
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 28 July 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.906322
This article is part of the Research Topic Duetting and Turn-Taking Patterns of Singing Mammals: From Genes to Vocal Plasticity, and Beyond View all 14 articles
 Chiara De Gregorio1*
Chiara De Gregorio1* Anna Zanoli1
Anna Zanoli1 Filippo Carugati1
Filippo Carugati1 Teresa Raimondi1
Teresa Raimondi1 Daria Valente1
Daria Valente1 Valeria Torti1
Valeria Torti1 Longondraza Miaretsoa1
Longondraza Miaretsoa1 Andry Rajaonson2
Andry Rajaonson2 Marco Gamba1
Marco Gamba1 Cristina Giacoma1
Cristina Giacoma1Parent-offspring interactions are essential to interpret animal social evolution and behavior, but their role in mediating acoustic communication in animals that interact vocally is still unclear. Increasing evidence shows that primate vocal communication is way more flexible than previously assumed, and research on this topic can provide further information on how the social environment shaped vocal plasticity during the evolution of the Primate order. Indris communicate through elaborated vocal emissions, usually termed songs. Songs are interactive vocal displays in which all members of the family group alternate their emissions, taking turns during chorusing events. We aimed to understand whether specific rules regulate the turn-taking of different group members and investigate the flexibility of indris’ vocal behavior when co-singing with their offspring. We found that social factors can influence the turn-taking organization in a chorus, as offspring were more likely to drop out from the parents’ duet than join in, and we speculate that overlap might signal competition by members of the same-sex. The duet between the reproductive pair was the most common type of singing organization, followed by a duet between mothers and sons and the triadic interaction between mother, father, and son. Interestingly, parents’ solo singing seems to stimulate offspring to vocalize, and we also found that mothers and fathers simplify, at least in part, song elaboration when chorusing with offspring. Our results indicate that indris can perform short-time adjustments to the number of co-emitters and their identity: our approach is advantageous in highlighting the multilevel influences on primate vocal flexibility. Moreover, it provides evidence that some aspects of our vocal plasticity were already present in the lemur lineage.
Animals of different species interact vocally in their natural environment (Tobias et al., 2016; De Gregorio et al., 2022). Individuals can adapt their vocal behavior to other emitters during these interactions to produce coordinated vocal displays, such as duets or choruses (Gamba et al., 2016). The interplay between emitters is a crucial feature of human conversations, but the level of non-human animals’ flexibility in vocal exchanges is still debated (Levinson, 2016). This topic has attracted great interest because of its possible implications for language evolution and similarity with human conversational rules (Chow et al., 2015; Pika et al., 2018).
Increasing evidence shows that the ability to take turns is widespread in different groups of primates. In New World monkeys, for example, squirrel monkeys (Saimiri sciureus) adjust the timing of their vocal exchange depending on the co-caller identity (Masataka et al., 1986), while in marmosets (Callithrix jacchus), the emission of different call types can be affected by the timing of another individual’s vocalization (Liao et al., 2018). Chimpanzees (Pan troglodytes) modify their vocalizations to promote chorusing with social partners (Fedurek et al., 2013a), and duetting gibbons can adapt their contribution to that of the other pair-member (Hylobates pileatus, Traeholt et al., 2006; Nomascus leucogenys, Deputte, 1982). Moreover, gibbons’ ability to adapt their vocal behavior to an external factor (e.g., forced partner exchange or predator presence) also emerged in siamangs (Symphalangus syndactylus, Geissmann, 1999) and white-handed gibbons (Hylobates lar, Clarke et al., 2006).
In particular, vocal interactions can occur between adults and juveniles of many species and are often crucial for developing adult-like vocal communication (humans, Goldstein and Schwade, 2008; birds, Chen et al., 2016; primates, Koda et al., 2013), enhancing vocal production learning (i., the ability to change the structure of vocalizations due to hearing others). In birds, for example, this process can occur by listening to a tutor (Mennill et al., 2018) or during direct interactions between older and younger animals (Rivera-Cáceres et al., 2018; Carouso-Peck et al., 2020).
While there is extensive work on birds’ juvenile-tutor vocal interactions, these mechanisms have been scarcely investigated in primates. Previous studies examined the antiphonal calling of the common marmoset (C. jacchus, Chow et al., 2015; Takahashi et al., 2015) and the co-singing interaction of gibbons (Hylobates agilis, Koda et al., 2013). These works suggest that parents could instantaneously influence juvenile/infants’ vocalizations. Nevertheless, many of these investigations focused on the offspring side of the interaction (e.g., Takahashi et al., 2016), highlighting infant vocal developmental trajectories shaped by adult feedback, while parents’ vocal behavior remained almost unexplored. Koda et al. (2013) provided an interesting case, showing that, in gibbons, mothers had a more stereotyped singing pattern when singing together (co-singing) with daughters than when singing alone. This evidence suggests that the identity of a co-singer can shape individual vocal behavior, but this is not the only feature to consider when investigating individual contributions in collective displays.
Human conversations can occur between more than two people, and the number of people participating can influence turn-taking dynamics (Sacks et al., 1974). As in humans, animal vocal interactions can occur with many participating individuals and varying degrees of overlap between emitters (Passilongo et al., 2015; Torti et al., 2018). In birds, for example, chorusing can often involve two males and one female or two females and one male, and the temporal organization of individuals’ contribution may favor or avoid overlap (Monias benschi, Seddon, 2002; Pheugopedius euophrys, Mann et al., 2006). The composition of the social group can also influence chorus structure and duration (Dacelo novaeguineae, Reyer and Schimdl, 1988).
More than two group members’ simultaneous emission of utterances occurs in different primate species, such as the pant-hoot chorusing of chimpanzees (P. troglodytes, Fedurek et al., 2013b) or the roaring bouts of howler monkeys (Alouatta pigra, Horwich and Gebhard, 1983). Despite chorusing occurring quite commonly in many singing primate species, the majority of work on this behavior is still rather descriptive (De Gregorio et al., 2022), and the extent to which the number of conspecifics in a choral display can influence the individual contribution remains pretty much unexplored. Taken altogether, these pieces of evidence suggest that many animals can adjust their utterances to external factors, such as the vocal behavior of co-emitter (e.g., sexual partner, offspring, preferred social partner), but it is unclear whether also the number of those co-emitters can regulate the structure of vocalizations in interacting animals.
We aimed to fill the gap about understanding adult changes during singing with offspring by investigating parent-offspring singing interactions in the indris (Indri indri). Besides possessing a rich vocal repertoire (Valente et al., 2019), indris are the only singing lemurs. They live in family groups (Bonadonna et al., 2019; Rolle et al., 2021) in the eastern rainforest of Madagascar, where every member can simultaneously participate in the choral display (Torti et al., 2017, 2018). Units of different types composing indri’s songs can be emitted alone (single notes) or organized in phrases of two to six units (Zanoli et al., 2020), with shorter phrases (i.e., including two and three units) more likely to be included in the songs (Valente et al., 2021). Indris emit units and phrases with a precise rhythmic pattern (De Gregorio et al., 2021a). Songs serve different functions, such as inter-and intra-group communication and territory defense (Torti et al., 2013; Bonadonna et al., 2020). Indris’ songs are sex-specific duets between males and females (Giacoma et al., 2010), where the calls can be given alternated or simultaneously. One or two additional individuals may participate in the vocal displays (De Gregorio et al., 2019) so that animals can take turns within the same song. Thus, individuals join in, and others drop out during the same song.
Field observations suggest that sexual competition between parents and offspring of the same-sex can occur (Bonadonna et al., 2014), as observed in birds (Seddon et al., 2002), and it could be of interest to understand if chorusing dynamics can reflect this competition. Therefore, we hypothesize that singing behavior in the indris can be regulated by balancing the competition in singing among group members to advertise their identity or mated/unmated status. Furthermore, avoiding excess overlapping between singers allows for maintaining the communicative function of the vocal display. Therefore, we predict that turn-taking behavior among individuals will not be random. However, it will show specific trajectories as, for example, adult individuals are more likely to sing together than with juveniles. Our second hypothesis is that parent’s vocal behavior can enhance offspring’s vocal development: as social factors and auditory feedback seems to mediate the development of singing behavior (De Gregorio et al., 2021b), we predict that (I) co-singing interactions would affect the temporal structure of parents’ songs, in line with the idea that social influences might shape temporal regulation of utterances (Henry et al., 2015). We also predicted that (II) parents will utter less elaborated songs when co-singing with their offspring, agreeing with previous gibbons’ findings (Koda et al., 2013). Our approach allows disentangling different aspects of social influences on parents’ contribution, as we will consider not only the identity of co-singers (pair mate, male offspring, and female offspring) but also their numerosity, as previous work showed that the number of singers in a chorus might influence the individual performance (Gamba et al., 2016; De Gregorio et al., 2019).
We collected data in the Maromizaha New Protected Area (Eastern Madagascar: 18° 56′ 49″ S, 48° 27′ 53″ E), with field observations conducted between 6:00 am and 1:00 pm, from 2010 to 2020, for a total of 63 months. We recorded spontaneous songs from a close distance (between 2 and 10 m) of 8 reproductive pairs from 8 habituated groups of indris. We performed the recordings using Sound Devices 702T, Olympus LS-100 and LS05, and Tascam DR-100, DR-40, and DR-05 with semi-directional microphones (ME 67 and AKG CK 98) oriented toward the vocalizing individuals. We set the recorders at a sampling rate of 44.1 kHz and an amplitude resolution of 16 bit during all the recording sessions. Files were saved in wav format. We were able to recognize all animals individually based on their natural marks. Our dataset comprised 440 duets and choruses (of two or more than two individuals, respectively), resulting in 826 individual contributions uttered by 16 individuals within eight reproductive pairs. Indris uttered 260 of the contributions during cosinging interactions with offspring (female offspring, Nsongs = 84; male offspring, Nsongs = 176).
Indris’ songs usually start with a series of roars, harsh emissions that are supposed to have an “attention gather” function (e.g., Hopkins et al., 2007). After that, indris emit a variable number of “long notes” (LN), which are longer and less modulated than the subsequent units. After those, we can find isolated units (or “single notes,” SN) or units organized in phrases of descending fundamental frequency (“descending phrases,” DP) that can include 2–6 units. We analyzed the indris’ choruses using Praat 6.0.56 (Boersma and Weenink, 2007).
We identified the contribution to the song of each singer using annotations in Praat TextGrids via visual inspection of the spectrograms and fieldwork notes. Spectrograms had a 0–7,000 dB view range, with a window of 0.006 s and 60.0 dB of dynamic range. First, we annotated the onset and offset of each unit for each contribution and labeled it according to the singer’s identity (Mother, Father, Son, Daughter). We labeled each unit according to its type and position (e.g., being part of a phrase or not, position within the phrase). Since the core of the indris’ songs relies on descending phrases and single notes, we focused our analysis on these vocal types: SN and DPs (DP2, DP3, DP4, DP5, DP6 based on the number of units forming the phrase). Next, we labeled silent gaps within units according to their position (Supplementary Figure 1): “inter” for silent intervals between different DPs, and “intra” for silent intervals between units of the same DP (De Gregorio et al., 2019). We used a custom Praat script to extract each interval duration (Gamba et al., 2015). To evaluate the rhythmic structure of parents’ contributions, we imported the duration of the intervals in R (R Core Team, 2020; version 3.4.3) to calculate the inter-onset intervals (IOI) within (WP) and between phrases (BP; De Gregorio et al., 2019, 2021a).
We then focused on parents’ contributions (Mothers and Fathers), and we labeled each overlapping part of the song according to the number and identity of vocalizing individuals. We did it by annotating when an animal would join the song and the exact timing in which it would stop singing. When an offspring started vocalizing during a silent interval between two units of a parent’s contribution, we considered the whole interval part of the singing interaction. We did the same when an individual stopped singing in between the silent gap of one of the parents’ contributions. We obtained eight types of co-singing associations (Figure 1): M-F for mothers’ contribution when duetting with fathers, F-M for fathers’ contributions when duetting with mothers, M-S for mothers’ contribution when duetting with sons, F-D for fathers’ contributions when duetting with daughters, M-F-S for mothers’ contribution when singing with fathers and sons, F-M-S for fathers’ contribution when singing with mothers and sons, and the same for M-F-D and F-M-D. We used the code F for fathers and M for mothers for the portion where parents sang “solo phrases” without overlapping with other family members. We then transformed each contribution into a string of consecutive co-singing types.
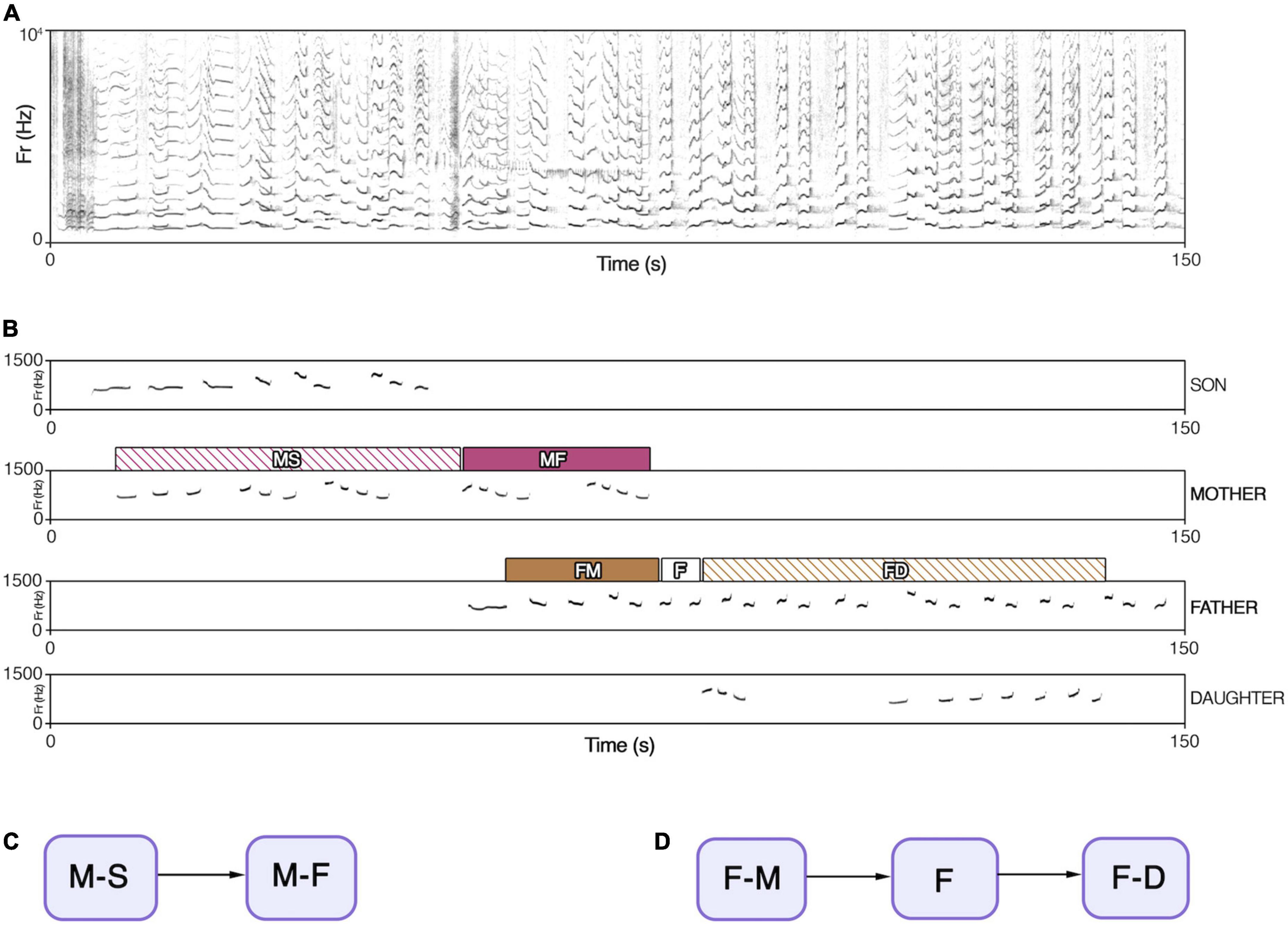
Figure 1. (A) A spectrogram of an indri chorus. (B) Individual contributions extracted from panel (A); the parents’ type of co-singing is highlighted by different color bands. MS, mother duetting with son; MF, mother duetting with father; FM, father duetting with mother; F, fathers’ solo units, FD, father duetting with daughter. (C) Flow diagram representing mother’s co-singing transitions, extracted from panel (B). (D) Flow diagram representing fathers’ co-singing transition, extracted from panel (B).
We transformed each parents’ song into a string of labels representing the phrases’ concatenation within an individual contribution, separated by a break symbol (e.g., SN| DP2| DP3| DP3| DP4). Then, we separated each string into different co-singing types, and we obtained 483 strings for males and 663 for females. To investigate if co-singing with offspring would affect parents’ song features, we used two measures of song elaboration: (a) the Levenshtein distance (hereafter, LD): a logic distance expressing the minimum cost to convert a sequence into another one (Kohonen, 1985), which has already been proven to be a robust quantitative approach for investigating animal acoustic sequences (Kershenbaum and Garland, 2015); (b) The Normalized Phrase Diversity: an index indicating the diversity of the individual contribution, calculated as the number of different vocal types emitted during a particular co-singing interaction, normalized for the total number of elements uttered during that interaction.
To investigate if singing with offspring would influence the song’s rhythmic structure (between phrase Inter-onset intervals, or bpIOI), we used a linear mixed model (LMM, lmer function of lme4 package, Bates et al., 2015). Before fitting the model, bpIOI was log-transformed since it did not show a normal distribution and then used as a response variable; we used the interaction between parent identity and the type of co-singing as a fixed factor. In addition, we included the singer’s identity and the specific song contribution from which we extracted the IOIs as nested random factors. Finally, we used the Tukey test (within the multiple contrast package multcomp in R) to perform all pairwise comparisons for all levels of the interaction (Bretz et al., 2010). To investigate if singing with offspring would influence the phrase rhythmic structure (within-phrase IOI, or wpIOI), we used a generalized linear mixed model (GLMM, glmmTMB package, Brooks et al., 2017), fitting a beta distribution as suggested by the package fitdistrplus (Delignette-Muller and Dutang, 2015) as a suitable theoretical distribution. We used wpIOI as the response variable and the interaction between parent identity and co-singing as a fixed factor. In addition, we included the singer’s identity and the specific song contribution from which we extracted the IOIs as nested random factors. We verified the assumptions of normality and homogeneity of residuals for both models via visual inspection of the qqplot and the residuals’ distribution (a function provided by R. Mundry). We also excluded the presence of collinearity among predictors considering variance inflation factors (vif package, Fox and Weisberg, 2011). To test for the significance of our full models (Forstmeier and Schielzeth, 2011), we compared them against null models containing only the random factors, with a likelihood ratio test (Anova with argument test “Chisq”, Dobson, 2002). We report estimates, standard error (SE), z-, and p-values for the Tukey test.
To understand the mechanisms governing the process of taking turns in indri choruses, we ran strings representing the dynamics of each singing event in Behatrix software (version 0.9.13, Friard and Gamba, 2021). This software independently generates the code for a flowchart representing the transitions between behaviors and performs a permutation test to indicate the statistical significance associated with the different transitions. We considered only the cases in which there is at least an alternation between singers, thus excluding songs consisting of only one type of duet or one type of co-singing with three individuals. Thus, our dataset comprised 203 parents’ contributions for this analysis, 135 for mothers and 68 for fathers. We used a 5% cut-off on the total number of transitions. First, we generated a flow diagram with the transitions from one co-singing condition to the next, with the percentage values of transition relative occurrences. Then, we ran a permutation test based on observed counts of the transitions between different co-singing types (Random permutation test in Behatrix). We permuted the strings 10,000 times, providing an accuracy of 0.001 of the probability values, and we obtained p-values for each transition between different co-singing conditions. Finally, we analyzed mothers’ and fathers’ co-singing transitions separately to evaluate how chorusing dynamics would influence each parents’ singing, and we calculated the frequency of different co-singing types for mothers and fathers.
To investigate differences in the combinatorics (i.e., the concatenation of phrases) between mothers and fathers in each co-singing type, we calculated the LD for each pair of strings in Behatrix (version 0.9.13, Friard and Gamba, 2021). First, we obtained a squared matrix composed of the distances between each pair of strings in the dataset. Next, we labeled the files according to the identity of the mother/father and the type of co-singing (e.g., fathers: FM, FD, FMS, MFD; mothers MF, MS, MFS, MFD) during which it was emitted. Then, we investigated whether mothers and fathers differed in their degree of variability depending on the co-singing type. Due to the juvenile singing variability and sample size (De Gregorio et al., 2021b), we did not consider offspring sex (i.e., MFO: the contribution of a mother singing with her pair-mate and an offspring). We averaged the LDs by labels to calculate the within- and between-labels average for each mother and father in each co-singing type using R (R Core Team, 2020). Finally, we performed four Mantel tests (9,999 randomizations) using in each test a reduced matrix with the mean LDs for the labels of interest against a matrix containing zero when the matching labels were of the same co-singing type (Krull et al., 2012), and one when they were of different co-singing type (vegan R-package; Oksanen et al., 2013). We checked the admissible number of permutations for our matrices through the function numPerms of the same R-Package. We then analysed differences between the mothers/fathers’ LDs in different co-singing types and between mothers’ and fathers’ LDs in the same co-singing type (MF vs. FM, MF vs. MFO, FM vs. FMO, MFO vs. FMO) by using the paired sample t-test. Finally, we verified the normal distribution of each label with a Shapiro-Wilk test for normality (built-in R-package stats) and computed the test’s power with the pwr.t.test function (pwr R-package; Champely et al., 2018).
To investigate differences in the composition of parents’ contributions in the different co-singing types, we calculated the Normalized Diversity for each contribution as the sum of each DP type normalized on the total number of DPs composing the string (stringr R-package; Wickham, 2019). Then, we ran a linear mixed model (LMM, lme4 R-package; Bates et al., 2015) to investigate whether the co-singing type influenced the contributions’ diversity. The model included log-transformed normalized diversity as the response variable, the co-singing type as the fixed factor and song and individual identity as the nested random factors. The co-singing type was a categorical variable indicating in which of the 16 co-singing categories (8 for fathers, 8 for mothers) each individual contribution was emitted. First, we verified the normal distribution and homogeneity of the model residuals through a qqplot and residuals against fitted values plot (a function provided by R. Mundry). Next, we compared the full model with a null model comprising only the random factor, we used a likelihood ratio test (Anova test with the “Chisq” argument; Dobson, 2002) and we calculated the p-values for predictors using a likelihood ratio test between the full and the null model (Barr et al., 2013). Finally, we performed all pairwise comparisons for the levels of the factor co-singing type with the Tukey test (R-package multcomp Bretz et al., 2010).
The sequential analysis of co-singing types indicated that both fathers and mothers showed non-random turn-taking behavior in co-singing dynamics. In particular, six out of ten possible transitions occurred above chance for fathers (solid lines in Figure 2A). Co-singing with mothers only followed co-singing with sons and pair-mates (F-M-S→F-M, p < 0.001). After this duetting, fathers’ solo phrases (F) take place (F-M→F, p = 0.002), followed by duets with the daughters (F→F-D, p = 0.005). Daughters can join the duet between the reproductive couple (F-M→F-M-D, p = 0.001), and, from that singing organization, they usually drop out from the interaction (F-M-D→F-M, p < 0.001), leaving only fathers and mothers singing (F-M). Additionally, after duetting only with mothers (F-M), fathers would duet with mothers and their son (F-M→F-M-S, p = 0.016). The other possible transitions did not occur significantly more than chance (dotted lines in Figure 2). Moreover, fathers most commonly duetted with mothers (52% of cases, F-M), followed by co-singing with mothers and their sons (F-M-S, 20% of cases) and then by co-singing interaction with mothers and daughters (M-F-D, 15% of cases). On the other hand, duetting with daughters (F-D, 7% of cases) and solo phrases (F, 6% of cases) were less frequent.
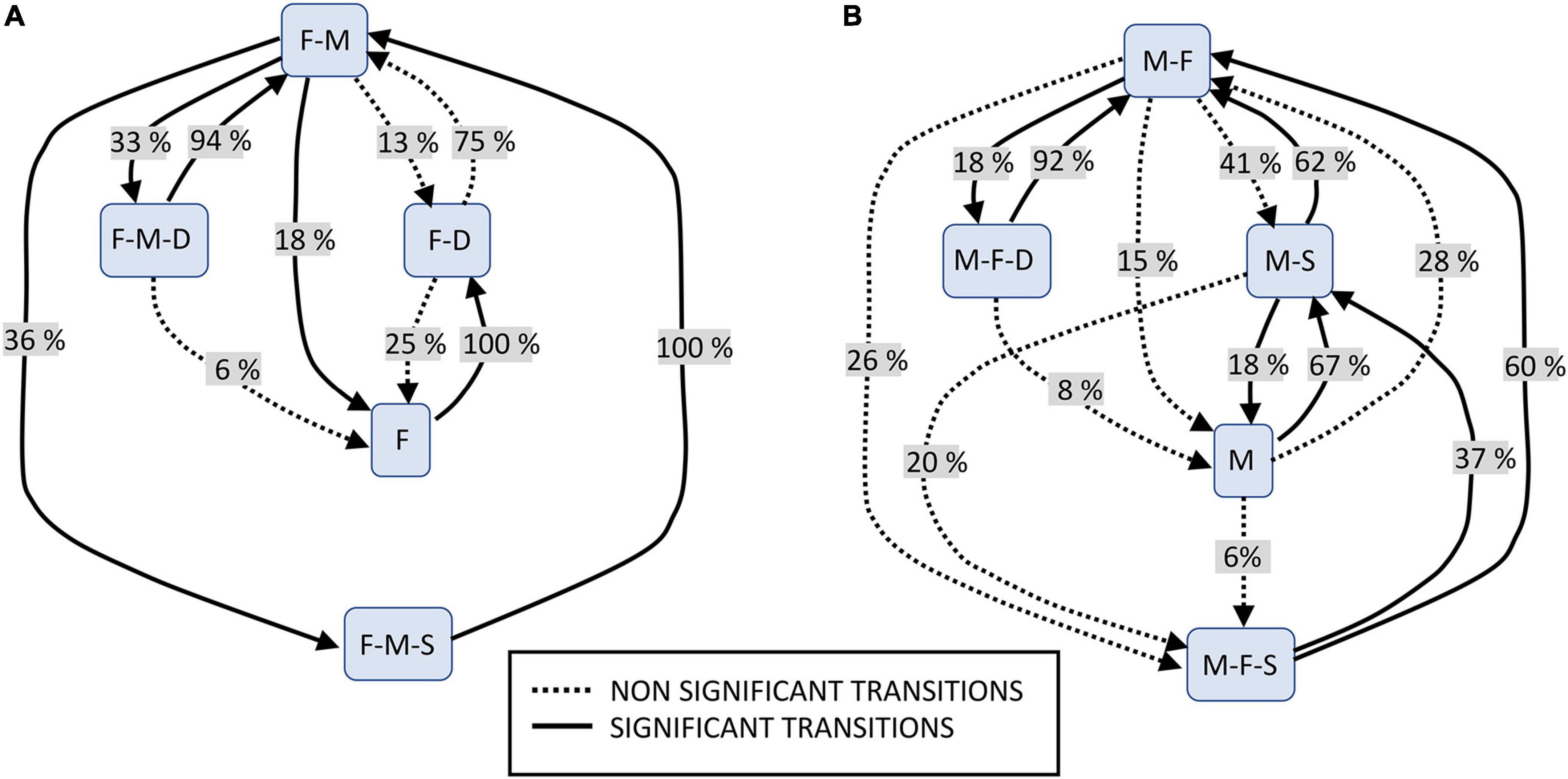
Figure 2. Flow diagram showing the occurrence of transitions (%) between different types of co-singing. (A) Fathers: FM, fathers’ contribution when duetting with mothers; FMS, when duetting with mothers and sons; FD, when duetting with daughters; FMS, when duetting with mothers and sons; F, fathers’ phrases with no overlap with any group members. (B) Mothers: MF, mothers’ contribution when duetting with fathers; MFD, when duetting with fathers and daughters; FMS, when duetting with fathers and sons; MS, when duetting with sons; M, mothers’ phrases with no overlap with any group member.
For mothers, seven out of 15 possible transitions occurred above chance (solid lines in Figure 2B). Co-singing with fathers and their offspring (both sons and daughters) was followed by duetting with fathers only (M-F-S→M-F, p < 0.001; M-F-D→M-F, p < 0.001), meaning that either daughters or sons ceased singing while their parents kept vocalizing. Still, mothers would also sing with their daughters and partners after duetting only with their partners (M-F→M-F-D, p < 0.001), even if this transition was less likely to occur than the opposite one. Moreover, mothers’ solo phrases (M) occurred before (M→M-S, p = 0.002), but also after duetting with their sons (M-S→M, p = 0.008). As for fathers, also for mothers, the most common co-sing type was duetting with their pair mate (42% of cases), followed by duetting with their sons (30% of cases). Singing with both sons and fathers occurred in 14% of co-singing interaction, while the involvement of daughters was more unusual (M-F-D), occurring in 5% of cases, even less than solo phrases (M, 9%).
The comparison between the full and null model for the between-phrases IOI showed that the two models were significantly different (χ2 = 38.877, df = 13, p < 0.001). The Tukey test indicated that mothers had significantly longer inter-onset intervals between phrases (bpIOI) when co-singing with their sons, compared to co-singing with their pair-mates (p < 0.003). The same was not true for fathers since we found no differences in the bpIOI values between co-singing with daughters or their pair-mates. Moreover, the Tukey test did not show any differences in bpIOI depending on the number of individuals involved. We reported the detailed results for the models and the Tukey tests in Supplementary Table 1.
On the other hand, the comparison between the full and null models for the within-phrase IOI (wpIOI) did not reach statistical significance (χ2 = 8.637, df = 13, p = 0.471) and thus, the fixed factors did not affect the duration of the inter-onset intervals between units given within a particular phrase.
We analyzed 1,051 parents’ contributions composed of 17,326 phrases. We found a significant difference between the LDs calculated for mothers and fathers when duetting between parents (MF vs FM Mantel test: r = 0.125, p = 0.013). Mothers showed higher average individual means (mean LD = 16 ± 1.92) than fathers (mean LD = 11.8 ± 0.89; Paired t-test: t = 5.0407, df = 7, p = 0.001). When considering parents’ phrase combinations when singing with other two individuals (the other pair-member plus one offspring), we found that mothers and fathers did not differ from each other (Figure 3B; MFO vs FMO, Mantel test: r −0.03467, p = 0.968). We also found that mothers showed a more stereotyped singing pattern when singing with their partner and one offspring than when singing with their partners only (Figures 3A, 4, MF vs MFO, Mantel test: r = 0.3478, p < 0.001), with higher average individual means when duetting with fathers (mean LD: 16 ± 1.92) than in the chorus including the offspring (mean LD = 6.1 ± 3.68; t-test: t = −4.2556, df = 7, p = 0.004; Figure 3A). The same was true for fathers, whose LD values were significantly different when duetting with their pair mate than when co-singing with their pair mate and one offspring (Figure 3B; FM vs MFO, Mantel test: r = 0.2303, p = 0.005). As for mothers, fathers had higher average individual means when duetting with their pair (mean LD: 11.8 ± 0.89) than in the chorus with also the offspring (mean LD = 6.1 ± 3.68; t-test: t = 11.293, df = 7, p < 0.001; Figure 3A).
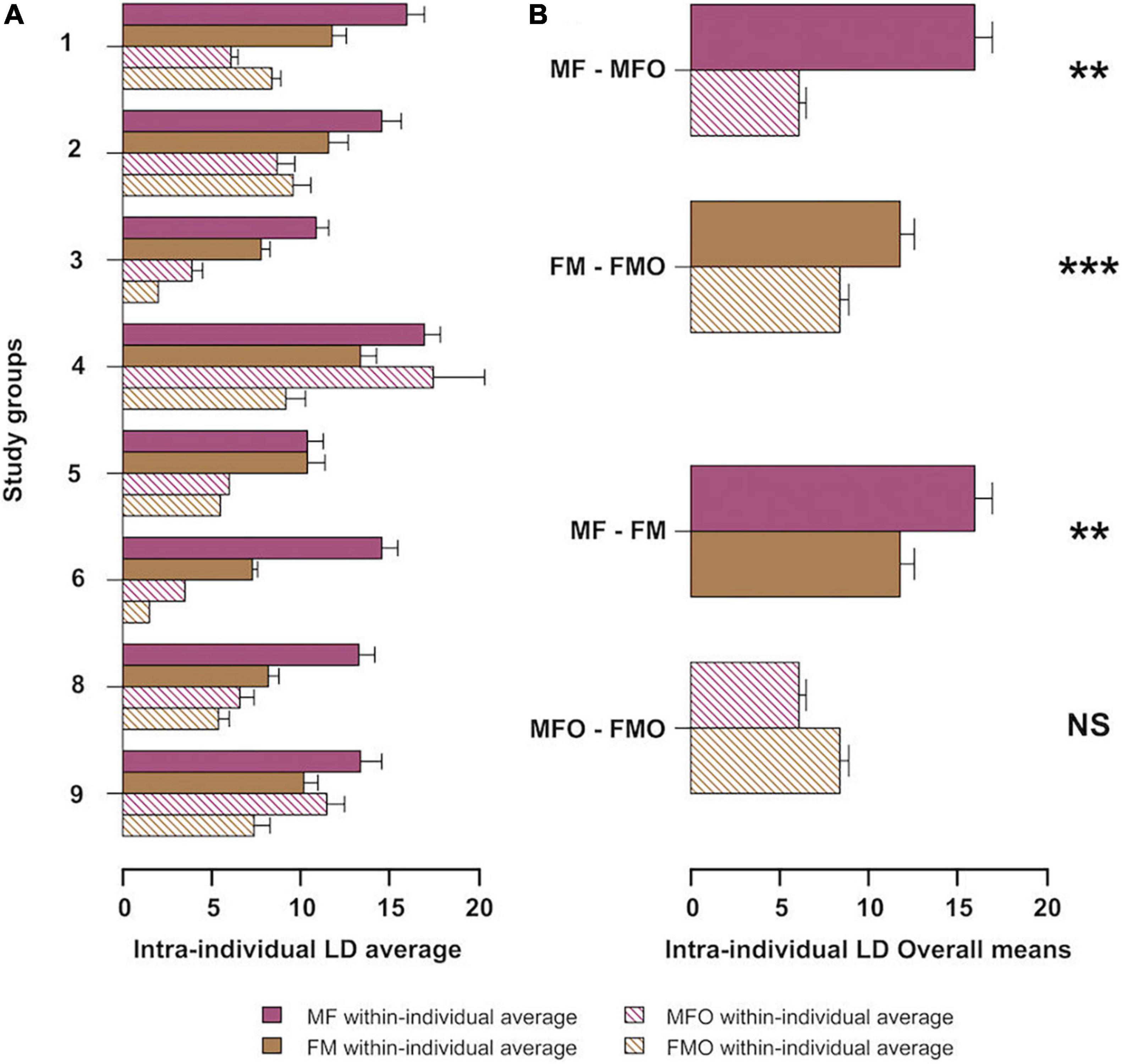
Figure 3. The average Levenshtein distance (LD) for mothers and fathers in the two co-singing conditions: singing only with the pair-mate (MF, mothers’ contribution when duetting with fathers, FM, fathers’ contribution when duetting with mothers; color-filled barplot) and singing with the pair-mate and an offspring (MFO, mothers’ contribution when duetting with fathers and offspring; FMO, fathers’ contribution when duetting with mothers and offspring; striped barplot). Capped lines represent standard deviation. (A) LD within mothers and fathers for the eight studied groups (B) Overall LD average between mothers and fathers; t-test significance at p < 0.001 is denoted by ***, at p = 0.005, and p = 0.004 is denoted by **.
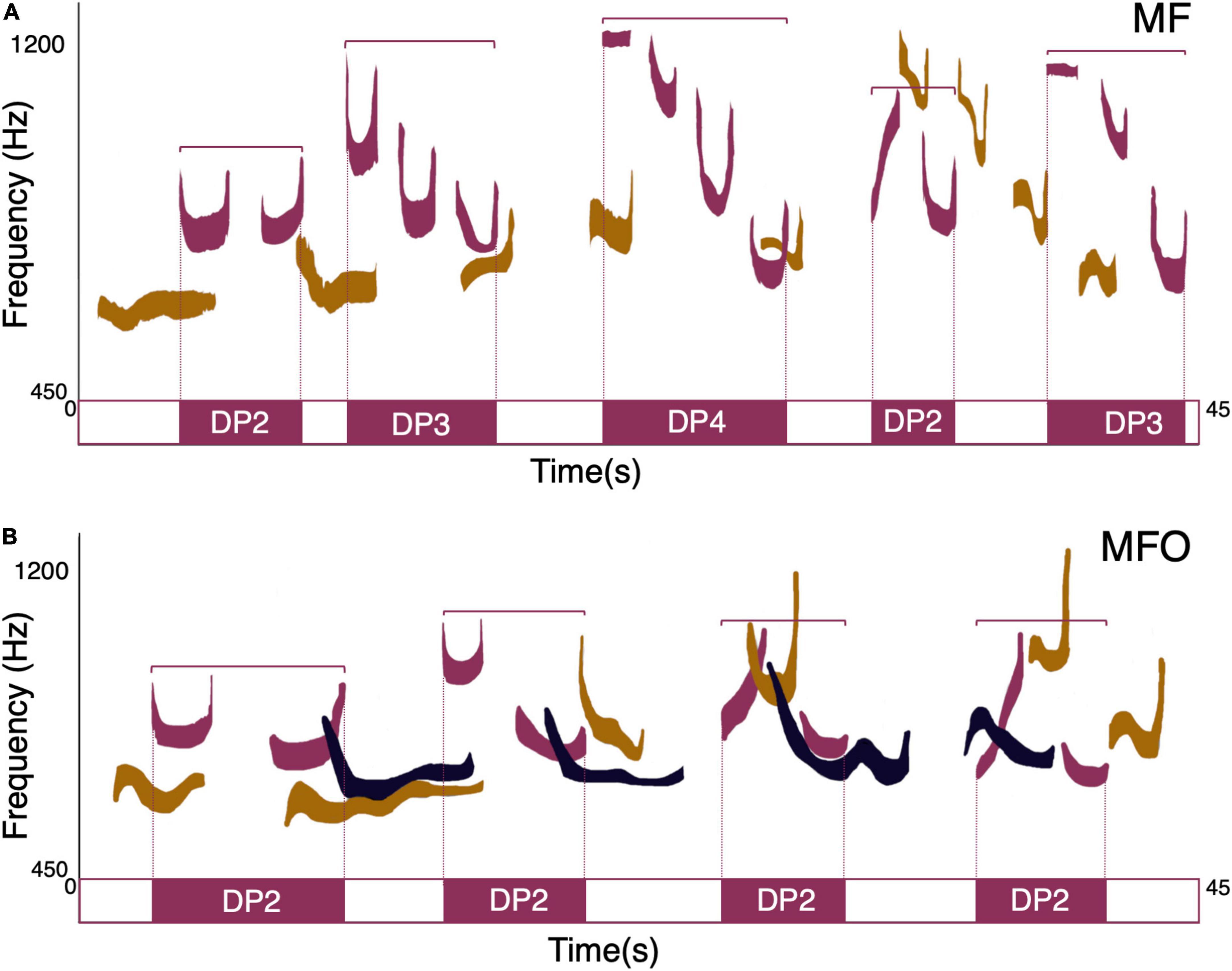
Figure 4. Schematic representation of mothers’ different phrases’ concatenation in two different cosigning types. (A) Mother (in purple) duetting with their pair-mate (MF). (B) Mother singing with the pair-mate and one offspring (MFO). The fundamental frequency profile of mother’s, father’s, and offspring’s contribution to the song is highlighted in purple, light brown, and blue, respectively.
When investigating the diversity of phrases forming the individual contributions of mothers and fathers in the different co-singing types, we found that the full model significantly differed from the null model (χ2 = 69.692, df = 7, p < 0.001). We reported estimate, SE, z- and p-values for all the pairwise comparisons of the Tukey test in Supplementary Table 2. When considering duets between mothers and fathers, we found that mothers showed less diversity than fathers (FM-MF, Figure 5). Moreover, mothers had a higher diversity when singing with their pair and an offspring than in a duet. In other words, we found an effect of the numerosity of individuals singing together, with mothers being more diverse when singing in a chorus of three individuals including the pair and one offspring (whichever its sex) than when in a duet (either with the other member of the reproductive pair or with an offspring, regardless of its sex).
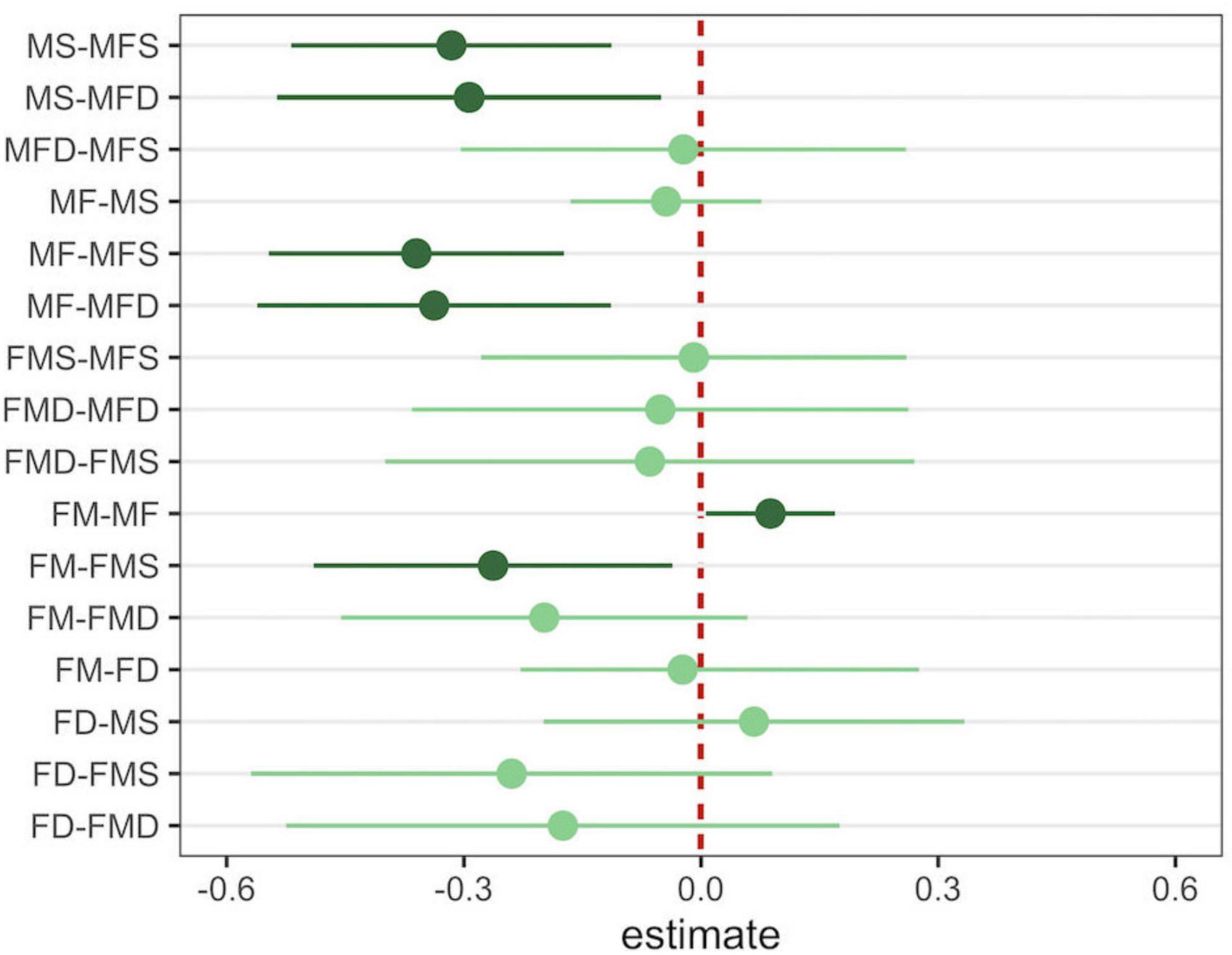
Figure 5. Plot showing all pairwise comparisons resulting from the Tukey test. Dark green points and lines represent the estimate and 95% confidence interval of significant comparisons. Light green points and lines represent the estimate and 95% confidence interval of non-significant comparisons. M, mothers; F, fathers; D, daughters; S, sons.
We found a different pattern for fathers, with a partial influence of the number of co-singers on the phrase diversity. In particular, we found a significant difference when comparing fathers singing with the other member of the reproductive pair and fathers singing in a triadic chorus including the pair and a son (FM-FMS, Figure 5). We found no difference in phrase diversity when comparing a father singing with the other member of the pair or when a daughter joined the chorus (FM-FMD, Figure 5). Lastly, when considering three individuals singing together, we found no effect of the co-singing types on the phrase diversity regardless of the chorus’s composition for both mothers and fathers (i.e., MFD vs MFS, FMD vs MFS).
We examined turn-taking dynamics in the choruses emitted by the indris’ family groups, and we found that the alternation between different singers is not casual but follows specific trajectories. Moreover, we investigated whether co-singing interactions with sons and daughters affected the song structure of adult indris, and we found that co-singing would influence both the rhythmic structure and the song elaboration.
Our work indicated that, within chorusing dynamics, the duet was the most common type of song organization for parents. However, we also found that duetting with the opposite-sex offspring is quite common for mothers but not fathers. A possible explanation for this difference is that sons remain in their natal group longer than daughters (De Gregorio et al., 2021b).
In particular, we found that offspring were more likely to drop out from the parents’ duet than join in. The inverse dynamic was infrequent and occurred when fathers concluded their singing and left sons duetting with their mothers. Also, the emission of parents’ solo phrases was always linked to offspring joining the song. This confirms our first prediction: turn-taking behavior among indris is not random but shows specific trajectories. Our results align with black-crested gibbons’ singing dynamics, in which the majority of duet bouts are given by the adult pair, with the adult male initiating the song. Still, occasionally the juvenile male starts calling first and duets with the adult female before giving up the turn to the adult male (Nomascus concolor, Fan et al., 2009). Offspring of both sexes are more likely to drop out from triadic singing interactions with parents in the indris. This evidence is in line with the idea that overlap between same-sex singers might involve singing competition, similar to what studies on gibbons suggested. When the juvenile male sang first, the adult male promptly started singing before the adult female (Fan et al., 2009).
Indri duets are composed of sex-specific song contributions (Giacoma et al., 2010). Thus, a new participant joining the song would emit the same unit types of one of the singers while coordinating the emission with the other. In human conversations, the overlap between participants has been considered a troublesome feature (Schegloff, 2000), and it could manifest one person’s willingness to take the floor (Sacks et al., 1974). Similarly, the overlap between two birds of the same-sex has been considered a signal of aggression (Naguib and Kipper, 2006) and threat by the animal that starts singing before the other has finished vocalizing (Mennill and Ratcliffe, 2004; Baker et al., 2012). In kookaburras, for example, parent-offspring sexual conflicts would manifest through aggressive interactions linked to the participation of offspring in the adult chorus (Parry, 1973; Reyer and Schimdl, 1988). Therefore, we argue that the overlap of indris’ utterances might signal an individual willingness to prevail over the other in singing. In siamangs, young individuals join the parents’ chorus more frequently as they mature (S. syndactylus, Chivers, 1976) and, although these mechanisms have not been investigated in indris yet, we can suppose that the older the offspring gets, the more competition in singing with parents of the same-sex can take place before the offspring disperse. In the case of the replacement of the dominant female, the dominant male and his mature son would compete for mating with the new female, by singing or by physical aggressions (De Gregorio, pers. obs.).
Parents’ solo singing can also be critical in regulating and motivating offspring to sing. Our results suggest that both mothers and fathers sang alone before duetting with the offspring of the opposite-sex. This finding aligns with the idea that adults might provide offspring with vocal stimulation for their song development, as auditory feedback and practicing might be essential for song maturation (De Gregorio et al., 2021b). Similarly, Merker and Cox (1999, Nomascus gabriellae) reported that, in gibbons, the mother-juvenile co-singing interactions were always initiated by mothers. Hence, if there might be some competition in singing, why do parents encourage sons and daughters to sing? For birds, it has been suggested that group singing might be involved in territorial defense (Mann et al., 2006), as chorusing can inform how many individuals are present in a given territory and, implicitly, their willingness to defend it.
Duetting birds may communicate their ability to engage in aggressive interactions through the degree of vocal coordination (Diniz et al., 2021). Furthermore, during territorial encounters between different indris’ groups, both parents and offspring participate in the territorial song (Torti et al., 2013). Thus, maintaining the vocal coordination in the chorus could be essential to indicate the ability or motivation of the family group to defend their resources cooperatively. Also, chorusing behavior might be favored in a territorial context as the overlap of vocalizations can enhance their transmission (Rehberg-Besler et al., 2017). Finally, indris can discriminate between neighboring and non-neighboring singing family groups (Spezie et al., 2022), suggesting that they might vocally recognize members of the nearby groups. Thus, the offspring’s presence, identity, and status should be regularly broadcast to other indris’ families.
Summarizing, turn-taking dynamics in the indris’ choruses consist in a trade-off between the need for young animals to participate in the chorus in order to practice and to broadcast their unmated status (Gamba et al., 2016; De Gregorio et al., 2021b), and the willingness of their same-sex-parents to renounce to their possibility to advertise their mated status and their presence. Therefore, we can conclude that the probability of an animal singing seems to be influenced by family’s social dynamics.
We found that mothers, but not fathers, had longer bpIOI when duetting with sons than when duetting with their pair-mate, thus partially confirming our second prediction. Indeed, mothers slowed down the rate of phrase emissions only when duetting with their sons might indicate that variation in song temporal structure can result from a vocal adjustment performed to facilitate offspring vocal development. This interpretation agrees with what was suggested for gibbons’ mother-daughter vocal interactions (H. agilis, Koda et al., 2013) and marmosets, whose mothers offered a vocal reinforcement to offspring exhibiting appropriate turn-taking (C. jacchus, Chow et al., 2015). It is interesting to notice that in humans, slowed infant-directed speech benefits child language learning (Raneri et al., 2020), and human caregivers can improve vocal articulation in stuttering children by slowing down their child-directed speech (Sawyer et al., 2017).
If this evidence suggests that this behavior might enhance sons’ vocal development, it remains unclear why there are such differences with father-daughter co-singing. An alternative explanation would be that, given that indri females are notably more flexible than males (Torti et al., 2017; De Gregorio et al., 2019; Zanoli et al., 2020), mothers are simply adjusting their timing to sons’ utterances to improve synchronization, even with a less experienced singer. Still, we found that duets between fathers and daughters occur more rarely than mother-son duets. Moreover, in line with previous work (De Gregorio et al., 2019), for both parents, the duration of the inter-onset intervals between two different phrases was not affected by the number of individuals (either one or two) singing simultaneously, independently of the identity and sex of co-singers. Considering the inter-onset intervals between units, the type of co-singing did not have a statistical effect on its duration. This result is in line with previous work on the indris’ song evidencing how notes within a phrase are more constrained than the organization of phrases within a song, as this trait does not change during ontogeny (Gamba et al., 2016; De Gregorio et al., 2021b).
We examined differences in parents’ song elaboration during different co-singing interactions, and we found that both the number and identity of co-singers can influence the sequential organization of phrases and their diversity. In particular, our results confirm what was previously found by Zanoli et al. (2020), namely that adult females are more variable in their phrases’ combination than adult males when singing together. We also found that fathers and mothers did not differ when a third individual joined the chorus, but their contributions became most stereotyped. Moreover, mothers had lower phrase diversity than fathers during duetting interactions. Nevertheless, mothers uttered more diverse contributions when offspring of both sexes joined the chorus. The same was true for fathers only when the male offspring joined the pair’s duet.
According to changes in adults’ phrase concatenation, the increase in chorus size is in line with previous work showing that the number of singers can influence the duration of both contribution (the total time spent singing) and phonation (the cumulative duration of the emitted notes, without considering silent gaps) of a female individual in a chorus (De Gregorio et al., 2019). Still, in our case, a variation of phrase combination occurs in both sexes. We suggest two possible, non-mutually exclusive, explanations: first, that it could be an effect of adults adjusting their singing behavior to maintain the coordination of utterances when a third animal joins the chorus (De Gregorio et al., 2019); second, mothers and fathers emit more stereotyped contributions when co-singing with offspring to facilitate them (Koda et al., 2013). Since the third animal joining the pair is always an offspring in our dataset, this analysis did not allow us to separate the influence of the number of co-singers and their identity and/or sex on parents’ phrase organization. On the other hand, the number of co-singers seems to have a stronger impact on the diversity of phrases uttered by adult indris. Indeed, mother and father did not vary this feature during duets with the pair-mate compared to duets with the opposite-sex offspring. This is in contrast to findings on humans, where parents simplify their speech during vocal interactions with children by using fewer unique words (Elmlinger et al., 2019). On the one hand, parents increased their phrase’s diversity when co-singing with two individuals. On the other hand, fathers’ contributions were less influenced by chorus size: they showed a more extensive phrase repertoire only when co-singing with their pair-mate and their sons, in agreement with previous studies (Torti et al., 2017). This result is interesting as it suggests that fathers could face major pressure when singing with an individual of the same-sex so that they might differentiate their singing to better broadcast their individuality. Thus, we can only partially confirm our third prediction: parents uttered less elaborated songs when co-singing with offspring. Parents’ phrases were more stereotyped in terms of combination but were more diverse in terms of phrases type during vocal interactions with their pair-mate and offspring.
Future works might consider the longitudinal development of co-singing with parents to understand whether these interactions influence vocal development. A focus on the acoustic resemblance between parents and offspring over time would also be beneficial to understanding whether parent-offspring similarity increases or decreases during ontogeny and whether the sexes show similar trajectories. These findings would be useful for further investigating co-singing conditions and understanding which particular traits are typical of juvenile phases.
In conclusion, our work indicates that indris might regulate parents-offspring turn-taking dynamics in family choruses by different degrees of motivation for conflict or cooperation between parents and offspring. If overlap might signal competition by members of the same-sex, parents also seem to stimulate offspring singing behavior. Moreover, indris perform rapid adaptation not only to the number of co-emitters, as it strongly influences the elaboration of parents’ songs, but also to their identity, as it affected mothers’ rhythmic structure and fathers’ phrase diversity. This mechanism is similar to what happens in human language, where speakers can adapt their speech to their interlocutors (Lee et al., 2021). Our work demonstrates that the interplay between different emitters is a fundamental aspect to consider when investigating short-term flexibility in animals’ vocal behavior, and that the social environment is among the major determinants of indris’ song structure. Finally, we provide strong evidence that some of the traits that characterize human vocal plasticity were already in place in the lemur lineage, possibly providing a foundation for further evolutionary paths leading to the emergence of human language.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The non-invasive methods used for data collections of wild indris adhere to the International Primatological Society (IPS) ‘Principles for the Ethical Treatment of Non-Human Primates’. Field data collection protocols were reviewed and approved by Madagascar’s Ministere de l’Environnement, de l’Ecologie et des Forets. Field data collection protocols were also approved by GERP (Groupe d’Etude et de Recherche sur les Primates de Madagascar), the association governing research in the Maromizaha New Protected Area.
CDG: conceptualization. CDG, DV, AZ, TR, FC, AR, and MG: methodology. CDG, DV, VT, TR, and LM: investigation. CDG and MG: writing–original draft. VT, AZ, DV, LM, AR, and CG: writing–review and editing. FC, TR, AZ, and DV: visualization. MG and CG: supervision. All authors contributed to the article and approved the submitted version.
This research was supported by the University of Torino and the Parco Natura Viva—Centro Tutela Specie Minacciate, with the financial assistance of the European Union, through the Project BIRD (ACP SandT Program, contract FED/2009/217077).
We thank the local field guides and the field assistants helping during the data collection. We are also grateful to GERP (Groupe d’Etude et des Recherche sur les Primates de Madagascar) for their support during the field activities and to Cesare Avesani Zaborra and Caterina Spiezio for the financial and technical support. We also thank the editors and reviewers for their helpful suggestions during the revision process. We have received permits for this research, each year, from “Direction des Eaux et Forêts” and “Madagascar National Parks” (formerly ANGAP) [2010 (N°118/10/MEF/SG/DGF/DCB.SAP/SCBSE and N°293/10/MEF/SG/DGF/DCB.SAP/SCB), 2011 (N°274/11/MEF/SG/DGF/DCB.SAP/SCB), 2012 (N° 245/12/MEF/SG/DGF/DCB.SAP/SCB), 2014 (N°066/14/MEF/SG/DGF/DCB.SAP/SCB), 2015 (N°180/15/MEEMF/SG/DGF/DAPT/SCBT), 2016 (N°98/16/MEEMF/SG/DGF/DAPT/SCB.Re and N° 217/16/MEEMF/SG/DGF/DSAP/SCB.Re)], 2017 (73/17/MEEF/SG/DGF/DSAP/SCB.RE). 2018: 91/18/MEEF/SG/DGF/DSAP/SCB.Re; 2019: 118/19/MEDD/SG/DGEF/DSAP/DGRNE and 284/19/MEDD/SG/DGEF/DSAP/DGRNE; 2019/2020: 338/19/MEDD/SG/DGEF/DSAP/DGRNE]. Data collection did not require a permit for 2013 because it has been performed by Malagasy citizens only.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.906322/full#supplementary-material
Supplementary Figure 1 | Schematic representation of the spectrogram of the isolated fundamental frequencies of three Descending Prhases. The sound spectrogram displays time (s) on the x-axis, frequency (Hz) on the vertical axis. We describe acoustic parameter collection of bpIOI and wpIOI.
Baker, T. M., Wilson, D. R., and Mennill, D. J. (2012). Vocal signals predict attack during aggressive interactions in black-capped chickadees. Anim. Behav. 84, 965–974. doi: 10.1016/j.anbehav.2012.07.022
Barr, D. J., Levy, R., Scheepers, C., and Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 68, 255–278. doi: 10.1016/j.jml.2012.11.001
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed- effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bonadonna, G., Torti, V., De Gregorio, C., Valente, D., Randrianarison, R. M., Pozzi, L., et al. (2019). Evidence of genetic monogamy in the lemur Indri (Indri indri). Am. J. Primatol. 81:e22993. doi: 10.1002/ajp.22993
Bonadonna, G., Torti, V., Randrianarison, R. M., Martinet, N., Gamba, M., and Giacoma, C. (2014). Behavioral correlates of extra-pair copulation in Indri indri. Primates 55, 119–123. doi: 10.1007/s10329-013-0376-0
Bonadonna, G., Zaccagno, M., Torti, V., Valente, D., De Gregorio, C., Randrianarison, R. M., et al. (2020). Intra- and intergroup spatial dynamics of a pair-living singing primate, Indri indri: a multiannual study of three indri groups in Maromizaha forest, Madagascar. Int. J. Primatol. 41, 224–245. doi: 10.1007/s10764-019-00127-5
Bretz, F., Hothorn, T., and Westfall, P. (2010). Multiple Comparisons Using R. Boca Raton, FL: Chapman & Hall/CRC Press.
Brooks, M. E., Kristensen, K., Van Benthem, K. J., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.3929/ethz-b-000240890
Carouso-Peck, S., Menyhart, O., DeVoogd, T. J., and Goldstein, M. H. (2020). Contingent parental responses are naturally associated with zebra finch song learning. Anim. Behav. 165, 123–132. doi: 10.1016/j.anbehav.2020.04.019
Champely, S., Ekstrom, C., Dalgaard, P., Gill, J., Weibelzahl, S., Anandkumar, A., et al. (2018). Package ‘pwr’. Available online at: https://cran.r-project.org/web/packages/pwr/pwr.pdf (accessed February 10, 2022).
Chen, Y., Matheson, L. E., and Sakata, J. T. (2016). Mechanisms underlying the social enhancement of vocal learning in songbirds. Proc. Natl. Acad. Sci. U.S.A. 113, 6641–6646. doi: 10.1073/pnas.1522306113
Chivers, D. J. (1976). Communication within and between family groups of siamang (Symphalangus syndactylus). Behaviour 57, 116–135. doi: 10.1163/156853976X00136
Chow, C. P., Mitchell, J. F., and Miller, C. T. (2015). Vocal turn-taking in a non-human primate is learned during ontogeny. Proc. R. Soc. B 282:20150069. doi: 10.1098/rspb.2015.0069
Clarke, E., Reichard, U. H., and Zuberbühler, K. (2006). The syntax and meaning of wild gibbon songs. PLoS One 1:e73. doi: 10.1371/journal.pone.0000073
De Gregorio, C., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., Friard, O., et al. (2021a). Categorical rhythms in a singing primate. Curr. Biol. 31, R1379–R1380. doi: 10.1016/j.cub.2021.09.032
De Gregorio, C., Carugati, F., Estienne, V., Valente, D., Raimondi, T., Torti, V., et al. (2021b). Born to sing! Song development in a singing primate. Curr. Zool. 67, 585–596. doi: 10.1093/cz/zoab018
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. 1:15. doi: 10.1080/03949370.2021.2015451
De Gregorio, C., Zanoli, A., Valente, D., Torti, V., Bonadonna, G., Randrianarison, R. M., et al. (2019). Female indris determine the rhythmic structure of the song and sustain a higher cost when the chorus size increases. Curr. Zool. 65, 89–97. doi: 10.1093/cz/zoy058
Delignette-Muller, M. L., and Dutang, C. (2015). fitdistrplus: an R package for fitting distributions. J. Stat. Soft. 64, 1–34. doi: 10.18637/jss.v064.i04
Deputte, B. L. (1982). “Duetting in male and female songs of the white-cheeked gibbon (Hylobates concolor leucogenys),” in Primate Communication, eds C. H. Snowdon and M. R. Petersen (Cambridge: Cambridge University Press), 67–93.
Diniz, P., Ramos, D. M., Webster, M. S., and Macedo, R. H. (2021). Rufous horneros perceive and alter temporal coordination of duets during territorial interactions. Anim. Behav. 174, 175–185. doi: 10.1016/j.anbehav.2021.02.007
Dobson, A. J. (2002). An Introduction to Generalized Linear Models, 2nd Edn. Boca Raton, FL: Chapman and Hall.
Elmlinger, S. L., Schwade, J. A., and Goldstein, M. H. (2019). The ecology of prelinguistic vocal learning: parents simplify the structure of their speech in response to babbling. J. Child Lang. 46, 998–1011. doi: 10.1017/S0305000919000291
Fan, P. F., Xiao, W. E. N., Huo, S., and Jiang, X. L. (2009). Singing behavior and singing functions of black-crested gibbons (Nomascus concolor jingdongensis) at Mt. Wuliang, Central Yunnan, China. Am. J Primatol. 71, 539–547. doi: 10.1002/ajp.20686
Fedurek, P., Machanda, Z. P., Schel, A. M., and Slocombe, K. E. (2013b). Pant hoot chorusing and social bonds in male chimpanzees. Anim. Behav. 86, 189–196. doi: 10.1016/j.anbehav.2013.05.010
Fedurek, P., Schel, A. M., and Slocombe, K. E. (2013a). The acoustic structure of chimpanzee pant-hooting facilitates chorusing. Behav. Ecol. Sociobiol. 67, 1781–1789. doi: 10.1007/s00265-013-1585-7
Forstmeier, W., and Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 65, 47–55. doi: 10.1007/s00265-010-1038-5
Fox, J., and Weisberg, S. (2011). An R Companion to Applied Regression, 2nd Edn. Thousand Oaks, CA: SAGE Publications, Inc.
Friard, O., and Gamba, M. (2021). Behatrix: Behavioral Sequences Analysis with Per-Mutations Test. Available online at: http://www.boris.unito.it/pages/behatrix (accessed February 04, 2022).
Gamba, M., Friard, O., Riondato, I., Righini, R., Colombo, C., Miaretsoa, L., et al. (2015). Comparative analysis of the vocal repertoire of Eulemur: a dynamic time warping approach. Int. J. Primatol. 36, 894–910. doi: 10.1007/s10764-015-9861-1
Gamba, M., Torti, V., Estienne, V., Randrianarison, R. M., Valente, D., Rovara, P., et al. (2016). The Indris have got rhythm! Timing and pitch variation of a primate song examined between sexes and age classes. Front. Neurosci. 10:249. doi: 10.3389/fnins.2016.00249
Geissmann, T. (1999). Duet songs of the siamang, Hylobates syndactylus: II. Testing the pair-bonding hypothesis during a partner exchange. Behaviour 136, 1005–1039. doi: 10.1163/156853999501694
Giacoma, C., Sorrentino, V., Rabarivola, C., and Gamba, M. (2010). Sex differences in the song of Indri indri. Int. J. Primatol. 31, 539–551. doi: 10.1007/s10764-010-9412-8
Goldstein, M. H., and Schwade, J. A. (2008). Social feedback to infants’ babbling facilitates rapid phonological learning. Psychol. Sci. 19, 515–523. doi: 10.1111/j.1467-9280.2008.02117.x
Henry, L., Craig, A. J. F. K., Lemasson, A., and Hausberger, M. (2015). Social coordination in animal vocal interactions. Is there any evidence of turn-taking? The starling as an animal model. Front. Psychol. 6:1416. doi: 10.3389/fpsyg.2015.01416
Hopkins, W. D., Taglialatela, J., and Leavens, D. A. (2007). Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Anim. Behav. 73, 281–286. doi: 10.1016/j.anbehav.2006.08.004
Horwich, R. H., and Gebhard, K. (1983). Roaring rhythms in black howler monkeys (Alouatta pigra) of Belize. Primates 24, 290–296. doi: 10.1007/BF02381093
Kershenbaum, A., and Garland, E. C. (2015). Quantifying similarity in animal vocal sequences: which metric performs best? Methods Ecol. Evol. 6, 1452–1461. doi: 10.1111/2041-210X.12433
Koda, H., Lemasson, A., Oyakawa, C., Pamungkas, J., and Masataka, N. (2013). Possible role of mother-daughter vocal interactions on the development of species-specific song in gibbons. PLoS One 8:e71432. doi: 10.1371/journal.pone.0071432
Kohonen, T. (1985). Median strings. Pattern Recognit. Lett. 3, 309–313. doi: 10.1016/0167-8655(85)90061-3
Krull, C. R., Ranjard, L., Landers, T. J., Ismar, S. M., Matthews, J. L., and Hauber, M. E. (2012). Analyses of sex and individual differences in vocalizations of Australasian gannets using a dynamic time warping algorithm. J. Acoust. Soc. Am. 32, 1189–1198. doi: 10.1121/1.4734237
Lee, Y., Goldstein, L., Parrell, B., and Byrd, D. (2021). Who converges? Variation reveals individual speaker adaptability. Speech Commun. 131, 23–34. doi: 10.1016/j.specom.2021.05.001
Levinson, S. C. (2016). Turn-taking in human communication–origins and implications for language processing. Trends Cogn. Sci. 20, 6–14. doi: 10.1016/j.tics.2015.10.010
Liao, D. A., Zhang, Y. S., Cai, L. X., and Ghazanfar, A. A. (2018). Internal states and extrinsic factors both determine monkey vocal production. Proc. Natl. Acad. Sci. U.S.A. 115, 3978–3983. doi: 10.1073/pnas.1722426115
Mann, N. I., Dingess, K. A., and Slater, P. J. B. (2006). Antiphonal four-part synchronized chorusing in a Neotropical wren. Biol. Lett. 2, 1–4. doi: 10.1098/rsbl.2005.0373
Masataka, N., Biben, M., and Symmes, D. (1986). Temporal and structural analysis of affiliative vocal exchanges in squirrel monkeys (Saimiri sciureus). Behaviour 98, 259–273. doi: 10.1163/156853986X00991
Mennill, D. J., and Ratcliffe, L. M. (2004). Overlapping and matching in the song contests of black-capped chickadees. Anim. Behav. 67, 441–450. doi: 10.1016/j.anbehav.2003.04.010
Mennill, D. J., Doucet, S. M., Newman, A. E., Williams, H., Moran, I. G., Thomas, I. P., et al. (2018). Wild birds learn songs from experimental vocal tutors. Curr. Biol. 28, 3273–3278. doi: 10.1016/j.cub.2018.08.011
Merker, B., and Cox, C. (1999). Development of the female great call in Hylobates gabriellae: a case study. Folia Primatol. 70, 97–106. doi: 10.1159/000021680
Naguib, M., and Kipper, S. (2006). Effects of different levels of song overlapping on singing behaviour in male territorial nightingales (Luscinia megarhynchos). Behav. Ecol. Sociobiol. 59, 419–426. doi: 10.1007/s00265-005-0066-z
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R. B., et al. (2013). Package ‘vegan’. Community Ecology Package, Version 2. Available online at: http://CRAN.R-project.org/package=vegan (accessed February 15, 2022).
Parry, V. (1973). The auxiliary social system and its effect on territory and breeding in kookaburras. Emu 73, 81–100. doi: 10.1071/MU973081
Passilongo, D., Mattioli, L., Bassi, E., Szabó, L., and Apollonio, M. (2015). Visualizing sound: counting wolves by using a spectral view of the chorus howling. Front. Zool. 12:22. doi: 10.1186/s12983-015-0114-0
Pika, S., Wilkinson, R., Kendrick, K. H., and Vernes, S. C. (2018). Taking turns: bridging the gap between human and animal communication. Proc. R. Soc. Lond. B 285:20180598. doi: 10.1098/rspb.2018.0598
R Core Team (2020). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Raneri, D., Von Holzen, K., Newman, R., and Bernstein Ratner, N. (2020). Change in maternal speech rate to preverbal infants over the first two years of life. J. Child Lang. 47, 1263–1275. doi: 10.1017/S030500091900093X
Rehberg-Besler, N., Doucet, S. M., and Mennill, D. J. (2017). Overlapping vocalizations produce far-reaching choruses: a test of the signal enhancement hypothesis. Behav. Ecol. 28, 494–499. doi: 10.1093/beheco/arw176
Reyer, H. U., and Schimdl, D. (1988). Helpers have little to laugh about: group structure and vocalization in the laughing kookaburra Dacelo novaeguineae. Emu 88, 150–160. doi: 10.1071/MU9880150
Rivera-Cáceres, K. D., Quirós-Guerrero, E., Araya-Salas, M., Templeton, C. N., and Searcy, W. A. (2018). Early development of vocal interaction rules in a duetting songbird. R. Soc. Open Sci. 5, 171791. doi: 10.1098/rsos.171791
Rolle, F., Torti, V., Valente, D., De Gregorio, C., Giacoma, C., and Von Hardenberg, A. (2021). Sex and age-specific survival and life expectancy in a free ranging population of Indri indri (Gmelin, 1788). Eur. Zool. J. 88, 796–806. doi: 10.1080/24750263.2021.1947398
Sacks, H., Schegloff, E. A., and Jefferson, G. (1974). The simplest systematics for the organization of turn taking for conversations. Language 50, 696–735.
Sawyer, J., Matteson, C., Ou, H., and Nagase, T. (2017). The effects of parent-focused slow relaxed speech intervention on articulation rate, response time latency, and fluency in preschool children who stutter. J. Speech Lang. Hear. Res. 60, 794–809. doi: 10.1044/2016_JSLHR-S-16-0002
Schegloff, E. A. (2000). Overlapping talk and the organization of turn-taking for conversation. Lang. Soc. 29, 1–63. doi: 10.1017/S0047404500001019
Seddon, N. (2002). The structure, context and possible functions of solos, duets and choruses in the subdesert mesite (Monias benschi). Behaviour 139, 645–676.
Seddon, N., Butchart, S. H., and Odling-Smee, L. (2002). Duetting in the subdesert mesite Monias benschi: Evidence for acoustic mate defence? Behav. Ecol. Sociobiol. 52, 7–16. doi: 10.1007/s00265-002-0488-9
Spezie, G., Torti, V., Bonadonna, G., De Gregorio, C., Valente, D., Giacoma, C., et al. (2022). Evidence for acoustic discrimination in lemurs: a playback study on wild indris (Indri indri). Curr. Zool. zoac009. doi: 10.1093/cz/zoac009
Takahashi, D. Y., Fenley, A. R., and Ghazanfar, A. A. (2016). Early development of turn-taking with parents shapes vocal acoustics in infant marmoset monkeys. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 371:20150370. doi: 10.1098/rstb.2015.0370
Takahashi, D. Y., Fenley, A. R., Teramoto, Y., Narayanan, D. Z., Borjon, J. I., Holmes, P., et al. (2015). The developmental dynamics of marmoset monkey vocal production. Science 349, 734–738. doi: 10.1126/science.aab1058
Tobias, J. A., Sheard, C., Seddon, N., Meade, A., Cotton, A. J., and Nakagawa, S. (2016). Territoriality, social bonds, and the evolution of communal signaling in birds. Front. Ecol. Evol. 4:74. doi: 10.3389/fevo.2016.00074
Torti, V., Bonadonna, G., De Gregorio, C., Valente, D., Randrianarison, R. M., Friard, O., et al. (2017). An intra-population analysis of the indris’ song dissimilarity in the light of genetic distance. Sci. Rep. 7:10140. doi: 10.1038/s41598-017-10656-9
Torti, V., Gamba, M., Rabemananjara, Z. H., and Giacoma, C. (2013). The songs of the indris (Mammalia: Primates: Indridae): contextual variation in the long-distance calls of a lemur. Ital. J. Zool. 80, 596–607. doi: 10.1080/11250003.2013.845261
Torti, V., Valente, D., De Gregorio, C., Comazzi, C., Miaretsoa, L., Ratsimbazafy, J., et al. (2018). Call and be counted! Can we reliably estimate the number of callers in the indri’s (Indri indri) song? PLoS One 13:e0201664. doi: 10.1371/journal.pone.0201664
Traeholt, C., Bonthoeun, R., Virak, C., Samuth, M., and Vutthin, S. (2006). Song activity of the pileated gibbon, Hylobates pileatus, in Cambodia. Primate Conserv. 2006, 139–144. doi: 10.1896/0898-6207.21.1.139
Valente, D., De Gregorio, C., Favaro, L., Friard, O., Miaretsoa, L., Raimondi, T., et al. (2021). Linguistic laws of brevity: conformity in Indri indri. Anim. Cogn. 24, 897–906. doi: 10.1007/s10071-021-01495-3
Valente, D., De Gregorio, C., Torti, V., Miaretsoa, L., Friard, O., Randrianarison, R. M., et al. (2019). Finding meanings in low dimensional structures: stochastic neighbor embedding applied to the analysis of Indri indri vocal repertoire. Animals 9:243. doi: 10.3390/ani9050243
Wickham, H. R. (2019). Package ‘stringr.’ CRAN. Available online at: https://cran.rproject.org/web/packages/stringr/stringr.pdf (accessed February 9, 2022).
Keywords: chorus, lemur, primate, flexibility, elaboration, duet, rhythm, song
Citation: De Gregorio C, Zanoli A, Carugati F, Raimondi T, Valente D, Torti V, Miaretsoa L, Rajaonson A, Gamba M and Giacoma C (2022) Parent-offspring turn-taking dynamics influence parents’ song structure and elaboration in a singing primate. Front. Ecol. Evol. 10:906322. doi: 10.3389/fevo.2022.906322
Received: 28 March 2022; Accepted: 11 July 2022;
Published: 28 July 2022.
Edited by:
Dena Jane Clink, Cornell University, United StatesReviewed by:
Nora Hengst Prior, University of Maryland, College Park, United StatesCopyright © 2022 De Gregorio, Zanoli, Carugati, Raimondi, Valente, Torti, Miaretsoa, Rajaonson, Gamba and Giacoma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiara De Gregorio, Y2hpYXJhLmRlZ3JlZ29yaW9AdW5pdG8uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.