
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 11 April 2022
Sec. Ecophysiology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.870081
This article is part of the Research Topic Nutritional Ecology of Wild and Managed Bees View all 8 articles
Pollen serves as an essential protein source for honey bee larvae. The nutrients in pollen greatly influence larval growth and development. Here, the survival, prepupal weight, developmental stage, pollen digestibility and midgut cells in honey bee (Apis mellifera L.) larvae were evaluated by performing in vitro and 5-ethynyl-2′-deoxyuridine (EdU) assays on larvae reared on three single pollens (Brassica napus L., Armeniaca sibirica L., and Pyrus bretschneideri Rehd.) and a pollen mixture (mixture of the three pollens in equal proportions). The results showed that the survival rate of larvae fed 10 mg of rape pollen was lowest (P < 0.05), but there were no notable differences in the survival rate among the groups receiving the other types and doses of pollen (P > 0.05). The prepupal weight of larvae fed apricot pollen was significantly lower than those of the other groups (P < 0.05). The digestibility of rape pollen and the pollen mixture were dramatically higher than those of apricot and pear pollen (P < 0.05). Pear and mixed pollen exerted negative effects on the nuclear area of midgut cells in the early larval stage (P < 0.05). In conclusion, detection of larval midgut cells using the EdU assay might be an effective method to assess the pollen nutritive value in honey bees. Compared to apricot and pear pollen, rape pollen was more beneficial in larval honey bee growth and development.
Pollen provides almost all the protein requirements of the colony and plays an important function in honey bee growth and development (Heinrich, 1979; Roulston and Cane, 2000). Bees consume a large amount of pollen during the larval stage to meet the requirements of metamorphosis (Plowright and Pendrel, 1977; Ribeiro, 1994; Pernal and Currie, 2000). Pollen quality and diversity directly affect larval honey bee development. Larvae may be ejected from the colony to compensate for a nutrient deficit and to sustain the quality of offspring under the conditions of food shortage (Tasei and Aupinel, 2008; Brodschneider and Crailsheim, 2010; Roger et al., 2017; Bortolotti et al., 2020). A shortage of pollen could also lead to a decrease in the colony population (Keller et al., 2005). To obtain adequate nutrition from pollen, foragers usually collect various types of pollen to feed larvae; pollen from different plants have different nutrient contents that have a certain influence on larval growth and development (Roulston and Cane, 2000; Tasei and Aupinel, 2008; Roger et al., 2017). High-quality pollen extends the honey bee life span. However, pollen shortage and low-quality pollen have negative effects on larval body size and cause a delay in larval development (Schmidt et al., 1987; Sutcliffe and Plowright, 1990; Tasei and Aupinel, 2008; Roger et al., 2017).
The survival rate, the prepupal weight, the development stage, and pollen digestibility have been used as indicators of larval growth and development, which are affected by the quality of pollen (Hendriksma et al., 2011b,2012; Steijven et al., 2016). Low-quality pollen, such as Helianthus annuus L., Pinus, and Zea mays L. pollen, can hinder larval development and have a negative effect on longevity (Wu et al., 2009; Nicolson and Human, 2013). Some pollen, such as Ambrosia, Uromyces (a rust spore), Typha, and Kallstroemia pollen, can decrease the life span of bees (Schmidt et al., 1987). It has been confirmed that the weights of larvae reared on Castanea, Papaver, and Rubus pollen were heavier than those of larvae reared on Helianthus and Cistus pollen. Moreover, the body size of larvae fed Brassica pollen was larger than those fed Picris, Hedera, Amaranthus, Solanum, Helianthus, and Graminaceae pollen (Tasei and Aupinel, 2008). Some pollen, such as Taraxacum officinale Weber ex Wigg pollen, is not suitable for brood rearing due to a lack of amino acids (Herbert and Bickley, 1970). Larvae can digest a variety of pollen, which benefits growth and development (Génissel et al., 2002; Tasei and Aupinel, 2008; Watrous et al., 2019). It was found that larvae fed monofloral pollen for a prolonged period of time tended to develop more slowly, have a lower larval weight, and have a smaller body size than those fed polyfloral pollen (Génissel et al., 2002; Tasei and Aupinel, 2008; Di Pasquale et al., 2013; Han et al., 2014; Watrous et al., 2019). In contrast, larvae fed polyfloral pollen were less susceptible to Aspergillus fumigatus infection (Foley et al., 2012). The digestibility of pollen by larvae varies largely among different floral species. It was demonstrated that the digestibility of Melastomataceae pollen was easier than that of Fabaceae pollen (Ueira-Vieira et al., 2013). Therefore, investigating the effects of different types of pollen on the growth and development of larvae will help improve the health of colonies, benefiting honey bee management.
The midgut is an important organ in the honey bee, as it is the main tissue exposed to toxins, food and water (Terra, 1990; Hakim et al., 2010). The midgut epithelium includes three types of cells: digestive cells, endocrine cells and regenerative cells. The main function of digestive cells is the synthesis of digestive enzymes and the absorption of nutrients. Endocrine cells produce hormones. Regenerative cells are located in the regenerative nests scattered among the digestive cells and differentiate into digestive and endocrine cells (Martins et al., 2006). Digestion and absorption of the diet also affect development of midgut (Wang et al., 2014). Ingested substance, such as colchicine, methoxyfenozide, tetracycline, and a combination of coumaphos and tau-fluvalinate, significantly reduce the regenerative cell proliferation rates in the midguts of honey bees (Forkpah et al., 2014). The growth and proliferation of cells in the midgut are measured using proliferation assays. The bromodeoxyuridine (BrdU) proliferation assay was used to observe the proliferation of midgut cells in honey bees and stingless bees, and the results showed that the digestive cells were prismatic and increased in size and the regenerative cells increased in number with the development of larvae. The replacement of larval epithelial cells during metamorphosis can be observed using the BrdU assay (Martins et al., 2006; Li, 2011). In contrast to the usual BrdU assay, the 5-ethynyl-2′-deoxyuridine (EdU) assay can be performed easily and quickly to detect DNA synthesis in cells and tissues without affecting the cellular structure or protein antigenicity (Salic and Mitchison, 2008). The EdU assay has been reported to be able to label and detect DNA synthesis and proliferating cells successfully. EdU has been successfully used to label and trace rat mesenchymal stromal cells, and also to evaluate the proliferation of chicken T lymphocyte and embryo tissue (Lin et al., 2009; Warren et al., 2009; Ye et al., 2019). In addition, EdU has been used to label enteroendocrine cells in zebrafish intestines. In insects, EdU was applied to label cells in adult Drosophila testis (Zhang et al., 2017). However, the EdU assay has never been used to visualize the midgut cells of honey bee larvae.
With the large-scale and regional development of facility agriculture, many different monoculture plants have been grown in large areas. In particular, when the plant blooming period is relatively concentrated and the number of plants is large, the honey bee may selectively collect more pollen from some plants. This causes other plants to be inadequately pollinated. Brassica napus, Armeniaca sibirica, and Pyrus bretschneideri plants are monocularized plants grown in a large area in China that are also collected by the honey bee colony during the period of spring multiplication. Therefore, investigating the effects of different types of monofloral pollen and polyfloral pollen on larval growth and development may help us understand the effects of large area monoculture plants on the colony, which is beneficial to improve plant pollination and colony management.
In this study, the effect of three pollen types (Brassica napus, Armeniaca sibirica, and Pyrus bretschneideri pollen) on the survival rate, prepupal weight, developmental stage, and pollen digestibility in honey bee (A. mellifera) larvae was determined by an in vitro larval rearing assay. The nuclear areas of midgut cells in the larval midgut were detected by the EdU assay. The following questions were investigated: (1) Does the type and dosage of pollen affect the growth and development of larvae? (2) Can the EdU assay be used to reflect pollen nutritive value? (3) Does polyfloral pollen have more advantages than monofloral pollen in larval development?
This study was conducted from June to August 2021 at the Institute of Apicultural Research, Chinese Academy of Agricultural Sciences (IAR-CAAS). The in vitro larvae rearing method was adopted (Aupinel et al., 2005, 2007; Hendriksma et al., 2011a; Tavares et al., 2015; Steijven et al., 2016). To obtain first instar larvae, empty combs were placed inside a hive for oviposition by the queen. We harvested the eggs within 24 h and enclosed the queen in a queen cage for the following 72 h. First instar larvae were collected and transferred to sterile 48-well culture plates. Each larva received 20 μL of diet on the first day, D4. The culture plate with larvae was maintained in an incubator [35°C and 95% relative humidity (RH)].
Larvae were fed with Diet I and Diet II according to the feeding protocol of Aupinel et al. (2005) and Steijven et al. (2016). The artificial diets consisted of 50% royal jelly and 50% sugar solution. The sugar solution of Diet I contained 12% glucose, 12% fructose, and 2% yeast extract; the sugar solution of Diet II contained 18% glucose, 18% fructose, and 4% yeast extract. On the first day (D4), larvae received 20 μL of Diet I; on D5, larvae were not fed; and from D6 to D9, larvae received an increasing amount of Diet II (20, 30, 40, and 50 μL, respectively) supplemented with pollen.
Different doses (1, 2, 5, and 10 mg) of pollen were supplemented starting in the third instar stage, and potential dose-dependent effects were observed (Malone et al., 2002; Hendriksma et al., 2011b; Steijven et al., 2016). Any residues were carefully removed daily by a vacuum pump, and the fresh diet was added to the cells. The survival of larvae during the experiment was recorded daily and calculated at the end of the experiment, on D11. Dead larvae were identified by discoloration and a lack of food consumption. The fresh weight of the prepupae was measured to the nearest 0.001 g after defecation on D11. The number of larvae reaching the prepupal stage was recorded, and whether the larvae reached prepupal stage was estimated according to the size and shape of the larvae and defecation.
Three single pollen types (rape, apricot, and pear pollen) and a pollen mixture (mixture of the three pollens in equal proportions) were fed to the larvae. Pollen was collected from Western honey bee colonies with pollen traps in 2021. Pear pollen was collected in a large-scale planting orchard in Yuncheng, Shanxi, China. Apricot pollen was collected in Beijing during the blooming season. Rape pollen was purchased from a beekeeper. Pollens were diluted and examined the purity under microscope with 10 replicates and more than 100 pollen grains per time. The purity of pollen was confirmed to more than 99%. All pollen samples were freshly stored at −80°C until use. Pollen was added to Diet II to ensure that the larvae in the four pollen groups were fed 0, 1, 2, 5, and 10 mg of pollen from D6 to D9. Each larva was provided a fresh portion of pollen every day. A 0-mg pollen (no pollen, NP) diet was used as the control. We conducted the rearing experiment with 48 larvae in each pollen and each dose.
A subsample of twelve larvae per experimental treatment was collected 12 h after the last feeding on D9 according to the Steijven’s methodology (Steijven et al., 2016). Larvae were euthanized by freezing them at −20°C, and careful dissection was performed in 1 × PBS (10 mM NaH2PO4/Na2HPO4 and 175 mM NaCl, pH 7.4, Solarbio, Beijing, China). The midgut of each larva was dissected entirely and suspended in 200 μL of 0.5 M glucose solution and stored at −20°C. The content of each sample was stained with lactophenol cotton blue (LPCB) and gently pipetted up and down 20 times to rupture the gut tissue and create a suspension with a uniform distribution before the pollen count. A Neubauer hemocytometer (Hemocytometer, Millipore) was used to quantify the digestion rate. Using the first 100 pollen grains, we scored digestion according to the remaining pollen grains in the exine; 0–10% remaining pollen grain was scored as “fully digested,” 10–90% remaining pollen grain was scored as “partly digested,” and >90% remaining pollen grain was scored as “undigested” (Babendreier et al., 2004). Two samples per larval gut were collected, and a total of 200 pollen grains were used to score pollen digestion. Aborted and non-aborted pollen grains were also evaluated before they were digested by the larvae (Brice et al., 1989). Non-aborted pollen grains were considered digested, and the percentage was determined using the following formula:
Larvae were divided into pollen groups as described in sections in vitro larvae rearing and pollen EdU reagent (Click-iT™ EdU Cell Proliferation Kit, Invitrogen, C10337, 10 μM) was added into the Diet II and used to feed larvae on D6, D7, D8, and D9. We selected the larvae fed the highest pollen dose to observe the most obvious effect of pollen on the midgut cells. Ten samples were taken from each day after pollen consumption (D7 represents D6–D7, D8 represents D7–D8, D9 represents D8–D9, and D10 represents D9–D10) were collected through the end of the prepupal stage. Larvae were fixed in 4% paraformaldehyde for 24 h. The larval midguts were dissected, and pollen was isolated under a stereomicroscope. The midguts were washed twice with 3% bovine serum albumin (BSA), incubated in 0.5% Triton X-100 at room temperature for 20 min, and then washed once with 3% BSA and PBS. The midgut samples were stained using an EdU Cell Proliferation kit and incubated with a freshly prepared Click-iT reaction cocktail containing azide-conjugated Alexa Fluor 488 for 30 min at room temperature in the dark. Samples were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; 1 μg/ml; Sigma) and mounted on blank slides with an anti-fluorescence quenching agent. The nucleus embedded with EdU emits green fluorescence, and the DAPI-positive nuclei emit blue fluorescence. Images were acquired using inverted confocal fluorescence microscopy (Leica TCS SP8).
Five fields in the anterior midgut region were randomly photographed. The number of EdU-positive cells in each randomly photographed region was counted. The large nuclear areas of EdU-positive cells, excluding the small nuclear areas of regenerative cells, were measured. Regenerative cells were detected by clustering in the regenerative nests and the significantly smaller size than other midgut cells. Only the area of the nucleus with clear edges was selected for statistical analysis. The area of the nucleus was measured by ImageJ software (Version 1.8.0).
All statistical analyses were performed using R project software (Version 3.6.2). Dynamic survival analysis was not applicable when all individuals in a group survived; in such cases, chi-square analysis was used (Hendriksma et al., 2011b). Prepupal weight and pollen digestion were analyzed with linear mixed-effects models using the package nlme. The developmental stages of the larvae were analyzed with chi-square analysis. The number and nuclear areas of EdU-positive cells were assessed for normality with the Kolmogorov–Smirnov test and subjected to analysis of variance (ANOVA) for normally distributed data that were log- or square root-transformed when necessary. Data are presented as means ± SD.
We conducted a complete pre-experiment and five and more than five pollen feeding experiments on larvae in the laboratory. In this experiment, we raised a total of 960 larvae. There was no significant difference among the larval survival rate in the four pollen groups (P > 0.05). Among the dose groups, the larval survival rate in the rape pollen subgroup of the 10-mg dose group was significantly lower than those of the other pollen groups (χ2 = 10.365, df = 3, P < 0.05, P = 0.029). The survival rates of larvae in the pear, apricot and mixed pollen groups were not affected by the dosage (Figure 1).
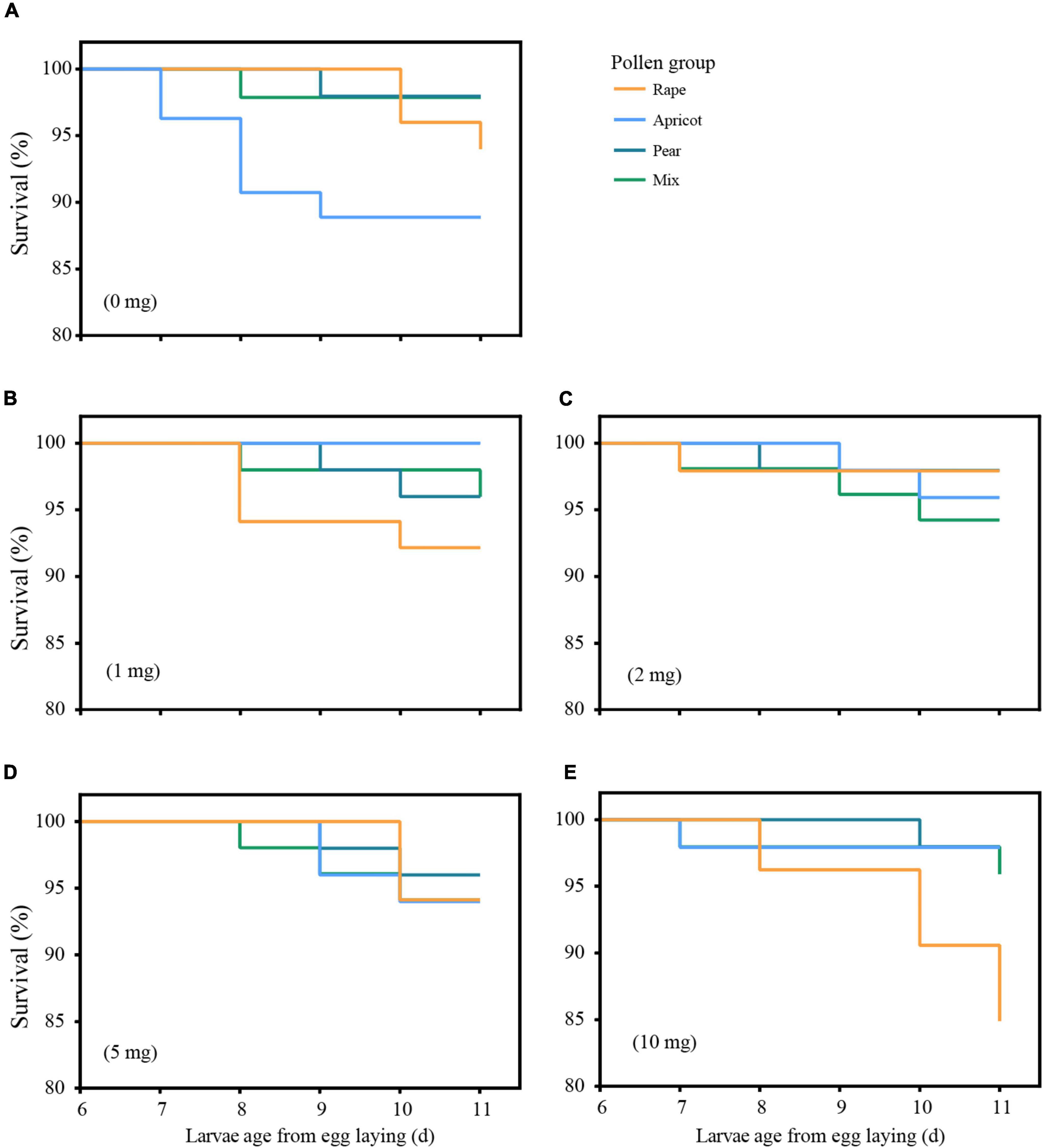
Figure 1. Survival curves of honey bee larvae fed four types of pollen at five doses. Pollen supplementation was administered from D6 until D9. Mortality was recorded until D11 (prepupal stage). (A) represents the NP group; (B) represents the 1-mg dose group; (C) represents the 2-mg dose group; (D) represents the 5-mg dose group; (E) represents the 10-mg dose group.
There was no interaction between pollen type and dose (F = 2.125, df = 9, P = 0.091). The prepupal weight in the apricot pollen group was significantly lower than those in the other pollen groups at 1, 2, and 5 mg (P < 0.05). The weights of the larvae fed a 10-mg dose of pollen were significantly lower than those in the NP group (P < 0.05). With the increase in dosage, the prepupal weight of larvae decreased significantly (F = 60.561, df = 4, P < 0.01). The prepupal weight of larvae in the NP group was significantly higher than those fed 1, 2, 5, and 10 mg of pollen (P < 0.01); the prepupal weight of those fed a 1-mg dose was significantly higher than the prepupal weights of those fed 5- and 10-mg doses (P < 0.01), and the prepupal weights of those fed 2- and 5-mg doses were significantly higher than those fed a 10-mg dose (P < 0.01) (Figure 2A).
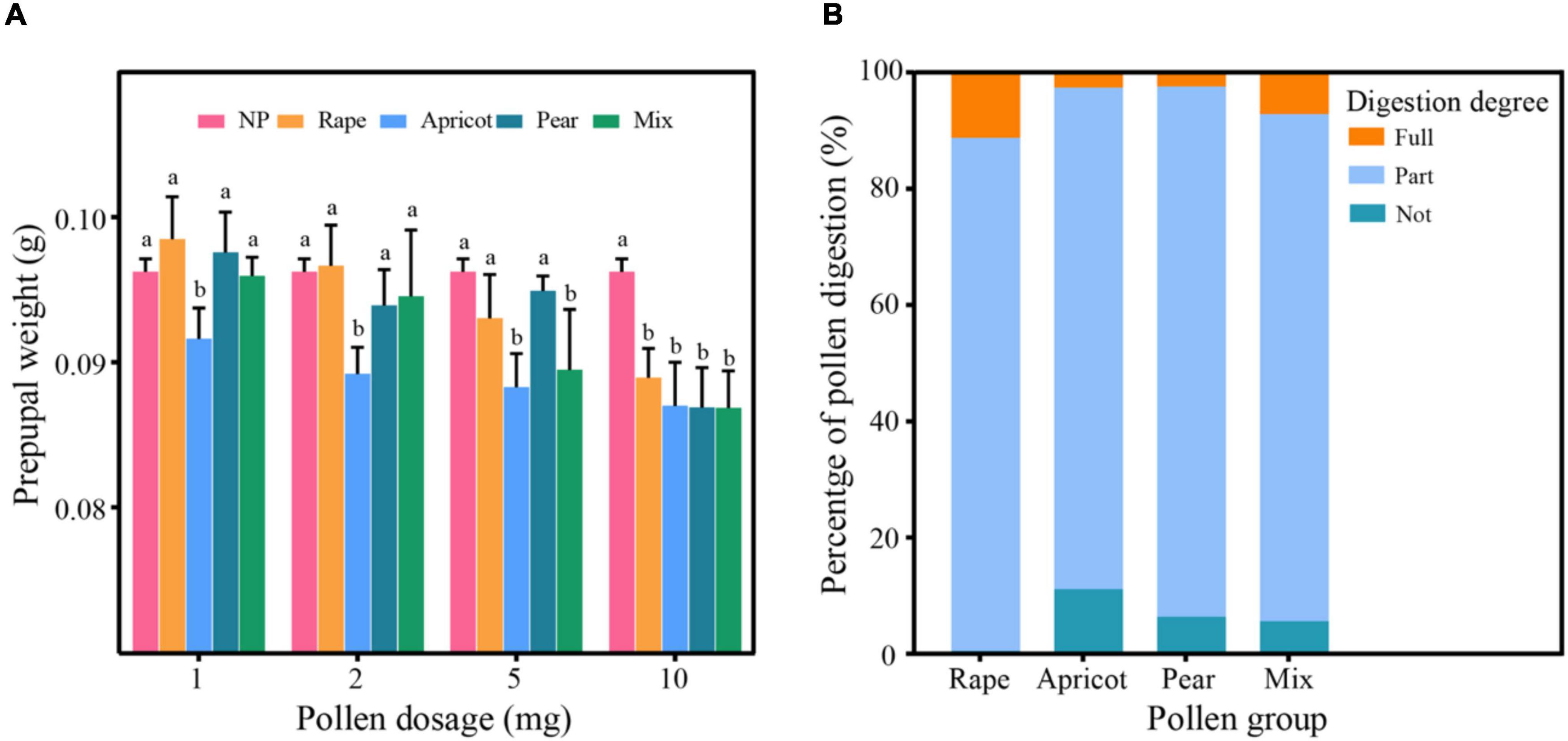
Figure 2. (A) Prepupal weight (g) of larvae in different pollen groups. Identical letters indicate no significant difference (P > 0.05); different lowercase letters indicate a significant difference (P < 0.05). (B) Digestion rates of different types of pollen. “Fully” represents fully digested pollen grains; “Partly” represents partly digested pollen grains; “Not” represents undigested pollen grains.
There was no significant difference in the developmental stage of larvae at D11 among the different pollen groups (χ2 = 2.126, df = 4, P > 0.05, P = 0.713), but there was a significant difference among the different dose groups (χ2 = 11.520, df = 4, P < 0.05, P = 0.021). The percentages of larvae reaching the prepupal stage in the 1-, 2-, and 5-mg dose groups were significantly higher than that in the 10-mg dose group (χ2 = 3.910, df = 1, P < 0.05, P = 0.048; χ2 = 5.517, df = 1, P < 0.05, P = 0.019; χ2 = 5.517, df = 1, P < 0.05, P = 0.019). In the NP group, 94.97–95.61% of larvae reached the prepupal stage. In the 1-mg dose group, 95.53–100% of larvae reached the prepupal stage. In the 2-mg dose group, 95.56–100% of larvae reached the prepupal stage. In the 5-mg dose group, 95.56–100% of larvae reached the prepupal stage. In the 10-mg dose group, 92.5–93.62% of larvae reached the prepupal stage.
The percentages of “fully digested,” “partly digested,” and “undigested” pollen grains compared to the reference pollen grains were significantly different among the different groups (Table 1 and Figure 2B). Moreover, there was an interaction between pollen type and dose (F = 9.094, df = 9, P < 0.01). The percentages of “fully digested” pollen grains in the rape pollen and mixed pollen groups were significantly higher than those in the apricot and pear pollen groups (F = 60.658, df = 3, P < 0.01). With increasing dose, there was no difference in larval digestion (F = 2.529, df = 3, P = 0.730). According to the pollen digestion formula, the percentages of each type of pollen digested by larvae were as follows: rape 11.30%, apricot 1.12%, pear 0.59%, and mixed pollen 5.27%.
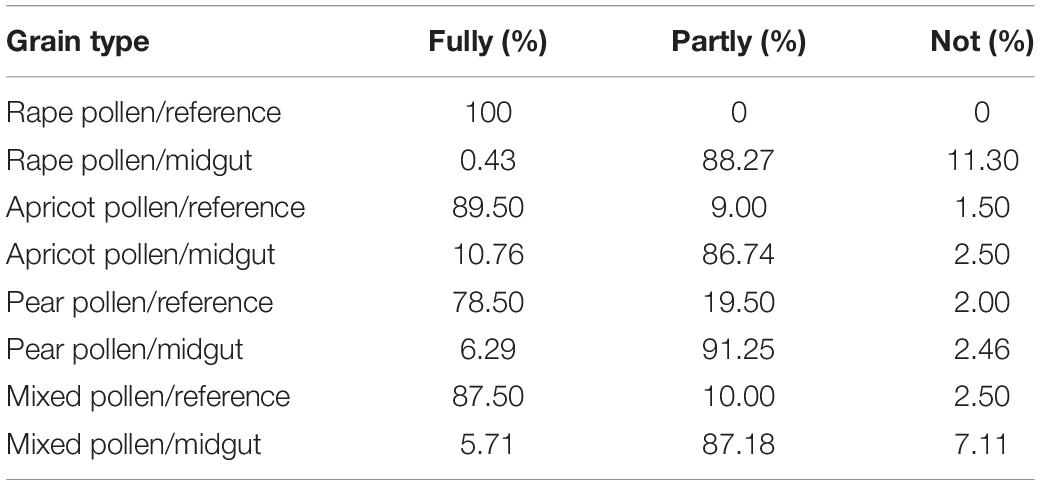
Table 1. Digestion percentage of pollen in the midgut of larvae fed different pollen types and doses.
With increasing larval age, the number of EdU-positive cells gradually decreased and the mean nuclear area of EdU-positive cells gradually increased in each group (Figure 3). There was an effect of the interaction between pollen type and developmental stage (F = 3.927, df = 12, P < 0.01) on cell proliferation of the midgut. The average number of EdU-positive cells in the midgut of larvae fed the different pollen types was significantly different at the D7 stage (F = 5.568, df = 4, P < 0.05). The average number of EdU-positive cells in per microscope field of the rape pollen group was dramatically less than those in the other pollen groups at the D7 stage (NP: 36.63 ± 6.99; rape: 29.45 ± 5.75; apricot: 35.75 ± 6.24; pear: 45.80 ± 5.38; mixed: 35.5 ± 4.03) (P < 0.05). No obvious difference in the number of EdU-positive cells in per microscope field was observed between each group at the D8 stage (NP: 23.86 ± 2.10; rape: 22.10 ± 3.18; apricot: 21.75 ± 1.30; pear: 25.50 ± 2.50; mixed: 21.00 ± 2.83), D9 stage (NP: 14.56 ± 3.43; rape: 14.45 ± 3.08; apricot: 15.42 ± 5.41; pear: 16.73 ± 2.33; mixed: 15.10 ± 2.66), and D10 stage (NP: 7.13 ± 4.05; rape: 7.00 ± 3.00; apricot: 6.86 ± 4.81; pear: 7.29 ± 3.90; mixed: 7.14 ± 2.91) (P > 0.05). The mean nuclear area of EdU-positive cells in the rape pollen group was significantly larger than those in the other pollen groups at the D7 stage (NP: 990.52 ± 12.98 μm2; rape: 1268.19 ± 48.06 μm2; apricot: 1011.33 ± 55.99 μm2; pear: 867.20 ± 55.37 μm2; mixed: 1078.57 ± 36.71 μm2) (P < 0.05). The mean nuclear areas of EdU-positive cells in the pear and mixed pollen groups were significantly smaller than those in the other pollen groups at the D9 stage (NP: 2598.64 ± 107.56 μm2; rape: 2485.25 ± 62.31 μm2; apricot: 2529.50 ± 120.96 μm2; pear: 1592.75 ± 177.77 μm2; mixed: 1759.89 ± 117.41 μm2) (P < 0.01) (Figure 4). Additionally, EdU-positive regenerative cells were obvious observed in the midgut of larvae from the rape pollen group at the D10 stage (Figure 5). Regenerative cells were clustered in the regenerative nests and significantly smaller than other midgut cells (Figure 5).
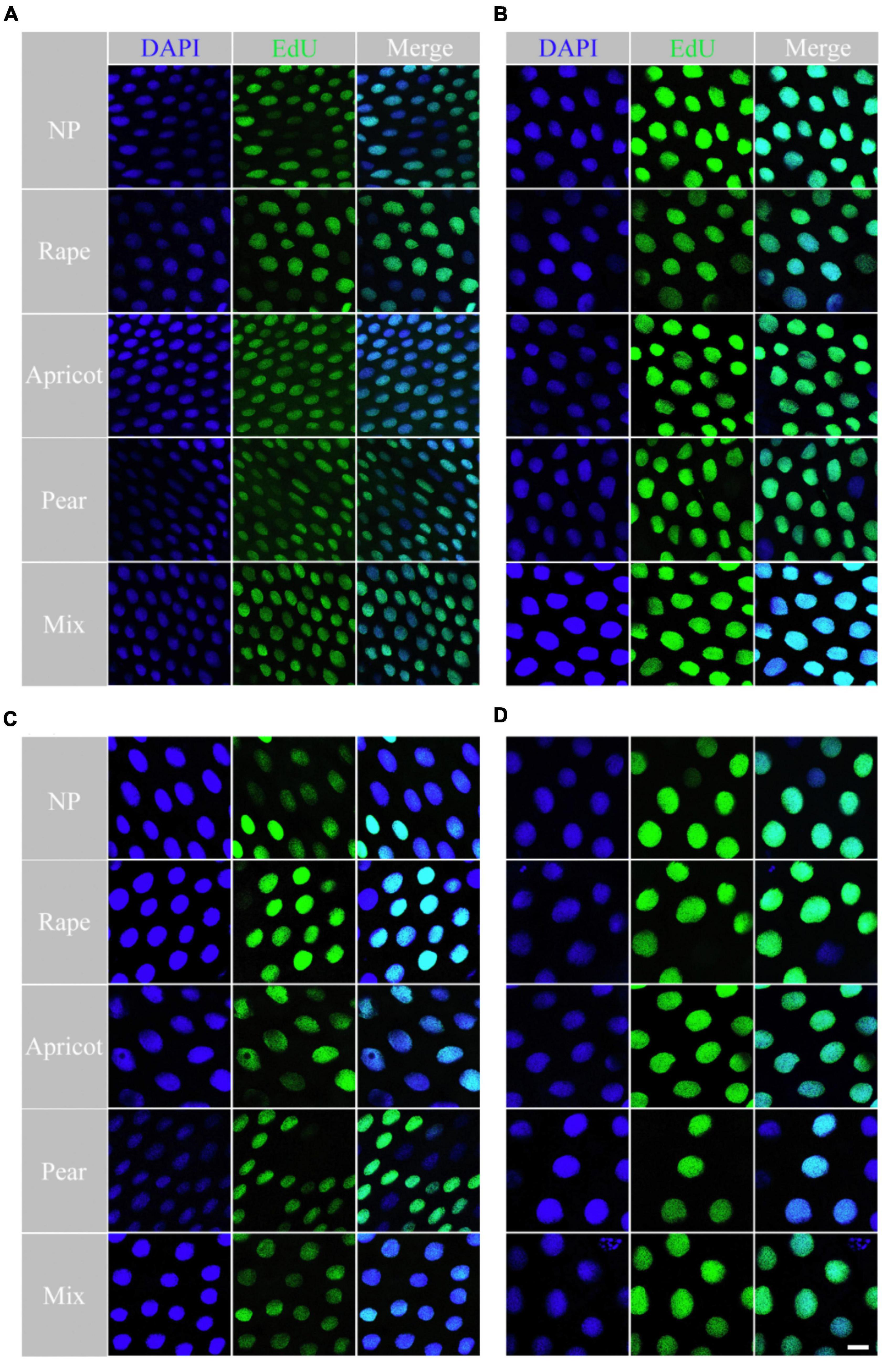
Figure 3. The nuclei difference of larval midgut cell was determined using the EdU assay. Cell nuclei labeled with EdU and stained with Alexa-Fluorescent 488 (green fluorescence) and DAPI (blue fluorescence) among the rape, apricot, pear, mixed pollen and NP groups. (A) represents midgut nuclei labeled on D6; (B) represents midgut nuclei labeled on D7; (C) represents midgut nuclei labeled on D8; represents midgut nuclei labeled on D9.
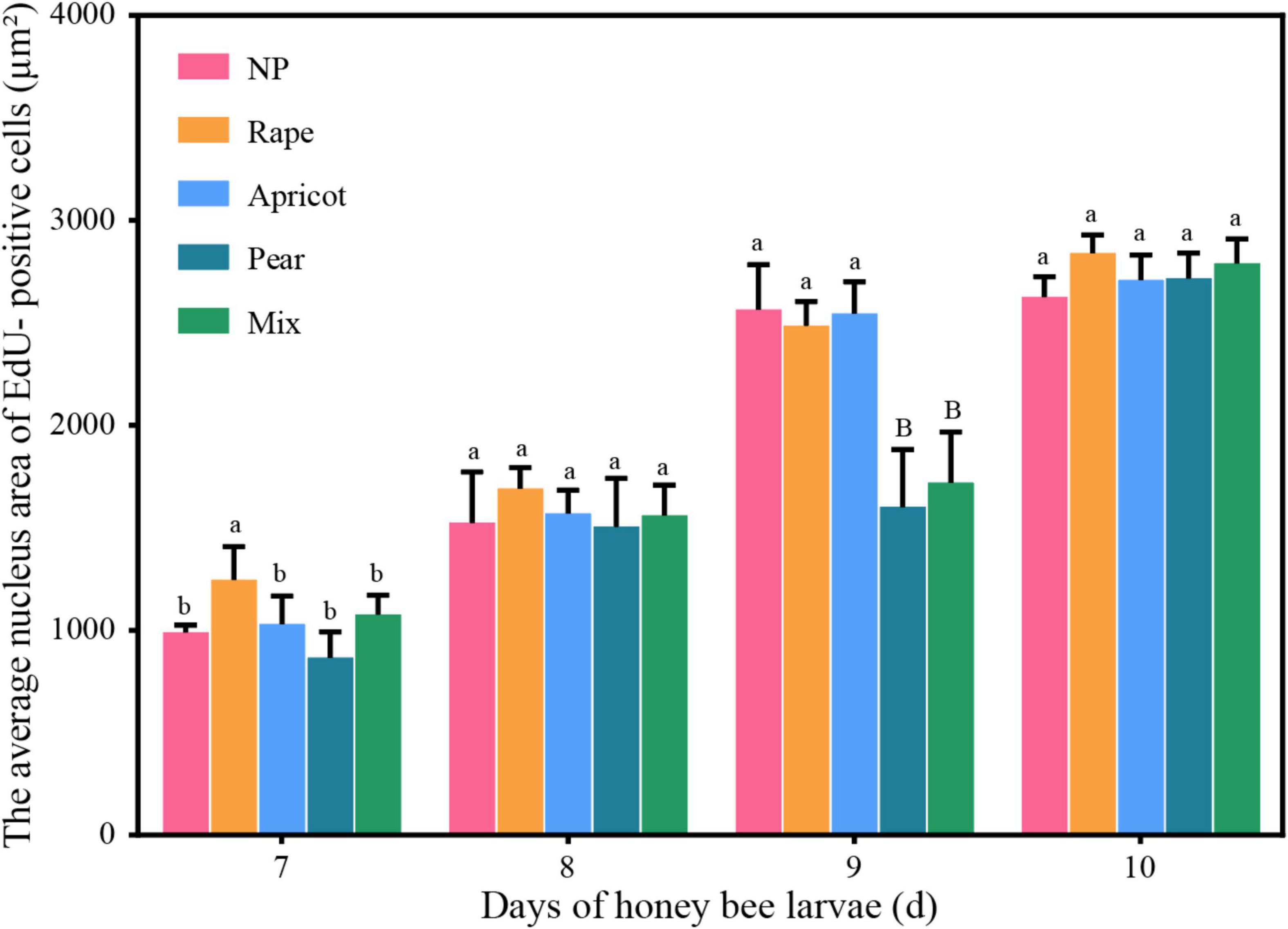
Figure 4. The mean nuclear areas of the midgut stained by fluorescence was compared among the different pollen groups. Identical letters indicate no significant difference (P > 0.05); different lowercase letters indicate a significant difference (P < 0.05); different capital letters indicate a highly significant difference (P < 0.01).
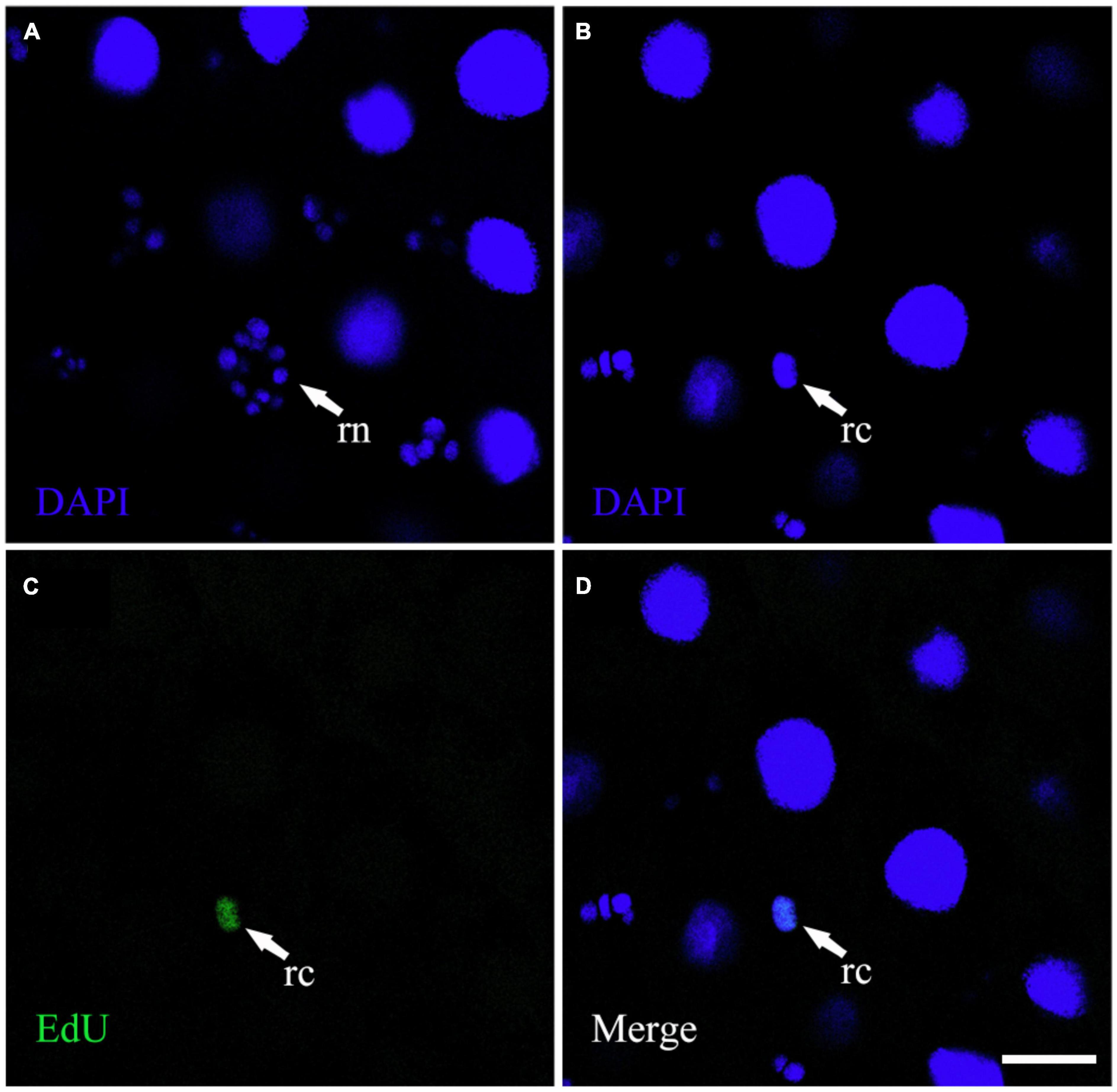
Figure 5. The EdU-positive regenerative cells were observed in the larval midgut. (A) Represents the image of the DAPI channel. (B) Represents the image of the DAPI channel at the same location as (A) on different horizontal planes. (C) Represents the image of the EdU channel on the same horizontal plane as (B). (D) Represents the merged image of the DAPI and EdU channels. “rn” represents a regenerative nest; “rc” represents regenerative cells. Scale bars, 25 μm.
Pollen, as the essential protein source of honey bees, influences the growth and health of colonies. In honey bees, larvae consume large amounts of pollen before metamorphosis. Therefore, the nutrients in pollen will have a substantially larger effect on growth and development in the larval stage than in other stages. The nutrients in pollen vary greatly among plant species (Roulston et al., 2000). Our study showed that three types of spring plant pollen significantly affected larval survival, the prepupal weight, pollen digestibility, and the number and nuclear area of EdU-positive cells in the midgut. Moreover, the pollen dose affected the speed of larval development. Among the test pollens, rape pollen had the greatest advantage in larval development, whereas apricot pollen had detrimental effects on the prepupal weight. The nuclear area of EdU-positive cells in the midgut of larvae fed pear pollen was smaller than those in the midgut of larvae fed the other pollen. This study improves our understanding of the effects of these three types of pollen on larval growth and development, which will be helpful in guiding the breeding of colonies.
Rape pollen is beneficial in increasing the total weights of pupae and newly emerged workers and extending the life span of honey bees (Gai et al., 2015; Gao et al., 2019). Our study confirmed that rape pollen promoted the growth and development of larvae, especially the prepupal weight and the nutrition absorption in the midgut cells. However, the highest dose of rape pollen was unsuitable for the survival of larvae. Even though the highest dose of rape pollen (10 mg) was associated with a lower larval survival rate (approximately 85%), the survival rate was higher than that previously associated with corn pollen (approximately 20–50%) (Steijven et al., 2016). It was demonstrated that the phagostimulant effects of linoleic acid and linolenic acid in lipids in pollen stimulated the larvae to consume more rape pollen (Singh et al., 1999). Bees that are forced to consume excessive concentrations of protein often have decreased longevity (Pirk et al., 2010; Vaudo et al., 2016). This may be the reason for the lower survival rate in the high-dose rape pollen group.
With increasing pollen amounts, the prepupal weight decreased (Steijven et al., 2016). It was also observed that with increasing pollen dose, the prepupal weight of larvae decreased. Compared to apricot and pear pollen, rape pollen had the smallest effect on the prepupal weight of larvae. The prepupal weight in the apricot pollen group was the lightest compared to those in the other pollen groups. This may be caused by the low pollen digestibility observed in our study. The low digestion rate of apricot and pear pollen may be caused by pollenkitt or chemicals in pollen, which influence digestion efficiency and nutrient assimilation, as found in Asteraceae pollen (Human et al., 2007). The pollen size and pollen wall characteristics were also documented to be related to pollen digestibility in honey bees (Roulston and Cane, 2000; Roulston et al., 2000). Moreover, according to the literature, a certain concentration of the fungicide Pristine® reduces the longevity of colonies and increases their consumption of pollen in field, but it does not affect the survival or reduce the consumption of pollen in the laboratory (Fisher et al., 2021). The opposite results obtained from the field and laboratory, suggest that, it is important to detect the effects of different pollens on larval growth and development in colonies, which may produce different results.
It was observed that the nuclear area of the midgut cells varied largely at different locations in honey bee larvae. This phenomenon was also found in the midgut of the bumble bee Bombus morio (Gonçalves et al., 2014). Furthermore, the size of midgut cells was significantly changeable in different larval stage. The nuclei number of EdU-positive cells in one field was observed to decrease gradually with increasing larval age. This decrease was due to the gradual enlargement of midgut cells. Interestingly, there was no evidence of mitosis observed in our results. EdU incorporation occurs only in replicating cells, and newly synthesized DNA is labeled and exhibits green fluorescence (Lin et al., 2009). Therefore, the large nucleus of EdU-positive cells is suspected to be attributed to endopolyploidy, which is the process of nuclear DNA amplification in the absence of typical mitosis (Edgar and Orr-Weaver, 2001; Lee et al., 2009; Rangel et al., 2015). No mitosis was observed in the midgut cells of B. morio, supporting the hypothesis the adult Hymenoptera midgut cells do not proliferate (Gonçalves et al., 2014). However, the literature has only reported that regenerative cells reproduce mitotically, and these cells proliferate rapidly before each molt differentiated into larval midgut cells in the bees’ larval stage (De Priester, 1971; Baldwin and Hakim, 1991; Hakim et al., 2010). The increases in the number and size of digestive cells in the midgut of Melipona quadrifasciata anthidioides were due to differentiation of regenerative cells, supporting the hypothesis that mitosis only replaces regenerative cells in bees’ larvae (Serrão and Da Cruz-Landim, 2000; Martins et al., 2006). Furthermore, endopolyploidy is commonly observed in the digestive cells of insects and associated with the intense protein synthesis, and the polyploidy of the midgut cells is presumed to be important in the rapid post-feeding synthesis of digestive proteinases (Billingsley, 1989; Gonçalves et al., 2014). Thus, midgut cells in larvae of the rape pollen group exhibit larger nuclei, which are beneficial for the absorption and storage of nutrients in the midgut (Lee et al., 2009).
Pollen nutrient deficiency can significantly affect honey bee midgut development in the larval developmental stage. This is reflected by the thinning of the midgut wall (Wang et al., 2014). Our EdU assay showed that the three types of pollen significantly altered the number and size of EdU-positive cells nucleus in the midgut at different larval stages. The nuclear area of EdU-positive cells in the rape pollen group was significantly larger than those in the other pollen groups at the D7 stage. Based on this result, the more sensitive characteristics of young larvae make the differences in protein synthesis of midgut cells more obvious (Yao et al., 2008). Therefore, the D7 stage better reflects the effect of pollen on the nuclear area of midgut cells in larvae. There was no significant difference in the nuclear area of EdU-positive cells at the D10 stage. The reason may be that larvae consumed a sufficient amount of pollen to gain weight rapidly within 90 h after hatching (Gong and Yang, 2013).
The nutritional value of pollen in bees is also affected by digestion. The digestibility of the three types of pollen was significantly different. Rape pollen was demonstrated to have greater digestibility than apricot and pear pollen. It has been reported to be digested easily by honey bees (Wang et al., 2020). Therefore, rape pollen was beneficial to larval development. Larvae fed polyfloral pollen were heavier and lived longer than those fed monofloral pollen (Tasei and Aupinel, 2008; Piou et al., 2018). However, our results showed that in larvae, the digestibility of rape pollen alone was greater than that of the mixture of rape, apricot and pear pollen. Monofloral pollen may have a more suitable nutritive value than polyfloral pollen (Di Pasquale et al., 2013; Moerman et al., 2017). Therefore, the nutritional value of the pollen mixture was poorer than that of rape pollen alone in our experiment.
In conclusion, (1) the three types and five dosages of pollen exerted significantly different effects on the growth and development of honey bee larvae. A high dose of pollen will affect the survival rate, prepupal weight, developmental stage. Compared with apricot and pear pollen, rape pollen was more advantageous for larval development. However, a high dose of rape pollen will affect the survival rate of larvae. (2) The EdU assay suggested that the number and nuclear area of midgut cells might be used as indicators of the pollen nutritive value for larvae. (3) Polyfloral pollen may not have more advantages than monofloral pollen in promoting larval development. The mixture of rape, apricot and pear pollen was not the best pollen for larvae, according to the results of larval survival, prepupal weight, pollen digestibility, the number and nuclear area of EdU-positive cells in the midgut. Our results confirmed that pollen had different effects on larval development. However, further experiments are needed to determine which substance in pollen affects honey bee larval development.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
CP, YL, JW, and JH: conceptualization. CP, YG, GD, and KD: methodology. CP, YG, GD, and JH: software. CP, YG, and KD: data curation. CP, YG, and GD: writing – original draft. CP, YG, and JH: visualization. JH and JW: supervision and funding. CP, YG, GD, KD, YL, JW, and JH: writing – reviewing and editing. All authors contributed to the article and approved the submitted version.
This project was financially supported in part by the National Natural Science Foundation of China (31870211), China Agriculture Research System of MOF and MARA (CARS-44), and Agricultural Science and Technology Innovation Program (CAAS-ASTIP-2022-IAR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Qi Zhang, Hongcai Chang, Wenting Su, Jun Lan, and Xiao Hu for their help with sampling and experiments.
Aupinel, P., Fortini, D., Dufour, H., Tasei, J. N., Michaud, B., Odoux, J. F., et al. (2005). Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 58, 107–111.
Aupinel, P., Fortini, D., Michaud, B., Marolleau, F., Tasei, J. N., and Odoux, J. F. (2007). Toxicity of dimethoate and fenoxycarb to honey bee brood (Apis mellifera), using a new in vitro standardized feeding method. Pest Manage. Sci. 63, 1090–1094. doi: 10.1002/ps.1446
Babendreier, D., Kalberer, N., Romeis, J., Fluri, P., Bigler, F., Kalberer, N., et al. (2004). Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants. Apidologie 35, 293–300. doi: 10.1051/apido:2004016
Baldwin, K. M., and Hakim, R. S. (1991). Growth and differentiation of the larval midgut epithelium during molting in the moth. Manduca sexta. Tissue Cell 23, 411–422. doi: 10.1016/0040-8166(91)90058-2
Billingsley, P. F. (1989). Endpolyploidy and digestion in the midgut of Rhodnius prolixus Stål (Hemiptera: Reduviidae). Ann. Trop. Med. Parasit. 83, 93. doi: 10.1080/00034983.1989.11812316
Bortolotti, L., Pošćić, F., and Bogo, G. (2020). Comparison of different pollen substitutes for the feeding of laboratory reared bumble bee (Bombus terrestris) colonies. J. Apic. Sci. 64, 91–104. doi: 10.2478/jas-2020-0013
Brice, A. T., Dahl, K. H., and Grau, C. R. (1989). Pollen digestibility by hummingbirds and psittacines. Condor 91, 681–688. doi: 10.2307/1368120
Brodschneider, R., and Crailsheim, K. (2010). Nutrition and health in honey bees. Apidologie 41, 278–294. doi: 10.1051/apido/2010012
De Priester, W. (1971). Ultrastructure of the midgut epithelial cells in the fly Calliphora erythrocephala. J. Ultrastruct. Res. 36, 783–805. doi: 10.1016/s0022-5320(71)90031-1
Di Pasquale, G., Salignon, M., Le Conte, Y., Belzunces, L. P., Decourtye, A., Kretzschmar, A., et al. (2013). Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One 8:e72016. doi: 10.1371/journal.pone.0072016
Edgar, B. A., and Orr-Weaver, T. L. (2001). Endoreplication cell cycles: more for less. Cell 105, 297–306. doi: 10.1016/S0092-8674(01)00334-8
Fisher, A., Cogley, T., Ozturk, C., Degrandi-Hoffman, G., Smith, B. H., Kaftanoglu, O., et al. (2021). The active ingredients of a mitotoxic fungicide negatively affect pollen consumption and worker survival in laboratory-reared honey bees (Apis mellifera). Ecotoxicol. Environ. Saf. 226, 112841. doi: 10.1016/j.ecoenv.2021.112841
Foley, K., Fazio, G., Jensen, A. B., and Hughes, W. O. H. (2012). Nutritional limitation and resistance to opportunistic Aspergillus parasites in honey bee larvae. J. Invertebr. Pathol. 111, 68–73. doi: 10.1016/j.jip.2012.06.006
Forkpah, C., Dixon, L. R., Fahrbach, S. E., and Rueppell, O. (2014). Xenobiotic effects on intestinal stem cell proliferation in adult honey bee (Apis mellifera L.) workers. PLoS One 9:e91180. doi: 10.1371/journal.pone.0091180
Gai, Q. B., Zhou, Z. Y., Zhang, H., Huang, J. X., and An, J. D. (2015). Optimizing supplementary pollen mixtures for bumblebee Bombus terrestris colonies based on colony reproductive variables. Chin. J. Appl. Entomol. 52, 333–342. doi: 10.7679/j.issn.2095?1353.2015.036
Gao, L. J., Liu, J. L., Luo, W. H., Yang, J. L., Cao, L., Ji, C. H., et al. (2019). Effects of different bee pollens on colony reproduction and worker development of Apis mellifera ligustica. Chin. J. Anim. Nutr. 31, 4630–4636. doi: 10.3969/j.issn.1006-267x.2019.10.027
Génissel, A., Aupinel, P., Bressac, C., Tasei, J. N., and Chevrier, C. (2002). Influence of pollen origin on performance of Bombus terrestris micro-colonies. Entomol. Exp. Appl. 104, 329–336. doi: 10.1023/A:1021279220995
Gonçalves, W. G., Fernandes, K. M., Barcellos, M. S., Silva, F. P., Magalhães-Junior, M. J., Zanuncio, J. C., et al. (2014). Ultrastructure and Immunofluorescence of the midgut of Bombus morio (Hymenoptera: Apidae: Bombini). C. R. Biol. 337, 365–372. doi: 10.1016/j.crvi.2014.04.002
Gong, F. Q., and Yang, M. X. (2013). The relationship between instars and weight growing during the larval development of honeybee queens (Apis cerana). Apic. China 64, 14–16. doi: 10.3969/j.issn.0412-4367.2013.04.003
Hakim, R. S., Baldwin, K., and Smagghe, G. (2010). Regulation of midgut growth, development, and metamorphosis. Annu. Rev. Entomol. 55, 593–608. doi: 10.1146/annurev-ento-112408-085450
Han, L. W., Zeng, Z. H., Zheng, Y. K., Yang, G., and You, M. S. (2014). Effects of pollen on colony development of Bombus lucorum L. J. Environ. Entomol. 36, 343–347. doi: 10.3969/j.issn.1674-0858.2014.03.9
Hendriksma, H. P., Härtel, S., Babendreier, D., Ohe, W., and Steffan-Dewenter, I. (2012). Effects of multiple Bt proteins and GNA lectin on in vitro reared honey bee larvae. Apidologie 43, 549–560. doi: 10.1007/s13592-012-0123-3
Hendriksma, H. P., Härtel, S., and Steffan-Dewenter, I. (2011a). Honey bee risk assessment: new approaches for in vitro larvae rearing and data analyses. Methods Ecol. Evol. 2, 509–517. doi: 10.1111/j.2041-210X.2011.00099.x
Hendriksma, H. P., Härtel, S., Steffan-Dewenter, I., and Smagghe, G. (2011b). Testing pollen of single and stacked insect-resistant Bt-maize on in vitro reared honey bee larvae. PLoS One 6:e28174. doi: 10.1371/journal.pone.0028174
Herbert, E. W., and Bickley, W. E. (1970). The brood-rearing capability of caged honey bees fed dandelion and mixed pollen diets. J. Econ. Entomol. 63, 215–218. doi: 10.1093/jee/63.1.215
Human, H., Nicolson, S. W., Strauss, K., Pirk, C. W., and Dietemann, V. (2007). Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata). J. Insect Physiol. 53, 649–655. doi: 10.1016/j.jinsphys.2007.04.002
Keller, I., Fluri, P., and Imdorf, A. (2005). Pollen nutrition and colony development in honey bees-Part II. Bee World 86, 27–34. doi: 10.1080/0005772X.2005.11099650
Lee, H. O., Davidson, J. M., and Duronio, R. J. (2009). Endoreplication: polyploidy with purpose. Genes Dev. 23, 2461–2477. doi: 10.1101/gad.1829209
Li, Z. Y. (2011). Replacement of midgut epithelium in Apis mellifera ligustica (Hymenoptera: Apidae) during postembryonic development. Acta Entomol. Sin. 54, 1127–1132.
Lin, G. T., Huang, Y. C., Shindel, A. W., Banie, L., Wang, G. F., Lue, T. F., et al. (2009). Labeling and tracking of mesenchymal stromal cells with EdU. Cytotherapy 11, 864–873. doi: 10.3109/14653240903180084
Malone, L. A., Tregidga, E. L., Todd, J. H., Burgess, E. P. J., Philip, B. A., Markwick, N. P., et al. (2002). Effects of ingestion of a biotin-binding protein on adult and larval honey bees. Apidologie 33, 447–458. doi: 10.1051/apido:2002030
Martins, G. F., Neves, C. A., Campos, L. A. O., and Serrão, J. E. (2006). The regenerative cells during the metamorphosis in the midgut of bees. Micron 37, 161–168. doi: 10.1016/j.micron.2005.07.003
Moerman, R., Vanderplanck, M., Fournier, D., Jacquemart, A. L., and Michez, D. (2017). Pollen nutrients better explain bumblebee colony development than pollen diversity. Insect. Conserv. Diver. 10, 171–179. doi: 10.1111/icad.12213
Nicolson, S. W., and Human, H. (2013). Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 44, 144–152. doi: 10.1007/s13592-012-0166-5
Pernal, S. F., and Currie, R. W. (2000). Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31, 387–409. doi: 10.1051/apido:2000130
Piou, V., Tabart, J., Hemptinne, J.-L., and Vétillard, A. (2018). Effect of pollen extract supplementation on the varroatosis tolerance of honey bee (Apis mellifera) larvae reared in vitro. Exp. Appl. Acarol. 74, 25–41. doi: 10.1007/s10493-017-0198-7
Pirk, C. W. W., Boodhoo, C., Human, H., and Nicolson, S. W. (2010). The importance of protein type and protein to carbohydrate ratio for survival and ovarian activation of caged honeybees (Apis mellifera scutellata). Apidologie 41, 62–72. doi: 10.1051/apido/2009055
Plowright, R. C., and Pendrel, B. A. (1977). Larval growth in bumble bees (Hymenoptera: Apidae). Can. Entomol. 109, 967–973. doi: 10.4039/Ent109967-7
Rangel, J., Strauss, K., Seedorf, K., Hjelmen, C. E., and Johnston, J. S. (2015). Endopolyploidy Changes with Age-Related Polyethism in the Honey Bee, Apis mellifera. PLoS One 10:e0122208. doi: 10.1371/journal.pone.0122208
Ribeiro, M. F. (1994). Growth in bumble bee larvae: relation between development time, mass, and amount of pollen ingested. Can. J. Zool. 72, 1978–1985. doi: 10.1139/z94-270
Roger, N., Michez, D., Wattiez, R., Sheridan, C., and Vanderplanck, M. (2017). Diet effects on bumblebee health. J. Insect Physiol. 96, 128–133. doi: 10.1016/j.jinsphys.2016.11.002
Roulston, T. H., and Cane, J. H. (2000). Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 222, 187–209. doi: 10.1007/BF00984102
Roulston, T. H., Cane, J. H., and Buchmann, S. L. (2000). What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny. Ecol. Monogr. 70, 617–643. doi: 10.2307/2657188
Salic, A., and Mitchison, T. J. (2008). A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. USA 105, 2415–2420. doi: 10.1073/pnas.0712168105
Schmidt, O. J., Thoenes, C. S., and Levin, D. M. (1987). Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. J. Econ. Entomol. 80, 176–183. doi: 10.1093/aesa/80.2.176
Serrão, J. E., and Da Cruz-Landim, C. (2000). Ultrastructure of the midgut epithelium of Meliponinae larvae with different developmental stages and diets. J. Apic. Res. 39, 9–17. doi: 10.1080/00218839.2000.11101016
Singh, S., Saini, K., and Jain, K. L. (1999). Quantitative comparison of lipids in some pollens and their phagostimulatory effects in honey bees. J. Apic. Res. 38, 87–92. doi: 10.1080/00218839.1999.11100999
Steijven, K., Steffan-Dewenter, I., and Härtel, S. (2016). Testing dose-dependent effects of stacked Bt maize pollen on in vitro-reared honey bee larvae. Apidologie 47, 216–226. doi: 10.1007/s13592-015-0392-8
Sutcliffe, G. H., and Plowright, R. C. (1990). The effects of pollen availability on development time in the bumble bee Bombus terricola K. (Hymenoptera: Apidae). Can. J. Zool. 68, 1120–1123. doi: 10.1139/z90-166
Tasei, J. N., and Aupinel, P. (2008). Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39, 397–409. doi: 10.1051/apido:2008017
Tavares, D. A., Roat, T. C., Carvalho, S. M., Silva-Zacarin, E. C. M., and Malaspina, O. (2015). In vitro effects of thiamethoxam on larvae of Africanized honey bee Apis mellifera (Hymenoptera: Apidae). Chemosphere 135, 370–378. doi: 10.1016/j.chemosphere.2015.04.090
Terra, W. R. (1990). Evolution of digestive systems of insects. Annu. Rev. Entomol. 35, 181–200. doi: 10.1146/annurev.en.35.010190.001145
Ueira-Vieira, C., Nunes-Silva, C. G., Absy, M. L., Da Costa Pinto, M. D. F. F., Kerr, W. E., Bonetti, A. M., et al. (2013). Pollen diversity and pollen ingestion in an Amazonian stingless bee, Melipona seminigra (Hymenoptera, Apidae). J. Apic. Res. 52, 173–178. doi: 10.3896/IBRA.1.52.3.09
Vaudo, A. D., Stabler, D., Patch, H. M., Tooker, J. F., Grozinger, C. M., and Wright, G. A. (2016). Bumble bees regulate their intake of the essential protein and lipid pollen macronutrients. J. Exp. Biol. 219, 3962–3970. doi: 10.1242/jeb.140772
Wang, Y., Ma, L. T., Hang, X. B., Yang, W. R., Liu, F., and Xu, B. H. (2014). Digestion of protein of two pollen types in China by the honeybee (Apis mellifera L.). Apidologie 45, 590–600. doi: 10.1007/s13592-014-0278-1
Wang, Y., Ma, L. T., Liu, Z. G., Wang, H. F., and Xu, B. H. (2020). Processing time of three kinds of bee pollen in the digestive tract of Apis mellifera L. Chin. J. Appl. Entomol. 57, 1111–1119. doi: 10.7679/j.issn.2095-1353.2020.112
Warren, M., Puskarczyk, K., and Chapman, S. C. (2009). Chick embryo proliferation studies using EdU labeling. Dev. Dynamics 238, 944–949. doi: 10.1002/dvdy.21895
Watrous, K. M., Duennes, M. A., and Woodard, S. H. (2019). Pollen diet composition impacts early nesting success in queen bumble bees Bombus impatiens Cresson (Hymenoptera: Apidae). Environ. Entomol. 48, 711–717. doi: 10.1093/ee/nvz043
Wu, J., Huang, J. X., An, J. D., and Hu, F. L. (2009). Effects of different diets on worker colony development of the bumblebee Bombus hypocrita Pérez (Hymenoptera: Apidae). Acta Entomol. Sin. 52, 1115–1121. doi: 10.16380/j.kcxb.2009.10.017
Yao, H., Jiang, C., Ye, G., Hu, C., and Peng, Y. (2008). Toxicological assessment of pollen from different Bt rice Lines on Bombyx mori (Lepidoptera: Bombyxidae). Environ. Entomol. 37, 825–837. 825:TAOPFD 2.0.CO;2 doi: 10.1603/0046-225X200837
Ye, L. H., Mueller, O., Bagwell, J., Bagnat, M., Liddle, R. A., and Rawls, J. F. (2019). High fat diet induces microbiota-dependent silencing of enteroendocrine cells. Elife 8:e48479. doi: 10.7554/eLife.48479
Keywords: honey bee, Apis mellifera, pollen, cell proliferation, midgut
Citation: Pang C, Dong K, Guo Y, Ding G, Lu Y, Guo Z, Wu J and Huang J (2022) Effects of Three Types of Pollen on the Growth and Development of Honey Bee Larvae (Hymenoptera, Apidae). Front. Ecol. Evol. 10:870081. doi: 10.3389/fevo.2022.870081
Received: 05 February 2022; Accepted: 16 March 2022;
Published: 11 April 2022.
Edited by:
Ying Wang, Shandong Agricultural University, ChinaReviewed by:
Qiang Huang, Jiangxi Agricultural University, ChinaCopyright © 2022 Pang, Dong, Guo, Ding, Lu, Guo, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Wu, YXBpc0B2aXAuc2luYS5jb20=; Jiaxing Huang, aHVhbmdqaWF4aW5nQGNhYXMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.