- 1Behavioral Ecology and Sociobiology Unit, German Primate Center - Leibniz-Institute for Primate Research, Göttingen, Germany
- 2Primate Genetics Laboratory, German Primate Center - Leibniz-Institute for Primate Research, Göttingen, Germany
- 3Laboratory of Comparative Ethology and Biocommunication, Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Moscow, Russia
Coordinated singing, performed as duets by mated pairs and often joined by offspring to form choruses, is a distinctive behavioral attribute of the social system of pair-living and pair-bonded Neotropical titi monkeys. Duets and choruses are presumed to be associated with mate or territorial defense, but no consensus has yet been reached regarding their function. Here, we examined temporal and spatial patterns of coordinated singing in eight wild groups of coppery titi monkeys, Plecturocebus cupreus, in Peruvian Amazonia to test predictions of the joint resource and mate defense. We investigated singing rates in relation to female reproductive state, fruit consumption and demographic context using a dataset based on 227 observation days and analyzed temporal and spatial distribution of songs using a dataset based on 150 songs, collected between June 2017 and September 2021. Titi monkeys sang least frequently when females were likely to be sexually receptive and most frequently when females were likely to be pregnant. Groups also sang slightly more often when fruits were consumed more intensively, although this association did not reach statistical significance. The duration of songs was not associated with female reproductive state or fruit consumption, but songs were longer during inter-group encounters compared to non-encounter contexts. Songs were not concentrated in the core areas of home ranges; rather, they were distributed throughout the home ranges in concordance with its use. Finally, songs were concentrated around dawn. Our results provide support for a function in joint resource defense and inter-group communication of coordinated songs in coppery titi monkeys. The function of coordinated songs for mate defense in the form of paternity guarding, on the other hand, was not supported by our findings.
Introduction
Duets are joint acoustic displays where two individuals coordinate their songs so that they alternate or overlap (Hall, 2009, 2004; De Gregorio et al., 2022). Among vertebrates, duetting is taxonomically most widespread among birds (although only a minority of birds duet; Hall, 2009). In mammals, duetting is mainly, but not exclusively, found in primates (Tilson and Norton, 1981; Paula and Monticelli, 2021). Both in birds and in primates, duetting is most common in pair-living species with long-term pair bonds and year-round territoriality (Malacarne et al., 1991; Benedict, 2008; Adret et al., 2018). Duetting primates include the Neotropical titi monkeys (genera Callicebus, Plecturocebus, and Cheracebus), south-east Asian gibbons (all species except Hylobates moloch and Hylobates klossii), Malagasy indris (Indri indri), Mentawai langurs (Presbytis potenziani), and Sulawesi tarsiers (genus Tarsius) (Tilson and Tenaza, 1976; Haimoff, 1986; Nietsch, 1999; Méndez-Cárdenas and Zimmermann, 2009; Adret et al., 2018; Bonadonna et al., 2020).
Duets are usually produced by paired females and males (although there are exceptions, such as male-male duets in lekking manakins of the genus Chiroxiphia: Snow, 1977) (Hall, 2004). In some species, offspring can join the adults’ duets to form choruses — e.g., in titi monkeys (Adret et al., 2018) and yellow-cheeked gibbons, Nomascus gabriellae (Merker and Cox, 1999). It has been suggested that the participation of the offspring in the adult singing may represent a form of practicing toward a development of adult-like song (De Gregorio et al., 2022).
Although duets have been studied extensively in birds, it remains unclear why in some species an individual coordinates its song with those of its partner instead of singing independently (Hall, 2009). Most avian studies agree that duets are largely cooperative displays, where song coordination provides one or several benefits to both individuals (Mennill and Vehrencamp, 2008; Hall, 2009). Several hypotheses have been proposed to describe the nature of these benefits.
According to the joint resource defense hypothesis, duets function as a cooperative display to outsiders, advertising an ownership of a home range and/or resources (Hall, 2004; Logue, 2005; Rasoloharijaona et al., 2006; Caselli et al., 2015). According to the mate defense hypothesis, an individual participates in coordinated singing to advertise its partner’s or its own mated status to outsiders and repel potential rivals (Robinson, 1981; Levin, 1996; Appleby et al., 1999; Hall, 2004). Within a mate defense function, an individual joins its partner’s song in a duet to advertise a partner’s mated status to outsiders and repel potential rivals (Robinson, 1981; Levin, 1996; Appleby et al., 1999; Hall, 2004). There are various forms of mate defense: an individual may prevent same-sex rivals from pairing with its partner or reinforce a partner’s position within the pair by advertising its status to opposite-sex outsiders. One specific type of mate defense is paternity guarding, where a male joins his mate’s singing to repel rival males seeking extra-pair copulations (Hall, 2004). In addition, an individual contribution to duetting has been proposed to be directed at a partner to function for pair-bond reinforcement or signaling commitment to a partner (Hall, 2004; Méndez-Cárdenas and Zimmermann, 2009).
Predictions for these hypotheses can be divided into two groups, one explaining the function of singing regardless of whether it is coordinated or not and the other explaining why duetting in each of these contexts is more effective than solo or uncoordinated singing in achieving the corresponding effect on listeners. For example, if duetting or uncoordinated singing functions for joint resource defense, it can be expected to be more frequent when defensible and valuable resources, such as fruits, are available. However, there are many features of duets that are consistent with a joint resource defense but do not distinguish duetting from uncoordinated singing — i.e., duets are often loud and performed in counter-singing interactions with neighbors or in response to intrusion (Hall, 2004, 2009). To show that duetting itself has a function for joint resource defense, it is necessary to show that coordination of songs plays a role in this function over and above that achieved by uncoordinated songs (Hall, 2009). Specifically, duets should be more threatening displays than uncoordinated songs and partners should be more likely to coordinate their songs into duets than to sing alone when faced with outsiders (Hall, 2004). Similarly, if singing functions, for example, for male mate defense in the form of paternity guarding, it should be more frequent when females are sexually receptive. However, to show that duetting is more efficient in achieving this function than uncoordinated singing, it is necessary to demonstrate that duets are initiated by females and that males join more of their partners’ songs to form duets when females are sexually receptive (Hall, 2004).
Titi monkeys are Neotropical primates living in groups comprising one reproductive pair and one to three young (Kinzey, 1981; Kinzey and Robinson, 1983; Bicca-Marques and Heymann, 2013; Fernandez-Duque et al., 2013). They exhibit strong long-term pair bonds, year-round territoriality and biparental infant care (Anzenberger, 1988; Bicca-Marques and Heymann, 2013; Fernandez-Duque et al., 2013; Van Belle et al., 2016). Titi monkey pairs regularly sing in duets that are often joined by juvenile and subadult group members to form choruses (Caselli et al., 2014; Adret et al., 2018). Duets are composed of partially overlapping sequences sung by both mates with no sex-specificity in song components (Robinson, 1979a; Müller and Anzenberger, 2002). However, as shown by research on captive Plecturocebus cupreus (previously Callicebus cupreus), there is individuality in duet contributions of each mate, with moderate heritability of some song characteristics, and duets are pair-specific as a result of a summation of individual attributes of the two mates (Müller and Anzenberger, 2002; Lau et al., 2020; Clink et al., 2022). There is also evidence for vocal convergence among mates in some features of duets and for changes in duet elements correlated with pair-bond duration (Müller and Anzenberger, 2002; Clink et al., 2019).
So far, few studies investigated the function duets and choruses in wild titi monkeys. Generally, the results of these studies seem to be more compatible with the joint resource defense rather than the mate defense hypothesis. In observational and playback studies of black-fronted titi monkeys (Callicebus nigrifrons), coordinated songs were produced more often in months with higher fruit availability, while groups did not sing more often during receptive periods of females; duets were initiated by either partner, and individuals did not show sex-specific responses to the playback of solo songs (Caselli et al., 2014, 2015). In Plecturocebus toppini (previously Callicebus brunneus), males reacted stronger to playbacks in the high-used versus low-used parts of the home range (Lawrence, 2007), also supporting the resource defense hypothesis. On the other hand, Plecturocebus ornatus (previously Callicebus moloch) showed sex-specific reactions to playbacks, and males often sang alone, lending some support to the mate defense hypothesis (Robinson, 1981).
Here, we examine temporal and spatial patterns of coordinated singing in eight wild groups of coppery titi monkeys, Plecturocebus cupreus, to test some of the predictions of the joint resource and mate defense hypotheses for the function of coordinated singing. If singing is more important in joint resource defense, we predict that songs would be produced more frequently and/or be longer when defensible and valuable resources, such as fruits, are consumed more intensively; and/or when groups need more resources, e.g., when females are pregnant or lactating and/or when there are more group members. If, on the other hand, singing is more important in mate defense (in the form of paternity guarding), we predict that songs would be produced more frequently and/or be longer when females are sexually receptive. In addition, we analyzed the spatial distribution of coordinated songs to see whether they are concentrated at the territory borders or are produced throughout the home range in concordance with its use. Finally, we examined temporal distribution of singing throughout the day to see whether songs are concentrated round dawn when sound propagation is optimal (Brown and Handford, 2003). If songs are concentrated around dawn, it would suggest that singing is used for inter-group communication, as opposed to pair-bond reinforcement where songs would be expected to be produced throughout the day. Because this study was observational, we could not analyze the reaction of listeners to different types of songs in different contexts to test the functions of duetting itself as opposed to uncoordinated singing. However, in the Discussion we put our findings in the context of previous experimental studies on wild and captive titi monkeys in order to explore the functions of coordinated singing in this taxon.
Methods
Study Site and Animals
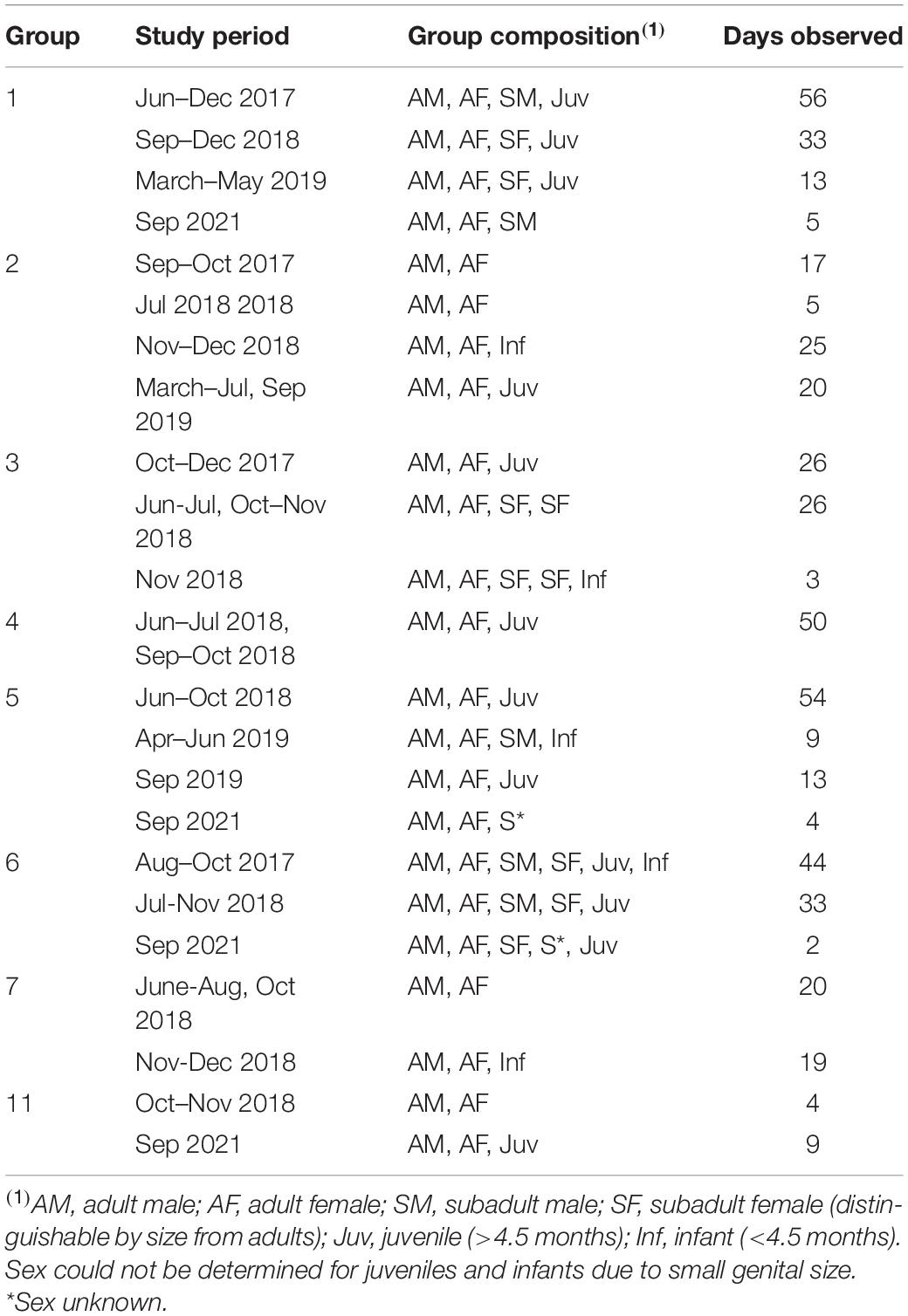
The study was conducted at the Estacioìn Bioloìgica Quebrada Blanco (EBQB) in the Peruvian Amazon (4°21′S, 73°09′W; for details of EBQB see Heymann et al., 2021). We studied eight habituated titi monkey groups in June–December 2017, June–December 2018, March–July and September 2019, and September 2021 (see Table 1 for observation periods, group compositions, and observation times for each group). Group 1 had been studied intermittently since 1997 (e.g., Tirado Herrera and Heymann, 2004) and was well habituated to the presence of humans by the start of this study; the other groups were habituated in 2017–2018.
Data Collection
Each group was followed by a team of two observers in blocks of 5–6 days. We followed a group from the early morning when the animals left a sleeping tree (most often between 5:30 h and 6:30 h) or from when we located the group until the late afternoon when the animals retired to a sleeping tree (most often between 16:00 h and 17:00 h) or until we lost sight of them. During follows, we used instantaneous scan sampling to record the activity of all non-infant (i.e., independently moving) group members at 10-min intervals, allowing 2 min for the location of the animals. During feeding, when possible, we specified the type of food as fruits, leaves, flowers, arthropods, or soil (from termite nests). During each scan point, we recorded the location of a focal group using a handheld GPS unit (Garmin GPSMAP 62s or 64s).
Starting from June 2018, we recorded duration, location, and context (whether a song was produced during an intergroup encounter or not) of all coordinated songs (duets and choruses) produced by focal groups, i.e., groups followed at the moment. A coordinated song was defined as overlapping stereotyped singing of two or more individuals (Adret et al., 2018; Clink et al., 2019). A singing bout was defined as singing interrupted by pauses of less than 5 min; when all group members were silent for ≥ 5 min in between singing bouts, we scored two singing bouts as independent. When calculating the duration of each singing bout, we did not exclude the duration of pauses because when the pauses were shorter than 5 min (our cut-off for scoring two independent bouts) animals usually stayed agitated and continued singing intermittently. We scored encounters whenever the focal group came within a visual contact with another group and responded to its presence by singing and/or chasing. We considered two encounters to be independent when all participants stopped singing and chasing for more than 30 min. In addition, in June–December 2017, we opportunistically recorded duration and location of some (but not all) of songs made by focal groups.
Data Analysis
We made two datasets for our analyses. Dataset 1 comprised data on presence or absence of singing during observation days. This dataset was used to examine whether songs were more frequent on days when fruits were consumed more intensively, when females were sexually receptive, or when groups had unweaned infants or more group members. We included data from June–December 2018, September 2019, and September 2021 in this dataset, as data on presence/absence of singing was only collected systematically during these periods. As animals were active for 10 h a day on average, we excluded days with less than 5 h of observation (less than 30 scan points) from our analyses. We further excluded days for which observations started after 10:00 h as most of songs occurred before this time. This resulted in a dataset of 227 days across eight study groups. Dataset 2 was used to analyze the temporal and spatial distribution of songs and to examine whether songs were longer on days when fruits were consumed more intensively, when females were sexually receptive, or when groups had unweaned infants or more group members, or during intergroup encounters. This dataset included 159 singing bouts across eight study groups for which data were collected starting from June 2017.
To estimate female reproductive state, we used data on infant birth dates (N = 15 births in seven study groups between June 2017 and September 2021) and observed copulations. An infant’s date of birth was estimated as the midpoint between the dates when a group was last seen without and first seen with an infant. The difference between these dates varied between 0 and 26 days. We supplemented these data with visual estimation of infant age based on its body size and tail coloration. To estimate periods when females were likely pregnant, we counted back from an estimated birth date using the average gestation length of 128 days in captive P. cupreus (Valeggia et al., 1999). We estimated that females started to be sexually receptive again 196 days after an estimated birth date as 196 days was the average duration of lactational anovulation in captive P. cupreus (Valeggia et al., 1999).
To estimate when fruits were consumed more intensively, we used a mean monthly proportion of feeding time allocated to fruits by each study group. We first calculated the daily proportions of feeding time allocated to fruits by each adult animal by dividing the number of scan points allocated to fruits by the total daily number of scan points. We then averaged these values for each month and each group. To make these data comparable between groups and months, we used data only for breeding adults because group composition varied between different groups and periods.
What Affected the Probability of Singing?
To test whether the probability of singing was affected by female receptivity, the presence of unweaned infant in a group, the proportion of feeding time allocated to fruits, or group size, we used Dataset 1 and ran a generalized linear mixed model (GLMM; Baayen, 2008) with binomial error structure and logit link function. As a response variable, we used the presence or absence of singing (yes/no) on an observation day. As test predictors, we used female reproductive state (receptive/pregnant/lactating), mean monthly proportion of feeding time allocated to fruits by each study group, and group size (number of non-infants, i.e., independently moving group members). To control for the possible effect of seasonality, we included rainfall data [monthly averages in mm at the nearest meteorological station in Tamshiyacu (4°00′10.7″S 73°09′38.2″W), ca. 40 km north of EBQB, data available at1 ] as a control predictor. To account for repeated observations, we used group ID as a random-effect predictor. The sample size for this model was 171 observation days across seven groups.
Prior to fitting the model, we z-transformed fruit proportion, group size, and rainfall to make a model more easily interpretable (Schielzeth, 2010) and make model convergence more likely. To rule out collinearity, we determined Variance Inflation Factors [VIF, Quinn and Keough (2002)] for a standard linear model excluding the random effects; all VIFs were close to 1 and thus not of issue. After fitting the model, we assessed model stability by excluding the levels of a random effect one by one from the full model. As an overall test of the effect of the test predictors, we compared the full model with a null model lacking the fixed-effect predictors but otherwise having the same structure as the full model (Forstmeier and Schielzeth, 2011) using a likelihood ratio test (Dobson et al., 2008). To test the effect of the individual predictors, we applied likelihood ratio tests (Barr et al., 2013) using R function drop1. We fitted the model in R [version 4.1.1; R Core Team (2021)] using the function lmer of the package lme4 [version 1.1–27.1; Bates et al. (2015)]. We determined VIFs using the function vif of the package car [version 3.0–11; Fox and Weisberg (2011)]. We assessed model stability and bootstrapped model estimates using functions kindly provided by Roger Mundry. The model was fairly stable for all the estimates.
What Affected the Duration of Singing Bouts?
To test whether the duration of singing bouts was affected by female fertility, the presence of an unweaned infant in a group, the proportion of feeding time allocated to fruits, or the context of singing (song produced during an encounter or not), we used Dataset 2 and ran a linear mixed model (LMM; Baayen, 2008). As a response variable, we used the duration of singing bouts (in min). As test predictors, we used female reproductive state (receptive/pregnant/lactating), mean monthly proportion of feeding time allocated to fruits by each study group, group size, and the context of singing (encounter vs. non-encounter). As in the previous model, we included rainfall data as a control predictor. To account for the repeated observations, we used group ID as a random-effect predictor. The sample size for this model was 114 songs across seven groups.
Prior to fitting the model, we square-root-transformed the response to achieve an approximately symmetrical distribution and z-transformed fruit proportion and rainfall to make a model more easily interpretable (Schielzeth, 2010) and make model convergence more likely. To rule out collinearity, we determined Variance Inflation Factors [VIF, Quinn and Keough (2002)] for a standard linear model excluding the random effects; all VIFs were close to 1 and thus not of issue. After fitting the model, we assessed model stability by excluding the levels of a random effect one by one from the full model. As an overall test of the effect of the test predictors, we compared the full model with a null model lacking the fixed-effect predictors but otherwise having the same structure as the full model (Forstmeier and Schielzeth, 2011) using a likelihood ratio test (Dobson et al., 2008). To test the effect of the individual predictors, we applied likelihood ratio tests [Barr et al. (2013)] using R function drop1. We fitted the model in R [version 4.1.1; R Core Team (2021)] using the function lmer of the package lme4 [version 1.1–27.1; Bates et al. (2015)]. We determined VIFs using the function vif of the package car [version 3.0–11; Fox and Weisberg (2011)]. We assessed model stability and bootstrapped model estimates using functions kindly provided by Roger Mundry. The model was fairly stable with the exception for the estimate of the effect of reproductive state.
Spatial Distribution of Songs
To analyze the spatial distribution of songs, we first estimated the home range and core areas of each group using the fixed kernel density method and the reference smoothing factor h (Erran Seaman and Powell, 1996) in the R package adehabitatHR (Calenge, 2006). We defined home ranges as the 95% fixed kernel contour and the core areas as the 50% fixed kernel contour (Asensio et al., 2012; Holzmann et al., 2012). In addition, we drew an inner 25 m border area within the 95% home range of each group using QGIS 3.22.3 (QGIS Development Team, 2022). We then mapped the locations of singing bouts onto the home ranges and used Fisher’s exact tests to compare the frequency of singing in the core area and in the rest of the 95% home range with expected values for each group separately. We did not analyze the border areas separately because the number of songs given in these areas was too low (N = 1–6 songs per group). Expected values were calculated under the null hypothesis of songs being evenly distributed across the home ranges, taking into account the time spent in each area; the time spent in each area was calculated in QGIS 3.22.3 as the proportion of GPS points taken within each area. First, we did these analyses for all songs; next, we repeated the analyses excluding songs given during the intergroup encounters (N = 52) because many of these songs were concentrated near the border areas and could potentially bias the result. Statistical tests were 2-tailed, with p ≤ 0.05.
Temporal Distribution of Songs
To see how songs were distributed over the activity period and whether songs were concentrated in the morning, we divided each observation day into 1-h intervals relative to the time of sunrise to account for seasonal variability in sunrise times. We then calculated the number of recorded singing bouts that began in each interval.
Results
What Affected the Probability of Singing?
The probability of singing was affected by female reproductive state (comparison of full model with reduced model not comprising reproductive state: χ2 = 15.697, df = 2, P = 0.0004; Table 2 and Figures 1, 2). Specifically, the probability of singing was lowest when females were sexually receptive, slightly higher when females were lactating (i.e., when there was an unweaned infant in a group), and considerably higher when females were pregnant. The probability of singing was also somewhat affected by fruit proportion, with groups singing slightly more frequently when fruit proportion in their diet was higher, although this effect did not reach statistical significance (comparison of full model with reduced model not comprising fruit proportion: χ2 = 3.442, df = 1, P = 0.064; Table 2 and Figures 1, 2). Group size and rainfall did not affect singing probability (full-reduced model comparisons for group size: χ2 = 1.471, df = 1, P = 0.225; for rainfall: χ2 = 0.189, df = 1, P = 0.664).
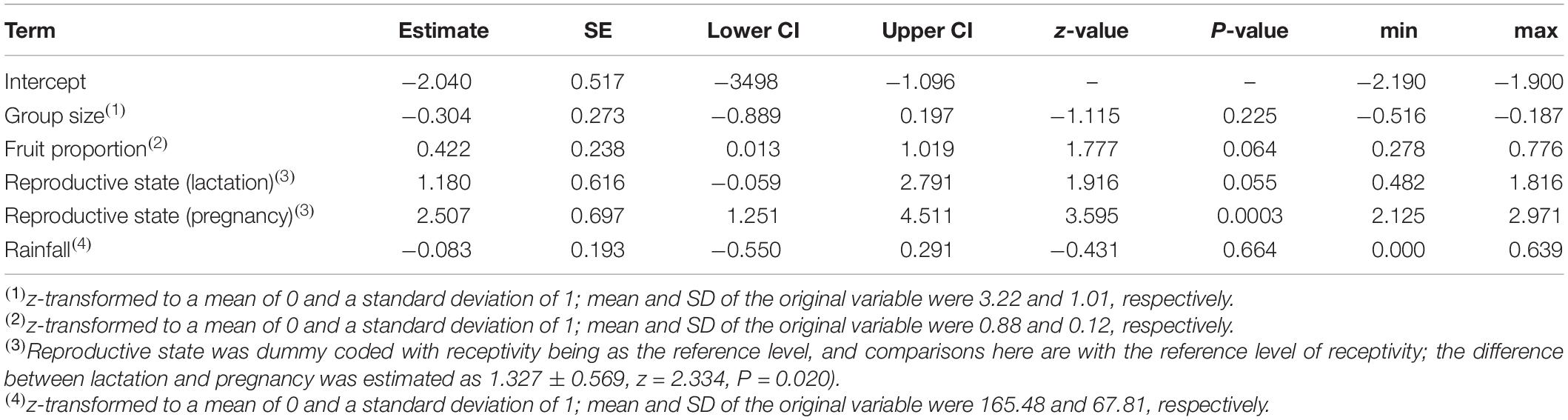
Table 2. Results of the model of the effects of female reproductive state, fruit proportion in the diet, and group size on the probability to of singing: estimates, together with standard errors, confidence intervals, test results, and minimum and maximum of model estimates derived by dropping levels of random effects one at a time.
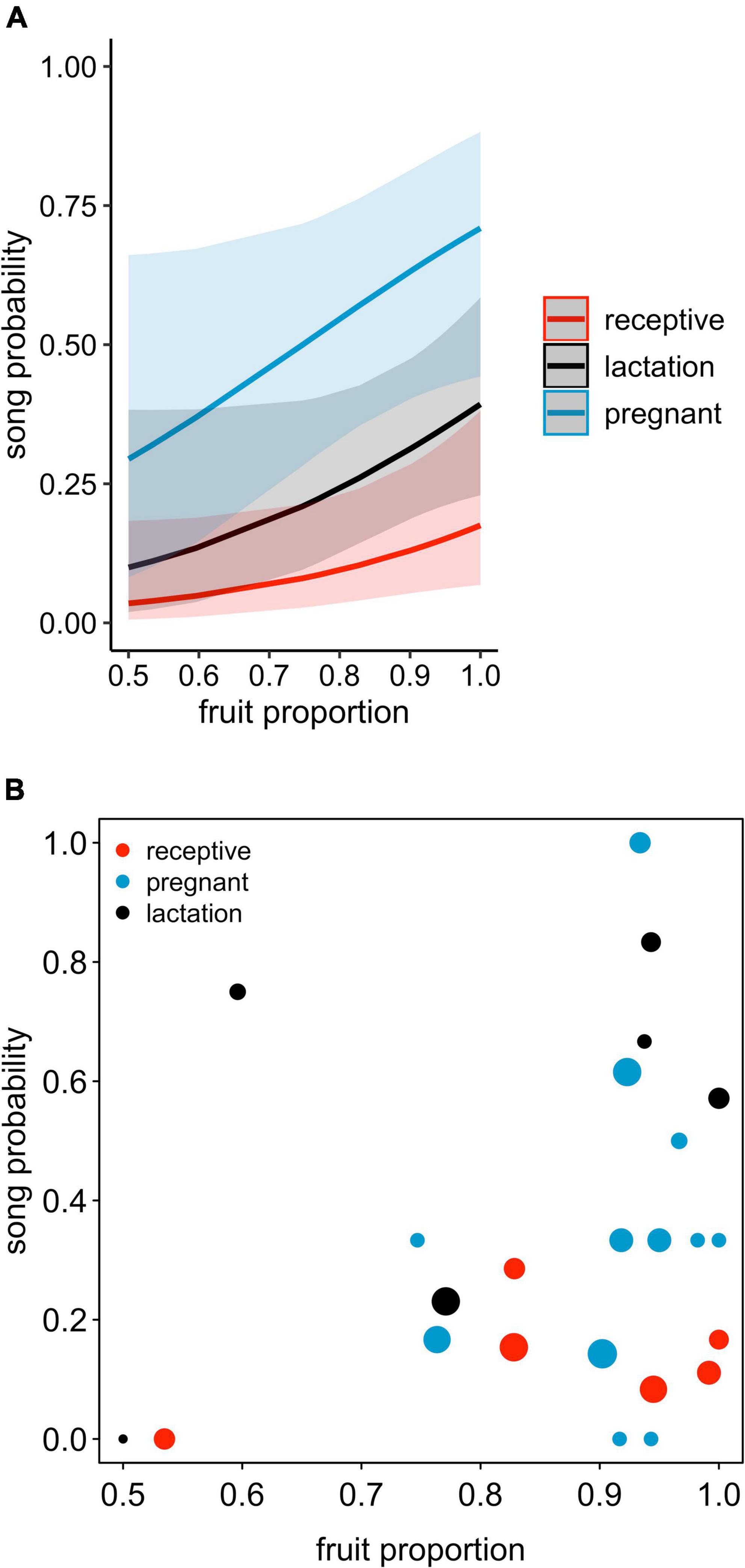
Figure 1. (A) Song probability as a function of female reproductive state and proportion of feeding time allocated to fruits, shown separately for each female reproductive state. The lines depict the fitted model (based on group size at its average), and blue, black, and red areas show the corresponding 95% confidence intervals. (B) Proportions of singing days for different female reproductive states and different fruit proportions, with the area of the dots increasing linearly with the respective sample size (1 to 14 observation days per each combination of reproductive state and fruit proportion, total N = 171 observation days).
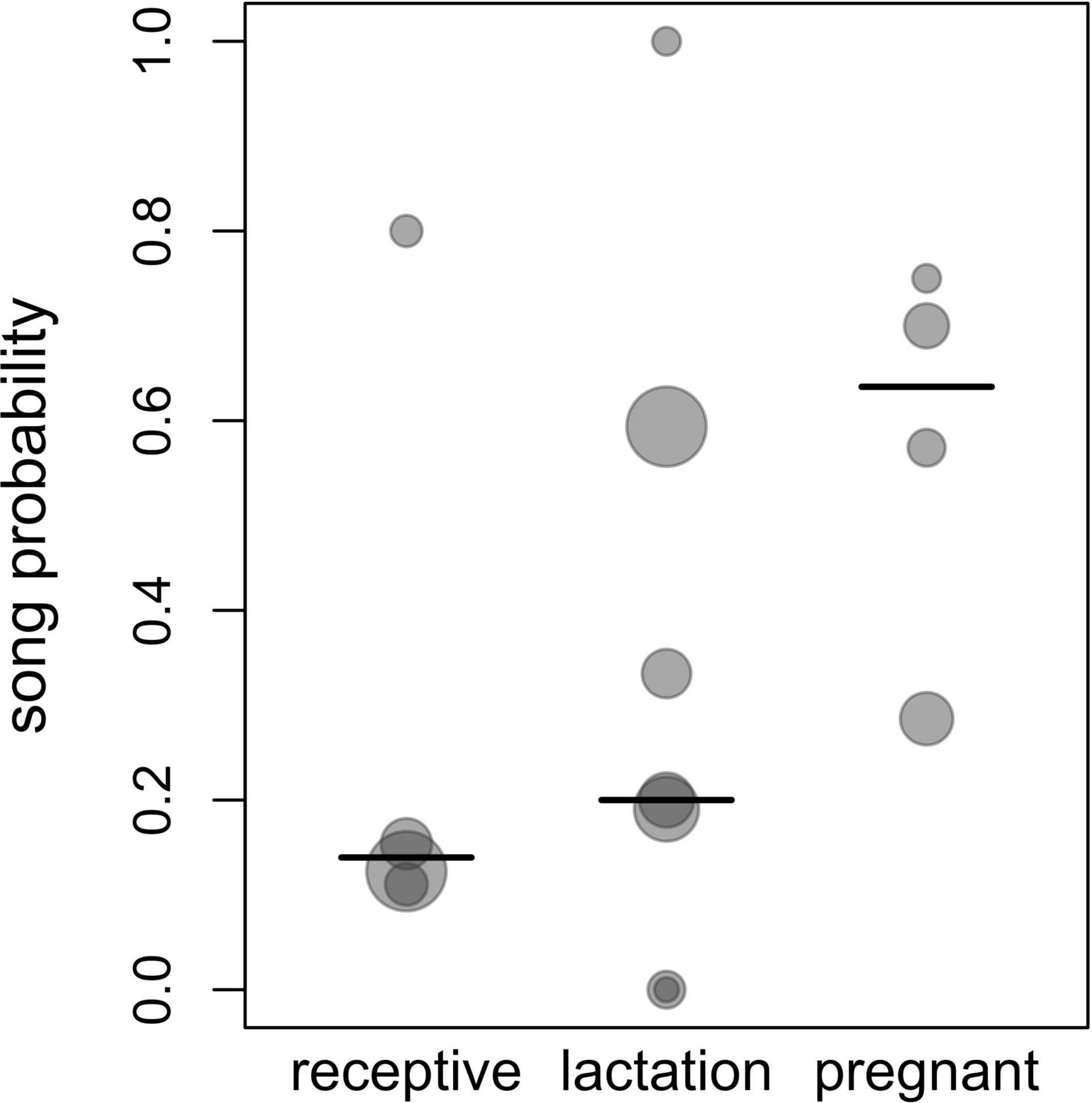
Figure 2. Song probability for different female reproductive states. Shown are proportions of singing days of all observation days, with each dot corresponding to one group ID and the area of the dots increasing linearly with the respective sample size for a given group and a given reproductive state (3 to 32 observation days per each combination of reproductive state and group ID, total N = 171 observation days). The lines depict the median values for each reproductive state.
What Affected the Duration of Singing?
The duration of singing bouts was affected only by context, with bouts being longer during encounters (comparison of full model with reduced model not comprising context: χ2 = 14.555, df = 1, P = 0.0001; Table 3 and Figure 3). Fruit proportion or female reproductive state did not affect the duration of singing bouts (comparisons of full model with reduced model not comprising fruit proportion: χ2 = 0.948, df = 1, P = 0.330, reproductive state: χ2 = 1.024, df = 2, P = 0.599; Table 3).
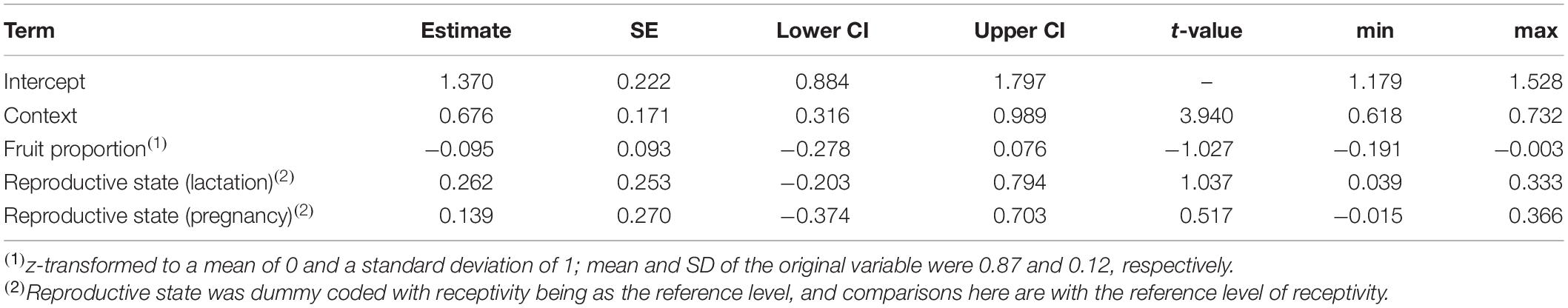
Table 3. Results of the model of the effects of song context, female reproductive state, and fruit proportion in the diet, on the duration of singing bouts: estimates, together with standard errors, confidence intervals, test results, and minimum and maximum of model estimates derived by dropping levels of random effects one at a time.
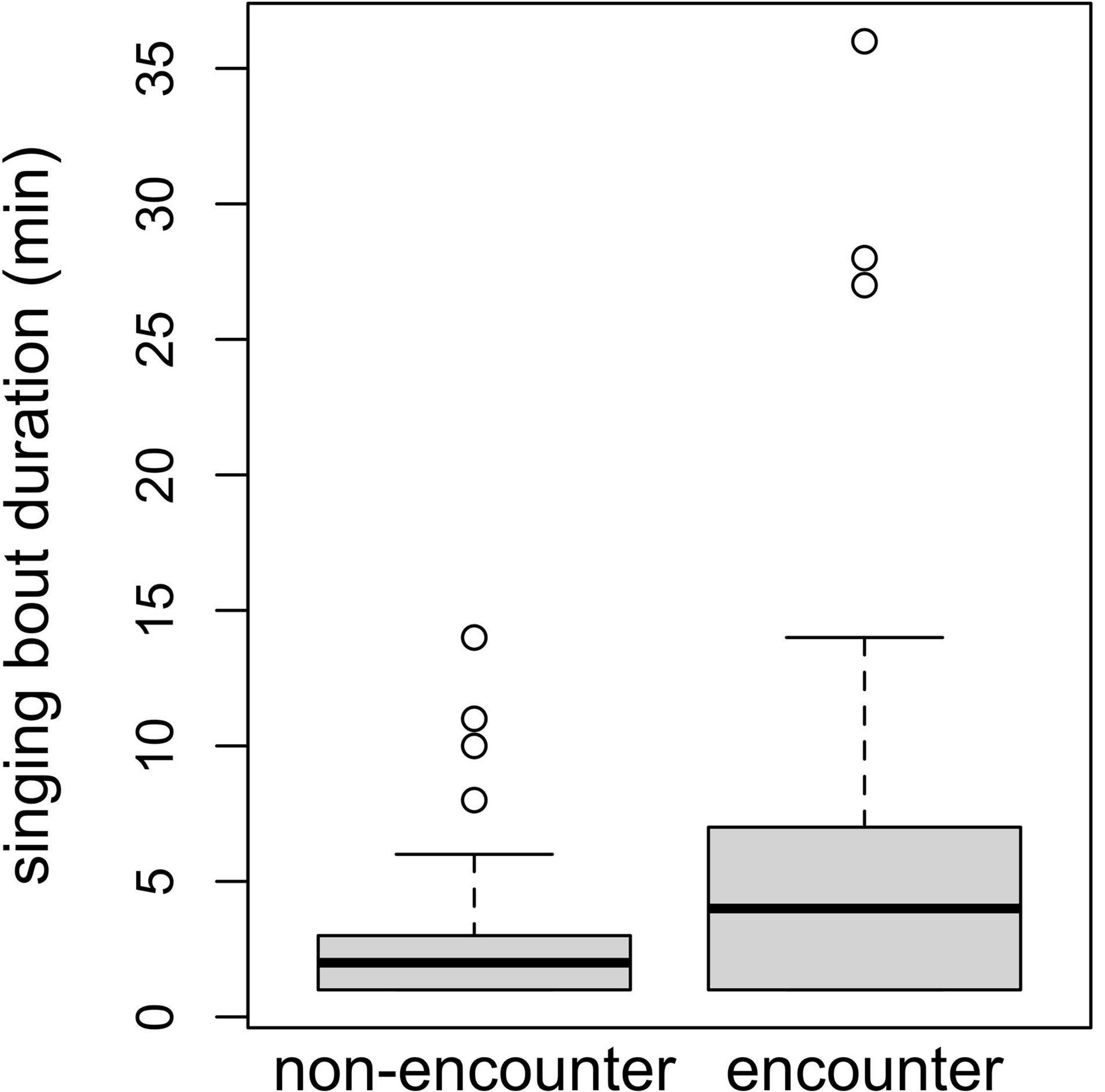
Figure 3. Singing bout durations (in minutes) for encounter and non-encounter contexts. Boxes depict median and lower and upper quartiles.
Spatial Distribution of Songs
Song produced outside of the intergroup encounter context were distributed throughout the home ranges in concordance with its use (Figure 4). The observed frequencies of singing within core areas and the rest of the home ranges did not significantly differ from the expected frequencies calculated under the null hypothesis of songs being distributed throughout the home range in concordance with its use, both when analyzing all songs and when excluding songs given during the intergroup encounters from the analyses (Fisher’s exacts tests for all songs: group 1, P = 0.094; group 2, P = 0.176; group 3, P = 1; group 4, P = 1; group 5, P = 1; group 6, P = 0.417; group 7, P = 0.608; group 11, P = 0.444).
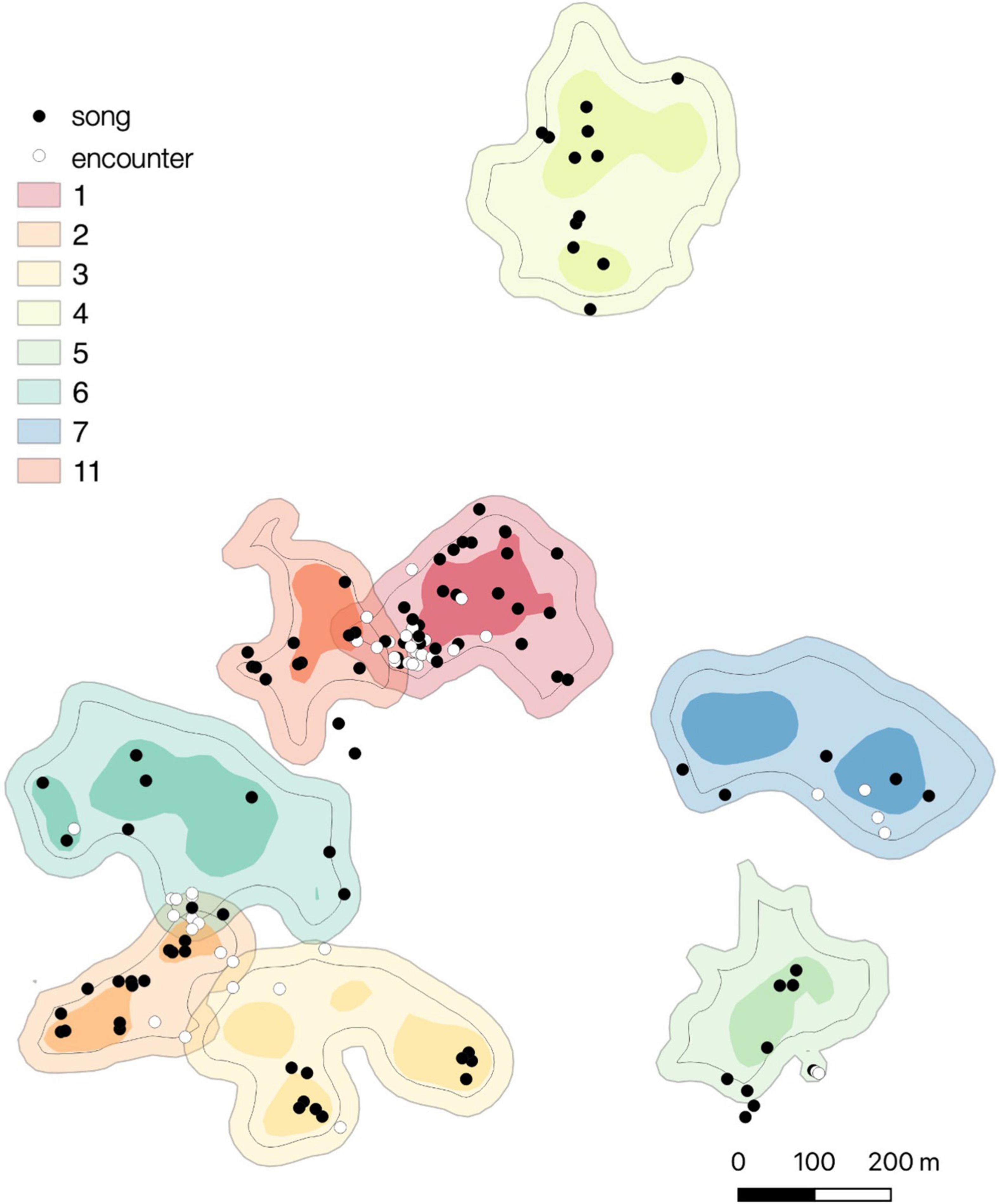
Figure 4. Spatial distribution of songs produced in the intergroup encounter context (white dots) and non-intergroup encounter contexts (black dots) for eight study groups. Light areas depict the 95% fixed kernel home ranges, darker areas depict the 50% fixed kernel core areas. Black lines within each home range depict 25-m inner border areas.
Temporal Distribution of Songs
Singing showed a clear peak around dawn (Figure 5). Approximately half from all 159 recorded singing bouts (75 bouts, 47%) started within an hour before or after sunrise, and most singing bouts (131 bouts, 82%) started within 1 h before or 3 h after sunrise. When analyzing songs produced in non-encounter context separately (N = 107), the dawn peak became even more pronounced: more than half of singing bouts (66 bouts, 62%) started within an hour before or after the sunrise and almost all singing bouts (101 bouts, 94%) started within 1 h before or 3 h after the sunrise; only six bouts were recorded later than 3 h after the sunrise and only one bout was recorded after midday. Songs produced during encounters (N = 52) were more evenly distributed throughout the day, with only a slight increase around dawn. Around half of the encounter singing bouts (29 bouts, 55%) were produced within 3 h after the sunrise and then frequency of encounter songs decreased slowly toward the end of the day.
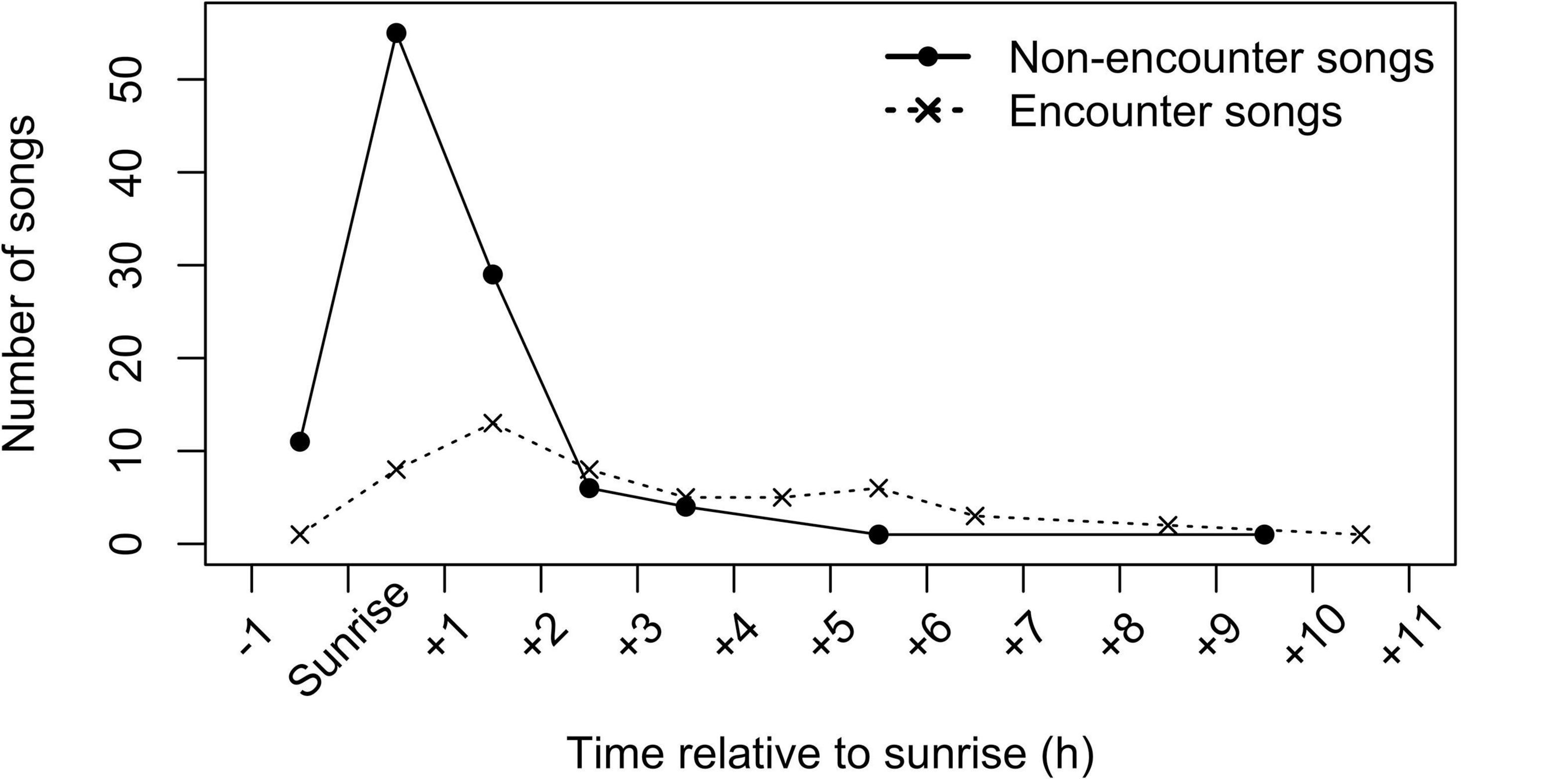
Figure 5. Temporal distribution of songs. Shown are numbers of songs that started in each 1-h interval relative to sunrise time on a given day, separately for the encounter (total N = 52 songs) and non-encounter (total N = 107 songs) contexts, for eight study groups.
Discussion
Our findings suggest that coordinated songs in coppery titi monkeys function for joint resource defense and inter-group communication. The function of coordinated songs for mate defense in the form of paternity guarding, on the other hand, was not supported by our results. Groups sang least frequently when females were likely to be sexually receptive, more frequently when females were lactating, and most frequently when females were likely to be pregnant. There was also a tendency for groups to sing more often when fruits were consumed more intensively, although this trend was not statistically significant. The duration of songs was not associated with female reproductive state or fruit consumption, but songs were longer during inter-group encounters compared to non-encounter contexts. Songs were not concentrated in the core areas of home ranges; rather, they were distributed throughout the home ranges in concordance with its use. Finally, songs were concentrated around dawn, supporting their function for inter-group communication.
Joint Resource Defense Hypothesis
In line with our predictions of the joint resource defense, songs were more frequent when females were lactating or likely to be pregnant—i.e., when groups were expected to have increased energetic demands. Higher duetting rates during lactation were also demonstrated in Milne Edwards’ sportive lemurs, Lepilemur edwardsi (Méndez-Cárdenas and Zimmermann, 2009). Lactation is considered to be the most energetically costly part of mammal reproduction (Clutton-Brock et al., 1989; Altmann and Samuels, 1992). Indeed, in our previous study on the same population, females increased their feeding time and consumed more arthropods (presumably rich in proteins) during lactation (Dolotovskaya and Heymann, 2020), suggesting increased requirements for energy and nutrients. Gestation is generally not as energetically demanding as lactation (Gittleman and Thompson, 1988), and in some primate species, pregnant and cycling females do not even differ in energy intake (e.g., white-faced capuchins, Cebus capucinus: McCabe and Fedigan, 2007). However, energy intake in pregnant females is still often increased compared to cycling or anestrous non-cycling females, and in several primate species pregnant females consume more or higher quality food (e.g., chimpanzees, Pan troglodytes: Murray et al., 2009); red-ruffed lemurs, Varecia rubra: Vasey, 2004, 2005). If the frequency of singing reflects the animals’ need of resources and motivation to defend them, then singing can be expected to be more frequent during lactation, not pregnancy. It is possible in titi monkeys, where infants are carried not by females but by males most of the time (Wright, 1984; Tirado Herrera and Heymann, 2004; Lawrence, 2007; Fernandez-Duque et al., 2013; Spence-Aizenberg et al., 2016), the male’s contribution relieves a mother from some of the costs of infant care, making an increase in energetic demands during lactation less pronounced than in species where infants are carried by mothers.
To date, no studies have assessed energy demands of lactation vs. pregnancy in titi monkeys. In Azara’s owl monkeys (Aotus azarae), who are similar to titi monkeys in size, social system, and patterns of biparental care (infants are carried by males most of the time: Fernandez-Duque et al., 2020), fecal cortisol in both sexes was highest during gestation compared to lactation, possibly indicating higher energetic costs of gestation compared to lactation in this species (Corley et al., 2021). However, this effect might have been due to seasonality, as gestation in A. azarae takes place during the southern winter in the Argentinean Chaco, where the study was conducted (Corley et al., 2021). At our site reproduction is not strictly seasonal, with births having been recorded in July, August, and September (dry season) as well as October through February (rainy season). To see whether our finding of higher singing frequency during gestation is explained by higher energetic demands of pregnant females, it will be necessary to study activity budgets or variation in cortisol levels during different reproductive periods and to have a larger dataset to separate the effects of seasonality and reproductive stage.
Our study groups also sang slightly more frequently when their fruit consumption was higher, although this result did not reach statistical significance. Average monthly feeding time allocated to fruits was used in this study as a proxy for seasonal changes in fruit availability, as we did not have direct measures of fruit availability available for our site. The use of this proxy is reasonable because, according to the optimal foraging theory, high-quality food items should increase in the diet as their abundance increases (Stephens and Krebs, 1986). And indeed, higher fruit consumption in times of higher fruit availability was shown for black-fronted titi monkeys, Callicebus nigrifrons (Caselli and Setz, 2011), as well as other primates, e.g., Hoolock hoolock (Neha et al., 2020).
Higher singing rates in months with higher fruit availability was also shown in C. nigrifrons (Caselli et al., 2014), P. toppini (Wright, 2013), in Hylobates gibbons (Cowlishaw, 1996), and in Milne Edwards’ sportive lemurs (Méndez-Cárdenas and Zimmermann, 2009). More frequent singing during higher fruit availability is usually interpreted as a defense of valuable resources (Cowlishaw, 1996). Another possible explanation is that since singing is likely to be energetically costly (Cowlishaw, 1996; Wich and Nunn, 2002), animals sing less when less high-quality food is available. During months with lower fruit availability, groups of P. toppini not only sang less but also had shorter daily path lengths (DPL) (Wright, 2013). Shorter DPL during fruit scarcity was also shown in C. nigrifrons (Nagy-Reis and Setz, 2017) and in Coimbra-Filho’s titi monkeys (Callicebus coimbrai) (although only in a small forest fragment, whereas in a larger fragment this association was reversed (Souza-Alves et al., 2021). The hypothesis that singing is energetically costly is also indirectly supported by findings that gibbons sing less often at higher altitudes (i.e., at lower temperatures: (Cowlishaw, 1996), after cold nights and after rainy nights (Hylobates klossii: (Whitten, 1982). However, decreased singing rates after rainy nights might be also related to decreased sound transmission due to the background noise produced by dripping water rather than to energetic constraints. In this study, we showed that average monthly rainfall did not affect singing rates. We did not, however, record daily rainfall and temperature and our data was not sufficient to analyze the relationship between DPL and fruit consumption. To better understand the links between singing rates, fruits consumption, and rainfall, it will be necessary to estimate energetic costs of singing compared to other daily activities and relative to the energy input and environmental conditions.
While findings from our and other observational studies support the joint resource defense function of singing, they do not address a function of coordinated singing specifically. This issue can be disentangled by playback studies testing whether duets are more threatening to listeners than solo songs and whether partners are more likely to coordinate their songs into duets than to sing alone when faced with outsiders (Hall, 2004). In line with the latter prediction, wild C. nigrifrons and P. ornatus pairs consistently replied with duets to simulated intruders (although they did not react differently to playbacks of duets vs. solo male or female songs) (Robinson, 1981; Caselli et al., 2014). The coordinated nature of response to outsiders’ songs is further supported by the behavior of young animals who join the adults to produce choruses both in our study population and in an observational and in a playback studies on C. nigrifrons (Caselli et al., 2014, 2015) and by coordinated behaviors displayed by captive P. cupreus in intruder tests (Mercier et al., 2020).
Mate Defense Hypothesis
Our findings did not support the mate defense hypothesis in the form of paternity guarding, as singing was not more frequent when females were estimated to be sexually receptive — in fact, singing was the least frequent during these periods. Similarly, in C. nigrifrons, groups did not sing more frequently when females were likely to be sexually receptive (Caselli et al., 2014). Moreover, predictions for this hypothesis are that duets are initiated by females (while males answer more of their partners’ songs to form duets when females are receptive) (Hall, 2004). However, in C. nigrifrons duets were started either simultaneously by both individuals or with a short time difference (Caselli et al., 2014). Unfortunately, in our study, we were not able to identify the individual that initiated duetting (in C. nigrifrons, too, it was only possible using the spectrogram inspection of recorded songs).
However, paternity guarding is not the only form of mate defense, other forms being mate defense by either males or females via the defense of their own positions or their mates’ positions within the partnership and commitment signaling, in which an individual prevents its partner from deserting (Hall, 2004, 2009). In these contexts, individuals are expected to show sex-specific responses to songs, and solo songs should be more threatening to listeners than duets (Hall, 2004). These predictions, impossible to test in an observational study, were addressed in a playback study in wild C. nigrifrons (Caselli et al., 2014). The study did not provide support for these forms of mate defense: individuals did not show sex-specific responses to duets or male and female solos and did not react stronger to duets than to solo songs. However, in an earlier playback study on P. ornatus, reactions to solo playbacks were sex-specific: males initiated duetting more often in response to male solo song, while females initiated duetting more often in response to female solo song, possibly indicating both male and female mate defense (Robinson, 1981). Moreover, in P. ornatus, males often sang alone (Robinson, 1981). The differences between the two playback studies might be related to population characteristics. P. ornatus were studied in a much higher-density population than C. nigrifrons, which could have increased intrasexual competition and enhanced potential for extra-pair copulations (Caselli et al., 2014).
On the other hand, intruder tests with captive P. cupreus showed that males react more consistently to same-sex intruders than females and show more behavioral arousal to strangers compared to females (Cubicciotti and Mason, 1978; Fernandez-Duque et al., 2000, 1997). Similar sex differences were demonstrated in three wild titi monkey populations, including our study population, where males were more active in inter-group encounters (Robinson, 1981; Wright, 1984; Lawrence, 2007; Dolotovskaya et al., 2020). Whether this sex-specific defense results from conflicting male and female interests regarding male and female intruders, or to common benefits of division of labor (related, for example, to body size dimorphism) (Marshall-Ball et al., 2006), remains an open question. It should be noted, however, that at least in two titi monkey species, males are also more active in anti-predator behaviors (P. cupreus: Dolotovskaya et al., 2019; P. discolor: De Luna et al., 2010), suggesting that they might be generally more involved in defense of their territory and their group. In P. toppini, for example, males reacted stronger to playbacks in the high-use versus low-use parts of the home range (Lawrence, 2007), supporting the resource defense hypothesis and possibly indicating more active male involvement in resource defense.
Spatial and Temporal Distribution of Songs
Our study groups sang throughout their home ranges proportional to its use. The same pattern was observed in four other titi monkey species, P. discolor (Van Belle et al., 2021), Plecturocebus modestus and Plecturocebus olallae (Martinez and Wallace, 2017), and Callicebus personatus (Price and Piedade, 2001). This suggests that intergroup spacing mechanism of titi monkeys involves regular advertisement of the occupancy of the entire home range, as shown also in indris, Indri indri (Bonadonna et al., 2020) and black howler monkeys, Alouatta caraya (da Cunha and Byrne, 2006; Van Belle et al., 2013).
An alternative spacing mechanism involves signaling visitation to either border or core areas and was demonstrated, e.g., in brown howlers, Alouatta guariba, where howling was concentrated almost exclusively at the home range borders (Da Cunha and Jalles-Filho, 2007). Singing mostly from border areas of the home range was also shown in P. ornatus (Robinson, 1979b,1981). Robinson (1979b) hypothesized that the spatial distribution of songs reflects spatial tensions between neighboring groups, where groups with small home ranges would engage in patrolling and singing at home range borders, while in groups with larger home ranges, border patrolling would be too energy-demanding and therefore, these groups would sing from more central areas. A preliminary study comparing titi monkey groups with different home range sizes (P. ornatus with smaller home ranges and Cheracebus cf. lucifer (previously Callicebus torquatus) with a larger home range) suggested that groups with smaller home ranges seem to sing and participate in intergroup encounters at the borders more often (Kinzey and Robinson, 1983). The study, however, included only one group with “large” home range and three groups with “small” home ranges. A larger sample will be needed to see whether titi groups with smaller home ranges are indeed more involved in patrolling behavior. It also remains to be studied whether songs of neighboring groups regulate movement decisions in listeners, as shown, for example, in black howlers (Van Belle and Estrada, 2020).
In our study, songs produced outside of the intergroup encounter context were concentrated around dawn. At this time of day, background noise in an Amazonian lowland forest is reduced, increasing communication distance (Ellinger et al., 2003). This further supports the notion that intergroup spacing mechanism of coppery titis involves regular advertisement of the occupancy of the entire home range rather than vocal border patrolling. Interestingly, in brown howlers, where howling is concentrated at home range borders, no dawn peak in howling was observed (Da Cunha and Jalles-Filho, 2007).
While songs produced during encounters did not show such a clear morning peak as songs produced outside of the encounters, around half of them were still produced within 3 h after the sunrise. Since most songs are produced around dawn, it is possible that neighboring groups are attracted to them and approach the borders of their home range to engage in intergroup encounters. It remains to be studied whether groups change their movement patterns in response to the songs of their neighbors. In this study, we did not distinguish between spontaneous songs and songs produced in response to neighbors’ songs, because it was not always possible, while following a focal group, to determine unequivocally which of the neighboring groups was singing. Moreover, we cannot exclude the possibility that hearing distance might be higher for titis than for human observers and that a song which appears spontaneous to human observers might be in fact given in response to another group’s song. Nevertheless, by following several neighboring groups in parallel, it should be possible in the future to study how groups react to their neighbors’ singing.
Other Possible Functions of Songs: Pair-Bond Reinforcement?
Pair-bond reinforcement has been proposed as another possible function of coordinated singing, and in Milne Edwards’ sportive lemur, pair mates were shown to synchronize their activities after duetting (Méndez-Cárdenas and Zimmermann, 2009). However, sportive lemurs live in dispersed pairs, foraging solitary but sleeping together, and duetting likely helps pair mates to localize each other and coordinate activities. Titi monkey pairs, on the other hand, are highly cohesive, and pair mates spend most of the day within a few meters from each other (Kinzey and Wright, 1982; Spence-Aizenberg et al., 2016; Dolotovskaya et al., 2020). Even if songs in titis function partly as a pair-bond reinforcing behavior, it is unlikely to be its main function. This is further supported by our finding that songs are concentrated around dawn and not produced randomly throughout the day, as could be expected if they were primarily serving for pair-bond reinforcement.
Conclusion
In sum, our findings, as well as results of other observational and playback studies, generally provide more support for the joint resource defense function of duetting in titi monkeys than for the mate defense function. However, there are several issues that still need to be explored by future studies. First, although pair mates in playback studies consistently replied with duets to simulated intruders, supporting the joint resource defense hypothesis, they did not react differently to playbacks of duets vs. solo songs as can be expected under this hypothesis. This issue can be disentangled by playback experiments comparing responses to duets with its temporal coordination artificially manipulated or by comparing playback of duets and playback of solo songs, using songs by the same two individuals. Second, playback studies that investigated the mate defense function of duets provided conflicting results: sex-specific responses were observed in P. ornatus but not in C. nigrifrons. To address this issue, future studies should evaluate the influence of population density on singing and on listeners’ responses to it. And third, the more active male reaction to outsiders, observed both in wild and in captive titi monkeys, still needs to be explained within either joint resource or mate defense functions. To address this question, male and female vocal strategies (initiating song or joining a mate to form duets) in response to playback should be studied in more detail.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
This work was conducted under all necessary permissions and ethical guidelines from the relevant authorities of Peru (research permit no. 249-2017-SERFOR/DGGSPFFS from the Servicio Nacional Forestal y de Fauna Silvestre of the Peruvian Ministry of Agriculture) and the German Primate Center.
Author Contributions
SD collected and analyzed the data and prepared the tables and figures. Both authors designed the research and wrote the manuscript.
Funding
This work was supported by the German Primate Center, the Leakey Foundation, the Deutsche Forschungsgemeinschaft (DFG) (grant HE 1870/29-1), the International Primatological Society, and the Primate Action Fund.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Camilo Flores Amasifuén, Migdonio Huanuiri Arirama, Ney Shahuano Tello, Sarah Walker, Mathieu Maréchal, and all other assistants who worked with us in the field. We are also grateful to Ilya Filippov (Yugra State University, Russia) for his invaluable help with the spatial analyses. We thank the Servicio Forestal y de Fauna Silvestre (SERFOR) of the Ministry of Agriculture in Lima for the research permit (no. 249-2017-SERFOR/DGGSPFFS). We are also grateful to two reviewers and to the guest editor CS for their helpful feedback.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.898509/full#supplementary-material
Footnotes
References
Adret, P., Dingess, K., Caselli, C., Vermeer, J., Martínez, J., Luna Amancio, J., et al. (2018). Duetting patterns of titi monkeys (Primates, Pitheciidae: Callicebinae) and relationships with phylogeny. Animals 8:178. doi: 10.3390/ani8100178
Altmann, J., and Samuels, A. (1992). Costs of maternal care: infant-carrying in baboons. Behav. Ecol. Sociobiol. 29, 391–398. doi: 10.1007/BF00170168
Anzenberger, G. (1988). The pairbond in the titi monkey (Callicebus moloch): intrinsic versus extrinsic contributions of the pairmates. Folia Primatol. 50, 188–203.
Appleby, B. M., Yamaguchi, N., Johnson, P. J., and Macdonald, D. W. (1999). Sex-specific territorial responses in Tawny Owls Strix aluco. Ibis 141, 91–99. doi: 10.1111/j.1474-919x.1999.tb04267.x
Asensio, N., Schaffner, C. M., and Aureli, F. (2012). Variability in core areas of spider monkeys (Ateles geoffroyi) in a tropical dry forest in Costa Rica. Primates 53, 147–156. doi: 10.1007/S10329-011-0288-9/FIGURES/6
Barr, D. J., Levy, R., Scheepers, C., and Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: keep it maximal. J. Mem. Lang. 68, 255–278. doi: 10.1016/j.jml.2012.11.001
Bates, D., Mächler, M., Bolker, B. M., and Walker, S. C. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Benedict, L. (2008). Occurrence and life history correlates of vocal duetting in North American passerines. J. Avian Biol. 39, 57–65. doi: 10.1111/j.2008.0908-8857.04103.x
Bicca-Marques, J. C., and Heymann, E. W. (2013). “Ecology and behavior of titi monkeys (genus Callicebus),” in Evolutionary Biology and Conservation of Titis, Sakis and Uacaris, eds A. Barnett, L. M. Veiga, S. F. Ferrari, and M. A. Norconk (New York, NY: Cambridge University Press), 196–207.
Bonadonna, G., Zaccagno, M., Torti, V., Valente, D., De Gregorio, C., Randrianarison, R. M., et al. (2020). Intra- and intergroup spatial dynamics of a pair-living singing primate, Indri indri: a multiannual study of three indri groups in Maromizaha Forest, Madagascar. Int. J. Primatol. 41, 224–245. doi: 10.1007/s10764-019-00127-5
Brown, T. J., and Handford, P. (2003). Why birds sing at dawn: the role of consistent song transmission. Ibis 145, 120–129. doi: 10.1046/j.1474-919X.2003.00130.x
Calenge, C. (2006). The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol. Modell. 197, 516–519. doi: 10.1016/J.ECOLMODEL.2006.03.017
Caselli, C. B., Mennill, D. J., Bicca-Marques, J. C., and Setz, E. Z. F. (2014). Vocal behavior of black-fronted titi monkeys (Callicebus nigrifrons): acoustic properties and behavioral contexts of loud calls. Am. J. Primatol. 76, 788–800. doi: 10.1002/ajp.22270
Caselli, C. B., Mennill, D. J., Gestich, C. C., Setz, E. Z. F., and Bicca-Marques, J. C. (2015). Playback responses of socially monogamous black-fronted titi monkeys to simulated solitary and paired intruders. Am. J. Primatol. 77, 1135–1142. doi: 10.1002/ajp.22447
Caselli, C. B., and Setz, E. Z. F. (2011). Feeding ecology and activity pattern of black-fronted titi monkeys (Callicebus nigrifrons) in a semideciduous tropical forest of southern Brazil. Primates 52, 351–359. doi: 10.1007/s10329-011-0266-2
Clink, D. J., Lau, A. R., and Bales, K. L. (2019). Age-related changes and vocal convergence in titi monkey duet pulses. Behaviour 156, 1471–1494. doi: 10.1163/1568539X-00003575
Clink, D. J., Lau, A. R., Kanthaswamy, S., Johnson, L. M., and Bales, K. L. (2022). Moderate evidence for heritability in the duet contributions of a South American primate. J. Evol. Biol. 35, 51–63. doi: 10.1111/jeb.13962
Clutton-Brock, T. H., Albon, S. D., and Guinness, F. E. (1989). Fitness costs of gestation and lactation in wild mammals. Nature 337, 260–262. doi: 10.1038/337260a0
Corley, M. K., Perea-Rodriguez, J. P., Valeggia, C., and Fernandez-Duque, E. (2021). Associations between fecal cortisol and biparental care in a pair-living primate. Am. J. Phys. Anthropol. 176, 295–307. doi: 10.1002/ajpa.24368
Cowlishaw, G. (1996). Sexual selection and information content in gibbon song bouts. Ethology 102, 272–284. doi: 10.1111/j.1439-0310.1996.tb01125.x
Cubicciotti, D. D., and Mason, W. A. (1978). Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behav. Ecol. Sociobiol. 3, 311–322. doi: 10.1007/BF00296316
da Cunha, R. G. T., and Byrne, R. W. (2006). Roars of black howler monkeys (Alouatta caraya): evidence for a function in inter-group spacing. Behaviour 143, 1169–1199. doi: 10.1163/156853906778691568
Da Cunha, R. G. T., and Jalles-Filho, E. (2007). The roaring of southern brown howler monkeys (Alouatta guariba clamitans) as a mechanism of active defence of borders. Folia Primatol. 78, 259–271. doi: 10.1159/000105545
De Gregorio, C., Carugati, F., Valente, D., Raimondi, T., Torti, V., Miaretsoa, L., et al. (2022). Notes on a tree: reframing the relevance of primate choruses, duets, and solo songs. Ethol. Ecol. Evol. 34, 205–219. doi: 10.1080/03949370.2021.2015451
De Luna, A. G., Sanmiguel, R., Di Fiore, A., and Fernandez-Duque, E. (2010). Predation and predation attempts on red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador. Folia Primatol. 81, 86–95. doi: 10.1159/000314948
Dobson, A. J., Barnett, A. G., and Barnett, A. G. (2008). An Introduction to Generalized Linear Models. New York, NY: Chapman and Hall. doi: 10.1201/9781584889519
Dolotovskaya, S., Flores Amasifuen, C., Haas, C. E., Nummert, F., and Heymann, E. W. (2019). Active anti-predator behaviour of red titi monkeys (Plecturocebus cupreus). Primate Biol. 6, 59–64. doi: 10.5194/pb-6-59-2019
Dolotovskaya, S., Walker, S., and Heymann, E. W. (2020). What makes a pair bond in a Neotropical primate: female and male contributions. R. Soc. Open Sci. 7:191489. doi: 10.1098/rsos.191489
Dolotovskaya, S., and Heymann, E. W. (2020). Do less or eat more: strategies to cope with costs of parental care in a pair-living monkey. Anim. Behav. 163, 163–173. doi: 10.1016/j.anbehav.2020.03.012
Ellinger, N., Hödl, W., and Ellinger, N. (2003). Habitat acoustics of a neotropical lowland rainforest. Bioacoustics 13, 297–321. doi: 10.1080/09524622.2003.9753503
Erran Seaman, D., and Powell, R. A. (1996). An evaluation of the accuracy of kernel density estimators for home range analysis. Ecology 77, 2075–2085. doi: 10.2307/2265701
Fernandez-Duque, E., Di Fiore, A., and de Luna, A. G. (2013). “Pair-mate relationships and parenting in equatorial saki monkeys (Pithecia aequatorialis) and red titi monkeys (Callicebus discolor) of ecuador,” in Evolutionary Biology and Conservation of Titis, Sakis and Uacaris, eds L. M. Veiga, A. A. Barnett, S. F. Ferrari, and M. A. Norconk (New York, NY: Cambridge University Press), 295–302.
Fernandez-Duque, E., Huck, M., Van Belle, S., and Di Fiore, A. (2020). The evolution of pair-living, sexual monogamy, and cooperative infant care: insights from research on wild owl monkeys, titi monkeys, sakis, and tamarins. Am. J. Phys. Anthropol. 171, 118–173. doi: 10.1111/j.1365-2958.2003.03935.x
Fernandez-Duque, E., Mason, W. A., and Mendoza, S. P. (1997). Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch). Am. J. Primatol. 43, 225–237.
Fernandez-Duque, E., Valeggia, C. R., and Mason, W. A. (2000). Effects of pair-bond and social context on male-female interactions in captive titi monkeys (Callicebus moloch, Primates: Cebidae). Ethology 106, 1067–1082. doi: 10.1046/j.1439-0310.2000.00629.x
Forstmeier, W., and Schielzeth, H. (2011). Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 65, 47–55. doi: 10.1007/s00265-010-1038-5
Gittleman, J. L., and Thompson, S. D. (1988). Energy allocation in mammalian reproduction. Integr. Comp. Biol. 28, 863–875. doi: 10.1093/icb/28.3.863
Haimoff, E. H. (1986). Convergence in the duetting of monogamous old world primates. J. Hum. Evol. 15, 51–59. doi: 10.1016/S0047-2484(86)80065-3
Hall, M. L. (2004). A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430. doi: 10.1007/s00265-003-0741-x
Hall, M. L. (2009). A review of vocal duetting in birds. Adv. Study Behav. 40, 67–121. doi: 10.1016/S0065-3454(09)40003-2
Heymann, E. W., Dolotovskaya, S., and Herrera, E. R. T. (2021). Estación Biológica Quebrada Blanco. Ecotropica 23, 4–7. doi: 10.30427/ecotrop202101
Holzmann, I., Agostini, I., and Di Bitetti, M. (2012). Roaring behavior of two syntopic howler species (Alouatta caraya and A. guariba clamitans): evidence supports the mate defense hypothesis. Int. J. Primatol. 33, 338–355. doi: 10.1007/s10764-012-9583-6
Kinzey, W. G. (1981). “The titi monkeys, genus Callicebus,” in Ecology and Behavior of Neotropical Primates, eds A. F. Coimbra-Filho and R. A. Mittermeier (Rio de Janeiro: Academia Brasileira de Ciencias), 241–276.
Kinzey, W. G., and Robinson, J. G. (1983). Intergroup loud calls, range size, and spacing in Callicebus torquatus. Am. J. Phys. Anthropol. 60, 539–544.
Kinzey, W. G., and Wright, P. C. (1982). Grooming behavior in the titi monkey (Callicebus torquatus). Am. J. Primatol. 3, 267–275. doi: 10.1002/ajp.1350030124
Lau, A., Clink, D. J., and Bales, K. L. (2020). Individuality in the vocalizations of adult and infant coppery titi monkeys (Plecturocebus cupreus). J. Acoust. Soc. Am. 82:e23134. doi: 10.1121/1.5136592
Lawrence, J. (2007). Understanding the Pair Bond in Brown Titi Monkeys (Callicebus brunneus): Male and Female Reproductive Interests. Ph.D. thesis. New York, NY: Columbia University.
Levin, R. N. (1996). Song behaviour and reproductive strategies in a duetting wren, Thryothorus nigricapillus: II. Playback experiments. Anim. Behav. 52, 1107–1117. doi: 10.1006/ANBE.1996.0258
Malacarne, G., Cucco, M., and Camanni, S. (1991). Coordinated visual displays and vocal duetting in different ecological situations among western palearctic non-passerine birds. Ethol. Ecol. Evol. 3, 207–219. doi: 10.1080/08927014.1991.9525369
Marshall-Ball, L., Mann, N., and Slater, P. J. B. (2006). Multiple functions to duet singing: hidden conflicts and apparent cooperation. Anim. Behav. 71, 823–831. doi: 10.1016/J.ANBEHAV.2005.05.021
Martinez, J., and Wallace, R. B. (2017). Ecological and behavioural factors influencing territorial call rates for the bolivian titi monkeys, Plecturocebus modestus and Plecturocebus olallae. Folia Primatol. 87, 279–290. doi: 10.1159/000448710
McCabe, G. M., and Fedigan, L. M. (2007). Effects of reproductive status on energy intake, ingestion rates, and dietary composition of female Cebus capucinus at Santa Rosa, Costa Rica. Int. J. Primatol. 28, 837–851. doi: 10.1007/s10764-007-9159-z
Méndez-Cárdenas, M. G., and Zimmermann, E. (2009). Duetting — a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol. 139, 523–532. doi: 10.1002/ajpa.21017
Mennill, D. J., and Vehrencamp, S. L. (2008). Context-dependent functions of avian duets revealed by microphone-array recordings and multispeaker playback. Curr. Biol. 18, 1314–1319. doi: 10.1016/J.CUB.2008.07.073
Mercier, F., Witczak, L. R., and Bales, K. L. (2020). Coppery titi monkey (Plecturocebus cupreus) pairs display coordinated behaviors in response to a simulated intruder. Am. J. Primatol. 82:e23141. doi: 10.1002/ajp.23141
Merker, B., and Cox, C. (1999). Development of the female great call in Hylobates gabriellae: a case study. Folia Primatol. 70, 97–106. doi: 10.1159/000021680
Müller, A. E., and Anzenberger, G. (2002). Duetting in the titi monkey Callicebus cupreus: structure, pair specificity and development of duets. Folia Primatol. 73, 104–115. doi: 10.1159/000064788
Murray, C. M., Lonsdorf, E. V., Eberly, L. E., and Pusey, A. E. (2009). Reproductive energetics in free-living female chimpanzees (Pan troglodytes schweinfurthii). Behav. Ecol. 20, 1211–1216. doi: 10.1093/beheco/arp114
Nagy-Reis, M. B., and Setz, E. Z. F. (2017). Foraging strategies of black-fronted titi monkeys (Callicebus nigrifrons) in relation to food availability in a seasonal tropical forest. Primates 58, 149–158. doi: 10.1007/s10329-016-0556-9
Neha, S. A., Habiba Khatun, M. U., and Ul Hasan, M. A. (2020). Feeding behavior of the western hoolock gibbon (Hoolock hoolock) in Bangladesh: response to temporal variation of food sources. Primate Conserv. 2020, 185–194.
Nietsch, A. (1999). Duet vocalizations among different populations of Sulawesi tarsiers. Int. J. Primatol. 204, 567–583. doi: 10.1023/A:1020342807709
Paula, B., and Monticelli, P. (2021). Maned wolf duet? The first record of two maned wolves vocalizing simultaneous. Acad. Lett. doi: 10.20935/al1720
Price, E. C., and Piedade, H. M. (2001). Ranging behavior and intraspecific relationships of masked titi monkeys (Callicebus personatus personatus). Am. J. Primatol. 53, 87–92.
QGIS Development Team (2022). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available Online at: http://qgis.osgeo.org (accessed January 11, 2022)
Quinn, G. P., and Keough, M. J. (2002). Experimental Designs and Data Analysis for Biologists. Cambridge: Cambridge University Press.
R Core Team (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rasoloharijaona, S., Randrianambinina, B., Braune, P., and Zimmermann, E. (2006). Loud calling, spacing, and cohesiveness in a nocturnal primate, the Milne Edwards’ sportive lemur (Lepilemur edwardsi). Am. J. Phys. Anthropol. 129, 591–600. doi: 10.1002/ajpa
Robinson, J. G. (1979a). An analysis of the organization of vocal communication in the titi monkey Callicebus moloch. Z. Tierpsychol. 49, 381–405.
Robinson, J. G. (1979b). Vocal regulation of use of space by groups of titi monkeys Callicebus moloch. Behav. Ecol. Sociobiol. 5, 1–15. doi: 10.1007/BF00302691
Robinson, J. G. (1981). Vocal regulation of inter- and intragroup spacing during boundary encounters in the titi monkey, Callicebus moloch. Primates 22, 161–172. doi: 10.1007/BF02382607
Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. doi: 10.1111/j.2041-210X.2010.00012.x
Snow, D. W. (1977). “Duetting and other synchronised displays of the blue-backed manakins,Chiroxiphia spp.,” in Evolutionary Ecology, eds B. Stonehouse and C. Perrins (London: Palgrave), 239–250 doi: 10.1007/978-1-349-05226-4_20
Souza-Alves, J. P., Chagas, R. R. D., Santana, M. M., Boyle, S. A., and Bezerra, B. M. (2021). Food availability, plant diversity, and vegetation structure drive behavioral and ecological variation in Endangered Coimbra-Filho’s titi monkeys. Am. J. Primatol. 83:e23237. doi: 10.1002/ajp.23237
Spence-Aizenberg, A., Di Fiore, A., and Fernandez-Duque, E. (2016). Social monogamy, male–female relationships, and biparental care in wild titi monkeys (Callicebus discolor). Primates 57, 103–112. doi: 10.1007/s10329-015-0489-8
Stephens, D. W., and Krebs, J. R. (1986). Foraging theory. Princeton, NJ: Princeton University Press.
Tilson, R. L., and Norton, P. M. (1981). Alarm duetting and pursuit deterrence in an African antelope. Am. Nat. 118, 455–462.
Tilson, R. L., and Tenaza, R. R. (1976). Monogamy and duetting in an old world monkey. Nature 263, 320–321. doi: 10.1038/263320a0
Tirado Herrera, E. R., and Heymann, E. W. (2004). Does mom need more protein? Preliminary observations on differences in diet composition in a pair of red titi monkeys (Callicebus cupreus). Folia Primatol. 75, 150–153. doi: 10.1159/000078304
Valeggia, C. R., Mendoza, S. P., Fernandez-Duque, E., Mason, W. A., and Lasley, B. (1999). Reproductive biology of female titi monkeys (Callicebus moloch) in captivity. Am. J. Primatol. 47, 183–195.
Van Belle, S., and Estrada, A. (2020). The influence of loud calls on intergroup spacing mechanism in black howler monkeys (Alouatta pigra). Int. J. Primatol. 41, 265–286. doi: 10.1007/s10764-019-00121-x
Van Belle, S., Estrada, A., and Garber, P. A. (2013). Spatial and diurnal distribution of loud calling in black howlers (Alouatta pigra). Int. J. Primatol. 34, 1209–1224. doi: 10.1007/s10764-013-9734-4
Van Belle, S., Fernandez-Duque, E., and Di Fiore, A. (2016). Demography and life history of wild red titi monkeys (Callicebus discolor) and equatorial sakis (Pithecia aequatorialis) in Amazonian Ecuador: a 12-year study. Am. J. Primatol. 78, 204–215. doi: 10.1002/ajp.22493
Van Belle, S., Porter, A., Fernandez-Duque, E., and Di Fiore, A. (2021). Ranging behavior and the potential for territoriality in pair-living, sexually monogamous titi monkeys (Plecturocebus discolor). Am. J. Primatol. 83:e23225. doi: 10.1002/ajp.23225
Vasey, N. (2004). Circadian rhythms in diet and habitat use in red ruffed lemurs (Varecia rubra) and white-fronted brown lemurs (Eulemur fulvus albifrons). Am. J. Phys. Anthropol. 124, 353–363. doi: 10.1002/ajpa.10357
Vasey, N. (2005). Activity budgets and activity rhythms in red ruffed lemurs (Varecia rubra) on the Masoala Peninsula, Madagascar: seasonality and reproductive energetics. Am. J. Primatol. 66, 23–44. doi: 10.1002/ajp.20126
Whitten, A. J. (1982). The ecology of singing in Kloss gibbons (Hylobates klossii) on Siberut Island, Indonesia. Int. J. Primatol. 3, 33–51. doi: 10.1007/BF02693489
Wich, S. A., and Nunn, C. L. (2002). Do male “long-distance calls” function in mate defense? A comparative study of long-distance calls in primates. Behav. Ecol. Sociobiol. 52, 474–484. doi: 10.1007/s00265-002-0541-8
Wright, P. C. (1984). “Biparental care in Aotus trivirgatus and Callicebus moloch,” in Female Primates: Studies by Women Primatologists, ed. M. F. Small (New York, NY: Alan R. Liss), 59–75.
Keywords: duets, coordinated singing, titi monkeys, Plecturocebus, resource defense, mate defense
Citation: Dolotovskaya S and Heymann EW (2022) Coordinated Singing in Coppery Titi Monkeys (Plecturocebus cupreus): Resource or Mate Defense? Front. Ecol. Evol. 10:898509. doi: 10.3389/fevo.2022.898509
Received: 17 March 2022; Accepted: 26 April 2022;
Published: 26 May 2022.
Edited by:
Charles T. Snowdon, University of Wisconsin-Madison, United StatesReviewed by:
Allison Lau, University of California, Davis, United StatesSarie Van Belle, University of Texas at Austin, United States
Copyright © 2022 Dolotovskaya and Heymann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sofya Dolotovskaya, cy5kb2xvdG92c2theWFAZ21haWwuY29t
 Sofya Dolotovskaya
Sofya Dolotovskaya Eckhard W. Heymann
Eckhard W. Heymann