
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 09 June 2022
Sec. Paleontology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.907903
This article is part of the Research TopicA Fossil View of Insect Evolution: Integrating Paleontological Evidence to Explore the Origins of Insect BiodiversityView all 10 articles
Two species of psocids discovered from the Mid-Cretaceous Burmese amber, Latempheria kachinensis Li, Yoshizawa, and Yao, gen. et sp. nov. and Burmempheria curvatavena Li, Yoshizawa, and Yao, sp. nov., are described and assigned to the Empheriidae (Trogiomorpha: Atropetae) family. A phylogenetic analysis of the infraorder Atropetae is conducted based on 38 morphological characters of three outgroups and fifteen ingroups, which supported the monophyly of Atropetae including fossil and extant taxa. In the phylogenetic result, all the genera of fossil families Empheriidae and Archaeatropidae form a monophyletic group, sister to the extant members of Atropetae. The two fossil families also share a lot of similarities in morphology, locality, and geological period. Recently discovered fossil species exhibited combined morphological characters of both families. Based on these observations and the results of the phylogenetic analysis, Archaeatropidae is treated here as a new junior synonym of Empheriidae.
The infraorder Atropetae Pearman, 1936 represents the largest group in the suborder Trogiomorpha, with more than 400 species mainly occurring in warm and humid regions (Lienhard and Smithers, 2002). These species are classified into two extinct families (Empheriidae and Archaeatropidae) (Smithers, 1972; Baz and Ortuño, 2000, 2001; Azar and Nel, 2004; Azar et al., 2010, 2014; Li et al., 2020) and three extant families (Psoquillidae, Lepidopsocidae, and Trogiidae) (Roesler, 1944; Badonnel, 1951). Fossil Psocodea have been studied for over 100 years; however, because of limitations in fossil specimens and incomplete preservation, and different interpretations of taxonomic characteristics, many species were classified into uncertain positions. For example, based on morphological characters, two families in Trogiomorpha, i.e., Empheriidae and Archaeatropidae, are hard to distinguish (Baz and Ortuño, 2000, 2001; Perrichot et al., 2003; Li et al., 2020).
Atropetae fossils are known in five families (including three extant families) and thirty-five genera. Their geological history ranged from Cretaceous to Paleogene, and they were distributed in Lebanon (Azar and Nel, 2004), Spain (Baz and Ortuño, 2000, 2001), Myanmar (Azar and Nel, 2004; Li et al., 2020; Liang et al., in press), France SW (Azar et al., 2014), New Jersey (Azar et al., 2010), France Oise (Nel et al., 2005), Siberia (Vishnyakova, 1975; Hakim et al., 2021), and Baltic region (Hagen, 1856; Enderlein, 1911). Most of the fossil species are placed in the extinct families Empheriidae and Archaeatropidae. Empheriidae was established by Kolbe (1884), with eight genera and eleven species (Hagen, 1856, 1882; Baz and Ortuño, 2001; Nel et al., 2005; Azar et al., 2010; Li et al., 2020; Hakim et al., 2021). Archaeatropidae was established by Baz and Ortuño (2000) based on amber specimens from Spain. The family is now composed of eight genera with twelve species recorded mainly from Lebanon, Myanmar, and France SW (Baz and Ortuño, 2000; Perrichot et al., 2003; Azar and Nel, 2004; Azar et al., 2014; Cumming and Le Tirant, 2021; Liang et al., in press). Li et al. (2020) mentioned that based on high similarity in morphological characters, living period, and fossil locality, Empheriidae and Archaeatropidae are likely to be synonyms. Previous phylogenetic analyses showed that Atropetae is a monophyletic group that comprises three extant families (Smithers, 1972; Yoshizawa et al., 2006; de Moya et al., 2021). However, these analyses did not include the extinct families of Atropetae, Empheriidae, and Archaeatropidae. Consequently, the phylogenetic placement of the two families remains unsolved to date.
In this study, all ambers were collected from the Hukawng Valley, Myitkyina District, Kachin State, Myanmar (Lin et al., 2019; Zhao et al., 2020). Herein, a new genus, Latempheria Li, Yoshizawa and Yao, gen. nov., distinguished by broad external valves and a new species of Burmempheria Li et al., 2020 in Empheriidae are described. Based on present observations and published information, 38 morphological characters for three outgroups and fifteen ingroups are coded. Using this data matrix, phylogenetic analyses were conducted to estimate the phylogenetic relationships among the families of Atropetae, including fossil taxa, for the first time. The result suggests that Empheriidae and Archaeatropidae, together form a monophyletic group, and their morphological differences are obscure. Therefore, we synonymize the latter family with Empheriidae.
The amber specimens were collected from Kachin (Hukawng Valley) in Northern Myanmar. The specimens are housed in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China (CNU, Curator: Yunzhi Yao). The specimens were acquired before 2013 and then studied in 2019, with no conflict (Wang et al., 2016; Engel, 2020; Shi et al., 2021).
The specimens were examined and photographed under a Nikon SMZ25 microscope with an attached Nikon DS-Ri2 digital camera system. The morphological terminology mainly follows Yoshizawa (2005). Abbreviations: mx, maxillary palp; la, labium; Sc, subcostal vein; Sc’, distal part of the subcostal vein; R, radius vein; Rs, radial sector; M, median vein; Cu, cubital vein; A, anal vein; ep, epiproct; p, paraproct; pa, parameres; s, subgenital plate; V3, external valve; mt: tarsus of mid leg; ht: tarsus of hind leg.
The outgroups were selected from Prionoglarididae, Psyllipsocidae, and Cormopsocidae, and the ingroups from Atropetae (Supplementary Table 1). For coding the morphological data from the fossil taxa, we selected those that were widespread and that had extensive information available on morphology. Thirty-eight multistate characters were treated as non-additive (= unordered) and equally weighted. The data matrix used in this phylogenetic analysis is provided in Supplementary Data Sheet 1. Inapplicable states were assigned as a gap value (“−”) and treated as equivalent to missing data (“?”).
The character matrix (Supplementary Datasheet) was compiled using Nexus Data Editor v0.5.0 (Page, 2001). A phylogenetic analysis was conducted by maximum parsimony analysis in WINCLADA v1.00.08 (Nixon, 2002) with NONA script (Goloboff, 1997; Nixon, 2002) and TNT v1.5 (Goloboff and Catalano, 2016). The analysis conducted in WINCLADA was set to keep 10,000 maximum trees, 1,000 replications, and 100 starting trees per replication. A repeated analysis was run in TNT using “Traditional Search.”
The maximum parsimony analyses using TNT and WINCLADA both yielded 6 most parsimonious trees (tree length of 86 steps, consistency index (CI) of.51, and retention index (RI) of.6), and a strict consensus tree with a tree length of 93 steps, consistency index (CI) of.47, and retention index (RI) of.54 (Figure 1 and Supplementary Figure 1). Bremer Supports are shown in Figure 1. The result supported that Empheriidae + Archaeatropidae constitutes a monophyletic group, and that Lepidopsocidae + Psoquillidae + Trogiidae constitutes a monophyletic group. These two groups formed a monophyletic group, the infraorder Atropetae.
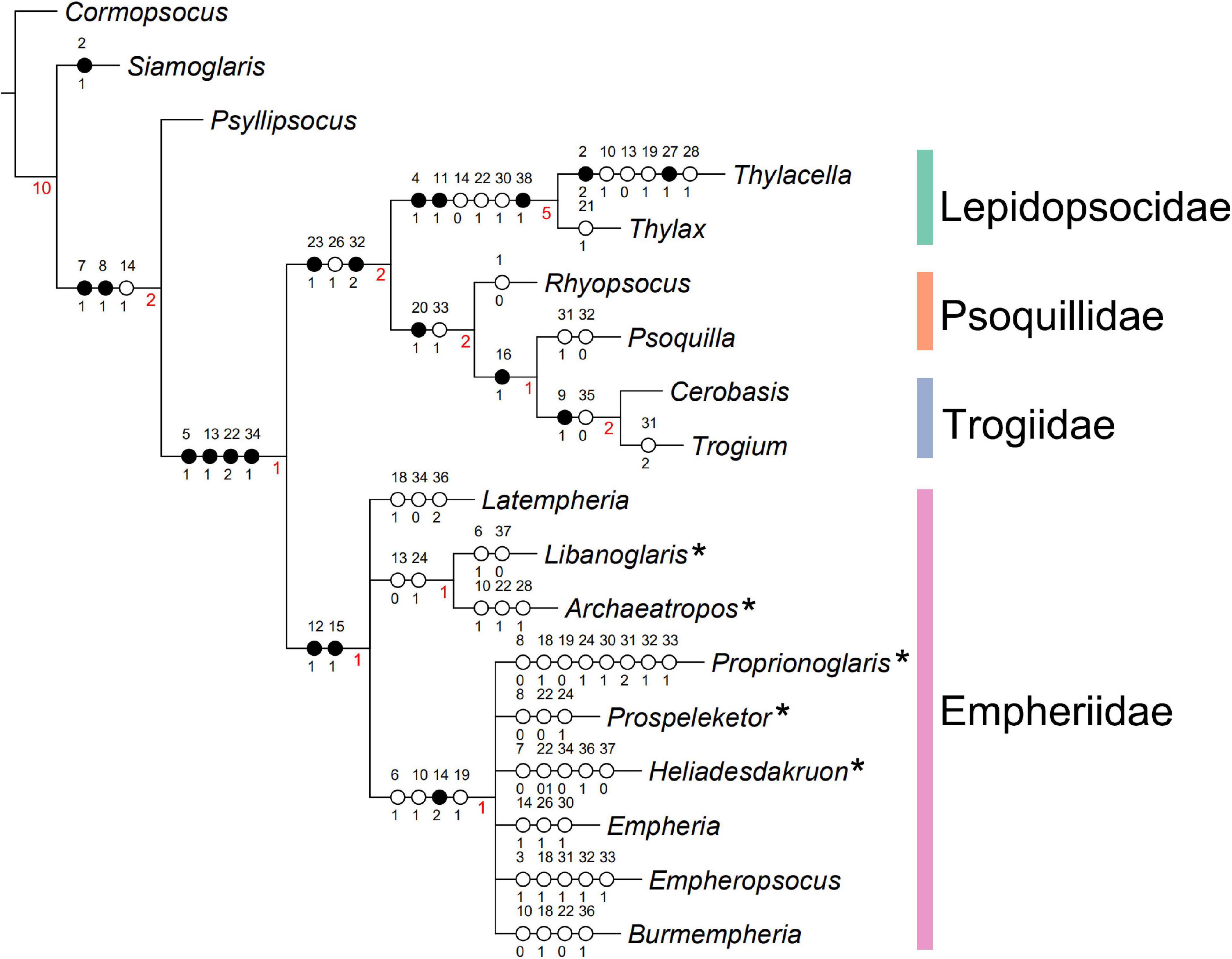
Figure 1. Strict consensus tree of the most parsimonious tree. Phylogeny of Atropetae based on 18 species and 38 characters, with characters being non-additive, under equal weighting. Analyzed with WINCLADA v1.00.08 with NONA script (length = 93, CI = 0.47, RI = 0.54). * Represents the genus originally placed in Archaeatropidae.
The monophyly of Atropetae including fossil and extant taxa was demonstrated for the first time, with the following four character states supporting its monophyly: mx2 with sensillum (character 5, state 1); Areola Postica long (character13, state 1); M1+2 longer than the second section of M (character 22, state 2); long and thin female external valves (character 34, state 1).
In Atropetae, the families Lepidopsocidae, Psoquillidae, and Trogiidae, i.e., the extant members of the infraorder, formed a monophyletic group, with two character states supporting this clade: (1) Radial cell absent in forewing (character 23, state 1); (2) pulvillus broad (character 32, state 2). In this clade, Psoquillidae + Trogiidae formed a monophyletic group, the supporting character for this clade was: forewing Rs and R1 not connected by a short crossvein (character 20, state 1). In the present analysis, the monophyly of Lepidopsocidae has been well-supported by three character states: (1) ocelli arranged far apart (character 4, state 1); (2) forewing pointed (character 11, state 1); (3) body covered by scales (character 38, state 1).
Empheriidae and Archaeatropidae formed a monophyletic group (Figure 1), with two characters supporting this clade: (1) forewing membrane with setae (character 12, state 1); (2) forewing veins with setae (character 15, state 1). In this lineage, the relationship of each genus remained unclear.
Suborder Trogiomorpha Roesler, 1940.
Infraorder Atropetae Pearman, 1936.
Family Empheriidae Kolbe, 1884.
Empheriidae Kolbe, 1884: 37 [Type genus: Empheria Hagen, 1856: Berliner Entomologische Zeitschrift. 28, 35–38].
Archaeatropidae Baz and Ortuño, 2000: 369 [Type genus: Archaeatropos Baz and Ortuño, 2000: Annals of the Entomological Society of America. 93, 367–373]. New junior synonymy of Empheriidae.
Included genera (* represents this genus originally placed in Archaeatropidae): Empheropsocus Baz and Ortuño, 2001; Preempheria Baz and Ortuño, 2001; Jerseyempheria Azar et al., 2010; Eoempheria Nel et al., 2005; Empheria Hagen, 1856; Trichempheria Enderlein, 1911; Burmempheria Li et al., 2020; Empherium Hakim et al., 2021; *Archaeatropos Baz and Ortuño, 2000; *Bcharreglaris Azar and Nel, 2004; *Libanoglaris Perrichot et al., 2003; *Proprionoglaris Perrichot et al., 2003; *Prospeleketor Perrichot et al., 2003; *Setoglaris Azar and Nel, 2004; *Heliadesdakruon Cumming and Le Tirant, 2021; *Longiantennum Liang et al., in press*; Latempheria Li, Yoshizawa, and Yao, gen. nov.
Genus: Latempheria Li, Yoshizawa and Yao, gen. nov.
Etymology: The generic name is a combination of Latin words “lat” (broad) and “empheria” (type genus of Empheriidae), referring to the broad external valves of the type species. The gender is feminine.
Type species: Latempheria kachinensis Li, Yoshizawa and Yao, gen. et sp. nov.
Diagnosis: Forewing Sc long, almost half length of forewing, ended in the middle of Radial cell; all tibiae with two apical spurs, and tibiae covered with two rows of obvious setae; external valves broad and elongate, lobate-liked.
Remarks: Latempheria shares a series of characters with Trogiomorpha: (1) antennae with more than 20 segments; (2) tarsi with three segments; (3) forewing pterostigma slightly opaque; (4) ventral and dorsal valves of gonapophyses strongly reduced (or absent), external valves well-developed and setose; (5) subgenital plate short, covering at most basal part of external valves (Yoshizawa et al., 2006). In addition, Latempheria can be classified into Atropetae by the following characters: (1) forewing basal segment of Sc well-developed; (2) hind wing A simple; (3) external valves of gonapophyses elongated and partially joined together on midline by membrane; (4) paraproct with anal spine (Yoshizawa et al., 2006). Latempheria can be assigned to Empheriidae according to (1) wings well-developed, rounded at apex; (2) forewing Sc bend to R; (3) venation covered with setae; (4) claws without preapical tooth (Baz and Ortuño, 2001).
In the family, Latempheria shares similar characteristics with Burmempheria Li et al., 2020 in forewing venation, legs, and antennae. The main difference between Latempheria and Burmempheria are: (1) Latempheria with broad and elongated external valves (vs. rod-like external valves in Burmempheria); (2) all tibiae with two rows of obvious spur in Latempheria (vs. Burmempheria with tibia bearing setae or bare).
Latempheria kachinensis Li, Yoshizawa and Yao, gen. et sp. nov. (Figures 2, 3).
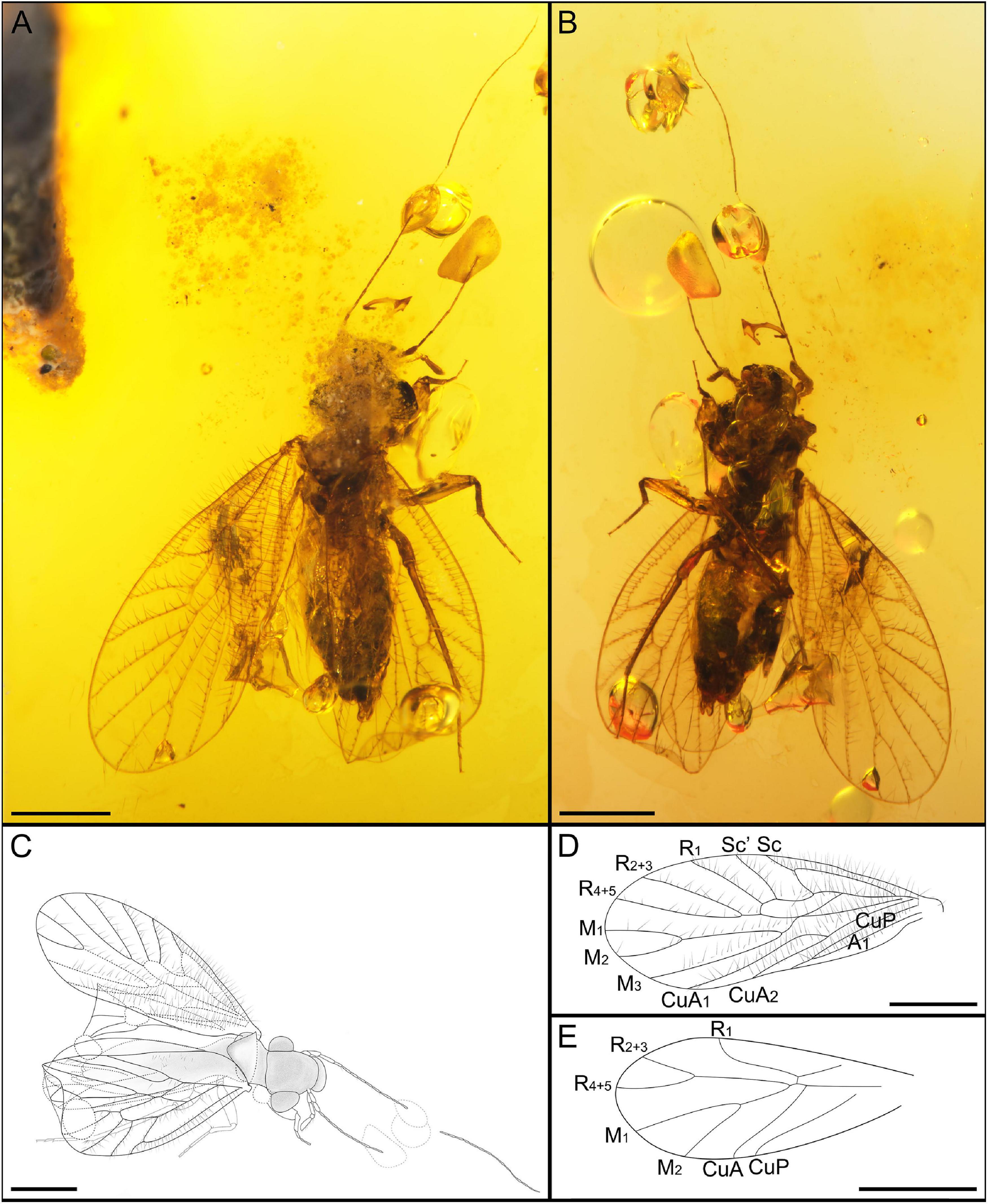
Figure 2. Habitus of Latempheria kachinensis Li, Yoshizawa and Yao, gen. et sp. nov. CNU-PSO-2015001. (A) Photograph in dorsal view; (B) photograph in ventral view; (C) line drawing in dorsal view; (D) line drawing of forewing; (E) line drawing of hind wing. Scale bars:0.5 mm.

Figure 3. Detailed photograph of Latempheria kachinensis Li, Yoshizawa and Yao, gen. et sp. nov. (A) Photograph of antennae; (B) line drawing of antennae; (C) photograph of mouthpart; (D) line drawing of right maxillary palps (dorsal view); (E) line drawing of labial palps; (F) photograph of left hind leg (dorsal view); (G) line drawing of left hind leg (dorsal view); (H) photograph of CuP and A1 in left forewing; (I) photograph of female terminalia; (J) line drawing of female terminalia. Scale bars:0.1 mm.
urn:lsid:zoobank.org:act:8500CE76-8F78-402B-AB7D-376B939FC0A9.
Etymology: “Kachin” indicates the type locality of the new species.
Material: Holotype, CNU-PSO-MA2015001 (female, head covered by impurities). Paratype, CNU-PSO-MA2015002 (female with head well preserved, gonapophyses distorted).
Locality and horizon: Hukawng Valley, Kachin State, Northern Myanmar; mid-Cretaceous, lowermost Cenomanian.
Diagnosis: All tibiae with two rows of obvious setae, with two apical spurs; external valves without setae; CuP and A1 fused for long distance before wing margin; CuA1 obviously curved, nearly 2 times longer than CuA2.
Description: Female, body completely preserved, head with some impurities (Figures 2A,B), antennae broken (Figure 2C); mouthpart well-preserved; three ocelli present, arranged in inverted triangle. Body length 1.71 mm (measured from frons to terminalia). Forewing length 1.812 mm, width.809 mm; hind wing length 1.46 mm, width.509 mm. IO/d = 2.23. Mt = 307 mm; ht = 0.361 mm.
Head: head narrow, compound eyes small, diameter less than 1/2 the length of interorbital distance; antennae long, obviously broken, left 25 segments preserved, right 7 segments preserved, distal part of flagellum with soft and thin setae, secondary annulations absent (Figures 3A,B). Maxillary palps with four segments (Figure 3C), second segments longest, terminal article hatchet-shaped, with sensillum (Figure 3D). Labium palps with two articles, terminal augment rounded (Figure 3E).
Thorax: prothorax broad, mesothorax well-developed, and mesonotum triangular.
Legs: All legs covered with setae, all tibiae with two rows of obvious setae, with two apical spurs (Figures 3F,G); tarsus three segmented, claws without pulvillus and preapical tooth.
Forewings: Macropterous, forewing oval (Figure 2D), margin glabrous, veins with long setae except CuP, membrane glabrous except vannal region. Veins with Sc, M, Cu, and A with long setae arranged along both edges of veins; other veins with one row of long setae; Sc long, basally fused with R for short distance, distally curved strongly, ended at R1 vein, short vein arises from top of curved Sc reaching to anterior margin; Sc’ long, pterostigma quadrilateral, not thickened; Rs and M fused for short distance; Rs fork distal to M fork; M1+2 longer than second section of M, almost 2.5 times longer with it. CuA1 and CuA2 long, CuA1 curved, nearly 2 times longer than CuA2; CuP weaker than other veins; CuP and A1 fused for long distance before reaching wing margin (Figure 3H).
Hind wing oval (Figure 2E). Hind wing margin and membrane glabrous (Figure 2E). Not preserved well. Distal part of R1 curved; R1 and Rs + M connected by a short crossvein; CuA unforked; A not visible.
Abdomen: Epiproct and paraproct covered with setae; epiproct with anal spine; dorsal valves degraded, external valves elongate, broad, lobate-liked, and partially joined together on midline by membrane (Figures 3I,J).
Genus: Burmempheria Li et al., 2020.
Type species: Burmempheria densuschaetae Li et al., 2020.
Burmempheria curvatavena Li, Yoshizawa and Yao, sp. nov. (Figures 4, 5).
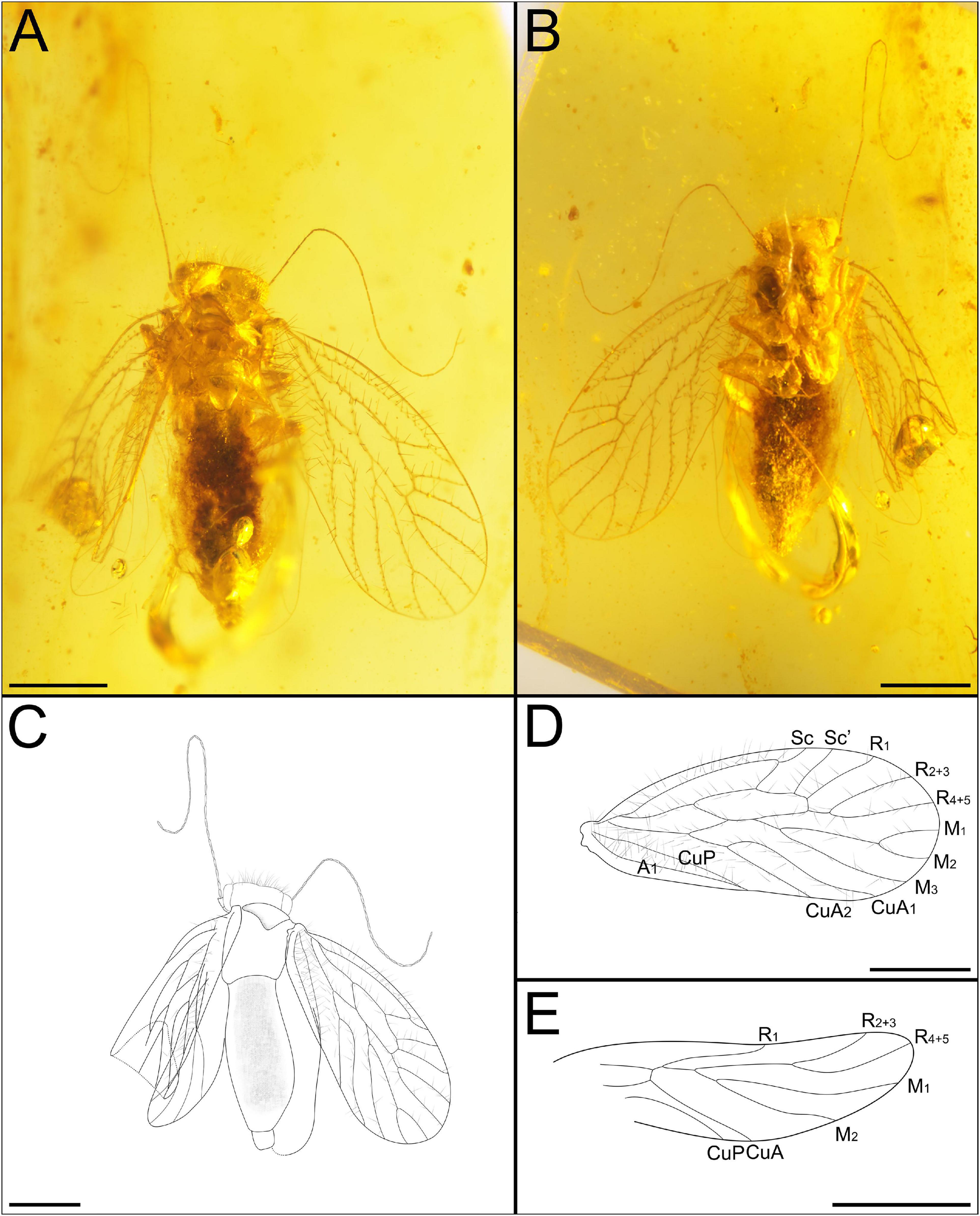
Figure 4. Habitus of Burmempheria curvatavena Li, Yoshizawa and Yao, sp. nov. CNU-PSO-2015003. (A) Photograph in dorsal view; (B) photograph in ventral view; (C) line drawing in dorsal view; (D) line drawing of forewing; (E) line drawing of hind wing. Scale bars:0.5 mm.
Figure 5. Detailed photograph of Burmempheria curvatavena Li, Yoshizawa and Yao, sp. nov. (A) Photograph of right antenna; (B) line drawing of right antenna; (C) photograph of mouthpart; (D) line drawing of mouthpart; (E) photograph of right hind leg (dorsal view); (F) line drawing of right hind leg (dorsal view); (G) photograph of CuP and A1 in left forewing. Scale bars:0.1 mm.
urn:lsid:zoobank.org:act:89231F37-E4CC-44BA-85F0-CCDE453FB3AA.
Etymology: A combination of Latin words “curvata” (bent) and “vena” (vein), indicating that CuA1 is obviously curved.
Material: Holotype, CNU-PSO-MA2015003, male, well-preserved, with genitalia covered by impurities.
Locality and horizon: Hukawng Valley, Kachin State, Northern Myanmar; mid-Cretaceous, lowermost Cenomanian.
Diagnosis: All tibiae with two rows of sparse and short setae; tibia with three apical spurs; distal part of forewing CuA1 obviously curved; M1+2 extremely long, almost 3 times longer than the distal section of M.
Description: Male, well -preserved, with folded distal left forewing. Body length 1.71 mm (measured from frons to terminalia). Forewing length 1.887 mm, width.762 mm; hind wing length 1.354 mm, width.453 mm. IO/d = 2.16. Mt = 0.375 mm; ht = 0.417 mm.
Head: vertex broad, covered with sparse and long setae (Figures 4A,B); compound eyes large, diameter longer than 1/2 the length of interorbital distance; three ocelli present, arranged in inverted triangle. Antennae long (Figure 4C), right flagellum with 30 segments, left with 32 flagellomeres, basal 10 segments covered with setae, secondary annulations absent (Figures 5A,B). Maxillary palps with four segments, covered with setae, terminal article hatchet-shaped, second article longest (Figures 5C,D).
Thorax: slight deformation, prothorax invisible, mesothorax well-developed, mesonotum triangular.
Legs. All legs long, all tibiae covered two rows of obvious setae, with three apical spurs (Figures 5E,F); tarsus three segmented, terminal segment with two claws, without pulvillus and preapical tooth.
Forewing: Macropterous, forewing oval (Figure 4D), margin glabrous, all veins with long setae except CuP, membrane glabrous except vannal region. Sc, R, M, and Cu with setae along both edges of veins, other veins with single row of setae; Sc long, basally fused with R for short distance and distally curved, short vein arises from top of curved Sc, reaching to the anterior margin; Sc’ long, not curved; distal R1 slightly curved; pterostigma quadrilateral, not thickened; M1+2 very long, almost 3 times longer than the second section of M; CuA with two branches, CuA1 is twice as CuA2, CuA1 distinctly curved; CuP weaker than other veins, CuP and A1 merged for short distance before reaching wing margin (Figure 5G).
Hind wing oval (Figure 4E), hind wing margin and membrane glabrous, right hind wing folded; R1 slightly curved; M1 and M2 relatively long; CuA unforked; A not visible.
Abdomen: genitalia covered by impurities, not well-preserved.
Remarks: Burmempheria curvatavena can be assigned into Burmempheria based on: (1) flagellum more than 30 segments; (2) Sc long and reaching to the anterior margin; (3) lack of pulvillus. There are obvious differences between B. curvatavena and other species: (1) tibiae with three apical spurs (vs. two apical spurs in B. densuschaetae and B. raruschaetae); (2) M1+2 extremely long, almost thrice the distal section of M (vs. M1+2 nearly twice the distal section of M in B. densuschaetae and B. raruschaetae).
The monophyly of Atropetae, including the fossil taxa, is recovered in the current analysis for the first time. Based on the extant species, Smithers (1972) mentioned that the reduction of female genitalia to setose, lobar external valves is the main apomorphy of Atropetae. Previous molecular research also supported the monophyly of Atropetae (Yoshizawa et al., 2006; de Moya et al., 2021). Based on their result, Yoshizawa et al. (2006) proposed two autapomorphies of extant Atropetae: (1) external valves of gonapophyses elongated and partially joined together on midline by membrane, composing the ovipositor; (2) spermathecal sac with one or two glandular accessory bodies.
The present analyses recovered the monophyly of Lepidopsocidae + Psoquillidae + Trogiidae, extant members of Atropetae, as recovered by Smithers (1972) and Yoshizawa et al. (2006). The autapomorphies of this clade identified by the present analyses are: forewing Radial cell absent (character 23, state 1); pulvillus broad (character 32, state 2). In this clade, Psoquillidae + Trogiidae formed a monophyletic group. This relationship had already been discussed by Smithers (1972) based on the presence of spermathecal accessory bodies, but Mockford (1993) suggested that this character is an autapomorphy of Atropetae (Yoshizawa et al., 2006). Mockford (1993) and Lienhard (1998) proposed two synapomorphies supporting the close relationship between Psoquillidae and Trogiidae: (1) pretarsal claw without preapical tooth; (2) pulvillus distinctly enlarged through its whole length. The autapomorphies of this clade identified by the present analyses are: R1 and Rs not connected by a short crossvein (character 20, state 1).
The current research suggested that Psoquillidae is paraphyletic, and Psoquilla Hagen, 1865 was placed to be the sister taxon of Trogiidae supported by reduced forewing veins (character 16, state 1). Based on Hagen (1856) and Smithers (1972), Psoquilla forewing veins are distinct but much simpler or more reduced than other genera. In Yoshizawa et al. (2006) and de Moya et al. (2021), both analyses are based on a single species of Psoquillidae. According to Yoshizawa et al. (2006), Psoquillidae and Trogiidae are treated as sister taxa, and in de Moya et al. (2021), Psoquillidae was placed as the sister group of Lepidopsocidae. The relationships among Psoquillidae, Lepidopsocidae, and Trogiidae remain unclear while the monophyly of Psoquillidae still requires further studies. The monophyly of Trogiidae is well-supported by an apparent apomorphic character, i.e., reduced forewing (character 9, state 1), as also suggested by a previous study (Mockford, 1993). Mockford (1993) and Yoshizawa et al. (2006) suggested the monophyly of Lepidopsocidae was supported by body covered by scales, which was also recovered by the present analyses.
Based on the differences in the forewing setae arrangement and nodulus condition, Empheriidae and Archaeatropidae have been treated as two different families. However, these families share a lot of similarities in amber deposit (Spain, France, Myanmar), living period, and morphological characters such as number of flagellomeres, shape of maxillary palps, wing shape and venation, and shape of external valves (Baz and Ortuño, 2001). The main differences between them are the rows of forewing veins setae (Empheriidae with two rows of setae vs. Archaeatropidae with one row of setae) and forewing nodulus (Empheriidae without nodulus vs. Archaeatropidae with nodulus) (Baz and Ortuño, 2000, 2001; Mockford et al., 2013). However, the forewing veins setae are not stable (Li et al., 2020). For example: Prospeleketor albianensis Perrichot et al., 2003 and Proprionoglaris axioperierga Azar et al., 2014 have been assigned to Archaeatropidae, but they have two rows of vein setae, and the Empheriidae species: Preempheria antiqua Baz and Ortuño, 2001 has one row of vein setae; Burmempheria has two rows of vein setae in Sc, A and Cu, but other veins have only one row; Jerseyempheria grimaldii Azar et al., 2010 with the setae covering the membrane of forewing. The forewing nodulus is also an unstable character (Wang et al., 2019) and cannot be used to distinguish these two families (Li et al., 2020). Li et al. (2020) mentioned that Empheriidae and Archaeatropidae are presumed to be closely related because of shared morphological conditions in the forewing veins setae and the setose anal area. The present phylogenetic analysis suggests for the first time that Empheriidae and Archaeatropidae form a monophyletic group supported by the membranous region of forewing with setae (character 12, state 1); forewing veins with setae (character 15, state 1). Based on these results, we conclude that Archaeatropidae Baz and Ortuño, 2000 should be treated as a junior synonymy of Empheriidae (Kolbe, 1884). As a result, Empheriidae now contains sixteen genera and twenty-five species in total (including this study) (Table 1).
This is the first study to explore the phylogenetics of the atropine families including the fossil taxa. To avoid reduction of tree resolution, we only selected well-preserved fossils and did not include highly autapomorphic genera (e.g., Jerseyempheria Azar et al., 2010 with the forewing membrane covered with setae). However, due to the limitations of fossil preservation, some important characters could not be observed from the fossils, and the phylogenetic placement of poorly preserved fossil taxa remains unknown. Further research is still needed to obtain more data using techniques, i.e., CT scanning, to obtain more information and to elucidate their relationship. Over the past 40 years, the phylogeny and taxonomy of Psocodea including their higher level of classification have been studied extensively based on the evidence of extant insects, including morphological and molecular systematics (Smithers, 1972; Yoshizawa, 2002; Perrichot et al., 2003; Yoshizawa and Johnson, 2003, 2006; Johnson et al., 2004, 2018; de Moya et al., 2021), but there are still some disagreement (Yoshizawa and Saigusa, 2001; Misof et al., 2014). The bias of sampling, the homogeneity of evidence, and the instability of taxonomic characteristics may be the important reasons for the above arguments. Therefore, it is important to integrate the fossil evidence and the modern insects to construct a phylogenetic tree in order to elucidate the morphological characteristics, origin, and evolutionary history of these insect groups.
The fossil records of Atropetae are mainly from Mesozoic, and twenty-four genera with twenty-nine species have been recorded during Cretaceous from Lebanon, Spain, France SW, Myanmar, New Jersey, and Siberia (Vishnyakova, 1975; Baz and Ortuño, 2000, 2001; Perrichot et al., 2003; Azar and Nel, 2004, 2011; Azar et al., 2010, 2014, 2017; Hakim et al., 2018, 2021; Wang et al., 2019; Li et al., 2020; Corentin et al., 2021; Cumming and Le Tirant, 2021; Liang and Liu, 2021). Only ten genera with thirteen species are known during Cenozoic from the Baltic region, France Oise, FuShun, and Tanzania (Hagen, 1856, 1865, 1866, 1882; Enderlein, 1911; Nel et al., 2005; Azar et al., 2018). The earliest fossil record was from Lebanon (Cretaceous, Lower Barremian). Most extant species of Atropetae live in tropical and subtropical regions (Smithers, 1972, 1999; New, 1975; Mockford, 1991; Lienhard, 2000). During the Cretaceous and Cenozoic, environment about the locality of the fossil record is mainly under a warm and humid tropical or subtropical climate (Scotese, 2002). Research showed that Myanmar had a tropical forest palaeoenvironment during the Cretaceous (Grimaldi et al., 2002; Shi et al., 2022), which also met their requirements for living environment. Over the 100 million years of evolution, the distributional range of Atropetae is probably mainly restricted to warm and humid areas. The Atropetae insects may be an indication of an ancient environment.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Zoobank, https://zoobank.org/52D5D725-1731-4855-86CB-4C2714781C65, https://zoobank.org/e490780d-ac0e-4bf7-a9f1-5ee8f0b01316, (urn:lsid:zoobank.org:act:8500CE76-8F78-402B-AB7D-376B939FC0A9) and (urn:lsid:zoobank.org:act:89231F37-E4CC-44BA-85F0-CCDE453FB3AA). Further enquires can be directed to the corresponding author(s).
SL, YY, and DR designed the research. SL conceived the study with support from KY, DR, and YY. DR and YY provided the materials. SL and QW took the photographs and prepared the line drawings. SL, KY, and QW performed the morphological analysis. SL and KY conducted the phylogenetic analyses. KY and MB revised the draft. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Nos: 31970436, 31730087, 32101239, and 32020103006), Joint Fund of the Beijing Municipal Natural Science Foundation and Beijing Municipal Education Commission (KZ201810028046), and Japan Society for the Promotion of Science (19H03278).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Charles Lienhard, editor, and reviewers for constructive criticism and valuable comments on the manuscript. We thank Yongjie Wang, Ruiqian Wang, and Xiangbo Guo of Capital Normal University, Chaofan Shi of Sun Yat-sen University, Xiaodan Lin of Hainan University, Xinyu Li of China Agricultural University, Feiyang Liang of Hunan University of Science and Technology, and Josh Jenkins Shaw of Natural History Museum of Denmark for helping with data collection and phylogenetic analysis, and providing valuable comments and suggestions.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.907903/full#supplementary-material
Supplementary Figure 1 | The most parsimonious tree obtained by TNT analysis. Phylogeny of Atropetae based on 18 species and 38 characters, with characters being nonadditive, under equal weighting. Analyzed with TNT v1.5. * Representsthe genus originally placed in Archaeatropidae.
Azar, D. A., Nel, A., and Perrichot, V. (2014). Diverse barklice (Psocodea) from Late Cretaceous Vendean amber. Paleontol. Contribut. 10, 9–15.
Azar, D., and Nel, A. (2004). Four new Psocoptera from Lebanese amber (Insecta: Psocomorpha: Trogimorpha). Ann. Soc. Entomol. Fr. 40, 185–192. doi: 10.1080/00379271.2004.10697415
Azar, D., and Nel, A. (2011). The oldest psyllipsocid booklice, in Lower Cretaceous amber from Lebanon (Psocodea, Trogiomorpha, Psocathropetae, Psyllipsocidae). Zookeys 130, 153–165. doi: 10.3897/zookeys.130.1430
Azar, D., Huang, D. Y., El-Hajj, L., Cai, C. Y., Nel, A., and Maksoud, S. (2017). New Prionoglarididae from Burmese amber (Psocodea: Trogiomorpha: Prionoglaridetae). Cretac. Res. 75, 146–156. doi: 10.1016/j.cretres.2017.03.028
Azar, D., Maksoud, S., Nammour, C., Nel, A., and Wang, B. (2018). A new trogiid genus from lower Eocene Fushun amber (Insecta: Psocodea: Trogiomorpha). Geobios 51, 101–106. doi: 10.1016/j.geobios.2018.02.002
Azar, D., Nel, A., and Petrulevièius, V. (2010). First Psocodean (Psocadea, Empheriidae) from the Cretaceous Amber of New Jersey. Acta Geol. Sin.-Engl. Ed. 84, 762–767. doi: 10.1111/j.1755-6724.2010.00255.x
Badonnel, A. (1951). “Psocoptères,” in Traité de Zoologie, ed. P. P. Grassé (Paris: Masson), 1301–1340.
Badonnel, A. (1986). Psocoptères de Colombie (Insecta, Psocoptera). Missions écologiques du Professeur Sturm (1956 à 1978). Spixiana 9, 179–223. doi: 10.5962/bhl.part.82381
Baz, A. (1993). Revision of the Cerobasis annulata group (Psocoptera: Trogiidae) from the Canary Islands. Zoo. Med. 67, 487–504.
Baz, A., and Ortuño, V. M. (2000). Archaeatropidae, a new family of Psocoptera from the Cretaceous amber of Alava, northern Spain. Ann. Entomol. Soc. Am 93, 367–373. doi: 10.1603/0013-87462000093[0367:AANFOP]2.0.CO;2
Baz, A., and Ortuño, V. M. (2001). New genera and species of empheriids (Psocoptera: Empheriidae) from the Cretaceous amber of Alava, northern Spain. CretacRes 22, 575–584. doi: 10.1006/cres.2001.0275
Casasola González, J. A. (2006). Phylogenetic relationships of the genera of Epipsocetae (Psocoptera: Psocomorpha). Zootaxa 1194, 1–32.
Corentin, J., Yoshizawa, K., Hakim, M., Huang, D. Y., and Nel, A. (2021). New psocids (Psocodea: Prionoglarididae, Psyllipsocidae) from Cretaceous Burmese amber deposits. CretacRes 126:104890. doi: 10.1016/j.cretres.2021.104890
Cumming, R. T., and Le Tirant, S. (2021). Review of the Cretaceous †Archaeatropidae and †Empheriidae and description of a new genus and species from Burmese amber (Psocoptera). Faunitaxys 9, 1–11.
de Moya, R. S., Yoshizawa, K., Kimberly, K. O. W., Andrew, D. S., Christipher, H. D., and Johnson, K. P. (2021). Phylogenomics of parasitic and non-parasitic lice (Insecta: Psocodea): Combining sequence data and Exploring compositional bias solutions in Next Generation Datasets. Syst. Biol. 70, 719–738, doi: 10.1093/sysbio/syaa075
Engel, M. S. (2020). Myanmar: palaeontologists must stop buying conflict amber. Nature 584, 525. doi: 10.1038/d41586-020-02432-z
Goloboff, P. A. (1997). NoName (NONA), Version 2.0. Fundación Instituto Miguel Lillo, Tucumán: Program and Documentation.
Goloboff, P. A., and Catalano, S. A. (2016). TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238. doi: 10.1111/cla.12160
Grimaldi, D. A., and Engel, M. S. (2006). Fossil Liposcelididae and the lice ages (Insecta: Psocodea). Proc. Royal Soc. B. 273, 625–633. doi: 10.1098/rspb.2005.3337
Grimaldi, D. A., Engel, M. S., and Nascimbene, P. C. (2002). Fossiliferous Cretaceous Amber from Myanmar (Burma): Its Rediscovery, Biotic Diversity, and Paleontological Significance. Am. Mus. Novit. 3361, 1–71. doi: 10.1206/0003-008220023612.0.CO;2
Hagen, H. (1856). “Die im Bernstein befindlichen Neuropteren der Vorwelt,” in Die im Bernstein Befindlichen Organischen Reste der Vorwelt, eds G. C. Berendt, F. J. Pictet de la Rive, and H. A. Hagen (Berlin: Gerhard), 1–122.
Hagen, H. (1876). Contributions to the natural history of Kerguelen Island, made in connection with the United States transit of Venus Expedition, 1874-75. II. Bull. U. S. Natl. Museum 2, 1–122.
Hagen, H. (1882). Beitrage zur Monographie der Psociden. über Psociden im Bernstein. Stettin. Entomol. Zeitung 43, 217–237.
Hakim, M., Azar, S., Maksoud, S., Huang, D. Y., and Azar, D. (2018). New polymorphic psyllipsocids from Burmese amber (Psocodea: Psyllipsocidae). Cretac. Res. 84, 389–400. doi: 10.1016/j.cretres.2017.11.027
Hakim, M., Huang, D. Y., and Azar, D. (2021). New fossil psocids from Cretaceous Siberian ambers (Psocodea: Trogiomorpha: Atropetae). Palaeoentomology 4, 186–198. doi: 10.11646/palaeoentomology.4.2.8
Huang, D. Y., Bechly, G., Nel, P., Engel, M. S., Prokop, J., Azar, D., et al. (2016). New fossil insect order Permopsocida elucidates major radiation and evolution of suction feeding in hemimetabolous insects (Hexapoda: Acercaria). Sci. Rep. 6:23004. doi: 10.1038/srep23004
Illiger, J. C. W. (1798). Kugelann Verzeuchniss der Käfer Preussens, entworfen von J. G. Kugelann, ausgearbeitet von Illiger, mit einer Vorrede von Hellwig und dem angehängten Versuch einer natürlichen Ordnung und Gattungsfolge der Insecten. Halle: Bei Johann Jacob Gebauer.
Johnson, K. P., Dietrich, C. H., Friedrich, F., Beutel, R. G., Wipfler, B., Peters, R. S., et al. (2018). Phylogenomics and the evolution of hemipteroid insects. PANS 115, 12775–12780. doi: 10.1073/pnas.1815820115
Johnson, K. P., Yoshizawa, K., and Smith, S. V. (2004). Multiple origins of parasitism in lice. Proc. Royal Soc. B. 271, 1771–1776. doi: 10.1098/rspb.2004.2798
Kolbe, H. J. (1882). Neue Psociden der paläarktischen Region. Entomol. Nachrichten Berlin. 8, 207–212.
Kolbe, H. J. (1884). Der Entwickelungsgang der Psociden im Individuum und in der Zeit. Berlin. Entomol. Zeitschrift 28, 35–38.
Kolbe, H. J. (1885). Zur Kenntniss der Psociden-Fauna Madagaskars. Berlin. Entomol. Zeitschrift 29, 183–192.
Li, F. S. (2002). Psocoptera of China. Beijing: National Natural Science Foundation of China, Science Press.
Li, S., Wang, Q. Z., Ren, D., and Yao, Y. Y. (2020). New genus and species of Empheriidae (Psocodea: Trogiomorpha) from mid-Cretaceous amber of northern Myanmar. CretacRes 110:104421. doi: 10.1016/j.cretres.2020.104421
Liang, F. Y., and Liu, X. Y. (2021). A new species of Psyllipsocus (Psocodea: Trogiomorpha: Psyllipsocidae) from the mid-Cretaceous amber of Myanmar. Zootaxa 5072, 081–087. doi: 10.11646/zootaxa.5072.1.9
Liang, F. Y., Li, S., Liu, X. Y., Bai, M., and Yao, Y. Z. (in press). A new genus and species of the family Archaeatropidae (Psocodea: Trogiomorpha) from mid-Cretaceous amber of Northern Myanmar. CretacRes 105233. doi: 10.1016/j.cretres.2022.105233
Lienhard, C. (2000). A new desert psocid from Namibia (Insecta: Psocoptera: Trogiidae). Rev. Suisse Zool. 107, 277–281.
Lienhard, C. (2004). Siamoglaris zebrina gen. n., sp. n., the first representative of Prionoglarididae from the Oriental Region (Insecta: Psocoptera). Rev. Suisse Zool. 111, 865–875.
Lienhard, C. (2011). A new species of Siamoglaris from Thailand with complementary description of the type species (Psocodea: ‘Psocoptera’: Prionoglarididae). Rev. Suisse Zool. 118, 293–306.
Lienhard, C., and Smithers, C. N. (2002). Psocoptera (insecta): World Catalogue and Bibliography. Geneva: Muséum d’Histoire Naturelle de Genève.
Lin, X. D., Labandeira, C. C., Shih, C. K., Hotton, C. L., and Ren, D. (2019). Life habits and evolutionary biology of new two-winged long-proboscid scorpionflies from mid-Cretaceous Myanmar amber. Nat. Commun. 10:1235. doi: 10.1038/s41467-019-09236-4
Misof, B., Liu, S. L., Meusemann, K., Peters, R. S., Donath, A., Mayer, C., et al. (2014). Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767. doi: 10.1126/science.1257570
Mockford, E. L. (1991). New species and records of Psocoptera (Insecta) From Roraima State, Brazil. Acta Amazon 21, 211–318.
Mockford, E. L. (1993). North American Psocoptera (Insecta). Flora and Fauna Handbook. Gainesville, FL: Sandhill Crane Press.
Mockford, E. L. (2005). New Genus of Perientomine Psocids (Psocoptera: Lepidopsocidae) with a Review of the Perientomine Genera. Trans. Am. Entomol. Soc. 131, 201–215.
Mockford, E. L., and Garcia Aldrete, A. N. (2010). Psoquilla infuscata Badonnel (Psocoptera: Psoquillidae) in the Western Hemisphere with description of the male and brachypterous form. Zootaxa 2618, 61–68. doi: 10.11646/zootaxa.2618.1.4
Mockford, E. L., Charles, L., and Yoshizawa, K. (2013). Revised classification of “Psocoptera” from Cretaceous amber, a reassessment of published information. Insect. Matsumurana N. S. 69, 1–26.
Nel, A., Prokop, J., De Ploeg, G., and Millet, J. (2005). New Psocoptera (Insecta) from the lowermost Eocene amber of Oise, France. J. Syst. Palaeontol. 3, 371–391. doi: 10.1017/S1477201905001598
New, T. R. (1975). Lepidopsocidae and Aamphientomidae (Psocoptera) from Malaysia and Singapore. Orient. Insects 9, 177–194. doi: 10.1080/00305316.1975.10434490
Nixon, K. C. (2002). WinClada, Version 1.00.08. New York, NY: Program and Documentation. Cornell University Press.
Pearman, J. V. (1936). The taxonomy of the Psocoptera: preliminary sketch. Proc. Royal Soc. B. 5, 58–62. doi: 10.1111/j.1365-3113.1936.tb00596.x
Perrichot, V., Azar, D., Néraudeau, D., and Nel, A. (2003). New Psocoptera in the Early Cretaceous amber of SW France and Lebanon (Insecta: Psocoptera: Trogiomorpha). Geol. Mag. 140, 669–683. doi: 10.1017/S0016756803008355
Pictet-Baraban, F., and Hagen, H. (1856). “Die im Bernstein befindlichen Neuroptera der Vorwelt,” in Die im Bernstein Befindlichen Organischen Reste der Vorwelt, ed. G. Berendt (Berlin: Nicholaische Buchhandlung), 57–64.
Scotese, C. R. (2002). Goal of the PALEOMAP Project. Available online at: http://www.scotese.com (accessed October 20, 2020).
Selys-Longchamps, E. D. (1872). Note on two new genera of Psocidae. Entomol. Month. Magazine 9, 145–146.
Shi, C., Cai, H. H., Jiang, R. X., Wang, S., Engel, M. S., Yuan, J., et al. (2021). Balance scientific and ethical concerns to achieve a nuanced perspective on ‘blood amber’. Science 351, 926–926.
Shi, C., Wang, S., Cai, H. H., Zhang, H. R., Long, X. X., Tihelka, E., et al. (2022). Fire-prone Rhamnaceae with South African affinities in Cretaceous Myanmar amber. Nat. Plants 8, 125–135. doi: 10.1038/s41477-021-01091-w
Smithers, C. N. (1972). The classification and phylogeny of the Psocoptera. Memo. Aust. Museum 14, 1–349.
Smithers, C. N. (1999). First records of Pteroxanium marrisi n.sp. and Haplophallus maculatus (Tillyard) for the Chatham Islands and list of Psocoptera from the subantarctic islands of New Zealand. Nz. Entomol. 22, 9–13. doi: 10.1080/00779962.1999.9722050
Vishnyakova, V. N. (1975). Psocoptera in Late Cretaceous insect-bearing resins from Taimyr. Entomol. Obozrenie 54, 94–96.
Wang, R. Q., Li, S., Ren, D., and Yao, Y. Z. (2019). New genus and species of the Psyllipsocidae (Psocodea: Trogiomorpha) from mid-Cretaceous Burmese amber. Cretac. Res. 104:104178. doi: 10.1016/j.cretres.2019.07.008
Wang, S., Shi, C., Zhang, Y. J., Hu, G. X., and Gao, L. Z. (2016). Trading away ancient amber’s secrets. Science 351, 926–926. doi: 10.1126/science.351.6276.926-a
Yoshizawa, K. (2002). Phylogeny and higher classification of suborder Psocomorpha (Insecta: Psocodea: ‘Psocoptera’). Zool. J. Linn. Soc. 136, 371–400. doi: 10.1046/j.1096-3642.2002.00036.x
Yoshizawa, K. (2005). Morphology of Psocomorpha (Psocodea: ‘Psocoptera’). Ins. matsum. N. S. 62, 1–44.
Yoshizawa, K., and Johnson, K. P. (2003). Phylogenetic position of Phthiraptera (Insecta: Paraneoptera) and elevated rate of evolution in mitochondrial 12S and 16S rDNA. Mol. Phylogenet. Evol. 29, 102–114. doi: 10.1016/S1055-7903(03)00073-3
Yoshizawa, K., and Johnson, K. P. (2006). Morphology of male genitalia in lice and relatives and phylogenetic implications. Syst. Entomol. 31, 350–361. doi: 10.1111/j.1365-3113.2005.00323.x
Yoshizawa, K., and Lienhard, C. (2020). †Cormopsocidae: A new family of the suborder Trogiomorpha (Insecta: Psocodea) from Burmese amber. Entomol. Sci. 23, 208–215. doi: 10.1111/ens.12414
Yoshizawa, K., and Saigusa, T. (2001). Phylogenetic analysis of paraneopteran orders (Insecta: Neoptera) based on forewing base structure, with comments on monophyly of Auchenorrhyncha (Hemiptera). Syst. Entomol. 26, 1–13. doi: 10.1046/j.1365-3113.2001.00133.x
Yoshizawa, K., Lienhard, C., and Johnson, K. P. (2006). Molecular systematics of the suborder Trogiomorpha (Insecta: Psocodea: “Psocoptera”). Zool. J. Linn. Soc. 146, 287–299. doi: 10.1111/j.1096-3642.2006.00207.x
Keywords: Archaeatropidae, new taxon, phylogeny, synonymy, Trogiomorpha, psocoptera
Citation: Li S, Yoshizawa K, Wang Q, Ren D, Bai M and Yao Y (2022) New Genus and Species of Empheriidae (Insecta: Psocodea: Trogiomorpha) and Their Implication for the Phylogeny of Infraorder Atropetae. Front. Ecol. Evol. 10:907903. doi: 10.3389/fevo.2022.907903
Received: 30 March 2022; Accepted: 04 May 2022;
Published: 09 June 2022.
Edited by:
Chenyang Cai, Nanjing Institute of Geology and Paleontology (CAS), ChinaReviewed by:
Dany Azar, Lebanese University, LebanonCopyright © 2022 Li, Yoshizawa, Wang, Ren, Bai and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunzhi Yao, eWFveXoxMDBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.