
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Ecol. Evol. , 19 May 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.860854
This article is part of the Research Topic Sex and Gender Effects on Power, Status, Dominance, and Leadership – An Interdisciplinary Look at Human and Other Mammalian Societies View all 22 articles
Infanticide by adult females includes any substantial contribution to the demise of young and inevitably imposes fitness costs on the victim’s genetic fathers, thereby generating sexual conflict with them. Few if any studies have quantified the impact of infanticide by females on male reproductive success, the magnitude of sexual conflict this causes and possible counterstrategies males use against infanticidal females. We examine these topics in spotted hyena (Crocuta crocuta) clans, where females socially dominate breeding males and strong female mate-choice is independent of male social status. We consider two causes of infanticide by females, violent attacks on cubs and fatal maternal neglect. Violent attacks are predicted during periods of social instability at the top of the female linear dominance hierarchy and victims are expected to predominantly have mothers above median rank. Fatal maternal neglect, when starving litters are abandoned, is associated with monopolization of food in clan territories by high-ranking females, and victims are predicted to have mothers below median rank. Female perpetrators of violent attacks are expected to reduce the reproductive success of the fathers of their victims more than perpetrators of fatal maternal neglect. We tested these predictions using 30 + years of data (54 recorded violent attacks, 43 cases of fatal maternal neglect, DNA profiling of 1,671 individuals). Using long-term observations at communal dens we investigated whether males use counterstrategies against infanticide reported in other mammals. Due to female social dominance over breeding males, strong female mate-choice and prolonged offspring dependence on lactation in spotted hyenas, we predicted that these counterstrategies were unlikely to be used by males against females, thus no incidences of them were likely to be observed. Our results revealed that breeding males lost cubs to violent attacks at all stages of their reproductive tenure and to perpetrators with whom they did not sire offspring. Amongst known sources of paternity loss, violent attacks comprised 12.2% and maternal neglect 9.8% of cases. Violent attacks significantly reduced offspring production rates of breeding males, suggesting that infanticide by females generates sexual conflict. As predicted, no evidence of males using counterstrategies against infanticide by females were observed.
Infanticide in mammals is widespread and has been reported from rodents, bats, lagomorphs, cetaceans, pinnipeds, terrestrial carnivores and primates (Hoogland, 1985; Agrell et al., 1998; Blumstein, 2000; Digby, 2000; Knörnschild et al., 2011; Towers et al., 2018; Lowe et al., 2020; Brown et al., 2021). In adult mammals, intra-sexual reproductive competition is thought to favor the evolution of infanticide by providing perpetrators with the benefit of increased reproductive success (Hrdy, 1979; van Schaik, 2000; Lukas and Huchard, 2014, 2019; Palombit, 2015). Adult males are thought to use infanticide to gain additional mating partners, whereas infanticide by females is thought to increase access to resources required for successful reproduction (Digby, 2000; Stockley and Campbell, 2013; Lukas and Huchard, 2014, 2019; Walker et al., 2021). Perpetrators of infanticide exercise power over their victim and the victim’s parents, and by killing offspring, perpetrators generate sexual conflict with the victim’s parent of the opposite sex (Trivers, 1972; Arnqvist and Rowe, 2005). Parents of victims may evolve behavioral, reproductive or physiological counterstrategies to reduce their loss of reproductive success to infanticide (Palombit, 2015).
Perpetrators of infanticide may be either males or females, and in some species they may be individuals of both sexes, as in chimpanzees (Pan troglodytes) (Lowe et al., 2020; Walker et al., 2021) and killer whales (Orcinus orca) (Towers et al., 2018). Consideration of sexual conflict generated by infanticide has predominantly focused on the conflict between male perpetrators and the mothers of their victims (Palombit, 2015). Far less attention has been given to the sexual conflict generated by adult female perpetrators with the fathers of their victims whose reproductive success they undermine [see Lukas and Huchard (2019) for comparative analyses of the determinants of female infanticide]. In particular, to our knowledge, few studies based on free-ranging social mammals have attempted to quantify the fitness costs of losing paternity to infanticide by females. Similarly, discussion of counterstrategies against infanticide in mammals has mostly focused on female counterstrategies against infanticide by adult males and/or females (Hrdy, 1979; van Schaik and Kappeler, 1997; Digby, 2000) rather than male counterstrategies against infanticide by females. Our study aims to redress this imbalance by investigating the loss of reproductive success by breeding spotted hyena (Crocuta crocuta) males to infanticide by adult females within their clan, the level of sexual conflict infanticide by females generates and possible counterstrategies by males to limit paternity losses to infanticide.
In many social mammals, the reproductive success of females holding high social status is higher than that of subordinates (Pusey et al., 1997; Wasser et al., 2004; Stockley and Bro-Jørgensen, 2011; Wright et al., 2020). This is because socially dominant females have priority of access to resources necessary for reproduction (Clutton-Brock and Huchard, 2013) such as food or access to communal dens or burrows (Digby, 2000). This also applies to the spotted hyena (Holekamp et al., 1996; Hofer and East, 2003), a social carnivore which lives in multi-female, multi-male, fission-fusion groups called clans where all immigrant males are socially subordinate to natal females and their offspring (Kruuk, 1972; Frank, 1986).
Although both adult male and female spotted hyenas have been observed killing juveniles within their clan (Kruuk, 1972; Hofer and East, 1995; East et al., 2003; White, 2005; Brown et al., 2021), the incidence of infanticide by adult males (one observed case, plus one case of a male digging in a den, apparently intent on infanticide), was considered too low to be a male reproductive tactic (East et al., 2003). The findings of a recent study (Brown et al., 2021) and the results of this study indicate that the vast majority of infanticides in spotted hyenas are committed by adult females. Brown et al. (2021) reported that one in ten offspring in their study died of infanticide by adult females and that infanticide was most likely a response to competition over social status between matrilines. The effect of infanticide by females on the reproductive success of breeding males was not reported.
Infanticide results from actions by conspecifics that substantially contribute to the death of young of the same species (Hrdy, 1992). This has led to a focus on violent attacks by adults on juveniles, but conceptually need not be limited to such actions. For instance, one source of juvenile death in spotted hyenas is a form of infanticide termed facultative siblicide, when the dominant cub in a twin litter monopolizes access to maternal milk during nursing bouts, thereby starving its subordinate sib to death (Hofer and East, 1997, 2008; Golla et al., 1999; Benhaiem et al., 2012). Facultative siblicide will not be considered further, as we focus on two less studied causes of infanticide in spotted hyenas: (i) Violent attack, when aggression by an adult female results in the immediate death of a cub or substantial damage and death ensuing soon after. (ii) Fatal maternal neglect, when a mother fails to provide her offspring with sufficient milk to prevent its death from starvation, usually because long foraging excursions result in mothers not nursing their offspring for many days (Hofer et al., 2016). In such circumstances, females should suspend continued investment in current offspring if their own survival is at risk and investment in future offspring is likely to yield higher benefits (Williams, 1966; Trivers, 1972).
As spotted hyenas give birth throughout the year, infanticides may occur in any month of the year. Litters are small, normally one or two cubs, rarely three cubs, which in our study population all three cubs never survive, and maternal input into offspring is high, in terms of highly nutritious milk produced throughout a long lactation period of 12–20 months (Holekamp et al., 1996; Hofer and East, 2003; Hofer et al., 2016). By contrast, the contribution of fathers to provisioning offspring is negligible. In our study population, high-ranking females monopolized food resources within clan territories, resulting in low-ranking females using long-distance commuting trips (to distant locations containing abundant migratory ungulates) throughout most of the year, to fuel the high cost of lactation, whereas high-ranking females generally only commuted when prey density in the clan territory was at its lowest (Hofer and East, 1993c; Gicquel et al., 2022). Cubs remain at the communal den for at least their first 12 months of life, and when threatened by aggressive individuals in the clan, they retreat into narrow and deep underground burrows where adults cannot reach them (Golla et al., 1999).
All immigrant males are socially subordinate to adult females and their offspring in their clan (Frank, 1986). At immigration, males acquire the lowest social position in a clan, by taking the lowest rank in the male linear dominance hierarchy which functions as a stable social queue (East and Hofer, 2001). Males increase in social rank as more dominant males die or disperse elsewhere. Physical contests between immigrant males to increase their status are rare, thus high-ranking immigrant males are those with the longest tenure (East and Hofer, 2001). Although most breeding males in a clan are immigrants, occasionally males do not disperse and become breeding males in their natal clan, in which case they join the breeding male hierarchy ahead of the immigrant males (East and Hofer, 2001; Höner et al., 2007).
In our study population, all breeding males conduct long-distance foraging excursions when large aggregations of migratory herbivores are absent from their territory (Hofer and East, 1993a,b). High-ranking males have priority of access to food resources in the clan territory over low-ranking males (Frank, 1986) which may explain why high-ranking males are more often in our study clan territories than low-ranking males (East and Hofer, 1991), particularly at the communal den, which is an important social center of the clan (East et al., 2013). Low-ranking males are present at communal dens less often, typically remain at dens for shorter periods than high-ranking males, and rarely venture close to communal dens, unlike high-ranking males (East et al., 2013). This predicts that high-ranking males are more likely to witness violent attacks by females on cubs, and are better placed to use counterstrategies to protect their offspring against these violent attacks, than low-ranking males.
The unusual anatomy of female reproductive organs (Matthews, 1939), in particular the penile clitoris positioned between the hind legs, ensures that copulation cannot be successful without the complete cooperation of females (East et al., 1993). Genetic studies on the paternity of offspring provide strong evidence that female mate-choice preferences do not necessarily match that of breeding males (Engh et al., 2002; East et al., 2003; Höner et al., 2007). For example, high-ranking males attempt to monopolize access to high-ranking females (East and Hofer, 2001; Szykman et al., 2001) but genetic paternity of cubs produced by high-ranking mothers is not skewed toward high-ranking males (Engh et al., 2002; East et al., 2003; Höner et al., 2007) and there is little evidence that coercion of females is an effective tactic to secure paternity (East et al., 2003). Thus female mate-choice is a likely source of sexual conflict in spotted hyenas (East et al., 2003).
Currently, little is known about the male traits that females prefer, beyond those associated with avoidance of inbreeding with close male relatives in the clan (Höner et al., 2007). This chiefly entails young females, regardless of their social status, selecting males that joined the clan after the females was born, to sire their cubs.
Breeding males foster associations with females in their clan (Szykman et al., 2001). Female mate-choice to avoid inbreeding is apparent in male-female associations in that older adult females are more tolerant of approaches by longer-tenured than shorter-tenured males, and younger adult females are more tolerant of approaches by shorter-tenured than longer-tenured males (East and Hofer, 2001). Throughout their lives, female produce offspring sired by several males and there is evidence that females mate with multiple males to promote sperm competition and confuse paternity (East et al., 2003).
Using direct observations of infanticide during observation sessions primarily at clan communal dens, from an ongoing long-term study in the Serengeti National Park initiated in 1987, coupled with microsatellite DNA profiling of 1,671 individually known spotted hyenas (adult males, adult females and offspring), we determined the genetic sires of cubs that died from infanticide by adult females, the level of sexual conflict the actions of these females generated with the victims’ fathers and potential male counterstrategies.
We predicted that the cost of infanticide to individual breeding males, in terms of reduced reproductive success, would depend on the type of infanticide (violent attack by females or fatal maternal neglect) and the social status of mothers, and we explored whether it varied with female age. High-ranking females have a higher reproductive success than low-ranking females because they give birth to their first litter at an earlier age, and their cubs have higher growth rates and chances of survival to adulthood than those of low-ranking females (Holekamp et al., 1996; Hofer and East, 2003; Ferreira et al., 2019). Thus siring cubs with high-ranking females provides breeding males with fitness benefits. As violent infanticide by adult females is considered an expression of female-female competition for resources essential for reproduction (Lukas and Huchard, 2019) and resource competition within our clan territories is most intense among high-ranking females (Hofer and East, 1993c; Goymann et al., 2001), we predicted that offspring sired with high-ranking mothers should be particularly vulnerable to fatal violent attacks by high-ranking females, and infanticide by violent attacks should increase during periods of social unrest between competing coalitions of high-ranking females.
As food resources within the clan territory are monopolized by high-ranking females, low-ranking females fuel the high energetic cost of lactation (Hofer et al., 2016) by regularly commuting long distances between their offspring at the clan communal den and distant aggregations of migratory ungulates throughout many months of the year (Hofer and East, 1993c; Gicquel et al., 2022). As a result of these long-distance commuting trips, cubs of low-ranking mothers have longer (in terms of days) intervals between nursing bouts and a lower intake of milk than high-ranking cubs (Hofer et al., 2016). Regular long-distance commuting trips increase fecal glucocorticoid levels in adult females (Goymann et al., 2001) and are associated with elevated infection loads of costly gastrointestinal parasites in lactating, low-ranking females, which may be indicative of down regulation of immune processes (East et al., 2015). At communal dens, harassment by high-ranking females disrupts attempts by low-ranking females to nurse their offspring and can prevent low-ranking females from attempting to nurse their offspring (Golla et al., 1999). For these reasons we predicted that males that sire offspring with low-ranking mothers are particularly vulnerable to infanticide by maternal neglect.
As infanticide by violent attack has been estimated to be a relatively important source of cub mortality (Brown et al., 2021), we predicted that infanticide by violent attacks should reduce male reproductive success more than by maternal neglect. Thus the magnitude of sexual conflict between female perpetrators and the genetic fathers of their victims would be higher for infanticide by violent attacks than fatal maternal neglect.
Various possible counterstrategies against violent infanticide have been reported in social mammals. These include counter attacks by the victim’s parent(s), and the formation of alliances with individuals in a group (Packer and Pusey, 1983; Agrell et al., 1998; Fruteau et al., 2010; Palombit, 2015), as well as dispersal, typically by adult females (Pusey and Packer, 1994; Sterck and Korstjens, 2000; Zhao et al., 2011). Infanticide by maternal neglect in species with biparental provisioning of offspring might be prevented by increased paternal provisioning of food to compensate undernourished offspring. More generally, bet-hedging in which males sire cubs with many females so that paternity lost due to infanticide incurred by one female partner is compensated by the survival to adulthood of offspring sired with other partners, might be a potential counterstrategy against various causes of infanticide. It is also conceivable that males could prefer or avoid specific females with whom to sire offspring if this helped to minimize paternity losses from infanticide. Social dominance of females over breeding males, strong female mate-choice, and prolonged offspring dependence on lactation in spotted hyenas predict that breeding males are unlikely to use any of these counterstrategies against infanticide of their offspring by adult females.
Infanticides were observed in five clans (Isiaka, Pool, Mamba, Songore, Campsite) located in the center of the Serengeti National Park (Serengeti NP) in Tanzania during observations periods primarily at the communal dens (Hofer and East, 1993a). Den observation periods at dawn and dusk lasted for 2 h or more. Incidences of infanticide, DNA profiling data and data used in the analysis of the effect of infanticides on the reproductive success of breeding males were obtained from the three main study clans (East et al., 2003) regularly monitored between 1987 and 2020 (Isiaka: May 1987–March 2020; Pool: November 1989–March 2020, Mamba: August 1999–March 2020). Dens in the Songore and Campsite clans were monitored less intensively between 1988 and 1995.
Individuals were identified by unique spot patterns, markings, and features using well established methods (Frank, 1986; Frank et al., 1990; Hofer and East, 1993a). The main study clans were well habituated to the presence of observers in vehicles, which permitted us to record detailed behavioral and life-history data at the clan communal dens and elsewhere. Females remained in their natal clan throughout their life, thus clans had several overlapping female generations (matrilineal society). Most males dispersed after they reached adulthood and immigrated into another clan. Some males became reproductively active in their natal group (East and Hofer, 2001). The term breeding male includes reproductively active natal males and immigrant males in a clan. Breeding tenure for natal males started on the date they first displayed behaviors of a reproductively active male. For immigrant males, breeding tenure started on the date they were first seen in a study clan (East and Hofer, 2001; Höner et al., 2007).
Females give birth to one or two cubs, very rarely three (Hofer and East, 2008) throughout the year (Hofer and East, 1995; Holekamp et al., 1996) after a gestation period of 110 days (Matthews, 1939). Their energetic and time investment per litter is amongst the highest in the order Carnivora, with cubs being completely dependent on maternal milk for the first 6 months and weaned at the age of between 12 and 20 months (Holekamp et al., 1996; Hofer et al., 2016). Cubs remain at the clan communal den until approximately 12 months old where they shelter in underground burrows that are too narrow for adults to access (Hofer and East, 1993c; Golla et al., 1999). Cubs were animals < 12 months old, subadults were those between 12 and < 24 months old and adults were those that were 24 months or older (Hofer and East, 2003; Hofer et al., 2016). We refer to offspring when we do not refer to specific age categories of individuals below 24 months.
Birth dates were either known or derived using several developmental characteristics of pelage, locomotion and aspects of physical appearance (Golla et al., 1999; East et al., 2003). The conception date of cubs was calculated by subtracting 110 days from their birth date. Cubs less than approximately 10 weeks old rarely emerged from the clan den during their mother’s absence, but they can be located in an underground burrow close enough to the den entrance to permit infanticidal females to grab them. Adult spotted hyenas cannot enter the narrow underground chambers of a clan den, thus cubs that remain deep underground cannot be grabbed by infanticidal females.
For each clan, the linear dominance hierarchies for adult females and breeding males were constructed from submissive behaviors recorded ad libitum during dyadic interactions between adults of the same sex (see East and Hofer, 2001; East et al., 2003). Dominance hierarchies were adjusted after each loss or recruitment of an adult and when dyadic interaction data revealed that an individual had increased or fallen in rank. To facilitate the comparison of ranks across clans of different sizes we computed standardized ranks. This measure placed the rank of an individual evenly between the highest (standardized rank + 1) and lowest (standardized rank −1) rank (Goymann et al., 2001; East et al., 2003). Low-ranking individuals in both the adult female and breeding male dominance hierarchies were termed low-ranking when they held a standardized rank between −1 and 0, and high-ranking when they held a rank above 0.
Female social status determined access to food within a clan’s territory and the proportion of the year during which females undertook regular long-distance foraging trips outside the clan territory to forage in areas containing large aggregations of migratory herbivores (Hofer and East, 1993b,2003). In brief, when a clan territory only contained resident herbivores, prey abundance was low (∼7.2 animals/km2) and adult females, regardless of social status, predominantly foraged elsewhere. When resident herbivores plus low numbers of migratory herbivores were present in the clan territory, prey abundance (∼31.0 animals/km2) was sufficient to support females in the upper third of the female dominance hierarchy, whereas lower-ranking females needed to forage elsewhere. When territories contained large numbers of migratory herbivores (∼238.5 animals/km2) all adult females foraged within their clan territory, mothers nursed their cubs daily, and thus maternal den attendance was high (Hofer and East, 1993b,c). Milk-dependent offspring remained at the clan communal den when their mother was foraging (Hofer et al., 2016). Long-distance commuting trips outside the clan territory resulted in maternal absences from their offspring of between usually 2–9 days, a substantial decrease in maternal den attendance (Hofer and East, 1993c). As a result, lower-ranking mothers spent a larger proportion of the year commuting and therefore transferred much less milk to their cubs than higher-ranking females (Hofer and East, 2003; Hofer et al., 2016; Gicquel et al., 2022).
We categorized the level of certainty we placed on records of violent attacks as follows: (i) “Observed” infanticides: Aggression causing the death, or in two cases substantial damage (with death following subsequently) of cubs, witnessed by a member of the project. As reported by Brown et al. (2021), violent attacks typically involved a bite to the head of a young cub. (ii) “Likely” infanticides: Presence of a recently dead cub (or part of a cub’s carcass) which had shown no recent clinical signs of disease, no signs of starvation and there was no evidence that death had resulted from other possible causes (e.g., wounds consistent with predation by large cats or being hit by a vehicle). If a cub carcass was carried or consumed by a clan member, this individual was not necessarily assumed to have committed infanticide. In some of these cases, females were observed with a recently killed carcass of a cub, and these females were the only individuals present with blood on their mouths. (iii) “Suspected” infanticides: The sudden disappearance of an apparently healthy and well-nourished cub during a period of social instability (see below) among high-ranking females.
We defined fatal maternal neglect as cases of cubs that were visibly extremely undernourished prior to their disappearance, with subsequent confirmation that the mother had survived, so litter death was not caused by the death of the mother. It takes on average 3 weeks for cubs devoid of any nutrition to starve to death following the disappearance of their mother (Hofer and East, unpublished data). Cubs markedly changed in their behavior during the last few days prior to death, when they became listless and lethargic, and frequently called for their mother (whoop, East and Hofer, 1991). Litters recorded as dying of fatal maternal neglect did not show clinical signs associated with pathogen infection such as labored breathing, discharge from eyes or nose, neurological disorders and lack of response to social stimulation (East et al., 2008; Marescot et al., 2018). Undernourished members of a twin litter were only classified as having died from maternal neglect if the high intensity of sibling aggression and the substantial difference in body size associated with facultative siblicide was not observed (Hofer and East, 1997, 2008). Cases of facultative siblicide (Golla et al., 1999; Hofer and East, 2008) were not included in either category of infanticide and are not considered further in this study. It is possible that the cubs we classified as dying from maternal neglect could have disappeared because of an unobserved violent attack by other females, in which case our records of neglect are a maximum estimates for fatal maternal neglect.
Our definition of fatal maternal neglect is a functional definition in that it solely focuses on the question whether the mother substantially contributed to the death of the cub because the mother did not nurse its offspring sufficiently frequently to prevent death by starvation. Maternal den attendance is an index of the frequency at which cubs receive milk (Hofer et al., 2016). To indicate the low rate of den attendance by females whose cubs died of neglect, we compared maternal den attendance in the 4 weeks prior to offspring death for cubs which died from fatal maternal neglect with that for cubs which died from violent attacks. The median proportion of observation periods when mothers of neglected cubs were at the den was 13.2% [mean 17.5%, 95% confidence limits (CL) 12.0%–23.0%, n = 38, with a median of 25.5 observation periods in these 4 weeks], or approximately 1 in 8 observation periods, equivalent to one visit to the communal den per 4 days. For mothers of victims of violent attacks the median proportion of observation periods when mothers were at the den was 24.0% [mean 26.3%, 95% CL 20.5%–32.1%, n = 49, with a median of 22 observation periods in these 4 weeks], approximately 1 in 4 observation periods or a visit to the communal den every 2 days, a significant difference (Mann–Whitney U-test, U = 673, nneglect = 38, nviolent = 49, p = 0.027).
In all three main study clans, coalitions of females regularly challenged the reigning alpha female and her coalition partners, typically daughters, and when successful, took over the top ranks (“coups”). During such periods of social unrest, serious fights were observed between participating females, much more intense than during normal dominance interactions, causing injuries (some severe) to the head, neck, shoulders and feet of participants (Figure 1). We termed these “periods of social instability,” and the remaining time “periods of social stability.” There were a total of 58 periods of social instability with coups or attempted coups. These periods occurred regularly, although not frequently, in all three study clans. Although these periods involved a decisive and typically short period of aggressive fighting between two opposing coalitions when the outcome of the challenge is decided, the build-up and the after-effects are not necessarily limited to that particular day but may take longer, as females return from commuting trips to a new social order. We therefore defined narrow and broad time periods of social instability as ± 2 days and ± 30 days around the date of decision. If a second coup or coup attempt followed soon after a previous coup and fell within the definition of broad time periods around the previous coup event, then both coups were amalgamated to a single period of instability. This amalgamation is responsible for smaller sample sizes for the broad definition periods. In order to test the prediction that periods of social instability and the associated increase in aggression elevate the likelihood of infanticide, we compiled annual mortality rates of offspring for both narrow and broad definitions of periods of social instability and the intervening periods of stability.

Figure 1. Damaging physical contests between competing high-ranking female coalitions during a period of social instability (coup). Photo: MLE and HH.
Genetic samples (gut epithelial layer from feces, feces or tissue) were obtained from hyenas and genotyped using up to nine microsatellite loci for microsatellite DNA profiling as previously described (East et al., 2003, 2009; Wilhelm et al., 2003; Höner et al., 2007). Maternity and paternity were determined using maximum likelihood methods as implemented in CERVUS (Marshall et al., 1998; Kalinowski et al., 2007). Adopted cubs were therefore correctly allocated, in terms of mate-choice to their genetic mother. Candidate fathers included reproductively active natal males and immigrant males throughout the duration of their breeding tenure in a clan plus a 90 day period before and after the start and end of their tenure. Maternity and paternity were accepted if they exceeded the threshold for a 95% assignment. This resulted in the successful assignment of fathers (in addition to mothers) for 1,245 cubs. The mean expected heterozygosity was 0.8514, mean polymorphic information content (PIC) was 0.8346, combined non-exclusion probability for a parental pair was 4.119 × 10–9, mean proportion of alleles typed was 0.9775 and the error rate, set at 1%, was 0.008.
We used non-parametric tests, in some cases with exact P values approximated by 10,000 Monte Carlo simulations, following the procedures recommended by Conover (1999) and Hollander et al. (2014). We applied the Mann–Whitney U-test to compare two independent groups. For the comparison of annual mortality rates we used the permutation test with general scores for paired samples, as this is the most powerful test for paired samples, to investigate differences between adjacent (paired) periods of social stability preceding periods of social instability.
Records of the tenures of immigrant males were complete provided they were observed to enter the study clan and had completed their tenure by either dying or dispersing to another clan before the end of the study period. The tenure of natal breeding males started on the date they were first observed exhibiting behaviors typical of immigrant males toward females in their clan and their tenures were complete when they died or dispersed to another clan. Some males were already members of the clan at the beginning of the study, or were members of the study clans and still alive at the end of the study. Their recorded tenures are therefore minimum estimates and constitute right-censored data (Kalbfleisch and Prentice, 1980). Males present at the beginning of the study were excluded from those comparisons when the exact length of tenure on the conception date of a victim was required in an analysis. In order to compare tenures between groups of males, we conducted a survival analysis and calculated non-parametric survivorship functions as Kaplan–Meier estimates, which incorporated right-censored data. We compared the tenures of males who lost cubs from violent attacks with males who did not with the non-parametric generalized Wilcoxon test known as Breslow–Gehan (or Gehan–Breslow) test.
Estimates are given as means with their 95% confidence limits (CL) unless otherwise stated, and probabilities are for two-tailed tests. Statistical analyses were performed with SYSTAT 13.0 (Systat Inc., San Jose, CA, United States) and StatXact 11.0 (Cytel Inc., Cambridge, MA, United States). Figures 2, 3 were plotted in R 4.1.2 (R Core Team., 2021) using the packages ggplot2 version 3.3.5 (Wickham, 2016) and ggprism version 1.0.3 (Dawson, 2021).
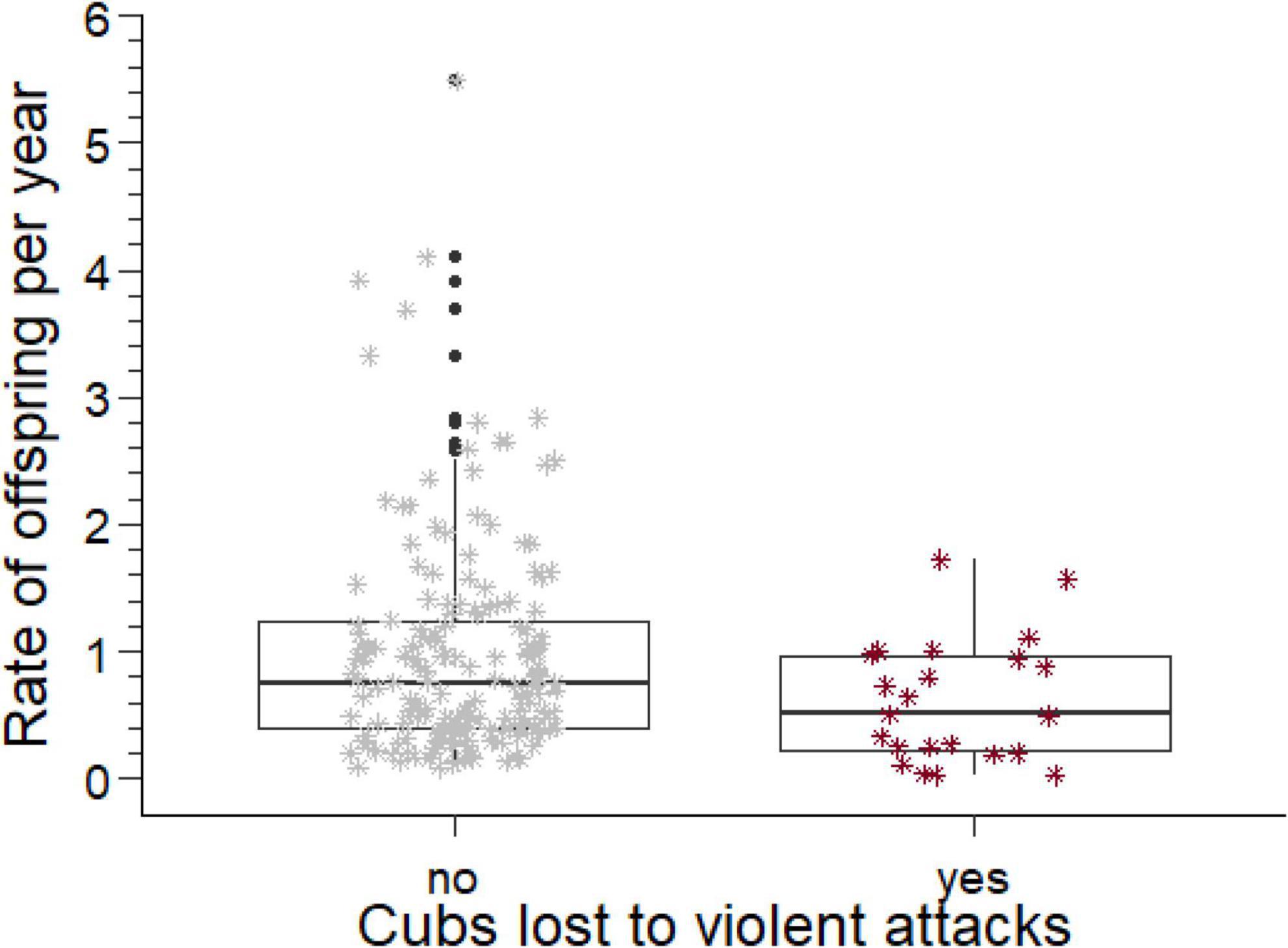
Figure 2. Rates of offspring produced per year by males who did and did not lose cubs to violent attack, adjusted for losses of cubs to the estimated rate of violent attack. The box comprises the interquartile range, the horizontal line is the median, the whiskers cover the range of values 1.5 times the value of the interquartile range, the asterisks indicate data points in the range between 1.5 and 3 times the value of the interquartile range, and open circles values beyond 3 times the value of the interquartile range.
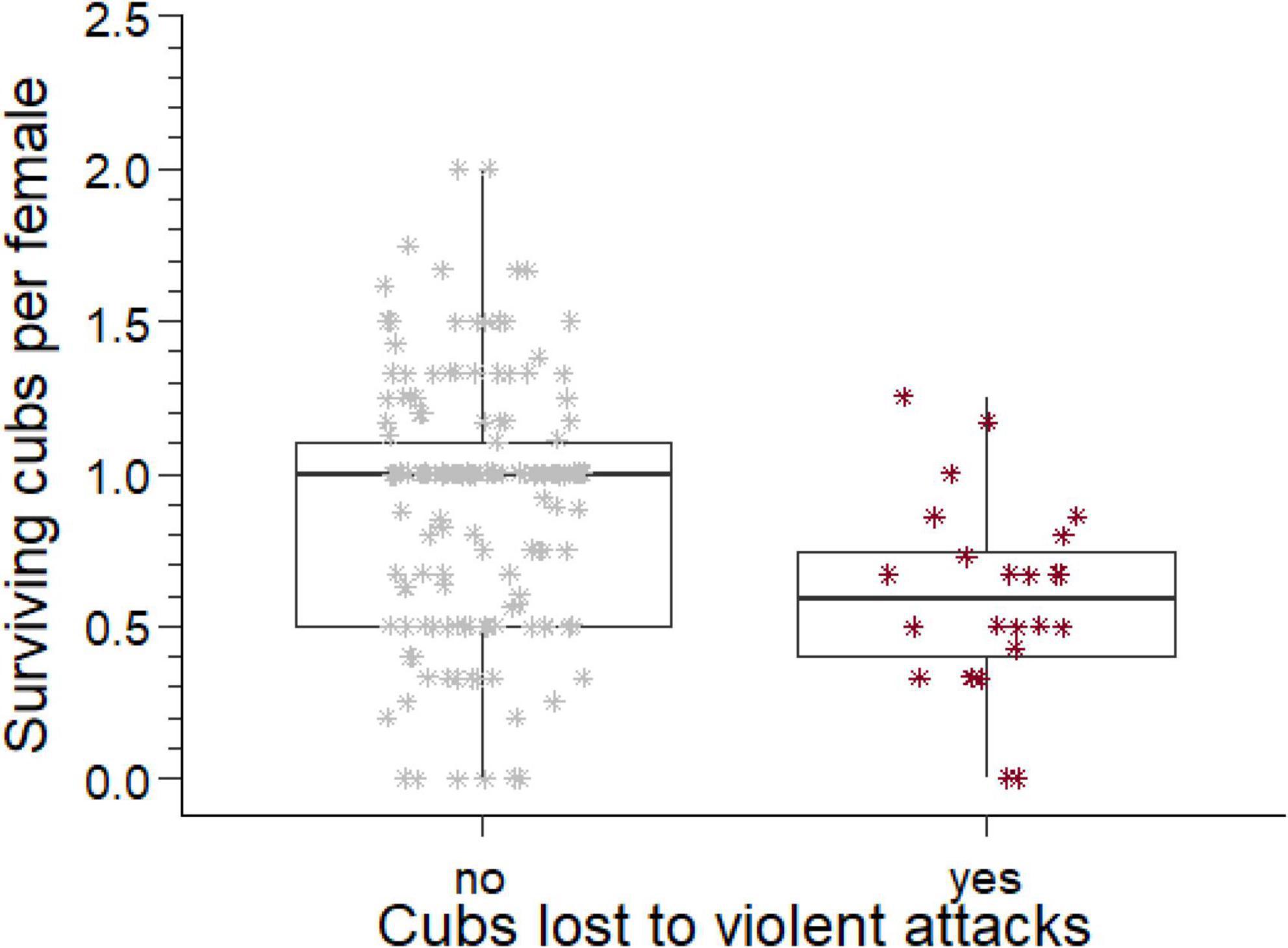
Figure 3. The number of cubs produced per mother by males who lost cubs to violent attack and those who did not. The box comprises the interquartile range, the horizontal line is the median, the whiskers cover the range of values 1.5 times the value of the interquartile range.
Of the 1,346 offspring which died in the Isiaka, Mamba and Pool clans before reaching adulthood (at the age of 24 months), 54 died from violent attacks, 43 died from neglect by their surviving mothers, 345 died from other known sources of mortality (e.g., disease, predation, traffic accidents), and 904 died of unknown causes. The overall incidence of violent attacks and fatal maternal neglect in all offspring, including deaths from unknown causes, was therefore 4.0% and 3.2%, respectively. Within the 442 offspring with known sources of mortality, the incidence of violent attacks was 12.2%, and for death from fatal maternal neglect it was 9.8%.
As predicted, annual rates of overall offspring mortality were substantially, and significantly, higher during periods of social instability than during periods of social stability (Table 1). These increases were mostly driven by cases of violent attacks, as demonstrated by their high incidence amongst known sources of mortality (Table 1), for both narrow and broad definitions of periods of instability.
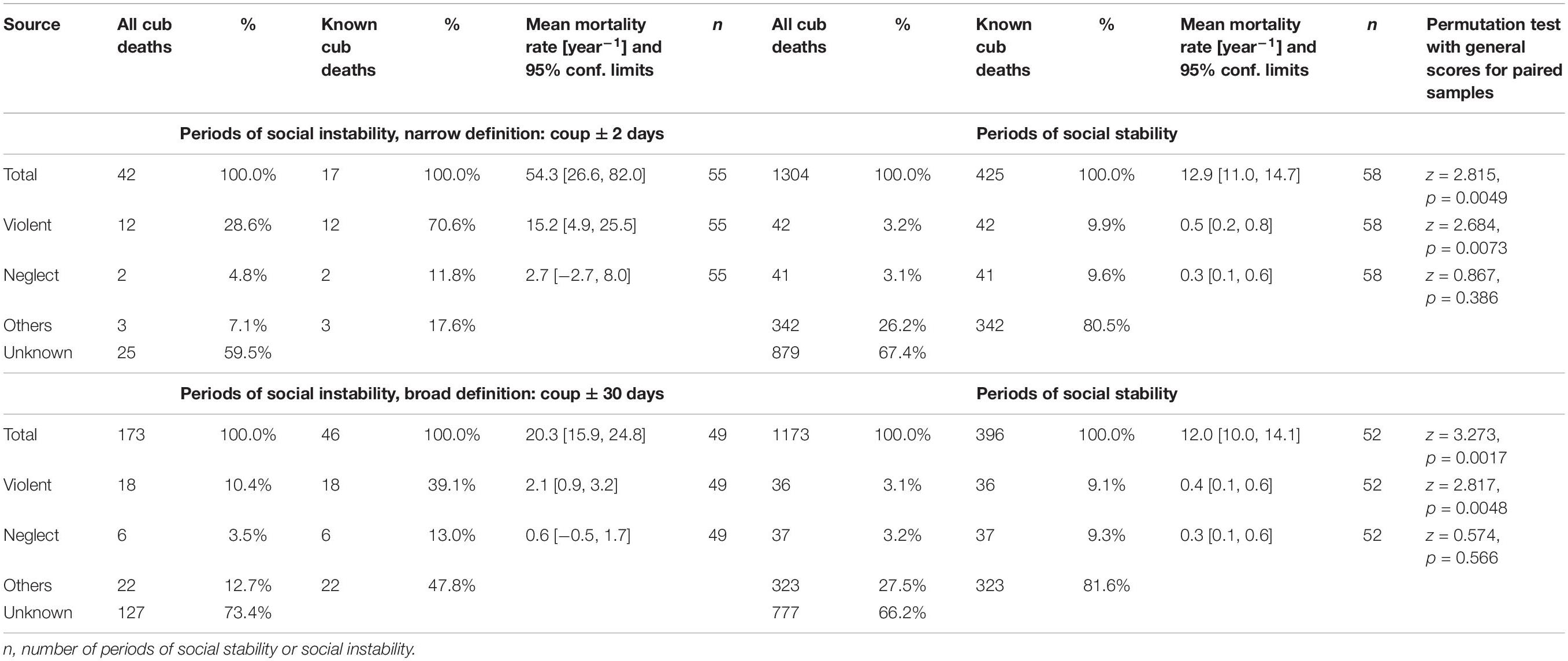
Table 1. Number of deaths, incidences and rates of annual mortality for all spotted hyena offspring (Total) which died before reaching adulthood, as well as for those experiencing violent attacks (Violent) and infanticide by fatal maternal neglect (Neglect), during periods of social instability (coups) and stability in the three main study clans.
The mean age of the 54 cubs in the main study clans when killed by violent attacks by females was 115.6 days (95% CL 90.3–140.9 days). Most cubs were killed by violent attacks during periods when prey abundance was low (n = 25 cases) or medium (n = 25 cases), few violent attacks occurred when prey was abundant (n = 4 cases). There were 43 cases of fatal maternal neglect in the three main study clans. The mean age of cubs when killed by maternal neglect was 151.5 days (95% CL 116.2–186.8 days). Most cubs killed by fatal maternal neglect occurred in periods when prey abundance was low (n = 21 cases) or medium (n = 16 cases), few cubs were killed by maternal neglect when prey was abundant (n = 3 cases).
In total, 56 violent attacks were recorded in five clans, including the main study clans (Isiaka, Mamba, Pools), and one attack each in the Songore and Campsite clans (Hofer and East, 1993a). These included: (i) 24 “observed” cases when an adult female in a clan was witnessed (by a member of the project) to lethally damage the cub of another female member of the clan. In 23 cases this involved crushing bites to the head, including one case when a mother grabbed her cub when she was aggressively harassed by a coalition of females, and the mother (probably accidentally) crushed its skull. (ii) 20 “likely” cases when a cub’s death was judged to be caused by a violent attack by a conspecific because of the occurrence of a crushed skull and the absence of the deep canine puncture wounds typical of predation by lions (Panthera leo) or leopards (Panthera pardus). (iii) 12 “suspected” cases of healthy cubs of high-ranking females that disappeared unexpectedly either during or shortly after (within 2 days) of a coup or a failed coup that involved intense aggression between competing female coalitions. For the 32 cases, for which we knew (24) or suspected (8) the identity of the killer, the perpetrators were adult females of the same clan in 30 cases. One case involved an immigrant male killing a starving, moribund cub, and one case was a subadult male offspring (of a high-ranking mother) which killed and consumed an offspring of a lower-ranking female.
Does infanticide by females reduce offspring production rates of breeding males and thereby generate sexual conflict? During the study period, there were 372 breeding males (immigrants and reproductively active natal males) in the three study clans, of which 281 males (75.5%) were genetically profiled, and 197 (70.1%) sired at least one cub. On average these 197 males sired a mean of 6.3 cubs (95% CL 5.5–7.2) during a mean tenure of 7.7 years (95% CL 6.8–7.8), and on average 3.7 of their offspring survived to adulthood (95% CL 3.2–4.3).
Considering the 197 males with at least one genetically typed cub, these males lost on average 0.16 offspring to violent attacks (95% CL 0.09–0.23) and 0.08 to maternal neglect (95% CL 0.03–0.13). These data originate from 31 of the recorded 54 cubs experiencing violent attacks, and 15 of the 43 cases of fatal maternal neglect for which we obtained genetic samples and identified the father with 95% confidence. As we did not establish the genetic fathers of all cubs lost to violent attacks and maternal neglect, our calculated losses are minimum estimates that underestimate the true losses. An estimate of the true loss (“adjusted”) for the situation when the identities of all fathers would be known for the cubs experiencing violent attacks or maternal neglect is the recorded loss multiplied by the total number of cases divided by the genetically typed cases. For cubs lost to violent attacks this is 0.16 × 54/31, equal to 0.28 cubs lost to violent attacks, and for cubs lost to maternal neglect this is 0.08 × 43/15, equal to 0.23 cubs lost to maternal neglect.
As predicted, violent attacks reduced male reproductive success more than fatal maternal neglect. The recorded losses of offspring are equivalent to an average of 3.3% (95% CL 1.6%–4.9%) of offspring deaths to violent attacks and 1.2% (95% CL 0.4%–2.0%) to maternal neglect. Adjusted for the incomplete identification of fathers of offspring that died from violent attacks and maternal neglect, these losses for each male on average amount to 5.7% (95% CL 2.9%–8.5%) of offspring deaths from violent attacks, to 3.5% (95% CL 1.2%–5.9%) to fatal maternal neglect and the combined value for violent attacks and fatal maternal neglect to 9.2% (95% CL 5.6–12.8%).
The annual rates at which breeding males sired offspring, their sired offspring reached adulthood, the rates at which their offspring were killed by violent attacks or died from fatal maternal neglect, and the rate of loss when both causes of paternity loss were combined are summarized in Table 2. If losses caused by violent attacks or fatal maternal neglect were sufficiently high to affect the rate of offspring production by males, then males with and without such losses should differ in the annual rate of offspring production after the losses had been subtracted from the overall rate of production. We therefore removed the adjusted (see above) rates of losses from the total rate of offspring production and then compared annual rates of offspring production between males with and without losses to violent attack or fatal maternal neglect. These offspring production rates (Table 2) were significantly lower (Figure 2) in males with losses from violent attacks than in males without (Mann–Whitney U test, U = 2,717, ninfanticide = 24, nno infanticide = 173, p = 0.014, Table 2), and also lower in males with losses from fatal maternal neglect than in males without (U = 1,600, ninfanticide = 11, nno infanticide = 186, p = 0.002, Table 2).
Considering all males for which we have complete reproductive tenures (n = 265), 135 males produced at least one offspring. For these males, we collated the total number of offspring they sired, the number of different females they sired offspring with, and the total number of offspring that survived to adulthood. The ratio of the number of offspring that survived to adulthood divided by the number of females each male sired offspring with is an indicator of the reproductive success of each male per female partner. For males with losses to violent attacks this reproductive success was approximately half of the reproductive success for males who did not suffer such losses (U = 2,871, ninfanticide = 24, nno infanticide = 173, p = 0.002, Figure 3).
We predicted that the fitness cost of infanticide by females to fathers depended on the social status of the mothers of victims. To assess whether offspring of high-ranking females were particularly vulnerable to violent attacks, we compared the mean standardized ranks and age of mothers of cubs killed in violent attacks with those lost to maternal neglect. On the day of conception for cubs killed in violent attacks, the mean standardized rank of their mothers was + 0.25 (95% CL 0.08–0.42), and for cubs lost to maternal neglect, it was −0.17 (95% CL −0.30 to −0.04). These standardized ranks differed significantly (Mann–Whitney U-test, U = 1581.5, nviolent = 54, nneglect = 43, p = 0.001).
On the day of conception of cubs lost to violent attacks, mean maternal age was 6.63 years (95% CL 5.72–7.53 years), and for cub lost to neglect, mean maternal age was 7.13 years (95% CL 6.10–8.18 years). These ages did not differ (U = 728.5, nviolent = 48, nneglect = 35, p = 0.30).
For secondary dispersal to be a potential counterstrategy by males, the chance of paternity loss to infanticide would need to increase with male tenure, as the longer a male stays in a clan, the chance that infanticide will kill one of his offspring is expected to increase. This was indeed the case for males who had lost cubs to violent attacks by females. Their mean tenure was 10.7 years (survival analysis, Kaplan–Meier estimate, n = 24, 95% CL 9.1–12.2 years), significantly higher than the mean tenure of 8.3 years of males who had not (n = 173, 95% CL 7.6–9.1 years, Breslow-Gehan test, χ2 = 5.66, df = 1, p = 0.017). For males who had lost cubs to fatal maternal neglect, mean tenure was 11.0 years (survival analysis, Kaplan–Meier estimate, n = 11, 95% CL 8.6–13.4 years), which tended to be higher than the mean tenure of 8.4 years of males who had not (n = 186, 95% CL 7.7–9.1 years, Breslow-Gehan test, χ2 = 2.08, df = 1, p = 0.15). However, breeding males lost offspring they had sired to infanticide (violent attack and fatal maternal neglect) by adult females regardless of the duration of breeding tenure and dominance status on the date of conception (Figure 4). Violent attacks affected 24 of the 197 males (12.2%), fatal maternal neglect affected 11 (5.6%) of these males. There were 32 males affected by either of these causes of lost paternity (16.2%) and three by both (1.5%). Amongst these 197 males, total tenure was complete for 135 individuals (mean 6.9 years, 95% CL 6.2–7.5 years), for 62 males, their tenure was ongoing at the beginning or the end of the study, with a mean of 8.3 years (95% CL 7.5–9.1 years). There was no difference in the incidence of infanticide amongst males whose tenure was complete (11.9%) and those with an ongoing tenure (12.9%, likelihood ratio test, G = 0.044, non–going = 65, ncomplete = 135, p = 0.835). Total tenures of males that lost cubs to either violent attacks or fatal maternal neglect were approximately 2.5 years longer than tenures of males who had not.
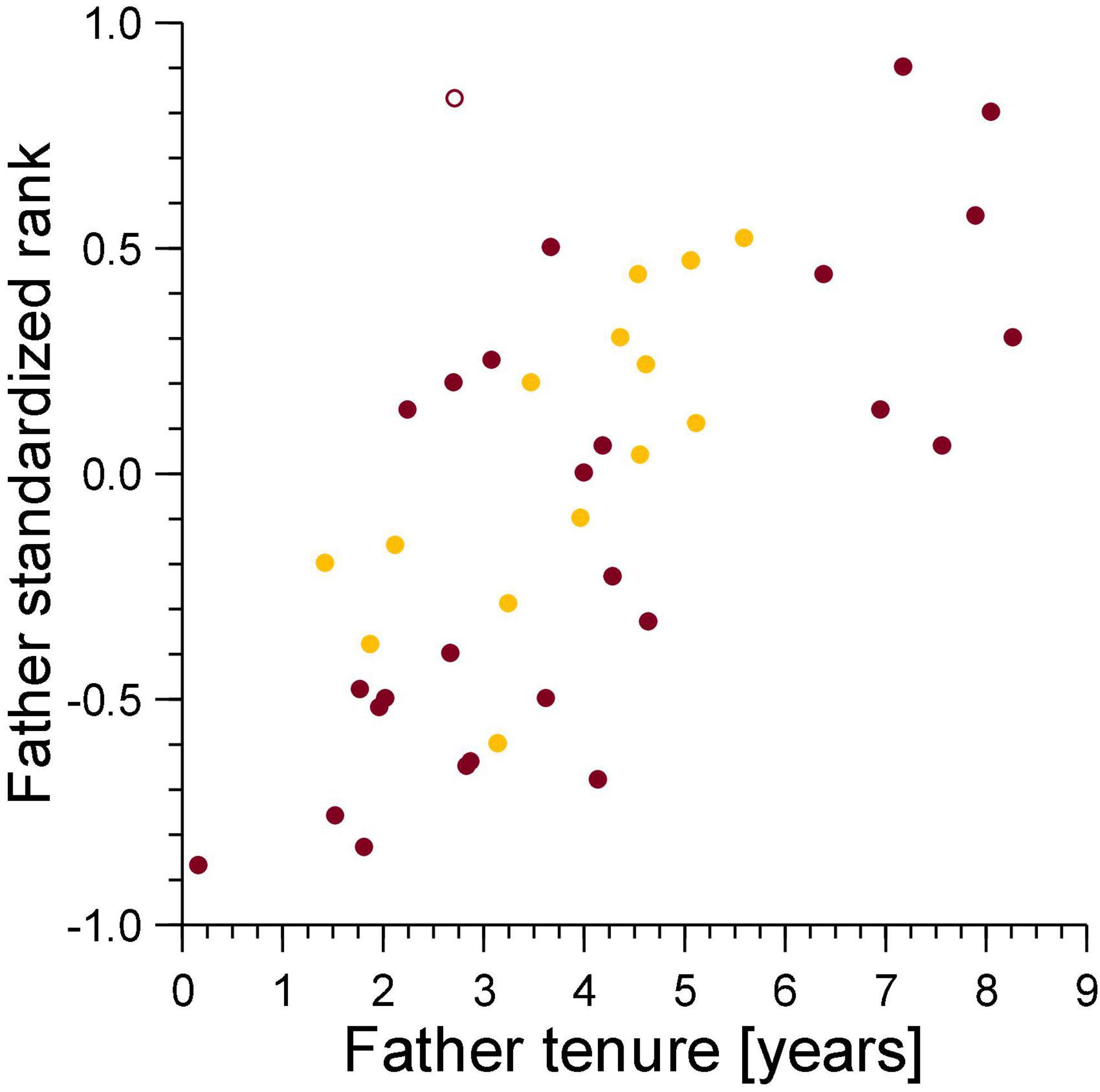
Figure 4. Male standardized rank in relation to breeding tenure for males that lost cubs to violent attacks (burgundy) and maternal neglect (amber) on the day of the conception of the victim. Filled symbol: immigrant males, open symbol: natal male.
Do males attempt to reduce their chance of losing offspring to infanticide through siring cubs with a particularly large or small number of different females (bet-hedging)? Males who lost cubs to violent attacks sired offspring with a similar number of different females (mean number 0.64 year–1, 95% CL 0.49–0.78 year–1) as those males who did not lose cubs to violent attacks (mean number 0.69 year–1, 95% CL 0.60–0.79 year–1, U = 1,928, ninfanticide = 24, nno infanticide = 173, p = 0.57). Similarly, males who lost cubs to fatal maternal neglect sired offspring with a similar number of different females (mean number 0.55 year–1, 95% CL 0.44 –0.66 year–1) as those who did not (mean number 0.69 year–1, 95% CL 0.60–0.78 year–1, U = 1,011, nneglect = 11, nno neglect = 186, p = 0.95). These results suggest that a male’s chance of losing paternity to violent attacks or fatal maternal neglect is not affected by the number of different females it sired offspring with.
We predicted that the genetic parents of the victims of infanticide should conform to expected female mate-choice patterns to avoid inbreeding, as we expected mothers of infanticide victims to have selected males that immigrated into their clan after they were born. Most females (78.4%, n = 37) applied this mate choice rule (Figure 5, points to the right of the line), and this was the case for both mothers that lost a cub to violent attack (79.2%, n = 24) and fatal maternal neglect (78.6%, n = 11, Figure 5). The tenure of fathers on the conception date of their cub which died from fatal maternal neglect grew with maternal age at the conception date of the victim (ρ = 0.615, n = 13, p < 0.05, Figure 5) as expected from female mate-choice based on male tenure.
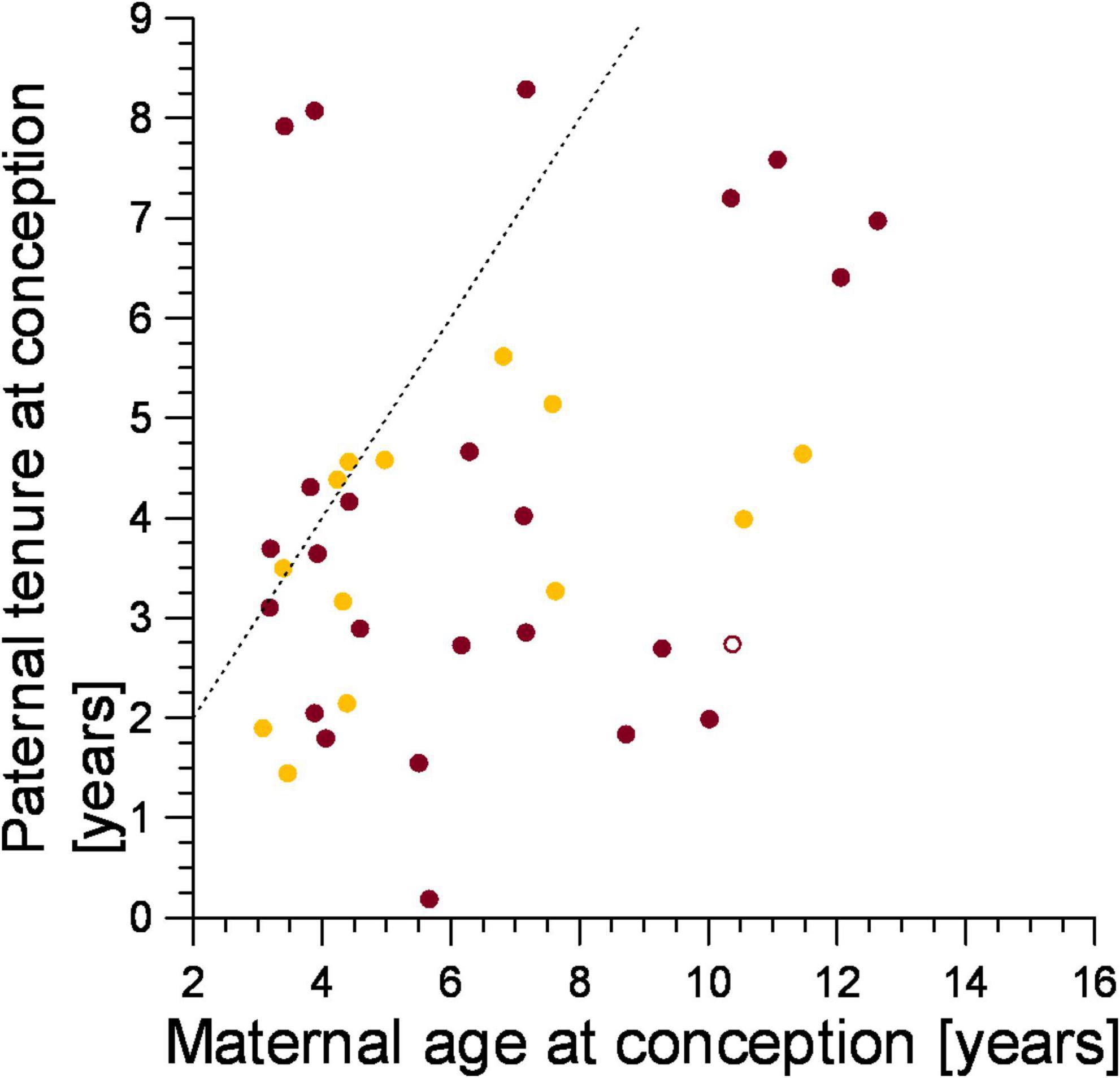
Figure 5. The tenure of fathers in relation to the age of mothers on the date they conceived an offspring that was later a victim of either infanticide by violent attack (burgundy) or maternal neglect (amber). Filled symbol: immigrant males, open symbol: natal male. Points to the right of the broken line represent males which immigrated into the clan after the female was born, those to the left of the broken line immigrated before the female was born.
The tenure of fathers on the conception date of their cub which died from a violent attack was unrelated to the age of the female perpetrator of the violent attack (Spearman’s correlation coefficient, ρ = 0.066, n = 15, Figure 6), thus adult female perpetrators, regardless of age, generated sexual conflict with breeding males.
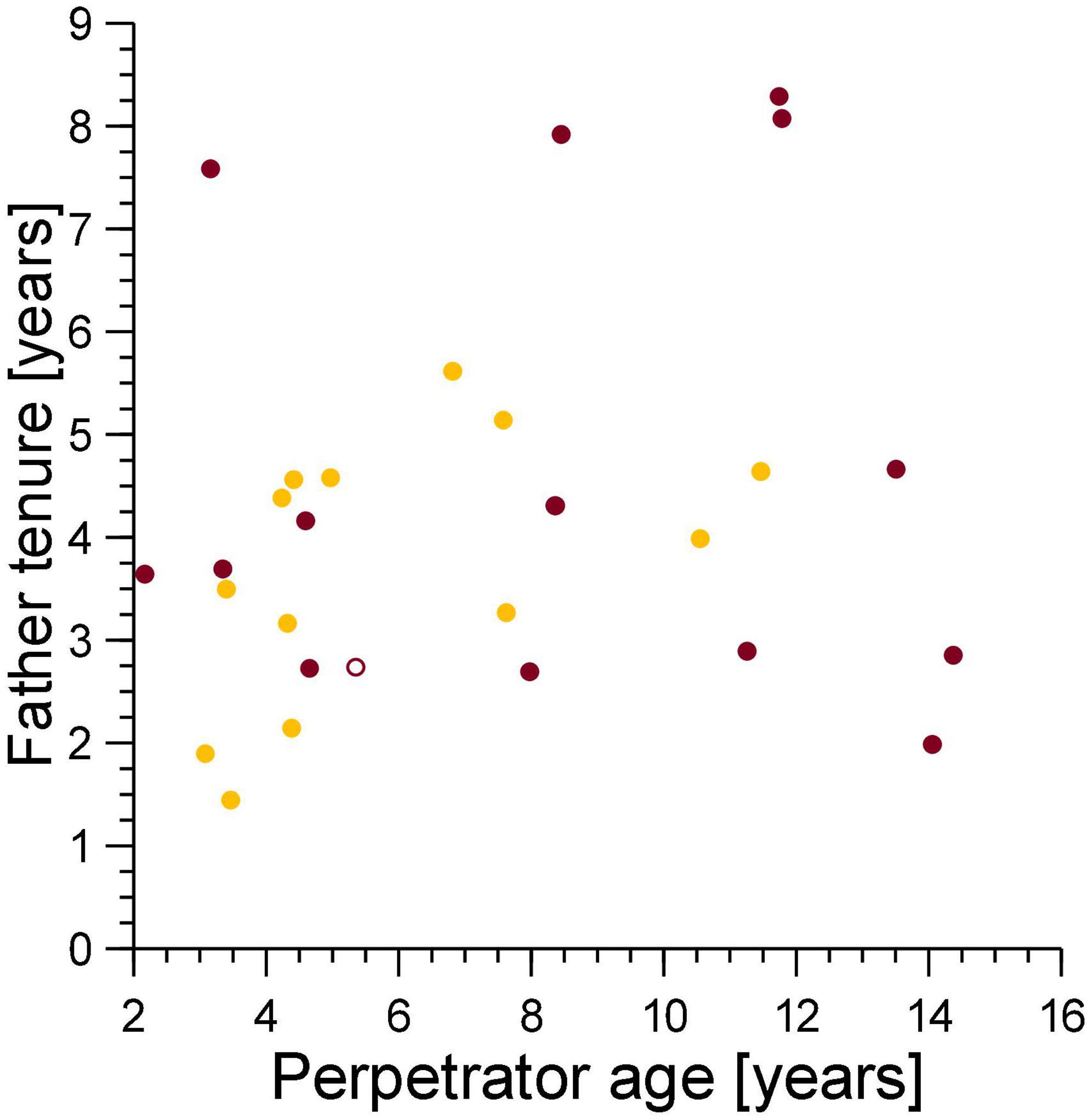
Figure 6. The tenure of the fathers of cubs lost to violent attacks (burgundy) and maternal neglect (amber) on the date when the cub was conceived in relation to the age of the female perpetrator of the violence on the date of cub death (burgundy) or the age of the mother at conception of the victim if the victim died from fatal maternal neglect (amber). Filled symbol: immigrant males, open symbol: natal male.
There was no relationship between the standardized rank of the genetic mother in the female dominance hierarchy and that of the genetic father in the breeding male dominance hierarchy on the date of conception for cubs lost to violent attacks (ρ = 0.14, n = 31, NS, Figure 7), and those lost to fatal maternal neglect (ρ = −0.19, n = 15, NS, Figure 7). This indicates that sexual conflict generated by infanticide by adult females is not higher for breeding males above median rank, as would be expected in species where paternity of offspring in a social group is skewed toward dominant male(s).
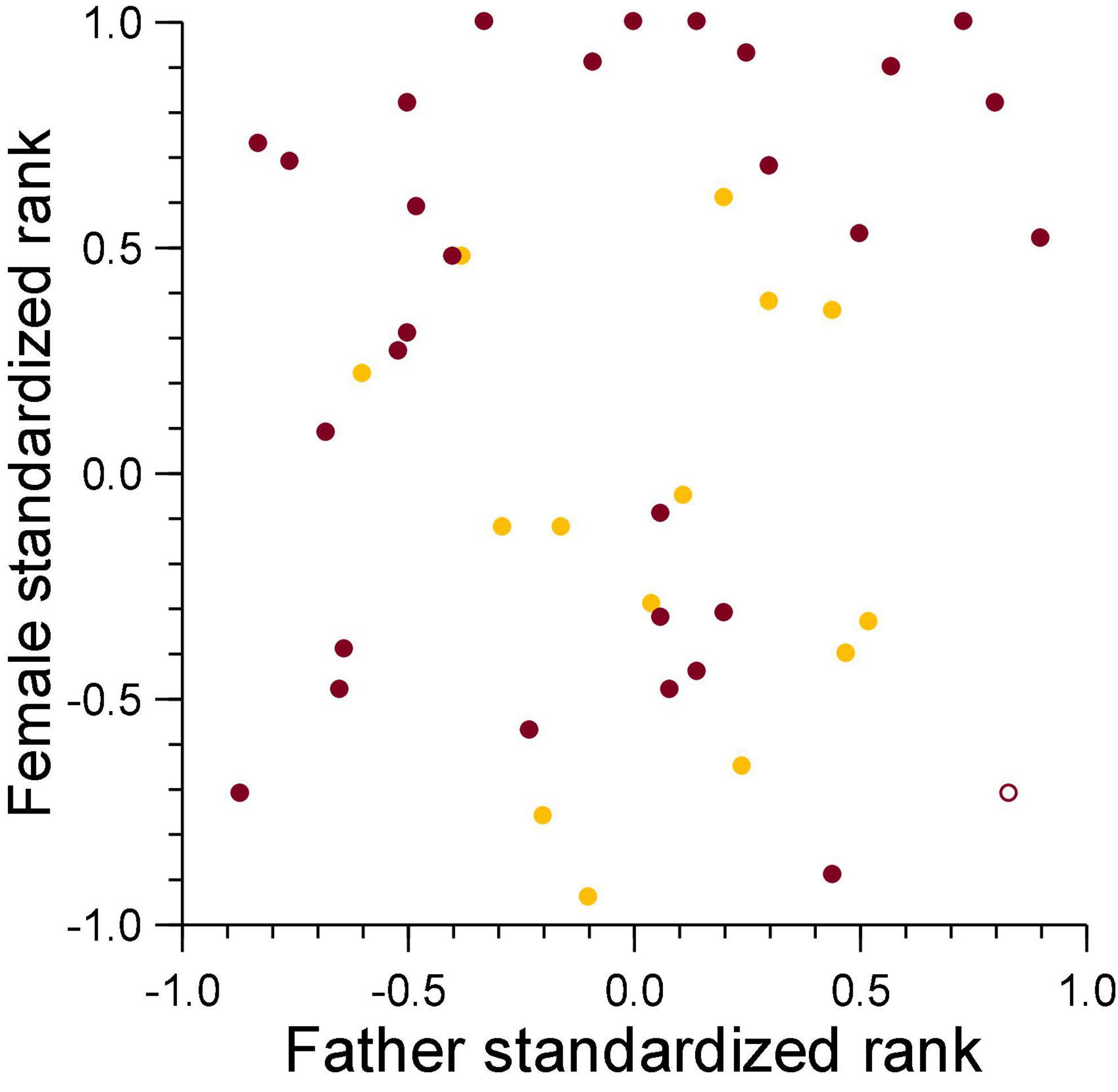
Figure 7. The standardized rank of mothers of cubs lost to violent attacks (burgundy) and maternal neglect (amber) in relation to the standardized rank of their fathers at conception of the victim. Filled symbol: immigrant males, open symbol: natal male.
Finally, we asked whether victims of infanticide by violent attacks and the dependent offspring of the perpetrator shared the same genetic father. For 32 infanticides by violent attack for which we knew or suspected the identity of the female perpetrator, we identified in 15 cases the genetic father of both the victim of the attack and the genetic father of the dependent offspring of the perpetrator when it killed the victim. There was not a single case in which the victim of infanticide and the offspring of the female perpetrator shared a father.
Despite substantial research on within-group infanticide in social mammals, to our knowledge this is the first study to gauge the reproductive cost of infanticide by adult females to breeding males in their group. Based on more than 30 years of data, our study provides evidence that infanticide by adult females in spotted hyena clans significantly reduced the reproductive success of breeding males whose offspring died of violent attacks or fatal maternal neglect. Because our recorded paternity losses incurred by males for both these sources of infanticide are probably minimum estimates, actual losses from infanticide are likely to be substantially larger. Both these sources of infanticide generated sexual conflict between breeding males whose reproductive success was reduced and adult females that comitted infanticide. We show that violent attacks on cubs by adult females during periods of social instability (aggressive conflict amongst competing females at the top of the female dominance hierarchy, Figure 1), have a larger negative impact on the reproductive success of affected males, thus generate a higher level of sexual conflict than cub deaths caused by fatal maternal neglect (Tables 1, 2). We found little evidence that breeding males have effective counterstrategies against infanticide by females. This suggests that breeding males are mostly powerless in preventing the detrimental fitness outcome of infanticide by females in their clan.
Research on infanticide in large wild mammals, such as the spotted hyena, is challenging because observations of violent infanticide are rare, attacks are swift, and evidence of infanticide, including material for genotyping the victim, may be swiftly consumed. For these reasons, our results on the fitness cost to fathers of violent attacks by adult females on the cubs they sired are minimum estimates, and the true effects on the fitness of fathers are likely to be substantially higher. A long-term study on infanticide in a population of spotted hyenas in Kenya also concluded that observed incidences of infanticide are likely to underestimate the true occurrence of this behavior (Brown et al., 2021).
Our results indicate that violent infanticide accounted for the death of between 3.3–5.7% of cubs born during our study. We predicted that periods of social instability in our three study clans would be associated with an increase in the incidence of violent infanticides. Our results are consistent with this prediction as periods of social instability were associated with a higher rate of offspring mortality (Table 1) than periods of social stability, and this increase was driven by the records of observed and suspected infanticides. Even during these short periods of social instability, there was a substantial proportion of offspring mortality for which the cause was unknown, which may have included undetected cases of infanticide.
As predicted, violent attacks by adult females represented a larger reduction of the rate at which breeding males produced offspring than fatal maternal neglect (Table 2). This suggests that violent attacks are a more important source of sexual conflict between adult female perpetrators and the genetic fathers of killed cubs than fatal maternal neglect. Fathers that lost cubs to violent attacks by adult females produced fewer offspring that survived to adulthood than those who did not (Figure 2). Thus violent attacks on cubs by females reduced fitness of affected breeding males, and was a source of sexual conflict between infanticidal females and affected breeding males. Infanticides by violent attack were perpetrated by adult females from a wide range of ages and the genetic fathers of their victims held widely different breeding tenures (Figure 6).
Violent attacks mostly killed cubs sired with higher-ranking females (Figure 7). As offspring of mothers above median rank had a higher survival to adulthood than those with mothers below median rank (Holekamp et al., 1996; Hofer and East, 2003; Höner et al., 2010; Ferreira et al., 2019), losses of offspring sired with high-ranking females may be compensated to some degree by the higher likelihood of survival to adulthood of other offspring sired with higher-ranking than lower-ranking females.
Female dominance hierarchies within spotted hyena clans can be stable for long periods, but periods of social instability do occur in our study population (Goymann et al., 2001) and we identified numerous periods of social instability in our three main study clans during the study (58 periods in total). As predicted, periods of social unrest were associated with intense fights between high-ranking females in competing coalitions (Figure 1) and increased cub losses to violent attacks by adult females (Table 1). These attacks mostly involved offspring sired by breeding males below or around median rank with high-ranking mothers.
The fission-fusion structure of clans is accentuated in our study population by long-distance foraging trips outside the clan territory. Low-ranking females meet the high energetic costs of lactation by allocating a large proportion of their protracted nursing period to commuting long distances between the clan communal den and distant locations containing large numbers of migratory ungulates (Hofer and East, 1993c; Gicquel et al., 2022). As commuting results in lengthy maternal absence intervals from milk-dependent cubs (Hofer and East, 1993c) and a reduced milk intake by cubs (Hofer et al., 2016), we expected males that sire offspring with low-ranking females should be more likely to lose paternity to maternal neglect. Evidence that long-distance commuting by lactating low-ranking females might undermine maternal body condition and reserves, thereby increasing the chance that a female might abandon a litter, is supported by evidence of significantly higher intestinal parasite burdens in low-ranking than high-ranking lactating females (East et al., 2015). As expected, infanticide by fatal maternal neglect resulted in the death of more offspring sired with lower-ranking than higher-ranking females, and we have no evidence that females at the top of the hierarchy (those above standardized ranks + 0.7) committed infanticide by fatal maternal neglect (Figure 7).
We predicted that strong female mate-choice would constrain any pattern of male mate-choice that might reduce paternity losses to infanticide. We expected females to avoid the fitness cost associated with inbreeding with close male relatives by choosing sires that started their reproductive tenure after the female was born (Höner et al., 2007). In line with this, most mothers of victims of infanticide (by violent attack and fatal maternal neglect) had selected a sire that adhered to the expected pattern of female mate-choice (Figure 5, points to the right of the dotted line). From this female mate-choice rule, we expected a positive association between female perpetrator age on the date of cub death due to fatal maternal neglect and the tenure of the genetic fathers, as was the case. We would not expect an association between the age of female perpetrators of violent attacks on the date of infanticide and the tenure of the fathers of the cubs they killed, and indeed there was no such association (Figure 6).
It is unlikely that young mothers are proficient in long-distance commuting trips to distant migratory herds when rearing their first few litters (East et al., 2015). As a result, the first few litters of low ranking-females may be particularly prone to infanticide by fatal maternal neglect, as young, inexperienced mothers have to fuel lactation for many months by long-distance foraging that can involve regular 140 km round trips (Hofer and East, 1993b). Consistent with this suggestion, most infanticides by fatal maternal neglect involved younger rather than older mothers (Figure 6). This suggests that by perpetrating infanticide by fatal maternal neglect, inexperienced breeding females caused sexual conflict with relatively short tenured, mostly low-ranking males. By contrast, and regardless of their age, adult females of a wide range of ages generated conflict with males that differed widely in their length of tenure (and hence social status) by perpetrating infanticides through violent attacks (Figure 6).
Although various counterstrategies against violent infanticide have been reported in mammals, we predicted that few if any would be implemented by male spotted hyenas because of female social dominance, strong female mate-choice and prolonged offspring dependence on lactation and the negligible contribution by fathers to rearing offspring.
The defense of offspring from violent infanticide, by counterattacking perpetrators and by forming alliances with other individuals to increase protection of offspring, has been reported in several species (Packer and Pusey, 1983; Agrell et al., 1998; Fruteau et al., 2010; Palombit, 2015). Our long-term observations provided no evidence of male spotted hyenas forming alliances to prevent violent infanticide or counterattacking adult females during or shortly after they perpetrated violent infanticide. There are several reasons why males would not be expected to do this. The fission-fusion structure of spotted hyena clans substantially reduces the chance that fathers would be present at the communal den to defend their offspring from a violent infanticide. In our long-distance commuting population, high-ranking breeding males are present at the communal den more often than other breeding males (East and Hofer, 1991), but even top-ranking males are much more frequently absent from dens than lactating females, which in turn can be absent for up to 9 days (Hofer and East, 1993c). Low-ranking males typically visit communal dens infrequently and only for short periods (East et al., 2013), thus their chance of being present at the communal den when violent infanticide of their offspring occurs is very low. High-ranking females aggressively harass, chase and even carry (by their necks) cubs of lower-ranking females at the communal den. Breeding males witness these acts, plus the attempts by mothers to assist their cubs, but we have never observed breeding males intervening, on their own or to support mothers, to protect cubs. When violent infanticides occur, the action of the perpetrator is typically swift, as perpetrators grab and crush the skulls of their victims, as also reported in other populations (Brown et al., 2021). Given that victims are mostly swiftly dispatched, an aggressive counterattack by a father would be very unlikely to prevent the death of its offspring. Finally, during periods of social instability when conflict between females is intense, breeding males—including high-ranking ones—usually keep themselves away from the communal dens, in order to avoid being targeted by aggressive acts of females (East and Hofer, unpublished observations).
Dispersal is a counterstrategy typically used by females in some mammals to avoid violent infanticide (Pusey and Packer, 1994; Sterck and Korstjens, 2000; Zhao et al., 2011). We assessed whether strategic timing of secondary dispersal by breeding males reduces their chance of paternity loss to infanticide by adult females, as we expected the likelihood of such losses to increase with a male’s breeding tenure. As expected, males which lost offspring to violent attacks or to fatal maternal neglect had longer total tenures, by approximately 2.5 years, than those that did not. This difference was significant for males with losses to violent attacks but not for males with losses caused by fatal maternal neglect. In the latter cases, the lack of significance is probably best explained by the small sample size, thus the statistical power of the test was too small to demonstrate significance. In principle, our result are consistent with the idea that secondary dispersal to another clan at a shorter breeding tenure than that held by males who suffered losses is a potential counterstrategy, but it is not one that is guaranteed to provide fitness benefits.
This is because secondary dispersal entails additional costs to those incurred when males remain in their current clan. These include the costs of dispersal and integration into a new clan and the losses of relationships formed with adult females during the years of tenure in the former clan (East and Hofer, 2001; Szykman et al., 2001). These negative effects of secondary dispersal on male fitness may be greater than those of infanticide by females in the former clan. Additionally, access of immigrant males to food in their clan territory increases with social status (Frank, 1986), and as this is determined by the immigrant male social queue, access to food in the clan territory also increases with male tenure. By placing an immigrant male at the bottom rank in the dominance hierarchy, secondary dispersal would therefore result in reducing access to food in the current clan territory. In our long-distance commuting population, this would also require the male to spend a greater proportion of the year on long-distance commuting than in its previous clan, thereby reducing time spent in the territory of a new clan, building relationships with females and locating females in estrus. As males of a wide range of tenures experience reproductive losses from infanticide (Figure 6), it is doubtful that secondary dispersal substantially reduces uncertainty about the future prospect of losses from infanticide by females. It therefore does not appear to be an effective counterstrategy to reduce the fitness cost of infanticide by females.
A third counterstrategy could be bet-hedging, in terms of males siring cubs with numerous females to ensure that potential losses of paternity from infanticide are compensated by the survival of offspring sired with other females. Effective application of such a counterstrategy is probably constrained by strong female mate-choice (Engh et al., 2002; East et al., 2003; Höner et al., 2007). The extensive fission-fusion nature of clans, in particular the long-distance commuting by both adult females and breeding males that, limits the number of females in estrus which a breeding male is likely to encounter when it is present in the clan territory. As females apparently confuse paternity of their offspring by copulating with multiple males when in estrus and when not in estrus, and—as genetic paternity analyses reveal—only a limited number of male copulations that occur are detected (Engh et al., 2002; East et al., 2003), observed male copulations do not accurately reflect genetic paternity. For these reasons, a test of whether males that were observed to copulate with multiple females lose fewer offspring to infanticide or have overall more surviving offspring than males that do not is not possible in our study system.
Could males flexibly respond in terms of bet-hedging if they recognized their offspring and were aware that they died from infanticide? If males could recognize their offspring, spreading their risk through bet-hedging might make sense regardless of the cause of death, so this would not be specific to offspring lost to infanticide. As argued above, the fission-fusion nature of Serengeti clans and the commuting system would ensure that there is a low likelihood that males would witness their offspring’s death, regardless of the cause of death. Confusion of paternity (East et al., 2003) ensures that males cannot use their own copulation success to know which cubs at a communal den they have sired. Alternatively, could males directly recognize their own offspring? There is no behavioral evidence that males recognize the offspring they sire (Van Horn et al., 2004) and even if males could recognize their offspring and/or were aware that their offspring had suffered infanticide, we found no evidence that they sought to increase the number of females they sired offspring with. Rather, males sired offspring with similar numbers of females, regardless of whether they had or had not lost paternity to infanticide by females. This result suggests that bet-hedging to avoid losses from infanticide is unlikely to be important for any breeding male in our population.
A fourth strategy might be that males choose to or avoid siring cubs with specific females. Did they do so? In the cases when we knew both the genetic fathers of cubs that died from violent attacks and the identity of the female perpetrator, the victims were not related through the paternal line to the offspring of the perpetrators and the males which lost cubs did not sire offspring with the perpetrators before or after the losses from violent attacks. This might be the fortuitous outcome of the large group sizes of our study clans, with a large number of breeding females (between 15 and 50) and a similar number of males (Olarte-Castillo et al., 2016), so even in the absence of mate-choice and random mating, the likelihood of any one combination of mating partners is low. This result therefore does not provide evidence of active choice or avoidance of females by males.
Furthermore, the slow rate of reproduction of females caused by high post-natal maternal input throughout the long lactation period (Hofer et al., 2016) probably creates a skewed operational sex ratio in favor of breeding males to females in estrus. To sire offspring, males must successfully compete for mating opportunities in a system where females exercise strong mate-choice, hence male counterstrategies to infanticide are constrained by this framework. Given the relatively low cost to males of siring offspring because of their negligible contribution to the rearing of offspring (Table 2), it is doubtful that breeding males reject any opportunity of mating offered by a female, even if the prospects of the resulting offspring reaching adulthood is low, such as offspring sired with low-ranking, inexperienced females (Figures 6, 7).
Instead, the best option for males to maximize reproductive success, and thereby minimize the possible consequences of paternity losses from infanticide is probably to start their breeding tenure in the clan containing the largest number of females likely to accept them as a mate. This would be the clan with the largest number of young females, which on occasion might be their own natal clan. If males do so, they substantially increase their reproductive success (Höner et al., 2007), which may compensate to some extent potential reproductive losses to infanticide.
Our study reveals that infanticide by adult females reduces the fitness of affected breeding males and generates sexual conflict to which breeding males apparently have no effective counterstrategies. In this respect, female perpetrators exercise power to obtain an outcome that is thought to be advantageous to them and costly to affected breeding males. Currently, little is known about the fitness costs of infanticide by females to breeding males in other social mammals. We hope our study encourages further research on this topic.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was approved by the Committee for Ethics and Animal Welfare of the Leibniz Institute for Zoo and Wildlife Research (2018-11-01).
ME and HH designed the study and wrote the manuscript. ME, DT, SB, SM, and HH collected the data. ME, DT, and HH conducted the analysis. All authors edited the manuscript.
We would like to thank the Deutsche Forschungsgemeinschaft DFG (grants EA 5/3-1, KR 4266/2-1, DFG-Graduiertenkollegs 1121, 2046), the Leibniz-Institute for Zoo and Wildlife Research, the Leibniz-Gemeinschaft (grant SAW-2018-IZW-3-EpiRank), the Fritz-Thyssen-Stiftung, the Stifterverband der deutschen Wissenschaft and the Max-Planck-Gesellschaft for past and current financial assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to the Commission for Science and Technology of Tanzania (COSTECH), the Tanzania Wildlife Research Institute (TAWIRI) and Tanzania National Parks (TANAPA) for their support of our research. We would like to thank Annie Francis, Malvina Andris, Nelly Boyer, Traudi Golla, Katja Goller, Nicole Gusset-Burgener, Stephan Karl, Berit Kostka, Michelle Lindson, Agnes Türk and Kerstin Wilhelm for assistance, and the editor and two reviewers for very helpful and constructive comments.
Agrell, J., Wolff, J. O., and Ylönen, H. (1998). Counterstrategies to infanticide in mammals: costs and consequences. Oikos 83, 507–517. doi: 10.2307/3546678
Benhaiem, S., Hofer, H., Kramer-Schadt, S., Brunner, E., and East, M. L. (2012). Sibling rivalry: training effects, emergence of dominance and incomplete control. Proc. R. Soc. Lond. B 279, 3727–3735. doi: 10.1098/rspb.2012.0925
Blumstein, D. T. (2000). “The evolution of infanticide in rodents: a comparative analysis,” in Infanticide by Males and its Implications, eds C. P. van Schaik and C. H. Janson (Cambridge: Cambridge University Press), 178–197.
Brown, A. K., Pioon, M. O., Holekamp, K. E., and Strauss, E. D. (2021). Infanticide by females is a leading source of juvenile mortality in a large social carnivore. Am. Nat. 198, 642–652. doi: 10.1086/716636
Clutton-Brock, T. H., and Huchard, E. (2013). Social competition and its consequences in female mammals. J. Zool. 289, 151–171. doi: 10.1111/jzo.12023
Conover, W. J. (1999). Practical Nonparametric Statistics, 3rd Edn. New York, NY: John Wiley & Sons.
Dawson, C. (2021). ggprism: A ‘ggplot2’ Extension Inspired by ‘GraphPad Prism’. R Package Version 1.0.3. Available online at: https://CRAN.R-project.org/package=ggprism (accessed February 15, 2022).
Digby, L. (2000). “Infanticide by female mammals: implications for the evolution of social systems,” in Infanticide by Males and its Implications, eds C. P. van Schaik and C. H. Janson (Cambridge: Cambridge University Press), 423–446. doi: 10.1101/cshperspect.a017640
East, M. L., and Hofer, H. (1991). Loud-calling in a female-dominated society: II. Behavioural contexts and functions of whooping of spotted hyaenas, Crocuta crocuta. Anim. Behav. 42, 651–669. doi: 10.1016/s0003-3472(05)80247-7
East, M. L., and Hofer, H. (2001). Male spotted hyenas (Crocuta crocuta) queue for status in social groups dominated by females. Behav. Ecol. 12, 558–568. doi: 10.1093/beheco/12.5.558
East, M. L., Burke, T., Wilhelm, K., Greig, C., and Hofer, H. (2003). Sexual conflict in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc. R. Soc. Lond. B 270, 1247–1254. doi: 10.1098/rspb.2003.2363
East, M. L., Gusset-Burgener, N., and Hofer, H. (2013). “Sex differences in olfactory behaviours reflect the importance of scent marking for social integration in adult females and competition between reproductively active males in the spotted hyena,” in Chemical Signals in Vertebrates 12, eds M. L. East and M. Dehnhard (Heidelberg: Springer), 149–160. doi: 10.1007/978-1-4614-5927-9_11
East, M. L., Hofer, H., and Wickler, W. (1993). The erect ‘penis’ is a flag of submission in a female dominated society: greetings in Serengeti spotted hyenas. Behav. Ecol. Sociobiol. 33, 355–370.
East, M. L., Höner, O. P., Wachter, B., Wilhelm, K., Burke, T., and Hofer, H. (2009). Maternal effects on offspring social status in spotted hyenas. Behav. Ecol. 20, 478–483. doi: 10.1038/ncomms1059
East, M. L., Otto, E., Helms, J., Thierer, D., Cable, J., and Hofer, H. (2015). Does lactation lead to resource allocation trade-offs in the spotted hyaena? Behav. Ecol. Sociobiol. 69, 805–814. doi: 10.1007/s00265-015-1897-x
East, M. L., Wibbelt, G., Lieckfeldt, D., Ludwig, A., Goller, K., Wilhelm, K., et al. (2008). A Hepatozoon species genetically distinct from H. canis infecting spotted hyenas in the Serengeti ecosystem, Tanzania. J. Wildl. Dis. 44, 45–52. doi: 10.7589/0090-3558-44.1.45
Engh, A. L., Funk, S. M., Van Horn, R. C., Scribner, K. T., Bruford, M. W., Libants, S., et al. (2002). Reproductive skew among males in a female-dominated mammalian society. Behav. Ecol. 13, 193–200. doi: 10.1093/beheco/13.2.193
Ferreira, S. C. M., Hofer, H., Madeira de Carvalho, L., and East, M. L. (2019). Parasite infections in a social carnivore: evidence of their fitness consequences and factors modulating infection load. Ecol. Evol. 9, 8783–8799. doi: 10.1002/ece3.5431
Frank, L. G. (1986). Social organization of the spotted hyaena Crocuta crocuta. II. Dominance and reproduction. Anim. Behav. 34, 1510–1527. doi: 10.1016/S0003-3472(86)80221-4
Frank, L. G., Glickman, S. E., and Powch, I. (1990). Sexual dimorphism in the spotted hyaena (Crocuta crocuta). J. Zool. Lond. 221, 308–313. doi: 10.1111/j.1469-7998.1990.tb04001.x
Fruteau, C., Range, F., and Noë, R. (2010). Infanticide risk and infanticide defence in multi-male free-ranging sooty mangabeys. Behav. Process. 83, 113–118.
Gicquel, M., East, M. L., Hofer, H., Cubaynes, S., and Benhaiem, S. (2022). Climate change does not decouple interactions between a central place foraging predator and its migratory prey. Ecosphere 13:e4012. doi: 10.1002/ecs2.4012
Golla, W., Hofer, H., and East, M. L. (1999). Within-litter sibling aggression in spotted hyaenas: effect of maternal nursing, sex and age. Anim. Behav. 58, 715–726. doi: 10.1006/anbe.1999.1189
Goymann, W., East, M. L., Wachter, B., Höner, O., Möstl, E., Van’t Hof, T. J., et al. (2001). Social, state-dependent and environmental modulation of faecal corticosteroid levels in free-ranging female spotted hyaenas. Proc. R. Soc. Lond. B 268, 2453–2459. doi: 10.1098/rspb.2001.1828
Hofer, H., and East, M. (1995). “Population dynamics, population size, and the commuting system of Serengeti spotted hyenas,” in Serengeti II: Dynamics, Management, and Conservation of an Ecosystem, eds A. R. E. Sinclair and P. Arcese (Chicago, IL: University of Chicago Press), 332–363.
Hofer, H., and East, M. L. (1993a). The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. I. Social organization. Anim. Behav. 46, 547–557. doi: 10.1006/anbe.1993.1222
Hofer, H., and East, M. L. (1993b). The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. II. Intrusion pressure and commuters’ space use. Anim. Behav. 46, 559–574. doi: 10.1006/anbe.1993.1223
Hofer, H., and East, M. L. (1993c). The commuting system of Serengeti spotted hyaenas: how a predator copes with migratory prey. III. Attendance and maternal care. Anim. Behav. 46, 575–589. doi: 10.1006/anbe.1993.1224
Hofer, H., and East, M. L. (1997). Skewed offspring sex ratios and sex composition of twin litters in Serengeti spotted hyaenas (Crocuta crocuta) are a consequence of siblicide. Appl. Anim. Behav. Sci. 51, 307–316. doi: 10.1016/s0168-1591(96)01113-6
Hofer, H., and East, M. L. (2003). Behavioural processes and costs of co-existence in female spotted hyenas: a life history perspective. Evol. Ecol. 17, 315–331. doi: 10.1023/A:1027352517231
Hofer, H., and East, M. L. (2008). Siblicide in Serengeti spotted hyenas: a long-term study of maternal input and cub survival. Behav. Ecol. Sociobiol. 62, 341–351. doi: 10.1007/s00265-007-0421-3
Hofer, H., Benhaiem, S., Golla, W., and East, M. L. (2016). Trade-offs in lactation and milk intake by competing siblings in a fluctuating environment. Behav. Ecol. 27, 1567–1578. doi: 10.1093/beheco/arw078
Holekamp, K. E., Smale, L., and Szykman, M. (1996). Rank and reproduction in the female spotted hyaena. J. Reprod. Fert. 108, 229–237. doi: 10.1530/jrf.0.1080229
Hollander, M., Wolfe, D. A., and Chicken, E. (2014). Nonparametric Statistical Methods, 3rd Edn. New York, NY: John Wiley & Sons.
Höner, O. P., Wachter, B., East, M. L., Streich, W. J., Wilhelm, K., Burke, T., et al. (2007). Female mate choice drives the evolution of male-biased dispersal in a social mammal. Nature 448, 798–801. doi: 10.1038/nature06040
Höner, O. P., Wachter, B., Hofer, H., Wilhelm, K., Thierer, D., Trillmich, F., et al. (2010). The fitness of dispersing spotted hyaena sons is influenced by maternal social status. Nat. Commun 1:60. doi: 10.1038/ncomms.1059
Hoogland, J. L. (1985). Infanticide in prairie dogs: lactating females kill offspring of close kin. Science 230, 1037–1040. doi: 10.1126/science.230.4729.1037
Hrdy, S. B. (1979). Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1, 13–40. doi: 10.1016/0162-3095(79)90004-9
Hrdy, S. B. (1992). Fitness tradeoffs in the history and evolution of delegated mothering with special reference to wet-nursing, abandonment, and infanticide. Ethol. Sociobiol. 13, 409–442. doi: 10.1016/0162-3095(92)90011-r
Kalbfleisch, J. D., and Prentice, R. L. (1980). The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons.
Kalinowski, S. T., Mark, L., Taper, M. L., and Marshall, T. C. (2007). Revising how the computer program cervus accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106. doi: 10.1111/j.1365-294X.2007.03089.x
Knörnschild, M., Ueberschaer, K., Helbig, M., and Kalko, E. K. V. (2011). Sexually selected infanticide in a polygynous bat. PLoS One 6:e25001. doi: 10.1371/journal.pone.0025001
Kruuk, H. (1972). The Spotted Hyena: A Study of Predation and Social Behavior. Chicago, IL: University of Chicago Press.
Lowe, A. E., Hobaiter, C., Asiimwe, C., Zuberbühler, K., and Newton-Fisher, N. E. (2020). Intra-community infanticide in wild, eastern chimpanzees: a 24-year review. Primates 61, 69–82. doi: 10.1007/s10329-019-00730-3
Lukas, D., and Huchard, E. (2014). The evolution of infanticide by males in mammalian societies. Science 346, 841–844. doi: 10.1126/science.1257226
Lukas, D., and Huchard, E. (2019). The evolution of infanticide by females in mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180075. doi: 10.1098/rstb.2018.0075
Marescot, L., Benhaiem, S., Gimenez, O., Hofer, H., Lebreton, J. D., Olarte-Castillo, X. A., et al. (2018). Social status mediates the fitness costs of infection with canine distemper virus in Serengeti spotted hyenas. Funct. Ecol. 32, 1237–1250. doi: 10.1111/1365-2435.13059
Marshall, T. C., Slate, J., Kruuk, L. E. B., and Pemberton, J. M. (1998). Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 7, 639–655. doi: 10.1046/j.1365-294x.1998.00374.x
Matthews, L. H. (1939). Reproduction in the spotted hyaena, Crocuta crocuta (Erxleben). Philos. Trans. R. Soc. Lond. B Biol. Sci. 230, 1–78. doi: 10.1098/rstb.1939.0004
Olarte-Castillo, X. A., Hofer, H., Goller, K. V., Martella, V., Moehlman, P. D., and East, M. L. (2016). Divergent sapovirus strains and infection prevalence in wild carnivores in the Serengeti Ecosystem: a long-term study. PLoS One 11:e0163548. doi: 10.1371/journal.pone.0163548
Packer, C., and Pusey, A. E. (1983). Adaptations of female lions to infanticide by incoming males. Am. Nat. 121, 716–728. doi: 10.1086/284097
Palombit, R. A. (2015). Infanticide as sexual conflict: coevolution of male strategies and female counterstrategies. Cold Spring Harb. Perspect. Biol. 7:a017640. doi: 10.1101/cshperspect.a017640
Pusey, A. E., and Packer, C. (1994). “Infanticide in lions: consequences and counterstrategies,” in Infanticide and Parental Care, eds S. Parmigiani and F. vom Saal (Chur: Harwood), 277–299.
Pusey, A., Williams, J., and Goodall, J. (1997). The influence of dominance rank on the reproductive success of female chimpanzees. Science 277, 828–831. doi: 10.1126/science.277.5327.828
R Core Team. (2021). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Sterck, E. H. M., and Korstjens, A. H. (2000). “Female dispersal and infanticide avoidance in primates,” in Infanticide by Males and its Implications, eds C. P. van Schaik and C. H. Janson (Cambridge: Cambridge University Press), 293–321. doi: 10.1017/cbo9780511542312.015
Stockley, P., and Bro-Jørgensen, J. (2011). Female competition and its evolutionary consequences in mammals. Biol. Rev. 86, 341–366. doi: 10.1111/j.1469-185X.2010.00149.x
Stockley, P., and Campbell, A. (2013). Female competition and aggression: interdisciplinary perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 368:20130073. doi: 10.1098/rstb.2013.0073
Szykman, M., Engh, A. L., Van Horn, R. C., Funk, S. M., Scribner, K. T., and Holekamp, K. E. (2001). Association patterns among male and female spotted hyenas (Crocuta crocuta) reflect male mate choice. Behav. Ecol. Sociobiol. 50, 231–238. doi: 10.1007/s002650100356
Towers, J. R., Muriel, J., Symonds, H. K., Sutton, G. J., Alexandra, B. M., Spong, P., et al. (2018). Infanticide in a mammal-eating killer whale population. Sci. Rep. 8:4366. doi: 10.1038/s41598-018-22714-x
Trivers, R. L. (1972). “Parental investment and sexual selection,” in Sexual Dimorphism and the Descent of Man, ed. B. Campbell (Chicago, IL: Aldine), 136–179. doi: 10.4324/9781315129266-7
Van Horn, R. C., Wahaj, S. A., and Holekamp, K. E. (2004). Role-reversed nepotism among cubs and sires in the spotted hyena (Crocuta crocuta). Ethology 110, 413–426. doi: 10.1111/j.1439-0310.2004.00984.x
van Schaik, C. P. (2000). “Infanticide by male primates: the sexual selection hypothesis,” in Infanticide by Males and its Implications, eds C. P. van Schaik and C. H. Janson (Cambridge: Cambridge University Press), 27–60. doi: 10.1017/cbo9780511542312.004
van Schaik, C. P., and Kappeler, P. M. (1997). Infanticide risk and the evolution of male-female association in primates. Proc. Biol. Sci. 264, 1687–1694. doi: 10.1098/rspb.1997.0234
Walker, K. K., Foerster, S., Murray, C. M., Mjungu, D., and Pusey, A. E. (2021). Evaluating adaptive hypotheses for female-led infanticide in wild chimpanzees. Anim. Behav. 180, 23–36. doi: 10.1016/j.anbehav.2021.07.025
Wasser, S. K., Norton, G. W., Kleindorfer, S., and Rhine, R. J. (2004). Population trend alters the effects of maternal dominance rank on lifetime reproductive success in yellow baboons (Papio cynocephalus). Behav. Ecol. Sociobiol. 56, 338–345.
White, P. A. (2005). Maternal rank is not correlated with cub survival in the spotted hyena Crocuta crocuta. Behav. Ecol. 16, 606–613. doi: 10.1093/beheco/ari033
Wilhelm, K., Dawson, D. A., Gentle, L. K., Greig, C., Horsefield, G., Schlötterer, C., et al. (2003). Characterisation of spotted hyena Crocuta crocuta microsatellite loci. Mol. Ecol. Notes 3, 360–362. doi: 10.1046/j.1471-8286.2003.00450.x
Williams, G. C. (1966). Adaptation and Natural Selection. A Critique of Some Current Evolutionary Thought. Princeton, NJ: Princeton University Press.
Wright, E., Galbaney, J., McFarlin, S. C., Ndayishimiye, E., Stoinski, T. S., and Robbins, M. M. (2020). Dominance rank but not body size influences female reproductive success in mountain gorillas. PLoS One 15:e0233235. doi: 10.1371/journal.pone.0233235
Keywords: infanticide, sexual conflict, resource competition, spotted hyena, DNA profiling, social instability
Citation: East ML, Thierer D, Benhaiem S, Metzger S and Hofer H (2022) Infanticide by Adult Females Causes Sexual Conflict in a Female-Dominated Social Mammal. Front. Ecol. Evol. 10:860854. doi: 10.3389/fevo.2022.860854
Received: 23 January 2022; Accepted: 20 April 2022;
Published: 19 May 2022.
Edited by:
Elise Huchard, UMR 5554 Institut des Sciences de l’Evolution de Montpellier (ISEM), FranceReviewed by:
Eli Strauss, Max Planck Institute of Animal Behavior, GermanyCopyright © 2022 East, Thierer, Benhaiem, Metzger and Hofer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marion L. East, ZWFzdEBpenctYmVybGluLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.