- 1College of Life Sciences and Academy for Multidisciplinary Studies, Capital Normal University, Beijing, China
- 2Science and Technology Research Center of China Customs, Animal Quarantine Intitute, Beijing, China
- 3Division of Entomology, Natural History Museum and Department of Ecology and Evolutionary Biology, University of Kansas, Lawrence, KS, United States
Burrowing bugs are distinctive, beetle-like insects of the pentatomoid family Cydnidae, noteworthy for their morphological specializations for digging and a hemiedaphic life history. However, less is known about their biological significance and the early origin of soil dwelling. Direct fossil evidence illuminating the evolutionary history of soil dwelling in cydnids is extremely rare. In this study, we report four new species of the burrowing bug subfamily Amnestinae from mid-Cretaceous Burmese amber, including two exhibiting specialized bulldozing and digging morphological traits on the anterior of the head and forelegs. Associated morphological features and phylogenetic placement indicate that Acanthamnestus represents the earliest unequivocal soil-dwelling cydnids and pushes back the geological record of hemiedaphic true bugs to 99 Ma. Environmental evidence, the distribution of host plants, and the fossils provide a new window for understanding the early origin of soil habits in Amnestinae and may be linked to the appearance of Moraceae.
Introduction
Soils are dynamic and rich environments that play a dramatic role in shaping the terrestrial biota we observe above their surfaces. To no small extent, soil-dwelling insects play an integral role in edaphic ecosystems, and any number of specializations are associated with living within, even partially so, the ground. Iconic soil-dwelling insects include scarab beetles (Coleoptera: Scarabaeidae), mole crickets (Orthoptera: Gryllotalpidae), some termites (Isoptera), and nymphal cicadas (Hemiptera: Cicadidae), among many others (Frost, 1959; Snodgrass, 1967; Richards and Davies, 1977; Gullan and Cranston, 2000). Often, such species have modified morphological structures unique to particular environments that permit them to invade and thrive within the soil ecosystem. The true bugs (Heteroptera) are principally phytophagous insects that include some of the most prolific agricultural pests. Among these, the most noticeable soil-dwelling bugs are those of the family Cydnidae, often dubbed as burrower bugs (Schuh and Slater, 1995). The hemiedaphic species of Cydnidae typically burrow into the soil using anatomical specializations of the head and forelegs, where they lay eggs and often spend much of their life cycle, before emerging to mate. Some species can become pestiferous, such as those feeding on crops ranging from peanuts to spinach. Not all cydnids are strictly soil dwelling, with some living fully above the soil layer and either on or in close association with their host plants. Those species not living as burrowers lack the phenotypic specializations otherwise found in the hemiedaphic lineage. The following characters are known specializations among Cydnidae for digging and living hemiedaphically: head flattened with circular crown setae (especially peg-like setae) on anterior margin; protibiae broadened apically with several strong spines at apical and lateral margins; protibiae cultrate and metatibiae clavate; and tarsi reduced or absent (Dolling, 1981; Schwertner and Nardi, 2015).
Cydnidae have a long geological history, with fossil species ranging from the Early Cretaceous to Early Miocene (Shcherbakov and Popov, 2002; Grimaldi and Engel, 2005; Yao et al., 2007; Lis et al., 2018). Nonetheless, evidence of their transition and evolution to hemiedaphic life histories is lacking owing to an absence of definitive fossil species with digging traits, presumably because of a taphonomic bias away from taxa whose lives are spent mostly below the soil surface. Accordingly, understanding the early evolution of soil habits in this major lineage of insects has been severely hindered. To date, the only attempt at reconstructing the early biology of Cydnidae has been that of Lis et al. (2018) who explored their association with several plant syninclusions in mid-Cretaceous amber from Myanmar. In this study, we reported four new species and three new genera of the subfamily Amnestinae (Cydnidae) and combined the morphological and phylogenetic evidence to demonstrate that the mid-Cretaceous cydnids had already evolved specialized characters for subterranean life. Additionally, climatic history and vegetative distribution patterns also provide clues that amnestine burrowing bugs may have had a lengthy association with the plant family Moraceae (mulberry and figs) and their early stem group.
Materials and Methods
Materials and Photography
The Burmese amber specimens investigated in this study were collected from the Hukawng Valley in Tanaing Township, Myitkyina District of Kachin State, Myanmar, and were acquired prior to June 2017. The Burmese amber deposits contain a diversity of fossil insects, such as termites (Zhao et al., 2020), hymenopterans (Cao et al., 2020), winged stick insects (Yang et al., 2020), and mecopterans (Lin et al., 2019). The age of this deposit has been dated radiometrically to a maximum of ca. 98.8 ± 0.6 Ma, using 206Pb/238U isotopes in magmatically derived zircon crystals taken from the volcaniclastic matrix of the amber (Shi et al., 2012). All specimens are housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNUB; Yunzhi Yao, Curator).
The amber samples were examined and photographed using a Nikon SMZ25 dissecting microscope and a Nikon ECLIPSE Ni microscope, both of them with a Nikon DS-Ri 2 digital camera system (Nikon, Japan), and illustrated with the aid of a camera attached to the microscope. The figures were drawn using Sai, Adobe Illustrator CC, and Photoshop CC graphics software packages. Morphological terminology mainly follows Schuh and Slater (1995). Body length was measured from the apex of the head to the apex of the abdomen. Body width was measured at the maximal width of the body. The lengths of the pronotum and scutellum were measured at the midline. The length of the forewings was measured from the base of the hemelytron to its apex. The length of the corium was measured from the base of the hemelytron to the apex. The missing dimensions were marked “?”.
Taxon Sampling, Morphological Characters
We carried out phylogenetic analyses for Amnestinae to clarify and confirm the position of our new taxa. Due to the phylogenetic relationships at the superfamily level in the superfamily Pentatomoidea, a lot of controversies about the monophyly of the Cydnidae and phylogenetic relationships with other pentatomoid families are still there (Schwertner and Nardi, 2015). Lis et al. (2017) indicated the non-monophyly of the “cydnoid” complex within Pentatomoidea. At the same time, 18 ingroup terminal taxa include 12 fossil taxa and 6 extant taxa. The earliest known Amnestinae were from the Early Cretaceous (Yao et al., 2007). All fossil species are from the Cretaceous (Supplementary Table S1). In addition, in the family Cydnidae, only Amnestinae are monophyletic groups, which can be confirmed by the presence of claval commissure, and have two genera. Hence, we used a hypothetical “all-zero” taxon as outgroup to polarize the individual characters. A complete list of the taxa used in the phylogenetic analyses is shown in Table 1. A total of 43 morphological characters were used in the analyses (characters 0–42), of which 26 are binary and 17 are multistate. All the characters are unordered and with equal weight, and the missing data were scored as unknown. The character matrix is provided in Table 1, and the description of morphological characters used in the phylogenetic analysis is provided in Supplementary Material.
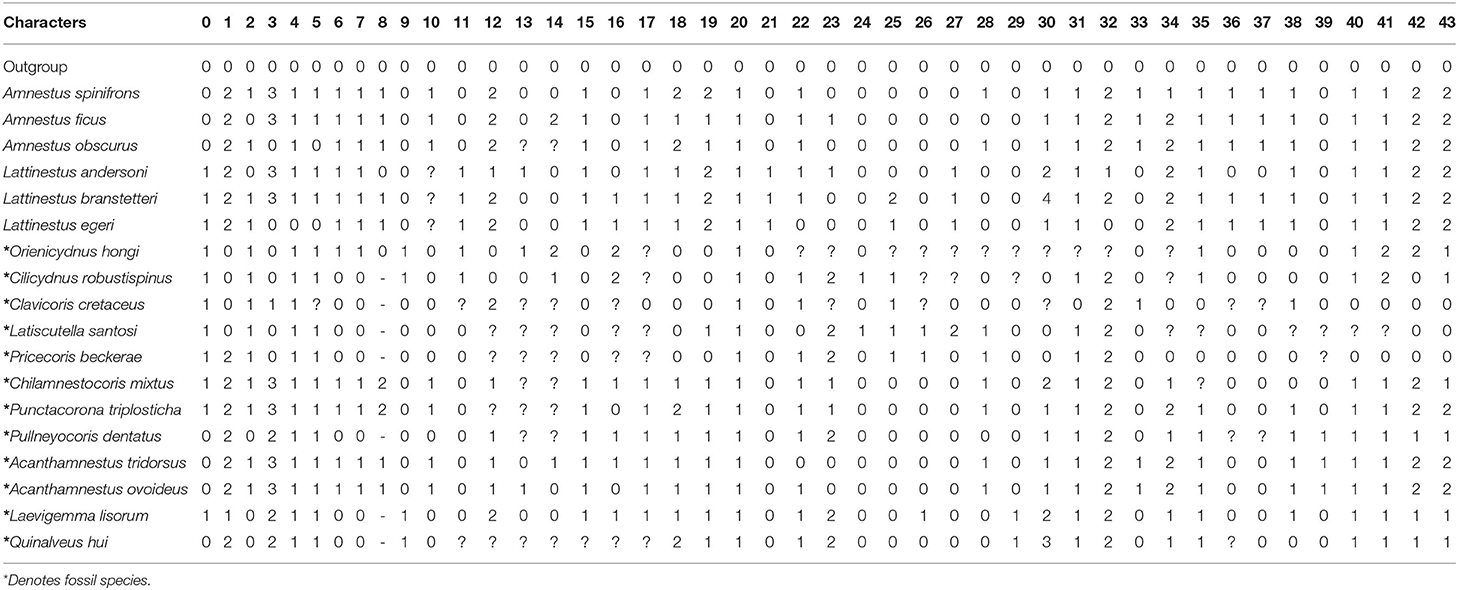
Table 1. Character matrix for phylogenetic analyses, including 43 morphological characters for 19 taxa.
Phylogenetic Analysis
Analysis of the character matrix (characters 0–41) was performed using the NONA version 2.0 (Goloboff, 1999) and the WinClada version 1.00.08 interface (Nixon, 2002). Runs were conducted using the following commands: multiple TBR+TBR search strategy, maximum trees to keep (hold) = 10,000; number of replications (mult*N) = 1,000; starting trees per rep (hold/) = 100. Bremer support values were obtained using TNT (Goloboff and Catalano, 2016). Ancestral character state reconstruction of soil-dwelling characteristics was conducted using equally weighted parsimony methods in Mesquite 2.75 (Maddison and Maddison, 2011). We coded the data for head and protibiae of terminal taxa based on morphological data from the corresponding species (Table 1, characters 42 and 43).
Results and Discussion
Systematic Paleontology
Order: Hemiptera Linnaeus, 1758
Suborder: Heteroptera Latreille, 1810
Superfamily: Pentatomoidea Leach, 1815
Family: Cydnidae Billberg, 1820
Subfamily: Amnestinae Hart, 1919
Genus: Acanthamnestus Du, Yao, and Engel, gen. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:661DEEDE-6906-420F-BCA7-531749C09A1A
Type species: Acanthamnestus atridorsus Du, Yao, and Engel, sp. nov.
Included species: Acanthamnestus atridorsus Du, Yao, and Engel, sp. nov.; Acanthamnestus ovoideus Du, Yao, and Engel, sp. nov.
Etymology: The generic name is a combination of the Greek word akanthos (meaning, “similar”) and the extant genus Amnestus of the subfamily Amnestinae. The gender is masculine.
Diagnosis: Body black with dense coarse punctures; clypeus with four peg-like setae, paraclypei with five peg-like setae marginally; rostrum not reaching metacoxae; fourth rostral segment shortest. Claval commissure nearly 1/2 length of scutellum; clavus with three rows of coarse punctures; end of corium curved; membrane almost translucent without veins. Meso- and metacoxae closer to each other than to procoxae; protibia flattened and broadened apically with a row of strong spines at the apical margin.
Remarks: The new genus shares with Amnestus a clypeus with four peg-like setae, the presence of ocelli, and a well-developed membrane. It is easy to distinguish from Amnestus in the following characteristics: membrane nearly 1/2 length of forewing, membranal suture distally curved near the edge of forewing (vs. membrane as long as 1/3 of forewing, base of membranal suture curved near claval suture or straight); profemur without a long, bifid spine on the posterior mesial region of ventral surface (vs. profemur usually with a long, bifid spine). Acanthamnestus gen. nov. is distinguished from Chilamnestocoris (Lis et al., 2018) by the following characteristics: three rows of coarse punctures in clavus and apex of protibia broadened with a row of strong spines along the apical margin with each marginal spine arising from its own well-developed tubercle (vs. with four rows of coarse punctures and simple spines); clypeus slightly longer than paraclypei (vs. clypeus as long as paraclypei); apex of clypeus with four long sharpened peg-like setae (vs. apex of clypeus with four short peg-like setae). It can also be excluded from Punctacorona (Wang et al., 2019) mainly by: paraclypei each with a row of five sharp peg-like setae and four to five hair-like setae (vs. paraclypei with six peg-like setae and one hair-like seta); pronotum with small, indistinct punctures, and posterior margin of calli without a distinct transverse cicatrice (vs. pronotum with large, coarse punctures and posterior margin of calli developed with a distinct transverse cicatrice), base of protibia slender, of even width (vs. protibia conspicuously thickened).
Acanthamnestus atridorsus Du, Yao, and Engel, sp. nov. (Figures 1, 2, 9A–C).
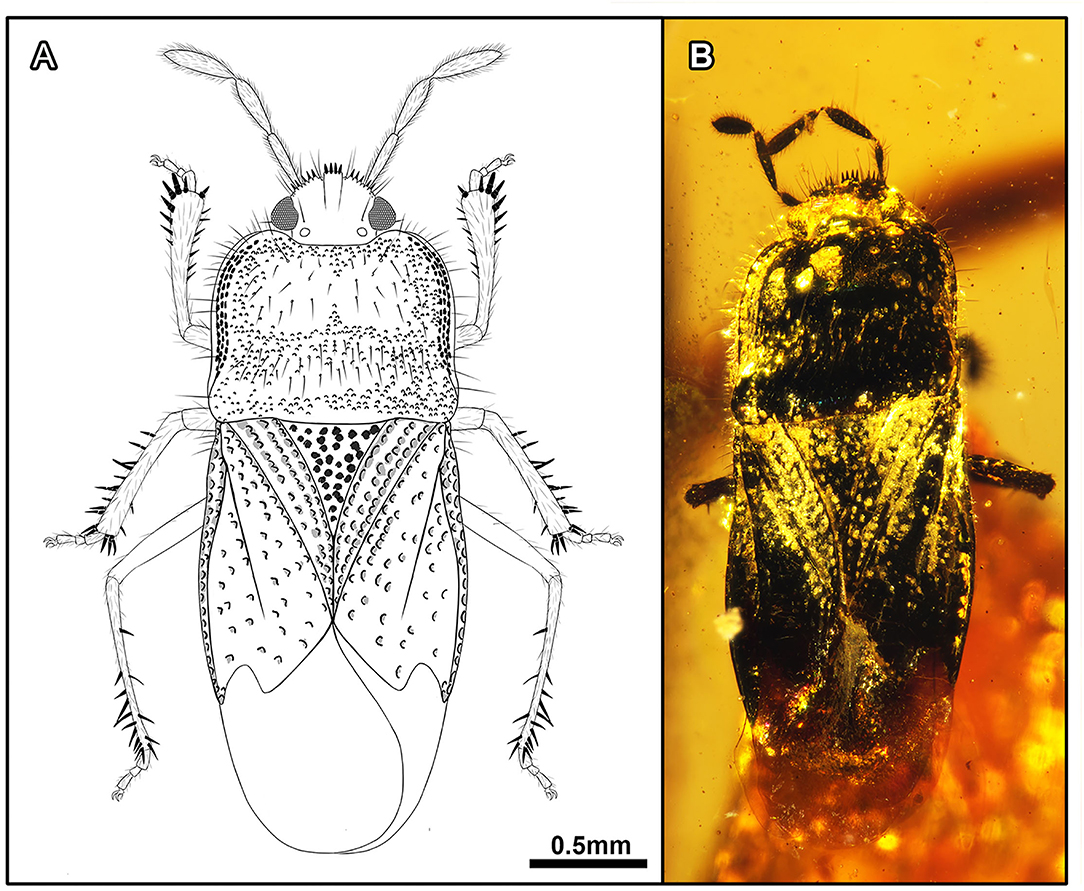
Figure 1. Acanthamnestus atridorsus gen. et sp. nov., in mid-Cretaceous amber from Myanmar, Holotype CNU-HET-MA2019002 (male). (A) Line drawing habitus in dorsal view. (B) Photograph habitus in dorsal view. Scale bars: A = B = 0.5 mm.
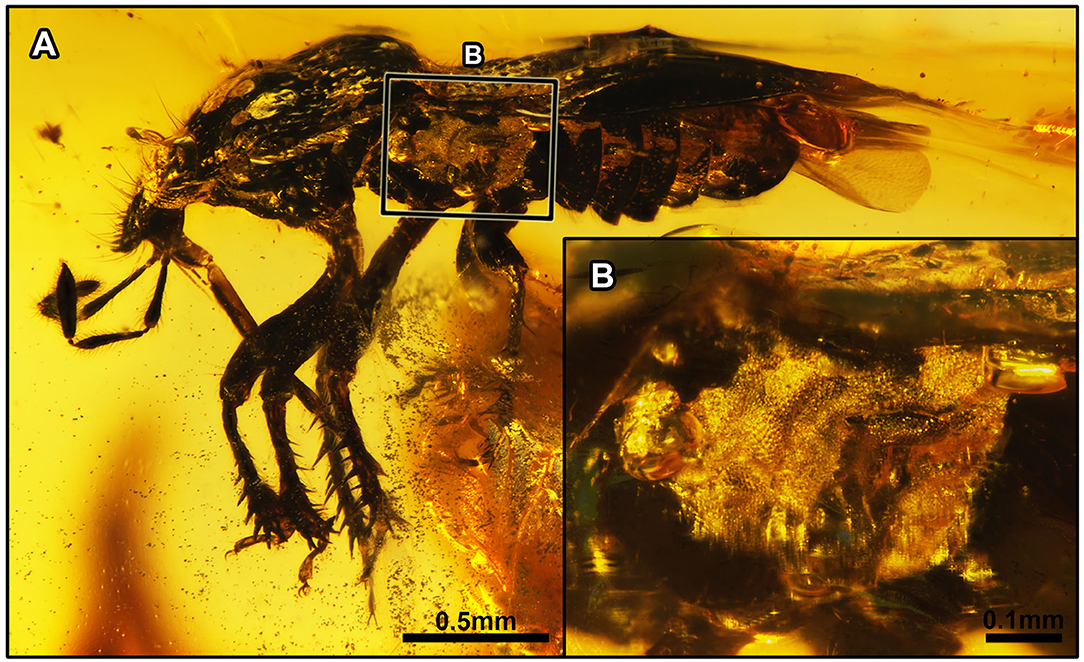
Figure 2. Acanthamnestus atridorsus gen. et sp. nov., in Burmese amber. Holotype, CNU-HET-MA2019002 (male). (A) Habitus in left lateral view. (B) Detail of metathoracic scent gland, as located on (A). Scale bars: A = 0.5 mm; B = 0.1 mm.
http://zoobank.org/urn:lsid:zoobank.org:act:43EF19B4-6558-4F3A-A67B-E2E7BFEAC819
Etymology: The specific epithet is a combination of the Latin words ater (meaning, “black”) and dorsum (meaning, “back”).
Material: Holotype: CNU-HET-MA2019002 (male), housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China.
Horizon and Locality: Hukawng Valley, Kachin State, northern Myanmar; mid-Cretaceous, lowermost Cenomanian.
Diagnosis: Body elongate. Evaporatorium with mycoid microsculpture, with elongate and strongly recurved peritreme. Apex of scutellum tapering. Forewing with C + Sc and R + M diverging in proximal fifth of corium. Tibial comb present.
Description: Completely preserved male specimen. Body flattened and elongate, length about 3 × as long as maximum pronotal width, almost unicolorous, dorsal surface slightly polished; head, antennae, pronotum, scutellum, corium, femora, tibiae, tibial spines, and ventral body surface black, rostrum and membrane pale brown; dorsal surfaces of head, pronotum, scutellum, corium, and clavus with dense coarse punctures.
Head: Nearly semicircular, dorsally with clearly visible punctures, and with slightly developed oblique striae in posterior part of paraclypei; clypeus slightly longer than paraclypei, apex of clypeus broad, with four blackish brown, long, sharp, peg-like setae; paraclypei each with a submarginal row of five more or less sharpened, peg-like setae, and five hair-like setae; each marginal setigerous puncture arising from its own well-developed tubercle; compound eyes large and reniform, contiguous with anterior margin of pronotum, each with a short apical seta and a long hair-like seta on inner margin of compound eyes; ocelli present, interocellar distance about 3 × greater than distance between ocellus and compound eye; antennae with five antennomeres, each with short, thick setae; first antennomere short, stout, barrel-shaped; second antennomere shortest and hardly visible; fifth antennomere fusiform, longer than third or fourth antennomeres; rostrum four-segmented, surpassing mesocoxae, not reaching metacoxae, first segment stout and shortest, second segment longest, third segment subequal to fourth segment.
Thorax: Pronotum nearly rectangular, almost parallel-sided, with dense setigerous punctures, without collar; anterior margin concave medially, anterior angles bulging forward, forming a distinct arc, posterior margin slightly convex medially, almost parallel with anterior margin, with 10–11 hair-like setae on the left side and nine hair-like setae on the right side, two long and inflexible hair-like setae on both sides of anterior margin; calli large, half of the protonal surface, without punctures, posterior margin of calli with dense large punctures; anterior margin of pronotum with midsized punctures, posterior margin of pronotum with small-sized punctures and about three rows of larger punctures on both sides. Mesopleuron and metapleuron with evaporatorium, covering most of the metapleuron, large, with mycoid microsculpture, with elongate and strongly recurved peritreme. Scutellum triangular, large, one-third length of hemelytron, lateral margin of scutellum with large coarse punctures; apex of scutellum tapering. Hemelytron: macropterous, clavus broad, with three rows of coarse punctures parallel to the lateral margin of scutellum; claval commissure present, nearly one-half length of scutellum; corium with a single row of large punctures close to clavus and anterior margin of forewing, with few punctures in between two rows; corium elongate, junction with membrane angled, base of corium with four short setae; anterior margin of forewing angulate, C + Sc and R + M diverging in one-fifth length of corium, R + M in forewing raised and keel-like, extending to apex of corium; membrane almost translucent, faintly infumate, distinctly surpassing tip of the abdomen. Legs: meso- and metacoxae closer to each other than to procoxae; femora stout and robust with short setae; protibia flattened, broadened apically, and stouter than meso- and metatibiae, with row of nine stout spines along apical margin, each marginal spine arising from its own well-developed tubercle, protibial comb present (visible on right protibia), consisting of a row of eight setae (Figure 9C); apex of mesotibia as thick basally, with five spines along the apical margin, lateral margin with about 19 stout, relatively short spines, internal margin with scarcely any spines; metatibia slender, with four to five spines along apical margin, lateral margin with about 16 long, relatively slender spines; tarsi trimerous, tarsomeres equal in thickness, second tarsomere shortest, first tarsomere almost as long as the third tarsomere; pretarsal claws elongate, pulvilli narrowed, capitate apically, as long as claws.
Abdomen: Abdomen of equivalent width with thorax; sterna dark brown, third to eight sterna with numerous distinct small punctures and shiny semierect setae.
Dimensions (in mm): Body length: 2.93, maximal width of body: 1.10; length of head: 0.24, width 0.53; length of compound eyes: 0.14, width 0.08; distance of ocelli: 0.24; length of antennomeres I–V: 0.15, 0.05, 0.23, 0.25, 0.33; length of rostral segments I–IV: 0.18, 0.37, 0.27, 0.29; length of pronotum: 0.80, width: 0.97; length of scutellum: 0.46, width: 0.48; length of anterior margin of corium: 1.08; length of hemelytron: 1.84, width: 0.65; length of claval commissure: 0.40; length of clavus: 0.89; length of fore leg: profemur: 0.59, protibia: 0.55, protarsomeres I–III: 0.09, 0.03, 0.08; length of mid leg: mesofemur: 0.50, mesotibia: 0.59, mesotarsomeres I–III: 0.09, 0.05, 0.08; length of hind leg: metafemur: ?, metatibia: 0.69, metatarsomeres I–III: 0.07; 0.03; 0.08.
Acanthamnestus ovoideus Du, Yao, and Engel, sp. nov. (Figures 3, 4).
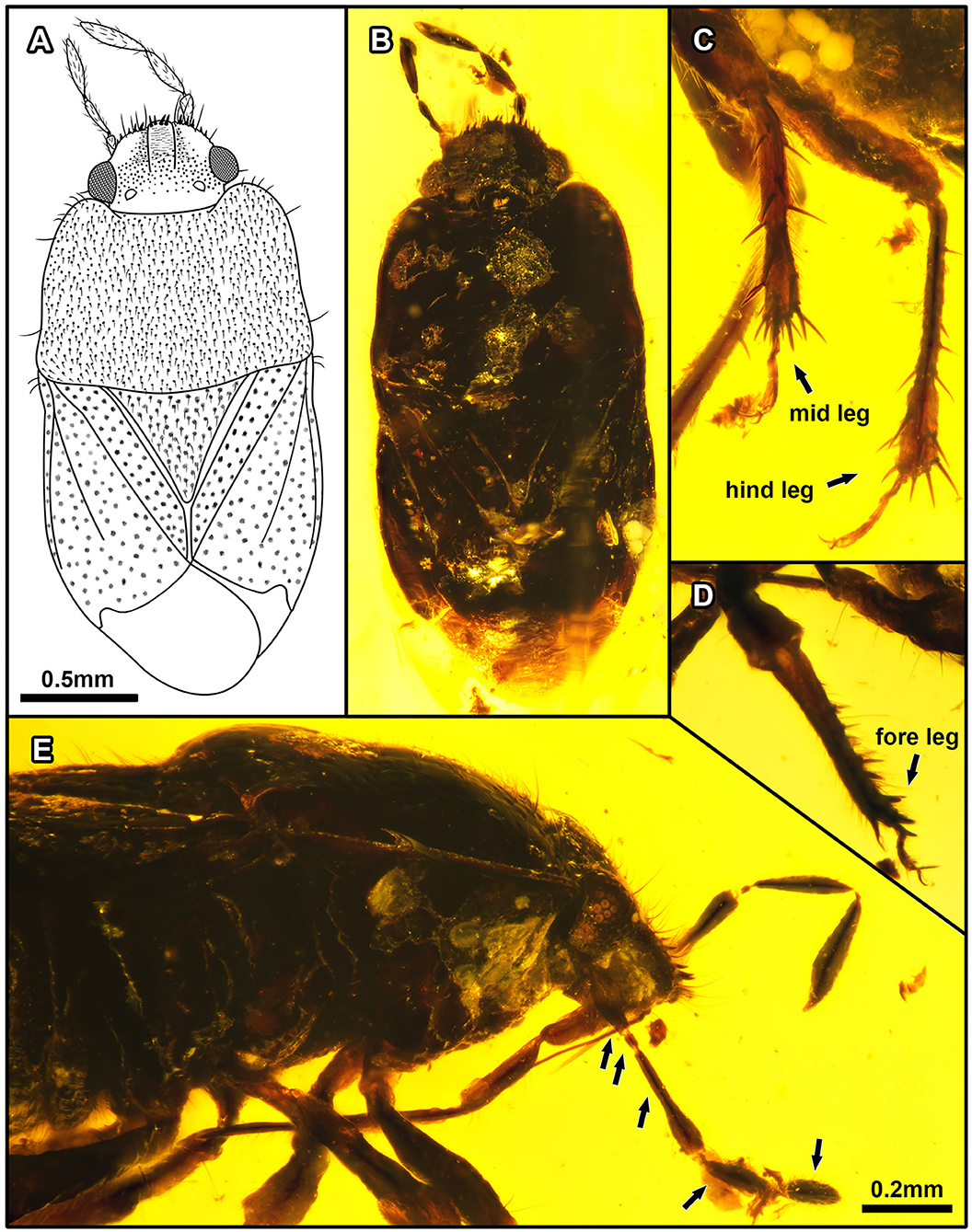
Figure 3. Acanthamnestus ovoideus gen. et sp. nov., in Burmese amber. Holotype, CNU-HET-MA2019003 (female). (A) Line drawing in dorsal view. (B) Habitus in dorsal view. (C) Meso- and metatibiae. (D) Protibiae. (E) Habitus in right lateral view of head, arrows indicate five antennomeres. Scale bars: A = B = 0.5 mm; C = D = E = 0.2 mm.

Figure 4. Acanthamnestus ovoideus gen. et sp. nov., in Burmese amber. Holotype, CNU-HET-MA2019003 (female). (A) Habitus in left lateral view. (B) Habitus in right lateral view. Scale bars: A = B = 0.5 mm.
http://zoobank.org/urn:lsid:zoobank.org:act:60753144-B0EB-4475-B2C6-CFCBCCCD8D7D
Etymology: The specific epithet is taken from the Latin word ovoideus (meaning “egg-shaped” or “ovoid”).
Material: Holotype: CNU-HET-MA2019003 (female), housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNUB; Yunzhi Yao, Curator).
Horizon and Locality: Hukawng Valley, Kachin State, northern Myanmar; mid-Cretaceous, lowermost Cenomanian.
Diagnosis: Body oval. Evaporatorium lacking mycoid microsculpture. Apex of scutellum blunt. Forewing with C + Sc and R + M diverging in the proximal sixth of corium. Tibial comb absent.
Description: Completely preserved female specimen. Body flattened and oval, almost parallel-sided, length more than 2 × as long as maximum pronotal width, overall blackish brown, rostrum brown, membrane castaneous, dorsum slightly polished; dorsal surfaces of head, pronotum, scutellum, clavus, and corium with dense small setae.
Head: Nearly semicircular, dorsally with small punctures; clypeus slightly longer than paraclypei and raised, apex of clypeus broad with four black, short, sharpened, peg-like setae; paraclypei each with a submarginal row of five short, stout, peg-like setae, and four hair-like setae; each marginal setigerous puncture arising from its own well-developed tubercle; compound eyes large and semicircular, contiguous with anterior margin of pronotum, each with a short apical seta on the surface of the compound eyes; ocelli present, round, interocellar distance more than 3 × than distance between ocellus and compound eye; antenna with antennomeres, with dense short, thick setae, first antennomere short, stout, barrel-shaped; second antennomere shortest and clavate; third antennomere subequal to fifth antennomere, longer than fourth antennomere, fourth and fifth antennomeres fusiform; rostrum four-segmented, surpassing mesocoxae but not reaching to metacoxae, first segment stout and subequal to second segment, longer than fourth segment, third segment longest, apex of fourth segment tapering.
Thorax: Pronotum nearly rectangular, almost parallel-sided, with dense punctures bearing hair-like setae tigerous, without collar; anterior margin concave medially, anterior angles bulging forward, forming a distinct arc, posterior margin slightly convex medially, almost parallel with anterior margin, with seven hair-like setae on the left side and six hair-like setae on the right side; calli not visible; lateral view of pronotum with dense small punctures. Mesopleuron and metapleuron with evaporatorium covering most of the metapleuron, peritreme not visible. Scutellum triangular, large, about one-third length of hemelytron, with large, indistinct coarse punctures; apex of scutellum blunt. Hemelytron: macropterous, clavus broad, with three rows of coarse punctures parallel to lateral margin of scutellum; claval commissure present, nearly one-half length of scutellum; corium elongate with same size punctures as those of clavus, junction with membrane angled, base of corium with two short setae; anterior margin of forewing angulate, C + Sc and R + M diverging at one-sixth length of corium, R + M in forewing raised and keel-like; membrane almost translucent, faintly infumate, distinctly surpassing tip of the abdomen. Legs: meso- and metacoxae closer to each other than to procoxae; femora stout and robust with short setae; protibia flattened and stout, broadened apically, with about five long spines along the apical margin, each marginal spine arising from its own well-developed tubercle, lateral margin with about six stout, relatively short spines; mesotibia as thick basally, with about eight long spines along the apical margin, lateral margin with about 12 stout, relatively short spines, internal margin with scarcely any spines; metafemur and metatibia more slender than other segments, metatibia with about 10 spines along the apical margin, lateral margin with about eight long, relatively slender spines; tarsi trimerous, tarsomeres equal in thickness, second tarsomere shortest, first tarsomere almost as long as the third tarsomere; pretarsal claws elongate, pulvilli narrowed, capitate apically, as long as claws.
Abdomen: Equivalent width with thorax; sterna dark brown, third to eight sterna with numerous distinct small punctures and shiny semierect setae; ovipositor platelike.
Dimensions (in mm): Body length: 2.53; maximal width of body: 1.21; length of head: ?, width: 0.68; length of compound eyes: 0.17, width: 0.14; distance of ocelli: 0.33; length of antennomeres I–V: 0.13, 0.04, 0.28, 0.23, 0.30; length of rostral segments I–IV: 0.23, 0.23, 0.36, 0.21; length of pronotum: 0.85, width: 1.19; length of scutellum: 0.49, width: 0.65; length of anterior margin of corium: 1.10; length of hemelytron: 1.69, width: 058; length of claval commissure: 0.28; length of clavus: 1.03; length of foreleg: profemur: 0.55, protibia: 0.46, protarsomeres I–III: 0.05, 0.03, 0.11; length of midleg: mesofemur: 0.67, mesotibia: 0.83, mesotarsomeres I–III: 0.05, 0.04, 0.06; length of hind leg: metafemur: 0.64, metatibia: 0.85, metatarsomeres I–III: 0.07, 0.04, 0.16.
Genus: Laevigemma Du, Yao, and Engel, gen. nov. (Figures 5, 6, 9D,E).
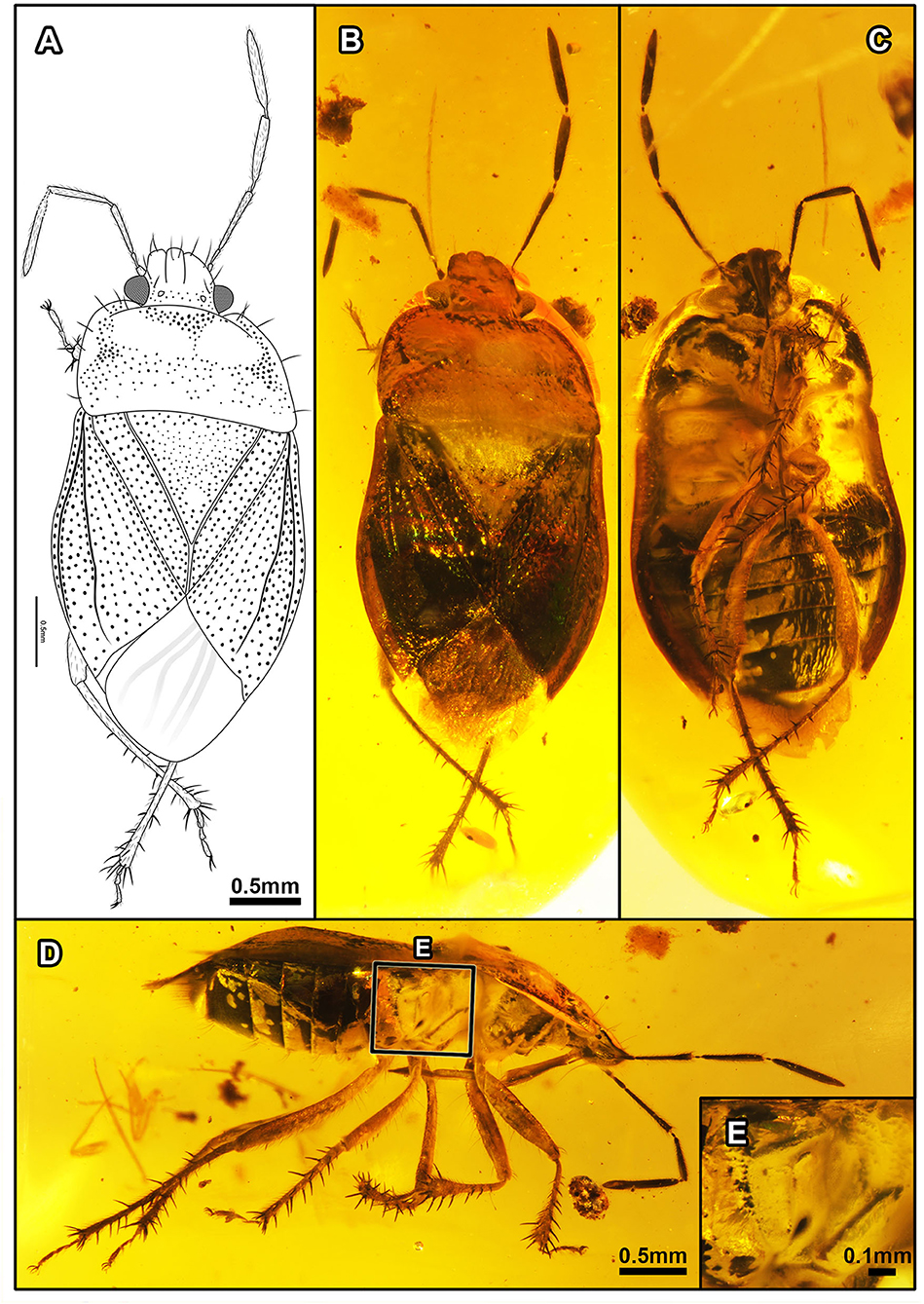
Figure 5. Laevigemma lisorum gen. et sp. nov., in Burmese amber. Related to Figure 2. Holotype, CNU-HET-MA2019004 (male). (A) Line drawing in dorsal view. (B) Habitus in dorsal view. (C) Habitus in ventral view. (D) Habitus in right lateral view. (E) Detail of metathoracic scent gland, as located on (D). Scale bars: A = B = C = 0.5 mm; D = 0.5 mm; E = 0.1 mm.
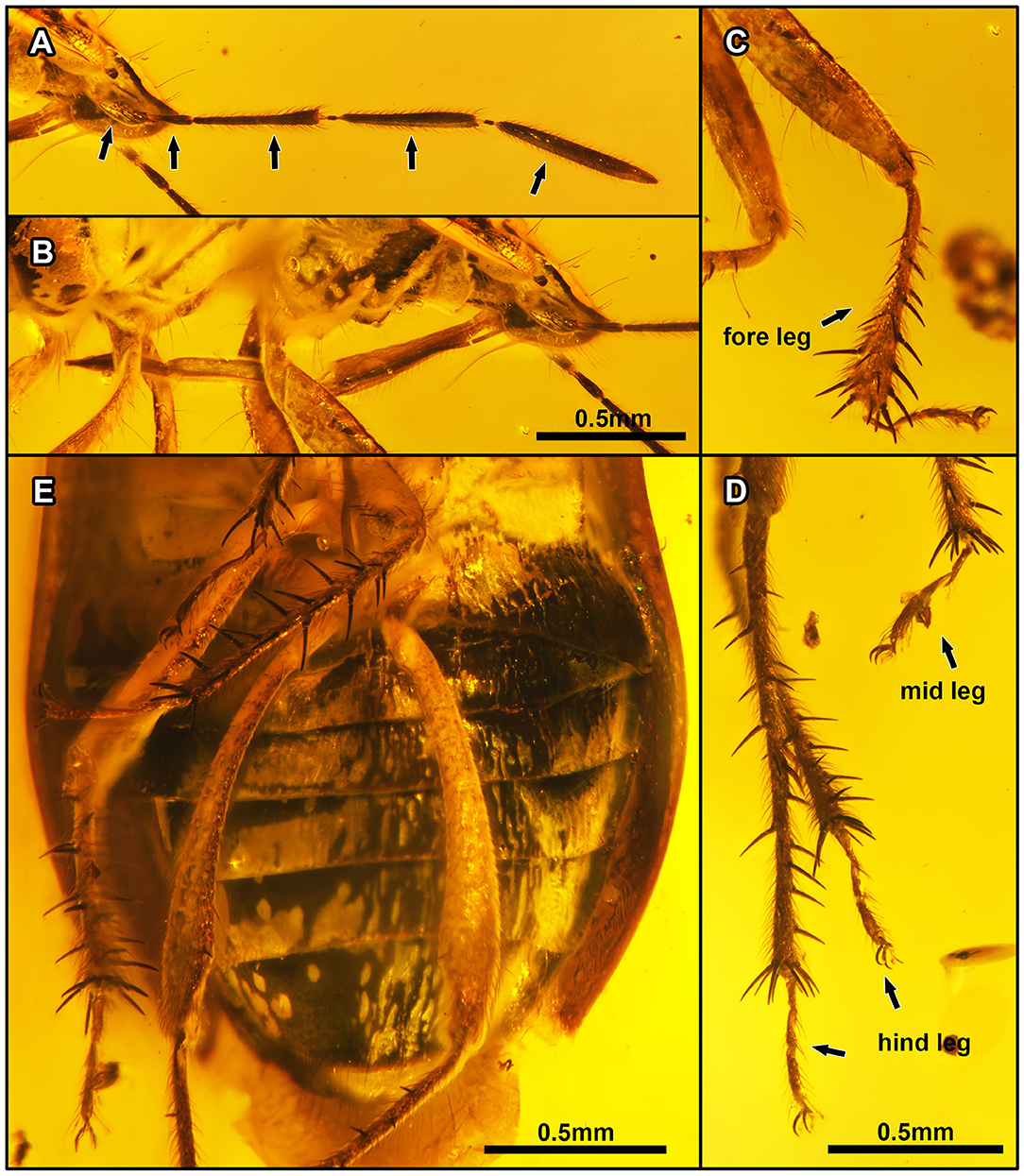
Figure 6. Laevigemma lisorum gen. et sp. nov., in Burmese amber. Related to Figure 2. Holotype, CNU-HET-MA2019004 (male). (A) Habitus in right lateral view of head, arrows indicate five antennomeres. (B) Rostrum in right lateral view. (C) Right foreleg. (D) Mesotarsi and right metatarsus. (E) Abdomen in ventral view. Scale bars: A = B = 0.5 mm; C = D = 0.5 mm; E = 0.5 mm.
http://zoobank.org/urn:lsid:zoobank.org:act:B91285D6-13B7-4468-88E8-D9B0A126F5DB
Type species: Laevigemma lisorum Du, Yao, and Engel, sp. nov.
Included species: Type species only.
Etymology: The generic name is a combination of the Latin words laevis (meaning “smooth”) and gemma (meaning “gemstone”), referencing the nearly smooth pronotum that differs from the large and coarse punctures of most Amnestinae. The gender of the name is masculine.
Diagnosis: Body relatively smooth dorsally. Clypeus and paraclypei without peg-like setae apically; rostrum reaching metacoxae. Clavus broad, with four rows of coarse punctures parallel to the lateral margin of scutellum; end of corium curved; claval commissure shorter than half of scutellar length; R+M separated from C+Sc at base of corium, membrane with four simple veins. Coxae of all three pairs of legs equally distant from each other; protibia with only longer weak spines at the apical margin.
Remarks: The new genus can be classified within the subfamily Amnestinae owing to the presence of a distinct claval commissure. It is excluded from the other genera of Amnestinae by the following combination of characters: clypeus and paraclypei without peg-like setae; membrane with four simple veins; protibiae not broadened apically; postiolar peritreme branching laterally, with anterior and lateral projections. This species is quite special as it does not have the typical coarse punctures or dark body of typical Amnestinae, and is instead relatively smooth dorsally. When the light hits its dorsum, the pronotum and forewings are shiny. The new genus is more like Quinalveus gen. nov.; however, the two genera have many differences, such as the relatively smooth body dorsally (vs. body with distinct large punctures); clavus with four rows of coarse punctures parallel to lateral margin of scutellum (vs. clavus with five rows of coarse punctures); end of corium curved (vs. end of corium straight); claval commissure shorter than half of scutellar length (vs. length of claval commissure subequal to half of scutellar length); membrane with four simple veins (vs. membrane with three simple veins).
Laevigemma lisorum Du, Yao, and Engel, sp. nov. (Figures 5, 6, 9D,E).
http://zoobank.org/urn:lsid:zoobank.org:act:79EA6BC3-242B-49CD-85B8-631233C09E65
Etymology: The specific epithet honors Prof. Jerzy A. Lis and his wife Barbara Lis for their considerable contributions to our knowledge of Cydnidae.
Material: Holotype: CNU-HET-MA2019004 (male), housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China.
Horizon and Locality: Hukawng Valley, Kachin State, northern Myanmar; mid-Cretaceous, lowermost Cenomanian.
Diagnosis: Same as generic diagnosis.
Description: Completely preserved male specimen. Body oval, almost unicolorous, relatively smooth dorsally, length about 2 × as long as maximum body width. Head, pronotum, and scutellum whitish shiny; antennae, rostrum, and corium castaneous; legs and membrane yellowish brown. Venter of body black.
Head: Flattened, 2 × as long as wide, with small punctures on posterior margin of head; clypeus slightly longer than paraclypei, apex blunt, not obviously broadened, without peg-like setae apically, with a pair of hair-like setae subapically; each paraclypei with three hair-like setae apically; compound eyes large, contiguous with anterior margin of pronotum, each with a short apical seta on inner margin of compound eyes; ocelli present, interocellar distance about 3 × greater than the distance between ocellus and compound eye; five antennomeres with dense setae, second antennomere shortest, fifth antennomere longest, third antennomere subequal with fourth antennomere; rostrum with four segments, extending to metacoxae, first segment shortest, subequal with fourth segment, second segment longest, subequal with third segment.
Thorax: Pronotum nearly rectangular, almost parallel-sided without collar; anterior margin upward in middle with coarse punctures, anterior angles bulging forward, forming a distinct arc with a hair-like seta and several coarse punctures, posterior margin slightly swollen and concave medially without punctures, pronotum with four hair-like setae on both sides of edge; calli large, smooth, posterior margin of calli with dense small punctures. Metathoracic scent gland grooves present on metapleuron, metapleuron with large evaporatory area and peritreme, postiolar peritreme branching laterally, with anterior and lateral projections. Scutellum large, triangular, sides of more-or-less equal length, depressed basomedially, basal and lateral of margin of scutellum with large and coarse punctures, small punctures in middle of scutellum, scutellar tip narrow, slightly elongate. Hemelytron: macropterous, clavus broad, with four rows of coarse punctures parallel to lateral margin of scutellum; claval commissure present, shorter than half length of scutellum; corium large, two-thirds length of hemelytron, with a single row of distinct punctures close to clavus and anterior margin of forewing, with dense punctures in between two rows; corium elongate, junction with membrane curvilinear, base of corium without seta; anterior margin of forewing angulate, R+M separating from C+Sc at base of corium, R + M in forewing raised and keel-like; membrane almost translucent, faintly infumate, with four simple veins, not branching, distinctly surpassing tip of the abdomen. Legs: coxae of all three pairs of legs equally distant from each other; profemur stout and robust with dense long setae, stouter than meso- and metafemora, and a short spine apically on profemur; protibia slightly broadened apically, shorter than meso- and metatibiae, with about seven long spines along apical margin, each marginal spine arising from its own well-developed tubercle, lateral margin with about 12 stout and relatively short spines; mesofemur straight with four short spines on lateral margin apically; mesotibia as thick basally, with about nine long spines along apical margin, lateral margin with about 17 stout, relatively short spines, internal margin with scarcely any spines; meta-femur slender with a short spine at a distance from distal end, metatibia slender, with about 10 spines along apical margin, lateral margin with about 16 long, relatively slender spines; tarsi trimerous, tarsomeres equal in thickness, third tarsomere longest, first tarsomere almost as long as second tarsomere; pretarsal claws elongate, pulvilli narrowed, capitate apically, as long as claws.
Abdomen: Equivalent width with thorax; sterna black, third to eight sterna with numerous distinct small punctures and shiny semierect setae and distinctive longer setae medially.
Dimensions (in mm): Body length: 3.79, maximal width of body: 1.88; length of head: 0.46, width: 0.89; length of compound eyes: 0.21, width: 0.20; distance of ocelli: 0.43; length of antennomeres I–V: 0.27, 0.13, 0.40, 0.46, 0.62; length of rostral segments I–IV: 0.37, 0.54, 0.51, 0.39; length of pronotum: 0.76, width: 1.64; length of scutellum: 0.89, width: 1.10; length of anterior margin of corium: 1.50; length of hemelytron: 2.46, width: 0.87; length of claval commissure: 0.39; length of clavus: 1.36; length of fore leg: profemur: 1.06, protibia: 0.77, protarsomeres I–III: 0.16, 0.06, 0.14; length of mid leg: mesofemur: 0.93, mesotibia: 0.88, mesotarsomeres I–III: 0.15, 0.06, 0.19; length of hind leg: metafemur: 1.29, metatibia: 1.41, metatarsomeres I–III: 0.13, 0.08, 0.14.
Genus: Quinalveus Du, Yao, and Engel, gen. nov. (Figures 7, 8).
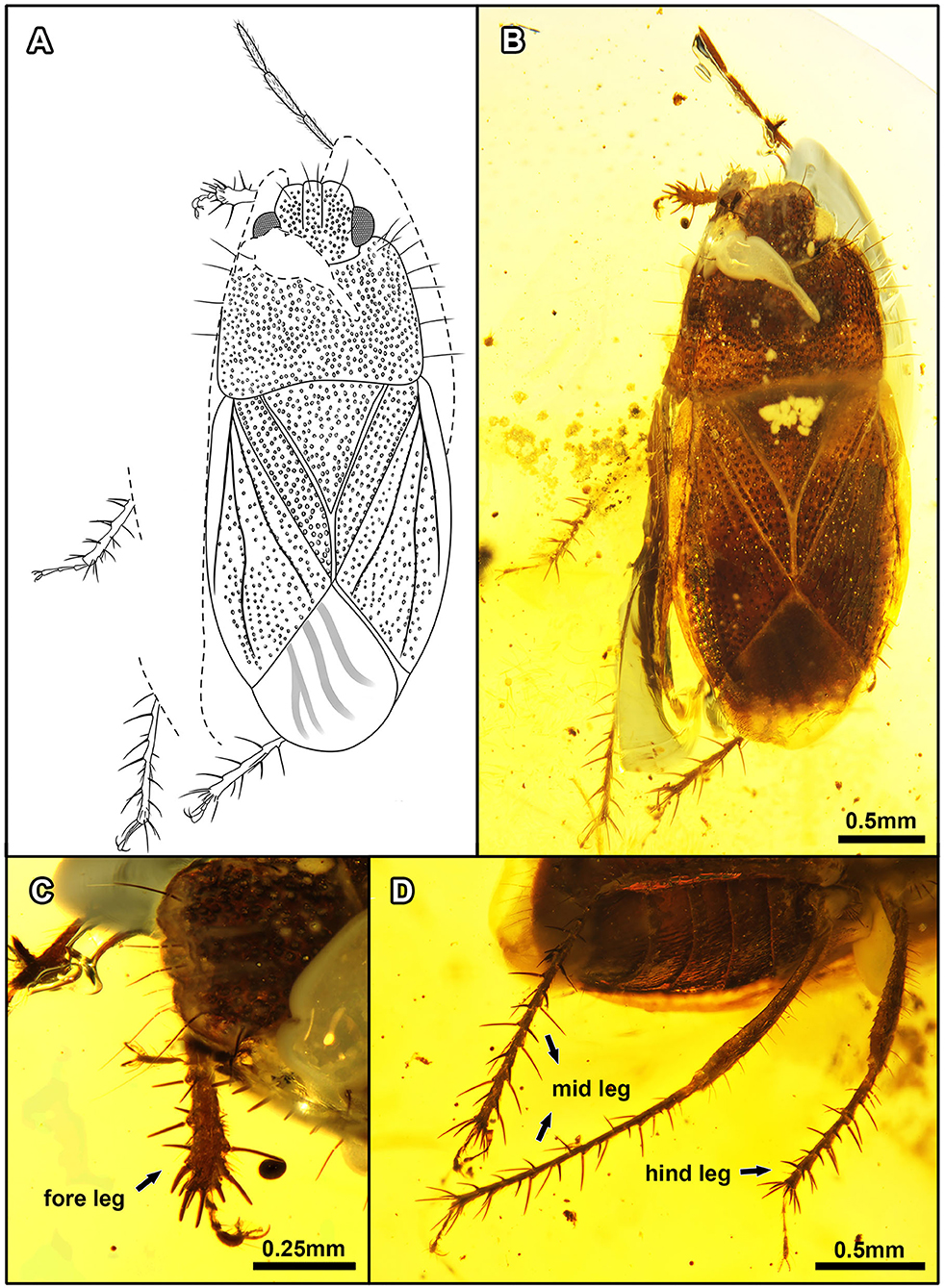
Figure 7. Quinalveus hui gen. et sp. nov., in Burmese amber. Holotype, CNU-HET-MA2019005 (male). (A) Line drawing in dorsal view. (B) Habitus in dorsal view. (C) Protibia. (D) Meso- and metatibiae. Scale bars: A = B = 0.5 mm; C = 0.25 mm; D = 0.5 mm.
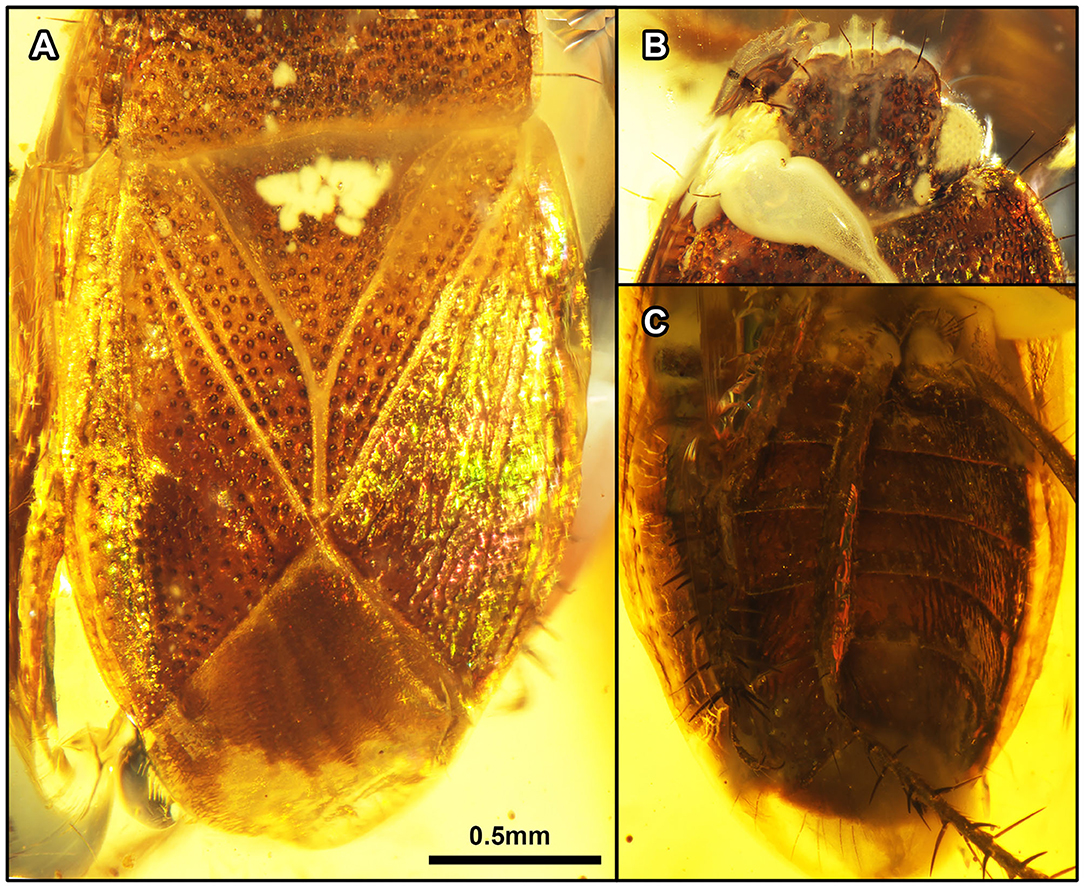
Figure 8. Quinalveus hui gen. et sp. nov., in Burmese amber. Holotype, CNU-HET-MA2019005 (male). (A) Scutellum and forewings in dorsal view. (B) Head in dorsal view. (C) Abdomen in ventral view. Scale bars: A = B = C = 0.5 mm.
http://zoobank.org/urn:lsid:zoobank.org:act:2F245C37-D6D0-4D53-A639-A53DF7B692C1
Type species: Quinalveus hui Du, Yao, and Engel, sp. nov.
Included species: Type species only.
Etymology: The generic name is a combination of the Latin words quini (meaning, “five”) and alveus (meaning, “hollow” or “pit”), referring to the five rows of coarse punctures on the clavus. The gender is masculine.
Diagnosis: Body with large distinct punctures. Clypeus and paraclypei without peg-like setae apically. Clavus broad, with five rows of coarse punctures parallel to lateral margin of scutellum; end of corium straight; length of claval commissure subequal to half of scutellar length; R +M separated from C + Sc at basal fifth of corium, membrane with three simple veins. Protibia with only longer weak spines at the apical margin.
Remarks: The new genus is similar to the extinct genus Pullneyocoris (Lis et al., 2020). However, it also has two autapomorphies: clavus broad and clavus with five rows of coarse punctures; this is the first time it appears in fossil species. Meanwhile, the membrane has three simple veins.
Quinalveus hui Du, Yao, and Engel, sp. nov. (Figures 7, 8).
http://zoobank.org/urn:lsid:zoobank.org:act:62E65192-ED0F-4F4B-B5B4-D372D38EC9D5
Etymology: The specific epithet honors Mr. Zhengkun Hu for his assistance and contribution in collecting Burmese amber.
Material: Holotype: CNU-HET-MA2019005 (male), housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNUB; Yunzhi Yao, Curator).
Horizon and Locality: Hukawng Valley, Kachin State, northern Myanmar; mid-Cretaceous, lowermost Cenomanian.
Diagnosis: Same as generic diagnosis.
Description: Nearly complete preserved male specimen. Body flattened and elongate with large coarse punctures. Length about 2 × as long as maximum pronotal width, overall brown, ventral view of thorax broken, not visible.
Head: Flattened and broad, nearly quadrate not 2 × wider than long, with large punctures on posterior margin of head and clypeus; clypeus slightly longer than paraclypei, apex blunt, not obviously broadened, without peg-like setae apically, with a pair of hair-like setae subapically; each paraclypei with a hair-like seta apically; compound eyes large, contiguous with anterior margin of pronotum, each with a short apical seta on inner margin of compound eyes; ocelli not visible; base of antenna not visible, last three antennomeres subequal with each other; rostrum incompletely preserved.
Thorax: Pronotum nearly trapezoidal with dense coarse punctures, collar absent; anterior margin raised upward medially, anterior angles bulging forward, forming a distinct arc with a hair-like seta, posterior margin slightly swollen and concave medially, pronotum with seven hair-like setae on both sides of edge at least; calli large in the middle of pronotum. Metathoracic scent gland not visible. Scutellum large, triangular, width subequal to length, depressed basomedially, margin of scutellum with relatively large and coarse punctures, fewer punctures medially on scutellum, apex of scutellum tapering. Hemelytron: macropterous, clavus broad, with five rows of coarse punctures parallel to lateral margin of scutellum; claval commissure present, length subequal to half of scutellar length; corium large, about two-thirds length of hemelytron, with a single row of distinct punctures close to clavus and anterior margin of forewing; corium elongate, junction with membrane straight, base of corium without seta; C entirely fused with Sc, R + M separated from C + Sc at about one-fifth length of corium, R + M in forewing raised and keel-like; Cu basally close to R + M, apex of Cu widely separated from the apex of R + M; membrane almost translucent, faintly infumate, with three simple veins, last vein branching at apex; forewing distinctly surpassing tip of the abdomen. Legs: meso- and metacoxae closer to each other than procoxae, meso- and metacoxae each with four long spines; femora thicker than tibiae with long setae; protibia flattened, broadened apically, shorter than meso- and metatibiae, with about eight long spines along apical margin, lateral margin with about 12 slender and relatively shorter spines; mesotibia as thick basally, with about seven long spines along apical margin, lateral margin with about 17 dense, slender, long spines; metatibia slender, with about seven long spines along the apical margin, lateral margin with more than 20 long, relatively slender spines; tarsi trimerous, tarsomeres equal in thickness, third tarsomere longest, first tarsomere almost as long as the second tarsomere; pretarsal claws elongate, pulvilli narrowed, capitate apically, as long as claws.
Abdomen: Abdomen of equivalent width with thorax; sterna brown, third to eight sterna with numerous distinct small punctures and shiny semierect setae and distinctive longer, dense setae medially.
Dimensions (in mm): Body length: 3.30; maximal width of body: 1.44; length of head: 0.49, width: 0.77; length of compound eyes: 0.17, width: 0.23; distance of ocelli: ?; length of antennomeres I–V: ?, ?, ?, 0.27, 0.24; length of rostral segments I–IV: ?, ?, 0.24, 0.28; length of pronotum: 0.82, width: 1.23; length of scutellum: 0.75, width: 0.77; length of anterior margin of corium: 1.49; length of hemelytron: 2.02, width: 0.81; length of claval commissure: 0.38; length of clavus: 1.18; length of fore leg: profemur: 0.69, protibia: 0.60, protarsomeres I–III: 0.13, 0.04, 0.11; length of mid leg: mesofemur: 0.82, mesotibia: 0.68, mesotarsomeres I–III: 0.11, 0.09, 0.13; length of hind leg: metafemur: 1.03, metatibia: 1.30, metatarsomeres I–III: 0.12, 0.07, 0.10.
Phylogenetic Analysis
Analyses of the character matrix yielded only one most parsimonious tree [tree length = 115, consistency index (CI) = 0.52, retention index (RI) = 0.66] as shown in Supplementary Figure S1. Pertinent results of the phylogenetic analysis include subfamily Amnestinae as monophyletic; the extant genus Amnestus with new genus Acanthamnestus being the sister group.
Monophyly of Amnestinae
The phylogenetic analyses are the first cladistic test of Amnestinae including fossil and extant taxa. Among all of the subfamilies of Cydnidae, the Amnestinae are easily recognized based on the claval commissure present, and this character state only appears in Amnestinae. Therefore, we regard Amnestinae as monophyletic.
Amnestus + Acanthamnestus
The results of our analysis show that the new genus Acanthamnestus and extant genus Amnestus have a sister-group relationship, with two character states supporting this clade: Body elongate, length more than 2 × but <4 × as long as maximum body width (character 0, state 0); forewing much longer than abdomen (character 33, state 1). Within this clade, the monophyly of Amnestus was supported by the following characteristics: Labium very long, reaching to metacoxae or abdomen (character 12, state 2); profemur and metafemur with a bifid spine on the ventral surface (character 36, state 1; character 37, state 1). The supporting character for the monophyly of Acanthamnestus is mesotibia broadened apically (character 39, state 1).
The Early Evolution of Soil Habits Among Amnestinae
The results of ancestral state reconstructions of soil-dwelling characters (Figure 11) indicated that soil-associated traits evolved once among Amnestinae. The lack of peg-like setae and strong protibial spines are considered plesiomorphies, and these features appear at different times independently on the tree. The appearance of peg-like setae on the head evolved later than the spines on the tibiae.
Discussion
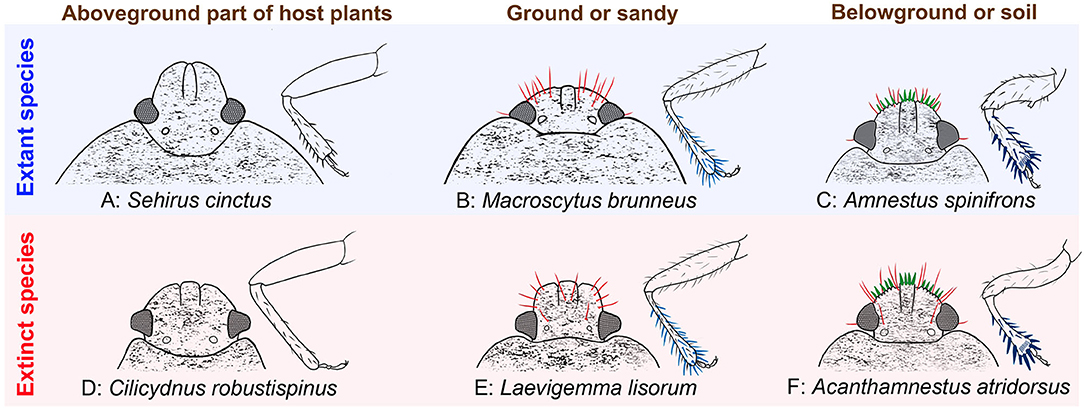
Among modern Heteroptera, two structures are closely linked to soil habits, situated on the head and foreleg, respectively: the anterior margins of the head with a circular crown consisting of hair- and peg-like setae; protibiae flattened and swollen, with thick spines or toe-like structures for pushing and digging. Lis and Pluot-Sigwalt (2002) noted that the rounded and flattened head with a circular crown of setae in Cydnidae suggested that such species are well suited for pushing and digging soil and are therefore bulldozers or diggers. They usually feed on roots, fallen fruits, or seeds, such as Amnestus ficus (Mayorga and Cervantes, 2001) (Amnestinae), Schiodtella secundus (Lis, 1991) (Cephalocteinae), and Chilocoris heissi (Lis, 1994) (Cydninae). Those lineages lacking peg-like setae on the head, such as the tribe Sehirini (Sehirinae), are commonly collected from the host-plant leaves above ground (Schwertner and Nardi, 2015), feeding on the fluids of plant stems and leaves, such as Sehirus cinctus albonotatus (Dallas, 1851) and Canthophorus dubius (Say, 1825; Hertzel and Scharmann, 1973). It is interesting that in Ochteridae such structures and behaviors are ontogenetically separated, with nymphs and adults differing. In this family, the fossorial nymphs possess the crown of setae and construct cavities in the soil in which they rest or molt (Dufour, 1833; Bobb, 1951; Boulard and Coffin, 1991; Lis and Pluot-Sigwalt, 2002), while the adults lack such structures and live aboveground and do not burrow. In addition, the flattened and swollen legs with stout thick spines or toe-like structures on the apical and lateral margins are similarly indicative of soil-dwelling insects, and similar burrowing/digging structures are found across numerous unrelated lineages, such as scarab beetles, mole crickets, and nymphal cicadas (Frost, 1959; Snodgrass, 1967; Richards and Davies, 1977; Gullan and Cranston, 2000).
Based on fossil records of Cretaceous Cydnidae (Supplementary Table S1), we can recognize three distinct morphotypes. In the first morphotype, the head lacks broad and flattened clypeus and paraclypei, peg-like setae are absent (Figure 10D), and the protibiae are relatively slender and not broadened apically, lacking stout spines on the apical and lateral margins. Such a type can be found in Clavicoris cretaceus (Popov, 1986), Pricecoris beckerae (Pinto and Ornellas, 1974), and Latiscutella santosi (Pinto and Ornellas, 1974), among many others. Species of the second morphotype have the surface of the body relatively smooth, with tiny and distinct punctures, the clypeus and paraclypei broad and flattened without peg-like setae, instead with only 4–6 hair-like setae (Figures 9D,E, 10E), and the femora and tibiae bearing some slender, weakspines on the outer margin, and with the protibial apex slightly enlarged—as may be observed for Pullneyocoris dentatus (Lis et al., 2020), Laevigemma lisorum gen. et sp. nov. and Quinalveus hui gen. et sp. nov. In the last morphotype (Figures 9A–C, 10F), the clypeus has four long, stout, sharp peg-like setae; and the paraclypei each bears a submarginal row of five more or less sharp-ended peg-like setae and five hair-like setae, with each marginal setigerous puncture arising from its own well-developed tubercle, and collectively, these form a circular crown; the profemur is relatively stout; the apex of the protibia is flattened and broadened, with distinct multiple short and stout spines in a longitudinal series from about mid-length of the lateral margin to the apex; and the apices of the meso- and metatibiae are slightly broadened, with many strong spines (Figure 9B). This third morphotype is found in Acanthamnestus gen. nov. It is interesting to note that the three morphotypes correspond to three different habitat preferences among modern cydnids (Figure 10).

Figure 9. Two different morphotypes in Burmese amber. Photograph of Acanthamnestus ovoideus gen. et sp. nov. in (A–C). (A) Head in dorsal view. (B) Protibiae in left lateral view. (C) Detail of protibial comb. Photograph of Laevigemma lisorum gen. et sp. nov. in (D,E). (D) Head in dorsal view. (E) Protibiae in left lateral view. Scale bars: A = B = 0.2 mm; C = 0.1 mm; D = E = 0.2 mm.
Representatives of the first morphotype (“surface dwellers”) are found from the outgroup and the earliest fossil cydnids. All of the known cydnid fossils in the Early Cretaceous belong to this type (Figure 10D), and they are generally similar to the extant subfamily Sehirinae (Figure 10A), which typically live on the aboveground portions of their host plants (Mcdonald, 1968; Rider, 2012; Schwertner and Nardi, 2015), and lack corresponding structures suitable for soil-dwelling habits. Fossils of the second and third morphotypes are both found in Burmese amber (Figure 9). The second morphotype (Figures 9D,E, 10E) resembles the extant genus Macroscytus (Fieber, 1860) (Figure 10B) in characters of the head and protibia, suggesting that these taxa already had some ability to burrow and therefore represent the first of the hemiedaphic cydnids, albeit still living the majority of their life on the surface (“minor burrowers”). The final morphotype (Figures 9A,B, 10F) is most consistent with the majority of extant cydnids which dig in and spend much of their life cycle in the soil (“true burrowers”), such as Amnestus spinifrons (Say, 1825) and Cyrtomenus teter (Spinola, 1837; Schwertner and Nardi, 2015). Within this lineage, there is often found a comb of setae at the protibial apex, a structure used to clean dust or soil from the antennae and/or labium. Referred to as a “grooming” or “cleaning” comb, such a structure is also present in Acanthamnestus atridorsus gen. et sp. nov (Figure 9C). Such grooming structures are particularly indicative of taxa spending much of their life within the soil, and its presence in A. atridorsus gen. et sp. nov. further supports the indication that this species lived a considerable part of its life in the soil, perhaps emerging as an adult only to mate and lay eggs, much like many modern cydnids (García and Bellotti, 1980; Riis et al., 2005; Nardi et al., 2008; Schwertner and Nardi, 2015). It further emphasizes that the origin of such soil-dwelling specializations among cydnids dates from at least the mid-Cretaceous. Reconstruction of character transitions across the phylogeny implies that soil-dwelling habits evolved once in Amnestinae and likely independently from other such occurrences elsewhere in Cydnidae as the basalmost amnestines lack such structures (Figure 11). Characters relating to soil habits first appeared during the Early Cretaceous, but only of the “minor burrowers” morphotype and were therefore not yet truly subterranean. Orienicydnus hongi (Yao et al., 2007) and Cilicydnus robustispinus (Yao et al., 2007) were found in the Early Cretaceous Yixian Formation in northeastern China, and both possessed characters for some limited burrowing but living principally on the soil surface. The true burrowers first appear in the record in the mid-Cretaceous. The time of appearance of different elements of the overall hemiedaphic suite of features is interesting, with the origin of peg-like setae on the head arising subsequent to the protibial spines. This pattern of transition is intuitive as the protibial structures are certainly necessary for digging a burrow and are present in many taxa that burrow into the soil, but do not necessarily live and feed within the soil substrate. The characters of the head, however, permit some degree of forward movement through the soil matrix for taxa not only burrowing into the soil but also simultaneously bulldozing their way through the substrate. Basically, the former one is simply removing soil and then moving into this space, while the latter one is digging but also penetrating forward into the matrix.
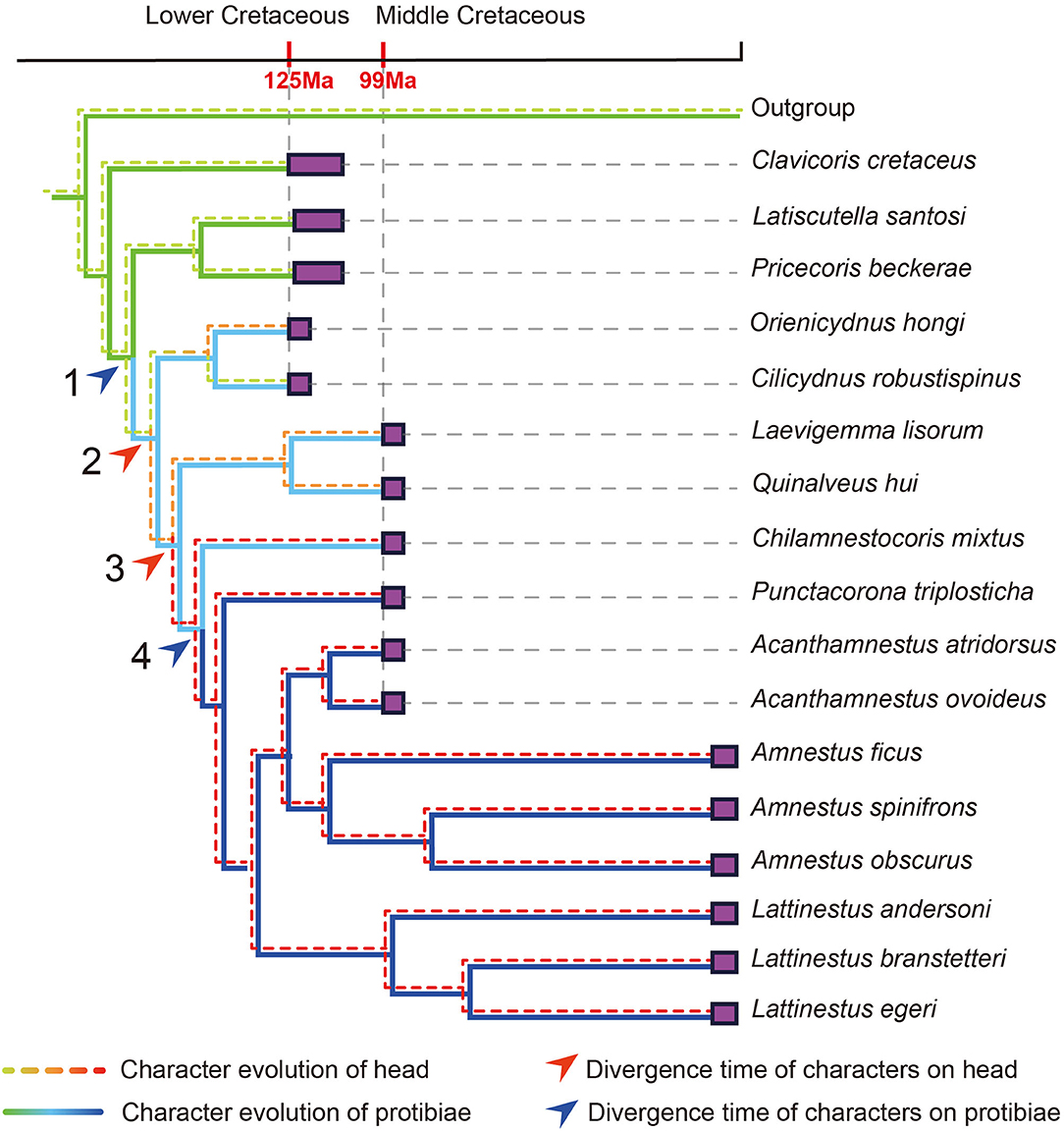
Figure 11. Phylogeny of subfamily Amnestinae, including fossil species and two extant genera. Reconstructions of character evolution derived from phylogenetic analysis. At the top are geological periods calibrated to a time scale; arrows indicate the divergence time of characters on head and protibiae; purple blocks represent the occurrence time of species. Branches are color coded to represent the evolution of different head and protibial characters (see legend; see also Supplementary Figure S1).
Acanthamnestus gen. nov. was recovered as a sister to the extant genus Amnestus (Dallas, 1851) (Figure 11), suggesting that they may perhaps share further biological similarities. Modern Amnestinae are found in the Western Hemisphere, living in warm, tropical or subtropical, moist deciduous forests. Perhaps not surprisingly, when looking at the paleoenvironments where fossil Amnestinae have been discovered (Supplementary Table S1), these same conditions are repeated in different geological periods—all were warm and humid, with rich tropical and subtropical vegetation. Species of the subfamily have preferred these same environmental conditions over a vast expanse of geological time. Myanmar in the mid-Cretaceous was also a warm, humid tropical forest and hosted a rich diversity of arthropods (Grimaldi et al., 2002; Grimaldi and Ross, 2017). This particular niche restriction suggests that, at least to some degree, the subfamily could be an indicator insect for the paleoenvironment. Some extant species of Amnestinae live in the soil near and feed on plants of the family Moraceae, such as Amnestus ficus (Mayorga and Cervantes, 2001), Amnestus obscurus (Mayorga and Cervantes, 2005), and Amnestus calakmulensis (Mayorga and Cervantes, 2005). Mayorga and Cervantes (2001) have reported that species of Ficus (Moraceae) were the primary food of A. ficus, especially when the fruits were mature. When large numbers of fruits and seeds fall to the ground, individuals of A. ficus would feed on these and females sometimes laid individual eggs inside the fruits of Ficus. Interestingly, fossil and modern occurrences of Amnestinae are consistently found within the historical range of Moraceae, or, during the Early and mid-Cretaceous, stem groups to Moraceae (Zerega et al., 2005; Zhang et al., 2018; Figure 12; Supplementary Table S2). Such a correlation suggests that the emergence of soil habits in Amnestinae may be related to the appearance of Moraceae, and there seems to have been a lengthy association between them. This would seem to suggest that early ancestors of Moraceae may have already been present by the mid-Cretaceous.
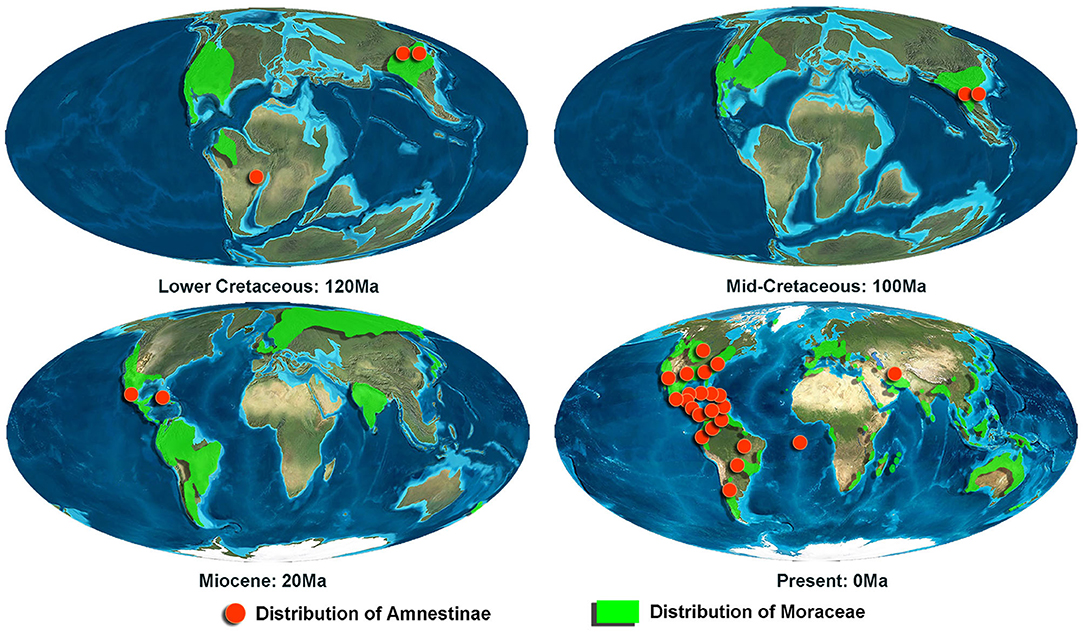
Figure 12. Distribution of Amnestinae and Moraceae in different geological ages. Red dots represent the distribution of Amnestinae; green outlines represent the distribution of Moraceae.
The discovery of Acanthamnestus from the mid-Cretaceous represents the earliest unequivocal soil-dwelling cydnids and pushes back the geological record of hemiedaphic true bugs to 99 Ma. The morphology and phylogenetic position of A. atridorsus indicate that it used its protibiae and crown of setae to dig and shovel through the soil as it lived beneath the surface. Environmental evidence, the pattern of distribution for host plants, and the present fossils provide new insight into the early origin of soil habits and that their emergence may be linked to the appearance of Moraceae, with a subsequent lengthy association between these plants and Amnestinae (Figure 13).

Figure 13. Three-dimensional ecological reconstruction of Acanthamnestus atridorsus gen. et sp. nov.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: ZooBank (urn:lsid:zoobank.org:pub:383D9EDF-02C3-4565-BED1-8C72E13E6FB9).
Author Contributions
SD and YY: conceptualization. YY: methodology and project administration. SD: software, formal analysis, investigation, data curation, writing—original draft preparation, and visualization. YY and ME: validation. YY and DR: resources, supervision, and funding acquisition. ME, YY, and LG: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (Nos. 31970436, 31730087, 32101239, and 32020103006), Joint Fund of the Beijing Municipal Natural Science Foundation, and Beijing Municipal Education Commission (KZ201810028046).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to Xiao Zhang, Yijia Wang, and Mao Zhang for their comments on the manuscript and Mr. Zhengkun Hu for his assistance and contribution in collecting Burmese amber. We also thank Xiaoran Zuo for assistance with reconstruction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.908044/full#supplementary-material
References
Bobb, M. L. (1951). Life history of Ochterus banksi Barber (Hemiptera: Ochteridae). Bull. Brookl. Entomol. Soc. 46, 92–100.
Boulard, M., and Coffin, J. (1991). Sur la biologie juvénile d'Ochterus marginatus (Latreille, 1804). Camouflage et construction (Hemiptera: Ochteridae). Travaux Lab. Biol. Evol. Insectes EPHE. 4, 57–68.
Cao, H. J., Boudinot, B. E., Shih, C. K., Ren, D., and Gao, T. P. (2020). Cretaceous ants shed new light on the origins of worker polymorphism. Sci. China Life Sci. 63, 1085–1088. doi: 10.1007/s11427-019-1617-4
Dallas, W. S. (1851). List of the specimens of hemipterous insects in the collection of the British Museum. J. British Astron. Assoc. 2, 1368.
Dolling, W. R. (1981). A rationalized classifi cation of the burrower bugs (Cydnidae). Syst. Entomol. 6, 61–67 doi: 10.1111/j.1365-3113.1981.tb00016.x
Dufour, L. (1833). Recherches anatomiques et physiologiques sur les Hémiptères accompagnées de considérations relatives à l'histoire naturelle et à la classification de ces insectes. Mém. Savants Etrang. Acad. Sci. Paris. 4, 123–432. doi: 10.5962/bhl.title.12418
Fieber, F. X. (1860). Die europäischen Hemiptera, Halbflüger (Rhynchota, Heteroptera), I-II. Wien: Gerold's Sohn Press, 1–112.
Frost, S. W. (1959). Insect Life and Insect Natural History, 2nd Edn. New York, NY: Dover Publications.
García, C. A., and Bellotti, A. C. (1980). Estudio preliminar de la biología y morfología de Cyrtomenus bergi F. Nueva plaga de la yuca. Rev. Colombiana de Ento. 6, 55–61.
Goloboff, P. A. (1999). NoName (NONA), Version 2.0. Program and Documentation. Tucumán: Fundación Instituto Miguel Lillo.
Goloboff, P. A., and Catalano, S. A. (2016). TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 32, 221–238. doi: 10.1111/cla.12160
Grimaldi, D., and Engel, M. S. (2005). Evolution of the Insects. Cambridge: Cambridge University Press.
Grimaldi, D. A., Engel, M. S., and Nascimbene, P. C. (2002). Fossiliferous Cretaceous amber from Myanmar (Burma): its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Nov. 3361, 1–71. doi: 10.1206/0003-0082(2002)3612.0.CO
Grimaldi, D. A., and Ross, A. J. (2017). “Extraordinary Lagerstätten in amber, with particular reference to the Cretaceous of Burma,” in Terrestrial Conservation Lagerstätten: Windows into the Evolution of Life on Land, eds N.C. Fraser, H-D. Sues (Edinburgh: Dunedin Academic Press), 287–342.
Gullan, P. J., and Cranston, P. S. (2000). The Insects: An Outline of Entomology. Oxford: Wiley-Blackwell, Press.
Hertzel, G., and Scharmann, K. H. (1973). Beitrag zur Kenntnis der Biologie und Verbreitung von Canthophorus dubius (Scopoli, 1765) (Het.: Cydnidae). Entomol. Ber. 33–39.
Lin, X. D., Labanderira, C. C., Shih, C. K., Hotton, C. L., and Ren, D. (2019). Life habits and evolutionary biology of new two-winged long-proboscid scorpionflies from mid-Cretaceous Myanmar amber. Nat. Commun. 10, 1235. doi: 10.1038/s41467-019-09236-4
Lis, J. A. (1991). Studies on Oriental Cydnidae. IV. New species, new synonyms and new records (Heteroptera: Pentatomoidea). Ann. Upper Silesian Mus. Entomol. 2, 165–190.
Lis, J. A. (1994). A Revision of Oriental Burrower Bugs (Heteroptera: Cydnidae). Bytom: Upper Silesian Museum, Department of Natural History, 349.
Lis, J. A., Lis, B., and Ernest, H. (2018). Chilamnestocoris mixtus gen. et spec. nov., the first burrower bug (Hemiptera: Pentatomoidea: Cydnidae) in Upper Cretaceous Burmese amber. Cretaceous Res. 91, 257–262. doi: 10.1016/j.cretres.2018.06.017
Lis, J. A., and Pluot-Sigwalt, D. (2002). Nymphal and adult cephalic chaetotaxy of the Cydnidae (Hemiptera: Heteroptera), and its adaptive, taxonomic and phylogenetic significance. Eur. J. Entomol. 99, 99–109. doi: 10.14411/eje.2002.017
Lis, J. A., Roca-Cusachs, M., Lis, B., and Jung, S. H. (2020). Pullneyocoris dentatus gen. et sp. nov. (Hemiptera: Pentatomoidea: Cydnidae), the third representative of the subfamily Amnestinae from mid-Cretaceous amber of northern Myanmar. Cretaceous Res. 115, 104562. doi: 10.1016/j.cretres.2020.104562
Lis, J. A., Ziaja, D. J., Lis, B., and Gradowska, P. (2017). Non-monophyly of the “cydnoid” complex within Pentatomoidea (Hemiptera: Heteroptera) revealed by Bayesian phylogenetic analysis of nuclear rDNA sequences. Arthropod Syst. Phylo. 75, 481–496.
Maddison, W. P., and Maddison, D. R. (2011). Mesquite: A Modular System for Evolutionary Analysis Version 2.75. Available online at: http://mesquiteproject.org
Mayorga, C. M., and Cervantes, L. P. (2005). Description of six new species of Amnestus Dallas (Hemiptera: Heteroptera: Cydnidae) from Mexico. J N Y Entomol. Soc. 113, 159–173. doi: 10.1664/0028-7199(2005)113[0159:DOSNSO]2.0.CO;2
Mayorga, M. C., and Cervantes, P. L. (2001). Life cycle and description of a new species of Amnestus Dallas (Hemiptera-Heteroptera: Cydnidae) associated with the fruit of several species of Ficus (Moraceae) in Mexico. J N Y Entomol. Soc. 109, 392–402. doi: 10.1664/0028-7199(2001)109[0392:LCADOA]2.0.CO;2
Mcdonald, F. J. D. (1968). Some observations on Sehirus cinctus (Palisot de Beauvois) (Heteroptera: Cydnidae). Can. J. Zool. 46, 855–858. doi: 10.1139/z68-122
Nardi, C., Fernandes, P. M., and Bento, J. M. S. (2008). Wing polymorphism and dispersal of Scaptocoris carvalhoi (Hemiptera: Cydnidae). Ann. Entomol. Soc. Am. 101, 551–557. doi: 10.1603/0013-8746(2008)101[551:WPADOS]2.0.CO;2
Nixon, K. C. (2002). WinClada ver. 1.00.08. Published by the author, Ithaca, New York, Renner and Weerasooriya, USA. Available online at: http://www.cladistics.com/ (accessed August 6, 2019).
Pinto, I. D., and Ornellas, L. P. (1974). “New Cretaceous Hemiptera (Insecta) from Codó Formation—Northern Brazil,” in Anais 28th Congresso Brasileiro de Geologia (Brazil), 289–304.
Popov, Y. A. (1986). “Peloridiina (=Coleorrhyncha) et Cimicina (=Heteroptera),” in Insects in Early Cretaceous Ecosystems of Western Mongolia, ed A. P. Rasnitsyn, Vol. 28, Transactions of the Joint Soviet-Mongolian Paleontological Expedition, 47–84.
Richards, O. W., and Davies, R. G. (1977). Imms' General Textbook of Entomology, vol. I: Structure, Physiology and Development, 10th Edn. London: Chapman and Hall.
Rider, D. A. (2012). The heteroptera (Hemiptera) of North Dakota I: pentatomomorpha: pentatomoidea. Gr. Lakes. Entomol. 45, 312–380.
Riis, L., Esbjerg, P., and Bellotti, A. C. (2005). Influence of temperature and soil moisture on some population growth parameters of Cyrtomenus bergi (Hemiptera: Cydnidae). Fla. Entomol. 88, 11–22. doi: 10.1653/0015-4040(2005)088[0011:IOTASM]2.0.CO;2
Say, T. (1825). Description of new hemipterous (and orthopterous) insects collected in the expedition to the Rocky Mountains, performed by order of Mr. Calhoun, Secretary of War, under command of Major Long. Journ. Acad. Nat. Sci. 4, 307–345.
Schuh, R. T., and Slater, J. A. (1995). True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History. New York, NY: Cornell University Press, 336.
Schwertner, C. F., and Nardi, C. (2015). “Chapter 21. Burrower bugs (cydnidae),” in True Bugs (Heteroptera) of the Neotropics, eds A. R. Panizzi, and J. Grazia (Dordrecht-Heidelberg-New York-London: Springer Netherland), XXII + 901.
Shcherbakov, D. E., and Popov, Y. A. (2002). “Superorder Cimicidea Laicharting, 1781. Order Hemiptera Linne, 1758. The bugs, cicadas, plant lice, scale insects, etc,” in History of Insects, eds A. P. Rasnitsyn, and D. L. J. Quicke (Dordrecht: Kluwer), 143–157.
Shi, G. H., Grimaldi, D. A., Harlow, G. E., Wang, J., Wang, J., Yang, M. C., et al. (2012). Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Res. 37, 155–163. doi: 10.1016/j.cretres.2012.03.014
Spinola, M. (1837). Essai sur les genres d'insectes appartenants à l'ordre des Hémiptéres, Lin. ou Rhyngotes, Fab. et à la section des Hètéroptères, Dufour. Genoa: Yves Graviers, p. 383.
Wang, Y. J., Du, S. L., Yao, Y. Z., and Ren, D. (2019). A new genus and species of burrower bugs (Heteroptera: Cydnidae) from the mid-Cretaceous Burmese amber. Zootaxa. 4585, 351–359. doi: 10.11646/zootaxa.4585.2.8
Yang, H. R., Shi, C. F., Engel, M., Zhao, Z. P., Ren, D., and Gao, T. P. (2020). Early specializations for mimicry and defense in a Jurassic stick insect. Natl. Sci. Rev. 8, nwaa056. doi: 10.1093/nsr/nwaa056
Yao, Y. Z., Cai, W. Z., and Ren, D. (2007). The first fossil Cydnidae (Hemiptera: Pentatomoidea) from the Late Mesozoic of China. Zootaxa. 1388, 59–68. doi: 10.11646/zootaxa.1388.1.5
Zerega, N. J. C., Clement, W. L., Datwyler, S. L., and Weiblen, G. D. (2005). Biogeography and divergence times in the mulberry family (Moraceae). Mol. Phylogenet. Evol. 37, 402–416. doi: 10.1016/j.ympev.2005.07.004
Zhang, Q., Onstein, R. E., Little, S. A., and Sauquet, H. (2018). Estimating divergence times and ancestral breeding systems in Ficus and Moraceae. Ann. Botany. 20, 1–14. doi: 10.1093/aob/mcy159
Keywords: fossorial insect, Pentatomomorpha, Ficus, Moraceae, angiosperm, modification of head and fore legs
Citation: Du S, Gu L, Engel MS, Ren D and Yao Y (2022) Morphological Phylogeny of New Cretaceous Fossils Elucidates the Early History of Soil Dwelling Among Bugs. Front. Ecol. Evol. 10:908044. doi: 10.3389/fevo.2022.908044
Received: 30 March 2022; Accepted: 19 April 2022;
Published: 23 May 2022.
Edited by:
Chenyang Cai, Nanjing Institute of Geology and Paleontology (CAS), ChinaCopyright © 2022 Du, Gu, Engel, Ren and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunzhi Yao, eWFveXoxMDBAMTI2LmNvbQ==
 Sile Du1,2
Sile Du1,2 Michael S. Engel
Michael S. Engel Yunzhi Yao
Yunzhi Yao