- 1Department of Life Sciences and Systems Biology, University of Turin, Turin, Italy
- 2Independent Researcher, Pistoia, Italy
Dung beetle functional ecology has traditionally focused on studying the relation between traits and ecosystem functions in multispecies assemblages, often ignoring the contribution of behavioral interactions and trait variability within species. Here we focus on the factors that affect dung removal at an intraspecific level in two horned dung beetle species with dimorphic males (Onthophagus taurus and Onthophagus verticicornis). By setting treatments for each species with single individuals (one female, F; one major male, M; one minor male, m) or with pairs of individuals (MF, mF, MM, mm, FF), we examined the effect on dung removal of morphological traits (head, pronotum, leg, horn), sex, and interactions between individuals. Our results showed that dung removal at an intraspecific level depended more on sex and behavioral interactions than on the underlying morphological traits, whose effects on dung removal were negligible. Single females generally removed more dung than single males, which suggests that females are more effective than males. In both species, pairs with at least one female (MF, mF, FF) showed high dung removal efficiency, but did not perform differently from the sum of single treatments (M + F, m + f, F + F). This suggests an additive effect: males and females (or two females) join their efforts when they are together. The pairs with only males (MM and mm) removed less dung than the sum of the single individuals (M + M and m + m), which indicates a mutual inhibition of males. In both species, male morphs performed similarly as they removed the same amount of dung. Despite our results are limited to two Onthophagus species, we suggest that the intraspecific functional ecology of dung beetles might be more influenced by behavioral interactions and sex rather than by morphological traits.
Introduction
In the last few decades, trait-based ecology has undergone large developments to advance our understanding of the effects of biological communities on ecosystem processes and services (Cadotte, 2017; Malaterre et al., 2019). The performance-related morphological, physiological, phenological, or behavioral features (growth, survival, and/or reproduction) of different species (Violle et al., 2007; Brousseau et al., 2018) have been used to link biodiversity with a suite of functions (Hooper et al., 2005; Gagic et al., 2015). The use of traits can help to predict species’ responses to environmental variables, to interactions with other species (Vandewalle et al., 2010; Brown et al., 2014; D’Amen et al., 2018; Burner et al., 2021) and to, ultimately, understand ecosystem functioning. Measurements of morphological traits are quite common in functional ecology because they are easy to collect (Violle et al., 2007) and are often used to better understand the processes structuring natural systems (McGill et al., 2006). However, employing an easy-to-measure proxy to describe ecological functions or other traits without experimentally testing these links can lead to incorrect inferences (Hortal et al., 2015; Malaterre et al., 2019).
Not only interspecific trait variability, but also intraspecific trait variability, are increasingly recognized as important components of diversity that drive ecosystem functioning (Lecerf and Chauvet, 2008) and functional responses to disturbances (Jung et al., 2014). Morphological and behavioral traits differ among individuals of the same species (Esperk et al., 2007; Gouws et al., 2011), and these trait variants can change the way that individuals interact with conspecifics (Pruitt and Ferrari, 2011). Interactions between individuals at an intraspecific level may influence certain activities or processes, such as reproduction, competition, or cooperation with individuals, which can significantly affect the provisioning of ecosystem functions and services (Uvarov, 2009; Fernandes et al., 2011).
In this context, dung beetles (Coleoptera: Scarabaeoidea) are considered excellent model organisms because they provide a range of ecosystem functions and services (Nichols et al., 2008), most of which derive directly or indirectly from dung removal, which is consumed or buried in the soil for feeding and breeding. By exploiting vertebrate feces, multispecies assemblages of dung beetles play an essential role in nutrient cycling through the removal, relocation and burial of mammalian dung (Nervo et al., 2014; Piccini et al., 2018; Gotcha et al., 2022), the stimulation of microbial activity through bioturbation (Slade et al., 2016), reductions in greenhouse gas emissions (Penttilä et al., 2013; Piccini et al., 2017), parasite suppression (Nichols et al., 2008), and secondary seed dispersal (Piccini et al., 2020; Almeida et al., 2021). A growing literature body shows that interspecific differences in the traits linked with dung beetle morphology and reproduction can influence dung removal and burial rates. Three main reproductive strategies are normally identified: tunnelers bury brood balls in vertical chambers below the pat; rollers transport dung ball some distance before burial in soil; dwellers lay eggs and breed their larvae inside the dung mass itself, or just at the soil-dung interface. Tunnelers play a more relevant role in dung removal compared to rollers and dwellers (Slade et al., 2007; Nervo et al., 2017; Noriega et al., 2021). Of all the morphological traits, body size has a positive effect on dung removal amounts (Nervo et al., 2014; Piccini et al., 2018). Furthermore, some other traits have been positively related to dung removal (head area and width, pronotum length and width, prothorax height and volume, and fore and hind tibiae size) and dung burial (prothorax height and volume, and protibia area) (deCastro-Arrazola et al., 2020).
Although understanding the effects of the variability of morphological traits among species is increasing, fewer studies have focused on variability within species (Griffiths et al., 2016; Raine et al., 2018). Moreover, factors other than morphological ones may significantly affect dung beetles’ ecological functions at the intraspecific level. The role of sex and social factors may, for instance, be important because several traits linked with reproductive behavior (clutch size, burrow depth and branching, parental care, etc.) depend on sex and interactions between individuals (Akamine, 2016, 2019). Male and female cooperation in nesting may lead to intraspecific dung removal and burial differences (Sowig, 1996; Hunt and Simmons, 2002). Biparental care is common in several dung beetle genera, although restricted to nest construction and food provisioning of the offsprings. In the genus Onthophagus, females dig a branched tunnel system in soil with a brood chamber at the terminal end of each branch, whereas males are active mostly on the surface by moving portions of dung to the tunnel system entrance (Sowig, 1996; Hunt and Simmons, 1998, 2000, 2002; Moczek and Emlen, 1999).
Other than differences between males and females, dung removal may also be strongly affected by male dimorphism, which leads to different behaviors and highly divergent phenotypes. Males in most Onthophagus species can be grouped as majors (large males with fully developed horns and elongated front legs) and minors (small males with only rudimentarily developed horns) (Moczek and Emlen, 1999, 2000) even if intermediate individuals also exist (Laini et al. unpublished data). Guarding major males block tunnels with their horns and by bracing their legs against tunnel walls (Moczek and Emlen, 2000). Forelegs, which provide digging power, and horns are important in these contests (Tomkins et al., 2005). Major males tend to engage in pushing contests, and expel rivals from the tunnels that they defend, while hornless males have been found to generally avoid physical contact with other males because they rely on alternative reproductive tactics to gain access to females (side tunnels and sneak copulations) (Emlen, 1997; Moczek and Emlen, 2000). Only major males usually help during the production of brood masses (Hunt and Simmons, 2002).
We focused on intraspecific functional ecology and investigated the extent to which morphological traits, sex and behavioral interactions affect dung removal in two horned-dung beetle species with dimorphic males in relation to horn expression: O. taurus (Schreber, 1759) and O. (Paleonthophagus) verticicornis (Laicharting, 1781). By creating treatments for each species with single individuals (one female, F; one major male, M; one minor male, m) or with pairs of individuals (MF, mF, MM, mm, FF), we examined the effect on dung removal of (1) morphological traits, (2) sex, and (3) interactions between individuals. Based on a previous study (see deCastro-Arrazola et al. (2020)), we expected greater dung removal efficiency for those treatments characterized by larger morphological traits (especially body size) or with a wider trait variability because of the complementarity of different traits (functional complementarity hypothesis; see Gagic et al. (2015)). We also assumed that females, single or pairs would be more efficient in dung removal than males given the biological need of laying eggs. By assuming biparental cooperation, we expected different sex pairs (MF and mF) to better perform in dung removal terms than same sex pairs (FF, MM, and mm).
Materials and Methods
Species Collection and Laboratory Experiment
Adult individuals from two different dung beetle species (O. taurus and O. verticicornis) were collected in cattle dung in May–June 2021. The O. taurus individuals were collected in the pastures of the Istituto per le Piante da Legno e l’Ambiente (IPLA) in Turin (Piedmont region, Italy) (45°05′18.5″N, 7°44′28.5″E, 300 m a.s.l), while those of O. verticicornis were collected in the pastures of Cascina di Spedaletto, Cantagallo, the Prato and Pistoia province (Tuscany region, Italy) (43°59′52.95″N; 11°01′08.52″E, 900 m a.s.l.). Collected beetles were transferred to terraria, separated per species, sex, and male morphology, and kept for at least 2 weeks until the experiments began. Since mating attempts between individuals were observed, we assumed the females and males were sexually mature. During this period, individuals of both species were acclimatized by exposing them to the same conditions of temperature (20–21°C). Overall, 177 terraria (a plastic glass of 7 cm diameter and 12 cm height filled with 10 cm of soil) were set in June–July 2021. In each terrarium, 80 g of fresh cattle dung were placed on top of a squared plastic mesh of 1 cm × 1 cm holes to weigh dung more easily at the end of the experiment with a minimum of soil residue. Eight different treatment types with single dung beetle individuals or pairs were arranged to assess the effect of morphological traits, sex, and interactions between individuals on dung removal: single female (F); single major male (M); single minor male (m); different sex pair with a major male and female (MF); different sex pair with a minor male and female (mF); pair with two females (FF); pair with two major males (MM); pair with two minor males (mm). The single individual treatments and different sex pair treatments were tested for 15 days at the end of June, while pairs of the same sex were tested for 15 days at the beginning of July. We arranged 7–11 replicates per treatment based on the availability of individuals. As the replicates where dung beetles remained inactive or died were discarded, our final data set comprised 150 terraria with dung beetles, 10 terraria with only dung as the controls of the single and different sex pairs (C1), and 10 terraria as the controls of the pairs of the same sex (C2) (see Table 1).
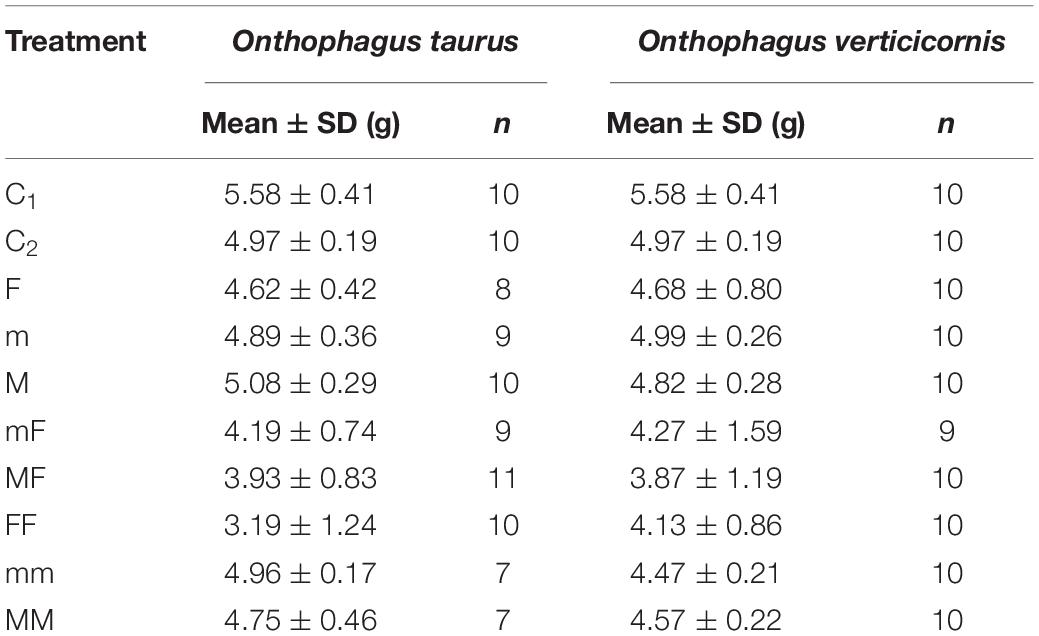
Table 1. Mean dry weight of residual dung (g) and number of replicates for each species and treatment (C1, control at time 1; C2, control at time 2; F, one female; m, one minor male; M, one major male; mF, one female and one minor male; MF, one female and one major male; FF, two females; mm, two minor males; MM, two major males).
Fifteen days after the experiment began, dung beetles were retrieved for the morphological analysis, and the remaining dung in both treatments and controls was dried at 60°C for 2 days (VWR INCU-Line 250R Premium) and weighed to measure dung removal. In each terrarium, dung removal was measured as the percentage of removed dung compared to the controls: [(dry weight of the initial dung pat – dry weight of the remaining dung)/dry weight of the initial dung pat] × 100. The controls C1 and C2 were used to calculate the dry weight of the initial dung pat, respectively, for the first and the second set of experiments. The dry weight of the initial dung pat was obtained by calculating the average of the dry weight of the dung pat in the controls.
Morphological Measurements
Morphological measurements were taken for all the individuals at the end of the experiment. For each species, five morphological traits were selected. Previous studies have suggested to correlate them with digging power or removal ability (head width, maximum pronotum width, fore femur length, foretibia length, outer basal row of tibial teeth length), and one that widely varied among individuals (horn length). The measurement of the outer basal row of foretibia teeth was chosen because of its relevant sexual dimorphism in O. verticicornis. Images of head (up and side views), pronotum and left foreleg (Figure 1) were taken by the LAS-Leica Application Suite software (Leica Microsystems AG, Wetzlar, Germany) using a Leica® DMC4500 (Leica Microsystems AG, Wetzlar, Germany) digital camera connected to a stereoscopic dissecting scope Leica® Z16 APO (Leica Microsystems AG, Wetzlar, Germany). Measurements (Figure 1) of maximum pronotum width (pro_W) [considered a reliable index of body size (Knell, 2009)], head width (head_W), fore femur length (femur_L), foretibia length (tibia_L), outer basal row of tibial teeth length (teeth_L) and cephalic horn length (horn_L) were taken by the LAS Measurement Module of the Leica Application Suite (LAS) Software. The female homolog of male horn (the vertex carina) was measured in females. In O. taurus males, characterized by a pair of large, curved head horns, measurements were taken following Moczek (2006). All the measurements were expressed as mm.
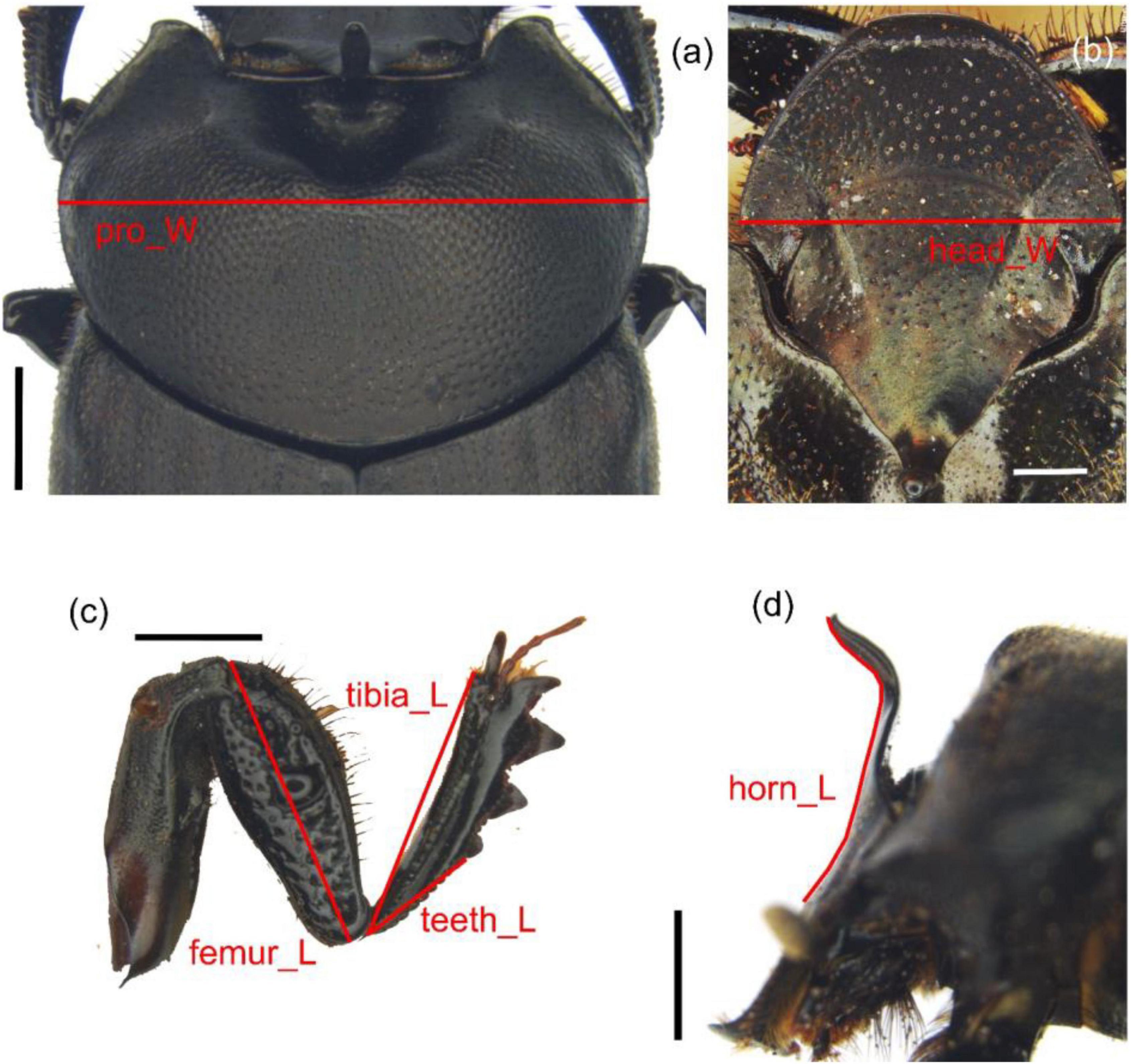
Figure 1. Measurements of the various morphological traits: (a) pronotum width, (b) head width (above), (c) left foreleg with tibia length, femur length, and teeth length, and (d) head with horn length (side view) of O. verticicornis. Scale bar = 1 mm for (a,c,d); scale bar = 0.5 mm for (b).
Statistical Analysis
Linear regression models (LM) and linear mixed models (LMM) were fitted within a Bayesian framework using the INLA package (Rue et al., 2009) for the R statistical computing software (R Core Team, 2017). The results for the effects of interest were expressed as 95% credible intervals (CI), the smallest interval with 95% probability; the lower and upper limit of the 95% CI correspond to the 2.5 and 97.5 percentiles of the posterior distribution for the parameter of interest. Parameter estimates whose 95% CI did not include zero were denoted as significant; those effects for which the 95% CI includes zero but the posterior probability that the treatment effect falls above/below zero is very large, were considered as clear evidence that an effect is present.
Effect of Morphological Traits
Differences in the traits among females, minor and major males for both O. taurus and O. verticicornis were tested with a LM. For this analysis, horn length was transformed with the natural logarithm and outliers were removed whenever necessary to fulfill the assumptions of the analysis.
The potential effect of morphology on dung removal was investigated with principal component analyses (PCA). A first PCA was performed on the morphological traits from the treatments with one individual or two. The first two principal components (PCs) were used to determine the hypervolume enclosing all the observations of either single individuals (F, M, m) or pairs (FF, MM, mm, MF, mF). Hypervolumes were computed to estimate the area of morphological space occupied by each treatment. A bigger area is related to increased morphological diversity and can be associated with greater dung-removing capacity. Based on this hypothesis, heterotypic pairs were expected to have higher morphological diversity and to, thus, remove more dung than single individuals and same sex pairs. Calculations were done with the hypervolume package (Blonder et al., 2014).
A second PCA was performed on the traits of the single individuals and pairs. For each pair, the traits of the two individuals were summed according to the hypothesis that dung removal scales linearly with traits; two same sized individuals remove twice the amount that a single individual removes. Loadings were used to quantify the importance of traits for determining the first two PCs (higher loadings mean making more contribution to the PC). A generalized additive model (GAM) was fitted for each species by taking the dung removal percentage as the dependent variable and the interaction between the first two PCs as the independent variable. GAM models were fitted with the mgcv package (Wood, 2011). Their results were used to predict dung removal in the morphological space identified by the first two PCs. Predictions were plotted as color gradient and contour lines to highlight the dung removal trends in the morphological space produced by the second PCA. The dung removal percentage was log-transformed and square root-transformed for O. taurus and O. verticicornis, respectively. The tidyverse package (Wickham et al., 2019) was used to manage data and ggplot2 (Wickham, 2009) to plot the results.
The Effect of Sex and Interactions Between Individuals on Dung Removal
The effect of treatments, morphological traits and time (e.g., experiments were carried out in two different time sets) on dung removal was tested with linear regression models and linear mixed models. The traits of the two individuals in the treatments with two individuals were summed. Time was included as fixed effect in the model to control for different conditions (e.g., dung moisture) between the first and the second experiment. For each species, the models assuming equal and unequal variances between observations in each treatment were implemented. While models with equal variance represent a classical LM, models with unequal variances imply using an LMM. Deviance Information Criterion (DIC; Spiegelhalter et al., 2002) was used for model selection. Given two competing models, the model with the lowest DIC points to the model that best fits the data; the bigger the difference between the DIC of two competing models, the better supported the best model was. As with Akaike’s Information Criterion, DIC differences of <7, between 7 and 14, and >14 provide plausible, equivocal and implausible support for the model with a larger DIC (Burnham et al., 2011). We tested the difference between treatments by setting specific linear combinations of individual treatment effects. The same approach was followed to test the difference between treatments with two individuals and the sum of the individual treatments to test the presence of interactions between individuals. For instance, the 95% CI of the linear combination MF – (M + F) will inform whether dung removed by the MF pair significantly differs from dung removed by M plus dung removed by F.
Results
Effect of Morphological Traits on Dung Removal
As expected, the characterization of morph categories indicated that major males had greater morphological traits than minor males in both species (Supplementary Figure 1). The maximum pronotum width in majors was on average 4.6 ± 0.1 mm (O. verticicornis) and 5.0 ± 0.2 mm (O. taurus) compared to 3.9 ± 0.3 mm (O. verticicornis) and 4.3 ± 0.4 mm (O. taurus) in minors. Major males generally had greater traits than females, which were larger than minor males in both species (Supplementary Figure 1).
The first two axis of the first PCA of the morphological traits data explained 89.8 and 95.3% of variance in O. taurus and O. verticicornis, respectively, for females and the two male morphs grouped separately in both species. The hypervolumes calculated in the PCA showed a similar area between the sex and male type morphs in O. taurus (F = 2.03, M = 1.78, m = 1.85) and O. verticicornis (F = 1.40, M = 1.09, m = 1.23). The area of the hypervolumes of the females paired with major males was bigger (O. taurus = 4.44; O. verticicornis = 4.22) than those of the females paired with minor males (O. taurus = 3.89; O. verticicornis = 2.50). Combining two individuals from the same morph category (FF, MM, mm) did not determine any increase in the volume area compared to the single individual treatments (F, M, m), which suggests that there was no significant increase in trait diversity in the pairs of individuals of the same sex.
The first two axis of the second PCA explained 98.5 and 98.8% of variance in O. taurus and O. verticicornis, respectively (Supplementary Figures 2a,c). PCA loadings (Supplementary Table 1) suggested that PC2 represented horn length and PC1 depicted the other traits. PC1 represented most traits (positive loadings). The GAM results indicated a significant PC1–PC2 interaction to explain the dung removal percentage in O. taurus (F = 2.778, p-value = 0.006). According to the GAM model, dung removal tended to decrease by moving from the lower-right to the upper-left quadrant of the morphological space (Supplementary Figure 2b). This meant that dung removal was more efficient in the individuals or pairs of individuals with greater traits, but with smaller horns. In O. verticicornis, neither the interaction nor the individual PC1 and PC2 variables had a significant effect on dung removal (Supplementary Figure 2d).
Effect of Social Context on Dung Removal
Table 1 shows the means and standard deviations of the residual dung dry weight for each treatment and species. The linear models assuming unequal variances (LMM) performed better than those assuming equal variances for both O. taurus (Δ DIC = 165.9) and O. verticicornis (Δ DIC = 220.0). Figure 2 reports 95% CI’s, obtained under the LMM, for the linear combinations on the treatments with one and two individuals, for both O. taurus and O. verticicornis.
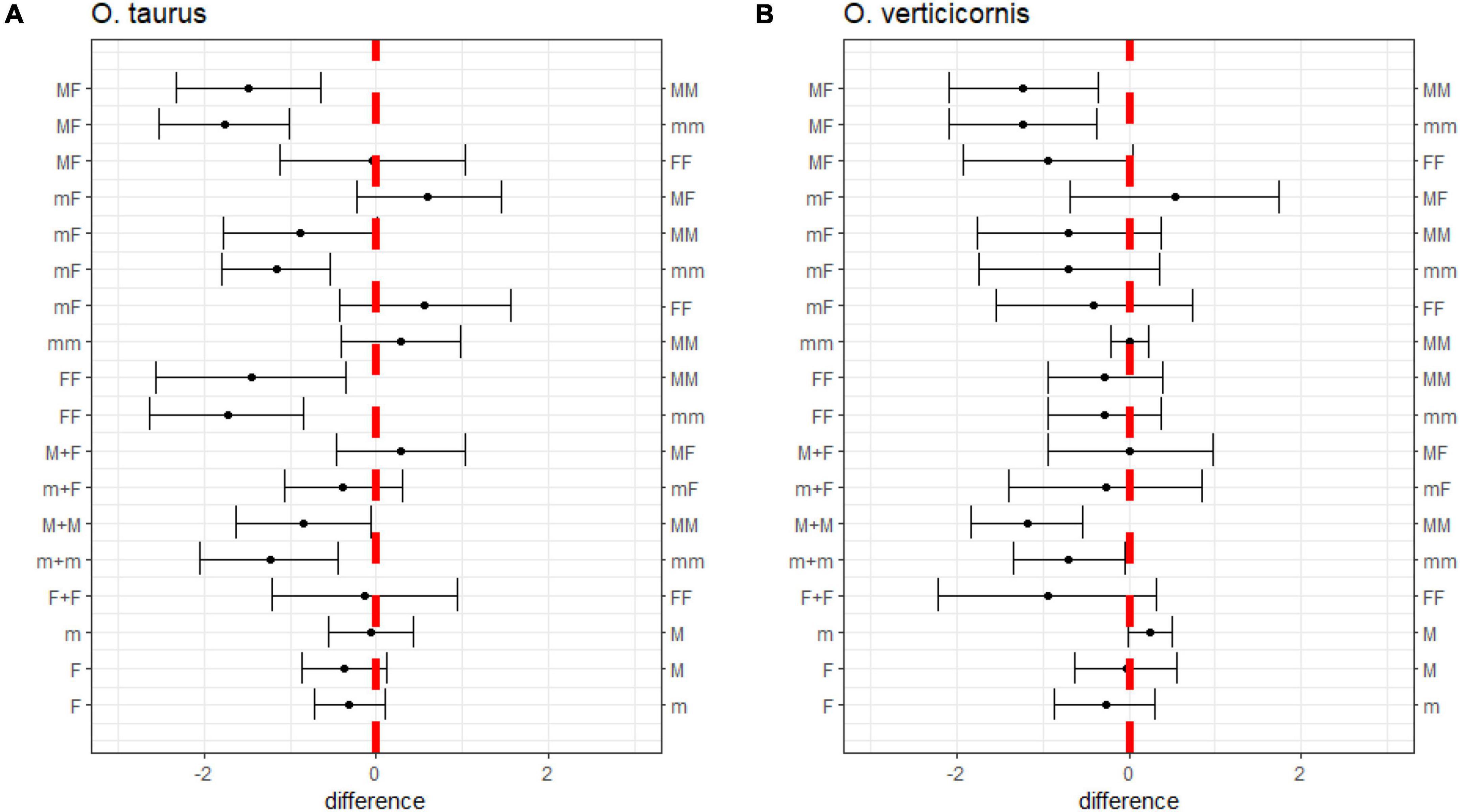
Figure 2. Differences in dung removal among treatments for (A) O. taurus and (B) O. verticicornis. Each bar represents the 95% CI of the difference between the treatments reported on the left and right axis. The comparisons whose bar encloses 0 are not significantly different.
Single females tended to remove more dung than males in both species. However, these differences in females and males were more relevant for major males (mean = −0.37, CI = −0.86, −0.11) than minor males in O. taurus (mean = −0.31, CI = −0.71, 0.10) (Figure 2), and were less evident in O. verticicornis (major: mean = −0.02, CI = −0.63, 0.55; minor: mean = −0.27, CI = −0.86, 0.30). Minor and major males performed similarly in both species (O. taurus: mean = −0.06, CI = −0.55, 0.42); while major males tended to perform better than minors O. verticicornis (mean = 0.24, CI = −0.01, 0.50).
The pairs of females (FF) removed more dung than the pairs of males (MM, mm) in O. taurus (FF-MM = −1.45, CI = −2.56, −0.35; FF-mm: mean = −1.72, CI = −2.63, −0.84). The MF pairs removed more dung than MM (O. taurus: mean = −1.49, CI = −2.33, −0.64; O. verticicornis: mean = −1.22, CI = −2.08, −0.36) and the mm pairs (O. taurus: mean = −1.76, CI = −2.52, −1.00; O. verticicornis: mean = −1.22, CI = −2.08, −0.37) in both species. Similarly, mF in O. taurus (mF-MM: mean = −0.89, CI = −1.78, −0.00; mF-mm: mean = −1.16, CI = −1.78, −0.53) removed more dung than the MM and mm pairs. Treatments mF, MF, and FF performed similarly in dung removal terms, as the treatment MM performed similarly to the mm in both species (Figure 2).
The dung removal performance of the treatments with at least one female (MF, mF, FF) did not significantly differ from that achieved by the sum of single individuals (M + F, m + F, F + F) in both species. However, the FF pairs removed less dung than the sum of the females F + F in O. verticicornis (Figure 2). The MM and mm pairs removed less dung than the M + M and m + m treatments in both O. taurus (M + M – MM: mean = −0.84, CI = −1.62, −0.07; m + m – mm: mean = −1.23, CI = −2.05, −0.44) and O. verticicornis (M + M – MM: mean = −1.18, CI = −1.82, −0.54; m + m – mm: mean = −0.69, CI = −1.33, −0.05).
Discussion
Dung beetle functional ecology has traditionally focused on studying the relation between morphological traits and ecosystem functions in multispecies assemblages. This community trait-based focus is reasonable because ecosystem functioning is the joint effect of the activities of the individuals of different species that co-exist in dung pats. In this context, previous studies have revealed the importance of morphological traits as drivers of dung removal and consequent functions (Nervo et al., 2014; deCastro-Arrazola et al., 2020; Gotcha et al., 2022).
However, functional ecology of dung beetle assemblages derives from integrating the functional contributions of the different species occurring in such assemblages, and often ignores intraspecific variations in traits (Hortal et al., 2015; Malaterre et al., 2019; Noriega et al., 2021). Here we focused on the intraspecific functional ecology of two dung beetle species by evaluating not only the morphological traits that affect dung removal, but also the sex and other factors that affect reproductive behavior and, consequently, the provisioning of ecosystem functions. By taking into account two male dimorphic species, we assumed that the morphological differences between sexes and morphs would indicate the importance of morphological traits at the intraspecific level. Despite the limitations of our study that focuses on only two species, our results showed, quite surprisingly, that dung removal at an intraspecific level depends more on sex and interactions between individuals than on the underlying morphological traits.
The Contribution of Morphological Traits
In multispecies assemblages, dung removal usually increases with body size (Nervo et al., 2014). However, this relation was not confirmed within the present intraspecific framework. Our linear models showed that body size (measured as pronotum width) was not related to dung removal in both O. taurus and O. verticicornis when contemplating single individual treatments. This result was not linked with morphological uniformity in the two species because females, major and minor males differed for most of the investigated morphological traits (which were larger in major males). Based on morphological traits, we expected the categories with larger traits (F, M, FM, FF, MM) to be the most efficient in dung removal. However, our results do not fall in line with these assumptions; in particular, the pairs of major males, characterized by the largest traits, were not the most efficient in dung removal (see the effect of sex and interactions between individuals below).
A PCA was used to describe the females and males within a multitrait framework. The hypervolume analyses based on the first PCA suggested higher functional diversity (i.e., variability in traits) in the females paired with major males (MF) than the females paired with minor males (mF) or females (FF). However, the dung removal efficiency of these three treatments was similar (see the effect of sex and interactions between individuals below). The community level studies revealed that ecosystem functions are underpinned by a combination of trait identities and trait complementarity (Gagic et al., 2015). Here, no variability in the traits measured in each species was found to be linked with ecosystem functions. The GAM analyses based on the second PCA showed a weak relation between morphological traits and dung removal in O. taurus, and no relation in O. verticicornis. In O. taurus, the results suggested that the individuals or pairs of individuals characterized by larger traits (pronotum, head, femur, and tibial teeth), but with smaller horns (females and minor males), were more efficient in dung removal terms.
Both the univariate and multivariate morphological analyses indicated that the contribution of morphological traits to dung removal was negligible. The morphological features that represent important traits in driving dung removal at the interspecific level (deCastro-Arrazola et al., 2020) may play a minor role if considered in an intraspecific context, where interactions between individuals may be more relevant. Nevertheless, our findings do not underestimate the importance of previous studies that have revealed consistent patterns in morphological traits across different species (at the community level); for example, body size correlates with physiology or ecosystem processes (Gotcha et al., 2021, 2022; Nervo et al., 2021). Instead, they stress the importance of also considering other factors at an intraspecific level in functional analyses.
The Effect of Sex and Interactions Between Individuals
Females Are More Effective Than Males
We found that single females were more efficient in dung removal than single males in O. taurus. Even though we found no significant differences in O. verticicornis, our data suggests that females tend to remove more dung than minor males. These differences between females and males can be explained by considering that female dung beetles are biologically predisposed to spawning and to take care of offspring, while dung removal is the sine qua non-condition for performing such activities. This result also coincides with previous observations showing that parental care in brood mass production and tunnel construction terms is provided mostly by single females prior to oviposition (Clutton–Brock, 1991).
Males and Females Join Their Efforts When They Are Together
The presence of a male that cooperates with a female in parental care may be advantageous for not only reproductive success, but also for the dung removed from the surface. Biparental care commonly appears in the dung beetles of the genus Onthophagus (Hunt and Simmons, 2002). Tunnelers usually exhibit a sex-specific division of labor, in which both sexes work apart. Male parental care may directly enhance reproductive success by cooperating in moving dung, which increases the number of brood masses and/or by increasing the amount of dung that each brood mass is provided with (Hunt and Simmons, 1998).
Our results showed that the pairs with at least one female (FF, MF, mF) performed similarly in dung removal terms. The different sex pairs with major males and females (MF) performed better in dung removal terms than the pairs of males (MM, mm) in both species. In O. taurus, the pairs of minor males and females (mF), and pairs of females (FF) also removed larger amounts of dung, which suggests that the presence of females is pivotal in driving dung removal. However, no significant differences were found when comparing dung removal in the pairs with females (FF, MF, mF) to that which derived from the sum of single individuals (F + F, M + F, m + F). These results showed that the presence of another male (MF, mF) or another female (FF) did not boost or reduce dung removal and, hence, suggest an additive effect on FF, MF, and mF due to the presence of two individuals. On the one hand, males and females may cooperate by providing more dung for brood masses (Hunt and Simmons, 2000). In contrast, the presence of males may incur a direct reproductive cost for females which, by spending more time on mating activities, may reduce the total number of produced offspring (Hunt and Simmons, 1998). The additive effect could also depend on the fact that males and females in pairs can adjust their behavior in accordance to their partner investment (Hunt and Simmons, 2002). The parental care provided by males and females in a cooperative pair is usually coordinated, with males adjusting their parental behaviors based on that of females. For example, it has been found that males in O. taurus perform more parental care when paired with small females than with large ones (Hunt and Simmons, 2002).
Males Mutually Inhibit One Another
The presence of two males together (MM, mm) decreased the amount of removed dung compared to the sum of single individuals (M + M, m + m), which hints at mutual inhibition due to possible competition. This effect was absent, or poorly expressed, in females, for which no significant differences were found between the dung removed in FF, and F + F. Mutual male inhibition may also be a reason to explain the greater efficiency noted in the pairs with females (FF, MF, mF). Sexual competition for females drives direct fights (major males) or alternative mate tactics, such as sneaking behavior (minor males) (Emlen et al., 2007), which may engage males in different activities other than feeding on dung.
Male Morphs Perform Similarly
We found that the male morphs removed the same amount of dung in both species and in both single treatments and pairs. However, previous studies have examined the role of males in O. taurus and shown that only major males generally provide assistance during the production of brood masses and would, consequently, be heavier than single females (Hunt and Simmons, 1998, 2000; Moczek and Emlen, 1999). Hunt and Simmons (2002) reported a partial compensation in the parental investment provided by cooperative pair members. This means that one parent’s shortfall is partially compensated for by the other. This finding suggests that even though major and minor males may provide different assistance types in pairs with females, these differences were not detected in our experiments because they were probably compensated for by females’ higher parental investment. Another possibility is that some minors or majors were actually intermediates (Laini et al., unpublished paper), which would explain why majors were only 15% (O. verticicornis) and 20% (O. taurus) larger than minors.
Caveat on Interpretation
Some caveats need to be made when interpreting the results. For technical reasons, experiments with pairs of the same sex were run after those with one individual or with pairs with different sexes. A better choice would have been to run all the treatments in both dates to control for confounding factors (e.g., individual’ performance decrease with time laboratory conditions). We did not consider the presence of females with eggs that could increase their propensity to remove dung. On the other hand, individuals were randomly assigned to each treatment, and we controlled for differences in dung characteristics between the first and the second experiment. Moreover, we included morphological traits in the statistical model to control for their potential confounding effect on the characterization of sex and behavioral interactions. Considering both limitations and strengths, we believe that the results of this work are relevant because they suggest a prominent role played by sex and interaction between individuals, opposed to a non-significant effect due to morphological traits. Further studies controlling for a more comprehensive set of possible confounders and using a larger number of replicates per treatments would increase our capacity to detect significant effects of sex and behavioral interaction at an intra-specific level.
Conclusion
Our research supports recent calls for increased reporting and use of intraspecific variation in traits (Bolnick et al., 2011; Laughlin et al., 2012; Violle et al., 2012), particularly in terrestrial animal groups (see Griffiths et al. (2016)). However, in the two examined dung beetle species, the role of morphological traits in dung removal seems secondary to that of sex and interactions between individuals. As dung removal represents one of the main functions performed by dung beetles, our results suggest that studying behavioral and parental interactions at the species level is one of the key points to understand dung beetle functional ecology. By way of conclusion, while the relations between traits and functions may be remarkably consistent across different taxa, they weaken when evaluating lower ecological hierarchy levels; for example, populations within a community (Malaterre et al., 2019; Gentile et al., 2021). Although our study refers to only two Onthophagus species, we suggest that ignoring interactions between individuals at an intraspecific level can bias our understanding of natural processes. We, therefore, encourage future studies to focus on within-species variability and to consider the effect of sex and behavioral interactions other than morphological traits on the ecosystem functions provided by other dung beetle species.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
BN, AtR, and CP conceived the ideas and designed methodology. BN and FF collected the data in the field. BN and AgR collected the data in the laboratory. AgR conducted the morphological analysis. AL analyzed the data. BN, AL, AtR, and CP led the writing of the manuscript. All authors contributed critically to the drafts, and gave final approval for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was made possible thanks to the facilities of the Geometric Morphometrics Laboratory at Department of Life Sciences and Systems Biology of University of Turin, equipped by funds from the CRT Foundation, Research and Education Section (Turin, Italy). We thank Massimo Ventrucci for his invaluable help in performing statistical analyses. We are also indebted to the two reviewers, whose comments greatly contributed to improve our manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.863669/full#supplementary-material
References
Akamine, M. (2016). Size–and context–dependent nest–staying behaviour of males of the Japanese dung beetle, Copris acutidens (Coleoptera: Scarabaeidae). Eur. J. Entomol. 113, 207–211. doi: 10.14411/eje.2016.025
Akamine, M. (2019). Size–dependent seasonal activity for males of the dung beetle Copris acutidens (Coleoptera: Scarabaeidae). Can. Entomol. 151, 757–767. doi: 10.4039/tce.2019.55
Almeida, H. A., Antonini, Y., Tavares Junior, C., Braga, R. F., da Silva, P. G., and Beiroz, W. (2021). Dung beetles can sow: the potential of secondary seed dispersers to assist ecological restoration. Ecol. Entomol. 47, 181–191. doi: 10.1111/een.13100
Blonder, B., Lamanna, C., Violle, C., and Enquist, B. J. (2014). The n–dimensional hypervolume. Global Ecol. Biogeogr. 23, 595–609. doi: 10.1111/geb.12146
Bolnick, D. I., Amarasekare, P., Araújo, M. S., Bürger, R., Levine, J. M., Novak, M., et al. (2011). Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. doi: 10.1016/j.tree.2011.01.009
Brousseau, P. M., Gravel, D., and Handa, I. T. (2018). On the development of a predictive functional trait approach for studying terrestrial arthropods. J. Anim. Ecol. 87, 1209–1220. doi: 10.1111/1365-2656.12
Brown, A. M., Warton, D. I., Andrew, N. R., Binns, M., Cassis, G., and Gibb, H. (2014). The fourth-corner solution–using predictive models to understand how species traits interact with the environment. Methods Ecol. Evol. 5, 344–352. doi: 10.1111/2041-210x.12163
Burner, R. C., Stephan, J. G., Drag, L., Birkemoe, T., Muller, J., Snäll, T., et al. (2021). Traits mediate niches and co-occurrences of forest beetles in ways that differ among bioclimatic regions. J. Biogeogr. 48, 3145–3157. doi: 10.1111/jbi.14272
Burnham, K. P., Anderson, D. R., and Huyvaert, K. P. (2011). AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav. Ecol. Sociobiol. 65, 23–35. doi: 10.1007/s00265-010-1029-6
Cadotte, M. W. (2017). Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 20, 989–996. doi: 10.1111/ele.12796
D’Amen, M., Mod, H. K., Gotelli, N. J., and Guisan, A. (2018). Disentangling biotic interactions, environmental filters, and dispersal limitation as drivers of species co-occurrence. Ecography 41, 1233–1244. doi: 10.1111/ecog.03148
deCastro-Arrazola, I., Hortal, J., Noriega, J. A., and Sánchez-Piñero, F. (2020). Assessing the functional relationship between dung beetle traits and dung removal, burial, and seedling emergence. Ecology 101:e03138. doi: 10.1002/ecy.3138
Emlen, D. J. (1997). Alternative reproductive tactics and male–dimorphism in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 41, 335–341. doi: 10.1007/s002650050393
Emlen, D. J., Lavine, L. C., and Ewen–Campen, B. (2007). On the origin and evolutionary diversification of beetle horns. P. Natl. Acad. Sci. U.S.A 104, 8661–8668. doi: 10.1073/pnas.0701209104
Esperk, T., Tammaru, T., Nylin, S., and Teder, T. (2007). Achieving high sexual size dimorphism in insects: females add instars. Ecol. Entomol. 32, 243–256. doi: 10.1111/j.1365-2311.2007.00872.x
Fernandes, I., Pascoal, C., and Cássio, F. (2011). Intraspecific traits change biodiversity effects on ecosystem functioning under metal stress. Oecologia 166, 1019–1028. doi: 10.1007/s00442-011-1930-3
Gagic, V., Bartomeus, I., Jonsson, T., Taylor, A., Winqvist, C., Fischer, C., et al. (2015). Functional identity and diversity of animals predict ecosystem functioning better than species–based indices. P. Roy. Soc. B–Biol. Sci. 282:20142620. http://doi.org/10.1098/rspb.2014.2620, doi: 10.1098/rspb.2014.2620
Gentile, G., Bonelli, S., and Riva, F. (2021). Evaluating intraspecific variation in insect trait analysis. Ecol. Entomol. 46, 11–18. doi: 10.1111/een.12984
Gotcha, N., Cuthbert, R. N., Machekano, H., and Nyamukondiwa, C. (2022). Density–dependent ecosystem service delivery under shifting temperatures by dung beetles. Sci. Total Environ. 807:150575. doi: 10.1016/j.scitotenv.2021.150575
Gotcha, N., Machekano, H., Cuthbert, R. N., and Nyamukondiwa, C. (2021). Low-temperature tolerance in coprophagic beetle species (Coleoptera: Scarabaeidae): implications for ecological services. Ecol. Entomol. 46, 1101–1112. doi: 10.1111/een.13054
Gouws, E. J., Gaston, K. J., and Chown, S. L. (2011). Intraspecific Body Size Frequency Distributions of Insects. PLoS One 6:16606. doi: 10.1371/journal.pone.0016606
Griffiths, H. M., Bardgett, R. D., Louzada, J., and Barlow, J. (2016). The value of trophic interactions for ecosystem function: dung beetle communities influence seed burial and seedling recruitment in tropical forests. P. Roy. Soc. B–Biol. Sci. 283:20161634. doi: 10.1098/rspb.2016.1634,
Hooper, D. U., Chapin Iii, F. S., Ewel, J. J., Hector, A., Inchausti, P., Lavorel, S., et al. (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. doi: 10.1890/04-0922
Hortal, J., de Bello, F., Diniz–Filho, J. A. F., Lewinsohn, T. M., Lobo, J. M., and Ladle, R. J. (2015). Seven shortfalls that beset large–scale knowledge of biodiversity. Ann. Rev.Ecol., Evol. Syst. 46, 523–549. doi: 10.1146/annurev-ecolsys-112414-054400
Hunt, J., and Simmons, L. W. (1998). Patterns of parental provisioning covary with male morphology in a horned beetle (Onthophagus taurus) (Coleoptera: Scarabaeidae). Behav. Ecol. Sociobiol. 42, 447–451. doi: 10.1007/s002650050459
Hunt, J., and Simmons, L. W. (2000). Maternal and paternal effects on offspring phenotype in the dung beetle Onthophagus taurus. Evolution 54, 936–941. doi: 10.1111/j.0014-3820.2000.tb00093.x
Hunt, J., and Simmons, L. W. (2002). Behavioural dynamics of biparental care in the dung beetle Onthophagus taurus. Anim. Behav. 64, 65–75. doi: 10.1006/anbe.2002.3036
Jung, V., Albert, C. H., Violle, C., Kunstler, G., Loucougaray, G., and Spiegelberger, T. (2014). Intraspecific trait variability mediates the response of subalpine grassland communities to extreme drought events. J. Ecol. 102, 45–53. doi: 10.1111/1365-2745.12177
Knell, R. J. (2009). On the analysis of non-linear allometries. Ecol. Entomol. 34, 1–11. doi: 10.1111/j.1365-2311.2008.01022.x
Laughlin, D. C., Joshi, C., van Bodegom, P. M., Bastow, Z. A., and Fulé, P. Z. (2012). A predictive model of community assembly that incorporates intraspecific trait variation. Ecol. Lett 15, 1291–1299. doi: 10.1111/j.1461-0248.2012.01852.x
Lecerf, A., and Chauvet, E. (2008). Intraspecific variability in leaf traits strongly affects alder leaf decomposition in a stream. Basic Appl. Ecol. 9, 598–605. doi: 10.1016/j.baae.2007.11.003
Malaterre, C., Dussault, A. C., Rousseau–Mermans, S., Barker, G., Beisner, B. E., Bouchard, F., et al. (2019). Functional diversity: An epistemic roadmap. BioScience 69, 800–811. doi: 10.1093/biosci/biz089
McGill, B. J., Enquist, B. J., Weiher, E., and Westoby, M. (2006). Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. doi: 10.1016/j.tree.2006.02.002
Moczek, A. P. (2006). A matter of measurements: challenges and approaches in the comparative analysis of static allometries. Am. Nat. 167, 606–611. doi: 10.1086/501075
Moczek, A. P., and Emlen, D. J. (1999). Proximate determination of male horn dimorphism in the beetle Onthophagus taurus (Coleoptera: Scarabaeidae). J. Evol. Biol. 11, 27–37. doi: 10.1046/j.1420-9101.1999.00004.x
Moczek, A. P., and Emlen, D. J. (2000). Male horn dimorphism in the scarab beetle. Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Anim. Behav. 59, 459–466. doi: 10.1006/anbe.1999.1342
Nervo, B., Caprio, E., Celi, L., Lonati, M., Lombardi, G., Falsone, G., et al. (2017). Ecological functions provided by dung beetles are interlinked across space and time: evidence from 15N isotope tracing. Ecology 98, 433–446. doi: 10.1002/ecy.1653
Nervo, B., Roggero, A., Isaia, M., Chamberlain, D., Rolando, A., and Palestrini, C. (2021). Integrating thermal tolerance, water balance and morphology: An experimental study on dung beetles. J. Thermal Biol. 101:103093. doi: 10.1016/j.jtherbio.2021.103093
Nervo, B., Tocco, C., Caprio, E., Palestrini, C., and Rolando, A. (2014). The effects of body mass on dung removal efficiency in dung beetles. PloS One 9:e107699. doi: 10.1371/journal.pone.0107699
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M. E., et al. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 141, 1461–1474. doi: 10.1016/j.biocon.2008.04.011
Noriega, J. A., March–Salas, M., Castillo, S., García–Q, H., Hortal, J., and Santos, A. M. C. (2021). Human perturbations reduce dung beetle diversity and dung removal ecosystem function. Biotropica 53, 753–766. doi: 10.1111/btp.12953
Penttilä, A., Slade, E. M., Simojoki, A., Riutta, T., Minkkinen, K., and Roslin, T. (2013). Quantifying beetle–mediated effects on gas fluxes from dung pats. PLoS One 8:e71454. doi: 10.1371/journal.pone.0071454
Piccini, I., Arnieri, F., Caprio, E., Nervo, B., Pelissetti, S., Palestrini, C., et al. (2017). Greenhouse gas emissions from dung pats vary with dung beetle species and with assemblage composition. PLoS One 12:e0178077. doi: 10.1371/journal.pone.0178077
Piccini, I., Caprio, E., Palestrini, C., and Rolando, A. (2020). Ecosystem functioning in relation to species identity, density, and biomass in two tunneller dung beetles. Ecol. Entomol. 45, 311–320. doi: 10.1111/een.12802
Piccini, I., Nervo, B., Forshage, M., Celi, L., Palestrini, C., Rolando, A., et al. (2018). Dung beetles as drivers of ecosystem multifunctionality: Are response and effect traits interwoven? Sci. Total Environ. 616, 1440–1448. doi: 10.1016/j.scitotenv.2017.10.171
Pruitt, J. N., and Ferrari, M. C. (2011). Intraspecific trait variants determine the nature of interspecific interactions in a habitat-forming species. Ecology 92, 1902–1908. doi: 10.1890/11-0701.1
R Core Team. (2017). A Language and Environment for Statistical Computing. Vienna: R Foundation forStatistical Computing.
Raine, E. H., Gray, C. L., Mann, D. J., and Slade, E. M. (2018). Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses. Ecol. Evol. 8, 8686–8696. doi: 10.1002/ece3.4218
Rue, H., Martino, S., and Chopin, N. (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. Roy. Stat. Soc. B Met. 71, 319–392. doi: 10.1111/j.1467-9868.2008.00700.x
Slade, E. M., Mann, D. J., Villanueva, J. F., and Lewis, O. T. (2007). Experimental evidence for the effects of dung beetle functional group richness and composition on ecosystem function in a tropical forest. J. Anim. Ecol. 76, 1094–1104. doi: 10.1111/j.1365-2656.2007.01296.x
Slade, E. M., Roslin, T., Santalahti, M., and Bell, T. (2016). Disentangling the ‘brown world’faecal–detritus interaction web: dung beetle effects on soil microbial properties. Oikos 125, 629–635. doi: 10.1111/oik.02640
Sowig, P. (1996). Duration and benefits of biparental brood care in the dung beetle Onthophagus vacca (Coleoptera: Scarabaeidae). Ecol. Entomol. 21, 81–86. doi: 10.1111/j.1365-2311.1996.tb00269.x
Spiegelhalter, D. J., Best, N. G., Carlin, B. P., and Van Der Linde, A. (2002). Bayesian measures of model complexity and fit. J. Roy. Stat. Soc. B Met. 64, 583–639. doi: 10.1111/1467-9868.00353
Tomkins, J. L., Kotiaho, J. S., and LeBas, N. R. (2005). Phenotypic plasticity in the developmental integration of morphological trade–offs and secondary sexual trait compensation. P. Roy. Soc. B–Biol. Sci. 272, 543–551. doi: 10.1098/rspb.2004.2950
Uvarov, A. V. (2009). Inter–and intraspecific interactions in lumbricid earthworms: their role for earthworm performance and ecosystem functioning. Pedobiologia 53, 1–27. doi: 10.1016/j.pedobi.2009.05.001
Vandewalle, M., de Bello, F., Berg, M. P., Bolger, T., Dolédec, S., Dubs, F., et al. (2010). Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodiv. Conserv. 19, 2921–2947. doi: 10.1007/s10531-010-9798-9
Violle, C., Enquist, B. J., McGill, B. J., Jiang, L. I. N., Albert, C. H., Hulshof, C., et al. (2012). The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 27, 244–252. doi: 10.1016/j.tree.2011.11.014
Violle, C., Navas, M. L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., et al. (2007). Let the concept of trait be functional! Oikos 116, 882–892. doi: 10.1111/j.0030-1299.2007.15559.x
Wickham, H., Averick, M., Bryan, J., Chang, W., McGowan, L. D. A., and François, R. (2019). Welcome to the Tidyverse. J. Open Sour. Soft. 4:1686. doi: 10.21105/joss.01686
Keywords: Onthophagus, male morphs, social context, tunnelers, cooperation, inhibition, ecosystem service
Citation: Nervo B, Laini A, Roggero A, Fabbriciani F, Palestrini C and Rolando A (2022) Interactions Between Individuals and Sex Rather Than Morphological Traits Drive Intraspecific Dung Removal in Two Dung Beetle Species. Front. Ecol. Evol. 10:863669. doi: 10.3389/fevo.2022.863669
Received: 27 January 2022; Accepted: 16 March 2022;
Published: 11 April 2022.
Edited by:
Fabricio Beggiato Baccaro, Federal University of Amazonas, BrazilReviewed by:
Jorge Ari Noriega, University of the Andes, ColombiaPedro Giovâni Da Silva, Federal University of Minas Gerais, Brazil
Copyright © 2022 Nervo, Laini, Roggero, Fabbriciani, Palestrini and Rolando. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Roggero, YW5nZWxhLnJvZ2dlcm9AdW5pdG8uaXQ=
 Beatrice Nervo1
Beatrice Nervo1 Angela Roggero
Angela Roggero Fabrizio Fabbriciani
Fabrizio Fabbriciani