- 1Independent Researcher, North Freedom, WI, United States
- 2Department of Integrative Biology, University of South Florida, St. Petersburg, FL, United States
- 3Centre for Conservation Ecology and Genomics, Institute for Applied Ecology, University of Canberra, Bruce, ACT, Australia
Rainfall following turtle nest construction has long been believed to increase nest survival by its effects on reducing the location cues used by nest predators. However, it is unclear if this is generally the case and if nesting turtles actively use this mechanism to increase their reproductive fitness by deliberately timing nesting to occur before or during rainfall. To address this question, we reviewed studies that examined freshwater turtle nesting behavior and nest predation rates in relation to rainfall. We supplemented our review with data on rainfall and nesting patterns from a 12-year study of two nesting populations of Ouachita Map Turtles (Graptemys ouachitensis). Our review revealed a diversity of responses in rainfall effects on predation and in the propensity for turtles to nest in association with rain. Our mixed findings could reflect a diversity of species- or population-specific responses, local adaptations, species composition of predator community, confounding abiotic factors (e.g., temperature decreases after rainfall) or methodology (e.g., most studies did not quantify rainfall amounts). Our case study on map turtles found very high yearly predation rates (75–100%), precluding our ability to rigorously analyze the association between nest predation and rainfall. However, close examination of the exact timing of both rainfall and predation revealed significantly lower predation rates when rain fell within 24 h after nesting, indicating that rainfall during or after nesting may reduce nest predation. Despite this effect, the best fitted model explaining the propensity to nest found that map turtles were more likely to nest after dry days than after days with rainfall, suggesting that rainfall was not a major factor driving turtles to nest in our populations. In both our review and in our map turtle populations there was little evidence that turtles can anticipate rainfall and nest prior to it occurring (e.g., in response to falling barometric pressure).
Introduction
Turtles in the modern world face mounting challenges to their continued existence. In addition to population losses due to habitat loss and degradation, reproductively valuable adults are exploited as commodities for human consumption and the international pet trade while being concurrently exposed to increased mortality from introduced predators, roadways, pollution, and disease (reviewed in Lovich et al., 2018). At the other end of the life cycle, eggs and hatchlings are threatened by elevated nest predation levels due to both anthropogenically increased densities of nest predators (Mitchell and Klemens, 2000; Prugh et al., 2009) and potentially increased nest-detection efficiencies of predators concentrating their efforts on nesting areas reduced in size from habitat loss and vegetational succession (Temple, 1987; Oehler and Litvaitis, 1996; Jackson and Walker, 1997; Marchand and Litvaitis, 2004).
Emerging threats from human-induced climate change present yet another threat to turtle populations on a pervasive, worldwide scale. Climate change is expected to bring changes to rainfall patterns due to higher global temperatures producing a more active hydrological cycle and increasing atmospheric water-holding capacities (Collins et al., 2013). Precipitation has increased about 2% since the beginning of the 20th century, although its distribution is neither spatially or temporally uniform (reviewed in Dore, 2005). As nest predation rates may be affected by rainfall and its timing relative to nesting events, this climatic variation may impact turtle populations in different ways: in some cases, potentially increasing nest success and hatching recruitment, and in others, reducing nest success (Czaja et al., 2018). Understanding how rainfall and correlated factors affect turtle reproduction is thus important in placing their potential impacts into an ecological context and in predicting how these effects may be altered by ongoing anthropogenic climate change.
Unlike mammals and birds which typically invest large amounts of time and resources in the protection and nurturing of their young, turtles lack direct knowledge of the eventual fate of their reproductive efforts, eliminating the chance of increasing nest success and offspring survival via adult learning and experience that may occur in other taxa (e.g., in some songbirds; Zanette, 2001). Nonetheless, mechanisms to increase nest and hatchling survival are presumed to be under strong selection pressure, including those which reduce nest predation (e.g., Spencer, 2002; Refsnider and Janzen, 2010; Schwanz et al., 2010; Bernstein et al., 2015; Czaja et al., 2020) and, thus, may be expected to evolve via natural selection (Spencer, 2002) given sufficient heritable variation in the relevant behavioral traits (Dochtermann et al., 2019). However, as nest location cues available to above-ground predators may be diverse and may arise as necessary components of nest construction (e.g., odors from recently disturbed soil; reviewed in Geller and Parker, 2022), turtles may be limited in their ability to significantly reduce the nest location signals of their subterranean nests (Voves et al., 2016). Nonetheless, the role of significant rainfall in reducing the signal strength of nest location cues and increasing nest survival has long been recognized (e.g., Legler, 1954), with some authors suggesting that, in some contexts, little hatchling recruitment would take place without it (Carr, 1952). Thus, one of the few ways turtles may be able to mitigate nest predation risks is by nesting during or just before significant rainfall (e.g., Burke et al., 1994; Bowen and Janzen, 2005).
Herein we test the hypothesis that turtles can use rainfall as a cue to reduce nest predation rates. Specifically, we surveyed the literature to synthesize our present understanding of (1) the propensity for turtles to preferentially nest either before or after significant rainfall; (2) how nest predation rates of freshwater turtle nests are affected by varying amounts of rainfall; and (3) the degree to which female turtles appear physiologically able to anticipate rainfall and accordingly time nest construction activities to enhance their own fitness. To supplement our literature review, we investigated associations of rainfall with nesting activity and nest survival using a 12-year data set from two populations of Ouachita Map Turtles (Graptemys ouachitensis) from Wisconsin, during 2008–2021. This data set, among the first to use trail cameras as primary data collection tools, provided fine-scale resolution on nesting, rainfall, and nest depredation timelines as well as on predator species involved and allowed us to assess nesting propensity and nest predation risk in the proper temporal contexts. We discuss our results within the context of expectations based on our literature review.
Methods
Literature Reviews
We surveyed over 60 papers providing data or speculating on the relationship between rainfall and nest predation rates in freshwater turtles. The papers were found by searching the references sections of published work and by on-line searches using Google, Google Scholar, and the academic research databases of the University of Wisconsin Library System (>1100 e-collection content selections from Primo Central Index [PCI] from Ex Libris [ProQuest] including Web of Science and Scopus) using the keywords: “turtle:nest:predation:rain;precipitation.” The papers were mostly peer-reviewed publications but some unpublished M.Sc. and Ph.D. theses were also included. Papers that only cited previous publications without providing new data were not included or are distinguished as such in this review.
Case Study From a Ouachita Map Turtle (Graptemys ouachitensis) Metapopulation
To supplement literature reviews, we also analyzed data on rainfall, nesting activity, and nest survivorship from two populations of Ouachita Map Turtles (Graptemys ouachitensis) located on the lower Wisconsin River within 10 km of Spring Green, WI, United States (43°10′38′′N and 90°04′02′′W). Both nesting sites are on sand terraces approximately 15 and 52 m from the main river channel, respectively, and are comprised of various xerophytic herbaceous vegetation covering approximately 20% of the surface, with the remainder being open sand (for a more complete description of these sites, see Geller, 2012a).
The study was carried out over 12 years from 2008 to 2021 excluding the years 2012 and 2018. Newly constructed turtle nests were located beginning in late May of each study year by review of images from pole-mounted trail cameras (RECONYX®, Inc.; Holmen, WI, United States) monitoring each nesting area. Cameras were programmed to take continuous time-lapse images at 1-min intervals to document nesting events along with motion-triggered series of more closely timed photographs to provide detailed documentation and timing of nest predator (all Northern raccoons, Procyon lotor) visits. Surveillance ended each year with either the documented predation or hatchling emergence of the last monitored nest in each year.
We obtained total daily rainfall amounts (in mm) from two sources: (1) data from the Lone Rock Tri-County Airport, Sauk County, Wisconsin, approximately 9.7 km northwest of the study sites (downloaded from NOAA National Climate Data Center1, station id: GHCND:USW00014921); and (2) from an on-site rain gauge. Where the two sources differed, we used the rainfall amounts from the on-site gauge, although these were not always available due to logistic constraints. In some years, rainfall timing on the site was determined by use of a funnel-driven waterwheel device enclosed within a Lucite®-fronted housing placed within the peripheral camera field-of-view (Geller, 2012a). Date-stamped time-lapse images indicating waterwheel movement delineated rain event timelines. These units were accurate to within 0.2 h of rainfall duration (sprinkles to heavier amounts), as determined by field tests and camera-visible nocturnal rainfall. In all years, within-day rainfall event amounts and timing were estimated using hourly rainfall tracking charts from Lone Rock, Wisconsin via Weather Underground historical weather charts2. We also used this database to derive metrics on daily high, low, and mean temperatures; and daily high, low, and mean air pressures.
Statistical Analysis
Our unit of observation was a day on which turtle activity was monitored for which we had rainfall data. For modeling purposes, we defined the nesting season as starting one day prior to the first recorded turtle nest and ending one day after the last recorded turtle nest for each year (12 years from 2008-2021, excluding 2012 and 2018). We excluded 26 days when the sites were flooded so nesting could not take place and, to be conservative, also excluded 28 nests constructed within 5 days after flooding because the timing of these nests may have been affected by females retaining eggs during flooded periods. In some years, some nests were protected from predation using nest cages. Protected nests were included in the analysis of nesting behavior in relation to rainfall but not in examining how rainfall affects nest predation risk. Reported sample sizes reflect varying numbers of camera records available for analysis, as influenced by camera position, intervening vegetation, and other variables.
We modeled the number of nests constructed each day (counts from 0 to 6) as a function of four variables that capture how turtle nesting might respond to daily rainfall variation: (1) rain on the day of nesting or not; (2) rain on two consecutive days (high rain frequency periods); (3) no rain for two consecutive days (low rain frequency periods); and (4) no rain on the day and rain the next day (nesting in anticipation of oncoming rain). Although we had data on the amount of rainfall for each day, using rainfall presence/absence provided a better fit to the data as indicated by lower values of Akaike’s Information Criteria (AIC).
We fitted four models to the data, with the number of nests constructed each day as the response variable and one of the four rainfall variables above as a univariate predictor in each model. We specified a Poisson error distribution (appropriate for count data) and included an observation level factor and year as random effects in each model. The observation level factor accounted for any overdispersion in the data, while the inclusion of year as a random effect accounted for the possibility that the probability of nesting per day was higher, on average, in some years than others. We compared the fit of the four models to the data using AIC, with better fitting models having lower AIC values. The models were fitted using the lme4 package (Bates et al., 2015) in the statistical analysis software R (R Core Team, 2017). We also tested for relationships the between number of nests constructed and the measures of daily temperature and air pressure by including these as univariate predictors as described above.
Results
Literature Review of Association of Turtle Nesting Activity and Rainfall
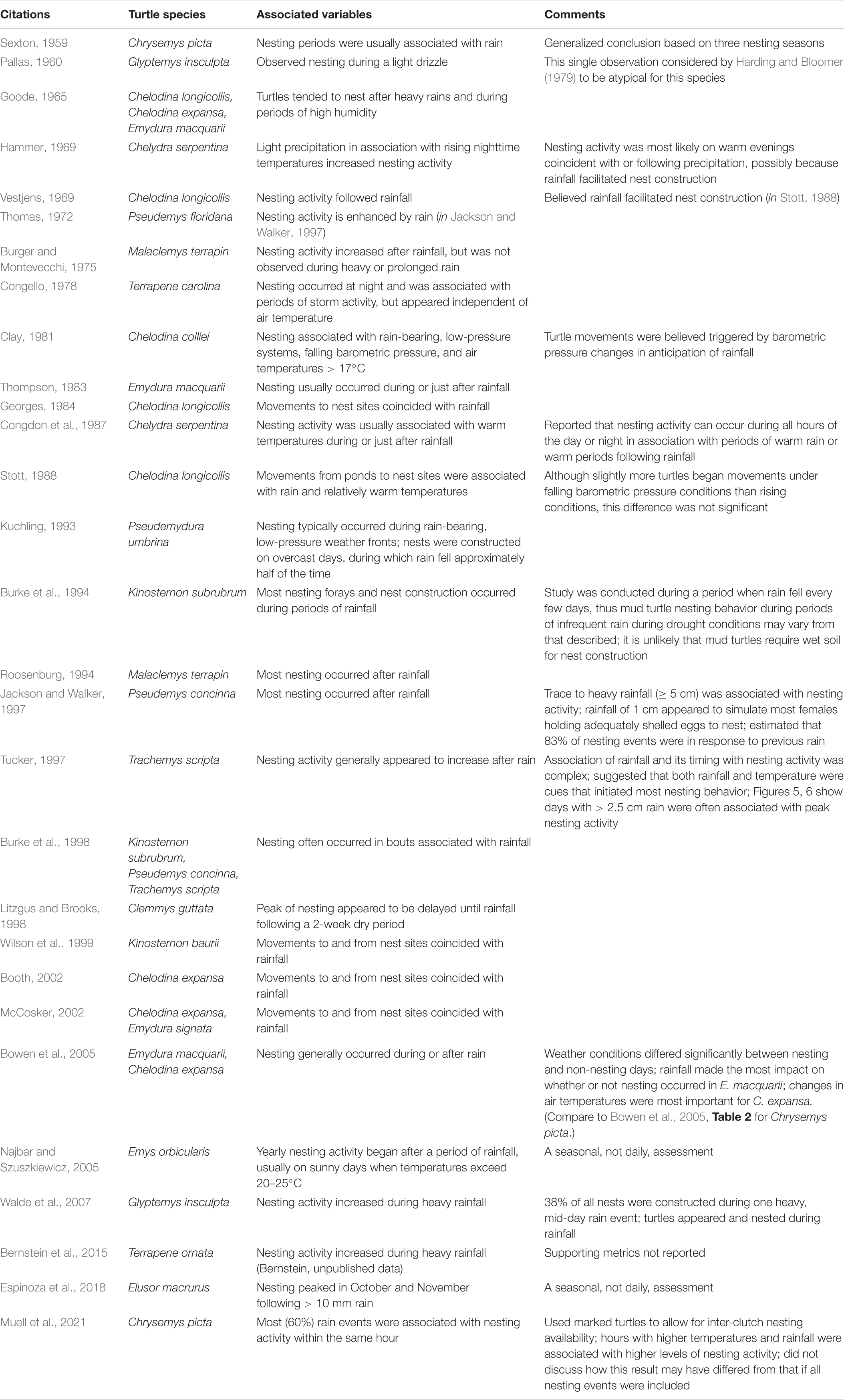
We reviewed 42 studies that quantified or addressed the potential association of nesting with rainfall in 23 species of freshwater turtles. Of these, 29 (69%) studies demonstrated or suggested that the propensity to nest was associated with rainfall, compared to 13 (31%) studies that found no association (Tables 1, 2). The research to-date is, thus, somewhat equivocal with regards to the association of rainfall and turtle nesting activity.
However, when associations have been found, our review revealed a general consensus for turtle nesting to occur during or after rainfall, rather than before it (Table 1). In addition, the few studies that systematically examined the effect of rainfall amount on nesting activity found larger rainfall amounts more likely to stimulate turtle nesting during or after the rain event than lesser amounts (≥10 mm, Jackson and Walker, 1997; >2.5 cm, Tucker, 1997; see also Walde et al., 2007; Bernstein et al., 2015; Table 1). However, Buckardt et al. (2020) found no relationship between rainfall amount and the propensity to nest, while others noted that heavy rainfall suppresses nesting activity (Legler, 1954; Burger and Montevecchi, 1975), although in some cases this is likely due to a concurrent decrease in air temperatures to non-optimal levels rather than rainfall amount effects per se (e.g., Harding and Bloomer, 1979; Vogt, 1980).
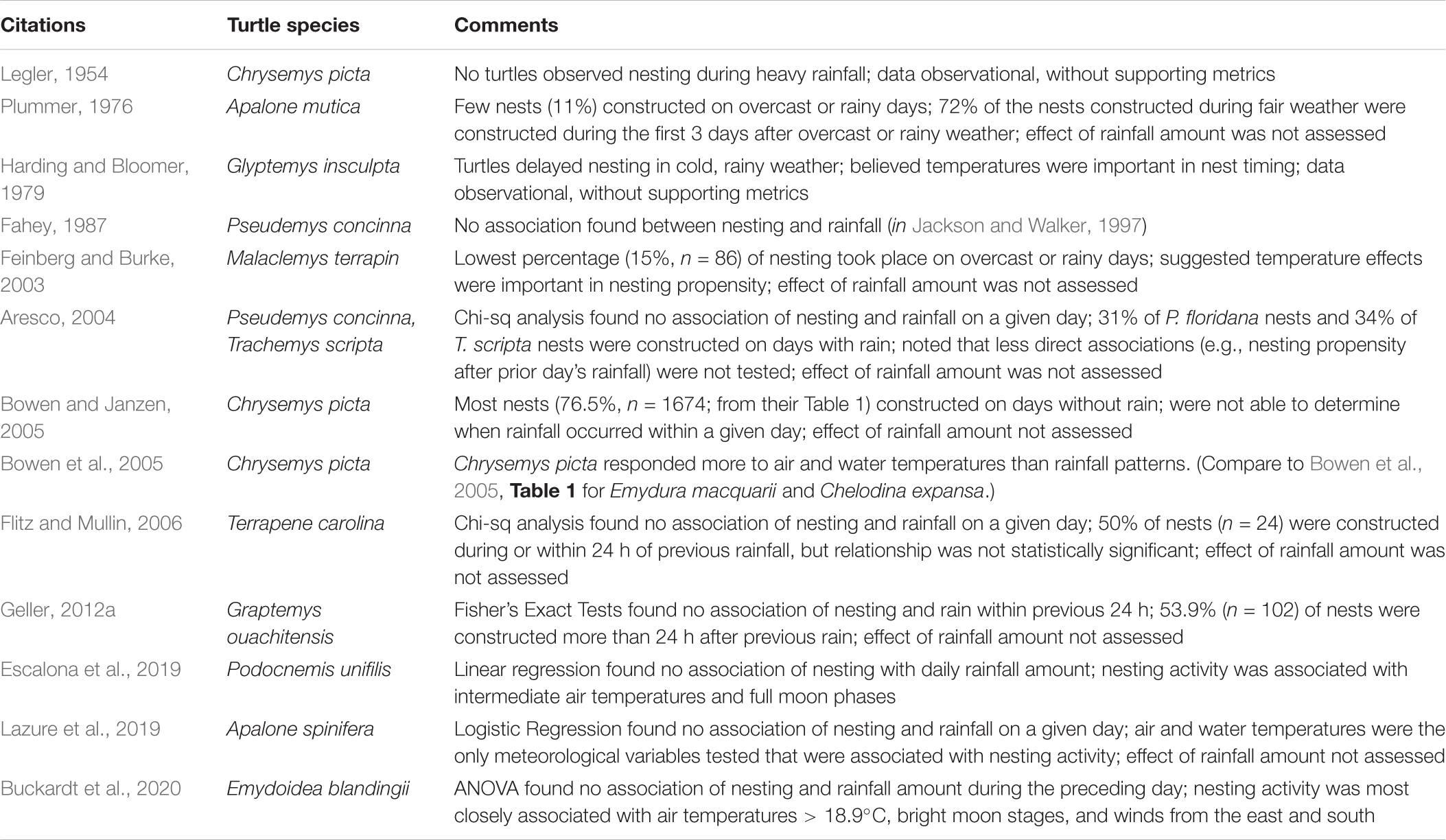
Some early reports (e.g., Pallas, 1960) are primarily anecdotal observations about particular rainfall events and do not provide the ecological context in which to evaluate the uniqueness of the association, such as the numbers of turtles nesting in other conditions. Limitations in study designs or data acquisition have restricted some other studies to basic reports on the association of nesting with rainfall on a simple binary, rain present/absent basis within a given calendar day, and do not allow assessments of the impact of rainfall within different temporal periods (e.g., previous 24 h) on nesting propensity or, importantly, how rainfall amounts were quantitatively distributed in relation to the timing of nesting events. For example, 77% of the reviewed studies that failed to find an association of nesting activity and rainfall did not record and include rainfall amounts in their analyses (Table 2). These methodological differences may have contributed to some of the disparity in findings regarding the association of rainfall and freshwater turtle nesting activity.
However, even better-quantified studies sometimes report different responses of turtles to rainfall patterns. For example, Bowen et al. (2005) noted that Chrysemys picta nested on virtually every day during the nesting season and suggested that timing of nesting in that species was primarily a function of physiological readiness and sufficiently warm temperatures, rather than of rainfall or other environmental variables. However, Bowen et al. (2005) were unable to provide explanations for why this behavior contrasted with those for Emydura macquarii and Chelodina expansa in that same study, whose nesting was correlated with rainfall and associated changes in air temperature. Disparity in study findings sometimes appear even for the same species at the same nesting sites (e.g., for Chrysemys picta at the Thompson Causeway, Illinois [Bowen and Janzen, 2005; Muell et al., 2021]; see Tables 1, 2).
Nonetheless, when associations have been found, many studies have concluded that rainfall on a given day is an important determinant of nesting propensity in freshwater turtles, either when considered as a single factor (Congello, 1978; Georges, 1984; Burke et al., 1994, 1998; Roosenburg, 1994; Wilson et al., 1999; Bowen et al., 2005, in part; Walde et al., 2007; Bernstein et al., 2015; Espinoza et al., 2018) or in conjunction with other meteorological variables such as rising or relatively high air temperatures and falling barometric pressures above certain minimums (Hammer, 1969; Burger and Montevecchi, 1975; Clay, 1981; Congdon et al., 1987; Stott, 1988; Kuchling, 1993; Tucker, 1997; Najbar and Szuszkiewicz, 2005; Muell et al., 2021).
Still other studies have found air and/or water temperatures alone to be principal cues to initiate nesting, with a preference for nesting on days with the relatively high daily temperatures that optimize locomotor performance and reduce the time spent nesting (e.g., for Chrysemys picta, Bowen et al., 2005; Frye et al., 2017) or that allow turtles that nest during the evening to maintain their body temperatures at functioning levels (e.g., for Emydoidea blandingii, Buckardt et al., 2020). Pig-nosed turtles (Carettochelys insculpta) ceased nesting for up to several days during cool periods in the tropical (winter) dry season (Doody et al., 2003). Rather than rainfall, additional studies have noted associations of nesting activity with bright moon phases, possibly a function of social facilitation and/or as a mechanism to reduce individual or nest predation (Escalona et al., 2019; Buckardt et al., 2020). These complexities show that single-factor analyses of rainfall frequencies alone (e.g., Aresco, 2004; Geller, 2012a) are not likely to be as informative for a given species and context as studies incorporating rainfall amounts and a broader array of meteorological parameters in their analyses, such as the concurrent effects of air and water temperatures, and time since last rainfall (noted by Jackson and Walker, 1997; Muell et al., 2021). Overall, the literature thus shows significant variation in the assessment of whether or not freshwater turtles appear to nest in greater numbers after rainfall, suggesting that this association is not a generalizable aspect of chelonian reproductive biology.
A Case Study of the Association of Rainfall and Nesting Activity With Ouachita Map Turtles (Graptemys ouachitensis)
A total of 245 G. ouachitensis nests were constructed on 147 of the 320 days on which activity was monitored, giving an average of 1.7 nests constructed on days when nesting occurred.
Rain fell on 142 of the 320 days (44.3%) when activity was monitored across all study years. Most nests (57.0%, n = 139) were constructed on calendar days without rainfall. Similarly, most nests were constructed more than 24 h after previous rainfall (54.9%, n = 134), more than 24 h before the next rainfall (63.1%, n = 154), and many (38.1%, n = 93) were without rain during both pre- and post-nest construction periods. A small number of nests (2.5%, n = 6) were constructed during rainfall itself. The all-year mean number of nests constructed on calendar days without rain (0.84 nests/d) did not differ from the mean number constructed on days with rain (Model 1 in Table 3 and Figure 1). Similarly, there was no association between the number of nests constructed on a given day and (any) rainfall amount (p = 0.81) or on calendar days with ≥ 20 mm of rain (p = 0.84).
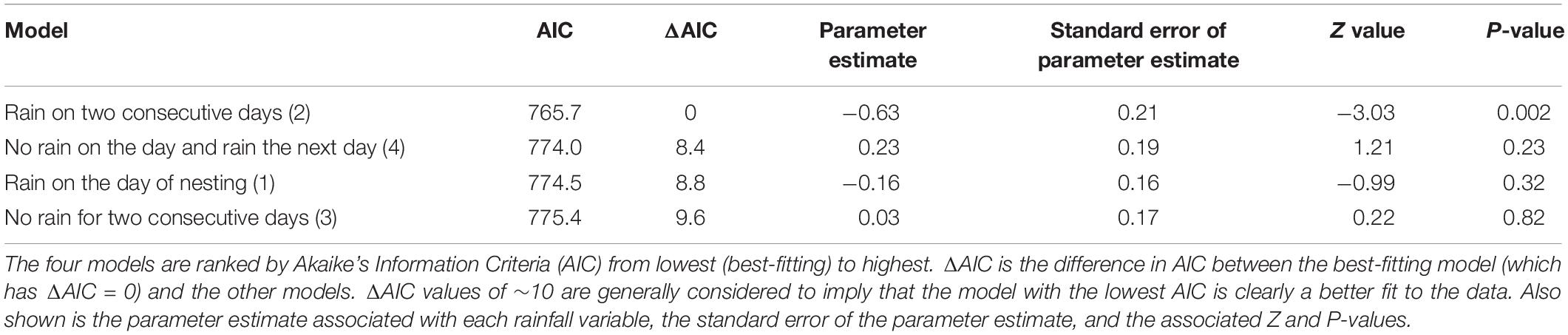
Table 3. Summary of the four regression models fitted to the data, each including a different rainfall variable as predictor (see section Methods).
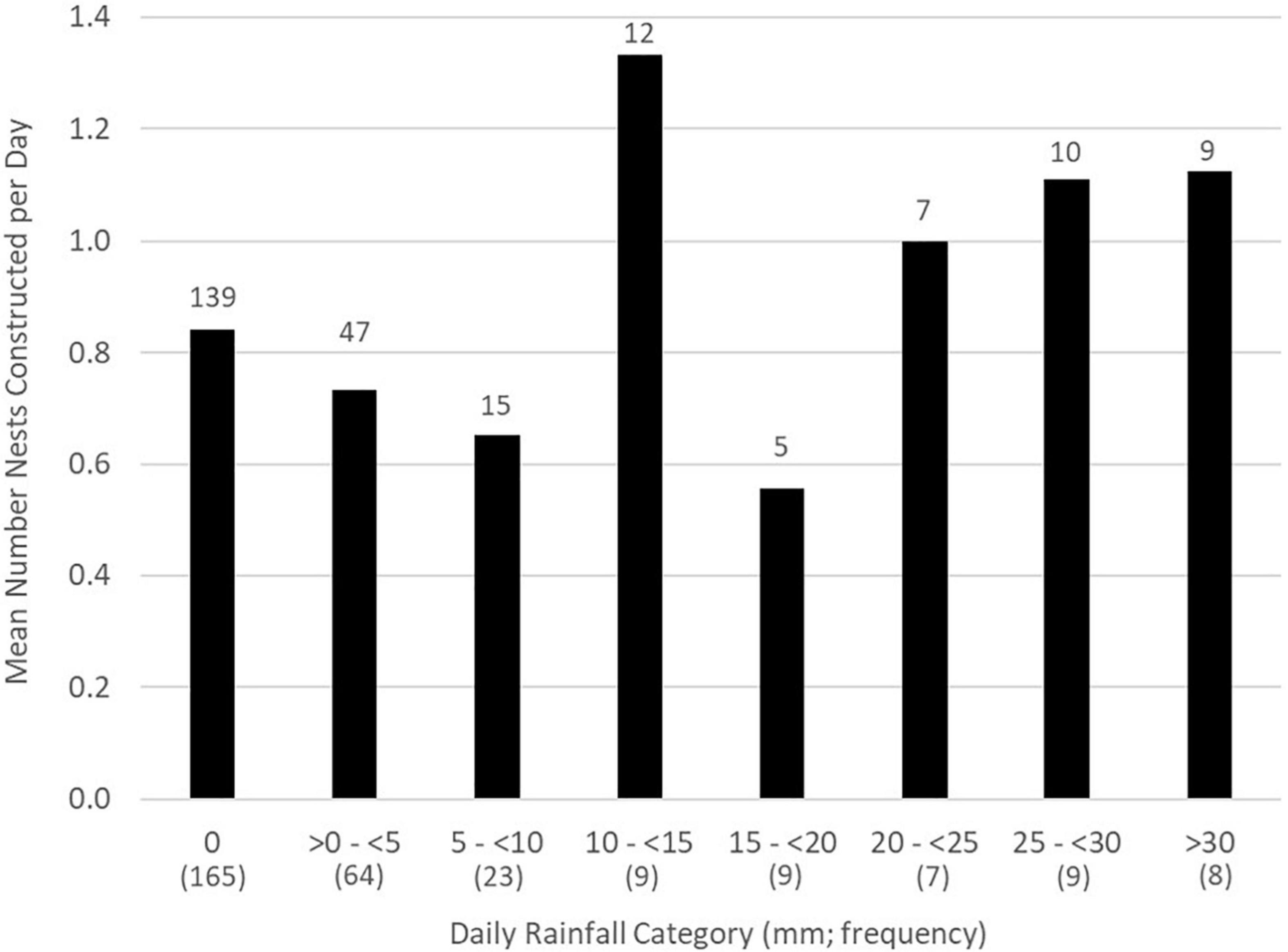
Figure 1. All-year mean number of Graptemys ouachitensis nests constructed/d by daily rainfall category along the Wisconsin River, WI, United States, during 12 study years from 2008 to 2021 (n = 244 nests). Overall number of nests per rainfall category appear above each bar; number of days within each category appear below, in parentheses.
On a calendar day basis, Model 2 (rain on the day of nesting and rain on the previous day) provided the best fit to the data on the number of nests constructed per day (Table 3). The negative parameter estimate for this model indicated fewer nests were constructed when it had rained two days in a row. There was strong evidence that the parameter estimate for Model 2 was negative: the 95% confidence interval, given by ±2 times the standard error, did not include zero and the outcome was associated with a low P-value. Figure 2 summarizes the pattern of turtle nesting in relation to whether it rained for two days or not. While there was a much lower probability of nesting given rain two days in a row (nesting probability = 0.31 relative to 0.51 if this did not occur), there was less of a difference in the mean number of nests constructed if nesting occurred on either occasion (1.7 versus 1.48, Figure 2). There were no significant effects of temperature or barometric pressure on the propensity to nest.
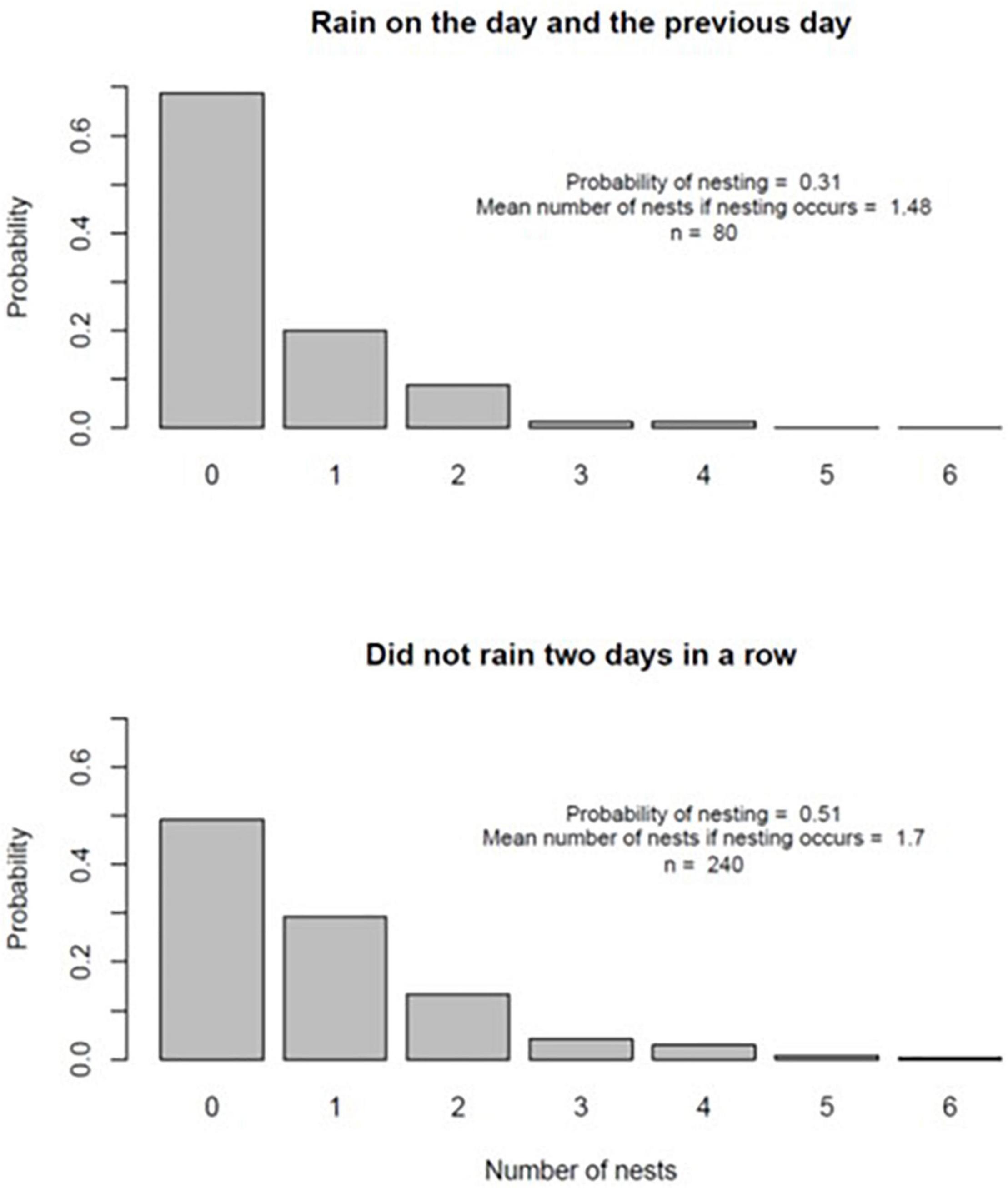
Figure 2. Probability distributions for the number of nests constructed on days where it had rained two days in a row (top panel) and days where it had not (bottom panel).
Literature Review of Effect of Rainfall on Turtle Nest Predation Rates
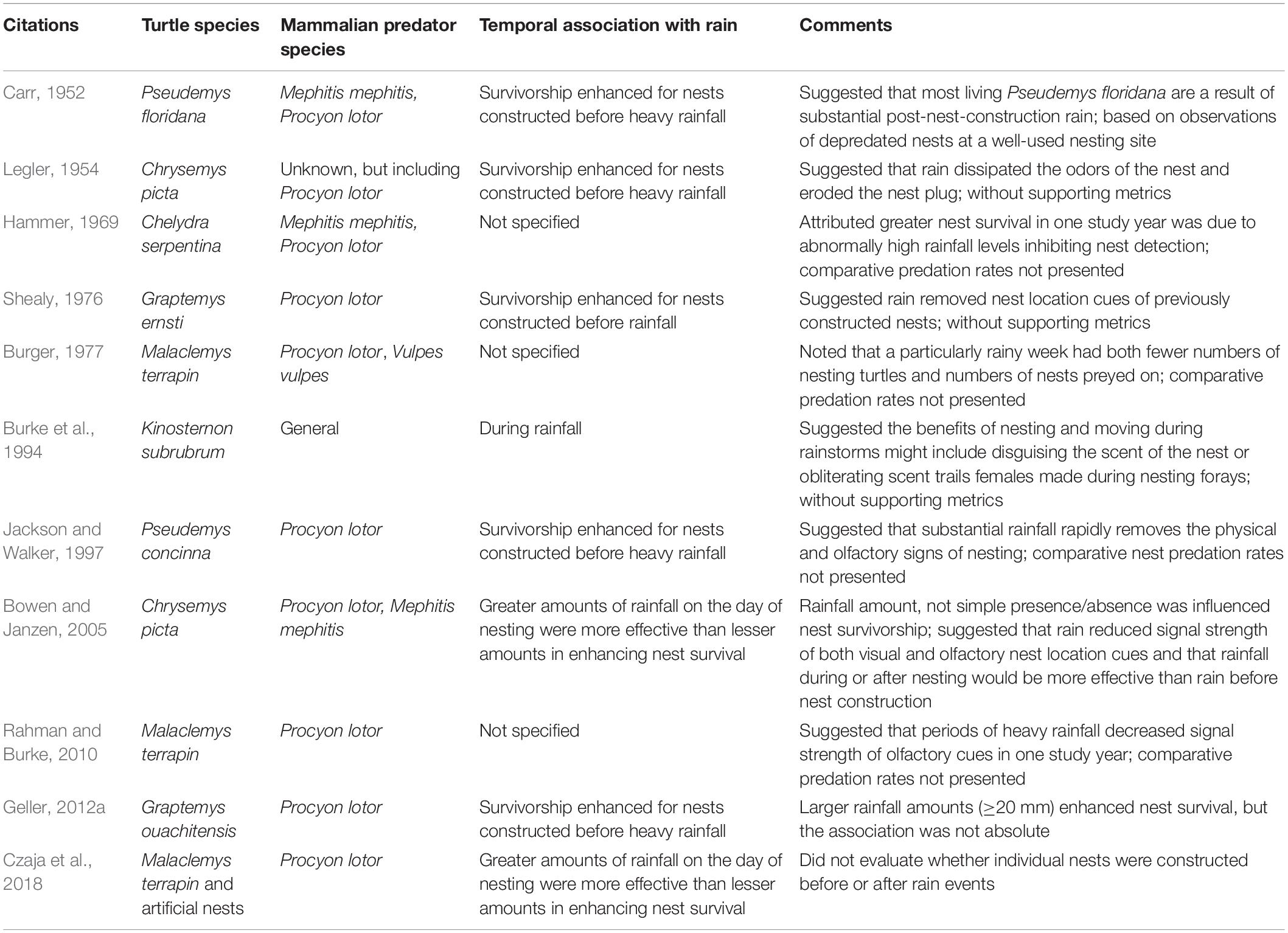
We reviewed 21 studies that quantified or addressed the potential for rainfall to increase nest survival in 7 turtle species. In our review, 11 (52%) of the studies demonstrated or suggested that rainfall increased nest survival, compared to 10 (48%) studies that found no effect (Tables 4, 5).
The primary cues used by predators to locate newly constructed freshwater turtle nests are believed to be largely olfactory for mammalian predators, with varying degrees of visual use based on predator species (reviewed in Geller and Parker, 2022); largely olfactory for certain nest-depredating lizards (e.g., Soanes et al., 2015); and largely visual by bird predators (e.g., Jackson and Walker, 1997). Whether visual or olfactory, many researchers have suggested that rainfall may dilute these nest location cues (Table 4). One proposed mechanism by which rainfall diminishes olfactory cues suggests that water percolating through the soil column flushes out the odors produced by soil microbes (Lindbo et al., 2012), thereby reducing what were formerly point-source olfactory signals of disturbed soil at nest locations (Geller, 2015; see also, Buzuleciu et al., 2016). Greater rainfall amounts, percolating to greater soil depths (reviewed in Hess et al., 2018), are likely more thorough in aerosolizing the microbe-produced compounds within newly constructed nest cavities as well as from surrounding substrates, reducing the olfactory gradient. Both the amount of rain and its timing relative to nest construction are, thus, potentially important influences on the degree to which rain reduces nest predation. As a corollary, rainfall during and after nest construction is likely to be more effective in reducing both olfactory and visual nest location cues than when rainfall precedes nesting (Bowen and Janzen, 2005).
These inferences are supported our review, in that all of those which noted an effect of rain in reducing nest predation, either qualitatively or quantitatively, found larger rainfall amounts on the day of or soon after nest construction to be more effective in reducing nest predation than smaller amounts or none (Table 4); No studies suggested that rainfall in advance of nesting would decrease nest predation rates. In contrast, it is difficult to fully evaluate studies that did not show an enhancing effect of post-construction rainfall on nest survival (Table 5) because (like some that did show an effect) none reported the relevant rainfall amounts except for the 7.3 mm reported by Wilhoft et al. (1979) and the experimental work of Buzuleciu et al. (2016), wherein 2 cm of dechlorinated tap water was applied to individual artificial nests. However, as pointed out by Czaja et al. (2018), experimentally applied water at nests themselves does not scale in effect to that of larger areas affected during natural rainfall and possibly explains why these negative results differ from other research where the effects of natural rainfall were assessed.
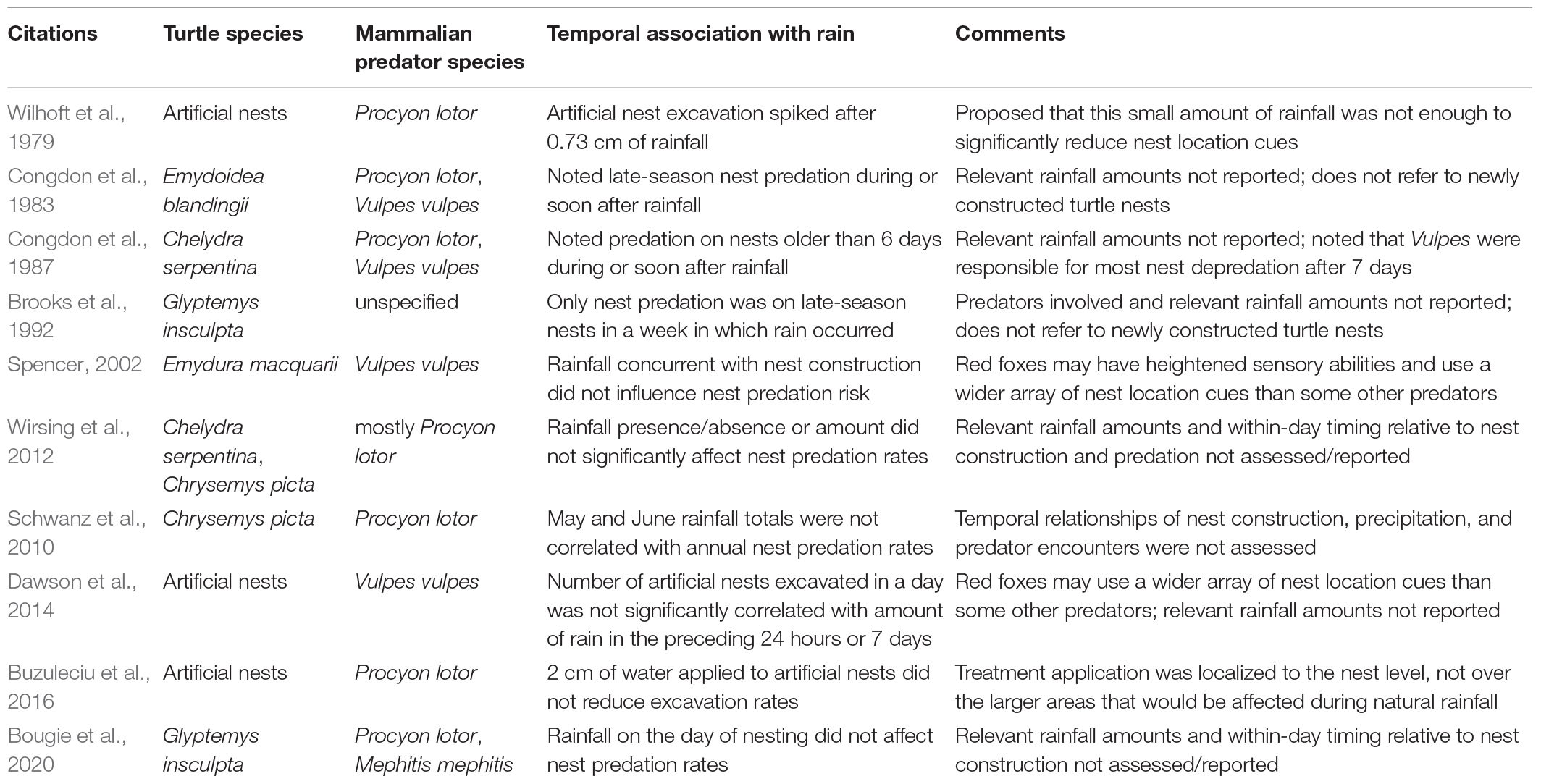
Importantly, none of the studies finding no effect of rainfall on predation rates on newly constructed natural turtle nests with raccoons as predominant predators (Schwanz et al., 2010; Wirsing et al., 2012; Bougie et al., 2020) assessed the temporal association between rainfall and individual nesting events, which is likely central to the dynamics of how rainfall affects the strength of nest location signals (see above). Some other studies that discounted the role of rainfall in reducing turtle nest predation rates recorded red foxes (Vulpes vulpes) as nest predators (Congdon et al., 1983, 1987; Spencer, 2002; Dawson et al., 2014), which may utilize a wider array of nest location cues than some other predators (e.g., raccoons), including, potentially, the scent of eggs themselves (see Congdon et al., 1987; Geller and Parker, 2022). However, whether these differences in sensory abilities explain the apparent lack of rainfall effect on nest survival is unclear. Similarly unexplained are reported increases in nest predation during or soon after rainfall for late-season turtle nests (Congdon et al., 1983; Brooks et al., 1992). However, late-season nests may have a different suite of nest location cues than those at recently constructed nests (see reviews in Riley and Litzgus, 2014; Geller and Parker, 2022), including, potentially, hatchling vocalizations (e.g., Ferrara et al., 2012; Geller and Casper, 2019). As hatchling emergence onto the surface is often associated with rainfall and warm or rising ambient temperatures (e.g., Tucker, 1997; Nagle et al., 2004; Geller et al., 2020), it is possible, although speculative, that rain during late, pre-emergence stages may increase overall hatchling activity, leading to increases in odors from disturbed soils or from the hatchlings themselves, hatchling-produced sounds, or other surface-detectable cues to nest locations.
A Case Study of the Interaction of Precipitation and Nest Predation With Ouachita Map Turtles (Graptemys ouachitensis)
Nest predation rates on our two nesting sites were high (overall 93.0%, yearly range 75–100%, n = 128) and only 9 unprotected (uncaged) nests escaped predation across all study years. All predation was from raccoons, which were present nearly every night during nesting periods (Geller, 2012b). Ninety-five percent of the nests depredated by raccoons were destroyed within 24 h of construction (mean nest survival = 11.8 h, range 0.0–7.6 days, n = 111), typically by the first raccoon within approximately 1 meter of a given nest (ca. 90% of the time; Geller, 2012b). Most depredated nests had no post-construction rain before predation (84.5%, n = 116 total depredated nests), and all of those without intervening rain were found by raccoons before or during the first night. Raccoons depredated 94.3% of the nests constructed without rain in the previous 24 h (n = 70) and 90.7% of the nests made when rain (median = 4.3 mm, mean = 9.5 mm, range 0.3–78.5 mm) did occur within 24 h before (n = 50) or during nest construction (n = 4) (Yate’s χ2 = 0.164, p = 0.685, n = 124), suggesting that rain falling before nest construction had little effect in reducing nest predation rates.
Given the high rate of predation and short nest survival timelines, we did not have sufficient data to reliably identify the characteristics of nests that escaped predation. However, surviving nests tended to have larger amounts of rainfall during both the first night after nest construction (mean = 7.8 mm, SD = 10.27, range 0.0–25.0, n = 9) and through the first four nights after nest construction (mean = 19.4 mm, SD = 14.63, range 0.8–35.1, n = 9) than did depredated nests (first night mean = 0.3 mm, SD = 1.23, range 0.0–10.4, n = 109) (Figure 3), and all surviving nests had either single or multiple rainfall bouts during the 4 days following nest construction. Moreover, raccoons depredated fewer nests having post-nest construction rain within 24 h (85.7%, n = 42 initial nests) than those without rainfall shortly after nest construction (96.4%, n = 83 initial nests), a difference unlikely to arise by chance alone (Fisher’s exact test; p = 0.060, n = 125). Further review of the camera data showed that 58.3% (21 of 36) of the depredated nests constructed within 24 h of the next rain event were detected by raccoons before any of this rainfall occurred. When these nests are removed from the depredated nests within 24 h of post-construction rain counts, the depredation rate of nests actually exposed to post-construction rain decreases to 71.4% (n = 21), strengthening evidence for a non-random difference (Fisher’s exact test; p = 0.002, n = 104). This result supports the idea that raccoons depredate a smaller proportion of nests when rainfall follows nest construction and highlights the importance of determining the exact timing of rainfall and nest predation in the field rather than using metrics based on the simple presence or absence of rain on a given day (see also Bowen and Janzen, 2005).
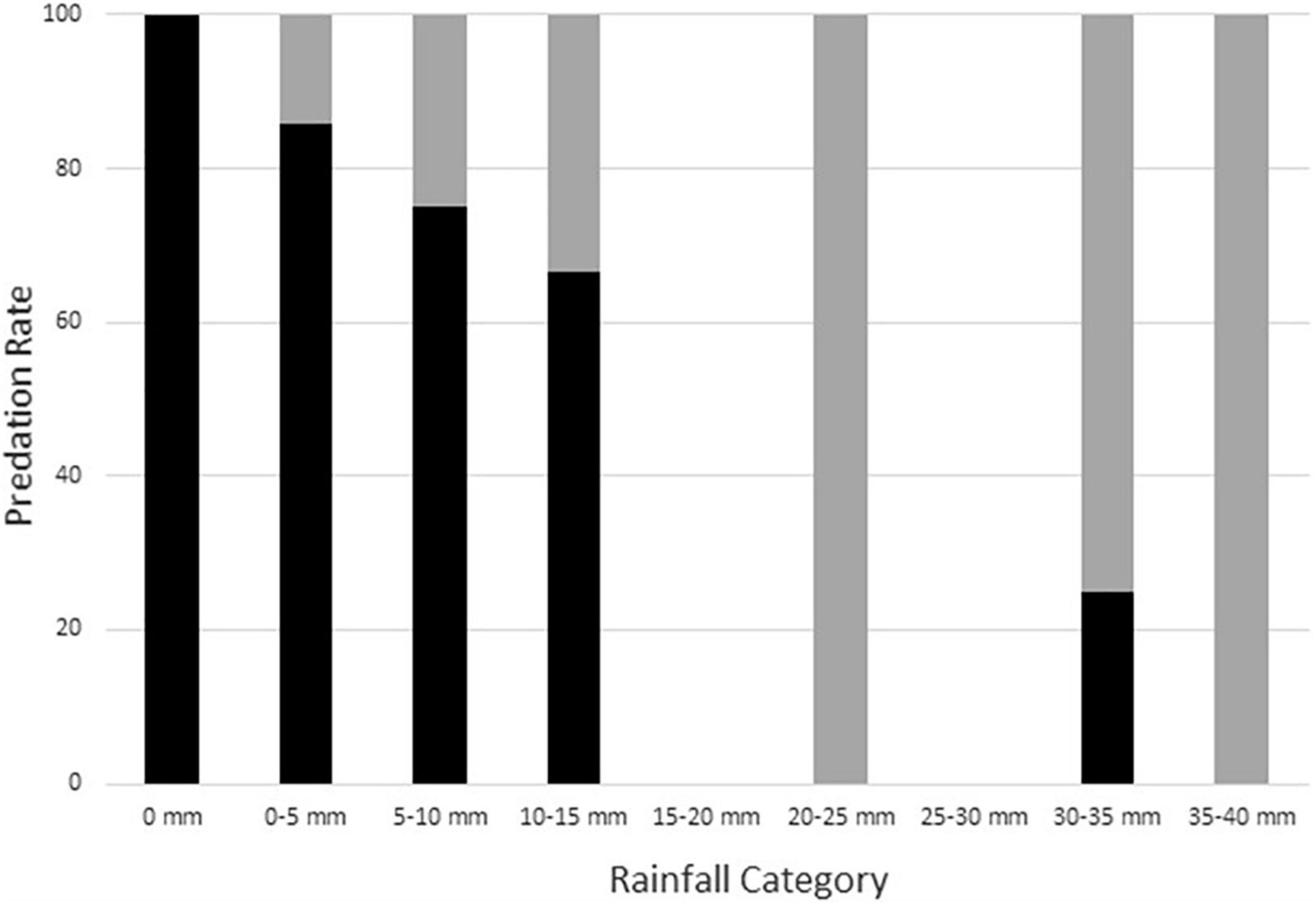
Figure 3. Association of 4-day cumulative rainfall amount with rates of nest depredation (black bars; n = 116) and nest survival (gray bars; n = 9) for natural Graptemys ouachitensis nests along the Wisconsin River, WI, United States, during 12 study years from 2008 to 2021. Most nests depredated during the first night (see text for details).
Literature Review and Case Study of Degree to Which Freshwater Turtles Appear Able to Predict Rainfall
To date, most research on the role of changing barometric pressure—declines in which are a key component of oncoming rainfall that could be a nest initiation cue to ovipositing turtles—has involved marine species. Drops in air pressure, in conjunction with other meteorological variables, have been suggested to be a cue to deteriorating habitat conditions for loggerhead sea turtles (Caretta caretta), resulting in movements away from nesting areas (Schofield et al., 2010). In other studies, higher barometric pressures were positively associated with higher rates of successful nesting (i.e., non-aborted attempts) by C. caretta and leatherback sea turtles (Dermochelys coriacea) (Pike, 2008; Palomino-González et al., 2020; respectively) and, although correlative in nature, were considered cues promoting nesting behavior. Within freshwater turtles, movements to nesting areas were believed triggered by falling barometric pressures in advance of rainfall by Chelodina colliei (then as C. oblonga) (Clay, 1981). In contrast, research on the allopatric congener, C. longicollis, indicated that, while there was a correlation of increased nesting-related movements out of ponds with falling barometric pressure, the association was not statistically significant and was weaker than that of rainfall itself, and was thus considered indirect and corollary (Stott, 1988, M.Sc. Thesis).
Our review of the literature reveals that air pressure data have rarely been collected in chelonian studies and we, thus, lack basic understanding of its potential role as a cue to nest initiation in freshwater turtles or how this environmental variable may affect turtles at different taxonomic and geographic scales. However, results from our case study indicated no relationship between decreasing barometric pressure or temperature and nesting activity, suggesting that turtles on our sites were not responding to these potential indicators of future rainfall.
Discussion
Rainfall has been found to stimulate nesting behavior in a variety of taxa, mostly those inhabiting arid or semi-arid ecosystems, including birds (reviewed in Cavalcanti et al., 2016), and, within reptiles, in certain lizards (e.g., Polychrus acutirostris, Vitt and Lacher, 1981; Oplurus cuvieri, Randriamahazo and Mori, 2001). Similarly, rainfall is believed to trigger the onset of the nesting season for some freshwater turtles in regions that recurringly experience strong drought/monsoon cycles (e.g., in Australia, within genera Chelodina [Goode, 1965; Clay, 1981; Georges, 1984] and Emydura [Espinoza et al., 2018]; in India, for Lissemys punctata and Melanochelys trijuga [Premkishore and Chandran, 1996]) and may be a recurring aspect of the annual reproductive biology of turtles in certain regions. Doody et al. (2003) found that the onset of nesting, during the dry season, was related to the magnitude of rainfall during the previous wet season in Carettochelys insculpta. Even in parts of the world without pronounced seasonality in rainfall regimes, rainfall following drought periods within a nesting season sometimes stimulates nesting activity (e.g., in North America, for Pseudemys concinna suwanniensis, Jackson and Walker, 1997; for Clemmys guttata, Litzgus and Brooks, 1998).
On a within-season basis, however, the relationship between rainfall and nest initiation in turtles has been historically unclear, despite its potential effects on both nesting turtles themselves and on nest predation risk. Our review found a diversity of responses: while most (69%) studies found that the propensity to nest was positively associated with rainfall (Table 1), others (31%) found either no association or a negative association (Table 2). Similarly, rainfall during or after nesting apparently increased nest survival by reducing predation in some species or populations (52%; Table 4), but not in others (48%; Table 5). Our mixed findings may reflect a diversity of species- or population-specific responses, local adaptations, confounding abiotic factors (e.g., temperature decreases after rainfall), and methodology (e.g., most studies did not quantify rainfall amounts). Disparate research findings resulting from differences in study designs or when relationships between interacting variables vary under different ecological and spatiotemporal contexts are widespread in ecological studies (Catford et al., 2022).
However, when sufficient data are available on rainfall amounts and its timing relative to nest construction, results reveal that predation rates on newly constructed turtle nests are typically reduced when significant rainfall occurs during or soon after nest construction, in accord with a long-suspected effect of post-nest-construction rain in reducing the olfactory/visual signals of nest presence (Carr, 1952; Legler, 1954; Table 4). In contrast, nest predation rates are maximal and unaffected when rainfall precedes nesting or when little or no rain occurs soon after nest construction. Our case study on map turtles found high predation rates and nest survival times were short, precluding our ability to rigorously analyze the association between nest predation and rainfall amounts. However, close examination of the exact timing of both rainfall and predation revealed significantly lower predation rates when rain fell within 24 h after nesting and before the first predator encounter, indicating that rainfall during or after nesting may reduce nest predation.
Despite potential benefits in reducing nest predation rates, we found no marked propensity of turtles to nest during or before oncoming rain, either in the reviewed literature or in our case study, where the best fitted model explaining the propensity to nest found that map turtles were more likely to nest after dry days and that rainfall was not a major factor driving turtles to nest in our populations. Previous authors (e.g., Aresco, 2004; Muell et al., 2021) have suggested that nesting associations with rainfall vary among turtle populations and are likely context specific. It is recognized that factors other than rain on the day of nesting are implicated in nest construction timing, as many turtles nest on days without rainfall. Other abiotic influences on nest timing, such as air and water temperatures and time since last rainfall, tend to obscure the role of rainfall on nesting propensity as a single variable (Jackson and Walker, 1997). Conditions on nesting substrates (e.g., soil type, compaction, and moisture retention), impacting the effort, time, and feasibility of making nest excavations (Doody et al., 2003) may also influence local associations between rainfall amounts and subsequent nest construction activity. For example, while the softening of nesting substrates by rain was believed important in nest timing by Chelydra serpentina (Hammer, 1969), the Ouachita map turtle study sites in our case study are comprised of easily worked sand and nesting durations are short (median = 34.0 min, n = 221) and diurnal, reducing the chances of direct predation by nocturnal mammals on nesting females.
Biotic factors also influence nest construction timing and further obscure the isolated effect of rainfall as a single variable. For example, multiple-clutching female turtles are physiologically unable to respond to appropriate nesting conditions for an interval of time after a prior nesting event, thus reducing an individual turtle’s ability to time her nesting efforts to optimal conditions including, perhaps, periods before rainfall (Pike, 2008; Czaja et al., 2018; Muell et al., 2021). In a novel approach to isolate the impact of abiotic factors on nesting propensity in Chrysemys picta, Muell et al. (2021) attempted to control for this physiological variable by removing those turtles putatively unable to nest (individually marked turtles known to be within inter-nesting intervals) from various analyses. While this approach requires a population of marked turtles and may not be an option for some studies, it is a promising method to reduce some of the confounding variables underlying efforts to understand the responses of nesting turtles to meteorological conditions.
In studies where an association has been found, turtle nesting tends to occur not before rainfall, but during and after it, concurrent with relatively warm temperatures. Although nesting during rainfall also likely enhances nest survival, this timing may be due to factors more related to female turtle survivorship than to predation risk—such as reduced time on land, when freshwater turtles are most vulnerable to terrestrial predators (Spencer, 2002). A similar conclusion, based on the evolutionary trade-offs between adult and nest survival, was reached by Spencer (2002) for Emydura macquarii, in which nesting females appear to make nest location choices that maximize their own survival by nesting closer to water when they perceive the risk of fox predation to be high, at the cost of less-than-optimal incubation conditions for their eggs and greater levels of nest depredation. As long-lived, iteroparous adults, female turtles, maximizing their own survival, are likely to nest before significant rainfall at some point in their reproductive life by chance alone, and may have enhanced nest success as a result (Carr, 1952; Czaja et al., 2018).
To-date, research on the propensity of turtles to nest in temporal associations with rain and of the effect of rainfall on nest predation rates has involved only a small number of turtle species. Even within this small scope, these studies have sometimes produced conflicting results, potentially due to limitations in obtaining the necessary resolution on rainfall timing and amounts relative to individual nesting events (e.g., Aresco, 2004; Bowen and Janzen, 2005), variations in study rigor (from a few observed events to multi-year studies using statistical analyses), and other methodological differences. Comparative, interspecific studies using similar methodologies and analyses (time-lapse camera use and datasets amenable to meta-analysis), as well as intraspecific studies between populations of species with wide geographic ranges, would be especially useful in delineating the phylogenetic variation and factors underlying patterns of meteorological cueing in given contexts, especially when conducted over several years (Bowen and Janzen, 2005). However, the apparent variation in the association of nest timing and rainfall suggests that attempts to generalize its tendency or impacts on turtle hatchling recruitment will be problematic.
While the ability to sense barometric pressure change has been established for many organisms (e.g., for birds, Metcalfe et al., 2013; for anurans, Oseen and Wassersug, 2002), this review indicates a scarcity of related research for either marine or freshwater turtles. Several reviewed studies (almost all involving Chelodina and Emydura inhabiting hot and arid environments) reported turtle movements toward nesting areas before or concurrent with rainfall, suggesting the theoretical possibility of barometric changes in triggering the nesting response (e.g., Clay, 1981). However, barometric pressure changes preceding rainfall do not occur in isolation, but are accompanied by changes in air temperature, humidity levels, cloud cover, wind (Glickman, 2000), and perhaps even olfactory signals; all of which could be perceived by turtles, making the association of nesting behavior and barometric pressure indirect and correlative. In addition, many other studies, including ours, reported no relationship between nesting and decreasing barometric pressure (or with rainfall in general), suggesting that turtles were not responding to these potential indicators of future rainfall. Clearly more research—especially perhaps, laboratory-based studies where the behavioral responses of turtles to manipulated changes in air pressure can be isolated from the impacts of other meteorological variables that occur in nature—is needed before firm conclusions can be reached on the ability of turtles to detect changes in barometric pressure, including how this ability, or lack of it, may reflect phylogenic and genetic constraints (e.g., Oostra et al., 2018) and influence nest timing at differing taxonomic and geographic scales.
Ongoing anthropogenic climate change is predicted to introduce more stochastic variation into global temperature and precipitation patterns (Collins et al., 2013) and is expected to impact various parameters of turtle life history. For example, potential increases in nest success in areas with increased rainfall via reduced nest predation rates and decreases in nest success where rainfall frequencies and amounts decrease (Czaja et al., 2018), may continue to occur as functions of rainfall influences on nesting female behavior. However, changing rainfall patterns also impact air and substrate temperatures, nest site flooding potentials, and the vegetational cover on nesting areas, all of which affect hatchling recruitment. Whether turtles will be able to mount adaptive responses to changes in global precipitation patterns depends on rates of environmental and genomic change relative to inter-generation length, the amount of trait plasticity and heritable genetic variation within populations, and phylogenic constraints (e.g., Urban et al., 2014; Oostra et al., 2018; Dochtermann et al., 2019; Scheiner et al., 2020; Patrício et al., 2021). As animals of relatively low vagility, the potential for adaptive change in turtle populations also depends on the quality of available habitats and their distribution on the landscape (e.g., Valenzuela et al., 2019). While these variables are currently unknown for turtles overall, and will likely be highly context specific, their interplay and ultimate effects on turtle reproductive biology represent additional influences and challenges to chelonian persistence in the Anthropocene.
Author Contributions
GG wrote the first draft, conducted the literature search, and provided the Figures 1, 3, and Tables 1, 2, 4, 5. JD edited the first draft, wrote parts of subsequent drafts, and provided additional citations. SC and RD provided statistical analysis, Figure 2 and Table 3, and manuscript edits. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The senior author would like to thank A. Pidgeon for assistance with literature searches and the landowners of the nesting sites for allowing access to their properties. We would also like to acknowledge the efforts of several reviewers in improving the manuscript.
Footnotes
- ^ https://www.ncdc.noaa.gov/cdo-web
- ^ https://www.wunderground.com/history/daily/us/wi/spring-green/KLNR/date/2008-6-1
References
Aresco, M. J. (2004). Reproductive ecology of Pseudemys floridana and Trachemys scripta (Testudines: Emydidae) in Northwestern Florida. J. Herpetol. 38, 249–256. doi: 10.1670/169-03a
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using Lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bernstein, N. P., McCollum, S. A., and Black, R. W. (2015). How do predators locate nests of ornate box turtles (Terrapene ornata)? A field experiment. Herpetol. Conserv. Biol. 10, 44–53.
Booth, D. T. (2002). The breaking of diapause in embryonic broad-shelled river turtles (Chelodina expansa). J. Herpetol. 36, 592–596. doi: 10.2307/1565218
Bougie, T. A., Byer, N. W., Lapin, C. N., Peery, M. Z., Woodford, J. E., and Pauli, J. N. (2020). Wood turtle (Glyptemys insculpta) nest protection reduces depredation and increases success, but annual variation influences its effectiveness. Can. J. Zool. 98, 715–724. doi: 10.1139/cjz-2020-0064
Bowen, K. D., and Janzen, F. J. (2005). Rainfall and depredation of nests of the painted turtle, Chrysemys picta. J. Herpetol. 39, 649–652. doi: 10.1670/34-05n.1
Bowen, K. D., Spencer, R.-J., and Janzen, F. J. (2005). A comparative study of environmental factors that affect nesting in Australian and North American freshwater turtles. J. Zool. 267, 397–404. doi: 10.1017/s0952836905007533
Brooks, R. J., Shilton, C. M., Brown, G. P., and Quinn, N. W. S. (1992). Body size, age distribution, and reproduction in a northern population of wood turtles (Clemmys insculpta). Can. J. Zool. 70, 462–469. doi: 10.1139/z92-070
Buckardt, E., Glowacki, G. A., and Gibbs, J. P. (2020). Environmental cues that trigger nesting activity by Blanding’s Turtles (Emydoidea blandingii). Chelonian Conserv. Biol. 19, 67–71. doi: 10.2744/ccb-1393.1
Burger, J. (1977). Determinants of hatching success in diamond-back terrapin, Malaclemys terrapin. Am. Midl. Nat. 97, 444–464. doi: 10.2307/2425108
Burger, J., and Montevecchi, W. A. (1975). Nest site selection in the terrapin Malaclemys terrapin. Copeia 1975, 113–119. doi: 10.2307/1442413
Burke, V. J., Gibbons, J. W., and Green, J. L. (1994). Prolonged nesting forays by common mud turtles (Kinosternon subrubrum). Am. Midl. Nat. 131, 190–195. doi: 10.2307/2426622
Burke, V. J., Rathbun, S. L., Bodie, J. R., and Gibbons, J. W. (1998). Effect of density on predation rate for turtle nests in a complex landscape. Oikos 83, 3–11. doi: 10.2307/3546540
Buzuleciu, S. A., Crane, D. P., and Parker, S. L. (2016). Scent of disinterred soil as an olfactory cue used by raccoons to locate nests of diamond-backed terrapins (Malaclemys terrapin). Herpetol. Conserv. Biol. 11, 539–551.
Carr, A. F. (1952). Handbook of Turtles: The Turtles of the United States, Canada, and Baja California. Ithaca, NY: Comstock Publishing Associates, 542.
Catford, J. A., Wilson, J. R. U., Pyšek, P., Hulme, P. E., and Duncan, R. P. (2022). Addressing context dependence in ecology. Trends Ecol. Evol. 37, 158–170. doi: 10.1016/j.tree.2021.09.007
Cavalcanti, L. M. P., Paiva, L. V. D., and Franca, L. F. (2016). Effects of rainfall on bird reproduction in a semi-arid neotropical region. Zoologia 33:e20160018.
Clay, B. T. (1981). Observations on the breeding, biology, and behavior of the Long-necked tortoise, Chelodina oblonga. J. R. Soc. West. Aust. 4, 27–32.
Collins, M., Knutti, R., Arblaster, J., Dufresne, J.-L., Fichefet, T., Friedlingstein, P., et al. (2013). “Long-term climate change: projections, commitments and irreversibility,” in Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge: Cambridge University Press).
Congdon, J. D., Breitenbach, G. L., van Loben Sels, R. C., and Tinkle, D. W. (1987). Reproduction and nesting ecology of snapping turtles (Chelydra serpentina) in Southeastern Michigan. Herpetologica 43, 39–54.
Congdon, J. D., Tinkle, D. W., Breitenbach, G. L., and van Loben Sels, R. C. (1983). Nesting ecology and hatching success in the turtle Emydoidea blandingi. Herpetologica 39, 417–429.
Congello, K. (1978). Nesting and egg laying behavior in Terrapene carolina. Proc. Pa. Acad. Sci. 52, 51–56.
Czaja, R. A., Kanonik, A., and Burke, R. L. (2018). The effect of rainfall on predation of diamond-backed terrapin (Malaclemys terrapin) nests. J. Herpetol. 52, 402–405. doi: 10.1670/17-167
Czaja, R. A., Scholz, A. L., Figuertas, M. P., and Burke, R. L. (2020). The role of nest depth and site choice in mitigating the effects of climate change on an oviparous reptile. Diversity 12:151. doi: 10.3390/d12040151
Dawson, S. J., Adams, P. J., Huston, R. M., and Fleming, P. A. (2014). Environmental factors influence nest excavation by foxes. J. Zool. 294, 104–113. doi: 10.1111/jzo.12158
Dochtermann, N. A., Schwab, T., Berdal, M. A., Dalos, J., and Royauté, R. (2019). The heritability of behavior: a meta-analysis. J. Hered. 110, 403–410. doi: 10.1093/jhered/esz023
Doody, J. S., West, P., and Georges, A. (2003). Beach selection in pig-nosed turtles, Carettochelys insculpta. J. Herpetol. 37, 178–182. doi: 10.1670/0022-1511(2003)037[0178:bsinpn]2.0.co;2
Dore, M. H. I. (2005). Climate change and changes in global precipitation patterns: what do we know? Environ. Int. 31, 1167–1181. doi: 10.1016/j.envint.2005.03.004
Escalona, T., Valenzuela, N., and Adams, D. C. (2019). Do local environmental factors and lunar cycle influence timing and synchrony of oviposition of a turtle with strict nocturnal nesting? Diversity 11:78. doi: 10.3390/d11050078
Espinoza, T., Connell, M., Marshall, S., Beukeboom, R., and McDougal, A. (2018). Nesting behaviour of the endangered Mary River turtle: monitoring and modelling to inform e-flow strategies. Aust. J. Zool. 66, 15–26. doi: 10.1071/zo17044
Fahey, K. M. (1987). Aspects of the Life History of the River Cooter, Pseudemys concinna (LeConte), in the Tallapoosa River, Tallapoosa County, Alabama. Ph.D. thesis. Auburn, AL: Auburn University.
Feinberg, J. A., and Burke, R. L. (2003). Nesting ecology and predation of diamondback terrapins, Malaclemys terrapin, at gateway national recreation area, New York. J. Herpetol. 37, 517–526. doi: 10.1670/207-02a
Ferrara, C. R., Vogt, R. C., and Sousa-Lima, R. S. (2012). Turtle vocalizations as the first evidence of posthatching parental care in chelonians. J. Comp. Psychol. 127, 24–32. doi: 10.1037/a0029656
Flitz, B. A., and Mullin, S. J. (2006). Nest-site selection in the eastern box turtle, Terrapene carolina carolina, in Illinois. Chelonian Conserv. Biol. 5, 309–312. doi: 10.2744/1071-8443(2006)5[309:nsiteb]2.0.co;2
Frye, A., Hardy, K., Hedrick, A. R., and Iverson, J. B. (2017). Factors affecting nesting times in the painted turtle Chrysemys picta in Nebraska. Chelonian Conserv. Biol. 16, 44–51. doi: 10.2744/ccb-1208.1
Geller, G. A. (2012a). Notes on the nesting ecology of Ouachita map turtles (Graptemys ouachitensis) at two Wisconsin sites using trail camera monitoring. Chelonian Conserv. Biol. 11, 206–213. doi: 10.2744/ccb-0990.1
Geller, G. A. (2012b). Notes on the nest predation dynamics of Graptemys at two Wisconsin sites using trail camera monitoring. Chelonian Conserv. Biol. 11, 197–205. doi: 10.2744/ccb-0992.1
Geller, G. A. (2015). A test of substrate sweeping as a strategy to reduce raccoon predation of freshwater turtle nests, with insights from supplemental artificial nests. Chelonian Conserv. Biol. 14, 64–72. doi: 10.2744/ccab-14-01-64-72.1
Geller, G. A., and Casper, G. S. (2019). Late term embryos and hatchlings of Ouachita map turtles (Graptemys ouachitensis) make sounds within the nest. Herpetol. Rev. 50, 449–452.
Geller, G. A., Casper, G. S., and Halstead, B. J. (2020). Hatchling emergence ecology of Ouachita map turtles (Graptemys ouachitensis) on the lower Wisconsin River, Wisconsin. Chelonian Conserv. Biol. 19, 217–235.
Geller, G. A., and Parker, S. L. (2022). What are the primary cues used by mammalian predators to locate freshwater turtle nests? A critical review of the evidence. Front. Ecol. Evol. 9:784786. doi: 10.3389/fevo.2021.784786
Georges, A. (1984). Observations on the nesting and natural incubation of the long-necked tortoise Chelodina expansa. Herpetofauna 15, 27–31.
Hammer, D. A. (1969). Parameters of a marsh snapping turtle population at Lacreek Refuge, South Dakota. J. Wildl. Manag. 33, 995–1005. doi: 10.2307/3799337
Harding, J. H., and Bloomer, T. J. (1979). The wood turtle, Clemmys insculpta…a natural history. Bull. N. Y. Herpetol. Soc. 15, 9–26.
Hess, L. J. T., Hinckley, E.-L. S., Robertson, G. P., Hamilton, S. K., and Matson, P. A. (2018). Rainfall intensification enhances deep percolation and soil water content in tilled and no-till cropping systems of the US Midwest. Vadose Zone J. 17, 1–12. doi: 10.2136/vzj2018.07.0128
Jackson, D. R., and Walker, R. N. (1997). Reproduction in the Suwannee cooter, Pseudemys concinna suwanniensis. Bull. Fla. Mus. Nat. Hist. 41, 69–167.
Kuchling, G. (1993). Nesting of Pseudemydura umbrina (Testudines: Chelidae): the other way round. Herpetologica 49, 479–487.
Lazure, L., Pare, P., Bouthillier, L., and Galois, P. (2019). Nesting biology and conservation of a northern population of spiny softshell turtles (Apalone spinifera). Herpetol. Conserv. Biol. 14, 659–667.
Legler, J. M. (1954). Nesting habits of the Western painted turtle, Chrysemys picta bellii (Gray). Herpetologica 10, 137–144.
Lindbo, D. L., Kozlowski, D. A. and Robinson, C. (2012). Know Soil, Know Life. Madison, WI: Soil Science Society of America, 206.
Litzgus, J. D., and Brooks, R. J. (1998). Reproduction in a northern population of Clemmys guttata. J. Herpetol. 32, 252–259. doi: 10.2307/1565305
Lovich, J. E., Ennen, J. R., Agha, M., and Gibbons, J. W. (2018). Where have all the turtles gone, and why does it matter? BioScience 68, 771–781. doi: 10.1093/biosci/biy095
Marchand, M. N., and Litvaitis, J. A. (2004). Effects of landscape composition, habitat features, and nest distribution on predation rates of simulated turtle nests. Biol. Conserv. 117, 243–251. doi: 10.1016/j.biocon.2003.07.003
McCosker, J. (2002). Chelodina expansa (broad shell river turtle) and Emydura signata (Brisbane shortneck turtle) Reproduction. Herpetol. Rev. 33, 198–199.
Metcalfe, J., Schmidt, K. L., Kerr, W. B., Guglielmo, C. G., and MacDougall-Shackleton, S. A. (2013). White-throated sparrows adjust behaviour in response to manipulations of barometric pressure and temperature. Anim. Behav. 86, 1285–1290. doi: 10.1016/j.anbehav.2013.09.033
Mitchell, J. C., and Klemens, M. W. (2000). “Primary and secondary effects of habitat alteration,” in Turtle Conservation, ed. M. W. Klemens (Washington, DC: Smithsonian Institution Press), 5–32.
Muell, M. R., Carter, A. L., and Janzen, F. J. (2021). Modeling onset of hourly nesting activity in a freshwater turtle using abiotic variables and physiological capability. J. Herpetol. 55, 11–20.
Nagle, R. D., Lutz, C. L., and Pyle, A. L. (2004). Overwintering in the nest by hatchling map turtles (Graptemys geographica). Can. J. Zool. 82, 1211–1218. doi: 10.1139/z04-096
Najbar, B., and Szuszkiewicz, E. (2005). Reproductive ecology of the European pond turtle Emys orbicularis (Linnaeus, 1758) (Testudines: Emydidae) in western Poland. Acta Zool. Cracov. 48, 11–19. doi: 10.3409/173491505783995752
Oehler, J. D., and Litvaitis, J. A. (1996). The role of spatial scale in understanding responses of medium-sized carnivores to forest fragmentation. Can. J. Zool. 74, 2070–2079. doi: 10.1139/z96-235
Oostra, V., Saastamoinen, M., Zwaan, B. J., and Wheat, C. W. (2018). Strong phenotypic plasticity limits potential for evolutionary responses to climate change. Nat. Commun. 9:1005. doi: 10.1038/s41467-018-03384-9
Oseen, K. L., and Wassersug, R. J. (2002). Environmental factors influencing calling in sympatric anurans. Oecologica 133, 616–625. doi: 10.1007/s00442-002-1067-5
Pallas, D. C. (1960). Observations on a nesting of the wood turtle, Clemmys insculpta. Copeia 1960, 155–156. doi: 10.2307/1440225
Palomino-González, A., López-Martínez, S., and Rivas, M. L. (2020). Influence of climate and tides on the nesting behaviour of sea turtles. J. Exp. Mar. Biol. 527:151378. doi: 10.1016/j.jembe.2020.151378
Patrício, A. R, Hawkes, L. A., Monsinjon, J. R., Godley, B. J., and Fuentes, M. M. P. B. (2021) Climate change and marine turtles: recent advances and future directions. Endang. Species Res. 44, 363–395. doi: 10.3354/esr01110
Pike, D. A. (2008). Environmental correlates of nesting in loggerhead turtles, Caretta caretta. Anim. Behav. 76, 603–610. doi: 10.1016/j.anbehav.2008.04.010
Plummer, M. V. (1976). Some aspects of nesting success in the turtle, Trionyx muticus. Herpetologica 36, 353–359.
Premkishore, G., and Chandran, M. R. (1996). Nesting studies of two freshwater turtles (Lissemys punctata punctata and Melanochelys trijuga trijuga) of Tamil Nadu, India, in the context of their conservation. Ann. Sci. Nat. Zool. Biol. Anim. 17, 99–104.
Prugh, L. R., Stoner, C. J., Epps, C. W., Bean, W. T., Ripple, W. J., Laliberte, A. S., et al. (2009). The rise of the mesopredator. Bioscience 59, 779–791. doi: 10.1525/bio.2009.59.9.9
R Core Team (2017). R: A Language and Environment for Statistical Computing. Available Online at: https://www.R-project.org/ (accessed November 2021).
Rahman, S., and Burke, R. L. (2010). “Evaluating nest protectors for turtle conservation,” in Final Reports of the Tibor T. Polgar Fellowship Program, 2009, eds D. J. Yozzo, S. H. Fernald, and H. Andreyko (New York, NY: Hudson River Foundation), 1–23.
Randriamahazo, H. J. A. R., and Mori, A. (2001). Egg-laying activities and reproductive traits in females of Oplurus cuvieri cuvieri. J. Herpetol. 35, 209–217. doi: 10.2307/1566110
Refsnider, J. M., and Janzen, F. J. (2010). Putting eggs in one basket: ecological and evolutionary hypothesis for variation in oviposition-site choice. Annu. Rev. Ecol. Evol. Syst. 41, 39–57. doi: 10.1146/annurev-ecolsys-102209-144712
Riley, J. L., and Litzgus, J. D. (2014). Cues used by predators to detect freshwater turtle nests may persist late into incubation. Can. Field Nat. 128, 179–188. doi: 10.22621/cfn.v128i2.1583
Roosenburg, W. M. (1994). Nesting habitat requirements of the Diamondback Terrapin: a geographic comparison. Wetl. J. 6, 8–11.
Scheiner, S. M., Barfield, M., and Holt, R. D. (2020). The genetics of phenotypic plasticity. XVII. Response to climate change. Evol. Appl. 13, 388–399. doi: 10.1111/eva.12876
Schofield, G., Hobson, V. J., Lilley, M. S., Katselidis, K. A., Bishop, C. M., Brown, P., et al. (2010). Inter-annual variability in the home range of breeding turtles: implications for current and future conservation management. Biol. Conserv. 143, 722–730. doi: 10.1016/j.biocon.2009.12.011
Schwanz, L. E., Spencer, R.-J., Bowden, R. M., and Janzen, F. J. (2010). Climate and predation dominate juvenile and adult recruitment in a turtle population with temperature-dependent sex determination. Ecology 91, 3016–3026. doi: 10.1890/09-1149.1
Sexton, O. J. (1959). Spatial and temporal movements of a population of the painted turtle, Chrysemys picta marginata (Agassiz). Ecol. Monogr. 29, 113–140. doi: 10.2307/1942200
Shealy, R. M. (1976). The natural history of the Alabama map turtle, Graptemys pulchra Baur, in Alabama. Bull. Fla. State Mus. Biol. Sci. 21, 47–111.
Soanes, R., Peters, A., Delhey, K., and Doody, J. S. (2015). The influence of nest-site choice and predator sensory cues on nesting success in the Crimson Finch (Neochmia phaeton). Emu Aust. Ornithol. 115, 317–325. doi: 10.1071/mu14046
Spencer, R.-J. (2002). Experimentally testing nest site selection: fitness trade-offs and predation risk in turtles. Ecology 83, 2136–2144. doi: 10.1890/0012-9658(2002)083[2136:etnssf]2.0.co;2
Stott, P. (1988). Terrestrial Movements of the Freshwater tortoise Chelodina longicollis. M.Sc. thesis. Adelaide: University of Adelaide.
Temple, S. A. (1987). Predation on turtle nests increases near ecological edges. Copeia 1987, 250–252. doi: 10.2307/1446069
Thomas, K. (1972). The Annual Cycle of Reproduction of the Emydine Turtle, Pseudemys floridana floridana (Testudinata, Testudinidae) with Observations on its Ecology. M.Sc. thesis. Auburn, AL: Auburn University.
Thompson, M. B. (1983). The Physiology and Ecology of the Eggs of the Pleurodiran Tortoise Emydura macquarii (Gray), 1831. Ph.D. thesis. Adelaide: University of Adelaide.
Tucker, J. K. (1997). Natural History Notes on Nesting, Nests, and Hatchling Emergence in the Red-Eared Slider Turtle, Trachemys scripta elegans, in West-Central Illinois, Vol. 140. Champaign, IL: Illinois Natural History Survey, 1–13.
Urban, M. C., Richardson, J. L., and Freidenfelds, N. A. (2014). Plasticity and genetic adaptation mediate amphibian and reptile responses to climate change. Evol. Appl. 7, 88–103. doi: 10.1111/eva.12114
Valenzuela, N., Literman, R., Neuwald, J. L., Mizoguchi, B., Iverson, J. B., Riley, J. L., et al. (2019). Extreme thermal fluctuations from climate change unexpectedly accelerate demographic collapse of vertebrates with temperature-dependent sex determination. Sci. Rep. 9:4254. doi: 10.1038/s41598-019-40597-4
Vestjens, W. J. M. (1969). Nesting, egg-laying, and hatching of the snake-necked tortoise at Canberra, A.C.T. Aust. Zool. 15, 141–149.
Vitt, L. J., and Lacher, T. E. Jr. (1981). Behavior, habitat, diet, and reproduction of the Iguanid lizard, Polychrus acutirostris, in the caatinga of Northeastern Brazil. Herpetologica 31, 53–63.
Vogt, R. C. (1980). Natural history of the map turtles Graptemys pseudogeographica and Graptemys ouachitensis in Wisconsin. Tulane Stud. Zool. Bot. 22, 17–48.
Voves, K. C., Mitchell, T. S., and Janzen, F. J. (2016). Does natural nest camouflage reduce turtle nest predation? Am. Midl. Nat. 176, 166–172. doi: 10.1674/0003-0031-176.1.166
Walde, A. D., Bider, J. R., Masse, D., Saumure, R., and Titman, R. D. (2007). Nesting ecology and hatching success of the wood turtle, Glyptemys insculpta, in Quebec. Herpetol. Conserv. Biol. 2, 49–60.
Wilhoft, D. C., Del Baglivo, M. G., and Del Baglivo, M. D. (1979). Observations on mammalian predation of snapping turtle nests (Reptilia, Testudines, Chelydridae). J. Herpetol. 13, 435–438. doi: 10.2307/1563478
Wilson, D. S., Mushinsky, H., and McCoy, E. (1999). Nesting behavior of the striped mud turtle (Kinosternon baurii) (Testudines: Kinosternidae). Copeia 1999, 958–968. doi: 10.2307/1447971
Wirsing, A. J., Phillips, J. R., Obbard, M. E., and Murray, D. L. (2012). Incidental nest predation in freshwater turtles: inter- and intraspecific differences in vulnerability are explained by relative crypsis. Oecologia 168, 977–988. doi: 10.1007/s00442-011-2158-y
Keywords: nest predation, predator cues, nesting, rain, barometric pressure, Reptilia, Testudines
Citation: Geller GA, Doody JS, Clulow S and Duncan RP (2022) Do Freshwater Turtles Use Rainfall to Increase Nest Success? Front. Ecol. Evol. 10:852175. doi: 10.3389/fevo.2022.852175
Received: 10 January 2022; Accepted: 13 April 2022;
Published: 19 May 2022.
Edited by:
Jordi Figuerola, Doñana Biological Station (CSIC), SpainReviewed by:
Tom Langen, Clarkson University, United StatesRonel Nel, Nelson Mandela University, South Africa
Copyright © 2022 Geller, Doody, Clulow and Duncan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gregory A. Geller, Z2dlbGxlcjU0QGdtYWlsLmNvbQ==
 Gregory A. Geller
Gregory A. Geller J. Sean Doody
J. Sean Doody Simon Clulow
Simon Clulow Richard P. Duncan
Richard P. Duncan