- Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China
Urban expansion often accompanies a loss of natural habitats and arable lands but an increase in urban population. In China, vegetable-dominant small farmlands are common in urban/peri-urban areas. Some farmlands are also associated with government policy that aims to enhance local farmers’ livelihoods as well as increase food availability for city citizens. While small urban farmlands create open greenery cover that may provide birds with resources such as food and shelter, little attention has been given to understanding bird diversity in urban farmlands. Using two hierarchical models (multi-species occupancy model and N-mixture model), we examined how species richness and abundance of birds were associated with environmental characteristics within and surrounding urban farmlands in Guangzhou, one of the largest cities in China. We conducted crop and bird surveys at urban farmlands during two winter seasons between December 2019 and January 2021. Species richness increased with non-woody (herbaceous) vegetation cover within a farmland. Abundance of three species was also positively associated with the local non-woody vegetation variable. Two species were more abundant at farmlands with higher crop diversity. Compositional features of matrix surrounding a farmland (a 500-m circular area) did not affect species richness. However, species richness and abundance of one species tended to decrease with increasing farmland fragmentation (patch density of farmlands) within a 1-km circular area. These findings suggest that (1) birds could be more influenced by environmental features within farmlands than matrix features surrounding farmlands, (2) local uncultivated herbaceous vegetation is an important environmental feature, and (3) diverse crops in farmlands may benefit some birds. They also indicate that the landscape pattern of farmlands, such as degree of fragmentation, could affect bird diversity in urban farmlands.
Introduction
Urbanization is a significant driver of land cover changes, causing habitat loss and fragmentation and consequently altering ecosystems and associated biological communities (Grimm et al., 2008; McDonald et al., 2008, 2013; Seto et al., 2012). Urbanization also raises a concern for food security, particularly in a city with high food demand, as urban development produces declines in arable land (Satterthwaite et al., 2010). A recent study forecasts that approximately 80% of global agricultural land loss from urban expansion would take place in Asia and Africa, with the highest loss in Asia (d’Amour et al., 2017). China and India are also expected to be centers of global urban expansion by 2030 (Seto et al., 2012). This land use change is critical to China because food security in China has been under pressure due to its large population size (Veeck, 2013; Grumbine, 2014).
There has been growing interest in urban or peri-urban agriculture as one of the options that can create “productive” cities, increasing the accessibility of food and supporting local farmers’ livelihoods (De Bon et al., 2010; Lin et al., 2015). Urban agriculture is broadly defined as agricultural production in any built environment, i.e., the production of a variety of crops (mostly vegetables and fruits) and livestock goods within cities, suburban, and peri-urban areas (Mougeot, 2000; McClintock, 2010). A majority of urban agriculture research has been centered on “food production” per se or human well-being (e.g., healthy food). Recent emphasis on urban agriculture is shifting to its ability to “greening cities” (WinklerPrins, 2017). Urban farmland is increasingly considered as a main component of non-built areas that may improve the sustainability of cities (La Rosa et al., 2014). However, relatively little attention has been given to the potential value of urban farmlands as semi-natural habitat for animals and their role in urban biodiversity conservation (but see urban garden studies; Egerer et al., 2020; Van Helden et al., 2020).
Green space is an essential component of urban infrastructure important to both human inhabitants and urban biodiversity, especially bird diversity (Sandström et al., 2006; Tzoulas et al., 2007; Aronson et al., 2014; Beninde et al., 2015; Filazzola et al., 2019). The positive effect of amount of greenery cover on diversity and abundance of birds is well-known (Chace and Walsh, 2006; Ortega-Álvarez and MacGregor-Fors, 2009; MacGregor-Fors and Schondube, 2011; among others). However, most studies have been conducted in tree/shrub-dominant green spaces such as forest remnants and urban parks. Recent studies suggest that in urban and peri-urban areas, open green space (e.g., recreational grassland and mixed weedy vegetation in vacant lots) can also be species-rich and benefit open habitat birds (Villaseñor et al., 2020; Pithon et al., 2021). Urban farmlands are frequently used for the cultivation of vegetables, which are herbaceous plants and structurally different from trees and shrubs. Some farmlands contain small patch of trees as well as uncultivated herbaceous vegetation (non-woody or weedy vegetation) between crops, along edges, and in unused part of farmlands. These farmlands may provide birds with a number of resources such as food and shelter. Vegetable-dominant farmlands may also be favored by open habitat species, i.e., birds preferring open habitats such as grasslands, early successional habitats, and other open areas.
In China, small-scale farming is common in most cities. Urban farming often occurs in the vicinity of residential areas and riverbanks, and urban fringes (Horowitz and Liu, 2017). Herbaceous edible crops, i.e., a variety of vegetables including leafy greens, starchy vegetables, and fruit, are major crops in urban farmlands (Horowitz and Liu, 2017; Lee et al., 2019). Several urban farmlands are also permanently preserved and supported by government policies such as “Vegetable Basket” and “urban modern agriculture.” These policies have been initiated to ensure both food supplies for city citizens and local farmers’ livelihoods (Peng et al., 2015; Zhong et al., 2019). Crop diversity (richness and evenness of crops) also somewhat varies by farmlands. As far as we know, only one study (Lee et al., 2019) specifically considered urban farmlands as part of open green spaces. Lee et al. (2019) report that herbaceous vegetation in weedy patches and crops in urban farmlands may serve as open habitats for birds in a subtropical city of southern China. While this implies a positive effect of urban farmlands on birds, the effect remains speculative because the study did not distinguish between crop cover and weedy vegetation cover. There is also a considerable lack of knowledge on the bird diversity–environment relationship in urban farmlands of China.
Our study aimed to quantify environmental characteristics affecting bird community (species richness) and population (abundance) in urban farmlands in Guangzhou, a subtropical city of Guangdong province, China. South Guangdong including Guangzhou and its peri-urban areas is part of the Indo-Burma biodiversity hotspot. Based on 2,000 data, approximately 92% of total urban areas across all biodiversity hotspots in China take place in the Indo-Burma biodiversity hotspot (Güneralp and Seto, 2013). A major portion of the total urban area is concentrated in south Guangdong. The largest urban expansion is also expected to take place in Guangdong and Guangxi in China (Güneralp et al., 2015). It is thus a pressing matter to develop sound management plans that minimize biodiversity loss in the city but at the same time ensure food availability for city citizens. Urban farmland may contribute to accomplishing both, i.e., urban biodiversity conservation and crop production. To assess the possibility, it is critical to understand biodiversity patterns in urban farmlands and environmental factors associated with those patterns.
We choose birds as focal taxon because they are relatively easy to survey and commonly used in urban biodiversity studies, and they contribute both direct and indirect ecosystem services such as pest control, seed dispersal, and pollination, and provide recreational activity, e.g., birdwatching (Sekercioglu, 2006; Beninde et al., 2015; Whelan et al., 2015). We also focused on winter bird community because urban winter bird diversity is less frequently studied (Lepczyk et al., 2017). Moreover, in southern China, bird diversity is higher in winter than summer due to a great number of winter migrants. A previous study also found a significant effect of crop features (e.g., crop diversity) on species richness and occupancy of birds in agricultural landscapes of southern China during winter (Lee and Goodale, 2018). Two hierarchical models, multi-species occupancy model and N-mixture model, were employed to estimate species richness and abundance, respectively. Most urban farmlands in China are small and embedded in a heterogeneous matrix composed of different extents of built-up structure, natural and semi-natural vegetation (weeds, trees/shrubs), open water, and other farmlands in some areas. Characteristics of matrix surrounding urban parks can have an impact on birds (Jokimäki, 1999; MacGregor-Fors and Ortega-Alvarez, 2011; Claudia and Christian, 2015; Canedoli et al., 2018). Similarly, birds in urban farmlands may be influenced by environment characteristics within farmlands and their matrix features as well. Thus, we adopted a multiple scale approach, considering both environmental features within a farmland (local scale) and matrix features surrounding urban farmlands (landscape scale). We expected that natural and semi-natural vegetation within and surrounding an urban farmland would have a significant effect on species richness of birds given the importance of greenery cover to bird diversity in urbanized landscapes. In particular, open habitat species should show a strong response to uncultivated non-woody vegetation cover because herbaceous vegetation is known to have a positive impact on grassland and farmland birds in agricultural landscapes (Henderson et al., 2000; Conover et al., 2009; Vickery et al., 2009; Josefsson et al., 2013). Crop diversity at a local scale may also benefit some species by increasing the availability of more and more diverse resources required by the species (Lee and Goodale, 2018).
Materials and Methods
Study Area
Our study was performed in vegetable-dominant farmlands in the city of Guangzhou, the capital of Guangdong province, China (Figure 1). Guangzhou is home to 18.7 million people.1 Guangzhou has a subtropical climate with hot and humid summer and mild and dry winter. Annual rainfall and mean annual temperature are 1,720 mm and 22.2°C, respectively.2
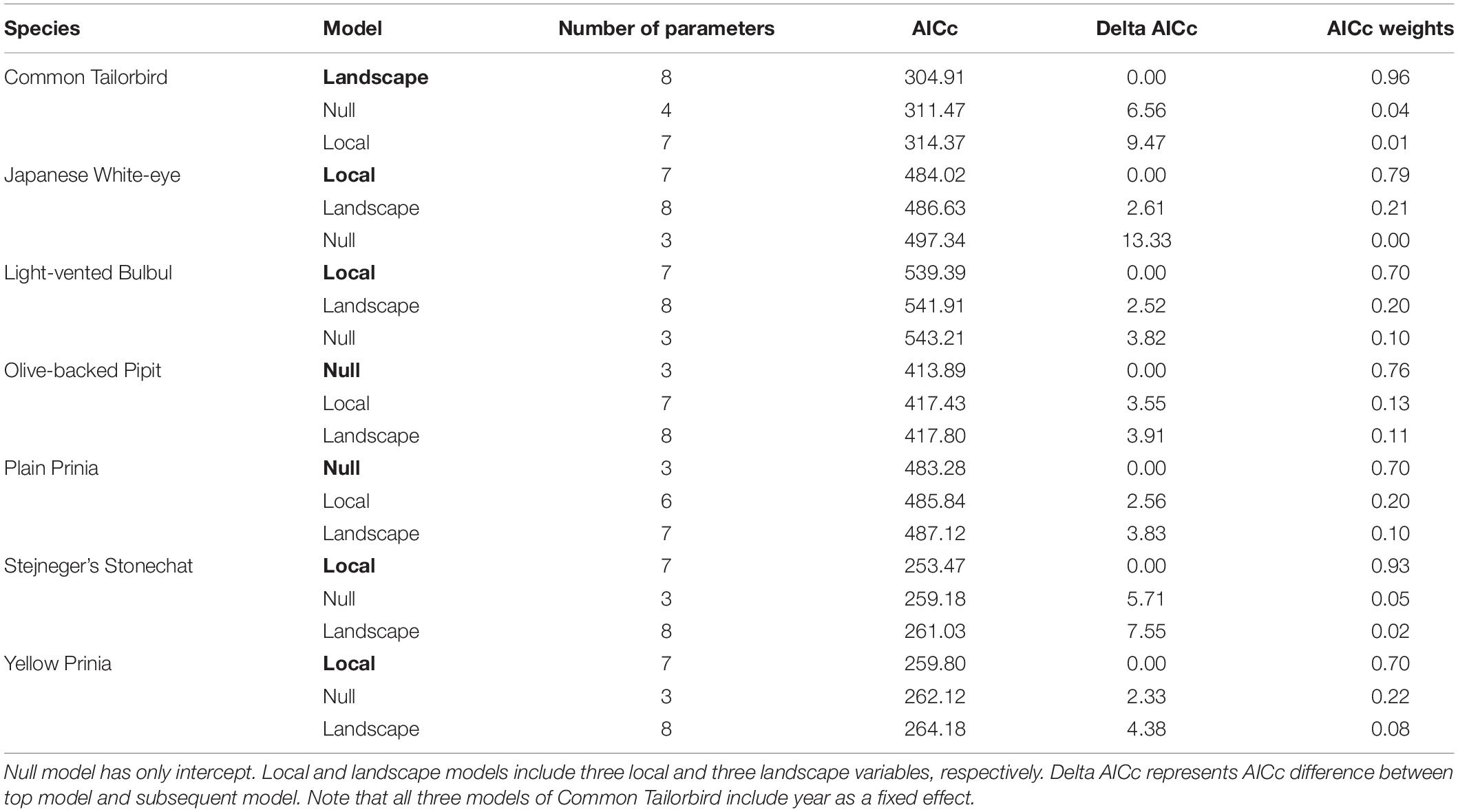
Figure 1. Location of study region (Guangzhou inset map) and urban farmlands (closed circles) sampled. Years 1, 2 represent study sites surveyed in the first winter and in the second winter, respectively. Both indicates farmlands surveyed in the first and second winters.
We selected 37 farmlands and established one sample point at the center or > 50 m from an edge of each farmland in 2019 (Figure 1). These farmlands were chosen considering their size, accessibility, and distance between farmlands to ensure spatial independence. In 2020, 7 farmlands could not be surveyed due to development, abandonment, or other accessibility issues. While maintaining the other 30 farmlands, we established another seven new farmlands (Figure 1). All farmlands were large enough to cover a 50 m circular area (7,850 m2) used for bird surveys: 29,900 ± 21,400 m2 (mean ± standard deviation), ranging from 8,900 to 108,800 m2. Distance between points was 1.66 ± 0.99 km. Percent cover of built-up structure (e.g., building, house, road, and other impervious surfaces) within a 500 m radius area surrounding a sample point was 59.69 ± 14.33%.
Bird Survey
We performed bird surveys during winter for 2 years: December 2019–January 2020 and December 2020–January 2021. We used a combination of 5-min point count and 5-min area search methods to increase detectability (Lee and Goodale, 2018; Lee et al., 2019). Each point was visited twice in the morning between 7:30 and10:30 a.m. during each winter. Survey orders of points between visits were alternated to avoid an effect of time-of-day. One observer recorded birds seen or heard within a 50-m circular area (site, hereafter) of a sample point. Before starting a bird survey, the observer collected survey-specific information that could be related to the detectability of birds by influencing bird activities or the observer: temperature, the number of people seen within a site for 1 min, and noise level (decibel meter reading every 10 s for 1 min, a total of 7 readings). All bird surveys were conducted by the same observer.
Environmental Data at Multiple Scales
We collected local scale environmental data within a 50-m circular area, i.e., the survey site. At each site, crop and non-crop features such as non-woody vegetation (weedy or herbaceous vegetation including grasses/forbs, Bidens spp., and other herbaceous vegetation), woody vegetation (trees and tall shrubs), open water (small pond, water channel, etc.), plowed area, and built-up structure (small storage and shelter) were identified and marked on a printed satellite image of Google Earth, which was taken between 2018 and 20203. Many farmlands were composed of a diversity of crops, but each crop was planted in a regular-size strip. Thus, we divided the site into blocks considering the size of strips. Within a block, we identified crop types, counted the number of strips of each crop, and calculated the relative frequency of each crop type from the sum of all counts. The frequency data were converted to “area” by multiplying each with the size of the block. Block and non-crop features were delineated in ArcGIS to calculate their area and percent cover of each crop and non-crop features at the local scale.
As a main landscape scale that could capture compositional characteristics of matrix in which urban farmlands are embedded, we used a 500-m circular area surrounding a sample point (Rottenborn, 1999; Pennington et al., 2008; Lee et al., 2019). To create the land cover map of the 500-m scale, satellite images were downloaded from Google Earth and georeferenced. The images were taken between 2018 and 2020. Using the georeferenced images as a basemap, we delineated 3 main green cover types that could be used by birds and calculated a percentage of each type in ArcGIS: cropland (vegetable-dominant farmland), non-woody vegetation (largely herbaceous vegetation at abandoned lands and construction sites, and lawn), and woody vegetation (trees and tall shrubs and in some cases, fruit trees including longan, citrus, and tree-like plants such as banana and papaya). We also delineated farmlands within a 1-km radius circular area (the 1-km scale). As a measurement of fragmentation of farmlands within a landscape, we calculated farmland patch density (PD), which is the number of farmlands per unit area, using Fragstats v 4.2.1 (McGarigal et al., 2012). Although patch density is a simple metric, it is often used to indicate habitat connectivity and fragmentation (e.g., McGarigal and McComb, 1995; Grand et al., 2004; Chambers et al., 2016; Bosco et al., 2019). Patch density values at the 500-m and 1-km scales were correlated (Pearson’s correlation, r = 0.8). However, we chose PD at the 1-km scale because percent cover of farmland was less correlated with PD at that scale (r = 0.39) than PD at the 500-m scale (r = 0.42). A 1-km circular area was also large enough to contain any farmland (including farmlands with the size of 1,500 m2) other than the sample site.
Based on the genus of each crop, we calculated crop diversity (Shannon-Wiener diversity index). Although there were over 110 crop types across study sites, most of them were very minor and shared similar biological features because some vegetables are variants of the same plant species. Thus, genus-level classification was used to minimize a potential bias associated with minor crops (see Supplementary Table 1 for the list of 78 genera and their mean percent cover per site). We included crop diversity as an indicator of environmental heterogeneity at the local scale given the finding of a positive relationship between crop diversity and species richness of winter birds in agricultural landscapes (Lee and Goodale, 2018). As environmental variables for analysis, we focused on three local variables [crop diversity (CropD), percent cover of non-woody vegetation (Non-wood), and percent cover of woody vegetation including orchard (Wood)], 3 matrix variables representing the compositional aspect of landscape matrix [percent cover of farmland (Crop500), non-woody vegetation (Non-wood500), and woody vegetation (Wood500)], and 1 other landscape variable [patch density of farmlands (PD1km)]. Pearson correlations between 3 local variables and between 4 landscape variables were all ≤ 0.45, which indicates low chance of having a multicollinearity problem in our analysis. The mean and standard deviation of each variable are summarized in Supplementary Table 2.
Data Analysis
To account for imperfect detection (i.e., species or individuals may not be detected even if they are present at a site) in our bird data, we employed two hierarchical models: multi-species occupancy model (Zipkin et al., 2009) and N-mixture model (Royle, 2004). Ignoring imperfect detection can lead to biased parameter estimates. The importance of imperfect detection is well recognized in wildlife surveys and numerous models have been developed (Williams et al., 2002; Royle and Dorazio, 2008). Both models have been widely adopted to incorporate imperfect detection into the estimation of species richness and occupancy (multi-species occupancy model) and abundance (N-mixture model). Multi-species occupancy models based on Bayesian modeling framework also provide several advantages over single species occupancy models because multi-species occupancy model deals with heterogeneous detection among species. The model also produces both community-level response (species richness) and species-level response (species occupancy), allows for occupancy estimates of rare species, and improves the estimate of species richness (Royle and Dorazio, 2008; Zipkin et al., 2009). Multi-species occupancy model uses presence/absence data of multiple species, whereas N-mixture model uses count data of species, i.e., abundance data.
The two hierarchical models have a similar model structure, linking two regression models: a state process model that estimates the latent state such as occupancy or abundance, and an observation model that the observed state is estimated as a product of detection probability (the probability of detecting species or individual when it is present) and the latent state (Kéry and Schaub, 2012). The main difference is whether a state process model is based on binomial or Bernoulli distribution (multi-species occupancy model) vs. Poisson distribution (N-mixture model). Both hierarchical models also require repeated surveys, i.e., replicates during a certain period, and assume a closed population. Our bird surveys were completed within 3–4 weeks with 10–13 days apart between 2 visits and thus our data satisfied the assumption.
We pooled 2 years of data to increase the effective sample size, resulting in a total of 74 samples (30 sites × 2 years + 7 sites in 2019 + 7 sites in 2020). For multi-species occupancy modeling, we considered 31 bird species detected at more than one site across all visits (Supplementary Table 3 for the list of species). For N-mixture modeling, we selected seven species abundant enough to perform the analysis as well as showing variations in abundance across sites: Common Tailorbird (Orthotomus sutorius), Japanese White-eye (Zosterops japonicus), Light-vented Bulbul (Pycnonotus sinensis), Olive-backed Pipit (Anthus hodgsoni), Plain Prinia (Prinia inornata), Stejneger’s Stonechat (Saxicola stejnegeri), and Yellow-bellied Prinia (Prinia flaviventris). Although Scaly-breasted Munia (Lonchura punctulata) was the most abundant, we did not include the species due to relatively imprecise count in several cases due to flushed flocks during surveys.
We built a model following two steps to avoid overparameterization. First, we chose survey-specific variables (detection covariates: noise, the number of people, and temperature) potentially affecting the detection of birds. We ran a model with these three detection covariates but without any environmental variables. Second, with the detection covariate(s) that showed an effect, we constructed two models, a local model including 3 variables (CropD, Non-wood, and Wood) and a landscape model including 4 variables (Crop500, Non-wood500, Wood500, and PD1km). Both models also included one additional variable, “year” (the first winter = 1 and the second winter = 2). We incorporated “year” into a model as a random or fixed effect to minimize the issue associated with temporal autocorrelation that could violate the assumption of independence (see below subsections). Our inferences were made based on these two models. We standardized all local and landscape variables to a mean of 0 and a standard deviation of 1 to improve model convergence.
Spatial dependence was also examined before final analyses. We performed Moran’s I test with the residuals of each of two generalized linear models (local model and landscape model) using observed detection of each species except Red-billed Starling (Spodiopsar sericeus) and White-rumped Munia (Lonchura striata). These two species were excluded from the test due to an error that may be caused by few detections. Spatial dependency was found in only 2 of 58 models (29 species × 2 models). For seven species selected for N-mixture modeling, we conducted another Moran’s I test with the residuals of each linear model using observed abundance (a maximum count across two visits). Spatial dependency was negligible (P > 0.06) in all cases. Thus, we assumed that the effect of spatial autocorrelation on our inferences would be minimal. Moran’s I test was carried out in SAM (Rangel et al., 2010).
Multi-Species Occupancy Modeling
While 95% Bayesian Credible Intervals (BCIs) of all three detection covariates widely overlapped 0, the number of people had a somewhat stronger effect on detection probability compared to the other two variables: the number of people, −0.031 ± −0.130 to 0.068 (estimated coefficient ± 95% BCI); temperature, 0.019 ± −0.079 to 0.115; noise, 0.009 ± −0.089 to 0.108. Thus, the number of people was included for final analysis. It should also be noted that the detection part of our multi-species occupancy models had a species-level intercept that was modeled as a random effect, which accounts for heterogeneous detection among species.
In our model, the latent occupancy state is represented by Zij, which is defined as a binary variable: if species i is present at site j, Zij = 1 and Zij = 0 otherwise. Zij is assumed to follow a binomial distribution with occupancy probability ψij (the occurrence of species i at site j). The observed occupancy state is indicated by yijk, which is also binary: if species i is detected at site j during a survey (replicate) k, yijk = 1 and yijk = 0 otherwise. It is assumed to be drawn from a binomial distribution with Zij and detection probability pijk (the probability of detecting species i at site j during survey k): yijk ∼ binomial (Zij, pijk). Species-, site-, or survey-specific factors that could affect ψij and pijk are incorporated into the model through a logit-link function. In our study, ψij and pijk are modeled as follows:
for local model
for landscape model
for both models
where ui and vi are species-level intercepts that are modeled as species-specific random effects, β is a coefficient related to the overall effect of the number of people on the detection of species at site j during survey k, εk(j) is a random effect of year in which site j was surveyed, and α1i,α 2i,α 3i, α 4i, α 5i, α 6i, and α 7i are coefficients representing the effects of the relevant environmental variables on the occupancy probability of species i.
All seven coefficients in ψij are also random effects that have their own mean and variance, called hyper-parameters (see Supplementary Material for model code). The hyper-parameters describe the community-level response. In particular, the mean value indicates a mean effect of the relevant environmental variable across all species, which can be interpreted as an effect of the variable on species richness. Our inferences are largely based on the mean value. We added a random year effect in the models to account for potential temporal autocorrelation between years. Model convergence and mode fit were also investigated with the Gelman–Rubin statistic (R) and Bayesian P-value, respectively. We ran three parallel chains for 80,000–90,000 iterations and thinned by 2. Half of the iterations were discarded. Both local model and landscape model converged (R < 1.1) and their model fit was adequate (Bayesian P-value = ∼0.48). We conducted all analyses using “rjags” package (Plummer, 2016) in R (version 4.0.2; R Core Team, 2020).
N-Mixture Modeling
Abundance analysis was carried out in R with “unmarked” package (Fiske and Chandler, 2011). The Poisson N-mixture model is the basic form of N-mixture modeling. The latent abundance at site j is described by a Poisson distribution with local abundance λj: Nj ∼ Poisson (λj). Environmental variables are incorporated into λj through a log-link function. Like multi-species occupancy model, the observed state is assumed to follow a binomial distribution: yjk = binomial (Nj, pjk). However, Poisson N-mixture models cannot handle overdispersion that is common in count data, which influences model fit and “uncertainty assessments” (Kéry and Royle, 2016). Zero-inflated Poisson (ZIP) and negative binomial (NB) N-mixture models are often considered as alternatives to take into account overdispersion. We built a Poisson N-mixture model for each species and performed a goodness of fit (GOF) test on the model using “AICcmodavg” package (Mazerolle, 2020). None of the models fit the data well (P < 0.05). Thus, we ran ZIP and NB N-mixture models that are implemented in unmarked. We chose a model with lower AICc (Akaike Information Criterion adjusted for small sample size) and again conducted a GOF test on the model. NB N-mixture models showed good fit in all cases (P > 0.07). Thus, we used NB N-mixture model for all analyses of abundance.
The relationship between detection covariates and detection of birds varied by species and 5 species showed a significant response (see Supplementary Table 4 for detail). During the bird surveys, detection probability of Light-vented Bulbul and Stejineger’s Stonechat was associated with noise, Olive-backed Pipit and Yellowed-bellied Prinia with temperature, and Japanese White-eye with the number of people. Final models of each of these five species included the significant detection covariate.
Both local models and landscape models were also compared with null model (intercept only model) based on differences in AICc values (Burnham and Anderson, 2002). If the null model was the top model or AICc difference between the null model and the top model (local or landscape model) was < 2, we concluded that there was no association between the species’ abundance and local or landscape variables considered. Unlike multi-species occupancy models, “year” was considered as a fixed effect because random effect is not allowed in unmarked. We included year in all models (null, local, and landscape models) and tested its effect. Year did not have an impact on abundance of any species except Common Tailorbird. Relationships between abundance of six other species and environmental features remained the same whether year was considered or not. Given that year was not the interest of our study, we excluded year from the final analyses of the six species.
Results
General Patterns
A total of 2,437 individuals of birds were observed across all sites for two winters. White Wagtail (Motacilla alba), Plain Prinia, Light-vented Bulbul, and Common Tailorbird were detected at over 65% of sites (Supplementary Table 3). However, the most abundant species were Scaly-breasted Munia (4.22 individuals per site) and Japanese White-eye (2.01 individuals per site). Among 78 genera of crops, 73 were composed largely of vegetables and several fruits, and 5 were used for medicine (Supplementary Table 1).
Species Richness–Environment Relationship
Estimated species richness from multi-species occupancy model was greater than observed species richness (Supplementary Figure 1). Mean and standard deviation of observed species richness per site were 8.9 and 2.2, respectively. Mean estimated species richness was 19.5 with a standard deviation of 1.1. However, wide 95% BCIs of estimated species richness indicated that the precision of estimates was relatively low, which could be affected by scarce detection of some species and minimum sampling efforts (two repeated visits per site).
Among environmental variables, percent cover of non-woody vegetation at the local scale showed a consistently positive effect on species occupancy within a community: that is, species richness increased with percent cover of weedy herbaceous vegetation within a farmland (Table 1). In particular, occupancy probability of open habitat species, Stejneger’s Stonechat, Eurasian Tree Sparrow (Passer montanus), and Yellow-bellied Prinia consistently increased as non-woody vegetation increased within a farmland (Figure 2). Ten other species also showed a tendency of positive response given that their 95% BCIs only slightly crossed 0 (Figure 2). Most landscape variables did not have a strong effect on species richness; however, species richness tended to decrease with increasing patch density of farmlands within a 1-km circular area (Table 1).
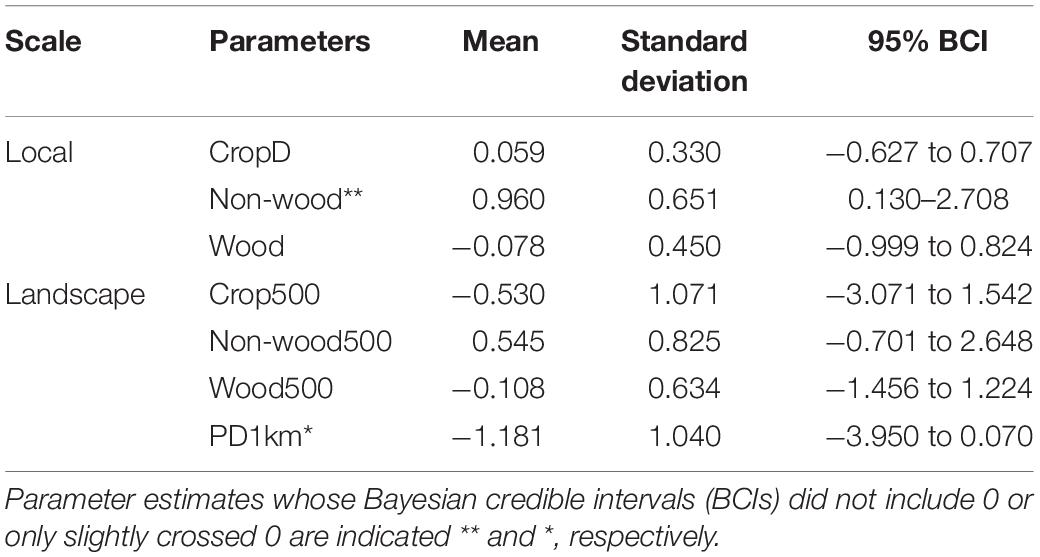
Table 1. Summary of community-level parameters for the occupancy covariates, which indicate effects of local and landscape variables on species richness.
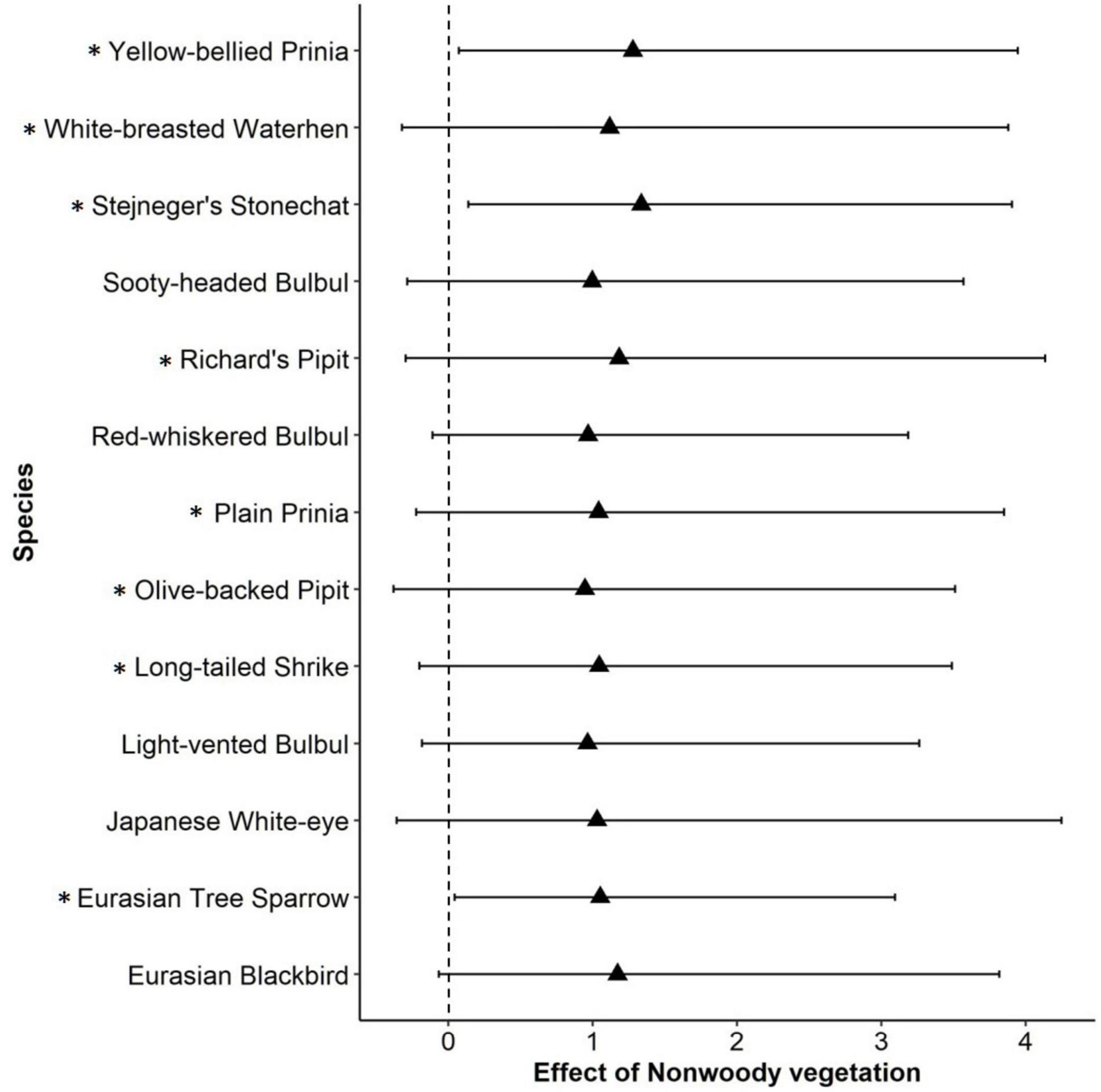
Figure 2. Species-level responses generated from multi-species occupancy model. The estimate is on the logit scale, that is, the value is not back-transformed. Species whose 95% Bayesian credible intervals (error bars on the graph) did not cross 0 or slightly overlapped with 0 are shown. Asterisk (*) indicates open habitat species (grassland species, early successional species, and other species preferring open areas).
Japanese White-eye was the only species showing a consistent response to crop diversity: its occupancy probability was high at farmlands with diverse crops (1.48 and 0.13–3.35, estimate and 95% BCI). Woody vegetation cover at the local and landscape scales was not strongly associated with occupancy of species except Fork-tailed Sunbird (Aethopyga christinae), which responded weakly to woody vegetation cover at the local scale (1.53 and −0.67 to 4.80). Species preferring open habitat such as Richard’s Pipit (Anthus richardi) and Zitting Cisticola (Cisticola juncidis) showed a tendency of positive response to landscape scale crop cover and negative response to patch density of farmlands (Supplementary Figure 2).
Abundance-Environment Relationship
Abundance of most species was related to local variables more than landscape variables: the local model was a top model in 4 species and the landscape model in 1 species (Table 2). Two species, Olive-backed Pipit and Plain Prinia, showed no clear association with environmental variables considered: their top model was the null model and AICc differences between the null model and the other models were >2 (Table 2). Crop diversity had a positive effect on abundance of Japanese White-eye and Yellow-bellied Prinia (Figure 3). Light-vented Bulbul, Stejnerger’s Stonechat, and Yellow-bellied Prinia were abundant in farmlands covered with more non-woody vegetation, which was similar to the patterns found in occupancy of these species (Figures 2, 3). Abundance of Common Tailorbird was strongly negatively affected by patch density of farmlands but positively by woody vegetation cover at the landscape scale. Although explanatory power of the landscape model was low compared to the local model, abundance of Japanese White-eye decreased with percent cover of farmlands within a landscape.
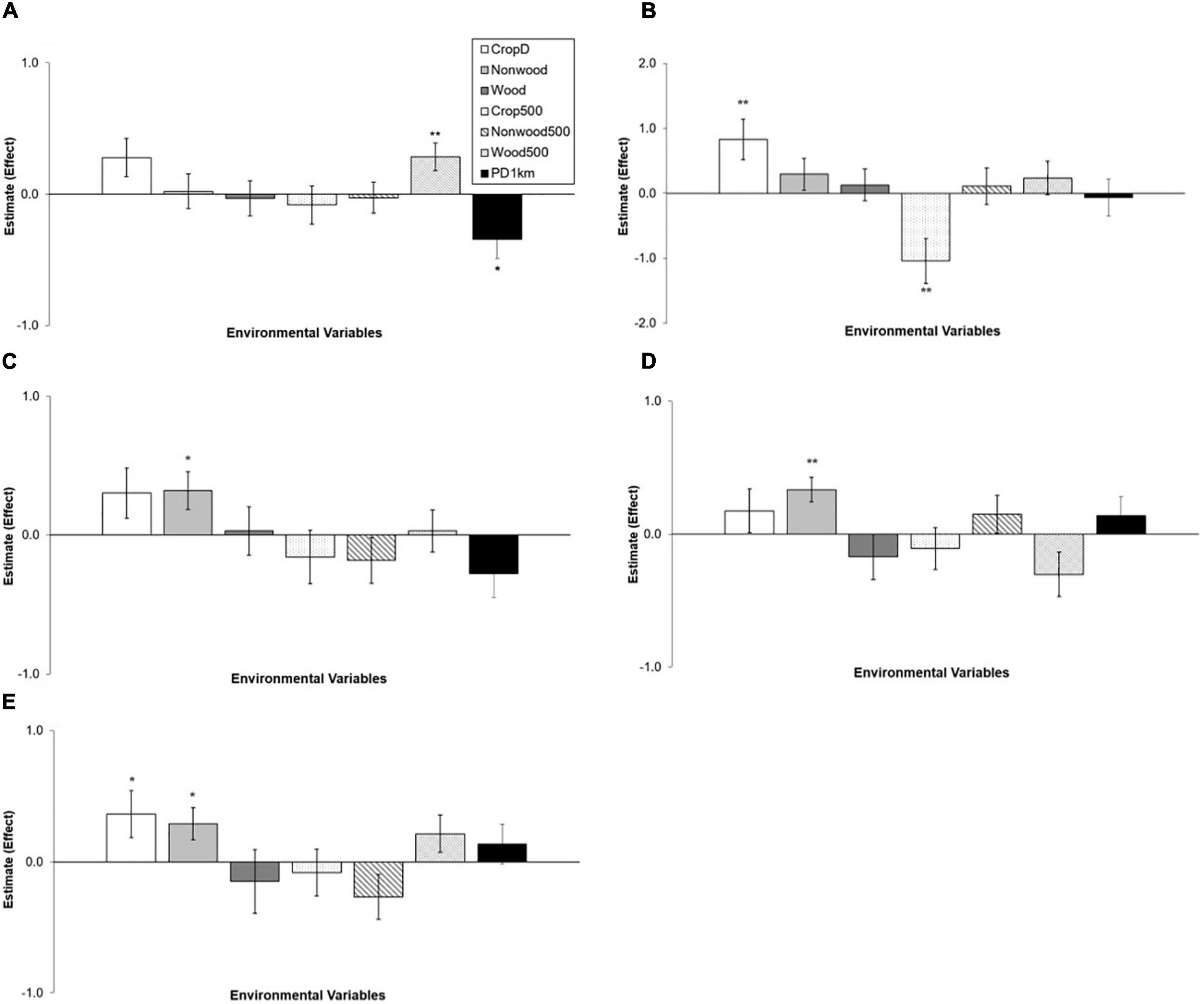
Figure 3. Relationships between environmental variables and abundance of five species: (A) Common Tailorbird, (B) Japanese White-eye, (C) Light-vented Bulbul, (D) Stejneger’s Stonechat, (E) Yellow-bellied Prinia. Estimates are from each of local and landscape models. They are on the log scale and error bars represent standard errors. See the methods for the full name of local (CropD, Non-wood, and Wood) and landscape (Crop500, Non-wood500, Wood500, and PD1km) variables. Significance: *0.01 ≤ P < 0.05; **P < 0.01.
Discussion
Our study suggests that species richness and abundance of birds in urban farmlands are more influenced by local environmental features than characteristics of the landscape matrix in which urban farmlands are embedded. In particular, the strong positive effect of non-woody vegetation indicates the importance of open natural and semi-natural vegetation to birds in cities where green spaces are otherwise dominated by trees and shrubs. The positive effect of crop diversity on abundance of two species also implies that diverse crops contribute to environmental heterogeneity that may benefit some birds. Although the relationships between birds and matrix features are weak, the tendency of decreasing bird species richness with increasing patch density of farmlands shows that birds may be susceptible to farmland fragmentation.
Given the well-known positive relationship between greenery cover and birds in urbanized landscapes (Chace and Walsh, 2006; Beninde et al., 2015), we expected that natural and semi-natural vegetation cover could have a positive impact on birds. The significant effect of local non-woody (herbaceous) vegetation cover partly supports the prediction. It is also consistent with the positive role of herbaceous vegetation for the conservation of birds, especially grassland birds in intensively managed agricultural landscapes (Conover et al., 2009; Josefsson et al., 2013; Evans et al., 2014; among others). However, woody vegetation (trees and tall shrubs) cover did not have an effect on most species despite little difference between percent covers of non-woody and woody vegetations at the local scale. One may point out that species’ habitat preference could influence this pattern. Compared to tree/shrub bird species (species favoring trees and tall shrubs), open habitat species are more related with herbaceous and short shrubby vegetation (Lee et al., 2019). Our surveys were conducted within a vegetable-dominant farmland that could serve as open habitat. Local woody vegetation cover is unlikely a main resource required by open habitat species. Tree/shrub species would also prefer woody patches. However, it is important to note that tree/shrub species (e.g., Japanese White-eye and Red-whiskered Bulbul, Pycnonotus jocosus) showed positive responses to local non-woody vegetation cover and no species showed a negative response to this variable. This indicates the importance of herbaceous vegetation cover, i.e., open natural and semi-natural vegetation cover to both open habitat and tree/shrub species in a city.
Another local variable, crop diversity, was positively associated with abundance of two species, Japanese White-eye and Yellow-bellied Prinia. Recently, a number of studies have explored the possibility that crop diversity or crop heterogeneity can contribute to environmental heterogeneity in agricultural landscapes (Fahrig et al., 2011; Sirami et al., 2019 for review). Environmental heterogeneity is often assumed to influence animal diversity positively by increasing the availability of different resources required by species or complimentary/supplementary resources available to different species (Rosenzweig, 1995; Tews et al., 2004). Thus, crop diversity may be a promising option to improve biodiversity without reducing croplands or agricultural production (Fahrig et al., 2011). Findings from previous studies are not conclusive. More studies have also reported a stronger association of biodiversity with spatial arrangement of farmlands or structural diversity of crops than crop diversity per se (Fahrig et al., 2015; Josefsson et al., 2017). In southern China, Lee and Goodale (2018) tested this possibility in rice- and sugarcane-dominant agricultural landscapes. They found that species richness and occupancy increased with crop diversity during winter when natural resources were relatively scarce. In the current study, although species richness of birds was not affected by crop diversity, the positive response of two insectivorous species suggests that diverse crops at urban farmlands can benefit some bird species. Yellow-bellied Prinia generally prey on a wide range of small insects including flies, ants, lepidopteran caterpillars, and butterflies (Madge et al., 2020). Abundance of cabbage white butterfly (Pieris rapae) tended to increase with crop diversity across our study sites (unpublished data). We also often observed many small flies and caterpillars at farmlands that contained weeds and different crops. These patterns could explain the positive response of Yellow-bellied Prinia. However, one may ponder the response of Japanese White-eye, usually considered as a tree/shrub species. During our surveys, we observed Japanese White-eyes foraging for insects at flowers of green onions and moving around or perching on trellis and vine crops, which are relatively common in farmlands with diverse crops. Japanese white-eyes may also use flowering crops (the mallow family Malvaceae) and fruit trees in farmlands although we did not find a significant relationship between percent cover of fruit trees and their abundance. In some farmlands, bananas and trees are common along the edge and used as a fence. This woody vegetation could attract Japanese White-eyes; Japanese white-eye often detected during surveys can be individuals moving from these trees to other trees or crops within a farmland (spillover effect; Tscharntke et al., 2012). Crop diversity may be intercorrelated with all of these aspects. Understanding the response of Japanese White-eyes to crop diversity will require more detailed observation of their behaviors and resource use patterns in urban farmlands.
Weak or no association of birds with landscape variables is somewhat unexpected as characteristics of matrix surrounding a patch can affect individuals, populations, and communities of animals and plants (Prevedello and Vieira, 2010). Matrix type and quality influence birds’ movement, dispersal, extinction threshold, and occurrence (Antongiovanni and Metzger, 2005; Boesing et al., 2018). Considering that urban farmlands are largely surrounded by built-up structure, we expected that an increasing amount of greenery cover within a landscape would have a positive effect on birds. The low explanatory power of landscape variables, especially matrix features, may be related to landscape complexity. In agroecosystems, landscape complexity based on the percentage of non-crop area can influence the relative effectiveness of local and landscape characteristics or management on ecosystem services and local biodiversity (the intermediate landscape complexity hypothesis; Tscharntke et al., 2012). Local characteristics are more effective in simple (1% non-crop area) or complex (>20% non-crop area) landscapes, whereas landscape characteristics can be stronger in intermediately complex landscapes (1–20% non-crop area). In urban ecosystems, non-crop area other than built-up structures consists of greenery cover (woody and non-woody vegetations) and open water. In our study, mean greenery cover, i.e., the sum of mean non-woody and woody vegetation covers is > 20% and mean of open water cover is 3.38%, indicating the complexity of our landscapes may not be considered intermediate under that hypothesis. Most landscapes contain all of the non-built-up structures. These aspects seem to explain the stronger effect of local environmental features than landscape matrix features. It is also possible that the landscape size of our study may be related to the weak effects of landscape variables. We selected landscape sizes (e.g., a 500-m circular area) by considering not only scales used in previous studies (Rottenborn, 1999; Concepción et al., 2008; Pennington et al., 2008; Lee and Goodale, 2018) but also logistics, especially manual creation of land cover map (onscreen digitization) to increase accuracy and precision of the map. While high correlation between landscape variables across scales is common (Lee and Carroll, 2014), the relative effects of landscape variables can be scale-dependent and inconsistent among species (Smith et al., 2011). Birds may respond to environmental features at larger scales than ours. It is unclear whether the weak effects of landscape variables on birds would remain similar at larger scales.
However, it is still puzzling that occupancy and abundance of even tree/shrub species were rarely related to percent cover of woody vegetation within a landscape. Only one case, abundance of Common Tailorbird, was influenced by landscape woody vegetation cover. In another study (in review), we examined winter bird diversity at woody patches and urban farmlands in two subtropical cities. Similar patterns were found: richness and abundance of tree/shrub species were not associated with landscape woody vegetation cover. Although we do not have a clear explanation of this pattern, environmental conditions of woody vegetation cover may influence the pattern in our study. Heterogeneity in vegetation composition and structure is one of the key features affecting birds in urban areas and forests (Freemark and Merriam, 1986; Gil-Tena et al., 2007; Evan et al., 2009; Lee and Carroll, 2018). Some woody patches surrounding urban farmlands have dense vegetation with little herbaceous ground cover. This would lower structural diversity of vegetation within the patch, which may not be favored by birds. Small patches of tree/shrub vegetation are often found in residential areas. However, local people heavily use these patches for daily recreational activities (singing, playing instrument, and dancing) and increase the degree of human disturbance, which can negatively affect occupancy of birds (Lee et al., 2019). Another possibility is a seasonal effect. We performed our surveys in winter, i.e., the non-breeding period. Birds can show seasonal variations in their habitat preference or use (Whelan and Maina, 2005; Šálek et al., 2018). Woody vegetation may be important to tree/shrub species during the breeding period as it could provide critical resources such as nest sites and foods (e.g., more flowers and small fruits in trees during spring and summer).
It is noteworthy that species richness in farmlands tended to be low in landscapes with highly fragmented farmlands (i.e., high patch density), whereas species richness was not influenced by percent cover of farmlands. This suggests that birds may be susceptible to the fragmentation of urban farmlands. Patch density is one of landscape metrics representing habitat fragmentation (e.g., McGarigal and McComb, 1995; Gorresen and Willig, 2004; Wang et al., 2014; Bosco et al., 2019). Patch density is often correlated with the habitat amount available in a landscape lineally or parabolically (Neel et al., 2004; Wang et al., 2014). In our study, the correlation was not strong at the 500-m scale. The correlation between percent cover of farmland at the 500-m scale and patch density of farmland at the 1-km scale was also low. Wang et al. (2014) found a weak correlation between habitat amount and patch density when the habitat amount is 10–30%, which is similar to our case. While this in part supports the effect of patch density independent from the amount of farmland, we need further investigation to verity the effect, especially at different scales.
Overall, our study provides valuable insights into how environmental characteristics within and surrounding an urban farmland would affect bird diversity and abundance. With rapid urban growth, small farmlands scattered throughout a city in China have been increasingly converted to built-up structure. While slightly larger-scale farmlands at city fringe areas are less susceptible to development, these farmlands often have only 1–3 types of cash crops with little herbaceous vegetation. Our findings suggest that creating herbaceous vegetation at field margins or unused areas of the farmlands could improve bird diversity. Crop diversification may also contribute to maintain populations of some species. The habitat value of small farmlands, which often contains different types of crops mixed with uncultivated herbaceous vegetation, needs to be considered in urban planning. However, we acknowledge that our study did not assess the relative importance of urban farmlands and other green spaces (e.g., urban forest, park, and weedy area) for the conservation of urban bird diversity. Our study was centered on identifying environmental drivers associated with birds in urban farmlands. We also focused on winter bird community. Although urban farmlands could be important to open habitat species, we do not know how good they are at improving urban bird diversity compared to other green spaces, how birds respond different types of green spaces, and whether there is seasonal variation in the responses. We recommend that a future study consider both urban farmlands and other green spaces, by adopting a landscape design such as sampling multiple sites within a landscape (e.g., 2 km × 2 km in size) and surveying multiple seasons. The landscape design requires intensive sampling (Brennan et al., 2002); however, it will enable us to partition diversity, i.e., α (within community) and β (among communities) diversity as well as to compare bird diversity between different types of green spaces. We also emphasize a need for future research on environmental conditions of urban farmlands, e.g., agrochemical usage and heavy metal accumulation in soil and crop, which could be linked to air pollutants and reuse of wastewater in a city. These conditions influence not only human health but also birds, e.g., their chick survival, brood size, and body fat (Brown and Jameton, 2000; Boatman et al., 2004; Mineau and Palmer, 2013; Antisari et al., 2015). This is of particular importance to China as the country has experienced serious environmental problems of high air pollution and heavy usage of fertilizer, pesticide, and herbicide (Kan et al., 2012; Lam et al., 2013). Developing eco-friendly environmental conditions of urban farmlands could contribute to increasing the habitat quality of these farmlands for birds and multifunctionality of urban agriculture, improving the sustainability of urban agroecosystems that balances agricultural production and biodiversity conservation in a city.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
M-BL conceived the idea of the research presented in this article, designed the study, conducted environmental surveys, created spatial data (GIS data), performed all analyses, and wrote the manuscript. DC carried out bird and environmental surveys. FZ provided additional funding and resources to support this project. All authors read and agreed on the last version of the manuscript.
Funding
This study was supported by the GDAS Special Project of Science and Technology Development (Grant No. 2020GDASYL-20200103089), GDAS Special Project of Top Institute (Grant No. 2019GDASYL-0203001), Science and Technology Program of Guangzhou (Grant No. 202103000065), and DFGP Project of Fauna of Guangdong (Grant No. 202115).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Yang Zhang and Cheng Ding for their assistance in collecting environmental data, Min Zhang for helping to deal with logistic issues, and local farmers for allowing us to perform our surveys. We also thank John Rotenberry for edits and comments on the draft of our manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.859199/full#supplementary-material
Footnotes
References
Antisari, L. V., Orsini, F., Marchetti, L., Vianello, G., and Gianquinto, G. (2015). Heavy metal accumulation in vegetables grown in urban gardens. Agron. Sustain. Dev. 35, 1139–1147.
Antongiovanni, M., and Metzger, J. P. (2005). Influence of matrix habitats on the occurrence of insectivorous bird species in Amazonian forest fragments. Biol. Conser. 122, 441–451.
Aronson, M. F. J., La Sorte, F. A., Nilon, C. H., Katti, M., Goddard, M. A., Lepczyk, C. A., et al. (2014). A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B 281:20133330. doi: 10.1098/rspb.2013.3330
Beninde, J., Veith, M., and Hochkirch, A. (2015). Biodiversity in cities needs space: a meta analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 18, 581–592. doi: 10.1111/ele.12427
Boatman, N. D., Brickle, N. W., Hart, J. D., Milsom, T. P., Morris, A. J., Murray, A. W. A., et al. (2004). Evidence for the indirect effects of pesticides on farmland birds. Evid. Indirect Eff. Pestic. Farmland Birds 146, 131–143. doi: 10.1371/journal.pone.0057457
Boesing, A. L., Nichols, E., and Metzger, J. P. (2018). Biodiversity extinction thresholds are modulated by matrix type. Ecography 41, 1520–1533.
Bosco, L., Wan, H. Y., Cushman, S. A., Arlettaz, R., and Jacot, A. (2019). Separating the effects of habitat amount and fragmentation on invertebrate abundance using a multi-scale framework. Landsc. Ecol. 34, 105–117. doi: 10.1007/s13744-016-0461-3
Brennan, J. M., Bender, D. J., Contreras, T. A., and Fahrig, L. (2002). “Focal patch landscape studies for wildlife management: optimizing sampling effort across scales,” in Integrating Landscape Ecology into Natural Resource Management, eds J. Liu and W. W. Taylor (Cambridge: Cambridge University Press), 68–91.
Brown, K., and Jameton, A. (2000). Public health implications of urban agriculture. J. Public Health Policy 21, 20–39.
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multimodel Inference: a Practical Information-Theoretic Approach, 2nd Edn. New York, NY: Springer-Verlag.
Canedoli, C., Manenti, R., and Padoa-Schioppa, E. (2018). Birds biodiversity in urban and periurban forests: environmental determinants at local and landscape scales. Urban Ecosyst. 21, 779–793.
Chace, J. F., and Walsh, J. J. (2006). Urban effects on native avifauna: a review. Landsc. Urban Plan. 74, 46–69.
Chambers, C. L., Cushman, S. A., Medina-Fitoria, A., Martínez-Fonseca, J., and Chávez-Velásquez, M. (2016). Influences of scale on bat habitat relationships in a forested landscape in Nicaragua. Landsc. Ecol. 31, 1299–1318.
Claudia, S., and Christian, S. (2015). Functional diversity of urban bird communities: effect of landscape composition, green space area and vegetdation cover. Ecol. Evol. 5, 5230–5239. doi: 10.1002/ece3.1778
Concepción, E. M., Díaz, M., and Baquero, R. A. (2008). Effects of landscape complexity on the cological effectivenness of agri-environmenta schemes. Landsc. Ecol. 23, 135–148.
Conover, R. R., Burger, L. W. Jr., and Linder, E. T. (2009). Breeding bird response to field border presence and width. Wilson J. Ornithol. 121, 548–555.
De Bon, H., Parrot, L., and Moustier, P. (2010). Sustainable urban agriculture in developing country: a review. Agron. Sustain. Dev. 30, 21–32.
d’Amour, C. B., Reitsma, F., Baiocchi, G., Barthel, S., Güneralp, B., Erb, K.-H., et al. (2017). Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. U.S.A. 114, 8939–8944. doi: 10.1073/pnas.1606036114
Egerer, M., Cecala, J. M., and Cohen, H. (2020). Wild bee conservation within urban gardens and nurseries: effects of local and landscape management. Sustainability 12:293.
Evan, K. L., Newson, S. E., and Gaston, K. J. (2009). Habitat influences on urban avian assemblages. Ibis 151, 19–39.
Evans, K. O., Burger, L. W. Jr., Riffell, S. K., Smith, M. D., Twedt, D. J., Wilson, R. R., et al. (2014). Avian response to conservation buffers in agricultural landscapes during winter. Wildl. Soc. Bull. 38, 257–264.
Fahrig, L., Baudry, J., Brotons, L., Burel, F. G., Crist, T. O., Fuller, R. J., et al. (2011). Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 14, 101–112. doi: 10.1111/j.1461-0248.2010.01559.x
Fahrig, L., Girard, J., Duro, D., Pasher, J., Smith, A., Javorek, S., et al. (2015). Farmlands with smaller crop fields have higher within-field biodiversity. Agric. Ecosyst. Environ. 200, 219–234.
Filazzola, A., Shrestha, N., and MacIvor, J. S. (2019). The contribution of constructed green infrastructure to urban biodiversity: a synthesis and meta-analysis. J. Appl. Ecol. 56, 2131–2143.
Fiske, I., and Chandler, R. (2011). Unmarked: an R package for fitting hierarchical models of wildlife occurrence and abundance. J. Stat. Softw. 43, 1–23.
Freemark, K. E., and Merriam, H. G. (1986). Importance of area and habitat heterogeneity to bird assemblages in temperate forest fragments. Biol. Conserv. 36, 115–141.
Gil-Tena, A., Saura, S., and Brotons, L. (2007). Effects of forest composition and structure on bird species richness in a Mediterranean context: implications for forest ecosystem management. For. Ecol. Manag. 242, 470–476.
Gorresen, P. M., and Willig, M. R. (2004). Landscape responses of bats to habitat fragmentation in Atlantic forest of Paraguay. J. Mammal. 85, 688–697.
Grand, J., Buonaccorsi, J., Cushman, S. A., Griffin, C. R., and Neel, M. C. (2004). A multiscale landscape approach to predicting bird and moth rarity hotspots in a threatened pitch pine–scrub oak community. Conserv. Biol. 18, 1063–1077.
Grimm, N. B., Faeth, S. H., Golubiewski, N. E., Redman, C. L., Wu, J. G., Bai, X. M., et al. (2008). Global change and the ecology of cities. Science 319, 756–760. doi: 10.1126/science.1150195
Grumbine, R. E. (2014). Assessing environmental security in China. Front. Ecol. Environ. 12:403–411. doi: 10.1890/130147
Güneralp, B., Perlstein, A. S., and Seto, K. C. (2015). Balancing urban growth and ecological conservation: a challenge for planning and governance in China. Ambio 44, 532–543. doi: 10.1007/s13280-015-0625-0
Güneralp, B., and Seto, K. C. (2013). “Sub-regional assessment of China: urbanization in biodiversity hotspots,” in Urbanization, Biodiversity and Ecosystem Services: Challenges and Opportunities, eds T. Elmqvist, M. Fragkias, J. Goodness, P. J. Marcotullio, R. I. McDonald, S. Parnell, et al. (Dordrecht: Springer), 57–63.
Henderson, I. G., Cooper, J., Fuller, R. J., and Vickery, J. (2000). The relative abundance of birds on set-aside and neighbouring fields in summer. J. Appl. Ecol. 37, 335–347.
Horowitz, S. S., and Liu, J. (2017). “Urban agriculture and the reassembly of the city: lessons from Wuhan, China,” in Global Urban Agriculture: Convergence of Theory and Practice Between North and South, ed. A. M. G. A. WinklerPrins (Wallingford, CT: CABI International), 207–219.
Jokimäki, J. (1999). Occurrence of breeding bird species in urban parks: effects of park structure and braod-scale variables. Urabn Ecosyst. 3, 21–34.
Josefsson, J., Berg, Å, Hiron, M., Pärt, T., and Eggers, S. (2013). Grass buffer strips benefit invertebrate and breeding skylark numbers in a heterogeneous agricultural landscape. Agric. Ecosyst. Environ. 18, 101–107.
Josefsson, J., Hiron, M., Pärt, T., and Eggers, S. (2017). Sensitivity of the farmland bird community to crop diversification in Sweden: does the CAP fit? J. Appl. Ecol. 54, 518–526.
Kan, H., Chen, R., and Tong, S. (2012). Ambient air pollution, climate change, and population health in China. Environ. Int. 42, 10–19. doi: 10.1016/j.envint.2011.03.003
Kéry, M., and Royle, A. (2016). Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS. San Diego: Academic Press.
Kéry, M., and Schaub, M. (2012). Bayesian Population Analysis Using WinBUGS: a Hierarchical Perspective. Cambridge, MA: Academic Press.
La Rosa, D., Barbarossa, L., Privitera, R., and Martinico, F. (2014). Agriculture and the city: a method for sustainable planning of newforms of agriculture in urban contexts. Land Use Policy 41, 290–303.
Lam, H. M., Remais, J., Fung, M. C., Xu, L. Q., and Sun, S. S. M. (2013). Food supply and food safety issues in China. Lancet 381, 2044–2053. doi: 10.1016/S0140-6736(13)60776-X
Lee, M.-B., and Carroll, J. P. (2014). Relative importance of local and landscape variables on site occupancy by avian species in a pine forest, urban, and agriculture matrix. For. Ecol. Manag. 320, 161–170.
Lee, M.-B., and Carroll, J. P. (2018). Effects of patch size and basal area on avian taxonomic and functional diversity in pine forests: implication for the influence of habitat quality on the species-area relationship. Ecol. Evol. 8, 6909–6920. doi: 10.1002/ece3.4208
Lee, M.-B., and Goodale, E. (2018). Crop heterogeneity and non-crop habitat features can enhance avian diversity in a tropical agricultural landscape in southern China. Agric. Ecosyst. Environ. 265, 254–263.
Lee, M.-B., Peabotowage, I., Gu, H., Zhou, W., and Goodale, E. (2019). Factors affecting avian species richness and occupancy in a tropical city in southern China: importance of human disturbance and open green space. Basic Appl. Ecol. 39, 48–56.
Lepczyk, C. A., La Sorte, F. A., Aronson, M. F. J., Goddard, M. A., MacGregor-Fors, I., Nilon, C. H., et al. (2017). “Global patterns and drivers of urban bird diversity,” in Ecology and Conservation of Birds in Urban Environments, eds E. Murgui and M. Hedblom (Cham: Springer International Publishing AG), 13–33.
Lin, B. B., Philpott, S. M., and Jha, S. (2015). The future of urban agriculture and biodiversity-ecosystem service: challenges and next steps. Basic Appl. Ecol. 16, 189–201.
MacGregor-Fors, I., and Ortega-Alvarez, R. (2011). Fading from the forest: bird community shifts related to uban park site-specific and landscape traits. Urban For. Urban Green. 10, 239–246.
MacGregor-Fors, I., and Schondube, J. E. (2011). Gray vs green urbanization: relative importance of urban features for urban bird communities. Basic Appl. Ecol. 12, 372–381.
Madge, S., del Hoyo, J., Kirwan, G. M., and Collar, N. (2020). “Yellow-bellied Prinia (Prinia flaviventris), version 1.0,” in Birds of the World, eds S. M. Billerman, B. K. Keeney, P. G. Rodewald, and T. S. Schulenberg (New York, NY: Cornell Lab of Ornithology). doi: 10.2173/bow.yebpri1.01
Mazerolle, M. J. (2020). AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R package version 2.3-0.
McClintock, N. (2010). Why farm the city? Theorizing urban agriculture through a lens of metabolic rift. Cambridge J. Reg. Econ. Soc. 3, 191–207.
McDonald, R., Marcotullio, P. J., and Güneralp, B. (2013). “Urbanization and global trends in biodiversity and ecosystem services,” in Urbanization, Biodiversity and Ecosystem Services: Challenges And Opportunities, eds T. Elmqvist, M. Fragkias, J. Goodness, P. J. Marcotullio, R. I. McDonald, S. Parnell, et al. (Dordrecht: Springer), 31–52.
McDonald, R. I., Kareiva, P., and Forman, R. T. T. (2008). The implications of current and future urbanization for global protected area and biodiversity conservation. Biol. Conserv. 141, 1695–1703.
McGarigal, K., Cushman, S. A., and Ene, E. (2012). FRAGSTATS v4.2.1: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Amherst, MA: University of Massachusetts, Amherst.
McGarigal, K., and McComb, W. C. (1995). Relationship between landscape structure and breeding birds in the Oregon Coast Range. Ecol. Monogr. 65, 235–260.
Mineau, P., and Palmer, C. (2013). Neonicotinoid Insecticides and Birds: the Impact of the Nation’s Most Widely used Insecticides on Birds. The Plains: American Bird Conservancy.
Mougeot, L. J. A. (2000). Urban Agriculture: Definition, Presence, Potentials and Risks and Policy Challenges. Cities Feeding People Series Report 31. Ottawa: International Development Research Centre.
Neel, M. C., McGarigal, K., and Cushman, S. A. (2004). Behavior of class level landscape metrics across gradients of class aggregation and area. Landsc. Ecol. 19, 435–455.
Ortega-Álvarez, R., and MacGregor-Fors, I. (2009). Living in the big city: effects of urban land-use on bird community structure, diversity, and composition. Landsc. Urban Plan. 90, 189–195.
Peng, J., Liu, Z., Liu, Y., Hu, X., and Wang, A. (2015). Multifunctionality assessment of urban agriculture in Beijing City China. Sci. Total Environ. 537, 343–351. doi: 10.1016/j.scitotenv.2015.07.136
Pennington, D. N., Hansel, J., and Blair, R. B. (2008). The conservation value of urban riparian areas for landbirds during spring migration: land cover, scale, and vegetation effects. Biol. Conserv. 14, 1235–1248.
Pithon, J. A., Duflot, R., Beaujouan, V., Jagaille, M., Pain, G., and Daniel, H. (2021). Grasslands provide diverse opportunities for bird species along an urban-rural gradient. Urban Ecosyst. online first 24, 1281–1294.
Prevedello, J. A., and Vieira, M. V. (2010). Does the type of matrix matter? A quantitative review of the evidence. Biodivers. Conserv. 19, 1205–1223.
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rangel, T. F., Diniz-Filho, J. A. F., and Bini, L. M. (2010). SAM: a comprehensive application for spatial analysis in macroecology. Ecography 33, 46–50.
Rosenzweig, M. L. (1995). Species Diversity in Space and Time. New York, NY: Cambridge University Press.
Rottenborn, S. C. (1999). Predicting the impacts of urbanization on riparian bird communities. Biol. Conserv. 88, 289–299.
Royle, J. A. (2004). N-mixture models for estimating population size from spatially replicated counts. Biometrics 60, 108–115. doi: 10.1111/j.0006-341X.2004.00142.x
Royle, J. A., and Dorazio, R. M. (2008). Hierarchical Modeling and Inference in Ecology. San Diego: Academic Press.
Šálek, M., Bažant, M., and żmihorski, M. (2018). Active farmsteads are year-round strongholds for farmland birds. J. Appl. Ecol. 55, 1908–1918.
Sandström, U. G., Angelstam, P., and Mikusiński, G. (2006). Ecological diversity of birds in relation to the structure of urban green space. Landsc. Urban Plan. 77, 39–53.
Satterthwaite, D., McGranahan, G., and Tacoli, C. (2010). Urbanization and its implications for food. Phil. Trans. R. Soc. B 365, 2809–2820.
Sekercioglu, C. H. (2006). Increasing awareness of avian ecological function. Trends Ecol. Evol. 21, 464–471. doi: 10.1016/j.tree.2006.05.007
Seto, K. C., Güneralp, B., and Hutyra, L. R. (2012). Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. Proc. Natl. Acad. Sci. U.S.A. 109, 16083–16088. doi: 10.1073/pnas.1211658109
Sirami, C., Gross, N., Baillod, A. B., Bertrand, C., Carrié, R., Hass, A., et al. (2019). Increasing crop heterogeneity enhances multitrophic diversity across agricultural regions. Proc. Natl. Acad. Sci. U.S.A. 116, 16442–16447. doi: 10.1073/pnas.1906419116
Smith, A. C., Fahrig, L., and Francis, C. M. (2011). Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography 34, 103–113. doi: 10.1890/1051-0761(2006)016[1076:ieofof]2.0.co;2
Tews, J., Brose, U., Grimm, V., Tielbörger, K., Wichmann, M. C., Schwager, M., et al. (2004). Animal species diversity driven by habitat heterogeneity/diversity: the importance of key structures. J. Biogeogr. 31, 79–92.
Tscharntke, T., Tylianakis, J. M., Rand, T. A., Didham, R. K., Fahrig, L., Batary, P., et al. (2012). Landscape moderation of biodiversity patterns and processes–eight hypotheses. Biol. Rev. 87, 661–685. doi: 10.1111/j.1469-185X.2011.00216.x
Tzoulas, K., Korpela, K., Venn, S., Yli-Pelkonen, V., Kazmierczak, A., Niemela, J., et al. (2007). Promoting ecosystem and human health in urban areas using green infrastructure: a literature review. Landsc. Urban Plan. 81, 167–178. doi: 10.1016/j.scitotenv.2018.09.030
Van Helden, B. E., Close, P. G., and Steven, R. (2020). Mammal conservation in a changing world: can urban gardens play a role? Urban Ecosyst. 23, 555–567.
Veeck, G. (2013). China’s food security: past success and future challenges. Eurasian Geogr. Econ. 54, 42–56.
Vickery, J. A., Feber, R. E., and Fuller, R. J. (2009). Arable field margins managed for biodiversity conservation: a review of food resource provision for farmland birds. Agric. Ecosyst. Environ. 133, 1–13.
Villaseñor, N. R., Chiang, L. A., Hernández, H. J., and Escobar, M. H. A. (2020). Vacant lands as refuges for native birds: an opportunity for biodiversity conservation in cities. Urban For. Urban Green. 49:126632.
Wang, X., Blanchet, F. G., and Koper, N. (2014). Measuring habitat fragmentation: an evaluation of landscape pattern metrics. Methods Ecol. Evol. 5, 634–646.
Whelan, C. J., and Maina, G. G. (2005). Our findings could be used to improve bird diversity and population persistence in a city whilst maintaining agricultural production for citizens. Funct. Ecol. 19, 529–536.
Whelan, C. J., Sekercioðlu, C. H., and Wenny, D. G. (2015). Why birds matter: from economic ornithology to ecosystem services. J. Ornithol. 156 (Suppl. 1), S227–S238.
Williams, B. K., Nichols, J. D., and Conroy, M. J. (2002). Analysis and Management of Animal Populations. San Diego: Academic Press.
WinklerPrins, A. M. G. A. (2017). Defining and Theorizing Global Urban Agriculture in Global Urban Agriculture: Convergence of Theory and Practice Between North and South. Wallingford: CABI International.
Zhong, T., Si, A., Crush, J., Scott, S., and Huang, X. (2019). Achieving urban food security through a hybrid public-private food provisioning system: the case of Nanjing China. Food Secur. 11, 1071– 1086.
Keywords: abundance, bird diversity, farmland, fragmentation, greenery cover, hierarchical model, scale, urban agriculture
Citation: Lee M-B, Chen D and Zou F (2022) Winter Bird Diversity and Abundance in Small Farmlands in a Megacity of Southern China. Front. Ecol. Evol. 10:859199. doi: 10.3389/fevo.2022.859199
Received: 21 January 2022; Accepted: 22 March 2022;
Published: 04 May 2022.
Edited by:
Tibor Magura, University of Debrecen, HungaryReviewed by:
Zoltan Elek, Hungarian Academy of Sciences (MTA), HungaryRoland Horváth, University of Debrecen, Hungary
Copyright © 2022 Lee, Chen and Zou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myung-Bok Lee, Ym9rLmVjb2xvZ3lAb3V0bG9vay5jb20=; Fasheng Zou, em91ZnNAZ2lhYnIuZ2QuY24=
 Myung-Bok Lee
Myung-Bok Lee Daojian Chen
Daojian Chen Fasheng Zou
Fasheng Zou