- Department of Experimental Psychology, University of Oxford, Oxford, United Kingdom
Explanations for the evolution of social monogamy in mammals typically emphasise one of two possibilities: females are overdispersed (such that males cannot defend access to more than one female at a time) or males provide a service to the female. However, the first claim has never been formally tested. I test it directly at three levels using population-level data from primates and ungulates. First, I show that the females of monogamous genera do not have territories that are significantly larger, either absolutely or relatively, than those of polygynous genera. Second, using two indices of territorial defendability, I show that, given their typical day journey lengths, males of most monogamous species could easily defend an area large enough to allow them to monopolise as many as 5–10 females if they ranged solitarily. Finally, I use a model of male mate searching strategies to show that the opportunity cost incurred by pairbonded males is typically 5–10 times the reproductive success they actually obtain by being obligately monogamous. This suggests that the selection pressure dissuading them from pursuing a roving male strategy must be very considerable. At present, the evidence is undecided as to whether mitigating predation or infanticide risk is the primary function, but estimates of their impacts suggest that both are in fact plausible.
Introduction
All male mammals face a choice between being social (i.e., living with a group of one or more females) or pursuing a roving male strategy that might give them access to many more females (Dunbar, 1988a; Komers, 1996). A roving male strategy would allow a male to range more widely, mating with individual females as and when they come into oestrus, but not staying with any of them longer than necessary to ensure fertilisation. In mammals, a roving strategy is common among some (but not all) insectivores, some miniature antelope, most felids and some strepsirrhines. A male’s preference will reflect the fitness-related costs and benefits of these two options, with these in turn being determined largely by the way females are distributed in the landscape, the male’s capacity to search for and locate female groups, and the females’ species-specific reproductive characteristics. Given that male mammals cannot easily contribute to the processes of reproduction and that, as a result, the male’s fitness is limited, all else being equal, by the number of females with whom he can mate, social systems in which males limit themselves to a single female (pairbonded monogamy) continue to puzzle behavioural ecologists–not least because pairbonded monogamy is a conspicuous feature of the social arrangements of such a wide variety of mammals and birds (Shultz and Dunbar, 2007).
Historically, three principal explanations have been proposed for such living arrangements, namely (1) female overdispersion, (2) biparental care (including indirect provisioning through the defence of food sources for the female and young), and (3) protection against external threats (notably predation or infanticide). The first implies that the male is constrained into monogamy by the fact that females are too widely distributed for males to be able to defend more than one (or, conversely, for a roving male to locate more than one unmated female in oestrus during a breeding season); the second and third imply that the male provides a service for the female. However, there has, as yet, been no satisfactory resolution to this debate, and most recent analyses (Dobson et al., 2010; Shultz et al., 2011; Lukas and Clutton-Brock, 2013; Opie et al., 2013; Kappeler and Pozzi, 2019) disagree with each other.
Although a possible explanation in the case of birds (Shultz and Dunbar, 2010), biparental care can be excluded for mammals since every substantive analysis has agreed that biparental care evolves after, or independently of, the adoption of pairbonding (Garber, 1994; Dunbar, 1995a; Komers and Brotherton, 1997; Brotherton and Komers, 2003; Lukas and Clutton-Brock, 2013; Opie et al., 2013). Beyond that, however, there seems to be little agreement as to whether the main factor selecting for monogamy has been male mating strategies or other services offered by the male (van Schaik and Dunbar, 1990; Komers and Brotherton, 1997; Brotherton and Komers, 2003; Lukas and Clutton-Brock, 2013; Opie et al., 2013; Kappeler and Pozzi, 2019).
The only consistent finding is that the females of monogamous species usually have separate, non-overlapping ranges. But this obviously has to be true by definition: if females lived in groups of two or more, this would constitute polygyny or polygynandry (depending on how many males are associated with the female group). The observation that females live alone (i.e., are overdispersed) has mainly been responsible for the claim that, when females are forced to disperse as a result of ecological competition, males are constrained into monogamy because the large size of female territories makes it impossible for them to defend more than one female. This hypothesis rests on two assumptions: that monogamous females are forced to forage alone in large territories for nutritional reasons, and that males are unable to defend larger territories that would give them access to more females. Surprisingly, neither of these assumptions have actually been formally tested.
Although many studies have claimed that female overdispersion is due to foraging competition (e.g., Schülke, 2003; Overdorff and Tecot, 2006), all the evidence to support this is at best circumstantial. In contrast, Brotherton and Manser (1997) found that there was no difference between the monogamous and polygynous groups of dikdik antelope (genus Madoqua) in either the size or the resource quality of their territories. More broadly, comparative analyses have suggested that monogamous female mammals do not necessarily live in larger territories than species where females live in groups, even though their densities might be lower (Dunbar, 1988a; Lukas and Clutton-Brock, 2013). That densities are lower might imply that habitat quality is lower (although there is no direct evidence for this) or that females are trying to avoid conspecifics for other, non-nutritional reasons. More importantly, time budget models consistently indicate that, except on the margins of their biogeographical distributions, there is nothing in their ecology to prevent most primate species living in significantly larger social groups than they actually do, with this being as true of monogamous species (gibbons: Dunbar et al., 2019) as of species that live in large polygynous social groups (colobines: Korstjens and Dunbar, 2007; guenons: Korstjens et al., 2018; baboons: Bettridge et al., 2010). A more likely explanation is that, when predation risk is low, females prefer to forage alone because the stresses of living with other females adversely affects their fertility (Dunbar, 2018a; Dunbar and Shultz, 2021a). The only question is how far apart they need to be to minimise the costs of spatial proximity.
Irrespective of the reasons why females live alone (and the reasons are not relevant to our present concerns), the crucial question is whether this is also a sufficient explanation for pairbonded monogamy. The dispersed females hypothesis rests explicitly on the claim that males maximise their fitness by being social rather than pursuing a roving male strategy because this maximises their mating opportunities. Brotherton and Manser (1997) suggested that, since defence of a feeding territory could not explain monogamy in dikdik, this genus’ social system might be more plausibly explained as mate-guarding. An alternative version of this hypothesis suggests that males are risk averse and seek to minimise the variance in sirings by opting for the certainty of one female rather than the risk of ending up with no matings at all if they pursue a roving male strategy (Komers and Brotherton, 1997; Brotherton and Komers, 2003). Other studies have suggested that males provide a service to the female by minimising the risk of infanticide (e.g., van Schaik and Dunbar, 1990; Opie et al., 2013), defending a food supply by keeping conspecifics out of the territory (Williams et al., 2004), or providing protection from predators (Dunbar and Dunbar, 1980).
In this paper, I test the assumptions of the dispersed females hypothesis at three successive levels. First, I ask whether the females of monogamous species really do occupy larger territories than the females of polygynously mating territorial and non-territorial species, either in absolute terms or relative to their metabolic demands (Hypothesis H1). Second, I ask whether the males of pairbonded monogamous species could, in principle, defend territories larger than those they actually occupy, such that they would have access to more than the one reproductive female that monogamy offers them (Hypothesis H2). Finally, I test whether, in terms of the expected number of fertilisations gained, it would pay males to search for females without necessarily having to defend a territory (Hypothesis H3). Hypothesis H3 takes into account not just the number of females that a roving male has access to but also the risk that, when he does find a female, she is not available to be fertilised (either because she is not in oestrus or because she has already been mated by another roving male). The latter issue is, of course, a particular problem for species like primates that have very long interbirth intervals and whose females are only in oestrus for a very short proportion of that period. In both the latter two analyses, I compare the payoffs that accrue to a male who adopts a roving strategy with the baseline payoff provided by being social (i.e., living permanently with females).
It is important to be clear about the logic of the second and third analyses. I start from an observed fact–that the males of some species adopt a form of pairbonded monogamy. Species undoubtedly differ in the degree of bondedness (Dunbar and Shultz, 2010, 2021b) that they exhibit, but broadly speaking these males are spatially and socially closely associated with one female (very occasionally two) on a long term basis. What we have to explain, from an evolutionary point of view, is why the male should behave in this way rather than pursue an alternative, potentially more profitable, strategy (in the limit, a roving male strategy in which the male associates with many individual females only briefly in order to mate with them, as some species in fact do).
We are not concerned with the ancestral state that precedes the evolution of monogamy, as many phylogenetic analyses have been (e.g., Opie et al., 2013; Kappeler and Pozzi, 2019). Our only precondition is that females have to be overdispersed (i.e., live alone), otherwise males cannot be monogamous (although humans are an exception, as are colonially nesting birds). Nor are we concerned with the behaviours that make this close association between a male and a female possible, or which member of the pair works hardest to maintain the pairbond once formed. It is crucial to distinguish between the selection pressures that act in favour of the male being social (i.e., living with a female) and those traits that evolve subsequently that lock the male and female into a pairbond. The latter include exploiting windows of opportunity that arise once the pairbond is in place (e.g., biparental care), reducing the birth interval and/or increasing litter size (callitrichid primates: Dunbar, 1995a) or the loss of visual cues of oestrus (making male detection of ovulation from a distance difficult), as well as various forms of pairbond reinforcement and mate guarding behaviour (gibbons: Palombit, 1996; some pitheciids: Menzel, 1993; Caselli et al., 2015; some miniature antelope: Dunbar and Dunbar, 1980). These are questions about how the pairbond is maintained after one has become established (i.e., mechanisms questions): they are not questions about why pairbonds have formed. The failure to distinguish between these two questions risks conflating two of Tinbergen’s Four Why’s (Dunbar, 2019) and has been one reason why this issue has continued to be unresolved.
In essence, the female dispersal hypothesis implicitly asserts that monogamy is a best-of-a-bad-job strategy for the male: being pairbonded is the best that he can do, since any alternative strategy in which he tries to monopolise access to several females results in reduced sirings either because the male would fail to locate a female when she was in oestrus or because he would lose sirings to rival males who got to the female first. For perhaps obvious reasons, this key assumption has never formally been tested: males in monogamous systems are unlikely to adopt a less profitable strategy, and hence there is rarely, if ever, an adequate basis for comparison. The only way to approach the question is by reverse engineering to determine the likely benefits of alternative strategies from first principles.
A reverse engineering approach asks whether, given the observed behaviour of the males and some general principles determining territory defendability, it would pay males to opt for a roving-male mating strategy as opposed to being social (i.e., staying permanently with the female). In doing so, this approach strips to the bare minimum the assumptions hypothesised to produce the observed behavioural outcome; thereafter, it progressively builds in whatever necessary additional assumptions are required, step by step. Hence, I begin with the simplest possible version of the hypothesis: the females of monogamous species have unusually large territories and that alone limits what males can do. I then ask whether, given the ranging patterns of the males, they could in fact defend a large enough territory to encompass the ranges of more than one female when these are overdispersed (i.e., the females live alone). In other words, in terms of female territory size and dispersion, when would it pay these males to abandon pairbonded monogamy in favour of roving male polygyny? Finally, I ask whether the problem the male faces is not the number of females he has access to but whether a roving strategy would leave him vulnerable to lost opportunities for fertilisations because he fails to find enough of the females when they are in oestrus.
I make the most conservative assumptions possible: roving males simply search randomly across their territory, without exploiting any knowledge they might have of where individual females prefer to forage or sleep. Such knowledge would significantly increase, rather than decrease, the advantages of roving by reducing the male’s search time costs, thus exaggerating any differences in the fitness benefits of the two strategies. The capacity to gain such knowledge is likely to arise only after adopting a roving male strategy, since there would be no point in paying the cost for a large memory store in the absence of any need for one.
If it turns out that males could do better by roving, then we have two important novel pieces of information: (1) the male is paying a price to be monogamous, and hence is choosing to do so, and (2) the difference between the payoffs for the two strategies (the selection ratio) gives us an estimate of the strength of the selection pressure against roving (the default strategy).
I test these hypotheses on the 12 genera of anthropoid primates that exhibit pairbonded monogamy (mainly hylobatids and the New World callitrichids, aotids, and pitheciids), two nominally monogamous strepsirrhines (Phaner and Lepilemur) whose social systems have been described as “dispersed monogamy” (a male and female live together but do not necessarily forage together: Schülke, 2003; Hilgartner et al., 2012) and two genera of monogamous ungulates (Oreotragus and Ourebia, one an obligate, the other a facultative monogamist). A form of dispersed monogamy is also found in some miniature antelope (e.g., dikdik: Hendrichs, 1975; duiker: Dunbar and Dunbar, 1979), but no data are available on their ranging patterns. Note that, rather than focussing on species averages as most comparative analyses invariably do, all my analyses are at the level of the population on the grounds that, under the conventional socioecological model, animals’ behaviour is a response to local ecological and demographic conditions rather than being rigidly inherited species-typical traits (see Dunbar, 1995b; Dunbar et al., 2009).
To ensure that the models really do make the correct predictions, I benchmark them against data from four group-living social primate genera (i.e., ones where males live with a group of females) and a set of primate genera known to be characterised by roving male polygyny. The first set includes two genera of Old World monkeys characterised by territorial unimale (but occasionally multimale) polygynous social groups (Cercopithecus and Colobus), one genus characterised by non-territorial unimale polygynous groups (Gorilla) and one genus characterised by multimale/multifemale (polygynandrous) social groups (Papio). The second group includes 11 genera of nocturnal strepsirrhines (mainly lorisids) that have a roving male mating system. The models should correctly predict that the males in the first set will prefer to be social, and the males in the second set will prefer a roving strategy. I also include among the comparator taxa two ungulate genera: a cervid, Muntiacus, with a roving male mating and a solitary social system, and a caprid, Capra, with a roving male mating system where females associate in small groups. Again, the models should predict that both genera should prefer a roving male strategy.
Two taxa (the callitrichids and the Cercopithecus guenons) are of particular interest in this context because of the unusual degree of variability in their social and mating systems. Although the callitrichids have traditionally been considered to be monogamous, their mating systems are in fact more complex and variable, with individual groups switching between monogamy, polygamy, polyandry, and polygynandry over time (Goldizen, 1988; Dunbar, 1995a,b; Digby et al., 2007). This is especially true of Saguinus, but may be less true of Callithrix (Ferrari and Ferrari, 1989). Nonetheless, I include them in the monogamous sample because they rarely have more than one breeding female, irrespective of the composition of the social group, due to puberty being suppressed in maturing females (Abbott, 1984). Although the typical social and mating system of the guenons (whom I include as a polygynous comparator genus) is a territorial one-male harem group, in some populations groups are subject to temporary influxes of males when there are many females simultaneously in oestrus in the group (Henzi and Lawes, 1987; Cords, 2004; Gao and Cords, 2020); these “bachelor” males sire up to 40% of the offspring produced by the group’s females on these occasions (Roberts et al., 2014). The genus thus exhibits both strategies in the same population: some males are social while others pursue a roving male strategy. The analyses may help illuminate why this is so, while at the same time providing a valuable form of post hoc support for the models.
To avoid misunderstanding, it is necessary to draw attention to two important caveats. First, all mammal species exhibit some variability in the size and composition of their social groups. Most of the species generally considered to be monogamous sometimes have groups with two, or even three, reproductively active females, occasionally even two or more adult males (Kappeler and Pozzi, 2019). Similarly, most of the species generally identified as having unimale harem-type social/mating systems occasionally have groups with a single female and a single male as well as groups with several females and several males (Colobus: Dunbar, 1988b; Fashing, 2007; Cercopithecus: Enstam and Isbell, 2007; Gorilla: Robbins, 2007), while some species that normally have large multimale/multifemale groups (polygynandry) occasionally have only a single male (Papio: Hamilton and Bulger, 1992). In large measure, this structural variability is simply a consequence of group size (Andelman, 1987). This variability has been known about since the earliest field studies, and forms the background for the rich social complexity of anthropoid primate social behaviour. The issue in the present context is simply whether a species typically adopts a particular social or mating system, not how consistently it does so. If species are inconsistent in their behaviour (sometimes adopting one system, sometimes another), then the reasons why this is so is an important follow-up question. But we need, first, to establish that there really is an issue worth investigating.
Second, genetic monogamy in mammals can range from complete (dikdik: Brotherton et al., 1997) to varying degrees of partial (Phaner: Schülke et al., 2004) overlap with social monogamy, just as it does in birds. The male should be willing to tolerate some level of non-paternity if they can be sure of paternity on the rest of the female’s offspring: it is simply a tradeoff between the number of future sirings he gains by continuing to stay with the female (with a fixed risk of non-paternity) and what he would gain by switching to roving. Griffin et al. (2013) have shown, for a wide taxonomic range, that males only become concerned about non-paternity when the cost of parental care and the risk of non-paternity are both high. Those costs are obviously reduced if the male himself takes advantage of any extrapair matings that come his way, because, within the range of the area he can cover, he is engaged in a zero-sum game. The female is simply interested in getting the best deal she can. The only question of interest is whether the proportion of fertilisations by extrapair males is so great that a socially monogamous male would do better to go roving. If this is the case, then our question remains: why do males continue to be pairbonded to a female?
Materials and Methods
Data
I sourced data on day journey length, territory size, mean social group size, mean number of females per group, group density (groups per km2) and interbirth interval (in days) for individual populations from the primary sources listed in Campbell et al. (2007), with additional data from a search of the post-2007 primary literature. Campbell et al., do not provide compilations for all genera, so the data for Gorilla populations derive from Doran and McNeilage (1998), Doran-Sheehy and Boesch (2004), and Lehmann et al. (2008) while data for gibbons are taken from Dunbar et al. (2019) and data for Papio from Dunbar (1992), Hill et al. (2000), Bettridge et al. (2010), and Dunbar et al. (2018a). Altogether, 100 populations from 48 species representing 15 genera are represented in the anthropoid sample, with a mean of 5.6 ± 2.8 (range 2–12) populations per genus. In most cases, the data are averages for several groups living in the same location. Data on interbirth intervals are used only if they derive from wild populations. Where possible, the value given for each specific study site is used; where these are not available, the taxon mean value is used. Data for the nocturnal strepsirrhines are species means obtained from Campbell et al. (2007). The data for two monogamous strepsirrhines (Lepilemur and Phaner) derive from Hilgartner et al. (2012), and for Phaner from Schülke (2003) and Schülke et al. (2004). Field studies of antelope almost never provide data on day journey length, and in any case many antelope genera have lek social systems. Nonetheless, I was able to source data for four populations of monogamous miniature antelope [three for klipspringer (Oreotragus) and one for the socially more variable oribi (Ourebia): Dunbar and Dunbar, 1980; Adamczak and Dunbar, 2008], one population of a miniature cervid, the muntjac (Muntiacus) with a roving male type system (Chapman et al., 1993) and one population of socially living, non-territorial feral goats (Capra) with a roving male mating system and foraging groups that average 3.3 (range 1–12) adult females (Dunbar et al., 1990).
Aside from the gorilla, the species included in these analyses do not vary significantly in body mass. Excluding gorilla, mean body mass is 2.84 ± 3.19 kg for the monogamous primates and 5.56 ± 2.59 kg for the comparator polygynous anthropoids: though monogamous taxa tend to be smaller, body mass does not differ significantly between the two groups (t12 = −1.33, p = 0.346). There is, thus, no reason to correct for body mass in respect of any ecological variables other than those where body mass forms a specific variable of interest.
The data are provided in the Supplementary Dataset.
Analyses
I tested the first hypothesis by comparing absolute territory size per adult female between monogamous and polygynous species. In order to check whether the results could be explained by differences in intrinsic energy demand, I also calculated relative territory size as area per female metabolic body weight (km2/kg0.75).
To test the second hypothesis, I determined the maximum territory size that a male could defend, and then estimated the number of females that could live in that territory given the observed mean size of female territories in that particular habitat. To determine the maximum size of territory that a male could defend, I exploited two alternative measures of territory defendability, namely, the Mitani and Rodman (1979) index and Lowen and Dunbar (1994) index. These differ in the assumptions they make about how males detect intruders, but, both predict whether primate species are territorial or not with surprisingly high accuracy despite their conceptual simplicity.
Mitani and Rodman (1979) showed empirically that primate species are territorial so long as:
where d is the day journey length (in km), A the home range area (in km2), and the territory is assumed to be a perfect circle. Note that this defines the minimum ratio for successful territory defence: a species can have a ratio >1 and not defend its territory, but no species with a ratio <1 defends a territory. Although the ratio of 1 has no particular significance (it is simply an empirical observation that differentiates territorial from most non-territorial species), it does tell us something about the minimum mean interval required between successive visits to a given location within the territory that is necessary to detect (and presumably evict) an intruder since the territorial male last checked it out, assuming that the territorial male ranges at random in search of food or other resources (the minimalist assumption). The maximum size of territory the male can defend, Amax, can be determined by inverting Eq. 1 and setting it equal to 1 (the minimum criterion for successful territorial defence) to obtain:
Lowen and Dunbar (1994) reformulated the Mitani–Rodman inequality on the assumption that the critical issue is how often a male can check the boundary of his territory rather than the whole area enclosed within this boundary (as the Mitani–Rodman inequality assumes). Using the Maxwell-Boltzmann gas dynamics equation, they found, again empirically, that primate species are territorial whenever:
when the arc of the territory boundary, s, within which a male can detect an invading intruder is taken to be s = 0.5 km (i.e., 250 m either side of the point at which the male arrives at the boundary of his territory). Lowen and Dunbar (1994) noted that, while providing an improvement on the accuracy with which territorial species were differentiated from non-territorial species, this formulation does little more than confirm the validity of the much simpler Mitani–Rodman inequality. The value of s (the width of the boundary detection arc) is crucial, since it affects how much of the territory’s circumference the male can monitor on any given visit, and hence how long it will take him to check the whole perimeter while travelling randomly about his territory (in effect, the time between successive visits to the same boundary location). The Lowen–Dunbar index is a monotonic function of s: as s decreases, the criterion on the right hand side increases proportionately, and correspondingly decreases as s increases. Subject to this constraint, we can determine the maximum defendable territory size in exactly the same way as we did with the Mitani–Rodman inequality, by inverting Eq. 3 and setting it equal to 0.08 * (0.5 s–1) such that:
Following van Schaik and Dunbar (1990) and Dunbar (1995a), I used Eqs 2, 4 to determine the number of reproductive females a male could expect to include within his maximally defendable territory by dividing Amax (less the share of the observed territory that the male needs for his own nutritional requirements, Amale) by the size of the observed territory required to support one female and her offspring (Afemale + young), where Amale + Afemale + young = A (with dependent young counted as the energetic equivalent of half an adult, on average). The number of females a male can expect to monopolise, E(f), is then given by:
where M is the number of males in the group (in the present sample of genera, M = 1 in all cases except Gorilla and Papio), F is the mean number of females per group and N is the mean size of the group. Except for Gorilla, males and females are treated as being of equivalent body mass. Because Gorilla are the most sexually dimorphic of all primates, I treated adult males as equivalent to two females.
The two indices are highly correlated in their prediction of the maximum size of territory that a male could defend (for the 81 populations in the sample for which there are day journey length data, r = 0.946, p ≪ 0.0001). The relationship between the two indices does, however, depend on the value of s in Eq. 4. The consequence of this is best illustrated by the effect it has on the number of females that a male would have access to in his maximum defendable territory. Compared to the Mitani–Rodman index, the Lowen–Dunbar index underestimates the maximum number of defendable females when s is small (s = 0.05 km: Supplementary Figure 1A) and overestimates it when s is large (s = 0.50 km: Supplementary Figure 1C). The two indices converge at s ≈ 0.20 (Supplementary Figure 1B). Although Lowen and Dunbar (1994) used a detection arc of s = 0.5 km, a value of s = 0.2 (i.e., 100 m either side of the male) is probably a more realistic limit for animals that live in forest, as all the genera in our sample except Papio do. Because of the uncertainty surrounding s and the fact that the Mitani–Rodman index is always more conservative for species that have small day journeys and territories (as most monogamous species do), I use this index in preference for illustrating the results; however, I give the equivalent results for the Lowen–Dunbar inequality in the Supplementary Material.
For perhaps obvious reasons, data on day journey length are not available for any of the nocturnal strepsirrhine species. I therefore used the observed mean territory sizes for each of the sexes and, given that they all pursue a roving male strategy, assumed that the males are already defending the largest territories they can. At least we can be sure that they cannot do worse than they already actually do. In some populations, males expand their territories or travel further during the mating season (as also happens with feral goats). Since this will simply exaggerate the magnitude of any advantage for the roving male strategy, non-mating season territory size is more conservative, I will use these values: any capacity to increase range size or travel distance will necessarily favour a roving strategy. For each species, I calculated E(f) as male territory size divided by female territory size (less the male’s share estimated as a third, since female territories do not usually overlap).
I then calculate the selection ratio of the two strategies, so that the male’s decision rule is:
where Fadj is the mean number of females living together in a social group (one in the case of monogamous genera, and the mean observed number of females in the group for polygamous genera). Because most callitrichids twin and some produce two litters a year, I use the genus-specific number of infants born to a female as the social baseline in these cases. This is the equivalent of four females producing one offspring a year for Callithrix, Saguinus, and Cebuella (whose females habitually produce twins twice a year) and two for Callimico (who produce one infant twice a year: Porter et al., 2001) and Leontopithecus (who produce twins once a year: Bales et al., 2001). The magnitude of ratio [6] is a measure of the strength of selection favouring roving.
The third hypothesis takes the analysis one step further towards measuring fitness directly by asking whether, in terms of actual numbers of offspring sired, a roving male would be better off than a social male, even if he risks missing some fertilisation opportunities if, as a result, he misses the fertile period of some or all of the females because he cannot check on their oestrous state with sufficient regularity, or because another male fertilises a female between his successive visits. To calculate the number of sirings such a male could expect to obtain, I used a model originally developed for feral goats (Dunbar et al., 1990) and later extended to great apes (Dunbar, 2000, 2001). The model assumes that males search randomly, subject to their own daily ranging limits. The number of progeny, p, that a roving male can expect to sire during the average female reproductive cycle, E(p), is:
where K is the average interbirth interval for the population (reproductive cycle length, in days), r is the detection distance, d the day journey length (in km) (so 2rd is the search path that the male carves out each day as he forages), Nf the density of female groups (groups/km2, defined as A–1 assuming, conservatively, no overlap in territories), F the average number of adult (i.e., reproductive) females in a female group, and g is the probability that a female will be in oestrus on any given day. Using the length of the reproductive cycle (i.e., interbirth interval) as the time base simplifies the calculations since it means that the payoff to a social male is simply the number of females he has in his group, since each will produce precisely one baby during that interval.
Equation 7 is made up of two components: the frequency with which a roving male can expect to encounter a group of females each day as he travels about the habitat, K2rdNf, and the expected number of fertilisable females in the group when he does find one, Fg. The first component is adapted from the Maxwell-Boltzmann gas dynamics equation and consists of two parts: the probability that a randomly searching male will encounter a female group during an average reproductive cycle (calculated as the area that he can search during the course of a reproductive cycle of length K days, given a daily search path of d km length and a detection distance either side of his path of travel of r km width) and the density of female groups, Nf. In the light of Supplementary Figure 1, I use 2r = 0.200 km. It does not matter whether a male searches the same locations repeatedly as the female groups are also mobile, and so have a random chance of occupying a space that the male has previously searched by the time of his next visit. The fact that there were no females in oestrus in the group last time he checked them tells him nothing about whether or not there will be females in oestrus in the group when he next encounters them (or checks the location where they had previously been found).
The second part of Eq. 7 gives the mean number of females in oestrus (i.e., that are fertilisable) that a male can expect to find in a group of F females. Primate females are typically receptive for about 5 days on each of three successive menstrual cycles during any given reproductive cycle, hence g = 15/K. Since miniature ungulates are fertile for just 2 days in a 14-day oestrous cycle with an annual reproductive cycle, I use g = 6/K (with K = 365) in their case. Goats have a 39-h oestrus period on a 21-day cycle, and in this case I assume g = 4.5/365.
Conveniently, a social female will produce just one offspring in each reproductive cycle, so that the number off offspring sired by social males is equivalent to the number of females in his group. As in inequality [6], the ratio of the payoff for a roving male to that for a social male, E(p)/Fadj, is an index of the selection pressure favouring roving.
This model predicts with almost no error the proportion of males that are social (i.e., are found associating with groups of females on any given day) in individual populations of chimpanzees, gorillas, orang utans, and humans (Dunbar, 2000), as well as feral goats (Dunbar et al., 1990). Most importantly, the regression line for both the model predictions and the data pass through the point of equilibration where males should be undecided whether or not to be social because the payoffs are equal (see Figure 1 in Dunbar, 2000). This indicates that, notwithstanding its simplifications and the fact that it ignores both the number and behaviour of rival males, the model correctly predicts what males actually do across a range of mammalian genera. That the model suggests that males ignore the presence of competing males (until, of course, they actually have to fight them) is, of course, interesting: it says something about how focussed the males of at least some mammal species can be during the mating season. However, it is particularly convenient for present concerns because it means we do not need to take the behaviour of other males into account.
Three other simplifications made in constructing the model in Eq. 7 should be noted. First, it assumes that female reproductive cycles are not in synchrony, so that females become available for mating at random with respect to each other. I therefore check whether this makes any difference by incorporating a correction for synchrony. Second, I have not included a correction for the fact that, if he finds a female in oestrous, the male should stay to mate with her long enough to ensure she is fertilised. With a fertilisation window of 5 days, he should expect to stay 2.5 days on average, so that his total “time out” would reduce the total number of fertilisations, E(p), by about 2.5 * E(p)/K. Because K is large, this correction would be very small and can be ignored. Third, Eq. 7 does not correct for the likelihood that the male encounters a female he has already fertilised. It is possible to correct for this (see Dunbar, 2000), but the effect is small given the time scales and the frequencies with which males contact individual females (it reduces payoffs by about 5% in most cases), and so for present purposes can be ignored.
Komers and Brotherton (1997) suggested that the problem might be that when females are overdispersed, males are unable to prevent rivals mating with at least some females if two or more are in oestrus simultaneously; as a result, males might opt for monogamy as a risk-averse strategy (the bird-in-the-hand strategy). How likely two or more females in a population will be in oestrus at the same time, given the species reproductive parameters, can be determined from the binomial expansion:
where P(x ≥ 2) the probability that there will be two or more females simultaneously in oestrus within the area the male can search out during a complete reproductive cycle, x is the number of females who are co-cycling, n is the total number of females in the area he can search during a full reproductive cycle, and p is the probability that any one of them will be in oestrus on any given day (i.e., p = 15/K), summed across the range 2 ≥ x = F, where F is the total number of females in the population. We can then take account of this by reducing the payoff to roving males by the fraction P(x ≥ 2).
Statistical Analysis
It is conventional to apply phylogenetic controls when undertaking comparative analyses so as to avoid the problem of inflated sample sizes that results when closely related species share traits by descent from a common ancestor. Phylogenetic methods are not used in the present analyses for four reasons. First, none of the analyses test functional (i.e., causal) hypotheses that compare correlated changes on two dimensions: I simply ask how a given taxon performs on a particular behavioural task. There are no phylogenetic methods available for one-dimensional comparisons of this kind, even if this were desirable (see Dunbar et al., 2018b). Second, most of the analyses are explicitly within-population comparisons: they compare alternative outcomes for a particular species at a particular location. Phylogenetic inertia cannot be an issue in such cases, especially as the variance in most of the variables of interest are subject to strong environmental and demographic influence (Dunbar et al., 2009). Third, all the comparisons presented here are at the within-genus level. This effectively removes the phylogenetic inertia between species of the same genus that is the main cause of the statistical problem that phylogenetic methods are designed to alleviate (Harvey and Clutton-Brock, 1985). I follow Dunbar et al. (2009) and treat species within a genus as ecological populations whose behaviour is mainly driven by environmental conditions rather than biological constraints (see also Strier et al., 2014). Finally, notwithstanding the two previous points, the phylogenetic signals for all behavioural and ecological measures for primates are close to λ = 0 (Kamilar and Cooper, 2013). Indeed, for no comparative analysis of primate behavioural or group size data so far reported (N ≈ 35) has the use of phylogenetic methods yielded results that differ from analyses that do not use phylogenetic methods (or, more importantly, produce non-significant results where uncorrected analyses are significant).
In testing whether males do better by roving compared to being social, I use a within-population one-sample t-test, separately for each genus. The tests are one-tailed positive because we are only interested in whether males do better by roving than by being social; by definition, a significant negative result (a male does significantly worse by roving) is as much evidence against the hypothesis as a non-significant result.
Results
Comparative Territory Size (H1)
I tested whether monogamous genera have larger territories than polygynous genera in two ways. Figure 1A plots absolute territory size per adult female for the monogamous genera and the comparator polygynous genera, as well as the three territorial ungulates (shown on the right of the graph). Also plotted are territory size per adult female for the comparator genera. It may be that the females of monogamous genera are obliged to live in territories that are relatively larger, given that large-bodied females will be able to exploit energy savings of scale because they can survive on relatively less energy (Schmidt-Nielsen, 1984). Figure 1B tests this by plotting territory size adjusted for female metabolic mass (km2/female * kg0.75).
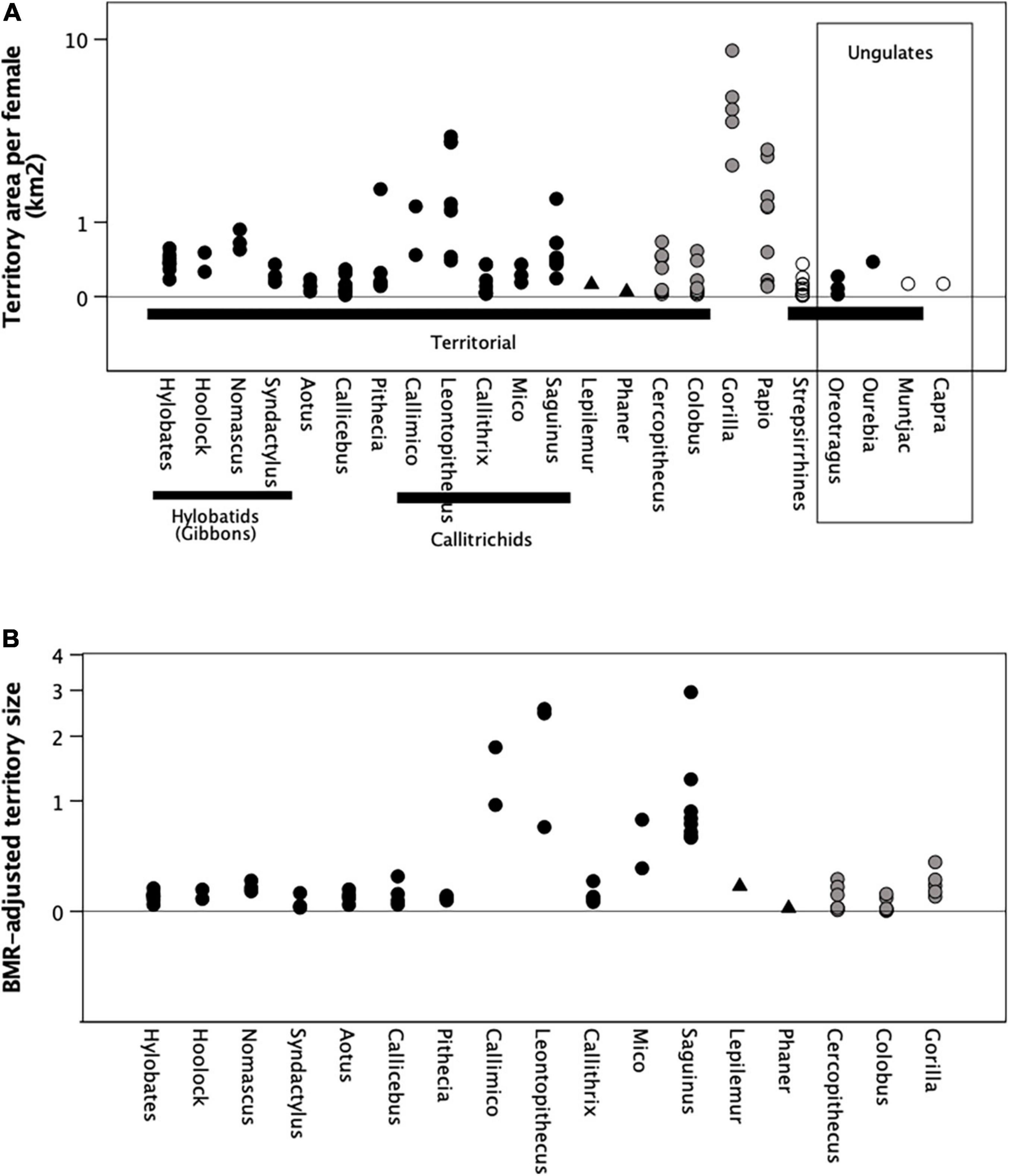
Figure 1. (A) Observed absolute territory size per adult female (km2) and (B) Relative territory size per female adjusted for female metabolic weight (km2/kg0.75). The main higher taxonomic groupings are indicated at the base of panel (A). Territorial taxa are indicated by the black bar across the lower part of the graph. Filled symbols: social or dispersed monogamous groups; grey symbols: stable polygynous groups; unfilled symbols: roving male polygyny. Ungulate genera are enclosed in the box on the right; all other genera are primates. Y-axis is log10 scaled.
While Callimico, Leontopithecus, and Saguinus have relative territory sizes that are certainly larger than other genera, the monogamous species do not differ, as a set, from the polygynous genera. This is true not just of the primates but also of the ungulates. In other words, with the possible exception of three of the five callitrichid genera, it seems that none of the monogamous genera are forced to overdisperse by the high energy demands of their females or the poor quality of their habitats.
On balance, then, there is no compelling evidence to support the suggestion that monogamous species have larger territories than territorial polygynous species. Hypothesis 1 may be rejected.
Maximally Defendable Territory Size (H2)
To determine whether males in monogamous genera are obliged to associate with one female because they cannot defend a larger territory, I used the Mitani–Rodman inequality (Eq. 2) to calculate the maximum size of territory that a male could defend, given his typical day journey length, and then determined (using Eq. 5) how many females could be encompassed within this territory. Figure 2 plots the predicted number of females that a territorial male could expect to have within his maximally defendable territory as a ratio of the baseline number he would have if he was social (i.e., lived with his female group) (adjusted for the high fertility of the callitrichids). The dashed line demarcates a ratio of 1 where the payoffs of the two strategies are equal. Males should prefer a roving strategy if the payoff ratio is >1, but prefer being social if the ratio is <1.
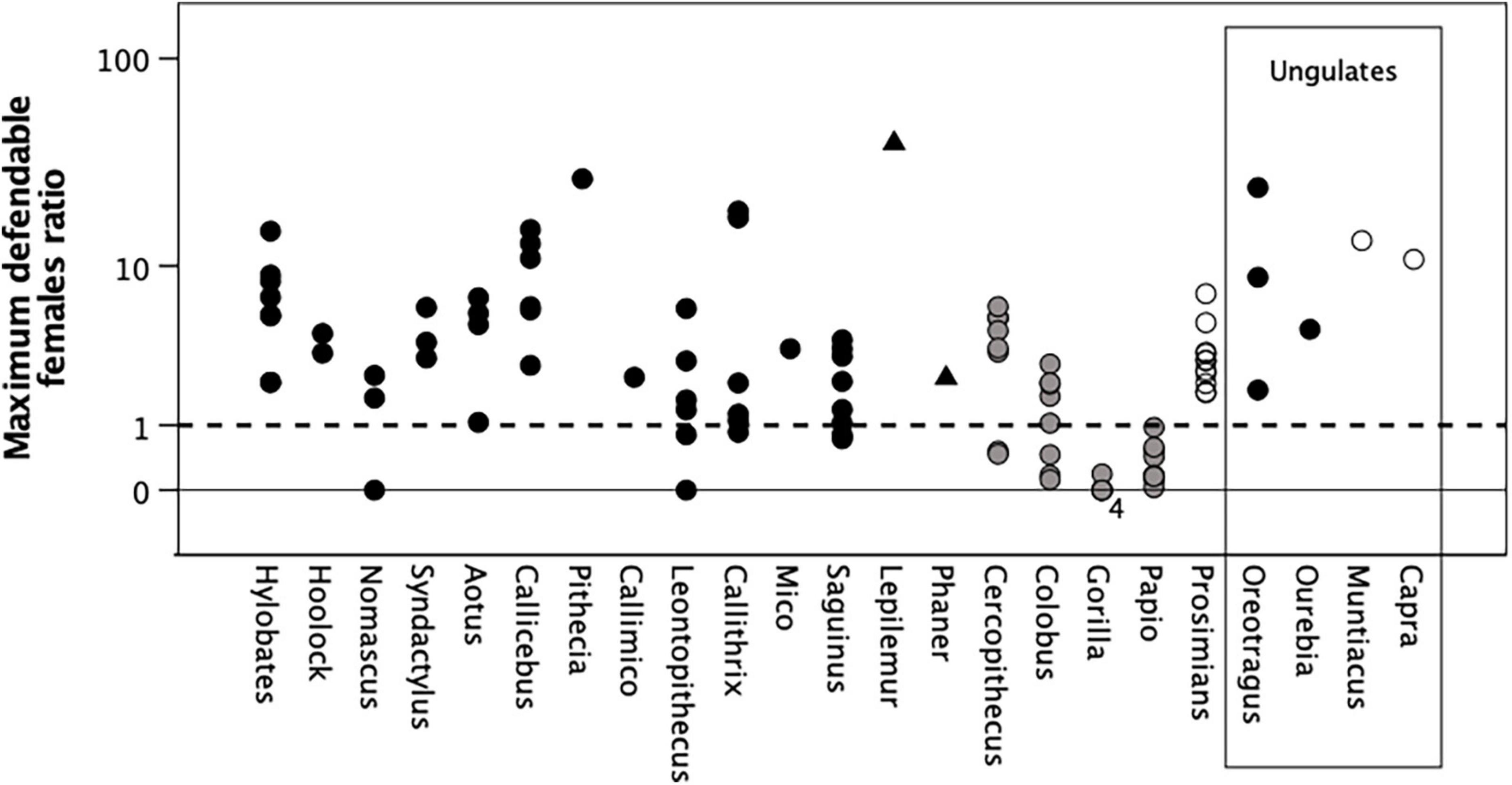
Figure 2. Ratio of maximum number of defendable females, as predicted by the Mitani–Rodman equation, to the default baseline of observed mean number of females per group, for individual populations. The baseline is one female for all monogamous taxa, except Callithrix, Mico, and Saguinus for whom the equivalent baseline is four females (pairbonded females produce twins twice a year) and Callimico and Leontopithecus for whom it is two females (see text). For the polygamous genera, the mean number of females per group is 7.1 for Cercopithecus, 3.8 for Colobus, and 4.4 for Gorilla, 16.5 for Papio, 1.0 for the strepsirrhines, and 3.3 for Capra. When the ratio = 1 (heavy dashed line), the payoffs are equal and males should be ambivalent about their preferred strategy. There are four datapoints at 0 for Gorilla, as indicated by the number just below the line. Filled symbols: social or dispersed monogamous groups; grey symbols: stable polygynous groups; unfilled symbols: roving male polygyny. Ungulate genera are enclosed in the box on the right; all other genera are primates. Y-axis is log10 scaled.
The model correctly predicts that Colobus, Gorilla, and Papio, but not Cercopithecus, should prefer to be social (mainly because of the large size and low density of their groups) and that all the nocturnal strepsirrhines should prefer the roving male strategy (Table 1). Cercopithecus excepted, the model thus makes the correct predictions for our benchmark taxa, confirming its reliability. For most of the monogamous populations, however, males would be able to monopolise access to significantly more females by pursuing a roving strategy (Table 1). For the monogamous genera as a set, the distribution of p-values is more significant than would be expected by chance (Fisher’s meta-analysis: χ2 = 63.21, df = 2 * 9 = 18, p ≪ 0.0001), indicating a consistent underlying pattern. Nomascus and Leontopithecus are the least significant (both occupy marginal or degraded habitats, and have absolutely larger territories than the other genera in their respective taxonomic families: Figure 1A), with Callithrix on the margin. Even so, in at least some populations of even these genera, it would pay males to pursue a roving male strategy.

Table 1. One sample t-tests against H0 = 1 for each of the main indices for each genus for Hypotheses H2 and H3.
We can invert Eq. 2 to calculate the day journey length that would make the ratio of defendable females equal to 1, the point at which the dispersed females hypothesis might explain why males are monogamous in these genera. Figure 3 plots these values against the observed day journey length for each population of the monogamous primate genera. There is a significant positive monotonic relationship (r = 0.661, N = 50, p ≪ 0.0001). Males would typically need to have day journeys that were about a third of their actual observed distances if they were to be constrained in the way suggested by the dispersed females hypothesis. Clearly, they are not constrained by travel considerations.
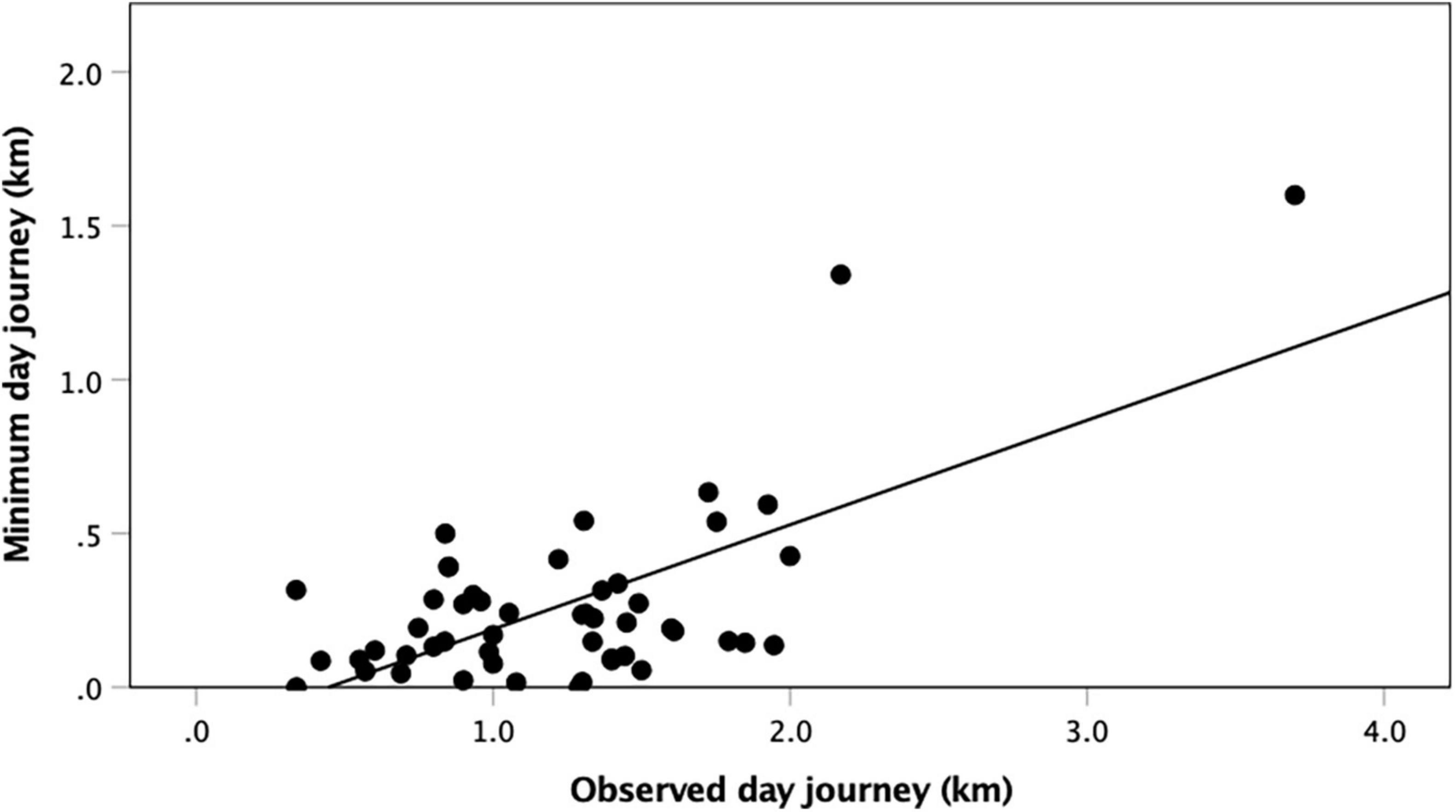
Figure 3. Minimum day journey length required to make pairbonded monogamy worth a male’s while plotted against observed day journey length, for individual monogamous primate populations. The equation for the regression line is DJmin = −0.15 + 0.34 * DJobs (r2 = 0.437, F(1,49) = 38.08, p ≪ 0.0001).
Figure 2 also plots the number of defendable females given by Eq. 5 for the four ungulate genera. In both monogamous cases, males could easily defend territories large enough to monopolise access to 5–10 females (the size of most harem groups in medium-sized antelope: Dunbar and Shultz, 2021a). Yet, like many of the miniature antelope, these species are monogamous, and especially so in the case of Oreotragus–the one antelope species that comes closest to primate levels of social attentiveness and bondedness (the modal separation between the male and female is just 3–5 m and the pair constantly monitor each other, with one moving or resting whenever the other moves or rests: unpublished data; see also Dunbar and Dunbar, 1980; Dunbar and Shultz, 2010). In contrast, both muntjac and goats do exactly what they should do (pursue a roving male strategy). Despite the fact that female goats are always found in groups (which might prompt males to be social), the mobility of male goats and high female group density means that males can check out many female groups on a near-daily basis (Dunbar et al., 1990).
A possible confound is that Eq. 5 assumes that the habitat is completely packed with female territories. If the density of territories (i.e., females) is significantly less than that assumed by continuous packing, then polygamy might not be a viable strategy. Figure 4 plots the mean density of groups for the primate taxa, based on actual field estimates. The median observed group density (indicated by the solid line) is 3.30 groups per km2; the median estimated density based on Eq. 5 assuming no overlap between the ranges of neighbouring females is 2.91 groups per km2 (indicated by the dashed line). Although some genera (such as Callithrix) have higher densities and some (such as Leontopithecus) have lower, on average actual density is not consistently lower than the maximum packing assumed by the model. This makes it very unlikely that Figure 2 seriously overestimates the payoff to promiscuous males.

Figure 4. Observed density of groups for monogamous genera, for individual populations. The solid line indicates the median value for the observed data; the dashed line indicates the median value estimated by the model (based only on day journey length and assuming no overlap of adjoining territories). The model does not overestimate the density of groups. Filled symbols: social or dispersed monogamous groups; grey symbols: stable polygynous groups. Multiple datapoints at the same position are indicated by the numbers adjacent to symbols. Y-axis is log10 scaled.
Supplementary Figure 2 plots the equivalent results for the anthropoid primates using the Lowen–Dunbar inequality (Eq. 4). Since night journey length data are not available for any of the strepsirrhines, it is not possible to calculate the maximally defendable territory size for these taxa. However, the model correctly predicts that the males of the four polygynous comparator genera should all prefer to be social (notwithstanding the fact that males in some individual Cercopithecus and Colobus populations might prefer to rove). The results across individual populations are significantly correlated with those in Figure 2 (r = 0.843, N = 96, p < 0.0001 1-tailed). The distribution of datapoints across genera is broadly similar to that for the Mitani–Rodman inequality, though p-values are lower in some, but not all, cases (Table 1).
Taken together, these results suggest that even though the males of some populations of the monogamous genera might do better by being monogamous, most would be significantly better off pursuing a roving male strategy and this is as true for antelopes as for primates. In sum, Hypothesis 2 does not receive convincing support, at least as a universal explanation for monogamy, even though it might explain some individual cases.
Fertility Payoff to Roving (H3)
Merely being able to control a large territory containing many females does not guarantee higher fitness. What is important is the likelihood that a female will be in oestrus when the male finds her. With interbirth intervals as long as they are in primates (up to 39 months in the case of gibbons), a randomly searching male would come across most females when they are not in oestrus. I determined the fitness payoff to a roving male using Eq. 7 to estimate how many females he could find when they were actually in oestrus. As the time base, I use the interbirth interval for the population so that the baseline payoff for a social male is exactly one offspring for each female in his group.
Figure 5A plots the payoff ratio to a roving male, E(p)/Fadj, in terms of the expected number of sirings he would gain relative to that obtained by a social male for each sampled population (adjusted, as in Figure 2, for the higher reproductive rates of the callitrichids). The dashed line corresponds to a payoff ratio of 1 (the outcomes for the two strategies are equal). As with Figure 2, males should prefer a roving strategy if the payoff ratio E(p)/Fadj > 1, but prefer to be social if E(p)/Fadj ≤ 1. There are no day journey data for nocturnal strepsirrhines, so no predictions are possible for these populations, but the model correctly predicts that Papio and Gorilla should prefer to be social, although both Cercopithecus and Colobus males might be better off roving.
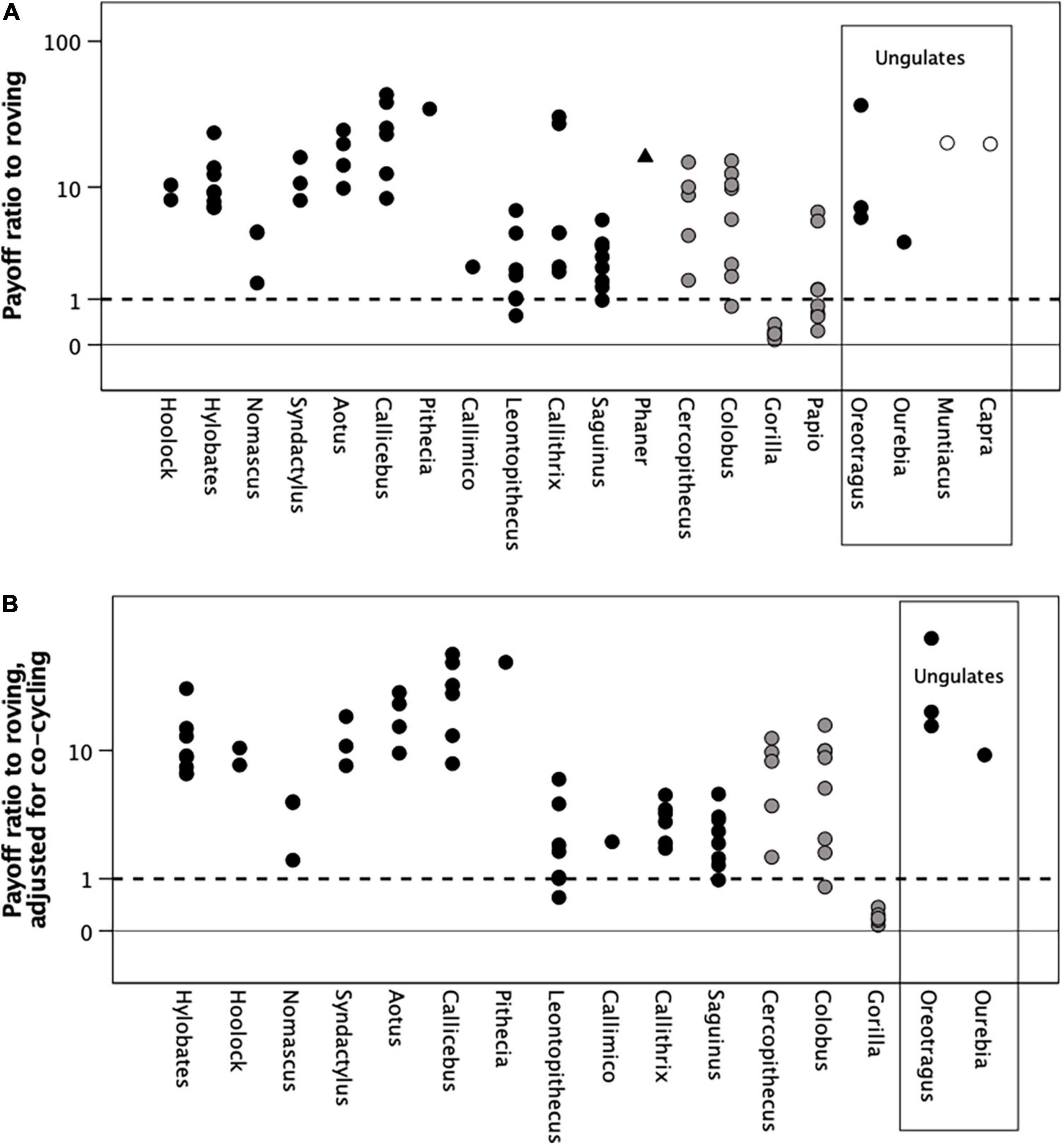
Figure 5. (A) Payoff ratio to promiscuous males (“roving male strategy”) predicted by the male mating strategies model, for individual populations. The payoff ratio is the number of offspring sired during the average population-specific reproductive cycle (i.e., interbirth interval) by a promiscuous roving male divided by that expected for a social male (equivalent to female group size, as in Figure 2). The dashed line at a ratio of 1 indicates the point of equilibration where the payoffs are equal and males should be ambivalent about which strategy to adopt. When the ratio lies above this line, males would do better by opting for roving male polygamy; when it falls below, they would do better by opting to be social (i.e., permanently attaching themselves to a group of females). (B) The same values as in panel (A) adjusted for the probability that two or more females would be in oestrus at the same time (see Supplementary Figure 4), making it impossible for the male to mate with both. Filled symbols: social or dispersed monogamous groups; grey symbols: stable polygynous groups; unfilled symbols: roving male polygyny. Ungulate genera are enclosed in the box on the right; all other genera are primates. Y-axis is log10 scaled.
Although the males in some individual monogamous genera should prefer to be social, in most of the populations they would benefit by opting for a roving strategy (payoff ratios significantly >1) (Table 1). Among the monogamous genera, only Nomascus, Callithrix, and Leontopithecus are not significant, and then only marginally (In fact, the non-significance in these cases is simply due to the high error variance in their cases; the majority of their populations would actually benefit by roving). The ungulate genera would all also clearly benefit by roving, although the small sample size for these data means that the difference is not significant in the one case where this can be tested.
I reran this analysis for detection distances of 2r = 0.05 km and 2r = 0.5 km. Although the payoff ratios are slightly smaller or larger, respectively, the conclusion are broadly the same (Supplementary Figure 3 and Supplementary Table 1). With 2r = 0.5, even Gorilla would almost do better to rove; at 2r = 0.05, most of the genera would do best to be social (Supplementary Figure 3A). It would, however, be necessary to reduce detection distances below 2r = 0.02 (10 m either side of the male) to remove the advantage of roving for all the monogamous populations.
A further possible confound, highlighted by Brotherton and Manser (1997), is that a male cannot monopolise two or more females that are simultaneously in oestrus, since it is not possible to be in two places at the same time. Equation 8 yields a mean likelihood of two or more co-cycling females of P(x ≥ 2) = 0.056 ± 0.018 across the 71 sampled monogamous populations (Supplementary Figure 4). Adjusting the payoff to rovers by Eq. 8 yields results (Figure 5B) that correlate significantly with those from Figure 5A (r = 0.982, N = 71 populations, p < 0.0001), with a very limited effect on payoff ratios (Table 1). The differences are marginal: across the 71 anthropoid populations, the mean loss due to reproductive synchrony is approximately one siring per reproductive cycle (means of 10.73 ± 13.9 vs. 9.85 ± 11.5 sirings). Note that Hoolock only fails to reach significance because its very small sample size (N = 2) results in a very large error variance estimate.
Some genera in this sample (mainly platyrrhine primates) are seasonal breeders, although even their mating seasons typically span as much as 6 months of the year. Since this is likely to increase the chances of females in different groups being in oestrus simultaneously, I reran Eq. 7 with p = 15/183 days (equivalent to a 6 months mating season). Adjusting the payoff to rovers by the corresponding probability of co-cycling has a relatively small effect on the significance of the payoff ratios for all genera (Supplementary Figure 5 and Table 1). A 6-month birth season would reduce the mean payoff ratio by approximately one further siring compared to Figure 5B (means of 9.85 ± 11.5 vs. 8.95 ± 9.6 sirings). Note that it is only the starred genera in Table 1 for whom this is an issue: the other genera do not have breeding seasons.
Finally, in both Figures 2, 5, the payoff ratios for the Callitrichids were compared against a baseline of their current reproductive performance. Since this involves a number of tactical adjustments that help lock the male into monogamy (biparental care, twinning twice a year by the females), and these necessarily must have evolved after the males had become pairbonded (a female could not take the risk of ramping up her fertility unless she could be sure the male would be around to provide the necessary paternal care), I re-ran the results from Figure 5 against their presumed ancestral baseline fertility (the standard anthropoid rate of one offspring each year) (Supplementary Figure 6). The corrected payoffs favouring roving are broadly similar to those for the other platyrrhine and hylobatid pairbonded genera.
In sum, the males of all the monogamous genera would do better to adopt a roving male strategy. Taken as a whole, then, Hypothesis H3 is not supported.
Discussion
All three sets of analyses suggest that the males of a wide range of monogamous primate and ungulate genera would do better by pursuing a roving male mating strategy. Contrary to conventional wisdom, females of monogamous genera do not have larger foraging territories than females in polygynous and polygynandrous social groups, even when controlling for the number of adult females in the group and female metabolic weight. Moreover, comparisons with territorial genera where males adopt either area-defence or female-defence polygyny (Emlen and Oring, 1977) suggest that there is no reason why the males of monogamous genera could not defend larger territories that would give them access to more females. In short, the males are not constrained into being monogamous by female ranging patterns. This implies that these males are foregoing considerable additional mating opportunities, suggesting that monogamy must offer them some benefit. The models suggest that the marginal benefit to monogamous males is in the order of 5–10 extra offspring in a typical female reproductive cycle (i.e., interbirth interval). Even if the male loses some of these to rival males, this represents a very substantial selection pressure against roving. That the models’ predictions are robust is confirmed by the fact that they correctly predict the mating strategies of the polygynous comparator taxa: in general, genera that should be social (Colobus, Papio, and Gorilla) are social and those that should have roving males (the nocturnal semi-solitary strepsirrhines) do so.
The models do not take into account competition from rival males, and one reason why monogamy seems to be difficult to reverse, at least in primates (Pérez-Barbería et al., 2007; Opie et al., 2013), might be that pressure from neighbouring males prevents a male from expanding his territory. However, it is important in this context not to fall foul of Dobzhansky’s Mazxim (Dobzhansky, 1973) by confusing “being adapted” with “becoming adapted” (in effect, where we are now versus where we were in the past before the trait evolved). Even in pairbonded species, males differ slightly in resource holding powers, and those males able to expand their ranges against neighbours by even a small amount will gain a fitness advantage from every extra female they can access by doing so. This advantage will accumulate through successive generations and eventually give rise to the levels of sexual dimorphism observed in polygynous species. However, the fact that the models (and especially Eq. 8) seem to work extremely well without considering competition from other males (Dunbar, 2000) implies that the presence of rivals is not a significant factor in males’ calculations.
Another criticism is that the models ignore the females’ interests. Callithrichid females’ ability to produce twins twice a year, for example, might be seen as an important counterweight to the temptation for males to rove. There is no doubt that this is advantageous for the females, but it alone does not explain why roving is disadvantageous for the males. Not only would roving benefit the males and more than offset their losses if the female had to abandon twinning, but it seems that Saguinus males actually do opt for a roving strategy in some circumstances (Garber, 1994; Dunbar, 1995b). More importantly, it is clear that, in the callitrichids, twinning (and the biparental care that makes this possible) evolved after the adoption of pairbonding and not before it (Dunbar, 1995a), and hence cannot have been causal in the evolution of pairbonding. In any case, twinning cannot explain the evolution of pairbonding in other anthropoid primates, and so cannot be a general explanation for pairbonding. It is necessary to distinguish adaptations that arise as a consequence of pairbonding (windows of evolutionary opportunity) from those that are genuinely causal. Females will, of course, always seek to find the optimal conditions for reproduction, but these will generally not be the same as those that influence male social and mating strategies since, except in the callitrichids, primate males do not contribute significantly to offspring rearing. Females will be more interested in minimising the constraints that act on them, most of which are associated with the reproductive costs of the psychosocial stresses of living in groups (Dunbar and Shultz, 2021a).
There are a few cases where the evidence against the dispersed females hypothesis is more marginal. Nomascus and Leontopithecus are the most obvious exceptions in this respect (Table 1). Both genera occupy more marginal habitats than the other members of their respective taxonomic families (Rylands, 1989; Kierulff and Rylands, 2003; Dunbar et al., 2019), and, as a result, typically have larger territories (Figure 1). Nonetheless, it is clear that a roving male strategy is a viable option in at least some of their populations, and that should be enough to impose selection pressure favouring a facultative roving strategy. Eulemur rubiventer might be another possible exception: Tecot et al. (2016) estimated that its Mitani–Rodman defendability index was ∼0.9, suggesting that males might be constrained into pairbonded monogamy. Of course, a single population may just be an outlier or, as seems to be the case with many of the lemurine populations, subject to temporal fluctuations in demographic or ecological circumstances at the time the population was sampled.
I included the genus Cercopithecus as a comparator taxon because they are territorial and have a unimale/multifemale group structure. However, as I noted in the Introduction, their behaviour is known to be anomalous in that their unimale groups are subject to periodic invasions by bands of roving males whenever there are several females in oestrus simultaneously (Henzi and Lawes, 1987; Cords, 2004; Roberts et al., 2014; Gao and Cords, 2020). A similar phenomenon has been noted in the Asian langur, Semnopithecus (Borries, 2000) and immigration by males into groups with higher than average number of females in oestrus has been reported for Papio (Clarke et al., 2008) and Lophocebus (Olupot and Waser, 2001).
The genetic data tell us that, in the guenon case, the roving males gain up to 40% of the sirings between them (Roberts et al., 2014). One reason for this may have to do with their demography. Cercopithecus groups typically contain ∼7 females, compared to the 3–4 characteristic of the unimale groups of Colobus and Gorilla (Dunbar et al., 2018b) but well below the 10–20 females typically found in Papio groups where resident males are simply unable to prevent other males joining their groups (Dunbar, 2000). Guenon groups seem to lie on the cusp of what a single male can successfully defend against intruders (Andelman, 1987). So long as there is only one female in oestrus, the male can keep rivals out; but when several females’ cycles coincide, he is unable to prevent other males from gaining access to some of the females (Dunbar, 2000), and so switches from group defence to defending individual females in oestrus. Note that he still gets 60% of the sirings, even though he loses some to the invading males (Roberts et al., 2014).
In fact, we find similar switches in strategy in many of the species that live in polygynandrous social groups. Pawlowski et al. (1998) found that the dominant males in large multimale/multifemale groups are only able to monopolise matings with all the females providing there are no more than four adult males in the group; when there are more males, they switch to a strategy of defending individual females in oestrus, much as the Cercopithecus males do. Such facultative plasticity in response to local circumstances is far from unusual in anthropoid primates, and may even reflect males’ ability to assess the likely interests of the females involved. Theropithecus baboon males, for example, explicitly target harem males with larger than the average numbers of females when trying to acquire a harem of their own through takeover; they seem to be aware that females are more likely to switch allegiance to them when there are more than about four females in the harem (Dunbar, 1984). Similarly, Kummer et al. (1974) showed, in an elegant series of experiments, that Papio hamadryas males are only willing to try to take over another male’s female if the female exhibits signs of disinterest in her male.
This should remind us that many of the behaviours that underpin mating and social strategies in mammals are highly flexible (Strier et al., 2014). Both Brotherton (1994) and Komers (1996) concluded that dikdik males in their respective populations could easily defend territories large enough to encompass the ranges of two females, and sometimes did so when the opportunity offered (e.g., when a neighbouring female was widowed). In an elegant series of field experiments, Kummer (1970) showed that female baboons can switch within a matter of weeks (and with equal facility in either direction) between the male-imposed harem-like social system of Papio hamadryas and the more relaxed female clique structure of Papio anubis. Similarly, callitrichid (and especially Saguinus) males switch facultatively from pairbonded monogamy to roving male polygyny and back again whenever there are subadult helpers-at-the-nest who can take over the paternal care duties that the male normally provides (Goldizen, 1987, 1988; Ferrari and Digby, 1996)–providing there is a high density of females in the local population (Dunbar, 1995b).
In sum, while solitary foraging by females is clearly a necessary condition for the evolution of monogamy, it is not a sufficient explanation: in particular, it does not, of itself, explain why males should find it worth their while to form a stable pairbond with a single female. The suggestion that monogamous males are engaged in mate guarding (Brotherton and Komers, 2003) is not convincing for anthropoid primates, given interbirth intervals that can be as long as 39 months. If breeding is highly synchronised, males might, of course, be forced into monogamy simply because they cannot both search for and keep rivals away from two dispersed females simultaneously (Knowlton, 1979). However, comparison of Figure 4 and Supplementary Figure 5 suggests that this is only likely to work if breeding is strongly seasonal. Most of the genera in this sample breed throughout the year (Colobus: Fashing, 2007; Cercopithecus: Isbell et al., 2004, except perhaps Cercopithecus mitis; gibbons: Leighton, 1987; Savini et al., 2008; Gorilla: Stewart and Harcourt, 1987), and those that don’t (callitrichids: Dietz and Baker, 1993; Digby et al., 2007; Aotus: Fernandez-Duque, 2007; pitheciids: Norconk, 2007; klipspringer: Norton, 1980) have birth (and therefore mating) seasons that occupy as much as half the year.
The fact that primates, in particular, have a long period of offspring dependency suggests that the problem is more likely to be associated with offspring survival. The models provide us with an estimate of the magnitude of this cost, suggesting that it may be as high as 5–10 offspring per reproductive cycle–equivalent to the typical average lifetime reproductive output for most primates. In contrast, the nocturnal strepsirrhines are willing to opt for roving male promiscuity even though the benefits of doing so are quite modest (on average, just 0.72 extra offspring per reproductive cycle: Figure 2), perhaps because most of them park their vulnerable young in nests. In addition, brain size is significantly larger in monogamous species of birds and mammals than is the case for promiscuous species (Shultz and Dunbar, 2007; Dunbar, 2010), probably because of the cognitive demands of maintaining stable relationships (see Dunbar, 2018b; Dunbar and Shultz, 2021b). The males of pairbonded species thus bear a double cost: they sacrifice sirings and they incur an expensive neuro-cognitive cost that the males of asocial roving males such as the strepsirrhines avoid.
The magnitude of these costs suggests that the fitness advantages of monogamy must be very substantial indeed. Identifying what those advantages might be is beyond the scope of this paper because we do not have sufficient data to model the impact on lifetime reproductive output. However, it is possible to provide some pointers for more detailed examination. If the defence of feeding resources can be ruled out on the grounds that most primate territories are nowhere close to their carrying capacities (Dunbar et al., 2009, 2019; Korstjens et al., 2018) and the fact that there is little convincing evidence that male primates defend feeding territories, then the only two costs known to be high enough to function as a countervailing cost to roving are predation risk (Cheney and Wrangham, 1987; Isbell, 1994; Shultz et al., 2004; Shultz and Finlayson, 2010; see also Burnham et al., 2012) and infanticide risk (van Schaik and Dunbar, 1990; van Schaik and Kappeler, 1997; Borries et al., 2011; Opie et al., 2013; Lukas and Huchard, 2014; Lowe et al., 2019).
Predation risk will probably always play some role, since it is the principal driver for group-living in mammals (Shultz et al., 2004; Shultz and Finlayson, 2010). The fact that the females of monogamous genera are willing to forage in groups of minimal size suggests that the risk of predation must be generally low for them. Indeed, these species often incur lower predation rates than those, like the nocturnal strepsirrhines, that have a completely solitary lifestyle (Cheney and Wrangham, 1987). However, we cannot rule predation out entirely. No diurnal primate lives entirely alone. There is some evidence to suggest that predation rates are significantly higher while individuals are transferring alone between groups than when living in groups. One estimate for adult male baboons (normally the least vulnerable animals) has suggested that the mortality rate due to predation while alone was 11 times greater than that faced by group-living males, equivalent to a mortality rate of 6.5% per month spent alone, or 78% per annum (Alberts and Altmann, 1995). At that rate, no male could expect to last more than 15 months on its own, with the much smaller-bodied females doing much less well. With offspring taking 3–4 years to reach puberty, this would effectively mean a solitary animal of either sex would have no surviving offspring even if it mated successfully. Solitary antelope are also significantly more susceptible to felid predation than are group-living species (Fitzgibbon, 1990; Shultz et al., 2004). In other words, both males and females would be under intense selection pressure to form groups. Although these calculations are based on data for species inhabiting a much riskier habitats than those typically occupied by any monogamous species, the risk of predation would have to be very low indeed to ensure that a roving male and a solitary female survived long enough to rear more than the five or so offspring that most social individuals can expect to produce in a lifetime.
The alternative cost is the risk of infanticide. In contrast to most other mammals, primates run a high risk of infanticide because of their greatly extended interbirth intervals, in turn a direct consequence of their unusually large brains. Infanticide is significantly and consistently lower in monogamous primates than among primates that live in polygamous groups (Borries et al., 2011; Opie et al., 2013; Lukas and Huchard, 2014), suggesting there has been selection to minimise this risk by adopting monogamy (The solitary nocturnal strepsirrhines are an exception because they park their infants in nests where they are relatively immune to both predation and infanticide, which may be one reason why their males unanimously prefer a roving strategy). In a wide-ranging review of infanticide in gibbons, Ma et al. (2019) found that 50% of gibbon infants died or disappeared within 2 months of a pair male being replaced. On top of natural pre-puberty mortality (itself often as high as 50% in anthropoid primates: Cheney et al., 2004), this represents a very substantial fitness cost to a male who may only sire around five offspring in a reproductive career. If 50% of the male’s infants die from natural causes and the other 50% from infanticide, a male who fails to stay with his female risks ending up with zero fitness, and so does the female, irrespective of any risk to themselves from predation. Infanticide would not, however, explain pairbonding in antelope, for whom predation risk might be a more likely explanation (Dunbar and Dunbar, 1980).
In sum, we have shown, using a reverse engineering approach, that the dispersed females hypothesis cannot be the explanation for monogamy in mammals. In virtually all populations which have useable data, males would do better to adopt a roving male strategy if the only reason was for being monogamous was to ensure access to sirings. I used several conceptually different models to test this, and they all agree with each other. This suggests that males must be providing the female with a service of some kind. We can exclude biparental care, since this always evolves after pairbonding in mammals in general, and primates in particular. Resource defence for the benefit of the female cannot be the answer because the models clearly show that males could do this for several females at the same time–in fact, they do not need to be pairbonded (i.e., attached) to a female to achieve this. Moreover, by staying with the female the male inevitably hampers his ability to detect and exclude ecological competitors. The most likely options thus seem to be that pairbonded males provide protection from predation or infanticide.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.905298/full#supplementary-material
References
Abbott, D. H. (1984). Behavioral and physiological suppression of fertility in subordinate marmoset monkeys. Am. J. Primat. 6, 169–186. doi: 10.1002/ajp.1350060305
Adamczak, V., and Dunbar, R. I. M. (2008). Variation in the mating system of oribis and their ecological determinants. Afr. J. Ecol. 45, 197–206. doi: 10.1111/j.1365-2028.2007.00833.x
Alberts, S. C., and Altmann, J. (1995). Balancing costs and opportunities: dispersal in male baboons. Am. Nat. 145, 279–306. doi: 10.1086/285740
Andelman, S. J. (1987). “Ecological and social determinants of cercopithecine mating patterns,” in Ecological Aspects of Social Evolution, eds D. I. Rubenstein and R. W. Wrangham (Princeton, NJ: Princeton University Press), 201–217. doi: 10.2307/j.ctt7zvwgq.14
Bales, K., O’Herron, M., Baker, A. J., and Dietz, J. M. (2001). Sources of variability in numbers of live births in wild golden lion tamarins (Leontopithecus rosalia). Am. J. Primatol. 54, 211–221. doi: 10.1002/ajp.1031
Bettridge, C., Lehmann, J., and Dunbar, R. I. M. (2010). Trade-offs between time, predation risk and life history, and their implications for biogeography: a systems modelling approach with a primate case study. Ecol. Model. 221, 777–790. doi: 10.1016/j.ecolmodel.2009.11.017
Borries, C. (2000). “Male dispersal and mating season influxes in Hanuman langurs living in multi-male groups,” in Primate Males: Causes and Consequences of Variation in Group Composition, ed. P. Kappeler (Cambridge, MA: Cambridge University Press), 146–140.
Borries, C., Savini, T., and Koenig, A. (2011). Social monogamy and the threat of infanticide in larger mammals. Behav. Ecol. Sociob. 65, 685–693. doi: 10.1007/s00265-010-1070-5
Brotherton, P. N., and Manser, M. B. (1997). Female dispersion and the evolution of monogamy in the dik-dik. Anim. Behav. 54, 1413–1424. doi: 10.1006/anbe.1997.0551
Brotherton, P. N., Pemberton, J. M., Komers, P. E., and Malarky, G. (1997). Genetic and behavioural evidence of monogamy in a mammal, Kirk’s dik–dik (Madoqua kirkii). Proc. R. Soc. Lond. 264B, 675–681. doi: 10.1098/rspb.1997.0096
Brotherton, P. N. M. (1994). The evolution of monogamy in the dik-dik. PhD thesis. Cambridge, MA: Cambridge University.
Brotherton, P. N. M., and Komers, P. E. (2003). “Mate guarding and the evolution of social monogamy in mammals,” in Monogamy: Mating Strategies and Partnerships in Birds, Humans and Other Mammals, eds U. H. Reichard and C. Boesch (Cambridge, MA: Cambridge University Press), 42–58. doi: 10.1017/cbo9781139087247.003
Burnham, D., Bearder, S., Cheyne, S., Dunbar, R. I. M., and Macdonald, D. (2012). A taste of predation past: is nocturnal behaviour in primates explained by the threat of predation by cats? Folia Primatol. 83, 236–251.
Campbell, C. J., Fuentes, A., Mackinnon, K., Panger, M., and Bearder, S. K. (eds) (2007). Primates in Perspective. Oxford: Oxford University Press.
Caselli, C. B., Mennill, D. J., Gestich, C. C., Setz, E. Z., and Bicca-Marques, J. C. (2015). Playback responses of socially monogamous black-fronted titi monkeys to simulated solitary and paired intruders. Am. J. Primat. 77, 1135–1142. doi: 10.1002/ajp.22447
Chapman, N. G., Claydon, K., Claydon, M., Forde, P. G., and Harris, S. (1993). Sympatric populations of muntjac (Muntiacus reevesi) and roe deer (Capreolus capreolus): a comparative analysis of their ranging behaviour, social organization and activity. J. Zool. 229, 623–640. doi: 10.1111/j.1469-7998.1993.tb02660.x
Cheney, D. L., Seyfarth, R. M., Fischer, J., Beehner, J., Bergman, T., Johnson, S. E., et al. (2004). Factors affecting reproduction and mortality among baboons in the Okavango Delta, Botswana. Internat. J. Primat. 25, 401–428. doi: 10.1023/b:ijop.0000019159.75573.13
Cheney, D. L., and Wrangham, R. W. (1987). “Predation,” in Primate Societies, eds B. Smuts, D. Cheney, R. Seyfarth, R. W. Wrangham, and T. Struhsaker (Chicago: Chicago University Press), 227–239.
Clarke, P. M. R., Henzi, S. P., Barrett, L., and Rendall, D. (2008). On the road again: competitive effects and condition-dependent dispersal in male baboons. Anim. Behav. 76, 55–63. doi: 10.1016/j.anbehav.2008.01.009
Cords, M. (2004). “When are there influxes in blue monkey groups?,” in The Guenons: Diversity and Adaptation in African Monkeys, (Boston, MA: Springer), 189–201. doi: 10.1007/0-306-48417-x_14
Dietz, J. M., and Baker, A. J. (1993). Polygyny and female reproductive success in golden lion tamarins, Leontopithecus rosalia. Anim. Behav. 46, 1067–1078. doi: 10.1006/anbe.1993.1297
Digby, L. J., Ferrari, S. F., and Saltzman, W. (2007). “The role of competition in cooperatively breeding species,” in Primates in Perspective, eds C. Campbell, A. Fuentes, K. Mackinnon, S. K. Bearder, and S. K. Stumpf (New York, NY: Oxford University Press), 85–106.
Dobson, F. S., Way, B. M., and Baudoin, C. (2010). Spatial dynamics and the evolution of social monogamy in mammals. Behav. Ecol. 21, 747–752. doi: 10.1186/s40462-019-0170-8
Dobzhansky, T. (1973). Nothing in biology makes sense except in the light of evolution. Am. Biol. Teach. 35, 125–129. doi: 10.2307/4444260
Doran-Sheehy, D. M., and Boesch, C. (2004). Behavioral ecology of western gorillas: new insights from the field. Am. J. Primat. 64, 139–143. doi: 10.1002/ajp.20068
Dunbar, R. I. M. (1984). Reproductive Decisions: An Economic Analysis of Gelada Baboon Social Strategies. Princeton NJ: Princeton University Press.
Dunbar, R. I. M. (1988b). Habitat quality, population dynamics and group composition in colobus monkeys (Colobus guereza). Internat. J. Primat. 9, 299–329. doi: 10.1007/bf02737386
Dunbar, R. I. M. (1992). Time: a hidden constraint on the behavioural ecology of baboons. Behav. Ecol. Sociobiol. 31, 35–49. doi: 10.1007/bf00167814
Dunbar, R. I. M. (1995a). The mating system of Callitrichid primates. I. Conditions for the coevolution of pairbonding and twinning. Anim. Behav. 50, 1057–1070. doi: 10.1016/0003-3472(95)80106-5
Dunbar, R. I. M. (1995b). The mating system of Callitrichid primates. II. The impact of helpers. Anim. Behav. 50, 1071–1089. doi: 10.1016/0003-3472(95)80107-3
Dunbar, R. I. M. (2000). “Male mating strategies: a modelling approach,” in Primate Males, ed. P. Kappeler (Cambridge, MA: Cambridge University Press), 259–268.
Dunbar, R. I. M. (2001). “The economics of male mating strategies among primates,” in Economic Models of Animal and Human Behaviour, eds J. van Hooff, R. Noë, and P. Hammerstein (Cambridge, MA: Cambridge University Press), 245–269. doi: 10.1017/cbo9780511752421.016
Dunbar, R. I. M. (2010). “Deacon’s dilemma: the problem of pairbonding in human evolution,” in Social Brain, Distributed Mind, eds R. I. M. Dunbar, C. Gamble, and J. A. J. Gowlett (Oxford: Oxford University Press), 159–179.
Dunbar, R. I. M. (2018a). Social structure as a strategy to mitigate the costs of group-living: a comparison of gelada and guereza monkeys. Anim. Behav. 136, 53–64. doi: 10.1016/j.anbehav.2017.12.005
Dunbar, R. I. M. (2018b). The anatomy of friendship. Trends Cogn. Sci. 22, 32–51. doi: 10.1016/j.tics.2017.10.004
Dunbar, R. I. M., Buckland, D., and Miller, D. (1990). Mating strategies of male feral goats: a problem in optimal foraging. Anim. Behav. 40, 653–667. doi: 10.1016/s0003-3472(05)80695-5
Dunbar, R. I. M., Cheyne, S., Lan, D., Korstjens, A. H., Lehmann, J., and Cowlishaw, G. (2019). Environment and time as constraints on the biogeographical distribution of gibbons. Am. J. Primat. 81:e22940. doi: 10.1002/ajp.22940
Dunbar, R. I. M., and Dunbar, P. (1979). Observations on the social organisation of the common duiker in Ethiopia. Afr. J. Ecol. 17, 249–252. doi: 10.1111/j.1365-2028.1979.tb00262.x
Dunbar, R. I. M., Korstjens, A. H., and Lehmann, J. (2009). Time as an ecological constraint. Biolog. Rev. 84, 413–429. doi: 10.1111/j.1469-185x.2009.00080.x
Dunbar, R. I. M., MacCarron, P., and Robertson, C. (2018a). Tradeoff between fertility and predation risk drives a geometric sequence in the pattern of group sizes in baboons. Biol. Lett. 14:20170700. doi: 10.1098/rsbl.2017.0700
Dunbar, R. I. M., MacCarron, P., and Shultz, S. (2018b). Primate social group sizes exhibit a regular scaling pattern with natural attractors. Biol. Lett. 14:20170490. doi: 10.1098/rsbl.2017.0490
Dunbar, R. I. M., and Shultz, S. (2010). Bondedness and sociality. Behaviour 147, 775–803. doi: 10.1163/000579510x501151
Dunbar, R. I. M., and Shultz, S. (2021a). The infertility trap: the costs of group-living and mammalian social evolution. Front. Ecol. Evol. 2021:634664.
Dunbar, R. I. M., and Shultz, S. (2021b). Social complexity and the fractal structure of social groups in primate social evolution. Biol. Rev. 96, 1889–1906. doi: 10.1111/brv.12730
Emlen, S. T., and Oring, L. W. (1977). Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223. doi: 10.1126/science.327542
Enstam, K. L., and Isbell, L. A. (2007). “The guenons (genus Cercopithecus) and their allies: behavioural ecology of polyspecific associations,” in Primates in Perspective, eds C. J. Campbell, A. Fuentes, K. Mackinnon, M. Panger, and S. K. Bearder (Oxford: Oxford University Press), 252–273.
Fashing, P. J. (2007). “African colobine monkeys: patterns of between-group interaction,” in Primates in Perspective, eds C. J. Campbell, A. Fuentes, K. Mackinnon, M. Panger, and S. K. Bearder (Oxford: Oxford University Press), 201–223.
Fernandez-Duque, E. (2007). “Aotinae: social monogamy in the only nocturnal haplorhines,” in Primates in Perspective, eds C. J. Campbell, A. Fuentes, K. Mackinnon, M. Panger, and S. K. Bearder (Oxford: Oxford University Press), 139–154.
Ferrari, S. F., and Digby, L. J. (1996). Wild Callithrix groups: stable extended families? Am. J. Primat. 38, 19–27. doi: 10.1002/(SICI)1098-2345(1996)38:1<19::AID-AJP3>3.0.CO;2-W
Ferrari, S. F., and Ferrari, M. A. L. (1989). A re-evaluation of the social organisation of the Callitrichidae, with reference to the ecological differences between genera. Folia Primatologica 52, 132–147. doi: 10.1159/000156392
Fitzgibbon, C. D. (1990). Why do hunting cheetahs prefer male gazelles? Anim. Behav. 40, 837–845. doi: 10.1016/s0003-3472(05)80984-4
Gao, L., and Cords, M. (2020). Effects of female group size on the number of males in blue monkey (Cercopithecus mitis) groups. Internat. J. Primat. 41, 665–682. doi: 10.1007/s10764-020-00174-3
Garber, P. A. (1994). Phylogenetic approach to the study of tamarin and marmoset social systems. Am. J. Primat. 34, 199–219. doi: 10.1002/ajp.1350340210
Goldizen, A. W. (1987). Facultative polyandry and the role of infant-carrying in wild saddle-back tamarins (Saguinus fiscicollis). Behav. Ecol. Sociob. 20, 89–109.
Goldizen, A. W. (1988). Tamarin and marmoset mating systems: unusual flexibility. Trends Ecol. Evol. 3, 36–40. doi: 10.1016/0169-5347(88)90045-6
Griffin, A. S., Alonzo, S. H., and Cornwallis, C. K. (2013). Why do cuckolded males provide paternal care? PLoS Biol. 11:e1001520. doi: 10.1371/journal.pbio.1001520
Hamilton, W. J., and Bulger, J. (1992). Facultative expression of behavioral differences between one-male and multimale savanna baboon groups. Am. J. Primat. 28, 61–71. doi: 10.1002/ajp.1350280106
Harvey, P. H., and Clutton-Brock, T. H. (1985). Life history variation in primates. Evolution 39, 559–581. doi: 10.2307/2408653
Hendrichs, H. (1975). Changes in a population of dikdik, Madoqua (Rhynchotragus) kirki (Gunther 1880). Zeitschrift für Tierpsychologie 38, 55–69. doi: 10.1111/j.1439-0310.1975.tb01992.x
Henzi, S. P., and Lawes, M. (1987). Breeding season influxes and the behaviour of adult male samango monkeys (Cercopithecus mitis albogularis). Folia Primatol. 48, 125–136. doi: 10.1159/000156290
Hilgartner, R., Fichtel, C., Kappeler, P. M., and Zinner, D. (2012). Determinants of pair-living in red-tailed sportive lemurs (Lepilemur ruficaudatus). Ethology 118, 466–479. doi: 10.1111/j.1439-0310.2012.02033.x
Hill, R. A., Lycett, J., and Dunbar, R. I. M. (2000). Ecological determinants of birth intervals in baboons. Behav. Ecol. 11, 560–564. doi: 10.1093/beheco/11.5.560
Isbell, L. A. (1994). Predation on primates: ecological patterns and evolutionary consequences. Evol. Anthropol. 3, 61–71. doi: 10.1002/evan.1360030207
Isbell, L. A., Cheney, D. L., and Seyfarth, R. M. (2004). “Why vervet monkeys (Cercopithecus aethiops) live in multimale groups,” in The Guenons: Diversity and Adaptation in African Monkeys, (Boston, MA: Springer), 173–187. doi: 10.1007/0-306-48417-x_13
Kamilar, J. M., and Cooper, N. (2013). Phylogenetic signal in primate behaviour, ecology and life history. Philosoph. Transac. R. Soc. 368B:20120341. doi: 10.1098/rstb.2012.0341
Kappeler, P. M., and Pozzi, L. (2019). Evolutionary transitions toward pair living in nonhuman primates as stepping stones toward more complex societies. Sci. Adv. 5:eaay1276. doi: 10.1126/sciadv.aay1276
Kierulff, M. C. M., and Rylands, A. B. (2003). Census and distribution of the golden lion tamarin (Leontopithecus rosalia). Am. J. Primat. 59, 29–44. doi: 10.1002/ajp.10064
Knowlton, N. (1979). Reproductive synchrony, parental investment, and the evolutionary dynamics of sexual selection. Anim. Behav. 27, 1022–1033. doi: 10.1016/0003-3472(79)90049-6
Komers, P. E. (1996). Obligate monogamy without paternal care in Kirk’s dikdik. Anim. Behav. 51, 131–140. doi: 10.1006/anbe.1996.0011
Komers, P. E., and Brotherton, P. N. M. (1997). Female space use is the best predictor of monogamy in mammals. Proc. R. Soc. Lond. 264B, 1261–1270. doi: 10.1098/rspb.1997.0174
Korstjens, A. H., and Dunbar, R. I. M. (2007). Time constraints limit group sizes and distribution in red and black-and-white colobus monkeys. Internat. J. Primat. 28, 551–575. doi: 10.1007/s10764-007-9148-2
Korstjens, A. H., Lehmann, J., and Dunbar, R. I. M. (2018). Time constraints do not limit group size in arboreal guenons but do explain community size and distribution patterns. Internat. J. Primat. 39, 511–531. doi: 10.1007/s10764-018-0048-4
Kummer, H. (1970). “Cross-species modification of social behaviour in baboons,” in Old World Monkeys, eds J. H. Napier and P. Napier (London: Academic Press), 353–363.
Kummer, H., Götz, W., and Angst, W. (1974). Triadic differentiation: an inhibitory process protecting pair bonds in baboons. Behaviour 49, 62–87. doi: 10.1163/156853974x00408
Lehmann, J., Korstjens, A. H., and Dunbar, R. I. M. (2008). Time management in great apes: implications for gorilla biogeography. Evolut. Ecol. Res. 10, 515–536.
Leighton, D. M. (1987). “Gibbons: territoriality and monogamy,” in Primate Societies, eds B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, and T. T. Strusaker (Chicago: Chicago University Press), 135–145.
Lowe, A. E., Hobaiter, C., Asiimwe, C., Zuberbühler, K., and Newton-Fisher, N. E. (2019). Intra-community infanticide in wild, eastern chimpanzees: a 24-year review. Primates 2019:3. doi: 10.1007/s10329-019-00730-3
Lowen, C. B., and Dunbar, R. I. M. (1994). Territory size and defendability in primates. Behav. Ecol. Sociob. 35, 347–354. doi: 10.1007/bf00184423
Lukas, D., and Clutton-Brock, T. H. (2013). The evolution of social monogamy in mammals. Science 341, 526–530. doi: 10.1126/science.1238677
Lukas, D., and Huchard, E. (2014). The evolution of infanticide by males in mammalian societies. Science 346, 841–844. doi: 10.1126/science.1257226
Ma, C. Y., Brockelman, W. Y., Light, L. E., Bartlett, T. Q., and Fan, P. F. (2019). Infant loss during and after male replacement in gibbons. Am. J. Primat. 81:e23036. doi: 10.1002/ajp.23036
Menzel, C. R. (1993). “Coordination and conflict in Callicebus social groups,” in Primate Social Conflict, eds W. A. Mason and S. P. Mendoza (Albany NY: State University of New York Press), 253–290.
Mitani, J. C., and Rodman, P. (1979). Territoriality: the relation of ranging pattern and home range size to defendability, with an analysis of territoriality among primate species. Behav. Ecol. Sociob. 5, 241–251. doi: 10.1007/bf00293673
Norconk, M. A. (2007). “Sakis, uakaris, and titi monkeys: behavioral diversity in a radiation of primate seed predators,” in Primates in Perspective, eds C.J. Campbell, A. Fuentes, K. Mackinnon, M. Panger, and S.K. Bearder (Oxford: Oxford University Press) 123–138.
Norton, P. M. (1980). The habitat and feeding ecology of the klipspringer Oreotragus Oreotragus (Zimmermann, 1973) in two areas of the Cape Province. MSc dissertation, University of Pretoria.
Olupot, W., and Waser, P. M. (2001). Correlates of intergroup transfer in male grey-cheeked mangabeys. Internat. J. Primat. 22, 169–187.
Opie, C., Atkinson, Q., Dunbar, R. I. M., and Shultz, S. (2013). Male infanticide leads to social monogamy in primates. PNAS 110, 13328–13332. doi: 10.1073/pnas.1307903110
Overdorff, D. J., and Tecot, S. R. (2006). “Social pair-bonding and resource defense in wild red-bellied lemurs (Eulemur rubriventer), in Lemurs,” in Ecology and Adaptation, eds L. Gould and M. L. Sauther (Boston MA: Springer), 235–254.
Palombit, R. A. (1996). Pair bonds in monogamous apes: a comparison of the siamang Hylobates syndactylus and the white-handed gibbon Hylobates lar. Behaviour 133, 321–356. doi: 10.1163/156853996x00486
Pawlowski, B. P., Lowen, C. B., and Dunbar, R. I. M. (1998). Neocortex size, social skills and mating success in primates. Behaviour 135, 357–368. doi: 10.1163/156853998793066285
Pérez-Barbería, J., Shultz, S., and Dunbar, R. I. M. (2007). Evidence for intense coevolution of sociality and brain size in three orders of mammals. Evolution 61, 2811–2821. doi: 10.1111/j.1558-5646.2007.00229.x
Porter, L. M., Hanson, A. M., and Becerra, E. N. (2001). Group demographics and dispersal in a wild group of Goeldi’s monkeys (Callimico goeldii). Folia Primatol. 72, 108–110. doi: 10.1159/000049933
Robbins, M. (2007). “Gorillas: diversity in ecology and behaviour,” in Primates in Perspective, eds C. J. Campbell, A. Fuentes, K. Mackinnon, M. Panger, and S. K. Bearder (Oxford: Oxford University Press), 305–321.
Roberts, S. J., Nikitopoulos, E., and Cords, M. (2014). Factors affecting low resident male siring success in one-male groups of blue monkeys. Behav. Ecol. 25, 852–861. doi: 10.1093/beheco/aru060
Rylands, A. B. (1989). Sympatric Brazilian callitrichids: the black tufted-ear marmoset, Callithrix kuhli, and the golden-headed lion tamarin, Leontopithecus chrysomelas. J. Hum. Evol. 18, 679–695. doi: 10.1016/0047-2484(89)90100-0
Savini, T., Boesch, C., and Reichard, U. H. (2008). Home-range characteristics and the influence of seasonality on female reproduction in white-handed gibbons (Hylobates lar) at Khao Yai National Park, Thailand. Am. J. Phys. Anthropol. 135, 1–12. doi: 10.1002/ajpa.20578
Schmidt-Nielsen, K. (1984). Scaling: Why Is Animal Size So Important?. Cambridge, MA: Cambridge University Press.
Schülke, O. (2003). To breed or not to breed—food competition and other factors involved in female breeding decisions in the pair-living nocturnal fork-marked lemur (Phaner furcifer). Behav. Ecol. Sociob. 55, 11–21. doi: 10.1007/s00265-003-0676-2
Schülke, O., Kappeler, P. M., and Zischler, H. (2004). Small testes size despite high extra-pair paternity in the pair-living nocturnal primate Phaner furcifer. Behav. Ecol. Sociob. 55, 293–301. doi: 10.1007/s00265-003-0709-x
Shultz, S., and Dunbar, R. I. M. (2007). The evolution of the social brain: Anthropoid primates contrast with other vertebrates. Proc. R. Soc. Lond. 274B, 2429–2436. doi: 10.1098/rspb.2007.0693
Shultz, S., and Dunbar, R. I. M. (2010). Social bonds in birds are associated with brain size and contingent on the correlated evolution of life-history and increased parental investment. Biolog. J. Linn. Soc. 100, 111–123. doi: 10.1111/j.1095-8312.2010.01427.x
Shultz, S., and Finlayson, L. V. (2010). Large body and small brain and group sizes are associated with predator preferences for mammalian prey. Behav. Ecol. 21, 1073–1079. doi: 10.1093/beheco/arq108
Shultz, S., Noe, R., McGraw, S., and Dunbar, R. I. M. (2004). A community-level evaluation of the impact of prey behavioural and ecological characteristics on predator diet composition. Proc. R. Soc. Lond. 271B, 725–732. doi: 10.1098/rspb.2003.2626
Shultz, S., Opie, C., and Atkinson, Q. D. (2011). Stepwise evolution of stable sociality in primates. Nature 479, 219–222. doi: 10.1038/nature10601
Stewart, K. J., and Harcourt, A. H. (1987). “Gorillas: variation in female relationships,” in Primate Societies, eds B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, and T. T. Strusaker (Chicago: Chicago University Press), 155–164.
Strier, K. B., Lee, P. C., and Ives, A. R. (2014). Behavioral flexibility and the evolution of primate social states. PLoS One 9:e114099. doi: 10.1371/journal.pone.0114099
Tecot, S. R., Singletary, B., and Eadie, E. (2016). Why “monogamy” isn’t good enough. Am. J. Primat. 78, 340–354. doi: 10.1002/ajp.22412
van Schaik, C., and Dunbar, R. I. M. (1990). The evolution of monogamy in large primates: a new hypothesis and some critical tests. Behaviour 115, 30–62. doi: 10.1163/156853990x00284
van Schaik, C. P., and Kappeler, P. M. (1997). Infanticide risk and the evolution of male–female association in primates. Proc. R. Soc. Lond. 264B, 1687–1694. doi: 10.1098/rspb.1997.0234
Keywords: pairbonding, primates, ungulates, territory size, predation risk, infanticide
Citation: Dunbar RIM (2022) Female Dispersion Is Necessary, but Not Sufficient, for Pairbonded Monogamy in Mammals. Front. Ecol. Evol. 10:905298. doi: 10.3389/fevo.2022.905298
Received: 26 March 2022; Accepted: 19 April 2022;
Published: 17 May 2022.
Edited by:
Javier Perez-Barberia, University of Castilla-La Mancha, SpainReviewed by:
Iain James Gordon, Australian National University, AustraliaFloyd Weckerly, Texas State University, United States
Copyright © 2022 Dunbar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. I. M. Dunbar, cm9iaW4uZHVuYmFyQHBzeS5veC5hYy51aw==
 R. I. M. Dunbar
R. I. M. Dunbar