspecifics (Voigt-Heucke et al., 2010). The common noctule (Nyctalus noctula), which is migratory as well, occurs also during migration in sympatry with P. nathusii, shows comparable foraging strategies but a different dietary composition and is three times larger in body-size (Dietz et al., 2009; Voigt et al., 2016). Both species are aerial hawking foragers, which catch their prey on flight (Norberg and Rayner, 1987), but isotopic data suggests that P. nathusii and N. noctula are using different habitats during migration compared to the pre-migration period (Voigt et al., 2016). Furthermore the diet of N. noctula contains predominantly larger non-tympanate insects such as Trichoptera, Epheneroptera, Coleoptera and Hemiptera, whereas the main diet of P. nathusii consists of Diptera, Lepidoptera and Neuroptera, which are only opportunistically catched by N. noctula (Krüger et al., 2014). Furthermore N. noctula is an open space aerial hawking bat whereas P. nathusii is an edge space forager (Schnitzler and Kalko, 2001; Denzinger and Schnitzler, 2013). By using a playback of 100 FB/min, we simulated a relatively high feeding activity which might also be interpreted by passing bats as a high density of conspecifics to. Thus, while a high number of FB may indicate a dense cluster of insects on the one side, it could also expose the approaching bat to higher levels of conspecific interference, i.e., aggression on the other side (Racey and Swift, 1985).
We further observed that the EC activity of P. nathusii bats decreased in response to the playback of conspecific courtship calls. Most previous studies documented that broadcasting SC will attract target bats or lead to an increase in acoustic activity. For example, in a group cohesion context, Wilkinson and Boughman (1998) demonstrated that social calls of neotropical Phyllostomus hastatus attracted conspecifics at roosts and on feeding sites. In a courtship context, some species of bats use calls or even complex songs to attract potential mates. In another neotropical, yet strictly insectivorous bat, Saccopteryx bilineata, it was shown that simulating the presence of singing males attracted dispersing females (Knörnschild et al., 2017). But in another case, social vocalizations such as song have also been shown to cause no response in conspecifics of Tadarida brasiliensis (Bohn et al., 2013). In our study, we observed a decrease in EC activity in response to the playback of conspecific courtship calls, suggesting two possible explanations: (1) the stimulus elicited an avoidance behavior of the simulated courtship area. Negative phonotaxis could be the result of bats listening to other bat calls, i.e., eavesdropping. If bats fly a detour around the loudspeaker, a temporary reduction in call activity would be measurable near the playback site. (2) Eavesdropping could continue as long as bats are in the vicinity of the speaker. It may be that the bats are listening but still flying near the microphone. In line with our results in P. nathusii, Barlow and Jones (1997) found that during the non-mating or migration phase, P. pipistrellus reduced their EC activity in response to the broadcasting of conspecific social calls. Barlow and Jones suggested that social calls similar to courtship vocalizations could be used to scare off individuals when used outside of the mating season (Barlow and Jones, 1997). Voigt-Heucke et al. (2016) however found that during the late mating season, the playback of con-and heterospecific social calls did not lead to a change in general EC activity, but a change in the social call rate of wild P. pipistrellus. During our experiments, the number of social calls from free flying P. nathusii was very low (0.02%). Thus, playback responses to social calls in Pipistrellus bats in general seem to depend on the season, and also on the calling rate with which the playback was constructed. This remains to be tested.
In our study, we were unaware about the sex of the individuals that listened to our playback treatments. In a study on tropical Saccopteryx bilineata, Knörnschild and colleagues showed that playback of male song elicited approach flights of mostly subadult females (Knörnschild et al., 2017). Moreover, female S. bilineata preferred songs from the local population over songs from foreign locations, demonstrating that song familiarity influences female phonotaxis. Here, we speculate that similar to birds (Kroodsma and Miller, 1996), courtship vocalizations could also serve to repel potentially competing males. Accordingly, migrating male bats might have been repelled by conspecific social vocalization because they are motivated to cover distances instead of engaging in territorial encounters that might lead to aggressive encounters. Female P. nathusii might as well ignore social vocalizations because social interactions (mating) might prolong their migratory journey. This scenario argues for an avoidance behavior of migratory bats when conspecific social vocalizations are heard in an otherwise ideal spatio-temporal context (i.e., feeding or mating opportunities in a migration corridor). Interestingly, a playback-study on the function and context of vocalization in a primate species, the mangabey (Cercocebus atys) also revealed that test groups moved away from neighboring and unknown calls, but approached those of their own males (Waser, 1977). Indeed, Barlow and Jones (1997) found in P. pipistrellus that the playback of conspecific SC led to a reduction of acoustic activity when broadcast outside of the mating season. An alternative interpretation to our results comes from another long-distance mammal migrant, Pacific humpback whales (Megaptera novaeangliae). Similar to P. nathusii, humpback whales also combine mating and migration. In playback experiments, Tyack (1983) observed approaches of male whales to songs and social sounds, but avoidance in females, respectively. Female humpback whales may have tried to protect their social group, and in particular the young, by avoiding conflicts, whereas males approached to defend their group. Moreover, playbacks mediated inter-group avoidance in a study on forest monkey, Cercocebus albiena, e.g., to circumvent conflicts (Waser, 1975). However, it is not known whether P. nathusii migrates in large groups and/or with their offspring. Thus, behavior related to group cohesion, protection or an association with offspring may not play a role for migratory P. nathusii, and remains speculative.
In conclusion, we found that P. nathusii avoided simulated courtship sites of conspecifics and did not respond to comparatively simulated heterospecific mating aggregations at a major European bat migration corridor. In contrast, we found that P. nathusii seemed to be attracted by simulated feeding sites of heterospecifics and did not respond to comparatively simulated conspecific aggregations. Our findings argue against a generalized increase of bat activity in response to playbacks of vocalizations of con-or heterospecifics. We therefore conclude advertent or inadvertent information received from calling con-or heterospecifics does not necessarily play a role for P. nathusii on migratory transit flights, even though foraging opportunities and mating partners are important in the general context of migration.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All procedures performed in this study involving animals were in accordance with the ethical standards of the University of Latvia, Laboratory of Ornithology (license number 10/2015 of the Latvian Nature Conservation Agency) and adhered to the ASAB/ABS Guidelines for the use of animals in research.
SV-H, LM, and OL designed the experiments. LM carried out the fieldwork, conducted the data analysis, and wrote the first draft of the manuscript. CV and GP supported the fieldwork, organization and administration of the project. CV and SV-H provided materials and supervized the project. All authors reviewed and edited the manuscript.
The project was funded by grants from the Leibniz Institute for Zoo and Wildlife Research.
We would like to thank Stefanie Zimmer and Olga Heim for allowing us to use their recordings of echolocation and social calls. Furthermore, we are grateful to Oskars Keišs for his support during field work at PBRS, and Kerstin Kraemer and Peter Busse for their support of the work of LM. We thank Lasse Jakobsen for discussing our data and calculations on the effective range of emitted stimuli. OL is grateful to the Deutsche Forschungsgemeinschaft (Projektnummer 395940726-SFB) for generous financial support. The project was funded by grants from the Leibniz Institute for Zoo and Wildlife Research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, A. M., Jantzen, M. K., Hamilton, R. M., and Fenton, M. B. (2012). Do you hear what I hear? Implications of detector selection for acoustic monitoring of bats. Methods Ecol. Evol. 3, 992–998. doi: 10.1111/j.2041-210X.2012.00244.x
Amichai, E., Blumrosen, G., and Yovel, Y. (2015). Calling louder and longer: how bats use biosonar under severe acoustic interference from other bats. Proc. R. Soc. B 282:20152064. doi: 10.1098/rspb.2015.2064
Barataud, M. (2015). Acoustic Ecology of European Bats. Biotope and National Museum of Natural History, Paris
Barclay, R. M. R. (1982). Interindividual use of echolocation calls: eavesdropping by bats. Behav. Ecol. Sociobiol. 10, 271–275. doi: 10.1007/BF00302816
Barlow, K. E., and Jones, G. (1997). Function of pipistrelle social calls: field data and a playback experiment. Anim. Behav. 53, 991–999. doi: 10.1006/anbe.1996.0398
Bohn, K., Shmarsh, G. C., and Smotherman, M. (2013). Social context evokes rapid changes in bat song syntax. Anim. Behav. 85, 1485–1491. doi: 10.1016/j.anbehav.2013.04.002
Budaev, S., Jørgensen, C., Mangel, M., Eliassen, S., and Giske, J. (2019). Decision-making from the animal perspective: bridging ecology and subjective cognition. Front. Ecol. Evol. 7:164. doi: 10.3389/fevo.2019.00164
Clark, C. W., and Mangel, M. (1984). Foraging and flocking strategies: information in an uncertain environment. Am. Nat. 123, 626–641. doi: 10.1086/284228
Costantini, D., Lindecke, O., Pētersons, G., and Voigt, C. C. (2019). Migratory flight imposes oxidative stress in bats. Current. Zoology 65, 147–153. doi: 10.1093/cz/zoy039
Currie, S. E., Boonman, A., Troxell, S., Yovel, Y., and Voigt, C. C. (2020). Echolocation at high intensity imposes metabolic costs on flying bats. Nat. Ecol. Evol. 4, 1174–1177. doi: 10.1038/s41559-020-1249-8
Cvikel, N., Berg, K. E., Levin, E., Hurme, E., Borissov, I., Boonman, A., et al. (2015). Bats aggregate to improve prey search but might be impaired when their density becomes too high. Curr. Biol. 25, 206–211. doi: 10.1016/j.cub.2014.11.010
Dechmann, D. K., Heucke, S. L., Giuggioli, L., Safi, K., Voigt, C. C., and Wikelski, M. (2009). Experimental evidence for group hunting via eavesdropping in echolocating bats. Proc. R. Soc. B Biol. Sci. 276, 2721–2728. doi: 10.1098/rspb.2009.0473
Dechmann, D. K., Wikelski, M., van Noordwijk, H. J., Voigt, C. C., and Voigt-Heucke, S. L. (2013). Metabolic costs of bat echolocation in a non-foraging context support a role in communication. Front. Physiol. 4:66. doi: 10.3389/fphys.2013.00066
Denzinger, A., and Schnitzler, H. U. (2013). Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 4:164. doi: 10.3389/fphys.2013.00164
Dietz, C., von Helversen, O., and Nill, D. (2009). Bats of Britain, Europe and Northwest Africa. London: A&C Black Publishers, 400.
Dorado-Correa, A. M., Goerlitz, H. R., and Siemers, B. M. (2013). Interspecific acoustic recognition in two European bat communities. Front. Physiol. 4:192. doi: 10.3389/fphys.2013.00192
Fenton, M. B. (2003). Eavesdropping on the echolocation and social calls of bats. Mammal Rev. 33, 193–204. doi: 10.1046/j.1365-2907.2003.00019.x
Furmankiewicz, J. (2003). The vocal activity of Pipistrellus nathusii (Vespertilionidae) in SW Poland. Acta Chiropterol. 5, 97–105. doi: 10.3161/001.005.0109
Furmankiewicz, J., Ruczynski, I., Urban, R., and Jones, G. (2011). Social calls provide tree dwelling bats with information about the location of conspecifics at roosts. Ethology 117, 480–489. doi: 10.1111/j.1439-0310.2011.01897.x
Gager, Y. (2019). Information transfer about food as a reason for sociality in bats. Mammal Rev. 49, 113–120. doi: 10.1111/mam.12146
Gillam, E. H. (2007). Eavesdropping by bats on the feeding buzzes of conspecifics. Can. J. Zool. 85, 795–801. doi: 10.1139/Z07-060
Goodale, E., Beauchamp, G., Magrath, R. D., Nieh, J. C., and Ruxton, G. D. (2010). Interspecific information transfer influences animal community structure. Trends Ecol. Evol. 25, 354–361. doi: 10.1016/j.tree.2010.01.002
Hedenström, A. (2009). Optimal migration strategies in bats. J. Mammal. 90, 1298–1309. doi: 10.1644/09-MAMM-S-075R2.1
Hutterer, R., Ivanova, T., Meyer-Cords, C. H., and Rodrigues, L. (2005). Bat migrations in Europe. A review of banding data and literature. BfN-Schriftenvertrieb im Landwirtschaftsverlag.
Jahelková, H., and Horáček, I. (2011). Mating system of a migratory bat, Nathusius' pipistrelle (Pipistrellus nathusii): different male strategies. Acta Chiropterol. 13, 123–137. doi: 10.3161/150811011X578679
Jones, G., and Siemers, B. M. (2011). The communicative potential of bat echolocation pulses. J. Comp. Physiol. A 197, 447–457. doi: 10.1007/s00359-010-0565-x
Jonker, M. N., de Boer, W. F., Kurvers, R. H. J. M., and Dekker, J. J. A. (2010). Foraging and public information use in common pipistrelle bats (Pipistrellus pipistrellus): a field experiment. Acta Chiropterol. 12, 197–203. doi: 10.3161/150811010X504699
Knörnschild, M., Blüml, S., Steidl, P., Eckenweber, E., and Nagy, M. (2017). Bat songs as acoustic beacons–male territorial songs attract dispersing females. Sci. Rep. 7:13918. doi: 10.1038/s41598-017-14434-5
Kroodsma, D. E., and Miller, E. H. (1996). Ecology and Evolution of Acoustic Communication in Birds. Cornell University Press, Ithaca, NY.
Krüger, F., Clare, E. L., Symondson, W. O. C., Keišs, O., and Pētersons, G. (2014). Diet of the insectivorous bat Pipistrellus nathusii during autumn migration and summer residence. Mol. Ecol. 23, 3672–3683. doi: 10.1111/mec.12547
Lindecke, O., Elksne, A., Holland, R. A., Pētersons, G., and Voigt, C. C. (2019). Orientation and flight behaviour identify the soprano pipistrelle as a migratory bat species at the Baltic Sea coast. J. Zool. 308, 56–65. doi: 10.1111/jzo.12654
Lindecke, O., Voigt, C. C., Pētersons, G., and Holland, R. A. (2015). Polarized skylight does not calibrate the compass system of a migratory bat. Biol. Lett. 11:20150525. doi: 10.1098/rsbl.2015.0525
Milner-Gulland, E. J., Fryxell, J. M., and Sinclair, A. R. E. (2011). Animal Migration: A Synthesis. Oxford: Oxford University Press.
Norberg, U. M., and Rayner, J. M. V. (1987). Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Phil. Trans. Soc. Lond. B 316, 335–427. doi: 10.1098/rstb.1987.0030
Pētersons, G. (2004). Seasonal migrations of north-eastern populations of Pipistrellus nathusii. Myotis 41–42, 29–56.
Racey, P. A., and Swift, S. M. (1985). Feeding ecology of Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during pregnancy and lactation. I. Foraging behaviour. J. Anim. Ecol. 54, 205–215. doi: 10.2307/4631
Reyes, G. A., and Szewczak, J. M. (2022). Attraction to conspecific social-calls in a migratory, solitary, foliage-roosting bat (Lasiurus cinereus). Sci. Rep. 12:9519. doi: 10.1038/s41598-022-13645-9
Roeleke, M., Blohm, T., Hoffmeister, U., Marggraf, L., Schlägel, U. E., Teige, T., et al. (2020). Landscape structure influences the use of social information in an insectivorous bat. Oikos 129, 912–923. doi: 10.1111/oik.07158
Roeleke, M., Johannsen, L., and Voigt, C. C. (2018). How bats escape the competitive exclusion principle- seasonal shift from intraspecific to interspecific competition drives space use in a bat ensemble. Front. Ecol. Evol. 6:101. doi: 10.3389/fevo.2018.00101
Roeleke, M., Schlägel, U. E., Gallagher, C., Pufelski, J., Blohm, T., Nathan, R., et al. (2022). Insectivorous bats form mobile sensory networks to optimize prey localization: The case of the common noctule bat. Ecology 119:e2203663119. doi: 10.1073/pnas.2203663119
Ruczyński, I., Kalko, E. K., and Siemers, B. M. (2007). The sensory basis of roost finding in a forest bat, Nyctalus noctula. J. Exp. Biol. 210, 3607–3615. doi: 10.1242/jeb.009837
Russ, J. M., and Racey, P. A. (2007). Species-specificity and individual variation in the song of male Nathusius’ pipistrelles (Pipistrellus nathusii). Behav. Ecol. Sociobiol. 61, 669–677. doi: 10.1007/s00265-006-0295-9
Rydell, J., Bach, L., Duborg-Savage, M. J., Green, M., Rodrigues, L., and Hedenstrom, A. (2010). Bat mortality at wind turbines in northwestern Europe. Acta Chiropterol. 12, 261–274. doi: 10.3161/150811010X537846
Schmidt, A. (1994a). Phänologisches verhalten und populationseigenschaften der Rauhhautfledermaus, Pipistrellus nathusii (Keyserling und Blasius, 1839), in Ostbrandenburg, Teil 1. Nyctalus 5, 77–100.
Schmidt, A. (1994b). Phänologisches Verhalten und populationseigenschaften der Rauhhautfledermaus, Pipistrellus nathusii (Keyserling und Blasius, 1839), in Ostbrandenburg, Teil 2. Nyctalus 5, 123–148.
Schnitzler, H. U., and Kalko, E. K. (2001). Echolocation by insect-eating bats. Bioscience 51, 557–569. doi: 10.1641/0006-3568(2001)051[0557:EBIEB]2.0.CO;2
Schoener, T. W. (1971). Theory of feeding strategies. Annu. Rev. Ecol. Syst. 2, 369–404. doi: 10.1146/annurev.es.02.110171.002101
Troxell, S. A., Holderied, M. W., Pētersons, G., and Voigt, C. C. (2019). Nathusius' bats optimize long- distance migration by flying at maximum range speed. J. Exp. Biol. 222:jeb176396. doi: 10.1242/jeb.176396
Tyack, P. (1983). Differential response of humpback whales, Megaptera novaeangliae, to playback of song or social sounds. Behav. Ecol. Sociobiol. 13, 49–55. doi: 10.1007/BF00295075
Übernickel, K., Tschapka, M., and Kalko, E. K. V. (2013). Selective eavesdropping behaviour in three neotropical bat species. Ethology 119, 66–76. doi: 10.1111/eth.12038
Voigt, C. C., Lindecke, O., Schönborn, S., Kramer-Schadt, S., and Lehmann, D. (2016). Habitat use of migratory bats killed during autumn at wind turbines. Ecol. Appl. 26, 771–783. doi: 10.1890/15-0671
Voigt, C. C., Rehnig, K., Lindecke, O., and Pētersons, G. (2018). Migratory bats are attracted by red light but not by warm-white light: implications for the protection of nocturnal migrants. Ecol. Evol. 8, 9353–9361. doi: 10.1002/ece3.4400
Voigt, C. C., Sörgel, K., Šuba, J., Keišs, O., and Pētersons, G. (2012). The insectivorous bat Pipistrellus nathusii uses a mixed-fuel strategy to power autumn migration. Proc. R. Soc. B 279, 3772–3778. doi: 10.1098/rspb.2012.0902
Voigt-Heucke, S. L., Taborsky, M., and Dechmann, D. K. N. (2010). A dual function of echolocation: bats use echolocation calls to identify familiar and unfamiliar individuals. Anim. Behav. 80, 59–67. doi: 10.1016/j.anbehav.2010.03.025
Voigt-Heucke, S. L., Zimmer, S., and Kipper, S. (2016). Does interspecific eavesdropping promote aerial aggregations in European pipistrelle bats during autumn? Ethology 122, 745–757. doi: 10.1111/eth.12519
Waser, P. M. (1975). Experimental playbacks show vocal mediation of intergroup avoidance in a forest monkey. Nature 255, 56–58. doi: 10.1038/255056a0
Waser, P. M. (1977). Individual recognition, intragroup cohesion and intergroup spacing: evidence from sound playback to forest monkeys. Behaviour 60, 28–74. doi: 10.1163/156853977X00270
Keywords: playback, phonotaxis, bats, acoustic communication, animal migration, eavesdropping, echolocation, Pipistrellus nathusii
Citation: Marggraf LC, Lindecke O, Voigt CC, Pētersons G and Voigt-Heucke SL (2023) Nathusius’ bats, Pipistrellus nathusii, bypass mating opportunities of their own species, but respond to foraging heterospecifics on migratory transit flights. Front. Ecol. Evol. 10:908560. doi: 10.3389/fevo.2022.908560
Received: 30 March 2022; Accepted: 19 December 2022;
Published: 10 January 2023.
Edited by:
Mirjam Knörnschild, Leibniz Institut für Evolutions und Biodiversitätsforschung, GermanyReviewed by:
Joanna Furmankiewicz, University of Wrocław, PolandCopyright © 2023 Marggraf, Lindecke, Voigt, Pētersons and Voigt-Heucke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lara C. Marggraf, ✉ bGFyYS5tYXJnZ3JhZkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.