
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Ecol. Evol. , 26 April 2022
Sec. Behavioral and Evolutionary Ecology
Volume 10 - 2022 | https://doi.org/10.3389/fevo.2022.858859
This article is part of the Research Topic Sex and Gender Effects on Power, Status, Dominance, and Leadership – An Interdisciplinary Look at Human and Other Mammalian Societies View all 22 articles
The extant primates of Madagascar (Lemuriformes) represent the endpoints of an adaptive radiation following a single colonization event more than 50 million years ago. They have since evolved a diversity of life history traits, ecological adaptations and social systems that rivals that of all other living primates combined. Their social systems are characterized by a unique combination of traits, including the ability of adult females to dominate adult males. In fact, there is no other group of mammals in which female dominance is so widespread. Yet, recent research has indicated that there is more interspecific variation in lemur intersexual relationships than previously acknowledged. Here, we therefore review and summarize the relevant literature, quantifying the extent of sex-bias in intersexual dominance relations documented in observational and experimental studies in captivity and the wild. Female dominance is often, but not always, implemented by spontaneous male submission in the absence of female aggression and linked to female sexual maturation. We connect the available evidence to the hypotheses that have been proposed to explain the evolution of female dominance among lemurs. The occurrence of female dominance in all lemur families and the interspecific variation in its extent indicate that it has evolved soon after lemurs colonized Madagascar – presumably in response to particular ecological challenges – and that it has since been reduced in magnitude independently in some taxa. Our study contributes important comparative information on sex roles from an independent primate radiation and provides general insights into the conditions, opportunities and obstacles in the evolution of female-biased power.
Repeated interactions among the same two individuals involve various combinations of affinitive, affiliative and agonistic interactions, generating diverse patterns of dyadic social relationships (Hinde, 1976). Agonistic interactions involve the exchange of aggressive and/or submissive acts and signals (Hausfater, 1975). Dyadic agonistic interactions in which only one individual exhibits only submissive behavior are clearly decided and can be used to unanimously determine dominance relationships and hierarchies (Hausfater, 1975; Pereira and Kappeler, 1997); all other agonistic interactions are undecided. Based on the direction and consistency of decided agonistic interactions, pairs of individuals can establish a dominance relationship between them (Drews, 1993), and the emergent structure resulting from all dyadic dominance relationships among group members can be represented as a dominance hierarchy (Allee, 1938; Landau, 1951; Tibbetts et al., 2022). As in humans, where social hierarchies need not rest exclusively on dominance relations and where group perceptions can be important instead (Redhead and Power, 2022), animals can integrate multiple overlapping social networks of different interaction types (Finn et al., 2019), but it is unknown whether they also navigate multiple social hierarchies, so that their social ranks have been primarily based on dyadic relationships and could also not be determined any other way because inferring the perceptions of bystanders would be extremely challenging to measure. First order intentionality is required from individuals to keep track of all their individual dominance relationships (Gallup, 1998). Experimental evidence indicates that individuals in many group-living species also dispose of second order intentionality, i.e., they are able to infer and monitor dominance relationships between third parties and to adjust their behavior accordingly (Jolly, 1966a; Humphrey, 1976; Cheney and Seyfarth, 1990; Bergman et al., 2003; Range and Noë, 2005). It is therefore meaningful to study the properties, drivers and consequences of dyadic dominance relationships at the group level to characterize the resulting hierarchy as it does not just reflect an artifact of human transposition.
Why two individuals establish a dominance relationship is easily explained if one focuses on its adaptive benefits. Every agonistic interaction is costly, especially in terms of a greater than zero risk of suffering an injury or worse. Even small scratches can develop into dangerous infections, and wound healing can draw energy from other energetic demands for days or weeks (Archie et al., 2012; MacCormick et al., 2012; Archie, 2013). It is therefore always advantageous to minimize the potential costs of fighting for both opponents. Two principal mechanisms are available for this purpose. First, signals of physical strength, agility and other species-specific determinants of fighting ability can be assessed and used to evaluate the potential costs and benefits of an agonistic interaction (Arnott and Elwood, 2009). Second, whenever individuals recognize individual conspecifics and are able to remember the outcome of previous agonistic interactions, an established agonistic asymmetry can be acknowledged by a subordinate by evading a confrontation altogether by an early retreat or by displaying formal signals of submission or by either terminating an interaction with submissive behavior (Reddon et al., 2021). The benefits and the other costs of a dominance relation are always asymmetrical, however, with the dominant enjoying priority of access to resources and mates whenever the features of a resource generate a potential for contest competition (Isbell, 1991; Wrangham et al., 1993; Sterck et al., 1997).
Proximate explanations of why two given individuals establish a dominance relation and how they subsequently maintain it differ accordingly. First, in many cases there is an asymmetry in agonistic power based on physical superiority, aggression, age or motivation that consistently predicts the outcome of dyadic agonistic interactions (Giles et al., 2015; Holekamp and Strauss, 2016; Bonanni et al., 2017; Deniz et al., 2021; Tibbetts et al., 2022). Second, it has been argued that some individuals have greater leverage or power because they control a resource that cannot be taken away by force, and this advantage can also predict the outcome of any given conflict (Young et al., 2017; Lewis, 2018, 2022). Power may be based on fighting ability, but also on knowledge or control over a mating opportunity, making it practically challenging to identify its base, however (Hobson, 2020; Hobson et al., 2021). Third, memories of previous interactions with known individuals can promote a learning effect that leads individuals toward exhibiting submissive behavior – either in response to received aggression or spontaneously – toward certain other conspecifics (Johnsson and Åkerman, 1998; Leimar, 2021). This learning process is further reinforced by the winner-loser effect or other self-organizing social dynamics, according to which winning or losing a fight increases the probability of the same outcome in the next agonistic interaction between the two opponents (Dugatkin, 1997; Hsu and Wolf, 1999; Franz et al., 2015; Lerena et al., 2021; Tibbetts et al., 2022). Finally, in some species, such as spotted hyenas and some catarrhine monkeys, the dominance relationship between two individuals does not exist as a result of prior interactions, but because these species have evolved a convergent social convention of maternal rank inheritance (Walters, 1980; Donabedian and Cords, 2021; Ilany et al., 2021). Accordingly, philopatric matriarchs and their female offspring maintain a life-long dominance hierarchy among matrilines in which the youngest daughter occupies the highest rank just below her mother and the oldest daughter is eventually pushed to the bottom of the within-matriline hierarchy. This system of rank inheritance has the evolutionary benefit of reducing the number of costly fights further because there is no need for an initial establishment of dyadic dominance relationships.
Several types of hierarchies have been reported for various animal societies (Chase et al., 2002). For example, a linear hierarchy is the simplest possibility, but it is unlikely if individuals vary little in agonistic power, and its likelihood decreases with increasing group size (Appleby, 1983). Hierarchies with intransitive relationships are therefore more common (Chase et al., 2002). In some species, there are only one or two individuals that dominate all other group members, whereas no consistent and clear dominance relationships can be discerned among the remaining group members (e.g., in wolves: Mech, 1999). In addition, not all individuals may interact with all others, resulting in various numbers of unresolved relationships. Accordingly, hierarchies in different taxa may differ in their steepness, linearity or other properties (e.g., uncertainty, repeatability), and dyadic relationships within hierarchies may differ in the intensity of aggression, the likelihood of counter-aggression or their conciliatory tendency (de Vries et al., 2006; Sánchez-Tójar et al., 2018; Strauss and Holekamp, 2019; Levy et al., 2020). Importantly, sex plays a key role in structuring hierarchies, because males and females differ in fighting strategies and agonistic power (Pandolfi et al., 2021) and are therefore often not distributed randomly across a dominance hierarchy, whether it is linear or not (Kappeler, 1990a; Smuts and Smuts, 1993; Hemelrijk et al., 2008, 2020). Thus, individuals often cluster within a group’s hierarchy as a function of their sex; in group-living mammals with typically either all or most males outranking all females.
Much of the research on animal hierarchies was conducted on non-human primates. They exhibit a rich diversity of social systems, with stunning variation in group size and composition, kinship structures, mating systems and patterns of (allo-)parental care, providing a rich source for interspecific comparisons in studies of social evolution (Smuts et al., 1987; Kappeler and van Schaik, 2002; Campbell et al., 2010; Mitani et al., 2012; for definitions see Kappeler, 2019). However, what sets the social systems of primates apart from those of other orders of mammals is the diversity and complexity of their social structures, defined as the patterning and nature of social relationships (Silk and Kappeler, 2017). Descriptions of and explanations for the evolution of the diversity of female social relationships have been in the center of socio-ecological research for decades, resulting in a profound understanding of their ecological and phylogenetic determinants, behavioral mechanisms, developmental processes and fitness consequences (Wrangham, 1980; van Schaik and van Hooff, 1983; Sterck et al., 1997; Isbell and Young, 2002; Clutton-Brock and Janson, 2012; Schülke and Ostner, 2012; Thierry, 2013; Strier, 2018; Moscovice et al., 2020). Similarly, the causes and consequences of variation in male–male relationships have been studied in detail (van Hooff and van Schaik, 1994; Alberts, 2012). In contrast, most studies of intersexual interactions focused on functions in the immediate context of reproduction, i.e., mate choice, parental care and infanticide (Fernandez-Duque et al., 2009; Kappeler, 2012a; Lukas and Huchard, 2014; but see e.g., Baniel et al., 2016). Dominance relationships between the sexes, in particular, have not enjoyed the same theoretical and empirical attention as same-sex dominance relations (Muller and Wrangham, 2009; Stumpf et al., 2011; Baniel et al., 2017; Kunz et al., 2021). Because male-biased sexual dimorphism is widespread among mammals (Lindenfors et al., 2007), the ability of males of these species to dominate females was presumably considered an unavoidable side-effect of male physical superiority and greater aggressiveness, and male dominance over females was considered to not require specific attention and explanation (Lewis, 2018).
Yet, the endemic primate radiation of Madagascar (Lemuriformes) offers a remarkable exception to the widespread primate pattern of larger males dominating females (Jolly, 1984; Richard, 1987; Kappeler, 1993; Wright, 1999; Dunham, 2008; Lewis, 2020). Recent research has challenged the long-held assertion that ubiquitous female dominance characterizes all lemur species by revealing considerable interspecific variation in this respect, however. Here, we offer an up-to-date appraisal of the relevant studies and reports of intersexual dominance relationships in lemurs and evaluate existing hypotheses about the evolution of female dominance in light of these new insights.
Today, more than 120 species of lemurs are recognized (Rowe and Myers, 2016). Phylogenetic analyses revealed that they represent the living endpoints of an adaptive radiation following a single successful colonization event of Madagascar more than 50 million years ago (Karanth et al., 2005; Herrera, 2017). Representing only one of four groups of terrestrial mammals that successfully colonized Madagascar (Poux et al., 2005; Kappeler et al., 2019), they subsequently diversified into 5 families and 15 genera plus at least 17 species from 8 additional genera that went extinct within the last few centuries (Godfrey, 2016). This adaptive radiation generated diversity in all fundamental adaptations. Their body sizes span several orders of magnitude (from 30 g – @ 150 kg), and the corresponding life history traits vary accordingly (Catlett et al., 2010). Lemurs evolved diurnal activity at least twice (Santini et al., 2015), and the diversity of their diets matches that of all other primates combined (Richard and Dewar, 1991). Their social systems are equally distinctive, with a wide range of socially diverse solitary species, at least two types of pair-living species, and group-living species in two separate families (Kappeler, 1997, 2012b; Kappeler and Pozzi, 2019). Yet, despite this stunning diversity in fundamental traits, lemurs differ from many better-studied anthropoid primates in that their groups are on average smaller, even after controlling for body size and phylogeny, the average adult sex ratio of their groups is not female-biased, they lack male-biased sexual dimorphism in body and canine size, females have masculinized external genitalia and female dominance is widespread (Richard, 1987; Kappeler and Fichtel, 2015).
Prominent reports of female dominance in ringtailed lemurs (Lemur catta; Jolly, 1966b), sifakas (Propithecus verreauxi and P. coquereli, Richard, 1974) and indris (Indri indri, Pollock, 1979), which were the subjects of some of the first extended field studies, contributed to the widespread notion that all lemurs exhibit female dominance (but see Pereira et al., 1990). Being apparently largely confined to lemurs, female dominance never became a mainstream topic in primatology because it appeared to require special explanation and generated several hypotheses that invoked lemur- or Madagascar-specific factors to explain the evolution of this sex role “reversal.” However, recent studies revealed several instances where lemur females only win a proportion of agonistic interactions with males, or where females dominate only some, but not all males, and even male dominance has been indicated in one report, suggesting the action of diverse selective forces. Below, we first summarize this variation in detail before we link it to the hypotheses proposed to explain the evolution of female dominance to facilitate the connection of this body of literature to studies of sex-based effects on power, status, dominance and leadership in other mammalian societies that have begun to enter mainstream ethology and primatology as a result of a more general recent interest in sex roles in human and animal societies (Gowaty et al., 2012; Schärer et al., 2012; Janicke et al., 2016).
In this section, we summarize the current knowledge about patterns of intersexual dominance relations in all genera of lemurs. We proceed taxonomically (by genus), summarizing relevant details of male–female interactions and relationships, including the proportion of decided conflicts and whether males and females are able to dominate some or all members of the opposite sex. We also note whether studies were observational or experimental, whether they took place in captivity or the wild, and whether they covered periods of reproduction. To provide context for these details, we also ask whether males and females are permanently associated and report the degree of sexual size dimorphism. The main variables are also summarized in Table 1.
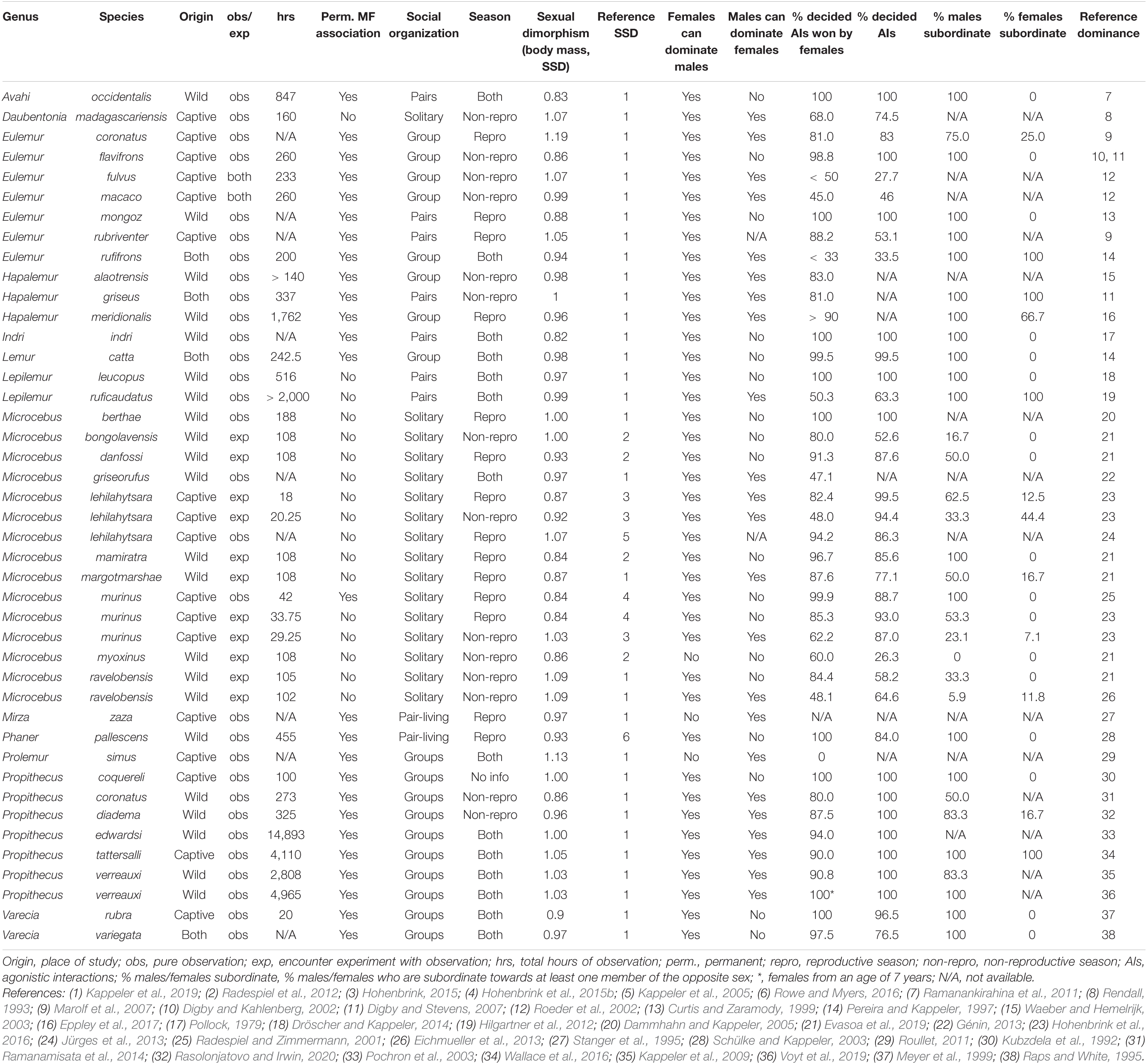
Table 1. Life style, sexual body mass dimorphism, conflict characteristics, and intersexual dominance relationships in lemurs (Lemuriformes).
No data are available on intersexual conflicts or dominance relationships in the hairy-eared dwarf lemur, Allocebus trichotis, the only member of this genus.
No reports on intersexual conflicts and their outcomes are available for any of the about 10 species of dwarf lemurs in the genus Cheirogaleus.
Among the currently recognized 25 species of mouse lemurs, genus Microcebus, some evidence on the outcomes of intersexual conflicts and dominance relationships is available for 10 species (40%). Among these, direct observational, quantitative data on the outcomes of intersexual conflicts from natural forest environments is only available for two species (M. berthae, M. griseorufus). The intersexual conflict behavior of the other eight species was either quantified in captivity (M. lehilahytsara, M. murinus) or in wild animals that were observed during social encounter experiments (M. bongolavensis, M. danfossi, M. mamiratra, M. margotmarshae, M. myoxinus, M. ravelobensis). For these experiments, one male and one female were temporarily (up to 1 week) kept in one or two connected cages, observed, and subsequently released at their point of capture.
This general scarcity of published, quantitative data for wild mouse lemurs is due to their social organization and difficult observation conditions for these small, arboreal, nocturnal solitary foragers. Social encounters occur only infrequently at night, and the identity as well as sex of the interaction partner cannot always be ascertained, even if some focal animals are equipped with radio-collars. The experimental encounter paradigm solves these problems, since the confinement of two animals to one cage setting increases social interaction rates and eliminates the identification problem. The determination of dominance relationships between two animals is therefore largely facilitated, although potential habituation problems and the confinement to limited space constrain the external validity of the results.
In one field study on M. berthae (188 h observation time before and during the mating season in Kirindy Forest), females won all (100%) observed conflicts with males, which occurred during feeding and in the sexual context (Dammhahn and Kappeler, 2005). The underlying number of conflicts was low (n < 10), however, and no further details were provided. Social encounters in wild M. berthae were rare in general (males: 0.93 encounters/h, females: 0.49 encounters/h), but intersexual encounters were more frequent than encounters between females or between males (Dammhahn and Kappeler, 2005).
A study on M. griseorufus in Berenty Reserve reported a mixed pattern during intersexual conflicts (Génin, 2013). Females won 47% of all observed conflicts with males (n = 34 conflicts). Although adult females were typically able to dominate males, three heavy males were reported to win conflicts with subadult females. In addition, females but not males were able to monopolize fruiting trees, and females fed significantly longer than males at these trees (Génin, 2013).
Outcomes of intersexual conflicts and intersexual dominance were determined in social encounter experiment with six male-female dyads of M. bongolavensis in Marosely Forest (Evasoa et al., 2019). During 108 observation hours in the non-reproductive season (July – August), females won 80% of all decided conflicts, but conflicts were generally rare (0.35 conflicts/h, n = 38), only one of six females was dominant over her male partner, and only 52.6% of all conflicts were decided (Evasoa et al., 2019). In fact, experimental pair partners were relatively often observed in mutual proximity and body contact.
Outcomes of intersexual conflicts and intersexual dominance in six male-female dyads of M. danfossi were also determined experimentally in Anjiamangirana Forest during 108 observation hours in the reproductive season (September – October). Two females were in estrus, two had recently been in estrus, and one was pregnant during the observations. The overall conflict rate was not very high (0.97 conflicts/h, n = 105). Still, most conflicts were decided (87.6%), females won significantly more conflicts than their male partners, and half of the females were dominant over their temporary mates (Evasoa et al., 2019).
Six male-female dyads of M. mamiratra were subjected to the same social encounter experiment in Lokobe National Park during the reproductive season (July). One female was swollen, one had been in estrus recently, and one was pregnant during the observations. Overall, conflicts occurred very frequently (8.8 conflicts/h, n = 954), significantly more often than in any other species tested with this paradigm, and most conflicts were decided (85.6%). All six females were dominant over their male partner and won 96.7% of all decided conflicts (Evasoa et al., 2019).
Using the same experimental design, six male–female dyads of M. margotmarshae were observed during the reproductive season (August–September) in Ankaramibe Forest (Evasoa et al., 2019). Two females had recently been in estrus and one female gave birth and lactated during the study. Conflicts occurred frequently (2.7 conflicts/h, n = 114) but less often than in their sister species M. mamiratra. Three of six females were dominant over their male partner, but one case of male dominance was also observed. Still, females won most decided conflicts (87.6%) and 77.1% of all conflicts were decided.
In M. myoxinus, the social encounter experiment was conducted in Bombetoka Forest in the non-reproductive season (September – October). Conflicts were generally rare (0.28 conflicts/h, n = 30) and intersexual dominance could not be determined in any dyad (Evasoa et al., 2019). Females still won the majority (60%) of all decided conflicts, but most (73.7%) conflicts were undecided. In this species, the authors suspected a high degree of behavioral disturbance by nocturnal human visitors passing nearby, since the animals were often jointly hiding in the sleeping site during the observation.
Intersexual dominance was also assessed experimentally in M. ravelobensis in Ampijoroa Forest in two studies during the non-reproductive season (Eichmueller et al., 2013: June – August, Evasoa et al., 2019: May – June). Eichmueller et al. (2013) observed 17 male–female dyads that could each interact only during 6 h of supervised encounter time distributed over four consecutive nights, adding up to a total observation time of 102 h. This study documented 206 intersexual conflicts but found female dominance in only one and male dominance in two out of 17 male–female dyads. This rather low incidence of female dominance coincided with a low rate of winning for females (48.1%) and a low rate of decided conflicts (64.6%), despite a moderately high overall conflict rate (2.0 conflicts/h). Evasoa et al. (2019) observed six male-female dyads for 105 h and detected female dominance in two out of six male-female dyads. Conflict rates (0.52 conflicts/h) and decision rates (58.2% of n = 55) were both rather low, but females still won 84.4% of all decided conflicts.
These six mouse lemur species were studied with the exact same experimental paradigm by the same observer, but differed significantly in their levels of mutual tolerance and patterns of intersexual dominance (Evasoa et al., 2019). The multivariate analyses on this multi-species dataset revealed that neither phylogenetic proximity nor habitat type (dry vs. humid forest) could explain the observed interspecific variation in intersexual relationships. However, reproductive activity did coincide with a higher incidence of female dominance, and an impact of seasonal reproduction on the frequency of intersexual conflicts in mouse lemurs was suggested.
Significant seasonal differences in intersexual conflict rates and the outcome of conflicts were also detected in captivity (Hannover colony, Germany) in M. lehilahytsara. Hohenbrink et al. (2016) employed a social encounter paradigm and conducted a series of encounters (=2.25 h/pair) with eight dyads during the reproductive season and with nine dyads during the non-reproductive season. Intersexual conflicts were significantly more frequent in the reproductive season (60.2 conflicts/h, n = 1,084) than in the non-reproductive season (19.4 conflicts/h, n = 393). Although the vast majority of conflicts was decided in both seasons (reproductive season: 99.5%, non-reproductive season: 94.4%), females won relatively more conflicts in the reproductive season (82.4%) than in the non-reproductive season (48%), which was also reflected in the higher proportion of dominant females in the reproductive season (62.5%) than in the non-reproductive season (33.3%). Conversely, males dominated females in four of nine dyads (44.4%) during the non-reproductive season. The seasonal change in dominance from the reproductive to non-reproductive season happened even in individual dyads (1x from female-dominant to male-dominant, 1x from female-dominant to undecided, 1x from undecided to male-dominant).
Another captive study on M. lehilahytsara was conducted in the Masoala Hall of Zurich Zoo (Switzerland) during 2 months after animals reappeared from seasonal torpor at the start of the reproductive season (Jürges et al., 2013). Focal animals had relatively frequent social encounters (defined as proximity of < 10 m) with a median of 10 times/h, although the majority of encounters (median = 75%) did not involve social interactions (Jürges et al., 2013). A total of 80 conflicts were observed between males and females in different contexts (feeding, social, sexual, unspecific), the vast majority of them being decided (86.3%, n = 69). Females won most decided conflicts (94.2%, n = 65), in fact significantly more than males (Jürges, 2010).
A study on agonistic intersexual conflicts and dominance in captive M. murinus documented unambiguous female dominance (i.e., all females were dominant over all males) in four study groups that were each composed of two adult males and two adult females (Radespiel and Zimmermann, 2001) and were observed for a total of 42 h after group formation during the reproductive season. The study period also included the first seasonal estrus of the females. Overall, 865 agonistic interactions were observed with an overall rate of 20.6 conflicts/h. Most of them were decided (88.7%) and in all but one case in favor of females (99.9%). Intersexual conflicts occurred in various behavioral contexts (sexual, feeding, sleeping, social, spatial).
Significant seasonal differences in intersexual conflict rates and outcomes of conflicts were detected in a more recent study on captive M. murinus. Hohenbrink et al. (2016) studied experimental encounters between 15 dyads during the reproductive season and 13 dyads during the non-reproductive season. Intersexual conflicts were significantly more frequent in the reproductive season (26.9 conflicts/h, n = 909) than in the non-reproductive season (5.8 conflicts/h, n = 169). The vast majority of conflicts was decided in both seasons (reproductive season: 93%, non-reproductive season: 87%). Females won relatively more decided conflicts in the reproductive season (85.3%) than in the non-reproductive season (62.2%). This seasonal impact was also reflected in the higher proportion of dominant females in the reproductive season (53.3%) compared to the non-reproductive season (23.1%). During the non-reproductive season, one male even dominated his female partner (same dyad: undecided during reproductive season); an outcome that was never observed during the reproductive season. The difference in the proportion of dominant females in both captive studies on M. murinus was argued to be the result of the different test paradigms (permanent group formation during estrus vs. temporary encounters outside estrus).
Although many studies have been conducted on wild M. murinus over the last 60 years, quantitative data on the frequency and outcomes of intersexual conflicts have not been published for this species to the best of our knowledge. However, two studies at Kirindy Forest documented that some females spatially monopolized food resources (in particular gum trees) by chasing males, but not other females, out of gum trees (Génin, 2003, 2013). One additional early study from the reproductive season (September – November) in Ankarafantsika National Park reported that although gray mouse lemurs spent an overall 11% of the observation time within 10m of conspecifics (n = 195 encounters), only 11.8% (n = 23) of the encounters included aggressive behaviors or “rejections of contact” (Pagès-Feuillade, 1988). Among these, the sex was known for both partners in 18 cases, and 83.3% (n = 15) of these occurred between the sexes. During these intersexual conflicts, females mostly rejected contacts attempted by males, suggesting that M. murinus females were dominant over males, which may have been facilitated by a female-biased body mass dimorphism (Pagès-Feuillade, 1988).
No quantitative data are available on intersexual conflicts or dominance relationships in Mirza coquereli. Only very few data are available on intersexual conflicts in Mirza zaza. One captive study reported on social interactions between one adult male and two adult females at the Duke Lemur Center (Stanger et al., 1995). The authors observed social interactions between the sexes during one estrous cycle of both females, and aggressive behavior was only displayed by the male but not by the females. This observation is suggestive of male dominance, but the lack of systematic data precludes a conclusive assessment.
Of the four recognized species of the genus Phaner, the social system, including intersexual conflict and dominance, has only been studied in Phaner pallescens (formerly referred to as P. furcifer), which live in family groups comprised of an adult pair and their offspring (Schülke and Kappeler, 2003). This study was conducted in Kirindy Forest and involved 455h of focal observations on 15 male and 15 female pair partners. Agonistic conflicts occurred at relatively low rates (0.49 conflicts/h, n = 225) but still more frequently than affiliative interactions. Conflicts occurred mostly in the feeding context (60.4%), but also during mate guarding, meetings with neighbors, resting, and during immigration of a new male (Schülke and Kappeler, 2003). The majority of conflicts (84%, n = 189) were decided, and all (100%) were won by females. Submissive behaviors of males occurred spontaneously (i.e., without aggression by females) in more than half of all decided conflicts (55.6%, n = 105).
Data on intersexual conflicts and dominance relationships of the 26 species of nocturnal sportive lemurs are available from field studies on only two of them.
Social interactions between eight pairs of redtailed sportive lemurs (Lepilemur ruficaudatus) were studied in Kirindy Forest for 24 months (Hilgartner et al., 2012). Due to the nature of their social organization as dispersed pairs, only 255 social encounters between pair partners were observed. Almost half (47.3%) of these encounters involved the exchange of agonistic behavior. Of the 120 agonistic interactions, 63.3% were decided. On average, half (49.7%) of them were won by males, but during the mating season, this rate increased to 87.1% (n = 31). In contrast, males lost most of the conflicts (78.9%, n = 38) during the birth season. During the rest of the non-mating season, agonistic encounters between pair partner were rare (n = 7) and wins were equally distributed between pair partners. Notably, not a single affiliative interaction between pair partners was ever observed.
Twenty nocturnal white-footed sportive lemurs (L. leucopus) were observed at Berenty Reserve for a complete annual cycle. Despite parallel observations of the members of 7 dispersed pairs, only 15 agonistic interactions were observed at all during 516h of focal observations (0.03 conflicts/h) (Dröscher and Kappeler, 2013). Most of them involved unidentified neighbors, and only three agonistic interactions between members of a pair were observed, and in all of them (100%) the female displaced the male from a food patch (Dröscher and Kappeler, 2014).
Thus, in sportive lemurs, rates of (agonistic) interactions are extremely low. They appear to lack unambiguous submissive behavior, contributing to relatively high proportions of undecided conflicts. Sex did not predict the outcome of decided conflicts in L. ruficaudatus, except during periods of reproduction. Interestingly, during the mating season males prevailed more often whereas females dominated males when they had small infants. In L. leucopus, females appear to be dominant over males, but the sample size is extremely small and agonistic interactions are so rare that dominance may not be a useful concept to describe the outcome of these few interactions.
Intersexual dominance relationships in solitary nocturnal aye-aye, Daubentonia madagascariensis, were studied in a captive colony at the Duke Lemur Center (Rendall, 1993). Two pairs of wild-caught individuals were observed for 160h, but only one of them consisted of adult individuals. Of 55 agonistic interactions between the members of the adult pair, 74.5% were decided, and 68% of the decided (and 42.9% of 14 undecided) conflicts were won by the female. Undecided interactions in this study were defined as those in which one individual exhibited only aggressive behavior, while the other responded with a combination of aggressive and submissive behavior. In the juvenile pair, the male was older, heavier and larger than the female. He managed to elicit submissive behavior by the female in 87% of the decided conflicts (n = 120) between them. However, the young female managed to displace the male in the majority (83.4%) of 66 undecided conflicts, most of which involved access to food. Thus, female aye-ayes appear to be able to dominate males, but larger sample sizes and data on intersexual encounter rates in their very large home ranges of hundreds of hectares (Sefczek et al., 2020) are required to establish the external validity of this preliminary study.
Intersexual relationships in the Indriidae have been studied mainly in the course of observational studies in the wild, except for Coquerel’s and golden-crowned sifakas (Propithecus coquereli, P. tattersalli).
The genus Avahi consists of nine species but information on intersexual conflicts is only available for one species, the Western woolly lemur (Avahi occidentalis). Western woolly lemurs are nocturnal and live in cohesive pairs. Six pairs were observed for 847 h over a period of 8 months in the National Park Ankarafantsika (Ramanankirahina et al., 2011). In total, 21 agonistic interactions with a median rate of 0.01/h were observed. Of those conflicts, 15 were decided and 5 were incompletely observed. All 15 decided conflicts between pair partners were won by females and males showed submissive behavior, suggesting that females are dominant over males.
Indri indri are diurnal and organized into pairs. Three pairs with their offspring were observed over a period of 15 months in Andasibe-Mantadia National Park (Pollock, 1979). In total, 135 social displacement including aggressive displacements were observed in two groups, whereas 107 social displacements – mainly in small feeding trees - were observed in the third group. Adult female indris always displaced adult males, and are, hence, dominant over males.
The genus Propithecus consists of nine species, which are all diurnal and group-living. Information on intersexual conflicts is available for six species. Coquerel’s sifakas (P. coquereli) were observed in outdoor enclosures of the Duke Lemur Center (Kubzdela et al., 1992). Two pairs of an adult female and male were observed for 100 h each outside the reproductive season. In total, 26 aggressive interactions were observed in feeding contexts. Females initiated and addressed aggression toward males during 23 events, with males responding three times with counter-aggression. Since only females initiated and addressed aggression toward males, females were considered to be dominant over males, at least in the feeding context.
Three groups of crowned sifakas (P. coronatus) were observed for 273 h in the Antrema Forest Station in north-west Madagascar. Out of 39 agonistic interactions, female initiated 80% agonistic interactions toward males, but could only dominate 50% of males (Ramanamisata et al., 2014).
In diademed sifakas (P. diadema) three groups were observed for 325 h outside the reproductive season at Tsinjoarivo. In total, 21 agonistic interactions were observed, of which 11 occurred between the sexes and 10 between males. Females won 88% of conflicts with males and dominated 83% of males (Rasolonjatovo and Irwin, 2020).
In Milne Edwards sifakas (P. edwardsi), data on intersexual relationships were obtained from observations of four groups over a period of 15 years at Ranomafana National Park. Each focal animal was observed for about 7.9 ± 2.9 h. Milne Edwards sifakas exhibited an aggression rate of 0.22 interactions/h. Out of 1,426 agonistic interactions 1,410 were decided (98.9%). 825 agonistic interactions occurred between the sexes and females won 94% of all conflicts with males, but information on the number of males dominated by males was not provided (Pochron et al., 2003).
Four adult wild-caught golden-crowned sifakas (P. tattersalli) were observed for 4,110 h at the Duke Lemur Center. Females won 90% of conflicts over males and could dominate all males (Wallace et al., 2016).
Ten groups of Verreaux’s sifakas (P. verreauxi) were observed throughout the year for 2,808 h at Kirindy Forest. A total of 383 agonistic interactions, of which 345 were decided (90.1%) and 38 undecided, were observed in a feeding context. Females won 91% of intersexual conflicts and could dominate 83% of males (Kappeler et al., 2009). In another study, four groups of Verreaux’s sifakas were observed for 4,965 h over a period of 9 years in Kirindy Mitea National Park (Voyt et al., 2019). A total of 483 decided agonistic interactions were observed. In 342 agonistic interactions, the age of the two conflicting partners was known. Young females of 3 years won only 30% of conflicts with males, females between an age of four to six won on average more than 50% of intersexual conflicts, whereas adult females older than 7 years won 100% of their conflicts with males. These data suggest that female dominance becomes more unambiguous with female reproductive maturity.
Although sifaka males are occasionally able to win individual conflicts over females, females win the majority of fights and are able to dominate most males in their groups. Hence, sifakas can be clearly classified as exhibiting widespread female dominance. Overall, all species of the indriids which have been studied so far, exhibit female dominance.
The family of lemurs contains 21 species in 5 genera, of which information on intersexual dominance relations exists for 14 species from mostly observational studies in captivity and the wild.
The social behavior of greater bamboo lemurs, Prolemur simus, has not been systematically studied. In the wild, they live in groups with multiple adult males and females (Frasier et al., 2015). In captive colonies in France, however, unsystematic behavioral observations revealed that two adult males cannot be kept together. The same is true for unrelated adult females and related females with offspring. According to Roullet (2011), males can become aggressive toward keepers and also dominate females. If confirmed by specific behavioral studies, P. simus would be the only known lemur species with exclusive male dominance.
Information on intersexual dominance relations in pair-living bamboo lemurs is available from three out of five species. In Hapalemur alaotrensis, four wild groups were observed for 4 months outside the reproductive season (Waeber and Hemelrijk, 2003). All but one intersexual agonistic interaction were decided, and the vast majority of them was over access to food. Of 260 male–female conflicts, 77% involved only submissive behavior, and in 83% of those only males exhibited submission. Nonetheless, in some conflicts females submitted to males, but it remains unknown how many different females exhibited submission, whether this included some of the juvenile females included in the study, and whether females that exhibited submission in a conflict with a male were able to elicit submission from the same male in other conflicts. Despite this lack of detail, it appears fair to conclude, based on the presently available data, that female Alaotran bamboo lemurs exhibit female dominance in the vast majority of agonistic interactions with adult males.
A total of 428 agonistic interactions were observed in two small groups and one pair of wild southern bamboo lemurs, H. meridionalis, during 1,762 h distributed across a full year (Eppley et al., 2017). Females initiated and won more than 90% of these conflicts (79.8% over access to food) and were twice as likely to target a male than males that initiated agonistic interactions. All three study units contained one adult male and two or one adult females, and a female took the dominant position in all three units.
In three groups of wild gray bamboo lemurs, H. griseus, observed over 3 months outside the reproductive season, only seven agonistic interactions between males and females were recorded in 337 h of focal animal observations, and in 3 (42.9%) of them, females directed aggression toward males (Foreit, 2016), but submissive behavior was not recorded in this study. Thus, sex did not predict the direction of aggression, but the number of observed conflicts is very low. A behavioral study of five captive pairs or small family groups lasting over a year yielded details on 42 intersexual agonistic interactions, of which the females decided 81% in their favor (Digby and Stevens, 2007). Thus, rates of agonistic interactions in these specialized folivores are low, and females win most, but by far not all agonistic interactions with adult males.
Ringtailed lemurs, Lemur catta, were the first lemur species for which female dominance was reported (Jolly, 1966b). They form the largest groups of all lemurs with multiple adult males and females. Numerous studies of wild and captive populations have since confirmed that all adult females unconditionally dominate all adult males. We therefore refer to only one captive and two different wild studies with large sample sizes.
Studying two semi-free ranging captive groups at the Duke Lemur Center, Pereira and Kappeler (1997) observed 495 agonistic interactions between males and females in more than a year of observations, and 99.5% of them were decided in favor of females. Most interactions consisted of spontaneous male submission whenever males and females came into close proximity. In a study of two wild groups at Beza Mahafaly, Sauther (1993) recorded 2,301 agonistic interactions during 1,800 h of observations, with 86% of them occurring over food resources. In 35 of intersexual agonistic interactions, a male displaced a female, but the latter were all young and nulliparous. In contrast, females won 96.9% of their agonistic interactions with males, and the rates of their conflicts peaked during the late lactation period. A 4-month study of two groups at Berenty Reserve during the birth season confirmed the pattern of unambiguous female dominance (Nakamichi and Koyama, 1997). Females were winners and males were losers in all of the 709 decided agonistic interactions between females and males, and females were dominant over males in 90 of 91 possible female–male dyads in these two groups; the remaining dyad was never observed to interact. Thus, ringtailed lemurs exhibit ubiquitous female dominance under variable environmental conditions in all behavioral contexts.
One field study focused on intersexual dominance relations in black and white ruffed lemurs, Varecia variegata. Overdorff et al. (2005) observed two groups for more 17 months, but they were not able to consistently decide which animals were winners and losers. Moreover, one group was socially instable for most of the study period. In a stable group of two females and two males, one female dominated all other group members, but submissive signals were rarely exchanged, and 45 of 49 submissive signals were given by one male toward the dominant female. In a year-long study of a captive pair living in a natural habitat enclosure at the Duke Lemur Center with their three offspring, 76.5% of 47 agonistic interactions between all group members were decided, and 97.1% of the decided interactions were won by females (Raps and White, 1995). Only 25.7% of females’ 35 wins lacked female aggression, however; i.e., spontaneous male submission is not common. A 2-month study of a semi-free ranging pair and their 5 offspring at Duke Lemur Center recorded 46 agonistic interactions, of which all but one (97.8%) were won by females (Kaufman, 1991).
Two captive groups of red ruffed lemurs, V. rubra, consisting of a breeding pair and their offspring, were studied at the Duke Lemur Center between September and April (i.e. including the mating and birth season; Raps and White, 1995). All 348 agonistic interactions were decided, 96.5% of them were won by females, and 69.5% of those involved female aggression. A 20 h study of a captive group of three females and two males reported 25 agonistic interactions, of which 96% were decided; all of them in favor of females (Meyer et al., 1999).
Thus, despite a lack of longer studies of multiple groups, the available evidence indicates that ruffed lemur females are generally able to dominate males and that female aggression appears to be often required to elicit male submission.
Of the 12 species of true lemurs, data on intersexual dominance relations are available for seven of them; most of them from studies in captivity. Between September and May (i.e. including the mating and birth season), four groups of crowned lemurs, Eulemur coronatus, were studied at Mulhouse Zoo (Marolf et al., 2007). A total of 83% of 424 intersexual agonistic interactions were decided, and females won 81% of them. Females were able to elicit much more submissive behavior from males than vice versa, but in one of the groups, the only male dominated the only adult female. The intersexual agonistic interaction rate increased during the breeding season in only one group. In a captive group at the Duke Lemur Center studied across a year, females won 97% of all decided agonistic interactions (N = 105) with males (Pereira et al., 1990).
In two family groups of redbellied lemurs, E. rubriventer, at Mulhouse Zoo, 53.1% of 64 agonistic intersexual conflicts were decided, and females won 88.2% of those (Marolf et al., 2007). Females never showed spontaneous submission toward males. In one of the groups, most conflicts occurred during the breeding season. The majority of intersexual agonistic conflicts in a year-long study of two wild family groups at Ranomafana National Park occurred in the context of infant transfer, when the mother cuffed the male in the process of transferring an infant on his back for carrying (Overdorff and Tecot, 2006).
Seven groups of blue-eyed black lemurs, E. flavifrons, were studied at the Duke Lemur Center for 260 h in June and July (i.e., during the non-reproductive season; Digby and Kahlenberg, 2002). Of 293 agonistic interactions between males and females, females won 99% of them. Males elicited submissive behavior from a female on only four occasions. Only 19% of agonistic interactions involved spontaneous male submission. In a subsequent study of the same population, females won 98.6% of 506 intersexual agonistic interactions, and 65.7% of 589 dominance interactions initiated by females involved aggression on their part (Digby and Stevens, 2007).
In one captive group of black lemurs, E. macaco, studied for 2 months after the mating season at the Strasbourg Primate Center, 46% of 81 intersexual conflicts were decided and the majority (95.6%) of decided conflicts were won by the aggressor independent of its sex (Roeder et al., 2002). In an experimental dominance study with black lemurs, females were dominant over males in a competitive drinking test, but no details on the nature of their conflicts are available (Fornasieri et al., 1993).
Two groups of brown lemurs, E. fulvus, were studied at the Strasbourg Primate Center for 233 h outside the mating season (Roeder et al., 2002). Of 102 intersexual conflicts, only 27.7% were decided and in 92.4% of the decided conflicts, the initiator prevailed, independent of sex. Similarly, in an experimental dominance study with brown lemurs, sex had no effect on the outcome of conflicts in a competitive drinking test (Roeder and Fornasieri, 1995).
In two groups of redfronted lemurs, E. rufifrons, studied in natural habitat enclosures at Duke Lemur Center, only 33.5% of 474 conflicts were decided, and less than 33% of those were decided in favor of females (Pereira and Kappeler, 1997). Similar results were obtained in an independent study of the same captive population (Pereira et al., 1990), with only 31% of agonistic interactions being decided. In a wild group at Ranomafana National Park, this percentage was at 61% (N = 279), and females won only 13% of the 172 decided agonistic interactions with males (Pereira et al., 1990). Thirty-four percent of conflicts at Ranomafana included female submission toward males and 65% male aggression toward females. A field study of two groups at Kirindy Forest between April and August (i.e. including the mating season) revealed that male-male agonistic interactions were most frequent and that more than 80% (N = 258) of them were decided (Ostner and Kappeler, 1999). Intersexual interactions were much rarer and only 27% of them were decided. Only one conflict between females was observed in 1,023 observation hours. Based on decided agonistic interactions, one male appeared on top of the dominance hierarchy of both groups.
Finally, in two wild family groups of mongoose lemurs, E. mongoz, females were reported to have priority of access to food in all conflicts with males, but no details on the number or nature of conflicts was provided (Curtis and Zaramody, 1999).
Thus, intersexual dominance relations among true lemurs are highly variable. Females are dominant in some species, but sex has no effect on the outcome of agonistic interactions in others. Aggression appears to be required to win agonistic interactions and exclusive submissive behavior is rare. Feeding and reproduction are contexts in which conflict rates are high, but most studies suffer from either short duration, few conflicts or a lack of relevant detail.
The available quantitative data offer very few opportunities for explorative statistical analyses. To assess potential impacts of study type (observation vs. experiment), setting (captivity vs. wild) or season (mating season included or not), not enough studies of the same species under different conditions are available. One strong prediction is that the ability to win agonistic interactions with members of the opposite sex is based on physical superiority. However, average sexual size dimorphism among lemurs is close to 1 with a mean ± SD of 0.97 ± 0.09, a minimum of 0.82 and a maximum of 1.19 (Table 1). Sexual size dimorphism is nonetheless negatively correlated with the average proportion of conflicts won by females (Figure 1; Pearson, N = 36 species, r = –0.38, p = 0.022), but only if Prolemur simus is included (with a value of 0% female wins; Roullet, 2011). After excluding P. simus, this correlation is no longer significant (Figure 1; Pearson, N = 35 species, r = –0.25, p = 0.149), however. Hence, quantitative data from Prolemur, but also additional species, on intersexual dominance are required to determine whether sexual size dimorphism covaries indeed with the proportion of conflicts females win over males.
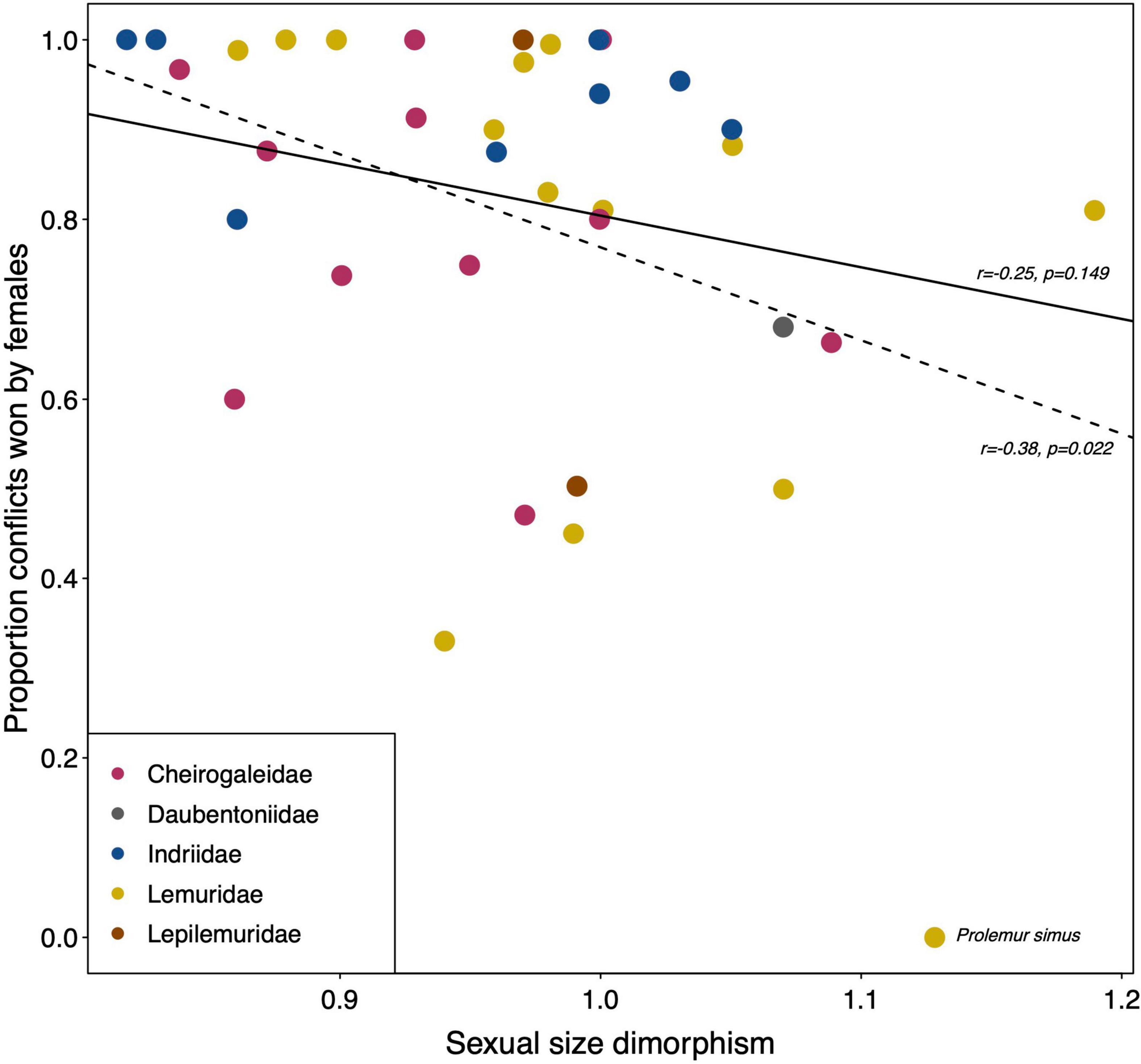
Figure 1. Relationships between the average sexual size dimorphism in body mass and the average proportion of conflicts females won over males across the members of different lemur families. Dashed line indicates the Pearson correlation including Prolemur simus; solid line presents the correlation excluding P. simus. All data from Table 1.
In striking contrast to most other primates and mammals, the ability of adult females to consistently dominate adult males is clearly widespread among the primates of Madagascar. However, there is more variation in intersexual dominance relations among and within species than previously acknowledged, ranging from well-documented empirical support for invariable female dominance in some species to possible male dominance in others. Given the nature of the available evidence, however, only a broad qualitative assessment of the dimensions and causes of this variation is currently possible.
First, females in all five families and all the genera studied so far are able to evoke submissive behavior from adult males. Prolemur may be an exception, but only systematic quantitative data will allow a firm assessment of this species in the future. Given this taxonomic distribution of female dominance, a phylogenetic reconstruction conducted for purely illustrative purposes (Figure 2) revealed that this ability has already characterized ancestral lemurs – perhaps even the first colonizers – than to postulate multiple evolutionary origins of female dominance (see also Petty and Drea, 2015; Lewis, 2018). The issue of whether the absence of male dominance and male-biased sexual size dimorphism in lemurs is just an idiosyncrasy of that lineage, or whether the colonization or ecology of Madagascar have prompted adaptations that are only rarely found in other mammals remains difficult to resolve, but some comparative evidence suggests that a combination of these factors might be implicated (Kappeler et al., 2019).
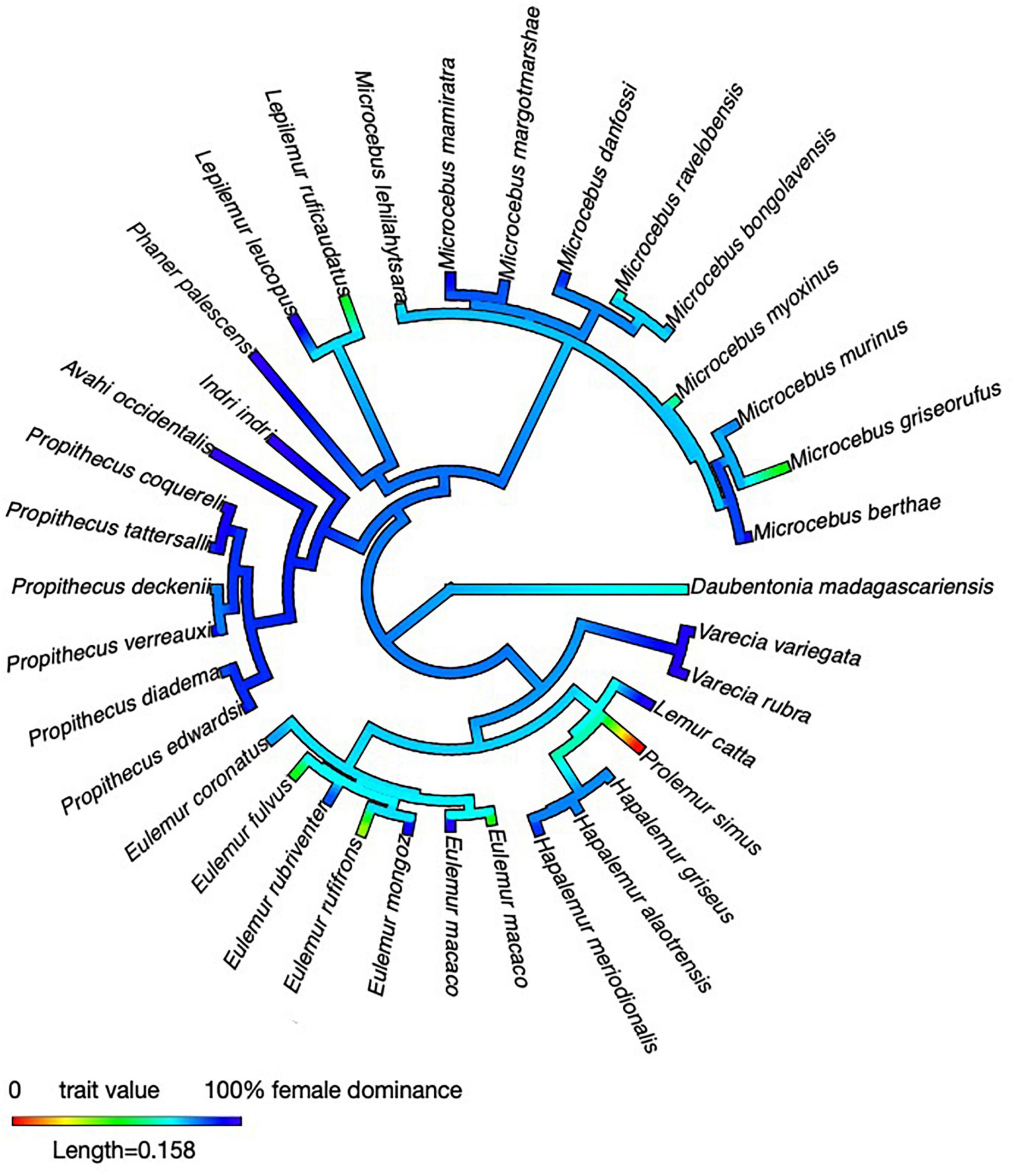
Figure 2. Phylogenetic reconstruction of intersexual dominance across the Lemuriformes. We conducted an ancestral state reconstruction using the package “phytools” (Revell, 2012) in R (version 4.0.3; R Core Team, 2020). For illustrative purposes, we mapped female dominance, i.e., the percentage of conflicts won by females, on a consensus tree obtained from the 10k trees website (Arnold et al., 2010). Female dominance varies between 0 and 100%, making a formal reconstruction formally challenging, also because no obvious criteria for a discrete classification (female dominance: “yes” or “no” exist). All data were taken from Table 1.
Second, substantial variation in the nature of intersexual dominance among closely-related species within the same genus indicates that environmental and/or social factors have shaped the variation in social structure seen among contemporary lemur species. The standardized experiments with Microcebus, but also the various observational studies of Eulemur and Propithecus revealed intriguing variation that is not obviously linked to a particular habitat, season or reproductive phase. Observations of wild species have emphasized feeding competition as a frequent context in which females dominate males, but captive and experimental studies have indicated that intersexual agonistic interactions are neither limited to this situation nor particular to any species. The phylogenetic reconstruction also indicates that the average proportion of intersexual conflicts won by females has been reduced independently in some taxa in all families, except for the Daubentoniidae (Figure 1), suggesting that intersexual dominance relations continue to evolve in response to selective factors that remain obscure for the time being.
The same applies to contexts related to reproduction. Female rejections of unwanted male advances and defense of vulnerable infants provide contexts in which male and female interests collide, and where females have greater power because they control the resource males want (fertilizable eggs), but the studies included in this review did not reveal a systematic increase in female dominance during the mating season. Furthermore, the available studies do not report data for the mating season and non-mating season separately, precluding formal statistical comparisons. We could only document a possible trend for a reduction in the proportion of conflicts won by females with an increasing size advantage of males, suggesting that physical superiority is involved in determining intersexual dominance relations. Using a different measure of female dominance, Hemelrijk et al. (2008) found no correlation with sexual size dimorphism across 22 primate species. Also, in spotted hyenas female dominance is independent of body mass (Vullioud et al., 2019). Ideally, however, year-round studies of multiple groups, pairs or large samples of known individuals should be conducted to record variation in the frequency, nature and contexts of agonistic interactions between opposite- and same-sex opponents to have a quantitative basis for more systematic comparisons in the future.
Finally, and related to this last point, the level of detail with which different studies have reported their results is highly heterogeneous, also hampering comparative analyses aimed at understanding the levels and sources of variation in intersexual dominance relations. Whereas many studies of dominance in lemurs have reported more details about the actual interactions than studies of other primates and mammals – especially with respect to the occurrence of submissive behavior - our review revealed that there is no generally agreed-upon standard for reporting data on agonistic interactions. For example, it would be desirable – in our view – to report for each sex combination of dyads the rate of conflicts, the proportion of decided and undecided conflicts, the proportion of conflicts with submissive signals and the social context of interactions. Interactions with juveniles should be reported separately because size differences may distort intersexual dominance relations. Moreover, at the level of summarizing and analyzing these data for group-living species, it should be reported whether and how these interactions were used to generate a hierarchy, as well as their basic properties like linearity and transitivity (see Levy et al., 2020). From dominance hierarchies, it is also possible to determine the number or proportion of members of the opposite sex dominated by any female or male to calculate a corresponding summary statistic (see e.g., Kappeler, 2022). Finally, whereas social network analyses may provide additional insights about the structure of intersexual dominance relations, the groups of most lemur species were too small to apply these methods in meaningful ways (see e.g., Eppley et al., 2017), but new methods for small groups are now available (Coelho et al., 2020).
As always, a Tinbergian perspective is most helpful for illuminating the evolution of a social phenomenon like intersexual dominance (Bergman and Beehner, 2021; Smith et al., 2021). First, the ability of female lemurs to evoke submission from adult males per se is proximately not dependent on physical superiority, but might be modulated by relatively small variation in sexual size dimorphism, and it is also not restricted to any particular social context like feeding or reproduction (see also Kappeler, 1990b). The relative roles of female aggression and male submission in generating decided agonistic interactions in different species remains unresolved, however, until more studies report details on the proportion of conflicts with (spontaneous) male submission. In particular, it would be of interest to have a more comprehensive understanding of the distribution of submissive signals as well as their importance in intersexual, but also same-sex interactions (Reddon et al., 2021). Focusing on the proximate control of female aggression and masculinization in lemurs, several studies have explored the possible role of androgenic steroid hormones in shaping female aggressive phenotypes (Petty and Drea, 2015). Whereas lemur females are strikingly masculinized in their genital morphology, this line of research has not suggested a uniform ultimate reason why female dominance might be adaptive.
Second, an ontogenetic perspective suggests that the ability of females to win agonistic interactions with males emerges in close temporal proximity with sexual maturity because young females in ringtailed lemurs (Pereira, 1993), Verreaux’s sifakas (Voyt et al., 2019) and gray mouse lemurs (Hohenbrink et al., 2015a,b) begin eliciting male submission at that developmental stage. Because these species represent different families, this functional relationship between female dominance and female reproduction is presumably ancestral for lemurs, but additional studies on the ontogeny of female dominance would be welcome.
Third, compared to other mammals, where unanimous dyadic female dominance is limited to spotted hyenas and less comprehensive forms of female dominance to a handful of other species (Kappeler, 1993; Koren et al., 2006; Watts and Holekamp, 2007; Dunham, 2008; Koren and Geffen, 2009; French et al., 2013; Surbeck and Hohmann, 2013; Kappeler and Fichtel, 2015; Holekamp and Sawdy, 2019; Vullioud et al., 2019), lemurs represent an unusual taxon from a phylogenetic perspective. This concentration of species with female dominance has engendered evolutionary explanations that focus on idiosyncrasies of either lemurs or Madagascar, or both. In some cases, these hypotheses have incorporated functional aspects that offer an ultimate explanation.
The predominant explanation for the prevalence of female dominance among lemurs is based on a combination of phylogenetic and functional considerations. It postulates that lemur females face higher energetic costs of reproduction in combination with food scarcity than other primates, so that they benefit from priority of access to contested food resources during energetic bottlenecks (‘energy conservation hypothesis’: Jolly, 1984; Wright, 1999), and males are selected to defer to them because they avoid the costs of escalated fighting by doing so (‘cost-asymmetry hypothesis’, Dunham, 2008). Empirical tests of the assumptions and predictions of this hypothesis have focused on lemur life histories (Young et al., 1990; Meyers and Wright, 1993; Kappeler, 1996) and Madagascar’s climate and phenology (Dewar and Richard, 2007), but have not produced unanimous support (Federman et al., 2017). In addition, it does neither explain the absence of male-biased sexual size dimorphism and dominance nor the even adult sex ratios of group-living lemurs. For a more conclusive evaluation of this group of hypotheses, future research should generate data sets that combine details of male–female interactions, developmental and reproductive schedules as well as climatic and phenological variables from a broad range of species, ideally including sets of sympatric species.
The ‘evolutionary disequilibrium hypothesis’ explained female dominance and a complex of functionally related traits as the result of largely non-adaptive consequences of human-induced environmental changes in the last few millennia, creating an evolutionary disequilibrium between current ecological conditions and lemur traits. It posited that female dominance is part of a complex of traits of diurnal group-living lemurs that persisted after very recent evolutionary transitions from nocturnal, pair-living ancestors following the Holocene extinction of large predatory eagles and large-bodied subfossil lemurs (van Schaik and Kappeler, 1996). Because the absence of sexual size dimorphism and consistent male dominance also characterize many pair-living mammals, their prevalence among group-living lemurs does not represent an adaptation to current ecological conditions but rather an example of phylogenetic inertia because a few centuries have not provided enough time for adaptations to the new ecological niche. This hypothesis has provoked several studies challenging its core assumption (e.g., Kirk, 2006), and it does not explain the prevalence of female dominance among the solitary lemur species. It is not incompatible with the energy conservation hypotheses, however, and also highlights links between lemur ecology and behavior.
The most recent attempt to take into account and reconcile the existing hypotheses about underlying adaptive function and proximate causation added a potential developmental mechanism linking maternal stress and filial masculinization to outline an evolutionary scenario for its canalization (Kappeler and Fichtel, 2015). Accordingly, lemur females are assumed to be subject to significant and unique patterns of resource limitation, especially during reproduction, creating recurrent energetic limitations. Lemur females, and in particular those of larger species with gestation and lactation periods spanning several months, are therefore potentially exposed to massive environmental stress during many, if not most, of their lifetime reproductive events because the exact timing of food availability is poorly predictable. The resulting physiological stress response would be exacerbated by feeding competition and leads to the masculinization of daughters which ought to be better prepared to compete with other females in adverse environments. Thus, natural selection will enhance the effects of maternal programming and synergistic epistasis, resulting in canalization in competitive traits that also allow female dominance as a by-product over evolutionary times, but the generality of the proposed underlying processes awaits additional empirical study.
The behavioral studies summarized in this review have not contributed new insights about the ecological and physiological factors implicated in shaping the evolution of female dominance. Yet, the confirmation that it has evolved in representatives of all 5 extant families indicates that it has either characterized the first lemurs colonizing Madagascar or that it has evolved very soon after the colonization. Given that female dominance is not known to occur in the lineage representing the last shared common ancestors on the African mainland (Bearder, 1999), it seems most parsimonious to assume that it evolved soon after the colonization under unique ecological conditions characterizing the various Malagasy forest habitats. The interspecific variation in the extent of female dominance highlighted here suggests, however, that intersexual dominance relations are subject to adaptations to variable social and ecological factors.
The ability of adult females to consistently dominate adult males is widespread among the primates of Madagascar, suggesting that it has evolved soon after the colonization of the island by lemurs. There is much more interspecific variation in intersexual dominance relationships than previously acknowledged, however, and variation even exists within some species. Female dominance is typically achieved via male submission, and male aggression toward females is relatively rare. Female lemurs do not consistently enjoy physical superiority over males, and other proximate bases of their power remain unknown. Female dominance emerges ontogenetically along with female sexual maturity, suggesting some functional link to sex-specific reproductive strategies, but it is not limited to the context of mating where females have greater power. Lemur- and Madagascar-specific explanations for the evolution of female dominance have emphasized links between ecology and behavior and the energetics of reproduction, but a lack of comparative data, also from other Malagasy and Southern African mammals, has hampered progress with testing assumptions and predictions of existing hypotheses.
PK drafted the manuscript to which CF and UR made substantial contributions. All authors contributed to the article and approved the submitted version.
This research was not supported by any specific funding sources, but the authors acknowledge the long-term support of their lemur research by the German Science Foundation (DFG).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Charlotte Hemelrijk and the other participants of the Lorentz Center workshop on Dynamics of Dominance of Females Relative to Males in a Group (January 2020 in Leiden, The Netherlands) for discussion and inspiration. UR and CF thank the organizers for the invitation to this workshop.
Alberts, S. C. (2012). “Magnitude and sources of variation in male reproductive performance,” in The Evolution of Primate Societies, eds J. C. Mitani, J. Call, P. M. Kappeler, R. Palombit, and J. B. Silk (Chicago, IL: The University of Chicago Press), 412–431.
Appleby, M. C. (1983). The probability of linearity in hierarchies. Anim. Behav. 31, 600–608. doi: 10.1016/s0003-3472(83)80084-0
Archie, E. A. (2013). Wound healing in the wild: stress, sociality and energetic costs affect wound healing in natural populations. Par. Immunol. 35, 374–385. doi: 10.1111/pim.12048
Archie, E. A., Altmann, J., and Alberts, S. C. (2012). Social status predicts wound healing in wild baboons. Proc. Natl. Acad. Sci. U.S.A. 109, 9017–9022.
Arnold, C., Matthews, L. J., and Nunn, C. L. (2010). The 10kTrees website: a new online resourcefor primate phylogeny. Evol. Anthropol. 19, 114–118. doi: 10.1002/evan.20251
Arnott, G., and Elwood, R. W. (2009). Assessment of fighting ability in animal contests. Anim. Behav. 77, 991–1004. doi: 10.1016/j.anbehav.2009.02.010
Baniel, A., Cowlishaw, G., and Huchard, E. (2016). Stability and strength of male-female associations in a promiscuous primate society. Behav. Ecol. Sociobiol. 70, 761–775. doi: 10.1007/s00265-016-2100-8
Baniel, A., Cowlishaw, G., and Huchard, E. (2017). Male violence and sexual intimidation in a wild primate society. Curr. Biol. 27, 2163–2168.e3. doi: 10.1016/j.cub.2017.06.013
Bearder, S. K. (1999). Physical and social diversity among nocturnal primates: a new view based on long term research. Primates 40, 267–282. doi: 10.1007/BF02557715
Bergman, T. J., and Beehner, J. C. (2021). Leveling with Tinbergen: four levels simplified to causes and consequences. Evol. Anthropol. *, doi: 10.1002/evan.21931
Bergman, T. J., Beehner, J. C., Cheney, D. L., and Seyfarth, R. M. (2003). Hierarchical classification by rank and kinship in baboons. Science 302, 1234–1236. doi: 10.1126/science.1087513
Bonanni, R., Cafazzo, S., Abis, A., Barillari, E., Valsecchi, P., and Natoli, E. (2017). Age-graded dominance hierarchies and social tolerance in packs of free-ranging dogs. Behav. Ecol. 28, 1004–1020. doi: 10.1093/beheco/arx059
Campbell, C., Fuentes, A., MacKinnon, K., Bearder, S. K., and Stumpf, R. M. (2010). Primates in Perspective, 2nd Edn. Oxford: Oxford University Press.
Catlett, K. K., Schwartz, G. T., Godfrey, L. R., and Jungers, W. L. (2010). ‘Life history space’: a multivariate analysis of life history variation in extant and extinct Malagasy lemurs. Am. J. Phys. Anthropol. 142, 391–404. doi: 10.1002/ajpa.21236
Chase, I. D., Tovey, C., Spangler-Martin, D., and Manfredonia, M. (2002). Individual differences versus social dynamics in the formation of animal dominance hierarchies. Proc. Natl. Acad. Sci. U.S.A. 99:5744. doi: 10.1073/pnas.082104199
Cheney, D. L., and Seyfarth, R. M. (1990). How Monkeys See the World. Chicago, IL: Chicago University Press.
Clutton-Brock, T. H., and Janson, C. H. (2012). Primate socioecology at the crossroads: past, present, and future. Evol. Anthropol. 21, 136–150. doi: 10.1002/evan.21316
Coelho, D., Chase, I., and Mueller, K. (2020). PeckVis: a visual analytics tool to analyze dominance hierarchies in small groups. IEEE Trans. Vis. Comput. Grap. 26, 1650–1660. doi: 10.1109/TVCG.2020.2969056
Curtis, D. J., and Zaramody, A. (1999). Social structure and seasonal variation in the behaviour of Eulemur mongoz. Fol. Primatol. 70, 79–96. doi: 10.1159/000021679
Dammhahn, M., and Kappeler, P. M. (2005). Social system of Microcebus berthae, the World’s smallest primate. Int. J. Primatol. 26, 407–435. doi: 10.1007/s10764-005-2931-z
de Vries, H., Stevens, J. M. G., and Vervaecke, H. (2006). Measuring and testing the steepness of dominance hierarchies. Anim. Behav. 51, 585–592. doi: 10.1002/ajp.20999
Deniz, M., de Sousa, K. T., do Vale, M. M., and Dittrich, J. R. (2021). Age and body mass are more important than horns to determine the social position of dairy cows. J. Ethol. 39, 19–27. doi: 10.1007/s10164-020-00667-x
Dewar, R. E., and Richard, A. F. (2007). Evolution in the hypervariable environment of Madagascar. Proc. Natl. Acad. Sci. U.S.A. 104, 13723–13727. doi: 10.1073/pnas.0704346104
Digby, L. J., and Kahlenberg, S. M. (2002). Female dominance in blue-eyed black lemurs. Primates 43, 191–199. doi: 10.1007/bf02629647
Digby, L. J., and Stevens, A. M. (2007). Maintenance of female dominance in blue-eyed black lemurs (Eulemur flavifrons) and gray bamboo lemurs (Hapalemur griseus) under semi-free-ranging and captive conditions. Zoo Biol. 26, 345–361. doi: 10.1002/zoo.20140
Donabedian, R., and Cords, M. (2021). Maternal rank acquisition in a primate with low aggression and coalition rates. Behav. Ecol. Sociobiol. 75:90. doi: 10.1007/s00265-021-03021-4
Drews, C. (1993). The concept and definition of dominance in animal behaviour. Behaviour 125, 283–313. doi: 10.1163/156853993x00290
Dröscher, I., and Kappeler, P. M. (2013). Defining the low end of primate social complexity: the social organization of the nocturnal white-footed sportive lemur (Lepilemur leucopus). Int. J. Primatol. 34, 1225–1243. doi: 10.1007/s10764-013-9735-3
Dröscher, I., and Kappeler, P. M. (2014). Competition for food in a solitarily foraging folivorous primate (Lepilemur leucopus)? Am. J. Primatol. 76, 842–854. doi: 10.1002/ajp.22272
Dugatkin, L. A. (1997). Winner and loser effects and the structure of dominance hierarchies. Behav. Ecol. 8, 583–587. doi: 10.1093/beheco/8.6.583
Dunham, A. E. (2008). Battle of the sexes: cost asymmetry explains female dominance in lemurs. Anim. Behav. 76, 1435–1439. doi: 10.1016/j.anbehav.2008.06.018
Eichmueller, P., Thorén, S., and Radespiel, U. (2013). The lack of female dominance in golden-brown-mouse lemurs suggests alternative routes in lemur social evolution. Am. J. Phys. Anthropol. 150, 158–164. doi: 10.1002/ajpa.22189
Eppley, T. M., Watzek, J., Hall, K., and Donati, G. (2017). Climatic, social and reproductive influences on behavioural thermoregulation in a female-dominated lemur. Anim. Behav. 134, 25–34. doi: 10.1016/j.anbehav.2017.10.003
Evasoa, M. R., Zimmermann, E., Hasiniaina, A. F., Rasoloharijaona, S., Randrianambinina, B., and Radespiel, U. (2019). Sources of variation in social tolerance in mouse lemurs (Microcebus spp.). BMC Ecol. 19:20. doi: 10.1186/s12898-019-0236-x
Federman, S., Sinnott-Armstrong, M., Baden, A. L., Chapman, C. A., Daly, D. C., Richard, A. R., et al. (2017). The paucity of frugivores in Madagascar may not be due to unpredictable temperatures or fruit resources. PLoS One 12:e0168943. doi: 10.1371/journal.pone.0168943
Fernandez-Duque, E., Valeggia, C. R., and Mendoza, S. P. (2009). The biology of paternal care in human and nonhuman primates. Ann. Rev. Anthropol. 38, 115–130. doi: 10.1146/annurev-anthro-091908-164334
Finn, K. R., Silk, M. J., Porter, M. A., and Pinter-Wollman, N. (2019). The use of multilayer network analysis in animal behaviour. Anim. Behav. 149, 7–22. doi: 10.1016/j.anbehav.2018.12.016
Foreit, K. (2016). Leading Behavior, Positioning, and Group Spacing as Indicators of Dominance in Hapalemur griseus. Unpublished MA thesis. De Kalb, IL: University of Northern Illinois.
Fornasieri, I., Caubèrt, M., and Roeder, J. J. (1993). Social dominance and priority of access to drinking in Lemur macaco. Aggr. Behav. 19, 455–464. doi: 10.1002/1098-2337(1993)19:6<455::aid-ab2480190606>3.0.co;2-#
Franz, M., McLean, E., Tung, J., Altmann, J., and Alberts, S. C. (2015). Self-organizing dominance hierarchies in a wild primate population. Proc. Roy. Soc. Lond. B Biol. Sci. 282, 20151512. doi: 10.1098/rspb.2015.1512
Frasier, C., Rakotonirina, J., Razanajatovo, L., Nasolonjanahary, T., Rasolonileniraka, Mamiaritiana, S., et al. (2015). Expanding knowledge on life history traits and infant development in the greater bamboo lemur (Prolemur simus): contributions from Kianjavato, Madagascar. Prim. Cons. 2015, 75–86. doi: 10.1896/052.029.0110
French, J. A., Mustoe, A. C., Cavanaugh, J., and Birnie, A. K. (2013). The influence of androgenic steroid hormones on female aggression in atypical mammals. Phil. Trans. Roy. Soc. B Biol. Sci. 368:0130084. doi: 10.1098/rstb.2013.0084
Gallup, G. G. Jr. (1998). Self-awareness and the evolution of social intelligence. Behav. Proc. 42, 239–247. doi: 10.1016/s0376-6357(97)00079-x
Génin, F. (2003). Female dominance in competition for gum trees in the grey mouse lemur Microcebus murinus. Rev. d’ Écol. Terre Vie 58, 397–410.
Génin, F. (2013). “Venus in fur: female power in mouse lemurs Microcebus murinus and M. griseorufus,” in Leaping Ahead: Advances in Prosimian Biology, eds J. Masters, M. Gamba, and F. Génin (New York, NY: Springer), 121–126. doi: 10.1007/978-1-4614-4511-1_14
Giles, S. L., Nicol, C. J., Harris, P. A., and Rands, S. A. (2015). Dominance rank is associated with body condition in outdoor-living domestic horses (Equus caballus). Appl. Anim. Behav. Sci. 166, 71–79. doi: 10.1016/j.applanim.2015.02.019
Godfrey, L. R. (2016). Subfossil lemurs. Int. Encyclopedia Primatol. 1–5. doi: 10.1002/9781119179313.wbprim0473
Gowaty, P. A., Kim, Y.-K., and Anderson, W. W. (2012). No evidence of sexual selection in a repetition of Bateman’s classic study of Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 109, 11740–11745. doi: 10.1073/pnas.1207851109
Hausfater, G. (1975). Dominance and reproduction in baboons Papio cyncephalus: a quantitative analysis. Contr. Primatol. 7, 1–150.
Hemelrijk, C. K., Wantia, J., and Isler, K. (2008). Female dominance over males in primates: self-organisation and sexual dimorphism. PLoS One 3:e2678. doi: 10.1371/journal.pone.0002678
Hemelrijk, C. K., Wubs, M., Gort, G., Botting, J., and van de Waal, E. (2020). Dynamics of intersexual dominance and adult sex-ratio in wild vervet monkeys. Front. Psychol. 11:839. doi: 10.3389/fpsyg.2020.00839
Herrera, J. P. (2017). Testing the adaptive radiation hypothesis for the lemurs of Madagascar. Roy. Soc. Op. Sci. 4:161014. doi: 10.1098/rsos.161014
Hilgartner, R. D., Fichtel, C., Kappeler, P. M., and Zinner, D. P. (2012). Determinants of pair-living in red-tailed sportive lemurs (Lepilemur ruficaudatus). Ethology 118, 466–479. doi: 10.1111/j.1439-0310.2012.02033.x
Hinde, R. A. (1976). Interactions, relationships and social structure. Man 11, 1–17. doi: 10.2307/2800384
Hobson, E. A. (2020). Differences in social information are critical to understanding aggressive behavior in animal dominance hierarchies. Curr. Op. Psychol. 33, 209–215. doi: 10.1016/j.copsyc.2019.09.010
Hobson, E. A., Mønster, D., and DeDeo, S. (2021). Aggression heuristics underlie animal dominance hierarchies and provide evidence of group-level social information. Proc. Natl. Acad. Sci. U.S.A. 118:e2022912118. doi: 10.1073/pnas.2022912118
Hohenbrink, S. (2015). Female Dominance in Mouse Lemurs. Ph.D. thesis. Hannover: University of Veterinary Medicine Hannover, Foundation.
Hohenbrink, S., Koberstein-Schwarz, M., Zimmermann, E., and Radespiel, U. (2015a). Shades of gray mouse lemurs: ontogeny of female dominance and dominance-related behaviors in a nocturnal primate. Am. J. Primatol. 77, 1158–1169. doi: 10.1002/ajp.22452
Hohenbrink, S., Schaarschmidt, F., Bünemann, K., Gerberding, S., Zimmermann, E., and Radespiel, U. (2016). Female dominance in two basal primates, Microcebus murinus and Microcebus lehilahytsara: variation and determinants. Anim. Behav. 122, 145–156. doi: 10.1016/j.anbehav.2016.10.008
Hohenbrink, S., Zimmermann, E., and Radespiel, U. (2015b). Need for speed: sexual maturation precedes social maturation in gray mouse lemurs. Am. J. Primatol 77, 1049–1059. doi: 10.1002/ajp.22440
Holekamp, K. E., and Sawdy, M. A. (2019). The evolution of matrilineal social systems in fissiped carnivores. Phil. Trans. Roy. Soc. B Biol. Sci. 374:20180065. doi: 10.1098/rstb.2018.0065
Holekamp, K. E., and Strauss, E. D. (2016). Aggression and dominance: an interdisciplinary overview. Curr. Op. Behav. Sci. 12, 44–51. doi: 10.1016/j.cobeha.2016.08.005
Hsu, Y., and Wolf, L. L. (1999). The winner and loser effect: integrating multiple experiences. Anim. Behav. 57, 903–910. doi: 10.1006/anbe.1998.1049
Humphrey, N. K. (1976). “The social function of intellect,” in Growing Points in Ethology, eds P. P. G. Bateson and R. A. Hinde (Cambridge: Cambridge University Press), 303–317.
Ilany, A., Holekamp, K. E., and Akçay, E. (2021). Rank-dependent social inheritance determines social network structure in spotted hyenas. Science 373:348. doi: 10.1126/science.abc1966
Isbell, L. A. (1991). Contest and scramble competition: patterns of female aggression and ranging behavior among primates. Behav. Ecol. 2, 143–155. doi: 10.1002/ajp.22429
Isbell, L. A., and Young, T. P. (2002). Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behaviour 139, 177–202. doi: 10.1163/156853902760102645
Janicke, T., Häderer, I. K., Lajeunesse, M. J., and Anthes, N. (2016). Darwinian sex roles confirmed across the animal kingdom. Sci. Adv. 2:e1500983. doi: 10.1126/sciadv.1500983
Johnsson, J. I., and Åkerman, A. (1998). Watch and learn: preview of the fighting ability of opponents alters contest behaviour in rainbow trout. Anim. Behav. 56, 771–776. doi: 10.1006/anbe.1998.0824
Jolly, A. (1966a). Lemur social behavior and primate intelligence. Science 153, 501–506. doi: 10.1126/science.153.3735.501
Jolly, A. (1984). “The puzzle of female feeding priority,” in Female Primates: Studies by Woman Primatologists, ed. M. F. Small (New York, NY: A.R. Liss), 197–215.
Jürges, V. (2010). Social Organisation of the Goodman’s mouse Lemur (Microcebus lehilahytsara) in the Masoala Hall of Zoo Zurich. MSc thesis. Hanover: University of Veterinary Medicine Hannover.
Jürges, V., Kitzler, J., Zingg, R., and Radespiel, U. (2013). First insights into the social organisation of Goodman’s mouse lemur (Microcebus lehilahytsara) - testing predictions from socio-ecological hypotheses in the Masoala Hall of Zurich Zoo. Fol. Primatol. 84, 32–48. doi: 10.1159/000345917
Kappeler, P., and Fichtel, C. (2015). Eco-evo-devo of the lemur syndrome: did adaptive behavioral plasticity get canalized in a large primate radiation? Front. Zool. 12:S15. doi: 10.1186/1742-9994-12-S1-S15
Kappeler, P. M. (1990a). Social status and scent marking behaviour in Lemur catta. Anim. Behav. 40, 774–776. doi: 10.1016/s0003-3472(05)80706-7
Kappeler, P. M. (1990b). Female dominance in Lemur catta: more than just female feeding priority? Fol. Primatol. 55, 92–95. doi: 10.1159/000156504
Kappeler, P. M. (1993). “Female dominance in primates and other mammals,” in Perspectives in Ethology. Vol 10: Behaviour and Evolution, eds P. P. G. Bateson, P. H. Klopfer, and N. S. Thompson (New York, NY: Plenum Press), 143–158.
Kappeler, P. M. (1996). Causes and consequences of life history variation among strepsirhine primates. Am. Nat. 148, 868–891. doi: 10.1086/285960
Kappeler, P. M. (1997). Determinants of primate social organization: comparative evidence and new insights from Malagasy lemurs. Biol. Rev. 72, 111–151. doi: 10.1017/s0006323196004999
Kappeler, P. M. (2012a). “Mate choice,” in Evolution of Primate Societies, eds J. Mitani, J. Call, P. M. Kappeler, R. Palombit, and J. B. Silk (Chicago, IL: University of Chicago Press), 367–386.
Kappeler, P. M. (2012b). “The behavioral ecology of strepsirrhines and tarsiers,” in The Evolution of Primate Societies, eds J. C. Mitani, J. Call, P. M. Kappeler, R. Palombit, and J. B. Silk (Chicago, IL: University of Chicago Press), 17–42. doi: 10.1016/j.ympev.2014.02.023
Kappeler, P. M., et al. (2022). Sex and dominance: how to assess and interpret intersexual dominance relationships in mammalian societies. Front. Ecol. Evol.
Kappeler, P. M., Mass, V., and Port, M. (2009). Even adult sex ratios in lemurs: potential costs and benefits of subordinate males in Verreaux’s sifaka (Propithecus verreauxi) in the Kirindy Forest CFPF, Madagascar. Am. J. Phys. Anthropol. 140, 487–497. doi: 10.1002/ajpa.21091
Kappeler, P. M., Nunn, C. L., Vining, A. Q., and Goodman, S. M. (2019). Evolutionary dynamics of sexual size dimorphism in non-volant mammals following their independent colonization of Madagascar. Sci. Rep. 9:1454. doi: 10.1038/s41598-018-36246-x
Kappeler, P. M., and Pozzi, L. (2019). Evolutionary transitions toward pair living in nonhuman primates as stepping stones toward more complex societies. Sci. Adv. 5:eaay1276. doi: 10.1126/sciadv.aay1276
Kappeler, P. M., Rasoloarison, R. M., Razafimanantsoa, L., Walter, L., and Roos, C. (2005). Morphology, behaviour and molecular evolution of giant mouse lemurs (Mirza spp.) Gray, 1870, with description of a new species. Prim. Rep. 71, 3–26.
Kappeler, P. M., and van Schaik, C. P. (2002). Evolution of primate social systems. Int. J. Primatol. 23, 707–740.
Karanth, K. P., Delefosse, T., Rakotosamimanana, B., Parsons, T. J., and Yoder, A. D. (2005). Ancient DNA from giant extinct lemurs confirms single origin of Malagasy primates. Proc. Natl. Acad. Sci. U.S.A. 102, 5090–5095. doi: 10.1073/pnas.0408354102
Kaufman, R. (1991). Female dominance in semifree-ranging black-and-white ruffed lemurs, Varecia variegata. Fol. Primatol. 57, 39–41. doi: 10.1159/000156562
Kirk, E. C. (2006). Eye morphology in cathemeral lemurids and other mammals. Fol. Primatol. 77, 27–49. doi: 10.1159/000089694
Koren, L., and Geffen, E. (2009). Androgens and social status in female rock hyraxes. Anim. Behav. 77, 233–238. doi: 10.1016/j.anbehav.2008.09.031
Koren, L., Mokady, O., and Geffen, E. (2006). Elevated testosterone levels and social ranks in female rock hyrax. Horm. Behav. 49, 470–477. doi: 10.1016/j.yhbeh.2005.10.004
Kubzdela, K. S., Richard, A. F., and Pereira, M. E. (1992). Social relations in semi-free-ranging sifakas (Propithecus verreauxi coquereli) and the question of female dominance. Am. J. Primatol. 28, 139–145. doi: 10.1002/ajp.1350280206
Kunz, J. A., Duvot, G. J., Willems, E. P., Stickelberger, J., Spillmann, B., Utami Atmoko, S. S., et al. (2021). The context of sexual coercion in orang-utans: when do male and female mating interests collide? Anim. Behav. 182, 67–90. doi: 10.1016/j.anbehav.2021.09.012
Landau, H. G. (1951). On dominance relations and the structure of animal societies: I. Effects of inherent characteristics. Bull. Math. Biophys. 13, 1–19. doi: 10.1016/j.prevetmed.2021.105549
Leimar, O. (2021). The evolution of social dominance through reinforcement learning. Am. Nat. 197, 560–575. doi: 10.1086/713758
Lerena, D. A. M., Antunes, D. F., and Taborsky, B. (2021). The interplay between winner–loser effects and social rank in cooperatively breeding vertebrates. Anim. Behav. 177, 19–29. doi: 10.1016/j.anbehav.2021.04.011
Levy, E. J., Zipple, M. N., McLean, E., Campos, F. A., Dasari, M., Fogel, A. S., et al. (2020). A comparison of dominance rank metrics reveals multiple competitive landscapes in an animal society. Proc. Roy. Soc. B Biol. Sci. 287:20201013. doi: 10.1098/rspb.2020.1013
Lewis, R. J. (2018). Female power in primates and the phenomenon of female dominance. Ann. Rev. Anthropol. 47, 533–551. doi: 10.1146/annurev-anthro-102317-045958
Lewis, R. J. (2020). Female power: a new framework for understanding “female dominance” in lemurs. Fol. Primatol. 91, 48–68. doi: 10.1159/000500443
Lewis, R. J. (2022). Aggression, rank and power: why hens (and other animals) do not always peck according to their strength. Phil. Trans. Roy. Soc. B Biol. Sci. 377:20200434. doi: 10.1098/rstb.2020.0434
Lindenfors, P., Gittleman, J., and Jones, K. (2007). “Sexual size dimorphism in mammals,” in Sex, Size, and Gender Roles: Evolutionary Studies of Sexual Dimorphism, eds D. J. Fairbairn, W. Blanckenhorn, and T. Szekely (Oxford: Oxford University Press), 16–26. doi: 10.1093/acprof:oso/9780199208784.003.0003
Lukas, D., and Huchard, E. (2014). The evolution of infanticide by males in mammalian societies. Science 346, 841–844. doi: 10.1126/science.1257226
MacCormick, H. A., MacNulty, D. R., Bosacker, A. L., Lehman, C., Bailey, A., Anthony Collins, D., et al. (2012). Male and female aggression: lessons from sex, rank, age, and injury in olive baboons. Behav. Ecol. 23, 684–691. doi: 10.1093/beheco/ars021
Marolf, B., McElligott, A. G., and Müller, A. E. (2007). Female social dominance in two Eulemur species with different social organizations. Zoo Biol. 26, 201–214. doi: 10.1002/zoo.20135
Mech, L. D. (1999). Alpha status, dominance, and division of labor in wolf packs. Can. J. Zool. 77, 1196–1203. doi: 10.1139/z99-099
Meyer, C., Gallo, T., and Schultz, S. T. (1999). Female dominance in captive red ruffed lemurs, Varecia variegata rubra (Primates, Lemuridae). Fol. Primatol. 70, 358–361. doi: 10.1159/000021718
Meyers, D. M., and Wright, P. C. (1993). “Resource tracking: food availability and Propithecus seasonal reproduction,” in Lemur Social Systems and Their Ecological Basis, eds P. M. Kappeler and J. U. Ganzhorn (New York, NY: Plenum Press), 179–192. doi: 10.1007/978-1-4899-2412-4_13
Mitani, J., Palombit, R., Kappeler, P., Call, J., and Silk, J. (2012). The Evolution of Primate Societies. Chicago, IL: Univ. of Chicago Press.
Moscovice, L. R., Sueur, C., and Aureli, F. (2020). How socio-ecological factors influence the differentiation of social relationships: an integrated conceptual framework. Biol. Lett. 16:20200384. doi: 10.1098/rsbl.2020.0384
Muller, M., and Wrangham, R. (2009). Sexual Coercion in Primates and Humans. Cambridge, MA: Harvard Univ. Press.
Nakamichi, M., and Koyama, N. (1997). Social relationships among ring-tailed lemurs (Lemur catta) in two free-ranging troops at Berenty Reserve, Madagascar. Int. J. Primatol. 18, 73–93. doi: 10.1023/A:1026393223883
Ostner, J., and Kappeler, P. M. (1999). Central males instead of multiple pairs in redfronted lemurs, Eulemur fulvus rufus (Primates, Lemuridae)? Anim. Behav. 58, 1069–1078. doi: 10.1006/anbe.1999.1222
Overdorff, D., and Tecot, S. (2006). “Social pair-bonding and resource defense in wild red-bellied lemurs (Eulemur rubriventer),” in Lemurs, eds L. Gould and M. Sauther (Berlin: Springer), 235–254. doi: 10.1007/978-0-387-34586-4_11
Overdorff, D. J., Erhart, E. M., and Mutschler, T. (2005). Does female dominance facilitate feeding priority in black-and-white ruffed lemurs (Varecia variegata) in southeastern Madagascar? Am. J. Primatol. 66, 7–22. doi: 10.1002/ajp.20125
Pagès-Feuillade, E. (1988). Modalités de l’occupation de l’espace et relations interindividuelles chez un prosimien nocturne malgache (Microcebus murinus). Fol. Primatol. 50, 204–220. doi: 10.1159/000156346
Pandolfi, M., Scaia, M. F., and Fernandez, M. P. (2021). Sexual dimorphism in aggression: sex-specific fighting strategies across species. Front. Behav. Neurosci. 15:659615. doi: 10.3389/fnbeh.2021.659615
Pereira, M. E. (1993). “Agonistic interaction, dominance relations, and ontogenetic trajectories in ringtailed lemurs,” in Juvenile Primates: Life History, Development, and Behavior, eds M. E. Pereira and L. A. Fairbanks (New York, NY: Oxford University Press), 285–305.
Pereira, M. E., and Kappeler, P. M. (1997). Divergent systems of agonistic behaviour in lemurid primates. Behaviour 134, 225–274. doi: 10.1163/156853997x00467
Pereira, M. E., Kaufman, R., Kappeler, P. M., and Overdorff, D. J. (1990). Female dominance does not characterize all of the Lemuridae. Fol. Primatol. 55, 96–103. doi: 10.1159/000156505
Petty, J. M. A., and Drea, C. M. (2015). Female rule in lemurs is ancestral and hormonally mediated. Sci. Rep. 5:9631. doi: 10.1038/srep09631
Pochron, S. T., Fitzgerald, J., Gilbert, C. C., Lawrence, D., Grgas, M., Rakotonirina, G., et al. (2003). Patterns of female dominance in Propithecus diadema edwardsi of Ranomafana National Park, Madagascar. Am. J. Primatol. 61, 173–185. doi: 10.1002/ajp.10119
Pollock, J. I. (1979). Female dominance in Indri indri. Fol. Primatol. 31, 143–164. doi: 10.1159/000155877
Poux, C., Madsen, O., Marquard, E., Vieites, D. R., de Jong, W. W., and Vences, M. (2005). Asynchronous colonization of Madagascar by the four endemic clades of primates, tenrecs, carnivores, and rodents as inferred from nuclear genes. Syst. Biol. 54, 719–730. doi: 10.1080/10635150500234534
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Radespiel, U., Ratsimbazafy, J. H., Rasoloharijaona, S., Raveloson, H., Andriaholinirina, N., Rakotondravony, R., et al. (2012). First indications of a highland specialist among mouse lemurs (Microcebus spp.) and evidence for a new mouse lemur species from eastern Madagascar. Primates 53, 157–170. doi: 10.1007/s10329-011-0290-2
Radespiel, U., and Zimmermann, E. (2001). Female dominance in captive gray mouse lemurs (Microcebus murinus). Am. J. Primatol. 54, 181–192. doi: 10.1002/ajp.1029
Ramanamisata, R., Pichon, C., Razafindraibe, H., and Simmen, B. (2014). Social behavior and dominance of the crowned sifaka (Propithecus coronatus) in Northwestern Madagascar. Prim. Conserv. 2014, 93–97. doi: 10.1896/052.028.0117
Ramanankirahina, R., Joly, M., and Zimmermann, E. (2011). Peaceful primates: affiliation, aggression, and the question of female dominance in a nocturnal pair-living lemur (Avahi occidentalis). Am. J. Primatol. 73, 1261–1268. doi: 10.1002/ajp.20998
Range, F., and Noë, R. (2005). Can simple rules account for the pattern of triadic interactions in juvenile and adult female sooty mangabeys? Anim. Behav. 69, 445–452. doi: 10.1016/j.anbehav.2004.02.025
Raps, S., and White, F. J. (1995). Female social dominance in semi-free ranging ruffed lemurs, Varecia variegata. Fol. Primatol. 65, 163–168. doi: 10.1159/000156883
Rasolonjatovo, S. M., and Irwin, M. T. (2020). Exploring social dominance in wild diademed sifakas (Propithecus diadema): females are dominant, but it is subtle and the benefits are not clear. Fol. Primatol. 91, 385–398. doi: 10.1159/000503345
Reddon, A. R., Ruberto, T., and Reader, S. M. (2021). Submission signals in animal groups. Behaviour 159, 1–20. doi: 10.1163/1568539X-bja10125
Redhead, D., and Power, E. A. (2022). Social hierarchies and social networks in humans. Philos. Trans. R. Soc. B Biol. Sci. 377:20200440. doi: 10.1098/rstb.2020.0440
Rendall, D. (1993). Does female social precedence characterize captive aye-ayes (Daubentonia madagascariensis)? Int. J. Primatol. 14, 125–130. doi: 10.1007/bf02196507
Revell, L. J. (2012). phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. doi: 10.1111/j.2041-210X.2011.00169.x
Richard, A. F. (1974). Intra-specific variation in the social organization and ecology of Propithecus verreauxi. Fol. Primatol. 22, 178–207. doi: 10.1159/000155624
Richard, A. F. (1987). “Malagasy prosimians: female dominance,” in Primate Societies, eds B. B. Smuts, D. L. Cheney, R. M. Seyfarth, R. W. Wrangham, and T. T. Struhsaker (Chicago, IL: University of Chicago Press), 25–33.
Richard, A. F., and Dewar, R. E. (1991). Lemur ecology. Ann. Rev. Ecol. Syst. 22, 145–175. doi: 10.1146/annurev.es.22.110191.001045
Roeder, J.-J., and Fornasieri, I. (1995). Does agonistic dominance imply feeding priority in lemurs? A study in Eulemur fulvus mayottensis. Int. J. Primatol. 16, 629–642. doi: 10.1007/bf02735285
Roeder, J.-J., Fornasieri, I., and Gosset, D. (2002). Conflict and postconflict behaviour in two lemur species with different social organizations (Eulemur fulvus and Eulemur macaco): a study on captive groups. Aggr. Behav. 28, 62–74. doi: 10.1002/ab.90006
Roullet, D. (2011). The role of the captive population of greater bamboo lemurs Prolemur simus in the conservation of the species. Lemur News 16, 20–25.
Sánchez-Tójar, A., Schroeder, J., and Farine, D. R. (2018). A practical guide for inferring reliable dominance hierarchies and estimating their uncertainty. J. Anim. Ecol. 87, 594–608. doi: 10.1111/1365-2656.12776
Santini, L., Rojas, D., and Donati, G. (2015). Evolving through day and night: origin and diversification of activity pattern in modern primates. Behav. Ecol. 26, 789–796. doi: 10.1093/beheco/arv012
Sauther, M. L. (1993). “Resource competition in wild populations of ringtailed lemurs (Lemur catta): implications for female dominance,” in Lemur Social Systems and Their Ecological Basis, eds P. M. Kappeler and J. U. Ganzhorn (New York, NY: Plenum Press), 135–152. doi: 10.1007/978-1-4899-2412-4_10
Schärer, L., Rowe, L., and Arnqvist, G. (2012). Anisogamy, chance and the evolution of sex roles. Trends Ecol. Evol. 27, 260–264. doi: 10.1016/j.tree.2011.12.006
Schülke, O., and Kappeler, P. M. (2003). So near and yet so far: territorial pairs but low cohesion between pair partners in a nocturnal lemur, Phaner furcifer. Anim. Behav. 65, 331–343. doi: 10.1006/anbe.2003.2018
Schülke, O., and Ostner, J. (2012). “Ecological and social influences on sociality,” in The Evolution of Primate Societies, eds J. Mitani, R. Palombit, P. M. Kappeler, J. Call, and J. B. Silk (Chicago, IL: University of Chicago Press), 195–219.
Sefczek, T. M., Hagenson, R. A., Randimbiharinirina, D. R., Rakotondrazandry, J. N., and Louis, E. E. (2020). Home range size and seasonal variation in habitat use of aye-ayes (Daubentonia madagascariensis) in Torotorofotsy, Madagascar. Fol. Primatol. 91, 558–574. doi: 10.1159/000508620
Silk, J. B., and Kappeler, P. M. (2017). “Sociality in primates,” in Comparative Social Evolution, eds D. Rubenstein and P. Abbott (Cambridge: Cambridge University Press), 253–283. doi: 10.1017/9781107338319.010
Smith, J. E., von Rueden, C. R., van Vugt, M., Fichtel, C., and Kappeler, P. M. (2021). An evolutionary explanation for the female leadership paradox. Front. Ecol. Evol. 9:676805. doi: 10.3389/fevo.2021.676805
Smuts, B. B., Cheney, D. L., Seyfarth, R. M., Wrangham, R. W., and Struhsaker, T. T. (1987). Primate Societies. Chicago, IL: Chicago University Press.
Smuts, B. B., and Smuts, R. W. (1993). Male aggression and sexual coercion of females in nonhuman primates and other mammals: evidence and theoretical implications. Adv. Stud. Behav. 22, 1–63. doi: 10.1016/s0065-3454(08)60404-0
Stanger, K. F., Coffman, B. S., and Izard, M. K. (1995). Reproduction in Coquerel’s dwarf lemur (Mirza coquereli). Am. J. Primatol. 36, 223–237. doi: 10.1002/ajp.1350360306
Sterck, E. H. M., Watts, D. P., and van Schaik, C. P. (1997). The evolution of female social relationships in nonhuman primates. Behav. Ecol. Sociobiol. 41, 291–309. doi: 10.1007/s002650050390
Strauss, E. D., and Holekamp, K. E. (2019). Inferring longitudinal hierarchies: framework and methods for studying the dynamics of dominance. J. Anim. Ecol. 88, 521–536. doi: 10.1111/1365-2656.12951
Stumpf, R. M., Martinez-Mota, R., Milich, K. M., Righini, N., and Shattuck, M. R. (2011). Sexual conflict in primates. Evol. Anthropol. 20, 62–75. doi: 10.1002/evan.20297
Surbeck, M., and Hohmann, G. (2013). Intersexual dominance relationships and the influence of leverage on the outcome of conflicts in wild bonobos (Pan paniscus). Behav. Ecol. Sociobiol. 67, 1767–1780. doi: 10.1007/s00265-013-1584-8
Thierry, B. (2013). Identifying constraints in the evolution of primate societies. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 368, 20120342. doi: 10.1098/rstb.2012.0342
Tibbetts, E. A., Pardo-Sanchez, J., and Weise, C. (2022). The establishment and maintenance of dominance hierarchies. Phil. Trans. Roy. Soc. Lond. B Biol. Sci. 377:20200450. doi: 10.1098/rstb.2020.0450
van Hooff, J. A. R. A. M., and van Schaik, C. P. (1994). Male bonds: affiliative relationships among nonhuman primate males. Behaviour 130, 309–337. doi: 10.1163/156853994x00587
van Schaik, C. P., and Kappeler, P. M. (1996). The social systems of gregarious lemurs: lack of convergence with anthropoids due to evolutionary disequilibrium? Ethology 102, 915–941. doi: 10.1111/j.1439-0310.1996.tb01171.x
van Schaik, C. P., and van Hooff, J. A. R. A. M. (1983). On the ultimate causes of primate social systems. Behaviour 85, 91–117. doi: 10.1163/156853983x00057
Voyt, R. A., Sandel, A. A., Ortiz, K. M., and Lewis, R. J. (2019). Female power in Verreaux’s sifaka (Propithecus verreauxi) is based on maturity, not body size. Int. J. Primatol. 40, 417–434. doi: 10.1007/s10764-019-00096-9
Vullioud, C., Davidian, E., Wachter, B., Rousset, F., Courtiol, A., and Höner, O. P. (2019). Social support drives female dominance in the spotted hyaena. Nat. Ecol. and Evol. 3, 71–76. doi: 10.1038/s41559-018-0718-9
Waeber, P. O., and Hemelrijk, C. K. (2003). Female dominance and social structure in Alaotran gentle lemurs. Behaviour 140, 1235–1246. doi: 10.1163/156853903771980576
Wallace, G. L., Paquette, L. B., and Glander, K. E. (2016). A comparison of activity patterns for captive Propithecus tattersalli and Propithecus coquereli. Zoo Biol. 35, 128–136. doi: 10.1002/zoo.21258
Walters, J. (1980). Interventions and the development of dominance relationships in female baboons. Fol. Primatol. 34, 61–89. doi: 10.1159/000155948
Wrangham, R. W. (1980). An ecological model of female-bonded primate groups. Behaviour 75, 262–300. doi: 10.1163/156853980x00447
Wrangham, R. W., Gittleman, J. L., and Chapman, C. A. (1993). Constraints on group size in primates and carnivores: population density and day-range as assays of exploitation competition. Behav. Ecol. Sociobiol. 32, 199–209.
Wright, P. C. (1999). Lemur traits and Madagascar ecology: coping with an island environment. Ybk. Phys. Anthropol. 42, 31–72. doi: 10.1002/(sici)1096-8644(1999)110:29+<31::aid-ajpa3>3.0.co;2-0
Young, A. L., Richard, A. F., and Aiello, L. C. (1990). Female dominance and maternal investment in strepsirhine primates. Am. Nat. 135, 473–488. doi: 10.1086/285057
Keywords: female dominance, intersexual relationships, social structure, lemurs, primates
Citation: Kappeler PM, Fichtel C and Radespiel U (2022) The Island of Female Power? Intersexual Dominance Relationships in the Lemurs of Madagascar. Front. Ecol. Evol. 10:858859. doi: 10.3389/fevo.2022.858859
Received: 20 January 2022; Accepted: 05 April 2022;
Published: 26 April 2022.
Edited by:
Patricia Izar, University of São Paulo, BrazilReviewed by:
Louise Barrett, University of Lethbridge, CanadaCopyright © 2022 Kappeler, Fichtel and Radespiel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter M. Kappeler, cGthcHBlbEBnd2RnLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.