- 1Hamilton College, Clinton, NY, United States
- 2Florida Geological Survey, Tallahassee, FL, United States
- 3Florida Museum of Natural History, Gainesville, FL, United States
- 4Coastal Plains Institute, Crawfordville, FL, United States
Florida’s freshwater spring and river ecosystems have been deteriorating due to direct and indirect human impacts. However, while the conservation and restoration strategies employed to mitigate these effects often rely on faunal surveys that go back several decades, the local ecosystem shifts tend to have much deeper roots that predate those faunal surveys by centuries or millennia. Conservation paleobiology, an approach which enhances our understanding of the past states of ecosystems, allows for comparison of modern faunal communities with those prior to significant human impacts. This study examines the historical record of freshwater mollusk assemblages from two spring-fed river systems, the Wakulla and Silver/Ocklawaha Rivers. Specifically, we compared fossil assemblages (latest Pleistocene - early Holocene) and live mollusk assemblages in the two targeted river systems. Bulk sampling of the fossil record (20 sites; 70 samples; 16,314 specimens) documented relatively diverse mollusk assemblages that consist of a suite of native freshwater species that is similar across the studied systems. In contrast, sampling of live communities (24 sites; 138 samples; 7,572 specimens) revealed depauperate species assemblies characterized by the absence of multiple native freshwater species commonly found in fossil samples, the widespread presence of introduced species, and dominance of brackish-tolerant species at the lower Wakulla River sites. Unlike fossil mollusk assemblages, live mollusk assemblages differ notably between the two river systems due to differences in relative abundance of introduced species (Melanoides tuberculata and Corbicula fluminea) and the presence of brackish-tolerant mollusks in the coastally influenced Wakulla River. The diverse, exclusively freshwater mollusk associations comparable across multiple river systems documented in the fossil record provide a historical perspective on the past state of freshwater river ecosystems complementing data provided by modern surveys. The conservation paleobiology approach used in this study reinforces the importance of considering the historical ecology of an ecosystem and the utility of the fossil record in providing a historical perspective on long-term faunal changes.
Introduction
Humans have lived around and exploited Florida’s springs and rivers for at least 13,000 years (Martin, 1966; Milanich, 1994). Modern development around freshwater springs began as early as the 1820s, initiating a long interval of gradually intensifying human impacts on water quality and ecosystem health (Revels, 1990; King, 2004). Today, these spring-fed river systems are increasingly threatened by invasive/introduced species (Bogan, 2006; Wingard et al., 2008; Nico et al., 2009; Kusnerik et al., 2020), excessive nutrient inputs (Turner and Rabalais, 1991; Turner et al., 2006; Liu et al., 2009; Heffernan et al., 2010; Bricker et al., 2014), vegetation loss/shifts (Brainwood et al., 2006; Lauretta et al., 2019), decreasing waterflow (Weber and Perry, 2006), sea level rise (Donoghue, 2011; Hong et al., 2014), and salinity fluctuations (Donoghue, 2011; Hong et al., 2014).
Recognizing these threats has prompted a renewed effort in recent years to study, conserve, and restore Florida’s spring and river ecosystems through environmental surveys, restoration plans, and management actions by various state and private agencies (Howard T. Odum Florida Springs Institute, 2014; Wetland Solutions Inc, 2014; Florida Department of Environmental Assessment and Restoration, 2015). However, although anthropogenic impacts to spring ecosystems have been ongoing for centuries, live faunal surveys encompass only the last few decades (Odum, 1957; Knight, 1980, 1983; Munch et al., 2006). These recent efforts have documented conditions in already-altered ecosystems and, as such, provide critical data on changes that have taken place over the last several decades. However, we still lack critical information on the historical state of these ecosystems as they existed prior to substantial human impacts.
Conservation paleobiology is a rapidly emerging discipline that uses fossil or subfossil assemblages to provide historical ecological context for altered ecosystems. These approaches use fidelity or discordance between live, dead, and fossil assemblages, and other related methods, to recognize spatial and temporal biotic changes and provide long-term baseline assessments that can assist conservation and restoration efforts (e.g., Kowalewski et al., 2000; Jackson et al., 2001; Kidwell, 2007, 2013; Yanes, 2013; Hyman et al., 2019). Whereas these approaches have been used extensively in marine (e.g., Kidwell, 2007, 2013; Hyman et al., 2019), terrestrial (e.g., Yanes, 2013; Barnosky et al., 2017), estuarine/lagoonal (e.g., Barbieri et al., 2020), and freshwater (e.g., Alin and Cohen, 2004; Brown et al., 2005; Czaja et al., 2019) settings, spring-fed fluvial systems have been comparatively understudied. The primary goal of this study was to apply conservation paleobiological approaches to two spring-fed, fluvial systems in Florida. By comparing the compositional fidelity between live, dead, and fossil assemblages, the study aimed to document the faunal composition of freshwater molluscan communities prior to substantial human impacts, assess long-term ecological changes to those communities, and provide historical context for restoration/conservation efforts in local freshwater ecosystems.
Mollusks were used because their biomineralized shells have high potential for preservation in the freshwater fossil record, and their larger populations make collection of meaningful sample sizes feasible (Boardman et al., 1987; Kusnerik et al., 2020). Radiocarbon dates on individual fossil specimens (Kusnerik et al., 2020) revealed that freshwater mollusk shells from the studied rivers came predominantly from individuals that were late Pleistocene to early Holocene in age (18,217 to 7,087 cal BP), coinciding with hydrologic activation of flow in freshwater springs throughout Florida (Balsillie and Donoghue, 2011; Donoghue, 2011; O’Donoughue, 2015; Kusnerik et al., 2020). The radiocarbon dating efforts also indicate that mollusk assemblages were time-averaged over hundreds to thousands of years, constituting an averaged archive of long-term paleoecological conditions in the study system (Kusnerik et al., 2020). In more general terms, we focus here on mollusks because they are often used as environmental indicators (Williams et al., 2014), are sensitive to changes in water conditions (Montagna et al., 2008; Williams et al., 2014), can archive geochemical changes in aquatic habitats in their shell structure (Brown et al., 2005; Brainwood et al., 2006; Williams et al., 2014), and often provide ecosystem services including water filtration, substrate consolidation, and habitat structuring (Williams et al., 2014; Vaughn, 2018).
Materials and Methods
Study Area and Field Methods
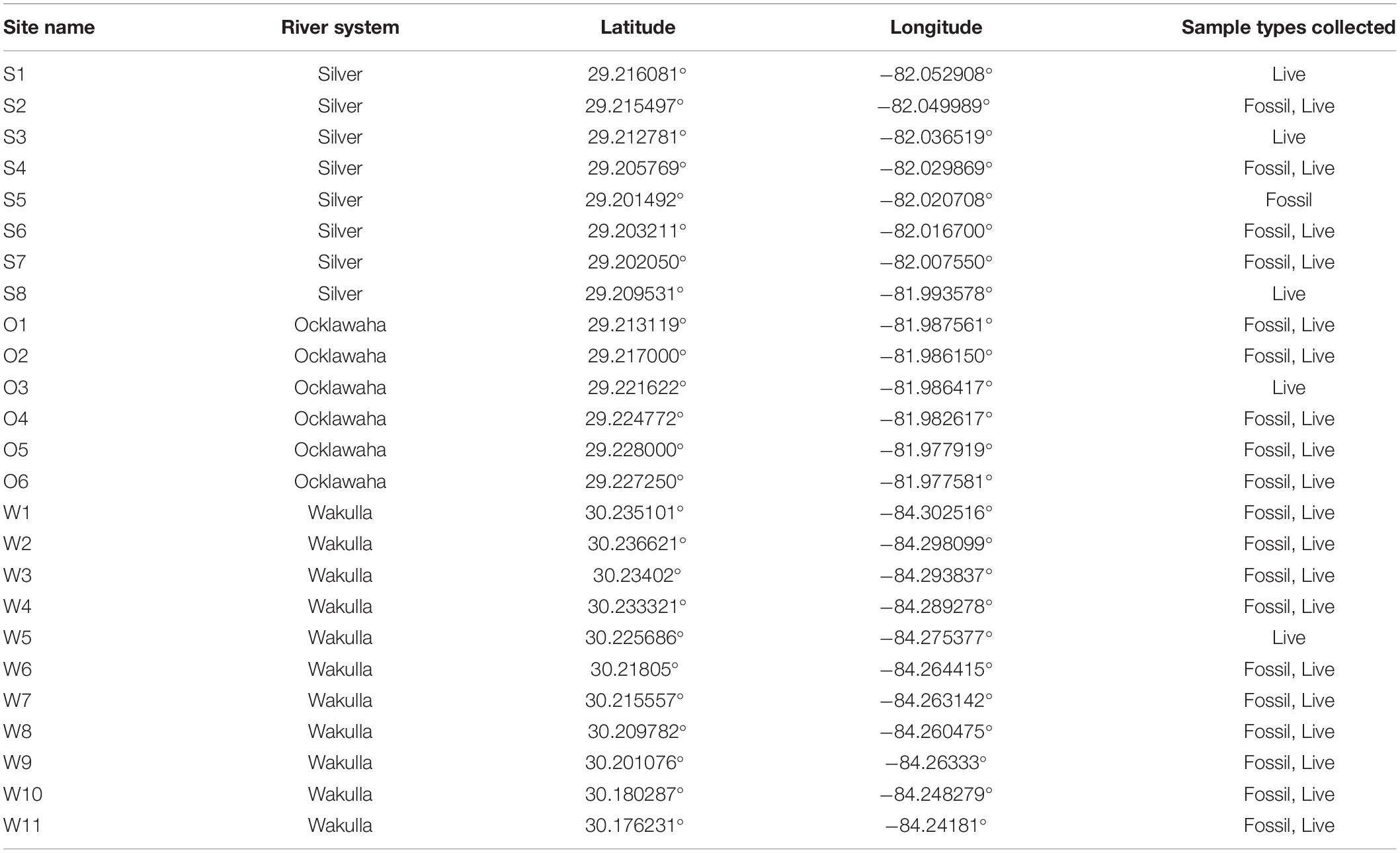
Samples were collected from two spring-fed river systems in Florida (Figure 1). These two systems, the Wakulla River and the Silver and Ocklawaha Rivers, were selected because they (1) vary in magnitude of human impacts, from severe (Silver River) to reduced (portions of the Wakulla River); (2) represent a range of salinity regimes, from fully freshwater (Silver/Ocklawaha Rivers) to coastally influenced (Wakulla River); and (3) afford easy logistical access.
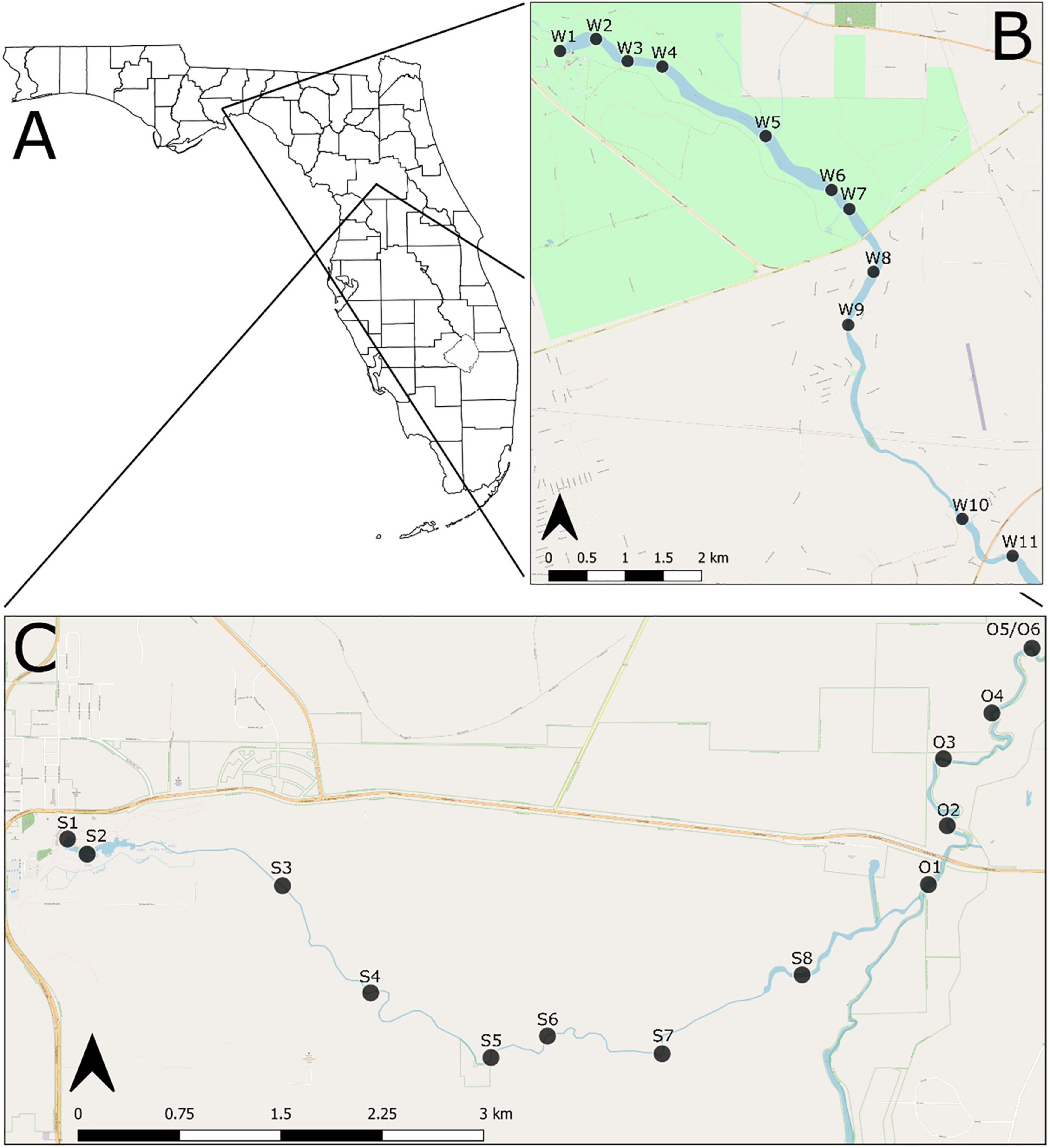
Figure 1. (A) General location of the study area; (B) sampling sites along the Wakulla River; (C) sampling sites along the Silver and Ocklawaha Rivers. Sites along the Wakulla River begin with a “W,” along the Silver River begin with an “S,” and along the Ocklawaha River begin with an “O”.
The Wakulla River, located in Wakulla County, was sampled at 11 sites along the uppermost 11 km of the river (Figure 1B). Sites were distributed longitudinally starting at Wakulla Spring and continuing downstream to approximately 4 km upriver of the confluence of the Wakulla and St. Marks Rivers. Seven sites were located within Edward Ball Wakulla Springs State Park, which includes the headspring and uppermost 5 km of the river. The remaining four sites were in a publicly accessible portion of the lower river. In the sampled area, the Wakulla River is primarily meandering, with a short, braided section in the lower park. The river flows over undifferentiated Holocene and Pleistocene sediments, which overlie limestone deposits of the lower Miocene St. Marks Formation. Bottom substrates include peat, mud, silt, quartz sand, and limestone hardgrounds.
The Silver River, located in north-central Florida, Marion County, was sampled at eight sites along its 7.5 km length, from Mammoth Spring (main spring of the Silver Springs group) to the confluence with the Ocklawaha River (Figure 1C). An additional six sites were sampled on the Ocklawaha River, along a 3.5 km stretch downstream of the confluence point. The headspring and upper portion of the Silver River are located within Silver Springs State Park. Both the Silver and Ocklawaha are meandering rivers within the sampled regions. Both flow over undifferentiated Holocene and Pleistocene sediments, which overlie the upper Eocene Ocala Limestone. Bottom substrates include silts, clays, muds, limestone hardgrounds, and limestone cobbles.
Two types of samples were collected using SCUBA: live and fossil assemblages. Live assemblages represent timed (5, 10, or 15 min) hand-collection of living specimens by divers at all habitat types present at each site. Habitats typically included leaf litter, submerged logs, submerged vegetation, floating vegetation, mid-river channel sands and muds, and emergent shoreline vegetation. Live surveys were conducted between 2016 and 2019 (Table 1).
Fossil samples were acquired through in situ, bulk collections of shell material from submerged vertical outcrops or small marl exposures on the riverbed. Outcrops and marls were composed of white to tan shell-rich clays with occasional layers of carbonate silt. Some outcrops contained a poorly indurated, dark-colored, organic-rich silt layer. When possible, outcrop exposures were sampled along a vertical profile, with each sample representing a rectangular cuboid spanning 5-10 cm vertically, 50-100 cm horizontally along the outcrop wall, and 10–20 cm perpendicularly into the outcrop exposure. Because of their size, smaller marl exposures on the riverbed were not sampled by cuboids, but rather, were sampled until one gallon of sediment was obtained. All samples were wet-sieved using 1 mm mesh sieves. Samples containing larger numbers of specimens were subsampled using strategies designed to minimize size-selection biases (sample splitters or multiple random subsets).
Death assemblages, the loose collection of shell material that accumulates in scours, divots, or similar erosional depressions on the river bottom, are also commonly used in conservation paleobiological research (e.g., Brown et al., 2005). While molluscan death assemblages were collected for this study, their complicated, multi-sourced provenance (Kusnerik et al., 2020) led to their exclusion from further analyses.
Specimen Description and Analysis
A total of 208 samples (live = 138, fossil = 70) were collected. Specimens were identified to the lowest taxonomic level, yielding 24,030 specimens that represent 20 taxa. Live samples from each site were pooled together into a single assemblage, regardless of their time of collection, to mitigate the effects of seasonal or annual variations in community composition. Bivalve counts were corrected to a minimum number of individuals by halving specimen counts. Two terrestrial gastropods (Polygyra septemvolva and Daedalochila auriculata) present in fossil assemblages were removed as live, terrestrial species were not sampled. The brackish-tolerant gastropod Vitta usnea, not observed in fossil samples, was removed as an ecological outlier in ordination analyses. Unionid mussels were also removed from ordination and pairwise comparisons because of taphonomic concerns, as their nacreous-aragonite shells do not preserve well in the fossil record (Simpson, 1899; Hinch and Green, 1988; Roper and Hickey, 1994; Wolverton et al., 2010; Williams et al., 2014). Exclusion of terrestrial species, freshwater mussels, and the brackish-tolerant V. usnea did not result in major changes to diversity analyses.
Samples with fewer than 20 specimens were removed from analyses to mitigate the effects of small sample sizes. Taxon abundance counts were standardized using double relativization (Wisconsin standardization). All analyses were performed in R (R Core Team, 2018) using custom written scripts and the package “vegan” (Oksanen et al., 2019). Sample-standardized richness was calculated using the “rarefy” function in the package “vegan” with the sample cutoff of 20 specimens. Ordination analyses were used to visualize compositional differences among samples and sample groups. Data were ordinated using Non-metric Multidimensional Scaling (nMDS) fit into k = 2 dimensions with 50 random restarts. Performance was assessed using stress values, with values < 0.2 deemed acceptable. Bray-Curtis similarity was used to quantify the compositional similarity between and within samples of both assemblage types and river systems. Differences in mean pairwise similarity between sample sets were statistically evaluated using randomization with distances resampled from pooled data to generate a sampling distribution under the null model of no difference in means. All p values were computed using the percentile approach with p = IE/IT, where IE is the number of extreme iterations (i.e., number of replicate samples in which the resampled absolute difference in means exceeded the true absolute difference in means observed in the data) and IT is a total number of iterations. When IE = 0, p was reported as p < 1/IT. Each randomization estimate was based on 10,000 iterations (pilot analyses indicated that estimates of p values stabilized at IT < 8,000 iterations). Permutational multivariate analysis of variance (PERMANOVA), based on pairwise Bray-Curtis dissimilarities between samples, was conducted to assess whether sample groups were distinguishable statistically between assemblage types and river systems.
Results
Fossil samples contained 16,314 specimens representing at least 14 species of freshwater mollusks (12 gastropods, one pea clam, and at least one species of unionid mussel) (Table 2). No introduced or brackish-tolerant species were present. Sample-standardized richness (n = 20 specimens) ranged from 2 to 6.38 (mean = 4.53). Whereas all fossil species were present in the samples from the Silver and Ocklawaha Rivers, only 11 were present in Wakulla River samples. The Wakulla samples were missing unionid mussels, indeterminate ancylid gastropods, and Physella sp., all of which were rare (n = 9, 3, and 2, respectively) in Silver/Ocklawaha fossil samples. Common species (> 10%) in both systems included Planorbella duryi, Notogillia wetherbyi, and Elimia floridensis. Physella hendersoni was also common in Silver/Ocklawaha samples. Pairwise similarities between fossil sites, estimated as Bray-Curtis similarity, ranged from 0.06 to 0.9 (mean = 0.51) for Wakulla fossil sites (Figure 2A) and from 0.04 to 0.88 (mean = 0.36) for Silver/Ocklawaha fossil sites (Figure 2B). Pairwise similarities between fossil sites compared across the two river systems ranged from 0 to 0.87 (mean = 0.3) (Figure 2C). The difference in mean pairwise similarity was statistically significant between within-Wakulla and within-Silver/Ocklawaha comparisons (Supplementary Figure 1B1,B2 and Table 3). The difference was also statistically significant when comparing pairwise similarities within rivers to those between rivers (Supplementary Figures 1F1,F2 and Table 3).
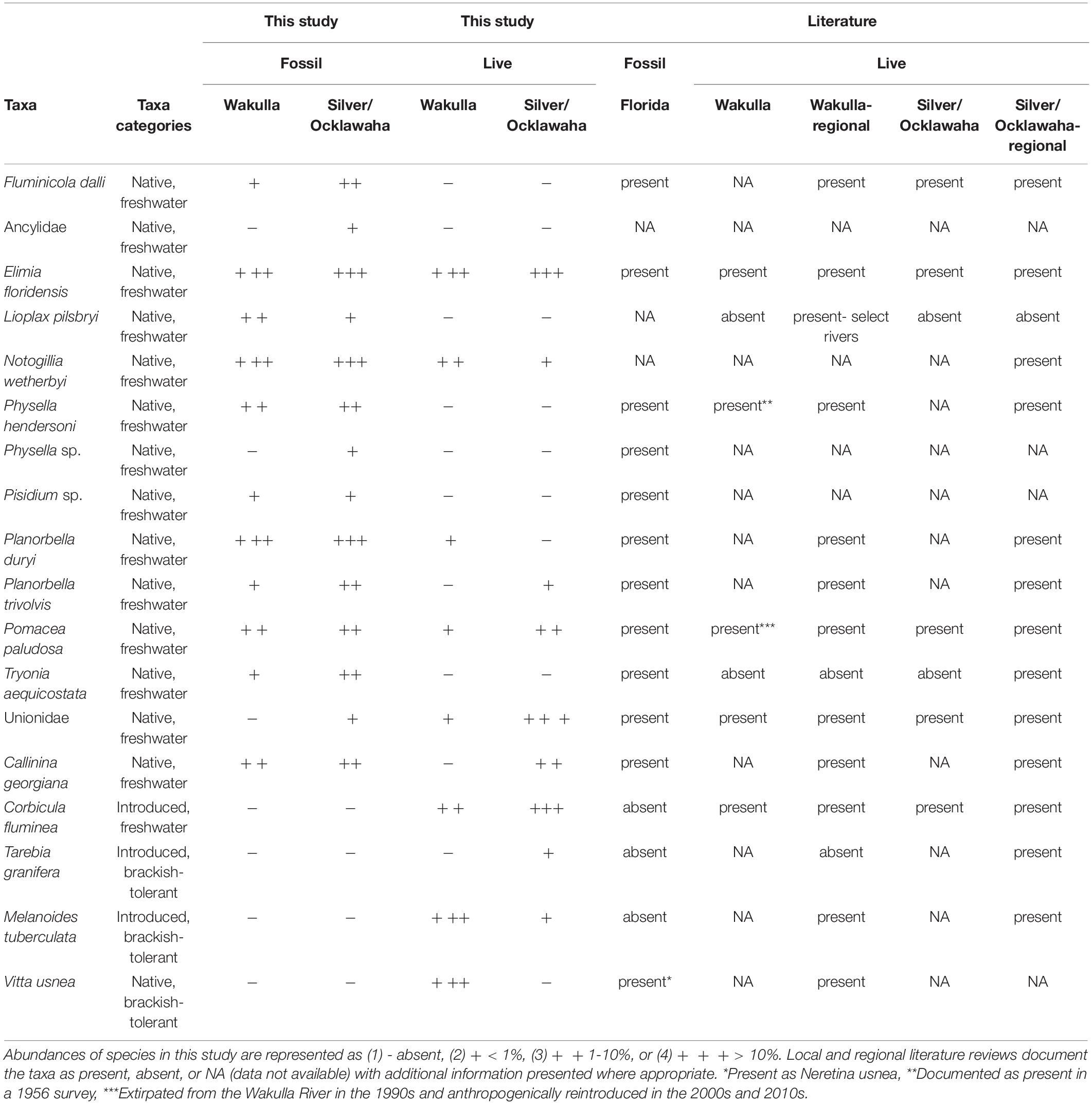
Table 2. Abundances of aquatic species present in fossil and live samples compared to local and regional surveys.
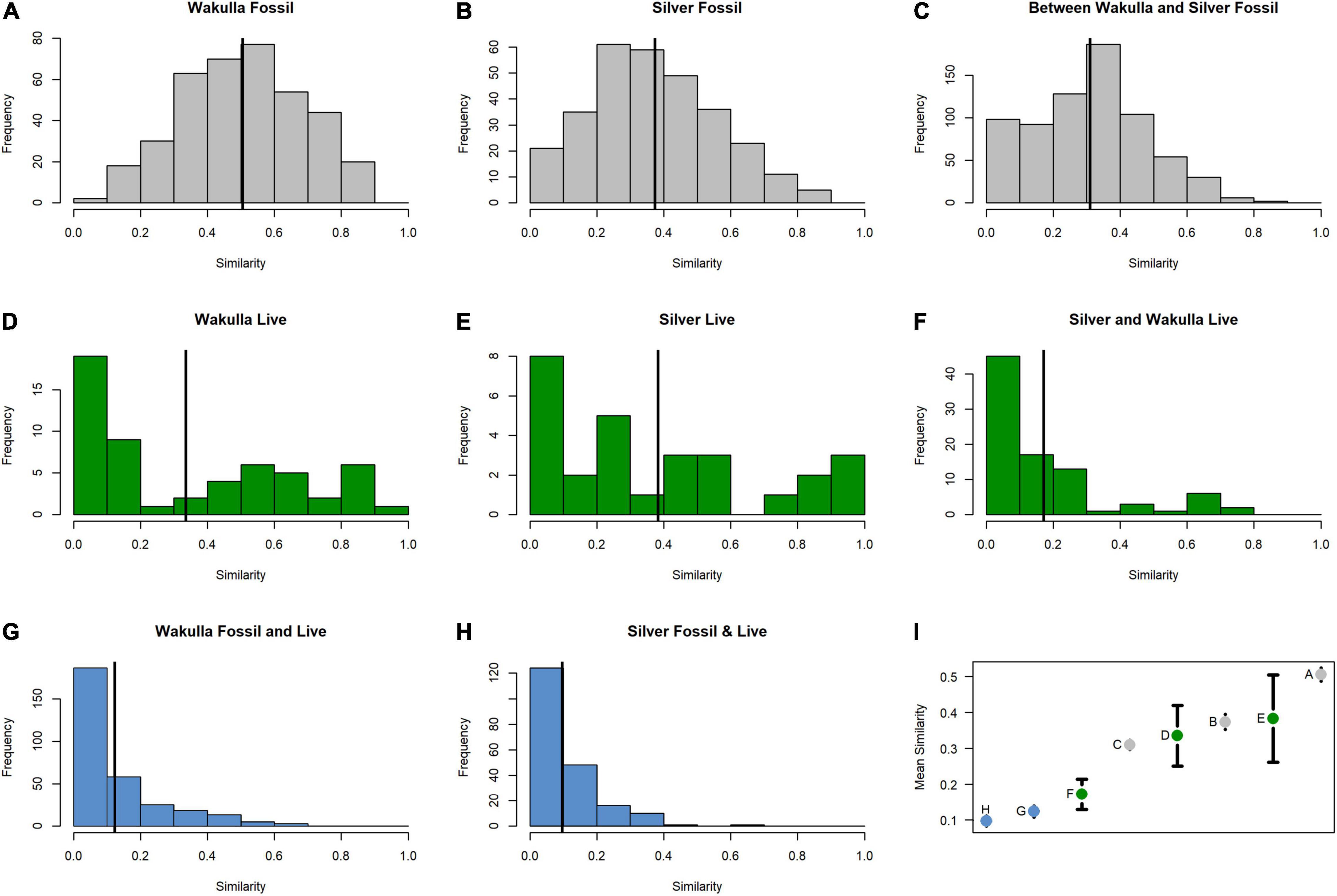
Figure 2. Pairwise comparisons of Bray-Curtis similarities between fossil (gray) samples (A) within the Wakulla River, (B) within the Silver and Ocklawaha Rivers, (C) between the Wakulla, Silver, and Ocklawaha Rivers, between live (green) samples (D) within the Wakulla River, (E) within the Silver and Ocklawaha Rivers, (F) between the Wakulla, Silver, and Ocklawaha Rivers, and between fossil and live (blue) samples (G) within the Wakulla River and (H) within the Silver River. (I) Mean similarities of the eight pairwise comparisons with 95% confidence intervals. (A–H) represent their respective panels in Figure 2.

Table 3. Probability of observed mean pairwise similarity between sample sets evaluated using randomly generated null model of no difference in means.
Pooling live samples by site produced a total of 25 samples, representing 7,572 specimens of 11 species (Table 2). These included eight native, freshwater mollusks (seven gastropods and one mussel), two non-native freshwater species (one gastropod and one clam), and one brackish-tolerant gastropod species (V. usnea). Sample-standardized species richness at sites (n = 20) ranged from 1 to 5.63 (mean = 2.63). Seven species were shared between the live communities of the Silver/Ocklawaha and Wakulla Rivers. An additional three species were present only in the Silver/Ocklawaha Rivers (Callinina georgiana, Planorbella trivolvis, and Tarebia granifera), whereas one species, the brackish-tolerant V. usnea was present only in the Wakulla River. Only one species was well represented (> 10%) in both systems, E. floridensis (Silver/Ocklawaha = 15.11% and Wakulla = 42.45%). In Silver/Ocklawaha River samples, other well represented species included unionid mussels (57.59%) and Corbicula fluminea (18.53%). In the Wakulla River, the common taxa were Melanoides tuberculata (29.56%) and V. usnea (23.19%). Between site Bray-Curtis similarity for live Wakulla samples ranged from 0 to 0.98 (mean = 0.36) and for the Silver/Ocklawaha samples ranged from 0.04 to 0.88 (mean = 0.36) (Figures 2D,E). Within-river mean similarity was not significantly different between the two systems (Supplementary Figures 1A1,A2 and Table 3). Between-river similarity estimates ranged from 0 to 0.8 (mean = 0.17) (Figure 2F) and was significantly different from within-river similarity estimates (Supplementary Figures 1E1,E2 and Table 3).
Live and fossil samples shared seven species (E. floridensis, P. duryi, C. georgiana, N. wetherbyi, P. trivolvis, P. paludosa, and unionid mussels) (Table 2). An additional seven freshwater species were present only in fossil samples (P. hendersoni, Physella sp., Fluminicola dalli, Tryonia aequicostata, Lioplax pilsbryi, Pisidium sp., and indeterminate ancylid gastropods). Four species were absent from the fossil record but present in live samples: the introduced C. fluminea, T. granifera, and M. tuberculata and the native, brackish-tolerant V. usnea. Compared to fossil samples, live samples were generally characterized by higher dominance, lower richness, and lower rarefied diversity (Figure 3). Similarity between fossil and live Wakulla samples ranged from 0 to 0.64 (mean = 0.11), and between fossil and live Silver/Ocklawaha samples from 0 to 0.62 (mean = 0.09) (Figures 2G,H). Mean similarity was statistically distinct between Wakulla live and Wakulla fossil samples, with fossil samples more similar and live samples less similar than expected (Supplementary Figures 1C1,C2). However, Silver/Ocklawaha live samples and Silver/Ocklawaha fossil samples were not statistically distinguishable in terms of pairwise similarities (Supplementary Figures 1D1,D2). Mean similarity between fossil samples from the two river systems was significantly more similar than expected, whereas live samples were significantly less similar (Figures 4A,B and Table 3).
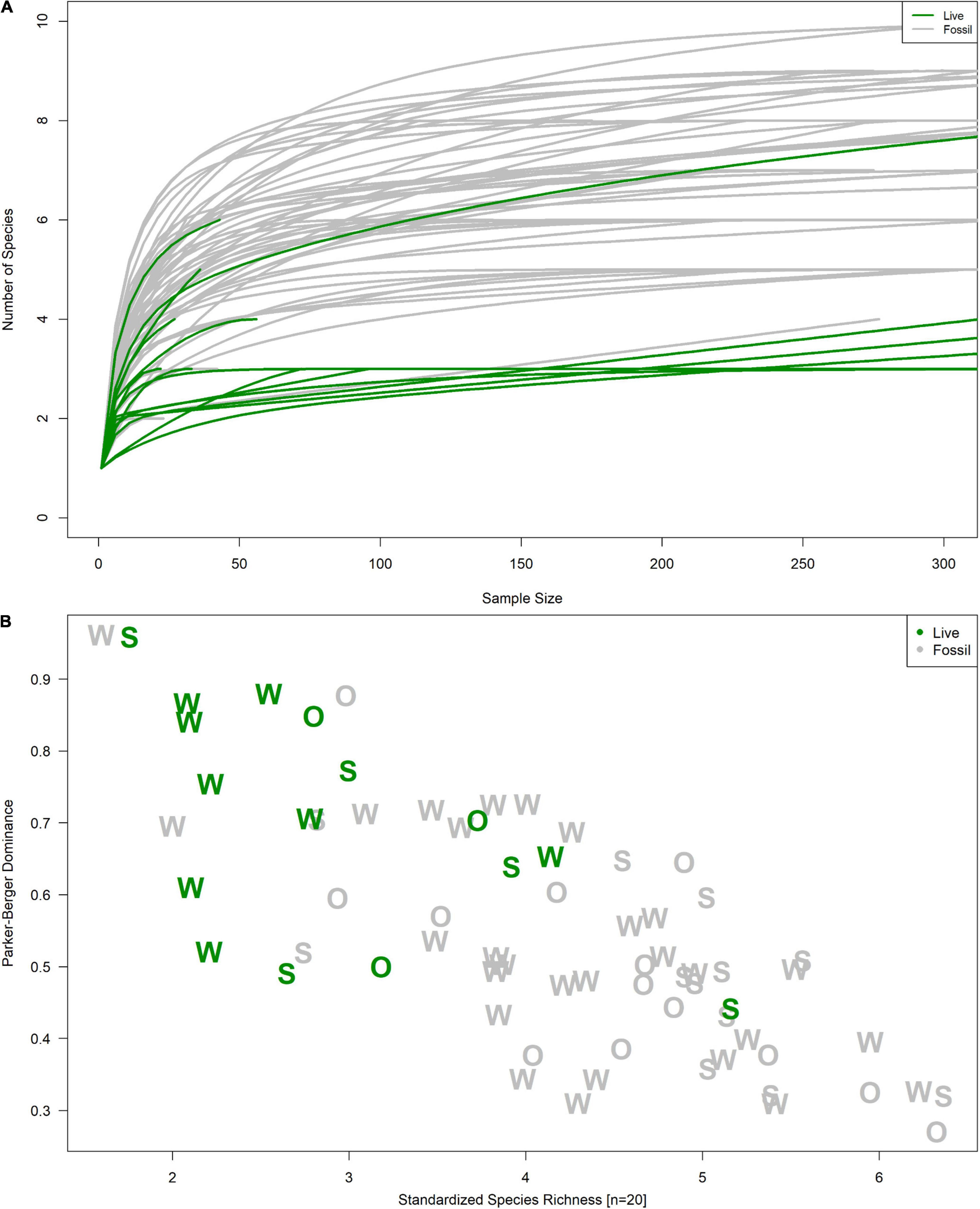
Figure 3. (A) Rarefaction curves and (B) richness-dominance plot and of fossil (gray) and live (green) samples. Letters in (B) correspond to a sample’s river system: “W” for Wakulla, “S” for Silver, and “O” for Ocklawaha.
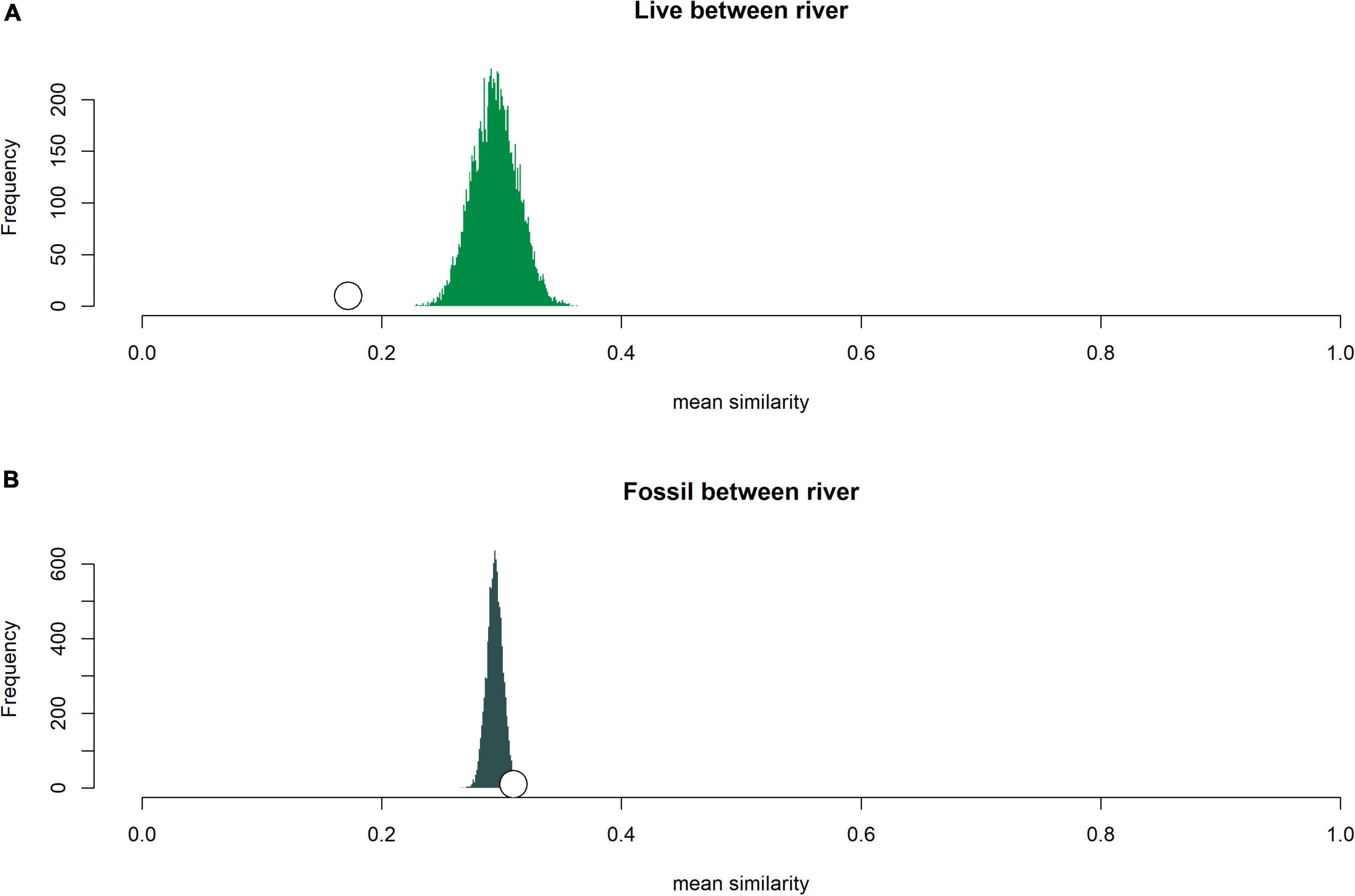
Figure 4. Comparison of expected mean similarity distributions (based on randomization under the null model that all pairwise comparisons came from the same underlying statistical population) with observed mean similarity values for within river (A) live samples and (B) fossil samples. Open white circle is the observed mean similarity for the given set of comparisons.
To account for the impact of introduced species only in the modern (as they are absent from fossil assemblages), similar analyses were conducted with their exclusion. Although removing introduced species does not change pairwise comparisons of Bray-Curtis similarity for fossil samples, similarity among live samples within the same river system increases (Supplementary Figures 5A,B). Bray-Curtis similarity increases in the Silver/Ocklawaha Rivers (min = 0.27, max = 0.85, mean = 0.51). In the Wakulla, although the lower and upper river remains dissimilar from one another, overall mean similarity increases in live samples because of the high agreement in the upper river (min = 0.03, max = 1, mean = 0.71). Removing introduced species does not dramatically change similarity between rivers (min = 0.03, max = 0.8, mean = 0.27) (Supplementary Figure 5C).
In ordination space, fossil samples plot as a cloud with some separation along nMDS1 and tight clustering along nMDS2 (Figure 5). Whereas there is some clustering by river system within the cloud, fossil samples from the different rivers broadly overlap. Live Wakulla River samples plot at high nMDS1 and high nMDS2 scores, distinct from nearly all fossil and live Silver/Ocklawaha River samples (Figure 5). Live Silver/Ocklawaha River samples mostly plot at high nMDS1 and low nMDS2 scores, distinct from all fossil and live Wakulla River samples. Whereas the majority of species plot within the cluster of fossil samples, three species do not. Two species, the introduced M. tuberculata and native E. floridensis, plot at higher nMDS1 and nMDS2 scores, similar to the Wakulla live samples, in which both taxa are abundant. The introduced C. fluminea scores are similar to most live Silver/Ocklawaha samples, at high nMDS1 and low nMDS2 scores. PERMANOVA indicates that the two assemblage types and two river systems are significantly different (F = 7.3, p < 0.001).
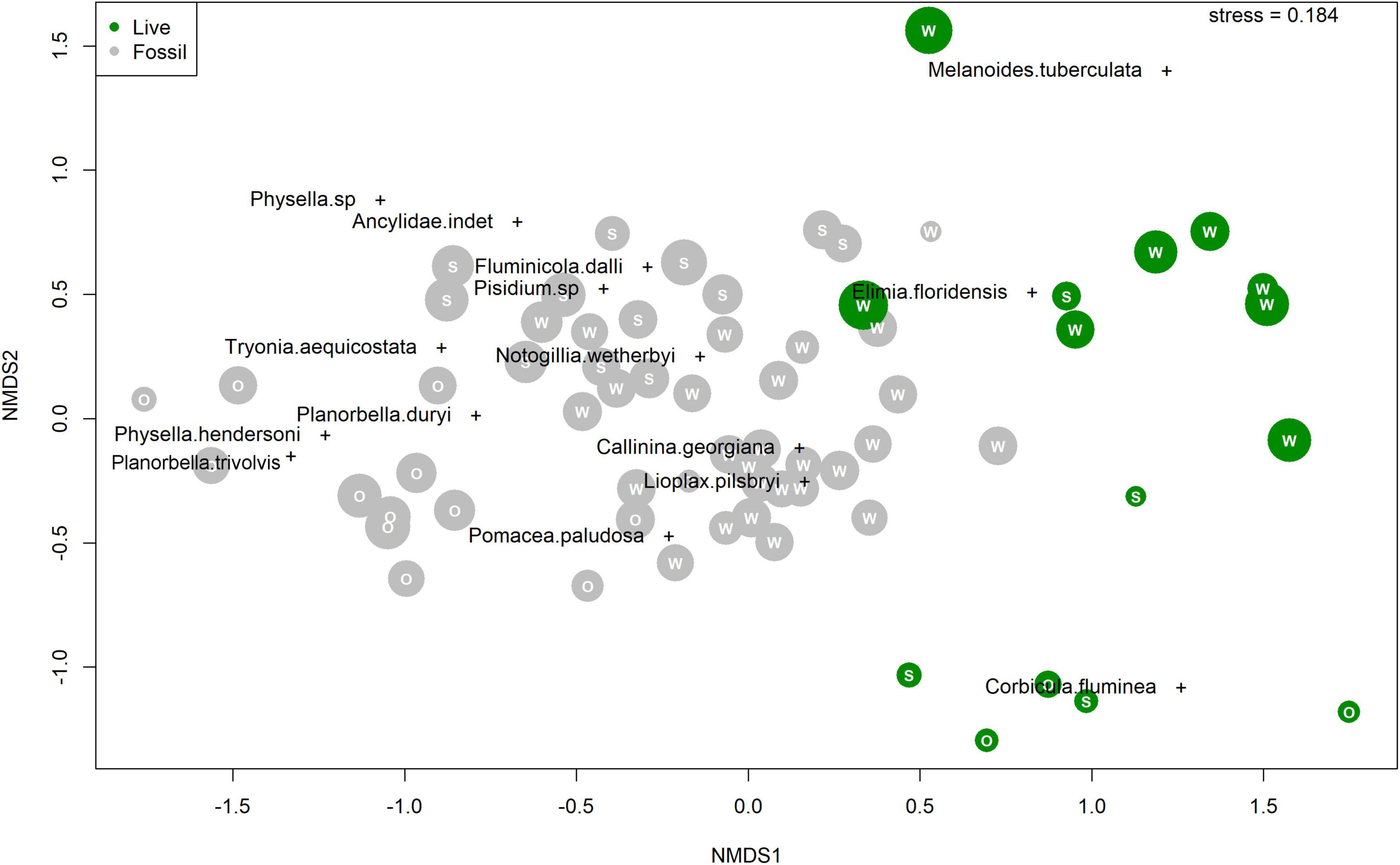
Figure 5. Non-metric Multidimensional Scaling (nMDS) species and sample scores. Fossil samples in gray and live samples in green. Letters correspond to a sample’s river system: “W” for Wakulla, “S” for Silver, and “O” for Ocklawaha. Points scaled by number of specimens in each sample.
When ordination is restricted to only fossil assemblages, samples plot as a broadly overlapping cloud (Figure 6). Wakulla and Silver River samples show substantial overlap with one another, with a weak separation along nMDS2. Most Ocklawaha River samples plot within a distinct cluster, separating from the broader Silver and Wakulla River cluster along nMDS1. A number of Ocklawaha River samples, however, still overlap with those of the other rivers. Species scores for L. pilsbryi, Pomacea paludosa, and C. georgiana are generally similar and overlap with the largest cluster of fossil Wakulla River samples. Two species, E. floridensis and N. wetherbyi, plot in a region of ordination space where Wakulla and Silver River samples overlap. Planorbella duryi, P. trivolvis, and P. hendersoni plot at similar scores as the cluster of Ocklawaha River samples.
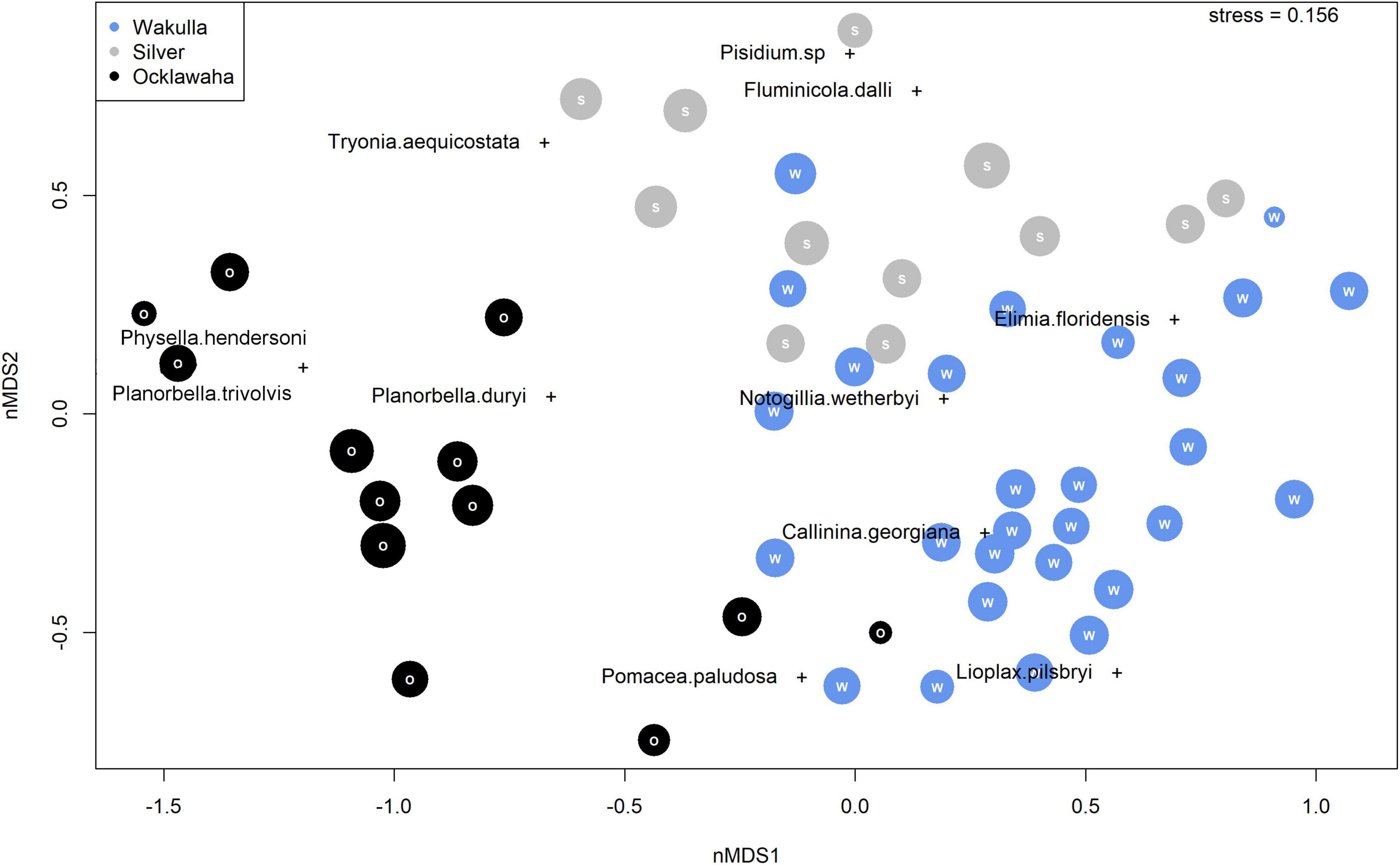
Figure 6. Non-metric Multidimensional Scaling (nMDS) species and sample scores for fossil assemblages exclusively. Wakulla River samples in blue, Silver River samples in gray, and Ocklawaha River samples in black.
If introduced species are removed from analyses and ordinations, live samples still exhibit higher dominance, lower richness, and lower rarefied diversity than fossil samples (Supplementary Figures 2, 3). In ordination space, there is still separation between the live samples of both river systems primarily along nMDS2 (Supplementary Figure 4). Whereas like Wakulla samples still separate from fossil samples along nMDS1, many live Silver/Ocklawaha samples instead now separate along a combination of nMDS1 and nMDS2. Among species scores, E. floridensis plots distinctly separate, roughly coinciding with the clustering of live Wakulla samples, likely due to their heavy abundance in these samples. Three species, C. georgianus, P. paludosa, and L. pilsbryi separate from other species scores, plotting similarly to one another in the same ordination space as a heavy cluster of fossil Wakulla samples and relatively near live Silver and Ocklawaha samples.
Comparative Local and Regional Faunal Context
Regional, county-level, or watershed-level occurrences are often recorded for live mollusks. However, except for species of special ecological interest (i.e., endangered species, harmful invasives, critical food source for endangered birds, etc.), live mollusks are rarely documented via quantitative sampling and are often relegated to passing mentions in larger faunal surveys. Because their shells have high preservation potential, however, mollusks are more readily recorded in the freshwater fossil record of Florida, and often documented in detail when found in fossil deposits (Portell et al., 1995; Karrow et al., 1996; Auffenberg et al., 2006; Kittle and Portell, 2010; Portell and Kittle, 2010). Below, we compare general abundances of species in our study with their previously published records in present-day local and regional river systems as well as in fluvial (or fluvial-adjacent) Pleistocene fossil deposits of Florida.
The comparative data from other fossil sites in the region includes three sites that are summarized here briefly. The first fossil locality, the Page-Ladson site, lies on the Aucilla River in Jefferson County, approximately 30 km SE of the sampled Wakulla River sites (Auffenberg et al., 2006). Fossiliferous deposits containing freshwater mollusks were radiocarbon dated to between ∼12,500 and 9,950 BP (Auffenberg et al., 2006; Webb and Dunbar, 2006). The paleoenvironment was interpreted as having been shallow (< 3 m) and freshwater (Auffenberg et al., 2006). The second locality, the Oldsmar site in Pinellas County, contains a non-marine, fossiliferous clay and sand layer of the late Pleistocene Fort Thompson Formation (Karrow et al., 1996) and lies approximately 150 km SW of sites sampled along the Silver and Ocklawaha Rivers. This non-marine layer was interpreted as having formed in near-coastal, poorly drained marsh, pond, and stream paleoenvironments (Karrow et al., 1996). The third locality, the basal layer in the Leisey Shell Pits in Hillsborough County, contains dark, organic-rich sediment with freshwater and estuarine mollusk fossils of the middle Pleistocene Bermont Formation (Bogan and Portell, 1995; Portell et al., 1995). The site lies about 175 km SW of the sampled Silver and Ocklawaha River sites. Paleoenvironment is interpreted as having been a protected bay or lagoon fed by an adjoining large river, likely the source of the freshwater fossil material (Portell et al., 1995).
Compared to their fossil presence, most native, freshwater species saw a reduction in their modern geographic ranges with many undergoing local extirpation from the Silver, Ocklawaha, and Wakulla Rivers, though persisting within the broader region. Two prominent introduced, freshwater species (C. fluminea and M. tuberculata) were documented first appearing in the Florida Gulf and St. Johns River watershed between 1960 and 1973 (Heard, 1964; Schneider, 1967; Clench, 1969; Daniel et al., 2021). The euryhaline V. usnea has also been documented in the fossil record of Florida and nearby regions (Kittle and Portell, 2010; Czaja et al., 2019). A full listing and discussion of all species is available in Table 2 and Supplemental Discussion (“Expanded Comparative Local and Regional Faunal Context”).
Discussion
Diverging Live Communities
Changes in the live communities of these rivers appear to reflect environmental and anthropogenically-related impacts to these fluvial ecosystems. In both river systems, the faunal composition of live mollusk associations has diverged from that observed in fossil assemblages, as demonstrated by statistically significant (Table 3) separation of live and fossil samples in the ordination space (Figure 5). These rivers have also become faunally dissimilar from one another through the differential loss of native freshwater species, the regionally variable spread of the introduced species M. tuberculata and C. fluminea, and encroachment of V. usnea into the coastally influenced lower Wakulla River. Whereas the decline in freshwater mollusk diversity was previously suggested based on fossils in Florida (Auffenberg et al., 2006), this study demonstrates that different river systems experienced diverging compositional shifts that resulted in increased faunal heterogeneity on a regional scale.
The compositional divergence in live communities of the two rivers is not solely a consequence of the modern presence of introduced species, but also represents changes in the remaining native, freshwater community. When introduced species are excluded, most live samples still separate by river system along nMDS2 (Supplementary Figure 4). Live samples also separate from fossil samples in the ordination space. As most of the species lost from modern assemblages were shared between the two river systems, divergence documented in live samples is likely caused by changes in the presence and relative abundance of the remaining native species. When introduced species are excluded, the freshwater communities of the two rivers are increasingly dominated by either E. floridensis, N. wetherbyi, or a combination of the two. In the Silver and Ocklawaha Rivers, C. georgiana also remains a notable component of the community at a relatively limited number of sites compared to the more widely dispersed E. floridensis.
In addition to invasive species, the native freshwater community may be responding to climate and anthropogenic factors including habitat loss, decreasing waterflow, excessive nutrient input, salinity fluctuations, and other impacts (Turner and Rabalais, 1991; Loper et al., 2005; Brainwood et al., 2006; Turner et al., 2006; Lysne et al., 2008; Liu et al., 2009; Heffernan et al., 2010; Donoghue, 2011; Bricker et al., 2014; Camp et al., 2014; Hong et al., 2014; Liebowitz et al., 2014; Freshwater Mollusk Conservation Society, 2016; Lauretta et al., 2019; Reaver et al., 2019). Invasive species (molluscan and non-molluscan) may directly or indirectly affect the native freshwater community through competition for resources, habitat alteration, or increased predation pressures (Isom, 1986; Leff et al., 1990; Wingard et al., 2008; Nico et al., 2009; United States Fish and Wildlife Service, 2014; Valentine-Darby et al., 2015; Ferreira-Rodriguez et al., 2016).
Fossil Community Composition
The composition of fossil communities provides a historical perspective to spring and river ecosystems critical for making informed restoration and conservation decisions. Fossil assemblages show similarity in faunal composition, despite the considerable distance (> 250 km) between the two studied river systems. Almost all fossil species present are shared between the two systems, with those absent from the Wakulla relatively rare in Silver/Ocklawaha samples. Given that radiocarbon dates on mollusk fossils coincide with the activation of spring flow across much of Florida (Balsillie and Donoghue, 2011; Donoghue, 2011; O’Donoughue, 2015; Kusnerik et al., 2020), the observed faunal resemblance of the two relatively distant river systems suggests that Florida springs and rivers were initially colonized by a similar suite of mollusks, reflected in the similarity of their fossil assemblages. Many fossil species identified in this survey have also been documented in freshwater fossil assemblages elsewhere in Florida (Karrow et al., 1996; Auffenberg et al., 2006), further suggesting that similar mollusk assemblages populated multiple Florida springs and rivers during the late Pleistocene-early Holocene.
Fossil samples from the Silver and upper Wakulla Rivers show a high degree of compositional fidelity, with many samples overlapping in ordination space (Figures 5, 6) suggesting the headsprings and upper reaches of the two rivers hosted similar mollusk communities in the past. Species shared in relatively high abundances between the two regions include E. floridensis, N. wetherbyi, and C. georgiana, all endemic to the southeastern United States (Figures 5, 6; Clench, 1962; Chambers, 1990; Thompson, 1999). Ocklawaha River samples form a more distinct cluster in the ordination space, perhaps because its fossil assemblages record more downstream communities rather than those of the headspring and upper river reaches. The interpretation of the Ocklawaha River samples as representing downstream mollusk associations, distinct from those of the headspring is further supported by their proximity in the ordination space (Figures 5, 6) not only to fossil samples from the lower Silver River (as expected given their close geographic proximity), but also those of the lower Wakulla River. These Silver and Ocklawaha Rivers samples are similar in terms of comparably moderate proportional presence of T. aequicostata and P. duryi. Compositionally similar Ocklawaha and Wakulla Rivers samples share high relative abundances of P. paludosa and C. georgiana, perhaps reflecting a preference of both species for soft substrates and slower water flows than typically found in upstream or headspring environments (Duch, 1976; Katoh and Foltz, 1994).
Whereas the changes in assemblage diversity documented in this study (reduced live species richness, increased fossil beta diversity, etc.) may be attributed to temporal- and spatial-averaging of fossil samples, the magnitude of those changes make this explanation unlikely. Fossil assemblages reflect long-term accumulations of shell material formed through time-averaging and post-mortem transport (Eagar, 1978; Kusnerik et al., 2020). These patterns would inflate alpha diversity, with a more pronounced effect in downstream areas that receive post-mortem accumulations from upstream regions. This would also reduce beta diversity between sites, as greater spatial and temporal mixing would make sites appear more similar to one another. Whereas this explanation (temporal- and spatial-averaging of fossil samples) for the perceived patterns is possible, the effects of these biases is expected to be much less dramatic (Tomašových and Kidwell, 2009, 2010) than those observed in this study, suggesting the effects documented in this study should not be attributed primarily to time-averaging and post-mortem transport.
Conservation and Restoration Management Suggestions
Conservation managers should evaluate the benefits and feasibility of targeted conservation or reintroduction of critical, freshwater species. In particular, unionid mussels are a bellwether of environmental or ecological conditions in freshwater systems, being sensitive to changes in salinity, water chemistry, and other environmental factors (Williams et al., 2014). A robust, diverse mussel population in a system is often evidence of a healthy environment, while their loss indicates the opposite. Even if an ecosystem is restored, any loss of unionid mussels also sees the loss of related ecosystem services including enhanced water filtration, nutrient recycling and storage, and habitat and substrate modification (Vaughn, 2018). The fossil and death assemblages may provide critical information on past occurrences of unionid species, documenting their historical ranges and providing a context to where reintroduction may prove most successful (Haag and Williams, 2013).
Many freshwater species documented in this study were once present in these river systems, either historically or prehistorically, but are missing from the living record due to a local extirpation. As many of these mollusk species persist regionally, their return would bolster biodiversity in these river systems. Anthropogenic reintroductions of macroinvertebrate populations may improve restoration outcomes, particularly in systems suffering from depauperate local populations where self-recolonization is difficult (Jourdan et al., 2018). These efforts have already been documented at the Wakulla River, anthropogenic reintroduction of P. paludosa populations in the early-to-mid 2000 (Darby et al., 1997; Loper et al., 2005; Wakulla Springs Alliance, 2021). Fossil and death assemblage records may provide the most complete assessment of which species might be suitable targets for reintroduction, based on their previous occurrences in these freshwater systems.
Restoration, conservation, and reintroduction efforts will also benefit from ongoing efforts to reduce harmful environmental stressors. Impacts to Florida’s freshwater systems are many and varied but notably include increasing withdrawal of groundwater, nutrient runoff and related harmful algal blooms, and human-induced changes in shore erosion and sedimentation rates (Florida Department of Environmental Protection, 2007, 2014). The negative impacts from these stressors degrade the environmental health of these systems, making them more susceptible to larger perturbations and decreasing the success of any reintroduction efforts (Jourdan et al., 2018). Continued monitoring of conditions in the springs, rivers, and adjacent terrestrial settings, as well maintaining many ongoing efforts to mitigate these effects, will enhance the long-term success of conservation and restoration efforts among freshwater faunal communities.
Finally, this study provides a historical perspective on the emerging ecological role of introduced species in spring-fed Florida rivers, thus emphasizing the importance of continued monitoring of those species and mitigating their impacts on freshwater systems. Florida has more non-native wildlife species than any other state (Hardin, 2007), and their effects on native populations and ecosystems should be monitored to prevent negative effects. Among introduced mollusks, C. fluminea populations must be maintained at low enough abundances to avoid negatively impacting populations of unionid mussels through competition for resources and overcrowding (Isom, 1986; Leff et al., 1990; Vaughn and Spooner, 2006; Ferreira-Rodriguez et al., 2016, 2018). Other introduced faunas may not directly compete for resources but cause negative impacts on native populations through habitat alteration, as in the burrows of the suckermouth armored catfish (Loricarridae) causing destructive shoreline erosion and sedimentation (Nico et al., 2009).
The impacts of invasive species mitigation must also be considered by conservation managers. Combating the spread of invasive aquatic plants such as Hydrilla has been an ongoing effort in many Florida freshwater environments, including Wakulla Springs. Unfortunately, more traditional removal methods such as mechanical harvesting has been documented to negatively impact native mollusk and other macroinvertebrate populations (Van Dyke, 2019). Alternative control methods, such as herbicide drip systems, reduced this impact but also highlighted the effects that some invasive species mitigation efforts have on other components of freshwater ecosystems (Van Dyke, 2019).
Effects of Sea-Level Changes
Rising sea levels in the Gulf of Mexico (Donoghue, 2011) leave the tidally influenced lower Wakulla River increasingly susceptible to increased salinization (Hong et al., 2014) and more vulnerable to storm surges from hurricane and storm events (Bromirski and Kossin, 2008; Knutson et al., 2019, 2020; Kossin et al., 2020). Storm surges and salinity disruptions like those documented in the modern Wakulla River are hypothesized to have previously caused the local loss of many freshwater taxa at the Page-Ladson site sometime after 9,950 cal BP, the youngest recorded deposits at the site, leading to the depauperate mollusk faunas of the modern Aucilla River (Auffenberg et al., 2006). In the lower Wakulla River, the mollusk community has been altered by the encroachment of saline waters, which displaced saline-intolerant native species that were replaced by taxa more tolerant of oligohaline conditions (Lewis et al., 2009; Hong et al., 2014).
The Value of State Parks
The upper Wakulla River may serve as a partial refugium for less saline-resilient freshwater species, as it is buffered from the effects of sea level rise and increasing salinity, and more protected from direct human impacts by the surrounding Edward Ball Wakulla Springs State Park. Numerous strategies for preserving the headspring and Wakulla River are being considered or implemented (Loper et al., 2005; Florida Department of Environmental Protection, 2007, 2020; Howard T. Odum Florida Springs Institute, 2014).
The Silver River has experienced substantial, protracted, anthropogenic impacts over at least two centuries. Although development of the springs as a tourist destination began as early as the 1820s, large changes between 1924 and 2013, including the addition of gas-powered glass bottom boat tours, exotic animal exhibits, a waterpark, and other attractions likely left lasting impacts on the aquatic communities of the headspring and river (Berson, 2011). In 2013, the Florida Park Service took control of property around the headspring, merging it with an existing, adjacent park to form Silver Springs State Park. With the addition of the headsprings, the Silver River and much of the Ocklawaha River now lie within a continuous stretch of State Park or state-managed lands, enabling more effective conservation efforts to address environmental challenges related to decreasing water flow, excessive nutrient runoff, and the impact of the Rodman/Kirkpatrick Dam on the Ocklawaha River and its tributaries (Shuman, 1995; Munch et al., 2006; Noll and Tegeder, 2011; Florida Department of Environmental Protection, 2014; Bi et al., 2019). Although no studies have documented how the recent incorporation of the headspring into the larger state park has impacted the aquatic communities, the removal of many of the attractions will likely reduce the direct anthropogenic impact on the river, possibly enabling recovery and restoration of the freshwater mollusk species that remain.
This study supports the importance of evaluating, maintaining, and expanding of the ongoing conservation and restoration strategies for Florida’s freshwater spring and river ecosystems, including connection of protected lands/waterways, management of invasive species, and preservation of existing fluvial and riparian buffers (Castillo et al., 2016). The State Parks, and similarly managed lands, likely enhance the resilience and long-term diversity of freshwater habitats. Protection of these regions should be maintained and, if possible, enhanced through increased safeguards and expansion. The creation or expansion of managed areas around critical headspring regions can provide increased buffer zones, mitigating the impacts of harmful human or natural perturbations (De Freese, 1995). Expansion of these areas may also enhance connections between managed lands. These connections provide critical aquatic wildlife corridors, reducing habitat fragmentation and ensuring populations can move freely along and between waterways. Aquatic wildlife corridors and interconnected waterways are critical for population flow among fluvial species and may allow for natural recolonization following local extirpation events (Jourdan et al., 2018). Fluvial connections may also be restored through the removal of human-related impediments such as dams, artificially channeled rivers, and similar features which prevent freshwater species from accessing potential habitats. Reducing these barriers will become increasingly important, allowing populations to move within and between waterways, as available habitable space shrinks due to sea level rise, salinity fluctuations, pollutant perturbations, and other direct and indirect anthropogenic influences already affecting the springs and rivers in the region (Loper et al., 2005; Hong et al., 2014) and across other parts Florida (Walsh, 2001; Endries et al., 2009; White and Crisman, 2014).
Spatial, Temporal and Fossil Record Considerations
The results reported here should be considered in the context of the spatiotemporal resolution of the fossil record. While radiocarbon dating (Kusnerik et al., 2020) suggests that a continuous record of mollusk associations from the Pleistocene to today is absent from sample intervals, the presence of mid-to-late Holocene shells in death assemblages suggests these younger fossil deposits are present but less abundant than the more heavily sampled late Pleistocene-early Holocene deposits (Kusnerik et al., 2020).
Because of time-averaging, fluvial fossil samples are temporally mixed over hundreds to thousands of years, thereby combining specimens of dramatically different ages (Kusnerik et al., 2020). Pooling of live data from multiple seasons and years into a single sample, as was done in this study, mimics to some extent the time-averaging that characterizes fossil samples (Peterson, 1977; Martin et al., 2002). Additionally, the results reported here represent a regional case study and their broader applicability needs to be validated and refined in future studies in other river systems before this approach can be applied on larger geographic scales and in a more diverse array of habitats. For a more detailed discussion on resolution consideration, refer to Supplemental Discussion “Extended Spatial, Temporal, and Fossil Record Considerations.”
Summary
Analyses of live and fossil freshwater assemblages demonstrated that past mollusk associations were more diverse and more homogeneous in faunal composition, compared to depauperate and spatially heterogeneous mollusk associations that exist in Wakulla, Silver, and Ocklawaha Rivers today. This shift in composition reflects increasing presence of non-native species (especially M. tuberculata and C. fluminea), extirpation of many native freshwater species, fundamental changes in the relative abundance of surviving native taxa, and increasing salinity disruptions in coastally influenced regions of Florida rivers. The findings support strategies for mitigating these impacts through preservation and restoration of critical springs and rivers including: (1) expansion of protected zones such as State Parks and state-managed lands, (2) reintroduction and/or conservation of critical native species including P. paludosa and unionid mussels, (3) reduction of stressors including input of nutrient-laden runoff and groundwater withdrawals, and (4) evaluation of the impact of invasive species on native communities (Loper et al., 2005; Munch et al., 2006; Florida Department of Environmental Protection, 2007, 2014, 2020; Howard T. Odum Florida Springs Institute, 2014; Wetland Solutions Inc, 2014; Florida Department of Environmental Assessment and Restoration, 2015). The conservation paleobiology approach used in this study reinforces the importance of considering the long-term history of local ecosystems and highlights the utility of the fossil record in providing a historical perspective that extends farther than provided by most modern surveys or written records. Comparison of fossil and modern communities can provide a perspective that enables the recognition of long-term faunal changes in altered, imperiled, or at-risk ecosystems to provide guidance for restoration, conservation, and/or mitigation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
KK and MK contributed to the study conception and design, involved in draft manuscript preparation. KK, MK, RP, GM, and RM conducted fieldwork and sample collection. KK, MM, and AK conducted laboratory processing and analysis. All authors reviewed the results, provided editorial feedback, and approved the final version of the manuscript.
Funding
Felburn Foundation, University of Florida, Florida Museum of Natural History, Florida Paleontological Society Inc., Southwest Florida Fossil Society Inc., Jon A. and Beverly L. Thompson Foundation, all provided funding toward aspects of the research including fieldwork and laboratory support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Felburn Foundation, the Florida Museum of Natural History (FLMNH) especially Invertebrate Paleontology’s Jon and Beverly Thompson Endowment, The Florida Paleontological Society, Inc., and Southwest Florida Fossil Society, Inc., for funding. We gratefully acknowledge C. Albin, A. Bard, A. Stiles, S. Lieb, P. Wilbur, A. Conyers, and others at the Florida Park Service for assistance with permitting, access, and collecting within the Florida state parks. We thank S. Casebolt (FLMNH), C. Pedrozo (University of Florida (UF)/FLMNH), J. Slapcinsky (FLMNH), J. Williams (FLMNH/Florida Fish and Wildlife Conservation Commission), and M. Ziegler (UF/FLMNH) for assistance in the field and laboratory. We thank M. Brenner (UF), B. Pine (UF), G. Paulay (FLMNH) and D. Jones (FLMNH) for insight and contributions to the research project and manuscript. We also thank two reviewers and GW (USGS) for useful suggestions and editorial insight that improved the quality and clarity of this manuscript. Collection was conducted under Florida Park Service permit numbers 05121613, 16100711, and 12071810.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2022.851499/full#supplementary-material
References
Alin, S., and Cohen, A. S. (2004). The live, the dead, and the very dead: Taphonomic calibration of the recent record of paleontological change in Lake Tanganyika. East Africa. Paleobiol. 30, 44–81. doi: 10.1666/0094-8373(2004)030<0044:tltdat>2.0.co;2
Auffenberg, K., Quitmyer, I. R., Williams, J. D., and Jones, D. S. (2006). “Non-marine Mollusca,” in First Floridians and Last Mastodons: The Page-Ladson Site in the Aucilla River. Topics in Geobiology, Vol. 26, ed. S. D. Webb (Netherlands: Springer), 247–261.
Balsillie, J. H., and Donoghue, J. F. (2011). “Northern Gulf of Mexico sea-level history for the past 20,000 years,” in Gulf of Mexico: Origin, Waters, and Biota, eds N. A. Buster and C. W. Holmes (Texas: Texas A&M University Press), 53–69.
Barbieri, G., Rossi, V., Ghosh, A., and Vaiani, S. C. (2020). Conservation paleobiology as a tool to define reference conditions in naturally stressed transitional settings: Micropaleontological insights from the Holocene of the Po Coastal Plain (Italy). Water 12:3420 doi: 10.3390/w12123420
Barnosky, A. D., Hadly, E. A., Gonzalez, P., Head, J., Polly, P. D., and Lawing, A. M. (2017). Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355, 594–604. doi: 10.1126/science.aah4787
Berson, T. R. (2011). Silver Springs: The Florida interior in the American imagination. [Ph.D. thesis] Florida: University of Florida, 330.
Bi, X., Borisova, T., and Hodges, A. W. (2019). Economic value of visitation to free-flowing and impounded portions of the Ocklawaha River in Florida: implications for management of river flow. Rev. Region. Stud. 49, 244–267.
Boardman, R. S., Cheetham, A. H., and Rowell, A. J. (1987). Fossil Invertebrates. California: Blackwell Scientific Publications, 713.
Bogan, A. (2006). “Conservation and extinction of the freshwater molluscan fauna of North America,” in The Mollusks: A Guide to Their Study, Collection, and Preservation. American Malacological Society, eds C. F. Sturm, T. A. Pearce, and A. Valdes (Canda: Universal Publishers, Inc), 373–383.
Bogan, A. E., and Portell, R. W. (1995). Early Pleistocene freshwater bivalves (Mollusca: Unionidae) from the Leisey Shell Pits, Hillsborough County, Florida. Bull.Florida Museum Nat. Hist. 37, 165–176.
Brainwood, M., Burgin, S., and Byrne, M. (2006). Is the decline of freshwater mussel populations in a regulated coastal river in south-eastern Australia linked with human modification of habitat? Aqua. Conserv. 16, 501–516. doi: 10.1002/aqc.758
Bricker, S. B., Rice, K. C., and Bricker, O. P. (2014). From headwaters to coast: Influence of human activities on water quality of the Potomac River estuary. Aquat. Geochem. 20, 291–323. doi: 10.1007/s10498-014-9226-y
Bromirski, P. D., and Kossin, J. P. (2008). Increasing hurricane wave power along the U.S. Atlantic and Gulf coasts. J. Geophys. Res. 113:C07012.
Brown, M. E., Kowalewski, M., Neves, R. J., Cherry, D. S., and Schreiber, M. E. (2005). Freshwater mussel shells as environmental chronicles: Geochemical and taphonomic signatures of mercury-related extirpations in the North Fork Holston River, Virginia. Environ. Sci. Technol. 39, 1455–1462. doi: 10.1021/es048573p
Camp, E. V., Staudhammer, C. L., Pine, W. E. III, Tetzlaff, J. C., and Frazer, T. K. (2014). Replacement of rooted macrophytes by filamentous macroalgae: Effects on small fishes and macroinvertebrates. Hydrobiologia 722, 159–170. doi: 10.1007/s10750-013-1694-3
Castillo, D., Kaplan, D., and Mossa, J. (2016). A synthesis of stream restoration efforts in Florida (USA). River Res. Appl. 32, 1555–1565. doi: 10.1016/j.scitotenv.2011.10.014
Chambers, S. M. (1990). The genus Elimia (= Goniobasis) in Florida and adjoining drainage basins (Prosobranchia: Pleuroceridae). Walkerana 4, 237–270.
Clench, W. J. (1962). A catalogue of the Viviparidae of North America with notes on the distribution of Viviparus georgianus. Occasional Papers on Mollusks 2, 261–287.
Czaja, A., Covich, A. P., Estrada-Rodriguez, J. L., Romero-Mendez, U., Saenz-Mata, J., and Meza-Sanchez, I. G. (2019). Fossil freshwater gastropods from northern Mexico - a case of a “silent” local extirpation, with the description of a new species. Boletin Soc. Geologica Mexicana 71, 607–627.
Daniel, W. M., Benson, A. J., and Neilson, M. E. (2021). Melanoides tuberculata (Muller, 1774): U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, FL. Available at online :https://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=1037 (accessed December 10, 2022).
Darby, P. C., Valentine-Darby, P. L., Bennetts, R. E., Croop, J. D., Percival, H. F., and Kitchens, W. M. (1997). Ecological studies of applesnails (Pomacea paludosa, Say). Florida Cooperative Fish and Wildlife Research Unit, Special Report. Gainesville: University of Florida, 152.
De Freese, D. E. (1995). Land acquisition: a tool for biological diversity protection in the Indian River Lagoon. Florida. Bull. Mar. Sci. 57, 14–27.
Donoghue, J. F. (2011). Sea level history of the northern Gulf of Mexico coast and sea level rise scenarios for the near future. Clim. Change 107, 17–33. doi: 10.1007/s10584-011-0077-x
Eagar, R. M. C. (1978). Shape and function of the shell: A comparison of some living and fossil bivalve molluscs. Biol. Rev. 53, 169–210. doi: 10.1111/j.1469-185x.1978.tb01436.x
Endries, M., Stys, B., Mohr, G., Kratimenos, G., Langley, S., Root, K., et al. (2009). Wildlife habitat conservation needs in Florida: updated recommendations for Strategic Habitat Conservation Areas. Reports TR-15 (Petersburg: Fish and Wildlife Research Institute Technical).
Ferreira-Rodriguez, N., Sousa, R., and Pardo, I. (2016). Negative effects of Corbicula fluminea over native freshwater mussels. Hydrobiologia 810, 85–95. doi: 10.1007/s10750-016-3059-1
Ferreira-Rodriguez, N., Fandino, L., Pedreira, A., and Pardo, I. (2018). First evidence of asymmetric competition between the non-native clam Corbicula fluminea and the native freshwater mussel Unio delphinus during a summer heat wave. Aquat. Conserv. 28, 1105–1113. doi: 10.1002/aqc.2964
Florida Department of Environmental Assessment and Restoration. (2015). Basin Management Action Plan for the Implementation of Total Maximum Daily Loads adopted by the Florida Department of Environmental Protection in the Silver Springs Basin Management Area for Silver Springs, Silver Springs Group, and Upper Silver River. Available online at: https://floridadep.gov/sites/default/files/SilverSprings-BMAP.pdf (accessed June 18, 2021).
Florida Department of Environmental Protection. (2007). Edward Ball Wakulla Springs State Park Unit Management Plan. Available online at :https://floridadep.gov/sites/default/files/Edward%20Ball%20Wakulla%20Springs%202007%20Approved%20Plan.pdf (accessed June 18, 2021).
Florida Department of Environmental Protection. (2014). Silver Springs State Park Unit Management Plan Amendment. Available online at https://floridadep.gov/sites/default/files/2014%20Silver%20Springs%20State%20Park%20Approved%20UMP%20-%20USE.pdf (accessed June 18, 2021).
Florida Department of Environmental Protection. (2020). Florida Forever Five-Year Plan Summary of Recommendations and Status as of December 2019 Wakulla Springs Protection Zone. 2020. Available online at https://floridadep.gov/sites/default/files/FLDEP_DSL_OES_FF_WakullaSpringsProtectionZone.pdf (accessed June 18, 2021).
Freshwater Mollusk Conservation Society (2016). A national strategy for the conservation of native freshwater mollusks. Freshwater Mollusk Biol. Conserv. 19, 1–21. doi: 10.31931/fmbc.v19i1.2016.1-21
Haag, W. R., and Williams, J. D. (2013). Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 735, 45–60. doi: 10.1007/s10750-013-1524-7
Hardin, S. (2007). Managing non-native wildlife in Florida: State perspective, policy, and practice. Manag. Vertebrate Invasive Species 14, 43–52.
Heffernan, J. B., Liebowitz, D. M., Frazer, T. K., Evans, J. M., and Cohen, M. J. (2010). Algal blooms and the nitrogen-enrichment hypothesis in Florida springs: Evidence, alternatives, and adaptive management. Ecol. Appl. 20, 816–829. doi: 10.1890/08-1362.1
Hinch, S. G., and Green, R. H. (1988). Shell etching on clams from low-alkalinity Ontario lakes: A physical or chemical process? Can. J. Fish. Aquat. Proc. 45, 2110–2113. doi: 10.1139/f88-245
Hong, X., Huang, W., Johnson, E., Lou, S., and Wan, W. (2014). Effects of sea level rise on salinity intrusion in St. Marks River Estuary, Florida, U.S.A. J. Coastal Res. 68, 89–96. doi: 10.2112/si68-012.1
Howard, T. Odum Florida Springs Institute (2014). Wakulla Spring: A Plan for the Future. https://floridaspringsinstitute.org/wp-content/uploads/2018/07/2014.11-Wakulla-Restoration-Executive-Summary.pdf (accessed June 18, 2021).
Hyman, A. C., Fraser, T. K., Jacoby, C. A., Frost, J. R., and Kowalewski, M. (2019). Long-term persistence of structured habitats: Seagrass meadows as enduring hotspots of biodiversity and faunal stability. Proc.R. Soc. B 286:20191861 doi: 10.1098/rspb.2019.1861
Isom, B. G. (1986). Historical review of Asiatic bivalve (Corbicula) invasion and biofouling of waters and industries in the Americas. Am. Malacol. Bull. Special Edition 2, 95–98.
Jackson, J. B., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., and Bourque, B. J. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–637. doi: 10.1126/science.1059199
Jourdan, J., Plath, M., Tonkin, J. D., Ceylan, M., Dumeier, A. C., and Gellert, G. (2018). Reintroduction of freshwater macroinvertebrates: Challenges and opportunities. Biol. Rev. 94, 368–387. doi: 10.1111/brv.12458
Katoh, M., and Foltz, D. W. (1994). Genetic subdivision and morphological variation in a freshwater snail species complex formerly referred to as Viviparus georgianus (Lea). Biol. J.Linnean Soc. 53, 73–90. doi: 10.1111/j.1095-8312.1994.tb01002.x
Karrow, P. F., Morgan, G. S., Portell, R. W., Simons, E., and Auffenberg, K. (1996). “Middle Pleistocene (early Rancholebrean) vertebrates and associated marine and non-marine invertebrates from Oldsmar, Pinellas County, Florida,” in Palaeoecology and Palaeoenvironments of Late Cenozoic Mammals, Tributes to the Career of C.S. (Rufus) Churcher, eds K. M. Stewart and K. L. Seymour (Canada: University of Toronto Press), 97–133. doi: 10.3138/9781487574154-009
Kidwell, S. M. (2007). Discordance between living and death assemblages as evidence for anthropogenic ecological change. Proc.Natl. Acad. Sci. U.S.A 104, 17701–17706. doi: 10.1073/pnas.0707194104
Kidwell, S. M. (2013). Time-averaging and fidelity of modern death assemblages: Building a taphonomic foundation for conservation paleobiology. J. Paleontol. 56, 487–522. doi: 10.1111/pala.12042
King, W. A. (2004). Through the looking glass of Silver Springs: Tourism and the politics of vision. Americana. J. Am. Popul. Cult. 3:1. doi: 10.5790/hongkong/9789622099821.003.0001
Kittle, B. A., and Portell, R. W. (2010). Mollusca: Fort Thompson Formation (Late Pleistocene). Florida Fossil Invertebrates Part 12, 1–32.
Knight, R. L. (1980). Energy basis of control in aquatic ecosystems. [Ph.D thesis] Gainesville: University of Florida.
Knight, R. L. (1983). “Energy basis of ecosystem control at Silver Springs, Florida,” in Dynamics of Lotic Ecosystems, eds T. D. Fontaine and S. M. Bartell (Michigan: Ann Arbor Science Publishers), 161–179.
Knutson, T., Camargo, S. J., Chan, J. C. L., Emanuel, K., Ho, C., Kossin, J., et al. (2019). Tropical cyclones and climate change assessment: Part I: Detection and attribution. Bull.Am. Meteorol. Soc. 100, 1987–2001. doi: 10.1175/bams-d-18-0189.1
Knutson, T., Camargo, S. J., Chan, J. C. L., Emanuel, K., Ho, C., Kossin, J., et al. (2020). Tropical cyclones and climate change assessment: Part II: Projected response to anthropogenic warming. Bull.Am. Meteorol. Soc. 101, E303–E322.
Kossin, J. P., Knapp, K. R., Olander, T. L., and Velden, C. S. (2020). Global increase in major tropical cyclone exceedance probability over the past four decades. PNAS 117, 11975–11980. doi: 10.1073/pnas.1920849117
Kowalewski, M., Serrano, G. E. A., Flessa, K. W., and Goodfriend, G. A. (2000). Dead delta’s former productivity: Two trillion shells at the mouth of the Colorado River. Geology 28, 1059–1062. doi: 10.1130/0091-7613(2000)028<1059:ddsfpt>2.3.co;2
Kusnerik, K. M., Means, G. H., Portell, R. W., Brenner, M., Hua, Q., Kannai, A., et al. (2020). Live, dead, and fossil mollusks in Florida freshwater springs and spring-fed rivers: Taphonomic pathways and the formation of multisourced, time-averaged death assemblages. Paleobiology 46, 356–378. doi: 10.1017/pab.2020.25
Lauretta, M. V., Pine, W. E., Walters, C. W., and Frazer, T. K. (2019). Plant mediated community structure within spring-fed, coastal rivers. PLoS One 14:e0219236. doi: 10.1371/journal.pone.0219236
Leff, L. G., Burch, J. L., and McArthur, J. V. (1990). Spatial distribution, seston removal, and potential competitive interactions of the bivalves Corbicula fluminea and Elliptio complanata in a coastal plain stream. Freshwater Biol. 24, 409–416. doi: 10.1111/j.1365-2427.1990.tb00720.x
Lewis, G., Wooten, N. D., and Bartel, R. L. (2009). Lower St. Marks River/Wakulla River/Apalachee Bay Resource Characterization. Northwest Florida Water Management District, Water Resources Special Report 2009-01. (Havana), 130.
Liebowitz, D. M., Cohen, M. J., Heffernan, J. B., Korhnak, L. V., and Frazer, T. K. (2014). Environmentally mediated consumer control of algal proliferation in Florida springs. Freshwater Biol. 59, 2009–2023. doi: 10.1111/fwb.12403
Liu, Z., Choudhury, S. H., Xia, M., Holt, J., Wallen, C. M., Yuk, S., et al. (2009). Water quality assessment of coastal Caloosahatchee River watershed. Florida. J. Environ. Sci. Health 44, 972–984. doi: 10.1080/10934520902996872
Loper, D. E., Landing, W. M., Pollman, C. D., and Chan Hilton, A. B. (2005). Degradation of water quality at Wakulla Springs, Florida: Assessment and recommendations. Report of the Peer Review Committee on the Workshop Solving Water Pollution Problems in the Wakulla Springshed of North Florida. http://aquaticcommons.org/978/1/WakullaPeerReportFinal.pdf (accessed June 18, 2021).
Lysne, S. J., Perez, K. E., Brown, K. M., Minton, R. L., and Sides, J. D. (2008). A review of freshwater gastropod conservation: Challenges and opportunities. J.North Am. Benthol. Soc. 27, 463–470. doi: 10.1899/07-061.1
Martin, R. A. (1966). Eternal spring: man’s 10,000 years of history at Florida’s Silver Springs. Great Outdoors Publishing Company. Florida: St. Petersburg, 264.
Martin, R. E., Hippensteel, S. P., Nikitina, D., and Pizzuto, J. E. (2002). Artificial time-averaging of marsh foraminiferal assemblages: Linking the temporal scales of ecology and paleoecology. Paleobiology 28, 263–277. doi: 10.1666/0094-8373(2002)028<0263:ataomf>2.0.co;2
Milanich, J. T. (1994). Archaeology of Precolumbian Florida. Gainesville: University of Florida Press, 502.
Montagna, P. A., Estevez, E. D., Palmer, T. A., and Flannery, M. S. (2008). Meta-analysis of the relationship between salinity and molluscs in tidal river estuaries of southwest Florida, U.S.A. Am. Malacol. Bull. 24, 101–115. doi: 10.4003/0740-2783-24.1.101
Munch, D. A., Toth, D. J., Huang, C., Davis, J. B., Fortich, C. M., Osburn, W. L., et al. (2006). Fifty-year retrospective study of the ecology of Silver Springs, Florida. Special Publication SJ2007-SP4. Tallahassee: Florida Department of Environmental Protection.
Nico, L. G., Jelks, H. L., and Tuten, T. (2009). Non-native suckermouth armored catfishes in Florida: Description of nest borrows and burrow colonies with assessment of shoreline conditions. Aquat. Nuisance Species Res. Prog. 9, 1–30. doi: 10.11646/zootaxa.4264.1.1
Noll, S., and Tegeder, D. (2011). The wicked ditch will never die: The on-going controversy over Rodman Reservoir. J. Florida Stud. 1.
O’Donoughue, J. M. G. (2015). The archaeology of northeast Florida springs. [Ph.D thesis] Florida: University of Florida, 391.
Odum, H. T. (1957). Trophic structure and productivity of Silver Springs. Florida. Ecol. Monogr. 27, 55–112. doi: 10.2307/1948571
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., McGlinn, D., et al. (2019). Vegan: Community Ecology Package. R package version. 2. 5–6. https://CRAN.R-project.org/package=vegan.
Peterson, C. H. (1977). The paleoecological significance of undetected short-term temporal variability. J. Paleontol. 51, 976–981.
Portell, R. W., and Kittle, B. A. (2010). Mollusca: Bermont Formation (middle Pleistocene). Florida Fossil Invertebrates Part 13, 1–40.
Portell, R. W., Schindler, K. S., and Nicol, D. (1995). Biostratigraphy and paleoecology of the Pleistocene invertebrates from the Leisey Shell Pits, Hillsborough County, Florida. Bull.Florida Museum Natural Hist. 37, 165–176.
R Core Team (2018). A Language and Environment for Statistical Computing. Vienna, Aus: R Foundation for Statistical Computing.
Reaver, N. G. F., Kaplan, D. A., Mattson, R. A., Carter, E., Sucsy, P., and Frazer, T. K. (2019). Hydrodynamic controls on primary producer communities in spring-fed rivers. Geophys. Res. Lett. 46, 4715–4725. doi: 10.1029/2019gl082571
Revels, T. J. (1990). Watery Eden: A history of Wakulla Springs. [Ph.D thesis] Florida: Florida State University, 163.
Roper, D. S., and Hickey, C. W. (1994). Population structure, shell morphology, age and condition of the freshwater mussel Hyridella menziesi (Unioncea: Hyriidae) from seven lake and river sites in the Waikato River system. Hydrobiologia 284, 205–217. doi: 10.1007/bf00006690
Shuman, J. R. (1995). Environmental considerations for assessing dam removal alternatives for river restoration. River Res. Appl. 11, 249–261. doi: 10.1007/s002679900111
Simpson, C. T. (1899). The pearly fresh-water mussels of the United States: Their habits, enemies, and diseases, with suggestions for their protection. Bull.U.S. Fish Commission 18, 279–288.
Thompson, F. G. (1999). An identification manual for the freshwater snails of Florida. Walkerana 10, 1–96.
Tomašových, A., and Kidwell, S. M. (2009). Fidelity of variation in species composition and diversity partitioning by death assemblages: time-averaging transfers diversity from beta to alpha levels. Paleobiology 35, 94–118. doi: 10.1666/08024.1
Tomašových, A., and Kidwell, S. M. (2010). Predicting the effects of increasing temporals scale on species composition, diversity, and rank-abundance distributions. Paleobiology 36, 672–695. doi: 10.1666/08092.1
Turner, R. E., and Rabalais, N. N. (1991). Changes in Mississippi River water quality this century. BioScience 41, 140–147. doi: 10.2307/1311453
Turner, R. E., Rabalais, N. N., Fry, B., Atilla, N., Milan, C. S., Lee, J. M., et al. (2006). Limnol. Oceanogr. 51, 518–533.
United States Fish and Wildlife Service. (2014). Vermiculated Sailfin Catfish (Pterygoplichthys disjunctivus) Ecological Risk Screening Summary. https://fws.gov/media/ecological-risk-screening-summary-vermiculated-sailfin-catfish-pterygoplichthys-disjunctivus (accessed June 18, 2021).
Valentine-Darby, P. L., Kell, S. E., and Darby, P. C. (2015). Predation on Florida apple snails (Pomacea paludosa) by native and non-native aquatic fauna, and predator-prey size relationships. Florida Sci. 78, 47–56.
Van Dyke, J. (2019). Controlling Hydrilla at Wakulla Springs State Park (1997-2007). http://wakullaspringsalliance.org/wp-content/uploads/2020/04/Jess-Van-Dyke.Wakulla-Herbicide-Treatment.2019.pdf (accessed June 18, 2021).
Vaughn, C. C. (2018). Ecosystem services provided by freshwater mussels. Hydrobiologia 810, 15–27. doi: 10.1007/s10750-017-3139-x
Vaughn, C. C., and Spooner, D. E. (2006). Scale-dependent associations between native freshwater mussels and invasive Corbicula. Hydrobiologia 568, 331–339. doi: 10.1007/s10750-006-0210-4
Wakulla Springs Alliance. (2021). Wakulla Springs and River Ecosystem Timeline. http://wakullaspringsalliance.org/information/wakulla-springs-and-river-ecosystem-timeline/ (accessed June 18, 2021).
Walsh, S. J. (2001). Freshwater macrofauna of Florida karst habitats. US Geological Survey Karst Interest Group Proceedings. Florida: St. Petersburg, 78–88.
Webb, S. D., and Dunbar, J. S. (2006). “Carbon dates,” in First Floridians and Last Mastodons: The Page-Ladson Site in the Aucilla River. Topics in Geobiology, 26, ed. S. D. Webb (Berlin: Springer), 333–341.
Weber, K. A., and Perry, R. G. (2006). Groundwater abstraction impacts on spring flow and base flow in the Hillsborough River Basin, Florida, U.S.A. Hydrogeol. J. 14, 1252–1264. doi: 10.1007/s10040-006-0040-5
White, W. R., and Crisman, T. L. (2014). Headwater streams of Florida: distribution and a framework for conservation. River Res. Appl. 32, 452–461.
Williams, J. D., Butler, R. S., Warren, G. L., and Johnson, N. A. (2014). Freshwater Mussels of Florida. Tuscaloosa: University of Alabama Press, 528.
Wingard, G. L., Murray, J. B., Schill, W. B., and Phillips, E. C. (2008). Red-Rimmed Melania (Melanoides tuberculatus)- A snail in Biscayne National Park, Florida- harmful invader or just a nuisance? USGS Fact Sheet 2008-3006. https://pubs.usgs.gov/fs/2008/3006/pdf/fs2008-3006.pdf (accessed June 18, 2021).
Wolverton, S., Randklev, C. R., and Kennedy, J. H. (2010). A conceptual model for freshwater mussel (family: Unionidae) remain preservation in zooarchaeological remains. J. Arch. Sci. 37, 164–173. doi: 10.1016/j.jas.2009.09.028
Keywords: mollusk, conservation paleobiology, fluvial, spring-fed, fossil, Quaternary, Florida
Citation: Kusnerik KM, Means GH, Portell RW, Kannai A, Monroe MM, Means R and Kowalewski M (2022) Long-Term Shifts in Faunal Composition of Freshwater Mollusks in Spring-Fed Rivers of Florida. Front. Ecol. Evol. 10:851499. doi: 10.3389/fevo.2022.851499
Received: 10 January 2022; Accepted: 25 March 2022;
Published: 03 May 2022.
Edited by:
G. Lynn Wingard, Florence Bascom Geoscience Center, United States Geological Survey (USGS), United StatesReviewed by:
Marden Seabra Linares, Federal University of Minas Gerais, BrazilKristina Barclay, University of Victoria, Canada
Copyright © 2022 Kusnerik, Means, Portell, Kannai, Monroe, Means and Kowalewski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristopher M. Kusnerik, S3VzbmVyaWtQYWxlb0xhYkBnbWFpbC5jb20=
 Kristopher M. Kusnerik
Kristopher M. Kusnerik Guy H. Means
Guy H. Means Roger W. Portell3
Roger W. Portell3 Michal Kowalewski
Michal Kowalewski