- Department of Environmental Sciences, Texas Christian University, Fort Worth, TX, United States
For urban environments to support bat communities, resources need to be readily available. For example, bats typically use urban water sources such as drainage ditches and ponds; however, these sources can be ephemeral. During these periods, bats have utilized residential swimming pools, although they only appear to drink at pools when access to more natural equivalents are limited. This posed the question “can we make residential swimming pools friendlier for a diversity of bat species?” Using citizen science to determine which pool characteristics influenced bat activity, we distributed a questionnaire to residents in a suburban neighborhood in Fort Worth, TX, United States. It focused on observations of bat activity and the features of the pools and immediate surroundings. We distributed the questionnaire through social media, local presentations, and by mail throughout 2019 and 2020. We then used classification trees to determine which characteristics in combination influenced bat activity at the pools. We generated three different trees for bats observed (1) flying around the property and backyard, (2) above the swimming pool, and (3) drinking at the pool. We found that more bats were observed at unlit pools without bush or shrub borders. Furthermore, among pools with borders, activity was lowest at pools with textured interiors and ≥6 trees visible. The presence of features, such as fountains, then contributed to a reduction in bat observations in backyards and the presence of pets appeared to further reduce activity specifically over the pools. Where bats were observed drinking, this activity was reported the least at pools with bush or shrub borders, textured interiors, and trees <5 m and >10 m from the edge of the pools. Our study revealed that certain characteristics of residential swimming pools encouraged bat activity, while others discouraged them. Thus, it may be possible to make swimming pools more bat-friendly. For example, turning lights off in the evening when backyards are not in use and reducing clutter around pools could have an immediate positive impact on local bat populations. The implementation of such recommendations could improve urban habitats for bats overall and alleviate some of the negative implications of continued urbanization.
Introduction
Citizen science has proven to be a useful tool for monitoring the abundance and distribution of species, establishing population trends, and informing wildlife management and conservation practices (Brown et al., 2020; Adesh et al., 2021; Lewanzik et al., 2021; Hughes et al., 2022). These citizen or community-based studies solicit volunteers to report wildlife sightings as, when, and where they are observed (such as iNaturalist1, a collaborative project from the California Academy of Sciences and the National Geographic Society, and eBird.org from the Cornell Lab of Ornithology) or answer a series of questions associated with wildlife sightings or events that have occurred (Chauhan and Gallacher, 2021; Gutierrez-Munoz et al., 2021; Niemiller et al., 2021; Edwards et al., 2022). Typically, citizen science studies are more successful and informative when they have received a higher volume and diversity of responses, as increased variability reduces biases and enhances the overall reliability of the information received (Wilson et al., 2020; Coomber et al., 2021; Richter et al., 2021). Studies that have the opportunity to involve more volunteers or are able to reach more respondents are those that cover a larger geographic range and are conducted in areas with a higher concentration of potential volunteers (Border et al., 2017; Fischer et al., 2021; Owens et al., 2021). Citizen science studies conducted in urban areas are, therefore, likely to have more volunteer involvement and yield higher return rates. For example, the greatest number of wildlife sightings tend to be clustered within cities and large towns (Spear et al., 2017).
Generally, urban areas are considered less than ideal for wildlife (Murray et al., 2019; Ng et al., 2020; Wilk et al., 2020; Jensen et al., 2021). Many studies have shown that urban and suburban environments do not provide sufficient resources for wildlife, they filter or hinder movement, and subsequently lead to declines in species abundance and diversity recorded across most taxa (Threlfall et al., 2012; Moretto and Francis, 2017; Palheta et al., 2020; Correa et al., 2021). Moreover, with urban sprawl predicted to progressively continue, urbanization is expected to have a greater impact on more species (Candido et al., 2021). However, contrary to this ideology an increasing number of studies have shown that urban areas can support healthy stable wildlife populations (Goncalves et al., 2021; van Helden et al., 2021; Liu and Slik, 2022). In particular, studies have revealed that certain bat species can potentially thrive in urban environments (Jara-Servin et al., 2017; Jung and Threlfall, 2018; Gili et al., 2020). For example, established roosting colonies for Tadarida brasiliensis are now commonly found in cities and urban settings throughout the southern United States and Mexico, which has been attributed to a range expansion by this species over the last two decades (Li and Wilkins, 2015; Kasper and Yancey, 2018; McCracken et al., 2018). Moreover, studies have shown that if managed effectively urban areas can be improved to provide the resources necessary for diverse array of species (Li and Wilkins, 2014; Threlfall et al., 2016). For example, the presence of green roofs in urban neighborhoods can enhance bat activity by increasing prey availability and providing foraging opportunities (Partridge et al., 2020). This in turn can aid dispersal, increase local biodiversity, and suppress or even reverse population declines (de Araujo and Bernard, 2016; Moretto et al., 2019). These improvements not only benefit bats, but also environmental health and resource availability by facilitating species that undertake essential ecosystem services (Parkins and Clark, 2015). Bats, for example, provide much needed pest control, pollination, and seed dispersal services (Aguiar et al., 2021; Ramirez-Francel et al., 2022). Supporting bats within urban environments can, therefore, benefit community health and welfare.
For bat populations to thrive in an urban environment they need a variety of resources to be available and readily accessible, including roosting sites, foraging opportunities, access routes (i.e., commuting corridors), mating opportunities, and water sources (Altringham, 2011; Lim et al., 2021). Moreover, studies have shown that in urban areas bats can use resources that do not necessarily visually resemble traditional resources (defined here as those preferential selected in more natural habitats) as long as they provide similar conditions and/or functions (Neubaum, 2018; Bergeson et al., 2020). For example, in natural or semi-natural environments, bats can typically roost in caves, trees, rock faces and crevices, and gullies (Ammerman et al., 2012). Yet in urban areas, bats can take advantage of anthropogenic structures, such as culverts, bridges, buildings, mines, wine cellars, loft spaces, and basements (Hoffmann et al., 2016; Jung and Threlfall, 2016; Kelly et al., 2016). Such resource selection is based on the notion that animals rely on a set of innate and/or learned criteria that dictate habitat quality and resource preference (Robertson et al., 2018). For bats, these criteria can be visual, auditory, tactile, olfactory, and/or electromagnetic cues (Tian et al., 2010; Bonsen et al., 2015; Cabral et al., 2016; Boerma et al., 2019; McGowan and Kloepper, 2020). Thus, if an anthropogenic structure or feature meets the “search” criteria, bats (depending on species) may utilize them. The use of such resources will also depend on their abundance and distribution (Li and Wilkins, 2014; Moretto et al., 2019). Urban areas, for example, can be improved for bats by providing more water resources (Gili et al., 2020; O’Malley et al., 2020; Thomas et al., 2021).
Typically, urban environments have a limited abundance of natural water sources, with semi-natural water sources representing more available alternatives (Ancillotto et al., 2019a), such as drainage ditches and retention ponds (Hintze et al., 2016; Ayala-Berdon et al., 2017; Nystrom and Bennett, 2019). However, in areas with persistently high temperatures and limited rainfall for long periods (such as summer months), these types of water resources can be ephemeral and as such not readily available to bats throughout their summer activity season (Hall and Bennett, 2021). During these resource-limited periods, bats may potentially move to areas with an available water supply or seek water from more anthropogenic alternatives (Loumassine et al., 2020). A recent study in a suburban neighborhood in north central Texas revealed that bats would drink water from residential swimming pools (Nystrom and Bennett, 2019). This study also demonstrated that bats did not readily utilize this alternative water source. Instead, they tended to drink at pools only when access to a more natural equivalent appeared to be limited. These findings suggested that, despite swimming pools being available throughout their activity season (March–October), bats preferentially selected not to use them when natural or semi-natural water sources were available. This posed the question “can residential swimming pools be made more bat-friendly for a wide range of bat species in an urban environment?” To address this, we distributed a questionnaire to residents with swimming pools in suburban neighborhoods within the Dallas-Fort Worth Metroplex, Texas, United States. This questionnaire focused on observations of bat activity, pool features, and characteristics associated with the immediate surrounding area. From this, we determined a set of features and characteristics that would enable and encourage a diversity of bat species to use residential swimming pools, along with a set of features and characteristics that might hinder bats from using such pools. Thus, by determining how we can increase water resource availability for bats in urban areas, we can make recommendations that potentially contribute to the conservation of bats.
Materials and Methods
Study Area
For our study, we targeted residents with swimming pools in a suburban neighborhood in Fort Worth, TX, United States (32°41′04.69″ N, 97°22′28.33″ W). The neighborhood comprised single-story ranch housing with 35 mph, two-lane, tarmacked roads. Mature trees were found on the majority of properties, as well as in tree-lined roads, including bur oaks (Quercus macrocarpa), live oaks (Q. virginiana), American elms (Ulmus americana), cedar elms (U. crassifolia), green ashes (Fraxinus pennsylvanica), common hackberries (Celtis occidentalis), pecans (Carya illinoinensis), and magnolia (Magnolia grandiflora; FOFPA, 2014). In addition, the neighborhood was centered around four interconnecting parks operated by the City of Fort Worth Parks and Recreation Department. These parks comprised 420 km2 of manicured grass bordering a tree-lined drainage ditch with a similar tree composition to the surrounding neighborhood. Along with a retention pond in the park system, the drainage ditch represented a semi-natural source of water for bats in the immediate area. Within a 2.5 km radius of the park system (representing our study area and a distance equivalent to the home range size of local bats, Hall and Bennett, 2021), only a few other natural and semi-natural water sources were available, including Willow Creek Lake, the Trinity River, and no more than five ornamental ponds (Figure 1). Yet, this area had >650 residential swimming pools that bats could potentially utilize as a water resource (Nystrom and Bennett, 2019; Hall, 2020). Previous studies showed that both the drainage ditch and an associated retention pond were ephemeral, more often than not drying up over the summer months (June–August) when temperatures regularly exceeded 30°C and precipitation rarely exceeded 70 mm per month (Nystrom and Bennett, 2019; Hall, 2020). These studies further showed that bats from the park system, including Lasiurus borealis, L. cinereus, Lasionycteris noctivagans, Perimyotis subflavus, Nycticeius humeralis, and Tadarida brasiliensis (Bienz, 2016), would access residential swimming pools to drink in the surrounding neighborhood when preferred water sources were not available to them.
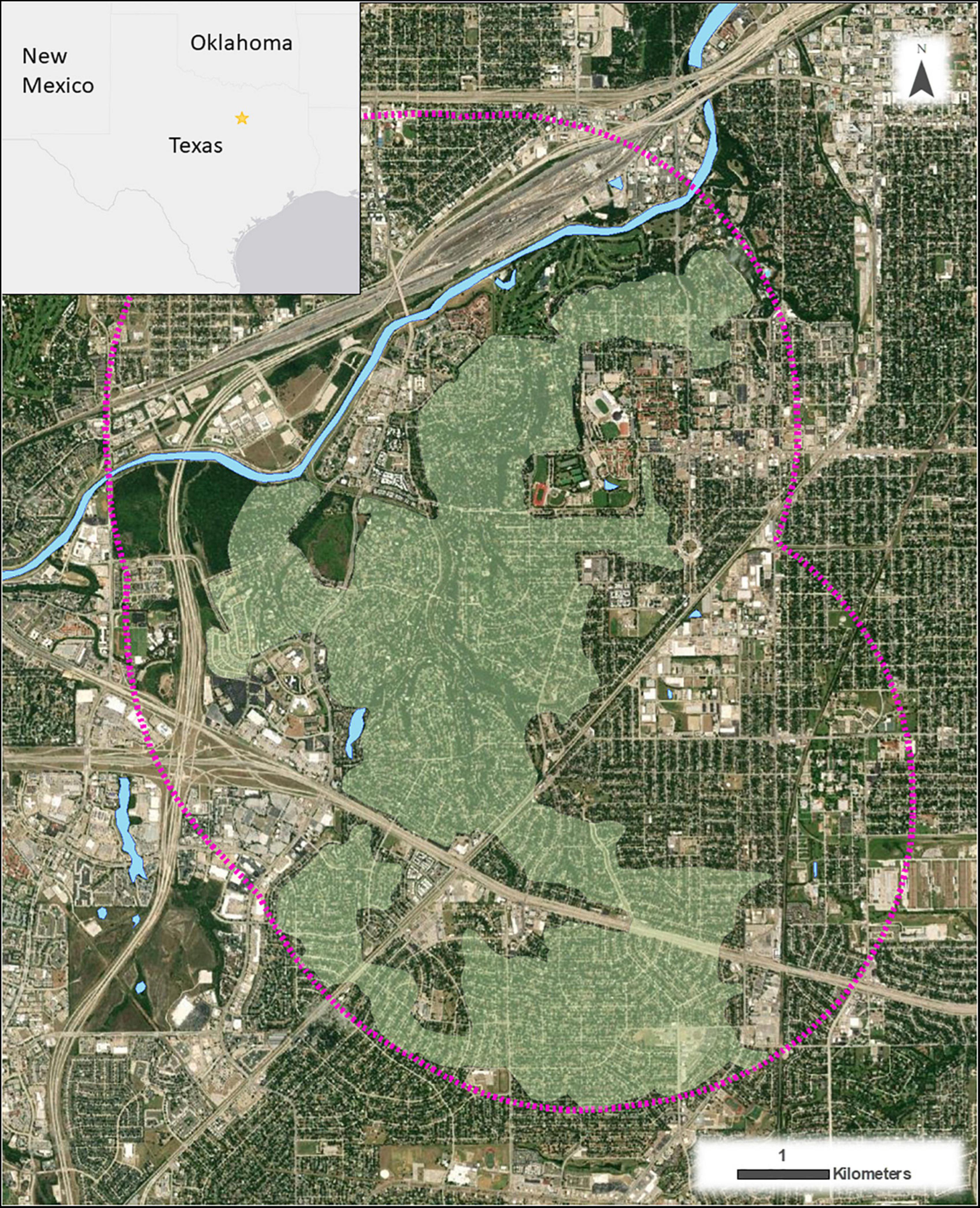
Figure 1. All residents with swimming pools within a suburban neighborhood in Fort Worth, TX, United States (shown as a translucent green polygon and star on the map of Texas) received a questionnaire between 2019 and 2020. Pink dotted outline shows total study area considered prior to the search and identification of residents with swimming pools. Light blue polygons display natural and semi-natural water sources.
Questionnaire
To determine which aspects of the residential swimming pools encouraged bats to use them as a water source, we distributed a questionnaire to residents with swimming pools within our study area which included questions regarding observations of bat activity and a description of their pools and the immediate surrounding area (see Supplementary Material; Agpalo, 2021). We predicted that the presence of bats in proximity to a swimming pool may be due to (1) emergence from nearby roost sites, (2) commuting, (3) foraging, and (4) drinking. Thus, we separated observed bat activity into four groups; (1) no bats were observed flying on the property, (2) bats were observed flying on the property, (3) bats were observed flying above the swimming pool, and (4) bats were observed drinking from the pool (defined as a bat swooping down close to or touching the surface of the water (Tuttle et al., 2006; McAlexander, 2013). We also acknowledged that bat observations were dependent on whether residents were present in their backyards from dusk onward, so we included (5) residents did not go in the backyard at or after dusk.
Based on available peer-reviewed literature, we then devised a series of questions regarding swimming pool attributes that could potentially influence bat activity at or near a pool. For example, we included questions that referred to the size and shape of the pool. We hypothesized smaller pools would have less bat activity, as species with limited maneuverability (whether it be due to wing morphology and/or larger body size) would be restricted in their ability to access such pools (Hall et al., 2016; Suarez-Rubio et al., 2018; Bailey et al., 2019; Moretto et al., 2019). Similarly, the presence of borders or objects surrounding (such as fencing, walls, or vegetation) or overhanging a pool (such as ledges and diving boards) could limit the available area in which bats maneuver as they approach the surface of the water (Tuttle et al., 2006). Moreover, as we considered that bats would preferentially select swimming pools with curved-edges (i.e., circular, oval, and kidney shaped) that were fully recessed in the ground, as they resembled natural sources, such as ponds and lakes (Ayala-Berdon et al., 2017; Ancillotto et al., 2019a), we included questions regarding pool shape.
Water quality has also been shown to impact resource use in some instances (Li et al., 2015; Li and Kalcounis-Rueppell, 2018), so we included questions regarding pool features and characteristics that could influence water quality. Firstly, as residential swimming pools are chemically treated, we considered that water quality and therefore use may have been influenced by the type and frequency of treatment. For example, Nystrom and Bennett (2019) revealed that while bats used both mineral- and chlorine-treated residential swimming pools as a water source, the rate of usage appeared to vary between these treatment types. Moreover, while bats predominantly used echolocation to identify resources, we proposed that the color and texture of a pool might provide visual cues that bats could use, especially at dusk when many bats emerged from their roosts to drink (Adams and Thibault, 2006). Thus, we anticipated that colors, such as turquoise and blue, would have indicated favorable water quality, while colors, such as greens, whites, and grays, may have resembled water sources with vegetation cover and/or higher suspended sediment (USGS, 2018). Similarly, studies have shown that bats typically drink from clutter-free surfaces, avoiding water sources with vegetation and exposed rocks (Ciechanowski et al., 2007; Jackrel and Matlack, 2010). We, therefore, expected that regular maintenance, such as cleaning and filtering of debris (such as petals, leaves, pollen, and dust) from a swimming pool, would keep the level of clutter to a minimum enabling bats to drink from that pool. We also considered that other features, such as fountains, connected hot tubs, and floaties (i.e., pool inflatables, pool noodles, and other floating devices) would create either ripples that disturb the surface of the water or create clutter, both of which would potentially deter bats from drinking (Rydell et al., 1999; Zsebok et al., 2013; Todd and Williamson, 2019).
There are also a number of features and activities that could disturb bats from readily using a swimming pool as a resource. For example, many studies have shown that the presence of light (both from moon illumination and artificial sources) influences bat activity (Rowse et al., 2016; Russo et al., 2017, 2019a). Such light sensitivity is species-specific and thought to be predominately associated with risk of predation (Frank et al., 2019; Russo et al., 2019b). As five of our six local species are considered to be light-sensitive (Haddock et al., 2019), we hypothesized that any illumination at or near residential swimming pools would lower overall bat activity. Similarly, anthropogenic activity in or near a swimming pool could deter bats from drinking or flying in proximity to the pool (Ancillotto et al., 2019b). We, therefore, generated a series of questions that explored pool and backyard usage by owners and their pets. We also assumed that as bats use ultrasonic echolocation to forage, drink, and navigate in the dark, any high frequency noises generated by mechanical equipment in proximity to the swimming pools could disrupt or mask bat echolocation (Bonsen et al., 2015; Moretto and Francis, 2017; Finch et al., 2020). We, therefore, considered that filtration systems, pool vacuums, sprinkler systems, and AC units operating in proximity to a swimming pool when the bats were active could interfere with their ability to successfully forage or drink at a swimming pool.
Accessibility to a swimming pool, whether it be associated with the pool itself, the immediate vicinity, or the surrounding area could also dictate bat activity at or near a pool (Threlfall et al., 2012). Thus, we expected that the presence of netting and pool covers would restrict access, while linear features, such as a tree-lines, walls, and fences, would potentially offer protection from aerial predators, enabling bats to more readily access a pool (Straka et al., 2016; Avila-Flores et al., 2019). As previously mentioned, maneuverability varies between bat species, which in turn influences their ability to access specific habitats and resources (Suarez-Rubio et al., 2018; Bailey et al., 2019; Moretto et al., 2019). For instance, none of the species commonly found in north central Texas are adapted to very cluttered environments (Ammerman et al., 2012). They prefer, for example, to forage, commute, and roost along forest edges and tree-lines. We would, therefore, expect that the presence of uninterrupted tree-lines from other areas across the neighborhood would increase access to a particular property (Le Roux et al., 2018). However, we would also expect bat abundance to be lower on residential properties with higher levels of clutter due to dense canopy cover, tall shrubs, or other structures, such as fences, walls, and buildings, in close proximity to each other (Threlfall et al., 2011).
A total of 20 pool characteristics were identified (see Table 1 for full details) from which 80 questions were devised (see Supplementary Material; Agpalo, 2021). To distribute the questionnaire to residents, we used Qualtrics XM, online survey software. We then circulated the link to Qualtrics among five different social networking websites for neighborhoods associated with our study area, including Nextdoor Networks and Facebook pages. In addition, we distributed questionnaires at six local community events from March to October 2019 and further targeted residents with swimming pools in our study area by mailing 645 questionnaires to them with a stamped addressed return envelope from January to April 2020 (Agpalo, 2021).
After collating the Qualtrics and written questionnaire responses (IRB #2021-233), we treated the characteristics of the pools and surrounding area as our independent variables and the bat activity observed as our dependent variables (see Table 1). We identified a total of three categorical dependent variables. Each of these was based on the type of bat activity observed, thus we included responses in which (1) bats were observed (including all observations of bats flying around the property and backyard, above the swimming pool, and drinking at the pool), (2) bats were observed above the swimming pool and drinking at the pool, and (3) bats were observed drinking at the pool only. Note that responses in which residents did not go outside near their pools at or after dusk were excluded from the analysis as these potentially represented false negatives (i.e., bats may have been active, but were not observed). In addition, we conducted a preliminary analysis of the data to assess whether the frequency at which respondents were outside in proximity to their swimming pools influenced their ability to observe bats effectively. For this, we compared pool usage with each of our three dependent variables and found a significant difference in bat activity with more bats being observed with increasing usage (χ2 = 9.819, df = 4, P = 0.044). As this pattern appeared to be autocorrelated (i.e., observed bat activity was contingent on frequency of pool use), we opted to also remove all responses where residents were in their backyards less frequently (>weekly intervals) and had not observed bat activity.
Analysis
To determine which characteristics in combination influenced bat activity at residential swimming pools, we conducted a series of Classification and Regression Tree (CART) analyses using Salford Predictive Modeler’s CART® in Minitab (version 19, State College, PA, United States). This statistical method uses recursive partitioning to stratify the frequency of bat observations (categorical dependent variables) under different combinations of characteristics, such as a curved-edged, chlorine-treated pool with lights on. In other words, CART explicitly identifies which characteristics in combination potentially encourage bat activity at pools and which do not. We opted to use this method as it was able to handle complex and context dependent multivariate data, had no distributional assumptions, and effectively generated predictions that can be used to inform management recommendations (Bennett et al., 2011). As all the independent variables were defined as categorical predictors (see Table 1), we generated three different classification trees, one for each of the aforementioned categorical dependent variables. After the trees were generated, we used the receiver operating characteristic (ROC) curve and R-squared values to optimally prune each tree and identify the important predictors of bat activity (McCune et al., 2002).
Results
Between 5 January 2019 and 3 July 2020, a total of 184 questionnaires were received, of which 109 were mail returns and 75 were Qualtrics responses. The mail returns alone represented a 16.9% return rate. Of the 184 questionnaires, 8 respondents indicated that they did not go outside near their pools at or after dusk, 98 respondents indicated they had observed bats, and 78 indicated that they had not observed bats. Of the latter, 63 respondents indicated that they infrequently went outside near their pools (>week). A total of 113 questionnaires were, therefore, considered in the following analysis, including 15 respondents that had indicated no bats were observed flying around the property and backyard, 33 indicating that bats were observed flying around the property and backyard, 36 that had observed bats flying above the swimming pool, and 29 that observed bats drinking at their pools. From an initial inspection of the data, we removed a further three independent variables prior to the analysis; (1) pool type, as all but one pool was in-ground/recessed, (2) frequency of pool treatment, as this typically occurred on a weekly basis at >90% of pools, and (3) pool access to bats, as all pools were available from March to September when bats are typically active in north central Texas (Hall and Bennett, 2021). We also did not include categories with less than five responses, which included extra-large pools from pool size, as only two pools were within this size bracket; combined from pool shapes, as only four were identified; and mineral and bromide, as only three respondents implemented these types of pool treatment.
Among swimming pools where bats were observed flying around the property and backyard (i.e., at all pools with bat activity recorded; n = 113), our CART analysis revealed that nine of the 16 independent variables included in the analysis were considered important predictors of bat activity (ROC = 0.85; R-squared = 0.33; Figure 2). Five of these variables were determined to influence bat activity, including the presence of bush or shrub borders, pool lighting, pool interior texture number of trees visible, and the presence of connected hot tub, fountain, and/or waterfall. The presence of bush or shrub borders in proximity to the pool edge was identified as the main variable determining whether bats were observed flying around the property and backyard, with less bats observed at pools with borders. The mean number of observations then decreased further at pools with intermediate and rough textured interiors as opposed to smooth. At textured pools, even fewer bats were observed when <6 trees were visible from the pool and a connected hot tub, fountain, and/or waterfall was present. In contrast, at pools without bush or shrub borders, more bats were observed when the pool area was not lit or only sporadically lit.
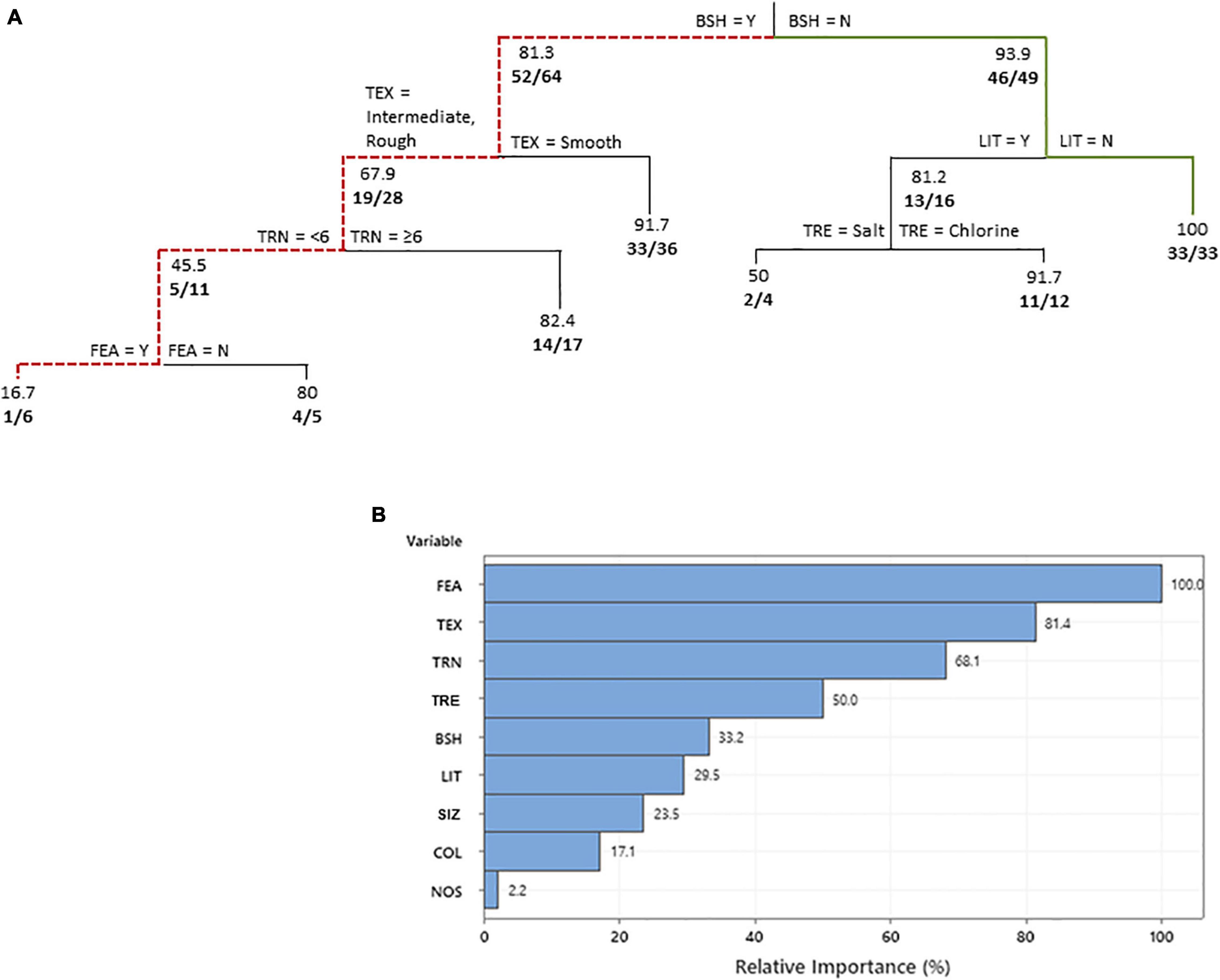
Figure 2. Classification tree (A) predicting the swimming pool characteristics that influenced bat activity observed around the properties and backyards of residents in a suburban neighborhood in Fort Worth, TX, United States. The variable predictors that create a split are labeled at each branch split. Below each node values represent the percentage of responses in which bats were observed and number of positive responses along with the total number of responses considered are given in bold. A bold solid green line shows the combination of independent variables that led to the most observations and a red dotted line shows the variables resulting in the least observations. Relative variable importance chart (B) with relative importance defined as % improvement with respect to the top predictor. FEA, connected hot tub/fountain/waterfall feature present; TEX, pool interior texture; TRN, number of trees visible from the pool; TRE, pool treatment; BSH, bushes/shrubs and structures (<2 m) close to or bordering pool; LIT, pool lighting (LIT); SIZ, surface area of swimming pool m2; COL, color of pool interior; and NOS, high frequency noises generated between 7 pm and 6 am.
Among swimming pools where bats were observed flying over the swimming pool (i.e., at all pools where bats were recorded flying over and drinking at the pools; n = 80), our CART analysis revealed that all 16 independent variables were considered important predictors of bat activity (ROC = 0.90; R-squared = 0.45; Figure 3). Five of these variables were determined to influence bat activity over the pool, including the presence of bush or shrub borders, pool lighting, the presence of pets, pool interior texture, and number of trees visible. The presence of bush or shrub borders in proximity to the pool edge was identified as the main variable dictating whether bats were observed flying over the swimming pool, with less bats observed at pools with borders. Observations decreased further at swimming pools when pets were allowed outside at or after dusk, the pools had intermediate and rough textured interiors as opposed to smooth, and <6 trees visible from the pool. In contrast, at pools without bush or shrub borders, more bats were observed over the pool when the pool area was not lit or only sporadically lit.
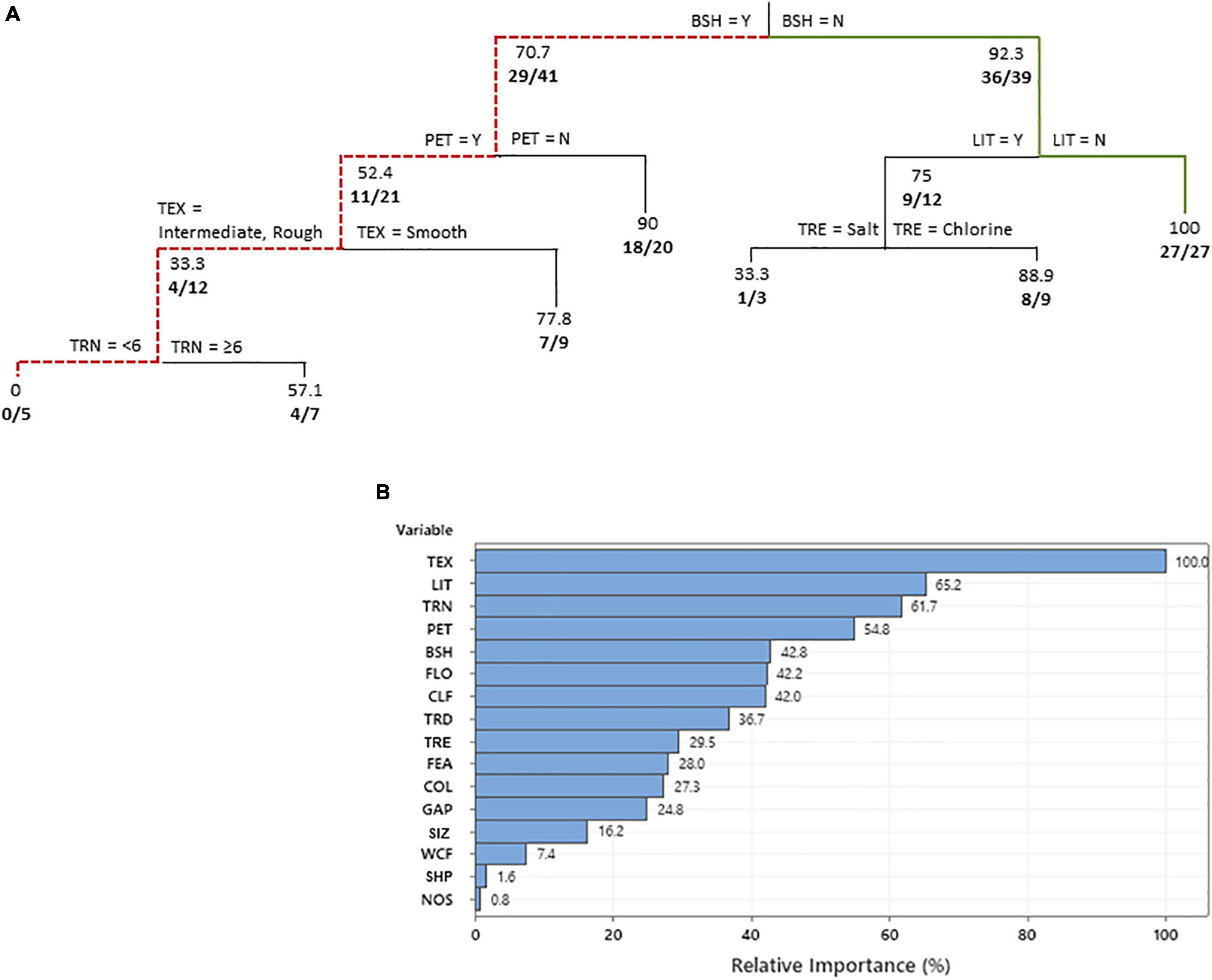
Figure 3. Classification tree (A) predicting the swimming pool characteristics that influenced bat activity observed over the pools of residents in a suburban neighborhood in Fort Worth, TX, United States. The variable predictors that create a split are labeled at each branch split. Below each node values represent the percentage of responses in which bats were observed and number of positive responses along with the total number of responses considered are given in bold. A bold solid green line shows the combination of independent variables that led to the most observations and a red dotted line shows the variables resulting in the least observations. Relative variable importance chart (B) with relative importance defined as % improvement with respect to the top predictor. TEX, pool interior texture; LIT, pool lighting; TRN, number of trees visible from the pool; PET, pets in backyard between 7 pm and 6 am; BSH, bushes/shrubs and structures (<2 m) close to or bordering pool; FLO, are pool floaties left overnight; CLF, how often is pool cleaned; TRD, distance (m) of trees from pool; TRE, pool treatment; FEA, connected hot tub/fountain/waterfall feature present; COL, color of pool interior; GAP, distance of gap between tree canopies; SIZ, surface area of swimming pool m2; WCF, walled edge/cliff/fence present; SHP, pool shape; and NOS, high frequency noises generated between 7 pm and 6 am.
Among swimming pools where bats were observed drinking at the pools (n = 44), our CART analysis revealed that 12 of the 16 independent variables were considered important predictors of bat activity (ROC = 0.85; R-squared = 0.37; Figure 4). Four of these variables were determined to influence bat drinking activity, including the distance of trees from the pool, presence of bush or shrub borders, pool interior texture, and the size of the pool. The distance of trees from the pool was identified as the main variable dictating whether bats were observed drinking from the swimming pools, with more bats observed drinking where trees were between 5 and 10 m from the edge of a pool. The presence of bush or shrub borders in proximity to the pool edge presence then decreased drinking activity further at pools 50 m2 or more in size with intermediate and rough textured interiors as opposed to smooth.
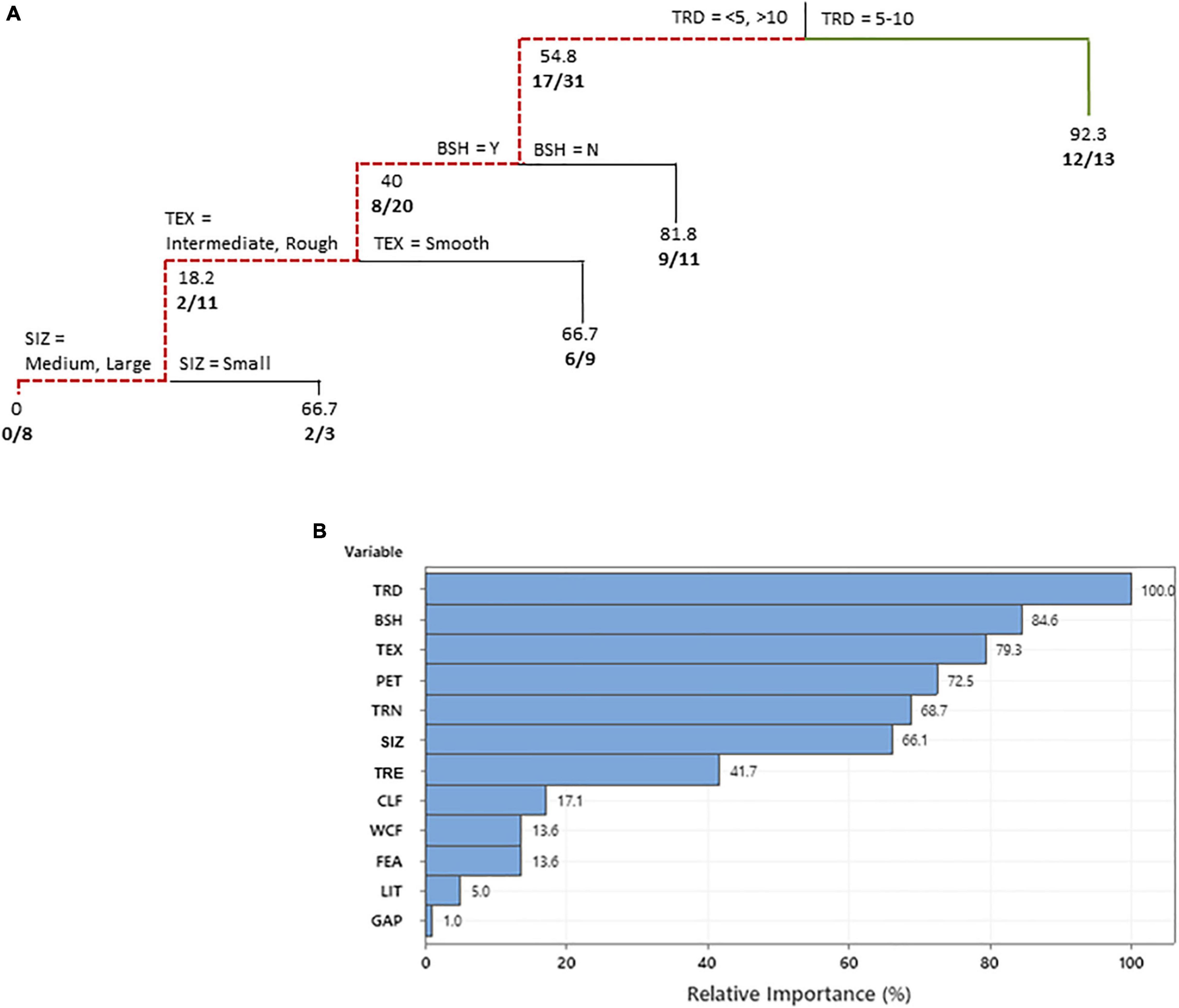
Figure 4. Classification tree (A) predicting the swimming pool characteristics that influenced bats observed drinking at the pools of residents in a suburban neighborhood in Fort Worth, TX, United States. The variable predictors that create a split are labeled at each branch split. Below each node values represent the percentage of responses in which bats were observed and number of positive responses along with the total number of responses considered are given in bold. A bold solid green line shows the combination of independent variables that led to the most observations and a red dotted line shows the variables resulting in the least observations. Relative variable importance chart (B) with relative importance defined as% improvement with respect to the top predictor. TRD, distance (m) of trees from pool; BSH, bushes/shrubs and structures (<2 m) close to or bordering pool; TEX, pool interior texture; TRN, number of trees visible from the pool; SIZ, surface area of swimming pool m2; TRE, pool treatment; CLF, how often is pool cleaned; WCF, walled edge/cliff/fence present; FEA, connected hot tub/fountain/waterfall feature present; LIT, pool lighting; and GAP, distance of gap between tree canopies.
Discussion
Our citizen science-based study revealed that there are features and characteristics of residential swimming pools that influence bat activity. One predominant characteristic that discouraged bats from actively flying in backyards, over pools, and drinking at the pools (i.e., all three of our dependent variables) was the presence of bushes, shrubs, and other structures close to or bordering a pool. More specifically, the presence of such borders reduced bat activity and supported our hypothesis that they could prevent bats from effectively approaching swimming pools to drink (i.e., reduced the amount of area available for maneuvering). In other words, such structures are likely to increase clutter and reduce the area that bats have to effectively maneuver in order to drink from pools. As all seven species known to be in our study area were not adapted to closed or cluttered environments, it is not surprising that features that have the potential to increase clutter and reduce the total area available, discouraged bat activity at swimming pools (Jackrel and Matlack, 2010; Ammerman et al., 2012).
Another characteristic that influenced the presence of bats on a property and over a swimming pool was lighting. From the responses to the questionnaire, we found that when lights were turned on bats avoided the area. This result supported our hypothesis that the presence of lighting could negatively affect bats. Moreover, six of the seven bat species known to be in our study area were considered to be light sensitive and, therefore, were likely to avoid areas with lights (Haddock et al., 2019; Voigt et al., 2020). Such avoidance behavior among the majority of species may have led to the decrease in the number of bats observed at lit swimming pools. However, as L. borealis is reportedly not sensitive to artificial lighting and even forages at street lights (Mager and Nelson, 2001). It would be interesting to determine whether the bats observed flying over and drinking at swimming pools with lighting comprise this species or whether any of the other local species can utilize lit pools.
We also found that the texture of the interior of the pool was an important predictor of all three dependent variables. More specifically, pools with textured surfaces (i.e., rough or intermediate) had up to 73% less bat activity reported. Studies have shown that smooth surfaces, including the surface of water, can act as acoustic mirrors, enabling bats to echolocate more effectively (Genzel et al., 2015). This is because smooth surfaces can reflect and focus echoes more clearly to improve foraging and drinking abilities (Siemers et al., 2005; Greif and Siemers, 2010; Zsebok et al., 2013). In contrast, textured surfaces scatter sound, essentially generating more noise, making it more challenging to distinguish prey items and to drink. We acknowledge that only a relatively small area of the interior is exposed (<0.25 m) around the top edge of the pool, however, studies have shown the echoes returning from the edges of water sources can create background noise for echolocating bats when they are attempting to drink (Greif and Siemers, 2010; Clare and Holderied, 2015). It is, therefore, possible that textured edges generate more background noise than smooth edges, hindering bats from coming in and drinking at swimming pools.
The fourth feature we identified as a predictor in this study was the number of trees visible from the swimming pool. It was an important feature in both the presence of bats on a property and the number of bats observed over the swimming pool. More specifically, we found that bats observed on the property decreased by ∼45% and no activity was recorded over pools with least than five trees visible. These findings support our hypothesis that connected tree-lines could provide bats with covered access to the residential swimming pools. Moreover, a number of studies have shown that contiguous trees and linear features increase resource access and availability for bats (Froidevaux et al., 2019; Moretto et al., 2019; Straka et al., 2019; Gili et al., 2020). Such studies also support our results and highlight the importance of the landscape connectivity in improving urban areas for bats.
A fifth characteristic of swimming pools that influenced the number of bats observed was the presence of connected hot tubs, fountains, and waterfalls. We found that these additional swimming pool features reduced bat observations on the property by ∼80%, but they were not identified as important predictors of bat activity over the swimming pools or drinking. We initially hypothesized that less bats would be active at pools with such features, as the disturbance they caused to the water surface could be challenging for echolocating bats and, therefore, impede them when approaching swimming pool surfaces to drink. Studies have shown that the bat species known to be in the study area prefer still water sources, such as ponds and lakes, as opposed to moving sources, such as rivers and streams (Ammerman et al., 2012). However, disruptions to the water surface should not necessarily cause bats to avoid the property. It is more likely that the noise generated by these features could have resulted in bats avoiding the area. As previously mentioned with regards to mechanical equipment, echolocation can be masked by high frequency sounds and the movement of water trickling or dripping can potentially generate high frequency noises that could hinder bats from echolocating effectively (Todd and Williamson, 2019). If this is the case, then turning connected hot tubs, fountains, and waterfalls off at dusk onward when they are not in use could lead in an increase in bat activity on a property, which in turn, could result in more bats drinking from swimming pools.
In comparison, we found the presence of pets to be an important predictor of bat activity over the swimming pools. More specifically, >40% less bats were reported flying over pools when pets were outside in backyards from dusk onward. This result may be due to the presence of potential predators in the vicinity of a pool being enough to dissuade bats from getting closer to the pools (i.e., the perceived risk of predation) and is supported by similar observations of avoidance behavior reported in other studies (Lima and O’Keefe, 2013; Ancillotto et al., 2019b).
Finally, we identified two features that exclusively influenced the number of bats that were observed drinking from the surfaces of swimming pools. The first was the distance of trees from pools and the second was pool size. The former also represented the most important predictor of drinking activity among bats, showing that when trees where too close to the pool (<5 m) or too far away (>10 m), the amount of drinking observed decreased by >40%. Given that most of the local bat species in the area do not fly in closed or cluttered environments, nor particularly open environments, but prefer to fly along tree-lines and woodland edges, this result is not surprising (Oprea et al., 2009; Moore and Best, 2018). Similarly, the decrease in drinking activity we observed at swimming pools with surface areas of 50 m2 or more in size may be an artifact of species-specific activity. N. humeralis are the most commonly recorded species in the study area both acoustically and caught in mist nets (Bienz, 2016; Nystrom and Bennett, 2019; Hall, 2020). We also know that this species exhibits a higher level of maneuverability in comparison to other local species of the same size (Bienz, 2016; Smith, 2019). For example, behavioral observation studies conducted in a controlled environment revealed that evening bats could drink effectively in more confined areas because they approach the water surface at tighter angles (i.e., a V-shape; Bienz, 2016). Thus, we speculate that the high abundance of N. humeralis, along with their ability and preference to drink water from sources with smaller surface areas, resulted in pools 50 m2 or over having significantly less bats reported drinking at them. Thus, while smaller swimming pools may have N. humeralis actively drink at them, such pools are unlikely to be accessible to a diversity of bat species. In contrast, while pool treatment was identified as an important predictor for bat activity, this characteristic did not overtly encourage or discourage bats. A recent study by Laverty and Berger (2020) support our findings suggesting bat activity is more correlated to water surface area than water quality.
We acknowledge that other characteristics of residential swimming pools, backyards, and surrounding areas, not included in this study, could have encouraged bat activity. The surrounding areas connectivity and canopy height, for example, may have affected the accessibility and approachability of swimming pools (Jackrel and Matlack, 2010; Bailey et al., 2019). While distance from, number of, and quality of natural or semi-natural water sources in the area could be driving the need for bats to use residential swimming pools as a foraging and/or drinking resource (Fern et al., 2018; Suarez-Rubio et al., 2018). We, therefore, recommend further studies to explore how these and other characteristics might influence bat activity, foraging, and drinking at residential swimming pools.
Other caveats associated with our study that should be acknowledged, include the limitations of citizen science, species composition, and study location. Whenever data is collected through citizen science there will always be concerns related to the quality of that data. With our study, in particular, the occurrence of false negatives (i.e., reports of bats not being observed when they are present) could have had a substantial influence on the results. These inaccurate responses could have easily come about, because residents have never acknowledged the presence of bats, do not recognize bats to be actively flying in proximity to them, and/or do not know what a bat or a bat in flight looks like. To accommodate these issues, we addressed the potential for false negatives in our analysis and subsequently discarded 63 of the 78 respondents (>80%) that indicated that they had not observed bats. Thus, of the 133 questionnaires included in this study only 13% represented negative responses. Further, as we have been raising bat awareness in this neighborhood for 8 years, at the time of survey, through outreach and community research involvement, we are confident that all 15 remaining negative responses represent true negatives.
Another major consideration in any citizen science study is the reduction of inaccurate responses. During the design of our questionnaire, we had to be cognizant of devising questions that limited “guesstimates” and uncertainty. For example, we could not ask residents what height they observed bats flying. Nor could be ask if bats were observed foraging or whether high frequency noises were generated by any electrical equipment near to their pools. Consequently, this resulted in many of our questions referencing rather broad, somewhat arbitrary categories. We acknowledge that this type of study does not advance our ecological understanding of water resource use by bats in urban areas, but it does (1) identify a number of avenues for further research, (2) support studies that logistically could not be conducted at the same scale (such as Nystrom and Bennett, 2019), and (3) revealed strategies, albeit broad, that the general public could easily implement to improve their backyards for bats. For example, “are lights on or off at or after dusk” represents a clearly defined question that is unlikely to cause confusion and lead to inaccurate responses. However, from an ecological prospective, it does not consider the influence of various types of illumination, light levels, or extent of illumination. By identifying that bats are more likely to be observed on properties when lights are turned off, our study revealed that lights on residential properties have a negative influence on bats and indicated that turning off lights could have an immediate positive effect on bat activity. Beyond this, it supports the need for additional research to determine the specific influence of lights on bats at residential swimming pools and potentially other water sources. Similarly, our study highlights that the influence of pets on bat activity warrants further research.
Species composition and location are also factors that are likely to be driving our results. Even within the bat community included in this study, there were species-specific differences in abundance, distribution, and ecology, which would have resulted in the more dominant species, such as N. humeralis, influencing our findings (Bienz, 2016; Nystrom and Bennett, 2019). Thus, we recommend that equivalent studies are conducted in areas with different bat communities to determine and identify the features and characteristics of residential swimming pools that influence bat activity among these communities. Similarly, we acknowledge that as our study area represents a more affluent neighborhood in the Dallas-Fort Worth Metroplex, this will have had an impact on species composition (see Li et al., 2019). However, as affluent neighborhoods have the majority of the residential swimming pools, perhaps species composition in these areas reflects the species (such as N. humeralis) that can utilize residential swimming pools as a water resource and potentially thrive in such areas as a result. One potential rationale may that the specific resources required by other species does not include residential swimming pools, thus determining their preferred water resources requires more investigation.
Overall, our results demonstrate that urban and suburban areas (green spaces and similar areas notwithstanding) can provide a resource for bats. Yet access and potential availability are dependent on the quality of those resources (i.e., the characteristics of the swimming pool, backyard, and surrounding area). In this study, we have highlighted some of these characteristics, such as lighting, landscape connectivity, the presence of bushes and shrubs bordering the swimming pool, interior texture, the presence of hot tubs, fountains and waterfalls, and the presence of pets when bats are actively flying over the pool at night. Furthermore, in areas similar to north central Texas, which experience hot and dry conditions for long periods of time, local bat communities could benefit from the presence of swimming pools if they were accessible. Even bat populations in urban areas with limited natural and semi-natural resources in wetter cooler climates may need to utilize swimming pools as a water resource.
Conclusion
There is the potential to make residential swimming pools more bat-friendly. Specifically, our study highlighted that reducing clutter, such as bushes and shrubs bordering swimming pools, would increase accessibility to a pool. In addition, turning lights off in the evening when the pools and backyards are not in use could have an immediate positive impact on urban bat populations. Reducing acoustic noise is also likely to encourage bats to use swimming pools more regularly, thus, we recommend turning off hot tubs, fountains, and waterfalls when not in use and, if possible, consider converting textured pool interiors, or at least the top edges of the pool, to smooth surfaces, such as tiles. Similarly, residents can encourage bats to use swimming pools by keeping pets inside after dusk. Finally, we recommend that trees are preserved where possible. Trees not only represent important roosting resources for bats, their connected canopies allow bats to access resources, such as swimming pools and foraging sites, throughout a neighborhood. Urban landscaping is, therefore, an essential factor in promoting a healthy stable bat community (Bailey et al., 2019). By implementing any or all of the aforementioned recommendations, we may be able to enhance the urban environment for bats (Parker et al., 2019) and alleviate some of the negative implications of continued urbanization.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Texas Christian University IRB Committee. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
VB conceived the presented idea and wrote the final manuscript with support from EA. VB and EA designed the study. EA performed the surveys. Both authors processed and analyzed the data.
Funding
Internal funding was received for the research including a Department Graduate Grant and a College Level Graduate Research Grant.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The content of this manuscript is based on a M.S. thesis research conducted by EA and the research was supported by funding received from the College of Science and Engineering Graduate SERC Grant Award and Department of Environmental Sciences Graduate Research Grant Award. We thank Becky Johnson, Kelly Rodgers Gant, and Shay Guerin who aided the data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.860523/full#supplementary-material
Footnotes
References
Adams, R. A., and Thibault, K. M. (2006). Temporal resource partitioning by bats at water holes. J. Zool. 270, 466–472. doi: 10.1111/j.1469-7998.2006.00152.x
Adesh, K., Ankit, S., and Amita, K. (2021). GIS mapping and distribution of Indian Peafowl (Pavo cristatus) in India by using citizen science data. Int. J. Zool. Invest. 7, 339–351. doi: 10.33745/ijzi.2021.v07i02.004
Agpalo, E. J. (2021). Improving urban habitats for bats: What makes a bat-friendly residential swimming pool? [M.S. thesis]. Fort Worth, TX: Texas Christian University.
Aguiar, L. M. S., Bueno-Rocha, I. D., Oliveira, G., Pires, E. S., Vasconcelos, S., Nunes, G. L., et al. (2021). Going out for dinner-the consumption of agriculture pests by bats in urban areas. PLoS One 16:258066. doi: 10.1371/journal.pone.0258066
Ammerman, L. K., Hice, C. L., and Schmidly, D. J. (2012). Bats of Texas. College Station: Texas A&M University Press.
Ancillotto, L., Bosso, L., Salinas-Ramos, V. B., and Russo, D. (2019a). The importance of ponds for the conservation of bats in urban landscapes. Landsc. Urban Plan. 190:103607. doi: 10.1016/j.landurbplan.2019.103607
Ancillotto, L., Venturi, G., and Russo, D. (2019b). Presence of humans and domestic cats affects bat behaviour in an urban nursery of greater horseshoe bats (Rhinolophus ferrumequinum). Behav. Proc. 164, 4–9. doi: 10.1016/j.beproc.2019.04.003
Avila-Flores, R., Bolaina-Badal, A., Gallegos-Ruiz, A., and Sánchez-Gómez, W. (2019). Use of linear features by the common vampire bat (Desmodus rotundus) in a tropical cattle-ranching landscape. Therya 10, 229–934. doi: 10.12933/therya-19-890
Ayala-Berdon, J., Vazquez-Fuerte, R., Rodriguez-Pena, N., and Gomez, M. M. (2017). Bat fauna associated with artificial ponds in La Malinche National Park, a mountain ecosystem of Mexico. Mammalia 81, 573–581. doi: 10.1515/mammalia-2016-0055
Bailey, A. M., Ober, H. K., Reichert, B. E., and McCleery, R. A. (2019). Canopy cover shapes bat diversity across an urban and agricultural landscape mosaic. Environ. Conserv. 46, 193–200. doi: 10.1017/S0376892919000109
Bennett, V. J., Fernandez-Juricic, E., Zollner, P. A., Beard, M. J., Westphal, L., and Fisher, C. L. L. (2011). Modelling the responses of wildlife to human disturbance: An evaluation of alternative management scenarios for black-crowned night-herons. Ecol. Model. 222, 2770–2779. doi: 10.1016/j.ecolmodel.2011.04.025
Bergeson, S. M., Holmes, J. B., and O’Keefe, J. M. (2020). Indiana bat roosting behavior differs between urban and rural landscapes. Urban Ecosyst. 23, 79–91. doi: 10.1007/s11252-019-00903-4
Bienz, C. (2016). Surface texture discrimination by bats: Implications for reducing bat mortality at wind turbines. [M.S. thesis]. Fort Worth, TX: Texas Christian University.
Boerma, D. B., Barrantes, J. P., Chung, C., Chaverri, G., and Swartz, S. M. (2019). Specialized landing maneuvers in Spix’s disk-winged bats (Thyroptera tricolor) reveal linkage between roosting ecology and landing biomechanics. J. Exp. Biol. 222:9. doi: 10.1242/jeb.204024
Bonsen, G., Law, B., and Ramp, D. (2015). Foraging strategies determine the effect of traffic noise on bats. Acta Chiropt. 17, 347–357. doi: 10.3161/15081109acc2015.17.2.010
Border, J. A., Newson, S. E., White, D. C. J., and Gillings, S. (2017). Predicting the likely impact of urbanisation on bat populations using citizen science data, a case study for Norfolk. UK. Landsc. Urban Plan. 162, 44–55. doi: 10.1016/j.landurbplan.2017.02.005
Brown, B. B., Hunter, L., and Santos, S. (2020). Bird-window collisions: different fall and winter risk and protective factors. PeerJ 8:e9401. doi: 10.7717/peerj.9401
Cabral, T. C., Dellagnese, D. G., Bordignon, S. A. D., Forneck, E. D., and Cademartori, C. V. (2016). The use of Piper amalago L. Volatile oil to attract fruit-eating bats. Cienc. Florest. 26, 949–955.
Candido, M. E. M. B., Miranda, P. N., and Morato, E. F. (2021). Orchid bees in riparian and terra-firme forest fragments in an urban matrix in southwestern Brazilian Amazonia. Acta Amazon. 51, 214–223. doi: 10.1590/1809-4392202003781
Chauhan, H. K., and Gallacher, D. (2021). Can citizen reporting apps plug the data gap in the Himalayan wildlife trade? Trees For. People 6:100150. doi: 10.1016/j.tfp.2021.100150
Ciechanowski, M., Zajac, T., Bitas, A., and Dunajski, R. (2007). Spatiotemporal variation in activity of bat species differing in hunting tactics: effects of weather, moonlight, food abundance, and structural clutter. Can. J. Zool. 85, 1249–1263. doi: 10.1139/z07-090
Clare, E. L., and Holderied, M. W. (2015). Acoustic shadows help gleaning bats find prey, but may be defeated by prey acoustic camouflage on rough surfaces. eLife 4:e07404. doi: 10.7554/eLife.07404
Coomber, F. G., Smith, B. R., August, T. A., Harrower, C. A., Powney, G. D., and Mathews, F. (2021). Using biological records to infer long-term occupancy trends of mammals in the UK. Biol. Conserv. 264:109362. doi: 10.1016/j.biocon.2021.109362
Correa, C. M. A., da Silva, P. G., Ferreira, K. R., and Puker, A. (2021). Residential sites increase species loss and cause high temporal changes in functional diversity of dung beetles in an urbanized Brazilian Cerrado landscape. J. Insect. Conserv. 25, 417–428. doi: 10.1007/s10841-021-00310-1
de Araujo, M., and Bernard, E. (2016). Green remnants are hotspots for bat activity in a large Brazilian urban area. Urban Ecosyst. 19, 287–296. doi: 10.1007/s11252-015-0487-z
Edwards, T., Jones, C. B., and Corcoran, P. (2022). Identifying wildlife observations on twitter. Ecol. Inform. 67:101500. doi: 10.1016/j.ecoinf.2021.101500
Fern, R. R., Davis, H. T., Baugardt, J. A., Morrison, M. L., and Campbell, T. A. (2018). Summer activity patterns of four resident south Texas bat species. Glob. Ecol. Conserv. 16:e00500. doi: 10.1016/j.gecco.2018.e00500
Finch, D., Schofield, H., and Mathews, F. (2020). Traffic noise playback reduces the activity and feeding behaviour of free-living bats. Environ. Pollut. 263:7. doi: 10.1016/j.envpol.2020.114405
Fischer, H. A., Gerber, L. R., and Wentz, E. A. (2021). Evaluating the fitness for use of citizen science data for wildlife monitoring. Front. Ecol. Evol. 9:620850. doi: 10.3389/fevo.2021.620850
FOFPA (2014). Overton & Foster Park master plan, final draft. Fort Worth, TX: Friends of the Foster and Overton Parks Association.
Frank, T. M., Gabbert, W. C., Chaves-Campos, J., and LaVal, R. K. (2019). Impact of artificial lights on foraging of insectivorous bats in a Costa Rican cloud forest. J. Trop. Ecol. 35, 8–17. doi: 10.1017/s0266467418000408
Froidevaux, J. S. P., Boughey, K. L., Hawkins, C. L., Broyles, M., and Jones, G. (2019). Managing hedgerows for nocturnal wildlife: do bats and their insect prey benefit from targeted agri-environment schemes? J. Appl. Ecol. 56, 1610–1623. doi: 10.1111/1365-2664.13412
Genzel, D., Hoffmann, S., Prosch, S., Firzlaff, U., and Wiegrebe, L. (2015). Biosonar navigation above water II: exploiting mirror images. J. Neurophysiol. 113, 1146–1155. doi: 10.1152/jn.00264.2014
Gili, F., Newson, S. E., Gillings, S., Chamberlain, D. E., and Border, J. A. (2020). Bats in urbanising landscapes: habitat selection and recommendations for a sustainable future. Biol. Conserv. 241:108343. doi: 10.1016/j.biocon.2019.108343
Goncalves, S. F., de Paula Lourenco, A. C., de Sousa Bueno, Filho, J. S., Barbosa, and de Toledo, M. C. (2021). Characteristics of residential backyards that contribute to conservation and diversity of urban birds: a case study in a Southeastern Brazilian city. Urban For. Urban Gree. 61:127095. doi: 10.1016/j.ufug.2021.127095
Greif, S., and Siemers, B. M. (2010). Innate recognition of water bodies in echolocating bats. Nat. Comms. 1:107. doi: 10.1038/ncomms1110
Gutierrez-Munoz, P., Walters, A. E. M., Dolman, S. J., and Pierce, G. J. (2021). Patterns and trends in cetacean occurrence revealed by Shorewatch, a land-based citizen science program in Scotland (United Kingdom). Front. Marine Sci. 8:642386. doi: 10.3389/fmars.2021.642386
Haddock, J. K., Threlfall, C. G., Law, B., and Hochuli, D. F. (2019). Light pollution at the urban forest edge negatively impacts insectivorous bats. Biol. Conserv. 236, 17–28. doi: 10.1016/j.biocon.2019.05.016
Hall, E. M. (2020). Home range expansion by evening bats (Nycticeius humeralis) in an urban environment. [M.S. thesis]. Fort Worth, TX: Texas Christian University.
Hall, E. M., and Bennett, V. J. (2021). Seasonal variation in home range size of evening bats (Nycticeius humeralis) in an urban environment. J. Mammal. 102, 1497–1506. doi: 10.1093/jmammal/gyab106
Hall, L. K., Lambert, C. T., Larsen, R. T., Knight, R. N., and McMillan, B. R. (2016). Will climate change leave some desert bat species thirstier than others? Biol. Conserv. 201, 284–292. doi: 10.1016/j.biocon.2016.07.020
Hintze, F., Duro, V., Carvalho, J. C., Eira, C., Rodrigues, P. C., and Vingada, J. (2016). Influence of reservoirs created by small dams on the activity of bats. Acta Chiropt. 18, 395–408. doi: 10.3161/15081109acc2016.18.2.007
Hoffmann, R., Ungureanu, M., and Covaciu-Marcov, S. D. (2016). A large abandoned cellar as roost for bats in western Romania. Period. Biol. 118, 139–141.
Hughes, J. M., Meadows, N. M., Stewart, J., Booth, D. J., and Fowler, A. M. (2022). Movement patterns of an iconic recreational fish species, mulloway (Argyrosomus japonicus), revealed by cooperative citizen-science tagging programs in coastal eastern Australia. Fish. Res. 247:106179. doi: 10.1016/j.fishres.2021.106179
Jackrel, S. L., and Matlack, R. S. (2010). Influence of surface area, water level and adjacent vegetation on bat use of artificial water sources. Am. Midl. Nat. 164, 74–79. doi: 10.1674/0003-0031-164.1.74
Jara-Servin, A. M., Saldana-Vazquez, R. A., and Schondube, J. E. (2017). Nutrient availability predicts frugivorous bat abundance in an urban environment. Mammalia 81, 367–374. doi: 10.1515/mammalia-2015-0039
Jensen, J. K., Jayousi, S., von Post, M., Isaksson, C., and Persson, A. S. (2021). Contrasting effects of tree origin and urbanization on invertebrate abundance and tree phenology. Ecol. Apps. 2021:e02491. doi: 10.1002/eap.2491
Jung, K., and Threlfall, C. G. (2016). “Urbanisation and its effects on bats – a global meta-analysis,” in Bats in the Anthropocene: Conservation of Bats in a Changing World, eds C. Voigt and T. Kingston (Cham: Springer), 13–33. doi: 10.1007/978-3-319-25220-9_7
Jung, K., and Threlfall, C. G. (2018). Trait-dependent tolerance of bats to urbanization: a global meta-analysis. Proc. Royal Soc. B 283:20181222. doi: 10.1098/rspb.2018.1222
Kasper, S., and Yancey, F. D. (2018). Year-round bridge colony of Mexican free-tailed bats (Tadarida brasiliensis mexicana) in Trans-Pecos Texas. Texas J. Sci. 70, 57–69. doi: 10.32011/txjsci_70_1_Article4
Kelly, R. M., Kitzes, J., Wilson, H., and Merenlender, A. (2016). Habitat diversity promotes bat activity in a vineyard landscape. Agric. Ecosyst. Environ. 223, 175–181. doi: 10.1016/j.agee.2016.03.010
Laverty, T. M., and Berger, J. (2020). Do bats seek clean water? A perspective on biodiversity from the Namib Desert. Biol. Conserv. 248:108686. doi: 10.1016/j.biocon.2020.108686
Le Roux, D. S., Ikin, K., Lindenmayer, D. B., Manning, A. D., and Gibbons, P. (2018). The value of scattered trees for wildlife: contrasting effects of landscape context and tree size. Divers. Distrib. 24, 69–81. doi: 10.1111/ddi.12658
Lewanzik, D., Straka, T. M., Lorenz, J., Marggraf, L., Voigt-Heucke, S., Schumann, A., et al. (2021). Evaluating the potential of urban areas for bat conservation with citizen science data. Environ. Pollut. 297:118785. doi: 10.1016/j.envpol.2021.118785
Li, H., and Kalcounis-Rueppell, M. (2018). Separating the effects of water quality and urbanization on temperate insectivorous bats at the landscape scale. Ecol. Evol. 8, 667–678. doi: 10.1002/ece3.3693
Li, H., and Wilkins, K. T. (2014). Patch or mosaic: bat activity responds to fine-scale urban heterogeneity in a medium-sized city in the United States. Urban Ecosyst. 17, 1013–1031. doi: 10.1007/s11252-014-0369-9
Li, H., and Wilkins, K. T. (2015). Selection of building roosts by Mexican free-tailed bats (Tadarida brasiliensis) in an urban area. Acta Chiropt. 17, 321–330. doi: 10.3161/15081109ACC2015.17.2.007
Li, H., Parker, K. A., and Kalcounis-Rueppell, M. C. (2019). The luxury effect beyond cities: bats respond to socioeconomic variation across landscapes. BMC Ecol. 19:46. doi: 10.1186/s12898-019-0262-8
Li, J. H., Wang, Z. H., Zhu, X. J., Deng, Z. H., Cai, C. X., Qiu, L. Q., et al. (2015). Health effects from swimming training in chlorinated pools and the corresponding metabolic stress pathways. PLoS One 10:14. doi: 10.1371/journal.pone.0119241
Lim, B. K., Brock Fenton, M., Brigham, R. M., Mistry, S., Kurta, A., Gillam, E. H., et al. (2021). 50 years of bat research: foundations and new frontiers. Berlin: Springer.
Lima, S. L., and O’Keefe, J. M. (2013). Do predators influence the behaviour of bats? Biol. Rev. 88, 626–644. doi: 10.1111/brv.12021
Liu, J., and Slik, F. (2022). Are street trees friendly to biodiversity? Landsc. Urban Plan. 218:104304. doi: 10.1016/j.lurbplan.2021.104304
Loumassine, H. E., Bonnot, N., Allegrini, B., Bendjeddou, M. L., Bounaceur, F., and Aulagnier, S. (2020). How arid environments affect spatial and temporal activity of bats. J. Arid. Environ. 180:104206. doi: 10.1016/j.jaridenv.2020.104206
Mager, K. J., and Nelson, T. A. (2001). Roost-site selection by eastern red bats (Lasiurus borealis). Am. Midl. Nat. 145, 120–126. doi: 10.1674/0003-0031(2001)145[0120:rssber]2.0.co;2
McAlexander, A. (2013). Evidence that bats perceive wind turbine surfaces to be water. [M.S. thesis]. Fort Worth, TX: Texas Christian University.
McCracken, G. F., Bernard, R. F., Gamba-Rios, M., Wolfe, R., Krauel, J. J., Jones, D. N., et al. (2018). Rapid range expansion of the Brazilian free-tailed bat in the southeastern United States, 2008-2016. J. Mammal. 99, 312–320. doi: 10.1093/jmammal/gyx188
McCune, B., Grace, J., and Urban, D. (2002). Analysis of Ecological Communities. Oregon: Wild Blueberry Media.
McGowan, K. A., and Kloepper, L. N. (2020). Different as night and day: wild bats modify echolocation in complex environments when visual cues are present. Anim. Behav. 168, 1–6. doi: 10.1016/j.anbehav.2020.07.025
Moore, L. H., and Best, T. L. (2018). Impact of vegetation on activity of bats over wetlands in coastal South Carolina. J. Mammal. 99, 1082–1092. doi: 10.1093/jmammal/gyy086
Moretto, L., and Francis, C. M. (2017). What factors limit bat abundance and diversity in temperate, North American urban environments? J. Urban Ecol. 3:16. doi: 10.1093/jue/jux016
Moretto, L., Fahrig, L., Smith, A. C., and Francis, C. M. (2019). A small-scale response of urban bat activity to tree cover. Urban Ecosyst. 22, 795–805. doi: 10.1007/s11252-019-00846-w
Murray, M. H., Sanchez, C. A., Becker, D. J., Byers, K. A., Worsley-Tonks, K. E. L., and Craft, M. E. (2019). City sicker? A meta-analysis of wildlife health and urbanization. Front. Ecol. Environ. 17, 575–583. doi: 10.1002/fee.2126
Neubaum, D. J. (2018). Unsuspected retreats: autumn transitional roosts and presumed winter hibernacula of little brown myotis in Colorado. J Mammal 99, 1294–1306. doi: 10.1093/jmammal/gyy120
Ng, A. M. B., Pontius, M. L., De Ruiter, S. L., and Proppe, D. S. (2020). Noise, avian abundance, and productivity at banding stations across the Continental United States. Avian Conserv. Ecol. 15:4. doi: 10.5751/ACE-01633-150204
Niemiller, K. D. K., Davis, M. A., and Niemiller, M. L. (2021). Addressing ‘biodiversity naivety’ through project-based learning using iNaturalist. J. Nat. Conserv. 64:126070. doi: 10.1016/j.jnc.2021.126070
Nystrom, G. S., and Bennett, V. J. (2019). The importance of residential swimming pools as an urban water source for bats. J. Mammal. 100, 394–400. doi: 10.1093/jmammal/gyz020
O’Malley, K. D., Kunin, W. E., Town, M., Mgoola, W. O., and Stone, E. L. (2020). Roost selection by Mauritian tomb bats (Taphozus mauritianus) in Lilongwe city, Malawi - importance of woodland for sustainable urban planning. PLoS One 15:e0240434. doi: 10.1371/journal.pone.0240434
Oprea, M., Mendes, P., Vieira, T. B., and Ditchfield, A. D. (2009). Do wooded streets provide connectivity for bats in an urban landscape? Biodivers. Conserv. 18, 2361–2371. doi: 10.1007/s10531-009-9593-7
Owens, E., Heard, S. B., and Johns, R. C. (2021). Having it all: hybridizing conventional and community science monitoring for enhanced data quality and cost savings. Facets 6, 2028–2041. doi: 10.1139/facets-2021-0013
Palheta, L. R., Urbieta, G. L., Brasil, L. S., Dias-Silva, K., da Silva, J. B., Graciolli, G., et al. (2020). The effect of urbanization on bats and communities of bat flies (Diptera: Nycteribiidae and Streblidae) in the Amazon, northern Brazil. Acta. Chiropt. 22, 403–416. doi: 10.3161/15081109ACC2020.22.2.014
Parker, K. A., Springall, B. T., Garshong, R. A., Malachi, A. N., Dorn, L. E., Costa-Terryll, A., et al. (2019). Rapid increases in bat activity and diversity after wetland construction in an urban ecosystem. Wetlands 39, 717–727. doi: 10.1007/s13157-018-1115-5
Parkins, K. L., and Clark, J. A. (2015). Green roofs provide habitat for urban bats. Glob. Ecol. Conserv. 4, 349–357. doi: 10.1016/j.gecco.2015.07.011
Partridge, D. R., Parkins, K. L., Elbin, S. B., and Clark, J. A. (2020). Bat activity correlates with moth abundance on an urban green roof. Northeast. Nat. 27, 77–89. doi: 10.1656/045.027.0107
Ramirez-Francel, L. A., Garcia-Herrera, L. V., Losada-Prado, S., Reinoso-Florez, G., Sanchez-Hernandez, A., Estrada-Villegas, S., et al. (2022). Bats and their vital ecosystem services: a global review. Integr. Zool. 17, 2–23. doi: 10.1111/1749-4877.12552
Richter, A., Comay, O., Svenningsen, C. S., Larsen, J. C., Hecker, S., Tottrup, A. P., et al. (2021). Motivation and support services in citizen science insect monitoring: a cross-country study. Biol. Conserv. 263:109325. doi: 10.1016/j.biocon.2021.109325
Robertson, B. A., Keddy-Hector, I. A., Shrestha, S. D., Silverberg, L. Y., Woolner, C. E., Hetterich, I., et al. (2018). Susceptibility to ecological traps is similar among closely related taxa but sensitive to spatial isolation. Anim. Behav. 135, 77–84. doi: 10.1016/j.anbehav.2017.10.023
Rowse, L., Lewanzik, D., Stone, E., Harris, S., and Jones, G. (2016). “Dark matters: The effects of artificial lighting on bats,” in Bats in the Anthropocene: Conservation of Bats in a Changing World, eds C. Voigt and T. Kingston (Cham: Springer), 187–213.
Russo, D., Ancillotto, L., Cistrone, L., Libralato, N., Domer, A., Cohen, S., et al. (2019a). Effects of artificial illumination on drinking bats: a field test in forest and desert habitats. Anim. Conserv. 22, 124–133. doi: 10.1111/acv.12443
Russo, D., Cistrone, L., Libralato, N., Korine, C., Jones, G., and Ancillotto, L. (2017). Adverse effects of artificial illumination on bat drinking activity. Anim. Conserv. 20, 492–501. doi: 10.1111/acv.12340
Russo, D., Cosentino, F., Festa, F., De Benedetta, F., Pejic, B., Cerretti, P., et al. (2019b). Artificial illumination near rivers may alter bat-insect trophic interactions. Environ. Pollut. 252, 1671–1677. doi: 10.1016/j.envpol.2019.06.105
Rydell, J., Miller, L. A., and Jensen, M. E. (1999). Echolocation constraints of Daubenton’s Bat foraging over water. Funct. Ecol. 13, 247–255. doi: 10.1046/j.1365-2435.1999.00304.x
Siemers, B. M., Baur, E., and Schnitzler, H. U. (2005). Acoustic mirror effect increases prey detection distance in trawling bats. Naturwissenschaften 92, 272–276. doi: 10.1007/s00114-005-0622-4
Smith, K. (2019). Assessing the potential impacts of radio transmitters on bat flight and behavior in a controlled environment. [M.S. thesis]. Fort Worth, TX: Texas Christian University.
Spear, D. M., Pauly, G. B., and Kaiser, K. (2017). Citizen science as a tool for augmenting museum collection data from urban areas. Front. Ecol. Evol. 5:86. doi: 10.3389/fevo.2017.00086
Straka, T. M., Lentini, P. E., Lumsden, L. F., Wintle, B. A., and van der Ree, R. (2016). Urban bat communities are affected by wetland size, quality, and pollution levels. Ecol. Evol. 6, 4761–4774. doi: 10.1002/ece3.2224
Straka, T. M., Wolf, M., Gras, P., Buchholz, S., and Voigt, C. C. (2019). Tree cover mediates the effect of artificial light on urban bats. Front. Ecol. Evol. 7:11. doi: 10.3389/fevo.2019.00091
Suarez-Rubio, M., Ille, C., and Bruckner, A. (2018). Insectivorous bats respond to vegetation complexity in urban green spaces. Ecol. Evol. 8, 3240–3253. doi: 10.1002/ece3.3897
Thomas, J. P., Kukka, P. M., Benjamin, J. E., Barclay, R. M. R., Johnson, C. J., Schmiegelow, F. K. A., et al. (2021). Foraging habitat drives the distribution of an endangered bat in an urbanizing boreal landscape. Ecosphere 12:e03457. doi: 10.1002/ecs2.3457
Threlfall, C. G., Law, B., and Banks, P. B. (2012). Influence of landscape structure and human modifications on insect biomass and bat foraging activity in an urban landscape. PLoS One 7:10. doi: 10.1371/journal.pone.0038800
Threlfall, C. G., Williams, N. S. G., Hahs, A. K., and Livesley, S. J. (2016). Approaches to urban vegetation management and the impacts on urban bird and bat assemblages. Landsc. Urban Plan. 153, 28–39. doi: 10.1016/j.landurbplan.2016.04.011
Threlfall, C., Law, B., Penman, T., and Banks, P. B. (2011). Ecological processes in urban landscapes: mechanisms influencing the distribution and activity of insectivorous bats. Ecography 34, 814–826. doi: 10.1111/j.1600-0587.2010.06939.x
Tian, L. X., Lin, W., Zhang, S. Y., and Pan, Y. X. (2010). Bat head contains soft magnetic particles: Evidence from magnetism. Bioelectromagnetics 31, 499–503. doi: 10.1002/bem.20590
Todd, V. L. G., and Williamson, L. D. (2019). Habitat usage of Daubenton’s bat (Myotis daubentonii), common pipistrelle (Pipistrellus pipistrellus), and soprano pipistrelle (Pipistrellus pygmaeus) in a North Wales upland river catchment. Ecol. Evol. 9, 4853–4863. doi: 10.1002/ece3.5085
Tuttle, S. R., Chambers, C. L., and Theimer, T. C. (2006). Potential effects of livestock water-trough modifications on bats in northern Arizona. Wildl. Soc. Bull. 3, 602–608. doi: 10.2193/0091-7648(2006)34[602:peolwm]2.0.co;2
USGS (2018). Sediment and suspended sediment. Available online at: www.usgs.gov/special-topics/water-science-school/science/sediment-and-suspended-sediment (accessed December 29, 2020).
van Helden, B. E., Close, P. G., Stewart, B. A., and Speldewinde, P. C. (2021). Managing gardens for wildlife: features that predict mammal presence and abundance in gardens vary seasonally. Ecosphere 12:e03453. doi: 10.1002/ecs2.3453
Voigt, C. C., Scholl, J. M., Bauer, J., Teige, T., Yovel, Y., Kramer-Schadt, S., et al. (2020). Movement responses of common noctule bats to the illuminated urban landscape. Landsc. Ecol. 35, 189–201. doi: 10.1007/s10980-019-00942-4
Wilk, A. J., Donlon, K. C., and Peterman, W. E. (2020). Effects of habitat fragment size and isolation on the density and genetics of urban red-backed salamanders (Plethodon cinereus). Urban Ecosyst. 23, 761–773. doi: 10.1007/s11252-020-00958-8
Wilson, J. S., Pan, A. D., General, D. E. M., and Koch, J. B. (2020). More eyes on the prize: an observation of a very rare, threatened species of Philippine Bumble bee, Bombus irisanensis, on iNaturalist and the importance of citizen science in conservation biology. J. Inect. Conserv. 24, 727–729. doi: 10.1007/s10841-020-00233-3
Keywords: bat activity, Chiroptera, drinking behavior, resource use, questionnaire, water source
Citation: Bennett VJ and Agpalo EJ (2022) Citizen Science Helps Uncover the Secrets to a Bat-Friendly Swimming Pool in an Urban Environment. Front. Ecol. Evol. 10:860523. doi: 10.3389/fevo.2022.860523
Received: 23 January 2022; Accepted: 05 May 2022;
Published: 20 May 2022.
Edited by:
Rusty Gonser, Indiana State University, United StatesReviewed by:
Han Li, University of North Carolina at Greensboro, United StatesNicole Abaid, Virginia Tech, United States
Copyright © 2022 Bennett and Agpalo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victoria J. Bennett, di5iZW5uZXR0QHRjdS5lZHU=
 Victoria J. Bennett
Victoria J. Bennett Elizabeth J. Agpalo
Elizabeth J. Agpalo