
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol., 16 June 2022
Sec. Paleontology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.892530
This article is part of the Research TopicA Fossil View of Insect Evolution: Integrating Paleontological Evidence to Explore the Origins of Insect BiodiversityView all 10 articles
Two new genera of velvety shore bugs, Arcochterus Zhang, Ren and Yao gen. nov. and Parvochterus Zhang, Ren and Yao gen. nov. are described, with three new species between them—Arcochterus fuscus Zhang, Ren and Yao sp. nov., Parvochterus reticulatus Ren and Yao sp. nov., and P. lanceolarus Zhang, Ren and Yao sp. nov. Based on the combination of fossil and extant taxa, a cladistic analysis is conducted to confirm the phylogenetic position of these species and allows reconstruction of the inter-genus relationships within the superfamily Ochteroidea. Major conclusions of the phylogenetic analysis: (1) these new species and Grimaldinia pronotalis belong to Ochteridae; (2) Ochteroidea is a monophyletic group, Ochteridae and Gelastocoridae are sister group and monophyletic, respectively. (3) The ancestral character state reconstruction (ACSR) shows that the length of the rostrum has occurred in at least three independent transitions during the evolution of the superfamily Ochteroidea.
The family Ochteridae are commonly known as “velvety shore bugs”, together with its sister group, Gelastocoridae (the toad bugs), jointly form the extent superfamily Ochteroidea, belonging to the infraorder Nepomorpha which is a small riparian group (Kment et al., 2020; Schuh and Weirauch, 2020). Ochteridae consist of three extant genera (Ochterus Latreille, 1807, Megochterus Jaczewski, 1934 and Ocyochterus Drake and Gómez-Menor, 1954) and 91 species (Kment et al., 2020; Polhemus, 2021). Gelastocoridae includes two subfamilies, Gelastocorinae Champion (1901) and Nerthrinae Kirkaldy (1906) with two extant genera (Gelastocoris Kirkaldy, 1897 and Nerthra Say, 1832) and 106 species (Estévez and Ruf, 2006; Poinar and Brown, 2016; Schuh and Weirauch, 2020).
Fossil Ochteroidea are scarce, including Ochteridae, Gelastocoridae, Propreocoridae, and Pseudonerthridae (Kment et al., 2020; Schuh and Weirauch, 2020). The fossil ochterids definitely contains four genera and five species (Kment et al., 2020), including the oldest on the fossil record—three genera and four species from the Early Cretaceous of China: Pristinochterus zhangi Yao et al., 2007, P. ovatus Yao et al., 2011, Angulochterus quatrimaculatus Yao et al., 2011, and Floricaudus multilocellus Yao et al., 2011, and one genus and species from Miocene Dominican amber: Riegerochterus baehri Popov and Heiss, 2014a. Fossil gelastocorids include three genera and five species, with two species from Early Cretaceous of Brazil (Ruf et al., 2005; Xie and Liu, 2018), two species from the mid-Cretaceous of Myanmar (Poinar and Brown, 2016) and one species from the late Holocene of Chile (Faúndez and Ashworth, 2015). Only one genus and species of Pseudonerthridae was reported: Pseudonerthra gigantea (Ruf et al., 2005), from the Early Cretaceous of Brazil. Propreocoris maculatus Popov et al., 1994, from the Early Jurassic of Britain, was considered the earliest fossil record of Ochteridae, nonetheless, Shcherbakov and Popov (2002) supposed it as the endemic subfamily Proprepocorinae, later Grimaldi and Engel (2005) and Yao et al. (2011) believed it should be as a common ancestor of the Ochteridae and Gelastocoridae; eventually, Kment et al. (2020) formally raised Proprepocorinae to family rank. Grimaldinia pronotalis Popov and Heiss, 2014b, from mid-Cretaceous Myanmar, was assigned to the Leptosaldinae (Leptopodomorpha: Leptopodidae) and regarded as a controversial species, was revised to Ochteroidea by Schuh and Weirauch (2020).
Recent phylogenetic analyses of Nepomorpha indicated superfamily Ochteroidea is a monophyletic group, Ochteridae and Gelastocoridae are sister group and monophyletic, respectively (Andersen and Weir, 2004; Hebsgaard et al., 2004; Yao et al., 2011; Ye et al., 2020). The main diagnostic characters of the family Ochteridae are as follows: eyes large, with inner margins emarginated; ocelli present; antennae 4-segmented, partially visible dorsally; rostrum long, segment III longest; legs adapted for walking, without swimming hairs, profemora not enlarged; tarsal formula 2-2-3; membrane of forewings with several closed cells (Chen et al., 2005; Schuh and Weirauch, 2020). Herein, we reported five Kachin amber ochterids with short rostrum from Hukawng Valley in northern Myanmar. These newly described taxa supplement Ochteridae and provided new evidence for clarifying the phylogeny of Ochteridae.
The amber specimens are from the Hukawng Valley (Kachin State, Northern Myanmar), near Tanai Village (26°21′33.41′′ N, 96°43′11.88′′ E) (Zhang et al., 2018; Wang et al., 2019) approximately from the Turonian or Cenomanian, 98.79 ± 0.62 Ma (Du et al., 2019; Li et al., 2020). There are five specimens, in one of which the amber embeds two individuals at once, including a female and a male. All these specimens herein are deposited in the Key Laboratory of Insect Evolution and Environmental Changes from the College of Life of Science at Capital Normal University, Beijing, China (CNU; Yunzhi Yao, Curator).
Morphological terminology and taxonomy mainly follow Andersen and Weir (2004) and Schuh and Weirauch (2020). All measurements are in millimeters (mm).
Two representatives from the family Belostomatidae (Belostoma lutarium Stål, 1856) and Naucoridae (Idiocarus minor La Rivers, 1971) are selected as the outgroup taxa (Hebsgaard et al., 2004; Yao et al., 2011; Ye et al., 2020; Wang et al., 2021), and 16 species of Ochteroidea are as the ingroup (nine extant taxa and three fossil taxa of Ochteridae; two extant taxa and two fossil taxa of Gelastocoridae). A total of 32 morphological characters from adults were used, most of the characters are from Hebsgaard et al. (2004) and Yao et al. (2011) (see Appendix 1 for the definitions of characters) and all the characters are equal-weighted (Table 1).
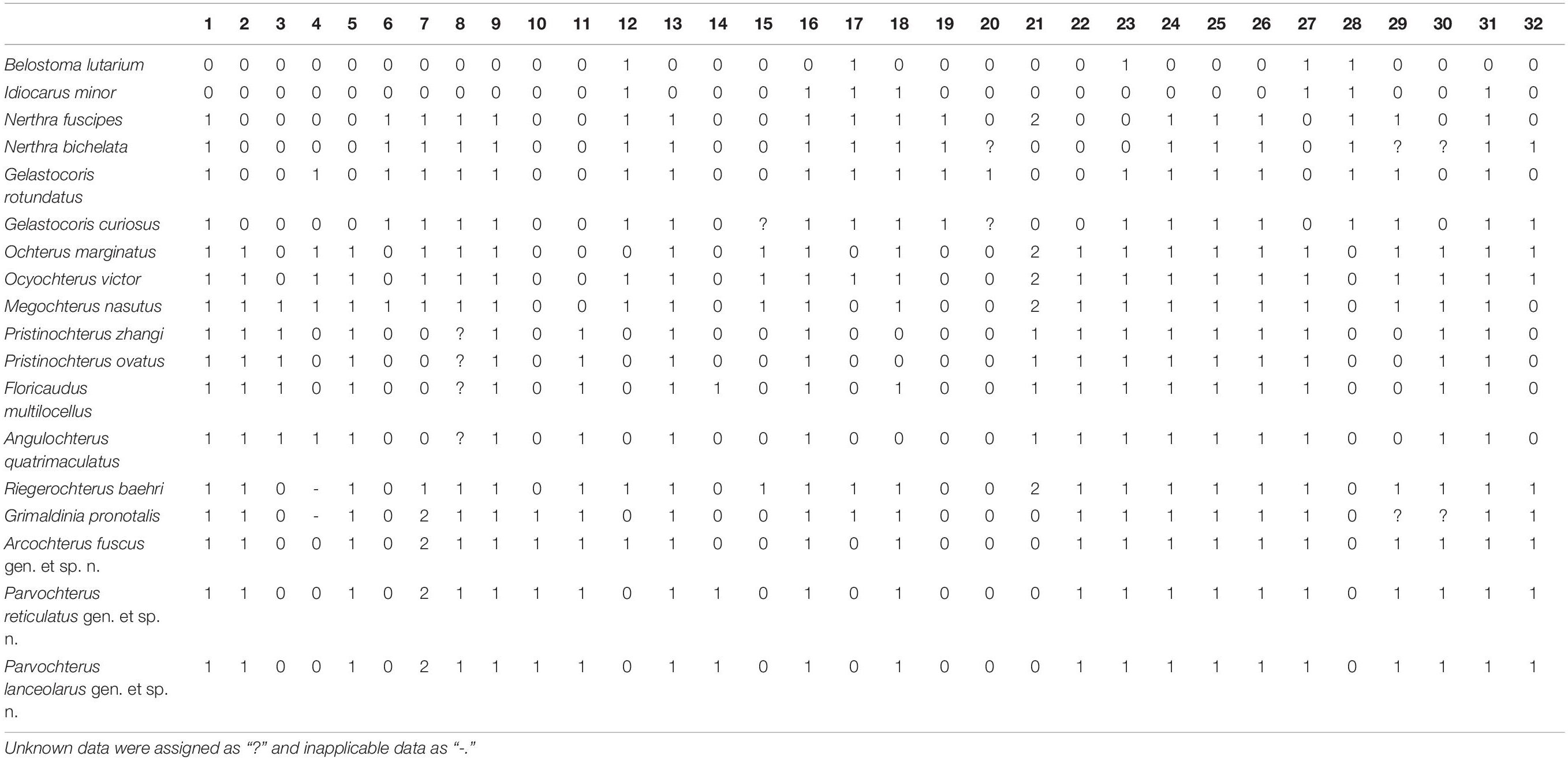
Table 1. Character state matrix of 32 characters for the 18 taxa included in the phylogenetic study.
The matrix (Appendix 1) was compiled using Nexus Data Editor (Version 0.5.0) (Page, 2001). Phylogenetic analysis based on the maximum parsimony was conducted in Winclada (Version 1.00.08) with NONA script and a repeated verification in TNT (Version 1.5) (Goloboff, 1999; Nixon, 2002; Goloboff and Catalano, 2016). Heuristic searches using Winclada were performed with a parameter of 10,000 maximum trees, 1,000 random-taxa-addition replicates, and 100 starting trees per replication. The repeated analysis in TNT was using “traditional search” and the Bremer Support values (BS) were calculated through the script “Bremer. Run.”
We reconstructed the ancestral state of rostrum length in Ochteroidea based on the phylogenetic analysis, using the Mesquite (Version 3.70) (Maddison and Maddison, 2021). The rostrum length of each of the 18 species of Ochteroidea is selected from character 21 in the matrix (Table 1). Three kinds of rostrum lengths were identified: (0) reaching procoxae, (1) reaching mesocoxae, and (2) reaching metacoxae.
The phylogenetic analysis returned the two most parsimonious trees [tree length = 52, consistency index (CI) = 0.65, retention index (RI) = 0.83]. The strict consensus tree [tree length = 53, consistency index (CI) = 0.64, retention index (RI) = 0.83] is shown in Figure 1, with unambiguous characters and Bremer support values (BS) marked. The major results of our phylogenetic analysis are as follows: the superfamily Ochteroidea (BS = 7) is well supported as a monophyletic; the monophyly of Ochteridae (BS = 4) and Gelastocoridae (BS = 2) are strongly supported, they are the sister group, and the new fossils all belong to Ochteridae.
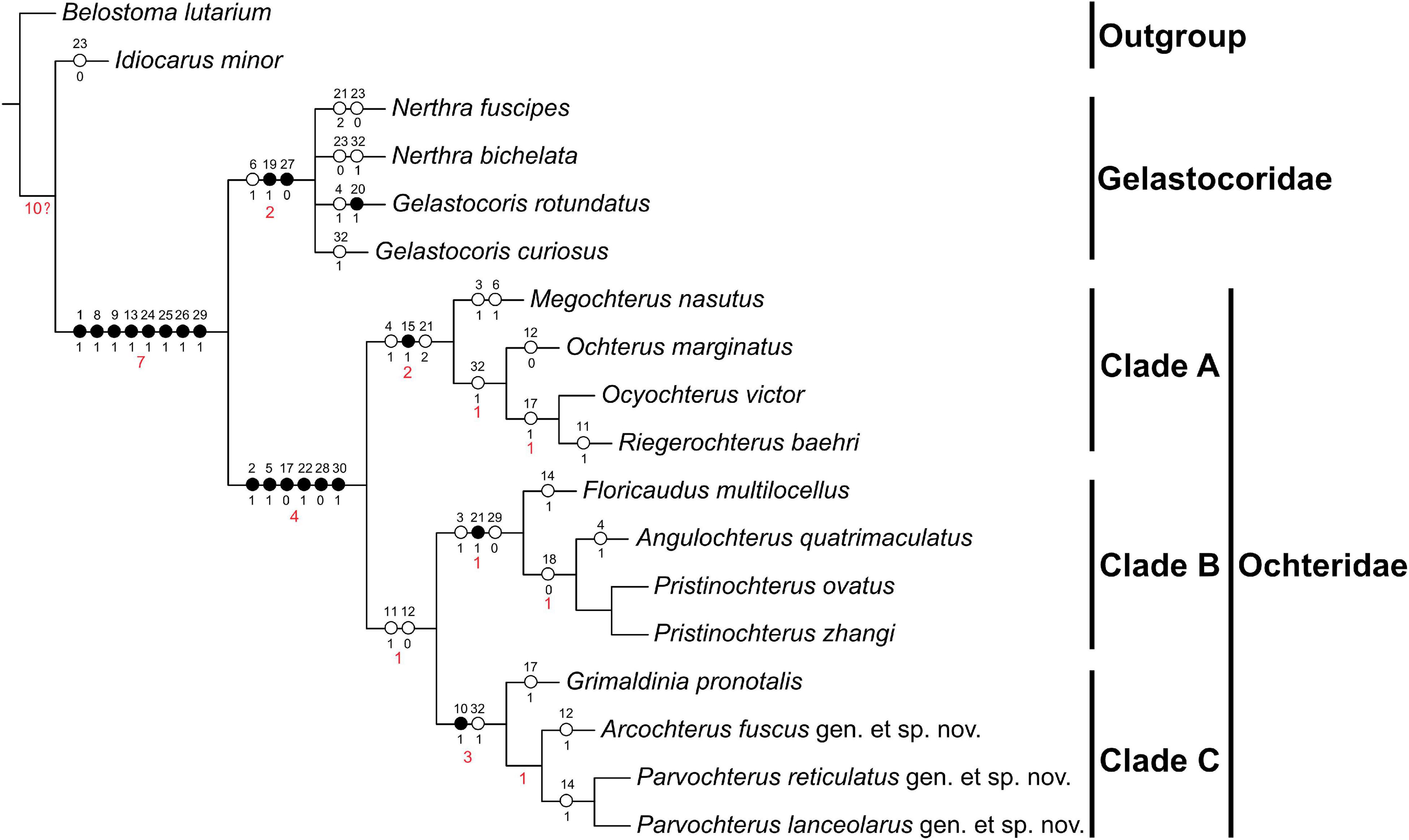
Figure 1. The strict consensus tree for the analyzed Ochteridae: tree length = 53, consistency index (CI) = 0.64, retention index (RI) = 0.83. •, non-homoplastic changes; °, homoplastic changes. The red numbers below the branches are Bremer support values (BS).
The results reconfirm the monophyly of Ochteroidea, Ochteridae, and Gelastocoridae, consistent with most previous studies (Rieger, 1976; Mahner, 1993; Hebsgaard et al., 2004; Li et al., 2012, 2014; Ye et al., 2020). The monophyly of Ochteroidea is supported by eight synapomorphies: clypeus divided by a cross fold (character 1, state 1), ocelli present (character 8, state 1), head and prothorax discretely (character 9, state 1), propleuron undeveloped (character 13, state 1), metacoxae short (character 24, state 1), metatibia without swimming hairs (character 25, state 1), tarsal formula not 2:3:3 (character 26, state 1), distal of sternum VII in males asymmetrical (character 29, state 1). The monophyly of Gelastocoridae is supported by two synapomorphies: the surface of thorax and forewings with wartlike sculpturation (character 19, state 1), tarsal formula: 1:2:3 (character 27, state 0). Ochteridae has 6 synapomorphies: clypeus with a pattern of ridges (character 2, state 1), antennae short, but visible dorsally, projecting (character 5, state 1), forewing with spots (character 17, state 0), forelegs cursorial (character 22, state 1), tarsal formula 2:2:3 (character 28, state 0), abdominal sternum VIII in males divided into two lobes (character 30, state 1). The monophyly of Clade A is supported by one synapomorphic character and two homoplastic characters: clavus not broad (character 15, state 1) and clypeus distinctly transversely rugose (character 4, state 1), rostrum generally extended beyond metacoxae (character 21, state 2). Clade B and Clade C are the sister group. Clade B is supported by one synapomorphy and two homoplastic characters: rostrum extending beyond procoxae, but never extended beyond metacoxae (character 21, state 1) and frontal plate produced above the base of the rostrum strongly (character 3, state 1), distal of sternum VII in males symmetrical (character 29, state 0). Clade C is supported by one synapomorphy and one homoplastic character: eye outer margin exceeding pronotal costal margin distinct (character 10, state 1) and body length less than 7 mm (character 32, state 2). The taxonomic location of Grimaldinia pronotalis has long been controversial (Popov and Heiss, 2014b; Schuh and Weirauch, 2020), but as shown according to our phylogenetic results, this species has six synapomorphies of Ochteridae. Grimaldinia with Arcochterus gen. nov. and Parvochterus gen. nov. as a monophly form clade C.
Phylogenetic reconstruction indicates the length of the rostrum has occurred at least three times independent transitions from exceeding to procoxa to meso- and metacoxa during the evolution of Ochteroidea: twice transitions occurred during the evolution of Ochteridae, once transition in Gelastocoridae (Figure 2). We hence conclude the different evolutionary trends in the length of rostrum of two groups: in Ochteridae, it gradually evolved from short to long, and in Gelastocoridae, more representatives retain the ancestral form.
Suborder Heteroptera Latreille, 1810.
Infraorder Nepomorpha Popov, 1968.
Superfamily Ochteroidea Kirkaldy, 1906.
Family Ochteridae Kirkaldy, 1906.
Genus Arcochterus Zhang, Ren and Yao gen. nov.
Type species: Arcochterus fuscus Zhang, Ren and Yao sp. nov. (Figure 3).
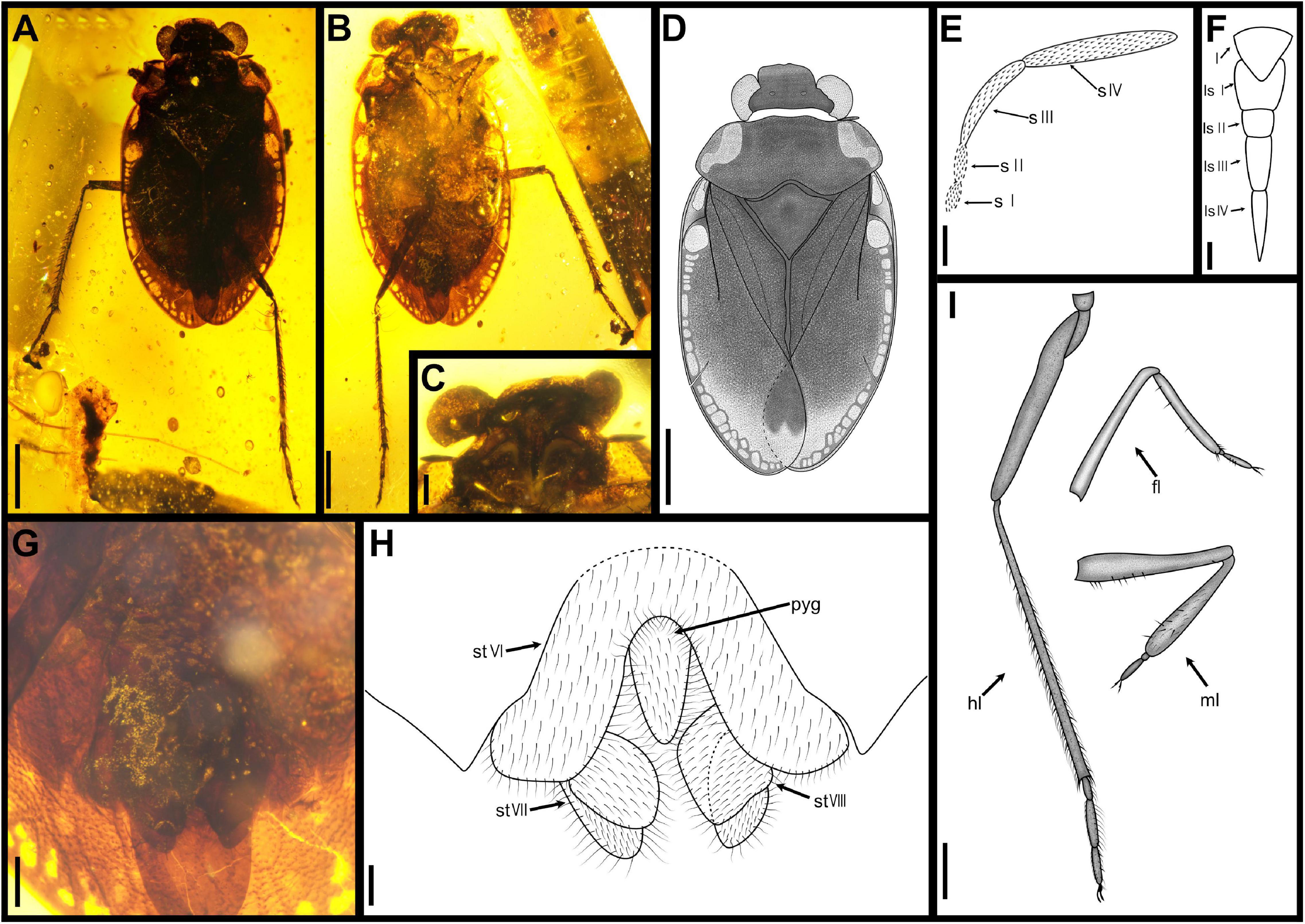
Figure 3. Arcochterus fuscus gen. et sp. nov., holotype, CNU-HET-MA2015003, male. (A) photograph in dorsal view; (B) photograph in ventral view; (C) rostrum; (D) line drawing habitus in dorsal view; (E) antenna, outline; (F) rostrum, outline; (G) abdomen apex; (H) abdomen apex, outline; (I) legs, outline. fl, foreleg; hl, hindleg; l, labrum; ls I-IV, labial segment I to IV; ml, midleg; pyg, pygophore; s I-IV, segment I to IV; st VI-VIII, sternum of segments VI to VIII. Scale bars: panels (A,B,D,I) = 0.5 mm, panels (C,F) = 0.1 mm, panels (E,G,H) = 0.2 mm.
Included species: Arcochterus fuscus Zhang, Ren and Yao sp. nov.
Etymology: The generic name is a combination of the Latin “arcus” (arch) and “ochterus” (type genera of Ochteridae) because the forewings costal margin with one-row arch cells. Gender: masculine.
Diagnosis: Eyes reniform. Two distal segments of antennae are longer than two basal segments (Figures 3C,E). Rostrum 4-segmented, short, reaching to procoxae, segment III in length subequal to segment IV (Figures 3C,F). Outer margins of the pronotum strongly explanate, the extended part wider than the eyes in width. Scutellum longer than pronotum at midline. Clavus broad, about two-thirds as long as forewing, with a long anal vein. Claval commissure is subequal to scutellum in length. Forewing with median fracture and deep costal fracture, costal fracture length about one-third of forewing width, rest of costal margins with over 20 cells (Figures 3A,D). SC curved, fused with C at one fifth basally, C reaching forewing apex. Tibia with dense, thin setae, and stout spines. Male abdominal sternum VI arched, sternum VII and VIII split into two large asymmetrical lobes (Figures 3G,H).
Remarks: The new genus is assigned to Ochteridae based on the following shared characters: body ovoid; eyes large, with inner margins emarginated; ocelli present; antennae 4-segmented, partially visible dorsally; tarsal formula 2-2-3; abdominal end of male asymmetrical.
Arcochterus gen. nov. can be distinguished from the same locality extinct genus Grimaldinia Popov and Heiss, 2014b, by the following characters: antennae distal three segments slender (vs. segment III larger than segments I and II); rostrum 4-segmented (vs. rostrum 3-segmented); pronotum anterior margins concave (vs. pronotum anterior margin convex); portion of mesoscutum invisible (vs. portion of mesoscutum visible); claval commissure subequal to scutellum length (vs. claval commissure insignificantly longer than scutellum length), clavus with a long anal vein (vs. clavus without vein); forewing with deep costal fracture (vs. costal fracture short), costal margin with two spots (vs. costal margin without spots); forewing with more than 20 serried cells (vs. forewing with 4 larger elongate and 2 smaller round cells).
Arcochterus fuscus Zhang, Ren and Yao sp. nov. (Figure 3).
Etymology: The new specific name is from a Latin “fuscus” (fuscous), referring to the particularly deep black presented by the dorsal view. Gender: masculine.
Material: Holotype, CNU-HET-MA2015001 (male), a well-persevered specimen.
Distribution: Hukawng Valley, Kachin State, Northern Myanmar (mid-Cretaceous amber: one species).
Diagnosis: Body length is about 5.50 mm, about 1.86 times as long as the width. Body dorsally very dark brown. Head width about half as long as body width. Antennae segment IV longest, subequal to segment III (Figures 3C,E). Rostrum segment I shortest, segment III subequal in length to segment IV (Figures 3C,F). Scutellum length and width almost equal. Clavus width 0.56 times as long as scutellum, with one anal vein. Costal fracture slender and straight. Medial fractures length about one-third of forewing. Basal of costal margins with two large and transparent spots. Profemur longer than mesofemur. Protibia longer than mesotibia. Pro- and mesotibia with few spurs; metatibia with two-row spurs and long setae dorsally, apex with a row of spines around (Figures 3B,I). Male abdomen apex covered by forewing. Sternum VI-VIII with densely and thin setae. The lobe in sternum VIII lager than sternum VII (Figures 3G,H).
Description: Body dorso-ventrally flattened with dense small points, without spines and setae. Surface except for head densely punctate.
Head broad, about 2.33 times as wide as long. Eyes large, smallest interocular distance 0.44 times of head width, prominent, with inner margins emarginated lightly and eyes stylate small. Ocelli separate, width of ocellus subequal to length of ocelli to eyes. Frontal plate declivent, not protruded anteriorly. Clypeus with pattern of ridges and divided by a cross fold and without distinct transverse wrinkles. Antennae 4-segmented, with dense setae, inserted below eyes, only segment IV partly visible in dorsal view; three distal segments of antennae slender, segment III twice as long as segment II, segment IV longest and subequal in length to segments II and III. Rostrum 4-segmented, short, reaching to procoxae, segment I shortest, segment III longest, segment III and segment IV almost equal length, segment III dark color.
Pronotum strongly transverse, 2.80 times as wide as long, moderately punctured. Anterior margin emarginate. Lateral margins attenuated, and widely rounded. Posterior margin strongly convex at the middle. Scutellum triangular, 1.15 times as wide as long, anterior margin slightly convex, elevated weakly baso-medially, tip pointed, punctured.
Forewings macropterous, in dark color, produced laterally and posteriorly over abdomen, with punctateed surface. Forewing 2.23 times as long as wide, nearly 0.72 times as long as body. Clavus wide and large, with claval suture distinct. Costal fracture extension line not reaching median fracture. Narrow membrane of right forewing overlapping left one.
Legs all walking legs and profemur strong. All legs surface densely setae. Tarsus with two curved claws, two setiform parempodium. Pro- and mesofemora with long setae, without spines; Pro- and mesotibia clavate, with some long spines and short spines mixed; tarsi two segments with some spines and long setae, segment I short. Metafemora thick and strong; tarsi three segments with long setae, segment I shortest, segment II subequal to segment III in length, tip of segment II with a row of spines.
Abdomen apex covered by forewing, abdominal segment VI bent, sternum VII and VIII divided into two independent plates, slightly stout, asymmetrical, with long setae. Sternum VII and VIII slightly smaller and subequal in length.
Dimensions (in mm): Body length 5.45, width 2.92; head length 0.66, width 1.54; antennae length 0.80 (I 0.07, II 0.11, III 0.26, IV 0.36); eye length 0.67, diameter of eye 0.31, interocular space 0.68; interocellar space 0.32; rostrum length 0.52, segment I 0.05, II 0.10, III 0.19, IV 0.18; pronotum length 0.83, width 2.32; scutellum length 1.00, width 1.15; hemelytron length 3.92, width 1.76; clavus length 2.62 width 0.56; abdomen width 1.83; length of foreleg: femur 1.52, tibia 0.91, tarsi 0.32 (I 0.06, II 0.26), claw 0.15; length of midleg: femur 1.36, tibia 1.12, tarsi 0.34 (I 0.05, II 0.29), claw 0.12; length of hindleg: femur 1.83, tibia 2.19, tarsi 0.82 (I 0.11, II 0.39, III 0.32), claw 0.15.
Genus Parvochterus Zhang, Ren and Yao gen. nov.
Type species: Parvochterus reticulatus Zhang, Ren and Yao sp. nov. (Figures 4, 5).
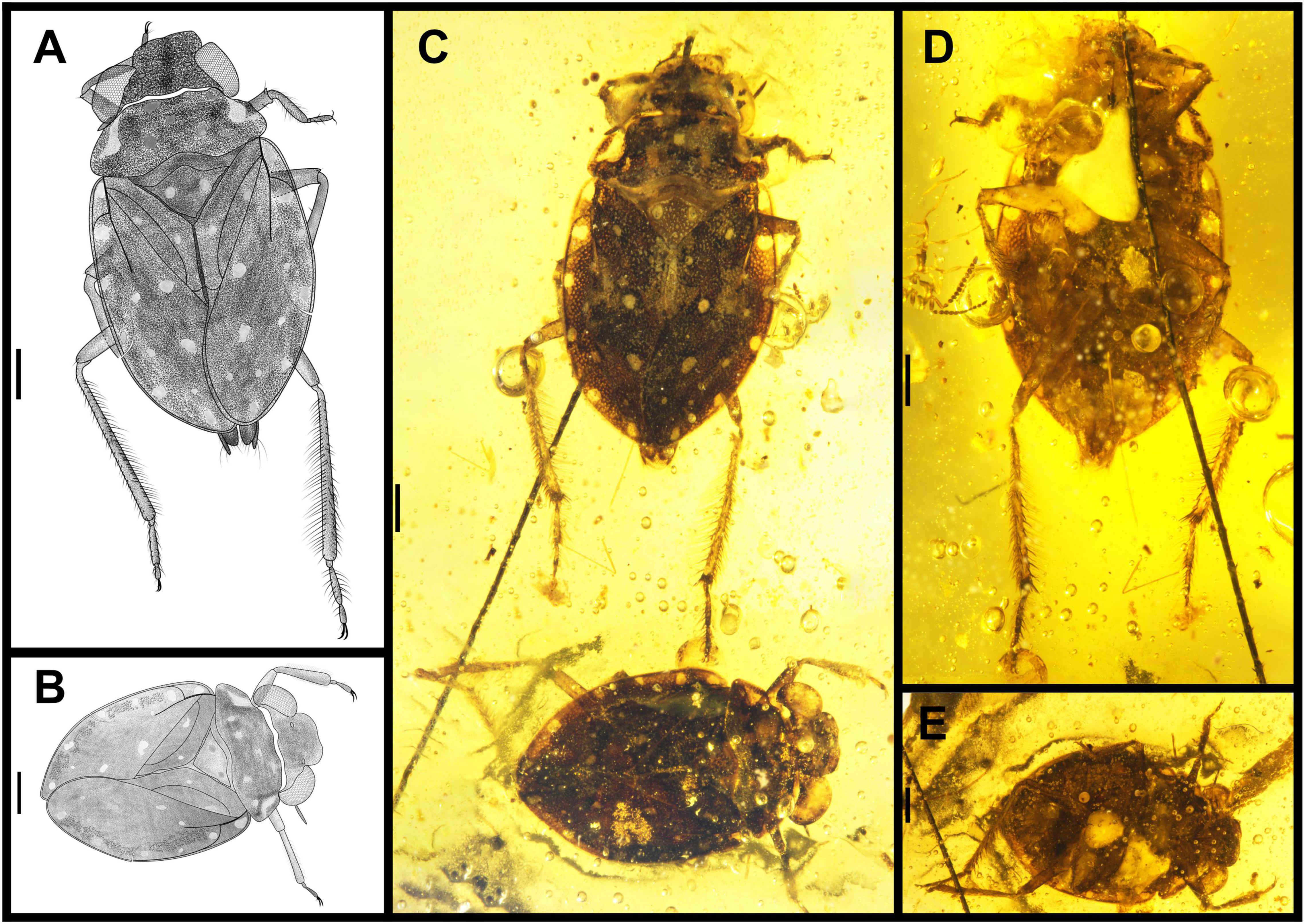
Figure 4. Parvochterus reticulatus gen. et sp. nov. (A) holotype, CNU-HET-MA2015001, male, Line drawing habitus in dorsal view; (B) allotype, CNU-HET-MA2015002, female, Line drawing habitus in dorsal view; (C) holotype (upper), allotype (lower), photograph in dorsal view; (D) holotype, photograph in ventral view; (E) allotype, photograph in ventral view. Scale bars: 0.5 mm.
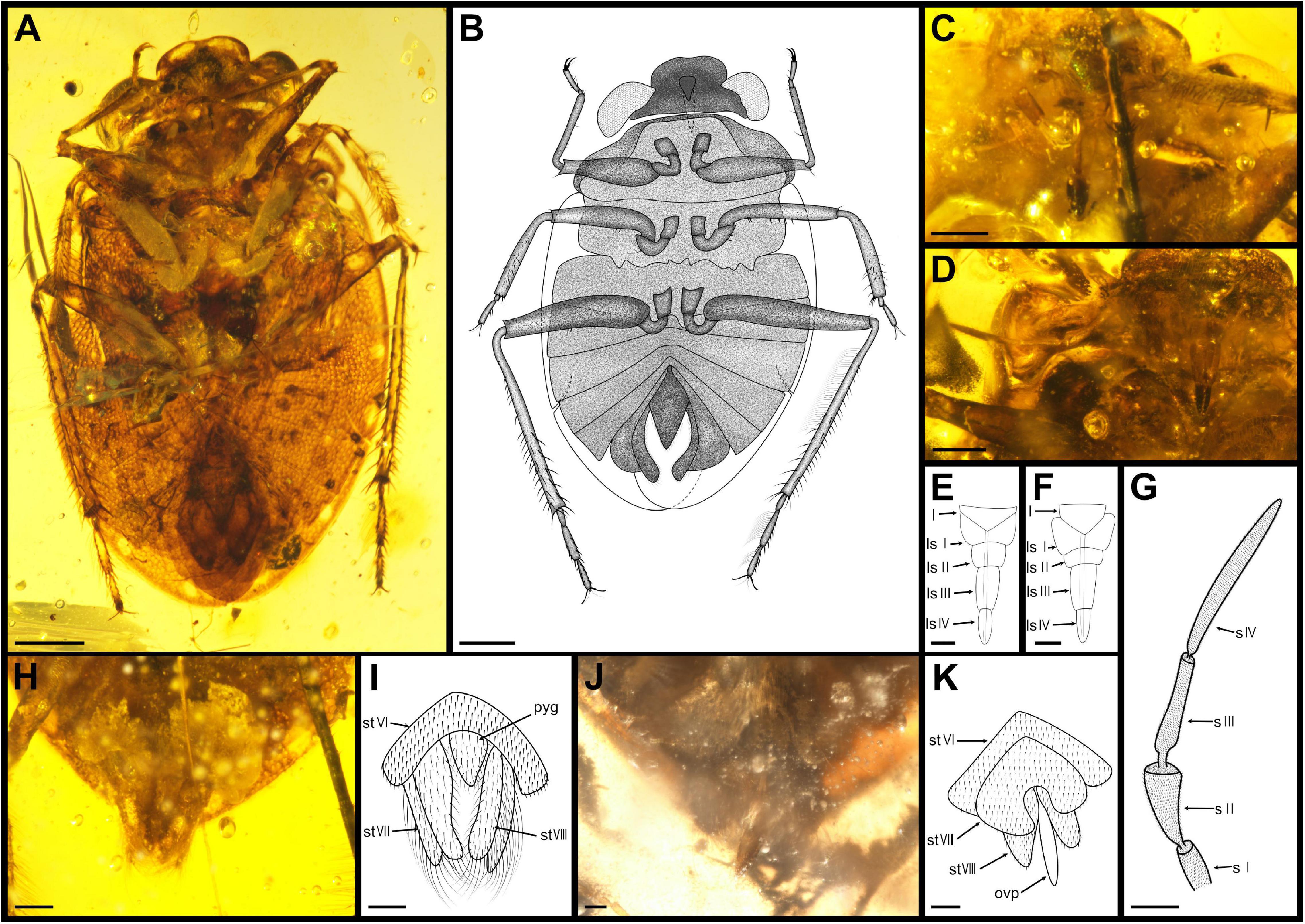
Figure 5. Parvochterus reticulatus gen. et sp. nov. (A) paratype, CNU-HET-MA2015005, male, photograph in ventral view. (B) Line drawing habitus in ventral view. (C) holotype, rostrum; (D) allotype, rostrum, and antenna. (E) holotype, rostrum, outline. (F) allotype, rostrum, outline. (G) allotype, antenna, outline. (H) holotype, abdomen apex. (I) allotype, abdomen apex. (J) holotype, abdomen apex, outline. (K) allotype, abdomen apex, outline. fl, foreleg; hl, hindleg; l, labrum; ls I-IV, labial segment I to IV; ml, midleg; ovp, ovipositor; pyg, pygophore; s I-IV, segment I to IV; st VI-VIII, sternum of segments VI to VIII. Scale bars: panels (A,B) = 0.5 mm, panels (C,D,G–K) = 0.2 mm, panels (E,F) = 0.1 mm.
Included species: Parvochterus reticulatus Zhang, Ren and Yao sp. nov., and P. lanceolarus Zhang, Ren and Yao sp. nov. (Figure 6).
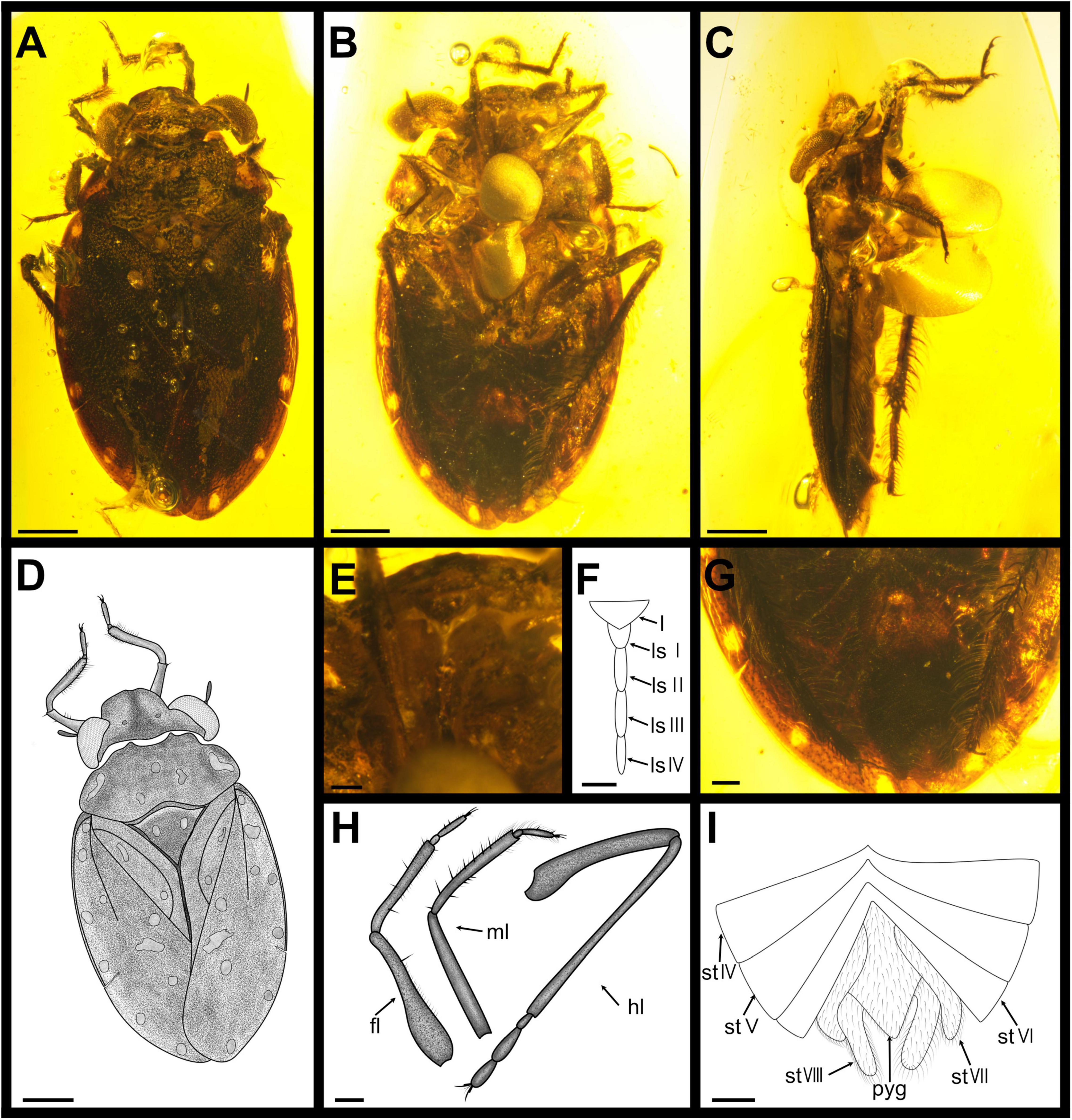
Figure 6. Parvochterus lanceolarus gen. et sp. nov., Holotype, CNU-HET-MA2015004, ♂. (A) photograph in dorsal view; (B) photograph in ventral view; (C) photograph in lateral view; (D) line drawing habitus in dorsal view; (E) rostrum; (F) rostrum, outline; (G) abdomen; (H) legs, outline; (I) abdomen, outline. fl, foreleg; hl, hindleg; l, labrum; ls I-IV, labial segment I to IV; ml, midleg; pyg, pygophore; st VI-VIII, sternum of segments VI to VIII. Scale bars: panels (A–D,I) = 0.5 mm, panels (E,F) = 0.1 mm, panels (G,H) = 0.2 mm.
Etymology: The generic name is a combination of the Latin “parv-”(small) and “ochterus” (type genera of Ochteridae) because the shape of scutellum is significantly smaller than the others. Gender: masculine.
Diagnosis: Head anterior margin emarginated. Eyes oblong, mesal margin emarginate lightly. Antennae segment IV length longer than segment III, segment III length subequal to segment I and segment II (Figures 5D,G). Rostrum 4-segmented, short, reaching to procoxae, segment III longer than segment IV (Figures 5E,F). Pronotum anterior margin depressed, outer margins explanate part in width narrower than width of an eye. Scutellum small, away from pronotum, shorter than pronotum in length at midline. Clavus broad, width subequal to scutellum length, length about 0.50 times as long as forewing. Claval commissure length longer than scutellum. Forewing SC arcuate, fused with C one fifth basally, with costal fracture and medial fracture. Membrane without cells. Pronotum, scutellum, and forewing with silvery-white spots. Tibia with stout spines and long setae. Femur apex with 1–2 spines. Profemur sturdy. Abdominal sterna VI sinuate; male abdominal sternum VII and VIII subdivided into two separate asymmetrical plates (Figures 5A,H,J).
Remarks: Parvochterus gen. nov. resembles Arcochterus gen. nov., for example the rostrum 4-segmented, short, reaching to procoxae; clavus broad; forewing SC arcuate, fused with C, with costal fracture and medial fracture; tibia with spines and setae. abdominal sterna VI sinuate; male abdominal sternum VII and VIII subdivided into two separate asymmetrical plates. The main differences between Parvochterus gen. nov. and Arcochterus gen. nov. are eyes oblong (vs. eyes reniform); rostrum segment length longer than segment IV (vs. rostrum segment III length subequal to segment IV); pronotum outer margins explanate part in width narrower than width of an eye (vs. pronotum extended part in width wider than width of an eye); scutellum small, shorter than pronotum in length at midline (vs. scutellum longer than pronotum at midline); forewings without cells (vs. forewings over 20 cells); Pronotum, scutellum, and forewing with silvery-white spots (vs. pronotum, scutellum, and forewing without spots); femur apex with 1–2 spines (vs. femur apex without spines).
Parvochterus reticulatus Zhang, Ren and Yao sp. nov. (Figures 4, 5).
Etymology: The new specific name is from a Latin word “reticulatus” (reticular), referring to the surface of hemelytron with many reticular punctuations. Gender: masculine.
Material: Holotype, CNU-HET-MA2015002 (male); allotype, CNU-HET-MA2015003 (female); paratype, CNU-HET-MA2015005 (male); three well-persevered specimens, the holotype and allotype in the same amber.
Distribution: Hukawng Valley, Kachin State, Northern Myanmar (mid-Cretaceous amber: one species).
Diagnosis: Antennae apical two segments slender, segment II expanded apically. Rostrum segment II shortest, segment III longest (Figure 5C–F). Pronotum with seven spots in male, and four spots in female. Scutellum with two spots. Forewings with two larger and four smaller ellipse silvery-white spots, clavus with two round silvery-white spots on both sides of anal vein, costal margins with six transparent spots. Costal fracture arched. Medial fractures extension and costal fracture without connection. Fore wing costal margin with dense protrusion notches like tiny grids. Protibia with dense long hairs. Metatibia with two-row thorns and one row of long hairs on each side. Male abdominal segments VII and VIII exposed in dorsal view, sternum VII plates slightly thinner, sternum VIII slightly expanded, with dense long hairs. Female abdomen apex covered by forewings.
Description: Male. Body small-sized, ovoid, about 3.38 mm, 1.92 times as long as wide, dorso-ventrally flattened with dense points, without spines and setae.
Head broad, about 3.08 times as wide as long. Eyes large, ellipse porrect, smallest interocular distance 0.47 times head width, with eyes stylate. Ocelli small, widely separated, close to eyes. Frontal plate declivent, not protruded anteriorly. Clypeus with a pattern of ridges and divided by a cross fold, anterior margin concave. Antennae 4-segmented, segment IV 1.33 times longer than segment III, apical two segments thicker than basal two segments, with dense setae, inserted below eyes, only segment IV partly visible in dorsal view. Labrum small triangle with dense setae, reaching to base of rostrum. Rostrum 4-segmented, short and stout, reaching to procoxae, segment I shortest, segment III 1.8 times longer than segment IV, segment IV dark color.
Pronotum strongly transverse, 3.33 times as wide as long at middle, moderately punctured, with 7 spots. Anterior margin emarginated lightly, posterior margin strongly convex at middle. Scutellum triangular, with two large and silvery-white spots, anterior margin convex, elevated weakly baso-medially, tip blunt, punctured.
Forewings macropterous or submacropterous, surface punctate. Forewing 2.06 times as long as wide, nearly 0.65 times as long as body. Clavus wide and large, with distinct vein penetration, with two spots on each side. Claval commissure, length longer than scutellum length. Costal fracture present, extension line reaching nearly to median fracture. Narrow membrane of right forewing overlapping left one.
Legs all walking legs. Procoxae close to mesocoxae, mesocoxae apart from metacoxae. Metafemora are longer than mesofemora and mesofemora longer than profemora. Tarsus with two curved claws and two setiform parempodium. Pro- and mesofemur stout. Protibia clavate with some rows of spines and dense short hairs. Pro- and mesotibia shorter than femur. Pro- and mesotarsi 2-segmented without spines and hair. Metatibia longer than others tibia, longer than metafemur, with some rows of short to long thin spines and long setae. Metatarsi 3-segmented, segment I shortest, other segments equal length, with hairs and short setae.
Male abdomen apex exceeding forewings submacropterous, not over macropterous, with seven visible segments, abdominal segments V-VI sinuate, segments VII and VIII divided into two long independent plates, asymmetrical, with dense long hairs. Pygophore with long hairs. Segment VII in length subequal to VIII.
Female. The structure and color similar to male, differences: body size of male is larger than female, but female abdomen width is longer than male; male hind femora are longer than middle femora, and middle femora longer than profemora, however, female has three pairs of similar length femora. Abdomen covered by forewings, sternum VII posterior margin strongly hollow, with dense setae, and ovipositor long, exceeding to last abdominal segment.
Dimensions (in mm): Male: body length 4.16, width 2.16; head length 0.48, width 1.48; eye length 0.63, diameter of eye 0.29, interocular space 0.70; interocellar space 0.39; rostrum length 0.58 (I 0.15, II 0.10, III 0.18, IV 0.15); pronotum length 0.55, width 1.83; scutellum length 0.53, width 0.95; hemelytron length 2.72, width 1.32; clavus length 1.53, width 0.45; abdomen width 1.63; length of foreleg: femur 1.01, tibia 0.60, tarsi 0.28 (I 0.06, II 0.22),claw 0.15; length of midleg: femur 1.10, tibia 0.81, tarsi 0.36 (I 0.08, II 0.28), claw 0.13; length of hindleg: femur 1.45, tibia 1.71, tarsi 0.63 (I 0.10, II 0.31, III 0.23), claw 0.12. Female: body length 3.38, width 2.06; head length 0.43, width 1.52; eye length 0.54, diameter of eyes 0.27, interocular space 0.67; interocellar space 0.39; antennae length 0.60 (I 0.07, II 0.15, III 0.21, IV 0.38); rostrum length 0.51 (I 0.15, II 0.07, III 0.17, IV 0.12); pronotum length 0.55, width 1.70; scutellum length 0.40, width 0.78; hemelytron length 2.36, width 0.95; clavus length 1.24, width 0.41; abdomen width 1.76; length of foreleg: femur 0.90, tibia 0.64, tarsi 0.22 (I 0.08, II 0.14), claw 0.15; length of midleg: femur 0.95, tibia 0.72, tarsi 0.27 (I 0.05, II 0.22), claw 0.11; length of hindleg: femur 1.62, tibia 1.64, tarsi 0.64 (I 0.08, II 0.29, III 0.27), claw 0.12.
Parvochterus lanceolarus Zhang, Ren and Yao sp. nov. (Figure 6).
Etymology: The new specific name is from the Latin word “lanceolarus” (lanceolar) by forming two lanceolar bulges with anterior margin of the pronotum. Gender: masculine.
Material: Holotype, CNU-HET-MA-2015003 (male), a well-persevered specimen.
Distribution: Hukawng Valley, Kachin State, Northern Myanmar (mid-Cretaceous amber: one species).
Diagnosis: Rostrum segment I shortest, segment III and segment IV almost equal length (Figures 6E,F). Pronotum with 3 spots. Scutellum with 2 spots. Clavus wide, with 2 spots on anal vein side, claval commissure longer than scutellum. Forewing costal margin with 6 transparent spots, membrane without cells. Costal fracture short and straight, about one-fifth of forewing width, medial fractures long, about two-fifth of forewing length, far from costal fracture. Protibia with dense long hairs; mesotibia latter part gradually coarsens with long setae and apical spines; metabiba with long setae and long, bent and rigidity spines; mesotarsi with two straight claws; metatarsi with two curved claws, with long setae and a row of spines (Figures 6B,C,H). Abdomen apex covered by forewings, sternum IV-VI bent strongly, sternum VII and VIII significantly reduced and retracted into sternum VI, divided into two independent plates, slightly stout, asymmetrical, with long setae (Figures 6G,I).
Description: Small size, ovoid, about 3.82 mm, about 1.80 times as long as wide. Body dorso-ventrally flattened with dense points, without spines and setae.
Head broad, about 3.14 times as wide as long. Eyes large, inner margins emarginated lightly, stylate smaller, smallest interocular distance 0.43 times of head width. Ocelli small, widely separated, close to eyes. Frontal plate declivent, not protruded anteriorly. Clypeus with pattern of ridges and divided by a cross fold. Antennae 4-segmented, slender and short, surface densely setae, inserted below eyes, only segment IV partly visible in dorsal view, fusiformis. Rostrum 4-segmented, short, reaching to procoxae, segment I shortest, segment III segment III longest, and segment IV almost equal length, about 2 times longer than segment I, segment IV dark color.
Pronotum strongly transverse, 2.38 times as wide as long at middle, moderately punctured; Anterior margin concave with two lanceolar bulges, Lateral margins flimsy and widely rounded, Posterior margin strongly convex at middle. Scutellum triangular, with one large marking on each side, anterior margin slightly convex, elevated weakly baso-medially, tip blunt, punctured.
Forewings macropterous, dark color, produced laterally and posteriorly over abdomen, surface punctate. Forewing 2.05 times as long as wide, nearly 0.68 times as long as body. Clavus wide and large, with distinct anal vein and claval suture. Claval commissure not straight, longer than scutellum length. Costal fracture obvious, median fracture extension line without gear into Costal fracture. Narrow membrane of right forewing overlapping left one.
Legs all walking legs. All legs with dense setae. Tarsus with two claws, two setiform parempodium. Metafemur sturdy, with some thin spines and long setae; protibia clavate, with some rows of spines; protarsus 2-segmented, with some spines and long setae, and with two curved claws. Mesotibia latter part gradually coarsens with long setae. Mesotarsi with two straight claws. Metatarsi with two curved claws.
Abdomen apex covered by forewings, sternum VII and VIII significantly reduced and retracted into sternum VI, divided into two long independent plates, slightly stout, asymmetrical, with dense long setae. Sternum VII longer than VIII.
Dimensions (in mm): Male: body length 3.82, width 2.03; head length 0.43, width 1.35; eye length 0.50, diameter of eyes 0.25, interocular space 0.58; interocellar space 0.28; antennae length, segment I ?, II ?, III ?, IV 0.32; rostrum length 0.46, segment I 0.08, II 0.13, III 0.14, IV 0.11; pronotum length 0.70, width 1.65; scutellum length 0.58, width 0.77; hemelytron length 2.61, width 1.27; clavus length 1.45 width 0.38; abdomen width 1.76; length of foreleg: femur 0.61, tibia 0.52, tarsi 0.30 (I 0.09, II 0.21), claw 0.12; length of midleg: femur 0.94, tibia 0.70, tarsi 0.33 (I 0.08, II 0.25), claw 0.12; length of hindleg: femur 1.11, tibia 1.48, tarsi 0.82 (I 0.11, II 0.39, III 0.32), claw 0.15.
The datasets presented in this study can be found online at Zoobank.org [https://zoobank.org] with the below accession number(s). Arcochterus Zhang, Ren and Yao; 8D0187BE-8202-4320-89AF-35B312A67381; Arcochterus fuscus Zhang, Ren and Yao; 85E72289-7F18-4479-9620-1F3A18B1ED0A; Parvochterus Zhang, Ren and Yao; FE840D9F-2F7B-46B3-A1A6-2FC2084C5EB8; Parvochterus lanceolarus Zhang, Ren and Yao; 19E8C1CB-3C96-4BE7-9FBF-30EC7A945467; Parvochterus reticulatus Zhang, Ren and Yao; 4B8B8279-E101-4516-AEE8-E331BC3D75F5.
MZ conceived the study with support from DR and YY. DR and YY provided materials. MZ drew the figures and wrote the draft. ZZ, DR, and YY revised the draft many times. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (Nos. 31970436, 31730087, 32101239, and 32020103006), Joint Fund of the Beijing Municipal Natural Science Foundation and Beijing Municipal Education Commission (KZ201810028046), and Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2020TD11).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to the editor and reviewers for constructive criticism and valuable comments on the manuscript.
Andersen, N. M., and Weir, T. A. (2004). Australian water bugs. Their biology and identification (Hemiptera-Heteroptera, Gerromorpha & Nepomorpha). Collingwood, CAN: Apollo Books, doi: 10.1163/187631204788920185
Champion, G. C. (1901). “Insecta Rhynchota. Hemiptera-Heteroptera, Vol II,” in Biologia Centrali-Americana: Zoology, Botany and Archaeology, eds F. D. Godman and O. Salvin (London, UK: Taylor & Francis), 345–416.
Chen, P. P., Nieser, N., and Zettel, H. (2005). The aquatic and semi-aquatic bugs (Heteroptera: Nepomorpha & Gerromorpha) of Malesia. Leiden-Boston, NL: Brill Academic Publishers, doi: 10.1163/9789047416807
Drake, C. J., and Gómez-Menor, J. (1954). A new genus of American Ochteridae (Hemiptera). Eos Rev. Española de Entomol. 30, 157–159.
Du, S. L., Hu, Z. K., Yao, Y. Z., and Ren, D. (2019). New genus and species of the Yuripopovinidae (Pentatomomorpha: Coreoidea) from mid-Cretaceous Burmese amber. Cretac. Res. 94, 141–146. doi: 10.1016/j.cretres.2018.10.022
Estévez, A. L., and Ruf, M. (2006). Separación de subfamilias en la familia de chinches Gelastocoridae (Hemiptera). Rev. Biol. Trop. 54, 1319–1322. doi: 10.15517/rbt.v54i4.3107
Faúndez, E. I., and Ashworth, A. C. (2015). Notas sobre la familia Gelastocoridae (Hemiptera: Heteroptera) en el extremo sur de Chile, con descripción de un subgénero y especie nuevos. An. Inst. Patagon. 43, 69–74. doi: 10.4067/S0718-686X2015000200005
Goloboff, P. A. (1999). NONA (No Name), Version 2.0. Program and Documentation. Tucumán, Argentina: Fundación Instituto Miguel Lillo.
Goloboff, P. A., and Catalano, S. A. (2016). TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238. doi: 10.1111/cla.12160
Grimaldi, D., and Engel, M. S. (2005). Evolution of the Insects. New York, USA: Cambridge University Press.
Hebsgaard, M. B., Andersen, N. M., and Damgaard, J. (2004). Phylogeny of the true water bugs (Nepomorpha: Hemiptera-Heteroptera) based on 16S and 28S rDNA and morphology. Syst. Entomol. 29, 488–508. doi: 10.1111/j.0307-6970.2004.00254.x
Henry, T. J. (1997). Phylogenetic analysis of family groups within the infraorder Pentatomomorpha (Hemiptera: Heteroptera), with emphasis on the Lygaeoidea. Ann. Entomol. Soc. Am. 90, 275–301.
Jaczewski, T. (1934). Notes on the Old World species of Ochteridae (Heteroptera). Ann. Mag. Nat. Hist. 10, 597–613. doi: 10.1080/00222933408654862
Kirkaldy, G. W. (1906). List of the pagiopodous Hemiptera-Heteroptera, with their type species, from 1758 to 1904 (and also of the aquatic and semi-aquatic Trochalopoda). Acta Ent. Mus. Nat. Pra. 32, 117–156.
Kment, P., Carapezza, A., and Jindra, Z. (2020). Taxonomic catalogue of the family Ochteridae with description of Ochterus papaceki sp. nov. from Socotra Island and Tanzania (Hemiptera: Heteroptera). Acta Ent. Mus. Nat. Pra. 60, 23–64. doi: 10.37520/aemnp.2020.003
Latreille, P. A. (1807). Genera Crustaceorum Et Insectorum Secundem Ordinem Naturalem In Familias Disposita Iconibus Exemplisque Plurimis Explicata. France: Apud Amand Kænig.
Latreille, P. A. (1810). Considérations Générals sur I’ordre Naturel des Animaux. Composant les Classes des Crustacés, des Arachnides, et des Insectes. Paris: F. Schoell.
Li, M., Wang, J., Tian, X. X., Xie, Q., Liu, H. X., and Bu, W. J. (2012). Phylogeny of the true water bugs (Hemiptera-Heteroptera: Nepomorpha) based on four Hox genes. Entomotaxonomia 34, 35–44.
Li, S., Wang, Q. Z., Ren, D., and Yao, Y. Z. (2020). New genus and species of Empheriidae (Psocodea: Trogiomorpha) from mid Cretaceous amber of northern Myanmar. Cretac. Res. 110:104421. doi: 10.1016/j.cretres.2020.104421
Li, T., Hua, J. M., Wright, A. M., Cui, Y., Xie, Q., Bu, W. J., et al. (2014). Long-branch attraction and the phylogeny of true water bugs (Hemiptera: Nepomorpha) as estimated from mitochondrial genomes. BMC Evo. Biol. 14:99. doi: 10.1186/1471-2148-14-99
Maddison, W. P., and Maddison, D. R. (2021). Mesquite: a modular system for evolutionary analysis. Version 3.70. Available Online at: http://www.mesquiteproject.org/ (accessed Aug 31, 2021).
Mahner, M. (1993). Systema cryptoceratorum phylogeneticum (Insecta, Heteroptera). Zoologica 143:302.
Nixon, K. C. (2002). WinClada ver. 1.00.08. Available Online at: http://www.cladistics.com/ (accessed Apr 11, 2009).
Poinar, G. O., and Brown, A. E. (2016). Toad bugs (Hemiptera: Gelastocoridae) in Myanmar amber. Cretac. Res. 63, 39–44. doi: 10.1016/j.cretres.2016.02.013
Polhemus, D. A. (2021). Two new species of Ocyochterus (Heteroptera: Ochteridae) from Ecuador and Panama. Zootaxa 4958, 34–44. doi: 10.11646/zootaxa.4958.1.5
Popov, Y. A. (1968). “Origin and main evolutionary trends of Nepomorpha bugs,” in Proceedings of the 13th international Congress of Entomology, Moscow, Vol. 1., (Leningrad: Nauka), 282–283.
Popov, Y. A., Dolling, W. R., and Whalley, P. E. S. (1994). British Upper Triassic and Lower Jurassic Heteroptera and Coleorrhyncha (Insecta: Hemiptera). Genus 5, 307–347.
Popov, Y. A., and Heiss, E. (2014a). Riegerochterus baehri gen. nov. and spec. nov., the first fossil velvety bug (Hemiptera: Heteroptera, Ochteridae) from Dominican Amber. Andrias 20, 185–190.
Popov, Y. A., and Heiss, E. (2014b). Grimaldinia pronotalis n. gen., n. sp. from Mid-Cretaceous Burmese Amber (Hemiptera: Heteroptera, Leptopodidae, Leptosaldinae). Zootaxa 3878, 444–450. doi: 10.11646/zootaxa.3878.5.2
Rieger, C. (1976). Skelett und Muskulatur des Kopfes und Prothorax von Ochterus marginatus Latreille. Zoomorphologie 83, 109–191. doi: 10.1007/BF00993483
Ruf, M. L., Goodwyn, P. P., and Martins-Neto, R. G. (2005). New Heteroptera (Insecta) from the Santana Formation, Lower Cretaceous (Northeastern Brazil), with description of a new family and new taxa of Naucoridae and Gelastocoridae. Gaea 1, 68–74.
Say, T. (1832). Descriptions of new species of Heteropterous Hemiptera of North America Dec. 1831:(Annual report of New-York state agricultural society). New Harmony, Ind: Erscheinungsort nicht ermittelbar.
Schuh, R. T., and Weirauch, C. (2020). True bugs of the World (Hemiptera: Heteroptera) Classification and Natural history, 2nd Edn. Manchester, UK: Siri Scientific Press.
Shcherbakov, D. E., and Popov, Y. A. (2002). “Superorder Cimicidea Laicharting, 1781. Order Hemiptera Linné, 1758. The bugs, cicadas, plantlice, scale insects, etc,” in History of Insects, eds A. P. Rasnitsyn and D. L. J. Quicke (Dordrecht, NL: Kluwer Academic Publishers), 143–157.
Stål, C. (1856). Hemiptera från Kafferlandet. Ofversigt af Kongliga Svenska Vetenskaps-Akademiens Förhandlingar 12, 89–100.
Wang, Y., Moreira, F. F. F., Rédei, D., Chen, P. P., Kuechler, S. M., Luo, J. Y., et al. (2021). Diversification of true water bugs reveled by transcriptome-based phylogenomics. Syst. Entomol. 46, 339–356. doi: 10.1111/syen.12465
Wang, Y. J., Du, S. L., Yao, Y. Z., and Ren, D. (2019). A new genus and species of burrower bugs (Heteroptera: Cydnidae) from the mid-Cretaceous Burmese amber. Zootaxa 4585, 351–359. doi: 10.11646/zootaxa.4585.2.8
Xie, T. Y., and Liu, G. Q. (2018). Notes on some toad bugs from china (Hemiptera, Heteroptera, Gelastocoridae). Zookeys 759, 137–147. doi: 10.3897/zookeys.759.21627
Yao, Y. Z., Cai, W. Z., and Ren, D. (2007). Pristinochterus gen. n. (Hemiptera: Ochteridae) from the Upper Mesozoic of northeastern China. Eur. J. Entomol. 104, 827–835. doi: 10.14411/eje.2007.103
Yao, Y. Z., Zhang, W. T., Ren, D., and Shih, C. K. (2011). New fossil Ochteridae (Hemiptera: Heteroptera: Ochteroidea) from the Upper Mesozoic of north-eastern China, with phylogeny of the family. Syst. Entomol. 36, 589–600. doi: 10.1111/j.1365-3113.2011.00578.x
Ye, Z., Damgaard, J., Yang, H. H., Hebsgaard, M. B., Weir, T., and Bu, W. J. (2020). Phylogeny and diversification of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha). Cladistics 36, 72–87. doi: 10.1111/cla.12383
Zhang, X., Ren, D., and Yao, Y. Z. (2018). A new genus and species of Mimarachnidae (Hemiptera: Fulgoromorpha: Fulgoroidea) from mid-Cretaceous Burmese amber. Cretac. Res. 90, 168–173. doi: 10.1016/j.cretres.2018.04.012
Appendix 1 Character states.
A description of the 32 morphological characters compiled from literature sources.
1. Clypeus: (0) not so modified; (1) divided by a cross fold (Hebsgaard et al., 2004). State 1 occurs only in Ochteridae and Gelastocoridae.
2. Clypeus: (0) without such pattern; (1) with pattern of ridges (Hebsgaard et al., 2004). State 1 occurs only in Ochteridae.
3. Frontal plate produced above base of rostrum: (0) slightly; (1) strongly (Yao et al., 2011). All of the Mesozoic fossils with broad frontal plate, sides subparallel.
4. Clypeus: (0) not transversely rugose; (1) distinctly transversely rugose (Yao et al., 2011). The transversely rugose clypeus is relatively common in all extant Ochteridae. In all fossil taxa and outgroup, this trait only occurs in Angulochterus.
5. Antennae: (0) short, not visible from above; (1) short, visible dorsally, projecting; (2) long (Hebsgaard et al., 2004; Yao et al., 2011). Antennal apex visible in dorsal is only found in Ochteridae.
6. Antennal segment IV: (0) normal; (1) broad (Hebsgaard et al., 2004). Antennal segment IV broad occurs only in Gelastocoridae.
7. Eye: (0) without eyestalk; (1) reniform, with distinct eyestalk; (2) ellipse, with small eyestalk (Henry, 1997). Eye without eyestalk occurs only in the Early Cretaceous species, the remaining Ochteridae with eyestalk, but the mid-Cretaceous Ochteridae with small eyestalk and eye ellipse, the extent Ochteridae and Riegerochterus baehri with distinct eyestalk.
8. Ocelli: (0) absent; (1) present (Hebsgaard et al., 2004). Ocelli absence occurs in the outgroup and the Early Cretaceous Ochteridae.
9. Head and prothorax: (0) tight junction; (1) discretely (Hebsgaard et al., 2004). Head and prothorax tight junction occur only in the outgroup.
10. Eye outer margin exceeding pronotal costal margin: (0) not obvious; (1) distinct. Eye outer margin exceeding pronotal costal margin is only found in the mid-Cretaceous Ochteridae.
11. Pronotum: (0) with transverse sulcus; (1) without transverse sulcus (Yao et al., 2011). The pronotum with transverse sulcus is the common condition in the outgroup and is also found in all extant Ochteridae. Whereas transverse sulci are absent in all fossil taxa.
12. Pronotum: (0) with spots; (1) without spots (Yao et al., 2011). In the extent and the mid-Cretaceous Ochteridae, spots on the Pronotum can be treated as being different between species.
13. Propleuron: (0) developed; (1) simple (Hebsgaard et al., 2004). Propleuron undeveloped occurs in the Ochteridae and Gelastocoridae.
14. Scutellum: (0) without spots; (1) with spots (Yao et al., 2011). Only Floricaudus multilocellus, Parvochterus reticulatus gen. et sp. nov., and P. lanceolarus gen. et sp. nov., spots on the Scutellum can be treated as being different from other species.
15. Clavus: (0) broad; (1) normal. The broad clavus occurs only in the mid-Cretaceous Ochteridae.
16. Forewing: (0) with thorny fields (“Dornfelder”); (1) without thorny fields (Hebsgaard et al., 2004). Forewing with thorny fields occurs only in the Belostomatidae.
17. Forewing: (0) with spots; (1) without spots (Yao et al., 2011). Forewing without spots is usually found in the outgroup, Ocyochterus victor, Riegerochterus baehri, Grimaldinia pronotalis and Parvochterus gen. nov. have some different pattern spots on the forewings.
18. Costal fracture: (0) absent; (1) present (Yao et al., 2011). Forewing with distinct costal fracture is a very stable character in extant velvety shore bugs.
19. Surface of thorax and forewings: (0) smooth; (1) with wartlike sculpturation (Hebsgaard et al., 2004). Surface of thorax and fore wings with wartlike sculpturation occurs only in the Gelastocoridae.
20. Rostrum: (0) inserted distally on head; (1) inserted posteriorly on head (Hebsgaard et al., 2004). Rostrum inserted distally on head occurs only in the Gelastocoris.
21. Rostrum: (0) short, only reaching beyond procoxae; (1) extending beyond procoxae, but never extended beyond metacoxae; (2) generally extended beyond metacoxae (Yao et al., 2011). The short rostrum occurs in the mid-Cretaceous Ochterids and outgroup, which is assigned as a plesiomorphy. State 1 occurs only in Early Cretaceous fossils. State 2 occurs in all extant Ochteridae and Riegerochterus.
22. Forelegs: (0) raptorial; (1) cursorial (Hebsgaard et al., 2004; Yao et al., 2011). Raptorial forelegs are only found in the outgroup and Gelastocoridae.
23. Protarsus and protibia: (0) fused; (1) normal (Schuh and Weirauch, 2020: ha 24). Foretarsus and foretibia fused are found in the Naucoridae and Nerthra.
24. Metacoxae: (0) conical, firmly united with metapleuron; (1) short, free (Hebsgaard et al., 2004). Metacoxae conical and firmly united with metapleuron occur in the Belostomatidae.
25. Metatibia: (0) flattened, with swimming hairs; (1) simple (Hebsgaard et al., 2004). Hind tibiae flattened with swimming hairs occurs in the Belostomatidae.
26. Tarsal formula: (0) 2:3:3; (1) different (Yao et al., 2011). Naucoridae and Belostomatidae with 2-3-3 tarsal formula.
27. Tarsal formula: (0) 1:2:3; (1) different (Yao et al., 2011). Gelastocoridae with 1-2-3 tarsal formula.
28. Tarsal formula: (0) 2:2:3; (1) different (Yao et al., 2011). Ochteridae with 2-2-3 tarsal formula.
29. Distal sternum VII in males: (0) symmetrical; (1) asymmetrical (Yao et al., 2011). Asymmetry of distal segment VII in males is found in all extant Ochteridae and Gelastocoridae. In our fossils, we found mid-Cretaceous Ochteridae with this character.
30. Sternum VIII in males: (0) undivided; (1) split into two lobes (Yao et al., 2011). Sternum VIII undivided in males is only found in the outgroup.
31. Respiratory siphon: (0) present; (1) absent (Hebsgaard et al., 2004; Yao et al., 2011). The respiratory siphon only occurs in Belostoma.
32. Body length: (0) over 7 mm; (1) less than 7 mm (Yao et al., 2011). The body length over 7 mm is only found in all extinct Ochteridae from Early Cretaceous and extant Megochterus. The longer bodies in the Early Cretaceous are the same as the outgroup taxa, as a plesiomorphy in Ochteri dae.
Keywords: Ochteridae, Gelastocoridae, new species, morphological phylogenetics, ancestral character state reconstruction, independent evolution
Citation: Zhang M, Zhao Z, Ren D and Yao Y (2022) Three New Species of Velvety Shore Bugs (Hemiptera: Heteroptera: Ochteroidea) From Mid-Cretaceous Kachin Amber Shed Light on the Evolution of Rostrum Length in Ochteroidea. Front. Ecol. Evol. 10:892530. doi: 10.3389/fevo.2022.892530
Received: 09 March 2022; Accepted: 20 April 2022;
Published: 16 June 2022.
Edited by:
Chenyang Cai, Nanjing Institute of Geology and Paleontology (CAS), ChinaReviewed by:
Tian Jiang, China University of Geosciences, ChinaCopyright © 2022 Zhang, Zhao, Ren and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunzhi Yao, eWFveXoxMDBAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.