- 1City of Edmonton Pest Management Lab, Edmonton, AB, Canada
- 2Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada
- 3Applied Biology, Centre for Sustainable Food Systems, University of British Columbia, Vancouver, BC, Canada
The provision of nutritional resources for beneficial insects that support pest control, such as parasitoid wasps, is one tactic in conservation biological control. This tactic could be an important element for the development of a biological control program to help control the ash leaf-cone roller, Caloptilia fraxinella Ely (Lepidoptera: Gracillariidae), an introduced pest of horticultural ash trees (Fraxinus) in Canadian Prairie cities, including Edmonton, AB, Canada. In the current study, we test the efficacy of carbohydrate food provision to support parasitism of C. fraxinella by its primary parasitoid, Apanteles polychrosidis Viereck (Hymenoptera: Braconidae). Laboratory experiments compared the longevity, parasitism rate and offspring fitness of wasps fed sucrose solutions at one of two concentrations (10 and 25% v/v) or flowers of buckwheat, Fagopyrum esculentum (Polygonaceae). Fed wasps lived longer than wasps with access to water only. Mated, but not unmated, males and females lived longer when fed 25% than 10% sucrose. Female A. polychrosidis had similar longevity when fed 25% sucrose or buckwheat flowers. Egg load did not change with sucrose provision. Adult longevity of fed unmated female A. polychrosidis was negatively correlated with egg load. Female A. polychrosidis fed 25% sucrose produced offspring with a slightly female-biased sex ratio and higher fecundity than offspring from female A. polychrosidis fed the 10% sucrose solution.
Introduction
The “parasitoid nectar provision hypothesis” (Heimpel and Jervis, 2005) contends that supplemented nutrition provided to target parasitoids can enhance biological control. Carbohydrate resources can increase wasp longevity and fecundity (Benelli et al., 2017), and increase retention in treated patches (Jamont et al., 2014; Tena et al., 2015). To maximize fitness, animals should spend more time in habitat patches where foraging time is optimized (Pyke et al., 1977). For adult parasitoids, optimal foraging occurs when nectar food sources are in close proximity to patches of susceptible insect hosts that provide oviposition sites. Artificial enhancement of carbohydrate resources can increase patch retention time in parasitoids. Aphytis melinus DeBach (Hymenoptera: Aphelinidae), a parasitoid of the California red scale, Aonidiella aurantii (Maskell) (Hemiptera: Diaspididae), remains in orchard habitat augmented with a carbohydrate solution (Tena et al., 2015). More of the aphid parasitoid, Diaeretiella rapae (McIntosh) (Hymenoptera: Braconidae) are attracted to and retained in patches of aphid-infested Brassica napus (Brassicaceae) supplemented with plantings of Vicia faba (Fabaceae) with extrafloral nectaries (Jamont et al., 2014).
Flowers release volatile compounds that attract parasitoid wasps (Belz et al., 2012), and can be manipulated to concentrate parasitoids where pest control is desired. Flower selection permits a more targeted approach to parasitoid attraction and retention than sugar feeding, as flower shape, color, and volatile profile influence which wasps are attracted and can access the nectar source (Wäckers, 2004). Flower morphology affects nectar availability for parasitic wasps. The width and depth of the corolla determines nectar access for Microctonus hyperodae Loan (Hymenoptera: Braconidae) (Vattala et al., 2006). The scent of yarrow, Achillea millefolium (Asteraceae) is repellant to Cotesia glomerata, Pimpla turionellae Linnaeus (Hymenoptera: Ichneumonidae), and Heterospilis prosopidis Vierek (Hymenoptera: Braconidae) (Wäckers, 2004). Buckwheat (Fagopyrum esculentum, Polygonaceae) flowers are accessible and attractive to M. hyperodae, and access to buckwheat as compared to other flower species increases wasp longevity (Vattala et al., 2006).
In some systems, carbohydrate provision with flower nectar or sugar solutions enhances female parasitoid fecundity. The amount and type of carbohydrate resource required for egg development differs depending on the reproductive strategy of the wasp. Synovigenic parasitoids continuously produce eggs after adult eclosion, whereas pro-ovigenic wasps have a full egg complement at adult eclosion (Flanders, 1950). There is a continuum of ovigenic strategies across the parasitic Hymenoptera that can be described with an index based on the proportion of developed eggs at eclosion (Jervis et al., 2001). Within the Braconidae studied to date, this index ranges from 0-1 (Jervis et al., 2001). Nutrients acquired post eclosion provide energy for egg building in synovigenic parasitoids, whereas nutrient allocation to flight and longevity may pervade in pro-ovigenic parasitoids (Jervis et al., 2008). The fecundity of female Cotesia marginiventris (Cresson) (Hymenoptera: Braconidae), a parasitoid of noctuid moths, increases when wasps have access to a honey solution (Riddick, 2007). Sugar feeding by Neochrysocharis formosa (Hymenoptera: Eulophidae) prevents egg resorption and promotes new egg production (Wang et al., 2014). Access to nectar of the sesame flower, Sesamum indicum (Pedaliaceae), promotes lifetime egg production of the synovigenic parasitoid Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae), a parasitoid of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae) (Liu et al., 2017). Provision of buckwheat flowers in planted borders around host habitat increases fecundity and decreases egg maturation time in the parasitoid Diadegma insulare Cresson (Hymenoptera: Ichneumonidae) (Lee and Heimpel, 2008).
Parasitoid efficacy is most commonly limited by adult female longevity rather than lifetime fecundity per se (Rosenheim, 2011). The role of carbohydrate nutrition in extending the lifespan of adult parasitoids, therefore, may be as or more important than nutritional enhancement to egg production. In protrandrous parasitoid species, the length of the adult stage will affect the timing and encounter rates between males and females (Kant et al., 2016). Parasitoid wasps access carbohydrates by feeding on flower nectar, extrafloral nectaries, and honeydew (Quicke, 2015). Manipulation of carbohydrate sources in managed ecosystems through the provision of sugar solutions or floral resources has the potential to extend parasitoid lifespan which can increase the access to the appropriate stage of the host (Jacob and Evans, 2000; Irvin and Hoddle, 2015; Ashraf et al., 2017).
The types of carbohydrates available to parasitoids vary in nature and may differ in their effect on parasitoid longevity. Consumption of glucose, fructose, and sucrose, the sugars most abundant in flower nectar, extends adult longevity of C. glomerata and allows wasps to recover after starvation events (Wäckers, 2001; Hausmann et al., 2005). Provision with honey water lengthens the lifespan of an ichneumonid parasitoid, Batheplectes curculionis, of Hypera postica (Gyllenhal) (Coleoptera: Curculionidae) (Jacob and Evans, 2000). The longevity of adult braconid wasps Apanteles ruficrus (Haliday) and Cotesia chilonis Munakata that parasitize larvae of the rice pest, Sesamia inferens Walker (Lepidoptera: Noctuidae), is increased when wasps have access to sesame flowers, compared to those given water (Zhu et al., 2015). Provision of certain Brassica flowers to D. insulare increases wasp longevity to a similar extent as access to honey-water (Idris and Grafius, 1995). The lifespan of B. curculionis (Thomson) (Hymenoptera: Ichneumonidae) is equally increased by feeding on a sucrose solution or on honeydew (England and Evans, 1997). In contrast, sucrose-feeding enhanced the longevity of the fruit fly parasitoid, Diachasmimorpha tryoni (Cameron) (Hymenoptera: Ichneumonidae), more than consumption of honey or golden syrup solutions (Zamek et al., 2013). The concentration of sugars can also impact parasitoid physiology. Food molecule transport in the insect gut can be disrupted by high sugar concentration (Chippendale, 1978). Consumption of sucrose solutions with greater than 25% sucrose decreases both the longevity and fecundity of Bracon hebetor (Say) (Hymenoptera: Braconidae), a parasitoid of moth pests in the family Pyralidae (Lepidoptera) (Ashraf et al., 2017).
The goal of the current study is to assess the effect of carbohydrate provisioning on the braconid parasitoid, Apanteles polychrosidis, a native parasitoid currently exploiting an introduced pest of ash (Fraxinus), the ash leaf-cone roller, Caloptilia fraxinella (Lepidoptera: Gracillariidae), in Prairie communities of western Canada. Ash are desirable boulevard and park trees in urban centers on the Canadian Prairies as they are drought tolerant and cold hardy (Lane et al., 2016). In Edmonton, AB, Canada, almost 40% of the boulevard trees are ash trees, with the vast majority being green ash, Fraxinus pennsylvanica (Oleaceae) (City of Edmonton, 2022). The leaf-mining moth, C. fraxinella, was first recorded on ash trees in Edmonton in 1999 (Pohl et al., 2004). The larvae emerge from eggs laid in spring and are leaf-miners for the first three larval instars (Pohl et al., 2004; Evenden, 2009). After dispersal on silken threads, the fourth larval instar rolls a leaflet into a cone shelter, inside which it completes development and pupates.
The primary parasitoid of a complex of parasitoids that have adopted C. fraxinella as a host in Edmonton is a generalist, solitary endoparasitoid, A. polychrosidis Vierek (Hymenoptera: Braconidae) (Wist and Evenden, 2013). A. polychrosidis is a koinobiont parasitoid, as the parasitized host larva continues to grow and develop as the parasitoid develops within it (Quicke, 2015). This wasp has a wide Nearctic distribution (Fernández-Triana and Huber, 2010) and is native to the Edmonton area. It has been recorded from larval hosts in the moth family Tortricidae, including Choristoneura rosaceana (Harris), Platynota idaeusalis (Walker), and Endopiza viteana Clemens (all Lepidoptera: Tortricidae) (Seaman et al., 1990; Biddinger et al., 1994; Li et al., 1999; Cossentine et al., 2004). In British Columbia, A. polychrosidis is bivoltine and overwinters in first or second instar larvae of C. rosaceana with a second generation in the summer (Cossentine et al., 2004). In Edmonton, the native larval hosts of A. polychrosidis have not been identified. A. polychrosidis has shifted hosts to exploit the introduced C. fraxinella as its larval host in the summer generation (Wist and Evenden, 2013) but the overwinter host is not known. Adult A. polychrosidis, like the majority of parasitoid wasp adults, are nectar feeders (Quicke, 2015) but it is unknown if carbohydrate provision will enhance biological control in this system. If access to sucrose solution or easily grown flowers enhances parasitism, these could potentially be provided in urban environments to support biological control of C. fraxinella in ash trees.
Materials and methods
Insects
For all laboratory feeding experiments, A. polychrosidis were collected between 13 and 28 June 2015 and 2016 from green ash trees infested with C. fraxinella at sites across Edmonton, AB, Canada. Leaf-rolls containing wasp pupae were identified by the lack of a “window” in the leaflet tissue that is normally excavated by unparasitized C. fraxinella larvae before pupation (Wist and Evenden, 2013). Leaf-rolls containing wasp pupae were placed individually in 36 ml plastic cups on cafeteria trays and stacked by a window (2015) or in plastic bags with moistened paper towels to prevent desiccation (2016), and maintained at room temperature (19–21°C) until wasp eclosion. Cups were checked daily for adult wasp emergence, and wasps were transferred to feeding treatments within 24 h of eclosion.
Larval hosts used in Experiment 3 were colony-raised C. rosaceana (Harris) (Lepidoptera: Tortricidae), reared from egg masses obtained from the Pacific Agri-Food Research Centre, Agriculture and Agri-Food Canada, in Summerland, BC, Canada. Larvae hatched, and were reared at a density of 2 larvae per 30 ml plastic cup on a pinto bean-based diet (Shorey and Hale, 1965) until use in trials. The colony was maintained at 24°C, L16:D8 photoperiod.
Experiments 1–3: Laboratory sucrose-feeding experiments
Laboratory feeding experiments tested the hypothesis that provision with sucrose solution impacts longevity and egg load of unmated wasps (Experiment 1), and the longevity of mated wasps (Experiment 2). Newly eclosed wasps were transferred individually to 36 ml plastic cups before (Experiment 1) or after (Experiment 2) having the opportunity to mate (Wist et al., 2015). Feeding treatments for both experiments consisted of two sucrose solutions: 10 and 25% (v/v) sucrose in deionized water and a deionized water control. Each wasp (n = 41–55 per treatment) was provided with a feeding treatment through a dental wick. A no-water control treatment was also included in Experiment 1. Wasps were maintained at room temperature (19–21°C) and food sources were refreshed every 5 days until death. In Experiment 1, dead females were frozen at −20°C until dissection to count eggs. Dissections were conducted in Ringer’s solution to remove the ovaries. Ovaries were placed on a glass slide under a glass coverslip, which was tapped to liberate eggs. Mature chorionated eggs were counted under 60× magnification, using a dissecting microscope (Leica Microsystems Inc., ON, Canada). Hind tibia length of each female wasp was measured as a metric of body size (Visser, 1994) using an ocular micrometer attached to a Leica MZ95 dissecting microscope at 32× magnification.
To test the hypothesis that the level of carbohydrate nutrition affects parasitism rate and offspring fitness, a third laboratory feeding experiment (Experiment 3) was conducted using females that had access to males and were presumed to be mated females fed either 25% (n = 11) or 10% (n = 11) (v/v) sucrose in deionized water. As successful parasitism first occurs when A. polychrosidis females are ∼4 days old (Cossentine et al., 2005), a water control treatment could not be included in this experiment because wasps would not survive long enough to parasitize hosts. After 9–15 days in 36 ml cups with access to one of the two sucrose solutions, individual female wasps were transferred to 160 ml chambers. Females were provided with the same feeding treatment and had access to 10, first to third instar C. rosaceana larvae as potential oviposition hosts on a 1 × 1 cm cube of pinto bean diet (Shorey and Hale, 1965; Cossentine et al., 2005). After 48 h, larvae were transferred in pairs to individual 36 ml cups containing pinto bean diet, and each female wasp received fresh larvae. This process was repeated until the female wasp died. Larvae that had been exposed to female wasps were maintained in a growth chamber at 24°C, L16:D8 and checked daily for signs of parasitism and wasp emergence. Wasp offspring that eclosed as adults were removed from feeding containers within 24 h of eclosion, and were frozen at −20°C until dissection and measurement. Sex of the emerged offspring was recorded, and the tibia length was measured using an ocular micrometer under a dissecting scope at 40× magnification. Female offspring were dissected as described in Experiment 1 to assess egg-load.
Experiment 4: Laboratory flower-feeding experiment
Experiment 4 tested the effect of access to buckwheat flowers compared to a sucrose solution on female A. polychrosidis longevity. The 25% sucrose solution was prepared as explained previously, except that distilled water was used in place of deionized water as the solvent. Buckwheat seeds (Apache Seeds, Edmonton, AB, Canada) were sown in seedling trays (cells 4.0 × 3.0 and 5.0 cm deep), in Sunshine Mix 2 soil (SunGro Horticulture Canada, Ltd., Seba Beach, AB, Canada) at a density of 3 seeds per cell. Trays were positioned in a growth room with full spectrum light (24°C, L16:D8), and watered every 2 days. Plants were fertilized with 1 g/L of 20-20-20 fertilizer (Plant-Prod All Purpose Fertilizer 20-20-20, Home Depot, Edmonton, AB, Canada) on two occasions before the start of the feeding trial.
Newly emerged, unmated female wasps were transferred individually to 36 ml plastic cups and supplied with one of four feeding treatments: (1) a cluster of 5–20 buckwheat blossoms on an intact plant (n = 22); (2) a single buckwheat leaf on an intact plant (n = 13); (3) 25% sucrose solution in distilled water (n = 35); or (4) distilled water (n = 37). Trays with the variously treated wasps were positioned within the same growth chamber in which plants were reared. For plant-based feeding treatments, wasps were transferred every 2 days to new blossoms or leaves. Other feeding treatments were changed every 5 days.
Statistical analyses
All statistical analyses were conducted in R (version 3.4.2) (R Core Team, 2017). The effect of feeding treatment on the longevity of unmated (Experiment 1) and mated (Experiment 2) wasps was compared using separate generalized linear models with negative binomial error distributions, to account for overdispersion of the data, using MASS package (version 7.3-47; Venables and Ripley, 2002). Explanatory variables included wasp sex and feeding treatment and the sex × treatment interaction term in each case. In Experiment 1, subsequent multiple comparisons were conducted using a pairwise Tukey’s test in the lsmeans package (version 2.27-2; Lenth, 2016). In Experiment 2, the interaction term between sex and feeding treatment was not significant and was removed from the final model. In Experiment 1, the effect of nutrition treatment on egg load in unmated females was analyzed with a linear model. The effect of wasp size and longevity on egg load was examined using a multiple regression model. Egg load was specified as the dependent variable, and body size (hind tibia length), and longevity were specified as independent variables.
In Experiment 3, a general linearized model (lme4 package, version 1.1-14; Bates et al., 2015) with a binomial error distribution was used to compare the rate of parasitism among females fed different concentrations of sucrose. A vector, y, bound the number of parasitized larvae, and the number of unparasitized larvae into a single object (Crawley, 2015) for the parasitism analysis. The total number of offspring produced by females fed different levels of sucrose was compared with a generalized linear model with a Poisson error distribution (lme4 package, version 1.1-11; Bates et al., 2015).
The proportion of male and female offspring produced by females fed different levels of sucrose solution was compared by constructing a vector (y) that bound the number of female offspring and the number of male offspring into a single object (Crawley, 2015). The vector was used as the response variable in a generalized linear mixed model with a binomial distribution. Maternal feeding treatment was specified as the explanatory variable and individual maternal wasp identity was considered a random factor (lme4 package, version 1.1-11; Bates et al., 2015). Tibia size of the offspring was compared with a linear model that specified maternal feeding treatment and wasp sex as the explanatory variables and maternal wasp identity as a random factor. The effect of maternal diet on the egg load of the female offspring was tested using a generalized linear mixed model with a Poisson error distribution. Maternal feeding treatment and offspring tibia length were specified as the explanatory variables and individual female wasp identity was treated as a random factor. There was no significant interaction between the main effects, so the interaction term was removed from the final model.
The longevity of wasps exposed to the various feeding treatments in Experiment 4 was compared using a generalized linear model with a negative binomial error distribution to account for overdispersion. Feeding treatment was specified as the explanatory variable (MASS package, version 7.3-47; Venables and Ripley, 2002).
Results
In Experiment 1, both wasp sex and carbohydrate diet source affected the longevity of unmated wasps (sex: χ2 = 11.49, df = 1, p = 0.0007, treatment: χ2 = 1444.9, df = 3, p < 0.0001). There was a marginally significant interaction between sex and carbohydrate source (sex × treatment: χ2 = 7.35, df = 3, p = 0.0614) that affected wasp longevity. Unmated male and female wasps lived on average 13.38 ± 0.88 and 16.04 ± 1.04 days, respectively when fed 10% sucrose solution. Wasps fed the 25% sucrose solution lived on average 14.88 ± 0.88 and 20.70 ± 1.22 days for males and females, respectively (Figure 1). Both male and female unmated wasps lived significantly longer when fed either sucrose solution compared to wasps given only water, or those held in empty containers with no access to food or water. As food is required for wasps to live to 4 days post eclosion when parasitism starts (Cossentine et al., 2005), nectar feeding appears to be a prerequisite to parasitism in this species. Unmated male wasps lived significantly longer when given water (2.46 ± 0.12 days), compared to those in empty containers (1.17 ± 0.05 days) (z = −4.43, p = 0.0002). Unmated males and females fed 10% sucrose solution had similar longevity (z = 2.121, p = 0.4010), as did male wasps fed either 10 or 25% sucrose solution (z = −1.267, p = 0.9110). Females fed 25% sucrose solution lived, on average, 4.66 days longer than females fed 10% sucrose solution (z = −2.881, p = 0.0765).
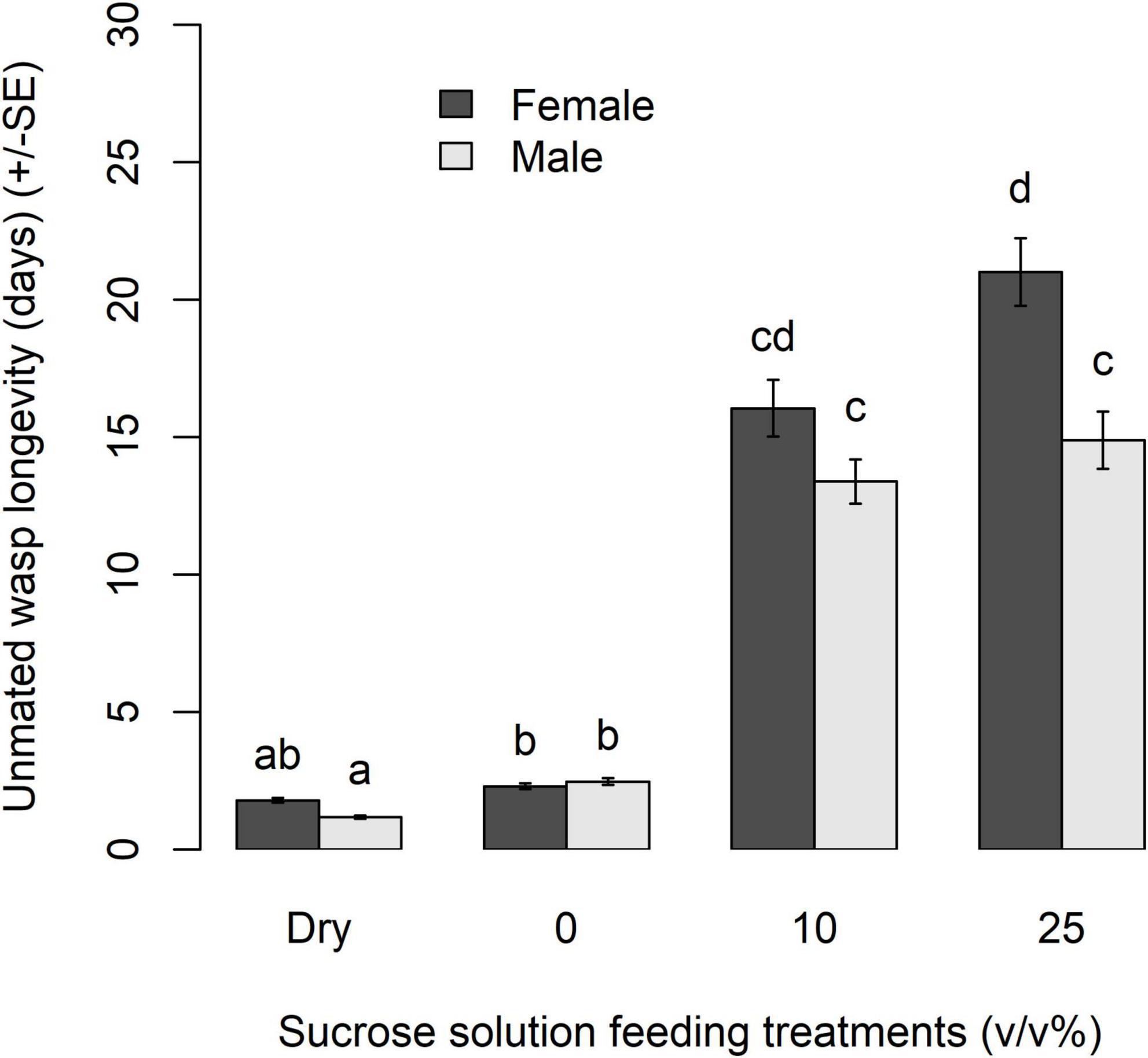
Figure 1. Mean longevity (d) of unmated Apanteles polychrosidis fed various sucrose feeding treatments. Each wasp (n = 41–55 per treatment) was provided with a feeding treatment of either 10 or 25% (v/v) sucrose in deionized water, a deionized water control (0), or a dry feeder control dry. Bars marked with different letters are significantly different (p < 0.05) [compact letter display (cld) of multiple pairwise comparisons].
The sucrose feeding treatment had a significant effect on the egg load of unmated female A. polychrosidis (F = 5.438, df = 3, p = 0.0016) (Figure 2). At dissection, wasps had between 5 and 52 eggs in their ovaries. Egg load was highest in females maintained on water (30.97 ± 1.4 eggs) or fed the lower concentration of sucrose (10%) (30.04 ± 1.68 eggs). Interestingly, the egg load of females fed the 25% sucrose solution was significantly lower than that in wasps provided with either 10% sucrose solution, or water alone (22.96 ± 1.71 days). The egg load of females maintained in dry containers was intermediate. Egg load increased with female wasp size, but decreased with wasp age [Egg load = −52.77 + 92.58 (tibia) −0.26 (age), R2 = 0.1816, tibia slope p-value = 0.0001, age slope p-value = 0.0032] (Figure 3).
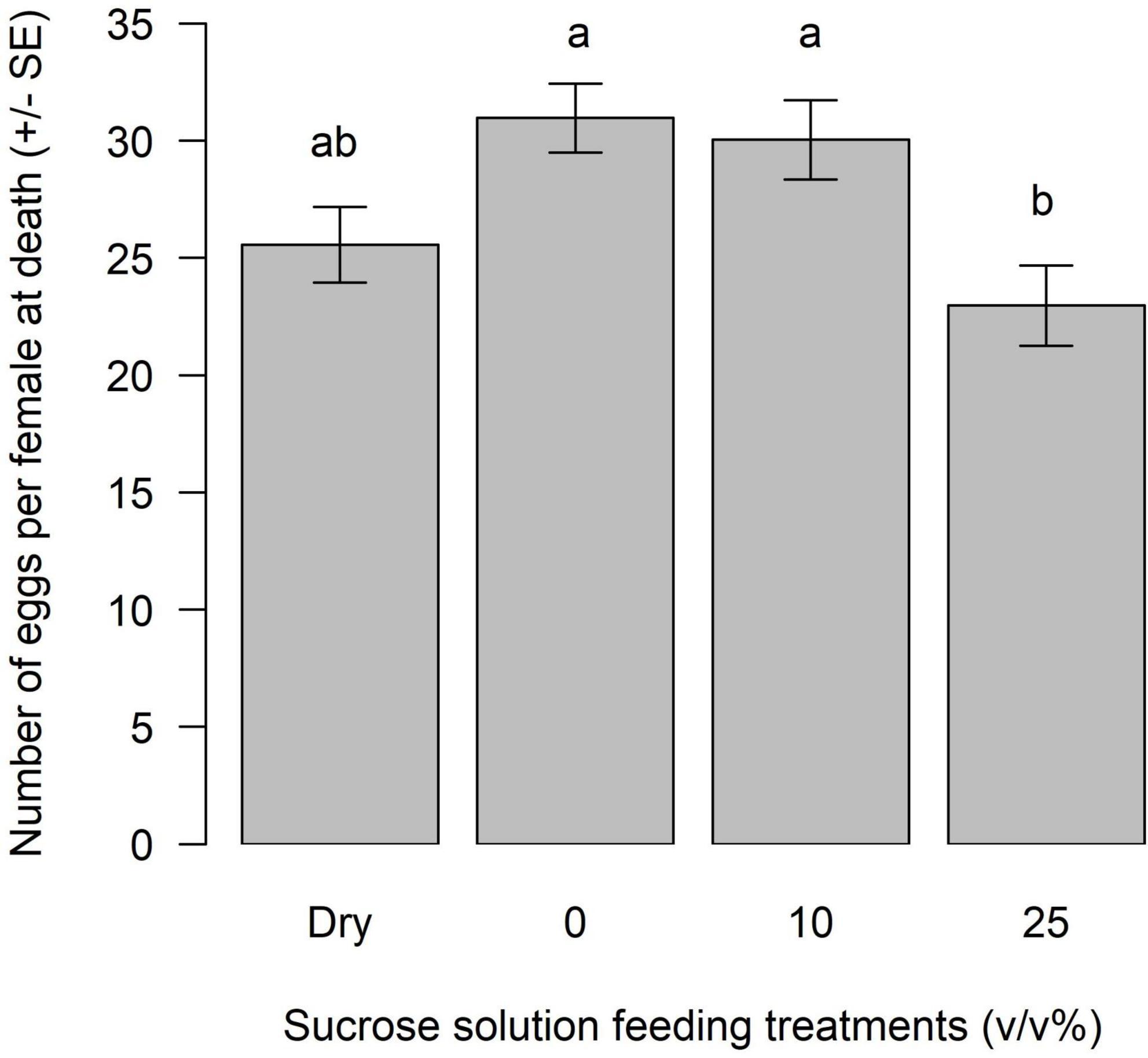
Figure 2. Mean egg load at death of unmated female Apanteles polychrosidis. Each wasp (n = 20–28) was provided with a feeding treatment of either 10 or 25% (v/v) sucrose in deionized water and a deionized water control (0), or a dry feeder control (Dry). Wasps were maintained at 21°C, and feeding treatments were changed every 5 days until death. Dead females were frozen at –20°C until dissection to count eggs. Bars marked with different letters are significantly different (p < 0.05) [compact letter display (cld) of multiple pairwise comparisons].
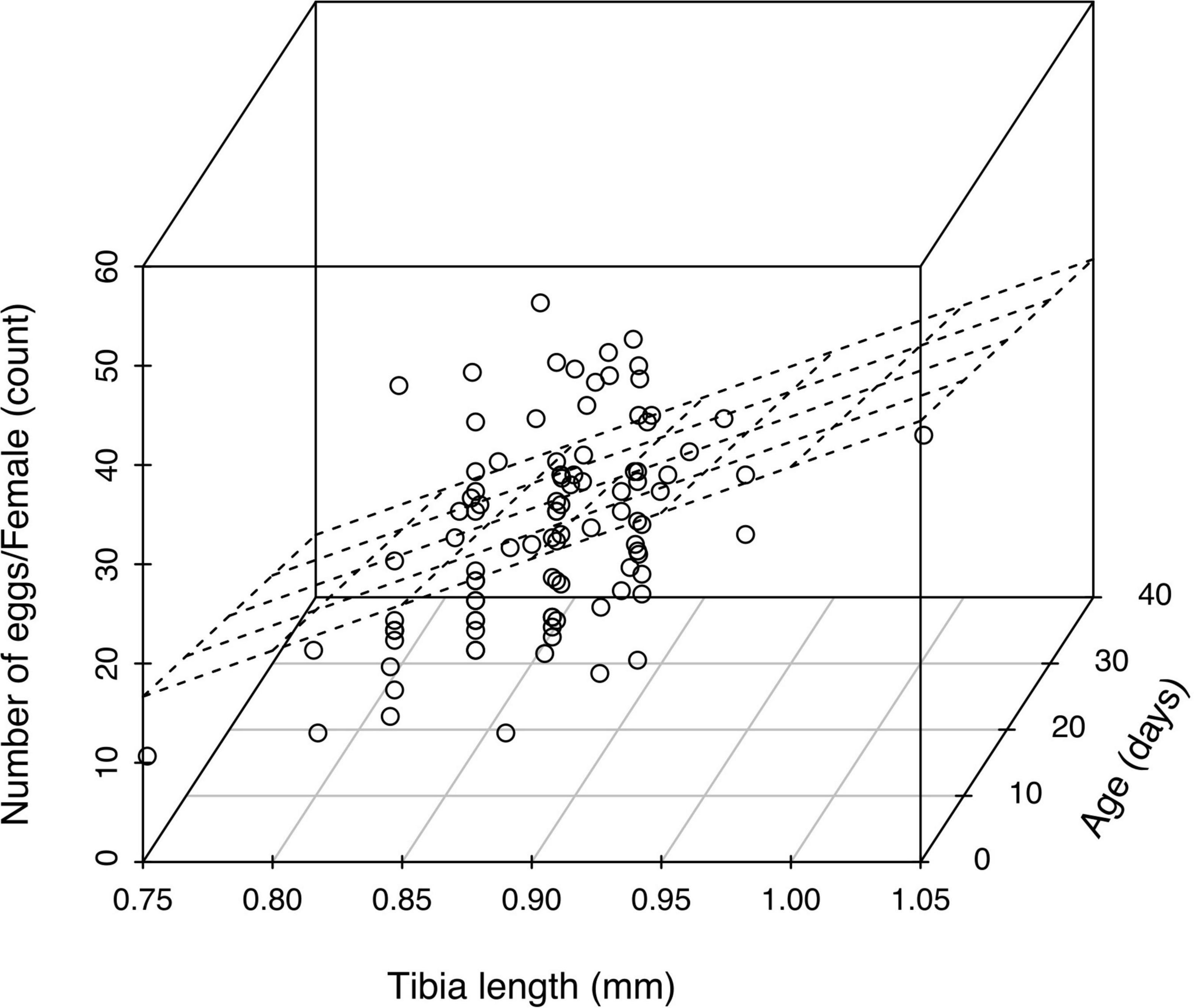
Figure 3. Three-dimensional scatterplot with regression plane of the effect of tibia length and longevity on egg load. Egg load = –52.77 + 92.58 (tibia) –0.26 (age), R2 = 0.1816, tibia p = 0.0001, age p = 0.0032.
In Experiment 2, mated wasp longevity was affected by wasp sex and feeding treatment (sex: χ2 = 9.74, df = 1, p = 0.0018, treatment: χ2 = 431.38, df = 2, p < 0.0001). Mated male and female wasps lived on average 12.83 ± 1.04 and 17.6 ± 0.97 days, respectively, when fed the 10% sucrose solution, and 17.76 ± 0.34 and 20.63 ± 2.17 days when fed the 25% sucrose solution. Male and female mated wasps fed the 25% sucrose solution lived significantly longer than those fed the 10% sucrose solution (z = −3.131, p = 0.0048), or those given water (z = −19.064, p < 0.0001) (Figure 4). When fed 10% sucrose solution, both sexes lived significantly longer than those fed water only (z = 17.00, p < 0.0001).
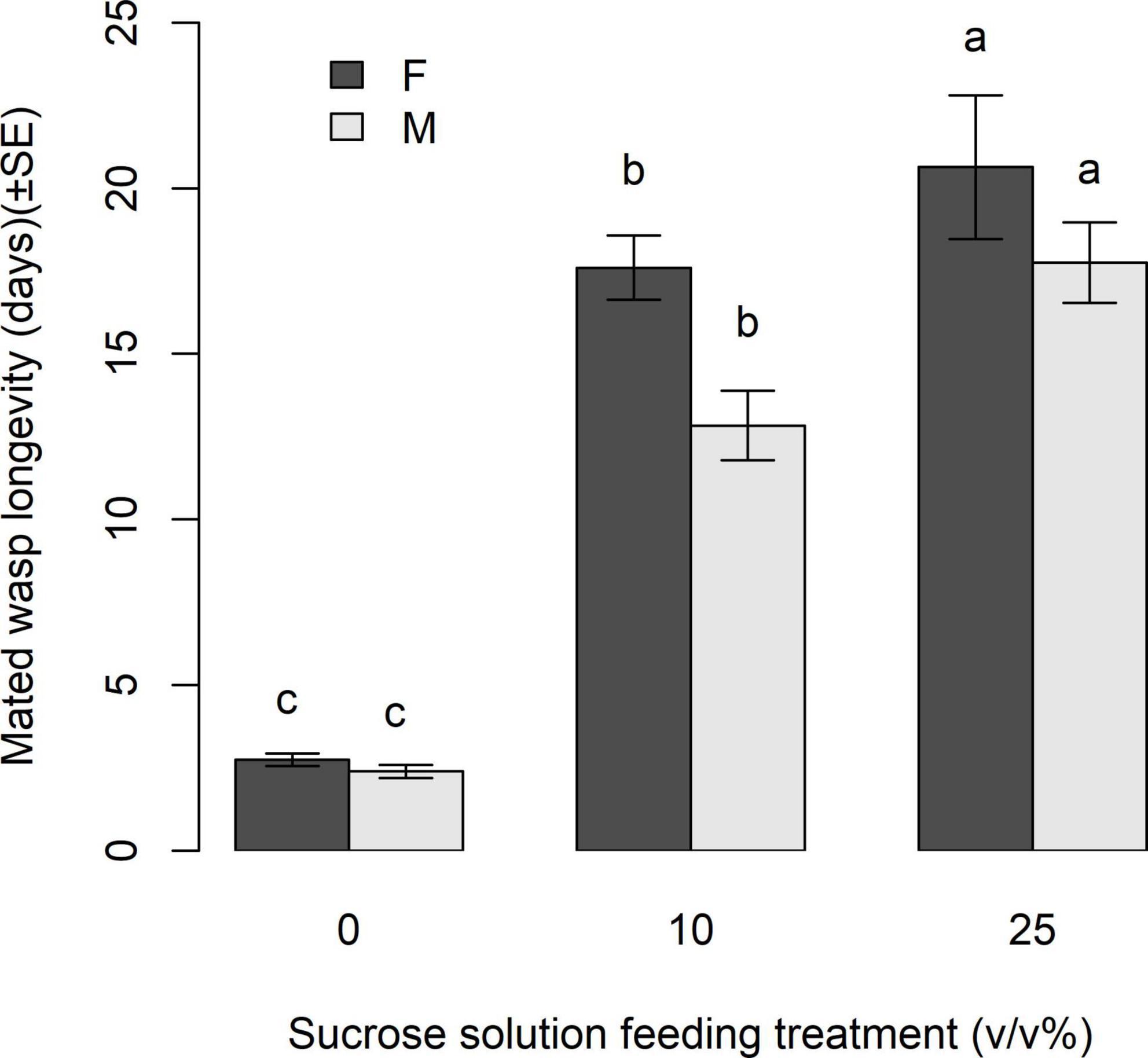
Figure 4. Mean longevity (d) of mated Apanteles polychrosidis. Each wasp (n = 30–42 per treatment) was provided with a feeding treatment of either 10 or 25% (v/v) sucrose in deionized water, or a deionized water control (0). Bars marked with different letters are significantly different (p < 0.05) [compact letter display (cld) of multiple pairwise comparisons].
The parasitism rate did not differ among female wasps fed 10% (12.5% parasitism) or 25% (13.05% parasitism) sucrose solutions in Experiment 3 (χ2 = 0.16, df = 1, p = 0.6886). The total number of larvae parasitized (10% sucrose n = 67; 25% sucrose n = 78) did not differ with wasp feeding treatment (χ2 = 1.35, df = 1, p = 0.2456), although larvae could have been parasitized more than once, as the experiment did not control for superparasitism. Females fed 25% sucrose solution lived marginally longer (25.25 ± 2.99 days) than those fed 10% sucrose solution (18.5 ± 2.26 days) (F = 3.382, df = 1, p = 0.0845).
The offspring sex ratio did not differ with maternal feeding treatment (χ2 = 1.42, df = 1, p = 0.2326). When mothers were fed the 10% sucrose solution there was an 8.5 male to female offspring ratio, whereas, those fed 25% sucrose solution produced offspring in a 1.8 male to female ratio. Interestingly, 7/11 of the female wasps fed the 10% sucrose solution and 4/11 of the females fed the 25% sucrose solution produced only male offspring, suggesting that a potential lack of mating may have resulted in the male-biased sex ratio in this study. The number of eggs counted in female offspring from mothers fed 10 and 25% sucrose solution was not significantly different (χ2 = 1.40, df = 1, p = 0.2370), but female offspring from mothers fed 25% sucrose contained, on average, more eggs than the offspring of those fed 10% sucrose. Overall offspring body size (males and females), as measured by tibia length, was marginally affected by maternal diet (χ2 = 3.2732, df = 1, p = 0.0664), with offspring from females fed the higher sucrose concentration (25%) being slightly larger than those from mothers fed the 10% sucrose solution. Female offspring had longer tibiae than males (χ2 = 45.77, df = 1, p < 0.0001). Body size of female offspring had a positive, significant effect on egg load (χ2 = 6.37, df = 1, p = 0.0116).
In Experiment 4, unmated female longevity was affected by feeding treatment (Dev = 265.42, df = 3, p < 0.0001). A. polychrosidis fed either 25% sucrose solution or buckwheat flowers, lived significantly longer than females provided with distilled water, or a buckwheat leaf. Female wasps fed nectar from buckwheat had statistically similar longevity as females fed 25% sucrose solution (χ2 = 93.79, df = 4, p = 0.2205), although flower-fed females lived on average, 4 days longer than sugar-fed wasps (Figure 5).
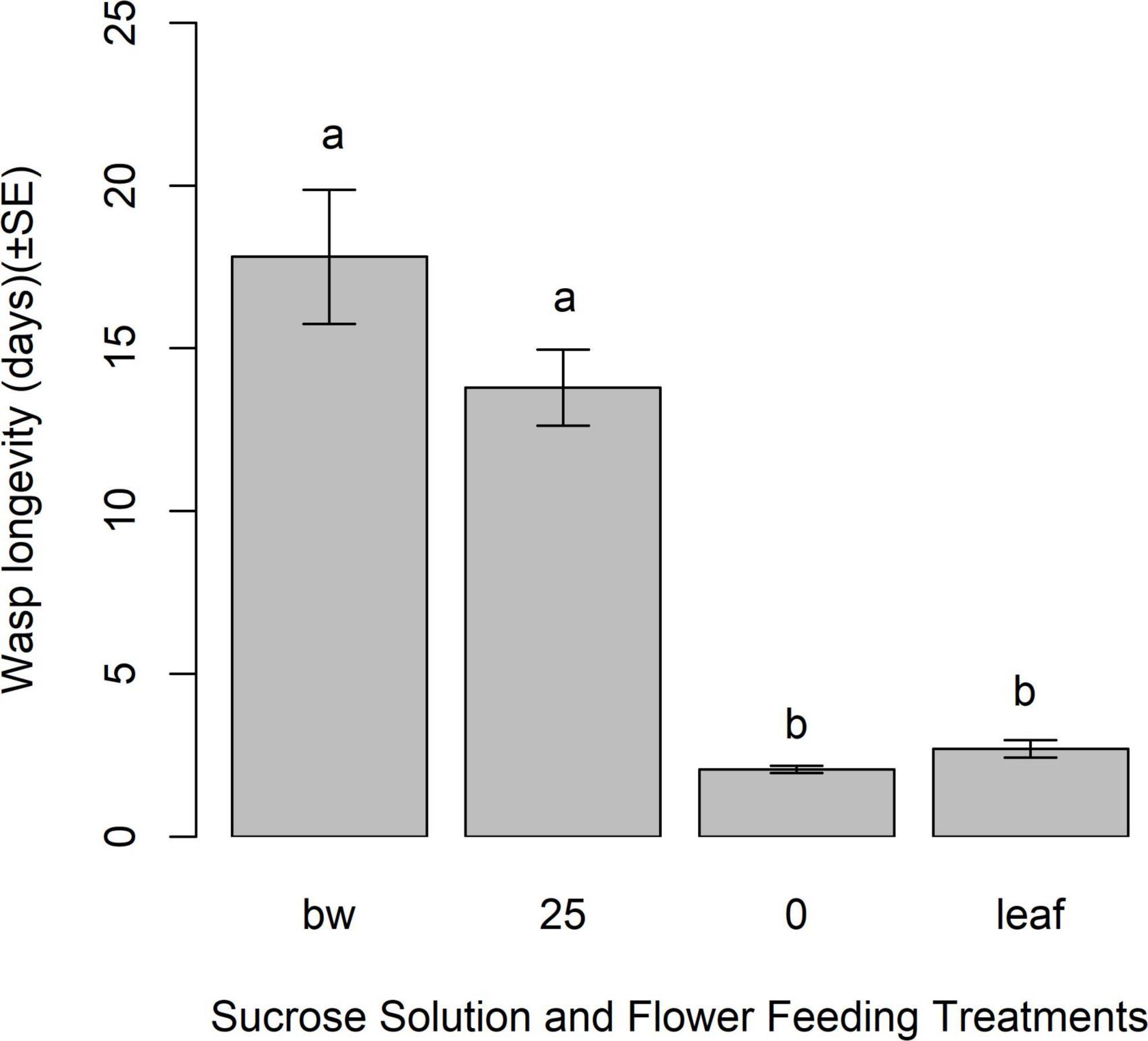
Figure 5. Mean longevity (d) of unmated Apanteles polychrosidis with buckwheat nectar or sucrose feeding. Each wasp (n = 13–41) was provided with a feeding treatment of either 25% (v/v) (25) sucrose in deionized water, a deionized water control (0), 5–20 buckwheat blossoms, or a buckwheat leaf control. Results were compared using a generalized linear model with a negative binomial error distribution. Differences between treatments, determined by compact letter display (cld) of multiple pairwise comparisons, shown with lower case letters (p < 0.05).
Discussion
Sucrose feeding at both concentrations increased longevity of both unmated and mated male and female A. polychrosidis compared to starved wasps. Female wasps live longer than males when fed on similar carbohydrate sources. In general, female parasitoids live longer than males (Kant et al., 2016; Ashraf et al., 2017) and are typically larger than males (King, 1987). In female Itoplectis naranyae Ashmead (Hymenoptera: Ichneumonidae), a parasitoid of lepidopteran pupae, large body size is correlated with increased longevity (Lui and Ueno, 2012). Longevity and oviposition opportunities increase with female body size in a bean weevil parasitoid, Lariophagus distinguendus (Forster) (Hymenoptera: Pteromalidae) (van den Assem et al., 1989). Selection for larger females may, therefore, lead to increased longevity compared to males in A. polychrosidis.
Female A. polychrosidis with and without access to mates live longer when fed the 25% sucrose solution, compared to those fed on the 10% sucrose solution. This difference is significant in females with access to mates and marginally significant for unmated females. Interestingly, unmated female wasps had marginally increased longevity when fed on buckwheat flowers compared to those fed the 25% sucrose solution. Female A. polychrosidis acquire more energy from the higher sucrose concentration, allowing them to live longer, but there is a nutritional difference between sucrose solution and nectar. C. glomerata Linnaeus (Hymenoptera: Braconidae) has greater flight capacity after nectar feeding, as compared to sugar feeding (Wanner et al., 2006). The sugar content in buckwheat nectar consists of a combination of mono, di, and trisaccharides, as well as sugar alcohols. The most concentrated sugars in buckwheat nectar are fructose, glucose, and sucrose (Nešović et al., 2021). Access to food sources post eclosion also affects male wasp longevity. Males with access to mates live longer when fed the higher concentration of sucrose but this effect of diet on longevity does not occur in unmated males. In male Nasonia vitripennis (Walker) (Hymenoptera: Pteromalidae), parasitoids of dipteran pupae, males that mate repeatedly experience decreased longevity, indicating an energetic cost of mating (Burton-Chellew et al., 2007). Resource expenditure involved with copulation and insemination, therefore, may be compensated with carbohydrate replenishment in A. polychrosidis. Access to a buckwheat leaf did not provide enough carbohydrate resources to A. polychrosidis wasps to extend wasp longevity beyond the water only control treatment. Although buckwheat leaf tissue contains high concentrations of glucose, fructose and sucrose (Nešović et al., 2021), wasps apparently could not access these nutrients from the leaf. We did not observe A. polychrosidis pollen feeding in our experiment, but pollen could also provide important nutrients, including additional carbohydrates (Nešović et al., 2021) to adult wasps.
Apanteles polychrosidis females with access to water but no carbohydrate contain a similar number of eggs as those females fed the 10% sucrose solution. This indicates that egg development may halt before wasps are 2 days post eclosion but more experiments are required to confirm this. Females with access only to water live to ∼2 days, compared to a life span of ∼16 days for females fed the 10% sucrose solution. As A. polychrosidis is a konobiont parasitoid (Quicke, 2015), egg development likely occurs mostly prior to adult eclosion (Jervis et al., 2001). As we did not dissect newly eclosed wasps, it is also possible that wasps develop some eggs during the first few days of the adult stage. This is the case for Fopius arisanus (Hymenoptera: Braconidae) that reach maximum egg load after 4 days of feeding as an adult (Wang and Messing, 2003).
Eggs can be resorbed to fuel somatic maintenance in female wasps as they age, and in Eupelmus vuilletti (Hymenoptera: Eupelmidae), one egg resorbed supports about 10% of the energy required for daily somatic maintenance (Casas et al., 2005). Egg resorption is an example of a trade-off between reproductive potential and somatic maintenance (Richard and Casas, 2009). Our findings suggest that egg resorption could occur in A. polychrosidis, as long-lived unmated females had fewer eggs than females that died early in the adult stage. Egg resorption occurs frequently in parasitoids with a synovigenic strategy, in which eggs are continuously produced throughout adulthood and are normally rich with yolk, but is less commonly reported in parasitoids with a pro-ovigenic egg development (Jervis et al., 2001). Sucrose provides energy that extends life and can fuel flight and locomotion (Casas et al., 2003; Amat et al., 2012), but it does not directly supply molecules for physical maintenance, such as amino acids and fats that are not produced by parasitoid wasps (Visser and Ellers, 2008). More study is needed to assess the number of eggs in the ovaries of A. polychrosidis at adult eclosion as compared to various time points throughout the adult life stage to determine if and when egg resorption occurs in this species. Additional studies could analyze the presence of anhydropic (yolk rich) eggs in the ovaries of A. polychrosidis and egg biochemistry, which could provide more insight on the likelihood of egg resorption as a resource allocation strategy (Jervis et al., 2001) in this species. Water availability, not surprisingly, also influences wasp success. Unmated male wasps with access to water live significantly longer than those without water. The egg count, but not the longevity, of unmated female wasps was enhanced with access to water. Lipids are metabolized for water production in water-stressed beetles and flies (Marron et al., 2003).
The parasitism rate of females fed the two sucrose treatments did not differ under laboratory conditions, but females fed 25% sucrose solution had slightly more offspring as a result of increased longevity. This was also found to be the case in honey-fed Dinarmus basalis Ashmead (Pteromalidae) and Anisopteromalus calandrae (Howard) (Pteromalidae), parasitoids of Acanthoscelides obtectus (Say) (Coleoptera: Bruchidae) (Schmale et al., 2001).
Our results illustrate that diet quality influences adult traits of wasp offspring. The carbohydrate concentration of the diet skewed offspring sex ratio, as more female offspring were produced by female wasps fed the high sucrose concentration. The low numbers of total female offspring and the high number of females that produced only male offspring, however, may have skewed the analysis. In general, “well-fed” female parasitoids produce more female offspring (Benelli et al., 2017). In the current study, there were more male than female offspring in total. Mating, however, was not directly observed in our study and 12/22 female wasps in Experiment 3 produced only male offspring, indicating that mating may not have occurred in over half of the female wasps in the parasitism study. The potential for a low mating rate and the resulting production of only male offspring in a large portion of females is likely the reason that our results differ from earlier female-biased field collections of A. polychrosidis (Wist and Evenden, 2013). As haplodiploid organisms, hymenopteran females can alter the sex ratio of their offspring through selective fertilization of eggs (Godfray, 1994). The quality of the host parasitized by the female parasitoid can determine offspring sex ratio, with more unfertilized eggs that result in male offspring being laid in low quality hosts (Ode and Hardy, 2008). The larval hosts used in the present study (C. rosaceana) were not the natal host species of the mothers that were reared from C. fraxinella. Some parasitoids prefer to lay eggs in the natal host (Jones et al., 2015). The novel host used in this bioassay may have been perceived as an inferior host to A. polychrosidis reared from C. fraxinella, though A. polychrosidis is a broad generalist that can switch hosts and must use different hosts at different times of year (Wist and Evenden, 2013). The diet of host insects influences host quality and can affect the sex allocation by female parasitoids exploiting the host. Broods of Apanteles galleriae (Wilkinson) (Hymenoptera: Braconidae), a parasitoid of the lesser wax moth, Achroia grisella (Fabricus) (Lepidoptera: Pyralidae) become significantly male-biased when larval hosts feed on an inferior diet (Uckan and Ergin, 2002). Sex ratio could also be affected by the use of artificial diet as a food source for C. rosaceana, and the temperature that insects were reared under. In the laboratory, parasitism experiments were conducted at 24°C, which is warmer the natural conditions in Edmonton during the summer. Increased temperature can increase the number of male offspring produced by parasitoids through effects on male sterilization and reduced mating (King, 1987). Low mating rates appear to have contributed to the male-biased sex ratio in this experiment.
Little work has been done on the maternal effects of nutrition on offspring fitness in parasitoids. The marginally larger offspring produced by females fed 25% sucrose solution in our study could have a fitness advantage, as larger female parasitoids have higher fecundity, and longevity (Lui and Ueno, 2012). Female offspring whose mothers fed on the high sucrose diet produced slightly more eggs than daughters of females fed the low sucrose diet. The inability of parasitoids to synthesize lipids from carbohydrate resources (Visser et al., 2010) means that the nutritional support through carbohydrate feeding by mothers does not result in additional lipid production for egg development in offspring. Instead, carbohydrate consumption supports ATP production for somatic maintenance in the mother and reduces the need for diversion of capital resources that sustain viable eggs (Wang et al., 2014). Larger male wasps seem to have an advantage in conspecific fights. This is the case for two parasitoids in the superfamily Chalcidoidea: Eurytoma sp. (Hymenoptera: Eurytomidae) (Macedo et al., 2013), and Melittobia acasta (Hymenoptera: Eulophidae) (Innocent et al., 2007). Local mate competition is not as common in Ichneumonoidea (Quicke, 2015), however, and it remains unclear if larger male A. polychrosidis have increased fitness.
It is clear that A. polychrosidis require carbohydrate sources to enhance longevity. Further, there is evidence that food quality influences wasp fitness and possibly offspring sex ratio and fitness. This work demonstrates that the provision of 25% sucrose solution or buckwheat flowers both have potential to enhance the performance of this parasitoid to aid in the control of C. fraxinella in urban plantings of green ash trees. Green ash trees infested with C. fraxinella that are located in park settings in Edmonton, would benefit from access to floral resources when A. polychrosidis adults are active in the summer. Whereas, provision of feeders containing 25% sucrose solution in green ash trees on boulevards would provide carbohydrate resources to foraging A. polychrosidis in areas that are deplete of nectar resources. The prolonged longevity of wasps with access to adequate carbohydrate provisioning would increase the likelihood of A. polychrosidis accessing the appropriate stage of C. fraxinella for parasitism (Wist and Evenden, 2013). Future studies on dispersal capacity of A. polychrosidis and availability of other hosts for the second wasp generation, will help to better amend the environment to support biological control in this system.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
SM: experimental design, laboratory experimentation, data collection and analysis, and manuscript writing. RP: laboratory experimentation, data collection and analysis, and manuscript writing. ME: project concept, experimental design, funding acquisition, supervision, and manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
Funding for this study was provided by the Alberta Crop Industry Development Fund (2015F034R). SM was supported through a TAship from the Department of Biological Sciences at the University of Alberta. In-kind support was provided by the City of Edmonton.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank members of the Evenden Lab in the Department of Biological Sciences at the University of Alberta. Ronald Batallas advised on statistical analyses. The City of Edmonton provided in-kind support, and project input from Mike Jenkins was greatly appreciated.
References
Amat, I., Bernstein, C., Besnard, S., Desouhant, E., Foray, V., and Pelosse, P. (2012). Fuelling flight in a parasitic wasp: which energetic substrate to use? Ecol. Entomol. 37:6. doi: 10.1111/j.1365-2311.2012.01388.x
Ashraf, S., Abbas, S. K., Abdin, Z. U., Anwar, M., Hussain, F., Khan, R. S. A., et al. (2017). Effect of different diet concentrations on longevity and fecundity of parasitic wasp Bracon hebetor (Say.) (Hymenoptera: Braconidae). Pakistan J. Zool. 49, 761–767. doi: 10.17582/journal.pjz/2017.49.3.761.767
Bates, D., Bolker, B., Maechler, M., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67:1. doi: 10.18637/jss.v067.i01
Belz, E., Kölliker, M., and Balmer, O. (2012). Olfactory attractiveness of flowering plants to the parasitoid Microplitis mediator: potential implications for biological control. BioControl 58:2. doi: 10.1007/s10526-012-9472-0
Benelli, G., Canale, A., Caselli, A., Desneux, N., Giulia, G., and Tena, A. (2017). The impact of adult diet on parasitoid reproductive performance. J. Pest Sci. 90, 807–823. doi: 10.1007/s10340-017-0835-2
Biddinger, D. J., Felland, C. M., and Hull, L. A. (1994). Parasitism of tufted apple bud moth (Lepidoptera: Tortricidae) in conventional insecticide and pheromone-treated Pennsylvania apple orchards. Environ. Entomol. 23, 1568–1579. doi: 10.1093/ee/23.6.1568
Burton-Chellew, M. N., Patterson, S., Shuker, D. M., Sykes, E. M., and West, S. A. (2007). The cost of mating and the relationship between body size and fitness in males of the parasitoid wasp Nasonia vitripennis. Evol. Ecol. Res. 9, 921–934.
Casas, J., Alphen, J. V., Bernstein, C., Christides, J. P., Desouhant, E., Driessen, G., et al. (2003). Energy dynamics in a parasitoid foraging in the wild. J. Anim. Ecol. 72, 691–697. doi: 10.1046/j.1365-2656.2003.00740.x
Casas, J. C., Giron, D., Mandon, N., Pincebourde, S., Poujol, R., and Vannier, F. (2005). Lifetime nutrient dynamics reveal simultaneous capital and income breeding in a parasitoid. Ecology 86, 545–554. doi: 10.1890/04-0812
Chippendale, G. M. (1978). “The functions of carbohydrates in insect life processes,” in Biochemistry of Insects, ed. M. Rockstein (New York, NY: Academic Press), 1–55. doi: 10.1016/B978-0-12-591640-0.50006-6
City of Edmonton (2022). Trees- count distribution| Edmonton- Open Data Portal. Available online at: https://data.edmonton.ca/EnvironTrees-mental-Services/TREES-COUNTDISTRIBUTION/evea-3md8 (accessed 19 February, 2022).
Cossentine, J. E., Jensen, L. B. M., Deglow, E. K., Bennett, A., Goulet, H., Huber, J., et al. (2004). The parasitoid complex affecting Choristoneura rosaceana and Pandemis limitata in organically managed apple orchards. Biol. Control 49, 359–372. doi: 10.1023/B:BICO.0000034603.56877.0b
Cossentine, J. E., Deglow, E. K., Goulet, H., and Jensen, L. B. M. (2005). Biological assessment of Macrocentrus linearis and Apanteles polychrosidis (Hymenoptera: Braconidae) as parasitoids of the obliquebanded leafroller, Choristoneura rosaceana (Lepidoptera: Tortricidae). Biocontrol. Sci. Technol. 15, 711–720. doi: 10.1080/09583150500136022
England, S., and Evans, E. W. (1997). Effects of pea aphid (Homoptera: Aphididae) honeydew on longevity and fecundity of the alfalfa weevil (Coleoptera: Curculionidae) parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ. Entomol. 26, 1437–1441. doi: 10.1093/ee/26.6.1437
Evenden, M. (2009). Biology of Caloptilia fraxinella (Lepidoptera: Gracillariidae) on ornamental green ash, Fraxinus pennsylvanica (Oleaceae). Can. Entomol. 141, 31–39. doi: 10.4039/n08-036
Fernández-Triana, J. L., and Huber, J. T. (2010). Braconid parasitoids (Hymenoptera: Braconidae) of nearctic Choristoneura species (Lepidoptera: Tortricidae), with a summary of other parasitoid families attacking Choristoneura. Can. Entomol. 142, 295–343. doi: 10.4039/n10-025
Flanders, S. E. (1950). Regulation of ovulation and egg disposal in the parasitic hymenoptera. Can. Entomol. 82, 134–140. doi: 10.4039/Ent82134-6
Godfray, H. J. C. (1994). Parasitoids: Behavioural and Evolutionary Ecology.. Princeton, NJ: Princeton University Press. doi: 10.1515/9780691207025
Hausmann, C., Dorn, S., and Wäckers, F. L. (2005). Sugar convertibility in the parasitoid Cotesia glomerata (Hymenoptera: Braconidae). Arch. Insect Biochem. Physiol. 60:4. doi: 10.1002/arch.20093
Heimpel, G. E., and Jervis, M. A. (2005). “Does floral nectar improve biological control by parasitoids?,” in Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications, eds F. L. Wäckers, P. C. J. van Rijn, and J. Bruin (Cambridge: Cambridge University Press), 67–304. doi: 10.1017/CBO9780511542220.010
Idris, A. B., and Grafius, E. (1995). Wildflowers as nectar sources for Diadegma insulare (Hymenopter: Ichneumonidae), a parasitoid of diamondback moth (Lepidoptera: Yponomeutidae). Environ. Entomol. 24, 1726–1735. doi: 10.1093/ee/24.6.1726
Innocent, T. M., Reece, S. E., Savage, J., and West, S. A. (2007). Lethal combat and sex ratio evolution in a parasitoid wasp. Behav. Ecol. 18, 709–715. doi: 10.1093/beheco/arm034
Irvin, N. A., and Hoddle, M. S. (2015). The effect of buckwheat flowers and Cahaba vetch extrafloral nectaries on fitness of the vine mealybug parasitoid Anagyrus pseudococci (Hymenotpera: Encyrtidae). Fla. Entomol. 98, 237–242. doi: 10.1653/024.098.0140
Jacob, H. S., and Evans, E. W. (2000). Influence of carbohydrate foods and mating on longevity of the parasitoid Bathyplectes curculionis (Hymenoptera: Ichneumonidae). Environ. Entomol. 29, 1088–1095. doi: 10.1603/0046-225X-29.5.1088
Jamont, M., Dubois-Pot, C., and Jaloux, B. (2014). Nectar provisioning close to host patches increases parasitoid recruitment, retention and host parasitism. Basic Appl. Ecol. 15, 151–160. doi: 10.1016/j.baae.2014.01.001
Jervis, M. A., Ellers, J., and Harvey, J. A. (2008). Resource acquisition, allocation, and utilization in parasitoid reproductive strategies. Annu. Rev. Entomol. 53, 361–385. doi: 10.1146/annurev.ento.53.103106.093433
Jervis, M. A., Harvey, J. A., Heimpel, G. E., Ferns, P. N., and Kidd, N. A. C. (2001). Life-history strategies in parasitoid wasps: a comparative analysis of ovigeny. J. Anim. Ecol. 70, 442–458. doi: 10.1046/j.1365-2656.2001.00507.x
Jones, T. S., Bilton, A. R., Mak, L., and Sait, S. M. (2015). Host switching in a generalist parasitoid: contrasting transient and transgenerational costs associated with novel and original host species. Ecol. Evol. 5, 459–465. doi: 10.1002/ece3.1333
Kant, R., Minor, M. A., and Trewick, S. (2016). Asymmetric effects of adult nutrition on reproductive success of male and female Diaeretiella rapae (Hymenoptera: Aphidiidae). Physiol. Entomol. 41, 91–95. doi: 10.1111/phen.12126
King, B. H. (1987). Sexual size dimorphism in parasitoid wasps. Biol. J. Linn. Soc. 30, 63–89. doi: 10.1111/j.1095-8312.1987.tb00290.x
Lane, T., Best, T., Carlson, J. E., Coggeshall, M. V., Davitt, J., Henry, N., et al. (2016). The green ash transcriptome and identification of genes responding to abiotic and biotic stresses. BMC Genom. 17:702. doi: 10.1186/s12864-016-3052-0
Lee, J. C., and Heimpel, G. E. (2008). Floral resources impact longevity and oviposition rate of a parasitoid in the field. J. Anim. Ecol. 77, 565–572. doi: 10.1111/j.1365-2656.2008.01355.x
Lenth, R. V. (2016). Least-squares means: the R package lsmeans. J. Stat. Softw. 69:1. doi: 10.18637/jss.v069.i01
Li, S., Barron, J., Fitzpatrick, S., O’Hara, J., Sharkey, M., and Troubridge, J. (1999). Parasitoids reared from the obliquebanded leafroller (Lepidoptera: Tortricidae) infesting raspberries. Can. Entomol. 131, 399–404. doi: 10.4039/Ent131399-3
Liu, K., Lu, Z., Chen, G., Lu, Y., Lü, Y., Zhang, J., et al. (2017). Effects of sesame nectar on longevity and fecundity of seven lepidoptera and survival of four parasitoid species commonly found in agricultural ecosystems. J. Integr. Agric. 16, 2534–2546. doi: 10.1016/S2095-3119(17)61665-4
Lui, H. Y., and Ueno, T. (2012). The importance of food and host on the fecundity and longevity of a host-feeding parasitoid wasp. J. Fac. Agric. Kyushu Univ. 57, 121–125. doi: 10.5109/22058
Macedo, M. V., Mayhew, P. J., Monteiro, R. F., and Silveira, M. P. (2013). Male-male contests for mates, sexual size dimorphism, and sex ratio in a natural population of a solitary parasitoid. Behav. Process. 100, 1–8. doi: 10.1016/j.beproc.2013.07.003
Marron, M., Gibbs, A., Kain, K., and Markow, T. (2003). Effects of starvation and desiccation on energy metabolism in desert and mesic Drosophila. J. Insect Physiol. 49, 261–270. doi: 10.1016/S0022-1910(02)00287-1
Nešović, M., Gašić, U., Tosti, T., Horvacki, N., Nedić, N., Sredojević, M., et al. (2021). Distribution of polyphenolic and sugar compounds in different buckwheat plant parts. RSC Adv. 11, 25816–25829. doi: 10.1039/D1RA04250E
Ode, P., and Hardy, C. (2008). “Parasitoid sex ratios and biological control,” in Behavioral Ecology of Insect Parasitoids: From Theoretical Approaches to Field Applications, eds E. Wajnberg, C. Bernstein, and J. van Alphen (Malden, MA: Blackwell), 261–265.
Pohl, G. R., Barr, W. B., Fownes, S. L., Saunders, C., and Wartenbe, M. D. (2004). Caloptilia fraxinella (Lepdoptera: Gracillariidae), a new pest of ash (Oleaceae: Fraxinus spp.) on the Canadian Prairies. Can. Entomol. 136, 733–736. doi: 10.4039/n03-101
Pyke, G. H., Charnov, E. L., and Pulliam, H. R. (1977). Optimal foraging: a selective review of theory and tests. Q. Rev. Biol. 52, 137–154. doi: 10.1086/409852
Quicke, D. L. J. (2015). The Braconid and Ichneumonid Parasitoid Wasps: Biology, Systematics, Evolution and Ecology. Oxford: Wiley-Blackwell. doi: 10.1002/9781118907085
Richard, R., and Casas, J. (2009). Stochasticity and controllability of nutrient sources in foraging: host-feeding and egg resorption in parasitoids. Ecol. Monogr. 79, 465–483. doi: 10.1890/08-1566.1
Riddick, E. W. (2007). Influence of honey and maternal age on egg load of lab-cultured Cotesia marginiventris. BioControl 52, 613–618. doi: 10.1007/s10526-006-9059-8
Rosenheim, J. A. (2011). Stochasticity in reproductive opportunity and the evolution of egg limitation in insects. Evolution 65, 2300–2312. doi: 10.1111/j.1558-5646.2011.01305.x
Schmale, I., Wäckers, F. L., Cardona, C., and Dorn, S. (2001). Control potential of three hymenopteran parasitoid species against the bean weevil in stored beans: the effect of adult parasitoid nutrition on longevity and progeny production. Biol. Control. 21, 134–139. doi: 10.1006/bcon.2000.0911
Seaman, A. J., Dennehy, T. J., and Nyrop, J. P. (1990). Egg and larval parasitism of the grape berry moth (Lepidoptera: Tortricidae) in three grape habitats in New York. Environ. Entomol. 19, 764–770. doi: 10.1093/ee/19.3.764
Shorey, H. H., and Hale, R. L. (1965). Mass-rearing of the larvae of nine noctuid species on a simple artificial medium. J. Econ. Entomol. 58, 522–524. doi: 10.1093/jee/58.3.522
Tena, A., Cano, D., Pekas, A., Urbaneja, A., and Wäckers, F. L. (2015). Sugar provisioning maximizes the biocontrol service of parasitoids. J. Appl. Ecol. 52, 795–804. doi: 10.1111/1365-2664.12426
Uckan, F., and Ergin, E. (2002). Effect of host diet on the immature developmental time, fecundity, sex ratio, adult longevity, and size of Apanteles galleriae (Hymenoptera: Braconidae). Environ. Entomol. 31, 168–171. doi: 10.1603/0046-225X-31.1.168
van den Assem, J., Los-den Hartogh, R. L., and van Iersel, J. J. A. (1989). Is being large more important for female than for parasitic wasps? Behaviour 108, 160–195. doi: 10.1163/156853989X00114
Vattala, H., Wratten, S., Phillips, C., and Wäckers, F. (2006). The influence of flower morphology and nectar quality on the longevity of a parasitoid biological control agent. Biol. Control 39, 179–185.
Venables, W. N., and Ripley, B. D. (2002). Modern Applied Statistics with S. New York, NY: Springer. doi: 10.1007/978-0-387-21706-2
Visser, B., and Ellers, J. (2008). Lack of lipogenesis in parasitoids: a review of physiological mechanisms and evolutionary implications. J. Insect Physiol. 54, 1315–1322. doi: 10.1016/j.jinsphys.2008.07.014
Visser, B., Lann, C. L., Blanken, F. J. D., Harvey, J. A., van Alphen, J. J. M., and Ellers, J. (2010). Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. Proc. Natl. Acad. Sci. U.S.A. 107, 8677–8682.
Visser, M. E. (1994). The importance of being large: the relationship between size and fitness in females of the parasitoid Aphaereta minuta (Hymenoptera: Braconidae). J. Anim. Ecol. 63, 963–978. doi: 10.2307/5273
Wäckers, F. (2004). Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol. Control. 29, 307–314. doi: 10.1016/j.biocontrol.2003.08.005
Wäckers, F. L. (2001). A comparison of nectar and honeydew sugars with respect to their utilization by the hymenopteran parasitoid Cotesia glomerata. J. Insect Physiol. 47, 1077–1084. doi: 10.1016/S0022-1910(01)00088-9
Wang, W., Cheng, L.-S., Liu, W.-X., Lu, S.-L., Wan, F.-H., and Zhang, Y.-B. (2014). Effects of five naturally occurring sugars on the longevity, oogenesis, and nutrient accumulation pattern in adult females of the synovigenic parasitoid Neochrysocharis formosa (Hymenoptera: Eulophidae). Neotrop. Entomol. 43, 564–573. doi: 10.1007/s13744-014-0247-4
Wang, X.-G., and Messing, R. H. (2003). Egg maturation in the parasitoid Fopius arisanus (Hymenoptera: Braconidae): do host-associated stimuli promote ovarian development? Ann. Entomol. 96, 571–578. doi: 10.1603/0013-8746(2003)096[0571:EMITPF]2.0.CO;2
Wanner, H., Gu, H., and Dorn, S. (2006). Nutritional value of floral nectar sources for flight in the parasitoid wasp, Cotesia glomerata. Physiol. Entomol. 31, 127–133. doi: 10.1111/j.1365-3032.2006.00494.x
Wist, T., and Evenden, M. (2013). Parasitoid complex and bionomics of Apanteles polychrosidis (Hymenoptera: Braconidae) on the ash leaf-cone roller (Lepidoptera: Gracillariidae). Can. Entomol. 145, 416–429. doi: 10.4039/tce.2013.26
Wist, T. J., Evenden, M. L., and Greis, R. (2015). Differential parasitism by a generalist parasitoid is mediated by volatile organic chemicals of the herbivore’s host. Arthropod Plant Interact. 9, 515–527. doi: 10.1007/s11829-015-9393-9
Zamek, A. L., Gurr, G. M., Mansfield, S., Micallef, J. L., and Reynolds, O. L. (2013). Carbohydrate diet and reproductive performance of a fruit fly parasitoid, Diachasmimorpha tryoni. J. Insect Sci. 13, 1–11. doi: 10.1673/031.013.7401
Keywords: parasitoid, carbohydrate provision, conservation biological control, horticulture, urban forest, integrated pest management, Fraxinus pennsylvanica, Fraxinus nigra
Citation: McPike SM, Pain RA and Evenden ML (2022) Provision of carbohydrate resources to support Apanteles polychrosidis, to increase parasitism of Caloptilia fraxinella in horticultural ash trees. Front. Ecol. Evol. 10:888527. doi: 10.3389/fevo.2022.888527
Received: 03 March 2022; Accepted: 04 July 2022;
Published: 22 July 2022.
Edited by:
Francisco José Cabrero-Sañudo, Complutense University of Madrid, SpainReviewed by:
Tyler Wist, Agriculture and Agri-Food Canada, CanadaEduardo Gabriel Virla, Fundación Miguel Lillo, Argentina
Copyright © 2022 McPike, Pain and Evenden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. M. McPike, U2FyYWgubWNwaWtlQGVkbW9udG9uLmNh
 S. M. McPike
S. M. McPike R. A. Pain
R. A. Pain M. L. Evenden
M. L. Evenden